Note: Descriptions are shown in the official language in which they were submitted.
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
1
TITLE OF THE INVENTION
Isolation of Growth and Differentiating Factors from Colostrum
FIELD OF THE INVENTION
The present invention relates to a novel process for isolating growth and
differentiating factors from colostrum. This process is characterized by
maturation steps (controlled mild acid hydrolysis) and physical steps
(molecular
filtration). The invention further includes the use of the growth and
differentiating
factors derived from this process in prophylactic, therapeutic, cosmetic,
cosmeceutical, dermatological, pharmaceutical, medical, veterinary or surgical
(burn wounds, wounds, etc.) applications.
BACKGROUND OF THE INVENTION
Colostrum is a thick, yellow fluid produced by mammary glands during the first
few days after birth. It provides life-supporting immune (gamma globulin) and
growth factors that ensure the health and vitality of a newborn.
The identities and functions of many of the bioactive principles of colostrum
milk
remain to be elucidated. However, colostrum is known to be a source of
numerous bioactive hormones and growth factors, many of which have been
demonstrated to influence intestinal growth, cell differentiation, and the
development of the immune and enteroendocrine systems when administered in
isolation.
Growth factors may be defined as proteins of 5 to 680 kDa that possess growth
modulating bioactivities. Their biological actions also include the modulation
and
facilitation of the expression of cellular phenotype. To exert biological
effects,
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
2
growth factors must interact with specific high-affinity membrane receptors
that
activate appropriate signal transduction/second messenger cascades.
In their natural state, most growth factors are inert on human cells and have
very
high molecular weights (340-580 kDa). In order to become active, these growth
factors need to be released from their inactive original forms either through
hydrolysis or temperature change, or both.
Interestingly, even growth factors from non-human origin, such as those
derived
from porcine or bovine colostrum, when converted into their active forms, have
been found to be active on human cells. This can be explained by the fact that
the active forms of smaller molecular weight are almost completely homologous
to the corresponding human growth factors. This has been found to be the case,
for example, for the following families of factors: IGFs (1-3), TFGs 3 (1-3),
PDGFs
(AA, AB, BB), BMPs (1-24) and FGFs (1-16). These factors, when in active form,
are recognized for their ability to proliferate and/or differentiate the stem
cells of a
newborn.
United States Patent No. 6,277,813 (Kelly) describes the extraction of a novel
growth factor from porcine colostrum. The process for extracting this growth
factor, identified as CDGF for "Colostrum Derived Growth Factor", includes the
following steps: (1) separating all components of colostrum having a molecular
weight below 200 kDa and discarding all components having a lower molecular
weight; (2) treating the product of step 1 with dithiothreitol and boiling for
10
minutes; and (3) centrifuging the mixture of step (2) to spin down any
precipitated
matter and recovering the CDGF located in the supernatant.
United States Patent No. 5,500,229 (Aalto et al.) discloses a colostral
fraction
having a low endotoxin, protein and immunoglobulin concentration. The
colostral
fraction is obtained through ultrafiltration of defatted colostrum using a
membrane
having a molecular weight cut off of 100 kDa and is intended for use as a
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
3
supplement in cell culture media. The colostral fraction is said to be
extremely
useful either alone or when complemented by other supplements for replacing
partially or completely fetal bovine serum in widely used cell culture media.
The
patent describes the effectiveness of the colostral fraction in the
cultivation of
hybridoma cells. (This invention is also described in Appl Microbiol
Biotechnol
(1992) 37: 451-456.)
European Patent No. 918464 (Adler etal.) discloses a process for preparing a
colostral milk product from which casein has been largely removed and the
colostrum has been defatted. The defatted and largely decaseinated colostrum
is
passed through an ultrafiltration column with an exclusion molecular mass of
approximately 106. The product obtained can be further filtered using columns
with exclusion molecular masses of 300 kDa and/or 150 kDa and/or 50 kDa
and/or 30 kDa and/or 20 kDa and/or 10 kDa and/or 5 kDa and/or 1 kDa and/or 0.5
kDa. The resulting products are said to be suitable for use as an additive for
drugs, food supplements, beverages, baby food, animal food, beverages in
intensive sport for muscle protection or for reducing the muscular recovery
phase,
and for the prevention and treatment of bacterial, viral and mycotic
infections.
Chinese Patent No. 1557837 (Gao Chunping) describes a process to separate
insulin-like growth factor, immunoglobulins and casein from bovine colostrum.
Colostrum is defatted and acidified to separate the insulin-like growth factor
from
binding, and the insulin-like growth factor is isolated through
ultrafiltration,
concentrated and freeze dried to obtain a powder. lmmunoglobulins are
separated through ultrafiltration and concentrated to prepare a powdered
product.
Casein is obtained through ultrafiltration or pH regulation, heat solidified
and
reacted with hydrolase to prepare casein phosphate polypeptide. The process is
said to greatly lower production costs.
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
4
Chinese Patent No. 1557340 (also to Gao Chunping) describes a method of
preparing a high bioreactivity growth factor and immunoglobulin from bovine
colostrum. The method involves collecting colostrum 72 hours after
parturition,
defatting the colostrum through centrifugation, acidifying the solution,
heating to
solidify casein, centrifugally filtering or filtering the solution with cloth
to eliminate
casein, diluting the resulting solution, collecting the supernatant,
concentrating
with low molecular weight ultrafiltration membranes, and processing further in
order to produce a dry powder preparation, a spray preparation, and the like.
The
product is intended for use in the treatment of various bacterial and viral
infections.
United States Patent No. 6,875,459 (Kopf et al.) discloses a method and
apparatus for separation of milk, colostrum and whey components. In a
preferred
embodiment, the apparatus and method employ cross-flow filtration,
chromatography and fermentation to separate the components of milk, colostrum
and whey. The apparatus and method allow the extraction of immunoglobulins,
among other factors.
European Patent No. 711171 (Laato at al.) describes a method for the
improvement of wound healing in mammals, including humans, by using a
colostral fraction. The colostral fraction is prepared by subjecting
colostrum, from
which part of the fat and cellular debris have been removed by conventional
methods such as centrifugation, to ultrafiltration by using a membrane having
a
cutoff of 100 kDa and recovering the filtrate. The method for promoting wound
healing consists of administering the colostral fraction locally.
PCT Publication No. WO 9811910 describes the use of a composition containing
at least one compound with Growth Factor-like activity for the prevention or
treatment of a gastrointestinal condition that is characterized at least
partially by
damage to epithelial cells and caused by the administration of a non-steroidal
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
anti-inflammatory drug. Compositions for use in the invention may contain an
IGF
(e.g. IGF-1 or 2), a transforming growth factor (e.g. TGF1, TGF2 or TGF3), a
keratinocyte growth factor, a fibroblast growth factor and/or a platelet-
derived
growth factor. The compositions containing the TGFs are preferably, though not
5 exclusively, derived from colostrum. Similarly, PCT Publication No. WO
9811904
describes the use of colostrum or a derivative thereof for the prevention or
treatment of a gastrointestinal condition that is characterized at least
partially by
damage to epithelial cells and caused by the administration of a non-steroidal
anti-inflammatory drug. Derivatives suitable for use include ultrafiltered or
microfiltered fractions of colostral whey (colostrum from which casein
proteins
have been removed), which are said to contain more concentrated Growth
Factors relative to remaining colostral proteins and nutrients. Colostral whey
may
be used in liquid form (which may be defatted if desired) or may be further
treated
(such as being spray dried).
Other methods for the extraction of growth factors are known in the art, but
surprisingly, no process appear to exist for deliberately and simultaneously
isolating growth factors with highly disparate molecular weights. In addition,
a
number of methods rely on temperature conditions that have the effect of
destroying the activity of the growth factors that are sought to be extracted.
There is therefore a need for a method of isolating growth and differentiating
factors from colostrum that permits the separation of a great number of these
factors (or "pools" of factors) in a manner that is efficient, reproducible
and non-
deleterious to their activities.
SUMMARY OF THE INVENTION
In accordance with the present invention, there is provided a novel process
for
isolating growth and differentiating factors from colostrum. More
specifically, this
CA 02580372 2010-12-09
6
process is characterized by maturation steps (controlled mild acid hydrolysis)
and
physical steps (molecular filtration) which optimize recovery of measured
growth
factors and their ability to entice a response on human cells.
In contrast to processes that are known in the art, the process of the present
invention is neither performed at boiling temperatures. What results from this
process are novel filtrate "pools" containing factors that are active on human
cells,
even if the colostrum is of bovine origin.
