Note: Descriptions are shown in the official language in which they were submitted.
CA 02580509 2007-03-15
WO 2006/031193 PCT/SE2005/001343
Powder metal composition comprising secondary
amides as lubricant and/or binder.
Field of the invention
The present invention concerns a powder metal
composition. Specifically the invention concerns a powder
metal composition including a lubricant and/or binder
comprising at least one secondary amide. The invention
further concerns a method of producing a green body, a
method of producing a bonded iron-based powder
composition and use of the lubricant and/or binder.
Background of the invention
Metal powders are used in industry for the
manufacture of metal products by compacting the metal
powder in a die under high pressures, ejecting the
compact from the die and optionally sintering the
product. In the majority of powder metallurgical (PM)
applications a lubricant is comprised in the powder in
order to provide the necessary lubrication action between
powder particles during compaction and between the die
and the compact during ejection from the die. Lubrication
achieved by a lubricant included in the metal powder is
referred to as internal lubrication in contrast to
external lubrication, which is achieved by applying a
lubricant to the walls of the die, wherein the powder is
compacted. Insufficient lubrication during ejection
results in excessive friction between the compact and the
die resulting in high ejection energies and damage of die
surfaces and product surfaces.
Internal lubrication is achieved by using special
lubricants. Normally these lubricants are admixed with
the iron or iron-based powder in the form of a powder.
Some lubricants may also be used for binding additives,
such as e.g. alloying elements, to the iron or iron-based
particles. In these cases the lubricants thus work as
binding agents and reduce or eliminate segregation of the
additives during shipping and handling.
CA 02580509 2007-03-15
WO 2006/031193 PCT/SE2005/001343
2
Commonly used lubricants for PM applications are
metal soaps, such as lithium and zinc stearate. A
disadvantage with this type of lubricant is that oxides
of the metals in the lubricant contaminate the inside of
the sintering furnace as a result of release of metals
from the lubricant during sintering, another problem is
that stains may be formed on the component after
sintering. Another commonly used lubricant is ethylene
bis stearamide (EBS). Stains may also be formed on the
component after sintering when using this lubricant, but
to a lesser extent compared with using e.g. zinc
stearate. As lubricants strongly affect compacting and
sintering properties of metal powders optimization of
amount, composition and structure of the used lubricant
is of vital importance to obtain high and consistent
densities and good surface finishes of the produced
parts.
Objects of the invention
An object of the present invention is to provide a
new powder metal composition comprising a lubricant
and/or binder that reduces or eliminates the problems
with high ejection forces and stained surfaces of the
sintered parts.
Further objects of the invention are to provide a
method of producing compacted products and sintered or
heat treated parts, a method of producing a bonded powder
metal composition and use of the lubricant and/or binder.
Summary of the invention
These objects are accomplished by a powder metal
composition comprising an iron based powder and a
lubricant and/or binder comprising at least one secondary
amide. The invention further concerns a method of
producing a green body by subjecting the above mentioned
composition to compaction.
CA 02580509 2009-03-10
31457-35
2a
According to another aspect of the present
invention, there is provided powder metal composition
comprising an iron based powder selected from pure iron,
iron powder pre-alloyed, diffusion annealed, or mixed with
one or more alloying elements selected from Cu, Mo, Cr, Mn,
P, C, Ni, Si, B, V, Ti, Al, Co, and W, and mixtures thereof,
and a lubricant and/or binder comprising 0.05-2% by weight
of at least one secondary amide of the general formula:
R1-NH-CO-R2
wherein R1 and R2 are the same or different, straight or
branched, saturated or unsaturated aliphatic hydrocarbon
groups.
CA 02580509 2007-03-15
WO 2006/031193 PCT/SE2005/001343
3
The method of producing a bonded powder metal
composition comprises: mixing an iron-based powder with
at least one secondary amide and heating the mixture to a
temperature above the melting point of the at least one
secondary amide.
Additionally, the invention concerns the use of the
at least one secondary amide as a lubricating and/or
binding agent for iron-based powders, and its use for die
wall lubrication.
