Note: Descriptions are shown in the official language in which they were submitted.
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
ARENE RUTHENIUM (II) COMPOUNDS AND THEIR USE IN CANCER THERAPY
This invention relates to ruthenium (II) compounds, to their use in medicine,
particularly
for the treatment and/or prevention of cancer, and to a process for their
preparation.
WO 01/30790 and WO 02/02572 disclose ruthenium (II) compounds for use in the
treatment of cancer. These compounds can be described as half-sandwich
compounds,
having an arene ring bound to the ruthenium, as well as other non-arene
ligands. The
compounds exemplified in these applications have as one of the ligands a halo
atom.
Without wishing to be bound by theory, it is thought that the hydrolysis of
the halo atom
activates the complexes and allows them to bind to DNA.
The present inventors have studied the hydrolysis rates of a number of
different ligands
including halo and have surprisingly found that complexes containing ligands
that have
longer hydrolysis times still exhibit anti-tumour activity.
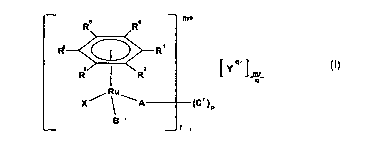
According to the present invention there is provided a ruthenium (I1) compound
of
formula (I):
RS R6 m+
4 R
R RZ L Y Q 1 mr
9
Ru
X~ 1 \A (C' )p
B
r
(I)
or a solvate or prodrug thereof, wherein:
R', R2, R3, R4, R5 and R6 are independently selected from H, Cl_7 alkyl, C5_20
aryl, C3_2o
heterocyclyl, halo, ester, amido, acyl, sulfo, sulfonamido, ether, thioether,
azo, amino, or
R' and R2 together with the ring to which they are attached form a saturated
or
unsaturated carbocyclic or heterocyclic group containing up to three 3- to 8-
membered
carbocyclic or heterocyclic rings, wherein each carbocyclic or heterocyclic
ring may be
fused to one or more other carbocyclic or heterocyclic rings;
X is a neutral or negatively charged N- or S- donor ligand;
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
2
Y is a counterion;
mis0or1;
qis1,2or3;
C' is CI_12 alkylene bound to two A groups;
pis0orl and ris 1 when pis0and ris2when pisl; and
A and B are each independently 0-donor, N-donor or S-donor ligands.
When p is 1, the ligand A is bound to another ligand A such that the compound
comprises two ruthenium atoms. Such complexes are called dinuclear complexes.
Ligands A and B may be connected to one another, but they cannot be bound to
ligand
X.
A second aspect of the present invention provides a composition comprising a
compound of the first aspect and a pharmaceutically acceptable carrier or
diluent.
A third aspect of the invention provides the use of a compound of the first
aspect in a
method of therapy.
A fourth aspect of the invention provides the use of a compound of the first
aspect in the
preparation of a medicament for the treatment of cancer.
A fifth aspect of the invention provides a method of treatment of a subject
suffering from
cancer, comprising administering to such a subject a therapeutically-effective
amount of
a compound of the first aspect, preferably in the form of a pharmaceutical
composition.
Definitions
N-donor ligands: N-donor ligands are ligands which bind to a metal atom via a
nitrogen
atom. They are well known in the art and include: nitrile ligands (N=C-R); azo
ligands
(N=N-R); aromatic N-donor ligands; amine ligands (NR"'RN2RN); azide (N3 );
cyanide
(N=C-); isothiocyanate (NCS-).
In both nitrile and azo ligands R may be selected from Ci_7 alkyl and C5_20
aryl.
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
3
Aromatic N-donor ligands include optionally substituted pyridine, pyridazine,
pyrimidine,
purine and pyrazine. The optional substituents may be selected from cyano,
halo and
CI-7 alkyl.
R"', R"2 and RN3 may be independently selected from H and CI-7 alkyl, or if A
and B are
both amine ligands, R"' on each ligand join together to form a CI-7 alkylene
chain.
When p is 1, RN2 on each A ligand join together form the group C' which is
CI_12 alkylene.
S-donor ligands: S-donor ligands are ligands which bind to a metal atom via a
sulphur
atom. They are well known in the art and include: thiosulfate (S203Z-);
isothiocyanate
(NCS"); thiocyanate (CNS"); sulfoxide ligands (Rs'RS2SO); thioether ligands
(RS'RSZS);
thiolate ligands (RS'S"); sulfinate ligands (Rs'S02 ); and sulfenate ligands
(Rs'SO"),
wherein RS' and RS2 are independently selected from CI-7 alkyl and C5_20 aryl,
which
groups may be optionally substituted.
0-donor ligands: 0-donor ligands are ligands which bind to a metal atom via an
oxygen
atom. They are well known in the art and include: carbonate (C03 );
carboxylate ligands
(RcCOz ); nitrate (N03 ); sulfate (S042-) and sulphonate (RS'03 ), wherein Rc
is selected
from CI-7 alkyl and C5_20 aryl and RS' is as defined above.
Cl_7 Alkyl: The term "Cl_7 alkyl", as used herein, pertains to a monovalent
moiety
obtained by removing a hydrogen atom from a carbon atom of a hydrocarbon
compound
having from 1 to 7 carbon atoms, which may be aliphatic or alicyclic, and
which may be
saturated or unsaturated (e.g., partially unsaturated, fully unsaturated).
Thus, the term
"alkyl" includes the sub-classes alkenyl, alkynyl, cycloalkyl, cycloalkyenyl,
cylcoalkynyl,
etc., discussed below.
Examples of saturated Cl_7 alkyl groups include, but are not limited to,
methyl (CI), ethyl
(CZ), propyl (C3), butyl (C4), pentyl (C5), hexyl (C6) and heptyl (CA
Examples of saturated linear CI-7 alkyl groups include, but are not limited
to, methyl (CI),
ethyl (C2), n-propyl (C3), n-butyl (C4), n-pentyl (amyl) (C5), n-hexyl (C6),
and n-heptyl (C7).
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
4
Examples of saturated branched Cl_7alkyl groups include iso-propyl (C3), iso-
butyl (C4),
sec-butyl (C4), tert-butyl (C4), iso-pentyl (C5), and neo-pentyl (C5).
C2_7 Alkenyl: The term "Ca_7 alkenyl", as used herein, pertains to an alkyl
group having
one or more carbon-carbon double bonds. Examples of C2_7 alkenyl groups
include, but
are not limited to, ethenyl (vinyl, -CH=CH2), 1-propenyl (-CH=CH-CH3), 2-
propenyl (allyl,
-CH-CH=CH2), isopropenyl (1-methylvinyl, -C(CH3)=CH2), butenyl (Ca), pentenyl
(C5),
and hexenyl (Cs).
C2_7 Alkynyl: The term "C2_7 alkynyl", as used herein, pertains to an alkyl
group having
one or more carbon-carbon triple bonds. Examples of C2_7 alkynyl groups
include, but
are not limited to, ethynyl (ethinyl, -C CH) and 2-propynyl (propargyl, -CHa-
C=CH).
C3_7 Cycloalkyl: The term "C3_7 cycloalkyl", as used herein, pertains to an
alkyl group
which is also a cyclyl group; that is, a monovalent moiety obtained by
removing a
hydrogen atom from an alicyclic ring atom of a carbocyclic ring of a
carbocyclic
compound, which carbocyclic ring may be saturated or unsaturated (e.g.,
partially
unsaturated, fully unsaturated), which moiety has from 3 to 7 carbon atoms.
Thus, the
term "C3_7 cycloalkyl" includes the sub-classes cycloalkyenyl and
cycloalkynyl. Examples
of cycloalkyl groups include, but are not limited to, those derived from:
saturated hydrocarbon compounds:
cyclopropane (C3), cyclobutane (C4), cyclopentane (C5), cyclohexane (C6),
cycloheptane
(CA methylcyclopropane (C4), dimethylcyclopropane (C5), methylcyclobutane
(C5),
dimethylcyclobutane (C6), methylcyclopentane (C6), dimethylcyclopentane (CA
methylcyclohexane (C7); and
unsaturated hydrocarbon compounds:
cyclopropene (C3), cyclobutene (C4), cyclopentene (C5), cyclohexene (CO,
methylcyclopropene (C4), dimethylcyclopropene (C5), methylcyclobutene (C5),
dimethylcyclobutene (C6), methylcyclopentene (C6), dimethylcyclopentene (C7).
The alkyl groups in the compounds of the invention may optionally be
substituted.
Substituents include one or more further alkyl groups and/or one or more
further
substituents, such as, for example, C5_20 aryl (e.g. benzyl), C3_20
heterocyclyl, cyano (-
CN), nitro (-NO2), hydroxyl (-OH), ester, halo, thiol (-SH), thioether and
sulfonate (-
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
S(=O)z)OR, where R is wherein R is a sulfonate substituent, for example, a
Ci_7 alkyl
group, a C3-2o heterocyclyl group, or a C5-2o aryl group, preferably a CI-7
alkyl group).
