Note: Descriptions are shown in the official language in which they were submitted.
CA 02578858 2007-03-05
DEMANDES OU BREVETS VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVETS
COMPREND PLUS D'UN TOME.
CECI EST LE TOME DE 2
NOTE: Pour les tomes additionels, veillez contacter le Bureau Canadien des
Brevets.
JUMBO APPLICATIONS / PATENTS
THIS SECTION OF THE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME I OF 2
NOTE: For additional volumes please contact the Canadian Patent Office.
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
ARYLPYRIDAZINONES AS PROSTAGLANDIN ENDOPEROXIDE H SYNTHASE BIOSYNTHESIS
INHIBITORS
This application is a divisional application of
application Serial No. 2,299,300, filed August 10, 1998.
Technical Field
The present invention encompasses. novel pyridazinone compounds useful
in the treatment of cyciooxygenase-2 mediated diseases. More particularly,
this
invention concerns a method of inhibiting prostaglandin biosynthesis,
particularly
the induced prostagiandin endoperoxide H synthase (PGHS-2, cyclooxygenase-2,
COX-2) protein.
Background of the Invention
The prostagiandins are extremely potent substances which produce a wide
variety of biological effects, often in the nanomolar to picomolar
concentration
range. The discovery of two forms of prostagiandin endoperoxide H synthase,
isoenzymes PGHS-1 and PGHS-2, that catalyze the oxidation of arachidonic acid
leading to prostaglandin biosynthesis has resuited in renewed research to
delineate the role of these two isozymes in physiology and pathophysiology.
These isozymes have been shown to have different gene regulation and represent
distinctly different prostaglandin biosynthesis pathways. The PGHS-1 pathway
is
expressed constitutively in most cell types. It responds to produce
prostaglandins
that regulate acute events in vascular homeostasis and also has a role in
maintaining normal stomach and renal function. The PGHS-2 pathway involves an
induction mechanism which has been linked to inflammation, mitogenesis and
ovulation phenomena.
Prostaglandin inhibitors provide therapy for pain, fever, and inflammation,
and are useful therapies, for example in the treatment of rheumatoid arthritis
and
osteoarthritis. The non-steroidal anti-inflammatory drugs (NSAIDs) such as
ibuprofen, naproxen and fenamates inhibit both isozymes. Inhibition of the
constitutive enzyme PGHS-1 results in gastrointestinal side effects including
ulcers
and bleeding and incidence of renal problems with chronic therapy. Inhibitors
of
the induced isozyme PGHS-2 may provide anti-inflammatory activity without the
side effects of PGHS-1 inhibitors.
-1-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
The problem of side-effects associated with NSAID administration has never
completely been solved in the past. Enteric coated tablets and co-
administration
with misoprostol, a prostagiandin derivative, have been tried in an attempt to
minimize stomach toxicity. It would be advantageous to provide compounds which
are selective inhibitors of the induced isozyme PGHS-2.
The present invention discloses novel compounds which are selective
inhibitors of PGHS-2.
Summary of the Invention
The present invention discloses pyridazinone compounds which are
selective inhibitors of cyclooxygenase-2 (COX-2). The compounds of the present
invention have the formula 1:
R3 N~NR
R ;X
R7
where
X is selected from the group consisting of 0, S, NR4, N-ORa, and
N-NRbRc, wherein R4 is selected from the group consisting of alkyl, alkenyl,
cycloalkyl, cycloalkenyl, cycloalkylalkyl, cycloalkenylalkyl, aryl,
heterocyclic,
heterocyclic alkyl, and arylalkyl; and Ra, Rb, and Rc are independently
selected
from the group consisting of alkyl, cycloalkyl, cycloalkylalkyl, aryl, and
arylalkyl;
R is selected from the group consisting of hydrogen, alkyl, alkenyl,
alkynyl, alkylcarbonylalkyl, alkylsulfonylalkyl, alkylsulfonylarylalkyl,
alkoxy,
alkoxyalkyl, carboxy, carboxyalkyl, cyanoalkyl, haloalkyl, haloalkenyl,
haloalkynyl, hydroxyalkyl, cycloalkyl, cycloalkylalkyl, cycloalkenyl,
cycloalkenylalkyl, aryl, arylalkyl, arylaikenyl, arylalkynyl, arylalkoxy,
arylhaloalkyl, arylhydroxyalkyl, aryloxy, aryloxyhydroxyalkyl,
aryloxyhaloalkyl,
arylcarbonylalkyl, haloalkoxyhydroxyalkyl, heterocyclic, heterocyclic alkyl,
heterocyclic alkoxy, heterocyclic oxy, -C(O)R5, -(CH2)nC(O)R5, -R6-R7,
-(CH2)nCH(OH)R5, -(CH2)nCH(ORd)R5, -(CH2)nC(NORd)R5,
-(CH2)nC(NRd)R5, -(CH2)nCH(NORd)R5, -(CH2)nCH(NRdRe)R5,
-2-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
-(CH2)nC=C-R7, -(CH2)n[CH(CX'3)Jm-(CH2)n-CX'3, -(CH2)n(C X'2)m-(CH2)n
-CX'3, -(CH2)n[CH(CX'3)lm-(CH2)n -R8 , -(CH2)n(C X'2)m-(CH2)n R8
-(CH2)n(CHX')m-(CH2)n - CX'3, -(CH2)n(CHX')m-(CH2)n -R8, and -
(CH2)n-R20,
wherein R5 is selected from the group consisting of alkyl, alkenyl,
alkynyl, cycloalkyl, cycloalkenyl, aryl, arylalkyl, haloalkyl, haloalkenyl,
haloalkynyl, heterocyclic, and heterocyclic alkyl;
wherein R6 is alkylene or alkenylene, or halo-substituted alkylene
halo-substituted alkenylene;
R7 and R8 are independently selected from the group consisting of
hydrogen, alkyl, alkenyl, alkynyl, haloalkyl, cycloalkyl, cycloalkenyl, aryl,
arylalkyl, heterocyclic, and heterocyclic alkyl;
R20 is selected from the group consisting of alkyl, alkenyl, haloalkyl,
cycloalkyl, cycloalkenyl, aryl, heterocyclic, and heterocyclic alkyl;
Rd and Re are independently selected from the group consisting of
hydrogen, alkyl, alkenyl, alkynyl, haloalkyl, cycloalkyl, cycloalkenyl, aryl,
arylalkyl, heterocyclic, and heterocyclic alkyl;
X' is halogen;
n is from 0 to about 10, and m is 0 to about 5;
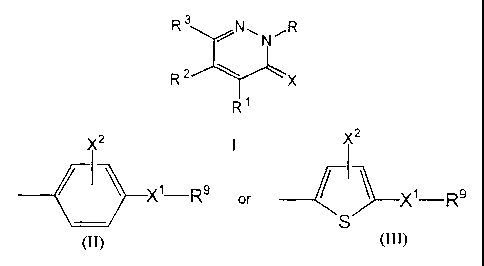
at Ieast one of R1, R2 and R3 is
2 X2
X1 Rs or )-X'-R9
S
where X1 is selected from the group consisting of -S02-, -SO(NR10)-, -SO-, -
Se02-, PO(OR11)-, and -PO(NR12R13)-,
R9 is selected from the group consisting of alkyl, alkenyl, alkynyl,
cycloalkyl, cycloalkenyl, amino, -NHNH2, -N-CH(NR10 R11), dialkylamino,
alkoxy, thiol, alkylthiol, protecting groups, and protecting groups attached
to X'
by an alkylene;
-3-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
X2 is selected from the group consisting of hydrogen, halogen, alkyl,
alkenyl, and alkynyl;
R10, R11, R12, and R13 are independently selected from the group
consisting of hydrogen, alkyl, and cycloalkyl, or R 12 and R13 can be taken
together, with the nitrogen to which they are attached, to form a heterocyclic
ring
having from 3 to 6 atoms.
The remaining two of the groups of R1, R2, and R3, are independently
selected from the group consisting of hydrogen, hydroxy, hydroxyalkyl,
halogen, alkyl, alkenyl, alkynyl, alkylamino, alkenyloxy, alkylthio,
alkylthioalkoxy, alkoxy, alkoxyalkyl, alkoxyalkylamino, alkoxyalkoxy, amido,
amidoalkyl, haioalkyl, halolkenyloxy, haloalkoxy, cycloalkyl, cycloalkylaikyl,
cycloalkenyl, cycloalkenylalkyl, cycloalkenylalkoxy, cycloalkylalkoxy,
cycloalkylalkylamino, cycloalkylamino, cycloalkyloxy, cycloalkylidenealkyl,
amino, aminocarbonyl, aminoalkoxy, aminocarbonylalkyl, alkylaminoaryloxy,
diaikylamino, dialkylaminoaryloxy, arylamino, arylalkyiamino, diarylamino,
aryl,
arylalkyl, arylalkyfthio, arylalkenyl, arylalkynyl, arylalkoxy, aryloxy,
heterocyclic,
heterocyclic alkyl, heterocyclic(alkyl) amino, heterocyclic alkoxy,
heterocyclic
amino, heterocyclic oxy, heterocyclic thio, hydroxy, hydroxyalkyl,
hydroxyalkylamino, hydroxyalkylthio, hydroxyalkoxy, mercaptoalkoxy,
oxoalkoxy, cyano, nitro, and -Y-R14, wherein Y is selected from the group
consisting of -0-, -S-, -C(R16) (R1 7)-, -C(O)NR21 R22-, -C(O)-, -C(O)O-, -NH-
, -
NC(O)-, -N=C R21 R22, N- R21 R22, and -NR19-. R14 is selected from the group
consisting of hydrogen, halogen, alkyl, alkoxyalkyl, alkylthioalkyl, alkenyl,
alkynyl, hydroxy, cycloalkyl, cycloalkylalkyl, cycloalkenylalkyl,
cycloalkenyl,
amino, cyano, aryl, arylalkyl, heterocyclic, and heterocyclic(alkyl),
R16, R17, and R19 are independently selected from the group
consisting of hydrogen, alkyl, alkenyl, cycloalkyl, cycloalkenyl, alkoxy,
aryl,
arylalkyl, heterocyclic, heterocyclic alkyl, or cyano; and
R21 and R22 are independently selected from the group consisting of
hydrogen, alkyl, alkenyl, cycloalkyl, cycloalkenyl, alkoxy, aryl, arylalkyl,
heterocyclic, heterocyclic alkyl, or cyano;
or their pharmaceutically acceptable salts, esters, or prodrugs thereof.
-4-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Detailed Description of the Invention
The present invention discloses pyridazinone compounds which are
cyclooxygenase (COX) inhibitors and are selective inhibitors of cyclooxygenase-
2
(COX-2). COX-2 is the inducible isoform associated with inflammation, as
opposed
to the constitutive isoform, cyclooxygenase-1 (COX-1) which is an important
"housekeeping" enzyme in many tissues, including the gastrointestinal (GI)
tract
and the kidneys.
In one embodiment , the compounds of the present invention have the
formula 1:
R3 N~R
R X
where
X is selected from the group consisting of 0, S, NR4, N-ORa, and
N-NRbRc, wherein R4 is selected from the group consisting of alkyl, alkenyl,
cycioalkyl, cycloalkenyl, cycloalkylalkyl, cycloalkenylalkyl, aryl,
heterocyclic,
heterocyclic alkyl, and arylalkyl; and Ra, Rb, and Rc are independently
selected
from the group consisting of alkyl, cycloalkyl, cycloalkylalkyl, aryl, and
arylalkyl;
R is selected from the group consisting of hydrogen, alkyl, alkenyl,
alkynyl, alkylcarbonylalkyl, alkylsulfonylalkyl, alkylsulfonylarylalkyl,
alkoxy,
alkoxyalkyl, carboxy, carboxyalkyl, cyanoalkyl, haloalkyl, haloalkenyl,
haloalkynyl, hydroxyalkyl, cycloalkyl, cycloalkylalkyl, cycloalkenyl,
cycloalkenylalkyl, aryl, arylalkyl, arylaikenyl, arylalkynyl, arylalkoxy,
arylhaloalkyl, arylhydroxyalkyl, aryloxy, aryloxyhydroxyalkyl,
aryloxyhaloalkyl,
arylcarbonylalkyl, halolkoxyhydroxyalkyl, heterocyclic, heterocyclic alkyl,
heterocyclic alkoxy, heterocyclic oxy, -C(O)R5. -(CH2)nC(O)R5, -R6-R7,
-(CH2)nCH(OH)R5, -(CH2)nCH(ORd)R5, -(CH2)nC(NORd)R5,
-(CH2)nC(NRd)R5, -(CH2)nCH(NORd)R5, -(CH2)nCH(NRdRe)R5,
-(CH2)nC=C-R7, -(CH2)n[CH(CX'3))m-(CH2)n-CX'3, -(CH2)n(C X'2)m-(CH2)n
-5-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
-CX'3, -(CH2)n[CH(CX'3))m-(CH2)n -R8, -(CH2)n(C X'2)m-(CH2)n R8 -(CH2)n(CHX')m-
(CH2)n - CX'3, -(CH2)n(CHX')m-(CH2)n -R8, and -
(CH2)n-R20,
wherein R5 is selected from the group consisting of alkyl, alkenyl,
alkynyl, cycloalkyl, cycloalkenyl, aryl, arylalkyl, haloalkyl, haloalkenyl,
haloalkynyl, heterocyclic, and heterocyclic alkyl;
wherein R6 is alkylene or alkenylene, or halo-substituted alkylene
halo-substituted alkenylene;
R7 and R8 are independently selected from the group consisting of
hydrogen, alkyl, alkenyl, alkynyl, haloalkyl, cycloalkyl, cycloalkenyl, aryl,
arylalkyl, heterocyclic, and heterocyclic alkyl;
R20 is selected from the group consisting of alkyl, alkenyl, haloalkyl,
cycloalkyl, cycloalkenyl, aryl, heterocyclic, and heterocyclic alkyl;
Rd and Re are independently selected from the group consisting of
hydrogen, alkyl, alkenyl, alkynyl, haloalkyl, cycloalkyl, cycloalkenyl, aryl,
arylalkyl, heterocyclic, and heterocyclic alkyl;
X' is halogen;
n is from 0 to about 10, and m is 0 to about 5;
at least one of R1, R2 and R3 is
X2 X2
I / I
}X1_R9 or 1_R9
X
S
where X1 is selected from the group consisting of -S02-, -SO(NR10)-, -SO-, -
Se02-, PO(OR11)-, and -PO(NR12R13)-,
R9 is selected from the group consisting of alkyl, alkenyl, alkynyl,
cycloalkyl, cycloalkenyl, amino, -NHNH2, -N=CH(N R10 R11), dialkylamino,
alkoxy, thiol, alkylthiol, protecting groups, and protecting groups attached
to X'
by an alkylene;
X2 is selected from the group consisting of hydrogen, halogen, alkyl,
alkenyl, and alkynyl;
-6-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
R10, R11, R12, and R13 are independently selected from the group
consisting of hydrogen, alkyl, and cycloalkyl, or R12 and R13 can be taken
together, with the nitrogen to which they are attached, to form a heterocyclic
ring
having from 3 to 6 atoms.
The remaining two of the groups of R1, R2, and R3, are independently
selected from the group consisting of hydrogen, hydroxy, hydroxyalkyl,
halogen, alkyl, alkenyl, alkynyl, alkylamino, alkenyloxy, alkylthio,
alkylthioalkoxy, alkoxy, alkoxyalkyl, alkoxyalkylamino, alkoxyalkoxy, amido,
amidoalkyl, haloalkyl, halolkenyloxy, haloalkoxy, cycloalkyl, cycloalkylalkyl,
cycloalkenyl, cycloalkenylalkyl, cycloalkenylalkoxy, cycloalkylalkoxy,
cycloalkylalkylamino, cycloalkylamino, cycloalkyloxy, cycloalkylidenealkyl,
amino, aminocarbonyl, aminoalkoxy, aminocarbonylalkyl, alkylaminoaryloxy,
dialkylamino, dialkylaminoaryloxy, arylamino, arylalkylamino, diarylamino,
aryl,
arylalkyl, arylalkylthio, arylalkenyl, arylalkynyl, arylalkoxy, aryloxy,
heterocyclic,
heterocyclic alkyl, heterocyclic alkylamino, heterocyclic alkoxy, heterocyclic
amino, heterocyclic oxy, heterocyclic thio, hydroxy, hydroxyalkyl,
hydroxyalkylamino, hydroxyalkylthio, hydroxyalkoxy, mercaptoalkoxy,
oxoalkoxy, cyano, nitro, and -Y-R14, wherein Y is selected from the group
consisting of -0-, -S-, -C(R16) (R1 7)-, -C(O)NR21 R22-, -C(O)-, -C(O)O-, -NH-
, -
NC(O)-, -N=C R21R22, N- R21R22, and -NR19-. R14 is selected from the group
consisting of hydrogen, halogen, alkyl, alkoxyalkyl, alkylthioalkyl, alkenyl,
alkynyl, hydroxy, cycloalkyl, cycloalkylalkyl, cycloalkenylalkyl,
cycloalkenyl,
amino, cyano, aryl, arylalkyl, heterocyclic, and heterocyclic(alkyl),
R16, R17, and R19 are independently selected from the group
consisting of hydrogen, alkyl, alkenyl, cycloalkyl, cycloalkenyl, alkoxy,
aryl,
arylalkyl, heterocyclic, heterocyclic alkyl, or cyano; and
R21 and R22 are independently selected from the group consisting of
hydrogen, alkyl, alkenyl, cycloalkyl, cycloalkenyl, alkoxy, aryl, arylalkyl,
heterocyclic, heterocyclic alkyl, or cyano;
or a pharmaceutically acceptable salt, ester, or prodrug thereof.
In another embodiment, compounds of the present invention have the
formula II:
-7-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
R3 R
Rl
I I
wherein Z is a group having the formula:
X2 X2
a or xi R9
S
where X1 is selected from the group consisting of -S02-, -SO-, -Se02-
,SO(NR10)-, and R9 is selected from the group consisting of alkyl, alkenyl,
alkynyl, cycloalkyl, cycloalkenyl, amino, -NHNH2, dialkylamino, alkoxy, thiol,
alkylthiol, protecting groups, and protecting groups attached to X' by an
alkylene;
R10 is selected from the group consisting of hydrogen, alkyl, and
cycloalkyl;
X2 is selected from the group consisting of hydrogen, halogen, alkyl,
alkenyl, and alkynyl;
R is selected from the group consisting of hydrogen, alkyl, alkenyl,
alkynyl, alkylcarbonylalkyl, alkylsulfonylalkyl, alkylsulfonylarylalkyl,
alkoxy,
alkoxyalkyl, carboxy, carboxyalkyl, cyanoalkyl, haloalkyl, haloalkenyl,
haloalkynyl, hydroxyalkyl, cycloalkyl, cycloalkylalkyl, cycloalkenyl,
cycloalkenylalkyl, aryl, arylalkyl, arylalkenyl, arylalkynyl, arylalkoxy,
arylhaloalkyl, arylhydroxyalkyl, aryloxy, aryloxyhydroxyalkyl,
aryloxyhaloalkyl,
aryicarbonylalkyl, haloalkoxyhydroxyalkyl, heterocyclic, heterocyclic alkyl,
heterocyclic alkoxy, heterocyclic oxy, -C(O)R5, -(CH2)nC(O)R5, -R6-R7,
-(CH2)nCH(OH)R5, -(CH2)nCH(ORd)R5, -(CH2)nC(NORd)R5,
-(CH2)nC(NRd)R5, -(CH2)nCH(NORd)R5, -(CH2)nCH(NRdRe)R5,
-(CH2)nC=C-R7, -(CH2)n[CH(CX'3)1m-(CH2)n-CX'3, -(CH2)n(C X'2)m-(CH2)n
-CX'3, -(CH2)n[CH(CX'3)1m-(CH2)n -R8, -(CH2)n(C X'2)m-(CH2)n R8
-(CH2)n(CHX')m-(CH2)n - CX'3, -(CH2)n(CHX')m-(CH2)n -R8, and -
(CH2)n-R20,
-8-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
wherein R5 is selected-from the group consisting of alkyl, alkenyl,
alkynyl, cycloalkyl, cycloalkenyl, aryl, arylalkyl, haloalkyl, haloalkenyl,
haloalkynyl, heterocyclic, and heterocyclic alkyl;
wherein R6 is alkylene or alkenylene, or halo-substituted alkylene
halo-substituted alkenylene;
R7 and R8 are independently selected from the group consisting of
hydrogen, alkyl, alkenyl, alkynyl, haloalkyl, cycloalkyl, cycloalkenyl, aryl,
arylalkyl, heterocyclic, and heterocyclic alkyl;
R20 is selected from the group consisting of alkyl, alkenyl, haloalkyl,
cycloalkyl, cycloalkenyl, aryl, heterocyclic, and heterocyclic alkyl;
Rd and Re are independently selected from the group consisting of
hydrogen, alkyl, alkenyl, alkynyl, haloalkyl, cycloalkyl, cycloalkenyl, aryl,
arylalkyl, heterocyclic, and heterocyclic alkyl;
X' is halogen;
n is from 0 to about 10, and m is 0 to about 5;
R1, and R3 are independently selected from the group consisting of
hydrogen, hydroxy, hydroxyalkyl, halogen, alkyl, alkenyl, alkynyl, alkylamino,
alkenyloxy, alkylthio, alkylthioalkoxy, alkoxy, alkoxyalkyl, alkoxyalkylamino,
alkoxyalkoxy, amido, amidoalkyl, haloalkyl, halolkenyloxy, haloalkoxy,
cycloalkyl, cycloalkylalkyl, cycloalkenyl, cycloalkenylalkyl,
cycloalkenylalkoxy,
cycloalkylaikoxy, cycloalkylalkylamino, cycloalkylamino, cycloalkyloxy, amino,
aminocarbonyl, aminoalkoxy, aminocarbonylalkyl, alkylaminoaryloxy,
dialkylamino, dialkylaminoaryloxy, arylamino, arylalkylamino, diarylamino,
aryl,
arylalkyl, arylalkylthio, arylaikenyl, arylalkynyl, arylalkoxy, aryloxy,
heterocyclic,
heterocyclic alkyl, heterocyclic(alkyl) amino, heterocyclic alkoxy,
heterocyclic
amino, heterocyclic oxy, heterocyclic thio, hydroxy, hydroxyalkyl,
hydroxyalkylamino, hydroxyalkoxy, mercaptoalkoxy, oxoalkoxy, cyano, nitro,
and -Y-R14, wherein Y is selected from the group consisting of -0-, -S-, -
C(R16)
(R17)-, -C(O)NR21 R22-, -C(O)-, -C(O)O-, -NH-, -NC(O)-, -N=C R21 R22, N-
R21 R22, and -NR19-. R14 is selected from the group consisting of hydrogen,
halogen, alkyl, alkoxyalkyl, alkylthioalkyl, alkenyl, alkynyl, hydroxy,
cycloalkyl,
cycloalkylalkyl, cycloalkenylalkyl, cycloalkenyl, amino, cyano, aryl,
arylalkyl,
heterocyclic, and heterocyclic(alkyl),
-9-
CA 02578858 2007-03-05
WO 99/10331 PC.'T/US98/16479
R16, R17, and R19 are independently selected from the group
consisting of alkyl, alkenyl, cycloalkyl, cycloalkenyl, alkoxy, aryl,
arylalkyl,
heterocyclic, heterocyclic alkyl, or cyano; and
R21 and R22 are independently selected from the group consisting of
hydrogen, alkyl, alkenyl, cycloalkyl, cycloalkenyl, alkoxy, aryl, arylalkyl,
heterocyclic, heterocyclic alkyl, or cyano;
or a pharmaceutically acceptable salt, ester, or prodrug thereof.
In yet another embodiment, compounds of the present invention have the
formula 111:
R3 N R
X
R9X1 R'
X2
III
wherein X, X1, X2, R, R1, R3, and R9 are as defined in Formula I; or a
pharmaceutically acceptable salt, ester, or prodrug thereof.
In a preferred embodiment, compounds of the present invention have
the formula III, wherein X1 is selected from the group consisting of -S02-, -
SO-, -
Se02-, and -SO(NR10)-, and R9 is selected from the group consisting of alkyl,
alkenyl, alkynyl, cycloalkyl, cycloalkenyl, amino, alkylamino, or
dialkylamino;
X2 is selected from the group consisting of hydrogen and halogen;
X is selected from the group consisting of 0, S, NR4, N-ORa, and
N-NRbRc, wherein R4 is selected from the group consisting of alkyl, alkenyl,
cyclo-
alkyl, cycloalkenyl, cycloalkylalkyl, cycloalkenylalkyl, aryl, heterocyclic,
heterocyclic
alkyl, and arylalkyl; and Ra, Rb, and Rc.are independently selected from the
group
consisting of alkyl, cycloalkyl, cycloalkylalkyl, aryl, and arylalkyl;
R is selected from the group consisting of hydrogen, alkyl, alkenyl,
alkynyl, alkyfcarbonylalkyl, alkylsulfonylalkyl, alkylsulfonylarylalkyl,
carboxyalkyl,
-10-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
cyanoalkyl, haloaikyl, hydroxyalkyl, cycloalkyl, cycloalkylalkyl, aryl,
arylalkenyl,
arylalkynyl, heterocyclic, heterocyclic alkyl, arylalkyl, -(CH2)nC(O)R5, -
(CH2)nC=C-
R7, -(CH2)n[CH(CX'3)]m(CH2)n- R8, and -(CH2)n-R20;
wherein R5 is selected from the group consisting of alkyl, alkenyl,
alkynyl, cycloalkyl, cycloalkenyl, aryl, arylalkyl, haloalkyl, heterocyclic,
and
heterocyclic alkyl;
R7 and R8 are independently selected from the group consisting of
hydrogen, alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkenyl, aryl, arylalkyl,
haloalkyl, heterocyclic, and heterocyclic alkyl,
R20 is selected from the group consisting of alkyl, alkenyl, haloalkyl,
cycloalkyl, cycloalkenyl, aryl, heterocyclic, and heterocyclic alkyl;
X' is halogen;
n is from 0 to about 10, m is from 0 to about 5;
R1and R3 are independently selected from the group consisting of
hydrogen, hydroxy, hydroxyalkyl, halogen, alkyl, alkenyl, alkynyl, alkoxy,
alkenyloxy, alkoxyalkyl, amido, amidoalkyl, haloalkyl, cycloalkyl,
cycloalkylalkyl,
cycloalkenyl, cycloalkenylalkyl, amino, aminocarbonyl, aminocarbonylalkyl,
alkylamino, dialkylamino, arylamino, arylalkylamino, diarylamino, aryl,
aryloxy,
heterocyclic, heterocyclic alkyl, cyano, nitro, and -Y-R14, wherein Y is
selected
from the group consisting of, -0-, -S-, -C(R16) (R1 7)-, -C(O)NR21 R22-, -C(O)-
, -
C(O)O-, -NH-, -NC(O)-, -N=C R21 R22, N- R21 R22, and -NR19-. R14 is selected
from the group consisting of hydrogen, halogen, alkyl, alkoxyalkyl,
alkylthioalkyl,
alkenyl, alkynyl, hydroxy, cycloalkyl, cycloalkylalkyl, cycloalkenylalkyl,
cycloalkenyl, amino, cyano, aryl, arylalkyl, heterocyclic, and
heterocyclic(alkyl),
R16, R17, and R19 are independently selected from.the group
consisting of alkyl, alkenyl, cycloalkyl, cycloalkenyl, alkoxy, aryl,
arylalkyl,
heterocyclic, heterocyclic alkyl, or cyano; and
R21 and R22 are independently selected from the group consisting of
hydrogen, alkyl, alkenyl, cycloalkyl, cycloalkenyl, alkoxy, aryl, arylalkyl,
heterocyclic, heterocyclic alkyl, or cyano;
or a pharmaceutically acceptable salt, ester, or prodrug thereof.
-11-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
In another preferred embodiment, compounds of the present invention
have the formula III, wherein X1 is selected from the group consisting of -S02-
,
-SO-, -Se02-, and -SO(NR10)-, and R9 is selected from the group consisting of
alkyl, alkenyl, alkynyl, cycloalkyl, cycloaikenyl, amino, alkylamino, or
dialkylamino;
X2 is selected from the group consisting of hydrogen and halogen;
X is selected from the group consisting of 0, S, NR4, N-ORa, and
N-NRbRc, wherein R4 is selected from the group consisting of alkyl, alkenyl,
cyclo-
alkyl, cycloatkenyl, cycloalkytalkyl, alkylcycloalkenyl, aryl, heterocyclic,
and
arylalkyl; and Ra, Rb, and Rc.are independently selected from the group
consisting
of alkyl, cycloalkyl, cycloalkylalkyl, aryl, and arylalkyl;
R is selected from the group consisting of hydrogen, alkyl, alkenyl, alkynyl,
alkylcarbonylalkyl, alkylsulfonylalkyl, alkylsulfonylarylalkyl, carboxyalkyl,
cyanoalkyl, hatoalkyl, hydroxyalkyl, cycloalkyl, cycloalkylalkyl, aryl,
arylalkenyl,
arylalkynyl, heterocyclic, heterocyclic alkyl, arylalkyl, -(CH2)nC(O)R5, and -
(CH2)n-R20;
wherein R5 is selected from the group consisting of alkyl, alkenyl,
alkynyl, cycloalkyl, cycloalkenyl, aryl, arylalkyl, haloalkyl, heterocyclic,
and
heterocyclic alkyl;
R20 is selected from the group consisting of alkyl, alkenyl, haloalkyl,
cycloalkyl, cycloalkenyl, aryl, heterocyclic, and heterocyclic alkyl;
n is from 0 to about 10;
R1and R3 are independently selected from the group consisting of
hydrogen, hydroxy, hydroxyalkyl, hatogen, alkyl, alkenyl, alkynyl, alkoxy,
alkoxyalkyl, alkylthioalkyl, aryloxyalkyl, arylthioalkyl, amido, amidoalkyl,
haloalkyl,
cycloalkyl, cycloalkylalkyl, cycloalkenyl, cycloalkenylalkyl, amino,
aminocarbonyl,
aminocarbonylalkyl, alkylamino, alkylaminoalkyl, dialkylamino, arylamino,
arylalkylamino, diarylamino, aryl, heterocyclic, heterocyclic(alkyl), cyano,
nitro, and
-Y-R1 4, wherein Y is selected from the group consisting of, -0-, -S-, -C(R16)
(R1 7)-,
-C(0)NR21 R22-, -C(O)-, -C(O)0-, -NH-, -NC(O)-, and -NR19-. R14 is selected
from
the group consisting of hydrogen, halogen, alkyl, alkoxyalkyl, alkylthioalkyl,
alkenyl,
alkynyl, hydroxy, cycloalkyl, cycloalkylalkyl, cycloalkenylalkyl,
cycloalkenyl, amino,
cyano, aryl, arylalkyl, heterocyclic, and heterocyclic(alkyl), and
-12-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
R16, R1 7, and R19 are independently selected from the group
consisting of alkyl, alkenyl, cycloalkyl, cycloalkenyl, alkoxy, aryl,
arylalkyl,
heterocyclic, heterocyclic alkyl, or cyano;
R21 and R22 are independently selected from the group consisting of
hydrogen, alkyl, alkenyl, cycloalkyl, cycloalkenyl, alkoxy, aryl, arylalkyl,
heterocyclic, heterocyclic alkyl, or cyano;
or a pharmaceutically acceptable salt, ester, or prodrug thereof.
In another preferred embodiment, compounds of the present invention
have the formula III, wherein X1 is selected from the group consisting of -S02-
,
-SO-, -Se02-, and -SO(NR10)-, and R9 is selected from the group consisting of
alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkenyl, amino, alkylamino, or
dialkylamino;
X2 is selected from the group consisting of hydrogen and halogen;
X is selected from the group consisting of 0, S, NR4, N-ORa, and
N-NRbRc, wherein R4 is selected from the group consisting of alkyl, alkenyl,
cyclo-
alkyl, cycloalkenyl, cycloalkylalkyl, alkylcycloalkenyl, aryl, heterocyclic,
and
arylalkyl; and Ra, Rb, and Rc.are independently selected from the group
consisting
of alkyl, cycloalkyl, cycloalkylalkyl, aryl, and arylalkyl;
R is selected from the group consisting of hydrogen, alkyl, alkenyl,
alkynyl, alkylcarbonylalkyl, alkylsulfonylalkyl, alkylsulfonylarylalkyl,
carboxyalkyl,
cyanoalkyl, haloalkyl, hydroxyalkyl, cycloalkyl, cycloalkylalkyl, aryl,
arylalkenyl,
arylaikynyl, heterocyclic, heterocyclic alkyl, arylalkyl, and -(CH2)nC(O)R5,;
wherein R5 is selected from the group consisting of alkyl, alkenyl,
alkynyl, cycloalkyl, cycloalkenyl, aryl, arylalkyl, haloalkyl, heterocyclic,
and
heterocyclic alkyl; and
n is from 0 to about 10;
R1and R3 are independently selected from the group consisting of
hydrogen, hydroxy, hydroxyalkyl, halogen, alkyl, alkenyl, alkynyl, alkoxy,
alkoxyalkyl, alkylthioalkyl, aryloxyalkyl, arylthioalkyl, amido, amidoalkyl,
haloalkyl,
cycloalkyl, cycloalkylalkyl, cycloalkenyl, cycloalkenylalkyl, amino,
aminocarbonyl,
aminocarbonylalkyl, alkylamino, alkylaminoalkyl, dialkylamino, arylamino,
arylalkylamino, diarylamino, aryl, heterocyclic, heterocyclic(alkyl), cyano,
nitro, and
-Y-R14, wherein Y is selected from the group consisting of, -0-, -S-, -C(R16)
(R17)-,
-13-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
-C(O)NR21 R22-, -C(O)-, -C(O)O-, -NH-, -NC(O)-, and -NR19-. R14 is selected
from
the group consisting of hydrogen, halogen, alkyl, alkoxyalkyl, alkylthioalkyl,
alkenyl,
alkynyl, hydroxy, cycloalkyl, cycloalkylalkyl, cycloalkenylalkyl,
cycloalkenyl, amino,
cyano, aryl, arylalkyl, heterocyclic, and heterocyclic(alkyl);
R15, R16, R1 7, and R19 are independently selected from the group
consisting of alkyl, alkenyl, cycloalkyl, cycloalkenyl, alkoxy, aryl,
arylalkyl,
heterocyclic, heterocyclic alkyl or cyano;
R21 and R22 are independently selected from the group consisting of
hydrogen, alkyl, alkenyl, cycloalkyl, cycloalkenyl, alkoxy, aryl, arylalkyl,
heterocyclic, heterocyclic alkyl, or cyano;
or a pharmaceutically acceptable salt, ester, or prodrug thereof.
In another preferred embodiment, compounds of the present invention have
the formula III, wherein X1 is selected from the group consisting of -S02-, -
SO-, -
Se02-, and -SO(NR10)-, and R9 is selected from the group consisting of alkyl,
alkenyl, alkynyl, cycloalkyl, cycloalkenyl, amino, alkylamino, or
dialkylamino;
X2 is selected from the group consisting of hydrogen and halogen;
X is selected from the group consisting of 0, S, NR4, N-ORa, and N-NRbRc,
wherein R4 is selected from the group consisting of alkyl, alkenyl,
cycloalkyl,
cycloalkenyl, cycloalkylalkyl, alkyicycloalkenyl, aryl, heterocyclic, and
arylalkyl; and
Ra, Rb, and Rc.are independently selected from the group consisting of alkyl,
cycloalkyl, cycloalkylalkyl, aryl, and arylalkyl;
R is selected from alkyl, haloalkyl, aryl, heterocyclic, heterocyclic alkyl,
and -
(CH2)n-R20 where is R20 is substituted and unsubstituted aryl wherein the
substituted aryl compounds are substituted with halogen;
n is from 0 to about 10;
Rl is selected from the group consisting of alkoxy, alkenyloxy,
hydroxyalkoxy, aryloxy, aryl, arylalkyl, heterocyclic, heterocyclic alkyl, and
-Y-R14,
wherein Y is selected from the group consisting of, -0-, -S-, -C(R16) (R17)-, -
C(O)-, -
C(0)0-, -NH-, -NC(O)-, and -NR19-. R14 is selected from the group consisting
of
hydrogen, halogen, alkyl, alkenyl, alkynyl, hydroxy, cycloalkyl, cycloalkenyl,
amino,
cyano, aryl, arylalkyl, heterocyclic, and heterocyclic alkyl,
R3 is hydrogen;
-14-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
R15, R16, R17, and R19 are independently selected from the group
consisting of alkyl, alkenyl, cycloalkyl, cycloalkenyl, alkoxy, aryl,
arylalkyl,
heterocyclic, heterocyclic alkyl, or cyano; and
R21 and R22 are independently selected from the group consisting of
hydrogen, alkyl, alkenyl, cycloalkyl, cycloalkenyl, alkoxy, aryl, arylaikyl,
heterocyclic, heterocyclic alkyl, or cyano:
or a pharmaceutically acceptable salt, ester, or prodrug thereof.
In another preferred embodiment, compounds of the present invention have
the formula III, wherein X1 is selected from the group consisting of -S02-, -
SO-,
and -SO(NR 10)-, and R9 is selected from the group consisting of alkyl,
alkenyl,
alkynyl, cycloalkyl, cycloalkenyl, amino, alkylamino, or dialkylamino;
X2 is selected from the group consisting of hydrogen and halogen;
X is selected from the group consisting of 0, S, NR4, N-ORa, and N-NRbRc,
wherein R4 is selected from the group consisting of alkyl, alkenyl,
cycloalkyl,
cycloalkenyl, cycloalkylalkyl, alkylcycloalkenyl, aryl, heterocyclic, and
arylalkyl; and
Ra, Rb, and Rc.are independently selected from the group consisting of alkyl,
cycloalkyl, cycloalkylalkyl, aryl, and arylalkyl;
R is selected from alkyl, haloalkyl, aryl, heterocyclic, heterocyclic alkyl,
and -
(CH2)n-R20 where is R20 is substituted and unsubstituted aryl wherein the
substituted aryl compounds are substituted with halogen;
n is from 0 to about 10;
R1 is selected from the group consisting of alkoxy, alkenyloxy,
hydroxyalkoxy, aryloxy, aryl, arylalkyl, heterocyclic, and heterocyclic alkyl;
and
R3 is hydrogen;
or a pharmaceutically acceptable salt, ester, or prodrug thereof.
In another preferred embodiment, compounds of the present invention have
the formula III, wherein X1 is -SO2- and and R9 is selected from the group
consisting of alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkenyl, amino,
alkylamino, or
dialkylamino;
X2 is selected from the group consisting of hydrogen and halogen;
-15-
CA 02578858 2007-03-05
WO 99/10331 PCT/US9S/16479
X is selected from the group consisting of 0, S, NR4, N-ORa, and N-NRbRc,
wherein R4 is selected from the group consisting of alkyl, alkenyl,
cycloalkyl,
cycloalkenyl, cycloalkylalkyl, alkyicycloalkenyl, aryl, heterocyclic, and
arylalkyi; and
Ra, Rb, and Rc.are independently selected from the group consisting of alkyl,
cycloalkyl, cycloalkylalkyl, aryl, and arylalkyl;
R is selected from haloalkyl, aryl, heterocyclic, heterocyclic alkyl, and -
(CH2)n-R20 where is R20 is substituted and unsubstituted aryl wherein the
substituted aryl compounds are substituted with halogen;
n is from 0 to about 10;
R 1 is selected from the group consisting of unsubstituted aryl, and
substituted aryl with one, two, or three substituents selected from the group
consisting of fluorine and chlorine including, but not limited to, p-
chlorophenyl, p-
fluorophenyl, 3,4-dichlorophenyl, 3-chloro-4-fluoro-phenyl, and the like; and
R3 is hydrogen;
or a pharmaceutically acceptable salt, ester, or prodrug thereof.
In another preferred embodiment, compounds of the present invention
have the formula III, wherein X1 is -S02-,and R9 is selected from the group
consisting of alkyl and amino;
X2 is selected from the group consisting of hydrogen and halogen;
X is O;
R is selected from the group consisting of alkyl, alkenyl, alkynyl,
haloalkyl, aryl, and arylalkyl;
R 1 is selected from the group consisting of alkoxy, aryl, alkenyloxy,
hydroxyalkoxy, halalkoxy, arylalkyl, alkyl, and aryloxy; and
R3 is hydrogen;
or a pharmaceutically acceptable salt, ester, or prodrug thereof.
In another preferred embodiment, compounds of the present invention have
the formula III, wherein X1 is -S02-, and R9 is selected from the group
consisting of
alkyl and amino
-16-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
X2 is selected from hydrogen and fluorine;
R is selected from haloalkyl, aryl, and alkyl;
n is from 0 to about 10;
R1 is selected from the group consisting of isobutyloxy, isopentyloxy, 1-(3-
methyl-3-butenyl)oxy, 2-hydroxy-2-methyl-propyloxy, 3-hydroxy-3-methyl-
butyloxy,
neopentyloxy, isopentyl, aryloxy including 4-fluorophenoxy, unsubstituted
aryl, and
substitued aryl with one, two, or three substituents selected from the group
consisting of fluorine and chlorine including, , 4-fluorophenyl, 4-
chlorophenyl, 4-
chloro-3-fluoro-phenyl, 3-chloro-4-fluoro-phenyl and the like; and
R3 is hydrogen;
or a pharmaceutically acceptable salt, ester, or prodrug thereof.
In another preferred embodiment, compounds of the present invention
have the formula III, wherein X1 is selected from the group consisting of -S02-
, and
-SO(NR10)-, and R9 is alkyl,
X2 is selected from the group consisting of hydrogen and fluorine;
XisO;
R is selected from the group consisting of alkyl, alkenyl, alkynyl,
haloalkyl, aryl, and arylalkyl;
R1 is selected from the group consisting of alkoxy, aryl, alkenyloxy,
hydroxyalkoxy, alkyl, and aryloxy; and
R3 is hydrogen;
or a pharmaceutically acceptable salt, ester, or prodrug thereof.
In another preferred embodiment, compounds of the present invention have
the formula III, wherein X1 is -S02-, R9 is amino;
X2 is selected from the group consisting of hydrogen and fluorine;
X is O;
R is selected from the group consisting of alkyl, alkenyl, alkynyl,
haloalkyl, aryl, and arylalkyl;
-17-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
R1 is selected from the group consisting of alkoxy, aryl, alkenyloxy,
hydroxyalkoxy, alkyl, and aryloxy; and
R3 is hydrogen;
or a pharmaceutically acceptable salt, ester, or prodrug thereof.
In another preferred embodiment, compounds of the present invention have
the formula III, wherein X1 is -S02-, and R9 is methyl;
X2 is selected from the group consisting of hydrogen;
X is O;
R is selected from the group consisting t-butyl, 3-chlorophenyl, 3,4-
difluorophenyl, 4-fluorophenyl, 4-chloro-3-fluoro-phenyl, and CF3CH2-1 ;
R1 is selected from the group consisting of aryloxy including 4-
fiuorophenoxy, isobutyloxy, isopentyloxy, 1-(3-methyl-3-butenyl)oxy, 2-hydroxy-
2-
methyl-propyloxy, 3-hydroxy-3-methyl-butyloxy, neopentyloxy, isopentyl, 4-
fluorophenyl, 4-chlorophenyl, 4-chloro-3-fluoro-phenyl, 3-chloro-4-fluoro-
phenyl;
and
R3 is hydrogen;
or a pharmaceutically acceptable salt, ester, or prodrug thereof.
In another preferred embodiment, compounds of the present invention have
the formula Ili, wherein X1 is -S02-, and R9 is amino;
X2 is selected from the group consisting of hydrogen;
XisO;
R is selected from the group consisting t-butyl, 3-chlorophenyl, 3,4-
difluorophenyl, 4-fluorophenyl, 4-chloro-3-fluoro-phenyl, 3-chloro-4-fluoro-
phenyl
and CF3CH2-1 ;
R1 is selected from the group consisting of aryloxy including 4-
fluorophenoxy, isobutyloxy, isopentyloxy, 1-(3-methyl-3-butenyl)oxy, 2-hydroxy-
2-
methyl-propyloxy, 3-hydroxy-3-methyl-butyloxy, neopentyloxy, isopentyl, 4-
fluorophenyl, 4-chlorophenyl, 4-chloro-3-fluoro-phenyl, 3-chloro-4-fluoro-
phenyl;
and
-18-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
R3 is hydrogen;
or a pharmaceutically acceptable salt, ester, or prodrug thereof
Definitions of Terms
As used throughout this specification and the appended claims, the following
terms have the meanings specified.
The term "protecting groups includes "carboxy protecting group" and "N-
protecyting groups" . "Carboxy protecting group" as used herein refers to a
carboxylic acid protecting ester group employed to block or protect the
carboxyiic
acid functionality while the reactions involving other functional sites of the
compound are carried out. Carboxy protecting groups are disclosed in Greene,
"Protective Groups in Organic Synthesis" pp. 152-186 (1981).
In addition, a carboxy protecting group can be
used as a prodrug whereby the carboxy protecting group can be readily cleaved
in
vivo , for example by enzymatic hydrolysis, to release the biologically active
parent.
T. Higuchi and V. Stella provide a thorough discussion of the prodrug concept
in
"Pro-drugs as Novel Delivery Systems", Vol 14 of the A.C.S. Symposium Series,
American Chemical Society (1975),
Such carboxy protecting groups are well known to those skilled in the
art, having been extensively used in the protection of carboxyl groups in the
penicillin and cephalosporin fields, as described in U.S. Pat. No. 3,840,556
and
3,719,667.
Examples of esters useful as prodrugs for compounds containing carboxyl groups
can be found on pages 14-21 of "Bioreversible Carriers in Drug Design: Theory
and Application", edited by E.B. Roche, Pergamon Press, New York (1987).
Representative carboxy protecting
groups are C1 to C8 alkyl (e.g., methyl, ethyl or tertiary butyl and the
like); haloalkyl;
alkenyl; cycloalkyl and substituted derivatives thereof such as cyclohexyl,
cylcopentyl and the like; cycloalkylalkyl and substituted derivatives thereof
such as
cyclohexylmethyl, cylcopentylmethyl and the like; arylalkyl, for example,
phenethyl
or benzyl and substituted derivatives thereof such as alkoxybenzyl or
nitrobenzyl
groups and the like; arylalkenyl, for example, phenylethenyl and the like;
aryl and
substituted derivatives thereof, for example, 5-indanyl and the like;
-19-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
dialkylaminoalkyl (e.g., dimethylaminoethyl and the like); alkanoyloxyalkyl
groups
such as acetoxymethyl, butyryloxymethyl, valeryloxymethyl,
isobutyryloxymethyl,
isovaleryloxymethyl, 1-(propionyloxy)-1-ethyl, 1-(pivaloyloxyl)-1-ethyl, 1-
methyl-l-
(propionyloxy)-1-ethyl, pivaloyloxymethyl, propionyloxymethyl and the like;
cycloalkanoyloxyalkyl groups such as cyclopropylcarbonyloxymethyl,
cyclobutylcarbonyloxymethyl, cyclopentylcarbonyloxymethyl,
cyclohexylcarbonyloxymethyl and the like; aroyioxyalkyl, such as
benzoyloxymethyl, benzoyloxyethyl and the like; arylalkylcarbonyloxyalkyl,
such as
benzylcarbonyloxymethyl, 2-benzylcarbonyloxyethyl and the like;
alkoxycarbonylalkyl, such as methoxycarbonylmethyl,
cyclohexyloxycarbonylmethyl, 1-methoxycarbonyl-1-ethyl, and the like;
alkoxycarbonyloxyalkyl, such as methoxycarbonyloxymethyl, t-
butyloxycarbonyloxymethyl, 1-ethoxycarbonyloxy-1-ethyl,
1-cyclohexyloxycarbonyloxy-l-ethyl and the like; alkoxycarbonylaminoalkyl,
such
as t-butyloxycarbonylaminomethyl and the like; alkylaminocarbonylaminoalkyl,
such as methylaminocarbonylaminomethyl and the like; alkanoylaminoaikyl, such
as acetylaminomethyl and the like; heterocycliccarbonyloxyalkyl, such as 4-
methylpiperazinylcarbonyloxymethyl and the like; dialkylaminocarbonylalkyl,
such
as dimethylaminocarbonylmethyl, diethylaminocarbonylmethyl and the like; (5-
(loweralkyl)-2-oxo-1,3-dioxolen-4-yl)alkyl, such as (5-t-butyl-2-oxo-1,3-
dioxolen-4-
yl)methyl and the like; and (5-phenyl-2-oxo-1,3-dioxolen-4-yl)alkyl, such as
(5-
phenyl-2-oxo-1,3-dioxolen-4-yl)methyl and the like.
The term "N-protecting group" or "N-protected" as used herein refers to those
groups intended to protect the N-terminus of an amino acid or peptide or to
protect
an amino group against undersirable reactions during synthetic procedures.
Commonly used N-protecting groups are disclosed in Greene, "Protective Groups
In Organic Synthesis," (John Wiley & Sons, New York (1981)).
N-protecting groups comprise acyl groups such as
formyl, acetyl, propionyl, pivaloyl, t-butylacetyl, 2-chloroacetyl, 2-
bromoacetyl,
trifiuoroacetyl, trichloroacetyl, phthalyl, o-nitrophenoxyacetyl, a-
chlorobutyryl,
benzoyl, 4-chlorobenzoyl, 4-bromobenzoyl, 4-nitrobenzoyl, and the like;
sulfonyl
groups such as benzenesulfonyl, p-toluenesulfonyl and the like; carbamate
forming
groups such as benzyloxycarbonyl, p-chlorobenzyloxycarbonyl, p-
methoxybenzyloxycarbonyl, p-nitrobenzyloxycarbonyl, 2-nitrobenzyloxycarbonyl;
p-
bromobenzyloxycarbonyl,
-20-
CA 02578858 2007-03-05
WO 99/10331 PCTIUS98/16479
3,4-dimethoxybenzyloxycarbonyl, 3,5-dimethoxybenzyloxycarbonyl,
2,4-dimethoxybenzyloxycarbonyl, 4-methoxybenzyloxycarbonyl, 2-nitro-4,5-
dimethoxybenzyloxycarbonyl, 3,4,5-trimethoxybenzyloxycarbonyl, 1-(p-
biphenylyl)-
1-methylethoxycarbonyl, a,a-dimethyl-3,5-dimethoxybenzyloxycarbonyl,
benzhydryloxycarbonyl, t-butyloxycarbonyl, diisopropylmethoxycarbonyl,
isopropyioxycarbonyl, ethoxycarbonyl, methoxycarbonyl, allyloxycarbonyl,
2,2,2,-
trichloroethoxycarbonyl, phenoxycarbonyl, 4-nitrophenoxycarbonyl, fluorenyl-9-
methoxycarbonyl, cyclopentyloxycarbonyl, adamantyloxycarbonyl,
cyclohexyloxycarbonyl, phenylthiocarbonyl and the like; alkyl groups such as
benzyl, triphenylmethyl, benzyloxymethyl and the like; and silyl groups such
as
trimethylsilyl and the like. Preferred N-protecting groups are formyl, acetyl,
benzoyl,
pivaloyl, t-butylacetyl, phenyisulfonyl, benzyl, t-butyloxycarbonyl (t-Boc)
and
benzyloxycarbonyl (Cbz).
The term "alkanoyl" as used herein refers to an alkyl group as previously
defined appended to the parent molecular moiety through a carbonyl (-C(O)-)
group. Examples of alkanoyl include acetyl, propionyl and the like.
The term "alkanoylamino" as used herein refers to an alkanoyl group as
previously defined appended to an amino group. Examples alkanoylamino include
acetamido, propionylamido and the like.
The term "alkenyl" as used herein refers to a straight or branched chain
hydrocarbon radical containing from 2 to 15 carbon atoms and also containing
at
least one carbon-carbon double bond. Alkenyl groups include, for example,
vinyl
(ethenyl), allyl (propenyl), butenyl, 1-methyl-2-buten-1-yl and the like.
The term "alkenylene" denotes a divaient group derived from a straight or
branched chain hydrocarbon containing from 2 to 15 carbon atoms and also
containing at least one carbon-carbon double bond. Examples of alkenylene
include -CH=CH-, -CH2CH=CH-, -C(CH3)=CH-, -CH2CH=CHCH2-, and the like.
The term "alkenyloxy" as used herein refers to an alkenyl group, as
previously defined, connected to the parent molecular moiety through an oxygen
(-0-) linkage. Examples of alkenyloxy include isopropenoxy, butenyloxy and the
like.
The term "alkoxy" as used herein refers to R41 O- wherein R41 is a loweralkyl
group, as defined herein. Examples of alkoxy include, but are not limited to,
ethoxy,
isobutyloxy, isopentyloxy, tert-butoxy, and the like.
-21-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
The term "alkoxyalkylamino" as used herein refers to an alkoxy as defined
herein appended to the parent molecular moiety through an alkylamino as
defined
herein. Examples of alkoxyalkylamino include, but are not limited to,
ethoxymethylamino, isobutyloxyethylamino and the like
The term "alkoxyalkoxy" as used herein refers to R800-R81 0- wherein R80
is loweralkyl as defined above and R81 is alkylene. Representative examples of
alkoxyalkoxy groups include methoxymethoxy, ethoxymethoxy, t-butoxymethoxy
and the like.
The term "alkoxycarbonyl" as used herein refers to an alkoxyl group as
previously defined appended to the parent molecular moiety through a carbonyl
group. Examples of alkoxycarbonyl include methoxycarbonyl, ethoxycarbonyl,
isopropoxycarbonyl and the like.
The term "alkoxycarbonylalkenyl" as used herein refers to an alkoxycarbonyl
group as previously defined appended to the parent molecular moiety through an
alkenylene. Examples of alkoxycarbonylaikenyl include
methoxycarbonylethenylene, ethoxycarbonylpropenylene, and the like.
The term "alkoxyalkoxyalkyl" as used herein refers to an alkoxyalkoxy group
as previously defined appended to an alkyl radical. Representative examples of
alkoxyalkoxyalkyl groups include methoxyethoxyethyl, methoxymethoxymethyl, and
the like.
The term "alkoxyalkoxyalkenyl" as used herein refers to an alkoxyalkoxy
group as previously defined appended to an alkenyl radical. Representative
examples of alkoxyalkoxyalkenyl groups include methoxyethoxyethenyl,
methoxymethoxymethenyl, and the like.
The term "alkoxyalkyl" as used herein refers to an alkoxy group as previously
defined appended to an alkyl radical as previously defined. Examples of
alkoxyalkyl include, but are not limited to, methoxymethyl, methoxyethyl,
isopropoxymethyl and the like.
The term "(alkoxycarbonyl)thioalkoxy" as used herein refers to an
alkoxycarbonyl group as previously defined appended to a thioalkoxy radical.
Examples of (alkoxycarbonyl)thioalkoxy include methoxycarbonylthiomethoxy,
ethoxycarbonylthiomethoxy and the like.
The terms "alkyl" and "loweralkyl" as used herein refer to straight or
branched chain alkyl radicals containing from 1 to 15 carbon atoms including,
but
-22-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
not limited to, methyl, ethyl, n-propyl, iso-propyl, n-butyl, iso-butyl, sec-
butyl, t-butyl,
n-pentyl, 1-methylbutyl, 2,2-dimethylbutyl, 2-methylpentyl, 2,2-
dimethyipropyl, n-
hexyl and the like.
The term "alkylamino" as used herein refers to R51 NH- wherein R51 is a
loweralkyl group, for example, ethylamino, butylamino, and the like.
The term "alkylaminoalkyl" as used herein refers to a loweralkyl radical to
which is appended an alkylamino group.
The term "alkylaminocarbonyl" as used herein refers to an alkylamino group,
as previously defined, appended to the parent molecular moiety through a
carbonyl
(-C(O)-) linkage. Examples of alkylaminocarbonyl include methylaminocarbonyl,
ethylaminocarbonyl, isopropylaminocarbonyl and the like.
The term "alkylaminocarbonylalkenyl" as used herein refers to an alkenyl
radical to which is appended an alkylaminocarbonyl group.
The term "alkylcarbonylalkyl" as used herein refers to R40-C(O)- R41-
wherein R40 is an alkyl group and R41 is an alkylene group.
The term "alkylene" denotes a divalent group derived from a straight or
branched chain saturated hydrocarbon having from 1 to 15 carbon atoms by the
removal of two hydrogen atoms, for example -CH2-, -CH2CH2-, -CH(CH3)-, -
CH2CH2CH2-, -CH2C(CH3)2CH2- and the like.
The term "alkylsulfonyl" as used herein refers to an alkyl group as previously
defined appended to the parent molecular moiety through a sulfonyl (-S(O)2-)
group. Examples of alkylsulfonyl include methylsulfonyl, ethylsulfonyl,
isopropylsulfonyl and the like.
The term "alkylsulfonylalkyl" as used herein refers to an alkyl group as
previously defined appended to the parent molecular moiety through a
sulfonylalkyl
(-S(O)2-R-) group. Examples of alkylsulfonylalkyl include
methylsulfonyimethyl,
ethylsulfonylmethyl, isopropyisulfonylethyl and the like.
The term "alkylsulfonylamino" as used herein refers to an alkyl group as
previously defined appended to the parent molecular moiety through a
sulfonylamino (-S(O)2-NH-) group. Examples of alkylsulfonylamino include
methylsulfonylamino, ethylsulfonylamino, isopropylsulfonylamino and the like.
The term "alkylsulfonylarylalkyl" as used herein refers to an alkyl group as
previously defined appended to the parent molecuiar moiety through a
sulfonylalkyl
-23-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
(-S(O)2-R45R33-) group wherein R45 is aryl and R33 is alkylene. Examples of
alkylsulfonylarylalkyl include methylsulfonyiphenyimethyl
ethylsulfonylphenylmethyl, isopropylsulfonylphenylethyl and the like.
The term "aikylthio" as used herein refers to R53S- wherein R53 is alkyl.
The term "alkylthioalkyl" as used herein refers to alkylthio as defined herein
appended to the parent molecular moiety through an alkylene group.
The term "alkylthioalkoxy" as used herein refers to alkylthio as defined
herein appended to the parent molecular moiety through an alkoxyl group as
defined herein.
The term "alkynyl" as used herein refers to a straight or branched chain
hydrocarbon radical containing from 2 to 15 carbon atoms and also containing
at
least one carbon-carbon triple bond. Examples of alkynyl include -C=C-H, H-
C=_C-
CH2-, H-C=C-CH(CH3)- and the like.
The term "amido" as used herein refers to R54-C(O)-NH- wherein R54 is an
alkyl group.
The term "amidoalkyl" as used herein refers to R34-C(O)-NHR35- wherein
R34 is alkyl and R35 is alkylene.
The term "amino" as used herein refers -NH2.
The term "aminoalkoxy" as used herein refers to an amino group appended
to the parent molecular moiety through an alkoxyl group as defined herein.
The term "aminocarbonyl" as used herein refers to H2N-C(O)-.
The term "aminocarbonylalkyl " as used herein refers to an aminocarbonyl
as described above appended to the parent molecular moiety through an
alkylene.
The term "aminocarbonylalkenyl" as used herein refers to an alkenyl radical
to which is appended an aminocarbonyl (NH2C(O)-) group.
The term "aminocarbonylalkoxy" as used herein refers to H2N-C(O)-
appended to an alkoxy group as previously defined. Examples of
aminocarbonylaikoxy include aminocarbonylmethoxy, aminocarbonylethoxy and
the like.
The term "aroyloxyalkyl" as used herein refers to R32-C(O)-O-R33- wherein
R32 is an aryl group and R33 is an alkylene group. Examples of aroyloxyalkyl
include benzoyloxymethyl, benzoyloxyethyl and the like.
-24-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
The term "aryl" as used herein refers to a mono- or bicyclic carbocyclic ring
system having one or two aromatic rings including, but not limited to, phenyl,
naphthyl, tetrahydronaphthyl, indanyl, indenyl and the like. Aryl groups can
be
unsubstituted or substituted with one, two or three substituents independently
selected from loweralkyl, halo, haloalkyl, haloalkoxy, hydroxy, oxo (=0),
hydroxyalkyl, alkenyloxy, alkoxy, alkoxyalkoxy, alkoxycarbonyl,
alkoxycarbonylaikenyl, (alkoxycarbonyl)thioalkoxy, thioalkoxy, alkylimino
(R"N=
wherein R* is a loweralkyl group), amino, alkylamino, alkylsulfonyl,
dialkylamino,
aminocarbonyl, aminocarbonylaikoxy, alkanoylamino, aryl, arylalkyl,
arylaikoxy,
aryloxy, mercapto, cyano, nitro, carboxy, carboxaldehyde, carboxamide,
cycloalkyl,
carboxyalkenyl, carboxyalkoxy, alkylsulfonyiamino, cyanoalkoxy, heterocyclic
alkoxy, -SO3H, hydroxyalkoxy, phenyl and tetrazolylalkoxy. In the case of
halo, aryl
may have up to five halo substituents. Examples of substituted aryl include 3-
chlorophenyl, 3-fluorophenyl, 4-chlorophenyl, 4-fluorophenyl, 3,4-
dichlorophenyl,
3-chloro-4-fluoro-phenyl, 4-methylsulfonylphenyl, pentaflurophenyl, and the
like.
The term "arylalkenyl" as used herein refers to an alkenyl radical to which is
appended an aryl group, for example, phenylethenyl and the like.
The term "arylalkynyl" as used herein refers to an alkynyl radical to which is
appended an aryl group, for example, phenylethynyl and the like
The term "arylaikoxy" as used herein refers to R420- wherein R42 is an
arylalkyl group, for example, benzyloxy, and the like.
The term "arylaikoxyalkyl" as used herein refers to a loweralkyl radical to
which is appended an arylalkoxy group, for example, benzyloxymethyl and the
like.
The term "arylalkyl" as used herein refers to an aryl group as previously
defined, appended to a loweralkyl radical, for example, benzyl and the like.
The term "arylalkylamino" as used herein refers to an arylalkyl group as
previously defined, appended to the parent molecular moiety through an amino
group.
The term "arylalkylthio" as used herein refers to an arylalkyl group as
previously defined, appended to the parent molecular moiety through an thiol
group.
The term "arylamino" as used herein refers to R45NH2- wherein R45 is an
aryl.
-25-
CA 02578858 2007-03-05
WO 99/10331 PCT/i3S98/16479
The term "arylcarbonylalkyP" as used herein refers to R45C(O)R33- wherein
R45 is an aryl group and R33 is an alkylene group.
The term "arylhaloalkyl" as used herein refers to an aryl group as previously
defined, appended to the parent molecular moiety through a haloalkyl as
defined
herein. Examples of arylhaloalkyl include, phenyl-2-fluoropropyl, and the
like.
The term "arylhydroxyalkyl" as used herein refers to an aryl group as
previously defined, appended to the parent molecular moiety through a
hydroxyalkyl as defined herein. Examples of arylhydroxyalkyl include, phenyl-2-
hydroxypropyl, and the like.
The term "aryloxy" as used herein refers to R450- wherein R45 is an aryl
group, for example, phenoxy, and the like.
The term "aryloxyalkyl" refers to an aryloxy group as previously defined
appended to an alkyl radical. Examples of aryloxyalkyl include phenoxymethyl,
2-
phenoxyethyl and the like.
The term "aryloxyhaloalkyl" as used herein refers to an aryioxy group as
previously defined, appended to the parent molecular moiety through a
haloalkyl
as defined herein. Examples of aryloxyhaloalkyl include, phenyloxy-2-
fluoropropyl,
and the like.
The term "aryloxyhydroxyalkyl" as used herein refers to an aryloxy group as
previously defined, appended to the parent molecular moiety through a
hydroxyalkyl as defined herein. Examples of aryloxyhydroxyalkyl include,
phenyoxy-2-hydroxypropyl, and the like.
The term "carboxaldehyde" as used herein refers to a formaldehyde radical,
-C(O)H.
The term "carboxamide" as used herein refers to -C(O)NH2.
The term "carboxy" as used herein refers to a carboxylic acid radical,
-C(O)OH.
The term "carboxyalkyl" as used herein refers to a carboxy group as
previously defined appended to an alkyl radical as previously defined.
Examples
of carboxyalkyl include 2-carboxyethyl, 3-carboxy-l-propyl and the like.
The term "carboxyalkenyl" as used herein refers to a carboxy group as
previousiy defined appended to an alkenyl radical as previously defined.
-26-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Examples of carboxyalkenyl include 2-carboxyethenyl, 3-carboxy-l-ethenyl and
the
like.
The term "carboxyalkoxy" as used herein refers to a carboxy group as
previously defined appended to an alkoxy radical as previously defined.
Examples
of carboxyalkoxy include carboxymethoxy, carboxyethoxy and the like.
The term "cyano" as used herein refers a cyano (-CN) group.
The term "cyanoalky" as used herein refers to an alkyl radical as previously
defined to which is appended a cyano (-CN) group. Examples of cyanoalkyl
include 3-cyanopropyl, 4-cyanobutyl, and the like.
The term "cyanoalkoxy" as used herein refers to a cyano (-CN) group
appended to the parent molecular moiety through an alkoxy radical. Examples of
cyanoalkoxy include 3-cyanopropoxy, 4-cyanobutoxy and the like.
The term "cycloalkanoyloxyalkyl" as used herein refers to a loweralkyl
radical to which is appended a cycloalkanoyloxy -group (i.e., R60-C(O)-O-
wherein
R60 is a cycloalkyl group).
The term "cycloalkyl" as used herein refers to an aliphatic ring system having
3 to 10 carbon atoms and 1 to 3 rings including, but not limited to,
cyclopropyl,
cyclopentyl, cyclohexyl, and the like. Cycloalkyl groups can be unsubstituted
or
substituted with one, two or three substituents independently selected from
hydroxy, halo, oxo (=0), alkylimino (R*N= wherein R* is a loweralkyl group),
amino,
alkylamino, dialkylamino, alkoxy, alkoxyalkoxy, alkoxycarbonyl, thioalkoxy,
haloalkyl, mercapto, carboxy, carboxaldehyde, carboxamide, cycloalkyl, aryl,
arylalkyl, -SO3H, nitro, cyano and loweralkyl.
The term "cycloalkenyl" as used herein refers to an aliphatic ring system
having 3 to 10 carbon atoms and 1 to 3 rings containing at least one double
bond
in the ring structure. Cycloalkenyl groups can be unsubstituted or substituted
with
one, two or three substituents independently selected hydroxy, halo, oxo (=O),
alkylimino (R*N= wherein R* is a loweralkyl group), amino, alkylamino,
dialkylamino, alkoxy, alkoxyalkoxy, alkoxycarbonyl, thioalkoxy, haloalkyl,
mercapto,
carboxy, carboxaldehyde, carboxamide, cycloalkyl, aryl, arylalkyl ,-SO3H,
nitro,
cyano and loweralkyl.
The term "cycloalkylalkyl" as used herein refers to a cycloalkyl group
appended to a loweralkyl radical, including but not limited to
cyclohexylmethyl.
-27-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
The term "cycloalkylalkoxy" as used herein refers to a cycloalkyl group
appended to an alkoxyl group as defined herein, including but not limited to
cyclohexylmethyoxy.
The term "cycloalkylamino" as used herein refers to a cycloalkyl group
appended to the parent molecular moiety through an amino group as defined
herein, including but not limited to cyclohexylamino and the like.
The term "cycloalkylalkylamino" as used herein refers to a cycloalkyl group
appended to the parent molecular moiety through an alkylamino group as defined
herein, including but not limited to cyclohexylmethylamino and the like.
The term "cycloalkylidenealkyl" as used herein refers to a cycloalkyl group
appended to the parent molecular moiety through a double bond which connects
to
an alkylene (=(CHz)n-). Examples include cyclopropylideneethyl,
cyclobutylidenepropyl and the like.
The term "cycloalkyloxy" as used herein refers to a cycloalkyl group
appended to the parent molecular moiety through an oxygen atom, including but
not limited to cyclohexyloxy and the like.
The term "cycloalkenylalkyl" as used herein refers to a cycloalkenyl group
appended to a loweralkyl radical, including but not limited to
cyclohexenylmethyl.
The term "cycloalkenylalkoxy" as used herein refers to a cycloalkenyl group
appended to a alkoxyl group as defined herein, including but not limited to
cyclohexenylmethyoxy and the like.
The term "dialkylamino" as used herein refers to R56R57N- wherein R56 and
R57 are independently selected from loweralkyl, for example diethylamino,
methyl
propylamino, and the like.
The term "dialkylaminoaryloxy" as used herein refers a dialkylamino as
defined herein appended to the parent molecular moiety through an aryloxy as
defined herein.
The term "diarylamino" as used herein refers to (R45)(R46)N- wherein R45
and R46 are independently aryl, for example diphenylamino and the like.
The term "dialkylaminoalkyl" as used herein refers to a loweralkyl radical to
which is appended a dialkylamino group.
The term "dialkylaminocarbonyl" as used herein refers to a dialkylamino
group, as previously defined, appended to the parent molecular moiety through
a
-28-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
carbonyl (-C(O)-) linkage. Examples of dialkylaminocarbonyl include
dimethylaminocarbonyl, diethylaminocarbonyl and the like.
The term "dialkylaminocarbonylalkenyP" as used herein refers to an alkenyl
radical to which is appended a dialkylaminocarbonyl group.
The term "dialkyiaminocarbonylalkyl" as used herein refers to
R50-C(O)-R51- wherein R50 is a dialkylamino group and R51 is an alkylene
group.
The term "halo" or "halogen" as used herein refers to I, Br, Cl or F.
The term "haloalkyl" as used herein refers to an alkyl radical, as defined
above, which has at least one halogen substituent, for example, chloromethyl,
fluoroethyl, trifluoromethyl or pentafluoroethyl, 2,3-difluoropentyl, and the
like.
The term "haloalkenyl" as used herein refers to an alkenyl radical which has
at least one halogen substituent, for example, chloromethenyl, fluoroethenyl,
trifluoromethenyl or pentafluoroethenyl, 2,3-difluoropentenyl, and the like.
The term "haloalkenyloxy" as used herein refers to an haloalkenyl group as
defined herein appended to the parent molecular moiety through an oxygen atom.
The term "haloalkynyl" as used herein refers to an alkynyl radical which has
at least one halogen substituent, for example, chloromethynyl, fluoroethynyl,
trifluoromethynyl or pentafluoroethynyl, 2,3-difluoropentynyl, and the like.
The term "haloalkoxy" as used herein refers to an alkoxy radical as defined
above, bearing at least one halogen substituent, for example, 2-fluoroethoxy,
2,2,2-trifluoroethoxy, trifluoromethoxy, 2,2,3,3,3-pentafluoropropoxy and the
like.
The term "haloalkoxyalkyl" as used herein refers to a loweralkyl radical to
which is appended a haloalkoxy group.
The term "haloalkoxyhydroxyalkyl" as used herein refers to a haloalkoxy
group as defined herein appended to the parent molecular moiety through a
hydroxyalkyl, as defined herein.
The term "heterocyclic ring" or "heterocyclic" or "heterocycle" as used herein
refers to any 3- or 4-membered ring containing a heteroatom selected from
oxygen,
nitrogen and sulfur; or a 5-, 6- or 7-membered ring containing one, two or
three
nitrogen atoms; one oxygen atom; one sulfur atom; one nitrogen and one sulfur
atom; one nitrogen and one oxygen atom; two oxygen atoms in non-adjacent
positions; one oxygen and one sulfur atom in non-adjacent positions; or two
sulfur
atoms in non-adjacent positions. Examples of heterocycles include, but are not
-29-
CA 02578858 2007-03-05
WO 99/10331 PCTIUS98/16479
limited to, thiophene, pyrrole, and furan. The 5-membered ring has 0-2 double
bonds and the 6- and 7-membered rings have 0-3 double bonds. The nitrogen
heteroatoms can be optionally quaternized. The term "heterocyclic" also
includes
bicyclic groups in which any of the above heterocyclic rings is fused to a
benzene
ring or a cycloalkane ring or another heterocyclic ring (for example, indolyl,
dihydroindolyl, quinolyl, isoquinolyl, tetrahydroquinolyl,
tetrahydroisoquinolyl,
decahydroquinolyl, decahydroisoquinolyl, benzofuryl, dihydrobenzofuryl or
benzothienyl and the like). Heterocyclics include: aziridinyl, azetidinyl,
pyrrolyl,
pyrrolinyl, pyrrolidinyl, pyrazolyl, pyrazolinyl, pyrazolidinyl, imidazolyl,
imidazolinyl,
imidazolidinyl, pyridyl, piperidinyl, homopiperidinyl, pyrazinyl, piperazinyl,
pyrimidinyl, pyridazinyl, oxazolyl, oxazolidinyl, isoxazolyl, isoxazolidinyl,
morpholinyl, thiomorpholinyl, thiazolyl, thiazolidinyl, isothiazolyl,
isothiazolidinyl,
indolyl, quinolinyl, isoquinolinyl, benzimidazolyl, benzothiazolyl,
benzoxazolyl,
oxetanyl, furyl, tetrahydrofuranyl, thienyl, thiazolidinyl, isothiazolyl,
triazolyl,
tetrazolyi, isoxazolyl, oxadiazolyl, thiadiazolyl, pyrrolyl, pyrimidyl and
benzothienyl.
S.S X.
I ~ .
Y+
Heterocyclics also include compounds of the formula / where X* is
-CH2- or -0- and Y* is -C(O)- or [-C(R")2-jv where R" is hydrogen or C1-C4-
alkyl
and v is 1, 2 or 3 such as 1,3-benzodioxolyl, 1,4-benzodioxanyl and the like.
Heterocyclics also include bicyclic rings such as quinuclidinyl and the like.
Heterocyclics can be unsubstituted or be substituted with one, two , or three
substituents independently selected from hydroxy, halo, oxo (=0), alkylimino
(R*N=
wherein R* is a loweralkyl group), amino, alkylamino, dialkylamino, alkoxy,
alkoxyalkoxy, alkoxycarbonyl, thioalkoxy, haloalkyl, mercapto, carboxy,
carboxaldehyde, carboxamide, cycloalkyl, aryl, arylalkyl, -SO3H, nitro, cyano
and
loweralkyl. In addition, nitrogen containing heterocycles can be N-protected.
The term "heterocyclic alkoxy" as used herein refers to a heterocyclic group
as defined above appended to an alkoxy radical as defined above. Examples of
heterocyclic(alkoxy) include 4-pyridylmethoxy, 2-pyridylmethoxy and the like.
The term "heterocyclic amino" as used herein refers to a heterocyclic group
as defined above appended to an amino as defined above. Examples of
heterocyclic amino include 4-pyridylamino, 2-pyridylamino and the like
-30-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
The term "heterocyclic oxy" as used herein refers to a heterocyclic group as
defined above appended to the parent molecular moiety through an oxygen.
Examples of heterocyclic oxy include 4-pyridyloxy, 2-pyridyloxy and the like.
The term "heterocyclic alkyl" as used herein refers to a heterocyclic group as
defined above appended to a loweralkyl radical as defined above.
The term "heterocyclic alkylamino" as used herein refers to a heterocyclic
group as defined above appended to a alkylamino as defined above
The term "heterocyclic carbonyloxyalkyl" as used herein refers to
R46-C(O)-O-R47- wherein R46 is a heterocyclic group and R47 is an alkylene
group.
The term "heterocyclic thio" as used herein refers to a heterocyclic group as
defined above appended to the parent molecular moiety through an thiol.
Examples of heterocyclic thio include 4-pyridylthio, 2-pyridylthio and the
like
The term "hydroxy" as used herein refers to -OH.
The term "hydroxyalkenyl" as used herein refers to an alkenyl radical to
which is appended a hydroxy group. Examples of hydroxyalkenyl include 3-
hydroxypropenyl, 3, 4-dihydroxybutenyl and the like
The term "hydroxyalkoxy" as used herein refers to an alkoxy radical as
previously defined to which is appended a hydroxy (-OH) group. Examples of
hydroxyalkoxy include 3-hydroxypropoxy, 4-hydroxybutoxy and the like.
The term "hydroxyalkyl" as used herein refers to a loweralkyl radical to which
is appended a hydroxy group. Examples of hydroxyalkyl include 1-hydroxypropyl,
4-hydroxybutyl, 1,3-dihydroxyisopentyl, and the like.
The term "hydroxyalkylamino" as used herein refers to a hydroxyalkyl group
appenmded to the parent molecular moiety through an amino. Examples of
hydroxyalkylamino include 1 -hydroxypropylamino, 4-hydroxybutylamino, 1,3-
dihydroxyisopentylamino,.and the like.
The term "hydroxyalkylthio" as used herein refers to a hydroxyalkyl group
appenmded to the parent molecular moiety through an thiol. Examples of
hydroxyalkylamino include 1-hydroxypropylthio, 4-hydroxybutylthio, 1,3-
dihydroxyisopentylthio, and the like
The term. "mercapto" or "thioP' as used herein refers to -SH.
-31-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
The term "nitro" as used herein refers to -N02.
The term oxoalkoxy refers to a carbonyl group attached to the parent
molecular moiety through an alkoxy group.
The term "mercaptoalkoxy" or "thioalkoxy" as used herein refers to R70S-
wherein R70 is alkoxy. Examples of thioalkoxy include, but are not limited to,
methylthio, ethylthio and the like.
The term "tetrazolyl" as used herein refers to a radical of the formula
K
N-N
A4 ~~N
or a tautomer thereof.
The term "tetrazolylaikoxy" as used herein refers to a tetrazolyl radical as
defined above appended to an alkoxy group as defined above. Examples of
tetrazolylalkoxy include tetrazolylmethoxy, tetrazoiyiethoxy and the like.
The term "thioalkoxyalkoxy" as used herein refers to R8OS-R81O- wherein
R80 is loweralkyl as defined above and R81 is alkylene. Representative
examples
of alkoxyalkoxy groups include CH3SCH2O-, EtSCH2O-, t-BuSCH2O- and the like.
The term "thioalkoxyalkoxyalkyl" as used herein refers to a thioalkoxyalkoxy
group appended to an alkyl radical. Representative exampies of
alkoxyalkoxyalkyl
groups include CH3SCH2CH2OCH2CH2-, CH3SCH2OCH2-, and the like.
The compounds of the present invention can be used in the form of salts
derived from inorganic or organic acids. These salts include but are not
limited to
the following: acetate, adipate, alginate, citrate, aspartate, benzoate,
benzenesulfonate, bisulfate, butyrate, camphorate, camphorsulfonate,
digluconate,
cyclopentanepropionate, dodecylsulfate, ethanesulfonate, glucoheptanoate,
glycerophosphate, hemisulfate, heptanoate, hexanoate, fumarate, hydrochloride,
hydrobromide, hydroiodide, 2-hydroxy-ethanesulfonate, lactate, maleate,
methanesulfonate, nicotinate, 2-naphthalenesuifonate, oxalate, pamoate,
pectinate, persulfate, 3-phenylpropionate, picrate, pivalate, propionate,
succinate,
tartrate, thiocyanate, p-toluenesuifonate and undecanoate. Also, the basic
nitrogen-containing groups can be quaternized with such agents as loweralkyl
halides, such as methyl, ethyl, propyl, and butyl chloride, bromides, and
iodides;
dialkyl sulfates like dimethyl, diethyl, dibutyl, and diamyl sulfates, long
chain
halides such as decyl, lauryl, myristyl and stearyl chlorides, bromides and
iodides,
-32-
CA 02578858 2007-03-05
WO 99/10331 PCTIUS98/16479
aralkyl halides like benzyi and phenethyl bromides, and others. Water or oil-
soluble or dispersible products are thereby obtained.
Examples of acids which may be employed to form pharmaceutically
acceptable acid addition salts include such inorganic acids as hydrochloric
acid,
sulphuric acid and phosphoric acid and such organic acids as oxalic acid,
maleic
acid, succinic acid and citric acid.
Basic addition salts can be prepared in situ during the final isolation and
purification of the compounds of formula (I), or separately by reacting a
carboxylic
acid function with a suitable base such as the hydroxide, carbonate or
bicarbonate
of a pharmaceutically acceptable metal cation or with ammonia, or an organic
primary, secondary or tertiary amine. Such pharmaceutically acceptable salts
include, but are not limited to, cations based on the alkali and alkaline
earth metals,
such as sodium, lithium, potassium, calcium, magnesium, aluminum salts and the
like, as well as nontoxic ammonium, quaternary ammonium, and amine cations,
including, but not limited to ammonium, tetramethylammonium,
tetraethylammonium, methylamine, dimethylamine, trimethylamine, triethylamine,
ethylamine, and the like. Other representative organic amines useful for the
formation of base addition salts include diethylamine, ethylenediamine,
ethanolamine, diethanolamine, piperazine and the like.
The term "pharmaceutically acceptable ester" as used herein refers to esters
which hydrolyze in vivo and include those that break down readily in the human
body to leave the parent compound or a salt thereof. Suitable ester groups
include,
for example, those derived from pharmaceutically acceptable aliphatic
carboxylic
acids, particularly alkanoic, alkenoic, cycloalkanoic and alkanedioic acids,
in which
each alkyl or alkenyl moiety advantageously has not more than 6 carbon atoms.
Examples of particular esters includes formates, acetates, propionates,
butyates,
acrylates and ethylsuccinates.
The term "pharmaceutically acceptable prodrug" as used herein refers to
those prodrugs of the compounds of the present invention which are, within the
scope of sound medical judgement, suitable for use in contact with the tissues
of
humans and lower animals without undue toxicity, irritation, allergic
response, and
the like, commensurate with a reasonable benefit/risk ratio, and effective for
their
intended use, as well as the zwitterionic forms, where possible, of the
compounds
of the invention. The term "prodrug" refers to compounds that are rapidly
transformed in vivo to provide the parent compound having the above formula,
for
-33-
CA 02578858 2007-03-05
WO 99/10331 PCTIUS98/16479
example by hydrolysis in blood. A thorough discussion is provided in T.
Higuchi
and V. Stella, Pro-drugs as Novelalivery Svst' ems, Vol. 14 of the A.C.S.
Symposium Series, and in Edward B. Roche, ed., Bioreversible Carriers in Drug
pes'gn, American Pharmaceutical Association and Pergamon Press, 1987.
As used throughout this specification and the appended claims, the term
metabolically cleavable group denotes a moiety which is readily cleaved in
vivo
from the compound bearing it, wherein said compound, after cleavage remains or
becomes pharmacologically active. Metabolically cleavable groups form a class
of
groups reactive with the carboxyl group of the compounds of this invention are
well
known to practitioners of the art. They include, but are not limited to groups
such
as, for example, alkanoyl, such as acetyl, propionyl, butyryl, and the like;
unsubstituted and substituted aroyl, such as benzoyl and substituted benzoyl;
alkoxycarbonyl, such as ethoxycarbonyl; trialkylsilyl, such as trimethyl- and
triethysilyl; monoesters formed with dicarboxylic acids, such as succinyl, and
the
like. Because of the ease with which the metabolically cleavable groups of the
compounds of this invention are cleaved in vivo, the compounds bearing such
groups act as pro-drugs of other prostagiandin biosynthesis inhibitors. The
compounds bearing the metabolically cleavable groups have the advantage that
they may exhibit improved bioavailability as a result of enhanced solubility
and/or
rate of absorption conferred upon the parent compound by virtue of the
presence of
the metabolically cleavable group.
Asymmetric centers may exist in the compounds of the present invention.
The present invention contemplates the various stereoisomers and mixtures
thereof. Individual stereoisomers of compounds of the present invention are
made
by synthesis from starting materials containing the chiral centers or by
preparation
of mixtures of enantiomeric products followed by separation as, for example,
by
conversion to a mixture of diastereomers followed by separation by
recrystallization
or chromatographic techniques, or by direct separation of the optical
enantiomers
on chiral chromatographic columns. Starting compounds of particular
stereochemistry are either commercially available or are made by the methods
detailed below and resolved by techniques well known in the organic chemical
arts.
-34.
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Preferred Embodiments
Compounds useful in practicing the present invention include, but are not
limited to:
2-(2,2,2-Trifiuoroethyl)-4-(4-chlorophenyl)-5-[4-(aminosulfonyl)phenyl]-3(2H)-
pyridazinone;
2-(4-Fluorophenyl)-4-(3-fluorophenyl)-5-[4-(methylsulfony!)phenyl]-3(2 H)-
pyridazinone;
2-(3-Chlorophenyl)-4-(3-methyl-3-butenoxy)-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone;
2-(2,2,2-Trifluoroethyl)-4-(4-chtoro-3-fluorophenyl)-5-[4-
(aminosulfonyl)phenyl]-
3(2H)-pyridazinone;
2-(4-fluorophenyl)-4-(4-flurophenyl)-5-[4-(aminosulfonyl)phenyl]-3(2H)-
pyridazinone;
2-(3,4-Difluorophenyl)-4-(3-fluorophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone;
2-(3,4-Difluorophenyl)-4-(2-hydroxy-2-methyl-l-propoxy)-5-[4-
(methylsulfonyl)phenyl]-3(2H)-pyridazinone;
2-(4-Fluorophenyl)-4-(3-hydroxy-3-methylbutoxy)-5-[4-(methylsulfonyl)phenyl]-
3(2H)-pyridazinone;
2-(t-Butyl)-4-(3-methylbutoxy)-5-[4-(aminosulfonyl)phenyl]-3(2H)-pyridazinone;
2-(t-Butyl)-4-(3-methylbutoxy)-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone;
2-(2,2,2-Trifluoroethyl)-4-(2,2-dimethytpropoxy)-5-[4-(aminosulfonyl)phenyl]-
3(2H)-
pyridazinone;
2-(2,2,2-Trifiuoroethyl)-4-(2-methylpropoxy)-5-[4-(aminosulfonyl)phenyl]-3(2H)-
pyridazinone;
-35-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
2-(3,4-Difluorophenyl)-4-(3-methyibutoxy)-5-[4-(aminosuifonyl)phenyl]-3(2H)-
pyridazinone;
2-(4-Fluorophenyl)-4-(3-methylbutyl)-5-[4-(aminosulfonyl)phenyl]-3(2H)-
pyridazinone;
2-(3-Chiorophenyl)-4-(3-methylbutoxy)-5-[4-(aminosulfonyl)phenyl]-3(2H)-
pyridazinone;
2-(4-Fluorophenyl)-4-(3-methylbutoxy)-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone;
2-(3-Chlorophenyl)-4-(3-methylbutoxy)-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone;
2-(3,4-Difluorophenyl)-4-(2-methylpropoxy)-5-[4-(aminosulfonyl)phenyl]-3(2H)-
pyridazinone;
2-(3-Chlorophenyl)-4-(2-methylpropoxy)-5-[4-(aminosulfonyl)phenyl]-3(2H)-
pyridazinone;
2-(4-Fluorophenyl)-4-(3-methyIbutoxy)-5-[4-(aminosuIfonyl)phenyl]-3(2H)-
pyridazinone;
2-(4-Fiuorophenyl)-4-(2-methyfpropoxy)-5-[4-(aminosulfonyl)phenyl]-3(2H)-
pyridazinone;
2-(4-Fluorophenyl)-4-(2-methylpropoxy)-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone;
2-(3,4-Difluorophenyl)-4-(2-methylpropoxy)-5-[4-(methylsuIfonyl)phenyl]-3(2H)-
pyridazinone;
2-(3,4-Difluorophenyl)-4-(4-fIuorophenoxy)-5-[4-(aminosuIfonyl)phenyi]-3(2H)-
pyridazinone;
-36-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
2-(3,4-Difluorophenyl)-4-(3-methylbutoxy)-5-[4-(methylsuIfonyl)phenyl]-3(2H)-
pyridazinone;
2-(4-Fluorophenyl)-4-(4-fluorophenoxy)-5-[4-(aminosuIfonyl)phenyl]-3(2H)-
pyridazinone;
2-(2,2,2-Trifluoroethyl)-4-(2,2-dimethylpropoxy)-5-[4-(methylsuIfonyl)phenyl]-
3(2H)-
pyridazinone;
2-(4-Chioro-3-fluorophenyi)-4-(4-fluorophenyl)-5-[4-(methylsutfonyi)phenyl]-
3(2H)-
pyridazinone;
2-(3,4-Difluorophenyi)-4-(4-fluorophenoxy)-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone;
2-(3,4-Difluorophenyl)- -4-(4-fluorophenyl)-5-[4-(aminosulfonyl)phenyl]- 3(2H)-
pyridazinone;
2-(3,4-Difluorophenyl)-4-(4-fluorophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone;
2-(4-Fluorophenyi)-4-(3-methylbutoxy)-5-[4-(methylsulfonyl)phenyi]-3(2H)-
pyridazinone;
2,4-Bis(4-fluorophenyl)-5-(4-methylsulfonylphenyl)-3(2H)-pyridazinone;
2-(4-fluorophenyl)-4-(4-flurophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone; and
2-(3,4-Difluorophenyl)-4-(2-hydroxy-2-methylpropoxy)-5-[4-
(aminosutfonyl)phenyl]-
3(2H)-pyridazinone;
2-(3,4-Difluorophenyl)-4-(2-oxopropoxy)-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone;
-37-
CA 02578858 2007-03-05
WO 99/10331 PCTIUS98/16479
2-(3,4-Difluorophenyl)-4-(2-methoxy-imino-propoxy)-5-[4-
(methylsulfonyl)phenyl]-
3(2H)-pyridazinone;
(R)-2-(3,4-Difluorophenyl)-4-(3-hydroxy-2-methylpropoxy)-5-[4-(methylsulfonyl)-
phenyl]-3(2H)-pyridazinone;
(S)- 2-(3,4-Difluorophenyl)-4-(3-hydroxy-2-methyipropoxy)-5-[4-
(methylsulfonyl)-
phenyl]-3(2H)-pyridazinone;
(R)-2-(3,4-Difluorophenyl)-4-(3-hydroxy-2-methylpropoxy)-5-[4-(aminosulfonyl)-
phenyl]-3(2H)-pyridazinone;
(S)- 2-(3,4-Difluorophenyl)-4-(3-hydroxy-2-methylpropoxy)-5-[4-(aminosulfonyl)-
phenyl]-3(2H)-pyridazinone;
2-(3,4-Difluorophenyl)-4-(3-oxo-butoxy)-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone;
2-(4-Fluorophenyl)-4-(3-oxo-butoxy)-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone; and
2,4-Bis(4-Flurophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone;
or a pharmaceutically acceptable salt, ester, or prodrug thereof.
More preffered compounds of the present invention include, but are not
limited to:
2-Phenyl-4-(4-fluorophenyl)-5-(4-methylsulfonylphenyl)-3(2H)-pyridazinone;
2-(2,2,2-Trifluoroethyl)-4-(4-fluorophenyl)-5-(4-methylsulfonylphenyl)-3(2H)-
pyridazinone;
-38-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
2-(2,2,2-Trifluoroethyl)-4-(4-chlorophenyl)-5-(4-methylsulfonylphenyl)-3(2H)-
pyridazinone;
2-(4-Fluorophenyl)-4-(3-methylbutoxy)-5-[4-(methylsulfonyl)phenyl]-3(2 H)-
pyridazinone;
2-(3,4-Difluorophenyl)-4-(2-methylpropoxy)-5-[4-(aminosulfonyl)phenyl]-3(2H)-
pyridazinone; and
2,4-Bis(4-fluorophenyl)-5-(4-methylsulfonylphenyl)-3(2H)-pyridazinone;
or a pharmaceutically acceptable salt, ester, or prodrug thereof.
Preparation of Compounds of the Invention
The compounds of the invention may be prepared by a variety of synthetic
routes. Representative procedures are outlined in Schemesl-3, below.
A general route to the compounds of the invention having Formula III, where
the aryl group at the 5-position on the pyridazinone ring is substituted with
a
sulfonyl group is described in Scheme 1, below. The dichloro-3(2H)-
pyridazinone
is reacted with benzyl chloride and potassium carbonate in methanol. The 2-
benzyl-4-chloro-5-methoxy-3(2H)-pyridazinone is then treated with a boronic
acid
such as 4-fluorobenzeneboronic acid (shown) and a palladium catalyst. The
methoxy group was hydrolyzed with 48% hydrobromic acid to furnish the 5-
hydroxypyridazinone compound. The 5-hydroxypyridazinone product was treated
with triflic anhydride followed by substitution on the pyridazinone ring using
4-
methylthiobenzeneboronic acid. This furnished the methyl thioether compound
which was reacted with peracetic acid in acetic acid and methylene chloride to
provide the methyl sulfone. The benzyl group is removed using aluminum bromide
or another suitable Lewis acid. The R group can be added via substitution
using an
appropriate alkylating agent and base.
