Note: Descriptions are shown in the official language in which they were submitted.
CA 02579528 2007-02-26
METHOD FOR HYDROCRACKING OF PETROLEUM HEAVY OIL
BACKGROUND OF THE INVENTION
1. Field of the Invention:
The present invention relates to a method for
hydrocracking of petroleum heavy grade oil and, more
particularly, to a method for hydrocracking of petroleum
heavy grade oil containing heavy metals. The method
consists of hydrogenating topped crude or vacuum residue in
the presence of a catalyst to give highly cracked products.
2. Description of the Related Art:
The petroleum industry is rapidly changing such that
heavy grade oil dominates on the one hand and demands for
lighter products increase on the other. This situation has
directed people's attention to the cracking technology to
produce lighter products in short supply from heavy grade
oil in oversupply. The cracking technology is becoming
important more and more in the present state of affairs
where the petroleum reserve is inevitably decreasing.
Although there have been proposed many methods for
thermal cracking and hydrocracking of heavy grade oil, they
still have some problems with cracking of heavy grade oil
such as vacuum residue.
Such heavy grade oil usually contains a large amount
of nitrogen compounds and sulfur compounds and hence its
cracking in the presence of a catalyst gives rise to
products containing extremely harmful organometallic
1
CA 02579528 2007-02-26
impurities. These impurities are dominated by nickel (Ni)
and vanadium (V). Being chemically combined with
comparatively high molecular weight organic compounds such
as asphaltene in heavy grade oil, they retard the catalytic
activity to decompose and remove nitrogen-, sulfur-, and
oxygen-containing compounds.
One method of treating vacuum residue without
resorting to catalysts is thermal cracking known as coking
process. This method, however, suffers the disadvantage of
disposing of a large amount of coke as a by-product.
Another disadvantage is low yields due to overcracking that
gives more gas than necessary, with recovered oil
containing much aromatic and olefinic components
detrimental to quality.
The disadvantage of the conventional method is
overcome by performing hydrocracking on heavy grade oil
which is supplied to a slurry bed reactor together with a
cheap disposable iron catalyst and recycled heavy reaction
products. This method achieves a high conversion rate
exceeding 90% regardless of the type of heavy grade oil
under the condition that the reaction pressure is higher
than 15 MPa, the temperature is 440-450 C, the reaction
time is 60-90 min, and the flow rate of recycled heavy
residues (boiling point (b.p.) >525 C) is 10-50 wt% (of the
amount of heavy grade oil supplied as the feed stock), on
the assumption that the selected iron catalyst is not
exceptionally poor in catalytic activity. The
2
CA 02579528 2007-02-26
aforementioned method (called hydrocracking with an iron
catalyst in a slurry bed reactor) is disclosed in Patent
Document 1 (Japanese Patent Laid-open No. 2001-89772).
OBJECT AND SUMMARY OF THE INVENTION
Needing high pressure, the hydrocracking method just
mentioned above is economically inferior to the thermal
cracking method mentioned earlier. Reduction of pressure
is essential for this method. In fact, this object is
achieved by employing a cheap active iron catalyst such as
natural limonite (iron ore). This catalyst allows for
hydrocracking at 10 MPa to attain a conversion rate
exceeding 90% under the above-mentioned condition (reaction
temperature, reaction time, and flow rate of recycled
residues). Nevertheless, the hydrocracking at a low
pressure still has the disadvantage of yielding a large
amount of coke when applied to heavy grade oil containing
asphaltene with more than 13 condensed rings. (This coke
is insoluble in toluene and hence it will be referred to as
toluene insolubles or TI hereinafter.) This causes an
increase of Ti concentration in the bottom or heavy
residues (b.p. >525 C) after repeated recycling and the
heavy residues (bottom) eventually become hard to decompose
because TI is very little reactive to decomposition. The
result is ineffective bottom recycling and low conversion
rates and yields.
An effective way of suppressing TI is to raise the
reaction pressure. However, this is economically
3
CA 02579528 2007-02-26
unfavorable. In order to keep low the concentration of TI
in the recycled heavy residues even though the amount of TI
increases under the low-pressure condition, it is necessary
to selectively remove TI from the system.
