Note: Descriptions are shown in the official language in which they were submitted.
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
USE OF N-DESMETHYLCLOZAPINE TO TREAT HUMAN
NEUROPSYCHIATRIC DISEASE
Field of the Invention
[0001] The present invention relates to the discovery of potent muscarinic
receptor agonist properties of the dibenzodiazepine compound N-
desmethylclozapine, 8-
chloro-l1-(1-piperazinyl)-5H-dibenzo[b,e][1,4]diazepine, which supports the
clinical use of
this drug as a stiperior therapeutic agent for the treatment of pain,
glaucoma, dementia,
affective disease, and psychosis.
Background of the Invention
[0002] The physiological actions of the hormone/neurotransmitter acetylcholine
are mediated, in part, by muscarinic acetylcholine receptors. Muscarinic
receptors
comprise a family of five (MI-M5) transmembrane proteins that mediate slow,
modulatory
signalling in cells and tissues expressing these genes. Muscarinic receptors
are the targets
of a number of therapeutically useful agents (1, 2). Peripherally, muscarinic
receptors
mediate the actions of acetylcholine in the parasympathetic nervous system.
Peripherally
acting muscarinic receptor agonists are therapuetically useful in lowering
intra-ocular
pressure in patients with glaucoma (3). Compounds that potentiate the central
actions of
acetylcholine as well as centrally acting muscarinic receptor agonists have
both
demonstrated clinical utility in the treatment of a number of neuropsychiatric
diseases (1, 2,
4-7).
[0003] The actions of acetylcholine are terminated by degradation of the
molecule by acetylcholinesterase enzymes. Inhibition of these enzymes within
the central
nervous system leads to increased concentrations of acetylcholine at
muscarinic receptors.
A number of acetylcholinesterase inhibitors have been developed and are in
routine clinical
use as cognitive enhancing agents in dementia (4).
[0004] A number of centrally acting muscarinic agonist have been the subject
of
clinical testing. One of these, Xanomeline, has been shown to possess efficacy
in
controlling psychosis and related behavioral disturbances observed in
Alzheimer's Disease
patients (5). Further, it has recently been demonstrated that xanomeline is
efficacious in
treating schizophrenia (6). Interestingly, it displayed efficacy against both
positive and
negative symptoms, and did not induce adverse motoric effects in initial
clinical studies in
-1-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
schizophrenics. These data suggest that compounds with muscarinic receptor
agonist
properties are likely to be efficacious in treating the behavioral
disturbances common to
neurodegenerative disease such as Alzheimers Disease and as antipsychotics to
treat human
psychoses, but only if they are tolerated in these patient populations.
Additionally,
muscarinic receptor agonists have shown activity in pre-clinical models of
neuropathic pain
states (7).
Summary of the Invention
[0005] Disclosed herein is a method of treating psychosis comprising:
identifying a subject suffering from one or more symptoms of psychosis; and
contacting the
subject with a therapeutically effective amount of N-desmethylclozapine;
whereby the one
or more symptoms of psychosis are ameliorated. In one embodiment, the subject
is human.
In some embodiments, the therapeutically effective amount of N-
desmethylclozapine is
administered as a single dose. In other embodiments, the therapeutically
effective amount
of N-desmethylclozapine is administered as a plurality of doses. In one
embodiment, the
method further comprises contacting the subject with an additional therapeutic
agent. In
one embodiment, the subject is contacted with the additional therapeutic agent
subsequent
to the contacting with N-desmethylclozapine. In another embodiment, the
subject is
contacted with the additional therapeutic agent prior to the contacting with N-
desmethylclozapine. In still another embodiment, the subject is contacted with
the
additional therapeutic agent substantially simultaneously with N-
desmethylclozapine. In
some embodiments, the additional therapeutic agent is selected from the group
consisting
of monoamine repuptake inhibitiors, selective serotonin reuptake inhibitors,
norepinephrine
reuptake inhibitors, dual serotonin and norepinephrine reupake inhibitors,
dopamine
agonists, antipsychotic agents, inverse serotonin agonists, serotonin
antagonists, serotonin
2 inverse agonists, serotonin 2 antagonists, serotoninlA agonists,
antiepileptic and
peripherally acting muscarinic antagonists.
[0006] Also disclosed herein is a method of treating affective disorders
comprising: identifying a subject suffering from one or more symptoms of an
affective
disorder; and administering a therapeutically effective amount of N-
desmethylclozapine to
the subject, whereby the one or more symptoms of the affective disorder are
ameliorated.
In one embodiment, the subject is human. In one embodiment, the affective
disorder is
depression. In another embodiment, the affective disorder is mania. In some
embodiments,
the therapeutically effective amount of N-desmethylclozapine is administered
as a single
-2-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
dose. In other embodiments, the therapeutically effective amount of N-
desmethylclozapine
is administered as a plurality of doses. In one embodiment, the method further
comprises
administering to the subject an additionaltherapeutic agent. In one
embodiment, the
subject is contacted with the additional therapeutic agent subsequent to the
contacting with
N-desmethylclozapine. In another embodiment, the subject is contacted with the
additional
therapeutic agent prior to the contacting with N-desmethylclozapine. In still
another
embodiment, the subject is contacted with the additional therapeutic agent
substantially
simultaneously with N-desmethylclozapine. In some embodiments, the additional
therapeutic agent is selected from the group consisting of monoamine reuptake
inhibitors,
selective serotonin reuptake inhibitors, norepinephrine reuptake inhibitors,
dual serotonin
and norepinephrine reuptake inhibitors, dopamine agonists, antipsychotic
agents, inverse
serotonin agonists, serotonin antagonists, serotonin 2 inverse agonists,
serotonin 2
antagonists, serotoninlA agonists, antiepileptic and peripherally acting
muscarinic
antagonists.
[0007] Also disclosed herein is a method of treating dementia, comprising:
identifying a subject suffering from one or more symptoms of dementia; and
administering
a therapeutically effective amount of N-desmethylclozapine to said subject,
whereby a
desired clinical effect is produced. In one embodiment, the subject is human.
In some
embodiments, the therapeutically effective amount of N-desmethylclozapine is
administered as a single dose. In other embodiments, the therapeutically
effective amount
of N-desmethylclozapine is administered as a plurality of doses. In one
embodiment, the
dementia manifests as a cognitive impairment. In another embodiment, the
dementia
manifests as a behavioral disturbance. In one embodiment, the method further
comprises
administering to the subject an additional therapeutic agent. In one
embodiment, the
subject is contacted with the additional therapeutic agent subsequent to the
contacting with
N-desmethylclozapine. In another embodiment, the subject is contacted with the
additional
therapeutic agent prior to the contacting with N-desmethylclozapine. In still
another
embodiment, the subject is contacted with the additional therapeutic agent
substantially
simultaneously with N-desmethylclozapine. In some embodiments, the additional
therapeutic agent is selected from the group consisting of monoamine reuptake
inhibitors,
selective serotonin reuptake inhibitors, norepinephrine reuptake inhibitors,
dual serotonin
and norepinephrine reuptake inhibitors, dopamine agonists, antipsychotic
agents, inverse
serotonin agonists, serotonin antagonists, serotonin 2 inverse agonists,
serotonin 2
-3-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
antagonists, serotoninlA agonists, antiepileptic and peripherally acting
muscarinic
antagonists.
[0008] Also disclosed herein is a method of treating neuropathic pain
comprising: identifying a subject suffering from one or more symptoms of
neuropathic
pain; and contacting said subject with a therapeutically effective amount of N-
desmethylclozapine, whereby the symptoms of neuropathic pain are reduced. In
one
embodiment, the subject is human. In some embodiments, the therapeutically
effective
amount of N-desmethylclozapine is administered as a single dose. In other
embodiments,
the therapeutically effective amount of N-desmethylclozapine is administered
as a plurality
of doses. In one embodiment, the method further comprises contacting the
subject with an
additional therapeutic agent. In one embodiment, the subject is contacted with
the
additional therapeutic agent subsequent to the contacting with N-
desmethylclozapine. In
another embodiment, the subject is contacted with the additional therapeutic
agent prior to
the contacting with N-desmethylclozapine. In still another embodiment, the
subject is
contacted with the additional therapeutic agent substantially simultaneously
with N-
desmethylclozapine. In some embodiments, the additional therapeutic agent is
selected
from the group consisting monoamine reuptake inhibitors, selective serotonin
reuptake
inhibitors, norepinephrine reuptake inhibitors, dual serotonin and
norepinephrine reuptake
inhibitors, dopamine agonists, antipsychotic agents, inverse serotonin
agonists, serotonin
antagonists, serotonin 2 inverse agonists, serotonin 2 antagonists,
serotoninlA agonists,
antiepileptic and peripherally acting muscarinic antagonists.
[0009] Also disclosed herein is a method of treating glaucoma comprising:
identifying a subject suffering from one or more symptoms of glaucoma; and
contacting
said subject with a therapeutically effective amount of N-desmethylclozapine,
whereby the
symptoms of glaucoma are reduced. In one embodiment, the subject is human. In
some
embodiments, the therapeutically effective amount of N-desmethylclozapine is
administered as a single dose. In other embodiments, the therapeutically
effective amount
of N-desmethylclozapine is administered as a plurality of doses. In some
embodiments, the
symptoms of glaucoma are selected from the group consisting of elevated
intraocular
pressure, optic nerve damage, and decreased field of vision. In one
embodiment, the
method further comprises contacting the subject with an additional therapeutic
agent. In
one embodiment, the subject is contacted with the additional therapeutic agent
subsequent
to the contacting with N-desmethylclozapine. In another embodiment, the
subject is
-4-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
contacted with the additional therapeutic agent prior to the contacting with N-
desmethylclozapine. In still another embodiment, the subject is contacted with
the
additional therapeutic agent substantially simultaneously with N-
desmethylclozapine. In
some embodiments, the additional therapeutic agent is selected from the group
consisting
of monoamine reuptake inhibitors, selective serotonin reuptake inhibitors,
norepinephrine
reuptake inhibitors, dual serotonin and norepinephrine reuptake inhibitors,
dopamine
agonists, antipsychotic agents, inverse serotonin agonists, serotonin
antagonists, serotonin 2
inverse agonists, serotonin 2 antagonists, serotoninlA agonists,
antiepileptics, prostenoids
and alpha and beta adrenergic agonists.
[0010] Also disclosed herein is a pharmaceutical composition comprising a
pharmaceutically effective amount of N-desmethylclozapine and an additional
therapeutic
agent. In some embodiments, the additional therapeutic agent is selected from
the group
consisting of monoamine reuptake inhibitors, selective serotonin reuptake
inhibitors,
norepinephrine reuptake inhibitors, dual serotonin and norepinephrine reuptake
inhibitors,
dopamine agonists, antipsychotic agents, inverse serotonin agonists, serotonin
antagonists,
serotonin 2 inverse agonists, serotonin 2 antagonists, serotoninlA agonists,
antiepileptic
and peripherally acting muscarinic antagonists. In some embodiments, the
additional
therapeutic agent is selected from the group consisting of a phenothiazine,
phenylbutylpiperadine, debenzapine, benzisoxidil, and salt of lithium. In some
embodiments, the additional therapeutic gent is selected from the group
consisting of
chlorpromazine (Thorazine ), mesoridazine (Serentil ), prochlorperazine
(Compazine ),
thioridazine (Mellaril ), haloperidol (Haldol ), pimozide (Orap ), clozapine
(Clozaril ),
loxapiine (Loxitane ), olanzapine (Zyprexa(g), quetiapine (Seroquel ),
risperidone
(Risperidal ), ziprasidone (Geodon ), lithium carbonate, Aripiprazole
(Abilify),
Clozapine, Clozaril, Compazine, Etrafon, Geodon, Haldol, Inapsine, Loxitane,
Mellaril,
Moban, Navane, Olanzapine (Zyprexa), Orap, Permitil, Prolixin, Phenergan,
Quetiapine
(Seroquel), Reglan, Risperdal, Serentil, Seroquel, Stelazine, Taractan,
Thorazine, Triavil,
Trilafon, Zyprexa, and pharmaceutically acceptable salts thereof. In some
embodiments the
selective serotonin reuptake inhibitor is selected from the group consisting
of fluoxetine,
fluvoxamine, sertraline, paroxetine, citalopram, escitalopram, sibutramine,
duloxetine,
venlafaxine, and pharmaceutically acceptable salts and prodrugs thereof. In
some
embodiments, the norepinephrine reuptake inhibitor is selected from the group
consisting of
thionisoxetine and reboxetine. In some embodiments, the dual serotonin and
-5-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
norepinephrine reuptake inhibitor is selected from the group consisting of
duloxetine,
milnacripran and fluvoxamine. In some embodiments, the dopamine agonist is
selected
from the group consisting of cabergoline, amantadine, lisuride, pergolide,
ropinirole,
pramipexole, L-DOPA and bromocriptine. In one embodiment, the inverse
serotonin
agonists selected from the group consisting of N-(1-methylpiperidin-4-yl)-N-(4-
flourophenylmethyl)-N'-(4-(2-methylpropyloxy)phenylmethyl) carbamide, MDL
100,907,
SR-43694B (eplivanserin), ritanserin, ketanserin, mianserin, cinanserin,
mirtazepine,
cyproheptadine and cinnarizine.
[0011] One embodiment of the present invention includes, a method of treating
cognitive impairment comprising identifying a subject in need of improvement
of cognition
and administering an amount of N-desmethylclozapine to said subject, which is
therapeutically effective in improving the cognition of said subject.
[0012] In some aspects of this embodiment, the subject is human. In some
aspects of this embodiment, the therapeutically effective amount of N-
desmethylclozapine
is administered as a single dose. In other aspects of this embodiment, the
therapeutically
effective amount of N-desmethylclozapine is administered as a plurality of
doses.
[0013] In further aspects of this embodiment, the method further comprises
contacting the subject with an additional therapeutic agent. For example, the
subject may
be contacted with said additional therapeutic agent subsequent to said
contacting with N-
desmethylclozapine. Alternatively, the subject may be contacted with said
additional
therapeutic agent prior to said contacting with N-desmethylclozapine.
[0014] In some cases, the subject is contacted with said additional
therapeutic
agent substantially simultaneously with N-desmethylclozapine. In some cases,
the
additional therapeutic agent is selected from the group consisting of
monoamine reuptake
inhibitors, selective serotonin reuptake inhibitors, norepinephrine reuptake
inhibitors, dual
serotonin and norepinephrine reuptake inhibitors, dopamine agonists,
antipsychotic agents,
inverse serotonin agonists, serotonin antagonists, serotonin 2 inverse
agonists, serotonin 2
antagonists, serotoninlA agonists, antiepileptic and peripherally acting
muscarinic
antagonists. In some aspects of this embodiment, the subject suffers from a
condition
selected from the group consisting of hallucinations, delusions, disordered
thought,
behavioral disturbance, aggression, suicidality, mania, anhedonia, flattening
of affect,
affective disorders, depression, mania, dementia, neuropathic pain, glaucoma
and two or
more any of the foregoing conditions.
-6-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
[0015] Another embodiment of the present invention includes method of
ameliorating at least one symptom of a condition where it is beneficial to
increase the level
of activity of an Ml muscarinic receptor comprising determining that a subject
would
benefit from an increased level of activity of an M1 muscarinic receptor and
administering
an amount of N-desmethylclozapine which is therapeutically effective to
increase the level
of activity of the M 1 muscarinic receptor and to ameliorate said at least one
symptom to the
subject. In some aspects of this embodiment, the therapeutically effective
amount of N-
desmethylclozapine is administered as a single dose. In other aspects of this
embodiment,
the therapeutically effective amount of N-desmethylclozapine is administered
as a plurality
of doses. In further aspects of this embodiment, the method further comprises
contacting
the subject with an additional therapeutic agent. For example, the subject may
be contacted
with said additional therapeutic agent subsequent to said contacting with N-
desmethylclozapine. Alternatively, the subject may be contacted with said
additional
therapeutic agent prior to said contacting with N-desmethylclozapine. In some
cases, the
subject is contacted with said additional therapeutic agent substantially
simultaneously with
N-desmethylclozapine. In some cases, the additional therapeutic agent is
selected from the
group consisting of monoamine reuptake inhibitors, selective serotonin
reuptake inhibitors,
norepinephrine reuptake inhibitors, dual serotonin and norepinephrine reuptake
inhibitors,
dopamine agonists, antipsychotic agents, inverse serotonin agonists, serotonin
antagonists,
serotonin 2 inverse agonists, serotonin 2 antagonists, serotoninlA agonists,
antiepileptic
and peripherally acting muscarinic antagonists. In some aspects of this
embodiment, the
subject suffers from a condition selected from the group consisting of
hallucinations,
delusions, disordered thought, behavioral disturbance, aggression,
suicidality, mania,
anhedonia, flattening of affect, affective disorders, depression, mania,
dementia,
neuropathic pain, glaucoma and two or more any of the foregoing conditions.
[0016] Another aspect of the present invention is a method for ameliorating
one
or more symptoms of psychosis, comprising administering to a subject
exhibiting one or
more symptoms of psychosis a therapeutically effective amount of N-
desmethylclozapine
essentially free of clozapine. One embodiment further comprises identifying a
subject
exhibiting one or more symptoms of psychosis. In one embodiment, the psychosis
is
induced by exposure of the subject to one or more medications. In one
embodiment, the
subject is human. In one embodiment, the N-desmethylclozapine is administered
as a
-7-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
single daily dose or administered in divided doses. In one embodiment, the N-
desmethylclozapine is administered two, three or four times daily.
[0017] Another aspect of the present invention is a method of ameliorating one
or more symptoms of an affective disorder, comprising administering to a
subject
exhibiting one or more symptoms of an affective disorder a therapeutically
effective
amount of N-desmethylclozapine essentially free of clozapine. One embodiment
further
comprises identifying a subject exhibiting one or more symptoms of an
affective disorder.
In one embodiment, the affective disorder is depression. In one embodiment,
the affective
disorder is mania.
[0018] Another aspect of the present invention is a method of ameliorating one
or more symptoms of dementia, comprising administering to a subject exhibiting
one or
more symptoms of dementia a therapeutically effective amount of N-
desmethylclozapine
essentially free of clozapine. One embodiment further comprises identifying a
subject
exhibiting one or more symptoms of dementia. In one embodiment, the dementia
comprises cognitive impairment. In one embodiment, the dementia comprises
behavioral
disturbances.
[0019] Another aspect of the present invention is a method of ameliorating one
or more symptoms of neuropathic pain, comprising administering to a subject
exhibiting
one or more symptoms of neuropathic pain a therapeutically effective amount of
N-
desmethylclozapine essentially free of clozapine. One embodiment further
comprises
identifying a subject exhibiting one or more symptoms of neuropathic pain.
[0020] Another aspect of the present invention is a method of ameliorating one
or more symptoms of glaucoma, comprising administering to a subject exhibiting
one or
more symptoms of glaucoma a therapeutically effective amount of N-
desmethylclozapine
essentially free of clozapine. One embodiment further comprises identifying a
subject
exhibiting one or more symptoms of glaucoma.
