Note: Descriptions are shown in the official language in which they were submitted.
CA 02578667 2007-02-13
LESION ASSESSMENT BY PACING
BACKGROUND OF THE INVENTION
Field of the Invention
This invention relates to methods and systems for the treatment of conduc-
tion disturbances of the heart. More particularly, this invention relates to
validat-
ing and monitoring percutaneous cardiac ablation procedures.
Description of the Related Art
Atrial fibrillation is a well-known disorder of the heart, which causes
hemodynamic efficiency to be reduced and which, in serious cases, can lead to
cardiac embolization, stroke, ventricular arrhythmias and other potentially
fatal
complications. Atrial fibrillation is frequently engendered by abnormal
electrical
conduction paths within the heart muscle. Normally, electrical activation
signals
are conducted in an orderly way through the atrium and into the ventricle,
passing
each point in the heart only once in each heart cycle. Electrical activation
signals
at different locations in the heart are well correlated, taking into account
normal
propagation delays from one region of the heart to another. In response to
local
activation signals, the atrial muscle fibers contract in proper synchrony, to
pump
blood through the atrium. In atrial fibrillation, however, this orderly
contraction is
lost, it is believed, as multiple, changing, spatially disorganized activation
wave-
lets sweep across the surface of the atria, resulting in irregular patterns of
electri-
cal activation. A given atrial muscle fiber is activated to contract multiple
times in
each heart cycle, and fibrillation takes the place of normal contraction.
These
phenomena are described in detail by Gregory W. Botteron and Joseph M. Smith
in an article entitled, "A Technique for Measurement of the Extent of Spatial
Or-
ganization of Atrial Activation During Atrial Fibrillation in the Intact Human
Heart," in IEEE Transactions on Biomedical Engineering, 12 (June 1995),
pages 579-586, and in a second article entitled, "Quantitative Assessment of
the
Spatial Organization of Atrial Fibrillation in the Intact Human Heart," in
Circula-
CA 02578667 2012-09-26
tion 93 (Feb. 1, 1996), pages 513-518.
Invasive cardiac ablation techniques for the treatment of arrhythmias such
as described above are well known in the art. For example, U.S. Patent
Nos. 5,443,489 and 5,480,422, issued to Ben-Haim describe systems for ablating
cardiac tissue. U.S. Patent No. 5,807,395, issued to Mulier et al., and U.S.
Patent
No. 6,190,382, issued to Ormsby et al., describe systems for ablating body
tissue
using radiofrequency energy. U.S. Patent Nos. 6,251,109 and 6,090,084, issued
to
Hassett et al., 6,117,101, issued to Diederich et al., 5,938,660 and
6,235,025, is-
sued to Swartz et al., 6,245,064, issued to Lesh et al., 6,164,283, 6,305,378
and 5,971,983, issued to Lesh, 6,004,269, issued to Crowley et al., and
6,064,902,
issued to Haissaguerre et al., describe apparatus for tissue ablation to treat
atrial
arrhythmia. U.S. Patent No. 5,366,490, issued to Edwards et al., describes a
method for applying destructive energy to a target tissue using a catheter.
Radiofrequency ablation using multiple contiguous circumferential points,
guided by electro-anatomical mapping is proposed in the document, Circumferen-
tial Radiofrequency Ablation of Pulmonary Vein Ostia: A New Anatomic Ap-
proach for Curing Atrial Fibrillation, Pappone C, et al., Circulation 102:2619-
2628 (2000).
U.S. Patent No. 6,743,225, issued to Sanchez et al., proposes to measure
electrical activity of the cardiac tissue proximate a lesion site during an
ablation
treatment, and then to compare the measurements in order to determine whether
the lesion is clinically efficacious so as to be able to block myocardial
propaga-
tion. For example, the standard deviation of the amplitude of the
electrocardio-
gram signal has been used as a metric.