Selective removal of TI from the system is
accomplished by the method of sedimentation solid-liquid
separation with solvent addition, which consists of adding
light solvent to heavy reaction products (containing heavy
residues and solids) in a settling tank and discharging
soluble matter from the overflow and insoluble matter
(mostly TI and catalyst) from the underflow. Unfortunately,
this method cannot use a light product arising from
hydrocracking as the light solvent because of low
solubility for heavy organic matter. The result of low
solubility is coagulation and clogging with solids in the
underflow. This problem does not exist with an aromatic
light solvent (such as toluene) capable of readily
solubilizing heavy organic matter.
However, an aromatic light solvent poses another
problem with very slow sedimentation of solids, and
addressing this problem needs a huge settling tank with a
large equipment cost. Moreover, an aromatic light solvent
is several times higher in production cost than a naphtha
fraction having approximately the same boiling point. When
used alone for solid-liquid separation, its loss results in
greatly increasing of the process cost.
The foregoing has aroused a demand for an inexpensive
4
CA 02579528 2007-02-26
method for selective TI removal (solid-liquid separation)
that permits the economical treating of heavy grade oil
with a high TI yield.
The present invention was completed in view of the
foregoing. It is an object of the present invention to
provide a method for hydrocracking of heavy grade oil which
permits selective removal of TI by sedimentation solid-
liquid separation in slurry bed hydrocracking with an iron
catalyst, without causing clogging in the underflow of the
settling tank and requiring a large settling tank.
The present invention relates to a method for
hydrocracking of petroleum heavy grade oil as defined below.
The first aspect of the present invention is directed
to a method for hydrocracking of petroleum heavy grade oil
which comprises steps of supplying a slurry bed reactor
with a petroleum heavy grade oil containing heavy metal
components together with an iron catalyst to perform hydro-
cracking on the petroleum heavy grade oil, transferring the
reaction products from the slurry bed reactor to a gas-
liquid separator to separate the gas fluid from the liquid
fluid (containing solids), recycling part of the liquid
fluid to the slurry bed reactor and mixing the remainder
with a light solvent for solid-liquid separation and
performing solid-liquid separation on the resulting mixture
in a sedimentation solid-liquid separator, and recovering
the light solvent for solid-liquid separation from the
fluid extracted from the overflow in the solid-liquid
CA 02579528 2007-02-26
separator and subsequently recycling the fluid partly or
entirely to the slurry bed reactor, wherein the solvent for
solid-liquid separation is a mixture of an aromatic light
solvent and a light solvent arising from the hydrocracking
and this mixed solvent is added in amount two to five times
the amount of the remainder of the liquid fluid, with the
sedimentation solid-liquid separator kept at 130-250 C.
The second aspect of the present invention is directed
to a method for hydrocracking of petroleum heavy grade oil
as defined in the first aspect, wherein the aromatic light
solvent is composed of a single component with a boiling
point lower than 150 C or a mixture of such components.
The third aspect of the present invention is directed
to a method for hydrocracking of petroleum heavy grade oil
as defined in the first or second aspect, wherein the light
solvent arising from the hydrocracking, which is mixed with
the aromatic light solvent, has a boiling point in the
range of 80 to 180 C.
The fourth aspect of the present invention is directed
to a method for hydrocracking of petroleum heavy grade oil
as defined in any of the first to third aspects, wherein
the mixing ratio of the aromatic light solvent and the
light solvent arising from the hydrocracking is from 30/70
to 60/40.
The fifth aspect of the present invention is directed
to a method for hydrocracking of petroleum heavy grade oil
as defined in any of the first to fourth aspects, wherein
6
CA 02579528 2007-02-26
reaction in the slurry bed reactor is carried out under the
condition that the reaction pressure is 6 to 14 MPaG, the
reaction temperature is 430 to 450 C, and the reaction time
is 30 to 120 min.
The sixth aspect of the present invention is directed
to a method for hydrocracking of petroleum heavy grade oil
as defined in any of the first to fifth aspects, wherein
the iron catalyst is a powdery limonite iron ore in the
form of fine particles with an average particle diameter
smaller than 2 m which has been prepared by mechanical
pulverizing in a petroleum solvent and it is added in an
amount of 0.3 to 2 wt% (in terms of iron) of the amount of
the petroleum heavy grade oil.
The seventh aspect of the present invention is
directed to a method for hydrocracking of petroleum heavy
grade oil as defined in any of the first to sixth aspects,
wherein the fluid which has been extracted from the
overflow in the solid-liquid separator and which remains
after removal of the light solvent for solid-liquid
separation is recycled to the slurry bed reactor in such an
amount that its fraction having a boiling point higher than
525 C accounts for 10 to 100 wt% of the amount of the pe-
troleum heavy grade oil being supplied to the slurry bed
reactor.