[0021] Another aspect of the present invention is a method of ameliorating one
or more symptoms of psychosis, comprising administering to a subject N-
desmethylclozapine in combination with another anti-psychotic agent, wherein
at least a
portion of the N-desmethylclozapine is administered by directly introducing N-
desmethylclozapine to the subject. In one embodiment, directly introducing N-
desmethylclozapine to the subject comprises orally administering N-
desmethylclozapine.
In one embodiment, directly introducing N-desmethylclozapine to the subject
comprises
-8-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
intravenous injection of N-desmethylclozapine. In one embodiment, the other
anti-
psychotic agent is selected from the group consisting of a phenothiazine,
phenylbutylpiperadine, debenzapine, benzisoxidil, and a salt of lithium. In
one
embodiment, the phenothiazine is selected from the group consisting of
chlorpromazine
(Thorazine ), mesoridazine (Serentil ), prochlorperazine (Compazine ), and
thioridazine
(Mellaril ). In one embodiment, the phenylbutylpiperadine is selected from the
group
consisting of haloperidol (Haldol ) and pimozide (Orap ). In one embodiment,
the
debenzapine is selected from the group consisting of clozapine (Clozaril ),
loxapine
(Loxitane ), olanzapine (Zyprexa ) and quetiapine (Seroquel ). In one
embodiment, the
benzisoxidil is selected from the group consisting of resperidone (Resperidal
) and
ziprasidone (Geodon(E). In one embodiment, the salt of lithium is lithium
carbonate. In
one embodiment, the antipsychotic agent is selected from the group consisting
of
Aripiprazole (Abilify), Clozapine, Clozaril, Compazine, Etrafon, Geodon,
Haldol, Inapsine,
Loxitane, Mellaril, Moban, Navane, Olanzapine (Zyprexa), Orap, Permitil,
Prolixin,
Phenergan, Quetiapine (Seroquel), Reglan, Risperdal, Serentil, Seroquel,
Stelazine,
Taractan, Thorazine, Triavil, Trilafon, and Zyprexa, or pharmaceutically
acceptable salts
thereof.
[0022] Another aspect of the present invention is a method of ameliorating one
or more symptoms of psychosis, including administering to a subject exhibiting
one or
more symptoms of psychosis a therapeutically effective amount of a
pharmaceutical
composition comprising N-desmethylclozapine and a pharmaceutically acceptable
excipient
or diluent, wherein the amount of any clozapine administered is low enough
such that the
combined N-desmethylclozapine and clozapine result in a net agonism at
muscarinic
receptors.
[0023] Another aspect of the present invention is a method of ameliorating one
or more symptoms of an affective disorder, including administering to a
subject exhibiting
one or more symptoms of an affective disorder a therapeutically effective
amount of a
pharmaceutical composition comprising N-desmethylclozapine and a
pharmaceutically
acceptable excipient or diluent, wherein the amount of any clozapine
administered is low
enough such that the combined N-desmethylclozapine and clozapine result in a
net agonism
at muscarinic receptors.
[0024] Another aspect of the present invention is a method of ameliorating one
or more symptoms of dementia, including administering to a subject exhibiting
one or more
-9-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
symptoms of dementia a therapeutically effective amount of a pharmaceutical
composition
comprising N-desmethylclozapine and a pharmaceutically acceptable excipient or
diluent,
wherein the amount of any clozapine administered is low enough such that the
combined
N-desmethylclozapine and clozapine result in a net agonism at muscarinic
receptors.
[0025] Another aspect of the present invention is a method of ameliorating one
or more symptoms of neuropathic pain, including administering to a subject
exhibiting one
or more symptoms of neuropathic pain a therapeutically effective amount of a
pharmaceutical composition comprising N-desmethylclozapine and a
pharmaceutically
acceptable excipient or diluent, wherein the amount of any clozapine
administered is low
enough such that the combined N-desmethylclozapine and clozapine result in a
net agonism
at muscarinic receptors.
[0026] Another aspect of the present invention is a method of ameliorating one
or more symptoms of glaucoma, including administering to a subject exhibiting
one or
more symptoms of glaucoma a therapeutically effective amount of a
pharmaceutical
composition comprising N-desmethylclozapine and a pharmaceutically acceptable
excipient
or diluent, wherein the amount of any clozapine administered is low enough
such that the
combined N-desmethylclozapine and clozapine result in a net agonism at
muscarinic
receptors.
Brief Description of the Drawings
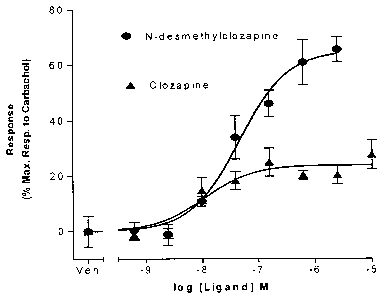
[0027] Figure 1 is a graph showing the results. of agonist activity of N-
desmethylclozapine at Ml muscarinic acetylcholine receptors in R-SAT Assays.
[0028] Figure 2 is a graph showing the results of agonist activity of N-
desmethylclozapine at Ml musacrinic acetylcholine receptors in Phosphatidyl
Inositol
Assay.
[0029] Figure 3 shows photographs of MAP kinase activation in rat
hippocampus following parenteral administration of N-desmethylclozapine.
[0030] Figure 4A shows a graph of the muscarinic M 1 receptor agonist activity
of a library of 462 compounds as determined by R-SAT assays. M 1 receptor
efficacy data
shown are derived from the 1-micromolar concentration of compound, and are
reported as
percentage efficacy relative to the maximal response observed for a saturating
40-
micromolar concentration of carbachol (100%). Figures 4B-D shows a graph of PI
hydrolysis data utilizing Chinese Hamster Ovary cells stably transfected with
the human
Ml receptor gene. Panel B depicts agonist responses reported as the percentage
response
-10-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
observed for carbachol. Drugs depicted are carbachol (squares), clozapine
(triangles), and
N-desmethylclozapine (circles), with observed potencies (pEC50) of: carbachol
(5.7), N-
desmethylclozapine (6.7), and clozapine (no response). Panel C depicts
competitive
antagonist responses obtained in the presence of a 3-micromolar concentration
of
carbachol, and are reported as the percentage response observed for atropine
(100%).
Drugs depicted are atropine (squares), clozapine (triangles), and N-
desmethylclozapine
(circles), with observed potencies (pKi) of: atropine (8.5), N-
desmethylclozapine (no
response), and clozapine (7.1). Panel D depicts competitive antagonist
responses obtained
in the presence of a 0.15-micromolar concentration of N-desmethylclozapine,
and are
reported as the percentage response observed for atropine (100%). Drugs
depicted are
atropine (squares), and clozapine (triangles), with observed potencies (pKi)
of: atropine
(8.4), and clozapine (7.6).
[0031] Figure 5 shows M1 muscarinic receptor agonist activity of N-
desmethylclozapine in mouse hippocampus. Phospho-MAPK immunoreactivity in the
cell
bodies and proximal dendrites of CA1 pyramidal cells (highlighted by arrows)
is shown
following the administration of vehicle (A), clozapine at 30 mg/kg (B), N-
desmethylclozapine at 10 (C), 30 (D), 100 (E), or N-desmethylclozapine
(30mg/ kg) and
scopolamine (0.3 mg/kg, i.p.)(F).
[0032] Figure 6 shows the quantification of M1 muscarinic receptor agonist
activity of N-desmethylclozapine in mouse hippocampus. Quantification of
phospho-
MAPK immunoreactivity was performed via computer calculated optical 'density
measurements of the CA1 region of the hippocampus from four mice, where (*)
indicates a
significant difference to vehicle treatment using a one factor ANOVA post-hoc
Dunnett's
test (F (5,23) =10.88: P<0.0001).
[0033] Figure 7 shows the results of an R-SAT assay with a combination of 150
nM NDMC and varying concentrations of clozapine.
[0034] Figure 8 shows the results of a PI hydrolysis assay with a combination
of
150 nM NDMC and varying concentrations of clozapine.
~
-11-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
Detailed Description of the Preferred Embodiment
Definitions
[0035] N-desmethylclozapine, 8- chloro -11- (1-piperazinyl) -5H- dibenzo [b,e]
[1,4] diazepine, also known as NDMC, is defined as the compound having the
molecular
structure depicted in Formula (I).
[0036] An "agonist" is defined as a compound that increases the basal activity
of a
receptor (i.e. signal transduction mediated by the receptor).
[0037] An "antagonist" is defined as a compound that competes with an agonist
or inverse agonist for binding to a receptor, thereby blocking the action of
an agonist or
inverse agonist on the receptor. However, an antagonist (also known as a
"neutral"
antagonist) has no effect on constitutive receptor activity.
[0038] A partial agonist is defined as an agonist that displays limited, or
less than
complete, activity such that it fails to activate a receptor in vitro,
functioning as an antagonist
in vivo.
[0039] The term "subject" refers to an animal, preferably a mammal, and most
preferably a human, who is the object of treatment, observation or experiment.
[0040] The term "therapeutically effective amount" is used to indicate an
amount of an active compound, or pharmaceutical agent, that elicits the
biological or
medicinal response indicated. This response may occur in a tissue, system,
animal or
human that is being sought by a researcher, veterinarian, medical doctor or
other clinician,
and includes alleviation of the symptoms of the disease being treated.
[0041] In certain embodiments, the method disclosed herein includes
administering a therapeutically effective amount of NDMC to a subject for the
purpose of
treating psychosis.
[0042] In certain embodiments, the above method for treating psychosis
comprises identifying a subject suffering from one or more symptoms of
psychosis; and
contacting the subject with a therapeutically effective amount of N-
desmethylclozapine;
whereby the one or more symptoms of psychosis are ameliorated.
[0043] In some embodiments, the symptom is cognitive impairment associated
with psychosis. In other embodiments, the subject suffering from psychosis
exhibits more
than one symptom of psychosis. In certain embodiments, one of the symptoms is
cognitive
impairment while another symptoms is one or more of hallucinations, delusions,
disordered
-12-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
thought, behavioral disturbance, aggression, suicidality, mania, anhedonia, or
flattening of
affect.
[0044] In a further embodiment, the method includes administering a
therapeutically effective amount of NDMC to a subject for the purpose of
treating
depression or mania.
[0045] In a still further embodiment, the method includes administering a
therapeutically effective amount of NDMC to a subject for the purpose of
treating the
psychiatric and other behavioral disturbances characteristic of dementia or
cognitive
impairment of any origin.
[0046] In a still further embodiment, the method includes administering a
therapeutically effective amount of NDMC to a subject for the purpose of
treating
neuropathic pain.
[0047] The present inventors have profiled a large series of drugs that have
utility in treating human disease for functional activity at the five human
muscarinic
receptor subtypes. With the exception of known muscarinic drugs, only two
agents studied
(out of more than 500) displayed muscarinic receptor agonist activity. One was
the atypical
antipsychotic clozapine (8). In vitro, this compound has been shown to possess
weak
partial agonist/antagonist activity at muscarinic M1, M2, and M4 receptors (9,
10), while in
vivo it is generally considered to display muscarinic receptor antagonist
properties. The
other was the related compound N-desmethylclozapine.
[0048] Administration of clozapine to human subjects results in the formation
of two major metabolites N-desmethylclozapine (NDMC) and clozapine-N-oxide
(11).
However, clozapine-N-oxide is a polar metabolite that is rapidly excreted and
likely does
not contribute to the biological activity of the parent compound. A
correlation exists
between the dose of clozapine administered to a subject, and the serum levels
of total
clozapine moieties, yet the levels of NDMC can vary widely between individual
subjects
(12). Generally, NDMC constitutes 40-75% of the total serum clozapine
concentrations
during steady state kinetics in humans (13). Conflicting data exists as to the
ability of
NDMC to penetrate the blood brain barrier and impart centrally mediated
activity (14, 15).
These observations demonstrate that NDMC has been routinely administered to
human
subjects, and is well tolerated. Few data exist as to the molecular properties
of NDMC.
-13-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
NDMC has been shown to possess antagonist activity at 5HT2C and D2 receptors
(16), but
no data on its interaction with muscarinic receptors has been reported.
[0049] Surprisingly, and unlike the closely related compound clozapine, it has
been found that the compound N-desmethylclozapine (NDMC) possesses heretofore
unappreciated functional activity as a muscarinic receptor agonist. Ex vivo
experiments
have demonstrated that NDMC crosses the blood brain barrier and acts as an
agonist at
central muscarinic receptors in rats. These observations have practical
applications that
support the use of NDMC as an antipsychotic, antimania agent, antidementia
agent, and as
a therepeutic agent to treat glaucoma or neuropathic pain. Thus, in one
aspect, disclosed
herein is a method of agonizing the activity of a muscarinic receptor
comprising contacting
the receptor with an effective amount of NDMC. In another aspect, disclosed
herein is a
method of treating a subject suffering from a muscarinic receptor related
disorder
comprising indentifying a subject in need thereof and administering to the
subject a
therapeutically effective amount of NDMC.
[0050] By "muscarinic related disorder," it is meant a disorder whose symptoms
are ameliorated by agonizing a muscarinic receptor.
[0051] In another aspect, disclosed herein is a method of ameliorating one or
more symptoms associated with schizophrenia or psychosis of any origin in a
subject,
comprising administering to the subject a therapeutically effective amount of
NDMC. In
some embodiments, the method comprises contacting a subject with a
pharmacologically
active dose of NDMC, for the purpose of controlling the positive
(hallucinations and
delusion) and negative (apathy, social withdrawal, anhedonia) symptoms of
schizophrenia
or related psychosis. In one embodiment, the NDMC administered to ameliorate
one or
more symptoms associated with schizophrenia or psychosis is essentially free
of clozapine.
By "essentially free of clozapine," it is meant that no appreciable amount of
clozapine may
be detected in the blood stream of the subject at the same time that NDMC is
detectable in
the blood stream of the subject. In one embodiment, the amount of any
clozapine
administered with the NDMC is low enough such that the combined NDMC and
clozapine
administered result in a net agonism at muscarinic receptors. In one
embodiment, some
amount of clozapine is administered but it is low enough such that the
combined NDMC
and clozapine administered result in a net agonism at muscarinic receptors. In
one
embodiment, the ratio of NDMC to clozapine is high enough to have a beneficial
effect due
-14-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
to net agonism at muscarinic receptors. In various embodiments, the ratio of
NDMC to
clozapine is at least about 100:1, 50:1, 10:1, 9:1, 7:1, 5:1, or 3:1.
[0052] In another aspect, disclosed herein is a method of ameliorating one or
more symptoms associated with affective disorders, including major depression,
mania,
bipolar disorder, and suicide, in a subject, comprising administering to the
subject a
therapeutically effective amount of NDMC. In some embodiments, the method
comprises
contacting a subject with a pharmacologically active dose of NDMC, for the
purpose of
controlling the symptoms observed during major depression or manic depression.
In one
embodiment, the NDMC administered to ameliorate one or more symptoms
associated with
affective disorders is essentially free of clozapine. In one embodiment, the
amount of any
clozapine administered with the NDMC is low enough such that the combined NDMC
and
clozapine administered result in a net agonism at muscarinic receptors. In one
embodiment,
some amount of clozapine is administered but it is low enough such that the
combined
NDMC and clozapine administered result in a net agonism at muscarinic
receptors.
[0053] In another aspect, disclosed herein is a method of ameliorating one or
more symptoms associated with Alzheimer's Disease and related
neurodegenerative
disorders in a subject, comprising administering to the subject a
therapeutically effective
amount of NDMC. In some embodiments, the method comprises contacting a subject
with
a pharmacologically active dose of NDMC, for the purpose of improving the
cognitive
deficits, and controlling the associated behavioral abnormalities, observed in
degenerative
dementias. In one embodiment, the NDMC administered to ameliorate one or more
symptoms associated with dementia is essentially free of clozapine. In one
embodiment,.
the amount of any clozapine administered with the NDMC is low enough such that
the
combined NDMC and clozapine administered result in a net agonism at muscarinic
receptors. In one embodiment, some amount of clozapine is administered but it
is low
enough such that the combined NDMC and clozapine administered result in a net
agonism
at muscarinic receptors.
[0054] In another aspect, disclosed herein is a method of ameliorate one or
more
symptoms associated with neuropathic pain in a subject, comprising identifying
a subject in
need thereof and administering to the subject a therapeutically effective
amount of NDMC.
In some embodiments, the method comprises contacting a subject with a
pharmacologically
active dose of NDMC, for the purpose of controlling the dysthesthetic,
hyperalgesic, and
other altered nociceptive symptoms observed in neuropathic pain states
regardless of their
-15-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
etiology. In one embodiment, the NDMC administered to ameliorate one or more
symptoms associated with neuropathic pain is essentially free of clozapine. In
one
embodiment, the amount of any clozapine administered with the NDMC is low
enough
such that the combined NDMC and clozapine administered result in a net agonism
at
muscarinic receptors. In one embodiment, some amount of clozapine is
administered but it
is low enough such that the combined NDMC and clozapine administered result in
a net
agonism at muscarinic receptors.
[0055] In another aspect, disclosed herein is a method of ameliorating one or
more symptoms associated with glaucoma in a subject, comprising administering
to the
subject a therapeutically effective amount of NDMC. In some embodiments, the
method
comprises contacting a subject with a pharmacologically active dose of NDMC,
for the
purpose of controlling the raised intra-ocular pressure observed in glaucoma,
regardless of
its etiology. In one embodiment, the NDMC administered to ameliorate one or
more
symptoms associated with glaucoma is essentially free of clozapine. In one
embodiment,
the amount of any clozapine administered with the NDMC is low enough such that
the
combined NDMC and clozapine administered result in a net agonism at muscarinic
receptors. In one embodiment, some amount of clozapine is administered but it
is low
enough such that the combined NDMC and clozapine administered result in a net
agonism
at muscarinic receptors.
[0056] Surprisingly, NDMC possesses potent agonist activity at the human
muscarinic receptors. It is further disclosed herein that NDMC can cross the
blood brain
barrier, and function in vivo as a muscarinic receptor agonist measured via
the activation of
MAP kinase activity in rat hippocampus. The molecular activities of NDMC, as
identified
by the present methods, combined with the known clinical efficacy of compounds
that
possess a similar molecular pharmacological profile, indicate that NDMC can be
used to
alleviate or treat disorders or conditions associated with human psychosis,
affective disease,
degenerative dementia, glaucoma, and neuropathic pain.
[0057] In another aspect, disclosed herein is a method of activating an Ml
muscarinic receptor comprising contacting the receptor with N-
desmethylclozapine.
[0058] In a further aspect, disclosed herein is a method of ameliorating at
least
one symptom of a condition where it is beneficial to increase the level of
activity of an Ml
muscarinic receptor comprising administering N-desmethylclozapine to a subject
in need
thereof.
-16-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
Preparation of 1V-desmeth lc~ lozapine (NDMC)
[0059] N-desmethylclozapine (NDMC) has the structure of Formula (I).
a
N-
CI ~ I \
N
H
[0060] NDMC is prepared as previously described (17). The dibenzo-diazepine-
lactam precursor (II) is converted to the thiolactam (III) using phosphorus
pentasulfide,
followed by alkylation with e.g. dimethyl sulfate to give the imino thioether
(IV).
Aminolysis of the thioether with an excess of piperazine gives the desired N-
desmethylclozapine (I). Alternatively, the dibenzo-diazepine-lactam (II) may
be converted
into the imino-chloride (V) by treatment with a halogenating agent such as
phosphorus
pentachloride and the product V is converted to N-desmethylclozapine (I) by
reaction with
piperazine.
