Note: Descriptions are shown in the official language in which they were submitted.
CA 02577861 2007-02-21
Alkylidene-Tetrahydronaphthalene Derivatives, Process for Their Production and
Their Use as 4ntiInflammatory Agents
The'invention relates to alkylidene-tetrahydronaphthalene derivatives, process
for their production and their use as anti-inflammatory agents.
Open-chain, non-steroidal anti-inflammatory agents are known from the prior
art
WO 02/10143 and WO 03/082827. In the experiment, these compounds show
,_.. .l
dissociations of action between anti-inflammatory and undesirable metabolic
actions and
are superior fo the previously described nonsteroidal glucocorticoids or
exhibit at least
just as good an action.
The selectivity of the compounds of the prior art compared to the other
steroid
receptors still requires improvement, however.
It was therefore the object of this invention to make available compounds
whose
selectivity is improved but is at least comparable relative to the other
steroid receptors.
f.... ,~. This object is achieved by the compounds of this invention,
explained in the
~ . ~ claims.
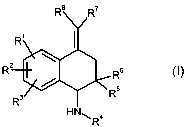
This inventionlelates to compounds of general formula (I)
. '~.
in which
R' and R2, independently of one another, mean a hydrogen atom, a hydroxy
CA 02577861 2007-02-21
= 2
group, a halogen atom, an optionally substituted (Cl-Cl )-alkyl group, a
(Ci-Ci )-alkoxy group, ~ (CI-Ci )-alkylthio group, a (CI-C5)-
perfluoroalkyl group, a;;Yano group, or a nitro group, or R' and R2
together mean a group that is selected from the groups -O-(CHZ)n-O-, -0-
(CH2)n-CH2-, -O-CH=CH-, -(CH2)n+2-, NH-(CH2)n+l, N(Cl-C3-alkyl)-
(CH2)õ+i, and -NH-N=CH-, whereby n=1 or 2, and the terminal atoms
are linked to directly adjacent ring-carbon atoms,
or NR9R10, whereby R9 and R'0, independently of one another, can be
~..
'. l hydrogen, CI-C5-allcyl or (CO)-CI-CS-alkyl,
R3 means a hydrogen atom, a hydroxy group, a halogen atom, an optionally
substituted (Ci-Clo)-alkyl group, a(Ci-Cl )-alkoxy group, a(Ci-C10)-
alkylthio group, a(CI-Cs)-perlluoroalkyl group, or a cyano group,
R4 means a Cl-C10--alkyl group, a Ci-ClO--alkyl group that is substituted by
one or more groups selected from 1-3 hydroxy groups, halogen atoms, or
1-3 (CI-C5)-alkoxy groups, an optionally substituted (C3-C7)-cycloalkyl
group, an optionally substituted heterocyclyl group, an optionally
; := 4
substituted aryl group; a monocyclic or bicyclic heteroaryl group
} . .
that optionally is substituted by one or more groups selected from
(CI-CS)-alkyl groups (which optionally can be substituted
by 1=3 hydroxy or 1-3 COOR6groups), (Ci-CS)-alkoxy groups, hydroxy
groups, halogen atoms, or (Ci-C3)exoalkylidene groups and that
optionally contains 1-4 nitrogen atoms and/or 1-2 oxygen atoms and/or
1-2 sulfur atoms and/or 1-2 keto groups, whereby this group can be linked
to the amine of the tetrahydronaphthalene system via any position and
optionally can be hydrogenated at one or more sites, and R12 means a
CA 02577861 2007-02-21
{
3
(CI-CS)-alkyl group or a benzyl group,
Rs means a hydroxy group;' a group OR", or an O-(CO)R" group, whereby
R~ 1 means any hydroxy,protective group or a CI-Ci -alkyl group,
R6 means a(CI-Cs)-alkyl group or an optionally partially or completely
fluorinated (CI-CS)-alkyl group, a(C3-C7)cycloalkyl group, a(C3-
C7)cycloalkyl(Ci-Cg)alkyl group, a (C3-C7)cycloalkyl(C2-Ce)alkenyl
group, a heterocyclyl group, a heterocyclyl(Ct-Cg)alkyl group, a
heterocyclyl(CZ-C8)alkenyl group, an aryl group, an aryl(CI-Ce)alkyl
~~.
) group, an aryl(CrCB)alkenyl group, an aryl(C2-Ca)alkinyl group; a
monocyclic or bicyclic heteroaryl group that optionally is substituted by
one or more keto groups, (Ci-CS)-alkyl groups, (Ci-Cs)-alkoxy groups,
halogen atoms, or (Ci-C3)exoalkylidene groups and that optionally
contains one or more nitrogen atoms andlor oxygen atoms and/or sulfur
atoms; a heteroaryl(CI-Ca)alkyl=group or a heteroaryl(CZ-C8)alkenyl
group, whereby these groups can be linked to the tetrahydronaphthalene
system via any position and optionally can be hydrogenated at one or
=~~~y
vr t more sites,
R7 and R8, independently of one another, mean a hydrogen atom, a halogen atom,
a(CI-CS)alkyl group, which can be substituted with OR10, SR", or
N(R9R1), or together with the carbon atom of the methylene group mean
a (C3-C6)-cycloalkyl ring, or
R' and Rg together mean an annelated five- to eight-membered, saturated or
unsaturated carbocyclic compound or heterocyclic compound, which
optionally is substituted by 1-2 keto groups, 1-2 (Cl-CS)-alkyl groups, 1-2
(Cl-Cs)-alkoxy groups, l-2 hydroxy groups, or 1-4 halogen atoms.
CA 02577861 2007-02-21
4
Compounds of general formula I, in which
R' and R2, independently of one another, mean a hydrogen atom, a hydroxy
group, a halogen atom, an optionally substituted (Cl-C10)-alkyl group, a
(Ci-Cio)-alkoxy group, a (CI-Clo)-alkylthio group, a (CI-Cs)-
perEluoroalkyl group, a cyano group, or a nitro group or
R' and RZ together mean a group that is selected from the groups -0-
(CHZ)õ-0-, -O{CH2)õ-CH2-, -O-CH=CH-, -(CH2)q+2-, -NH-(CHZ)a+I,
N(Cl-C3-atkyl)-(CH2)õ+I, NH N=CH-, whereby n=1 or 2, and the
terminal atoms are linked to directly adjacent ring-carbon atoms, or
NRgR10, whereby R9 and R10, independently of one another, can be
hydrogen, Cl-Cs-alkyl or (CO)-CI-CS-alkyl,
R3 means a hydrogen atom, a hydroxy group, a halogen atom, an optionally
substituted (Ci-Cio)-alkyl group, a(CI-Clo)-allcoxy group, a(CI-CIo)-
alkylthio group, a(Cl-Cs)-perfluoroalkyl group, or a cyano group,
R4 means a Cl-Cio-allcyl group, a Cl-Cl -alkyl group that is substituted by
one or more groups selected from 1-3 hydroxy groups, halogen atoms, or
1-3 (Ci-CS)-alkoxy groups, an optionally substituted (C3-C+cycloallcyl
group, an optionally substituted heterocyclyl group, an optionally
substituted aryl group; a monocyclic or bicyclic heteroaryl group that
optionally is substituted by one or more groups selected from (Cl-Cs)-
alkyl groups (which optionally can be substituted by 1-3 hydroxy groups
or 1-3 COOR1Z groups), (CI-CS)-alkoxy groups, hydroxy groups, halogen
atoms, or (CI-C3)exoalkylidene groups and that optionally contains 174
nitrogen atoms and/or 1-2 oxygen atoms and/or 1-2 sulfur atoms and/or I -
2 keto groups, whereby these groups can be linked to the amine of the
CA 02577861 2007-02-21
tetrahydronaphthalene system via any position and optionally can be
hydrogenated at one or more sites, and R12 means a(Cl-CS)-alkyl group
or a benzyl group,
RS means a hydroxy group, a gioup OR' 1 or an O-(CO)R' 1 group, whereby
Ri' means any hydroxy protective group or a CI-C10-alkyl group,
R6 means a(Ci-C5)-alkyl group or an optionally partially or completely
fluorinated (CI-Cs)-alkyl group, a (C3-C7)cycloalkyl group, a(C3-
C7)cycloalkyl(Cj-Cg)alkyl group, a (C3-C7)cycloalkyl(CZ-C$)alkenyl
=~:~~
group, a heterocyclyl group, a heterocyclyl(CI-Ca)alkyl group, a
heterocyclyl(C2-C8)alkenyl group, an aryl group, an aryl(Ci-CS)alkyl
group, an aryl(CZ-C$)alkenyl group, an aryl(C2-Ca)alkinyl group; a
monocyclic or bicyclic heteroaryl group that optionally is substituted by
one or more keto groups, (Cl-CS)-aIlcyl groups, (C!-CS)-alkoxy groups,
halogen atoms, (CI-C3)exoalkylidene groups and that contains one or
more nitrogen atoms and/or oxygen atoms and/or sulfur atoms;
a heteroaryl(Cl-C8)alkyl group or a heteroaryl(C2-C8)alkenyl group,
~}? whereby these groups ~;;' ;~=f can be linked to the tetrahydronaphthalene
system
via any position and optionally can be hydrogenated at one or more sites,
R7 and Rg, independently of one another, mean a hydrogen atom, a halogen atom,
a(Cl-CS)alkyl group that cari be substituted with OR10, SR'0 or N(R4R'),
or together with the carbon atom of the methyiene group mean a(C3-C6)-
cycloalkyl ring, or
R' and R$ together mean an annelated five- to eight-membered, saturated or
CA 02577861 2007-02-21
6
unsaturated carbocyclic compound or heterocyclic compound, which
optionally is substituted by 1-2 keto groups, 1-2 (CI-CS)-alkyl groups, 1-2
(CI-C5)-alkoxy groups, 1-2 hydroxy groups, or 1-4 halogen atoms,
are another subject of the invention.
Compounds of general formula I, in which
R' and RZ, independently of one another, mean a hydrogen atom, a hydroxy
group, a halogen atom, an optionally substituted (CI-CIo)-alkyl group, a
(CI-Clo)-alkoxy group, a (CI-Cio)-alkylthio group, a (CI-C5)-
.~~
Q0
perfluoroalkyl group, a cyano group, or a nitro
group, or
R' and RZ together mean a group that is selected from the groups -0-
(CH2)õ-0-, --0-(CHZ)o-CHZ-, -0-CH=CH-, -(CH2)6+z-,-NH-(CH2),,.,-,,
N(CI-C3-alkyl}(CH2)p+I, or -NH-N=CH-, whereby n=1 or 2, and the
terminal atoms are linked to directly adjacent ring-carbon atoms,
or NR9Rl0, whereby R9 and R10, independently of one another, can be
hydrogen, CI-CS-alkyl or (CO)-Cj-CS-allcyl,
R3 means a hydrogen atom, a hydroxy group, a halogen atom, an optionally
~;~;;~ s>-.= substituted (Ci-Clo)-alkyl group, a(CI-Clo)-alkoxy group, a(CI-
C,o)-
.-
alkylthio group, a(Ci-CS)-perfluoroalkyl group, or a cyano group,
R4 means a Ci-Cio-atkyl group, a Cl-Cio-alkyl group that is substituted by
one or more groups selected from 1-3 hydroxy groups, halogen atoms, 1-
3(CI-C5)-alkoxy groups; an optionally substituted (C3-C7)-cycloalkyl
group, an optionally substituted heterocyclyl group, an optionally
substituted aryl group; a monocyclic or bicyclic heteroaryl group that
optionally is substituted by one or more groups selected from (CI -CS)-
allcyl groups (which optionally can be substituted by 1-3 hydroxy or 1-3
CA 02577861 2007-02-21
7
COOR12 groups), (C1-C5)-alkoxy groups, hydroxy groups, halogen atoms,
or (Cl-C3)exoalkylidene groups and that optionally contains 1-4 nitrogen
atoms and/or 1-2 oxygen atoms and/or 1-2 sulfur atoms and/or 1-2 keto
groups, whereby this group can be linked to the amine of the
tetrahydroriaphthalene system via any position and optionally can be
hydrogenated at one or more sites,
RS means a hydroxy group, a group OR~ 1 or an O-(CO)R" group, whereby
R' 1 means any hydroxy protective group or a Ci-Cio-alkyl group,
. .,:,
V1k~'='}
\=.'
R6 means a(CI-CS)-alkyl group or an optionally partially or completely
fluorinated (CI-C5)-alkyl group, a(C3-C7)cycloalkyl group, a(C3-
C7)cycloalkyl(Cj-C8)alkyl group, a (C3-C7)cycloalkyl(C2-Ca)alkenyl
group, a heterocyclyl group, a heterocyclyl(C1 -Ce)alkyl group, a
heterocyclyl(C2-Cg)alkenyl group, an aryl group, an aryl(Cl-Ca)alkyl
group, an aryl(C2-Cs)alkenyl group, an aryl(CZ-C8)alkinyl group; a
monocyclic or bicyclic heteroaryl group that optionally is substituted by
one or more keto groups, (CI-CS)-alkyl groups, (CI-CS)-alkoxy groups,
halogen atoms, or (CI-C3)exoalkylidene groups and that contains one or
more nitrogen atoms and/or oxygen atoms and/or sulfur atoms; a
heteroaryl(CI-Ca)a1ky1 group or a heteroaryl(C2-C8)alkenyl group,
whereby these groups can be linked to the tetrahydronaphthalene system
via any position and optionally can be hydrogenated at one or more sites,
R7 and R8, independently of one another, mean a hydrogen atom, a halogen atom,
a(Cl-CS)atkyl group, which can be substituted with OR10, SR'O, or
N(R9R1), or, together with the carbon atom of the methylene group,
mean a (C3-C6)-cycloalkyl ring, or
CA 02577861 2007-02-21
8
R' and R8 together mean an annelated five- to eight-membered saturated or
unsaturated carbocyclic compound or heterocyclic compound, which
optionaUy is substituted,by 1-2 keto groups, 1-2 (CI-Cs)-alkyl groups, 1-2
(CI-Cs)-alkoxy groups, or 1-4 halogen atoms,
are another subject of the invention.
Stereoisomers of general formula (I), in which
R' and RZ, independently of one another, mean a hydrogen atom, a hydroxy
group, a halogen atom, an optionally substituted (CI-Clo)-alkyl group, a
(Cl-Cio)-alkoxy group, a(Cr-Clo)-alkylthio group, a(C--Cs)-
perfluoroalkyl group, a cyano group, or a nitro group, or R' and RZ
together mean a group that is selected from the groups -O-(CHZ)o-O-,
-O-(CH2)n-CH2-, -O-CH=CH-, or -(CH2)o+2-, whereby n=1 or 2, and the
terminal atoms are linked to directly adjacent ring-carbon atoms,
or NR9R10, whereby R9 and R'O, independently of one another, can be
hydrogen, Cl-Cs-alkyl or (CO)-CI-Cs-alkyl,
R3 means a hydrogen atom, a hydroxy group, a halogen atom, an optionally
h?~
substituted (CrCio)-alkyl group, a(Ci-Clo)-alkoxy group, a(Cj-Clo)-
alkylthio group, a(Cl-Cs)-perfluoroalkyl group, or a cyano group,
R4 means a Cl-Cio-alkyl group, a Cl-Clo-alkyl group that is substituted by
one or more groups selected from 1-3 hydroxy groups, halogen atoms, or
1-3 (CI-Cs)-alkoxy groups; an optionally substituted phenyl group; a
monocyclic or bicyclic heteroaryl group that optionally is substituted by
1-2 keto groups, 1-2 (C,-Cs)-alkyl groups, 1-2 (Cl-Cs)-alkoxy groups, 1-
3 hydroxy groups, 1-3 halogen atoms, or 1-2 (Cl-C3)-exoalkylidene
groups and that contains 1-3 nitrogen atoms and/or 1-2 oxygen atoms
CA 02577861 2007-02-21
9
and/or 1-2 sulfur atoms and/or 1-2 keto groups, whereby these groups can
be linked to the aniine of the tetrahydronaphthalene system via any
position, and optionally can be hydrogenated at one or more sites,
i=
RS means a hydroxy group, R6 means a(CI-C5)-alkyl group or an optionally
partially or completely
fluorinated (Cl-CS)-alkyl group, an aryl group, an aryl(Ci-C8)alkyl group,
an aryi(C2-C8)alkenyl group, a (C3-C7)cycloalkyl group, a(C3-
C7)cycloalkyl(Ci-C8)alkyl group, or a (C3-C7)cycloallcyl(CZ-C8)aikenyl
:.S C.
group,
R7 and Rg, independently of one another, mean a hydrogen atom, a halogen
atoni,
a methyl or ethyl group, which should be substituted with OR10, SR'0, or
N(R9R1), or together with the carbon atom of the methylene group mean
a (C3-C6)-cycloalkyl ring, or
R' and Rg together mean an annelated five- to eight-membered, saturated or
unsaturated carbocyclic compound or heterocyclic compound, which
optionally is substituted by 1-2 keto groups, 1-2 (CI-C5)-alkyl groups, 1-2
(CI-CS)-alkoxy groups, or 1-4 halogen atoms,
,-~
'. r
are another subject of the invention.
Stereoisomers of general formula (1), in which
R' and R2, independently of one another, mean a hydrogen atom, a hydroxy
group, a halogen atom, an optionally substituted (C1-Cs)-alkyl group, a
(Ci-Cs)-alkoxy group, or R' and RZ together mean a group that is selected
from the groups -O-(CHZ)o-O-, -O-(CHZ)6-CH2-, -O-CH=CH-, or
-(CHZ)õ+Z-, whereby n=1 or 2, and the terminal atoms are linked to
directly adjacent ring-carbon atoms,
CA 02577861 2007-02-21
R3 means a hydrogen atom, a hydroxy group, a halogen atom, an optionally
substituted (CvCl )-alkyl group, or a(CI-Cl )-alkoxy group,
R4 means a Cl-Clo-alkyl group, a Cl-Cl -alkyl group that is substituted by
1-3 hydroxy groups or halogen atoms; a phenyl, naphthyl, phthalidyl,
isoindolyl, dihydroindolyl, dihydroisoindolyl, dihydroisoquinolinyl,
thiophthalidyl, benzoxazinonyl, phthalazinonyl, quinolinyl, isoquinolinyl,
quinolonyl, isoquinolonyl, indazolyl, benzothiazolyl, quinazolinyl,
quinoxalinyl, cinnolinyl, phthalazinyl, 1,7- or 1,8-naphthyridinyl,
dihydroindolonyl, dihydroisoindolonyl, benzimidazole or indolyl group
that optionally is substituted by one or more groups selected from 1-2
keto groups, 1-2 (CI-CS)-alkyl groups, 1-2 (CI-CS)-alkoxy groups, 1-3
hydroxy groups, 1-3 halogen atoms, or 1-2 (Cl-C3)-exoalkylidene groups,
whereby these groups can be linked to the amine of the
tetrahydronaphthalene system via any position and optionally can be
hydrogenated at one or more sites,
RS means a hydroxy group,
R6 means a(C,-CS)-alkyl group or an optionally partially or completely
fluorinated (Cl-CS)-a1kyI group,
R7 and R8, independently of one another, mean a hydrogen atom, a halogen atom,
a methyl or ethyl group, which should be substituted with OR10, SR'O, or
N(R9R1), or together with the carbon atom of the methylene group mean
a (C3-C6)-cycloalkyl ring, or
R' and R8 together mean an annelated five- to eight-membered, saturated or
CA 02577861 2007-02-21
;
unsaturated carbocyclic compound or heterocyclic compound, which
optionally is substituted by 1-2 keto groups, 1-2 (CI-CS)-alkyl groups,1-2
(CI-CS)-alkoxy groups, or 1-4 halogen atoms,
are a subject of this invention.
Stereoisomers of general formula (1), in which
R' and R1, independently of one another, inean a hydrogen atom, a hydroxy
group, a halogen atom, a(Cl-Cs)-alkyl group, a(Cl-CS)-alkoxy group, or
together a group that is selected from the groups -O-(CH2).-O-, -O-
(CH2)o-CHZ-, -O-CH=CH-, or -(CHZ)Q+Z-,
whereby n=1 or 2, and the terminal atoms are linked to directly adjacent
ring-carbon atoms,
R3 means a hydrogen atom, a hydroxy group, a halogen atom, an optionally
substituted (Ci-Clo)-alkyl group, or a(CI-Cio)-alkoxy group,
R means a phenyl, naphthyl, phthalidyl, isoindolyl, dihydroindolyl,
dihydroisoindolyl, dihydroisoquinolinyl, thiophthalidyl, benzoxazinonyl,
phthalazinonyl, quinolinyl, isoquinolinyl, quinolonyl, isoquinolonyl,
...:~~.
