Note: Descriptions are shown in the official language in which they were submitted.
CA 02578169 2007-02-22
WO 2006/023930 PCT/US2005/029989
NOVEL DISTAL PORTIONS FOR MEDICAL ELECTRICAL LEADS
The present invention pertains to medical electrical leads and more
particularly to
pre-shaped distal lead portions.
Cardiac stimulation systems commonly include a pulse-generating device, such
as
a pacemaker or implantable cardioverter/defibrillator that is electrically
connected to the
heart by at least one medical electrical lead. A medical electrical lead
delivers electrical
pulses emitted by the pulse generator to the heart, stimulating the myocardial
tissue via
electrodes included on the lead. Cardiac signals may also be sensed by lead
electrodes
and conducted, via the lead, back to the device to monitor the electrical
activity of the
heart. These leads are coupled to the devices via connector terminals carrying
one or more
contact surfaces, which are in turn coupled to corresponding lead electrodes
by elongate
conductors extending within the lead.
In recent years, with the development of cardiac resynchronization therapy,
pacing
of the left ventricle has been achieved by implanting transvenous lead
electrodes in the
coronary venous system of the heart to stimulate an epicardial surface of the
left ventricle.
Precise placement of lead electrodes through the coronary veins is often
difficult, forcing
clinicians to work around sub-optimal pacing thresholds and/or unwanted extra-
cardiac
stimulation, for example phrenic nerve stimulation. Transvenous leads
including a
plurality of electrodes can provide an increased opportunity to provide more
optimal
pacing in that, once the lead is best positioned within a coronary vein, a
choice of pacing
sites is provided by the plurality of electrodes. Furthermore, pre-shaped
distal portions of
leads can enable stable placement of electrodes and enhance contact between
the
electrodes and electrically active cardiac muscle.
The following drawings are illustrative of particular embodiments of the
invention
and therefore do not limit its scope, but are presented to assist in providing
a proper
understanding of the invention. The drawings are not to scale (unless so
stated) and are
intended for use in conjunction with the explanations in the following
detailed description.
The present invention will hereinafter be described in conjunction with the
appended
drawings, wherein like numerals denote like elements, and:
CA 02578169 2007-02-22
WO 2006/023930 PCT/US2005/029989
-2-
Figure lA is a plan view of a medical electrical lead according to one
embodiment
of the present invention;
Figure 1B is a schematic of the lead of Figure lA implanted in a coronary
venous
system from an anterior perspective;
Figure 1 C is an enlarged view of a distal portion of the lead shown in Figure
1 A
implanted within a coronary vein;
Figure 2 is an enlarged detailed plan view of a lead electrode assembly
according
to one embodiment of the present invention; and
Figure 3 is an enlarged detailed section view of another lead electrode
assembly
according to another embodiment of the present invention.
The following detailed description is exemplary in nature and is not intended
to
limit the scope, applicability, or configuration of the invention in any way.
Rather, the
following description provides a practical illustration for implementing
exemplary
embodiments of the invention.
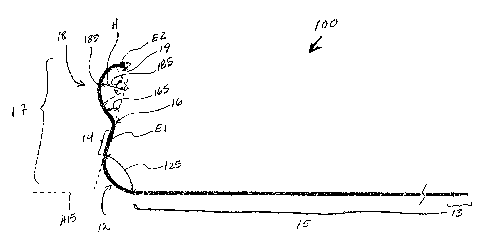
Figure lA is a plan view of a medical electrical lead 100 according to one
embodiment of the present invention. Figure lA illustrates lead 100 including
an
approximately straight proximal lead body portion 15, which is terminated at a
proximal
end by a lead connector 13, and a pre-formed distal lead body portion 17
extending
distally from proximal portion 15. Figure lA further illustrates distal lead
body portion 17
including a first arcuate segment 12 bending in a first direction, an
approximately straight
segment 14 extending from first arcuate segment 12, a second arcuate segment
16
extending from straight segment 14 and bending in a second, generally distal,
direction, a
third arcuate segment 18 bending in a third, generally proximal, direction,
and a distal tip
segment 19 extending from the third arcuate segment 18. According to the
illustrated
embodiment of the present invention, lead 100 further includes a first
electrode El
coupled to approximately straight segment 14 and second electrode coupled to
distal tip
segment 19; the position of pre-formed curves of arcuate segments of distal
portion 17
with respect to electrodes E1 and E2 provide for epicardial contact of
electrodes E1 and
E2 when implanted in a coronary vessel, as will be further described below.