-39-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
SCHEME 1
"-1NH E(2C03, PhCH2CI ~NCH2Ph
ci CH3OH, reflux, 10 h CH,
Ci ci
CsF, Pd(Ph3P)4, N" CHZPh
CHZPh F CH3 0
\ I B OH)2
CH3 O (
CI
anhyd. DME, 100 C, 18 h
F
NCH2Ph NCHZPh
CH3 O H \ O
48% HBr
~ AcOH, refiux, 7 h
F F
NCHZPh CH2Ph
H 0 T \ O
(CF3SO2)20
Pyridine, 0 C to RT, 24 h
F F
~
NCH2Ph 1. Et3N, Pd(Ph3P)4
/
CHZPh
CH3
Tt 0
I / ~ \ O
B(OH)2
Toluene,100 C, 20 min cH'O
F 2. CH3CO3H in CH3CO2H,
CH2C12, 0 C, 1 h F
NCHZPh ~ \ NH
/ I \ 0 AIBr3
CH30 Toluene, 80 C, 15 min CH,o
F
F
-40-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
N
NH NR
K2C03, RX
cH3o anhyd. DMF C"'O \ I~
F F
Another route to the compounds of the invention having Formula III is
described in Scheme 2, below. The 4-bromothioanisole or other suitable
thioether
is reacted with a trialkoxyborate, such as trimethoxyborate or
triisopropylborate to
convert it to 4-(Methylthio)benzeneboronic acid . The boronic acid is reacted
with
2-benzyl-4,5-dibromo-3(2H)-pyridazinone using tetrakis(triphenylphosphine)-
palladium (0) in dimethoxyethane. The product is then coupled with a second
boronic acid such as 4-fluorobenzeneboronic acid (shown) and a palladium
catalyst to provide the thioether. This furnished the methyl thioether
compound
which was reacted with meta-chloroperoxybenzoic acid (MCPBA) in methylene
chloride to provide the methyl sulfone. The benzyl group is removed using
aluminum bromide or another suitable Lewis acid. The R group can be added via
substitution using an appropriate alkylating agent and base.
SCHEME 2
1) n-BuLi, (MeO)3B,
B~ THF, -78 C B(OH)2
2 10% NaOH, water,
CH3S ) CH3S
THF, RT
N ~ ~NH K2CO3' R'X f;- N NR'
Br o DMF Br o
B r Br
-41-
CA 02578858 2007-03-05
WO 99/10331 PCT/[7S98/16479
B(OH)2
CH3S ):::~ 1% NR'
Pd(PPh3)4, Na2CO3
+ DME, EtOH, H20
Br
N ~ CH3S
NR'
Br O
Br
% , NR'
NR' R ' / g(pH)2 C
0 Pd PPh Na CO
~ 3)4~ 2 3 CH3S (
- - _ -- _-,
CH3S Br DME, EtOH, H20
F
NR' NR'
MCPBA o
CH3S CH2CI2, 0~ C CH302
F F
A third route to the compounds of the invention having Formula III is
described in Scheme 3, below. (4-Thiomethylphenyl)dimethylthioketene acetal,
mono-S-oxide was prepared by reaction of 4-thiomethylbenzaidehyde (Y is CH3S)
with methyl(methylsulfinylmethyl)sulfide and sodium hydroxide. The thioketene
acetal and methyl 4-fluorophenylacetate or suitable ester (X is fluorine) were
treated with a strong base such as sodium hexamethyldisilazide in THF to
provide
the butyrate ester. The dithioacetal ketene was directly cyclized to the
unsubstituted pyridazinone using hydrazine and a sait_ The pyridazinone was
oxidized with peroxyacetic acid to provide the sulfonyl pyridazone. In an
alternate
route, Scheme 3-A, the thioacetal ketene was treated with perchloric acid to
provide an ester-aldehyde as a mixture of diastereomers. The oxidation
products
were treated with hydrazine and then oxidized with peroxyacetic acid to obtain
the
-42-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
sulfonyl dihydropyridazinone. The dihydropyridazinone can be dehydrogenated to
form the pyridazinone by treatment with reagents such as bromine in acetic
acid.
The R group may be added via substitution using an appropriate alkylating
agent
and base.
SCHEME 3
~ HO S~ O
+,,S NaOH, 70 C S~
O ~
~ ~ NaHMDS, C C [y1~a
/ C02CH3 THF
OC H3
O 1\ H
Na THF, 0 C to RT / 02CH3
OCH3 + / S
\ I H S
Y 0' ,
0
H 02CH3 NH2NH2- HCI I/ NH
i
s 8:2 DMSO/H20 N
Y ,S,
H !
Y ~
0 0
NH K2CO3, RX tvR
N DMF, RT N
Y' Y'
SCHEME 3A
~
I/ H 02CH3 HC104 I H 02CH3
/ S~ CH3CN, 0 C H CHO
Y ~ O:S", 56%
-43-
CA 02578858 2007-03-05
WO 99/10331 FGT/US98/16479
H
H 02cH3 NH2NH2(5 eq.) I~ NH
H CHO EtOH, reflux, 18 h H
53% Y
x'
CH3CO3H
NH 3 3 NH
H N CH2CI2 H N
Y'
X
NH Br2 I~ NH
~ N AcOH, 95 C N
~IH
Y'
x
NH K2CO3, RX NR
i I 'N DMF,RT Y, :~-" Y,
The preparation of the 5-hydroxy-2(5H)-furanones can be accomplished by
the application of methodologies published in a variety of sources, including:
J.
Med. Chem., 1987, 30, 239-249 and WO 96/36623,
and are shown in Scheme 4.
-44-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
SCHEME 4
X O X~~ O
NH K2C03, RX ~ (VR
i f N DMF, RT ~ I ~N
Y' \ Y' \
Method IV:
F I~ O F O F
0
~ NH2NH2.H20
N
H Na2CO3, RX N R
I~ 3 O H n-BuOH 4R3 N DMF - N
3
Me02S Me02S Me02S R
A general route to the compounds of the invention having Formula III, where
the aryl group at the 5-position on the pyridazinone ring is substituted with
a
sulfonyl group ring is described in Scheme 5, below. A mucohalo acid, such as,
for
example, mucobromic or mucochioric acid, is reacted with an hydrazine having
the
desired R group to provide the dihalopyridazinone compound, 5A. Treatment of
the dihalo-compound with an alcohol in the presence of a base, such as, for
example, sodium or potassium hydride, will provide an alkoxide, 5B. (If the
alkoxy
group is to be removed at a.later time then methanol is the preferred
alcohol.)
Reaction of the alkoxy-halide with a methylthiophenyl boronic will provide the
alkoxy-pyridazinone 5C. The alkoxy group can be converted to a hydrocarbyl
group by treatment with a Grignard reagent to provide the thioether 5D. The
thioether can be oxidized with an oxidizing agent, such as, for example,
peracetic
acid, meta-chloroperoxybenzoic acid and the like, to form the sulfinyl
compound
5G, or the methylsulfone compound 5E. Rearrangement and hydrolysis of the
sulfinyl compound, 5G, provides the thiophenol. The thiophenol is then
oxidized,
activated and aminated to convert it to the amino-sulfonyl compound 5H.
Alternatively, the methylsulfonyl compound, 5E, can be converted to the amino-
sulfonyl compound by 5H by treatment of the methylsulfonyl compound with a
diazodicarboxylate, such as, for example, DBAD, DIAD, DEAD and the like, and a
disilazane anion, such as, for example, lithium HMDS and the like, followed by
-45-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
treatment with sodium acetate and hydroxylamine-O-sulphonic acid in water
provides the aminosulfonyl compound, 5H.
SCHEME 5
o O o
AcOH R R97OH R97
N.R
Ji + R-NHNH2
NaH
OH
5A 5B
0 0
AcOH N,R R97OH R97 N.R
+ R-NHNH2 ~ = N ~N
OH NaH )
5A 5B
0
R97 0 R B(OH)2 Pd(PPh3)4 R
N- + ~ .00 \ I \ (PPh3)4 , N~
,
S CsF
~SI~
5B 5C
O 0 0
Rs~ I N,R R96M9X R96 R [OX]R96 R
~ N o)J THF o~ JC
S S SO
5C 5D 5E
O O 0
Rss R Rgg R 1. TFA Rss R
N- [OxJ N" 2. NaOH/MeOH N-
I\
S 3. C12 4
0 4. NH4OH H2N- 00
5E 5G 5H
-46-
CA 02578858 2007-03-05
WO 99/10331 PCTIUS98/16479
0 0
R96 R 1. DBAD/HMDS R~ R
N~ 2.. NaOH N~
N N
O
,~ 3. NaOAc/hydroxylamine- ,S~
H3~ 0 0-sulphonic acid /H20 H2N 0
5E 5H
Alternatively, the alkoxy-pyridazinone 5C can be oxidized, as shown in
Scheme 5A. The first step is employing an oxidizing agent, such as, for
example,
peracetic acid, meta-chloroperoxybenzoic acid and the like, to form the
sulfinyl
compound 5G', or the methylsulfone compound 5E'. Rearrangement and
hydrolysis of the sulfinyl compound provides the thiophenol. The thiophenol is
then
oxidized, activated and aminated to convert it to the amino-sulfonyl compound
5H'.
Finally, the methylsulfonyl compound can be converted to the aminosulfonyl
compound by 5H' by treatment of the methylsulfonyl compound 5E' with a
diazodicarboxylate, such as, for example, DBAD, DIAD, DEAD and the like, and a
disilazane anion, such as, for example, lithium HMDS and the like, followed by
treatment with sodium acetate and hydroxylamine-O-sulphonic acid in water
provides the aminosulfonyl compound, 5H'.
SCHEME 5A
0 0
R97 R R97 R
I N" [Ox] I N,
J ~ ~N I ~ ~
~S ~ 4 /
H3C"0
5C 5E'
-47-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
0 0 1. TFA 0
97
R97 N"R [OX] R N-R 2. NaOH/MeOH R97 N-R
N I \ N
S S i 3. C12 QS
~ 4. NH4OH H2N" 0
5C 5G' 5H'
0 0
J;:: R 1. DBAD/HMDS R97 ,- R
~ N~ 2. NaOH N
O ~ \ ~ /N
H3C~~~ 3. NaOAc/hydroxylamine- H NS ~
0 0-sulphonic acid /H20 Z O
5E' 5H'
Preparation of compounds of the invention having Formula III, where the
group at the 4-position on the pyridazinone ring is a substituted alkyl or
alkenyl
group is described in Scheme 6A, below. The thioether 5E, where R96 is alkyl,
e.g., methyl shown, is halogenated with a halogenating reagent, such as, for
example, NBS and peroxide, to provide the bromo compound 6A. The bromo
compound can be reacted with an alcohol and a weak base, such as, for example,
sodium or potassium carbonate to provide the 4-alkyl-ether, 6B. The bromo
compound can be reacted with a thio compound in the presence of a base, such
as, for example, silver carbonate, to provide the 4-alkyl-thioether, 6C. The
bromo
compound can be reacted with an amine and a weak base, such as, for example,
sodium or potassium carbonate to provide the 4-alkyl amino-alkyl compound 6D.
SCHEME 6
0 0
CH3 N"R NBS BrCH2 N,R
O, I AO O, I
0 0
4E 5A
-48-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
O O
BrCH2 N,R R950H Rs5OCH N R
N N
base
0 0
5A 5B
O O
BrCHZ R R94SCH R
N 1. Nal N
!ON ~N
4S I 2. R94SH/ base
O 0
5A 5C
O O
BrCH2 N,R 93 R93NHCH N,R
R NH2
Q I~ N Q\ ~N
S base S
O 0
5A 5D
A general route to the compounds of the invention having Formula III, where
the group at the 4-position on the pyridazinone ring can be readily
substituted is
illustrated in Scheme 6, above. The synthesis starts with the alkoxide, 5E',
where
R97 is methyl. The methoxy compound is treated with a base, such as, for
example, sodium or potassium hydroxide, to provide the 4-hydroxy-pyradizinone,
6A. The alcohol is treated with p-toluenesulfonyl chloride to provide the
tosyloxy
compound, 6B. The tosyloxy compound can be readily substituted with a
compound R92Z' that*can undergo an SN2 reaction. Examples of these
compounds are compounds such as alcohols, thiols, amines or hydrocarbyl
anions.
-49-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
SCHEME 6A
O p
R97 .R HO N. R SO2C1 .'S'0
N O
base ~ p
N \ I ~N O N' R
( ~N
CH3SO2 CH3S0 , ~ ! \
5E' 6A CH3S02 6B
OO'O p R R92Z, R92Z N,R
'
CH3SO2
CH3SO2 ~ 6B 6C
As used throughout this specification and the appended claims, the following
abbreviations have been used:
ACD for acid citrate dextrose, CAP for carrageenan induced air pouch
prostaglandin, CIP for rat carrageenan pleural inflammation model, COX-2 for
cyclooxygenase-2, CPE for carrageenan induced paw edema in rats, DBAD for di-t-
butylazodicarboxylate, DEAD for diethyl azodicarboxylate, DIAD for disopropyl
azodicarboxylate, DMAP for 4-(dimethylamino)pyridine, DME for
1,2-dimethoxyethane, DMF for N,N-dimethylformamide, DMSO for dimethyl
sulfoxide, DMSO for dimethyl suifoxide, EDTA for ethylenediaminetetraacetic
acid,
EIA for enzyme immunoassay, FAB for fast atom bombardment, GI for
gastrointestinal, HMDS, lithium or Li HMDS for lithium 1,1,1,3,3,3-
hexamethyldisilazide, HWPX for Human Whole Platelet Cyclooxygenase-1,
MCPBA for meta-chloroperoxybenzoic acid, NSAIDs for non-steroidal anti-
inflammatory drugs, PEG 400 for polyethyleneglycol, PGE2 for prostagiandin E2,
PGHS for prostaglandin endoperoxide H synthase, RHUCX1 for recombinant
human cyclooxygenase-1, RHUCX2 for recombinant human cyclooxygenase-2, r-
hu Cox1 for recombinant human Cox-1, TEA for Triethylamine, TFA for
Trifluoroacetic acid, and THF for Tetrahydrofuran and WISH for human amnionic
whole cell cyclooxygenase-2. The following examples illustrate the process of
the
invention, without limitation.
-50-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Compounds of the present invention include, but are not intended to be
limited to, the following Examples:
Example 1
4-(Methvlthio)benzeneboronic acid
A stirred solution of 4-bromothioanisole (5.0 g, 0.0246 mol) in anhydrous
tetrahydrofuran (THF) was chilled to -78 C under a nitrogen atmosphere. A 2.5
M
solution of n-butyl lithium (12 mL, 0.030 mol) in hexanes was added dropwise
to
the chilled solution. When the addition was complete, the reaction mixture was
stirred at -78 C for about 45 minutes. Trimethylborate (8.5 mL, 0.0748) was
introduced via syringe. The reaction mixture was then allowed to warm to room
temperature overnight. The room temperature solution was treated successively
with 10% aqueous sodium hydroxide solution (50 mL) and water (33.5 mL) and
stirred at room temperature for 1 hour. The reaction mixture was lowered to
about
pH = 4-5 using 10% aqueous citric acid and the THF was removed under reduced
pressure. The aqueous residue was saturated with sodium chloride and extracted
with ethyl acetate. The organic extract was dried over MgSO4 and filtered. The
filtrate was concentrated under reduced pressure to provide a white solid
which
was washed with hexanes to provide the product as a white solid (yield: 1.5 g;
36%). M.p. 170 C. 1 H NMR (300 MHz, DMSO-d6) 8 2.47 (s, 3H), 7.20 (d, J = 8
Hz,
2H), 7.71 (d, J = 8 Hz, 2H), 7.96 (br s, 2H).
Example 2
2-Benzyl-4.5-d'bromo-3 2H)-Rvridazinone
Benzyl bromide (0.59 mL, 0.005 mol) was added to a stirred solution of 4,5-
dibromo-3(2H)-pyridazinone (1.27 g, 0.005 mol) and potassium carbonate (0.76
g,
0.0055 mol) in 20 mL of anhydrous dimethylformamide (DMF). The solution was
stirred overnight at room temperature, and partitioned between aqueous citric
acid
and ethyl acetate. The aqueous layer was extracted twice with ethyl acetate.
The
combined organic extracts were washed with brine, dried over MgSO4 and
filtered.
The filtrate was concentrated under reduced pressure to provide a beige solid,
which was purified by column chromatography (silica gel, 9:1 hexanes/ethyl
acetate). The product was obtained as a white solid (yield: 1.32 g, 76.7%).
M.p.
95-96 C. 1 H NMR (300 MHz, CDCI3) 5 5.31 (s, 2H), 7.29-7.37 (m, 3H), 7.41-
7.47
(m, 2H), 7.79 (s, 1 H). MS (DCI-NH3) m/z 345 (M+H)+. IR (KBr) 1645 cm-1.
-51-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 3
2-Benzyl-4-bromo-5-14-(methylthio)Rhenyl]-3(2H)-oyridazinone
A solution of the boronic acid (0.318 g, 0.001889 mol), prepared according
to the method of Example 1, the dibromopyridazinone (0.975 g, 0.002834 mol),
prepared according to the method of Example 2, and
tetrakis(triphenylphosphine)-
palladium (0) (0.16 g, 0.0142 mol), in dimethoxyethane (30 mL) was prepared. A
2
M aqueous solution of sodium carbonate (2.83 mL, 0.005668 mol) was added to
the dimethoxyethane solution and the mixture was heated at reflux. After 16
hours,
a chromatographic (TLC) check (9:1 hexanes/ethyl acetate) indicated that both
starting materials were still present and a fresh aliquot of palladium
catalyst was
added. The reaction mixture was stirred at reflux for an additional 5 hours,
allowed
to cool to room temperature and stand over the weekend. The volatile materials
were removed under reduced pressure and the residue was partitioned between
water and ethyl acetate. The aqueous layer was extracted with ethyl acetate.
The
combined organic extracts were washed with brine, dried over MgSO4, and
filtered.
The filtrate was concentrated under reduced pressure to provide an oil which
was
purified by column chromatography (silica gel, 95:5 hexanes/ethyl acetate).
Fractions containing the desired product were combined and concentrated under
reduced pressure. This material was rechromatographed (95:5 hexanes/ethyl
acetate) to furnish 0.200 g of a beige solid. The solid was crystallized from
ether/hexanes to provide white crystals (yield: 110 mg, 15%) M.p. 115-118 C.
1 H
NMR (300 MHz, CDC13) S 2.53 (s, 3H), 5.40 (s, 2H), 7.30-7.42 (m, 7 H), 7.49-
7.54
(m, 2H), 7.65 (s, 1 H). MS (DCI-NH3) m/z 387 (M+H)+.
Example 4
2-Benzyl-4-(4-fluoroRhenyl)-5-(4-(met"hylthio)ohenvlj-3(2y)-p,yridazinone
A solution of the product prepared in Example 3, (0.100 g, 0.000258 mol), 4-
fluorobenzeneboronic acid (0.072 g, 0.000516 mol),
tetrakis(triphenylphosphine)-
palladium (0) (0.015 g, 0.000013 mol), and a 2 M aqueous solution of sodium
carbonate (0.64 mL, 0.001291 mol) in 30 mL of dimethoxyethane (DME) was
stirred
at reflux for 16 hours. A fresh aliquot of palladium catalyst was added with
an addi-
tional equivalent of the boronic acid. The reaction was maintained at reflux
for 24
hours. The volatile materials were removed under reduced pressure and the
residue was partitioned between water and ethyl acetate. The aqueous layer was
extracted with ethyl acetate. The combined organic layers were washed with
brine,
dried over MgSO4, and filtered. The filtrate was adsorbed onto silica gel. The
-52-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
silica gel/product was placed at the top of a column of silica gel and the
product
eluted with 93:7 hexanes/ethyl acetate. Fractions containing product were
combined and concentrated under reduced pressure. The residue was purified
further by a second column chromatography (silica gel, 95:5 hexanes/ethyl
acetate). Fractions containing product were concentrated under reduced
pressure
to provide a viscous oil (yield: 0.028 g, 27%). 1 H NMR (300 MHz, CDCI3) S
2.46 (s,
3H), 5.39 (s, 2H), 6.95 (t, J = 9 Hz, 2H), 6.99 (d, J = 9 Hz, 2H), 7.11 (d, J
= 9 Hz, 2H),
7.16-7.23 (m, 2H), 7.30-7.40 (m, 3H), 7.52-7.57 (m, 2H), 7.86 (s, 1 H). MS
(DCI-
NH3) m/z 403 (M+H)+.
Example 5
2-Benzyl-4-(4-fluorophenyl)-5-(4-(meth Isy ulfonY1)phenyll 3(2H)-Rvridazinorle
A solution of meta-chloroperoxybenzoic acid (MPCBA) (0.039 g, 0.00013
mol) in dichloromethane (5 mL) was added dropwise to a stirred solution of the
sulfide (0.027 g, 0.000067 mol), prepared according to the method of Example
4, in
chilled (0 C) dichloromethane (10 mL). After 5 minutes, TLC (1:1
hexanes/ethyl
acetate) indicated that the starting sulfide had been consumed. The reaction
was
quenched with aqueous sodium sulfite. The organic layer was washed twice with
aqueous sodium hydroxide and o.nce with brine. The dichloromethane solution
was dried over MgSO4, and filtered. The filtrate was concentrated under
reduced
pressure. The residue was purified by column chromatography (silica gel, 7:3
hexanes/ethyl acetate) to provide the desired sulfone product. Further elution
with
100% ethyl acetate removed the sulfoxide from the column. The sulfoxide
product
was re-subjected to the MCPBA oxidant (0.04 g, 1 hour, 0 C) and worked-up as
described above. The residue obtained was combined with the sulfone from the
first column and the mixture was purified by column chromatography (silica
gel, 7:3
hexanes/ethyl acetate). Fractions containing product were combined and
concentrated under reduced pressure. The residue was crystallized from
ether/hexanes to provide the product as white crystals (yield: 13 mg, 44.6%).
M.p.
101-103 C. 1 H NMR (300 MHz, CDC13) S 3.05 (s, 3H), 5.40 (s, 2H), 6.95 (t, J=
9
Hz, 2H), 7.12-7.20 (m, 2H), 7.28-7.41 (m, 3H), 7.31 (d, J = 9 Hz, 2H), 7.58-
7.53 (m,
2H), 7.84 (s, 1 H), 7.87 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 435 (M+H)+. MS
(FAB,
high res.) calculated: m/z 435.1179 (M+H)+, found: m/z 435.1184 (M+H)+.
-53-
CA 02578858 2007-03-05
WO 99/10331 P(.'T/US98/16479
Example 6
2-Benzvj (4-fluorophenY - -methoXy-3(2H)-Qyrid H)-Qy
To a mixture of 2-be nzyl-5-methoxy-4-bro mo-3 (2 H)-pyridazi none, prepared
according to the method of S. Cho et aL described in J. Het. Chem., 1996,33,
1579-1582, ,(2.94 g; 10 mmol), 4-fluorobenzeneboronic acid (1.54 g; 11 mmol),
and CsF (3.04 g; 22 mmol) in 25 mL of anhydrous DME, under N2, was added
Pd(Ph3P)4 (347 mg 0.3 mmol). After addition, the mixture was heated at reflux
for
at 100 C, for 18 hours. The mixture was concentrated in vacuo and the residue
partitioned between ethyl acetate and water. The acetate layer was washed with
brine, dried over MgSO4 and concentrated in vacuo. The solid residue was
suspended in ethyl ether-hexanes and filtered to provide a solid product
(yield: 3.1
g; about 100%; > 95% purity). 1 H NMR (300 MHz, CDCI3) S 3.90 (s, 3H), 5.36
(s,
2H), 7.09 (t, J = 9 Hz, 2H), 7.31 (m, 3H), 7.50 (m, 4H), 7.91 (s, 1 H). MS
(DCI-NH3)
m/z 311 (M+H)+, 328 (M+NH4)+.
Example 7
2-Benzvl-4-(4-fluorophenY1)-5-hy,droxy.3(2 )-Ryridazinnnp
A mixture of the product prepared according to the method of Example 6
(1.24 g; 4 mmol) in 20 mL of acetic acid was treated with aqueous 48% HBr (25
mL). The mixture was heated at reflux for about 5 to about 8 hours (TLC
analysis).
The mixture was concentrated in vacuo. The product was dissolved in ethyl
acetate, washed with 10% bicarbonate, brine and concentrated in vacuo. The
residue was treated with diethyl ether-hexanes (2:1) and the solid was
filtered to
provide an almost pure product (yield: 1.16 g; 98%). 1 H NMR (300 MHz, DMSO-
d6) S 5.24 (2H), 7.21 (m, 2H), 7.30 (m, 5H), 7.55 (m, 2H), 7.85 (s, 1 H),
11.31 (broad
s, 1 H). MS (DCI-NH3) m/z 296 (M+H)+, 314 (M+NH4)+.
Example 8
2-Benzvl-4-(4-fluorooõhenyl)-~(trifluoromethyls uI y,loxy)-3(2H)-Ryridazi none
A solution of the product prepared according to the method of Example 7,
(89 mg, 0.3 mmol) in 2.5 mL of anhydrous pyridine was prepared under a N2
atmosphere and maintained at 0 C. Triflic anhydride (Tf20; 0.06 mL; 0.32 mmol)
was added to the solution, dropwise. The resulting mixture was stirred at 0 C
for 5
minutes and at room temperature for 16 hours. (The pyridine and Tf20 should be
pure for good results. Occasionally an additional amount of Tf20 is necessary
to
force the reaction to completion.) The mixture was then poured to a cold
solution of
-54-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
citric acid and extracted with ethyl acetate to obtain an almost pure product
(yield:
127 mg, about 99%). 1 H NMR (300 MHz, DMSO-d6) S 5.34 (s, 2H), 7.35 (m, 7H),
7.60 (m, 2H), 8.48 (s, 1 H). MS (DCI-NH3) m/z 429 (M+H)+, 446 (M+NH4)+.
Example 9
2-Benzyl-4-(4-fiuoroohenyl)-5-[4-(methvlthio)ghe nyl]-3(2 H):pY1' dazi
A mixture of the product prepared according to the method of Example 8
(154 mg, 0.36 mmol), 4-(methylthio)benzeneboronic acid (67 mg, 0.4 mmol), Et3N
(0.11 mmol; 0.8 mmol) and Pd(Ph3P)4 (30 mg, 0.025 mmol) in 15 mL of toluene
was heated at reflux, about 100 C for about 45 minutes. The mixture was
concentrated in vacuo and the residue purified by column chromatography
(hexanes-ethyl acetate 3:1) to provide the title compound (yield: 98 mg, 68%).
1 H
NMR (300 MHz, CDCI3) S 2.47 (s, 3H), 5.38 (s, 2H), 6.98 (m, 4H), 7.12 (m, 2H),
7.20
(m, 2H), 7.35 (m, 3H), 7.54 (rr.m, 2H), 7.86 (s, 1 H). MS (DCI-NH3) m/z 403
(M+H)+,
420 (M+NH4)+.
Example 10
2-Benzvl-4-(4-fluoronhenyl)-5-[4- (meth isulfo 1)12henyjJ_3 (2 H)-pyridazi
nnnP
To a solution of the product prepared according to the method of Example 9
(140 mg, 0.348 mmol), in 10 mL of CH2CI2, at 0 C was added peracetic acid
(CH3COOOH; 0.5 mL; 30%). The mixture was stirred at 0 C for 90 minutes. The
dichloromethane was then removed in vacuo. The residue was dissolved in ethyl
acetate, washed with 10% NaHCO3, and brine. The ethyl acetate was removed
under reduced pressure. The residue was chromatographed (silica gel, CH2CI2-
diethyl ether 19:1) to provide the title compound (yield: 130 mg, 86%). 1 H
NMR
(300 MHz, CDCI3) 6 3.04 (s, 3H), 5.40 (s, 2H), 6.95 (m, 2H), 7.16 (m, 2H),
7.33 (m,
5H), 7.55 (m, 2H), 7.86 (m, 3H). MS (DCI-NH3) m/z 434 (M+H)+, 452 (M+NH4)+.
Example 11
4-(4-Fluoroohenyl)-5-j4-(meth ts uIf. nyl),henx(l-3(2H)-Ryridazinnna
A mixture of the product prepared according to the method of Example 10
(37 mg, 0.085 mmol) and AIBr3 (70 mg, 0.26 mmol) in 10 mL of toluene was
heated
at reflux, about 80 C for about 15 minutes and cooled to 0 C. The cooled
mixture
was treated with 1 N HCI and extracted with ethyl acetate. The acetate layer
was
washed with water, brine and concentrated in vacuo. Purification of the
residue on
silica gel column (ethyl acetate as an eluent) provided the title compound
(yield: 22
-55-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
mg, 76%). 1 H NMR (300 MHz, CDCI3) 5 3.07 (s, 3H), 7.00 (t, J = 9 Hz, 2H),
7.20 (m,
2H), 7.56 (d, J = 9 Hz, 2H), 7.86 (s, 1 H), 7.91 (d, J = 9 Hz, 2H), 10.94
(broad s, 1 H).
MS (DCI-NH3) m/z 345 (M+H)+, 362 (M+NH4)+.
Example 12
2-Phenyl-4-(4-fluorophen}l)-5-j4-(methylsulfonyl) henyll-3(2H)-Dyridazinone
12A. 2-Phenyl-4-chloro-5-methoxy-3(2d)-Rylidazinone
The 2-phenyl-4-chioro-5-methoxy-3(2H)-pyridazinone compound was
prepared according to the method of S. Cho et al. described in J. Het. Chem.,
1996, 33, 1579-1582, , starting with the N-phenyl-dichloropyridazinone. A
mixture
of 2-phenyl-4,5-dichloro-3(2H)-pyridazinone (1 g, 4.1 mmol) and finely
powdered,
anhydrous K2C03 (580 mg, 4.2 mmol) in 50 mL of methanol was heated at reflux
for 5 hours and concentrated in vacuo. The residue was partitioned between
water
and ethyl acetate. The acetate layer was washed with water, and brine to
provide
2-phenyl-4-chforo-5-methoxy-3(2H)-pyridazinone (yield: 920 mg, 95%). 1 H NMR
(300 MHz, DMSO-d6) S 4.15 (s, 3H), 7.50 (m, 5H), 8.43 (s, 1 H). MS (DCI-NH3)
m/z
237 (M+H)+, 254 (M+NH4)+.
12B. 2-Phenvl-4-(4-fluoro h nxj)-5-methoxX-3(2H)-oyridazinone
The 2-phenyl-4-chloro-5-methoxy-3(2H)-pyridazinone product was coupled
with 4-fluorophenylboronic acid according to the method of Example 6 to
provide 2-
phenyl-4-(4-fluorophenyl)-5-methoxy-3(2H)-pyridazinone (yield: 1.1 g; 96%). 1
H
NMR (300 MHz, CDCI3) 8 4.00 (s, 3H), 7.10 (t, J = 9 Hz, 2H), 7.45 (m, 3H),
7.60 (m,
4H), 8.06 (s, 1 H). MS (DCI-NH3) m/z 297 (M+H)+.
12C. 2-Phenyl-4-(4-fluoro heny,j)-5-hvdroxy-3(2 H)-pvridazinone
The 2-phenyl-4-(4-fluorophenyl)-5-methoxy-3(2H)-pyridazinone product was
treated with 48% HBr according to the method of Example 7 to furnish 2-phenyl-
4-
(4-fiuorophenyl)-5-hydroxy-3(2H)-pyridazinone (yield: 957 mg, 92%). MS (DCI-
NH3) m/z 283 (M+H)+, 300 (M+NH4)+.
12D. 2-Phenyi-4-(4-fluoroohenyl)-5-trifli,nrnmathanPsulfoqY1Qxy-3(2H)-
Ryridazinone
The 2-phenyl-4-(4-fluorophenyi)-5-hydroxy-3(2H)-pyridazinone product was
sulfonylated according to the method of Example 8 to furnish 2-phenyl-4-(4-
fluorophenyl)-5-trifluoromethanesulfonyioxy-3(2H)-pyridazinone (yield: 1.35 g;
96%) MS (DCI-NH3) m/z 415 (M+H)+, 432 (M+NH4)+.
12E. 2-Phenvl-4-(4-fluorooh .nvl)~14-(methylsulfonY1)ohenvJJõ2(2 H)-
oyridazinone
-56-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
The 2-phenyl-4-(4-fluorophenyl)-5-trifluoromethanesulfonyioxy-3(2H)-
pyridazinone was coupled with 4-(methylthio)phenylboronic acid as in Example 9
to provide 2-phenyt-4-(4-Fluorophenyl)-5-[4-(methylthio)phenyl]-3(2H)-
pyridazinone (yield: 915 mg, 92%) which was immediately oxidized with
peracetic
acid as in Example 9 to provide the title compound after column chromatography
(silica gel, 1:1 hexanes-ethyl acetate) and crystallization from diethyl ether-
hexanes
(yield: 288 mg, 69%). M.p. 219-220 C. 1 H NMR (300 MHz, DMSO-d6) b 3.25 (s,
3H), 7.15 (t, J = 9 Hz, 2H), 7.30 (m, 2H), 7.46 (m, 1 H), 7.56 (m, 4H), 7.64
(m, 2H),
7.90 (d, J = 9 Hz, 2H), 8.24 (s, 1 H). MS (DCI-NH3) m/z 421 (M+H)+, 438
(M+NH4)+.
Example 13
4-FluoroRhenylacetic acid. meth, ly ester
A catalytic amount (0.5 mL) of concentrated sulfuric acid was added to a
solution of 4-fluorophenylacetic acid (30.8 g, 0.20 mol) in 500 mL of
methanol. The
solution was stirred at reflux for 4 hours. The volatile materials were
removed
under reduced pressure to furnish a colorless oil which was dissolved in
ether/ethyl
acetate and washed with 2 N aqueous Na2CO3, brine, dried over MgSO4, and
filtered. The filtrate was concentrated under reduced pressure to provide an
oil
which was dried overnight under high vacuum (yield: 33.6 g; 95%). 1 H NMR (300
MHz, CDCI3) 8 3.59 (s, 2H), 3.65 (s, 3H), 7.01 (t, J = 9 Hz, 2H), 7.20-7.28
(m, 2H).
MS (DCI-NH3) m/z 186 (M+NH4)+.
Example 14
I4-(Meth I~thio) henyl]dime ylthioketene acetal. mono-S-oxide
A mixture of methyl(methylsulfinylmethyl)sulfide (50 g, 0.40 mol), and finely
powdered sodium hydroxide (3.12 g, 0.078 mol) was stirred at 70 C for 4
hours. 4-
(Methylthio)benzaldehyde (27.4 mL, 0.195 mol) was then added in one lot and
the
reaction mixture was stirred at 70 C for an additional 4 hours. The mixture
was
cooled to room temperature and partitioned between 10% aqueous citric acid and
dichloromethane. The organic layer was dried over MgSO4 and filtered. The
filtrate was concentrated under reduced pressure to provide a brown oil. The
oil
was purified by column chromatography (7:3 hexanes/ethyl acetate) to provide a
solid. The solid was crystallized from ether/hexanes (yield: 24.7 g; 72%).
M.p. 52-
53 C. 1 H NMR (300 MHz, CDCI3) S 2.33 (s, 3H), 2.53 (s, 3H), 2.77 (s, 3H),
7.17 (d,
-57-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
J = 9 Hz, 2H), 7.57 (s, 1 H), 7.86 (d, J = 9 Hz, 2H). MS (DCI-NH3) mlz 259
(M+H)+
and m/z 276 (M+NH4)+.
Example 15
2-(4-Fluoro enxJ)-3-(4-(mgtbylthio)12heny(j-4-methyithio-4-meth Isy ulfinyl-n-
butyric
acid. methyl ester
A solution of the ester product, prepared according to the method of Example
13, (16.24 g, 0.0966 mol) in 50 mL of THF was added dropwise to a stirred
solution
of 1.0 M sodium hexamethyldisilazide in THF (96.6 mL, 0.0966 mol), maintained
at
0 C, under an atmosphere of dry nitrogen. After 30 minutes, a solution of the
ketene thioacetal, prepared according to the method of Example 14 (20.8 g,
0.0805
mol), in 50 mL of THF, was added dropwise to the reaction mixture maintained
at 0
C. After 4 hours, the reaction mixture was acidified with 10% aqueous citric
acid.
The aqueous layer was washed twice with ethyl acetate. The organic extracts
were
combined, washed with brine, dried over MgSO4 and filtered. The filtrate was
concentrated under reduced pressure to provide a brown oil which was purified
by
column chromatography (85:15 to 1:1 dichloromethane/ethyl acetate gradient).
Several products having different Rf values and NMR spectra were isolated.
These
compounds had identical mass spectra. The mixture of compounds was carried on
in the following reactions (yield: 22.4 g; 65%). MS (DCI-NH3) m/z 444
(M+NH4)+.
Example 16
2-(4-Fluorophenyl)-3-j4-(methylthio) enylj-3-forrnyl-n-~ro,eanoic acid, methyl
ester
The mixture of compounds, prepared according to Example 17, (9.0 g, 0.021
mol) was dissolved in acetonitrile (80 mL) and cooled to 0 C. Perchioric acid
(60%; 1.06 g, 0.006 mol) was added to the stirred solution. The reaction
mixture
was stirred at 0 C for 8 hours, and quenched with 2 N aqueous Na2CO3. The
acetonitrile was removed under reduced pressure and the resulting aqueous
mixture was extracted with ethyl acetate. The organic solution was dried over
MgSO4 and filtered. The filtrate was concentrated under reduced pressure to
give
a yellow oil which was purified by column chromatography (silica gel, 7:3
hexanes/ethyl acetate). Fractions containing the highest Rf diastereomers from
the
product mixture were concentrated in vacuo and the residue was crystallized
from
methanol to furnish the title aldehyde-ester compound as white crystals
(yield: 0.27
g, 4.0%). M.p. = 112-113 C. 1 H NMR (300 MHz, CDCI3) S 2.49 (s, 3H), 2.46 (s,
3H), 4.39 (s, 2H), 7.03 (t, J = 9 Hz, 1 H), 7.21 (d, J = 9 Hz, 1 H), 7.25 (d,
J = 9 Hz, 2H),
-58-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
7.40-7.47 (m, 2H). MS (DCI-NH3) m/z 333 (M+H)+ and m/z 350 (M+NH4)+.
Fractions containing lower Rf compounds from the product mixture were
concentrated in vacuo and the residue was identified as the hydrate of the
aldehyde-ester (yield: 2.6 g, 35.2%). 1 H NMR (300 MHz, CDCI3) 8 2.44 & 2.46
(2 s,
3H), 3.56 & 3.48 (2 s, 3H), 3.55 & 3.76 (2 dd, J = 6 Hz, J = 6 Hz, 1 H), 3.98
& 4.26 (2
d, J = 12 Hz, 1 H), 5.41 & 5.47 (2 d, J = 6 Hz, 1 H), 6.96 & 7.00 (t, J = 9
Hz, 2H), 7.11 -
7.26 (m, 6H). MS (DCI-NH3) m/z 333 (M+H)+ and m/z 350 (M+NH4)+.
The lowest Rf compound was identified as the hydroxy lactone formed when
a hydroxy group from the hydrate displaces the methoxy group from the ester
(yield:
1.1 g, 16.4%). 1 H NMR (300 MHz, CDCI3) S 2.45 (s, 3H), 3.54-3.71 (m, 1 H),
3.98-
4.21 (m, 1 H), 4.61 (broad s, 1 H), 5.85-6.01 (m, 1 H), 6.98 (t, J = 9 Hz,
2H), 7.12-7.27
(m, 6H). MS (DCI-NH3) m/z 336 (M+NH4)+.
Example 17
4-(4-Fluoronhenvl)-544-(methylthio)phenyll-4 5-dihvdro-3(2H) -ovridazi nonP
The aldehyde-ester, hydrate, and hydroxy lactone prepared in Example 16
(0.10 g, 3 mmol), were dissolved in 100 mL of ethanol. This solution was
treated
with hydrazine monohydrate (0.15 mL, 30 mmol) and the resulting solution was
stirred at reflux in a Soxhelet apparatus containing molcular sieves. After 18
hours,
the reaction mixture was cooled and the volatile materials removed under
reduced
pressure. The residue was partitioned between ethyl acetate and aqueous HCI.
The aqueous layer was washed twice with ethyl acetate. The combined organic
extracts were washed twice with brine, dried over MgSO4, and filtered. The
filtrate
was concentrated under reduced pressure and the residue was purified by column
chromatography (4:1 hexanes/ethyl acetate) to obtain the title compound
(yield: 50
mg, 53%). 1 H NMR (300 MHz, CDCI3) S 2.46 (s, 3H), 3.75 (d, J = 12 Hz, 1 H),
3.87
(d, J = 12 Hz, 1 H), 6.93-7.08 (m, 6H), 7.16 (d, J = 9 Hz, 2H), 8.71
(S(broad), 1 H).
MS (DCI-NH3) m/z 315 (M+H)+ and m/z 332 (M+NH4)+.
Example 18
4-(4-Fluoro enyl)-5-(4-(rr hy suI yI)~henIl-y. 4 5-dihydro- (2H)-Ryridazinone
A solution of peracetic acid, 32% in acetic acid, (0.4 mL, 1.6 mmol) was
added to a stirred solution of the sulfide, prepared according to the method
of
Example 17, (0.050 g, 0.16 mmol) in dichloromethane, and maintained at 0 C.
The reaction mixture was stirred for 5 hours at 0 C then diluted with water.
The
organic layer was dried over MgSO4 and filtered. The filtrate was concentrated
-59-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
under reduced pressure to provide an oil which solidified on trituration with
ether
(yield: 47 mg, 85%). 1 H NMR (300 MHz, CDCI3) S 3.05 (s, 3H), 3.77 (d, J= 12
Hz,
1 H), 4.05 (d, J = 12 Hz, 1 H), 6.95-7.08 (m, 4H), 7.28 (d, J = 9 Hz, 2H),
7.90 (d, J = 9
Hz, 2H), 8.75 (s, broad, 1 H). MS (DCI-NH3) m/z 364 (M+NH4)+.
Example 19
4-(4-FluoroRhenvl)-5-[4-(methvlsulfonyl)phenY1]-3(2H)-RYridazinone
The dihydropyridazinone product prepared according to the method of
Example 18 (47 mg, 0.136 mmol) was dissolved in acetic acid (25 mL). Bromine
(0.025 mL, 0.16 mmol) was added to the solution and the reaction mixture was
stirred at 95 C for 20 minutes. The reaction mixture was concentrated under
reduced pressure. The residue was partitioned between ethyl acetate and water.
The organic layer was washed with brine, dried over MgSO4 and filtered. The
filtrate was concentrated under reduced pressure to provide a solid which was
eluted through a short pad of silica gel with ethyl acetate. The title
compound was
crystallized from ethyl acetate/hexanes (yield: 35 mg, 75%). M.p. 255-256 C 1
H
NMR (300 MHz, CDC13) B 3.07 (s, 3H), 6.98 (t, J = 9 Hz, 2H), 7.16-7.23 (m,
2H),
7.35 (d, J = 9 Hz, 2H), 7.86 (s, 1 H), 7.91 (d, J = 9 Hz, 2H). MS (DCI-NH3)
m/z 345
(M+H)+ and m/z 362 (M+NH4)+.
Example 20
2-(4-Fluorobenzvl)-4-(4-fluoroohenyl)-5-[4- ethylsuffonyl)phenvl]-3(2Hl-
Ryridazinone
A solution of the nitrogen-unsubstituted pyridazinone product, prepared in
Example 19 (160 mg, 0.465 mmol), K2C03 (193 mg, 1.4 mmol), 4-fluorobenzyl-
bromide (0.09 mL, 0.7 mmol) and Nal (catalytic) in 10 mL of anhydrous
N,N-dimethylformamide (DMF) was stirred at room temperature for 18 hours. The
reaction mixture was quenched with 2N HCI, extracted with ethyl acetate (2 x
20
mL), washed with brine and water, dried over MgSO4, filtered and concentrated
in
vacuo. The residue was purified by column chromatography (2:2:6 ethyl
acetate/dichloromethane/pentanes). Crystallization from ether/pentanes
provided
white crystals (yield: 110 mg, 52%). M.p. 153-154 C. 1 H NMR (CDCI3, 300 MHz)
b 3.06 (s, 3H), 5.36 (s, 2H), 6.96 (t, J = 8.4 Hz, 2H), 7.04 (t, J = 8.7 Hz,
2H), 7.16 (dd,
J = 9.1 Hz, J = 5.4 Hz, 2H), 7.31 (d, J = 8.5 Hz, 2H), 7.54 (dd, J = 8.8 Hz,
5.5 Hz, 2H),
7.84 (s, 1 H), 7.87 (d, J = 8.8 Hz, 2H). MS (DCI-NH3) m/z 453 (M+H)+.
-60-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 21
2-( Phenvl, orooarqxl)-4-(4-fluoro2henyl)-5-(4-(methylsulfonyl)phenylJ-3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 20,
substituting phenylpropargyl bromide for 4-fiuorobenzyl bromide. M.p. 100-103
C.
1 H NMR (CDCI3, 300 MHz) S 3.06 (s, 3H), 5.26 (s, 2H), 6.97 (t, J = 9 Hz, 2H),
7.20
(dd, J = 9 Hz, J = 6 Hz, 2H), 7.31 (m, 3H), 7.34 (d, J = 9 Hz, 2H), 7.48 (m,
2H), 7.89
(d, J = 9 Hz, 2H), 7.9 (s, 1 H). MS (DCI-NH3) m/z 459 (M+H)+.
Example 22
2-(2.4-Difluorobenzyl)-4-(4-fluoro Inyl)_ 5-j4-(methylsulfony,J)phenyll-3
(2Fi)-
~yridazinone
The title compound was prepared according to the method of Example 20,
substituting 2,4-difluorobenzyl bromide for 4-fluorobenzyl bromide. M.p. 179-
182
C. 1 H NMR (CDCI3, 300 MHz) S 3.06 (s, 3H), 5.45 (s, 2H), 6.87 (m, 2H), 6.96
(t, J
9 Hz, 2H), 7.17 (dd, J = 9 Hz, J = 6 Hz, 2H), 7.32 (d, J = 9 Hz, 2H), 7.54 (m,
1 H), 7.86
(s, 1 H), 7.88 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 471 (M+H)+.
Example 23
2_ (Methvl-2-oro nyl)-4-(4-fluorophenyj)-5-[4-(methylsuifonyl)Qhenyll-3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 20,
substituting 3-chioro-2-methyipropene for 4-fluorobenzyl bromide. M.p. 140-142
C. 1 H NMR (CDCI3, 300 MHz) S 1.86 (s, 3H), 3.08 (s, 3H), 4.83 (s, 2H), 4.94
(t, J
1 Hz, 1 H), 5.05 (t, J = 1 Hz, 1 H), 6.98 (t, J = 9 Hz, 2H), 7.21 (dd, J = 9
Hz, J = 6 Hz,
2H), 7.37 (d, J = 9 Hz, 2H), 7.89 (s, 1 H), 7.91 (d, J = 9 Hz, 2H). MS (DCI-
NH3) m/z
399 (M+H)+.
Example 24
2_( -Methyl-2-butenyl)-4-(4-fluorol2hgnyl)-5-(4-(methylsulfonyl)phen,ylj;3(?H)-
Ryridazinone
The desired compound was prepared according to the method of Example
20 substituting 4-bromo-2-methyl-2-butene for 4-fluorobenzyl bromide. M.p. 169-
172 C. 1 H NMR (CDCI3, 300 MHz) S 1.78 (s, 3H), 1.85 (s, 3H), 3.06 (s, 3H),
4.86
(d, J = 7.5 Hz, 2H), 5.47 (t, J = 7.5 Hz, 1 H), 6.96 (t, J = 9 Hz, 2H), 7.18
(dd, J = 9 Hz,
-61-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
J = 6 Hz, 2H), 7.33 (d, J = 9 Hz, 2H), 7.84 (s, 1 H), 7.88 (d, J = 9 Hz, 2H).
MS (DCI-
N H3) m/z 413 (M+H)+.
Example 25
2-(2-Trifluorometh ly benzyl)-4-(4-fluoro henyl)-5-[4-(methylsulfonyl)phenylJ-
3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 20,
substituting 2-(trifluoromethyl)benzyl bromide for 4-fluorobenzyl bromide.
M.p. 87-
90 C. 1 H NMR (CDCI3, 300 MHz) 5 3.07 (s, 3H), 5.66 (s, 2H), 6.97 (t, J = 9
Hz, 2H),
7.21 (dd, J = 9 Hz, J = 6 Hz, 2H), 7.26 (d, J = 7.7 Hz 1 H), 7.37 (d, J = 9
Hz, 2H), 7.42
(t J = 7.7 Hz, 1 H), 7.53 (t, J = 7.7 Hz, 1 H), 7.73 (d J = 7.7 Hz, 1 H), 7.9
(s, 1 H), 7.91
(d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 503 (M+H)+.
Example 26
2-(Cvclonrooylmethvl)-4-(4-fluoropjgny!)-5-[4-(methylsulfonyi)phgny,ll-3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 20,
substituting 2-(bromomethyl)cyclopropane for 4-fluorobenzyl bromide. M.p. 118-
121 C. 1 H NMR (CDCI3, 300 MHz) S 0.45-0.52 (m, 2H), 0.54-0.63 (m, 2H), 1.40-
1.52 (m, 1 H), 3.07 (s, 3H), 4.07 (d, J = 7 Hz, 2H), 6.97 (t, J = 9 Hz, 2H),
7.19 (dd, J
9 Hz, J = 6 Hz, 2H), 7.35 (d, J = 9 Hz, 2H), 7.83 (s, 1 H), 7.88 (d, J = 9 Hz,
2H). MS
(DCI-NH3) m/z 399 (M+H)+ and m/z 416 (M+NH4)+.
Example 27
212-Pyridvlmethvll-4-(4-fluorophenyl)-~[4-(methylsulfony-,)oheny1J-3(2H)
Ryridazinone
The title compound was prepared according to the method of Example 20,
substituting 2-(bromomethyl)pyridine for 4-fluorobenzyl bromide. M.p. 182-184
C.
1 H NMR (CDCI3, 300 MHz) S 3.07 (s, 3H), 5.56 (s, 2H), 6.95 (m, 2H), 7.17 (m,
2H),
7.26 (m, 1 H), 7.35 (m, 2H), 7.46 (m, 1 H), 7.71 (m, 1 H), 7.90 (m, 3H), 8.63
(m, 1 H).
MS (DCI-NH3) m/z 436 (M+H)+.
-62-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 28
2- (4- Pyridvlmethyl~4-( -4 fluoroohenyl)-5-[4-(methyisulfonyl)phenyl)-3(2h1)-
pyridazinone
The title compound was prepared according to the method of Example 20,
substituting 4-(bromomethyl)pyridine for 4-fluorobenzyl bromide. M.p. 153-156
C.
1 H NMR (CDCI3, 300 MHz) S 3.07 (s, 3H), 5.40 (s, 2H), 6.97 (m, 2H), 7.17 (m,
2H),
7.34 (m, 2H), 7.42 (m, 2H), 7.90 (m, 3H), 8.63 (m, 2H). MS (DCI-NH3) m/z 436
(M+H)+.
Example 29
2-(3-Pyridylmetilyl)-4-(4-fluorophenyl)-5-[4-(methylsulfonyJ)uhenyll-3(2H)-
Qyrid~
The title compound was prepared according to the method of Example 20,
substituting 3-(bromomethyl)pyridine for 4-fluorobenzyl bromide. M.p. 160-161
C.
1 H NMR (CDC13, 300 MHz) S 3.07 (s, 3H), 5.43 (s, 2H), 6.97 (m, 2H), 7.15 (m,
2H),
7.34 (m, 4H), 7.35 (m, 2H), 7.87 (m, 2H), 7.97 (s, 1 H), 8.60 (m, 1 H), 8.81
(m, 1 H).
MS (DCI-NH3) m/z 436 (M+H)+.
Example 30
2-(6-Fluoroauinolin-2-ylmethyl)-4-(4-fluoroRhenyl)-5-[4-
(methylsulfonyl)oheny~l
3(2H)-Ryridazinone
The title compound was prepared according to the method of Example 20,
substituting 2-(chloromethyl)-6-fluoroquinoline for 4-fluorobenzyl bromide.
M.p.
116-119 C. 1H NMR (CDCI3, 300 MHz) 83.07 (s, 3H), 5.73 (s, 2H), 6.96 (m, 2H),
7.18 (m, 2H), 7.34 (m, 4H), 7.35 (m, 2H), 7.46 (m, 2H), 7.58 (m, 3H), 7.90 (m,
3H),
8.12 (m, 2H). MS (DCI-NH3) m/z 504 (M+H)+.
Example 31
2-(Quinolin-2-ylmethyl)-4-(4-fluorophenyl)-5-[4-(sneth Isulfoayl)uhenYll-3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 20,
substituting 2-(chloromethyl)-quinoline for 4-fluorobenzyl bromide. M.p. 97-
100 C.
1 H NMR (CDCI3, 300 MHz) S 3.06 (s, 3H), 5.75 (s, 2H), 6.95 (m, 2H), 7.19 (m,
2H),
7.35 (m, 2H), 7.55 (m, 2H), 7.73 (m, 1 H), 7.82 (m, 1 H), 7.90 (m, 3H), 8.15
(m, 2H).
MS (DCI-NH3) m/z 386 (M+H)+.
-63-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 32
2-Benzyl-4-(4-fluoroohenyl)-5-(4-(methvls_y1)nhenyll-3(2H)-Ryridazinethione
A mixture of 2-benzyl-4-(4-fluorophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone, prepared according to the method of Example 5, (109 mg, 0.25
mmol) and Lawesson's reagent (202 mg, 0.5 mmol) in 15 mL of toluene was
stirred
at reflux for 48 hours. The mixture was concentrated in vacuo and the residue
was
chromatographed (silica gel, ethyl acetate) to provide the title compound
(yield: 100
mg, 88%). M.p. 88-90 C. 1 H NMR (300 MHz, CDCI3) S 3.04 (s, 3H), 6.05 (s,
2H),
6.96 (m, 2H), 7.08 (m, 2H), 7.26 (m, 2H), 7.37 (m, 3H), 7.61 (m, 2H), 7.84 (d,
J = 9
Hz, 2H), 8.13 (s, 1 H). MS (DCI-NH3) m/z 451 (M+H)+.
Example 33
2-Benzyl-4-(4-fluorophenyl)-5-[4-(aminosulfonyl) henyll-3(2H)-pyridazinone
33A. Preparation of 2-Benzyl-4-(4-fluorophenyl)-5-[4-(aminosulfonyl)ohenyll-
3 (2 H)-pyridazi none
A solution of 2-benzyl-4-(4-fluorophenyl)-5-[4-(methylthio)phenyl]-3(2H)-
pyridazinone, prepared according to the method of Example 4, (450 mg, 1.12
mmol) in CH2CI2 (10 mL) was added dropwise to a suspension of
hydroxy(tosyloxy)iodobenzene (439 mg, 1.12 mmol) in CH2CI2 (15 mL) and the
mixture was stirred until a clear solution was obtained (about 1 hour). The
reaction
mixture was then washed with water and dried with MgSO4. Removal of solvent in
vacuo provided the corresponding sulfoxide (yield: 485 mg, about 100%). 1 H
NMR
(300 MHz, CDCI3) 8 2.72 (s, 3H), 5.40 (s, 2H), 6.90 (m, 2H), 7.15 (m, 3H),
7.33 (m,
3H), 7.57 (m, 3H), 7.71 (m, 1 H), 7.86 (s, 1 H). MS (DCI-NH3) m/z 419 (M+H)+,
436
(M+NH4)+.
33B. Preparation of 2-benzyl-4-(4-fluorophenyl)-5-
(acetoxymethylsulfonylphenvl)-
3(2H)-pyridazinone
The sulfoxide was transformed into the sulfonamide according to a
procedure described by M. De Vleeschauwer and J. V. Gauthier in Syn. Lett.,
1997,375 with the following modifications:
A suspension of the sulfoxide, prepared according to the method of Example
33A, (485 mg, 1.12 mmol) and AcONa (1.4 g) in 15 mL of Ac20 was stirred at
reflux
for 2 hours and concentrated in vacuo. The residue was distilled twice with
toluene, dissolved in 25 mL of CH2CI2, cooled to 0 C, and treated with CH3CO3H
(1 mL). After 1 hour, the mixture was washed, successively, with saturated
NaHCO3 and brine. The solvent was removed in vacuo. The residue was
-64-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
chromatographed (silica gel, 1:1 hexanes-ethyl acetate) to provide the desired
product, 2-benzyl-4-(4-fluorophenyl)-5-(acetoxymethylsulfonylphenyl)-3(2H)-
pyridazinone (yield: 150 mg, 27%). MS (DCI-NH3) m/z 493 (M+H)+.
33C. Preparation of 2-Benzyl-4-(4-fluoroohenyl)-5-(4-(sodiumsulfinate)ohenyJj-
3(2H)- Ryridazinone
To a solution of the acetoxymethylsulfone, prepared according to the method
of Example 33B, (150 mg, 0.305 mmol), in 10 mL of THF and 5 mL of methanol at
0
C, was added 1 N NaOH (0.305 mL, 0.305 mmol). The mixture was stirred at 0 C
for 1 hour. The mixture was concentrated in vacuo, the residual water was
removed via an EtOH/toluene azeotrope followed by a toluene azeotrope. The
residue was dried under high vacuum for 48 hours to provide the sodium
sulfinate
(yield: 140 mg, 96%). MS (DCI-NH3) m/z 443 (M+H)+
33D. Preparation of 2-Benzvl-4-(4-fluorophenx1)-5-[4-(chlnrnsulfonY1)nvll-
3(2H)-Ryridazinone
The sodium sulfinate (about 0.31 mmol) in CH2CI2 (10 mL) was treated at 0
C with SOCI2 (0.033 mL, 0.4 mmol) for 2 hours. The mixture was washed with
brine, dried with MgSO4 and concentrated in vacuo to provide the crude
suifonyl
chloride (yield: 145 mg, about 100%). MS (DCI-NH3) m/z 455 (M+H)+.
33E. Preparation of 2-Benzyl-4-(4-fluorophenyj)-5-j4-(aminosulfonyJ)oh nv-1-
3(2H)-pvridazinone
The crude chloride prepared according to the method of Example 33D, in 10
mL of THF, was added to a solution of 50% NH4OH, in 10 mL of THF, maintained
at
0 C. The mixture was allowed to warm to room temperature over 3.5 hours. The
THF was removed in vacuo and the product was extracted with ethyl acetate. The
ethyl acetate was removed in vacuo and the residue was treated with diethyl
ether-
hexanes 2:1 to provide the sulfonamide (yield: 113 mg, 84%). M.p. 188-191 C.
1 H NMR (300 MHz, DMSO-d6) 8 2.70 (dd, J = 15 Hz, 2H), 5.36 (s, 2H), 7.13 (t,
J = 9
Hz, 2H), 7.22 (m, 2H), 7.40 (m, 7H), 7.73 (d, J = 9 Hz, 2H), 8.11 (s, 1 H). MS
(DCI-
NH3) m/z 436 (M+H)+.
Example 34
2-(2.2.2-Trifluoro .thvl)-4-(4-fluorophenY[)-5 j4-(methvlsõ uIfonvl)phenvll-
3(2H1
Rvridazinone
The title compound was prepared according to the method of Example 20,
substituting 2-iodo-1,1,1-trifluoroethane for 4-fluorobenzyl bromide. M.p. 177-
179
-65-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
C. 1 H NMR (CDCI3, 300 MHz) S 3.06 (s, 3H), 4.88 (q, J = 9 Hz, 2H), 6.98 (t, J
= 9
Hz, 2H), 7.18 (dd, J = 9 Hz, J = 6 Hz, 2H), 7.35 (d, J = 9 Hz, 2H), 7.89 (s, 1
H), 7.91
(d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 427 (M+H)+ and m/z 444 (M+NH4)+.
Example 35
2-(3.3-Dichloro-2-Rropenyl)-4-(4-fluoroohenyl)-5-[4-(methytsulfonvl)uhenylj-
3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 20,
substituting 1,1,3-trichloropropene for 4-fluorobenzyl bromide. M.p. 150-152
C.
1 H NMR (CDCI3, 300 MHz) S 3.06 (s, 3H), 4.98 (d, J = 7 Hz, 2H), 6.25 (t, J =
7 Hz,
1 H), 6.98 (t, J = 9 Hz, 2H), 7.18 (dd, J = 9 Hz, J = 6 Hz, 2H), 7.33 (d, J =
9 Hz, 2H),
7.85 (s, 1 H), 7.89 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 453 (M+H)+ and m/z 470
(M+NH4)+.
Example 36
2-(3-Phenvl-2-propeny1)-4-(4- orol2jeoY1);5-[4-( et ylsulfonY1), henXlj-3(2H)-
p,yridazinone
The title compound was prepared according to the method of Example 20,
substituting cinnamyl bromide for 4-fluorobenzyl bromide. M.p. 165-167 C. 1 H
NMR (CDCI3, 300 MHz) 5 3.06 (s, 3H), 5.01 (d, J = 7 Hz, 2H), 6.48 (dt, J = 15
Hz, 7
Hz, 1 H), 6.79 (d, J = 15 Hz, 1 H), 6.97 (t, J = 9 Hz, 2H), 7.19 (dd, J = 9
Hz, J = 6 Hz,
2H), 7.25-7.44 (m, 5H), 7.37 (d, J = 9 Hz, 2H), 7.86 (s, 1 H), 7.89 (d, J = 9
Hz, 2H).
MS (DCI-NH3) m/z 461 (M+H)+ and m/z 478 (M+NH4)+.
Example 37
2-(4-Carboxy hR enacyl)-4-(4-fluoroQhenul)-5-[4-(methy su yl)pbgClylj-3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 20,
substituting methyl 4-(bromomethyl)benzoate for 4-fluorobenzyl bromide and
hydrolysis of the resulting ester. M.p. 239-241 C. 1 H NMR (CDC13, 300 MHz) S
3.06 (s, 3H), 5.46 (s, 2H), 6.96 (t, J = 9 Hz, 2H), 7.17 (dd, J = 9 Hz, 6 Hz,
2H), 7.33
(d, J = 9 Hz, 2H), 7.63 (d, J = 9 Hz, 2H), 7.87 (s, 1 H), 7.89 (d, J = 9 Hz,
2H), 8.08 (d,
J = 9 Hz, 2H). MS (DCI-NH3) m/z 479 (M+H)+ and m/z 496 (M+NH4)+.
-66-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 38
2- (5-Methyfthiazol-2-ylmethyl)-4-(4-fluoroohenyl)-5-(4-(methylsulfonyl) ha
env_Il-
3(2H)-ovridazinone The title compound was prepared according to the method of
Example 20,
substituting 2-(bromomethyl)-5-methylthiazole for 4-fluorobenzyl bromide. M.p.
114-116 C. 1H NMR (d6-DMSO, 300 MHz) 52.64 (s, 3H), 3.23 (s, 2H), 5.37 (s,
2H), 7.13 (m, 2H), 7.23 (m, 2H), 7.40 (s, 1 H), 7.47 (d, J = 8 Hz, 2H), 7.87
(d, J = 8
Hz, 2H), 8.10 (s, 1 H). MS (DCI-NH3) m/z 356 (M+H)+.
Example 39
2-(5-Chlorothiazol-2-ylmethyl)-4-(4-fluoroohenyJ)-5-(4-(methls,
ulfonyl)ohenvll-
3(2 H)-ovridazinone
The title compound was prepared according to the method of Example 20,
substituting 2-(bromomethyl)-5-chlorothiazole for 4-fluorobenzyl bromide. M.p.
185-186 C. 1 H NMR (d6-DMSO, 300 MHz) S 2.32 (s, 3H), 5.50 (s, 2H), 7.15 (m,
2H), 7.24 (m, 2H), 7.47 (m, 2H), 7.87 (m, 3H), 8.14 (s, 1 H). MS (DCI-NH3) m/z
476
(M+H)+ and m/z 493 (M+NH4)+.
Example 40
2-(2.3.3.4.4.4-Hexafluoro-n-buten-1-yl)-4-(4-fluorophgpxl)-5-[4-
(methylsulfonyi)-
henyll-3(2H)-Ryridazinone
The title compound was prepared according to the method of Example 20,
substituting 2,2,3,3,4,4,4-heptafluoro-l-iodobutane for 4-fluorobenzyl
bromide.
Under the alkylation conditions, elimination of HF provided the unsaturated
product. M.p. 167-169 C. 1 H NMR (CDC13, 300 MHz) 5 3.07 (s, 3H), 7.00 (t, J
= 9
Hz, 2H), 7.17 (dd, J = 9 Hz, 6 Hz, 2H), 7.33 (d, J = 9 Hz, 2H), 7.68 (d, J =
24 Hz, 1 H),
7.93 (d, J = 9 Hz, 2H), 8.01 (s, 1 H). MS (DCI-NH3) m/z 507 (M+H)+ and m/z 524
(M+NH4)+.
Example 41
2-(2.4-Difluoro h na vl)-4-(4-fluoroohenyl)-5-(4-(methy su y,l)phgOYlj-3j2FL-
pyridazinone
The title compound was prepared according to the method of Example 20,
substituting 2-chloro-2',4'-difluoroacetophenone for 4-fluorobenzyl bromide.
M.p.
191-192 C. 1H NMR (CDCI3, 300 MHz) 53.08 (s, 3H), 5.57 (d, J = 3 Hz, 2H),
6.94-
7.07 (m, 2H), 6.96 (t, J = 9 Hz, 2H), 7.39 (dd, J 9 Hz, 6 Hz, 2H), 7.91 (s, 1
H), 7.91
-67-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
(d, J = 9 Hz, 2H), 8.03-8.12 (m, 1 H). MS (DCI-NH3) m/z 499 (M+H)+ and m/z 516
(M+NH4)+.
Example 42
2-(5-Chlorothien-2-vlmethvl)-4-(4-fluoro henyl)-5-[4-(methylsulfonvl)nhenyll-
3(2H)-
põvridazinone
The title compound was prepared according to the method of Example 20,
substituting 2-(bromomethyl)-5-chlorothiophene for 4-fluorobenzyl bromide.
M.p.
139-141 C. 1 H NMR (d6-DMSO, 300 MHz) 5 3.23 (s, 3H), 5.43 (s, 2H), 7.03 (d,
J
4 Hz, 1 H), 7.09-7.29 (m, 5H), 7.47 (d, J = 8 Hz, 2H), 7.87 (d, J = 8 Hz, 3H),
8.13 (s,
1 H). MS (DCI-NH3) m/z 474 (M+H)+ and m/z 492 (M+NH4)+.
Example 43
2-(5-Methvlthien-2-vlmethvl)-4-(4-fluoronhenyl)-5-[4-(methy su
y!)phenyl]õ3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 20,
substituting 2-(bromomethyl)-5-methylthiophene for 4-fluorobenzyl bromide.
M.p.
172-175 C. 1 H NMR (d6-DMSO, 300 MHz) S 3.22 (s, 3H), 5.49 (s, 2H), 7.03 (m,
1 H), 7.14 (m, 2H), 7.23 (m, 3H), 7.48 (m, 3H), 7.86 (m, 2H), 8.11 (s, 1 H).
MS (DCI-
NH3) m/z 441 (M+H)+ and m/z 458 (M+NH4)+.
Example 44
214-Diethvlaminoph .narvl)-4-(4-fluoroDhenyl)-5-[4-(methylsulfonY1)ohenvlJ-
3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 20,
substituting 2-chloro-4'-diethylaminoacetophenone for 4-fluorobenzyl bromide.
M.p. 105-108 C. 1 H NMR (CDC13, 300 MHz) S 1.23 (t, J = 7 Hz, 3H), 3.07 (s,
3H),
3.44 (q, J = 7 Hz, 2H), 5.61 (s, 2H), 6.66 (d, J = 9 Hz, 2H), 6.94 (t, J = 9
Hz, 2H), 7.21
(dd, J = 9 Hz, 6 Hz, 2H), 7.38 (d, J = 9=Hz, 2H), 7.87-7.94 (m, 4H), 7.90 (s,
1 H). MS
(DCI-NH3) m/z 534 (M+H)+.
Example 45
2-12 3 4 5 6-Pentafi u robenzyl)-4-(4-fluorophenyl)-5-[4-(methvlsulfonyl)nyll
312H1-Ryridazinone
The title compound was prepared according to the method of Example 20,
substituting 2,3,4,5,6-pentafluorobenzyl bromide for 4-fluorobenzyl bromide.
M.p.
-68-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
115-116 C. 1 H NMR (CDCI3, 300 MHz) 3.06 (s, 3H), 5.50 (s, 2H), 6.96 (t, J =
9 Hz,
2H), 7.17 (dd, J = 9 Hz, 6 Hz, 2H), 7.33 (d, J = 9 Hz, 2H), 7.82 (s, 1 H),
7.89 (d, J = 9
Hz, 2H). MS (DCI-NH3) m/z 525 (M+H)+ and m/z 542 (M+NH4)+.
Example 46
2-(Phenacy I)-4-(4-fluoroohenyl)-5-[4-(methylsulfonyl)Qhenyl]-3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 20,
substituting 2-bromoacetophenone for 4-fluorobenzyl bromide. M.p. 228-230 C.
1 H NMR (CDCI3, 300 MHz) 3.07 (s, 3H), 5.68 (s, 2H), 6.95 (t, J = 9 Hz, 2H),
7.20
(dd, J = 9 Hz, 6 Hz, 2H), 7.38 (d, J = 9 Hz, 2H), 7.53 (t, J = 7 Hz, 2H), 7.65
(t, J = 7
Hz, 1 H), 7.90 (d, J = 9 Hz, 2H), 7.91 (s, 1 H), 8.04 (d, J = 7 Hz, 2H). MS
(DCI-NH3)
m/z 463 (M+H)+ and m/z 480 (M+NH4)+.
Example 47
2-(4-Chloro henacyl)-4-(4-fluorophenyl)-5-[4-(methylsulfonyl)phenvl]-3(2H);
p,yridazinone
The title compound was prepared according to the method of Example 20,
substituting 2-bromo-4'-chloroacetophenone for 4-fluorobenzyl bromide. M.p.
186-
188 C. 1 H NMR (CDCI3, 300 MHz) 3.07 (s, 3H), 5.63 (s, 2H), 6.95 (t, J = 9
Hz, 2H),
7.19 (dd, J = 9 Hz, 6 Hz, 2H), 7.38 (d, J = 9 Hz, 2H), 7.51 (d, J = 9 Hz, 2H),
7.65 (t, J
= 7 Hz, 1 H), 7.90 (d, J = 9 Hz, 2H), 7.91 (s, 1 H), 7.98 (d, J = 9 Hz, 2H).
MS (DCI-
NH3) m/z 497 (M+H)+ and m/z 514 (M+NH4)+.
Example 48
2-(ProparAtil)-4-(4-fluoro h~enyl)-5-j4-(methyjsulfonkl)phenyll-3(2H)-
pXridazinone
The title compound was prepared according to the method of Example 20,
substituting propargyl bromide for 4-fluorobenzyl bromide. M.p. 196-198 C. 1
H
NMR (CDCI3, 300 MHz) 2.42 (t, J = 3 Hz, 1 H), 3.06 (s, 3H), 5.04 (d, J = 3 Hz,
2H),
6.97 (t, J = 9 Hz, 2H), 7.19 (dd, J = 9 Hz, 6 Hz, 2H), 7.34 (d, J = 9 Hz, 2H),
7.90 (s,
1 H), 7.91 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 383 (M+H)+ and m/z 400
(M+NH4)+.
-69-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 49
2-(4-Cyano h~yl)-4-(4-fluoroohenyl)-5-j4-(methylsulfonyJ)ohenylJ-3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 20,
substituting 2-bromo-4'-cyanoacetophenone for 4-fluorobenzyl bromide. M.p. 188-
189 C. 1 H NMR (CDCI3, 300 MHz) 3.08 (s, 3H), 5.64 (s, 2H), 6.96 (t, J = 9
Hz, 2H),
7.19 (dd, J = 9 Hz, 6 Hz, 2H), 7.38 (d, J = 9 Hz, 2H), 7.84 (d, J = 9 Hz, 2H),
7.91 (d, J
= 9 Hz, 2H), 7.93 (s, 1 H), 8.14 (d, J = 9 Hz, 2H). MS (DCI-NH3) mlz 488
(M+H)+ .
Example 50
2-(a-Methyl-4-ffuorobenzyl)-4-(4-fluorophepyl)-5-[4-(methylsulfonvl)ohenkl
3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 20,
substituting a-methyl-4-fluorobenzyl bromide for 4-fluorobenzyl bromide. M.p.
162-
164 C. 1 H NMR (CDCI3, 300 MHz) 3.06 (s, 3H), 6.40 (t, J = 9 Hz, 2H), 6.95
(t, J = 9
Hz, 2H), 7.05 (t, J = 9 Hz, 2H), 7.15 (dd, J = 9 Hz and 6 Hz, 2H), 7.31 (d, J
= 9 Hz,
2H), 7.53 (dd, J = 9 Hz and 6 Hz, 2H), 7.87 (d, J = 9 Hz, 2H), 7.88 (s, 1 H).
MS (DCI-
NH3) m/z 467 (M+H)+ and m/z 484 (M+NH4)+.
Example 51
2-Phenethvl-4-(4-fluoropheny1)3-j4-(methy su y,j)glheIlXJj-3(2H)-Ryridazinone
The title compound was prepared according to the method of Example 20,
substituting (2-bromoethyl)benzene for 4-fluorobenzyl bromide. M.p. 170-171
C.
1 H NMR (CDC13, 300 MHz) 3.07 (s, 3H), 3.20 (t, J = 9 Hz, 2H), 4.28 (t, J = 9
Hz,
2H), 6.98 (t, J = 9 Hz, 2H), 7.18 (dd, J = 9 Hz and 6 Hz, 2H), 7.22-37 (m, 5
H), 7.34
(d, J = 9 Hz, 2H), 7.83 (s, 1 H), 7.89 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 449
(M+H)+ and m/z 466 (M+NH4)+.
Example 52
2-Benzyl-4-(3-chloro-4-fluoro henyl)-S-[4-(nethxlsulfonvl henyjj-3(2H)-
Ryridazinone
The title compound was prepared according to the method described in
Examples 6-10 substituting 3-chloro-4-fluorobenzeneboronic acid for 4-fluoro-
benzeneboronic acid in Example 6. M.p. 134-136 C. 1 H NMR (CDCI3, 300 MHz)
3.06 (s, 3H), 5.41 (s, 2H), 6.96-7.02 (m, 2H), 7.29-7.41 (m, 3H), 7.33 (d, J =
9 Hz,
-70-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
2H), 7.51-7.56 (m, 2H), 7.85 (s, 1 H), 7.91 (d, J = 9 Hz, 2H). MS (DCI-NH3)
m/z 469
(M+H)+ and m/z 486 (M+NH4)+.
Example 53
2-Benzyl-4-(4-chtoro henyl)-5-[4-(methy(sulfony()~he_ vll-3(2H)-pyridazinone
The title compound was prepared according to the method described in
Examples 6-10 except substituting 4-chlorobenzeneboronic acid for 4-fluoro-
benzeneboronic acid in Example 6. M.p. 157-159 C. 1 H NMR (CDC13, 300 MHz)
3.05 (s, 3H), 5.40 (s, 2H), 7.11 (d, J = 9 Hz, 2H), 7.24 (d, J = 9 Hz, 2H),
7.28-7.40 (m,
2H), 7.31 (d, J = 9 Hz, 2H), 7.51-7.57 (m, 2H), 7.84 (s, 1 H), 7.88 (d, J = 9
Hz, 2H).
MS (DCI-NH3) m/z 451 (M+H)+ and m/z 468 (M+NH4)+.
Example 54
2-(2 2 2-Trifluoroethyl)-4-(3-chloro-4-fluorophenyl)-5-[4-
(methylsulfon,yl)nhenyll
3(2H)-pyridazinone
The title compound was prepared by N-debenzylation of the product,
prepared in Example 52 according to the method of Example 11, followed by
alkylation with 2-iodo-1,1,1-trifluoroethane according to the method of
Example 20.
M.p. 165-166 C. 1 H NMR (CDCI3, 300 MHz) 3.07 (s, 3H), 4.89 (q, J= 9 Hz, 2H),
7.00-7.06 (m, 2H), 7.31-7.35 (m, 1 H), 7.37 (d, J = 9 Hz, 2H), 7.90 (s, 1 H),
7.94 (d, J
9 Hz, 2H). MS (DCI-NH3) m/z 461 (M+H)+ and m/z 478 (M+NH4)+.
Example 55
2-(4-Trifluoromethoxyphenacyi)-4-(4-fluorophenyl)-5-[4-(methylsulfony,I)Qhenkl-
3(2H)-pyridazinone
The title compound was prepared according to the method of Example 20,
substituting 2-bromo-4'-trifluoromethoxyacetophenone for 4-fluorobenzyl
bromide.
M.p. 160-161 C. 1 H NMR (CDCI3, 300 MHz) 3.08 (s, 3H), 5.65 (s, 2H), 6.96 (t,
J = 9
Hz, 2H), 7.20 (dd, J = 9 Hz, 6 Hz, 2H), 7.37 (d, J = 9 Hz, 2H), 7.91 (d, J = 9
Hz, 2H),
7.93 (s, 1 H), 8.11 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 547 (M+H)+ and m/z 564
(M+NH4)+.
-71-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 56
2-(4-Triffuoromethyl enacyl)-4-(4-ftuoro henyl)-5-[4-(methylsulfonyl)oh~ envll-
3(2H)-ovridazinone
The title compound was prepared according to the method of Example 20,
substituting 2-bromo-4'-trifluoromethylacetophenone for 4-fluorobenzyl
bromide.
M.p. 205-206 C. 1 H NMR (CDCI3, 300 MHz) 3.07 (s, 3H), 5.66 (s, 2H), 6.96 (t,
J
9 Hz, 2H), 7.20 (dd, J = 9 Hz, 6 Hz, 2H), 7.38 (d, J = 9 Hz, 2H), 7.80 (d, J =
9 Hz,
2H), 7.91 (d, J = 9 Hz, 2H), 7.92 (s, 1 H), 8.15 (d, J = 9 Hz, 2H). MS (DCI-
NH3) m/z
531 (M+H)+ and m/z 548 (M+NH4)+.
Example 57
2-[2-(Benzo[b]fhien-3-vl)-2-oxoethyl]-4-(4-fluorol2henyl)-5-[4-(meth
Isulfony,J)-
Rhenyll 3(2H)-Qyridazinone
The title compound was prepared according to the method of Example 20,
substituting 3-chforoacetylbenzo[b]thiophene for 4-fluorobenzyl bromide. M.p.
183-
184 C. 1 H NMR (CDCI3, 300 MHz) 3.08 (s, 3H), 5.68 (s, 2H), 6.96 (t, J = 9
Hz, 2H),
7.21 (dd, J = 9 Hz, 6 Hz, 2H), 7.39 (d, J = 9 Hz, 2H), 7.42-7.54 (m, 2H), 7.91
(d, J = 9
Hz, 2H), 7.91 (d, J = 7 Hz, 1 H), 7.94 (s, 1 H), 8.53 (s, 1 H), 8.72 (d, J = 7
Hz, 1 H). MS
(DCI-NH3) m/z 519 (M+H)+.
Example 58
2-(2.2.2-Trifluoroethvl)-4-(4-chloroQh nyl -5-[4-(methytsulfonyl)phenyl]-3(2H)-
Ryridazinone
The title compound was prepared by N-debenzylation of the product,
prepared in Example 54 according to the method of Example 12, followed by
alkylation with 2-iodo-1,1,1-trifluoroethane according to the method of
Example 20.
M.p. 55-57 C. 1 H NMR (CDC13, 300 MHz) 3.07 (s, 3H), 4.88 (q, J = 9 Hz, 2H),
7.13
(d, J = 9 Hz, 2H), 7.26 (d, J = 9 Hz, 2H), 7.36 (d, J = 9 Hz, 2H), 7.89 (s, 1
H), 7.92 (d,
J = 9 Hz, 2H). MS (DCI-NH3) m/z 443 (M+H)+ and m/z 460 (M+NH4)+.
Example 59
2-(3.3-Dimethyl-2-oxobutvl)-4-(4-fluorophenvl)-5-[4-(meth Isi Y1)phenvl]-3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 20,
substituting 1-bromopinacolone for 4-fluorobenzyl bromide. M.p. 168-170 C. 1
H
NMR (CDCI3, 300 MHz) 1.31 (s, 9H), 3.06 (s, 3H), 5.21 (s, 2H), 6.95 (t, J = 9
Hz,
-72-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
2H), 7.17 (dd, J = 9 Hz, 6 Hz, 2H), 7.35 (d, J = 7 Hz, 2H), 7.86 (s, 1 H) 7.89
(d, J = 9
Hz, 2H). MS (DCI-NH3) m/z 443 (M+H)+ and m/z 460 (M+NH4)+.
Example 60
2-(3-Thienyimethyl)-4-(4-fluorophenyl)-5-[4-(meth Isy ulfonyl)phenyll-3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 20,
substituting 3-(chloromethyl)thiophene for 4-fluorobenzyl bromide. M.p. 169-
172
C. 1 H NMR (300 MHz, DMSO d6) 8 3.22 (s, 3H), 5.36 (s, 2H), 7.18 (m, 5H), 7.51
(m, 4H), 7.88 (m, 2H); 8.08 (s, 1 H). MS (DCI-NH3) m/z 441 (M+H)+ and m/z 458
(M+NH4)+ .
Example 61
2-( -B n o[b]thienytmethYl)-4-(4-fluorophgnyl)-5-j4-(methylsulfonyl)12henyll-
3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 20
substituting 2-(chloromethyl)benzo[b]thiophene for 4-fluorobenzyl bromide.
M.p.
93-96 C. 1 H NMR (300 MHz, CDCI3) S 3.05 (s, 3H), 5.64 (s, 2H), 6.97 (m, 2H),
7.18 (m, 2H), 7.33 (m, 5H), 7.78 (m, 2H), 7.86 (m, 3H). MS (DCI-NH3) m/z 491
(M+H)+ and m/z 508 (M+NH4)+.
Example 62
2.4-Bis(4-fluoroQhenyj)-5-[4-(methylsulfonyl) he tyll-3(2H)-Ryridazinone
A mixture of 4-(4-fluorophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone (172 mg, 0.5 mmol), prepared according to the method of Example
10, Cu powder (32 mg), anhydrous K2C03 (207 mg, 1.5 mmol) and 4-
fluoroiodobenzene (0.12 mL, 1 mmol) was prepared in 20 mL of pyridine . The
solution was stirred at reflux for 14 hours. The mixture was then cooled to
room
temperature and partitioned between water and ethyl acetate. The ethyl acetate
layer was washed with 10% citric acid, water, brine and concentrated in vacuo.
Separation by column chromatography (silica gel, CH2CI2-diethyl ether 15:1)
provided 190 mg of crude product. Crystallization from CH2CI2-diethyl ether-
hexanes furnished the title compound (yield: 175 mg, 79.9%). M.p. 168-169 C.
1 H NMR (300 MHz, CDCI3) 8 3.07 (s, 3H), 6.98 (t, J = 9 Hz, 2H), 7.20 (m, 4H),
7.40
(d, J = 9 Hz, 2H), 7.69 (m, 2H), 7.92 (d, J = 9 Hz, 2H), 7.98 (s, 1 H). MS
(DCI-NH3)
-73-
CA 02578858 2007-03-05
WO 99/10331 PCTIUS98/16479
m/z 439 (M+H)+, 456 (M+NH4)+. Anal. calc. for C23H16F2N2O3S-0.25 H20: C,
62.36; H, 3.75; N, 6.32. Found: C, 62.23; H, 3.55; N, 6.26.
Example 63
4-(4-FluoroRhenyl)-5-j4-(methylsulfonXl)phepyl]-6-methYL-3(2H)-pyridazinone
The 5-hydroxy-5-methyl-2(5H)-furanone prepared via above cited methods
(454 mg, 1.25 mmol) was dissolved in n-butanol (10 mL) and treated with
hydrazine hydrate (0.3 mL, 6.2 mmol) and stirred at reflux for 18 hours. On
cooling,
white crystals (224 mg, 50%) were obtained. M.p. 290 C (dec.) 1 HNMR (300
MHz, d6-DMSO) 5 1.99 (s, 3H), 3.10 (s, 3H), 7.05 (t, J = 9 Hz, 2H), 7.15 (dd,
J = 6
Hz, J= 9 Hz, 2H), 7.48 (d, J= 9 Hz, 2H), 7.85 (d, J= 9 Hz, 2H), 13.10 (br s, 1
H). MS
(DCI/NH3) 376 (M+NH4)+. Anal. calc. for C18H15N2FSO3 0.25 H2O: C, 59.57; H,
4.30; N, 7.71. Found: C, 59.28; H, 4.39; N, 8.39
Example 64
2-(2.2.2-Trifluoroethyl)-4-(4-fluorophenyl)-5-[4-(plgthylsulfonyl) henylJ-6-
methyl-
3j2H1 pvridazinone
The product of Example 63 (100 mg, 0.28 mmol) was dissolved in anhydrous
DMF (3 mL) and treated with 1,1,1-trifluoro-2-iodoethane (27.5 mL, 280 mmol)
in
presence of anhydrous sodium carbonate (130 mg, 1.2 mmol) at 50-60 oC for 2
hours. The reaction mixture was partitioned between water and ethyl acetate to
provide the desired compound as an amorphous solid (60 mg, 48%). 1 HNMR (300
MHz, CDCI3) 5 2.10 (s, 3H), 3.10 (s, 3H), 4.85 (q, J 9 Hz, 2H), 6.90 (m, 2H),
7.10
(dd, J = 6 Hz, J = 9 Hz, 2H), 7.25 (m, 2H), 7.95 (d, J 9 Hz, 2H),. MS
(DCI/NH3)
458 (M+NH4)+ Anal. calc. for C20H16N2F4SO3: C, 54.54; H, 3.66; N, 6.36.
Found: C, 54.41; H, 3.56; N, 6.35.
Example 65
2-Benzyl-4-(3.4-dichloronhenyl)-5-(4-(methv Su Ifo Y1)p hen y1 -J 3(2H)-
Ryridazinone
The title compound was prepared by coupling 3,4-dichlorophenylboronic
acid with 2-benzyl-4-bromo-5-methoxy-3(2H)-pyridazinone (J. Het. Chem., 1996,
33, 1579-1582) according to the method of Example 6. This product was
converted
to the 5-hydroxy-derivative according to the method of Example 7. The 5-
hydroxy
compound was converted to the 5-trifluoromethylsufonyloxy-derivative according
to
the method of Example 8. Coupling of 4-(methylthio)phenylboronic acid to the
triflate according to the method of Example 9 provided the 5-[4-
(methylthio)phenyl]-
-74-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
intermediate which was oxidized according to the method of Example 10 to
provide
the final product (yield: 780 mg, 84%). M.p. 161-163 C. 1 H NMR (300 MHz,
DMSO-d6) S 3.22 (s, 3H), 5.35 (s, 2H), 7.08 (dd, J = 9 Hz, 3 Hz, 1 H), 7.32-
7.44 (m,
5H), 7.47 (dd, J = 9 Hz, 3 Hz, 3H), 7.48 (d, J = 3 Hz, 1 H), 7.90 (d, J = 9
Hz, 2H), 8.13
(s, 1 H). MS (DCI-NH3) m/z 485 (M+H)+. Anal. caic. for C24H18C12N2O3S: C,
59.38; H, 3.73; N, 5.77. Found: C, 59.28; H, 3.92; N, 5.42.
Example 66
2-(2.2.2-Trifluoroethyl)-4-(4-n-plQeylplienyl)-5-[4-(meth,ylsulfonvl)nhenyll-
3(2,Hl,-_
pyridazinone
The title compound was prepared by coupling 4-(n-propyl)phenylboronic
acid with 2-benzyl-4-bromo-5-methoxy-3(2H)-pyridazinone (J. Het. Chem., 1996,
33, 1579-1582) according to the method of Example 6. This product was
converted
to the 5-hydroxy- derivative according to the method of Example 7. This
product
was converted to the 5-trifluoromethylsufonyloxy-derivative according to the
method of Example 8. Coupling of 4-(methylthio)phenylboronic acid to the
triflate
according to the method of Example 9 provided the 5-[4-(methylthio)phenyl]-
intermediate which was oxidized according to the method of Example 10 to
provide
the final product (yield: 220 mg, 70%). M.p. 64-66 C. 1 H NMR (300 MHz,
CDCI3)
S 0.91 (t, J = 7.5 Hz, 3H), 1.6 (h, J- 7.5 Hz, 2H), 2.55 (q, J = 7.5 Hz, 2H),
3.05 (s,
3H), 4.88 (q, J = 9 Hz, 2H), 7.08 (s, 4H), 7.35 (d, J = 9 Hz, 2H), 7.86 (d, J
= 9 Hz,
2H), 7.87 (s, 1 H). MS (DCI-NH3) m/z 451 (M+H)+. Anal. calc. for
C22H21 F3N203S: C, 58.65; H, 4.69; N, 6.21. Found: C, 58.71; H, 4.72; N, 6.20.
Example 67
2-(2.2.2-Trifluoroethvl)-4-(4-chloro-3-fluoroohenyl)-5-j4-(methy suI y1) nyL
3(2H)-Ryridazinone
The title compound was prepared by first coupling 3-fluoro-4-
chlorophenylboronic acid with 2-benzyl-4-chloro-5-methoxy-3(2H)-pyridazinone
according to the method of Example 6. The product was converted to the 5-
hydroxy
compound according to the method of Example 7. This 5-hydroxy compound was
converted to the 5-trifluoromethylsufonyloxy-derivative according to the
method of
Example 8. Coupling of 4-(methylthio)phenylboronic acid to the triflate
according to
the method of Example 9 provided the 5-[4-(methylthio)phenyl]-intermediate
which
was oxidized according to the method of Example 10 to provide the final
product
(yield: 170 mg, 84%). M.p. 174-175 C. 1 H NMR (300 MHz, CDC13) S 3.09 (s,
3H),
-75-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
4.89 (q, J = 9 Hz, 2H), 6.87 (dm, J = 9 Hz, 1 H), 7.09 (dd, J = 9 Hz, 3 Hz, 1
H), 7.30 (t.
J = 9 Hz, 1 H), 7.39 (d, J = 9 Hz, 2H), 7.91 (s, 1 H), 7.95 (d, J = 9 Hz, 2H).
MS (DCI-
NH3) m/z 461 (M+H)+. Anal. calc. for C1 gH13CIF4N2O3S: C, 49.52; H, 2.84; N,
6.07. Found: C, 49.66; H, 2.70; N, 5.96.
Example 68
2-(2.2.2-Trifluoroethyl)-4-fluoroohenvlLj4-(aminosulfonvl)phenyjl-3(-I)-
Qyridazinone
A solution of 2-(2,2,2-trifluoroethyl)-4-(4-fluorophenyl)-5-[4-
(methylsulfinyl)-
phenyl] -3(2H)-pyridazinone (680 mg, 1.53 mmol) in trifluoroacetic anhydride
(30
mL) was stirred at room temperature for 1 hour. The excess solvent was
evaporated in vacuo and the residue was treated with a deoxygenated 1 N
solution
of methanol-NaOH (50 mL, 4:1) at 0 C. The solution was stirred at room
temperature for 2 hours and quenched with dilute HCI solution until acidic.
The
white suspension formed was concentrated in vacuo to evaporate the methanol.
THF was added to the resulting suspension until a clear solution was obtained.
Chlorine gas was slowly bubbled into the solution, maintained at 0 C. After 10
minutes, nitrogen gas was bubbled into the solution for a few minutes to
displace
residual chlorine. Ammonium hydroxide solution (30%, 5 to 10 mL), at 0 C, was
slowly added to the solution (to consume all starting sulfonyl chloride) and
stirred at
room temperature for 5 minutes The solution was partitioned between water and
ethyl acetate. The organic layer was washed first with water, then brine, and
dried
over MgSO4, and filtered. The filtrate was concentrated in vacuo. The residue
was
purified. by chromatography on silica gel (40:60 ethyl acetate/hexanes) to
provide
the title compound (yield: 500 mg, 75%). M.p. 193-195 C. 1 H NMR (300 MHz,
CDCI3) S 4.82 (s, 2H), 4.88 (q, J = 9 Hz, 2H), 6.98 (t, J = 9 Hz, 2H), 7.19
(dd, J = 9
Hz, 6 Hz, 2H), 7.30 (d, J = 9 Hz, 2H), 7.88 (d, J = 9 Hz, 2H), 7.90 (s, 1 H).
