Note: Descriptions are shown in the official language in which they were submitted.
CA 02578684 2006-12-12
WO 2006/002338
PCT/US2005/022378
SYSTEMS AND METHODS FOR MONITORING COUGH
1. FIELD OF THE INVENTION
[0001] The present invention provides systems and methods for real-time
physiological
monitoring, particularly of a sleeping subject in a home environment, and more
particularly
of cough frequency and EEG arousals during sleep. The invention is also useful
for
monitoring awake and/or ambulatory subjects.
2. BACKGROUND OF THE INVENTION
[0002] Cough is a frequent complaint of COPD (chronic obstructive pulmonary
disease)
patients (and other patients) that can significantly impact quality of life at
both a functional
and a nuisance level. It is expected that understanding cough in disease
progression and
treatment will enable more targeted treatments and better understanding of the
patient's
disease experience. However, true cough frequency and its circadian
distribution remain
relatively unknown because it has been difficult to objectively quantify cough
in the 'real
world environment' for a number of technical reasons leaving. Objective
quantification of
cough by other routine has been difficult and time consuming for both
researchers and
subjects.
[0003] Moreover, the art lacks portable and easy-to-use monitoring methods and
systems that
provide objective and quantitative data on cough and, for cough during sleep,
accompanying
EEG arousals. In the inventor(s) experience, no portable device has heretofore
demonstrated
an ability to recognize coughs and to monitor cough frequency or to provide
concurrent
cough and EEG data. Although a number of portable devices for assessing
daytime and night
time cough have been reported, none has been reported to assess night time
cough together
with its influence on sleep architecture as revealed by electroencephalography
(EEG). See,
e.g., Cox et al., 1984, An electromyographic method of objectively assessing
cough intensity
CA 02578684 2006-12-12
WO 2006/002338
PCT/US2005/022378
and use of the method to assess effects of codeine on the dose-response curve
to citric acid.
British Journal of Clinical Pharmacology 18: 377-382, 1984; Munyard et al.,
1994, A new
device for ambulatory cough recording. Pediatric Pulnionology 18: 178-186,
1994; and
Subburaj et al., 1996, Methods of recording and analyzing cough sounds.
Pulnzonaty
Pharmacology 9: 269-279, 1996.
[0005] Considerable confusion in the art has resulted from this lack of
objective methods and
systems for monitoring cough and sleep. On one hand, it has been previously
reported that
sleep suppresses cough. See, e.g., Hsu et al., Coughing frequency in patients
with persistent
cough: assessment using a 24 hour ambulatory recorder. European Respiratory
Journal 7:
1246-1253, 1994. Studies from EEG laboratories have reported that cough is
almost
completely absent in stage 3 and 4 sleep (deep sleep) and is further not
thought to be
accompanied by night time awakenings. See, e.g., Power et al., 1984, Nocturnal
cough in
patients with chronic bronchitis and emphysema. American Review of Respiratory
Disease
130: 999-1001, 1984. On the other hand, it has also been reported that the
nocturnal cough
and wheezing associated with asthma may impact sleep quality. In the study of
Selby et al.,
1997, Inhaled salmeterol or oral theophylline in nocturnal asthma? American
Journal of
Respiratory & Critical Care Medicine 155: 104-108, 1997, patients either
received 50 fig
salmeterol or individually dose-titrated sustained-release oral theophylline.
Post sahneterol
treatment, patients reported an improved quality of life. The authors did
observe fewer
nocturnal arousals, but they did not indicate whether the arousals were due to
airway
obstruction or to cough. Sleep architecture did not appear to differ pre/post
treatment.
[0006] On the other hand, others report that sleep in patients with a number
of sleep
disorders, pulmonary disorders, and in some elderly is punctuated with
frequent, brief
arousals. The arousals are transient and generally do not result in behavioral
awakening,
reoccurring in some conditions as often as once per minute. The arousing
stimulus differs in
the various disorders and can be identified in some cases (i.e. cough, apnea,
leg movements,
pain), whereas in other cases (i.e. "normal" sleep of elderly, some insomnias)
it is idiopathic.
EEG data during sleep reveals patients arouse to cough. Thus, multiple cough
bouts over the
course of the night yield multiple arousals and, therefore, may ultimately
influence over all
sleep quality. The important fact is that the arousals result in fragmented
sleep rather than
shortened sleep. Just as with shortened sleep, it now is clear that sleep
fragmentation leads to
increased daytime sleepiness and other deleterious effects.
- 2 -
CA 02578684 2013-07-09
WO 2006/002338
PCT/US2005/022378
[0007] This lack of objective and quantitative cough and sleep monitoring
methods and
systems has thus led to confusion in the art and has hindered management of
COPD, asthma,
and similar conditions. Such methods and systems would therefore benefit
medical research
and medical practice.
[0008] None of the references cited herein, regardless of how
characterized above, is admitted as prior to the invention of the subject
matter claimed therein.
3. SUMMARY OF THE INVENTION
[0009] The objects of this invention include objective and quantitative cough
monitoring
methods and systems in waking and sleeping subjects. Further methods and
systems monitor
sleep disturbance due to cough by also processing EEG data. This invention
will aid in
management of COPD (chronic obstructive pulmonary disease), asthma, and
similar
conditions (e.g., cystic fibrosis (CF)) and will also promote medical
research.
[0010] The systems and methods of this invention monitor subjects and gather
respiratory
and electroencephalographic (EEG) data. This respiratory data is processed to,
inter alia,
objectively recognize cough occurrences. In controlled research environments,
accuracies up
to 99% have been verified by application of the methods of this invention to
subjects also
observed by simultaneous video recording. Similar accuracies are also achieved
and
evidenced in "real life" situations, both waking and sleeping. The EEG data is
processed to,
inter alia, recognize abrupt changes in frequency that reflect brief arousals
(suggestive of an
awake state) similar to those that can be manually identified on routine
polysomnograms. If
electromyographic (EMG) data is available in an embodiment, such arousals can
be
corroborated by brief increases in EMG amplitude. These arousals are brief and
transient, and
therefore can cause uncertainties reading the standard 20 or 30-second epoch
sleep stage
scoring system or be overlooked entirely. See, e.g., Bonnet et al., 1992, EEG
arousals:
scoring rules and examples - a preliminary report from the sleep disorders
atlas task force of
the American sleep disorders association, Sleep 15: 173484, 1992.
[00111 The processed monitoring data is preferably then combined to determine
new
clinically relevant outcome variables, the cough arousal index (CAI) and a
cough disturbance
index (CDT). This CAI reflects the number of nocturnal coughs associated with
an EEG
-3-
CA 02578684 2006-12-12
WO 2006/002338
PCT/US2005/022378
arousal during each hour of sleep. If nocturnal coughs are not associated with
an EEG
arousal, they are counted in a cough disturbance index (CDI) which is defined
by the number
of coughs per hour of sleep not associated with an arousal. These new indices
are for medical
management of individual patients and also for medical research, for example,
for the
understanding of the anti-tussive and/or pro-tussive profiles of
pharmacological compounds.
[0012] In more detail, the present invention provides methods for monitoring a
subject during
sleep by recording respiratory and EEG data, by recognizing the occurrences of
coughs from
the respiratory data, by recognizing the occurrences of transient EEG arousals
from the EEG
data; and by detecting and cough-arousal event when a recognized event occurs
in association
with a recognized EEG arousal. The methods further determine a cough arousal
index as the
number of cough-arousal events per time period during sleep. The present
invention also
provides systems for monitoring a subject during sleep that preferably include
garments
comprising sensor for respiratory and EEG signals, and a computer system in
data
communication with the garment for performing the methods of this invention.
The present
invention also provides a program product with a computer readable medium on
which is
encoded instructions for performing the methods of this invention. Further
embodiments
provides methods for use of a cough arousal index: for treating a patient
subject to cough by
determining the patient cough arousal index; and administering medication in
order that the
patient's cough arousal index is within selected bounds; and for evaluating a
therapeutic agent
by administering the therapeutic agent to a subject; and monitoring the
subject's cough
arousal index.
