Note: Descriptions are shown in the official language in which they were submitted.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
Actelion 64A/TI1
New bicyclic antibiotics
The present invention concerns novel antibiotics, pharmaceutical antibacterial
compositions
containing them and the use thereof in the manufacture of a medicament for the
treatment of
infections (e.g. bacterial infection). These compounds are useful
antimicrobial agents
effective against a variety of human and veterinary pathogens including among
others Gram
positive and Gram negative aerobic and anaerobic bacteria and mycobacteria.
The intensive use of antibiotics has exerted a selective evolutionary pressure
on
micro-organisms to produce genetically based resistance mechanisms. Modern
medicine and
socio-economic behaviour exacerbates the problem of resistance development by
creating
slow growth situations for pathogenic microbes, e.g. artificial joints-related
infections, and by
supporting long-term host reservoirs, e.g. in immuno-compromised patients.
In hospital settings, an increasing number of strains of Staphylococcus
aureus, Streptococcus
pneumoniae, Enterococcus spp., and Pseudomonas aeruginosa, major sources of
infections,
are becoming multi-drug resistant and therefore difficult if not impossible to
treat:
- S. aureus is resistant to 13-lactam, quinolone and now even to vancomycin;
- S. pneumoniae is becoming resistant to penicillin, quinolone and even to new
macrolides;
- Enteroccocci are quinolone and vancomycin resistant and 13-lactams are
inefficacious against
these strains;
- Enterobacteriacea are cephalosporin and quinolone resistant;
- P. aeruginosa are 13-lactam and quinolone resistant.
Further new emerging organisms like Acinetobacter spp., which have been
selected during
therapy with the currently used antibiotics, are becoming a real problem in
hospital settings.
In addition, microorganisms that are causing persistent infections are
increasingly being
recognized as causative agents or cofactors of severe chronic diseases like
peptic ulcers or
heart diseases.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-2-
A new type of quinoline or naphthyridine derivatives having antibacterial
activity and
therefore useful for treating infections in mammals, particularly in humans,
have been
reported.
WO 99/37635, WO 00/21948, WO 00/21952, WO 00/43383, WO 03/101138,
WO 01/025227, WO 02/040474 and WO 2004/011454 disclose quinoline,
naphthyridine and
quinazoline derivatives containing a 4-methylpiperidinyl spacer.
WO 00/78748, WO 02/50040 and WO 02/050061 disclose quinoline and naphthyridine
derivatives containing a piperazinyl spacer.
WO 01/07432, WO 01/07433, WO 02/08224, WO 02/056882, WO 03/064421,
WO 03/064431, WO 2004/02490 and WO 2004/058144 disclose quinoline, quinoxaline
and
naphthyridine derivatives containing a 4-aminopiperidinyl spacer.
WO 04/035569 discloses quinoline and naphthyridine derivatives containing a
3-aminomethylpiperidinyl spacer.
WO 2004/002992, WO 03/087098, WO 2004/014361 and WO 2004/035569 disclose
quinoline, quinoxaline and naphthyridine derivatives containing a 4-
aminocyclohexyl spacer.
It has now been found that certain novel bicyclic derivatives are useful
antimicrobial agents
and effective against a variety of multi-drug resistant bacteria. Thus, the
present invention
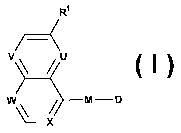
relates to novel bicyclic derivatives of the formula
Ri
T__<
V U
W \ M-D
'--X
wherein R' represents alkyl, alkoxy, haloalkoxy, halogen or cyano;
one or two of U, V, W and X represent(s) N, the remaining represent CH, or, in
the case of U,
V and/or W, may also represent CRa and, in the case of X, may also represent
CRb;
Ra represents halogen;
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-3-
Rb represents halogen or alkoxy;
D represents alkyl, aryl or heteroaryl;
M is selected from the group consisting of Ml, M2, M3 and M4:
H (CH2)p
(CH2)n I-= 1B2 - A2
A B ~
M +
(CH2)CH2)p
wherein
A' represents NHCO, OCH2, CH2CH2, CH=CH or CH(OH)CH2;
A2 represents NHCH2, NHCO, NHCH2CONH, NHCH2CH=CH, CH2CH2, CH2CO, COCH2,
CH2CH(OH) or CH2CH(OCONH2);
B 1 and B2 each represent independently from each other N or CH;
when A' represents OCH2, B1 represents CH;
n is 1; or n is also 0 when BI is CH; and
p is 1; or p is also 0 when B2 is CH;
R2
O R5
M2_ A3 1
N-A4_
R3 R4
wherein
A3 represents NHCO, CH2CH2, CH=CH, COCH2, CH(OH)CH2, CH2CH(OH),
CH(OH)CH(OH) or OCH2;
A4 represents CH2, CO, CH2CH=CH, COCH=CH or CH2CONH;
R2 represents hydrogen, alkyl, hydroxyalkyl, alkylcarbonyloxyalkyl,
carbamoyloxyalkyl,
carboxyalkyl or carbamoylalkyl;
R3 and R4 each independently represent hydrogen, hydroxy or alkylcarbonyloxy;
or R3 and R4
together represent a bridged dimethylmethylenedioxy chain attached to the
carbons bearing R3
and R4;
RS represents hydrogen, alkyl or hydroxyalkyl; and
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-4-
the dotted line represents a single bond or, when R3 and R4 represent
hydrogen, also a double
bond;
M3 = 4-- 5Bs - qs - wherein
A5 represents CH2CH2, CH=CH, COCH2, CH(OH)CH2, NHCO(CH2),,,, NHCOCH2O,
NHCOOCH2 or O(CH2)q;
m is 0, 1 or 2;
qis 1,2or3 and
B6 represents N and A6 represents CH2, CH2CH2, CH2CH=CH or CH2CH2S; or
B6 represents CH and A6 represents CH2NHCH2, CH2NHCH2CONH or CH2NHCH2CH=CH;
HN Rs
4= 7 I -
M -- A
N-A8
wherein
A' represents NHCO, CH2CH2 or OCH2;
A8 represents CH2; and
R6 represents hydrogen or alkyl.
In particular, the compounds of forrnula I may be compounds of formula ICE
Ri
T--~
V\ /U
W\ ~ M-D
X ICE
wherein Rl represents alkyl, alkoxy, haloalkoxy, halogen or cyano;
one or two of U, V, W and X represent(s) N, the remaining represent CH, or,
for one of U, V,
W or X, may also represent CRa;
Ra represents halogen;
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-5-
D represents an alkyl radical, a phenyl radical optionally substituted one or
twice by
substituents independently selected frorn the halogen atoms or a heteroaryl
radical;
M is selected from the group consisting of Ml, M2, M3 and M4:
(CH2)n H (CH2)p
=
1g2 - Q2f
=
M +A1_B (CH2)n H (CH2)p
wherein
A' represents NHCO, OCH2, CH2CH2, CH=CH or CH(OH)CH2;
A2 represents NHCH2, NHCO, NIRCH2CONH, NHCH2CH=CH, CHZCH2, CH2CO,
CH2CH(OH) or CH2CH(OCONH2);
B1 and B2 each represent independently from each other N or CH;
when A' represents OCH2, B1 represents CH;
n is 1; or n is also 0 when B' is CH; and
p is 1; or p is also 0 when B2 is CH;
R2
O R5
M2 4 -A3 N-q4-
~
R3 R4
wherein
A3 represents NHCO, CH2CH2, CH=CH, COCH2, CH(OH)CH2, CH2CH(OH),
CH(OH)CH(OH) or OCH2;
A4 represents CH2, CO, CH2CH=CH, COCH=CH or CH2CONH;
R2 represents hydrogen or hydroxyalkyl;
R3 and R4 each independently represent hydrogen or hydroxy; or R3 and R4
together represent
a bridged dimethylmethylenedioxy chain attached to the carbons bearing R3 and
R4;
R5 represents hydrogen; and
the dotted line represents a single bond or, when R3 and R4 represent
hydrogen, also a double
bond;
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 6 -
M 3 = - - A5 B6 -'_' A 6 - ~ -
wherein
A5 represents NHCO(CH2)m, NHCOCH2O or O(CH2)q;
mis0,1or2;
qis1,2or3and
B6 represents N and A6 represents CH2, CH2CH2, CHzCH=CH or CH2CH2S; or
B6 represents CH and A6 represents CH2NHCH2or CH2NHCH2CH=CH;
HN Rs
-~.
4= -~-Ar I $
M
N - A
wherein
A7 represents NHCO, CH2CH2 or OCH2,
Ag represents CH2; and
R6 represents hydrogen.
The compounds of formula I may also be compounds of formula IcEP2
R'
/--X
V U
W M-D
X ICEP2
wherein Rl represents alkyl, alkoxy, haloalkoxy, halogen or cyano;
one or two of U, V, W and X represent(s) N, the remaining represent CH, or, in
case of U, V
and/or W, may also represent CRa and, in the case of X, may also represent
CRb;
Ra represents halogen;
Rb represents halogen or alkoxy;
D represents alkyl, phenyl or heteroaryl;
M is selected from the group consisting of MI, M2, M3 and M4:
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-7-
H (CH2)p
(CH2)n =
1 1 \2_A2f
+
= A B (CH2)n H (CH2)p
wherein
A' represents NHCO, OCH2, CH2CH2, CH=CH or CH(OH)CH2;
A2 represents NHCH2, NHCO, NHCH2CONH, NHCH2CH=CH, CIKH2, CH2CO,
CH2CH(OH) or CH2CH(OCONH2);
B1 and B2 each represent independently from each other N or CH;
when Al represents OCH2, B1 represents CH;
n is 1; or n is also 0 when B1 is CH; and
p is 1; or p is also 0 when B2 is CH;
R2
O R5
M2 _ _ A3 1
N_A4_~.
R3 R4
wherein
A3 represents NHCO, CH2CH2, CH=CH, COCH2, CH(OH)CH2, CH2CH(OH),
CH(OH)CH(OH) or OCH2;
A4 represents CH2, CO, CH2CH=CH or CH2CONH;
RZ represents hydrogen or hydroxyalkyl;
R3 and R4 each independently represent hydrogen or hydroxy; or R3 and R4
together represent
a bridged dimethylmethylenedioxy chain attached to the carbons bearing R3 and
R4;
R5 represents hydrogen; and
the dotted line represents a single bond or, when R3 and R4 represent
hydrogen, also a double
bond;
M3 = - - A5 Bs - A6 - ~ _
~
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-8-
wherein
A5 represents NHCO(CH2)m, NHCOCH2O or O(CH2)q;
mis0,1or2;
qis 1,2or3 and
B6 represents N and A6 represents CH2, CH2CH2, CH2CH=CH or CH2CH2S; or
B6 represents CH and A6 represents CH2NHCH2 or CH2NHCH2CH=CH;
HN Rs
M4 -~-A7 I _~.
N-A$
wherein
A7 represents NHCO, CH2CH2 or OCH2;
A8 represents CH2; and
R6 represents hydrogen.
Another aspect of this invention relates to compounds of formula IP2
Ri
f --~
V\ ~U
w\ M-D
X IP2
wherein R' represents alkyl, alkoxy, haloalkoxy, halogen or cyano;
one or two of U, V, W and X represent(s) N, the remaining represent CH, or, in
case of U, V
and/or W, may also represent CRa and, in the case of X, may also represent
CRb;
Ra represents halogen;
Rb represents halogen or alkoxy;
D represents alkyl, aryl or heteroaryl;
M is selected from the group consisting of M', Mz, M3 and M4:
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-9-
H (GH2)p
(CH2)n =
B2 - l42
M7 +AI-BI f
(CH2)n H (CH2)p
wherein
A' represents NHCO, OCH2, CH2CH2, CH=CH or CH(OH)CH2;
A 2 represents NHCH2, NHCO, NHCH2CONH, NHCH2CH=CH, CH2CH2, CH2CO, COCH2,
CH2CH(OH) or CH2CH(OCONH2);
B I and B2 each represent independently from each other N or CH;
when A' represents OCH2, B1 represents CH;
n is 1; or n is also 0 when B1 is CH; and
p is 1; or p is also 0 when B'' is CH;
R2
O R5
1 _~-
M - -
2= A3 N R3 R4
wherein
A3 represents NHCO, CH2CH2, CH=CH, COCH2, CH(OH)CH2, CH2CH(OH),
CH(OH)CH(OH) or OCH2;
A4 represents CH2, CO, CH2CH=CH or CH2CONH;
R2 represents hydrogen, alkyl, hydroxyalkyl, alkylcarbonyloxyalkyl,
carbamoyloxyalkyl,
carboxyalkyl or carbamoylalkyl;
R3 and R4 each independently represent hydrogen, hydroxy or alkylcarbonyloxy;
or R3 and R~
together represent a bridged dimethylmethylenedioxy chain attached to the
carbons bearing R3
and R4;
RS represents hydrogen, alkyl or hydroxyalkyl; and
the dotted line represents a single bond or, when R3 and R4 represent
hydrogen, also a double
bond;
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 10-
M3= -~-q5 Bs-A6-~-
wherein
A5 represents CH2CH2, CH=CH, COCH2, CH(OH)CH2, NHCO(CH2),,,, NHCOCHzO,
NHCOOCH2or O(CH2)q;
m is 0, 1 or 2;
q is 1, 2 or 3 and
B6 represents N and A6 represents CHz, CH2CHz, CH2CH=CH or CH2CH2S; or
B6 represents CH and A6 represents CH2NHCH2, CH2NHCH2CONH or CH2NHCH2CH=CH;
HN Rs
4= + -N-
M +Aw- A$
wherein
A7 represents NHCO, CH2CH2 or OCH2;
A$ represents CH2; and
R6 represents hydrogen or alkyl.
A further aspect of this invention relates to compounds of formula IPl
V\ U
W M --- D
X IPi
wherein Rl represents alkyl, alkoxy, halogen or cyano;
one or two of U, V, W and X represent(s) N, the remaining represents CH, or,
in case of U
and/or X, also CR;
R represents alkoxy or halogen;
D represents alkyl, aryl or heteroaryl;
M is one of the spacers M1, M2 and M3:
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-11-
H (CH2)p
(CH2)n =
1 B2-A2
Mi +A1 = B f
(CH2)n = (CH2)p
wherein
A' represents NHCO, OCH2, CH2CH2, CH=CH or CH(OH)CH2;
A' represents NHCH2, NHCH2CONH, NHCH2CH=CH, CH2CH2, CH2CO, CH2CH(OH) or
CH2CH(OCONH2);
B1 and B2 each represent independently from each other N or CH;
when A' represents OCH2, B1 represents CH;
n is the integer 1; or n is also 0 when B1 is CH; and
p is the integer 1; or p is also 0 when B2 is CH;
R2
0 R5
M2= -~-A3 I -~-
N-A4 c~
R3 R4
wherein
A3 represents NHCO, CH2CH2, CH=CH, COCH2, CH(OH)CH2, CH(OH)CH(OH) or OCH2;
A4 represents CH2, CH2CH=CH or CH2CONH;
RZ represents hydrogen, alkyl, hydroxyalkyl, alkylcarbonyloxyalkyl,
carbamoyloxyalkyl,
carboxyalkyl or carbamoylalkyl;
R3 and R4 each independently represent hydrogen, hydroxy or alkylcarbonyloxy;
R5 represents hydrogen or alkyl; and
the dotted line represents a single bond or, when R3 and R4 represent
hydrogen, also a double
bond;
M 3 AS B6 -- A 4-
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-12-
wherein
A5 represents CH2CH2, CH=CH, COCHz, CH(OH)CH2, NHCO(CH2),,,, NHCOCH2O,
NHCOOCH2 or O(CH2)q;
m is an integer between 0 and 2;
q is an integer between 1 and 3 and
B6 represents N and A6 represents CH2, CH2CH2, CH2CH=CH or CH2CH2S; or
B6 represents CH and A6 represents CH2NHCH2, CH2NHCH2CONH or CH2NHCH2CH=CH.
A further embodiment of the bicyclic derivatives of the above formula I, ICE
or lPl relates to
their prodrugs, their tautomers, their optically pure enantiomers, mixtures of
enantiomers,
racemates, optically pure diastereoisomers, mixtures of diastereoisomers,
diastereoisomeric
racemates, mixture of diastereoisomeric racemates, meso forms,
pharmaceutically acceptable
salts, solvent complexes and morphological forms thereof. Particularly
preferred are the
optically pure enantiomers, optically pure diastereoisomers, meso forms,
pharmaceutically
acceptable salts, solvent complexes and morphological forms.
The following paragraphs provide definitions of the various chemical moieties
for the
compounds of formula IP1 and are intended to apply to those compounds unless
an otherwise
expressly set out definition provides a broader definition:
= The term "alkyl" refers to a saturated straight or branched chain alkyl
group, containing
from one to ten, preferably one to six, in particular one to four carbon
atoms, for example
methyl, ethyl, propyl, iso-propyl, butyl, iso-butyl, sec-butyl, tert-butyl, n-
pentyl, iso-
pentyl, n-hexyl, 2,2-dimethylbutyl, n-octyl. Any alkyl group as defined herein
may be
substituted with one, two or more substituents, for example F, Cl, Br, I, NHz,
OH, SH,
COOH or NO2. Examples for substituted alkyl groups are trifluoromethyl,
trifluoroethyl,
hydroxyrnethyl, hydroxyethyl, carboxymethyl and carboxyethyl. Alkyl groups may
also
be substituted by alkylcarbonyloxy, such as in acetoxymethyl, acetoxyethyl,
propionyloxymethyl or propionyloxyethyl; by carbamoyloxy, such as in
carbamoyloxymethyl or carbamoyloxyethyl; or by carbamoyl, such as in
carbamoylmethyl or carbamoylethyl.
= Alkyl groups combined to form alkylcarbonyloxy are exemplified e. g. as
acetoxy or
propionyloxy.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-13-
= The term "alkoxy" is an "alkyl-O" group, where "alkyl" has the above
significance.
Examples for substituted alkoxy groups are trifluoromethoxy and
trifluoroethoxy.
= The term "halogen" refers to fluorine, chlorine, bromine or iodine,
preferably to fluorine
or chlorine.
d= The term "aryl" refers to an aromatic cyclic group with one, two or three
rings, having
five to 14 carbon ring-atoms preferably from five or six to ten carbon ring-
atoms, for
example phenyl or naphthyl groups. Any aryl group as defined herein may be
substituted
with one, two or more substituents, for example F, Cl, Br, I, OH, NH2, SH, N3,
NO2, alkyl
groups such as methyl or ethyl, perfluoroalkyl groups such as trifluoromethyl
or
trifluoroethyl, alkoxy groups such as methoxy, amino groups such as
methylamino or
dimethylamino, or cyano. Specific examples are 4-fluoro-phenyl, 4-chloro-
phenyl,
4-methoxy-phenyl, 4-methyl-phenyl, 4-trifluoromethyl-phenyl, 4-
trifluoromethoxy-
phenyl, 2,4-difluoro-phenyl, 2,4-dichloro-phenyl, 2,4-dimethoxy-phenyl, 2,4-
dimethyl-
phenyl, 2,4-ditrifluoromethyl-phenyl and 2,4-ditrifluoromethoxy-phenyl.
= The term "heteroaryl" refers to an aryl group as defined herein where one,
two or more
ring-carbon atoms are replaced by an oxygen, nitrogen or sulphur atom, for
example
pyridyl, imidazolyl, pyrazolyl, quinolinyl, isoquinolinyl, pyrrolyl, oxazolyl,
isoxazolyl,
thiazolyl, isothiazolyl, 1,2,3-triazolyl, 1,2,4-triazolyl, oxadiazolyl,
thiadiazolyl, indolyl,
indazolyl, tetrazolyl, pyrazinyl, pyrimidinyl and pyridazinyl groups. The term
"heteroaryl" also covers bicyclic structures such as benzo[1,3]dioxol-5-yl,
2,3-dihydro-
benzo[1,4]dioxin-6-yl, 4H-benzo[1,4]oxazin-3-one-6-yl, 4H-benzo[1,4]thiazin-3-
one-6-yl,
3-oxo-3,4-dihydro-2H-benzo[1,4]thiazin-6-yl, 1H-pyrido[2,3-b][1,4]thiazin-2-
one-7-yl,
2,3-dihydro-[1,4]dioxino[2,3-c]pyridin-7-yl, 2,3-dihydro-[1,4]dioxino[2,3-
b]pyridin-7-yl,
4H-pyrido[3,2-b][1,4]oxazin-3-one-6-yl, 3,4-dihydro-2H-pyrido[3,2-b]thiazin-6-
yl,
3-oxo-3,4-dihydro-2H-pyrido[3,2-b]thiazin-6-yl, 3,4-dihydro-lH-quinolin-2-one-
7-yl,
3,4-dihydro-lH-quinoxalin-2-one-7-yl, 2-oxo-3,4-dihydro-lH-[1,8]naphtyridin-6-
yl,
6,7-dihydro-[1,4]dioxino[2,3-d]pyrimidin-2-yl, 2-oxo-2,3-dihydro-
1H-pyrido[3,4-b][1,4]oxazin-7-yl, 2-oxo-2,3-dihydro-lH-pyrido[2,3-
b][1,4]oxazin-7-yl,
benzo[1,2,5]thiadiazol-5-yl, benzofuran-3-yl and 7-fluoro-4H-benzo [
1,4]thiazin-3 -one-
6-yl. Any heteroaryl group as defined herein may be substituted with one, two
or more
substituents, for example F, Cl, Br, I, OH, NH2, SH, N3, NO2, alkyl groups
such as methyl
or ethyl, perfluoroalkyl groups such as trifluoromethyl or trifluoroethyl,
alkoxy groups
such as methoxy, amino groups such as methylamino or dimethylamino, or cyano.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 14-
= The present invention also relates to pro-drugs that are composed of a
compound of
formula IPI having a free carboxylic acid and at least one pharmacologically
acceptable
protective group that will be cleaved off under physiological conditions. Such
prodrugs
have been reviewed by Beaumont, Kevin; Webster, Robert; Gardner, lain; Dack,
Kevin in
Current Drug Metabolism (2003), 4(6), 461-485. Examples of such promoities are
alkoxy-, aralkyloxy-, OCH(Ra)OCORb (e.g. pivaloyloxymethyloxy), OCH(Ra)ORb,
2-alkyl-, 2-aryl- or 2-aralkyl-oxycarboriyl-2-alkylidene-ethoxy group, 5-
alkyl[1,3]dioxol-
2-one-4-yl-methoxy, dialkylamino-alkyloxy or acyloxy as defined herein, e.g.
ethoxy,
benzyloxy, acetyl or acetyloxy wherein Ra and Rb are hydrogen, C1-C6 alkyl, C2-
C6
alkenyl, C2-C6 alkynyl, C2-C6 heteroalkyl, C2-C6 cycloalkyl, C2-C6
heterocycloalkyl,
alkylaryl, alkylheteroaryl, heteroalkylcycloalkyl, heteroalkylheteroaryl,
aryl, heteroaryl,
heterocycloalkylaryl or heterocycloalkylheteroaryl. Furthermore, if a free
hydroxy group
is present on a compound of Formula I. it can be protected as a prodrug of the
type sulfate
(OS03H), phosphate (OP03H2), oxyrnethylene phosphate (OCH2OPO3H2), succinate
(OCOCH2CH2COOH), or ester of naturally occurring amino acids or a derivative
thereof
(e.g. dimethylaminoglycine).
The following paragraphs provide definitions of the various chernical moieties
for the
compounds according to the invention and are intended to apply uniformly
throughout the
specification and claims (except for the compounds of formula IPi that have
their own
definitions), unless an otherwise expressly set out definition provides a
broader or narrower
definition:
= The term "alkyl" refers to a saturated straight or branched chain alkyl
group, containing
from one to ten, preferably one to six, and in particular one to four carbon
atoms.
Representative examples of alkyl groups include, but are not linzited to,
methyl, ethyl,
propyl, iso-propyl, n-butyl, iso-butyl, sec-butyl, tert-butyl, n-pentyl, iso-
pentyl, n-hexyl,
2,2-dimethylbutyl, n-heptyl or n-octyl. The term "(C1-Cx)a1ky1" (x being an
integer) refers
to a straight or branched chain alkyl group containing 1 to x carbon atoms.
= The term "alkoxy" refers to a saturated straight or branched chain alkoxy
group,
containing from one to ten, preferably one to six, and in particular one to
four carbon
atoms. Representative examples of alkoxy groups include, but are not limited
to, methoxy,
ethoxy, propoxy, iso-propoxy, n-butoxy, iso-butoxy, sec-butoxy, tert-butoxy or
n-
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-15-
hexyloxy. The term "(C1-C,,)alkoxy" refers to a straight or branched chain
alkoxy group
containing 1 to x carbon atoms.
=e= The term (C1-C6)alkoxy-(C1-C6)alkyl refers to a(C1-C6)alkyl group as
previously defined
itself substituted with a(C1-C6)alkoxy group as previously clefined.
= The term "haloalkoxy" refers to a saturated straight or branched chain
alkoxy group,
containing from one to six and preferably one to four carbon atoms, in which
at least one
hydrogen atom (and possibly all) has been replaced by a halogen atom.
Representative
examples of haloalkoxy groups include, but are not lirnited to,
trifluoromethoxy o:r
difluoromethoxy. The term "(C1-CX)haloalkoxy" (x being an integer) refers to a
straight of
branched chain haloalkoxy group containing 1 to x carbon a.toms.
= The term "hydroxyalkyl" refers to a saturated straight or branched chain
alkyl group
substituted once by hydroxy and containing from one to six, and in particular
one to four,
carbon atoms.
d= The term "alkylcarbonyloxyalkyl" refers to an alkylcarboriyloxyalkyl
wherein each alkyl
group is independently a saturated straight or branched chaL-in alkyl group
containing frorn
one to six, and in particular one to four, carbon atoms.
= The term "carbamoyloxyalkyl" refers to a carbamoyloxyalkyl wherein the alkyl
group is a
saturated straight or branched chain alkyl group contaixiing from one to six,
and in
particular one to four, carbon atoms.
= The term "carboxyalkyl" refers to a carboxyalkyl whereirn the alkyl group is
a saturaterd
straight or branched chain alkyl group containing from one to six, and in
particular one to
four, carbon atoms.
=e= The term "carbamoylalkyl" refers to a carbamoylalkyl wherein the alkyl
group is a
saturated straight or branched chain alkyl group containing from one to six,
and in
particular one to four, carbon atoms.
= The term "alkylcarbonyloxy" refers to an alkylcarbonyloxy wherein the alkyl
group is a
saturated straight or branched chain alkyl group contai.ning from one to six,
and in
particular one to four, carbon atoms.
= The term "alkoxycarbonylalkyl" refers to an alkoxycarbornylalkyl wherein the
alkyl grotip
is a saturated straight or branched chain alkyl group corirtaining from one to
six, and in
particular one to four, carbon atoms and the alkoxy gxoup is a saturated
straight or
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-16-
branched chain alkoxy group, containing from one to six, and in particular one
to four
carbon atoms.
= The term "halogen" refers to fluorine, chlorine, bromine or iodine,
preferably to fluorine
or chlorine.
= The term "cycloalkyl", used alone or in combination, refers to a saturated
cyclic
hydrocarbon moiety containing 3 to 7 carbon atoms. The term "(Cy
CZ)cycloalkyl", used
alone or in combination, refers to a saturated cyclic hydrocarbon moiety
containing y to z
carbon atoms. Representative examples of cycloalkyl groups include, but are
not limited
to, cyclopropyl, cyclopentyl and cyclohexyl.
= The term "cycloalkylalkyl", used alone or in combination, refers to an alkyl
group as
previously defined itself substituted by a cycloalkyl group as previously
defined.
Representative examples of cycloalkylalkyl groups include, but are not limited
to,
cyclopropylmethyl and cyclohexylmethyl.
= The term "(C2-C6)alkenyl" refers to a straight or branched hydrocarbon chain
containing 2
to 6 carbon atoms with at least one carbon-carbon double bond. Representative
examples
of alkenyl include, but are not limited to, ethenyl, 2-propenyl, 3-butenyl, 4-
pentenyl or
5-hexenyl.
= The term "aryl", used alone or in combination, refers to an aromatic cyclic
group with
one, two or three rings, having five to 14 carbon ring-atoms preferably from
five or six to
ten carbon ring-atoms, for example phenyl or naphthyl groups. Any aryl group
as defined
herein may be substituted with one, two or more substituents, each of which is
independently selected from the group consisting of halogen, alkyl, alkoxy,
trifluoromethyl and trifluoromethoxy. Specific examples of aryl are phenyl,
naphtyl,
4-fluoro-phenyl, 4-chloro-phenyl, 4-methoxy-phenyl, 4-methyl-phenyl, 4-
trifluoromethyl-
phenyl, 4-trifluoromethoxy-phenyl, 2,4-difluoro-phenyl, 2,4-dichloro-phenyl,
2,4-
dimethoxy-phenyl, 2,4-dimethyl-phenyl, 2,4-ditrifluoromethyl-phenyl and
2, 4-ditrifluoromethoxy-phenyl .
= The term "heteroaryl" refers to an aryl group as defined herein where one,
two or more
ring-carbon atoms are replaced by an oxygen, nitrogen or sulphur atom, for
example
pyridyl, imidazolyl, pyrazolyl, quinolinyl, isoquinolinyl, pyrrolyl, thienyl,
oxazolyl,
isoxazolyl, thiazolyl, isothiazolyl, 1,2,3-triazolyl, 1,2,4-triazolyl,
oxadiazolyl, thiadiazolyl,
indolyl, indazolyl, tetrazolyl, pyrazinyl, pyrimidinyl and pyridazinyl groups.
The term
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-17-
"heteroaryl" also covers bicyclic structures selected from the group
consisting of
benzo[1,3]dioxol-5-yl, 2,3-dihydro-benzo[1,4]dioxin-6-yl, 2,3-dihydro-
[1,4]dioxino[2,3-b]pyridin-6-yl, 2,3-dihydro-[1,4]dioxino[2,3-c]pyridin-7-yl,
3-oxo-
3,4-dihydro-2Fl-benzo[1,4]thiazin-6-yl, 3-oxo-3,4-dihydro-2H-benzo[1,4]oxazin-
6-yl,
3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazin-6-yl, 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]oxazin-6-yl, 2-oxo-2,3-dihydro-lH-pyrido[3,4-
b][1,4]oxazine-7-yl,
2-oxo-3,4-dihydro-lH-quinolin-7-yl, benzo[1,2,5]thiadiazol-5-yl, chroman-7-yl
and
benzofuran-3-yl. Any heteroaryl group as defined herein may be substituted
with one, two
or more substituents on its aromatic ring(s), said substituents being from the
group
consisting of halogen, alkyl and alkoxy; preferably, any heteroaryl group as
defined herein
may be substituted with one halogen substituent. Hence, exemples of heteroaryl
groups
include, but are not limited to, benzo[1,3]dioxol-5-yl, 2,3-dihydro-
benzo[1,4]dioxin-6-yl,
2,3-dihydro-[1,4]dioxino[2,3-b]pyridin-6-yl, 2,3-dihydro-[1,4]dioxino[2,3-
c]pyridin-7-yl,
3-oxo-3,4-dihydro-2H-benzo[1,4]thiazin-6-yl, 7-fluoro-3-oxo-3,4-dihydro-
2H-benzo[1,4]thiazin-6-yl, 3-oxo-3,4-dihydro-2H-benzo[1,4]oxazin-6-yl,
3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazin-6-yl, 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]oxazin-6-yl, 2-oxo-2,3-dihydro-lH-pyrido[3,4-
b][1,4]oxazine-7-yl,
2-oxo-3,4-dihydro-lH-quinolin-7-yl, benzo[1,2,5]thiadiazol-5-yl, thiophen-2-
yl, thiazol-
2-yl, 2,2-dimethyl-chroman-7-yl and benzofuran-3-yl.
= When in the formula
Ri
v\ u
W\ / M-D
'-X
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-18-
M represents the radical
R2
O R5
2 A3 1
M _- -~-
N-A4
R3 R4
this means specifically that the radical A3 is attached to the
Ri
V U
w X
group whereas the radical A4 is attached to the D group.
This is applicable mutatis niutandis to all other variants of the M radical
(i.e. Ml, M3 and
M4) and to all radicals that make M radicals (e.g. A3 and A4). As a further
example, in the
substructure M2, if it is stated that A4 represents CH2CONH, it is thereby
meant that the
CH2 part of the CH2CONH radical is attached to the nitrogen bearing the
radical RS and
that the CONH part of the CH2CONH is attached to the D group. In other words,
the left
part of a radical is always attached to the right part of the radical that is
next to the left.
=e= When in a rest
M31
_ - As 'OOC As _
the two bonds linking the cyclohexyl ring to the radicals A5 and A6 are
represented by two
bold lines (and not by wedged bonds that would then depict an absolute
stereochemistry),
this means that the two bonds are in a cis configuration relatively to said
cyclohexyl ring
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-19-
(i.e. the radicals A5 and A6 are either both above the median plan of the
cyclohexyl ring or
both under said median plan).
This is applicable mutatis mutandis to all other cyclohexyl, pyrane or
piperidine ring
representations of compounds of formula I or ICE.
Compounds of formula I, ICE, IPZ, ICEPZ or IPl carrying a double bond in AI-A6
are present as
ZIE (cisltrans) isomer mixtures or as Z (cis) or E (trans) isomers. Preferred
are the E(tNans)
isomers.
Preferably, the radical R' will be alkyl, alkoxy, haloalkoxy or cyano. More
preferably, the
radical R' will be (Cl-C3)alkyl, (C1-C3)alkoxy, (C1-C2)haloalkoxy or cyano (in
particular
methyl, methoxy or cyano, and notably methoxy or cyano).
Preferably also, D will be aryl or heteroaryl, notably phenyl (which may
optionally be
substituted once or twice by substituents independently selected from halogen,
methyl and
methoxy) or heteroaryl. More preferably, D will be phenyl substituted once or
twice by
substituents independently selected from halogen atoms (especially 2,5-
difluorophenyl) or
heteroaryl. In particular, D will be heteroaryl.
Besides, the radical Ra will preferably be fluorine. Preferably also, the
radical Rb will be
fluorine.
Preferably, U, V, W and X will be such that one or two of U, V, W and X
represent(s) N and
the remaining represent CH. According to another preferred embodiment, U, V, W
and X will
be such that one or two of U, V, W and X represent(s) N, one of U, V, W and X
represents CF
and the remaining represent(s) CH.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-20-
Preferred combinations for the symbols U, V, W and X in the compounds of
formula I, ICE or
IPl are evident from the following particular structures:
I I
~VUL nJWL
R1 R, N\ R1 N
/
N N N
R1 R1 N\ R, N\
N N
wherein R' is preferably (C1-C3)alkyl, methoxy, ethoxy, trifluoromethyl,
trifluoromethoxy or
cyano, and in particular methyl, methoxy or cyano.
Other preferred combinations for the symbols U, V, W and X in the compounds of
formula I,
ICE or IPI are evident from the following particular structures:
I I
~ ru
F
R, R,
N N
~ ruLrLr%j
R, CI R, N\ F
N N
wherein R' is preferably (C1-C3)alkyl, methoxy, ethoxy, trifluoromethyl,
trifluoromethoxy or
cyano, and in particular methyl, methoxy or cyano.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-21-
Preferred embodiments of D for the compounds of formula I, ICE, IPZ, ICEP2 or
IPi are
(C1-C9) linear alkyl, phenyl, benzofuran-3-yl, thiazol-2-yl or heteroaryl of
the formula
Y-!4
z P
wherein P is a ring selected from
Q c")
Q N ~
H
QisOorS;
K, Y and Z are each independently N or CR3; and
R3 is hydrogen or halogen (and in particular hydrogen or fluorine).
More preferably, D will be a heteroaryl of the formula
Y-fC
Z P
wherein P is a ring selected from
(Q TQ N Q
> ;
Q 4 N N 0
H
QisOorS;
K, Y and Z are each independently N or CR3; and
R3 is hydrogen or halogen (and in particular hydrogen or fluorine).
Particularly preferred embodiments of D for the compounds of formula IPl are:
= 2,3-dihydro-benzo[1,4]dioxin-6-yl;
= benzo[1,3]dioxol-5-yl;
= 4H-benzo [ 1,4]oxazin-3-one-6-yl;
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-22-
= 4H-benzo[1,4]thiazin-3-one-6-yl;
= 7-fluoro-4H-benzo[1,4] thiazin -3-one-6-yl;
= 2,3-dihydro[1,4]dioxino[2,3-c]pyridin-7-yl;
= 2,3-dihydro[1,4]dioxino[2,3-b]pyridin-6-yl;
= 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazin-6-yl;
= 2-oxo-lH-pyrido[2,3-b][1,4]thiazin-7-yl; and
= benzo[1,2,5]thiadiazol-5-yl.
Particularly preferred embodiments of D for the compounds of formula I, ICE,
IP2a ICEP2 or IPl
are:
= 2,3-dihydro-benzo[1,4]dioxin-6-yl;
= 4H-benzo[1,4]oxazin-3-one-6-yl;
=e= 4H-benzo[1,4]thiazin-3-one-6-yl;
=++= 7-fluoro-4H-benzo[1,4] thiazin -3-one-6-yl;
= 2,3-dihydro[1,4]dioxino[2,3-c]pyridin-7-yl;
= 2,3-dihydro[1,4]dioxino[2,3-b]pyridin-6-yl;
= 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazin-6-yl;
e1.'= benzo[1,2,5]thiadiazol-5-yl.
According to a first variant of the invention, the spacer M of the compounds
of formula I, ICE,
IPZ, ICEPZ or IPlwill be the spacer Ml.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-23-
For the spacer Ml, preferred embodiments are the follo-wing three structures
(the group of
which will be called M"):
H
iAh1oo- N-CH2-A21 M111
H
~A11 ....1111IN-A22~ M112
H
H
OH
H
I
N-p22_~_ M113
+HC-CH2-N
l>---,
H
wherein
A" represents NHCO, OCH2, CH(OH)CH2 or CI-I2CH2i
A'1 represents CH2, CO, CH(OH) or CH(OCONH2);
A22 represents CH2, CO, CH2CONH or CH2CH=CH.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-24-
Particularly preferred spacers MI are the following:
0
H \C
-NH -0 ; CH 0
c~ 2~-
~ ~nnNH inINH N
\
O H CHZ~ -~ -NH
H H
HO H
H H
CH~ O CHZ ~- '
0 CH2
-NH +NH ~ H ~
H H
0 H
H H2N ~-O
N
' --0 ; CH~
' ~
-O H ~/ HO
0 H
OH H
H N
I-CH N
--N NH CH2
CH2 ~ H ~
H ~
Other preferred spacers MI are the following:
/ OH OH
H / H
-CH CH
~N N\ \-~N NH HN ~
C
H H
0
O
OH
/ H
-~-CH
~N NH
\
CHZ
H ~ 2
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-25-
Preferred compounds of formula I having the spacer Ml are the following:
= (2,3-dihydro-[1,4]dioxino[2,3-b]pyridin-6-ylmethyl)-[(1 a,5a,6a)-6-(6-
methoxy-
[ 1,5]naphthyridin-4-yloxymethyl)-bicyclo[3.1.0]hex-3-yl]-amine;
= (2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-[(1 a,5a,6a)-6-(6-methoxy-
[1,5]naphthyridin-
4-yloxymethyl)-bicyclo[3.1.0]hex-3-yl]-amine;
= 7-fluoro-6-{(1 a,5 a,6a)-[6-(6-methoxy-[1,5]naphthyridin-4-yloxymethyl)-
bicyclo[3.1.0]hex-3-ylamino]-methyl}-4H-benzo[ 1,4]thiazin-3-one;
= 6-{[(1 a,5a,6a)-6-(6-methoxy-[1,5]naphthyridin-4-yloxymethyl)-
bicyclo[3.1.0]hex-
3-ylamino]-methyl } -4H-benzo [ 1,4] oxazin-3-one;
= 6-{[(1 a,5a,6a)-6-(6-methoxy-[1,5]naphthyridin-4-yloxymethyl)-
bicyclo[3.1.0]hex-
3 -ylamino] -methyl } -4H-benzo[ 1, 4]thiazin-3 -one;
= (1 a,3Q,5a,6a)-{3-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-
bicyclo[3.1.0]hexane-6-carboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-
amide;
= (1 a,3,8,5 a,6a)-3-[(3-oxo-3,4-dihydro-2H-benzo[1,4]oxazin-6-ylmethyl)-
amino]-
bicyclo[3.1.0]hexane-6-carboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-
amide;
= (1 a,3,(3,5 a,6a)-3-[(3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazin-6-
ylmethyl)-amino]-
bicyclo[3.1.0]hexane-6-carboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-
amide;
= (1 a,3,(3,5a,6a)-3-[(7-fluoro-3-oxo-3,4-dihydro-2H-benzo[1,4]thiazin-6-
ylmethyl)-amino]-
bicyclo[3.1.0]hexane-6-carboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-
amide;
= 1-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-2-[(1 a,5a,6a)-6-(6-methoxy-quinazolin-
4-yloxymethyl)-3-aza-bicyclo [3.1.0]hex-3-yl]-ethanone;
= rac-1-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-2-[(1 a,5 a,6a)-6-(6-methoxy-
quinazolin-
4-yloxymethyl)-3-aza-bicyclo [3.1.0]hex-3-yl]-ethanol;
= rac-carbamic acid 1-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-2-[(la,5a,6a)-6-(6-
methoxy-
quinazolin-4-yloxymethyl)-3-aza-bicyclo[3.1.0]hex-3-yl]-ethyl ester;
= 4-{(1 a,5a,6a)-3-[2-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethyl]-3-aza-
bicyclo[3.1.0]hex-
6-ylmethoxy } -6-methoxy-quinoline;
= 4-{(1 a,5a,6a)-3-[2-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethyl]-3-aza-
bicyclo[3.1.0]hex-
6-ylmethoxy} -6-methoxy-quinazoline;
= 8-{(1 a,5a,6a)-3-[2-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethyl]-3-aza-
bicyclo[3.1.0]hex-
6-ylmethoxy} -2-methoxy-[ 1, 5]naphthyridine;
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-26-
= 1-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-2-[(1 a,5 a,6a)-6-(6-methoxy-
[1,5]naphthyridin-
4-yloxymethyl)-3 -aza-bicyc lo [3 .1.0]hex-3 -yl] -ethanone;
= rac- 1 -(2,3 -dihydro -benzo [ 1,4] dioxin-6-yl)-2 - [(1 a,5 a, 6 a)-6 -(6 -
methoxy-
[1,5]naphthyridin-4-yloxymethyl)-3-aza-bicyclo[3.1.0]hex-3-yl]-ethanol;
= rac-carbamic acid 1-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-2-[(1a,5a,6a)-6-(6-
methoxy-
[ 1,5]naphthyridin-4-yloxymethyl)-3-aza bicyclo[3.1.0]hex-3-yl]-ethyl ester;
= rac-(1 a,5 a,6a)-4-{3-[2-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethyl]-3-aza-
bicyclo [3.1.0]hex-6-ylmethoxy} -quinoline-6-carbonitrile;
= 3-chloro-4-{(1 a,5a,6a)-3-[2-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethyl]-3-
aza-
bicyclo[3.1.0]hex-6-ylmethoxy}-6-methoxy-quinoline;
= rac-4-{(1 a,5a,6a)-3-[2-hydroxy-2-(3-oxo-3,4-dihydro-2H-benzo[1,4]oxazin-6-
yl)-ethyl]-
3-aza-b icyc lo [3 .1.0] hex-6-ylmethoxy }-quino line-6-carbonitrile;
= rac-2-[(1 a,5a,6a)-6-(3-chloro-6-methoxy-quinolin-4-yloxymethyl)-3-aza-
bicyclo[3.1.0]hex-3-yl]-1-(2,3-dihydro-benzo [ 1,4]dioxin-6-yl)-ethanol;
= rac-carbamic acid 2-[(1a,5a,6a)-6-(3-chloro-6-methoxy-quinolin-4-
yloxymethyl)-3-aza-
bicyclo[3.1.0]hex-3-yl]-1-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethyl ester;
= 6-{2-[(1 a,5 a,6a)-6-(3-chloro-6-methoxy-quinolin-4-yloxymethyl)-3-aza-
bicyclo[3.1.0]hex-3-y1]-1-hydroxy-ethyl} -4H-benzo[ 1,4]oxazin-3-one;
= 5-{(1 a,5 a,6a)-3-[2-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethyl]-3-aza-
bicyclo[3.1.0]hex-
6-ylmethoxy}-3-methoxy-quinoline;
= 6-{(1 a,5 a,6a)-2-[6-(3-methoxy-quinolin-5-yloxymethyl)-3-aza-
bicyclo[3.1.0]hex-3-yl]-
acetyl}-4H-benzo[1,4]oxazin-3-one;
= 6-{ 1-hydroxy-2-[(1 a,5a,6a)-6-(3-methoxy-quinolin-5-yloxymethyl)-3-aza-
bicyclo[3.1.0]hex-3-yl]-ethyl}-4H-benzo[ 1,4] oxazin-3 -one;
= (1 a,5 a,6a)-3-[2-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethyl]-3-aza-
bicyclo[3.1.0]hexane-
6-carboxylic acid (2-cyano-quinolin-8-yl)-amide;
= (1 a,5a,6a)-3-[2-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethyl]-3-aza-
bicyclo[3.1.0]hexane-
6-carboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-amide;
= (1 a,5 a,6a)-3-[2-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethyl]-3-aza-
bicyclo[3.1.0]hexane-
6-carboxylic acid (3-chloro-6-methoxy-quinolin-4-yl)-amide;
= (1 a,5 a,6a)-3-[2-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethyl]-3-aza-
bicyclo[3.1.0]hexane-
6-carboxylic acid (3-methoxy-quinoxalin-5-yl)-amide;
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-27-
= 3-[(1 a,5a,6a)-2-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-2-oxo-ethyl]-3-aza-
bicyclo[3.1.0]hexane-6-carboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-
amide;
= rac-3-[(1 a,5 a,6a)-2-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-2-hydroxy-ethyl]-3-
aza-
bicyclo[3.1.0]hexane-6-carboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-
amide;
= rac-4-{(1 a,5 a,6a)-3-[2-hydroxy-2-(3-oxo-3,4-dihydro-2H-benzo[1,4]thiazin-6-
yl)-ethyl]-
3-aza-bicyclo[3.1.0]hexane-6-carboxylic acid (6-methoxy-[1,5]naphthyridin-4-
yl)-amide;
= rac-(1 a,5a,6a)-6-({3-[2-hydroxy-2-(6-methoxy-quinolin-4-yl)-ethyl]-3-aza-
bicyclo [3.1.0]hex-6-ylamino } -methyl)-4H-pyrido[3,2-b] [ 1,4]thiazin-3-one;
= rac-(1 a,5 a,6a)-6-({3-[2-hydroxy-2-(6-methoxy-quinolin-4-yl)-ethyl]-3-aza-
bicyclo[3.1.0]hex-6-ylamino}-methyl)-4H-benzo[1,4]oxazin-3-one;
= rac-(1 a,5a,6a)-2-{6-[(2,3-dihydro-[1,4]dioxino[2,3-e]pyridin-7-ylmethyl)-
amino]-3-aza-
bicyclo [3.1.0]hex-3-yl } -1-(6-methoxy-quinolin-4-yl)-ethanol;
= rac-2-{(1 a,5 a,6a)-6-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-3-
aza-
bicyclo [3.1.0]hex-3-yl} -1-(6-methoxy-quinolin-4-yl)-ethanol;
= rac-2-{(1 a,5 a,6a)-6-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-3-
aza-
bicyclo [3.1.0]hex-3-yl} -1-(6-methoxy-[ 1, 5]naphthyridin-4-yl)-ethanol;
= rac-6-({(1 a,5a,6a)-3-[2-hydroxy-2-(6-methoxy-[1,5]naphthyridin-4-yl)-ethyl]-
3-aza-
bicyclo[3.1.0]hex-6-ylamino} -methyl)-4H-benzo[ 1,4]thiazin-3-one;
= rac-2-{(1 a,5 a,6a)-6-[(2,3-dihydro-[1,4]dioxino[2,3-b]pyridin-6-ylmethyl)-
amino]-3-aza-
bicyclo[3.1.0]hex-3-yl}-1-(6-methoxy-[1,5]naphthyridin-4-yl)-ethanol;
= rac-6-({(1 a,5 a,6a)-3-[2-hydroxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-3-aza-
bicyclo[3.1.0]hex-6-ylamino}-methyl)-4H-benzo[ 1,4]oxazin-3-one;
= rac-2-{(1 a,5a,6a)-6-[(2,3-dihydro-[1,4]dioxino[2,3-b]pyridin-6-ylmethyl)-
amino]-3-aza-
bicyclo [3.1.0]hex-3-yl} -1-(3-methoxy-quinolin-5-yl)-ethanol;
= rac-6-({(1 a,5a,6a)-3-[2-hydroxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-3-aza-
bicyclo[3.1.0]hex-6-ylamino}-methyl)-4H-pyrido[3,2-b] [ 1,4]thiazin-3-one;
= rac-6-({(1 a,5a,6a)-3-[2-hydroxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-3-aza-
bicyclo[3.1.0]hex-6-ylamino}-methyl)-4H-benzo[ 1,4]thiazin-3-one;
= rac-2-{(1 a,5 a,6a)-6-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-3-
aza-
bicyclo[3.1.0]hex-3-yl}-1-(3-methoxy-quinolin-5-yl)-ethanol;
= 6-({(1 a,5 a,6a)-3-[(2R)-2-hydroxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-3-aza-
bicyclo[3. 1. 0]hex-6-ylamino } -methyl)-4H-benzo [ 1,4]thiazin-3 -one;
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-28-
= (2R)-2-{(1 a,5a,6a)-6-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-3-
aza-
b icyc lo [3 .1. 0] hex-3 -yl }-1-(3 -m ethoxy-quino lin-5 -yl)-ethano l;
= 3-oxo-3,4-dihydro-2H-pyrido[3,2-b] [ 1,4]thiazine-6-carboxylic acid
{(1 a,5 a,6a)-3-[(2R)-2-hydroxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-3-aza-
bicyclo[3.1.0]hex-6-yl}-amide;
= 6-({(1 a,5 a,6a)-3-[(2R)-2-hydroxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-3-aza-
bicyclo[3.1.0]hex-6-ylamino} -methyl)-4H-benzo[ 1,4]oxazin-3-one;
= rac-2-{(1 a,5 a,6a)-3-[2-hydroxy-2-(6-methoxy-quinolin-4-yl)-ethyl]-3-aza-
b icyclo [3.1.0] hex-6-ylamino } -N-thiazol-2-yl-acetamide;
= rac-(1a,5a,6a)-2-{6-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-3-aza-
bicyclo[3.1.0]hex-3-yl} -1-(3-methoxy-quinoxalin-5-yl)-ethanol;
= rac-(1 a,5 a,6a)-6-({3-[2-hydroxy-2-(3-methoxy-quinoxalin-5-yl)-ethyl]-3-aza-
bicyclo[3.1.0]hex-6-ylamino} -methyl)-4H-benzo [ 1,4]thiazin-3-one;
= rae-(1 a,5 a,6a)-6-({3-[2-hydroxy-2-(3-methoxy-quinoxalin-5-yl)-ethyl]-3-aza-
bicyclo[3.1.0]hex-6-ylamino}-methyl)-4H-benzo[1,4]oxazin-3-one;
= rac-(1 a,5a,6a)-2-{6-[(benzo[1,2,5]thiadiazol-5-ylmethyl)-amino]-3-aza-
bicyclo[3.1.0]hex-3-yl}-1-(3-methoxy-quinoxalin-5-yl)-ethanol;
= rac-(1 a,5 a,6a)-6-({3-[2-hydroxy-2-(3-methoxy-quinoxalin-5-yl)-ethyl]-3-aza-
bicyclo[3.1.0]hex-6-ylamino} -methyl)-4H-pyrido [3,2-b] [ 1,4]thiazin-3-one;
= rac-(la,5a,6a)-2-{6-[(benzofuran-2-ylmethyl)-amino]-3-aza-bicyclo[3.1.0]hex-
3-yl}-1-
(3 -methoxy-quinoxalin-5 -yl)-ethanol;
= rac-(1 a,5 a,6a)-1-(3-methoxy-quinoxalin-5-yl)-2-[6-(3-phenyl-allylamino)-3-
aza-
bicyclo [3.1.0]hex-3-yl]-ethanol;
= rac-(1 a,5 a,6a)-2-{6-[(2,2-dimethyl-chroman-7-ylmethyl)-amino]-3-aza-
bicyclo[3.1.0]hex-3-yl}-1-(3-methoxy-quinoxalin-5-yl)-ethanol;
= 6-{2-[(1 a,5a,6a)-6-(6-methoxy-quinazolin-4-yloxymethyl)-3-aza-
bicyclo[3.1.0]heK--
3-yl]-acetyl} -4H-benzo[1,4]thiazin-3-one;
= 6-{ 1-hydroxy-2-[(1 a,5a,6a)-6-(6-methoxy-quinazolin-4-yloxymethyl)-3-aza-
bicyclo[3.1.0]hex-3-yl]-ethyl}-4H-benzo[ 1,4]thiazin-3-one.