MS (DCI-
NH3) m/z 428 (M+H)+. Anal. caic. for C18H13F4N303S: C, 50.58; H, 3.06; N,
9.83.
Found: C, 51.04; H, 3.26; N, 9.63.
Example 69
2-(2.2.2-Trifluoroethvl)-4-(4-chlorophg6X()~(4-(aminosulfonyJ)ohenyll-3~?
."õ
Ryridazinone
69A. 2-Benzvl-4-chloro-5-[4-(methylthio)phenY1l-3(2j)-Ryridazinone
The title compound was prepared according to the method of Example 77.
The product was coupled with 4-chlorophenylboronic acid following the method
of
-76-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 6. The product was N-debenzylated according to the method of Example
11 and N-alkylated with 2-iodo-1,1,1-trifluoroethane according to the method
of
Example 20 to provide the sulfice compound.
69B. 2-Benzyl-4-chloro-5-[4-(methylsulfi nyl)phenX[J-3 (2 H)-Ryridazi none
The sulfide was oxidized to the corresponding sulfoxide with one equivalent
of meta-chloroperoxybenzoic acid to provide the corresponding methylsulfoxide
which was converted to the sulfonamide final product according to the method
of
Example 68 (yield: 540 mg, 70%). M.p. 154-156 C. 1 H NMR (300 MHz, CDCI3) 8
4.86 (s, 2H), 4.87 (q, J = 9 Hz, 2H), 7.14 (d, J = 9 Hz, 2H), 7.29 (d, J = 9
Hz, 2H),
7.31 (d, J = 9 Hz, 2H), 7.89 (d, J = 9 Hz, 2H), 8.00 (s, 1 H). MS (DCI-NH3)
m/z 444
(M+H)+. Anal. calc. for C18H13CIF3N3O3S: C, 48.71; H, 2.95; N, 9.46. Found: C,
49.05; H, 3.01; N, 9.15.
Example 70
2-(2.2.2-Trifluoroethyl)-4-(2-~ro o~xv)-5-[4-(aminosulfonyl)phenyll-3(2H)-
Ryridazinone
The methyl sulfide intermediate prepared in Example 83 was oxidized with
one equivalent of ineta-chloroperoxybenzoic acid to provide the
methylsulfoxide
which was converted to the sulfonamide final product according to the method
of
Example 68 (yield: 396 mg, 60%). M.p. 158-160 C. 1 H NMR (300 MHz, CDCI3) 8
1.21 (d, J = 6 Hz, 6H), 4.83 (q, J = 7.5 Hz, 2H), 4.86 (s, 2H), 5.46 (p, J = 6
Hz, 1 H),
7.72 (d, J = 9 Hz, 2H), 7.82 (s, 1 H), 8.03 (d, J = 9 Hz, 2H). MS (DCI-NH3)
m/z 392
(M+H)+. Anal. caic. for C15H16F3N304S: C, 46.03; H, 4.12; N, 10.73. Found: C,
46.08; H, 4.22; N, 10.52.
Example 71
2-(2.2.2-Trifluoroethyl)-4-(4-fluoro h~y)-5-[4-(aminosulfonyl)nhenyll-3(2H)-
Ryridazinone
The methyl sulfide intermediate of Example 76 was oxidized with one
equivalent of ineta-chloroperoxybenzoic acid to provide the methylsulfoxide
which
was converted to the sulfonamide final product according to the method of
Example
68 (yield: 180 mg, 37%). M.p. 150-152 C. 1 H NMR (300 MHz, CDCI3) S 4.71 (q,
J
= 7.5 Hz, 2H), 4.72 (s, 2H), 6.88 (dd, J = 9 Hz, 4.5 Hz, 2H), 7.0 (t, J = 9
Hz, 2H), 7.73
(d, J = 9 Hz, 2H), 7.98 (s, 1 H), 8.05 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 444
(M+H)+. Anal. calc. for C18H13F4N304S: C, 48.76; H, 2.95; N, 9.47. Found: C,
48.49; H, 2.8; N, 8.95.
-77-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 72
2i4-Bis-(4-fluorophgIIX1)-5-f3-fluoro-4-(aminosulfonvl)QhgnY11-3(2H)-
Ryridazinone
72A-1. 2-Fluorothioanisole
A deoxygenated solution of 2-fluorothiophenol (10 g, 78 mmol) in anhydrous
DMF (10 mL) was treated with iodomethane (4.9 mL, 78 mmol) and potassium
carbonate (10.8 g, 78 mmol). The reaction mixture was stirred at room
temperature
for 1 hour. A thin layer chromotography (100% hexanes) sample indicated that
the
reaction had not gone to completion, so an additional equivalent of base and
iodomethane were added and the reaction mixture was stirred overnight at room
temperature. The reaction was acidified with 10% aqueous citric acid and
extracted with hexanes (2 X 125 mL). The combined organic extracts were washed
with brine, dried over MgSO4, and filtered. The filtrate was concentrated
under
reduced pressure to provide the desired compound as a pale yellow oil (yield:
6.68
g; 60 !0).
72A-2. 2-Fluorothioanisole
An alternative method for preparing 2-fluorothioanisole begins with a
solution of 1,2-difluorobenzene (0.79 mL, 8 mmol) in anhydrous DMF (50 mL) was
treated with sodium thiomethoxide (0.59 g, 8 mmol). The reaction mixture was
stirred at room temperature for 6 hours, and partitioned between hexanes and
water. The organic layer was washed with brine, dried over MgSO4, and
filtered.
The filtrate was concentrated under reduced pressure to provide the desired
compound (1.1 g, 100%) slightly contaminated with 1,2-bis(methylthio)benzene,
a
lower Rf material, which was removed by chromatography with 100% hexanes (0.9
g, 80%). 1 H NMR (300 MHz, CDCI3) S 2.46 (s, 3H), 6.98-7.19 (m, 3H) 2.26 (dt,
J
9 Hz, 3 Hz, 1 H).
72B. 4-Bromo-2-fluorothioanisole
A solution of 2-fluorothioanisole (1.42 g, 10 mmol) and iron powder (0.03 g,
0.5 mmol) in dichloromethane (20 mL) was chilled to C and treated dropwise
with
Bromine (0.5 mL, 10 mmol). Upon completion of the Bromine treatment, the
reaction was sampled for TLC (100% hexanes). A new, higher Rf material was
present but the reaction had not gone to completion so another equivalent of
-78-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
bromine was added along with a catalytic amount of aluminum chloride. The
reaction mixture was stirred overnight at room temperature. Aqueous sodium
sulfite was added to the reaction mixture and the organic layer was isolated,
dried
over MgSO4, and filtered. The filtrate was filtered through a pad of silica
gel to
remove color then concentrated under reduced pressure to provide the product
as
a clear, colorless oil (yield: 1.3 g; 60%). 1 H NMR (300 MHz, DMSO-d6) S 2.48
(s,
3H), 7.31 (t, J = 9 Hz, 1 H), 7.43 (dd, J= 9 Hz, 3 Hz, 1 H) 7.54 (dd, J = 9
Hz, 3 Hz,
1 H).
72C. 3-Fluoro-4-(methylthio)benzeneboronic acid
A solution of 4-bromo-2-fluorothioanisole (0.5 g, 22.6 mmol) in dry THF (20
mL) was chilled to -78 C under a nitrogen atmosphere. The reaction mixture
was
treated with 1.6 M n-butyllithium in hexanes (1.7 mL, 27.1 mmol), and the
mixture
was warmed to -40 C where it was maintained for 0.5 hours. The reaction
mixture
was then chilled to -78 C and three equivalents of triisopropyl borate (1.56
mL,
67.8 mmol) were added. The reaction mixture was allowed to warm to room
temperature and stirred for 1.5 hours. At this point, 10% aqueous KOH (200 mL,
360 mmol) was added and the mixture was stirred overnight at room temperature.
The reaction mixture was then poured into an ice/concentrated HCI mixture with
stirring to yield a white precipitate. This solid was dried in a vacuum oven
(65 C,
29 in Hg) overnight to provide the title compound (yield: 0.22 g; 52.4%). 1 H
NMR
(300 MHz, DMSO-d6) S 2.48 (s, 3H), 7.31 (t, J = 9 Hz, 1 H), 7.49 (dd, J = 12
Hz, 1.5
Hz, 1 H) 7.54 (dd, J = 9 Hz, 1.5 Hz, 1 H).
72D. 2.4-Bis-(4-fluoroti~ henyl)-5-'[3-fh,oro-4-(aminosulfonY1)Rhenylj-ti(2H)-
Rvridazinone
2-Benzyl-4-chloro-5-methoxy-3(2H)-pyridazinone (J. Het. Chem., 1996, 33,
1579-1582) was converted to the 5-hydroxy-analog according to the method of
Example 7 and then to the 5-trifluoromethylsulfonyloxy-analog following the
method of Example 8. Subsequent coupling to 3-fluoro-4-(methylthio)phenyl-
boronic acid, according to the method of Example 9, provided 2-benzyl-4-chloro-
5-
[3-ffuoro-4-(methylthio)phenyl]-3(2H)-pyridazinone. This intermediate was
coupled
in the 4-position with 4-fluorophenylboronic acid following the method of
Example
6. This product was N-debenzylated according to the method of Example 11 and
N-arylated with 4-fluoroiodobenzene according to the method of Example 62. The
-79-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
resulting sulfide was oxidized with one equivalent of meta-chloroperoxybenzoic
acid to provide the methylsulfoxide which was converted to the sulfonamide
final
product according to the method of Example 68 (yield: 500 mg, 75%). M.p. 222-
224 C. 1 H NMR (300 MHz, CDCI3) S 5.06 (s, 2H), 7.01 (t, J = 9 Hz, 2H), 7.06
(d, J
= 9 Hz, 2H), 7.10 (d, J = 9 Hz, 2H), 7.18 (t, J = 9 Hz, 2H), 7.69 (dd, J = 9
Hz, 3 Hz,
2H), 7.88 (t, J = 9 Hz, 1 H), 7.95 (s, 1 H). MS (DCI-NH3) m/z 458 (M+H)+.
Anal. calc.
for C22H14F3N303S: C, 57.76; H, 3.08; N, 9.18. Found: C, 57.5; H, 3.15; N,
8.8.
Example 73
2-(2.2.2-Trifluoroeth, I)-4-(3-fluoro-4-chloroohenyl)-5-[4-
(aminosulfony,()phenyll-
3(2H)-Ryridazinone
The methyl sulfide intermediate prepared in Example 67 was oxidized with
one equivalent of ineta-chloroperoxybenzoic acid, according to the method of
Example 68 to provide the methyl sulfoxide. The methyl suifoxide was converted
to
the sulfonamide product according to the method of Example 68 (yield: 1.5 g,
63%).
M.p. 180-183 C. 1 H NMR (300 MHz, DMSO-d6) 6 5.09 (q, J 9 Hz, 2H), 7.01 (dd,
J = 9 Hz, 3 Hz, 1 H), 7.15 (dd, J = 9 Hz, 3 Hz, 1 H), 7.39 (dd, J 9 Hz, 3 Hz,
1 H), 7.47
(dd, J = 9 Hz, 3 Hz, 1 H), 7.55 (t, J = 9 Hz, 1 H), 7.71 (t, J = 9 Hz, 1 H),
7.78 (s, 2H),
8.37 (s, 1 H). MS (DCI-NH3) m/z 480 (M+H)+. Anal. calc. for C18H11 CIF5N3O3S:
C, 45.05; H, 2.31; N, 8.75. Found: C, 46.19; H, 3.02; N, 7.43.
Example 74
2-Benzyl-4-(2-propoxY)-5-[4- (IDethY su Y1)phe nXIl-3 (2 H)-pyridazinone
2-Benzyl-4-chloro-5-methoxy-3(2H)-pyridazinone (J. Het. Chem., 1996, 33,
1579-1582) was converted to the 5-hydroxy-analog according to the method of
Example 7 and then to the 5-trifluoromethylsulfonyloxy-analog following the
method of Example 8. Subsequent coupling to 4-(methylthio)phenylboronic acid
according to the method of Example 9 provided 2-benzyl-4-chloro-5-[4-(methyl-
thio)phenyl]-3(2H)-pyridazinone. This 4-chloro-intermediate thus prepared was
treated with 2-propanol (20 mL, 261 mmol) and potassium t-butoxide (110 mg,
0.98
mmol) at refiux for 45 minutes furnished 2-benzyl-4-(2-propoxy)-5-[4-
(methylthio)pentyl]-3(2H)-pyridazinone This methyl sulfide was oxidized
according
to the method of Example 10 to provide the title compound (yield: 180 mg,
80%).
M.p. 109-111 C. 1 H NMR (300 MHz, CDC13) S 1.18 (d, J = 6 Hz, 6H), 3.12 (s,
3H),
5.36 (s, 2H), 5.49 (h, J = 6 Hz, 1 H), 7.35 (m, 3H), 7.47 (dd, J = 9 Hz, 3 Hz,
2H), 7.74
(d, J = 9 Hz, 2H), 7.79 (s, 1 H), 8.03 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 399
-80-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
(M+H)+. Anal. caic. for C21 H22N204S: C, 63.29; H, 5.56; N, 7.03. Found: C,
63.17; H, 5.57; N, 6.95.
Example 75
2-Benzyl-4-(4-fluoro h~y)-5-[4-(methylsulfonyl)QhenyIJ-3(2H)-oyridazinone
The title compound was prepared according to the method of Example 74
substituting 4-fluorophenol in place of 2-propanol (yield: 180 mg, 99%). M.p.
188-
190 C. 1 H NMR (300 MHz, CDCI3) S 3.12 (s, 3H), 5.26 (s, 2H), 6.86 (dd, J = 9
Hz,
6 Hz, 2H), 6.99 (t, J = 9 Hz, 2H), 7.34 (m, 3H), 7.46 (dd, J = 9 Hz, 3 Hz,
2H), 7.72 (d,
J = 9 Hz, 2H), 7.92 (s, 1 H), 8.02 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 451
(M+H)+.
Anal. caic. for C24H1 9FN204S: C, 63.98; H, 4.25; N, 6.21. Found: C, 63.74; H,
4.2;
N, 6.12.
Example 76
2-(2.2.2-Trifiuoroethyl)-4-(4-fluoroRh enoxy)-5-[4-(methylsulfonXl)ohenX,l-
3(2H)-
~,vridazinone
The title compound was prepared according to the method of Example 75
substituting 2-(2,2,2-trifluoroethyl)-4-chloro-5-[4-(methyfsulfonyl)phenyl]-
3(2H)-
pyridazinone in place of 2-benzyl-4-chloro-5-[4-(methyisulfonyl)phenyl]-3(2H)-
pyridazinone (yield: 180 mg, 63%). M.p. 161-164 C. 1 H NMR (300 MHz, CDCI3) S
3.09 (s, 3H), 4.81 (q, J = 9 Hz, 2H), 6.88 (dd, J = 9 Hz, 4.5 Hz, 2H), 7.0 (t,
J = 9 Hz,
2H), 7.78 (d, J= 9 Hz, 2H), 7.79 (s, 1 H), 8.06 (d, J = 9 Hz, 2H). MS (DCI-
NH3) m/z
443 (M+H)+. Anal. calc. for C1 gH14F4N204S: C, 51.58; H, 3.18; N, 6.33. Found:
C, 51.8; H, 3.3; N, 6.22.
Example 77
2-(2.2.2-Trifluoroethyl)-4-(4-chlorophenyl)-5-[4-(methylsulfinyl)nhenyll-3
(2H)-
Ryridazinone
2-Benzyl-4-chloro-5-methoxy-3(2H)-pyridazinone (J. Het. Chem., 1996, 33,
1579-1582) was converted to the 5-hydroxy-analog according to the method of
Example 7 and then to the 5-trifluoromethylsulfonyloxy-analog according to the
method of Example 8. Subsequent coupling to 4-(methylthio)phenylboronic acid,
according to the method of Example 9, provided 2-benzyl-4-chloro-5-[4-(methyl-
thio)phenyl]-3(2H)-pyridazinone. This intermediate was coupled with 4-
chlorophenylboronic acid according to the method of Example 6. This product
was
N-debenzylated according to the method of Example 11 and N-alkylated with 2-
-81-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
iodo-1,1,1-trifluoroethane according to the method of Example 20. The
resulting
sulfide was oxidized to the corresponding sulfoxide with one equivalent of
ineta-
chloroperoxybenzoic acid, according to the method of Example 5 to provide the
title
compound (yield: 130 mg, 70%). M.p. 154-155 C. 1 H NMR (300 MHz, CDCI3) S
2.74 (s, 3H), 4.88 (q, J = 9 Hz, 2H), 7.14 (d, J = 9 Hz, 2H), 7.26 (d, J = 9
Hz, 2H),
7.31 (d, J = 9 Hz, 2H), 7.61 (d, J = 9 Hz, 2H), 7.82 (s, 1 H). MS (DCI-NH3)
m/z 427
(M+H)+. Anal. calc. for C19H14CIF3N202S: C, 53.46; H, 3.3; N, 6.56. Found: C,
53.58; H, 3.34; N, 6.42.
Example 78
2-Benzyl-4-chloro-5-[4-(methvls~yl) enY1]-3 (2H)-pyridazinnnp
The title compound was prepared by oxidizing 2-benzy{-4-chloro-5-[4-
(methylthio)phenyl]-3(2H)-pyridazinone, (prepared as an intermediate in
Example
77) according to the method of Example 10 (yield: 180 mg, 83%). M.p. 166-167
C.
1 H NMR (300 MHz, CDCI3) 8 3.12 (s, 3H), 5.41 (s, 2H), 7.37 (m, 3H), 7.53 (dd,
J = 9
Hz, 3 Hz, 2H), 7.68 (d, J = 9 Hz, 2H), 7.74 (s, 1 H), 8.08 (d, J = 9 Hz, 2H).
MS (DCI-
NH3) m/z 375 (M+H)+. Anal. calc. for C18H15CIN203S: C, 57.67; H, 4.03; N,
7.47.
Found: C, 57.43; H, 4.06; N, 7.35.
Example 79
2-(2.2.2-Trifluoroethyl)-4-(4-methyiQhenyl)-5-[4-(methylsulfonyj)ohenyl]-3(2H)-
Ryridazinone
2-Benzyl-4-bromo-5-methoxy-3(2H)-pyridazinone (J. Het. Chem., 1996, 33,
1579-1582) was converted to the 5-hydroxy-analog according to the method of
Example 7 and then to the 5-(trifluoromethyl)sutfonyloxy-analog according to
the
method of Example 8. Subsequent coupling to 4-(methylthio)phenylboronic acid,
according to the method of Example 9, provided 2-benzyl-4-bromo-5-[4-(methyl-
thio)phenyl]-3(2H)-pyridazinone. This intermediate was coupled with 4-
methylphenylboronic acid according to the method of Example 6. This product
was
N-debenzylated according to the method of Example 11 and N-alkylated with 2-
iodo-1,1,1-trifluoroethane according to the method of Example 20. The
resulting
sulfide was oxidized to the title compound according to the method of Example
10
(yield: 210 mg, 98%). M.p. 154-156 C. 1 H NMR (300 MHz, CDCI3) S 2.33 (s,
3H),
3.07 (s, 3H), 4.89 (q, J = 9 Hz, 2H), 7.08 (s, 4H), 7.37 (d, J = 9 Hz, 2H),
7.88 (s, 1 H),
7.89 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 423 (M+H)+. Anal. calc. for
C20H17F3N203S: C, 56.86; H, 4.05; N, 6.63. Found: C, 56.59; H, 4.11; N, 6.53.
-82-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 80
2-(2 2 2-TrifluoroethY1)-4-(4-chloro-3-fluoronhenyl)-5-[4-(aminosulfonyl
henvl]-
3 (2 H)-uvridazinone
2-Benzyl-4-chloro-5-methoxy-3(2H)-pyridazinone (J. Het. Chem., 1996, 33,
1579-1582) was converted to the 5-hydroxy-analog according to the method of
Example 7 and then to the 5-(trifluoromethyl)sulfonyloxy-analog according to
the
method of Example 8. Subsequent coupling to 4-(methylthio)phenylboronic acid,
according to the method of Example 9, provided 2-benzyl-4-chloro-5-[4-(methyl-
thio)phenyl]-3(2H)-pyridazinone. This intermediate was coupled with 4-chloro-3-
fluorophenylboronic acid according to the method of Example 6. This product
was
N-debenzylated according to the method of Example 11 and N-alkylated with 2-
iodo-1,1,1-trifluoroethane according to the method of Example 20. The
resulting
sulfide was oxidized to the corresponding sulfoxide with one equivalent of
meta-
chloroperoxybenzoic acid to provide the methylsulfoxide which was converted to
the sulfonamide final product according to the method of Example 68 (yield:
500
mg, 75%). M.p. 214-215 C. 1 H NMR (300 MHz, CDCI3) S 4.82 (s, 2H), 4.88 (q, J
9 Hz, 2H), 6.88 (m, 1 H), 7.09 (dd, J = 9 Hz, 3 Hz, 1 H), 7.31 (d, J = 9 Hz, 1
H), 7.32 (d,
J = 9 Hz, 2H), 7.90 (s, 1 H), 7.92 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 462
(M+H)+.
Anal. calc. for C18H12F4CIN303S: C, 46.81; H, 2.61; N, 9.09. Found: C, 46.79;
H,
2.59; N, 8.86.
Example 81
2-(2.2.2-Trifluoroethyl)-4-(3.4-dichloroghenyl)-5-[4-(rrethylsulfonyl)]2henyll-
3(
pyridazinone
The product described in Example 65 was N-debenzylated according to the
method of Example 11. The intermediate was N-alkylated according to the method
of Example 20, substituting 2-iodo-1,1,1-trifluoroethane in place of 4-
fluorobenzyl
bromide to provide the title compound (yield: 165 mg, 55%). M.p. 197-198 C. 1
H
NMR (300 MHz, CDC13) S 3.09 (s, 3H), 4.88 (q, J = 9 Hz, 2H), 6.98 (dd, J = 9
Hz, 3
Hz, 1 H), 7.37 (d, J = 9 Hz, 4H), 7.91 (s, 1 H), 7.95 (d, J = 9 Hz, 2H). MS
(DCI-NH3)
m/z 477 (M+H)+. Anal. calc. for C19H13F3CI2N203S: C, 47.81; H, 2.74; N, 5.86.
Found: C, 47.94; H, 2.87; N, 5.83.
-83-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 82
2-Benzyi-4-(2-prQRylamino)-5-[4-(met ylsulfo yI)ohenY11-3(2H)-pyridazinone
2-Benzyl-4,5-dibromo-3(2H)-pyridazinone (2 g, 6 mmol) was reacted with
2-aminopropane (2 mL, 23.5 mmol) and potassium t-butoxide (910 mg, 6.6 mmol)
in toluene (40 mL) at reflux for 18 hours to provide the 4-(2-propylamino)-
derivative
after column chromatography (silica gel, 92:8 hexanes/ethyl acetate). The
intermediate was coupled in the 5-position with 4-(methylthio)phenylboronic
acid
according to the method of Example 6. The methyl sulfide was oxidized,
according
to the method of Example 10, to provide the title compound (yield: 120 mg,
48%).
M.p. 146-147 C. 1 H NMR (300 MHz, CDCI3) S 0.92 (d, J = 6 Hz, 6H), 3.11 (m, 1
H),
3.13 (s, 3H), 5.34 (s, 2H), 5.59 (m, 1 H), 7.33 (m, 3H), 7.42 (s, 1 H), 7.48
(dd, J = 9 Hz,
3 Hz, 2H), 7.56 (d, J = 9 Hz, 2H), 8.00 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z
399
(M+H)+. Anal. calc. for C21 H23N303S: C, 63.45; H, 5.83; N, 10.57. Found: C,
63.31; H, 5.87; N, 10.44.
Example 83
2-(2.2.2-TrifluoroethYl)-4-(2- roooxv)-5-(4-(methksulfonyl)Rhenvi1-3(2)_
ovridazinone
83A. 2-(2.2.2-TrifluoroethylZ-4.5-dibromo-3(2 H)-pyridazinone
A solution of mucobromic acid (10 g, 38.8 mmol) and trifluoroethyl hydrazine
(70% in water, 4.88 mL, 38.8 mmol) in 100 mL of methanol was prepared and
heated at reflux for 3 hours. The reaction mixture was concentrated in vacuo
and
partitioned between ethyl acetate and water. The ethyl acetate layer was dried
over MgSO4, filtered, passed through a silica gel pad, and concentrated in
vacuo.
The product was obtained as yellowish solid (yield: 8.8 g, 68%) . 1 H NMR (300
MHz, CDCI3) 8 4.78 (q, J = 9 Hz, 2H), 7.87 (s, 1 H). MS (DCI-NH3) m/z 337
(M+H)+.
83B. 2-(2.2.2-TrifluoroethYl)-4-(2-~r poxy)-5-bromo-3(2H)-Ryridazinone
A solution of 2-(2,2,2-trifluoroethyl)-4,5-dibromo-3(2H)-pyridazinone (2 g, 6
mmol), isopropyl alcohol (3 mL) and sodium hydride (60% dispersed in oil, 290
mg,
7.2 mmol) in toluene (40 mL) was heated at reflux for 5 hours. The reaction
mixture
was partitioned between ethyl acetate and water. The ethyl acetate layer was
filtered, and concentrated in vacuo. The residue was purified by
chromatography
(95:5 hexanes/ethyl acetate) to provide the product as a greenish oil (yield:
1.22 g,
-84-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
65%). 1 H NMR (300 MHz, CDCI3) 61.46 (d, J = 7.5 Hz, 6H), 5.48 (h, J = 6 Hz, 1
H),
7.87 (s, 1 H). MS (DCI-NH3) m/z 316 (M+H)+.
83C. 2-(2.2.2-Trifluoroethyl)-4- -oro oxy)-5-f4-(methylthiolphenyll-3j2H1-
p,vridazinone
A solution of 2-(2,2,2-trifluoroethyl)-4-(2-propoxy)-5-bromo-3(2H)-
pyridazinone (1.2 g, 3.8 mmol), 4-(methylthio)phenylboronic acid (704 mg, 4.19
mmol), tetrakis(triphenylphosphine)palladium(0) (220 mg, 5% mmol) and cesium
carbonate (2.72 g, 8.3 mmol) in 20 mL of ethylene glycol dimethyl ether was
heated
to reflux for 5 hours. The mixture was partitioned between ethyl acetate and
water.
The ethyl acetate layer was washed with water, brine, dried over MgSO4 and
concentrated in vacuo. The residue was purified by chromatography on silica
gel
(94:6 hexanes/ethyl acetate). The product was obtained as a greenish solid
(yield:
1.1 g, 81 %). 1 H NMR (300 MHz, CDCI3) S 1.19 (d, J= 7.5 Hz, 6H), 2.55 (s,
3H),
4.83 (q, J = 9 Hz, 2H), 5.28 (h, J = 6 Hz, 1 H), 7.32 (d, J = 9 Hz, 2H), 7.52
(d, J = 9 Hz,
2H), 7.85 (s, 1 H). MS (DCI) m/z 359 (M+H)+.
83D. 2-(2.2.2-Trifluoroethyl)-4-(2-~roRoxy)5-[4-(methylsulfonY1)ohenvl]-3(2H)-
R,vridazinone
The title compound was prepared according to the method of Example 10,
substituting 2-(2,2,2-trifiuoroethyl)-4-(2-propoxy)-5-[4-(methylthio)phenyl]-
3(2H)-
pyridazinone in place of 4-(4-fluorophenyl)-5-[4-(methytthio)phenyl]-3(2H)-
pyridazinone (yield: 220 mg, 100%). M.p. 152-153 C. 1 H NMR (300 MHz, CDCI3)
S 1.2 (d, J 6 Hz, 6H), 3.13 (s, 3H), 4.84 (q, J = 9 Hz, 2H), 5.49 (p, J = 6
Hz, 1 H),
7.78 (d, J 9 Hz, 2H), 7.82 (s, 1 H), 8.05 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z
391
(M+H)+. Anal. caic. for C16H17F3N204S: C, 49.22; H, 4.38; N, 7.17. Found: C,
49.34; H, 4.25; N, 7.01.
Example 84
2-(2.2.2-Trifluoroethti -4-cyclohexyloxy-5-j4-(meth Isy ulfonyl)ohenyl]-3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 83,
substituting cyclohexanol in place of 2-propanol (yield: 250 mg, 52%). M.p.
129-
130 C. 1 H NMR (300 MHz, CDC13) S 1.1-1.6 (m, 8H), 1.84 (m, 2H), 3.12 (s,
3H),
4.83 (q, J = 9 Hz, 2H), 5.21 (h, J = 4.5 Hz, 1 H), 7.77 (s, 1 H), 7.80 (d, J =
9 Hz, 2H),
-85-
CA 02578858 2007-03-05
WO 99/10331 PCT/C]S98/16479
8.06 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 431 (M+H)+. Anal. caic. for
C1 9H21 F3N204S: C, 53.01; H, 4.91; N, 6.50. Found: C, 52.96; H, 4.84; N,
6.45.
Example 85
2-(2.2.2-Trifluoroethyl)-4-cyclooentyloxy-5-j4-(methylsulfonyl)phenyjl 3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 83,
substituting cyclopentanol in place of 2-propanol (yield: 250 mg, 52%). M.p.
148-
150 C. 1 H NMR (300 MHz, CDC13) S 1.35-1.55 (m, 4H), 1.68-1.75 (m, 4H), 3.12
(s,
3H), 4.83 (q, J = 9 Hz, 2H), 5.89 (h, J = 4.5 Hz, 1 H), 7.75 (d, J = 9 Hz,
2H), 7.83 (s,
1 H), 8.04 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 417 (M+H)+. Anal. calc. for
C18H1 gF3N204S: C, 51.91; H, 4.59; N, 6.72. Found: C, 52.04; H, 4.50; N, 6.65.
Example 86
2-(2.2.2-Trifluoroethyl)-4-(2-RroRy mino)-5-[4-(methvl ,Ifon MI)Phe
86A. 2-(2.2.2-Trifluoroethvl)-4-(2 -proRylamino)-5-bromo-3(2H)-Ryridazinone
The title compound was prepared according method of the Example 83B,
substituting 2-propylamine in place of 2-propanol (yield: 70%). MS (DCI-NH3)
m/z
315 (M+H)+.
86B. 212.2.2-Trifluoroethyl)-4-(2;prQpylamino)-5-[4-(methylthio)phgIlyij;3(2H)-
Qyridazinone
The title compound was prepared according method of the Example 83C,
substituting 2-(2,2,2-trifluoroethyl)-4-(2-propylamino)-5-bromo-3(2H)-
pyridazinone
in place of 2-(2,2,2-trifluoroethyl)-4-isopropoxy-5-bromo-3(2H)-pyridazinone
(yield:
80%). MS (DCI-NH3) m/z 358 (M+H)+.
86C. 2-(2.2.2-Trifluoroethvl)-4-(2-RroRylamino)-5-[4-(methy s, nvl~ henyll-
3(2H1-
pyridazinone
The title compound was prepared according to the method of Example 10 ,
substituting 2-(2,2,2-Trifluoroethyl)-4-(2-propylamino)-5-[4-
(methylthio)phenyl]-
3(2H)-pyridazinone in place of 4-(4-fluorophenyl)-5-[4-(methylthio)phenyl]-
3(2H)-
pyridazinone (yield: 180 mg, 83%). M.p. 173-174 C. 1 H NMR (300 MHz, CDC13) b
0.95 (d, J = 6 Hz, 6H), 3.13 (s, 3H), 4.81 (q, J = 9 Hz, 2H), 5.97 (s, 1 H),
7.45 (s, 1 H),
-86-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
7.59 (d, J = 9 Hz, 2H), 8.03 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 340 (M+H)+.
Anal.
caic. for C16H18F3N304S: C, 49.35; H, 4.65; N, 10.79. Found: C, 49.29; H,
4.52;
N, 10.65.
Example 87
2- nzyl-4-(4-mor h{olino)-5-[4-(methylsulfonyl){Zhenyl]-3(2H)-Ryridazinone
2-Benzyl-4,5-dichloro-3(2H)-pyridazinone, prepared following the procedure
in Example 2, was reacted with morpholine following the procedure of Example
86
to provide the 4-morpholino-derivative. The morpholino intermediate was
coupled
at the 5-position with 4-(methylthio)phenylboronic acid according to the
method of
Example 6. The resutting methyl sulfide was oxidized to the title compound
according to the method of Example 10 (yield: 150 mg, 69%). M.p. 158-160 C. 1
H
NMR (300 MHz, CDC13) S 3.06 (t, J = 4.5 Hz, 3H), 3.12 (s, 3H), 3.69 (t, J =
4.5 Hz,
3H), 5.33 (s, 2H), 7.35 (m, 3H), 7.5 (m, 4H), 7.58 (s, 1 H), 8.05 (d, J = 9
Hz, 2H). MS
(DCI-NH3) m/z 426 (M+H)+. Anal. calc. for C22H23N304S: C, 62.10; H, 5.44; N,
9.87. Found: C, 61.74; H, 5.47; N, 9.59.
Example 88
2-(2.3.3-Trifluoro-2-~ro e~n=1-yl)]-4-(4-fluoro henyl)-5-[4-(methylsulfonyl)
heny 1-
3 j2Hj-12yridazinone
88A. 1-Methylsulfonyloxy-2.3.3-trifluoro-2-prooene
2,3,3-Trifluoro-2-propen-l-ol was prepared as reported in J.
Org.Chem.,1989, 54, 5640-5642. The mesylate was obtained by reacting 2,3,3-
trifluoro-2-propen-l-ol with mesyl chloride in diethyl ether. Standard workup
provided the product, which was used without purification.
88B. 2-(2.3.3-Trifluoro-2-~ro e~ n=1-yl)-4-(4-fluoro henyl)-544-Imethylthio)
ph
3(2H)-Ryridazinone.
4-(4-Fluorophenyl)-5-14-(methvlthio)phenvll-3(2H)-pyridazinone is prepared
starting with the 2-benzyl-pyridazinone from Example 9 and debenzylating the
compound according to the procedure of Example 11.
A mixture of 4-(4-fluorophenyl)-5-[4-(methylthio)phgnyll-3(2H)-pyridazinone
(250 mg, 0.8 mmol), Cs2CO3 (650 mg, 2 mmol), and 3-methyisufonyloxy-1,1,2-
trifluoropropene (mesylate, 250 mg, 1.19 mmol) in ethyl acetate (30 mL) was
stirred
at 55 C for 1.5 hours. The mixture was partitioned between ethyl acetate and
water. The organic layer was washed with brine, dried with MgSO4 and filtered.
The filtrate was concentrated in vacuo. The residue was purified by column
-87-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
chromatography on silica gel eluted with 15% ethyl acetate/hexanes, to provide
the
methyl sulfide, 2-(2,3,3-trifluoro-2-propen-1-yl)-4-(4-fluoropheny!)-5-[4-
(methylthio)phenyl]-3(2H)-pyridazinone as a greenish oil (yield: 175 mg, 53%).
88C. 2-(2.3.3-Trifluoro-2-prooen-1-yl)]-4-(4-fluoroohenyJ)-5 j4-(meth ly
sulfonxJ)-
12h e nX(1:3 (2 H)-12vridazinone
The methyl sulfide, prepared above, was oxidized to the title compound
according to the method of Example 10 (yield: 125 mg, 68%). M.p. 154-156 C. 1
H
NMR (300 MHz, CDCI3) 8 3.07 (s, 3H), 5.1 (ddd, J = 21 Hz, 3 Hz, 1.5 Hz, 2H),
6.98
(t, J = 9 Hz, 2H), 7.19 (dd, J = 9 Hz, 6 Hz, 2H), 7.35 (d, J = 9 Hz, 2H), 7.89
(s, 1 H),
7.9 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 439 (M+H)+. Anal. calc. for
C20H14F4N203S: C, 54.79; H, 3.21; N, 6.38. Found: C, 54.52; H, 3.15; N, 6.21.
Example 89
24-Bis(4-fluoroohenvl)-5-(4- (aminosulfonyl)ohenylL-3(2 H)-Ryridazi nnnp
The title compound was prepared according to the method of Example 68
substituting 2,4-bis(4-fluorophenyl)-5-[4-(methylsulfinyI)phenyl)-3(2H)-
pyridazinone
in place of 2-(2,2,2-trifluoroethyl)-4-(4-fluorophenyl)-5-[4-
(methylsulfinyl)phenyl]-
3(2H)-pyridazinone (yield: 118 mg, 43%). M.p. 213-216 C. 1 H NMR (300 MHz,
DMSO-d6) S 7.15 (t, 2H), 7.27 (m, 2H), 7.4 (m, 6H), 7.7 (dd, 2H), 7.76 (d, J =
9 Hz,
2H), 8.2 (s, 1 H). MS (DCI-NH3) m/z 440 (M+H)+, 439.44 (M+NH4)+. Anal. caic.
for
C21 H15FN203S2: C, 60.13; H, 3.44; N, 9.56. Found: C, 59.94; H, 3.37; N, 9.46.
Example 90
2-(2.2.2-Triftuoroethvl)-4-cv~.Inp roRylmethoxy- 5-[4- (methylsulfony,j)
ip~nyll-3 2 H2 :
Ryridazinone
90A. 2-(2.2.2-Triffuoroethvl)-4-methoxy-5-bromo-3(2H)-Pyridazinone
The title compound was prepared according method of the Example 83B,
substituting methanol in place of isopropanol (yield: 78%). 1 H NMR (300 MHz,
CDCI3) S 4.3 (s, 3H), 4.76 (q, J = 9 Hz, 2H), 7.85 (s, 1 H). MS (DCI-NH3) m/z
288
(M+H)+.
90B. 2-(2.2.2-Trifluoroethy,I)-4-methoxy-544-(methylthio)phenXll-3 (2H)-
12yridazinone
The title compound was prepared according method of the Example 83C,
substituting 2-(2,2,2-trifluoroethyl)-4-methoxy-5-bromo-3(2H)-pyridazinone in
place
-88-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
of 2-(2,2,2-trifluoroethyl)-4-(2-propoxy)-5-bromo-3(2H)-pyridazinone (yield:
80%).
1 H NMR (300 MHz, CDCI3) S 2.54 (s, 3H), 4.11 (s, 3H), 4.82 (q, J = 9 Hz, 2H),
7.33
(d, J = 9 Hz, 2H), 7.48 (d, J = 9 Hz, 2H), 7.84 (s, 1 H). MS (DCI-N.H3) m/z
331
(M+H)+.
90C. 2-(2.2.2-Trifluoroethyl)-4-hydroxy-5-f4-(meth I~thio)nhenyll-3(2H)z
pyridazinone
A solution of 2-(2,2,2-Trifluoroethyl)-4-methoxy-5-[4-(methylthio)phenyl]-
3(2 H)-pyridazi none (2 g, 6.1 mmol) and hydrobromic acid (40% in water, 20
mL) in
acetic acid (40 mL) was heated at reflux for 3 hours. The reaction mixture was
cooled to room temperature and water (50 mL) was added. The crystals formed
were filtered, washed with water and 5% ethyl acetate in hexanes, and dried to
constant weight. The product was obtained as a white solid (yield: 1.75 g, 91
%).
1 H NMR (300 MHz, CDCI3) 6 2.54 (s, 3H), 4.82 (q, J- 9 Hz, 2H), 7.47 (d, J- 9
Hz,
2H), 7.65 (d, J = 9 Hz, 2H), 7.73 (br s, 1 H), 8.00 (s, 1 H). MS (DCI) m/z 317
(M+H)+.
90D. 2-(2.2.2-Trifluoroethyl)-4-cyclo r~Rylmethoxy-5-f4-(methylthio) h~ylJ-
3(2H)-
Ryridazinone
A solution of 2-(2,2,2-trifiuoroethyl)-4-hydroxy-5-[4-(methyfthio)phenyl]-
3(2H)-pyridazinone (150 mg, 0.47 mmol), cyclopropyl methanol (43 mL, 0.52
mmol)
and triphenylphosphine (124 mg, 0.47 mmol) in freshly distilled THF was
prepared
and added dropwise to diethyl azodicarboxylate (75 mL, 0.52 mmol) at 0 C. The
mixture was allowed to warm to room temperature, stirred for 5 hours and
concentrated in vacuo. The residue was purified by chromatography on silica
gel
(95:5 hexanes/ethyl acetate) to provide the product as a colorless oil (yield:
140
mg, 81 %). 1H NMR (300 MHz, C DC13) S 0.22 (m, 2H), 0.48 (m, 2H), 1.6 (m, 1H),
2.53 (s, 3H), 4.26 (d, J = 7.5 Hz, 2H), 4.72 (q, J = 9 Hz, 2H), 7.32 (d, J- 9
Hz, 2H),
7.55 (d, J = 9 Hz, 2H), 7.87 (s, 1 H). MS (DCI-NH3) m/z 371 (M+H)+.
90E. 2-(2.2.2-TrifluoroethvIl-4-cyl~Q prQRy th xy-5 f4-(meth Iy
sulfonyllohenyll
3(2H)-Rvridazinone
The title compound was prepared according to the method of example 10,
substituting 2-(2,2,2-trifluoroethyl)-4-cyclopropylmethoxy-5-[4-
(methylthio)phenyl]-
3(2H)-pyridazinone in place of 4-(4-fluorophenyl)-5-[4-(methylthio)phenyl]-
3(2H)-
pyridazinone (yield: 130 mg, 85%). M.p. 133-135 C. 1 H NMR (300 MHz, CDCI3) 8
0.22 (m, 2H), 0.5 (m, 2H), 1.07 (m, 1 H), 3.12 (s, 3H), 4.4 (d, J = 9 Hz, 2H),
4.83 (q, J
-89-
CA 02578858 2007-03-05
WO 99/10331 PC1'/US98/16479
= 9 Hz, 2H), 7.79 (s, 1 H), 7.83 (d, J = 9 Hz, 2H), 8.07 (d, J = 9 Hz, 2H). MS
(DCI-
NH3) m/z 403 (M+H)+. Anal. caic. for C17H17F3N204S: C, 50.74; H, 4.25; N,
6.96.
Found: C, 50.56; H, 4.09; N, 6.88.
Example 91
2-(2.2.2-TrifluoroethY1)-4-(3-Rropen-1-oxy)-5-[4-(meth Is~ ulfonyl)nhenyl)-
3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 90,
substituting 2-propen-l-ol in place of cyclopropylmethanol (yield: 120 mg,
77%).
M.p. 121-123 C. 1 H NMR (300 MHz, CDCI3) S 3.12 (s, 3H), 4.84 (q, J = 12 Hz,
2H), 5.07 (d, J = 6 Hz, 2H), 5.21 (dd, J = 13.5 Hz, 1 Hz, 1 H), 5.27 (dd, J =
15 Hz, 1
Hz, 1 H), 5.85 (m, 1 H), 7.25 (d, J = 9 Hz, 2H), 7.83 (s, 1 H), 8.06 (d, J = 9
Hz, 2H). MS
(DCI-NH3) m/z 389 (M+H)+. Anal. caic. for C16H15F3N204S: C, 49.48; H, 3.89; N,
7.21. Found: C, 49.24; H, 3.77; N, 7.16.
Example 92
212.2.2-Trifluoroethyl)-4-(4-fluoro-a/pha-methylbenzvlo y)-5-[4-
(methylsulfnnvl)-
.}Zhenyl]-3(2H)-pyridazinone
The title compound was prepared according to the method of Example 90,
substituting 4-fluoro-a/pha-methylbenzyl alcohol in place of
cyclopropylmethanol
(yield: 155 mg, 76%). M.p. 133-135 C. 1 H NMR (300 MHz, CDCI3) 8 1.57 (d, J 6
Hz, 3H), 3.13 (s, 3H), 4.75 (q, J = 7.5 Hz, 1 H), 4.87 (q, J = 7.5 Hz, 1 H),
6.34 (q, J 6
Hz, 1 H), 6.83 (t, J = 9 Hz, 2H), 6.98 (dd, J = 9 Hz, 6 Hz, 2H), 7.59 (d, J =
9 Hz), 7.70
(s, 1 H), 8.03 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 471 (M+H)+. Anal. caic. for
C21 H1 8F4N204S: C, 53.61; H, 3.85; N, 5.95. Found: C, 53.54; H, 3.73; N,
5.86.
Example 93
2-[4-(Methvlthio)ohenvll-4-(4-fluoronhenyl)-5-[4-(methvlau y,j)pheny~l-3(Z,N-
Ryridazinone
A solution of the product from Example 11, 4-(4-Fluorophenyl)-5-[4-(methyl-
sulfonyl)phenyl]-3(2H)-pyridazinone (344 mg, 1.0 mmol), 4-bromothioanisole
(812
mg, 4.0 mmol), and copper (70 mg, 1.1 mmol) in 20 mL of pyridine was stirred
at
reflux under a nitrogen atmosphere for 18 hours. After cooling to room
temperature, the reaction mixture was diluted with a mixture of water and
ethyl
acetate. The two layers were filtered through Celite , and separated. The
organic
layer was washed with 10% aqueous citric acid, with brine, dried over MgSO4,
and
-90-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
filtered. The filtrate was concentrated in vacuo and the residue purified by
column
chromatography (silica gel, 93:7 dichloromethane/ethyl acetate) to provide the
title
compound as a foam (yield: 380 mg, 81.5%). 1 H NMR (300 MHz, CDCI3) 8 2.55 (s,
3H), 3.05 (s, 3H), 6.98 (t, J = 9 Hz, 2H), 7.22 (dd, J = 9 Hz, 6 Hz, 2H), 7.38
(dd, J = 8
Hz, 2 Hz, 4H), 7.64 (d, J = 9 Hz, 2H), 7.91 (d, J = 9 Hz, 2H), 7.98 (s, 1 H).
MS (DCI-
NH3) m/z 467 (M+H)+. Anal. calc. for C24H19FN203S2-0.5 H20: C, 60.63; H,
4.21; N, 5.90. Found: C, 60.72; H, 3.96; N, 5.70.
Example 94
2.5-BiBj4-(me bylsulfonyl)phepyl]-4-(4- IuoroQhenyI)-3(2H)-oyridazinone
The title compound was prepared by oxidizing the product of Example 93,
according to the method of Example 10 (yield: 156 mg, 78%). 1 H NMR (300 MHz,
CDCI3) S 3.10 (s, 3H), 3.12 (s, 3H), 7.02 (m, 2H), 7.24 (m, 2H), 7.42 (br d, J
= 9 Hz,
2H), 7.94 (dd, J = 9 Hz, 2 Hz, 2H), 8.02 (dd, J = 9 Hz, 2 Hz, 2H), 8.10 (m,
3H). MS
(DCI-NH3) m/z 499 (M+H)+, 516 (M+NH4)+. Anal. calc. for C24H19FN205S2-0.5
H20: C, 56.80; H, 3.94; N, 5.53. Found: C, 56.50; H, 3.88; N, 5.38.
Example 95
2-(3-Methyl-2-thienyl)-4-(4-fluorophenyl)-5-(4-(methvlsulfonvl) henyl]-3(2H)-
pyridazinone
The title compound was prepared according to Example 93, substituting 2-
bromo-3-methylthiophene in place of 4-bromothioanisole (yield: 190 mg, 43%).
M.p. 215-217 C. 1 H NMR (300 MHz, CDCI3) S 2.21 (s, 3H), 3.08 (s, 3H), 6.90
(d, J
= 9 Hz, 1 H), 6.98 (t, J = 9 Hz, 2H), 7.24 (dd, J = 9 Hz, 6 Hz, 3H), 7.41 (d,
J = 9 Hz,
2H), 7.94 (d, J = 9 Hz, 2H), 7.98 (s, 1 H). MS (DCI-NH3) m/z 441 (M+H)+, 458
(M+NH4)+. Anal. calc. for C22H17FN2O3S2-0.5 H20: C, 58.80; H, 4.01; N, 6. 24.
Found: C, 58.85; H, 3.78; N, 5.99.
Example 96
2-(2-Trifluorometh I-5-ni r Rhenyl)-4-(4-fluoro i nyl)-5-[4-
(methvls_ulfonyl)nhenv[L-
3(2H)-Ryridazinone
The title compound was prepared according to Example 93, substituting 2-
bromo-5-nitrobenzotrifluoride in place of 4-bromothioanisole (yield: 390 mg,
73%).
1 H NMR (300 MHz, CDCI3) 8 3.08 (s, 3H), 6.98 (t, J = 9 Hz, 2H), 7.21 (dd, J =
9 Hz,
6 Hz, 2H), 7.43 (d, J = 9 Hz, 2H), 7.80 (d, J = 9 Hz, 1 H), 7.96 (d, J = 9 Hz,
2H), 8.02
(s, 1 H), 8.61 (dd, J = 9 Hz, 3 Hz, 1 H), 8.75 (d, J = 3 Hz, 1 H). MS (DCI-
NH3) m/z 534
-91-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
(M+H)+, 551 (M+NH4)+. Anal. calc. for C24H15F4N305S-0.75 H20: C, 52.70; H,
3.02; N, 7.69. Found: C, 52.42; H, 3.04; N, 6.82.
Example 97
? (3-(Methvlthio)Pnyl]-4-(4-fluoronhenyl)-5-j4-(methylsulfonvl)nhenyl]-3(2Hl-
pyridazinone
The title compound was prepared according to Example 93, substituting 3-
bromothioanisole in place of 4-bromothioanisole (yield: 355 mg, 76%). M.p. 196
C. 1 H NMR (300 MHz, CDC13) 5 2.55 (s, 3H), 3.08 (s, 3H), 6.99 (t, J = 9 Hz,
2H),
7.23 (dd, J = 9 Hz, 6 Hz, 2H), 7.28-7.33 (m, 1 H), 7.37-7.49 (m, 2H), 7.40 (d,
J = 9 Hz,
2H), 7.58 (m, 1 H), 7.92 (d, J = 9 Hz, 2H), 7.99 (m, 1 H). MS (DCI-NH3) m/z
467
(M+H)+, 484 (M+NH4)+. Anal. calc. for C24H19FN203S2: C, 61.80; H, 4.08; N,
6.01. Found: C, 61.56; H, 3.93; N, 5.86.
Example 98
2-[3-(Methvlsulfonyl)r~henvl]-4-(4-fluoroohenyl)-5-[4-(methyisulfonvl)ohenyl]-
3 (2 H)-
l2yridazinone
The title compound was prepared by oxidizing the product of Example 97,
according to the method of Example 10 (yield: 98 mg, 65.6%). M.p. 141-142 C.
1 H NMR (300 MHz, DMSO-d6) 8 3.25 (s, 3H), 3.35 (s, 3H), 7.18 (t, J = 9 Hz,
2H),
7.32 (dd, J = 9 Hz, 6 Hz, 2H), 7.52 (d, J = 9 Hz, 2H), 7.83 (t, J = 9 Hz, 1
H), 7.95 (d, J
= 9 Hz, 2H), 8.05 (m, 1 H), 8.25 (t, J = 1.5 Hz, 1 H), 8.33 (s, 1 H). MS (DCI-
NH3) m/z
516 (M+NH4)+. Anal. calc. for C24H1 gFN2O5S2-H2O: C, 55.81; H, 4.07; N, 5.43.
Found: C, 56.24; H, 4.29; N, 5.10.
Example 99
2- (4-Fluoro heny I)-4- (4-chforoj2henyl)-5-[4-(methylsulfonyl)nhenyj]-3 (2
H)~
R,yridazinone
4-(4-Chlorophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone is
prepared starting with the 2-benzyfpyridazinone from Example 53 and
debenzylating the compound according to the method of Example 11.
The title compound was prepared according to the method of Example 93,
starting with 4-(4-chlorophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone in
plaae of 4-(4-fluorophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone
and
substituting 1-fluoro-4-iodobenzene in place of 4-bromothioanisole (yield: 245
mg,
54%). M.p. 195-197 C. 1 H NMR (300 MHz, CDCI3) 8 3.08 (s, 3H), 7.19 (m, 4H),
-92-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
7.25 (m, 2H), 7.41 (d, J = 9 Hz, 2H), 7.70 (m, 2H), 7.95 (d, J = 9 Hz, 2H),
8.01 (s,
1 H). MS (DCI-NH3) m/z 455 (M+H)+, 472 (M+NH4)+. Anal. calc. for
C23H16CIFN203S: C, 60.78; H, 3.52; N, 6.17. Found: C, 60.81; H, 3.53; N, 5.93.
Example 100
2-(5-Chloro-2-thienyl)-4-(4-chloro henyl)-5-[4-(meth Isy ulfonyl)ohenylJ-3(2H)-
pyridazinone
The title compound was prepared according to Example 93, substituting 4-
(4-chlorophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone in place of 4-
(4-
fluorophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone and substituting
2-
bromo-5-chlorothiophene in place of 4-bromothioanisole (yield: 150 mg, 45%).
M.p. 249-251 C. 1 H NMR (300 MHz, CDCI3) S 3.05 (s, 3H), 6.92 (d, J = 9 Hz, 1
H),
7.18 (d, J = 9 Hz, 2H), 7.31 (d, J = 9 Hz, 2H), 7.39 (d, J = 9 Hz, 2H), 7.58
(d, J = 6 Hz,
1 H), 7.94 (d, J = 9 Hz, 2 Hz, 2H), 8.04 (s, 1 H). MS (DCI-NH3) m/z 477
(M+H)+, 494
(M+NH4)+. Anal. calc. for C21 H14C12N2O3S2-H2O: C, 50.9; H, 3.03; N, 5.60.
Found: C, 50.5; H, 2.79; N, 5.26.
Example 101
2-(3-Trifluoromethyl2henyl)-4-(4-chloroRhenyl)-5-[4-(methylsulfoOYl)ohenyll-
3(2H)-
pyridazinone
The title compound was prepared according to Example 93, starting with 4-
(4-chlorophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone in place of 4-
(4-
fluorophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone and substituting
3-
iodobenzotrifluoride in place of 4-bromothioanisole (yield: 210 mg, 59.5%).
M.p.
103-105 C. 1 H NMR (300 MHz, CDC13) S 3.08 (s, 3H), 7.18 (d, J = 9 Hz, 2H),
7.28
(d, J = 9 Hz, 2H), 7.41 (d, J = 9 Hz, 2H), 7.65 (m, 2H), 7.95 (m, 3H), 8.04
(m, 2H).
MS (DCI-NH3) m/z 505 (M+H)+, 525 (M+NH4)+. Anal. calc. for
C24H16CIF3N203S: C, 57.14; H, 3.17; N, 5.56. Found: C, 56.61; H, 3.28; N,
5.38.
Example 102
2-13-Chloro-4-fluorophgnylL(4-chloronhenyl)-5-f4-(methylsulforlyl)ohenyll-
3(2H)-
Rvridazinone
The title compound was prepared according to Example 93, starting with 4-
(4-chlorophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone (described in
Example 99) in place of 4-(4-fluorophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone and substituting 1-bromo-3-chloro-4-fluorobenzene in place of 4-
-93-
CA 02578858 2007-03-05
WO 99/10331 PCT/11S98/16479
bromothioanisole (yield: 330 mg, 58.8%). M.p. 205 C. 1 H NMR (300 MHz, CDC13)
S 3.10 (s, 3H), 7.17 (d, J = 9 Hz, 2H), 7.23-7.31 (m, 1 H), 7.28 (d, J = 9 Hz,
2H), 7.41
(d, J = 9 Hz, 2H), 7.65 (ddd, J = 9 Hz, 3 Hz, 1.5 Hz, 1 H), 7.85 (dd, J = 9
Hz, 3 Hz,
1 H), 7.93 (d, J = 9 Hz, 2H), 8.01 (s, 1 H). MS (DCI-NH3) m/z 489 (M+H)+, 508
(M+NH4)+. Anal. calc. for C23H15CI2N203S: C, 56.44; H, 3.17; N, 5.73. Found:
C, 56.37; H, 3.19; N, 5.64.
Example 103
2-(3-F luorophelyj)-4-(4-fiuorophenyl)_5-[4-(methylsulfonyl)ohenyjl-3(2H)-
pyridazinone
The title compound was prepared according to Example 93, substituting 1-
fluoro-3-iodobenzene in place of 4-bromothioanisole (yield: 310 mg, 70.8%).
M.p.
245-247 C. 1 H NMR (300 MHz, CDC13) 5 3.08 (s, 3H), 6.98 (t, J = 9 Hz, 2H),
7.14
(m, 1 H), 7.24 (dd, J = 9 Hz, 6 Hz, 2H), 7.40 (m, 2H), 7.52 (m, 3H), 7.92 (d,
J = 9 Hz,
2H), 8.01 (s, 1 H). MS (DCI-NH3) m/z 439 (M+H)+, 456 (M+NH4)+. Anal. calc. for
C23H16F2N203S-0.25 H20: C, 62.34; H, 3.67; N, 6.38. Found: C, 62.33; H, 3.68;
N, 6.22.
Example 104
2-[2-(Methylthio)henyl]-4-(4-fluoroRhenvl)-5-[4- ethylsulfonyl)phenY11 3(2H,
Ryridazinone
The title compound was prepared according to Example 93, substituting 2-
bromothioanisole in place of 4-bromothioanisole (yield: 280 mg, 60%). M.p. 206-
208 C. 1 H NMR (300 MHz, CDCI3) 8 2.49 (s, 3H), 3.08 (s, 3H), 6.95 (t, J = 9
Hz,
2H), 7.25 (dd, J = 9 Hz, 6 Hz, 2H), 7.29-7.51 (m, 4H), 7.43 (d, J = 9 Hz, 2H),
7.92 (d,
J = 9 Hz, 3H), 8.01 (s, 1 H), 7.98 (s, 1 H). MS (DCI-NH3) m/z 467 (M+H)+, 484
(M+NH4)+. Anal. calc. for C24H1 9FN203S2=H2O: C, 59.50; H, 4.13; N, 5.79.
Found: C, 59.62; H, 4.15; N, 5.52.
Example 105
2-(5-Nitro-2-thien~)-4-(4-fluorop henyl) -5-[4-(methvlsulfonyl)2he11y11-3L2H~
Ryridazinone
The title compound was prepared according to Example 93, substituting 2-
bromo-5-nitrothiophene in place of 4-bromothioanisole (yield: 330 mg, 70%).
M.p.
252-253 C. 1 H NMR (300 MHz, CDC13) 5 3.06 (s, 3H), 7.05 (t, J = 9 Hz, 2H),
7.25
(dd, J = 9 Hz, 6 Hz, 2H), 7.40 (d, J = 9 Hz, 2H), 7.71 (d, J = 6 Hz, 1 H),
7.95 (m, 3H),
-94-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
8.14 (s, 1 H). MS (DCI-NH3) m/z 472 (M+H)+, 489 (M+NH4)+. Anal. calc. for
C21 H14FN305S2=0.5 H20: C, 52.50; H, 3.02; N, 8.75. Found: C, 52.79; H, 3.18;
N,
8.74.
Example 106
2-(3.4-Difluoroahenyl)-4-(4-chloroohenyl)-5 j4-(methyJsulfonyl)phenyll-3(2H)-
p-yridazinone
The title compound was prepared according to Example 93, starting with 4-
(4-chlorophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone in place of 4-
(4-
fluorophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone and substituting
1-
bromo-3,4-difluorobenzene in place of 4-bromothioanisole (yield: 310 mg,
65.7%).
M.p. 187-188 C. 1 H NMR (300 MHz, CDCI3) S 3.09 (s, 3H), 7.18 (d, J = 9 Hz,
2H),
7.29 (m, 3H), 7.41 (d, J = 9 Hz, 2H), 7.52 (m, 1 H), 7.65 (m, 1 H), 7.92 (d, J
= 9 Hz,
2H), 8.01 (s, 1 H). MS (DCI-NH3) m/z 473 (M+H)+, 490 (M+NH4)+. Anal. calc. for
C23H15CIF2N203S-0.5 H20: C, 57.38; H, 3.33; N, 5.82. Found: C, 57.44; H, 3.38;
N, 5.52.
Example 107
2-(3-Benzothienyl)-4-(4-fluoroRhenyl)-5-[4-(methylsulfonyl)phenyll-3(2H,)-
pyridazinone
The title compound was prepared according to Example 93, substituting
3-bromobenzothiophene in place of 4-bromothioanisole (yield: 185 mg, 41 %).
M.p.
265-267 C. 1 H NMR (300 MHz, CDCI3) 6 3.09 (s, 3H), 7.0 (t, J = 9 Hz, 2H),
7.27
(dd, J = 9 Hz, 6 Hz, 2H), 7.39-7.47 (m, 2H), 7.44 (d, J = 9 Hz, 2H), 7.75-7.82
(m, 1 H),
7.87-7.94 (m, 2H), 7.94 (d, J = 9 Hz, 2H), 8.05 (s, 1 H). MS (DCI-NH3) m/z 477
(M+H)+, 494 (M+NH4)+. Anal. calc. for C25H17FN203S2: C, 63.03; H, 3.57; N,
5.88. Found: C, 62.89; H, 3.55; N, 5.71.
Example 108
2-(4-Fluorouhenyl -L4-j4-fluoro n xyl-5-[4-(methylsulfonyl ohenyll-3(2H)-
Ryridazinone
108A. 4-(4-Fluorochenoxy)-54 4-(methylsulforlYl)ohenylJ-3(2HLpyridazinone
The title compound was prepared by treating 2-benzyl-4-(4-fluorophenoxy)-
5-[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone (Example 75) with AIBr3 in
toluene according to the procedure in Example 11 (yield: 1.8 g, 95%).
-95-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
108B. 2: (4-F luorophenYl)-4-(4-fluoroohenoxy)-5-[4-(methylsulfonvllohenylj-
3(2H):
pyridazinone
The title compound was prepared according to Example 93, starting with 4-
(4-fluorophenoxy)-5-[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone and
substituting
1-fluoro-4-iodobenzene in place of 4-bromothioanisole (yield: 60 mg, 53%).
M.p.
83-85 C. 1 H NMR (300 MHz, CDCI3) 5 3.10 (s, 3H), 6.89-7.03 (m, 4H), 7.15 (t,
J
9 Hz, 2H), 7.65 (dd, J = 9 Hz, 6 Hz, 2H), 7.83 (d, J = 6 Hz, 2H), 8.07 (d, J =
9 Hz,
2H), 8.08 (s, 1 H). MS (DCI-NH3) m/z 455 (M+H)+, 472 (M+NH4)+.
.10 Example 109
2-(3.4-Difluorophenyl)-4-(4-fluoronhenoxy)-5-[4-(methylsulfonyl)phenyll-3(2F,~-
ovridazinone
The title compound was prepared according to Example 93, substituting 1-
bromo-3,4-difiuorobenzene in place of 4-bromothioanisole and 4-(4-
ftuorophenoxy)-5-[4-(methylsulfony!)phenyl]-3(2H)-pyridazinone (Example 108A)
in
place of 4-(4-fluorophenyl)-5-[4-(methylsulfonyl)phenyt]-3(2H)-pyridazinone
(yield:
185 mg, 39%). M.p. 178-180 C. 1 H NMR (300 MHz, CDC13) S 3.11 (s, 3H), 6.89-
7.04 (m, 4H), 7.45-7.52 (m, 1 H), 7.45-7.52 (m, 1 H), 7.61 (dt, J = 6 Hz, 3
Hz, 1 H),
7.82 (d, J = 9 Hz, 2H), 8.07 (d, J = 9 Hz, 2H), 8.08 (s, 1 H). MS (DCI-NH3)
m/z 473
(M+H)+, 490 (M+NH4)+. Anal. calc. for C23H15F3N204S-0.5 H20: C, 57.38; H,
3.33; N, 5.83. Found: C, 57.17; H, 3.13; N, 5.62.
Example 110
2-(3-Bromoohenvl)-4-(4-fiuoro henoxy)_5-[4-(methyisulfonxOohgnyll-3(2T
pyridazinone
The title compound was prepared according to Example 93, substituting 1,3-
dibromobenzene in place of 4-bromothioanisole and 4-(4-fluorophenoxy)-5-[4-
(methytsulfonyl)phenyl]-3(2H)-pyridazinone (Example 108A) in place of 4-(4-
f{uorophenyl)-5-[4-(methyisulfonyl)phenyf)-3(2H)-pyridazinone (yield: 260 mg,
50.5%). M.p. 208-210 C. 1 H NMR (300 MHz, CDCI3) S 3.09 (s, 3H), 6.89-7.04
(m,
4H), 7.34 (t, J = 9 Hz, 1 H), 7.53 (br d, J = 9 Hz, 1 H), 7.64 (br d, J = 9
Hz, 1 H), 7.82 (d,
J = 9 Hz, 2H), 7.87 (t, J = 1.5 Hz, 1 H), 8.08 (d, J = 9 Hz, 2H), 8.09 (s, 1
H). MS (DCI-
NH3) m/z 517 (M+H)+, 534 (M+NH4)+. Anal. caic. for C23H16BrFN204S: C, 53.7;
H, 3.11; N, 5.45. Found: C, 53.46; H, 2.88; N, 5.18.
-96-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 111
? (3 5-DifluoroQhen IY)-4-(4-fluorooh enoxv)-5-[4-(methylsulfonyl)nhenyl)-
3(2H)-
Ryridazinone
The title compound was prepared according to Example 93, substituting 1-
bromo-3,4-difluorobenzene in place of 4-bromothioanisole and 4-(4-
fluorophenoxy)-5-[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone (Example 108A)
in
place of 4-(4-fluorophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone
(yield:
175 mg, 37%). M.p. 209-211 C. 1 H NMR (300 MHz, CDC13) S 3.10 (s, 3H), 6.85
(tt, J = 9 Hz, 3 Hz, 1 H), 6.90-7.04 (m, 4H), 7.38 (dd, J = 9 Hz, 3 Hz, 2H),
7.81 (d, J =
9 Hz, 2H), 8.07 (d, J = 9 Hz, 2H), 8.10 (s, 1 H). MS (DCI-NH3) m/z 473 (M+H)+,
490
(M+NH4)+. Anal. calc. for C23H15F3N204S=H20: C, 58.47; H, 3.18; N, 5.94.
Found: C, 58.31; H, 3.15; N, 5.82.
Example 112
2-(3-Chlorophenyl)-4-(4-fluoro h~ enoxy)-5-j4-(methylsulfonvl)phenylJ-3(2H)-
Ryridazinone
The title compound was prepared according to Example 93, substituting 1-
bromo-3-chlorobenzene in place of 4-bromothioanisole and 4-(4-fluorophenoxy)-5-
[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone (Example 108A) in place of 4-(4-
fluorophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone (yield: 25 mg,
5.3%).
M.p. 211-213 C. 1 H NMR (300 MHz, DMSO-d6) 6 3.30 (s, 3H), 7.15 (d, J 9 Hz,
4H), 7.51-7.64 (m, 3H), 7.71-7.75 (m, 1 H), 7.91 (d, J = 9 Hz, 2H), 8.06 (d, J
9 Hz,
2H), 8.41 (s, 1 H). MS (DCI-NH3) m/z 471 (M+H)+, 488 (M+NH4)+. Anal. caic. for
C23H16CIFN2O4S=0.5 H20: C, 57.62; H, 3.44; N, 5.85. Found: C, 57.62; H, 3.52;
N, 5.48.
Example 113
2- (4-Nitrobenzyl)-4-(4-fluorop henyl)-5-[4-(methylsulfonY1)oheIly~l-3(2 H)-
pyridazinone
The title compound was prepared according to the method of Example 20,
substituting 4-nitrobenzyl bromide in place of 4-fluorobenzyl bromide (yield:
164
mg, 58.9%). M.p. 183-184 C. 1 H NMR (300 MHz, CDC13) 8 3.05 (s, 3H), 5.47 (s,
2H), 6.96 (t, J = 9 Hz, 2H), 7.16 (dd, J = 9 Hz, 3 Hz, 2H), 7.32 (d, J = 9 Hz,
2H), 7.70
(d, J = 9 Hz, 2H), 7.87 (s, 1 H), 7.88 (d, J = 9 Hz, 2H), 8.22 (d, J = 9 Hz,
2H). MS
(DCI-NH3) m/z 480 (M+H)+, m/z 497 (M+NH4)+. Anal. calc. for C24H18FN305S:
C, 60.12; H, 3.78; N, 8.76. Found: C, 59.89; H, 3.83; N, 8.61.
-97-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 114
2-(4-Acetoxvbenzyl)-4-(4-fluorophenY1)-5-[4-(meth lsx ulfonyl) henx11-3(2),
Ryridazinone
The title compound was prepared according to the method of Example 20,
substituting 4-(chloromethyl)phenyl acetate in place of 4-fluorobenzyl bromide
(yield: 220 mg, 76.9%). M.p. 172-174 C. 1 H NMR (300 MHz, CDCI3) S 2.30 (s,
3H), 3.05 (s, 3H), 5.38 (s, 2H), 6.95 (t, J = 9 Hz, 2H), 7.06 (d, J = 9 Hz,
2H), 7.16 (dd,
J = 9 Hz, 5 Hz, 2H), 7.31 (d, J = 9 Hz, 2H), 7.60 (d, J = 9 Hz, 2H), 7.81 (s,
1 H), 7.87
(d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 510 (M+NH4)+. Anal. caic. for
C26H21 FN2O5S: C, 63.40; H, 4.30; N, 5.69. Found: C, 63.28; H, 4.41; N, 5.39.
Example 115
2-(4-Hvdroxybenzvl)-4-(4-fluoropbeoY1)-5-[4-(methY su K()Rbgnyl1-3(ZH1-
Qvridazinone
A solution of 2-(4-acetoxybenzyl)-4-(4-fluorophenyl)-5-[4-(methylsulfonyl)-
phenyl]-3(2H)-pyridazinone (0.2 g, 4.06 mmol) (Example 114) in THF (20 mL) was
treated with a solution of lithium hydroxide monohydrate (0.05 g, 1.22 mmol)
in
water (5 mL). Methanol (2 mL) was added to provide a homogeneous solution
which was stirred at room temperature overnight. The reaction mixture was then
acidified with 10% aqueous citric acid and extracted with ethyl acetate. The
ethyl
acetate layer was dried over MgSO4 and filtered. The filtrate was concentrated
in
vacuo to provide a white foam which was purified by column chromatography
(silica gel, 65:35 hexanes/ethyl acetate). Product fractions were combined and
concentrated in vacuo. The residue was crystallized from ethyl acetate/hexanes
(yield: 195 mg, 70%). M.p. 225-226 C. 1 H NMR (300 MHz, CDCI3) S 3.05 (s,
3H),
4.86 (s, 1 H), 5.33 (s, 2H), 6.80 (d, J = 8.5 Hz, 2H), 6.95 (t, J = 9 Hz, 2H),
7.15 (dd, J
9 Hz, 5 Hz, 2H), 7.30 (d, J = 8.5 Hz, 2H), 7.46 (d, J = 8.5 Hz, 2H), 7.83 (s,
1 H), 7.87
(d, J = 8.5 Hz, 2H). MS (DCI-NH3) m/z 451 (M+H)+. Anal. calc. for
C24H19FN204S: C, 63.99; H, 4.25; N, 6.22. Found: C, 63.73; H, 4.16; N, 6.11.
Example 116
2-(3-Nitroben yl)-4-(4-fluorop (methy Sulfonyl) henyl]-3(2);j)-
Ryridazinone
The title compound was prepared according to the method of Example 20,
substituting 3-nitrobenzyl bromide in place of 4-fluorobenzyl bromide (yield:
195
mg, 70%). M.p. 156-157 C. 1 H NMR (300 MHz, CDCI3) 5 3.05 (s, 3H), 5.48 (s,
-98-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
2H), 6.96 (t, J = 9 Hz, 2H), 7.16 (dd, J = 9 Hz, 5 Hz, 2H), 7.33 (d, J = 8.5
Hz, 2H),
7.54 (t, J = 7 Hz, 1 H), 7.88 (s, 1 H), 7.90 (d, J = 8.5 Hz, 2H), 8.19 (br d,
J = 7 Hz, 1 H),
8.37 (t, J = 1.7 Hz, 1 H). MS (DCI-NH3) m/z 480 (M+H)+, m/z 497 (M+NH4)+.
Anal.
calc. for C24H18FN305S: C, 60.12; H, 3.78; N, 8.76. Found: C, 59.98; H, 3.73;
N,
8.67.
Example 117
2-(3.4.4-Trifluoro-3-butenyl)-4-(4-fluoroohenyl)-5-j4-
(methylsulfonyI)ohenyl,]3 (2 H) -
pyridazinone
The title compound was prepared according to the method of Example 20,
substituting 4-bromo-1,1,2-trifluoro-l-butene in place of 4-fluorobenzyl
bromide
(yield: 38 mg, 14.5%). M.p. 131-132 C. 1 H NMR (300 MHz, CDCI3) 5 2.92 (br d,
J
= 21.7 Hz, 2H), 3.06 (s, 3H), 4.47 (t, J = 6.6 Hz, 2H), 6.98 (t, J = 9 Hz,
2H), 7.17 (dd,
J = 9 Hz, 5 Hz, 2H), 7.35 (d, J = 8.5 Hz, 2H), 7.85 (s, 1 H), 7.89 (d, J = 8.5
Hz, 2H).
MS (DCI-NH3) m/z 453 (M+H)+, m/z 470 (M+NH4)+. Anal. calc. for
C21 H16F4N203S: C, 55.75; H, 3.56; N, 6.19. Found: C, 55.63; H, 3.62; N, 6.10.
Example 118
2-(2-Hexvnvl)-4-(4-fiuoroohenyj);544- (meth S~ yl)pheny,jl-3(2H)-R~~~idazinone
The title compound was prepared according to the method of Example 20,
substituting 1-chloro-2-hexyne in place of 4-fluorobenzyl bromide (yield: 170
mg,
69%). M.p. 79-80 C. 1 H NMR (300 MHz, CDCI3) S 0.99 (t, J = 7.5 Hz, 3H), 1.56
(h,
J = 7.5 Hz, 2H), 2.21 (m, 2H), 3.06 (s, 3H), 5.01 (t, J = 3 Hz, 2H), 6.96 (t,
J = 9 Hz,
2H), 7.18 (dd, J = 9 Hz, 6 Hz, 2H), 7.34 (d, J = 9 Hz, 2H), 7.88 (s, 1 H),
7.89 (d, J = 9
Hz, 2H). MS (DCI-NH3) m/z 425 (M+H)+. Anal. calc. for C23H21 FN2O3S: C,
65.07; H, 4.98; N, 6.59. Found: C, 64.87; H, 4.90; N, 6.58.
Example 119
2-(3.3-Dichloro-2-orooenvl)-4-(4-fluorophenyj)-5- [4- (aminosulfony!)Phgnyll-3
(,H1-
pyridazinone
The title compound was prepared according to the method of Example 20,
substituting 1,1,3-trichloropropene in place of 4-fluorobenzyl bromide (yield:
1.15 g,
68%). M.p. 184-185 C. 1 H NMR (300 MHz, DMSO-d6) S 4.39 (d, J = 7.5 Hz, 2H),
6.43 (t, J = 7.5 Hz, 1 H), 7.14 (t, J = 9 Hz, 2H), 7.23 (dd, J = 9 Hz, 6 Hz,
2H), 7.38 (d, J
= 9 Hz, 2H), 7.43 (s, 2H), 7.73 (d, J = 9 Hz, 2H), 8.11 (s, 1 H). MS (DCI-NH3)
m/z
-99-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
454 (M+H)+. Anal. caic. for C1 gH14C12F4N303S: C, 50.23; H, 3.1; N, 9.24.
Found: C, 50.28; H, 3.29; N, 9.19.
Example 120
2-Cvclohexyl-4-(4-fluorophgnyl)-5Z-[4-(methylsulfopyl)ohenylJ-3(2H) -
12yridazinone
The title compound was prepared according to the method of Example 20
substituting cyclohexyl bromide in place of 4-fluorobenzyl bromide (yield: 163
mg,
76%). M.p. 169-171 C. 1 H NMR (DMSO-d6, 300 MHz) 5 1.23 (m, 1 H), 1.41 (m,
2H), 1.71 (m, 3H), 1.87 (m, 4H), 3.23 (s, 3H), 4.85 (m, 1 H), 7.11 (m, 2H),
7.22 (m,
2H), 7.46 (d, J = 9 Hz, 2H), 7.85 (d, J = 9 Hz, 2H), 8.11 (s, 1 H). MS (DCI-
NH3) m/z
427 (M+H)+ and m/z 444 (M+NH4)+. Anal. calc. for C23H23FN203SØ5 H20: C,
63.43; H, 5.55; N, 6.43. Found: C, 63.25; H, 5.28; N, 6.28.
Example 121
2-Cvciooentvl-4-(4-fluoronhenyl)-5-[4-(met ylSuIf onyl) h{~enyll-3(2J;j)-
Ryridazinnr,P
The title compound was prepared according to the method of Example 20,
substituting cyclopentyl bromide in place of 4-fluorobenzyl bromide (yield:
165 g,
80%). M.p. 191-193 C. 1 H NMR (DMSO-d6, 300 MHz) S 1.67 (m, 2H), 1.85 (m,
4H), 2.05 (m, 2H), 3.23 (s, 3H), 5.36 (m, 1 H), 7.12 (t, J = 9 Hz, 2H), 7.22
(m, 2H),
7.45 (d, J = 9 Hz, 2H), 7.85 (d, J = 9 Hz, 2H), 8.13 (s, 1 H). MS (DCI-NH3)
m/z 413
(M+H)+ and m/z 430 (M+NH4)+. Anal. caic. for C22H21 FN2O3S=0.5 H20: C,
62.69; H, 5.26; N, 6.57. Found: C, 62.53; H, 4.93; N, 6.50.
Example 122
2-Cvclobutvl-4-(4-fluoroohenyl)-5-[4-(met y suIfonyl)~h nyll-3(2H)-
Ryridaz~none
The title compound was prepared according to the method of Example 20,
substituting cyclobutyl bromide in place of 4-fluorobenzyl bromide (yield: 270
g,
68%). M.p. 202-203 C. 1 H NMR (DMSO-d6, 300 MHz) S 1.85 (m, 2H), 2.32 (m,
2H), 2.50 (m, 2H), 5.40 (quintet, J = 7 Hz, 1 H), 7.11 (t, J = 9 Hz, 2H), 7.21
(m, 2H),
7.47 (d, J = 9 Hz, 2H), 7.86 (d, J = 9 Hz, 2H), 8.16 (s, 1 H). MS (DCI-NH3)
m/z 399
(M+H)+ and m/z 416 (M+NH4)+. Anal. calc. for C21 H1 9FN203S-0.75 H20: C,
61.22; H, 5.01; N, 6.80. Found: C, 61.19; H, 4.62; N, 6.73.
-100-
CA 02578858 2007-03-05
WO 99/10331 PCTIUS98/16479
Example 123
? (3-Methvl- -b rt nyl)-4-(4-fluorophenyl)-5-[3-fluoro-4-
(aminosulfonyl)ohenvll-
3(2H)-Ryridazinone
2-Benzy{-4-(4-fluorophenyl)-5-[3-fluoro-4-(aminosulfonyl)phenyl]-3(2H)-
pyridazinone prepared according to the method of Example 68 was N-
debenzylated according to the method of Example 11. The intermediate was N-
alkylated according to the method of Example 20, substituting 1-bromo-3-methyl-
2-
butene in place of 4-fluorobenzyl bromide, to provide the title compound
(yield: 50
mg, 30%). M.p. 134-136 C. 1 H NMR (300 MHz, CDC13) S 1.79 (s, 3H), 1.86 (s,
3H), 4.78 (s, 2H), 4.85 (d, J = 7.5 Hz, 2H), 5.48 (t, J = 6 Hz, 1 H), 6.96 (t,
J = 9 Hz,
2H), 7.18 (dd, J = 9 Hz, 6 Hz, 2H), 7.28 (d, J = 9 Hz, 2H), 7.83 (s, 1 H),
7.85 (d, J = 9
Hz, 2H).'MS (DCI-NH3) m/z 414 (M+H)+. Anal. cafc. for C21 H2OFN303S: C, 61;
H, 4.87; N, 10.16. Found: C, 60.98; H, 4.66; N, 9.95.
Example 124
2-(2.4-Difluorobenzyl)-4-(4-fluoro henvl)-5-[4-(aminosulfonyl)nhenvl]-3(2H)-
Qyridazinone
The title compound was prepared according to the method of Example 123,
substituting 2,4-difluorobenzyl bromide in place of 1-bromo-3-methyl-2-butene
(yield: 65 mg, 24%). M.p. 236-238 C. 1 H NMR (300 MHz, CDCI3) S 4.78 (s, 2H),
5.43 (s, 2H), 6.88 (m, 2H), 6.97 (t, J = 9 Hz, 2H), 7.18 (dd, J = 9 Hz, 6 Hz,
2H), 7.38
(d, J = 9 Hz, 2H), 7.55 (m, 1 H), 7.85 (s, 1 H), 7.86 (d, J = 9 Hz, 2H). MS
(DCI-NH3)
m/z 472 (M+H)+. Anal. calc. for C23H16F3N303S: C, 58.59; H, 3.42; N, 8.91.
Found: C, 58.44; H, 3.47; N, 8.72.
Example 125
2=(Pentafluorobenzyl)-4-(4-fluoroRhenyl)-5-(4-(aminosulfonyl)nhenxl]-3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 123,
substituting 2,3,4,5,6-pentafluorobenzyl bromide in place of 1-bromo-3-methyl-
2-
butene (yield: 105 mg, 35%). M.p. 201-203 C. 1 H NMR (300 MHz, CDC13) S 4.8
(s, 2H), 5.5 (s, 2H), 6.98 (t, J = 9 Hz, 2H), 7.18 (dd, J = 9 Hz, 6 Hz, 2H),
7.28 (d, J = 9
Hz, 2H), 7.32 (s, 1 H), 7.37 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 526 (M+H)+.
Anal.
calc. for C23H13F6N303S: C, 52.57; H, 2.49; N, 7.99. Found: C, 52.66; H, 2.68;
N,
7.8.
-101-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 126
2-(3-Cyclohexeny()-4_( - -o Rliapyj)-5-j4-(aminosulfonyl)phen_vjl-3( -1)-
Ryridazinone
The title compound was prepared according to the method of Example 123,
substituting 3-bromocyclohexene in place of 1 -bromo-3-methyl-2-butene (yield:
30
mg, 10%). M.p. 206-208 C. 1 H NMR (300 MHz, CDCI3) S 1.75-1.85 (m, 3H), 2.1-
2.3 (m, 3H), 4.8 (s, 2H), 5.75 (m, 2H), 6.1 (m, 1 H), 6.97 (t, J = 9 Hz, 2H),
7.20 (dd, J
9 Hz, 6 Hz, 2H), 7.28 (d, J = 9 Hz, 2H), 7.86 (d, J = 9 Hz, 2H), 7.90 (s, 1
H). MS (DCI-
NH3) m/z 426 (M+H)+. Anal. caic. for C22H20FN303S: C, 62.10; H, 4.73; N, 9.87.
Found: C, 61.27; H, 4.75; N, 9.56.
Example 127
2-(3.4-Difluorobenzvl)-4-(4-fluoronheny,J)õ5-[4-(aminosulfonyj)Rhgpy,j]_3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 123,
substituting 3,4-difluorobenzyl bromide in place of 1-bromo-3-methyl-2-butene
and
running the reaction in DMSO instead of DMF to prevent formation of byproducts
(yield: 210 mg, 62%). M.p. 253-255 C. 1 H NMR (300 MHz, DMSO-d6) 5 5.33 (s,
2H), 7.13 (t, J = 9 Hz, 2H), 7.22 (dd, J = 9 Hz, 6 Hz, 2H), 7.28 (m, 1 H),
7.39 (d, J = 9
Hz, 2H), 7.42 (s, 2H), 7.47 (m, 2H), 7.73 (d, J = 9 Hz, 2H), 8.12 (s, 1 H). MS
(DCI-
NH3) m/z 472 (M+H)+. Anal. calc. for C23H1 6F3N303S: C, 58.59; H, 3.42; N,
8.91.
Found: C, 58.05; H, 3.55; N, 8.49.
Example 128
2-(2.3-Dihvdro-1 H-inden-2-yl)-4-(4-fiuoropienyl)-5-[4-(methvisuI xj) nyll-
3(2H)-Ryridazinone
A solution of 4-(4-fluorophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone (172 mg, 0.5 mmol), prepared in Example 11, 2-indanol (67 mg, 0.5
mmol) and Ph3P (262 mg, 1 mmol) in toluene (20 mL) and ethyl acetate (5 mL)
was
prepared and added dropwise a solution of DIAD (0.2 mL, 1 mmol) in toluene (10
mL). The mixture was stirred at room temperature for 6 hours and concentrated
in
vacuo. The residue was chromatographed (silica gel, 19:1 CH2CI2-ethyl acetate)
to provide 200 mg of product (contaminated with reduced DIAD). A second column
chromatography (hexanes-ethyl acetate 1:1) furnished the title product (yield:
170
mg, 74%). M.p. 97-100 C. 1 H NMR (DMSO-d6, 300 MHz) 5 3.22 (s, 3H), 3.32 (m,
2H), 3.44 (dd, J = 9 Hz and 15 Hz, 2H), 5.83 (m, 1 H), 7.25 (m, 4H), 7.34 (m,
4H),
-102-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
7.46 (d, J = 9 Hz, 2H), 7.85 (d, J = 9 Hz, 2H), 8.06 (s, 1 H). MS (DCI-NH3)
m/z 461
(M+H)+ and m/z 478 (M+NH4)+.
Example 129
2-(2.3-Dihydro-1 H-inden-l-yl)-4-(4-fluoropheny!)-5-j4-(methylsulfonxl)phenyll-
3 (2H)-12yridazinone
The title compound was prepared according to the method of Example 128
substituting 1-indanol in place of 2-indanol (yield: 110 mg, 48%). M.p. 128-
130 C.
1 H NMR (DMSO-d6, 300 MHz) 5 2.40 (m, 1 H), 2.60 (m, 1 H), 3.00 (m, 1 H), 3.22
(s+m, 4H), 6.60 (dd, J = 9 Hz, 6 Hz, 1 H), 7.16 (m, 4H), 7.27 (m, 4H), 7.47
(d, J = 9
Hz, 2H), 7.85 (d, J = 9 Hz, 2H), 8.02 (s, 1 H). MS (DCI-NH3) m/z 461 (M+H)+
and
m/z 478 (M+NH4)+.
Example 130
214-Tetrahydro-2H-pyran-4-yi1-4-(4-fluorophenyl)-5-[4-(methylsulfon r~l
phenyll-
3(2H)-pyridazinone
The title compound was prepared according to the method of Example 128
substituting 4-tetrahydropyranol in place of 2-indanol (yield: 140 g, 65%).
M.p. 230-
231 C. 1 H NMR (300 MHz, DMSO-d6) 6 1.75 (m, 2H), 1.93 (m, 2H), 3.14 (s, 3H),
3.46 (m, 2H), 3.93 (m, 2H); 5.02 (m, 1 H), 7.05 (t, J = 9 Hz, 2H), 7.15 (m,
2H), 7.40 (d,
J = 9 Hz, 2H), 7.80 (d, J = 9 Hz, 2H), 8.08 (s, 1 H). MS (APCI-) m/z 428 (M-H)-
and
m/z 463 (M+CI)- . Anal. calc. for C22H21 FN204S-1.25 H20: C, 58.59; H, 5.25;
N,
6.21. Found: C, 58.31; H, 4.75; N, 6.05.
Example 131
212-Methylcyclopentyll-4-(4-fluoropheny!)-5-j4-(methylsulfonyl)phenyl)-3(2H1
pyridazinone
The title compound was prepared according to the method of Example 128
substituting 2-methylcyclopentanol in place of 2-indanol (yield: 230 g, 86%).
M.p.
180-181 C. 1 H NMR (300 MHz, DMSO-d6) b 0.75 (d, J = 7 Hz, 3H), 1.60 (m, 2H),
1.89 (m, 2H), 2.10 (m, 1 H), 2.21 (m, 1 H), 2.40 (m, 1 H), 3.23 (s, 3H), 5.37
(q, J = 7
Hz, 1 H), 7.12 (t, J = 9 Hz, 2H), 7.21 (m, 2H), 7.47 (d, J = 9 Hz, 2H), 7.86
(d, J = 9 Hz,
2H), 8.11 (s, 1 H). MS (APCI+) m/z 427 (M+H)+ and (APCI-) m/z 461 (M+CI)-.
Anal.
calc. for C23H23FN203S: C, 64.77; H, 5.43; N, 6.56. Found: C, 64.71; H, 5.34;
N,
6.28.
-103-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 132
2-(2-Adamantvl)-4-(4-fluoro enyl)-5-[4-(meth Isy ulfony[)c~henyl]-3(2EL-
pyridazinone
The title compound was prepared according to the method of Example 128
substituting 2-adamantanol in place of 2-indanol, (yield: 75 g, 25%).M.p. 195-
197
C. 1 H NMR (300 MHz, DMSO-d6) 81.60 (m, 2H), 1.77 (m, 2H), 1.94 (m, 6H), 2.35
(m, 4H), 3.23 (s, 3H), 4.83 (m, 1 H), 7.11 (t, J = 9 Hz, 2H), 7.22 (m, 2H),
7.47 (d, J = 9
Hz, 2H), 7.87 (d, J = 9 Hz, 2H), 8.11 (s, 1 H). MS (APCI+) m/z 479 (M+H)+ and
(APCI-) m/z 478 (M-H)-, m/z 513 (M+CI)-. Anal. calc. for C27H27FN2O3S-0.25
H20: C, 67.13; H, 5.73; N, 5.79. Found: C, 67.06; H, 5.76; N, 5.06.
Example 133
2-(3-Methylcyclopentyl)-4-(4-fluoroohenyl)-5-[4-(meth Isy ulfonyl)oheny,j]-
3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 128
substituting 3-methylcyclopentanol in place of 2-indanol (yield: 155 g, 73%).
M.p. 169-171 C. 1 H NMR (300 MHz, DMSO-d6) S 1.05 (dd, 2:1, 3H), 1.24 (m,
1 H), 1.63 (m, 1 H), 2.00 (m, 3H), 2.22 (m, 2H), 3.23 (s, 3H), 5.43 (m, 1 H),
7.1 (t, J = 9
Hz, 2H), 7.21 (m, 2H), 7.46 (d, J = 9 Hz, 2H), 7.86 (d, J = 9 Hz, 2H), 8.12
(two s, 2:1,
1 H). MS (APCI+) m/z 27 (M+H)+ and (APCI-) m/z 426 (M-H)-, m/z 461 (M+CI)-.
Anal. calc. for C27H27FN2O3S-0.25 H20: C, 64.09; H, 5.49; N, 6.49. Found: C,
64.27; H, 5.62; N, 6.46.
Example 134
2-(1-MethylcycloRentyl)-4-(4-fluoroRhenyl)-5-[4-(methylsulfonyj)ohen,}l1-3(2H)-
pyridazinone
A solution of 4-(4-fluorophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone (206 mg, 0.6 mmol), prepared according to the method of Example
11, 1-methyl-1-cyclopentanol (60 mg, 0.6 mmol), DMAP (18 mg, 0.12 mmol) and
Ph3P (262 mg, 1 mmol) in toluene (30 mL) in ethyl acetate (5 mL) was prepared
and added dropwise to a solution of DIAD (0.2 mL, 1 mmol) in 10 mL of toluene.
The mixture was stirred at room temperature for 6 hours and then concentrated
in
vacuo. The residue was chromatographed (silica gel, 19:1 CH2CI2-ethyl acetate)
to provide 80 mg of product (contaminated with reduced DIAD). A second column
chromatography (hexanes-ethyl acetate 1:1) furnished the title product,
(yield: 50
mg, 19%). M.p. 107-110 C. 1 H NMR (DMSO-d6, 300 MHz) S 1.55 (s, 3H), 1.70 (m,
-104-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
4H), 2.08 (m, 2H), 2.32 (m, 2H), 3.22 (s, 3H), 7.10 (t, J- 9 Hz, 2H), 7.20 (m,
2H),
7.45 (d, J = 9 Hz, 2H), 7.86 (d, J = 9 Hz, 2H), 8.03 (s, 1 H). MS (APCI+) m/z
427
(M+H)+ and (APCI-) m/z 426 (M-H)-, m/z 461 (M+CI)-.