[0013] This invention includes the following embodiments. In a first
embodiment, this
invention includes a computer-implemented method for monitoring cough in a
subject that
processes tidal volume (VT) data obtained from said subject in order to
recognize a
respiratory event when a peak-to-peak amplitude of a breath exceeds a
threshold; processes
sound data obtained from said subject in order to recognize a sound event when
a sound
envelope exceeds a threshold; processes each recognized event respiratory to
determine if it
temporally overlaps a sound event and further to determine if it has an
expiration-inspiration
pattern characteristic of a cough; and selects as a cough event each
respiratory event that
overlaps a sound event and that has said characteristic expiration-inspiration
pattern.
[0014] Selected aspects of this embodiment include obtaining sound data from a
sensor in
contact with, or in close proximity to, said subject's throat; and further
processing
- 4 -
CA 02578684 2006-12-12
WO 2006/002338
PCT/US2005/022378
accelerometer data obtained from said subject in order to recognize motion of
said subject; to
retain said selected cough event if no subject motion is recognized during
said cough; and
otherwise to discard said cough event if subject motion is recognized during
said cough.
[0015] In a second embodiment, this invention includes a computer-implemented
method for
monitoring cough in a subject that processes respiratory data and sound data
obtained from
said subject in order to recognize cough events; processes said EEG data
obtained from said
subject in order to recognize transient arousal events; and detects a cough-
arousal (CA) event
when a recognized cough event occurs in association with a recognized EEG
arousal event.
[0016] Selected aspects of this embodiment include processing accelerometer
data obtained
from said subject in order to recognize motion of said subject; retain said
selected cough
event if no subject motion is recognized during said cough; and otherwise
discard said cough
event if subject motion is recognized during said cough; and further
comprising determining
a CA index (CM) for a selected period of time as the number of CA events
during said
selected period of time and a plurality of CAIs for selected periods of time
spanning a period
of sleep of said subject.
[0017] In a third embodiment, this invention includes a computer-implemented
method for
monitoring cough in a subject that processes tidal volume (VT) data and sound
data in order
to recognize coughs and further processes each cough event to determine a
ratio of the depth
of said cough event to a mean expiratory volume during a period of quiet
breathing. Selected
aspects of this embodiment then classify as a cough of cystic fibrosis if said
ratio is in a range
determined to be characteristic of cystic fibrosis coughs, or as a post-
infectious cough if said
ratio is in a range determined to be characteristic of post-infectious coughs,
said post-
infectious range being less than said cystic fibrosis range; or as a cough of
chronic
obstructive pulmonary disease (COPD) if said ratio is in a range determined to
be
characteristic of COPD coughs, said COPD range being less than said post-
infectious range.
[0018] In a fourth embodiment, this invention includes a system for monitoring
a subject
during sleep having a monitoring garment comprising sensors providing
respiratory signals,
sound signals, and EEG signals from said subject; and a computer system
comprising a
computer-readable memory comprising encoded instructions for receiving said
sensor
signals; processing said respiratory signals and said sound signals in order
to recognize cough
events; processing said EEG signals in order to recognize transient arousal
events; detecting a
- 5 -
=
CA 02578684 2016-08-04
WO 2006/002338
PCT/US2005/022378
cough-arousal (CA) event when a recognized cough event occurs in association
with a
recognized EEG arousal event; and determining a CA index (CAI) for a plurality
of selected
time periods as the number of CA events during said selected period of time.
[0019] Selected aspects of this embodiment include processing said
accelerometer signals in
order to recognize motion of said subject; retain said selected cough event if
no subject
motion is recognized during said cough; and otherwise discard said cough event
if subject
motion is recognized during said cough; and a sensor providing sound signals
is in contact
with, or in close proximity to, said subject's throat.
[0020] This invention also includes program products comprising computer
readable media
on which are encoded instructions for practicing the methods of this invention
in all their
aspects. Further applications of this invention include methods directed to
solving medical
and pharmaceutical problems. For example, one such method is for treating
cough in a
subject that determines cough disturbance indices (CDI) for said subject for
selected periods
of time as the number of cough events during said selected periods of time;
and administers an anti-tussive therapeutic agent
to said subject in order that said CDIs are within selected bounds.
[0021] Another such method is for treating disordered sleep in a subject due
to cough during
sleep that determines cough arousal indices (CM) for selected periods when the
subject is
sleeping as the number of cough arousal events during said selected periods of
time during
sleep, and administers
an anti-tussive therapeutic agent to said subject in order that said CAIs are
within selected
bounds. A further such method is for.evaluating a therapeutic agent in a
subject that
determines prior cough disturbance indices (CDI) for said subject for selected
periods of time
administers said therapeutic agent to said subject; determines subsequent CDIs
for said
subject for further selected periods of time; and compares said prior CDIs
with said
subsequent CDIs to determine an effect of said therapeutic agent on cough of
said subject.
[0022] This invention also includes further aspects of this method and further
embodiments
that will be recognized from the following description, figures, and claims.
[0023] Specific embodiments of this invention will be appreciated from the
following
detailed descriptions and attached figures, and various of the described
embodiments are
recited in appended claims.
-6-
CA 02578684 2006-12-12
WO 2006/002338
PCT/US2005/022378
4. BRIEF DESCRIPTION OF THE DRAWINGS
[0024] The present invention may be understood more fully by reference to the
following
detailed description of preferred embodiments of the present invention,
illustrative examples
of specific embodiments of the invention, and the appended figures in which:
[0025] Fig. 1 illustrates a wearable monitoring device and associated
processing system;
[0026] Fig. 2 illustrates general methods of this invention;
[0027] Fig. 3 illustrates an example of cough event detection;
[0028] Fig. 4 illustrates an example of cough-arousal detection;
[0029] Fig. 5 illustrates an example diurnal cough variability in a subject
with COPD;
[0030] Fig. 6 illustrates an example of disturbed sleep architecture in
subjects with COPD;
[0031] Figs. 7A-B illustrate an example of the relation of the CM index on
pulmonary
function and an example of the lack of a similar relation in the prior art;
[0032] Fig. 8 illustrates an exemplary cough signal;
[0033] Fig. 9 illustrates methods of cough detection;
[0034] Figs. 10A-B illustrate preferred filter responses;
[0035] Fig. 11 illustrates exemplary data recorded during a cough;
[0036] Fig. 12 illustrates methods of pitch determination;
[0037] Figs. 13A-D illustrate an example of pitch determination;
[0038] Figs. 14A-B illustrate examples of coughs in a subject with COPD;
[0039] Figs. 15A-B illustrate examples of coughs in a subject with CF; and
[0040] Figs. 16A-B illustrate exemplary coughs in a subject with post-
infectious cough
(PIC).
-7-
CA 02578684 2006-12-12
WO 2006/002338
PCT/US2005/022378
5. DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
[0041] Preferred embodiments of the systems and methods of this invention are
described in
the following. In the following, and in the application as a whole, headings
are used to clarity
and convenience only.
5.2 SYSTEMS AND METHODS OF THIS INVENTION
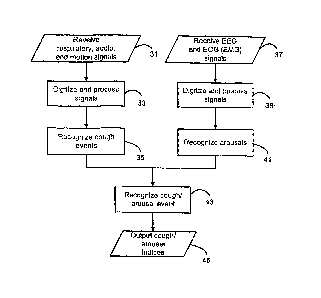
[0042] Fig. 2 generally illustrates the methods of this invention. Briefly,
these methods
process and combine two separate streams of physiological data in order to
determine novel
cough-arousal indices. Subject respiratory, audio, and motion data 31, after
pre-processing
33, are used in an objective and automatic procedure 35 to detect occurrences
of subject
coughs. Subject electroencephalogram (EEG) and electrooculogram data (EOG)
(generally,
selected electromyogram (EMG) data) 37, after pre-processing 39, are used in
an objective
and automatic procedure 41 to detect occurrences of subject arousals.