U.S. Patent No. 5,954,665, issued to Ben-Haim, describes a cardiac catheter
having two elec-
2
CA 02578667 2007-02-13
trodes, spaced apart. In operation, there is a measurable propagation delay be-
tween activation signals at the two electrodes under conditions of normal
conduc-
tion. The catheter is manipulated so as to position the ablation device in
contact
with the endocardium at the site of a suspected abnormal conduction path.
First
and second pre-ablation signals, responsive to the heart's activation signals,
are
received from the two electrodes, respectively, preferably simultaneously, or
al-
ternatively successively. A correlation coefficient of the first and second
pre-
ablation signals is computed. An ablation device is then activated so as to
ablate
the endocardium at the site, preferably by applying radiofrequency energy
thereto. After the ablation is completed, and the ablation device is
deactivated,
first and second post-ablation signals are respectively received from the
first and
second electrodes, and the correlation coefficient is again computed. If the
pre-
and post-ablation correlation coefficients are substantially the same, the
ablation
is determined to have been insufficient to interrupt the abnormal conduction
path.
If the post-ablation correlation coefficient is substantially less than or
greater than
the pre-ablation coefficient, however, the ablation is considered to have been
ef-
fective in interrupting the abnormal path.
It has also been proposed to produce circumferential ablative lesions using
ultrasound delivered through a balloon. This technique is described, for
example,
in the document, First Human Experience With Pulmonary Vein Isolation Using
a Through-the-Balloon Circumferential Ultrasound Ablation System for Recur-
rent Atrial Fibrillation, Natale A, et al., Circulation 102:1879-1882 (2000).
SUMMARY OF THE INVENTION
It is often difficult to determine the proper dosage of energy, e.g., radiofre-
quency energy, that should be applied in an ablation procedure in order to
achieve
the desired result. When the dosage is insufficient, the non-conducting lesion
does not extend deeply enough through the heart wall to disrupt the abnormal
3
CA 02578667 2007-02-13
conduction, so that arrhythmia may persist or return after the procedure is
com-
pleted. On the other hand, excessive dosage may cause dangerous damage to the
tissue at and around the ablation site. The proper dosage is known to vary
from
case to case depending on various factors, such as catheter geometry,
thickness of
the heart wall, quality of the electrical contact between the catheter
electrode and
the heart wall, and blood flow in the vicinity of the ablation site. Blood
flow car-
ries away heat generated by the radiofrequency energy.
A safe, simple method for monitoring ablation progress in near real time is
provided by disclosed embodiments of the invention, in which capture of a
pacing
signal is evaluated while ablation energy is concurrently directed to a target
site.
Using this technique, a practitioner can determine when a sufficient lesion
has
been created without interrupting the ablation procedure. The same catheter
and
electrode that are used for ablation are simultaneously used to test pacing
capture.
According to aspects of the invention, the practitioner immediately knows when
to stop ablating, indicated by loss of capture of a pacing signal at a
predetermined
maximum voltage. Thus, the danger of excessive ablation is mitigated.
An embodiment of the invention provides a method for ablating tissue
within a heart of a subject, which is carried out by inserting a probe into a
cham-
ber of the heart, disposing the probe in proximity to a target in the chamber
for
ablation of the target, pacing the heart by transmitting a pacing signal
through the
probe, and directing energy from the probe toward the target to ablate tissue
therein until the pacing signal is no longer captured in the heart.
In one aspect of the method, pacing the heart and directing energy from the
probe are performed simultaneously.
In a further aspect of the method, pacing the heart and directing energy
from the probe are iteratively performed in alternation.
4
CA 02578667 2007-02-13
Yet another aspect of the method includes determining whether the pacing
signal is captured following each performance of pacing the heart.
In an aspect of the method, directing energy comprises conducting an en-
ergy signal through the probe on a common channel with the pacing signal.
According to still another aspect of the method, the pacing signal and the
energy signal have different frequencies.
According to a further aspect of the method, the energy is radiofrequency
energy.
In yet another aspect of the method, after the pacing signal is no longer cap-
tured in the heart, the magnitude of the pacing signal is increased until the
pacing
signal is recaptured in the heart, after which the step of directing energy is
per-
formed a second time.