[Effect of the invention]
The method according to the present invention allows
for hydrocracking of petroleum heavy grade oil with the
7
CA 02579528 2007-02-26
help of an iron catalyst in a slurry bed reactor, which
involves selective TI removal by sedimentation solid-liquid
separation, without causing clogging with the underflow in
the settling tank and requiring a large settling tank.
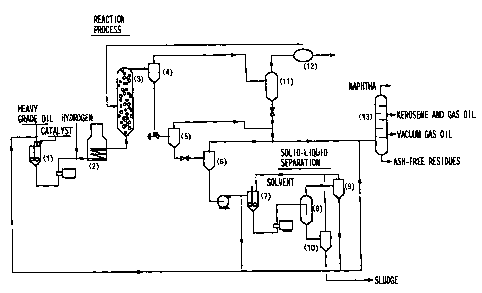
BRIEF DESCRIPTION OF THE DRAWING
Fig. 1 is a flow diagram illustrating the method for
hydrocracking of petroleum heavy grade oil according to the
present invention.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
After their extensive investigation to achieve the
above-mentioned object, the present inventors devised an
improved method for selective TI removal by sedimentation
solid-liquid separation in hydrocracking by the slurry bed
system that employs an iron catalyst. The improved method
is characterized in that the liquid fluid containing solids
is given for solid-liquid separation of a mixture of
aromatic light solvent and self-supplied light solvent
arising from hydrocracking and that the amount of the
mixture is 2 to 5 times the amount of the liquid fluid and
the sedimentation solid-liquid separation is carried out at
130 to 250 C. It permits selective TI removal while
allowing the underflow in the settling tank (sedimentation
solid-liquid separator) to flow smoothly without clogging
and also requiring no large settling tank.
The foregoing improved method for hydrocracking of
petroleum heavy grade oil is defined by the first aspect of
the present invention as follows. It comprises steps of
8
CA 02579528 2007-02-26
supplying a slurry bed reactor with a petroleum heavy grade
oil containing heavy metal components together with an iron
catalyst to perform hydrocracking on the petroleum heavy
grade oil, transferring the reaction products from the
slurry bed reactor to a gas-liquid separator to separate
the gas fluid from the liquid fluid (containing solids),
recycling part of the liquid fluid to the slurry bed
reactor and mixing the remainder with a light solvent for
solid-liquid separation and performing solid-liquid
separation on the resulting mixture in a sedimentation
solid-liquid separator, and recovering the light solvent
for solid-liquid separation from the fluid extracted from
the overflow in the solid-liquid separator and subsequently
recycling the fluid partly or entirely to the slurry bed
reactor, wherein the solvent for solid-liquid separation is
a mixture of an aromatic light solvent and a light solvent
arising from the hydrocracking and this mixed solvent is
added in an amount two to five times the amount of the
remainder of the liquid fluid, with the sedimentation
solid-liquid separator kept at 130-250 C.
Incidentally, the step of gas-liquid separation (to
separate the reaction product from the slurry bed reactor
into a gas fluid and a liquid fluid containing solids)
should preferably be carried out in three stages at high
pressure, low pressure, and vacuum pressure.
According to the first aspect of the present invention,
the method for hydrocracking of petroleum heavy grade oil
9
CA 02579528 2007-02-26
offers the advantage of removing TI selectively without
causing clogging with the underflow in the settling tank
(sedimentation solid-liquid separator) and requiring any
large settling tank.
According to the first aspect of the present invention,
the method for hydrocracking of petroleum heavy grade oil
is characterized in that the solvent for solid-liquid
separation is added in an amount two to five times the
amount of the liquid fluid (or part of the liquid fluid
separated by the reduced pressure gas-liquid separator)
from which solids are to be separated. The reason for this
is that the amount less than the lower limit does not let
solid components (coke and catalyst) settle at a desirable
rate and the amount more than the upper limit makes the
process uneconomical because of the necessity of
refurnishing solvent lost from the solvent recycling system.
According to the first aspect of the present invention,
the method for hydrocracking of petroleum heavy grade oil
is characterized in that the sedimentation solid-liquid
separator is operated at 130-250 C. The reason for this is
that operation at temperatures below 130 C does not let
solid components (coke and catalyst) settle at a desirable
rate and operation at temperatures above 250 C needs
expensive facilities which withstands a pressure exceeding
2 MPa in order to keep the solvent (having a high vapor
pressure) in a liquid state.