In one preferred embodiment, the process includes:
= Diluting the colostrum and subjecting it to partial hydrolysis by
adjusting the
pH to about 3.75-3.85;
= vortexing the resulting colostral solution 60 minutes (30-90 minutes);
= precipitating casein by adjusting the pH of the colostral solution to
about 4.52-
4.55;
= centrifuging the new colostral solution, and setting aside the resulting
supernatant; and
= running the supernatant through a filtration system comprising one or
more
filtration columns (ceramic membranes) in order to obtain a fraction
containing
pools of growth and differentiating factors,
all the while ensuring that the reaction temperature never exceeds (about) 38
C.
In another embodiment, the process is performed all the while ensuring that
the
reaction temperature never exceeds about 37 C.
In another embodiment, the process further comprises lyophilizing the pools of
derived growth and differentiating factors.
Generally, the process includes a filtration system which is comprised of one
or
more filtration columns selected from the following filtration sizes: 0.2pm,
300
kDa, 150 kDa, 50 kDa, 15 kDa and 5 kDa. More specifically, and depending on
the content and concentration of pooled growth and differentiating growth
factors
that are sought, the filtration system is selected from one of the following:
a 0.2pm
column; a 300 kDa column; a 150 kDa column; a 50 kDa column; a 15 kDa
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
7
column; a 0.2pm column linked with a 150 kDa column; a 0.2pm column linked
with a 15 kDa column; and a 150 kDa column linked with a 15 kDa column.
The invention further includes colostral fractions isolated from the process
of the
present invention. Such fractions may include one or more fractions selected
from the following: LP1, LP2, LP3, LP4, LP5, LP1-LP3, LP1-LP5 and LP3-LP5
(see the compositions of these fractions in Table 5). Depending on the
application, these fractions may be used in their native form or they may be
combined with an excipient or carrier.
Advantageously, this process allows the derivation and isolation of growth and
differentiating factors, with the result that a number of factors with highly
disparate
sizes (or molecular weights) can be separated in pools from one another and
used in select and varied ways, such as in cosmetic, cosmeceutical,
nutraceutical, dermatological, pharmaceutical, medical and veterinary
applications.
Other objects, advantages and features of the present invention will become
apparent upon reading of the following non restrictive description of
preferred
embodiments thereof, given by way of example only with reference to the
accompanying drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1: Schematic view of the process steps for the isolation of growth
factors
from colostrum, including (A) controlled mild acid hydrolysis and (B)
molecular
filtration.
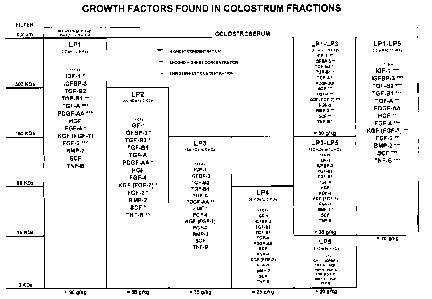
Figure 2: Growth factors found in colostral fractions.
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
8
Figure 3: Human ELISA test results for growth factors found in colostral
fractions.
Figure 4: Fibroblast growth (Hoechst) during 72 hr exposure.
Figure 5: Fibroblast growth (new pools, Hoechst) during 72 hr exposure.
Figure 6: Proliferation of Human Umbilical Vein Endothelial Cells (HUVECs)
(Cyquane).
Figure 7: Percent of proline integrated into collagen synthesis.
Figure 8: Collagen Synthesis and Deposition in monolayer cell cultures as a
function of cell number.
Figure 9: Collagen synthesis and deposition by fibroblasts in fibrin gel (new
LP
pools).
Figure 10: Fibroblasts grown in fibrin gel for 7-9 days. In the presence of
3.3
mg/ml LP1-LP3, fibroblasts formed a dense matrix as observed on phase contrast
(A) whereas the cell density was limited as observed after Hoescht staining
(B).
Conversely, the control culture in serum-free resulted in poor matrix density
(C&D), compared to A & B. At day 8, fibroblast-containing fibrin gels were
released from the culture wells and observed the next day for potential
contraction as observed in the presence of 3.3 mg/ml LP1-LP3 (E) compared to
control culture (F). In the presence of LP1-LP5, at 1 mg/ml, fibroblasts
reorganized into a network as observed by phase contrast (G) and at 3.3 mg/ml
fibrin liquefied and agregated (H). Magnification at 20X.
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
9
figure 11: Human fibroblast proliferation assay (Cyquant ); 0.33 mg/ml, 1
mg/ml
and 3.30 mg/ml of growth factor pools LP1-LP3, LP1-LP5 and LP3-LP5 were
tested.
Figure 12: Effect on chondrocyte proliferation of 1 mg/ml and 3 mg/ml LP1-LP5
incubated 3 days.
Figure 13: Effect on chondrocyte proliferation of 1 mg/ml and 3 mg/ml LP1-LP5
incubated 7 days.
Figure 14: Effect on chondrocyte proliferation of 1 mg/ml and 3 mg/ml LP1-LP5
incubated 10 days.
Figure 15: Average number of chondrocytes treated with LP1-LP5 over three
different periods of time.
Figure 16: Epidermal covering (epidermization) at day 7 due to LP1-LP3, LP1-
LP5 and LP3-LP5.
Figure 17: Diminution of wound areas (granulation tissue) caused by LP1-LP3,
LP1-LP5 and LP3-LP5 after 7 days, 14 days and 28 days.
Figure 18: Wound (dermal) thickness 5 after 7 days and 14 days resulting from
LP1-LP3, LP1-LP5 and LP3-LP5.
Figure 19: Formation of collagen fibers due to LP1-LP3, LP1-LP5 and LP3-LP5
after 7 days, 14 days and 28 days.
Figure 20: Ratio of epidermization for fractions LP1 and LP1-LP3, and LP1-LP5,
after 5, 7 and 10 days.
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
Figure 21: Specific activity of alkaline phosphatase at days 0, 6 and 12 for
brush
cells exposed to LP1, LP1-LP3, LP1-LP5 and LP3-LP5.
5 Figure 22: Specific activity of sucrase at days 0, 6 and 12 for brush
cells exposed
to LP1, LP1-LP3, LP1-LP5 and LP3-LP5.
Figure 23: Specific activity of lactase at days 0, 6 and 12 for brush cells
exposed
to LP1, LP1-LP3, LP1-LP5 and LP3-LP5.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
Definitions: Unless defined otherwise, all technical and scientific terms used
herein have the same meaning as commonly understood by one of ordinary skill
in the art to which this invention belongs.
"Cosmeceutical": A cosmetic product claimed to have medicinal or drug-like
benefits. Cosmeceutical products are marketed as cosmetics, but reputedly
contain biologically active ingredients. Examples include anti-wrinkle skin
creams
with ingredients such as alpha lipoic acid and dimethylaminoethanol.
"Brush cell": A brush cell has rootlet like projections as a tuft that form
squat
microvilli with filaments that stretch into the cell's cytoplasm; about 120-
140
microvilli may be found on each cell, and the cell has a skewed or tilted
position in
tissue sections. Brush cells have been identified in the gastrointestinal
(about
0.3% cells) and respiratory tracts. Identification of brush cells has relied
primarily
on morphology with electron microscopy; they have a distinctive pear shape
with
a wide base, and a narrow microvillous apex. The function of brush cells is to
activate the digestion and absorption of sugars, amino acids and small chain
carbohydrates in the bowel.
CA 02580372 2010-12-09
11
"Digestive epithelium": The digestive tube, which is comprised of comprised of
the
oral cavity (mouth), pharynx (throat), esophagus, stomach, small intestine,
large
intestine and rectum is lined by a simple (1 cell thick) epithelium that is
continuous at either end with the epidermis of the skin. This digestive
epithelium
is a mucosa i.e. an epithelium that secretes watery mucus for the purpose of
lubrication.
The following is a list of all growth and differentiating factors detected by
human
ELISA tests built in with factors of human origin as standards. (See also
Figure
3.)
BMP-2: bone morphogenic protein 2
BMP-4: bone morphogenic protein 4
EGF: epidermal growth factor
FGF-2: (basic) fibroblast growth factor basic
FGF-4: fibroblast growth factor 4
HGF: hepatocyte growth factor
IGF-1: insuline-like growth factor 1
IGFBP-1: insuline-like growth factor binding protein 1
IGFBP-3: insuline-like growth factor binding protein 3
KGF (FGF-7): keratinocyte growth factor (fibroblast growth factor-7)
PDGF-AA: platelet-derived growth factor-AA
PDGF-AB: platelet-derived growth factor-AB
PDGF-BB: platelet-derived growth factor-BB
PLGF: placenta growth factor
SCF: stem cell factor c-kit ligand
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
12
TGF-a: transforming growth factor alpha
TGF-131: activated transforming growth factor beta 1
TGF-132: activated transforming growth factor beta 2
TNFa: tumor necrosis factor alpha
TNFI3: tumor necrosis factor beta
VEGF: vascular endothelial growth factor
Example 1: Isolation of Growth and Differentiating Factors from
Commercially Available Bovine Colostrum
The process of the invention, shown schematically in Figures 1 (A) & (B), will
now
be described.
