Note: Descriptions are shown in the official language in which they were submitted.
CA 02575978 2007-02-02
~s+i at~R' DEPI.OYMENT ~~s9nA-A,'i'UB
T}T,CUNTrAT. FJEI,D
This invention relates generally to implants for
repairing ducts and passageways in the body. More
specifically, the invention relates to implant deployment
apparatus.
This is a divisional application of Can.adian Patent
Application 2,275,921, filed on December 9, 2007, which
is a national entry of International Application
PCT/US97/21641, filed on December 9, 2007.
HACKGROLTNjZ ART
Treatment or isolation of vascular aneurysms or of
vessel walls which have been thickened or thinned by
disease has traditionally been performed via surgical
bypassing with vascular grafts. Shortcomings of this
procedure include the morbidity and mortality associated
with surgery, long recovery times after surgery, and the
high incidence of repeat intervention needed due to
limitations of the graft or of the procedure.
Vessels thickened by disease may be treated less
invasively with stents which mechanically hold vessels
open. In some instances, stents may be used subsequent to
or as an adjunct to a balloon angioplasty procedure.
Stents also have been described in conjunction with grafts
where the graft is intended to provide a generally smooth
interface with blood flowing through the vessel.
Generally, it is important that the stent or
stent-graft be accurately deployed so that it may be
positioned at the desired location. Endovascular stent or
stent-graft deployment can be swannarized as a two-step
process. The first step is moving the stent within the
vasculature to a desired location. The stent or stentgraft
may be self-expanding or balloon expandable. In both
cases, the implant is typically delivered in a collapsed
state to facilitate delivery through relatively small
vessel lumens. The second step involves some method of
"locking" the stent or stent-graft into its final geometry
I
CA 02575978 2007-02-02
WO 98/27894 PCT/US97121641
so that it will remain implanted in the desired location.
A number of techniques for delivering self-expanding
or balloon expandable stents and stent-grafts are known.
In the case of a self-expanding stent or stent-graft, a
restraining mechanism typically is used to keep the stent
or stent-graft in its collapsed state during delivery. The
restraining mechanism is later removed to allow the stent
or stent-graft to expand and engage the vessel wall at the
desired implantation site. In the case of a balloon
expandable stent or stent-graft, a restraining mechanism
typically keeps the expandable device in a collapsed
position during delivery with an inflatable balloon
positioned within the collapsed device. The restraining
mechanism is later removed to allow for inflation of the
balloon which causes the stent or stent-graft to expand so
that it engages the vessel wall. Generally, tubular
sheaths or tying elements, which may be in the form of a
filament or thread, have been described to restrain the
collapsed devices.
U.S. Patent No. 4,878,906, to Lindemann et al.,
discloses balloon expandable stent-grafts which are
deployed through a tubular sheath. The stent grafts are
forwarded in a collapsed state along the vessel until they
are in the correct location where the sheath is withdrawn,
allowing expansion of the balloon within the stent-graft.
After the balloon has expanded the stent-graft into final
position, the balloon is deflated and drawn back into the
tubular sheath. An alternative deployment method disclosed
Lindemann et al. dispenses with the tubular sheath and
uses a "thread" wrapped around the stent-graft and balloon
which can be withdrawn when balloon inflation is desired.
Pinchuk, U.S. Patent No. 5,019,090, shows a helically
wrapped spring stent which is deployed with a balloon
expansion catheter through a "sheath" which holds the
stent and balloon catheter in a generally compressed
2
SUBSTITUTE SHEET (rule 26)
CA 02575978 2007-02-02
WO 98/27894 PGTIUS97/21641
state. Once the stent and balloon have been forwarded into
the correct position along a lumen, the sheath is
withdrawn. The balloon is then inflated, deflated, and
withdrawn, leaving the stent in final implantation
position.
U.S. Patent No. 5,246,452, to Sinnott, discloses a
porous vascular graft which is implanted with a tear-away
removable nonporous sheath. Once the graft has been
forwarded into the desired position, circulation is
restored to the area and blood is allowed to clot inside
of the porous graft. After five minutes of clotting, the
nonporous sheath can be removed by cutting or by pulling a
string which tears the sheath and pulls it away.
U.S. Patent No. 5,344,426, to Lau et al., discloses
an expandable stent which is preferably self locking when
expanded. The stent is positioned over an expandable
member such as a balloon catheter and covered by a one or
two layer sheath which is connected to a guidewire. When
the assembly of sheath, stent, and expandable member has
been forwarded to the desired position, the sheath is
removed by moving the guidewire distally. With the sheath
pulled off of the stent, the expandable member can be
activated to expand the stent into its final position.
U.S. Patent No. 5,366,473, to Winston et al.,
discloses an assembly in which a vascular graft is held in
a compressed state over a pair of stents by a sheath. The
stents take the form of flexible sheets wound around a
spool. After the spool has been inserted to the correct
endovascular site, the sheath is withdrawn allowing the
stents to unwind and press the graft against the vessel
walls.
Strecker, U.S. Patent No. 5,405,378, discloses an
expandable prosthesis which is held in radially compressed
condition by a releasable sheath. The sheath can be a
strippable meshwork which allows the compressed prosthesis
3
SUBSTITUTE SHEET (rule 26 )
CA 02575978 2007-02-02
to expand when the meshwork is controllably unravelled.
Generally, the mechanisms described above involve a
number of components that may increase operational
complexity. In addition, the size and mechanical
properties of these mechanisms may limit deliverability of
implants in small vessels. Delivery accuracy also may be a
problem as discussed.
The diameter of conventional telescoping stent
sheaths may contribute to undesirable friction with the
delivery catheter as the sheath is pulled from the stent
and over a push rod during deployment. This may make
deployment accuracy difficult to control. Push rods, which
are used to push the stent through the delivery catheter
and which typically have a length of up to about 100 cm,
also may contribute to undesirable friction with the
catheter. This problem may be exacerbated where the
catheter bends along its path in the vasculature The
sheath may also reposition the stent as it is retracted.
DISCLOSURE OF THE INVENTION
The present invention generally involves a delivery
apparatus for an implant, such as a stent or stent-graft. The
delivery system generally comprises a sheet of material
adapted to extend around at least a portion of a collapsed
implant, such as a collapsed stent or stent-graft. The
sheet of material may form a tubular member when extending
around at least a portion of a collapsed member. The
system also may include a coupling member for coupling
portions of the sheet together to maintain the implant in
its collapsed state during delivery to a desired site in a
mammalian body. With this construction a smooth interface
between the collapsed stent and a vessel lumen, as
compared to thread-like restraining members, may be
achieved.
According to another aspect of the invention, the
EOrEAEo V.p 15 m
4
40155765.1
CA 02575978 2007-02-02
sheet may be constructed of a thin material which does not
significantly contribute to the structural rigidity or
cross-sectional profile to the delivery assembly. This
construction may also eliminate the need for external
sheathing or a guide catheter and is believed to
advantageously increase the ability of the surgeon to
deliver the device to relatively remote sites and through
small tortuous vasculature. In addition, the sheet may
comprise implantable material so that after release it may
remain with the stent at the desired site.
According to another embodiment of the invention, an
assembly comprising a stent and a restraining member
coupled to the stent is provided. The stent has a
collapsed and an expanded state and the restraining member
comprises a sheet of material adapted to be wrapped around
at least a portion of the stent when the stent is in the
collapsed state. Portions of the sheet are adapted for
coupling to one another to maintain the sheet wrapped
around at least a portion of the stent in its collapsed
state. Thus, in one configuration, portions of the sheet
are releasably coupled to one another so that the sheet
maintains the stent in its collapsed state.
According to another aspect of the invention, the
portions of the sheet that may be coupled to one another
may be coupled with a filament or thread-like member. The
stent may be expanded (or allowed to expand when a self-
expanding stent is used) after the thread-like coupling
member is removed such as by being remotely pulled by a
pull line, which may be an extension of the coupling
member. Since the pull line may also have a thread-like
low profile, friction with the catheter, through
which the pull line is pulled, and the pull line is
minimized. It is believed that such construction may
further facilitate deployment accuracy.
According to another aspect of the invention,
5 ENTERED SEP 2 5 2001
40155765.1
CA 02575978 2007-02-02
multiple restraining members may be used. Alternatively,
multiple coupling members may be used to couple multiple
portions of one or more restraining members. These
constructions can reduce deployment time and may reduce
the time in which fluid flow may disturb the position of
the implant as it is deployed.
According to another aspect of the invention, an
assembly comprises a stent and a restraining member
coupled to the stent. The stent has a collapsed and an
expanded state and first and second portions that move
relative to one another when said stent moves between its
collapsed and expanded states. The said restraining member
comprises a sheet of material adapted to be wrapped around
at least a portion of the stent when it is in its
collapsed state, and portions of the sheet being adapted
for coupling to one another to maintain said sheet wrapped
around at least a portion of the stent in its collapsed
state. The said assembly further includes a member having
a first portion coupled to the restraining member and a
second portion coupled to one of the stent first and
second portions.
According to another aspect of the invention, an
expandable stent, which is restrained in a collapsed state
with a restraining member, is released and the restraining
member urged against the wall of the lumen in which the
stent is placed. Since the restraining member remains at
the site, the number of deployment steps can be reduced as
compared to other techniques (e.g. pushing a
self-expanding implant out the end of a radially
constraining sheath and retracting the sheath).
According to another aspect of the invention, a
method of preparing a stent for delivery comprises
restraining a collapsed stent in a sheet of material which
may be in the form of a tube and coupling side margins of
the tube.