Example 135
2-(3.4-Difluoroohenyl)-4-(4-fluoro-3-vinylphenyl)-5-[4-(meth Isy
uifony,))oheny,jl-
3(2H)-Ryridazinone.
135A. 5-Bromo-2-fluorostyrene.
A mixture of inethyltriphenylphosphonium bromide (2.14 g, 6 mmol) and
potassium t-butoxide (672 mg, 6 mmol) in 50 mL of THF was refluxed for 30
minutes under N2 and then cooled to room temperature. 5-Bromo-2-
fluorobenzaldehyde (1.02 g, 5 mmol) was added and the resulting mixture was
refluxed for 2 hours (until the TLC showed the disappearance of starting
aldehyde).
The reaction was concentrated in vacuo and partitioned between water and ethyl
acetate. The acetate layer was washed with water and brine. The solution was
dried over MgSO4 and concentrated in vacuo. The residue was purified by
chromatography (silica gel, 15:1 hexanes-diethyl ether) to provide 900 mg
(90%) of
5-bromo-2-fluorostyrene.
135B. 2-(3.4-Difiuoroohenvl)-4-(4-fluoro-3-vinylQh1a1L)-5;(4-
(methvlthio)Rhenyll-
3(2H)-Ryridazinone.
The bromo-styrene compound, prepared above, in 10 mL of THF was added
dropwise to a heated mixture of magnesium turnings (120 mg, 5 mmol) and a few
drops of 1,2-dibromoethane in THF (20 mL) at a rate to maintain a gentle
reflux.
The mixture was refluxed for the next 30 minutes and cooled to room
temperature.
The Grignard reagent solution was cooled to -78 C and added, dropwise, to a
solution of 2-(3,4-difluorophenyl)-4-methoxy-5-[4-(methylthio)phenyl]-3(2H)-
pyridazinone (540 mg, 1.5 mmol) in THF (20 mL). The reaction mixture was
allowed to warm to room temperature for 12 hours. Afterwards, a saturated
solution
of NH4CI was added and the mixture was extracted with ethyl acetate to provide
320 mg of crude sulfide.
135C. 2-(3.4-Difluoroohen ly )-4-(4-fluoro-3-vinylnhenxJ)-5-[4-
(methvlsulfnnvll
phenY(J-3(2-Fi)-Ry(idazinone.
The sulfide, prepared above, was dissolved in CH2CI2 (20 mL) and at 0 C
was treated with 30% CH3CO3H in CH3CO2H (0.5 mL). After 1.5 hours, 10%
NaHCO3 was added and the mixture extracted with CH2CI2. The extract was
concentrated in vacuo and the residue purified by chromatography (silica gel,
1:1
-105-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
hexanes-ethyl acetate) to provide the title compound (yield: 270 mg, 37%). 1 H
NMR (DMSO-d6, 300 MHz) S 3.22 (s, 3H), 5.37 (d, J = 12 Hz, 1 H), 5.65 (d, J 18
Hz, 1 H), 6.77 (dd, J = 12 Hz and 18 Hz, 1 H), 7.15 (m, 2H), 7.57 (m, 5H),
7.90 (m,
3H), 8.28 (s, 1 H). MS (APCI+) m/z 483 (M+H)+ and (APCI-) m/z 517 (M+CI)-.
Anal.
calc. for C25H17F3N2O3S=0.5 H20: C, 61.09; H, 3.69; N, 5.69. Found: C, 61.04;
H,
3.71; N, 5.34.
Example 136
? [3 4-Difluoropheny.1)-4- -methyl-3-hepjenyl)-5-[4-(methylsulfonyI), ghenXll -
3 (2H)-
pyridazinone
A Grignard, prepared as described in Example 135, substituting 2-(2-bromo-
ethyl)-1,3-dioxane (586 mg, 3 mmol) in place of 5-bromo-2-fluorostyrene, was
added to a solution of 2-(3,4-difluorophenyl)-4-methoxy-5-[4-
(methylthio)phenyl]-
3(2H)-pyridazinone (720 mg, 2 mmol) in THF (30 mL) at -78 C. The mixture was
left at room temperature for 14 hours, quenched with a saturated solution of
NH4CI
and extracted with ethyl acetate to obtain 900 mg of crude sulfide.
The intermediate sulfide product was dissolved in CH2CI2 (10 mL) and
treated at 0 C with 33% solution of CH3CO3H in CH3CO2H (0.7 mL) for 1 hour.
The mixture was concentrated in vacuo and the residue was partitioned between
saturated NaHCO3 and ethyl acetate. The acetate layer was dried over MgSO4
and concentrated in vacuo to provide 950 mg of crude sulfonyl derivative.
The sulfonyl compound, prepared above, was dissolved in acetone (50 mL)
and treated with 2 N HCI (10 mL). The resulting mixture was refluxed for 16
hours
and concentrated in vacuo. The residue was extracted with ethyl acetate to
provide
900 mg of 2-(3,4-difluorophenyl)-4-(2-formylethyl)-5-[4-
(methylsulfonyl)phenyl]-
3(2H)-pyridazinone (crude aldehyde, contaminated with some unreacted starting
dioxane derivative).
A mixture of isoamyltriphenylphosphonium bromide (414 mg, 1 mmol) and
potassium t-butoxide (112 mg, 1 mmol) in toluene (25 mL) was refluxed for 30
minutes and then cooled to room temperature. The crude aldehyde was added
and the mixture was refluxed for 14 hours. The reaction mixture was then
cooled to
room temperature and concentrated in vacuo. The residue was dissolved in ethyl
acetate and was washed with water, 10% citric acid, brine, dried over MgSO4
and
concentrated in vacuo. Purification by column chromatography (silica gel, 1:1
hexanes-ethyl acetate) provided the title compound as an oil (yield: 120 mg,
13%).
1 H NMR (300 MHz, DMSO-d6) S 0.74 (d, J- 7 Hz, 6H), 1.44 (m, 1 H), 1.70 (t, J
= 7
-106-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Hz, 2H), 2.22 (m, 2H), 2.54 (m, 2H); 3.30 (s, 3H), 5.29 (m, 2H), 7.51 (m, 1
H), 7.63
(m, 1 H), 7.74 (d, J = 9 Hz, 2H), 7.82 (m, 1 H), 8.02 (s, 1 H), 8.10 (d, J = 9
Hz, 2H). MS
(APCI+) m/z 473 (M+H)+ and (APCI-) m/z 471 (M-H)-, m/z 507 (M+CI)-. Anal.
calc.
for C25H26F2N203S: C, 63.54; H, 5.54; N, 5.92. Found: C, 63.74; H, 5.67; N,
5.58.
Example 137
2-(3.4-Difluorouhenvl)-4-(3-cvcloproRy ~I.deneproaY1)-5-(4-
(methylsulfonyl)nhenyll
3 (2H)-pvridazinone
The title compound was prepared according to the method of Example 136
substituting cyclopropyltriphenylphosphonium bromide in place of
isoamyltriphenylphosphonium bromide (yield: 55 mg, 12%). M.p. 128-129 C. 1H
NMR (300 MHz, DMSO-d6) S 0.81 (m, 2H), 0.97 (m, 2H), 2.34 (m, 2H), 2.65 (m,
2H),
3.32 (s, 3H), 5.64 (m, 1 H), 7.52 (m, 1 H), 7.63 (m, 1 H), 7.73 (d, J = 9 Hz,
2H), 7.81
(m, 1 H), 8.02-(s, 1 H), 8.10 (d, J= 9 Hz, 2H). MS (APCI+) m/z 443 (M+H)+ and
(APCI-) m/z 441 (M-H)-, m/z 477 (M+CI)-. Anal. caic. for C23H2OF2N203S-0.5
H20: C, 61.18; H, 4.68; N, 6.20. Found: C, 61.48; H, 4.60; N, 6.02.
Example 138
2-(3.4-Difiuoroohenvl)-4-(5-methyl- -hexenyl)-5-(4-(methylsulfonvl)phenyll-
3(2H)-
Ryridazinone
The title compound, an oil, was prepared according to the method of
Example 136 substituting isobutyltriphenylphosphonium bromide in place of
isoamyltriphenylphosphonium bromide (yield: 170 mg, 74%). 1 H NMR (300 MHz,
DMSO-d6) S 0.75 (d, J = 7 Hz, 6H), 2.22 (m, 3H), 2.54 (m, 2H), 3.32 (s, 3H),
5.12 (m,
2H), 7.52 (m, 1 H), 7.60 (m, 1 H), 7.72 (d, J = 9 Hz, 2H), 7.80 (m, 1 H), 8.02
(s, 1 H),
8.10 (d, J = 9 Hz, 2H). MS (APCI+) m/z 459 (M+H)+ and (APCI-) m/z 457 (M-H)-,
m/z 493 (M+CI)-. Anal. calc. for C24H24F2N203S: C, 62.86; H, 5.27; N, 6.10.
Found: C, 62.57; H, 5.32; N, 5.81.
Example 139
2-(3.4-Difluoroohenvl)-4-(5-methvlhexYl)-[4-(methylsu y1)phgnyl]-3(2)_
Ryridazinone
The title compound, an oil, was prepared according to the method of
Example 135B, substituting 5-methylhexyimagnesium bromide for 3-fluoro-4-
vinylphenylmagnesium bromide, (yield: 28 mg, 10%). 1 H NMR (300 MHz, DMSO-
d6) S 0.77 (d, J = 7 Hz, 6H), 0.88 (m, 1 H), 1.03 (m, 2H), 1.20 (m, 1 H), 1.46
(m, 5H),
-107-
CA 02578858 2007-03-05
WO 99/10331 PCTIUS98/16479
3.32 (s, 3H), 7.52 (m, 1 H), 7.62 (m, 1 H), 7.75 (d, J = 9 Hz, 2H), 7.82 (m, 1
H), 8.02 (s,
1 H), 8.11 (d, J = 9 Hz, 2H). MS (APCI+) m/z 461 (M+H)+ and (APCI-) m/z 459 (M-
H)-, m/z 495 (M+CI)-.
Example 140
2-(3-Chloro-1-methvl-~ 2E-progenyl)-4-(4-fluoronhenyl)-5-j4-
(aminosulfonyl),henylj-
3(2H) -pvridazinone
The title compound was prepared according to the method of Example 127,
substituting 1,3-dichloro-l-butene in place of 3,4-difluorobenzyl bromide
(yield: 55
mg, 30%). M.p. 152-154 C. 1 H NMR (300 MHz, CDCI3) fi 4.71 (dt, J = 15 Hz,
7.5
Hz, 2H), 2.28 (d, J = 1.5 Hz, 3H), 4.8 (s, 2H), 4.99 (d, J = 1 Hz, 1 H), 5.02
(d, J = 1 Hz,
1 H), 5.85 (td, J = 4 Hz, 1 Hz, 1 H), 6.98 (t, J = 9 Hz, 2H), 7.19 (dd, J = 9
Hz, 6 Hz, 2H),
7.28 (d, J = 9 Hz, 2H), 7.86 (s, 1 H), 7.87 (d, J = 9 Hz, 2H). MS (DCI-NH3)
m/z 434
(M+H)+. Anal. calc. for C20H17CIFN3O3S: C, 55.36; H, 3.94; N, 9.68. Found: C,
54.99; H, 3.83; N, 9.34.
Example 141
2-(2.3.3-Trifluoro-2-orQpS0-1-yi)-4-(4-fluoro h~eqyl)-5-(4-(aminosulfonY1)~n,
vll
3(2H)-Rvridazinone
The title compound was prepared according to the method of Example 127,
substituting 1-methylsufonyloxy-2,2,3-trifluoro-1-propene (mesylate), prepared
in
Example 88, in place of 3,4-difluorobenzyl bromide (yield: 10 mg, 4%). M.p.
173-
175 C. 1 H NMR (300 MHz, CDCI3) S 4.39 (s, 2H), 5.09 (ddd, J = 26 Hz, J = 3
Hz, J
= 1 Hz, 2H), 6.98 (t, J = 9 Hz, 2H), 7.19 (dd, J = 9 Hz, J = 6 Hz, 2H), 7.29
(d, J = 9 Hz,
2H), 7.78 (s, 1 H), 7.78 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 440 (M+H)+, MS
(FAB,
high res.) m/z caic. for C1 gH14F4N303S: 440.0692 (M+H)+. Found: 440.0695
(M+H)+, (0.7 ppm error).
Example 142
2-(1.1.2-Trifluoro-2-~r penyl)-4õ(4-fluorophgnyl)-5-(4-(aminosulfonyl nvll-
3(2H)-Ryridazinone
The title compound was isolated from the same reaction mixture (Example
141) that was used to prepare 2-(2,3,3-trifluoro-2-propen-1-yl)-4-(4-
fluorophenyl)-5-
(4-(aminosulfonyl)phenyl)-3(2H)-pyridazinone (The title product is a result of
an
SN2' attack.) (yield: 50 mg, 20%). M.p. 230-232 C. 1 H NMR (300 MHz, CDCI3) 8
4.7 (s, 2H), 5.28 (dd, J = 15 Hz, 4.5 Hz, 1 H), 5.39 (dd, J = 45 Hz, 4.5 Hz, 1
H), 6.98 (t,
-108-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
J = 9 Hz, 2H), 7.19 (dd, J = 9 Hz, 6 Hz, 2H), 7.31 (d, J = 9 Hz, 2H), 7.9 (d,
J = 9 Hz,
2H), 7.92 (s, 1 H), . MS (DCI-NH3) m/z 440 (M+H)+. Anal. calc. for
C19H13F4N3O3S: C, 51.93; H, 2.98; N, 9.56. Found: C, 51.88; H, 3.01; N, 9.15.
Example 143
2-(3.3-Difluoro-2-orooenyl)-4-(4-fluoroohenyl)-5-(4-(aminosuifonyi)o, henylJ-
3(2H)-
~yridazinone
The title compound was prepared according to the method of Example 127,
substituting 1,3-dibromo-1,1-difluoropropane in place of 3,4-difiuorobenzyl
bromide
and employing 5 equivalents of potassium carbonate (yield: 220 mg, 65%). M.p.
191-194 C. 1 H NMR (300 MHz, DMSO-d6) S 4.77 (d, J = 7.5 Hz, 2H), 4.95 (dtd,
J
24 Hz, 7.5 Hz, 1 Hz, 1 H), 7.12 (t, J = 9 Hz, 2H), 7.23 (dd, J = 9 Hz, 6 Hz,
2H), 7.49 (d,
J = 9 Hz, 2H), 7.50 (s, 2H), 7.74 (d, J = 9 Hz, 2H), 8.1 (s, 1 H). MS (DCI-
NH3) m/z
422 (M+H)+. Anal. calc. for C1 gH14F3N303S: C, 54.15; H, 3.34; N, 9.97. Found:
C, 53.88; H, 3.42; N, 9.76.
Example 144
2=(a-Methyl-3-fluorobenzyl)-4-(4-fluoroghenyl)-5-f4-(aminosulfony henyl]-3(2H)-
R,yridazinone
The title compound was prepared according to the method of Example 127,
substituting 3-fluoro-a-methylbenzyl chloride in place of 3,4-difluorobenzyl
bromide (yield: 220 mg, 65%). M.p. 192-194 C. 1 H NMR (300 MHz, DMSO-d6) S
1.76 (d, 6 Hz, 3H), 6.27 (q, J = 7 Hz, 1 H), 7.1 (t, J = 9 Hz, 2H), 7.22 (dd,
J = 9 Hz, 6
Hz, 2H), 7.49 (d, J = 9 Hz, 2H), 7.51 (s, 2H), 7.72 (d, J = 9 Hz, 2H), 8.18
(s, 1 H). MS
(DCI-NH3) m/z 468 (M+H)+. Anal. caic. for C24H1 9F2N303S: C, 61.66; H, 4.09;
N,
8.98. Found: C, 61.36; H, 3.96; N, 8.86.
Example 145
2-(1-Cvclohexenvlmethvl)-4-(4-fluorophenyl)-5-14-(aminosulfonyl hPny(1 3(2H1-
pyridazinone
The title compound was prepared according to the method of Example 127,
substituting 1-bromomethylcyclohexene in place of 3,4-difluorobenzyl bromide
(yield: 70 mg, 28%). M.p. 192-193 C. 1 H NMR (300 MHz, DMSO-d6) S 1.55 (m,
4H), 1.98 (m, 4H), 4.64 (s, 2H), 5.53 (s, 1 H), 7.12 (t, J = 9 Hz, 2H), 7.22
(dd, J = 9 Hz,
6 Hz, 2H), 7.39 (d, J = 9 Hz, 2H), 7.39 (s, 2H), 7.72 (d, J = 9 Hz, 2H), 8.07
(s, 1 H).
-109-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
MS (DCI-NH3) m/z 440 (M+H)+. Anal. calc. for C23H22FN303S: C, 62.85; H, 5.04;
N, 9.56. Found: C, 62.47; H, 5.23; N, 9.14.
Example 146
2-((x-Methyl-2.3.4-trifluorobenzvl)-4-(4-fluorophenyl -5-f4-
(aminosulfonyl)nheny,l]-
3 (2H)-Rvridazinone
The title compound was prepared according to the method of Example 127,
substituting 2,3,4-trifluoro-a-methylbenzyl chloride in place of 3,4-
difluorobenzyl
bromide (yield: 70 mg, 50%). M.p. 192-194 C. 1 H NMR (300 MHz, CDC13) S 1.84
(d, J = 6 Hz, 3H), 4.8 (s, 2H), 6.54 (q, J = 7 Hz, 1 H), 6.96 (t, J = 9 Hz,
2H), 6.99 (m,
1 H), 7.18 (dd, J = 9 Hz, 6 Hz, 2H), 7.2 (m, 1 H), 7.38 (d, J = 9 Hz, 2H),
7.86 (d, J = 9
Hz, 2H), 7.88 (s, 1 H). MS (DCI-NH3) m/z 504 (M+H)+. Anal. caic. for
C24H17F4N303S: C, 57.25; H, 3.4; N, 8.34. Found: C, 56.84; H, 3.52; N, 7.91.
Example 147
2-(a-Methyl-3.5-difluorobenzyl)-4-(4-fluorophenyl)-5-[4-(aminosulfonyl)phenylt
3 (2H)-pyridazinone
The title compound was prepared according to the method of Example 127,
substituting 3,5-difluoro-a-methylbenzyl chloride in place of 3,4-
difluorobenzyl
bromide (yield: 80 mg, 45%). M.p. 139-141 C. 1 H NMR (300 MHz, CDC13) S 1.83
(d, J = 6 Hz, 3H), 4.79 (s, 2H), 6.32 (q, J = 7 Hz, 1 H), 6.84 (m, 1 H), 6.97
(t, J= 9 Hz,
2H), 7.02 (dd, J = 6 Hz, 1.5 Hz, 2H), 7.18 (dd, J = 9 Hz, 6 Hz, 2H), 7.28 (d,
J = 9 Hz,
2H), 7.85 (s, 1 H), 7.9 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 486 (M+H)+. Anal.
calc.
for C24H18F3N303S: C, 59.37; H, 3.73; N, 8.65. Found: C, 59.00; H, 3.70; N,
8.35.
Example 148
2-(a-Methvl-3.4-difluorobenzvl)-4-(4-fluorophenYl)-5-[4-(aminosulfonyl)phenyll-
3(2H)-Rvridazinone
The title compound was prepared according to the method of Example 127,
substituting 3,4-difluoro-a-methylbenzyl chloride in place of 3,4-
difluorobenzyl
bromide (yield: 200 mg, 58%). M.p. 214-215 C. 1 H NMR (300 MHz, CDCI3) 8
1.82 (d, J = 6 Hz, 3H), 4.7 (s, 2H), 6.35 (q, J = 7 Hz, 1 H), 6.96 (t, J = 9
Hz, 2H), 7.16
(m, 4H), 7.28 (d, J = 9 Hz, 2H), 7.37 (m, 1 H), 7.84 (d, J = 9 Hz, 2H), 7.90
(s, 1 H). MS
(DCI-NH3) m/z 486 (M+H)+. Anal. caic, for C24H18F3N303S: C, 59.37; H, 3.73; N,
8.65. Found: C, 59.13; H, 3.73; N, 8.54.
-110-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 149
2-(3-Fluorobenzyl)-4-(4-fluorootlen)tl)-5-[4-(aminosulfonyj)ohenyl]-3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 127,
substituting 3-fluorobenzyl bromide in place of 3,4-difluorobenzyl bromide
(yield:
160 mg, 61 %). M.p. 220-222 C. 1 H NMR (300 MHz, DMSO-d6) 5 5.37 (s, 2H),
7.12 (t, J = 9 Hz, 2H), 7.22 (m, 5H), 7.39 (m, 5H), 7.73 (d, J = 9 Hz, 2H),
8.11 (s, 1 H).
MS (DCI-NH3) m/z 454 (M+H)+. Anal. calc. for C23H17F2N303S: C, 60.92; H,
3.77; N, 9.26. Found: C, 61.06; H, 4.22; N, 8.88.
Example 150
2-(4-Fluorobenzyl)-4-(4-fluoronhenyl).5-[4-(aminosulfonY1)phenylj-3(2H)-
p,yridazinone
The title compound was prepared according to the method of Example 127,
substituting 4-fluorobenzyl bromide in place of 3,4-difluorobenzyl bromide
(yield:
85 mg, 34%). M.p. 237-239 C. 1 H NMR (300 MHz, DMSO-d6) S 5.32 (s, 2H), 7.12
(t, J = 9 Hz, 2H), 7.22 (m, 4H), 7.38 (m, 4H), 7.47 (dd, J = 9 Hz, 6 Hz, 2H),
7.72 (d, J
= 9 Hz, 2H), 8.10 (s, 1 H). MS (DCI-NH3) m/z 454 (M+H)+. Anal. caic. for
C23H17F2N303S: C, 60.92; H, 3.77; N, 9.26. Found: C, 60.61; H, 3.96; N, 8.74.
Example 151
luorop henyl)-5-[4-(aminosulfonyl)12henyll-3(2 HL-
Ryridazinone
The title compound was prepared according to the method of Example 127,
substituting 2,4,6-trifluorobenzyl bromide in place of 3,4-difluorobenzyl
bromide
(yield: 255 mg, 73%). M.p. 201-203 C. 1 H NMR (300 MHz, DMSO-d6) S 5.38 (s,
2H), 7.13 (t, J = 9 Hz, 2H), 7.23 (m, 4H), 7.38 (d, J = 9 Hz, 2H), 7.42 (s,
2H), 7.70 (d,
J = 9 Hz, 2H), 8.08 (s, 1 H). MS (DCI-NH3) m/z 490 (M+H)+. Anal. caic. for
C23H1 5F4N303S: C, 56.44; H, 3.08; N, 8.58. Found: C, 56.31; H, 3.09; N, 8.40.
Example 152
2-(2.4.5-Triftuorobenzyl)-4-(4-fluoronhenyl)~44-(aminosulfonyl)phenY11-3(2 IJL-
Ryridazinone
The title compound was prepared according to the method of Example 127,
substituting 2,4,5-trifluorobenzyl bromide in place of 3,4-difluorobenzyl
bromide
(yield: 180 mg, 49%). M.p. 236-238 C. 1 H NMR (300 MHz, DMSO-d6) S 5.35 (s,
-111-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
2H), 7.13 (t, J = 9 Hz, 2H), 7.23 (dd, J = 9 Hz, 6 Hz, 2H), 7.39 (d, J = 9 Hz,
2H), 7.41
(s, 2H), 7.6 (m, 2H), 7.72 (d, J = 9 Hz, 2H), 8.11 (s, 1 H). MS (DCI-NH3) m/z
490
(M+H)+. Anal. caic. for C23H15F4N303S: C, 56.44; H, 3.08; N, 8.58. Found: C,
56.38; H, 3.28; N, 8.41.
Example 153
2-(2.3.4-Trifluorobenzyl)-4-(4-fluorouhenyl)-5-[4-(aminosulfonyl) henylJ-3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 127,
substituting 2,3,4-trifluorobenzyl bromide in place of 3,4-difluorobenzyl
bromide
(yield: 220 mg, 63%). M.p. 218-220 C. 1 H NMR (300 MHz, DMSO-d6) S 5.40 (s,
2H), 7.13 (t, J = 9 Hz, 2H), 7.22 (dd, J = 9 Hz, 6 Hz, 2H), 7.34 (m, 2H), 7.39
(d, J = 9
Hz, 2H), 7.42 (s, 2H), 7.73 (d, J = 9 Hz, 2H), 8.12 (s, 1 H). MS (DCI-NH3) m/z
490
(M+H)+. Anal. calc. for C23H15F4N303S: C, 56.44; H, 3.08; N, 8.58. Found: C,
56.32; H, 3.24; N, 8.31.
Example 154
2-(4.4.4-Trifiuoro-3-methyl-2E-butenyl)-4-(4-fluoropheny,O-5-[4-(aminosulfona-
phenylj-3 (2 H )-ovridazinone
The title compound was prepared according to the method of Example 123,
substituting 1-bromo-3-methyl-4,4,4-trifluoro-2-butene in place of 1-bromo-3-
methyl-2-butene (yield: 160 mg, 48%). M.p. 155-157 C. 1 H NMR (300 MHz,
CDCI3) S 2.00 (s, 3H), 4.8 (s, 2H), 4.96 (d, J = 7.5 Hz, 2H), 6.33 (m, 1 H),
6.99 (t, J
9 Hz, 2H), 7.19 (dd, J = 9 Hz, 6 Hz, 2H), 7.29 (d, J= 9 Hz, 2H), 7.95 (s, 1
H), 7.97 (d,
J = 9 Hz, 2H). MS (DCI-NH3) m/z 468 (M+H)+. Anal. calc. for C21 H17F4N303S:
C, 53.96; H, 3.66; N, 8.98. Found: C, 53.84; H, 3.51; N, 8.77.
Example 155
2_-(4-Binhenvl)-4-(4-fluoro eny1)_5-[4-(pethy su y1)io ~enyj -3(2 );
pyridazinone
The title compound was prepared according to the method of Example 62
substituting 4-bromobiphenyl in place of 4-iodo-l-fluorobenzene (yield: 0.275
g,
100%). M.p. 249-251 C. 1 H NMR (300 MHz, DMSO d6) 8 3.24 (s, 3H), 7.16 (m,
2H), 7.30 (m, 2H), 7.42 (m, 1 H), 7.48-7.58 (m, 4H), 7.75 (m, 4H), 7.84 (m,
2H), 7.91
(m, 2H), 8.27 (s, 1 H). MS (DCI-NH3) m/z 497 (M+H)+, 514 (M+NH4)+. Anal. calc.
for C23H21 FN2O3S: C, 70.15; H, 4.26; N, 5.64. Found: C, 69.81; H, 4.42; N,
5.41.
-112-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 156
2-(4- B ro moRbSly.l)-4-(4-flu4lophenX1)-t-14.(IDet11lsulf4QX1)1?h8 IlX1)-
3(2H)-
~,yridazinone
The title compound was prepared according to the method of Example 62
substituting 1,4-dibromobenzene in place of 4-iodo-l-fluorobenzene (yield:
0.337
g, 93%). 1 H NMR (300 MHz, DMSO d6) 8 3.24 (s, 3H), 7.14 (m, 2H), 7.28 (m,
2H),
7.64 (m, 2H), 7.75 (m, 2H), 7.90 (m, 2H), 8.25 (s, 1 H). MS (DCI-NH3) m/z 499
(M+H)+, 518 (M+NH4)+. Anal. calc. for C23H16BrFN2O3S-0.75 H20: C, 53.86; H,
3.43; N, 5.46. Found: C, 53.92; H, 3.16; N, 5.34.
Example 157
2-(4-Nitroohenvl)-4-(4-fluoroQhenyl)-5-j4-(methylsulfonyJ)pheayJ -3(2H)-
~yridazinone
The title compound was prepared according to the method of Example 62
substituting 1-iodo-4-nitrobenzene in place of 4-iodo-l-fluorobenzene (yield:
0.45
g, 100%). M.p. 110-116 C. 1 H NMR (300 MHz, DMSO d6) S 3.24 (s, 3H), 7.17 (m,
2H), 7.32 (m, 2H), 7.53 (m, 2H), 7.91 (m, 2H), 8.03 (m, 2H), 8.34 (s, 1 H),
8.40 (m,
2H). MS (DCI-NH3) m/z 466 (M+H)+, 483 (M+NH4)+. Anal. caic. for
C23H16FN305S: C, 59.35; H, 3.46; N, 9.03. Found: C, 59.02; H, 3.62; N, 8.82.
Example 158
2-(4-Phenoxvohenvl)-4-(4-fluoroohenyl)-5-j4-(methylsulfony,j)phenylj-3(2H)-
Ryridazinone
The title compound was prepared according to the method of Exampie 62
substituting 4-bromodiphenylether in place of 4-iodo-l-fluorobenzene (yield:
0.667
g, 22%). M.p. 118-125 C. 1 H NMR (300 MHz, DMSO d6) S 3.24 (s, 3H), 7.12 (m,
5H), 7.15-7.33 (m, 4H), 7.46 (m, 2H), 7.52 (m, 2H), 765 (m, 2H), 7.90 (m, 2H),
8.23
(s, 1 H). MS (DCI-NH3) m/z 513 (M+H)+. Anal. calc. for C25H21 FN2O4S-0.75
H20: C, 66.21; H, 4.31; N, 5.32. Found: C, 65.98; H, 4.25; N, 5.27.
Example 159
2-(4-t-Butvlohenvll-4-(4-fluoroQtignyU-5-(4-(methy sW yj)pherayJ]-3(ZH):
Ryridazinone
The title compound was prepared according to the method of Example 62
substituting 1-bromo-4-t-butyl-benzene in place of 4-iodo-l-fluorobenzene. No
product was observed. The solution was concentrated in vacuo. The resulting
-113-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
solid was dissolved in DMF (5 mL) and Cul (13.3 mg, 0.07 mmol) was added. The
solution was allowed to reflux overnight. Upon completion, the mixture was
poured
into 10% citric acid and extracted with ethyl acetate. The organic layer was
washed
with water, dried over MgSO4 and concentrated in vacuo. The crude solid was
purified using flash chromatography (Si02), eluting with 5% diethyl
ether/CH2CI2
to provide the desired product (yield: 0.292 g, 84%). M.p. 132-136 C. 1 H NMR
(300 MHz, DMSO d6) S 1.34 (s, 9H), 3.24 (s, 3H), 7.14 (m, 2H), 7.29 (m, 2H),
7.54
(m, 6H), 7.90 (m, 2H), 8.23 (s, 1 H). MS (DCI-NH3) m/z 477 (M+H)+, 494
(M+NH4)+.
Anal. calc. for C27H25FN203S: C, 68.05; H, 5.29; N, 5.88. Found: C, 67.94; H,
5.31;N,5.67.
Example 160
2-(4-Chloroohenvl)-4-(4-fluorophenyl)-5-(4-(methy su Y()phenylj-3 H1-
R,yridazinone
The title compound was prepared according to the method of Example 62
substituting 4-bromo-l-chlorobenzene in place of 4-iodo-l-fluorobenzene
(yield:
0.254 g, 83.5%). M.p. 214-216 C. 1 H NMR (300 MHz, DMSO d6) S 3.24 (s, 3H),
7.16 (m, 2H), 7.29 (m, 2H), 7.52 (m, 2H), 7.61 (m, 2H), 7.71 (m, 2H), 7.91 (m,
2H),
8.26 (s, 1 H). MS (DCI-NH3) m/z 455 (M+H)+, 472 (M+NH4)+. Anal. caic. for
C23H16CIFN2O3S: C, 60.73; H, 3.55; N, 6.16. Found: C, 60.45, H, 3.41; N, 6.05.
Example 161
2-(3-Methvlohenvl)-4-(4-fluorophenyl)-5-(4lmethvlsulfonyl)oheny,jJ-3(2H)-
gyridazinone
The title compound was prepared according to the method of Example 62
substituting 3-bromotoluene in place of 4-iodo-l-fluorobenzene (yield: 0.262
g,
83%). M.p. 213-216 C. 1 H NMR (300 MHz, DMSO d6) S 2.39 (s, 3H), 3.24 (s,
3H),
7.14 (m, 2H), 7.28 (m, 3H), 7.43 (m, 3H), 7.53 (m, 2H), 7.80 (m, 2H), 8.22 (s,
1 H).
MS (DCI-NH3) m/z 435 (M+H)+, 452 (M+NH4)+. Anal. caic. for C24Hl9FN2O3S:
C, 66.35; H, 4.41; N, 6.45. Found: C, 66.00, H, 4.16; N, 6.23.
Example 162
2-(3-Vinvlohenvl)-4-(4-fluorophenyl)_5-j4-(met su y1)R11ii,py~l-3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 62
substituting 3-bromostyrene in place of 4-iodo-l-fluorobenzene (yield: 0.202
g,
-114-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
62%). M.p. 182-183 C. 1 H NMR (300 MHz, DMSO d6) S 3.25 (s, 3H), 5.35 (d, J
12 Hz, 1 H), 5.92 (d, J = 15 Hz, 1 H), 6.82 (m, 1 H), 7.15 (m, 2H), 7.30 (m,
2H), 7.50-
7.60 (m, 4H), 7.74 (m, 1 H), 7.91 (m, 2H), 8.24 (s, 1 H). MS (DCI-NH3) m/z 447
(M+H)+, 464 (M+NH4)+. Anal. calc. for C25H1 9FN203S=0.50 H20: C, 65.92; H,
4.42; N, 6.14. Found: C, 65.86; H, 4.40; N, 6.07.
Example 163
2-(2-Formylphenyl)-4-(4-fluorophenvl)-5-[4-(methylsulfonXl)ghenyl]-3(2H)-
pyridazinone
The title was prepared according to the method of Example 62 substituting 2-
bromobenzaidehyde in place of 4-iodo-l-fluorobenzene (yield: 0.196 g, 60%).
M.p.
234-236 C. 1 H NMR (300 MHz, DMSO d6) S 3.24 (s, 3H), 7.15 (m, 2H), 7.27 (m,
2H), 7.54 (m, 2H), 7.64-7.75 (m, 2H), 7.86-7.95 (m, 3H), 8.01 (m, 1 H), 8.29
(s, 1 H),
10.02 (s, 1 H). MS (DCI-NH3) m/z 449 (M+H)+. Anal. calc. for
C24H17FN204S=0.50 H20: C, 63.01; H, 3.96; N, 6.12. Found: 63.04; H, 3.82; N,
5.88.
Example 164
2-(2-Nitroohenvl)-4-(4-fluorochenyl)-5-(4-(methvlsulfonyl) he I, I-3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 62
substituting 1-bromo-2-nitrobenzene in place of 4-iodo-l-fluorobenzene (yield:
0.307 g, 90.8%). M.p. 236-239 C. 1 H NMR (300 MHz, DMSO d6) S 3.24 (s, 3H),
7.12-7.27 (m, 4H), 7.56 (m, 2H), 7.7-8.01 (m, 5H), 8.18 (m, 1 H), 8.35 (s, 1
H). MS
(DCI-NH3) m/z 466 (M+H)+, 483 (M+NH4)+. Anal. caic. for C23H16FN3O5S=0.25
H20: C, 58.78; H, 3.53; N, 8.94. Found: C, 58.63; H, 3.54; N, 8.88.
Example 165
2-(3-Chlorophenyl)-4-(4-fluoro henyl)-5-[4-(methylsulfonyl)phenyll-3(2H)-
R,vridazinone
The title compound was prepared according to the method of Example 62
substituting 1-bromo-3-chlorobenzene in place of 4-iodo-l-fluorobenzene
(yield:
0.255 g, 77%). M.p. 232-235 C. 1 H NMR (300 MHz, DMSO d6) S 3.23 (s, 3H),
7.14 (m, 2H), 7.29 (m, 2H), 7.49-7.58 (m, 4H), 7.66 (m, 1 H), 7.79 (m, 1 H),
7.90 (m,
2H), 8.25 (s, 1 H). MS (DCI-NH3) m/z 455 (M+H)+, 472 (M+NH4)+. Anal. caic. for
C23H16CIFN2O3S: C, 60.73; H, 3.55; N, 6.16. Found: C, 60.40; H, 3.43; N, 5.98.
-115-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 166
? -(romoRhenxl)-4-(4-fluoroRhenvl, )-5-[4-(methyisulfoO,Yl)oheny,j]-3(2H1
Rvridazi .none
The title compound was prepared according to the method of Example 62
substituting 1,3 dibromobenzene in place of 4-iodo-l-fluorobenzene (yield:
0.216
g, 60%). M.p. 210-212 C. 1 H NMR (300 MHz, DMSO d6) 8 3.23 (s, 3H), 7.15 (m,
2H), 7.29 (m, 2H), 7.48-7.55 (m, 3H), 7.69 (m, 2H), 7.90 (m, 3H), 8.26 (s, 1
H). MS
(DCI-NH3) m/z 499 (M+H)+, 519 (M+NH4)+. Anal. calc. for C23H116BrFN2O3S: C,
55.32; H, 3.23; N, 5.61. Found: C, 55.12; H, 3.12; N, 5.51.
Example 167
2;(4-Cyanophenyl)-4-(4-fluoroohenyl)-5-[4-(methvlsulfonyj)Qheny-(J-3(2H);
R,yridazinone
The title compound was prepared according to the method of Example 62
substituting 4-bromobenzonitrile in place of 4-iodo-l-fluorobenzene (yield:
0.349 g,
100%). M.p. 273-278 C. 1 H NMR (300 MHz, DMSO d6) S 3.24 (s, 3H), 7.11-7.21
(m, 2H), 7.25-7.35 (m, 2H), 7.52 (m, 2H), 7.88-7.96 (m, 4H), 8.04 (m, 2H),
8.31 (s,
1 H). MS (DCI-NH3) m/z 445 (M+H)+. Anal. calc. for C24H16FN303S: C, 64.71; H,
3.62; N, 9.43. Found: C, 64.50; H, 3.53; N, 9.35.
Example 168
2-(5-Methyl-2-thienyl))-4-(4-fluoro henyl)-5-[4-(me ylsulfonyl)Rhenxll-3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 62
substituting 2-bromo-5-methylthiophene in place of 4-iodo-l-fluorobenzene
(yield:
0.200 g, 62%). M.p. 219-224 C. 1 H NMR (300 MHz, DMSO d6) S 2.45 (s, 3H),
3.23 (s, 3H), 6.80 (m, 1 H), 7.17 (m, 2H), 7.29 (m, 2H), 7.52 (m, 3H), 7.89
(m, 2H),
8.33 (s, 1 H). MS (DCI-NH3) m/z 441 (M+H)+, 458 (M+NH4)+. Anal. caic. for
C22H17FN203S2: C, 59.99; H, 3.89; N, 6.36. Found: C, 59.90; H, 3.91; N, 6.26.
Example 169
2-(3-BiRhenyl)-4-(4-fluoro henY1)_5-[4-(methyl ulfonyj)nhenyl]-3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 62
substituting 3-bromobiphenyl in place of 4-iodo-l-fluorobenzene (yield: 0.28
g,
78%). M.p. 126-134 C. 1 H NMR (300 MHz, DMSO d6) 5 3.24 (s, 3H), 7.15 (m,
-116-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
2H), 7.31 (m, 2H), 7.37-7.45 (m, 1 H), 7.51 (m, 4H), 7.64 (m, 2H), 7.68-7.79
(m, 3H),
7.92 (m, 3H), 8.27 (s, 1 H). MS (DCI-NH3) m/z 497 (M+H)+, 514 (M+NH4)+. Anal.
caic. for C29H21 FN2O3S: C, 70.15; H, 4.26; N, 5.64. Found: C, 69.91; H, 4.33;
N,
5.74.
Example 170
2-(3.5-Dimethylphepx()-4-(4-fluoroohenyl)-5-[4-(meth Isy ulfony,j)uhenylj-
3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 62
substituting 5-bromo-m-xylene in place of 4-iodo-l-fluorobenzene (yield: 0.152
g,
46.5%). M.p. 130-134 C. 1 H NMR (300 MHz, DMSO d6) S 2.34 (s, 6H), 3.23 (s,
3H), 7.07-7.12 (m, 2H), 7.15 (m, 1 H), 7.21-7.32 (m, 4H), 7.52 (m, 2H), 7.90
(m, 2H),
8.29 (s, 1 H). MS (DCI-NH3) m/z 449 (M+H)+, 466 (M+NH4)+. Anal. calc. for
C25H21 FN2O3S: C, 66.95; H, 4.72; N, 6.25. Found: C, 66.81; H, 4.57; N, 6.07.
Example 171
2-(3.4-DifIuoroohenyl)-4-(4-fiuorobenzY[)-5-[4-(methy Su Ifo yj)o, heny1j-3 (2
H) -
Ryridazinone
4-(4-Fluorophenyimethyl)-5-[4-(methyisulfonyl)phenyl]-3(2H)-pyridazinone
was prepared according to the method of Example 11, starting with 2-benzyl-4-
(4-
fiuorophenyimethyl)-5-[4-(methyisulfonyl)phenyl]-3(2H)-pyridazinone in place
of 2-
benzyl-4-(4-fluorophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone
(yield:
0.3319 g, 83%).
The title compound was prepared according to the method of Example 62
substituting 4-(4-fluorophenylmethyl)-5-[4-(methyisulfonyl)phenyl]-3(2H)-
pyridazinone in place of 4-(4-fluorophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone and substituting 1-bromo-3,4-difluorobenzene in place of 4-iodo-l-
fiuorobenzene (yield: 0.085 g, 54%). M.p. 157-159 C. 1 H NMR (300 MHz, DMSO
d6) 5 3.30 (s, 3H), 3.88 (bs, 2H), 7.04 (m, 4H), 7.49-7.66 (m, 2H), 7.70 (m,
2H), 7.81
(m, 1 H), 8.12 (s, 1 H). MS (DCI-NH3) m/z 471 (M+H)+, 488 (M+NH4)+. Anal.
caic.
for C24H17F3N203S=0.25 H20: C, 60.69; H, 3.71; N, 5.84. Found: C, 6.39; H,
3.76; N, 5.81.
-117-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 172
2-(3-Chioro-4-fluoroohenyl)-4-(4-fluorobenzyl)-5-[4-(methylsulfo I)ohe 113(2H)-
~yridazinone
The title compound was prepared according to the method of Example 62
substituting 4-(4-fluorophenylmethyl)-5-[4-(methylsulfonyl)phenylJ-3(2H)-
pyridazinone in place of 4-(4-fluorophenyl)-5-[4-(methylsutfonyl)phenyll-3(2H)-
pyridazinone and substituting 4-bromo-2-chloro-l-fluorobenzene in place of 4-
iodo-l-fluorobenzene (yield: 0.110 g, 74%). M.p. 153-156 C. 1 H NMR (300 MHz,
DMSO d6) 5 3.30 (s, 3H), 3.89 (bs, 2H), 7.02-7.07 (m, 4H), 7.59 (m, 1 H), 7.65-
7.72
(m, 4H), 8.07 (m, 2H), 8.12 (s, 1 H). MS (DCI-NH3) m/z 487 (M+H)+, 504
(M+NH4)+.
Anal. calc. for C24H17CIF2N203S=0.25 H20: C, 58.65; H, 3.58; N, 5.64. Found:
C,
58.41; H, 3.56; N, 5.36.
Example 173
2-(2-Thienvl)-4-(4-fluorophen,y,j)-5-[4-(methylsulfonyj)o_henY11.3 (2 H)-
pvridazinone
The title compound was prepared according to the method of Example 62
substituting 2-bromothiophene in place of 1-bromo-4-fluorobenzene (yield: 98
mg,
40%). M.p. 215-217 C. 1 H NMR (300 MHz, DMSO-d6) S 3.25 (s, 3H), 7.18 (m, J
9 Hz, 3H), 7.29 (m, 2H), 7.42 (d, 2H), 7.75 (d, 1 H), 7.93 (d, J = 9 Hz), 8.4
(s, 1 H).
MS (DCI-NH3) m/z 427 (M+H)+, 444 (M+NH4)+. Anal. caic. for C21 H15FN203S2:
C, 59.14; H, 3.54; N, 6.57.
Example 174
2-(4-Trifluoromethyl enyl)-4-(4-fluorophenyl)-5-j4-(methy au )n henyll-3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 62
substituting 1-bromo-4-trifluoromethylbenzene in place of 1-bromo-4-
fluorobenzene (yield: 185 mg, 64%). M.p. 171-173 C. 1 H NMR (300 MHz, DMSO-
d6) S 3.25 (s, 3H), 7.18 (t, 2H), 7.29 (m, 2H), 7.52 (d, J = 9 Hz 2H), 7.91
(d, J = 9 Hz,
2H), 7.93 (s, 4H), 8.32 (s, 1 H). MS (DCI-NH3) m/z 489 (M+H)+, 506 (M+NH4)+.
Anal. caic. for C24H16F4N203S: C, 59.02; H, 3.3; N, 5.74. Found: C, 58.75; H,
3.35; N, 5.69.
-118-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 175
? (4-(1-Pyrroyl)ohenyll-4-(4-fluorophenyl)-5-[4-(methylsulfonyl)phgnyl]-3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 62
substituting 1-(4-iodophenyl)pyrrole in place of 1-bromo-4-fluorobenzene
(yield:
140 mg, 50%). M.p. 229-231 C. 1 H NMR (300 MHz, DMSO-d6) S 3.25 (s, 3H), 6.3
(t, 2H), 7.18 (t, 2H), 7.29 (m, 2H), 7.46 (t, 2H) 7.53 (d, J = 9 Hz 2H), 7,75
(s, 4H), 7.91
(d, J= 9 Hz, 2H), 8.27 (s, 1 H). MS (DCI-NH3) m/z 486 (M+H)+, 504 (M+NH4)+.
Anal. calc. for C27H20FN303S-0.5 H20: C, 66.79; H, 4.15; N, 8.65. Found: C,
65.21; H, 4.29; N, 8.12.
Example 176
2-(5-Chloro-2-thienyl)-4-(4-fluorophenyl)-5-j4-(methyisulfonyl) henyll,_3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 62
substituting 1-bromo-5-chlorothiophene in place of 1-bromo-4-fluorobenzene
(yield: 225 mg, 93%). M.p. 190-192 C. 1 H NMR (300 MHz, DMSO-d6) S 2.38 (s,
3H), S 3.25 (s, 3H), 7.15 (t, 2H), 7.29 (m, 4H), 7.5 (D, 4H) 7.91 (d, J = 9
Hz, 2H), 8.21
(s, 1 H). MS (DCI-NH3) m/z 435 (M+H)+, 452 (M+NH4)+. Anal. calc. for C24H19F
N203S: C, 66.35; H, 4.41; N, 6.45. Found: C, 66.15; H, 4.37; N, 6.3.
Example 177
2-(4-Methyinhenv{)-4-(4-fluoronhenyl)-5-[4-(methylsulfonyl)phenxl]-3(2H,-,)-
pyridazinone
The title compound was prepared according to the method of Example 62
substituting 1-bromo-4-methylbenzene in place of 1-bromo-4-fluorobenzene
(yield:
79 mg, 31 %). M.p. 190-192 C. 1 H NMR (300 MHz, DMSO-d6) 8 2.38 (s, 3H), S
3.25 (s, 3H), 7.15 (t, 2H), 7.29 (m, 4H), 7.5 (D, 4H) 7.91 (d, J = 9 Hz, 2H),
8.21 (s,
1 H). MS (DCI-NH3) m/z 435 (M+H)+, 452 (M+NH4)+. Anal. caic. for C24H1 9F
N203S: C, 66.35; H, 4.41; N, 6.45. Found: C, 66.15; H, 4.37; N, 6.3.
-119-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 178
2-(4-F furoRl]eny1)-4-(2-ethyl-1-hexyloxy)-5-[4-(methylsulfonyl)12henyl]-3(2H)-
pyridazinone
To a solution of 2-ethyl-1 -hexanol (65 mg, 0.5 mmol) in THF (15 mL) at room
temperature was added NaH (60% oil suspension) (20 mg, 0.5 mmol) and after 10
minutes 2-(4-fluorophenyl)-4-tosyloxy-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone (193 mg, 0.5 mmol) was added. The resulting mixture was stirred
at
room temperature for the next 2 hours. The mixture was quenched with 10%
citric
acid and extracted with ethyl acetate. The extract was washed with water,
brine,
dried with MgSO4, and purified by chromatography (silica gel, 2:1 hexanes-
ethyl
acetate) to provide the desired product (yield: 140 mg, 60%). M.p. 120-122 C.
1 H
NMR (300 MHz, DMSO-d6) S 0.75 (m, 6H), 1.1 (m, 6H), 1.20 (quintet, J = 7 Hz,
2H),
1.44 (m, 1 H), 3.27 (s, 3H), 4.30 (d, J = 6 Hz, 2H), 7.37 (t, J = 9 Hz, 2H),
7.65 (m, 2H),
7.89 (d, J = 9 Hz, 2H), 8.06 (d, J = 9 Hz, 2H), 8.18 (s, 1 H). MS (APCI+) m/z
473
(M+H)+; (APCI-) m/z 507 (M+CI)-. Anal. calc. for C25H29FN2O4S=0.5 H20: C,
62.35; H, 6.27; N, 5.87. Found: C, 62.22; H, 6.14; N, 6.22.
Example 179
2-(3-Thienyl)-4-(4-fluoropheIIy,j)-5-[4-(methylsulfony,l)phgpyl]-3(2H)-
ovridazin ne
The title compound was prepared according to the method of Example 62
substituting 3-bromothiophene in place of 1-bromo-4-fluorobenzene (yield: 225
mg,
93%). M.p. 200-202 C. 1 H NMR (300 MHz, DMSO-d6) 8 3.25 (s, 3H), 7.15 (t,
2H),
7.29 (m, 2H), 7.5 (d, J = 9 Hz, 2H), 7.6 (M, 1 H) 7.66 (dd, 1 H), 7.91 (d, J =
9 Hz, 2H),
8.13 (dd, 1 H), 8.25 (s, 1 H). MS (DCI-NH3) m/z 427 (M+H)+, 444 (M+NH4)+.
Anal.
caic. for C21 H15FN203S2: C, 55.07; H, 4.07; N, 6.11. Found: C, 54.63; H,
3.47; N,
6.01.
Example 180
2-(3.5-Difluorophenyl)-4-(4-fluoro henxl)- -[4-(methylsulfonyl)ohenvll-3(2H1-
pyridazinone
The title compound was prepared according to the method of Example 62
substituting 3,5-difluorobromobenzene in place of 1-bromo-4-fiuorobenzene
(yield:
-120-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
250 mg, 96%). M.p. 166-168 C. 1 H NMR (300 MHz, DMSO-d6) 8 3.25 (s, 3H), S
7.15 (t, 2H), 7.27 (m, 2H), 7.4 (m, 1 H), 7.41 (m, 2H), 7.51 (d, J = 9 Hz,
4H), 7.9 (d, J
9 Hz, 2H), 8.3 (s, 1 H). MS (DCI-NH3) m/z 457 (M+H)+, 474 (M+NH4)+. Anal.
calc.
for C23H1 5F3N203S: C, 60.13; H, 3.31; N, 6.14. Found: C, 60.49; H, 3.31; N,
6.03.
Example 181
2-(2.4-Difluorophenvl)-4-(4-ftuorophenyl)-5-j4-(methvlsulfonyl) henyll-3(2H)-
~,vridazinone
The title compound was prepared according to the method of Example 62
substituting 2,4-difluorobromobenzene in place of 1-bromo-4-fluorobenzene
(yield:
40 mg, 15%). M.p. 245-247 C. 1 H NMR (300 MHz, DMSO-d6) S 3.23 (s, 3H), 8
7.15 (t, 2H), 7.3 (t, 2H), 7.54 (m, 2H), 7.57 (m, 2H), 7.75 (m, 1 H), 7.9 (d,
J = 9 Hz,
2H), 8.27 (s, 1 H). MS (DCI-NH3) m/z 457 (M+H)+, 474 (M+NH4)+. Anal. calc. for
C28H15F3N203S: C, 60.52; H, 3.31; N, 6.03.
Example 182
2-(3.4-Difluoro henyl)-4-(4-fluoro~heny()-5-j4-(methylsulfonvl)~he6k1,3( -I -
R,yridazinone
The title compound was prepared according to the method of Example 62
substituting 3,4-difluorobromobenzene in place of 1-bromo-4-fluorobenzene
(yield:
170 mg, 70%). M.p. 109-110 C. 1 H NMR (300 MHz, DMSO-d6) S 3.23 (s, 3H), 5
7.15 (t, 2H), 7.3 (t, 2H), 7.25 (m, 2H), 7.59 (m, 4H), 7.83 (m, 1 H), 7.9 (d,
J = 9 Hz,
2H), 8.27 (s, 1 H). MS (DCI-NH3) m/z 457 (M+H)+, 474 (M+NH4)+. Anal. calc. for
C23H15F3N303S: C, 60.52; H, 3.31; N, 6.14. Found 60.60; H, 3.48; N, 5.89
Example 183
2-(3-Furyl)-4-(4-fluoro h~enyl)-5-j4-(methy suIfnnyl)nhenyjl-3(2H)-
Rvridazinone
The title compound was prepared according to the method of Example 62
substituting 3-bromofuran in place of 1-bromo-4-fluorobenzene (yield: 175 mg,
73%). M.p. 239-242 C. 1 H NMR (300 MHz, DMSO-d6) S 3.25 (s, 3H), 7.09 (d, 1
H),
7.15 (t, 2H), 7.29 (m, 2H), 7.5 (d, J = 9 Hz 2H), 7.8 (t, 1 H) 7.91 (d, J = 9
Hz, 2H), 8.3
(s 1 H), 8.58 (s, 1 H). MS (DCI-NH3) m/z 411 (M+H)+, 428 (M+NH4)+. Anal. calc.
for
C21 H15F N204S=0.5 H20: C, 61.46; H, 3.68; N, 6.83. Found: C, 59.91; H, 3.54;
N,
6.54.
-121-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 184
2-(3-Ftuoro-4-methoxyohenyl)-4-(4-fluorophenyl)-5-[4-(methylsulfonyl)oh, envll-
3,(2H)-Ryridazinone
The title compound was prepared according to the method of Example 62
substituting 3-fiuoro-4-methoxybromobenzene in place of 1-bromo-4-
ftuorobenzene (yield: 230 mg, 85%). M.p. 97-101 C. 1 H NMR (300 MHz, DMSO-
d6) S 3.25 (s, 3H), 3.9 (s, 3H), 7.16 (d, 1 H), 7.29 (m, 3H), 7.5 (m, 4H),
7.91 (d, J = 9
Hz, 2H), 8.23 (s 1 H). MS (DCI-NH3) m/z 469 (M+H)+, 491 (M+NH4)+. Anal. catc.
for C24H18F2N2O4S-0.5 H20: C, 61.53; H, 3.87; N, 5.98. Found: C, 61.18; H,
4.01;N,5.58.
Example 185
2-(2-Fluoro enyl)-4-(4-fluoro enyl)-5-j4-(methyJsutfonyj)ohenvll-3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 62
substituting 2-fluorobromobenzene in place of 1-bromo-4-ftuorobenzene (yield:
195 mg, 75%). M.p. 96-103 C. 1 H NMR (300 MHz, DMSO-d6) fi 3.23 (s, 3H), S
7.15 (t, 2H), 7.3 (m, 3H), 7.55 (m, 5H), 7.9 (d, J = 9 Hz, 2H), 8.27 (s, 1 H).
MS (ESI)
mlz 437 (M-H)+). Anal. calc. for C23H16F2N203S: C, 63.01; H, 3.68; N, 6.39.
Found, C, 62.91; H, 4.06; N, 5.99.
Example 186
2-[4-(Aminosulfonyl)phenyl]-4-(4-ftuorophenyl)-5-(4-(m t ylsulfonyl)pheny(j-
3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 62
substituting 4-aminosulfonyl-l-bromobenzene in place of 1-bromo-4-
fluorobenzene. M.p. 213-216 C. 1 H NMR (300 MHz, DMSO-d6) 8 3.25 (s, 3H),
7.15 (t, 2H), 7.29 (m, 2H), 7.53 (s, 2H) 7.55 (s, 1 H), 7.7 (dd, 2H) 7.91 (t,
4H), 7.98 (d,
2H), 8.3 (s, 1 H). MS (DCI-NH3) m/z 499 (M+H)+, 517 (M+NH4)+. Anal. calc. for
C23H18FN305S2-0.5 H20: C, 55.30; H, 3.63; N, 8.41. Found: C, 54.4; H, 3.79; N,
7.78.
-122-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 187
2-(3-Chloro-4-fluoroohenyl)-4-(4-fluorophenyl)-5-[4-(methxlsulfo yI)ohenvl]-
3(2H)-
gyridazinone
The title compound was prepared according to the method of Example 62
substituting 3-chloro-4-fluoro-l-bromobenzene in place of 1-bromo-4-
fluorobenzene (yield: 320 mg, 78%). M.p. 155-157 C. 1 H NMR (300 MHz, DMSO-
d6) 8 3.23 (s, 3H), 8 7.15 (t, 2H), 7.3 (t, 2H), 7.25 (m, 2H), 7.53 (d, J = 9
Hz, 2H),
7.59 (t, 1 H), 7.73 (m, 1 H), 7.9 (d, J = 9 Hz, 2H) 7.96 (m, 1 H), 8.27 (s, 1
H). MS (DCI-
NH3) m/z 473 (M+H)+, 490 (M+NH4)+. Anal. calc. for C23H15CIF2N203S: C,
58.42; H, 3.2; N, 5.92. Found 58.23; H, 2.87; N, 5.70
Example 188
2-(3.5-Dichlorophenyl)-4-(4-fluoro2henyl)-5-[4-(methylsulfonyl)oheny,ll-3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 62
substituting 3,5-dichlorobenzene in place of 1-bromo-4-fluorobenzene (yield:
360
mg, 78%). M.p. 289-294 C. 1 H NMR (300 MHz, DMSO-d6) S 3.25 (s, 3H), S 7.15
(t, 2H), 7.27 (m, 2H), 7.51 (d, J = 9 Hz, 4H), 7.75 (t, 1 H), 7.83 (d, 2H),
7.9 (d, J = 9
Hz, 2H), 8.3 (s, 1 H). MS (DCI-NH3) m/z 490 (M+H)+, 507 (M+NH4)+. Anal. caic.
for
C23H15C12FN2O3S-0.5 H20: C, 56.45; H, 3.09; N, 5.72. Found: C, 55.36; H, 3.00;
N, 5.50.
Example 189
2-(4-Fluoro-3-methvl henyl)-4-(4-fluoroohenyl)-5 j4-(methylsulfonyJ)nhenyl]-
3(2H)-
Rvridazinone
The title compound was prepared according to the method of Example 62
substituting 1-bromo-4-fluoro-3-methylbenzene in place of 1-bromo-4-
fluorobenzene (yieid: 275 mg, 71 %). M.p. 168-170 C. 1 H NMR (300 MHz, DMSO-
d6) S 2.3 (s, 3H), S 3.25 (s, 3H), 7.15 (t, 2H), 7.3 (m, 3H), 7.56 (m, 4H),
7.9 (d, 2H),
8.23 (s, 2H). MS (DCI-NH3) m/z 453 (M+H)+, 471 (M+NH4)+. Anal. calc. for
C24H1 8F2N203S: C, 63.71; H, 4.01; N, 6.01. Found: C, 63.53; H, 4.06; N, 5.92.
-123-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 190
2-(4-Chloro-3-fluoro enyl)-4-(4-fluoronhenyl)-5-j4-(methylsulfonyi)ohenyl)-
3(2H)-
~,vridazinone:
The title compound was prepared according to the method of Example 62
substituting 4-bromo-l-chloro-2-fiuorobenzene in place of 1-bromo-4-
fluorobenzene (yield: 220 mg, 80%). M.p. 102-110 C. 1 H NMR (300 MHz, DMSO-
d6) S 3.23 (s, 3H), 7.11-7.19 (m, 2H), 7.25-7.32 (m, 2H), 7.51 (d, J = 5.6 Hz,
2H),
7.58-7.64 (m, 1 H), 7.75-7.87 (m, 2H), 7.91 (d, J = 5.6 Hz, 2H), 8.28 (s, 1
H). MS
(APCI+) m/z 473 (M+H)+.
Example 191
2-(4-Chloro-2-fluoroohenyl)-4-(4-fluorophep,y~,)--5-[4-(methylsulfonyl)ohenyjj
3(2H);
Ryridazinone:
The title compound was prepared according to the method of Example 62
substituting 1-bromo-4-chloro-2-fiuorobenzene in place of 1-bromo-4-
fluorobenzene (yield: 65 mg 24%). M.p. 250-260 C. 1 H NMR (300 MHz, DMSO-
d6) 5 3.21 (s, 3H), 7.12-7.19 (m, 2H), 7.25-7.32 (m, 2H), 7.49-7.58 (m, 3H),
7.68-
7.78 (m, 2H), 7.91 (d, J = 8.7 Hz, 2H), 8.29 (s, 1 H). MS (APCI+) m/z 473
(M+H)+.
Anal. calc. for C23H15CIF2N203S: C, 58.41; H, 3.19; N, 5.92. Found: C, 58.69;
H,
3.45; N, 5.78.
Example 192
2-(1-Adamantvloxvcarbonvl)-4-(4-fluoro henyl)~[4-(methylsuifonyl)phenY1,J-
3(2H)-
Ryridazinone
A solution of 4-(4-fluorophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone, prepared according to the procedure of Example 11 (200 mg, 0.58
mmol) in CH2CI2 (8 ml) was prepared and stirred. 1-Adamantylfluoroformate (172
mg, 0.87 mmol), dimethylaminopyridine (14 mg, 0.011 mmol) and triethylamine
(0.12 ml, 0.87 mmol) were added. The reaction mixture was stirred at room
temperature overnight. The reaction mixture was diluted with CH2CI2 (50 ml)
and
washed with 10% citric acid (50 ml), brine (50 ml) and dried over MgSO4, and
concentrated in vacuo. The resulting crude residue was purified using flash
chromatography (Si02, eluting with 15:1 CH2CI2:diethyl ether) to provide the
desired product (yield: 55 mg, 18%). 1 H NMR (300 MHz, DMSO-d6) b 1.66 (bs,
6H), 2.25 (bd, 10H), 3.21 (s, 3H), 7.15 (t, 2H), 7.24 (m, 2H), 7.6 (dd, 2H),
7.88 (d, J
-124-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
9 Hz, 2H), 8.15 (s, 1 H). MS (ESI) m/z 521 (M-H)+. Anal. calc. for C21 H15F
N203S2: C, 64.35; H, 5.20; N, 5.36.
Example 193
? (2.2.2-Trifluoroethyl)-4-(4-fluorobenzyl)-5-[4-(methylsulfon}i)Rhenyl)-3(2H)-
pyridazinone
193A. 2-(2.2.2-Trifluoroethyl)-4.5-dichloro-3(2H)-Ryridazinone
2,2,2-Trifluoroethylhydrazine (70% solution in water, 35.0 g, 0.307 mol) was
treated with mucochloric acid (51.88 g, 0.307 mol) in ethanol (300 mL) and
refluxed
for 5 hours. The solvent was concentrated in vacuo. The crystals obtained were
washed with water and air dried (yield: 50 g; 67.5%). 1 H NMR (300 MHz, CDCI3)
d
4.8 (q, J = 9 Hz, 2H), 7.85 (s, 1 H). MS (DCI-NH3) m/z 264 (M+NH4)+.
193B. 2-(2.2.2-Trifluoroethyl)-4-chloro-5-hydroxy-3(2H)-pyridazinone
2-(2,2,2-Trifluoroethyl)-4,5-dichloro-3(2H)-pyridazinone (15.0 m 60.7 mmol),
and potassium carbonate (10 g, 72.4 mmol.) were mixed with water (500 mL) and
stirred at reflux for 6 hours. TLC (1:1:2 CH2CI2/hexanes/ethyl acetate)
indicated
that all starting material was consumed.) The reaction mixture was cooled to
room
temperature. The pH of the reaction mixture was adjusted to about 4 with
hydrochloric acid (15%). The product was extracted with ethyl acetate (700
mL).
The organic phase was washed with brine, dried over anhydrous MgSO4 and
filtered. The filtrate was concentrated under reduced pressure. The hydroxy
compound was obtained as a light brown solid (yield: 13.1 g, 94%). 1 H NMR
(300
MHz, DMSO-d6) S 4.92 (q, J = 9 Hz, 2H), 7.9 (s, 1 H). MS (DCI-NH3) m/z 229
(M+H)+.
193C. 212.2.2-Trifluoroethyl)-4-chloro-5-(trifluoromethXl u_ Ifonyjoxy)-3(2H)_
R,y,ridazinone
Anhydrous Na2CO3 (9.04 m, 85.32 mmol) was placed in a 500 mL round
bottom flask and anhydrous CH2CL2 (200 mL) was added. The reaction mixture
was cooled to 0 C under N2. The halohydroxy pyridazinone prepared in Example
193B was dissolved in CH2CL2 (100 mL) and added slowly to the flask and
stirred
overnight. The reaction slowly warmed to room temperature. (TLC (2: 1
hexanes/ethyl acetate) indicated completion of the reaction.) The reaction was
quenched with H20. The organic phase containing the product was separated,
washed with brine and dried over MgSO4. The resulting filtrate was
concentrated
under reduced pressure. The crude product viras isolated as deep red-brown
-125-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
residue. Purification using a silica gel column (30:70 ethyl acetate/pentanes)
provided the title compound as a dark, reddish residue (14.3 m, 70%). 1 H NMR
(300 MHz, CDCI3) S 4.85 (q, J = 9 Hz, 2H), 7.9 (s, 1 H). MS (DCI-NH3) m/z 378
(M+NH4)+.
193D. 2-(2.2.2-Trifluoroethvl)-4-chloro-5-[4-(met )phenY11-3(2H)-
pyridazinone
A solution of the triflate prepared in Example 193C (1.56 g 4.3 mmol), 4-
(methylthio)phenylboronic acid (870 mg, 5.16 mmol),
tetrakis(triphenylphosphine)-
palladium(0) (250 mg, 5% mmol) and triethylamine (1.44 ml, 10.32 mmol) in
toluene was heated at reflux for 1 hour. The mixture was partitioned between
ethyl
acetate and water. The ethyl acetate layer was washed with water, then brine,
followed by drying over MgSO4 and fittration. The filtrate was concentrated in
vacuo. The residue was purified by column chromatography (silica gel, 92:8
hexanes/ethyl acetate) to provide the coupled intermediate as a pale, greenish-
yellow solid (yield: 500 mg, 35%). M.p. 130-139 C. 1H NMR (300 MHz, CDCI3) 5
2.55 (s, 3H), 4.87 (q, J = 9 Hz, 2H), 7.37 (d, J = 9 Hz, 2H), 7.48 (d, J = 9
Hz, 2H),
7.82 (s, 1 H). MS (DCI-NH3) m/z 335 (M+H)+.
193 E. 2-(2.2.2-Trifluoroethyl)-4-ch lo ro-5-[4-(methylsulfonyl)ph enyl]-3 (2
H)-
pyridazinone
The title compound was prepared according to the method of Example 10,
substituting the coupled intermediate prepared in Example 193D in place of 2-
benzyl-4-(4-fluorophenyl)-5-[4-(methylthio)phenyl]-3(2H)-pyridazinone (yield:
440
mg, 81 %). M.p. 221-222 C. 1 H NMR (300 MHz, DMSO-d6) S 3.33 (s, 3H), 5.10
(q,
J = 9 Hz, 2H), 7.90 (d, J = 9 Hz, 2H), 8.12 (d, J = 9 Hz, 2H), 8.20 (s, 1 H).
MS (DCI-
NH3) m/z 367 (M+H)+.
193 F. 2-(2.2.2-Trifluoroethyl)-4-(4-fluorobenzyI)-5-[4-(methylsu
Ifonyl)Ahenvlt-
3(2H)-pyridazinone
Magnesium turnings (500 mg) were placed in a dry 250 mL round bottom
flask. Anhydrous ether (20 mL) was added under N2 at room temperature then
fluorobenzyl bromide (3 mL) was added and stirred. The reaction was heated at
40
C for 2 hours. All magnesium was consumed resulting in a pale brownish-yellow
solution. The 2-(2,2,2-trifluoroethyl)-4-chloro-5-[4-(methylsulfonyl)phenyl]-
3(2H)-
pyridazinone prepared in Example 193E was dissolved in dry THF (25 mL) and
transferred to the Grignard solution. The mixture was heated for 3 hours. TLC
(2:1
hexanes/ethyl acetate) indicated that the pyridazinone starting material was
consumed.) The reaction was cooled to room temperature then quenched with a
-126-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
saturated NH4CI solution. The product was extracted with ethyl acetate (250
mL);
and the organic layer was washed with saturated NH4CI, and brine. The ethyl
acetate solution was dried over MgSO4 and fiftered. The filtrate was
concentrated
under reduced pressure. The product was isolated as an orange residue.
Purification using a silica gel column (20:80 ethyl acetate/pentanes) provided
the
title compound as a pale yellow powder (yield: 140 mg, 28%). 1 H NMR (300 MHz,
CDCI3) 8 3.13 (s, 3H), 4.85 (m, 2H), 6..93 (m, 4H), 7.49 (d, J = 9 Hz, 2H)
7.72 (s,
1 H), 8.08 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 441 (M+H)+. Anal. calc. for
C20H16F4N2O3S-0.5 H20: C, 53.45; H, 3.81; N, 6.23. Found C, 53.45; H, 3.81; N,
6.23.
Example 194
2-(4-Fluorophenyl)-4-(4-fluorophenoxvmethyl)-5-(4-
(methylsulfonyl)ohenyl]_3(2H)-
pyridazinone
194A. 2-(4-Fluorophenyl)-4.5-dibromo-3(2H)-pvridazinone
Mucobromic acid (5.0 g, 19.4 mmol) dissolved in acetic acid (110 mL) was
treated with 4-fluorophenyl hydrazine=HCI, and the heterogeneous mixture
brought
to refiux at a bath temperature of 115 C for 15 hours. During the course of
reaction, the mixture became a homogeneous deep red solution, and upon cooling
to 23 C, a crystalline precipitate formed. The solution was poured into ice
water
(1000 mL) and stirred for 20 minutes. The yellow/brown crystals were filtered
off,
washed with additional cold water, and dried in vacuo to provide 5.8 g (86%)
of
product. (J. Het. Chem.., 1993, 30, 1501; Heterocycles 1985, 23, 2603) 1 H NMR
(300 MHz, DMSO-d6) S 7.31-7.41 (m, 2H), 7.57-7.64 (m, 2H), 8.29 (s, 1 H). MS
(DCI+) m/z 347 (Br79Br79 M+H)+, m/z 349 (Br7gBr81 M+H)+, m/z 364 (Br79Br79
M+NH4)+, and m/z 366 (Br79Br81 M+NH4)+.
194B. 2-(4-Fluorophenyl)-4-methoxy-5-bromo-3(2H)-pyridazinone
A 23 C homogeneous solution of 2-(4-fluorophenyl)-4,5-dibromo-3(2H)-
pyridazinone (7.18 g, 20.6 mmol) prepared above in tetrahydrofuran (322 mL)
was
treated with methanol (0.843 mL, 20.8 mmol) and after 5 minutes with NaH
(0.833
g, 20.8 mmol, 60% oil dispersion). The reaction exothermed for several minutes
and then was continued for 8 hours at 23 C (Note: several reactions have run
to
completion at this point). The reaction did not run to completion, and so the
temperature was raised to reflux for 4 hours more. The reaction was still not
completed. An additional 0.1 equivalent of NaOMe solution was prepared in a
separate flask as above with the quantities: 32 mL of tetrahydrofuran, 0.084
mL of
-127-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
methanol, and 83 mg of 60% NaH oil dispersion. This NaOMe solution was added
via syringe to the reaction mixture cooled to 23 C, and then the temperature
raised
to refiux for 4 hours The reaction was still not complete, and so another 0.1
equivalent NaOMe solution was prepared, added, and the reaction brought to
reflux, as above. After this 4 hours, the reaction was completed. The mixture
was
cooled to 23 C and diluted to 2000 mL with water. The yellow/white
precipitate
that formed was fittered off, washed with additional water, and concentrated
in
vacuo to provide 5.39 g (88%) of product. (J. Het. Chem., 1988, 25, 1757) 1 H
NMR
(300 MHz, DMSO-d6) S 4.13 (s, 3H), 7.30-7.40 (m, 2H), 7.56-7.62 (m, 2H), 8.22
(s,
1 H). MS (APCI+) m/z 299 (Br79 M+H)+ and m/z 301 (Br81 M+H)+.
194C. 2-(4-Fluoro henvl)-4-methoxv-5-[4-(meth ly thio)12henylJ-3(2b)-
pyridazinnnP
The title compound was prepared according to the method of Example 6
starting with 2-(4-fluorophenyl)-4-methoxy-5-bromo-3(2H)-pyridazinone in place
of
2-benzyl-4-bromo-5-methoxy-3(2H)-pyridazinone and substituting 4-(methylthio)-
benzeneboronic acid in place of 4-fluorobenzeneboronic acid (yield: 70 mg, 61
%).
1 H NMR (500 MHz, DMSO-d6) S 2.54 (s, 3H), 4.02 (s, 3H), 7.35 (dd, J = 9.0,
9.0 Hz,
2H), 7.39 (d, J = 8.5 Hz, 2H), 7.61 (d, J = 8.5 Hz, 2H), 7.65 (dd, J = 9.0,
5.0 Hz, 2H),
8.14 (s, 1 H). MS (APCI+) m/z 343 (M+H)+.
194D. 2-(4-Fluoroahenyl -4-methyl-5-j4-(methylthio h nY11-3(2 H)-põyridazi
nnnP
The title compound was prepared according to the method of Example 228
substituting methyl magnesium bromide in place of cyclohexylmagnesium chloride
(yield: 0.83 g, 87%). 1 H NMR (300 MHz, CDC13) S 2.25 (s, 3H), 2.55 (s, 3H),
7.17
(dd, J = 8.8, 8.8 Hz, 2H), 7.31 (d, J = 8.7 Hz, 2H), 7.38 (d, J = 8.7 Hz, 2H),
7.61-7.68
(m, 2H), 7.82 (s, 1 H). MS (APCI+) m/z 327 (M+H)+.
1 94E. 2-(4-Fluoropherlyl -4- hyl-5-[4-(methylsulfopyl)oheny1l-'~~,?H1-
Ryridazinone
The title compound was prepared according to the method of Example 10
substituting 2-(4-fluorophenyl)-4-methyl-5-[4-(methylthio)phenyl]-3(2H)-
pyridazinone in place of 2-benzyl-4-(4-fluorophenyl)-5-[4-(methylthio)phenyl]-
3(2H)-pyridazinone (yield: 473 mg, 86%). 1 H NMR (300 MHz, CDCI3) S 2.24 (s,
3H), 3.14 (s, 3H), 7.19 (dd, J = 8.8, 8.8 Hz, 2H), 7.61 (d, J = 8.4 Hz, 2H),
7.63-7.69
(m, 2H), 7.80 (s, 1 H), 8.12 (d, J = 8.4 Hz, 2H). MS (APCI+) m/z 359 (M+H)+
and m/z
376 (M+NH4)+.
-128-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
194F. 2-(4-Fluorophepy11-4-bromomethyl-5-[4-(methvlsulfonyj)ohenyll-3(2H)-
p,vridazinone
To a heterogeneous, refluxing solution of 2-(4-fluorophenyl)-4-methyl-5-[4-
(methylsulfonyl)phenyl]-3(2H)-pyridazinone (590 mg, 1.65 mmol) and carbon
tetrachloride (24 mL) was quickly added N-bromosuccinimide (yield: 308 mg,
1.73
mmol) followed by benzoyl peroxide (12 mg, 0.05 mmol). After 1 hour the
reaction
had only run to near 50% completion. Additional benzoyl peroxide (12 mg, 0.05
mmol) was added, and the reaction checked after another 1 hour. The reaction
was still not complete, and so more benzoyl peroxide (4 mg, 0.017 mmol) was
added. After 30 minutes, the reaction was completed. The mixture was cooled to
23 C and diluted with ethyl acetate. The acetate solution was washed with
saturated NaHCO3, water, and brine. The solution was dried over MgSO4,
filtered,
and concentrated in vacuo. The residue was chromatographed (flash silica gel,
ethyl acetate/hexanes gradient 1:1 to 4:1) to provide the product (yield: 530
mg,
74%). 1 H NMR (300 MHz, CDCI3) S 3.16 (s, 3H), 4.34 (s, 2H), 7.20 (dd, J 8.8,
8.8
Hz, 2H), 7.67-7.74 (m, 2H), 7.82 (d, J = 8.7 Hz, 2H), 7.86 (s, 1 H), 8.17 (d,
J 8.7 Hz,
2H). MS (APCI+) m/z 437 (M+H)+.
194G. 2-(4-Fluoronhenvl)-4-(4-fluoro h noxymethvl)-5-[4-(methylsulfony1)
henyll-
3(2H)- pyridazinone
To a homogeneous solution of 2-(4-fluorophenyl)-4-bromomethyl-5-[4-
(methylsutfonyl)phenyl]-3(2H)-pyridazinone, prepared above, (107 mg, 0.246
mmol) and 4-fluorophenol (30.3 mg, 0.270 mmol) dissolved in acetone (4 mL) was
added powdered K2C03 (37.3 mg, 0.270 mmol). The mixture was stirred at 23 C
for 2 hours, filtered through a bed of Celite , and concentrated in vacuo. The
residue was chromatographed (flash.silica gel, ethyl acetate/hexanes 3:2) to
provide the product (yield: 83 mg, 72%). M.p. 65-80 C. 1 H NMR (300 MHz,
CDCI3) S 3.12 (s, 3H), 4.94 (s, 2H), 6.78-6.86 (m, 2H), 6.91-7.00 (m, 2H),
7.15-7.24
(m, 2H), 7.65-7.72 (m, 2H), 7.74 (d, J = 8.7 Hz, 2H), 7.93 (s, 1 H), 8.08 (d,
J = 8.7 Hz,
2H). MS (APCI+) m/z 469 (M+H)+. Anal. caic. for C24H18F2N204S: C, 61.53; H,
3.87; N, 5.97. Found: C, 61.22; H, 3.63; N, 5.64.
-129-
CA 02578858 2007-03-05
WO 99/10331 PCTIUS98/16479
Example 195
2-(4-Fluoroohenyl)-4-(3-fluoro hp enoxvmethvl)-5-[4-(methylsulfonx() heny!]-
3(2H)-
~yridazinone
The title compound was prepared according to the method of Example 194G
substituting 3-fluorophenol in place of 4-fluorophenol (yield: 94 mg, 88%).
M.p.
142-144 C. 1 H NMR (300 MHz, CDCI3) S 3.12 (s, 3H), 4.98 (s, 2H), 6.49-6.56
(m,
1 H), 6.60-6.73 (m, 2H), 7.15-7.25 (m, 3H), 7.65-7.75 (m, 4H), 7.93 (s, 1 H),
8.07 (d, J
= 8.7 Hz, 2H). MS (APCI+) m/z 469 (M+H)+. Anal. calc. for C24H18F2N204S: C,
61.53; H, 3.87; N, 5.97. Found: C, 61.20; H, 3.92; N, 5.86.
Example 196
2-(4-Fluoronhenyl)-4-ohenoxymethy1-5-[4-(methylSu I.fr nvl)nhen ly 3 (2 H)-
Ryridazinone
The title compound was prepared according to the method of Example 294G
substituting phenol in place of 4-fluorophenol (yield: 67 g, 93%). M.p. 42-75
C.
1 H NMR (300 MHz, DMSO-d6) S 3.28 (s, 3H), 4.92 (s, 2H), 6.83-6.90 (m, 2H),
6.91-
6.99 (m, 1 H), 7.22-7.30 (m, 2H), 7.35-7.44 (m, 2H), 7.66-7.73 (m, 2H), 7.81-
7.88 (m,
2H), 8.02-8.08 (m, 2H), 8.21 (s, 1 H). MS (APCI+) m/z 451 (M+H)+.
Example 197
2-(4-Fiuoroahenvl)-4-(t-butytthiomethyl)-5-(4-(met Isu f y1)phenyl]-3( H~)-
Rvridazinone
A 0 C solution of the 2-(4-fluorophenyl)-4-bromomethyl-5-[4-(methyl-
sulfonyl)phenyl]-3(2H)-pyridazinone prepared in Example 194F (92.5 mg, 0.212
mmol) in acetone (2.5 mL) was treated with Nal (35 mg, 0.233 mmol), and after
5
minutes, the cooling bath was removed and the reaction warmed to 23 C. After
30
minutes, conversion to the 2-(4-fluorophenyl)-4-iodomethyl-5-[4-
(methylsulfonyl)-
phenyl]-3(2H)-pyridazinone was complete (thin layer chromatography, ethyl
acetate/hexanes 4:1). The NaBr and residual Nal were filtered off through a
pad of
Celite . Additional acetone (2 mL) was added along with 2-methyl-2-
propanethiol
(20.5 mg, 0.227 mmol), and the solution cooled to 0 C before addition of
A92CO3
(63 mg, 0.227 mmol). After 5 minutes, the cooling bath was removed and the
solution warmed to 23 C for 5 hours. The reaction mixture was filtered
through
Celite and concentrated in vacuo. The residue was chromatographed (flash
silica
gel, ethyl acetate/hexanes gradient 1:1 to 3:2) to provide the product (yield:
57 mg,
60%). M.p. 50-70 C. 1 H NMR (300 MHz, CDCI3) b 1.34 (s, 9H), 3.14 (s, 3H),
3.65
-130-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
(s, 2H), 7.13-7.21 (m, 2H), 7.63-7.70 (m, 2H), 7.79 (s, 1 H), 7.84 (d, J = 8.7
Hz, 2H),
8.13 (d, J = 8.7 Hz, 2H). MS (APCI+) m/z 447 (M+H)+. Anal. calc. for
C22H23FN203S2: C, 59.17; H, 5.19; N, 6.27. Found: C, 59.48; H, 5.36; N, 5.90.
Example 198
2-(4-Fluoronhenvl)-4-(2-methyl{ZroOYlthiomethvj)-544-(methylsulfonyJ) henyll-
3 (2H)12,vridazinone
The title compound was prepared according to the method of Example 197
substituting 2-methyl-l-propanethiol in place of 2-methyl-2-propanethiol
(yield: 66
mg, 70%). M.p. 45-60 C. 1 H NMR (300 MHz, CDCI3) S 0.95 (d, J = 6.6 Hz, 6H),
1.67-1.82 (m, 1 H), 2.62 (d, J = 6.6 Hz, 2H), 3.15 (s, 3H), 3.61 (s, 2H), 7.19
(dd, J =
8.2, 8.2 Hz, 2H), 7.62-7.71 (m, 2H), 7.75 (d, J = 8.4 Hz, 2H), 7.79 (s, 1 H),
8.13 (d, J
8.4 Hz, 2H). MS (APCI+) m/z 447 (M+H)+. Anal. calc. for C22H23FN203S2: C,
59.17; H, 5.19; N, 6.27. Found: C, 59.35; H, 5.25; N, 6.05.
Example 199
2-(4-Fluoronhenvl)-4-(2- roooxy)-5-(4-(methvlthio)Ri~nyll-3(2H)-Rvridazinone
The title compound was prepared by the following sequence of reactions.
Mucobromic acid and 4-fluorophenylhydrazine hydrochloride were reacted to
provide 2-(4-fluorophenyl)-4,5-dibromo-3(2H)-pyridazinone following the
procedure in Example 194A. The dibromo-intermediate was reacted according to
the procedure described in Example 194B, substituting isopropanol in place of
methanol, to selectively react at the 4-position and provide 2-(4-
fluorophenyl)-4-(2-
propoxy)-5-bromo-3(2H)-pyridazinone.
The 5-bromo-compound was coupled to 4-(methylthio)phenylboronic acid
according to the method of Example 6 to provide the title compound (yield: 435
mg,
53.9%). M.p. 135-137 C. 1 H NMR (300 MHz, CDCI3) S 1.21 (d, J = 6 Hz, 6H),
2.55
(s, 3H), 5.26 (sept, J = 6 Hz, 1 H), 7.17 (t, J = 9 Hz, 2H), 7.34 (d, J = 9
Hz, 2H), 7.57
(d, J= 9 Hz, 2H), 7.58-7.66 (m, 2H), 7.95 (s, 1 H). MS (DCI-NH3) m/z 371
(M+H)+.
Example 200
2-(4-Fluoroohenvl)-4-(2-propoxti)-5-j4-(methyls uIfnnyl)phenyl]-3(?~t )-
pyridazinone
The methyl sulfide compound prepared in Example 199 was oxidized
according to the method of Example 10 to provide the title compound (yield:
240
mg, 92%). ,M.p. 160-162 C. 1 H NMR (300 MHz, DMSO-d6) 81.30 (d, J= 6 Hz,
6H), 3.41 (s, 3H), 5.41 (m, 1 H), 7.48 (t, J = 9 Hz, 2H), 7.77 (dd, J = 9 Hz,
6 Hz, 2H),
-131-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
8.05 (d, J = 9 Hz, 2H), 8.19 (d, J = 9 Hz, 2H), 8.31 (s, 1 H). MS (DCI-NH3)
m/z 403
(M+H)+, 420 (M+NH4)+. Anal. calc. for C20H19FN2O4S: C, 59.70; H, 4.73; N,
6.97. Found: C, 59.40; H, 4.86; N, 6.69.
Example 201
2-(3-Chlorophenyl)-4-(2-~r po1cX)-5-[4-(methvlsulfonvl)nhenyl]-3(2H)-
pyridazinone
2-(3-Chlorophenyl)-4-(2-propoxy)-5-[4-(methylthio)phenyl]-3(2H)-
pyridazinone was prepared according to the method of Example 199, substituting
3-chlorophenylhydrazine hydrochloride in place of 4-fluorophenylhydrazine
hydrochloride, in the first step. The resulting methyl sulfide was oxidized
according
to the method of Example 10 to provide the title compound (yield: 260 mg,
80%).
M.p. 134-136 C. 1 H NMR (300 MHz, CDCI3) 81.24 (d, J = 6 Hz, 6H), 3.13 (s,
3H),
5.48 (sept, J = 6 Hz, 1 H), 7.37-7.48 (m, 2H), 7.59 (dt, J = 7 Hz, 1.5 Hz, 1
H), 7.70 (br
s, 1 H), 7.84 (d, J = 9 Hz, 2H), 7.93 (s, 1 H), 8.06 (d, J = 9 Hz, 2H). MS
(DCI-NH3)
m/z 419 (M+H)+, 436 (M+NH4)+. Anal. calc. for C20H1 9CIN2O4S: C, 57.42; H,
4.55; N, 6.70. Found: C, 57.08; H, 4.59; N, 6.44.
0
Example 202
2-(3-Fluoroohenvl)-4-(2- ro poxy)-5-[4-(methvlsulfonyl)ohenylJ-3(2H)-
pyridazinone
The methyl sulfide intermediate was prepared according to the method of
Example 199, substituting 3-fluorophenylhydrazine hydrochloride in place of
4-fluorophenylhydrazine hydrochloride in the first step. The resulting methyl
sulfide
compound was oxidized according to the method of Example 10 to provide the
title
compound (yield: 290 mg, 72%). M.p. 110-112 C. 1 H NMR (300 MHz, CDCI3) S
1.31 (d, J = 6 Hz, 6H), 3.11 (s, 3H), 5.47 (sept, J = 6 Hz, 1 H), 7.09-7.18
(m, 1 H),
7.41-7.52 (m, 3H), 7.83 (d, J = 9 Hz, 2H), 7.93 (s, 1 H), 8.08 (d, J = 9 Hz,
2H). MS
(DCI-NH3) m/z 403 (M+H)+, 447 (M+NH4)+. Anal. calc. for C20H1 gFN2O4S: C,
59.70; H, 4.73; N, 6.97. Found: C, 59.54; H, 4.87; N, 6.70.
Example 203
2-(3-Bromophenyl)-4-(2-propoxy)-5-14-(methyisulfonyl henylJ 3(2H) pyridazinone
The methyl sulfide intermediate was prepared according to the method of
Example 199, substituting 3-bromophenylhydrazine hydrochloride in place of
4-fluorophenylhydrazine hydrochloride. The resulting methyl sulfide compound
was oxidized according to the method of Example 10 to provide the title
compound
(yield: 75 mg, 77.6%). M.p. 130-132 C. 1 H NMR (300 MHz, CDCI3) 5 1.23 (d, J
-132-
CA 02578858 2007-03-05
WO 99/10331 PCTIUS98/16479
6 Hz, 6H), 3.15 (s, 3H), 5.48 (sept, J = 6 Hz, 1 H), 7.38 (t, J = 9 Hz, 1 H),
7.55 (br d, J
= 7 Hz, 1 H), 7.65 (br d, J = 7 Hz, 1 H), 7.79-7.87 (m, 1 H), 7.83 (d, J = 9
Hz, 2H), 8.13
(s, 1 H), 8.06 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 465 (M+H)+, 480 (M+NH4)+.
Anal. calc. for C20H1 gBrN2O4S: C, 51.84; H, 4.10; N, 6.05. Found: C, 51.95;
H,
4.18; N, 5.74.
Example 204
2-(2.5-DifluoroRhenyl)-4-(2-propoxY)-5-[4-(methylsulfonyl)ohenyll-3(2-1)-
pyridazinone
2-(2,5-Difluorophenyl)-4-(2-propoxy)-5-[4-(methylthio)phenyl]-3(2H)-
pyridazinone was prepared according to the method of Example 199, substituting
2,5-difluorophenylhydrazine hydrochloride in place of 4-fluorophenylhydrazine
hydrochloride .
The resulting methyl sulfide compound was oxidized according to the
method of Example 10 to provide the title compound (yield: 390 mg, 90%). M.p.
161-164 C. 1 H NMR (300 MHz, CDCI3) S 1.23 (d, J = 6 Hz, 6H), 3.12 (s, 3H),
5.55
(sept, J = 6 Hz, 1 H), 7.12-7.29 (m, 3H), 7.82 (d, J = 9 Hz, 2H), 7.92 (s, 1
H), 8.07 (d, J
= 9 Hz, 2H). MS (DCI-NH3) m/z 421 (M+H)+, 438 (M+NH4)+. Anal. caic. for
C20H18 F2N2O4S-0.5 H20: C, 55.94; H, 4.31; N, 6.53. Found: C, 55.86; H, 4.19;
N, 6.38.
Example 205
2-(3-Chloro-4-fluoro h yj)-4-(2-methy,ll2ropoxy)-5-[3-fluoro-4-
(methylsulfonvll-
henyl]-3(2H)-2Yridazinone
The title compound was prepared by the following sequence of reactions.
Mucobromic acid and 3-chloro-4-fluorophenylhydrazine hydrochloride were
reacted to provide 2-(3-chloro-4-fluorophenyl)-4,5-dibromo-3(2H)-pyridazinone
according to the method of Example 194A. The intermediate was selectively
reacted at the 4-position with isobutanol and base to provide 2-(4-
fluorophenyl)-4-
[1-(2-methylpropoxy)]-5-bromo-3(2H)-pyridazinone according to the method of
Example 194B. The 5-bromo-compound was coupled to 3-fluoro-4-(methylthio)-
phenylboronic acid prepared in Example 194C according to the method of
Example 6 to produce the intermediate methyl sulfide. The sulfide compound was
oxidized to the title methyl sulfone according to the method of Example 10
(yield:
810 mg, 83.8%). M.p. 142-144 C. 1 H NMR (300 MHz, CDC13) 8 0.90 (d, J = 6 Hz,
6H), 1.95 (sept, J = 6 Hz, 1 H), 3.30 (s, 3H), 4.37 (d, J = 6 Hz, 2H), 7.26
(t, J = 9 Hz,
-133-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
1 H), 7.52-7.61 (m, 3H), 7.75 (dd, J = 9 Hz, 3 Hz, 1 H), 7.89 (s, 1 H), 8.10
(t, J = 9 Hz,
1 H). MS (DCI-NH3) m/z 469 (M+H)+, 486 (M+NH4)+.
Example 206
2-(3.4-Difluorophenyl)-4-(4-fluorop he nyl)-5-(3-methy -L4-
(methylsulforlyl)oh, envll
3(2H)-pyridazinone
206A. 2-Methylthioanisole
A solution of 2-bromothioanisole (10.53 g, 52 mmol) in tetrahydrofuran (173
mL) was prepared and cooled to -78 C. n-BuLi (21.8 mL, 54.5 mmol, 2.5 M
solution in hexanes) was slowly added along the interior wall of the reaction
vessel.