Detailed description of the invention
The lubricant and/or binder in the powder metal
composition according to the invention is at least one
secondary amide that may be defined by the general
formula:
R1-NH-CO-R2,
wherein the R1- and R2-groups, which may be the same
or different, are straight or branched, saturated or
unsaturated aliphatic hydrocarbon groups.
Preferably, R1 and R2 independently include 10 to 24
carbon atoms.
Preferably R1 and R2 are selected from the group con-
sisting of alkyl and alkenyl.
The alkyl groups may be chosen from decyl, undecyl,
dodecyl, tridecyl, tetradecyl, pentadecyl, hexadecyl,
heptadecyl, octadecyl, nonadecyl, eicosyl, heneicosyl,
docosyl, tricosyl, tetracosyl.
The alkenyl groups may be chosen from decenyl,
undecenyl, dodecenyl, tridecenyl, tetradecenyl,
pentadecenyl, hexadecenyl, heptadecenyl, octadecenyl,
nonadecenyl, eicosenyl, heneicosenyl, docosenyl,
tricosenyl, tetracosenyl.
Examples of preferred secondary amides are shown in
Table 1.
CA 02580509 2007-03-15
WO 2006/031193 PCT/SE2005/001343
a) v
a)
E a) -a 2
E a) E v E E at i
E EE 2 a) E ro E c0 cu
E CU cu 0
o m c
i o a m
E Q O o 0
E ai 2 0 " 0 CI p rn 0)
U O U) O c~ O cn O W W W _J _,
= U
U 0
V N
N N N =
U
co m
_ _ = U = U = U U
0
U U = V = _
F- cli
o U U V U U U U U
u U U U M
z =O U O= O= _ I Z Z Z U U
ai ^a0 Zao = . ;: m N _ N
n
= N = N = N = = _ = N N
rd U 0 U 0 U 0 U_ U_ U_ U U_ U_
= O = O = O = _ _ = O O
U U U U U U U U U U U U
44 U Z U Z U z U U U U Z Z
= n= = _ _ = co M
n .` n ^ gyn ^ n n n N N
N N N N N N N N N N N N
U = _ _ _ _ _ _ _ _ _ _ _
~ U U U U U U U U U U U U
M M M M M M M M M M M M
C7 U U U U U U U U U U U U U
a) a)
:a v a)
v E a)E v a) a)
E a) a) -a (u -a E
(U E ac) E a E v E
E aa) a~i a~i
a)
c: 0 r- U
a) U a) 0
U 'O .0 U U C U
N a) cv a) a3 O c0 O O U U N
a) X ~a U D O a) -O
E O o U O U -0 !2 0
-51
U O O O O O O O U U U 0 0
E O O v O
(6 t0
N a) a) a) a) a ~C0 m 0 0 0
U U U
U O O O O O O O in
O O la-~ H
()(c:)
U U U U U U U U U U U
O a O a U U -O .O U_ j
LC0Q Q 02 c}0 a) a) a) 42
Q Q C C Q Q C a Q
N a) a) a) a) a) a) a) a) a) a) C a)
_ _ _ _
C
C C
> > >' >
m a) C aO O
a) a) a) a) a) a) a)
U U U U U
N a0 N (U a0 L~0 O O O I`0 N
U U U V U U U o 0 0
0 0 0 0 0 0 0 o a 0 I--
O U)
C U E Lo N- N- N- ti r I- ti - - I-
4- O s- r- r- t- r- N N r- N ~-
O N
N cn
LC V E 00 co co 00 00 oO co N N N ~1 -t
O r- r- ~- r r- N N N N N
H O 15
CA 02580509 2007-03-15
WO 2006/031193 PCT/SE2005/001343
The amount of secondary amides may constitute 0.05-
2.0 % by weight of the powder metal composition,
preferably 0.05-1.0% by weight.
One embodiment of the invention concerns a powder
5 metal composition comprising a lubricant and/or binder
further comprising at least one primary amide in addition
to the at least one secondary amide. The at least one
primary amide is preferably a saturated or unsaturated
fatty acid amide having 12-24, preferably 14-22 C-atoms
and most preferably 16-22 C-atoms.