;'~.c~:t'='
indazolyl, benzothiazolyl, quinazolinyl, quinoxalinyl, cinnolinyl,
phthalazinyl, 1,7- or 1,8-naphthyridinyl, dihydroiirndolonyl,
dihydroisoindolonyl, benzimidazole or indolyl group that optionally is
substituted with Cl-Cs-alkyl, halogen, hydroxy, CI-C5-alkoxy, keto
groups, or (CI-C3)-exoalkylidene groups,
whereby these groups can be linked to the amine of the
tetrahydronaphthalene system via any position and optionally can be
hydrogenated at one or more sites,
RS means a hydroxy group,
CA 02577861 2007-02-21
a
12
R6 means a(CI-CS)-alkyl group or an optionally partially or completely
fluorinated (Ci-Cs)-alkyl grouP,
R7 and Rg, independently of one another, mean a hydrogen atom, a halogen atom,
a methyl or ethyl group, or together with the carbon atom of the
methylene group mean a(C3-C6)-cycloalkyl ring or
R' and Rg together mean an annelated five- to eight-membered, saturated or
unsaturated carbocyclic compound or heterocyclic compound,
are another subject of this invention.
Especially preferred are compounds of general formula I, in which
R' and R2, independently of one another, mean a hydrogen atom, a hydroxy
group, a halogen atom, or a(Cl-CS)-alkoxy group,
R3 means a hydrogen atom, or a halogen atom,
R4 means a quinolinyl, quinolonyl, quinazolinyl or phthalazinonyl group that
is optionally substituted with Cl-CS-alkyl, halogen, or keto groups,
whereby these groups can be linked to the amine of the
tetrahydronaphthalene system via any position and optionally can be
hydrogenated at one or more sites,
RS means a hydroxy group,
R6 means an optionally partially or completely fluorinated (CI-CS)-alkyl
group,
R7 and R8, independently of one another, mean a bydrogen atom, a methyl or
ethyl group, or
R' and R$ together mean an annelated five- to eight-membered saturated or
unsaturated carbocyclic compound or heterocyclic compound.
Quite especially preferred are compounds of general fonnula I, in which
CA 02577861 2007-02-21
13
R' and RZ, independently of one another, mean a hydrogen atom, a hydroxy
group, a fluorine atom or a chlorine atom, or a methoxy group,
R3 means a hydrogen atomor a chlorine atom,
R4 means a quinolinyl, quinolonyl, quinazolinyl or phthalazinonyl group that
is optionally substituted with one or more groups selected from a methyl,
hydroxy, or keto group or a fluorine atom,
whereby these groups can be linked to the amine of the
tetrahydronaphthalene system via any position and optionally can be
hydrogenated at one or more sites,
RS means a hydroxy group,
R6 means a trifluoromethyl group,
R7 and Ra, independently of one another, mean a hydrogen atom, a methyl group
or an ethyl group, or
R' and Re together mean an annelated, six-membered heterocyclic compound,
which optionally is substituted by a hydroxy group.
Stereoisomers of general formula (1) according to claim 1, in which R' and R$
together mean an annelated six-membered heterocyclic compound, which contains
an
oxygen atom and a boron atom and which optionally is substituted by a hydroxy
group,
are another subject of the invention.
The designation halogen atom or halogen means a fluorine, chlorine, bromine or
iodine atom. A fluorine, chlorine or bromine atom is preferred. The fluorine
atom and
the chlorine atom are especially preferred.
The alkyl groups that are mentioned in the claims, in particular R', R2, R3,
R6,
R7, 0, Rg, RtO, R", R'2, and R", can be straight-chain or branched and stand
for, for
example, a methyl, ethyl, n-propyl, iso-propyl, n-butyl, iso-butyl, tert-
butyl, or n-pentyl,
CA 02577861 2007-02-21
+, a
14
2,2-dimethylpropyl, 2-methylbutyl or 3-methylbutyl group. A CI-C3-alkyl group
is
preferred.
They can optionally be substituled by a group that is selected from 1-3
hydroxy
groups, 1-3 halogen atoms, 1-3 (Cf-C3)-alkoxy groups andlor 1-3 COORI' groups.
Hydroxy groups are preferred.
Alkyl group R4 has the meaning that is mentioned in the preceding paragraph,
but
the possible substituents are selected from the group of hydroxy, halogen and
(CI-CS)-
allcyloxy.
Alkyl groups R7 and R8 have the meaning that is mentioned in the first
paragraph
that relates to. alkyl groups, but the possible substituents are selected from
the group
OR10, SR'O and N(R9R'), whereby R9 and R' 0 mean hydrogen, Ci-C5-alkyl or
(CO)Ci-
Cs-alkyl, and alkyl is also defined as above.
The alkoxy groups can be straight-chain or branched and stand for a methoxy,
ethoxy, n-propoxy, iso-propoxy, n-butoxy, iso-butoxy, tert.-butoxy or n-
pentoxy, 2,2-
dimethylpropoxy, 2-methylbutoxy or 3-methylbutoxy group. A methoxy or ethoxy
group is preferred.
The alkylthio groups can be straight-chain or branched and stand for a
methylthio, ethylthio, n-propylthio, iso-propylthio, n-butylthio, iso-
butylthio, tert.-
butylthio or n-pentylthio, 2,2-dimethylpropylthio, 2-methylbutylthio or 3-
methylbutylthio group. A methylthio or ethylthio group is preferred.
For a partially or completely fluorinated alkyl group, which can be straight-
chain
or branched, for example, the following partially or completely fluorinated
groups are
considered: fluoromethyl, difluoromethyl, trifluoromethyl, fluoroethyl, 1,1-
difluoroethyl, 1,2-difluoroethyl, 1, 1, 1 -trifluoroethyl, tetrafluoroethyl,
pentafluoroethyl,
C3F7, C3HZF5, C4F9, and CSFI 1. Of the latter, the trifluoromethyl group or
the
CA 02577861 2007-02-21
pentafluoroethyl group is preferred. The reagents are commercially available,
or the
published syntheses of the corresponding reagents belong to the prior art.
Aryl substituents R~ and R2 can, form a ring by two aryl substituents together
meaning a chain selected from the groups --0-(CH2)õ-0-, -O-(CH2)õ-CH2-, -O-
CH=CH-,
-(CHz)õ+z-, NH-(CHZ)õ+i; N(CI-C3-allcyl)-(CH2)õ+i, and -NH-N=CH-, whereby n=1
or
2. The terminal atoms of the above-cited groups are linked to directly
adjacent aryl-
ring-carbon atoms, such that an annelat,ed ring is produced.
The substituent NR9R10 means, for example, NH2, NH(CH3), N(CH3)Z,
NH(CaHs), N(C2H5)2, NH(C3H7), N(C3Hr)2, NH(C4H9), N(C4H9)2, NH(CsHI i),
N(CSHI i)zi NH(CO)CH3, NH(CO)C21-15i NH(CO)C3H7, NH(CO)C4H9, or
NH(CO)CSH .
The cycloalkyl group means a saturated cyclic group, optionaily substituted by
one or more groups selected from hydroxy groups, halogen atoms, (C,-CS)-alkyl
groups,
or (CI-Cs)-alkoxy groups, with 3 to 7 ring-carbon atoms, such as, for example,
cyclopropyl, methylcyclopropyl, cyclobutyl, methylcyclobutyl, cyclopentyl,
methylcyclopentyl, cyclohexyl, methylcyclohexyl, cycloheptyl, and
methylcycloheptyl.
The cycloalkylalkyl group means, for example, -(CH2)-cycloalkyl, -(C2H4)-
~~
cycloaIlcyl, -(C3H6)-cycloalkyl, -(C4H8)-cycloalkyl, or -(CsHlo)-cycloalkyl,
whereby
cycloalkyl is defined as described above.
Cycloalkylalkenyl group means, for example, -(CH=CH)-cycloalkyl,
-[C(CH3)=CH]-cycloalkyl, -[CH=C(CH3)]-cycloalkyl, -(CH=CH-CH2)-cycloalkyl,
-(CHz-CH=CH)-cycloalkyl, -(CH=CH-CHz-CHz)-cycloalkyl, -(CHz-CH=CH-CHz)-
cycloalkyl, -(CH2-CHi-CH=CH)-cycloalkyl, -(C(CH3)=CH-CH2)-cycloalkyl, or
-(CH=C(CH3)-(CH2)-cycloalkyl.
CA 02577861 2007-02-21
16
A(CI-C3)-exoallcylidene group is defined as a group that is bonded to the
system
(ring or chain) via an exo-double bond. Exomethylene is preferred.
The heterocyclyl group is not aromatic and can be, for example, pyrrolidine,
imidazolidine, pyrazolidine, or piperidine. As substituents, hydroxy groups,
halogen
atoms, (CI-C5)-alkyl groups or (CI-CS}alkoxy groups are suitable.
Heterocyclylalkyl groups are defined as heterocyclyl groups that are bonded to
the skeleton via a CI-CS-alkyl group, whereby the alkyl group=can be straight-
chain or
branched.
.'~
Heterocyclylalkenyl groups are heterocyclyl groups that are bonded to the
skeleton via an unsaturated C2-CS-alkyl group, whereby the alkenylene groups
can be
straight-chain or branched.
Aryl groups R4 and R6 can be phenyl or naphthyl.
As substituents for both groups, CI-C3-alkyl, hydroxy, Ci-C3-alkoxy, Cl-C3-
alk.ylthio, halogen, cyano, COO(Ci-CS)allcyl, COOH, N(R9R1), and nitro are
considered. The degree of substitution can be single or multiple and can
contain several
substituents that are the same or different. Mono- or di-substituted phenyl
and naphthyl
groups R4 are preferred.
The aryl groups can be partially hydrogenated and then, in addition to or as
an
alternative to the above-cited substituents, can also cany keto, (CI-
C3}exoalkylidene. A
partially hydrogenated phenyl is defined as, e.g., cyclohexadienyl,
cyclohexenyl, or
cyclohexyl. A partially hydrogenated substituted naphthalene system is, for
example, 1-
tetralone or 2-tetralone.
The arylalkyl group is an aryl group that is bonded to a skeleton via a CI-Cg-
alkyl group, whereby the alkyl group can be straight-chain or branched. For
example,
benzyl or phenethylene can be mentioned.
CA 02577861 2007-02-21
17
An arylalkenyl group is an aryl group that is bonded to a skeleton via a Cz-CB-
alkenyl group, whereby the alkenyl group can be straight-chain or branched.
The arylalkinyl group is an aryl, group that is bonded to the skeleton via a
C2-C$-
alkinyl group, whereby the alkinyl group can be straight-chain or branched.
Monocyclic or bicyclic heteroaryl groups R' and R6, which can be hydrogenated
at one or more sites, are defined as all monocyclic or bicyclic aromatic ring
systems that
contain at least one heteroatom and at most seven heteroatoms. Ring systems
with 1-5
heteroatoms are preferred. As heteroatoms, 1-4 nitrogen atoms, 1-2 oxygen
atoms and
<=..:
.. ,.r .
1-2 sulfur atoms are suitable, which can occur in the ring system in all
subcombinations,
as long as they do not exceed the number specified for the respective
heteroatom and, in
the sum, the highest number of seven heteroatoms. For example, compounds of
formula
I in which R4 or R6 means ftiranyl, thiophenyl, pyrazolyl, pyrrolyl, oxazolyl,
thiazolyl,
imidazolyl, isoxazolyl, isothiazolyl, oxadiazolyl, triazolyl, tetrazolyl,
thiadiazolyl,
pyridyl, pyridiminyl, pyridazinyl, pyrazinyl, triazinyl, azaindolizinyl,
phthalidyl,
thiophthalidyl; indolyl, isoindolyl, dihydroindolyl, dihydroisoindolyl,
indazolyl,
benzothiazolyl, indolonyl, dihydroindolonyl, isoindolonyl,
dihydroisoindolonyl,
benzofuranyl, benzimidazolyl, indolizinyl, isobenzofuranyl, azaindolyl,
azaisoindolyl,
furanopyridyl, furanopyrimidinyl, furanopyrazinyl, fiuanopyridazinyl,
dihydrobenzofiuanyl, dihyrofuranopyridyl, dihydrofuranopyrimidinyl,
dihydrofuranopyrazinyl, dihydrofuranopyridazinyl, dihydrobenzofuranyl,
dihydroisoquinolinyl, dihydroquinolinyl, benzoxazinonyl, phthalazinonyl,
quinolinyl,
isoquinolinyl, quinolonyl, isoquinolonyl, quinazolinyl, quinoxalinyl,
cinnolinyl,
phthalazinyl, and 1,7- or 1,8-naphthyridinyl are thus part of this invention
and represent
a special embodiment of the invention.
CA 02577861 2007-02-21
_ 18
If the heteroaryl groups are partially or completely hydrogenated, compounds
of
formula I in which R4 means tetrahydropyranyl, 2H-pyranyl, 4H-pyranyl,
piperidyl,
tetrahydropyridyl, dihydropyridiyl,lH-pyridin-2-ony1,1H-pyridin-4-onyl, 4-
aminopyridyl, l H-pyridin-4-ylideneaminyl, chromanyl, thiochromanyl,
decahydroquinolinyl, tetrahydroquinolinyl, dihydroquinolinyl, 5,6,7,8-
tetrahydro-lH-
quinolin-4-onyl, decahydroisoquinolinyl, tetrahydroisoquinolinyl,
dihydroisoquinolinyl,
3,4-dihydro-2H-benz[1,4]oxazinyl, 1,2-dihydro[1,3]benzoxazin-4-onyl, 3,4-
dihydrobenz[1,4]oxazin-4-onyl, 3,4-dihydro-2H-benzo[1,4]thiazinyl, 41-1-
..JN~.~
benzo[1,4]thiazinyl, 1,2,3,4tetrahydroquinoxalinyl,lH-cinnolin-4-onyl, 3H-
quinazolin-
4-onyl, IH-quinazolin-4-onyl, 3,4-dihydro-lH-quinoxalin-2-onyl, 2,3-1,2,3,4-
tetrahydro[1,5]naphthyridinyl, dihydro-lH-[1,5]naphthyridyl,lH-[1,5]naphthyrid-
4-
onyl, 5,6,7,8-tetrahydro-]H-naphthyridin-4-onyl, 1,2-dihydropyrido[3,2-
d][1,3]oxazin-
4-onyl, octahydro-lH-indolyl, 2,3-dihydro-1H-indolyl, octahydro-2H-
isoindoly1,1,3-
dihydro-2H-isoindolyl,1,2-dihydroindazolyl, IH-pynrolo[2,3-b]pyridyl, 2,3-
dihydro-
1H-pyrrolo[2,3-b]pyridyl, or 2,2-dihydro-lH-pyrrolo[2,3-b]pyridin-3-onyl are
part of
this invention.
e=.c=:,;
If this is a heteroarylalkyl group, it is understood to include an optionally
also
'.;.
partially hydrogenated heteroaryl group as described above, which is bonded to
the
skeleton via a CI-Cg-allcyi group, which can be straight-chain or branched.
A heteroarylalkenyl group is defined as an optionally also partially
hydrogenated
heteroaryl group, as described above, which is bonded to the skeleton via a C2-
C8-
alkenyl group, which can be straight-chain or branched.
As hydroxy protective group R' 1, the protective groups that are known to one
skilled in the art, such as, e.g., trimethylsilyl, tert-butyldimethylsilyl,
phenyld'unethylsilyl, or benzyl, can be present as needed.
CA 02577861 2007-02-21
19
The hydroxy group in RS can be protected by one of the commonly used hydroxy
protective groups as, e.g., benzyl ether, silyl ether, such as, e.g., (CH3)3Si-
O-,
= (phenyl)(CH3)2Si-O-, (tert-butylxCH3)2Si-O- or can be present as CI-C5-alkyl
ether or
Ci-CS-alkyl ester or benzyl ester.
As radical Rs, the hydroxy group is preferred.
If R' and Ra form a five- to eight-membered carbocyclic compound or
heterocyclic compound (also substituted), a tricyclic system then is present.
As heteroatoms, nitrogen, oxygen, sulfur or boron are suitable.
For the case that the formed heterocyclic compound contains boron, this is a
special aspect of this invention. If a six-membered heterocyclic compound is
present,
radicals R' and R8 form two links, while the already existing tetraline system
forms the
other 4 links. For this purpose, Rl is bonded to the carbon atom that is
directly adjacent
to the bridge-carbon atom, and R8 is then the Z substituent of the exo-double
bond.
Compounds of general formula I in which R6 means a(Cl-C5)-alkyl group or an
optionally par6ally or completely fluorinated (C1-Ci)-alkyl group, a(C3-
C7)cycloalkyl
group, a(C3-C7)cycloalkyl(C1-C8)alkyl group, a(C3-C7)cycloalkyl(C2-C8)alkenyl
group,
a heterocyclyl group, a heterocyclyl(CI -Ca)alkyl group, a hetero cyclyl(C2-
C$)alkenyl
group, an aryl group, an aryl(CI-Cg)alkyl group, or an aryl(C2-C8)alkenyl
group are
another subject of the invention.
Compounds of general formula I in which R4 is a phenyl or naphthyl,
phthalidyl,
thiophthalidyl, benzoxazinonyl, phthalazinonyl, quinolinyl, isoquinolinyl,
quinolonyl,
isoquinolonyl, indazolyl, benzothiazolyl, quinazolinyl, quinoxalinyl,
cinnolinyl,
phthalaziny1,1,7- or 1,8-naphthyridinyl, dihydroindolonyl,
dihydroisoindolonyl,
benzimidazole or indolyl group that optionally is substituted with Ci-CS-
alkyl, halogen,
hydroxy or CI-CS-alkoxy are a preferred subject.
CA 02577861 2007-02-21
Compounds of genera( formula I, in which R4 means a quinolinyl group, a
quinolonyl group, a quinazolinyl group, a quinazolonyl group, a phthalazinyl
group or a
phthalazinonyl group, are another prefgrred subject of the invention.
Especially
preferred are compounds of general formula I, in which R4 means a quinolinyl,
quinolony), quinazolinyl or a phthalazinonyl group.
If an above-mentioned heterocyclic compound contains a keto group, all
chemically useful regioisomers, such as, for example 2H-phthalazin-l-one and
phthalazin-2-one, are also subjects of the invention.
Compounds of general formula I in which R6 means a(CI-C5)-alkyl group or an
optionally partially or completely fluorinated (Cl -C5)-alkyl group, an aryl
group, an
aryl(CI-Ca)alkyl group, an aryl(CZ-C$)alkenyl group, a(C3-C+cycloalkyl group,
a(C3-
C7)cycloalkyl(Ci-Ca)alkyl group, or a(C3-C7)cycloalkyl(C2-Cg)alkenyl group are
a
special subject of the invention.
Compounds of general formula I in which R6 represents a(Ci-C3)-alkyl group or
an optionally partially or completely fluorinated (CI-C3)-alkyl group are
another subject
of the invention. The completely fluorinated alkyl groups, in particular the
CF3 group,
are especially preferred.
~,: :.Y =~.
.,.:.
Compounds of formula I in which R" means a Cl-Cio-alkyl group, which
optionally can be substituted by 1-3 hydroxy groups, halogen atoms, an
optionally
substituted phenyl group; a monocyclic or bicyclic heteroaryl group that
optionally is
substituted by 1-2 keto groups, 1-2 (Cl-CS)-alkyl groups, 1-2 (Ci-CS)-alkoxy
groups, 1-3
halogen atoms, 1-2 (CI-C3)exoalkylidene groups and/or that contains 1-4
nitrogen atoms
and/or 1-2 oxygen atoms and/or 1-2 sulfur atoms, whereby these groups can be
linked to
the nitrogen atom via any position and optionally can be hydrogenated at one
or more
sites, are another subject of the invention.