H S H p CI
CI N - CI N - C I N
_
H~i
H~/ H
(III) (II) (V)
H
N SR CD
CI (~ ~ - CI I~ N~ D
H
H\~ (IV) (I)
-17-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
[0061] NDMC may be formulated in pharmaceutical compositions comprising
NDMC together with a pharmaceutically acceptable dilutant or excipient. Such
compositions may be formulated in an appropriate manner and in accordance with
accepted
practices such as those disclosed in Remington's Pharmaceutical Sciences,
Gennaro, Ed.,
Mack Publishing Co., Easton PA, 1990. In some embodiments, a pharmaceutical
composition comprising NDMC is provided that is essentially free of clozapine.
[0062] Advantageously, NDMC may be administered in a single daily dose, or
the total daily dosage may be administered as a plurality of doses, (e.g.,
divided doses two,
three or four times daily). Furthermore, compound for the present invention
may be
administered in intranasal form via topical use of suitable intranasal
vehicles, or via
transdermal routes, or via topical use of ocular formulations, or using those
forms of
transdermal skin patches well known to persons skilled in the art.
[0063] The dosage regimen of NDMC can be selected in accordance with a
variety of factors. These include type, species, age, weight, sex and medical
condition of
the patient; the severity of the condition to be treated; the route of
administration; the renal
and hepatic function of the patient; and the particular compound employed. A
physician of
ordinary skill can readily determine and prescribe the effective amount of the
drug required
to prevent, counter or arrest the progress of the disease or disorder that is
being treated.
[0064] The daily dosage of the products may be varied over a wide range from
0.01 to 1000 mg per adult human per day. An effective amount of the drug is
ordinarily
supplied at a dosage level of about 0.0001 mg/kg to about 25 mg/kg body weight
per day.
Preferably, the range is from about 0.001 to 10 mg/kg of body weight per day,
and
especially from about 0.001 mg/kg to 1 mg/kg of body weight per day. The
compounds
may be administered on a regimen of 1 to 4 times per day.
[0065] NDMC may be used alone at appropriate dosages defined by routine
testing in order to obtain optimal pharmacological effect, while minimizing
any potential
toxic or otherwise unwanted effects. In addition, it is believed that NDMC may
be used as
adjunctive therapy with known drugs to reduce the dosage required of these
traditional
drugs, and thereby reduce their side effects.
[0066] In some embodiments, NDMC is administered in combination with one
or more additional therapeutic agents. The additional therapeutic agents can
include, but
are not limited to, a neuropsychiatric agent. As used herein, a
"neuropsychiatric agent"
refers to a compound, or a combination of compounds, that affects the neurons
in the brain
-18-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
either directly or indirectly, or affects the signal transmitted to the
neurons in the brain.
Neuropsychiatric agents, therefore, may affect a person's psyche, such as the
person's
mood, perception, nociception, cognition, alertness, memory, etc. In certain
embodiments,
the neuropsychiatric agent may be selected from the group consisting of
monoamine
reputkate inhibitiors, selective serotonin reuptake inhibitors, norepinephrine
reuptake
inhibitors, dual serotonin and norepinephrine reupake inhibitors, dopamine
agonists,
antipsychotic agents, inverse serotonin agonists, serotonin antagonists,
serotonin 2 inverse
agonists, serotonin 2 antagonists, serotoninlA agonists, antiepileptic and
peripherally
acting muscarinic antagonists.
[0067] In some embodiments, the antipsychotic agent may be selected from the
group consisting of a phenothiazine, phenylbutylpiperadine, debenzapine,
benzisoxidil, and
salt of lithium. The phenothiazine group of compounds may be selected from the
group
consisting of chlorpromazine (Thorazine ), mesoridazine (Serentil ),
prochlorperazine
(Compazine ), and thioridazine (Mellaril ). The phenylbutylpiperadine group of
compounds may be selected from the group consisting of haloperidol (Haldol ),
and
pimozide (Orap(g). The debenzapine group of compounds may be selected from the
group
consisting of clozapine (Clozaril ), loxapine (Loxitane ), olanzapine (Zyprexa
) and
quetiapine (Seroquel ). The benzisoxidil group of compounds may be selected
from the
group consisting of resperidone (Resperidal ) and ziprasidone (Geodon ). The
salt of
lithium may be lithium carbonate. In some embodiments, the antipsychotic agent
may be
selected from the group consisting of Aripiprazole (Abilify), Clozapine,
Clozaril,
Compazine, Etrafon, Geodon, Haldol, Inapsine, Loxitane, Mellaril, Moban,
Navane,
Olanzapine (Zyprexa), Orap, Permitil, Prolixin, Phenergan, Quetiapine
(Seroquel), Reglan,
Risperdal, Serentil, Seroquel, Stelazine, Taractan, Thorazine, Triavil,
Trilafon, and
Zyprexa, or pharmaceutically acceptable salts thereof.
[0068] In certain embodiments, the selective serotonin reuptake inhibitor is
selected from the group consisting of fluoxetine, fluvoxamine, sertraline,
paroxetine,
citalopram, escitalopram, sibutramine, duloxetine, and venlafaxine, and
pharmaceutically
acceptable salts or prodrugs thereof.
[0069] In other embodiments, the norepinephrine reuptake inhibitor is selected
from the group consisting of thionisoxetine and reboxetine.
-19-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
[0070] In further embodiments, the dopamine agonist is selected from the group
consisting of cabergoline, amantadine, lisuride, pergolide, ropinirole,
pramipexole, and
bromocriptine.
[0071] In another embodiment, the inverse serotonin 2A agonist is N-(1-
methylpiperidin-4-yl)-N-(4-flourophenylmethyl)-N' -(4-(2-
methylpropyloxy)phenylmethyl)carbamide, MDL 100,907, SR-43694B (eplivanserin),
rtianserin, ketanserin, mianserin, cinanserin, mirtazepine, cyproheptadine and
cinnarizine.
[0072] In another aspect, the present disclosure is directed to a method of
treating neuropsychiatric disorder in a patient comprising identifying a
patient in need
thereof and administering to said patient a therapeutically effective amount
of a
pharmaceutical composition comprising a compound of Formula (I) and a
neuropsychiatric
agent. In yet another aspect, the present disclosure is directed to a method
of treating
neuropsychiatric disorder in a patient comprising identifying a patient in
need thereof and
administering to said patient a therapeutically effective amount of a compound
of Formula
(I) and a therapeutically effective amount of a neuropsychiatric agent.
[0073] In some embodiments, NDMC and additional therapeutic agent(s) are
administered nearly simultaneously. These embodiments include those in which
the
compounds are in the same administrable composition, i.e., a single tablet,
pill, or capsule,
or a single solution for intravenous injection, or a single drinkable
solution, or a single
dragee formulation or patch, contains the compounds. The embodiments also
include those
in which each compound is in a separate administrable composition, but the
patient is
directed to take the separate compositions nearly simultaneously, i.e., one
pill is taken right
after the other or that one injection of one compound is made right after the
injection of
another compound, etc.
[0074] In other embodiments, one of NDMC and an additional therapeutic
compound is administered first and then the other one of NDMC and the
additional
therapeutic compound is administered second. In these embodiments, the patient
may be
administered a composition comprising one of the compounds and then at some
time, a few
minutes or a few hours later, be administered another composition comprising
the other one
of the compounds. Also included in these embodiments are those in which the
patient is
administered a composition comprising one of the compounds on a routine or
continuous
basis while receiving a composition comprising the other compound
occasionally.
-20-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
[0075] In some embodiments of combination administration, NDMC is
administered in combination with another therapeutic agent, wherein at least a
portion of
the NDMC is administered by directly introducing NDMC to a subject. Thus, for
example,
clozapine may be administered in combination with NDMC wherein both clozapine
and
NDMC are directly administered to a subject. A portion of the NDMC
administered to the
patient will be due to metabolism of clozapine. However, another portion of
NDMC will
be due to direct administration of NDMC. In one embodiment, directly
introducing NDMC
to a subject may be accomplished by the subject orally ingesting NDMC. In one
embodiment, directly introducing NDMC to a subject may be accomplished by
intravenously injecting NDMC into the subject.
[0076] Defining the functional pharmacological activity of NDMC at a given
receptor can be achieved by a variety of methodologies. A currently favored
assay is the
Receptor Selection and Amplification Technology (R-SAT) assay disclosed in US
5,707,798, the content of which is hereby incorporated by reference in its
entirety.
[0077] Defining the functional pharmacological activity of NDMC at a given
receptor can be achieved by a variety of methodologies. Another currently
favored assay is
the PI Hydrolysis assay (18).
[0078] Defining the ability of NDMC to penetrate the blood brain barrier and
elicit a meaningful biological response can be achieved by a variety of
methodologies. A
currently favored assay is the hippocampal MAP kinase activation assay (19).
[0079] The present invention is further disclosed in the following examples,
which are not in any way intended to limit the scope of the invention as
claimed.
Examples
Example 1: Receptor Selection and Amplification Technology
[0080] The functional receptor assay, Receptor Selection and Amplification
Technology (R-SAT), was used (essentially as disclosed in US 5,707,798,
incorporated by
reference herein in its entirety) to investigate the functional
pharmacological properties of
known drugs, including many of their metabolites. These experiments have
provided a
molecular profile, or fingerprint, for each of these agents. Of all of the
agents tested, only
one, NDMC, displayed potent M1 acetylcholine receptor agonist activity. Figure
1 shows
the concentration response relationship of clozapine (filled triangles) and N-
desmethylclozapine (filled circles) to activate human Ml muscarinic receptors.
Data was
-21-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
derived from R-SAT assays as previously previously described (20). Data is
plotted as the
percentage activation relative to the full muscarinic receptor agonist
carbachol versus drug
concentration. Veh denotes vehicle.
[0081] As shown in Figure 1, clozapine displays high potency (pEC50 of 7.2)
yet limited intrinsic efficacy (<25% relative efficacy) at human M1 receptors.
Clozapine is
thus defined as a weak partial agonist. Partial agonists lack sufficient
intrinsic agonist
activity to stimulate the receptor in a manner similar to full agonists. They
thus behave as
antagonists in vivo. In contrast, NDMC also displays high potency (pEC50 of
7.2) at human
M1 receptors, yet it displays significantly greater intrinsic agonist activity
at MI receptors
(65% relative efficacy to carbachol), behaving as a robust agonist in R-SAT
assays. This
increased efficacy suggests that NDMC will act as an agonist in vivo, a
functional profile
distinct from that observed for clozapine.
[0082] To confirm the observation that NDMC displays increased agonist
efficacy at M1 receptors, a PI hydrolysis assay was performed, the results of
which are
disclosed in Figure 2 and Table 1. The data in Figure 2 is derived from PI
assays as
described in (18). In Figure 2, the concentration response relationship of
carbachol (filled
squares), clozapine (filled triangles), and N-desmethylclozapine (filled
circles) to activate
human M1 muscarinic receptors is shown. Data are plotted as a radioactivity
measured in
counts per minute versus drug concentration.
Table 1
Compound M,
%Efficacy pEC50 n
Carbachol 100% 6.04 0.05 5
Clozapine No Activity
N-desmethylclozapine 65 10 7.01 0.06 5
[0083] In Table 1, potency is reported as pEC50 values and efficacy is
reported
as that relative to the full agonist carbachol, both +/- standard deviation.
"n" denotes
number of experimental determinations. NDMC displays high potency as an M 1
agonist in
this system (pEC50 = 7.0), with full efficacy (>65% relative efficacy to
carbachol). Thus,
two distinct functional assays confirm that NDMC possesses previously
unappreciated
potent and fully efficacious agonist activity at human M1 muscarinic
acetylcholine
-22-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
receptors. This significantly greater positive intrinsic activity of NDMC
suggests that it
behaves as an Ml receptor agonist in vivo.
[0084] Clozapine and NDMC were tested at the remaining muscarinic receptor
subtypes. These data are disclosed in Table 2. The data in Table 2 are derived
from R-SAT
assays as previously described (20). Potency is reported as pEC50 values and
efficacy is
reported as that relative to the full agonist carbachol, both +/- standard
deviation. N
denotes number of experimental determinations.
Table 2
Compound M1 M2 M3
Efficacy pEC50 Efficacy pEC50 Efficacy pEC50
Clozapine 24 3 7.63 0.37 65 8 6.23 0.14 No response
N-desmeth Icloza ine 72f5 7.26 0.07 106 19 6.47 0.21 27 4 6.49 0.18
Olanzapine No response No response No response
N-desmeth lolanza ine No response No response No response
Xanomeline 121 6 7.20 0.08 106 9 6.30 0.23 66 6 6.63 0.21
Carbachol 101 2 6.11 0.03 101 5 6.23 0.09 102 3 6.53f0.04
Compound M4 M5
Efficacy pECso Efficacy pEC50
Clozapine 57 5 7.35 0.10 No response
N-desmeth lcloza ine 87 8 6.87 0.17 48 6 7.63 0.25
Olanzapine No response No response
N-desmeth lolanza ine No response No response
Xanomeline 116 9 7.46 0.14 86 12 6.59 0.22
Carbachol 96 3 6.53 0.05 105f3 6.76 0.12
[0085] NDMC displays increased intrinsic activity at all five muscarinic
receptor subtypes when compared to clozapine. The profile of NDMC at human
muscarinic receptors is most similar to that observed for the investigational
agent
Xanomeline, with one important distinction, a significantly lower efficacy at
human m3
receptors.
[0086] To confirm aspects of this molecular profile in vivo, and to assess the
ability of NDMC to access the central nervous system, NDMC was administered
- 23 -
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
parenterally to rats, and the M1 receptor mediated activation of hippocampal
MAP kinase
(MAPK) activity was determined, and this is disclosed in Figure 3. NDMC
treatment
activates MAPK in CA1 pyramidal neurons. C57BL6 mice were treated s.c with
vehicle, N-
desmethylclozapine, clozapine, or NDMC and scopolamine (i.p.) at the doses
described in
Figure 3, and then subjected to labeling via immunohistochemistry. With NDMC
treatment,
cell bodies and proximal dendrites of CA1 pyramidal neurons showed increased
phospho-
MAPK immunoreactivity compared to either vehicle or clozapine treatment.
Furthermore,
scopolamine reduced NDMC induced MAPK activation in the CAl region indicative
of a
muscarinic receptor mediated mechanism. Robust activation was observed, at a
dose of 30
mg/kg. This confirms that NDMC penetrates the blood brain barrier, and
function as a
muscarinic receptor agonist in vivo.
Example 2: Nonclinical Pharmacology of NDMC
[0087] A comprehensive functional pharmacological screen of nearly all known
antipsychotics, and many of their metabolites, at a majority of the known
biogenic amine
G-protein-coupled receptors (GPCRs) identified NDMC as pharmacologically
unique.
NDMC is an antagonist of D2 dopamine receptors and a potent inverse agonist of
5HT2A
receptors. However, unlike any other compound tested, NDMC is a potent and
efficacious
muscarinic receptor agonist. Specifically, NDMC is a potent partial agonist of
M1
(Ki=50nM) and M5 receptors (K;=25nM). NDMC also displays agonism of M2, M3,
and M4
receptors, however this interaction is 10-fold less potent than the
interaction with other
subtypes and indeed, under physiological conditions NDMC is able to
competitively
antagonize M3 receptors. In comparison, clozapine is a potent competitive
antagonist of M1,
M3, and M5 receptors, a weak agonist of M2 receptors, and a potent partial
agonist of M4
receptors. Furthermore, olanzapine, an antipsychotic structurally related to
NDMC and
clozapine is an antagonist of all 5 muscarinic subtypes. Haloperidol,
risperidone, and
ziprasidone do not interact with any of these receptors at concentrations up
to 1 M. Thus,
the agonist activity of NDMC at muscarinic receptors, particularly M, and M5
receptors, is
unique among antipsychotic drugs.
[0088] In addition to its activity at D2, 5HT2A, and muscarinic receptors,
NDMC
has affinity for aI, az, D1, Hi, S2, 5HTIA, 5HTIB, 5HT3, 5HT6, and 5HT7
receptors, and Ca2+
channels in ligand binding assays. Functionally it is a potent competitive
antagonist of
5HT2C, HI, and aIA receptors and an inverse agonist of 5HT6A and 5HT7A
receptors.
-24-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
[0089] NDMC is orally active in two models thought to be predictive of
antipsychotic activity. Like clozapine, NDMC attenuates both MK-801-induced
and
amphetamine-induced hyperactivity in mice at doses lower or similar to those
that reduce
spontaneous activity. Unlike clozapine and haloperidol, NDMC does not
attenuate
apomorphine-induced climbing in mice. This may reflect the reduced affinity of
NDMC
for D2 receptors compared to these other antipsychotics. NDMC administration
results in a
dose-dependent activation of mitogen-activated protein kinase (MAPK) in the
CA1 region
of hippocampus and this activation can be blocked by the non-selective
muscarinic
antagonist scopolamine. Given that M, receptors are the predominant subtype of
muscarinic receptor responsible for MAPK activation in the CAl region of the
hippocampus, this finding supports the in vivo agonism of M1 receptors by
NDMC.
Clozapine administration does not result in MAPK activation. Additional
evidence of
pharmacological activity of NDMC comes from the observation that NDMC
administration
increases cFOS expression in the prefrontal cortex and nucleus accumbens, but
not in the
striatum. The lack of cFOS expression in the striatum suggests that NDMC is
unlikely to
produce extrapyramidal side effects.
Example 3: Nonclinical Pharmacokinetics and Metabolism of NDMC
[0090] The pharmacokinetics of NDMC and clozapine were investigated in rats
and dogs. In both species, a single dose of NDMC was administered orally (10
mg/kg) or
intravenously (1 mg/kg) and blood samples were taken at regular intervals post-
dose. The
data showed that the oral bioavailability of NDMC is 25% and 44% in rats and
dogs,
respectively. In comparison, the oral bioavailability of clozapine is 1.5% and
7% in rats
and dogs, respectively. Thus these data indicate that NDMC has superior oral
bioavailability relative to clozapine.
[0091] In animals that received clozapine, appreciable levels of NDMC were
detected. In rats, NDMC levels at Cmax were approximately 20-fold higher than
the levels
of clozapine at its Cmax. In dogs, peak NDMC levels were approximately 16% of
the peak
clozapine levels. These data confirm published studies that demonstrate the
metabolism of
clozapine to NDMC in several species including mice, rabbit, dog, pig, monkey,
and
human.
[0092] The brain-to-plasma ratio of NDMC was calculated in rats. The ratio
was 1.0 at 240 minutes after oral administration of NDMC and 2.6 at 240
minutes after oral
- 25 -
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
administration of clozapine. Together with data available in the literature,
these results
show that NDMC distributes into the CNS.
Example 4: In Vitro Pharmacology of NDMC
[0093] The affinity of NDMC for 50 receptors, ion channels, and transporters
was evaluated at a single high dose (10 M). This screen identified 16 sites
at which
NDMC caused 90% or greater inhibition of binding and these were al, U2, D1,
DzS, Hi, Mi,
M2, M3, 82, 5HTIA, 5HTIB, 5HT2A, 5HT3, 5HT6, and 5HT7 receptors, and CaZ+
channels.