6 ENTERED SEP 2 5 2001
40155765.1
CA 02575978 2007-02-02
According to another aspect of the invention, an
expandable stent (or stent-graft) is collapsed into a
generally cylindrical or tubular restraining by pulling
the stent through a tapered member and into a tubular
restraining member.
The above is a brief description of some deficiencies
in the prior art and advantages of the present invention.
Other features, advantages, and embodiments of the
invention will be apparent to those skilled in the art
from the following description, accompanying drawings, and
appended claims.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a perspective view of a mammalian implant
that is restrained in a collapsed state in accordance with
the principles of this invention.
Figure 2 is an end view of the restrained implant of
Figure 1.
Figure 3 is a perspective view of the assembly of
Figure 1 with the restraint released and the implant in an
expanded state.
Figure 4A is an end view of the assembly of Figure 3.
Figure 4B is a bottom plan view of the restraining
member of Figure 4A.
Figure 5A shows a restraining member retraction
mechanism according to another embodiment of the invention
where the mechanism is in an unactuated state.
Figure 5B shows the mechanism of Figure 5A in an
actuated state.
Figure 5C shows a retraining member retraction
mechanism according to yet another embodiment of the
invention where the mechanism is in an unactuated state
Figure 5D shows the mechanism of Figure 5C in an
actuated state.
ENTERED SFo 2 5 2001
40155765.1
CA 02575978 2007-02-02
WO 98/27894 PCT/US97/21641
Figure 6A is a perspective view of another embodiment
of the implant in conjunction with the restraining member
of Figure 1.
Figure 6B is a perspective view of a further
embodiment of the implant in conjunction with the
restraining member of Figure 1.
Figure 7A illustrates the restraining and coupling
member of Figure 1 and the pull direction for removing the
coupling member from the restraining member.
Figure 7B shows the assembly of Figure 7A with the
coupling member loosened to illustrate the chain knots
used according to one embodiment of the invention.
Figure 7C diagrammatically represents release of the
assembly of Figure 7A or 7B as the coupling member is
pulled in the direction shown.
Figures BA, 8B, 8C, 8D, 8E and 8F diagrammatically
show a procedure for loading an expandable stent-graft
into a restraining member in accordance with the present
invention prior to endolumenal delivery.
Figure 9A diagrammatically shows delivering a
restrained implant to a desired site in a mammalian body
lumen in accordance with the present invention with the
coupling member configured as shown in Figures 7A-7C.
Figure 9B is a sectional view of Figure 9A taken
along line 9B-9B.
Figure 9C shows an alternate multiple restraining
member arrangement for that shown in Figure 9A.
Figure 10A diagrammatically shows partial deployment
of the implant assembly illustrated in Figure 9A showing
progressive expansion in a direction away from the distal
end of the illustrated guidewire (i.e., toward the
illustrated hub).
Figure lOB is a sectional view of Figure 1OA taken
along line lOB-lOB.
Figure 11 A diagrammatically shows full deployment of
8
SUBSTITUTE SHEET { rule 26 )
CA 02575978 2007-02-02
the implant assembly illustrated in Figure 9A.
Figure 11B is a sectional view of Figure 11A taken along
the line 11B-11B.
Figures 12A, 128, 12C and 12D diagrammatically show
deployment of a restrained implant according to another
embodiment of the invention where the coupling member
configuration provides release from the middle portion of
the implant outward toward the implant ends.
Figure 13 illustrates one coupling member
configuration for deployment as shown in Figures 12A-12D.
Figure 14A is a perspective view of a bifurcated
stent-graft that can be used with the illustrated delivery
systems.
Figure 14B is a top plan view of the bifurcated
stent-graft of Figure 14A.
Figure 14C is a cross-section view taken along
section line 14C-14C depicted in Figure 14A.
Figure 14D is a cross-sectional view taken along
section line 14D-14D depicted in Figure 14A showing an
alternate embodiment.
Figure 15 is a front view of the assembled bifurcated
stent-graft of Figure 14A placed at a bifurcation site
within the vasculature of a body.
Figure 16 is a perspective break-away view showing a
close-up of one construction of stent anchoring apexes.
Figure 17 is a perspective break-away view showing a
close-up of a preferred construction of the stent
anchoring-apexes.
Figure 18 is a cross-sectional view of the
stent-graft of Figure 14B taken along section line 18-18.
Figure 19 is a cross-sectional view of the
stent-graft of Figure 14A taken along section line 19-19.
Figure 20 is an enlarged partial cross-sectional view
of the contralateral leg connection depicted in Figure 18
having a localized zone of decreased diameter.
Figure 21 and Figure 22 are enlarged partial
cross-sectional views of alternative constructions of the
9
ENTERED ~Fp 2 5 2001
40155765.1
CA 02575978 2007-02-02
receiving lumen.
Figure 23 is a partial perspective view of an
alternate scalloped construction of the distal region of
the contralateral leg component.
Figures 24A and 24B are cross-sectional views taken
along section line 24A-24A as shown in Figure 14A
depicting a free state and a forced state respectively.
Figures 25A and 25B are cross-sectional views taken
along section line 25A-25A as shown in Figure 23 depicting
a free state and a forced state respectively.
Figure 26A is a front view of preassembled graft
components.
Figures 26B and 26C are respectively the front view
and top view of the assembled graft of Figure 26A.
Figure 27A is a front view of the unassembled
components of an alternate construction of the graft
element.
Figure 27B is a front view of the assembled graft
element according to the alternative construction of
Figure 27A.
Figures 28A through 28E diagrammatically show
deployment of a bifurcated stent-graft.
Figures 29A, through 29D diagrammatically show
deployment of a bifurcated stent-graft using an alternate
delivery system.
DETAILED DESCRIPTION
Referring to the drawings in detail wherein like
numerals indicate like elements, delivery systems for
delivering implants or devices, such as stents or
stent-grafts, to a desired site in mammalian vasculature
are shown in accordance with the principles of the present
invention. The delivery systems of the present invention
generally include a restraining member that is adapted and
configured for surrounding at least a portion of a
collapsed or compressed implant and a coupling member(s)
for releasably coupling portions of the restraining member
ENTFRFn cc'3 2 ~ 9001
40155765.1
CA 02575978 2007-02-02
to one another to maintain the implant in its collapsed or
compressed state.
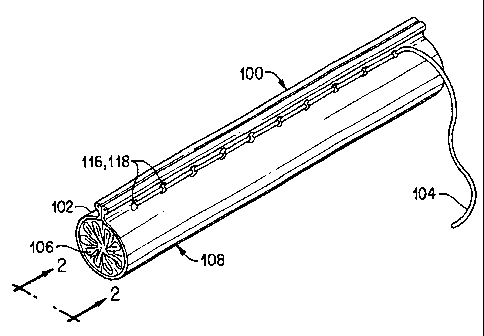
Referring to Figures 1-4, an implant delivery system
constructed in accordance with the present invention is
shown. Delivery system (100), generally includes a
restraining member (102), which as shown may be in the
form of a sheet of material, and a coupling member (104)
for releasably coupling portions of the restraining member
to one another. The restraining member portions that are
coupled may differ from those illustrated, but preferably
are selected to maintain the implant, such as
self-expanding stent-graft (106), in a collapsed or
compressed state as shown in Figures 1 and 2 where the
restraining member (102) is shown in the form of a tube.
In the illustrative embodiment, the coupling member (104)
is shown as a filament or thread-like element which
prevents the restraining member (102) from rearranging to
a configuration where the stent-graft (106) could expand
to its expanded state.
The implant may be collapsed in any suitable manner
for placement within the restraining member (102). For
example, the implant may be folded or radially crushed
before placement within the restraining member (102) as
will be described in more detail below. As shown in
Figures 9-11, a delivery assembly (108), which includes
the restraining member (102) and the stent-graft (106),
has relatively small cross-sectional dimensions which
facilitate endolumenal delivery of the assembly to a site
where the natural lumen diameter may be smaller than the
expanded diameter of the stent-graft (106).
Referring to Figures 3 and 4A, stent graft (106) and
restraining member (102) are shown in a deployed state after
removal of the coupling member (104). The restraining member
(102) may be fixedly secured to the stent-graft (106) so that
the two components remain attached after expansion at the
desired deployment site. The attachment between the
restraining member and the stent-graft preferably is made to
11
ENTERED SEP 2 5 2001
40155765.1
CA 02575978 2007-02-02
prevent significant movement between the restraining member
and stent-graft after deployment which could disrupt
endovascular fluid flow. Referring to Figures 4A and 4B
multiple sutures (110) may be used to fixedly attach the
restraining member (102) to the stent-graft (106). More
specifically, the sutures can form loops that pass through
the restraining member and around portions of the stent as
shown in Figure 4A. It is further noted that although one
arrangement of the sutures (110) is shown in Figure 4B
other arrangements may be used.
Although other configurations of the restraining
member (102) can be used, a preferred configuration is a
generally rectangular one having constant width as shown
in Figure 4B. For example, in the case where the
restraining member is used in conjunction with a modular
bifurcated stent as will be described below, the
restraining member may have a similar rectangular
configuration as that shown in Figure 4B. Alternatively,
it may have two differently sized rectangular portions
arranged to mate with the regions of different diameter
(trunk and leg) or another configuration that would
maintain the implant in a collapsed state when secured.
