Note: Descriptions are shown in the official language in which they were submitted.
CA 02580304 2007-03-05
APPLICATION FOR PATENT
TITLE: CURABLE RESIN COATED LOW APPARENT SPECIFIC GRAVITY
BEADS AND METHOD OF USING THE SAME
SPECIFICATION
Field of the Invention
This invention relates to methods and compositions useful for subterranean
formation treatments, such as hydraulic fracturing treatments and sand
control. In
particular, this invention relates to use of curable resin coated plastic
beads having low
specific gravity (ASG) in sand control methods such as gravel packing, frac
pack
treatments, etc., as well as proppant material in hydraulic fracturing
treatments.
Background of the Invention
Typically, it is necessary, when producing oil and/or gas from an
unconsolidated
subterranean formation, to prevent sand grains and/or other formation fines
from
migrating into the wellbore and being produced from the well. The production
of such
particulates can reduce the rate of hydrocarbon production from the well and
can cause
serious damage to well tubulars and to well surface equipment.
Gravel packs are often used to control particulate migration in such producing
formations. A gravel pack typically consists of a mass of particulates which
are packed
around the exterior of a screening device. Such screening devices, typically
positioned in
an open hole or inside the well casing, have very narrow openings which are
large
enough to permit the flow of formation fluid but small enough to allow the
particulates to
pass through. The particulates operate to trap, and thus prevent the further
migration of,
formation sand and fines which would otherwise be produced along with the
formation
fluid.
In order to be useful in gravel packing applications, such particulates must
exhibit
high strength and be capable of functioning in low permeability formations.
Ultra
lightweight (ULW) particulate materials have been proposed for use in gravel
packing
applications to improve transport, placement, and packing efficiency. Concerns
exist
1
CA 02580304 2007-03-05
however that ULW particulate materials do not demonstrate the acid and
chemical
resistance properties required of particulates for use in gravel packing.
U.S. Patent No. 5,531,274 reports the use of polystyrene divinylbenzene
(PSDVB) beads for use in hydraulic fracturing at temperatures up to about 150
F.
PSDVB beads have been reported to reduce fluid velocity required to maintain
proppant
transport within the fracture. This, in turn, provides for a greater fracture
area to be
propped. When used as a proppant, PSDVB beads, while offering excellent
compressive
strength, often soften and loose their compressive strength especially at high
temperature
and high pressure conditions.
While PSDVB beads have sufficient strength, acid resistance and low ASG for
use as ULW in gravel packing treatments, they are unfortunately subject to
fluidization
and flowback and thus are unacceptable for such use.
Alternative ULW materials of low ASG which exhibit high particle strength,
acid
resistance and which are not subject to fluidization and flowback have been
sought to
improve transport, placement and packing efficiency.
Summary of the Invention
Plastic beads provide highly desirable results in gravel packing when the
beads
are coated with a curable resin. The beads preferably have an apparent
specific gravity
(ASG) less than about 2.0, more preferably less than or equal to 1.5, and
exhibit high acid
and chemical resistance. Polystyrene beads crosslinked with divinylbenzene
(PSDVB) as
well as polyamide beads are preferred as the plastic beads.
The curable resin coating facilitates consolidation of the plastic beads once
the
pack is in place. Preferred curable resins include phenolic resins, epoxy
resins, furan
resins, phenolic formaldehyde resins, melamine formaldehyde resins, urethane
resins and
phenolic and furan resin mixtures.
In a preferred embodiment, the plastic beads are substantially neutrally
buoyant in
the carrier fluid introduced into the wellbore. (As used herein, the term
"carrier fluid"
shall include pumping and fracturing fluids.)
The plastic particulates are useful in gravel packing procedures wherein a
screening device is placed in the wellbore and the plastic beads are then
introduced such
2
CA 02580304 2007-03-05
that they are packed around the exterior of the screening device. The packed
plastic
beads provide a fluid-permeable barrier around the screening device which is
operable for
preventing the migration of formation particulates into the screening device.