According to the first aspect of the present invention,
CA 02579528 2007-02-26
the method for hydrocracking of petroleum heavy grade oil
is characterized in that the light solvent used for solid-
liquid separation is a mixture of aromatic light solvent
and self-supplied light solvent arising from hydrocracking.
According to the second aspect of the present invention,
the aromatic light solvent should be one having a boiling
point lower than 150 C in pure or mixed form. It includes,
for example, benzene and toluene.
According to the third aspect of the present invention,
the self-supplied light solvent (arising from
hydrocracking) to be mixed with the aromatic light solvent
should be one having a boiling point of 80-180 C. The
reason for this is that the one having a boiling point
lower than 80 C raises the vapor pressure of the mixed
solvent (for solid-liquid separation), which necessitates
expensive facilities, and the one having a boiling point
higher than 180 C uneconomically needs a large amount of
heat for solvent recovery from the fluid discharged from
the overflow and underflow in the solid-liquid separator.
According to the fourth aspect of the present
invention, the light solvent for solid-liquid separation
should be composed of an aromatic light solvent and a self-
supplied light solvent in a ratio of from 30/70 to 60/40.
The reason for this is that the mixed solvent for solid
liquid separation in which the self-supplied light solvent
accounts for more than 70 wt% or the mixing ratio is
smaller than 30/70 is liable to cause clogging with solids
11
CA 02579528 2007-02-26
in the bottom of the sedimentation solid-liquid separator,
disabling normal operation, and that the mixed solvent for
solid liquid separation in which the self-supplied light
solvent accounts for less than 40 wt% or the mixing ratio
is larger than 60/40 is liable to retard solids from
settling in the sedimentation solid-liquid separator,
resulting in uneconomical operation with an enlarged
sedimentation solid-liquid separator and a larger amount of
aromatic light solvent added thereto.
According to the fifth aspect of the present invention,
the reaction in the slurry bed reactor should be carried
out under the condition that the reaction pressure is 6 to
14 MPaG, the reaction temperature is 430 to 450 C, and the
reaction time is 30 to 120 min. Incidentally, the reaction
pressure is expressed in terms of gauge pressure. 1 MPaG
is equal to 1.1 MPa in terms of absolute pressure. Normal
pressure is 0 MPa in terms of gauge pressure and 0.101 MPa
in terms of absolute pressure. 1 MPaG = 1 x 106 Pa and
9.080665 x 104 Pa = 1 kgf/cmZ (or 0.980665 x 105 Pa =
1 kgf/cm2), and hence 0.980665 MPa = 10 kgf/cm2. This
means that the pressure of 6 to 14 MPaG is equivalent to
6 x 10/0.980665 to 14 x 10/0.980665 kgf/cm2.
According to the sixth aspect of the present invention,
the method for hydrocracking of heavy grade oil employs as
an iron catalyst a powdery limonite iron ore in the form of
fine particles with an average particle diameter smaller
than 2 m which has been prepared by mechanical pulverizing
12
CA 02579528 2007-02-26
in a petroleum solvent and it is added in an amount of 0.3
to 2 wt% (in terms of iron) of the amount of the petroleum
heavy grade oil. Limonite iron ore as a catalyst is more
active than hematite (Fe203), pyrite (Fe52), and iron
sulfate (FeSO4), and is a cheap naturally occurring
catalyst. With an amount less than 0.3 wt%, it causes coke
to occur in large amounts. With an amount more than 2 wt%,
it makes production cost high without increasing oil yields.
According to the seventh aspect of the present
invention, the method for hydrocracking of petroleum heavy
grade oil includes the steps of separating the light
solvent for solid liquid separation from the fluid
discharged from the overflow in the solid-liquid separator
and recycling the separated fluid partly or entirely to the
slurry bed reactor. The amount of the fluid to be recycled
to the slurry bed reactor should be such that the amount of
heavy residues (bottom) with a boiling point higher than
525 C in the fluid is 10 to 100 wt% of the amount of the
petroleum heavy grade oil supplied.
The reason for this is that recycling with an amount
less than 10 wt% does not increase oil yields and is poor
in the effect of bottom recycling and that recycling with
an amount more than 100 wt% does not increase oil yield so
much as recycling with an amount specified above although
it increases oil yields more than recycling with an amount
less than 10 wt%.