More preferably, the compounds of formula I having the spacer Ml will be
selected from the
first 48 compounds mentioned in the list hereabove.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-29-
In particular, the following compounds of formula I having the spacer Mi are
preferred:
= 2-{rac-(1a,5a,6a)-6-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-3-aza-
bicyclo [3.1.0]hex-3-yl} -1-(3-methoxy-quinoxalin-5-yl)-ethanol;
= rac-(1 a,5a,6a)-6-({3-[2-hydroxy-2-(3-methoxy-quinoxalin-5-yl)-ethyl]-3-aza-
bicyclo[3.1.0]hex-6-ylamino}-methyl)-4H-benzo[1,4]oxazin-3-one;
= rac-(1 a,5a,6a)-6-({3-[2-hydroxy-2-(3-methoxy-quinoxalin-5-yl)-ethyl]-3-aza-
bicyclo[3.1.0]hex-6-ylamino} -methyl)-4H-benzo[ 1,4]thiazin-3-one;
= rac-(1a,5a,6a)-6-({3-[2-hydroxy-2-(3-methoxy-quinoxalin-5-yl)-ethyl]-3-aza-
b icyclo [3 .1.0] hex-6-ylamino } -methyl)-4H-pyrido [3,2-b] [ 1,4]thiazin-3 -
one.
According to a second variant of the invention, the spacer M of the compounds
of formula I,
ICg, IPZ, ICEP2 or Ip1will be the spacer M2.
Preferred compounds of formula I wherein M is M' are those wherein at least
one of the
following further characteristics is present:
=2= A3 representing OCH2, NHCO, CH2CH2, CH=CH, COCH2, CH(OH)CH2 or CH2CH(OH),
or also, provided U is N, CH(OH)CH(OH);
A4 representing CH2 or CO, or also, provided D is a non-annelated aryl or
heteroaryl
group, CH2CH=CH or CH2CONH;
= R2 representing hydrogen, alkyl or hydroxyalkyl;
= R5 representing hydrogen, alkyl or hydroxyethyl.
More preferred compounds of formula I wherein M is Mz are those wherein at
least one of the
following further characteristics is present:
= A3 representing NHCO, CH2CH2, 'CH=CH, CH(OH)CH2 or CH2CH(OH), or also,
provided U is N, CH(OH)CH(OH);
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-30-
= A4 representing CH2 or CO, or also, provided D is a non-annelated aryl or
heteroaryl
group, CH2CH=CH or CH2CONH;
= RZ representing hydrogen or hydroxyalkyl (and preferably hydrogen or
hydroxyethyl);
d= R3 and R4 each independently representing hydrogen or hydroxy;
= R5 representing hydrogen or hydroxyethyl;
= the dotted line of M2 representing a single bond.
Particularly preferred compounds of formula I wherein M is M' are those
wherein at least one
of the following further characteristics is present:
= A3 representing CH(OH)CH2 or CH2CH(OH), or also, provided U is N,
CH(OH)CH(OH);
d= A4 representing CH2 or CO;
= RZ representing hydrogen;
= each of R3 and R4 representing hydrogen;
=e= R5 representing hydrogen;
= the dotted line of M2 representing a single bond.
Besides, among the compounds of formula I wherein M is M2, those wherein the
substituents
A3 and N(R5)-A4 have the trans stereochemistry are preferred (in particular
when each of R2,
R3 and R4 is hydrogen).
With regard to the spacer M2, its stereochemistry will preferably be the
following (called MZI
hereafter):
R2
R5
O I
M21 = - - q3 N - q4 - ~ =
~
R3 R4
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-31-
In particular, when one of R2, R3 and R4 is not hydrogen, the stereochemistry
of M2 will
preferably be the following (called MZ11 hereafter):
R2 R5
M211 0 (R)'1, 1 \ N-A
(S)
~ A3 R4
R3
Preferred spacers M2 are the following:
0 H
H O .~~NN/~CH-
0 .\\ NH- O /
O
0
H
0
H NH-
N
0
Also preferred are the following spacers M2:
0
H H
~ 0 .~\N~
.~\N~~CH~ p NH CH ~'~
~/\/ c'
C~~ ~C' '
H H H
C N ~.~ H 0 .~\N '
40"\
~\N
-~-
0 ~~~'~
0
OH
J H
H H
O \N40 4-OLOH
OH
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-32-
H H
H .~~N .~~N 1 _
O .~N 4CH2 4CH O
~
~
CHz
~ ,j~
Other preferred spacers M2 are the spacers M22 1, M222, M2z3, M224 and M225
represented
below:
OH
Mzz1 _ -~ O
OH
H
HO 222
Mz2a
N-A4-~-
Mzzz O HO ~"~ 222
..,1~mN-A4-~-
H ???
HO
OH Mzzs _ .'
N-A4-~-
_~ H ???
Mzzs = HO
QlIlJNA4+
H
and in particular the spacers M221, M222 and M''z3.
Preferred compounds of formula I having the spacer M2 are the following:
= (2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-[(3R' 6S)-6-(6-methoxy-quinolin-
4-yloxymethyl)-3,6-dihydro-2H-pyran-3-yl]-amine;
= (2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-[(3R,6S)-6-(6-methoxy-quinolin-
4-yloxymethyl)-tetrahydro-pyran-3-yl]-amine;
= (2,3-dihydro-[1,4]dioxino[2,3-c]pyridin-7-ylmethyl)-[(3R,6S)-6-(6-methoxy-
quinolin-
4-yloxymethyl)-tetrahydro-pyran-3-yl]-amine;
= 6- { [(3R, 6S)-6-(6-methoxy-quinolin-4-yloxymethyl)-tetrahydro-pyran-3 -
ylamino]-
methyl}-4H-benzo[1,4]oxazin-3-one;
= (2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-[(3R,6S)-6-(6-methoxy-
[1,5]naphthyridin-
4-yloxymethyl)-tetrahydro-pyran-3-yl]-amine;
= 6-{[(3R,6S)-6-(6-methoxy-[1,5]naphthyridin-4-yloxymethyl)-tetrahydro-pyran-
3-ylamino] -methyl }-4H-b enzo [ 1, 4] oxazin-3 -one;
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-33-
= 6-{ [(3R,6S)-6-(6-methoxy-quinolin-4-yloxymethyl)-tetrahydro-pyran-3-
ylamino]-
methyl} -4H-benzo [ 1,4]thiazin-3-one;
= 6-{[(3R,6S)-6-(6-methoxy-[1,5]naphthyridin-4-yloxymethyl)-tetrahydro-pyran-
3-ylamino]-methyl} -4H-benzo[ 1,4]thiazin-3-one;
= 6-{[(3R,6S)-6-(6-methoxy-[1,5]naphthyridin-4-yloxymethyl)-tetrahydro-pyran-
3-ylamino]-methyl} -4H-pyrido[3,2-b] [ 1,4]thiazin-3-one;
= [(3R,6S)-6-(6-methoxy-[ 1,5]naphthyridin-4-yloxymethyl)-tetrahydro-pyran-3-
yl]-
(3-phenyl-allyl)-amine;
= benzofuran-2-ylmethyl-[(3R,6S)-6-(6-methoxy-[ 1,5]naphthyridin-4-
yloxymethyl)-
tetrahydro-pyran-3-yl]-amine;
= (2S,5R)-5-[(2,3-dihydro-benzo[ 1,4]dioxin-6-ylmethyl)-aminoj -tetrahydro-
pyran-
2-carboxylic acid (2-methyl-quinolin-8-yl)-amide;
= 8-{5-[(3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazin-6-ylmethyl)-amino]-
tetrahydro-
pyran-2-ylmethoxy} -quinoline-2-carbonitrile;
= (2S,5R)-5-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-aminoj -tetrahydro-
pyran-
2-carboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-amide;
= (2S,5R)-5-[(2,3-dihydro-[1,4]dioxino[2,3-c]pyridin-7-ylmethyl)-amino]-
tetrahydro-pyran-
2-carboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-amide;
= (2S,5R)5-[(3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazin-6-ylmethyl)-amino]-
tetrahydro-pyran-2-carboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-amide;
= (2S,5R)-5-[(2,3-dihydro-[1,4]dioxino[2,3-c]pyridin-7-ylmethyl)-amino]-
tetrahydro-pyran-
2-carboxylic acid (2-cyano-quinolin-8-yl)-amide;
= (2S,5R)-5-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-tetrahydro-pyran-
2-carboxylic acid (2-cyano-quinolin-8-yl)-amide;
= (2S,5R)-5-[(3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazin-6-ylmethyl)-
amino]-
tetrahydro-pyran-2-carboxylic acid (2-cyano-quinolin-8-yl)-arnide;
= (2S,5R)-5-[(3-oxo-3,4-dihydro-2H-benzo[1,4]thiazin-6-ylmethyl)-amino]-
tetrahydro-
pyran-2-carboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-amide;
= (2S,5R)-5-[(2,3-dihydro-[1,4]dioxino[2,3-b]pyridin-6-ylmethyl)-amino]-
tetrahydro-pyran-
2-carboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-amide;
= 2-[(2R,3R,6S)-3-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-6-(6-
methoxy-
quinolin-4-yloxymethyl)-tetrahydro-pyran-2-yl]-ethanol;
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-34-
= 6-{ [(2R,3R,6S)-2-(2-hydroxy-ethyl)-6-(6-methoxy-quinolin-4-yloxymethyl)-
tetrahydro-
pyran-3-ylamino]-methyl} -4H-benzo[ 1,4]thiazin-3 -one;
= 6-{ [(2R,3R,6S)-2-(2-hydroxy-ethyl)-6-(6-methoxy-quinolin-4-yloxymethyl)-
tetrahydro-
pyran-3-ylamino]-methyl} -4H-pyrido [3,2-b] [ 1,4]thiazin-3-one;
= 6-{ [(3R,6S)-6-(3-methoxy-quinolin-5-yloxymethyl)-tetrahydro-pyran-3-
ylamino]-
methyl} -4H-pyrido [3,2-b] [ 1,4]thiazin-3-one;
= 6-{[(3R,6S)-6-(3-methoxy-quinolin-5-yloxymethyl)-tetrahydro-pyran-3-ylamino]-
methyl}-4H-benzo [ 1,4]thiazin-3-one;
= benzo[1,3]dioxol-5-ylmethyl-[(3R,6S)-6-(3-methoxy-quinolin-5-yloxymethyl)-
tetrahydro-
pyran-3-yl]-amine;
= (2,3-dihydro-benzo[1,4]dioxin-6-yhnethyl)-[(3R,6S)-6-(3-methoxy-quinolin-
5-yloxymethyl)-tetrahydro-pyran-3 -yl]-amine;
= 6-{[(3R,6,S)-6-(3-methoxy-quinolin-5-yloxymethyl)-tetrahydro-pyran-3-
ylamino]-
methyl} -4H-benzo[ 1,4] oxazin-3 -one;
= (2,3-dihydro-[1,4]dioxino[2,3-b]pyridin-6-ylmethyl)-[(3R,6S)-6-(3-methoxy-
quinolin-
5-yloxymethyl)-tetrahydro-pyran-3-yl]-amine;
= (2,3-dihydro-[ 1,4]dioxino[2,3-c]pyridin-7-ylmethyl)-[(3R,6S)-6-(3-methoxy-
quinolin-
5-yloxymethyl)-tetrahydro-pyran-3-yl]-amine;
= 7-fluoro-6-{[(3R,6,S)-6-(3-methoxy-quinolin-5-yloxymethyl)-tetrahydro-pyran-
3 -ylamino] -methyl} -4H-benzo [ 1,4]thiazin-3 -one;
= benzofuran-2-ylmethyl-[(3R,6S)-6-(3-methoxy-quinolin-5-yloxymethyl)-
tetrahydro-
pyran-3-yl]-amine;
= [(3R,6S)-6-(3-methoxy-quinolin-5-yloxymethyl)-tetrahydro-pyran-3-yl]-(3-
phenyl-allyl)-
amine;
= benzo[1,2,5]thiadiazol-5-ylmethyl-[(3R,6S)-6-(3-methoxy-quinolin-5-
yloxymethyl)-
tetrahydro-pyran-3-yl]-amine;
= (3R,6S)-heptyl-[6-(3-methoxy-quinolin-5-yloxymethyl)-tetrahydro-pyran-3-yl]-
amine;
= 2-[(3R,6S)-6-(3-methoxy-quinolin-5-yloxymethyl)-tetrahydro-pyran-3-ylamino]-
1V-thiazol-2-yl-acetamide;
= (2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-[(3R,6S)-6-(3-methoxy-quinoxalin-
5-yloxymethyl)-tetrahydro-pyran-3-yl]-amine;
= 6-{ [(3R,6S)-6-(3-methoxy-quinoxalin-5-yloxymethyl)-tetrahydro-pyran-3-
ylamino]-
methyl} -4H-pyrido[3,2-b] [ 1,4]thiazin-3-one;
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-35-
= (2,3-dihydro-benzo[ 1,4]dioxin-6-ylmethyl)-[(3R,6S)-6-(6-trifluoromethoxy-
quinolin-
4-yloxymethyl)-tetrahydro-pyran-3-yl]-amine;
= 6-{ [(3R,6S)-6-(6-trifluoromethoxy-quinolin-4-yloxymethyl)-tetrahydro-pyran-
3-ylamino]-
methyl} -4Fl-pyrido[3,2-b] [ 1,4]thiazin-3-one;
= 8-{(2S,5R)-5-[(3-oxo-3,4-dihydro-2H-benzo[1,4]thiazin-6-ylmethyl)-amino]-
tetrahydro-
pyran-2-ylmethoxy} -quinoline-2-carbonitrile;
= 6-{ [(3R,6S)-6-(6-methoxy-quinolin-4-yloxymethyl)-tetrahydro-pyran-3-
ylamino]-
methyl}-4H-pyrido[3,2-b] [ 1,4]thiazin-3-one;
= 6-{ [(3R,6S)-6-(2-methoxy-quinolin-8-yloxymethyl)-tetrahydro-pyran-3-
ylamino]-
methyl} -4H-pyrido [3,2-b] [ 1,4]thiazin-3-one;
= 6-{ [(3R,6S)-6-(6-methoxy-quinazolin-4-yloxymethyl)-tetrahydro-pyran-3-
ylamino]-
methyl} -4H-pyrido[3,2-b] [ 1,4]thiazin-3-one;
= 6-{[(3R,6S)-6-(8-fluoro-6-methoxy-quinolin-4-yloxymethyl)-tetrahydro-pyran-
3-ylamino]-methyl } -4Fl-pyrido[3,2-b] [ 1,4]thiazin-3-one;
= 6-{ [(3R,6S)-6-(8-fluoro-6-methoxy-quinolin-4-yloxymethyl)-tetrahydro-pyran-
3-ylamino]-methyl} -4H-pyrido[3,2-b] [1,4]oxazin-3-one;
= 6-{(3R,6S)-[6-(6-methoxy-quinazolin-4-yloxymethyl)-3,6-dihydro-2H-pyran-3-
ylamino]-
methyl} -4H-pyrido [3,2-b] [ 1,4]thiazin-3-one;
= 6- { [(3R, 6S)-6-(6-difluoromethoxy-quinolin-4-yloxymethyl)-tetrahydro-pyran-
3 -ylamino] -
methyl}-4H-pyrido[3,2-b][1,4]thiazin-3-one;
= 6-{(3R,6S)-[6-(3-methoxy-quinoxalin-5-yloxymethyl)-tetrahydro-pyran-3-
ylamino]-
methyl}-4HHpyrido[3,2-b] [ 1,4]oxazin-3-one;
= 6-{(3R,6S)-[6-(3-methoxy-quinoxalin-5-yloxymethyl)-tetrahydro-pyran-3-
ylamino]-
methyl}-4H-benzo[1,4]oxazin-3-one;
= 6-{(3R,6S)-[6-(3-methoxy-quinoxalin-5-yloxymethyl)-tetrahydro-pyran-3-
ylamino]-
methyl } -4H-benzo[ 1,4]thiazin-3 -one;
= 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid
(3R, 6S)-[6-(3-methoxy-quinoxalin-5-yloxymethyl)-tetrahydro-pyran-3-yl]-amide;
= 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid
(3R,6S)-[6-(2-methoxy-quinolin-8-yloxymethyl)-tetrahydro-pyran-3-yl]-amide;
= 4-{(2S,5R)-5-[(2,3-dihydro-[1,4]dioxino[2,3-c]pyridin-7-ylmethyl)-amino]-
tetrahydro-
pyran-2-ylmethoxy} -quinoline-6-carbonitrile;
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-36-
= 4- {(2S, 5R)-5- [(2, 3-dihydro-benzo [ 1, 4] dioxin-6-ylmethyl)-amino]-
tetrahydro-pyran-
2-ylmethoxy} -quinoline-6-carbonitrile;
= 4- {(2S,5R)-5-[(3-oxo-3,4-dihydro-2H-benzo[ 1,4]thiazin-6-ylmethyl)-amino] -
tetrahydro-
pyran-2-ylmethoxy} -quinoline-6-carbonitrile;
= 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid (3R,6S)-[6-
(6-cyano-
quinolin-4-yloxymethyl)-tetrahydro-pyran-3-yl]-amide;
= 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid
(4R,7S)-[4-(6-methoxy-quinolin-4-yloxymethyl)-cis-(4RS, 5RS)-2,2-dimethyl -
tetrahydro-
[ 1,3 ] dioxo lo [4, 5 -c]pyran-7-yl] -amide;
= 6-{(4R,7S)-[4-(6-methoxy-quinolin-4-yloxymethyl)-(4S,5S)-2,2-dimethyl-
tetrahydro-
[ 1,3]dioxolo[4, 5-c]pyran-7-ylamino]-methyl} -4H-pyrido[3,2-b] [ 1,4]thiazin-
3-one;
= 6-{(4R,7S)-([4-(6-methoxy-quinolin-4-yloxymethyl)-(4R,5R)-2,2-dimethyl-
tetrahydro-
[ 1,3]dioxolo[4, 5-c]pyran-7-ylamino]-methyl}-4Fl-pyrido[3,2-b] [ 1,4]thiazin-
3-one;
= 6-{(3S,4S,5S,6R)-[4,5-dihydroxy-6-(6-methoxy-quinolin-4-yloxymethyl)-
tetrahydro-
pyran-3 -ylamino]-methyl} -4H-pyrido[3,2-b] [ 1,4]thiazin-3 -one;
= 6-{(3S,4R,5R,6R)-[4,5-dihydroxy-6-(6-methoxy-quinolin-4-yloxymethyl)-
tetrahydro-
pyran-3-ylamino]-methyl} -4H-pyrido[3,2-b] [ 1,4]thiazin-3-one;
= 8-{(2S,5R)-5-[(3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]oxazin-6-ylmethyl)-
amino]-
tetrahydro-pyran-2-ylmethoxy} -quinoline-2-carbonitrile;
= 6-{[(3S,6R)-6-(6-methoxy-quinazolin-4-yloxymethyl)-tetrahydro-pyran-3-
ylamino]-
methyl}-4H-pyrido[3,2-b] [ 1,4]thiazin-3-one;
= (2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-[(3S,6R)-6-(3-inethoxy-quinolin-
5-yloxymethyl)-tetrahydro-pyran-3-yl]-amine;
= 6-{ [(3S,6R)-6-(3-methoxy-quinolin-5-yloxymethyl)-tetrahydro-pyran-3-
ylamino]-
methyl}-4H-pyrido[3,2-b][1,4]oxazin-3-one;
= 6-{[(3S,6R)-6-(3-methoxy-quinolin-5-yloxymethyl)-tetrahydro-pyran-3-ylamino]-
methyl } -4H-pyrido [3, 2-b] [ 1,4]thiazin-3 -one;
= 6-({(3R,6R)-6-[2-(6-methoxy-[1,5]naphthyridin-4-y1)-ethyl]-tetrahydro-pyran-
3 -ylamino } -methyl)-4H-pyrido [3,2-b] [ 1,4]thiazin-3 -one;
= 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid
{ (3R,6R)-6-[2-(6-methoxy-[ l, 5]naphthyridin-4-yl)-ethyl]-tetrahydro-pyran-3 -
yl} -amide;
= 6-({(3R,6R)-(6-[2-(6-methoxy-[1,5]naphthyridin-4-yl)-ethyl]-tetrahydro-pyran-
3-ylamino} -methyl)-4H-pyrido[3,2-b] [ 1,4]oxazin-3-one;
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-37-
= 6-((3R,6R)-{6-[2-(6-methoxy-[1,5]naphthyridin-4-yl)-ethyl]-tetrahydro-pyran-
3-ylamino } -methyl)-4H-benzo [ 1,4]thiazin-3-one;
= 6-({(3R,6R)-6-[2-(6-methoxy-quinazolin-4-yl)-ethyl]-tetrahydro-pyran-3-
ylamino}-
methyl)-4H-pyrido [3,2-b] [ 1,4]thiazin-3-one;
= 6-((3R,6R)-{6-[2-(6-methoxy-quinazolin-4-yl)-ethyl]-tetrahydro-pyran-3-
ylamino}-
methyl)-4H-benzo[ 1,4]thiazin-3-one;
= 6-({(3R,6S)-6-E-[2-(3-methoxy-quinolin-5-yl)-vinyl]-tetrahydro-pyran-3-
ylamino}-
methyl)-4H-pyrido[3,2-b] [ 1,4]thiazin-3-one;
= 6-({(3R,6R)-6-[2-(3-methoxy-quinolin-5-yl)-ethyl]-tetrahydro-pyran-3-
ylamino}-methyl)-
4H-pyrido[3,2-b][1,4]thiazin-3-one;
= 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid
(3R, 6R)- { 6-[2-(3 -methoxy-quinolin-5-yl)-ethyl]-tetrahydro-pyran-3 -yl } -
amide;
= 6-((3R,6R)-{6-[2-(3-methoxy-quinolin-5-yl)-ethyl]-tetrahydro-pyran-3-
ylamino}-methyl)-
4H-pyrido [3, 2-b] [ 1,4] oxazin-3 -one;
= 6-((3R,6R)-{6-[2-(6-methoxy-quinolin-4-yl)-ethyl]-tetrahydro-pyran-3-
ylamino}-methyl)-
4H-pyrido[3,2-b] [ 1,4]thiazin-3-one;
= (3R,6R)-6-({6-[2-(6-methoxy-quinolin-4-yl)-ethyl]-tetrahydro-pyran-3-
ylamino}-methyl)-
4FI-benzo [ 1, 4]thiazin-3 -one;
= 6-((3R,6R)-{6-[2-(6-methoxy-quinolin-4-yl)-ethyl]-tetrahydro-pyran-3-
ylamino}-methyl)-
4H-benzo [ 1,4]oxazin-3 -one;
= 6-({3R,6S)-6-[(1R,2R)-1,2-dihydroxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-
tetrahydro-
pyran-3 -ylamino } -methyl)-4H-pyrido [3,2-b] [ 1,4]thiazin-3-one;
= 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid
{ (3R, 6S)-6-[(1R,2R)-1,2-dihydroxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-
tetrahydro-pyran-
3-yl}-amide;
= 6-({(3R,6S)-6-[(1R,2R)-1,2-dihydroxy-2-(6-methoxy-[1,5]naphthyridin-4-yl)-
ethyl]-
tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b] [ 1,4]thiazin-3 -one;
= 3-oxo-3,4-dihydro-2H-pyrido[3,2-b] [ 1,4]thiazine-6-carboxylic acid
{(3R,6S)-6-[(1R,2R)-1,2-dihydroxy-2-(6-methoxy-[ 1,5]naphthyridin-4-yl)-ethyl]-
tetrahydro-pyran-3-yl}-amide;
= (3R,6S)-6-({6-[(1R,2R)-1,2-dihydroxy-2-(2-methoxy-quinolin-8-yl)-ethyl]-
tetrahydro-
pyran-3 -ylamino } -methyl)-4H-pyrido [3,2-b] [ 1,4]thiazin-3 -one;
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-38-
3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid
{(3R,6S)-6-[(1R,2R)-1,2-dihydroxy-2-(2-methoxy-quinolin-8-yl)-ethyl]-
tetrahydro-pyran-
3-yl}-amide;
= (3R,6S)-6-({6-[(1R,2R)-1,2-dihydroxy-2-(3-methoxy-quinoxalin-5-yl)-ethyl]-
tetrahydro-
pyran-3 -ylamino} -methyl)-4H-pyrido [3,2-b] [ 1,4]thiazin-3 -one;
= 6-({(3R,6S)-6-[(1S,2S)-1,2-dihydroxy-2-(6-methoxy-[1,5]naphthyridin-4-yl)-
ethyl]-
tetrahydro-pyran-3-ylamino} -methyl)-4H-pyrido[3,2-b] [ 1,4]thiazin-3-one;
= 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid
(3R,6S)-{6-[(1R,2R)-1,2-dihydroxy-2-(3-methoxy-quinoxalin-5-yl)-ethyl]
tetrahydro-
pyran-3 -yl } -am ide;
= 6-({(3R,6S)-6-[(1R,2R)-1,2-dihydroxy-2-(3-methoxy-quinoxalin-5-yl)ethyl]-
tetrahydro-
pyran-3 -ylamino} -methyl)-4H-pyrido [3,2-b] [ 1,4]oxazin-3-one;
= 8-((1R,2R)-1,2-dihydroxy-2-{(2S,5R)-[(3-oxo-3,4-dihydro-2H-pyrido[3,2-
b][1,4]thiazin-
6-ylmethyl)-amino] -tetrahydro-pyran-2-yl } -ethyl)-quino line-2-carbonitrile;
= 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid
{(3R,6S)-6-[(1R,2R)-2-(2-cyano-quinolin-8-yl)-1,2-dihydroxy-ethyl]-tetrahydro-
pyra.n-
3-yl}-amide;
= 8-((1R,2R)-1,2-dihydroxy-2-{(2S,5R)-5-[(3-oxo-3,4-dihydro-
2H-pyrido[3,2-b] [ 1,4] oxazin-6-ylmethyl)-amino] -tetrahydro-pyran-2-yl} -
ethyl)-
quinoline-2-carbonitrile;
= 6-((3R,6S)-{6-[trans-(1RS,2RS)-1,2-dihydroxy-2-(3-methoxy-quinolin-5-yl)-
ethyl]-
tetrahydro-pyran-3-ylamino } -methyl)-4H-pyrido[3,2-b] [ 1,4]thiazin-3-one;
= 6-((3R,6S)-{6-[(1R,2R)-1,2-dihydroxy-2-(6-methoxy-quinolin-4-yl)-ethyl]-
tetrahydro-
pyran-3-ylamino} -methyl)-4H-pyrido[3,2-b] [ 1,4]thiazin-3 -one;
= 6-((3R,6S)-{6-[(1S,2R)-1,2-dihydroxy-2-(6-methoxy-quinolin-4-yl)-ethyl]-
tetrahydro-
pyran-3 -ylamino } -methyl)-4H-pyrido [3, 2-b] [ 1,4]thiazin-3 -one;
= (1RS)-1-{(2S,5R)-5-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-
tetrahydro-pyran-
2-yl} -2-(6-methoxy-quinolin-4-yl)-ethanol;
= 6-({(3R,6S)-6-[(1RS)-1-hydroxy-2-(6-methoxy-quinolin-4-yl)-ethyl]-tetrahydro-
pyra.n-
3 -ylamino} -methyl)-4H-pyrido [ 1,4]thiazin-3 -one;
= 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid
{ (3R, 6S)-6-[(1 RS)-1-hydroxy-2-(6-methoxy-quinolin-4-yl)-ethyl] -tetrahydro-
pyran-3 -yl } -
amide;
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-39-
= 6-({(3R,6S)-6-[(1S)-1-hydroxy-2-(6-methoxy-quinolin-4-yl)-ethyl]-tetrahydro-
pyran-
3-ylamino} -methyl)-4H-pyrido[3,2-b] [ 1,4]thiazin-3-one;
= 6-((3R, 6S)-{6-[1-hydroxy-2-(6-methoxy-quinolin-4-yl)-ethyl]-tetrahydro-
pyran-
3 -ylamino } -methyl)-4H-pyrido [3,2-b] [ 1,4] oxazin-3 -one;
= 7-((3R,6S)-{6-[(1S)-1-hydroxy-2-(6-methoxy-quinolin-4-yl)-ethyl]-tetrahydro-
pyran-
3-ylamino} -methyl)-1H-pyrido[3,4-b] [ 1,4]oxazin-2-one;
= 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid
(3R,6S)-{ 6-[(1 S)-1-hydroxy-2-(6-methoxy-quinolin-4-yl)-ethyl]-tetrahydro-
pyran-3-yl} -
amide;
= 6-({(3R,6S)-6-[(1R)-1-hydroxy-2-(6-methoxy-quinolin-4-yl)-ethyl]-tetrahydro-
pyran-
3-ylamino } -methyl)-4H-pyrido[ 1,4]thiazin-3-one;
= 6-({(3R,6S)-6-[(1RS)-1-hydroxy-2-(6-methoxy-[1,5]naphthyridin-4-yl)-ethyl]-
tetrahydro-
pyran-3-ylamino} -methyl)-4H-pyrido[3,2-b] [ 1,4]thiazin-3-one;
= 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid (3R,6S)-
{6-[(1S)-1-hydroxy-2-(6-methoxy-[1,5]naphthyridin-4-yl)-ethyl]-tetrahydro-
pyran-3-yl}-
amide;
= (3R, 6S)-6-({6-[(1S)-1-hydroxy-2-(6-methoxy-[1,5]naphthyridin-4-yl)-ethyl]-
tetrahydro-
pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b] [ 1,4]thiazin-3-one;
= 6-((3R, 6S)-{6-[(1S)-1-hydroxy-2-(6-methoxy-[1,5]naphthyridin-4-yl)-ethyl]-
tetrahydro-
pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b][1,4]oxazin-3-one;
= (3S,6R)-(6-({6-[(1S,2S)-1,2-dihydroxy-2-(6-methoxy-[1,5]naphthyridin-4-yl)-
ethyl]-
tetrahydro-pyran-3 -ylamino } -methyl)-4H-pyrido [3, 2-b] [ 1,4]thiazin-3 -
one;
= 6-((3R,6S)-{6-[(1S)-1-hydroxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-tetrahydro-
pyran-
3-ylamino } -methyl)-4H-pyrido [3,2-b] [ 1,4]oxazin-3-one;
= 6-((3R, 6S)-{6-[(1S)-1-hydroxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-tetrahydro-
pyran-
3-ylamino}-methyl)-4H-pyrido [3,2-b] [ 1,4]thiazin-3-one;
= (1S)-1-((2S,5R)-5-heptylamino-tetrahydro-pyran-2-yl)-2-(6-methoxy-quinolin-4-
yl)-
ethanol;
= 6-((3R,6S)-{6-[(2R)-2-hydroxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-tetrahydro-
pyran-
3-ylamino}-methyl)-4H-pyrido[3,2-b][1,4]thiazin-3-one;
= 6-((3R,6S)-{6-[(2S)-2-hydroxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-tetrahydro-
pyran-
3-ylamino}-methyl)-4H-pyrido [3,2-b] [ 1,4]thiazin-3-one;
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-40-
= 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid (3R,6S)-{6-
[(2RS)-2-
hydroxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-tetrahydro-pyran-3-yl} -amide;
= 6-((3R,6S)-{6-trans-[2-(3-fluoro-6-methoxy-[1,5]naphthyridin-4-yl)-vinyl]-
tetrahydro-
pyran-3-ylamino}-methyl)-4.H-pyrido[3,2-b] [ 1,4]thiazin-3-one;
= 6-(3R,6S)-{6-[(1R,2R)-2-(3-fluoro-6-methoxy-[1,5]naphthyridin-4-yl)-1,2-
dihydroxy-
ethyl]-tetrahydro-pyran-3-ylainino}-methyl)-4H-pyrido[3,2-b] [1,4]thiazin-3-
one;
= 6-({6-[2-(8-fluoro-6-methoxy-quinolin-4-yl)-vinyl]-tetrahydro-pyran-3-
ylamino}-
methyl)-4H-pyrido [3,2-b] [ 1, 4]thiazin-3-one;
= 6-((3R, 6S)-{6-[2-(8-fluoro-6-methoxy-quinolin-4-yl)-ethyl]-tetrahydro-pyran-
3-ylamino}-methyl)-4H-pyrido[3,2-b][1,4]thiazin-3-one;
= 6-((3R,6S)-{6-[(1R,2R)-2-(8-fluoro-6-methoxy-quinolin-4-yl)-1,2-dihydroxy-
ethyl]-
tetrahydro-pyran-3-ylamino l -methyl)-4H-pyrido[3,2-b] [ 1,4]thiazin-3-one;
= 6-((3R,6S)-{6-[(1R,2R)-2-(8-fluoro-6-methoxy-quinolin-4-yl)-1, 2-dihydroxy-
ethyl]-
tetrahydro-pyran-3-ylamino } -methyl)-4H-pyrido [3,2-b] [ 1,4]oxazin-3-one;
= 6-(3R,6S)-{6-[(1R,2R)-2-(3-fluoro-6-methoxy-[1,5]naphthyridiri-4-yl)-1,2-
dihydroxy-
ethyl]-tetrahydro-pyran-3-ylamino}-methyl)-4Fl-pyrido[3,2-b] [1,4]oxazin-3-
one;
= (3S,6R)-6-({6-[(1S,2S)-1,2-dihydroxy-2-(6-methoxy-[1,5]naphthyridin-4-yl)-
ethyl]-
tetrahydro-pyran-3-ylamino } -methyl)-4H-pyrido [3,2-b] [ 1,4]oxazin-3-one;
= (3S,6R)-(6-({6-[(1R,2R)-1,2-dihydroxy-2-(6-methoxy-[1,5]naplhthyridin-4-yl)-
ethyl]-
tetrahydro-pyran-3-ylamino} -methyl)-4H-pyrido [3,2-b] [ 1,4]thia.zin-3 -one;
= (3S,6R)-6-({6-[(1R,2R)-1,2-dihydroxy-2-(6-methoxy-[1,5]naphthyridin-4-yl)-
ethyl]-
tetrahydro-pyran-3-ylamino} -methyl)-4H-pyrido[3,2-b] [ 1,4]oxazin-3-one;
= (3S,6R)-6-({(6-[(1R)-1-hydroxy-2-(6-methoxy-[1,5]naphthyridin-4-yl)-ethyl]-
tetrahydro-
pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b] [ 1,4]thiazin-3-one;
= (3S,6R)-6-({6-[(1R)-1-hydroxy-2-(6-methoxy-[1,5]naphthyridin-4-yl)-ethyl]-
tetrahydro-
pyran-3-ylamino } -methyl)-4H-pyrido [3,2-b] [ 1,4]oxazin-3-one;
= (3S,6R)-6-({(6-[(1S)-1-hydroxy-2-(6-methoxy-[1,5]naphthyridin-4-yl)-ethyl]-
tetrahydro-
pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b] [ 1,4]thiazin-3-one;
= 6-({(3R,6S)-6-[(1S,2S)-1,2-dihydroxy-2-(3-methoxy-quinoxalin-5-yl)-ethyl]-
tetrahydro-
pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b][1,4]thiazin-3-one;
= 6-({(3S,6R)-6-[(1R,2R)-1,2-dihydroxy-2-(3-methoxy-quinoxalin-5-yl)-ethyl]-
tetrahydro-
pyran-3-ylamino} -methyl)-4H-pyrido[3,2-b] [ 1,4]thiazin-3-one;
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-41-
= 6-({(3R,6S)-6-[(1S)-2-(8-fluoro-6-methoxy-quiraolin-4-yl)-1-hydroxy-ethyl]-
tetrahydro-
pyran-3-ylamino }-methyl)-4H-pyrido[3,2-b] [ 1,4]thiazin-3-one;
= 6-{[6-(6-fluoro-quinolin-4-yloxymethyl)-tetrahydro-pyran-3-ylamino]-methyl}-
4H-pyrido [3,2-b] [ 1,4]thiazin-3 -one;
= 6-{[(3R,6S)-6-(6,8-difluoro-quinolin-4-yloxyrnethyl)-tetrahydro-pyran-3-
ylamino]-
methyl} -4H-pyrido[3,2-b] [ 1,4]thiazin-3-one;
= 3-oxo-3,4-dihydro-2H-pyrido [3,2-b] [ 1,4]oxazirie-6-carboxylic acid
(3R,6S)- {6-[(1S)-1-hydroxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-tetrahydro-
pyran-3-yl} -
amide;
= (1S)-1-{(2S,5R)-5-trans-[3-(2,5-difluoro-phenyl)-allylamino]-tetrahydro-
pyran-2-yl}-
2-(6-methoxy-quinolin-4-yl)-ethanol;
= 6-({(3R,6S)-6-[(2RS)-2-hydroxy-2-(6-methoxy- [1,5]naphthyridin-4-yl)-ethyl]-
tetra.hydro-
pyran-3-ylamino} -methyl)-4H-pyrido[3,2-b] [ 1,4]thiazin-3-one;
= 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid
{(3R,6S)-6-[(2RS)-2-hydroxy-2-(6-methoxy-[1, 5]naphthyridin-4-yl)-ethyl]-
tetrahydro-
pyran-3-yl}-amide;
= 2- { (2S, 5R)-5-[tr ans-3-(2,5-difluoro-phenyl)-allylamino]-tetrahydro-pyran-
2-yl} -
1-(6-methoxy-[ 1, 5]naphthyridin-4-yl)-ethanol;
= trans-3-(2, 5-difluoro-phenyl)-N- { (3R, 6S)-6- [(2. RS')-2-hydroxy-2-(6-
methoxy-
[1,5]naphthyridin-4-yl)-ethyl]-tetrahydro-pyran_-3-yl}-acrylamide;
= trans-3-(2,5-difluoro-phenyl)-N-{(3R,6S)-6-[(1,S)-1-hydroxy-2-(6-methoxy-
quinolin-
4-yl)-ethyl]-tetrahydro-pyran-3-yl} -acrylamide ;
= (1R,2S)-1-{(2S,5R)-5-[trans-3-(2,5-difluoro-phenyl)-allylamino]-tetrahydro-
pyran-2-yl}-
2-(6-methoxy-[ 1, 5]naphthyridin-4-yl)-ethane-12-diol;
= 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazilne-6-carboxylic acid
{(3R,6S)-6-[(1S,2R)-2-(3-fluoro-6-methoxy-[1, 5]naphthyridin-4-yl)-1,2-
dihydroxy-ethyl]-
tetrahydro-pyran-3 -yl } -am ide;
= 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]oxazine-6-carboxylic acid
{(3R,6,S')-6-[(1S,2R)-2-(3-fluoro-6-methoxy-[1, 5]naphth-yridin-4-yl)-1,2-
dihydrox'y-
ethyl]-tetrahydro-pyran-3-yl}amide;
= (3,4-dichloro-benzyl)-{6-[2-(8-fluoro-6-methoxy-quinolin-4-yl)-ethyl]-
tetrahydro-pyran-
3-yl}-amine;
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-42-
= 3-oxo-3,4-dihydro-2H-pyrido [3,2-b] [ 1,4]thiazine-6-carboxylic acid
[(2R,3R,6S)-6-[(1S,2R)-1,2-dihydroxy-2-(6-methoxy-[ l, 5]naphthyridin-4-yl)-
ethyl ]-
2-(2-hydroxy-ethyl)-tetrahydro-pyran-3-yl]-amide;
= 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid
(2R,3R,6R)-{2-(2-hydroxy-ethyl)-6-[2-(6-methoxy-[1,5]naphthyridin-4-yl)-ethyl]-
tetrahydro-pyran-3-yl} -amide;
= 6-((2R,3R,6R)-{2-(2-hydroxy-ethyl)-6-[2-(6-methoxy-[1,5]naphthyridin-4-yl)-
ethy l]-
tetrahydro-pyran-3 -ylam ino } -methyl)-4H-pyrido [3,2-b] [ 1,4]thiazin-3 -
one;
= tr~ans-3-(2,5-difluoro-phenyl)-N-{(2R,3R,6R)-2-(2-hydroxy-ethyl)-6-[2-(6-
methoxy-
[1,5]naphthyridin-4-yl)-ethyl]-tetrahydro-pyran-3-yl}-acrylamide;
= tr ans-3-(2,5-difluoro-phenyl)-N-{(2R,3R,6R)-6-[2-(6-methoxy-quinolin-4-yl)-
ethyl]-
tetrahydro-pyran-3-yl} -acrylamide;
= 6-({(3R,6S)-6-[(2R)-2-(3-fluoro-6-methoxy-quinolin-4-yl)-2-hydroxy-ethyl]-
tetrahydro-
pyran-3-ylamino } -methyl)-4H-pyrido [3,2-b] [ 1,4]thiazin-3-one;
= 6-({(3R,6S)-6-[(2S)-2-(3-fluoro-6-methoxy-quinolin-4-yl)-2-hydroxy-ethyl]-
tetraliydro-
pyran-3-ylamino } -methyl)-4H-pyrido [3,2-b] [ 1,4]thiazin-3-one;
= N-(2,5-difluoro-phenyl)-2-{(3R,6R)-6-[2-(6-methoxy-quinolin-4-yl)-ethyl]-
tetrahydro-
pyran-3 -ylamino } -acetamide.
More preferably, the compounds of formula I having the spacer MZ will be
selected from the
first 134 compounds mentioned in the list hereabove (and even more preferably
from the first
24 compounds mentioned in the list hereabove).
