Note: Descriptions are shown in the official language in which they were submitted.
CA 02574972 2013-01-11
WO 20061015161
PC17US2005/026875
Title of the Invention
[001] Metallic Drug-Releasing Medical Devices and Method of Making Same
Cross-Reference to Related Applications
[0021 This application is related to U.S. Patent Publication No. 2004-007449
filed November 17, 2002, which is related to U.S. Patent No. 8,252,044
tiled November 17, 2000. This application is also related to prior co-pending
and commonly
assigned U.S. Patent No. 7,300,457 filed April 29, 2002 and U.S Patent No.
6,936,066 filed
April 29, 2002, both of which claim priority to U.S. Provisional Application
Serial No. 60/310,617 filed August 7, 2001, and to co-pending and commonly
assigned
U.S. Patent Publication No. 2004-0024449, filed October 17, 2002 which
corresponds to
PCT International Publication No. Vv'002060506A I published on August 8, 7007.
Backaround of the Invention
[003] The present invention relates generally to iraplantable metallic medical
devices capable of releasing pharinacologicatly active, agents. More
specifically, the present
invention relates to implantable drug-releasing medical devices, including,
for example,
surgical and endoliuninal vascular grafts, stents, stent-grafts, covered
Stents, skin grafts,
shunts, bone grafts, surcal patches, non-vascular conduits, valvular leaflets,
filters,
occlusion menthranes, sphincters, artificial tendons and ligaments, vascular
plugs, and
orthopedic and dental implants. More specifically, the present invention
relates to
implantable medic,a1 grafts fabricated of metallic or pseuclornetallic films
of biocompatible
-materials having a plurality of microperforations passing through the film
and having a
plurality of drug-releasing pockets defined within the fihn and positioned
between adjacent
pairs of microperforations. The plurality of microperforations impart both
compliance and a
-fabric-like quality to the metallic, or pseudometallic film of biocompatible
material and permit
geometric deformation of the film to permit, for example ciretunferential or
longitudinal
expansion of a tubular film.
[OM For purposes of this application both metallic and pseudometaltie
films of
biocompatible materials will be referred to collectively as "metal fihn" or
"metallic fihn,"
The inventive metal films may be fabricated by conventional wrought metal
processing
CA 02574972 2007-01-24
WO 2006/015161
PCT/US2005/026875
techniques, or may be made by nanofabrication techniques such as physical
vapor deposition
or chemical vapor deposition.
1005] For purposes of this application the terms "microperforation" or "micro-
opening" when used in either the singular or the plural are intended to mean
openings having
open surface area in the sub-millimeter to nanometer-scale openings.
[006] The metal film may have a generally tubular geometry, may have a
generally
planar geometry, or may be formed into complex geometric shapes. Those skilled
in the art
will understand, however, that regardless of the particular geometric shape of
the metal film,
the metal film has generally two opposing surfaces, hereinafter termed first
and second metal
film surfaces.
[007] The drug-releasing pockets are enclosed entirely within the metal film,
except
for at least one opening in the pocket of sufficient size to allow drug-
release therethrough by
diffusion, pumping or other active or passive means without being bound to or
contained by a
polymeric matrix. The drug-releasing pockets are bounded on all sides by the
metal film and
have a generally pillow-chamber-like or box-chamber-like geometry, with the at
least one
opening passing through the metal film at either or both of the first and
second surfaces of
the metal film.
[008] In addition to serving as boundaries for the drug-releasing pockets, the
plurality of microperforations may serve multiple purposes, including, for
example,
permitting geometric deformation of the film, imparting a fabric-like quality
to the film, and
imparting flexibility to the film. The term "fabric-like" is intended to mean
a quality of being
pliable and/or compliant in a manner similar to that found with natural or
synthetic woven
fabrics.
[009] The inventive implantable grafts are =fabricated entirely of self-
supporting
fihns made of biocompatible metals or biocompatible pseudometals. The metal
films may
either be single layer metal films or plural layer films. The terms "metal
film," "thin metallic
film" and "metal thin film" are used in this application synonymously to refer
to single or
plural layer films fabricated of biocompatible metals or biocompatible
pseudometals having
thicknesses greater than 0 mu and less than about 125 um. Heretofore in the
field of
implantable medical devices, it is unknown to fabricate an implantable medical
device that
comprises a graft at least as one of its elements, such as a covered stent or
stent-graft, entirely
of self-supporting metal or pseudometal materials. As used herein the term
"graft" is
-2-
CA 02574972 2007-01-24
WO 2006/015161
PCT/US2005/026875
intended to indicate any type of device or part of a device that comprises
essentially a
material delimited by two surfaces where the distance between said surfaces is
the thickness
of the graft and that exhibits integral dimensional strength and that has
microperforations that
pass through the thickness of the graft. The inventive grafts may be formed in
planar sheets,
toroids, and in other shapes as particular applications may warrant. However,
for purposes of
illustration only, the present application will refer to tubular grafts. For
purposes of this
application, the terms "pseudometal" and "pseudometallic" are intended to mean
a
biocompatible material which exhibits biological response and material
characteristics
substantially the same as biocompatible metals. Examples of pseudometallic
materials
include, for example, polymers, composite materials and ceramics. Composite
materials are
composed of a matrix material reinforced with any of a variety of fibers made
from ceramics,
metals, carbon, or polymers.
Iob7"When implanted into the body, metals are generally considered to have
superior biocompatibility than that exhibited by polymers used to fabricate
commercially
available polymeric grafts. It has been found that when prosthetic materials
are implanted,
integrin receptors on cell surfaces interact with the prosthetic surface. The
integrin receptors
are specific for certain ligands in vivo. If a specific protein is adsorbed on
a prosthetic
surface and the ligand exposed, cellular binding to the prosthetic surface may
occur by
integrin-ligand docking. It has also been observed that proteins bind to
metals in a more
permanent fashion than they do to polymers, thereby providing a more stable
adhesive
surface. The conformation of proteins coupled to surfaces of most medical
metals and alloys
appears to expose greater numbers of ligands and preferentially attract
endothelial cells
having surface integrin clusters to the metal or alloy surface relative to
leukocytes. Finally,
metals and metal alloys exhibit greater resistance to degradation of metals
relative to
polymers, thereby providing greater long-term structural integrity and stable
interface
conditions.
[pout Because of their relatively greater adhesive surface
profiles, metals are
also susceptible to short-term platelet activity and/or thrombogenicity. These
deleterious
properties may be offset by administration of pharmacologically active
antithrombogenic
agents in routine use today. Surface thrombogenicity usually disappears 1-3
weeks after
initial exposure. Antithrombotic coverage is routinely provided during this
period of time for
coronary stenting. In non-vascular applications such as musculoskeletal and
dental, metals
have also greater tissue compatibility than polymers because of similar
molecular
-3-
CA 02574972 2007-01-24
WO 2006/015161
PCT/US2005/026875
considerations. The best article to demonstrate the fact that all polymers are
inferior to metals
is van der Giessen, WJ. et al. Marked inflammatory sequelae to implantation of
biodegradable and non-biodegradable polymers in porcine coronary arteries,
Circulation,
1996:94(7):1690-7.