According to the present invention, the light solvent
13
CA 02579528 2007-02-26
used for solid-liquid separation is a mixture of aromatic
light solvent and self-supplied light solvent arising from
the hydrocracking process. In the initial stage of
operation, the self-supplied light solvent is light oil,
such as naphtha, obtained by distillation of the gas fluid
and/or the liquid fluid which has been separated in the gas
separator placed downstream the reactor. (This solvent
will be referred to as "light oil A obtained by
distillation" hereinafter.) It is mixed with aromatic
light solvent and the resulting mixture is used as the
light solvent for solid liquid separation. In the
subsequent stages, the light solvent for solid liquid
separation is obtained by separation from the fluid
discharged from the overflow in the solid-liquid separator.
(The resulting solvent is referred to as "solvent a"
hereinafter.) The light solvent for solid liquid
separation is also obtained by separation from the fluid
discharged from the underflow in the solid-liquid separator.
(The resulting solvent is referred to as "solvent b"
hereinafter.) The light solvent for solid-liquid
separation is also prepared by separating the light solvent
for solid-liquid separation from the fluid discharged from
the overflow and/or the underflow in the solid-liquid
separator and then distilling the separated light solvent
to give light oil, such as naphtha, (which is referred to
as "light oil B obtained by distillation" hereinafter) and
mixing it with purchased aromatic light oil. (The
14
CA 02579528 2007-02-26
resulting solvent is referred to as "solvent c"
hereinafter.) Two or more of solvent a, solvent b, and
solvent c mentioned above are used as the light solvent for
solid-liquid separation. When in short, the thus prepared
light solvent for solid-liquid separation is supplemented
with light oil A obtained by distillation and aromatic
light solvent individually or in combination.
According to the present invention, the light solvent
used for solid-liquid separation is a mixture of aromatic
light solvent and self-supplied light solvent arising from
the hydrocracking process. The latter includes light oil A
and light oil B as well as light solvents (excluding
aromatic light solvent) selected from solvent a, solvent b,
and solvent c mentioned above.
According to the present invention, the method for
hydrocracking of petroleum heavy grade oil includes the
step of supplying the reaction product from the slurry bed
reactor to the high-pressure gas-liquid separator for
separation into gas fluid and liquid fluid (containing
solids). The liquid fluid contains heavy oil components
and solids (coke and catalyst) as well as light oil
components. The heavy oil components are those having a
boiling point higher than 525 C and the light oil
components are those having a boiling point lower than that
of the heavy oil components.
The liquid fluid is subsequently supplied to the low-
pressure gas-liquid separator for separation into gas fluid
CA 02579528 2007-02-26
and liquid fluid (containing solids). The liquid fluid is
supplied to the vacuum gas-liquid separator for separation
into gas fluid and liquid fluid (containing solids). The
liquid fluid contains heavy oil components and solids as
well as light oil components. Incidentally, the liquid
fluid is composed of light oil components and heavy oil
components dissolved therein and it also contains solids.
The liquid fluid is partly recycled to the slurry bed
reactor and the remainder of the liquid fluid is mixed with
the light solvent for solid-liquid separation and the
resulting mixture is supplied to the sedimentation solid-
liquid separator for sedimentation of solids. The fluid
containing a less amount of solids is discharged from the
upper part of the solid-liquid separator and the fluid
containing a larger amount of solids is discharged from the
lower part of the solid-liquid separator. The fluid
discharged from the upper part of the solid-liquid
separator contains heavy oil components, light oil
components, and light solvent for solid-liquid separation.
It is composed of light oil components and light solvent
for solid-liquid separation and heavy oil components
dissolved in the former two. On the other hand, the fluid
discharged from the lower part of the solid-liquid
separator contains solids (coke and catalyst) insoluble in
light solvent for solid-liquid separation as well as oil
components. It is a slurry composed of oil components and
solid components.
16
CA 02579528 2007-02-26
According to the present invention, the method for
hydrocracking of petroleum heavy grade oil is accomplished
by using the apparatus shown in Fig. 1, which is comprised
of (1) slurry preparation vessel, (2) preheater, (3) slurry
bed reactor, (4) high pressure gas-liquid separator, (5)
low-pressure gas-liquid separator, (6) vacuum gas-liquid
separator, (7) slurry preparation vessel, (8) sedimentation
solid-liquid separator (settling tank), (9) apparatus to
recover solvent from overflow, (10) apparatus to recover
solvent from underflow, (11) high-pressure low-temperature
gas-liquid separator, (12) gas purification unit, and (13)
distillation column. The foregoing apparatus is operated
as follows for the hydrocracking of petroleum heavy grade
oil according to the present invention.