CA 02577861 2007-02-21
21
Compounds of formula I in which R4 means a monocyclic or bicyclic heteroaryl
group that optionally is substituted by one or more groups selected from (Ci-
CS)-alkyl
groups (which optionally can be substituted by 1-3 hydroxy groups or 1-3 COOR6
groups), (CI-CS)-alkoxy groups, hydroxy groups, halogen atoms, or (CI -
C3)exoalkylidene groups and that optionally contains 1-3 nitrogen atoms and/or
1-2
oxygen atoms and/or 1-2 sulfur atoms and/or 1-2 keto groups, whereby this
group can be
linked to the amine of the tetrahydronaphthalene system via any position and
optionally
can be hydrogenated at one or more sites, and R1Zmeans a(CI-CS)-alkyi group or
a
..~..
benzyl group, are especially preferred.
Compounds of general formula I in which R4 means a phenyl, phthalidyl,
isoindolyl, dihydroindolyl, dihydroisoindolyi, dihydroisoquinolinyl,
thiophthalidyl,
benzoxazinonyl, phthalazinonyl, quinolinyl, isoquinolinyl, quinolonyl,
isoquinolonyl,
indazolyl, benzothiazolyl, quinazolinyl, quinoxalinyl, cinnolinyl,
phthalaziny1,1,7- or
1,8-naphthyridinyl, dihydroindolonyl, dihydroisoindolonyl, benzimidazole or
indolyl
group are a preferred subject of the invention. Compounds of general fonnula
I, in
which R4 means a quinolinyl, quinolonyl, quinazolinyl or phthalazinonyl group,
are
.~;~.
quite especially preferred.
The compounds of general formula I according to the invention can be present
as
stereoisomers because of the presence of asymmetry centers. All possible
diastereomers
(e.g.: RR, RS, SR, SS) both as racemates and in enantiomer-pure form are
subjects of
this invention.
In addition, the compounds according to the invention can be present as E-/Z-
isomers. Both the separate E or Z isomers and mixtures thereof are subjects of
this
invention. E-/Z-Mixtures can be separated with commonly used methods, such as,
for
example, chromatography.
CA 02577861 2007-02-21
= 22
The compounds according to the invention can also be present in the form of
salts with physiologically compatible anions, for example in the form of
hydrochloride,
sulfate, nitrate, phosphate, pivalate, maleate, fumarate, tartrate, benzoate,
mesylate,
citrate or succinate.
The compounds according to the invention are produced
a) by the open-chain precursors of general fon;nula (!I) being generated
according to methods that are known in the prior art, for example by
cyclopropanation of
the compounds of formula IV, in which radicals R', R2, R3, R4, Rs and R6 have
the
meanings that are indicated in claim I
N~ M
R'
(I.ewis acid)
which then are cyclized and rearranged to form the compounds of general
formula (1)
either without additional reagent or by adding inorganic or organic acids or
Lewis acids
under temperatures in the range of-70 C to +80 C (preferably in the range of -
30 C to
+80 C),
b) by the styrenes of general formula (III), produced according to methods
known in the prior art, being converted by an optionally enantioselectively
conducted
En-reaction with chiral Lewis acids into the compounds of general formula
(IV),
whereby RS means a hydroxy group, which optionally can be converted into a
protective
group according to the other meanings that are defined for R5 in claim I in a
way that is
known to one skilled in the art. By reduction and amination, the imine (V) is
produced
according to the method that is known to one skilled in the art,
CA 02577861 2007-02-21
. = 23
= = ~ R R'
M oR
-- - #
= ~ R
t~ =
~y f. ~M011kl011 R~
!. 1!'NMI
R
~ ,=f
~~
*~ ~/~M ~1w
R' , ~ ~ ~rf
M
[Key: Katalysator = Catalyst; Reduktion = Reduction; Lewis Slure = Lewis Acid]
which then is cyclized to form the compounds of general fonrnula (1) either
without
additional reagent or by adding inorganic or organic acids or Lewis acids
under
temperatures in the range of -70 C to +80 C (preferably in the range of -30 C
to
+80 C). Radicats R', R2, R3, R4, R5, R6, R7 and R8 that are defined in general
in the
above-cited formulas have the meanings that are indicated in claim I and with
R12 in the
meaning of (Cl-Cs)-alkyl, or benzyl.
The binding of substances to the glucocordcoid receptor (OR) and other steroid-
hormone receptors (mineral corticoid receptor (MR), progesterone receptor (PR)
and
androgen receptor (AR)) is examined with the aid of recombinantly produced
receptors.
Cytosol preparations of Sfzl cells, which had been infected with recombinant
baculoviruses that code for the GR, are used for the binding studies. In
comparison to
CA 02577861 2007-02-21
24
the reference substance [3H]-dexamethasone, the substances show a high
affinity to the
GR IC50(GR) = 20 nM and IC5a(PR) > I lvl were measured for the compound from
Example 1, and ICso(GR) = 30 nM and= IC5o(PR) > I M were measured for the
compound from Example 2.
The GR-mediated inhibition of the transcription of cytokines, adhesion
molecules, enzymes and other pro-inflammatory factors is considered to be an
essential,
molecular mechanism for the anti-inflammatory action of glucocorticoids. This
inhibition is produced by an interaction of the GR with other transcription
factors, e.g.,
AP-1 and NF-kappa-B (for a survey, see Cato, A. C. B., and Wade, E., BioEssays
18,
37I-378, 1996).
The compounds of general formula I according to the invention inhibit the
secretion of cytokine IL-8 into the human monocyte cell line THP-1 that is
triggered by
lipopolysaccharide (LPS). The concentration of the cytokines was determined in
the
supernatant by means of commercially available ELISA kits. The compound of
Example I showed an inhibition ICso(IL8) = 6.4 nM at a 90% efficiency relative
to [3H1-
dexamethasone as a standard.
The anti-inflammatory action of the compounds of general fonnula I was tested
in the animal experiment by tests in the croton oil-induced inflammation in
rats and mice
(J. Exp. Med. (1995),182, 99-108). To this end, croton oil in ethanolic
solution was
applied topically to the animals' ears. The test substances were also applied
topically or
systemically at the same time or two hours before the croton oil. After 16-24
hours, the
ear weight was measured as a yardstick for inflammatory edema, the peroxidase
activity
as a yardstick for the invasions of granulocytes, and the elastase activity as
a yardstick
for the invasion of neutrophilic granulocytes. In this test, the compounds of
general
CA 02577861 2007-02-21
- 25
formula I inhibit the three above-mentioned inflammation parameters both after
topical
administration and after systemic administration.
One of the most frequent undesirable actions of a glucocorticoid therapy is
the
so-called "steroid diabetes" [cf., Hatz, H. J., Glucocorticoide:
Immunologische
Grundlagen, Pharmakologie und Therapierichtlinien, [Glucocorticoids:
Immunological
Bases, Phannacology and Therapy Guidelines], Wissenschaftliche
Verlagsgesellschaft
mbH, Stuttgart, 1998]. The reason for this is the stimulation of
gluconeogenesis in the
liver by induction of the enzymes responsible in this respect and by free
amino acids,
which are produced from the degradation of proteins (catabolic action of
glucocorticoids). A key enzyme of the catabolic metabolism in the liver is
tyrosinamino
transferase (TAT). The activity of this enzyme can be determined from liver
homogenates by photometry and represents a good measurement of the undesirable
metabolic actions of glucocorticoids. To measure the TAT induction, the
animals are
sacrificed 8 hours after the test substances are administered, the livers are
removed, and
the TAT activity is measured in the homogenate. In this test, at doses in
which they
have an anti-inflammatory action, the compounds of general formula I induce
little or no
tyrosinamino transferase.
Because of their anti-inflammatory action, and, in addition, anti-allergic,
immunosuppressive and antiproliferative action, the compounds of general
formula I
according to the invention can be used as medications for treatment or
prophylaxis of the
following pathologic conditions in mammals and humans: In this case, the term
"DISEASE" stands for the following indications:
(i) Lung diseases that are accompanied by inflammatory, allergic and/or
proliferative
processes:
- Chronic, obstructive lung diseases of any origin, primarily bronchial
CA 02577861 2007-02-21
26
asthma
- Bronchitis of different origins
- Adult respiratory distress syndrome (ARDS)
Bronchiectasis
- All forms of restrictive lung diseases, primarily allergic alveolitis,
- All fonms of pulmonary edema, primarily toxic pulmonary edema, e.g.,
radiation pneumonitis
- Sarcoidoses and granulomatoses, especially Boeck's d.isease
.,,.a
(ii) Rheumatic diseases/autoimmune diseases/joint diseases that are
accompanied by inflanunatory, allergic and/or proliferative processes:
- All forms of rheumatic diseases, especially rheumatoid arthritis, acute
rheumatic fever, polymyalgia rheumatica, Behqet's disease
- Reactive arthritis
- Inflammatory soft-tissue diseases of other origins
- Arthritic symptoms in the case of degenerative joint diseases (arthroses)
- Traumatic arthritides
Vitiligo
- Collagenoses of any origin, e.g., systemic lupus erythematodes,
sclerodermia, polymyositis, dermatomyositis, SjBgren's syndrome, Still's
syndrome, Felty's syndrome
- Sarcoidoses and granulomatoses
- Rheumatic soft-tissue diseases
(iii) Allergies or pseudoallergic diseases that are accompanied by
inflammatory and/or
proliferative processes:
CA 02577861 2007-02-21
27
- All forms of allergic reactions, e.g., Quincke's edema, hay fever, insect
bites, allergic reactions to pharmaceutical agents, blood derivatives,
contrast media, etc., anaphylactic shock, urticaria, allergic and irritative
4
,
contact dermatitis, allergic vascular diseases
- Vasculitis allergica
(iv) Vascular inflammations (vasculitides)
- Panarteritis nodosa, temporal arteritis, erythema nodosum
- Polyarteritis nodosa
- Wegner's granulomatosis
- Giant cell arteritis
(v) Dennatological diseases that are accompanied by inflammatory, allergic
and/or
proliferative processes:
- Atopic dermatitis (primarily in children)
- All forms of eczenZa, such as, e.g., atopic eczema (primarily in children)
- Rashes of any origin or dermatoses
- Psoriasis and parapsoriasis group
s~, t - Pityriasis rabra pilaris
- Erythematous diseases, triggered by different noxae, e.g., radiation,
chemicals, bums, etc.
- Bullous dermatoses, such as, e.g., autoimmune pemphigus vulgaris,
bullous pemphigoid
- Diseases of the lichenoid group,
- Pruritis (e.g., of allergic origin)
- Seborrheal eczema
- Rosacea group
CA 02577861 2007-02-21
28
- Pemphigus vulgaris
- Erythema exudativum multiforme
- Balanitis
1 ,
- Vulvitis
- Manifestation of vascular diseases
- Hair loss such as alopecia areata
- Cutaneous lymphoma
- Parapsoriasis
(vi) Kidney diseases that are accompanied by inflammatory, allergic and/or
proliferative processes:
- Nephrotic syndrome
- All nephritides, e.g., glomerulonephritis
(vii) Liver diseases that are accompanied by inflammatory, allergic and/or
proliferative
processes:
- Acute liver cell decomposition
- Acute hepatitis of different origins, e.g., viral, toxic, pharmaceutical
agent-induced
- Chronic aggressive hepatitis and/or chronic intermittent hepatitis
(viii) Gastrointestinal diseases that are accompanied by inflammatory,
allergic and/or
proliferative processes:
- Regional enteritis (Crohn's disease)
- Colitis ulcerosa
- Gastritis
- Reflux esophagitis
- Ulcenitive colitis of other origins, e.g., native sprue
CA 02577861 2007-02-21
29
(ix) Proctologic diseases that are accompanied by inflammatory, allergic
and/or
proliferative processes:
- Anal eczema
- Fissures
- Hemorrhoids
- Idiopathic proctitis
(x) Eye diseases that are accompanied by inflammatory, allergic and/or
proliferative
processes:
- Allergic keratitis, uveitis, iritis
- Conjunctivitis
- Blepharitis
- Optic neuritis
- Chorioiditis
- Sympathetic ophthalmia
(xi) Diseases of the ear-nose-throat area that are accompanied by
inflammatory, allergic
and/or proliferative processes:
Allergic rhinitis, hay fever
- Otitis externa, e.g., caused by contact dermatitis, infection, etc.
- Otitis media
(xii) Neurological diseases that are accompanied by inflammatory, allergic
and/or
proliferative processes:
- Cerebral edema, primarily tumor-induced cerebral edema
- Multiple sclerosis
- Acute encephalomyelitis
- Meningitis,
CA 02577861 2007-02-21
- Various forms of convulsions, e.g., infantile nodding spasms
- Acute spinal cord injury
- Stroke
(xiii) Blood diseases that are accompanied by inflammatory, allergic and/or
proliferative processes, such as, e.g.:
- Acquired hemolytic anemia
- Idiopathic thrombocytopenia
(xiv) Tumor diseases that are accompanied by inflammatory, allergic and/or
.:,..
proliferative processes, such as, e.g.:
- Acute lymphatic leukemia
- Malignant lymphoma
- Lymphogranulomatoses
- Lymphosarcoma
- Extensive metastases, mainly in breast, bronchial and prostate cancers
(xv) Endocrine diseases that are accompanied by inflammatory, allergic and/or
proliferative processes, such as, e.g.:
A - Endocrine orbitopathy
..:~ f
- Thyreotoxic crisis
- De Quervain's thyroiditis
- Hashimoto's thyroiditis
- Basedow's disease
- Endocrine ophthalmopathy
- Granulomatous thyroiditis
- Lymphadenoid goiter
- Graves' disease
CA 02577861 2007-02-21
31
(xvi) Organ and tissue transplants, graft-versus-host disease
(xvii) Severe shock conditions, e.g., anaphylactic shock, systemic
inflammatory
response syndrome (SIRS)
(xviii) Substitution therapy in:
- Innate primary suprarenal insufficiency, e.g., congenital adrenogenital
syndrome
- Acquired primary suprarenal insufficiency, e.g., Addison's disease,
autoinunune adrenalitis, meta-infective tumors, metastases, etc.
- Innate secondary suprarenal insufficiency, e.g., congenital
hypopituitarism
- Acquired secondary suprarenal insufficiency, e.g., meta-infective tumors,
etc.
(xix) Vomiting that is accompanied by inflammatory, allergic and/or
proliferative
processes:
- e.g., in combination with a 5-HT3 antagonist in cytostatic-agent-induced
vomiting
; A.
(xx) Pains of inflammatory origins, e.g., lumbago
(xxi) Various diseases.
Moreover, the compounds of general formula I according to the invention can be
used for treatment and prophylaxis of additional pathologic conditions that
are not
mentioned above, for which synthetic glucocorticoids are now used (see in this
respect
Hatz, H. J., Glucocorticoide: Immunologische Grundlagen, Pharmakologie und
Therapierichtlinien, Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart,
1998).
CA 02577861 2007-02-21
32
The invention also relates to a combination therapy, whereby one or more
compounds of this invention, or their pharmaceutically acceptable salt or a
pharmaceutical agent that contains one. or more compounds or a formulation
that
contains one or more compounds is administered at the same time or in
succession or as
a combination preparation with one or more other therapeutically active agents
or active
agents for treating one or more conditions.
All previously mentioned indications (i) to (xx) are described in more detail
in
Hatz, H. J., Glucocorticoide: Immunologische Grundlagen, Pharmakologie und
Therapierichtlinien, WissenschafRliche Verlagsgesellschaft mbH, Stuttgart,
1998.
For the therapeutic actions in the above-mentioned pathologic conditions, the
suitable dose varies and depends on, for example, the active strength of the
compound of
general formula I, the host, the type of administration, and the type and
severity of the
conditions that are to be treated, as well as the use as a prophylactic agent
or therapeutic
agent.
In addition, the invention provides:
(i) The use of one of the compounds of general formula I according to the
,;..
invention or mixture thereof for the production of a medication for treating
a DISEASE;
(ii) A process for treating a DISEASE, said process comprises an
administration of an amount of the compound according to the invention,
whereby the amount suppresses the disease and whereby the amount of
compound is given to a patient who requires such a medication;
(iii) A pharmaceutical composition for treating a DISEASE, said treatment
comprises one of the compounds according to the invention or mixture
thereof and at least one pharmaceutical adjuvant and/or vehicle.
CA 02577861 2007-02-21
33
In general, satisfactory results can be expected in animals when the daily
doses
comprise a range of 1 g to 100,000 g of the compound according to the
invention per
kg of body weight. In the case of larger mammals, for example the human, a
recommended daily dose lies in the range of I g to 100,000 pg per kg of body
weight.
Preferred is a dose of 10 to 30,000 pg per kg of body weight, and more
preferred is a
dose of 10 to 10,000 g per kg of body weight. For example, this dose is
suitably
administered several times daily. For treating acute shock (e.g., anaphylactic
shock),
individual doses can be given that are significantly above the above-mentioned
doses.
The formulation of the pharmaceutical preparations based on the new compounds
is carried out in a way that is known in the art by the active ingredient
being processed
with the vehicles, fillers, substances that influence decomposition, binding
agents,
moisturizers, lubricants, absorbents, diluents, flavoring correctives,
coloring agents, etc.,
that are commonly used in galenicals and converted into the desired form of
administration. In this case, reference is made to Remington's Pharmaceutical
Science,
15'h Edition, Mack Publishing Company, East Pennsylvania (1980).
For oral administration, especially tablets, coated tablets, capsules, pills,
powders, granulates, lozenges, suspensions, emulsions or solutions are
suitable.
For parenteral administration, injection and infusion preparations are
possible.
For intra-articular injection, correspondingly prepared crystal suspensions
can be
used.
For intramuscular injection, aqueous and oily injection solutions or
suspensions
and corresponding depot preparations can be used.
For rectal administration, the new compounds can be used in the form of
suppositories, capsules, solutions (e.g., in the form of enemas) and ointments
both for
systemic and for local treatment.
CA 02577861 2007-02-21
. 34
For pulmonary administration of the new compounds, the latter can be used in
the form of aerosols and inhalants.
For local application to eyes, outer ear channels, middle ears, nasal
cavities, and
paranasal sinuses, the new compounds can be used as drops, ointments and
tinctures in
corresponding pharmaceutical preparations.
For topical application, formulations in gels, ointments, fatty ointments,
creams,
pastes, powders, milk and tinctures are possible. The dosage of the compounds
of
general formula I should be 0.01 %-20% in these preparations to achieve a
sufficient
pharmacological action.
The invention also comprises the compounds of general formula I according to
the invention as therapeutic active ingredients. In addition, the compounds of
general
formula I according to the invention are part of the invention as therapeutic
active
ingredients together with pharmaceutically compatible and acceptable adjuvants
and
vehicles.
The invention also comprises a pharmaceutical composition that contains one of
the
pharmaceutically active compounds according to the invention or mixtures
thereof or a
pharmaceutically compatible salt thereof and pharmaceutically compatible
adjuvants and
vehicles.
CA 02577861 2007-02-21
Experiments
Example 1
't .
(cis.Z)-4-Ethvlidene-6-fluoro-l-[(2-nieth-õylquinolin-5 ;yl)aminol-2-(tnt
fluorometh,yl)-
1,2.3,4-tetrahydronaphthalen-2-ol
3-[]-(3-Fluoro-2-methoxyphenyl)-cyclopropy41-2-oxopropionic acid ethyl ester
396 ml of a 0.5 molar (198 mrnol) solution of bis-(trimethylsilyl)-potassium
amide in toluene is added in drops to 26 g (180 mmol) of 2,6-difluoroanisole
and 14.6
:,=.,.,~
ml (198 mmol) of cyclopropylcyanide in 500 m of toluene at 0 C over 40
minutes. It is
stirred for 18,hours at room temperature and mixed with water and 1 M sulfuric
acid
while being cooled with ice. The organic phase is separated, and the aqueous
phase is
extracted several times with ethyl acetate. It is washed with brine, dried
with sodium
sulfate and concentrated by evaporation in a vacuum. After chromatographic
purification on silica gel (hexane/ethyl acetate 10%-20%),12.7 g of 1-(3-
fluoro-2-
methoxyphenyl)-cyclopropylnitrile is obtained. 12.7 g (66.1 mmol) of the
nitrile is
slowly mixed in toluene at -78 C with 82.7 ml (99.2 mmol) of diisobutyl
aluminum
hydride solution (20% in toluene), and after 3 hours at -78 C, 11.1 ml of
isopropanol
was added in drops. It is allowed to heat to -5 C, and 150 ml of a 10% aqueous
tartaric
acid solution is added. After dilution with ether, it is stirred vigorously,
the organic
phase is separated, and the aqueous phase is extracted several times with
ethyl acetate.