Occurrences of
recognized coughs and arousals are correlated 43 and the determined cough-
arousal and
cough-disturbance indices 45 are output. These steps and accompanying systems
are
described in more detail below.
5.2.2 RECOGNITION OF COUGH EVENTS
[0043] Data processed by this invention is preferably obtained by a wearable
monitoring
garment, such as garment or shirt 1 illustrated in Fig. 1, which is
sufficiently comfortable and
unobtrusive so that subject sleep is (substantially) not disturbed. Such a
garment carries, has
embedded, or integrally included sensors for gathering necessary subject
monitoring data,
and permits physiological recording during sleep in a home setting of up to a
full night's
duration and/or daytime recordings in an unrestricted ambulatory setting.
[0044] This garment is a preferred example of the monitoring equipment used to
provide data
for this invention. It does not limit the invention, and in other embodiments
the data
processed by this invention can be gathered by other sensor technologies known
in the art,
and by other dispositions and arrangements of such sensors on the monitored
subject.
However, for conciseness only, the following description is largely in terms
of this preferred
embodiment of the monitoring garment and associated system components.
[0045] Respiratory, audio, and motion signals 31 are obtained, respectively,
from inductive
plethysmographic (IP) respiratory sensor bands 5 and 7 (or other sensor types
providing
- 8 -
CA 02578684 2006-12-12
WO 2006/002338
PCT/US2005/022378
respiratory rate and volume information), one or more accelerometers and the
like for sensing
body posture and motion, for example exemplary accelerometer 11 illustrated as
within the
shirt, and one or more microphones for detecting cough sounds, such as throat
microphone
14. Gaiment 1 (also referred to herein as a "shirt") is made of stretchable
material that fits
sufficiently snugly to expand and contract with a subject's body so that
embedded IP sensor
bands (which, for respiratory measurement, are known as respiratory inductive
plethysmographic, or RIP, bands) can measure cross sectional areas or
circumferences of the
subject's torso. One RIP band is adequate, but preferably two RIP bands are
used: band 5 at
the level of the rib cage, and band 7 at the level of the abdomen. Details of
IP technology
(and of alternative sensor technologies known in the art) are described
following Sec. 5.3 and
in the references included therein.
[0046] EMG and EOG signals 37 are obtained from EEG and EOG sensors, such as
single
bipolar (parietally-located) EEG sensor 15 and single lead EOG sensor 13. The
EEG and
EOG sensors are preferably in electrical communication with shirt 1, for
example by means
of conductive connector 17. Additional sensors, optional for this invention,
may be in or in
communication with the shirt, and include pulse oximeters, capnographs, EEG
electrodes
(illustrated at 9a and 9b), and the like. In the hospital, clinic, or
laboratory, other signals may
be obtained from a wide range of physiological sensors.
[0047] Associated locally with preferred garment 1 is local data recording
unit 3 operatively
connected to subject sensors of the garment by data cable 2 (or by short range
radio link).
Data recoding unit 3 is preferred for ambulatory use and is preferably compact
and
lightweight so that it can be worn on a belt, put in a pocket, or embedded in
shirt 1. This unit
stores sensor data with sufficient accuracy and precision for full medical
disclosure and off-
line analysis, and may include a touch screen (or other user input facility)
for implementing a
digital diary whose data may also be transferred to the analysis computer for
correlation with
the sensor readings.
[0048] The methods of this invention are implemented by analysis software that
is executed
on analysis computers, such as computer 21. Analysis can be done either
concurrently with
signal recording (online) or at a later time (off line). For offline analysis,
sensor data can be
transferred from data recording unit 3 to analysis computer 21 on memory card
19, such as a
compact flash card. Data may alternatively be transferred by wireless links,
such as
transmission using cell phone technologies and the like. All or part of this
analysis software
- 9 -
CA 02578684 2006-12-12
WO 2006/002338
PCT/US2005/022378
implementing this invention's methods may be made available as a program
product on a
computer readable medium, such as optical disk 23. For sleep monitoring,
sensors carried by
garment 1 can be directly linked to data analysis and storage system 21.
Alternatively, a data
recording unit for use during sleep can include additional capability and
processing at the cost
of decreased portability.
[0049] Referring again to Fig. 2, initial digitization and processing of
sensor data 33 includes
as necessary digitization of analog sensor signals, filtering digitized signal
to remove noise
and artifacts. Further processing of respiratory signals includes calibration
and combination
of signals from one or more RIP bands into respiratory rate and tidal volume
(VT) signals
(and, optionally, processing to remove further remaining artifacts), and their
analysis to
determine baselines and trends. Details of the processing steps described
below.
[0050] Further processing of microphone data includes identification of lower
frequency
sound components and their temporal variability which are combined in order to
recognize
audio events characteristic of coughs. In a preferred embodiment, likely cough
events are
then identified when the lower frequency sound components exceed determined
thresholds
for determined times. These thresholds and times are preferably adjusted to
reflect the
variations of individual subjects.
[0051] Next, objective, computer implemented processes 35 combine and
correlate
preprocessed respiratory, audio, and motion signals in order to recognize
likely cough events.
These events are recognized when data is indicative of individual forceful
exhalations
occurring against a partially closed glottis in a single breath. In
particular, a likely cough is
indicated by respiratory signals with a high expiratory flow preferably
substantially above a
(temporally) locally-determined baseline expiratory flow. Further, because a
cough is an
exhalation against a partially closed glottis, they are often associated with
sound events
having lower frequency components that are substantially constant for a
certain time
intervals. A further indicator of a likely cough is observation of sound with
these
characteristics from processed microphone data. Coughs are recognized by the
occurrence of
a characteristic breath event, and likely coughs are recognized by a
coincidence of a
characteristic breath event and a characteristic sound event.
[0052] Fig. 3 illustrates likely coughs detected according to these methods.
This figure has
eight concurrent traces of, from top to bottom, a tidal volume (VT) signal, a
rib-cage RIP
- 10 -
CA 02578684 2006-12-12
WO 2006/002338
PCT/US2005/022378
band (RC) signal, an abdominal RIP band (AB) signal (the VT is a combination
of the RC
and AB signals), an electrocardiogram signal (ECG), a microphone signal (MIC),
occurrences of recognized sound events (EVT) (recognized from the MIC signal),
occurrences of recognized coughs (CGH), and the accelerometer signal (ACC). In
all traces,
time increases from left to right. The VT signal is a calibrated combination
of the RC and AB
signals, and the EVT signal indicates occurrences of sound events from the MIC
signal.
[0053] Fig. 3 illustrates three recognized likely coughs 51c, 53c, and 55c.
Cough 51c is
recognized because EVT 51b is coincident with high expiratory flow indicated
by the large
negative slope 51a in the VT signal. Similarly, coughs 53c and 55c represent
coincidences in
EVTs 53b and 55b with large negative VT slopes 53a and 55a. EVTs 57a, 57b, and
57c are
not recognized as coughs because they do not correspond negative slopes in the
VT signals.
Finally EVT 59a is not a cough because it corresponds to inspiration (positive
slope) 59a in
the VT signal.
5.2.3 RECOGNITION OF EEG AROUSALS
[0054] Returning to Fig. 2, received 37 EEG and EOG signals (alternatively,
selected EMG
signals) are preprocessed 39 and then used to recognize transient arousals 41.
Preferred EEG
sensor locations, defined using the positioning notation common in the EEG
arts, includes
central bipolar placements at C4/A1 or C3/ A2, and optional bipolar occipital
referential
placements such as 01/A2, 02/A1 or OZ/A1 or A2. Preferred bipolar EOG
electrode
placements are LOC/A1 and/or ROC/A2. In alternative embodiments, the EOG
signals may
be supplemented or replaced by submental or other EMG signals.