The plastic beads may further be introduced into a portion of the wellbore
extending into the subterranean formation such that the beads are packed in
the wellbore
to provide a fluid-permeable barrier which is operable for preventing the
migration of
formation particulates. Formation fluid is then produced through the packed
particulate
bed.
The plastic beads may further be used to stimulate a subterranean formation
such
that they are deposited in the fracture and thus provide a fluid permeable
region within
the formation.
The curable resin coated plastic beads provide permeability levels and
production
rates substantially superior to those provided by the ULW particulates of the
prior art
while providing excellent control of formation sand and formation fines.
Brief Description of the Drawings
In order to more fully understand the drawings referred to in the detailed
description of the present invention, a brief description of each drawing is
presented, in
which:
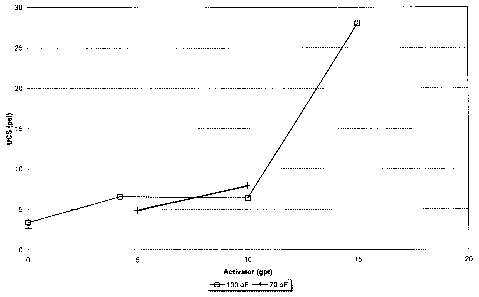
FIG. 1 contrasts the compressive strength and permeability of curable resin
coated
PSDVB beads at 100 and 70 F.
Detailed Description of the Preferred Embodiments
Plastic beads having a coating of a curable resin exhibit sufficient strength
for use
as ultra lightweight particulates in sand control methods, such as gravel
packing and frac
pack treatments, as well as hydraulic fracturing. The curable resin coated
plastic beads
exhibit high acid and chemical resistance.
The plastic beads typically have an apparent specific gravity (ASG) of 2.0 or
less,
preferably less than or equal to 1.5. preferably less than about 1.15 and most
preferably
less than about 1.07. In a particularly preferred mode, the beads are
polystyrene
divinylbenzene beads having an ASG of about 1.05. The beads are typically
highly
3
CA 02580304 2007-03-05
spherical. In a preferred embodiment, the curable resin coated plastic beads
as those
commercially available from Sun Drilling Products Corp. of Belle Chasse,
Louisiana.
The resin coating on the plastic beads is not fully cured. The resin
principally
functions in proppant flowback control. When pumped downhole, the resin
typically
completes the curing process at temperatures of about 100 F, as proppant
particulates
adhere to each other. The presence of the curable resin on the plastic beads
facilitates
consolidation of the pack once the beads are introduced into the formation.
Preferred curable resins include phenolic resins, epoxy resins, furan resins,
phenolic formaldehyde resins, melamine formaldehyde resins, urethane resins
(especially
low volatile urethane resins) and phenolic and furan resin mixtures.
The resin coating is generally present in the curable resin coated plastic
bead in an
amount of from about 4% to about 10% by weight of total weight. The thickness
of the
resin coating is generally between from about 0.5 to about 4 microns.
The curable resin coated plastic beads exhibit crush resistance under
conditions of
high stress, API RP 56 or API RP 60, generally at conditions greater than
2,000 psi
closure stress.
The plastic beads are beads of crosslinked polymers derived from monomers
containing an ethylenic bond, such as acrylate esters, methacrylate esters,
vinyl acetate,
styrene, vinylnaphthalene, vinyltoluene, allyl esters, olefins, vinyl
chloride, allyl alcohol,
acrylonitrile, acrolein, acrylamides, methacrylamides, vinyl fluoride,
vinylidene
difluoride, polyfunctional acrylates, methacrylates, acrylamides,
methacylamides and
polyunsaturated hydrocarbons, etc.