It is washed with brine, dried with sodium sulfate, and concentrated by
evaporation in a
vacuum. 11.8 g of aldehyde is obtained as a yellow oil. A solution of 16.3 g
(60.7
mmol) of 2-diethylphosphono-2-ethoxyacetic acid ethyl ester in 60 ml of
tetrahydrofuran is mixed while being cooled with ice within 20 minutes with
33. 4 ml
(66.8 mmol) of a 2 M solution of lithium diiospropylamide in tetrahydrofuran-
heptane-
CA 02577861 2007-02-21
36
toluene, and it is stirred for 30 minutes at 0 C. Within 30 nzinutes, a
solution of 11.8 g
(60.7 mmol) of 1 in 61 ml of tetrahydrofuran is added in drops at 0 C. After
20 hours at
room temperature, ice water is added, fnd it is extracted several times with
ether and
ethyl acetate. It is washed with saturated ammonium chloride solution, dried
on sodium
sulfate and concentrated by evaporation. The crude product is saponified with
170 ml of
2 M sodium hydroxide solution in 170 ml of ethanol over 15 hours at room
temperature.
13.9 g of acid, which is stirred with 87 ml of 2N sulfiiric acid at 90 C over
16 hours, is
obtained. After cooling, it is made basic with potassium carbonate, washed
with ether
and acidified with hydrochloric acid. After extraction with ethyl acetate,
washing with
saturated sodium chloride solution and removal of the solvent, 10.2 g of the
crude keto
acid is obtained. 10.2 g (40.6 mmol) of 3-[]-(3 fluoro-2-methoxyphenyl)-
cyclopropylJ-2-
oxopropionic acid and 4.5 ml (85.3 mmol) of sulfuric acid (96%) are refluxed
in 200 ml
of ethanol for one hour. The batch is concentrated by evaporation in a vacuum,
the
residue is added to ice water and made basic with saturated sodium bicarbonate
solution.
It is extracted several times with ethyl acetate, washed with saturated sodium
chloride
solution, dried (sodium sulfate), and concentrated by evaporation in a vacuum.
After
chromatographic purification on silica gel (hexane%thyl acetate 20%), 9.6 g of
3-[1-(3-
fluoro-2-methoxyphenyl)-cyclopropyl]-2-oxopropionic acid ethyl ester is
obtained.
'H-NMR (CDCI3): S= 0.90 (m, 41-), 1.29 (t, 311), 3.09 (s, 2H), 3.99 (d, 3H),
4.20 (q, 2H), 6.87 (ddd, 1 H), 6.95 (ddd,1 H), 7.07 (d, 114).
3-[]-(3-Fluoro-2-methoxyphenyl)-cyclopropylJ-2-hydrozy-2-
(trifluoromethyl)propanal
9.6 g (34.3 mmol) of 3-[1-(3-fluoro-2-methoxyphenyl)-cyclopropyl] -2-
oxopropionic acid ethyl ester and 34.5 ml (233 mmol) of (trifluoromethyl)-
trimethylsilane in 343 ml of DMF are mixed with 46.9 g of cesium carbonate at
0 C. It
CA 02577861 2007-02-21
37
is stirred for 2 hours at 0 C, and then the reaction mixture is added to
water. It is
extracted several times with ethyl acetate, washed with saturated sodium
chloride
solution, dried with sodium sulfate and.concentrated by evaporation in a
vacuum. After
chromatographic purification on silica gel (hexane/ethyl acetate 10%-40%),
10.4 g of 3-
[1-(3-fluoro-2-methoxyphenyl)-cyclopropylj-2-hydroxy-2-(trifluoromethyl)-
propanoic
acid ethyl ester is obtained as a yellow oil. This oil is mixed in 297 ml of
diethyl ether
at 0 C with 2.25 g (59.4 mmol) of lithium aluminum hydride and stirred for I
more hour
at room temperature. 20 ml of saturated ammonium chloride solution is
carefully added
to the batch at 0 C, and vigorous stirring is continued for 15 minutes. It is
extracted
several times with diethyl ether, washed with saturated sodium chloride
solution, dried
with sodium sulfate and concentrated by evaporation in a vacuum. After
chromatographic purification on silica gel (hexane/ethyl acetate 10%-50%), 5.6
g of 3-
[1-(3-fluoro-2-methoxyphenyl)-cyclopropyl]-2-(trifluoromethyl)-propane-l,2-
diol is
obtained. 12.4 ml (89 mmol) of t,riethylamine and, in portions over 10
minutes, 11 g (70
mmol) of pyridine SO3 complex are added to 5.6 g (18.1 mmol) of diol in 100 ml
of
dichloromethane and 61 ml of DMSO. It is stirred over 3 hours, and saturated
ammonium chloride solution is added. The mixture is stirred for another 15
minutes, the
phases are separated, and it is extracted with dichloromethane. It is washed
with water
and dried on sodium sulfate. The solvent is removed in a vacuum, and after
chromatographic purification on silica gel (hexane/ethyl acetate, 0-50%), 5.9
g of
product is obtained.
'H-NMR (CDC13): S= 0.68-0.76 (m, 2H), 0.90-1.02 (m, 2H), 2.03 (d, 1H), 2.91
(d, IH), 3.85 (s, IH), 4.03 (s, 3H), 6.80 (d, 1H), 6.87 (ddd,1H), 6.98
(dd,1H), 9.26 (s,
1H).
CA 02577861 2007-02-21
38
200 mg (0.65 mmol) of 3-[1-(3-fluoro-2-methoxyphenyl)-cyclopropyl]-2-
hydroxy-2-trifluoromethylpropanal, 120 mg (0.76 mmol) of 5-amino-2-
methylquinoline
and 0.3 ml of titanium tetraethylate are,stirred in 25 ml of toluene for 2
hours at 100 C.
t
After cooling, it is poured into water, and vigorous stirring is continued.
The suspension
is filtered through Celite, and it is thoroughly rewashed with ethyl acetate.
The phases
of the filtrate are separated, and it is extracted again with ethyl acetate.
lt is dried on
sodium sulfate, and the solvent is removed in a vacuum, and 270 mg of 3-[1-(3-
fluoro-2-
methoxyphenyl)-cyclopropyl]-1-(2-methyl-quinolin-5-yl)imino]-2-
.,:,..,
=,=..~.
(trifluoromethyl)propan-2-ol is obtained as a crude product. 5.5 ml (5.5 mmol)
of a I M
boron tribromide solution is added in drops at -30 C to 270 mg (0.6 mmol) of
imine in
27 ml of CH2C12. It is allowed to heat to room temperature and stirred for 20
hours. The batch is mixed with saturated NaHCO3, the phases are separated, the
aqueous phase
is extracted with CH2CI2i the combined organic phases are dried (Na2SO4) and
concentrated by evaporation in a vacuum. Column chromatography on silica gel
(hexane/ethyl acetate 0-70%) and subsequent HPLC (Kromasil C18,
water/acetonitrile
30-60%) yield 24 mg of product.
1H-NMR (300 MHz, CD3OD): 8 =1.74 (d, 3H), 2.52 (d, 1 H), 2.69 (s, 314), 3.12
(d, I H), 4.55 (s, I H), 5.81 (q, 1 H), 6.34 (d, I H), 6.73 (dd, 1 H), 6.94
(dd, 1 H), 7.26 (d,
1H), 7.35 (d,1H), 7.42 (t,1H).
Example 2
(cis.Z)-5-{j4-Ethylidene-6-fluoro-2,5-dihyiroxy-2-(trifluoromethyll-1,2 3 4-
tet rahvdronaphthalen-1-vl]amino} -quinolin-2(1 H)-one
S Aminoisoquinolin-2(ItV-one:
CA 02577861 2007-02-21
39
4.5 g of 5-nitroquinolin-2(lH)-one (Chem. Pharm. Bull. (1981), 29, pp. 651-56)
is hydrogenated in 200 rnl of ethyl acetate and 500 ml of inethanol in the
presence of
450 mg of palladium on activated carbon as a catalyst under normal pressure
with
hydrogen until the reaction is completed. The catalyst is removed by
filtration through
diatomaceous earth, and the reaction solution is concentrated by evaporation
in a
vacuum. 3.8 g of the title compound is obtained as a yellow solid.
1H-NMR (DMSO): S= 5.85 (bs, 2H), 6.27 (d,1H), 6.33 (d, IH), 6.43 (d, IH),
7.10 (t, l H), 8.07 (d, l H),11.39 (br,1H)
\:': 'Nn
0.41 ml (2.0 mmol) of titanium tetraethylate is added to 300 mg (0.98 mmol) of
3-[1-(3-fluoro-2-methoxyphenyl)-cyclopropyl]-2-hydroxy-2-
(trifluoromethyl)propanal
and 188 mg (1.18 mmol) of 5-aminoquinolin-2(1H)-one in 3 ml of toluene, and
the
mixture is heated over one hour to 100 C. After cooling, it is poured into
water, and
vigorous stirring is continued. The suspension is filtered through Celite, and
it is
thoroughly rewashed with ethyl acetate. The phases of the filtrate are
separated, and it is
extracted again with ethyl acetate. It is dried on sodium sulfate, and the
solvent is
removed in a vacuum. The 5-({3-[1-(3-fluoro-2-methoxyphenyl)-cyclopropyl]-2-
(trifluoromethyl)propan-l-yl}-imino)-quinolin-2(IH)-one that is thus obtained
in crude
form is purified by column chromatography on silica gel (hexane%thyl acetate
S0%).
The thus obtained 235 mg (0.52 mmol) of imine is taken up in 10 ml of
dichloromethane
and cooled to -60 C. 8.9 ml (8.9 mmol) of a 1 M boron tribromide solution in
dichloromethane is added in drops over 10 minutes, and it is allowed to heat
ovemight.
The solution is poured into a mixture of ice and saturated sodium bicarbonate
solution
and vigorously stirred for 15 minutes. It is extracted with dichloromethane,
washed with
saturated sodium chloride solution and dried on sodium sulfate. After
concentration by
CA 02577861 2007-02-21
evaporation and chromatography on silica gel (hexane%thyl acetate 0-50%) and
subsequent HPLC (Kromasil C18, water/acetonitrile 30-60%), 10.3 mg of the
desired
product is obtained.
'H-NMR (300 MHz, CD3OD): 8 =1.76 (d, 311), 2.53 (d, IH), 3.13 (d, 1H), 4.53
(s, IH), 5.82 (q, 1 H), 6.19 (d, l I-i), 6.57 (d, l H), 6.69 (d, 1 H), 6.77
(dd, IH), 6.98 (dd,
IH), 7.29 (t, IH), 8.24 (d,1H)
Example 3
5-([2-Hydroxy-4-propYlidene-2-(trifluoromethyl)-1.2,3.4-tetrahydronaphthalen-l-
y11amino}-quinolin-2(1 H)-one
2-Hydrozy-4 phenyl-2-(trifluoromethyl)-hept-4-enal
1.4 ml (0.7 mtnol) of a 0.5 M titanium tetraisopropylate solution in toluene
is
added to 400 mg (1.4 mmol) of 1,1'-bi-2-naphthol, and the red solution is
stirred for 2
hours at room temperature. 1.1 g (7.6 mmol) of 2-phenyl-1 -pentene and 2.5 g
(15.2
mmol) of ethyl trifluoropyruvate are added, and the mixture is heated over 8
hours to
100 C. After cooling, it is immediately purified by column chromatography on
silica
gel (hexane%thyi acetate 15%), and 1.85 g of 2-hydroxy-4-phenyl-2-
(trifluoromethyl)-
hept-4-enoic acid ethyl ester is obtained as an E/Z mixture. 0.75 g (2.3 mmol)
of (E)-2-
hydroxy-4-phenyl-2-(trifluoromethyl)-hept-4-enoic acid ethyl ester is cooled
in 20 ml of
diethyl ether to -5 C, and 175 mg (4.6 mmol) of lithium aluminum hydride is
added in
solid form in portions over 10 minutes. It is stirred for 1 hour at 0 C and
poured into
saturated ammonium chloride solution. The suspension is filtered through
Celite, and it
is thoroughly rewashed with ethyl acetate. The phases of the filtrate are
separated, and it
is extracted again with ethyl acetate. It is washed with saturated sodium
chloride
solution, dried on sodium sulfate, and the solvent is removed in a vacuum. The
CA 02577861 2007-02-21
41
separation by column chromatography on silica gel (hexane%thyl acetate 0-15%)
yields
0.42 g of (E)-2-hydroxy-4-phenyl-2-(trifluoromethyl)-hept-4-enal and 0.12 g of
alcohol.
'H-NMR (300 MHz, CDCl3): S=1.06 (t, 311), 2.26 (dq, 2I-), 3.26 (d, 1H), 3.33
(d, 1 H), 3.69 (s, 1 H), 5.79 (t, 1 H), 7.20-7.33 (m, 5H), 9.22 (s, 1 H)
0.3 ml (1.5 mrnol) of titanium tetraethylate is added to 200 mg (0.73 mmol) of
2-
hydroxy-4-phenyl-2-(trifluoromethyl)-hept-4-enal and 120 mg (0.73 mmol) of 5-
amino-
quinolin-2(1 H)-one in 5 ml of toluene, and the mixture is heated over 2 hours
to 100 C.
After cooling, it is poured into water, and vigorous stirring is continued.
The suspension
is filtered through Celite, and it is thoroughly rewashed with ethyl acetate.
The phases
of the filtrate are separated, and it is extracted again with ethyl acetate.
It is dried on
sodium sulfate, and the solvent is removed in a vacuum, and 310 mg of 5-{[2-
hydroxy-
4-phenyl-2-(trifluoromethyl)-hept-4-en-lyl)imino)-quinolin-2(1H)-one is
obtained as a
crude product. 155 mg (0.36 mmol) thereof in 5 ml of dichloromethane is taken
up and
cooled to -78 C. 1.8 ml (1.8 mmol) of a 1 M titanium tetrachloride solution in
dichloromethane is added in drops over 5 minutes, and the cooling bath is
removed after
;.h
".' 10 minutes. After another 30 minutes, the solution that is heated to room
temperature is
poured into a mixture of ice and saturated sodium bicarbonate solution and
vigorously
stirred for 10 minutes. It is extracted with ethyl acetate, washed with
saturated sodium
chloride solution and dried on sodium sulfate. After concentration by
evaporation and
chromatography on silica gel (hexane/ethyl acetate 0-50%), 12 mg of the
desired product
is obtained.
iH-NMR (300 MHz, CD3OD): 8 =1.17 (t, 3H), 2.32 (dq, 2H), 2.85 (d, 1H), 3.07
(d, 1H), 5.18 (s, 1 H), 6.32 (t, l H), 6.52 (d, 1 H), 6.60 (d, 1 H), 6.71 (d,
IH), 7.18 (d, 1H),
7.26 (t, 2H), 7.37 7.69 (d,1H), 8.25 (d,1H)
CA 02577861 2007-02-21
42
Example 4
(cfs.Z)-5-{f4-Ethvlidene-6-fluoro-2,5-dihvdroxy-2-(trifluoromethyi)-1.2.3.4-
tetrahydronaphthalen-l-yl]amino l -2-methylphthalazin-l-one
S Amino-2-rnethyl phthalazin-l-one:
3-Bromo-4-nitro-phthalide
5.37 g of 4-nitrophthalide (Tetrahedron Lett. (2001), 42, pp. 1647-50), 8.04 g
of
N-bromosuccinimide and 196 mg of benzoyl peroxide are refluxed in 80 ml of
benzotrifluoride and heated by exposure to light until the reaction is
completed. It is
added to water, extracted with dichloromethane, washed several times with
water, dried,
and the solvent is removed in a vacuum. 7.24 g of 3-bromo-4-nitro-phthalide is
obtained
as a solid.
'H-NMR (300 MHz, CDC13), 6= 7.26 (s, l H), 7.88 (t, 1 H), 8.3 (d, 1I4), 8.56
(d,
IH)
5-Nitro-phthalazin-l -one:
18.25 g of hydrazine sulfate and 14.88 g of sodium carbonate are stined in 300
ml of DMF at 100 C for 1 hour. Then, 7.24 g of 3-bromo-4-nitro-phthalide in
100 ml of
==.:;z;:
DMF is added, and it is stirred for another 4 hours at 100 C. It is added to
water,
extracted several times with ethyl acetate, and the organic phase is washed
with water
and brine. It is dried, and the solvent is removed in a vacuum. After
recrystallization
from ethyl acetate, 2.35 g of 5-nitro-phthalazin-l-one is obtained as a solid.
'H-NMR (300 MH4 DMSO-db), S= 8.05 (t,1H), 8.57-8.66 (m, 211), 8.73 (s,
1H),13.13(bs,lA)
CA 02577861 2007-02-21
43
2-Methyl-5-nitro-phthalazin-l-one
1.6 g of 5-nitro-phthalazin-l-one and 2.31 g of potassium carbonate are
stirred
for 10 minutes at room temperature in 60 ml of DMF. 1.1 ml of methyl iodide is
added,
and it is stirred overnight. It is added to water, extracted several times
with ethyl
acetate, and the organic phase is washed with water and brine. It is dried,
and the
solvent is removed in a vacuum. 1.57 g of 2-methyl-5-nitro-phthalazin-l-one is
obtained as a yellow solid.
'H-NMR (300 MHz, DMSO-d6), S= 3.73 (s, 3H), 8.05 (t, 1H), 8.62 (d, 2H), 8.75
4= .
5-Amino-2-methyl-phthalazin-l-one
1.57 g of 2-methyl-5-nitro-phthalazin-l-one and 130 mg of palladium on
activated carbon are suspended in 45 ml of ethyl acetate and hydrogenated with
hydrogen under normal pressure. It is filtered through diatomaceous earth, and
the
solvent is removed in a vacuum. 1.26 g of 5-amino-2-methyl-phthalazin-l-one is
obtained as a yellow solid.
~~ 1H-NMR (300 MHz, CDC13), = 3.81 (s, 311), 7.0 (d, 1 H), 7.5 (t, l H), 7.8
(d, 1 H),
8.16 (s, I H)
0.6 ml (2.4 mmol) of titanium tetraethylate is added to 430 mg (1.4 mmol) of 3-
[1-(3-fluoro-2-methoxyphenyl)-cyclopropyl]-2-hydroxy-2-
(trifluoromethyl)propanal and
253 mg (1.44 mmol) of 5-amino-2-methyl-phthalazin-l-one in 50 ml of toluene,
and the
mixture is heated to 100 C over 2 hours. After cooling, it is poured into
water and
vigorous stirring is continued. The suspension is filtered through Celite, and
it is
thoroughly rewashed with ethyl acetate. The phases of the filtrate are
separated, and it is
extracted again with ethyl acetate. It is dried on sodium sulfate, and the
solvent is
CA 02577861 2007-02-21
44
removed in a vacuum. The 650 mg of 5-({3-[1-(3-fluoro-2-methoxyphenyl)-
cyclopropyl]-2-(trifluoromethyl)propan-1-yl}-imino)-2-methyl-phthalazin-l-one
that is
thus obtained in crude form is taken up.in 55 ml of dichloromethane and cooled
to
-30 C. 12 ml (12 mmol) of a I M boron tribromide solution in dichloromethane
is
added in drops over 10 minutes, and the solution is subsequently refluxed over
8 hours.
The cooled solution is poured into a mixture of ice and saturated sodium
bicarbonate
solution and vigorously stirred for 15 minutes. It is extracted with
dichloromethane,
washed with saturated sodium chloride solution and dried on sodium sulfate.
After
;; ;; =~t :
concentration by evaporation and chromatography on silica gel (hexane/ethyl
acetate 0-
50%) and subsequent HPLC (Kromasil C18, water/acetonitrile 30-60%), 75 mg of
the
desired product is obtained.
'H-NMR (300 MHz, CD3OD): S= 1.76 (d, 3H), 2.55 (d, 1H), 3.13 (d, 1H), 3.84
(s, 3H), 4.59 (s, 1H), 5.85 (q, IH), 6.74-6.84 (m, 3H), 7.56 (t, 1H), 7.63 (d,
1H), 8.56 (s,
1 H).
Eaample 5
(cis.Z)-4-Ethylidene-6-fluoro-l-jS2-methylquinazolin-5-yllamino)-2-
(trifluoromethyl)-
1 2,3,4-tetrahydronaphthalene-2.5-diol
5-Amino-2-methylquinazoline
12.7 g (62 mmol) of 2-methyl-5-nitro-3H-quinazolin-4-one (M. T. Bogert, V. J.