The resultant light yellow solution was stirred for 30 minutes before methyl
iodide
(8.10 g, 57.1 mmol) diluted with tetrahydrofuran (6 mL) was slowly added along
the
interior wall of the reaction vessel. The mixture was stirred for another 30
minutes
at -78 C. The cooling bath was removed, and the mixture stirred for 1 hour.
The
solution was cooled to 0 C and a saturated aqueous NH4CI solution added. The
resultant solution was extracted several times with ethyl acetate, and the
combined
acetate layers washed with brine, dried over MgSO4, filtered, and concentrated
in
vacuo. The residue was chromatographed (flash silica gel, ethyl
acetate/hexanes
1:19) to provide the product (yield: 6.74 g, 94%). 1 H NMR (300 MHz, CDCI3) S
2.34
(s, 3H), 2.46 (s, 3H), 7.02-7.09 (m, 1 H), 7.12-7.22 (m, 3H).
206B. 4-Bromo-2-meth,ylthioanisole.
To a 0 C solution of 2-methylthioanisole (0.50 g, 3.57 mmol) in methylene
chloride (40 mL) was added powdered Fe (20 mg, 0.36 mmol) followed by
dropwise addition of bromine (0.58 g, 3.54 mmol). After 30 minutes, the
starting
material had been consumed (thin layer chromatography, hexanes). The excess
bromine was quenched by adding a solution of NaHSO3 and stirring for several
minutes. The methylene chloride layer was separated, and the aqueous phase
extracted with additional methylene chloride. The combined methylene chloride
solution was dried over MgSO4, filtered, and concentrated in vacuo. The
resultant
oil was chromatographed (flash silica gel, ethyl acetate/hexanes 1:49) to
provide
the product (yield: 0.74 g, 96%). 1 H NMR (300 MHz, CDCI3) S 2.30 (s, 3H),
2.45 (s,
3H), 7.00 (d, J = 8.4 Hz, 1 H), 7.27-7.33 (m, 2H).
C 206C. 3-Methyl-4-(meth Ithio)benzenehnrnnic acid.
3-Methyl-4-(methylthio)benzeneboronic acid was prepared according to the
method of Example 1, substituting 4-bromo-2-(methylthio)anisole in place of 4-
-134-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
bromothioanisole (yield: 5.3 g, 67%). M.p. 208-210 . 1 H NMR 2.28 (s, 3H),
2.46
(s, 3H), 7.20 (d, J= 8.4 Hz, 1 H), 7.62 (s, 1 H), 7.70 (d, J = 8.4 Hz, 1 H).
206D. 2-(3.4-Difluorophenyl)-4.5-dibromo-3(2H)-pvridazinone.
The title compound was prepared according to the method of Example 194A,
substituting 3,4-difluorophenyl hydrazine=HCI in place of 4-fluorophenyl
hydrazine=HCI (yield: 39 g, 78%). 1 H NMR (300 MHz, DMSO-d6) 5 7.45 (m, 1 H),
7.61 (m, 1 H), 7.75 (m, 1 H), 8.30 (s, 1 H). MS (DCI-NH3) m/z 382 (M+NH4)+.
206E. 2-(3 4-Difluorophenyl)-4-methoxy-5-bromo-3(2H)-pvridazinone
The title compound was prepared according to the method of Example 194B,
substituting 2-(3,4-difluorophenyl)-4,5-dibromo-3(2H)-pyridazinone in place of
2-(4-
fluorophenyl)-4,5-dibromo-3(2H)-pyridazinone (yield: 15 mg, 88%). 1 H NMR (300
MHz, DMSO-d6) S 4.14 (s, 3H), 7.45 (m, 1 H), 7.60 (m, 1 H), 7.74 (m, 1 H),
8.24 (s,
1 H). MS (DCI-NH3) m/z 317 (M+H)+ and m/z 334 (M+NH4)+.
206F. 2-(3.4-Difluoroohenyl)-4-methoxy-5-[3-methyl-4-(meth ly thio)phenylj-
3(2H)-
pyridazinone.
The title compound was prepared according to the method of Example 6
starting with 2-(3,4-difluorophenyl)-4-methoxy-5-bromo-3(2H)-pyridazinone in
place of 2-benzy1-4-bromo-5-methoxy-3(2H)-pyridazinone and substituting 3-
methyl-4-(methylthio)benzeneboronic acid in place of 4-fluorobenzeneboronic
acid
(yield: 2.0 g, 85%). 1 H NMR (300 MHz, CDCI3) 8 2.39 (s, 3H), 2.53 (s, 3H),
4.11 (s,
3H), 7.22-7.32 (m, 2H), 7.34 (s, 1 H), 7.42-7.50 (m, 2H), 7.55-7.64 (m, 1 H),
7.92 (s,
1 H). MS (APCI+) m/z 375 (M+H)+.
206G. 2-(3.4-Difluoroohenvl)-4-(4-fluorophenyl)-5-[3-methvl-4_(meth Iy
thio)phenkl-
3(2H)-pvridazinone.
2-(3,4-Difluorophenyl)-4-(4-fluorophenyl)-5-[3-methyl-4-(methylthio)phenyl]-
3(2H)-pyridazinone, was prepared according to the method of Example 228,
starting with 2-(3,4-difluorophenyl)-4-methoxy-5-[3-methyl-4-
(methylthio)phenyl]-
3(2H)-pyridazinone in place of 2-(4-fluorophenyl)-4-methoxy-5-[4-(methylthio)-
phenyl]-3(2H)-pyridazinone and substituting 4-fluorophenyl magnesium bromide
in
place of cyclohexylmagnesium chloride (yield: 330 mg, 56%). 1 H NMR (300 MHz,
CDCI3) 8 2.24 (s, 3H), 2.47 (s, 3H), 6.90-7.03 (m, 6H), 7.22-7.31 (m, 2H),
7.49-7.54
(m, 1 H), 7.60-7.68 (m, 1 H), 8.02 (s, 1 H). MS (APCI+) m/z 439 (M+H)+.
206H. 2-(3.4-Difluorophenyl)-4-(4-fluorophen IY )-5-[3-methyl-4-
(methylsulfonyll-
ghenyl)-3(2H)-pyridazinone.
The title compound was prepared according to the method of Example 10,
substituting 2-(3,4-difluorophenyl)-4-(4-fluorophenyi)-5-[3-methyl-4-
(methylthio)-
-135-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
phenyl]-3(2H)-pyridazinone in place of 2-benzyl-4-(4-fluorophenyl)-5-[4-
(methyl-
thio)phenyl]-3(2H)-pyridazinone (yield: 251 mg, 82%) M.p. 80-100 C. 1 H NMR
(300 MHz, DMSO-d6) S 2.59 (s, 3H), 3.25 (s, 3H), 7.13-7.34 (m, 5H), 7.45 (s, 1
H),
7.52-7.69 (m, 2H), 7.81 (d, J = 8.4 Hz, 1 H), 7.81-7.90 (m, 1 H), 8.27 (s, 1
H). MS
(APCI+) m/z 471 (M+H)+ and m/z 488 (M+NH4)+. Anal. calc. for
C24H17F3N203S: C, 61.27; H, 3.64; N, 5.95. Found: C, 61.53; H, 3.92; N, 5.67.
Example 207
2-(3-Chloroohen ly )-4-(4-fluoroQhenoxymethyl)-5-14- et I us IfonY1)12heay1]-
3(2H)-
pyridazinone
207A. 2-(3-Chlorol2hgpy,J)-4.5-dibromo-3(2j)-Ryridazinone.
The title compound was prepared according to the method of Example 194A,
substituting 3-chlorophenyl hydrazine=HCI in place of 4-fluorophenyl
hydrazine=HCI
(yield: 24.8 g, 88%). 1 H NMR (300 MHz, DMSO-d6) 6 7.53-7.57 (m, 3H), 7.67-
7.70
(m, 1 H), 8.29 (s, 1 H). MS (DCI-NH3) m/z 365 (M+H)+ and m/z 382 (M+NH4+)+.
207B. 2-(3-ChlorophenY1)-4-methoxy-5-bromo-3(2j)-Ryridazinnnp
The title compound was prepared according to the method of Example 194B,
substituting 2- (3-chlorophenyl)-4,5-dibromo-3 (2H)-pyridazi none in place of
2-(4-
fluorophenyl)-4,5-dibromo-3(2H)-pyridazinone (yield: 12.4 g, 95%). 1 H NMR
(300
MHz, DMSO-d6) S 4.21 (s, 3H), 7.58-7.62 (m, 3H), 7.73-7.76 (m, 1 H), 8.28 (s,
1 H).
MS (DCI-NH3) m/z 317 (M+H)+ and m/z 334 (M+NH4+)+.
207C. 2-(3-Chlorophe Ilyl)-4-methoxy-5-14-(methylthio)ohenyl]-3(2H)-
Ryridazinone.
The title compound was prepared according to the method of Example 6
starting with 2-(3-chlorophenyl)-4-methoxy-5-bromo-3(2H)-pyridazinone in place
of
2-benzyl-4-bromo-5-methoxy-3(2H)-pyridazinone and substituting 4-(methylthio)-
benzeneboronic acid in place of 4-fluorobenzeneboronic acid (yield: 3.3 g,
68%).
1 H NMR (300 MHz, DMSO-d6) S 2.54 (s, 3H), 4.03 (s, 3H), 7.40 (d, J = 9.0 Hz,
2H),
7.50-7.64 (m, 5H), 7.73-7.77 (m, 1 H), 8.18 (s, 1 H). MS (DCI-NH3) m/z 359
(M+H)+.
207D. 2-(3-Chforoohenyl)-4-methyl-5-[3-methyJ-4-(methyfthyQ)Rhgnyl]-312BL-
Ryridazinone.
2-(3-Chlorophenyl)-4-(4-fluorophenyl)-5-[3-methyl-4-(methylthio)phenyl]-
3(2H)-pyridazinone, was prepared according to the method of Example 228,
starting with 2-(3-chlorophenyl)-4-methoxy-5-[3-methyl-4-(methylthio)phenyl]-
3(2H)-pyridazinone in place of 2-(4-fluorophenyl)-4-methoxy-5-[4-(methylthio)-
phenyl]-3(2H)-pyridazinone and substituting 4-fluorophenyl magnesium bromide
in
place of cyclohexylmagnesium chloride (yield: 180 mg, 94%). 1 H NMR (300 MHz,
-136-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
CDCI3) S 2.25 (s, 3H), 2.56 (s, 3H), 7.28-7.45 (m, 6H), 7.58-7.63 (m, 1 H),
7.71-7.74
(m, 1 H), 7.82 (s, 1 H). MS (APCI+) m/z 343 (M+H)+ and m/z 360 (M+NH4)+.
207E. 2-j3-Chloro henyl -4-methyl-5-[4-(methylsulfonyl h~epyll-3(2H)=
Ryridazinone
The title compound was prepared according to the method of Example 10,
substituting 2-(3-chlorophenyl)-4-methyl-5-[4-(methylthio)phenyl]-3(2H)-
pyridazinone for 2-benzyl-4-(4-fluorophenyl)-5-[4-(methylthio)phenyl]-3(2H)-
pyridazinone (yield: 125 mg, 67%). M.p. 164-168. 1 H NMR (300 MHz, CDCI3) S
2.23 (s, 3H), 3.13 (s, 3H), 7.37-7.46 (m, 2H), 7.61 (m, 3H), 7.71-7.74 (m, 1
H), 7.81
(s, 1 H), 8.13 (d, J = 8.7 Hz, 2H). MS (APCI+) m/z 343 (M+H)+ and m/z 360
(M+NH4)+.
207F. 2-(3-Chloroohenyl)-4-bromomethyl-5-j4-(methylsulfonyl)QhenylJ-3(2H)-
pyridazinone
2-(3-Chlorophenyl)-4-bromomethyl-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone was prepared according to the method of Example 194F,
substituting
2-(3-chlorophenyl)-4-methyl-5-[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone in
place of 2-(4-fluorophenyl)-4-methyl-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone (yield: 90 mg, 99%). 1 H NMR (300 MHz, CDCI3) b 3.13 (s, 3H),
4.33
(s, 2H), 7.40-7.47 (m, 2H), 7.66 (ddd, J = 2.4, 2.4, 7.2 Hz, 1 H), 7.76-7.78
(m, 1 H),
7.81 (d, J = 8.7 Hz, 2H), 7.86 (s, 1 H), 8.17 (d, J = 8.7 Hz, 2H). MS (APCI+)
m/z 453
(M+H)+ and m/z 470 (M+NH4)+.
207G. 2-(3-Chloroohenvl)-4-(4-fluoro f~oxymethyji-5-j4-(methylsulfonyl)~he4y11-
3(2H)-Ryridazinone
The title compound was prepared according to the method of Example
1 94G, substituting 2-(3-chlorophenyl)-4-bromomethyl-5-[4-
(methylsulfonyl)phenyl]-
3(2H)-pyridazinone in place of 2-(4-fluorophenyl)-4-bromomethyl-5-[4-
(methylsulfonyl)phenyl]-3(2H)-pyridazinone (yield: 30 mg, 31 %). M.p. 50-80
C.
1 H NMR (300 MHz, CDCI3) S 3.11 (s, 3H), 4.94 (s, 2H), 6.78-6.85 (m, 2H), 6.91-
6.99 (m, 2H), 7.39-7.48 (m, 2H), 7.64 (ddd, J = 7.5, 1.9, 1.9 Hz, 1 H), 7.71-
7.77 (m,
3H), 7.93 (s, 1 H), 8.08 (d, J = 8.7 Hz, 2H). MS (APCI+) m/z 485 (M+H)+.
Example 208
2-13-Chloroohenvll-4-(benzoyloxvmethyl)-5-[4-(met y suI yl)phenyl]-3(2H)-
~yridazinone
The title compound was prepared according to the method of Example 207
substituting benzoic acid in place of 4-fluorophenol (yield: 33 mg, 34%). M.p.
50-70
-137-
CA 02578858 2007-03-05
WO 99/10331 PCTNS98/16479
C. 1 H NMR (300 MHz, CDCI3) S 3.00 (s, 3H), 5.36 (s, 2H), 7.36-7.48 (m, 4H),
7.52-
7.59 (m, 1 H), 7.61-7.68 (m, 3H), 7.75-7.78 (m, 1 H), 7.83-7.88 (m, 2H), 7.89
(s, 1 H),
8.02 (d, J = 8.7 Hz, 2H). MS (APCI+) m/z 495 (M+H)+.
Example 209
2-(2.2.2-Trifluoroethyl)-4-(3-methylbutvl)-5-[4-(meth Isy ulfonyl)ohenyll-
3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 193 ,
substituting 1-bromo-4-methylpentane in place of 4-fluorobenzyl bromide
(yield: 80
mg, 19%). 1 H NMR (300 MHz, CDCI3) 5 0.81 (d, J = 7.5 Hz, 6H), 1.3-1.6 (m,
3H),
2.52 (m, 2H), 3.14 (3 H, s) 4.85 (q, J = 9 Hz, 2H), 7.55 (d, J = 9 Hz, 2H)
7.67 (s, 1 H),
8.1 (d, J = 9 Hz, 2H). MS (DCI-NH3), m/z 403 (M+H)+. Anal. calc. for
C18H21 F3N2O3S-0.25 H20: C, 53.12; H, 5.32; N, 6.88. Found C, 52.90; H, 5.14;
N, 6.43.
Example 210
2-(2.2.2-Trifluoroethyl)-4- (4-fluoro-3-methyjDhe nyl)-5-[4- (methy su I)Phe
nvll-
3(2H)-pvridazinone
210A. Preparation of boronic acid:
2-Fluorotoluene-5-Bromo (6 g, 31.7 mmol) was dissolved in dry THF (50 mL)
and cooled to -78 C under N2. n-BuLi (14 mL, 2.5M solution in THF) was added
slowly using a dry syringe. Cloudiness appeared. The reaction was stirred for
40
minutes at -78 C. Triisopropyl borate (22 mL, 95 mmol) was slowly added while
stirring. The reaction was allowed to warm to room temperature. Stirring
continued
for an additional 2 hours. A pale yellow, cloudy solution formed. (TLC (1:2
ethyl
acetate /hexanes)) indicated disappearance of the starting material. The
reaction
was quenched by adding 10% aqueous NaOH (200 mL). After stirring for 45
minutes, 10% citric acid solution (300 mL) was added until, pH -5Ø The
product
was extracted with ethyl acetate (500 mL). The organic phase was washed with
brine and dried over MgSO4, and filtered. The filtrate was concentrated under
reduced pressure to provide an off white solid (yield: 4.1 g, 84%).
210B. Suzuki Cou In ing:
The boronic acid (231 mg, 1.5 mmol), prepared in example 210A, 2-(2,2,2-
trifluoroethyl)-4-chloro-5-[4-(methylsulfonyl)pheny11-3(2H)-pyridazinone (500
mg,
1.36 mmol), tetrakis-(triphenylphosphine)-palladium(0) (47 mg, 0.041 mmol),
and
CsF (413 mg, 2.72 mmol) were stirred at reflux in DME (20 mL) under N2 for 5
hours. TLC (1:1 hexanes/ethyl acetate) indicated that all the starting
material was
-138-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
consumed. Volatiles were removed in vacuo. The residue was partitioned
between water and ethyl acetate. The organic layer was washed with brine,
dried
over MgSO4, and filtered. The filtrate was concentrated in vacuo. An off white
powder was obtained (yield: 275 mg, 46%). M.p. 88-91 C; 1 H NMR (300 MHz,
CDC13, a mixture of rotamers) S 2.2, 2.25 (2d, J = 1.5 Hz, 3H) 3.05, 3.09 (2
s, 3H)
4.78-4.92 (m, 2H) 6.61-6.8 (m, 1 H) 6.82-6.98 (m, 1 H) 7.35 (d, J = 9 Hz, 1 H)
7.78 (d,
J = 9 Hz, 1 H) 7.86-8.09 (m, 4H). MS (DCI-NH3), m/z 441 (M+H)+. Anal. calc.
for
C20H16F4N203S=0.5 H20: C, 53.45; H, 3.81; N, 6.23. Found C, 53.17; H, 3.65; N,
5.88.
Example 211
2-(2.2.2-Trifluoroethyl)-4-(3.5-dichlorophenY1)~[4-(met Isulfonl)oheny_(1
3(2HL-
pyridazinone
2-(2,2,2-Trifluoroethyl)-4-chloro-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone (150 mg, 0.409 mmol) (Example 193E) was dissolved in anhydrous
DME (8 mL) and heated to reflux with 3,5-dimethylbenzeneboronic acid in
presence of CsF (150 mg, 0.98 mmol) and tetrakis(triphenylphosphine)-palladium
(17.38 mg, 0.015 mmol) for 6 hours. After cooling to room temperature the
reaction
mixture was diluted with water and extracted with ethyl acetate (100 mL). The
organic layer was washed with brine, dried over MgSO4, and evaporated in
vacuo.
The compound was purified on a silica gel column, eluting with 30% ethyl
acetate
in pentanes, to provide the title compound (yield: 110 mg, 58%). 1 H NMR (300
MHz, CDCI3) 5 3.08 (s, 3H), 4.88 (q, J = 9 Hz, 2H), 7.06 (d, J = 1.5 Hz, 9 Hz,
2H),
7.31 (t, J = 1.5 Hz, 1 H), 7.36 (d, J = 9 Hz, 2H), 7.94 (s, 1 H), 7.96 (d, J =
9 Hz, 2H).
MS (DCI-NH3) m/z 496 (M+NH4)+. Anal. calc. for C1 gH13C12F3N203S: C, 47.81;
H, 2.75; N, 5.87. Found: C, 47.77; H, 2.75; N, 5.65
Example 212
2-(2.2.2-Trifluoroethvl)-4-(3-ethoxyohenyl)-5-[4-(methvlsulfonyl)phenyj]-3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 211,
substituting 3-ethoxyphenylboronic acid for 3,5-dimethylbenzeneboronic acid
(yield: 155 mg, 86%). 1 H NMR (300 MHz, CDCI3) b 1.42 (t, J = 7.5 Hz, 3H),
3.06 (s,
3H), 3.90 (q, J = 7.5 Hz, 2H), 4.88 (q, J = 9 Hz, 2H), 6.65 (d, J = 7.5 Hz, 1
H), 6.75 (t,
J = 1.5 Hz, 1 H), 6.85 (dd, J = 1.5 Hz, 9 Hz, 1 H), 7.15 (t, J = 9 Hz, 1 H),
7.38 (d, J = 9
Hz, 2H), 7.88 (d, J = 9 Hz, 2H), 7.90 (s, 1 H). MS (DCI-NH3) m/z 470 (M+NH4)+.
-139-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Anal. calc. for C21 H19CI2F3N204S: C, 55.75; H, 4.23; N, 6.19. Found: C,
55.62;
H, 4.30; N, 5.99
Example 213
2-(2 2 2-Trifluoroethyl)-4-(4-tri luoromethylphenyl)-5-(4-
(mgthylsulfonvl)ohenyll-
3 (2H)-.oyridazinone
The title compound was prepared according to the method of Example 211,
substituting 4-(trifluoromethyl)benzeneboronic acid in place of 3,4-dimethyl-
benzeneboronic acid (yield: 85 mg, 44%). 1 H NMR (300 MHz, CDCI3) 5 3.08 (s,
3H),4.90(q,J=9Hz,2H),7.35(t,J=9Hz,4H),7.58(d,J=9Hz,2H),7.90(d,J=
9 Hz. 3H). MS (DCI-NH3) m/z 494 (M+NH4)+. Anal. calc. for C20H14F6N203S:
C, 50.42; H, 2.96; N, 5.88. Found: C, 50.20; H, 3.02; N, 5.70
Example 214
2-(r? 2 2-Trifiuoroethyl)-4_(3-nitropheny_I)-5-j4-(methylsulfonvl) henylJ-
3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 211,
substituting 3-nitrobenzeneboronic acid in place of 3,4-dimethylbenzeneboronic
acid (yield: 40 mg, 22%). 1 H NMR (300 MHz, CDCI3) S 3.05 (s, 3H), 4.92 (q, J
= 9
Hz, 2H), 7.36 (d, J = 9 Hz, 2H), 7.45-7.60 (m, 2H), 7.91 (d, J = 9 Hz, 2H),
7.95 (s,
1 H), 8.05 (m, 1 H), 8.15-8.21 (m, 1 H). MS (DCI-NH3) m/z 471 (M+NH4)+. Anal.
caic. for C1 gH14C12F3N3O5S-0.5 EtOAc: C, 50.70; H, 3.64; N, 8.44. Found: C,
50.61; H, 3.58; N, 8.53
Example 215
2-(2 2 2-Trifluoroethyl)-4 ;(2-methylphenyl)-5 44-(methylsulfony1)phenyll-
3(2H);
p,yridazinone
The title compound was prepared according to the method of Exampie 211,
substituting 2-methylbenzeneboronic acid in place of 3,4-
dimethylbenzeneboronic
acid (yield: 45 mg, 27%). .1 H NMR (300 MHz, CDCI3) b 2.05, 2.12 (2s, 3H),
3.01 (s,
3H), 4.75-5.05 (m, 2H), 6.88 (d, J = 9 Hz, 1 H), 7.03-7.25 (m, 3H), 7.31 (d, J
= 9 Hz,
2H), 7.85 (d, J = 9 Hz, 2H), 7.95 (s, 1 H). MS (DCI-NH3) m/z 440 (M+NH4)+.
Anal.
calc. for C20H17F3N203S: C, 55.10; H, 4.27; N, 6.42. Found: C, 55.17; H, 4.18;
N,
6.10
-140-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 216
2 12.2.2-Trifluoroethyl)-4-(4-vinvlohenyJ)-5-[4-(methyJsulfonyl)phenYll-3( 2H1-
Ryridazinone
The title compound was prepared according to the method of Example 211,
substituting 4-vinylbenzeneboronic acid in place of 3,4-dimethylbenzeneboronic
acid (yield: 56 mg, 32%). 1 H NMR (300 MHz, CDCI3) 5 3.06, 3.08 (2s, 3H), 4.78-
4.95 (m, 2H), 5.30 (t, J = 6 Hz, 1 H), 5.65, 5.75(2d, J = 18 Hz, 1 H), 6.58-
6.92 (m, 1 H),
7.1-7.4 (m, 6H), 7.75-8.08 (m, 3H). MS (DCI-NH3) m/z 452 (M+NH4)+. Anal. calc.
for C21 H17F3N203S: C, 58.06; H, 3.94; N, 6.45. Found: C, 57.82; H, 4.01; N,
6.09
Example 217
2-(2.2.2-Trifluoroethyl)-4-[3-(trifluoromethyl)nhenyll-5-[4-
(methylsulfony[)phenvlJ-
3(2H)-pyridazinone
The title compound was prepared according to the method of Example 211,
substituting 3-trifluoromethylbenzeneboronic acid in place of 3,4-
dimethylbenzene-
boronic acid (yield: 120 mg, 63%). 1 H NMR (300 MHz, CDCI3) S 3.03, 3.08 (2s,
3H), 4.75-4.98 (m, 2H), 7.30-7.60 (m, 6H), 7.75-8.10 (m, 3H). MS (DCI-NH3) m/z
494 (M+NH4)+. Anal. caic. for C20H14F6N203S: C, 50.42; H, 2.96; N, 5.88.
Found: C, 50.38; H, 2.97; N, 5.74
Example 218
2-(2.2.2-Trifluoroethvl)-4-(3-fluoro-4-methox henyl)-5-[4-
(methvlsulfonyl)phenvll-
3(2H)-Ryridazinone
The title compound was prepared according to the method of Example 211,
substituting 3-fluoro-4-methoxybenzeneboronic acid in place of 3,4-dimethyl-
benzeneboronic acid (yield: 32 mg, 18%). 1 H NMR (300 MHz, CDCI3) S 3.05, 3.09
(2s, 3H), 3.85, 3.87 (2s, 3H), 4.78-4.90 (m, 2H), 6.60-7.10 (m, 3H), 7.30-8.15
(m,
5H). MS (DCI-NH3) m/z 474 (M+NH4)+. Anal. calc. for C20H16F4N204S-0.5
H20: C, 51.61; H, 3.68; N, 6.01. Found: C, 51.52; H, 3.65; N, 5.93
Example 219
2-(2.2.2-Trifluoroethyl)-4-(3-fluoro-4-methyl enyl)-5-[4-(m t
ylsulf.onyl)phenrll-
3(2H)-Rvridazinone
The title compound was prepared according to the method of Example 211
substituting 3-fluoro-4-methylbenzeneboronic acid in place of 3,4-dimethyl-
benzeneboronic acid (yield: 58 mg, 33%). 1 H NMR (300 MHz, CDCI3) 8 2.21, 2.25
-141-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
(2d, J = 1.5 Hz, 3H), 3.50,3.55 (2s, 3H), 4.75-4.95 (m, 2H), 6.56-7.15 (m,
3H), 7.30-
8.10 (m, 5H). MS (DCI-NH3) m/z 458 (M+NH4)+. Anal. calc. for
C20H16F4N203S-0.5 H20: C, 53.45; H, 3.81; N, 6.23. Found: C, 53.14; H, 3.80;
N,
5.97
Example 220
2-(2.2.2-Trifluoroethyj)-4-(3.5-difluoro-4-methoxy enyl)-5-(4-(methylsulfonvll-
Rhe - (2H)-Rxridazinone
The title compound was prepared according to the method of Example 211,
substituting 3,5-difluoro-4-methoxybenzeneboronic acid in place of 3,4-
dimethylbenzeneboronic acid. 1 H NMR (300 MHz, CDCI3) 5 2.9, 3.1 (2s, 3H),
3.92,
4.01 (2s, 3H), 4.78-4.95 (m, 2H), 6.25-6.80 (m, 1 H), 7.30-7.5 (m, 2H), 7.7-
8.15 (m,
4H). MS (DCI-NH3) m/z 492 (M+NH4)+. Anal. calc. for C20H15F5N204S: C,
50.64; H, 3.19; N, 5.90. Found: C, 50.542; H, 3.41; N, 5.67
Example 221
2-(2.2.2-Trifluoroethyl)-4-(1.3-dihydro-1-oxo-5-isobenzofuranyl)-5-[4-(methvl-
suffonyl)phe IlY11-3(2 H)-Ryridazinone
6-Bromophthalide (300 mg, 1.40 mmol, Teppema et al Recl. Trav. Chim.
Pays-Bays ,1923, 42, 47) and hexamethyiditin (326 L, 1.55 mmol) were
dissolved
in toluene (5 mL), degassed with a nitrogen stream for 5 minutes, treated with
(Ph3P)4Pd (79 mg) and heated at reflux for 1 hour. The reaction was cooled and
directly purified by chromatography on a Biotage*40S column (pretreated with
hexanes-TEA 400:1 then rinsed with hexanes) eluted with 4:1 hexanes-ethyl
acetate. The product fractions were combined and evaporated to provide the
intermediate, 6-(trimethyltin)phthalide (yield: 362 mg, 87%). * trade-mark
The tin reagent (180 mg, 0.61 mmol), prepared above, and 2-(2,2,2-trifluoro-
ethyl)-4-chloro-5-(4-(methylsulfonyl)phenyl)-3(2H)-pyridazinone, prepared in
Example 193E, (223 mg, 0.61 mmol) were dissolved in dry toluene (10 mL),
degassed with an nitrogen stream for 5 minutes, treated with (Ph3P)4Pd (34 mg)
and heated at reflux for 1 day. The reaction was cooled and directly purified
by
chromatography on a Biotage 40S column eluted with 4:1 hexanes-ethyl acetate.
The product fractions were combined and evaporated to provide the title
compound
along with the 4-(1,3-dihydro-l-oxo-6-isobenzofuranyl)-isomer in a 9:1 ratio.
Further manipulations to attempt to remove the minor isomer (ie
chromatography,
recrystallization from ethyl acetate-hexanes) failed (yield: 176 mg, 62%).
M.p. 237-
-142-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
239 C. 1 H NMR (300 MHz, CDCI3) S 3.07 (s, 3H), 4.91 (q, J = 8 Hz, 2H), 5.30
(s, 2
H, major isomer), 5.33 (s, 2 H, minor isomer), 7.20 (dd, J = 1 Hz, 7 Hz, 1 H),
7.36 (d,
J = 8 Hz, 2H), 7.52 (s, 1 H), 7.79 (d, J = 7 Hz, 1 H), 7.92 (d, J = 8 Hz, 2H),
7.96 (s, 1 H).
MS (DCI-NH3) m/z 482 (M+NH4)+. Anal, caic. for C21 H15F3N205S: C, 54.31; H,
3.26; N, 6.03. Found: C, 54.15; H, 3.12; N, 5.76.
Example 222
2; (2.2.2-TrifluoroethY1)-4-(2-oroRenyll-5 f4-(methylsulfony.I)phenyl)-3(H) ;
nvridazinone
A suspension of 2-(2,2,2-trifluoroethyl)-4-chloro-5-[4-(methylsulfonyi)phenyl]-
3(2H)-pyridazinone (200 mg, 0.546 mmol), prepared according to the method of
Example 193E, in THF (27 mL) was cooled to -78 C. A solution of isopropenyl-
magnesium bromide (2.8 mL, 0.5 M in THF, Aldrich) was added. The reaction was
warmed to room temperature and stirred for 30 minutes. The reaction was
quenched at 0 C by the addition of saturated ammonium chloride solution and
partitioned between ethyl acetate and additional ammonium chloride solution.
The
organic layer was washed with brine, dried over sodium sulfate, filtered, and
concentrated under reduced pressure to provide a reddish brown solid. The
crude
material was dissolved in methylene chloride and adsorbed onto silica gel (2
g).
Solvent was removed under reduced pressure, the adsorbed silica gel layered
over an Extract-Clean Cartridge (Alltech, packing: 5 g silica gel) and the
cartridge
eluted with a hexanes/acetone step gradient consisting of 40 mL of the
following
mixtures: hexanes, 8:1 hexanes/acetone, 4:1, 2:1, and 1:1. Fractions
containing
desired product were combined, concentrated, and further purified using HPLC
(Technikrom Kromasil 60-5sil column, 20 mm x 25 cm). The column was eluted
with a linear gradient consisting of 30% ethyl acetate/hexanes to 100% ethyl
acetate at 10 mUmin over 50 minutes. Fractions containing the title product
were
combined and concentrated under reduced pressure to provide a pale yellow
solid
(yield: 99.3 mg, 49%). M.p. 192-195 C. 1 H NMR (300 MHz, CDCI3) 5 8.03 (d, J
17.4 Hz, 2H), 7.76 (s, 1 H), 7.55 (d, 2H, J = 17.4 Hz), 5.23 (br s, 1 H), 4.84
(m, 3H),
3.11 (s, 3H), 1.98 (s, 3H). MS (DCI-NH3) m/z 373 (M+H)+, m/z 390 (M+NH4)+.
Anal. calc. for C16H15F3N203S: C, 51.61; H, 4.06; N, 7.52. Found: C, 51.72; H,
4.24; N, 7.35. Kromasil is a trade-mark
-143-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 223
? (2.2.2- rifluoroethX()-4-(2-buten-2-Y1}-5-[4-(methylsulfonyl)nhenY1)-3( 2H)-
Ryridazinone
The product was prepared according to the method of Example 222
substituting 1-methyl-l-propenylmagnesium bromide in place of isopropenylmag-
nesium bromide to provide a mixture of geometric isomers (-3:1 ratio) as an
off-
white solid (yield: 44.8 mg, 21 %). M.p. 175-180 C. 1 H NMR (300 MHz, CDCI3)
S
8.03 (d, J = 18.0 Hz, 1.5H), 8.01 (d, J = 18.0 Hz, 0.5H), 7.29 (s, 0.75H),
7.28 (s,
0.25H), 7.56 (d, J- 17.4 Hz, 1.5H), 7.51 (d, J- 17.4 Hz, 0.5H), 5.55 (m,
0.75H),
5.33 (m, 0.25H), 5.86 (q, J= 17.4 Hz, 2H), 3.12 (s, 2.25H), 3.11 (s, 0.75H),
2.88 (m,
2H), 2.85 (m, 1 H), 1.27 (m, 3H). MS (DCI-NH3) m/z 387 (M+H)+, m/z 404
(M+NH4)+, m/z 421 (M+2NH4-H)+. Anal. caic. for C17H17F3N203S: C, 52.85; H,
4.43; N, 7.25. Found: C, 53.16; H, 4.68; N, 6.92.
Example 224
2-(2.2.2-Trifluoroethyl)-4-(3-fluorobenzyl)-5-[4-(meth lsy ulfonyl,)phe0y11-3~
2H1-
Qyridazinone
224A. 3-Fluorobenzyl magnesium bromide.
3-Fluorobenzyl bromide (613 L, 5 mmol), followed by dibromoethane (10
L), was added dropwise to an oven-dried flask containing small pieces of
magnesium ribbon (134 mg, 5.5 mmol) and diethyl ether (12 mL). Gas evolution
was noted followed by gentle reflux of the ether. The reaction was stirred
until gas
evolution ceased and most of the magnesium had dissolved. The resulting pale
yellow solution of 3-fluorobenzylmagnesium bromide was used directly in the
next
reaction.
224B. 2-(2.2.2-Trifluoroethyl)-4-(3-fluorobenzyl)-5-[4-(methylsulfonyJ) henvll-
3(2H)-Ryridazinone.
A suspension of 2-(2,2,2-trifluoroethyl)-4-chloro-5-[4-(methylsulfonyl)phenyl]-
3(2H)-pyridazinone (200 mg, 0.546 mmol), prepared according to the method of
Example 193E, in THF (10 mL) was cooled to 0 C. A solution of 3-fluorobenzyl
magnesium bromide (4.0 mL, -0.42 M in diethyl ether), prepared above was
added. The reaction was stirred at 0 C for 3 hours, quenched by the addition
of
saturated ammonium chloride solution, and partitioned, between ethyl acetate
and
additional ammonium chloride solution. The organic layer was washed with
brine,
dried over sodium sulfate, filtered, and concentrated under reduced pressure
to
provide a yellow oil. The crude material was dissolved in methylene chloride
and
-144-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
adsorbed onto silica gel (2 g). Solvent was removed under reduced pressure,
the
silica gel with the product adsorbed was layered over an Extract-Clean
Cartridge
(Alitech, packing: 10 g silica gel) and the cartridge eluted with a
hexanes/acetone
step gradient consisting of 60 mL of each of the following mixtures: hexanes,
8:1
hexanes/acetone, 4:1, 2:1, and 1:1. Fractions containing desired product were
combined, concentrated, and further purified using HPLC (Technikrom Kromasil
60-5 sil silica column, 20 mm x 25 cm). The column was eluted with a linear
gradient consisting of 30% ethyl acetate/hexanes to 100% ethyl acetate at 10
mL/min. for 50 minutes. Fractions containing the title product were combined
and
concentrated under reduced pressure to provide a pale yellow solid (yield:
130.9
mg, 54%). M.p. 58-62 C. 1 H NMR (300 MHz, CDCI3) 5 8.07 (d, J- 18.0 Hz, 2H),
7.73 (s, 1 H), 7.47 (d, J- 17.4 Hz, 2H), 7.18 (m, 1 H), 6.88 (m, 1 H), 6.76
(br d, J =
15.6 Hz, 1 H), 6.68 (br d, J = 18.6 Hz, 1 H), 4.86 (q, J- 17.4 Hz, 2H), 3.93
(s, 2H),
3.12 (s, 3H). MS (DCI-NH3) m/z 441 (M+H)+, m/z 458 (M+NH4)+, m/z 475
(M+2NH4-H)+. Anal. calc. for C20H16F4N203S: C, 54.54; H, 3.66; N, 6.36.
Found: C, 54.52; H, 3.81; N, 6.17.
Example 225
2-(2.2.2-Trifluo roethyl)-4-(1-cyclohexenyl)-544-(methvlsu lfo nyl)ohe n,yõ(]-
3(2 H)-
Ryridazinone
225A. 1-Cyclohexenyltriflate.
n-Butyllithium (2.5M in hexanes, 2.20 mL, 5.50 mmol) was added to a
solution of diisopropylamine (0.77 mL, 5.50 mmol) in THF (20 mL) at-78 C. The
resulting pale yellow solution was warmed to 0 C for 30 minutes then was
cooled
to -78 C. Cyclohexanone (0.52 mL, 5.0 mmol) was added and the nearly
colorless
solution was warmed to 0 C for 1 hour. N-Phenyltrifluoromethanesulfonimide
(1.79 g, 5.5 mmol) was added as a solid. The solution was stirred at room
temperature for 12 hours. The reaction mixture was then partitioned between
diethyl ether and saturated sodium bicarbonate solution. The ether layer was
washed with water then brine, dried over sodium sulfate, filtered, and
concentrated
under reduced pressure. The crude material was purified by flash
chromatography
(20:1 hexanes/ethyl acetate) to provide the triflate as a pale yellow oil
(yield: 0.73 g,
64%).
225B. 1-Cyclohexenyltrimethyltin
A solution of 1-cyclohexenyltriflate (412 mg, 1.79 mmol), prepared according
to the method of Example 225A, and LiCI (380 mg, 8.95 mmol) in THF (9 mL) was
-145-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
deoxygenated by bubbling a stream of N2 through the solution. Hexamethylditin
(339 L, 1.61 mmol) and tetrakis(triphenylphosphine)palladium(0) (414 mg, 0.36
mmol) were added and the reaction heated at reflux for 12 hours. The reaction
was
cooled to room temperature and partitioned between diethyl ether and saturated
sodium bicarbonate solution. The ether layer was washed with water then brine,
dried over sodium sulfate, filtered, and concentrated under reduced pressure.
The
crude material was dissolved in hexanes (1 mL) and loaded onto an Extract-
Clean
Cartridge (Alitech, packing: 10 g silica gel) which had been wetted with 10%
triethylamine in hexanes. The cartridge was eluted with hexanes and fractions
containing the triflate combined and concentrated under reduced pressure to
provide 1-cyclohexenyltrimethyltin as a clear oil (yield: 150 mg, 34%).
225C. 2-(2.2.2-Trifluoroethvl)-4-(1-cyclohexenY1)-5-[4-(methvlSu yJ)p .nvll-
3(2H)-pyridazinone.
A solution of 1-cyclohexenyitrimethyltin (150 mg, 0.61 mmol), prepared
according to the method of Example 225B, and 2-(2,2,2-trifluoroethyl)-4-chloro-
5-
[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone (172 mg, 0.47 mmol), prepared
according to the method of Example 193E, in anhydrous N-methylpyrrolidinone
(1 mL) was deoxygenated with nitrogen. Dichlorobis(triphenylphosphine)
palladium(II) (6.6 mg, 0.009 mmol) and [1,1'-bis(diphenylphosphino)ferrocene]
dichloropalladium(II) (7.7 mg, 0.009 mmol) were added and the reaction heated
at
80 C for 16 hours. The reaction mixture was cooled to room temperature and
partitioned between diethyl ether and water. The ether was washed with two
additional portions water then brine, dried over sodium sulfate, filtered, and
concentrated under reduced pressure. The crude material was dissolved in
acetone and adsorbed onto silica gel (1 g). Solvent was removed under reduced
pressure, the adsorbed silica gel layered over an Extract-Clean Cartridge
(Ailtech, packing: 10 g silica gel) and the cartridge eluted with a
hexanes/acetone
step gradient consisting of the following mixtures: hexanes (60 mL), 8:1
hexanes/acetone (80 mL), 4:1 hexanes/acetone (150 mL). Fractions containing
desired product were combined, concentrated, and further purified using HPLC
(Technikrom Kromasil 60-5 sil silica column, 20 mm x 25 cm). The column was
eluted with a linear gradient consisting of 30% ethyl acetate/hexanes to 100%
ethyl
acetate at 10 mL/min. over 50 minutes. Fractions containing the title product
were
combined and concentrated under reduced pressure to provide a pale yellow foam
(yield: 95.0 mg, 49%). M.p. 75-81 C. 1 H NMR (300 MHz, CDCI3) S 8.02 (d, J-
17.4 Hz, 2H), 7.76 (s, 1 H), 7.55 (d, J- 17.4 Hz, 2H), 5.51 (br s, 1 H), 4.83
(br q, J-
-146-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
16.2 Hz, 3H), 3.11 (s, 3H), 2.18 (br, 2H), 1.96 (br, 2H), 1.70-1.50 (m, 4H).
MS (DCI-
NH3) m/z 413 (M+H)+, m/z 430 (M+NH4)+, m/z 447 (M+2NH4-H)+. Anal. calc. for
C19H1 gF3N2O3S: C, 55.33; H, 4.64; N, 6.79. Found: C, 55.53; H, 4.71; N, 6.55.
Example 226
2-(2.2.2-TrifluoroethXl)-4- - ethylbutyl)-5-[3-fluoro-4-(aminosu(fonY1) ho
env11-
3(2H)-pyridazinone
226A. 3-Fluoro-4-(methylthio)benzeneboronic acid.
3-Fluoro-4-(methylthio)benzeneboronic acid was prepared according to the
method of Examplel, substituting 4-bromo-3-fluorothioanisole in place of 4-
bromo-
thioanisole.
226B. 2-Benzvl-4-methoxy-5-bromo-3(2 H)-pyridazinone
2-Benzyl-4-methoxy-5-bromo-3(2H)-pyridazinone is prepared according to
the method of Example 83B starting with 2-benzyl-4,5-dibromo-3(2H)-
pyridazinone,
in place of 2-(2,2,2-trifluoroethyl)-4,5-dibromo-3(2H)-pyridazinone and
substituting
methanol in place of isopropanol.
226C. 2-Benzyl-4-methoxy-5-[3-fiuoro-4-(meth 1Y thio)Rhenyl]-3(2H)-
oyridazinone
3-Fluoro-4-(methylthio)benzeneboronic acid and 2-benzyl-4-methoxy-5-
bromo-3(2H)-pyridazinone were coupled according to the method of Example 83C
to provide 2-benzyl-4-methoxy-5-[3-fluoro-4-(methylthio)phenyll-3(2H)-
pyridazinone as a yellow solid (yield: 4.98 g, 91 %). 1 H NMR (300 MHz, CDCI3)
a
7.76 (s, 1 H), 7.47 (m, 2H), 7.39-7.21 (m, 7H), 5.34 (s, 2H), 4.13 (s, 3H),
2.51 (s, 3H).
MS (DCI-NH3) m/z 357 (M+H)+, m/z 374 (M+NH4)+.
226D. 3-Meth, ly butylmaanesium bromide
An oven-dried flask containing small pieces of magnesium ribbon (134 mg,
5.5 mmol) was charged with diethyl ether (12 mL). 1-Bromo-3-methylbutane (600
L, 5 mmol) was added dropwise, followed by dibromoethane (10 L). The
reaction required heating at gentle reflux before gas evolution was observed.
The
reaction was refluxed for 3 hours and cooled to room temperature. The pale
gray
solution of 3-methylbutylmagnesium bromide was used in the next reaction.
226E. 2-Benzyl-4-(3-methylbutyl)-5 j3-fluoro-4-(methylthio)phenyl]-3(2H)-
~,tiridazinone
A solution of 2-benzyl-4-methoxy-5-[3-fluoro-4-(methylthio)phenyl]-3(2H)-
pyridazinone (500 mg, 1.40 mmol), prepared according to the method of Example
226C, in THF (20 mL) was cooled to-78 C. 3-Methylbutylmagnesium bromide (5
-147-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
mL, 1.96 mmol), prepared in Example 226D, was added, dropwise. Upon
completion of the addition, the reaction mixture was placed in an ice bath.
After 2.5
hours, the reaction was quenched by adding saturated ammonium chloride
solution. The crude reaction mixture was partitioned between ethyl acetate and
additional ammonium chloride solution. The organic layer was washed with
brine,
dried over sodium sulfate, filtered, and concentrated under reduced pressure
to
provide a yellow oil (yield: 550 mg, 99%). 1 H NMR (300 MHz, CDCI3) a 7.67 (s,
1 H), 7.49 (m, 2H), 7.39-7.25 (m, 4H), 7.02 (m, 2H), 5.35 (s, 2H), 2.57-2.49
(m, 2H),
2.52 (s, 3H), 1.62-1.36 (m, 3H), 0.83 (d, 6H, J = 12.0 Hz). MS (DCI-NH3) m/z
397
(M+H)+, m/z 414 (M+NH4)+. MS (DCI-NH3) m/z 397 (M+H)+, m/z 414 (M+NH4)+.
226F. 4- -M thylbutyl)õ543-fluoro-4-(methvlthio)phgpyIl-3(2 H)-pyridazinnnP.
2-Benzyl-4-(3-methylbutyl)-5-[3-fluoro-4-(methylthio)phenyl]-3(2H)-
pyridazinone (550 mg, 1.39 mmol), prepared in Example 226E, was debenzylated
according to the method of Example 11 to provide 4-(3-methylbutyl)-5-[3-fluoro-
4-
(methylthio)phenyl]-3(2H)-pyridazinone as a pale yellow solid (yield: 375 mg,
88%). 1H NMR (300 MHz, CDCI3) S 7.65 (s, 1H), 7.34 (dd, 1 H, J= 16.2, 16.2
Hz),
7.11-6.98 (m, 2H), 2.60-2.50 (m, 2H), 2.54 (s, 3H), 1.65-1.37 (m, 3H), 0.83
(d, 6H, J
= 12.0 Hz). MS (DCI-NH3) m/z 307 (M+H)+, m/z 324 (M+NH4)+ MS (DCI-NH3) m/z
307 (M+H)+, m/z 324 (M+NH4)+.
226G. 2-(2.2.2-Trifluoroethvl)-4-(3-methvl-~yl)-5-[3-fh,oro-4-(methylthio) hp
env_Il
3(2H) -Ryridazinone.
4-(3-Methylbutyl)-5-[3-fluoro-4-(methylthio)phenyl]-3(2H)-pyridazinone (375
mg, 1.23 mmol), prepared in Example 226F, was alkylated according to the
method
of Example 20 to provide 2-(2,2,2-trifluoroethyl)-4-(3-methylbutyl)-5-[3-
fluoro-4-
(methylthio)phenyl]-3(2H)-pyridazinone as a clear oil (yield: 331 mg, 69%). 1
H
NMR (300 MHz, CDCI3) S 7.67 (s, 1 H), 7.34 (dd, 1 H, J = 16.8, 16.8 Hz), 7.11-
6.98
(m, 2H), 4.82 (dd, 2H, J = 17.4, 17.4 Hz), 2.60-2.51 (m, 2H), 2.53 (s, 3H),
1.61-1.32
(m, 3H), 0.85 (d, 6H, J = 12.0 Hz). MS (DCI-NH3) m/z 389 (M+H)+, m/z 406
(M+NH4)+. MS (DCI-NH3) m/z 389 (M+H)+, m/z 406 (M+NH4)+.
226H. 2-(2.2.2-Trifluoroethvl)-4-(3-methylbutyl)-5-[3- unrc,-4- th, Isulf'
yl,L
phenyQ-3(2H)-pyridazinone.
2-(2,2,2-Trifluoroethyl)-4-(3-methylbutyl)-5-[3-fluoro-4-(methylthio)phenyl]-
3(2H)-pyridazinone (331 mg, 0.85 mmol), prepared in Example 226G, was oxidized
according to the method of Example 5 using only one equivalent of MCPBA to
provide 2-(2,2,2-trifluoroethyl)-4-(3-methylbutyl)-5-[3-fluoro-4-
(methyisulfinyl)-
phenyl]-3(2H)-pyridazinone as an off-white solid (yield: 240 mg, 69%). 1 H NMR
-148-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
(300 MHz, CDCI3) S 8.02 (dd, 1 H, J = 15.0, 15.0 Hz), 7.67 (s, 1 H), 7.37 (dd,
1 H, J
17.4, 3.0 Hz), 7.11 (dd, 1 H, J = 18.6, 3.0 Hz), 4.84 (dd, 2H, J = 17.4, 17.4
Hz), 2.91
(s, 3H), 2.53 (m, 2H), 1.60-1.35 (m, 3H), 0.57 (d, 6H, J = 12.0 Hz). MS (DCI-
NH3)
m/z 405 (M+H)+, m/z 422 (M+NH4)+. MS (DCI-NH3) m/z 405 (M+H)+, m/z 422
(M+NH4)+.
2261. 2-(2.2.2-Trifluoroethvl)-4-(3-methylbutyl)-5-j3-fluoro-4-(aminosulfonvlj-
phenylJ-3(2H)-pyridazinone
2-(2,2,2-Trifluoroethyl)-4-(3-methylbutyl)-5-[3-fluoro-4-(methylsulfinyl)-
phenyl]-3(2H)-pyridazinone (240 mg, 0.594 mmol), prepared in Example 226H,
was converted to the sulfonamide according to the procedure of Example 68 to
provide the title compound as a white solid (yield: 109 mg, 44%). M.p. 153-156
C.
1 H NMR (300 MHz, CDCI3) S 8.07 (dd, J = 15.0, 15.0 Hz, 1 H), 7.74 (s, 1 H),
7.27-
7.19 (m, 2H), 5.14 (br s, 2H), 4.83 (q, J = 18.0 Hz, 2H), 2.52 (m, 2H), 1.55
(m, 1 H),
1.41 (m, 2H), 0.85 (d, J = 12.6 Hz, 6H). MS (ESI (-)) m/z 420 (M-H)-. Anal.
calc. for
C17H19F4N303S: C, 48.45; H, 4.54; N, 9.97. Found: C, 48.24; H, 4.56; N, 9.80.
Example 227
2-(2.2.2-Trifluoroethyl}-4-benzvl-5-[4-(methvlsulfonyl)12henyll-3(2H)-
pyridazinone
The title compound was prepared by adding 1.0 M benzylmagnesium
chloride in ether (0.53 mL, 0.53 mmol) to a THF (20 mL) solution of 2-(2,2,2-
trifluoroethyl)-4-chloro-5-[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone (150
mg,
0.41 mmol), prepared according to the method of Example 193E, at 0 C, then
allowing the mixture to warm to room temperature over 2 hours. After an
aqueous
work-up, the crude material was purified by column chromatography (silica gel,
65:35 hexanes/ethyl acetate) and crystallized from ethyl acetate/hexanes to
provide
white, crystalline product (yield: 74 mg, 43%). M.p. 112-114 C. 1 H NMR (300
MHz, CDCI3) S 3.12 (s, 3H), 3.94 (s, 2H), 4.85 (q, J = 12 Hz, 2H), 6.99 (dd, J
= 7.5
Hz, 3 Hz, 2H), 7.2 (m, 3H), 7.48 (d, J = 9 Hz, 2H), 7.72 (s, 1 H), 8.06 (d, J
= 9 Hz, 2H).
MS (DCI-NH3) m/z 423 (M+H)+. Anal. caic. for C20H17F3N203S: C, 56.86; H,
4.05; N, 6.63. Found: C, 56.60; H, 4.13; N, 6.57.
Example 228
2-(4-Fluorophenvl)-4-cyclohexyl-5-[4-(methylsulfonyl)12henyll-3(2H)-
pyridazinone
A solution of 2-(4-fluorophenyl)-4-methoxy-5-[4-(methylthio)phenyl]-3(2H)-
pyridazinone, prepared in Example 194C, (200 mg, 0.51 mmol) in THF (8 ml) was
cooled to -78 C and treated with cyclohexylmagnesium chloride, 2 M solution
in
-149-
CA 02578858 2007-03-05
WO 99/10331 PCTIUS98/16479
ether (0.31 ml, 0,7 mmol). The reaction mixture was stirred at -78 C for 2
hours
and then was warmed up to room temperature by removing the cooling bath.
Stirred at room temperature for 2 hours water (50 ml) was added to the
reaction
mixture and extracted with ethyl acetate (50 ml). The organic layer was dried
over
MgSO4 and concentrated in vacuo. The resulting methyl sulfide compound was
purified by flash chromatography (Si02, eluting with 9:1 hexanes:ethyl
acetate) to
provide the desired product (yield: 128 mg, 69%). MS (DCI-NH3) m/z 395 (M+H)+,
412 (M+NH4)+.
The methyl sulfide compound, prepared above, (122 mg, 0.3 mmol) in
CH2CI2 (10 ml) at 0 C, was treated with CH3CO3H (0.3 ml, 1 mmol). The reaction
was complete in 2 hours. The reaction mixture was diluted with CH2CI2 and
washed with saturated NaHCO3 and brine respectively. The resulting crude
residue was purified by flash chromatography (Si02, eluting with 1:1
hexanes:ethyl
acetate) to provide the desired product (yield: 110 mg, 93%). M.p. 231-233 C.
1 H
NMR (300 MHz, DMSO-d6) 8 1.1 (m, 3H), 1.6 (m, 6H), 2.15 (m, 2H), 7.35 (t, 2H),
7.65 (m, 2H), 7.73 (dd, 2H) 7.93 (s, 1 H), 8.1 (d, 2H). MS (DCI-NH3) m/z 427
(M+H)+, 444 (M+NH4)+. Anal. caic. for C23 H23FN2O3S=0.75 H20: C, 64.77; H,
5.44; N, 6.57. Found: C, 62.86; H, 5.53; N, 5.78.
Example 229
2-(4-Fluorophenyl)-4-(4-met hylphe (2H)-
Ryridazinone
The title compound was prepared according to the method of Example 228,
substituting p-tolylmagnesium bromide in place of cyclohexylmagnesium chloride
(yield: 90 mg, 39%). M.p. 242-244 C. 1 H NMR (300 MHz, DMSO-d6) 8 2.25 (s,
3H), 8 3.25 (s, 3H), 7.1 (t, 4H), 7.35 (t, 2H), 7.5 (d, J = 9 Hz, 2H), 7.7
(dd, 2H) 7.9 (d,
J = 9 Hz, 2H), 8.2 (s, 1 H). MS (DCI-NH3) m/z 435 (M+H)+, 452 (M+NH4)+. Anal.
caic. for C24H19FN203S=0.5 H20: C, 66.34; H, 4.41; N, 6.45. Found: C, 64.61;
H,
4.57; N, 6.10.
Example 230
2-(4-FluoroohenY1)-4-benzyl-5-[4-(methylsulfonyj)phenyll-3(2H)-pyridazinone
The title compound was prepared according to the method of Example 228,
substituting benzylmagnesium bromide in place of cyclohexyimagnesium chloride
(yield: 179 mg, 81%). M.p. 180-182 C. 1 H NMR (300 MHz, DMSO-d6) 8 3.3 (s,
3H), 7.0 (d, 2H), 7.2 (m, 3H), 7.35 (t, 2H), 7.65 (m, 2H)7.72 (d, 2H) 8.05 (m,
3H). MS
-150-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
(DCI-NH3) m/z 435 (M+H)+, 452 (M+NH4)+. Anal. caic. for C24H19FN2O3S-0.5
H20: C, 66.34; H, 4.41; N, 6.45. Found: C, 66.48; H, 4.17; N, 6.36.
Example 231
2-(4-Fluoronhenyl)-4-(ohenylethynyl)-5-[4-(methylsulfonY[)ohenyll-3l2HL
pyridazinone
The title compound was prepared according to the method of Example 228,
substituting phenylacetylene magnesium bromide in place of
cyclohexylmagnesium chloride (yield: 150 mg, 55.5%). M.p. 203-204 C. 1 H NMR
(300 MHz, DMSO-d6) S 3.3 (s, 3H), 7.4 (m, 8H), 7.7 (m, 2H), 8.16 (m, 4H) ;
8.35 (s,
1 H). MS (DCI-NH3) m/z 435 (M+H)+, 452 (M+NH4)+. Anal. catc. for
C25H17FN203S: C, 67.56; H, 3.86; N, 6.30. Found: C, 67.63; H, 3.86; N, 6.30.
Example 232
2-(3.4-DifluoroRhenyl)-4-cyclohexyl-5-[4-(methylsulfonyl)ohenyll-3(2H)=
pyridazinone
The title compound was prepared according to the method of Example 228,
starting with 2-(3,4-difluorophenyl)-4-methoxy-5-[4-(methylthio)phenyl]3(2H)-
pyridazinone in place of 2-(4-fluorophenyl)-4-methoxy-5-[4-
(methylsulfonyl)phenyl]-
3(2H)-pyridazinone (yield: 245 mg, 80%). M.p. 80-83 C. 1 H NMR (300 MHz,
DMSO-d6) 8 1.1 (m, 3H), 1.6 (m, 6H), 2.15 (m, 2H), 7.5 (m, 1 H), 7.6 (m, 2H),
7.7 (d,
2H), 7.78 (m, 2H), 7.93 (s, 1 H), 8.1 (d, 2H). MS (DCI-NH3) m/z 445 (M+H)+,
462
(M+NH4)+. Anal. calc. for C23H22F2N203S: C, 62.15; H, 4.99; N, 6.30. Found: C,
62.65; H, 5.25; N, 5.97.
Example 233
2-(3.4-Difluoronhenyl)-4-benzvl-5-[4-(methylsulfonyl)phenxll-3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 228,
starting with 2-(3,4-difluorophenyl)-4-methoxy-5-[4-(methylsulfonyl)phenyl]-
3(2H)-
pyridazinone in place of 2-(3,4-difluorophenyl)-4-methoxy-5-[4-
(methylthio)phenyl]-
3(2H)-pyridazinone and substituting benzylmagnesium bromide in place of
cyclohexylmagnesium chloride (yield 206 mg, 66%). M.p. 166-168 C. 1 H NMR
(300 MHz, DMSO-d6) 8 3.3 (s, 3H), 3.9 (s, 2H), 7.0 (d, 2H), 7.2 (m, 3H), 7.6
(m, 2H),
7.72 (d, 2H), 7.8 (d, 1 H), 8.05 (d, 2H), 8.12 (s, 1 H). MS (DCI-NH3) m/z 453
(M+H)
470 (M+NH4)+. Anal. caic. for C24H1 9F2N203S: C, 63.71; H, 4.01; N, 6.19.
Found: C, 63.53; H, 4.33; N, 5.76.
-151-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 234
? (3 4-DifluoroRhenyl)-4-(4-methyll2hgrlyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 228,
starting with 2-(3,4-difiuorophenyl)-4-methoxy-5-[4-(methylsulfonyl)phenyl]-
3(2H)-
pyridazinone in place of 2-(3,4-difluorophenyl)-4-methoxy-5-[4-
(methylthio)phenyl]-
3(2H)-pyridazinone and substituting cyclohexylmagnesium chloride in place of p-
tolylmagnesium bromide (yield: 140 mg, 56%) . M.p. 190-192 C. 1 H NMR (300
MHz, DMSO-d6) S 2.28 (s, 2H), S 3.25 (s, 3H), 7.1 (s, 4H), 7.5 (m, 4H), 7.89
(m, 3H),
8.05 (d, 2H), 8.23 (s, 1 H). MS (DCI-NH3) m/z 453 (M+H)+, 470 (M+NH4)+. Anal.
calc. for C24F2H18N203S: C, 63.71; H, 4.01; N, 6.19. Found: C, 63.69; H, 4.29;
N,
5.96.
Example 235
2-(3.4-Difluoroohenvl)-4-(4-fluoro-3-methyjphenyl)-5-[4-
(methylsulfonyl)ohenyl]-
3 (2H)-pyridazinone
The title compound was prepared according to the method of Example 228,
starting with 2-(3,4-difluorophenyl)-4-methoxy-5-[4-(methylsulfonyl)phenyl]-
3(2H)-
pyridazinone in place of 2-(4-fluorophenyl)-4-methoxy-5-[4-(methylthio)phenyl]-
3(2H)-pyridazinone and substituting 4-fluoro-3-methylbenzenemagnesium bromide
in place of cyclohexylmagnesium chloride (yield: 180 mg, 72.5%) . M.p. 166-168
C. 1 H NMR (300 MHz, DMSO-d6) 8 2.15 (s, 3H), 8 3.25 (s, 3H), 7.01 (m, 2H),
7.25
(d, 1 H), 7.6 (m, 4H), 7.9 (m, 3H), 8.26 (s, 2H). MS (DCI-NH3) m/z 471 (M+H)+,
488
(M+NH4)+. Anal. calc. for C24F3H17N203S: C, 61.27; H, 3.64; N, 5,95. Found: C,
61.47; H, 3.84; N, 5.67.
Example 236
2-(3.4-Difluoronhenyl)-5-j4-(me ylsulfonyl)pjjgnyll- -vinyl-3(2H)-Ryridazinone
The titie compound was prepared according to the method of Example 228,
starting with 2-(3,4-difluorophenyl)-4-methoxy-5-[4-(methylsulfonyl)phenyl]-
3(2H)-
pyridazinone in place of 2-(4-fluorophenyl)-4-methoxy-5-[4-(methylthio)phenyl]-
3(2H)-pyridazinone and substituting vinyl magnesium bromide in place of
cyclohexylmagnesium chloride (yield: 85 mg, 31.8%). 1 H NMR (300 MHz, DMSO-
d6) S 2.15 (s, 3H), 8 3.3 (s, 3H), 5.7 (dd, 1 H), 6.4 (dd, 1 H), 6.7 (dd, 1 H)
7.01 (m, 2H),
-152-
CA 02578858 2007-03-05
WO 99/10331 PCT/[IS98/16479
7.5 (m, 1 H), 7.65 (m, 1 H), 7.8 (m, 3H), 8.1 (s, 3H). MS (DCI-NH3) m/z 389
(M+H)+,
406 (M+NH4)+.
Example 237
2=(3.4-Difluoroph ethyl su yj)ohenylj-3(2 H) -
p,yridazinone
The title compound was prepared according to the method of Example 228,
starting with 2-(3,4-difluorophenyl)-4-methoxy-5-[4-(methylsulfonyl)phenyl]-
3(2H)-
pyridazinone in place of 2-(4-fluorophenyl)-4-methoxy-5-[4-(methylthio)phenyl]-
3(2H)-pyridazinone and substituting 2-thienylmagnesium bromide in place of
cyclohexylmagnesium chloride (yield: 66 mg, 28%). M.p. 189-191 C 1 H NMR (300
MHz, DMSO-d6) S 3.3 (s, 3H), 6.95 (m, 2H), 7.55 (m, 1 H), 7.7 (m, 5H), 7.85
(m, 1 H),
8.03 (d, J = 9 Hz, 2H), 8.13 (s, 1 H). MS (DCI-NH3) m/z 445 (M+H)+, 462
(M+NH4)+. Anal. caic. for C21 H14F2N203S2: C, 56.75; H, 3.17; N, 6.30. Found:
C, 56.92, H, 3.92, N, 5.79.
Example 238
2-(3.4-Difluoropheny!)-4-(1-~rol2ynyl)-5-r4_(0]ethylsulforlgj)nhenyjl-3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 228,
starting with 2-(3,4-difluorophenyl)-4-methoxy-5-[4-(methylsuifonyl)phenyl]-
3(2H)-
pyridazinone in place of 2-(4-fluorophenyl)-4-methoxy-5-[4-(methylthio)phenyl]-
3(2H)-pyridazinone and substituting methylacetylenemagnesium bromide in place
of cyclohexylmagnesium chloride (yield: 65 mg, 24%). M.p. 149-150 C. 1 H NMR
(300 MHz, DMSO-d6) S 2.1 (s, 3H), 3.3 (s, 3H), 7.51 (m, 1 H), 7.65 (m, 1 H),
7.8 (m,
1 H), 8.1 (m, 4H) ; 8.3 (s, 1 H). MS (DCI-NH3) m/z 463M+H)+, 480 (M+NH4)+.
Anal.
calc. for C20H14F2N203S=0.25 H20: C, 59.94; H, 3.52; N, 7.00. Found: C, 59.49;
H, 3.63; N, 6.34.
Example 239
2-(3.4-Difluoroohenvl)-4-t-butyJ-5~:[L-(meth culf nyl)ohenkl]-3(2H)-
oyridazinnne
The title compound was prepared according to the method of Example 228,
starting with 2-(3,4-difluorophenyl)-4-methoxy-5-[4-(methylsuIfonyl)phenyl]-
3(2H)-
pyridazinone in place of 2-(4-fluorophenyl)-4-methoxy-5-[4-(methylthio)phenyl]-
3(2H)-pyridazinone and substituting t-butyimagnesium bromide in place of
cyclohexylmagnesium chloride (yield: 60 mg, 24%). M.p. 158-161 C. 1 H NMR
-153-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
(300 MHz, DMSO-d6) S 1.21, (s, 9H), 3.3 (s, 3H), 7.51 (m, 1 H), 7.45 (m, 1 H),
7.75
(m, 4H), 8.02 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 419 (M+H)+, 436 (M+NH4)+.
Anal. calc. for C21 H20F2N203S: C, 60.27; H, 4.82; N, 6.69. Found: C, 60.15;
H,
5.10; N, 6.39
Example 240
2-(2.2.2-Trifluoroethyl)-4-cyclohexyl-5-[4-(methylsulfonyl)phenyl]-3(2H)-
Ryrda~ zinone
The title compound was prepared according to the method of Example 228,
starting with 2-(2,2,2-trifluoroethyl)-4-chloro-5-[4-(methylsulfonyl)phenyl]-
3(2H)-
pyridazinone, prepared in Example 193E, in place of 2-(4-fluorophenyl)-4-
methoxy-
5-[4-(methylthio)phenyl]-3(2H)-pyridazinone, (yield: 120 mg, 53%). M.p. 215-
218
C. 1 H NMR (300 MHz, CDCI3) S 1.1 (tt, J = 9 Hz, J = 4.5 Hz, 2H), 1.25 (tt, J
= 9 Hz,
4.5 Hz, 1 H), 1.49 (d, J = 12 Hz, 2H), 1.63 (d, J = 12 Hz, 1 H), 1.75 (dt, J =
12 Hz, 3
Hz, 2H), 2.21 (qd, J = 9 Hz, 4.5 Hz, 2H), 2.51 (tt, J = 12 Hz, 3 Hz, 1 H),
3.17 (s, 3H),
4.83 (q, J = 12 Hz, 2H), 7.49 (d, J = 9 Hz, 2H), 7.6 (s, 1 H), 8.09 (d, J = 9
Hz, 2H). MS
(DCI-NH3) m/z 415 (M+H)+. Anal. calc. for C19H21 F3N203S: C, 55.06; H, 5.1; N,
6.75. Found: C, 55.08; H, 5.10; N, 6.70.
Example 241
2-(3-Chloroohenvl)-4-(3-fluorobenzvl)-5-[4-(methylsulfonyl)phenx(l-3(2H)-
p~rridazinone
2-(3-Chlorophenyl)-4-(3-fluorobenzyl)-5-[4-(methylthio)phenyl]-3(2H)-
pyridazinone was prepared according to the method of Example 228, starting
with
2-(3-chlorophenyl)-4-methoxy-5-[4-(methylthio)phenyl]-3(2H)-pyridazinone,
prepared in Example 331, and substituting 3-fluorobenzylmagnesium chloride in
place of cyclohexylmagnesium chloride to provide the methyl sulfide compound.
The methyl sulfide compound was oxidized according to the method of
Example 10 to provide the title compound (yield: 180 mg, 55%). M.p. 142-143
C.
1 H NMR (300 MHz, CDCI3) S 3.14 (s, 3H), 3.98 (s, 2H), 6.75 (br d, J = 9 Hz, 1
H),
6.82 (br d, J = 9 Hz, 1 H), 6.88 (br t, J 9 Hz, 1 H), 7.15-7.23 (m, 1 H), 7.37-
7.47 (m,
2H), 7.54 (d, J = 9 Hz, 2H), 7.63 (dt, J 9 Hz, 2 Hz, 1 H), 7.75 (t, J = 2 Hz,
1 H), 7.82
(s, 1 H), 8.10 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 469 (M+H)+, 486 (M+NH4)+.
Anal. caic. for C24H18CIF2N203S-0.5 H20: C, 60.38; H, 3.88; N, 5.87. Found: C,
60.62; H, 3.89; N, 5.82.
-154-
CA 02578858 2007-03-05
WO 99/10331 PCTIUS98/16479
Example 242
2-(4-Fluorophenyl)-4-(3-fluorobenzyl)-5-[4-(methylsulfonyllohenvll-3(2H)-
Ryridazinone
2-(4-Fluorophenyl)-4-(3-fluorobenzyl)-5-[4-(methylthio)phenyl]-3(2H)-
pyridazinone was prepared according to the method of Example 228, starting
with
2-(4-fluorophenyl)-4-methoxy-5-[4-(methylthio)phenyl]-3(2H)-pyridazinone,
prepared in Example 194C, and substituting 3-fluorobenzylmagnesium chloride in
place of cyclohexylmagnesium chloride to provide the methyl sulfide compound.
The methyl sulfide compound was oxidized according to the method of
Example 10, to provide the title compound (yield: 450 mg, 66.8%). M.p. 176-178
C. 1 H NMR (300 MHz, CDCI3) S 3.14 (s, 3H), 3.95 (s, 2H), 6.75 (br d, J = 9
Hz,
1 H), 6.82 (br d, J = 9 Hz, 1 H), 6.88 (br t, J = 9 Hz, 1 H), 7.14-7.23 (m,
3H), 7.54 (d, J
9 Hz, 2H), 7.67 (dd, J = 9 Hz, 6 Hz, 2H), 7.81 (s, 1 H), 8.10 (d, J = 9 Hz,
2H). MS
(DCI-NH3) m/z 516 (M+NH4)+. Anal. calc. for C24H1 9F2N205S=H20: C, 61.28; H,
4.04; N, 5.96. Found: C, 61.24; H, 4.09; N, 5.77.
Example 243
2-(3.4-Difluorowl)-4-(3-fluorobenzy1)-5-[4-(methylsulfonyl)ohenyJJ-3(2H)-
Qyridazinone
2-(3,4-Difluorophenyl)-4-(3-fluorobenzyl)-5-[4-(methylthio)phenyl]-3(2H)-
pyridazinone was prepared according to the method of Example 228, starting
with
2-(3,4-difluorophenyl)-4-methoxy-5-[4-(methylthio)phenyl]-3(2H)-pyridazinone
prepared in Example 206E, and substituting 3-fluorobenzylmagnesium chloride in
place of cyclohexylmagnesium chloride to provide the methyl sulfide compound.
The methyl sulfide compound was oxidized according to the method of
Example 10 to provide the title compound (yield: 390 mg, 68%). M.p. 161-163
C.
1 H NMR (300 MHz, CDC13) S 3.14 (s, 3H), 3.95 (s, 2H), 6.74 (br d, J = 9 Hz, 1
H),
6.82 (br d, J = 9 Hz, 1 H), 6.89 (br t, J = 9 Hz, 1 H), 7.15-7.33 (m, 2H),
7.48-7.57 (m,
1 H), 7.53 (d, J = 9 Hz, 2H), 7.59-7.67 (m, 1 H), 7.83 (s, 1 H), 8.10 (d, J =
9 Hz, 2H).
MS (DCI-NH3) m/z 471 (M+H)+, 488 (M+NH4)+. Anal. calc. for
C24H17F3N203S=0.5 H20: C, 60.13; H, 3.65; N, 5.85. Found: C, 60.08; H, 3.81;
N,
5.54.
-155-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 244
2-(3-Chloroohenyl)-4-(4-fluoro-3-meth henyj)-5-[4-(methylsulfony,j)12jgnyl]-
3(ZH)-
Ryridazinone
2-(3-Chlorophenyl)-4-(4-fluoro-3-methylphenyl)-5-[4-(methylthio)phenyl]-
3(2H)-pyridazinone was prepared according to the method of Example 228,
starting with 2-(3-chlorophenyl)-4-methoxy-5-[4-(methylthio)phenyl]-3(2H)-
pyridazinone, prepared in Example 207B, and substituting 4-fluoro-3-
methylphenylmagnesium bromide in place of cyclohexylmagnesium chloride to
provide the methyl sulfide compound.
The methyl sulfide compound was oxidized according to the method of
Example 10 to provide the title compound (yield: 620 mg, 57%). M.p. 228-230
C.
1 H NMR (300 MHz, CDCI3) S 2.20 (s, 3H), 3.06 (s, 3H), 6.83-6.93 (m, 2H), 7.19
(br
d, J = 9 Hz, 1 H), 7.37-7.47 (m, 2H), 7.40 (d, J = 9 Hz, 2H), 7.65 (dt, J = 7
Hz, 3 Hz,
1 H), 7.68 (t, J = 3 Hz, 1 H), 7.91 (d, J = 9 Hz, 2H), 7.98 (s, 1 H). MS (DCI-
NH3) m/z
469 (M+H)+, 486 (M+NH4)+. Anal. calc. for C24H18CIFN2O3S: C, 61.54; H, 3.85;
N, 5.99. Found: C, 61.39; H, 3.84; N, 5.82.
Example 245
2-(4-Fluoroohenvl)-4-(4-fluoro-3-methylphenyl)-5-j4- ethylsulfonyI)nhenY1]-
3(2H)-
R,yridazinone
2-(4-Fluorophenyl)-4-(4-fluoro-3-methylphenyl)-5-[4-(methylthio)phenyl]-
3(2H)-pyridazinone was prepared according to the method of Example 228,
starting with 2-(4-fluorophenyl)-4-methoxy-5-[4-(methylthio)phenyl]-3(2H)-
pyridazinone, prepared in Example 194C, and substituting 4-fluoro-3-methyl-
phenylmagnesium bromide in place of cyclohexylmagnesium chloride to provide
the methyl sulfide compound.
The methyl sulfide compound was oxidized according to the method of
Example 10 to provide the title compound (yield: 590 mg, 74.4%). M.p. 245-247
C. 1 H NMR (300 MHz, CDCI3) S 2.01 (s, 3H), 3.07 (s, 3H), 6.87 (m, 2H), 7.21
(m,
3H), 7.41 (d, J = 9 Hz, 2H), 7.68 (m, 2H), 7.92 (d, J = 9 Hz, 2H), 7.97 (s, 1
H). MS
(DCI-NH3) m/z 453 (M+H)+, 470 (M+NH4)+. Anal. calc. for C24H18F2N203S-0.5
H20: C, 62.47; H, 3.90; N, 6.08. Found: C, 62.11; H, 4.11; N, 5.81.
-156-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 246
2-(3-Chloro-4-fluorophenyl)-4-cyclohexXl-5-[4-(methylsulfo yl)ohenyl]-3(2H)-
~,vridazinone.
246A. 2-(3-Chloro-4-fluorophenyj)-4.5-dibromo-3(2H)-pyridazinone.
The title compound is prepared according to the method of Example 194A,
substituting 3-chloro-4-fluorophenyl hydrazine=HCI in place of 4-fluorophenyl
hydrazine=HCI (yield: 9.1 g, 9%). 1 H NMR (300 MHz, CDCI3) 7.22 (d, J = 9 Hz,
1 H), 7.53-7.58 (m, 1 H), 7.73 (dd, J = 9 Hz, 3 Hz, 1 H), 7.94 (s, 1 H). MS
(DCI-NH3)
m/z 383 (M+H)+, 400 (M+NH4)+
246B. 2-(3-Chloro-4-ffuorol2hgnY1)-4-methoxv-5-bromo-3(2H)-ovridazinone.
The title compound is prepared according to the method of Example 194B,
substituting 2-(3-chloro-4-fluorophenyl)-4,5-dibromo-3(2H)-pyridazinone in
place of
2-(4-fluorophenyl)-4,5-dibromo-3(2H)-pyridazinone (yield: 5.6 g, 84%). 1 H NMR
(300 MHz, CDCI3) 4.32 (s, 3H), 7.22-7.30 (m, 1 H), 7.45-7.55 (m, 1 H), 7.64-
7.74 (m,
1 H), 7.94 (d, J = 9 Hz, 1 H). MS (DCI-NH3) m/z 335 (M+H)+, 352 (M+NH4)+.
246C. 2-(3-Chloro-4-fluoro henyl)-4-methoxv-5-[4-(methylthio) henyjl-3(2H1-
Rvridazinone.
The title compound is prepared according to the method of Example 6
starting with 2-(3-chloro-4-fluorophenyl)-4-methoxy-5-bromo-3(2H)-pyridazinone
in
place of 2-benzyl-5-methoxy-4-bromo-3(2H)-pyridazinone and substituting 3-
methyl-4-(methylthio)benzeneboronic acid in place of 4-fluorobenzeneboronic
acid
(yield: 3.2 g, 63%). 1 H NMR (300 MHz, CDCI3) S 2.53 (s, 3H), 4.13 (s, 3H),
7.25 (t,
J = 9 Hz, 1 H), 7.35 (d, J = 9 Hz, 2H), 7.52 (d, J = 9 Hz, 2H), 7.55-7.64 (m,
1 H), 7.78
(dd, J = 9 Hz, 3 Hz, 1 H), 7.93 (s, 2H). MS (DCI-NH3) m/z 377 (M+H)+, 394
(M+NH4)+.
246D. 2-(3-Chloro-4-fluoroohenxl)-4-cy loc hexyl-5-[4-(methyfthjQ)
henyj];3(2H)-
Ryridazinone
The title compound is prepared starting with 2-(3-chloro-4-fluorophenyl)-4-
methoxy-5-[4-(methylthio)phenyl]-3(2H)-pyridazinone in place of 2-(4-
fluorophenyl)-4-methoxy-5-[4-(methylthio)phenyl]-3(2H)-pyridazinone by
treatment
of the methoxy-sulfide compound with cyclohexylmagnesium chloride according to
the method of Example 228 to provide the cyclohexyl sulfide compound.
246E. 2-(3-Chloro-4-fluoro I~r y(}-4-cvclohexyl-5-[4-(methylsulfonvI) henvll-
3(2H)-Ryridazinone.
The methyl sulfide compound was oxidized according to the method of
Example 10 to provide the title compound (yield: 150 mg, 53%). M.p. 180-181
C.
-157-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
1 H NMR (300 MHz, CDCI3) S 1.02-1.36 (m, 2H), 1.49-1.68 (m, 4H), 1.75 (br d, J
12 Hz, 2H), 2.28 (dq, J = 12 Hz, 3 Hz, 2H), 2.57 (tt, J = 12 Hz, 3 Hz, 1 H),
3.17 (s,
3H), 7.25 (t, J = 9 Hz, 1 H), 7.53 (d, J = 9 Hz, 1 H), 7.53-7.61 (m, 2H), 7.69
(s, 1 H),
7.78 (dd, J = 9 Hz, 3 Hz, 1 H), 8.12 (d, J= 9 Hz, 2H). MS (DCI-NH3) m/z 461
(M+H)+, 478 (M+NH4)+. Anal. calc. for C23H22CIFN203S: C, 60.01; H, 4.78; N,
6.09. Found: C, 59.85; H, 4.97; N, 5.79.
Example 247
2-(3-Chloro-4-fluoropheny] -)(4-fluoro-3-methylohenyl)-5-[4-
(met vlsulfonyl)ptianyJ)-3(2H)-Ryridazinone
2-(3-Chloro-4-fluorophenyl)-4-(4-fluoro-3-methylphenyl)-5-[4-(methylthio)-
phenyl]-3(2H)-pyridazinone was prepared according to the method of Example
228, starting with 2-(3-chloro-4-fluorophenyl)-4-methoxy-5-[4-
(methylthio)phenyl]-
3(2H)-pyridazinone, prepared in Example 246D, and substituting 4-fluoro-3-
methylphenylmagnesium bromide in place of cyclohexylmagnesium chloride to
provide the methyl sulfide compound.
The methyl sulfide was oxidized according to the method of Example 10 to
provide the title compound (yield: 118 mg, 53.7%). M.p. 207-208 C. 1 H NMR
(300
MHz, CDCI3) S 2.21 (br s, 3H), 3.08 (s, 3H), 6.81-6.93 (m, 2H), 7.15-7.30 (m,
2H),
7.41 (d, J = 9 Hz, 2H), 7.60-7.68 (m, 1 H), 7.85 (dd, J = 9 Hz, 3 Hz, 1 H),
7.93 (d, J = 9
Hz, 2H), 7.99 (s, 1 H). MS (DCI-NH3) m/z 487 (M+H)+, 504 (M+NH4)+. Anal. caic.
for C24H17CIF2N203S=0.25 H20: C, 58.75; H, 3.52; N, 5.72. Found: C, 58.74; H,
3.60; N, 5.32.
Example 248
2-(3-Chloro-4-fluoroohenyl)-4-benzy,l-5-[4-(methylsulfonyj)ohenyJ]-3(2H)-
~yJidazinone
2-(3-Chloro-4-fluorophenyl)-4-benzyl-5-[4-(methylthio)phenyl]-3(2H)-
pyridazinone was prepared according to the method of Example 228, starting
with
2-(3-chloro-4-fluorophenyl)-4-methoxy-5-[4-(methylthio)phenyl]-3(2H)-
pyridazinone, prepared in Example 246D, and substituting benzyimagnesium
chloride in place of cyclohexyimagnesium chloride to provide the methyl
sulfide
compound.
The methyl sulfide was oxidized according to the method of Example 10 to
provide the title compound (yield: 110 mg, 38.4%). M.p. 164-166 C. 1 H NMR
(300
MHz, CDCI3) S 3.11 (s, 3H), 3.99 (s, 2H), 7.01-7.06 (m, 2H), 7.17-7.28 (m,
4H), 7.53
-158-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
(d, J = 9 Hz, 2H), 7.59-7.66 (m, 1 H), 7.81 (s, 1 H), 7.82 (dd, J = 6 Hz, 3
Hz, 1 H), 8.09
(d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 473 (M+H)+, 490 (M+NH4)+. Anal. calc. for
C24H18CIFN2O3S: C, 61.54; H, 3.85; N, 5.99. Found: C, 61.40; H, 3.82; N, 5.54.
Example 249
2-(3-Chloro-4-fluoroohenyl)-4-(3-fluorobenzyl)-5-[4-(methylsulfonyl)ohenvl]-
3(2H)-
Rvridazinone
2-(3-Chloro-4-fluorophenyl)-4-(3-fluorobenzyl)-5-[4-(methylthio)phenyl]-
3(2H)-pyridazinone was prepared according to the method of Example 228,
starting with 2-(3-chloro-4-fluorophenyl)-4-methoxy-5-[4-(methylthio)phenyl]-
3(2H)-
pyridazinone, prepared in Example 246D, and substituting 3-fluorobenzyl-
magnesium chloride in place of cyclohexylmagnesium chloride to provide the
methyl sulfide compound.
The methyl sulfide was oxidized according to the method of Example 10 to
provide the title compound (yield: 33 mg, 15%). M.p. 101-103 C. 1 H NMR (300
MHz, CDCI3) S 3.15 (s, 3H), 3.95 (s, 2H), 6.73 (br d, J = 9 Hz, 1 H), 6.81 (br
d, J = 9
Hz, 1 H), 6.88 (br t, J = 9 Hz, 1 H), 7.15-7.28 (m, 2H), 7.51 (d, J = 9 Hz,
2H), 7.53
(ddd, J = 9 Hz, 3 Hz, 1.5 Hz, 1 H), 7.83 (dd, J = 6 Hz, 3 Hz, 1 H), 7.83 (s, 1
H), 8.10 (d,
J = 9 Hz, 2H). MS (DCI-NH3) m/z 487 (M+H)+, 504 (M+NH4)+. Anal. calc. for
C24H17CIF2N2O3S: C, 58.75; H, 3.52; N, 5.62. Found: C, 58.50; H, 3.65; N,
5.29.
Example 250
2-(4-Fluoroohenyl)-4-(3-fluoro-4-methyl henyl)-5-[4-(methyls_ulfonyl)OheoY11-
3(2H)-
Ry_ridazinone
2-(4-Fluorophenyl)-4-(3-fluoro-4-methylphenyl)-5-[4-(methylthio)phenyl]-
3(2H)-pyridazinone was prepared according to the method of Example 228,
starting with 2-(4-fluorophenyl)-4-methoxy-5-[4-(methylthio)phenyl]-3(2H)-
pyridazinone, prepared in Example 194C, and substituting 3-fluoro-4-methyl-
phenylmagnesium bromide in place of cyclohexylmagnesium chloride to provide
the methyl sulfide compound.
The methyl sulfide was oxidized according to the method of Example 10 to
provide the title compound (yield: 540 mg, 73%). M.p. 245-248 C. 1 H NMR (300
MHz, CDCI3) S 2.22 (br s, 3H), 3.05 (s, 3H), 6.83 (dd, J = 9 Hz, 1.5 Hz, 1 H),
6.96
(dd, J = 9 Hz, 1.5 Hz, 1 H), 7.06 (t, J = 9 Hz, 1 H), 7.18 (t, J = 9 Hz, 2H),
7.41 (d, J = 9
Hz, 2H), 7.65-7.72 (m, 2H), 7.91 (d, J = 9 Hz, 2H), 7.95 (s, 1 H). MS (DCI-
NH3) m/z
-159-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
452 (M+H)+, 470 (M+NH4)+. Anal. caic. for C24Hl8F2N203S: C, 63.86; H, 3.99;
N, 6.21. Found: C, 63.49; H, 4.13; N, 5.98.
Example 251
2-(3-Chloro-4-fluoropbgpyl)-4-(3.5-difluoro-4-methoxvo_ henY1)-5-[4-
ethylsulfonvl)pjgpyjj-3(2H)-pyridazinone
2-(3-C hloro-4-fluorophenyl)-4-13,5-difluoro-4-methoxyphenyl)-5-[4-(methyl-
thio)phenyl]-3(2H)-pyridazinone was prepared according to the method of
Example
228, starting with 2-(3-chloro-4-fluorophenyl)-4-methoxy-5-[4-
(methylthio)phenyl]-
3(2H)-pyridazinone, prepared in Example 246D, and substituting 3,5-difluoro-4-
methoxyphenylmagnesium bromide in place of cyclohexylmagnesium chloride to
provide the methyl sulfide compound.
The methyl sulfide was oxidized according to the method of Example 10 to
provide the title compound (yield: 590 mg, 65.7%). M.p. 195-197 C. 1 H NMR
(300
MHz, CDCI3) S 3.10 (s, 3H), 4.12 (s, 3H), 6.81 (br d, J = 9 Hz, 2H), 7.27 (t,
J = 9 Hz,
1 H), 7.43 (d, J = 9 Hz, 2H), 7.60-7.67 (m, 1 H), 7.83 (br d, J = 9 Hz, 1 H),
7.98 (d, J = 9
Hz, 2H), 7.98 (s, 1 H). MS (DCI-NH3) m/z 487 (M+H)+, 504 (M+NH4)+. Anal. caic.
for C24H16CIF3N203S-0.5 H20: C, 54.44; H, 3.12; N, 5.30. Found: C, 54.50; H,
3.12; N, 5.15.
Example 252
2- (3-Chloro-4-fluorophe nyl)-4-(3-methylbutyl)- 5-[3-fluoro-4-
(methylsulfonyl) henyll-
3(2H)-Ryridazinone
2-(3-Chloro-4-fluorophenyl)-4-(3-methylbutyl)-5-[4-(methylthio)phenyl]-
3(2H)-pyridazinone was prepared according to the method of Example 228,
starting with 2-(3-chloro-4-fluorophenyl)-4-methoxy-5-[4-(methylthio)phenyl]-
3(2H)-
pyridazinone, prepared in Example 246D, and substituting 1-(3-methylbutyl)-
magnesium bromide in place of cyclohexylmagnesium chloride to provide the
methyl sulfide compound.
The methyl sulfide was oxidized according to the method of Example 10 to
provide the title compound (yield: 425 mg, 54.4%). M.p. 102-104 C. 1 H NMR
(300
MHz, CDC13) 8 0.85 (d, J = 9 Hz, 6H), 1.41-1.62 (m, 1 H), 2.50-2.63 (m, 2H),
3.30 (s,
3H), 7.22-7.38 (m, 3H), 7.57-7.64 (m, 1 H), 7.72 (br s, 1 H), 7.80 (br d, J =
6 Hz, 1 H),
8.15 (t, J = 9 Hz, 1 H). MS (DCI-NH3) m/z 467 (M+H)+, 484 (M+NH4)+. Anal.
calc.
for C22H21 CIF2N203S: C, 56.65; H, 4.51; N, 6.01. Found: C, 56.25; H, 4.49; N,
6.06.
-160-
CA 02578858 2007-03-05
WO 99/10331 PCT/1JS98/16479
Example 253
2- (4-FluoroI2 he nyj)-4-(3-fluorobenZyu-5-j4-(aminosulfonvl) p henyl]-3(2H)-
Ryridazinone
The methyl sulfide intermediate from Example 242 was oxidized to the
methyl sulfoxide with one equivalent of ineta-chloroperoxybenzoic acid
according
to the procedure in Example 69B to provide the sulfinyl compound.
The sulfoxide was converted to the title sulfonamide according to the method
of Example 68 (yield: 120 mg, 31 %). M.p. 199-202 C. 1 H NMR (300 MHz, DMSO-
d6) S 3.92 (s, 2H), 6.85 (br t, J = 9 Hz, 2H), 6.99 (br t, J= 9 Hz, 1 H), 7.26
(q, J = 7 Hz,
1 H), 7.35 (t, J = 9 Hz, 2H), 7.50 (s, 2H), 7.62-7.71 (m, 4H), 7.95 (d, J = 9
Hz, 2H),
8.11 (s, 1 H). MS (DCI-NH3) m/z 454 (M+H)+, 471 (M+NH4)+. Anal. caic. for
C23H17F2N303S: C, 60.86; H, 3.75; N, 9.27. Found: C, 60.99; H, 3.76; N, 9.02.
Example 254
2-(3.4-DifluoroDhenyl)-4-(nhenylethvOYl)-5-j4-(methylsulfonyl)phenyll-3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 232
substituting phenylethynylmagnesium bromide in place of chloride (yield: 195
mg,
61 %). M.p. 211-213 C. 1 H NMR (300 MHz, DMSO-d6) S 7.46 (m, 5H), 7.65 (m,
2H), 8.18 (t, 4H) ; 8.4 (s, 1 H). MS (DCI-NH3) m/z 463M+H)+, 480 (M+NH4)+.
Anal.
ca(c. for C25H16F2N2O3S: C, 64.56; H, 3.49; N, 6.06. Found: C, 64.49; H, 3.68;
N,
5.86.