The slurry preparation vessel (1) is supplied with
petroleum heavy grade oil (containing heavy metals)
together with iron catalyst. After mixing, the resulting
mixture (slurry) is fed to the preheater (2), which is also
supplied with hydrogen. The preheated mixture (including
hydrogen) is fed to the slurry bed reactor (3), in which
hydrocracking of petroleum heavy grade oil takes place.
The reaction product from the slurry bed reactor (3)
is fed to the high-pressure gas-liquid separator (4), in
which separation of gas fluid and liquid fluid (containing
solids) takes place. The separated liquid fluid is fed to
the low-pressure gas-liquid separator (5), in which
separation of gas fluid and liquid fluid (containing
17
CA 02579528 2007-02-26
solids) takes place. The liquid fluid is fed to the vacuum
gas-liquid separator (6), in which separation of gas fluid
and liquid fluid (containing solids) takes place. The
liquid fluid is partly recycled to the slurry bed reactor
(3) mentioned above, and the remainder of the liquid fluid
is fed, together with the light solvent for solid-liquid
separation, to the slurry preparation vessel (7) for their
mixing.
Here, the light solvent used for solid-liquid
separation is a mixture of aromatic light solvent and self-
supplied light solvent arising from hydrocracking, and it
is mixed with the remainder of the liquid fluid supplied to
the slurry preparation vessel (7), with the mixing ratio
being two to five times. Incidentally, the self-supplied
light solvent is naphtha arising from the distillation
column (13). In the early stage of operation, a mixture of
this naphtha and aromatic light solvent is used as the
solvent for solid liquid separation. After that, it is
switched to "solvent a" separated from the apparatus (9) to
recover solvent from the overflow or "solvent b" separated
from the apparatus (10) to recover solvent from the
underflow (as mentioned later). When the amount is short,
these solvents for solid-liquid separation are supplemented
with aromatic light solvent and naphtha arising from the
distillation column (13).
The mixture (slurry) arising from the slurry
preparation vessel (7) is fed to the sedimentation solid-
18
CA 02579528 2007-02-26
liquid separator (8) which allows solids to settle, with
the fluid containing less solids being discharged from its
upper part and the fluid containing more solids being
discharged from its lower part. Incidentally, the
sedimentation solid-liquid separator (8) is run at 130 to
250 C .
The fluid discharged from the upper part of the solid-
liquid separator (8) is fed to the overflow solvent
recovery apparatus (9) from which the light solvent for
solid-liquid separation is removed. The removed fluid is
partly recycled to the slurry bed reactor (3) through the
slurry preparation vessel (1) and the preheater (2), with
the remainder being fed to the distillation column (13).
The light solvent for solid-liquid separation recovered in
the solvent recovery apparatus (9) is fed to the slurry
preparation vessel (7) in which it is used for solid-liquid
separation.
The fluid discharged from the lower part of the solid-
liquid separator (8) is fed to the underflow solvent
recovery apparatus (10) from which the light solvent for
solid-liquid separation is removed, with the residues
(sludge) being discharged from the system.
Incidentally, the gas fluid separated by the high-
pressure gas-liquid separator (4) is fed to the high-
pressure low-temperature gas-liquid separator (11) and the
gas separated in the separator (11) is supplied to the gas
purification unit (12). The gas fluid separated by the
19
CA 02579528 2007-02-26
low-pressure gas-liquid separator (5) and the vacuum gas-
liquid separator (6) is fed, together with liquid fluid
separated by the high-pressure low-temperature gas-liquid
separator (11), to the distillation column (13).
EXAMPLES
The invention will be described in more detail with
reference to the following Example and Comparative Examples,
which are not intended to restrict the scope thereof and
which may be modified in any manner within the scope
thereof.
Example 1
Hydrocracking was performed on petroleum heavy grade
oil containing heavy metals by using an apparatus
equivalent to that shown in Fig. 1. A detailed description
follows.
The slurry preparation vessel (1) is charged with
petroleum heavy grade oil containing heavy metals, an iron
catalyst, and a co-catalyst. After mixing, the resulting
mixture (slurry) is supplied to the preheater (2), which is
also fed with hydrogen. After preheating, the resulting
mixture is fed, together with hydrogen, to the slurry bed
reactor (3). Incidentally, the petroleum heavy grade oil
containing heavy metals is vacuum residues (VR for short
hereinafter). The iron catalyst is limonite iron ore, which
is used in an amount of 1 wt% (in terms of iron) for the
amount of the petroleum heavy grade oil. The co-catalyst is
sulfur, which is used in an amount of 1.2 times the amount
CA 02579528 2007-02-26
of the iron component. Hydrocracking in the slurry bed
reactor (3) is carried out under the following conditions.