The polymers may be crosslinked with divinylbenzene as well as
trimethylolpropane trimethacrylate, trimethylolpropane triacrylate,
trimethylolpropane
dimethacrylate, trimethylolpropane diacrylate, pentaerythritol
tetramethacryalate,
pentaerythritol trimethacrylate, pentaerythritol dimethacrylate,
pentaerythritol
tetraacrylate, pentaerythritol triacrylate, pentaerythritol diacrylate,
bis(methacrylamides),
polyolefins, polyethyleneglycol dimethylacrylates, polyethyleneglycol
diacrylates,
ethyleneglycol dimethacrylate, ethyleneglycol diacrylate, diethyleneglycol
dimethacrylate, diethyleneglycol diacrylate, triethyleneglycol dimethacrylate
and
triethyleneglycol diacrylate.
4
CA 02580304 2007-03-05
Suitable curable resin coated plastic beads include rigid chain entanglement
crosslinked polymers such as those set forth in U.S. Patent No. 6,248,838,
herein
incorporated by reference.
The plastic beads are preferably polystyrene beads crosslinked with a
crosslinker,
such as divinylbenzene (PSDVB). In another preferred embodiment, the plastic
beads are
polyamides, such as polyamide-6, 6 as well as polyamide 6, like the Technyl
polyamides from Rhodia Engineering Plastics. Further suitable polyamides are
polyamide 6,10; polyamide 6,12; polyamide 4,6, polyamide 11 and polyamide 12.
Preferably, such beads have a sphericity of about 0.9 from API RP 58, an
important parameter for gravel packing as higher sphericity equates to
relatively high
permeability. The increased propensity for flowback created by such sphericity
indices,
coupled with the low ASG of the particulates, is addressed by the presence of
the curable
resin coating which provides the requisite consolidation to the plastic bead.
Any plastic
bead of low ASG and high sphericity, such as those observed with PSDVB beads
and
polyamide beads, and exhibiting sufficient strength, are acceptable for use in
the
invention.
The plastic beads may contain varying amounts of the crosslinker to produce
materials having varying degree of elasticity. In this regard, any amount of
crosslinker
suitable for forming elastic material may be employed. Percentages of
crosslinker
employed may be selected based on the downhole conditions to which the plastic
beads
are to be used. Typically, the amount of crosslinker in the copolymer is from
about 1 to
about 30 weight percent, preferably less than or equal to about 10 weight
percent.
The curable resin coated plastic beads are prepared by mixing the resin and
plastic
beads in a vessel at elevated temperatures, typically from about 200 to about
350,
preferably around 250 F. An adherent, such as a resin adhesive or tackifying
resin, may
further be added to the vessel during mixing. The adherent serves to assist
the adhesion
of the curable resin onto the plastic beads. The heating is terminated when
the
temperature has reached 250 F. The curable resin coated plastic beads are
then cooled to
room temperature.
Alternatively, the curable resin coated plastic beads may be prepared by use
of
fluidized bed or spray coating techniques.
5
CA 02580304 2007-03-05
The curable resin coated plastic beads may further be prepared in the presence
of
a filler in order to increase the strength and/or temperature resistance of
the particulates.
Typically, the particle size of the filler range from about 100 nm to about
200 m.
Suitable as fillers are minerals (such as finely divided minerals or finely
divided
minerals and/or fibers) optionally bound by a suitable organic or inorganic
binder.
Suitable minerals include fly ash, silica and sand (including fumed silica,
quartz sand,
and silica flour), alumina, mica, silicates, such as orthosilicates and
metasilicates,
aluminum silicate and calcium silicate, kaolin, talc, zirconia, boron and
glass, such as
glass spheres (especially glass microspheres), glass powder, glass beads,
glass bubbles,
ground glass, borosilicate glass and fiberglass. Suitable fibers include
mineral fibers,
glass fibers, ceramic fibers, carbon fibers, polymeric fibers, coated fibers
(such as nickel
coated carbon fibers) and synthetic fibers. Further, suitable fillers include
clay, hematite,
alkali metal salts, molybdenum disulfide, oil, aluminum flake, stainless
steel, silicone,
polytetrafluoroethylene, cement, inorganic salts, carbon black, carbon
Buckminster
fullerenes, carbon nano-tubes, polyhedral oligomeric silsesquioxane, metals,
metallic
oxides (such as trimanganese tetraoxide), metallic salts (including alkali
metal salts),
phosphates, borates, calcium carbonate, calcium chloride, calcium bromide,
barium
sulfate, aluminum flakes, a modified naturally occurring material, crushed nut
shells,
ground or crushed seed shells, ground or crushed fruit pits, processed wood
and organic
polymeric materials. Further, the filler may contain a cation selected from
the group
consisting of alkali metals, alkaline earth metals, ammonium, manganese, and
zinc and an
anion selected from the group consisting of a halide, an oxide, a carbonate,
nitrate,
sulfate, acetate and formate.