An additional aspect of the method is carried out after disposing the probe
by monitoring temperature in the vicinity of the target.
One aspect of the method is carried out after disposing the probe by moni-
toring an electrical activation map of the heart.
Yet another aspect of the method includes obtaining an ultrasound image of
the target through the probe while directing energy to the target.
An embodiment of the invention provides a cardiac ablation system, includ-
ing a catheter adapted for insertion into a heart, and having a distal tip and
a dis-
tally disposed electrode thereon. The system includes a first generator for
produc-
ing a pacing signal, a second generator for producing an ablation energy
signal, a
conductor in the catheter for transmitting the pacing signal and the ablation
en-
5
CA 02578667 2007-02-13
ergy signal to the electrode, and a monitor operative to provide an indication
of
capture of the pacing signal by the heart while the ablation energy signal is
being
applied to the electrode.
The cardiac ablation system may include a position sensor in the catheter,
and electrical circuitry linked to the position sensor for determining a
location of
the distal tip of the catheter within the heart. The electrode can be exactly
one
common electrode for conducting the pacing signal and the ablation energy sig-
nal.
Another aspect of the present invention is a use of the cardiac ablation sys-
tem described above for ablating tissue in a heart of a subject.
BRIEF DESCRIPTION OF THE DRAWINGS
For a better understanding of the present invention, reference is made to the
detailed description of the invention, by way of example, which is to be read
in
conjunction with the following drawings, wherein like elements are given like
reference numerals, and wherein:
Fig. 1 is a pictorial illustration of a system for performing ablative proce-
dures on a heart of a living subject in accordance with a disclosed embodiment
of
the invention;
Fig. 2 is a block diagram of a portion of the system shown in Fig. 1, in
which the output of a radiofrequency power source is mixed with a pacing
signal,
in accordance with a disclosed embodiment of the invention;
Fig. 3 is a flow chart illustrating a method of assessing a lesion formed by
intracardiac ablation in accordance with a disclosed embodiment of the
invention;
6
CA 02578667 2007-02-13
Fig. 4 is a schematic of the distal tip of a catheter for use in the system
shown in Fig. 1 in accordance with an alternate embodiment of the invention;
Fig. 5 is an end view of a distal portion of a catheter having a fenestrated
tip, for use in the system shown in Fig. 1, in accordance with an alternate em-
bodiment of the invention;
Fig. 6 is a sectional view taken along line 6-6 of the catheter shown in
Fig. 5; and
Fig. 7 is an end view of a distal portion of a catheter having a multiply fen-
estrated tip, for use in the system shown in Fig. 1, in accordance with an
alternate
embodiment of the invention.
DETAILED DESCRIPTION OF THE INVENTION
In the following description, numerous specific details are set forth in order
to provide a thorough understanding of the present invention. It will be
apparent
to one skilled in the art, however, that the present invention may be
practiced
without these specific details. In other instances, well-known circuits,
control
logic, and the details of computer program instructions for conventional
algorithms and processes have not been shown in detail in order not to obscure
the present invention unnecessarily.
Software programming code, which embodies aspects of the present
invention, is typically maintained in permanent storage, such as a computer
readable medium. In a client-server environment, such software programming
code may be stored on a client or a server. The software programming code may
be embodied on any of a variety of known media for use with a data processing
7
CA 02578667 2007-02-13
system. This includes, but is not limited to, magnetic and optical storage
devices
such as disk drives, magnetic tape, compact discs (CD's), digital video discs
(DVD's), and computer instruction signals embodied in a transmission medium
with or without a carrier wave upon which the signals are modulated. For
example, the transmission medium may include a communications network, such
as the Internet. In addition, while the invention may be embodied in computer
software, the functions necessary to implement the invention may alternatively
be
embodied in part or in whole using hardware components such as application-
specific integrated circuits or other hardware, or some combination of
hardware
components and software.