[0055] The received signals are next digitized and preprocessed 39. Typically
EEG and EOG
signals exceeding about 50 Hz are of less interest, so adequate signal
digitization is 100 /sec
(the Nyquist frequency); more preferably digitization is at 150 /sec or
greater, and even more .. -
preferably at 200 /sec or greater. The digitized signals are next low pass
filtered to remove
less significant higher frequencies, for example above about 50 Hz. Finally,
the signals are
processed to provide a spectrogram-type output that is reflective of signal
frequency content
versus time, preferably accord'ng to the standard EEG frequency bands, namely,
the alpha
band, the beta band, the theta band, the delta band, and so forth. This
processing can be by,
for example, a bank of time-windowed band-pass filters or a multi-resolution
wavelet
- 11 -
CA 02578684 2006-12-12
WO 2006/002338
PCT/US2005/022378
decomposition, where the filter pass bands or wavelet resolutions are selected
according to
the EEG frequency bands.
[0056] Arousals are then recognized 41 from spectrogram-type output derived
from either the
central or occipital derivation EEG by, preferably automatically, applying
rules derived from
standard definitions of EEG arousal. See, for example, Bonnet et al., 1992,
EEG arousals:
scoring rules and examples - a preliminary report from the sleep disorders
atlas task force of
the American sleep disorders association, Sleep 15: 173-184, 1992. A preferred
rule
recognizes arousals when the spectrogram reveals an abrupt shift in EEG
frequency 3
seconds or greater duration at greater than 16 Hz (e.g., theta, alpha and/or
beta frequencies)
but without spindles. Because the 3 second criteria is primarily
methodological as opposed to
physiological, other durations may be used that permit reliable recognition of
EEG frequency
shifts in the circumstances.
[0057] This rule can be qualified according to certain subsidiary rules. Since
arousals are
considered periodic phenomena disrupting sleep, one subsidiary rule is that an
arousal is
recognized when a subject has been asleep in any sleep stage for 10 or more
seconds, and
further that a second arousal is recognized when 10 seconds or more of any
sleep stage
intervenes between a prior arousal. Generally, 10 seconds is chosen because
determination of
sleeping versus waking over an interval of less than 10 seconds is less
reliable. However, the
minimum amount of intervening sleep necessary to score independent arousals
will depend
on the background EEG and may vary in the circumstances.
[0058] A further subsidiary rule makes use of the known classification of
sleep according to
EEG characteristics into REM (rapid-eye-movement) sleep or NREM (non-REM)
sleep
(NREM sleep being further sub-classified into sleep stages 1, 3, 3, and 4). In
NREM sleep,
arousals can be recognized on the basis of EEG characteristics alone. But
because bursts of
alpha or theta EEG activity are common in REM sleep and may not reflect
physiological
arousal, reliable scoring of arousal from REM sleep preferably additionally
requires that
BOG (or EMG) amplitudes increase. However, arousals cannot be scored based
solely on
= changes in EMG amplitude. In essence, if REM sleep is recognized, then
such EOG or EMG
amplitude increases are required to recognize an arousal.
[0059] Further rules useful in recogni7ing arousals can be derived from the
further conditions
=
described in, for example, Bonnet et al.
- 12 -
CA 02578684 2006-12-12
WO 2006/002338
PCT/US2005/022378
5.2.4 RECOGNITION OF COUGH/AROUSAL EVENTS
[0060] Referred again to Fig. 2, cough-arousal events are recognized 43 during
sleep when a
recognized cough 35 is detected in association with a recognized EEG arousal
41. A cough
and an arousal are associated if the cough occurs during the arousal; also a
cough and an
arousal are associated if the cough occurs within a time window that includes
an arousal. A
preferred time window precedes the arousal and has a length of approximately
30 sec. (or up
to approximately 1 min). Another further preferred time window is
approximately 30 sec. (or
up to approximately 1 min) subsequent to the arousal. Other suitable time
windows can be
determined for individual subjects. A cough is not associated with an arousal
if it does not
occur during an arousal or during a time window associated with an arousal.
[0061] Fig. 4 illustrates an exemplary cough-arousal event. This figure has
eleven concurrent
traces of, from top to bottom, an EEG signal (EEG), an BOG signal (EOG),
recognized
arousals (ARS), a tidal volume (VT) signal, a rib-cage RIP band (RC) signal, a
abdominal
RIP band (AB) signal, a high-frequency filtered VT signal (HFB), a microphone
signal
(MIC), occurrences of recognized sound events (EVT), occurrences of recognized
coughs
(CGH), and the accelerometer signal (ACC). In all traces, time increases from
left to right.
Cough 61d is recognized as the coincidence of forced expiration 61b with sound
event 61c.
And because cough 61d occurs in association with (here, during) an EEG arousal
61a, a
cough-arousal event is recognized. Forces expiration 61b is more apparent at
61b' in the HFB
signal from which low frequency components have been removed. It is also
preferably to
monitor accelerometer data from a subject. High pass filtering this data
provides information
on subject motion; low pass filtered data provides information on subject
posture. Because
motion and/or posture change can cause artifacts in sensor signals, it is
advantageous to
discard those coughs and/or arousals associated with motion and/or posture
change
[0062] The cough-arousal index (CAI) is then determined as the number of cough
arousal
events (associated coughs and arousals) per hour (or per other appropriate
time period) during
sleep. The cough disturbance index (CDI) is determined as the number of coughs
per hour
(or per other appropriate time period) during sleep that are not part of a
cough arousal event
(that is, are associated with an EEG arousal). The sum of the CAI and the CDT
is the total
number of coughs per hour.
[0063] These indices are output for use by the monitored subject and
monitoring personal.
For example, the monitored subject may adjust medication doses so that the CAI
is less than
- 13 -
CA 02578684 2006-12-12
WO 2006/002338
PCT/US2005/022378
an acceptable threshold, or within an acceptable range so that abnormalities
in the subject's
sleep architecture are adequately reduced. Medical monitoring personnel may
monitor CAIs
and CDIs of a test population in the course of drug development, testing, or
evaluation.
5.2.5 COUGH/AROUSAL INDEX EXAMPLES
[0064] The systems and methods of this invention, characteristics of
disordered sleep, and the
clinical significance of the CAI have been ascertained by the following
measurements.
[0065] Ten patients with mild to severe COPD were monitored in their homes
performing
their normal daily activities (including sleep) using the LifeShirt
monitoring system from
VivoMetrics, Inc. (Ventura, CA). The LifeShirt system implemented the
preferred
monitoring garment and data recorder described above. In particular, the
monitoring garment
included an RC and an AB RIP band sensors, a modified limb II ECG sensor, an
accelerometer sensor filtered for posture and movement, a contact microphone
sensor at
thyroid cartilage to identify cough sounds. During sleep, data from associated
EEG and EOG
sensors was also recorded. This physiological monitoring data was processed by
the
preferred methods also described above. In addition, video (with audio) tape
recordings were
used to validate the preferred automatic cough recognition. A sensitivity of
0.78, a
specificity of 1.0, and an accuracy of 0.99 were observed.
[0066] Results of these measurements include the following. First, Fig. 5
illustrates mean
cough frequency per hour throughout each of two days. Cough frequency followed
similar
circadian patterns on both days, being characterized by cough frequency peaks
at
approximately 8:00 AM and during approximately the 2-4:00 PM period. Nocturnal
cough
occurred at a significant frequency throughout most of the night except the
early morning. A
number of these nocturnal coughs, one of which was illustrated in Fig. 4,
occurred during an
EEG arousal or within a permissible time window associated with an arousal,
and thus
contributed to subjects' CAIs (other coughs being counted in the CDI).