The amount of filler(s) in the composition is such as to impart the desired
ASG.
Typically, the amount of filler in the composition is between from about 1 to
about 85,
more typically between from about 25 to about 60, percent by volume. The
amount of
filler and polyamide particulate may be adjusted to tailor the composition to
achieve the
desirable physical properties, including particle density, bulk density, crush
strength, etc.
The particle size of the curable resin coated plastic beads may be selected
based
on anticipated downhole conditions. In this regard, larger particle sizes may
be more
desirable in situations where a relatively lower strength particulate material
is employed.
6
CA 02580304 2007-03-05
The plastic beads typically have a size ranging from about 4 mesh to about 100
mesh,
alternatively from about 20 mesh to about 40 mesh.
The particulates deform with stress and yet are sufficiently strong to be used
on
their own at high pressures, such as in excess of 4,000 psi. The curable resin
coating
prevents sand grains and/or other formation fines from migrating into the
wellbore.
The curable resin coated plastic beads may be employed with carrier or
treatment
fluids in order to facilitate placement of the composite to a desired location
within the
formation. The ASG of the coated beads is generally greater than or equal to
the ASG of
the carrier fluid. Any carrier fluid suitable for transporting the particulate
into a well
and/or subterranean formation fracture in communication therewith may be
employed
including, but not limited to, carrier fluids including a brine, salt water,
unviscosified
water, fresh water, potassium chloride solution, a saturated sodium chloride
solution,
liquid hydrocarbons, and/or a gas such as nitrogen or carbon dioxide. In a
preferred
embodiment, the carrier fluid is unviscosified water or brine.
The carrier fluid may be gelled, non-gelled or have a reduced or lighter
gelling
requirement. The latter may be referred to as "weakly gelled", i.e., having
minimum
sufficient polymer, thickening agent, such as a viscosifier, or friction
reducer to achieve
friction reduction when pumped downhole (e.g., when pumped down tubing, work
string,
casing, coiled tubing, drill pipe, etc.), and/or may be characterized as
having a polymer or
viscosifier concentration of from greater than 0 pounds of polymer per
thousand gallons
of base fluid to about 10 pounds of polymer per thousand gallons of base
fluid, and/or as
having a viscosity of from about 1 to about 10 centipoises. The non-gelled
carrier fluid
typically contains no polymer or viscosifer.
The use of a non-gelled carrier fluid eliminates a source of potential packing
and/or formation damage and enhancement in the productivity of the well.
Elimination
of the need to formulate a complex suspension gel may further mean a reduction
in
tubing friction pressures, particularly in coiled tubing and in the amount of
on-location
mixing equipment and/or mixing time requirements, as well as reduced costs. In
one
embodiment employing a substantially neutrally buoyant particulate and a brine
carrier
fluid, mixing equipment need only include such equipment that is capable of
(a) mixing
7
CA 02580304 2007-03-05
the brine (dissolving soluble salts), and (b) homogeneously dispersing in the
substantially
neutrally buoyant particulate.
Gelling agents for proppant carrier fluids may provide a source of proppant
pack
and/or formation damage, and settling of proppant may interfere with proper
placement
downhole. The resulting suspension preferably forms a pack of particulate
material that
is permeable to fluids produced from the wellbore and substantially prevents
or reduces
production of formation materials from the formation into the wellbore.