It should be appreciated here that while this process is specifically
described for
use with colostrum, substitutes for colostrum, namely other milks and milk
products, may also be used. The efficiency of the process is believed to be
enhanced with colostrum, because colostral milk contains a higher
concentration
of growth and differentiation factors than other milks and milk products.
Colostral
substitutes ¨ filter sterilized milk, modified milk (i.e., milk from which the
fatty
constituents have been wholly or partially removed, with or without the
addition of
vitamins or solid elements derived from milk), enriched milk (i.e., enriched
with
non-fat solids), vitaminized milk (milk with vitamins added), and lacto-serum
¨
may also be used as starting materials since they are known to contain growth
and differentiation factors of a similar nature. However, not all the factors
will be
found in milk, and then, not in the same concentration as in colostrum. When
using a colostral substitute, it will be necessary to modify the process
slightly to
maximize the yields of growth and differentiation factors. Such modifications
should be within the purview of one of skill in the art.
CA 02580372 2010-12-09
13
1. Preliminary Preparation
When starting with a lyophilized colostral preparation (freeze dried colostrum
exempt of fat, coliforms and antibiotics) it has been found that the best way
to
reconstitute colostrum is to dissolve 80 g per liter of water (18.2 Mega
Ohms).
The best pH for extraction is between about 3.75 and 3.85. The colostrum is
adjusted to this pH (with a 10 N HCI solution, for example) and then placed in
an
agitator (Hobartn"), at the first speed for 60 minutes. The pH of the solution
is
readjusted, with NaOH ION, to a pH of about 4.52-4.55 (precipitating casein)
and
the solution agitated for another 15 minutes before being centrifuged for 20
minutes at 9285 G. The best results were observed using a Beckmann" Avanti J-
20XPX-12 with rotor JLA 8.1 centrifuging 6 liters at a time for 20 minutes at
9285
G.
The combined precipitates are dissolved in water for re-extraction (12 liters
of
18.2 Mega Ohms for every kg of precipitate) and centrifuged again for about 20
minutes at 9285 G. The supernatants from each bottle are added to the pooled
supernatant (1) from the first centrifugation. The final pH of the solution
sometimes needs to be readjusted as it will be 4.35-4.40 instead of the 4.50-
4.65
required for optimal results.
The solution is now ready for filtration and lyophilization, as described
below.
2. Filtration using TAMILABC) Filter System
Using the solution obtained in step 1, filtration is conducted by passing the
supernatant through progressively smaller filtration columns, or molecular
sieves.
The choice of molecular sieve will depend on the fraction that is sought. As
shown in Table 1, these fractions are identified as LP1 to LP5, depending on
the
filtration column selected.
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
13
1. Preliminary Preparation
When starting with a lyophilized colostral preparation (freeze dried colostrum
exempt of fat, coliforms and antibiotics) it has been found that the best way
to
reconstitute colostrum is to dissolve 80 g per liter of water (18.2 Mega
Ohms).
=
The best pH for extraction is between about 3.75 and 3.85. The colostrum is
adjusted to this pH (with a 10 N HCI solution, for example) and then placed in
an
agitator (Hobartn"), at the first speed for 60 minutes. The pH of the solution
is
readjusted, with NaOH ION, to a pH of about 4.52-4.55 and the solution
agitated
for another 15 minutes before being centrifuged for 20 minutes at 9285 G. The
best results were observed using a Beckman TM Avanti J-20XPX-12 with rotor JLA
8.1 centrifuging 6 liters at a time for 20 minutes at 9285 G.
The combined precipitates are dissolved in water for re-extraction (12 liters
of
18.2 Mega Ohms for every kg of precipitate) and centrifuged again for about 20
minutes at 9285 G. The supernatants from each bottle are added to the pooled
supernatant (1) from the first centrifugation. The final pH of the solution
sometimes needs to be readjusted as it will be 4.35-4.40 instead of the 4.50-
4.65
required for optimal results.
The solution is now ready for filtration and lyophilization, as described
below.
2. Filtration using TAMILAB Filter System
Using the solution obtained in step 1, filtration is conducted by passing the
supernatant through progressively smaller filtration columns, or molecular
sieves.
The choice of molecular sieve will depend on the fraction that is sought. As
shown in Table 1, these fractions are identified as LP1 to LP5, depending on
the
filtration column selected.
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
14
Table 1: Correspondence between Filtration Column and Fraction
Filtration Size Retention Fraction
0.2 pm 5 kDa LP1
300 kDa 5 kDa LP2
150 kDa 5 kDa LP3
50 kDa 5 kDa LP4
15 kDa 5 kDa LP5
In accordance with one embodiment of the present invention, in order to obtain
fraction LP3 (150 kDa ¨ 5 kDa), a first filtration is performed using a 0.20
pm
column. A column of this size will eliminate unwanted factors quickly before
the
supernatant is passed through the 150 kDa column, which is the column that is
suitable for the LP3 fraction.
Moreover, in-between fractions may also be generated. For example, fraction
LP3 may be filtered on a 50 kDa molecular sieve (used to obtain LP4). The
result
will be a retentate having a cutoff molecular weight of 150 kDa to 50 kDa (LP3-
LP4). Similarly, LP4 may be filtered on a 15 kDa molecular sieve (used to
obtain
LP5). The result will be a retentate having a cutoff molecular weight of 50
kDa to
kDa (LP4-LP5).
As may be seen in Table 2, certain in-between fractions or pools were found to
be
especially interesting. These are LP1-LP3, LP1-LP5 and LP3-LP5. To prepare
the LP1-LP3 fraction or pool, the solution resulting from Step 1, above, is
run
through a column having a 0.2 pm cutoff and then through a column having a 150
kDa cutoff. Similarly, to prepare the LP1-LP5 fraction or pool, the solution
resulting from Step 1, above, is run through a column having a 0.2 pm cutoff
and
then through a column having a 15 kDa cutoff. Likewise, to prepare the LP3-LP5
fraction or pool, the solution resulting from Step 1, above, is run through a
column
having a 150 kDa cutoff and then through a column having a 15 kDa cutoff.
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
3. Lyophilization
This operation must be done very carefully in order to maximize efficiency.
The
different fractions are divided into samples of 2.5 liters per tray on
lyophilizer FTS
5 and frozen at about -35 C. This method permits rapid freezing without
liquid
nitrogen.
In a FTS tray lyophilizer the tray must be placed one at a time at 4 C without
vacuum then frozen to -35 C before applying vacuum (10-100 mThors) at -80 C
10 to -85 C. for approximately 36-48 hours; the lyophilized samples, once
in the form
of a fine powder (250-500 pm) are ready for encapsulation or ready to be
pooled
and conserved in storage bags (sterile freezer bags) at a temperature of
approximately -18 C to -20 C.
15 Using the process described above, it is possible to isolate growth and
differentiating factors from colostrum. Figure 2 shows the growth factors
found in
the following fractions, as verified through human ELISA testing: LP1, LP2,
LP3,
LP4, LP5, LP1-LP3, LP3-LP5 and LP1-LP5.
Figure 3 reveals the quantities of certain of the growth factors identified in
Figure
2. The quantities, measured through human ELISA, are per kg of colostrum.
Table 2 shows the quantity of isolated product per fraction for colostrum (1
kg; dry
matter basis).
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
16
Table 2: Quantity of Isolated Product per Fraction for Colostrum
Fraction Filter Weight (g/Kg)
LP1 F 0.2 pm ¨ R 5 kDa = 90
LP2 F 300 kDa ¨ R 5 kDa = 55
LP3 F 150 kDa - R 5 kDa =35
LP4 F 50 kDa - R 5 kDa =25
LP5 F 15 kDa - R 5 kDa =20
LP1-LP3 F 0.2 pm ¨ R 150 kDa = 50
LP1-LP5 F 0.2 pm ¨ R 15 kDa = 70
LP3-LP5 F 150 kDa ¨ R 15 kDa =30
Example 2: Isolation of Growth and Differentiating Factors from Natural
Colostrum (from dairy cows)
1. Preliminary Preparation
Frozen colostrum is thawed (storage temperature is -20 C) then centrifuged 6
liters at a time at 20 C. The layer of butter and other residues are filtered
first
through cheesecloth and then through a WhatmanTM 541 ashless filter. A
thorough removal of this layer of fat will facilitate filtration and enhance
the overall
isolation of the growth and differentiating factors.