[0067] Next, sleep was staged into NREM (stages 1-4) and REM sleep using the
previously
described rules to evaluate the recorded EEG signal, and the number of coughs
during each
sleep stage ascertained. Fig. 6 illustrates these measurements: the stippled
dark bars indicate
the mean number of coughs during the sleep stages in the COPD patients; the
black squares
indicate mean time duration the COPD patients spent in each sleep stage; and
the open
rectangles ("REF") indicate mean time normal, healthy age-matched controls
spend in each
=
- 14 -
CA 02578684 2006-12-12
WO 2006/002338
PCT/US2005/022378
sleep stage. All values are means with standard errors of the means being
conventionally
indicated by error bars (appearing as "I's"). This figure shows that these
COPD patients
experienced cough evenly distributed throughout both stages 3 and 4 of NREM
sleep and also
REM sleep. However, during NREM stage 1, coughs were somewhat increases; and
during
NREM stage 2, an exceptional number of coughs occurred. Thus, nocturnal cough
occurred
most frequently during the lighter sleep stages, and hence these COPD patients
spent a
greater than normal percentage of time in stage 1 sleep.
[0068] Thus, nocturnal cough is likely to be preventing these COPD patient
from progressing
naturally to deeper sleep stages, leading a disruption of sleep architecture
in which an unusual
percentage of time is spent in stage 1 and 2 sleep. This disruption is likely
to adversely affect
the daytime performance, decrease quality of life, and perhaps lead to further
problems. This
confirms the importance of monitoring and treating nocturnal coughs in
susceptible subjects.
[0069] Further, the CAI was determined for these monitored COPD patients
according to the
previously described methods, and each patient's CAI was correlated to that
patient's percent
predicted peak expiratory flow. The percent predicted expiratory flow, which
is the
percentage ratio of a patient's FEVI to the FEVI predicted for normal, age-
matched controls,
is a known measure of airway obstruction useful in monitoring COPD. Fig. 7A
illustrates the
results of this comparison: a bivariate fit of percent predicted peak
expiratory flow with CAI
shows a correlation strength of 0.64 at a significance of 0.05. This
correlation confirms the
utility of the CAI as a clinically variable linking observed pulmonary
function and sleep
quality.
[0070] It is significant that the measurements and indices of this invention
are determined
objectively by computer-implemented methods. These measurements do not rely on
patient
questioning and recollection. In contrast, prior determinations of cough and
disordered sleep
have relied on such patient recollection and reporting, both of which are
known to be
unreliable. Fig. 7B illustrates another evidently unreliable prior art
comparison of percent
predicted peak expiratory flow with an index of cough. The cough index used,
in the absence
of long term recording of cough occurrences, was sensitivity to capsaicin, a
cough inducing
irritant which is an active component of peppers and used in scale of
gustatory spiciness. In
comparison with Fig. 7A, which demonstrates an objectively-determined cough
cough-
arousal index that strongly correlates with percent predicted peak expiratory
flow, this figure
-15-
CA 02578684 2006-12-12
WO 2006/002338
PCT/US2005/022378
demonstrates no observable correlation in either COPD or asthma patients
between percent
predicted peak expiratory flow and this cough index.
5.3 PREFERRED SYSTEMS AND METHODS
[0071] This subsection further additional details of the previously described
systems and
methods.
5.3.2 PREFERRED SYSTEMS
[0072] Respiratory data preferably reflects time-varying cross-sectional areas
of the subject's
rig cage, and also advantageously the subject's abdomen. Techniques of signal
processing
and pattern recognition with reference to established physiological models
(such as the two-
compat __ (went model of respiratory volumes) can yield indicia or measures of
physiological
functions and times of occurrences of physiological events. For example, it is
possible to
obtain respiratory rate, tidal volume indicia, indicia of cardiac stroke
volumes, occurrence
times of respiratory apneas, and the like.
[0073] One preferred sensor technology fur such measurements is inductive
plethysmography (IP). This technology has been clinically confirmed to provide
reliable,
semi-quantitative and quantitative data on cardiac and respiratory functions.
Briefly, IP
measures the inductance of conductive loops (generally, configured as sensor
bands) that are
placed at various levels about the thorax, abdomen, and other body structures
of a monitored
subject. Such time-varying loop inductance measurements reflect primarily the
time-varying
cross-sectional areas enclosed by these loops.
[0074] However, this invention is not limited to IP-based sensors, and
alternative sensor
technologies can be employed. Possible alternative sensor technologies make,
similar to IP-
based sensors, measurements reflective of cross-sectional areas, or
circumferences, or their
geometric equivalents (for example, stress or strain), at one or more levels
through the thorax,
abdomen, or other body structures. Their signals can be processed by methods
already
developed for IP sensor signals. For example, alternative sensors can be based
on thread and
fabric technologies being and to be developed: a sensor may measure the
resistance of
conductive threads having strain-dependent resistance may be incorporated into
garments or
bands; or a sensor may measure by optical or electrical means the local strain
of a fabric
woven so that local strain is reflective of circumferential overall strain.
For another example,
-16-
CA 02578684 2013-07-09
WO 2006/002338
PCT/US2005/022378
alternative sensors may use energy radiation (such as ultrasound, or electric,
magnetic, or
electromagnetic fields) to measure geometric parameters (such as distances)
through body
structures.
[0075] Other sensors may be incorporated in this invention as needed and when
available.
These can include, for example, sensors for chemical exposures (CO, CH4, and
the like),
sensors for biological hazards (various kinds of radiation, of organisms, and
the like), and
other sensors. Details of IP-based wearable sensors and garments are disclosed
in the sensor
and garment patents and/or the cardiac function patents.
=
[0076] Physiological sensors are preferably disposed on monitored subjects in
various kinds
of garments, for example, in bands, or in partial-shirts, or in shirts, or on
partial body suits, or
. in full body suits, and the like that are unobtrusive, comfortable, and
non-restricting fabric.
This invention includes a variety of such garments and sensor dispositions
therein, the
particulars of which depend primarily on the type and extent of Physiological
monitoring.
These garments are preferably designed to allow sleep and/or ambulatory
activities without
significant disturbance
Details of the preferred IP technology, its disposition in garments, its
processing and
interpretation, and certain closely allied sensor technologies can be found in
the following
U.S. patents and applications (the "IP patents") currently assigned to the
current assignee of
this application. U.S. patents (the sensor and garment patents")
disclosing IP technology and its disposition in fabrics and garments include,
for example,
patent nos. 6,551,252; 6,341,504; 6,047,203; 5,331,968; 5,301,678; -and
4,807,640, issued
February 28, 1989 (stretchable IP transducer).
[0077] U.S. patents (the "data processing and interpretation patents")
disclosing processing of
IP signals, for example, patent nos. 6,413,225; 6,015,388; 5,159,935;
4,860,766; 4,834,109;
4,815,473; 4,777,962; 4,648,407; 4,373,534; and 4,308,872. Similar U.S. patent
applications
includes publications US 2005/0119586 and US 2004/0249299. U.S. patents
("cardiac functions
patents") disclosing processing of IP signals to obtain measures of cardiac
function include,
for example, 5,588,425; 5,178,151; 5,040,540; 4,986,277; 4,456,015; and
4,452,252, and U.S.
application publication no. US 2003/0187341.
- 17 -
CA 02578684 2006-12-12
WO 2006/002338
PCT/US2005/022378
5.3.3 METHODS OF COUGH EVENT RECOGNITION
[0078] Generally, these methods proceed by recognizing candidate respiratory
events from
input respiratory parameters including AB, RC, and VT signals and, optionally,
candidate
sound events from audio input. Then coughs events are detected from coincident
combinations of candidate respiratory events and associated candidate sound
events. Types
and severity of coughs may be discriminated by the values of the respiratory
and sound event
parameters.