Example 255
2-(3.4-Difluoropbenyl)-~(3.4-difluorobenzyl)-5-14-(methylsulfonyl)~henyl]-3( -
l -
Ryridazinone
3,4-Difluorobenzyl bromide (0.1 ml, 0.8 mmol) in ether (10 ml) was treated
with magnesium turnings (19.4 mg, 0.81 mmol) and the reaction mixture was
refluxed for 1 hour. The reaction mixture was cooled and added to a solution
of 2-
(3,4-difluorophenyl)-4-methoxy-5-[4-(methylthio)phenyl]-3(2H)-pyridazinone
(0.25
g, 0.7 mmol) in THF (10 ml) at -78 C. The reaction mixture was stirred at
room
temperature for 18 hours. Water (50 ml) was added to the reaction mixture and
extracted with ethyl acetate (50 ml). The organic layer was dried over MgSO4
and
concentrated in vacuo. The resulting crude residue was purified by flash
-161-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
chromatography (Si02, eluting with 9:1 hexanes:ethyl acetate) to provide 120
mg
of desired product and some starting material.
The methytthio compound (120 mg, 0.3 mmol) from above in CH2CI2 (10 ml)
at 0 C, was treated with CH3CO3H (0.3 ml, 1 mmol). The reaction was complete
in
2 hours. The reaction mixture was diluted with CH2CI2 and washed with
saturated
NaHCO3 and brine respectively. The resulting crude residue was purified by
flash
chromatography (Si02, eluting with 1:1 hexanes:ethyl acetate) to provide the
desired product (yield: 44 mg, 13%). M.p. 177-179 C. 1 H NMR (300 MHz,
DMSO-d6) S 3.3 (s, 3H), 3.9 (s, 2H), 6.85 (m, 1 H), 7.15 (m, 1 H), 7.25 (m,
2H), 7.6 (m,
7H), 8.15 (m, 3H). MS (DCI-NH3) m/z 489 (M+H)+, 506 (M+NH4)+. Anal. calc. for
C24H16F4N2O3S-0.25 H20: C, 59.01; H, 3.30; N, 5.74. Found: C, 58.16; H, 3.56;
N, 4.51.
Example 256
2-(3.4-Difluoroohenyl)-4-(3-methylbutyl)-5-[4-(methylsulfonyl)12henvll-3(2H):
pyridazinone
The title compound was prepared according to the method of Example 233,
starting with 2-(3,4-difluorophenyl)-4-methoxy-5-[4-(methylsulfonyl)phenyl]-
3(2H)-
pyridazinone in place of 2-(3,4-difluorophenyl)-4-methoxy-5-[4-
(methylthio)phenyl]-
3(2H)-pyridazinone and substituting 1-bromo-3-methylbutane in place of
3,4-difluorobenzyl bromide (yield: 198 mg, 48%). M.p. 55-58 C. 1 H NMR (300
MHz, DMSO-d6) S 0.75 (d, 6H), 1.4, (m, 3H), 2.48 (m, 2H), 3.3 (s, 3H), 7.51
(m, 1 H),
7.65 (m, 1 H), 7.75 (d, J = 9 Hz, 2H), 7.81 (m, 1 H) 8.05 (s, i H), 8.12 (d, J
= 9 Hz, 2H).
MS (DCI-NH3) m/z 433 (M+H)+, 450 (M+NH4)+. Anal. calc. for
C22H22F2N2O3SØ25 H20: C, 61.10; H, 5.13; N, 6.48. Found: C, 61.09; H, 5.23;
N, 6.36.
Example 257
2-(3-Chloro-4-fluoroohenvl)-4-(3-methylbutyl)-5-[4-(methXlsulfonyi)phenylj-3
(2 H)-
Qyridazinone
The title compound was prepared according to the method of Example 233,
procedure starting with 2-(3,4-difluorophenyl)-4-methoxy-5-[4-(methylsulfonyl)-
phenyl]-3(2H)-pyridazinone with 2-(3-chloro-4-fluorophenyi)-4-methoxy-5-[4-
(methylthio)phenyl]-3(2H)-pyridazinone and substituting 1-bromo-3-methyibutane
in place of 3,4-difluorobenzyl bromide (yield: 256 mg, 88%). M.p. 55-58 C. 1
H
NMR (300 MHz, DMSO-d6) 8 0.75 (d, 6H), 1.4, (m, 3H), 2.48 (m, 2H), 3.3 (s,
3H),
-162-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
7.62 (m, 2H), 7.75 (d, 2H), 7.93 (dd, 1 H), 8.05 (s, 1 H), 8.12 (d, J- 9 Hz,
2H). MS
(DCI-NH3) m/z 449 (M+H)+, 466 (M+NH4)+. Anal. caic. for
C22H22FN2O3SCI-0.25 H20: C, 58.86; H, 4.94; N, 6.24. Found: C, 59.23; H, 5.12;
N, 6.00.
Example 258
2-(3.4-Difluorophenyl)-4-(3-methvlbutyl)-5-[3-fluoro-4;(methylsulforlyI)Qh
envll;
3(2H1-R,yridazinone
The title compound was prepared according to the method of Example 233,
procedure starting with 2-(3,4-difluorophenyl)-4-methoxy-5-[4-(methylsulfonyl)-
phenyl]-3(2H)-pyridazinone in place of 2-(3-chloro-4-fluorophenyl)-4-methoxy-5-
[3-
fluoro-4-(methylthio)phenyl]-3(2H)-pyridazinone and substituting 1-bromo-3-
methylbutane in place of 3,4-difluorobenzyl bromide (yield: 100 mg, 20%). M.p.
119-121 C. 1 H NMR (300 MHz, DMSO-d6) 8 0.75 (d, 6H), 1.4, (m, 3H), 2.48 (m,
2H), 3.4 (s, 3H), 7.51 (m, 1 H), 7.8 (m, 2H), 7.81 (m, 2H). MS (DC(-NH3) m/z
451
(M+H)+, 468 (M+NH4)+. Anal. caic. for C22H21 F3N203S: C, 58.66; H, 4.7; N,
6.22.
Example 259
244-Fluoro-3-(meth ly thio) henyl]-4-(4-fluoroohenyl)-5-f4-(methxlsulfonxj)oh
nvll-
3(2H)-Ryridazinone
To a stirred solution of 2-(3,4-difluorophenyl)-4-(4-fluorophenyl)-5-[4-
(methylsulfonyl)phenyl]-3(2H)-pyridazinone (315 mg, 0.69 mmol) in DMF (10 ml)
at
room temperature was treated with sodium thiomethoxide (51 mg, 0.7 mmol). The
reaction mixture was stirred at room temperature for 3.15 hours. The reaction
was
poured into water (75 ml) and extracted into ethyl acetate. The organic layer
was
washed two times with brine, dried over MgSO4, and concentrated in vacuo. The
resulting crude residue was purified using flash chromatography (Si02, eluting
with
(15:1 CH2CI2:diethyl ether) to provide the desired product (yield: 30 mg, 8%)
.
M.p. 105-107 C. 1 H NMR (300 MHz, DMSO-d6) S 2.55 (s, 3H), 3.23 (s, 3H), 8
7.15
(m, 2H), 7.3 (m, 2H), 7.55 (m, 5H), 7.9 (d, 2H), 8.25 (s, 1 H). MS (DCI-NH3)
m/z 485
(M+H)+, 502 (M+NH4)+. Anal. caic. for C24H18F2N203S2: C, 59.49; H, 3.74; N,
5.78.
-163-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 260
2-Benzyl-4-(4-fluorophenyl)-5-[4-(trifluoromethylsulfonyl)ohenv_I]-3(2H)-
pyridazinone:
260A. 2-Benzyl-4-(4-fluoroohenyl)-5-[4-(methylsulfinyõj)phenyll-3(2H)-
12yridazinone
The title compound was prepared starting with 2-benzyl-4-(4-fluorophenyl)-
5-[4-(methytthio)phenyi]-3(2H)-pyridazinone and oxidizing the sulfide
according to
the procedure in example 69B.
260B. Bis(4-(5-(2-benzyl-4-(4-fluoro enyl)-3(2H)-
Ryridazinonelp,henylldisulfide:
A heterogeneous solution of 2-benzyl-4-(4-fluorophenyl)-5-[4-(methyl-
sulfinyl)phenyl]-3(2H)-pyridazinone (1.0 g, 2.39 mmol) in trifluoroacetic
anhydride
(10 mL, 70.8 mmol) was rapidly stirred at reflux for 2 hours with a bath
temperature
of 40-43 C. The reaction solution was cooled to 23 C, concentrated in vacuo,
and
azeotroped with toluene (2 x 5-7 mL). The resultant yellow/orange oil was
cooled
to 0 C, and methanol/triethylamine (1:1, 6 mL) was slowly added, along the
interior
wail of the reaction vessel with rapid stirring. The bright red-orange
solution was
stirred for 10 minutes at 0 C, the cooling bath removed, and the reaction
mixture
stirred an additional 1.5 hours warming to 23 C. The mixture was cooled back
to 0
C, and a saturated NH4CI solution (200 mL) slowly added followed by enough
aqueous 1 M HCI to adjust the solution to pH 1-2. The cooling bath was removed
and the solution stirred overnight. The mixture was extracted with ethyl
acetate.
The ethyl acetate solution was washed with water and brine, and concentrated
in
vacuo. The resultant yellow/brown oil (0.89 g) was a mixture of predominantly
the
mono-sulfide and desired di-sulfide. Subsequent rapid stirring of a portion of
the
crude reaction mixture (360 mg) in benzene (100 mL) with 12 (648 mg, 2.55
mmol)
at 23 C for 30 minutes completed the conversion of the mono-sulfide to the di-
sulfide. (Chem. Pharm. Bull., 1992, 40, 2842) The mixture was treated with a
0.1 M
Na2S2O3 solution to consume the excess 12. This solution was extracted with
ethyl
acetate, and the ethyl acetate layers dried over MgSO4, filtered, and
concentrated
in vacuo. The residue was dissolved in CH2CI2/hexanes and concentrated in
vacuo to provide the of product (yield: 347 mg, 90% for partial conversion). 1
H
NMR (300 MHz, CDCI3) S 5.38 (s, 4H), 6.91 (dd, J = 8.8, 8.8 Hz, 4H), 7.02 (d,
J = 8.7
Hz, 4H), 7.11-7.20 (m, 4H), 7.28-7.39 (m, 10H), 7.54 (dd, J = 6.9, 1.5 Hz,
4H), 7.83
(s, 2H).
-164-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
260C. 2-Benzyl-4-(4-fluoroohenyl)-5-[4-(trifluorometh I~thio)phenyl]-3{2H--
Ryridazinone:
A rapidly stirred mixture of bis[4-{5-[2-benzyl-4-(4-fluorophenyl)-3(2H)-
pyridazinone])-phenyl]-disulfide (140 mg, 0.181 mmol), potassium
trifluoroacetate
(55 mg, 0.361 mmol), and sulfolane (1.5 mL) was immersed in a 180 C pre-
heated
oil bath. The oil bath was heated to increase the temperature to 210 C, and
the
reaction flask was promptly removed from the oil bath after 10 minutes from
the
point of first immersion. During the course of the reaction, the mixture
changed
from colorless and heterogeneous to deep, blood red and homogeneous. After
cooling to 23 C, the mixture was diluted with ethyl acetate and washed with
aqueous 1 M HCI, water, and brine. The ethyl acetate solution was dried over
MgSO4, filtered and concentrated in vacuo. The residue was chromatographed
(fiash silica gel, ethyl acetate/hexanes 1:4) to provide the product (yield:
17 mg,
41 %). (Tetrahedron Lett.., 1996, 37, 9057) 1 H NMR (300 MHz, CDCI3) S 5.41
(s,
2H), 6.94 (dd, J = 8.2, 8.2 Hz, 2H), 7.11-7.20 (m, 4H), 7.31-7.42 (m, 3H),
7.52-7.61
(m, 4H), 7.86 (s, 1 H). MS (APCI+) m/z 457 (M+H)+ and m/z 474 (M+NH4)+.
260D. 2-Benzvl-4-(4-fiuorophenyl)-5-[4-jtrifluorometh su yl)pheIlyj,j-3(2H)-
Ryridazinone:
A solution of 2-benzyl-4-(4-fluorophenyl)-5-[4-(triffuoromethylthio)phenyl]-
3(2H)-pyridazinone (100 mg, 0.219 mmol), 3-chloroperoxybenzoic acid (380 mg,
1.3 mmol, 57-86%), and methylene chloride (5 mL) was brought to reflux at a
bath
temperature of 55 C. After 1.75 hours, 3.5 hours, 5 hours, and 6 hours, the
reaction was not complete and additional 3-chloroperoxybenzoic acid (380 mg,
1.3
mmol, 57-86%) was added each time. With the reaction completed after 7.75
hours, the mixture was cooled to 23 C and concentrated in vacuo. The residue
was diluted with ethyl acetate and carefully shaken with a NaHSO3 solution, 3
times, for several minutes to consume the excess 3-chloroperoxybenzoic acid.
The
ethyl acetate solution was subsequently washed with a saturated Na2CO3
solution
(3x), water, and brine and dried over MgSO4, filtered, and concentrated in
vacuo.
The residue was chromatographed (flash silica gel, ethyl acetate/methylene
chloride/hexanes 1:2:7) to provide of product (yield: 93 mg, 87%). (J. Med.
Chem.,
1990, 33, 2569) M.p. 80-115 C. 1 H NMR (300 MHz, DMSO-d6) S 5.36 (s, 2H),
7.11 (dd, J = 9.0, 9.0 Hz, 2H), 7.18-7.26 (m, 2H), 7.29-7.46 (m, 5H), 7.66 (d,
J = 8.7
Hz, 2H), 8.10 (d, J = 8.7 Hz, 2H), 8.18 (s, 1 H). MS (APCI+) m/z 489 (M+H)+
and m/z
-165-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
506 (M+NH4)+. Anal. caic. for C24H16F4N203S: C, 59.02; H, 3.30; N, 5.74.
Found: C, 59.30; H, 3.48; N, 5.59.
Example 261
2-(2.2.2-Trifluoroethyi)-4-(2.2-dimethyJp,ronoxy)-5;j4-
jmethylsulfonyllpheayll-3(2H)-
Ryridazinone
2-(2,2,2-Trifluoroethyl)-4-chloro-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone (150 mg, 0.41 mmol), prepared in Example 193E, and neopentyl
alcohol (43 mg, 0.49 mmol) were dissolved in DMF (2 mL) and NaH (25 mg, 0.62
mmol, 60% in mineral oil) was added with shaking and left overnight. The
reaction
mixture was carefully quenched with saturated NH4CI solution, diluted with
ethyl
acetate and extracted with 1 N HCI, twice, then water, 3 times, and then dried
over
M9SO4. After filtration of the drying agent and concentration of the filtrate
in vacuo,
the residue was purified by chromatography on silica gel (Biotage 40S) eluted
with
2:1 hexanes-ethyl acetate. The product fractions were combined and evaporated
to provide the title compound (yield: 137 mg, 76%). M.p. 145-146 C. 1 H NMR
(300 MHz, DMSO-d6) S 0.76 (s, 9H), 3.28 (s, 3H), 4.06 (s, 2H), 5.02 (q, J = 9
Hz,
2H), 7.88 (d, J = 8 Hz, 2H), 8.04 (d, J = 8 Hz, 2H), 8.13 (s, 1 H). MS (DCI-
NH3) m/z
419 (M+H)+, 436 (M+NH4)+. Anal. calc. for C18H21 F3N204S: C, 51.67; H, 5.06;
N, 6.69. Found: C, 51.47; H, 5.12; N, 6.48.
Example 262
2-(2.2.2-Trifluoro _thyI)-4-(4-methoxyohe_noxy)-5-[4-jmethylsuifonyORhenyl]-
a(2h)-
Ryridazinone
The title compound was prepared according to the method of Example 261,
substituting 4-methoxyphenol in place of neopentyl alcohol (yield: 130 mg,
54%).
M.p. 194-195 C. 1 H NMR (300 MHz, DMSO-d6) 5 2.24 (s, 3H), 3.26 (s, 3H), 5.00
(q, J = 9 Hz, 2H), 6.88 (d, J = 8 Hz, 2H), 7.09 (d, J- 8 Hz, 2H), 7.37 (d, J =
8 Hz, 2H),
8.03 (d, J- 8 Hz, 2H), 8.33 (s, 1 H). MS (ESI-) m/z 439 (M-H)-. Anal. caic.
for
C19H17F3N204S: C, 54.79; H, 3.91; N, 6.39. Found: C, 55.04; H, 4.00; N, 6.11.
Example 263
2-(2.2.2-Trifluoroethy I)-4-(2-fluoro-5-trifluorometh n xy -5-[4-(m t y
sõIfnnvl)henylj-3(2H)-Ryridazinone
The title compound was prepared according to the method of Example 261,
substituting 2-fluoro-5-trifluoromethylphenoi in place of neopentyl alcohol
(yield:
-166-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
155 mg, 89%). M.p. 133-135 C. 1 H NMR (300 MHz, DMSO-d6) S 3.28 (s, 3H),
5.03 (q, J = 9 Hz, 2H), 7.10-7.53 (m, 2H), 7.72 (dd, J = 1 Hz, 7 Hz 1 H), 7.92
(d, J = 8
Hz, 2H), 8.07 (d, J = 8 Hz, 2H), 8.38 (s, 1 H). MS (DCI-NH3) m/z 528 (M+NH4)+
Anal. caic. for C20H13F7N204S: C, 47.66; H, 3.09; N, 5.05. Found: C, 47.68; H,
2.95;N,5.16.
Example 264
2-(2.2.2-Trifluoroethyl -L4-(4-cyano hp enoxY)-5-[4-(methylsulfonyl)phenylj-
3(2H)-
gyridazinone
The title compound was prepared according to the method of Example 261,
substituting 4-cyanophenol in place of neopentyl alcohol (yield: 109 mg, 71%).
M.p. 179-181 C. 1 H NMR (300 MHz, DMSO-d6) 6 3.26 (s, 3H), 5.02 (q, J = 9 Hz,
2H), 7.25 (d, J = 9 Hz, 2H), 7.81 (d, J = 9 Hz, 2H), 7.86 (d, J = 8 Hz, 2H),
8.03 (d, J
8 Hz, 2H), 8.37 (s, 1 H). MS (DCI-NH3) m/z 467 (M+NH4)+. Anal. calc. for
C20H14F3N304S: C, 53.45; H, 3.14; N, 9.35. Found: C, 53.19; H, 3.01; N, 9.09.
Example 265
2-(2 2 2-Trifluoroethxl)-4-(3-pyridyloxy)-5-f4-(methyIsulfonyl)phenyll-3(2H)-
Qyridazinone
The title compound was prepared according to the method of Example 261,
substituting 3-hydroxypyridine in place of neopentyl alcohol (yield: 120 mg,
69%).
M.p. 191-193 C. 1 H NMR (300 MHz, DMSO-d6) 8 3.26 (s, 3H), 5.01 (q, J = 9 Hz,
2H), 7.36 (dd, J = 3 Hz, 8 Hz, 1 H), 7.55 (ddd, J = 1 Hz, 3 Hz, 8 Hz, 1 H),
7.88 (d, J = 8
Hz, 2H), 8.04 (d, J = 8 Hz, 2H), 8.31 (dd, J = 1 Hz, 5 Hz, 1 H), 8.36 (s, 1
H), 8.38 (d, J
= 3 Hz, 1 H). MS (DCI-NH3) m/z 426 (M+H)+, 443 (M+NH4)+. Anal. caic. for
C18H14F3N304S: C, 50.82; H, 3.32; N, 9.88. Found: C, 50.95; H, 3.57; N, 9.71.
Example 266
2-(2.2.2-Trifluoroethyl)-4-(4-n-propylphenoxy)-5-[4-(methylsulfonyl)phenylJ-
3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 261,
substituting 4-(n-propyl)phenol in place of neopentyl alcohol (yield: 147 mg,
77%).
M.p. 152-153 C. 1 H NMR (300 MHz, DMSO-d6) 6 0.87 (t, J = 7 Hz, 3H), 1.54 (h,
J
= 7 Hz, 2H), 3.25 (s, 3H), 5.00 (q, J = 9 Hz, 2H), 6.88 (d, J = 9 Hz, 2H),
7.09 (d, J = 9
Hz, 2H), 7.87 (d, J = 8 Hz, 2H), 8.02 (d, J = 8 Hz, 2H), 8.32 (s, 1 H). MS
(DCI-NH3)
-167-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
m/z 484 (M+H)+. Anal. calc. for C22H21 F3N204S: C, 56.33; H, 4.54; N, 6.01.
Found: C, 56.23; H, 4.75; N, 5.79.
Example 267
2-(2.2.2-Trifluoroethyl)-4-j4-(meth lsy ulfonyl)ohenoxy]-5-j4;(methy 5u yl)
nvll-
3(2H)-Ryrjdazinone
The title compound was prepared according to the method of Example 261,
substituting 4-(methylsulfonyl)phenol in place of neopentyl alcohol (yield:
115 mg,
56%). M.p. 212-213 C. 1 H NMR (300 MHz, DMSO-d6) S 3.21 (s, 3H), 3.27 (s,
3H),
5.03 (q, J = 9 Hz, 2H), 7.31 (d, J = 9 Hz, 2H), 7.83-7.89 (m, 4H), 8.04 (d, J
= 8 Hz,
2H), 8.40 (s, 1 H). MS (DCI-NH3) m/z 520 (M+NH4)+. Anal. caic. for
C20H17F3N206S2: C, 47.81; H, 3.41; N, 5.58. Found: C, 47.92; H, 3.18; N, 5.52.
Example 268
2-(2.2.2-Trifluoroethyl)-4-(4-nhenYlohenoxy)-5-r4- (methylsulfonyl)Pbgnyll
3(2HL
gyridazinone
The title compound was prepared according to the method of Example 261,
substituting 4-phenylphenol in place of neopentyl alcohol (yield: 105 mg,
51%).
M.p. 163-165 C. 1 H NMR (300 MHz, DMSO-d6) 5 3.26 (s, 3H), 5.02 (q, J = 9 Hz,
2H), 7.10 (d, J = 8 Hz, 2H), 7.33 (br t, J = 7 Hz, 1 H), 7.44 (t, J = 7 Hz,
2H), 7.57-7.63
(m, 4H), 7.92 (d, J = 8 Hz, 2H), 8.04 (d, J = 8 Hz, 2H), 8.37 (s, 1 H). MS
(DCI-NH3)
m/z 518 (M+NH4)+. Anal. calc. for C25H1 gF3N204S: C, 60.00; H, 3.83; N, 5.60.
Found: C, 60.18; H, 3.66; N, 5.52.
Example 269
2-(2.2.2-Trifluoro .thvl)-4-f2-(m .thvlthio) .th xy]-5-(4-
(methylsulfonv!)ohenyl]-3(2Hl-
Ryridazinone
The title compound was prepared according to the method of Example 261,
substituting 2-(methylthio)ethanol in place of neopentyl alcohol (yield: 105
mg,
61 %). M.p. 103-105 C. 1 H NMR (300 MHz, DMSO-d6) S 2.01 (s, 3H), 2.72 (t, J-
7
Hz, 2H), 3.29 (s, 3H), 4.59 (t, J = 7 Hz, 2H), 5.03 (q, J = 9 Hz, 2H), 7.91
(d, J = 8 Hz,
2H), 8.04 (d, J = 8 Hz, 2H), 8.15 (s, 1 H). MS (DCI-NH3) m/z 423 (M+H)+, 440
(M+NH4)+. Anal. calc. for C16H17F3N204S2: C, 45.49; H, 4.06; N, 6.33. Found:
C, 45.83; H, 4.11; N, 6.42.
-168-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 270
? (2.2.2-Trifluoroethvl)-4- eny,Jmethoxy)-5-[4-(methylsulfony1)12henYl1-3 (2
H)m
Ryridazinone
The title compound was prepared according to the method of Example 261,
substituting benzyl alcohol in place of neopentyl alcohol (yield: 137 mg,
76%). M.p.
121-123 C. 1 H NMR (300 MHz, DMSO-d6) S 3.28 (s, 3H), 5.06 (q, J = 9 Hz, 2H),
5.48 (s, 2H), 7.20-7.25 (m, 2H), 7.27-7.81 (m, 3H), 7.76 (d, J = 8 Hz, 2H),
7.98 (d, J
8 Hz, 2H), 8.12 (s, 1 H). MS (DCI-NH3) m/z 456 (M+H)+. Anal. caic. for
C20H17F3N204S: C, 54,79; H, 3.91; N, 6.39. Found: C, 55.10; H, 3.91; N, 6.13.
Example 271
2-(2.2.2-Trifluoroethyt)-4-(2-furylmethoxy)-5 -14-(methylsulfonY1loõ heny,l -
3(2H)m
tavridazinone
The title compound was prepared according to the method of Example 261,
substituting 2-(hydroxymethyl)furan in place of neopentyl alcohol (yield: 101
mg,
58%). M.p. 113-115 C. 1 H NMR (300 MHz, DMSO-d6) 5 3.28 (s, 3H), 5.07 (q, J
9 Hz, 2H), 5.52 (s, 2H), 6.41 (dd, J = 2 Hz, 3 Hz, 1 H), 6.45 (d, J = 4 Hz, 1
H), 7.62 (d,
J = 2 Hz, 1 H), 7.69 (d, J = 8 Hz, 2H), 7.97 (d, J = 8 Hz, 2H), 8.13 (s, 1 H).
MS (DCI-
NH3) m/z 446 (M+NH4)+. Anal. caic. for C18H15F3N205S: C, 50.66; H, 3.80; N,
6.21. Found: C, 51.02; H, 3.71; N, 6.23.
Example 272
2-(2.2.2-TrifluoroethXl)-4-[2-(3.4-dimethoxyohenyI)ethoxy)]-5-[4-
ethylsulfonyl
RJ]gnYl]-3 (2H)-pY i zin ng
The title compound was prepared according to the method of Example 261,
substituting 2-(3,4-dimethoxyphenyl)ethanol in place of neopentyl alcohol
(yield:
118 mg, 56%). M.p. 133-134 C. 1 H NMR (300 MHz, DMSO-d6) 5 2.82 (t, J = 7 Hz,
2H), 3.28 (s, 3H), 3.63 (s, 3H), 3.70 (s, 3H), 4.68 (t, J = 7 Hz, 2H), 5.01
(q, J = 9 Hz,
2H), 6.61 (dd, J = 2 Hz, 8 Hz, 1 H), 6.74 (d, J = 2 Hz, 1 H), 6.77 (d, J = 8
Hz, 1 H), 7.74
(d, J = 8 Hz, 2H), 7.93 (d, J = 8 Hz, 2H), 8.11 (s, 1 H). MS (DCI-NH3) m/z 530
(M+NH4)+. Anal. caic. for C23H23F3N206S: C, 53.90; H, 4.52; N, 5.47. Found: C,
53.87; H, 4.48; N, 5.45.
-169-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 273
2-(2 2 2-TrifluoroethyJ)-4-[2-(4-mor olino)ethoxy)]-5-[4-
(methvlsulfonyl)nhenvll-
3(2H)-Rvridazinone
The title compound was prepared according to the method of Example 261,
substituting 4-(2-hydroxyethyl)morpholine in place of neopentyl alcohol
(yield: 111
mg, 59%). M.p. 147-148 C. 1 H NMR (300 MHz, DMSO-d6) S 2.23 (m, 4H), 2.46 (t,
J = 5 Hz, 2H), 3.28 (s, 3H), 3.40 (m, 4H), 4.60 (t, J = 5 Hz, 2H), 5.02 (q, J
= 8 Hz, 2H),
7.96 (d, J = 8 Hz, 2H), 8.03 (d, J = 8 Hz, 2H), 8.17 (s, 1 H). MS (DCI-NH3)
m/z 462
(M+H)+. Anal. calc. for C1 9H22F3N305S: C, 49.45; H, 4.81; N, 9.11. Found: C,
49.59; H, 4.80; N, 8.88.
Example 274
2-(2 2 2-TrifluoroethyJ)-4-[2-(1- r inyj)ethoxy)]-5-[4-(methylsulfonvl)ohenvll-
3 (2 H)-ovridazinone
The title compound was prepared according to the method of Example 261,
substituting 1-(2-hydroxyethyl)piperidine in place of neopentyl alcohol
(yield: 103
mg, 55%). M.p. 117-118 C. 1 H NMR (300 MHz, DMSO-d6) 81.30 (br s, 6H), 2.20
(br s, 4H), 2.41 (t, J = 4 Hz, 2H), 3.28 (s, 3H), 4.60 (t, J = 5 Hz, 2H), 5.02
(q, J = 9 Hz,
2H), 7.97 (d, J = 8 Hz, 2H), 8.03 (d, J = 8 Hz, 2H), 8.15 (s, 1 H). MS (DCI-
NH3) m/z
460 (M+H)+. Anal. caic. for C20H24F3N304S: C, 52.28; H, 5.26; N, 9.15. Found:
C, 52.22; H, 5.08; N, 8.94.
Example 275
22.2.2-TrifluoroethyjL4-[4-(carboxamido) h2enoxy)J-5-[4-(methylsulfonyl)
henvll-
3(2H)-Qyridazinone
The title compound was prepared according to the method of Example 261,
substituting 4-hydroxybenzamide in place of neopentyl alcohol (yield: 50 mg,
26%).
M.p. > 250 C. 1 H NMR (300 MHz, DMSO-d6) S 3.26 (s, 3H), 5.02 (q, J = 8 Hz,
2H),
7.08 (d, J = 9 Hz, 2H), 7.30 (s, 1 H), 7.82 (d, J = 9 Hz, 2H), 7.88 (d, J = 8
Hz, 2H),
7.92 (s, 1 H), 8.03 (d, J = 8 Hz, 2H), 8.47 (s, 1 H). MS (DCI-NH3) m/z 468
(M+H)+,
485 (M+NH4)+. Anal. caic. for C20H16F3N305S: C, 51.39; H, 3.45; N, 8.99.
Found: C, 51.31; H, 3.28; N, 8.77.
-170-
CA 02578858 2007-03-05
WO 99/10331 PCTNS98/16479
Example 276
? (2.2.2-Trifluoroethyl)-4_(1-indanyloxy)-5-[4-(methylsulfonyI)Rhenti]-3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 261,
substituting 1-indanol in place of neopentyl alcohol (yield: 84 mg, 44%). M.p.
113-
114 C. 1 H NMR (300 MHz, DMSO-d6) 5 2.07-2.14 (m, 1 H), 2.22-2.35 (m, 1 H),
2.73 (dd, J = 5 Hz, 7 Hz, 2H), 3.24 (s, 3H), 5.00-5.22 (m, 2H), 6.48 (dd, J =
2 Hz, 6
Hz, 1 H), 7.12-7.24 (m, 2H), 7.21-7.28 (m, 2H), 7.44 (d, J = 8 Hz, 2H), 7.87
(d, J = 8
Hz, 2H), 8.09 (s, 1 H). MS (DCI-NH3) m/z 482 (M+NH4)+. Anal. calc. for
C22H19F3N204S: C, 57.19; H, 4.48; N, 5.80. Found: C, 57.36; H, 4.30; N, 5.78.
Example 277
2-(2.2.2-Trifluoroethyl)-4-[4-(acetamido) henoxy)]-5-j4-(meth Isy ulfonyl) hp
eny11-
3(2H)-pvridazinone
The title compound was prepared according to the method of Example 261,
substituting 4-acetamidophenol in place of neopentyl alcohol (yield: 45 mg,
23%).
M.p. 215-216 C. 1 H NMR (300 MHz, DMSO-d6) S 2.02 (s, 3H), 3.26 (s, 3H), 5.02
(q, J = 8 Hz, 2H), 6.61-6.65 (m, 1 H), 7.17-7.20 (m, 2H), 7.34 (br s, 1 H),
7.88 (d, J = 9
Hz, 2H), 8.03 (d, J = 8 Hz, 2H), 8.36 (s, 1 H), 9.97 (s, 1 H). MS (DCI-NH3)
m/z 499
(M+NH4)+. Anal. calc. for C21 H18F3N305S: C, 52.39; H, 3.77; N, 8.73. Found:
C,
52.57; H, 4.02; N, 8.37.
Example 278
2-(2.2.2-Trifluoroethyl)-4-(2-methyl~rooxy)-5-[4-(methylsulfonyl)n~yll 3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 261,
substituting 2-methylpropanol in place of neopentyl alcohol (yield: 111 mg,
50%).
M.p. 108-110 C. 1 H NMR (300 MHz, DMSO-d6) 8 0.77 (d, J = 6.4 Hz, 6H), 1.52
(sept, J = 6.4 Hz, 1 H), 3.28 (s, 3H), 4.17 (d, J = 6 Hz, 2H), 5.02 (q, J = 9
Hz, 2H),
7.88 (d, J= 9 Hz, 2H), 8.04 (d, J= 9 Hz, 2H), 8.14 (s, 1 H). MS (DCI-NH3) m/z
405
(M+H)+, 422 (M+NH4)+. Anal. calc. for C17H1 gF3N2O4S: C, 50.49; H, 4.74; N,
6.93. Found: C, 50.69; H, 4.89; N, 6.75.
Example 279
2-(2.2.2-Trifluoroethyl)-4-(1-methylcyclo}ropytmethoxy)-5-[4-(methylsutfony,J)-
henyl]-3 (2H)-Ryridazinone
-171-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
The title compound was prepared according to the method of Example 261,
substituting 1-methylcyclopropanemethanol in place of neopentyl alcohol
(yield:
360 mg, 75.5%). M.p. 98-99 C. 1 H NMR (300 MHz, CDCI3) S 0.35 (dt, J = 40 Hz,
5
Hz, 4H), 0.91 (s, 3H), 3.11 (s, 3H), 4.32 (s, 2H), 4.82 (q, J = 8.5 Hz, 2H),
7.80 (d, J
8.5 Hz, 2H), 7.84 (s, 1 H), 8.06 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 417
(M+H)+,
m/z 434 (M+NH4)+. Anal. calc. for C18H19F3N204S: C, 51.92; H, 4.60; N, 6.73.
Found: C, 51.87; H, 4.72; N, 6.69.
Example 280
2-(2.2.2-Tr'rfluoroethyl)-4-(3.3-dimethylbutoxx)-5-[4-
(methylsuffony,j)ohenõvll-3,(~
pyridazinone
The title compound was prepared according to the method of Example 261,
substituting 3,3-dimethyl-l-butanol in place of neopentyl alcohol (yield: 270
mg,
67.4%). M.p. 83-85 C. 1 H NMR (300 MHz, CDCI3) S 0.88 (s, 9H), 1.56 (t, J = 8
Hz,
2H), 4.60 (t, J = 8 Hz, 2H), 4.83 (q, J = 8.5 Hz, 2H), 7.73 (d, J = 8.5 Hz,
2H), 7.81 (s,
1 H), 8.05 (d, J = 8.5 Hz, 2H). MS (DCI-NH3) m/z 433 (M+H)+, m/z 450 (M+NH4)+.
Anal. calc. for C1 gH23F3N204S: C, 52.77; H, 5.36; N, 6.48. Found: C, 52.95;
H,
5.29; N, 6.35.
Example 281
2-(3.4-Difluoraohenvl).-4-(4-chloro henoxy)-5-[g_(methy su f XIl henyjl-3(2a-
põyridazinone
A mixture of 2-benzyl-4-chloro-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone (187 mg, 0.5 mmol).
prepared in Example 78, p-chlorophenol (129
mg, 0.5 mmol) and NaH (60% oil suspension) (40 mg, 1 mmol) in THF (25 mL) was
refiuxed at 50 C for 3 hours and then concentrated in vacuo. The residue was
partitioned between water and ethyl acetate. The acetate layer was washed with
brine, dried over MgSO4 and concentrated in vacuo. The residue was
chromatographed (silica gel, 1:1 hexanes-ethyl acetate) to provide 2-benzyl-4-
(4-
chlorophenoxy)-5-[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone (yield: 200 mg,
82%).
The above derivative was dissolved in toluene (25 mL) and was treated with
AIBr3 (400 mg, 1.5 mmol) for 20 minutes at 80 C. The mixture was cooled to
room
temperature and poured into ice-10% citric acid-ethyl acetate. The organic
layer
was separated, dried over MgSO4 and concentrated in vacuo to provide crude
desbenzyl derivative. This compound was immediately dissolved in pyridine (50
-172-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
mL) and was treated with 3,4-difluorobromobenzene (0.17 mL, 1.5 mmol), Cu (20
mg) and K2C03 (100 mg, 1.5 mmol) at refiux for 16 hours. After the mixture was
concentrated in vacuo, the residue was dissolved in ethyl acetate and was
washed
with water, 10% citric acid and brine. Purification by column chromatography
(silica
gel, 1:1 hexanes-ethyl acetate) provided the title compound (yield: 73 mg,
30%).
M.p. 192-194 C. 1 H NMR (300 MHz, DMSO-d6) S 3.22 (s, 3H), 7.13 (m, 2H), 7.35
(m, 2H), 7.50 (m, 1 H), 7.60 (m, 1 H), 7.75 (m, 1 H), 7.87 (d, J = 9 Hz, 2H),
8.05 (d, J =
9 Hz, 2H), 8.41 (s, 1 H). MS (APCI+) m/z 488 (M+H)+ and (APCI-) m/z 523 (M+CI)-
.
Example 282
2-(3.4-Difluoroehenvl)-4-(4-bromonh noxy)-5-j4-(methylsulfo4Y1) her),yl -3(2H)-
Rysidazinone.
The title compound was prepared according to the method of Example 281,
substituting p-bromophenol in place of p-chlorophenol (yield: 54 mg, 20%).
M.p.
196-199 C. 1 H NMR (300 MHz, DMSO-d6) S 3.25 (s, 3H), 7.09 (d, J = 9 Hz, 2H),
7.47 (d, J = 9 Hz, 2H), 7.52 (m, 1 H), 7.62 (m, 1 H), 7.78 (m, 1 H), 7.89 (d,
J = 9 Hz,
2H), 8.05 (d, J = 9 Hz, 2H), 8.41 (s, 1 H). MS (APCI+) m/z 533 (M+H)+ and
(APCI-)
m/z 569 (M+CI)-.
Example 283
2-(2.2. -Trifluoroethvl)-4-(cvclopenjvithio)-5:L4.-Jmethylsulfonxl)pheny!)-
3(2H)~
pvridazinone
To a solution of NaH (26 mg, 1.1 mmol) in acetonitrile (3.0 mL), under
nitrogen, was added cyclopentyl mercaptan (120 L, 1.1 mmol) dropwise via
syringe. The resulting solution was flushed with nitrogen for a period of 20
minutes;
after which 2-(trifluoroethyl)-4-chloro-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone, prepared in Example 193E, (200 mg, 0.52 mmol) was added in one
portion. The solution was stirred for an additional 20 minutes at which time,
all the
4-bromo pyridazinone was consumed. The solution was analyzed by TLC (1:1,
ethyl acetate-Hex). Water (5 mL) was carefully added and the reaction
partitioned
between ethyl acetate (125 mL) and saturated saline (50 mL). The organic layer
is
washed with saturated saline (50 mL), dried over MgSO4, and concentrated in
vacuo. Silica gel chromatography (20% ethyl acetate-80% hexanes) provided a
pale yellow solid (yield: 202 mg, 83.1 %). M.p. 149-151 C. I H NMR (300 MHz,
CDC13) 8 1.40-1.34 (m, 2H), 1.62-1.54 (m, 4H), 1.93-1.88 (m, 2H), 3.13 (s,
3H),
4.40-4.35 (m, 1 H), 4.85 (q, J = 8.2 Hz, 2H), 7.58 (d, J = 8.5 Hz, 2H), 7.66
(s, 1 H),
-173-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
8.06 (d, J = 8.4 Hz, 2H). MS (DCI-NH3) m/z 432 (M+H)+, (M+NH4)+. Anal. calc.
for
C18H1 gF3N203S2: C, 49.99; H, 4.43; N, 6.48. Found: C, 50.15; H, 4.39; N,
6.45.
Example 284
2-(2.2.2-TrifluoroethyJ)-4-(1 H-1.2.4-triazole-3-ylthio)-5-[4-(methylsulfon
yl) h{eOyl)-
3(2H)-Ryridazinone.
The title compound was prepared according to the method of Example 283,
substituting 1 H-1,2,4-triazole-3-thiol in place of cyclopentyl mercaptan
(yield: 164
mg, 93%). M.p. 197-200 C. 1 H NMR (300 MHz, CDCI3) S 3.14 (s, 3H), 4.84 (q, J
8.1 Hz, 2H), 7.41 (s, 1 H), 7.68 (d, J = 6.8 Hz, 2H), 7.83 (s, 1 H), 8.00 (d,
J = 7.1 Hz,
2H), 8.05 (s, 1 H). MS (DCI-NH3) m/z 431 (M+H)+, (M+NH4)+. Anal. calc. for
C15H12F3N203S2: C, 41.76; H, 2.80 ; N, 16.23. Found: C, 41.68; H, 2.85; N,
15.99.
Example 285
2-(2.2.2-Trifluoroethyl)-4-hen lY methxlthio-5-i4-(methylsulfon,y1)ohenyll-
3(2L,1-
Ryridazinone.
The title compound was prepared according to the method of Example 283,
substituting benzyl mercaptan in place of cyclopentyl mercaptan (yield: 141
mg,
76%). M.p. 108-111 C. 1 H NMR (300 MHz, CDCI3) S 3.01 (s, 3H), 4.38 (s, 2H),
4.87 (q, J = Hz, 2H), 7.10-7.06 (m, 2H), 7.22-7.20 (m, 5H), 7.59 (s, 1 H),
7.95 (d, J
8.5 Hz, 2H). MS (DCI-NH3) m/z 454 (M+H)+, (M+NH4)+. Anal. calc. for
C20H17F3N203S2, 0.75 EtOAc: C, 53.06; H, 4.45 ; N, 5.38. Found: C, 53.55; H,
4.16; N, 5.84.
Example 286
2=12.2.2-Trifluoroethvl)-4-(4-fluorophenylthio)-5-[4-(methylsulfonyl)ohenyI]-
3(~-I)-
Ryridazinone
The title compound was prepared according to the method of Example 283,
substituting 4-fluorophenylmethyl mercaptan in place of cyclopentyl mercaptan
(yield: 184 mg, 73.5%). M.p. 182-185 C. 1 H NMR (300 MHz, CDCI3) S 3.08 (s,
3H), 4.82 (q, J = 8.5 Hz, 2H), 6.87-6.81 (m, 2H), 7.19-7.11 (m, 2H), 7.48 (d,
J = 9.0
Hz, 2H), 7.68 (s, 1 H), 7.93 (d, J = 8.5 Hz, 2H). MS (DCI-NH3) m/z 458 (M+H)+,
(M+NH4)+. Anal. caic. for C1 gH14F4N203S2: C, 49.78; H, 3.08 ; N, 6.11. Found:
C, 49.89 ; H, 3.18 ; N, 5.86
-174-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 287
2õ(2 2 2-Trifluoroethxj)-4-(cvclohexvlthio)-5-[4-(methyisulfony1)Qhe0Y1]- l3
2H)-
12,yridazinone
The title compound was prepared according to the method of Example 283,
substituting cyclohexyl mercaptan in place of cyclopentyl mercaptan (yield:
189 mg,
78%). M.p. 165-167 C. 1 H NMR (300 MHz, CDC13) 8 1.28-1.17 (m, 5H), 1.64-1.56
(m, 3H), 1.82-1.79 (m, 2H), 3.13 (s, 3H), 4.08-4.05 (m, 1 H), 4.86 (q, J = 8.5
Hz, 2H),
7.58 (d, J = 8.4 Hz, 2H), 7.67 (s, 1 H), 8.06 (d, J = 8.5 Hz, 2H). MS (DCI-
NH3) m/z
446 (M+H)+, (M+NH4)+. Anal. caic. for C1 9H21 F3N203S2: C, 51.11; H, 4.74 ; N,
6.27. Found: C, 51.39 ; H, 4.72 ; N, 5.91.
Example 288
2(2 2 2-Trifluoroethyl)-4-(3-chloro-4-flubroohenylthio)-5-[4-(methvtsulfonyl)
ho envll-
3 (2 H)- pvridazinone
The title compound was prepared according to the method of Example 283,
substituting 3-chloro-4-fluorothiophenol in place of cyclopentyl mercaptan
(yield:
190 mg, 65%). M.p. 142-145 C. 1 H NMR (300 MHz, CDCI3) 8 3.18 (s, 3H), 4.85
(q, J = 8.4 Hz, 2H), 6.96 (ov. t, J = 8.5 Hz, 1 H), 7.14-7.10 (m, 1 H), 7.18
(dd, J = 2.1,
6.5 Hz, 1 H,), 7.53 (d, J = 8.4 Hz, 2H), 7.77 (s, 1 H), 7.96 (d, J = 8.0 Hz,
2H). MS (CI)
m/z 493 (M+1)+, (M+NH4)+. Anal. caic. for C1gH13CIF4N203S2Ø25 C6H020:
C, 47.36 ; H, 2.92; N, 5.41. Found: C, 47.88 ; H, 2.95; N, 5.24.
Example 289
2-(2.2.2-Trifluoroethvl)-4-(2.2.2-trifluoroethylthio)-5-[4-
(methylsulfonvl)ohenvll-
3(2H)_R,vridazinone.
The title compound was prepared according to the method of Example 283,
substituting 2,2,2-trifluoroethyl mercaptan in place of cyclopentyl mercaptan
(yield:
175 mg, 66%). M.p. 155-158 C. 1 H NMR (300 MHz, CDC13) S 3.14 (s, 3H), 3.98
(q, J = 9.8 Hz, 2H), 4.86 (q, J = 8.1 Hz, 2H), 7.58 (d, J = 8.4 Hz, 2H), 7.75
(s, 1 H),
8.10 (d, J = 8.4 Hz, 2H). MS (DCI-NH3) m/z 446 (M+H)+, (M+NH4)+. Anal. caic.
for
C15H12F6N203S2: C, 40.36 ; H, 2.71; N, 6.28. Found: C, 40.50; H, 2.72; N,
6.01.
Example 290
22.2.2-TrifluoroethylL(tert-butylthio)-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone.
-175-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
The title compound was prepared according to the method of Example 283,
substituting tert-butyl mercaptan in place of cyclopentyl mercaptan (yield:
212 mg,
85%). M.p. 186-189 C. 1 H NMR (300 MHz, CDCI3) S 1.25 (s, 9H), 3.13 (s, 3H),
4.87 (q, J = 8.1 Hz, 2H), 7.62 (d, J = 8.5 Hz, 2H), 7.67 (s, 1 H), 8.05 (d, J
= 8.1 Hz,
2H). MS (ESI) m/z 420 (M+H)+, (M+Na)+. Anal. caic. for C17H1 gF3N203S2: C,
48.56 ; H, 4.55; N, 6.66. Found: C, 50.15; H, 4.39; N, 6.45.
Example 291
2-(2.2.2-Trifluoroethyl)-4-(4-acetamidophenylthio)-5-[4-(me ylsulfonyl) henyiJ-
3(2H)-Ryridazinone.
The title compound was prepared according to the method of Example 283,
substituting 4-acetamidothiophenol in place of cyclopentyl mercaptan (yield:
100
mg, 37%). M.p. 191-193 C. 1 H NMR (300 MHz, CDC13) S 2.16 (s, 3H), 3.08 (s,
3H), 4.83 (q, J = 8.2 Hz, 2H), 7.00 (d, J = 8.8 Hz, 2H), 7.19 (d, J = 8.8 Hz,
2H), 7.31
(d, J= 8.1 Hz, 2H), 7.58 (s, 1 H), 7.78 (d, J= 8.1 Hz, 2H). MS (CI) m/z 497
(M+H)+,
(M+NH4)+. Anal. caic. for C21 H18F3N304S2-0.25H20, 0.25 C6H6: C, 52.83; H,
4.06; N, 7.70. Found: C, 52.97; H, 3.85; N, 7.65.
Example 292
2- (2.2.2-Trifluoroethyl)-4- (2-nroRy thi ) -5-[4- (methylsulfonyl)gheny 11 -
3(2H) -
Ryridazinone.
The title compound was prepared according to the method of Example 283,
substituting isopropyl mercaptan in place of cyclopentyl mercaptan (yield: 180
mg,
81 %). M.p. 165-167 C. 1 H NMR (300 MHz, CDCI3) 81.17 (d, J = 6.8 Hz, 6H),
3.13
(s, 3H), 4.33 (p, J = 6.8 Hz, 1 H), 4.86 (q, J = 8.5 Hz, 2H), 6.59 (d, J = 8.5
Hz, 2H),
7.68 (s, 1 H), 8.07 (d, J = 8.1 Hz, 2H). MS (DCI-NH3) m/z 406 (M+H)+,
(M+NH4)+.
Anal. calc. for C16H17F3N203S2, 0.75H20: C, 45.76 ; H, 4.4; N, 6.67. Found: C,
45.91; H, 3.98; N, 6.46.
Example 293
2=(2.2.2-Trifluoroethyl)-4- (2-met loro p-1-ylthio)-5-[4-(methylsulfony nvll-
3(2H)-Ryridazinone.
The title compound was prepared according to the method of Example 283,
substituting 2-methyl-l-propyl mercaptan in place of cyclopentyl mercaptan
(yield:
100 mg, 83%). M.p. 135-138 C. 1 H NMR (300 MHz, CDCI3) 6 0.87 (d, J = 6.4 Hz,
6H), 1.67-1.60 (m, 1 H), 3.00 (d, J = 6.7 Hz, 2H), 3.14 (s, 3H), 4.84 (q, J =
8.5 Hz,
-176-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
2H), 7.61 (d, J = 8.4 Hz, 2H), 7.67 (s, 1 H), 8.08 (d, J = 8.5 Hz, 2H). MS
(DCI-NH3)
m/z 420 (M+H)+, (M+NH4)+. Anal. calc. for C17H19F3N203S2: C, 48.56 ; H, 4.55;
N, 6.66. Found: C, 47.86; H, 4.57; N, 6.51.
Example 294
? (2 2 2-Trifluoroethvl)-4-amino-5-[4-(methXlsulfonyI)phenyll-3(2H)-
ovridazinone
2-(2,2,2-Trifluoroethyl)-4-chloro-5-[4-(methytthio)phenyl]-3(2H)-
pyridazinone, prepared according to Example 193E, (500 mg, 1.36 mmol) was
dissolved in DMF (10 mL) and treated with NaN3 (100 mg, 1.5 mmol). After 2
hours
at room temperature, the reaction was diluted with ethyl acetate and washed
with
water, 4 times, and dried over MgSO4. After filtration of the drying agent and
concentration of the filtrate in vacuo, the residue was purified by
chromatography
on silica gel (Biotage 40S) eluted with 2:1 hexanes-ethyl acetate. The product
fractions were combined and evaporated to provide the azido intermediate, 2-
(2,2,2-Trifluoroethyl)-4-azido-5-[4-(methylthio)phenyl]-3(2H)-pyridazinone
(yield:
481 mg, 95%).
The 4-azido-compound above (39 mg, 0.105 mmol) was dissolved in THF (3
mL) and MeOH (2 mL) and treated with excess NaBH4. After 15 minutes, the
reaction was quenched with saturated NH4CI solution and the product was
extracted into ethyl acetate. The organic layer was washed with water, 3
times, and
dried over MgSO4. Filtration of the drying agent and evaporation of the
solvent
provided the title compound (yield: 26 mg, 71 %). M.p. > 260 C. 1 H NMR (300
MHz, DMSO-d6) 8 3.26 (s, 3H), 4.93 (q, J = 9 Hz, 2H), 6.71 (s, 2H), 7.72 (s,
1.H),
7.76 (d, J = 8 Hz, 2H), 8.02 (d, J = 8 Hz, 2H). MS (ESI-) m/z 346 (M-H)-.
Anal. caic.
for C13H12F3N303S: C, 44.96; H, 3.48; N, 12.10. Found: C, 44.59; H, 3.52; N,
11.93.
Example 295
2-(2.2.2-Trifluoroethyl)-4-(3-methoxypropylamino)-5-[4-(methylsuffonyl)phenyl]-
3(2H)-pyridazinone
A solution of 2-(2,2,2-trifluoroethyl)-4-chloro-5-[4-(methylsulfonyl)phenyl]-
3(2H)-pyridazinone (200 mg, 0.546 mmol), prepared according to the method of
Example 193E, and 3-methoxypropylamine (145 mg, 1.64 mmol) in pyridine (4 mL)
was heated at 100 C for 16 hours. The reaction mixture was cooled to room
temperature, mixed with silica gel (2 g), and the solvent removed under
reduced
pressure. The adsorbed silica gel was layered over an Extract-Clean Cartridge
-177-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
(Alitech, packing: 10 g silica gel) and the cartridge eluted with a
hexanes/acetone
step gradient consisting of 60 mL of each of the following mixtures: hexanes,
8:1
hexanes/acetone, 4:1, 2:1, and 1:1. Fractions containing desired product were
combined, concentrated, and further purified using HPLC (Technikrom Kromasil
60-5 sil silica column, 20 mm x 25 cm). The column was eluted with a linear
gradient consisting of 30% ethyl acetate/hexanes to 100% ethyl acetate at 10
mL/min over 50 minutes. Fractions containing product were combined and
concentrated under reduced pressure to provide the product as off-white
crystals
(yield: 215 mg, 95%). M.p. 110-113 C. 1 H NMR (300 MHz, CDCI3) S 8.02 (d, J =
18.0 Hz, 2H), 7.55 (d, 2H, J = 18.0 Hz), 7.48 (s, 1 H), 6.57 (br t, 1 H, J =
9.0 Hz), 4.81
(q, J = 17.4 Hz, 2H), 3.33 (t, J 12.0 Hz, 2H), 3.28 (s, 3H), 3.12 (s, 3H),
2.76 (dt, J =
12.0, 12.0 Hz, 2H), 1.65 (tt, J=12.0, 12.0, Hz, 2H). MS (DCI-NH3) m/z 420
(M+H)+,
m/z 437 [M+NH4]+. Anal. caic. for C17H2OF3N304S: C, 48.68; H, 4.81; N, 10.02.
Found: C, 48.74; H, 4.69; N, 9.84.
Example 296
2-(2.2.2-Trifluoroethvl)-4-(cy~loRgnty inQ)-5-[4-(methylsulfonvi)nhenylk
3(2H,),:
~yridazinone
The product was prepared according to the method of Example 295,
substituting cyclopentylamine in place of 3-methoxypropylamine to provide
brown
crystals (yield: 195 mg, 86%). M.p. 134-139 C. 1 H NMR (300 MHz, CDCI3) S
8.03
(d, J = 18.0 Hz, 2H), 7.56 (d, J = 18.0 Hz, 2H), 7.45 (s, 1 H), 6.12 (br d, J
= 16.8 Hz,
1 H), 4.79 (q, J = 17.4 Hz, 2H), 3.33 (br m, 1 H), 3.12 (s, 3H), 1.64-1.23 (br
m, 8H).
MS (DCI-NH3) m/z 416 (M+H)+, m/z 433 (M+NH4)+. Anal. caic. for C18H20-
F3N303S: C, 52.04; H, 4.85; N, 10.11. Found: C, 52.40; H, 4.93; N, 10.03.
Example 297
2-(2.2.2-Trifluoroethyl)-4-(cy .lob u ylamino)-5-14-(methylsulfonyl) ohenyl -
H)-
~yridazinone
The product was prepared according to the method of Example 295,
substituting cyclobutylamine in place of 3-methoxypropylamine to provide an
off-
white solid (yield: 206 mg, 94%). M.p. 169-172 C. 1 H NMR (300 MHz, CDCI3) 5
8.03 (d, J = 17.4 Hz, 2H), 7.54 (d, J= 17.4 Hz, 2H), 7.45 (s, 1 H), 6.28 (br
d, J = 16.2
Hz, 1 H), 4.81 (q, J = 17.4 Hz, 2H), 3.42 (m, 1 H), 3.13 (s, 3H), 1.79 (m,
4H), 1.64 (m,
1 H), 1.39 (m, 1 H). MS (DCI-NH3) m/z 402 (M+H)+, m/z 419 (M+NH4)+. Anal calc.
-178-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
for C17H18F3N3O3S-0.25 CH3COCH3: C, 51.25; H, 4.72; N, 10.10; found: C,
51.38; H, 4.68; N, 10.25.
Example 298
2-(2.2.2-Trifluoroethyl)-4-(3.4-dimethoxy ethvlamino)-5-[4-(methylsulfonvl)-
pheny1l~3 (2 H)-R,yridazi none
The product was prepared according to the method of Example 295,
substituting 3,4-dimethoxyphenethylamine in place of 3-methoxypropylamine to
provide an off-white solid (yield: 206 mg, 94%). M.p. 163-165 C. 1 H NMR (300
MHz, CDCI3) S 8.02 (d, J = 18.0 Hz, 2H), 7.52 (d, J = 18.0 Hz, 2H), 7.45 (s, 1
H), 6.75
(d, J = 16.2 Hz, 1 H), 6.50 (m, 2H), 6.16 (br d, J = 11.4 Hz, 1 H), 4.79 (q, J
= 17.4 Hz,
2H), 3.84 (s, 3H), 3.83 (s, 3H), 3.11 (s, 3H), 2.91 (dt, J = 12.6, 12.6 Hz,
2H), 2.60 (t, J
= 13.8 Hz, 2H). MS (DCI-NH3) m/z 529 (M+NH4)+. Anal. caic. for
C23H24F3N305S: C, 54.01; H, 4.73; N, 8.21. Found: C, 54.30; H, 4.69; N, 8.16.
Example 299
2-(2.2.2-Trifluoroethvl)-4-(cyclohex Iay mino)-5-j4-(methylsulfony,J)ohenyl]-
3(2HL
pyridazinone
The product was prepared according to the method of Example 295,
substituting cyclohexylamine in place of 3-methoxypropylamine to provide an
off-
white solid (yield: 103 mg, 42%). 1 H NMR (300 MHz, CDCI3) S 8.04 (d, J = 18.0
Hz,
2H), 7.58 (d, J = 18.0 Hz, 2H), 7.44 (s, 1 H), 6.06 (br d, J = 18.6 Hz, 1 H),
4.81 (q, J
18.0 Hz, 2H), 3.11 (s, 3H), 2.70 (m, 1 H), 1.66-1.48 (m, 4H), 1.42 (m, 1 H),
1.07 (m,
3H), 0.76 (m, 2H). MS (DCI-NH3) m/z 430 (M+H)+, m/z 447 (M+NH4)+. Anal. calc.
for C1 gH22F3N303S: C, 53.14; H, 5.16; N, 9.78. Found: C, 52.86; H, 5.06; N,
9.52.
Example 300
2=(2 2 2-Trifluoroethyl)-4-[2-(1-oi ridi nyl)eth ylamino]-5-[4-(methy Gult
nyI) henvll
3(2H)-R,yridazinone
The product was prepared according to the method of Example 295,
substituting cyclopentylamine in place of 3-methoxypropylamine to provide an
off-
white solid (yield: 210 mg, 84%). 1H NMR (300 MHz, CDCI3) 8 8.02 (d, J= 18.0
Hz, 2H), 7.56 (d, J- 18.0 Hz, 2H), 7.49 (s, 1 H), 6.91 (br, 1 H), 4.82 (q, J-
18.0 Hz,
2H), 3.13 (s, 3H), 2.64 (br, 2H), 2.32 (br, 4H), 1.58 (br, 6H), 1.42 (br, 2H).
MS (DCI-
NH3) m/z 459 (M+H)+. Anal. caic. for C19H22F3N303S: C, 52.39; H, 5.50; N,
12.22. Found: C, 52.64; H, 5.59; N, 12.00.
-179-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 301
2-(2 2 2-Trifluoroethvl)-4-(Z-tP; hwdr9furfurylamino)-5-[4-
(methvlsulfonvl)uhenvll-
3(2H)-Ryridazinone
The product was prepared according to the method of Example 295,
substituting tetrahydrofurfurylamine in place of 3-methoxypropylamine to
provide
an off-white solid (yield: 150 mg, 64%). M.p. 128-129 C. 1 H NMR (300 MHz,
CDCI3) 5 8.03 (d, J- 18.0 Hz, 2H), 7.56 (d, J- 18.0 Hz, 2H), 7.47 (s, 1 H),
6.48 (br
t, J- 9.0 Hz, 1 H), 4.81 (q, J- 18.0 Hz, 2H), 3.84 (m, 2H), 3.72 (m, 1 H),
3.12 (s, 3H),
2.83 (m, 1 H), 2.64 (m, 1 H), 1.84 (m, 3H), 1.34 (m, 1 H). MS (DCI-NH3) m/z
432
(M+H)+, m/z 449 (M+NH4)+. Anal. calc. for C18H20F3N303S: C, 50.11; H, 4.67; N,
9.74. Found: C, 50.25; H, 4.68; N, 9.68.
Example 302
2-(2.2.2-Trifluoroethvl)-4-(cyclonroRylamino)-5-[4-(methvlsulfonyl)o, henyll-
3(2W-
pyridazinone
The product was prepared according to the method of Example 295,
substituting cyclopropylmethylamine in place of 3-methoxypropylamine to
provide
an off-white solid (yield: 130 mg, 59%). M.p. 145-146 C. 1 H NMR (300 MHz,
CDC13) S 8.01 (d, J- 18.0 Hz, 2H), 7.53 (d, J = 18.0 Hz, 2H), 7.48 (s, 1 H),
6.20 (br,
1 H), 4.82 (q, J = 18.0 Hz, 2H), 3.12 (s, 3H), 2.45 (br d, J = 13.2 Hz, 2H),
0.88 (m,
1 H), 0.51 (m, 2H), 0.10 (m, 2H). MS (DCI-NH3) m/z 402 (M+H)+, m/z 419
(M+NH4)+. Anal. caic. for C17H18F3N303S: C, 50.87; H, 4.52; N, 10.47. Found:
C, 51.00; H, 4.52; N, 10.44.
Example 303
2-(2.2.2-Trifluoroethvl)-4-(2.3-dihydro-1 H-inden-1-vlamino)-5-[4-
(methylsulfonvl)-
phenyll-3(2H)-ovridazinone
The product was prepared according to the method of Exarnpie 295,
substituting 1-indanylamine in place of 3-methoxypropylamine to provide an off-
white solid (yield: 82 mg, 32%). M.p. 155-158 C. 1 H NMR (300 MHz, CDC13) S
8.04 (d, J = 18.0 Hz, 2H), 7.68 (d, J- 18.0 Hz, 2H), 7.49 (s, 1 H), 7.27-7.14
(m, 4H),
6.30 (br d, J = 18.0 Hz, 1 H), 4.81 (q, J = 18.0 Hz, 2H), 4.57 (m, 1 H), 3.09
(s, 3H),
2.89 (m, 1 H), 2.60 (m, 1 H), 1.85 (m, 1 H), 1-.68 (m, 1 H). MS (ESI (-) m/z
462 (M-H)-.
Anal. calc. for C22H2OF3N303S: C, 57.01; H, 4.35; N, 9.07. Found: C, 57.30; H,
4.45; N, 8.86.
-180-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 304
2 f2.2.2-TrifluoroethyJl-~(1-D1D,-_erilirly,l)-5-(4-(methvls~ lfonyl)phenylj-
3( 2)_
avridazinone
The product was prepared according to the method of Example 295,
substituting piperidine in place of 3-methoxypropylamine to provide an off-
white
solid (yield: 180 mg, 79%). M.p. 160-161 C. 1 H NMR (300 MHz, CDCI3) S 8.04
(d,
J_ 18.0 Hz, 2H), 7.58 (s, 1 H), 7.46 (d,'J = 18.0 Hz, 2H), 4.80 (q, J = 18.0
Hz, 2H),
3.13 (s, 3H), 2.96 (m, 4H), 1.65-1.52 (m, 6H). MS (DCI-NH3) m/z 416 (M+H)+.
Anal. caic. for C18H2OF3N3O3S-H2O: C, 52.04; H, 4.85; N, 10.11. Found: C,
52.21; H, 5.02; N, 9.75.
Example 305
2-(2.2.2-Trifiuoroethyl)-4-(3-hydroxyproRvlamino)-5-(4-(methvls. u{fonyj) he~
Il-
3(2H)-Ryridazinone
The product was prepared according to the method of Example 295,
substituting 3-hydroxypropylamine in place of 3-methoxypropylamine to provide
a
white solid (yield: 109.6 mg, 50%). M.p. 152-154 C. 1 H NMR (300 MHz, CDCI3)
5
8.02 (d, J- 18.0 Hz, 2H), 7.56 (d, J = 18.0 Hz, 2H), 7.48 (s, 1 H), 6.48 (br,
1 H), 4.79
(q, J = 17.4 Hz, 2H), 3.63 (t, J- 12.0 Hz, 2H), 3.12 (s, 3H), 2.81 (dt, J =
12.0, 12.0
Hz, 2H), 1.65 (tt, J- 12.0, 12.0 Hz, 2H). MS (DCI-NH3) m/z 406 (M+H)+, m/z 423
(M+NH4)+. Anal. calc. for C16H18F3N304S: C, 47.41; H, 4.48; N, 10.37. Found:
C, 47.53; H, 4.33; N, 10.27.
Example 306
2-(2.2.2-Trifluoroethyl)-4-(3-(1 H-imidazol-1-yl), roovlaminoj-5-[4-(methy
sulfnnvl)-
phepyj)-3(2 H);p,yridazi none
The product was prepared according to the method of Example 295,
substituting (3-aminopropyl)imidazole in place of 3-methoxypropylamine. The
reaction mixture was concentrated to dryness and the residue purified using RP-
HPLC (Rainin Dynamax C-18 column, 60 A pore size, 21.4 mm i.d.). The column
was eluted with a linear gradient consisting of 20% acetonitrile (containing
0.1%
TFA)/80% water (containing 0.1 % TFA) to 100% acetonitrile (containing 0.1 %
TFA)
at 15 mUmin over 70 minutes. The peak corresponding to the title product was
collected and lyophilized to provide a tan hygroscopic foam (yield: 70.2 mg,
28%).
1 H NMR (300 MHz, DMSO) 5 8.95 (br s, 1 H), 7.97 (d, J = 16.8 Hz, 2H), 7.66
(d, J
* trade-mark
-181-
CA 02578858 2007-03-05
WO 99/10331 PCTIUS98/16479
16.2 Hz, 2H), 7.61 (s, 1 H), 7.58 (d, J = 15.0 Hz, 2H), 6.99 (br t, 1 H, J =
13.2 Hz),
4.97 (dt, J 18.0, 18.0 Hz, 2H), 3.97 (t, J = 13.2 Hz, 2H), 3.28 (s, 3H), 2.69
(m, 2H),
1.81 (tt, J 13.2, 13.2 Hz, 2H). MS (DCI-NH3) m/z 456 (M+H)+. Anal. caic. for
C1 gH2OF3N5O3S-1.4 CF3COOH: C, 42.57; H, 3.51; N, 11.39. Found: C, 42.78; H,
3.58; N, 11.24.
Example 307
2-(2.2.2-Trifiuoroethvl)-4-(2R-hydroxy,jRroQyjamipQ)-5-[4- (methv au Iton
yi)nhenvli-
3 (2H)-Ryridazjnone
The product was prepared according to the method of Example 295,
substituting (R)-(-)-2-propanolamine in place of 3-methoxypropylamine to
provide
an off-white solid (yield: 109.6 mg, 50%). M.p.- 140-142 C. 1 H NMR (300 MHz,
CDC13) S 8.04 (d, J = 18.0 Hz, 2H), 7.56 (d, J = 18.0 Hz, 2H), 7.49 (s, 1 H),
6.42 (br,
1 H), 4.79 (m, 2H), 3.80 (m, 1 H), 3.12 (s, 3H), 2.68 (m, 2H), 1.02 (d, J =
12.0 Hz, 3H).
MS (DCI-NH3) m/z 406 (M+H)+, m/z 423 (M+NH4)+. Anal. calc. for
C16H18F3N304S: C, 47.41; H, 4.48; N, 10.37. Found: C, 47.56; H, 4.41; N,
10.25.
Example 308
2-(2.2.2-Trifluoroethyl)-4-(2-cyanoethylamino)--5-[4-(methy,jsulfonyl)nhenyl -
3(2uL-
Ryridazinone
The product was prepared according to the method of Example 295,
substituting 1-cyanoethylamine in place of 3-methoxypropylamine to provide an
off-
white solid (yield: 27 mg, 12%). M.p. 172-174 C. 1 H NMR (300 MHz, CDCI3) 8
8.09 (d, J- 18.0 Hz, 2H), 7.63 (d, J 18.0 Hz, 2H), 7.51 (s, 1 H), 6.08 (br t,
1 H),
4.87 (q, J- 18.0 Hz, 2H), 3.17 (dt, J 13.2, 13.2 Hz, 2H), 3.13 (s, 3H), 2.39
(t, J
13.2 Hz, 2H). MS (DCI-NH3) m/z 418 (M+NH4)+. Anal. caic. for
C1 gH15F3N403S: C, 48.00; H, 3.78; N, 13.99. Found: C, 48.28; H, 3.77; N,
13.80.
Example 309
2-(2.2.2-Trifluoroethvl)-4-(4-cvanoanilino)-S-[4-(methvlsulfonxJ)ohenyl]-3j2H,-
Ryridazinone
A suspension of 2-(2,2,2-trifluoroethyl)-4-chloro-5-[4-(methylsulfonyl)phenyl]-
3(2H)-pyridazinone (300 mg, 0.820 mmol), prepared according to the method of
Example 193E, 4-aminobenzonitrile (290 mg, 2.46 mmol), and silver oxide (760
mg, 3.28 mmol) in pyridine (1.5 mL) was stirred at 80 C for 24 hours. The
reaction
was cooled to room temperature, adsorbed onto silica gel (2 g) and solvent
-182-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
removed under reduced pressure. The adsorbed silica gel was layered over an
Extract-Clean Cartridge (Alltech, packing: 10 g silica gel) and the cartridge
eluted
with a hexanes/acetone step gradient consisting of 60 mL of each of the
following
mixtures: hexanes, 8:1 hexanes/acetone, 4:1, 2:1, and 1:1. Fractions
containing
desired product were combined, concentrated, and further purified using HPLC
(Technikrom Kromasil 60-5sil column, 20 mm x 25 cm). The column was eluted
with a linear gradient consisting of 30% ethyl acetate/hexanes to 100% ethyl
acetate at 10 mL/min over 50 minutes. Fractions containing product were
combined and concentrated under reduced pressure to provide the product as a
tan solid (yield: 149.9 mg, 41 %). M.p.>230 C. 1 H NMR (300 MHz, DMSO) S 9.49
(s, 1 H), 8.00 (s, 1 H), 7.69 (d, J = 17.4 Hz, 2H), 7.43 (d, J- 16.8 Hz, 2H),
7.32 (d, J
= 18.0 Hz, 2H), 6.78 (d, J = 18.0 Hz, 2H), 5.06 (q, J = 18.0 Hz, 2H), 3.13 (s,
3H),
2.68 (m, 2H), 1.02 (d, J = 12.0 Hz, 3H). MS (DCI-NH3) m/z 466 (M+NH4)+. Anal.
calc. for C20H15F3N403S: C, 53.57; H, 3.37; N, 12.49. Found: C, 53.47; H,
3.49;
N, 12.35.
Example 310
2-(2.2.2-Trifluoroethyl)-4-j -methoxy-5-(triflunrnmpthy))anilino)-5-j4-(methvl-
suifonyl)pheny,[,jy3(2H)-Ryridazinone
The product was prepared according to the method of Example 309 ,
substituting 3-methoxy-5-(trifluoromethyl)aniline in place of 4-
aminobenzonitrile to
provide a brown solid (yield: 226.5 mg, 80%). M.p. 206-208 C. 1 H NMR (300
MHz, CDC13) S 7.90 (s, 1 H), 7.77 (s, 1 H), 7.71 (d, J- 18.0 Hz, 2H), 7.28 (d,
J - '
17.4 Hz, 2H), 6.61 (br s, 1 H), 6.46 (br s, 1 H), 6.31 (br s, 1 H), 4.90 (q,
J= 17.4 Hz,
2H), 3.72 (s, 3H), 2.94 (s, 3H). MS (DCI-NH3) m/z 539 (M+NH4)+. Anal. caic.
for
C21 H17F6N304S: C, 48.37; H, 3.29; N, 8.06. Found: C, 48.60; H, 3.33; N, 7.94.
Example 311
212.2.2-Trifluoroethy I)-4-anilino-5-[4-(methvlsulfonyl)1heny,jj-
3(2H)oyridazinone
The product was prepared according to the method of Example 309 ,
substituting aniline in place of 4-aminobenzonitrile to provide a tan solid
(yield: 90
mg, 53%). M.p. 154-156 C. 1 H NMR (300 MHz, CDCI3) S 7.89 (br s, 1 H), 7.72
(s,
1 H), 7.62 (d, J = 18.0 Hz, 2H), 7.19 (d, J= 18.0 Hz, 2H), 7.96-7.82 (m, 3H),
6.61 (d,
J = 14.4 Hz, 2H), 4.90 (q, J = 18.0 Hz, 2H), 2.94 (s, 3H). MS (DCI-NH3) m/z
424
(M+H)+, m/z 441 (M+NH4)+. Anal. calc. for C1 gH16F3N303S: C, 53.90; H, 3.81;
N,
9.92. Found: C, 53.87; H, 3.73; N, 9.89.
-183-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 312
2- (2.2.2 Trifluoroethv1) -4- (2 5-dimethoxvp hen yiamino)-5-(4-
(methylsulfonvl)oheoYL1=
3 (2 H)-ovridazinone
The product was prepared according to the method of Example 309 ,
substituting 2,5-dimethoxyaniline in place of 4-aminobenzonitrile to provide a
tan
solid (yield: 140 mg, 53%). M.p. 95-96 C. 1 H NMR (300 MHz, CDCI3) S 7.78 (br
s,
1 H), 7.72 (s, 1 H), 7.63 (d, J = 18.0 Hz, 2H), 7.18 (d, J = 18.0 Hz, 2H),
6.54 (d, J =
18.0 Hz, 1 H), 6.38 (dd, J- 6.0, 18.0 Hz, 1 H), 4.89 (q, J = 18.0 Hz, 2H),
3.73 (s, 3H),
3.47 (s, 3H), 2.96 (s, 3H). MS (DCI-NH3) m/z 484 (M+H)+, m/z 501 (M+NH4)+.
Anal. caic. for C21 H20F3N305S: C, 52.17; H, 4.17; N, 8.69. Found: C, 52.47;
H,
4.17; N, 8.43.
Example 313
2-(2 2 2-Trifluoroethylõ )-4-(3-fluoroanilino)-544-(methylsulfonvi)phenXll-
3(2H)-
pyridazinone
The product was prepared according to the method of Example 309 ,
substituting 3-fluoroaniline in place of 4-aminobenzonitrile to provide a tan
solid
(yield: 151.3 mg, 42%). M.p. 156-158 C. 1 H NMR (300 MHz, DMSO) S 9.18 (s,
1 H), 7.91 (s, 1 H), 7.62 (d, J = 17.4 Hz, 2H), 7.36 (d, J = 17.4 Hz, 2H),
6.88 (dd, J
15.0, 15.0 Hz, 1 H), 6.56 (m, 1 H), 6.49 (m, 2H), 5.04 (q, J- 18.0 Hz, 2H),
3.08 (s,
3H). MS (DCI-NH3) m/z 442 (M+H)+, m/z 459 (M+NH4)+, m/z 476 (M+2NH4-H)+.
Anal. calc. for C1 gH15F4N303S-0.5 CH3COCH3: C, 52.33; H, 3.85; N, 8.93.
Found: C, 52.51; H, 3.58; N, 8.81.
Example 314
2-(2.2.2-Trifluoroethyl)-4-(2.4-difluoroanilino)-5-j4-(methylsulfonyl)phenyfJ-
3(2H)-
pyridazinone
The product was prepared according to the method of Example 309 ,
substituting 2,4-difluoroaniline in place of 4-aminobenzonitrile to provide a
tan solid
(yield: 63.1 mg, 17%). M.p. 170-175 C. 1 H NMR (300 MHz, DMSO) S 9.00 (s, 1
H),
7.80 (s, 1 H), 7.57 (d, J = 17.4 Hz, 2H), 7.26 (d, J = 17.4 Hz, 2H), 7.05 (m,
1 H), 6.75
(m, 2H), 5.05 (q, J = 18.0 Hz, 2H), 3.09 (s, 3H). MS (DCI-NH3) m/z 460 (M+H)+,
m/z 477 (M+NH4)+. Anal. calc. for C1 gH14F5N303S: C, 49.68; H, 3.07; N, 9.15;
found: C, 50.00; H, 2.95; N, 9.10.
-184-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 315
2-(2.2.2-Trifluoroethyl)-4-(2.3.5-trifluoroanilino,)_5-[4- ethylsulfonyl)ohe
yl];3(2H1
Ryridazinone
The product was prepared according to the method of Example 309
substituting 2,3,5-trifluoroaniline in place of 4-aminobenzonitrile to provide
a pale
purple solid (yield: 85.3 mg, 22%). M.p. 190-194 C. 1 H NMR (300 MHz, DMSO) S
9.27 (s, 1 H), 7.90 (s, 1 H), 7.70 (d, J = 17.4 Hz, 2H), 7.39 (d, J = 17.4 Hz,
2H), 7.03
(m, 1 H), 6.76 (m, 1 H), 5.06 (q, J- 18.0 Hz, 2H), 3.14 (s, 3H). MS (DCI-NH3)
m/z
495 (M+NH4)+. Anal. caic. for C19H13F6N303S: C, 47.80; H, 2.74; N, 8.80.
Found: C, 47.51; H, 2.55; N, 8.63.
Example 316
2_(2.2.2-Trifluoroethyl)-4-(4-fluoroanilino)-544.(met hylsu Ifonyl)Pheny(]-3(2
H)-
Ryridazinone
The product was prepared according to the method of Example 309 ,
substituting 4-fluoroaniline in place of 4-aminobenzonitrile to provide a tan
solid
(yield: 15.8 mg, 4%). M.p. 158-160 C. 1 H NMR (300 MHz, CDCI3) S 7.80 (br s,
1 H), 7.69 (s, 1 H), 7.65 (d, J = 18.0 Hz, 2H), 7.18 (d, J:; 18.0 Hz, 2H),
6.63 (d, J-
3.6 Hz, 2H), 6.61 (s, 2H), 4.89 (q, J- 17.4 Hz, 2H), 2.96 (s, 3H). MS (DCI-
NH3) m/z
459 (M+NH4)+. Anal. caic. for C19H15F4N303S-1.25 H20: C, 49.19; H, 3.80; N,
9.05. Found: C, 59.57; H, 3.53; N, 8.70.
Example 317
2-Benzyl-4-(3-thienyl)-5-j4-(met ylsulfonyI)phenyl]-3 (2H)-nvridazinone
2-Benzyl-4-chloro-5-[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone prepared
in Example 78 (150 mg, 0.4 mmol), thiophene-3-boronic acid (66.5 mg, 0.52
mmol),
CsF (145.8 mg, 0.96 mmol), and tetrakis-(triphenylphosphine)-palladium(0)
(13.9
mg, 0.012 mmol) in DME (25 mL) were stirred at reflux for 6 hours'TLC (1
CH2CI2:1
hexanes:1.5 ethyl acetate) indicated that all starting materials were
consumed. The
reaction mixture was cooled to room temperature and concentrated under reduced
pressure. The residue was partitioned between water and ethyl acetate. The
organic layer was washed with brine, dried over MgSO4, and filtered. The
fiitrate
was concentrated under reduced pressure. The residue was purified using a
silica
gel column (0.5:2.5:0.5 CH2CI2/hexanes/ethyl acetate). A yellow powder was
obtained (yield: 50 mg, 31 %). 1 H NMR (300 MHz, CDC13) S 3.09 (s, 3H), 5.41
(s,
2H), 6.72 (dd, J = 1.5 Hz, 9 Hz, 1 H), 7.13 (dd, J- 3 Hz, 3 Hz, 1 H), 7.3-7.45
(m, 5H),
-185-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
7.5-7.6 (m, 3H), 7.78 (s, 1 H), 7.92 (d, 9 Hz, 2H). MS (DCI-NH3) m/z 423
(M+H)+.
Anal. calc. for C22 H18N203S2. 0.5 H20: C, 6.23; H, 4.43; N, 6.49. Found C,
61.29; H, 4.40; N, 6.16.
Example 318
2-Benz,vl-4-(2-benzofuranyl)-5-r4-(methvlsulfonyl)ohenyl1-3(2H)_Ryridazinone
The title compound was prepared according to the method of Example 317,
substituting 2-benzofuranboronic acid 3-thiopheneboronic acid (yield: 46 mg,
25%). 1 H NMR (300 MHz, CDCI3) S 3.13 (s, 3H), 5.5 (s, 2 H,), 6.85-6.92 (m, 1
H),
7.15-7.25 (m, 3H), 7.3-7.42 (m, 3H), 7.45-7.7 (m, 5H), 7.79 (s, 1 H) 8.0 (d, J
= 9 Hz,
2H), 8.08 (s, 1 H). MS (DCI-NH3), m/z 457 (M+H)+. Anal. calc. for
C26H2ON2O4S-H2O: C, 65.80; H, 4.67; N, 5.90. Found C, 65.44; H, 4.42; N, 6.14.
Example 319
2-Benzyl-4-(1.3-dihvdro-l-oxo-5-isobenzofuranyj)-5-[4-(methylsulfonyJ oheny~L-
3(2H)-ovridazinone
The title compound was prepared according to the method of Example 221
substituting 2-benzyl-4-chloro-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone,
prepared in Example 78, in place of 2-(2,2,2-trifluoroethyl)-4-chloro-5-[4-
(methylsulfonyl)-phenyl]-3(2H)-pyridazinone (yield: 112 mg, 44%). M.p. > 250
C.
1 H NMR (300 MHz, DMSO-d6) S 3.20 (s, 3H), 5.34 (s, 2H), 5.36 (s, 2H), 7.30-
7.44
(m, 6H), 7.48 (d, J = 8 Hz, 2H), 7.57 (s, 1 H), 7.73 (d, J = 8 Hz, 1 H), 7.85
(d, J = 8 Hz,
2H), 8.17 (s, 1 H). MS (DCI-NH3) m/z 473 (M+H)+, 490 (M+NH4)+. Anal. calc. for
C26H2ON205S: C, 65.46; H, 4.33; N, 5.87. Found: C, 65.56; H, 4.48; N, 5.75.
Example 320
2-Benzyl-4-(5-chloro-2-thienyl)-5-[4-(methylsulfonYl)phenyl]-3(2H)-
12vridazinone
The title compound was prepared according to the method of Example 317,
substituting 4-chloro-2-thiopheneboronic acid in place of 3-thiopheneboronic
acid
(yield: 21 mg, 17%). 1 H NMR (300 MHz, CDC13) S 3.15 (s, 3H), 5.45 (s, 2H),
6.51
(d, J = 4.5 Hz, 1 H), 6.7 (d, J = 4.5 Hz, 1 H), 7.3-7.4 (m, 3H), 7.5 = 7.6 (m,
4H), 7.6 (s,
1 H), 8.05 (d, J = 9 Hz, 2H). MS (DCI-NH3), m/z 457 (M+H)+. Anal. caic. for
C18H15CIN2O3S: C, 57.68; H, 4.03; N, 7.47. Found C, 57.61; H, 3.84; N, 7.14.
-186-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 321
2-BenzY1-4-(3-nitrop-henyl)-5-[4-(methylsulfonyl)phenyll-3(2H)-Ryridazinone
The title compound was prepared according to the method of Example 317,
substituting 3-nitrobenzeneboronic acid in place of 3-thiopheneboronic acid
(yield:
20 mg, 11 %). 1 H NMR (300 MHz, CDCI3) S 3.0 (s, 3H), 5.93 (s, 2H), 7.6-7.8
(m,
9H), 7.8 (t, J = 4.5 Hz, 3H), 8.04 (s, 1 H), 8.15 (m, 1 H). MS (DCI-NH3), m/z
462
(M+H)+. Anal. calc. for C24 H1 9N3O5S. 0.75 H20: C, 60.68; H, 4.35; N, 8.84.
Found C, 60.99; H, 3.97; N, 8.35.
Example 322
2-Benzyl-4-(4-vinylphenyl)-5-[4-(methylsulfonvl) henx(1 3(2H)-pvridazinone
The title compound was prepared according to the method of Example 317,
substituting 4-vinylbenzeneboronic acid in place of 3-thiopheneboronic acid
(yield:
40 mg, 23%). 1 H NMR (300 MHz, CDCI3) 8 3.05 (s, 3H), 5.28 (d, J = 12 Hz, 1
H),
5.41 (s, 2H), 5.74 (d, J = 18 Hz, 1 H) 6.65 (dd , J = 12 Hz, 18 Hz, 1 H), 7.1-
7.6 (m,
11 H) 7.83 (d, J = 3 Hz, 2H), 7.85 (s, 1 H). MS (DCI-NH3), m/z 443 (M+H)+.
Anal.
caic. for C26 H22N203S: C, 70.57; H, 5.01; N, 6.33. Found C, 70.34; H, 4.67;
N,
5.97.
Example 323
2-BenZyl-4-(4-trifluormethylphepyl)-5-[4-(methylsulfonyl)oheny!);3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 317,
substituting 4-(trifluoromethyl)benzeneboronic acid in place of 3-
thiopheneboronic
acid (yield: 101 mg, 52%). 1 H NMR (300 MHz, CDCI3) S 3.05 (s, 3H), 5.42 (s,
2H),
7.3-7.5 (m, 8H), 7.55-7.6 m, 3H), 7.85 (s, 2H), 7.9 (s, 1 H). MS (DCI-NH3) m/z
485
(M+H)+. Anal. caic. for C25H1 9F3N2O3S=0.25 H20: C, 61.40; H, 4.01; N, 5.72.
Found C, 61.26; H, 4.01; N, 5.35.
Example 324
2-Benzyt-4-(2-methoxyoheny1)-544-(methylsuffonyl)ohenyj];3 (2 H)-Ryridazi nnnA
The title compound was prepared according to the method of Example 317,
substituting 2-methoxybenzeneboronic acid in place of 3-thiopheneboronic acid
(yield: 75 mg, 42%). 1 H NMR (300 MHz, CDCI3) S 3.01 (s, 3H), 3.5 (s, 3H),
5.40
(dd, J = 12 Hz, 18 Hz, 2H), 6.76 (d, J = 9 Hz, 1 H), 6.85-6.95 (m, 1 H), 7.09
(dd, J =
1.5 Hz, 9 Hz, 1 H), 7.26-7.41 (m, 6H), 7.55 (dd, J= 1.5 Hz, 9 Hz, 2H), 7.82
(d, J= 9
-187-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Hz, 3H). MS (DCI-NH3) m/z 447 (M+H)+. Anal. calc. for C25H22N204S=0.5 H20:
C, 65.91; H, 5.08; N, 6.14. Found C, 65.86; H, 5.08; N, 5.58.
Example 325
2-Benzyl-4-(3.4-dimethylRhenyl)-5-[4-(methvlsulfonyl)Dhenyj,l-3(2 H)-
,ovridazinone
2-Benzyl-4-chloro-5-[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone (150 mg,
0.4 mmol) prepared in Example 78 was dissolved in anhydrous DME (10 mL) and
heated to reflux with 3,4-dimethylbenzeneboronic acid in presence of CsF (146
mg,
0.96 mmol) and tetrakis(triphenylphosphine)palladium (14 mg, 0.012 mmol) for 6
hours. After cooling to room temperature the reaction mixture was diluted with
water and extracted with ethyl acetate (100 mL). The organic layer was washed
with brine, dried over MgSO4, and evaporated in vacuo. The compound was
purified on a silica gel column, eluting with 30% ethyl acetate in pentanes,
providing the desired compound (yield: 100 mg, 56%). 1 H NMR (300 MHz, CDC13)
S 2.15, 2.20 (2s, 3H), 2.25, 2.30 (2s, 3H), 3.05, 3.08 (2s, 3H), 5.35, 5.40
(2s, 2H),
6.60-7.1 (m, 3H), 7.30-7.40 (m, 4H), 7.42-7.60 (m, 2H), 7.70-8, 02 (m, 4H). MS
(DCI-NH3) m/z 445 (M+H)+. Anal. calc. for C26H24N203S=H20: C, 67.51; H, 5.66;
N, 6.05. Found: C, 67.45;H, 5.56; N, 5.85.
Example 326
2-Benzvl-4-(3-fluoro-4-methoxyphenyl)-5-j4- et y su y,l)phenyl]-3(
Ryridazinone
The title compound was prepared according to the method of Example 325,
substituting 3-fluoro-4-methoxybenzeneboronic acid in place of 3,4-dimethyl-
benzeneboronic acid (yield: 35 mg, 19%). 1 H NMR (300 MHz, CDCI3) S 3.05 (s,
3H), 3.85 (s, 3H), 5.3, 5.4 (2s, 2H), 6.75-7.03 (m, 3H), 7.3-7.40 (m, 5H), 7.4-
7.55 (dd,
J = 1.5 Hz; 7.5 Hz, 2H), 7.8-7.95 (m, 3H). MS (DCI-NH3) m/z 465 (M+H)+. Anal.
calc. for C25H21 N204S-0.25 H20: C, 64.02; H, 4.62; N, 5.97. Found: C, 63.93;
H,
4.54; N, 5.43
Example 327
2-Benzvl-4-f3-(2-methoxyRyridyl)]-5õ[4-(methyJsulfony,j)phenyl]-3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 325,
substituting 2-methoxy-3-pyridylboronic acid in place of 3,4-dimethylbenzene-
boronic acid (yield: 35 mg, 19%). 1 H NMR (300 MHz, CDCI3) S 3.05 (s, 3H),
3.58
(s, 3H), 5.4 (dd, J = 15 Hz, 18 Hz; 2H), 6.88 (m, 1 H), 7.28-7.40 (m, 5H), 7.5-
7.6 (dd,
-188-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
J = 1.5 Hz; 7.5 Hz, 3H), 7.82 (s, 1 H), 7.85 (d, J = 18 Hz, 2H), 8.15 (br s, 1
H). MS
(DCI-NH3) m/z 448 (M+H)+. Anal. calc. for C24H21 N304S: C, 64.42; H, 4.73; N,
9.39. Found: C, 64.17; H, 5.11; N, 9.04
Example 328
2-Benzyl-4-(3-ethoxyphgny1)-5-j4-(methyisuI.faIlyl)phenyll:3(2H)-pyridazinnnA
The title compound was prepared according to the method of Example 325,
substituting 3-ethoxybenzeneboronic acid in place of 3,4-
dimethylbenzeneboronic
acid (yield: 115 mg, 67%). 1 H NMR (300 MHz, CDCI3) S 1.31 (t, J = 7.5 Hz,
3H),
3.05 (s, 3H), 3.89 (q, J 7.5 Hz, 2H), 5.14 (s, 2H), 6.65 (d, J = 9 Hz, 1 H),
6.72 (t, J
1.5Hz;1H),6.8(dd,J=1.5Hz,9Hz,1H),7.15(t,J=9Hz, 1 H), 7.3-7.4 (m, 5H),
7.5-7.6 (m, 2H), 7.85 (d, J = 9 Hz, 3H). MS (DCI-NH3) m/z 461 (M+H)+. Anal.
calc.
for C26H24N204S=0.5H20: C, 66.50; H, 5.36; N, 5.96. Found: C, 66.39; H, 5.02;
N,
5.77
Example 329
2-Benzvl-4-(4-fluorobenzY1)-5-14- (methY au Ifo y1)phenyl];(2 H)-Ryridazinone
329A. 2-Benzyl-4.5-dibromo-3(?.H)-pyridazi nnnp
The title compound was prepared according to the method of Example 194A,
substituting benzyl hydrazine hydrochloride in place of 4-fluorophenyl
hydrazine
hydrochloride (yield: 7.86 g, 60%). 1 H NMR (300 MHz, DMSO d6) S 5.27 (s, 2H),
7.26-7.41 (m, 5H), 8.19 (s, 1 H). MS (DCI-NH3) m/z 345 (M+H)+, 362 (M+H)+.
329B. 2-Benzvl-5-bromo-4-methoxy-3(2H)-Ryridazi none
The title compound was prepared according to the method described in
Example 194B, substituting 2-benzyl-4,5-dibromo-3(2H)-pyridazinone for 2-(4-
fluorophenyl)-4,5-dibromo-3(2H)-pyridazinone (yield: 2.877 g; 85%). 1 H NMR
(300
MHz, DMSO-d6) fi 4.14 (s, 3H), 5.23 (s, 2H), 7.26-7.38 (m, 5H), 8.11 (s, 1 H).