C2-12 alkylene: The term "C2-12 alkylene" is defined similarly to the
definition of the term
5"alkyP' but includes C2 to C12 groups and is a divalent species with radicals
separated by
two or more (e.g. from two to twelve) carbon atoms linked in a chain.
Preferably, the
alkylene groups are straight chain groups. C2-12 alkylene groups are
optionally
substituted in the akylene chain, preferably with one or more phenylene (eg, 1-
4-
phenylene) and/or -CONR'a- groups and/or -NR2a- groups, where R'a and R2a
independently represent H, CI-7 alkyl, C3-20 heterocyclyl or C5-ZO aryl.
Preferably, R'a and
R2a are H or C, to C3 alkyl.
C5_20 Aryl: The term "C5-ao aryl", as used herein, pertains to a monovalent
moiety
obtained by removing a hydrogen atom from an aromatic ring atom of an aromatic
compound, which moiety has from 3 to 20 ring atoms. Preferably, each ring has
from 5
to 7 ring atoms.
In this context, the prefixes (e.g., C3-20, C5a, C5-6, etc.) denote the number
of ring atoms,
or range of number of ring atoms, whether carbon atoms or heteroatoms. For
example,
the term "C5-6 aryl", as used herein, pertains to an aryl group having 5 or 6
ring atoms.
The ring atoms may be all carbon atoms, as in "carboaryl groups". Examples of
carboaryl groups include C3-20 carboaryl, C5-20 carboaryl, C5-15 carboaryl, C5-
12 carboaryl,
C5-10 carboaryl, C5-7 carboaryl, C5_6 carboaryl, C5 carboaryl, and C6
carboaryl.
Examples of carboaryl groups include, but are not limited to, those derived
from benzene
(i.e., phenyl) (C6), naphthalene (Clo), azulene (Clo), anthracene (C14),
phenanthrene
(C14), naphthacene (CI$), and pyrene (C16).
Examples of aryl groups which comprise fused rings, at least one of which is
an aromatic
ring, include, but are not limited to, groups derived from indane (e.g., 2,3-
dihydro-1 H-
indene) (C9), indene (C9), isoindene (C9), tetraline (1,2,3,4-
tetrahydronaphthalene (Clo),
acenaphthene (C12), fluorene (C13), phenalene (C13), acephenanthrene (C15),
and
aceanthrene (C16).
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
6
Alternatively, the ring atoms may include one or more heteroatoms, as in
"heteroaryl
groups". Examples of heteroaryl groups include C3_20 heteroaryl, C5-2o
heteroaryl, C5-15
heteroaryl, C5-12 heteroaryl, C5_10 heteroaryl, C5-7 heteroaryl, C5.6
heteroaryl, C5 heteroaryl,
and C6 heteroaryl.
Examples of monocyclic heteroaryl groups include, but are not limited to,
those derived
from:
N1: pyrrole (azole) (C5), pyridine (azine) (C6);
O1: furan (oxole) (C5);
S1: thiophene (thiole) (C5);
N1O1: oxazole (C5), isoxazole (C5), isoxazine (C6);
N201: oxadiazole (furazan) (C5);
N301: oxatriazole (C5);
N1S1: thiazole (C5), isothiazole (C5);
N2: imidazole (1,3-diazole) (C5), pyrazole (1,2-diazole) (C5), pyridazine (1,2-
diazine) (C6),
pyrimidine (1,3-diazine) (C6) (e.g., cytosine, thymine, uracil), pyrazine (1,4-
diazine) (C6);
N3: triazole (C5), triazine (C6); and,
N4: tetrazole (C5).
Examples of heteroaryl groups which comprise fused rings, include, but are not
limited
to:
C9 heteroaryl groups (with 2 fused rings) derived from benzofuran (O1),
isobenzofuran (01), indole (N1), isoindole (N1), indolizine (N1), indoline
(N1), isoindoline
(N1), purine (N4) (e.g., adenine, guanine), benzimidazole (NA indazole (N2),
benzoxazole
(N101), benzisoxazole (N101), benzodioxole (02), benzofurazan (N201),
benzotriazole
(N3), benzothiofuran (S1), benzothiazole (N1S1), benzothiadiazole (N2S);
C10 heteroaryl groups (with 2 fused rings) derived from chromene (01),
isochromene (O1), chroman (O1), isochroman (O1), benzodioxan (02), quinoline
(N1),
isoquinoline (N1), quinolizine (N1), benzoxazine (N101), benzodiazine (NZ),
pyridopyridine (N2), quinoxaline (NZ), quinazoline (N2), cinnoline (N2),
phthalazine (NZ),
naphthyridine (NZ), pteridine (N4);
C11 heteroaryl groups (with 2 fused rings) derived from benzodiazepine (N2);
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
7
C13 heteroaryl groups (with 3 fused rings) derived from carbazole (Ni),
dibenzofuran (01), dibenzothiophene (Sj), carboline (NA perimidine (NA
pyridoindole
(N2); and,
C14 heteroaryl groups (with 3 fused rings) derived from acridine (NJ),
xanthene
(O1), thioxanthene (SI), oxanthrene (02), phenoxathiin (O1S1), phenazine (Na),
phenoxazine (N101), phenothiazine (N1S1), thianthrene (SA phenanthridine (NJ),
phenanthroline (NZ), phenazine (N2).
Cs-zo aryl groups may optionally be substituted with one or more substituents
including,
for example, CI_7 alkyl, C5_20 aryl, C3_20 heterocyclyi, cyano, nitro,
hydroxyl, ester, halo,
thiol, thioether and sulfonate.
Ca-2o Heterocyclyl: The term "C3_2o heterocyclyi", as used herein, pertains to
a
monovalent moiety obtained by removing a hydrogen atom from a ring atom of a
heterocyclic compound, which moiety has from 3 to 20 ring atoms, of which from
1 to 10
are ring heteroatoms. Preferably, each ring has from 3 to 7 ring atoms, of
which from 1
to 4 are ring heteroatoms.
In this context, the prefixes (e.g., C3_20, Caa, C5-6, etc.) denote the number
of ring atoms,
or range of number of ring atoms, whether carbon atoms or heteroatoms. For
example,
the term "C5_6heterocyclyP", as used herein, pertains to a heterocyclyl group
having 5 or 6
ring atoms. Examples of groups of heterocyclyl groups include C3_2o
heterocyclyl, C5_2o
heterocyclyl, C3-15 heterocyclyi, C5_15 heterocyclyl, C3-12 heterocyclyl,
C5_12 heterocyclyl,
C3-10 heterocyclyl, C5_10 heterocyclyl, C3-7 heterocyclyl, C5_7 heterocyclyl,
and C5-6
heterocyclyl.
Examples of monocyclic heterocyclyl groups include, but are not limited to,
those derived
from:
N1: aziridine (C3), azetidine (C4), pyrrolidine (tetrahydropyrrole) (C5),
pyrroline (e.g.,
3-pyrroline, 2,5-dihydropyrrole) (C5), 2H-pyrrole or 3H-pyrrole (isopyrrole,
isoazole) (C5),
piperidine (C6), dihydropyridine (C6), tetrahydropyridine (C6), azepine A);
O1: oxirane (C3), oxetane (C4), oxolane (tetrahydrofuran) (C5), oxole
(dihydrofuran) (C5),
oxane (tetrahydropyran) (C6), dihydropyran (C6), pyran (C6), oxepin (C7);
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
8
Si: thiirane (C3), thietane (C4), thiolane (tetrahydrothiophene) (C5), thiane
(tetrahydrothiopyran) (C6), thiepane (C7);
02: dioxolane (C5), dioxane (C6), and dioxepane P);
03: trioxane (C6);
N2: imidazolidine (C5), pyrazolidine (diazolidine) (C5), imidazoline (C5),
pyrazoline
(dihydropyrazole) (C5), piperazine (C6);
N101: tetrahydrooxazole (C5), dihydrooxazole (C5), tetrahydroisoxazole (C5),
dihydroisoxazole (C5), morpholine (C6), tetrahydrooxazine (C6), dihydrooxazine
(C6),
oxazine (C6);
NjSj: thiazoline (C5), thiazolidine (C5), thiomorpholine (C6);
N201: oxadiazine (C6);
OjSj: oxathiole (C5) and oxathiane (thioxane) (C6); and
NjOjSj: oxathiazine (C6).
C3_20 heterocyclyl groups may optionally be substituted with one or more
substituents
including, for example, Cl_7 alkyl, C5_20 aryl, C3_2o heterocyclyl, cyano,
nitro, hydroxyl,
ester, halo, thiol, thioether and sulfonate.
Halo: -F, -Cl, -Br, and -I.
Ester (carboxylate, carboxylic acid ester, oxycarbonyl): -C(=0)OR, wherein R
is an ester
substituent, for example, a CI_7 alkyl group, a C3_20 heterocyclyl group, or a
C5_20 aryl
group, preferably a C1_7 alkyl group. Examples of ester groups include, but
are not
limited to, -C(=0)OCH3i -C(=0)OCH2CH3, -C(=0)OC(CH3)3, and -C(=0)OPh.