Especially preferred primary amides are stearic acid
amide (stearamide), behenic acid amide (behenamide),
eurcic acid amide (erucamide), palmitic acid amide
(palmitamide) and arachidic acid amide (arachidamide).
The primary and secondary amides according to the
invention are either commercially obtainable or may be
produced from commercially obtainable material by the use
of processes well known in the art.
The amount of primary and secondary amides may
constitute a total of 0.05-2.0 % by weight of the powder
metal composition, preferably 0.05-1.0% by weight.
The amount of the at least one primary amide may be
0.05-1.0% by weight and the amount of the at least one
secondary amide may be 0.05-1.0% by weight for the
embodiment of the invention comprising both types of
amides.
The lubricant and/or binder may be added to the
powder metal composition in the form of solid particles
of each amide. The average particle size may vary, but is
preferably less than 150 pm.
Alternatively, the lubricant and/or binder may be
added to the powder metal composition as a molten and
subsequently solidified particulate mixture of the
amides. This may be accomplished by mixing the amides in
a predetermined ratio, the mixture is then melted, cooled
and subsequently milled to a lubricant powder.
CA 02580509 2007-03-15
WO 2006/031193 PCT/SE2005/001343
6
The at least one secondary amide according to the
invention may be used as a binder for obtaining a bonded
mixture, wherein optional alloying elements and the at
least one secondary amide are bonded to the iron-based
powder. This may be achieved by mixing an iron-based
powder with at least one secondary amide according to the
invention, and heating the mixture to a temperature above
the melting point of the at least one secondary amide. At
least one primary amide may further be mixed into the
above mentioned mixture and the heating temperature may
then be lower than the melting point of the primary
amide.
Apart from the lubricant and/or binder disclosed
above, the powder metal composition according to the
invention may, if so desired, contain other lubricants,
such as zinc stearate, lithium stearate, EBS etc.
To accomplish a bonding of the powder metal
composition according to the invention other types of
bonding systems may be used such as alkydes, cellulose
ester resins, hydroxyalkyl cellulose resins having 1-4
carbon atoms in the alkyl group, or thermoplastic
phenolic resins.
As used in the description and the appended claims,
the expression "iron-based" powder encompasses powder
essentially made up of pure iron, iron powder that has
been pre-alloyed with other elements improving the
strength, the hardening properties, the electromagnetic
properties or other desirable properties of the end
products and particles of iron mixed with particles of
such alloying elements (diffusion annealed mixture or
purely mechanical mixture). Examples of alloying elements
are copper, molybdenum, chromium, manganese, phosphorous,
carbon in the form of graphite, nickel, silicon, boron,
vanadium, titanium, aluminium, cobalt and tungsten, which
are used either separately or in combination, e.g. in the
form of compounds (Fe3P and FeMo).
CA 02580509 2007-03-15
WO 2006/031193 PCT/SE2005/001343
7
The iron based powders may be used for the
preparation of soft magnetic parts and may, for this
application, be electrically insulated. Electrical
insulation of the powder particles may be made of an
inorganic material. Especially suitable are the type of
insulation disclosed in the US 6348265, which concerns
particles of a base powder consisting of essentially pure
iron having an insulating oxygen- and phosphorus-contai-
ning barrier. Insulated powder particles are available as
SomaloyTM 500 and 550 from Hoganas AB, Sweden.
Apart from the iron-based powder and the lubricant
and/or binder, the powder metal composition according to
the invention may contain one or more additives selected
from the group consisting of processing aids and hard
phases.
The processing aids used in the powder metal
composition may consist of talc, forsterite, manganese
sulphide, sulphur, molybdenum disulphide, boron nitride,
tellurium, selenium, barium difluoride and calcium
difluoride, which are used either separately or in
combination.
The hard phases used in the powder metal composition
may consist of carbides of tungsten, vanadium, molyb-
denum, chromium, A1203, B4C and various ceramic materials.
The invention further concerns a method of producing
a green body comprising: compacting the powder metal
composition according to the invention to a compacted
body, wherein the composition comprises an iron based
powder and a lubricant and/or binder comprising at least
one secondary amide having the general formula:
R1-NH-CO-R2, wherein R1 and R2 are the same or different,
straight or branched, saturated or unsaturated aliphatic
hydrocarbon groups. The compacted body may be sintered or
heat-treated.