Chambers J. Org Chem. 1905, 649-658) and 37.5 g of phosphorus pentachloride
are
refluxed in 75 ml ofphosphoryl chloride over 20 hours. Aftercooling, it is
poured into
saturated NaHCO3 solution and extracted with ethyl acetate. The organic phase
is dried,
and the solvent is removed. 14 g of 4-chloro-2-methyl-5-nitroquinazoline, of
which 4.5
g (20.2 nunol) in 225 ml of ethyl acetate and 22.5 ml of triethylamine are
dissolved, is
CA 02577861 2007-02-21
obtained. 2 g of palladium is added to carbon, and it is stirred while being
cooled with
ice for 4 hours under a hydrogen atmosphere at normal pressure. Catalyst is
removed
from the solution by means of filtration through Celite, whereby it is
rewashed with 200
j
ml of ethanol and concentrated by evaporation. After chromatography on silica
gel with
ethyl acetate-ethano! (0-10%), 530 mg of the product is obtained.
'H-NMR (300 MHz, CDC13); S= 2.87 (s, 3H), 4.52 (br., 2H), 6.77 (d, 1H), 7.33
(d, IH), 7.65 (t, IH), 9.40 (s, lH).
=;;:d~.
0.8 ml (3.2 mmol) of titanium tetraethylate is added to 500 mg (1.63 mmol) of
3-
[1-(3-fluoro-2-methoxyphenyl)-cyclopropyl]-2-hydroxy-2-
(trifluoromethyl)propanal and
300 mg (1.88 mmol) of 5-amino-2-methyl-quinazoline in 75 ml of toluene, and
the
mixture is heated to 100 C over 2 hours. After cooling, it is poured into
water and
vigorous stirring is continued. The suspension is filtered through Celite, and
it is
thoroughly rewashed with ethyl acetate. The phases of the filtrate are
separated, and it is
extracted again with ethyl acetate. It is dried on sodium sulfate, and the
solvent is
removed in a vacuum. After chromatography on silica gel (hexane/ethyl acetate
0-60%),
>>?;.
350 mg of 3-[l-(3-fluoro-2-methoxyphenyl)-cyclopropyl]-1-[(2-methyl-quinazol-5-
yl)imino]-2-(trifluoromethyl)propan-2-ol, which is taken up in 36 ml of
dichloromethane
and cooled to -30 C,.is obtained. 7.9 ml (7.9 mmol) of a 1 M boron tribromide
solution
in dichloromethane is added in drops over 10 minutes, and the solution is
subsequently
refluxed over 5 hours. The cooled solution is poured into a mixture of ice and
saturated
sodium bicarbonate solution and vigorously stirred for 15 minutes. It is
extracted with
dichloromethane, washed with saturated sodium chloride solution and dried on
sodium
sulfate. After concentration by evaporation and HPLC (Kromasil C 18,
water/acetonitrile 30-60%), 86 mg of the desired product is obtained.
CA 02577861 2007-02-21
46
'H-NMR (300 MHz, CDCl3); 8 = 1.83 (d, 311), 2.66 (d, IH), 2.81 (s, 3H), 3.22
(d,1H), 5.03 (d,1H), 4.67 (d,1H), 5.85 (d,1H), 5.95 (q, IH), 6.55 (d,1H), 6.85
(dd,
1H), 6.95 (d, 1H), 7.28 (d, 1H), 7.66 (t?:IH), 9.35 (s,1H).
Example 5A/5B (rac,cis,Z)-4-Ethylidene-6-fluoro-l-[(2-methylquinazolin-5-
yl)aminoJ-
2-(trifluoromethyl)-1,2,3,4-tetrahydronaphthalene-2,5-diol is cleaved into the
enantiomer-pure compounds by means of preparative chiral HPLC (Chiracel OD 20
):
(+)-Enantiomer: analytical HPLC: Ri = 8.0 min (Chiracel OD 10 , 250 x 4.6
mm, hexaneJethano110%,1 ml/min of flow)
(-}Enantiomer: analytical HPLC: Rt =11.1 min (Chiralcel OD 10 , 250 x 4.6
mm, hexane/ethanol 10%, 1 ml/min of flow)
Example 6
(cis.Z)-6-Chloro-4-ethylidene-l-[(2-methylquinazolin-5-yl aminoj-2-
(trifluoromethl)-
1.2,3.4-tetrahydronaQhthalene-2,5-diol
3, 3, 3-Trfjluoro-2-[]-(3-chloro-2-methoxyphenyl)-cyclopropylmethylJ-2-hydroxy-
..
propianaldehyde
1-(3-Chloro-2-methoxy-phenyl)-cyclopropanecarbonitrile:
5.50 g (30.36 mrnol) of (3-chloro-2-methoxy-phenyl)acetonitrile is dissolved
in
50 ml of DMF. At 0 C, 2.96 g (73.93 mmol) of a sodium hydride suspension (55-
60%)
is added under a cover gas within 30 minutes. After 30 more minutes of
stirring at 0 C,
7.16 g (38.09 mmol) of 1,5-dibromoethane is added in drops. After stirring
overnight at
room temperature, the reaction mixture is added to water and extracted three
times with
methyl tert-butyl ether. The combined organic extracts are washed as usual
with brine
and dried. The residue that remains after the desiccant is filtered off and
the solvent is
CA 02577861 2007-02-21
47
spun off is chromatographed on silica gel (mobile solvent: ethyl
acetate/hexane). 4.36 g
(69.2%) of the desired compound is isolated. 'H-NMR (300 MHz, CDC13): 6 = 1.34
(2H), 1.70 (3H), 4.13 (3H), 7.02 (IH), 7.18 (1H), 7.39 (IH).
1-(3-Chloro-2-methoxy-phenyl)-cyclopropanecarbaldehyde:
4.36 g (21 nnnol) of 1-(3-chloro-2-methoxy-phenyl)-cyclopropanecarbonitrile is
dissolved in 75 ml of toluene, and the reaction mixture is cooled to -70 C. At
this
temperature, 26.14 ml (31.50 mmol) of a 1.2 molar DIBAH solution in toluene is
added
in drops, and the batch is stirred for two more hours at this temperature.
Then, 239.2 ml
of a 10% tartaric acid is carefully added in drops, whereby the temperature
quickly rises
to -5 C to +5 C. After a brief continuation of stirring, methyl tert-butyl
ether is added,
and the batch is stirr-ed vigorously. The organic phase is separated, and the
aqueous
phase is subsequently re-extracted twice with methyl tert-butyl ether. The
combined
organic extracts are washed with brine and dried on sodium sulfate. After the
dessicant
is filtered off and the solvent is spun off, 5.42 & (> 100%) of the aldehyde
is obtained,
which is fiuther used in crude form.
'H-NMR (300 MHz, CDC13): 5 =1.39 (2H), 1.68 (3H), 3:86 (3H), 7.00-7.12
(2H), 7.37 (iH), 9.20 (1 H).
(E/Z)-3-[1-(3-Chloro-2-methoxy-phenyl)-cyclopropyl]-2-ethoxy-acrylic acid
ethyl ester:
At 0 C, 15.3 ml (24.25 mmol) of butyllithium (1.6 molar in hexane) is slowly
added in drops to 2.5 g (24.47 mmol) of diisopropylamine in 5.9 ml of
tetrahydrofuran,
and it is stirred for 30 more minutes. The thus produced LDA is added in drops
to 5.63
g (20.98 mmol) of (diethoxy-phosphoryl)-ethoxy-acetic acid ethyl ester,
introduced into
17.3 ml of tetrahydrofuran, specifically at 0 C. After 20 minutes of stirring,
4.42 g
CA 02577861 2007-02-21
48
(20.98 mmol) of the aldehyde that is described in the previous section,
dissolved in 17.3
ml of THF, is added in drops at 0 C to the deprotonated phosphonate. After
stirring
overnight at room temperature, the reaction mixture is mixed with water and
extracted
three times with methyl tert-butyl ether. The combined organic extracts are
further
worked up as usual, and the remaining residue is chromatographed on silica gel
(mobile
solvent: ethyl acetate/hexane). 4.83 g (70.9%) of the desired compound is
isolated as an
E/Z mixture. For this reason, only the position of the signals, and not the
integration, is
indicated in the 1H-NMR below. 'H-NMR (300 MHz, CDC13): S= 0.85-0.99;1.09,
's.
1.16-1.40, 3.49, 3.73, 3.94, 4.00, 4.10-4.29, 5.70, 6.08, 6.90-7.00, 7.19-
7.30.
(E/Z)-3-[1-(3-Chloro-2-methoxy-phenyl)-cyclopropyl]-2-ethoxy-acrylic acid:
4.83 g (14.87 mmol) of (EJZ)-3-[1-(3-chloro-2-methoxy-phenyl)-cyclopropyl]-2-
ethoxy-acrylic acid ethyl ester is mixed with 140 ml of a I M NaOH in
ethanol/water 2:1
and stirred overnight at room temperature. The ethanol is drawn off in a
rotary
evaporator, the mixture is diluted with water and extracted three times with
methyl tert-
butyl ether. The organic phases are discarded after TLC monitoring. The
aqueous phase
is acidified with a I M HCI and extracted three times with methyl tert-butyl
ether. The
combined organic extracts are washed with brine and dried on sodium sulfate.
The
residue that is obtained according to the usual procedure (4.33 g= 98.2%) is
used in
crude form in the next stage. For this reason, only the position of the
signals, and not
the integration, is indicated in the 'H-NMR below. 'H-NMR (300 MHz, CDC13): 8
0.88, 1.00,1.13-1.49, 3.50, 3.80, 3.95, 4.00, 5.93, 6.22, 6.92-7.00, 7.19-
7.30, 7.40.
CA 02577861 2007-02-21
49
3-[1-(3-Chloro-2-methoxy-phenyl)-cyclopropyl]-2-oxo-propionic acid:
4.33 g (14.59 mmol) of (E/Z)-3-[1-(3-chloro-2-methoxy-phenyl)-cyclopropyl]-2-
ethoxy-acrylic acid is added in 65 ml of sulfuric acid (I M) and stirred
overnight at
90 C. After cooling, it is diluted with some water and made basic with
potassium
carbonate. It is extracted three times with methyl tert-butyl ether, and the
combined
organic extracts are discarded after TLC monitoring. The aqueous phase is
acidified
with 1 M hydrochloric acid and extracted with methyl tert-butyl ether. After
additional
usual working-up, 3.40 g (86.7%) of the desired a-ketocarboxylic acid is
obtained. 1H-
NMR (300 MHz, CDC13): S= 0.97 (2H), 1.05 (2H), 3.19 (2H), 4.05 (3H), 6.99
(iH),
7.20-7.30 (2H).
3-[1-(3-Chloro-2-methoxy-phenyl)-cyclopropyl]-2-oxo-propionic acid ethyl
ester:
15.12 g (56.27 mmol) of 3-[1-(3-chloro-2-methoxy-phenyl)-cyclopropyl]-2-oxo-
propionic acid is dissolved in 350 ml of ethanol and mixed with 6.3 ml of
sulfuric acid
(conc.). After five hours of refluxing, the reaction mixture is spun in until
a dry state is
reached. The residue is taken up in 700 ml of saturated sodium bicarbonate
solution and
extracted three times with ethyl acetate. The combined organic extracts are
washed with
brine, dried on sodium sulfate, and the solvent is spun off after the
desiccant is filtered
off. After chromatography of the residue on silica gel (mobile solvent: ethyl
acetate/hexane), 12.36 g(74 /a) of the desired ester is isolated. 'H-NMR (300
MHz,
CDC13); S= 0.90-0.98 (411),1.30 (3H), 3.19 (2H), 3.97 (314), 4.20 (211), 6.95
(1 H),
7.20-7.30 (2H).
CA 02577861 2007-02-21
2-[ l -(3-Chloro-2-methoxy-phenyl)-cyclopropylmethyl]-3,3,3-trifluoro-2-
trimethylsilyloxy-propionic acid ethyl ester:
6.18 g (20.83 mmol) of 3-[1-(3-chloro-2-methoxy-phenyl)-cyclopropyl]-2-oxo-
propionic acid ethyl ester and 3.55 g(24.99 mmol) of (trifluoromethyl)-
trimethylsilane
are dissolved in 33 ml of tetrahydrofiuan. After 51 mg of tetrabutylammonium
fluoride
trihydrate is added, it is stirred overnight at room temperature. The reaction
mixture is
diluted with methyl tert-butyl ether, and washed first with water and then
with brine.
After the usual procedure (drying and filtering off of the solvent), the
residue is
chromatographed on silica gel (mobile solvent: ethyl acetate/hexane). 5.65 g
(66.4%)
of the desired, but contaminated compound is isolated.
2-[ 1-(3-Chloro-2-methoxy-phenyl)-cyclopropylmethyl]-3,3,3-ttifluoro-2-hydroxy-
propionic acid ethyl ester:
5.65 g (1.75 mmol) of the compound that is described in the previous section
is
dissolved in 76 ml of tetrahydrofuran and mixed with 4.34 g (13.75 mrnol) of
tetrabutylammonium fluoride trihydrate. After one hour of stirring at room
temperature,
it is worked up as usual, and the residue that remains after the solvent is
spun in is
chromatographed on silica gel (hexane/ethyl acetate 0-30%). 3.24 g(64.3%) of
the
desired compound is isolated. 'H-NMR (300 MHz, CDC13): S= 0.59-0.69 (IH), 0.73-
0.82 (IH), 0.98-1.20 (5H), 1.75 (1H), 3.09 (1H), 3.39-3.51 (iH), 3.80 (1H),
3.85-4.03
(4H), 6.92 (1 H), 7.08 (1 H), 7.25 (1 H).
CA 02577861 2007-02-21
51
3,3,3-Trifluoro-2-[1-(3-chloro-2-methoxyphenyl)-cyclopropylmethyl]-2-hydroxy-
propionaldehyde:
0.850 g (2.317 mmol) of the ester that is described in the preceding section
is
dissolved in 8 ml of diethyl ether and mixed in portions with 66 mg (1.74
mmol) of
lithium aluminum hydride at 0 C under a cover gas. After two more hours of
stirring at
0 to 5 C, 2.7 ml of saturated sodium bicarbonate solution is carefully added
in drops at
0 C. After being extracted three times with methyl tert-butyl ether, the
combined
organic extracts are washed as usual. The ultimately obtained residue is
chromatographed on a Flashmaster. 750 mg (65.5%) of a mixture that consists of
the
desired aldehyde to 64% and the starting ester to 36% is isolated.
0.39 ml (1.9 mmol) of titanium tetraethylate is added to 300 mg (0.93 mmol) of
3,3,3-trifluoro-2-[1-(3-chloro-2-methoxyphenyl)-cyclopropylmethyl]-2-hydroxy-
propionaldehyde and 155 mg (0.98 mmol) of 5-amino-2-methylquinazoline in 28 ml
of
toluene, and the mixture is heated to 100 C over 2 hours. After cooling, it is
poured into
water, and vigorous stirring is continued. The suspension is filtered through
Celite and
thoroughly rewashed with ethyl acetate. The phases of the filtrate are
separated, and it is
extracted again with ethyl acetate. It is dried on sodium sulfate, and the
solvent is
removed in a vacuum. The 3-[I-(3-chloro-2-methoxyphenyl)-cyclopropylj-l-[(2-
methyl-quinazol-5-yl)imino]-2-(trifluoromethyl)propan-2-ol that is thus
obtained in
crude form is taken up in 23 ml of dichloromethane and cooled to -20 C. 7.8 ml
(7.8
mmol) of a 1 M boron tribromide solution in dichloromethane is added in drops
over 10
minutes, and the solution is subsequently refluxed over 3 hours. The cooled
solution is
poured into a mixture of ice and saturated sodium bicarbonate solution and
vigorously
stirred for 15 minutes. It is extracted with dichloromethane, washed with
saturated
sodium chloride solution and dried on sodium sulfate. After concentration by
CA 02577861 2007-02-21
52
evaporation, chromatography on silica gel (1. separation: hexane/ethyl acetate
15-20%,
2. Separation: hexane/2-propano110%), 4 mg of the desired product is obtained.
'H-NMR (300 MHz, CD3OD); S=1.75 (d, 3H), 2.58 (d,1H), 2.83 (s, 3H), 3.15
(d, IH), 4.65 (s, 1H), 5.87 (q, 1H), 6.50 (d,lH), 6.84 (d,1H), 7.19 (d, IH),
7.25 (d, lH),
7.71(t,1H),9.65(s,1ID.
Example 7
(cis Z)-6-Chloro-4-ethylidene-l-f(7-fluoro-2-methvlauinazolin-5-yl)amino]-2-
(trifluoromethyl)-1,2,3,4-tetrah~ironaphthalene-2.5-diol
S Amino-7 fluoro-2-methylquinazoline
17 g (70.5 mmol) of 3,6-difluoro-2-N-pivaloylaminobenzaldehyde (L. Florvall,
I.
Fagervall, L.-G. Larsson, S. B. Ross, Eur. J. Med Chem. 34 (1999)137-151), 9.2
g of
acetamidine hydrochloride, 13.4 g of potassium carbonate and 10.4 g of
molecular sieve
(4A) are added together in 70 ml of butyronitrile. It is heated for 17 hours
to 145 C
while being stirred vigorously, and the solvent is removed in a vacuum. After
chromatography of the residue on silica gel with hexane/ethyl acetate (0-70%),
4.5 g of
7-fluoro-S-N-pivaloylamino-2-methylquinazoline is obtained.
1 g (3.82 mmol) of 7-fluoro-5-N-pivaloylamino-2-methylquinazoline is dissolved
in 74 ml of toluene and cooled to -70 C. Over 30 minutes, 9.5 ml (11.4 mmol)
of a 1.2
M diisobutyl aluminum hydride solution in toluene is added in drops. The
reaction
mixture is allowed to heat to -40 C and stirred for 4 hours at -40 C. Water is
slowly
added, and it is stirred for 30 minutes at room temperature until a
precipitate forms,
which is removed by means of filtrat.ion through Celite. The phases are
separated,
washed with saturated sodium chloride solution and dried on sodium sulfate.
After
CA 02577861 2007-02-21
53
chromatography on silica gel with hexane-ethyl acetate (0-100%), 64 mg of the
product
is obtained.
'H-NMR (300 MHz, CDC13); S= 2.83 (s, 3H), 4.67 (br., 2H), 6.50 (dd,1 H),
6.93 (dd, 1H), 9.23 (s, 1 H).
Analogously to Example 6,15 mg of the correspondingly formed 3-[1-(3-chloro-
2-methoxyphenyl)-cyclopropyl]-1-[(7-fluoro-2-methyl-quinazol-5-yl)imino]-2-
(trifluoromethyl)propan-2-ol is treated with BBr3 solution and refluxed for
one hour.
After worlcing-uP and chromatography on silica gel (ethyl acetate), 1.6 mg of
the desired
product is obtained.
'H-NMR (300 MHz, CDC13); S= 1.82 (d, 3H), 2.60 (d, lH), 2.81 (s, 3H), 3.24
(d, 1H), 4.60 (d, lH), 5.94 (q, 1H), 6.08 (d, IH), 6.24 (dd, IH), 6.88 (d,
1H), 6.90 (dd,
1H), 7.27 (d, IH), 9.25 (s, lH).
h:
Example 8
(cis. Z)-4-Ethylidene-6-fluoro-l- [!7-fluoro-2-methylquinazolin-S-~+I)aminot2-
:': 6rifluoromethvl}-1,2,3,,4-tetnahydronaphthalene-2,5 -diol
Analogously to Example 5, 1-[(7-fluoro-2-methyl-quinazol-5-yl)imino]-3-[1-(3-
fluoro-2-methoxyphenyl)-cyclopropylj-2-(trifluoromethyl)propan-2-ol is
produced
quantitatively from 800 mg (2.61 mmol) of 3-[1-(3-fluoro-2-methoxyphenyl)-
cyclopropyl]-2-hydroxy-2-(trifluoromethyl)propanal and 500 mg (2.82 mmol) of 5-
amino-7-fluoro-2-methyl-quinazoline. The treatment with 24 ml (24 nunol) of
BBr3
solution and subsequent refluxing over 14 hours produce 130 mg of desired
product
after analogous chromatography and HPLC.