Embodiment 1
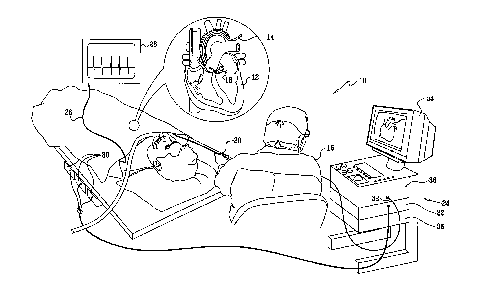
Turning now to the drawings, reference is initially made to Fig. 1, which is
a pictorial illustration of a system 10 for performing ablative procedures on
a
heart 12 of a living subject in accordance with a disclosed embodiment of the
in-
vention. The system comprises a probe, typically a catheter 14, which is
percuta-
neously inserted by an operator 16, who is typically a physician, through the
pa-
tient's vascular system into a chamber or vascular structure of the heart. The
op-
erator 16 brings the catheter's distal tip 18 into contact with the heart wall
at a
target site that is to be ablated. Radiofrequency electrical current is then
con-
ducted through wires in the catheter to one or more electrodes at the distal
tip 18,
which apply the radiofrequency energy to the myocardium. The energy is ab-
sorbed in the tissue, heating it to a point (typically about 50 C) at which it
per-
manently loses its electrical excitability. When successful, this procedure
creates
non-conducting lesions in the cardiac tissue, which disrupt the abnormal
electrical
pathway causing the arrhythmia.
The catheter 14 typically comprises a handle 20, having suitable controls to
enable the operator 16 to steer, position and orient the distal tip 18 of the
catheter
as desired during the ablation. To aid the operator 16, the distal portion of
the
catheter 14 contains position sensors (not shown) that provide signals to a
posi-
8
CA 02578667 2012-09-26
tioning processor 22, located in a console 24. ECG electrodes (not shown) on
the
patient's body surface conduct electrical signals via a cable 26 to an ECG
moni-
tor 28. The catheter 14, may be adapted, mutatis mutandis, from the ablation
catheter described in commonly assigned U.S. Patent No. 6,669,692.
The positioning processor 22 is an element of a positioning subsystem that
measures location and orientation coordinates of the catheter 14. Throughout
this
patent application, the term "location" refers to the spatial coordinates of
the
catheter, and the term "orientation" refers to its angular coordinates. The
term
"position" refers to the full positional information of the catheter,
comprising both
location and orientation coordinates.
In one embodiment, the positioning subsystem comprises a magnetic posi-
tion tracking system that determines the position and orientation of the cathe-
ter 14. The positioning subsystem generates magnetic fields in a predefined
work-
ing volume, and senses these fields at the catheter. The positioning subsystem
typically comprises a set of external radiators, such as field generating
coils 30,
which are located in fixed, known positions external to the patient. The coils
30
generate fields, typically electromagnetic fields, in the vicinity of the
heart 12.
In an alternative embodiment of the positioning subsystem, a radiator in the
catheter 14, such as a coil, generates electromagnetic fields, which are
received
by sensors (not shown) outside the patient's body.
The position sensor in the catheter 14 (not shown) transmits, in response to
the sensed fields, position-related electrical signals over a cable 32 running
through the catheter 14 to the console 24. Alternatively, the position sensors
in
the catheter 14 may transmit signals to the console 24 over a wireless link,
as de-
scribed in U.S. Patent Application Publication Nos. 2003/0120150
and 2005/0099290.
9
CA 02578667 2012-09-26
The positioning processor 22 calculates the location and orientation of the
cathe-
ter 14 based on the signals sent by the position sensor. The positioning
proces-
sor 22 typically receives, amplifies, filters, digitizes, and otherwise
processes sig-
nals from the catheter 14. The positioning processor 22 also provides a signal
out-
put to a display 34 that provides a visual indication of the position of the
distal
tip 18 of the catheter 14 relative to the site chosen for ablation.