A FIRST METHOD FOR COUGH RECOGNITION
[0079] The first preferred method for cough detection uses only respiratory
data and is thus
advantageous where sound data is not available. According to a first cough
detection
method, coughs must be recognized true breaths with expiratory periods greater
than a pre-
determined threshold having a range of from 0.25 to 3 secs. A useful and
preferred threshold
is approximately 1 sec, which may be individualized. Then, true breaths
meeting these
criteria are recognized as coughs if their peak expiratory flow (PEF) is
greater than a pre-
determined threshold of the running median baseline PEF value as determined
from a
leading, two minute window. A preferred PEF threshold is between 100 and 1000%
or
greater of the running median baseline PEF value; for many subjects, a PEF
threshold greater
than approximately 250% results in adequate cough recognition. The threshold
can be
individualized to particular subjects using past monitoring data.
[0080] Fig. 8 illustrates actual subject data containing coughs 94 and 98. PEF
is determined
from the dV/dt (labeled dVt/dt) curve as short, rapid exhalations. and in
which the same two
coughs 96 and 102 are readily visible as short sharp exhalations. In this
example, PEF for
cough 96 is approximately 400% of the running median PEF baseline, while for
cough 102,
the PEF is approximately 380% of the baseline.
A SECOND METHOD FOR COUGH RE. COGNITION
[0081] The second method for cough detection incorporates sound input as an
aid to cough
detection and is preferred if sound data is not available. In this subsection
and
accompanying figures, input data and derived data are often referred to by the
following
abbreviations:
RC Ribcage (RC) measurements (input data)
-18-
CA 02578684 2006-12-12
PCT/US2005/022378
WO 2006/002338
AB Abdominal (AB) measurements (input data)
VT Tidal Volume (method input data derived as described from the
RC
and AB measurements)
TIFB High frequency band pass filtered Vt (derived data)
LFB Low frequency band pass filtered Vt (derived data)
FAB High frequency band pass filtered AB (derived data)
M/C Microphone audio signal recorded from a throat microphone
(input
data)
SE Microphone audio signal envelope (derived data)
PITCH Maximum significant audio pitch level in a selected time
interval
(derived data)
PITCHm Mean audio pitch level in selected time intervals (derived
data)
EVT Audio event and duration detector (method step)
CGH Cough marker (method output data indicating presence of a
detected
cough)
[0082] Briefly, the Vt is first filtered into high frequency and low frequency
components.
The AB signal is also filtered into high frequency components. These further
are optionally
designed to further limit high frequency noise and low frequency movement
artifacts. If the
filtered signals have peak-to-peak power amplitude, or breath amplitudes (the
difference
between maximum expiration and maximum inspiration) exceeding a predefined
threshold, T,
then both respiratory and audio signals are examined in more detail to detect
the presence of a
likely cough event. If the threshold is not exceeded, a cough event is not
likely.
[0083] Audio signals (from, for example, a throat microphone) are processed
with a speech
recognition front-end to determine if an audio event contains voiced or
unvoiced speech.
Important to this determination is the derived signal PITCHm, which is the
mean of pitch
values over a finite duration in selected bands, m. This mean level should
increase
significantly if the subject is speaking or engaged in a conversation, and not
increase in the
case of a cough. The pitch value is computed by measuring the peak-to-peak
power present in
the Cepsturm or Mel Frequency Cepstral Coefficients (lVfFCCs). Another
important derived
signal is the PITCH signal. Output from audio signal processing are pulses, as
illustrated by
the EVT trace in Fig. 11, with timing and duration equal to that of
significant audio events
detected in the input sound data.
[0084] In the absence of a sound event, no cough is detected. If a sound event
is present, its
duration determines which filtered respiratory signals should be applied to
the cough
-19-
CA 02578684 2006-12-12
WO 2006/002338 PCT/US2005/022378
signature detector. If the duration of the sound event is relatively long
(that is longer than the
median significant sound event), e.g., >= 600 msec, the low frequency band
pass filtered
respiratory data, LFB, is analyzed by a cough signature detector. If the audio
duration is
relatively short (that is longer than the median signification sound event),
e.g. <= 600 msec.,
the high frequency band pass respiratory data, HFB, is analyzed. This signal
selection has
been found to lead to adequate filtering of movement and motion artifact so
that cough
signatures may be more clearly detected.
[0085] Fig. 9 illustrates in detail this second method for cough detection.
The tidal volume
trace VT, which has been previously determined as the linearly weighted sum of
the RC and
AB bands, is first passed through 2 FIR band pass filters in parallel and the
peak power (as
reflected by the maximum of the filtered signal) is measured to determine the
existence of a
possible cough event if the peak power exceeds threshold T. Filters for the
input respiratory
signals are preferably of the finite impulse response (FIR) design, although
infinite impulse
response (BR) filters with a minimal phase shift or time delay may be used.
Here, respiratory
signal phase must be sufficiently unperturbed so that it remains temporally
coincident with
the corresponding audio signals.
[0086] A filter length of 1024 was determined as the preferable in order to
achieve the
sufficiently sharp frequency and flat phase characteristics illustrated in
Fig. 10A for the high
frequency band filter in Fig. 10B for the low frequency band filter. Table 1
lists the
parameters of these preferred respective filters, which have been selected to
filter to the
extent possible subject physical movement while retaining sufficient
respiratory movement
captured from the rib cage and abdomen (RC and AB).
Signal Stop 1 Pass 1 Stop 2 Pass 2 Stop 1 Pass Stop 2
Freq Freq Freq Freq Attenuation Attenuation Attenuation
(Hz) (Hz) (Hz) (Hz) (dB) (dB) (dB)
LFB 0.4 0.5 4.9 5.0 80 0.5 80
HFB 1.0 1.1 4.9 5.0 80 0.5 80
Table 1. FIR filter design parameters.
[0087] Next the peak-to-peak power is measured and compared to a threshold.
The peak-to-
peak power is preferably taken to be the difference between the maximum on a
positive going
signal to the minimum on a negative going signal. If it meets a predetermined
threshold, a
candidate cough event is considered likely present in the filtered respiratory
signal. If this
threshold is not met, a cough event is not considered likely and no further
processing of this
-20 -
CA 02578684 2006-12-12
WO 2006/002338
PCT/US2005/022378
portion of the signal is performed. Signals LFB, HFB, and FAB are measured to
make this
determination. Signal FAB is the filter residual from the AB filtered trace,
and is
advantageous in the event that RC and AB are out of phase and a have a
subtraction effect on
Vt decreasing the true effort in the bands.
[0088] The threshold T is preferably selected so that normal breaths are not
passed for further
examination. It can be a median or mean or other measure the subject's current
breaths.
Alternatively, a fixed threshold can be used. Generally, approximately 200 ml
expired
volume is suitable for resting or sleeping subjects., Preferably, a fixed
threshold is selected
for a particular subject population or more preferably for a particular
subject, in which can a
wide range of volumes may be suitable. A threshold can also be selected as a
percent of the
subject's current expiratory volume.
[0089] The next steps process the input microphone signal (MIC). Fig. 11
illustrates at an
enlarged scale an exemplary sound envelope - trace SE - derived from an
exemplary
microphone input - trace M/C. The sound envelope is preferably down sampled to
the same
sample frequency as all respiratory bands, that is preferably 50 Hz to
minimize the effects of
filter residuals and derivations of the respiratory signals (also preferably
sampled at 50 Hz).
This down sampling involves averaging every 30 samples from the microphone
stream,
which is initially sampled at 1500 Hz to yield the 50 Hz sound envelope. The
figure also
illustrates the determined audio event ¨trace EVT ¨ and the accompanying high
frequency
filtered Vt signal ¨ trace HFB.
[0090] Next, the sound envelope signal is processed for audio event detection
and duration
determination. The start of an audio event is recog,nind when the sound
envelope passes a
threshold determined to be a selected multiple of the calibrated background
noise threshold.
Preferably, the noise threshold is calibrated from local or long terra
microphone recordings
(up to 240 hours has been used). This signal is scaled to a variation of
between +1 and ¨1
and represents a level of 30 (arbitrary units) on the sound envelope signal
scale. An
advantageous event threshold has been found to be twice the noise threshold,
or a value of 60.