The inhibition of ligand binding in these assays provides information
regarding the binding
of NDMC to these receptors, however does not indicate the nature of the
interaction.
Example 5: Functional Screen of NDMC Against Multiple G-Protein-Coupled
Receptors
(GPCRs)
[00941 The pharmacological profile of NDMC was extensively studied in a
wide range of functional GPCR assays using proprietary Receptor Selection and
Amplification Technology (R-SAT; 2, 3). Table 3 reports the functional
pharmacological
activity of NDMC and leading typical and atypical antipsychotics at a subset
of human
monoaminergic receptor at which these drugs demonstrate the highest potencies.
-26-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
Table 3 Antagonist and Inverse Agonist Activity of NDMC and Reference
Antipsychotics in R-SAT Assays
Compound NDMC Clozapine Olanzapine Haloperidol Risperidone Ziprasidone
Competitive Antagonist
Receptor pKi pKi pKi pKi pKi pKi
DZ 7.2 0.1 7.7 0.1 8.4 0.2 10.0 0.1 9.3 0.1 8.3 0.3
5-HTZA 8.3 0.2 8.3 0.2 8.6 0.1 7.3 0.1 9.7 0.1 8.6 0.1
5-HTIA nrI nr nr nr nr nr*2
5-HTZC 7.8 0.2 7.4 0.2 7.4 0.1 nr 7.2 0.3 7.4 0.2
H, 8.2 0.2 9.5 0.2 8.4 0.1 nr 7.0 0.2 nr
M, nr* 7.8 0.2 7.2 0.2 nr nr nr
M2 nr* nr* 6.9 0.1 nr nr nr
M3 6.8 0.7 8.2 0.2 6.7 0.5 nr nr nr
M4 nr* nr* 7.4 0.3 nr nr nr
M5 nr* 7.5 0.3 7.2 0.2 nr nr nr
D3 nr 6.3 0.1 7.6 0.4 9.7 0.1 7.9 0.4 7.5 0.3
aIA 7.3 0.1 8.1 0.1 7.4 0.2 7.4 0.1 8.5 0.1 7.4 0.2
aZA nr nr nr nr 7.7 0.1 nr
Inverse Agonist
pEC50 pEC50 pEC50 pEC50 pEC50 pEC50
5HT2A 8.0 0.3 8.0 0.3 7.8 0.1 6.8 0.1 9.0 0.3 8.8 0.3
5HT6A 6.9 0.1 7.0 0.2 7.4 0.2 nr nr nr
5HT7A 7.3 0.1 7.4 0.1 nr nr 9.1 0.2 7.3 0.1
I nr = no significant antagonist or inverse agonist activity up to 1 M.
2 nr* = no significant antagonist or inverse agonist activity up to 1 M;
significant agonist activity (see Table
2).
[0095] The pharmacological activity of NDMC was similar to that of existing,
clinically efficacious atypical antipsychotics. Like all atypical
antipsychotics, NDMC
showed high potency, competitive antagonist and inverse agonist activity at 5-
HT2A
receptors. It displayed lower potency as a dopamine D2 receptor antagonist,
than clozapine
and therefore has a higher 5-HT2A/D2 receptor potency ratio. NDMC also
displayed lower
potency as an Hi and aIA receptor antagonist than clozapine, suggesting that
it may have
less of a propensity to induce adverse clinical effects, including sedation
and orthostatic
hypotension, mediated by these receptor subtypes. Consistent with these data,
published
reports confirm the potent competitive antagonist activity of NDMC at D2 and 5-
HT2C
receptors in vitro (Kouppamaki M, Syvalahti E and Hietala J (1993). Clozapine
and N-
-27-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
desmethylclozapine are potent 5-HTIC receptor antagonists. Eur J Pharm, 245:
179-182),
the lack of potent activity at histamine H3 receptors (Alves-Rodriques A,
Leurs R, Willems
E and Timmerman H (1996). Binding of clozapine metabolites and analogues to
the
histamine H3 receptor in rat brain cortex. Arch Pharm Pharm Med Chem, 329: 413-
416;
Schlicker E and Marr I(1996). The moderate affinity of clozapine at H3
receptors is not
shared by its two major metabolites and by structurally related and unrelated
atypical
neuroleptics. Naunyn-Sch Arch Pharmacol, 353: 290-294), and only low potency
interactions with GABAA receptors (Wong G, Kuoppamaki M, Hietala J, Luddens H,
Syvalahti E and Korpi ER (1996). Effects of clozapine metabolites and chronic
clozapine
treatment on rat brain GABAA receptors. EurJPharm, 314: 319-323).
[0096] Of the antipsychotics screened, only NDMC and clozapine possessed
muscarinic receptor agonist properties (Table 2; Sur C, Mallorga PJ, Wittmann
M,
Jacobsen MA, Pascarella D, Williams JB, Brandish PE, Pettibone DJ, Scolnick EM
and
Conn PJ (2003). N-desmethylclozapine, an allosteric agonist at muscarinic 1
receptor,
potentiates N-methyl-D-aspartate receptor activity. PNAS, 100: 13674-13679).
NDMC was
a potent, partial agonist of human M1 and M5 receptors and a less potent, full
agonist of
human M2 and M4 receptors (Table 2); it lacked antagonist activity at these
receptors under
similar conditions (Table 1). The physiological significance of M2 and M5
agonism in
schizophrenia is unknown. However, agonism of M, and M4 receptors is
associated with
antipsychotic activity (Bymaster FP, Felder C, Ahnmed S and McKinzie D (2002).
Muscarinic Receptors as a Target for Drugs Treating Schizophrenia. Curr Drug
Targ CNS
Neurol Dis, 1: 163-181; Felder CC, Bymaster FP, Ward J and DeLapp N (2000).
Therapeutic Opportunities for Muscarinic Receptors in the Central Nervous
System. J Med
Chem, 43: 4333-4353). Furthermore, agonism of M, receptors may confer
cognition-
enhancing activity on NDMC (Bymaster FP, Felder C, Ahmned S and McKinzie D
(2002).
Muscarinic Receptors as a Target for Drugs Treating Schizophrenia. Curr Drug
Targ CNS
Neurol Dis, 1: 163-181). NDMC displays minimal, low potency agonist activity
at M3
receptors and behaves as an antagonist at this site (Tables 3 and 4).
Muscarinic M3
receptors are the predominant receptor subtype that mediate cholinergic
effects of
parasympathetic activation in humans, such that significant agonist activity
would likely
result in treatment-limiting parasympathetic side effects including sweating,
ocular, and
gastrointestinal dysfunction. The antagonist activity of NDMC at M3 suggests
that severe
parasympathetomimetic effects will not be observed in clinical testing. The
- 28 -
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
pharmacological activity of NDMC at the muscarinic receptors has been observed
by others
(Sur et al. PNAS 2003).
Table 4 Muscarinic Receptor Agonist Activity of Dibenzodiazepine
Antipsychotics
M1 M2 M3 M4 M5
Compound Efficacy' pECso Efficacy pEC50 Efficacy pECso r87 ficacy pECso
Efficacy pECso
NDMC 72t52 7.3t 106 6.5 27+4 6.5t 8 6 .948t6 7=60.1 19 0.2 0.2 0.2 0.3
Clozapine 7.3 6.5 7.4
24 3 0.4 65 8 0.1 nr 7 5 0.1 nr
Olanzapine nr nr nr nr nr
Carbachol 6.1 t 6.5 6.5 t 6.8 t
101t2 01 lOltS 6.3v0.1 ]02 3 01 96t3 01 1O5t3 01
t Efficacy is % carbachol activation of the receptor
2 Data are mean S.E.M.
3 nr=no significant agonist activity up to 10 M
[0097] The pharmacological profile of NDMC at the muscarinic receptors is
distinct from that of clozapine. Clozapine displayed potent agonist activity
at M1 receptors,
however the efficacy of this interaction was very low (Table 4) and under
similar conditions
clozapine was a potent antagonist of M1 receptor activation (Table 3). Also in
contrast to
NDMC, clozapine demonstrated potent M3 and M5 antagonism. At the M2 and M4
receptors
clozapine demonstrated partial agonism. These data predict that, whereas it is
likely that
NDMC will behave as an Ml agonist in vivo, clozapine is likely to act as an Ml
antagonist.
Example 6: Effect of NDMC on Spontaneous Locomotion and Reversal of MK-801-
Induced Hyperactivity in Non-Swiss Albino Mice
[0098] NDMC was administered subcutaneously (s.c.) or orally (p.o.) to male,
adult Non-Swiss Albino (NSA) mice at 1, 10, or 30 mg/kg. Upon both s.c. and
p.o.
administration, NDMC significantly reduced spontaneous activity at 10 and 30
mg/kg. At
mg/kg s.c. the maximal reduction was achieved at 30 minutes post-
administration and
was maintained for the duration of the experiment, 120 minutes. This effect of
NDMC was
similar to that seen with clozapine, which reduced spontaneous locomotion at 3
and 10
mg/kg s.c. and p.o.
[0099] Clinically effective antipsychotic drugs can block the behavioral
effects
of non-competitive N-methyl-D-aspartate agonists, such as MK-801. NDMC was
evaluated
for its ability to attenuate MK-801-induced hyperactivity in male, adult, NSA
mice and its
activity in this assay was compared to that of clozapine. NDMC attenuated MK-
801-
-29-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
induced hyperactivity with a minimal effective dose of 1 mg/kg s.c. and 10
mg/kg p.o.,
consistent with antipsychotic-like efficacy. These doses were lower than or
similar to those
that reduced spontaneous locomotion, suggesting that the antipsychotic-like
effects can be
differentiated from general locomotor behavioral disruption. Similarly,
clozapine reduced
MK-801-induced hyperactivity with a minimal effective dose of 1 mg/kg s.c. and
3 mg/kg
P.O.
Example 7: Effect of NDMC on the Reversal of Amphetamine-induced Locomotor
Behaviors in Non-Swiss Albino Mice
[0100] Similar to attenuation of hyperactivity induced by N-methyl-D-aspartate
agonists, clinically effective antipsychotics also attenuate dopamine-mediated
hyperactivity
in rodents. Amphetamine-induced hyperactivity in mice is, therefore, a
commonly used
assay for in vivo antipsychotic-like activity. NDMC attenuated amphetamine-
induced
hyperactivity in male, adult NSA mice at 10 mg/kg after s.c. or p.o.
administration.
Clozapine also reduced amphetamine-induced hyperactivity with a minimal
effective dose
of 3 mg/kg p.o. These results are predictive of antipsychotic-like efficacy in
humans.
Example 8: Effect of NDMC on Reversal of apomorphine-induced climbing in Non-
Swiss Albino Mice
[0101] Another way to assess the blockade of dopamine-mediated behavior in
rodents is the attenuation of apomorphine-induced climbing in mice. Direct D2
receptor
antagonists most effectively block climbing induced by the dopamine receptor
agonist
apomorphine. Haloperidol, a typical neuroleptic antipsychotic drug with high
affinity for
dopamine D2 receptors, completely attenuated the apomorphine-induced climbing
in male,
adult, NSA mice at 0.1 mg/kg s.c. Clozapine also reduced apomorphine-induced
climbing
in a dose-dependent manner with the minimal effective dose at 10 mg/kg s.c. In
contrast
NDMC did not attenuate apomorphine-induced climbing at doses up to 100 mg/kg
s.c. This
may reflect the reduced affinity of NDMC for D2 receptors as compared to
clozapine and
haloperidol.
Example 9: Effect of NDMC on MAPK Activation in Brain in C57BL/6 Mice
[0102] In an effort to confirm the muscarinic agonist properties of NDMC in
vivo, the activation of mitogen-activated protein kinase (MAPK) in CA1 region
of the
hippocampus was examined. NDMC was administered s.c. at doses of 3, 10, 30,
and 100
mg/kg to C57BL/6 mice. The animals were killed two hours later; whole brains
were
removed and subjected to immunodetection of MAPK activity in hippocampus. NDMC
-30-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
administration resulted in the stimulation of MAPK activity at all doses in a
dose-
dependent manner. In contrast, clozapine at 30 mg/kg did not result in MAPK
activation in
CA 1 region of brain. The stimulation of MAPK activity induced by NDMC was
blocked by
the non-selective muscarinic receptor antagonist scopolamine (0.3 mg/kg,
i.p.), confirming
that NDMC acts as a muscarinic receptor agonist in vivo. It has been
demonstrated in vitro
that M, receptors are the predominant subtype of muscarinic receptor that is
responsible for
activation of MAPK in the forebrain (Hamilton SE and Nathanson NM (2001). The
M,
Receptor is required for Muscarinic Activation of Mitogen-activated Protein
(MAP) Kinase
in Murine Cerebral Cortical Neurons. J Biol Chem, 276: 15850-15853; Berkeley
JL,
Gomeza J, Wess J, Hamilton SE, Nathanson NM and Levey Al (2001). M1 Muscarinic
Acetylcholine Receptors Activate Extracellular Signal-Regulated Kinase in CAl
Pyramidal
Neurons in Mouse Hippocampal Slices. Mol Cell Neurosci, 18: 512-524; Berkeley
JL and
Levey Al (2003). Cell-Specific Extracellular Signal-regulated Kinase
Activation by
Multiple G Protein-coupled receptor Families in Hippocampus. Mol Pharm, 63:
128-135).
Hence these data support the in vivo agonism of muscarinic M1 receptors by
NDMC.
Example 10: Effects of Desmethylclozapine on Fos Protein Expression in the
Forebrain:
In vivo Biological Activity of the Clozapine Metabolite
[0103] The first in vivo demonstration of pharmacological activity of NDMC
(desmethylclozapine) was a dose-dependent induction of the expression of the
immediate
early gene cFOS in rat brain (Young CD, Meltzer HY and Deutch AY (1997).
Effects of
desmethylclozapine on Fos protein expression in the forebrain: In vivo
biological activity of
the clozapine metabolite. Neuropsychopharm, 19: 99-103). NDMC was administered
to
adult male Sprague-Dawley rats s.c. at doses of 7.5 and 30.0 mg/kg; the
animals were
sacrificed two hours later and homogenized tissue from various brain regions
was subjected
to immunodetection of cFOS by western blotting. NDMC resulted in the induction
of cFOS
expression in the pre-frontal cortex and nucleus accumbens, but not in
striatum, and these
effects were similar in magnitude and regional selectivity to those observed
for clozapine.
The lack of cFOS expression in the striatum of NDMC-treated animals may
indicate a low
propensity for NDMC to cause EPS.
-31-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
Example 11: Pharmacokinetic Evaluation of Clozapine and N-Desmethylclozapine
following Administration of a Single Intravenous Dose or Oral Dose to
Conscious Sprague
Dawley Rats
[0104] The pharmacokinetics of clozapine and N-desmethylclozapine (NDMC)
was evaluated in rats after intravenous (i.v.) and oral (p.o.) dosing. Cmax,
Tmax and
bioavailability after p.o. dosing and the volume of distribution (Vss),
terminal plasma half-
life (Tyz) and clearance (CLs) after i.v. dosing were determined. The brain-to-
plasma ratio
of NDMC after both intravenous and oral administration was also determined. A
total of 18
male Sprague-Dawley rats were dosed with clozapine p.o. (N=6, 10 mg/kg), NDMC
p.o.
(N=6, 10 mg/kg), clozapine i.v. (N=6, 1 mg/kg), or NDMC i.v. (N=6, 1 mg/kg),
and serum
samples for bioanalytical analysis were obtained at regular intervals at
between 0 and 240
minutes post dose. Animals were euthanised and brain and plasma samples
obtained at 60
or 240 minutes post-dose, depending on study group. The levels of NDMC and
clozapine
were measured in each sample. Pharmacokinetic data for NDMC is presented in
tables 5-8.
Table 5 Plasma Concentration (ng/mLt) of NDMC in Rat after NDMC
Administration2
Compound
Measured (route) Time (min)
30 60 120 180 240
NDMC (p.o.) 305 101 582 265 481 181 227 75 170 26 122 54
NDMC (p.o.) 277 57 576 161 614 60 NS3 NS NS
NDMC (i.v.) 540 46 276 30 126 38 33.7 11.4 11.7 3.8 5.3 0.3
'Mean SD; Z Dosages for oral administration were 10 mg/kg and lmg/kg for
intravenous administration;
3 NS = no sample taken because study terminated at 60 minutes
- 32 -
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
Table 6 Plasma Concentration (ng/mL') of NDMC and Clozapine in Rat after
Clozapine Administration2
Time (min)
Compound
Measured (route) 10 30 60 120 180 240
Clozapine (p.o.) 3.8 1.5 10.2 5.2 10.8 6.0 5.2 2.0 2.8 0.8 2.2 0.3
Clozapine (p.o.) 4.9 1.7 35.8 30.8 38.0 39.0 NS3 NS NS
Clozapine (i.v.) 1124 75.1 6.3 44.5 4.0 24.8 1.8 13.6 2.6 9.5 1.5
NDMC (p.o.) 77.1 88.7 194 161 147 86.6 42.5 15.1 13.4 2.54 7.1 0.5
NDMC (p.o.) 241 21.3 576 135 510 247 NS NS NS
NDMC (i.v.) 3.5 2.8 1.2 4.0 1.5 2.3 1.0 0.7 0.1 0.8 0.6
' Mean SD; 2 Dosages for oral administration were 10 mg/kg and 1mg/kg for
intravenous administration;
3 NS = no sample taken because study terminated at 60 minutes; N=2
Table 7 Pharmacokinetic Parameters' of NDMC in Rat after NDMC
Administration
Average CLs
Compound AUC I Cmax Tmax T'/Z (min BAZ Vss (mLi min
Measured (route) (min.ng (ng/mL) (min) ) (%) (L/kg)
.mL-') .kg')
NDMC (i.v.) 27331 756 0 39.3 - 1.47 36.2
NDMC (p.o.) 68227 582 60 ND3 25.0 ND ND
Mean SD; Z BA=ora1 bioavailability; 3 ND=not determined
Table 8 Pharmacokinetic Parameters' of NDMC and Clozapine in Rat after
Clozapine Administration
Average CLs
Compound AUC Cmax T""x BA 2 Vss (mL i min"
Measured (route) (m'n'ng/ (ng/mL) (min) T'n (min) (%) (L/kg)
mL) .kg')
NDMC (i.v.) 489.7 3.99 60 - - - -
NDMC (p.o) 16199 194 30 - - - -
Clozapine (i.v.) 8836 137 0 79.4 - 9.88 101
Clozapine (p.o.) 1347 10.8 60 ND3 1.5 0.6 ND ND
' Mean SD; Z BA=oral bioavailability; 3ND=not determined
[0105] These data demonstrate that NDMC was rapidly absorbed from the
gastrointestinal tract following oral administration; a Cmax of 582 ng/mL was
achieved by
-33-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
30 minutes. NDMC had low clearance from the circulation, a low volume of
distribution,
and was approximately 25% orally bioavailable. Clozapine reached much lower
peak drug
levels (10.8 ng/mL; 1150'" that of NDMC), had higher clearance, and poorer
bioavailability
(1.5%) following oral administration. These data suggest that NDMC may have
acceptable
pharmacokinetic properties after oral administration in humans and may indeed
have
improved pharmacokinetic properties as compared to clozapine.