Some position tracking systems that may be used for this purpose are de-
scribed, for example, in U.S. Patents 6,690,963, 6,618,612 and 6,332,089, and
U.S. Patent Application Publications 2002/0065455, 2004/0147920, and
2004/0068178. Although the positioning subsystem shown in Fig. 1 uses mag-
netic fields, the methods described below may be implemented using any other
suitable positioning subsystem, such as systems based on electromagnetic
fields,
acoustic or ultrasonic measurements.
Alternatively, the system 10 can be realized as the Carto-Biosense Navi-
gation System, available from Biosense Webster, Inc., 3333 Diamond Canyon
Road, Diamond Bar, CA 91765, suitably modified to execute the procedures de-
scribed herein.
Embodiments of the present invention combine simultaneous ablation and
pacing so that an ablation lesion can be assessed in near real time, without
inter-
rupting the procedure. For this purpose, the console 24 includes a
radiofrequency
power source 36 that generates a radiofrequency ablation power signal. A power
output of 50 watts at a frequency of 13.56 MHz is suitable. The console 24 is
provided with a low frequency pacing generator 38 that produces a cardiac
pacing
signal. The pacing generator 38 typically has circuitry for varying its output
volt-
age under control of the operator 16, e.g., from 3-6 volts, while maintaining
a
constant current output. Alternatively, the pacing generator 38 may maintain a
constant voltage, while varying its current output. The outputs of the
radiofre-
CA 02578667 2012-09-26
quency power source 36 and the pacing generator 38 are conducted to the cathe-
ter 14 via the cable 32.
Reference is now made to Fig. 2, which is a block diagram of a portion of
the system 10 (Fig. 1) in which the output of the radiofrequency power source
36
is mixed with the pacing signal produced by the pacing generator 38 in a
mixer 40, in accordance with a disclosed embodiment of the invention. Since
the
radiofrequency and pacing signals are at different, widely spaced frequencies,
the
pacing signal does not substantially affect the ablation power, and the
ablation
signal has no effect on pacing of the heart. The combined waveform is
conducted
through the catheter 14 along a wire 42 that acts as a common channel for the
combined waveform. The combined waveform is applied to a common elec-
trode 44 at the distal tip 18 of the catheter 14 to simultaneously pace the
patient's
heart and deliver ablation energy to the target. The electrode 44 can be con-
structed in accordance with U. S. Patent Application Publication
No. 2004/0158141, of common assignee herewith. The ECG monitor 28 (Fig. 1)
indicates whether the heart has actually captured the pacing signal. A
position
sensor 46 is typically located within the distal tip 18, adjacent to the
electrode 44.
The position sensor 46 can be an ultrasound position sensor of the type
described
in U.S. Patent No. 6,751,492, issued to Ben-Haim.
Alternatively, the output of the radiofrequency power source 36 can be in-
terlaced with the output of the pacing generator 38. In this mode of
operation, the
radiofrequency power source 36 is periodically disabled for a short time, typi-
cally 5-60 milliseconds. During this interval, the pacing generator 38 is
operative
to generate a pacing signal.
As a further alternative, the radiofrequency power source 36 remains dis-
abled for a period of time, during which the pacing generator 38 is enabled
for a
period that is sufficiently long for a determination to be made, automatically
or
11
CA 02578667 2007-02-13
by the operator, whether the pacing signal has been captured. After this
determi-
nation, the radiofrequency power source 36 is re-enabled if additional
ablation is
necessary.
Although the electrode 44 is shown as a single electrode as shown in Fig. 2,
the catheter 14 may comprise any number of electrodes in any form. For
example,
the catheter 14 may comprise two or more ring electrodes, a plurality or array
of
point electrodes, or any combination of these types of electrodes for
performing
the therapeutic functions described herein.
Monitoring Ablation
The approach taken according to embodiments of the invention for assess-
ing the extent of lesions created by ablation is to attempt to pace the heart
concur-
rently with ablation through an electrode that is applied to the ablated
region. If
the pacing signal is "captured", i.e., the heartbeat synchronizes with the
pacing
signal, then lesion formation is considered to be incomplete. In the past it
was
necessary to stop the ablation procedure in order to test for capture of the
pacing
signal, and then resume the procedure afterwards if it was determined that
further
ablation was required.