In particular, the following compounds of formula I having the spacer M2 are
preferred:
= (2RS)-{(2S,5R)-5-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-
tetrahydro-pyran-
2-yl } -1-(6-methoxy-quino lin-4-yl) -ethano l;
= (2RS)-2-{(2S,5R)-{5-[(2,3-dihydro-[1,4]dioxino[2,3-c]pyridin-7-ylmethyl)-
amino]-
tetrahydro-pyran-2-yl} - l -(6-methoxy-quinolin-4-yl)-ethanol;
= (2RS)-2-{(2S,5R)-{5-[(2,3-dihydro-[1,4]dioxino[2,3-b]pyridin-6-ylmethyl)-
amino]-
tetrahydro-pyran-2-yl } -1-(6-methoxy-quinolin-4-yl)-ethanol;
= 6-({(3R,6S)-6-[(2RS)-2-hydroxy-2-(6-methoxy-quinolin-4-yl)-ethyl]-tetrahydro-
pyran-
3 -ylamino} -methyl)-4H-benzo [ 1,4]oxazin-3 -one;
= 6-({(3R,6S)-6-[(2R5)-6-[2-hydroxy-2-(6-methoxy-quinolin-4-yl)-ethyl]-
tetrahydro-pyran-
3-ylamino}-methyl)-4H-benzo[ 1,4]thiazin-3-one;
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-43-
= 6-({(3R,6S)-6-[(2RS)- [2-hydroxy-2-(6-methoxy-quinolin-4-yl)-ethyl]-
tetrahydro-pyran-
3-ylamino} -methyl)-4H-pyrido[3,2-b] [ 1,4]thiazin-3-one;
= (2RS)-{(2S,5R)-5-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-
tetrahydro-pyran-
2-yl} -1-(3-methoxy-quinolin-5-yl)-ethanol;
= (2RS')-2-{(2S,5R)-{5-[(2,3-dihydro-[1,4]dioxino[2,3-c]pyridin-7-ylmethyl)-
amino]-
tetrahydro-pyran-2-yl}-1-(3-methoxy-quinolin-5-yl)-ethanol;
= (2RS)-2-{(2S,5R)-{5-[(2,3-dihydro-[1,4]dioxino[2,3-b]pyridin-6-ylmethyl)-
amino]-
tetrahydro-pyran-2-yl} -1-(3-methoxy-quinolin-5-yl)-ethanol;
= 6-({(3R,6S)-6-[(2RS)-2-hydroxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-tetrahydro-
pyran-
3-ylamino}-methyl)-4H-benzo[1,4]oxazin-3-one;
= 6-({(3R,6S)-6-[(2RS)-6-[2-hydroxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-
tetrahydro-pyran-
3 -ylamino } -methyl)-4H-benzo [ 1,4]thiazin-3-one;
= 6-({(3R,6S)-6-[(2RS)-[2-hydroxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-
tetrahydro-pyran-
3-ylamino} -methyl)-4H-pyrido[3,2-b] [ 1,4]thiazin-3-one;
= (2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-{(3R,6S)-6-[2-(6-methoxy-quinolin-
4-yl)-
ethyl] -tetrahydro-pyran-3 -yl } -amine;
= (2,3-dihydro-[1,4]dioxino[2,3-c]pyridin-7-ylmethyl)-{(3R, 6S)-6-[2-(6-
methoxy-quinolin-
4-yl)-ethyl]-tetrahydro-pyran-3 -yl } -amine;
= (2,3-dihydro-[1,4]dioxino[2,3-b]pyridin-6-ylmethyl)-{(3R,6S)-6-[2-(6-methoxy-
quinolin-
4-yl)-ethyl]-tetrahydro-pyran-3-yl}-amine;
= 6-({(3R,6S)-6-[2-(6-methoxy-quinolin-4-yl)-ethyl]-tetrahydro-pyran-3-
ylamino}-methyl)-
4H-benzo[1,4]oxazin-3-one;
= 6-({(3R,6S)-6-[2-(6-methoxy-quinolin-4-yl)-ethyl]-tetrahydro-pyran-3-
ylamino}-methyl)-
4FI-benzo [ 1,4]thiazin-3-one;
= 6-({(3R,6S)-6-[2-(6-methoxy-quinolin-4-yl)-ethyl]-tetrahydro-pyran-3-
ylamino}-methyl)-
4H-pyrido [3,2-b] [ 1,4]thiazin-3-one;
= (2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-{(3R,6S)-6-[2-(3-methoxy-quinolin-
5-yl)-
ethyl] -tetrahydro-pyran-3 -yl} -amine;
= (2,3-dihydro-[1,4]dioxino[2,3-c]pyridin-7-ylmethyl)-{(3R,6S)-6-[2-(3-methoxy-
quinolin-
5-yl)-ethyl]-tetrahydro-pyran-3-yl}-amine;
= (2,3-dihydro-[1,4]dioxino[2,3-b]pyridin-6-ylmethyl)-{(3R,6S)-6-[2-(3-methoxy-
quinolin-
5-yl)-ethyl]-tetrahydro-pyran-3-yl} -amine;
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-44-
= 6-({(3R,6S)-6-[2-(3-methoxy-quinolyin-5-yl)-ethyl]-tetrahydro-pyran-3-
ylamino}-
methyl)-4H-benzo [ 1,4]oxazin-3-one;
= 6-({(3R, 6S)-6-[2-(3-methoxy-quinolyin-5-yl)-ethyl]-tetrahydro-pyran-3-
ylamino}-
methyl)-4H-benzo [ 1,4]thiazin-3-one;
= 6-({(3R, 6S)-6-[2-(3-methoxy-quinolyin-5-yl)-ethyl]-tetrahydro-pyran-3-
ylamino}-
methyl)-4H-pyrido [3,2-b] [ 1,4]thiazin-3-one;
= (2-{(2S,5R)-5-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-tetrahydro-
pyran-
2-yl} -1-(6-methoxy-quinolin-4-yl)-ethane-1,2-diol;
= 2-{(2S, 5R)-{5-[(2,3-dihydro-[1,4]dioxino[2,3-c]pyridin-7-ylmethyl)-amino]-
tetrahydro-
pyran-2-yl}-1-(6-methoxy-quinolin-4-yl)-ethane-1,2-diol;
= 2-{(2S,5R)-{5-[(2,3-dihydro-[1,4]dioxino[2,3-b]pyridin-6-ylmethyl)-amino]-
tetrahydro-
pyran-2-yl}-1-(6-methoxy-quinolin-4-yl)-ethane-1,2-diol;
= 6-({(3R,6S)-6-[1,2-dihydroxy-2-(6-methoxy-quinolin-4-yl)-ethyl]-tetrahydro-
pyran-
3 -ylamino } -methyl)-4H-benzo[ 1 , 4] oxazin-3 -one;
= 6-({(3R,6S)-6-[1,2-dihydroxy-2-(6-methoxy-quinolin-4-yl)-ethyl]-tetrahydro-
pyran-
3-ylamino} -methyl)-4H-benzo[ 1,4]thiazin-3-one;
= 6-({(3R,6S)-6-[1,2-dihydroxy-2-(6-methoxy-quinolin-4-yl)-ethyl]-tetrahydro-
pyran-
3-ylamino} -methyl)-4H-pyrido [3,2-b] [ 1,4]thiazin-3-one;
= 2-{(2S,5R)-5-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-tetrahydro-
pyran-2-yl}-
1-(3-methoxy-quinolin-5-yl)-ethane-1,2-diol;
= 2-{(2S,5R)-{5-[(2,3-dihydro-[1,4]dioxino[2,3-c]pyridin-7-ylmethyl)-amino]-
tetrahydro-
pyran-2-yl} -1-(3-methoxy-quinolin-5-yl)-ethane-1,2-diol;
= 2-{(2S,5R)-{5-[(2,3-dihydro-[1,4]dioxino[2,3-b]pyridin-6-ylmethyl)-amino]-
tetrahydro-
pyran-2-yl } -1-(3 -methoxy-quinolin-5-yl)-ethane-1,2-diol;
= 6-({(3R,6S)-6-[1,2-dihydroxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-tetrahydro-
pyran-
3-ylamino} -methyl)-4H-benzo[ 1,4] oxazin-3 -one;
= 6-({(3R,6S)-6-[1,2-dihydroxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-tetrahydro-
pyran-
3-ylamino }-methyl)-4H-b enzo [ 1, 4] thiazin-3 -one;
= 6-({(3R,6S)-6-[1,2-dihydroxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-tetrahydro-
pyran-
3-ylamino} -methyl)-4H-pyrido [3,2-b] [ 1,4]thiazin-3 -one;
= 6-({(3R,6S)-6-[1,2-dihydroxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-tetrahydro-
pyran-
3-ylamino } -methyl)-4H-pyrido [3,2-b] [ 1,4]thiazin-3-one;
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-45-
= (2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-{(3R,65)-E-6-[2-(6-methoxy-quinol
in-4-yl)-
vinyl] -tetrahydro -pyran-3 -yl } -amine;
= (2,3-dihydro-[1,4]dioxino[2,3-c]pyridin-7-ylmethyl)-{(3R,6S)-E-6-[2-(6-
methoxy-
[ 1, 5] naphthyri d in-4-yl)-viny l] -tetrahydro-pyran-3 -y l}-am ine;
= (2,3-dihydro-[1,4]dioxino[2,3-b]pyridin-6-yhnethyl)-{(3R,6S)-E-6-[2-(6-
methoxy-
[ 1, 5]naphthyridin-4-yl)-vinyl]-tetrahydro-pyran-3-yl } -amine;
= 6-({(3R,6S)-E-6-[2-(6-methoxy-quinolin-4-yl)-vinyl]-tetrahydro-pyran-3-
ylamino}-
methyl)-4H-benzo [ 1,4]oxazin-3 -one;
= 6-({(3R,6S)-E-6-[2-(6-methoxy-quinolin-4-y1)-vinyl]-tetrahydro-pyran-3-
ylamino}-
methyl)-4H-benzo [ 1,4]thiazin-3-one;
= 6-({(3R,6S')-E-6-[2-(6-methoxy-quinolin-4-yl)-vinyl]-tetrahydro-pyran-3-
ylamino}-
methyl)-4H-pyrido[3,2-b] [ 1,4]thiazin-3-one;
= (2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-{(3R,6S)-E-6-[2-(3-methoxy-quinol
in-5-yl)-
viny 1 ] -tetrahydro-pyran-3 -yl } -amine;
= (2,3-dihydro-[1,4]dioxino[2,3-c]pyridin-7-ylmethyl)-{(3R, 6S')-E-6-[2-(3-
metho,xy-
quinolyin-5-yl)-vinyl]-tetrahydro-pyran-3-yl} -amine;
= (2,3-dihydro-[1,4]dioxino[2,3-b]pyridin-6-ylmethyl)-{(3R,6S)-E-6-[2-(3-
methoxy-
quinolyin-5-yl)-vinyl]-tetrahydro-pyran-3-yl } -amine;
= 6-({(3R, 6S)-E-6-[2-(3-methoxy-quinolin-5-yl)-vinyl]-tetrahydro-pyran-3-
ylarnino}-
methyl)-4H-benzo [ 1,4] oxazin-3 -one;
= 6-({(3R,6S)-E-6-[2-(3-methoxy-quinolin-5-yl)-vinyl]-tetrahydro-pyran-3-
ylamino}-
methyl)-4H-benzo [ 1,4]thiazin-3 -one;
= 6-({(3R,6,S)-E-6-[2-(3-methoxy-quinolin-5-yl)-vinyl]-tetrahydro-pyran-3-
ylamino}-
methyl)-4H-pyrido[3,2-b] [ 1,4]thiazin-3-one;
= (2R,3R,6R)-{3-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-6-[2-(6-
methoxy-
quinolin-4-yl)-ethyl]-tetrahydro-pyran-2-yl}-acetic acid;
= (2R,3R,6R)-{3-[(2,3-dihydro-[1,4]dioxino[2,3-c]pyridin-7-ylmethyl)-amino]-
6-[2-(6-methoxy-quinolin-4-yl)-ethyl]-tetrahydro-pyran-2-yl}-acetic acid;
= (2R,3R,6R)-{3-[(2,3-dihydro-[1,4]dioxino[2,3-b]pyridin-6-ylmethyl)-amino]-
6-[2-(6-methoxy-quinolin-4-yl)-ethyl]-tetrahydro-pyran-2-yl}-acetic acid;
= (2R,3R,6R)-{6-[2-(6-methoxy-quinolin-4-yl)-ethyl]-3-[(3-oxo-3,4-dihydro-
2H-benzo[1,4]oxazin-6-ylmethyl)-amino]-tetrahydro-pyran-2-yl}-acetic acid;
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-46-
= (2R,3R,6R)-{6-[2-(6-methoxy-quinolin-4-yl)-ethyl]-3-[(3-oxo-3,4-dihydro-
2H-benzo[1,4]thiazin-6-ylmethyl)-amino]-tetrahydro-pyran-2-yl}-acetic acid;
= (2R,3R,6R)-{6-[2-(6-methoxy-quinolin-4-yl)-ethyl]-3-[(3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazin-6-ylmethyl)-amino]-tetrahydro-pyran-2-yl}-acetic
acid;
= (2R,3R,6R)-{3-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-6-[2-(3-
methoxy-
quinolin-5-yl)-ethyl]-tetrahydro-pyran-2-yl}-acetic acid;
= (2R,3R,6R)-{3-[(2,3-dihydro-[1,4]dioxino[2,3-c]pyridin-7-ylmethyl)-amino]-
6-[2-(3-methoxy-quinolin-5-yl)-ethyl]-tetrahydro-pyran-2-yl}-acetic acid;
= (2R,3R,6R)-{3-[(2,3-dihydro-[1,4]dioxino[2,3-b]pyridin-6-ylmethyl)-amino]-
6-[2-(3 -methoxy-quinolin-5-yl)-ethyl]-tetrahydro-pyran-2-yl} -acetic acid;
= (2R,3R,6R)-{6-[2-(3-methoxy-quinolin-5-yl)-ethyl]-3-[(3-oxo-3,4-dihydro-
2FI-benzo[1,4]oxazin-6-ylmethyl)-amino]-tetrahydro-pyran-2-yl}-acetic acid;
= (2R,3R,6R)-{6-[2-(3-methoxy-quinolin-5-yl)-ethyl]-3-[(3-oxo-3,4-dihydro-
2H-benzo[1,4]thiazin-6-ylmethyl)-amino]-tetrahydro-pyran-2-yl}-acetic acid;
= (2R,3R,6R)-{6-[2-(3-methoxy-quinolin-5-yl)-ethyl]-3-[(3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazin-6-ylmethyl)-amino]-tetrahydro-pyran-2-yl}-acetic
acid;
= 2-[(3R,6S)-6-(6-methoxy-quinolin-4-yloxymethyl)-tetrahydro-pyran-3-ylamino]-
N-thiazol-2-yl-acetamide.
According to a third variant of the invention, the spacer M of the compounds
of formula I, IcE,
IP2, IcEP2 or IPlwill be the spacer M3.
With regard to spacers M3 wherein B6 represents CH, the relative
stereochemistry is
preferably the following (called M31 hereafter):
M31
- A5 A6 - -
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-47-
With regard to spacers M3 wherein B6 represents CH, the relative
stereochernistry is more
preferably the following (called M311 hereafter):
M311
- A5 A6
Preferred spacers M3 are the following:
H H O
~-N N--0 N, L N~N? "-10~ CHa1 H
0
--
HzC
N
H -~-N
~H H H
-Tr~ -N N-CH2-~- -N
O O
0
CH2~ S + ~H2~ N
N N N
-~ N _~_N +O --0
jr""O 0 0
O
H H ~J N '1,00~~ I-N N'-"H~,~.
o r-l- H~-
O
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-48-
Even more preferred spacers M3 are the following:
H H H N N~
+O N'CH21 o "~
-r~ O
HZC, --
~
~
~-H N H N'CH2-- -~-N ~_N -l*\
O O
O
CHz~ --S CH2 ~~-
N N N
-~-N -~ N tO --0
O O
Preferred compounds of the forinula I having the spacer M3 are the following:
= rac-4-{3-[1-(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-piperidin-3-yl]-
propoxy}-
6-methoxy-quinoline;
= 6-methoxy-4-{3-[1-(trans-3-phenyl-allyl)-piperidin-3-yl]-propoxy}-quinoline;
= 4-[3-(1-benzofuran-2-ylmethyl-piperidin-3-yl)-propoxy]-6-methoxy-quinoline;
= rac-3-[1-(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-piperidin-3-yl]-N-(6-
methoxy-
[ 1,5]naphthyridin-4-yl)-propionamide;
= rac-N-(6-methoxy-[1,5]naphthyridin-4-yl)-3-[1-(3-phenyl-allyl)-piperidin-3-
yl]-
propionamide;
= rac-N-(6-methoxy-[1,5]naphthyridin-4-yl)-3-{ 1-[2-(thiophen-2-ylsulfanyl)-
ethyl]-
piperidin-3-yl}-propionarnide;
= rac-N-(6-methoxy-[1,5]naphthyridin-4-yl)-3-[1-(3-oxo-3,4-dihydro-2H-
benzo[1,4]oxazin-
6-ylmethyl)-piperidin-3-yl]-propionamide;
= rac-N-(6-methoxy-[ 1,5]naphthyridin-4-yl)-3-[ 1-(3-oxo-3,4-dihydro-
2H-pyrido[3,2-b] [ 1,4]thiazin-6-ylmethyl)-piperidin-3-yl]-propionarnide;
= rac-3-[1-(2,3-dihydro-[1,4]dioxino[2,3-c]pyridin-7-ylmethyl)-piperidin-3-yl]-
N-(6-methoxy-[ 1, 5 ]naphthyridin-4-yl)-propionamide;
= rac-3-{ 1-[2-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethyl]-piperidin-3-yl}-N-(6-
methoxy-
[1,5]naphthyridin-4-yl)-propionamide;
= rac-N-(2-cyano-quinolin-8-yl)-3-[1-(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-
piperidin-
3-yl]-propionamide;
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-49-
= rac-N-(2-cyano-quinolin-8-yl)-3-[ttrans-1-(3-phenyl-allyl)-piperidin-3-yl]-
propionamide;
= rac-N-(2-cyano-quinolin-8-yl)-3-[ 1-(3-oxo-3,4-dihydro-2H-benzo[1,4]thiazira-
6-ylmethyl)-piperidin-3-yl]-propionamide;
= rac-2-[1-(2,3-dihydro-benzo[1,4]dioxin-6-yhnethyl)-piperidin-3-yloxy]-N-(6-
methoxy-
[1,5]naphthyridin-4-yl)-acetamide;
= (2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-[3-(6-methoxy-quinolin-4-
yloxyrnethyl)-
cyclohexylmethyl]-amine;
= (2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-[(1R,3S')-3-(6-methoxy-quinolin-
4-yloxymethyl)-cyclohexylmethyl ] -ainine;
= rac-3-{[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-methyl}-
cyclohexanecarboxylic acid (2-methyl-quinolin-8-yl)-amide;
= rac-3-[trans-(3-phenyl-allylamino)-methyl]-cyclohexanecarboxylic acid (2-
rnethyl-
quinolin-8-yl)-amide;
= rac-3-{[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-methyl}-
cyclohexanecarboxylic acid (6-m(--thoxy-[1,5]naphthyridin-4-yl)-amide;
= rac-3-{[(3-oxo-3,4-dihydro-2H-benzo[1,4]oxazin-6-ylmethyl)-amino]-methyl}-
cyclohexanecarboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-amide;
= cis-3-{[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-methyl}-
cyclohexanecarboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-amide;
= cis-3-{[(3-oxo-3,4-dihydro-2H-benzo[1,4]thiazin-6-ylmethyl)-amino]-methyl}-
cyclohexanecarboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-amide;
= cis-3-{[(2,3-dihydro-[1,4]dioxino[2,3-b]pyridin-6-ylmethyl)-amino]-methyl} -
cyclohexanecarboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-amide;
= cis-3-{[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-methyl}-
cyclohexanecarboxylic acid (2-cyano-quinolin-8-yl)-amide;
= cis-3-{[(3-oxo-3,4-dihydro-2H-benzo[1,4]thiazin-6-ylmethyl)-amino]-methyl}-
cyclohexanecarboxylic acid (2-cyano-quinolin-8-yl)-amide;
= cis-3-{[(benzo[1,2,5]thiadiazol-5-ylmethyl)-amino]-methyl}-
cyclohexanecarboxylic acid
(2-cyano-quinolin-8-yl)-amide;
= cis-3-{[(3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazin-6-ylmethyl)-amino]-
methyl}-
cyclohexanecarboxylic acid (2-cyano-quinolin-8-yl)-amide;
= cis-3-[(trans-3-phenyl-allylamino)-methyl]-cyclohexanecarboxylic acid (2-
c3lano-
quinolin-8-yl)-amide;
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-50-
= cis-3-{[(3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazin-6-ylmethyl)-amino]-
methyl}-
cyclohexanecarboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-amide;
= 3-{[(3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]oxazin-6-ylmethyl)-amino]-
methyl}-
cyclohexanecarboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-amide.
More preferably, the compounds of formula I having the spacer M3 will be
selected from the
first 28 compounds mentioned in the list hereabove.
According to a fourth variant of the invention, the spacer M of the compounds
of for-mula I,
ICE, IP2, ICEP2 or Ipiwill be the spacer M4.
Among the compounds of formula I wherein M is 1VI4, those wherein the
substituents A7 and
N(R6)-A$ have the trans stereochemistry are preferred.
Preferred spacers M4 are the following:
H
H 2, ~ H HN .~\N
N
O
Preferred compounds of formula I having the spacer M4 are the following:
= rac-trans-(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-[6-(6-methoxy-quinolin-
4-yloxymethyl)-piperidin-3-yl]-amine;
= rac-trans-6-{[6-(6-methoxy-quinazolin-4-yloxymethyl)-piperidin-3-ylamino]-
metlzyl}-
4H-pyrido[3,2-b][1,4]thiazin-3-one;
= rac-trans-(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-[6-(6-methoxy-quinazolin-
4-yloxymethyl)-piperidin-3-yl]-amine;
= rac-trans-{2-(6-methoxy-quinazolin-4-yloxymethyl)-5-[(3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazin-6-ylmethyl)-amino]-piperidin-l-yl}-acetic acid
tert-butyl
ester;
= rac-trans-{2-(6-methoxy-quinazolin-4-yloxym(--thyl)-5-[(3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazin-6-ylmethyl)-amino]-piperidin-l-yl}-acetic acid;
= rac-trans-6-({6-[2-(6-methoxy-[1,5]naphthyridin-4-yl)-ethyl]-piperidin-3-
ylamino }-
methyl)-4H-pyrido [3,2-b] [ 1,4]thiazin-3-one;
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-51-
= rac-trans-5-[(3-oxo-3,4-dihydro-2H-pyrido[3,2-b][ 1,4]thiazin-6-ylmethyl)-
amino]-
piperidine-2-carboxylic acid (6-methoxy-[ 1, 5]naphthyridin-4-yl)-amide;
= rac-trans-5-[(3-oxo-3,4-dihydro-2H-benzo[ 1,4]thia.zin-6-ylmethyl)-amino]-
piperidine-
2-carboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-amide;
= rac-trans-5-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-piperidine-2-
carboxyLic
acid (6-methoxy-[ 1, 5]naphthyridin-4-yl)-amide.
Compounds of formula I are suitable for the use as chemotherapeutic active
compounc3s in
human and veterinary medicine and as substances for preserving inorganic and
organic
materials in particular all types of organic materials for example polymers,
lubricants, pa-ints,
fibres, leather, paper and wood.
These compounds according to the invention are particularly active against
bacteria and
bacteria-like organisms. They are therefore particularly suitable in human and
veterinary
medicine for the prophylaxis and chemotherapy of local and systemic infections
caused by
these pathogens as well as disorders related to bacte;rial infections
comprising pneumonia,
otitis media, sinusitis, bronchitis, tonsillitis, and mastoiditis related to
infection_ by
Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis,
Staphyloco ccus
aureus, Enterococcusfaecalis, E. faeciunz, E. casselfla-vus, S. epidermidis,
S. haemolyticus, or
Peptostreptococcus spp.; pharyngitis, rheumatic fever, and glomerulonephritis
related to
infection by Streptococcus pyogenes, Groups C and G streptococci,
Corynebate'l~ium
diphtheriae, or Actinobacillus haemolyticum; respiratory tract infections
related to infection
by Mycoplasma pneunzoniae, Legionella pneurnophila, Streptococcus pneurnoa-
iiae,
Haemophilus influenzae, or Chlanzydia pneumoniae; blood and tissue infections,
including
endocarditis and osteomyelitis, caused by S. aureus, S' haemolyticus, E.
faecalis, E. faecium,
E. durans, including strains resistant to known antibacterials such as, but
not limited to, beta-
lactams, vancomycin, aminoglycosides, quinolones, chloramphenicol,
tetracyclines and
macrolides; uncomplicated skin and soft tissue infections and abscesses, and
puerperal fever
related to infection by Staphylococcus aureus, coa.gulase-negative
staphylococci (i.e:., S.
epidermidis, S. haeinolyticus, etc.), Streptococcus pyogenes, Streptococcus
agalactiae,
Streptococcal groups C-F (minute colony streptococci), viridans streptocccci,
Corynebacterium minutissimum, Clostridiurn spp., or Bartonella henselae;
uncomplicated
acute urinary tract infections related to infection by Staphylococcus auweus,
coagulase-negative staphylococcal species, or Enterococcus spp.; urethritis
and cervicitis;
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-52-
sexually transmitted diseases related to infection by Clzlamydia trachomatis,
Haemophilus
ducreyi, Treponema pallidurn, Ureaplasma urealyticum, or Neiserria gonorrheae;
toxin
diseases related to infection by S. aureus (food poisoning and toxic shock
syndrome), or
Groups A, B, and C streptococci; ulcers related to infection by Helicobacter
pylori; systemic
febrile syndromes related to infection by Borrelia recurrentis; Lyme disease
related to
infection by Borrelia burgdorferi; conjunctivitis, keratitis, and
dacrocystitis related to
infection by Chlamydia trachomatis, Neisseria gonorrhoeae, S. aureus, S.
pneumoniae, S.
pyogenes, H. influenzae, or Listeria spp.; disseminated Mycobacterium avium
complex
(MAC) disease related to infection by Mycobacterium avium, or Mycobacterium
intracellulare; infections caused by Mycobacterium tuberculosis, M leprae, M.
paratuberculosis, M kansasii, or M. chelonei; gastroenteritis related to
infection by
Canapylobacter jejuni; intestinal protozoa related to infection by
Cryptosporidium spp.;
odontogenic infection related to infection by viridans streptococci;
persistent cough related to
infection by Bordetella pertussis; gas gangrene related to infection by
Clostridium
peifringens or Bacteroides spp.; and atherosclerosis or cardiovascular disease
related to
infection by Helicobacter pylori or Chlamydia pneumoniae.
Compounds of formula I according to the present invention are further useful
for the
preparation of a medicament for the treatment of infections that are mediated
by bacteria such
as E. coli, Klebsiella pneumoniae and other Enterobacteriaceae, Acinetobacter
spp.,
Stenothrophomonas maltophilia, Neisseria meningitidis, Bacillus cereus,
Bacillus anthracis,
Corynebacteriuni spp., Propionibacterium acnes and bacteroide spp.
Compounds of formula I according to the present invention are further useful
to treat
protozoal infections caused by Plasmodium malaria, Plasmodium falciparum,
Toxoplasnia
gondii, Pneumocystis carinii, Trypanosoma brucei and Leishmania spp.
The present list of pathogens is to be interpreted merely as examples and in
no way as
limiting.
As well as in humans, bacterial infections can also be treated in other
species like pigs,
ruminants, horses, dogs, cats and poultry.
The present invention also relates to pharmacologically acceptable salts, or
solvates and
hydrates, respectively, and to compositions and formulations of compounds of
formula I.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-53-
Examples of pharmacologically acceptable salts of sufficiently basic compounds
of formula I
are selected from the group consisting of salts of physiologically acceptable
mineral acids like
hydrochloric, hydrobromic, sulfuric and phosphoric acid; or salts of organic
acids like
methanesulfonic, p-toluenesulfonic, lactic, acetic, trifluoroacetic, citric,
succinic, fumaric,
maleic and salicylic acid. Further, a sufficiently acidic compound of formula
I may form
alkali or earth alkaline metal salts, for example sodium, potassium, lithium,
calcium or
magnesium salts; ammonium salts; or organic base salts, for example
methylamine,
dimethylamine, trimethylamine, triethylamine, ethylenediamine, ethanolamine,
choline
hydroxide, meglumin, piperidine, morpholine, tris-(2-hydroxyethyl)amine,
lysine or arginine
salts. Compounds of formula I may be solvated, especially hydrated. The
hydratation can
occur during the process of production or as a consequence of the hygroscopic
nature of the
initially water free compounds of formula I.
The pharmaceutical composition according to the present invention contains at
least one
compound of formula I (or a pharmaceutically acceptable salt thereof) as the
active agent and
optionally carriers and/or diluents and/or adjuvants, and may also contain
additional known
antibiotics.
The present invention also relates to pro-drugs that are composed of a
compound of formula I
or ICE having at least one pharmacologically acceptable protective group that
will be cleaved
off under physiological conditions. Such prodrugs have been reviewed by
Beaumont, Kevin;
Webster, Robert; Gardner, lain; Dack, Kevin in Current Drug Metabolism (2003),
4(6),
461-485. Examples of such promoities are, in case the compound of formula I or
IcE contains
a free carboxylic acid, alkoxy- (e.g. ethoxy), phenalkyloxy- (e.g. benzyloxy),
OCH(Ra)OCORb (e.g. pivaloyloxymethyloxy), OCH(Ra)OCOZRb (e.g.
[ [(1 -methylethoxy)carbonyl] oxy] ethyl ester; proxetil), OCH(Ra)ORb, 2-alkyl-
, 2-cycloalkyl-,
or 2-cycloalkylalkyl-oxycarbonyl-2-alkylidene-ethoxy groups, 5-
alkyl[1,3]dioxol-2-one-4y1-
methyloxy, dialkylamino-alkoxy or acyloxy wherein R' is hydrogen or (CI-
C4)alkyl and Rb is
hydrogen, (C1-C6)alkyl, (C2-C6)alkenyl, (C1-C6)alkoxy-(C1-C6)alkyl,
(C1-C6)haloalkoxy-(C1-C6)alkyl, (C3-C6)cycloalkyl or (C3-C6)cycloalkylmethyl.
Furthermore,
if a free hydroxy group is present on a compound of formula I or IcE, it can
be protected as a
prodrug of the type sulfate (OS03H), phosphate (OP03H2), oxymethylene
phosphate
(OCH2OPO3H2), succinate (OCOCH2CH2COOH), ester of dimethylaminoglycine or of a
naturally occurring amino acid, or as an inorganic salt of one of the latter.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-54-
As mentioned above, therapeutically useful agents that contain compounds of
formula I, their
solvates, salts or formulations are also comprised in the scope of the present
inverntion. In
general, compounds of formula I will be administered by using the known and
acceptable
modes known in the art, either alone or in combination with any other
therapeutic agent. Such
therapeutically useful agents can be administered by one of the following
routes: ora_l, e.g. as
tablets, dragee, coated tablets, pills, semisolids, soft or hard capsules, for
example soft and
hard gelatine capsules, aqueous or oily solutions, emulsions, suspensions or
syrups, parenteral
including intravenous, intramuscular and subcutaneous injection, e.g. as an
injectable solution
or suspension, rectal as suppositories, by inhalation or insufflation, e.g. as
a powder
formulation, as microcrystal or as a spray (e.g. liquid aerosol), transdermal,
for exaYnple via
an transdermal delivery system (TDS) such as a plaster containing the active
ingredient,
topical or intranasal. The substance of the present invention can also be used
to impregnate or
coated devices that are foreseen for implantation like catheters or artificial
joints.. The
pharmaceutically useful agents may also contain additives for conservation,
stabilisation, e.g.
W stabilizers, emulsifiers, sweetener, aromatisers, salts to change the
osmotic pressure,
buffers, coating additives and antioxidants.
Another aspect of the invention concerns a method for the treatment of disease
comprising the
administration to the patient of a pharmaceutically active amount of a
derivative according to
formula I.
Besides, any preferences indicated for the compounds of formula I (whether for
the
compounds themselves, salts thereof, compositions containing the compounds or
salts thereof,
uses of the compounds or salts thereof, etc.) apply mutatis mutandis to
compounds of
formula IP2, compounds of formula ICE, compounds of formula ICEP2 and
compounds of
formula IPI.
Moreover, the compounds of formula I or IcE may also be used for cleaning
purposes, e.g. to
remove pathogenic microbes and bacteria from surgical instruments or to make a
ro om or an
area aseptic. For such purposes, the compounds of formula I or IcE could be
conta-ined in a
solution or in a spray formulation.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-55-
PREPARATION OF COMPOUNDS OF FORMULA I
Abbreviations:
The following abbreviations are used throughout the specification and the
examples:
AcOH acetic acid
AD-mix a 1,4-bis(dihydroquinine)phthalazine, K3Fe(CN)6, K2C03 and
K20s04.2H20
AD-mix (3 1,4-bis(dihydroquinidine)phthalazine, K3Fe(CN)6, K2C03 and
K20s04.2H20
aq. aqueous
atm atmosphere
BINAP 2,2'-bis-(diphenylphosphino)-1,1'-binaphthaline
BOCzO di-tert-butyl dicarbonate
Cbz benzyloxycarbonyl
d day(s)
DCC dicyclohexyl carbodiimide
1,2-DCE 1,2-dichloroethane
DBU 1,8-diazabicyclo[5.4.0]undec-7-ene
DBN 1,5-diazabicyclo[4.3.0]non-ene
DCM dichloromethane
(DHQD)2PHAL 1,4-bis(dihydroquinidine)phthalazine
DIAD diisopropyl azo dicarboxylate
DIBAH diisobutylaluminium hydride
DIPEA N,N-diisopropylethylamine
DMAP 4-dimethylaminopyridine
1,2-DME 1,2-dimethoxyethane
DMF N,N-dimethylformamide
DMSO dimethylsulfoxide
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-56-
DMPU 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidone
DPPA diphenylphosphorylazide
EA ethyl acetate
ESI electron spray ionisation
Ether or Et20 diethyl ether
EtOH ethanol
h hour(s)
HATU O-(7-azabenzotriazol-1-yl)-N,N,N;N'-tetramethyluronium
hexafluorophosphate
Hex hexane
HMPA hexamethylphosphoramide
HV high vacuum conditions
LC liquid chromatography
LDA lithium diisopropylamide
LG leaving group
LiHMDS lithium hexamethyldisilane
MeOH methanol
min minute(s)
MCPBA meta-chloroperbenzoic acid
MeCN acetonitrile
MS mass spectroscopy
MsC1 methanesulfonyl chloride
NBS N-bromosuccinimide
NHS N-hydroxysuccinimide
n-BuLi n-butyl lithium
NMO 4-methylmorpholine-N-oxide
org. organic
PPh3 triphenylphosphine
PTSA para-toluene sulfonic acid
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 57 -
quant. quantitative
rac racemic
Rf retention factor
rt room temperature
TBAF tetrabutylammonium fluoride
TBDMSCI tert-butyldimethylsilyl chloride
TEA triethyl amine
TFA trifluoroacetic acid
THF tetrahydrofuran
TLC thin layer chromatography
TLC over Si02 thin layer chromatography over silica gel
wt% weight percent
General preparation methods:
The compounds of formula I (including the compounds of formula ICE or IPl) can
be
manufactured in accordance with the present invention by
a) reacting a compound of the general formula II
Li
R U
X
V W
II
with a compound of the general formula III
A M D
III
wherein R1, U, V, W, X and D are as before and A M is one of the spacers Ml,
M2, M3
and M4, in which A is Al, A3, A5 or A', respectively, functionally modified,
as well as the
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-58-
reactive group L', to connect the two moieties of formulas II and III, and M
is one of the
spacers M1, M2, M3 and M4 diminished by A', A3, A5 or A7 , respectively, and
M), M2, M3,
M4, A', A3, AS and A7 are as before, or
b) reacting a compound of the general formula IV:
M01- H
Ri U
X
V W
IV
wherein M01 is
(CHz)n (CH2)n (CH2)n H (CHx)o ~ s
O i
N
~A'-B'\ N4 +A'-B'\ _ ~NH 4 -~-A3 +
(CH2)n (CH2)o (CHz)n H (CH2o
R3 R4
M011 Mo12
Mota
HN Rs
As N-2 ~- -AS N+
222
Mou Mots Mols
with a compound of the general formula V:
L2D
V
wherein L2 is a reactive group yielding the group A2, A4, A6, A8, A2
diminished by 1"iH or
A6 diminished by CH2NH, respectively, and A'-A8, B1, R', R3, R4, R5, R6,U, V,
W, X, D,
n and o are as before,
and where required, transforming groups Al-Ag into other such groups,
and, if desired, may be converted into their pharmaceutically acceptable
salts.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-59-
In particular, the compounds of formula IPl can be manufactured in accordarnce
with the
present invention by
a) reacting a compound of the general formula II
L
R U
\ X
/
V W
II
with a compound of the general formula III
A M D
III
wherein R1, U, V, W, X and D are as before and A M is one of the spacers Ml,
M2 and
M3, in which A is Al, A3 or A5, respectively, functionally modified, as well
as the
reactive group L', to connect the two moieties of formulas II and III, and M
is one of the
spacers Ml, M2 and M3 diminished by Al, A3 or A5, respectively, and M', M:2,
M3, Al, A3
and A5 are as before, or
b) reacting a compound of the general formula IV:
M01-H
Ri U
X
/
V W
IV
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-60-
wherein M01 is
H
~CH2)n = (CHz\ (CHz)n H (CH2)o
+A1 _BN 4
+Al-BlNH
(CH2)n H (CH2)o (CHz)n H (CH2)o
M011 Mo12
R2
O R5
-~-A3 N+ 1-A5OJ- -~-AS J N ~
~
R3 R4
Mo13 Mo14 Mo15
with a compound of the general formula V:
LZD
V
wherein L2 is a reactive group yielding the group A2, A4, A6, A 2 diminished
by NH or A6
diminished by CH2NH, respectively, and Al-A6, B1, R', R3, R4, R5, U, V, W, X,
D, n and
o are as before,
and where required, transforming groups Al-A6 into other such groups,
and, if desired, may be converted into their pharmaceutically acceptable
salts.
In process alternative a) preferred reactive groups Ll and A and resulting
connections Al, A3,
A5 and/or A', as the case may be, are evident from the following Table 1:
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-61-
Table 1
Ll A Al/A3/A5/A' Subsequent
transformations
NH2 HOOC-(CH2). NHCO(CH2),n
NH2 HOOC-CH2O NHCOCH2O
OSO2CF3 or H2NCO-(CH2)rõ NHCO(CH2),,,
halogen
OSOZCF3 or NH2COOCH2 NHCOOCH2
halogen
OH or halogen HO-(CH2)n O(CH2)n
oxiranyl H (B1=N) CH(OH)CH2
Li or MgBr HCO-CH2 CH(OH)CH2
Li or MgBr CH3N(OCH3)COCH2 COCH2 CH(OH)CH2
Li or MgBr CF3SOZO-CH2CH2 CH2CH2
Li or MgBr halogen-CH2CH2 CH2CH2
OSO2CF3 HC C C= C CH=CH,
CH2CH2,
CH(OH)CH(OH),
CH(OH)CH2,
CH2CH(OH)
OSO2CF3 (HO) 2B-CH2CH2 CH2CH2
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-62-
Ll A Al/A3/A5/A' Subsequent
transformations
OSO2CF3 H2C=CH CH=CH CH2CH2,
CH(OH)CH(OH)
CH(OH)CH2,
CH2CH(OH)
CHO P(Ph)3=CH CH=CH CH2CH29
CH(OH)CH(OH),
CH(OH)CH2,
CH2CH(OH)
CHO PO(OR)2CH2 CH=CH CH2CH2,
(R = alkyl) CH(OH)CH(OH),
CH(OH)CH2,
CH2CH(OH)
CHO RSO2CH2- CH=CH CH2CH2,
R = aryl, heteroaryl CH(OH)CH(OH),
CH(OH)CH2,
CH2CH(OH)
In process alternative b) preferred reactive groups L2 and resulting
connections A2, NRSA4, A6
and/or NR6A8, as the case may be, are evident from the following Table 2:
Table 2
L 2 M01 A2/NRSA4/A6/NR6Ag Subsequent
transformations
OHC-CH=CH M012, Mo13 NHCHzCH=CH
OHC M012, M013 NHCH2
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-63-
L2 Moi A2/NR5A4/A,6/NR6A8 Subsequent
transformations
OHC M015 CHzNHCHz
OHC-CH=CH M015 CH2NHCH2CH=CH
OHC M01, M014 CH2
OHC-CH=CH Mo11, Mo14, Mo16 CH2CH=CH
XCH2 Moi2, Moi3, Mo16 NHCHZ
XCH2 Moli, Moi4 CHZ
XCH2CH2 Moli, Moi4 CH2CIH2
XCH2CO Moll, Moi4 CH2CO CH2CH(OH)
CH2CH(OCONH2)
XCH2CONH Moi2, Moi3, Mo16 NHCH2CONH
XCH2CONH M015 CH2NHCH2CONH
XCH2CH2S M011, M01~ CH2CH2S
TsOCH2 Moi2, Moi3, Moi6 NHCH2
TsOCH2 Moli, Mo14 CH2
TsOCHaCHz Moi2, Moi3, Moi6 NHCH2CHa
TsOCH2CH2 M011, M 14 CH2CTIZ
oxiranyl Moi2, Moi3, Moi6 NHCH2CH(OH)
oxiranyl M01, M01~ CHCH(OH)
X= halogen
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-64-
Ts= tosyl
The required quinoline, [1,5]-naphthyridine, quinazoline and quinoxaline
derivatives of
formula II are prepared following literature procedures. For example, 4-
hydroxy-[1,5]-
naphthyridines (L= OH, U= W= N and V= X= CH) and 4-hydroxy quinolines (L= OH,
W= N
and U= V= X= CH) can be prepared from the corresponding aminopyridines or
anilines by
reaction with diethyl ethoxymethylene malonate to produce the 4-
hydroxycarboxylic acid
ester derivative with subsequent hydrolysis to acid, followed by thermal
decomposition in
inert solvents (J.T. Adams, J. Am. Chem. Soc. (1946), 68, 1317). Others routes
to such
derivatives uses the condensation of substituted aminopyridines or anilines
with 2,2-dimethyl-
[1,3]dioxane-dione and triethylorthoformate followed by heating of the
resulting
2,2-dimethyl-5-[(arylamino)methylidene]-1,3-dioxane-4,6-dione intermediate in
refluxing
diphenyl ether. Quinazolines (L= OH, Cl, NHZ, W= -N= N and U= V= CH) may be
prepared
by standard routes as described by T.A. Williamson in Heterocyclic Compounds
(1957), 6,
324. 3-substituted quinoxalin-5-ol (L= OH, U= V= N and X= W= CH) can be
prepared as
described by Y. Abe et al. in J Med. Chem. 1998, 41, 4062.
The compounds of formula I can be prepared by different routes as illustrated
in Schemes 1-8
below (reference is made to Tables 1 and 2 above):
0
)~- M E
NH2 HN
R'U'X + M02E R'
\/U
V~ W~ T'~V~...TlllW
I-1 I-2 I-3
0
Y'-NI D
HN
U'
Rx
Tlv W
1-4
Scheme 1
In Scheme 1, M02 is the group M011 wherein BI is CFI, M012 wherein BI is CH,
or M013, M014,
M015 or M016, wherein A is HOOC(CHa)t and t is 0, 1 or 2 and E is a
protecting group; the
other symbols have their above meanings.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-65-
Compounds of formula I can for example be obtained from an amine I-1 and an
acid 1-2.
Thus, a 4-hydroxy-[1,5]-naphthyridine, a 4-hydroxyquinazoline, a 5-hydroxy
quinoxaline or a
4-hydroxy quinoline derivative can be converted into the corresponding chloro
derivative by
heating in phosphorous oxychloride between 40 C and 100 C neat or in an inert
solvent like
dichloroethane, or to the corresponding 4-trifluoromethanesulphonyloxy
derivative by
reaction with trifluoromethanesulphonic anhydride, in the presence of an
organic base
between -40 C and 80 C in an aprotic solvent like DCM or THF (K. Ritter,
Synthesis (1993),
735). 4-amino-[1,5]-naphthyridine, 4-aminoxyquinazoline, 5-amino quinoxaline
or 4-amino
quinoline derivatives can be obtained by reaction of the corresponding 4-
trifluoromethanesulphonyloxy derivatives with ammonia in a solvent like DCM or
THF, or
with n-propylamine hydrochloride in pyridine between -20 C and 100 C (R.
Radinov,
Synthesis (1986), 886). 4-aminoxyquinazolirne can also be obtained from its 4-
chloro analogue
by reaction with ammonia under the same conditions.
Carboxylic acids 1-2 may be prepared by Jones' oxidation of the corresponding
alcohols using
chromium acid and sulphuric acid in water/methanol between 40 C and 110 C (E.
R. H.
Jones et al, J Chem. Soc. (1946), 39). Other oxidising agents may be used for
this
transformation such as sodium periodate catalysed by ruthenium trichloride (G.
F. Tutwiler et
al, J. Med. Chem. (1987), 30, 1094), or potassium permanganate (D. E. Reedich
et al, J. Org.
Chem. (198:5), 50, 3535.
Derivatives 1-3 can be obtained by reacting the 4-amino derivative I-1 with a
carboxylic acid
derivative I-2, in the presence of an activating agent such as DCC, 1-
(dimethylaminopropyl)-
3-ethylcarbodiimide hydrochloride (EDC) or 1-hydroxybenzotriazole (HOBT) or
HATU (G.
Benz in C naprehensive Organic Syntlzesis, B.M. Trost, I. Fleming, Eds;
Pergamon Press:
New York ( 1991), vol. 6, p. 381) between -20 C and 60 C in an dry aprotic
solvent like DCM
acetonitrile or DMF. Alternatively, the carboxylic acid can be activated by
conversion into its
corresponding acid chloride by reaction with oxalyl chloride or thionyl
chloride neat or in a
solvent like DCM between -20 and 60 C.
Removal of the protecting group (E) such as Boc or Cbz on a nitrogen atom in 1-
3 is carried
out under standard acidic conditions to give the corresponding free amine.
Alternatively the
Cbz group can be removed under catalytic hydrogenation over palladium on
charcoal. The use
of protecting groups to mask reactive functionality is wellknown to those of
skill in the art,
and other protecting groups are listed in reference book such as P.J.
Kocienski 'Protecting
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-66-
Groups', Thieme (1994). The amine is then reacted with an (hetero)aryl
aldehyde and a
suitable reducing agent to provide the homologue 1-4. The intermediate imine
may be formed
in a variety of protic or aprotic solvents such as DMF, N,N-dimethylacetamide,
1,2-DCE,
MeOH, MeCN, in presence or not of a drying agent such as molecular sieves. The
imine is
reduced subsequently or simultaneously with a suitable reagent such a NaBH4,
sodium
triacetoxyborohydride or sodium cyanoborohydride (R.O. and 1VI.K. Hutchins
Conzprehensive
Organic Synthesis, B.M. Trost, I. Fleming, Eds; Pergamon Press: New York
(1991), vol. 8,
p. 25-78). Alternatively, the amine may also be homologa.ted to give product 1-
4 by
nucleophilic displacement of a suitable alkyl (hetero)aryl halide, mesylate or
tosylate between
-20 C and 100 C in a dry aprotic solvent like DCM, MeCN, DMF or THF in
presence of a
base such as K2C03 or DIPEA.
0
)~- M E
L HN
1 1
RY U. ~ X + MosE R Ux - X
-J r
v v
II-1 11-2 1-3
L= OTf, halogen
0
\~-M D
HN
--00- R7 U' '
-JX
-
v
I-4
Scheme 2
In Scheme 2, M03 is one of the group Mo" to M016, wherein Al, A3, A5 and A'
are
H2NC(O)(CH2),,, u is 0, 1 or 2 and E is a protecting group; the other symbols
have their above
meanings.
As illustrated in Scheme 2, the intermediate 1-3 can also be obtained from a
4-trifluoromethanesulfonate derivative 11-1 and an amide derivative II-2.
These amide
derivatives are obtained from a suitable carboxylic acid I-2, which is
converted into an
activated form using, for example, EDC and HOBt, SOC12 or NHS and DCC between -
20 C
and 60 C in a dry aprotic solvent like DCM, ethyl acetate or 'THF, and the
activated acid is
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-67-
subsequently reacted with aqueous ammonium hydroxide or gaseous ammonia, to
afford
amide 11-2 in an appropriate solvent such as THF o-r DCM between -20 C and 60
C. The
amide 11-2 and the 4-trifluoromethanesulphonate II-1 are coupled under
palladiutrn-catalyzed
Buchwald-Hartwig conditions J. Am. Chem. Soc. (1996), 118, 10333) or copper-
catalyzed
conditions (J. Am. Chem. Soc. (2002), 124, 7421) to afford the derivative 1-3.
Various
palladium sources and ligands may be used, as well as a variety of solvents,
including for
example dioxane, toluene. Other partners of Formula II-1, such as iodo (L= I),
broino (L=Br)
or chloro (L= CI) may be used in the metal-catalysed coupling reaction.
O -M E
R1 u(OH, CI) Ri
04 ~ X
~ w ~ X + ME v
, -
v - -J
III-1 111-2 111-3
O-M D
R1-j U 'X
v~ w-J
'
111-4
Scheme 3
In Scheme 3, M04 is the group M 1 wherein B' is CH, M012 wherein B' is CH or
M013 to M016,
wherein A', A3, A5 and A7 are HO(CHZ),,, v is 1, 2 or 3 and the other symbols
have their
above meanings.
As shown in Scheme 3, the compounds of formula I can also be obtained by
coupling, for
example, a substituted 4-hydroxy quinoline, 8-hydroxy quinoline 4-hyciroxy-
[1,5]-
naphthyridine, 4-hydroxy-[1,3]-quinazoline or 5-hydroxy quinoxaline III-1 and
an alcohol
derivative 111-2. The coupling reaction between III-1 and III-2 may be
achieved under
Mitsunobu conditions (as reviewed in O. Mitsunobu, Synthesis 1981, 1). For
example, an
alcohol III-2 and a 4-hydroxy derivative 111-1 are reacted to form ether III-3
in the presence
of diethyl or diisopropyl azodicarboxylate and triphenylphosphine. The
reaction may be
performed in a wide range of solvents such as N,N-dimethylfonnamide, THF, DCM
and at a
wide range of temperature (between -78 C and 50 C). An alternate route to III-
3 rnay require
the activation of the alcohol III-2 as for example a tosylate, a triflate or a
mesylate by
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-68-
treatment with tosyl chloride, trifluoromethanesulphonic anhydride or mesyl
chloride
respectively in the presence of an organic base such as triethylamine between -
40 C and 60 C
in a dry aprotic solvent like DCM, acetonitrile or THF. Once activated,
alcohol III-2 reacts
with the anion of the 4-hydroxy derivative, generated with a mineral base such
as sodiurn
hydride or potassium carbonate or an organic base suclh as lithium
hexamethyldisilazide, to
generate 111-3 between -20 C and 60 C. Alternatively, derivative III-3 can be
obtained by
reaction of a 4-halogeno quinazoline derivative with an alcohol derivative in
presence of a
strong base like alkali alkoxide like sodium or potassiurn methylate, metal
hydride like NaH,
DBU or DBN between -20 C and 60 C in a dry aprotic solvent like DMF, MeCN or
THF. Irn
a subsequent step, the protecting group is removed and tlhe free amine is
reacted with an alky I
(hetero)aryl halide in presence of a base or with an (hetero)aryl aldehyde in
presence of a
reducing reagent as previously described .
O HO M0sA01
Ri U ~
X + H_ MosAoI -~ R ( U. ' X
l - -J ~ - -J
v w v w
IV-1 IV-2
IV-3
HO MOSp
R 1 U N )1 '
-J
v w
~
IV-4
Scheme 4
In Scheme 4, M05 is the spacer Ml diminished by Al and A2 and B1 is N. The
remaining
symbols are as above.
Compounds of the formula I can also be obtained from a 4-oxiranyl derivative
IV-1 and an
amine derivative IV-2. The racemic epoxides IV-1 may be prepared from the
corresponding
4-carboxaldehydes using trimethylsulfonium iodide in presence of a base (G.A.
Epling and aL.
J. Het. Chem. (1987), 24, 853), or by epoxidation of a 4-vinyl derivatives
using an peroxyacid
derivative such as MCPBA as an oxidizing reagent between -20 C and 60 C in an
aprotic dry
solvent like DCM or THF (Somersekar Rao, A. in Cornprehensive Organic
Synthesis, B.M=.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 69 -
Trost, I. Fleming, Eds; Pergamon Press: New York (1991), vol. 7, p. 357). The
vinyl
derivatives can be obtained from the corresponding aldehyde by a Wittig
olefination reaction
using triphenylmethylene phosphorane (for example generated by treatment of
methyl
triphenylphosphonium bromide with n-BuLi in THF at -78 C) or by reaction of
the triflate II-
1 with a vinyl tributyl stannnane under Stille coupling reaction conditions.
The corresponding
chiral epoxides may be obtained using asymmetric catalytic dihydroxylation (AD-
mixtures)
followed by the chiral diol closure as reviewed by K.B. Sharpless et al. Chem.
Rev. (1994),
94, 2483. The optical purity is generally ranging between 10 and 98%, and may
be further
enhanced by recrystallisation. By such a method, both enantiomers are equally
available. The
reaction of the bicyclic amine IV-2 and the epoxide IV-1 may take place in
various solvents
such as ethanol, N,N-dimethylformamide, at a temperature generally ranging
between 0 C
and 80 C. The reaction is helped by the addition of lithium perchlorate. In a
further step, the
protecting group of IV-3 is removed and the free amine is reacted with an
alkyl (hetero)aryl
halide in presence of a base such as DIPEA or KZC03 in a solvent such as MeCN,
DMF or
EtOH at a temperature ranging between 0 C and 100 C. The free amine may also
be reacted
with an (hetero)aryl aldehyde in presence of a reducing reagent as previously
described.
M E
~ I I
1 1
R U ~ R U
~ ' X + M06E ~ ' X
l~
v~ W-J \/- N-
II-1 V-1
V-2
M E M D
1
~- R1 U \X RY U. X
v W
v w
V-3 V-4
Scheme 5
In Scheme 5, M06 is the group M011 wherein B1 is CH, M012 wherein B1 is CH or
M013 to M 16,
wherein Al, A3, A5 and A7 are HC=C, L is OSO2CF3 or a halogen atom and E is a
protecting
group; the other symbols have their above meanings.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-70-
Compounds of formula I can also be obtained from compound II-1 (Scheme 5)
Intermediate V-2 may be obtained from derivative II-1 and a ter-minal alkyne
derivative V-1.
These alkyne derivatives V-1 are generally obtained from a suitable alcohol
III-2 (see
Scheme 3) which is converted first into an aldehyde using for example the
Moffat-Swern (see
Synthesis 1981, 165), or the Dess-Martin periodinane (see J. Ana. Chem. Soc.
(1991), 113,
7277) oxidation protocols. The aldehyde is converted into the corresponding
alkyne using
either the Corey-Fuchs protocol (formation of the gem-dibromide then treatment
with n-BuLi)
as described in TetrahedNon Letters (1972), 3769 or using dimethyl-2-
oxopropylphosphonate
diazo derivative (so called Ohira's reagent, Synth. Com. (1989), 19, 561) or
dimethyldiazomethylphosphonate as described in Synlett (2003), 59 and Synlett
(1996), 521.
The alkyne V-1 and the 4-trifluoromethanesulfonate II-1 are coupled under
Sonogashira
conditions using catalytic amount of a palladium salt, an organic base such as
triethylamine
and a catalytic amount of a copper derivative (usually copper iodide) in a
solvent such a DMF
between 20 C to 100 C (see Sonogashira, K. in Metal-Catalyzed Reactions,
Diedrich, F.,
Stang, P.J., Eds; Wiley-VCH: New York 1998); alternatively, for example when U
= V = CH
and W = X = N, the 4-trifluoromethanesulfonate II-1 can be replaced by a
halogeno (e.g.
chloro) derivative II-1. The resulting alkyne V-2 is hydrogenated to the
alkane V-3 using
catalytic system such as palladium on charcoal or platinum oxide in a solvent
like EtOH or
EA in presence of hydrogen. Other methods may also be suitable as reviewed by
Siegel, S. et
al. in Comprehensive Organic Synthesis, B. M. Trost, I. Fleming, Eds; Pergamon
Press: New
York (1991), vol. 8, p. 417-488. The alkane V-3 is further transformed into
the compounds V-
4 using procedures previously described.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-71-
M E
H O
1 1
R U\ X R U
~ + M07E ON ~'r; X
v w-J ~v w"J
VI-1 VI-2
M Q VI-3
M E HO'
HO, GH
OH R Ob- U
X
R1 U
~ ' x ~ - -J
"J v w
v w
VI-4 VI-5
Scheme 6
In Scheme 6, M07 is the group M013, wherein A' is RSO2CH2 and E is a
protecting group; R
may be 1-phenyl-lH-tetrazol-5-yl or benzothiazol-2-yl and the other symbols
have their
above meanings.