[0106] High plasma levels of NDMC were observed following oral
administration of clozapine and peak plasma levels of NDMC were nearly 20-fold
greater
than those observed for clozapine (194 ng/mL versus 10.8 ng/mL). Similar
observations
have been made by others (Weigmann H, Harter S, Fischer V, Dahmen N and Hiemke
C
(1999). Distribution of clozapine and desmethylclozapine between blood and
brain in rats.
Eur Neuropsychopharm, 9: 253-256; Baldessarini RJ, Centorrino F, Flood JG,
Volpicelli
SA, Huston-Lyons D and Cohen BM (1993). Tissue concentrations of clozapine and
its
metabolite in the rat. Neuropsychopharm, 9: 117-124). Weigmann et al. (Eur
Neuropsychopharm 1999) showed that following oral administration of 5 doses
(20 mg/kg)
of clozapine at 1.5-hour intervals to male Sprague-Dawley rats, plasma
concentrations of
NDMC exceeded those of clozapine by up to 2.2-fold. In another study, high
levels of
circulating NDMC were observed following intraperitoneal (i.p.) administration
of varying
(1-60 mg/kg) doses of clozapine to Sprague-Dawley rats (Baldessarini et al;
Neuropsychopharm 1993). Thus, NDMC is a major chemical moiety formed after
oral
administration of clozapine in the rat. It is also been shown in vitro that
NDMC is the
primary clozapine metabolite formed by rat liver microsomes (Bun H, Disdier B,
Aubert C
and Catalin J (1999). Interspecies variability and drug interactions of
clozapine metabolism
by microsomes. Fund Clin Pharm, 13: 577-58 1).
[0107] The pharmacokinetic study described above included an initial
assessment of the distribution of NDMC into brain. The ratio of brain-to-
plasma levels of
NDMC was 0.36 0.16 at 60 minutes and 1.0 0.4 at 240 minutes following oral
administration of 10 mg/kg NDMC to Sprague-Dawley rats. Additionally, after
oral
administration of clozapine the brain-to-plasma ratio of NDMC was 0.26 0.07
at 60
minutes and 2.6 0.8 at 240 minutes. This latter result confirms previously
published
findings showing that oral administration of clozapine to male Sprague-Dawley
rats
resulted in NDMC levels in brain that were up to 3.9-fold higher than those
observed in
serum (Baldessarini et al.; Neuropsychopharm 1993) and intraperitoneal
administration of
-34-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
20, 30, and 60 mg/kg of clozapine to Sprague-Dawley rats resulted in the
detection of
NDMC in brain (Bun et al.; Fund Clin Pharm 1999). Together these in vivo data
clearly
document that NDMC distributes into the CNS after oral administration.
Example 12: Bioavailability Assessment of Clozapine and N-Desmethylclozapine
in Male
Beagle Dogs
[0108] The pharmacokinetics of clozapine and N-desmethylclozapine (NDMC)
were evaluated in dogs after intravenous (i.v.) and oral (p.o.) dosing. Cmax,
Tmax and
bioavailability after p.o. dosing and the volume of distribution (Vss),
terminal plasma half-
life (Tyz) and clearance (CLs) after i.v. dosing were determined. A total of 6
beagle dogs
were dosed with clozapine p.o. (N=3, 10 mg/kg), NDMC p.o. (N=3, 10 mg/kg),
clozapine
i.v. (N=3, 1 mg/kg), or NDMC i.v. (N=3, 1 mg/kg). Serum samples for
bioanalytical
analysis were obtained pre-dose and 10 min, 30 min, 1, 2, 3, 4, and 6 h post
dose after p.o.
administration and pre-dose, 2, 5, 10, 30 min, 1,2 3, and 4 h after i.v.
administration. The
levels of NDMC and clozapine were measured in each sample. Pharmacokinetic
data for
NDMC are presented in tables 9-12.
Table 9 Plasma Concentration (ng/mLt) of NDMC in Dog after NDMC
Administration2
Compound
Measured (route) Time (min)
- 10 30 60 120 180 240 360
NDMC (p o.) 1.0 14 12 z 67 37 155 249 274
261
95 44 44
2 5 10 30 60 120 180 240
NDMC (i.v.) 1890 73 t 22 50 10 35 2 32 6 28 4 27 7 27 4
Mean SD; 2 Dosages for oral administration were 10 mg/kg and 1mg/kg for
intravenous administration.
-35-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
Table 10 Plasma Concentration (ng/mL') of NDMC and Clozapine in Dog after Oral
of Intravenous Clozapine Administration2
Compound Time (min)
Measured (route)
- 10 30 60 120 180 240 360
NDMC (p.o.) - 0 2.45 25.4 5.8 10.29 19.23 46.7
Clozapine (p.o.) 0.46 9.53 611.8 103 35 20 57 16 100 213
33 91
2 5 10 30 60 120 180 240
NDMC (i.v.) 0.54 0.47 0.64 1.72 3.55 4.31 4.89 4.44
0.12 0.06 0.26 0.75 1.03 1.34 1.41 1.31
Clozapine (i.v.) 1 266 1136 98 24 75 10 76 7 61 8 58 11 41 6
Mean SD; 2 Dosages for oral administration were 10 mg/kg and 1mg/kg for
intravenous administration.
Table 11 Pharmacokinetic Parameters1 of NDMC in Dog after Oral or Intravenous
NDMC and Clozapine Adniinistration
Average CLs
Compound AUC ~ Cmax T""x B'4 z Vss (mLi min"
Measured (route) (min.ng (ng/mL) (min) TI/z (min) M (L/kg)
.mL"') .kg"I)
NDMC (i.v.) 134.8 353.2 13.2 7.0 - 28202.1 1850
21.3 242 4919.8 1060.4
NDMC o. 597.6 286.3 3.3 1.2 ND 44.3 ND ND
(p' ) 111.8 25
Clozapine (i.v.) 15.0 3.9 5.3 1.2 2.7 0.58
Clozapine (p.o.) 32.1 24 0 19.2 7.2 4.0 0.0
' Mean SD; 2 BA=oral bioavailability
-36-
CA 02576153 2007-02-05
WO 2006/017614 . PCT/US2005/027645
Table 12 Pharmacokinetic ParametersI of Clozapine in Dog after Clozapine
Administration
Average CLs
Compound AUC _~ C,,,ax T"'ax BAZ Vss (mLfmin
Measured (route) (min.ng (ng/mL) (min) T'n (min) (o~o) (L/kg)
.mL-') .kg')
Clozapine (i.v.) 266 33 189 18 - 3.3 - 10335 2190
0.63 1636 295.9
Clozapine (p.o.) 1186 09.5 124
58 3+ 3.0 1.7 ND 7.0 ND ND
Mean $D; 2 BA=oral bioavailability
[0109] NDMC was absorbed from the gastrointestinal tract following oral
administration with a Cm,,,, of 286.3 ng/mL achieved by 3.3 h. NDMC had low
clearance
from the circulation, a low volume of distribution, and was approximately 44%
orally
bioavailable. Clozapine had poorer oral bioavailability (7%). These data
suggest that
NDMC may have acceptable pharmacokinetic properties after oral administration
in
humans and may indeed have improved pharmacokinetic properties as compared to
clozapine.
[0110] NDMC was readily detectable in plasma following both intravenous and
oral administration of clozapine. The mean NDMC/clozapine AUC ratio was 0.056
after
i.v. administration of clozapine and 0.161 (i.e., 16%) after oral
administration. These data
confirm recent studies that demonstrated the metabolism of clozapine to N-
desmethylclozapine in dog both in vitro (Bun et al. Fund Clin Pharm 1999) and
in vivo
(Mosier KE, Song J, McKay G, Hubbard JW and Fang J (2003). Determination of
clozapine, and its metabolites, N-desmethylclozapine and clozapine N-oxide in
dog plasma
using high-performance liquid chromatography. J Chromat B, 783: 377-382).
Mosier and
colleagues showed that following oral administration of clozapine to a dog the
C,r,aX of
desmethylclozapine was approximately 20% that of clozapine (i.e., the
NDMC/clozapine
ratio was approximately 0.2). An early study did not detect N-
desmethylclozapine in dog
(Gauch R and Michaelis W (1970)). The metabolism of 8-chloro-ll-(4-mehtyl-l-
piperazinyl)-5H-dibenzo[b,e] [1,4] diazepine (Clozapine) in mice, dogs, and
human
subjects. Il Farmaco, 26: 667-681) after oral administration; however this may
have been
due to insensitive analytical techniques.
-37-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
Example 13: The role of Ml muscarinic receptor agonism of N-desmeth lc~pine in
the
unique clinical effects of clozapine
Methods
[0111] Molecular profiling of clinically relevant drugs was performed at all
known monoaminergic receptor subtypes except the Dopamine D4, Serotonin 5A,
and
Histamine H4 receptors using Receptor Selection and Amplification Technology
(R-SAT)
assays. Briefly, NIH/3T3 cells plated at 70-80% confluency were transfected
with various
receptor cDNA (10-100ng receptor and 20ng [3-Gal reporter/well of a 96 well
plate) using
the Polyfect Reagent (Qiagen Inc.) as described in the manufacture's protocol.
One day
after transfection, ligands were added in Dulbecco's modified Eagle's medium
supplemented with penicillin (100 U/ml), streptomycin (100 g/ml) and 2% Cyto-
SF3.
After four to six days, the media was aspirated off, the cells were lysed, O-
Nitrophenyl-
beta-D-Galactopyranoside (ONPG) was added and the resulting absorbance was
measured
spectrophotometrically. Concentration response curves were performed as eight-
point
concentration response experiments run in duplicate, where the maximal
antipsychotic
concentrations varied from 10-25 micromolar, and data were analyzed using
Excel fit and
Graph Pad Prism. Reported EC50 values represent the concentration of a ligand
that
produces a half-maximal response from a receptor in the absence of other
ligands, and IC50
values represent the concentration of a ligand that inhibits half of the
agonist-induced
activity. Competitive antagonist IC50 data were adjusted for agonist occupancy
using the
equation Ki = IC50/ { 1+[agonist]/EC50 agonist}. Data are reported as negative
log values
(pEC5o and pKi). Sources of the drugs utilized in this study are described in
Weiner et al.
(2001) and Wellendorph et al. (2002), with the exception of N-
desmethylclozapine, which
was acquired from Sigma, Inc., and N-desmethylolanzapine, which was
synthesized by
ACADIA Pharmaceuticals. A list of the compounds screened can be found as
supplemental information.
[0112] PI hydrolysis assays were performed on Chinese Hamster Ovary cells
stably transfected with the human M1 muscarinic receptor cDNA as described in
Spalding
et al (2002), and the data are derived from six or eight-point concentration
response
experiments performed in duplicate.
[0113] MAP Kinase assays utilized C57BL6 mice treated subcutaneously with
either vehicle, clozapine, or N-desmethylclozapine with or without
scopolamine, sacrificed
-38-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
two hours later, and phospho-MAPK immunoreactivity was assayed as described in
Berkeley et al (2001). Briefly, after treatments which were administered s.c.
at 60 min.,
mice were perfused with 100 ml of 4% paraformaldehyde followed with 100 ml of
10%
sucrose. Brains were removed and cryoprotected in 30% sucrose overnight at 4
C. The
next day, 50 m slices were cut on a sliding microtome. Slices were rinsed,
treated with
3% HZOz for 10 minutes at room temperature and rinsed again. Slices were
blocked in PBS
containing 10 g/ml avidin (Vector Laboratories Burlingame, CA), 0.1% triton-X
and 4%
normal goat serum (NGS) for 1 hour. Slices were rinsed and incubated in PBS
containing
50 g/ml biotin (Vector Laboratories Burlingame, CA), 2% NGS, and phospho-
ERK1/2
antibody (Cell signal Technologies, Beverly, MA) at a concentration of 1:250
and allowed
to incubate overnight at 4 C. The next day, slices were rinsed and placed in
PBS
containing 2% NGS and biotinylated goat anti-rabbit (Vector Laboratories
Burlingame,
CA) at a concentration of 1:100 for 1 hour at 4 C. Slices were rinsed and
placed in
horseradish peroxidase-conjugated avidin-biotin complex (Vector Laboratories
Burlingame,
CA) for 1 hour at 4 C. Slices were rinsed and incubated in TSA Fluorescein
tyramide for
min at room temperature. Slices were treated with 10 mM CuSO4 for 30 minutes,
mounted onto glass slides with Vectashield mounting media (Vector Laboratories
Burlingame, CA). Slides were visualized via a fluorescence microscope and
digital images
were analyzed with Scion image analysis software (Scion Corp. Frederick, MD).
[0114) Stepwise multiple-regression analysis, including the dependent measure,
dose, age, and gender was utilized to assess the contribution of NDMC to
treatment
response in schizophrenic subjects (Hasegawa et al 1993 and Lee et al 1999).
The analysis
was adjusted for baseline level of symptom severity, age, and dose, since dose
was not
fixed. The plasma samples chosen for the analyses were obtained at 6 weeks and
6 months
after initiation of therapy, were related to the clinical measures obtained at
those times, and
were drawn 12 hours after the last clozapine dose. Only subjects who had
received at least
100 mg of clozapine per day were included in the analysis, and some data were
unavailable
for these subjects at some time points. Regarding co-treatment with
anticholinergic agents,
only two subjects in this sample were treated with benztropine. The results
did not differ
when data from these two subjects were omitted (data not shown). Lastly, ten
of the
patients in this study were treated with benzodiazepines at the time the
levels of clozapine
-39-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
and NDMC were measured. Benzodiazepines have not been reported to affect the
metabolism of clozapine.
[0115] Drugs screened, grouped according to clinical class, included:
[0116] Antipsychotics: Amoxapine, Amisulpiride, Amperozide, Bromperidol,
Butaclamol, Chlorproethazine, Chlorpromazine, Chlorprothixene, Cis-
flupentixol,
Clothiapine, Clozapine, Droperidol, Fananserin, Fluphenazine, Fluspiriline,
Haloperidol,
Loxapine, Mazapertine, M100907, Melperone, Mesoridazine, Molindone, N-
Desmethyl
Clozapine, N-desmethylolanzapine, Ocaperidone, Octoclothepin, Olanzapine,
Perazine,
Perlapine, Pimozide, Pimpamperone, Promazine, Prothypendyl, Quetiapine,
Remoxipride,
Risperidone, Sertindole, Spiperone, Sulpride, Sultopride, Telfludazine,
Thioridazine,
Thiothixene, Tiapride, Moperone, Tiospirone, Trans-flupentixol,
Trifluoperazine,
Trifluoperidol, Triflupromazine, and Ziprasidone.
[0117] Antidepressants/Anxiolytics: Acetyltryptophan, Acetyltryptophanamide,
Alaprocate, Alprazolam, Amitriptyline, Barbital, Bromazepam, Buproprion,
Buspirone,
Chloral Hydrate, Clobazam, Clonazepam, Clomipramine, Clorgyline,
Chlordiazepoxide,
Chlormezanone, Continine, Compazine, Desipramine, Deprenyl, Desmethyldiazepam,
Diazoxide, Doxepin, Flumazenil, Flunitrazepam, Fluoxetine, Flurazepam,
Fluvoxamine,
Imipramine, Indatraline, Iproniazid, Maprotiline, Meprobamate, Milnacipram,
Minaprine,
Mirtazepine, Modafinil, Nitrazepam, Nomifensine, Nortriptyline, Oxazepam,
Pargyline,
Phenelzine, Prazepam, Protripytline, Rolipram, Tracazolate, Tranylcypromine,
Trazadone,
Triazolam, Trihexaphendyl, Trimipramine, Viloxazine, Zimelidine, Zolpidem, and
Zopiclone.
[0118] CNS Miscellaneous: 3PPP, 5-Aminopentanoic Acid, 5-Hydroxy MDA,
5-Methoxy DMT, 5-Methoxytryptamine, Acetaminophen, Acetylsalicylic Acid,
Alprenelol,
Amantadine, Amiodarone, AMPA, Apocodeine, Apomorphine, Atropine, Baclofen,
Balperidone, Benztropine, Bicuculline, Bradykinin, Bretylium, BRL 37344,
Bromocriptine,
Cannabidiol, Carbemazepine, Carbidopa, Cyproheptadine, Cirazoline, D-
Amphetamine,
(D-Ser2)-Leu Enkephalin-Thr, (Leu 5) Enkephalin, D-Phenylalanine, Dibucaine,
Diclofenac, Dihydroergotamine, DOI, Domperidone, Ebalzotan, Edrophonium,
Ephedrine,
Etadolac, Ethosuxamide, Felbamate, Fenbufen, GABA, Gabaxadol, Galanthamine,
Gamma-Vinyl GABA, Gabapentin, (-) GMC III, (+) GMC III, Heroin, Himbacine, I-4-
AA,
ICI 204448, Indoprofen, Isoguvacine, Ketamine, Ketaprofen, Labetalol,
Lamotrigine,
Levallorphan, Lidocaine, Lisuride, L-745-870, Melatonin, Metoclopromide,
Memantine,
-40-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
Mescaline, Naftopidil, Nalbuphine, N-Allyl SKF 38393, Naloxone, Naltrexone,
Naltrindole, Neostigmine, Nicotine, Nipecotic Acid, N-Methyl ICI 118-551, N-
Methyldopamine, N, N-Dimethyl MDA, Norapomorphine, Norcodeine,
Norfenfulramine,
Normetazocine, Oxethazine, Pemoline, Pergolide, PCP, Phaclofen, Phenacetin,
Phenteramine, Phenoxybenzamine, Phenytoin, Physostigmine, P-Iodoclonidine,
Pirenzepine, Prilocaine, Primodone, Procaine, Prochlorperazine, Propranolol,
Pseudoephedrine, Quinpirole, Raclopride, Rauwolscine, Reserpine, Rimcazole, RO-
05-
3663, RS 100329, RX 821002, Saclofen, Salicylamide, SCH 12679, SCH 23390,
Scopolamine, SKF 81297, SKF 38393, SKF 82948, SKF 82957, SKF 83566, SR
141716A,
SR 144528, Succinylcholine, Tenoxicam, Terguride, Tetracaine, Tolazoline,
Tropicamide,
UK 14304, Valproate, Vigabatrin, WIN 55212-2, Xylazine, Yohimbine, and
Zomepirac.
[0119] Monoaminergic: 7-OH-DPAT, 8-OH-DPAT, Alpha Methyl Serotonin,
Arecoline, Astemizole, Bethanacol, Carbachol, CGS 12066A, Cinanserin,
Chlorpheniramine, Cimetidine, Clobenpropit, CPP, Dihydroergocristine,
Dimaprit,
Diphenhydramine, Doxylamine, Eltoprazine, Famotidine, Histamine, Imetit,
Isomaltane,
Ketanserin, Loperamide, L-Tryptophan, LY 53857, mCPP, Mesulergine,
Metergoline,
Methergine, Methiothepin, Methysergide, Mexamine, Mianserin, MK 212,
Mepyramine,
Pheniramine, Phenylbiguanide, Pimethixene, Piperazine, Pirenpirone, Prazosin,
Promethazine, Pyrilamine, Quiapazine, Ranitidine, Ritanserin, SB 204741, SB
206553,
Serotonin, Spiroxatrine, Sumitriptan, Thioperamide, Tripellenamine,
Triprolidine,and WB
4101.