This preliminary filtration is followed by acid extraction at a pH of about
3.75-3.85.
It is convenient to use a 10 N HCI solution for this purpose. If needed, water
can
be added to the supernatant (to a maximum of about 10%) in order to increase
the fluidity of the supernatant for extraction. This greatly enhances
filtration on the
TAMILAB system of columns (0.20 pm, 300 kDa, 150 kDa, 50 kDa, 15 kDa and
5 kDa), as will be described below.
The solution is now ready for filtration and lyophilization, as described in
Example
1.
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
17
NB: As with Example 1, it should be appreciated here that while this process
is
specifically described for use with colostrum, substitutes for colostrum,
namely
other milks and milk products, may also be used. . When using a colostral
substitute, it may be necessary to modify the process slightly to maximize the
yields of growth and differentiation factors. Such modifications should be
within
the purview of one of skill in the art.
Example 3: Preferred Isolation Method for LP1-LP5
The following process is based on that shown schematically in Figures 1(A) and
(B).
1. Isolation
1.1 Starting with a lyophilized colostral preparation (freeze dried colostrum
exempt of fat, coliforms, E. coli, S. aureus, salmonella, clostridium and
antibiotics, colostrum is reconstituted by dissolving 1000 g of raw colostrum
per 12 liters of water (0.2 pm filtered), placed in a blending tank (HobartTM)
and agitated for 15 minutes;
1.2 The
pH is adjusted to between 3.75 and 3.85 with a 10 N HCI solution and
run at 400 rpm for 60 minutes;
1.3 The pH of the solution is readjusted with a 10N NaOH solution, to a
pH of
about 4.50-4.60, and the solution agitated for another 15 minutes at the
same speed;
1.4 The solution is centrifuged for 20 minutes at about 9285 G, 18 C using a
BeckmanTM Avanti J-20XPX-12 with rotor JLA 8.1, centrifuging 6 liters at a
time;
1.5 The supernatant is filtered on Whatman 541 Ashless filter and store
in a 25
liter bottle at 4 C until all centrifugation is completed and move on to
filtration; and
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
18
1.6 The total quantity of quantity of solution to be filtered is between 20.5
to
21.5 liters.
2. Filtration using TAMILAB Filter System
2.1 Before starting filtration, the machine is rinsed by running 7 liters of
alcohol
(70%) through the system, letting 2 liters out and letting stand for 5 minutes
before draining 5 liters;
2.2 The machine is rinsed again by running 5 liters of filtered water
(0.2 pm)
through the system, letting 2 liters out and draining from the system;
2.3 The solution from in step 1 is filtered by passing the supernatant through
two 0.2 pm Dahlia ceramic columns with 1000 cm2 surface (CeRAM from
TAMI Industries);
2.4 The temperature of the solution in the filtration system should never
exceed
approximately 37 C (if the temperature exceeds 37 C, the machine should
be stopped for 15 minutes while the tank is refrigerated);
2.5 The filtration process is stopped at 4 liters less than the total starting
quantity, the filtrate kept and stored at 4 C until the next day and the
system
thoroughly drained;
2.6 9.5 liters of filtered water are run through the system (0.45 pm)
heated at
100 C with 500 ml of 10 N NaOH until it reaches 50-70 C;
2.7 5 liters of filtered water are added and run until the tank is empty,
and then
drained from the system;
2.8 9 liters of filtered water are run through the system (0.45 pm),
heated at 60-
70 C with 1 liter of 10N HCI, stopped and drained from the system;
2.9 7 liters of filtered water (0.2 pm) and 3 liters are run through and
drained
from the system;
2.10 7 liters of alcohol (70%) are added and 2 liters run through and the
system
drained;
2.11 0.2 pm columns are exchanged for 15 kDa columns;
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
19
2.12 7 liters of alcohol (70%) are run through the system, letting 2 liters
out and
letting stand for 5 minutes before draining the system;
2.13 The system is rinsed by running 5 liters of filtered water (0.2 pm)
through it,
letting 2 liters out;
2.14 The 0.2 pm filtrate is passed through two 15 kDa Dahlia ceramic columns
with 1000 cm2 surface (CeRAM from TAMI Industries);
2.15 The temperature of the filtration system should never exceed 37 C (if it
does, the machine should be stopped for 15 minutes);
2.16 The filtration process should be stopped at 3.5 liters less than the
total
starting quantity;
2.17 The system should be drained and the retentate kept;
2.18 The retentate should be centrifuged at 9285 G for 20 minutes;
2.19 The supernatant is ready for lyophilisation; it is stored at 2-4 C until
ready
for processing;
2.20 To clean the filtering machine, points 2.6 to 2.10 are repeated;
2.21 In order to keep the system germ free, the process should always be
finished with adequate cleaning procedures and the columns stored in
alcohol.
3. Lyophilization
3.1 Using a FTS tray lyophilizer, the supernatant is processed according
to the
instructions for this equipment;
3.2 After the lyophilization is complete, the powder is passed through a
250 or
500 pm sieve under sterile environment;
3.3 The powder is conserved in sterile 50 ml centrifugation tubes, 20 grams
per
tube and stored at a temperatue of about -16 C to -24 C;
3.4 The powder could be irradiated up to 8 kGy without loss of activity
on human
cells; and
3.5 The powder is stored at a temperature of about -16 C to -24 C.
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
-
Example 4: Isolation Results for Growth Factors IGF-1 and TFG-132 (after
partial hydrolysis)
Growth Factors IGF-1 and TFG-132 were quantified in 2.5 ml fractions
5 (hydrogenated, pH 3.9 colostrum) that were purified on HPLC. Tables 3 and
4
show the results for growth factors IGF-1 and TFG-132, respectively.
Table 3: Quantification of IGF-1 in Retentate 21
Sample No. Concen- O.D. Correction Quantity Specific
(MW / % retentate) tration Factor * IGF-1 Activity
(mg/ml) Dilution (ng/ml) (pg/g
factor powder)
Fraction 2 X1 20,0 0,001 100 * 1 N/A N/A
(>1400kDa/2.8%)
Fraction 3 X2 20,0 0,005 100 * 1 N/A N/A
(1400kDa/5.6%)
Fraction 4 X3 20,0 0,007 100 * 1 N/A N/A
(950kDa/4.8%)
Fraction 5 X4 20,0 0,002 100 * 1 N/A N/A .
(680kDa/4.3%)
Fraction 6 X5 20,0 0,001 100 * 1 N/A N/A
(490kDa/4.0%)
Fraction 7 X6 20,0 0,002 100* 1 N/A N/A
(350kDa/3.2%)
Fraction 8 X7 20,0 0,002 100 * 1 N/A N/A
(250kDa/4.1%)
Fraction 9 X8 19,7 -0,001 100 * 1 N/A N/A
(180kDa/2.8%)
Fraction 10 X9 20,0 0,002 100 * 1 N/A N/A
(133kDa/16.6%)
Fraction 11 X10 20,1 0,005 100* 1 N/A N/A
(96kDa/11.3%)
Fraction 12 X11 20,0 0,061 100 * 1 48,4 2,42
(70KDa/5.5%) _
Fraction 13 X12 20,0 0,231 100 * 1 125,1 6,26
(>45kDa/2.8%)
Fraction 14 X13 20,0 0,081 100 * 1 58,5 2,93
(35kDa/7.4%)
Fraction 15 X14 20,0 0,058 100 * 1 46.9 2,35
(26kDa/4.1%)
Fraction 16 X15 20,0 0,056 100 * 1 45.9 2,29
(20kDa/3.0%)
Fraction 17 X16 20,0 0,113 100* 1 74,7 3,73
(13kDa/1.9%)
Fraction 18 X17 20,0 0,146 100 * 1 91,3 4,57
(10kDa/1.6%)
Fraction 19 X18 10,0 0,257 100 * 1 147,3 14,73
(7kDa/0.7%)
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
21
TABLE 4: Quantification of TGF-132from Retentate 21-CLAR in Fractions 2 to
25 purified with HPLC
Sample No. Concentration O.D. Over-
Correction bcorrected Specific
(MW! % retentate) (mg/ml) estimation Factor * TGF-132
Activity
0.D - 0.120 Dilution factor Quantity
(pg/g
(pg/ml) powder)
Fraction 2 XI 9,9 0,948 0,828 7.81 4161,1
420,32
(>1400Kda/2.0%)
Fraction 3 X2 10,1 1,061 0,941 7.81 4747,1
470,01
(1400Kda/4.7%)
Fraction 4 X3 9,8 1,607 1,487 7.81 7604,8
776,00
(950Kda/3.1%)
Fraction 5 X4 9,8 3,258 3,138 7.81 16410,2
1674,52
(680Kda/2.6%)
Fraction 6 X5 10,0 3,460 3,340 7.8*1 17499,2
1749,92
(490Kda/2.3%)
Fraction 7 X6 10,1 3,013 2,893 78*1 15092,3
1494,29
(350Kda/2.2%)
Fraction 8 X7 9,9 1,472 1,352 7.81 6894,7
696,44
(250Kda/2.4`)/0)
Fraction 9 X8 10,0 0,372 0,252 7.8*1 1222,2
122,22
(180Kdar7.0%)
Fraction 10 X9 10,0 0,435 0,315 7.81 1538,0
153,80
(I33Kda/12.2%)
Fraction 11 X10 9,9 1,725 1,605 7.81 8227,0
831,01
(96Kda/7.3%)
Fraction 12 X11 10,2 2,314 2,194 7.81 11351,6
1112,90
(70Kda/3.9%)
Fraction 13 X12 9,9 0,625 0,505 7.8*1 2500,7
252,59
(50Kda/3.6%)
Fraction 14 X13 9,9 0,117 -0,003 7.8*1 N.A. N.A.