Amino: -NR'R2, wherein R' and R2 are independently amino substituents, for
example,
hydrogen, a CI_7 alkyl group (also referred to as CI_7 alkylamino or di-CI_7
alkylamino), a
C3_20 heterocyclyl group, or a C5_20 aryl group, preferably H or a Cl_7 alkyl
group, or, in the
case of a "cyclic" amino group, R' and R2, taken together with the nitrogen
atom to which
they are attached, form a heterocyclic ring having from 4 to 8 ring atoms.
Amino groups
may be primary (-NH2), secondary (-NHR'), or tertiary (-NHR'R2), and in
cationic form,
may be quaternary (-+NR'RZR3). Examples of amino groups include, but are not
limited
to, -NH2, -NHCH3, -NHC(CH3)2, -N(CH3)2, -N(CH2CH3)2, -NHCH2Ph and -NHPh.
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
9
Examples of cyclic amino groups include, but are not limited to, aziridino,
azetidino,
pyrrolidino, piperidino, piperazino, morpholino, and thiomorpholino.
Amido (carbamoyl, carbamyl, aminocarbonyl, carboxamide): -C(=O)NR'R2, wherein
R'
and R2 are independently amino substituents, as defined for amino groups.
Examples of
amido groups include, but are not limited to, -C(=O)NH2, -C(=O)NHCH3, -
C(=O)N(CH3)2,
-C(=O)NHCHaCH3, and -C(=O)N(CH2CH3)2, as well as amido groups in which R' and
R2,
together with the nitrogen atom to which they are attached, form a
heterocyclic structure
as in, for example, piperidinocarbonyl, morpholinocarbonyl,
thiomorpholinocarbonyl, and
piperazinocarbonyl.
Acyl (keto): -C(=O)R, wherein R is an acyl substituent, for example, a Cl_7
alkyl group
(also referred to as Cl_7 alkylacyl or Cl_7 alkanoyl), a C3_20 heterocyclyl
group (also
referred to as C3_2o heterocyclylacyl), or a C5_20 aryl group (also referred
to as C5_20
arylacyl), preferably a CI_7 alkyl group. Examples of acyl groups include, but
are not
limited to, -C(=O)CH3 (acetyl), -C(=O)CH2CH3 (propionyl), -C(=O)C(CH3)3 (t-
butyryl), and
-C(=O)Ph (benzoyl, phenone).
Sulfo: -S(=O)20H, -SO3H.
Sulfonamido (sulfinamoyl; sulfonic acid amide; sulfonamide): -S(=O)2NR'R2,
wherein R'
and R2 are independently amino substituents, as defined for amino groups.
Examples of
sulfonamido groups include, but are not limited to, -S(=O)2NH2, -
S(=O)2NH(CH3),
-S(=O)2N(CH3)2, -S(=O)2NH(CH2CH3), -S(=O)ZN(CHaCH3)2, and -S(=O)ZNHPh.
Ether: -OR, wherein R is an ether substituent, for example, a Cl_7 alkyl group
(also
referred to as a Cl_7 alkoxy group), a C3_20 heterocyclyl group (also referred
to as a C3_20
heterocyclyloxy group), or a C5_20 aryl group (also referred to as a C5_20
aryloxy group),
preferably a Cl_7 alkyl group.
Thioether (sulfide): -SR, wherein R is a thioether substituent, for example, a
Cl_7 alkyl
group (also referred to as a Cl_7 alkylthio group), a C3_20 heterocyclyl
group, or a C5_20 aryl
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
group, preferably a Cl_7 alkyl group. Examples of Cl_7 alkylthio groups
include, but are
not limited to, -SCH3 and -SCH2CH3.
Azo: -N=N-R, where R is an azo substituent, for example a CI_7 alkyl group, a
C3_20
5 heterocyclyl group, or a C5_20 aryl group, preferably a Cl_7 alkyl group.
Examples of azo
groups include, but are not limited to, -N=N-CH3 and -N=N-Ph.
Heterocyclic ring: The term "heterocyclic ring" as used herein refers to a 3-,
4-, 5-, 6-, 7-,
or 8- (preferably 5-, 6- or 7-) membered saturated or unsaturated ring, which
may be
10 aromatic or non-aromatic, containing from one to three heteroatoms
independently
selected from N, 0 and S, e.g. indole (also see above).
Carbocyclic ring: The term "carbocyclic ring" as used herein refers to a
saturated or
unsaturated ring, which may be aromatic or non-aromatic, containing from 3 to
8 carbon
atoms (preferably 5 to 7 carbon atoms) and includes, for example,
cyclopropane,
cyclobutane, cyclopentane, cyclohexane and cycloheptane (also see above).
Includes Other Forms
Unless otherwise specified, included in the above are the well known ionic,
solvate, and
protected forms of these substituents. For example, a reference to carboxylic
acid
(-COOH) also includes the anionic (carboxylate) form (-COO") or solvate
thereof, as well
as conventional protected forms. Similarly, a reference to an amino group
includes the
protonated form (-N+HR'R2) or solvate of the amino group, as well as
conventional
protected forms of an amino group. Similarly, a reference to a hydroxyl group
also
includes the anionic form (-O") or solvate thereof, as well as conventional
protected
forms.
Isomers
Certain compounds may exist in one or more particular geometric, optical,
enantiomeric,
diasteriomeric, epimeric, atropic, stereoisomeric, tautomeric, conformational,
or anomeric
forms, including but not limited to, cis- and trans-forms; E- and Z-forms; c-,
t-, and r-
forms; endo- and exo-forms; R-, S-, and meso-forms; D- and L-forms; d- and I-
forms; (+)
and (-) forms; keto-, enol-, and enolate-forms; syn- and anti-forms; synclinal-
and
anticlinal-forms; a- and R-forms; axial and equatorial forms; boat-, chair-,
twist-,
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
11
envelope-, and halfchair-forms; and combinations thereof, hereinafter
collectively
referred to as "isomers" (or "isomeric forms").
Note that, except as discussed below for tautomeric forms, specifically
excluded from the
term "isomers," as used herein, are structural (or constitutional) isomers
(i.e., isomers
which differ in the connections between atoms rather than merely by the
position of
atoms in space). For example, a reference to a methoxy group, -OCH3, is not to
be
construed as a reference to its structural isomer, a hydroxymethyl group, -
CH2OH.
Similarly, a reference to ortho-chlorophenyl is not to be construed as a
reference to its
structural isomer, meta-chlorophenyl. However, a reference to a class of
structures may
well include structurally isomeric forms falling within that class (e.g.,
CI_7alkyl includes
n-propyl and iso-propyl; butyl includes n-, iso-, sec-, and tert-butyl;
methoxyphenyl
includes ortho-, meta-, and para-methoxyphenyl).
The above exclusion does not pertain to tautomeric forms, for example, keto-,
enol-, and
enolate-forms, as in, for example, the following tautomeric pairs: keto/enol
(illustrated
below), imine/enamine, amide/imino alcohol, amidine/amidine, nitroso/oxime,
thioketone/enethiol, N-nitroso/hydroxyazo, and nitro/aci-nitro.
H 0 ,OH H+ O-
-i-C~ ~ ~C=C~ ZZ=2= C=C
H+
keto enol enolate
Note that specifically included in the term "isomer" are compounds with one or
more
isotopic substitutions. For example, H may be in any isotopic form,
including'H, 2H (D),
and 3H (T); C may be in any isotopic form, including12C,13C, and'''C; 0 may be
in any
isotopic form, including160 and 180; and the like.
Unless otherwise specified, a reference to a particular compound includes all
such
isomeric forms, including (wholly or partially) racemic and other mixtures
thereof, for
example, a mixture enriched in one enantiomer. Methods for the preparation
(e.g., asymmetric synthesis) and separation (e.g., fractional crystallisation
and
chromatographic means) of such isomeric forms are either known in the art or
are readily
obtained by adapting the methods taught herein, or known methods, in a known
manner.
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
12
Solvates
It may be convenient or desirable to prepare, purify, and/or handle a
corresponding
solvate of the active compound. The term "solvate" is used herein in the
conventional
sense to refer to a complex of solute (e.g., active compound, salt of active
compound)
and solvent. If the solvent is water, the solvate may be conveniently referred
to as a
hydrate, for example, a mono-hydrate, a di-hydrate, a tri-hydrate, etc.
Unless otherwise specified, a reference to a particular compound also include
solvate
forms thereof.
Chemically Protected Forms
It may be convenient or desirable to prepare, purify, and/or handle the active
compound
in a chemically protected form. The term "chemically protected form" is used
herein in
the conventional chemical sense and pertains to a compound in which one or
more
reactive functional groups are protected from undesirable chemical reactions
under
specified conditions (e.g., pH, temperature, radiation, solvent, and the
like). In practice,
well known chemical methods are employed to reversibly render unreactive a
functional
group, which otherwise would be reactive, under specified conditions. In a
chemically
protected form, one or more reactive functional groups are in the form of a
protected or
protecting group (also known as a masked or masking group or a blocked or
blocking
group). By protecting a reactive functional group, reactions involving other
unprotected
reactive functional groups can be performed, without affecting the protected
group; the
protecting group may be removed, usually in a subsequent step, without
substantially
affecting the remainder of the molecule. See, for example, Protective Groups
in Organic
Synthesis (T. Green and P. Wuts; 3rd Edition; John Wiley and Sons, 1999).