The audio event ends when sound envelope drops back below the noise threshold
(here, a
value of 60). Use of a throat microphone minimizes background noise. An audio
event is
marked in the EVT trace as a pulse of amplitude 10 (arbitrary units) and
duration equal to the
length of the audio event. If no audio event if detected, a cough is not
likely to be present and
processing of this portion of the signal ends.
-21-
CA 02578684 2006-12-12
WO 2006/002338
PCT/US2005/022378
[0091] A cough signature is found by combining the processed respiratory and
the processed
sound signals. If a possible audio event coincides with a possible respiratory
event, one of
these signals is selected depending on the audio duration and further analysed
for further
cough signature detection. Having determined the duration of a significant
audio cough event
either the LFB signal or HFB signal is further analyzed for the presence of a
cough signature.
To select the frequency band to analyze, the audio event duration is measured.
For short
audio event durations, that is for events less than about 600 ms (preferably,
individually
adjusted), the HFB signal is analyzed, because shorter coughs as revealed by
the shorted
sound even time are likely to have higher respiratory frequency components (in
order to
expire a the shorter time). Conversely, audio events of longer time duration
are likely to have
respiratory signals of lower frequency signals so that the LFB signal is
chosen for further
cough signature detection.
[0092] A typical cough signature is shown in the HFB trace of Fig. 11. A cough
signature
preferably has a sharp expiration (corresponding to a high peak expiratory
flow) followed by
a sharp inspiration in either the HFB or LFB traces or both, that occur in
association with an
audio event classified as a cough event. The lowest sample value the HFB or
LFB traces is
preferably located close to the center region of the associated audio event.
The center region
is defined as those times that are greater than 33% of the audio event
duration from the start
of the audio event and less than 33% of the event duration from the event end.
Furthermore,
this minimum value must exceed the T value, which may be selected and
calibrated based on
the mean breath volume for the particular subject (measured during times of
identified quite
or relaxed breathing).
[0093] Moreover, the slopes of the HFB or LFB traces (and the gradients of
these slopes) on
either side of the minimum are preferably within the following constraints.
First, difference
between each sample [x(n)-x(n-1)] should therefore be negative before the
center of the
signature and positive after the center and before the end. Next, the
signature should be
reasonably symmetrical with similar slopes on each side of the center sample
of minimum.
The end points of each slope on either side of the center sample or minimum
are the points
where the signal reaches maximum amplitude before starting to decrease. These
end points
should not exceed a time duration greater than 50% of the event time duration
past the end of
the event or before the end of the event. By applying these tight constraints,
the possibly of
falsely detecting a cough like event as a cough has been found to be reduced.
Alternatively,
- 22 -
CA 02578684 2006-12-12
WO 2006/002338
PCT/US2005/022378
thresholds may be specified that must be exceeded by the peak expiratory flow
and the
succeeding peak inspiratory flow.
[0094] If the cough signature detector determines that a cough is not likely,
further
processing of this portion of the signal ends. If a cough signature.is
detected, in one
embodiment, the likely presence of a cough is finally output. However, in a
preferred
embodiment, the sound signal is further analyzed to separate cough sounds from
speech
sounds. The further analysis converts the input audio waveform to a compact
parametric
representation (preferably a form of frequency versus time representation) so
that cough
sounds may be distinguished from speech sounds, the former generally having
lower
frequencies and the latter higher frequencies. Accordingly, a frequency-
related threshold
may be defined in the compact representation so that signals below the
threshold are likely to
be cough sounds. If the pitch exceeds what is likely for a cough, P, the event
is not
considered to be likely to be a cough. If the pitch determination is
satisfactory (less than P),
this embodiment output the likely presence of a cough.
[0095] The following summarizes these tests. A candidate event that has the
respiratory
signature of a cough is not considered to be a cough if the associated sound
event is
determined not to include cough sounds and/or to include speech sounds.
Conversely, a
candidate event that has the sound signature of a cough is not a considered to
be a cough if
the associated respiratory event does not have cough characteristics. An
alternate test
depending on pitch accepts a sound event as cough if the signal power below
the cough-
speech threshold increases even if there is signal power above the cough-
speech threshold. A
candidate event is also not considered a true cough if the PITCH value is
above a certain
threshold (mel-frequency threshold of 1.5-2). Even if the PITCH value is just
below this
threshold, a candidate event will not be considered a cough if the PITCHm
value is above this
threshold, where PITCHm is the average of all PITCH values within a predefm.ed
time
duration. If the average of these PITCH values is above this threshold, it is
implied that there
is speech before and after this event, and therefore this event is probably
speech.
[0096] For these further tests, the characteristics of a speech audio signal
are considered to be
stationary over time increments of approximately 10 msec., and the pitch of
the audio signal
is therefore analyzed over such segments of such time duration. An example of
the stationary
portion of a speech signal is shown in Fig. 13A (time in msec.). Even though
over longer
time durations, speech signal characteristics certainly change to reflect the
different audio
-23 -
CA 02578684 2006-12-12
WO 2006/002338
PCT/US2005/022378
sounds being generated, short-time spectral analysis is a known way to so
characterize audio
signals.
[0097] Several techniques are known for parametrically extracting and
representing the pitch
characteristics of an audio signal, such as Linear Prediction Coding (LPC),
Mel-Frequency
Cepsturrn Coefficients (MFCC), and others. MFCCs have been found to be the
preferable
method. Generally, MFCCs are based on the known variation of the human ear's
critical
bandwidths so that these coefficients are expressed in a mel-frequency scale,
which is linear
at frequencies less than 1000 Hz and logarithmic at frequencies above 1000 Hz.
These filters
capture the phonetically important characteristics of speech.
MFCC DETERMINATION
[0098] Fig, 12 is a flowchart of the preferred process of computing MFCCs and
is now
described in this subsection. It process an audio input sampled at 1500 Hz, a
sampling
frequency chosen to resolve speech and cough components. The first step in
this process, the
frame blocking step, blocks the continuous audio input signal into frames of N
samples, with
adjacent frames being separated by M samples (M <N). The first frame consists
of the first
N samples. The second frame begins M samples after the first frame, and
overlaps it by N -
M samples. Similarly, the third frame begins 2M samples after the first frame
(or M samples
after the second frame) and overlaps it by N - 2M samples. This process
continues until the
entire audio has been blocked into one or more frames. Preferred blocking
parameters N and
Mare N= 64 (which is equivalent to ¨ 40 msec. windowing and facilitates the
fast radix-2
FFT) and M= 32.
[0099] The windowing step windows each individual frame to minimize signal
discontinuities at frames boundaries. Spectral distortion is minimized by
using a continuous
and smooth window to taper the signal to zero at the beginning and end of each
frame. If a
window is defined as w(n), 0 n N ¨1, where Nis the number of samples in each
frame,
then the result of windowing is the signal
y i(n) = xi(n)w(n), 0 n N-1 (1)
The Hamming window is preferably used in this invention. It is defined as:
- 24-
CA 02578684 2006-12-12
WO 2006/002338
PCT/US2005/022378
w(n) = 0.54 ¨ 0,46 cos 27rn 0 <n<N¨ 1 (2)
(N ¨1)
[0100] The next processing step is the Fast Fourier Transform, which converts
each frame of
N samples from the time domain into the frequency domain. The FFT is a well
known
algorithm for implementing the discrete Fourier transform (DFT), which is
defined on the set
of N samples {xõ}, as follows:
N-1
Xn = E xke-27r2kn I N , n = 0,1,2,...,N ¨ 1 (3)
k=0
In general Xõ's are complex numbers. The resulting sequence {Xõ} is
interpreted as follows:
the zero frequency corresponds to n = 0, positive frequencies 0 <f <F5 / 2
correspond to
values n N 12 ¨1, while negative frequencies ¨ P /2 <f <0 correspond to
N 12 +1 n N¨i. Here, F, denotes the sampling frequency. The result of this
step is
often referred to as spectrum or periodogram. Fig. 13B illustrates the
spectrum or
periodogram of the signal of Fig. 13A.