[0120] Cardiovascular: Acetazolamide, Adenosine, Albuterol, Atenolol,
Amiloride, Amrinone, Bepridil, Caffeine, Catopril, CGS-15943, CGS-21680, CGP-
12177A, Chlorothiazide, Clonidine, Debrisoquin, Digitoxin, Digoxin, Diltiazem,
Dipyridamole, Disopyramide, Dobutamine, Doxazosin, DPCPX, Epinephrine,
Enalapril,
Flunarizine, Furosemide, Guanabenz, Guanethidine, Hydralazine,
Hydrochlorothiazide,
Isoproterenol, Isosorbide, Lidocaine, Linisopril, Metaproterenol, Methoxamine,
Metrifudil,
Metolazone, Metoprolol, Midodrine, Minoxidil, N-Acethylpocainamide,
Nicardipine,
Nifedipine, Nimodipine, Nitrendipine, Norepinephrine, Nylidrin, Oxymetazoline,
Paraxanthine, Pentoxifylline, Phentolamine, Pinacidil, Pindolol, Procainamide,
Propranalol,
Quinidine, Spironolactone, Theophylline, Theoyphylline 1-3, Timolol,
Triamterene,
Urapidil, Verapamil, and Warfarin.
-41-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
[0121] Systemic Miscellaneous: Acyclovir, Adephenine, Allupurinol,
Amodiaquine, 6-bromo-APB, Artemisinin, Azathioprine, Azithromycin, Camphor,
Capsaicin, Carbetapentane, Carisoprodol, Cefotaxime, Cinchonidine,
Chloramphenicol,
Chloroquine, Chlorpropamide, Chlorzoxazone, Clarithromycin, Clofilium,
Clotrimazole,
Cyclobenzaprine, D-Cycloserine, Danazol, Dantrolene, Dextromethorphan,
Dimethadione,
Dropropizine, E-Capsaicin, Edoxudine, Ethinimate, Fipexide, Fluconazole,
Foscarnet,
Gallamine, Glibenclamide, Glipizide, Hypericin, lbuprofen, Ifenprodil,
Indomethacin,
Isobutylmethylxanthine, Kainic Acid, Ketoconazole, Levorphanol, Linopiridine,
Mazindol,
Meclizine, Mefexamide, Mefloquine, Mephenesin, Mesbeverine, Methocarbamol,
Metoclopramide, Metronidazole, MK 801, N-Aminohexyl-5-Chloronaphthalene-l-
Sulfonamide, N-Methyl-D-Aspartic Acid , NCS 382, Neophesperidin, Nixoxetine,
Nocapine, Octopamine, Omeprazole, Orphenadrine, Oxyphenbutazone, Papaverine,
Penicillamine, Pentamidine, Phenacemide, Picrotoxin, Pitrazepine, Piracetam,
Piroxicam,
Primaquine, Probenecid, Pyrimethamine, Quinine, Ritodrine, Saccharin,
Sulindac, Suramin,
SB 218795, Thalidomide, Tilorone, Trimeprazine, Tolazamide, Tolbutamide,
Tolperisone,
Uridine, Vidarabine, Zaleplon, and Zidovudine.
Results and Discussion
[0122] A library of 462 clinically relevant drugs were profiled for functional
activity at 33 of the 36 known human monoaminergic G-protein coupled receptors
using the
mammalian cell-based functional assay Receptor Selection and Amplification
Technology
(R-SAT). Table 13 illustrates data on representative antipsychotic agents for
receptors at
which the most potent activities were observed. Potency data for five
representative
antipsychotics and the clozapine metabolite N-desmethylclozapine (NDMC) at 13
human
monoamine receptor subtypes are shown. Potency data are reported as pKi values
for the
competitive antagonist studies, while inverse agonist data are reported as
pEC50 values,
both derived from three to eight separate determinations +/= standard error.
Asterixes (*)
indicate the presence of agonist activity where the muscarinic receptor
agonist potencies are
reported in Table 14. Ziprasidone displays limited but detectable agonist
efficacy at human
5-HTIA receptors (<30% relative to 8-OH-DPAT), and a Ki > 1-micromolar when
assayed
as a competitive antagonist. Abbreviations used: NDMC-N-desmethylclozapine, 5-
HT-
serotonin, H- histamine, M-muscarinic, D-dopamine, and Alpha-alpha adrenergic,
and nr-
-42-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
no response defined as no significant antagonist or inverse agonist activity
at concentrations
up to 1-micromolar.
Table 13 Pharmacological activities of antipsychotics at human monoamine
receptors.
lial 'ciol doi,e Zi done a ne a ne navlc
Antgaist
Fbcow
Cp 10.0+1-0.1 9.3H-0.1 &3+/-0.3 &4+/-0.2 7.7+/-0.1 7.2+/-0.1
54f12A 7.3+/-0.1 9.7+/-0.1 8.6+/-0.1 &6+/-0.1 8.3+/-0.2 &3+/-0.2
5-HT1A rr rr rri rr rr rr
5-H12C rr 7.2+/-0.3 7.4-q-0.2 7.4+/-0.1 7.4+/-0.2 7.8".2
H1 rr 7.0+1-0.2 rr &4+/-0.1 9.5+/-0.2 8.2+/-0.2
M1 rr rr rr 7.2+/-0.2 7.8+/-0.2 rr '
IVQ rr rr rr 6.9H-0.1 rr* rr
Mi rr rr rr 6.7+/-0.5 &2+1-0.2 6.8+/-0.7k
N# rr rr rr 7.4+/-0.3 nr" r-e
fV6 rr rr rr 7.2+/-0.2 7.5+/-0.3 rri'
D6 9.7+/-0.1 7.9+/-0.4 7.5+/-0.3 7.6+/-0.4 6.3+1-0.1 rr
Alpha 1A 7.4+/-0.1 &54-0.1 7.4+/-0.2 7.4+/-0.2 &1+1-0.1 7.3+/-0.1
AI 2A rr 7.7+/-0.1 rr rr rr rr
Irneise Agonist
5-FiT2A 6.8-q-0.1 9.0+/-0.3 &8".3 7.8+/-0.1 &0+1-0.3 &0+/-0.3
5+ff6A rr rr rr 7.4-q-0.2 7.0+/-0.2 6.9+/-0.1
5a-iT7A rr 9.1+/-0.2 7.3+/-0.1 rr 7.4+/-0.1 7.3+/-0.1
[0123] Competitive antagonism of D2 receptors, and inverse agonism of 5-HT2A
receptors was nearly uniform throughout this class, with typical agents
demonstrating low
5HTZA/DZ ratios, and atypical agents demonstrating high ratios (Meltzer et al
1989 and
Weiner et al 2001). Inverse agonism of H, receptors was commonly observed,
where
clozapine and olanzapine displayed particularly high potency (Weiner et al
2001). Many
compounds showed antagonist activity at alphal-adrenergic receptors, fewer
agents
exhibited potent 5-HT6 activity, while many, particularly risperidone,
displayed potent
inverse agonist activity at 5-HT7 receptors. Clozapine, olanzapine, and a
number of typical
agents (e.g. thioridazine, data not shown), were found to possess potent
muscarinic receptor
antagonist properties. Importantly, no single antagonist activity
differentiated clozapine
from all other agents.
[0124] In contrast to the widespread antagonist activity of these compounds,
very few agents possessed agonist activity. Figure 4A reports the results of
the functional
agonist screen of this compound library at the human M1 muscarinic
acetylcholine receptor.
Only four compounds, the known muscarinic receptor agonists arecoline and
carbachol,
-43-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
moperone and N-desmethylclozapine (NDMC), the major metabolite of clozapine
(Gauch
and Michaelis 1971), were identified. Moperone displayed only a very low
potency
(EC50>1-micromolar) interaction. In contrast, NDMC displayed an EC50 of 100 nM
with
80% efficacy (relative to carbachol) in this study. This result was further
confirmed in a
second functional assay, PI hydrolysis. As depicted in Figure 4B, clozapine
displays
limited agonist efficacy in this assay, precluding accurate potency
determinations, whereas
NDMC displayed high potency (93 +/- 22nM, n=3) and greater agonist efficacy
(56 +/- 8%,
n=3) relative to carbachol. In fact, when assayed against carbachol for
competitive
antagonist activity, clozapine behaved as an antagonist, while NDMC only
partially
reversed carbachol-induced PI hydrolysis (Figure 4C), consistent with the lack
of an
antagonistic response observed when NDMC was tested as a competitive
antagonist at M 1
receptors in R-SAT (Table 13). Finally, the agonist activity of NDMC was
blocked by both
atropine and clozapine (Figure 4D). These results confirm that NDMC is a
potent,
efficacious, Ml receptor agonist, distinguishing it from the M1 receptor
antagonist
properties of clozapine.
[0125] Having demonstrated the agonist activity of NDMC at human M 1
receptors in multiple in vitro functional assays, we then profiled carbachol,
clozapine,
NDMC, olanzapine, the major olanzapine metabolite N-desmethylolanzapine, and
the
muscarinic agonist xanomeline (Shannon et al 1994), at all five human
muscarinic receptor
subtypes using R-SAT (Table 14).
-44-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
Table 14 Muscarinic acetylcholine receptor agonist activity of antipsychotics.
Muscarinic receptor (M1-M5) agonist activity of clozapine, N-
desmethylclozapine,
olanzapine, N-desmethylolanzapine, xanomeline, and carbachol was determined
using R-
SAT as previously described (Spalding et al 2002). Average efficacy
(percentage relative
to carbachol) and potency (pEC50) +/- standard error are reported for 3 or
more replicate
determinations. No response denotes the lack of agonist activity at
concentrations up to 10-
micromolar.
Compound M1 M2 M3
Efficacy pEC50 Efficacy pEC50 Efficacy pEC50
Clozapine 24 3 7.63f0.37 65 8 6.23 0.14 No response
N-desmeth lcloza ine 72 5 7.26 0.07 106 19 6.47 0.21 27 4 6.49 0.18
Olanzapine No response No response No response
N-desmeth lolanza ine No response No response No response
Xanomeline 121 6 7.20 0.08 106 9 6.30 0.23 66 6 6.63 0.21
Carbachol 101t2 6.11 0.03 101 5 6.23 0.09 102 3 6.53 0.04
Compound M4 M5
Efficacy pEC50 Efficacy pEC50
Clozapine 57 5 7.35 0.10 No response
N-desmeth lcloza ine 87 8 6.87 0.17 48 6 7.63 0.25
Olanzapine No response No response
N-desmeth lolanza ine No response No response
Xanomeline 116 9 7.46 0.14 86 12 6.59 0.22
Carbachol 96 3 6.53 0.05 105 3 6.76 0.12
[0126] Clozapine was found to be a very weak partial agonist at Ml receptors,
a
more efficacious agonist at M2 and M4 receptors, and to lack agonist activity
at M3 and
M5 receptors. NDMC also displayed high potency interactions with all five
human
muscarinic receptors, but with increased agonist efficacy at M1, M4, and M5
receptors
when compared to clozapine (Table 14). In contrast, olanzapine and N-
desmethylolanzapine, both structurally related to clozapine and NDMC, lacked
agonist
activity at human muscarinic receptors. Interestingly, xanomeline displayed a
muscarinic
receptor profile that is similar to that observed for NDMC, with the notable
exception of
higher agonist efficacy at M3 receptors. The agonist activities of clozapine,
NDMC, and
xanomeline at human muscarinic receptor subtypes are unique among all
neuropsychiatric
agents tested (Figure 4, and Tables 13 and 14).
- 45 -
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
[0127] The present inventors discovered that muscarinic receptor agonism, and
M1 receptor agonism in particular, of NDMC can be achieved in vivo during
pharmacotherapy with clozapine. Clozapine and NDMC were tested for their
ability to
increase the phosphorylation of mitogen-activated protein kinase (MAP kinase)
in the CA1
region of mouse hippocampus, a response that has been shown to reflect Ml
receptor
activation (Berkeley et al 2001). As depicted in Figure 5, subcutaneous
administration of
vehicle (Figure 5A), clozapine (Figure 5B), or scopolamine alone (data not
shown) fails to
stimulate phosphorylation of hippocampal MAP kinase. In contrast, NDMC induced
phosphorylation of MAP kinase in hippocampal neurons in a dose dependent
manner
(Figures 5C, 5D, and E), an effect that was blocked by pretreatment with
scopolamine
(Figure 5F). Quantification of this effect demonstrates statistically
significant Ml receptor
activation at NDMC doses of 30 mg/kg and greater (Figure 6). Clozapine fails
to behave as
an agonist under these experimental conditions, which likely reflects either
insufficient
metabolism to NDMC after acute administration in mouse, or direct antagonist
effects at
the M1 receptor as demonstrated in the in vitro studies. These data confirm
that NDMC
passes the blood brain barrier and activates hippocampal M1 receptors in vivo.
[0128] It has long been appreciated that antagonism of central muscarinic
receptors can attenuate the EPS induced by antipsychotics (Miller and Hiley
1974). Initial
investigations of the anti-muscarinic properties of antipsychotics defined the
high potency
of clozapine for these receptors in rodent brain, and elucidated the inverse
correlation
between muscarinic receptor antagonism and propensity to induce EPS (Snyder et
al 1974).
Following the elucidation of five muscarinic acetylcholine receptor subtypes
(Bonner et al
1987), clozapine was described as a potent competitive antagonist (Bolden et
al 1991).
Functional studies in various cell lines subsequently documented that
clozapine has
significant agonist activity at M2 and M4 receptors, and low agonist efficacy
at M 1
receptors (Zorn et al 1994 and Olianas et al 1999), consistent with the
results reported
herein. In humans, clozapine has two major metabolites, NDMC and clozapine-N-
oxide
(Gauch and Michaelis 1971). After steady state dosing, NDMC represents a large
proportion of total detectable moieties, with concentrations ranging from 20-
150% of that
observed for clozapine, with mean values of 60-80% (Bondesson and Lindstrom
1988 and
Perry et al 1991). That NDMC is an active metabolite is supported by the
present data, as
well as by prior reports documenting D1, D2, and 5-HT2C receptor competitive
antagonist
activity (Kuoppamaki et al 1993), and a recent report of M1 receptor agonist
activity (Sur et
-46-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
al 2003). In contrast, the other major clozapine metabolite, clozapine-N-
oxide, displays
only very low potency (pKI's<6.0) functional activity at human monoaminergic
receptors
(data not shown). While varying degrees of brain penetration of NDMC have been
reported
in rodents (Baldessarini et al 1993 and Weigmann et al 1999), the present
results, the
observation that systemically administered NDMC activates cFOS expression in
rodent
brain (Young et al 1998), and the detection of NDMC in human cerebrospinal
fluid
following parenteral administration of clozapine (Nordin et al 1995),
demonstrate that
NDMC is brain penetrant and centrally active.
[0129] The present inventors have discovered that clozapine, acting through
its
predominant metabolite NDMC, functions as a direct acting muscarinic receptor
agonist in
vivo. During pharmacotherapy with clozapine, the agonist actions of NDMC is
attenuated
by the antagonistic actions of the parent compound. Thus, high NDMC levels,
and
particularly high NDMC/clozapine ratios, increases agonist efficacy at
muscarinic
receptors, as predicted by mass action and by agonist/antagonist mixing
studies (Brauner-
Osborne et al 1996). Clinical data support this notion. Not only does
clozapine therapy
usually lack the traditional anti-cholinergic side effects of dry mouth,
blurred vision, and
urinary retention common to classical muscarinic antagonists, it is unique in
its ability to
frequently produce sialorrhea (Baldessarini and Frankenburg 1991), an effect
that can be
blocked by the muscarinic antagonist pirenzepine (Fritze and Elliger 1995).
Thus, the
muscarinic receptor agonist activity of NDMC likely mediates this peripheral
effect, while
the muscarinic receptor subtype responsible is still unknown, receptor
subtypes in addition
to the M3 have been implicated (Bymaster et a12003).
[0130] The muscarinic agonist properties of NDMC reported herein underlies
some of the unique central effects of treatment with clozapine. Multiple lines
of evidence
support a pro-cognitive effect of potentiating central cholinergic
neurotransmission,
including the clinical effects of acetylcholinesterase inhibitors and direct
acting muscarinic
receptor agonists (Davis et al 1993). High dose clozapine therapy in treatment
refractory
schizophrenics may actually serve to raise brain levels of NDMC to achieve
central
muscarinic receptor agonist activity, particularly Ml receptor stimulation,
rather than
recruiting additional lower potency receptor interactions that clozapine and
NDMC possess
(Table 13). Thus, NDMC/clozapine ratios are a better predictor of therapeutic
response to
clozapine, particularly for cognition, than absolute clozapine levels.
- 47 -
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
[0131] The data on clozapine and NDMC plasma levels and clinical response
that were prospectively gathered as part of two clinical trials which included
59 neuroleptic
resistant patients (Hasegawa et al 1993), and 33 neuroleptic responsive
patients (Lee et al
1999) with schizophrenia were re-analyzed. Patients were classified as
treatment resistant
or not by standard criteria (Kane et al 1988), and clinical ratings and
neuropsychological
test scores were obtained by trained raters who were blinded to plasma drug
levels. The
mean daily dosages of clozapine, as well as clozapine and NDMC serum levels,
and
NDMC/Clozapine ratios after 6 weeks and 6 months of treatment are reported in
Table
15A.
Table 15 Serum N-desmethylclozapine levels and clinical response in
schizophrenia.
Statistical analysis of the correlation between clinical outcome and serum
levels of
clozapine and N-desmethylclozapine (NDMC) for a cohort of 92 clozapine treated
schizophrenics are reported. Table 15A reports the clozapine dose, clozapine
level, NDMC
levels, and NDMC/clozapine ratios for all treatment resistant (TR) subjects,
responders,
non-responders, and all subjects at 6 weeks and 6 months. P* reports
statistically
significant differences between responders and non-responders. Table 15B
reports the
major relationships of interest for the prediction of the contribution of NDMC
to response
to clozapine treatment, including quality of life, negative symptoms, and
cognition,
analyzed by multiple linear regression. R2** refers to the model applied.
Abbreviations
.used include: NS-not significant, BPRS-Brief Psychiatric Rating Scale, SANS-
Scale for the
Assessment of Negative Symptoms, SAPS- Scale for the Assessment of Positive
Symptoms, WISC-Wisconsin Card Sorting Test.