Returning to Figure 4B, the restraining member may be
described as having side margins (112) that extend between
the ends (114) of the member. Eyelets (116) are disposed
along the side margins so that the coupling member (104)
may be laced or threaded therethrough. The eyelets may be
in the form of through holes (118), which may be formed by
a uniform-diameter puncturing device or by other means
such as laser-drilling. Alternatively, the eyelets may be
formed by loops (120) which may be attached to the side
margins (112) or formed by other means as would be
12 ENTERFn c~~ 2 5 200~
40155765.1
CA 02575978 2007-02-02
WO 98/27894 PGT/US97121641
apparent to one of ordinary skill in the art.
It is further desirable to have structural
reinforcement at the side margins (112) to minimize or
eliminate the possibility of the coupling member (104)
from tearing the restraining member (102) when under load.
Reinforced side margins may be formed by folding a portion
of the restraining member (102) over a reinforcement
member (122), such as a small diameter suture, which may
be heat bonded between the two layers of sheet material.
With this construction, a relatively low profile bead of
material along the side margins (112) prevents or
minimizes the possibility of tear propagation and, thus,
accidental uncoupling of the restraining member (102). The
small diameter suture (122) may comprise ePTFE, for
example.
As the restraining member (102) constrains a
collapsed self-expanding stent-graft, for example, forces
resulting from stored spring energy in the collapsed
stent-graft (106) will be acting on the restraining member
(102) when it is configured for delivery. Thus, according
to another aspect of the invention the restraining member
(102) may comprise a material which is creep resistant and
can withstand required loads without stretching over time.
The restraining member (102) may comprise, for example,
ePTFE, which is believed to provide suitable creep
resistance, flexibility, and biocompatibility in a thin
sheet form which can be heat bonded. Other materials also
may be used including polyethers such as polyethylene
terephthalate (DACRON or MYLAR ) or polyaramids such as
KEVLAR .
The thread-like coupling member (104) may also
comprise ePTFE. Sutures of polyethers such as polyethylene
terephthalate (DACRONO or MYLAR ) or polyaramids such as
KEVLAR or metal wire comprising nitinol, stainless steel
or gold may also be used for the coupling member (104).
13
SUBSTITUTE SHEET (rule 26 )
CA 02575978 2007-02-02
WO 98/27894 PCT/US97/21641
The coupling member (104) may simply extend to form a
remote pull line as will be discussed below.
Alternatively, a metallic pull line, such as one
comprising stainless steel may be coupled to a nonmetallic
coupling member (104) such as one comprising ePTFE. The
coupling may be made by folding the end of the metallic
pull line back upon itself to form an eyelet and threading
the coupling member therethrough and securing it to the
eyelet with a knot.
It is further noted that the width of the restraining
member, when in a flat orientation as shown in Figure 4A,
preferably is less than the diameter of the implant.
Typically the restraining member width will be less than
about 40mm (typically about 25-40mm when the device is
sized for thoracic aorta applications), and typically less
than about 15mm in other applications where the lumen is
smaller. The sheet of material preferably has a thickness
less than .010 inch (0.254 mm) and more preferably less
than .005 inch (0.127 mm). In addition, the length of the
restraining member preferably is less than or equal to
that of the implant.
According to the present invention, a retraction
assembly may be provided to retract the restraining member
during expansion of the implant, so that the length of the
restraining member is maintained to be about equal to or
less than that of the implant. The expandable portion of
the implant may undergo minor amounts of shortening along
the axial direction due to the expansion thereof in the
radial direction, which may lead to an overlap of the
restraining member at the ends of the implant, but for the
use of some type of retraction assembly in these
situations. The retraction assembly minimizes or
eliminates the risk of the restraining member extending
beyond the implant and interfering with any channel formed
by the implant, or any fluid flowing therethrough after
14
SUBSTITUTE SHEET (rule 26 )
CA 02575978 2007-02-02
expansion.
Referring to Figures 5A-5D, retraction assemblies or
mechanisms constructed according to the principles of the
invention are shown. In Figure 5A, a retraction assembly
(340) is shown including a biocompatible filament (342),
which includes a portion that is stitched, tied or
otherwise fixed to the restraining member (102), as shown
at an attachment point (348), adjacent to one end of the
restraining member. Filament (342) is passed underneath
the members forming the first or end helical turn of the
stent (126) and looped under or otherwise slidably secured
to a portion of the second, third or another helical turn
other than the first helical turn such as an apex or bend
portion (344) in a second turn. The other end portion of
filament (342) is further fixed, by tying or other means,
to a portion of the stent that is circumferentially spaced
from the attachment point (348) or the apex or bend
portion (344), for example, such as an apex or bend
portion (346) of the same helical turn. Preferably, the
filament (342) is looped through an apex portion (344) of
the second helical turn and tied to an apex portion (346)
which is adjacent to the apex portion (344) as shown in
Figure 5A.
Figure 5A shows the stent in the compressed state.
Upon expansion of the stent, as mentioned above, the
members of the stent expand to effect the radial expansion
of the stent, as shown in Figure 5B. Because the distance
between apex portions (344) and (346) becomes greater upon
expansion of the stent, and because the filament (342) is
relatively unyieldable and inelastic, the distance between
the attachment point (348) and the apex portion (344)
necessarily decreases. The result is that the end of the
restraining member (102) is retracted with respect to the
stent (126), as shown in Figure SB. Thus, the retraction
along the longitudinal axis of the restraining member is
15 ENTERED SEP 2 5 2001
40155765.1
CA 02575978 2007-02-02
driven by the expanding distance between adjacent apexes
in this embodiment. Although only one retraction mechanism
is shown at one end of the restraining member, another
similarly configured and arranged retraction mechanism may
be used at the other end of the restraining member.
Figures 5C and 5D show another embodiment for a
retraction assembly. The views of this assembly (as are
those shown in Figures 5A and 5B) are taken from a
location between the generally cylindrical graft and stent
looking radially outward. In contrast to that shown above
where one end portion of a filament is secured to the
restraining member and another to a portion of the stent
that circumferentially moves during stent expansion, the
other end of the filament is secured to a portion of a
stent that moves generally parallel to the longitudinal
axis of the stent (axially) as the stent expands. In this
embodiment, at least one apex portion (364) of an end
helix of the stent member (126') (which differs from stent
(126) in that it includes eyelets or loops which may be
formed as shown in the drawings) is made shorter than the
majority of apex portions (366) . However, the apex
portions may be otherwise configured such as those shown
in Figures 5A and 5B. A filament (362) is tied or
otherwise fixed at one end to apex portion (364), and at
the other end, to one end portion of the restraining
member (102). As shown in Figure 5D, upon radial expansion
of the stent, inwardly positioned apex portion (364)
retracts to a greater extent in the longitudinal or axial
direction than the full height apex portions (366) which
are shown in the last or most outwardly positioned turn of
the stent. This relative greater retraction directly
translates through filament (362) such that the end of the
restraining member (102) is retracted relative to apex
portions (366). As described above, another similarly
constructed retraction mechanism may be provided at the
16 ENTERED CFO 2 2001
40155765.1
CA 02575978 2007-02-02
other end of the restraining member.
Returning to Figure 3, one stent-graft construction
that may be used in conjunction with the delivery systems
disclosed herein is shown. Stent-graft (106) generally
includes a thin-walled tube or graft member (124), a stent
member (126), which can be a self-expanding stent, and a
ribbon or tape member (128) for coupling the stent (126)
and graft (124) members together. The stent (126) and
graft (124) members may be heat bonded together, thus
sealing in portions of the stent member (126) that are
between the tape member (128) and the graft member (124).
The mechanical properties of the stent-graft (106) may be
customized, for example, through materials selection, by
varying the structural pattern of the stent member,
varying the thickness of the tape (128) and graft (124)
members, and varying the pattern with which the tape
member contacts the stent and graft members.
As shown in Figure 3, the tape member (128) may
cover only a portion of the stent member (126) as it
follows the helical turns of the undulating stent member.
With this construction, regions of the stent member do not
interface with the tape member when the stent-graft is in
an uncompressed state, for example. This is believed to
advantageously reduce shear stresses between the stent
member (126) and the tape member (128) when the
stent-graft undergoes bending or compression, thereby
reducing the risk of tearing the graft (124) or tape (128)
members or causing delamination between the stent (126)
and graft (124) members.
The tape member (128) also preferably has a generally
broad or flat surface for interfacing with the stent (126)
and graft (124) members as compared to filament or
thread-like structures such as sutures. This increases
potential bonding surface area between the tape member
(128) and the graft member (124) to enhance the structural
17 ENTERED SER 2 5 2001
40155765.1
CA 02575978 2007-02-02
WO 98/27894 PCT/[JS97/21641
integrity of the stent-graft. The increased bonding
surface area also facilitates minimizing the thickness of
the tape member (128). It has been found that a tape
member in the form of a generally flat ribbon as shown in
the drawings provides desired results.
Tape members having widths of 0.025, 0.050 and 0.075
inches applied to a stent member having a peak-to-peak
undulation amplitude of about 0.075 inch are believed to
provide suitable results. However, it has been* found that
as the tape member band width increases, the stent-graft
flexibility generally is diminished. It is believed that a
tape member width of about one-fourth to three-fourths the
amplitude of the stent member undulations, measured
peak-to-peak, may be preferred (may be more preferably
about one-third to two-thirds that amplitude) to optimize
flexibility. It also has been found that by positioning
one of the lateral margins of the tape member adjacent to
the apexes, the tape member width may be reduced without
significantly sacrificing apex securement. Varying the
width of the tape member (e.g., varying width of the tape
along the length of the stent graft) can also result in
the adjustment of other structural properties. Increasing
the width can also potentially increase the radial
stiffness and the burst pressure and decrease the porosity
of the device. Increasing band width can also diminish
graft member wrinkling between coupling member turns.
