Note: Descriptions are shown in the official language in which they were submitted.
CA 02576084 2007-02-01
WO 2006/013599 PCT/IT2005/000390
DISPOSABLE DEVICE FOR ONE OR MORE INTRODUCTIONS,
TREATMENT AND SAMPLING OF BIOLOGICAL MATERIAL FROM AT
LEAST ONE OF THE SEPARATION PHASES PRESENT WITHIN
THE DEVICE, UNDER STERILITY CONDITIONS AND CONSTANT
PRESSURE
The present invention concerns a disposable device for multiple
introductions, treatment and sampling of biological material from one of
the separation phases under sterility and constant pressure conditions.
More specifically, the invention concerns a device of the above
kind for example allowing to carry out ex-vivo marking of figuration blood
elements, such as leucocytes, red cells and platelets, with radioactive
isotopes or other marking material, under sterility and safety conditions for
the operator.
In the following, the specification will be mainly addressed to
the use of the device for carrying out the ex-vivo marking of leucocytes
with radioactive isotopes under sterility conditions, but it is well evident
that the device according to the invention can be used for each
application, such as extraction of nucleic acids and proteins, requiring
admission and sampling of substances from a container, under sterility
conditions, in two or more phases and by the sampling within the container
occurring at different levels.
Thus, referring particularly to the scintigraphy with marked
leucocytes, it is known that it is a diagnostic investigation aiming
identifying presence of possible infection focus in organism that cannot be
individuated by other techniques.
The above method is based on intravenous injection of patient
white cells to which a radio-mimetic agent has been previously legato in
vitro, 99mTc-HMPAO (Roca et al. 1998) or "'In-oxina (Thakur et al. 1977),
so as to identify seats wherein they are accumulated putting into evidence
the presence of an infection focus.
As it is well known, scintigraphy with labelled leucocytes is used
in clinical practice for diagnosis of pathologies characterised by the
presence of acute inflammation areas. Scintigraphy with labelled
leucocytes is considered very important for diagnosis and follow-up of
articular prosthesis (Larikka et al. 2001) and vascular (Liberatore et al.
1998) infections, as well as of osteomyelitis (Krznaric et al. 1996). It
further
CA 02576084 2007-02-01
WO 2006/013599 PCT/IT2005/000390
2
has an important role for intestinal chronic inflammatory diseases (Martin-
Comin et al. 1999) and for post-surgical neurological infections (Medina et
al. 2000).
At present, preparation of labelled leucocytes occurs by various
protocols, all very similar each other.
Mainly used protocol (Roca et al. 1998) provides that:
- 50cc of blood are taken from a forearm vein of the patient by a
syringe containing a suitable dose of anti-coagulant (ACD);
- under sterility conditions, by the use of a laminar flow hood
and of a centrifugal machine, white cells are purified by the other blood
cells and then labelled by a radioactive substance (99mTc-HMPAO or ll'In-
oxina or 99mTcSnF); said operation takes about 90 minutes;
- at the end of the previous operation, suspension containing
white cells is injected in patient by intravenous mode and then images are
obtained by a gamma-camera of the whole body or of single body
portions, after about 3-4 hours and about 24 hours from the injection.
Different protocols for labelling of white cells employing 99mTc-
HMPAO (Karesh et al. 1897; Solanki et al. 1988; Roca et al. 1998) or
"'In-oxina (Thakur et al. 1977) are different each other only for minor
aspects.
All the known protocols provide the use of a laminar flow hood
for carrying out the procedure and skilled technical personnel authorised
to handle biological substances potentially infect or cancerogen
substances.
Thus, at present, this procedure can be carried out only with
nuclear medicine divisions with trained personnel and provided with a
laminar flow hood able allowing that the different phases comprising the
method can be carried out under sterility and anti-pirogen conditions for
patient and safety conditions for operator.
Said conditions are indispensable since labelled white cells are
re-injected within the patient at the end of the same procedure and can be
carrier of pathogenic micro organism virus.
The above problem remarkably limits diffusion of scintigraphy
with labelled leucocytes, notwithstanding it can be a basic test for some
pathologies, such as for diagnosis of articular prosthesis and vascular
infections and for osteomyelitis.
CA 02576084 2007-02-01
WO 2006/013599 PCT/IT2005/000390
3
Impossibility of making the above procedure with a hospital
creates noticeable logistic problems for transferring the patients in
structures where it is practiced: further consequence of the reduced
availability is unavoidably a long waiting list for patients. The other
remarkable problem, relevant to the separation and labelling procedure of
white cells, is the potential infective risk for exposition of the operator
carrying out the procedure for handling the blood.
In view of the above, it is well evident the advantage of having a
device as the one proposed according to the present invention that allows
remarkably limiting the infection risk.