[0012] Normally, endothelial cells (EC) migrate and proliferate to cover
denuded
areas until confluence is achieved. Migration, quantitatively more important
than
proliferation, proceeds under normal blood flow roughly at a rate of 25 pnl/hr
or 2.5 times the
diameter of an EC, which is nominally 10iim. EC migrate by a rolling motion of
the cell
membrane, coordinated by a complex system of intracellular filaments attached
to clusters of
cell membrane integrin receptors, specifically focal contact points. The
integrins within the
focal contact sites are expressed according to complex signaling mechanisms
and eventually
couple to specific amino acid sequences in substrate adhesion molecules. An EC
has roughly
16-22% of its cell surface represented by integrin clusters. Davies, P.F.,
Robotewskyi A.,
Griem M.L. Endothelial cell adhesion in real time. J.Clin.Invest.1993; 91:2640-
2652, Davies,
P.F., Robotewski, A., Griem, M.L., Qualitiative studies of endothelial cell
adhesion,
J.Clin.Invest.1994; 93:2031-2038. This is a dynamic process, which implies
more than 50%
remodeling in 30 minutes. The focal adhesion contacts vary in size and
distribution, but 80%
of them measure less than 6 lim2, with the majority of them being about 1 gm2,
and tend to
elongate in the direction of flow and concentrate at leading edges of the
cell. Although the
process of recognition and signaling to determine specific attachment receptor
response to
attachment sites is incompletely understood, availability of attachment sites
will favorably
influence attachment and migration. It is known that materials commonly used
as medical
grafts, such as polymers, do not become covered with EC and therefore do not
heal after they
are placed in the arteries. It is therefore an object of this invention to
replace polymeric
implant materials with metallic film materials that have a greater healing
response, are more
hospitable for EC coverage and can heal completely. Furthermore,
heterogeneities in the
inventive metallic film materials that are in contact with blood flow are
preferably controlled
by exercising control over processing parameters employed during vacuum
deposition of
device-forming materials.
[0013] There have been numerous attempts to increase endothelialization of
implanted medical devices such as stents, including covering the stent with a
polymeric
material (U.S. Patent No. 5,897,911), imparting a diamond-like carbon coating
onto the stent
(U.S. Patent No. 5,725,573), covalently binding hydrophobic moieties to a
heparin molecule
-4-
CA 02574972 2007-01-24
WO 2006/015161
PCT/US2005/026875
(U.S. Patent No. 5,955,588), coating a stent with a layer of blue to black
zirconium oxide or
zirconium nitride (U.S. Patent No. 5,649,951), coating a stent with a layer of
turbostratic
carbon (U.S. Patent No. 5,387,247), coating the tissue-contacting surface of a
stent with a
thin layer of a Group VB metal (U.S. Patent No. 5,607,463), imparting a porous
coating of
titanium or of a titanium alloy, such as Ti-Nb-Zr alloy, onto the surface of a
stent (U.S. Patent
No. 5,690,670), coating the stent, under ultrasonic conditions, with a
synthetic or biological,
active or inactive agent, such as heparin, endothelium derived growth factor,
vascular growth
factors, silicone, polyurethane, or polytetrafiuoroethylene, U.S. Patent No.
5,891,507),
coating a stent with a silane compound with vinyl functionality, then forming
a graft polymer
by polymerization with the vinyl groups of the silane compound (U.S. Patent
No. 5,782,908),
grafting monomers, oligomers or polymers onto the surface of a stent using
infrared
radiation, microwave radiation or high voltage polymerization to impart the
property of the
monomer, oligomer or polymer to the stent (U.S. Patent No. 5,932,299).
However, all these
approaches do not address the lack of endothelialization of polymer grafts.
[0014] It is, therefore, desirable to fabricate the present invention of
metallic and/or
pseudometallic materials fabricated in such a manner as to control the
mechanical, physical
and chemical properties of the material. The inventive metal devices may be
fabricated of
pre-existing conventional wrought metallic materials, such as stainless steel
or nitinol
hypotubes, or may be fabricated by vacuum deposition techniques, such as
physical vapor
deposition or chemical vapor deposition. In accordance with the present
invention, it is
preferable to fabricate the inventive implantable devices by vacuum
deposition. Vacuum
deposition permits greater control over many material characteristics and
properties of the
resulting formed device. For example, vacuum deposition permits control over
grain size,
grain phase, grain material composition, bulk material composition, surface
topography,
mechanical properties, such as transition temperatures in the case of a shape
memory alloy.
Moreover, vacuum deposition processes will permit creation of devices with
greater material
purity without the introduction of large quantities of contaminants that
adversely affect the
material, mechanical or biological properties of the implanted device. Vacuum
deposition
techniques also lend themselves to fabrication of more complex devices than
those
susceptible of manufacture by conventional cold-working techniques. For
example, multi-
layer structures, complex geometrical configurations, extremely fine control
over material
tolerances, such as thickness or surface uniformity, are all advantages of
vacuum deposition
processing.
-5-
CA 02574972 2007-01-24
WO 2006/015161
PCT/US2005/026875
[0015] In vacuum deposition technologies, materials are formed directly in the
desired geometry, e.g., planar, tubular, etc. The common principle of vacuum
deposition
processes is to take a material in a minimally processed form, such as pellets
or thick foils,
known as the source material and atomize them. Atomization may be carried out
using heat,
as is the case in physical vapor deposition, or using the effect of
collisional processes, as in
the case of sputter deposition, for example. In some forms of deposition, a
process, such as
laser ablation, which creates microparticles that typically consist of one or
more atoms, may
replace atomization; the number of atoms per particle may be in the thousands
or more. The
atoms or particles of the source material are then deposited on a substrate or
mandrel to
directly form the desired object. In other deposition methodologies, chemical
reactions
between ambient gas introduced into the vacuum chamber, i.e., the gas source,
and the
deposited atoms and/or particles are part of the deposition process. The
deposited material
includes compound species that are formed due to the reaction of the solid
source and the gas
source, such as in the case of chemical vapor deposition. In most cases, the
deposited
material is then either partially or completely removed from the substrate, to
form the desired
product.
[0016] A first advantage of vacuum deposition processing is that vacuum
deposition
of the metallic and/or pseudometallic films permits tight process control and
films may be
deposited that have regular, homogeneous atomic and molecular pattern of
distribution along
their fluid-contacting surfaces. Different process parameters employed in
vacuum deposition
processing may be controlled to fabricate achieve metallic and/or
pseudometallic films with
controlled material properties, atomic and molecular constitution and
controlled surface
heterogeneities. Process parameters which may be controlled in exercising
control over the
properties of the resulting deposited film include, for example, target
composition, shape and
construction, target and/or substrate temperature, rate of deposition, shape
and construction
of the magnetron, shape and strength of the magnetic field, the strength of
the applied
electrical field, the partial pressure of gases during deposition, the chamber
pressure, the
substrate composition and/or topography, or vacuum chamber configuration.
Vacuum
deposition of device-forming fihns avoids the marked variations in surface
composition,
creating predictable oxidation and organic adsorption patterns and has
predictable
interactions with water, electrolytes, proteins and cells. Particularly, EC
migration is
supported by a homogeneous distribution of binding domains that serve as
natural or
implanted cell attachment sites, in order to promote unimpeded migration and
attachment.