The carrier fluid may further contain one or more conventional additives to
the
well service industry such as a gelling agent, crosslinking agent, gel
breaker, surfactant,
biocide, surface tension reducing agent, foaming agent, defoaming agent,
demulsifier,
non-emulsifier, scale inhibitor, gas hydrate inhibitor, polymer specific
enzyme breaker,
oxidative breaker, buffer, clay stabilizer, acid, buffer, solvent or a mixture
thereof and
other well treatment additives known in the art. The addition of such
additives to the
carrier fluids minimizes the need for additional pumps required to add such
materials on
the fly.
The curable resin coated plastic beads may be advantageously pre-suspended as
a
substantially neutrally buoyant particulate and stored in the carrier fluid
(e.g., brine of
near or substantially equal density), and then pumped or placed downhole as
is, or diluted
on the fly.
The term "substantially neutrally buoyant" refers to plastic bead particulates
that
have an ASG sufficiently close to the ASG of the selected ungelled or weakly
gelled
carrier fluid (e.g., ungelled or weakly gelled completion brine, other aqueous-
based fluid,
slick water, or other suitable fluid) which allows pumping and satisfactory
placement of
the proppant/particulate using the selected ungelled or weakly gelled carrier
fluid.
It may be preferred to pump an activator with the slurry into the formation in
order to assist bonding of the curable resin particulates and accelerate the
downhole
curing process. Suitable activators include those commercially available from
Santrol of
Fresno, Texas.
The curable resin coated plastic beads may be introduced into the wellbore at
any
concentration deemed suitable or effective for the downhole conditions to be
encountered.
8
CA 02580304 2007-03-05
In a preferred embodiment, the curable resin coated plastic beads and/or
substantially neutrally buoyant curable resin coated plastic beads are used in
a sand
control method. The beads may be introduced into the wellbore in a slurry with
a carrier
fluid. The beads are placed adjacent the subterranean formation to form a
fluid-
permeable pack. The fluid permeable pack is capable of reducing or
substantially
preventing the passage of formation particles from the subterranean formation
into the
wellbore while at the same time allowing passage of formation fluids from the
subterranean formation into the wellbore.
In a preferred gravel pack operation, a screen assembly may be placed or
otherwise disposed within the wellbore so that at least a portion of the
screen assembly is
disposed adjacent the subterranean formation. (The gravel pack operation may
further
proceed using a screenless pack.) A slurry containing the curable resin coated
plastic
beads may then be introduced into the wellbore and placed adjacent the
subterranean
formation by circulation or other suitable method. A fluid-permeable pack is
formed in
the annular area between the exterior of the screen and the interior of the
wellbore which
is capable of reducing or substantially preventing the passage of formation
particles from
the subterranean formation into the wellbore during production of fluids from
the
formation. At the same time, the permeable pack allows the passage of
formation fluids
from the subterranean formation through the screen into the wellbore. When the
flow is
reversed, the consolidated curable resin coated plastic beads will flow back
with minimal
formation sands. Particularly advantageous results are obtained in horizontal
gravel
packing which are large, such as those 6,000 ft long.
The curable resin coated plastic beads may be mixed with the carrier fluid in
any
manner suitable for delivering the mixture to a wellbore and/or subterranean
formation.
In one embodiment, the disclosed particulates may be injected into a
subterranean
formation in conjunction with a hydraulic fracturing treatment or other
treatment at
pressures sufficiently high enough to cause the formation or enlargement of
fractures, or
to otherwise expose the particles to formation closure stress. Such other
treatments may
be near wellbore in nature (affecting near wellbore regions) and may be
directed toward
improving wellbore productivity and/or controlling the production of fracture
proppant or
formation sand.
9
CA 02580304 2007-03-05
The curable resin coated plastic beads are further employed in frac-pack
operations, especially in unconsolidated and semi-consolidated formations in
order to
facilitate fluid recovery while preventing particulate migration. The frac-
pack operation
typically embodies the features of both a fracturing operation and a gravel
packing
operation. The unconsolidated formation may initially be fractured using the
particulate
materials. Additional proppant may then be held in place in the wellbore by
(a) packing
the material around a gravel packing screen and/or (b) consolidating the
proppant
material by means of a resin coating.