Compounds of formula I can also be obtained from compound VI-1 (Scheme 6).
Intermediate VI-3 may be obtained as an (E)-isomer from an aldehyde derivative
VI-1 and a
sulfone VI-2. The sulfone is generated from the corresponding sulphide via an
oxidation
reaction. A wide range of oxidizing agent may be used to perform such a
reaction, such as
MCPBA in a solvent such as DCM, oxone in a solvent such as aq. MeOH (see
Tetrahedron
Letters (1981), 22, 1287), or aq. hydrogen peroxide in presence of ammonium
heptamolybdate tetrahydrate in EtOH (see J Org. Chem. (1963), 28, 1140). The
sulphide is
obtained from a suitable alcohol III-2 (Scheme 3) via a Mitsunobu coupling (as
reviewed in
0. Mitsunobu Synthesis (1981), 1) with 1-phenyl-1H tetrazole-5-thiol in the
presence of
diethyl azodicarboxylate or DIAD and PPh3. The reaction may be performed in a
wide range
of solvents such as DMF, THF or DCM and within a wide range of temperatures
(between
-78 C and 50 C). An alternate route to form the intermediate sulphide requires
the activation
of the alcohol 111-2 as for example a tosylate, a triflate or a mesylate by
treatment with tosyl
chloride, trifluoromethanesulphonic anhydride or mesyl chloride respectively
in the presence
of an organic base such as TEA between -40 C and 60 C in a dry aprotic solvent
like DCM,
acetonitrile or THF. Once activated, alcohol III-2 reacts with sodium iodide
or potassium
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-72-
iodide in acetone at a temperature ranging between 0 C and 65 C, to form the
corresponding
iodide. The latter serves as an alkylating agent of the 1-phenyl-lH-tetrazole-
5-thiol. The
alkylation reaction is performed in presence of an inorganic base such as KOH
or NaOH in a
solvent such as EtOH at a temperature ranging between -20 C and 70 C.
The sulfone VI-2 and the aldehyde VI-1 are coupled in presence of a base such
as potassium-
or lithium-hexamethyldisilazide in a solvent such as 1,2-dimethoxyethane, DMF
or toluene as
revie-wed by Blakemore, P.R in JChem.Soc., Perkin Trans. 1 (2002), 2563-2585.
The
(E)-alkene VI-3 is transformed into the corresponding chiral cis-diol
derivative by treatment
with AD mixtures in presence of inethanesulfonainide in a water/2-methy-2-
propanol mixture
as described in Chem. Rev. (1994), 94, 2483. The sense of induction relies on
the chiral ligand
contained in the mixture, either a dihydroquinine-based ligand in AD-mix a or
a
dihydroquinidine-based ligand in AD-mix P. The chiral cis-diol VI-4 is further
transformed
into the chiral compounds VI-5 using procedures previously described.
An alternate route to obtain (E)-alkene VI-3 may be to couple a 4-
trifluoromethanesulfonate
derivative II-1 (Scheme 2) with an organostannane deriving from a terminal
alkyne
derivative V-1 (see Scheme 5). Indeed, the hydrostannation reaction of an
alkyne derivative
V-1 using tributyl tin hydride and a catalytic amount of either a palladium
salt or a
molybdenum complex generates an E: Z mixture of the vinylstannane intermediate
as
described in J. Org. Chem. (1990), 55, 1857. The vinylstannane is reacted with
a 4-
trifluoromethanesulfonate derivative II-1 under Stille coupling conditions (as
described in J.
Am. Chem. Soc. (1987), 109, 5478). Typical reaction conditions involve a
palladium salt such
as tetrakis(triphenylphosphine) palladium or dichloro bis(triphenylphophine)
palladium,
lithium chloride and a radical inhibitor such as 2,6-dimethyl-4-methyl phenol
in a solvent
such as DMF or dioxane at a temperature ranging between 0 C and 100 C, more
preferably at
a ternperature ranging between 20 C and 80 C. As the reaction proceeds
normally at a faster
rate using (E)-vinylstannane, the resulting (E)-alkene VI-3 is usually
obtained with a high
isorneric purity.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 73 -
M E 0 O
HO
R1 U OH O= MoE
X ~ Ri U~
J
v w v w
VI-4 VII-1
M E Mop
OH OH
R1 U X ---)00- R1 U ' X
. -J
v w v w
VII-2 VII-3
Scheme 7
As illustrated in Scheme 7, the previously mentioned chiral cis-diol VI-4 may
be transformed
in the corresponding cyclic carbonate VII-1, by treatment with either
phosgene, diphosgene
or triphosgene in presence of an organic base such as TEA or pyridine or
carbonyldimidazole
in an inert solvent such as DCM or THF at a tetnperature ranging between -78 C
and 50 C,
more conveniently at a temperature ranging between 0 C and 20 C. The cyclic
carbonate
VII-1 is subsequently transformed to the hornobenzylic alcohol VII-2 by
hydrogenolysis
using catalytic system such as palladium on cha.rcoal in presence of hydrogen
in a solvent
such as EA. The intermediate VII-2 is further transformed into the compounds
VII-3 using
procedures previously described.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-74-
M E
L HO
Ri U ~ ~ Ri U
~j ~ + M08E X
'V'w'
V w
II-1 VIII-1
VIII-2
M D
HO
_ Ri U X
- -J
v w
VIII-3
Scheme 8
In Scheme 8, M08 is the group M011 wherein B1 is CH, M 12 wherein B1 is CH or
M013 to M I6,
wherein Al is HCO(CH2)w, w is 1, 2 or 3 and E is a protecting group; the other
symbols have
their above meanings -
As illustrated in Scheme 8, the benzylic alcohol VIII-2 may be obtained by
addition of an
organometallic derivative of aromatic II-1 onto an aldeliyde VIII-1. The
aldehyde VIII-1 is
obtained from a suitable alcohol III-2 by a homologation reaction. Oxidation
of the
alcoholIII-2 into its corresponding aldehyde may be performed using one of the
aforementioned oxidation methods. The resulting aldehyde is further converted
to the
corresponding alkene using the phosphorane generated from
methyltriphenylphosphonium
bromide and a base like n-BuLi or potassium tert-butoxide in a solvent such as
THF at a
temperature between -80 C and 0 C (see Org. Synth. Coll. (1973), 5, 751). The
terminal
alkene is subsequently transformed into the primary alcohol via an
hydroboration reaction
using either BH3-dirnethylsulfide complex, or 9-borabicyclo[3.3.1]nonane (9-
BBN) (for a
review see Smith, K.; Pelter, A. G. Conzprehensive Organic Synthesis, B.M.
Trost, I. Fleming,
Eds; Pergamon Press: New York (1991), vol. 8, p. 703-731) followed by
oxidative workup
with aq. NaOH and 30% H202 (see also Pelter, A.; Srnith, K. G. Comprehensive
Organic
Syntlaesis, B.M. Trost, I. Fleming, Eds; Pergamon Press: New York (1991), vol.
7, p.
593-611). The alcohol is finally oxidized to the aldehyde VIII-1 as already
described.
Derivatives II-1 (L1=Br) are treated with an alkylithium such as n-BuLi at a
temperature
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-75-
ranging between -80 C and -30 C to generate a lithio specie that undergoes
nucleophilic
addition on to the aldehyde VIII-1 to from the benzylic alcohol VIII-2. The
intermediate
VIII-2 is further transformed into the compounds VIII-3 using procedures
previously
described.
Aldehydes VI-1 are prepared following literature procedures or from the
corresponding
derivatives II-1 (L1=Br) are after treatment with an alkyllithium such as n-
BuLi at a
temperature ranging between -80 C and -30 C and subsequent quenching of the
lithio specie
with DMF as described inJ. Org. Chem. (1980), 45, 1514.
An alternate route to generate aldehyde VI-1 consists in reacting derivative
II-1 (L=OTf, Br
or Cl) with trans-phenylvinyl boronic acid under typical Miyaura-Suzuki
coupling conditions
(see Synth.Commun. (1981), 11, 513) employing a palladium salt, an inorganic
base such as
K2C03 or NazCO3, in an aq. solvent such as a dioxane-water nzixture at a
temperature ranging
between 20 and 100 C. The corresponding alkene may be directly transformed
into the
aldehyde VI-1 by ozonolysis (03 stream then quenching with either
dimethylsulfide or PPh3)
or via a periodic cleavage of the intermediate diol using sodiurn periodate in
aq. acetone. The
diol is obtained using a catalytic amount of osmium tetroxide in the presence
a co-oxidant
such as NMO in aq. solvent such as acetone-water or DCM-water (see Cha, J.K.
Chem. Rev.
(1995), 95, 1761-1795).
However, the following compounds are novel intermediates useful in the
manufacture of the
bicyclic derivatives of formula I in accordance with the methods disclosed
above, viz. such
novel intermediates are of the general formula
H
lc>_ Rc o~ - Iin NHRd
H
VI
wherein R represents CHZOH, COORe or CONEZ;
Re represents hydrogen or alkyl; and
Rd represents hydrogen or a nitrogen protecting group.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-76-
Nitrogen protecting groups Rd are preferably allyloxycarbonyl, t-
butyloxycarbonyl,
benzyloxycarbonyl, p-nitrobenzyloxycarbonyl, benzyl or acetyl.
The intermediates of formula VI can be converted into the end products of
formula I by
reaction with the compounds of formula II in analogy to the reaction beween
the compounds
of formulas II and III; any nitrogen protecting group Rd is subsequently split
off as described
above to yield starting compounds of formula IV.
Processes involving the spacers N42, M3 and M4 proceed in quite analogous
fashion to those
involving spacer Ml. The above Tables 1 and 2 give suitable coupling reactions
for arriving at
each and every compound of formula I.
The required bicyclic systems M1 involved in these reactions were prepared
according to
literature procedures described in Synlett (1996), 1097-99, or from procedures
deriving from
J. Chenz. Soc Perkin Trans I(1994), 1891-92, and J Org. Cliem. (1997), 62,
4601-09.
The required tetrahydropyran derivatives M2 involved in these reactions were
prepared
according to literature procedures described in Eur. J Org. Chem. (2003), 2418-
2427.
The required systems M3 involved in these reactions were prepared according to
literature
procedures described in J. Med. Clzem. (1998), 41, 2175-79.
The required systems M4 involved in these reactions were prepared starting
from rac-trans-
piperidine-2,5-dicarboxylic acid 5-methyl ester (J. Heterocycl. Chem. (1 995),
32, 857) after
the transformations set out in Scheme 9 hereafter.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-77-
PG PG
H I
HOOC N N N 10 HO
~COOMe ' j
IX1 IX-2 ~COOMe IX 3 /COOMe
I
PG PG PG
N
HO PGO
IX-5 ~NH2 IX-6 ""'NH2 IX-4 ~COOMe
PGI PG
I
N PG N
CRV~ ~ PGO R~ ~ "''INH2
\ 'NHZ X
D I
/ H v W
IX-7 1
111-4 V-4
(Mo_ M016 ) (Mo_ M016
)
Scheme 9
The piperidine nitrogen of compound IX-1 is protected with a protecting group
PG (Cbz or
BOC). The carboxylic acid function is reduced into its corresponding a_lcohol
by reduction
with borane in THF in an organic solvent such as THF or dioxane between -20
and 50 C to
yield the compound IX-2. The alcohol is further transformed into the
corresponding aldehyde
IX-3 using Dess-Martin periodinane or Moffat-Swern protocols, protected as a
silyl or THP
ether using TBDMSCI in presence of an organic base such as TEA or pyridine in
a solvent
such as DCM or THF, or dihydropyran in an aprotic solvent such as DCM, THF or
ether
between -40 and +40 C, or directly used in coupling with compounds III-1 (see
Scheme 3)
wherein Ll is OH or Cl. The 5-methylester function is hydrolysed in presence
of an alkali
hydroxide such as NaOH, LiOH or KOH in a water/THF mixture between 0 and 40
C. The
resulting acids are subjected to a Curtius degradation in presence of
diphenylphosphoryl azide
in t-butanol in presence of an organic base such as TEA between 60 arid 140 C
to give the
intermediate t-butyl carbamate followed by acidic treatment with TFA or an
inorganic acid
such as HCl in an organic solvent such as THF or DCM to liberate the arnine IX-
5, IX-6 and
IX-7.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-78-
The intermediate aldehyde IX-3 is reacted with dimethyl
acetylmethylphosphonate,
p-toluenesulfonyl azide and MeOH in presence of an inorganic base such as
KZC03 in a polar
solvent such as acetonitrile between 0 and 50 C to give the intermediate
allcyne derivative
IX-4, which is subsequently subjected to coupling under Sonogashira
conditions. The
intermediates IX-4, IX-5 and IX-6 are further processed to deliver compounds
III-4
(Mo=Moi6) and V-4 (Mo=Mo16)
The following examples further illustrate the preparation of the
pharmacologically active
compounds of the invention but do not lirrrnit the scope thereof.
EXAMPLES
All temperatures are stated in C. All analytical and preparative HPLC
investigations on non-
chiral phases are performed using RP-C 18 based columns. Analytical HPLC
investigations
are performed on two different instrurnents with cycle-times of -2.5 min and -
3.5 inin
respectively.
Example 1: (2,3-dihydro-[1,4]dioxino [2,3-b]pyridin-6-ylmethyl)-
[(1 a,5 a,6a)-6-(6-methoxy-[1,5] naphthyridin-4-yloxymethyl)-bicyclo
[3.1.0]liex-3-yl]-
amine:
1.i. rac-(1 c;5c~ 6a)-bicyclo[3.1. O]hex-2-en-6-yl-methanol:
To a solution of rac-(la,5a,6a)- bicyclo[3.1.0]hex-2-ene-6-carbaldehyde <crude
product
obtained from bicyclo[2.2.1]hepta-2,5-diene (16 g) as described by M.E Jung et
al. in J Org.
Chem. (1997), 62, 4601-4609) in MeOH (300 ml) was added, at 0 C, sodium
borohydride
(13 g). The reaction was stirred at the same temperature for 1 h and water
(100 ml) was
added. The volatiles were removed in vacuo and the residue was extracted twice
with ether (2
x 300 ml). After drying over Na2SO4, and filtration, the solvent was removed
in vacuo. The
residue was purified over silica gel (EA-Hex 1-4 then 1-1) to afford the title
compound as an
oil (6.2 g).
'H NMR (CDC13) 8: 5.91 (m, 1H), 5.43 (m., 1H), 3.53 (dd, J= 6.7, 11.3 Hz, 1H);
3.42 (dd,
J= 7.4, 11.3 Hz, 1H); 2.61 (tdd, J= 1, 5, 15 Hz, 1H); 2.39 (dd, J= 2, 17.8 Hz,
1H); 1.80 (m,
1H); 1.51 (m, 2H), 0.57 (m, 1H).
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-79-
l.ii. rac-(1 c;5q6a)-(bicyclo[3.].OJhex-2-en-6 ylmethoxy)-tef=t-butyl-dimethyl-
silane:
To a solution of intermediate l.i (6.2 g) in DCM (250 ml) were added, at rt,
DMAP (10.3 g)
and tert-butylchlorodimethylsilane (8.5 g). The reaction was stirred at rt for
1 h, and the
solvent was removed under reduced pressure. The residue was purified over
silica gel (EA-
Hex 1-4) to afford the title compound (10.2 g) as an oil.
'H NMR (CDC13) S: 5.90 (m, 1H), 5.40 (m, 1H); 3.56 (dd, J= 6, 10.8Hz, 1H);
3.48 (dd, J= 6,
10.8 Hz, 1H); 2.51 (m, 1H); 2.31 (m, 1 H); 1.75 (m, 1 H); 1.46 (rn, 1 H); 0.9
(s, 9H); 0.07 (s,
6H).
l.iii. (1 c~3a,5e;6a)-6-(tert-butyl-dimethyl-silanyloxymethyl)-
bicyclo[3.1.O]hexan-3-ol:
To an ice-cooled solution of intermediate 1.ii (10.2 g) in T'HF (210 ml) was
added
borane.THF complex solution (1M in THF, 45 ml). The reaction was let under
stirring at the
same temperature for 11 h. After cooling down to 0 C, EtOH (10 rnl) was added
followed by
3M aq. NaOH (90 ml) and 50% aq. hydrogen peroxide (80 ml). After stirring for
40 min at
0 C, the reaction mixture was warmed to rt over 40 min. The two layers were
separated. The
aq. layer was extracted twice with EA (2 x 100 ml). The combined organic
layers were
washed with water (3 x 100 ml) and saturated sodium thiosulfate (100 ml).
After washing
with brine (100 ml), the organic phase was dried over NazSO4, filtered, and
concentrated to
dryness. The residue was purified by chromatography (EA-Hex 1-4 then 1-2) to
afford the
title compound (6.2 g) as an oil.
'H NMR (CDC13) 8: 4.01 (p, J= 7 Hz, 1H); 3.39 (d, J= 6.3 Iiz, 2H); 2.16 (dd,
J= 6.3,
12.6 Hz, 2H); 1.66 (m, 2H); 1.56 (br s, 1H); 1.19 (m, 2H); 0.9 (s, 9H); 0.74
(m, 1H); 0.07 (s,
6H).
13C NMR (CDC13) 8: 71.8, 65.9, 36.8, 25.9, 24.9, 20.4, 18.3, -4.3.
l.iv. (1 c;3[3 5c;6a)-(3-azido-bicyclo[3.]. OJhex-6 ylmethoxy)-tert-butyl-
dimethyl-silane:
To a solution of intermediate 1.iii (5 g) in DCM (100 ml) cooled to 0 C, were
added
successively TEA (5.8 ml) and then MsCl (1.91 ml). The reaction was stirred at
the same
temperature for 3 h. The reaction mixture was washed with diluted aq.
saturated NaHCO3 (2 x
100 ml). The organic layer was dried over Na2SO4, filtered and concentrated to
dryness. The
residue was taken up in pentane (300 ml). The resulting solid was filtered off
and the filtrate
was concentrated in vacuo. This substance (6.5 g) was taken up in DMF (80 ml)
and sodium
azide (2.6 g) was added. The reaction mixture was heated at 80 C overnight.
After cooling,
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 80 -
the reaction mixture was diluted with water (200 ml) and extracted with Hex (3
x 200 ml).
The combined extracts were washed with brine, dried over Na2SO4, filtered and
concentrated
to dryness. The oily residue was further dried under high vacuum to give the
title compound
(4.9 g) as an oil.
'H NMR (CDC13) 8: 4.12 (m, 1H); 3.46 (d, J= 6Hz, 2H); 2.09 (m, 2H); 1.86 (dd,
J= 1,
14.4 Hz, 2H); 1.25 (m, 2H); 1.19 (m, 1H); 0.9 (s, 9H); 0.07 (s, 6H).
l.v. (1 c~ 3Q 5c~ 6a) [6-(tert-butyl-dimethyl-silanyloxymethyl)-bicyclo[3-1.
OJhex-3 ylJ-
carbamic acid tert-butyl ester:
To a solution of intermediate l.iv (4.9 g) in THF (60 ml) and water (6 ml) was
added PPh3
(9.6 g). The reaction mixture was heated at 60 C for 2 h. After cooling to rt,
1M NaOH
(40 ml) was added and BOC2O (4.36 g) was added. The reaction was stirred 3 h
at rt and the
volatiles were removed under reduced pressure. The residue was then extracted
with EA (3 x
150 ml). The combined extracts were washed with brine, dried over Na2SO4,
filtered and
concentrated to dryness. The residue was chromatographed (EA-Hex 1-19) to
afford the title
compound (5.2 g) as a white solid.
'H NMR (CDC13) 8: 4.2 (br s, 1H); 4.12 (m, 1H); 3.46 (d, J= 6.3 Hz, 2H); 2.27
(m, 2H);
1.62 (m, 2H); 1.42 (s, 9H); 1.2 (m, 2H); 0.92 (s, 9H); 0.81 (m, 1H); 0.05 (s,
6H).
l.vi. (1 a, 3/3, 5c; 6a)-(6-hydroxymethyl-bicyclo[3.1. OJhex-3 yl)-carbam ic
acid tert-butyl ester:
To a solution of intermediate 1.v (5.2 g) in THF (60 ml) was added a solution
of TBAF (1M
in THF, 20 ml). The reaction was stirred at rt for 3 h. The reaction mixture
was then
concentrated in vacuo. The residue was chromatographed (EA-Hex 1-1) to afford
the title
compound (3.3 g) as an oil.
1H NMR (CDC13) 8: 4.2 (br s, 1H); 4.12 (m, 1H); 3.45 (d, J= 6 Hz, 2H); 2.29
(m, 2H); 1.62
(m, 2H); 1.42 (s, 9H); 1.25 (m, 2H); 0.93 (m, 1H).
MS (ESI, m/z): 228.3 [M+H+].
1.vii. (1 c;5c;6a)-[6-(6-methoxy-[1,5]naphthyridin-4 yloxymethyl)-
bicyclo[3.1:0]hex-3 yl.J-
carbamic acid tert-butyl ester:
To a solution of intermediate l.vi (1 g), 6-methoxy-[1,5]naphthyridin-4-ol
(0.775 g, prepared
as described in WO 03/010138) and PPh3 (1.73 g) in THF (26 ml), was added
dropwise DIA.D
(1.3 ml). The reaction mixture was stirred overnight at rt and concentrated to
dryness. The
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-81-
residue was purified over silica gel (DCM-MeOH 19-1) to afford the title
compound (1.5 g)
as a yellow foam.
MS (ESI, m/z): 386.5 [M+H+].
1.viii. (I c;5c~ 6a)-6-(6-methoxy-[1, 5]naphthyridin-4 yloxymethyl)-
bicyclo[3.]. OJhex-
3 ylamine:
A solution of intermediate l.vii (1.5 g) in TFA (7 ml) was stirred at rt for
30 min. The
reaction was concentrated under HV and the residue was partitioned between 2N
aq. NaOH
(10 ml) and a DCM-MeOH mixture (9-1, 40 ml). The organic layer was extracted
twice more
with the same mixture and the combined extracts were washed with brine and
dried over
Na2SO4. The residue was chromatographed over silica gel (DCM-MeOH 19-1 1%
NH4OH) to
afford the title compound (0.582 g) as a yellow oil.
MS (ESI, mlz): 286.2 (MH+).
1.ix. (2, 3-dihydro-[1, 4]dioxino[2, 3-bJpyridin-6 ylmethyl)-[(1 c;5 c;6a)-6-
(6-methoxy-
[1, 5]naphthyridin-4 yloxymethyl)-bicyclo[3.1.OJhex-3 ylJ-amine:
To a solution of intermediate l.viii (0.085 g) in 1,2-DCE (6 ml) a.nd MeOH (2
ml) were added
3A molecular sieves (1.5 g) and 2,3-dihydro-[1,4]dioxino[2,3-b]pyridine-6-
carbaldehyde
(0.054 g, prepared as described in WO 2004/014361). The reaction was stirred
at rt overnight.
NaBH4 (0.1 g) was added and the reaction was stirred for 2 h. The reaction
mixture was
filtered through Hydromatrix (pretreated with aq. NaHCO3). The filtrate was
concentrated in
vacuo and the residue was chromatographed (DCM-MeOH 19- 1 1% aq. NH4OH) to
afford
the title compound (0.071 g) as a white solid.
1H NMR (CDC13) 8: 8.58 (d, J= 5.2 Hz, 1H); 8.15 (d, J= 9.0 Hz, 1H); 7.12 (d,
J= 7.9 Hz,
1H); 7.1 1(d, J= 9.0 Hz, 11-1); 6.89 (d, J= 5.2 Hz, 1H); 6.83 (d, J= 7.9 Hz,
1H); 4.43 (m,
2H); 4.24 (m, 2H); 4.13 (s, 3H); 4.06 (d, J= 6.8 Hz, 2H); 3.68 (s, 2H); 3.3
8(m, 1 H); 2.18 (m,
2H); 1.86 (m, 1H); 1.84 (br s, 1H); 1.69 (dd, J= 3.0, 13.7 Hz, 2H- ; 1.26 (s,
2H).
MS (ESI, m/z): 435.3 [M+H+].
Example 2: (2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-[(1 a,5 a,6a)-6-(6-
methoxy-
[1,5] nap hthyridin-4-yloxymethyl)-bicyclo [3.1.0] hex-3-yl]-amine:
This compound (0.072 g) was prepared from intermediate l.viii (0.100 g) and
1,4-benzodioxan-6-carboxaldehyde (0.039 g) using the procedure of Example 1,
step l.ix.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 82 -
MS (ESI, m/z): 434.5 [M+H+].
Example 3: -7-fluoro-6-{(1a,5a,6a)-[6-(6-methoxy-[1,5]naphthyridin-4-
yloxymethyl)-
bicyclo [3.1.0] hex-3-ylami no]-methyl}-4H-benz o[ 1,4] thiazin-3-one:
This compound (0.099 g) was prepared from intermediate l.viii (0.100 g) and 7-
fluoro-3-oxo-
3,4-dihydro-2H-benzo[1,4]thiazine-6-carbaldehyde (0.081 g, prepared as
described in
WO 03/087098) using the procedure of Example 1, step l.ix.
MS (ESI, m/z): 481 .5 [M+H+].
Example 4: 6-{[(1 a,5a,6a)-6-(6-methoxy-[1,5]naphthyridin-4-yloxymethyl)-
bicyclo[3.1.0]hex-3-ylamino]-methyl}-4H-benzo[1,4]oxazin-3-one:
The title compound (0.081 g) was prepared from intermediate l.viii (0.100 g)
and 3-oxo-
3,4-dihydro-2H-benzo[1,4]oxazine-6-carbaldehyde (0.068 g, prepared as
described in
WO 02/34754) using the procedure of Example 1, step l.ix.
'H NMR (CDC13) S: 8.63 (d, J= 5.2 Hz, 1H); 8.21 (d, J= 9.0 Hz, 1H); 7.13 (d,
J= 9.0 Hz,
1H); 6.95 (d, J= 5.2 Hz, 1H); 6.87 (m, 2H); 4.57 (s, 2H); 4.13 (s, 3H); 4.12
(d, J= 7.1 Hz,
2H); 3.58 (s, 2H); 3.40 (m, 1H); 2.18 (m, 2H); 1.88 (m, 1H); 1 .67 (dd, J=
2.6, 13.6 Hz, 2H);
1.51 (s, 2H); 1.7 (br s, 1H).
MS (ESI, m/z): 447.5 [M+H+].
Example 5: 6-{[(1 a,5a,6a)- 6-(6-methoxy-[1,5]naphthyridin-4-yloxymethyl)-
bicyclo [3.1.0] hex-3-ylamino] -methyl}-4H-benzo [ 1,4] thiazin-3-one :
The title compoun_d (0.051 g) was prepared from intermedia_te l.viii (0.085 g)
and 3-oxo-
3,4-dihydro-2H-benzo[1,4]thiazine-6-carbaldehyde, using the procedure of
Example 1, step
1.ix.
MS (ESI, m/z): 463.5 [M+H+].
Example 6: (1a,3,6,5a,6a)-{3-[(2,3-dihydro-benzo[1,4]dioxii--6-ylmethyl)-
amino]-
bicyclo[3.1.0]hexane-6-carboxylic acid (6-methoxy-[1,5]naplithyridin-4-yl)-
amide:
6.i. (1c43/3,5c;6a) 3-tert-butoxycarbonylamino-bicyclo[3.1.0]hex-6-carboxylic
acid:
To an ice-chilled solution of intermediate l.vi (2.2 g) in DCM (22 ml), water
(22 ml) and
MeCN (22 ml) was added sodium periodate (9.5 g) and a solution of ruthenium
trichloride
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-83-
(22 mg) in water (9 ml). The rnixture was stirred at the same ternperature for
4 h. The reaction
mixture was diluted with EA (100 ml). The solids were filtered off and MeOH
(20 ml) was
added to the filtrate. The resulting precipitate was removed by filtration.
The filtrate was
treated with a diluted solution of sodium hydrogenosulfite (10%, 35 ml) and
the pH was
adjusted to 2 by addition of 1M aq. HCI. The organic layer was separated and
the aq. layer
was extracted with EA (2 x 100 ml). The combined extracts were washed with
brine, dried
over Na2SO4, and filtered. The filtrate was evaporated to dryne ss. The solid
was triturated in
Hex and filtered to yield the title compound (1.7 g) as a tan solid.
1H NMR (d6-DMSO) 8: 6.9 (br s, 1H); 3.91 (m, 1H), 2.15 (rn, 2H); 1.68 (m, 4H);
1.5 (t,
J= 2.9 Hz, 1H); 1.37 (s, 9H).
MS (ESI, m/z): 240.2.3 [M-I-3+].
6.ii. (1 c;3)6,5c;6a) [6-(6-me thoxy-[1, 5]naphthyridin-4 ylcarbczmoyl)-
bicyclo[3.1. OJhex-
3 yl_j-carbaniic acid tert-butyl ester:
To a solution of intermediate 6.i (0.725 g), DIPEA (0.5 ml) and HATU (1.15 g)
in DMF
(6 ml) was added 6-methoxy-[1,5]naphthyridin-4-ylamine (0.525 g, prepared as
described in
WO 03/010138). The reaction was stirred at rt for 22h. The volatiles were
removed under HV
and the residue was chromatographed over silica gel (DCM-MeOH 19-1) to afford
the title
compound (0.65 g) as a yello-wish oil.
MS (ESI, m/z): 399.7 [M+H+].
6.iii. (1c;3/3,5c;6a) 3-amino-bicyclo[3.1.0]hexane-6-carboxylic acid (6-
methoxy-
[1,5]naphthyridin-4 yl)-amide:
A solution of intermediate 6.ii (0.65 g) in TFA (5 mL) was stirred at rt for
20 min. The
reaction was concentrated under HV and the residue was partitioned between 2N
NaOH
(10 ml) and a DCM-MeOH (9-1, 40 ml). The organic layer was extracted twice
more with the
same mixture and the combined extracts were washed with brine and dried over
Na2SO4. The
residue was evaporated under reduced pressure and chromatographed over silica
gel (DCM-
MeOH 19-1 1% NH4OH) to afford the title compound (0.27 g) as a colourless
solid.
MS (ESI, m/z): 299.3 [M+H}].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 84 -
6. iv. (1 a,3/3, 5c~ 6a)-{3-[(2, 3-dihydro-benzo[1, 4]dioxin-6-ylmethyl)-
aminoJ-
bicyclo[3.1.OJhexane-6-carboxylic acid (6-methoxy-[1, 5Jnaphthy-idin-4 yl)-
amide:
To a solution of intermediate 6.iii (0.09 g) in 1,2-DCE (6 ml) arnd MeOH (2
ml) were added
1,4-benzodioxan-6-carboxaldehyde (0.058 g) and powdered 3A molecular sieves (2
g). The
resulting mixture was stirred at rt overnight. NaBH4 (0.1 g) was added and the
mixture was
stirred at rt for 1 h. The reaction mixture was filtered through a plug of
Hydromatrix ,
pretreated with NaHCO3 (6mL). The filtrate was concentrated in vacuo. The
residue was
chromatographed over silica gel (DCM-MeOH 19-1 1% concentrated NH4OH) to
afford the
title compound (0.12 g) as a foam.
MS (ESI, m/z): 447.6 [M+H+].
Example 7: (1a,3,1i,5a,6a)-3-[(3-oxo-3,4-dihydro-2H-benzo[1,4]oxazin-6-
ylmethyl)-
amino]-bicyclo[3.1.0]hexane-6-carboxylic acid (6-methoxy-[1,5]naphthyridin-4-
yl)-
amide:
The title compound (0.071 g) was obtained from 3-oxo-3,4-dilhydro-2Fl-
benzo[1,4]oxazine-
6-carbaldehyde (0.062 g) and intermediate 6.iii (0.090 g) by the rrnethod
described in Example
6, step 6.iv.
1H NMR (d6- DMSO) 8: 10.68 (s, 1H); 9.76 (s, 1H); 8.64 (d, J= 5.2 Hz, 1H);
8.37 (d,
J= 5.2 Hz, 1H); 8.25 (d, J= 9.0 Hz, 1H); 7.31 (d, J= 9.0 Hz, 1H); 6.90 (m,
3H); 4.55 (s, 2H);
4.09 (s, 3H); 3.54 (s, 2H); 3.26 (m, 1H); 2.63 (s, 1H); 2.09 (m, 214); 1.86
(s, br s, 2H); 1.73 (d,
J= 15 Hz, 1H).
MS (ESI, m/z): 460.4 [M+H+].
Example 8: (1a,3A5a,6a)-3-[(3-oxo-3,4-dihydro-2H-pyrido[3,2-b] [1,4]thiazin-
6-ylmethyl)-amino]-bicyclo[3.1.0]hexane-6-carboxylic acid (6-methoxy-
[1,5] naphthyridin-4-yl)-amide:
The title compound (0.025 g) was obtained from 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.018 g, prepared as described
in
WO 2004/002992) and intermediate 6.iii (0.024 g) by the method described in
Example 6,
step 6.iv.
MS (ESI, m/z): 477.3 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 85 -
Example 9: (1 a,3A5 a;6 a)-3-[(7-fluoro-3-oxo-3,4-dihydro-2H-benzo
[1,4]thiazin-
6-ylmethyl)-amino]-bicyclo[3.1.0]hexane-6-carboxylic acid (6-methoxy-
[1,5] naphthyridin-4-yl)-amide:
The title compound (0.071 g) was obtained from 7-fluoro-3-oxo-3,4-dihydro-
2H-benzo[1,4]thiazine-6-carbaldehyde (0.052 g) and intermediate 6.iii (0.062
g) by the
method described in Example 6, step 6.iv.
MS (ESI, m/z): 494.4 [M+H+].
Example 10: 1-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-2-[(1 a,5a,6a)-6-(6-methoxy-
quinazolin-4-yloxymethyl)-3-aza-bicyclo [3.1.0] hex-3-yl]-ethanone:
10.i. (1 c~5c;6a)-5-(6-methoxy-quinazolin-4 yloxyrnethyl)-3-aza-
bicyclo[3.1.0]hex-
3-carboxylic acid benzyl ester:
To an ice-chilled solution of (1a,5a,6a)-6-hydroxymethyl-3-aza-
bicyclo[3.1.0]hexane-
3-carboxylic acid benzyl ester (2 g) (obtained as described by K.E. Brighty et
aL in Synlett
(1996), 1097-1099) in DMF (40 ml) was added NaaI3 (60% dispersion in mineral
oil, 0.357 g).
After stirring for 15 min, 4-chloro-6-methoxy-quiriazoline (1.57 g) was added
in one portion.
The reaction was then stirred for 2 h at rt. Water (50 ml) was added and the
reaction mixture
was extracted with EA (2 x 100 ml). The combiried organic layers were washed
with brine
and dried over Na2S04. After filtration, the flltrate was concentrated to
dryness. The residue
was purified over silica gel (EA) to afford the title compound (3.1 g) as a
foam.
1H NMR (CDC13) S: 8.68 (s, 1H); 7.86 (d, J= 9. 1 Hz, 1H); 7.48 (dd, J= 2.9, 9-
1 Hz, 1H);
7.41 (d, J= 2.9 Hz, 1H); 7.31 (m, 5H); 5.12 (s, 2H); 4.48 (m, 2H); 3.98 (s,
3H); 3.76 (dd,
J= 11.8, 18.1 Hz, 2H); 3.49 (m, 2H); 1.70 (m, 2H) ; 1.32 (m, 1 H).
10.ii. 4-[(1c~5c~6a) 3-aza=bicyclo[3.].0Jhex-6 ylmethoxyJ-6-methoxy-
quinazoline :
To a solution of intermediate 10.i (3.05 g) in MeOH (100 ml) was added 20%
Pd(OH)2 on
charcoal (2 g). The reaction mixture was stirred under hydrogen atmosphere for
90 0 min. The
catalyst was removed by filtration and the flltrate was concentrated to
dryness to afford the
title compound (2.31 g) as a white solid.
'H NMR (CDC13) S: 8.68 (s, 1H); 7.86 (d, J= 9-1 Hz, 1H); 7.48 (dd, J= 2.9, 9_1
Hz, 1H);
7.41 (d, J= 2.9 Hz, 1H); 4.50 (d, J= 7.2 Hz, 211); 3.98 (s, 3H); 3.10 (d, J=
11-4 Hz, 2H);
2.96 (br d, J= 11.4 Hz, 2H); 1.60 (m, 2H); 1.21 (rn, 1H).
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-86-
MS (ESI, m/z): 272.2 [M+H+]
10.iii. 1-(2, 3-dihydro-benzo[1, 4]dioxin-6 yl)-2-[(1 c~ 5c~ 6a)-6-(6-methoxy-
quinazolin-
4 yloxymethyl)-3-aza-bicyclo[3.1.OJhex-3 ylJ-ethanone:
To a solution of intermediate l0.ii (0.8 g) in DMF (6 ml) were added DIPEA
(0.53 ml) and 2-
chloro-1-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethanone (0.744 g). The reaction
was heated at
70 C for 3 h. The volatiles were removed under reduced pressure. The residue
was
chromatographed (DCM-MeOH 19-1) to afford the title compound (1.15 g) as a
yellowish
solid.
MS (ESI, m/z): 448.6 [M+H+].
Example 11: rac-1-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-2-[(la,5a,6a)-6-(6-
methoxy-
quinaz olin-4-yloxymethyl)-3-aza-bicyclo [3.1.0] hex-3-yl]-ethanol:
To a solution of the compound of Example 10 (1.05 g) in MeOH (20 rnl) was
added at rt
NaBH4 (0.25 g). The reaction was stirred for 2 h. Water (10 ml) was added and
the volatiles
were removed under reduced pressure. The residue was dissolved in EA a.nd
filtered through a
plug of Hydromatrix (pretreated with aq. NaHCO3). The filtrate was
concentrated in vacuo
and the residue was purified by chromatography (DCM-MeOH 9-1) to afford the
title
compound (0.78 g) as a yellowish foam.
MS (ESI, m/z): 450.6 [M+H+].
Example 12: rac-carbamic acid 1-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-
2-[(ta,5a,6a)-6-(6-methoxy-quinazolin-4-yloxymethyl)-3-aza-bicyclo[3.1.0]hex-3-
yl]-
ethyl ester:
To a solution of the compound of Example 11 (0.53 g) in DCM (6 rnl) was added
at 0 C,
trichloroacetylisocyanate (0.18 ml). The reaction was stirred at the same
temperature for 1 h.
The volatiles were removed under reduced pressure and the residue was taken up
in MeOH
(6 ml), 2-methyl-2-propanol (6 ml) and THF (2 ml). Saturated aq. K2C03 (3 ml)
was added
and the mixture was refluxed for 3 h. The volatiles were removed under reduced
pressure and
the residue was extracted with DCM-MeOH (9-1, 2 x 50 ml). The corrnbined
extracts were
washed with brine and dried over Na2SO4. After filtration, the solvernt was
evaporated to
dryness. The residue was chromatographed (DCM-MeOH 19-1, 1% concentrated aq.
NH40H)
to afford the title compound (0.220 g) as a foam.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-87-
MS (ESI, m/z): 493.4 [M+H+]
Example 13: 4-{(1 a,5a,6a)-3-[2-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethyl]-3-
aza-
bicyclo [3.1.0] hex-6-ylmethoxy}-6-methoxy-quinoline:
13.i. (1c~5c;6a)-6-(6-methoxy-quinolin-4 yloxymethyl)-3-aza-
bicyclo[3.1.0]hexane-
3-carboxylic acid benzyl ester:
To a solution of (la,5a,6a)-6-hydroxymethyl-3-aza-bicyclo[3.1.0]hexane-3-
carboxylic acid
benzyl ester (1 g), 6-methoxy-quinolin-4-ol (0.85 g) and PPh3 (1.59 g) in THF
(3 0 ml) and
DMF (2 ml) was added dropwise DIAD (1.2 ml). The reaction mixture was stirred
at rt for
4 h. The reaction mixture was concentrated to dryness. The residue was diluted
with 0.2N HC1
(100 ml). The aq. layer was washed three times with ether (3 x 100 ml). The pH
was then
made basic using 1MNaOH (20 ml). The aq. layer was extracted with EA (2 x 150
ml). The
combined extracts were washed with brine and dried over NaZSO4. After
filtration, the solvent
was removed under reduced pressure and the residue was purified by
chromatography (EA-
MeOH 19-1) to afford the title compound (0.97 g) as a thick oil.
1H NMR (CDC13) 8: 8.60 (d, J= 5.8 Hz, 1H); 7.93 (d, J= 9.2 Hz, 1H); 7.43 (d,
,P= 2.8 Hz,
IH); 7.35 (m, 6H); 6.64 (d, J= 5.2 Hz, 1H); 5.12 (s, 2H); 4.10 (d, J= 6.8 Hz,
211); 3.97 (s,
3H); 3.80 (dd, J= 10.8, 20.1 Hz, 2H); 3.52 (m, 2H); 1.70 (m, 2H); 1.32 (m,
1H).
13.ii. 4-[(1 c; 5c;6a)-3-aza-bicyclo[3. 1. OJhex-6 ylmethoxy]-6-methoxy-
quinoline:
The title amine (0.63 g) was obtained from intermediate 13.i (0.97 g) using
the xnethod of
Example 10, step 10.ii.
1H NMR (CDC13) S: 8.60 (d, J= 5.2 Hz, 1H); 7.93 (d, J= 9.2 Hz, 1H); 7.46 (d,
J= 2.8 Hz,
1H); 7.35 (dd, J= 2.8, 9.2 Hz, 1H); 5.12 (s, 211); 4.10 (d, J= 6.8 Hz, 2H);
3.97 (s, 3H);
3.16 (d, J= 11.4 Hz, 2H); 3.01 (d, J= 11.4 Hz, 2H), 20.1Hz, 2H); 3.52 (m, 2H);
2.18 (br s,
1H); 1.70 (m, 2H); 1.32 (m, 1H).
13.iii. Toluene-4-sulfonic acid 2-(2,3-dihydro-benzo[1,4]dioxin-6 yl)-ethyl
ester:
To a solution of 2-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethanol (3.55 g;
prepared as described
in EP 350309) in DCM (70 ml) were added at 0 C, DMAP (4.2 g) and p-toluerie
sulfonyl
chloride (4.13 g). After stirring at this temperature for 20 min, the reaction
mixture was
warmed to rt. After 2 h, the reaction mixture was concentrated in vacuo and
the residue
partitioned between EA (150 mL) and a saturated solution of CuSO4 (50 mL).
T'he organic
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 88-
layer was further washed with the same solution (4 x 50 ml) and brine (50 m1).
After drying
over Na2SO4 and filtration, the filtrate was evaporated to dryness.
'H NMR (CDC13) b: 7.73 (m, 2H); 7.31 (m, 2H); 6.74 (d, J= 8.1 Hz, 1H); 6.59
(m, 2H);
4.23 (s, 414); 4.16 (t, J= 7.1 Hz, 2H); 2.84 (t, J= 7.1 Hz, 2H); 2.45 (s, 3H).
13. iv. 4-{(1 a,5 c~ 6a)-3-[2-(2, 3-dihydro-benzo[1, 4]dioxin-6yl)-ethylJ-3-
aza-
bicyclo[3.1.OJhex-6 ylmethoxy}-6-methoxy-quinoline:
To a solution of intermediate 13.ii (0.4 g) in DMF (6 ml) were added DIPEA,
(0.53 ml) and
intermediate 13.iii (0.535 g). The reaction was heated at 70 C overnight. The
volatiles were
removed under reduced pressure and the residue was chromatographed (DCN4-MeOH
19-1,
1% concentrated NH4OH) to afford the title compound (0.138 g) as a yellowislh
solid.
MS (ESI, m/z): 433.7 [M+H+].
Example 14: 4-{(1a,5a,6a)-3-[2-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethyl] -3-
aza-
bicyclo [3.1.0] hex-6-ylmethoxy}-6-methoxy-quinazoline:
The title compound (0.388 g) was obtained from intermediate 10.ii (0.4 g)
using the method
of Example 13, step U.N.
MS (ESI, in/z): 434.6 [M+H+].
Example 15: 8-{(1 a,5a,6a)-3-[2-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethyl]-3-
aza-
bicyclo [3.1.0] hex-6-ylmethoxy}-2-methoxy- [1,5] naphthyridine:
15.i. (1 c~Sc~6a)-6-(6-methoxy-[1,5]naphthyf=idin-4 yloxymethyl)-3-aza-
bicyclo[3.1.OJhexane-3-carboxylic acid benzyl ester:
This compound (0.98 g) was obtained from 6-methoxy-[1,5]naphthyridin-4-ol
(0.932 g) and
(la,5a,6a)-6-hydroxymethyl-3-aza-bicyclo[3.1.0]hexane-3-carboxylic acid benzyl
ester
(1.1 g) using the method of Example 13, step 13.i.
MS (ESI, m/z) : 406.5 [M+H+].
15.ii. 8-[(1 c;5c46a)-3-aza-bicyclo[3.1.0]hex-6 ylmethoxyJ-2-methoxy-
[1,5]naphthyridine:
This compound (0.68 g) was obtained from intermediate 15.i (0.98 g) using the
method of
Example 10, step 10.ii.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-89-
'H NMR (CDC13) 6: 8.60 (d, J= 5.2 Hz, 1H); 8.16 (d, J= 9.1 Hz, 11-1); 7.12 (d,
J= 9.1 Hz,
1 H); 6.90 (d, J= 5.2 Hz, 1 H); 4.20 (d, J= 6.7 Hz, 2H); 4.13 (s, 3H); 3.13
(d, J= 11.4 Hz,
2H); 2.99 (d, J= 11.4 Hz, 2H); 2.08 (br s, 1 H); 1.64 (m, 2H); 1.31 (m, 1 IH).
MS (ESI, m/z): 272.6 [M+H+]
15. iii. 8- {(1 c~ 5c;6a}3-[2-(2, 3-dihydro-benzo[1, 4]dioxin-6 yl)-ethylJ-3-
aza-
bicyclo[3.1.OJhex-6 ylmethoxy}-2-methoxy-[l,5Jnaphthyridine:
The title compound (0.103 g) was obtained from intermediate 15.ii (0.136 g)
using the
method of Example 13, step 13.iv.
'H NMR (CDC13) S: 8.60 (d, J= 5.2 Hz, 1H); 8.16 (d, J= 9.0 Hz, 1H3; 7.11 (d,
J= 9.0 Hz,
1H); 6.90 (d, J= 5.2 Hz, 1H); 6.77 (d, J= 8.2 Hz, 1H); 6.71 (d, J= 2.2 Hz,
1H); 6.65 (dd,
J= 2.2, 8.0 Hz, 1H); 4.24 (s, 4H); 4.13 (overlapped d, J= 7.0 Hz, 2H); 4.12
(s, 3H); 3.21 (br
d, J= 7.0 Hz, 2H); 2.67 (br s, 4H); 2.43 (br d, J= 7.0 Hz, 114); 1.90 (br s,
111); 1.60 (br s,
2H).
MS (ESI, m/z): 434.6 [M+H+].
Example 16 .: 1-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-2-[(1 a,5u,6a)-6-(6-
methoxy-
[1,5] naphthyridin-4-yloxymethyl)-3-aza-bicyclo [3.1.0] hex-3-yl]-etha none;
The title compound (0.38 g) was obtained from intermediate 15.ii (0.5 g) using
the method of
Example 10, step 10.iii.
MS (ESI, m/z): 448.6 [M+H+]
Example 17: rac-1-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-2-[(la,5c~6 a)-6-(6-
methoxy-
[ 1,5] nap lithyridin-4-yloxymethyl)-3-aza-bicyclo [3.1.0] hex-3-yl]-ethoLnol:
The title compound (0.286 g) was obtained from the compound of Exarnple 16
(0.37 g) using
the method of Example 11.
MS (ESI, m/z): 450.5 [M+H+]
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-90-
Example 18: rac-carbamic acid 1-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-
2-[(1 a,5a,6cr)-6-(6-methoxy-[1,5]naphthyridin-4-yloxymethyl)-3-aza
bicyclo[3.1.0]hex-
3-yl]-ethyl ester:
The title compound (0.045 g) was obtained from the compound of Example 17
(0.67 g) using
the method of Example 12.
'H NMR (d6-DMSO) 8: 8.56 (d, J= 5.2 Hz, 1H); 8.34 (br s, 1H); 8.16 (d, J= 9.0
Hz, 1H);
7.20 (d, J= 9.0 Hz, 1 H); 7.12 (d, J= 5.2 Hz, 1 H); 6.76 (m, 3H); 6.54 (br s,
1H); 5.47 (m, 1 H);
4.19 (s, 4H); 4.10 (d, J= 6.4 Hz, 2H); 4.00 (s, 3H); 3.02 (br t, J= 9.0 Hz,
2H); 2.75 (m, 1H);
2.58 (m, 1H); 2.39 (m, 2H); 1.74 (br s, 2H); 1.34 (s, 1H).
MS (ESI, m/z): 493.4 [M+H+].
Example 19 : rac-(1 a,5a,6a)-4-{3-[2-(2,3-dihydro-benzo [1,4]dioxin-6-yl)-
ethyl]-3-aza-
bicyclo [3.1.0] hex-6-ylm ethoxy}-quinoline-6-carbonitrile:
19.i. (1 a,5cr, 6a)-6-hydroxymethyl-3-aza-bicyclo[3.].OJhexczne-3-carboxylic
acid tert-butyl
ester:
To a solution of (la,5a,6a)-6-hydroxymethyl-3-aza-bicyclo[3.1.0]hexane-3-
carboxylic acid
benzyl ester (2.8 g) in MeOH (100 ml) was added 20% Pd(OH)2 on charcoal (0.6
g). The
reaction was stirred under hydrogen atmosphere for 1 h. 0.2N aq. HCl (50 ml)
was added and
the reaction mixture was filtered. The volatiles were removed under reduced
pressure and the
residue was partitioned between dioxane (50 ml) and 3M aq. NaOH (15 ml). BOC2O
(4 g)
was added and the reaction was stirred for 1 h. The volatiles were removed
under reduced
pressure and the residue was extracted twice with EA (2 x 150 ml). The
combined extracts
were washed with brine and dried over Na2SO4. After filtration, the solvent
was removed in
vacuo and the residue was purified by chromatography (EA-Hex 2-1 then 1-0) to
afford the
title compound (2.4 g) as an oil.
MS (ESI, m/z): 236.3 [M+Na].
19.ii. (1 c;Scr,6a)-6-(6-cyano-quinolin-4 yloxymethyl)-3-aza-bicyclo[3.1.