-6-
CA 02574972 2007-01-24
WO 2006/015161
PCT/US2005/026875
[0017] Secondly, in addition to materials and devices that are made of a
single metal
or metal alloy, henceforth termed a layer, the inventive grafts may be
comprised of a layer of
biocompatible material or of a plurality of layers of biocompatible materials
formed upon one
another into a self-supporting multilayer structure because multilayer
structures are generally
known to increase the mechanical strength of sheet materials, or to provide
special qualities
by including layers that have special properties such as superelasticity,
shape memory, radio-
opacity, corrosion resistance etc. A special advantage of vacuum deposition
technologies is
that it is possible to deposit layered materials and thus films possessing
exceptional qualities
may be produced (cf., H. Holleck, V. Schier: Multilayer PVD coatings for wear
protection,
Surface and Coatings Technology, Vol. 76-77 (1995) pp. 328-336). Layered
materials, such
as superstructures or multilayers, are commonly deposited to take advantage of
some
chemical, electronic, or optical property of the material as a coating; a
common example is an
antireflective coating on an optical lens. Multilayers are also used in the
field of thin film
fabrication to increase the mechanical properties of the thin film,
specifically hardness and
toughness.
[0018] Thirdly, the design possibilities for possible configurations and
applications of
the inventive graft are greatly enhanced by employing nanofabrication
methodologies.
Vacuum deposition is an additive technique that lends itself toward
fabrication of
substantially uniformly thin materials with potentially complex three
dimensional geometries
and structures that cannot be cost-effectively achieved, or in some cases
achieved at all, by
employing conventional wrought fabrication techniques. Additionally,
subtractive processes,
such as photolithography, etching, including, without limitation, chemical
etching and laser
etching or electrical discharge machining (EDM) may be employed to selectively
remove
materials from a pre-existing film and create very small scale, e.g., 10-8 to
10-10 features in the
film.
[0019] Conversely, conventional wrought metal fabrication techniques may
entail
smelting, hot working, cold working, heat treatment, high temperature
annealing,
precipitation annealing, grinding, ablation, wet etching, dry etching, cutting
and welding. All
of these processing steps have disadvantages including contamination, material
property
degradation, ultimate achievable configurations, dimensions and tolerances,
biocompatibility
and cost. For example conventional wrought processes are not suitable for
fabricating tubes
having diameters greater than about 20mm diameter, nor are such processes
suitable for
fabricating materials having wall thicknesses down to about 5 gm with sub-gm
tolerances.
-7-
CA 02574972 2007-01-24
WO 2006/015161
PCT/US2005/026875
[0020] While the inventive metal or pseudometal drug-releasing graft may be
fabricated of conventionally fabricated wrought materials, in accordance with
the best mode
contemplated for the present invention, the inventive drug-releasing graft is
preferably
fabricated by vacuum deposition techniques. By vacuum depositing the metal
and/or
pseudometallic film as the precursor material for the inventive drug-releasing
graft, it is
possible to more stringently control the material, biocompatibility and
mechanical properties
of the resulting film material and graft than is possible with conventionally
fabricated graft-
forming materials. The inventive self-supporting graft may be used alone, i.e.
, the whole
implantable device may be made of a single graft, or it may be a part of a
structure where the
graft is used in conjunction either with other grafts, or in conjunction with
other structural
elements, such as scaffolds, stents, and other devices. The term "in
conjunction" may mean
actual connection, such as that made by welding, fusing, or other joining
methods, as well as
being made from the same piece of material by forming some area of the piece
into a graft
and some other area of the piece into another member or part of the device.
Summary of the Invention
[0021] In accordance with a preferred embodiment of the invention, there is
provided
a self-supporting graft member having a wall thickness between about lmu to
about 75 MIA,
a plurality of microperforations passing through the wall thickness of the
graft and a plurality
of enclosed pockets positioned between adjacent pairs of microperforations and
formed
within the wall thickness of the graft or formed upon a surface of the graft
and having a
plurality of drug-releasing openings communicating between an enclosed chamber
within
each enclosed pocket and external the enclosed pocket. The graft member may
assume
virtually any geometric configuration, including sheets, tubes or rings, but
preferably is
provided as a generally tubular configuration. The plurality of
microperforations serve to
impart geometric compliance to the graft, geometric distendability to the
graft and/or limit or
permit the passage of body fluids or biological matter through the graft, such
as facilitating
transmural endothelialization while preventing fluid flow through the wall of
the graft under
normal physiological conditions. The plurality of microperforations also
impart a fabric-like
quality to the graft by imparting pliability and/or elastic, plastic or
superelastic compliance to
the graft, such as that required for longitudinal flexibility in the case of a
vascular graft.
-8-
CA 02574972 2007-01-24
WO 2006/015161
PCT/US2005/026875
[0022] In accordance with a preferred embodiment of the invention, the drug-
releasing pockets are positioned intermediate adjacent pairs of
microperforations and reside
entirely with the wall thickness of the graft material and are bounded
entirely by the graft
material, with at least one opening communicating between an internal chamber
within the
drug-releasing pocket and external the graft.
[0023] In accordance with an alternate embodiment of the invention, the drug-
releasing pockets are positioned on either a first or second surface of the
graft and the drug-
releasing pockets bounded on at least one surface, but not entirely, by the
graft material, with
at least one opening communicating between a chamber within the drug-releasing
pocket and
external the graft.
[0024] In a first embodiment, the graft may be made from plastically
deformable
materials such that upon application of a force, the microperforations
geometrically deform to
impart permanent enlargement of one or more axes of the graft, such as length
in the case of a
planar graft, e.g., a surgical patch graft, or diameter, such as in the case
of a tubular graft,
e.g., a vascular graft. In a second embodiment, the graft may be fabricated of
elastic or
superelastic materials. Elastic and/or superelastic materials will permit the
microperforations
to geometrically deform under an applied force in a manner that allows for a
recoverable
change in one or more axes of the graft.
[0025] The applied force may also be utilized to deform openings in the
implantable
material which communicate with drug-releasing chambers in the implantable
material and,
thereby, release the drug from the implantable material. For example, a
balloon mounted on
a catheter may be employed as the source of the applied force to the
implantable material.
[0026] The positioning and conformation of the drug-releasing pockets may be
controlled to enable the force applied from the geometric deformation of the
plurality of
microperforations to transfer to the drug-releasing pockets, thereby serving
to apply a
pumping-like force to release a metered dose of the agent within the drug-
releasing pockets.
Alternatively, the pockets may be isolated from the strain resulting from the
geometric
deformation of the plurality of microperforations to prevent release of the
agent during
geometric deformation of the drug-releasing graft.
[0027] It is desirable, in accordance with the preferred embodiments of the
invention,
to position the drug-releasing pockets in an even distribution about
circumferential and
longitudinal axes of the device so as to provide substantially uniform
coverage and release
-9-
CA 02574972 2007-01-24
WO 2006/015161
PCT/US2005/026875
about all axes of the device. However, in accordance with alternative
embodiments of the
invention, the drug-releasing pockets may be positioned such that different
longitudinal or
circumferential regions of the device have higher or lower densities and
distribution of the
drug-releasing pockets. By varying the position, density, size and
distribution of the drug-
releasing pockets relative to position along the axes of the device, drug
dosage may be
controlled as a function of position of the drug-releasing pockets.
[0028] With current polymer-coated drug-releasing stents, the drug releases
substantially uniformly about the circumference of the polymer, and along the
length of the
device. There is, however, a significant reduction in released drug
concentration at the
proximal and distal ends of the device where there is little polymer surface
area from which
the drug may release. In comparison, with the present invention, the positions
of the drug-
releasing pockets may be selected during device design and fabrication to
directionally
release drug, i.e., luminally, abluminally, at selected positions along either
the longitudinal or
circumferential axis of the device or from either or both of the proximal and
distal ends of the
device.