Reaction pressure: 12 MPa, reaction temperature: 450 C,
reaction time: 90 min, and amount of heavy residues (b.p.
>525 C) recycled: 50 wt%. The fractions of VR are shown in
Table 1 (in which "wt% on feed VR" means the ratio (in wt%)
of VR (by weight) fed.
Table 1
L Composition of distillate (wt% on feed VR)
<171 C 171-232 C 232-343 C 343-525 C >525 C
Feed VR -- -- -- 16.4 83.6
The slurry bed reactor (3) supplies its reaction
product to the high-pressure gas-liquid separator (4) for
separation into gas fluid and liquid fluid (containing
solids). The liquid fluid enters the low-pressure gas-
liquid separator (5) for separation into gas fluid and
liquid fluid (containing solids). The liquid fluid enters
the vacuum gas-liquid separator (6) for separation into gas
fluid and liquid fluid (containing solids). The high-
pressure gas-liquid separator (4) runs at 12 MPaG and 400 C,
the low-pressure gas-liquid separator (5) runs at 0.3 MPaG
and 380 C, and the vacuum gas-liquid separator runs at 10
mmHg and 350 C. The liquid fluid separated by the vacuum
gas-liquid separator (6) has the composition as shown in
Table 2. In Table 2, BTM (>525 C) denotes a mixture of
heavy organic matter having a boiling point higher than
525 C and inorganic matter (catalyst), HS components denote
21
CA 02579528 2007-02-26
those which are soluble in hexane, and HI-TS components
denote those which are insoluble in hexane and soluble in
toluene. These terms are also used in Tables 3 and 4 later.
Table 2
Composition of Composition of Composition of
liquid fluid from liquid fluid from liquid fluid from
Components vacuum gas-liquid upper part of lower part of
separator to settling tank (wt%) settling tank (wt%)
settlin tank (wt%)
Oil (b.p. 343-525 C) 45.81 53.18 29.47
BTM (>525 C) (54.19) (46.81) (70.54)
HS components 24.96 28.97 16.06
HI-TS components 14.65 15.64 12.45
TI components 9.77 2.13 26.71
Catalyst 4.81 0.07 15.32
The liquid fluid separated by the vacuum gas-liquid
separator (6) enters, together with the light solvent for
solid-liquid separation, the slurry preparation vessel (7)
for their mixing. The light solvent for solid-liquid
separation is a mixture of aromatic light solvent and self-
supplied light solvent arising from the hydrocracking
process. Its amount is four times the amount of the liquid
fluid supplied to the slurry preparation vessel (7). The
aromatic light solvent is toluene, and the self-supplied
light solvent is naphtha (b.p.: 100-170 C) arising from the
distillation column (13). They are mixed in a ratio of
40/60.
This mixture prepared in the slurry preparation vessel
(7) enters the sedimentation solid-liquid separator (8) in
....
which solids settle. The sedimentation solid-liquid
22
CA 02579528 2007-02-26
separator (8) discharges a fluid with less solids from its
upper part and discharges a fluid with more solids from its
lower part. It runs at 1.5 MPa and 220 C.
The fluid from the upper part of the solid-liquid
separator (8) has the light solvent for solid-liquid
separation separated. The fluid, after the separation of
the light solvent separated, has the composition as shown
in Table 2. The fluid from the lower part of the solid-
liquid separator (8) has the light solvent for solid-liquid
separation separated. The fluid, after the separation of
the light solvent, has the composition as shown in Table 2.
It is noted from Table 2 that the liquid fluid from the
lower part of the solid-liquid separator (8) contains
concentrated TI (toluene insolubles) and catalyst and that
the liquid fluid from the upper part of the solid-liquid
separator (8) contains less TI and catalyst.
Part of the fluid from the upper part of the solid-
liquid separator (8), after the separation of the light
solvent, is recycled to the reaction system together with
the liquid fluid from the vacuum gas-liquid separator (6).
The above-mentioned hydrocracking of petroleum heavy
grade oil successfully achieves the selective TI removal by
sedimentation solid-liquid separation without clogging in
the underflow of the settling tank (sedimentation solid-
liquid separator) (8) and without the necessity of
enlarging the settling tank.