O]hexane-
3-carboxylic acid tert-butyl ester:
To a solution of intermediate 19.i (1 g), 6-cyano-quinolin-4-ol (0.8 g,
prepared as described in
WO 2004/002992) and PPh3 (1.59 g) in THF (28 mL) anci DMF (2 ml) was added
dropwise
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-91-
DIAD (1.4 ml). The reaction mixture was stirred at rt overnight and
concentrated to dryness
under reduced pressure. The residue was chromatographed (DC1VI-MeOH 19-1) to
afford a
yellow oil (1.06 g).
MS (ESI, m/z): 366.2 [NI+H+].
19.iii. 4-[(1 a,5a,6a)-3-aza-bicyclo[3.1.0]hex-6-ylmethoxy]-quinoline-6-
carbonitrile:
A solution of intermediate 19.ii (1.06 g) in TFA (6 ml) was stirred at rt for
36 h. The volatiles
were removed under reduced pressure and the residue was taken up in saturated
NaHCO3. The
solids were filtered off and then dissolved in MeOH. The organic layer was
concentrated to
dryness and the residue was purified by chromatography (DCM-MeOH 19-1, 1%
concentrated NH4OH) to afford the title compound (0.143 g).
MS (ESI, m/z): 266.4 [M+H+]
19.iv. rac-(1 c;5a, 6a)-4- {3-[2-(2, 3-dihydro-benzo[1, 4]dioxin-6 yl)-ethylJ-
3-aza-
bicyclo[3.1.OJhex-6 ylmethoxy}-quinoline-6-carbonitrile:
The title compound (0.052 g) was obtained by the method of Example 13, step
13.iv from
intermediate 19.iii (0.100 g) and intermediate 13.iii (0.152 g).
MS (ESI, m/z): 428.4 [M+H+].
Example 20 : 3-chloro-4-{(1 a,5a,6a)-3-[2-(2,3-dihydro-benzo [1,4]dioxin-6-yl)-
ethyl]-
3-aza-bicyclo [3.1.0] hex-6-ylmethoxy}-6-methoxy-q uinoline:
20.i. (1 a,5a,6a)-6-(3-chloro-6-methoxy-quinolin-4 yloxymethyl)-3-aza-
bicyclo[3.1.0Jhexane-
3-carboxylic acid tert-butyl ester:
The title compound (2-6 g) was obtained by the method of Example 1, step l.vii
from
3-chloro-6-methoxy-quinolin-4-ol (1.65g, prepared as described in WO 02/40474)
and
intermediate 19.i (1.4 g)-
MS (ESI, m/z): 405.2 [M+H+].
20.ii. 4-[(1 c45c;6a)-3-a.za-bicyclo[3.1.0]hex-6 ylmethoxyJ-3-clhloro-6-
methoxy-quinoline:
This compound was prepared from (la,5a,6a)-6-(3-chloro-6-methoxy-quinolin-
4-yloxymethyl)-3-aza-bicyclo[3.1.0]hexane-3-carboxylic acid tert-butyl ester
(2.6 g)
according to the method described for Example 19, step 19.iii to afford a
yellow solid (1.4 g).
MS (ESI, m/z): 305.4 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-92-
20.iii. 3-chloro-4-{(1 a,5c~6a)-3-[2-(2,3-dihydro-benzo[1,4]dioxin-6 yl)-
ethylJ-3-aza-
bicyclo[3.1.O]hex-6 ylrnethoxy}-6-methoxy-quinoline:
The title compound (0.020 g) was obtained by the method of Example 13, step
13.iv from
intermediate 20.ii (0.200 g) and intermediate 13.iii (0.182 g).
MS (ESI, m/z): 467.5 [M+H+]
Example 21: rac-4-{(1 a,5 a,6 a)-3-[2-hydroxy-2-(3-oxo-3,4-dihydro-
2H-benzo [1,4] oxazin-6-yl)-ethyl]-3-aza-bicyclo [3.1.0] hex-6-ylmethoxy}-
quinoline-
6-carbonitrile:
To a solution of intermediate 19.iii (0.1 g) in DMF (3.5 ml) were added DIPEA
(0.132 ml)
and 2-chloro-l-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethanone (0.093 g). Tlze
reaction was
heated at 70 C for 1 h. The volatiles were removed under reduced pressure and
the residue
was taken up in MeOH (6 ml) and NaBH4 (0.03 g) was added at 0 C. The reaction
mixture
was stirred for 1 h and concentrated to dryness. The residue was
chromatographed (DCM-
MeOH 19-1 1% concentrated aq. ammonia) to afford the title compouncl (0.082 g)
as an
orange solid.
MS (ESI, m/z): 457.5 [M+H+].
Example 22: rac-2-[(la,5a,6a)-6-(3-chloro-6-methoxy-quinolin-4-yloxymethyl)-3-
aza-
bicyclo [3.1.0] hex-3-yl]-1-(2,3-dihydro-benzo [1,4] dioxin-6-yl)-ethanol:
According to the procedures described in Examples 10 and 11, the title
cornpound (0.410 g)
was obtained in two steps from intermediate 20.ii (0.5 g) as a foam.
MS (ESI, m/z): 483.6 [M+H+].
Example 23 : rac-carbamic acid 2-[(1ct,5a,6a)-6-(3-chloro-6-methoxy-quinolin-
4-yloxymethyl)-3-aza-bicyclo[3.1.0]hex-3-yl]-1-(2,3-dihydro-benzo[1,4] di oxin-
6-yl)-ethyl
ester:
This compound was prepared according to the method of Example 12 from the
compound of
Example 22 (0.35 g). A foam (0.290 g) was obtained.
MS (ESI, m/z): 526.4 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-93-
Example 24: 6-{2-[(1 a,5a,6a)-6-(3-chloro-6-methoxy-quinolin-4-yloxymethyl)-3-
aza-
bicyclo[3.1.0] hex-3-yl]-1-hydroxy-ethyl}-4H-benzo[1,4]oxazin-3-one:
To a solution of intermediate 20.ii (0.483 g) in DMF (8 ml) was added 6-(2-
chloro-acetyl)-
4H-benzo[1,4]oxazin-3-one (0.375 g) and DIPEA (0.56 ml). The mixture was
heated at 70 C
for 30 min. After the volatiles were removed under reduced pressure, the
residue was taken up
in MeOH (15 ml) and NaBH4 (0.19 g) was added at rt. The reaction was then
stirred for 30
min and the volatiles were removed under reduced pressure. The residue was
purified over
silica gel (DCM MeOH 19-1) to afford the title compound (0.380 g) as a
yellowish foam.
MS (ESI, m/z): 496.5 [M+H+].
Example 25 : 5-{(la,5a,6a)-3-[2-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethyl]-3-
aza-
bicyclo [3.1.0] hex-6-ylmethoxy}-3-methoxy-quinoline:
25.i. 3, 5-dibromoquinoline:
To concentrated H2SO4 (130 ml) was added dropwise at 0 C, over 80 min, 3-
bromoquinoline
(50 g) at a rate allowing the internal temperature to be maintained between 0
and 10 C. After
the addition was complete, NBS (48 g) was added portionwise and the reaction
mixture was
stirred at rt overnight. The reaction mixture was poured onto ice (2 1) and
the resulting solid
was dissolved in DCM (600 ml). The aq, layer was further extracted with DCM
(600 ml) and
the combined extracts were washed with 11V1NaOH (300 ml) and concentrated in
vacuo. The
residue was dispersed in silica gel and the resulting dispersal was loaded on
the top of a
column and eluted with a DCM-Hex (1-1, 3 1) then DCM (3 1) and finally DCM-
ether (1-1,
2 1). The title compound was recovered from the last fraction after
evaporation to yield 40 g of
a white solid.
1H NMR (CDC13) 8: 8.94 (d, J= 2.2 Hz, 11H); 8.73 (d, J= 2.2 Hz, 1H); 8.08 Cd,
J= 8.5 Hz,
1H); 7.88 (d, J= 7.5 Hz, 1H); 7.62 (dd, J= 7.5, 8.5 Hz, 1H).
25.ii. 5-bromo-3-methoxyquinoline:
To a mixture of sodium methoxide (14.5 g) in DMPU (350 ml) heated at 125 C.
was added in
one portion 3,5-dibromoquinoline (34.5 g). The reaction was then heated at the
same
temperature for 1 h. The reaction mixture was then cooled to rt and poured
onto ice (300 g).
After the ice melt, the solid was filtered off and dried under vacuum. Tlie
filtrate was
extracted with ether (4 x 150 ml). The combined extracts were washed with
brine and dried
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-94-
over Na2SO4. After filtration, the solvent was evaporated and the residue was
purified o ver
silica gel (Hex-EA 4-1) to afford a material that was pooled with the solid.
The material was
dissolved in DCM and dried over Na2SO4. After filtration and evaporation, the
solid vvas
further dried under ,IIV to afford the title compound (24.5 g) as a beige
solid.
'H NMR (CDC13) 8: 8.68 (d, J=2.8 Hz, 1H); 8.03 (d, J= 8.3 Hz, 1H); 7.80 (d, J=
7.5 IRz,
IH); 7.72 (d, J= 2.8 Hz, 1H); 7.42 (dd, J= 7.5, 8.3 Hz, IH); 4.02 (s, 3H).
MS (ESI, m/z): 239.7 [M+H+].
25.iii. 3-methoxy-S-(4, 4, 5, 5-tetramethyl-[1, 3, 2]dioxabof=olan-2 yl)-
quinoline:
To a mixture of bis(pinacolato)diboron (1.92 g), 1,1'-
bis(diphenylphosphino)ferrocene-
palladium(II)dichloride dichloromethane complex (0.5 g) and potassium acetate
(1.9 g) -%vas
added a solution of intermediate 25.ii (1.5 g) in DMSO (45 ml). The resulting
mixture -Nvas
stirred at 80 C overnight. After cooling, the reaction mixture was diluted
with water (100 ml)
and EA (100 ml). The two layers were decanted and the aq. layer was extracted
twice with EA
(2 x 100 ml). The combined organic layers were washed with brine, dried over
Na2SO4,
filtered and concentrated to dryness. The brown residue was chromatographed
(EA-Hex 1-4)
to afford the title boronate as a white solid (1.3 g).
'H NMR (CDC13) & 8.67 (d, J= 2.9 Hz, 1H); 8.49 (d, J= 2.9 Hz, 1H); 8.12 (m,
2H); 7.55 (m,
1H); 3.97 (s, 3H); 1.42 (s, 12H).
MS (ESI, m/z) : 285.8 [M+H+].
25.iv. 3-methoxy-quinolin-5-ol:
To an ice-chilled solution of intermediate 25.iii (1.35 g), in THF (35 ml)
were added 3111 aq.
NaOH (4.25 ml) and then 30% aq. hydrogen peroxide (2 ml). The reaction mixture
was stirred
at the same temperature for 1 h. Water (50 ml) and 3N aq. HCI was added until
the br-ight
yellow colour vanished to leave a colourless reaction mixture (pH 6). The
reaction miKture
was then diluted -with EA (100 ml). The two layers were decanted and the aq.
layer was
extracted twice more (2 x 100 ml). The combined organic layers were washed
with bxine,
dried over Na2SO4, filtered and concentrated to dryness. The residue was
triturated with Et20
and the solid was filtered to afford after drying the title compound (0.62 g).
'H NMR (d6-DMSO) 8: 10.34 (s, 1H); 8.60 (d, J= 3.0 Hz, 1H); 7.76 (d, J= 3.0
Hz, 1H);
7.39 (m, 2H); 6.92 (dd, J= 1.4, 7.2 Hz, IH); 3.92 (s, 3H)
MS (ESI, m/z): 175.8 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-95-
25.v. (1 cy, 5c4 6a}6-(3-methoxy-quinolin-5 yloxyrnethyl)-3-aza-
bicyclo[3.1.OJhexane-
3-carboxylic acid benzyl ester:
The title compound (0.7 g) was obtained as an oil using the method of Example
1, step l.vi i
and starting from 3-methoxy-quinolin-5-ol (0.62 g) and intermediate 19.i
(0.875 g).
'H NMR (CDC13) 8: 8.69 (d, J= 3.0 Hz, 1H); 7.81 (d, J = 3.0 Hz, 1H); 7.67 (d,
J= 8.4 Hz,
1 H); 7.44 (dd, J= 7.6, 8.4 Hz, 1H); 7.37 (m, 5H); 6.82 (d, J= 7.6 Hz, 1 H);
5.15 (s, 2H~ ;
4.08 (dd, J= 4.8, 6.9 Hz, 2H); 4.00 (s, 3H); 3.83 (d, J= 10.9 Hz, 1H); 3.77
(d, J= 10.9 Hz:,
1H); 3.53 (m, 2H); 1.70 (m, 2H); 1.31 (m, 1H).
MS (ESI, m/z): 406.2 [M+H+].
25.vi. 5-[(1 c; 5c; 6a)-3-aza-bicyclo[3.1.OJhex-6 ylmethoxyJ-3-methoxy-
quinoline:
This cornpound (0.35 g) was obtained as a colourless oil starting from
intermediate 25.-v
(0.7 g) and using the method of Example 10, step l0.ii.
MS (ESI, m/z): 271.3 [M+H+].
25.vii. S-{(1 a, 5c~ 6a)-3-[2-(2, 3-dihydro-benzo[1, 4]dioxiYi-6 yl)-ethylJ-3-
aza-
bicyclor3.1.OJhex-6 ylmethoxy}-3-methoxy-quinoline:
The title compound (0.094 g) was obtained as a colourless foam using the
procedure
described in Example 13, step 13.iv and starting from intermediate 25.vi (0.1
g) an_d
intermediate 13.iii (0.123 g).
'H NMR (d6-DMSO) b: 8.65 (d, J= 3.0 Hz, 1H); 7.77 (d, J= 3.0 Hz, 1H); 7.53
((J,
J= 8.2 Hz, 1 H); 7.48 (dd, J= 7.6, 8.2 Hz, 1H); 7.02 (d, J= 7.6 Hz, 1H); 6.69
(m, 3H:);
4.19 (s, 4H); 4.04 (d, J= 6.9 Hz, 2H); 3.95 (s, 3H); 2.89 (m, 2H); 2.56 (m,
2H); 2.34 (m, 21-1:);
1.63 (m, 2H); 1.54 (m, 2H); 1.27 (m, 1H).
M S(E S I, m/z) : 43 3.1 [M +H+] .
Example 26: 6-{(1 a,5a,6a)-2-[6-(3-methoxy-quinolin-5-yloxymethyl)-3-aza-
bicyclo[3.1.0]hex-3-yl]-acetyl}-4H-benzo[1,4]oxazin-3-one:
To a solution of intermediate 25.vi (0.125 g) in DMF (2 ml) were added DIPEA
(0.17 ml) and
2-chloro-l-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethanone (0.104 g). The
reaction mixture was
heated at 70 C for 1 h and then diluted with water (30 ml). The solid was
filtered off and
dried under HV to afford the title compound (0.093 g) as a yellowish foam.
MS (ESI, m/z): 460.5[M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-96-
Example 27 : 6-{1-liydroxy-2-[(1 a,5a,6a)-6-(3-methoxy-quinolin-5-yloxymethyl)-
3-aza-
bicyclo [3.1.0] hex-3-yl]-ethyl}-4H-benzo [ 1,4] oxazin-3-o ne :
To a solution of the compound of Example 26 (0.083 g) in MeOH (3 ml) was added
at rt
NaBH4 (0.1 g). The reaction was stirred for 30 min and the volatiles were
removed under
reduced pressure. The residue was purified over silica gel (DCM MeOH 19-1) to
afford the
title compound (0.071 g) as a white foam.
MS (ESI, m/z): 462.4 [M+H+].
Example 28 : (1a,5cx,6a)-3-[2-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethyl]-3-aza-
bicyclo[3.1.0]hexane-6-carboxylic acid (2-cyano-quinolin-8-yl)-amide:
28.i. (1 c;5c~ 6a)-6-carbamoyl-3-aza-bicyclo[3.1. O]hexane-3-carboxylic acid
benzyl estef=:
To a solution of (la,5a,6a)-3-aza-bicyclo[3.1.0]hexane-3,6-dicarboxylic acid 3-
benzyl ester
(4.65 g) (obtained as described by K.E. Brighty et al. in Synlett (1996), 1097-
1099) in DCM
(80 ml) were added DMF (0.6 ml) and oxalyl chloride (2.2 ml). The reaction was
stirred at rt
for 1 h, and the volatiles were removed under reduced pressure. The residue
was then diluted
with THF (80 ml) and DCM (10 ml) and concentrated aq. NH4OH (80 ml) was added
quickly.
After stirring at rt for 3 h, the volatiles were removed under reduced
pressure and the residue
was filtered off and thoroughly washed with water until neutral pH was
reached. The solid
was collected by filtration, and a recrystallisation from EA afforded the
title compound
(2.95 g) as a white solid.
MS (ESI, m/z): 261.4 [M+H+].
28.ii. (1 q5c;6a}6-(2-cyano-quinolin-8 ylcarbamoyl)-3-aza-
bicyclo[3.1.O,jhexane-
3-carboxylic acid benzyl ester:
A mixture of interrnediate 28.i (0.522 g), rac-2,2'-bis(diphenylphosphino)-
1,1'-binaphtyl
(0.089 g), tris (dibenzylideneacetone) dipalladium(0)-chloroform complex
(0.036 g) and
cesium carbonate (0. 8 g) was degassed. After 10 min, dioxane (25 ml) was
added and the
mixture was sonicated for 5 min. Trifluoro-methanesulfonic acid 2-cyano-
quinolin-8-yl ester
(0.602 g) (prepared as described in WO 03/010138) was added and the reaction
was refluxed
overnight. The reaction mixture was then filtered through celite (rinsed -with
THF). After
concentration to dryness, the residue was recrystallized from an EA-MeOH
mixture to give
the title compound (0.82 g) as a yellowish solid.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-97-
MS (ESI, m/z): 413.2 [M+H+].
28.iii. (1 c~5c;6a)-3-aza-bicyclo[3.1.0]hexane-6-carboxylic acid (2-cyano-
quinolin-8yl)-
amide:
A solution of interrnediate 28.ii (0.275 g) in TFA (6 ml) was stirred at rt
for 36 h. The
volatiles were removed under reduced pressure and the residue was taken up in
saturated
NaHCO3. The solids were filtered off and then dissolved in MeOI-I. The organic
layer was
concentrated to dryness and the residue was purified by chromatogra.phy (DCM-
MeOH 19-1,
1% concentrated aq. NH4OH) to afford the title compound (0.2 g) as a solid.
MS (ESI, m/z): 279.5 [M+H+].
28.iv. (1 c~ 5e;6a)-3-[2-(2, 3-dihydro-benzo[1, 4Jdioxin-6 yl)-ethylJ-3-erza-
bicyclo[3.1.OJhexane-6-carboxylic acid (2-cyano-quinolin-8yl)-amicfe:
The title compound (0.124 g) was obtained from intermediate 28.iii (0.143 g)
by the method
of Example 13, step 13.iv.
MS (ESI, m/z): 441-2 [M+H+].
Example 29: (1a,5 a,6a)-3-[2-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethyl]-3-aza-
bicyclo[3.1.0]hexane-6-carboxylic acid (6-methoxy-[1,5]naphthyriidin-4-yl)-
amide:
29.i. (1 c~5c~ 6a)-6-(6-methoxy-[1,5]naphthyridin-4 ylcarbamoyl)-3-aza-
bicyclo[3.1.O]hexane-3-carboxylic acid benzyl ester:
This compound was prepared from intermediate 28.i (0.776 g) and trifluoro-
methanesulfonic
acid 6-methoxy-[1,5]naphthyridin-4-yl ester (0.92 g) by the method of Example
28, step 28.ii.
A white solid (0.98 g) was obtained.
1H NMR (d6-DMSO) S: 10.06 (s, 1H); 8.65 (d, J= 5.2 Hz, 1H); 8.65 (d, J= 5.2
Hz, 1H);
8.24 (d, J= 9.0 Hz, IH); 7.39 (m, 5H); 7.29 (d, J= 9.0 Hz, 1H); 5- 08 (s, 2H);
4.17 (s, 3H);
3.67 (m, 2H); 3.54 (m, 2H); 2.36 (t, J= 3.3 Hz, 1H); 2.15 (br s, 1H).
MS (ESI, m/z): 419.5 [M+H+].
29.ii. (1c~5c;6a1-3-aza-bicyclo[3.1.0]hexane-6-carboxylic acid (6-rrzethoxy-
[1, 5Jnaphthyridin-4 yl)-amide:
To a solution of intermediate 29.i (0.98 g) in MeOH (30 ml) and THF (10 ml)
was added
palladium hydroxide (0.55 g). The reaction mixture was stirred under hydrogen
atmosphere
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-98-
for 3 h. After filtration, the solvent was evaporated to dryness to give the
title amine (0.56 g)
as a white solid.
1H NMR (d6-DMSO) 6: 9.79 (s, 1H); 8.64 (d, J= 5.2 Hz, 1H); 8.40 (d, J= 5.2 Hz,
1H);
8.26 (d, J= 9.0 Hz, 1H); 7.30 (d, J= 9.0 Hz, 1H); 4.15 (s, 3H); 3.01 (d, J=
11.3 Hz, 2H);
2.80 (D, J= 11.3 Hz, 2111); 2.46 (br s, 1H); 2.14 (t, J= 3.0 Hz, 1H); 1.98 (br
s, 2IR).
MS (ESI, m/z): 285.2 [M+H+].
29.iii. (1 e~ 5c;6a)-3-[2-(-~3-dihydro-benzo[1, 4]dioxin-6 yl)-ethylJ-3-aza-
bicyclo[3.1.O]hexane-6-carboxylic acid (6-methoxy-[1, 5]naphthyridin-4 yl)-arn
ide:
To a solution of interrnediate 29.ii (0.029 g) in DMF (1 ml) were added DIPEA
(0.035 ml)
and toluene-4-sulfonic acid 2-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethyl ester
(0.037 g). The
reaction mixture was stirred at 70 C overnight and then concentrated to
dryness. The residue
was purified by preparative TLC eluted with DCM-MeOH 19-1 containing 1%
concentrated
NH4OH, to give a yellow gum (0.013 g).
MS (ESI, m/z): 447.5 [M+H+].
Example 30: (1a,5a,6a)-3-[2-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethyl]-3-aza-
bicyclo [3.1.0] hexane-6-carboxylic acid (3-chloro-6-methoxy-quinolin-4-yl)-
amide:
30.i. (1 c;5c;6a)-6-(3-chloro-6-methoxy-quinolin-4ylcarbamoyl)-3-aza-
bicyclo[3.1.OJhexane-3-carboxylic acid benzyl ester:
This compound was prepared by the method of Example 28, step 28.ii f'rom 4-
bromo-
3-chloro-6-methoxy-quinoline (0.545 g, prepared as described in WO 02/40474)
and
intermediate 28.i (0.522 g). A yellow solid (1.08 g) was obtained.
MS (ESI, m/z): 452.4 [M+H+].
30.ii. (1a,5a,6a)-3-Aza-bicyclo[3.1.0]hexane-6-carboxylic acid (3-chloro-6-
rn.ethoxy-
quinolin-4-yl)-amide
This compound was prepared by the method of Example 28, step 28.iii from
ir3termediate 30.i
(1.05 g). A yellow solid (0.086 g) was obtained.
MS (ESI, m/z): 318.6 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-99-
30.iii. (1 c~5c~6a)-3-[2-(2,3-dihydr -benzo[1,4]dioxin-6yl)-ethylJ-3-aza-
bicyclo[3.1.O]hexane-6-carboxylic acid (3-chloro-6-methoxy-quinolin-4 yl)-
amide:
According to the procedure described in Example 13, step 13.iv, the title
compound (0.037 g)
was obtained from intermediate 30.ii (0.086 g).
MS (ESI, m/z): 480.3 [M+H+].
Example 31: (1 a,5a,6a)-3-[2-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethyl]-3-aza-
bicyclo[3.1.0]hexane-6-carboxylic acid (3-methoxy-quinoxalin-5-yl)-amide:
31J. (1 c~ 5c46a)-6-(3-methoxy-quinoxalin-S ylcarbamoyl)-3-aza-
bicyclo[3.1.0]hexane-3-
carboxylic acid benzyl ester:
This compound was prepared by the method of Example 28, step 28.ii fron-i
trifluoro-
methanesulfonic acid 3-methoxy-quinoxalin-5-yl ester (0.550 g) (prepared as
described in
WO 2004/002992) and intermediate 28.i (0.463 g). A yellow solid (0.550 g) was
obtained.
MS (ESI, m/z): 419.5 [M+H+].
31.ii. (1 c45g6a)-3-aza-bicyclo[3. I.O]hexane-6-carboxylic acid (3-methoxy-
quinox; alin-5 yl)-
amide:
This compound was prepared by the method of Example 10, step 10.ii from
interniediate 31.i
(0.5 5 g). A yellow solid (0.080 g) was obtained.
MS (ESI, m/z): 285.4 [M+H+].
31.iii. (1 a,5c;6a)-3-[2-(2,3-dihydro-benzo[1,4]dioxin-6 yl)-ethylJ-3-aza-
bicyclo[3.1.OJhexane-6-carboxylic acid (3-methoxy-quinoxalin-5 yl)-amide:
According to the procedure described in Example 13, step 13.iv, the title
compournd (0.035 g)
was obtained from intermediate 31-ii (0.078 g).
MS (ESI, m/z): 447.6 [M+H+].
Example 32: 3-[(1 a,5a,6a)-2-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-2-oxo-ethyl]-
3-aza-
bicyclo[3.1.0]hexane-6-carboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-
amide:
The title compound (0.16 g) was prepared from intermediate 29.ii (0.2 g)
according to the
procedure described for Example 10, step 10.iii.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 100 -
IH NMR (CDC13) 8: 9.60 (s, 1H); 8.68 (d, J= 5.2 Hz, 1H); 8.51 (d, J= 5.2 Hz,
1H); 8.24 (d,
J= 9.0 Hz, 1H); 7.52 (s, 1H); 7.51 (d, J= 7.6 Hz, 1H); 7.18 (d, J= 9.0 liz,
1H); 6.93 (d,
J= 7.6 Hz, 1H); 4.34 (m, 4H), 4.19 (s, 3H); 4.07 (s, 2H); 3.51 (m, 2H); 2.81
(rn, 2H); 2.46 (br
s, 1H); 2.40 (br s, 2H).
MS (ESI, m/z): 461.3 [M+H+].
Example 33: rac-3-[(1 a,5a,6a)-2-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-2-hydroxy-
ethyl]-
3-aza-bicyclo[3.1.0]hexane-6-carboxylic acid (6-methoxy-[1,5]naphthyridin-4-
yl)-amide:
The title compound (0.052 g) was prepared from the compound of Exainple 32
(0.1 g)
according to the procedure described for Example 11.
MS (ESI, m/z): 463.4 [M+H+].
Example 34: rac-4-{(1 a;5a,6a)-3-[2-hydroxy-2-(3-oxo-3,4-dihydro-
2H-benzo[1,4]thiazin-6-yl)-ethyl]-3-aza-bicyclo[3.1.0]hexane-6-carboxylic acid
(6-methoxy-[1,5] naphthyridin-4-yl)-amide:
To a solution of intermediate 29 _ii (0.2 g) in DMF (4 ml) were added DIPEA
(0.35 ml) and
2-chloro-l-(2,3-dihydro-benzo[1, 4]thiazin-6-yl)-ethanone (0.170 g). The
reaction was heated
at 70 C for 1 h. The volatiles were removed under reduced pressure and the
residue was taken
up in MeOH (6 ml) and NaBH4 (0.2 g) was added at 0 C. After stirring for 2 h,
the reaction
mixture was diluted with water (100 ml). The solid was filtered off and taken
up in EA
(50 ml). The insoluble material was filtered off and the filtrate was
concentra.ted in dryness to
afford the title compound (0.084 g) as a beige solid.
MS (ESI, m/z): 492.4 [M+H+].
Example 35: rac-(1 a,5a,6a)-6-({3-[2-hydroxy-2-(6-methoxy-quinolin-4-yl)-
ethyl]-3-aza-
bicyclo[3.1.0]hex-6-ylamino}-nmethyl)-4H-pyrido[3,2-b] [1,4]thiazin-3-one :
35.i. rac-(1 c;5c~6a)-{3-[2-hydroxy-2-(6-methoxy-quinolin-4 yl)-ethylJ-3-azcz-
bicyclo[3.1.O]hex-6 yl}-carbamic acid tert-butyl ester:
To a solution of (la,5a,6a)-(3-aza-bicyclo[3.1.0]hex-6-yl)-carbamic acid tert-
butyl ester
(0.587 g, obtained as described by K.E. Brighty et al. in Synlett (1996), 1097-
1099) and
6-methoxy-4-oxiranyl-quinoline (0.567 g, obtained from 6-methoxy-quinoline-
4-carbaldehyde as described in WO 2004/035569) in DMF (9 ml) were added K2C03
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-101-
(0.545 g) and lithium perchlorate (0.315 g). The reaction mixture was stirred
a_t 80 C
overnight. The solids were filtered off and the filtrate concentrated to
dryness. The residue
was chromatographed (DCM-MeOH 19-1) to afford a yellow foam (0.724 g).
'H NMR (CDC13) 8: 8.74 (d, J= 4.5 Hz, IH); 8.01 (s, 1H); 7.57 (d, J= 4.5 Hz,
1H); 7_35 (dd,
J= 2.7, 9.2 Hz, 1H); 7.18 (d, J= 2.7 Hz, 1H); 5.33 (dd, J= 3.6, 9.4 Hz, 1H);
4.71 (bs, 1H);
3.92 (s, 3H); 3.52 (d, J= 8.4 Hz, 1H); 3.16 (d, J= 9.1 Hz, 1H); 2.66 (m, 5H);
1.84 (bs, 1H),
1.67 (m,1H), 1.59 (m, 1H); 1.45 (s, 9H).
35.ii. rac-(Ic;5c;6a)-2-(6-amino-3-aza-bicyclo[3.1. OJhex-3 yl)-1-(6-methoxy-
quinolin-4 yl)-
ethanol;
A solution of intermediate 35.i (0.724 g) in TFA (4 ml) was stirred at rt for
30 ni in. The
solvent was removed under reduced pressure. The residue was basified with 1N
aq- NaOH
solution and extracted with a mixture DCM-MeOH 9-1 (5 x 20 ml). The combined
organic
layers were dried over MgSO4 and concentrated to dryness to afford a yellowish
foam
(0.532 g).
'H NMR (CDC13) 8: 8.76 (d, J=4.5 Hz, 11-1); 8.03 (d, J= 9.2 Hz, 1H); 7.60 (dd,
J= 0.66, 4.5 Hz, 1H); 7.36 (dd, J= 2.7, 9.2 Hz, 1H); 7.18 (d, J= 2.7 Hz, 1H);
5. 32 (dd,
J= 3.4, 10 Hz, 1H); 3.93 (s, 3H); 3.40 (d, J= 8.5 flz, 1H); 3.05 (d, J=8.9 Hz,
1H); 2.62 (m,
3H), 2.54 (dd, J= 3.8, 8.4 Hz, 1H), 1.73 (bs, 3I3), 1.43 (ddd, J= 1.9, 3.8,
9.9 Iiz, 1H),
1.35 (ddd, J= 1.9, 3.8, 9.9 Hz, 1H).
35.iii. rac-(1 c;5c~6a)-6-({3-[2-hydroxy-2-(6-methoxy-quinolin-4 yl)-ethylJ-3-
aza-
bicyclo[3.1. O]hex-6-ylainino}-nzethyl)-4H-pyrido[3, Z-bJ[l, 4]thiazin-3-one:
To a solution of intermediate 35.ii (0.1 g) in MeOH (2 ml) and DCM (5 ml) were
added 3 A
molecular sieves (1.5 g) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-
carbaldehyde
(0.058 g). The reaction mixture was stirred at rt overnight. NaBH4 (0.1 g) was
added and the
reaction was stirred for 2 h. The reaction mixture was filtered through
Hydroinatrix
(pretreated with saturated NaHCO3). The filtrate was concentrated to dryness
and the residue
was chromatographed (DCM-MeOH 19-1, 1% concentrated NH4OH) to afford a white
foam
(0.070 g).
'H NMR (CDC13) 6: 8.76 (d, J= 4.5 Hz, 1H); 8.34 (br s, 1H); 8.03 (d, J=
9.213z, 1H);
7.59 (m, 2H); 7.36 (dd, J= 2.7, 9.2 Hz, 1H); 7.17 (d, J= 2.7 Hz, 1H); 6.94 (d,
J= 7.8 Hz,
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-102-
1H); 5.36 (bd, J= 8.2 Hz, 1H); 3.93 (s, 3H); 3.85 (s, 2H); 3.49 (s, 2H); 3.40
(d, J= 8.5 Hz,
IH); 3.06 (d, J= 8.6 Hz, IH); 2.63 (m, 5H), 1.73 (bs, 2H), 1.43 (m, IH), 1.35
(m, 1FI).
MS (ESI, m/z): 478.3 [M+H+].
Example 36: rac-(1a,5a,6a)-6-({3-[2-hydroxy-2-(6-methoxy-quinolin-4-yl)-ethyl]-
3-aza-
bicyclo[3.1.0]hex-6-ylamino}-methyl)-4H-benzo[1,4]oxazin-3-one:
This was prepared by the method of Example 35, step 35.iii from 3-oxo-3,4-d
ihydro-
2H-benzo[1,4]oxazine-6-carbaldehyde (0.064 g) and intermediate 35.ii (0.100 g)
to afford a
pale yellow foam (0.099 g).
MS (ESI, m/z): 461.5 [M+H+].
Example 37: rac-(1a,5a,6a)-2-{6-[(2,3-dihydro-[1,4]dioxino[2,3-c]pyridin-7-
ylmethyl)-
am ino]-3-aza-bicyclo [3.1.0] hex-3-yl}-1-(6-m ethoxy-y uinolin-4-yl)-ethanol:
This was prepared by the method of Example 35, step 35.iii from 2,3-dihydro-
[1,4]dioxino[2,3-c]pyridine-7-carbaldehyde (prepared as described in WO
02/056882,
0.024 g) and intermediate 35.ii (0.049 g) to afford a white foam (0.060 g).
'H NMR (CDC13) 6: 8.76 (d, J= 4.5 Hz, 1H); 8.13 (s, 1H); 8.04 (d, J= 9.2 Hz,
1H); '7.59 (d,
J= 4.5 Hz, 1H); 7.46 (dd, J= 2.7, 9.2 Hz, 1H); 7- 18 (d, J= 2.7 Hz, 1H); 6.79
(:s, 1H);
5.33 (m, 114); 4.31 (m, 4H); 3.94 (s, 3H); 3.82 (s, 2H); 3.40 (d, J= 8.4 Hz,
1H); 3.05 (d,
J= 8.8 Hz, 1H); 2.67 (m, 3H); 2.55 (m, 2H); 1.92 (br s, 2H); 1.58 (m, 2H).
MS (ESI, mlz): 449.5 [M+H+]
Example 38: rac-2-{(1a,5a,6a)-6-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-
arnilno]-
3-aza-bicyclo [3.1.0] hex-3-yl}-1-(6-methoxy-quinolin-4-yl)-ethanol:
According to the procedure described in Example 16, the title compound (0.025
g) was
obtained from intermediate 35.ii (0.1 g) as a foam.
MS (ESI, m/z): 448.5 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-103-
Example 39: rac-2-{(1 a,5a,6a)-6-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-
amino]-
3-aza-bicyclo [3.1.0] hex-3-yl}-1-(6-methoxy-[ 1,5] naphthyrid in-4-yl)-
ethanol:
39.i. 2-methoxy-8-oxira7iyl-[1, S]naphthyridine:
To an ice-chilled solution of 2-bromo-l-(6-methoxy-[]L,5]naphthyridin-4-yl)-
ethanone
(prepared as described in WO 02/056882, 5.2 g) in EtOH (75 rnL), was added
NaBH4 (2.1 g).
The reaction was stirred 1 h at this temperature and then 15 rnin at rt. The
reaction mixture
was partitioned between water and EA. The aq. layer was extracted twice with
EA. The
combined organic extracts were washed with brine and dried over Na2SO4. After
filtration and
evaporation to dryness, the residue was taken up in MeOH ("75 ml), and K2CO3
(2.6 g) was
added. After stirring for 1 h, water (20 ml) was added and the volatiles were
removed in
vacuo. The residue was extracted with ether (2 x 50 ml) and the combined
ethereal layers
were dried over MgSO4, filtered and concentrated to dryness- The residue was
purified over
silica gel (Hex-EA 1-1) to afford the title epoxide (2.9 g).
'H NMR (d6-DMSO) 8: 8.77 (d, J= 4.5 Hz, 1H); 8.31 (d, J= 9.5 Hz, 1H), 7.39 (d,
J= 4.5 Hz, 1 H); 7.29 (d, J= 9.5 Hz, 1 H); 4.90 (dd, J= 2.5, 4.3 Hz, 1 H);
4.06 (s, 3H);
3.37 (dd, J= 4.3, 5.7 Hz, 1 H); 2.92 (dd, J= 2.5, 5.7 Hz, 1 H).
MS (ESI, m/z): 203.3 [M+H+].
39.ii. rac-(1c;5c;6a)-{3-[2-hydroxy-2-(6-methoxy-[1,S]naphtliyridin-4 yl)-
ethylJ-3-aza-
bicyclo[3.1.0Jhex-6 yl)-carbamic acid tert-butyl ester:
Starting from intermediate 39.i (0.350 g) and (la,5(x,6oc)-3-aza-
bicyclo[3.1.0]hex-6-yl)-
carbamic acid tert-butyl ester (0.360 g), the title compound (D.371 g) was
prepared according
to the procedure described in Example 35, step 35.i.
'H NMR (CDC13) S: 8.75 (d, J= 4.5 Hz, 1H); 8.20 (d, J= 9-1 Hz, 1H); 7.74 (d,
J= 4.5 Hz,
1H); 7.10 (d, J= 9.1 Hz, 1H); 5.62 (dd, J= 3.2, 9.8 Hz, 1I3); 4.61 (bs, 1H);
4.04 (s, 3H);
3.48 (m, 1H); 3.16 (m, 1H); 3.04 (dd, J= 3.5, 12.1 Hz, 1H); 2.60 (m, 4H); 1.83
(bs, 1H);
1.62 (m, 1H); 1.58 (m, 1H); 1.43 (s, 9H).
MS (ESI, m/z): 401.6 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 104 -
39.iii. rac-(la,5a,6(x)-2-(6-amino-3-aza-bicyclo[3.1.0]hex-3-yl)-1-(6-methoxy-
[1,5]naphthyridin-4-yl)-ethanol:
Starting from intermediate 39.ii (0.370 g), the title amine (0.223 g) was
obtained according to
the procedure described in Exarnple 35, step 35.ii.
MS (ESI, m/z): 301.4 [M+H+].
39.iv. rac-2-{(1 c;5a,6a)-6-[(2, 3-dihydro-benzo[1,4]dioxin-6 ylmethyl)-aminoJ-
3-aza-
bieyclo[3.1.OJhex-3 yl}-1-(6-methoxy-[1,5]naphthyridin-4 yl)-ethanol:
According to the procedure described in Example 35, step 35.iii, the title
compound (0.055 g)
was obtained from intermediate 39.iii (0.059 g) as a yellow gum.
MS (ESI, m/z): 449.5 [M+H}].
Example 40: rac-6-({(1 a,5a,6a)-3-[2-hydroxy-2-(6-methoxy-[1,5]naphthyridin-4-
yl)-
ethyl]-3-aza-bicyclo[3.1.0] hex-6-ylamino}-methyl)-4H-benzo[1,4]thiazin-3-one:
According to the procedure described in Example 35, step 35.iii, the title
compound (0.049 g)
was obtained from intermediate 39.iii (0.059 g) as a yellow foam.
MS (ESI, m/z): 478.3 [M+H+].
Example 41: rac-2-{(1 a,5a,6 a)-6-[(2,3-dihydro-[1,4]dioxino[2,3-b]pyridin-6-
ylm(--thyl)-
amino]-3-aza-bicyclo [3.1.0] hex-3-yl}-1-(6-methoxy- [1,5] naphthyridin-4-yl)-
ethanol:
The title compound (0.067 g) was prepared from intermediate 39.iii (0.100 g)
and
2,3-dihydro-[1,4]dioxino[2,3-b]pyridine-6-carbaldehyde (prepared as described
in
WO 2004/014361, 0.049 g) using the procedure of Example 35, step 35.iii.
'H NMR (CDC13) 8: 8.75 (d, J= 4.3 Hz, 1H); 8.20 (d, J= 9.1 Hz, 1H), 7.72 (d,
J= 4.3 Hz,
1H); 7.14 (d, J= 7.9 Hz, 1H); 7.10 (d, J= 9.1 Hz, 1H); 6.82 (d, J= 7.9 Hz,
1H); 5_61 (dd,
J= 3.2, 9.9 Hz, 1H); 4.45 (rn, 211); 4.26 (m, 2H); 4.04 (s, 3H); 3.77 (s, 2H);
3.34 (d,
J= 8.8 Hz, 1H); 3.03 (m, 2H); 2.56 (m, 3H); 2.47 (m, 1H); 1.88 (bs, 2H); 1.57
(rn, 1H);
1.50 (m, 1H).
MS (ESI, m/z): 450.4 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-105-
Example 42: rac-6-({(1 a,5 a,6 a)-3-[2-hydroxy-2-(3-methoxy-quinolin-5-yl)-
ethylJ-3-aza-
bicyclo [3.1.0] hex-6-ylamino}-methyl)-4H-benzo [1,4] oxazin-3-one:
42. i. 3-me thoxyquinol ine-5-caNbaldehyde :
To a solution of 5-bromo-3-methoxyquinoline (10 g) in THF (250 ml) cooled to -
78 C, was
added n-BuLi (22 ml). After 15 min, a solution of DMF (10 ml) in ether (20 ml)
vvas quickly
added. The solution was stirred 15 min and EtOH (5 ml), followed by 1MNaHSO4
(40 ml),
was added. After warming to rt, the organic layer was diluted with EA (100
mlD. The two
layers were separated and the aq. layer was extracted once with EA (100 ml).
The combined
organic layers were washed with brine and concentrated to dryness. The residue
was
chromatographed (EA-Hex 1-2 then 1-1) to afford the title compound (4.75 g) as
a yellowish
solid.
'H NMR (CDC13) 6: 10.32 (s, 1H); 9.02 (d, J= 2.9 Hz, 1H); 8.75 (d, J= 2.9 Hz,
1H); 8.31 (d,
J= 8.3 Hz, 1 H); 8.02 (d, J= 7.1 Hz, 1 H); 7.72 (dd, J= 7.1, 8.3 Hz, 1 H);
4.02 (s, 3H).
MS (ESI, m/z): 187.9 [M+H+]
42.ii. rac-3-methoxy-5-(oxiran-2-yl)quinoline:
To a solution of 3-methoxyquinoline-5-carbaldehyde (2.1 g) in MeCN (35 ml)
were added
successively water (20 drops), trimethylsulfonium iodide (2.4 g) and crushed
KOH (4.5 g).
The heterogenous mixture was heated at 60 C for 30 min. The solids were
filtered off and
water (20 ml) was added to the filtrate. The volatiles were removed under
reduced pressure
and the residue was extracted twice with EA (2 x 150 ml). The combined organic
layers were
washed with brine, dried over Na2SO4, filtered and concentrated to dryness.
The residue was
purified by chromatography (EA-Hex 1-1 then 2-1) to afford the title compound
(1.6 g) as a
beige solid.
'H NMR (CDC13) S: 8.72 (d, J= 2.9Hz, 1H); 8.02 (dd, J= 2, 7.2 Hz, 1FIE); 7.68
(d,
J= 2.9 Hz, 1H); 7.51 (m, 2IT); 4.40 (dd, J= 2.7, 3.7 Hz, 1H); 4.00 (s, 3H);
3.31 Cdd, J= 3.7,
5.6 Hz, IH); 2.91 (dd, J= 2.7, 5.6 Hz, 1H).
MS (ESI, m/z): 202.2 [M+H+]
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 106 -
42.iii. rac- {(1 c;5c; 6a)-3-[2-hydroxy-2-(3-methoxy-quinolin-5 yl)-ethylJ-3-
aza-
bicyclo[3.1.OJhex-6 yl}-carbarnic acid tert-butyl ester:
Starting from intermediate 42.ii (1.59 g) and (la,5(x,6a)-3-aza-
bicyclo[3.1.0]hex-6-yl)-
carbamic acid tert-butyl ester (1.48 g), the title compound (1.60 g) was
prepared according to
the procedure described in Example 35, step 35.i.
MS (ESI, m/z): 400- 5 [M+H+].
42.iv. rac-2-[(lc~5cr,6a)-6-amino-3-aza-bicyclo[3.1.0]hexan-3 ylJ-1-(3-
methoxyquinolin-
5 yl)ethanol:
Starting from intermediate 42.iii (1.6 g), the title amine was obtained in 75%
yield according
to the procedure described in Example 35, step 35.ii.
MS (ESI, m/z): 300.3 [M+H+].
42.v. rac-6-({(1 c45 e~ 6a) 3-[2-hydroxy-2-(3-methoxy-quinolin-5 yl)-ethylJ-3-
aza-
bicyclo[3.1.O]hex-6 ylamino}-methyl)-4H-benzo[l, 4Joxazin-3-one:
The title compound (0.082 g) was prepared from intermediate 42.iv (0.100 g)
and 3-oxo-3,4-
dihydro-2H-benzo[1,4]oxazine-6-carbaldehyde (0.065 g) using the procedure of
Example 35,
step 35.iii.
MS (ESI, m/z): 461.4 [M+H+].
Example 43: rac-2-{(1 a,5a,6a)-6-[(2,3-dihydro-[1,4]dioxino[2,3-b]pyridin-6-
ylmethyl)-
amino]-3-aza-bicyclo [3.1.0] hex-3-yl}-1-(3-methoxy-quinolin-5-yl)-ethanol:
The title compound (0.086 g) was prepared from intermediate 42.iv (0. 100 g)
and
2,3-dihydro-[1,4]dioxino[2,3-b]pyridine-6-carbaldehyde (0.061 g) using the
procedure of
Example 35, step 35.iii.
MS (ESI, m/z): 449.5 [M+H+].
Example 44: rac-6-({(1a,5a,6a)-3-[2-hydroxy-2-(3-methoxy-quinolin-5-yl)-e-
thyl]-3-aza-
bicyclo[3.1.0]hex-6-ylamino}-methyl)-4H-pyrido[3,2-b][1,4]thiazin-3-one:
The title compound (0.032 g) was prepared from intermediate 42.iv (0.100 g)
and 3-oxo-3,4-
dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.071 g) using the
procedure of
Example 35, step 3 5.iii.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 107 -
MS (ESI, m/z): 478.5 [M+H+].
Example 45: rac-6-({(1 a,5a,6a)-3-[2-hydroxy-2-(3-methoxy-quinolin-5-yl)-
ethyl]-3-aza-
bicyc lo [3.1.0] hex-6-ylani i no}-methyl)-4H-benzo [ 1,4] thiazin-3-one :
The title compound (0.072 g) was prepared from intermediate 42.iv (0.100 g:)
and 3-oxo-
3,4-dihydro-2Fl-benzo[1,4]thiazine-6-carbaldehyde (0.071 g) using the
procedure of Example
35, step 35.iii.
MS (ESI, m/z): 477.3 [M+H+].
Example 46: rac-2-{(1oc,5a,6a)-6-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-
amino]-
3-aza-bicyclo [3.1.0] hex-3-yl}-1-(3-methoxy-quinolin-5-yl)-ethanol:
The title compound (0.045 g) was prepared from intermediate 42.iv (0.100 g)
and
2,3-dihydro-benzo[1,4]dioxine-6-carbaldehyde (0.06 g) using the procedure of
Example 35,
step 35.iii.
MS (ESI, m/z): 448.5 [M+H+]
Example 47: 6-({(1 a,5cx,6a)-3-[(2R)-2-hydroxy-2-(3-methoxy-quinolin-5-yl)-
ethyl]-
3-aza-bicyclo[3.1.0]hex-6-ylamino}-methyl)-4H-benzo[1,4]thiazin-3-one:
47. i. 3-methoxy-5-vinyl-quino l ine :
To a solution of triphenyl methyl phosphonium bromide (10 g) in THF (110 nil)
was added
n-BuLi (2.5M, 2.6 ml) at -78 C. The mixture was stirred at the same
temperature for 15 min
and then 45 min at 0 C. After cooling to -78 C, a solution of 3-methoxy-
quinoline-
5-carbaldehyde (4.0 g) in THF (15 ml+ 5 ml rinse) was quickly added. The
resulting mixture
was then stirred at rt for 90 min. The volatiles were removed under reduced
pre ssure and the
residue was loaded on a silica gel column and eluted (Hex-EA 4-1 then 1-1) to
a_fford the title
compound (3.7 g) as an oil.
MS (ESI, m/z): 463.4 [M+H+].
47.ii. (1R)-1-(3-methoxy-quinolin-5 yl)-ethane-1,2-diol:
To an ice-chilled solution of intermediate 47.i (4.9 g) in 2-methyl-2-propanol
(130 ml) and
water (130 ml) was added AD mix [3 (38 g). The solution was stirred at this
temperature
overnight. Sodium metabisulfite (42 g) was added portion wise. After stirring
fuerther 30 min,
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 108 -
the two layers were separated. The aq. layer was extracted twice with EA (2 x
200 ml). The
combined organic phases were washed with brine and dried over NkISO4. After
filtration and
evaporation to dryness, the residue was purified over silica gel (EA-MeOH 19-
1) to afford the
title diol compound (5.2 g) as a white foam.
MS (ESI, m/z): 220.5 [M+H+].
47.iii. (2R)-toluene-4-sulfonic acid 2-hydroxy-2-(3-methoxy-quinolin-5 yl)-
ethyl ester:
To a mixture of intermediate 47.ii (3.0 g) in DCM (65 ml) were added TEA (3 _8
ml) and
DMAP (1.67 g). The resulting mixture was then cooled to -78 C and p-toluene
sulfonyl
chloride (2.60 g) was added in one portion. The reaction was then stored in a
fridge at -30 C
for 12 h. The solution was warmed up to rt and saturated NaHCO3 (50 ml) was
added. The
two layers were separated and the organic layer was evaporated to dryness. The
residue was
purified over silica gel (EA-Hex 1-2 then 2-1) to afford the title compound
(3.1 g) as a white
solid.
MS (ESI, m/z): 374.6 [M+IH+].