Unless otherwise specified, a reference to a particular compound also includes
chemically protected forms thereof.
A wide variety of such "protecting," "blocking," or "masking" methods are
widely used
and well known in organic synthesis. For example, a compound which has two
nonequivalent reactive functional groups, both of which would be reactive
under
specified conditions, may be derivatized to render one of the functional
groups
"protected," and therefore unreactive, under the specified conditions; so
protected, the
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
13
compound may be used as a reactant which has effectively only one reactive
functional
group. After the desired reaction (involving the other functional group) is
complete, the
protected group may be "deprotected" to return it to its original
functionality.
For example, a hydroxy group may be protected as an ether (-OR) or an ester
(-OC(=O)R), for example, as: a t-butyl ether; a benzyl, benzhydryl
(diphenylmethyl), or
trityl (triphenylmethyl) ether; a trimethylsilyl or t-butyidimethylsilyl
ether; or an acetyl ester
(-OC(=O)CH3, -OAc).
For example, an aidehyde or ketone group may be protected as an acetal (R-
CH(ORM
or ketal (RZC(OR)2), respectively, in which the carbonyl group (>C=O) is
converted to a
diether (>C(OR)2), by reaction with, for example, a primary alcohol. The
aldehyde or
ketone group is readily regenerated by hydrolysis using a large excess of
water in the
presence of acid.
For example, an amine group may be protected, for example, as an amide (-NRCO-
R) or
a urethane (-NRCO-OR), for example, as: a methyl amide (-NHCO-CH3); a
benzyloxy
amide (-NHCO-OCH2C6H5, -NH-Cbz); as a t-butoxy amide (-NHCO-OC(CH3)3, -NH-
Boc);
a 2-biphenyl-2-propoxy amide (-NHCO-OC(CH3)2C6H4C6H5r -NH-Bpoc), as a 9-
fluorenylmethoxy amide (-NH-Fmoc), as a 6-nitroveratryloxy amide (-NH-Nvoc),
as a
2-trimethylsilyiethyloxy amide (-NH-Teoc), as a 2,2,2-trichloroethyloxy amide
(-NH-Troc),
as an allyloxy amide (-NH-Alloc), as a 2(-phenylsulphonyl)ethyloxy amide (-NH-
Psec); or,
in suitable cases (e.g., cyclic amines), as a nitroxide radical (>N-O=).
For example, a carboxylic acid group may be protected as an ester for example,
as: an
CI_7alkyl ester (e.g., a methyl ester; a t-butyl ester); a Cl_7haloalkyl ester
(e.g., a
CI_7trihaloalkyl ester); a triCj_7alkylsilyl-Cj_7alkyl ester; or a C5_20aryl-
Cl_7alkyl ester (e.g., a
benzyl ester; a nitrobenzyl ester); or as an amide, for example, as a methyl
amide.
For example, a thiol group may be protected as a thioether (-SR), for example,
as: a
benzyl thioether; an acetamidomethyl ether (-S-CH2NHC(=O)CH3).
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
14
Prodrugs
It may be convenient or desirable to prepare, purify, and/or handle the active
compound
in the form of a prodrug. The term "prodrug," as used herein, pertains to a
compound
which, when metabolised (e.g., in vivo), yields the desired active compound.
Typically,
the prodrug is inactive, or less active than the active compound, but may
provide
advantageous handling, administration, or metabolic properties.
Unless otherwise specified, a reference to a particular compound also include
prodrugs
thereof.
For example, some prodrugs are esters of the active compound (e.g., a
physiologically
acceptable metabolically labile ester). During metabolism, the ester group (-
C(=O)OR) is
cleaved to yield the active drug. Such esters may be formed by esterification,
for
example, of any of the carboxylic acid groups (-C(=O)OH) in the parent
compound, with,
where appropriate, prior protection of any other reactive groups present in
the parent
compound, followed by deprotection if required.
Examples of such metabolically labile esters include those of the formula -
C(=O)OR
wherein R is:
Cl_7alkyl
(e.g., -Me, -Et, -nPr, -iPr, -nBu, -sBu, -iBu, -tBu);
CI_7aminoalkyl
(e.g., aminoethyl; 2-(N,N-diethylamino)ethyl; 2-(4-morpholino)ethyl); and
acyloxy-CI_7alkyl
(e.g., acyloxymethyl;
acyloxyethyl;
pivaloyloxymethyl;
acetoxymethyl;
1 -acetoxyethyl;
1 -(1 -methoxy-1 -methyl)ethyl-carbonxyloxyethyl;
1-(benzoyloxy)ethyl; isopropoxy-carbonyloxymethyl;
1-isopropoxy-carbonyloxyethyl; cyclohexyl-carbonyloxymethyl;
1-cyclohexyl-carbonyloxyethyl;
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
cyclohexyloxy-carbonyloxymethyl;
1-cyclohexyloxy-carbonyloxyethyl;
(4-tetrahydropyranyloxy) carbonyloxymethyl;
1-(4-tetrahydropyranyloxy)carbonyloxyethyl;
5 (4-tetrahydropyranyl)carbonyloxymethyl; and
1-(4-tetrahydropyranyl)carbonyloxyethyl).
Also, some prodrugs are activated enzymatically to yield the active compound,
or a
compound which, upon further chemical reaction, yields the active compound
(for
10 example, as in ADEPT, GDEPT, LIDEPT, etc.). For example, the prodrug may be
a
sugar derivative or other glycoside conjugate, or may be an amino acid ester
derivative.
Use of Compounds of the Invention
The invention provides compounds of formula (I), or solvates or prodrugs
thereof ("active
15 compounds"), for use in a method of treatment of the human or animal body.
Such a
method may comprise administering to such a subject a therapeutically-
effective amount
of an active compound, preferably in the form of a pharmaceutical composition.
The term "treatment" as used herein in the context of treating a condition,
pertains
generally to treatment and therapy, whether of a human or an animal (e.g. in
veterinary
applications), in which some desired therapeutic effect is achieved, for
example, the
inhibition of the progress of the condition, and includes a reduction in the
rate of
progress, a halt in the rate of progress, amelioration of the condition, and
cure of the
condition. Treatment as a prophylactic measure (i.e. prophylaxis) is also
included.
The term "therapeutically-effective amount" as used herein, pertains to that
amount of an
active compound, or a material, composition or dosage form comprising an
active
compound, which is effective for producing some desired therapeutic effect,
commensurate with a reasonable benefit/risk ratio.
Administration
The active compound or pharmaceutical composition comprising the active
compound
may be administered to a subject by any convenient route of administration,
whether
systemically/ peripherally or at the site of desired action, including but not
limited to, oral
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
16
(e.g. by ingestion); topical (including e.g. transdermal, intranasal, ocular,
buccal, and
sublingual); pulmonary (e.g. by inhalation or insufflation therapy using, e.g.
an aerosol,
e.g. through mouth or nose); rectal; vaginal; parenteral, for example, by
injection,
including subcutaneous, intradermal, intramuscular, intravenous,
intraarterial,
intracardiac, intrathecal, intraspinal, intracapsular, subcapsular,
intraorbital,
intraperitoneal, intratracheal, subcuticular, intraarticular, subarachnoid,
and intrasternal;
by implant of a depot, for example, subcutaneously or intramuscularly.
The subject may be a eukaryote, an animal, a vertebrate animal, a mammal, a
rodent
(e.g. a guinea pig, a hamster, a rat, a mouse), murine (e.g. a mouse), canine
(e.g. a
dog), feline (e.g. a cat), equine (e.g. a horse), a primate, simian (e.g. a
monkey or ape), a
monkey (e.g. marmoset, baboon), an ape (e.g. gorilla, chimpanzee, orang-utan,
gibbon),
or a human.
Formulations
While it is possible for the active compound to be administered alone, it is
preferable to
present it as a pharmaceutical composition (e.g. formulation) comprising at
least one
active compound, as defined above, together with one or more pharmaceutically
acceptable carriers, adjuvants, excipients, diluents, fillers, buffers,
stabilisers,
preservatives, lubricants, or other materials well known to those skilled in
the art and
optionally other therapeutic or prophylactic agents.
Thus, the present invention further provides pharmaceutical compositions, as
defined
above, and methods of making a pharmaceutical composition comprising admixing
at
least one active compound, as defined above, together with one or more
pharmaceutically acceptable carriers, excipients, buffers, adjuvants,
stabilisers, or other
materials, as described herein.
The term "pharmaceutically acceptable" as used herein pertains to compounds,
materials, compositions, and/or dosage forms which are, within the scope of
sound
medical judgement, suitable for use in contact with the tissues of a subject
(e.g. human)
without excessive toxicity, irritation, allergic response, or other problem or
complication,
commensurate with a reasonable benefit/risk ratio. Each carrier, excipient,
etc. must
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
17
also be "acceptable" in the sense of being compatible with the other
ingredients of the
formulation.