With the aid of conventional techniques, the iron-
based powder, the lubricant and/or binder and optional
CA 02580509 2007-03-15
WO 2006/031193 PCT/SE2005/001343
8
additives may be mixed to a substantially homogeneous
powder composition before the compaction step.
The powder metal composition and/or the die may be
preheated before the compaction.
The invention further concerns the use of at least
one secondary amide, defined as above, as a lubricating
and/or binding agent for iron or iron based powders.
A further embodiment of the invention concerns the
use of at least one secondary amide, defined as above, as
a die wall lubricant.
Detailed description of the figure
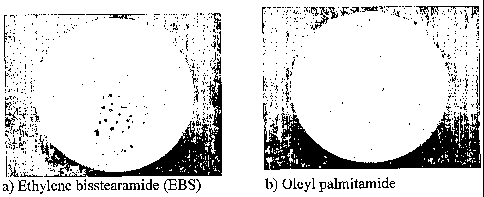
Figure 1 shows stain formation of components after
sintering due to the use of different lubricants.
la) Ethylene bisstearamide (EBS);
lb) Oleyl palmitamide (a secondary amide according to the
invention).
The invention will now be further described with the
following unlimiting examples.
CA 02580509 2007-03-15
WO 2006/031193 PCT/SE2005/001343
9
Examples
In the following examples lubricants having the formulas
disclosed in Table 2 below have been used.
Table 2.
Chemical name Structural formula* Amide
type
Ref: Ethylene CH3 (CH2) 16CONH (CH2) 2NHCO (CH2) 16CH3 bis amide
bis stearamide
(EBS)
Stearamide (S) CH3 (CH2) 16CONH2 Primary
Arachidamide (A) CH3 (CH2) 18CONH2 Primary
Erucamide (E) CH3 (CH2) 7CH=CH (CH2) 11CONH2 Primary
Behenamide (B) CH3 (CH2) 20CONH2 Primary
Stearyl R1=C18:0 R2=C17:0 Secondary
Stearamide (SS)
Erucyl R1=C22:1 R2=C17:0 Secondary
Stearamide (ES)
Oleyl Palmit- R1=C18:1 R2=C15:0 Secondary
amide (OP)
Stearyl R1=C18:0 R2=C21:1 secondary
Erucamide (SE)
Oleyl R1=C18:1 R2=C17:0 secondary
Stearamide (OS)
Stearyl R=C18:0 R2=C17:1 secondary
Oleamide (SO)
* The structural formulas for the secondary amides are
referring to R1-NH-CO-R2 as previously described.
CA 02580509 2009-03-10
31457-35
Example 1
This example demonstrates the lubrication properties of
different secondary amides and different combinations of
secondary and primary amides, which are added as a powder
5 in iron-based powder mixes.
Base powder ASC 100.29 (available from Hoganas AB,
Sweden) was mixed with 0.5% by weight of graphite (uf-4
from Kropfmuhl) and 0.8% by weight of lubricants,
10 according to Table 3 and 4, in a Lodige'mixer for 2
minutes. Ethylene bisstearamide (EBS, available as
Licowax TM from Clariant, Germany) was used as a
reference. The lubricants had a particle size less than
150 pm. Compositions comprising both a secondary and a
primary amide contained 50% of each amide (0.8% by weight
of the total composition).
In order to measure the lubricating properties rings with
an inner diameter of 45 mm, an outer diameter of 55 mm
and a height of 10 mm were compacted at ambient
temperature at three different compaction pressures (400,
600 and 800 MPa). During ejection of the compacted parts
the ejection force was recorded. The green density of the
parts was measured after ejection and the total ejection
energy/enveloping area needed in order to eject the
samples from the die was calculated.
The resulting ejection energies and densities are shown
in Table 3 and 4. Lower ejection energies where achieved
when using the powder metal composition according to the
invention compared with the use of the reference
composition comprising EBS.
CA 02580509 2007-03-15
WO 2006/031193 PCT/SE2005/001343
11
Table 3. Densities and ejection energies (secondary
amides and reference).