Reference is now made to Fig. 3, which is a flow chart illustrating a method
of assessing a lesion formed by intracardiac ablation in accordance with a dis-
closed embodiment of the invention. At initial step 48, the operator
introduces the
catheter 14 (Fig. 1) into the heart conventionally.
Next, at step 50, the operator positions the distal tip 18 at the target site,
us-
ing position indications provided by the display 34 to navigate the catheter
within
the cardiac chambers.
Next, at step 52, the operator activates the pacing generator 38 and in-
creases the pacing voltage until the pacing signal is captured.
12
CA 02578667 2007-02-13
Next, at step 54, the radiofrequency power source 36 is activated, and the
operator begins to ablate tissue at the target site.
Next, at delay step 56 it is expected that a lesion forms in the wall of the
heart 12. Ablation continues until one of the following events has occurred:
(1)
the extent of the lesion is such that the pacing signal is no longer captured
or (2) a
timeout interval has been exceeded, typically 10 cycles. The timeout interval
is
not critical, and a range of 2-10 cardiac cycles is suitable.
Control now proceeds to decision step 58, where it is determined if a time-
out has occurred. If the determination at decision step 58 is affirmative,
then con-
trol proceeds to decision step 60, which is described below.
If the determination at decision step 58 is negative, then control proceeds to
step 62, where the pacing voltage is increased. The increment is generally
based
on the experience of the operator and the condition of the patient. Prior to
abla-
tion the pacing threshold for capture is typically in a range of 0.3-1.0 mA.
After
ablation the pacing threshold may rise to about 10 mA. Initially a relatively
large
increment is used in step 62, approximately 0.5mA, in order to quickly locate
the
pacing threshold. Later in the procedure the increment may be reduced to
about 0.1 mA.
Control now proceeds to decision step 64, where it is determined if the pac-
ing signal has been recaptured as a result of the increase in pacing signal
strength.
If the determination at decision step 64 is affirmative, then control returns
to de-
lay step 56, and the ablation continues.
If the determination at decision step 64 is negative, then control proceeds to
decision step 66, where it is determined if a predetermined maximum level has
been reached. When constant current pacing is used, the maximum value is typi-
13
CA 02578667 2007-02-13
cally set at about 2-3 times the level of the initial pacing threshold. For
example,
if the initial threshold is 0.5 mA, ablation may be considered to be complete
once
the threshold rises to 1.5 mA. If the determination at decision step 64 is
negative,
then control returns to step 62.
If the determination at decision step 64 is affirmative, then it is concluded
that the extent of the lesion created by the ablation is sufficient. The
procedure is
terminated successfully at final step 68 and then terminated. Of course, when
re-
entrant loops or other abnormal conduction paths are complex, the sequence be-
ginning at step 50 can be iterated at another target site.
Decision step 60 is performed if the determination at decision step 58 is af-
firmative. At this point, it has not been possible to interrupt capture of the
pacing
signal by ablation. The operator now decides whether to reposition the
catheter
and make further attempts at ablation. If the determination at decision step
60 is
negative, then the procedure is declared to be unsuccessful and terminated at
final
step 70.
If the determination at decision step 60 is affirmative, then control proceeds
to step 72, where the radiofrequency power source 36 and pacing generator 38
are
reset. Control then returns to step 50 for adjustment in the position of the
distal
tip 18.
Embodiment 2
The method disclosed above with reference to Fig. 2 may be combined with
other lesion assessment techniques. Reference is now made to Fig. 4, which is
a
schematic of the distal tip 18 of the catheter 14 (Fig. 1) in accordance with
an al-
ternate embodiment of the invention. The distal tip 18, shown in juxtaposition
to
target tissue 74, is now provided with an array of ultrasound transducers 76
and a
temperature sensor 78, for additional lesion production and assessment as de-
scribed, for example, in U.S. Patent Nos., 5,443,489, 6,321,109, 6,083,170,
14
CA 02578667 2012-09-26
6,301,496 and U.S. Patent Application Publication Nos. 2004/0143258
and 2004/0147920. The transducers 76 and the temperature sensor 78 are con-
nected to suitable signal processing circuitry in the console 24 (Fig. 1),
which can
be realized as the above-noted Carto-Biosense Navigation System. Lesion as-
sessment may be conducted simultaneously using feedback from electrical ob-
tained using the electrode 44 and local temperature information obtained from
the
temperature sensor 78 in conjunction with information obtained using the trans-
ducers 76.