Suitable carriers, excipients, etc. can be found in standard pharmaceutical
texts, for
example, Remington's Pharmaceutical Sciences, 18th edition, Mack Publishing
Company, Easton, Pa., 1990.
The formulations may conveniently be presented in unit dosage form and may be
prepared by any methods well known in the art of pharmacy. Such methods
include the
step of bringing into association the active compound with the carrier which
constitutes
one or more accessory ingredients. In general, the formulations are prepared
by
uniformly and intimately bringing into association the active compound with
liquid carriers
or finely divided solid carriers or both, and then if necessary shaping the
product.
Formulations may be in the form of liquids, solutions, suspensions, emulsions,
elixirs,
syrups, tablets, losenges, granules, powders, capsules, cachets, pills,
ampoules,
suppositories, pessaries, ointments, gels, pastes, creams, sprays, mists,
foams, lotions,
oils, boluses, electuaries, or aerosols.
Formulations suitable for oral administration (e.g. by ingestion) may be
presented as
discrete units such as capsules, cachets or tablets, each containing a
predetermined
amount of the active compound; as a powder or granules; as a solution or
suspension in
an aqueous or non-aqueous liquid; or as an oil-in-water liquid emulsion or a
water-in-oil
liquid emulsion; as a bolus; as an electuary; or as a paste.
A tablet may be made by conventional means, e.g., compression or moulding,
optionally
with one or more accessory ingredients. Compressed tablets may be prepared by
compressing in a suitable machine the active compound in a free-flowing form
such as a
powder or granules, optionally mixed with one or more binders (e.g. povidone,
gelatin,
acacia, sorbitol, tragacanth, hydroxypropylmethyl cellulose); fillers or
diluents (e.g.
lactose, microcrystalline cellulose, calcium hydrogen phosphate); lubricants
(e.g.
magnesium stearate, talc, silica); disintegrants (e.g. sodium starch
glycolate, cross-
linked povidone, cross-linked sodium carboxymethyl cellulose); surface-active
or
dispersing or wetting agents (e.g. sodium lauryl sulfate); and preservatives
(e.g. methyl
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
18
p-hydroxybenzoate, propyl p-hydroxybenzoate, sorbic acid). Moulded tablets may
be
made by moulding in a suitable machine a mixture of the powdered compound
moistened with an inert liquid diluent. The tablets may optionally be coated
or scored
and may be formulated so as to provide slow or controlled release of the
active
compound therein using, for example, hydroxypropylmethyl cellulose in varying
proportions to provide the desired release profile. Tablets may optionally be
provided
with an enteric coating, to provide release in parts of the gut other than the
stomach.
Formulations suitable for topical administration (e.g. transdermal,
intranasal, ocular,
buccal, and sublingual) may be formulated as an ointment, cream, suspension,
lotion,
powder, solution, past, gel, spray, aerosol, or oil. Alternatively, a
formulation may
comprise a patch or a dressing such as a bandage or adhesive plaster
impregnated with
active compounds and optionally one or more excipients or diluents.
Formulations suitable for topical administration in the mouth include losenges
comprising
the active compound in a flavoured basis, usually sucrose and acacia or
tragacanth;
pastilles comprising the active compound in an inert basis such as gelatin and
glycerin,
or sucrose and acacia; and mouthwashes comprising the active compound in a
suitable
liquid carrier.
Formulations suitable for topical administration to the eye also include eye
drops wherein
the active compound is dissolved or suspended in a suitable carrier,
especially an
aqueous solvent for the active compound.
Formulations suitable for nasal administration, wherein the carrier is a
solid, include a
coarse powder having a particle size, for example, in the range of about 20 to
about 500
microns which is administered in the manner in which snuff is taken, i.e. by
rapid
inhalation through the nasal passage from a container of the powder held close
up to the
nose. Suitable formulations wherein the carrier is a liquid for administration
as, for
example, nasal spray, nasal drops, or by aerosol administration by nebuliser,
include
aqueous or oily solutions of the active compound.
Formulations suitable for administration by inhalation include those presented
as an
aerosol spray from a pressurised pack, with the use of a suitable propellant,
such as
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
19
dichlorodifluoromethane, trichlorofluoromethane, dichoro-tetrafluoroethane,
carbon
dioxide, or other suitable gases.
Formulations suitable for topical administration via the skin include
ointments, creams,
and emulsions. When formulated in an ointment, the active compound may
optionally be
employed with either a paraffinic or a water-miscible ointment base.
Alternatively, the
active compounds may be formulated in a cream with an oil-in-water cream base.
If
desired, the aqueous phase of the cream base may include, for example, at
least about
30% w/w of a polyhydric alcohol, i.e., an alcohol having two or more hydroxyl
groups
such as propylene glycol, butane-1,3-diol, mannitol, sorbitol, glycerol and
polyethylene
glycol and mixtures thereof. The topical formulations may desirably include a
compound
which enhances absorption or penetration of the active compound through the
skin or
other affected areas. Examples of such dermal penetration enhancers include
dimethylsulfoxide and related analogues.
When formulated as a topical emulsion, the oily phase may optionally comprise
merely
an emulsifier (otherwise known as an emulgent), or it may comprises a mixture
of at least
one emulsifier with a fat or an oil or with both a fat and an oil. Preferably,
a hydrophilic
emulsifier is included together with a lipophilic emulsifier which acts as a
stabiliser. It is
also preferred to include both an oil and a fat. Together, the emulsifier(s)
with or without
stabiliser(s) make up the so-called emulsifying wax, and the wax together with
the oil
and/or fat make up the so-called emulsifying ointment base which forms the
oily
dispersed phase of the cream formulations.
Suitable emulgents and emulsion stabilisers include Tween 60, Span 80,
cetostearyl
alcohol, myristyl alcohol, glyceryl monostearate and sodium lauryl sulphate.
The choice
of suitable oils or fats for the formulation is based on achieving the desired
cosmetic
properties, since the solubility of the active compound in most oils likely to
be used in
pharmaceutical emulsion formulations may be very low. Thus the cream should
preferably be a non-greasy, non-staining and washable product with suitable
consistency
to avoid leakage from tubes or other containers. Straight or branched chain,
mono- or
dibasic alkyl esters such as di-isoadipate, isocetyl stearate, propylene
glycol diester of
coconut fatty acids, isopropyl myristate, decyl oleate, isopropyl palmitate,
butyl stearate,
2-ethylhexyl palmitate or a blend of branched chain esters known as Crodamol
CAP may
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
be used, the last three being preferred esters. These may be used alone or in
combination depending on the properties required.
Alternatively, high melting point lipids such as white soft paraffin and/or
liquid paraffin or
5 other mineral oils can be used.
Formulations suitable for rectal administration may be presented as a
suppository with a
suitable base comprising, for example, cocoa butter or a salicylate.
10 Formulations suitable for vaginal administration may be presented as
pessaries,
tampons, creams, gels, pastes, foams or spray formulations containing in
addition to the
active compound, such carriers as are known in the art to be appropriate.
Formulations suitable for parenteral administration (e.g. by injection,
including
15 cutaneous, subcutaneous, intramuscular, intravenous and intradermal),
include aqueous
and non-aqueous isotonic, pyrogen-free, sterile injection solutions which may
contain
anti-oxidants, buffers, preservatives, stabilisers, bacteriostats, and solutes
which render
the formulation isotonic with the blood of the intended recipient; and aqueous
and non-
aqueous sterile suspensions which may include suspending agents and thickening
20 agents, and liposomes or other microparticulate systems which are designed
to target
the compound to blood components or one or more organs. Examples of suitable
isotonic vehicles for use in such formulations include Sodium Chloride
Injection, Ringer's
Solution, or Lactated Ringer's Injection. Typically, the concentration of the
active
compound in the solution is from about 1 ng/ml to about 10 pg/ml, for example
from
about 10 ng/ml to about 1 pg/mI. The formulations may be presented in unit-
dose or
multi-dose sealed containers, for example, ampoules and vials, and may be
stored in a
freeze-dried (lyophilised) condition requiring only the addition of the
sterile liquid carrier,
for example water for injections, immediately prior to use. Extemporaneous
injection
solutions and suspensions may be prepared from sterile powders, granules, and
tablets.
Formulations may be in the form of liposomes or other microparticulate systems
which
are designed to target the active compound to blood components or one or more
organs.
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
21
Dosage
It will be appreciated that appropriate dosages of the active compounds, and
compositions comprising the active compounds, can vary from patient to
patient.
Determining the optimal dosage will generally involve the balancing of the
level of
therapeutic benefit against any risk or deleterious side effects of the
treatments of the
present invention. The selected dosage level will depend on a variety of
factors
including, but not limited to, the activity of the particular compound, the
route of
administration, the time of administration, the rate of excretion of the
compound, the
duration of the treatment, other drugs, compounds, and/or materials used in
combination,
and the age, sex, weight, condition, general health, and prior medical history
of the
patient. The amount of compound and route of administration will ultimately be
at the
discretion of the physician, although generally the dosage will be to achieve
local
concentrations at the site of action which achieve the desired effect without
causing
substantial harmful or deleterious side-effects.