The tape member (or separate pieces thereof) also may
surround the terminal end portions of the stent-graft to
secure the terminal portions of the graft member to the
stent member.
Figures 6A and 6B illustrate further stent-graft
constructions that may be used with the delivery systems
described herein. Referring to Figure 6A, stent-graft
(200) is the same as stent-graft (106) with the exception
that stent-graft (200) includes a filament that couples
18
SUBSTITUTE SHEET (rule 26 )
CA 02575978 2007-02-02
stent undulations in adjacent turns. Filament (202) is
laced or interwoven between undulations of the stent
member and acquires a helical configuration (i.e., it
forms a secondary helix) in being laced as such. Such a
configuration is disclosed in PCT publication No. WO
95/26695 (International Application No. PCT/US95/04000).
The stent-graft (300) shown in Figure 6B is the
same as that shown in Figure 6A with the exception that
the filament (302) is interwoven between undulations in
the same helical turn of the stent member.
The filaments (202, 302) are of the same construction
and may be of any appropriate filamentary material which
is blood compatible or biocompatible and sufficiently
flexible to allow the stent to flex and not deform the
stent upon folding. Although the linkage may be a single
or multiple strand wire (platinum, platinum/ tungsten,
gold, palladium, tantalum, stainless steel, etc.), much
preferred is the use of polymeric biocompatible filaments.
The flexible link may be tied-off at either end of the
stent-graft (200, 300), for example, by wrapping its end
portion around the stent and tying it off at the point at
the beginning of the last turn as would be apparent to one
of ordinary skill.
A percutaneously delivered stent-graft must expand
from a reduced diameter, necessary for delivery, to a
larger deployed diameter. The diameters of these devices
obviously vary with the size of the body lumen into which
they are placed. For instance, the stents of this
invention may range in size from 2.0mm in diameter (for
neurological applications) to 40mm in diameter (for
placement in the aorta). A range of about 2.0mm to 6.5mm
(perhaps to 10.0mm) is believed to be desirable.
Typically, expansion ratios of 2:1 or more are required.
These stents are capable of expansion ratios of up to 5:1
19 ENTERED SEP 2 5 2001
40155765.1
CA 02575978 2007-02-02
WO 98/27894 PGT/US97/21641
for larger diameter stents. Typical expansion ratios for
use with the stents-grafts of the invention typically are
in the range of about 2:1 to about 4:1 although the
invention is not so limited. The thickness of the stent
materials obviously varies with the size (or diameter) of
the stent and the ultimate required yield strength of the
folded stent. These values are further dependent upon the
selected materials of construction. Wire used in these
variations are typically of stronger alloys, e.g., nitinol
and stronger spring stainless steels, and have diameters
of about 0.002 inches to 0.005 inches. For the larger
stents, the appropriate diameter for the stent wire may be
somewhat larger, e.g., 0.005 to 0.020 inches. For flat
stock metallic stents, thicknesses of about 0.002 inches
to 0.005 inches is usually sufficient. For the larger
stents, the appropriate thickness for the stent flat stock
may be somewhat thicker, e.g., 0.005 to 0.020 inches.
The following example is provided for purposes of
illustrating a preferred method of manufacturing a
stent-graft as shown in Figure 3. It should be noted,
however, that this example is not intended to limit the
invention. The stent member wire is helically wound around
a mandrel having pins positioned thereon so that the
helical structure and undulations can be formed
simultaneously. While still on the mandrel, the stent
member is heated to about 460'F for about 20 minutes so
that it retains its shape. Wire sizes and materials may
vary widely depending on the application. The following is
an example construction for a stent-graft designed to
accommodate a 6mm diameter vessel lumen. The stent member
comprises a nitinol wire (50.8 atomic % Ni) having a
diameter of about 0.007 inch. In this example case, the
wire is formed to have sinusoidal undulations, each having
an amplitude measured peak-to-peak of about 0.100 inch and
the helix is formed with a pitch of about 10 windings per
SUBSTITUTE SHEET (rule 26 )
CA 02575978 2007-02-02
WO 98/27894 PCTIUS97/21641
inch. The inner diameter of the helix (when unconstrained)
is about 6.8mm. (The filament when used as shown in
Figures 6A and 6B may have a diameter of about 0.006
inch.)
In this example, the graft member is porous expanded
polytetrafluorethylene (PTFE), while the tape member is
expanded PTFE coated with FEP. The tape member is in the
form of a flat ribbon (as shown in the illustrative
embodiments) that is positioned around the stent and graft
member as shown in Figure. 3. The side of the tape member
or ribbon that is FEP coated faces the graft member to
secure it to the graft member. The intermediate stent--
graft construction is heated to allow the materials of the
tape and graft member to merge and self-bind as will be
described in more detail below.
The FEP-coated porous expanded PTFE film used to form
the tape member preferably is made by a process which
comprises the steps of:
(a) contacting a porous PTFE film with another layer
which is preferably a film of FEP or alternatively of
another thermoplastic polymer;
(b) heating the composition obtained in step (a) to a
temperature above the melting point of the thermoplastic
polymer;
(c) stretching the heated composition of step (b)
while maintaining the temperature above the melting point
of the thermoplastic polymer; and
(d) cooling the product of step (c).
In addition to FEP, other thermoplastic polymers
including thermoplastic fluoropolymers may also be used to
make this coated film. The adhesive coating on the porous
expanded PTFE film may be either continuous (non-porous)
or discontinuous (porous) depending primarily on the
amount and rate of stretching, the temperature during
stretching, and the thickness of the adhesive prior to
21
SUBSTITUTE SHEET (rule 26 )
CA 02575978 2007-02-02
Stretching.
In constructing this example, the thin wall expanded
PTFE graft was of about 0.1mm (0.004 in) thickness and had
a density of about 0.5g/cc. The microstructure of the
porous expanded PTFE contained fibrils of about 25 micron
length. A 3cm length of this graft material was placed on
a mandrel having the same diameter as the inner diameter of
the graft. The nitinol stent member having about a 3cm length
was then carefully fitted over the center of the thin wall
graft.
The stent-member was then provided with a tape
coupling member comprised of the FEP coated film as
described above. The tape member was helically wrapped
around the exterior surface of the stent-member as shown
in Figure 3. The tape member was oriented so that its
FEP-coated side faced inward and contacted the exterior
surface of the stent-member. This tape surface was exposed
to the outward facing surface of the thin wall graft
member exposed through the openings in the stent member.
The uniaxially-oriented fibrils of the microstructure of
the helically-wrapped ribbon were helically-oriented about
the exterior stent surface.
The mandrel assembly was placed into an oven set at
315 C for a period of 15 minutes after which the
film-wrapped mandrel was removed from the oven and allowed
to cool. Following cooling to approximately ambient
temperature, the mandrel was removed from the resultant
stent-graft. The amount of heat applied was adequate to
melt the FEP-coating on the porous expanded PTFE film and
thereby cause the graft and coupling members to adhere to
each other. Thus, the graft member was adhesively bonded
to the inner surface of the helically-wrapped tape,member
through the openings between the adjacent wires of the
stent member. The combined thickness of the luminal and
exterior coverings (graft and tape members) and the stent
ENTERED SEP 2 5 2001
22
40155765.1
CA 02575978 2007-02-02
WO 98127894 PCT/fJS97121641
member was about 0.4mm.
Although the invention has been described with
reference to the stent-graft examples illustrated in the
drawings, it should be understood that it can be used in
conjunction with other devices, stents or stent-grafts
having constructions different than those shown. For
example, delivery systems described herein may be used in
conjunction with bifurcated stents or stent-grafts as will
be described in detail below. In addition, although a
self-expanding stent-graft has been described, balloon
expanding stent-grafts also may be used in conjunction
with the delivery systems described herein. These
stent-grafts require a balloon to expand them into their
expanded state as opposed to the spring energy stored in a
collapsed self-expanding stent.
Referring to Figures 7A-C, one slip knot
configuration that may be used in conjunction with the
thread-like coupling member (104) will be described. The
restraining member (102) is shown without an implant
positioned therein for purposes of simplification. Figure
7A illustrates the slip knot in a prerelease or
predeployment state. The series of knots are generally
flush with the restraining member (102) surface and add
very little profile to the construct which is preferred
during implant delivery. Figure 7B shows the assembly of
Figure 7A with the thread-like coupling member (104)
loosened to illustrate how the chain knots (130) may be
formed. Figure 7C diagrammatically represents release of
the assembly of Figure 7A or 7B. The illustrated stitch is
releasable by pulling one end of the line that results in
releasing of the cylindrical or tubular restraining member
and then deployment of the device. This particular stitch
is called a chain stitch and may be created with a single
needle and a single line. A chain stitch is a series of
loops or slip knots that are looped through one another
23
SUBSTITUTE SHEET (rule 26 )
CA 02575978 2007-02-02
such that one slip knot prevents the next slip knot from
releasing. When the line is pulled to release a slip knot,
the following slip knot is then released and that releases
the next slip knot . This process continues during pulling
of the line until the entire line is pulled out of the
restraining member.
Referring to Figures 7A-C, as the unknotted portion
or the lead (132) of the thread-like coupling member (104)
is pulled, such as in the direction shown by reference
arrow (134), each consecutive chain knot (130) releases
the next adjacent one. In the preferred embodiment, the
chain knots (130) of the coupling member (104) are
arranged to progressively release the collapsed implant in
a direction away from the distal portion of the delivery
catheter as shown in Figure l0A and as will be discussed
in detail below.