47.iv. 3-methoxy-5-[(2R)-oxiran-2 ylJquinoline:
To a solution of intermediate 47.iii (3.1 g) in MeOH (100 ml) was added K2C03
(2 g). The
reaction was stirred at rt for 1 h. Water (100 ml) was added and the volatiles
were removed in
vacuo. The residue was extracted three times with EA (3 x 100 ml). The
combined extracts
were washed with brine and dried over Na2SO4. After filtration and evaporation
to dryness,
the residue was chromatographed (EA-Hex 1-1) to afford the title epoxide as a
solid (1.6 g) of
96-98% enantiomeric excess.
The enantiomeric excess was measured by chiral HPLC on Chiralcel OD (detection
at
254 nm) using a THF-Hex mixture (3-7). The major enantiomer eluted after 13.0
rrnin and the
minor one after 14.1 min (column Chiralcel OD 4.6 x250 mm, 10 m; flow 0.8
ml/min;
eluent: 95% Hex and 5% EtOH with 0.1% diethanolamine).
47.v. {(1c;5c~6a)-3-[(2R)-2-hydroxy-2-(3-methoxy-quinolin-5 yl)-ethylJ-3-aza-
bicyclo[3.1.O]hex-6 yl}-carbamic acid tert-butyl ester:
Starting from intermediate 47.iv (0.995 g) and (la,5a,6a)-3-aza-
bicyclo[3.1.0]hex-6-yl)-
carbamic acid tert-butyl ester (1.0 g), the title compound (1.36 g) was
prepared according to
the procedure described in Example 35, step 35.i.
MS (ESI, m/z): 400.2 [M+HJ.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-109-
47.vi. (IR)-2-[(1 e;5c;6a)-6-amin -3-aza-bicyclo[3.1. O]hexan-3 yl]-1-(3-
naethoxyquinolin-
yl)ethanol:
Starting from intermediate 47.v (1.36 g), the title amine was obtained iri 98%
yield according
to the procedure described in Exarnple 35, step 35.ii.
5 MS (ESI, in/z): 300.3 [M+H+].
47.vii. 6-({(1a,5c~6a) 3-[(2R)-2-hydroxy-2-(3-methoxy-quinolin-5 yl)-ethylJ-3-
aza-
bicyclo[3.1.O]hex-6 ylamino}-rnetdiyl)-4H-benzo[1, 4Jthiazin-3-one:
The title compound (0.053 g) was prepared from intermediate 47.vi (0. 100 g)
and 3-oxo-3,4-
dihydro-2H-benzo[1,4]thiazine-6-carbaldehyde (0.071 g) using the procedure of
Example 35,
step 35.iii.
MS (ESI, m/z): 477.5 [M+H+].
Example 48: (2R)-2-{(la,5a,6a)-6-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-
amino]-
3-aza-bicyclo [3.1.0] hex-3-yl}-1-(3-methoxy-quinolin-5-yl)-ethanol:
The title compound (0.081 g) was prepared from intermediate 47.vi (0.100 g)
and 2,3-
dihydro-benzo[1,4]dioxine-6-carbaldehyde (0.061 g) using the procedure of
Example 35, step
35.iii.
MS (ESI, m/z): 448.8 [M+H+].
Example 49: rac-4-{3-[1-(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)o-piperidin-3-
yl]-
propoxy}-6-methoxy-quinoline:
49.i. rac-3-[3-(6-methoxy-quinolin-4 yloxy) propylJ piperidine-l-carboxylic
acid tert-butyl
ester:
To a solution of rac-3-(3-hydroxy-propyl)-piperidine-l-carboxylic acid tert-
butyl ester (1 g,
prepared as described in WO 94/12181), 6-methoxy-quinolin-4-ol (1.5 5 g) and
PPh3 (2.46 g)
in THF (30 ml) was added DIAD (1.86 ml). The reaction mixture was stirred at
rt for 4 h and
then concentrated to dryness. The residue was diluted with 0.2N aq. I3Cl (100
ml). The aq.
layer was washed three times with ether (3 x 100 ml). The pH was then made
basic using 1M
aq. NaOH (20 ml). The aq. layer was extracted with EA (2 x 150 ml). The
combined extracts
were washed with brine and dried over Na2SO4. After filtration, the solvent
was evaporated
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 110 -
and the residue was purified by chromatography (EA-MeOH 9-1) to afford the
title compound
(l.l g) as an oil.
'H NMR (CDC13) b: 8.60 (d, J= 5.2 Hz, 1H); 7.95 (d, J= 9.1 Hz, 1H); 7.44 (d,
J= 2.8 Hz,
1 H); 7.55 (dd, J= 2.8, 5.2 Hz, 1 H); 6.70 (d, J= 9.1 Hz, 1 H); 4.19 (t, J=
6.5 Hz, 1 H); 3.94 (s,
3H); 3.93 (overlapped m, 11-1); 2.84 (m, 1H); 2.60 (broad m, 1H); 2.04 (s, J=
6.5 Hz, 2H);
1.96 (m, 1H); 1.64-1.42 (m, 5H); 1.44 (s, 9H); 1.26 (m, 1H).
49.ii. rac-6-methoxy-4-(3piperidin-3 yl propoxy)-quinoline:
To a solution of intermediate 49. i(l.l g) in MeOH (10 mL) was added HCl (15
ml, 1.25N in
MeOH). After stirring at rt for 5 h, the reaction mixture was concentrated to
dryness, and the
residue was taken up in water (100 ml). The aq. layer was washed twice with EA
(2 x 50 ml).
Solid KOH (1 g) was added to reach pH 12. The aq. layer was extracted with EA
(2x 100 ml)
and the combined organic layers were washed with brine and dried over Na2SO4.
After
filtration and concentrated to dryness, the residue was purified over silica
gel (DCM-MeOH
19-1, 1% concentrated aq. NH4OH) to afford the title piperidine (0- 81 g) as
an oil.
'H NMR (CDC13) 8: 8.60 (d, J= 5.2 Hz, 1 H); 7.93 (d, J= 9.1 Hz, 1 H); 7.44 (d,
J= 2.8 Hz,
1H); 7.35 (dd, J= 2.8, 9.1 Hz, 1H); 6.69 (d, J= 5.2 Hz, 1H); 4.18 (t, J= 6.5
Hz, 2H); 3.93 (s,
3H); 3.13 (br d, J= 11.9 Hz, 1H); 3.03 (br d, J= 11.9 Hz, 1H); 2.54 (td, J=
2.9, 11.9 Hz,
1H); 2.31 (dd, J= 10.1, 11.9Hz, 1H); 2.04-1.88 (m, 4H); 1.68 (rn, 1H); 1.56-
1.40 (m, 3H);
1.11 (m, 1H).
49.iii. rac-4-{3-[1-(2,3-dihydro-benzo[1,4]dioxin-6 ylmethyl) piperidin-3-
ylJpropoxy}-
6-methoxy-quinol ine:
To a solution of intermediate 49.ii (0.15 g) in 1,2-DCE (3 ml) were added
successively
2,3-dihydro-benzo[1,4]dioxine-6-carbaldehyde (0.098 g) and sodium
triacetoxyborohydride
(0.137 g). The reaction was stirred overnight. The reaction mixture was
diluted with DCM
(15 ml) and washed once with saturated aq. NaHCO3 (10 ml). 'The organic layer
was dried
over Na2SO4, filtered and concentrated to dryness. The residue was purified
over silica gel
(DCM-MeOH 9-1) to afford the title compound (0.165 g) as a thick oil.
'H NMR (CDC13) 6: 8.60 (d, J= 5.2 Hz, 1H); 7.93 (d, J= 9.1 Hz, 1H); 7.44 (d,
J= 2.8 Hz,
1H); 7.35 (dd, J= 2.8, 9.1 Hz, 1 H); 6.89 (s, 1H); 6.82 (app s, 2FII); 6.69
(d, J= 5.2 Hz, 1H);
4.23 (s, 4H); 4.17 (t, J= 6.5 Hz, 2H); 3.96 (s, 3H); 3.50 (m, 2H); 3.02 (m,
2H); 2.08-1.71 (m,
6H); 1.48 (m, 3H); 1.05 (m, 2H)-
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-I11-
MS (ESI, m/z): 449.6 [M+H+].
Example 50: 6-methoxy-4-{3-[1-(trans-3-phenyl-allyl)o-piperidin-3-yl]-propoxy}-
quinoline:
Starting from intermediate 49.ii (0.1 g) and trans-cinnamaldehyde (0.053 ml),
the title
compound (0.105 g) was prepared as a white foam according to the procedure
described in
Example 49, step 49.iii.
MS (ESI, m/z) : 449.6 [M+Hi"].
Example 51: 4-[3-(1-benzofuran-2-ylmethyl-piperidin-3-yl)-propoxy]-6-methoxy-
quinoline:
Starting from intermediate 49.ii (0.15 g) and benzofuran-2-carbaldehyde (0.072
ml), the title
compound (0.183 g) was prepared as a white foam according to the procedure
described in
Example 49, step 49.iii.
MS (ESI, m/z): 431.5 [M+H+].
Example 52: rac-3-[l-(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-piperidin-3-yl]-
N-(6-methoxy-[1,5]naphthyridin-4-yl)-propionamide:
52.i. rac-3-[2-(6-methoxy-[1,5]naphthyridin-4-ylcarbamoyl)-ethylJ piperidine-1-
carboxylic
acid tert-butyl ester:
To a solution of 3-(2-carboxy-ethyl)-piperidine-l-carboxylic acid tert-butyl
ester (0.525 g),
DIPEA (0.338 ml) and HATU (0.776 g) in DMF (4 ml) was added 6-methoxy-
[1,5]naphthyridin-4-ylamine (0.357 g). The reaction was stirred at rt for 22
h. DMF was
removed under reduced pressure and the residue was purified by chromatography
(DCM-MeOH 19-1) to afford the title compound (0.51 g) as an oil.
MS (ESI, m/z): 415.3 [M+H+]
52.ii. rac-N-(6-methoxy-[1,5]naphthyridin-4-yl)-3-piperidin-3-yl-propionamide:
The title piperidine (0.374 g) was obtained as an oil starting from
intermediate 52.i (0.51 g),
using the procedure described in Example 49, step 49.ii.
MS (ESI, m/z): 315.4 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 112 -
52.iii. rac-3-[]-(2,3-dihydro-benzo[1,=l]dioxin-6 ylmethyl) piperidin-3-ylJ-N-
(6-methoxy-
[1,5]naphthyridin-4 yl) propionamide:
Starting from intermediate 52.ii (0.1 g) and 2,3-dihydro-benzo[1,4]dioxine-6-
carbaldehyde
(0.057 g), the title compound (0.078 g) was prepared as a gum according to the
procedure
described in Example 49, step 49.iii).
MS (ESI, m/z): 463.6 [M+H}].
Example 53: rac-N-(6-methoxy-[1,5]naphthyridin-4-yl)-3-[1-(3-phenyl-allyl)-
piperidin-
3-yl]-propionamide:
Starting from intermediate 52.ii (0.1 g) and trans-cinnamaldehyde (0.05 ml),
the title
compound (0.025 g) was prepared as a gum according to the procedure described
in Example
49, step 49.iii.
MS (ESI, m/z): 431.6 [M+H}].
Example 54: rac-N-(6-methoxy-[1,5]naphthyridin-4-yl)-3-{1-[2-(thiophen-2-
ylsulfanyl)-
ethyl]-piperidin-3-yl}-propionamide:
To a solution of intermediate 52.ii (0.1 g) in DMF (4 ml) were added 2-(2-
bromo-
ethylsulfanyl)-thiophene (0.112 g) and DIPEA (0.15 ml). The reaction was
heated at 70 C for
2 h. The volatiles were removed under HV and the residue was purified over
silica gel (DCM-
N4eOH 19-1, 1% concentrated aq. NH4OH) to afford the title compound (0.056 g)
as a thick
oil.
1VIS (ESI, m/z): 457.6 [M+H+].
Example 55: rac-N-(6-methoxy-[l,5]naphthyridin-4-yl)-3-[1-(3-oxo-3,4-dihydro-
2H-benzo [1,4] oxazin-6-ylmethyl)-piperidin-3-yl]-propionamide:
Starting from intermediate 52.ii (0.1 g) and 3-oxo-3,4-dihydro-213-
benzo[1,4]oxazine-6-
carbaldehyde (0.057 g), the title compound (0.026 g) was prepared as a_ foam
according to the
procedure described in Example 49, step 49.iii.
MS (ESI, m/z): 476.5 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 113 -
Example 56: rac-N-(6-methoxy-[1,5]naphthyridin-4-yl)-3-[1-(3-oxo-3,4-dihydro-
2FI-pyrido[3,2-b] [1,4]thiazin-6-ylmethyl)-piperidin-3-yl]-propionami4de:
Starting from intermediate 52.ii (0.1 g) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-
b][1,4]thiazine-
6-carbaldehyde (0.058 g), the title compound (0.038 g) was prepared as a foam
according to
the procedure described in Example 49, step 49.iii.
MS (ESI, m/z): 493.1 [M+H}].
Example 57: rac-3-[1-(2,3-dihydro-[1,4]dioxino[2,3-c]pyridin-7-ylmethyl)-
piperidin-
3-yl]-1V (6-methoxy-[1,5]naphthyridin-4-yl)-propionamide:
Starting from intermediate 52.ii (0.1 g) and 2,3-dihydro-[1,4]dioKino[2,3-
c]pyridine-7-
carbaldehyde (0.057 g), the title compound (0.075 g) was prepared as a'vhite
foam according
to the procedure described in Example 49, step 49.iii).
MS (ESI, m/z): 464.6 [M+H+].
Example 58: rac-3-{1-[2-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethyl]-piperidin-3-
yl}-
N-(6-methoxy- [ 1,5] naphthyridin-4-yl)-propion amide:
Starting from intermediate 52.ii (0.077 g) and intermediate 13.iii (0.1 g),
the title compound
(0.034 g) was prepared as a yellow foam according to the procedure described
in Example 54.
MS (ESI, m/z): 477.5 [M+H+] .
Example 59: rac-1V-(2-cyano-quinolin-8-yl)-3-[1-(2,3-dihydro-benzo [1,4]dioxin-
6-ylmethyl)-piperidin-3-yl]-propionamide:
59.i. rac-3-(2-carbanaoyl-ethyl) piperidine-l-carboxylic acid tert-butyl
ester:
To a solution of rac-3-(2-carboxy-ethyl)-piperidine-l-carboxylic acid tert-
butyl ester (5 g) in
EA (75 ml) were added NHS (2.14 g) and DCC (4.3 g). The reaction was stirred
for 13 h at rt,
whereupon the solids were filtered off and the filtrate concentrated to
dryness. The residue
was taken up in THF (200 ml) and gaseous NH3 was bubbled through tlhe mixture
for 10 min.
The resulting slurry was stirred for 2 h. The solvent was evaporated and the
residue directly
subjected to chromatography over silica gel (DCM-MeOH 9-1) to a..fford the
title amide
(4.4 g) as a thick oil.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 114 -
1H NMR (CDC13) 5: 5.60 (br s, 1H), 5.48 (br s, 1H); 3.86 (m, 2H); 2.86 (m,
1H); 2.58 (dd,
J= 9.3, 13.1 Hz, 1H); 2.28 (td, J= 1.2, 7.6 Hz, 2H); 1.84 (m, 11--!); 1.60 (m,
3H),
1.44 (overlapped m, 2H); 1.43 (s, 9H); 1.16 (m, 1H).
MS (ESI, m/z): 257.5 [M+H+].
59.ii. rac-3-[2-(2-cyano-quinolin-8 ylcarbamoyl)-ethylJ piperidine-l-
carboxylic acid
tert-butyl ester:
To a mixture of intermediate 59.i (1.02 g), cesium carbonate (1.6 g), rac-
BINAP (0.180 g)
and tris(dibenzilideneacetone) dipalladiuin(0)-chlorofonn complex (0.074 g)
was added
dioxane (41 ml). The mixture was sonicated 15 min and trifluoro-
methanesulfonic acid 2-
cyano-quinolin-8-yl ester (1.2 g) was added. The mixture was heated a.t 100 C
overnight.
After filtration, the filtrate was concentrated to dryness and the residue was
triturated in ether
to afford the title amide (1.1 g) as a white solid.
iH NMR (d6-DMSO) 8: 10.07 (s. 1H); 8.70 (partially overlapped dd, J = 2.7, 6.6
Hz, 11-1);
8.67 (d, J= 8.4 Hz, 1H); 8.11 (d, J= 8.4 Hz, 1H); 7.78 (m, 2H), 3.84 (br s,
IH); 3.75 (m, IH);
2.80 (ddd, J= 3.0, 11.1, 14.1 Hz, 1H); 2.65 (t, J= 7.5 Hz, 2H); 1.84 (m, 1H);
1.64-1.49 (m,
3H); 1.43-1.24 (overlapped m, 31H); 1.36 (s, 9H); 1.13 (m, 1H).
MS (ESI, m/z): 409.4 [M+H+].
59.iii. rac-N-(2-cyano-quinolin-8 yl)-3piperidin-3yl propionamide:
A solution of intermediate 59.ii (1.1 g) in TFA (5 ml) was stirred at rt for
10 min. After the
solvent was evaporated, the residue was partitioned between 3N aq. NaOH (20
ml) and a
DCM-MeOH mixture (9-1, 50 ml). The aq. layer was extracted twice more and the
combined
extracts were washed with brine, dried over Na2SO4, filtered, and concentrated
to dryness.
The residue was ehromatograplhed (DCM-MeOH 9-1, 1% concentrated aq. NH4OH) to
afford
the title compound (0.720 g) as a yellowish foam.
MS (ESI, m/z): 309.1 [M+H+].
59.iv. rac-N-(2-cyano-quinolin-8 yl)-3-[1-(2,3-dihydro-benzo[1,4]dioxin-6
ylmethyl)-
piperidin-3 ylJ propionanaide:
Starting from intermediate 59.iii (0.11 g) and 2,3-dihydro-benzo[1,4]dioxine-6-
carbaldehyde
(0.065 g), the title compound (0.073 g) was prepared as a white foam according
to the
procedure described in Example 49, step 49.iv.
MS (ESI, m/z): 457.1 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 115 -
Example 60: rac-N-(2-cyano-quinolin-8-yl)-3-[trans-l-(3-phenyl-allyl)-
piperidin-3-yl]-
propionamide:
Starting from intermediate 59.iii (0.11 g) and trans-cinnamaldehyde (0.055
ml), the title
compound (0.062 g) was prepared according to the procedure described in
Example 49, step
49.iv.
MS (ESI, m/z): 425.5 [M+H}].
Example 61: rac-N-(2-cyano-quinolin-8-yl)-3-[1-(3-oxo-3,4-dihydro-
2H-benzo [ 1,4] thiazin-6-ylm ethyl)-piperidin-3-yl] -propionam ide:
Starting from intermediate 59.iii (0.11 g) and 3-oxo-3,4-dihydro-2H-benzo[
1,4]thiazine-6-
carbaldehyde (0.076 g), the title cornpound (0.081 g) was prepared according
to the procedure
described in Example 49, step 49.iv.
MS (ESI, m/z): 486.1 [M+H+].
Example 62: rac-2-[1-(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-piperidin-3-
yloxy]-
N-(6-methoxy-[1,5] naphthyridin-4-yl)-acetamide:
62.i. rac-3-carboxymethoxy piperidine-l-carboxylic acid benzyl ester:
A solution of rac-3-tert-butoxycarbonylmethoxy-piperidine-l-carboxylic acid
benzyl ester
(2.8 g, prepared as described in WO 94/22835) in DCM (20 ml) was treated with
TFA
(10 ml). After stirring at rt for 1 h, the volatiles were removed under
reduced pressure. The
residue was purified over silica gel (EA-Hex 1-1) to afford the title compound
(2 g) as an oil.
MS (ESI, m/z): 294.4 [M+H+].
62.ii. rac-3-[(6-methoxy-[1,5]naphthyridin-4 ylcarbamoyl)-methoxyJpiperidine-
1-carboxylic acid benzyl ester:
Starting from intermediate 62.i (0.598 g) and 6-methoxy-[1,5]naphthyridin-4-
ylamine
(0.357 g), the title amide (0.21 g) was prepared as a gum, as described in
Example 52, step
52.i.
MS (ESI, m/z): 451.3 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 116 -
62.iii. N-(6-rnethoxy-[1,5]naphthyridin-4 yl)-2-(piperidin-3yloxy)-acetamide:
To a solution of intermediate 62.ii (0.21 g) in MeOH (6 ml) was added 20%
palladium
hydroxide on charcoal (0.1 g). The reaction mixture was stirred at rt under
hydrogen
atmosphere for 1 h. The catalyst was removed by filtration and the filtrate
was concentrated in
vacuo to afford after drying a semi solid (0.145 g).
MS (ESI, m/z): 317.4 [M+H+].
62.iv. rac-,-[I-(2,3-dihydro-benzo[1,4]dioxin-6 ylinethyl) piperidin-3yloxyJ-N-
(6-methoxy-
[1,5]naphthyridin-4 yl)-acetamide:
Starting frorrn intermediate 62.iii (0.14 g) and 2,3-dihydro-benzo[1,4]dioxine-
6-carbaldehyde
(0.090 g), the title compound (0.081 g) was prepared according to the
procedure described in
Example 49, step 49.iv.
MS (ESI, m/z): 465.5 [M+H+].
Example 63: (2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-[3-(6-methoxy-quinolin-
4-yloxymetliyl)-cyclohexylmethyl]-amine:
63.i. 5-(ethoxycarbonyl-nitro-methyl)-cyclohex-3-enecarboxylic acid methyl
ester:
To a degassed solution of tetrakis(triphenylphosphine)palladium (0.357 g) in
DCM (56 ml)
was added 5-methoxycarbonyloxy-cyclohex-3-enecarboxylic acid inethyl ester
(7.1 g) and
ethyl nitroacetate (3.67 ml). The reaction was stirred at rt for 30 min. The
volatiles were
removed under reduced pressure and the residue was purified over silica gel
(EA-Hex 1-5) to
afford the title compound (7.8 g) as an equimolar mixture of diasteroiners.
'H NMR (CDC13) &: 5.93 (m, 1H); 5.53 (m, 1H); 5.00 (app t, J= 8-9 Hz, 1H);
4.32 (m, 2H);
3.72 (s, 1.51H); 3.71 (s, 1.5H); 3.33 (m, 1H); 2.68 (m, 1H); 2.38-1.90 (m,
2.5H); 1.88 (m,
0.5H); 1.66 (q, J= 12.6 Hz, 0.5H); 1.55 (q, J= 11.6 Hz, 0.5H); 1.33 (m, 3H).
63.ii. 5-nitromethyl-cyclohex-3-enecarboxylic acid methyl ester:
A mixture of intermediate 63.i (7.8 g) in EtOH (17 ml) and 5Maq. NaOH (22 ml)
was heated
at 70 C overnight. The volatiles were removed under reduced pressure and 2M
aq. H2SO4 was
added to reach pH 3. The mixture was extracted three times with ether (3 x 100
ml). The
combined organic layers were washed with brine, dried over Na2SO4 and
evaporated under
reduced pressure. The residue was further dried under HV to afford the
intermediate acid
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 117 -
(3.1 g) as a pale solid. The latter was dissolved in ether (100 ml) and MeOH
(15 ml).
Trimethylsilyldiazomethane (2M in hexanes, 10 ml) vvas added. The reaction
mixture was
stirred for 30 min at rt and AcOH (1 ml) was added. The volatiles were removed
under
reduced pressure. The residue was purified over silicaa gel (EA-Hex 1-5) to
afford the title
compound as a 1.5:1 mixture of diastereomers (3.15 g).
'H NMR (CDC13) 8: 5.89 (m, 1H); 5.53 (m, 1H); 4.30 (m, 2H); 3.71 (s, 3H); 3.10
(m, 1H);
2.64 (m, 1H); 2.38-2.15 (m, 2.6H); 2.01 (m, 0.4H); 1.85 (m, 0.4H); 1.48 (m,
0.6H).
13C NMR (CDC13) 8: 174.75, 174.67, 128.92, 128.42, 124.91, 142.40, 79.64,
78.59, 51.56,
51.51, 38.61, 35.12, 34.74, 32.33, 28.95, 27.21, 26.84, 26.80.
63.iii. rac-3-(tert-butoxycarbonylamino-methyl)-cycloliexanecarboxylic acid
methyl ester:
To a mixture of intermediate 63.ii (3.15 g) in MeOH (73 ml) was added 10%
palladium on
charcoal (2.9 g). The reaction vessel was evacuated and the reaction was
filled with hydrogen.
The reaction mixture was stirred under hydrogen overnight. The reaction
mixture was diluted
with MeOH (50 ml) and 1M aq. HCI (20 ml) was add.ed. After filtration, the
volatiles were
removed under reduced pressure. The residue was taken up in THF (50 ml) and
NaHCO3 (5 g)
was added. BOC2O (4.5 g) was added and the reaction mixture was stirred at rt
for 1 h. The
volatiles were removed under reduced pressure and the residue was partitioned
between water
and EA (100 ml). The aq. layer was extracted once rnore and the combined
extracts were
washed -with brine and dried over Na2SO4. After filtration, the volatiles were
removed under
reduced pressure. The residue was purified over silica gel (EA-Hex 1-4) to
afford the title
compound (3.09 g) as a 1.5:1 mixture of diastereomers.
'H NMR (CDC13) 8: 4.50 (m, 1H); 3.68 (s, 1.8H); 3.67 (s, 1.2H); 3.02 (m, 2H);
2.68 (m,
0.4H); 2.31 (m, 0.6H); 2.02-1.12 (multiple overlapped m, 7H); 1.46 (s, 9H);
1.05-0.88 (m,
2H).
63.iv. rcac-(3-hydroxymethyl-cyclohexylmethyl)-carbamic acid tert-butyl ester:
To an ice-chilled mixture of intermediate 63.iii (0.63 g) in ether (10 ml) was
added, DIBAH
(4 ml, 1.M in Hex). The mixture was stirred at the same temperature for 45
min. Water
(0.45 ml) was added and the mixture was stirred at rt for 40 min. The solids
were filtered off
and the filtrate was concentrated in vacuo. The residue was purified over
silica gel (EA-Hex
1-1) to afford the title compound (0.350 g) as 1.5:1 mixture of diasteromers.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 118 -
1H NMR (CDC13) b: 4.59 (br s, 1H); 3.50 (m, 2H); 3.00 (m, 2H); 1.86-1.74 (m,
3H);
1.58-1.25 (m, 5H); 1.46 (s, 9H); 0.88 (m, 1.4H); 0.64 (app q, J= 12.1 Hz,
0.6H).
63.v. rac-[3-(6-methoxy-quinolin-4 yloxymethyl)-cyclohexylmethyZ]-carbamic
acid tert-butyl
ester:
This compound (0.168 g) was prepared as an oil starting from intermediate
63.iv (0.35 g) and
6-methoxy-quinolin-4-ol (0.302 g) using the procedure described in Example 1,
step 1.i.
MS (ESI, m/z): 401.3 [M+H+].
63.vi. rac-C-[3-(6-methoxy-quinolin-4 yloxymethyl)-cyclohexylJanethylamine:
The title amine (0.115 g) was obtained as an oil, starting from intermediate
63.v (0.165 g) and
using the procedure described in Example 1, step I.H.
MS (ESI, m/z): 301.4 [].VI+H+].
63.vii. (2, 3-dihydro-beaizo[1, 4]dioxin-6 ylmethyl)-[3-(6-methoxy-quinolin-
4yloxymethyl)-
cyclohexylmethylJ-amine:
To a solution of interrnediate 63.vi (0.115 g) in DCM (6 ml) and MeOH (2 ml)
were added
2,3-dihydro-benzo[1,4]dioxine-6-carbaldehyde (0.070 g) and 3 A molecular
sieves (2 g). The
resulting mixture was stirred at rt overnight. NaBH4 (0.1 g) was added and the
mixture was
stirred at rt for 2 h. The reaction mixture was filtered through a plug of
Hydromatrix ,
previously treated with NaHCO3 (6 ml). The filtrate was concentxated in vacuo.
The residue
was chromatographed (DCM-MeOH 19-1, 1% concentrated aq. NH4OH) to afford the
title
compound (0.109 g) as a thick oil. The compound was recovered as a 1:1 mixture
of
diastereomers.
MS (ESI, m/z): 449.7 (M+Hi"].
Example 64: (2,3-dihydro-benzo [1,4] dioxin-6-ylmethyl)-[(1R,3,S)-3-(6-methoxy-
quinolin-4-yloxymethyl)-cyclohexylmethyl]-amine:
64.i. (IS, 5S)-5-nitromethyl-cyclohex-3-enecarboxylic acid methyl ester:
To a solution of tris(dibenzilideneacetone) dipalladium chloroform complex
(0.06 g) and
(1R,2R)-(+)-1,2-diaminocyclohexane-N,N'-bis(2'-diphenylphospliinobenzoyl)
(0.12 g) in
DCM (14 ml) were added nitromethane (1.22 ml) and N,O-bis(trimethylsilyl)-
acetamide
(0.77 ml). After stirring at rt for 5 min, 6-oxa-bicyclo[3.2.1]oct-3-en-7-one
(0.35 g) was added
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 119 -
and the mixture was stirred at rt for 14 h. After concentration to dryness,
the residue was
quickly filtered though a plug of silica gel (ether 1% AcOH) and the filtrate
was concentrated
to dryness. The residue was taken up in ether (45 ml) and MeOH (5 ml) and
trimethylsilyldiazomethane (2M in Hex, 2.3 ml) was added. The reaction
proceeded for
30 min. AcOH (0.1 ml) was added and the reaction mixture was concentrated in
vacuo. The
residue was chromatographed (Hex-ether 3-1) to afford the title compound (0.3
5 g) as an oil.
'H NMR (CDC13) 8: 5.89 (m, 1H); 5.51 (rn, 1H); 4.35 (dd, J= 6.9, 12.0 Hz, 1H);
4.29 (dd,
J= 7.8, 12.0 Hz, IH); 3.71 (s, 3H); 3.10 (in, 1H); 2.65 (m, 1H); 2.36-2.17
(ni, 3H); 1.45 (td,
J= 11.1, 12.6 Hz, 1H).
64.ii. (JR, 3S)-(3-hydroxymethyl-cyclohexytmethyl)-carbamic acid tert-butyl
ester:
The title alcohol (0.148 g) was obtained as an oil starting from intermediate
64.i (0.35 g) and
using the procedures described in Example 63, steps 63.iii and 63.iv.
MS (ESI, m/z): 244.3 [M+I-+].
64.iii. (2,3-dihydro-benzo[1,4]dioxin-6 ylmethyl)-[(1R, 3S)-3-(6-methoxy-
quinolin-4-
yloxymethyl)-cyclohexylmethyl]-amine:
The title compound (0.08 g) was obtained as a colourless oil starting from
intermediate 64.ii
(0.148 g) and using the procedures described in Example 63, steps 63.v, 63.vi
and 63.vii.
'H NMR (CDC13) S: 8.60 (d, J= 5.2 Hz, lIR); 7.95 (d, J= 9.2 Hz, 1H); 7.47 (d,
J= 2.8 Hz,
1 H); 7.3 5(dd, J= 2.8, 9.2Hz, 1 H); 6. 8 6(d, J= 0.9Hz, 1H); 6.82 (rn, 2H);
6.70 (d,
J= 5.2 Hz, 1H); 4.25 (s, 4H); 4.00 (dd, J= 1, 5.9 Hz, 2H); 3.94 (s, 3H); 3.71
(s, 2H); 2.54 (d,
J= 6.7 Hz, 2H); 2.02 (m, 3H); 1.92 (m, 2H); 1.68 (m, 2H); 1.42 (m, 1H); 1.12
(m, 1H);
0.93 (m, 2H).
MS (ESI, m/z) : 449.4 [M+H+].
Example 65: rac-3-{[(2,3-dihydro-benzo [1,4]dioxin-6-ylmethyl)-amino]-methyl}-
cyclohexanecarboxylic acid (2-methyl-quiinolin-8-yl)-amide:
65.i. rac-5-nitromethyl-cyclohex-3-enecarboxylic acid (2-methyl-quinolin-8 yl)-
amide:
Starting from rac-5-nitromethyl-cyclohex-3-enecarboxylic acid (0.377 g,
prepared as
described in Example 63, step 63.ii) and 2-methyl-quinolin-8-ylamine (0.322
g), the title
compound (0.487 g) was obtained as a yellow oil using the procedure described
in Example
52, step 52.i.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 120 -
MS (ESI, m/z): 326.3 [M+H}].
65.ii. rcac-3-aminomethyl-cyclohexanecarboxylic acid (2-methyl-quinolin-8 yl)-
amide:
To a solution of intermediate 65.i (0.48 g) in MeOH (15 ml) was addecl 10%
palladium on
charcoa.l (0.5 g). The reaction was stirred under hydrogen atmosphere for 4 h.
The catalyst
was removed by filtration. The residue was purified over silica gel (DCM-MeOH
9-1, 1%
concentrated aq. NH3) to afford the title compound (0.230 g) as a yellow oil.
MS (ESI, m/z): 298.4 [M+H}].
65.iii. -ac-3-{[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-methyl }-
cyclohexanecarboxylic acid (2-methyl-quinolin-8-yl)-amide:
This compound (0.12 g) was prepared as a yellow oil from intermediate 65.ii
(0.115 g) and
2,3-dihydro-benzo[1,4]dioxine-6-carbaldehyde (0.069 g) using the procedure
described in
Example 63, step 63.vii.
MS (ESI, m/z): 446.5 [M+H+].
Example 66: rac-3-[trans-(3-phenyl-allylamino)-methyl]-cyclohexanecarboxylic
acid
(2-metliyl-quinolin-8-yl)-amide:
This compound (0.102 g) was prepared as a yellow oil from intermediate 65.ii
(0.115 g) and
trans-cinnamaldehyde (0.055 g) using the procedure described in Example 63,
step 63.vii.
MS (ESI, m/z): 414.3 [M+H+]
Example 67: rac-3-{[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-methyl}-
cyclohexanecarboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-amide:
67.i. nac-3-(tert-butoxycarbonylamino-methyl)-cyclohexanecarboxylic acid:
A solution of intermediate 63.iii (3.09 g) in dioxane (100 ml) was treated
with NaOH (3M,
12 ml). The mixture was refluxed overnight. The volatiles were removed under
reduced
pressure. The residue was washed twice with ether (2 x 100 ml). The pl3 of the
aq. layer was
adjusted to 3 using 0.2M aq. HCI. The aq. layer was extracted with EA (3 x 150
ml). The
combined organic layers were washed with brine and dried over Na2SO4. After
filtration, the
filtrate was concentrated in vacuo to afford the title acid (2.5 g) as a
colourless foam.
MS (ESI, m/z): 256.2 [M-H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-121-
67.ii. rac-[3-(6-methoxy-[1,5]naphthyridin-4-ylcarbamoyl)-cyclohexylmethyl]-
carbamic acid
tert-butyl ester:
This compound (0_493 g) was prepared as a yellow solid from intermediate 67.i
(0.78 g) and
6-methoxy-[1,5]naphthyridin-4-ylamine (0.531 g) according to the procedure
described in
Example 52, step 52.i.
MS (ESI, m/z): 415.6 [M-H+].
67.iii. rac-3-aminomethyl-cyclohexanecarboxylic acid (6-methoxy-
[1,5]naphthyridin-4-yl)-
amide
This compound (0.27 g) was obtained as a yellow oil starting from intermediate
6 7.ii
(0.493 g) and using the procedure described in Example 49, step 49.ii.
MS (ESI, m/z): 315.4 [M-H+].
67.iv. rac-3-{[(2,3-dihydro-benzo[1,4]dioxin-6 ylrnethyl)-aminoJ-methyl}-
cyclohexanecarboxylic acid (6-methoxy-[1,5]naphtlryridin-4yl)-amide:
This compound (0.093 g) was obtained, as a pale yellow foam, from intermediate
67.iii
(0.1 g) and 2,3-dihydro-benzo[1,4]dioxine-6-carbaldehyde (0.057 g), as
described in
Example 63, step 63.vii).
MS (ESI, m/z): 463.6 [MH+].
Example 68: rac-3-{[(3-oxo-3,4-dihydro-2H-benzo[1,4]oxazin-6-ylmethyl)-amino]-
methyl}-cyclohexanecarboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-amide:
This compound (0.080 g) was obtained, as a pale yellow foam, from intermediate
67.iii
(0.1 g) and 3-oxo-3,4-dihydro-2F7-benzo[1,4]oxazine-6-carbaldehyde (0.062 g),
as descri-bed
in Example 63, step 63.vii.
MS (ESI, m/z): 476.5 [MH+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 122 -
Example 69: cis-3-{[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-methyl}-
cyclohexanecarboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-amide:
69.i. cis-[(3-(6-methoxy-[1,5]naphthyridin-4ylcarbamoyl)-cyclohexylmethylJ-
carbamic acid
tert-butyl ester:
Using cis-3-(tert-butoxycarbonylamino-methyl)-cyclohexanecarboxylic acid (0.78
g, prepared
as described in J. Med. Chem. (1998), 41, 2175) and 6-methoxy-
[1,5]naphthyridin-4-ylamine
(0.531 g), the title compound (0.45 g) was prepared as described in Example
67, step 67. ii)
MS (ESI, m/z): 415.0 [M+H+].
69.ii. cis-3-aminomethyl-cyclohexanecarboxylic acid (6-methoxy-
[1,5]naphthyridin-4 yZ)-
eamide:
This compound (0.21 g) was prepared from interrnediate 69.i (0.45 g) as
described in
Example 67, step 67.iii.
1H NMR (CDC13) 8: 9.55 (s, IH); 8.70 (d, J= 5.1 Hz, 1H); 8.52 (d, J= 5.1 Hz,
1H); 8.22 (d,
.T= 9.1 Hz, 1H); 7.18 (d, J= 9.0 Hz, 1H); 4.13 (s, 311); 2.69 (br d, J= 5.85
Hz, 2H); 2.49 (tt,
J= 3.2, 11.9 Hz, 11-1); 2.21 (m, 2H); 1.69-1.35 (m, 511); 1.33-1.29 (m, 2H);
1.27 (q, J= 12 Hz,
1 H); 1.00 (qd, J= 3.7, 12.7 Hz, 1H).
MS (ESI, m/z): 315.3 [M-H+].
69.iii. cis- 3-{[(2,3-dihydro-benzo[1,4]dioxin-6 ylmerhyl)-aminoJ-methyl}-
cyclohexanecarboxylic acid (6-methoxy-[1, 5]naphthy.-idin-4 yl)-amide:
The title compound (0.072 g) was prepared from intermediate 69.ii (0.1 g)
following the
procedure described in Example 67, step 67.iv.
1H NMR (d6-DMSO) S: 9.75 (s, 1H); 8.66 (d, J= 5.1 Hz, 1H); 8.39 (d, J= 5.1
Hz:, 1H);
8.26 (d, J= 9.0 Hz, 1H); 7.31 (d, J= 9.0 Hz, 1H); 6.81 (s,1H); 6.75 (m, 2H);
4.19 (s, 4H);
4.10 (s, 3H); 3.55 (s, 2H); 2.70 (m, 1H); 2.37 (m, 2H); 2.15 (m, 1H); 1.97 (m,
2H); 1- 80 (m
2H); 1.61 (m, 1H); 1.39 (m, 2H); 1.10 (q, J= 12 Hz,1H); 0.89 (m, 1H).
MS (ESI, m/z): 463.5 [M-H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-123-
Example 70: cis-3-{[(3-oxo-3,4-dihydro-2H-benzo[1,4]thiazin-6-ylmetliyl)-
amino]-
methyl}-cyclohexanecarboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-amide:
The title compound (0.063 g) was prepared from intermediate 69.ii (0.1 g) and
3-oxo-
3,4-dihydro-211'-benzo[1,4]thiazine-6-carbaldehyde (0.074 g) following the
procedure
described in Example 67, step 67.iv.
MS (ESI, mlz): 492.4 [M+H+]
Example 71: cis-3-{[(2,3-dihydro-[1,4]dioxino[2,3-b]pyridin-6-ylmethyl)-amino]-
methyl}-cyclohexanecarboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-amide:
This compound (0.071 g) was obtained, as a yellow gum, from intermediate 69.ii
(0.100 g)
and 2,3-dihydro-[1,4]dioxino[2,3-b]pyridine-6-carbaldehyde (0.063 g), as
described in
Example 19, step 19.iv.
MS (ESI, m/z) : 464.6 [M+H+]
Example 72: cis-3-{[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-methyl}-
cyclohexanecarboxylic acid (2-cyano-quinolin-8-yl)-amide:
72.i. cis-(3-carbamoyl-cyclohexylmethyl)-carbamic acid tert-butyl ester:
This compound (2.78 g) was prepared as a white solid from cis-3-(tert-
biutoxycarbonylamino-
methyl)-cyclohexanecarboxylic acid (3.5 g) using the procedure described in
Example 59,
step 59.i.
MS (ESI, m/z): 257.4 [M+H+].
72.ii. [3-(2-cyano-quinolin-8 ylcarbamoyl)-cyclohexylmethylJ-carbamic ezcid
tert-butyl ester:
Starting from intermediate 72.i (1.02 g) and trifluoro-methanesulfonic acid 2-
cyano-quinolin-
8-yl ester (1.2 g), the title compound (1.1 g) was obtained as a white solid
using the procedure
described in Example 59, step 59.ii.
MS (ESI, m/z): 409.6 [M+H+].
72.iii. cis-3-aminomethyl-cyclohexanecarboxylic acid (2-cyano-quinolin-8 yl)-
amide:
This amine (0.66 g) was obtained as a yellow foam, starting from intermediate
72.ii (1.1 g)
and using the procedure described in Example 59, step 59.iii.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 124 -
MS (ESI, m/z) : 309.3 [M+IH+].
72.iv. cis-3-([(2,3-dihydro-benzo[I, 4]dioxin-6 ylmethyl)-aminoJ-methyl}-
cyclohexanecarboxylic acid (2-cyano-quinolin-8yl)-amide:
This compound (0.072 g) was obtained, as a yellow foam, from intermediate
72.iii (0.11 g)
and 2,3-dihydro-benzo[1,4]dioxine-6-carbaldehyde (0.065 g), as described in
Example 57,
step 67.iv.
MS (ESI, m/z): 457.2 [M+H+].
Example 73: cis-3-{[(3-oxo-3,4-diliydro-2H=benzo[1,4]thiazin-6-ylmethyl)-
amino]-
methyl}-cyclohexanecarboxylic acid (2-cyano-quinolin-8-yl)-amide:
This compound (0.054 g) was obtained, as a yellow foam, from intermediate
72.iii (0.11 g)
and 3-oxo-3,4-dihydro-2H-benzo[1,4]thiazine-6-carbaldehyde (0.075 g), as
described in
Example 67, step 67.iv.
MS (ESI, m/z): 486.2 [M+H+].
Example 74: cis-3-{[(benzo[1,2,5] thiadiazol-5-ylmethyl)-amino]-methyl}-
cyclohexanecarboxylic acid (2-cyano-quinolin-8-yl)-amide:
This compound (0.046 g) was obtained, as a yellow foam, from intermediate
72.iii (0.11 g)
and benzo[1,2,5]thiadiazole-5-carbaldehyde (0.064 g), as described in Example
67, step 67.iv.
MS (ESI, m/z): 457.1 [M+H+].
Example 75: cis-3-{[(3-oxo-3,4-dihydro-2H-pyrido[3,2-b] [1,4]thiazin-6-
ylmethyl)-
amino]-methyl}-cyclohexanecarboxylic acid (2-cyano-quinolin-8-yl)-amide:
This compound (0.058 g) was obtained, as a yellow foam, from intermediate
72.iii (0.11 g)
and 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.076 g),
as described
in Example 67, step 67.iv.
MS (ESI, m/z): 487.5 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 125 -
Example 76: cis-3-[(trans-3-phenyl-allylamino)-methyl]-cyclohexanecarboxylic
acid
(2-cyano-qui nolin-8-yl)-amide:
This compound (0.054 g) was obtained, as a yellow foam, from intermediate
72.iii (0.11 g)
and trans-cinnamaldehyde (0.052 g), as described in Example 67, step 67.iv.
MS (ESI, m/z): 426.5 [M+H+].
Example 77: (2,3-dihydro-benzo [1,4] dioxin-6-ylmethyl)-[(3R,6S)-6-(6-methoxy-
quinolin-4-yloxymethyl)-3,6-dihydro-2H-pyran-3-yl]-amine:
77.i. (3R, 6S) -(6-hydroxymethyl-3, 6-dihydro-2H-pyran-3 yl)-carbarnic acid
tert-butyl ester:
To a solution of [(3R,6S)-6-(tert-butyl-dimethyl-silanyloxymethyl)-3,6-dihydro-
2H-pyran-
3-yl]-carbamic acid tert-butyl ester (obtained from 3,4,6-tri-O-acetyl-D-
glucal as described by
H. S. Overkleeft et al. in Eur. J. Org. Chem. (2003), 2418-2427; 6.39 g) in
THF (100 ml) was
added TBAF (28 ml, 1M solution in THF) at 0 C. The reaction rnixture was
stirred at this
temperature for 30 min, then at rt for 1 h. After concentration to dryness,
the residue was
chromatographed (EA-Hex 1-1 to 1-0) to afford a white solid (3.52 g).
1H NMR (CDC13) b: 5.89 (d, J= 10.4 Hz, 1H); 5.76 (td, J= 1.9, 10.4 Hz, 1H);
4.57 (br. s,
1H); 4.20 (m, 2H); 4.11 (dd, J= 4.7, 11.1 Hz, 1H); 3.62 (d, J= 6.1 Hz, 2H),
3.41 (m, 1H),
2.00 (br. s, I IFI), 1.45 (s, 9H).
77.ii. [(3R, 6S)-6-(6-methoxy-quinolin-4 yloxymethyl)-3, 6-dihydro-2H-pyran-3
ylJ-carbamic
acid tert-butyl ester:
To a solution of intermediate 77.i (1 g), 6-methoxy-quinolin-4-ol (0.916 g)
and PPh3 (1.715 g)
in THF (32 rnl) and DMF (2.2 ml) was added dropwise DIAD (1.322 g). The
reaction mixture
was stirred at rt for 4h and concentrated to dryness. The residue was diluted
with 0.2N aq.
HCl (110 ml) and the aq. layer was washed three times with ether. The pH was
made basic by
addition of 1N aq. NaOH (22 ml) and the aq. layer was extracted twice with EA.
The
combined organic layers were washed with brine and dried over Na2SO4, filtered
and
concentrated. The residue, was crystallized from EA, was triturated in ether
to afford the title
compound a.s a yellow solid (0.5 g).
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 126 -
'H NMR (d6-DMSO) 6: 8.57 (d, J=5.2 Hz, 1H); 7.87 (d, J=9.8 Hz, 1H); 7.38 (m,
2H);
7.03 (br. s, 1 H); 7.02 (d, J=5.2 Hz, 1H); 5.94 (m, 2H); 4.57 (m, 1 H); 4.29
(m, 2H); 4.02 (br.
m, 1H); 3_98 (m, 1H); 3.89 (s, 3H); 3.81 (m, 1H); 1.40 (s, 9H).
77.iii. (3R, 6S)-6-(6-methoxy-quinolin-d yloxymethyl)-3, 6-dihydro-2H-pyran-3
ylamine:
A solution of intermediate 77.ii (0.3 g) in TFA (3 ml) was stirred for 30 min.
The re:action
mixture was concentrated to dryness. The residue was basified with 1N aq. NaOH
and
extracted six times with a mixture DCM-MeOH (9-1). The combined organic layers
were
dried over Na2SO4, filtered and concentrated to give a yellow gum (0.221 g).
'H NMR (CDC13) 8: 8.61 (d, J=5.2 Hz, 1H); 7.93 (d, J=9.2 Hz, 1H); 7.46 (d,
J=2.7 Hz, 1H);
7.34 (dd, J=2.7, 9.2 Hz, 1 H); 6.71 (d, J=5 _ 2 Hz, 1 H); 6.02 (m, 1H); 5.88
(m, 1 H); 4.463 (m,
1H); 4.26 (m, 1H); 4.16 (m, 2H); 3.93 (s, 3H); 3.52 (m, 1H); 3.35 (m, 1H),
1.54 (br. s, 211).
77.iv. (2, 3-dihydro-benzo[1, 4]dioxin-6 ylmethyl)-[(3R, 6S)-6-(6-methoxy-
quinolin-
4 yloxymethyl)-3, 6-dihydro-2H-pyran-3 yl]-amine:
To a solution of intermediate 77.iii (0.1 g) in DCM (6 ml) and MeOH (2 ml)
were added
2,3-dihydro-benzo[1,4]dioxine-6-carbaldehyde (0.064 g) and 3 A molecular
sieves (2 g). The
resulting mixture was stirred overnight at rt. NaBH4 (0.054 g) was added.
After 2 h, the
reaction rnixture was filtered though Hydromatrix (pretreated with aq.
NaHCO3). After the
filtrate was concentrated in vacuo, the residue was purified by column
chromatography over
silica gel (DCM-MeOH 19-1, 1% concentrated NH4OH) to give a yellow gum (0.040
g~o.
'H NMR (CDC13) 8: 8.60 (d, J= 5.2 Hz, 1 H); 7.93 (d, J= 9.2 Hz, 1H); 7.45 (d,
J= 2.8 Hz,
1H); 7.34 (dd, J= 2.8, 9.2 Hz, 1H); 6.84 (m, 3H); 6.70 (d, J= 5.2 Hz, 1H);
6.11 (rn, 1H);
5.91 (m, 1H); 4.65 (m, 1H); 4.25 (m, 2H); 4.24 (overlapped s, 4H); 4.15 (m,
1H); 3.92 (s,
3H); 3.78 (s, 2H); 3.51 (m, 1H); 3.38 (m, 1H), 1.66 (br. s, 1H).
MS (ESI, m/z): 435.6 [M+H+]
Example 78: (2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-[(3R,6S)-6-(6-methoxy-
quinolin-4-yloxymethyl)-tetrahydro-pyran-3-yl]-amine:
781 (3R, 6S)-(6-hydroxymethyl-tetrahydro pyran-3 yl)-carbamic acid tert-butyl
ester:
To a solution of (3R,6S)-(6-hydroxyrnethyl-3,6-dihydro-2H-pyran-3-yl)-
carbarnic acid
tert-butyl ester (prepared as described in Example 1, step l.i, 1.3 g) in MeOH
(70 rnl) was
added 20% palladium hydroxide on charcoal (0.16 g). The mixture was stirred
under
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 127 -
hydrogen for 3 h. The catalyst was removed by filtration and the filtrate
concentrated in
vacuo. The residue was purified by column chromatography (EA-Hex 4-1) to
afford the title
product as a white solid (0.7 g).