[0029] In each of the first and second embodiments of the invention, the graft
may be
fabricated in such a manner as to have fabric-like qualities by controlling
the film thickness,
material properties and geometry of the plurality of microperforations.
Furthermore, in such
cases where minimally invasive delivery is required, such as for endoluminal
delivery of
vascular grafts, the first and second embodiments allow for delivery using
balloon expansion
and self-expansion, respectively, or a combination of both. Minimally invasive
delivery may
also be accomplished by folding the graft for delivery similar to the manner
in which an
angioplasty balloon is creased and fluted or folded. The graft may be
delivered by unfolding
the device in vivo either by assistance such as by using a balloon, or by the
graft material's
plastic, elastic or superelastic properties or by a combination thereof. The
plurality of
microperforations may be patterned in such a manner as to allow for additional
dimensional
enlargement of the drug-releasing graft member in vivo by elastic or plastic
deformation such
as a radially expansive positive pressure.
[0030] For some applications it is preferable that the size of each of the
plurality of
microperforations be such as to permit cellular migration through each
opening, without
permitting fluid flow there through. In this manner, for example, blood cannot
flow through
the plurality of microperforations (in their deformed or un-deformed state),
but various cells
or proteins may freely pass through the plurality of microperforations to
promote graft
-10-
CA 02574972 2007-01-24
WO 2006/015161
PCT/US2005/026875
healing in vivo. For other applications, moderate amounts of fluid flow
through the plurality
of deformed or un-defonned microperforations may be acceptable. For example,
endoluminal
saphenous vein grafts may be fabricated with microperforations that serve the
dual function
of permitting transmural endothelialization while also excluding biological
debris, such as
thrombus from passing through the wall thickness of the graft, effectively
excluding
detrimental matter from entering the circulation. In this example, each of the
plurality of
microperforations in either their deformed or undeformed state, may exceed
several hundred
microns.
[0031] Those skilled in the art will understand that a direct relationship
exists
between the size of pores and the overall ratio of expansion or deformability
of an
implantable graft. Generally, therefore, it is appreciated that pore sizes
must increase in order
to increase the effective attainable degree of expansion or deformation of the
graft.
[0032] For applications where large deformation and small pore size are both
requirements, in accordance with another aspect of the inventive graft
embodiment, it is
contemplated that two or more graft members are employed such as diametrically
concentric
grafts for tubular configurations. The two or more graft members have a
pattern of a plurality
of microperforations passing there through, with the plurality of patterned
microperforations
being positioned out of phase relative to one another such as to create a
tortuous cellular
migration pathway through the wall of the concentrically engaged first and
second graft
members as well as a smaller effective pore size. The two or more graft
members may each
be drug-releasing grafts or a combination of drug-releasing and non-drug-
releasing grafts.
For example, a lumenal graft only may be drug-releasing to release a
pharmacologically
active agent into the blood-stream, while a concentrically positioned non-
releasing graft may
be an ablumenal graft, alternatively, the relative position of the lumenal and
ablumenal grafts
may be switched such that the ablumenal graft is drug-releasing and the
lumenal graft is non-
releasing.
[0033] In order to facilitate cellular migration through and healing of the
first and
second graft members in vivo, it may be preferable to provide additional
cellular migration
pathways that communicate between the plurality of microperforations in the
first and second
graft members. These additional cellular migration pathways, if necessary, may
be imparted
as 1) a plurality of projections formed on either the lutninal surface of the
second graft or the
abluminal surface of the first graft, or both, which serve as spacers and act
to maintain an
annular opening between the first and second graft members that permits
cellular migration
-11-
CA 02574972 2007-01-24
WO 2006/015161
PCT/US2005/026875
and cellular communication between the plurality of microperforations in the
first and second
graft members, 2) a plurality of microgrooves, which may be random, radial,
helical, or
longitudinal relative to the longitudinal axis of the first and second graft
members, the
plurality of microgrooves being of a sufficient size to permit cellular
migration and
propagation along the groove, the microgrooves serve as cellular migration
conduits between
the plurality of microperforations in the first and second graft members, or
3) where the
microperforations are designed to impart an out of plane motion of the graft
material upon
deformation, thereby keeping a well defined space between the planes
originally defining the
facing surfaces of the grafts.
[0034] Each of the drug-releasing graft or the non-releasing graft members may
be
formed as a monolayer film, or may be formed from a plurality of film layers
formed one
upon another. The particular material used to form each layer of biocompatible
metal and/or
pseudometal is chosen for its biocompatibility, corrosion-fatigue resistance
and mechanical
properties, i.e., tensile strength, yield strength. The metals include,
without limitation, the
following: titanium, vanadium, aluminum, nickel, tantalum, zirconium,
chromium, silver,
gold, silicon, magnesium, niobium, scandium, platinum, cobalt, palladium,
manganese,
molybdenum and alloys thereof, such as zirconium-titanium-tantalum alloys,
nitinol, and
stainless steel. Additionally, each layer of material used to form the graft
may be doped with
another material for purposes of improving properties of the material, such as
radiopacity or
radioactivity, by doping with tantalum, gold, or radioactive isotopes.
Alternatively,
pseudometallic materials may include polymers, carbon-fiber or ceramics.
Brief Description of the Drawings
[0035] Figure 1 is a perspective view of the inventive drug-releasing graft.
[0036] Figure 2 is a fragmentary cross-sectional view taken along line 2-2 of
Figure
1.
[0037] Figure 3 is a fragmentary plan view of an embodiment of the inventive
drug-
releasing graft depicting a pattern of chambers and openings in the graft.
[0038] Figure 4A is a fragmentary plan view of an alternative embodiment of
the
inventive drug-releasing graft depicting a pattern of chambers and openings in
the graft.
[0039] Figure 4B is a cross-sectional view taken along line 4B-4B of Figure
4A.
-12-
CA 02574972 2007-01-24
WO 2006/015161
PCT/US2005/026875
[0040] Figure 5 is a fragmentary cross-sectional view taken along line 5-5 of
Figure
4A.
[0041] Figure 6 is a fragmentary cross-sectional view taken along line 6-6 of
Figure
3.
[0042] Figure 7 is a fragmentary cross-sectional view of an alternative
embodiment of
the present invention.
[0043] Figure 8 is a process flow diagram illustrating methods of making the
inventive implantable drug-releasing medical device.
Detailed Description of the Preferred Embodiments
[0044] With the foregoing as background, we turn now to a description of the
present
invention with reference the preferred embodiments thereof and with reference
to the
accompanying figures. As noted above, the inventive microporous metallic
implantable
devices may assume a wide number of geometric configurations, including, for
example,
planar sheets, tubes, toroids or other geometric configurations. For ease of
reference,
however, the accompanying figures and the following description of the
invention will refer
to tubular implantable graft members. Those skilled in the art, however, will
understand that
this is merely an exemplary geometric configuration and is not intended to
limit the scope of
the invention to tubular members or be limited in application to graft
members.