Referring to Figures 8A through 8F, a method for
making an assembly comprising a restraining member with a
collapsed or compressed implant therein is shown for
purposes of example. Figure 8A shows the restraining
member (102) with its side margins releasably coupled to
one another and its left end dilated by a tapered
mechanical dilator (402). A small funnel (404) is then
inserted into the restraining member (102) as shown in
Figures 8B and 8C. The small funnel (404) and restraining
member (102) are then mounted onto a pulling frame (410),
and a large funnel (406) is fitted into the small funnel
(404) as shown in Figure 8D. Traction or pull lines (408),
which have been sutured to one end of the stent-graft,
(106) are pulled through the large funnel (406), small
funnel (404), and restraining member (102) with a tapered
mandrel (416). As shown in Figures 8F, the pull lines
(408) are fastened to a tie down post (412) located on a
tension screw (414) and then are pulled by the tension
screw (414). The stent-graft (106) is then pulled and
24 ENTERED SEP 2 S 2A01
40155765.1
CA 02575978 2007-02-02
WO 98/27894 PCTlUS97R1641
collapsed sequentially through the large (406) and small
(404) funnels, and then into the restraining member (102).
Once the stent-graft (106) has been radially collapsed
into the restraining member (102), which has its side
margins coupled together, the pull lines (408) can be
removed. The mandrel (416) may be inserted into the
restrained implant to facilitate introduction of another
component. In the preferred embodiment, a multilumen
catheter (136) (Figures 9 - 11) is introduced through the
center of the compressed stent-graft (106) and is used to
deliver the radially restrained stent-graft to the desired
endolumenal site.
It also is noted that the funnels may be chilled to
facilitate compression of the stent when the stent is made
of nitinol. That is, when the stent is made of nitinol,
the funnels may be chilled below 0'C or below the
transition temperature (Mf) where nitinol is in its
martensitic state. In addition, the stent-graft could be
folded first and then reduced in profile by pulling
through the funnel and into the restraining member.
Cooling may be accomplished by spray soaking the stent-
graft with chilled gas such as tetrafluroethane.
Micro-Dust' dry circuit duster manufactured by MicroCare
Corporation (Conn) provides' suitable results. The spray
canister preferably is held upside down to discharge the
fluid as a liquid onto the stent-graft.
A method of deploying an implant will be described
with reference to Figures 9-11. In general, an implant may
be delivered percutaneously with the delivery systems
described herein, typically through the vasculature, after
having been assembled in the reduced diameter form (see
e.g. Figure 1). At the desired delivery site, the implant
may be released from the restraining member, thus allowing
the implant to expand or be expanded against the lumen
wall at the delivery site. Although other devices
SUBSTITUTE SHEET ( ruie 26 )
CA 02575978 2007-02-02
including stents or stent-grafts may be used, such as
balloon expandable stents, the following example will be
made with reference to a self-expanding stent-graft, which
has the ability to fully expand itself into its final
predetermined geometry when unconstrained. More
particularly, the following example will be made using a
delivery system as shown in Figures 1 and 7A-C and a
stent-graft construction as shown in Figure 3.
Referring to Figures 9A and 9B, an implant delivery
assembly including a collapsed stent-graft (106) that is
confined within a restraining member (102) and, which
surrounds a distal portion of the multilumen delivery catheter
(136), is shown. The attending physician will select a device
having an appropriate size. Typically, the stent-graft
will be selected to have an expanded diameter of up to
about 20% greater than the diameter of the lumen at the
desired deployment site.
The delivery catheter preferably is a multilumen
catheter. The proximal portion of the catheter (136) is
coupled to a hub (140), which includes a guidewire port
(143) for a guidewire (142), and a deployment knob (144),
which is coupled to the lead (132) of the thread-like
coupling member (104). Accordingly, when the knob (144) is
retracted, the restraining member (102) is released so
that the stent-graft may expand. The hub (140) also may
include a flushing port (146) as is conventional in the
art. The stent-graft (106) is held axially in place prior
to deployment by a proximal barrier (148) and distal
barrier (150) which are positioned around multilumen delivery
catheter (136) adjacent to the proximal and distal
portions, respectively, of the restrained stent-graft. The
proximal and distal barriers (148, 150) may be fixedly
secured to the multilumen delivery catheter (136) to restrict
any axial movement of the restrained stent-graft. The barriers
preferably are positioned to abut against the stent-graft
26 ENTERED SEP 2 5 2001
40155765.1
CA 02575978 2007-02-02
or restraining member. The lead (132) of the coupling
member (104) is passed through an aperture (152) in the
proximal barrier (148) which is fluidly coupled to a lumen
in the multilumen delivery catheter (136) so that the coupling
member lead (132) can be coupled to the deployment knob (144).
Figures 9A and 9B show advancement of the catheter (136)
and the restrained implant through a vessel (154) toward a
desired site. Referring to Figures 10A and lOB, once the
restrained stent-graft reaches the desired site (156), the
deployment knob (144) is retracted so that the stent-graft
progressively expands as shown in the drawings as the
coupling member (104) is removed from the restraining
member. The coupling member preferably is arranged to
facilitate stent-graft expansion in a direction from the
distal to proximal ends of the stentgraft (i.e., in a
direction from the catheter tip to the catheter hub).
Figures 11A and 11B show the stent-graft (106) and
restraining member (102) in their final implantation
position after the coupling member and catheter have been
removed therefrom. In another embodiment, multiple
restraining members may be used as shown in Figure 9C.
When the multiple coupling members (104) are released
simultaneously implant deployment time may be reduced.
A method for deploying a balloon expandable
stent-graft may be the same as that described above, with
the exception that after the coupling member (104) has
been retracted from the eyelets (116), the balloon, which
may be positioned inside the stent-graft prior to
delivery, is inflated to expand the stent-graft (106) and
then deflated for removal through the catheter (136).
According to further embodiments of the invention,
multidirectional coupling member release or multiple
coupling members may be used. These configurations may
facilitate more rapid deployment of the implant than when
a single unidirectional coupling member is used. Figures
27 ENTERED SEP 2 5 2001
40155765.1
CA 02575978 2007-02-02
WO 98/27894 PCT/US97/21641
12A - 12D diagrammatically show multidirectional
deployment of a restrained implant according to the
principles of the invention where a coupling member
arrangement is provided to release the implant from its
middle portion, preferably its axial center, outward
toward the implant ends. Although a particular coupling
member configuration is not shown in these diagrammatic
representations, one suitable coupling configuration is
shown in Figure 13 where the leads (132) may be passed
through the aperture (152) and coupled to the deployment
knob (144) as shown in Figure 9A and described above.
Referring to Figure 12A, the restrained stent-graft,
which is positioned on the distal end portion of delivery
catheter (136), is advanced through a vessel (154) for
deployment in an aneurysm (158). The axial midpoint of the
restraining member (102) preferably is positioned at the
center of the aneurysmal sac. As the coupling member
arrangement unlacing propagates from middle of the
construct toward the proximal and distal ends of the
restraining member (102) and the stentgraft (106), the
stent-graft (106) progressively expands from its axial
midportion toward its ends as shown in Figures 12B and
12C. This may be accomplished by pulling the leads (132)
shown in Figure 13 simultaneously when the arrangement in
that figure is used. The stent-graft size is selected so
that when the restraining member is fully released and the
stent-graft fully deployed as shown in Figure 12D, the
proximal and distal portions of the stent-graft are
positioned against the proximal and distal necks of the
aneurysm. The delivery catheter may then be retracted.
As is apparent from the drawings, this embodiment
advantageously allows fluid flow through the aneurysmal
sac to remain substantially unobstructed during the
release of the restraining member. For example, the
stent-graft ends are still constrained at the deployment
28
SUBSTITUTE SHEET (rule 26 )
CA 02575978 2007-02-02
time shown in Figure 12C where the aneurysm neck regions
are shown minimally obstructed. In addition, this
simultaneous, multidirectional release of the restraining
member advantageously reduces the time in which fluid flow
in the vessel may disturb the implant position as it is
deployed as compared to a single directional release
mechanism such as that shown in Figures 9-11.
Referring to Figure 13, a multiple coupling member
configuration is shown. The illustrated arrangement
includes two lacing configurations (151) and (153). Except
for the placement of the lead (132) of thread-like
coupling member (104), configuration (153) is the mirror
image of configuration (151). Accordingly, description of
only one of.the configurations will be made for purposes
of simplification. Referring to the lacing configuration
(152), configuration (153) is the same as that shown in
Figures 7A-C with the exception that configuration (153)
further includes two additional slip knots, generally
designated with reference numeral (504), and tuck or loop
arrangement (506). The additional slip knots are not
interwoven in the restraining member and provide a delay
mechanism for release of the coupling member, as is
apparent from the drawings, when the lead (132) is pulled
in the direction of the arrow (134). Thus, inadvertent
pulling of the lead (132) will not immediately begin to
release the coupling member from the restraining member.
The tuck arrangement simply involves tucking the slack
from lead (132) under stitches at various intervals as
shown so that the additional slip knots (504) may be
pulled out of the way for delivery. In addition, the tuck
or loop arrangement (506) provides an additional delay
mechanism for release of the slip knots.
As discussed, the delivery systems described above
can be used with other implants or devices. These systems,
for example, can be used in conjunction with the
29 ENTEREO SFP 2 5 2001
40155765.1
CA 02575978 2007-02-02
bifurcated devices described below.