Administration in vivo can be effected in one dose, continuously or
intermittently (e.g. in
divided doses at appropriate intervals) throughout the course of treatment.
Methods of
determining the most effective means and dosage of administration are well
known to
those of skill in the art and will vary with the formulation used for therapy,
the purpose of
the therapy, the target cell being treated, and the subject being treated.
Single or
multiple administrations can be carried out with the dose level and pattern
being selected
by the treating physician.
In general, a suitable dose of the active compound is in the range of about
100 pg to
about 250 mg per kilogram body weight of the subject per day. Where the active
compound is a salt, an ester, prodrug, or the like, the amount administered is
calculated
on the basis of the parent compound and so the actual weight to be used is
increased
proportionately.
Cancers
Examples of cancers which may be treated by the active compounds include, but
are not
limited to, a carcinoma, for example a carcinoma of the bladder, breast, colon
(e.g.
colorectal carcinomas such as colon adenocarcinoma and colon adenoma), kidney,
epidermal, liver, lung, for example adenocarcinoma, small cell lung cancer and
non-small
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
22
cell lung carcinomas, oesophagus, gall bladder, ovary, pancreas e.g. exocrine
pancreatic
carcinoma, stomach, cervix, thyroid, prostate, or skin, for example squamous
cell
carcinoma; a hematopoietic tumour of lymphoid lineage, for example leukemia,
acute
lymphocytic leukemia, B-cell lymphoma, T-cell lymphoma, Hodgkin's lymphoma,
non-
Hodgkin's lymphoma, hairy cell lymphoma, or Burkett's lymphoma; a
hematopoietic
tumor of myeloid lineage, for example acute and chronic myelogenous leukemias,
myelodysplastic syndrome, or promyelocytic leukemia; thyroid follicular
cancer; a tumour
of mesenchymal origin, for example fibrosarcoma or habdomyosarcoma; a tumor of
the
central or peripheral nervous system, for example astrocytoma, neuroblastoma,
glioma
or schwannoma; melanoma; seminoma; teratocarcinoma; osteosarcoma; xenoderoma
pigmentoum; keratoctanthoma; thyroid follicular cancer; or Kaposi's sarcoma.
Examples of other therapeutic agents that may be administered together
(whether
concurrently or at different time intervals) with the compounds of the formula
(I) include
but are not limited to topoisomerase inhibitors, alkylating agents,
antimetabolites, DNA
binders and microtubule inhibitors (tubulin target agents), such as cisplatin,
cyclophosphamide, doxorubicin, irinotecan, fludarabine, 5FU, taxanes,
mitomycin C or
radiotherapy. For the case of active compounds combined with other therapies
the two
or more treatments may be given in individually varying dose schedules and via
different
routes.
The combination of the agents listed above with a compound of the present
invention
would be at the discretion of the physician who would select dosages using his
common
general knowledge and dosing regimens known to a skilled practitioner.
Where the compound of the formula (I) is administered in combination therapy
with one,
two, three, four or more, preferably one or two, preferably one other
therapeutic agents,
the compounds can be administered simultaneously or sequentially. When
administered
sequentially, they can be administered at closely spaced intervals (for
example over a
period of 5-10 minutes) or at longer intervals (for example 1, 2, 3, 4 or more
hours apart,
or even longer periods apart where required), the precise dosage regimen being
commensurate with the properties of the therapeutic agent(s).
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
23
The compounds of the invention may also be administered in conjunction with
non-
chemotherapeutic treatments such as radiotherapy, photodynamic therapy, gene
therapy; surgery and controlled diets.
Preferences
R1-R 6
In one group of embodiments of the present invention, R' and R2 together with
the ring to
which they are attached form a saturated or unsaturated carbocyclic or
heterocyclic
group containing up to 3- to 8- membered carbocyclic or heterocyclic rings,
wherein each
carbocyclic or heterocyclic ring may be fused to one or more other carbocyclic
or
heterocyclic rings.
In this group of embodiments, it is preferred that R3, R4, R5 and R6 are H.
R' and R2 together with the ring to which they are bound in compounds of
formula (I)
may represent an ortho- or peri-fused carbocyclic or heterocyclic ring system.
R' and R2 together with the ring to which they are bound may represent a
wholly
carbocyclic fused ring system such as a ring system containing 2 or 3 fused
carbocyclic
rings, e.g. optionally substituted, optionally hydrogenated naphthalene or
anthracene.
Alternatively, R' and R2 together with the ring to which they are bound in
compounds of
formula (I) may represent a fused tricyclic ring such as anthracene or a mono,
di, tri, tetra
or higher hydrogenated derivative of anthracene. For example, R' and R2
together with
the ring to which they are bound in formula (I) may represent anthracene, 1, 4-
dihydroanthracene or 1, 4, 9, 10-tetrahydroanthracene. '
R' and R2 together with the ring to which they are bound in formula (I) may
also
represent:
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
24
cc> (D-0
or
In another group of embodiments, R1, R2, R3, R4, R5 and R6 are independently
selected
from H, CI_7 alkyl, C5_20 aryl, C3_2o heterocyclyl, halo, ester, amido, acyl,
sulfo,
sulfonamido, ether, thioether, azo and amino. In this group of embodiments,
R', R2, R3,
R4, R5 and R 6 are preferably independently selected from H, CI_7 alkyl, C5_20
aryl and
ester. Of these H and Cl_7 alkyl (in particular C1_3 alkyl)are most preferred.
In this group of embodiments, four, five or six of R1, R2 , R3, R4, R5 and R 6
are preferably
hydrogen, with the other (if any) groups being selected from Cl_7 alkyl, C5_2o
aryl, C3_20
heterocyclyl, halo, ester, amido, acyl, sulfo, sulfonamido, ether, thioether,
azo and
amino, or more preferably CI_7 alkyl, C5_20 aryl and ester, and most
preferably Cl_7 alkyl (in
particular CI_3 alkyl). If two of R', R2, R3, R4, R5 and R6 are not H, then
these groups are
preferably meta or para to one another, and more preferably para to one
another.
Examples of particularly preferred substitutent patterns include, but are not
limited to:
phenyl; 1-methyl; and 4-iso-propyl.
A and B
It is that A and B to ether re resent NRNaRNS_ CRc'Rcz Ns N7 cl
preferred g p ( )õNR R, wherein R
and RC2 are independently selected from H and CI-4 alkyl, RNa, RN5, RNS and R
N7 are
independently selected from H and CI.a alkyl, and n is an integer from 1 to 4.
Preferably, R14 and R15 are both hydrogen. Preferably n is 2 or 3, more
preferably 2.
RN4~ RN5~ RNS and RN' are preferably H or methyl and, more preferably, all of
RN4, RN5RNS and RN7 are H.
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
When RN4 is present in A, then p is 0. When RN4 is absent, then p is I and C
takes the
place of RN4. In a group of embodiments, RN4 is absent from A, p is I and
preferably C' is
C4-10 alkylene with no substituents (e.g. hexylene).
5 Examples of dinuclear complexes of this group of embodiments are those in
which pairs
of A and B together with linker C' represent:
r --i r--
HZ NH-(CHZ)6-HN NHz H2N NH NH NH2
HZN ~NHz
CHz1-( H2N NH-(CHZ)x(NH)(CHz)u-HNH2
HZNJ ~NHz
H2N NH-(CH2)õ(NH)(CH2)y(NH)(CH2)õ-HN NH2
(CHz).
H2N NHz H2N NH2 H2N NH-(CHz)õ(CONH)(CHz)y(NHOC)(CHz)z HN NH2
p(CHz).
0 ~
HZN NH-C-(CHz)4 C-NH NHz -O NHz H2N 0-
0
NHz p(CHz)r-HNOC F ~CONH-(CHz)õ p
HzNH-(CHz)6-HN
-O NHZ HzN p-
wherein each n', n", x', x" and y' independently represents an integer from 1
to 12,
preferably 1 to 6.
x
When X is an N-donor ligand, it is preferably selected from azide,
isothiocyanate, and
optionally substituted pyridine ligands. Of these, azide and isothiocyanate
are preferred.
When X is an optionally substituted pyridine ligand, the ligand is preferably
at least
mono-substituted, and may be di-substituted. These substituents are preferably
selected
from halo (e.g. chloro, flouro), cyano, and lower alkyl (e.g. methyl). Of
these, chloro,
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
26
cyano and methyl are preferred. Preferred substituent patterns include, but
are not
limited to, 3-, 5-dichloro, 4-cyano and 3-methyl.
In some embodiments, X is selected from nitrile ligands (N=-C-R); azo ligands
(N=N-R);
amine ligands (NR"'RN2RN); azide (N3 ); cyanide (N=C") and isothiocyanate
(NCS").
If X is an S-donor ligand, it is preferably a thiolate ligand, for example,
PhS-.
yq-
yq- in compounds of formula (I) is a counterion and is only present in the
compound
when the complex containing the metal ion is charged. Yq" is preferably a non-
nucleophilic anion such as PFs , BF4 , BPh4 or CF302S0" ,for example.