The transducers 76 are typically realized as a phased array. In this embodi-
ment a segment 80 of the wall of the catheter that generally opposes the
transduc-
ers 76 is sonolucent, so that the transducers 76 have a field of view 82
directed
generally forward, as indicated by broken lines. As the electrode 44 is solid,
transfer of energy to the target tissue occurs efficiently. However, when
using this
embodiment, it may be necessary to adjust the position of the distal tip 18 in
or-
der to bring the ablation site into the field of view 82.
Alternatively, a 2-dimensional array of transducers, or even a single ele-
ment transducer can be used. The transducers 76 may be forward looking or have
other directional characteristics, for example they may be side looking, or
could
even be omnidirectional. Typically, the array comprises at least ten
transducers,
each of which is no more than 0.5 mm across. The console 24 drives the trans-
ducers 76 at a high frequency, typically in the range of 5-15 MHz. An array of
sixteen transducers under these conditions, for example, is capable of
producing
images (including Doppler images) of tissue with a resolution of about 0.1 mm.
The transducers 76 may be used in this manner to determine the thickness and
other qualities of the target tissue 74 prior to ablation, and to assess the
progress
and results of the ablation procedure.
In one embodiment, the transducers 76 can be used to determine the tem-
perature of the target tissue 74 as a measure of the extent of ablation, in
addition
CA 02578667 2007-02-13
to or instead of temperature measurements that may be made by the temperature
sensor 78. To determine the temperature, the propagation speed of ultrasonic
waves in a surface layer 84 is assessed, by measuring the round-trip time of
the
waves that are reflected from a far surface 86 of the target tissue 74 and
which
then return to the transducers 76. Generally, the propagation speed of the
ultra-
sonic waves increases with tissue temperature. In water, for example, the
speed of
ultrasonic waves varies by about 2 m/s per degree. Therefore, a temperature in-
crease is perceived as a thinning of the surface layer 84, relative to
underlying
layers, as the ultrasonic waves are reflected back to the transducers 76 in a
shorter
span of time. By measuring and comparing the apparent thickness of the target
tissue 74 before and after applying radiofrequency ablation, the temperature
changes in the tissue, and hence the extent of the ablation, can be assessed.
When
the transducers 76 emit and receive ultrasonic waves at frequencies in the
range
of 10-15 MHz, apparent thickness variations on the order of 0.1 mm or less may
be detected in this manner, corresponding to temperature variations on the
order
of a few degrees.
As another example, the transducers 76 may be used to observe creation of
microbubbles in the target tissue 74 due to cavitation during ablation. The
number
of microbubbles typically increases with the tissue temperature. The microbub-
bles can be most clearly observed by subtracting successive images formed by
the
transducers 76, wherein the orderly increase and decrease in the density of
micro-
bubbles over time can be used to distinguish the microbubbles from background
noise in an ultrasonic image developed in the console 24 using known methods.
The microbubble density thus observed gives a measure of the tissue
temperature.
In still a further example, the transducers 76 may be used in a Doppler im-
aging mode to measure the speed of blood flow in a deeper layer 88 of the
target
tissue 74. Ablation of overlying layers, such as the surface layer 84, is
expected to
cause blockage of blood vessels within the deeper layer 88, thus causing
changes
16
CA 02578667 2007-02-13
in blood flow velocity. The extent of ablation is thus assessed by measuring
the
change in velocity resulting from the ablation procedure.