(35Kda/7.3%)
Fraction 15 X14 10,2 0,115 -0,005 7.81 N.A. N.A.
(26Kda/4.8%)
Fraction 16 X15 10,1 0,131 0,011 7.81 N.A. N.A.
(20Kda/3.0%)
Fraction 17 X16 10,2 0,128 0,008 7.81 N.A. N.A.
(I3Kda/2.6%)
Fraction 18 X17 9,9 0,115 -0,005 7.81 N.A. N.A.
(I0Kda/1.7%)
Fraction 19 X18 10,0 ' 0,115 ' -0,005 7.81 N.A.
N.A.
(7Kda/1.2%)
Fraction 20 X19 10,0 0,116 -0,004 7.81 N.A. N.A.
(5Kda/1.2%)
Fraction 21 X20 10,0 0,090 -0,030 7.81 .N.A. N.A.
(3.5Kda/0.6%)
Fraction 22 X21 ' 10,0 0,098 -0,022 7.81 N.A. N.A.
(2.5Kda/0.8%)
Fraction 23 X22 10,0 0,086 -0,034 7.8*1 N.A. N.A.
(2Kda/0.3%)
Fraction 24 X23 10,0 0,128 0,008 7.8*1 N.A. N.A.
(1.5Kda/0.2%)
Fraction 25 X24 10,0 0,092 -0,028 7.81 N.A. N.A.
-
(1Kda/0.3%)
Discussion
The results in Tables 3 and 4 are but two examples showing the specific
activity
of the pools of factors derived using the process of the present invention.
Partial
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
22
hydrolysis converts many factors from their inactive (or "pro") forms (>450
kDa) to
their active forms. Significantly, these factors, which are present in pools
in the
various fractions, as verified through human ELISA testing (Figure 3), have
been
found to be active on human cells.
Example 5: Effect on Cell Behavior of a Variety of Purified Fractions
1. Objectives of the Study
The objectives of the study were to evaluate the effect on cell behavior of a
variety of fractions purified with the process of the present invention. The
pools
tested were termed LP1, LP2, LP3, LP4, LP5, LP1-LP3, LP3-LP5 and LP1-LP5.
The proliferation and growth of human fibroblasts as well as their collagen
synthesis were investigated in vitro in order to select optimal pools for
further
study. In addition, some studies were also performed with human vascular
endothelial cells.
2. Materials and Methods
2.1. Cells
Human fibroblasts, stored in liquid nitrogen, and derived from foreskin of
young
were used at passages 3-8. Fibroblasts were grown in Dulbecco's modified
Eagles medium with 5% fetal bovine serum (FBS). Ascorbic acid and 8-
aminoproprionitrile were added to the cultures dedicated to the collagen
synthesis
assessment.
Human vascular endothelial cells, stored in liquid nitrogen and derived from
umbilical veins (HUVECs), were used at passages 3-4. HUVECs were grown on
gelatin-adsorbed culture dishes in Medium 199 containing 10% FBS, L-glutamine
(2mM) and endothelial cells growth supplement (ECGS at 20 pg/ml). To test the
pools, serum-free Medium 199 was used with ECGS and L-glutamine to permit
cell survival. In a pilot experiment, endothelial cells died in less than 24
hrs when
grown in culture without ECGS and serum.
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
23
2.2. LP pool concentrations
In the first set of experiments, LP pools were diluted to final concentrations
of 0.1,
1.0, 10 mg/ml. In the following sets of experiments, the final concentrations
tested
were 0.33, 1.0, and 3.3 mg/ml. These conditions were compared to negative
control cultures free of serum. In some cases, serum-supplemented medium was
used in positive control cultures.
2.3. Proliferation Test (Cyquant Assay from Molecular Probes)
Cells were seeded in wells of 24 multiwell plates at a density of 5x103
fibroblast/well and a density of 1x104 endothelial cells/well and grown for 6-
24 hrs
to allow cell adhesion in the presence of serum (5% for fibroblasts, and 10%
for
endothelial cells). At time zero, medium was removed and cells were rinsed
twice
with Hank's balanced salt solution (HBSS), then replaced by culture serum-free
medium containing the LP pool to be tested at different concentrations.
Control
cultures were grown in parallel. After 12 or 24 hours of growth without
changing
medium, medium was removed, and the wells were rinsed twice in PBS. Multiwell
plates were frozen at -70 C. Two hours later, plates were thawed, the lysis
buffer
(solutions A and B, provided with the kit, revealing fluorescent solutions)
was
added with an incubation of 3-5 minutes, then fluorescence was read in a
cytoplate with a BioTek FL-600 fluorometer at 480nm excitation and 520nm
emission.
2.4. Cell growth (Hoechst)
Cells were seeded in wells of 24 multiwell plates at a density of 1x104
fibroblast or
endothelial cells/well and grown overnight (or 24 hrs in the first set of
experiment)
to allow cell adhesion in the presence of serum (5% for fibroblasts, and 10%
for
endothelial cells). The next day (time zero), medium was removed and cells
were
rinsed twice with HBSS, then replaced by culture serum-free medium containing
the LP pool to be tested at different concentrations. After 72 hours of growth
without changing medium, medium was removed, and the wells were rinsed twice
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
24
in PBS. Then, PBS was replaced by a 200p1 saline-sodium citrate buffer (SSC1).
solution containing 0.1% SDS, and incubated for 1 hour at 37 C. Twenty (20) pl
of
Hoechst 33258 solution (at 1mg/m1) was added to the SSCI solution. After
agitation (up-down), fluorescence was read in a cytoplate at 340nm excitation
and
460nm emission with a sensitivity set at 100-120.
In parallel, incrementing cell density was established, incubated with Hoechst
33258 solution (at 1mg/m1), then fluorescence was read to perform a standard
curve in which the cell number is plotted against the optical density.
2.5. Statistic analyses
One Way Analysis of Variance was used for the statistical analysis of
quantitative
data, with a p value 50.05. Bonferroni t-test method was used for all pairwise
comparison procedures.
2.6. Collagen synthesis in monolayer fibroblast cultures
Cells were seeded in wells of 24 multiwell plates at a density of 1X105
fibroblast/well and grown overnight to allow cell adhesion in the presence of
serum (5%), ascorbic acid (10pg/m1) and 6-aminoproprionitrile (10pg/m1). The
next day (time zero), medium was removed, rinsed with PBS, and cells were
exposed to medium containing LP pools, and radioactive proline (14 C or 3 H
proline). Control cultures were run in parallel. Cultures lasted for 7 days to
allow
collagen synthesis and deposition, for which fresh medium containing LP pools
and radioactive proline was changed every other day. At medium changes, media
of each condition were collected and pooled (i.e., soluble collagen). At the
end of
the 7 day culture period, cells and matrix were pooled (i.e., cellular,
insoluble and
deposited collagen), separately of the medium pools (i.e., soluble collagen).
Matrix-cells and media were promptly diluted in a protease cocktail inhibitor
solution. Matrix-cell pools were counted on a scintillation counter, whereas
medium pools were dialyzed to remove any free radioactive proline, then
counted.