General Synthesis Methods
The present invention also provides a process for preparing the compounds of
the
invention which comprises the reaction of a compound of formula [(r)6-
C6(R')(R2)(R3)(R4)(R5)(R6))RuABCI][Yq-], which may be in the form of a monomer
or a
dimer, with AgNO3 in a suitable solvent for the reaction, followed by removal
of AgCI and
reaction with MX, optionally in the presence, or with subsequent addition of,
Y4-, in a
suitable solvent for the reaction, wherein R', R2, R3, R4, R5, R6, X, A, B and
Y are as
defined above for the compounds of the invention, and M is an appropriate
cation, e.g.
Na+.
Preferred reaction conditions include:
(a) stirring the starting ruthenium complex, as described above, in a 1:1
mixture of
MeOH and H20 as a solvent with AgNO3;
(b) filtering off the AgCl precipitate formed;
(c) adding MX (which may be dissolved by heating, if necessary) and allowing
to
react;
(d) adding a source of yq-, such as a compound of formula (NH4+)Yq", e.g.,
NH4PF6,
and evaporating the filtrate to yield the product.
The filtrate may be purified, for example, by recrystalisation from acetone.
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
27
The following non-limiting examples illustrate the present invention.
Examples
General Methods
Electrospray lonisation Mass Spectrometry (ESI-MS): Positive-ion electrospray
ionisation
mass spectra were obtained with a Platform II mass spectrometer (Micromass,
Manchester, U.K.). For offline ESI-MS assays, the samples were prepared in 50%
CH3CN / 50% H20 (v/v) and infused directly into the mass spectrometer at 6 L
min'.
The ions were produced in an atmospheric pressure ionisation (API) / ESI ion
source.
For the online LC-ESI-MS assays, a Waters 2690 HPLC system was interfaced with
the
mass spectrometer, using the same column and gradients as described above for
the
HPLC assays with a flow rate of 1.0 mL min"' and a splitting ratio of 1/5. The
spray
voltage was 3.50-3.68 kV. The cone voltage was varied over the range of 15-30
V as
required. The capillary temperature was 338 K for direct infusion and 413 K
for the HPLC
sampling, with a 450 L h-' flow of nitrogen drying gas. The quadrupole
analyser, operated
at a background pressure of 2 x 10-5 Torr, was scanned at 300 Da s' for direct
infusion
and 750 Da s' for HPLC sampling. Data were collected (for 10 scans during the
direct
infusion assays) and analysed on a Mass Lynx (ver. 2.3) Windows NT PC data
system
using the Max Ent Electrospray software algorithm and calibrated versus an Nal
calibration file. The mass accuracy of all measurements was within 0.1 m/z
unit.
X-ray crystallography: All data were collected at 150 K on a Bruker Smart Apex
CCD
diffractometer equipped with an Oxford Cryosystems low-temperature device.
Following
application of a multi-scan absorption correction (SADABS)(Sheldrick, G.M.,
SADABS,
Program for carrying-out multiscan absorption corrections, University of
Gottingen,
Germany, 1998) the structures were all solved by direct methods (Shelxs,
SIR92, Dirdif)
(Sheldrick, G.M., SHELXS and SHELXL. Programs for the solution and refinement
of
crystal structures, University of Gottingen, Germany, 1998; Altomare, A., et
al., A. J.
Appl. Crystallogr., 26, 343-350 (1993); Beurskens, P.T., et al., The DIRDIF96
Program
System, Technical Report of the Crystallography Laboratory, University of
Nijmegen, The
Netherlands (1996)) and refined against F2 using all data (SHELXL)
(Betteridge, P.W., et
al., J. Appl. Cryst., 36, 1487 (2003))
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
28
Comparative Example 1: Synthesis of [(rls-CsH5C6H5)Ru(en)CI][PF6] (Cl)
PF6
\I
/
HZNH U'CI
/ N a
This compound was synthesised as described in Morris, R.E., et al., J. Med.
Chem., 44,
3616-3621 (2001) - compound 9.
Example 1: Synthesis of [(ris-CsH5C6H5)Ru(en)N3][PFs] (1)
PFs
/\ I
HZN_Ru"'N
~NH2
II
N
This complex was prepared by refluxing complex Cl (25.0 mg, 0.0496 mmol) and
AgNO3
(8.4 mg, 0.0494 mmol) in 2.5 mL of a 1:1 mixture of MeOH and H20 for one hour.
AgCl
was removed by filtration. NaN3 was added (163 mg, 2.51 mmol), dissolved by
heating,
and left overnight. NH4PF6 (250 mg) was added, leading to a microcrystalline,
yellow
precipitate. Recrystallization of the precipitate from acetone gave a yellow
crystalline
product. Yield of 1: 8.6 mg (34%).
Anal. Calcd for C14F6H1$NSPRu: C 33.47, H 3.61, N 13.94. Found: C 33.37, H
3.46, N
13.68. MS: m/z 357,7 for [M-PF6]+ (caic. 357.1)
Comparative Example 2: Synthesis of [(rls-C6(CH3)s)Ru(en)CI][PFs] (C2)
Me PF6
Me ~ Me
Me I Me
Me
HaNH CI
\/Na
This complex was prepared in an analogous manner to compound Cl in Comparative
Example 1 from [(r16-C6(CH3)6)RuCI2]2. Yield of C2: 68%. Anal. Calcd. for
C14F6HI2N2CIPRu : C 33.59, H 4.43, N 5.60 Found: C 33.55, H 4.57, N 5.54
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
29
Example 2: Synthesis of [(rls-Cs(CH3)s)Ru(en)(pyridine)][PFs]Z (2)
Me -1 (PF 6)2
Me Me
Me I Me Me
~Ru
HZs NH2 ' No-,
This complex was prepared by refluxing complex C2 (25.0 mg, 0.0496 mmol) and
AgNO3
(8.4 mg, 0.0494 mmol) in 2.5 mL of a 1:1 mixture of MeOH and H20 for one hour.
AgCI
was removed by filtration. Pyridine (101 i, 1.25 mmol) was added and the
mixture was
left overnight. The volume was reduced to ca. 1.5 mL by rotary evaporation and
100 mg
of NH4PF6 was added. The yellow precipitate was dissolved in acetone. The
solution
was then filtered and the acetone allowed to evaporate slowly, resulting in a
microcrystalline, yellow product. Yield of 2: 19.3 mg (56%). Anal. Calcd for
C19F1aH3jN3PaRu: C 32.96, H 4.51, N 6.07. Found: C 33.47, H 4.50, N 6.24.
Example 3: Synthesis of [(rls-Cs(CH3)s)Ru(en)(SCN)][PF6]a (3)
Me PFs
Me ~ Me
~
Me Me Me
H2 N IRul N
Z_~,NH2 s
This complex was prepared by refluxing complex C2 (25.0 mg, 0.0496 mmol) and
AgNO3
(7.0 mg, 0.0412 mmol) in 2.5 mL of a 1:1 mixture of MeOH and H20 for one hour.
AgCI
was removed by filtration. KSCN was added (243 mg, 2.50 mmol) and the solution
stirred for one day. 150 mg of KPF6 was added, and enough acetone was added to
dissolve the resulting precipitate. Slow evaporation of the acetone yielded
yellow
crystals, which were suitable for X-ray crystallography studies. Yield: 6.9 mg
(26 %)
X-ray crystal structure determination yielded the result shown below, from
which it can be
seen that the isothiocyanate is bound via the nitrogen atom.
Crystal data and structure refinement for compound 3
X-ray data:
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
Crystal Data
Empirical formula C15 H26 F6 N3 0 P Ru S
Formula weight 542.49
Crystal system Orthorhombic
5 Space group Pca2l
Unit cell dimensions a = 14.7411(12)A a = 90 deg.
b = 9.0154(7)A (3 = 90 deg.
c = 15.6070(12)A y = 90 deg.
Volume 2074.1(3)A3
10 Z 4
Data Collection
Instrument Bruker Smart Apex CCD
15 Solution and Refinement
Solution Patterson (Dirdif)
RI = 0.0619 [5064 data]
M1T} Rutt)
~t1Tf
S}iT7 NItE} ~3; N(2E)
Ct2E}
Ct1E}
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
31
Example 4: Synthesis of [(rls-Cs(CH3)6)Ru(en)(SPh)][PF6] (4)
M Me Me7 PF6
~~ e /
I
Me Me
Me
HaN----/ ullS~
Z~-Ha 'I I
This complex was prepared by refluxing complex C2 (25.0 mg, 0.0496 mmol) and
AgNO3
(8.4 mg, 0.0494 mmol) in 2.5 mL of a 1:1 mixture of MeOH and H20 for one hour.
AgCI
was removed by filtration. NaSPh was added (7.9 mg, 0.0595 mmol) and the
solution
was left overnight. 250 mg of NH4PF6 was added, leading to an orange
precipitate. Slow
evaporation of the acetone solution of the precipitate led to a crystalline
orange product
and a yellow powder, both of which, by mass spectrometry, seemed to be the
desired
compound. Yield: 10.2 mg (36 %). MS: m/z 433.0 for [M - PF6]+ (Calc. 433.1).