Table 15A
Drug Measure All TR Subjects Responders Non-Responders P*
(59) (26) (25)
Dose (mg/day) 468+/-190 485+/-205 433+/-178 NS
NDMC Level (ng/ml) 260+/-203 308+/-243 171+/-123 0.01
Clozapine Level (ng/ml) 393+/-301 453+/-328 268+/-207 0.02
NDMC/Clozapine 0.75+/-0.36 0.70+/-0.22 0.81+/-0.48 NS
Drug Measure All Subjects at 6 All Subjects at 6 Months
Weeks (86) (92)
Dose (mg/day) 369+/-169 417+/-197
NDMC Level (ng/ml) 194+/-136 235+/-190
Clozapine Level (ng/mi) 287+/-190 365+/-285
NDMC/Clozapine 0.83+/-1.08 0.71+/-0.30
Table 15B
- 48 -
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
Clinical Measure Beta F P df
Dependent Variable: 6 Weeks
BPRS-Withdrawal/Retardation -0.52 3.73 0.06 0.32 3.73
SANS Attentional Impairment -0.28 5.65 0.02 0.26 3.65
SAPS Global Delusions -1.00 3.87 0.05 0.60 3.55
Quality of Life Scale: Total 17.50 5.20 0.03 0.50 2.40
Quality of Life Scale: Objects and 2.91 7.10 0.01 0.43 2.40
Activities 13.80 14.84 0.01 0.54 2.39
Quality of Life Scale: Instrumentai Role 2.27 4.10 0.05 0.75 4.33
WISC-R Maze
Dependent Variable: 6 Months 7.45 6.75 0.01 0.47 4.47
Petersen's Consonant Trigram Test 1.35 3.67 0.06 0.47 3.48
WISC-Categories Formed
[0132] Both time points were analyzed because improvement in
psychopathology and cognition with clozapine may take six months or longer
(Hagger et al
1993). Thirteen of the 92 patients (14.1%) had NDMC/clozapine ratios >/=1. Of
these
thirteen patients, the highest ratio was 1.77 and the median was 1.05. The
Spearman rank
order correlation between clozapine and NDMC levels was 0.82 and 0.89 at 6
weeks and 6
months, respectively (P=0.0001). The correlation between NDMC/clozapine ratios
at 6
weeks and 6 months was 0.92 (P= 0.0001), indicating remarkable stability of
NDMC/clozapine ratios within subjects. Importantly, dose and NDMC/clozapine
ratios
were not significantly correlated at either time point (rho<0.10) in neither
the neuroleptic-
resistant nor neuroleptic-responsive patients.
[0133] Stepwise multiple-regression were utilized to determine the best
predictors of outcome from each of these measures, including baseline levels
of the
dependent measure, dose, age, and gender, since all have been shown to
significantly
predict response to clozapine (Table 15B).
[0134] In all the models tested, baseline levels of the dependent measure
predicted the largest share of the variance in the model. The NDMC/clozapine
ratio was
the next most frequent predictor of response; the ratio significantly
predicted response in
8/24 (33.3%) of the models, all in the expected direction: the higher the
ratio, the better the
outcome. This result contrasts with the lack of predictive power of clozapine
levels alone,
NDMC levels alone, or their sum. The exception was that higher NDMC levels
alone
predicted greater improvement in two subscales of the Quality of Life scale
(Heinrichs et al
-49-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
1984) (data not shown). As shown in Table 15B, higher NDMC/clozapine ratio
predicted
improvement in multiple measures of cognition, as well as the Scale for the
Assessment of
Negative Symptoms-Attention subscale, which has been suggested to be more
related to
cognition than negative symptoms. The ratio also predicted improvement in
Quality of
Life-total score, including the Instrumental Role Function factor, which has
been shown to
be dependent upon cognitive status (Green 1996), and negative symptoms, which
have been
found to correlate with cognition. The ratio also predicted improvement in
delusions, but
not hallucinations, with clozapine treatment. Dose did not contribute to the
prediction of
any of the models in Table 15B. Dose is significantly correlated with plasma
levels of
clozapine and NDMC (P=0.01-0.001) but not, as noted above, with the
NDMC/clozapine
ratio. This provides further evidence that the absolute levels of clozapine
and NDMC,
while important in identifying responders and non-responders (Fabrazzo et al
2002) are not
as important as their ratio when baseline levels of the dependent measure are
included in
the model. Although additional analyses in larger cohorts are necessary, this
analysis, as
well as recent reports (Frazier et al 2003 and Mauri et al 2003) all suggest
that the
NDMC/clozapine ratio is a better predictor of clinical response to clozapine
than clozapine
levels alone, and support the hypothesis that NDMC is a critical mediator of
clozapine
action.
[0135] The muscarinic receptor agonist properties of NDMC also contribute to
the efficacy of clozapine therapy against positive symptoms. Not only did high
NDMC/clozapine ratios predict response to delusions as noted above, but
additional
support comes from the observation that there are several similarities between
the central
effects of muscarinic receptor agonists and dopamine D2 receptor antagonists
(Pfeiffer and
Jenney 1957 and Mirza et al 2003). For example, behavioral pharmacological
experiments
with mice harboring targeted deletions of each of the five muscarinic receptor
subtypes
have shown that the M1 receptors plays a central role in DA-mediated behaviors
(Gerber et
al 2001). In addition, xanomeline (which displays some selectivity for M1 and
M4
receptors) inhibits amphetamine-induced locomotion (Shannon et al 2000).
Clinically,
xanomeline was found to diminish hallucinosis and aggression in Alzheimer's
Disease
patients (Bodick et al 1997), and has been shown to display activity against
both positive
and negative symptoms in a recent, small, Phase 2 study in schizophrenia
(Schekhar et al,
unpublished data).
-50-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
[0136] The central dopaminergic and muscarinic cholinergic systems are well
known to be functionally interrelated (Miller and Hiley 1974). The muscarinic
antagonist
properties of clozapine are thought to contribute to its low propensity to
cause EPS, yet the
anti-EPS effects of clozapine are more robust than those obtained by the
adjunctive use of
anticholinergics agents like trihexyphenidyl, and some EPS producing
antipsychotics, e.g.
thioridazine, also possess potent muscarinic receptor antagonist properties.
These
observations suggest that although antagonism of central muscarinic receptors
can confer
anti-EPS effects, cholinergic modulation of the motoric effects of D2 receptor
blockade are
more complex than previously appreciated. Present data show that agonism, not
antagonism, of certain muscarinic receptor subtypes expressed within critical
basal ganglia
structures (Weiner et al 1990), are a more efficacious mechanism to lessen
these adverse
motor effects. Further, the widespread use of adjunctive anticholinergics
should be
reevaluated in light of the present data on the pro-cognitive benefits
conferred by the central
muscarinic receptor agonist properties of NDMC.
[0137] In summary, functional characterization of therapeutically useful
neuropsychiatric drugs has revealed the potent, efficacious, muscarinic
receptor agonist
activity of NDMC. This activity was found to be unique among neuropsychiatric
agents as
a class. It is demonstrated that NDMC can cross the blood brain barrier and
function as an
Ml receptor agonist in vivo. Consideration of the contribution of NDMC to
improvement
in cognition and quality of life in clozapine treated patients shows that NDMC
mediates
clinically relevant aspects of treatment response that differentiate clozapine
from other
agents used to treat schizophrenia. These findings show that muscarinic
receptor agonism
mediates the unique clinical properties of clozapine, and that Ml muscarinic
receptor
agonists (Spalding et al 2002), including NDMC itself, may be efficacious
atypical
antipsychotic agents.
Example 14: Net Agonism in N-desmeth lcy lozapine/Clozapine Mixtures
[0138] The effect of mixtures of clozapine and N-desmethylclozapine was
evaluated using an R-SAT assay as described above. 150 nM of N-
desmethylclozapine was
provided with varying concentrations of clozapine. Figure 7 depicts the
results of the R-
SAT assay as a function of clozapine concentration. As indicated by the dotted
line in
Figure 7, net agonistic activity was observed for clozapine concentrations of
about 100 nM
and below. Thus, ratios of NDMC to clozapine of about 1.5 and greater provide
a net
agonistic effect.
-51-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
[0139] The results of the R-SAT assay were confirmed using a PI hydrolysis
assay as described above. 150 nM of N-desmethylclozapine was again provided
with
varying concentrations of clozapine. Figure 8 depicts the results of the assay
as a function
of clozapine concentration. The dotted line in Figure 8 indicates the maximum
concentration of clozapine for which a net agonistic effect is observed.
Similar to the
results of the R-SAT assay, net agonistic activity was observed for clozapine
concentrations
of about 100 nM and below, thus confirming that a ratio of NDMC to clozapine
of about
1.5 and greater provide a net agonistic effect.
Example 15: Administration of Single Doses of NDMC to Schizophrenic Patients
[0140] A single-center, in-patient, randomized, double blind, placebo
controlled, single dose study is conducted on two sequential group of
patients. Two
different groups of 6 patients each are enrolled. Each patient in the first
group of patients
receives single doses of placebo, 25 mg of NDMC, and 50 mg of NDMC
sequentially in
random order. Each patient in the second group of patients receives single
doses of
placebo, 75 mg of NDMC, and 100 mg of NDMC sequentially in random order. The
NDMC and placebo is administered orally as a powder in a gelatin capsule. Male
or female
patients, 20 to 50 years of age, with a history of schizophrenia or
schizoaffective disorder,
who are otherwise in good health are selected for the study. The patients are
not
experiencing acute exacerbation of severe psychosis, defined as a Positive and
Negative
Syndrome Scale (PANSS) score greater than 75.
[0141] Patients are withdrawn from all centrally active medications during a
lead-in period of 4-7 days prior to study start on Study Day -1. On Study Day -
1, patients
are randomized to a schedule of NDMC:placebo in a 2:1 manner. On Study Day 1,
patients
receive study drug or placebo, orally, in the morning, and serial blood
samples are collected
up to 24 h after dose administration. Patients are monitored for 8 hr post-
dose by
continuous lead II ECG monitoring. Clinical evaluation, clinical rating
scales, and frequent
assessments of safety including vital signs, ECGs, clinical labs, and adverse
event recording
are performed periodically throughout Study Day. No study drug is given on
Study Days 2
and 3. On Study Day 4, subjects once again receive study drug or placebo,
orally, in the
morning, and serial blood samples are collected up to 24 h after dose
administration.
Patients are monitored for 8 h post-dose by continuous lead II ECG monitoring.
Clinical
evaluation, clinical rating scales, and frequent assessments of safety
including vital signs,
ECGs, clinical labs, and adverse event recording are performed periodically
throughout the
-52-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
Study Day 4. No study drug or placebo is given on Study Days 5 and 6. On Study
Day 8,
patients receive study drug or placebo, orally, in the morning, and serial
blood samples are
collected up to 24 h after dose administration. Patients are monitored for 8 h
post-dose by
continuous lead II ECG monitoring. Clinical evaluation, clinical rating
scales, and frequent
assessments of safety including vital signs, ECGs, clinical labs, and adverse
event recording
are performed periodically throughout the Study Day 8. A final End of Study
evaluation is
performed 3-5 days following Study Day 8 and a clinical evaluation,
administration of
clinical rating scales, and a safety assessment are performed.
[0142] An interim analysis of the safety variables and pharmacokinetic data
obtained from Group 1 is conducted after the End of Study evaluation and
before the
randomization of Group 2. Safety variables are reviewed by the PI in order to
determine
the doses to be administered in Group 2. If NDMC administration during Group 1
is
deemed safe the second patient cohort is screened, randomized, and enrolled.
If the doses
of NDMC in Group 2 are greater than those administered in Group 1, then,
during the lead-
in period, a pre-conditioning dose of 25 mg of NDMC is given to each subject.
This test
dose is used to identify any patient who may be particularly sensitive to
higher doses of
NDMC, and is administered at least 3 days prior to Day -1. Study related
procedures for
Group 2 is identical to those of Group 1, with the exception of the NDMC dose.
Pharmacokinetic Analysis
[0143] Plasma samples are analyzed for concentrations of NDMC.
Pharmacokinetic parameters are calculated including C,,,ax (maximum plasma
concentration), tmax (time to maximum plasma concentration), AUCo_Z (area
under the
plasma concentration time curve from time zero to the last quantifiable
timepoint,
calculated by linear-log trapezoidal summation), AUC0_. (area under the plasma
concentration time curve from time zero to infinity, calculated by linear-log
trapezoidal
summation and extrapolated to infinity by addition of the last quantifiable
plasma
concentration divided by the elimination rate constant kz), kz (elimination
rate constant,
determined by linear regression of the terminal points of the log-linear
plasma
concentration-time curve), tliz (terminal half-life, determined as ln(2)/Xz),
and CLpo
(apparent oral clearance, calculated by Dose / AUC(0-oo)).
Tolerability
[0144] Tolerability of NDMS is determined by measuring extrapyramidal (EPS)
motor effect using the Simpson and Angus Sacle (SAS) and the Barnes Akathisia
Scale
-53-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
(BAS). These scales are administered at baseline (Study Day -1), 3-5 hours
after drug
administration on Study Days 1, 4, and 8, and at the End of Study evaluation.
Antipsychotic efficacy
[0145] Antipsychotic efficacy is measured using the PANSS and the Clinical
Global Impression Scale-Schisophrenia (CGI-S) measures. These scales are
administred at
baseline (Study Day -1), on Study Days 1, 4, and 8, and at the End of Study
evaluation.
Safety
[0146] Safety is evaluated by measuring vital signs including 3-positional
blood
pressure and pulse rate (5 minute supine, 1 minute sitting, 3 minutes
standing), respiratory
rate, and oral temperature except during screening and post-study procedures.
[0147] 12-lead ECGs are recorded and standard electrocardiogram parameters
including QRS, PR, QT, and QTc intervals are measured. In addition, continuous
lead-II
ECG monitoring is performed for the first 8 hours of Day 1, 4, and 8 following
each
NDMC or placebo dose administration.
[0148] A neurological screen is conducted by the clinically responsible
physician at the clinic. The neurological screen consists of a qualitative
assessment of
muscle tone in the extremities, the presence of tremors, fasiculations, and
nystagmus, and
various tests of cerebellar coordination (finger nose test,
dysdiadochokinesia, heel-shin test,
and gait).
[0149] Clinical laboratories are measured after at least an 8-hour fast on
Study
Days 1, 4, 8, and the End of Study evaluation and include the following:
[0150] Erythrocytes: RBC count, hematocrit, hemoglobin, mean corpuscular
hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), mean
corpuscular volume (MCV), RDW, and reticulocyte count.
[0151] Leukocytes: WBC count and differential (basophils, eosinophils,
lymphocytes, monocytes, and neutrophils) reported as absolute values.
[0152] Coagulation: platelet count, PT as INR, and aPTT.
[0153] Liver: alkaline phosphatase, ALT (serum glutamic-pyruvic transaminase
[SGPT]), AST (serum glutamic-oxaloacetic transaminase [SGOT]), bilirubin
(total, direct,
indirect), gamma-glutamyl transferase (GGTP), creatine phosphokinase (CPK) and
LDH.
[0154] Renal: blood urea nitrogen (BUN), creatinine, and uric acid.
[0155] Electrolytes: carbon dioxide, chloride, magnesium, potassium, and
sodium.
-54-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
[0156] General: albumin, calcium, glucose (fasting) phosphate, and protein
(total).
[0157] Endocrine: prolactin.
[0158] Lipids: cholesterol (total), HDL cholesterol, LDL cholesterol, and
triglycerides.
[0159] Macroscopic urinalysis: pH, specific gravity, glucose, ketones,
leukocyte
esterase, nitrites, occult blood, and protein.
[0160] Microscopic urinalysis: RBC/high powered field, WBC/high powered
field, bacteria, castes, epithelial cells, mucous threads and crystals.
Example 16: Administration of Multiple Doses of NDMC to Schizophrenic Patients
[0161] A single-center, in-patient, randomized, double blind, placebo
controlled, multiple dose study is conducted on two sequential groups. Twelve
patients, in
two different groups of six patients each are enrolled. Each patient receives
either placebo
or NDMC daily for five days. The NDMC and placebo is administered orally as a
powder
in a gelatin capsule. Male or female patients, 20 to 50 years of age, with a
history of
schizophrenia or schizoaffective disorder, who are otherwise in good health
are selected for
the study. The patients are not experiencing acute exacerbation of severe
psychosis,
defined as a Positive and Negative Syndrome Scale (PANSS) score greater than
75.
[0162] Patients are withdrawn from all centrally active medications during the
lead-in period of 4-7 days prior to study start on Study Day -1. If the safety
profile of
NDMC as determined by the single-dose study of Example 14 suggests that a
gradual dose
escalation is warranted, and if the pharmacokinetics properties of NDMC
demonstrate that
the t1i2 is less than 8 hr, then patients enrolled in the study receive pre-
conditioning doses of
NDMC prior to Study Day -1. If indicated, subjects receive, during the lead-in
portion of
the study, a single dose of NDMC that is 25% of the planned dose, followed by
a second
dose the following day which is 50% of the planned dose. If these doses are
deemed safe,
then subjects are randomized on Study Day -1 to either NDMC or placebo. The
dosages
and frequency of administration (QD or BID) of NDMC are determined based on
the safety
and pharmacokinetics observed during the single dose safety study of Example
14.
[0163] On Study Day 1, patients receive study drug or placebo, orally, and
serial
blood samples are collected up to 24 hours after dose administration. Patients
are
monitored for 8 hr post-dose by continuous lead II ECG monitoring. Clinical
evaluation,
clinical rating scales, and frequent assessments of safety including vital
signs, ECGs,
-55-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
clinical labs, and adverse event recording are performed periodically
throughout Study Day
1.
[0164] Patients receive study drug or placebo daily for the next four days. On
Study Days 2, 3, and 4, pre-dose serum sampling for pharmacokinetic analysis
are obtained,
and patients are monitored for 8 hr post-dose by continuous lead II ECG
monitoring.
Clinical evaluation, clinical rating scales, and frequent assessments of
safety including vital
signs, ECGs, clinical labs, and adverse event recording are performed
periodically
throughout Study Days 2-4.
[0165] On Study Day 5, pre-dose serum sampling as well as serial blood
samples collected up to 24 hours after study drug or placebo administration
are obtained for
pharmacokinetic analysis, and patients are monitored for 8 hr post-dose by
continuous lead
II ECG monitoring. Clinical evaluation, clinical rating scales, and frequent
assessments of
safety including vital signs, ECGs, clinical labs, and adverse event recording
are performed
periodically throughout Study Day 5.
[0166] A final End of Study evaluation is conducted 5-7 days after the
cessation
of active dosing on Study Day 5. A safety assessment is performed during this
clinical
evaluation, including vital signs, ECG, clinical labs, NDMC serum
determination, and
adverse event recording. All patients are followed clinically, as in-patients,
for as long as is
indicated following the cessation of active dosing of NDMC.
[0167] An interim analysis of the safety variables and pharmacokinetic data
from Group 1 is conducted after the End of Study evaluation, and before the
randomization
of Group 2. Safety variables are reviewed by the PI in order to determine the
doses to be
administered in Group 2. If NDMC administration during Group 1 is deemed safe,
the
second patient cohort is screened, randomized, and enrolled. Study related
procedures for
Group, 2 are identical to those of Group 1, with the exception of NDMC dose
and/or
frequency of administration.
Pharmacokinetic Analysis
[0168] Plasma samples are analyzed for concentrations of NDMC.
Pharmacokinetic parameters are calculated are calculated following the single
dose
administration on Day 1 including Cmax (maximum plasma concentration), tmax
(time to
maximum plasma concentration), AUCo_Z (area under the plasma concentration
time curv
e
from time zero to the last quantifiable timepoint, calculated by linear-log
trapezoidal
summation), AUCo_,,,, (area under the plasma concentration time curve from
time zero to
-56-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
infinity, calculated by linear-log trapezoidal summation and extrapolated to
infinity by
addition of the last quantifiable plasma concentration divided by the
elimination rate
constant ?,z), Xz (elimination rate constant, determined by linear regression
of the terminal
points of the log-linear plasma concentration-time curve), t1i2 (terminal half-
life, determined
as ln(2)/Xz), and CLpo (apparent oral clearance, calculated by Dose / AUC(0-
oo)).