Lubricant Premixed
Green Density Ejection Energy
(g/cm3) (J/cm2)
400 MPa 600 MPa 800 MPa 400 MPa 600 MPa 800 MPa
EBS (Ref) 6.70 7.04 7.17 19.2 26.1 28.2
SS 6.72 7.06 7.19 17.6 24.7 27.9
ES 6.78 7.12 7.23 16.3 20.3 20.8
OP 6.78 7.14 7.25 16.7 21.3 20.3
SE 6.78 7.13 7.24 16.8 21.9 21.8
OS 6.78 7.13 7.24 17.7 21.3 20.5
SO 6.79 7.13 7.23 15.9 21.4 20.4
Table 4. Densities and ejection energies (secondary +
primary amides 1:1 and reference)
Lubricant Premixed
Green Density Ejection Energy
(g/cm3) (J/cm2)
400 MPa 600 MPa 800 MPa 400 MPa 600 MPa 800 MPa
EBS (Ref) 6.70 7.04 7.17 19.2 26.1 28.2
SS+E 6.69 7.06 7.21 19.1 24.2 23.6
OP+S 6.70 7.06 7.19 18.2 22.1 22.3
ES+S 6.71 7.06 7.19 17.9 21.5 21.8
ES+E 6.72 7.11 7.23 17.8 20.7 19.0
Example 2
The base powder ASC 100.29 was mixed with 2% by weight of
Copper (-100pm), 0.8% by weight graphite and 0.8% by
weight of lubricants (a) EBS or b) oleyl palmitamide) in
a Lodige mixer for 2 minutes. The lubricants had a
particle size less than 150 pm. In order to measure the
CA 02580509 2007-03-15
WO 2006/031193 PCT/SE2005/001343
12
stain formation after sintering of components,
cylindrical components with a diameter of 64 mm and a
height of 32 mm were compacted to a green density of 7.1
g/cm3 at ambient temperature. The weight of one cylinder
was 700 g. The components were sintered in an atmosphere
containing 90/10 N2/H2 at 1120 C for 15 minutes.
Photos of the components are shown in Figure la) Ethylene
bisstearamide (EBS) and 1b) oleyl palmitamide, in which
figure la) show stain formation in contrast to the part
produced from the powder composition according to the
present invention (1b) which has no stains.
Example 3
This example demonstrates the lubrication properties of
different combinations of secondary and primary amides,
which have been melted together, cooled and milled before
being mixed with iron-based powder mixes.
The lubricant combinations were made according to
following method: The mixed lubricants, 50% primary and
50% secondary amide, were melted together at 80-110 C and
then cooled. Then the materials were milled to a mean
particle size of below 150pm.
The base powder ASC100.29 was mixed with 0.5 % by weight
of graphite and 0.8 % by weight of lubricant combination
(see Table 5), in a Lodige mixer for 2 minutes. In order
to measure the lubricating properties rings with inner
diameter of 45 mm, outer diameter 55 mm and a height 10
mm were compacted at three different compaction
pressures, 400, 600 and 800 MPa at ambient temperature.
The resulting ejection energies and densities are shown
in Table 5.
CA 02580509 2007-03-15
WO 2006/031193 PCT/SE2005/001343
13
Table S. Densities and ejection energies (secondary +
primary amides and reference).
Lubricant Melted and solidified
Green Density Ejection Energy
(g/cm3) (J/Cm2)
400 600 800 400 600 800
MPa MPa MPa MPa MPa MPa
EBS (Ref) 6.70 7.04 7.17 19.2 26.1 28.2
SS+E 6.70 7.06 7.20 18.8 22.4 22.6
OP+S 6.71 7.07 7.20 18.5 23.2 24.4
ES+S 6.71 7.07 7.20 18.9 22.7 23.5
ES+E 6.70 7.07 7.20 17.2 19.8 18.0
When comparing the test results in Table 5 it can be seen
that samples produced from the powder metal composition
according to the invention show lower ejection energies
compared to samples produced from the known lubricant
EBS.
Example 4
This example demonstrates the lubricating and binding
properties of different combinations of amides in powder
metal compositions.