MS
(DCI-NH3) m/z 295 (M+H)+, 312 (M+NH4)+.
329C. 2-Benzvl-4-methoxv-5-j4-(methvlthio henvl]-3(2H)-pyridazinone
The title compound was prepared according to the method described in
Example 6, substituting 2-benzyl-4-methoxy-5-bromo-3(2H)-pyridazinone for 2-
-189-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
benzyl-4-methoxy-5-bromo-3(2H)-pyridazinone (yield: 3.705 g). 1 H NMR (300
MHz, DMSO-d6) S 2.52 (s, 3H), 3.99 (s, 3H), 5.28 (s, 2H), 7.26-7.41 (m, 7H),
7.55
(m, 2H), 8.02 (s, 1 H). MS (DCI-NH3) m/z 339 (M+H)+, 356 (M+NH4)+.
329D. 2-Benzyl-4-(4-fluorobenzyl-5-j4-(methylsulforayl)ohenyl]-3(2 H)-
ovridazinone
The title compound was prepared according to the method of Example 233,
substituting 4-fluorobenzyl magnesium chloride in place of cyclohexylmagnesium
chloride and 2-benzyl-4-methoxy-5-[4-(methylthio)phenyl]-3(2H)-pyridazinone
was
substituted in place of 2-(4-fluorophenyl)-4-methoxy-5-[4-(methylthio)phenyl]-
3(2H)-pyridazinone.
329C. 2-Benzyl-4-methoxy-5-j4-(methylsulfonyl)12henyll-3(2 H)-pyridazinone
The sulfide compound (Example 329D) was oxidized to the methyl sulfonyl
compound according to the method of Example 10. M.p. 186-189 C. 1 H NMR
(300 MHz, DMSO d6) S 3.27 (s, 3H), 3.83 (s, 2H), 5.31 (s, 2H), 6.94-7.05 (m,
4H),
7.27-7.40 (m, 5H), 7.67 (m, 2H), 7.94 (s, 1 H), 8.03 (m, 2H). MS (DCI-NH3) m/z
449
(M+H)+, 466 (M+NH4)+. Anal. caic. for C25H21 FN2O3S: C, 66.95; H, 4.72; N,
6.25. Found: C, 66.68; H, 4.75; N, 6.14.
Example 330
2-(tert.-Butvl)-4-(3-methvlbutoxy)-5-[4-(methy suIfonyl)phenyll-3(2H)-
pyridazinone
330A. 2-(tert-Butyl)-4 5-dichloro-3(2H)-pyridazinone
A solution of mucochloric acid (33.8 g, 200 mmol) and tert.-butylhydrazine
hydrochloride (24.9 g, 200 mmol) in methanol (400 mL) was stirred at reflux
overnight. Methanol was removed in vacuo and the residue was partitioned
between ether and water. The organic layer was dried over MgSO4 and filtered.
The filtrate was concentrated in vacuo and the residue was purified by column
chromatography (silica gel, 100% hexanes). Product-containing fractions were
combined and the title compound was crystallized from ether/hexanes (yield:
10.0
g, 22.6%). M.p. 63-64 C. 1 H NMR (300 MHz, CDCI3) S 1.65 (s, 9H), 7.73 (s, 1
H).
MS (DCI-NH3) m/z 221 (M+H)+, 238 (M+NH4)+.
330B. 2-(tert.-Butyl)-4-(3-methvlbutoxy)-5-chloro-3(2H) pvridazinone
A stirred, room temperature solution of 3-methyl-1 -butanol (0.5 mL, 4.52
mmol) in tetrahydrofuran (10 mL) was treated with a 60% oil suspension of
sodium
hydride (0.24 g, 5.88 mmol). After 5 minutes, hydrogen gas evolution had
subsided, so the dichloro-intermediate from Example 330A (1.0 g, 4.52 mmol)
was
-190-
CA 02578858 2007-03-05
WO 99/10331 PCT/1JS98/16479
added and the reaction mixture was stirred at room temperature for 20 hours.
The
reaction was quenched with 10% aqueous citric acid and extracted with ethyl
acetate. The organic layer was washed with brine, dried over MgSO4, and
filiered.
The fiftrate was concentrated in vacuo, and the residue was purified by column
chromatography (silica gel, 100% hexanes). The title compound was obtained as
a
pale yellow oil (yield: 0.7 g, 56.7%). 1 H NMR (300 MHz, CDCI3) S 0.95 (d, J 6
Hz,
6H), 1.63 (s, 9H), 1.64 (q, J = 6 Hz, 2H), 1.85 (nonet, J = 6 Hz, 1 H), 4.49
(t, J 6 Hz,
2H), 7.64 (s, 1 H). MS (DCI-NH3) m/z 273 (M+H)+, 290 (M+NH4)+.
330C. 2-(tert.-Butyl); 4-(3-meth Iby utoxy -5-L4-(methylthio)pheny!]-3(2H)-
pyridazinone
A solution of the intermediate from Example 330B (700 mg, 2.57 mmol), 4-
(methylthio)benzeneboronic acid (560 mg, 3.34 mmol), cesium carbonate (2.17 g,
6.67 mmol), and tetrakis(triphenylphosphine)palladium(0) (210 mg, 0.18 mmol)
in
dimethoxyethane (40 mL) was heated at reflux for 5 hours. The heat source was
then removed and the reaction mixture was stirred at room temperature for 64
hours. The reaction mixture was filtered and the filtrate was concentrated in
vacuo
to provide a brown oil. This oil was purified by column chromatography twice
(silica
gel, 97:3 hexanes/ethyl acetate, then 96:4 hexanes/ethyl acetate) to provide a
semi-solid product (yield: 270 mg, 29.2%). 1 H NMR (300 MHz, CDCI3) S 0.81 (d,
J
= 6 Hz, 6H), 1.49 (q, J = 6 Hz, 2H), 1.63 (nonet, J = 6 Hz, 1 H), 1.69 (s,
9H), 2.52 (s,
3H), 7.32 (d, J = 9 Hz, 2H), 7.50 (d, J = 9 Hz, 2H), 7.73 (s, 1 H). MS (DCI)
m/z 361
(M+H)+.
330D. 2-(tert.-Butvl)-4-(3-methy but y)&:[A--(mgjhy suI j)nhenvj] 3(2FI
pyridazinone
The title compound was prepared according to the method of Example 10,
substituting 2-(tert.-butyl)-4-(3-methylbutoxy)-5-[4-(methylthio)phenyl]-3(2H)-
pyridazinone for 4-(4-fluorophenyl)-5-[4-(methylthio)phenyl]-3(2H)-
pyridazinone
(yield: 188 mg, 63.9%). M.p. 138-139 C. 1 H NMR (300 MHz, CDCI3) S 0.81 (d, J-
6 Hz, 2H), 1.48 (q, J = 6 Hz, 2H), 1.48-1.68 (m, 1 H), 1.69 (s, 9H), 3.10 (s,
3H), 4.38
(t, J = 6 Hz, 2H), 7.71 (s, 1 H), 7.74 (d, J = 9 Hz, 2H), 8.03 (d, J = 9 Hz,
2H). MS
(DCI-NH3) m/z 393 (M+H)+. Anal. calc. for C20H28N204S: C, 61.20; H, 7.19; N,
7.14. Found: C, 61.13; H, 7.23; N, 6.89.
-191-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 331
2_(3-Chlorol2heuyj)-4-methoxv-5-[4-(methylsulfonyl)ohenyl]-3(2H)-oyridazinone
The title compound was prepared according to the method of Example 10,
substituting 2-(3-chiorophenyl)-4-methoxy-5-[4-(methylthio)phenyl]-3(2H)-
pyridazinone (Example 207C) in place of 2-benzyl-4-(4-fluorophenyl)-5-[4-
(methyl-
thio)phenyl]-3(2H)-pyridazinone (yield: 3.31 g, 96%). M.p. 112-114 C. 1 H NMR
(300 MHz, DMSO d6) S 3.31 (m, 3H), 4.10 (m, 3H), 7.52-7.65 (m, 3H), 7.75 (m, 1
H),
7.90 (m, 2H), 8.07 (m, 2H), 8.21 (s, 1 H). MS (DCI-NH3) m/z 391 (M+H)+, 408
(M+NH4)+. Anal. caic. for: C18H15CIN204S=0.25 H20: C, 54.68; H, 3.95; N, 7.08.
Found: C, 54.59; H, 3.65; N, 6.98.
Example 332
2-(3-Chlorophenyl)-4-hydroxy-5-[4-(methylsulfonyl) henyl]-3(2H)-Ryridazinone
A suspension of 2-(3-chlorophenyl)-4-(methoxy)-5-[4-(methylsulfonyl)-
phenyl]-3(2H)-pyridazinone (6.26 g, 16 mmol) in 5% NaOH (54 mL) dioxane (39.4
mL) was heated at reflux and stirred for 1.5 hours. As the reaction proceeds,
the
solution becomes orange and homogeneous. The mixture was cooled and poured
into 1 N HCI, with constant stirring. The resulting white solid was filtered
and rinsed
with H20 and left to dry overnight. The mostly dry product was taken up in
CH2CI2
and azeotroped with toluene to remove any remaining H20, to provide the
desired
product as a white solid (yield: 6.79 g, >100%). 1 H NMR (300 MHz, DMSO d6) S
2.27 (s, 3H), 7.51-7.62 (m, 2H), 7.68 (m, 1 H), 7.79 (m, 1 H), 8.03 (m, 4H),
8.24 (s,
1 H). MS (DCI-NH3) m/z 377 (M+H)+, 396 (M+NH4)+.
Example 333
2-13-Chloronhenvl -4-tosvloxy-5-[4-(methy ulfonyj)ohenyjj-3(2H)-pyridazinone
To a 0 C solution of 2-(3-chlorophenyl)-4-hydroxy-5-[4-(methylsulfonyl)-
phenyl]-3(2H)-pyridazinone, prepared in Example 332, (6.79 g, 16 mmol) in
pyridine (160 mL) was added p-toluenesulfonyl chloride (3.06 g, 16 mmol). The
solution was left to warm slowly to room temperature with stirring under
nitrogen.
After 2.5 hours, the mixture was poured into H20 with constant stirring. The
resulting off-white solid was filtered, rinsed with H20 and dried to provide
the
desired product (yield: 6.26 g, 79%). M.p. 198-200 C. 1 H NMR (300 MHz, DMSO
d6) S 2.35 (s, 3H), 3.28 (s, 3H), 7.20 (m, 2H), 7.52-7.64 (M, 5H), 7.70 (m,
3H), 7.89
(m, 2H), 8.32 (s, 1 H). MS APCI+ 531 (M+H)+, 548 (M+H2O)+, APCI-493 (M+35)-.
-192-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Anal. calc. for C24H1 gCIN206S2: C, 54.29; H, 3.61; N, 5.28. Found: C, 54.55;
H,
3.46; N, 5.57.
Example 334
2-(~-~ Chloro h nyl)-4-chloro-5-[4-(methylsuifonyI)ohenyll-3(2H)-pvridazinone
A solution of 2-(3-chlorophenyl)-4-hydroxy-5-[4-(methylsulfonyl)phenyl]-
3(2H)-pyridazinone, prepared in Exampie 332, in POC13 was heated to reflux for
3
hours while stirring under nitrogen. The mixture was cooled to room
temperature
and poured into ice with constant swirling. The resulting white solid was
extracted
with ethyl acetate. The combined organics were washed with H20, dried over
MgS04, and concentrated to a solid. The crude product was purified using flash
chromatography (Si02, eluting with 1:1 ethyl acetate/hexanes) to provide the
desired product (yield: 0.151 g, 29%). M.p. 203-204 C. 1 H NMR (300 MHz,
DMSO d6) S 3.29-3.36 (3H, obstructed by H20), 7.60 (m, 3H), 7.76 (m, 1 H),
7.92
(m, 2H), 8.14 (m, 2H), 8.25 (s, 1 H). MS (DCI-NH3) m/z 395 (M+H)+, 412
(M+NH4)+.
Anal. caic. for C17H12C12N203S: C, 51.66; H, 3.06; N, 7.09. Found: C, 51.67;
H,
3.03; N, 6.93.
Example 335
2-(3-Chloro henvl)-4-(2-methylpropoxy)-5-[4-(methylsulfonyI)phenyll-3(2H)-
pyridazinone
To a stirred suspension of 2-(3-chlorophenyl)-4-tosyloxy-5-[4-(methyl-
sulfonyl)phenyl]-3(2H)-pyridazinone, prepared in Example 333, (0.175 g, 0.33
mmol) in THF (3.3 mL) was added isobutanol (0.03 mL, 0.33 mmol), and NaH
(0.0132 g, 0.33 mmol). The resulting solution was stirred under nitrogen for 1
hour.
The reaction was poured into H20 and extracted with ethyl acetate. The
combined
organics were dried over MgSO4 and concentrated in vacuo. The crude solid was
purified using flash chromatography (Si02, 2:1 hexanes:ethyl acetate) to
provide
the desired product (yield: 0.1088 g 76%). M.p. 166-169 C. 1 H NMR (300 MHz,
DMSO d6) 8 0.78 (d, J = 6 Hz, 6H), 1.84 (m, 1 H), 3.29 (s, 3H), 4.20 (d, J- 6
Hz, 2H),
7.51-7.63 (m, 3H), 7.76 (m, 1 H), 7.92 (m, 2H), 8.07 (m, 2H), 8.21 (s, 1 H).
MS (DCI-
NH3) m/z 433 (M+H)+, 450 (M+NH4)+. Anal. caic. for C21 H21 CIN204S: C, 57.07;
H, 5.01; N, 6.33. Found: C, 57.06; H, 4.78; N, 6.13.
-193-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 336
2-( - .hlorooheny()-4-(t-butoxy,)-5-j4-(methylsulfonyl)Qhenyl]-
3(2H)_oyridazinone
The title compound was prepared according to the method of Example 335,
substituting t-butanol in place of isobutanol (yield: 0.093 g, 66%). M.p. 232-
235 C.
1 H NMR (300 MHz, DMSO d6) 81.18 (s, 9H), 3.30 (s, 3H), 7.52-7.64 (m, 3H),
7.74
(m, 1 H), 7.92 (m, 2H), 8.08 (m, 2H), 8.20 (s, 1 H). MS (DCI-NH3) m/z 433
(M+H)+,
450 (M+NH4)+. Anal. calc. for C21 H21 CIN2O4S: C, 58.26; H, 4.89; N, 6.47.
Found: C, 58.21; H, 4.88; N, 6.28.
Example 337
2:(3- orophenXl)-4-(cyclohexyloxy)-5-[4-(methylsulfonyl)ohenyl]-3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 335,
substituting cyclohexanol in place of isobutanol (yield: 0.139 g, 92%). semi-
solid;
1 H NMR (300 MHz, CDCI3) S 1.09-1.50 (m, 6H), 1.57 (m, 2H), 1.88 (m, 2H), 3.13
(s,
3H), 5.19 (m, 1 H), 7.38-7.48 (m, 2H), 7.59 (m, 1 H), 7.70 (m, 1 H), 7.83 (m,
2H), 7.92
(s, 1 H), 8.07 (m, 2H). MS APCI+ 459 (M+H)+, 476 (M+H20)+, APCI-458 (M)-, 493
(M+35)-. Anal. calc. for C23H23CIN2O4S-0.25 H20: C, 59.60; H, 5.11; N, 6.04.
Found: C, 59.48; H, 4.86; N, 5.88.
Example 338
2-(3-Chlorophepyl)-4-(2.2-dimethylr~o oxy)-5-j4-(me ylsulfony )ohe ]-3(2H1-
Ryridazinone
The title compound was prepared according to the method of Example 335,
substituting neopentyl alcohol in place of isobutanol (yield: 0.109 g, 74%).
M.p.
151-153 C. 1 H NMR (300 MHz, DMSO d6) b 0.78 (s, 9H), 3.29 (s, 3H), 4.10 (s,
2H), 7.52-7.64 (m, 3H), 7.76 (m, 1 H), 7.92 (m, 2H), 8.07 (m, 2H), 8.20 (s, 1
H). MS
(DCI-NH3) m/z 447 (M+H)+, 464 (M+NH4)+. Anal. caic. for C22H23CIN2O4S: C,
59.12; H, 5.19; N, 6.27. Found C, 59.40; H, 5.31; N, 5.99.
Example 339
2-( -Chlorophenyl)-4-(3-methxlbutoxy)-5-[4-(methylsulfonyl)nheny!]-3 Hl-
Ryridazinone
The title compound was prepared according to the method of Example 335,
substituting 3-methyl-l-butanol was substituted in place of isobutanol (yield:
0.229
g, 80.5%). M.p. 134-135 C. 1 H NMR (300 MHz, DMSO d6) S 0.79 (d, J = 6 Hz,
6H), 1.42-1.64 (m, 3H), 3.30 (s, 3H), 4.43 (t, J = 6 Hz, 2H), 7.52-7.65 (m,
3H), 7.76
-194-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
(m, 1 H), 7.90 (m, 2H), 8.07 (m, 2H), 8.21 (s, 1 H). MS (DCI-NH3) m/z 447
(M+H)+,
464 (M+NH4)+. Anal. calc. for C22H23CIN2O4S: c, 59.12; H, 5.19; N, 6.27.
Found:
C, 58.91; H, 5.12; N, 6.01.
Example 340
2-(3-ChloroghenyI)-4-(3-octvn-1-yloxy)-5-[4-(methyisulfonyl)xLenyll-3(2H)-
~yridazinone
The title compound was prepared according to the method of Example 335,
substituting 3-octyn-l-ol in place of isobutanol (yield: 0.128 g, 77%). Oil. I
H NMR
(300 MHz, CDCI3) S 0.88 (m, 3H), 1.25-1.44 (m, 4H), 2.05 (m, 2H), 2.52 (m,
2H),
4.68 (t, J= 6 Hz, 2H), 7.43 (m, 2H), 7.59 (m, 1 H), 7.70 (m, 1 H), 7.86 (m,
2H), 7.92 (s,
1 H). MS (DCI-NH3) m/z 485 (M+H)+. Anal. ca1c. for C25H25CIN2O4S: C, 61.94;
H, 5.20; N, -5.78. Found: C, 61.82; H, 4.99; N, 5.57.
Example 341
2-(3-Chlorol2henyl)-4-[2-(dimethyiamino)ethoxy)-5-[4-(methvls ulfonyJ)phenvll-
3(2H)-Rytidazinone
The title compound was prepared according to the method of Example 335,
substituting N,N-(dimethyl)ethanolamine in place of isobutanol (yield: 0.111
g,
75%). M.p. 110-113 C. 1 H NMR (300 MHz, DMSO d6) S 2.29 (bs, 6H), 2.68 (bs,
2H), 4.68 (t, J = 5 Hz, 2H), 7.38-7.48 (m, 2H), 7.57 (m, 1 H), 7.68 (m, 1 H),
7.89 (m,
2H), 8.07 (m, 2H). MS (DCI-NH3) m/z 448 (M+H)+. Anal. caic. for
C21 H22CIN3O4S-0.50 H20: C, 55.19; H, 5.07; N, 9.19. Found: C, 55.24; H, 4.97;
N, 9.07.
Example 342
2-( -Chloroghenyl)-4-[2-methyl-1-(1-methylethyJ) RropoxyJ-5-
[4_(methylsulfonvl)-
henyll-3(2H)-pyridazinone
The title compound was prepared according to the method of Example 335,
substituting 2,4-dimethyl-3-pentanol in place of isobutanol (yield: 0.075 g,
48%).
Semi-solid; 1 H NMR (300 MHz, DMSO d6) S 0.79 (m, 12H), 1.78-1.92 (m, J = 6
Hz,
2H), 3.29 (s, 3H), 5.40 (t, J = 6 Hz, 1 H), 7.57 (m, 3H), 7.72 (m, 1 H), 7.91
(m, 2H),
8.07 (m, 2H), 8.17 (m, 1 H). MS (DCI-NH3) m/z 475 (M+H)+, 492 (M+NH4)+. Anal.
calc. for C24H27CIN2O4S (0.75 H20): C, 59.00; H, 5.88; N, 5.78. Found: C,
58.83;
H, 5.74; N, 5.52.
-195-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 343
? (3- hlorophgpyJ -L4-(nhenoxy)-5-f4-(methylsulfony1)o,, henyll-
3(2H)nyridazinone
The title compound was prepared according to the method of Example 335,
substituting phenol in place of isobutanol (yield: 0.053 g, 35%). M.p. 205-207
C.
1 H NMR (300 MHz, DMSO d6) S 3.28 (s, 3H), 7.08 (m, 3H), 7.31 (m 2H), 7.50-
7.64
(m, 3H), 7.73 (m, 1 H), 7.90 (m, 2H), 8.05 (m, 2H), 8.40 (s, 1 H). MS (DCI-
NH3) m/z
453 (M+H)+, 470 (M+NH4)+. Anal. calc. for C23H17CIN204S: C, 60.99; H, 3.78; N,
6.19. Found: C, 60.79; H, 3.65; N, 5.87.
Example 344
2-(3-Chlorophenyl)-4-j3-(dimethvlamino)}heny11-5-[}-(methylsulfonyj)ohenyll-
3J2H),-pyridazinone
The title compound was prepared according to the method of Example 335,
substituting 3-(dimethylamino)phenol in place of isobutanol (yield: 0.057 g,
60%).
M.p. 191-193; 1 H NMR (300 MHz, DMSO d6) S 2.85 (s, 6H), 3.27 (s, 3H), 6.36
(m,
3H), 7.05 (m, 1 H), 7.51-7.63 (m, 3H), 7.72 (m, 1 H), 7.90 (m, 2H), 8.05 (m,
2H), 8.39
(s, 1 H). MS APCI+ 495 (M+H)+, APCI-, 495 (M)-, 590 (M+35)-. Anal. calc. for
C25H22CIN304S: C, 60.54; H, 4.47; N, 8.47. Found: C, 60.04; H, 4.49; N, 8.26.
Example 345
2-(3-Chlorophglyl)-4-(4-methoxy h~ enoxy)-5-(4-(methylsulfonyl)ohenyll 3(2H)--
Ryridazinone
The title compound was prepared according to the method of Example 335,
substituting 4-methoxyphenol in place of isobutanol (yield: 0.080 g, 69%).
M.p.
182-184 C. 1 H NMR (300 MHz, DMSO d6) 5 3.27 (s, 3H), 3.70 (s, 3H), 6.84 (m,
2H), 7.00 (m, 2H), 7.56 (m, 3H), 7.72 (m, 1 H), 7.90 (m, 2H), 8.04 (m, 2H),
8.38 (s,
1 H). MS (DCI-NH3) m/z 483 (M+H)+, 500 (M+NH4)+. Anal. calc. for
C24H1 9CIN2O5S: C, 59.64; H, 3.97; N, 5.80. Found: C, 59.86; H, 3.94; N, 5.62.
Example 346
2-(3.4-Difluoroohenyl)-4-(2-methyloropoxy)-5-l4-(methylsulfonyi)nhenyjj-3(2HL-
Rvridazinone
The title compound was prepared according to the method of Example 335,
substituting 2-(3,4-difluorophenyl)-4-tosyloxy-5-(4-(methylsulfonyl)phenyl]-
3(2H)-
pyridazinone in place of 2-(3-chlorophenyl)-4-tosyloxy-5-[4-(methylsulfonyl)-
phenyl]-3(2H)-pyridazinone (yield: 150 mg, 61 %) . M.p. 116-117 C. 1 H NMR
(300
-196-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
MHz, DMSO-d6) S 0.78 (d, 6H), 1.84, (m, 1 H), 3.3 (s, 3H), 4.2 (d, 2H), 7.54
(m, 1 H),
7.6 (m, 1 H), 7.82 (m, 1 H), 7.91 (d, 2H), 8.07 (d, 2H), 8.21 (s, 1 H). MS
(DCI-NH3)
m/z 435 (M+H)+, 452 (M+NH4)+. Anal. calc. for C21 F2H2ON204S: C, 58.06; H,
4.64; N, 6.45.
Example 347
2-(3.4-Difluoroohen}l -4-(3-methyl-1-butoxy)-5-j4-(methvlsulfonyI)nhenyll-
3(2Hl--
Qvridazinone
The title compound was prepared according to the method of Example 346
substituting 3-methyl-1 -butanol in place of isobutanol (yield: 63 mg, 23%).
M.p.
121-123 C. 1 H NMR (300 MHz, DMSO-d6) 8 0.78 (d, 6H), 1.48, (m, 3H), 3.3 (s,
3H), 4.43 (t, 2H), 7.54 (m, 1 H), 7.6 (m, 1 H), 7.82 (m, 1 H), 7.91 (d, J = 9
Hz, 2H), 8.07
(d, J = 9 Hz, 2H), 8.2 (s, 1 H). MS (DCI-NH3) m/z 449 (M+H)+, 466 (M+NH4)+.
Anal. calc. for C22H22F2N204S: C, 58.92; H, 4.94; N, 6.25. Found, C, 59.22; H,
4.97;N,6.07.
Example 348
2-(3 4-DifluorouhenyI)-4-(4-fluoro henoxY)-5-[3-fluoro-4-(methylsulfonyl)
henY11
3 (2H)-Ryridazinone
The title compound was prepared according to the method of Example 346,
starting with 2-(3,4-difluorophenyl)-4-tosyloxy-5-[3-fluoro-4-
(methylsulfonyl)phenyl]-
3(2H)-pyridazinone in place of 2-(3-difluorophenyl)-4-tosyloxy-5-[4-(methyl-
sulfonyl)phenyl]-3(2H)-pyridazinone and substituting 4-fluorophenol in place
of
isobutanol M.p. 168-170 C. 1 H NMR (300 MHz, DMSO-d6) 8 3.39 (s, 3H), 7.15
(d,
4H), 7.51 (m, 1 H), 7.6 (m, 1 H) 7.75 (m, 3H), 7.97 (t, 1 H); 8.4 (s, 1 H). MS
(DCI-NH3)
m/z 491 (M+H)+, 508 (M+NH4)+. Anal. caic. for C23H14F4N2O4S: C, 56.33; H,
2.88; N, 5.71. Found, C, 56.07; H, 2.94; N, 5.33.
Example 349
2-(3.4-Difluoronhenvl)-4-(2.2-dimethyl~ro~oxy)-5-[4-(methylsulfonvl)ohenyll-
3(2H)-
Rvridazinone
The title compound was prepared according to the method of Example 346
substituting neopentyl alcohol in place of isobutanol (yield: 1.18 g, 94%).
M.p. 126-
128 C. 1 H NMR (300 MHz, DMSO-d6) 8 0.78 (s, 9H), 3.3 (s, 3H), 4.1 (s, 2H),
7.51
(m, 1 H), 7.6 (m, 1 H), 7.82 (m, 1 H), 7.91 (d, J = 9 Hz, 2H), 8.07 (d, J = 9
Hz, 2H), 8.21
-197-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
(s, 1 H). MS (DCI-NH3) m/z 449 (M+H)+, 466 (M+NH4)+. Anal. caic. for
C22H22F2N204S: C, 58.92; H, 4.94; N, 6.25. Found: C, 59.03; H, 5.03; N, 6.18.
Example 350
2-(3.4-Difluoroohenvl)-4-[2-(isoRroooxy)ethoxyl-5-[4-(methylsulfonY1)ohenylJ-
3(2H)-
~vridazinone
The title compound was prepared according to the method of Example 346
substituting 2-(isopropoxy)ethanol in place of isobutanol (yield: 432 mg,
72%). M.p.
105-107 C. 1 H NMR (300 MHz, DMSO-d6) S 0.95 (d, 6H), 3.3 (s, 3H), 3.43 (m,
1 H), 3.54 (m, 2H), 4.63 (m, 2H), 7.54 (m, 1 H), 7.6 (m, 1 H), 7.8 (m, 1 H),
8.01 (m, 4H),
8.2 (s, 1 H). MS (DCI-NH3) m/z 465 (M+H)+, 482 (M+NH4)+. Anal. caic. for
C22H22F2N205S: C, 56.89; H, 4.77; N, 6.03. Found, C, 57.03; H, 4.65; N, 5.83.
Example 351
2-(3.4-Difluoroohenyl)-4-(3-methYjPentyjQxy)-5-[4-(methyl us IfonX()nhe ly1J-
3(2w_-
pyridazinone
The title compound was prepared according to the method of Example 346
substituting 3-methylpentyl-l-ol in place of isobutanol (yield: 400 mg, 80%).
M.p.
100-102 C. 1H NMR (300 MHz, DMSO-d6) S 0.75 (m, 6H), 1.05 (m, 1H), 1.28 (m,
3H) 1.6 (m, 1 H), 3.3 (s, 3H), 4.45 (m, 2H), 7.5 (m, 1 H), 7.6 (m, 1 H), 7.8
(m, 1 H), 7.9
(d, J = 9 Hz, 2H) 8.05 (d, J = 9 Hz, 2H), 8.2 (s, 1 H). MS (DCI-NH3) mlz 463
(M+H)+,
480 (M+NH4)+. Anal. calc. for C23H24F2N204S: C, 59.73; H, 5.23; N, 6.06.
Found, C, 59.78; H, 5.31; N, 6.00.
Example 352
2-(3.4-Difluorophenyl)-4-(4-methyl-3-oenten-1-yloZc, )- -j4-(meti3, su yJ) nvn-
5-3(2H)-Ryridazinone
The title compound was prepared according to the method of Example 346
substituting 4-methyl-3-pentene-l-ol in place of isobutanol (yield: 405 mg,
67.8%).
M.p. 88-90 C. 1 H NMR (300 MHz, DMSO-d6) 81.5 (d, 6H), 2.27 (m, 2H) 3.3 (s,
3H), 4.43 (t, 2H), 4.95 (m, 1 H), 7.5 (m, 1 H), 7.6 (m, 1 H), 7.8 (m, 1 H),
7.9 (d, 2H), 8.06
(d, 2H), 8.2 (s, 1 H). MS (DCI-NH3) m/z 461 (M+H)+, 478 (M+NH4)+. Anal. caic.
for
C23H22F2N204S: C, 59.99; H, 4.82; N, 6.08. Found, C, 59.88; H, 4.76; N, 5.84.
-198-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 353
2-(3 a-Difluoroobgnyl)-4-r3-(methoxy)butoxy]-5-[4-(methylsulfonyl)phenvl]-
3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 346
substituting 3-methoxybutyl-l-ol in place of isobutanol (yield: 350 mg, 68%) .
M.p.
99-101 C. 1 H NMR (300 MHz, DMSO-d6) 8 0.97 (d, 3H), 1.7 (m, 2H), 3.05 (s,
3H),
3.2 (m, 1 H) 3.3 (s, 3H), 4.45 (m, 2H), 7.54 (m, 1 H), 7.6 (m, 1 H), 7.8 (m, 1
H), 7.9 (d, J
= 9 Hz, 2H) 8.01 (d, J = 9 Hz, 2H), 8.2 (s, 1 H). MS (DCI-NH3) m/z 465 (M+H)+,
482
(M+NH4)+. Anal. calc. for C22H22F2N205S: C, 56.89; H, 4.77; N, 6.03. Found, C,
56.60; H, 4.83; N, 5.96.
Example 354
2-(3-Chloroohenyl)-4-(N-methvlbenzylamino)-5-j4-(methylsul,~I)Qhenyl]-3(2H)-
pyridazinone:
To a rapidly stirred 0 C mixture of N-methylbenzylamine (67.5 mg, 0.56
mmol) and tetrahydrofuran (3.7 mL) was slowly added dropwise an n-BuLi
solution
(0.235 mL, 0.59 mmol, 2.5 M in hexanes). The reaction mixture was stirred for
10
minutes at 0 C and 1 hour at 23 C. The solution was cooled to -78 C, and a
tetrahydrofuran (10-15 mL) solution of the 2-(3-chlorophenyl)-4-methoxy-5-[4-
(methylthio)phenyl]-3(2H)-pyridazinone (200 mg, 0.56 mmol) slowly added along
the interior wall of the reaction vessel. This reaction mixture was stirred
overnight,
slowly warming to 23 C as the cooling bath evaporated. The reaction was
quenched with water and diluted with a large excess of ethyl acetate. The
layers
were separated, and the ethyl acetate layer washed with additional water and
brine
and dried over MgSO4, filtered, and concentrated in vacuo. The residue was
chromatographed (flash silica gel, ethyl acetate/hexanes 1:9) to provide 2-(3-
chlorophenyl)-4-(N-methyl benzylamino)-5-[4-(methylthio)phenyl]-3(2H)-
pyridazinone (yield: 145 mg, 58%).
The title compound was prepared according to the method of Example 10,
substituting 2-(3-chiorophenyl)-4-(N-methylbenzyiamino)-5-[4-
(methylthio)phenyl]-
3(2H)-pyridazinone in place of 2-benzyl-4-(4-fluorophenyl)-5-[4-(methylthio)-
phenyl]-3(2H)-pyridazinone (yield: 143 mg, 95%). M.p. 60-85 C. 1 H NMR (300
MHz, CDCI3) S 2.46 (s, 3H), 3.09 (s, 3H), 4.63 (s, 2H), 7.19 (d, J = 8.7 Hz,
2H), 7.24-
7.29 (m, 2H), 7.32-7.48 (m, 5H), 7.60 (ddd, J = 7.2, 1.8, 1.8 Hz, 1 H), 7.67
(s, 1 H),
7.70 (dd, J = 1.8, 1.8 Hz, 1 H), 7.91 (d, J = 8.7 Hz, 2H). MS (APCI+) m/z 480
(M+H)+.
-199-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 355
2-(4-Fluoronhenyl)-4-(1-ni inyl)-5-[4-(methvlsulfonyl) I yll;3(2H)-
pyridazinone
To a slightly heterogeneous solution of piperidine (99.7 mg, 1.17 mmoi) and
toluene (8 mL) cooled to -78 C was slowly added dropwise an n-BuLi solution
(0.235 mL, 0.59 mmol, 2.5 M in hexanes). After stirring at -78 C for 10
minutes, the
cooling bath was removed and the mixture stirred an additional 1 hour at 23
C.
The 2-(4-fluorophenyl)-4-methoxy-5-[4-(methylthio)phenyl]-3(2H)-pyridazinone
(400 mg, 1.17 mmol) was dissolved in portions in toluene (3 x 6-7 mL aliquots)
with
a heat gun and cooled to 0 C prior to transfer via syringe to the lithium
amide
solution (cooled to -78 C). The addition was made slowly along the interior
wall of
the reaction vessel. This reaction mixture was stirred overnight, slowly
warming to
23 C as the cooling bath evaporated. The reaction was quenched with water and
diluted with a large excess of ethyl acetate. The layers were separated, and
the
ethyl acetate layer washed with additional water and brine and dried over
MgSO4,
filtered, and concentrated in vacuo. The residue was chromatographed (flash
silica
gel, ethyl acetate/hexanes 1:2) to provide 440 mg (95%) of 2-(4-fluorophenyl)-
5-[4-
(methyithio)phenyl]-4-piperidino-3(2H)-pyridazinone.
The title compound was prepared according to the method of Example 10,
substituting 2-(4-fluorophenyl)-5-[4-(methylthio)phenyl]-4-piperidino-3(2H)-
pyridazinone in place of 2-benzyl-4-(4-fluorophenyl)-5-[4-(methylthio)phenyl]-
3(2H)-pyridazinone (yield: 165 mg, 98%). M.p. 80-100 C. 1 H NMR (300 MHz,
CDC13) S 1.59 (br s, 6H), 2.59 (br s, 4H), 3.14 (s, 3H), 7.17 (dd, J = 8.7,
8.7 Hz, 2H),
7.51 (d, J = 8.7 Hz, 2H), 7.55-7.62 (m, 2H), 7.68 (s, 1 H), 8.06 (d, J = 8.7
Hz, 2H).
MS (APCI+) m/z 428 (M+H)+. Powdered out in CH2CI2/C6H14. Anal. caic. for
C22H22FN303S-0.25C6H14: C, 62.85; H, 5.72; N, 9.35. Found: C, 62.46; H, 5.77;
N, 9.13.
Example 356
2-14-FluoroohenylL4-(1-Ryr iny1)-5-[4-(methylsulfonyl henyjlõ3(H)-
pyridazinone
The title compound was prepared according to the method of Example 355,
substituting pyrrolidine for piperidine (yield: 107 mg, 82%). M.p. 192-195 C.
1 H
NMR (300 MHz, CDC13) S 1.71-1.80 (m, 4H), 3.13 (s, 3H), 3.40-3.49 (m, 4H),
7.16
(dd, J = 8.7, 8.7 Hz, 2H), 7.47-7.60 (m, 5H), 7.99 (d, J = 8.7 Hz, 2H). MS
(APCI+)
-200-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
m/z 414 (M+H)+. Anal. calc. for C21 H2OFN3O3S: C, 61.00; H, 4.87; N, 10.16.
Found: C, 60.95; H, 4.94; N, 10.07.
Example 357
2-(3-ChioroR1lgnyl)-4-(4-methyl henylthio)-5-j4-(metFty su f p,y1) nvn-. ( H)-
Qvridazinone
To a stirred suspension of 2-(3-chlorophenyl)-4-tosyloxy-5-[4-(methyl-
sulfonyl)phenylj-3(2H)-pyridazinone, prepared in Exampie 333, (0.0802 g, 0.15
mmol) in EtOH (1.5 mL) was added thiocresol (0.019 g, 0.15 mmol) and K2C03
(0.0203 g, 0.15 mmol). The suspension was heated to 50 C with stirring for
2.5
hours. The mixture was poured into H20 with constant stirring. The resulting
precipitate was filtered, rinsed with H20 and dried to provide the desired
product
(yield: 0.060 g, 83%). M.p. 178-178 C. 1 H NMR (300 MHz, DMSO d6) S 2.19 (s,
3H), 3.23 (s, 3H), 6.95 (m, 2H), 7.08 (m, 2H), 7.52-7.66 (m, 3H), 7.72 (m, 1
H), 7.88
(m, 2H), 8.08 (s, 1 H). MS (DCI-NH3) m/z 483 (M+H)+, 500 (M+NH4)+. Anal. calc.
for: C24H19CIN203S2=0.75 H20: C, 58.05; H, 4.16; N, 5.64. Found: C, 57.99; H,
3.69; N, 5.76.
Example 358
2-(3-Chloroohenyf)-4-(2-R, ri y hio)-5-j4-(meth Isulfortyl) henyl]-3(,
Ryridazinone
The title compound was prepared according to the method of Example 357,
substituting 2-mercaptopyridine in place of thiocresol (yield: 0.061 g, 39%).
M.p.
110-114 C. 1H NMR (300 MHz, DMSO d6) 8 3.28 (s, 3H), 7.16 (m, 1H), 7.37 (m,
1 H), 7.51-7.71 (m, 5H), 7.81 (m, 2H), 8.03 (m, 2H), 8.27 (s, 1 H), 8.34 (m, 1
H). MS
(DCI-NH3) m/z 470 (M+H)+. Anal. calc. for C22H16CIN303S2-0.50 H20: C, 55.16;
H, 3.57; N, 8.77. Found: C, 54.88; H, 3.19; N, 8.59.
Example 359
2-(3-Chloronhenvl)-4-( t PnylmethylthiQ)-5-[4-(methy S, Ito yJ)RheIlY1]-3(2H)-
Rvridazinone
To a stirred suspension of 2-(3-chlorophenyl)-4-tosyloxy-5-[4-(methyl-
sulfonyl)phenyl]-3(2H)-pyridazinone, prepared in Example 333, (0.175 g, 0.33
mmol) in THF (3.3 mL) was added benzyl mercaptan (0.04 mL, 0.33 mmol) and
TEA (0.046 mL, 0.33 mmol). The resulting solution was stirred at room
temperature
under nitrogen for 1 hour. The mixture was poured into H20 and extracted with
-201-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
ethyl acetate. The combined organics were dried over MgSO4 and concentrated in
vacuo. The resulting crude product was purified using flash chromatography
(Si02, 2:1 hexanes:ethyl acetate) to provide the desired product (yield: 0.136
g
85%). M.p. 142-145 C. 1 H NMR (300 MHz, DMSO d6) S 3.31 (s, 3H), 4.36 (s,
2H),
7.17 (m, 2H), 7.21-7.33 (m, 3H), 7.51 (m, 2H), 7.57-7.64 (m, 3H), 7.74 (m, 1
H), 8.01
(m, 2H). MS (DCI-NH3) m/z 483 (M+H)+, 500 (M+NH4)+. Anal. calc. for
C24H1 9CIN2O3S2: C, 59.68; H, 3.96; N, 5.80. Found: C, 59.40; H, 4.11; N,
5.71.
Example 360
2-( -Chioro henyl)-4-(2-furvlmethyfthio)-5-[4-(methylsulfonyl)phenyl?.-3(2H)-
pyridazinone
The titie compound was prepared according to the method of Example 359,
substituting furfuryl mercaptan in place of benzyl mercaptan (yield: 0.162 g,
100%).
M.p. 140-149 C. 1 H NMR (300 MHz, DMSO d6) 8 3.31 (s, 3H), 4.46 (s, 2H), 6.20
(m, 1 H), 6.37 (m, 1 H), 7.50-7.67 (m, 6H), 7.77 (m, 1 H), 8.03 (m, 2H), 8.08
(s, 1 H).
MS (DCI-NH3) m/z 473 (M+H)+, 490 (M+NH4)+. Anal. calc. for C22H17CIN2O4S2:
C, 55.87; H, 3.62; N, 5.92. Found: C, 55.84; H, 3.61; N, 5.82.
Example 361
2-(3-Chlorophenyl)-4-j2-(methylpropyl)thiol-5-[4-(methylsulfony hpr1Yll3(2iH)
pyridazinone
The title compound was prepared according to the method of Example 359,
substituting 2-methyl-l-propanethiol in place of benzyl mercaptan (yield:
0.134 g,
91 %). Oil. 1 H NMR (300 MHz, DMSO d6) S 0.61 (d, J = 6 Hz, 6H), 1.54-1.69 (m,
1 H), 2.91 (d, J = 6 Hz, 2H), 3.33 (s, 3H), 7.52-7.64 (m, 3H), 7.74 (m, 1 H),
7.79 (m,
2H), 8.04 (m, 3H). MS (DCI-NH3) m/z 449 (M+H)+, 466 (M+NH4)+. Anal. calc. for
C21 H21 CIN2O3S2 (0.50 H20): C, 55.07; H, 4.84; N, 6.11. Found: C, 54.70; H,
4.64; N, 5.85.
Example 362
2-(3-Chloroahenvl)-4-(cyclopgratyl)-5-[4-(methylsulfonyl) henyll-3(2H)-
pyridazinone
To a -78 C solution of 2-(3-chlorophenyl)-4-tosyloxy-5-[4-(methylsulfonyl)-
phenyl]-3(2H)-pyridazinone, prepared in Example 333, (0.175 g, 0.33 mmol) in
THF
(3.3 mL) was added cyclopentyl magnesium chloride (0.17 mL, 1.0 M in diethyl
ether). The resulting solution was stirred under nitrogen less than 1 hour
with
-202-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
warming to room temperature. The reaction was poured into water and extracted
with ethyl acetate. The combined organics were dried over MgSO4 and
concentrated in vacuo. The resulting crude product was purified using flash
chromatography (Si02, 2:1 ethyl acetate:hexanes) to provide the desired
product
(yield: 0.1328 g, 94%). M.p. 155-157 C. 1 H NMR (300 MHz, DMSO d6) S 1.50 (m,
2H), 1.66 (m, 2H), 1.79 (m, 2H), 2.09 (m, 2H), 2.90 (m, J = 8 Hz, 1 H), 3.26-
3.37 (3H,
obstructed by H20), 7.49-7.63 (m, 3H), 7.71 (m, 3H), 7.97 (s, 1 H), 8.10 (m,
2H). MS
(DCI-NH3) m/z 429 (M+H)+, 446 (M+NH4)+. Anal. caic. for C22H21 CIN203S: C,
61.60; H, 4.93; N, 6.53. Found: C, 61.48; H, 4.81; N, 6.22.
Example 363
2-(3-Chloronhenvl)-4-(3-methy1~r RyJ)-5-(4-(methylsulfonyl)phenyll-3(2H)-
Rvridazinone
The title compound, an oil, was prepared according to the method of
Example 362, substituting isobutyl magnesium chloride in place of
cyclohexylmagnesium chloride, (yield: 0.132 g, 96%). 1 H NMR (300 MHz, CDCI3)
S 0.77 (d, J; 6 Hz, 6H), 2.08 (m, 1 H), 2.54 (d, J = 7 Hz, 2H), 7.36-7.46 (m,
2H), 7.56
(m, 2H), 7.62 (m, 1 H), 7.73 (m, 2H), 8.11 (m, 2H). MS (DCI-NH3) m/z 417
(M+H)+ ,
434 (M+NH4)+. Anal. calc. for C21 H21 CIN203S-0.50 H20: C, 59.21; H, 5.20; N,
6.57. Found: C, 59.27; H, 5.40; N, 6.12.
Example 364
2-(3-Chloronhenvl)-4-(cy Iohexylmethyl)-5-j4-(methylsulfonyOphgny11-3(
pyridazinone
The title compound, an oil, was prepared according to the method of
Example 362, substituting cyclohexylmethyl magnesium bromide in place of
cyclopentyl magnesium chloride (yield: 0.0579 g, 38%). 1 H NMR (300 MHz, DMSO
d6) 5 0.66 (m, 2H), 1.03 (m, 3H), 1.50 (m, 6H), 1.61 (m, 1 H), 2.46 (m, 1 H),
3.27-3.42
(3H, obstructed by H20), 7.50-7.66 (m, _3H), 7.75 (m, 3H), 7.99 (s, 1 H), 8.10
(m, 2H).
MS (DCI-NH3) m/z 457 (M+H)+, 474 (M+NH4)+. Anal. caic. for C24H25CIN203S:
C, 63.08; H, 5.51; N, 6.13. Found: C, 63.08; H, 5.47; N, 6.04.
-203-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 365
2-(3-Chloro henyl)-4-(2-c cly ohex ly ethY1)-5-j4-(methyjsulfonvl)chenylj-
3(zH)_
Ryridazinone
The title compound was prepared according to the method of Example 362,
substituting cyclohexylethyl magnesium bromide in place of cyclopentyl
magnesium chloride (yield: 0.165 g, 94%). 1 H NMR (300 MHz, DMSO d6) S 0.76
(m, 3H), 0.99-1.21 (m, 5H), 1.31-1.62 (m, 8H), 2.42-2.56 (1 H, obstructed by
DMSO),
3.25-3.34 (2H, obstructed by H20), 7.48-7.65 (m, 3H), 7.48-7.65 (m, 3H), 7.76
(m,
3H), 8.01 (s, 1 H), 8.10 (m, 2H). MS (DCI-NH3) m/z 471 (M+H)+, 488 (M+NH4)+.
Anal. calc. for C25H27CIN203S: C, 63.75; H, 5.78; N, 5.95. Found: C, 63.48; H,
5.70; N, 5.67.
Example 366
2-(3-Chloroohenyl)-4-(3-methylbutyJ)-5-[4-(methy Su yj)pjgqyjl-3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 362,
substituting 3-methylbutyl magnesium bromide in place of cyclohexylmagnesium
chloride (yield: 0.0221 g, 16%). M.p. 60-65 C. 1 H NMR (300 MHz, DMSO d6) S
0.75 (d, J = 7 Hz, 6H), 1.32-1.52 (m, 3H), 3.31 (s, 3H), 7.50-7.65 (m, 3H),
7.77 (m,
3H), 8.03 (s, 1 H), 8.11 (m, 2H). MS (DCI-NH3) m/z 431 (M+H)+, 448 (M+NH4)+.
Anal. caic. for C22H23CIN203S=0.25 H20: C, 60.68; H, 5.43; H, 6.43. Found C,
60.29; H, 5.60; N, 6.17.
Example 367
2-(3-Chloroohenyl)-4-benzyl-5-[4-(methylsu Y1)phepyj)-3(2H)-RyridazinnnA
The title compound was prepared according to the method of Example 362,
substituting benzyl magnesium chloride in place of cyclohexylmagnesium
chloride.
M.p. 174-177 C (yield: 25.9 g, 57%). 1 H NMR (300 MHz, DMSO d6) S 3.30 (s,
3H),
3.91 (bs, 2H), 7.02 (m, 2H), 7.12-7.25 (m, 31-1), 7.51-7.64 (m, 3H), 7.72 (m,
3H), 8.07
(m, 2H), 8.12 (s, 1 H). MS (DCI-NH3) m/z 451 (M+H)+, 468 (M+NH4)+. Anal. caic.
for C24H1 gCIN203S: C, 63.92; H, 4.25; N, 6.21. Found: C, 63.69; H, 4.28; N,
6.02.
Example 368
2-(3-Chloroohenyll-4-cvclohexy.J-5z[4-(methvisulonylln, henyll-3(2 j)-
RyridazinnnA
The title compound was prepared according to the method of Example 362
substituting cyclohexylmagnesium chloride in place of cyclopentylmagnesium
-204-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
chloride (yield: 0.099 g, 68%). M.p. 85-90 C. 1 H NMR (300 MHz, CDCI3) S 1.01-
1.30 (m, 3H), 1.48-1.69 (m, 3H), 1.75 (m, 2H), 2.28 (m, 21-1), 2.57 (m, 1 H),
3.16 (s,
3H), 7.35-7.46 (m, 2H), 7.50-7.62 (m, 3H), 7.68 (m, 2H), 8.11 (m, 2H). MS (DCI-
NH3) m/z 443 (M+H)+, 460 (M+NH4)+. Anal. calc. for C23H23CIN203S (1.25
H20): C, 59.34; H, 5.52; N, 6.01. Found: C, 59.02; H, 5.24; N, 5.65.
Example 369
2-(3-Chloroohenyl)-4-(4-fluorobenzyl)-5-[4-(methvlsulfony!)Q hgnyll-3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 228,
substituting 4-fluorobenzyl magnesium chloride in place of cyclopentyl
magnesium
chloride (yield: 0.1895 g, 41%). M.p. 183-185 C. 1 H NMR (300 MHz, DMSO d6) 5
3.25-3.36 (3H, obstructed by H20), 3.89 (bs, 2H), 6.97-7.09 (m, 4H), 7.50-7.64
(m,
3H), 7.71 (m, 3H), 8.06 (m, 2H), 8.11 (s, 1 H). MS (DCI-NH3) m/z 469 (M+H)+,
486
(M+NH4)+. Anal. caic. for C24H18CIFN203S: C, 61.47; H, 3.87; N, 5.97. Found:
C, 61.23; H, 3.84; N, 5.77.
Example 370
2-(3-Chloronhen,yf)-4-(4-methY1Rhenyl)-5-[4-(met Is uI yI)uhenyjl-3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 362
substituting p-tofylmagnesium bromide in place of cyclopentylmagnesium
chloride
(yield: 65 mg, 40.9%). M.p. 222-224 C. 1 H NMR (300 MHz, DMSO-d6) 5 2.28 (s,
3H), 3.25 (s, 3H), 7.12 (t, 4H), 7.6 (m, 5H), 7.79 (t, 1 H) 7.9 (d, J = 9 Hz,
21-1), 8.22 (s,
1 H). MS (DCI-NH3) m/z 451 (M+H)+, 468 (M+NH4)+. Anal. calc. for
C24H1 9CIN2O3S-0.25 H20: C, 63.92; H, 4.25; N, 6.21. Found: C, 62.99; H, 4.28;
N, 5.85.
Example 371
2-(3.4-Difluoroohenvl)-4-(3-flunrc-4-m thyl henyl)-5-[4-(methylsulfonyl)
h~PnyIl-
3(2H)-Ryridazinone
2-(3,4-Dif(uorophenyl)-4-(3-fluoro-4-methylphenyl)-5-[4-(methylthio)phenyl]-
3(2H)-pyridazinone was prepared according to the method of Example 362,
starting with 2-(3,4-difluorophenyl)-4-tosyloxy-5-[4-(methylsulfonyl)phenyl]-
3(2H)-
pyridazinone and substituting 3-fluoro-4-methylphenylmagnesium bromide in
place
of cyclohexylmagnesium chloride to provide the methyl sulfide compound.
-205-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
The methyl sulfide was oxidized according to the method of Example 10 to
provide the title compound (yield: 265 mg, 85.4%). M.p. 204-206 C. 1 H NMR
(300
MHz, CDCI3) S 2.25 (br s, 3H), 3.08 (s, 3H), 6.83 (dd, J = 9 Hz, 1.5 Hz, 1 H),
6.96
(dd, J = 9 Hz, 1.5 Hz, 1 H), 7.08 (t, J = 9 Hz, 1 H), 7.23-7.33 (m, 1 H), 7.41
(d, J = 9 Hz,
2H), 7.49-7.56 (m, 1 H), 7.61-7.69 (m, 1 H), 7.93 (d, J = 9 Hz, 2H), 7.99 (s,
i H). MS
(DCI-NH3) m/z 471 (M+H)+, 488 (M+NH4)+. Anal. calc. for C24H17F3N203S: C,
61.28; H, 3.62; N, 5.96. Found: C, 61.07; H, 3.95; N, 5.56.
Example 372
2-(3-ChloropheoY1)-4- ( nethyl)-5-[4-(methy_Isulfonyj)phepyll-3 (2 H)-
ovridazinnne
The title compound was prepared according to the method of Example 228,
starting with 2-(3-chlorophenyl)-4-methoxy-5-[4-(methylthio)phenylj-3(2H)-
pyridazinone in place of 2-(4-fluorophenyl)-4-methoxy-5-[4-(methylthio)phenyl]-
3(2H)-pyridazinone and substituting phenethyl magnesium chloride in place of
cyclohexylmagnesium chloride then oxidizing by the method of Example 10
(yield:
0.100 g, 39%). M.p. 142-145 C. 1 H NMR (300 MHz, DMSO d6) S 2.80 (m, 4H),
3.30 (s, 3H), 7.01 (m, 2H), 7.21 (m, 3H), 7.51-7.60 (m, 4H), 7.63 (m, 1 H),
7.78 (m,
1 H), 8.03 (m, 3H). MS (DCI-NH3) m/z 465 (M+H)+, 482 (M+NH4)+. Anal. calc. for
C25H21 CIN2O3S: C, 64.58; H, 4.55; N, 6.02. Found: C, 64.24; H, 4.50; N, 5.90.
Example 373
2-(3-ChloroQhenyl)-4-(2-methyl~ropoxy)-5-[3-fluoro-4- Pthy suI nvll-
3(2H)-pyridazinone
373A. 2-(3-ChloroQhenvl)-4-( -methylpropoxy)-5-brmmo-3(2 ( j)-Ryridazi nnnp
The title compound is prepared according to the method of Example 194B,
starting with 2-(3-chlorophenyl)-4,5-dibromo-3(2H)-pyridazinone (Example 207A)
in place of 2-(4-fluorophenyl)-4,5-dibromo-3(2H)-pyridazinone ans substituting
2-
methyl-l-propanol in place of methanol.
373B. 2-(3-Chlorooheny I)-4-(2-methY1propoxy)-543-fhunrco-4-
(methyfthjQ)nhenvll-
3(2H)-Ryridazinone
The title compound is prepared according to the method of Example 6,
starting with 2-(3-chlorophenyl)-4-(2-methylpropoxy)-5-bromo-3(2H)-
pyridazinone
in place of 2-benzyl-4-bromo-5-methoxy-3(2H)-pyridazinone and substituting 3-
fluoro-4-(methylthio)benzeneboronic acid (Example 72C) in place of 4-
fluorobenzeneboronic acid.
-206-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
373C. 2-(3-Chloroohenvl)-4-(2-methyjgropQxy)-5-(3-fluoro-4-(methylsulfonvl)-
phenXl]-3(2H)-Ryridazinone
The methyl sulfide compound was oxidized according to the method of
Example 10 to provide the title compound (yield: 0.73 g, 100%). M.p. 180-183
C.
1 H NMR (300 MHz, DMSO d6) S 0.82 (d, J = 6 Hz, 2H), 3.30-3.39 (3H, obstructed
by H20) 4.25 (d, J = 6 Hz, 2H), 7.57 (m, 3H), 7.75 (m, 1 H), 7.85 (m, 1 H),
8.00 (m,
1 H), 8.23 (s, 1 H). MS (DCI-NH3) m/z 451 (M+H)+, 468 (M+NH4)+. Anal. calc.
for
C21 H2OCIFN2O4S: C, 55.94; H, 4.47; N, 6.21. Found: C, 55.73; H, 4.58; N,
6.01.
Example 374
2-(3-ChlorophenY1)-4-(benzyloxy)-5-[4-(me yJ uIfonyJ ohenyjl 3(2H)-
12yridazinone
To a stirred solution of 2-(3-chlorophenyl)-4-hydroxy-5-[4-(methylsulfonyl)-
phenyl]-3(2H)-pyridazinone (Example 332) (0.100 g, 0.28 mmol) in DMF (2.8 mL)
was added benzyl chloride (0.32 mL, 0.28 mmol). The resulting solution was
stirred with heating to 60 C overnight. The solvent was removed in vacuo and
the
resulting residue partitioned between ethyl acetate and 10% citric acid. After
extracting with ethyl acetate, the combined organics were dried over MgSO4 and
concentrated in vacuo. The crude product was purified using flash
chromatography
(Si02, 1:1 ethyl acetate:hexanes) to provide the desired product (yield: 0.096
g,
76%). M.p. 110-113 C. 1 H NMR (300 MHz, DMSO d6) S 3.39 (s, 3H), 5.48 (s,
2H),
7.29 (m, 4H), 7.59-7.71 (m, 3H), 7.76 (m, 3H), 8.00 (m, 2H), 8.21 (s, 1 H). MS
(DCI-
NH3) m/z 467 (M+H)+, 484 (M+NH4)+. Anal. caic. for C24H1 9CIN204S: C, 61.73;
H, 4.10; N, 6.00. Found: C, 62.00; H, 4.18; N, 5.93.
Example 375
2-(4-FluoroohenY1)-4-(3-meth ly butoxy)-5-[4-(methY GuI Y1)12henyIL-3(2H)-
Ryridazinone
2-(4-Fluorophenyl)-4-methoxy-5-bromo-3(2H)-pyridazinone (Example 194B)
is converted into 2-(4-fluorophenyl)-4-methoxy-5-[4-(methylsulfonyl)phenyl]-
3(2H)-
pyridazinone according to the method of Example 194C followed by the oxidation
method in Example 10. The methoxy compound is converted to the 2-(3-
chlorophenyl)-4-hydroxy-5-[4-(methy(sulfonyl)phenyl]-3(2H)-pyridazinone, by
treatment with NaOH according to the procedure of Example 332. The hydroxy
compound is treated with p-toluenesulfonyl chloride according to the procedure
of
Example 333, to furnish 2-(4-fluorophenyl)-5-[4-(methylsulfonyl)phenyl]-4-
tosyloxy-
3(2H)-pyridazinone.
-207-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
The title compound was prepared according to the method of Example 335,
starting with 2-(4-fluorophenyl)-5-[4-(methylsulfonyl)phenyl]-4-tosyloxy-3(2H)-
pyridazinone in place of 2-(3-chlorophenyl)-5-[4-(methylsulfonyl)phenyl]-4-
tosyloxy-3(2H)-pyridazinone substituting 3-methyl-l-butanol in place of
isobutanol
(yield: 0.3932 g, 94%). M.p. 117-120 C. 1 H NMR (300 MHz, DMSO d6) S 0.79 (d,
J = 6 Hz, 6H), 1.41-1.59 (m, 3H), 3.30 (s, 3H), 4.42 (d, J = 5 Hz, 2H), 7.36
(m, 2H),
7.65 (m, 2H), 7.90 (m, 2H), 8.06 (m, 2H), 8.18 (s, 1H). MS (DCI-NH3) m/z 431
(M+H)+, 448 (M+NH4)+. Anal. calc. for C22H23FN204S: C, 61.38; H, 5.39; N,
6.51. Found: C, 61.42; H, 5.30; N, 6.40.
Example 376
2-(4-Fluoroehenvl)-4-(2-meth,ylpropoxy)-5-[4-(methylsulfonyl)ohenyll-3(2H1-
pyridazinone
The title compound was prepared according to the method of Example 335,
substituting 2-(4-fluorophenyl)-5-[4-(methylsulfonyl)phenyl]-4-tosyloxy-3(2H)-
pyridazinone (prepared as an intermediate in Example 375) in place of 2-(3-
chlorophenyl)-5-[4-(methylsulfonyl)phenyl]-4-tosyloxy-3(2H)-pyridazinone
(yield:
0.486 g, 100%). M.p. 121-128 C. 1 H NMR (300 MHz, DMSO d6) 8 0.78 (d, J = 7
Hz, 6H), 1.84 (m, 1 H), 3.30 (s, 3H), 4.20 (d, J = 6 Hz, 2H), 7.37 (m, 2H),
7.66 (m,
2H), 7.92 (m, 2H), 8.07 (m, 2H), 8.19 (s, 1 H). MS (DCI-NH3) m/z 417 (M+H)+,
434
(M+NH4)+. Anal. calc. for C21 H21 FN2O4S-0.50 H20: C, 59.28; H, 5.21; N, 6.58.
Found: C, 59.49; H, 4.97; N, 6.34.
Example 377
2-(4-Fluorophenvl)-4-(4-fluorobenzyl)-5-L4-(Qeth Isulfo yl) henvll- (2H)-
Ryridazinone
The title compound was prepared according to the method of Example 62,
starting with 4-(4-fluorophenylmethyl)-5-[4-(methylsulfonyi)phenyl]-3(2H)-
pyridazinone and reacting with 1-iodo-4-fluorobenzene (yield: 0.0881 g, 78%).
M.p. 175-177 C. 1 H NMR (300 MHz, DMSO d6) S 3.27-3.36 (3H, obstructed by
H20), 3.88 (bs, 2H), 6.98-7.09 (m, 4H), 7.34 (m, 2H), 7.65 (m, 2H), 7.71 (m,
2H),
8.06 (m, 3H). MS (DCI-NH3) m/z 453 (M+H)+, 470 (M+NH4)+. Anal. calc. for
C24H1 gF2N203S: C, 63.71; H, 4.01; N, 6.19. Found: C, 63.61; H, 4.26; N, 6.03.
-208-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 378
2-(4-FluoroDh2nvj)-4-(3-met IbutyI)-5-[4-(methvis~ ulfonyl)nhenyl]-3(2H1
pyridazinone
The title compound was prepared according to the method of Example 228,
substituting 3-methylbutyl magnesium bromide in place of cyclohexylmagnesium
chloride (yield: 0.325 g, 69%). M.p. 151-154 C. 1 H NMR (300 MHz, DMSO d6) S
0.75 (d, J = 7 Hz, 6H), 1.32-1.51 (m, 3H), 3.31 (s, 3H), 7.37 (m, 2H), 7.66
(m, 2H),
7.77 (m, 2H), 8.00 (s, 1 H), 8.10 (m, 2H). MS (DCI-NH3) m/z 415 (M+H)+, 432
(M+NH4)+. Anal. calc. for C22H23FN2O3S-0.50 H20: C, 62.39; H, 5.71; N, 6.61.
Found: C, 62.04; H, 5.78; N, 6.46.
Example 379
2-(Tetrahvdro-2H-Ryr no-2-yl)-4-(4-fluoronhenyl)-5-[4-(methyisulfonyl)phenvll-
3(2H)-Ryridazinone
To the solution of 4-(4-fluorophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone prepared according to Example 11 (172 mg, 0.5 mmol) and p-
toiuenesuifonic acid hydrate (19 mg, 0.1 mmol) in dioxane (10 mL) was added
2,3-
dihydropyran (2 mL). The mixture was stirred at room temperature for 6. hours.
The
mixture was then poured into a solution of saturated NaHCO3 and extracted with
ethyl acetate. The ethyl acetate was concentrated in vacuo and the residue was
chromatographed (silica gel, 1:1 hexanes-ethyl acetate) to provide the title
compound (yield: 25 mg, 11 %). 1 H NMR (DMSO-d6, 300 MHz) S 1.54 (m, 2H), 1.74
(m, 2H), 2.00 (m, 1 H), 2.17 (m, 1 H), 3.23 (s, 3H), 3.62 (m, 1 H), 4.00 (m, 1
H), 5.98 (m,
1 H), 7.13 (7, J = 9 Hz, 2H), 7.23 (m, 2H), 7.47 (d, J = 9 Hz, 2H), 7.86 (d, J
= 9 Hz,
2H), 8.12 (s, 1 H). MS (DCI-NH3) m/z 429 (M+H)+.
Example 380
2-(3-(4-FluoroghenY1)pbenyl)-4-(4-fiuoro h{enyl)-5-[4-(methylsuifonvl)phenyll~
3(2H)-oyridazinone
The title compound was prepared according to the method of Example 4,
starting with 2-(3-bromophenyl)-4-(4-fluorophenyl)-5-[4-
(methylsulfonyl)phenyl]-
3(2H)-pyridazinone (Example 166) in place of 2-benzyl-4-bromo-5-[4-
(methylthio)-
phenyl]-3(2H)-pyridazinone and substituting cesium fluoride for sodium
carbonate
(yield: 0.62g, 62%). M.p. 222-225 C. 1 H NMR (300 MHz, DMSO d6) S 3.24 (s,
3H), 7.16 (m, 2H), 7.36 (m, 3H), 7.53 (m, 2H), 7.64 (m, 2H), 7.73-7.81 (m,
3H), 7.93
(m, 3H), 8.27 (s, 1 H). MS (DCI-NH3) m/z 515 (M+H)+, 532 (M+NH4)+. Anal. caic.
-209-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
for C29H2OF2N2O3S-0.25 H20: C, 67.10; H, 3.98; N, 5.35. Found: C, 66.93; H,
3.99; N, 5.17.
Example 381
2-(2.2.2-Trifluoroethyl)-4-(2.2-dimethY1propoxy)-5-[3-fluoro-4-
(aminosulfony,J)nhenx(1~3(2H)-nvridazinone
2-(2,2,2-Trifluoroethyl)-4-(2,2-dimethylpropoxy)-5-[3-fluoro-4-(methylthio)-
phenyl]-3(2H)-pyridazinone was prepared according to the method of Example
261, substituting 2-(2,2,2-trifluoroethyl)-4-chloro-5-[3-fluoro-4-
(methylthio)phenyl]-
3(2H)-pyridazinone in place of 2-(2,2,2-trifluoroethyl)-4-chloro-5-[4-(methyl-
sulfonyl)phenyl]-3(2H)-pyridazinone.
The methyl sulfide was oxidized with one equivalent of meta-chloroperoxy-
benzoic acid to give the methyl sulfoxide. The sulfoxide was converted to the
title
compound according to the method of Example 68 (yield: 196 mg, 28%). M.p. 144-
145 C. 1 H NMR (300 MHz, CDCI3) S 0.86 (s, 9H), 4.23 (s, 2H), 4.82 (q, J = 8
Hz,
2H), 5.10 (s, 2H), 7.46 (s, 1 H), 7.48 (br s, 1 H), 7.79 (s, 1 H), 8.03 (t, J
= 8 Hz, 1 H).
MS (DCI-NH3) m/z 438 (M+H)+. Anal. caic. for C17H19F3N3O4S: C, 46.68; H,
4.38; N, 9.61. Found: C, 46.76; H, 4.30; N, 9.52.
Example 382
2-(2.2.2-Trifluoroethyl)-4-( -methylproo~xy)-5-[3-fluoro-4-
(aminosulfonyl)ohnvll-
3 (2H)-Ryridazinone
The title compound was prepared according to the method of Example 68
substituting 2-(2,2,2-trifluoroethyl)-4-(2-methylpropoxy)-5-[4-
(methylsulfinyl)phenyl]-
3(2H)-pyridazinone in place of 2-(2,2,2-trifluoroethyl)-4-(4-fluorophenyl)-5-
[4-
(methylsulfinyl)phenyl]-3(2H)-pyridazinone (yield: 260 mg, 26%). M.p. 163-164
C.
1 H NMR (300 MHz, CDC13) S 0.86 (d, J = 6.6 Hz, 6H), 1.91 (septet, J = 6.6 Hz,
1 H),
4.34 (d, J = 6.6 Hz, 2H), 5.11 (br s, 2H), 7.43-7.52 (m, 2H), 7.80 (s, 1 H),
8.02 (t, J = 8
Hz, 1 H). MS (DCI-NH3) m/z 424 (M+H)+, m/z 441 (M+NH4)+. Anal. calc. for
C16H17F4N304S: C, 45.39; H, 4.05; N, 9.92. Found: C, 59.89; H, 3.83; N, 8.61.
Example 383
2-Benzvl-4-(4-fluorobenzyl)-5-[4-(aminosu Ifonyl)phenyll-3(2H)-oyridazinone
The title compound was prepared according to the method of Example 384,
substituting 2-benzyl-4-(4-fluorophenylmethyl)-5-[4-(methylsulfonyl)phenyl]-
3(2H)-
pyridazinone in place of 2-(3,4-difluorophenyl)-4-(4-fluorophenyl)-5-[4-
(methyl-
-210-
CA 02578858 2007-03-05
WO 99/10331 PCTIUS98/16479
sulfonyl)phenyl]-3(2H)-pyridazinone (yield: 0.5723 g 34%). M.p. 120-123 C. 1
H
NMR (300 MHz, DMSO d6) S 3.83 (bs, 2H), 5.30 (bs, 2H), 6.95-7.06 (m, 4H), 7.28-
7.40 (m, 5H), 7.48 (m, 2H), 7.60 (m, 2H), 7.91 (m, 2H), 7.95 (s, i H). MS (DCI-
NH3)
m/z 450 (M+H)+, 467 (M+NH4)+. Anal. calc. for C24H20FN303S: C, 64.13; H,
4.48; N, 9.35. Found: C, 63.76; H, 4.71; N, 9.02.
Example 384
2-Benzyl-4-(4-fluorophenyl -5-f4-(aminosulfonvl)phenyll-3(2H):p,vridazinQne
To a solution of 2-benzyl-4-(4-fluorophenyl)-5-[4-(methylsuffonyl)phenyl]-
3(2H)-pyridazinone (130 mg, 0.3 mmol) and di-t-butylazodicarboxylate (DBAD)
(69
mg, 0.3 mmol) in THF (30 mL) at -78 C was added dropwise a 1 N solution of
lithium 1,1,1,3,3,3-hexamethyldisilazide (0.9 mL, 0.9 mmol) in THF. After
addition,
the reaction was stirred an additional 45 minutes at -78 C (or until the TLC
indicated a disappearance of starting material). The reaction was quenched
with a
saturated solution of NH4CI and extracted with ethyl acetate. The acetate
extract
was dried over MgSO4 and concentrated in vacuo to obtain 220 mg of crude
adduct.
The above adduct was dissolved in THF (30 ML) and was treated at room
temperature with 1 N NaOH (3 mL) for 5 hours. Sodium acetate (NaOAc-3 H20,
1.38 g, 10 mmol) was added followed by addition of hydroxyfamine-O-sulfonic
acid
(1.13 g, 10 mmol) and H20 (30 mL). The resulting mixture was stirred at room
temperature for 18 hours and then extracted with ethyl acetate. The extract
was
washed with water, brine, dried over MgSO4 and concentrated in vacuo. The
residue was purified by chromatography (silica gel, 1:1 hexanes-ethyl acetate)
to
provide the desired product (yield: 70 mg, 54%). M.p. 185-189 C. 1 H NMR
(DMSO-d6, 300 MHz) S 5.33 (s, 2H), 7.11 (m, 2H), 7.22 (m, 2H), 7.40 (m, 7H),
7.83
(d, J = 9 Hz, 2H), 8.10 (s, 1 H). MS (DCI-NH3) m/z 436 (M+H)+. Anal. caic. for
C23H18FN3O3S=0.75 H20: C, 61.65; H, 4.26; N, 9.04. Found: C, 61.67; H, 4.61;
N,
8.66.
Example 385
2-(4-Fluoro henyl); 4-(4-fluorQphenoxy)-5-j4-(aminosulfonyl)phenyll-3(2H)-
pyridazinone
The product from Example 108 was converted to the title sulfonamide
according to the method of Example 384, (yield: 65 mg, 28.8%). M.p. 227-229
C.
1 H NMR (300 MHz, DMSO-d6) 8 7.08-7.17 (m, 4H), 7.36 (t, J = 3 Hz, 2H), 7.47
(br s,
-211-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
2H), 7.61-7.69 (m, 2H), 7.83 (d, J = 9 Hz, 2H), 7.93 (d, J = 9 Hz, 2H), 8.40
(s, 1 H).
MS (DCI-NH3) m/z 469 (M+H)+, 486 (M+NH4)+. Anal. caic. for C24H15 F2N304S:
C, 58.02; H, 3.30; N, 9.24. Found: C, 57.84; H, 3.34; N, 9.01.
Example 386
2-(3.4-Difluorophenyl)-4-(3-fluoro-4-methyl i yl) 5-f4 (aminosulfonvl) f ],
3(2H)-p,yridazinone
The product from Example 371 was converted to the title sulfonamide
according to the method of Example 384 (yield: 45 mg, 28%). M.p. 198-200 C. 1
H
NMR (300 MHz, DMSO-d6) S 6.87 (dd, J = 9 Hz, 3 Hz, 1 H), 7.13 (dt, J = 9 Hz, 3
Hz,
1 H), 7.19 (t, J = 7 Hz, 1 H), 7.46 (d, J = 9 Hz, 2H), 7.47 (br s, 2H), 7.52-
7.69 (m, 2H),
7.79 (d, J = 9 Hz, 2H), 7.82-7.89 (m, 1 H), 8.25 (s, 1 H). MS (DCI-NH3) m/z
472
(M+H)+, 489 (M+NH4)+.
Example 387
2-(4-Fluoroohenvl)-4-13-fluoro-4-methylohenyi);~(4-(aminosulfonvj) henyll-
3(2H)-
Ryridazinone
The product from Example 250 was converted to the title sulfonamide
according to the method of Example 384 (yield: 185 mg, 46%). M.p. 187-188 C.
1 H NMR (300 MHz, DMSO-d6) S 2.22 (br s, 3H), 6.87 (dd, J = 9 Hz, 3 Hz, 1 H),
7.16
(q, J = 9 Hz, 2H), 7.38 (t, J = 9 Hz, 2H), 7.46 (br s, 2H), 7.47 (d, J = 9 Hz,
2H), 7.67-
7.73 (m, 2H), 7.77 (d, J = 9 Hz, 2H), 8.22 (s, 1 H). MS (DCI-NH3) m/z 454
(M+H)+,
471 (M+NH4)+. Anal. caic. for C23H17F2N303S-0.25 H20: C, 60.36; H, 3.87; N,
9.19. Found: C, 60.30; H, 4.26; N, 8.83.
Example 388
2-(3.4-Difluoronhenvl)-4-(4-fluorophenoxyl-5-[4-(aminosulfony()ghenyllõ3(2w-
gyridazinone
The product from Example 109 was converted to the title sulfonamide
according to the method of Example 384 (yield: 110 mg, 45.7%). M.p. 224-226
C.
1 H NMR (300 MHz, CDC13) S 4.86 (br s, 2H), 6.89-7.03 (m, 4H), 7.19-7.30 (m, 1
H),
7.45-7.52 (m, 1 H), 7.56-7.66 (m, 1 H), 7.79 (d, J = 9 Hz, 2H), 8.04 (d, J = 9
Hz, 1 H),
8.08 (s, 1 H). MS (DCI-NH3) m/z 474 (M+H)+, 491 (M+NH4)+. Anal. calc. for
C22H14 F3N304S-0.25 H20: C, 55.32; H, 2.93; N, 8.80. Found: C, 55.26; H, 3.11;
N, 8.58.
-212-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 389
2-(3-Chloro-4-fluorophenY1)-4-(4-fluoro-3-methY1oheny1-'- 4-
jaminosulfonyl ohenyl]-3(2H)-12yridazinone
The product from Example 247 was converted to the title sulfonamide
according to the method of Example 384 (yield: 230 mg, 38%). M.p. 243-245 C.
1 H NMR (300 MHz, DMSO-d6) 8 2.17 (br s, 3H), 6.94-7.09 (m, 2H), 7.25 (dd, J =
9
Hz, 3 Hz, 1 H), 7.41-7.48 (m, 4H), 7.60 (t, J = 9 Hz, 1 H), 7.68-7.75 (m, 1
H), 7.77 (d, J
= 9 Hz, 2H), 7.95 (dd, J = 6 Hz, 3 Hz, 1 H), 8.25 (s, 1 H). MS (DCI-NH3) m/z
469
(M+H)+, 486 (M+NH4)+. Anal. calc. for C23H16CIF2N303S: C, 56.67; H, 3.29; N,
8.63. Found: C, 56.81; H, 3.35; N, 8.95.
Example 390
2-(4-Fluoropheny,j)-4-(4-ffuoro-3-methylphenyl)-5-[4-(aminosulfonyl)ohenyl]-
3(2H)-
Rvridazinone
The methyl sulfone product of Example 245 was converted to the title
sulfonamide according to the method of Example 384 (yield: 78 mg, 28.3%). M.p.
202-204 C. 1 H NMR (300 MHz, CDCI3) S 2.22 (s, 3H), 4.86 (s, 2H), 6.83-6.91
(m,
2H), 7.14-7.25 (m, 3H), 7.36 (d, J = 9 Hz, 2H), 7.65-7.72 (m, 2H), 7.91 (d, J
= 9 Hz,
2H), 8.0 (s, 1 H). MS (DCI-NH3) m/z 454 (M+H)+, 471 (M+NH4)+. Anal. caic. for
C23H17F2N3O3S-0.25 H20: C, 60.36; H, 3.77; N, 9.19. Found: C, 60.24; H, 3.93;
N, 9.25.
Example 391
2-(3-ChloroRhenyl)-4-(4-fluoro-3-methylohenvl)-5-[4-(aminosu-Ifonyl)ohenYl]-
3(2h)-
pyridazinone
The methyl sulfone product of Example 244was converted to the title
sulfonamide according to the method of Example 384 (yield: 125 mg, 39%). M.p.
187-188 C. 1 H NMR (300 MHz, CDCI3) S 2.21 (s, 3H), 4.71 (s, 2H), 6.85-6.92
(m,
2H), 7.21 (d, J = 9 Hz, 1 H), 7.32-7.47 (m, 2H), 7.37 (d, J = 9 Hz, 2H), 7.64
(dt, J = 7
Hz, 3 Hz, 1 H), 7.77 (br s, 1 H), 7.91 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 470
(M+H)+, 487 (M+NH4)+. Anal. caic. for C23H17CIFN3O3SØ25 H20: C, 58.32; H,
3.65; N, 8.88. Found: C, 58.27; H, 3.91; N, 8.62.
-213-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 392
? (3- hlorophenyl)-4-(3-methvlbutyJ)-5-14-(aminosulfonvl)phepylj-3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 384,
substituting 2-(3-chlorophenyl)-4-(3-methylbutyl)-5-[4-(methylsulfonyl)phenyl]-
3(2H)-pyridazinone (Example 366) in place of 2-benzyl-4-(4-fluorophenyl)-5-[4-
(methylsulfonyl)phenyl]-3(2H)-pyridazinone (yield: 0.0756 g, 16%). M.p. 167-
170
C. 1 H NMR (300 MHz, DMSO d6) 5 0.78 (d, J = 6 Hz, 6H), 1.47 (5H, obstructed
by
hexanes), 7.51-7.65 (m, 4H), 7.68 (m, 2H), 7.75 (m, 1 H), 7.98 (m, 2H), 8.03
(s, 1 H),
8.60 (bs, 1 H). MS (DCI-NH3) m/z 432 (M+H)+, 449 (M+NH4)+. Anal. caic. for
C21 H22CIN303S (0.25 H20): C, 57.79; H, 5.19; N, 9.62. Found: C, 57.78; H,
5.02;
N, 9.40.
Example 393
2-(3-ChloronhenYl)-4-(oh, enethyl)-5-[4-(aminosulfonyl)ohenyll-3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 384,
substituting 2-(3-chlorophenyl)-4-(phenethyl)-5-[4-(methylsulfonyl)phenyl]-
3(2H)-
pyridazinone (Example 372) in place of 2-benzyl-4-(4-fluorophenyl)-5-[4-
(methyl-
sulfonyl)phenyl]-3(2H)-pyridazinone (yield: 0.075 g, 17%). semi-solid; 1 H NMR
(300 MHz, DMSO d6) S 2.80 (m, 4H), 3.29-3.42 (3H, obstructed by H20), 6.96 (m,
2H), 7.14-7.28 (m, 3H), 7.46-7.68 (m, 7H), 7.78 (m, 1 H), 7.92 (m, 2H), 8.01
(s, 1 H).
MS (DCI-NH3) m/z 466 (M+H)+, 483 (M+NH4)+. Anal. calc. for
C24H2OCIN2O3S=0.25 H20: C, 61.27; H, 4.39; N, 8.93. Found: 61.18; H, 4.68; N,
8.58.
Example 394
2-(3-ChtoroRhenyl)-4-(3-meth Iby utoxy)-5-[4-(aminosulfonyl)Rhenyll-3(2H)-,
Ryridazinone
The title compound was prepared according to the method of Example 384,
substituting 2-(3-chlorophenyl)-4-(3-methylbutoxy)-5-[4-
(methylsulfonyl)phenyl]-
3(2H)-pyridazinone (Example 339) in place of 2-benzyl-4-(4-fluorophenyl)-5-[4-
(methylsulfonyl)phenyl]-3(2H)-pyridazinone (yield: 0.575 g, 18%). M.p. 137-139
C. 1 H NMR (300 MHz, DMSO d6) 8 0.81 (d, J = 7 Hz, 6H), 1.49 (m, 2H), 1.57 (m,
1 H), 4.42 (t, J = 7 Hz, 2H), 7.44-7.65 (m, 5H), 7.76 (m, 1 H), 7.84 (m, 2H),
7.94 (m,
2H), 8.20 (s, 1 H). MS (DCI-NH3) m/z 448 (M+H)+, 465 (M+NH4)+. Anal. caic. for
C21 H22CIN3O4S: C, 56.31; H, 4.95; N, 9.38. Found C, 56.02; H, 4.82; N, 9.31.
-214-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 395
2-(3-Chloronhenyl)-4-(2-methyl~ro o~xy)-5-[4-(aminosulforlyl)ohenyll-
3(2H)Qyridazinone
The title compound was prepared according to the method of Example 384,
substituting 2-(3-chiorophenyl)-4-(2-methyipropoxy)-5-[4-
(methyisulfonyl)phenyl]-
3(2H)-pyridazinone (Example 335) in place of 2-benzyf-4-(4-fluorophenyl)-5-[4-
(methylsulfonyl)phenyl]-3(2H)-pyridazinone (yield: 0.0458 g, 25%). M.p. 80-85
C.
1 H NMR (300 MHz, DMSO d6) 5 0.80 (d, J = 6 Hz, 6H), 1.74-1.92 (m, 3H), 4.20
(d, J
= 6 Hz, 2H), 7.49-7.64 (m, 5H), 7.76 (m, 1 H), 7.85 (m, 2H), 7.95 (m, 2H),
8.21 (m,
1 H). MS (DCI-NH3) m/z 434 (M+H)+, 451 (M+NH4)+. Anal. calc. for
C20H2OCIN3O4S: C, 55.36; H, 4.65; N, 9.68. Found: C, 55.12; H, 4.58; N, 9.42.
Example 396
2-(4-Fiuoroohenvl)-4-(3-methylbutyl)-5-14-(aminosulfonyl)ghenyl]-3(
Ryridazinone
The title compound was prepared according to the method of Example 384,
substituting 2-(4-fluorophenyl)-4-(3-methylbutyl)-5-[4-(methylsulfonyl)phenyl]-
3(2H)-pyridazinone (Example 378) in place of 2-benzyl-4-(4-fluorophenyl)-5-[4-
(methylsutfonyl)phenyl]-3(2H)-pyridazinone (0.090 g 21 %). M.p. 180-183 C. 1
H
NMR (300 MHz, DMSO d6) S 0.78 (d, J = 6 Hz, 6H), 1.49 (m, 5H), 7.36 (m, 2H),
7.53
(m, 2H), 7.62-7.73 (m, 4H), 7.98 (m, 3H). MS (DCI-NH3) m/z 416 (M+H)+, 433
(M+NH4)+. Anal. calc. for C21 H22FN303S: C, 60.71; H, 5.34; N, 10.11. Found:
C,
60.37, H, 5.36, N, 9.84.
Example .397
2-(4-Fluoroohenvl)-4-(2-methylRropoxy)~j4-(aminosulfonyl) henx]-3(2H)-
Rvridazinone
The title compound was prepared according to the method of Example 384,
substituting 2-(4-fluorophenyl)-4-(2-methylpropoxy)-5-[4-
(methylsulfonyl)phenyl]-
3(2H)-pyridazinone (Example 376) in place of 2-benzyl-4-(4-fluorophenyl)-5-[4-
(methylsulfonyl)phenyl]-3(2H)-pyridazinone (yield: 0.024 g, 6%). M.p. 132-136
C.
1 H NMR (300 MHz, DMSO d6) S 0.79 (d, J = 6 Hz, 6H), 1.83 (m, 1 H), 4.19 (d, J
= 6
Hz, 2H), 7.36 (m, 2H), 7.50 (m, 2H), 7.66 (m, 2H), 7.84 (m, 2H), 7.95 (m, 2H),
8.18
(s, 1 H). MS (DCI-NH3) m/z 418 (M+H)+, 435 (M+NH4)+. Anal. calc. for
C20H2OFN3O4S: C, 57.54; H, 4.83; N, 10.07. Found C, 57.26; H, 5.00; N, 9.78.
-215-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 398
2-(4-Fluorop (jgpyl)-4-(3-meth Iby utoxy)-5-14-(aminosuffonyl ohenYll-3(2H)-
R,~ridazinone
The title compound was prepared according to the method of Example 384,
substituting 2-(4-fluorophenyl)-4-(3-methylbutoxy)-5-[4-
(methylsulfonyl)phenyl]-
3(2H)-pyridazinone (Example 375) in place of 2-benzyl-4-(4-fluorophenyl)-5-[4-
(methylsulfonyl)phenyl]-3(2H)-pyridazinone (yield: 0.051 g, 18%). Yellow oil.
1 H
NMR (300 MHz, DMSO d6) 5 0.80 (d, J = 5 Hz, 6H), 1.47 (m, 3H), 4.42 (t, J = 6
Hz,
2H), 7.37 (m, 2H), 7.50 (m, 1 H), 7.65 (m, 2H), 7.83 (m, 2H), 7.93 (m, 2H),
8.18 (s,
1 H), 8.60 (bs, 1 H). MS (DCI-NH3) m/z 432 (M+H)+, 449 (M+NH4)+. Anal. calc.
for
C21 H22FN304S: C, 58.46; H, 5.14; N, 9.74. Found: C, 58.16; H, 5.21; N, 9.57.
Example 399
2-(t-Butvl)-4-(3-methyl-l-butoxy)-5-[4-(aminosulfony,l)oheny1]-3(2H)-
ovridazinnnp
2-(t-Butyl)-4-(3-methyl-1-butoxy)-5-[4-(methylthio)phenyl]-3(2H)-
pyridazinone prepared in Example 330C was oxidized with one equivalent of
meta-chloroperoxybenzoic acid to the corresponding methyl sulfoxide. The
sulfoxide was converted to the title sulfonamide by the method of Example 68
(yield: 1.25 g, 54%). M.p. 153-155 C. 1 H NMR (300 MHz, CDCI3) S 0.82 (d, J =
6
Hz, 2H), 1.48 (q, J = 6 Hz, 2H), 1.49-1.69 (m, 1 H), 1.70 (s, 9H), 4.37 (t, J
= 6 Hz, 2H),
4.32 (s, 2H), 7.70 (d, J = 9 Hz, 2H), 7.72 (s, 1 H), 8.01 (d, J = 9 Hz, 2H).
MS (DCI-
NH3) m/z 394 (M+H)+. Anal. caic. for C19H27N304S: C, 57.99; H, 6.91; N, 10.67.
Found: C, 58.11; H, 6.71; N, 10.58.
Example 400
?-(3.4-Difluoroohenvl)-5-j4-(aminosulfonyOpheny!]-4-(4-fluorophenyj)-3(ZH)-
pyridazinone
The title compound was prepared according to Example 384 substituting 2-
(3,4-difluorophenyl)-4-(4-fluorophenyl)-5-[4-(methylsulfonyl)phenyf]-3(2H)-
pyridazinone (Example 182) in place of 2-benzyl-4-(4-fluorophenyl)-5-[4-
(methyl-
sulfonyl)phenyl]-3(2H)-pyridazinone (yield: 950 mg, 54%). M.p. 177-181 C. 1 H
NMR (300 MHz, DMSO-d6) S 7.15 (t, 2H), 7.29 (m, 2H), 7.43 (s, 1 H), 7.45 (bs,
2H),
7.59 (m, 2H), 7.76 (d, J = 9 Hz, 2H), 7.85 (m, 1 H), 8.27 (s, 1 H). MS (DCI-
NH3) m/z
458 (M+H)+, 475 (M+NH4)+. Anal. calc. for C22H14F3N303S: C, 57.77; H, 3.08;
N, 9.19. Found, C, 57.22; H, 3.28; N, 8.99.
-216-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 401
2-(3-Chloro-4-fluorophenyl)-4-(4-fluorophenyl)-5-(4-(aminosulfonyl)phenyl]-
3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 384,
substituting 2-(3-chloro-4-fluorophenyl)-4-(4-fluorophenyl)-5-[4-
(methylsulfonyl)-
phenyl]-3(2H)-pyridazinone in place of 2-benzyl-4-(4-fluorophenyl)-5-[4-
(methylsulfonyl)phenyl]-3(2H)-pyridazinone (yield: 380 mg, 47%). M.p. 208-210
C. 1 H NMR (300 MHz, DMSO-d6) 8 7.15 (t, 2H), 7.27 (m, 2H), 7.43 (s, 1 H),
7.45
(bs, 2H) 7.51 (d, J = 9 Hz, 4H), 7.6 (t, 1 H), 7.7 (m, 1 H), 7.75 (d, J = 9
Hz, 2H), 7.94
(dd, 1 H), 8.25 (s, 1 H). MS (DCI-NH3) m/z 474 (M+H)+, 491 (M+NH4)+. Anal.
calc.
for C22H14F2CI2N303S-0.5 H20: C, 55.76; H, 2.98; N, 8.87. Found: C, 56.05; H,
3.42; N, 8.65.
Example 402
2-(3.4-Difluorophenyl)-4-(4-fluoro-3-methyl henyl)-5-j4-
(aminosulfonyl)~herJyll-
3(2H)-Ryridazinone
The title compound was prepared according to the method of procedure
Example 384, substituting 2-(3,4-difiuorophenyl)-4-(4-fluoro-3-methylphenyl)-5-
[4-
(methylsulfonyl)phenyl]-3(2H)-pyridazinone in place of 2-benzyl-4-(4-
fiuorophenyl)-
5-[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone (yield: 105 mg, 27%). M.p. 243-
245 C. 1 H NMR (300 MHz, DMSO-d6) S 2.2 (s, 3H), 7.01 (m, 2H), 7.25 (m, 1 H),
7.45 (s, 1 H), 7.47 (bs, 2H), 7.6 (m, 2H), 7.77 (d, J = 9 Hz, 2H), 7.85 (m, 1
H), 8.26 (s,
2H). MS (DCI-NH3) m/z 472 (M+H)+, 489 (M+NH4)+. Anal. calc. for
C24H17F3N203S-0.5 H20: C, 58.59; H, 3.42; N, 8.91. Found: C, 57; H, 4.23; N,
8.89.
Example 403
2-(3.4-Difluoronhenvl)-4-(2-methylRro~oxy)-5-j4-(aminosulfonvl) henyl]-3(2Fj,L
Ryridazinone
The title compound was prepared according to the method of Example 384,
substituting 2-(3,4-difluorophenyl)-4-(2-methylpropoxy)-5-[4-(methylsulfonyl)-
phenyl]-3(2H)-pyridazinone in place of 2-benzyl-4-(4-fluorophenyl)-5-[4-
(methylsulfonyl)phenyl]-3(2H)-pyridazinone (yield: 35 mg, 42%). M.p. 169-171
C.