The modular stent-graft of Figures 14A through 14D
generally has two principal components; a main body (700)
and a contralateral leg (730) each generally having a
graft member attached to a stent member according to the
description above. The main body (700) generally has a
number of sections which have distinct overall
constructions. A distal trunk section (708) has a single
lumen structure beginning at a distal end (702) of the
main body (700) and continuing until a bifurcation point
(728). The bifurcation point (728) is the location within
the prosthesis where the single lumen of the distal trunk
section (708) bifurcates into internal two flow lumen.
An intermediate section (710) begins at the
bifurcation point (728) and continues to the receiving
hole (704). In the intermediate section (710), the stent-
graft has an internal graft structure which is bifurcated
into two lumen surrounded by a generally tubular,
single-lumen stent structure. Finally, a proximal section
(712) is a single lumen structure for both the stent
member and the graft member and includes an ipsilateral
leg (726) which terminates at an ipsilateral leg hole
(706).
The graft member of the intermediate section (710)
bifurcates the single lumen distal trunk section (708)
into the ipsilateral leg (726) and an internal female
receiving lumen (703). The receiving lumen (703)
terminates at a receiving hole (704). The receiving hole
(704) and receiving lumen (703) accommodate delivery and
attachment of the contralateral leg component (730).
Preferably, the graft material at the distal end (734) of
the contralateral leg component (730) is scalloped as
shown more clearly in Figure 23 discussed below.
The receiving hole (704) is supported by a wire
structure around a substantial portion of its periphery so
30 ENTERED SFP 2 5 2001
40155765.1
CA 02575978 2007-02-02
WO 98/27894 PGT/US97/21641
that the receiving hole (704) is held open after
deployment. In a preferred embodiment the wire structure
that supports the receiving hole (704) is an independent
wire ring (714).
The independent wire ring (714) is located in the
general area of the receiving hole (704) in the
intermediate section (710). The independent wire ring
(714) ensures that the graft material at the receiving
hole (704) is supported in an open position to receive the
distal end (734) of the contralateral leg (730). In
absence of such support, the receiving hole (704) may not
reliably open after delivery of the main body component
(700) because within the intermediate section (710) the
bifurcated graft member in the area of the receiving lumen
(703) does not have full stent support on its interior
periphery. This may be better seen in Figure 18 which
shows the absence of any internal stent support of the
interior graft periphery (785) in the area of the
receiving lumen (703).
The independent wire ring (714) may be comprised of
the same materials as the other stent-graft sections
discussed above and is preferably self-expanding. In a
preferred embodiment, the independent wire ring comprises
a single turn of an undulating wire stent material
surrounded by at least one layer of tape which is heat
bonded to the receiving hole (704). Alternatively, the
independent wire ring (714) could be formed as the last
turn of the main body (700).
A radiopaque marker may be used to make the receiving
hole (704) visible during implantation. Such a marker may
include a radiopaque wire adjacent to the independent wire
ring (714). Such markers make it easier to see the
location of the receiving hole (704) after deployment of
the main body (700) within the mammalian body.
This construction of the intermediate stent section
31
SUBSTITUTE SHEET (rule 26 )
CA 02575978 2007-02-02
WO 98/27894 PCP/US97121641
(710) as seen in crosssection in Figure 14C is
characterized by a single-lumen stent member and
bifurcated graft member and offers both a smaller
compressed profile as well as simplified manufacturing
over constructions which have discrete stented leg
features. The compressed profile is determined largely by
the physical amount of stent and graft material present in
a given section. This construction eliminates the stent
material that.would normally support the inside periphery
of the bifurcated graft section resulting in less stent
material to compress in that region. As the main body
component (700) is compressed for delivery as discussed
above, the compressed profile is significantly smaller
than would be a structure that had a section of bifurcated
stent over the section of bifurcated graft.
Even though bifurcated flow is supported,
manufacturing is simplified because there is no bifurcated
stent section. Winding a bifurcated stent section in one
piece, for example, is a more complex process. Likewise,
winding separate cylindrical stent structures and
connecting them to form a bifurcated stent structure is
complicated and ultimately may be less reliable. The
intermediate section (710) allows the entire stent member
that covers the main body component (700) to be made from
a single undulating wire arranged in multiple helical
turns. The result is a bifurcated- stent-graft device which
is simple to manufacture, easily compressible and which
expands reliably upon deployment.
An alternate construction of the intermediate stent
section (710), is shown in Figure 14D. The intermediate
stent section (710') has a shape characterized by the
indented regions (727). The shape could generally be
described as a'figure-8', except that the area between
the bifurcated graft member remains unsupported at its
centermost region. This construction is still a single
32
SUBSTITUTE SHEET ( rule 26 )
CA 02575978 2007-02-02
stiffness, flexibility and kink-resistance. With
complicated structures, such as those required for
treating a bifurcated site, it is increasingly difficult
to obtain the desired structural properties because
optimizing one may negatively effect the other.
For instance, optimizing the global axial stiffness
of a stent or stent-graft will necessarily make the device
significantly less flexible and consequently impair its
resistance to kinking and lessen its ability to conform to
the natural bends or curves the body's vasculature.
Conversely a device that has high flexibility with little
axial stiffness is difficult to properly deploy and does
not aid in anchoring the device in the desired location.
With these constraints in mind, it has been
discovered that having a bifurcated stent-graft which has
segments constructed with varying structural properties
offers improved deployability, is less susceptible to
kinking, and favorably tends to maintain its desired
position after deployment while allowing sufficient
flexibility to accommodate movement by the body. The exact
structural properties desired may depend on the location
where the prosthesis is to be deployed.
For these reasons, it is preferable that the
bifurcated stent or stent-graft be constructed with at
least two segments having structural properties different
from one another. For example, in Figure 14A, a length of
the distal section (708) and the intermediate section
(710) may be constructed with a higher axial stiffness for
improved deployment and positional stability while the
proximal section (712) may be constructed to have higher
flexibility to accommodate the geometry of the iliac
artery.
It may be further desirable to have a number of
segments that have different structural properties.
Accordingly, the main body component (700) and the
34 ENTERED sFp 2 g 200~
40155765.1
CA 02575978 2007-02-02
contralateral leg component (730) of the assembled
stent-graft (740) have segments constructed with
structural properties different from adjacent segments. In
one preferred embodiment shown in Figure 15, the main body
component (700) has four different segments constructed
with different structural properties. The distal segment
(742) is constructed to have higher axial stiffness than
the more flexible proximally adjacent segment (744). The
proximal section (748) is constructed to have a higher
flexibility than that of its distally adjacent segment
(746). Likewise the contralateral leg component (730) has
an axially stiffer distal segment (750) and a more
flexible proximal segment (749).
There are a number of ways to alter the structural
properties of stent or stent-graft components. One way of
selectively altering the structural properties of a
stent-graft segment is to use a tape member for that
segment that has different physical dimensions. Such a
tape member is discussed above with reference to the tape
member (128) of Figure 3. For example the tape member
width, thickness or spacing may be increased, from the
preferred dimensions discussed above, in a segment where
it is desirable to have increased or decreased stiffness.
For example, the use of wider tape wound with closer
spacing will increase the stiffness in that area.
Another way of selectively altering the structural
properties of a stent or stent-graft segment is shown in
Figures 14A and 15. Extended struts (718) and (719) may be
used to increase the axial stiffness of a stent-graft
segment. Extended struts are formed by extending an apex
on one turn of the undulating wire until it contacts an
apex on an adjacent turn. This contact between an extended
strut and the apex of an adjacent stent turn provides an
added amount of axial stiffness. In a preferred
embodiment, a layer of tape (not shown) is applied around
ENTFRED SEP 2 5 200t
40155765.1
CA 02575978 2007-02-02
the device in a helical pattern that covers each of the
apexes of the extended struts. This additional layer of
taping keeps the strut pairs together.
Referring to Figure 14A, a first helical stent turn
(720) and a second helical stent turn (721) have a
generally undulating shape having apexes. An extended
strut (718) of the stent turn (720) is formed having its
apex near or in contact with the apex of the stent turn
(721) directly below. The extended strut (719) is
similarly formed by extending an apex of the stent turn
(721) directly down to contact the apex in the turn below.
This pattern in continued, each time spacing the extended
strut over one undulation. This results in a helical
pattern of extended struts down the length of the device.
Of course, the extended struts may be arranged in patterns
other than the helical configuration described.
A number of these patterns may be employed in any one
segment or the extended strut pattern may be used in other
segments to increase axial stiffness. Preferably the
distally adjacent segment (746) on the main body component
(700) and the axially stiff distal segment (750) on the
contralateral leg component are constructed with extended
struts as shown in Figure 15.
Referring to Figure 15, the distal end (702) may be
sized to properly fit the inside diameter of the target
artery, in this case the abdominal aortic artery.
Typically the prosthesis is designed to have an
unconstrained diameter slightly larger than the inside of
the target vessel.
The ipsilateral and contralateral legs of the
assembled bifurcated stent graft (740) are typically the
same size at their distal ends regardless of the size of
the distal end (702) and undergo tapered sections (724)
and (738) that taper to a diameter which corresponds
approximately to the internal diameter of the iliac
36 ENTEREp 200
40155765.1
CA 02575978 2007-02-02
arteries. These tapered sections (724) and (738) are
preferable to abrupt changes in diameter as they tend to
produce superior flow dynamics.
After deployment, the assembled bifurcated
stent-graft (740) must establish sufficient contact with
the healthy vessel lumen on each side of the aneurysm
(758) so that the device does not migrate or dislodge when
subjected to the relatively high fluid pressures and flow
rates encountered in such a major artery, especially when
the body again becomes mobile after recovery. Further,
sufficient contact must be made so that there is no
leakage at the distal end (702), the ipsilateral leg hole
(706) or the proximal end (736) of the contralateral leg.