Alternatively or additionally, other methods for measuring tissue tempera-
ture and assessing the extent of ablated tissue may be used, as are known in
the
art. For example, the catheter 14 may comprise a miniature nuclear magnetic
resonance (NMR) sensor (not shown), which can be used to map the extent of
ablation in the vicinity of the catheter tip.
These techniques can be applied in many combinations in conjunction with
assessment of pacing signal capture as described above. For example, a
critical
local temperature may be reached before maximum pacing voltage has been rec-
ognized at decision step 66 (Fig. 3). This could lead the operator to
temporarily
cease ablation to prevent charring of tissue. Alternatively, the information
ob-
tained using the transducers 76 might reveal sufficient disruption of tissue
anat-
omy or blood flow to enable the procedure to be terminated early. For example,
when an ultrasound transducer is used in combination with the pacing and abla-
tion electrode, if the pacing and ablation electrode were touching the target
tissue,
the pacing electrode might not reach a level at which the procedure would be
re-
quired to be halted. However, the ultrasound transducer would detect changes
that
would then trigger a halt to the procedure. Alternatively, if the catheter tip
is not
directed toward the target, the forward-looking ultrasound transducer would
not
detect progress of the ablation. Nevertheless, attainment of the pacing
threshold
would alert the operator to halt the procedure.
Embodiment 3
Reference is now made to Fig. 5, which is an end view of a distal por-
tion 90 of a catheter suitable for use in the system 10 (Fig. 1), in
accordance with
an alternate embodiment of the invention. In this embodiment, the tip is
provided
with an ablation electrode 92 having a central aperture 94 measuring about 1-
1.5
mm in diameter.
17
CA 02578667 2007-02-13
Reference is now made to Fig. 6, which is a sectional view taken along
line 6-6 of the distal portion 90 of the catheter shown in Fig. 5. The
electrode 92
extends a short distance behind the tip of the catheter, being delimited by a
bro-
ken line 96. An ultrasound transducer 98 is positioned a short distance behind
the
aperture 94, being separated from the distal end of the catheter by a plug 100
of a
sonolucent material such as silicon, and having an interface 102 with the
plug 100. The transducer 98 is forward looking, and has a field of view 104,
indi-
cated by broken lines, with an operational range of about 8 mm. The field of
view 104 extends through the aperture 94 and encompasses the target tissue,
but
transfer of energy is somewhat reduced due to reduction in the contact area of
the
electrode 92 as compared with the electrode 44 (Fig. 4).
Embodiment 4
Reference is now made to Fig. 7, which is an end view of a distal por-
tion 106 of a catheter suitable for use in the system 10 (Fig. 1), in
accordance
with an alternate embodiment of the invention. The construction of this embodi-
ment is similar to the distal portion 90 (Fig. 6). However, in this
embodiment, the
tip is provided with an ablation electrode 108 having multiple small
perforations
or fenestrations 110, measuring about 0.1 mm in diameter, that are separated
by
solid areas 112. Typically, there are about 75 fenestrations 110. However, the
number is not critical. While round perforations are shown in the electrode
108 in
Fig. 7, other shapes may be equally effective.
This embodiment has characteristics that are intermediate between the de-
sign shown in Fig. 4 and the design shown in Fig. 5 and Fig. 6. The solid ar-
eas 112 partially block the field of view of the transducer, but energy
transfer
from the electrode is increased, as compared with embodiment of Fig. 5. Fur-
thermore, this embodiment has the advantage that the field of view of the
trans-
ducer extends through the fenestrations 110 and includes the target area.
Hence,
there is no need to revise the position of the catheter tip to obtain an
ultrasound
18
CA 02578667 2007-02-13
image of the target during operation, as may be the case with the embodiment
of
Fig. 4.
It will be appreciated by persons skilled in the art that the present
invention
is not limited to what has been particularly shown and described hereinabove.
Rather, the scope of the present invention includes both combinations and sub-
combinations of the various features described hereinabove, as well as
variations
and modifications thereof that are not in the prior art, which would occur to
per-
sons skilled in the art upon reading the foregoing description.
19