[0045] With particular reference to Figures 1-2, the inventive implantable
medical
device 10 is illustrated as a graft. It will be understood that the device 10
is described herein
only as a non-limiting example of the inventive implantable medical device,
and that the
inventive implantable medical may assume other geometries, and be used for
other
applications or indications. Device 10 consists generally of a body member 12
comprising a
coherent metal or pseudometallic material and having a first surface 14 and a
second surface
16 and a thickness 18 intermediate the first surface 14 and the second surface
16. A plurality
of microperforations 20 is provided that pass through at least one of the
first suiface 14, the
second surface 16 or the thickness 18 of the body member 12 with
interperforation regions 22
of the body member 12 being located between adjacent pairs of
microperforations 20. The
plurality of microperforations 20 each may have a geometric configuration that
is susceptible
of geometric change under the application or release of an externally applied
load, or upon a
phase change in the material, such as a shape memory or superelastic change.
Alternatively,
-13-
CA 02574972 2007-01-24
WO 2006/015161
PCT/US2005/026875
the plurality of microperforations 20 may have a pattern and geometric
configuration that
imparts a fabric-like quality and compliance to the material of device 10. A
plurality of drug-
releasing chambers 15 are provided in the interperforation regions 22
intermediate adjacent
pairs of microperforations 20 and retain a pharmacologically active agent 24
for release
through at least one microperforation 20 in fluid flow communication with the
drug-releasing
chambers 15.
[0046] Each of the plurality of microperforations 20 in the undeformed state
preferably has an open surface area less than about 2 II1M2, with the total
open surface area of
the graft in the undeformed state being between 0.001 to 99%. The open surface
area of the
plurality of microperforations and the open surface area of the graft may
change considerably
upon deformation of the plurality of microperforations 20. Both the size of
the
microperforations 20 in the deformed and undefomied state and the total open
area of the
body member 12 in the deformed and undeformed state may be selected in view of
the
following non-exclusive factors based on the graft application: 1) the desired
compliance of
the device 10, 2) the desired strength of the device 10, 3) desired stiffness
of the device 10, 4)
the desired degree of geometric enlargement of the microperforations 20 upon
deformation,
5) in some cases, such as with vascular grafts, the desired delivery profile
and post delivery
profile, and 6) the drug release profile for delivering the pharmacologically
active agent from
the drug-releasing pockets.
[0047] In accordance with a preferred embodiment of the present invention, the
plurality of microperforations 20 is patterned in such a manner as to define
regions of the
body member which permit, but do not require deformation of the device 10. The
thickness
18 is between 0.1Ixm and 75p.m, preferably between lpm and 50gm. When
fabricated within
these thickness ranges, the device 10 has a thickness 18 which is thinner than
the wall
thickness of conventional non-metallic implantable grafts and that of
conventional metal
endoluminal stents.
[0048] The plurality of microperforations 20 is patterned in a regular array
forming a
regular array of microperforations 20 in both the longitudinal and
circumferential axes of the
body member 12. For purposes of reference, the pattern of microperforations 20
will,
hereinafter, be described with reference to a planar X-Y axes, which in a
tubular member will
correspond to the longitudinal or circumferential axes of the tubular member.
Those of
ordinary skill in the art will understand that reference to X-axis or Y-axis
when applied to a
tubular member may be used such that the term "X-axis" may correspond to
either the
-14-
CA 02574972 2007-01-24
WO 2006/015161
PCT/US2005/026875
longitudinal axis or circumferential direction of the tubular member and the
term "Y-axis"
may refer to the corresponding circumferential direction or longitudinal axis
or the tubular
member.
[0049] It will be appreciated by those of ordinary skill in the art that
individual
different geometric patterns may have associated intended uses, function or
mechanical
requirements of a particular device. Thus, the particular intended use of the
inventive device
will be a consideration in the selection of the particular geometric pattern
for the plurality
of microperforations 20. For example, where the inventive device 10 has an
intended use as a
free-standing implantable endoluminal vascular graft, a large circumferential
expansion ratio
10 and longitudinal flexibility may be desirable. Thus, a particular
geometry of the plurality of
microperforations 20 that offers these properties will be selected. The
plurality of
microperforations 20 also affect the material properties of the inventive
device 10. For
example, the geometry each microperforation 20 may be altered so that each
microperforation 20 exhibits stress-strain relief capabilities or the
microperforations 20 may
control whether geometric deformation of the microperforations 20 are plastic,
elastic or
superelastic deformation. Thus, both the geometry of the individual
microperforations 20,
the orientation of the microperforations 20 relative to the X-Y axis of the
device 10 and the
pattern of the microperforations 20 may be selected to directly impart, affect
or control the
mechanical and material properties of the device 10.
[0050] The plurality of drug-releasing chambers 15 may reside entirely within
the
thickness 18 of the body member 12, may reside entirely without the thickness
18 of the body
member 12 and be proximate either the first surface 14 or the second surface
16, or both, of
the body member 12, or be defined by a recess in at least one of the first
surface 14 and the
second surface 16 and enclosed therebetween. One skilled in the art will
understand that the
first surface 14 and the second surface 16 may be opposing surfaces of a
single member, or
may be surfaces of plural members positioned adjacent one another as is
illustrated by
phantom line 37 in Figure 5.
[0051] Suitable pharmacologically active agents include, without limitation,
paclitaxel, taxol, rapamycin, rapamycin derivatives, such as those disclosed
in U.S. Patent
Application Publication 2003/0170287 published September 11, 2003, sirolimus,
rapamune,
tacrolimus, dexamethasone, everolimus, ABT-578 (a rapamycin analogue that
inhibits the
mTOR cell cycle regulatory protein), and growth factors, such as VEG-F. The
pharmacologically active agents may be loaded into the plurality of drug-
releasing chambers
-15-
CA 02574972 2007-01-24
WO 2006/015161
PCT/US2005/026875
15 by employing a pharmacologically acceptable carrier. The term
"pharmacologically active
agents" as used herein is used synonymously with "drug(s)".
[0052] The pharmacologically active agents may be incorporated into or affixed
to
the device 10 in a number of ways and utilizing any biocompatible materials;
it may be
incorporated into e.g. a polymer or a polymeric matrix and sprayed onto the
device. A
mixture of the pharmacologically active agents and the polymeric material may
be prepared
in a solvent or a mixture of solvents and applied to the device 10 also by dip-
coating, brush
coating and/or dip/spin coating, the solvent component being allowed to
evaporate to leave a
film with entrapped drugs. In the case of stents where the drug(s) is
delivered from
micropores, struts or channels, a solution of a polymer may additionally be
applied as an
outlayer to control the drug(s) release; alternatively, the active agent may
be comprised in the
micropores, struts, channels or internal chambers and the active co-agent may
be incorporated
in the outlayer, or vice versa. The active agent may also be affixed in an
inner layer of the
stent and the active co-agent in an outer layer, or vice versa. The drug(s)
may also be attached
by a covalent bond, e.g. esters, amides or anhydrides, to the stent surface,
involving chemical
derivatization. The drug(s) may also be incorporated into a biocompatible
porous ceramic
coating, e.g. a nanoporous ceramic coating. The medical device of the
invention is configured
to release the active co-agent concurrent with or subsequent to the release of
the active agent.