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
2.7. Cell cultures in 3-D fibrin gel and collagen synthesis/deposition
Fibrin gel was used instead of collagen gel in order to investigate the
collagen/deposition by fibroblasts, since collagen itself is known to inhibit
collagen
5 synthesis. Moreover, fibrin represents the primary extracellular matrix
during
wound healing. A 3 mg/ml fibrinogen solution was mixed with
5x104fibroblasts/m1
and polymerized by thrombin in the wells. The fibrin gels were then covered
with
culture medium containing the different LP pools, and radioactive proline. The
method to analyze collagen synthesis and deposition was similar to that
10 described earlier in Section 2.6, above.
3. Results
3.1. Fibroblast proliferation
Proliferation was measured at 12 and 24 hrs of cultures in the presence of LP
15 pools with incrementing concentrations.
At 24 hours, 1mg/m1 LP1 and LP1-LP3 induced a statistically significant higher
value compared to the other LPs and control culture with no serum (not shown).
At 10 mg/ml, the values with LP1 were significantly higher than those with LP1-
20 LP3 at the same concentration. The latter was not different
statistically with 10
mg/ml LP2, but different with 10 mg/ml LP3, LP4 and LP5. The values of LP1
were similar at 1 and 10 mg/ml. The values between 1 and 10 mg/ml of LP1-LP3
and LP2 were also similar. The values with 1 and 10 mg/ml LP1, LP2 and LP1-
LP3 were significantly higher than those at 0.1 and control. The values of LP4
and
25 LP3 were not significantly different. High doses of LP5 induced a
significant
inhibition compared to control and the other LP pools at 10 mg/ml.
At 12 hrs (not shown), the values of cell proliferation at 0.33, 1 and 3.3
mg/ml of
LP1-LP3 was statistically higher than the other conditions, except with 3.3
mg/ml
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
26
LP-2 which was similar to LP1-LP3. However, the values with 3.3 mg/ml LP2 were
not different than LP1, LP3, LP4 and LP5 at the same concentration.
3.2. Fibroblast growth
The first assay was performed with 0.1, 1.0 and 10 mg/ml of LP pools (not
shown). There was a statistically significant increase in the presence of LP1-
LP3
at 1 and 10 mg/ml and between 1 and 10 mg/ml LP1-LP3. LP1-LP3 did not reach
the number of cells found in the control cultures in serum-supplemented
medium,
which was 1.5-fold increase.
A significantly higher number of cells was found in the presence of 10 mg/ml
LP1,
LP2, LP3 and LP4 compared to those pools at lower doses, control without
serum, and to 10 mg/ml LP5. The presence of LP5 resulted in a significant
inhibition at the highest dose (10 mg/ml).
A second set of experiments was performed with 0.33, 1.0 and 3.3 mg/ml of LP
pools (Figure 4). The numbers of cells in the presence of 1.0 and 3.3 mg/ml
LP1-
LP3 were significantly higher than those of the other pools and the control
cultures without serum, except 3.3 mg/ml LP2, which resulted statistically in
a
similar number of cells than that with 3.3 mg/ml LP1-LP3. The cell number with
3.3 mg/ml LP2 was not significantly different than that with 3.3 mg/ml LP3. In
addition, LP1-LP3, LP2 and LP3 had a significant increase in cell numbers
between 1 and 3.3 mg/ml.
A third set of experiments was conducted with new pools LP1-LP3, LP3-LP5 and
LP5 (Figure 5). The number of cells in the presence of 3.3 mg/ml LP1-LP3 was
significantly higher than the other conditions. The cell number with 1.0 and
3.3
mg/ml LP3-LP5 was significantly different than 3.3 mg/ml LP5. The cell number
with 1.0 mg/ml LP1-LP3 and LP3-LP5 were not found to be statistically
different,
but different compared to LP5 and controls.
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
27
3.3. Proliferation of HUVECs
The Cyquant assay shows a significant increase in the proliferation within 12
hours in the presence of LP1-LP3 at 0.33, 1.0 and 3.3 mg/ml, as compared with
the other conditions (Figure 6). However, the values for 3.3 mg/ml LP1-LP3
were
close to those with 3.3 mg/ml LP2, and those for 1.0 mg/ml LP1-LP3 were not
different than those of 1.0 mg/ml LP 1, LP2 and LP5.
3.4. Growth of HUVECs
A drop in cell number (from 10,000 cells at seeding time to 3,700 cells after
more
than 72 hrs of incubation) was observed (not shown), due to the lack of serum
since these cells are very dependent on it. Once again, the exposure to LP1-
LP3
significantly enhanced cell growth at the 3 doses tested, compared to the
other
pools and the control cultures, recovering the initial number of cells.
However, the
cell number with 3.3 mg/ml LP1-LP3 was close to that of 3.3 mg/ml LP3. LP3
also
increased significantly the number of cells when used at 3.3 mg/ml.
Conversely, a
high dose of LP1 inhibited endothelial cell growth.
3.5. Collagen synthesis and deposition in monolayer =
The presence of LP1-LP3, particularly at 1.0 and 3.3 mg/ml, enhanced collagen
synthesis, as shown by increased radioactivity (Figure 7). Similar doses of
LP3
and LP2 also increased collagen synthesis, but to a lesser degree.
Phase contrast microscopy shows the extracellular matrix deposition and cells
(not shown). LP2 induced matrix between cells particularly with 3.3 mg/ml. LP3
also enhanced matrix deposition at all doses tested. The behavior of cells in
the
presence of 1.0 and 3.3 mg/ml LP1-LP3 appeared different from the others with
a
reorganization of cells into a network, rarely seen in monolayer cell
cultures.
In another set of experiments, the effects of LP1-LP3, LP1-LP5 and LP3-LP5
were compared. The CPM values were reported to the cell number at day 7.
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
28
Thus, collagen synthesis and deposition per cell was particularly enhanced in
the
presence of 0.33, 1.0 and 3.3 mg/ml of LP1-LP5, even above the value found in
the presence of serum (Figure 8). Moreover, the values of collagen synthesis
and
deposition were elevated in the presence of LP1-LP3 and LP3-LP5.
3.6. 3-0 cell cultures and collagen synthesis
In a first set of experiments, cell and matrix pools showed an increase in
collagen
synthesis and deposition with LP2 and LP3, particularly at 3.3.mg/m1(not
shown).
LP1-LP3 also enhanced collagen synthesis at 3.3 mg/ml, but less than LP2 and
LP3. Phase contrast microscopic observation (not shown) shows numerous cells
with extracellular matrix deposition at day 7, in the presence of 1 and 3
mg/ml
LP2 and LP3 and 1 mg/ml LP1-LP3, all compared to the control and LP1, LP4
and LP5.
A second set of experiments was performed with new LP1-LP3, LP3-LP5 and
LP5 pools. Afterwards, fibrin gels were detached from the wells to allow
contraction. LP1-LP3 induced an increase in collagen synthesis and deposition
in
the cell-matrix pools , which was close to that observed in the presence of
serum
(Figure 9). LP3-LP5 induced less collagen synthesis, higher than that in the
control without serum. Cell cultures were observed by phase contrast
microscopy
at day 8. One and 3.3 mg/ml LP1-LP3 resulted in a dense matrix with few cells,
when compared particularly with the control cultures and LP5 (Figure 10 A-D).
By
day 9, the contraction occurred that resulted in a floating fibrin gel. The
latter was
very dense in the presence of 3.3 mg/ml LP1-LP3 (Figure 10 E-F).
Another study was performed with LP1-LP5. In the presence of LP1-LP5,
fibroblasts in fibrin gels were reorganized into a network, particularly at 1
mg/ml,
as shown in Figure 10 G. Moreover, at a higher dose (3.3 mg/ml) of LP1-LP5,
the
fibrin gel was likely dissolved, perhaps by fribrinolysis, and some residual
fibrin
particles aggregated (Figure 10 H). Measurement of collagen synthesis and
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
29
deposition show less production than with LP1-LP3, with an increase at 3.3
mg/ml
(not shown). However, considering the decrease in cell density by day 9,
collagen production was more elevated in the presence of LP1-LP5.
4. Discussion and Conclusion
The data shows clearly that cell proliferation and growth are stimulated by
the
presence of LP1-LP3 (3.3-fold increase in cell number), even with doses as low
as 0.33 mg/ml as observed in some experiments, and this is incrementing as a
function of the dose. Similarly, but to a lesser degree, LP2, LP3, and LP3-LP5
stimulate cell growth and replication when 3.3 mg/ml is used. The stimulation
of
cell replication in the presence of LP1 appears only after 24 hours, and the
consequence on cell number is perceptible when high dose of 10 mg/ml is used.