The following examples will illustrate the practice of the present invention
in its
preferred embodiments. Other embodiments within the scope of the claims herein
will be
apparent to one skilled in the art from consideration of the specification and
practice of
the invention as disclosed herein. It is intended that the specification,
together with the
Examples, be considered exemplary only, with the scope and spirit of the
invention being
indicated by the claims which follow.
EXAMPLES
Example 1: This Example illustrates the ability of curable resin coated PSDVB
beads
withstand greater than 20 psi stress without breaking.
Curable phenolic resin coated polystyrene divinylbenzene (PSDVB) beads
(containing 10 weight percent divinylbenzene) were prepared by heating the
beads in the
presence of 7.5% (wt) of phenolic resin. As the temperature increased, the
resin melted.
The higher the temperature, the quicker the resin cured. At the point where
the phenolic
resin became to demonstrate signs of a "breakout" or "dryout" where the beads
separated,
heat was removed to limit the amount of curing.
A core was made by mixing 100 grams of the resulting curable resin coated
PSDVB beads with about 100 ml of 2% potassium chloride solution and from 0 to
15
gallons per thousand (gpt) (1.5 volume percent) of activator, commercially
available from
Santrol as SuperSet PTM. The resulting slurry was introduced into a two-inch
diameter
high pressure high temperature (HPHT) 500 ml stainless steel cell. Excess
fluid was then
displaced from the cell when nitrogen gas was applied to the movable piston.
500 psi
pressure was then applied to the piston and the cell was heated to either 70
F or 100 F.
CA 02580304 2007-03-05
After four hours, the external heat source was terminated and the pressure was
released.
After cooling and removing the formed core from the cell, the core was placed
between
two parallel platens until a stress was reached where the core failed. This
unconfined
compressive strength (UCS) of the samples is set forth in Table I.
Table I
Sample Activator Amount (gpt) Temperature, F UCS (psi)
A 15 100 28.00
B 10 100 6.41
C 4.2 100 6.60
D 0 100 3.40
E 10 70 7.90
F 5 70 4.90
G 0 70 2.70
This unconfined compressive strength (UCS) test showed the ability of the
curable resin coated PSDVB beads of the invention to exceed stress levels of
20 psi and
is a measurement of the ability of the beads to adhere together. The
compressive strength
and permeability of the curable resin coated PSDVB beads are shown to be
higher at
100 F than at 70 F. This is further illustrated in FIG. 1.
Example 2. This Example illustrates the insolubility of the curable resin
coated
particulates of the invention in acid media.
PSDVB beads (containing 10 weight percent divinylbenzene) having a phenolic
curable resin coating were prepared in a manner similar to that set forth in
Example 1
above. The particulate exhibited a tan color with fine particles ranging in
color from dark
brown to light orange. Samples were dried for one hour at 150 F in order to
obtain a
constant weight. Acid solubility tests were then conducted with the sample at
room
temperature in 15% HCl and 12% HCI-3% HF systems. Each acid solubility test
was run
in triplicate. In each test, a 5.0 gram sample of the particulate was added to
a plastic
bottle and 100 ml aliquot of acid was added. Each sample bottle was then
thoroughly
mixed and allowed to stand for one hour at 70 F. After the one-hour
incubation period,
11
CA 02580304 2007-03-05
the acid and the particulate solids were filtered through a Whatman 41 filter
paper to
collect the remaining solids. After complete filtration of the acid, the
solids were washed
with distilled water to remove any remaining acid. The collected solids were
placed in a
150 F oven for one hour for drying. After drying, the remaining solids were
removed
from the Whatman #1 filter paper and their weight was determined on a balance.
The
final weight of the solids was utilized to determine the percent acid
solubility. Acid
solubilities were calculated by the following formula:
Initial weight - final weight =% solids dissolved
Initial weight
The results are set forth in Table 11 below.