1H NMR (CDC13) 8: 4.25 (br. s, 1H); 4.11 (m, 1H); 3.60 (dd, J= 3.4, 1 1.5 Hz,
2H); 3.53 (m,
1H); 3.37 (m, l H); 3.02 (t, J= 10.7 Hz, 1 H); 2.10 (m, l H); 1.83 (br. s, 1
H); 1.62 (m, 1 H);
1.49 (m, 1H); 1.44 (s, 9H); 1.32 (m, 1H).
78.ii. [(3R, 6S)-6-(6-methoxy-quinolin-4 yloxymethyl)-tetrahydro pyran-3ylJ-
carbamic acid
tert-butyl ester:
This compound (0.920 g) was prepared as a yellow solid, starting from
intermediate 78.i (1 g)
and 6-methoxy-quinolin-4-ol (0-907 g), according to the procedure described in
Example 77,
step 77.ii.
'H NMR (CDC13) 8: 8.60 (d, J = 5.4 Hz, 1H); 7.95 (d, J= 9.3 Hz, 1H); 7.45 (d,
J= 2.7 Hz,
1 H); 7.35 (dd, J= 2.7, 9.2 Hz, 1 H); 6.71 (d, J= 5.4 Hz, 1 H), 4.26 (rn, 2H);
4.15 (m, 211);
3.94 (s, 3H); 3.81 (m, 1H); 3.12 (t, J= 10.5 Hz, 1H); 2.20 (m, 1H); 1-94 (m,
2H); 1.68 (m,
1H); 1.45 (s, 9H).
78.iii. (3R,6S)-6-(6-methoxy-quinolin-4yloxyzethyl)-tetrahydropyran-3 ylamine:
A solution of intermediate 78 - ii (0.9 g) in TFA (9 ml) was stirred for 30
min at rt. The
reaction mixture was evaporated to dryness and the residue basified with 1N
aq. NaOH
solution and extracted six times with a mixture DCM-MeOH 9-1. The combined
organic
layers were dried over Na2SO4, filtered and concentrated to give a yello-w gum
(0.662 g).
'H NMR (CDC13) 8: 8.60 (d, J= 5.1 Hz, 1H); 7.93 (d, J= 9.0 Hz, 113); 7.45 (d,
J= 3.0 Hz,
1H); 7.34 (dd, J= 3.0, 9.0 Hz, 111); 6.71 (d, J= 5.1 Hz, 1H), 4.25 (dd, J=
6.0, 10.1 Hz, 1H);
4.12 (dd, J= 4.5, 10.1 Hz, 1H); 4.03 (m, 1H); 3.94 (s, 3H); 3.81 (m, 1I3);
3.13 (t, J= 10.5 Hz,
1H); 2.91 (m, 1H); 2.16 (m, 1H); 1.91 (m, 1H); 1.88 (br. s, 1H); 1.63 (rn,
1H); 1.38 (m, 1H).
78.iv. (2, 3-dihydro-benzo[1, 4Jdioxin-6 ylrnethyl)-[(3R, 6S)-6-(6-methoxy-
quinolin-
4 yloxymetlhyl)-tetrahydro pyran-3 ylJ-amine:
Starting from intermediate 78-iii (0.1 g) and 2,3-dihydro-benzojl,4]dioxine-6-
carbaldehyde
(0.064 g), the title compound (0.073 g) was prepared as a yellow foam
according to the
procedure described in Example 77, step 77.iv.
IH NMR (CDC13) S: 8.60 (d, J= 5.2 Hz, 1H); 7.93 (d, J= 9.2 Hz, 1H); 7.46 (d,
J= 2.9 Hz,
1H); 7.34 (dd, J= 2.9, 9.2 Hz, 111); 6.84 (m, 1H); 6.80 (m, 2H); 6.70 (d, J=
5.2 Hz, 1H);
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 128-
4.23 (s, 4H); 4.22 (dd, J= 5.9, 10.0 Hz, 1H); 4.12 (ddd, J= 2.3, 4.3, 10.9 Hz,
1H); 3.94 (s,
3H); 3.84 (m, 1H); 3.73 (d, J= 1.7 Hz, 2H); 3.20 (t, J= 10.6 Hz, 1H); 2.76 (m,
1H); 2.19 (m,
1H); 1.55 (m, 1H); 1.60 (m, 2H); 1.59 (br. s, 1H); 1.43 (m, 1H).
MS (ESI, m/z): 437.7 [M+H}].
Example 79: (2,3-dihydro-[1,4]dioxino[2,3-c]pyridin-7-ylmethyl)-
[(3R,6S)-6-(6-methoxy-quinolin-4-yloxymethyl)-tetrahydro-pyran-3-yl]-amine:
Starting from intermediate 78.iii (0.115 g) and 2,3-dihydro-[1,4]dioxino[2,3-
c]pyridine-
7-carbaldehyde (0.059 g), the title compound (0.109 g) was prepared as a white
foam
according to the procedure described in Example 77, step 77.iv.
MS (ESI, m/z): 438.6 [M+H}].
Example 80: 6-{[(3R, 6S)-6-(6-methoxy-quinolin-4-yloxymethyl)-tetrahydro-pyran-
3-ylamino]-methyl}-4H-benzo[1,4]oxazin-3-one:
Starting from intermediate 78.iii (0.1 g) and 3-oxo-3,4-dihydro-2H-
benzo[1,4]oxazine-
6-carbaldehyde (0.069 g), the title compound (0.088 g) was prepared as a pale
yellow solid
according to the procedure described in Example 77, step 77.iv.
MS (ESI, m/z): 450.5 [M+H+].
Example 81: (2,3-dihydro-benzo[1,4]dioxin-6-ylrnethyl)-[(3R,6S)-6-(6-methoxy-
[1,5] naphthyridin-4-yloxymethyl)-tetrahydro-pyran-3-yl]-amine:
81.i. [(3R, 6S)-6-(6-methoxy-[1,5]naphthyridin-4 yloxymethyl)-tetrahydropyran-
3 ylJ-
carbamic acid tert-butyl ester:
This compound (0.77 g) was obtained as a yellow solid from (3R,6S)-(6-
hydroxyniethyl-
tetrahydro-pyran-3-yl)-carbamic acid tert-butyl ester (1 g, prepared as
described in
Example 2, step 2.i), and 6-methoxy-[1,5]naphthyridin-4-ol (0.913 g),
according to the
procedure of Example 77, step 77.ii.
MS (ESI, m/z): 390.2 [M+H+].
81. ii. (3A6S)-6-(6-methoxy-[1, 5]naphthyridin-4 yloxymethyl)-tetrahydro pyran-
3 ylafrzine:
Starting from intermediate 81.i (0.764 g), the title cornpound (0.669 g) was
prepared as a pale
yellow oil according to the procedure described in Example 77, step 77.iii.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-129-
MS (ESI, m/z): 290.4 [M+H+].
81. iii. (2, 3-dihydro-benzo[1, 4]dioxin-6-ylmethyl)-[(3R, 6S)-6-(6-methoxy-
[], .SJnaphthyridin-
4yloxymethyl)-tetrahydro pyran-3 ylJ-amine:
Starting from intermediate 81.ii (0.1 g) and 2,3-dihydro-benzo[1,4]dioxine-6-
carbaldehyde
(0.064 g), the title compound (0.056 g) was prepared as a pale yellow foam
according to the
procedure described in Example 77, step 77.iv.
1H NMR (CDC13) S: 8.60 (d, J= 5.2 Hz, 1H); 8.15 (d, J= 9.1 Hz, 1H); 7.1 1(d,
J= 9.1 Hz,
1H); 6.92 (d, J= 5.2 Hz, 1H); 6.80 (m, 3H); 4.29 (dd, J= 5.7, 10.0 Hz, lI-i);
4.24 (s, 4H);
4.14 (m, 1H); 4.10 (s, 3H); 3.87 (m, 1H); 3.73 (d, J= 1.2 Hz, 2H); 3.20 (t,
..J= 10.6 Hz, 1H);
2.76 (m, 1 H); 2.18 (m, 1 H); 1.90 (m, 1 H); 1.64 (br. s, 1 H); 1.63 (m, 2H);
1.42 (m, 1H).
MS (ESI, m/z): 438.6 [M+H+].
Example 82: 6-{[(3R,6S)-6-(6-methoxy-[1,5]naphthyridin-4-yloxymethyl)-
tetrahydro-
pyran-3-ylamin o]-methyl}-4H-benzo [1,4] oxazin-3-one:
Starting from intermediate 81.ii (0.1 g) and 3-oxo-3,4-dihydro-2H-
benzo[1,4]oxazine-
6-carbaldehyde (0.069 g), the title compound (0.069 g) was prepared as a white
solid
according to the procedure described in Example 77, step 77.iv.
MS (ESI, m/z): 451.5 [M+H+].
Example 83: 6-{[(3R,6b')-6-(6-methoxy-quinolin-4-yloxymethyl)-tetrabydro-pyran-
3-ylamino]-met hyl}-4H-benzo [1,4] thiazin-3-one:
Starting from intermediate 78.iii (0.11 g) and 3-oxo-3,4-dihydro-2H-
benzo[1,4]thiazine-
6-carbaldehyde (0.081 g), the title compound (0.056 g) was prepared as a
yellowish solid
according to the procedure described in Example 77, step 77.iv.
MS (ESI, m/z): 466.3 [M+H+].
Example 84: 6-{[(3R,6S)-6-(6-methoxy-[1,5]naphthyridin-4-yloxymethyl)-
tetrahydro-
pyran-3-ylamino]-methyl}-4H-benzo[1,4]thiazin-3-one:
Starting from intermediate 81.ii (0.1 g) and 3-oxo-3,4-dihydro-2H-
benzo[1,4]thiazine-
6-carbaldehyde (0.074 g), the title compound (0.091 g) was prepared as a white
solid
according to the procedure described in Example 77, step 77.iv.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-130-
MS (ESI, mlz): 467.3 [M+H+].
Example 85: 6-{[(3R,6S)-6-(6-methoxy-[1,5]naphthyridin-4-yloxymethyl)-
tetrahydro-
pyran-3-ylamino]-methyl}-4H-pyrido[3,2-b] [1,4]thiazin-3-one:
The title compound (0.086 g) was obtained as a white solid starting from
intermediate 81.ii
(0.1 g) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde
(0.073 g) using
the procedure described in Example 77, step 77.iv
MS (ESI, nz/z): 467.3 [M+H+].
Example 86: [(3R,6S)-6-(6-methoxy-[1,5]naphthyridin-4-yloxymethyl)-tetrahydro-
pyran-3-yl] -(3-phenyl-allyl)-amine:
The title compound (0.064 g) was obtained as a colourless oil starting from
intermediate 81.ii
(0.130 g) and trans-cinnamaldehyde (0.062 ml) using the procedure described in
Example 77,
step 77.iv.
MS (ESI, in/z): 406.5 [M+H+].
Example 87: benzofuran-2-ylmethyl-[(3R,6S)-6-(6-methoxy-[1,5]naphthyridin-
4-yloxymethyl)-tetrahydro-pyran-3-yl]-amine:
The title compound (0.072 g) was obtained as a colourless oil starting from
intermediate 81.ii
(0.130 g) and 2-benzofuran-carbaldehyde (0.06 ml) using the procedure
described in Example
77, step 77 . iv.
MS (ESI, rn/z): 420.2 [M+Hj.
Example 88: (2S,5R)-5-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-
tetrahydro-
pyran-2-carboxylic acid (2-methyl-quinolin-8-yl)-amide:
88.i. 2-methyl-quinolin-8 ylamine:
To a solution of 2-methyl-8-nitroquinoline (5 g) in MeOH (200 ml) and EA (50
ml) was
added 10% palladium on charcoal (1.6 g). The reaction was stirred under
hydrogen
atmosphere for 4 h. The catalyst was removed by filtration and the filtrate
was concentrated in
vacuo to afford after drying the title amine (3.94 g) as a red solid.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 131 -
1H NMR (CDC13) 6: 7.94 (d, J= 8.4 Hz, IH); 7.25 (m, 2H); 7.10 (dd, J= 1.3, 8.2
Hz, IH);
6.90 (dd, J= 1.3, 7.4 Hz, IH); 4.90 (br s, 2H); 2.71 (s, 3H).
MS (ESI, m/z): 159.3 [M+H+].
88.ii. [(3R,6S)-6-(2-methyl-quinolin-8ylcarbamoyl)-tetrahydropyran-3ylJ-
carbamic acid
tert-butyl ester:
To a solution of (2S,5R)-5-tert-butoxycarbonylamino-tetrahydro-pyran-2-
carboxylic acid
(0.5 g, prepared as described in Eur. J. Org. Chem. (2003), 2418-2427), DIPEA
(0.338 ml)
and HATU (0.776 g) in DMF (4 ml) was added 2-methyl-quinolin-8-ylamine (0.322
g). The
reaction was stirred at rt for 3 d. DMF was removed under reduced pressure and
the residue
was partitioned between water (100 ml) and EA (100 ml). The aq. layer was
extracted with
EA (100 rnl) and the combined extracts were washed with brine and dried over
Na2SO4. After
filtration and concentration to dryness, the residue was purified by
chromatography (EA-Hex
1-3) to afford the title compound (0.58 g) as a yellowish solid.
MS (ESI, m/z): 386.6 [M+H+].
88.iii. (2S5R)-5-amino-tetrahydro pyran-2-carboxylic acid (2-methyl-quinolin-8
yl)-amide:
A solution of intermediate 88.ii (0.58 g) in TFA (4 ml) was stirred at rt for
20 min. The
solvent was removed under reduced pressure and the residue was partitioned
between 1M aq.
NaOH (3 0 ml) and DCM-MeOH (9-1, 50 ml). The aq. layer was extracted twice
more with
the same solvent mixture. The extracts were washed with brine, dried over
Na2SO4 and
filtered. The filtrate was evaporated to dryness. The residue was triturated
with heptane and
the solid was dried under HV to yield the title amine (0.2 g) as a yello-wish
solid.
1H NMR (d6-DMSO) 6: 10.69 (s, 1H); 8.63 (dd, J= 1.5, 8.0 Hz, 113); 8.28 (d, J=
8.4 Hz,
IH); 7.62 (dd, J= 1.5, 8.0 Hz, 1H); 7.52 (d, J= 8.4 Hz, 1H); 7.50 (t, J= 8.0
Hz, 1H);
4.04 (ddd, J= 1.8, 4.5, 10.4 Hz, 1H); 3.96 (dd, J= 2.5, 11.5 Hz, 1H); 3.13 (t,
J= 10.6 Hz,
1H); 2.72 (overlapped m, IH); 2.71 (s, 3H); 2.13 (qd, J= 3.0, 13.1 Hz, 1H);
2.0 (m, IH);
1.48 (m, 1H); 1.47 (overlapped br s, 2H); 1.33 (m, IH).
MS (ESI, m/z): 286.4 [M+Hr].
88.iv. (2S, 5R)-5-[(2, 3-dihydro-benzo[1, 4]dioxin-6 ylmethyl)-arninoJ-
tetrahydro pyran-
2-carboxylic acid (2-methyl-quinolin-8 yl)-amide:
To a solution of intermediate 88.iii (0.1 g) in 1,2-DCE (6 ml) and MeOH (2 ml)
were added
2,3-dihydro-benzo[1,4]dioxine-6-carbaldehyde (0.064 g) and powdered 3 A
molecular sieves
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 132 -
(2 g). The resulting mixture was stirred at rt overnight. NaBIL (0.1 g) was
added and the
mixture was stirred at rt for 1 h. The reaction mixture was filtered through a
plug of
Hydromatrix (pretreated with NaHCO3). After concentration in vacuo, the
residue was
chromatographed (DCM-MeOH 9-1, 1% concentrated NH4OI3) to afford the title
compound
(0.055 g) as a thick oil.
'H NMR (CDC13) 8: 11.5 (s, 1H); 8.75 (m, 1H); 8.03 (d, J = 8.4 Hz, 1H); 7.46
(m, 2H);
7.32 (d, J= 8.4 Hz, 1H); 6.85 (m, 3H); 4.34 (ddd, J= 2.0, 4.5, 11.5 Hz, 1H);
4.25 (s, 4H);
4.00 (dd, J= 2.5, 11.5 Hz, 1H); 3.77 (dd, AB system, J= 13.0 Hz, 2H); 3.29 (t,
J= 10.5 Hz,
1H); 2.84 (m, 1H); 2.77 (s, 3H); 2.38 (qd, J= 2.9, 13.4 Hz, 1H); 2.22 (m, 1H);
1.67 (m, 1H);
1.50 (br s, 1H); 1.44 (m, 1H).
MS (ESI, m/z): 434.5 [M+H+].
Example 89: (2S,5R)-8-{5-[(3-oxo-3,4-dihydro-2H-pyrido[3,2-b] [1,4]thiazin-
6-ylmethyl)-amino]-tetrahydro-pyran-2-ylmethoxy}-quinolii ne-2-carbonitrile:
Starting from 8-(5-amino-tetrahydro-pyran-2-yhnethoxy)-quinoline-2-
carbonitrile (0.076 g)
and 3-oxo-3,4-dihydro-2Fl-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.057 g),
the title
compound (0.068 g) was prepared as a beige foam according to the procedure
described in
Example 88, step 88.iv.
'H NMR (CDC13) 8: 9.27 (br.s, 1H); 8.61 (br.s, 1H); 8.24 (d, J= 8.4 Hz, 1H);
7.75 (d,
J= 8.4 Hz, 1H); 7.62 (d, J= 2.3 Hz, 1H); 7.58 (dd, J= 1.1, 7-9 Hz, 1H); 7.44
(d, J= 8.4 Hz,
1H); 7.18 (dd, J= 1.1, 7.9 Hz, 1H); 6.99 (dd, J= 3.5, 7.9 Hz, 1H); 4.28 (dd,
J= 6.1, 10.1 Hz,
1H); 4.18 (m, 2H); 3.89 (d, J= 3.7 Hz, 2H); 3.47 (s, 2H); 3.28 (t, J= 10.8 Hz,
1 H); 2.80 (m,
1H); 2.21 (m, 1H); 1.98 (m, 1H); 1.60 (br. m, 3H).
MS (ESI, m/z): 462.2 [M+H+].
Example 90: (2S,5R)-5-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-
tetrahydro-
pyran-2-carboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-amide:
90.i. (3R,6S)-(6-carbamoyl-tetrahydropran-3 yl)-carbamic acid tert-butyl
ester:
To a solution of (2S,5R)-5-tert-butoxycarbonylamino-tetra.hydro-pyran-2-
carboxylic acid
(3 g) in EA (50 ml) was added NHS (1.5 g) and DCC (2.7 g). The reaction was
stirred
overnight at rt. The solids were removed by filtration. The filtrate was
concentrated in vacuo
and the residue was taken up in THF (180 ml). NH3 was bubbled through the
solution for 10
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-133-
min, and the resulting turbid mixture was stirred at rt for 1 h. Silica gel
(20 g)l was added in
the mixture, and the volatiles were removed by rotatory evaporation. The
material was
chromatographed over silica gel (DCM-MeOH 19-1) to afford the title compouund
(1.3 g) as a
white solid.
MS (ESI, mlz) : 245.3 [M+H+].
90.ii. [(3R,6S)-6-(6-methoxy-[1,5]naphthyridin-4 ylcarbamoyl)-tetrahydro pyran-
3 ylJ-
carbamic acid tert-butyl ester:
To a mixture of intermediate 90.i (0.8 g), cesium carbonate (1.3 g), rac-BINAP
(0.145 g) and
tris(dibenzilideneacetone) dipalladium(0)-chloroform complex (0.057 g) was
added dioxane
(41 ml). The mixture was sonicated 15 min and trifluoro-methanesulfonic acid 6-
methoxy-
[1,5]naphthyridin-4-yl ester (1.0 g) was added. The mixture was heated at 1 0
C overnight.
After filtration, the filtrate was concentrated to dryness and the residue was
purified over
silica gel (DCM-MeOH 19-1) to afford the title amide (1.3 g) as a foam.
1H NMR (CDC13) 8: 10.57 (s, 1H); 8.70 (d, J= 5.2 Hz, 1H); 8.51 (d, J= 5.2 H::,-
,, 1H); 8.22 (d,
J= 9.0 Hz, 1H); 7.16 (d, J= 9.0 Hz, 1H); 4.32 (m, 2H); 4.12 (s, 3H); 4.0 1
(dd, J= 2.5,
11.4 Hz, 1H); 3.72 (m, 1H); 3.23 (t, J= 10.6 Hz, 1H); 2.39 (qd, J= 2.8, 10.2
Hz, 1H);
2.22 (m. 111); 1.76 (m, 1H); 1.47 (overlapped m, 1H); 1.47 (s, 9H).
MS (ESI, tn/z) : 403.6 [M+W].
90.iii. (2S,5R)- 5-amino-tetrahydro pyran-2-carboxylic acid (6-methoxy-
[1,5]naphthyridin-
4 yl)-amide :
The title arnine (0.5 g) was obtained as a white solid, starting from
intermediate 90.ii (1.3 g)
and using the procedure described in Example 88, step 88.iii. The compound -
Nvas purified by
chromatography (DCM-MeOH 19-1, 1% concentrated aq. NH4OH).
MS (ESI, m/z): 303.2 [M+H+].
90.iv. (2S, SR)-5-[(2, 3-dihydro-benzo[1, 4]dioxin-6 ylmethyl)-aminoJ-
tetrahydw-o pyran-
2-carboxylic acid (6-methoxy-[1,5]naphthyridin-4 yl)-amide:
As described in Example 88, step 88.iv, the title compound (0.024 g) was
obtained as a
yellow gum, starting from intermediate 90.iii (0.1 g) and 2,3-dihydro-
benzo[1,4]dioxine-
6-carbaldehyde (0.059 g).
MS (ESI, m/z): 451.6 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 134 -
Example 91: (2S,5R)-5-[(2,3-dihydro-[1,4]dioxino[2,3-c]pyridin-7-ylmethyl)-
amino]-
tetrahydro-pyran-2-carboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-amide:
As described in Exanzple 88, step 88.iv, the title compound (0.089 g) was
obtained as a white
solid, starting from intermediate 90.iii (0.11 g) and 2,3-dihydro-
[1,4]dioxino[2,3-c]pyridine-7-
carbaldehyde (0.054 g).
'H NMR (d6-DMSO) S: 10.51 (s, 1H); 8.70 (d, J= 5.0 Hz, IH); 8.38 (d, J= 5.0
Hz, 1H);
8.28 (d, J= 9.0 Hz, 1 H); 7.32 (d, J= 9.0 Hz, 1 H); 7.25 (d, J= 7.9 Hz, IH);
6.97 (d,
J= 7.9 Hz, 1H); 4.38 (m, 2H); 4.22 (m, 2H); 4.18 (partially overlapped dd, J=
3.0, 10.6 Hz,
1H); 4.07 (partially overlapped m, 1H); 4.06 (s, 3H); 3.67 (dd, AB system, J=
14.6 Hz, 2H);
3.25 (t, J= 10.6 Hz, 1 H); 2.61 (m, 1H); 2.15 (m, 3H); 1.56-1.36 (m, 2H).
MS (ESI, m/z): 452.5 [M+W].
Example 92: (2S,5R)-5-[(3-oxo-3,4-dihydro-2H=pyrido[3,2-b] [1,4] thiazin-6-
ylmethyl)-
amino]-tetrahydro-pyran-2-carboxylic acid (6-methoxy-[1,5] naphthyridin-4-yl)-
amide:
92.i. [(3R,6S)-6-(6-methoxy-[1,5]naphthyridin-4 ylcarbamoyl)-tetrahydropyran-3
ylJ-
carbamic acid tert-butyl ester:
To a solution of (2S,5R)-5-tert-butoxycarbonylamino-tetrahydro-pyran-2-
carboxylic acid
(0.5 g), DIPEA (0.338 mL) and HATU (0.776 g) in DMF (4 ml) was added 6-methoxy-
[1,5]naphthyridin-4-ylamine (0.357 g) in DMF (4 ml). The reaction mixture was
stirred for
24 h at rt and the solvent was evaporated under reduced pressure. The; residue
was partitioned
between DCM and water and the phases separated. The aq. layer was extracted
twice more
with DCM. The combined organic layer were dried over Na2SO4, filtered and
concentrated in
vacuo. The residue was chromatographed (DCM-MeOH 19-1) to afford the title
product as a
yellow foam (0.614 g).
MS (ESI, m/z): 403.3 [MH+].
92.ii. (2S,5R)-5-amino-tetrahydro pyran-2-carboxylic acid (6-methoxy-
[1,5Jnaphthyridin-
4 yl)-amide:
The title amine (0.3 60 g) was obtained as a yellow foam, starting from
intermediate 92. i
(0.608 g) and using the procedure described in Example 88, step 88.iii.
MS (ESI, m/z): 303.4 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 135 -
92.iii. (2S, 5R)-5-[(3-oxo-3, 4-dihydro-2H pyrido[3, 2-bJ[l, 4]thiazin-
6ylmethyl)-aminoJ-
teti~ahydro pyran-2-carboxylic acid (6-methoxy-[1,5]naphthyridin-4 yl)-amide:
As described in Example 88, step 88.iv, the title compound (0.064 g) was
obtained as a white
solid, starting from intermediate 92.ii (0.145 g) and 3-oxo-3,4-dihydro-2H-
pyrido[3,2-
b] [1,4]thiazine-6-carbaldehyde (0.085 g).
MS (ESI, m/z): 481.6 [M+H+].
Example 93: (2S,5R)-5-[(2,3-dihydro-[1,4]dioxino[2,3-c]pyridin-7-ylmethyl)-
amino]-
tetrahydro-pyran-2-carboxylic acid (2-cyano-quinolin-8-yl)-amide:
93.i. [(3R, 6S)-6-(2-methoxy-quinolin-8 ylccrrbamoyl)-tetrahydropyran-3 ylJ-
carbarnic acid
te 7-t-butyl ester:
The title amide (0.780 g) was obtained as an orange foam, starting from
intennediate 90.i
(0.489 g) and trifluoro-methanesulfonic acid 2-cyano-quinolin-8-yl ester
(0.604 g) and using
the procedure described in Example 90, step 90.ii.
MS (ESI, m/z): 397.2 [M+H+].
93.ii. (2S,5R)-5-amino-tetrahydropyran-2-carboxylic acid (2-cyano-quinolin-
8yl)-amide:
The title amine (0.32 g) was obtained as a, colourless foam, starting from
intermediate 93.i
(0.780 g) and using the procedure described in Example 88, step 88.iii). The
compound was
purified by chromatography over silica gel (DCM-MeOH 19-1, 1% concentrated aq.
NH40H).
MS (ESI, m/z): 297.3 [M+H+].
93.iii. (2S,5R)-5-[(2,3-dihydro-[1,4]dioxino[2,3-cJpyridin-7 ylmethyl)-aminoJ-
tetrahydro-
pyran-2-carboxylic acid (2-cyano-quinolin-8yl)-amide:
The title compound (0.074 g) was obtained as a yellow foam, starting from
intermediate 93.ii
(0.11 g) and 2,3-dihydro-[1,4]dioxino[2,3-c]pyridine-7-carbaldehyde (0.067 g)
according to
the procedure described in Example 88, step 88.iv.
MS (ESI, m/z): 446.1 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 136 -
Exanz ple 94: (2S,5R)-5-[(2,3-dihydro-benzo [1,4] dioxin-6-ylmethyl)-amino]-
tetrahydro-
pyran-2-carboxylic acid (2-cyano-quinolin-8-yl)-amide:
The title compound (0.065 g) was obtained as a colourless foam, starting from
interrnediate
93.ii (0.11 g) and 2,3-dihydro-benzo[1,4]dioxine-6-carbaldehyde (0.064 g)
according; to the
procedure described in Example 88, step 88.iv.
MS (ESI, m/z): 445.4 [M+H}].
Exanple 95: (2S,5R)-5-[(3-oxo-3,4-dihydro-2H-pyrido[3,2-b] [1,4]thiazin-6-
ylmetliyl)-
amino]-tetrahydro-pyran-2-carboxylic acid (2-cyano-quinolin-8-yl)-amide:
The title compound (0.065 g) was obtained as a colourless foam, starting from
interrnediate 93.ii (0.11 g) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-
b][1,4]thia.zine-6-
carbaldehyde (0.064 g) according to the procedure described in Example 88,
step 88.iv_
'H NMR (CDC13) 6: 10.52 (s, 1H); 8.93 (dd, J= 1.2, 8.0 Hz, 1H); 8.34 (d, J=
8.4 BEz, 1H);
8.33 (br s, 1H); 7.77 (d, J= 8.4 Hz, 1 H); 7.70 (t, J= 8.0 Hz, 1H); 7.60 (m,
2H); 7.01 (d,
J= 8_ 0 Hz, 1H); 4.42 (ddd, J= 2. 0, 4.5, 11.0 Hz, 1H); 4.03 (dd, J= 2.3, 11.3
Hz, 1 H); 3.93 (s,
2H); 3.49 (s, 2H); 3.34 (t, J= 10.6 Iiz, 1H); 2.86 (m, 1H); 2.39 (m, 1 H);
2.26 (rn, 1 H);
1.82 (br,s, 1H); 1.68 (m, 1H); 1.54 (m, 1H).
MS (ESI, m/z): 475.5[M+H+].
Example 96: (2S,5R)-5-[(3-oxo-3,4-dihydro-2H-benzo[1,4]thiazin-6-ylmethyl)-
annino]-
tetrabydro-pyran-2-carboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-amide:
As described in Example 88, step 88.iv, the title compound (0.11 g) was
obtained as a white
foam, starting from intermediate 90.iii (0.1 g) and 3-oxo-3,4-dihydro-2H-
benzo[1,4]ti-iiazine-
6-carbaldehyde (0.07 g).
MS (ESI, m/z): 480.5 [M+H}].
Example 97: (2S,5R)-5-[(2,3-dihydro-[1,4]dioxino[2,3-b]pyridin-6-ylmethyl)-
amino]-
tetraliydro-pyran-2-carboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-amide:
As described in Example 88, step 88.iv, the title compound (0.1 g) was
obtained as a white
foam_, starting from intermediate 90.iii (0.1 g) and 2,3-dihydro-
[1,4]dioxino[2,3-b]pyridine-
6-carbaldehyde (0.06 g).
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-137-
MS (ESI, m/z): 452.5 [M+H+].
Example 98: 2-[(2R,3R,6S)-3-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-
6-(6-methoxy-q uinolin-4-yloxymethyl)-tetrahydro-pyran-2-yl]-ethanol :
98.i. (2R, 3R, 6S) -[6-(tert-butyl-dimethyl-silanyloxymethyl)-2-(2-hydroxy-
ethyl)-3, 6-dihydY-o-
2H-pyran-3 ylJ-carbamic acid tert-butyl ester:
A mixture of 2-rnethyl-2-propanol (185 ml) and water (185 ml), containing AD
mix (3 (50 g)
was stirred at rt until two clear phases were obtained. After cooling to 0 C,
(2R,3R,6S')-[2-allyl-6-(tert-butyl-diinethyl-silanyloxymethyl)-3,6-dihydro-2H-
pyran-3-yl] -
carbamic acid tert-butyl ester (obtained as described in Eur. J. Org. Chem.
(2003),
2418-2427, 12.8 g) was added, and the reaction mixture was stirred at the same
temperature
for 14 h. Sodiurri metabisulfite (54 g) was added portion wise and the mixture
was stirred 1 h.
The two layers were decanted and the aq. layer was extracted with EA (3 x 150
ml). The
combined organic layers were washed with brine, dried over Na2SO4, filtered
and
concentrated to dryness. The residue was taken up in acetone (160 ml) and a
solution of
sodium periodate (10.4 g) in hot water (30 ml) was quickly added. After
stirring for 1 h at rt,
the solids were removed by filtration, and the filtrate was concentrated in
vacuo. The residue
was taken up in MeOH (150 ml) and NaBH4 (2 g) was added. After stirring 1 h at
rt, vvater
(100 ml) was added and the volatiles were removed by evaporation. The residue
was extracted
with EA (3 x 150 ml). The combined extracts were washed with brine and dried
overNa2SO4,
filtered and concentrated in vacuo. The residue was purified over silica gel
(EA-Hex 3-1) to
afford the title alcohol (4 g) as a colourless oil.
'H NMR (CDC13) &: 6.0 (ddd, J= 2.1, 6.0, 10.2 Hz, 1H); 5.82 (dd, J= 3.0, 10.2
Hz, 1H);
4.62 (m, 1H); 4.23 (m, 1H); 4.09 (m, 1H); 3.91 (m, 1H); 3.80 (m, 1H), 3.62
(dd, J= 4.5,
11.1 Hz, 1H); 1.98 (broad s, 2H); 1.77 (m, 2H); 1.43 (s, 9H), 0.91 (s, 911),
0.09 (s, 611).
98.ii. (2R, 3R, 6S)-{6-hydroxymethyl-2-[2-(tetrahydro pyran-2 yloxy)-ethylJ-3,
6-dihydro-
2H-pyran-3 yl}-carbamic acid tert-butyl ester:
To a solution of intermediate 98.i (4 g) in DCM (80 ml) was added PTSA (0.083
g). After
stirring for 15 min, 3,4-dihydro-2H-pyran (2.3 mL) was added dropwise. The
reactiorn was
stirred at rt for 90 min. 1MNaHCO3 (10 ml) was added and the two layers were
sepa.rated.
The organic layers were collected, washed with brine, dried over Na2SO4,
filtered and
concentrated in vacuo. The residue was resuspended in THF (100 ml) and 1M TBAF
in THF
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 138 -
(13 ml) was added. The reaction mixture was stirred for 22 h and the volatiles
were removed
under reduced pressure. The residue was purified by chromatography over silica
gel (EA-Hex
3-1) to afford the title alcohol (2.7 g) as an oil.
1H NMR (CDC13) 8: 6.02 (m, 1H); 5.77 (m, 1H); 4.70 (m, 1H); 4.59 (m, 0.5H);
4.46 (m,
0.5H); 4.26 (m, 1H); 4.15 (m, 114); 3.98-3.70 (m, 4H); 3.5 8-3.70 (m, 2.5H);
2.99 (broad s,
0.5H); 1.86 (m, 4H); 1.54 (m, 4H); 1.47 (s, 9H).
98.iii. (2R,3R,6S)-{6-(6-methoxy-quinolin-4-yloxymethyl)-2-[2-(tetrahydro-
pyran-2-yloxy)-
ethyl]-3,6-dihydro-21Y pyran-3-yl}-carbamic acid tert-butyl ester
To an ice-chilled solution of intermediate 98.ii (2.7 g) in THF (80 ml) were
added
successively 6-methoxyquinolin-4-ol (1.32 g), PPh3 (3.96 g) and DIAD (3 ml).
The reaction
was stirred at rt for 14 h. Silica gel (20 g) was added and the volatiles were
removed under
reduced pressure. This material was chromatographed over silica gel (EA-MeOH
19-1) to
afford the title compound (1.6 g) as a brownish oil.
MS (ESI, m/z): 516.4 [M+H+].
98.iv. (2R, 3R, 6S)-[2-(2-hydroxy-ethyl)-6-(6-methoxy-quinolin-4 yloxymethyl)-
3, 6-dihydro-
2H-pyran-3 ylJ-carbamic acid tert-butyl ester:
To a solution of intermediate 98.iii (1.6 g) in MeOH (40 rnl) was added PTSA
(0.8 g). The
reaction mixture was refluxed for 36 h. After cooling, K2C03 (1 g) was added
and the solvent
was removed in vacuo. The residue was partitioned betweern water (100 ml) and
EA (100 ml).
The aq. layer was extracted with EA (100 ml) and the conzbined extracts were
washed with
brine and dried over Na2SO4. After evaporation, the residue was
chromatographed over silica
gel (DCM-MeOH 19-1) to afford the title alcohol (0.7 g) as a foam.
1H NMR (CDC13) S: 8.61 (d, J= 5.1 Hz, 1H); 7.86 (d, J= 9.1 Hz, 1H); 7.47 (d,
J= 2.8 Hz,
IH); 7.37 (dd, J= 2_ 8, 9.1 Hz, 1H); 6.71 (d, J= 5.1 Hz, 1H); 6.20 (ddd, J=
2.1, 5.9, 10.3 Hz,
1H); 6.00 (dd, J= 3.0, 10.3 Hz, 1H); 4.80 (m, 2H); 4.38 (dd, J= 8.1, 10.4 Hz,
1H); 4.22 (dd,
J= 3.8, 10.4 Hz, 211); 4.12 (m, 1H); 3.94 (s, 3H); 3.78 (m, 2H); 2.68 (br s,
1H); 1.85 (m, 2H);
1.46 (s, 9H).
MS (ESI, m/z): 431.4 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 139 -
98.v. (2R, 3R, 6S)-[2-(2-hyd.-oxy-ethyl)-6-(6-methoxy-quinotin-4 yloxymethyl)-
tetrahydro-
pyran-3 ylJ-carbamic acid tert-butyl ester:
To a solution of intermediate 98.iv (0.7 g) in EA (14 rn1) was added 10%
palladium on
charcoal (0.5 g). The reaction was stirred for 6 h under hydrogen atmosphere
and the catalyst
was removed by filtration. The filtrate was concentrated in vacuo to afford
the title compound
(0.45 g) as a white solid.
MS (ESI, m/z): 433.4 [M+H+].
98.vi. 2-[(2R, 3R, 6S)-3-amirro-6-(6-methoxy-quinolin-4 yloxymethyl)-
tetrahydro pyran-2 ylJ-
ethanol:
The title amine (0.36 g) was obtained as a white solid starting from
intermediate 98.v (0.45 g)
and using the procedure described in Example 12, step 12. iii. The compound
was purified by
chromatography (DCM-MeOH 9-1, 1 1o concentrated NH40H).
MS (ESI, m/z): 333.2 [M+H+].
98.vii. 2-[(2R, 3R, 6S)-3-[(2, 3-dihydro-benzo[1, 4]dioxin-6 ylmethyl)-aminoJ-
6-(6-methoxy-
quinolin-4yloxymethyl)-tet7-ahydro pyran-2 ylJ-ethanol:
To a solution of intermediate 98.vi (0.1 g) in 1,2-DCE (6 inl) and MeOH (2 ml)
were added
powdered 3 A molecular sieves (2 g) and 2,3-dihydro-benzo[1,4]dioxine-6-
carbaldehyde
(0.054 g). The reaction mixture was stirred at rt overnight and NaBH4 (0.1 g)
was added.
After stirring for 2 h, the reaction mixture was filtered through a plug of
Hydromatrix
(pretreated with NaHCO3) - After concentration in vacuo, the residue was
chromatographed
over silica gel (DCM-MeOH 19-1, 1% concentrated aq. NH4OH) to afford the title
compound
(0.075 g) as a white foam.
'H NMR (d6-DMSO) 8: 8.55 (d, J= 5.1 Hz, 1H); 7.85 (d, J= 9.1 Hz, 1H); 7.44 (d,
J= 2.8 Hz, 1H); 7.37 (dd, J= 2.8, 9.1 Hz, IH); 6.94 (d, J= 5.1 Hz, 1H); 6.84
(s, 1H); 6.78 (s,
2H); 4.43 (broad s, 1H); 4.21 (s, 4H); 4.19 (partially overlapped m, 1H); 4.04
(m, 3H);
3.90 (s, 3H); 3.68 (m, 2H); 3.58 (s, 2H); 2.71 (m, 1H); 2.04 (m, 2H); 1.70 (m,
3H); 1.58 (m,
1H); 1.36 (m, 1H).
MS (ESI, m/z): 481.6 [M+]H+]
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 140 -
Example 99: 6- { [(2R,3R,6S')-2-(2-hyd roxy-ethyl)-6-(6-methoxy-quinolin-
4-yloxymethyl)-tetrahydro-pyran-3-ylamino]-methyl}-4H-benzo [1,4] thiazi n-3-
one:
The title compound (0.05 g) was obtained as a yellowish solid, from
intermediate 98.vi (0.1 g)
and 3-oxo-3,4-dihydro-2H-benzo[1,4]thiazine-6-carbaldehyde (0.064 g), using
the procedure
described in Example 98, step 98.vii.
MS (ESI, m/z): 510.5 [M+H+].
Example 100: 6-{[(2R,3R,6S')-2-(2-lhydroxy-ethyl)-6-(6-methoxy-quinolin-
4-yloxymethyl)-tetrahydro-pyran-3-ylamino]-methyl}-4H-pyrido[3,2-b]
[1,4]thiazin-
3-one:
The title compound (0.10 g) was obtained as a yellowish solid, from
intermediate 98.vi (0.1 g)
and 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.064 g),
using the
procedure described in Example 98, step 98.vii.
MS (ESI, m/z) : 511.5 [M+H+].
Example 101: 3-oxo-3,4-dihydro-2ll-pyrido[3,2-b] [1,4]thiazine-6-carboxylic
acid
{(1 a,5a,6a)-3-[(2R)-2-hydroxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-3-aza-
bicyclo [3.1.0] hex-6-yl}-amide:
To a mixture of intermediate 47.vi and 3-oxo-3,4-dihydro-2H-pyrido[3,2-
b][1,4]thiazine-6-
carboxylic acid (0.110 g, 0.52 mmol) in DMF (2.5 ml) and DCM (2.5 ml) were
added DIPEA
(0.28 ml) and HATU (0.225 g, 0.59 mmol). The reaction was stirred at rt for 2
h. The solvents
were evaporated and the crude mixture was chromatographed over silica gel (DCM-
MeOH
17-3 containing 1% aq. NH4OH) affording the title amide (0.06 g, 0.12 mmol) as
a beige
foam.
MS (ESI, m/z): 492.3 [M+H+].
Example 102: 6-({(1 a,5 c;6 a)-3- [(2R)-2-hydroxy-2-(3-methoxy-quinolin-5-yl)-
ethyl]-
3-aza-bicyclo[3.1.0]hex-6-ylamino}-methyl)-4H-benzo[1,4]oxazin-3-one:
Starting from intermediate 47.vi (0.100 g) and 3-oxo-3,4-dihydro-2H-
berizo[1,4]oxazine-
6-carbaldehyde (0.065 g), the title compound (0.074 g) was prepared as a white
foam
according to the procedure describecl in Example 88, step 88.iv.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 141 -
MS (ESI, m/z): 461.4 [M+H+].
Example 103: rac-2-{(1 a,5a,6a)-3-[2-hydroxy-2-(6-methoxy-quinolin-4-yl)-
ethyl]-
3-aza-bicyclo [3.1.0] hex-6-ylamino}-N-th iazol-2-yl-acetamide:
To a solution of rac-(1 a,5 a,6a)-6-amino-3-aza-bicyclo[3.1.0]hex-3-yl)-1-(6-
methoxy-
quinolin-4-yl)-ethanol (0.400 g) in DMF (5 ml) were added DIPEA (0.465 irxl)
and 2-bromo-
N-thiazol-2-yl-acetamide (0.455 g). The resulting solution was heated at 80 C
for 2.5 h. The
reaction mixture was concentrated to dryness and purified by column
chrorrnatography over
silica gel (DCM-MeOH 19-1, 1% aq. NIi4OH) to afford the title compound as a
yellow foam
(0.581 g). The compound of 77% purity was contaminated with some diallcyla.ted
compound.
MS (ESI, m/z): 440.5 [M+H}].
Example 104: rac-(la,5a,6a)-2-{6-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-
amino]-
3-aza-bicyclo [3.1.0] hex-3-yl}-1-(3-methoxy-quinoxalin-5-yl)-ethanol:
104.i. 2-cyano-N-(2-methyl-6-nitro phenyl)-acetamide:
To a solution of 2-methyl-6-nitroaniline (25 g, 164.3 minol) in benzene (20c)
ml) were added
cyanoacetic acid (14.5 g, 170.46 mmol) and PCl5 (35 g, 168 mmol). The reaction
mixture was
heated at 60 C for 7 h. After cooling to rt, the reaction mixture was filtered
and the solid was
washed with benzene and water. The solid was dried under reduced pressure to
afford the title
acetamide (24 g, 109 mmol) as a yellow solid.
'H NMR (d6-DMSO) 8: 10.2 (s, 1H); 7.78 (d, J = 8.3 Hz, 1H); 7.65 (d, J = 8.3
Hz, 1H);
7.43 (t, J= 8.3 Hz, 1H); 3.95 (s, 211); 2.30 (s, 3H).
104.ii. 3-hydroxy-5-methyl-l-oxy-quinoxaline-2-carbonitrile:
To a solution mixture of intermediate 104.i (24 g, 109.5 mmol) in 1Maq. NaOH
(100 ml) was
added pyridine (100 ml). The reaction nzixture was stirred at rt for 4 h. The
pH was adjusted
to 6 by addition of 1M aq. HCI. The solid was filtered off and washed witlh
water. The solid
was triturated with EtOH. After drying under HV, the title nitrile (17.7 g,
87.9 mmol) was
obtained as a yellow solid.
MS (ESI, m/z): 202.1 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 142 -
104. iii. 8-methyl-quinoxalin-2-ol:
To a solution of intermediate 104.ii (17.7 g, 87.9 mmol) in water (300 ml) and
EtOl3 (24 ml)
was added sodium dithionite (35.4 g, 203.9 mmol). The reaction mixture was
heated at 60 C
for 1 h. The reaction mixture was filtered till warm, and the pH of the
filtrate was ad;usted to
2 by adding 1M aq. HCI. The pH of the solution was subsequently made basic by
adcl ing solid
NaOH (10 g). EA (150 ml) was added. The aq. layer was extracted twice more
with EA (2 x
150 ml). The combined organic extracts were dried over Na2SO4, filtered and
concentrated to
dryness. The residue was dried under HV to afford the title intermediate (11.1
g, 69 rnmol) as
a yellow solid.
'H NMR (d6-DMSO) S: 11.75 (br s, 1H); 8.17 (s, 1H); 7.62 (d, J= 8.4 Hz, 1H);
7.40 (d,
J= 8.4 Hz, 1H); 7.21 (t, J = 8.4 Hz, 1H); 2.42 (s, 3H).
MS (ESI, m/z): 161.1 [M+H+].
104.iv. 2-ehloNo-8-me thyl-quinoxaline:
A solution of intermediate 104.iii (11.1 g, 69.5 mmol) in phosphorus
oxychloride (80 ml) was
heated at 110 C during 2 h. After cooling to rt, the reaction mixture was
poured onto ice
(200 g). The aqueous layer was extracted with EA (2 x 200 ml). The combined
extracts were
washed with brine (100 ml), dried over Na2SO4, filtered and concentrated to
dryness. The
residue was chromatographed over silica gel (Hex-EA 1-1) to afford the title
intermediate
(12.5 g, 69.5 mmol) as a red solid.
'H NMR (d6-DMSO) 6: 8.99 (s, 1H); 7.97 (m, 1H); 7.80 (m, 2H); 2.68 (s, 3H).
MS (ESI, m/z): 179.2 [M+H+].
104.v. 2-methoxy-8-naethyl-quinoxal ine:
To a solution of intermediate 104.iv (12.5 g, 69.5 mmol) in DMF (80 ml) was
added sodium
methoxide (9 g, 166 mmol). The reaction mixture was heated at 45 C for 4h.
After cooling to
rt, the reaction mixture was partitioned between water (10 ml) and EA (200
ml). Tlae organic
layer was washed once with water (100 ml), dried over Na2SO4, filtered and
concentrated to
dryness. The residue was chromatographed over silica gel (Hex-EA 1-4) to
afford the title
intermediate (10.2 g, 58.55 mmol) as a yellow solid.
'H NMR (CDC13) S: 8.48 (s, 1H); 7.88 (d, J = 7.9 Hz, 1H); 7.55 (d, J = 7.9 Hz,
1HI); 7.47 (t,
J= 7.9 Hz, IH); 4.12 (s, 3H); 2.69 (s, 3H).
MS (ESI, m/z): 175.4 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-143-
104.vi. 8-dibromomethyl-2-nzethoxy-quinoxaline:
To a solution of intermediate 104.v (10.2 g) in CC14 (560 ml) were added AIBN
(0.96 g) and
NBS (25.9 g, 145.5 mmol). The reaction mixture was heated at 80 C for 3 h.
After cooling to
rt, the reaction mixture was washed with water (200 ml) and the organic layer
was dried over
Na2SO4, filtered and concentrated in vacuo. The residue was triturated with
MeOH to give,
after drying under HV, the title dibromide (14.4 g, 43.3 mmol) as a slightly
beige solid.
'H NMR (d6-DMSO) 8: 8.69 (s, 1H); 8.25 (dd, J = 1.3, 7.5 Hz, 1H); 8.07 (dd, J
= 1.3, 8.3 Hz,
1H); 8.02 (s, 1H); 7.74 (dd, J= 7.5, 8.3 Hz, 1H); 4.14 (s, 3H).
MS (ESI, m/z): 332.8 [M+H+].
104.vii. 3-methoxy-quinoxal ine-S-carbaldehyde :
To a solution of intermediate 104.vi (10.7 g, 32.2 mmol) in EtOH (330 ml) was
added, at rt, a
solution of silver nitrate (15 g) in water (70 ml). The reaction was stirred
at rt for 1 h. The
reaction mixture was diluted with MeCN (200 ml) and the solids were filtered
off and the
filtrate was concentrated in vacuo. The residue was filtered over a silica gel
pad (eluent: EA)
to afford the title aldehyde (6.2 g, 32.2 mmol) as a slightly yellow solid.
1H NMR (d6-DMSO) 6: 11.15 (s, 1H); 8.74 (s, 1H); 8.36 (dd, J= 1.3, 8.1 Hz,
1H); 8.21 (dd,
J = 1.3, 7.9 Hz, 1H); 7.80 (dd, J = 7.9, 8.1 Hz, 1H); 4.14 (s, 3H).
MS (ESI, m/z): 189.2 [M+H+].
104.viii. rac-2-methoxy-8-oxinanyl-quinoxaline:
To a solution of intermediate 104.vii (3 g, 15.9 mmol) in MeCN (120 ml) were
added at 60 C,
trimethylsulfonium iodide (3.4 g, 16.6 mmol) and KOH (6.4 g). The mixture was
heated at
this temperature for 1 h. The reaction mixture was filtered and the filtrate
was concentrated to
dryness. The residue was chromatographed over silica gel (Hex-EA 2-1) to
afford the title
epoxide (2.9 g, 14.3 mmol) as a slightly yellow solid.
1H NMR (CDC13) S: 8.53 (s, 1H); 7.97 (m, 1H); 7.56 (m, 2H); 4.94 (dd, J= 2.6,
4.1 Hz, 1H);
4.14 (s, 3H); 3.33 (dd, J= 4. 1, 5.7 Hz, 1H); 2.87 (dd, J= 2.6, 5.7 IFIz, 1H).