CA 02577861 2007-02-21
54
1H-NMR (300 MHz, CD30D); 8=1.77 (d, 314), 2.57 (d, 1H), 2.80 (s, 3H), 3.14
(d, 1H), 4.64 (s, 1H), 5.86 (q, 1H), 6.26 (dd, 1H), 6.77-6.97 (m, 2H), 7.02
(dd, 1H), 9.57
(s, IH)
Egample 8A/8B
(rac, cis, Z)-4-Ethylene-6-fluoro-l-[(7-fluoro-2-methylquinazolin-5-yl)amino]-
2-
(trifluoromethyl)-I,2,3,4-tetrahydronaphthalene-2,5-diol is cleaved into the
enantiomer-
pure compounds by means of preparative chiral HPLC (Chiracel OD-H 5p):
(+)-Enantiomer: analytical HPLC: R{ =10.4 min (Chiralcel OD 10 g, 250 x 4.6
mm, hexane/ethanol 7%, 1 mUmin of flow)
(-)-Enantiomer: analytical HPLC: R{ 16.5 min (Chiralcel OD 10 , 250 x 4.6
mm, hexane/ethanol 7%, 1 ml/min of flow)
Example 9
(cis.Z -4-Ethylidene-6-fluoro-l-[(8-fluoro-2-methvlguinazolin-5-yl)amino12-
(trifluoromethyl)-1,2,3,4-tetrahydronaphthalene-2,5-diol
5-Amino-8, fluoro-2-methylquinazoline
A solution of 2.4 g (18.6 mmol) of 2,5-difluoroaniline in 11 ml of water and
1.6
ml of concentrated hydrochloric acid (37%) that is 50 C and that was stirred
in advance
for 1 hour at this temperature is added to a solution of 3.35 g (20.25 mmol)
of chloral
hydrate and 21.27 g (149.7 mmol) of sodium sulfate in 72 ml of water. It is
stirred for
another 30 minutes at room temperature and after the addition of 4.09 g (58.9
mmol) of
hydroxylammonium chloride in 19 ml of water, it is heated over 45 minutes to
125 C
and kept at this temperature for 5 minutes. After cooling and after another
hour, the
deposited light-brown precipitate is filtered off, washed with water and
dried. 3.0 g
CA 02577861 2007-02-21
. ~
(15.0 mmol) of the hydroxylimine is obtained as an intermediate product, which
is
dissolved in portions in 15 ml of concentrated sulfuric acid at 60 C. After
the addition
is completed, it is heated for 2 hours to,80 C and for 4 hours to 90 C. It is
allowed to
cool off, and the solution is poured into 100 g of ice. It is extracted with
ethyl acetate,
the organic phase is washed with water, dried on sodium sulfate and
concentrated by
evaporation. After chromatography on silica gel with hexane-ethyl acetate (0-
45%), 1.2
g (7.1 mmol) of the 4,7-difluoroisatin is obtained. 1.8 ml of a 30% hydrogen
peroxide
solution is added in drops over 10 minutes to isatin in 30 ml of a I molar
sodium
!:w4
hydroxide solution. After 2 hours of stuTing at room temperature, it is cooled
to 0 C,
and 5 ml of a 4 molar hydrochloric acid is added and diluted with 50 ml of
water. It is
extracted with ethyl acetate, dried on sodium sulfate, concentrated by
evaporation and
1.27 g of the 3,6-difluoroanthranilic acid, which is reacted without further
purification,
is thus obtained quantitatively.
The 3,6-difluoroanthranilic acid is heated in 8 ml of acetic acid anhydride
for 45
minutes to 100 C. After cooling, the acetic acid that is produced and excess
acetic acid
anhydride are removed azeotropically with toluene in a vacuum. The residue is
mixed
>.. .
with 40 ml of a 25% ammonia solution while being cooled with ice, and it is
stirred for
72 hours. It is diluted with water and acidified with acetic acid. It is
extracted with
ethyl acetate, the organic phase is washed with water, dried on sodium sulfate
and
concentrated by evaporation. The thus obtained 1.03 g (5.25 mmol) of 5,8-
difluoro-2-
methyl-3H-quinazolin-4-one and 6 g of phosphorus pentachloride are heated in
20 ml of
phosphoryl chloride over 12 hours to 125 C. After cooling, it is poured into
saturated
NaHCO3 solution and extracted with ethyl acetate. The organic phase is dried,
and the
solvent is removed. 1.7 g of 4-chloro-5,8-difluoro-2-methylquinazoline, which
is
dissolved in 60 ml of ethyl acetate and 5 ml of triethylamine, is
quantitatively obtained.
CA 02577861 2007-02-21
56
600 mg of palladium on carbon is added and shaken for 2 hours (480 ml of
hydrogen
absorption) under a hydrogen atmosphere at normal pressure. Catalyst is
removed from
the solution by means of filtration on Celite, whereby it is rewashed with 100
ml of
ethanol and concentrated by evaporation. After chromatography on silica gel
with
hexane-ethyl acetate-ethanol (0-40%), 550 mg of 5,8-difluoro-2-
methylquinazoline is
obtained. 890 mg (13.7 mmol) of sodium azide is added to 240 mg (1.3 mmol) of
5,8-
difluoro-2-methylquinazoline, 300 mg (1.13 mmol) of 18-crown-6- in 10 ml of
DMF,
and the mixture is heated over 8 hours to 125 C. The solvent is removed in a
vacuum,
and it is chromatographed on silica gel with ethyl acetate, and 52 mg of
product is
obtained.
'H-NMR (3001VIHz, CDC13); S= 2.92 (s, 3H), 4.31 (br., 2H), 6.67 (dd, 1H),
7.38 (dd,1H), 9.37 (s, 1H).
0.62 ml (2.9 mmol) of titanium tetraethylate is added to 400 mg (1.3 mmol) of
3-
[1-(3-fluoro-2-methoxyphenyl)-cyclopropyl]-2-hydroxy-2-
(trifluoromethyl)propanal and
266 mg (1.5 mmol) of 5-amino-8-fluoro-2-methylquinazoline in 50 ml of toluene,
and
the mixture is heated over 2 hours to 100 C. After cooling, it is poured into
water, and
vigorous stirring is continued. The suspension is filtered through Celite and
thoroughly
rewashed with ethyl acetate. The phases of the filtrate are separated, and it
is extracted
again with ethyl acetate. It is dried on sodium sulfate, and the solvent is
removed in a
vacuum. The 600 mg of 1-[(7-fluoro-2-methyl-quinazol-5-yl)imino]-3-[l-(3-
fluoro-2-
methoxyphenyl)-cyclopropyl]-2-(trifluoromethyl)propan-2-ol that is thus
obtained in
crude form is taken up in 60 ml of dichloromethane and cooled to 0 C. 13.5 ml
(13.5
mmol) of a I M boron tribromide solution in dichioromethane is added in drops
over 10
CA 02577861 2007-02-21
57
minutes, and the solution is subsequently refluxed over 4.5 hours. The cooled
solution is
poured into a mixture of ice and saturated sodium bicarbonate solution and
vigorously
stirred for 15 minutes. It is extracted with dichloromethane, washed with
saturated
sodium chloride solution and dried on sodium sulfate. Afl.er concentration by
evaporation and chromatography on silica gel (hexane/2-propanol 0-15 /a), 300
mg of
the desired product is obtained.
'H-1VMR (300 MHz, CDC13); 8 =1.83 (d, 3H), 2.64 (d, 1H), 2.89 (s, 3H), 3.18
(d, IH), 4.60 (d, 1H), 5.53 (d,1H), 5.95 (q,1H), 6.45 (dd,1H), 6.80 (dd,1H),
6.97 (d,
1H), 7.41 (dd,1H), 9.34 (s, 1H).
Example 9A/9B (rac,cis,29-4-Ethylidene-6-fluoro-l-[(8-fluoro-2-
methylquinazolin-5-
yl)amino]-2-(trifluoromethyl)-1,2,3,4-tetrahydronaphthalene-2,5-diol is
cleaved into the
enantiomer-pure compounds by means of preparative chiral HPLC (Chiracel OD 10
}t):
(+)-Enantiomer: analytical HPLC: Rt= 15.7 min (Chiraicel OD 10 , 250 x 4.6
mm, hexane%thanol7%,1 ml/min of flow)
(-)-Enantiomer: analytical HPLC: 12~ = 26.1 min (Chiralcel OD 10 , 250 x 4.6
mm, hexane/ethanol 7%,1 ml/min of flow)
Example 10
(Cis, Z:4-Ethylidene-6-fluoro-l-[(8-fluoro-2-methylauinazolin-5=yllamino]-2-
(trifluoromethyl)-1.2.3.4-tetrahydronaphthalene-2.5-diol
Analogously to Bxample 6,420 mg of the correspondingly formed 3-[1-(3-
chloro-2-methoxyphenyl)-cyclopropyl]-1-[(8-fluoro-2-methyl-quinazol-5-
yl)imino]-2-
(trifluoromethyl)propan-2-ol is treated with BBr3 solution and refluxed for
two hours.
CA 02577861 2007-02-21
58
After working-up and chromatography on silica gel (hexanel2-propanol 20-
30%),13 mg
of the desired product is obtained.
'H-NMR (300 MHz, CD3OD); $=1.75 (d, 3H), 2.57 (d, 1H), 2.87 (s, 3H), 3.14
(d, 1I4), 4.60 (s, 1H), 5.86 (q,1H), 6.41 (dd, 1H), 6.85 (d,1H), 7.25 (d, 1H),
7.48 (dd,
IH), 9.68 (s, IH).
Example 11
(cis)-6-F'luoro-1-fl8-fluoro-2-methylauinazolin-5-yllamino]-4-isopropylidene-2-
(trifluoromethvll-12.3,4-tetrahydronaphthalene-2.5-diol
4-(3-Fluoro-4-methoxy-phenyl)-2-hydroxy-5-methyl-2-(tri, fluoromethyl)hex-4-
enal
24.5 ml (235 mmol) of 2-methyl-propionic acid chloride is added in drops at 0
C
to 20 ml (214 mmo)) of 2-fluorophenol in 150 ml of dichloromethane and 24 ml
of
pyridine. The mixture is stirred for two hours, and 200 ml of a 1 M
hydrochloric acid is
added. It is extracted with dichloromethane and washed with water. After being
dried
on sodium sulfate and after the solvent is removed in a vacuum, 43 g of 2-
methylpropionic acid-2-fluorophenyl ester is obtained quantitatively. 43 g
(214 mmol)
B
of 2-methylpropionic acid-2-fluorophenyl ester in 20 ml of 1,2-dichlorobenzene
is added
in drops to 28 g of aluminum trichloride in 25 ml of 1,2-dichlorobenzene, and
the
mixture is subsequently stirred for 24 hours at 100 C. It is allowed to cool,
diluted with
dichloromethane and carefully poured into a mixture of 4 M hydrochloric acid
and ice.
The phases are separated; it is extracted with dichloromethane, washed with
water and
dried on sodium sulfate. The crude product is purified by column
chromatography on
silica gel (hexane%thyl acetate 0-40%), and 26.5 g of l-(3-fluoro-2-
hydroxyphenyl)-2-
methyl-propan-l-one, and 12 g of 1-(3-fluoro-4-hydroxyphenyl)-2-methyl-propan-
l-one
are obtained. 4.4 g (24 mmol) of 1-(3-fluoro-4-hydroxyphenyl)-2-methyl-propan-
l-one
CA 02577861 2007-02-21
59
is dissolved in 30 ml of DMF, and 3.73 g (27 mmol) of potassium carbonate and
1.7 ml
(27 rnmol) of methyl iodide are added. The mixture is stirred for 20 hours at
room
temperature, and it is poured into water and extracted with diethyl
ether/hexane (1:1)
and ethyl acetate. It is washed with brine, dried on sodium sulfate, and after
the solvent
is removed in a vacuum, 4.8 g of 1-(3-fluoro-4-methoxyphenyl)-2-methyl-propan-
l-one
is obtained quantitatively.
3.25 g (50 mmol) of zinc dust and 139 mg (0.5 mmol) of lead(II) chloride are
suspended in 50 ml of THF, and 2.9 ml (26 mmol) of dibromomethane is added at
room
L.. ._
temperature. It is stirred for another 30 minutes, and 5.5 m1(5.5 mmol) of a 1
M
titanium(IV) chloride solution in dichloromethane is added in drops, and the
mixture is
slighely heated. After one hour at room temperature, 1.0 g (5.1 mmol) of 1-(3-
fluoro-4-
methoxyphenyl)-2-methyl-propan-l-one in 10 ml of TBF is added in drops. It is
stirred
for another 13 hours at room temperature. It is diluted with diethyl ether,
and the
reaction mixture is carefully added to a mixture of 4 M hydrochloric acid and
ice. The
phases are separated, extracted with diethyl ether, washed with water, dried
on sodium
sulfate, and the solvent is removed. The crude product is purified by column
r.s
chromatography on silica gel (hexane/isopropyl ether 0-20%), and 0.47 g of 2-
fluoro-I-
;~..=
methoxy-6-(2-methyl-l-methylenepropyl)-benzene is obtained.
'H-NMR (300 MHz, CDC13); S= 1.09 (d, 6H), 2.76 (m,1H), 3.89 (s, 3H), 5.00
(s, IH), 5.11 (s, 1H), 6.90 (dd, 1H), 7.08-7.13 (m, 2H), 9.38.
0.25 ml (0.12 mmol) of a 0.5 M titanium tetraisopropylate solution in toluene
is
added to 69 mg (0.24 mmol) of 1,1'-bi-2-naphthol in 0.5 ml of toluene, and the
red
solution is stured for 2 hours at room temperature. 0.45 g (2.3 mmol) of 2-
fluoro-l-
methoxy-6-(2-methyl-l-methylenepropyl)-benzene and 780 mg (4.6 mmol) of ethyl
CA 02577861 2007-02-21
trifluoropyruvate are added, and it is stirred for 24 hours at 0 C, for 24
hours at room
temperature, and the mixture is heated over 5 hours to 100 C. After cooling,
it is
purified immediately by column chromatography on silica gel (hexane/ethyl
acetate 0-
20%), and 250 mg of 4-(3-fluoro-4-methoxyphenyl)-2-hydroxy-5-methyl-2-
(trifluoromethyl)-hex-4-enoic acid ethyl ester is obtained. 250 g (0.7 mmol)
of 4-(3-
fluoro-4-methoxyphenyl)-2-hydroxy-5-methyl-2-(trifluoromethyl)-hex-4-enoic
acid
ethyl ester is cooled to -5 C in 10 ml of diethyl ether, and 56 mg (1.4 mmol)
of lithium
aluminum hydride is added in portions in solid fonn over 10 minutes. It is
stirred for 2
hours at 0 C and poured into saturated ammonium chloride solution. The
suspension is
filtered through Celite and thoroughly rewashed with ethyl acetate. The phases
of the
filtrate are separated, and it is extracted again with ethyl acetate. It is
washed with
saturated sodium chloride solution, dried on sodium sulfate, and the solvent
is removed
in a vacuum. The separation by column chromatography on silica gel
(hexane/ethyl
acetate 30%) yields 140 mg of 4-(3-fluoro-4-methoxyphenyl)-2-hydroxy-5-methyl-
2-
(trifluoromethyl)-hex-4-enal and 80 mg of alcohol.
'H-NMR (300 MHz, CDC13); 8 =1.56 (s, 3I-I), 1.88 (s, 3H), 3.09 (d, IH), 3.26
.: .k
(d, 1H), 3.67 (s, 1I-1), 3.89 (s, 3H), 6.68-6.90 (m, 3H), 9.14 (s, 1H).
0.2 ml (1.0 mmol) of titanium tetraethylate is added to 75 g (0.23 mmol) of 4-
(3-
fluoro-2-methoxyphenyl)-2-hydroxy-5-methyi-2-(trifluoromethyl)-hex-4-enal and
41
(0.23 mol) of 5-amino-8-fluoro-2-methylquinazoline in 4 ml of toluene, and the
mixture
is heated over 2 hours to 100 C. After cooling, it is poured into water, and
vigorous
stirring is continued. The suspension is filtered through Celite, and it is
thoroughly
rewashed with ethyl acetate. The phases of the filtrate are separated, and it
is extracted
again with ethyl acetate. It is dried on sodium sulfate, and the solvent is
removed in a
CA 02577861 2007-02-21
61
vacuum, and 115.5 mg of 4{3-fluoro-4-methoxyphenyl)-1-[(8-fluoro-2-
methylquinazolin-5-yl)-imino]-5-methyl-2-(trifluoromethyl)-hex-4-en-2-ol is
obtained
as a crude product. The imine is taken up in 7 ml of dichloromethane and
cooled to
-78 C. 1.3 ml (1.3 mmol) of a I M titanium tetrachloride solution in
dichloromethane is
added in drops over 5 minutes, and after 20 minutes, the cooling bath is
removed. After
another 15 minutes, the solution that is heated to 0 C is poured into a
mixture of ice and
saturated sodium bicarbonate solution and vigorously stirred for 10 minutes.
It is
extracted with ethyl acetate, washed with saturated sodium chloride solution
and dried
~ 45
- on sodium sulfate. After concentration by evaporation and chromatography on
silica gel
(hexane%thyl: acetate 50%), 11 mg of the desired product is obtained.
'H-NMR (300 MHz, CDC13); S= 1.93 (s, 3H), 2.03 (s, 3H), 2.69 (d, IH), 2.90
(s, 3H), 3.17 (d,1H), 3.68 (s, 3H), 4.68 (d, IH), 5.52 (d, 1H), 6.54 (dd, IH),
6.90 (d,
1 H), 7.10 (d, l H), 7.43 (dd, 114), 9.38 (s, I H).
Example 12
(cis,Z)-1-[(7,8-Difluoro-2-methvlquinazolin-5-yl)aminoJ-4ethvlidene-6-fluoro-2-
(trifluoromethyl)-1,2,3.4-tetrahvdronaphthalene-2.5-diol
S Amino-7,8-dffluoro-2.methylquinazotfne
156 ml (391 mmol) of a 2.5 M butyllithium solution in hexane is added in drops
at -70 C to 41.7 g(l80 mmol) of 2,2-dimethyl-N-(3,4,5-trifluorophenyl)-
propionamide
in 385 ml of THF. It is allowed to stir for one hour and then 38.6 ml of DMF
in 290 ml
of TIF is added in drops, and the solution must heat to -60 C. It is stirred
for another
hour at -70 C, and then the cold reaction solution is poured into a mixture of
2 kg of ice
and 400 ml of concentrated hydrochloric acid. It is stirred vigorously and
after one hour,
it is extracted several times with diethyl ether. The organic phase is washed
neutral with
CA 02577861 2007-02-21
62
water and dried on sodium sulfate. After concentration by evaporation, 49.3 g
(188
mmol) of crude 4,5,6-trifluoro-2-N-pivaloylaminobenzaldehyde, which is added
together with 26 g (275 mmol) of acetamidine hydrochloride, 38.3 g (277 mmol)
of
~
potassium carbonate and 30 g of molecular sieve (4A) in 206 ml of
butyronitrile, is
obtained. It is heated for 18 hours to 145 C while being stirred vigorously,
and the
solvent is removed in a vacuum. After the residue is chromatographed on silica
gel with
hexane/ethyl acetate (0-100%), 9.1 g of 7,8-difluoro-5 N-pivaloylamino-2-
methylquinazoline is obtained.
2.0 g (7.2 mmol) of 7,8-difluoro-5 N-pivaloylamino-2-methylquinazoline is
dissolved in 140 nil of toluene and cooled to -70 C. Over 30 minutes, 24 ml
(28.8
mmol) of a 1.2 M diisobutyl aluminum hydride solution in toluene is added in
drops.
The reaction mixture is allowed to heat to -25 C over 2 hours and stirred for
2 hours at
-25 C. Isopropanol and then water are slowly added and stirred for 12 hours at
room
temperature until a precipitate forms, which is removed by means of filtration
through
Celite. It is rewashed well with a methylene chloride-methanol mixture and
concentrated by evaporation. The residue is stirred vigorously in 200 ml of
ethyl acetate
>'= ' and 50 ml of methanol together with 100 g of silica gel and 20 g of
manganese dioxide.
It is filtered through Celite, rewashed well with a methylene chloride-
methanol mixture
and concentrated by evaporation. After chromatography on silica gel (hexane-
ethyl
acetate 0-100%), 370 mg of the product is obtained.
1H-NMR (300 MHz, CD3OD); S= 2.81 (s, 3H), 6.64 (dd, 1H), 9.52 (s,1H).