Furthermore, the proliferation and growth of vascular endothelial cells are
also
stimulated by the presence of LP1-LP3. Assessment of endothelial cell growth
shows an incrementing effect as a function of dose. LP3 and LP2 may also
enhance cell replication and growth, but to a lesser degree.
The observation and quantification of collagen synthesis and deposition show
different patterns in monolayer cell cultures versus 3-D cultures in fibrin
gel, more
specifically in the presence of LP2 and LP3. The two latter induce a
significant
increase in collagen synthesis and deposition by fibroblast in fibrin gel,
particularly
with 3.3 mg/ml. On the other hand, LP1-LP3 also increases, but at a less
degree,
collagen synthesis and deposition. LP1-LP3 also increases the organization of
fibroblasts in a monolayer and more specifically in a fibrin gel (since they
have a
matrix to attach and migrate), as observed on micrographs. This observation is
confirmed by the induction of a dense contracted matrix after days in culture.
This
suggests that newly formed collagen deposited in fibrin is remodeled by
fibroblasts. Conversely, LP3-LP5 is less efficient to induce newly formed
collagen,
compared to LP1-LP3. On the other hand, LP1-LP5 induces synthetic activity as
demonstrated in monolayer cultures. Whereas in 3-D fibrin gel, a
differentiation
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
activity is exhibited that involved protease activation as observed during
wound
remodeling.
Without wishing to be bound by any theory, the effect of LP1-LP3 on collagen
5 synthesis and deposition may be explained by the presence of high cell
density at
the start of the cell cultures, due to the stimulation of cell replication as
determined by the different assays. Although the LP pools are renewed at
medium change during the 7-9 day period of fibroblast cultures for collagen
synthesis assay, it appears that by 8 days the cell density is less than
expected,
10 and less than that observed in the control culture with serum. Thus, LP1-
LP3 not
only enhances fibroblast proliferation and growth, but also the biosynthetic
activity
of fibroblast towards the formation of collagen, its deposition, and its
remodeling.
In conclusion, selective LP pools such as LP1-LP3, LP2, LP3 and LP5 have
15 potential and specific effects on fibroblasts and endothelial cell
behaviour. These
pools may have a beneficial effects in wound healing and closure.
Example 6: Proliferation and Growth of Human Fibroblasts, and Collagen
Synthesis
1. Objectives of the Study
The objectives of the study were to evaluate the effect on cell behavior of
the
growth and differentiating factors present in three pools: LP1-LP3, LP3-LP5
and
LP1-LP5. The proliferation and growth of human fibroblasts as well as their
collagen synthesis were investigated in vitro for a comparative study.
2. Materials and Methods
2.1. Fibroblasts
Human fibroblasts were used in conditions similar to those described in
Example
5. They were derived from the same batch used in the previous experiments.
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
31
2.2. LP pools concentrations
LP pools were diluted to final concentrations of 0.33, 1.0, and 3.3 mg/ml.
These
conditions were compared to negative control cultures in serum-free medium and
positive control cultures in serum-supplemented medium.
2.3. Test of proliferation (Cyquant0 Assay); Cell growth (Hoechst); and
Collagen synthesis in monolayer and in fibrin gel cultures (14C-proline)
The experimental method used was similar to that described earlier, as was the
statistical comparison.
3. Results
3.1. Fibroblast proliferation (Figure 11)
Cell proliferation after 24hrs of culture was increased, more specifically
with 1.0
and 3.3 mg/ml of LP1-LP3 and LP3-LP5 pool. Statistical analyses show that the
values of 1.0 mg/ml LP1-LP3 and those of 3.3 mg/ml LP1-LP3 and LP3-LP5 were
significantly higher than those of the control with no serum. Due to large
variations in the values with LP1-LP5 pools, the cell proliferation values
were not
significantly different than those of the control.
3.2. Fibroblast growth
Cell growth increased as a function of the doses tested for the different
pools (not
shown). The values of 3.3 mg/ml LP1-LP3 were significantly higher than all the
other conditions, except with the control cultures in the presence of serum.
The
values of 3.3 mg/ml LP1-LP5 were significantly higher than all the other
conditions, except LP1- LP3 and LP3-LP5 both at 3.3 mg/ml (similar), and the
presence of serum (lower). The values of 3.3 mg/ml LP3-LP5 were significantly
different than those of the two control cultures. Statistically, the values of
1.0
mg/ml LP1 -LP3 were significantly different than those of the two control
cultures.
Moreover, the values at 0.33 mg/ml were different for LP1-LP3 and LP3-LP5,
compared to the control cultures with no serum.
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
32
3.3. Collagen synthesis and deposition in monolayer
After 7 days in cell culture, collagen synthesis and deposition was elevated
for
LP1-LP3 and LP3-LP5. However, when the values were reported with respect to
the cell number, collagen synthesis and deposition per cell was particularly
enhanced in the presence of 0.33, 1.0 and 3.3 mg/ml of LP1-LP5, even above the
value found in the presence of serum. Observation of the cell cultures shows
clearly less cells left in the presence of LP1-LP5, more specifically with the
highest concentration tested compared to the other conditions. In the presence
of
serum, a dense population of cells was seen, for little quantities of formed
collagen. Moreover, the values of collagen synthesis and deposition were
higher
in the presence of LP1-LP3 and LP3-LP5. Specifically, 3.3 mg/ml of LP3-LP5
enhanced collagen synthesis and deposition. The curve of LP1-LP3 resembles
that reported earlier in monolayer cell culture. The ratio of soluble collagen
versus
insoluble collagen was relatively constant in any conditions tested.
3.4. 3-D cell cultures and collagen synthesis and deposition
While experimental conditions were not optimal due to a weakness in fibrin gel
formation resulting from a limited number of cells (2 x105 cells/well instead
of 5 x
105 cells/well), some of the data generated is of interest. In fibrin gel, LP
1 -LP5
behaved differently compared to LP1-LP3 and LP3-LP5. Observation of cells
shows a clearly diminished number of cells as well an organisation of the
fibroblasts into a network in the presence of 1.1 mg/ml LP1-LP5. This has not
been observed with other components, and may correspond to dramatic cell
differentiation. Moreover, LP1-LP5 at 3.3 mg/ml appeared to induce the
dissolution of the fibrin gel, and it is accompanied by cell death and loss
after
each medium change (radioactivity value was not determined). The latter
phenomenon may be induced by excessive protease activation, in particular
plasminogen activators secreted by fibroblasts that have differentiated. In
one
instance (not shown), LP1-LP3 increased the formation of soluble and insoluble
collagen slightly. However, it did not show any stimulation when the values of
cpm
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
33
were reported to the number of cells. LP3-LP5 increased the collagen
production
per cell. On the other hand, LP1-LP5 appeared to enhance collagen synthesis
and deposition when the values were reported to the number of cells.
4. Discussion and conclusion
The three LP pools of pools stimulate cell proliferation and cell growth,
particularly
the LP1-LP3 at high dose of 3.3 mg/ml. Although the stimulation of cell growth
by
LP1-LP3 occurs, collagen synthesis and deposition was limited when compared
specifically with LP1-LP5. The latter induces a significant increase of
collagen
formation in monolayer cell culture and in 3D fibrin gel. Moreover, the
presence of
LP1-LP5 results in a cell differentiation into cord-like structures, but at
high doses
proteases are likely to be involved.
In conclusion, the LP1-LP5 pool of factors induces cell differentiation along
with
synthetic activity rather than proliferative and growth activity. The
synthetic activity
is accurately demonstrated in monolayer cultures, while the differentiation
activity
is exhibited in 3D fibrin gels.
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
34
Example 7: Cell Proliferation Effect of LP1-LP5 Pool of Growth Factors
on Chondrocytes
The effect on chondrocyte proliferation of the LP1-LP5 pool of growth factors
was
measured. Figures 12, 13 and 14 show the effect on chondrocyte proliferation
of
1 mg/ml and 3 mg/ml LP1-LP5 after 3 days, 7 days and 10 days, respectively.
Two and ten percent fetal bovine serum (FBS) served as controls. Figure 15
shows the proliferation (number of chondrocytes) due to 1 mg/ml of LP1-LP5
over
the same three periods of time.
As may be appreciated from the results, chondrocyte proliferation was enhanced
in the presence of both 1 mg/ml and 3 mg/ml LP1-LP5. Figures 12, 13 and 14
reveal that after three days the proliferation is similar to that for cells
incubated
with FBS. However, by days 7 and 10, chondrocyte proliferation is markedly
increased in the presence of LP1-LP5.