Another object of the present invention is that of providing a
device allowing the separation and labelling of white cells as one of each
commercially available radio-mimetic agent (99mTc-HMPAO or "'In-oxina
or others) without the need of having a laminar flow hood, while the
standard procedure described by Roca et al. (1998) provides the "open
work" labelling but under the laminar flow hood in order to maintain the
sterility conditions of the preparation that must be then re-injected within
the patient, requiring the use of disposable sterile materials (Pasteur
pipette, falcon test tubes, etc.), training of personnel skilled for working
under sterility conditions, execution of periodic sterility controls of hood
and of the other apparatuses employed, high risk of viral and bacterial
contamination of the preparation, and high risk of contamination of the
operator when handling infect blood. To the above, it is necessary adding
the investment and maintenance expenses for the laminar flow hood.
By the solution according to the present invention, it is on the
contrary possible obtaining:
- reduction of disposable material to be used;
- reduction of the risk of infecting the preparation;
- reduction of the infection risk for the operator;
- higher execution simplicity of the procedure not requiring
specialised personnel;
- reduction of quality controls to be carried out.
It is therefore specific object of the present invention a
disposable device for one or more introductions, treatment and sampling
of biological material from at last one of the separation phases under
sterility and constant pressure conditions comprising a sealed sterile test
tube, said sterile test tube, comprised of glass or plastic material,
providing
CA 02576084 2007-02-01
WO 2006/013599 PCT/IT2005/000390
4
a first upper opening, or sampling opening, a second opening or inlet
opening, and a third opening, or filtered opening for maintaining the sterile
atmospheric pressure, said first opening providing the passage of a
needle, said needle having a length sufficient to reach the bottom of the
test tube, the coupling between said needle and the first opening being a
sealing coupling obtained by an elastic element allowing the translation in
a substantially vertical direction and further allowing inclination of the
needle; said second opening being sealed by a membrane comprised of a
material allowing the piercing by a syringe needle and closing back after
the removal of the needle; said third opening providing a filter or sealing
valve balancing the pressure inside the test tube and the environmental
pressure, guaranteeing sterility of the content; said device having
dimensions and materials allowing its centrifugal.
Preferably, according to the invention; said test tube can be
provided with a plug coupable with the same test tube by a sealing
coupling.
Always according to the invention, said plug can be integral with
the same test tube.
Still according to the invention, coupling between said first
opening and said movable needle can be comprised of a bellow or of an
elastic sheath.
Furthermore, according to the invention, said membrane is
integral with said test tube or with the plug of said test tube.
Always according to the invention, said third opening can be
provided with a filter, in order to prevent penetration of bacteria within the
test tube.
The invention further concerns a method for labelling of
figuration elements of blood, particularly leucocytes, with radioactive
isotopes under sterility conditions employing a device as described in the
above and providing the following steps:
- sampling by a syringe containing anticoagulant agent and
sedimentation agent, an amount of blood using a butterfly
device and gently mixing the contents;
- leaving sedimentation the blood within the syringe for 30-60
minutes;
CA 02576084 2007-02-01
WO 2006/013599 PCT/IT2005/000390
- at the end of sedimentation, transferring the plasma rich of
cells from the syringe to the device, introducing the butterfly
needle within the second opening;
- centrifugating the device for 5 minutes, creating on the
5 bottom of the test tube a red coloured pellet containing the
leucocytes, and then introducing a disposable sterile syringe
within the needle introduced within the first opening and
removing the supernatant from the device;
- after having suspended again the cellular pellet gently
agitating the device, adding 99mTc-HMPAO, already
prepared, by a disposable sterile syringe through the second
opening of the device;
- at the end of the incubation time necessary (about 10
minutes), adding physiological sterile solution by disposable
sterile syringe through the first opening and centrifugating
for 5 minutes;
- introducing a disposable sterile syringe in the needle
introduced in the first opening and removing the supernatant
from the device;
- adding 2-3 ml of physiological sterile solution by disposable
sterile syringe through the second opening and gently
suspending again the pellet before taking again the labelled
cells to be injected in the patient, introducing a disposable
sterile syringe in the needle of the first opening and
sampling all the contents of the device.
The present invention will be now described, for illustrative but
not limitative purposes, according to its preferred embodiments, with
particular reference to the figures of the enclosed drawings, wherein:
Figure 1 is a perspective view of an embodiment of a device
according to the invention;
Figure 2 is a perspective view of a particular of the device of
figure 1; and
Figure 3 is a front view of the device of figure 1.
In the figures it is shown a prototype of a disposable device
according to the invention particularly realised for ex vivo labelling of
leucocytes with radioactive isotopes under sterility conditions. However, it
must be once more evidenced that, simply modifying material and
CA 02576084 2007-02-01
WO 2006/013599 PCT/IT2005/000390
6
dimensions of the device, it will be possible realising the same for a
specific different application.