Analogously to Example 6, 33 mg of the correspondingly formed 3-[1-(3-chloro-
2-methoxyphenyl)-cyclopropyl]-1-[(7,8-difluoro-2-methyl-quinazol-5-yl)imino]-2-
(trifluoromethyl)propan-2-ol is treated with BBr3 solution and refluxed for
one hour.
CA 02577861 2007-02-21
63
After working-up and chromatography on silica gel (hexane/2-propano115%), 4.5
mg of
desired product is obtained.
'H-NMR (300 MHz, CDC13); 8 =1.83 (d, 3H), 2.61 (d, IH), 2.87 (s, 3H), 3.22
(d,1H), 4.55 (d,1H), 5.90 (d, IH), 5.95 (q,1H), 6.30 (dd, 1H), 6.82 (dd,1H),
7.01 (dd,
1H), 9.31 (s, lH).
Example 13
(cis.E)-1-[{7,8-Difluoro-2-meth +} lguinazolin-5-Xl)aminol-4-ethvlidene-6-
fluoro-5-
~::; methoxy-2-(trifluoromethyll-1.2.3.4-tetrahydronayhthalen-2-ol
E-4-(3-Fluoro-2-methoxy phenyl)-2-hydroxy-2-(tr fluoromethyl) pent-4-enal:
19.6 ml (225 mmol) of propionic acid chloride is added in drops to 20 ml (214
mmol) of 2-fluorophenol in 150 ml of dichloromethane and 24 ml of pyridine at
0 C.
The mixture is stirred for two hours, and 200 ml of a I M hydrochloric acid is
added. It
is extracted with dichloromethane and washed with water. After being dried on
sodium
sulfate and after the solvent is removed in a vacuum, 36 g of propionic acid-2-
fluorophenyl ester is obtained quantitatively. 36 g (214 mmol) of propionic
acid-2-
..~,:.,
fluorophenyl ester in 20 ml of 1,2-dichlorobenzene is added in drops to 28 g
of
aluminum trichloride in 25 ml of 1,2-dichlorobenzene, and the mixture is
subsequently
stirred for 24 hours at 100 C. It is allowed to cool, diluted with
dichloromethane, and
carefully poured into a mixture of 4 M hydrochloric acid and ice. The phases
are
separated; it is extracted with dichloromethane, washed with water and dried
on sodium
sulfate. The crude product is purified by column chromatography on silica gel
(hexane/ethyl acetate 0-40%), and 17 g of 1-(3-fluoro-2-hydroxyphenyl)-propan-
l-one
and 12.7 g of 1-(3-fluoro-4-hydroxyphenyl)-propan-l-one are obtained. 17 g
(102
mmol) of 1-(3-fluoro-2-hydroxyphenyl)-propan-l-one is dissolved in 160 ml of
acetone,
CA 02577861 2007-02-21
64
and 26 g of potassium carbonate and 11.5 ml of methyl iodide are added. The
mixture is
stirred for 7 hours at 70 C, and the solvent is subsequently removed in one
large portion.
The residue is poured into water and extracted with diethyl ether. It is
washed with
water, dried on sodium sulfate, and after the solvent is removed in a vacuum,
18.5 g of
1-(3-fluoro-2-methoxyphenyl)-propan-l-one is obtained quantitatively. 38.5 g
(589
mmol) of zinc dust and 804 mg (2.9 mmol) of lead(II) chloride are suspended in
595 ml
of THF, and 36 ml (513 mmol) of dibromomethane is added at room temperature.
It is
stirred for another 30 minutes, and 68.8 ml (68.8 mmol) of a I M titanium(IV)
chloride
solution in dichloromethane is added in drops at 0oC. The cooling bath is
removed, and
after 30 minutes at room temperature,12.5 g of (1-(3-fluoro-2-methoxyphenyl)-
propan-
1-one in 128 ml of TBF is added in drops. It is stirred for another 5 hours at
room
temperature, whereby after 2 hours, a slight heating set in, which was cooled
by means
of a water bath. It is diluted with diethyl ether, and the reaction mixture is
carefully
added to a mixture of 4 M hydrochloric acid and ice. The phases are separated,
extracted with diethyl ether, washed with water, dried on sodium sulfate, and
the solvent
is removed. The crude product is purified by column chromatography on silica
gel
(hexane/isopropyl ether 0-3%), and 8.1 g of 2-fluoro-6-(1-methylenepropyl)-
phenol.is
w,.
obtained.
3.3 ml (1.65 nunol) of a 0.5 M titanium tetraisopropylate solution in toluene
is
added to 896 mg (3.3 mmol) of 1,1'-bi-2-naphthol, and the red solution is
stirred for 2
hours at room temperature. 5.07 g (28.1 mmol) of 2-fluoro-6-(1-
methylenepropyl)-
phenol and 7.3 ml (60.8 mmol) of ethyltrifluoropyruvate are added, and the
mixture is
heated over 17 hours to 140 C. After cooling, it is immediately purified by
column
chromatography on silica gel (hexane/ethyl acetate 0-5%), and 4.6 g of 4-(3-
fluoro-2-
methoxyphenyl)-2-hydroxy-2-(trifluoromethyl)-hex-4-enoic acid ethyl ester is
obtained
CA 02577861 2007-02-21
as an E/Z mixture. 2.0 g (5.7 mmol) of E-4-(3-fluoro-2-methoxyphenyl)-2-
hydroxy-2-
(trifluoromethyl)-hex-4-enoic acid ethyl ester is cooled in 100 ml of diethyl
ether to -
5 C, and 498 g (13.1 mmol) of lithium. aluminum hydride is added in portions
in solid
form over 10 minutes. It is stirred for 2 ho,urs at room temperature and
poured into
saturated ammonium chloride solution. Tlie suspension is filtered through
Celite and
thoroughly rewashed with ethyl acetate. The phases of the filtrate are
separated, and it is
extracted again with ethyl acetate. It is washed with saturated sodium
chloride solution,
dried on sodium sulfate, and the solvent is removed in a vacuum. The
separation on
silica gel (hexane/ethyl acetate 0-15%) by column chromatography yields 0.51 g
of E-4-
(3-fluoro-2-methoxyphenyl)-2-hydroxy-2-(trifluoromethyl)-hex-4-enal and 1.2 g
of
alcohol.
'H-NMR (300 MHz, CDC13); 8 =1.85 (d, 3H), 3.31 (d, 1H), 3.42 (d, 1H), 3.71
(s, 1 H), 3.89 (s, 3H), 5.78 (q, 1 H), 6.67 (d, 1 H), 6.92 (ddd, 1 H), 7.00
(ddd, 1 H), 9.31 (s,
IH).
Analogously to Example 11, 77 mg of the correspondingly formed E-1-[(7,8-
:=;..:.
difluoro-2-methylquinazolin-5-yl)-imino]-4-(3-fluoro-2-methoxyphenyl)-2-
(trifluoromethyl)-hex-4-en-2-ol is treated with titanium tetrachloride
solution at -78 C
for 30 minutes. After working-up and chromatography on silica gel
(hexane/ethyl
acetate 50%), 4 mg of the desired product is obtained.
'H-NMR (300 MHz, CDC13); 8 =1.94 (d, 3H), 2.89 (s, 314), 2.92 (d, 1H), 2.99
(d, 1H), 3.88 (s, 3H), 4.79 (d, 1H), 5.69 (d, IH), 6.45 (dd,1H), 6.77 (q, 1H),
6.92 (dd,
1H), 6.93 (dd, 111), 9.28 (s, 1H).
CA 02577861 2007-02-21
= 66
Example 14
(cis/trans E)-4-Ethylidene-6-fluoro-5-methoxy-l-[(2-meth +lquinolin-5-
yl)aminol-2-
(trifluoromethyl)-1,2.3.4-tetrahvdronanhthalen-2-ol
Analogously to Example 11, 150 mg (0.49 mmol) of E-4-(3-fluoro-2-
methoxyphenyl)-2-hydroxy-2-(trifluoromethyl)-hex-4-enal is reacted with 78 mg
(0.49
mmol) of 5-amino-2-methylquinoline to form the corresponding E-4{3-fluoro-2-
methoxyphenyl} 1-[(2-methylquinzaolin-5-yl)imino]2-(trifluoromethyl)-hex-4-en-
2-ol.
The imine is taken up in 15 ml of dichloromethane and treated at -30 C with 5
ml (5
mmol) of a 1 M boron tribromide solution. It is allowed to heat to room
temperature
over 30 minutes, and the solution is poured into a mixture of ice and
saturated sodium
bicarbonate solution after 4 hours and vigorously stirred for 15 minutes. It
is extracted
with dichloromethane, washed with saturated sodium chloride solution and dried
on
sodium sulfate. After concentration by evaporation and chromatography on
silica gel
(hexane/ethyl acetate 30-60%), 44 mg of the title compound and 82 mg of the
compound
of Example 15 are obtained.
'H-NMR (300 MHz, CDC13); 8 =1.86 (d, 1.5H), 1.96 (d, 1.5H), 2.65 (d, 0.51-1),
2.72 (s, 3H), 2.92-2.97 (m, 1H), 3.04 (d, 0.5H), 2.99 (d, IH), 4.70 (d, 0.51-
1), 4.77-4.85
(m, 1 H), 4.94 (d, 0.51-1), 5.99 (q, 0.5H), 6.61 (d, 0.5H), 6.67-6.96 (m,
311), 7.24 (d, 1 H),
7.51 (m, 2H), 8.01 (d, 1H).
Example 15
(cis)-9-Fluoro-3-methyl- l -[(2-methylquinolin-5-vllaminol-5-(trifluoromethyl)-
5,6-
dihydro-dH-1-oxa-2-boraphenalene-2,5-diol
Production is described in Example 14.
CA 02577861 2007-02-21
67
1H-NMR (300 MHz, CDC13); S= 2.06 (s, 311), 2.71 (s, 314), 3.15 (d, 1 H), 3.32
(d, IH), 4.89 (d, IH), 5.19 (d, lH), 6.81 (d, 1H), 7.05 (m, 2H), 7.23 (d, 1H),
7.48-7.56
(m, 2H), 8.08 (d,1H).
Example 16
(cfs)-9-Fluoro-6-[(2-meth ylquinolin-5-yl)amino]-5-(trifluoromethvl)-2,4,5,6-
tetrahydrobenzo[de]chromen-5-ol
3-(8-Fluoro-2H-chromen-4 yl)-2-hydroxy-2-(trifluoromethyl) propanal:
33.2 g (296 mmoi) of 2-fluorophenol and 18.4 ml (281 mmol) of acrylonitrile
are
stirred together with 5.0 g (29.6 mmol) of benzyltrimethylanunonium hydroxide
for 4
days at 80 C. At room temperature, it is mixed with ice, and 2N hydrochloric
acid is
added. It is stirred for 10 minutes, extracted with ethyl acetate, washed with
saturated
sodium bicarbonate and sodium chloride solution, dried on sodium sulfate, and
the
solvent is removed in a vacuum. The separation on silica gel (hexane/ethyl
acetate 5-
50%) by column chromatography yields 6.4 g of 3-(2-fluorophenoxy)-
propionitrile. 6.4
g (38.8 mmol) of 3-(2-fluorophenoxy)-propionitrile is refluxed in 38.6 ml of
concentrated hydrochloric acid over 2 hours. At room temperature, it is
diluted with ice
water and stirred for 10 minutes. It is extracted with ethyl acetate, washed
with
saturated ammonium chloride solution and dried on sodium sulfate. After the
solvent is
removed in a vacuum, 6.5 g of 3-(2-fluorophenoxy)-propionic acid is obtained.
6.5 g
(35.6 nunol) of 3-(2-fluorophenoxy)-propionic acid is added to 39 g of
polyphosphoric
acid and stirrrnd for four hours at 70 C. After cooling overnight, it is
poured into ice
water. It is extracted with ethyl acetate, washed with saturated sodium
bicarbonate and
sodium chloride solution and dried on sodium sulfate. After the solvent is
removed in a
vacuum, 5.5 g of 8-fluorochroman-4-one is obtained as a crystalline solid.
CA 02577861 2007-02-21
68
29.8 g (456 mmol) of zinc dust and 710 mg (2.5 mmol) of lead(II) chloride are
suspended in 450 ml of THF, and 28.6 ml (253 mmol) of dibromomethane is added
at
room temperature. It is stirred for another 60 minutes, and 50.7 ml (50.7
mmol) of a I.
M titanium(IV) chloride solution in dichloromethane is added in drops over 40
minutes
while being cooled with ice. Afler one hour, 8.4 g (50.7 mmol) of 8-
fluorochroman-4-
one in 500 ml of THF is added in drops at room temperature. It is stirred for
another 18
hours at room temperature. It is diluted with diethyl ether, and the reaction
mixture is
carefully added to a mixture of 4 M hydrochloric acid and ice. The phases are
separated,
extracted with diethyl ether, washed with water, dried on sodium sulfate, and
the solvent
is removed. The crude product is purified by column chromatography on silica
gel
(hexane/isopropyl ether 0-20%), and 0.81 g of 8-fluoro-4-methylene-chroman is
obtained.
0.98 ml (0.49 mmol) of a 0.5 M titanium tetraisopropylate solution in toluene
is
added to 281 mg (0.98 mmol) of 1,1'-bi-2-naphthol, and the red solution is
stirred for 2
hours at room temperature. 0.81 g (4.9 mmol) of 8-fluoro-4-methylene-chroman
and
1.21 ml (9.8 mmol) of ethyltrifluoropyruvate are added, and the mixture is
heated over 3
hours to 120 C. After cooling, it is immediately purified by column
chromatography on
S
silica gel (hexane%thyl acetate 0-20%), and 0.69 g of 3-(8-fluoro-2H-chromen-4-
yl)-2-
hydroxy-2-(trifluoromethyl)-propionic acid ethyl ester is obtained. 345 mg
(1.0 mmol)
of 3-(8-fluoro-2H-chromen-4-yl)-2-hydroxy-2-(trifluoromethyl)-propionic acid
ethyl
ester is cooled in 10 ml of diethyl ether to -5 C, and 78 mg (2.0 mmol) of
lithium
aluminum hydride is added in portions in solid form over 10 minutes. It is
stirred for 2
hours at room temperature and poured into saturated ammonium chloride
solution. The
suspension is filtered through Celite and thoroughly rewashed with ethyl
acetate. The
phases of the filtrate are separated, and it is extracted again with ethyl
acetate. It is
CA 02577861 2007-02-21
69
washed with saturated sodium chloride solution, dried on sodium sulfate, and
the solvent
is removed in a vacuum. The chromatographic separation on silica gel
(hexane/ethyl
acetate 0-15%) yields 46 g of 3-(8-fluoro-2H-chromen-4-yl)-2-hydroxy-2-
(trifluoromethyl)-propanol and 71 mg of alcohol.
'H-NMR (300 MHz, CDC13); S= 3.07 (d, 1H), 3.25 (d, IH), 3.89 (s, 1 H), 4.77
(m, 2H), 5.79 (t, IH), 6.85 (ddd, 1H), 6.95-7.01 (m, 2H), 9.64 (s, 1H).
Analogously to Example 11, 46 mg (0.16 mmol) of 3-(8-fluoro-2H-chromen-4-
yl)-2-hydroxy-2-(trifluoromethyl)-propanal is reacted with 26 mg (0.17 mmol)
of 5-
amino-2-methylquinoline to form the corresponding 3-(8-fluoro-2H-chromen-4-yl)-
1-
[(2-methylquinazolin-5-yl)imino)-2-(trifluoromethyl)-propan-2-ol. The imine is
taken
up in 1.9 ml of dichloromethane and treated at -30 C with 0.95 ml (0.95 mmol)
of a I M
boron tribroniide solution. It is allowed to heat over 30 minutes to room
temperature,
and the solution is poured into a mixture of ice and saturated sodium
bicarbonate
solution and stirred vigorously for 5 minutes. It is extracted with
dichloromethane,
washed with saturated sodium chloride solution and dried on sodium sulfate.
After
concentration by evaporation and chromatography on silica gel (hexane/ethyl
acetate
30%), 7 mg of the desired product is obtained.
'H-NMR (300 MHz, CDC13); S= 2.74 (s, 3H), 2.92 (d, 1H), 3.25 (d, 1H), 4.51
(d, 1H), 4.84-4.98 (m, 2H), 5.02 (d, 1H), 6.74 (d, 1H), 6.83 (dd, 1H), 6.86
(dd, lH), 7.00
(ddd, 1H), 7.28 (d, 1H), 7.55 (t, IH), 7.59 (d, 1H), 8.12 (d, IH).
CA 02577861 2007-02-21
Example 17
(cis.Z1-2-Fluoro-5-[(2-methvlquinazolin-5- +~1 aminoj-8-(propylidene)-6-
(trifluoromethyi)-5.6,7.8-tetrahvdronaphthalene-1.6-diol
'~ .
iH-NMR (300 MHz, CD3OD); 8 =1.03 (t, 3H), 2.05 (ddq, 1H), 2.20 (ddq, 1H),
2.49 (d, IH), 2.77 (s, 3H), 3.08 (d,1H), 4.59 (s,1H), 5.67 (dd, lH), 6.43 (d,
1H), 6.73
(dd, IH), 6.93 (dd, lH), 7.13 (d, 1H), 7.65 (t, 1H), 9.59 (s, 1 H).
Example 18
E::"=~;~
(cis.E)-2-Fluoro-5-[(2-methvlquinawlin-5-yl)aminoj-8-propylidene-6-
(trifluoromethyl)-
5.6.7, 8-tetrahydronaphthalene-l.6-diol
'H-NMR (300 MHz, CD3OD); 8=1.12 (t, 3H), 2.28 (m, 2H), 2.76 (d, 1H), 2.77
(s, 3H), 2.99 (d, 1H), 4.96 (d,1H), 6.69 - 6.73 (m, 3H), 6.84 (dd,1H), 7.14
(d,1H), 7.69
(t, 1H), 9.59 (s, IH).
Example 19
(cis -2-Fluoro-5-[ 7-fluoro-2-methylquinazolin-5-yl)aminoj-B-Qropvlidene-6-
::> (trifluoromethy)-5.6.7.8-tetrahydronaphthalene-1,6-diol
'H-NMR (300 MHz, CDCl3); 8 = 1.08 (t, 3H), 2.15 (ddq, 1H), 2.25 (ddq, IH),
2.63 (d, l H), 2.79 (s, 3H), 3.23 (d, l H), 4.56 (d, 1 H), 5.82 (dd, 1 H),
6.21 (m, 2H), 6.80
(dd, 1 H), 6.88 (d, 1 H), 6.93 (dd, IH), 9.29 (s, IH).
Example 20
Lcis.E)-2-Fluoro-5-j(7-fluoro-2-methylquinazolin-5- l+j )aminoj-8-propylidene-
6-
(trifluorometh +~1-5.6,7.8-tetrahvdronaphthalene-1.6-diol
CA 02577861 2007-02-21
71
'H-NMR (300 MHz, CDC13); 8=1.08 (t, 3H), 2.12 (ddq, 1H), 2.21 (ddq,1H),
2.93 (s, 3H), 3.00 (m, 2H), 4.93 (d, IH), 6.44 (m, 2H), 6.87 - 6.96 (m, 3H),
(d,1H), 7.01
(dd,1H), 9.28 (s, 111).
Example 21
jcis -3-Chloro-8-ethylidene-2-fluoro-5-[(2-methylguinolin-5-vl aminol-6-
(trifluorometh}+1)-5,6,7,8-tetrahYdronaphthalene-1 L6-diol
'H-NMR (300 MHz, CD3OD); 8 =1,90 (d, 311), 2.74 (s, 311), 2.80 (d, IH), 3.04
(d,1H), 4.92 (s,1H), 6.38 (d, 1H), 6.80 (d,1H), 6.82 (q, 1H), 7.33 (d, 1H),
7.43 (d, 1H),
7.49 (t, 1 H), ; 8.48 (d, 1H).
Example 22
(cis,Z)-3-Chloro-8-ethylidene-2-fluoro-5-f(2-methvlquinolin-5-yl)aminol-6-
(trifluoromethvl)-5,6,7,8-tetrah3grgnaphtWene-1.6-diol
'H-NMR (300 MHz, CD3OD); 8 =1.77 (d, 314), 2.54 (d,1H), 2.75 (s, 3H), 3.14
(d, 1 H), 4.56 (s, 1 H), 5.86 (q, 1 H), 6.61 (d, 1 H), 6.81 (d, l H), 7.34 (d,
1 H), 7.44 (d, 1 H),
'cn~r 7.53 (t, 1 H), 8.50 (s, 1 H).