[0053] Examples of polymeric materials include hydrophilic, hydrophobic or
biocompatible biodegradable materials, e.g. polycarboxylic acids; cellulosic
polymers; starch;
collagen; hyaluronic acid; gelatin; lactone-based polyesters or copolyesters,
e.g. polylactide;
polyglycolide; polylactide-glycolide; polycaprolactone; polycaprolactone-
glycolide;
poly(hydroxybutyrate); poly(hydroxyvalerate); polyhydroxy(butyrate-co-
valerate);
polyglycolide-co-trimethylene carbonate; poly(diaxanone); polyorthoesters;
polyanhydrides;
polyaminoacids; polysaccharides; polyphospoeters; polyphosphoester-uretha- ne;
polycyanoacrylates; polyphosphazenes; poly(ether-ester) copolymers, e.g. PEO-
PLLA, fibrin;
fibrinogen; or mixtures thereof; and biocompatible non-degrading materials,
e.g.
polyurethane; polyolefms; polyesters; polyamides; polycaprolactame; polyimide;
polyvinyl
chloride; polyvinyl methyl ether; polyvinyl alcohol or vinyl alcohol/olefin
copolymers, e.g.
vinyl alcohol/ethylene copolymers; polyacrylonitrile; polystyrene copolymers
of vinyl
monomers with olefms, e.g. styrene acrylonitrile copolymers, ethylene methyl
methacrylate
copolymers; polydimethylsiloxane; poly(ethylene-vinylacetate); acrylate based
polymers or
coplymers, e.g. polybutylmethacrylate, poly(hydroxyethyl methylmethacrylate);
polyvinyl
-16-
CA 02574972 2007-01-24
WO 2006/015161
PCT/US2005/026875
pyrrolidinone; fluorinated polymers such as polytetrafluoethylene; cellulose
esters e.g.
cellulose acetate, cellulose nitrate or cellulose propionate; or mixtures
thereof.
[0054] When a polymeric matrix is used, it may comprise 2 layers, e.g. a base
layer in
which the drug(s) is/are incorporated, e.g. ethylene-co-vinylacetate and
polybutylmethacrylate, and a top coat, e.g. polybutylmethacrylate, which is
drug(s)-free and
acts as a diffusion-control of the drug(s). Alternatively, the active agent
may be comprised in
the base layer and the active co-agent may be incorporated in the outlayer, or
vice versa.
[0055] The plurality of drug-releasing chambers 15 and the associated
plurality of
microperforations 20 may be similarly dimensioned and positioned generally
uniformly along
both the longitudinal axis and the circumferential axis of the device 10 to
achieve a generally
uniform drug delivery profile from the device 10. Alternatively, the drug-
releasing chambers
and the associated plurality of microperforations 20 may be sized and
positioned
differentially about the circumferential and longitudinal axes of the device
10 to provide
different dosage vs. position relationships or dosage vs. time relationships.
Moreover, at least
15 some of the plurality of microperforations 20 may be positioned on
either or both of the
luminal and abluminal surfaces of the device in order to release the drug from
the outer
circumference of the device 10 and/or from the inner circumference of the
deice 10. Drug
concentration releasing over time may be regulated by providing differentially
dimensioned
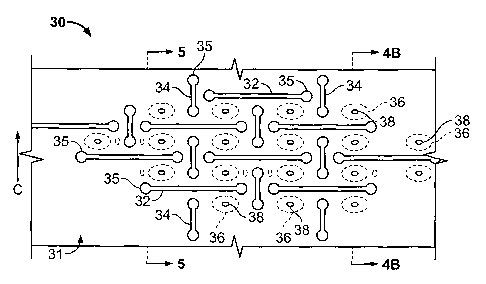
rnicroperforations 20 which permit different release rates for the drug and
may be controlled
to achieve desired concentration plateaus over an extended period of time.
[0056] Additionally, conventional polymer-coated drug-releasing stents have
release
profiles which release drug about the entire circumferential axis of the
coating, with a
significant drop off of concentration from the proximal and distal ends of the
device. This
concentration drop off is often associated with end restenosis known in the
art as the "candy-
wrapper effect." By permitting wide variability in positioning of the drug-
releasing chambers
15 and the associated microperforations 20, the directional orientation,
position and
concentration of drug release may be controlled in the device 10 of the
present invention.
[0057] Exemplary, non-limiting, geometric patterns for the plurality of
microperforations 20 in Figures 1-2 are shown in Figure 4A. Figures 4A and 4B
depict an
implantable drug-releasing device 30 having a longitudinal axis L and a
circumferential axis
C, denominated by directional arrows L and C, respectively in Figure 4A, with
Figure 4B
being a transverse cross-sectional view taken along the circumferential axis C
of device 30
-17-
CA 02574972 2013-01-11
WO 2006/415161
PCIMS20051016875
- depicted in Figure 4A. A plurality of first elongate micropeiforations 32
having a common
orientation parallel to the longitudinal axis L of the device 30 are provided
and pass through
the thickness of the device 30 and the first =liar second surfaces 31, thereof
Circumferentially adjacent pairs of first elongate microperforations 32 are
longitudinally
offset such that the terminus of eaCh first elongate microperfiaration 32
resides
circumferentially adjacent an intermediate region of the adjacent first
elongate
rnicroperforation 32.
[0058) A plurality of second elongate microperforations 34 are provided that
have a
common orientation parallel to the circumferential axis C of the device 30 and
pass through
the thickness of the device 30 and the first or second surfaces 31, thereof A
single second
elongate microperforation 34 is positioned intermediate adjacent pairs of the
first elongate
microperforations 32, with each terminus of the second elongate
naieroperforations 32
residing proximate an intermediate region of a circiunferentially adjacent
first elongate
microperforation 32.
[0059j Each of the first and second generally elongate microperforations 32,
34
preferably have terminal fillets 35 on each opposing ends of each
elongate.rnicroperforation
32, 34. The terminal fillets 35 serve a strain relief function that aids in
strain distribution
through the interperforation regions 22 between adjacent slots 32 and 34.
[04)601 Those skilled in the art will understand that each of the
microperforations 20
may have any of a wide variety of geometric configuration, dimension and
surface area, and
that the elongate slot configuration of the exemplary configuration in Figures
4A and 4B are
for illustration purposes only. Alternative microperforation 20
geornetries.are disclosed, for
example, in commonly assigned co-pending U.S. Patent Nos. 7,300,457 and
6,936,066,
both filed September 26, 2002 and U.S. Patent No. 7,704,274, filed
September 26, 2003, both entitled "Implantable Graft and Methods of Making
Same", both of
which are commonly assigned with the present application and illustrate a wide
variety
of suitable microperforation 20 geoinetries.
[0061j Of particular significance to the present invention is the provision of
a
plurality of dmg-releasing chambers 36. In accordance with one embodiment of
the
invention, as illustrated in Figures 4A and 5, the plurality of drug-releasing
chambers 36 may
reside entirely within the thickness of the device 30, intermediate the first
31 and second 33
-1S-
CA 02574972 2007-01-24
WO 2006/015161
PCT/US2005/026875
surfaces of the device 30. Alternatively, as illustrated in Figures 3 and 6,
the drag releasing
chambers 36 may reside adjacent either the first 21 or second 23 surfaces of
the device 10,
and be bounded by a second layer of device material 46 which acts as an
enclosing cap for
the drug releasing chamber 36. The plurality of openings 20 preferably pass
through the
second layer of device material 46 and communicate with the dru.g releasing
chamber 36 to
permit release of the drug therefrom. Finally, in accordance with a third
embodiment depicted
in Figure 7, the drag-releasing chambers may be formed as recesses 56 in a
first layer of a
device material 52, which is then covered by a second layer of a device
material 54 having a
plurality of openings 58 passing therethrough patterned such that at least one
opening 58
positionally corresponds to one of the recesses 56.