Anchoring or staying features that allow the stent or
stent-graft exterior to anchor itself to the vessel lumen
wall may be provided to help the device seal to the vessel
wall and maintain its deployed position. For example,
anchors (716) as seen in Figures 14A and 15 are provided
on the main body component (700) and could also be
provided on the contralateral leg component (730).
Preferably the top stent portion (717) is directed
angularly outward. This flared stent portion works to
force the anchors (716) into the vessel wall as the top
stent portion (717) expands under force into radial
interference with the vessel wall upon deployment.
A preferred construction for an anchor (716) is shown
in Figure 17. This construction involves extending two
wires from the upper stent turn (762) under an apex of an
adjacent lower stent turn (764). The two ends of stent
wires (760 and 761) are then bent out and away from the
graft material (768). Extended struts (771) are formed
adjacent to each anchor in the manner described above
except the extended struts extend under the adjacent lower
stent turn (764) down to a third stent turn (765). This
extended strut arrangement provides support for the
37 ENTERED SEP 2 5 2001
40155765.1
CA 02575978 2007-02-02
anchors (716) and provides for low stresses in the wires
(760 and 761) under the application of bending forces
encountered as the prosthesis expands into the vessel
wall. The extended struts (771) minimize the localized
deformation of the stent-graft structure in the area of
the anchors by providing broader support.
Another construction of the anchors (716') are shown
in Figure 16. An anchor (716') is formed in the same
manner except the ends of the anchor remain connected in a
'U-shape' configuration as shown. An anchor (716')'may be
formed at any location on the stent-graft. Most
preferably, the anchors are formed in an evenly spaced
pattern around the top stent portion (717) (Figure 14A).
It should be apparent that the anchors as described
above are not limited in use to the stent-graft
combination shown in the figures but indeed could be used
in any non-bifurcated or stent only construction that
require similar functionality.
Sealing at the vessel wall may also be enhanced by
the alternate construction shown in Figure 17 by way of a
sealing mechanism. A sealing mechanism can be used with
any type of implant, including any of the implants
discussed above. For purposes of illustration, the sealing
mechanism is shown with reference to the bifurcated
implant of Figure 14 and comprises seal member (772) as
seen in detail in Figures 16 and 17. The sealing mechanism
described below can be used with any of the implants
discussed above.
One preferred construction for seal member (772) in
the variations shown in Figures 16 and 17 may be similar
to the preferred construction for the tape member used in
constructing the stent-graft tubular member, as is
provided in reference to Figure 1 and Figure 3 above.
In general, a thin walled ePTFE tape is used for seal
member (772) similarly as that for tape member (128),
38 ENTERED SEP 2 5 2001
40155765.1
CA 02575978 2007-02-02
shown variously in the previous figures. The tape used for
seal member (772) is adhered to the outer surface of the
stent-graft, including over tape member (128), described
previously for bonding the stent and graft members. Seal
member (772) has an inner surface constructed of a similar
material for either the outer surface of the tape member
(128) or the outer surface of the graft-member (124),
depending upon which surface the seal member is desirably
adhered. -
First cuff end (767) is bonded to the stent-graft
outer surface and second cuff end (769) is not, in order
to form the unadhered flange to function as a oneway valve
against peri-stent-graft flow. Seal member (772) may be
selectively adhered along its length in this manner by
providing a variable inner surface to the seal member such
that, upon heating, only the surface in the region of
first cuff end (767) bonds to the outer surface of the
stent-graft. For example, the inner surface of seal member
(772) may have an FEP liner in the region of first cuff
end (767) but not in the region of second cuff end (769).
In this case, upon contacting an outer surface of the
stent-graft that has a uniform FEP outer surface, only
first cuff end (767) may be heat secured thereon.
Alternatively, seal member (772) may have a uniform
inner surface, such as constructed of FEP, and a variable
outer surface, such as with a selective portion of FEP,
may be provided either on the tape member (128) or on the
graft member (124) in the region where the heat bonding of
seal member (772) is desired. Still further, seal member
(772) may have a uniform surface and may be positioned
over tape member (128) and graft member (124) so that
variability between the outer surfaces of tape member
(128) and graft member (124) causes a selective bonding
with the first cuff end (767) over one of those surfaces.
Further to the construction of seal member (772), the
39 ENTERED SEP 2 5 209
40155765.1
CA 02575978 2007-02-02
particular wall of thickness of the tape which may be used
for this component should desirably be as thin as possible
to functionally provide the flange-one-way- valve function
for that member. This is because, since seal member (772)
is over the outer surface of the other stent and graft
components of the stent-graft, seal member (772) is
believed to be the profile-limiting feature of the overall
assembly. Therefore, in a particular design, seal member
(772) may desirably be a thinner wall than for the tape
member used to construct the stent-graft described in
reference to Figures 1 and 3.
Further referring to the particular constructions and
related methods just described for adhering seal member
(772) to the outer surface of the underlying stent-graft,
it should be apparent to one of ordinary skill in the art
that the desired construction and heat securing technique
for seal member (772) is premised upon the theory that,
where one polymer meets a like polymer (such as FEP
meeting FEP), heating under proper conditions will allow
for a selected heat bond. Any suitable means may be used
for securing a seal member to the outer surface of a given
tubular member, as would be apparent to one of ordinary
skill.
Further there is a plurality of circumferential strut
spaces between the struts of the stent member. It is
believed that these spaces may provide a path for leakage
flow around the outer surface of the graft member and
along the outside of the stent-graft. Second cuff end
(769), however, captures such leakage flow beneath its
flange, which can not propagate along the outer surface of
the stent graft because first cuff end (767) is secured to
the outer surface of that stent-graft. In other words,
flow over the stent-graft and into an aneurysm is
occluded.
Furthermore, when anchor (716) is anchored into
FNTERFn ;FP 2 5 20
40155765.1
CA 02575978 2007-02-02
the wall of abdominal aortic artery as shown in Figure 15,
it has been observed that the portion of main body
component (700) at and adjacent to the anchor (716)
may be forced away from the artery wall. This action
causes a separation between the outer surface of main body
(700) and the artery wall, which separation is believed to
create a leakage flow path. The flange of seal member
(772) captures that flow and occludes it from propagating
into the aneurysm (758).
In addition to maintaining a good contact with the
vessel lumen walls, the components of the stent-graft must
make sufficient contact with each other such that the
separate modules stay attached and do not leak at their
engagement interface. The stent-graft shown in Figure 18
illustrates several important features designed to
effectuate a leak-free and positionally stable seal at the
interface between the receiving lumen (703) of the main
body component (700) and contralateral leg component
(730).
Figure 18 shows a partial cross-section of the
assembled stent-graft. The contralateral leg component
(730) has been inserted into the receiving lumen (703) of
the main body component (700). This cross-sectional view
shows clearly that the main body component (700) includes
a main body graft member (780) and a main body stent
member (782). The contralateral leg component (730)
includes a contralateral graft member (784) and a
contralateral stent member (786).
At the interface between the contralateral leg
component (730) and the receiving lumen (703), the
assembly provides for an extending sealing region (790).
Preferably the extended sealing region (790) consists of a
generally cylindrical interfering friction fit between the
outside diameter of the contralateral leg component (730)
41 ENTERED SFP 2 S 2001
40155765.1
CA 02575978 2007-02-02
and the inside diameter of the receiving lumen (703). That
is, the natural or resting outside diameter of the self
expanding contralateral leg component (730) would be
larger than the natural inside diameter of the receiving
lumen (703). Thus the forces created by the interference
act to seal the two components and also serve to resist
movement of the two components.
The type of generally cylindrical extended sealing
region just described has many advantages. First, it
allows for the stent and graft structures in the extended
sealing region (790) to be constructed of relatively
simple generally cylindrical elements that are easily
manufactured. Because the extended scaling region (790)
extends over a large length it necessarily has a large
surface area to effectuate sealing between the components.
This larger sealing area typically provides that multiple
turns of the stent structures will be engaged in an
interfering and thus sealing relationship.
In one preferred embodiment, the extended sealing
region has a length in excess of one-half of the diameter
of the receiving lumen (703), more preferably the length
is greater than the diameter of the receiving lumen (703),
and most preferably the length is more than 2 times the
diameter of the receiving lumen (703).
Because the manufacturing tolerances of the
simplified shapes are easily controlled and because the
engagement of the extended sealing region (790) is quite
large, a highly reliable joint is formed between the
modular components. Even so it may be desirable to create
one or more localized zones of increased interference to
increase the sealing capability and positional stability.
Localized zones of interference may be created in a
number of ways. In a preferred embodiment, an annular ring
of decreased diameter is formed within the receiving
lumen. Such a localized decreased diameter causes a
42 ENTERED SEP 2 5 20M
40155765.1
CA 02575978 2007-02-02
greater interference with the outside diameter of the
contralateral leg component in a localized area while the
remainder of the engagement with the receiving lumen is
subject to the general interference friction fit described
above.
One way of creating a localized decreased diameter is
illustrated in Figure 20 which shows a partial
cross-section of the extended scaling region (790). A zone
of reduced diameter (799) is created by placing an
anchoring ring (798) between the graft member (780) and
the stent member (782) of the receiving lumen (703). The
anchoring ring may be made from any polymeric or wire
material, preferably a material that will not inhibit the
receiving lumen from selfexpanding to an open position.
Most preferably the material is a suture material,
typically ePTFE.