Example 23
(ci.s -3-Chloro-S-ethylidene-2-fluoro-5-[(2-methXlquinazolin-5-ylaminol-6-
(trifluoromethyl)-5,6.7,8-tetrahydronaphthalene- I .6-diol
1H-1VMR (300 MHz, CD3OD); 6 =1.78 (d, 311), 2.58 (d, 1H), 2.77 (s, 3H), 3.10
(d, 1H), 4.62 (s, 1H), 5.89 (q, 1H), 6.45 (d, 1H), 6.93 (d, 1H), 7.13 (d,
114), 7.65 (t, 1H),
9.59 (s, 1 H).
CA 02577861 2007-02-21
72
Example 24
(cis,Z)-3-Chloro-8-ethylidene-2-fluoro-5-1(7-fluoro-2-methylquinazolin-
5_yl)aminoL6-
(trifluoromethyl)-5.6,7,8-tetrahydronaphthalene-1,6-diol
1H-NMR (300 MHz, CD3OD); 8 =1.77 (d, 3H), 2.57 (d, lH), 2.81 (s, 3H), 3.14
(d, 1H), 4.64 (s,11-), 5.90 (q, 1H), 6.30 (d, 1H), 6.82 (d, 114), 6.88 (d,
1H), 7.13 (d,1H),
9.58 (s, 1H).
Example 25
(cis,Z)-3-Chloro-8-ethylidene-2-fluoro-5-[{8-fluoro-2-methylquinazolin-5-
yl)aminoj-6-
(trifluoromethyl)-5.6.7.8-tetrahydronaphthalene-l,6-diol
'H-NMR (300 MHz, CD30D); 8 =1.72 (d, 3H), 2.52 (d,1H), 2.84 (s, 3H), 3.10
(d, 1H), 4.54 (s, IH), 5.83 (q, IH), 6.39 (dd, IH), 6.86 (d, IH), 7.47
(dd,1H), 9.65 (s,
IH).
Example 26
cfsZ-3-Chloro-5-j(7,8-difluoro-2-methylguinazolin-5-vl)aminol-8-ethvlidene-2-
,
fluoroifluoromethvl)-5.6,7,8-tetrahydronaphthalene-l.6-diol
'H-NMR (300 MHz, CD30D); 8 =1.75 (d, 311), 2.54 (d, 1H), 2.80 (s, 3H), 3.11
(d, 1H), 4.58 (s, 1H), 5.82 (q, IH), 6.35 (dd, 1H), 6.86 (d, IH), 7.13 (d,
1H), 9.58 (s,
1 H).
Example 27
(crs 5-{[7-Chloro-2.5-dihydroxy-4-ethylidene-6-fluoro-2-trifluoromethyl-12 3 4-
tetrahydronanhthalen-l-yllaminol-quinolin-2( I H)-orie
CA 02577861 2007-02-21
73
'H-NMR (300 MHz, CD3OD); 8=1.74 (d, 31-1), 2.48 (d,1H), 3.07 (d, IH), 4.45
(s,1H), 5.82 (q, IH), 6.12 (d, 1H), 6.52 (d,1H), 6.66 (d, IH), 6.78 (d,1H),
7.26 (t, 1H),
8.20 (d, l H).
1:.
Example 28
tcis 5-{[7-Chloro-2.5-dihydroxy-4-ethylidene-6-fluoro-2-trifluoromethy1-
1,2,3,4-
tetrahydronaphthalen-l-vllaminol-quinolin-21 -one
'H-iVMR (300 MHz, CD3OD); 8=1.84 (d, 3H), 2.74 (d, 1H), 2.95 (d, IH), 4.82
(s, l H), 6.36 (d, 1 H), 6.52 (d, l H), 6.70 (d, l H), 6.78 (d, 1H), 6.80 (q,
l H), 7.32 (t, l H),
8.22 (d, IH).,
Example 29
jcis 5- j7-Chloro-2,5-dihydrox~thylidene-6-fluoro-2-trifluoromethyl-l.2,3 4-
tetrahydronWhthalen-l-Xll amino } -2-methy lphthalazin-l-one
'H-NMR (300 MHz, CD30D); 8 =1.71 (d, 3H), 2.50 (d, 1H), 3.09 (d, 1H), 3.81
(s, 3H), 4.52 (s, 1H), 5.81 (q, 1H), 6.76 (d, lH), 6.82 (d, 1H), 7.55 (t, 1H),
7.62 (d, IH),
8.53 (s, 1H).
Example 30
(cis -3-Chloro-8-ethylidene-2-fluoro-5-[(2-methvlquinazolin-5-yDaminoj-6-
(trifluoromethyl)-5,6,7, 8-tetrahydronaphthalene-1, 6-dioi
'H-NMR (300 MHz, CD3OD); 8 =1.86 (d, 314), 2.78 (s, 3H), 2.80 (d, IH), 2.97
(d, 1H), 4.97 (s, 1H), 6.71 (d, 1H), 6.78 (d, IH), 6.85 (q, 1 H), 7.17 (d,
1H), 7.71 (t, IH),
9.60 (s, 1H).
CA 02577861 2007-02-21
74
Example 31
(cis.E)-3-Chloro-8-ethylidene-2-fluoro-5-f(7-fluoro-2-meth +lauinazolin-5-
yl)aminol-6-
(trifluoromethyl)-5,6, 7.8-tetrahX~ ronaphthalene-l.6-diol
'H-NMR (300 MHz, CD3OD); 8 =1.91 (d, 3H), 2.80 (s, 311), 2.87 (d,1H), 3.01.
(d, 1 H), 5.07 (s, 1 H), 6.61 (d,1 H), 6.82 (d, 1 H), 6.83 (d, 1 H), 6.95 (q,
1 H), 9.59 (s, l H).
Example 32
(cis.E)-3-Chloro-5-[(7.8-difluoro-2-methylquinazolin-5A)aminol- S-ethvlidene-2-
;. .,~
fluoro-6-(trifluoromethyl)-5.6.7.8-tetrahvdronaphthalene-1,6-diot
'H-NMR (300 MHz, CD3OD); S= 1.87 (d, 3H), 2.78 (s, 314), 2.81 (d, 1H), 2.97
(d, 1H), 4.98 (s, 1H), 6.64 (dd, 1H), 6.79 (d, IH), 6.89 (q, 1H), 9.57 (s,1H).
Example 33
jcis -3-Chloro-8-ethvlidene-5-[(2-methylauinazolin-5-yl)aminol-l-methoxy-6-
(trifluoromethyl,)-5.6,7,8-tetrahydronaphthalen-6-ol
=::Y': 4
'H-NMR (300 MHz, CD3OD); 8 =1.89 (d, 3H), 2.78 (d, 1H), 2.80 (s, 3H), 3.08
(d, 1 H), 3.70 (s, 3H), 4.96 (s, l H), 6.68 (d, l H), 6.83 (q, l H), 6.98 (d,
1 H), 7.14 (d, 1 H),
7.18 (d,1H), 7.69 (t, 9.57 (s,1H).
Example 34
(cis.Z)-5,6-Difluoro-4-ethylidene-l-[(2-methylauinazolin-5-vl)amino12-
(trifluoromethy1L1.2,3.4-tetrahYdronaQhthalen-2-ol
CA 02577861 2007-02-21
'H-NMR (300 MHz, CDC13); 8 =1.83 (m, 3H), 2.72 (d,1H), 2.82 (s, 3H), 3.24
(d, 1H), 4.69 (d, 1H), 5.98 (q, 1H), 6.08 (d,1H), 6.54 (d, lH), 7.07 - 7.14
(m, 2H), 7.28
(d, 1H), 7.68 (t, 1H), 9.41 (s, 1H),
Example 35
(cis,E)-5,6-Difluoro-4-etliylidene-l-f(2-methylcuinazolin-5-vl)amino]-2-
(trifluoromethylkl ,2,3,4-tetrahydronaphthaten-2-ol
'H-NMR (300 MHz, CD30D); 8 =1.90 (d, 3H), 2.77 (s, 3H), 2.94 (m, 211), 5.15
~S'r ti!1
l H), 6.63 (q, l H), 6.80 (d,. l H), 6.98 - 7.09 (m, 2H), 7.17 (d, 1 H), 7.72
(t, 1 H), 9.60
(s, l H).
Example 36
rcis,Z1-5-Fluoro-4-ethylidene-l-[(2-methylquinazolin-5-vl)amino)-2-
(trifluorometh +
1,2,3,4-t.etrah~dronaphthalene-2.6-diol
'H-NMR (300 MHz, CD30D); 8 =1.74 (m, 3H), 2.57 (d, 1H), 2.77 (s, 3H), 3.08
(d, 1H), 4.71 (s,1H), 5.86 (q, 1H), 6.54 (d,1H), 6.80 (dd, IH), 6.92 (d, IH),
7.13 (d,
1H),7.67(t,1H),9.58(s,lH).
Example 37
cis Z}-5-{f2,6-Dihvdroxy-4-ethvlidene-5-fluoro-2-(trifluoromethvl 1 2 3 4-
tetrahydronaphthalen-l-yllamino} -quinolin-2(1 H)-one
'H-NMR (300 MHz, CD30D); 8 =1.66 (m, 311), 2.45 (d, 1H), 2.99 (d, 1H), 4.49
(s, 1 H), 5.76 (q, 1H), 6.14 (d, 1H), 6.43 (d, I H), 6.57 (d, 1H), 6.72 (t,
1H), 6.81 (d, 1 H),
7.18 (t, IH), 8.11(d, 1H).
CA 02577861 2007-02-21
76
Example 38
(cis,Z}-4-Ethlidene-7-fluoro-l-[(7-fluoro-2-methvlquinazolin-5-yl)amino]-2-
(trif luoromethy11,2,3,4tetrahydronaphthalene-2.6-diol
'H-NMR (300 MHz, CD3OD); 8 =1.97 (d, 3H), 2.66 (d, 1H), 2.74 (s, 3H), 2.94
(d, 1 H), 5.11 (s, 1H), 5.73 (q, I H), 6.62 (d, 1H), 6.73 (d, 1H), 6.95 (d, 1
H), 7.10 (d,1 H),
9.52 (s, 1H).
Example 39
~ ~..
jcis Ef-4-Ethvlidene-7-fluoro-l-[(7-fluoro-2-methyiquinazolin-5-vl)aminol-2-
(trifluoromefti)-1.2.3.4-tetrahydronanhthalene-2,6-diol
'H-NMR (300 MHz, CD3OD); 8= 1.83 (d, 3H), 2.73 (s, 3H), 2.76 (d,1H), 3.05
(d, 1 H), 5.22 (s, 1H), 6.27 (q,1 H), 6.69 (d, 1 H), 6.74 (d, 1 H), 6.86 (d,1
H), 7.18 (d, IH),
9.53 (s, 1H).
Example 40
(cis.Z)-5- ( I2.6-DihydroxY-4-ethylidene-7-fluoro-2-(trifluoromethvll-1.2,34-
~
: ~. tetrahYdronaphthalen-l- yllaminol-quinolin-2(lH)-one
'H-NMR (300 MHz, CD3OD); 8 =1.94 (d, 3H), 2.62 (d, 1H), 2.92 (d, iH), 4.90
(s, 114), 5.71 (q, IH), 6.49 (d, lH), 6.60 (d. iH), 6.68 (d, 1H), 6.92 (d,
1H), 7.03 (d, 1H),
7.33 (t, 1H), 8.18 (d, 1H).
Example 41
{cis,E)-5-{ f2.6-Dihydroxy-4-ethylidene-7-fluoro-2-(trifluoromethyl)-1.2.3.4-
tetrahydronaphthalen-l-vl] amino} -quinolin-2(1 H)-one
CA 02577861 2007-02-21
77
'H-NMR (300 MHz, CD3OD); 8 =1.84 (d, 3H), 2.74 (d, IH), 3.03 (d, 1 H), 5.06
(s,1 H), 6.24 (q, 1 H), 6.50 (d, 1 H), 6.57 (d, 1 H), 6.69 (d, 1 H), 6,87 (d,
1 H), 7.17 (d, IH),
7.35 (t, 1H), 8.20 (d, 11i).
t:.
Example 42
(cis, E)-7-Chloro-4-ethylidene-l-[(7-fluoro-2-methylquinazolin-5-yl)amino]-2-
(trifluoromethyl)-1.2.3 4-tetrahydronVhthalene-2 6-diol
'H-NMR (3001vII1z, CD3OD); 8 =1.85 (d, 3H), 2.69 (d, 1H), 2.75 (s, 3H), 3.04
(d,1H), 5.23 (s, 1H), 6.34 (q, 1H), 6.70 (d, IH), 6.78 (d, 1H), 7.11 (s, 1H),
7.18 (s, iH),
9.52 (s, 1H).,
Example 43
(cis. Z)-7-Chloro-4-ethylidene-l- [(8-fluoro-2-methylquinazolin-5-vl)amino)-2-
(trifluoromethyl)-1 2 3 4-tetrahydronaphthalene-2 6-diol
'H-NMR (300 MHz, CD3OD); S= 1.96 (d, 3H), 2.67 (d, 1H), 2.80 (s, 3H), 2.95
(d, 1 H), 5.00 (s, 1 H), 5.77 (q, 1 H), 6.72 (dd, 1 H), 7.07 (s, I H), 7.21
(s, I H), 7.51 (dd,
1H), 9.61 (s, IH).
Example 44
(cis.E)-7-Chloro-4-ethylidene-l-[(8-fluoro-2-meth +Iquinazolin-5-yl aminoL2-
(trifluoromethyl)-1.2 3.4-tetrahvdronaphthalene-2 6-diol
'H-NMR (300 MHz, CD3OD); S= 1.83 (d, 3H), 2.75 (d, IH), 2.80 (s, 3H), 3.01
(d, 1H), 5.12 (s, 1H), 6.31 (q, 1H), 6.78 (dd, 1H), 7.13 (s, 1H), 7.15 (s,
1H), 7.51 (dd,
1H), 9.62 (s,1H).
CA 02577861 2007-02-21
78
Example 45
(cis, Z)-7-Chloro-l-[(7.8-difluoro-2-methylquinazolin-5-vl)amino)-4-ethylidene-
2-
(trifluoromethvl)-i.2,3_4-tetrahvdronaphthalene-2 6-diol
'H-NMR (300 MHz, CD3OD); 8 =1.97 (d, 314), 2.68 (d, 1H), 2.78 (s, 3H), 2.94
(d, 1 H), 5.06 (s, 1 H), 5.78 (q, 1 H), 6.71 (dd, I H), 7.09 (s, 1 H), 7.19
(s, 111), 9.5 5(s, 1 H).
Example 46
(cis,E}-7-Chloro-l-[(7,8-difluoro-2-methvlquinaaolin-5-yl)amino]-4-ethylidene-
2-
.
(trifluoromethyl)-1,2,3,4-tetrahvdronaphthalene-2.6-diol
'H-NMR (300 MHz, CD3OD); 8 =1.84 (d, 3H), 2.77 (d, IH), 2.79 (s, 3H), 3.02
(d, I H), 5.16 (s, 1 H), 6.32 (q, 1 H), 6.79 (dd, 1H), 7.12 (s, 1 H), 7.17 (d,
1H); 9.56 (s,
1 H).
Example 47
(cis, Z)-5- { [7-Chloro-2,6-dihydroxy-4-ethylidene-2{trifluoromethyl)- I
,2,3,4-
tetrahydronaphthalen-l-yl]amino} -2-methylphthalazin-l-one
'H-NMR (300 MHz, CD3OD); 8 =1.96 (d, 3H), 2.65 (d, 1H), 2.94 (d, 111), 3.80
(s, 3H), 4.99 (s, l H), 5.77 (q, 1 H), 7.04 (s,1H), 7.06 (d, 1 H), 7.17 (s, 1
H), 7.59 (d, 1H),
7.60 (t, 1H), 8.46 (s, IH).
Example 48
(cis,E)-5- { [7-Chloro-2,6-dihydroxy-4-ethylidene-2-(trifluoromethyl)-1,2,3,4-
tetrahydronaphthalen-1-yl]amino}-2-methylphthalazin-l-one
CA 02577861 2007-02-21
79
'H-NMR (300 MHz, CD3OD); 8 = 1.82 (d, 3H), 2.72 (d,1H), 2.99 (d, 1H), 3.79
(s, 311), 5.12 (s, 1 H), 6.32 (q, 1 H), 7.06 (s, 1 H), 7.13 (s, 1 H), 7.14 (d,
1 H), 7.60 (m, 2H),
8.50 (s, 1H).
Example 49
(cis,E)-5-{[7-Chloro-2.6-dihydroxy-4-ethylidene-2-(trifluoromethyl)-1,2 3 4-
tetrahvdronaphthalen-1-L+llamino j -quinolin-2(1 H)-one
'H-NMR (300 MHz, DMSO-d6); 8 =1.75 (m, 3I3), 2.65 (d, 111), 2.81 (d,1H),
5.14 (d, 1 H), 6.08 - 6.19 (m, 2H), 6.37 (d, 1 H), 6.45 (d, 1 H), 6.55 (d, 1
H), 6.95 (s, 1 H),
7.18 (t, 1H), .7.19 (s, 1H), 8.14 (d, 1H),10.07 (s, IH), 11.54 (s, 1H).
Example 50
(cis)-2-Fluoro-8-isopropylidene-5-j(2-methylquinazolin-5-y)amino)-6-
(trifluoromethyl)-5,6.7.8-tetrahydronaphthalene-1 6-diol
1H-NMR (300 MI-Iz, CDC13); S= 1.86 (s, 311), 1.94 (s, 3H), 2.23 (d, 1H), 2.73
(s,
3H), 3.62 (d,1H), 4.53 (d, l H), 5.46 (d, 1 H), 6.34 (d, lH), 6.80 (dd, 1 H),
6.87 (dd, 1H),
Sk~~
7.20 - 7.42 (m, 3H), 8.21 (d, 1H).
Example 51
(cis)-2-Fluoro-8-isopropylidene-5-f (2-methvlquinazolin-5-vl)amino];6-
(trifluoromethvll-5.6 7 8-tetrahydronaphthalene-1 6-diol
'H-NMR (300 MHz, CD3OD); 8 =1.83 (s, 3H), 1.95 (s, 3H), 2.22 (d, 111), 2.83
(s, 314), 3.5 5 (d, I H), 4.55 (s, 1 H), 6.45 (d, 1 H), 6.77 (dd,1 H), 6.95
(dd, 1 H), 7.18 (d,
1H), 7.70 (t, 111), 9.65 (s, 1H).
CA 02577861 2007-02-21
Example 52
(cis)-8-Chloro-9-fluoro-3-methvl-1-j(2-methvlyuinolin-5-yllaminoJ-5-
(trifluoromethyl -5.6-dihydro-4H-l-oxa-2-boraphenalene-2 5-diol
'H-NMR (300 MHz, CD3OD); S= 2.03 (s, 3H), 2.74 (s, 3H), 3.05 (d,1H), 3.44
(d, 1H), 5.46 (s, i H), 6.84 (d, 1 H), 7.15 (d, 1 H), 7.36 (d, 1H), 7.42 (d,
1H), 7.58 (t, 1H),
8.51 (d, 111).
Without further elaboration, it is believed that one skilled in the art can,
using the
preceding description, utilize the present invention to its fullest extent.
The preceding '
preferred specific embodiments are, therefore, to be construed as merely
illustrative, and
not limitative of the remainder of the disclosure in any way whatsoever.
In the foregoing and in the examples, all temperatures are set forth
uncorrected in
degrees Celsius and, all parts and percentages are by weight, unless otherwise
indicated.
The entire disclosures of all applications, patents and publications, cited
herein
and of corresponding German application No. 10 2004 044 680.6, filed September
9,
2004, and U.S. Provisional Application Serial No. 60/615,604, filed October 5,
2004, are
incorporated by reference herein.
The preceding examples can be repeated with similar success by substituting
the
generically or specifically described reactants and/or operating conditions of
this
invention for those used in the preceding examples.
From the foregoing description, one skilled in the art can easily ascertain
the
essential characteristics of this invention and, without departing from the
spirit and
scope thereof, can make various changes and modifications of the invention to
adapt it to
various usages and conditions.