[0062] Thus, one embodiment of the present invention provides a new metallic
and/or
pseudometallic implantable graft that is biocompatible, geometrically
changeable either by
folding and unfolding or by application of a plastically, elastically or
superelastically
deforming force, and capable of endoluminal delivery with a suitably small
delivery profile.
Suitable metal materials to fabricate the inventive graft are chosen for their
biocompatibility,
mechanical properties, i.e., tensile strength, yield strength, and their ease
of fabrication. The
compliant nature of the inventive graft material may be employed to form the
graft into
complex shapes by deforming the inventive graft over a mandrel or fixture of
the appropriate
design. Plastic deformation and shape setting heat treatments may be employed
to ensure the
inventive implantable members 10 retain a desired conformation.
[0063] According to a first preferred method of making the graft of the
present
invention, the drug-releasing device is fabricated of at least two vacuum
deposited metallic
and/or pseudometallic films into which the plurality of microperforations are
formed. The at
least two films are conjoined in such a manner as to form a pattern of
internal chambers in an
interfacial region between the two films. The plurality of release openings
are then formed
through one or both of the at least two films and in communication with the
pattern of
internal chambers. Finally, a pharmacologically active agent is loaded through
the release
openings.
[0064] In accordance with a second preferred method of making the device of
the
present invention, a first layer of device-forming material is vacuum
deposited, a pattern of a
sacrificial material is then imparted onto a surface of the first layer of
device-forming
material, then a second layer of device-forming material is vacuum deposited
onto the first
layer and the sacrificial material. The plurality of microperforations is
formed through the
-19-
CA 02574972 2007-01-24
WO 2006/015161
PCT/US2005/026875
first and second layers of device-forming material between regions where the
sacrificial
material is present. A plurality of releasing-openings is then formed through
at least one of
the first and second layer of device-forming material and in communication
with the regions
of the sacrificial material. The sacrificial material is then removed, such as
by chemical
etching specific for the sacrificial material, through the release openings.
The
pharmacologically active agent may then be loaded through the releasing
openings.
[0065] The methods 50 of making the inventive drag-releasing device of the
present
invention are illustrated in Figure 8. A first material blank of either a
conventionally
fabricated or of a vacuum deposited biocompatible metal or pseudometallic
material is
provided at step 52. If a sacrificial material is to be employed to form the
drug-releasing
pockets of the device, it is deposited in a pattern corresponding to the
positions of the drug-
releasing pockets onto the first material blank at step 56, then a second
material blank is
provided at step 56 and conjoined at step 58 to the first material blank from
step 52. Methods
of depositing patterns of material onto another material surface are well-
known in the art of
semiconductor processing and may be accomplished by, for example,
photolithography. If
the drug-releasing pockets are not being formed by employing a sacrificial
material at step
56, then the first material blank from step 52 and the second material blank
from step 56 are
conjoined at step 58 without the intervening sacrificial material. The
conjoining step 58 may
be accomplished in at least one of two manners. First, the first material
blank from step 52
may be conjoined to the second material blank from step 54 by juxtaposing the
first material
blank and the second material blank and creating a pattern of welds 37 in the
interperforation
regions 22 that defme boundaries for the drug-releasing chambers 36 (See, e.g,
Fig. 4B and
Fig. 5). The pattern of welds 37 may be formed by spot welding, laser welding,
ultrasonic
welding, chemical adhesion or such other suitable methods of joining two
similar or
dissimilar biocompatible metals or pseudometals.
[0066] A second method for conjoining the first material blank from step 52
with the
second material blank from step 54 is to vacuum deposit the second material
blank onto the
first material blank. In this second method, it is desirable to employ the
pattern of sacrificial
material from step 56 since the second material blank will conform to the
topography of the
first material blank and it will not be technically feasible under currently
known fabrication
techniques to create the drug-releasing chambers between the deposited layers
of the first
material blank and the second material blank without subsequent removal of
portions of the
first and second material blanks. In this manner, the second material blank
from step 54 will
-20-
CA 02574972 2007-01-24
WO 2006/015161
PCT/US2005/026875
be formed on the first material blank from step 52 and over the sacrificial
material from step
56, and will conform to the topography of the first material blank and the
sacrificial material.
[0067] Under the first method, that being to join pre-existing first and
second material
blanks and circumscribe the drug-releasing chambers by the weld junctions, a
plurality of
openings communicating with the drug-releasing chambers may be formed at step
60 either
after the first and second material blanks are conjoined or may be formed at
step 60 prior to
conjoining the materials as reflected by the double-headed arrows between step
60 and steps
52 and 54, respectively. Under the second method, that being to vacuum deposit
a second
material blank at step 54 over the first material blank and the sacrificial
material, step 60, i.e.,
forming the plurality of openings communicating with the drug-releasing
chambers will need
to be conducted after the first and second material blanks are conjoined as
step 58, so that
deposition of either or both of the first and second material blanks, and
deposition of the
sacrificial material may occur without occluding or obstructing the plurality
of openings.
[0068] The plurality of internal chambers are created at step 62 either as an
integral
result of the conjoining step 58 where the first and second material blanks
are welded to one
another, or as a result of removing the sacrificial material through the
plurality of openings
formed in step 60. Finally, a pharmacologically active agent may be loaded at
step 64 into
the drug-releasing chambers as the final step in making the inventive drug-
releasing device.
[0069] The plurality of microperforations in either or both of the first and
second
material blanks may be formed either before or after the materials are
conjoined. Where a
vacuum deposition process is employed in practicing the method, those skilled
in the art will
find that it is better to form the plurality of microperforations after the
first and second
material blanks are conjoined at step 58. Where the first and second material
blanks are
conjoined by welding, those skilled in the art will fmd it more desirable to
form the plurality
of microperforations prior to conjoining the first and second material blanks.
[0070] The plurality of microperforations may be formed by masking the
material
blank to expose only those regions defming the plurality of microperforations.
The exposed
regions are then subjected to removal either by etching, such as by wet or dry
chemical
etching processing, with the etchant being selected based upon the material of
the precursor
blank, or by machining, such as by laser ablation or EDM. Alternatively, when
employing
the vacuum deposition techniques, a pattern mask corresponding to the
plurality of
micropeiforations may be interposed between the target and the source and the
metal or
-21-
CA 02574972 2007-01-24
WO 2006/015161
PCT/US2005/026875
pseudometal deposited through the pattern mask to form the patterned
microperforations.
Further, when employing the vacuum deposition, plural film layers maybe
deposited to form
a multilayer film structure of the film prior to or concurrently with forming
the plurality of
microperforations. Alternatively, plurality layers of material blanks may be
employed and
conjoined by welding. Those skilled in the art will understand, however, that
device profile
issues will likely be attendant to employing the method whereby non-vacuum
deposited
material blanks are employed due to the difficulty of producing ultra-thin
material blanks on
the order of between about 0.1 gm to about 15 tm using conventional non-vacuum
deposition
production methods.