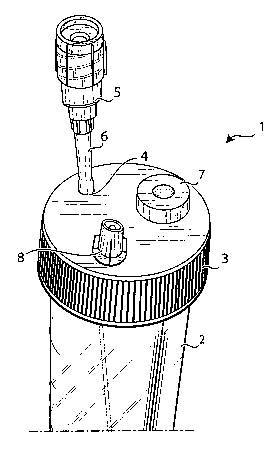
Observing now specifically figures 1 - 3, it is shown a
disposable device, generically indicated by reference number 1, providing
a sterile test tube, e.g. a test tube with a volume of 60 ml and a length of
12 cm, with a plug 3, that can be integral with the test tube 2 or sealingly
coupled with the same, provided with three openings, that will be
described in greater detail in the following.
Said test tube 2 provides a conical narrowing on its bottom for
collection of leucocytes pellet after centrifugations.
A needle 5 is provided in correspondence of the first-opening 4,
for example a needle having a diameter 19G and long 12 cm that can be
handled outside the test tube 2.
In correspondence of the coupling between needle 5 and
opening 4 a bellow or sheath 6 is provided, sealing coupled both to the
plug 3 of the test tube and to the needle 5, allowing mobility of needle 5
e.g. for 2 cm within the test tube 2, ensuring its sterility. In other words,
opening 4 is the outlet of the device according to the invention, through
which it is possible sampling contents of device 1 at different heights along
the test tube 2 thanks to the action of the bellow 6.
Projection of needle 5 and sheath 6 from the plug 3 of test tube
2 must be sufficient not to hinder the centrifugation phase.
Second opening 7 is comprised of a membrane, integrally
coupled with the plug 3, and thus not removable, said membrane being
comprised of a material having features allowing its disinfection with
alcohol and its piercing, even more times, by a needle.
Material of membrane 7 must re-close on itself after the piercing
by the needle and the removal of the same needle, so as to maintain
sterility of test tube 2.
Said opening 7 is used for introduction of materials inside the
test tube 2 by a needle coupled with a suitable syringe (not shown).
Third opening 8 provides a filter, for example a 0.2 filter,
guaranteeing the atmospheric pressure within the test tube 2, and at the
same time preventing penetration of bacteria within the same test tube.
As already said, device 1 according to the invention is a
disposable device, and it is realised in such a way to be introduced in
CA 02576084 2007-02-01
WO 2006/013599 PCT/IT2005/000390
7
every kind of centrifugal machine, since needle 5 is flexible and adaptable
to different rotors.
Coming now to describe, for exemplificative and not limitative
purposes, the steps of the use of device I according to the invention for
ex-vivo labelling of leucocytes with radioactive leucocytes, under sterility
conditions, the following sequence will be followed:
- by a 60 ml syringe containing 7.5 ml of anticoagulant agent
(ACD) and 7.5 ml of sedimentation agent (HES), 45 ml of
blood are sampled using a 19G butterfly device and gently
mixing the contents;
- leaving sedimentation the blood within the syringe for 30-60
minutes;
- at the end of sedimentation, transferring the plasma rich of
cells from the syringe to the device, introducing the butterfly
needle within the opening 7;
- centrifugating the device for 5 minutes at 150g. On the
bottom of the test tube 2 a red coloured pellet containing the
leucocytes will be created. Then a 20 cc disposable sterile
syringe is introduced within the needle introduced within the
opening 5 and the supernatant is removed from the device;
- after having suspended again the cellular pellet gently
agitating the device, adding 99mTc-HMPAO, already
prepared, by a disposable sterile syringe through the
opening 7 of the device;
- at the end of the incubation time necessary (about 10
minutes), adding physiological sterile solution by disposable
sterile syringe through the opening 7 and centrifugating for 5
minutes at 150g;
- introducing a 10 cc disposable sterile syringe in the needle 5
introduced in the opening 4 and removing the supernatant
from the device;
- adding 2-3 ml of physiological sterile solution by disposable
sterile syringe through the opening 7 and gently suspending
again the pellet before taking again the labelled cells to be
injected in the patient. Also the last operation is carried out
introducing a 5 cc disposable sterile syringe in the needle 5
of the opening 4 and sampling all the contents of the device.
CA 02576084 2007-02-01
WO 2006/013599 PCT/IT2005/000390
8
Summarising, the whole cellular labelling operation requires
four piercings of membrane 7 and three connections of a sterile syringe
with needle 5. The whole operation requires only seven disposable sterile
syringes, beside the device 1 according to the invention, thus obtaining a
remarkable save of disposable sterile material with respect to the standard
methods (Roca et al. 1998), but mainly difficulties are remarkably reduced
for the operator and consequently the possibilities of bacterial
contamination of preparation are strongly reduced.
The present invention has been described for illustrative but not
limitative purposes, according to its preferred embodiments, but it is to be
understood that modifications and/or changes can be introduced by those
skilled in the art without departing from the relevant scope as defined in
the enclosed claims.