Figure I A further illustrates angles 125, 165 and 185 of arcs included in
arcuate
segments 12, 16 and 18, respectively; according to some embodiments of the
present
invention, dimensions of the arcs are as indicated in Table 1.
CA 02578169 2007-02-22
WO 2006/023930 PCT/US2005/029989
-3-
Table 1: Arc Dimensions
Arcuate Segment Arc radius (inch) range Arc angle range
12 -0.2 - -0.3 Angle 125: -45 - -90
16 -0.2 - -0.4 Angle 165: -10 - -40
18 -0.1 - -0.4 Angle 185: -60 - -100
Furthermore, a length of straight segment 14, according to some embodiments,
is
from approximately 0.2 to approximately 0.7 inch and a length of distal tip
segment 19 is
from approximately 0.05 inch to approximately 0.2 inch. According to one
embodiment
electrode E2 terminates distal tip segment 19, which may or may not extend
proximally
from electrode; according to another embodiment a portion of distal tip
segment 19
extends distally from electrode E2 as illustrated by dashed lines in Figure 1
and this
extension may or may not be curved. Distal lead body portion 17 is alternately
described
as being canted, bending at angle 125 with respect to a longitudinal axis A15
of proximal
portion 15 and including a hump-like segment, corresponding to segment 18,
extending
from approximately straight segment 14 and having a distal apex 180. According
to one
embodiment of the present invention, the arc of segment 18 has a chord length
of
approximately 0.4 inch to approximately 0.7 inch and distal apex 180 of
segment 18 has a
height H of approximately 0.1 inch to approximately 0.3 inch.
General construction details concerning lead 100, for example of arrangement
of
conductors and insulation, coupling of electrodes to conductors, and assembly
of
connector 13, are well known to those skilled in the art. Conductors coupling
electrodes
El and E2 to connector contacts of connector 13 may be side-by-side cables or
coaxial
coils, either of which may be formed of wires made from MP35N alloy; and
insulation
formed about conductors for electrical isolation may formed of polyurethane,
fluoropolymers, silicone, polyimide or any combination thereof. Methods for
pre-forming
distal portion 17 include pre-forming of conductors extending therein and/or
sheaths
extending about the conductors; according to one method one or more sheaths
extending
between proximal lead body portion 15 and distal tip segment 17 are formed of
polyurethane, which is heat set into the preformed curve; such a method is
further
described in U.S. 5,999,858, which is incorporated herein by reference.
CA 02578169 2007-02-22
WO 2006/023930 PCT/US2005/029989
-4-
Figure 1B is a schematic of lead 100 implanted in a coronary venous system
193,
and Figure IC is an enlarged view of distal lead body portion 17 therein.
Figure 1B
illustrates lead 100 having been passed through a coronary sinus 191 into
coronary
vasculature 193 such that electrodes El and E2 are positioned for left
ventricular pacing.
According to some embodiments of the present invention both electrodes El and
E2 are
designed for pacing stimulation so that one of the two electrodes may be
selected for
ventricular pacing based on a preferred implant position; as illustrated in
Figure 1C, the
pre-formed curvature of distal lead body portion 17 assures that both
electrodes El and E2
contact a left ventricular epicardial surface 175. Electrodes E1 and E2 may
each have a
surface area ranging between approximately 2 square millimeters and
approximately 10
square millimeters and may be formed from any suitable material known to those
skilled
in the art, for example platinum-iridium and titanium. Dashed lines in Figure
1C show an
alternate distal lead body portion wherein a pre-formed hump (i.e. segment 18,
Figure lA)
is not included in order to illustrate a need for the hump when two electrodes
are included
in the distal lead body portion. Figure IC also shows how canted distal
portion 17 serves
to force electrode E2 into contact with epicardial surface 175.
Figure 1C further illustrates that pre-formed segments 12, 16 and 18 (Figure
IA) of
distal portion 17 are flexible to bend in compliance with external forces such
as that
applied by the vessel walls of coronary vasculature 193. These segments may
also be bent
in compliance with an internal force applied by a stylet inserted within a
lumen of lead
100.