[0071] Thus, the present invention provides a new metallic and/or
pseudometallic
implantable drug-releasing material for forming a wide variety of drug-
delivery devices that
is biocompatible, compliant, geometrically changeable either by folding and
unfolding or by
application of a plastically, elastically or superelastically deforming force,
and, in some cases,
capable of endoluminal delivery with a suitably small delivery profile and
suitably low post-
delivery profile. Suitable metal materials to fabricate the inventive graft
are chosen for their
biocompatibility, mechanical properties, i.e., tensile strength, yield
strength, and in the case
where vapor deposition is deployed, their ease of deposition include, without
limitation, the
following: titanium, vanadium, aluminum, nickel, tantalum, zirconium,
chromium, silver,
gold, silicon, magnesium, niobium, scandium, platinum, cobalt, palladium,
manganese,
molybdenum and alloys thereof, such as zirconium-titanium-tantalum alloys,
nitinol,
chromium-cobalt alloy and stainless steel. Examples of pseudometallic
materials potentially
useful with the present invention include, for example, composite materials
and ceramics.
[0072] The present invention also provides a method of making the inventive
drug-
delivery material by vacuum deposition of a metal or pseudometal material
blank and
formation of the microperforations either by removing sections of deposited
material, such as
by etching, EDM, ablation, or other similar methods, or by interposing a
pattern mask,
corresponding to the microperforations, between the target and the source
during deposition
processing. Alternatively, a pre-existing metal and/or pseudometallic material
blanks
manufactured by conventional non-vacuum deposition methodologies, such as
wrought
hypotube or sheet, may be obtained, and the microperforations formed in the
pre-existing
metal and/or pseudometallic film by removing sections of the film, such as by
etching, EDM,
ablation, or other similar methods. An advantage of employing multilayer film
structures to
form the inventive drug-releasing material is that differential
functionalities may be imparted
-22-
CA 02574972 2007-01-24
WO 2006/015161
PCT/US2005/026875
in the discrete layers. For example, a radiopaque material such as tantalum
may form one
layer of a structure while other layers are chosen to provide the graft with
its desired
mechanical and structural properties.
[0073] In accordance with the preferred embodiment of fabricating the
inventive
microporous metallic implantable drug-releasing device in which the device is
fabricated
from vacuum deposited nitinol tube, a cylindrical deoxygenated copper
substrate is provided.
The substrate is mechanically and/or electropolished to provide a
substantially uniform
surface topography for accommodating metal deposition thereupon. A cylindrical
hollow
cathode magnetron sputtering deposition device was employed, in which the
cathode was on
the outside and the substrate was positioned along the longitudinal axis of
the cathode. A
cylindrical target consisting either of a nickel-titanium alloy having an
atomic ratio of nickel
to titanium of about 50-50% and which can be adjusted by spot welding nickel
or titanium
wires to the target, or a nickel cylinder having a plurality of titanium
strips spot welded to the
inner surface of the nickel cylinder, or a titanium cylinder having a
plurality of nickel strips
spot welded to the inner surface of the titanium cylinder is provided. It is
known in the
sputter deposition arts to cool a target within the deposition chamber by
maintaining a
thermal contact between the target and a cooling jacket within the cathode. In
accordance
with the present invention, it has been found useful to reduce the thermal
cooling by
thermally insulating the target from the cooling jacket within the cathode
while still providing
electrical contact to it. By insulating the target from the cooling jacket,
the target is allowed
to become hot within the reaction chamber. Two methods of thermally isolating
the
cylindrical target from the cooling jacket of the cathode were employed.
First, a plurality of
wires having a diameter of 0.0381mm were spot welded around the outer
circumference of
the target to provide an equivalent spacing between the target and the cathode
cooling jacket.
Second, a tubular ceramic insulating sleeve was interposed between the outer
circumference
of the target and the cathode cooling jacket. Further, because the Ni-Ti
sputtering yields can
be dependant on target temperature, methods which allow the target to become
uniformly hot
are preferred.
[0074] The deposition chamber was evacuated to a pressure less than or about 2-
5 x
10-7 TOIT and pre-cleaning of the substrate is conducted under vacuum. During
the
deposition, substrate temperature is preferably maintained within the range of
300 and 700
degrees Centigrade. It. is preferable to apply a negative bias voltage between
0 and -1000
volts to the substrate, and preferably between -50 and -150 volts, which is
sufficient to cause
-23-
CA 02574972 2007-01-24
WO 2006/015161
PCT/US2005/026875
energetic species arriving at the surface of the substrate. During deposition,
the gas pressure
is maintained between 0.1 and 40 mTorr but preferably between 1 and 20 mTorr.
Sputtering
preferably occurs in the presence of an Argon atmosphere. The argon gas must
be of high
purity and special pumps may be employed to reduce oxygen partial pressure.
Deposition
times will vary depending upon the desired thickness of the deposited tubular
film.
[0075] After deposition of the first material, a copper sacrificial layer was
vacuum
deposited onto the first material. A photolithography mask corresponding to
the desired
pattern of the sacrificial material was applied onto the copper sacrificial
layer and the entire
assembly immersed in nitric acid which specifically etched the undesired
regions of the
copper sacrificial layer, leaving only the desired sacrificial regions
corresponding to the
position of the drag-releasing chambers. Other modes of chemical etching,
photoetching,
ablative techniques, such as laser etching or machining techniques, such as
electric discharge
machining (EDM) may be employed to remove undesired regions of the sacrificial
material.
[0076] A second layer of nickel-titanium was vacuum deposited following the
foregoing procedures onto the first material blank and the sacrificial copper.
After deposition
of the second nickel-titanium layer, a plurality of openings were laser cut
through the second
layer of nickel-titanium and into the sacrificial copper, then the entire
assembly was
immersed in nitric acid for a period of time sufficient to etch the copper
sacrificial material
through the plurality of openings, leaving a plurality of drag-releasing
chambers between the
first and the second layers of nickel-titanium alloy.
[0077] In accordance with the present invention, it has been found desirable
when
employing a non-biocompatible sacrificial metal, such as copper or hexavalent
chromium, for
example, to include a diffusion barrier between the first material and the
sacrificial material,
and then between the patterned sacrificial material and the second layer of
material deposited
onto the sacrificial material. It has been found that certain metals, such as
copper, tend to
diffuse into the surface of the first material blank and the second material
blank under
vacuum deposition conditions. Since the presence of non-biocompatible metals
is
undesirable, interposing a diffusion barrier which is removable with the
sacrificial material
serves to prevent metal diffusion and the presence of undesirable metals in
the finished
device. Suitable diffusion barriers may include, for example, titanium
nitrides, silicon
oxides, TiSiN or tantalum, which is, itself, biocompatible and would not
require removal.
-24-
CA 02574972 2007-01-24
WO 2006/015161
PCT/US2005/026875
[0078] After deposition, the plurality of microperforations are formed in the
tube by
removing regions of the deposited film by etching, such as chemical or
photoetching,
ablation, such as by excimer laser or by EDM, or the like. After the plurality
of
microperforations are formed, the formed microporous film is removed from the
copper
substrate by exposing the substrate and film to a nitric acid bath for a
period of time sufficient
to remove dissolve the copper substrate.
[0079] While the present invention has been described with reference to its
preferred
embodiments, those of ordinary skill in the art will understand and appreciate
that variations
in materials, dimensions, geometries, and fabrication methods may be or become
known in
the art, yet still remain within the scope of the present invention which is
limited only by the
claims appended hereto.
-25-