[0169] Pharmacokinetic parameters are also calculated following the last dose
on Day 5 including Cmax,ss (maximum steady-state plasma concentration),
Cmin,ss (minimum
steady-state plasma concentration), Cavg,ss (average steady-state plasma
concentration
calculated as AUC(0-i)sS divided by the dosing interval i), tmax,ss (time to
maximum steady-
state plasma concentration), tmin,ss (time to minimum steady-state plasma
concentration),
AUCo_Z (area under the plasma concentration time curve from time zero to the
last
quantifiable timepoint, calculated by linear-log trapezoidal summation),
AUCo_tss (area
under the plasma concentration time curve from time zero to the end of the
steady-state
dosing interval, calculated by linear-log trapezoidal summation), ?,z,ss
(steady-state
elimination rate constant, determined by linear regression of the terminal
points of the log-
linear plasma concentration-time curve), tli2,ss (steady-state terminal half-
life, determined as
ln(2)/Xz,ss), and CLpo,SS (apparent oral clearance, calculated by Dose / AUC(0-
i)ss).
Tolerability
[0170] Tolerability of NDMS is determined by measuring extrapyramidal (EPS)
motor effect using the Simpson and Angus Sacle (SAS) and the Barnes Akathisia
Scale
(BAS). These scales are administered at baseline (Study Day -1), 6 hours after
drug
administration on Study Days 1-5, and at the End of Study evaluation.
Antipsychotic efficacx
[0171] Antipsychotic efficacy is measured using the PANSS and the Clinical
Global Impression Scale-Schisophrenia (CGI-S) measures. These scales are
administred at
baseline (Study Day -1), on Study Days 1, 5, and at the End of Study
evaluation.
Safety
[0172] Safety is evaluated by measuring vital signs including 3-positional
blood
pressure and pulse rate (5 minute supine, 1 minute sitting, 3 minutes
standing), respiratory
rate, and oral temperature except during screening and post-study procedures.
[0173] 12-lead ECGs are recorded and standard electrocardiogram parameters
including QRS, PR, QT, and QTc intervals are measured. In addition, continuous
lead-II
-57-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
ECG monitoring is performed for the first 8 hours of Days 1-5 following each
NDMC or
placebo dose administration.
[0174] A neurological screen is conducted by the clinically responsible
physician at the clinic. The neurological screen consists of a qualitative
assessment of
muscle tone in the extremities, the presence of tremors, fasiculations, and
nystagmus, and
various tests of cerebellar coordination (finger nose test,
dysdiadochokinesia, heel-shin test,
and gait).
[0175] Clinical laboratories are measured after at least an 8-hour fast and
include the following:
[0176] Erythrocytes: RBC count, hematocrit, hemoglobin, mean corpuscular
hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), mean
corpuscular volume (MCV), RDW, and reticulocyte count.
[0177] Leukocytes: WBC count and differential (basophils, eosinophils,
lymphocytes, monocytes, and neutrophils) reported as absolute values.
[0178] Coagulation: platelet count, PT as INR, and aPTT.
[0179] Liver: alkaline phosphatase, ALT (serum glutamic-pyruvic transaminase
[SGPT]), AST (serum glutamic-oxaloacetic transaminase [SGOT]), bilirubin
(total, direct,
indirect), gamma-glutamyl transferase (GGTP), creatine phosphokinase (CPK) and
LDH.
[0180] Renal: blood urea nitrogen (BUN), creatinine, and uric acid.
[0181] Electrolytes: carbon dioxide, chloride, magnesium, potassium, and
sodium.
[0182] General: albumin, calcium, glucose (fasting) phosphate, and protein
(total).
[0183] Endocrine: prolactin.
[0184] Lipids: cholesterol (total), HDL cholesterol, LDL cholesterol, and
triglycerides.
[0185] Macroscopic urinalysis: pH, specific gravity, glucose, ketones,
leukocyte
esterase, nitrites, occult blood, and protein.
[0186] Microscopic urinalysis: RBC/high powered field, WBC/high powered
field, bacteria, castes, epithelial cells, mucous threads and crystals.
-58-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
Literature Cited
[0187] Each of the following references is incorporated by reference herein in
its entirety, including any drawings.
[0188] The following references are incorporated herein by reference in their
entireties, including any drawings.
[0189] Eglen, R., M., Choppin, A., and Watson, N., (2001) Therapeutic
opportunities from muscarinic receptor research. Trends Pharmacol. Sci. 22(8):
409-414.
[0190] Brown, J., H., and Taylor, P., (1996) Muscarinic receptor agonists
and antagonists, in The pharmacological basis of therapeutics. Hardiman, J.,
G., and
Limbird, L., E., editors, Mcgraw-Hill, New York, pp. 141-161.
[0191] Moroi, S., E., and Lichter, P., R. (1996) Ocular pharmacology, in
The pharmacological basis of therapeutics. Hardiman, J., G., and Limbird, L.,
E., editors,
Mcgraw-Hill, New York, pp. 1619-1647.
[0192] Davis, R E; Doyle, P D; Carroll, R T; Emmerling, M R; Jaen, J.
Cholinergic therapies for Alzheimer's disease: Palliative or disease altering?
Arzneimittel-
Forschung,.45, 425-431, 1995.
[0193] Shekhar, A., Potter, W., Z., Lienemann, J., et. al. (2001) Efficacy of
xanomeline, a selective muscarinic agonist, in treating schizophrenia: a
double blind
placebo controlled study. ACNP abstracts 135: 173.
[0194] Rodriquez, M.A., Whipple, B., Ocampo, G., et. al. (2002)
Muscarinic agonists in neuropathic and nociceptive pain assays in rats.
International
Association for the Study of Pain's 10'h World Congress, 1160-P76: 388.
[0195] Baldessarini, R., J., and Frankenburg, F., R. (1991) Clozapine. A
novel antipsychotic agent. New. Engl. J. Med., 324(11): 746-754.
[0196] Jann, M., W., Grimsley, S., R., Gray, E., C., and Chang, W. (1993)
Pharmacokinetic and pharmacodynamics of clozapine. Clin. Pharmacokinet. 24(2):
161-
176.
[0197] Centorrino, F., Baldessarini, R., J., Kando, J., C., et. al. (1994)
Clozapine and metabolites: concentrations in serum and clinical findings
during treatment
of chronically psychotic patients. J. Clin. Psychopharmacol. 14: 119-125.
-59-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
[0198] Hunziker F. Fisher, E., and Scmutz, J. (1967) 11-amino-5H-
dibenzo[b,e]-1,4-diazepine. Mitteilung uber siebenglienrige Heterocyclen.
Helv. Chim.
Acta, 50:1588-1599.
[0199] Jensen, A., A., Spalding, T., A., Burstein E., S., et. al. (2000)
Functional importance of the Ala(116)-Pro(136) region in the calcium-sensing
receptor.
Constitutive activity and inverse agonism in a family C G-protein-coupled
receptor. J Biol
Chem. 275(38): 29547-55.
[0200] Baldessarini RJ, Frankenburg FR (1991) Clozapine: a novel
antipsychotic agent. NEngl JMed 324:746-754.
[0201] Baldessarini RJ, Centorrino F, Flood JG, Volpicelli SA, Huston-Lyons
D, Cohen BM (1993) Tissue concentrations of clozapine and its metabolites in
the rat.
Neuropsychopharmacology 9:117-124.
[0202] Berkeley JL, Gomeza J, Wess J, Hamilton SE, Nathanson NM, Levey Al
(2001) M1 muscarinic acetylcholine receptors activate extracellular signal-
regulated kinase
in CA1 pyramidal neurons in mouse hippocampal slices. Mol Cell Neurosci 18:512-
524.
[0203] Bodick NC, Offen WW, Levey AI, Cutler NR, Gauthier SG, Satlin A,
Shannon HE, Tollefson GD, Rasmussen K, Bymaster FP, Hurley DJ, Potter WZ, Paul
SM
(1997) Effects of xanomeline, a selective muscarinic receptor agonist, on
cognitive function
and behavioral symptoms in Alzheimer disease. Arch Neurol 54:465-473.
[0204] Bolden C, Cusack B, Richelson E(1991) Clozapine is a potent and
selective muscarinic antagonist at the five cloned human muscarinic
acetylcholine receptors
expressed in CHO-K1 cells. Eur JPharmacol 192:205-206.
[0205] Bondesson U, Lindstrom LH (1988) Determination of clozapine and its
N-demethylated metabolite in plasma by use of gas chromatography-mass
spectrometry
with single ion detection. Psychopharmacology 95:472-475.
[0206] Bonner TI, Buckley NJ, Young AC, Brann MR (1987) Identification of a
family of muscarinic acetylcholine receptor genes. Science 237:527-532.
[0207] Brauner-Osborne H, Ebert B, Brann MR, Falch E, Krogsgaard-Larsen P
(1996) Functional partial agonism at cloned human muscarinic acetylcholine
receptors. Eur
JPharmacol 313:145-150.
[0208] Bymaster FB, Carter PA, Yamada M, Gomeza J, Wess J, Hamilton S,
Nathanson NM, McKinzie DL, Felder CC (2003) Role of specific muscarinic
receptor
-60-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
subtypes in cholinergic parasympathomimetic responses, in vivo
phosphoinositide
hydrolysis, and pilocarpine-induced seizure activity. Eur JNeurosci 17:1403-
1410.
[0209] Carlsson A (1978) Antipsychotic drugs, neurotransmitters, and
schizophrenia. Am JPsychiatry 135(2):165-173.
[0210] Davis RE, Emmerling MR, Jaen JC, Moos WH, Spiegel K (1993)
Therapeutic intervention in dementia. Crit Rev Neurobiol 7:41-83.
[0211] Fabrazzo M, La Pia S, Monteleone P, Esposito G, Pinto A, De Simone
L, Bencivenga R, Maj M (2002) Is time course of clozapine response correlated
to the time
course of plasma clozapine levels? A one-year prospective study in drug-
resistant patients
with schizophrenia. Neuropsychopharmacology 27:1050-1055.
[0212] Frazier JA, Glassner Cohen L, Jacobsen L, Grothe D, Flood J,
Baldessarini RJ, Piscitelli S, Kim GS, Rapoport JL (2003) Clozapine
pharmacokinetics in
children and adolescents with childhood-onset schizophrenia. J Clin
Psychopharmacol
23(1):87-91.
[0213] Fritze J, Elliger T (1995) Pirenzepine for clozapine-induced
hypersalivation. Lancet 346:1034.
[0214] Gauch R, MichaelisW (1971) The metabolism of 8-chloro-11-(4-methyl-
1-piperazinyl)-5H-dibenzo [b,e] [1,4] diazepine (clozapine) in mice, dogs, and
human
subjects. Farmaco 26:667-681.
[0215] Gerber DJ, Sotnikova TD, Gainetdinov RR, Huang SY, Caron MG,
Tonegawa S (2001) Hyperactivity, elevated dopaminergic transmission, and
response to
amphetamine in M1 muscarinic acetylcholine receptor-deficient mice. Proc Natl
Acad Sci
USA 98(26):15312-15371.
[0216] Green MF (1996) What are the functional consequences of
neurocognitive deficits in schizophrenia? Am JPsychiatry 153:321-330.
[0217] Hagger C, Buckley P, Kenny JT, Friedman L, Ubogy D, Meltzer HY
(1993) Improvement in cognitive functions and psychiatric symptoms in
treatment-
refractory schizophrenic patients receiving clozapine. Biol Pyschiatry 34:702-
712.
[0218] Hasegawa M, Gutierrez-Esteinou R, Way L, Meltzer HY (1993)
Relationship between clinical efficacy and clozapine concentrations in plasma
in
schizophrenia: effect of smoking. J Clin Psychopharmacol 13:383-390.
-61 -
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
[0219] Heinrichs DW, Hanlon TE, Carpenter WT (1984) The Quality of Life
Scale: an instrument for rating the schizophrenia deficit syndrome. Schizophr
Bull 10:388-
398.
[0220] Kane J, Honigfeld G, Singer J, Meltzer H, Clozaril Collaborative Study
Group (1988) Clozapine for the treatment-resistant schizophrenic. Arch Gen
Psychiatry
45:789-796.
[0221] Kuoppamaki M, Syvalahti E, Hietala J (1993) Clozapine and N-
desmethylclozapine are potent 5-HTIC receptor antagonists. Eur J Pharmacol
245:179-
182.
[0222] Lee MA, Jayathilake K, Meltzer HY (1999) A comparison of the effect
of clozapine with typical neuroleptics on cognitive function in neuroleptic-
responsive
schizophrenia. Schizophr Res 37:1-11.
[0223] Leucht S, Wahlbeck K, Hamann J, Kissling W (2003) New generation
antipsychotics versus low-potency conventional antipsychotics: a systematic
review and
meta-analysis. Lancet 361(9369):1581-1589.
[0224] Mauri MC, Volonteri LS, Dell'Osso B, Regispani F, Papa P, Baldi M,
Bareggi SR (2003) Predictors of clinical outcome in schizophrenic patients
responding to
clozapine. J Clin Psychopharmacol 23(6):660-664.
[0225] Meltzer HY, Matsubara S, Lee JC (1989) Classification of typical and
atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2
pKi values.
JPharmacol Exp Ther 251:238-246.
[0226] Meltzer HY, Alphs L, Green Al, Altamura AC, Anand R, Bertoldi A,
Bourgeois M, Chouinard G, Zahur Islam M, Kane J, Krishnan R, Lindenmayer JP,
Potkin S
(2003) Clozapine treatment for suicidality in schizophrenia. Arch Gen
Psychiatry 60:82-91.
[0227] Miller RJ, Hiley CR (1974) Anti-muscarinic properties of neuroleptics
and drug-induced Parkinsonism. Nature 248:596-597.
[0228] Mirza NR, Peters D, Sparks RG (2003) Xanomeline and the
antipsychotic potential of muscarinic receptor subtype selective agonists. CNS
Drug Rev
9(2):159-186.
[0229] Nordin C, Alme B, Bondesson U (1995) CSF and serum concentrations
of clozapine and its demethyl metabolite: a pilot study. Psychopharmacology
122:104-107.
-62-
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
[0230] Olianas MC, Maullu C, Onali P (1999) Mixed agonist-antagonist
properties of clozapine at different human cloned muscarinic receptor subtypes
expressed in
chinese hamster ovary cells. Neuropsychopharmacology 20(3):263-270.
[0231] Perry PJ, Miller DD, Arndt SV, Cadoret RJ (1991) Clozapine and
norclozapine plasma concentrations and clinical response of treatment-
refractory
schizophrenic patients. Am JPsychiatry 148(2):231-135.
102321 Pfeiffer CC, Jenney EH (1957) The inhibition of the conditioned
response and the counteraction of schizophrenia by muscarinic stimulation of
the brain. Ann
NYAcad Sci 66:753-764.
[0233] Shannon HE, Bymaster FP, Calligaro DO, Greenwood B, Mitch CH,
Sawyer BD, Ward JS, Wong DT, Olesen PH, Sheardown MJ, Swedberg MDB, Suzdak PD,
Sauerberg P (1994) Xanomeline: a novel muscarinic receptor agonist with
functional
selectivity for M1 receptors. JPharmacol Exp Ther 269(1):271-281.
[0234] Shannon HE, Rasmussen K, Bymaster FP, Hart JC, Peters SC, Swedberg
MD, Jeppesen L, Sheardown MJ, Sauerberg P, Fink-Jensen A (2000) Xanomeline, an
M(1)/M(4) preferring muscarinic cholinergic receptor agonist, produces
antipsychotic-like
activity in rats and mice. Schizophr Res 42:249-259.
[0235] Snyder S, Greenberg D, Yamamura HI (1974) Anti-schizophrenic drugs
and brain cholinergic receptors. Affinity for muscarinic sites predicts
extrapyramidal
effects. Arch Gen Psychiatry 31:58-61.
[0236] Spalding TA, Trotter C, Skjaerbaek N, Messier TL, Currier EA, Burstein
ES, Li D, Hacksell U, Brann MR (2002) Discovery of an ectopic activation site
on the M(l)
muscarinic receptor. Mol Pharmacol 61:1297-1302.
[0237] Spina E, Avenoso A, Facciola G, Salemi M, Scordo MG, Ancione M,
Madia AG, Perucca E (2001) Relationship between plasma risperidone and 9-
hydroxyrisperidone concentrations and clinical response in patients with
schizophrenia.
Psychopharmacology 153:238-243.
[0238] Sur C, Mallorga PJ, Wittmann M, Jacobson MA, Pascarella D, Williams
JB, Brandish PE, Pettibone DJ, Scolnick EM, Conn, PJ (2003) N-
desmethylclozapine, an
allosteric agonist at muscarinic 1 receptor, potentiates N-methyl-d-aspartate
receptor
activity. Proc Natl Acad Sci USA 100(23):13674-13679.
[0239] The Parkinson Study Group (1999) Low-dose clozapine for the treatment
of drug-induced psychosis in Parkinson's Disease. NEngl JMed 340:757-763.
- 63 -
CA 02576153 2007-02-05
WO 2006/017614 PCT/US2005/027645
[0240] Weigmann H, Hartter S, Fischer V, Dahmen N, Hiemke C (1999)
Distribution of clozapine and desmethylclozapine between blood and brain in
rats. Eur.
Neuropsychopharmaco19:253-256.
[0241] Weiner DM, Levey Al, Brann MR (1990) Expression of muscarinic
receptor acetylcholine and dopamine receptor mRNA's in rat basal ganglia. Proc
Natl
AcadSci USA. 87:7050-7054.
[0242] Weiner DM, Burstein ES, Nash N, Croston GE, Currier EA, Vanover
KE, Harvey SC, Donohue E, Hansen HC, Andersson CM, Spalding TA, Gibson DFC,
Krebs-Thomson K, Powell, SB, Geyer MA, Hacksell U, Brann MR (2001)5-
hydroxytryptamine2a receptor inverse agonists as antipsychotics. J Pharmacol
Exp Ther
299:268-276.
[0243] Weissman JT, Ma J, Essex A, Gao Y, Burstein ES (2003) G-protein-
coupled receptor-mediated activation of rap GTPases: characterization of a
novel Gi
regulated pathway. Oncogene 23(1):241-249.
[0244] Wellendorph P, Goodman MW, Burstein ES, Nash NR, Brann MR,
Weiner DM (2002) Molecular cloning and pharmacology of functionally distinct
isoforms
of the human histamine H3 receptor. Neuropharmacology 42:929-940.
[0245] Wong AH, Van Tol HH (2003) Schizophrenia: from phenomenology to
neurobiology. Neurosci Biobehav Rev 27(3):269-306.
[0246] Young CD, Meltzer HY, Deutch AY (1998) Effects of
desmethylclozapine on fos protein expression in the forebrain: in vivo
biological activity of
the clozapine metabolite. Neuropsychopharmacology 19:99-103.
[0247] Zorn SH, Jones SB, Ward KM, Liston DR (1994) Clozapine is a potent
and selective muscarinic M4 receptor agonist. Eur JPharmaco1269:R1-R2.
-64-