Example 8: Wound Healing Capabilities of LP1-LP3, LP1-LP5 and LP3-
LP5 Pools of Growth Factors
The wound healing capabilities of LP1-LP3, LP1-LP5 and LP3-LP5 were
investigated in a guinea pig model. Briefly, 9 male guinea pigs were used in
the
experiments. (The protocol was accepted by the Committee for the Protection of
Animals of the Centre hospitalier universitaire de Quebec (CHUQ).) Under
general anesthesia (isoflurane with oxygen) and using dermatological punches,
four 6-mm (diameter) punch biopsies were made in the backs of each animal.
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
The wounds were arranged so that three wounds were positioned on one side of
each animal's back in order to receive a sample of one of the three pools to
be
tested (2 mg per wound of LP1-LP3, LP1-LP5 or LP3-LP5), and one wound was
positioned on the other side of the back to receive physiological liquid (0.9%
5 saline solution). This arrangement was devised to minimize cross-
contamination
between the wounds. The animals were sacrificed after 7, 14 and 28 days
according to the following schedule: one animal given an LP1-LP3 dosage was
sacrificed after 7 days, a second after 14 days and a third after 28 days; one
animal given an LP1-LP5 dosage was sacrificed after 7 days, a second after 14
10 days and a third after 28 days; and one animal given an LP3-LP5 dosage
was
sacrificed after 7 days, a second after 14 days and a third after 28 days.
Figure 16 shows the epidermal covering (epidermization) at day 7 of the three
pools. Figure 17 reveals the diminution of wound areas (granulation tissue)
after
15 7 days, 14 days and 28 days. Figure 18 shows the wound or dermal
thickness
after 7 days and 14 days, while Figure 19 reveals the degree of newly-formed
collagen fibers after 7 days, 14 days and 28 days.
In a separate but related experiment, the ratio of epidermization resulting
from
20 pools LP1, LP1-LP3 and LP1-LP5 after 5, 7 and 10 days was investigated
(see
Figure 20). The results reveal that wound closure occurs much more rapidly in
the presence of these pools than they would otherwise (see percent
epidermization of pools compared to serum at 7 days, for example).
Interestingly,
the wound closures were devoid of keloids.
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
36
Quantification has allowed the demonstration of the reduction in surface area
occupied by granulation tissue, as well as a diminution in its thickness,
especially
early on (at days 7 and 14) with the LP1-LP5 pool of growth factors. This
reduction is accompanied by a rapid deposition of collagen (particularly at
day 7),
which does not occur to a significantly greater degree subsequently. It should
also be noted that at day 7, in the presence of LP1-LP5, wound contraction is
much augmented in comparison to the other conditions at this time. These
observations would suggest that LP1-LP5 has a moderate scarring activity,
while
avoiding excess tissue repair as is observed during foetal scarring, for
example.
No differences in the migration and epidermal covering have been found, which
leads to the supposition that LP1-LP5 acts preferentially on granulation
tissue,
under the assay conditions used (in vivo).
Example 9: Precocious Maturation (Differentiation) of Brush Cells
Brush cells incubated with 1% serum along with growth factor pools LP1-LP3,
LP1-LP5 and LP3-LP5 differentiate faster than cells incubated solely with 1%
or
10% serum. As may be seen from Figure 21, the specific activity of alkaline
phosphatase is significantly increased for cells exposed to LP1 and LP1-LP5,
even before confluence. An increase in the specific activities of sucrase and
lactase is also observed, post confluence, especially with pools LP1 and LP1-
LP5, as may be observed from Figures 22 and 23, respectively.
Interestingly, brush cells, pre-confluence, incubated with the different
pools, and
particularly with LP1 and LP1-LP5, demonstrated a degree of polarization that
is
significant when compared to cells incubated in 1% and 10% serum (not shown).
These cells exhibited a cuboidal morphology and appeared to be squeezed more
tightly against each other.
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
37
The above observations have significant implications as far as the digestive
epithelium is concerned. The use of the growth factor pools speeds up the
maturation and differentiation of brush cells, leading them to generate their
digestive enzymes (lipases, amylases and proteases) more rapidly. The pools
could therefore be used to treat compromised digestive systems, such as those
of
premature and mature newborns or individuals suffering from GI tract ailments
(inflammations and obstructions).
Example 10: Uses or Applications for Various Growth Factors
The growth factors that are isolated through the novel process of the present
invention may be used in a number of applications, including: cosmetics,
cosmeceuticals, nutraceuticals and food additives, as well as in
dermatological,
pharmaceutical, medical and veterinary applications. Suggested applications
for
the specific growth factors found in individual fractions (see Figure 1(B) and
Figure 3 for the factors found in the various fractions) are listed in Table
5.
Interestingly, fraction LP5, which is the filtrate passing through the
microfilter of 5
kDa (Figure 1(B)), may also be useful in a number of applications. LP5 has
been
found to contain a wealth of vitamins, trace elements, amino acids, natural
peptides and salts, among other pools. It can therefore be used as a diluent
in
the manufacture of cosmetic products and as an effluent in the preparation of
nutraceutical substances, among other applications.
CA 02580372 2007-03-13
WO 2006/029518 PCT/CA2005/001398
38
Table 5: Uses or Applications for Growth Factors in Specific Fractions
Fraction Applications
PPT (cheese) Emergency nutrient for prized calfs
born
by Caesarean section (IGMs and
primary casein), trace IgG and IgM
W541 - 0.2 pm Emergency nutrient pH 4.50 = soluble
casein and partially hydrolyzed globulins
For prized calfs born by Caesarean
section not having access to maternal
colostrum
LP1 Nutraceutical (digestive inflammation)
LP2 Nutraceutical (digestive inflammation)
(with traces of primary casein)
LP3 Cosmetic and
cosmeceutical;
Nutraceutical (digestive inflammation of
the bowel) (without casein and gamma-
globulin)
LP4 Dermal pool of mature growth factors
of
low molecular weight for transdermal
applications (for the manufacture of high
end cosmetics without bacteria or
viruses)
LP5* Food and beverage supplement;
vitamins, salts, amino acids, lactose,
oligoelements and small peptides
LP1 ¨ LP3 Cell proliferation and some
differentiation (collagen secretion and
maturation)
LP3 ¨ LP5 Cell proliferation but even more
differentiation (collagen secretion and
maturation) relative to LP1-LP3
LP1 ¨ LP5 Cell proliferation and differentiation
(collagen secretion and maturation);
contractility of the dermis; elaboration of
specific digestive enzymes, etc.
* NB: The LP5 extract includes the following: Lactose (13%); calcium (1.2%);
sodium (0.3%);
phosphorus (0.6%); magnesium (0.2%); potassium (0.8%); alanine (2.9 g/100 g
protein); arginine
(1.5 g/100 g protein); aspartic acid + asparagine (9.5 g/100 g protein);
cystein (1.9 g/100 g protein);
glutamic acid + glutamine (20.1 9/100 g protein); glycine (2 g/100 g protein);
histidine (1 g/100 g
protein); isoleucine (4.4 g/100 g protein); leucine (10 g/100 g protein);
lysine (4.5 g/100 g protein);
methionine (2.2 g/100 g protein); phenylalanine (6.1 g/100 g protein); proline
(3.6 g/100 g protein);
serine (7.2 g/100 g protein); threonine (7.3 g/100 g protein); tryptophan (0.9
g/100 g protein);
tyrosine (7.9 g/100 g protein); valine (6.9 g/100 g protein); IGF1 (monomer 7
kDa and dimer 14
CA 02580372 2007-03-13
WO 2006/029518
PCT/CA2005/001398
39
kDa) (0.1-0.7 mg/100 g); TGF-I32 (85% and TGF43) (0.06-0.46 g/100 g);
lactoferrin (0.16 g/100 g);
lactoperoxydase (9.1 mg/100 g); lysozyme (0.16 mg/100 g); vitamin A (5 pg/g
MG); vitamin B12 (23
pg/100 g); choline (0.3 mg/100 g); folic acid (3.8 pg/100 g); riboflavin (23
pg/100 g); thiamin (0.28
mg/100 g); biotin (13 pg/100 g); nicotinic acid (0.46 mg/100 g); ascorbic acid
(12 pg/100 g); and
pantothenic acid (0.8 mg/100 g).
Although the present invention has been described hereinabove by way of
preferred embodiments thereof, it can be modified without departing from the
spirit, scope and nature of the subject invention, as defined in the appended
claims.
=