The lubricants had a particle size less than 150 um. The
base powder ASC100.29 was mixed with 2% by weight Cu-100,
0.8 % by weight of graphite and 0,8 % by weight of
lubricant/binder combination according to Table 6, in a
Lodige mixer for 2 minutes. The mixture with EBS was kept
as reference while the mixtures comprising amides were
heated to a temperature above the melting point of the
secondary amide but below the melting point of the
primary amide during mixing in another mixer followed by
CA 02580509 2007-03-15
WO 2006/031193 PCT/SE2005/001343
14
cooling to accomplish bonding of the additives to the
iron powder. In this mixture the secondary amide will
thus act as a binder and the primary amide will act as a
lubricant. The melting temperatures of the amides are
disclosed in Table 7.
Further, the ejection energy was measured on rings having
an outer diameter of 55 mm and an inner diameter of 45 mm
and a height of 10 mm compacted at three different
compaction pressures, 400, 600 and 800 MPa at ambient
temperature. The resulting ejection energies and green
densities are shown in Table 8.
Table 6. Lubricant/binder combinations for example 4.
Secondary amide Primary amide
0.2% by weight 0.6% by wt
ES B
OP S
OP B
EBS (Ref 1)
(0.8% by wt)
Table 7. Melting temperatures of the amides.
Amide Melting temperature( C)
ES 72.9
OP 66.9
B 101.9
S 106.6
CA 02580509 2007-03-15
WO 2006/031193 PCT/SE2005/001343
Table 8. Densities and ejection energies (primary +
secondary amides and reference).
Binder/Lubricant Melt bonded
Green Density Ejection Energy
(g/cm3) (J/cm2)
400 600 800 400 600 800
MPa MPa MPa MPa MPa MPa
ES+B 6.75 7.06 7.19 18.5 24.6 28.1
OP+S 6.73 7.09 7.18 19.3 26.6 28.3
OP+B 6.77 7.08 7.19 19.9 25.3 27.1
EBS (Ref) 6.74 7.06 7.17 21.4 30.8 32.8
Samples produced with the aid of the lubricant/binder
5 according to the invention show lower ejection energies
compared to samples produced with the lubricant used as
reference, i.e. EBS. Use of the powder composition
comprising the lubricant/binder according to the
invention resulted in compacted sintered parts (sintered
10 in 90/10 N2/H2 at 1120 C for 30 minutes) with excellent
surface finishes, i.e. essentially without scratches and
no stain formation.
Example 5
15 A coarse soft magnetic iron-based powder, wherein the
particles are surrounded by an inorganic insulation was
mixed with secondary amide lubricant according to Table
9. As reference lubricants the known substances Zinc-
stearate and EBS were used. The particle size
distribution of the used iron-based powder is disclosed
in Table 10.
The obtained mixes were transferred to a die and
compacted into cylindrical test samples (50 g) having a
diameter of 25 mm, in an uniaxial press movement at a
CA 02580509 2007-03-15
WO 2006/031193 PCT/SE2005/001343
16
compaction pressure of 1100 MPa. The die material used
was conventional tool steel. During ejection of the com-
pacted samples the ejection force was recorded. The total
ejection energy/enveloping area needed in order to eject
the samples from the die was calculated.
The results of the measurements regarding ejection
energy, green density and surface appearance in the green
state are shown in Table 9. Use of the powder metal
compositions according to the invention resulted in that
compacted components with excellent surface appearance
and lower ejection energies were achieved compared with
the reference compositions.
Table 9. Densities, ejection energies and surface
appearance.
Mix no Lubricant Ejection Green Surface
(0.2 wt%) energy Density appearance
(J/cm2) (g/cm3)
1 ES 76 7.65 Perfect
2 SE 71 7.66 Perfect
3 SS 78 7.63 Perfect
4 OP 76 7.66 Perfect
Ref 1 Zinc stearate 117 7.66 Not acceptable
Ref 2 EBS 113 7.64 Perfect
Table 10
Particle size Coarse powder
(um) (wt o )
>425 0.1
425-212 64.2
212-150 34.1
150-106 1.1
106-75 0.3
45-75 0.2
<45 0