[0101] The next step is mel-frequency wrapping. Psychophysical studies have
shown that
human perception of the frequency contents of sounds does not follow a linear
scale. Thus
for each tone with an actual frequency, f, measured in Hz, a subjective pitch
is measured on a
scale called the 'mel' scale, which has a linear frequency spacing below 1000
Hz and a
logarithmic spacing above 1000 Hz. As a reference point, the pitch of a 1 kHz
tone, 40 dB
above the perceptual hearing threshold, is defined as 1000 mels. Therefore the
following
approximate formula computes mels. for a given frequencyf in Hz:
mel(f) = 2595 * logio(1 + f I 700) (4)
[0102] Simulating the subjective audio spectrum commonly is done by a filter
bank, with
filters spaced uniformly on the mel scale as illustrated in Fig. 13C. The
filter bank preferably
has a triangular band pass frequency response, and the spacing as well as the
bandwidth is
determined by a constant mel frequency interval. The mel-filtered spectrum of
an input
S(w), thus consists of the output power of these filters when S(o) is the
input. The
number of mel spectrum coefficients, K, is typically chosen as between 18 and
24. Note that
this filter bank is applied in the frequency domain, therefore it simply
amounts to multiplying
those triangle-shape window coefficients of Fig. 13C with the time frequency
spectrum of
-25 -
CA 02578684 2006-12-12
WO 2006/002338
PCT/US2005/022378
Fig. 13B. In this method, it has been found preferable to apply a K = 10 mel
scale filter banks
to the input signal frequency spectrum due to the low sample rate.
[0103] In the final step of cepsturm determination, the log mel spectrum is
transformed back
to time resulting in the mel frequency cepsturm coefficients (MFCC). The
cepstral
representation of the speech spectrum provides a representation of the local
spectral
properties of the signal for the given frame analysis. Because the mel
spectrum coefficients
(and so their logarithm) are real numbers, they can be converted to the time
domain using the
Discrete Cosine Transform (DCT). Therefore if the mel power spectrum
coefficients that are
the result of the last step are denoted by k, k = 1,2,...,K, the MFCC's, ,
may be calculated
as:
= E log (gk, ) cos [(k ¨ Y2) ¨n7r1 n = 1, , K (5)
k=1
Note the first component, ao, is advantageously excluded from the DCT since it
represents
the mean value of the input signal that carries little speaker specific
information.
[0104] Fig. 13D illustrates the cepsturm output for the speech signal already
presented in
Figs. 13A-C. Cough and unvoiced speech sounds have been found to generally
fall below a
me-frequency threshold of 1.5-2. It is evident that voiced speech is present
in the exemplary
signal because signal power is present above this threshold in the higher
pitches. The
PITCHm signal can be obtained as a simple mean, or a power-weighted mean, or
the like of
the mel-frequency spectrum. The PITCH signal can be obtained as the maximum
(significant
or having 5% or 10% or 20% of the total mel power) mel-frequency cepstral
coefficient
resultant from the discrete cosine transform.
COUGH SEVERITY AND CLASSIFICATION
[0105] Optionally, detected coughs may be analyzed for severity and type.
Cough severity
events can be analyzed by extracting particular characteristics of the band
pass filtered lung
volume data, the LFB and HFB signals. The characteristics include the depth or
amplitude of
the cough signature and the reflex inspiratory drive at the end of the cough
signature.
Measures that allow for a discrimination of the pathological causes of coughs
include a ratio
of the depth of cough with the mean expiratory volume calculated on a per
subject bases
during identified periods of quiet and relaxed breathing. This allows severity
to be
- 26 -
CA 02578684 2006-12-12
WO 2006/002338
PCT/US2005/022378
determined based in the individual calibration and therefore aids in
determining lung disease.
Further such measures include the rate of change of both expiratory and
inspiratory volume
during a cough event. Further measures analyze segments of the cough and
compare rates of
change of volume at different intervals of the cough event.
[0106] In simpler cases, the amplitude of these signals (cough volume) and
their slope
(airflow rate) can be combined into diagnostic criteria for classifying one
type of cough from
another. These criteria reflect, for example, the different depth of cough and
the reflex
inspiratory action at the end of the cough event. Appearance of a cough
signature in the
unfiltered Vt is further indicia of particular severe cough. Using these
simpler severity
criteria, it has been found that CF coughs can be recognized because they are
likely to be of a
higher severity; and COPD coughs because they are likely to be of a lower
severity. PIC
coughs are likely to be of an intermediate severity. Presence of a cough
signature in the
ri-nfiltered tidal volume trace Vt accompanies coughs of the highest severity.
5.3.4 COUGH EXAMPLES
[0107] Various types of cough signatures and preferred criteria for there
discrimination are
now described in connection with Figs. 14A-B, 15A-B, and 16A-B. Chronic
obstructive
pulmonary disease (COPD) generally refers to a group of pulmonary disorders
that lead to
progressively worsening respiratory function. Two common causes of COPD that
progressively impair airflow to the lungs are bronchitis and emphysema. In
chronic
bronchitis, the airways are blocked and inflamed, mucus producing glands in
the bronchi are
enlarged, and an excessive amount of mucus is secreted into the lungs.
Therefore, this form
of COPD leads to an increased need to cough in order to clear this excessive
mucus.
[0108] Figs. 14A-B illustrate COPD coughs that were identified by the systems
and methods
of this invention as implemented in a software application and confirmed by
audio and video
recording. The HFB and LFB traces illustrate that the true cough in Fig. 14A
is characterized
by sharp (short duration and high airflow) expiration followed by sharp
inspiration. Further =
an audio event was detected from throat microphone input that was
characterized as having a
low pitch and most likely to include cough sounds. Fig. 14B illustrates
several non-cough
events and one true cough event from a different COPD subject. The non-cough
events are
seen as low-pitched sound events that lacked accompanying respiratory cough
indicia (sharp
inspiration and expiration in the LFB or the HFB signals). On the other hand,
the true cough
- 27 -
CA 02578684 2013-07-09
WO 2006/002338
PCT/US2005/022378
event is characterized by associated sound and respiratory events having
proper
=
characteristics.
10109] Cystic Fibrosis (CF) is a life threatening multi-system condition that
primarily affects
the lungs and digestive systems. CF leads to the secretion of sticky mucus
obstructing the
airways, and causing a need to cough frequently in order to try to clear the
mucus from the
airways. Coughing can often loosen the mucus allowing easier breathing. Figs.
15A-B
illustrate coughs from two CF patients. It is apparent from examination of the
associated
traces, especially the F1FB and LFB traces, that these coughs are more severe
than the COPD
coughs, having greater amplitudes and/or higher airflows. Furthermore, the
amplitudes are
sufficient so that cough signatures are readily identified in the unfiltered
tidal volume (Vt)
trace.
[0110] Post-infectious cough (PIC) is most common after viral infections of
the upper
respiratory tract. These infections can induce coughing due to persisting
inflammation
regardless of any increased mucus secretion. Figs 16A-B illustrate two
examples of PIC
coughs. They are seen to be of a severity intermediate between CF and COPD
coughs.
[0111] The invention described and claimed herein is not to be limited in
scope by the
preferred embodiments herein disclosed, since these embodiments are intended
as
illustrations of several aspects of the invention. Any equivalent embodiments
are intended to
be within the scope of this invention. Indeed, various modifications of the
invention in
addition to those shown and described herein will become apparent to those
skilled in the art
from the foregoing description. Such modifications are also intended to fall
within the scope
of the appended claims.
[0112] A number of references are cited herein, but none of these references,
regardless of how
characterized above, is admitted as prior to the invention of the subject
matter claimed therein.
-28 -