Example 5: Synthesis of [(rl6-Cs(CH3)s)Ru (en)N3][PF6] (5)
Me PFs
Me / Me
Me /~ Me
HaN_Ru"I Me
V NHa NII~
N
This complex was prepared by refluxing complex C2 (25.0 mg, 0.0496 mmol) and
AgNO3
(8.4 mg, 0.0494 mmol) in 2.5 mL of a 1:1 mixture of MeOH and H20 for one hour.
AgCI
was removed by fiitration. NaN3 was added (163 mg, 2.51 mmol), dissolved by
heating,
and left overnight. NH4PF6 (250 mg) was added, leading to a microcrystalline,
yellow
precipitate. Recrystallization of the precipitate from acetone gave to a
yellow crystalline
product. Yield of 5: 16.4 mg (65%). Anal. Calcd for C14F6H26N5PRu: C 32.94, H
5.13, N
13.72. Found: C 32.32, H 4.45, N 12.63.
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
32
Example 6: Synthesis of [(r16-Cs(CH3)s)Ru(en)(3,5-dichloropyridine)][PFs]Z (6)
Me 7 (PF 6)2
Me \ Me
Me ( / Me
N~Ru Me
HNH~~ i CI
CI
This complex was prepared in an analogous manner to compound 2 in Example 2.
MS:
m/z 616.0 for [6 - PF6]' (Calc. 616.0)
Example 7: Synthesis of [(rls-Cs(CH3)6)Ru(en)(3,5-difluoropyridine)][PFs]2 (7)
Me -1 PF 6
Me \ Me
Me ( Me Me
~Ru
HZ N NHZ\ (
F
N
/
F
This complex was prepared in an analogous manner to compound 2 in Example 2.
MS:
m/z 583.9 for [7 - PF6]+ (Calc. 584.1)
Example 8: Synthesis of [(rls-Cs(CH3)s)Ru(en)(p-cyanopyridine)][PFs]Z (8)
Me -1 PF6
Me \ Me
Me ( Me Me
~Ru
Ha~NHZ~ i
CN
This complex was prepared in an analogous manner to compound 2 in Example 2.
MS:
m/z 572.9 for [8 - PF6]+ (Caic. 573.1)
X-ray data:
Crystal Data
Empirical formula C20 H30 F12 N4 P2 Ru
Formula weight 717.49
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
33
Crystal system Monoclinic
Space group P2(1)/n
Unit cell dimensions a= 8.6230(2)A oc = 900
b = 34.7990(10)A (3 = 114.4360(10)0
c = 9.8620(3)A y 90
Volume 2694.22(13) A3
Z 4
Data Collection
Absorption correction SADABS
Solution and Refinement
Solution direct (SHELXS-97)
Program used for refinement SHELXL-97
R1 = 0.0575 [4950 data]
pp,~
Ru
Ni N3
~
.<~ ~~~yrlt,r~/~?
N4
=w. -.~~
Example 9: Synthesis of [(rls-Cs(CH3)s)Ru(en)(3-methylpyridine)][PF6]Z (9)
Me -1 PF6
Me Me
Me Me Me
HZN~R \
N ~
NH2
~ /
Me
This complex was prepared in an analogous manner to compound 2 in Example 2.
MS:
m/z 562.1 for [9 - PF6]' (Calc. 562.1)
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
34
X-ray data:
Crystal Data
Empirical formula C20 H27 F12 N3 P2 Rul
Formula weight 700.45
Crystal system Orthorhombic
Space group P n a 21
Unit cell dimensions a = 21.3199(6)A a= 900
b = 7.7155(2)A R = 90
c = 16.1809(5)A y = 90
Volume 2661.66 A3
Z 4
Data Collection
Absorption correction SADABS
Solution and Refinement
Solution direct (SHELXS-97)
Program used for refinement SHELXL-97
R1 = 0.0444
ILO)
Ru
N1
N
N7
~.~
rX
C7
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
Example 10: Analysis of Compounds
Methods
Ultraviolet and Visible (UV-Vis) Spectroscopy: A Perkin-Elmer Lambda-16 UV-Vis
spectrophotometer was used with 1-cm path-length quartz cuvettes (0.5 mL) and
a PTP1
5 Peltier temperature controller. Spectra were processed using UVWinlab
software for
Windows' 95.
Kinetic Studies: Aliquots of stock solutions of the complexes to be tested (4 -
10 mM) in
methanol were diluted to 500 L with water, and the absorbance at selected
wavelengths
10 (determined by hydrolysis in an 19:1 mixture of water and methanol - see
table 1(A))
was then recorded at 6 to 20 second intervals depending on the hydrolysis rate
of each
complex at 298 K. The hydrolysis rate constant leH20 for each complex was
determined by
computer fit of the absorbance/time data for each complex to the first-order
rate equation
(eq.1),
15 A=Co+Cle"kt (1)
where Co and C, are computer-fitted constants, and A is the absorbance
corresponding
to time t, and the results are reported in table 1 as the half life (t~is).
Cytoxicity Studies
20 A2780 (1st Method): A2780 cells were plated on day zero, and the complexes
to be
tested were added on day 3. The complex was removed on day 4 (i.e., 24 h cell
exposure), and after growth in fresh medium in the absence of drug, the cells
were
counted on day 7. The complexes were stored in the dark at 277 K as a
precaution
against photochemical decomposition. The IC50 (dose of compound required to
cause
25 50% inhibition of cell growth) values are listed in Table 1.
A2780 (2"d method) and A549: Cell line A2780 (human ovarian carcinoma, ECACC
93112519) was maintained in medium comprising RPMI-1640 (Sigma) with 5% Fetal
30 Bovine Serum (Invitrogen), 2mM L-Glutamine (Sigma) and 1% Penicillin /
Streptomycin
(Invitrogen), in T-75 flasks (Costar). Cells were passaged at approximately 75-
90%
confluence (1:8 dilution) using 0.25% Trypsin / EDTA (invitrogen)
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
36
Cell Line A549 (human lung carcinoma, ECACC 86012804) was maintained in medium
comprising DMEM (Sigma) with 10% Fetal Bovine Serum (Invitrogen), 2mM L-
Glutamine
(Invitrogen) and 1% Penicillin / Streptomycin (invitrogen), in T-75 flasks
(Costar). Cells
were passaged at approximately 70-90% confluence (1:8 dilution) using 0.25%
Trypsin /
EDTA (invitrogen).
Both cell lines were incubated at 37 C, 5% COZ, in high humidity.
A2780 carcinoma cells were seeded (150taL) into 96 well plates (Nunc Maxisorp)
at 5000
( 10%) cells per well and incubated at 37 C, 5% CO2 in high humidity for 48
hours.
A549 carcinoma cells were seeded (150iaL) into 96 well plates (Nunc Maxisorp)
at 2000
( 10%) cells per well and incubated at 37 C, 5% CO2 in high humidity for 24
hours.
The compounds to be tested were solubilised by sonication in DMSO (Fisher
Scientific)
to provide 20mM solutions. Compounds were serially diluted with DMSO before
diluting
in cell culture medium to give concentrations four-fold greater than the final
concentrations required in the assay. The dilutions of compound in culture
medium were
added to the cell plates (50iaL) in triplicates to achieve flnal
concentrations of 100NM,
50pM, 10pM, 5pM, 1 NM and 0.1 pM. The final DMSO concentration in each well
was
0.5% (v/v). The plates were incubated for 24 hours at 37 C, 5% CO2, in high
humidity.
After 24 hours incubation, the cells were washed (200pL) twice with sterile
phosphate
buffered Saline (Sigma) and the cell culture medium replenished (200NL).
Plates were
incubated at 37 C, 5% C02, in high humidity for 96 hours. After the incubation
surviving
cells were fixed by the addition of 50%(w/v) Trichloroacetic acid (50pL) and
incubated at
4 C for 1 hour. Plates were washed three times with excess tap water and air-
dried.
Cells were dyed by the addition of (100NL) 0.4% sulforhodamine B (Sigma)
solution to
the plates followed by five washes (200p1) with 1% acetic acid solution to
remove excess
dye before air-drying. Dye was re-solubilised in (200pL) 10mM Tris buffer
(Fisher
Scientific) and the absorbance of each well read at both 565nm and 690nm using
a BMG
Fluorostar microplate reader. The reading at 690nm was subtracted from the
565nm
reading, and the IC50 values determined by plotting the corrected absorbance
value Vs.
CA 02578280 2007-02-08
WO 2006/018649 PCT/GB2005/003242
37
the compound concentration in the wells (XLfit version 4.0, ID Business
Solutions Ltd).
These are shown below in table 1
Results
The results of the above analyses are shown in table 1 below.
Table 1
ICe0 (NM)
Compound A (nm) t,i, (min) A2780 A2780 A549
(15' method) (2"d method)
C1 260 5.0 8 6.5 11
1 270 367 4 14 8.5
C2 254 0.44 9
2 a a
3 24
4 a a 23 23 38
5 270 21.3 18 7.9
6 270 537 23 18
7 270 555
8 270 43.9 6
9 254 a 50 50
a- no hydrolysis observed by UV-VIS