Alternately, localized zones of decreased diameter
may be created as shown in Figures 21 and 22 by folding a
portion of the graft member (780) back up into the
receiving lumen (703). In Figure 21, the zone of reduced
diameter (806) is formed by creating a folded flap (808)
of the graft member (780) around an anchoring ring
(802). The flap is heat bonded in place roughly at a
location (804) as shown. In Figure 22, the zone of reduced
diameter (809) is formed of flap (808) and heat bonded
roughly at a location (807) in a similar manner but
without any anchoring ring. The localized interference
using these methods tends to cover a larger area and the
flap (808) provides a more flexible member to seal against
the outside diameter of the contralateral leg component
(730).
One further aspect of ensuring a good seal between
the stent-graft components involves the use of a scalloped
stent-graft construction at the distal end of the
contralateral leg component (810). To create this
43
ENTERFR SFp 2 5 200~
40155765.1
CA 02575978 2007-02-02
scalloped construction, the graft material between the
apexes of the stent member is removed on the last turn of
the stent. For example scallop (812) may be formed by
removing (or cutting and folding under) the graft material
from between a first apex (814) and an adjacent apex
(816) as shown in Figure 23.
The advantages of using a scalloped arrangement are
illustrated in Figures 24A through 25B. Figure 24A shows a
cross-section of the fully expanded contralateral leg
component (730) having an unscalloped construction. A
first apex (822) and an adjacent apex (824) have
continuous graft material (784) in the area between them.
When the apex (822) and the adjacent apex (824) are forced
together in the directions of the arrows (820), the graft
material (784) forms a buckle or wrinkle (818) which is a
potential leak path or is a potential site for
thrombogenic material to build up as seen in Figure 24B.
The scalloped construction shown in Figures 25A and 25B,
on the other hand, have no graft material between the
first apex (814) and the adjacent apex (816) and there f ore
when forced together do not form a graft material wrinkle.
The wrinkle (818), mentioned above may also be formed
when the stent-graft is not allowed to expand to its
complete diameter. For instance it is quite common that
the receiving lumen or vessel wall internal diameter is
smaller than the fully expanded stent-graft outer
diameter. This being the case, it should be clear that the
scalloped construction may alternately be used at any of
the terminal openings of the main body component or the
contralateral leg component. Preferably, the distal end
(702) of the main body component (700) also has this
scalloped construction as shown in Figures 14A and 14B.
In the previous discussion we have referred generally
to a stent-graft that includes a graft member. While the
construction of such straight stent grafts are discussed
44
ENTEREn-
40155765.1
CA 02575978 2007-02-02
at length above, the construction of a bifurcated graft
member is illustrated in Figures 26, 27A and 27B. A
bifurcated graft member suitable for construction of the
main body component (700) discussed above is generally
formed of two graft members: the ipsilateral tapered graft
(840) and the contralateral tapered graft (842). The
separate contralateral leg graft component (844) is a
straight or tapered section and may be formed according to
the principles discussed in the first section above.
The ipsilateral tapered graft (840) has three
sections which are separated by tapers. A top section
(846), a middle section (848), and a bottom section (850).
The body component graft (854) is formed by heat bonding
the top section (846) of ipsilateral tapered graft (840)
to the top section (847) of contralateral tapered graft
(842). This heat bonding forms a common septum (856) which
in a preferred embodiment is subsequently cut away to
produce a smooth bifurcation. Cutting away the
septum material prevents fluid flow disturbance or
blockage that could result from deviation of the septum.
Such deviation is caused by the fluid pressure and is
aggravated if the stent-graft is radially compressed in a
manner which causes the septum to become loose or no
longer taut.
In another embodiment, a graft section may be
constructed in the manner illustrated in Figures 27A and
27B. According to this embodiment, the body component
graft (867) is constructed from two pieces. A tubular
graft section (860) is bent into a'U-shape'. A top hole
(864) is formed by notching the top of the 'U-shape'.
Upper graft section (862) is placed over the top hole
(864) of tubular graft section (860). The two pieces are
bonded together at the bonding interface (866).
Preferably, the two graft pieces are heat bonded while
supported by interior mandrels (not shown) to obtain the
ENTERM SFo 2
40155765.1
CA 02575978 2007-02-02
WO 98/27894 PCT1US97121641
desired shape and smooth interior. However, upper graft
section (862) may be attached to the tubular graft section
(860) at the bond interface (866) in any manner that
provides a sufficiently leak free seal. For example the
components may be sutured together or adhesive bonded.
In use, the modular bifurcated stent-graft is
typically delivered percutaneously through the vasculature
of the body. Preferably the prosthesis is delivered by way
of a restraining member as described in detail above.
Figures 28A though 28E diagrammatically illustrate
deployment of a bifurcated stent-graft with a restraining
member (902) using a percutaneous catheter assembly.
Referring to Figure 28A, a multilumen catheter assembly
(928) has been inserted to a selected site within a body
lumen. The main body component (700) of a bifurcated
stent-graft is held in a compressed state about a
guidewire (926) and a guidewire lumen (929) by a
restraining member (902) and a coupling member (906). The
collapsed main body component (700) is held axially in
place prior to deployment by a distal barrier (930) and a
proximal barrier (932). The distal (930) and proximal
(932) barriers are typically affixed to the cuidewire
lumen (929). The coupling member (906) extends through the
eyelets (920) of the restraining member (902) forming
chain knots and into the multilumen catheter (928).
Figure 28A shows advancement of the multilumen
catheter (928) with the distally located main body
component (700) and the restraining member (902) into
implantation position, typically at the bifurcation of a
major vessel. During deployment it is critical that the
surgeon align the main body component (700) so that the
ipsolateral leg (726) will extend down one branch of the
bifurcated vessel, and so the receiving hole (704) and the
receiving lumen (703) will be lined up with the other
branch of the bifurcated vessel so as to receive the
46
SUBSTITUTE SHEET (rule 26 )
CA 02575978 2007-02-02
WO 98/27894 PGT/US97/21641
contralateral leg component (730).
One way of facilitating this alignment is to provide
radiopaque markers so that the surgeon may readily
determine the rotational position of the main body
component (700) prior to deployment or release from the
restraining member (902). In a preferred embodiment, a
long marker (934) is located on the contralateral side of
the compressed assembly and a shorter marker (936) is
placed on the ipsolateral side. Preferably these markers
are placed on the stent prior to compression but may
alternatively be part of the restraining member. Having
one marker of a different length allows the surgeon to
identify the orientation of both the ipsolateral leg and
the receiving lumen relative to the bifurcated vessel.
Once the assembly is properly aligned and positioned
for implantation, the coupling member (906) is pulled and
the restraining member (902) begins to release the
implant, typically at the distal end first. in the
preferred embodiment, the restraining member (902) is
located down the side as shown because it is less likely
to interfere with deployment of the receiving lumen (703).
Figure 28B shows the main body component (700)
radially expanding as the coupling member (906) is
retracted through the eyelets (920) of the restraining
member (902) and into the catheter assembly (928). In the
preferred embodiment, the restraining member (902) has
been fixedly attached to the main body component (700)
with a number of sutures along the length of the main body
component to prevent any relative longitudinal movement
between the implanted prosthesis and the restraining
member (902). The restraining member may optionally employ
a retracting or pull-down mechanism as described at length
above.
Figure 28C shows the main body component (700) and
the restraining member (902) in final implantation
47
SUBSTITUTE SHEET (rule 26 )
CA 02575978 2007-02-02
WO 98/27894 PGT/US97/51641
position at the vessel bifurcation after the guidewire
(926) and the catheter assembly (928) have been retracted.
Figure 28D shows the contralateral leg component
(730) being delivered to the contralateral receiving hole
using a restraining member (942). The procedure for
positioning and releasing the contralateral leg component
(730) is the same as that described above for implantation
of a generally cylindrical stent-graft except that certain
radiopaque markers may be employed to ensure its proper
position relative to the bifurcation point (728) of main
body component (700).
Radiopaque markers may be located, for example, to
indicate the position of the receiving hole (704), the
distal end (734) of the contralateral leg component (730),
and the bifurcation point (728) of the main body component
(700). These markers serve to indicate the position of the
contralateral leg component as it enters the receiving
hole (704) and its ultimate position relative to the
receiving lumen (703) which begins at bifurcation point
(728). In a preferred embodiment illustrated in Figure 19,
the radiopaque wires (794) may be heat bonded or imbedded
into the graft material (780) around the periphery of the
receiving lumen. Such radiopaque wires could be used in
other places such as the contralateral leg component
lumen, the ipsolateral leg lumen or the lumen at the
distal end of the main body component (700).
Figure 28E shows the assembled bifurcated stent-graft
in its final implantation state with the contralateral leg
component expanded into and engaged with the receiving
lumen of the main body component (700).
Figures 29A through 29D diagrammatically show the
same stent or stent-graft components being deployed except
that the restraining member (902) is released from the
center out towards as the coupling member (906) is
retracted. This may provide more accurate placement
48
SUBSTITUTE SHEET (rule 26 )
CA 02575978 2007-02-02
WO 99/27894 rcr/US'97121aa1
. . .,~.~~
relative to the bifurcation point of the vessel instead of
relative to the distal end as with end release.
While this invention has been described with
reference to illustrative embodiments, this description is
not intended to be construed in a limiting sense. Various
modifications and combinations of the illustrative
embodiments, as well as other embodiments of the invention
will be apparent to persons skilled in the art upon
reference to the description. It is therefore intended
that the appended claims encompass any such modifications
or embodiments.
The disclosures of the publications and patents that
are cited in this application are hereby incorporated by
reference.
49
SUBSTITUTE SHEET (rule 26 )