Figure 2 is an enlarged detailed plan view of a lead electrode assembly,
corresponding to first electrode E1 illustrated in Figures lA-C, according to
one
embodiment of the present invention. Figure 2 illustrates approximately
straight segment
14 of distal lead body portion 17 extending away from electrode E1 toward
segment
12(Figure lA); EI may be positioned along segment 14 such that segment 14
further
extends in an opposite direction from electrode El, or such that electrode El
is in close
proximity or adjacent to second arcuate segment 16 (thus segment 14/16
indicated in
Figure 2). Figure 2 further illustrates electrode E1 including a central
portion having a
maximum diameter D2 that is greater than diameters D1 and D1' of segments 14
and
14/16, respectively, while either end of electrode El is approximately flush
with diameters
DI and Dl'. According to some embodiments of the present invention, a ratio of
diameter
D2 to diameters D1 and Dl' is from approximately 1.1 to approximately 1.6. It
is likely
CA 02578169 2007-02-22
WO 2006/023930 PCT/US2005/029989
-5-
that an active outer surface of electrode El in proximity to D2 will make best
contact with
epicardial tissue, for example epicardial surface 175 illustrated in Figure
1C.
According to the illustrated embodiment the active outer surface of electrode
El
has a generally arcuate profile and includes a recess 21, approximately
aligned with a
longitudinal center of electrode E 1 and in which a therapeutic or bioactive
agent 22 is
held, agent 22 being adapted to disperse out from recess 21 upon implantation
of electrode
El. According to an alternate embodiment, a recess holding an agent is offset
from the
longitudinal center of E1, as illustrated in Figure 2 with dashed lines in
proximity to
segment 14. Although Figure 1 illustrates recess extending about a
circumference of
electrode El, alternate embodiments of the present invention include recesses,
of a
generally macroscopic scale, which are discrete in nature and of various
orientations.
Other dashed lines in Figure 2 illustrate alternate profiles of agent 22
including arcuate
and flat profiles which may be either protruding, flush or recessed with
respect to adjacent
outer surface of electrode El. According to one set of embodiments of the
present
invention, agent 22 is embedded in a polymer matrix, and, according to a
particular
embodiment, agent 22 is an anti-inflammatory agent such as a steroid, for
example
dexamethasone sodium phosphate, dexamethasone acetate, or beclomethasone
diproprionate, embedded in a polyurethane or silicone matrix such that the
steroid may
elute from the matrix to prevent inflammation at the electrode contact site.
Methods for
forming such compounds for application in embodiments of the present invention
are well
known to those skilled in the art. According to another set of embodiments, a
surface of
recess 21 includes a microstructure in which agent 22 is embedded, for example
a
platinized surface in which beclomethasone is embedded.
Figure 3 is an enlarged detailed section view of another lead electrode
assembly,
corresponding to second electrode E2 illustrated in Figures lA-C, according to
another
embodiment of the present invention. Figure 3 illustrates lead 100 including a
lumen 30
formed by a conductor coil 31 and a core 33 to which conductor coil 31 and
electrode E2
are coupled; lumen 30 is terminated at a distal end of distal tip segment 19
with a resilient
element 34 mounted upon core 33 and adjacent to electrode E2. According to the
illustrated embodiment, element 34 is generally cup shaped and includes an
outer surface
302, which forms a portion of an external surface 32 of distal tip segment 19
of distal lead
body portion 17 (Figure IA), and an inner surface 300 adapted both to seal off
lumen 30
and to spread apart to allow passage of an elongate member, for example a
guide wire, by
nature of the resiliency of element 34. U.S. patent 6,192,280 describes in
part the
CA 02578169 2007-02-22
WO 2006/023930 PCT/US2005/029989
-6-
assembly illustrated in Figure 3 and is incorporated herein in its entirety.
According to
some embodiments of the present invention, element 34 further includes a
therapeutic or
bioactive agent embedded therein which is adapted to disperse out from outer
surface 302
upon implantation of lead 100. According to one embodiment, the agent is an
anti-
inflammatory agent such as a steroid, for example dexamethasone sodium
phosphate,
dexamethasone acetate, or beclomethasone diproprionate, and element 34 is
formed by
transfer molding a blend of the steroid (10%-50% by weight) and a silicone
rubber,
according to methods known to those skilled in the art of silicone molding.
In the foregoing detailed description, the invention has been described with
reference to specific embodiments. However, it may be appreciated that various
modifications and changes can be made without departing from the scope of the
invention
as set forth in the appended claims. For example, the inventive electrode
assemblies
described herein are not limited to the lead body embodiments described herein
and may
be incorporated in many types of medical electrical systems. Furthermore,
although
embodiments of the present invention have been described herein in the context
of cardiac
pacing from the coronary venous vasculature, the scope of the present
invention is not
limited to this particular application and embodiments of the present
invention may be
applied to other vessel-like environments.