The slurry bed reactor achieves reactions effectively -
23
CA 02579528 2007-02-26
such that the rate of conversion is 91%, the yield of heavy
residues (b.p. >525 C) is 7.5 wt% of the amount of feed VR,
and the yield of oil is 85 wt% of the amount of feed VR.
Here, the rate of conversion is calculated from the
following formula (1).
(A - B)
Rate of conversion (~) = x 100 ... (1)
A
(where A is the amount (wt%) of heavy residues (b.p.
>525 C) in feed VR and B is the yield of heavy residues.)
Comparative Example 1
The same procedure as in Example 1 was repeated for
hydrocracking o.f petroleum heavy grade oil except that the
light solvent for solid liquid separation is composed
solely of self-supplied light solvent, which is naphtha
(b.p.: 100-170 C) arising from the distillation column (13).
The result was that clogging occurred in the lower
part of the settling tank (sedimentation solid-liquid
separator) (8), which discontinued operation. The solid
substance that caused clogging was found to have the
composition shown in Table 3.
Table 3
Composition of solid
Components substance remaining
in lower part of
settling tank (wt%)
HS components 5.4
HI-TS components 19.3
TI components 46.2
Catalyst 29.1
24
CA 02579528 2007-02-26
Comparative Example 2
The same procedure as in Example 1 was repeated for
hydrocracking of petroleum heavy grade oil except that the
light solvent for solid liquid separation is composed
solely of aromatic light solvent, which is toluene (b.p.:
110 C) .
The result was that the solid-liquid separator (8)
allowed fluids to be discharged from its upper and lower
parts without clogging. The composition of the fluids,
after the separation of the light solvent for solid-liquid
separation is shown in Table 4.
Table 4
Composition of Composition of
liquid fluid from liquid fluid from
Components upper part of lower part of
settlin tank (wt%) settlin tank (wt%)
Oil (b.p. 343-525 C) 50.22 33.89
BTM (>525 C) (49.78) (66.12)
HS components 27.36 18.46
HI-TS components 15.58 12.14
TI components 6.70 18.09
Catalyst 0.14 17.43
Despite continued operation without clogging in the
lower part of the settling tank (sedimentation solid-liquid
separator) (8), the procedure in Comparative Example 2 is
less satisfactory in selective TI removal than that in
Example 1 as indicated by Table 4. That is, the fluid from
the lower part of the solid-liquid separator (8) contains
TI components and catalyst remaining with a low degree of
CA 02579528 2007-02-26
concentration and the fluid from the upper part of the
solid-liquid separator (8) contains TI components and
catalyst in large amounts (indicating a low ratio of TI
removal and insufficient selective TI removal).
The fluid discharged from the upper part of the solid-
liquid separator (8) enters the solvent recovery apparatus
(9) in which the light solvent for solid-liquid separation
is separated. Part of the fluid discharged from the
apparatus (9) is recycled to the reaction system together
with the liquid fluid separated by the vacuum gas-liquid
separator (6). The slurry bed reactor achieved reactions
such that the conversion rate is 87%, the yield of heavy
residues (b.p. >525 C) is 11.4 wt% on feed VR, and the
yield of oil is 82 wt% on feed VR.
It is noted from the foregoing that the procedure in
Example 1 and Comparative Example 2 permits selective TI
removal without clogging in the lower part of the settling
tank unlike the procedure in Comparative Example 1. The
procedure in Comparative Example 2, however, is poor in the
ratio of TI removal and insufficient in selective TI removal.
In addition, it is uneconomical because it relies on only
aromatic light solvent as the solvent for solid-liquid
separation. The procedure in Example 1 is superior to that
in Comparative Example 2 in that it performs selective TI
removal effectively and it is economical because it employs
a mixture of aromatic light solvent and self-supplied light
solvent as the solvent for solid-liquid separation.
26
CA 02579528 2007-02-26
The present invention provides a method for
hydrocracking petroleum heavy grade oil containing heavy
metals in a slurry bed reactor with the help of an iron
catalyst. This method includes a step of selectively
removing TI by sedimentation solid-liquid separation from
the liquid fluid arising from the reaction product. The
procedure for separation causes no clogging in the
underflow of the settling tank (sedimentation solid-liquid
separator) and requires no large settling tank. Therefore,
the method of the present invention is suitable for
hydrocracking of heavy grade oil containing heavy metals.
27