Table II
Sample Acid System Initial Weight Final Weight % Acid Solubility
A 15% HCI 5.00 4.97 0.006
B 15% HC1 5.00 4.96 0.008
C 15% HCI 5.00 4.96 0.008
D 12% HC1-3% HF 5.00 4.98 0.004
E 12% HCl-3% HF 5.00 4.95 0.01
F 12% HC1-3% HF 5.00 4.97 0.006
Table II shows that the curable resin coated PSDVB particulate exhibits very
low acid
solubility in 15% HCl and 12% HCl-3% HF systems.
Example 3.
A phenolic curable resin coated PSDVB particulate was prepared as set forth
above in Example 1. Conductivity tests were performed on the phenolic curable
resin
coated PSDVB particulate as well as the uncoated PSDVB particulate in
accordance with
a modified API RP 61 (1St Revision, Oct. 1, 1989) using an API conductivity
cell with
Ohio sandstone wafer side inserts to simulate the producing formation. A
multilayer
pack of the composite containing about 31.5 g of particulate was then loaded
between the
sealed sandstone wafers to increase the propped width. The conductivity cell
was then
placed on a press while stress was applied at 100 psi/minute until the target
temperature
was reached. Fluid was then allowed to flow through the test pack maintaining
Darcy
12
CA 02580304 2007-03-05
flow. The differential pressure was measured across 5 inches of the pack using
a
"ROSEMOUNT" differential pressure transducer (#3051 C). Flow was measured
using
Micromotion mass flow meters and data points were recorded every 2 minutes for
50
hours. An Isco 260D programmable pump applied and maintained effective closure
pressure. Experimental parameters and results are set forth in Tables III and
IV for the
curable resin coated PSDVB particulate and the uncoated PSDVB particulate,
respectively.
Table III
Temperature: 150 F
Particulate Size: 16/40
Time, Closure, Conductivity, Width, Permeability,
Hours Psi md-ft Inches Darcies
0 1008 1926 0.235 98
10 994 1565 0.232 81
24 994 1432 0.232 74
0 2013 1243 0.227 66
10 2012 822 0.220 45
2011 709 0.216 39
24 2014 684 0.216 38
Table IV
15 Temperature: 150 F
Particulate Size: 20/40
Time, Closure, Conductivity, Width, Permeability,
Hours Psi md-ft inches Darcies
0 992 1081 0.226 57
10 988 742 0.220 40
24 994 665 0.217 37
0 2005 544 0.212 31
10 2003 308 0.205 18
20 2003 270 0.200 16
24 2004 255 0.200 15
13
CA 02580304 2007-03-05
Tables III and IV illustrate increased permeability and conductivity with the
curable resin coated PSDVB beads versus the uncoated PSDVB beads,
respectively.
Example 4.
The phenolic curable resin coated PSDVB particulate and uncoated PSDVB
particulate of Example 3 were tested in accordance with Example 3.
Experimental
conditions and results are set forth in Tables V and VI, respectively.
Table V
Temperature: 100 F
Particulate Size: 16/40
Time, Closure, Conductivity, Width, Permeability,
Hours Psi md-ft inches Darcies
0 58 8021 0.282 341
10 46 7650 0.282 326
24 41 7936 0.282 338
0 496 7239 0.2350 370
10 496 6637 0.2350 339
497 6621 0.2320 342
24 496 6812 0.2320 352
15 Table VI
Temperature: 100 F
Particulate Size: 20/40
Time, Closure, Conductivity, Width, Permeability,
Hours Psi md-ft inches Darcies
0 52 3713 0.247 180
10 49 4398 0.247 214
24 40 4778 0.247 232
0 143 4393 0.247 213
10 513 3697 0.233 190
20 512 3427 0.228 180
24 513 3435 0.228 181
14
CA 02580304 2007-03-05
Tables V and VI illustrate increased permeability and conductivity with the
curable resin coated PSDVB beads versus the uncoated PSDVB beads at lower
temperatures and at 500 psi closure stress (as compared to the data set forth
in Tables III
and IV) above.
From the foregoing, it will be observed that numerous variations and
modifications may be effected without departing from the true spirit and scope
of the
novel concepts of the invention.