1 H NMR (300 MHz, DMSO-d6) 6 0.78 (d, 6H), 1.84, (m, 1 H), 4.2 (d, 2H), 7.54
(m,
3H), 7.6 (m, 1 H), 7.82 (m, 3H), 7.91 (d, 2H), 8.21 (s, 1 H). MS (DCI-NH3) m/z
436
-217-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
(M+H)+, 453 (M+NH4)+. Anal. caic. for C20H19F2N304S=0.25 H20: C, 55.17; H,
4.40; N, 9.65. Found: C, 54.19; H, 4.25; N, 9.35
Example 404
2-(3.4-DifluoropheoY1)-4-( -methylbutvl)-5-[4-(aminoGulfonY1)Qjjgny(j-3(~,H1-
Ryridazinone
The title compound was prepared according to the method of Example 384,
substituting 2-(3,4-difluorophenyl)-4-(3-methylbutyl)-5-[4-
(methylsulfonyl)phenyl]-
3(2H)-pyridazinone in place of 2-benzyl-4-(4-fluorophenyl)-5-[4-(methyl-
sulfonyl)phenyl]-3(2H)-pyridazinone (yield: 58 mg, 52%). M.p. 171-173 C. 1 H
NMR (300 MHz, DMSO-d6) S 0.75 (d, 6H), 1.4, (m, 3H), 2.48 (m, 2H), 3.3 (s,
3H),
7.51 (m, 1 H), 7.65 (m, 1 H), 7.75 (d, J = 9 Hz, 2H), 7.81 (m, 1 H) 8.05 (s, 1
H), 8.12 (d,
J = 9 Hz, 2H). MS (DCI-NH3) m/z 434 (M+H)+, 451 (M+NH4)+. Anal. calc. for
C21 H21 F2N3O3S-0.25 H20: C, 58.19; H, 4.88; N, 9.69. Found: C, 57.69; H,
5.01;
N, 9.18.
Example 405
2-(3-Chforo-4-fluoroohenvl)-4-(3-methylbuty,J)-5-[4-(aminosulfonyl)
henyj]_3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 384,
substituting 2-(3-chloro-4-fluorophenyl)-4-(3-methylbutyl)-5-[4-
(methylsulfonyl)-
phenyl]-3(2H)-pyridazinone in place of 2-benzyl-4-(4-fluorophenyl)-5-[4-
(methylsulfonyl)phenyl]-3(2H)-pyridazinone (yield: 102 mg, 61.8%). M.p. 154-
156
C. 1 H NMR (300 MHz, DMSO-d6) S 0.75 (d, 6H), 1.4, (m, 3H), 2.48 (m, 2H), 7.54
(s, 2H), 7.6 (m, 1 H), 7.69 (m, 2H), 7.93 (dd, 1 H), 8.05 (m, 2H). MS (DCI-
NH3) m/z
450 (M+H)+, 468 (M+NH4)+. Anal. calc. for C22H22FN2O3SCI-0.25 H20: C,
58.86; H, 4.94; N, 6.24. Found: C, 59.23; H, 5.12; N, 6.00.
Example 406
2-(3 4-DiflunrnnhAnyl)-4-f2 2-dimethyl~ro o~y]-5 j4 (aminosuIfonyl)Pheny1]
3(2H)
Ryridazinone
The title compound was prepared according to the method of Example 384
substituting 2-(3,4-difluorophenyl)-4-(2,2-dimethyipropoxy)-5-[4-
(methylsulfonyl)-
phenyl]-3(2H)-pyridazinone in place of 2-benzyl-4-(4-fluorophenyl)-5-[4-
(methylsulfonyl)phenyl]-3(2H)-pyridazinone (yield: 310 mg, 38%). M.p. 173-175
C. 1 H NMR (300 MHz, DMSO-d6) S 0.8 (s, 9H), 3.3 (s, 3H), 4.1 (s, 2H), 7.51
(m,
-218-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
3H), 7.6 (m, 1 H), 7.85 (m, 3H), 7.95 (d, J = 9 Hz, 2H), 8.21 (s, 1 H). MS
(DCI-NH3)
m/z 450 (M+H)+, 467 (M+NH4)+. Anal. calc. for C21 H21 F2N304S: C, 56.12; H,
4.71; N, 9.35. Found, C, 55.83; H, 4.73; N, 9.08.
Example 407
2-(3.4-Difluorophen yl)-4-(4-fluorophenoxy)-5-[3-fluoro-4-
(aminosulfonyl)phenyll-
3(2H)-pvridazinone
The title compound was prepared according to the method of Example 400
substituting 2-(3,4-difluorophenyl)-4-(4-fluorophenoxy)-5-[4-(methylsulfonyl)-
phenyl]-3(2H)-pyridazinone in place of 2-benzyl-4-(4-fluorophenyl)-5-[3-fluoro-
4-
(methylsulfonyl)phenyl] -3(2H)-pyridazinone (yield: 125 mg, 31%). M.p. 224-226
C. 1 H NMR (300 MHz, DMSO-d6) S 7.15 (d, 4H), 7.51 (m, 1 H), 7.6 (m, 2H) 7.75
(m, 4H), 7.9 (t, 1 H); 8.4 (s, 1 H). MS (DCI-NH3) m/z 492 (M+H)+, 509
(M+NH4)+.
Anal. calc. for C22H13F4N304S: C, 53.77; H, 2.67; N, 8.55. Found,; C, 53.33;
H,
2.84; N, 8.22
Example 408
2-(3.3-Difluoro-2-propenyl)J-4-(4-fluorophenyl)-5-[3-fluoro-4-
(aminosulfonyl)ohenyll-3(2H)-pyridazinone
The intermediate, 2-benzyl-4-(4-fluorophenyl)-5-[3-fiuoro-4-(methylthio)-
phenylj-3(2H)-pyridazinone prepared according to the method of Example 72, was
oxidized with one equivalent of ineta-chloroperoxybenzoic acid to provide the
methyl sulfoxide which was converted to the sulfonamide according to the
method
of Example 68. The sulfonamide material was N-debenzylated according to the
method of Example 11 and N-alkylated according to the method of Example 127,
substituting 1,3-dibromo-1,1-difluoropropane in place of 3,4-difluorobenzyl
bromide
and employing 4 equivalents of potassium carbonate to provide the title
compound
(yield: 120 mg, 27%). M.p. 180-183 C. 1 H NMR (300 MHz, CDCI3) 8 4.71 (dt, J
=
15 Hz, 7.5 Hz, 2H), 4.75 (d, J = 7.5 Hz, 2H), 5.06 (s, 2H), 7.02 (m, 2H), 7.19
(dd, J =
9 Hz, 6 Hz, 2H), 7.81 (s, 1 H), 7.87 (t, J = 7.5 Hz, 2H). MS (DCI-NH3) m/z 440
(M+H)+. Anal. calc. for C1 gH13F4N303S: C, 51.93; H, 2.98; N, 9.56. Found: C,
51.71;H,3.15;N,9.28.
-219-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 409
2-(3.4-Difluoro henyl)-4-j2-(2-Rro~oxy et y]-5-[4-(aminosulfonyJ)ohenyl]-
3(2H)Ryridazinone
The title compound was prepared according to the method of Example 384,
substituting 2-(3,4-difluorophenyl)-4-[2-(2-propoxy)ethoxy]-5-[4-
(methylsulfonyl)-
phenyl]-3(2H)-pyridazinone in place of 2-benzyl-4-(4-fluorophenyl)-5-[4-
(methyl-
sulfonyl)phenyl]-3(2H)-pyridazinone (yield: 110 mg, 34%). M.p. 54-56 C. 1 H
NMR
(300 MHz, DMSO-d6) 51.0 (d, 6H), 3.43 (m, 1 H), 3.54 (m, 2H), 4.63 (m, 2H),
7.5 (m,
3H), 7.6 (m, 1 H), 7.8 (m, 1 H)~, 7.95 (m, 4H), 8.2 (s, 1 H). MS (DCI-NH3) m/z
466
(M+H)+, 483 (M+NH4)+. Anal. calc. for C21 H21 F2N305S: C, 54.19; H, 4.55; N,
9.03. Found, C, 54.29; H, 4.67; N, 8.95.
Example 410
2-(3.4-Difluorop,hepYj)-4-(4-methyl-3- ep ntenyloxy)-5-[4-(aminosulfonyl) nvll-
3(2H)-Ryridazinone
The title compound was prepared according to the method of Example 384
substituting 2-(3,4-difluorophenyl)-4-(4-methyl-3-pentenyloxy)-5-[4-(methyl-
sulfonyl)phenyl]-3(2H)-pyridazinone in place of 2-benzyl-4-(4-fluorophenyl)-5-
[4-
(methylsulfonyl)phenyl]-3(2H)-pyridazinone. M.p. 70-73 C. 1 H NMR (300 MHz,
DMSO-d6) S 1.5 (d, 6H), 2.27 (m, 2H) 4.43 (t, 2H), 4.5 (m, 1 H), 7.5 (m, 2H),
7.6 (m,
1 H), 7.8 (m, 2H), 7.92 (d, J = 2 H, 2H), 8.2 (s, 1 H). MS (DCI-NH3) m/z 462
(M+H)+,
479 (M+NH4)+. Anal. calc. for C22H21 F2N304S: C, 57.26; H, 4.59; N, 9.11.
Found, : C, 56.96; H, 4.70; N, 9.01.
Example 411
2-(3-Chloroohenyl)-4-(3-fluoro henoxy)-5-[4-(methvlSulfonyj)phgnyl1-3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 335,
substituting 3-fluorophenol in place of isobutanol (yield: 0.034 g, 22%). M.p.
178-
180 C. 1 H NMR (300 MHz, DMSO d6) 8 3.27 (s, 3H), 6.88-7.00 (m, 2H), 7.10 (m,
1 H), 7.36 (m, 1 H), 7.59 (m, 3H), 7.74 (m, 1 H), 7.90 (m, 2H), 8.06 (m, 2H),
8.43 (s,
1 H). MS (DCI-NH3) m/z 488 (M+H)+. Anal. caic. for C23H16CIFN2O4S-0.25 H20:
C, 58.10; H, 3.49; N, 5.89. Found C, 58.04; H, 3.59; N, 5.80.
-220-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 412
2-(3-Chlorol2henY1)-4-(2-methylRro{2oxy -r-[3-fiuoro-4-(aminosulfonyl)phenyll-
3 (,2 Fi)-Ryli azinone
The title compound was prepared according to the method of Example 384
substituting 2-(3-chlorophenyl)-4-(2-methylpropoxy)-5-[3-fluoro-4-
(methylsulfonyl)-
phenyl]-3(2H)-pyridazinone in place of 2-benzyl-4-(4-fluorophenyl)-5-[4-
(methyl-
suifonyl)phenyl]-3(2H)-pyridazinone (yield: 0.019 g, 10%). M.p. 157-159 C. 1
H
NMR (300 MHz, DMSO d6) S 0.81 (d, J = 6 Hz, 6H), 1.86 (m, 1 H), 4.24 (d, J = 6
Hz,
2H), 7.75 (m, 3H), 7.66 (m, 1 H), 7.73 (m, 2H), 7.83 (m, 2H), 7.91 (m, 1 H),
8.23 (s,
1 H). Anal. calc. for C21 H1 9CIFN3O4S: C, 53.16; H, 4.24; N, 9.30. Found: C,
53.02; H, 4.43; N, 9.10.
Example 413
2-(3-Chloronhenyl)-4-(4-methylpentyloxy)-5-[4-(meth IsY...ulfonyl)phenXll-
3(2H)-
~yridazinone
The title compound was prepared according to the method of Example 335,
substituting 4-methyl-l-pentanol in place of isobutanol (yield: 0.137 g, 90%).
M.p.
139-140 C. 1 H NMR (300 MHz, DMSO d6) S 0.74 (d, J = 6 Hz, 6H), 1.03 (m, 2H),
1.39 (m, 1 H), 1.54 (m, 2H), 3.29 (s, 3H), 4.40 (t, J = 5 Hz, 2H), 7.51-7.60
(m, 3H),
7.75 (m, 1 H), 7.90 (m, 2H), 8.07 (m, 2H), 8.20 (s, 1 H). MS (DCI-NH3) m/z 461
(M+H)+, 478 (M+NH4)+. Anal. caic. for C23H25CIN204S: C, 59.95; H, 5.97; N,
6.08. Found: C, 59.62; H, 5.63; N, 5.86.
Example 414
2-(4-Fluoropheny!)-4-(4-methylpentyloxy)-5-(4-(methyisulfonyl)phenyll-3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 335,
starting with 2-(4-fiuorophenyl)-4-tosyioxy-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone in piace of 2-(3-chlorophenyl)-4-tosyloxy-5-[4-(methylsulfonyl)-
phenyl]-3(2H)-pyridazinone and substituting 4-methyl-l-pentanol in place of
isobutanol (yield: 0.128 g, 85%). M.p. 123-125 C. 1 H NMR (300 MHz, DMSO d6)
S 0.74 (d, J = 6 Hz, 6H), 1.03 (m, 2H), 1.39 (m, 1 H), 1.54 (m, 2H), 3.28 (s,
3H), 4.39
(t, J = 6 Hz, 2H), 7.37 (m, 2H), 7.66 (m, 2H), 7.91 (m, 2H), 8.07 (m, 2H),
8.18 (s, 1 H).
-221-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
MS (DCI-NH3) m/z 445 (M+H)+. Anal. calc. for C23H25FN204S: C, 62.14; H, 5.67;
N, 6.30. Found: C, 62.28; H, 5.59; N, 6.25.
Example 415
2-(4-Fluoropjepyl)-4-hydroxy-5-[4-(meth IsY ulfony!)nhenylJ-3(~I )-
Ryridazinone
The title compound was prepared according to the method of Example 332,
substituting 2-(4-fluorophenyl)-4-methoxy-5-[4-(methyisulfonyl)phenyl]-3(2H)-
pyridazinone for 2-(3-chlorophenyl)-4-methoxy-5-[4-(methylsulfonyl)phenyl]-
3(2H)-
pyridazinone (yield: 2.022 g, 97%). 1 H NMR (300 MHz, DMSO d6) S 3.28 (s, 3H),
7.38 (m, 2H), 7.70 (m, 2H), 8.03 (m, 4H), 8.22 (s, 1 H). MS (APCI-+Q1 MS) 361
(M+H)+, (-Q1 MS) 359 (M-H)-.
Example 416
2-(4-Fluoroohenvl)-4-cvcloproR,ylmethoxy_-5-[4-
(methylsulfonyl)Pheny(];3(2LuL'l--
Ryridazinone
The title compound was prepared according to the method of Example 335,
substituting 2-(4-fluorophenyl)-4-tosyloxy-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone in place of 2-(3-chlorophenyl)-4-tosyloxy-5-[4-(methylsulfonyl)-
phenyl]-3(2H)-pyridazinone and substituting cyclopropylmethanol in place of
isobutanol (yield: 0.117 g, 83%). M.p. 166-167 C. 1 H NMR (300 MHz, DMSO d6)
5 0.22 (m, 2H), 0.46 (m, 2H), 1.10 (m, 1 H), 3.31 (s, 3H), 4.30 (d, J = 7 Hz,
2H), 7.36
(m, 2H), 7.66 (m, 2H), 7.96 (m, 2H), 8.07 (m, 2H), 8.20 (s, 1 H). MS (DCI-NH3)
m/z
415 (M+H)+, 432 (M+NH4)+. Anal. calc. for C23H25CIN2O4S: C, 60.86; H, 4.62; N,
6.76. Found: C, 60.76; H, 4.72; N, 6.61.
Example 417
2-(4-Fluorophenyl)-4-(2-cyclopropyl-1-ethoxy)-5-[4-(methvlsulfonyl)phenylJ
3(2H)
pyridazinone
The title compound was prepared according to the method of Example 335,
substituting 2-(4-fluorophenyl)-4-tosyloxy-5-[4-(methylsulfonyl)phenyl]-3(2H)-
-222-
CA 02578858 2007-03-05
WO 99/10331 PCT/1JS98/16479
pyridazinone in place of 2-(3-chlorophenyl)-4-tosyloxy-5-[4-(methylsuifonyl)-
phenyl]-3(2H)-pyridazinone and substituting 2-cyclopropane ethanol in place of
isobutanol (yield: 0.1472 g, 100%). M.p. 111-117 C. 1 H NMR (300 MHz, DMSO
d6) 8 -0.01 (m, 2H), 0.31 (m, 2H), 0.60 (m, 1 H), 1.49 (q, J = 6 Hz, 2H), 3.29
(s, 3H),
4.48 (t, J = 6 Hz, 2H), 7.37 (m, 2H), 7.65 (m, 2H), 7.91 (m, 2H), 8.06 (m,
2H), 8.17 (s,
1 H). MS (DCI-NH3) m/z 429 (M+H)+, 446 (M+NH4)+. Anal. calc. for
C22H21 FN204S: C, 61.67; H, 4.94; N, 6.54. Found: C, 61.59; H, 5.02; N, 6.45.
Example 418
2-(3-Chloroohenyl)-4-cyclopro Qanemethoxy-5_-j4-(methylsulfonyi)phep, I-'~~]
~! H)-
R,yridazinone
The title compound was prepared according to the method of Example 335,
substituting cyclopropane methanol in place of isobutanol (yield: 0.0917 g,
64%).
M.p. 158-161 C. 1 H NMR (300 MHz, DMSO d6) 8 0.22 (m, 2H), 0.46 (m, 2H), 1.13
(m, 1 H), 3.31 (s, 3H), 4.31 (d, J = 7 Hz, 2H), 7.57 (m, 3H), 7.75 (m, 1 H),
7.96 (m, 2H),
8.08 (m, 2H), 8.23 (s, 1 H). MS (DCI-NH3) m/z 431 (M+H)+, 448 (M+NH4)+. Anal.
calc. for C21 H1 gC1N2O4S-0.25 H20: C, 57.92; H, 4.51; N, 6.43. Found: C,
57.86;
H, 4.35; N, 6.27.
Example 419
2-(3-Chloronhenyl)-4-(2-cyclooropane-l-ethoxy)-5-[4-(methylsulfonyl) heny~l
3(2H)-pyridazinone
The title compound was prepared according to the method of Example 335,
substituting 2-cyclopropane ethanol in place of isobutanol (yield: 0.114 g,
78%).
M.p. 124-128 C. 1 H NMR (300 MHz, DMSO d6) 6 0.00 (m, 2H), 0.32 (m, 2H), 0.61
(m, 1 H), 1.49 (q, J = 6 Hz, 2H), 3.30 (s, 3H), 4.50 (t, J = 6 Hz, 2H), 7.58
(m, 3H), 7.76
(m, 1 H), 7.91 (m, 2H), 8.07 (m, 2H), 8.21 (s, 1 H). MS (DCI-NH3) m/z 445
(M+H)+,
462 (M+NH4)+. Anal. calc. for C22H21 CIN204S: C, 59.39; H, 4.76; N, 6.30.
Found: C, 58.92; H, 4.94; N; 6.15.
-223-
CA 02578858 2007-03-05
WO 99/10331 PCTIUS98/16479
Example 420
2=(4-Fluorol2heny,])-4-(4-methyjpentY1)-5-[4-(methylsulfonyl)nhenyl]-3(2H)-
Qyridazinone
The title compound was prepared according to the method of Example 362,
substituting 2-(4-fluorophenyl)-4-tosyloxy-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone in place of 2-(3-chlorophenyl)-4-tosyfoxy-5-[4-(methylsulfonyl)-
phenyl]-3(2H)-pyridazinone and substituting 4-methylpentane-1 -magnesium
bromide for cyclopropyl magnesium chloride (yield: 0.165 g, 99%). M.p. 112-115
C. 1 H NMR (300 MHz, DMSO d6) S 0.75 (d, J = 7 Hz, 6H), 1.07 (q, J = 7 Hz,
2H),
1.32-1.53 (m, 3H), 2.45 (t, 2H), 3.31 (s, 3H), 7.37 (m, 2H), 7.66 (m, 2H),
7.76 (m,
2H), 8.00 (s, 1 H), 8.10 (m, 2H). MS (DCI-NH3) m/z 429 (M+H)+. 446 (M+NH4)+.
Anal. caic. for C23H25FN203S: C, 64.47; H, 5.88; N, 6.54. Found: C, 64.44; H,
5.90; N, 6.49.
Example 421
2-(3-Chlorophenyl)-4-(4-methyll2entyl);5-[4-(methylsulfonyl)phenyll-3(2H)-
12yridazinone
The title compound was prepared according to the method of Example 362,
substituting 4-methylpentane-1-magnesium bromide in place of cyclopropyl
magnesium chloride (yield: 165 mg, 98%). oil. 1 H NMR (300 MHz, DMSO d6) 8
0.76 (d, J = 6 Hz, 6H), 1.07 (m, 2H), 1.33-1.55 (m, 3H), 2.45 (m, 2H), 3.32
(s, 3H),
7.51-7.65 (m, 4H), 7.76 (m, 2H), 8.03 (s, 1 H), 8.11 (m, 2H). MS (DCI-NH3) m/z
445
(M+H)+, 462 (M+NH4)+. Anal. calc. for C23H25CIN2O3S: C, 62.06; H, 5.66; N,
6.30. Found: C, 61.86; H, 5.64; N, 6.18.
-224-
CA 02578858 2007-03-05
WO 99/10331 PCTIUS98/16479
Example 422
? (4 Fluorooeny_I)-4- (3 methyl 2 butenoxy)-5-(4-(methylsulfonyl)ohenyl,l:3
(~..~H)-
p,yridazinone
The title compound was prepared according to the method of Example 335,
substituting 2-(4-fluorophenyl)-4-tosyloxy-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone in place of 2-(3-chlorophenyl)-4-tosyloxy-5-[4-(methylsulfonyl)-
phenyl]-3(2H)-pyridazinone and substituting 3-methyl-2-buten-l-ol in place of
isobutanol (yield: 0.1284 g, 88%). M.p. 128-132 C. 1 H NMR (300 MHz, DMSO
d6) S 1.58 (s, 3H), 1.67 (s, 3H), 3.30 (s, 3H), 4.95 (d, J = 7 Hz, 2H), 5.31
(m, 1 H),
7.38 (m, 2H), 7.65 (m, 2H), 7.89 (m, 2H), 8.06 (m, 2H), 8.18 (s, 1 H). MS (DCI-
NH3)
m/z 429 (M+H)+, 446 (M+NH4)+. Anal. calc. for C22H21 FN2O4S: C, 61.67; H,
4.94; N, 6.54. Found: C, 61.41; H, 4.95; N, 6.47.
Example 423
2-(3-Chlorophenyl)-413-methyl-2-butenoxyL[4-(met yisulfonyl)ohenyl]-3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 335,
substituting 3-methyl-2-buten-1-ol in place of isobutanol (yield: 0.119 g,
81%). M.p.
113-115 C. 1 H NMR (300 MHz, DMSO d6) 8 1.58 (s, 3H), 1.67 (s, 3H), 3.31 (s,
3H), 4.96 (m, 2H), 5.32 (m, 1 H), 7.58 (m, 3H), 7.75 (m, 1 H), 7.89 (m, 2H),
8.07 (m,
2H), 8.21 (s, 1 H). MS (APCI+Q1 MS) 445 (M+H)+, (APCI-Q1 MS) 479 (M+35)-.
Anal. caic. for C22H21 CIN2O4S: C, 59.39; H, 4.76; N, 6.30. Found: C, 59.14;
H,
4.66; N, 6.16.
Example 424
2-(4-FluoroRhenyl)-4-(4-meth,yl_3-12e ntenyloxy)-5-[4-(met Isulfony_I)nhenyl]-
3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 335,
substituting 2-(4-fluorophenyl)-4-tosyloxy-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone in place of 2-(3-chlorophenyl)-4-tosyloxy-5-[4-(methylsulfonyl)-
-225-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
phenyl]-3(2H)-pyridazinone and substituting 4-methyl-2-penten-l-ol in place of
isobutanol (yield: 0.1165 g, 77%). M.p. 111-114 C. 1 H NMR (300 MHz, DMSO
d6) S 1.46 (s, 3H), 1.56 (s, 3H), 2.26 (m, 2H), 3.30 (s, 1 H), 4.43 (t, J = 7
Hz, 2H), 4.96
(m, 1 H), 7.37 (m, 2H), 7.65 (m, 2H), 7.91 (m, 2H), 8.06 (m, 2H), 8.18 (s, 1
H). MS
(DCI-NH3) m/z 443 (M+H)+, 460 (M+NH4)+. Anal. caic. for C23H23FN204S: C,
62.43; H, 5.24; N, 6.33. Found: C, 62.32; H, 5.30; N, 6.25.
Example 425
2-(4-Fluoro2henvl)-4-(3-methyl-3-butenoxy)-5-[4-(methylsulfonyj)phenylj-3(2H1
pyridazinone
The title compound was prepared according to the method of Example 335,
substituting 2-(4-fluorophenyl)-4-tosyloxy-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone in place of 2-(3-chlorophenyl)-4-tosyloxy-5-[4-
(methylsulfonyl)phenyl]-3(2H)-pyridazinone and substituting 3-methyl-3-butene-
l-
ol in place of isobutanol (yield: 0.1327 g, 91%). M.p. 109-111 C. 1H NMR (300
MHz, DMSO d6) 5 1.61 (s, 3H), 2.32 (t, J = 7 Hz, 2H), 3.30 (s, 3H), 4.56 (t, J
= 7 Hz,
2H), 4.63 (bs, 1 H), 4.68 (bs, 1 H), 7.37 (m, 2H), 7.66 (m, 2H), 7.90 (m, 2H),
8.05 (m,
2H), 8.19 (s, 1 H). MS (DCI-NH3) m/z 429 (M+H)+, 446 (M+NH4)+. Anal. caic. for
C22H21 FN204S: C, 61.67; H, 4.94; N, 6.54. Found: C, 61.50; H, 5.00; N, 6.45.
Example 426
2-(3-Chloroohenvl)-4-(4-methvl-3-oentenyloxy)-5-[4- ethy su yJ) henyl]-3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 335,
substituting 4-methyl-3-pentene-1-ol in place of isobutanol (yield: 0.1149 g,
76%).
M.p. 110-111 C. 1 H NMR (300 MHz, DMSO d6) 5 1.47 (s, 3H), 1.55 (s, 3H), 2.27
(m, 2H), 3.30 (s, 3H), 4.44 (t, J = 6 Hz, 2H), 4.96 (m, 1 H), 7.52-7.64 (m,
3H), 7.75 (m,
1 H), 7.91 (M, 2H), 8.06 (m, 2H), 8.21 (s, 1 H). MS (DCI-NH3) m/z 459 (M+H)+,
476
(M+NH4)+. Anal. calc. for C23H23CIN204S: C, 60.19; H, 5.05; N, 6.10. Found: C,
60.06; H, 4.90; N, 5.96.
-226-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 427
? ( -Chiorol2heLyJ)-4-(3-methyl-3-butenox,y)-5-j4-(methylsulfonyl)phenylJ-
3(2H)-
pyridazinorte
The title compound was prepared according to the method of Example 335,
substituting 3-methyl-3-butene-1-ol in place of isobutanol (yield: 0.1159 g,
79%).
M.p. 110-112 C. 1 H NMR (300 MHz, DMSO d6) 5 1.62 (s, 3H), 2.32 (t, J = 7 Hz,
2H), 3.30 (s, 3H), 4.57 (t, J = 6 Hz, 2H), 4.63 (bs, 1 H), 4.68 (bs, 1 H),
7.51-7.64 (m,
3H), 7.76 (m, 1 H), 7.90 (m, 2H), 8.05 (m, 2H), 8.21 (s, 1 H). MS (DCI-NH3)
m/z 445
(M+H)+, 462 (M+NH4)+. Anal. calc. for C22H21 CIN204S: C, 59.39; H, 4.76; N,
6.30. Found: C, 59.27; H, 4.68; N, 6.18.
Example 428
2-(4-Fluorophenxl)-4-(1.5-hexadieny)-3-oxv)-5-[4_(methylsulfonyl)ohnyl)-3(2H)-
R,yridazinone
The title compound was prepared according to the method of Example 178,
substituting 1,5-hexadien-3-ol in place of 2-ethyi-l-hexanol (yield: 150 mg,
85%).
M.p. 104-105 C. 1 H NMR (300 MHz, DMSO-d6) 5 2.42 (m, 2H), 3.30 (s, 3H), 5.00
(m, 2H), 5.17 (m, 2H), 5,64 (m, 2H), 7.36 (t, J = 9 Hz, 2H), 7.64 (m, 2H),
7.92 (d, J = 9
Hz, 2H), 8.06 (d, J = 9 Hz, 2H), 8.19 (s, 1 H). MS (APCI+) m/z 441 (M+H)+;
(APCI-)
m/z 475 (M+CI)-. Anal. calc. for C23H21 FN2O4S: C, 62.71; H, 4.80; N, 6.35.
Found: C, 62.96; H, 4.93; N, 5.85.
Example 429
2-(4-Fluoroohenyl -5-methyl-2-hexyloxy)-5-j4-(methylsulfony1)n, henyll ;3(2H)-
py~dazinone
The title compound was prepared according to the method of Example 178,
substituting 5-methyl-2-hexanol in place of 2-ethyl-l-hexanol (yield: 150 mg,
82%).
M.p. 102-103 C. 1 H NMR (300 MHz, DMSO-d6) S 0.73 (d, J = 7 Hz, 6H), 1.04 (m,
-227-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
2H), 1.14 (d, J = 7 Hz, 3H), 1.40 (m, 3H), 3.29 (s, 3H), 5.12 (m, 1 H), 7.36
(t, J = 9 Hz,
2H), 7.66 (m, 2H), 7.92 (d, J = 9 Hz, 2H), 8.07 (d, J = 9 Hz, 2H), 8.19 (s, 1
H). MS
(APCI+) m/z 459 (M+H)+; (APCI-) m/z 493 (M+CI)-. Anal. calc. for C24H27FN204S:
C, 62.86; H, 5.93; N, 6.10. Found: C, 62.83; H, 5.99; N, 6.07.
Example 430
2-(4-Fluoroohenvl)-4-(2-ethvl-1-butoxy)-5-(4-(methylsu Y1)phenyll-3(2H)-
pyridazinone
The title compound was prepared according to the method of Example 178,
substituting 2-ethyl-l-butanol in place of 2-ethyl-l-hexanol (yield: 140 mg,
80%).
M.p. 107-108 C. 1 H NMR (300 MHz, DMSO-d6) S 0.73 (t, J = 7 Hz, 6H), 1.20
(quintet, J = 7 Hz, 4H), 1.40 (m, 1 H), 3.29 (s, 3H), 4.29 (d, J = 7 Hz, 2H),
7.37 (t, J = 9
Hz, 2H), 7.66 (m, 2H), 7.90 (d, J= 9 Hz, 2H), 8.07 (d, J= 9 Hz, 2H), 8.19 (s,
1 H). MS
(APCI+) m/z 445 (M+H)+; (APCI-) m/z 479 (M+CI)-. Anal. caic. for C23H25FN204S:
C, 62.14; H, 5.66; N, 6.30. Found: C, 62.05; H, 5.86; N, 6.30.
Example 432
2-(4-Fluoronhenyl)-4-(2-thioisopropy i-l-ethoxx)-5-j4-(methy sulfnnyl)phenyl]-
~f~
Ryridazinone
The title compound was prepared according to the method of Example 178,
substituting 2-(isopropylthio)ethanol in place of 2-ethyl-l-hexanol (yield:
138 mg,
74%). M.p. 137-139 C. 1 H NMR (300 MHz, DMSO-d6) 81.13 (d, J = 7 Hz, 6H),
2.77 (t, J = 7 Hz, 2H), 2.88 (quintet, J = 7 Hz, 1 H), 3.29 (s, 3H), 4.58 (t,
J = 7 Hz, 2H),
7.37 (t, J = 9 Hz, 2H), 7.66 (m, 2H), 7.92 (d, J = 9 Hz, 2H), 8.06 (d, J = 9
Hz, 2H),
8.18 (s, 1 H). MS (APCI+) m/z 463 (M+H)+. Anal. calc. for C22H23FN204S2: C,
57.12; H, 5.01; N, 6.05. Found: C, 56.82; H, 4.91; N, 5.99.
-228-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 433
? (4-Fluorophenyl)-4-(3-methylthio-l-hexyioxy,-L5-(4-(methylsulfonyl)ohenyl]-
3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 178,
substituting 3-(methylthio)-1-hexanol in place of 2-ethyl-l-hexanol (yield:
155 mg,
79%). M.p. 90-92 C. 1 H NMR (300 MHz, DMSO-d6) S 0.78 (t, J = 7 Hz, 3H), 1.30
(m, 4H), 1.76 (m, 2H), 2.82 (s, 3H), 2.38 (m, 1 H), 3.29 (s, 3H), 4.55 (m,
2H), 7.37 (t, J
= 9 Hz, 2H), 7.66 (m, 2H), 7.92 (d, J = 9 Hz, 2H), 8.06 (d, J = 9 Hz, 2H),
8.18 (s, 1 H).
MS (APCI+) m/z 491 (M+H)+; (APCI-) m/z 525 (M+CI)-. Anal. calc. for
C24H27FN204S2: C, 58.75; H, 5.54; N, 5.70. Found: C; 58.66; H, 5.54; N, 5.66.
Example 434
2-( -Fluoroohenyl)-4-(2-methyl-4-nentenyl-l-oxy)-5-[4-
(methvlsulfonyl)pllPn,yll-
3 (2H)-nvridazinone
The title compound was prepared according to the method of Example 178,
substituting 2-methyl-4-penten-l-ol in place of 2-ethyl-l-hexanol (yield: 135
mg,
76%). M.p. 106-107 C. 1 H NMR (300 MHz, DMSO-d6) S 0.76 (d, J = 7 Hz, 3H),
1.78 (m, 2H), 2.00 (m, 1 H), 3.29 (s, 3H), 4.25 (m, 2H), 4.90 (m, 2H), 5.67
(m, 1 H),
7.37 (t, J = 9 Hz, 2H), 7.66 (m, 2H), 7.92 (d, J = 9 Hz, 2H), 8.06 (d, J = 9
Hz, 2H),
8.18 (s, 1 H). MS (APCI+) m/z 443 (M+H)+; (APCI-) m/z 477 (M+CI)-. Anal. caic.
for
C23H23FN204S: C, 62.42; H, 5.23; N, 6.33. Found: C, 62.13; H, 5.12; N, 6.22.
Example 435
2-(3.4-Diftuoroohenvl)-4-(3-trifluoromethyl-l-butoxy)-5-[4-
(methyisulfonyl)ohenyll-
3 (2H)-Ryridazinone
To a solution of 2-(3,4-difluorophenyl)-4-hydroxy-5-[4-
(methyfsulfonyl)phenyl]-3(2H)-pyidazinone (189mg, 0.5 mmol), Ph3P (262 mg, 1
mmol) and 3-trifluoromethyl-l-butanol (66 mg, 0.5 mmol) in THF (25 mL) was
added dropwise a solution of DIAD (0.2 mL, 1 mmol) in THF (5 mL) and the
resulting mixture was stirred at room temperature for 8 hours. The mixture was
-229-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
concentrated in vacuo and the residue was chromatographed (silica gel, 1:1
hexanes-ethyl acetate) to provide the desired product, (yield: 180 mg 71%).
M.p.
126-128 C. 1 H NMR (300 MHz, DMSO-d6) S 0.96 (d, J = 7 Hz, 3H), 1.55 (m, 1
H),
1.97 (m, 1 H), 2.30 (m, 1 H), 3.29 (s, 3H), 4.46 (m, 2H), 7.52 (m, 1 H), 7.62
(m, 1 H),
7.81 (m, 1 H), 7.90 (d, J = 9 Hz, 2H), 8.08 (d, J = 9 Hz, 2H), 8.22 (s, 1 H).
MS (APCI+)
m/z 503 (M+H)+; (APCI-) m/z 537 (M+CI)-. Anal. caic. for C22H1 9F5N204S: C,
52.59; H, 3.81; N, 5.57. Found: C, 52.70; H, 3.73; N, 5.63.
Example 436
2-(3.4-Difluoroohenyl)-4-ethoxy-5-[4-(meth,yls- lfonyl) (2 H)-Ryridazinone
The title compound was prepared according to the method of Example 178,
starting with 2-(3,4-difluorophenyl)-4-tosyloxy-5-[4-(methylsulfonyl)phenyl]-
3(2H)-
pyridazinone in place of 2-(4-fluorophenyl)-4-tosyloxy-5-[4-
(methylsuIfonyl)phenyl]-
3(2H)-pyridazinone and substituting ethanol in piace of 2-ethyl-l-hexanol
(yield: 25
mg, 12%). M.p. 121-123 C. 1 H NMR (300 MHz, DMSO-d6) 51.23 (t, J = 7 Hz, 3H),
3.30 (s, 3H), 4.51 (q, J= 7 Hz, 2H), 7.52 (m, 1 H), 7.62 (m, 1 H), 7.81 (m, 1
H), 7.90 (d,
J = 9 Hz, 2H), 8.08 (d, J = 9 Hz, 2H), 8.22 (s, 1 H). MS (APCI+) m/z 407
(M+H)+;
(APCI-) m/z 441 (M+Cl)-. Anal. calc. for C19H16F2N204S-0.25 H20: C, 55.53; H,
4.04; N, 6.81. Found: C, 55.58; H, 4.21; N, 6.61.
Example 437
2-(,3.4-Difluoroohenvl)-4-(4-methvl-l-oen yloxy)-5-14-(methy au yj)ghen}ll-
3(2H)-
Ryridazinone
The title compound was prepared according to the method of Example 178,
starting with 2-(3,4-difluorophenyl)-4-tosyioxy-5-[4-(methylsulfonyl)phenyl]-
3(2H)-
pyridazinone in place of 2-(4-fluorophenyl)-4-tosyloxy-5-[4-
(methylsulfonyl)phenyl]-
3(2H)-pyridazinone and substituting 4-methyl-1 -pentanol in place of 2-ethyl-l-
hexanol (yield: 120 mg, 52%). M.p. 98-99 C. 1 H NMR (300 MHz, DMSO-d6) b
0.73 (d, J = 7 Hz, 6H), 1.02 (m, 2H), 1.29 (m, 1 H), 1.54 (m, 2H), 3.30 (s,
3H), 4.40 (t,
J = 7 Hz, 2H), 7.52 (m, 1 H), 7.62 (m, 1 H), 7.81 (m, 1 H), 7.90 (d, J = 9 Hz,
2H), 8.08
(d, J = 9 Hz, 2H), 8.22 (s, 1 H). MS (APCI+) m/z 463 (M+H)+; (APCI-) m/z 497
-230-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
(M+CI)-. Anal. calc. for C23H24F2N204S: C, 59.72; H, 5.23; N, 6.05. Found: C,
59.57; H, 5.28; N, 6.01.
Example 438 2-(3.4-Difluorophenyl)-4-(4-methyl-2-oentyloxy)-5 -[4-
(methyJsuJfodyl)phg0Y1]-3(2H)-Ryridazinone
The title compound was prepared according to the method of Example 178,
starting with 2-(3,4-difluorophenyl)-4-tosy(oxy-5-[4-(methyfsulfonyl)phenyl]-
3(2H)-
pyridazinone in place of 2-(4-fluorophenyl)-4-tosyloxy-5-[4-
(methylsulfonyl)phenyl]-
3(2H)-pyridazinone and substituting 4-methyl-2-pentanol for 2-ethyl-l-hexanol
(yield: 115 mg, 50%). M.p. 132-133 C. 1 H NMR (300 MHz, DMSO-d6) S 0.80 (d, J
= 7 Hz, 3H), 0.87 (d, J = 7 Hz, 3H), 1.10 (d, J = 7 Hz, 3H), 1.26 (m, 1 H),
1.50 (m, 1 H),
1.63 (m, 1 H), 3.30 (s, 3H), 5.31 (m, 1 H), 7.52 (m, 1 H), 7.62 (m, 1 H), 7.81
(m, 1 H),
7.90 (d, J = 9 Hz, 2H), 8.08 (d, J = 9 Hz, 2H), 8.22 (s, 1 H). MS (APCI+) m/z
463
(M+H)+; (APCI-) m/z 497 (M+CI)-. Anal. calc. for C23H24F2N204S: C, 59.72; H,
5.23; N, 6.05. Found: C, 59.44; H, 5.26; N, 5.99.
Example 439 2-(3.4-Difluoroohenyl)-4-(2-cyclonenty -1-ethoxy)-5-14-
(meth Is~fonyl)phe nyll-3(2H)-ovridazinone
The title compound was prepared according to the method of Example 178,
starting with 2-(3,4-difluorophenyl)-4-tosyloxy-5-[4-(methylsulfonyl)phenyl]-
3(2H)-
pyridazinone in place of 2-(4-fluorophenyl)-4-tosyloxy-5-[4-
(methylsulfonyl)phenyl]-
3(2H)-pyridazinone and substituting 2-cyclopentyl-1-ethano! in place of 2-
ethyl-l-
hexanol (yield: 115 mg, 60%). M.p. 100-101 C. 1 H NMR (300 MHz, DMSO-d6) 8
1.00 (m, 2H), 1.38 (m, 2H), 1.57 (m, 7H), 3.30 (s, 3H), 4.42 (t, J = 7 Hz,
2H), 7.52 (m,
1 H), 7.62 (m, 1 H), 7.81 (m, 1 H), 7.90 (d, J = 9 Hz, 2H), 8.08 (d, J = 9 Hz,
2H), 8.22
(s, 1 H). MS (APCI+) m/z 475 (M+H)+; (APCI-) m/z 509 (M+CI)-. Anal. caic. for
C24H24F2N204S-0.25 H20: C, 60.17; H, 5.15; N, 5.84. Found: C, 60.12; H, 5.14;
N, 5.76.
-231-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 440
2-(3.4-Difluorophenyl)-4-(2-cvclooent-2-enyl-l-ethoxy -5-f4-
(methylsuIfonyl)phe nylJ-3(2H)-Ryridazinone
The title compound was prepared according to the method of Example 178,
starting with 2-(3,4-difluorophenyl)-4-tosyloxy-5-[4-(methylsulfonyl)phenyl]-
3(2H)-
pyridazinone in place of 2-(4-ftuorophenyl)-4-tosyloxy-5-[4-
(methyfsulfonyl)phenyl]-
3(2H)-pyridazinone and substituting 2-cyclopent-2-enyl-1-ethanol in place of 2-
ethyl-1 -hexanol (yield: 95 mg, 48%). M.p. 126-127 C. 1 H NMR (300 MHz, DMSO-
d6) 81.30 (m, 1 H), 1.57 (sextet, J= 7 Hz, 1 H), 1.69 (sextet, J = 7 Hz, 1 H),
1.87 (m,
2H), 2.57 (m, 1 H), 3.30 (s, 3H), 4.45 (m, 2H), 5.60 (m, 1 H), 5.68 (m, 1 H),
7.52 (m,
1 H), 7.62 (m, 1 H), 7.81 (m, 1 H), 7.90 (d, J = 9 Hz, 2H), 8.08 (d, J = 9 Hz,
2H), 8.22
(s, 1 H). MS (APCI+) m/z 473 (M+H)+; (APCI-) m/z 507 (M+CI)-. Anal. caic. for
C24H22F2N204S: C, 61.00; H, 4.69; N, 5.92. Found: C, 60.76; H, 4.65; N, 5.80.
Example 441
2-(2-Hvdroxy;2-12henylethyl)-4-(4-fluorophenyl)-5-[4-(meth }Is~ulfonyI)ohenyll-
3(2H)-
pyridazinone
A mixture of the product from Example 46, 2-phenacyl-4-(4-fluorophenyl)-5-
[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone (700 mg, 1.5 mmol), and sodium
borohydride (69 mg, 1.8 mmol) in ethanol (200 mL), was stirred at 40 C for 2
hours. The reaction mixture was then concentrated in vacuo and the residue was
partitioned between ethyl acetate and 2 N aqueous hydrochloric acid. The
organic
layer was washed with brine, dried over MgSO4, and filtered. The filtrate was
concentrated in vacuo to provide a pale yellow solid which was crystallized
from
ethyl acetate/hexanes to provide the title compound as white crystals (yield:
540
mg, 78%). M.p. 205-207 C. 1 H NMR (300 MHz, CDCI3) S 3.07 (s, 3H), 3.75 (br
s,
1 H), 4.63-4.47 (m, 2H), 5.33 (dd, J = 9 Hz, 3 Hz, 1 H), 7.00 (t, J = 9 Hz,
2H), 7.20 (dd,
J = 9 Hz, 3 Hz, 2H), 7.30-7.45 (m, 5H), 7.52 (d, J = 9 Hz, 2H), 7.91 (s, 1 H),
7.91 (d, J
= 9 Hz, 2H). MS (DCI-NH3) m/z 465 (M+H)+. Anal. calc. for C25H21 FN2O4S: C,
64.64; H, 4.55; N, 6.03. Found: C, 64.34; H, 4.66; N, 5.93.
-232-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 442
2-(2-Methoxy-2-phen ly ethyl)-4-(4-fluoroghenyl)-5-j4-(methvlsulfony1)phenyl]-
3(2H)-
~yridazinone
A mixture of the product from Example 441, 2-(2-hydroxy-2-phenylethyl)-4-
(4-fluorophenyl)-5-[4-(methyisuifonyl)phenyl]-3(2H)-pyridazinone (210 mg, 0.45
mmol), iodomethane (56 L, 0.90 mmol), and an 80% oil dispersion of sodium
hydride (18 mg, 0.59 mmol) in anhydrous DMF (16 mL) was stirred at room
temperature for 18 hours. The reaction mixture was partitioned between ethyl
acetate and 2 N aqueous hydrochloric acid. The organic layer was washed with
brine, dried over MgSO4, and filtered. The filtrate was concentrated in vacuo
to
provide a yellow oil which was purified by column chromatography (silica gel,
70:30 hexanes/ethyl acetate). Fractions containing product were combined and
concentrated in vacuo , and the residue was triturated with hexanes to provide
the
title compound (yield: 75 mg, 34.7%). M.p. 135-137 C. 1 H NMR (300 MHz,
CDCI3) S 3.07 (s, 3H), 3.26 (s, 3H), 4.33-4.52 (m, 2H), 4.91 (dd, J = 9 Hz, 3
Hz, 1 H),
6.99 (t, J 9 Hz, 2H), 7.20 (dd, J = 9 Hz, 3 Hz, 2H), 7.31-7.50 (m, 7H), 7.87
(s, 1 H),
7.89 (d, J 9 Hz, 2H). MS (DCI-NH3) m/z 479 (M+H)+. Anal. caic. for
C26H23FN204S: C, 65.25; H, 4.84; N, 5.85. Found: C, 64.98; H, 4.83; N, 5.81.
Example 443
2-(2-Methoxvimino-2-ohenvlethyl)-4-(4-fluoro h nvl)- -[4-(methy su-f
nyl)ohenyll-
3(2H)-Rvridazinone
A mixture of the product from Example 46, 2-phenacyl-4-(4-fluorophenyl)-5-
[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone (220 mg, 0.476 mmol),
methoxylamine hydrochloride (318 mg, 3.8 mmol), and sodium acetate (518 mg,
3.8 mmol) in methanol (100 mL) was stirred at reflux for 48 hours. The
reaction
mixture was concentrated in vacuo , and the residue was partitioned between
ethyl
acetate and saturated aqueous ammonium chloride. The organic layer was
washed with brine then dried over MgSO4, and filtered. The filtrate was
concentrated in vacuo to provide a brown oil which was purified by column
chromatography (silica gel, 70:30 hexanes/ethyl acetate). Fractions containing
product were combined and concentrated in vacuo . The residue was crystallized
-233-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
from methanoVwater to provide the title compound as a mixture of E and Z
oximes
(yield: 82 mg, 35%). M.p. 95-99 C. 1 H NMR (300 MHz, CDCI3) 8 3.03 (s, 3H),
4.07 (s, 3H), 5.57 (s, 2H), 6.94 (t, J = 9 Hz, 2H), 7.07 (dd, J = 9 Hz, 3 Hz,
2H), 7.24
(d, J = 9 Hz, 2H), 7.31-7.37 (m, 3H), 7.60-7.67 (m, 2H), 7.74 (s, 1 H), 7.83
(d, J = 9
Hz, 2H). MS (DCI-NH3) m/z 492 (M+H)+. Anal. caic. for C26H22FN304S: C,
63.53; H, 4.51; N, 8.54. Found: C, 63.40; H, 4.51; N, 8.31.
Example 444
2-(3.4-Difluoroohenvl)-4-(4-methytpentyl)-5-[3-fluoro-4-(methvlsulfony,]
nhenvll-
3 (ZH)-Ryridazinone
The title compound was prepared according to the method of Example 255,
substituting 1-bromo-4-methylpentane in place of 3,4-difluorobenzyl bromide
(yield:
145 mg, 58%). M.p. 111-113 C. 1 H NMR (300 MHz, DMSO-d6) fi 0.75 (d, 6H),
1.09 (m, 2H), 1.4 (m, 3H), 2.48 (m, 2H), 3.4 (s, 3H), 7.61 (m, 2H), 7.75 (d,
2H), 7.81
(m, 1 H), 8.02 (s, 1 H), 8.1 (d, 2H). MS (DCI-NH3) m/z 447 (M+H)+, 464
(M+NH4)+.
Anal. caic. for C23H24F2N203S: C, 61.87; H, 5.42; N, 6.27. Found: C, 61.76; H,
5.55; N, 6.11.
Example 445
2-(3.4-Difluoroohenvl)-4-(3-methvl-l-butoxy)-5-[4-(aminosulfonyl)phenyl]-3(2EL-
~yridazinone
The title compound was prepared as described in Example 384,-substituting
2-(3 ,4-difluo rophenyl)-4-(3-methyl-1-butoxy)-5-[4-(methylsulfonyl)phenyl]-
3(2 H)-
pyridazinone (Example 347) in place of 2-benzyl-4-(4-fluorophenyl)-5-[4-
(methylsulfonyl)phenyl]-3(2H)-pyridazinone (yield: 248 mg, 42%). M.p. 149-151
C. 1 H NMR (300 MHz, DMSO-d6) S 0.8 (d, J = 6 Hz, 6H), 1.48 (m, 2H), 1.54 (m,
1 H), 4.4 (t, 2H), 7.51 (m, 3H), 7.6 (m, 1 H), 7.85 (m, 3H), 7.95 (d, J = 9
Hz, 2H), 8.21
(s, 1 H). MS (DCI-NH3) m/z 450 (M+H)+, 467 (M+NH4)+. Anal. caic. for
C21 H21 F2N304S: C, 56.12; H, 4.71; N, 9.35. Found, C, 56.12; H, 4.67; N,
9.15.
-234-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 446
? (2.2.2-Trifluoroethyl)-4-(2.2-dimethylnro~axy)-5-[4-(aminosulfonyl)nhenylj-
3(2H)-
pyridazinone
The intermediate, 2-(2,2,2-trifluoroethyl)-4-hydroxy-5-[4-(methylthio)phenyl]-
3(2H)-pyridazinone prepared in Example 90C was reacted with 2,2-dimethyl-
propanol to provide 2-(2,2,2-trifluoroethyl)-4-(2,2-dimethylpropoxy)-5-[4-
(methylthio)phenyl]-3(2H)-pyridazinone according to the method of Example.
90D.
The product was oxidized with one equivalent of meta-chloroperoxybenzoic acid
to
provide the methyl sulfoxide. The sulfoxide was converted to the title
compound
according to the method of Example 68, substituting 2-(2,2,2-trifluoroethyl)-4-
(2,2-
dimethylpropoxy)-5-[4-(methylsulfinyl)phenyl]-3(2H)-pyridazinone for 2-(2,2,2-
trifluoroethyl)-4-(4-fluorophenyl)-5-[4-(methylsulfinyl)phenyl]-3(2H)-
pyridazinone
(yield: 125 mg, 53%). M.p. 123-124 C. 1 H NMR (300 MHz, CDCI3) S 0.82 (s,
9H),
4.18 (s, 2H), 4.82 (q, J = 9 Hz, 2H), 4.84 (s, 2H), 7.70 (d, J = 9 Hz, 2H),
7.81 (s, 1 H),
8.04 (d, J = 9 Hz, 2H). MS (DCI-NH3) m/z 420 (M+H)+. Anal. caic. for
C17H20F3N304S: C, 48.68; H, 4.80; N, 10.01. Found: C, 48.76; H, 4.77; N, 9.94.
Example 447
2-(2.2.2-Trifluoroethyl)-4-(3-methvlbutoxy)-5-14-(meth Isy ulfonyõl)ohenyl]-
3(2H)m
pyridazinone
The title compound was prepared according to the method of Example 83,
substituting 3-methyl-l-butanol in place of isopropanol (yield: 65 mg, 85%).
M.p.
111-113 C. 1 H NMR (300 MHz, CDCI3) 6 0.84 (d, J = 6 Hz, 6H), 1.51 (m, 2H),
1.63
(m, 1 H), 3.11 (s, 3H), 4.54 (t, J = 6 Hz, 2H), 4.83 (q, J = 9 Hz, 2H), 7.73
(d, J = 9 Hz,
2H), 7.82 (s, 1 H), 8.05 (d, J = 9 Hz, 2H); MS (DCI-NH3) m/z 419 (M+H)+. Anal.
caic. for C18H21 F3N204S: C, 51.66; H, 5.05; N, 6.69. Found: C, 51.91; H,
5.06; N,
6.56.
-235-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 448
2-(2.2.2-Trifluoroethyj)-4-(3-methy butoxy)-5-[4-(aminosulfonyj,,, henY[j-
3(2H)-
Ryridazinone
The intermediate, 2-(2,2,2-trifluoroethyl)-4-hydroxy-5-[4-(methylthio)phenyl]-
'3(2H)-pyridazinone prepared in Example 90C was reacted with 3-methyl-l-
butanol
to provide 2-(2,2,2-trifiuoroethyl)-4-(3-methylbutoxy)-5-[4-
(methylthio)phenyl]-
3(2H)-pyridazinone according to the method of Example 90D. The product was
oxidized with one equivalent of meta-chloroperoxybenzoic acid to provide the
methyl sulfoxide. The sulfoxide was converted to the title compound according
to
the method of Example 68, substituting 2-(2,2,2-trifluoroethyl)-4-(3-
methylbutoxy)-5-
[4-(methylsulfinyl)phenyl]-3(2H)-pyridazinone for 2-(2,2,2-trifluoroethyl)-4-
(4-
fluorophenyl)-5-[4-(methylsulfinyl)phenyl]-3(2H)-pyridazinone (yield: 65 mg,
50%).
M.p. 123-124 C. 1 H NMR (300 MHz, CDCI3) S 0.84 (d, J = 6 Hz, 6H), 1.52 (q, J
= 6
Hz, 2H), 1.60 (h, J = 7.5 Hz, 1 H), 4.52 (t, J = 6 Hz, 2H), 4.83 (q, J = 9 Hz,
2H), 4.90
(s, 2H), 7.69 (d, J = 9 Hz, 2H), 7.82 (s, 1 H), 8.04 (d, J = 9 Hz, 2H). MS
(DCI-NH3)
m/z 420 (M+H)+. Anal. calc. for C17H2OF3N304S: C, 48.68; H, 4.80; N, 10.01.
Found: C, 48.86; H, 4.83; N, 9.92.
Example 449
2-(2 2 2-Trifluoroethyl)-4-(2-meth,klpropoxy)-5-[4-(aminosulfony!)phenvl]-
3(2H)-
pyridazinone
The intermediate, 2-(2,2,2-trifluoroethyl)-4-hydroxy-5-[4-(methylthio)phenyl]-
3(2H)-pyridazinone prepared in Example 90C was reacted with 2-methyl-l-
propanol to provide 2-(2,2,2-trifluoroethyl)-4-(2-methylpropoxy)-5-[4-
(methylthio)-
phenyl]-3(2H)-pyridazinone according to the method of Example 90D. The product
was oxidized with one equivalent of meta-chloroperoxybenzoic acid to provide
the
methyl sulfoxide. The sulfoxide was converted to the title compound according
to
the method of Example 68, substituting 2-(2,2,2-trifluoroethyl)-4-(2-
methylpropoxy)-
5-[4-(methylsulfinyl)phenyl]-3(2H)-pyridazinone for 2-(2,2,2-trifluoroethyl)-4-
(4-
fluorophenyl)-5-[4-(methylsulfinyl)phenyl]-3(2H)-pyridazinone (yield: 120 mg,
40%).
M.p. 170-172 C. 1 H NMR (300 MHz, CDCI3) 5 0.83 (d, J = 6 Hz, 6H), 1.9 (m, 1
H),
4.3 (m, 2H), 4.82 (s, 2H), 4.88 (m, 2H), 7.70 (d, J = 9 Hz, 2H), 7.79 (s, 1
H), 8.03 (d, J
= 9 Hz, 2H); MS (DCI-NH3) m/z 406 (M+H)+. Anal. caic. for C16H18F3N304S: C,
47.4; H, 4.47; N, 10.36. Found: C, 47.48; H, 4.36; N, 10.25.
-236-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 450
2-(2.3.3-Trifluoro ro2enyl)-4-(4-fluorophenyl)-5-[4-(aminosulfonyl)nhenyl]-
3(2H)-
Ryridazinone
The product of Example 4, 2-benzyl-4-(4-fluorophenyl)-5-[4-
(methylthio)phenyl]-3(2H)-pyridazinone, was N-debenzylated by the method of
Example 11 to provide 4-(4-fluorophenyl)-5-[4-(methylthio)phenyl]-3(2H)-
pyridazinone. The intermediate was mixed with one equivalent of 1-
methylsufonyl-
oxy-2,3,3-trifluoro-2-propene, (Example 88A) in ethyl acetate, followed by one
equivalent of cesium carbonate. The reaction mixture was heated to 50 C for 5
hours. Aqueous work-up, followed by chromatography provided 2-(2,3,3-trifluoro-
propenyl)-4-(4-fluorophenyl)-5-[4-(methylthio)phenyl]-3(2H)-pyridazinone (650
mg,
63%). The product was oxidized with one equivalent of meta-chloroperoxybenzoic
acid to provide the methyl sulfoxide which was converted to the title compound
according to the method of Example 68, substituting 2-(2,3,3-
trifluoropropenyl)-4-
(4-fluorophenyl)-5-[4-(methylsulfinyl)phenyl]-3(2H)-pyridazinone for 2-(2,2,2-
trifluoroethyl)-4-(4-fluorophenyl)-5-[4-(methylsulfinyl)phenyl]-3(2H)-
pyridazinone
(yield: 65 mg, 35%). M.p. 190-193 C. 1 H NMR (300 MHz, CDCI3) S 5.07 (s, 2H),
5.10 (dt, J = 21 Hz, J = 3 Hz, 2H), 7.05 (m, 4H), 7.19 (dd, J = 9 Hz, J = 6
Hz, 2H),
7.84 (s, 1 H), 7.87 (t, J = 7.5 Hz, 1 H). MS (ESI-NH3) m/z 456 (M-H)+. Anal.
caic. for
C1 gH12F5N303S: C, 49.89; H, 2.64; N, 9.18. Found: C, 49.89; H, 2.73; N, 9.03.
Example 451
2-(4-Fluoronhenvl)-4-(3-hydroxy-3-met I-1-butox,y)-5;[4-(methylsulfonyl)phewl
3 (2 H)-Ryridazinone
The title compound was prepared according to the method of Example 178,
substituting 3-methyl-1,3-butandiol in place of 2-ethyl-1-hexanol (yield: 110
mg,
61 %), M.p. 133-134 C. 1H NMR (300 MHz, DMSO-d6) S 1.04 (s, 6H), 1.72 (t, J=
7
Hz, 2H), 3.29 (s, 3H), 4.32 (s, 1 H), 4.53 (t, J = 7 Hz, 2H), 7.37 (t, J = 9
Hz, 2H), 7.66
(m, 2H), 7.90 (d, J= 9 Hz, 2H), 8.07 (d, J= 9 Hz, 2H), 8.19 (s, 1 H). MS
(APCI+) m/z
447 (M+H)+; (APCI-) m/z 481 (M+CI)-. Anal. calc. for C22H23FN205S=0.25 H20:
C, 58.59; H, 5.25; N, 6.21. Found: C, 58.42; H, 5.00; N, 6.02.
-237-
CA 02578858 2007-03-05
WO 99/10331 PCTIUS98/16479
Example 452
2-(3.4-DifluorophepY1)-4-(2-hydroxy-2-methyl-1-nroDOXV)-544
(methylsulfon}l)phenyl]-3(2 H)-Ryridazinone
The title compound was prepared according to the method of Example 178,
starting with 2-(3,4-difluorophenyl)-4-tosyloxy-5-[4-(methylsulfonyl)phenyl]-
3(2H)-
pyridazinone in place of 2-(4-fluorophenyl)-4-tosytoxy-5-[4-
(methylsulfonyl)phenyl]-
3(2H)-pyridazinone and substituting 2-methyl-1,2-propandiol in place of 2-
ethyl-l-
hexanol (yield: 55 mg, 31 %). 1 H NMR (300 MHz, DMSO-d6) S 0.97 (s, 6H), 3.30
(s,
3H), 4.20 (s, 2H), 4.54 (s, 1 H), 7.52 (m, 1 H), 7.62 (m, 1 H), 7.81 (m, 1 H),
7.98 (d, J =
9 Hz, 2H), 8.05 (d, J = 9 Hz, 2H), 8.21 (s, 1 H). MS (APCI+) m/z 451 (M+H)+;
(APCI-)
m/z 485 (M+CI)-. Anal. calc. for C21 H20F2N205S: C, 55.99; H, 4.47; N, 6.21.
Found: C, 56.00; H, 4.48; N, 5.87.
uExample 453
2-(3.4-DifluoronhenY1)-4-methoxy-5-[4-(methvlsulfonyJ)lahgpyll-3 (2H)-
pyridazinone
The title compound was isolated from the reaction mixture in Example 233,
as a product of oxidation of unreacted starting material (yield: 22 mg, 8%).
M.p.
113-115 C. 1 H NMR (300 MHz, DMSO-d6) S 3.3 (s, 3H), 4.1 (s, 3H), 7.53 (m, 1
H),
7.63 (m, 1 H), 7.8 (m, 1 H), 8.15 (d, 2H), 8.2 (s, 2H). MS (DCI-NH3) m/z 393
(M+H)+,
410 (M+NH4)+. Anal. caic. for C18H14F2N204S: C, 55.10; H, 3.60; N, 7.14.
Example 454
2-(2.3.4.5.6-Pentafluorobenzyl)-4-(4-fluoro ,Dhenyl)-5-[4-[(di methylaminQ)-
methvlene]aminosulfonyll2jjgIlyl]-3 (2 H[)-Ryridazinone
The title compound was isolated from the reaction mixture in Example 125,
as a product resulting from a reaction with the solvent, N,N-dimethylformamide
(yield: 53 mg, 16%). M.p. 194-196 C. 1 H NMR (300 MHz, CDCI3) S 3.05 (s, 3H),
3.17 (s, 3H), 5.49 (s, 2H), 6.97 (t, J = 9 Hz, 2H), 7.18 (dd, J = 9 Hz, 6 Hz,
2H), 7.20
(d, J = 9 Hz, 2H), 7.81 (s, 1 H), 7.82 (d, J = 9 Hz, 2H), 8.14 (s, 1 H). MS
(DCI-NH3)
m/z 581 (M+H)+. Anal. caic. for C26H18F6N4O3S: C, 53.79; H, 3.12; N, 9.65.
Found: C, 53.50; H, 3.24; N, 9.56.
-238-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 455
2-(2.4-Difluorobenzy 1)-4-(4-fluoronhenyl)-5-(4-((dimethylamino methyle
nel-
am e nyj]--312 H)-Ryridazinone
The title compound was isolated from the reaction mixture in Example 124,
as a product resulting from a reaction with the solvent, N,N-dimethylformamide
(yield: 55 mg, 18%). M.p. 193-195 C. 1 H NMR (300 MHz, CDC13) S 3.03 (s, 3H),
3.16 (s, 3H), 5.43 (s, 2H), 6.88 (m, 2H), 6.95 (t, J = 9 Hz, 2H), 7.18 (dd, J
= 9 Hz, 6
Hz, 2H), 7.20 (d, J = 9 Hz, 2H), 7.52 (m, 1 H), 7.81 (d, J = 9 Hz, 2H), 7.84
(s, 1 H),
8.13 (s, 1 H). MS (DCI-NH3) m/z 527 (M+H)+. Anal. calc. for C26H21 F3N4O3S: C,
59.30; H, 4.02; N, 10.64. Found: C, 59.08; H, 3.97; N, 10.48.
Example 456
2-(4-FluoroRhenyl)-5-j4-(methvlselenortyl)phenylJ-3(2H)-pyridazinnnP
446A. 4-Bromoselenoanisole
Freshly crushed magnesium turnings (6.1 g, 0.25 mol) were suspended with
vigorous stirring in a solution of diethyl ether (360 mL) and 1,4-
dibromobenzene
(10 g, 0.04 mol). The solution was brought to reflux for 30 minutes, without
initiation. Several crystals of iodine were added which initiated the reaction
to a
self-sustained reflux. The reflux was maintained as the remainder of the 1,4-
dibromobenzene (49 g, 0.21 mol) was slowly added. The reaction was refluxed
for
an additional 2 hours after addition of the 1,4-dibromobenzene was completed.
When nearly all of the magnesium turnings had been consumed, the yellow/gray
heterogeneous solution was cooled to 23 C, and selenium (19 g, 0.24 mol) was
added in small portions via spatula so as to maintain a gentle reflux. The
selenium
that became stuck to the sides of the flask was washed iri with additional
diethyl
ether. After addition, the solution was stirred for 20 minutes at 23 C and
then was
cooled to 0 C. A diethyl ether (20 mL) solution of methyl iodide (35.5 g, 0.25
mol)
was slowly added dropwise to the reaction mixture. Upon completion of
addition,
the cooling bath was removed, and the solution stirred for 3 hours at 23 C.
The
reaction solution was slowly poured into ice water/1 M HCI , and then the
biphasic
solution filtered through a glass wool plug. The ethereal layer was separated
and
the aqueous phase extracted twice more with diethyl ether. The combined
ethereal
extracts were dried over MgSO4, filtered, and concentrated in vacuo to provide
a
semi-viscous orange oil. On standing overnight at -20 C, large yellow needles
formed. The residual oil was drawn off via pipette to provide 17 g (27%) of
-239-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
crystalline product. (J. Org. Chem., 1983, 48, 4169) 1 H NMR (300 MHz, CDC13)
S
2.46 (s, 3H), 7.12 (d, J = 8.7 Hz, 2H), 7.39 (d, J = 8.7 Hz, 2H). MS (APCI+)
m/z 248
(Se76 M+H)+, m/z 250 (Se78 M+H)+, m/z 252 (Se80 M+H)+, and m/z 254 (Se82
M+H)+.
446B. 2.4-Bis(4-fluorouhenyl)-5-14-(methylseleno)nheny,j)-3(2 H)-pyridazinnne
The title compound was prepared according to the method of Example 228,
substituting 2-(4-fluorophenyl)-4-methoxy-5-[4-(methylseleno)phenyl]-3(2H)-
pyridazinone [prepared according to the method of Example 194C, substituting
4-(methylseleno)benzeneboronic acid, (prepared according to the method of
Example 1, substituting 4-bromoselenoanisole in place of 4-bromothioanisole)
in
place of 4-(methylthio)benzeneboronic acid] in place of 2-(4-fluorophenyi)-4-
methoxy-5-[4-(methylthio)phenyl]-3(2H)-pyridazinone and substituting 4-
fluorophenyl magnesium bromide in place of cyclohexylmagnesium chloride
(yield:
44 mg, 69%). 1 H NMR (300 MHz, CDCI3) S 2.37 (s, 3H), 6.98 (dd, J= 8.8, 8.8
Hz,
2H), 7.05 (d, J = 8.7 Hz, 2H), 7.17 (dd, J = 8.7, 8.7 Hz, 2H), 7.23-7.31 (m,
2H), 7.32
(d, J = 8.7 Hz, 2H), 7.65-7.72 (m, 2H), 8.00 (s, 1 H). MS (APCI+) m/z 455
(M+H)+.
446C. 2.4-Bis(4-fiuoroohenyl)-5-[4-(methy SPf nor1Yl)o henvll-3 (2 H)-
Ryridazinone
A stirred solution of the 2,4-bis(4-fluorophenyl)-4-(4-fluorophenyl)-5-[4-
(methylseleno)phenyl]-3(2H)-pyridazinone (40 mg, 88.1 mmol) in methylene
chloride (2 mL) was treated with 3-chloroperoxybenzoic acid (100 mg, 342 mmol,
57-86%) at 23 C. After 2 hours, the reaction appeared to be only slightly
more
than 50% completed. Additional 3-chioroperoxybenzoic acid (80 mg, 274 mmol,
57-86%) was added. The reaction ran to completion over the next 16 hours of
stirring at 23 C. The solution was diluted with ethyl acetate and carefully
shaken
with a NaHSO3 solution (two times) for several minutes to consume the excess 3-
chloroperoxybenzoic acid. The ethyl acetate solution was subsequently washed
with a saturated Na2CO3 solution (two times), water, and brine and dried over
MgSO4, filtered, and concentrated in vacuo. The residue was chromatographed
(flash silica gel, acetone/methylene chloride/hexanes 2:2:1) to provide the
product
(yield: 40 mg, 93%). (J. Chem. Soc., Chem. Commun., 1985, 569). M.p. 110-150
C. 1 H NMR (300 MHz, CDC13) b 3.32 (s, 3H), 6.91 (dd, J = 8.7, 8.7 Hz, 2H),
7.14-
7.27 (m, 4H), 7.48 (d, J = 8.4 Hz, 2H), 7.65-7.73 (m, 2H), 7.97 (s, 1 H), 8.00
(d, J =
8.4 Hz, 2H). MS (APCI+) m/z 487 (M+H)+ and m/z 504 (M+NH4)+. Anal. caic. for
-240-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
C23H16F2N2O3Se=0.5 H20: C, 55.88; H, 3.46; N, 5.66. Found: C, 55.60; H, 3.61;
N, 5.29.
Example 457
2-(3.4-Difluoroohenyl)-4-(3-fluorophenY1)-5-[4-(meth suIfnnyl) h~enyl,l 3(2H)-
Ryridazinone
The title compound was prepared as described in Example 62, starting with
4-(3-fluorophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone in place of
4-(4-
fluorophenyl)-5-[4-(methylsutfonyl)phenyl]-3(2H)-pyridazinone and substituting
3,4-
difluorobromobenzene in place of 1-bromo-4-fluorobenzene (yield: 185 mg,
46.5%). M.p. 182-185 C. 1 H NMR (300 MHz, DMSO-d6) S 3.23 (s, 3 H), 6.98 (d,
J
= 9 Hz, 1 H), 7.18 (m, 2H), 7.32 (m, 1 H), 7.52 (d, J = 9 Hz, 2 H), 7.6 (m,
2H), 7.85 (m,
1 H), 7.9 (d, J = 9 Hz, 2H), 8.3 (s, 1 H). MS (DCI-NH3) m/z 457 (M+H)+, 474
(M+NH4)+.
Example 458
2-(4-Fluoroohenvl)-4-(3-fluorophepy1)-5-[4-(mg~jt y su yj)phenY1]-3(2H)-
pvridazinone
The title compound was prepared as described in Example 62, substituting
4-(3-fluorophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone in place of
4-(4-
fluorophenyl)-5-[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone (yield: 135 mg,
34%). M.p. 199-201 C. 1 H NMR (300 MHz, DMSO-d6) 5 3.24 (s, 3H), 6.98 (d, J
9 Hz, 1 H), 7.18 (m, 2H), 7.32 (m, 1 H), 7.39 (t, 1 H), 7.54 (d, J = 9 Hz, 2
H), 7.71 (m,
2H), 7.91 (d, J = 9 Hz, 2 H), 8.27 (s, 1 H). MS (DCI-NH3) m/z 439 (M+H)+, 456
(M+NH4)+.
-241-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 459
2; (3.4-Difluoropheny,l)-4-(2-jyslroxy- -methvlRropoxy)-5-[4-(aminosulfonvl)-
Rhp,py1L-3(2 H)-Ryridazinone
2-(3,4-Difluorophenyl)-4-(2-hydroxy-2-methytpropoxy)-5-[4-(methylsulfonyl)-
phenyl]-3(2H)-pyridazinone (Example 452) is converted to the title sulfonamide
according to the method of Example 384.
Example 460
2-(3.4-Difluoronhenyl)-4-12-oxo-l-gro o~xyl-5-[4-(methvls~yl)phenvl)-3(2Fil
pyridazinone
A solution of 2-(3,4-difluorophenyl)-4-hydroxy-5-[4-(methyisulfonyl)phenyl]-
3(2H)-pyridazinone (378 mg, 1 mmol), Ph3P (524 mg, 2 mmol) and acetol (74 mg,
1 mmol) in THF (25 mL) at room temperature was treated dropwise with a
solution
of DIAD (0.4 mL, 2 mmol) in THF (5 mL). The mixture was stirred at room
temperature for 6 hours and concentrated in vacuo. The residue was
chromatographed (silica gel, 1:1 hexanes-ethyl acetate) to provide the desired
product (yield: 205 mg, 48%). M.p. 169-170 C. 1 H NMR (300 MHz, DMSO-d6) 5
2.08 (s, 3H), 3.30 (s, 3H), 5.30 (s, 2H), 7.48 (m, 1 H), 7.62 (q, J = 10 Hz, 1
H), 7.75 (m,
1 H), 7.94 (d, J = 9 Hz, 2H), 8.05 (d, J = 9 Hz, 2H), 8.21 (s, 1 H). MS
(APCI+) m/z 435
(M+H)+, (APCI-) m/z 469 (M+CI)-. Anal. calc. for C20H1 6F2N205S=0.75H20: C,
53.62; H, 3.93; N, 6.25. Found: C, 53.26; H, 3.61; N, 6.08.
Example 461
2-(3.4DifluorophenY1)-4-j2-(methoxyjniino)propoxXJ-5-[4-(methy su yJ) nvll-
3(2H)-nvridazinone
A mixture of 2-(3,4-difluorophenyl)-4-(2-oxo-l-propoxy)-5-[4-
(methylsulfonyl)phenyl]-3(2H)-pyridazinone from Example 460 (150 mg, 0.3 mmol)
in H20 (10 mL) and dioxane (20 mL) was treated with methoxylamine
-242-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
hydrochloride (84 mg, 1 mmol) and sodium acetate trihydrate (138 mg, 1 mmol).
The mixture was stirred at room temperature for 6 hours. The reaction mixture
was
extracted with ethyl acetate and purified by column chromatography (silica
gel, 1:1
hexanes-ethyl acetate) to provide the title compound (yield: 20 mg, 15%). M.p.
143-145 C. 1 H NMR (300 MHz, DMSO-d6) 5 1.63 (s, 3H), 3.30 (s, 3H), 3.74 (s,
3H), 4.93 (s, 2H), 7.54 (m, 1 H), 7.65 (q, J = 10 Hz, 1 H), 7.82 (m, 1 H),
7.92 (d, J = 9
Hz, 2H), 8.07 (d, J = 9 Hz, 2H), 8.24 (s, 1 H). MS (APCI+) m/z 464 (M+H)+;
(APCI-)
m/z 498 (M+CI)-. Anal. calc. for C21 H1 9F2N305S: C, 54.42; H, 4.13; N, 9.06.
Found: C, 54.33; H, 3.93; N, 8.92.
Example 462
(S)-2-(3.4-DifluoroDhenyl)-4-(3-hydroxy-2-methyjp rQpoxx)-5-j4-(methyl-
sulfonyl)phenyll-3(2H)-pyridazinnne
462A (R -3->rButoxy-2-methyl-l-DfoDanal
A solution of (S)-(+)-methyl 3-hydroxy-2-methylpropionate (1.18 g, 10 mmol)
in t-butyl acetate (30 mL) was treated with 70% HCIO4 (0.1 mL), and the
reaction
mixture was left at room temperature in a tighthly closed flask for 24 hours.
The
mixture was poured into a saturated solution of sodium bicarbonate and
extracted
with diethyl ether. The ether was removed in vacuo and the residue was
dissolved
in THF (50 mL). To the resulting solution was added sodium borohydride (925
mg,
mmol) and at 55 C dropwise methanol (10 mL). The reaction was continued at
55 C for 1 hour, then cooled to room temperature, acidified with 10% citric
acid to
pH 5 and extracted with ethyl acetate. The acetate extract was washed with
water,
25 brine, dried over MgSO4 and concentrated in vacuo. The residue was
chromatographed (silica gel, 2:1 hexanes-ethyl acetate) to provide (R)-3-t-
butoxy-2-
methyl-1-propanol (yield: 1 g, 68%). 1 H NMR (300 MHz, CDC13) S 0.85 (d, J = 7
Hz, 3H), 1.20 (s, 9H), 2.03 (m, 1 H), 3.30 (t, J = 12 Hz, 1 H), 3.53 (dd, J =
12 Hz, 4.5
Hz, 1 H), 3.70 (m, 2H). MS (DCI-NH3) m/z 164 (M+NH4)+.
462B (S)-2-(3.4-DifluoroQhenyl)-4-(3-t-butox - -mP ylprol2oxy)-5-[4-
(methvlsul honyl)pheny1]-3(2H)-Ryridazinone
To a solution 2-(3,4-difluorophenyl)-4-hydroxy-5-[4-(methylsuffonyl)phenyl]-
3(2H)-pyridazinone (378 mg, 1 mmol), Ph3P (524 mg, 2 mmol) and the above
-243-
CA 02578858 2007-03-05
WO 99/10331 PCTlUS98/16479
alcohol, (R)-3-t-butoxy-2-methyl-l-propanol (146 mg, 1 mmol) in THF (25 mL) at
room temperature was added dropwise a solution of DIAD (0.4 mL, 2 mmol) in THF
(5 mL). The mixture was then stirred at room temperature for 6 hours and
concentrated in vacuo. The residue was passed through a silica gel pad
(hexanes-
ethyl acetate as an eluent) to provide 550 mg of roughly purified (S)-2-(3,4-
difluorophenyl)-4-(3-t-butoxy-2-methyipropoxy)-5-[4-(methylsulphonyl)phenyl]-
3(2H)-pyridazinone, still contaminated with reduced DIAD. MS (APCI+) m/z 507
(M+H)+; (APCI-) m/z 541 (M+CI)-.
462C (S)-2-(3.4-Difluorophenyl)-4-(3-hydroxy-2-methyl~ro~oxy)-5-j4-(methyl-
suifonyl ohenyll 3(2 H)-p,vridazinone
A mixture of the above product (100 mg, -0.2 mmol) in TFA (5 mL) was
stirred at room temperature for 24 hours and then concentrated in vacuo. The
residue was neutralized with saturated NaHCO3 and extracted with ethyl
acetate.
Purification by column chromatography (silica gel, 1:2 hexanes-ethyl acetate)
provided the title compound (yield: 51 mg, 56%). 1 H NMR (300 MHz, DMSO-d6) 5
0.75 (d, J = 7 Hz, 3H), 1.81 (septet, J = 7 Hz, 1 H), 3.21 (d, J = 6 Hz, 2H),
3.30 (s,
3H), 4.29 (dd, J = 12 Hz, 6 Hz, 1 H), 4.40 (dd, J = 12 Hz, 6 Hz, 1 H), 4.48
(br s, 1 H),
7.52 (m, 1 H), 7.61 (m, 1 H), 7.80 (m, 1 H), 7.91 (d, J = 9 Hz, 2H), 8.07 (d,
J = 9 Hz,
2H), 8.20 (s, 1 H). MS (APCI+) m/z 451 (M+H)+; (APCI-) m/z 485 (M+CI)-. Anal.
calc. for C21 H20F2N205S: C, 55.99; H, 4.47; N, 6.21. Found: C, 55.65; H,
4.65; N,
5.92.
Example 463
(R)-2-(3.4-Difluoronheny1)-4-(3-hydroxy- -m thylpropQxy)-5-[4-(meth sulfnnvll-
}Zhe nvl)pvridazi no ne
The desired material was prepared according to the procedure of Example
462 starting with (R)-(-)-methyl 3-hydroxy-2-methylpropionate in place of (S)-
(-)-
methyl 3-hydroxy-2-methytpropionate (yield: 65 mg, 61 %). 1 H NMR (300 MHz,
DMSO-d6) 8 0.75 (d, J = 7 Hz, 3H), 1.81 (septet, J = 7 Hz, 1 H), 3.21 (t, J =
6 Hz, 2H),
3.30 (s, 3H), 4.29 (dd, J = 6 Hz and 12 Hz, 1 H), 4.40 (dd, J = 6 Hz and 12
Hz, 1 H),
4.49 (t, J = 6 Hz, 1 H), 7.52 (m, 1 H), 7.61 (m, 1 H), 7.80 (m, 1 H), 7.91 (d,
J = 9 Hz,
2H), 8.07 (d, J = 9 Hz, 2H), 8.20 (s, 1 H). MS (APCI+) m/z 451 (M+H)+; (APCI-)
m/z
-244-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/1 b479
485 (M+CI)-. Anal. calc. for C21 H20F2N205S: C, 55.99; H, 4.47; N, 6.21.
Found:
C, 55.62; H, 4.52; N, 6.06.
Example 464
(S)-2-(3.4-Difluoroohenyl)-4;(3-hydroxv-2-methyl 2Roxy)-5-j4-(aminosulfonyl)-
henylJ-3(2H)-pvridazinone
To a solution of (S)-2-(3,4-difluorophenyl)-4-(3-hydroxy-2-methylpropoxy)-5-
[4-(methylsulfonyl)phenyl]-3(2H)-pyridazinone from Example 462 (450 mg, -0.9
mmol) and DBAD (207 mg, 0.9 mmol) in THF (25 mL) at -78 C was added
dropwise 1 M lithium bis(trimethylsilyl)amide solution in THF (3 mL,.3 mmol).
The
resulting mixture was stirred at -78 C for 2 hours. The mixture was warmed to
room temperature and 1 N NaOH was added (5 mL, 5 mmol). After 12 hours, at
room temperature, sodium acetate trihydrate (2.76 g, 20 mmol) and H20 (10 mL)
followed by hydroxylamine-O-sulphonic acid (2 g, 15 mmol) were added and the
mixture was stirred at room temperature for 5 hours. The product was extracted
with ethyl acetate and purified by chromatography (silica gel, 1:2 hexanes-
ethyl
acetate) to provide the desired intermediate (yield: 160 mg, 35%). MS (APCI+)
m/z
508 (M+H)+; (APCI-) m/z 542 (M+CI)-.
TFA (5 mL) was added to the above intermediate and the resulting solution
was stirred at room temperature for 24 hours. The TFA was removed in vacuo,
and
the residue was neutralized with saturated NaHCO3 and extracted with ethyl
acetate. The organic extract was dried over MgSO4 and filtered. The filtrate
was
concentrated in vacuo and the residue was chromatographed (silica gel, 1:2
hexanes-ethyl acetate) to provide the title compound (yield: 50 mg, 33%). 1 H
NMR
(300 MHz, DMSO-d6) 8 0.76 (d, J = 7 Hz, 3H), 1.81 (sextet, J = 7 Hz, 1 H),
3.22 (t, J
6 Hz, 2H), 4.28 (dd, J = 12 Hz, 6 Hz, 1 H), 4.40 (dd, J = 12 Hz, 6 Hz, 1 H),
4.50 (t, J =
6 Hz, 1 H), 7.51 (m, 3H), 7.61 (m, 1 H), 7.80 (m, 1 H), 7.84 (d, J = 9 Hz,
2H), 7.95 (d, J
= 9 Hz, 2H), 8.20 (s, 1 H). MS (APCI+) m/z 452 (M+H)+; (APCI-) m/z 486 (M+CI)-
.
-245-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
Example 465
(R)-2-(3 4-Difluoroo, henvj);4-(3-hydrox_v-2-methyJRroo~xv)-5-[4-(ami
osulfonvl)-
phenyl]-3(2H)-Ryridazinone
The title compound was prepared according to the procedure of Example
464 starting with (R)-2-(3,4-difiuorophenyl)-4-(3-hydroxy-2-methylpropoxy)-5-
[4-
(methylsulfonyl)phenyl]-3(2H)-pyridazinone in place of (S)-2-(3,4-
difluorophenyl)-
4-(3-hydroxy-2-methylpropoxy)-5-[4-(methylsulfonyl)phenyl]-3 (2H)-pyridazinone
(yield: 30 mg, 20%). 1 H NMR (300 MHz, DMSO-d6) S 0.76 (d, J = 7 Hz, 3H), 1.81
(sextet (J = 7 Hz, 1 H), 3.22 (t, J = 6 Hz, 2H), 4.28 (dd, J = 6 Hz and 12 Hz,
1 H), 4.40
(dd, J = 6 Hz and 12 Hz, 1 H), 4.50 (t, J= 6 Hz, 1 H), 7.51 (m, 3H), 7.61 (m,
1 H), 7.80
(m, 1 H), 7.84 (d, J = 9 Hz, 2H), 7.95 (d, J = 9 Hz, 2H), 8.20 (s, 1 H). MS
(APCI+) m/z
452 (M+H)+; (APCI-) m/z 486 (M+CI)-. Anal. caic. for C20H1 gF2N305S: C, 53.21;
H, 4.24; N, 9.30. Found: C, 53.45; H, 5.53; N, 9.50.
Example 466
2-(4-Fiuoroo, henyi)-4-(4-hydroxv-3-meth Iby utoxy)-5-[4-
(methylsuln_honyl)ohenylj-
3(2H)-pyridazinone.
The title compound was prepared according to the method of Example 178,
substituting 2-methyl-1,4-butanediol in place of 2-ethyl-l-hexanol and
separating
the regioisomeric products by preparative TLC using Silica Gel with ethyl
acetate:hexanes (4/1). 1 H NMR (300 MHz, CDCI3) S 0.87 (d, J = 8.1 Hz, 3H),
1.48-
1.87 (m, 4H), 3.13 (s, 3H), 3.41 (dd, J = 6.3, 13.5 Hz, 1 H), 3.46 (dd, J =
6.3, 13.5 Hz,
1 H), 4.48-4.63 (m, 2H), 7.15-7.24 (m, 2H), 7.58-7.66 (m, 2H), 7.79 (d, J=10.5
Hz,
2H), 7.91 (s, 1 H), 8.07 (d, J = 10.5 Hz, 2H). MS (APCI+) m/z 447 (M+H)+.
Example 467
213.4-difluoroohenvl)-4-(3-oxobutoxy)-5-j4-(methylsulfonyl)ohenxlJ-3(?H)-
Qytidazinone
The title compound is prepared according to the method of Example 460
f3substituting 4-hydroxy-2-butanone in place of acetol. (yield: 95.0 mg, 21
%). M.p.
134-135 C. 1 H NMR (300 MHz, CDCI3) S 2.06 (s, 3H), 2.81 (t, J = 9 Hz, 2H),
3.13
(s, 3H), 4.75 (t, J = 9 Hz, 2H), 7.30 (m, 1 H), 7.45 (m, 1 H), 7.58 (m, 1 H),
7.73 (d, J = 9
-246-
CA 02578858 2007-03-05
WO 99/10331 PG'T/US98/16479
Hz, 2H), 7.89 (s, 1 H), 8.05 (d, J= 9 Hz, 2H) . MS (DCI-NH3) m/z 449 (M+H)+,
466
(M+NH4)+. Anal. caic. for C21 H18F2N205S: C, 56.25; H, 4.02; N, 6.25. Found:
C,
55.97; H, 4.17; N, 6.11.
Example 468
2-(4-Fluoroohenvl(3-oxobutoxy)-5-[4-(methylsulfo I)phenyij-3(2H)-
Ryridazinone
The title compound is prepared according to the method of Example 460
starting with 2-(4-fluorophenyl)-4-hydroxy-5-[4-(methylsulfonyl)phenyl]-3(2H)-
pyridazinone in place of 2-(3,4-difluorophenyl)-4-hydroxy-5-[4-
(methylsulfonyi)-
phenylj-3(2H)-pyridazinone and substituting 4-hydroxy-2-butanone in place of
acetol. (yield: 85.0 mg, 20%). M.p. 133-136 C. 1 H NMR (300 MHz, CDC13) S
2.04
(s, 3H), 2.80 (t, J = 9 Hz, 2H), 3.13 (s, 3H), 4.76 (t, J = 9 Hz, 2H)., 7.20
(t, J = 9 Hz,
2H), 7.55 (m, 2H), 7.75 (d, J = 9 Hz, 2H), 7.91 (s, 1 H), 8.05 (d, J- 9 Hz,
2H) . MS
(DCI-NH3) m/z 431 (M+H)+, 448 (M+NH4)+. Anal. caic. for C21 H1 9FN205S: C,
58.60; H, 4.42; N, 6.52. Found: C, 58.87; H, 4.55; N, 6.51.
Prostaglandin Inhibition Determination
Compound Preparation and Administration
For oral administration, test compounds were suspended on the day of use
in 100% polyethyleneglycol (PEG 400) with a motorized homogenizer equipped
with a Teflon-coated pestle (TRI-R instrument, Jamaica, NY). Teflon is a trade-
mark
To compare the mean responses of the treatment groups, analysis of
variance was applied. Percent inhibition values were determined by comparing
the
individual treatment mean values to the mean of the control group. Linear
regression was used to estimate IC50'stED50's in appropriate assays.
EIA Determination of Prostaglandins
EIA reagents for prostaglandin determination were purchased from
Perseptive Diagnostics, (Cambridge, MA). Prostaglandin E2 (PGE2) levels in
lavage fluids were determined after the samples were dried under nitrogen and
-247-
CA 02578858 2007-03-05
WO 99/10331 PCT/US98/16479
reconstituted with assay buffer. PGE2 levels in enzyme assays or cell culture
media were measured against standards prepared in the same milieu. The
immunoassays were conducted as recommended by the manufacturer. The EIA
was conducted in 96 well microtiter plates (Nunc Roskilde, Denmark) and
optical
density was measured using a microplate reader (Vmax, Molecular Devices Corp.,
Menlo Park, CA).
Recombinant Human PGHS-1 and PGHS-2 Enzyme Assays
Inhibition of prostagiandin biosynthesis in vitro was evaluated using
recombinant human Cox-1 (r-hu Cox1) and Cox-2 (r-hu Cox2) enzyme assays.
Representative compounds dissolved in DMSO (3.3% v/v) were preincubated with
microsomes from recombinant human PGHS-1 or PGHS-2 expressed in the
baculovirus/Sf9 cell system (Gierse, J. K., Hauser,S. D., Creely, D. P.,
Koboldt, C.,
Rangwala, S., H., lsakson, P. C., and Seibert, K. Expression and selective
inhibition of the constituitive and inducible forms of cyclooxyg_nasP ,
Biochem J.
1995, 305: 479.), together with the cofactors phenol (2 mM) and hematin (1 M)
for
60 minutes prior to the addition of 10 M arachidonic acid. The reaction was
allowed to run for 2.5 minutes at room temperature prior to quenching with HCI
and
neutralization with NaOH. PGE2 production in the presence and absence of the
drug was determined by EIA analysis. The EIA was conducted in 96 well
microtiter
plates (Nunc Roskilde, Denmark) and optical density was measured using a
microplate reader (Vmax, Molecular Devices Corp., Menlo Park, CA). EIA
reagents
for prostaglandin determination were purchased from Perseptive Diagnostics
(Cambridge, MA). PGE2 levels were measured against standards prepared in the
same milieu. The immunoassays were conducted as recommended by the
manufacturer.
The data illustrating the inhibition of prostaglandin biosynthesis in vitro by
compounds of this invention is shown in Table 1. The compounds are designated
by the Example Number. Column 2 shows Cox-1 percent inhibition at the
particular
micromolar dose level and Column 3 shows Cox-2 percent inhibition at the
particular nanomolar dose level. Values for Cox-2 inhibition that are
parenthetical
indicate IC50 values.
SEE ATTACHED TABLE
-248-
CA 02578858 2007-03-05
DEMANDES OU BREVETS VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVETS
COMPREND PLUS D'UN TOME.
CECI EST LE TOME DE _2
NOTE: Pour les tomes additionels, veillez contacter le Bureau Canadien des
Brevets.
JUMBO APPLICATIONS / PATENTS
THIS SECTION OF THE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME OF _2
NOTE: For additional volumes please contact the Canadian Patent Office.