MS (ESI, m/z): 203.3 [M+W].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-144-
104.ix. rac-(1 a, 5a, 6a))-{3-[2-hydroxy-2-(3-meth xy-quinoxalin-5 yl)-ethylJ-
3-caza-
bicyclo[3.1.OJhex-6 yl}-carbainic acid tert-butyl ester:
To a solution of intermediate 104.viii (1 g, 4.94 mmol) and (la,5cr,6a)-(3-aza-
bicyclo[3.1.0]hex-6-yl)-carbamic acid tert-butyl ester (0.98 g, 4.94 mmol) in
DMF (20 ml)
were added K2C03 (0.72 g, 5.2 mmol) and lithium perchlorate (0.552 g, 5.2
mmol). The
reaction mixture was heated at 80 C overnight. After concentration to dryness,
the residue
was chromatographed over silica gel (DCM-MeOIH 19-1) to afford the title
compound (1.3 g,
3.24 mmol) as an oil.
MS (ESI, m/z): 401.1 [M+H+].
104.x. rac-(1 a,5a,6a)-2-(6-amino-3-aza-bicyclo[3.1.0Jhex-3yl)-1-(3-nzethoxy-
quinoxalin-
5 yl)-ethanol:
A solution of intermediate 104.ix (1.3 g) in TFA (4 ml) was stirred at rt for
30 min. The
solvent was removed under reduced pressure. The residue was basified with 1N
aq. NaOH
and extracted with a DCM-MeOH 9-1 mixture (5 x 20 ml). The combined organic
layers were
dried over MgSO4 and concentrated to dryness. The residue was purified over
silica gel
(DCM-MeOH 9-1, 1% aq. NH4OH) to afford the title amine (0.8 g, 2.66 mmol) as a
yellowish
foam.
MS (ESI, m/z): 301.3 [M+H+].
104.xi. rac-(1 a, 5c~ 6a}2-{6-[(2, 3-dihydro-benzo[l, 4Jdioxin-6 ylmethyl)-
aminoJ-3-aza-
bicyclo[3.1.O]hex-3yl}-1-(3-naethoxy-quinoxalin-5yl)-ethanol:
To a solution of intermediate 104.x (0.1 g, 0.33 rnmol) in MeOH (2 ml) and 1,
2-DCE (6 ml)
were added 3 A molecular sieves (2 g) and 2,3-dihydro-benzo[1,4]dioxine-6-
carbaldehyde
(0.06 g, 0.367 mmol). The reaction was stirred at room temperature overnight.
NaBH4 (0.1 g,
2.7 mmol) was added and the mixture was further stirred 1 h. The reactiorn
mixture was
filtered through Hydromatrix (pretreated with saturated NaHCO3). The filtrate
was
concentrated to dryness and the residue was chromatographed over silica gel
(DCM-MeOH
19-1 containing 1% aq. NH4OH) to afford the title compound as a white foam
(0.063 g,
0.14 mmol).
'H NMR (d6-DMSO) 8: 8.60 (s, 1H); 7.88 (d, J = 8.1 Hz, 1H); 7.84 (d, J = 7.9
Hz, 1H);
7.60 (dd, J= 7.9, 8.1 Hz, 1H); 6.80-6.69 (m, 31H); 5.63 (m, 1H); 5.02 (d, J=
4.4 Hz, 1H);
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-145-
4.21 (s, 4H); 4.04 (s, 3H); 3.51 (s, 2H); 3.06 (d, J = 8.6 Hz, 1H); 2.95 (d, J
= 8.6 Hz, 1H);
2.56 (m, 2H); 2.45 (m, 2H); 2.16 (br s, 1H); 1.26 (br s, 2H).
MS (ESI, m/z): 449.5 [M+H+].
Example 105: rac-(1 a,5a,6a)-6-({3-[2-hydroxy-2-(3-methoxy-quinoxalin-5-yl)-
ethyl]-
3-aza-bicyclo[3.1.0]hex-6-ylamino}-methyl)-4H-benzo[1,4]thiazin-3-one:
The title compound (0.055 g, 0.11 mmol) was obtained as a yellowish foam by
the method of
Example 35, step 35.iii, starting from 3-oxo-3,4-dihydro-2F3-
benzo[1,4]thiazine-
6-carbaldehyde (0.071 g) and intermediate 104.x (0.1 g).
MS (ESI, m/z): 478.5 [M+H+].
Example 106: rac-(1a,5a,6a)-6-({3-[2-hydroxy-2-(3-methoxy-quinoxalin-5-yl)-
ethyl]-
3-aza-bicyc lo [3.1.0] hex-6-ylam in o}-methyl)-4H-benzo [ 1,4] oxazin-3-o ne
:
The title cornpound (0.059 g, 0.13 mmol) was obtained as a yellowish foam by
the method of
Example 35, step 35.iii, starting from 3-oxo-3,4-dihydro-2H-benzo[1,4]oxazine-
6-carbaldehyde (0.065 g) and intermediate 104.x (0.1 g).
MS (ESI, rnlz): 462.2 [M+H+].
Example 107: rac-(1a,5a,6a)-2-{6-[(benzo[1,2,5]thiadiazol-5-ylmethyl)-amino]-3-
aza-
bicyclo [3.1 _ 0] hex-3-yl}-1-(3-methoxy-quinoxalin-5-yl)-ethanol:
The title compound (0.035 g) was obtained as a yellowish foairx by the method
of
Example 35, step 35.iii starting from benzo[1,2,5]thiadiazole-5-carba.ldehyde
(0.06 g) and
intermediate 104.x (0.1 g).
MS (ESI, ni/z): 449.5 [M+H+].
Example 108: rac-(1 a,5a,6a)-6-({3-[2-hydroxy-2-(3-methoxy-quinoxalin-5-yl)-
ethyl]-
3-aza-bicyclo[3.1.0]hex-6-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4]thiazin-3-
one:
The title compound (0.026 g) was obtained as a yellowish foarrn by the method
of
Example 35, step 35.iii, starting from 3-oxo-3,4-dihydro-2H-pyrido[3,2-
b][1,4]thiazine-
6-carbaldehyde (0.035 g) and intermediate 104.x (0.05 g).
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 146 -
1H NMR (d6-DMSO) b: 10.87 (s, 1H); 8.58 (s, 1H); 7.86 (d, J= 8.1 Hz, 1H); 7_
82 (d,
J = 7.9 Hz, 1 H); 7.72 (d, J = 7.8 Hz, 1H); 7.60 (dd, J = 7.9, 8.1 Hz, 1 H);
7.02 (d, J = 7.8 Hz,
1H); 5.63 (m, 1H); 5.02 (d, J= 4.4 Hz, 1H); 4.03 (s, 3H); 3.66 (s, 2H); 3.51
(s, 2H); 3.06 (d,
J = 8.6 Hz, 1 H); 2.96 (d, J = 8.6 Hz, 1 H); 2.56 (m, 2H); 2.45 (m, 2H); 2.09
(br s, 1 H);
1.28 (br s, 2H).
MS (ESI, m/z): 479.5 [M+H}].
Example 109: rac-(1 a,5 a,6 a)-2- {6-[(benzofuran-2-ylmethyl)-amino]-3-aza-
bicyclo [3.1.0] hex-3-yl}-1-(3-meth oxy-q uinoxalin-5-yl)-eth anol:
This compound (0.024 g) was obtained as a yellowish foam by the method of
Exarnple 35,
step 35.iii, using benzofuran-2-carbaldehyde (0.027 g) and intermediate 104.x
(0.05 g).
MS (ESI, m/z): 431.3 [M+H+].
Example 110: rac-(1 a,5a,6a)-1-(3-methoxy-quinoxalin-5-yl)-2-[6-(3-phenyl-
allylamino)-3-aza-bicyclo [3.1.0] h ex-3-yl]-ethanol:
This compound (0.059 g) was obtained as a yellowish foam by the method of
Exatrnple 35,
step 35.iii starting from trans-cinnamaldehyde (0.048 g) and intermediate
104.x (0.1 g) .
MS (ESI, m/z): 417.4 [M+H+].
Example 111: rac-(1 a,5a,6a)-2-{6-[(2,2-dimethyl-chroman-7-ylmethyl)-amino]-3-
aza-
bicyclo [3.1.0] h ex-3-yl}-1-(3-metli oxy-quinoxalin-5-yl)-ethanol:
This compound (0.068 g) was obtained as a white foam by the method of Exaniple
35,
step 35.iii, using 2,2-dimethyl-chroman-7-carbaldehyde (0.075 g) and
intermediate 104.x
(0.1 g).
MS (ESI, m/z): 475.3 [M+H+].
Example 112: 6-{2-[(1 a,5a,6a)-6-(6-methoxy-quinazolin-4-yloxymethyl)-3-aza-
bicyclo [3.1.0] hex-3-yl]-acetyl}-4I-I-benzo [1,4] thiazin-3-o ne:
This compound (0.21 g) was obtained as an orange solid by the method of
Exarrnple 10,
step 10.iii, using intermediate 10.ii (0.271 g, lmmol) and 6-(2-chloro-acetyl)-
4H-benzo[1,4]thiazin-3-one (0.241 g, 1 mmol).
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 147 -
MS (ESI, m/z): 477.1 [M+H+].
Example 113: 6-{1-hydroxy-2-[(1 a,5a,6q)-6-(6-methoxy-quinazolin-4-
yloxymethyl)-
3-aza-bicyclo [3.1.0] hex-3-yl]-ethyl}-4H-benzo [1,4]thiazin-3-one:
Starting from the compound of Example 112 (0.150 g, 0.315 mrnol), the title
compound
(0.09 g) was prepared as an orange solid using the procedure described in
Example 11.
'H NMR (d6-DMSO) 8: 10.56 (s, 1H); 8.66 (s, 1H); 7.85 (d, J= 9.0 Hz, 1H); 7.57
(dd,
J = 2.8, 9.0 Hz, 1H); 7.41 (d, J = 2.8 Hz, 1H); 7.21 (d, J = 7.6 Hz, 1H); 6.97
(s, 11-1); 6.90 (d,
J = 7.6 Hz, 11-1); 5.04 (d, J = 3.5 Hz, 1H); 4.51-4.34 (m, 3H); 3.93 (s, 3H);
3.42 (s, 2H);
3.05 (dd, J= 8.4, 22.4 Hz, 2H); 2.50 (overlapped m; 214); 2.38 (m, 2H); 1.71
(m, 1H);
1.65 (br s, 2H).
MS (ESI, m/z): 479.2 [M+H+].
Exatnple 114: 6-{[(3R,6S)-6-(3-methoxy-quinolin-5-yloxymethyl)-tetrahydro-
pyran-
3-ylamino]-methyl}-4H-pyrido[3,2-b] [1,4]thiazin-3-one:
114. i. [(3R, 6S)-6-(3-methoxy-quinolin-5yloxymethyl)-tetrahydro py.-an-3ylJ-
carbamic acid
tert-butyl ester:
This compound (3.33 g, 8.57 mmol) was obtained as a yellowish foam from
intermediate 78.i
(3.47 g, 15 mmol) and 3-methoxy-quinolin-5-ol (2.62 g, 15 mrnol), according to
the
procedure of Example 77, step 77.ii.
MS (ESI, m/z): 389.0 [M+H+].
114. ii. (3R, 6S)-6-(3-methoxy-quinolin-Syloxymethyl)-tetrahydro py.-an-3
ylamine:
Starting from intermediate 114.i (3.33 g), the title compound (2.20 g, 90%
yield) was
prepared as a yellowish oil according to the procedure described in Example
77, step 77.iii.
MS (ESI, m/z): 289.5 [M+H+].
114-iii. 6-{[(3R,6S)-6-(3-methoxy-quinolin-S yloxymethyl)-tetrahydY-o pyran-
3ylaminoJ-
methyl}-4H-pyrido[3, 2-b][I, 4Jthiazin-3-one:
The title compound (0.016 g) was obtained as a white foam by the method of
Example 88,
step 88.iv, using 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-
carbaldehyde (0.037 g)
and intermediate 114.ii (0.05 g).
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 148 -
MS (ESI, m/z): 467.5 [M+H']
Example 115: 6-{[(3R,6S)-6-(3-methoxy-quinolin-S-yloxymethyl)-tetrahydro-pyran-
3-ylamino]-methyl}-4H=benzo [1,4] thiazin-3-one:
Starting from intermediate 114.ii (0.11 g) and 3-oxo-3,4-dihydro-2H-
benzo[1,4]thiazine-
6-carbaldehyde (0.077 g), the title compound (0.03 g) was prepared as a pale
yellow solid
using the procedure described in Example 88, step 88.iv.
MS (ESI, m/z): 466.5 [M+H+].
Example 116: benzo[1,3]dioxol-5-ylmethyl-[(3R,6S')-6-(3-methoxy-quinolin-
5-yloxymethyl)-tetrahydro-pyran-3-yl]-amine hydrochloride:
To a solution of intermediate 114.ii (0.100 g) in MeOH (2 ml) and 1,2-DCE (5
ml) were
added 3 A molecular sieves (2 g) and benzo[1,3]dioxole-5-carbaldehyde (0.055
g). Th_e
mixture was stirred at rt overnight. NaBH4 (0.100 g) was added and the
reaction was stirre d
for 2 h. The reaction mixture was filtered through Hydromatrix (treated with
saturate d
NaHCO3 solution) and the filtrate was concentrated u_nder reduced pressure.
The residue wa.s
purified by column chromatography over silica gel (DCM-MeOH 19-1 containing 1%
aq.
NH4OH). The product was dissolved in ether and 2N HCl was added to form tlxe
hydrochloride salt as a strong yellow solid that was collected by filtration
(0.030 g).
IH N1VIR (d6-DMSO) S: 8.87 (s, 1H); 8.00 (s, 1H); 7.69 (d, J= 8.4 Hz, 1H);
7.61 Ct,
J= 7.8Hz, 1H); 7.28 (d, J= 1.4 Hz, 1H); 7.17 (d, J= 7.8 Hz, 1H); 7.08 (dd, J=
1.4, 8.0 Hz,
1H); 6.97 (d, J= 8.0 Hz, 1H); 4.28 (m, 1H); 4.21 (d, J= 4.7 Hz, 2H); 4.10 (m,
2H); 4.00 (s,
3H); 3.82 (m, 1H); 3.57 (t, J= 10.7 Hz, 1H); 3.17 (s, 2H); 2.34 (m, 1H); 1.99
(m, lif);
1.83 (rn, 1H); 1.54 (m, 1H), 1.21 (m, 1H).
MS (ESI, m/z): 423.6 [M+H+].
Example 117: (2,3-dihydro-benzo[1,4]dioxin-6-ylrnethyl)-[(3R,6S)-6-(3-methoxy-
quinolin-5-yloxymethyl)-tetrahydro-pyran-3-yl]-ainine hydrochloride:
Starting from intermediate 114.ii (0.102 g) and 2,3-dihydro-benzo[1,4]dioxine-
6-carbaldehyde (0.064 g), the title compound (0.061 g) was prepared as a white
foaan
according to the procedure described in Example 88, step 88.iv.
MS (ESI, m/z): 437.5 [M+H'].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 149 -
Example 118: 6-{[(3R,6S)-6-(3-methoxy-quinolin-5-yloxymethyl)-tetrahydro-pyran-
3-ylamino]-methyl}-4J-I-benzo [1,4] oxazin-3-one:
Starting from intermediate 114.ii (0.111 g) and 3-oxo-3,4-dihydro-2H-
berizo[1,4]oxazine-
6-carbaldehyde (0.075 g), the title compound (0.023 g) was prepared as a
yellow solid
according to the procedure described in Example 88, step 88.iv.
MS (ESI, m/z): 450.5 [M+H+].
Example 119: (2,3-dihydro-[1,4]dioxino[2,3-b]pyridin-6-ylmethyl)-
[(3R,6S)-6-(3-methoxy-q uinolin-5-yloxymethyl)-tetrahydro-pyran-3-yl] -a mine:
Starting from intermediate 114.ii (0.082 g) and 2,3-dihydro-[1,4]dioxino[2,3-
b]pyridine-
6-carbaldehyde (0.049 g), the title compound (0.075 g) was prepared as a white
foam
according to the procedure described in Example 88, step 88.iv.
1H NMR (CDC13) 6: 8.65 (d, J= 3.0 Hz, 1H); 7.80 (d, J= 3.0 Hz, 1H); 7.64 (d,
J= 8.6 Hz,
1H); 7.42 (dd, J= 7.8, 8.6 Hz, 1H); 7.14 (d, J= 7.8 Hz, 1H); 6.85 (m, 2Ii);
4.43 (m, 2H);
4.23 (m, 2H); 4.16 (m, 2H); 4.07 (dd, J= 4.3, 10.1 Hz, 1 H); 3.97 (s, 3H); 3.
82 (d, J= 2.4 Hz,
2H); 3.29 (t, J= 10.7 Hz, 1H); 2.79 (m, 1H); 2.22 (m, IH), 2.03 (m, 211E),
1.92 (m, 1H);
1.51 (m, 2H).
MS (ESI, m/z): 438.4 [M+H+].
Example 120: (2,3-clihydro-[1,4]dioxino[2,3-c]pyridin-7-ylmethyl)-
[(3R,6S)-6-(3-methoxy-quinolin-5-yloxymethyl)-tetrahydro-pyran-3-yl]-a.mine:
Starting from interrtnediate 114.ii (0.080 g) and 2,3-dhydro-[1,4]dioxirio[2,3-
c]pyridine-
7-carbaldehyde (0.048 g), the title compound (0.080 g) was prepared as a white
foam
according to the procedure described in Example 88, step 88.iv.
'H NMR (CDC13) 5: 8.65 (d, J= 2.8 Hz, 1H); 8.10 (s, 1H); 7.80 (d, J= 2.8 Hz,
1H); 7.60 (d,
J= 8.5 Hz, 1H); 7.44 (dd, J= 7.8, 8.5 Hz, 1H); 6.86 (d, J= 7.8 Hz, lE-i); 6.81
(s, 1H);
4.32 (m, 2H); 4.27 (rn, 2H); 4.16 (m, 2H); 4.07 (dd, J= 4.3, 10.1 Hz, 1Ii);
3.96 (s, 3H);
3.81 (d, J= 4.2 Hz, 2IH); 3.25 (t, J= 10.6 Hz, 1H); 2.76 (m, 1H); 2.20 (m, 1
H), 2.10 (m, 1H),
1.90 (m, 1H); 1.59 (m, 1H); 1.49 (m, 1H).
MS (ESI, m/z): 438.5 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 150 -
Example 121: 7-fluoro-6-{[(3R,6S)-6-(3-methoxy-quinolin-5-yloxymethyl)-
tetrahydro-
pyran-3-ylamino]-methyl}-4I3-benzo [ 1,4] thiazin-3-o ne:
Starting from intermediate 114.ii (0.100 g) and 6-fluoro-3-oxo-3,4-dihydro-
2H-benzo[1,4]thiazine-7-carbaldehyde (0.081 g), the title compound (0.054 g)
was prepared
as a white solid according to the procedure described in Example 88, step
88.iv.
MS (ESI, m/z): 484.4 [M+H+].
Example 122: benzofuran-2-ylmethyl-[(3R,6S)-6-(3-methoxy-quinolin-5-
yloxymethyl)-
tetrahydro-pyran-3-yl]-amin e:
The title compound (0.028 g) was obtained as a colourless oil by the method of
Example 88,
step 88.iv, using benzofuran-2-carbaldehyde (0.028 g) and intermediate 114.ii
(0.05 g).
MS (ESI, m/z): 419.4 [M+Hj -
Example 123: [(3R,6S)-6-(3-methoxy-quinolin-5-yloxymethyl)-tetrahydro-pyran-3-
yl]-
(3-phenyl-allyl)-amine:
The title compound (0.061 g, 0.15 mmol) was obtained as a colourless oil by
the method of
Example 88, step 88.iv, using trans-cinnamaldehyde (0.051 g) and intermediate
114.ii (0.1 g).
MS (ESI, m/z): 405.6 [M+H+].
Example 124: benzo[1,2,5]thiadiazol-5-ylmethyl-[(3R,6,S')-6-(3-methoxy-
quinolin-
5-yloxymethyl)-tetrahydro-pyran-3-yl]-amine:
This compound (0.096 g) was obtained as a colourless oil by the method of
Example 88,
step 88.iv, using benzo[1,2,5]thiadiazole-5-carbaldehyde (0.062 g) and
interlnediate 114.ii
(0.1 g).
MS (ESI, m/z): 437.4 [M+H+].
Example 125: (3R,6S)-heptyl-[6-(3-methoxy-quinolin-5-yloxymethyl)-tetrz~khydro-
pyran-3-yl]-amine:
Starting from intermediate 114. ii (0.146 g, 0.5 mmol) and n-heptaldehyde
(0.078 ml, 1.1 eq.),
the title compound (0.105 g, 53% yield) was prepared as yellow oil according
to the
procedure described in Example 88, step 88.iv.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 151 -
1H NMR (CDC13) d: 8.66 (d, J = 3.0 Hz, 1H); 7.81 (d, J = 2.9 Hz, 111); 7.65
(d, J = 8.5 Hz,
1H); 7.43 (t, J= 8.4 Hz, 1H); 6.87 (d, J= 7.68 Hz, IH); 4.21 (dd overlapped,
J= 6.1, 10.1 Hz,
1H); 4.15 (m overlapped, 2H); 4.09 (dd, J = 4.3, 10.1 Hz, 1H); 3.97 (s, 3H);
3.82 (m, 1H);
3.17 (t, J = 10.5 Hz, 1H); 2.68 (m, 3H); 2.17 (m, 1H); 1.89 (m, 1H); 1-63 (m,
1H); 1.50 (m,
2H); 1.29 (m, 911); 0.88 (t, J = 6.9 Hz, 3H).
MS (ESI, m/z): 387.4 [M+H+].
Example 126: 2-[(3R,6S)-6-(3-methoxy-quinolin-5-yloxymethyl)-tetrahydro-pyran-
3-ylamino]-N-thiazol-2-yl-acetamide:
To a solution of intermediate 114.ii (0.111 g) in DMF (2.85 ml) were added
DIPEA
(0.150 ml) and 2-bromo-N-thiazol-2-yl-acetamide (0.131 g). The resulting
solution was
heated at 80 C for 2.5 h. The reaction mixture was concentrated to dryness and
purified by
column chromatography over silica gel (DCM-MeOH 19-1 containing 1% aq. NH4OH)
to
afford the title compound as a yellow foam (0.059 g).
'H NMR (CDC13) S: 8.66 (d, J= 3.0 Hz, 1H); 7.79 (d, J= 3.0 Hz, 1 H); 7.66 (dd,
J= 2.3,
8.5 Hz, 1H); 7.46 (d, J= 3.6 Hz, 1H); 7.43 (d, J= 8.5 Hz, IH); 7.00 (d, J= 3.6
Hz, 1H);
6.86 (d, J= 7.1 Hz, 1 H); 4. 18 (m, 1 H); 4.12 (m, 2H); 3.97 (s, 3H); 3.83 (m,
1 H); 3.57 (d,
J= 5.0Hz, 2H); 3.22 (t, J= 10.6 Hz, 1H); 2.75 (m, IH); 2.23 (m, 1H); 1.92 (m,
1H); 1.60 (m,
1H), 1.43 (m, 1H):
MS (ESI, m/z): 429.2 [M+li+].
Example 127: (2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-[(3R,6S')-6-(3-methoxy-
quinoxalin-5-yloxymethyl)-tetrahydro-pyran-3-yl]-amine:
127.i. (2S, 5R)-methanesulfonic acid 5-tert-butoxycarbonylamino-tetrahydro
pyr=an-
2 ylmethyl ester:
To a solution of intermediate 78.i (1.9 g, 8.21 mmol) in DCM (50 ml) were
added, at 0 C,
TEA (2.2 ml) and MsCI (0.72 ml, 9.43 mmol). The reaction was stirred 20 min at
this
temperature and saturated NaHCO3 (30 ml) was added. The two layers were
separated. The
organic layer was dried over NazSO4, filtered and concentrated to dryness. The
residue was
chromatographed over silica gel (EA-Hex 2-1) to afford the title mesylate as a
white solid
(2.3 g).
MS (ESI, m/z): 310.3 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 152 -
127.ii. [(3R,6S)-6-(3-methoxy-quinoxalin-5 yloxyuaethyl)-tetrahydropyran-3 ylJ-
carbczmic
acid tert-butyl ester:
To a solution of intermediate 127.i (1.78 g, 5.75 mmol) in MeCN (25 ml) were
added
successively 3-methoxy-quinoxalin-5-ol (prepared as described in WO
2004/002490, 1.12 g,
6.33 mmol), K2C03 (1.39 g) and tetrabutylammonium iodide (0.2 g). The reaction
rnixture
was refluxed for 36 h. After cooling, the reaction mixture was concentrated to
dryness. The
residue was partitioned between water (50 ml) and EA (100 ml). The aq. layer
was ex'tracted
three times more. The combined organic layers were washed with brine, dried
over IJkISO4,
filtered and concentrated to dryness. The residue was chromatographed over
silica gel
(Hex-EA 2-1 then 0-1) to afford the title compound (1.1 g) as a white solid.
1H NMR (CDC13) 8: 8.49 (s, 1H); 7.68 (dd, J = 1.1, 8.4 Hz, 1H); 7.47 (tdd,
J=7.9, :8.4 Hz,
1H), 7.15 (dd, J = 1.1, 7.9 Hz, 1H); 4.32 (app dd, J = 5.7, 9.9 Hz, 2H); 4.22-
4.13 (overlapped
m, 2H); 4.15 (s, 3H); 3.82 (m, 1H); 3.70 (br s, 1H); 3.14 (t, J = 10.6 Hz,
1H); 2.22 (br d,
J= 12.3 Hz, 1 H); 2.02 (qd, J= 3.5, 13.1 Hz, 1 H); 1.71-1.63 (m, 1 H); 1.47
(s, 9H),
1.40 (partially overlapped m, 1H).
MS (ESI, m/z): 390.4 [M+H+].
127.iii. (3R, 6S)-6-(3-methoxy-quinoxalin-5 yloxyynethyl)-tetrahydro pyran-3-
ylamine:
The title amine (0.72 g) was prepared from interYnediate 127.ii (1.1 g, 2.82
mmol) according
to the procedure described in Example 77, step 77.iii.
1H NMR (CDC13) S: 8.49 (s, 1H); 7.68 (dd, J 1.1, 8.4 Hz, 1H); 7.47 (tdd,
J=7.9, 8.4 Hz,
1H), 7.15 (dd, J = 1.1, 7.9 Hz, 1H); 4.30 (dd, J 5.7, 9.9 Hz, 1H); 4.16
(partially overlapped
dd, J = 4.7, 9.9 Hz, 1H); 4.15 (s, 3H); 4.05 (ddd, J = 2.2, 4.5, 10.7 Hz, IH);
3.83 (rn, 1H);
3.13 (t, J = 10.7 Hz, 1H); 2.90 (tt, J = 4.4, 10.9 Hz, 1H); 2.15 (m, 1H); 2.00
(m, 1H); 1 .66 (m,
1H); 1.45-1.27 (m, 3H).
MS (ESI, m/z): 290.1 [M+H+].
127.iv. (2, 3-dihydro-benzo[1, 4]dioxin-6 ylmethyl)-[(3R, 6S)-6-(3-methoxy-
quinoxalin-
5 yloxymethyl)-tetrahydro pyran-3 ylJ-amine:
This compound (0.085 g) was obtained as a co lourless oil by the method of
Exarnple 88,
step 88.iv, using 2,3-dihydro-benzo[1,4]dioxine-6-carbaldehyde (0.062 g) and
intermediate 127.iii (0.1 g).
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 153 -
1H NMR (CDC13) 8: 8.39 (s, 1H); 7.57 (dd, J= 1. l, 8.4 Hz, 1H); 7.38 (dd, J=
7.9, 8.4 Hz,
1 H); 7.0 6 (dd, J= 1.1, 7.9 Hz, 1 H); 6.81-6.69 (m, 3H); 4.19 (partially
overlapped rn, 1 H);
4.17 (s, 4H); 4.09 (partially overlapped m, 2H); 4.05 (s, 3H); 3.76 (m, 11-1);
3.65 (s, 2H);
3.12 (app t, J= 10.5 Hz, 1 H); 2.69 (m, 1 H); 2.09 (m, 1H); 1.88 (m, 1 H);
1.57 (br s, 1 H);
1.55 (m, 1H); 1.35 (app qd, J = 3.5, 12.3 Hz, 1H).
MS (ESI, m/z): 438.1 [M+H+].
Example 128: 6-{[(3R,6S)-6-(3-methoxy-quinoxalin-5-yloxymethyl)-tetrahydro-
pyran-
3-ylamino]-methyl}-4Fl-pyrido[3,2-b] [1,4]thiazin-3-one:
This cornpound (0.06 g) was obtained as a colourless oil by the method of
Example 88,
step 88.iv, using 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4-]thiazine-6-
carbaldehyde (0.074 g)
and intermediate 127.iii (0.1 g).
MS (ESI, m/z): 468.3 [M+H+].
Example 129: (2,3-dihydro-benzo [1,4] dioxin-6-ylmethyl)-
[(3R,6S)-6-(6-trifluoromethoxy-quinolin-4-yloxymethyl:)-tetrahydro-pyran-3-y1]-
anm ine:
129.i. [(3R, 6S)-6-(6-trifluoromethoxy-quinolin-4yloxymethyl)-tetrahydro pyran-
3-ylJ-
carbamzc acid tert-butyl ester:
This compound (2.72 g) was obtained as a yellowish foam from intermediate 78.i
(1.5 g,
6.48 mmol) and 6-trifluoromethoxy-quinolin-4-ol (1.56 g, 6.81 mmol), according
to the
procedure of Example 77, step 77.ii.
MS (ESI, m/z): 443.0 [M+H+].
129.ii. (3R,6S)-6-(6-trifluoromethoxy-quinolin-4 yloxymethyl)-tetrahydro pyran-
3 ylarnine:
Starting from intermediate 129.i (2.72 g), the title compound (0.571 g) was
prepared as a pale
yellow oil according to the procedure described in Example 77, step 77.iii.
MS (ESI, m/z): 343.3 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-154-
129. iii. (2, 3-dihydro-benzo[Z, 4]dioxin-6 ylmethyl)-[(3R, 6S)-6-(6-tr
fluoromethoxy-quinolin-
4 yloxymethyl)-tetrahydro pyran-3 ylJ-amine:
Starting from intermediate 129.ii (0.104 g) and 2,3-dihydro-benzo[1,4]dioxine-
6-carbaldehyde (0.055 g), the title compound (0.067 g) was prepared as a
colourless oil
according to the procedure described in Example 88, step 88.iv.
'H NMR (CDC13) 5: 8.75 (ci, J= 5.2 Hz, 1H); 8.06 (d, J= 9.3 Hz, 1H); 8.02 (d,
J= 1.7 Hz,
1H); 7.55 (m, 1 H); 6.84 (m, 1 H); 6.80 (m, 1 H); 6.77 (m, 1H); 4.24 (s, 4H);
4.21 (m, 1H);
4.12 (dd, J= 5.6, 9.3 Hz, 2I3); 3.83 (m, 114); 3.74 (d, J= 1.9 Hz, 2H); 3.20
(t, J= 10.6 Hz,
1H); 2.77 (m, 1H); 2.19 (m, IH); 1.91 (m, 1H); 1.60 (m, 1H); 1.42 (m, 1H).
MS (ESI, m/z): 491.3 [M+H+].
Example 130: 6-{[(3R,6S')-6-(6-trifluoromethoxy-quinolin-4-yloxymethyl)-
tetrahydro-
pyran-3-ylamino]-methyl}-4H-pyrido[3,2-b] [1,4]thiazin-3-one:
Starting from interrnediate 129.ii (0.1 g) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.063 g), the title compound
(0.065 g) was
prepared as a white solid according to the procedure described in Example 88,
step 88.iv.
MS (ESI, m/z): 521.4 [M+H+].
Example 131: 8-{(2S,5R)-5-[(3-oxo-3,4-dihydro-2H-benzo[1,4]thiazin-6-ylmethyl)-
amino]-tetrahydro-pyran-2-ylmethoxy}-quinoline-2-carbonitrile:
Starting from (2S,5R)-8-(5-amino-tetrahydro-pyran-2-ylmethoxy)-quinoline-2-
carbonitrile
(0.036 g) and 3-oxo-3,4-dihydro-2H-benzo[1,4]thiazine-6-carbaldehyde (0.027
g), the title
compound (0.015 g) was prepared as a beige foam according to the procedure
described in
Example 88, step 88.iv.
'H NMR (CDC13) S: 8.25 (d, J= 8.4 Hz, 1H); 8.16 (br. s, 1H); 7.71 (d, J= 8.4
Hz, 1H);
7.60 (t, J= 8.0 Hz, 1H); 7.45 (dd, J= 1, 8.2 Hz, 1H); 7.27 (m, 1H); 7.18 (dd,
J= 1, 7.8 Hz,
1H); 6.97 (td, J= 1.6, 8.0 Hz, 1H); 6.86 (dd, J = 1.2, 10.7 Hz, 1H); 4.28 (dd,
J= 6.1, 10.1 Hz,
1H); 4.15 (m, 2H); 3.93 (ni, 111); 3.79 (dd, J= 4.3, 11.8 Hz, 2H); 3.22 (t, J=
10.8 Hz, 1H);
3.04 (s, 2H); 2.79 (m, 1H); 2.20 (m, 1H); 1.99 (m, 1H), 1.21 (m, 2H).
MS (ESI, m/z): 461.4 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-155-
Example 132: 6-{[(3R,6.S')-6-(6-methoxy-quinolin-4-yloxymethyl)-tetrahydro-
pyran-
3-ylamino]-methyl}-4H-pyrido[3,2-b] [1,4]thiazin-3-one:
Starting from (3R,6S)-6-(6-methoxy-quinolin-4-yloxymethyl)-tetrahydro-pyran-3-
ylamine
(0.089 g) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde
(0.060 g), the
title compound (0.054 g) -was prepared as a beige foam according to the
procedure described
in Example 88, step 88.iv-
1H NMR (CDC13) S: 8.60 (d, J= 5.3 Hz, 1H); 8.22 (br. s, 1H); 7.95 (d, J= 9.2
Hz, 1H);
7.58 (d, J= 7.8 Hz, 1H); 7.46 (d, J= 2.8 Hz, 1H); 7.35 (dd, J= 2.8, 9.2 Hz,
1H); 6.97 (d,
J= 7.8 Hz, 1 H); 6.72 (d, J= 5.3 Hz, 1 H); 4.25 (dd, J= 6.0, 10.2 Hz, 1 H);
4.16 (dd, J= 4.3,
10.2 Hz, 1H); 3.94 (s, 311); 3.88 (d, J= 2.2 Hz, 2H); 3.48 (s, 2H); 3.25 (t,
J= 10.6 Hz, 1H);
2.77 (m, 1H); 2.22 (m, 1 H); 1.94 (m, 1 H); 1.61 (m, 1 H), 1.50 (m, 1H).
MS (ESI, m/z): 467.5 [M+H+].
Example 133: 6-{[(3R,6,57-6-(2-methoxy-quinolin-8-yloxymethyl)-tetrahydro-
pyran-
3-ylamino]-methyl}-4H-pyrido[3,2-b][1,4]thiazin-3-one:
133.i. [(3R, 6S)-6-(2-methoxy-quinolin-8yloxyrnethyl)-tetrahydro pyran-3 ylJ-
carbamic acid
tert-butyl ester:
This compound (0.67 g, 36% yield) was prepared as a white solid using the
protocol
described in Example 127, step 127.ii and starting from intermediate 127.i
(1.5 g, 4.84 mmol)
and 2-methoxy-quinolin-8-ol (prepared as described in WCt 2004/002490, 0.934
g,
5.33 mmol).
MS (ESI, m/z): 389.5 [M+H+].
133.ii. (3R,6S)-6-(2-methoxy-quinolin-8yloxymethyl)-tetrahydro pyran-3
ylamine:
This compound (0.46 g, 92% yield) was obtained as yellow gum starting from
intermediate 133.i (0.67 g) and using the procedure described in Example 77,
step 77.iii.
MS (ESI, m/z): 289.3 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-156-
133.iii. 6-{[(3R,6S)-6-(2-methoxy-quinolin-8yloxymethyl)-tetrahydropyran-
3ylaminaJ-
methyl}-4H pyrido[3, ,-bJ[I, 4]thiazin-3-one:
Starting from intermediate 133.ii (0.214 g) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.151 g), the title compound
(0.175 g) was
prepared as a white foam according to the procedure described in Example 88,
step 88. iv.
MS (ESI, m/z): 467.0 [M+H+].
Example 134: 6-{[(3R,6S)-6-(6-methoxy-quinazolin-4-yloxymethyl)-tetrahydro-
pyran-
3-ylamino]-methyl}-4H-pyrido [3,2-b] [1,4]thiazin-3-one:
134.i. [(3R,6S)-6-(6-methoxy-qziinazolin-4 yloxymethyl)-tetrahydropyran-3ylJ-
carbazmie
acid tert-butyl ester:
This compound (1.25 g) was prepared as a white solid starting from
intermediate 78.i (1.28 g,
6.6 mmol) and 4-chloro-6-methoxy-quinazoline (1.51 g, 6.54 mmol) using the
procedure
described in Example 10, step 10. i.
IH NMR (CDC13) 6: 8.72 (s, 1 H); 7.93 (d, J = 9.1 Hz, 1H); 7.51 (dd, J = 2.8,
9.1 Hz, 1H);
7.45 (d, J= 2.8 Hz, 1H); 4.66 (dd, J= 3.7, 11.6 Hz, 1H); 4.59 (dd, J= 6.4,
11.6 Hz, 1H);
4.30 (br s, 1H); 4.16 (ddd, J = 2.1, 4.7, 10.9 Hz, 1H); 3.97 (s, 3H); 3.81 (m,
1H); 3.72 (m,
1H); 3.10 (app t, J= 10.7 Hz, 111); 2.21 (m, 1H); 1.89 (m, 1H); 1.65 (qd, J=
3.4, 12.9 Hz,
1H); 1.46 (s, 9H); 1.39 (partially overlapped qd, J= 3.9; 12.0 Hz, 1H).
MS (ESI, m/z): 390.1 [M+H+].
134.ii. (3R,6S)-6-(6-methoxy-quinazolin-4 yloxymethyl)-tetrahydropyran-3
ylamine:
This compound (0.245 g, 26% yield) was obtained as a white solid starting from
intermediate 134.i (1.25 g) using the procedure described in Example 10, step
10.ii.
MS (ESI, m/z): 290.3 [M+H+].
134.iii. 6-{[(3R,6S)-6-(6-methoxy-quinazolin-4 yloxymethyl)-tetrahydro pyran-3
ylarninoJ-
methyl}-4H pyrido[3, 2-bJ[1, 4Jthiazin-3-one:
Starting from intermediate 134.ii (0.100 g) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.070 g), the title compound
(0.054 g) was
prepared as a white solid according to the procedure described in Example 88,
step 89.iv.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 157 -
IH NMR (d6-DMSO) 6: 10.88 (s, 1H); 8.67 (s, 1H); 7.86 (d, J= 9.1 Hz, I H);
7.74 (d,
J= 7.8Hz, 1 H); 7.60 (dd, J= 2.9, 9.1 Hz, 1 H); 7.3 8 (d, J= 2.9 Hz, 1 H);
7.09 (ut, J= 7.8 Hz,
1H); 4.51 (d, J= 6.8 Hz, 2H); 3.98 (m, 1H); 3.92 (s, 3H); 3.77 (m, 3H); 3.53
(s, 2H); 3.02 (t,
J= 10.5 Hz, 1H); 2.09 (m, 2H); 1.81 (m, 1H); 1.39 (m, 1H); 1.28 (m, 1H).
MS (ESI, m/z): 468.4 [M+H}].
Example 135: 6-{[(3R,68)-6-(8-fluoro-6-methoxy-quinolin-4-yloxymethyl)-
tetrahydro-
pyran-3-ylamino]-methyl}-4H-pyrido[3,2-b] [1,4]thiazin-3-one:
135.i. (3R, 6S)-6-(8 fluoro-6-methoxy-quinolin-4 yloxymethyl)-tetrahydro pyran-
3 ylamine:
To a solution of intermediate 78.i (2.38 g, 10.3 mmol) in THF (53 ml) at 0 C
was added
8-fluoro-6-methoxy-quinolin-4-ol (prepared as described in WO 2004/050036;
2.09 g,
10.81 mmol), PPh3 (4.1 g, 15.44 rnmol) and DIAD (2.1 ml). The reaction mixture
was
allowed to reach rt and further stirred overnight at rt. The reaction mixture
was concentrated
to dryness. The residue was dissolved in TFA (10 ml) and the reaction mixture
was stirred
min. After evaporation to dryness, the residue was taken up in water (50 ml)
and washed
15 with DCM-MeOH 9-1 (4 x 100 ml)_ 8Maq. NaOH was added to the mixture until a
cloud was
forined (pH = 10 was reached). The aqueous layer was extracted twice with DCM-
MeOH
(2 x 100 ml). The combined organic layers were washed with brine (50 rnL),
dried over
NaZSO4, filtered and concentrated to dryness. The residue was chromatographed
over silica
gel (DCM-MeOH 97-3 containing 1% aq. NH40H to 6-1 containing 1% ac1. NH4OH) to
20 afford the title amine (1.22 g, 3.98 rnmol) as a white solid.
MS (ESI, m/z): 307.2 [M+H+].
135.ii. 6-{[(3R,6S)-6-(8 fluoro-6-naethoxy-quinolin-4 yloxymethyl)-tetrahydro
pyran-
3 ylaminoJ-methyl}-4H-pyrido[3,2-bJ[1,4]thiazin-3-one:
Starting from intermediate 135.i (0.2 g, 0.65 mmol) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.141 g, 0.729 mmol), the title
compound
(0.092 g) was prepared as a slightly pink foam according to the procedure;
described in
Example 88, step 88.iv.
MS (ESI, m/z): 484.9 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-158-
Exaniple 136: 6-{[(3R,6S)-6-(8-fluoro-6-methoxy-quinolin-4-yloxymethyl)-
tetrahydro-
pyran-3-ylamino]-methyl}-4Fl-pyrido[3,2-b] [1,4]oxazin-3-one:
Starting from intermediate 135.i (0.2 g, 0.65 mmol) and 3-oxo-3,4-dihydro. -
2H-pyrido[3,2-b][1,4]oxazine-6-carbaldehyde (0.129 g, 0.727 mmol), the title
compound
(0.120 g; contaminated with some starting amine (around 20%)) was prepared as
a slightly
pink foam according to the procedure described in Example 88, step 88.iv.
MS (ESI, m/z): 469.3 [M+H+].
Example 137: 6-{(3R,6b')-[6-(6-methoxy-quinazolin-4-yloxymethyl)-3,6-dihydro-
2H-pyran-3-ylamino]-methyl}-4H-pyrido[3,2-b] [1,4]thiazin-3-one:
137.i. (3R,6S)-[6-(6-methoxy-quinazolin-4-yloxymethyl)-3,6-dihydro-2H-pyran-3-
yl]-
carbamic acid tert-butyl ester:
This compound (1.25 g) was prepared as a white solid using the procedure
described in
Example 10, step 10.i and starting from (3R,6S)-(6-hydroxymethyl-3,6-dihydro-
2H-pyran-
3-yl)-carbamic acid tert-butyl ester (2.29 g, 10 mmol) and 4-chloro-6-methoxy-
quinazolirze
(prepared as in WO 96/09294; 1.94 g, 10 mm 1).
'H NMR (DMSO) 8: 8.67 (s, 1H); 7.86 (d, J= 9.1 Hz, 1H); 7.60 (dd, J= 2.9, 9.1
Hz, 1I1);
7.38 (d, J = 2.9 Hz, 1H); 7.02 (d, J = 8.1 Hz, 1H); 5.95-5.84 (m, 2H); 4.61-
4.56 (m, 3IL);
4.09 (br s, 1H); 3.96 (partially overlapped nz, 1H); 3.91 (s, 3H); 3.31 (dd,
J= 8.5, 10.7 Hz,
1H); 1.39 (s, 9H).
MS (ESI, m/z): 388.1 [M+H+].
137.ii. (3R,6S)-6-(6-methoxy-quinazolin-4-yloxymethyl)-3,6-dihydro-2Fl-pyran-3-
ylamine:
The title amine (1.32 g, 77% yield) was obtained as a yellowish oil starting
from intermedia_-te
137.i (2.32 g, 6 mmol) and using the procedure described in Example 77, step
77.iii. TL-le
compound was purified by chromatography (DCM-MeOH 9-1 containing 1%
concentrated
NH4OH).
MS (ESI, m/z): 288.3 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 159 -
137.iii. 6-{(3R, 6S)-[6-(6-methoxy-quinazolin-4 yloxymethyl) -3, 6-dihydro-2H-
pyran-
3 ylaminoJ-naethyl}-4H-pyrido[3,2-bJ[l,4Jthiazin-3-one:
This compound (0.065 g) was prepared as a pale yellow foam according to the
procedure
described in Example 88, step 88.iv, starting from intermediate 137.ii (0.100
g) and 3-oxo-
3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.071 g),
1H NMR (CDC13) S: 8-69 (s, 1H); 8.33 (br. s, 1H); 7.95 (d, J= 9.1 Hz, 1H);
7.58 (d,
J= 7.8 Hz, 1 H); 7.47 (dd, J= 2.9, 9.1 Hz, 1 H); 7.41 (d, J= 2.9 Hz, 1 H);
7.00 (d, J= 7.8 Hz,
1H); 6.12 (dd, J= 2.2, 12.7 Hz, 1H); 5.94 (d, J= 10.4 Hz, 1H); 4.70 (m, 1H);
4.64 (m, 2H);
4.17 (dd, J= 4.6, 11.3 Hz, 1H); 3.95 (s, 2H); 3.93 (s, 3H); 3.63 (dd, J= 6.6,
11.3 Hz, 1H);
3.46 (s, 2H); 3.44 (m, 1I3).
MS (ESI, m/z): 466.3 [M+H+].
Example 138: 6-{[(3R,6S')-6-(6-difluoromethoxy-quinoli n-4-yloxymethyl)-
tetrahydro-
pyran-3-ylamino]-methyl}-4H-pyrido[3,2-b] [1,4]thiazin-3-one:
138J. 6-difluoromethoxy-quinolin-4-ol:
To a solution of 4-difluoromethoxy-phenylamine (5 g, 31.42 mmol) in ethanol
(25 ml) were
added triethyl orthoforrnate (5.3 ml, 31.86 mmol) and Meldrum's acid (5 g,
34.69 mmol). The
reaction was refluxed for 3 h. Upon cooling, a yellowish solid formed. The
solid was filtered
off, washed with Hex and dried under HV. To a refluxing solution of phenyl
ether (120 ml)
was added portion-wise the latter solid. After heating for 2 min, the reaction
mixture was
cooled down to rt using an ice-water bath. The reaction mixture was diluted
with ether
(150 ml) and the solid was filtered off. This material was recrystallized from
MeOH to afford
after drying the title quinolinol as a beige solid (3.1 g)
1H NMR (d6 DMSO) S: 11.83 (br s, 1H); 7.94 (d, J= 7.4 IRz, 1H); 7.78 (d, J=
2.5 Hz, 1H);
7.62 (d, J= 8.9 Hz, 1H); 7.49 (dd, J= 2.8, 8.9 Hz, 1H); 7.33 (t, J= 73.9 Hz,
1H);
6.05 (d, J = 7.4 Hz, 1H)_
138.ii. (3R,6S)-[6-(6-difluoromethoxy-quinolin-4 yloxymethyl)-tetrahydro pyran-
3ylJ-
carbamic acid tert-butyl ester:
The title compound (2.04 g, 83% yield) was obtained as a yellow foam using the
procedure
described in Example 77, step 77.ii and starting from interrnediate 78.i (1.34
g, 5.8 mmol) and
intermediate 138.i (1.29 g, 6.1 mmol).
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-160-
MS (ESI, m/z): 425.0 [M+H}].
138.iii. (3R,6S)-6-(6-difluoromethoxy-quinolin-4 yloxymethyl)-tetrahydro pyran-
3 ylamine:
The title amine (0.554 g, 35% yield) was obtained as a white solid using the
procedure
described in Example 77, step 77.iii and starting from intermediate 138.ii
(2.04 g, 4.8 mmol>.
The compound was purified by chromatography over silica gel (DCM-MeOH 9-1
containing
1% concentrated NH4OH).
MS (ESI, m/z): 325.3 [M+H+].
138.iv. 6-[[(3R, 6S)-6-(6-difluoromethoxy-quinoZin-4 yloxymethyl)-tetrahydro
pyran-
3 ylaminoJ-methyl}-4H-pyrido[3,2-bJ[1,4Jthiazin-3-one:
Starting from intermediate 138.iii (0.100 g) and 3-oxo-3,4-dihydr -
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.063 g), the title compound
(0.048 g) was
prepared as a white foam according to the proceciure described in Example 88,
step 88.iv.
1H NMR (CDC13) S: 8.72 (d, 1H); 8.35 (br. s, 1H); 8.04 (d, J= 9.2Hz, 1H); 7.86
(d,
J= 2.6Hz, 1H); 7.59 (d, J= 7.8 Hz, 1H); 7.48 (dd, J= 2.6, 9.2 Hz, IH); 6.98
(d, J= 7.8 Hz,
1H); 6.75 (d, J= 5.2 Hz, 1H); 6.64 (t, J= 73.7 Hz, 1H); 4.18 (m, 3H); 3.90 (d,
J= 2.7 Hz,
2H); 3.84 (m, 1H); 3.47 (s, 2H); 3.28 (t, J = 10.6 Hz, 1H); 2.79 (m, 1H); 2.22
(m, 11T);
1.93 (m, 1H); 1.57 (m, 2H).
MS (ESI, m/z): 503.5 [M+H+].
Example 139: 6-{(3R,6S)-[6-(3-methoxy-quinoxalin-5-yloxymethyl)-tetrahydro-
pyran-
3-ylamino]-methyl}-4H-pyrido[3,2-b][1,4]oxazin-3-one:
The title compound (0.038 g) was obtained as a white solid by the method of
Example 8 8,
step 88.iv, starting from 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]oxazine-6-
carbaldehyde
(0.068 g, 0.38 mmol) and intermediate 127.iii (0-1 g, 0.34 mmol).
MS (ESI, m/z): 450.4 [M+H+].
Example 140: 6-{(3R,6S)-[6-(3-methoxy-quinoxalin-5-yloxymethyl)-tetrahydro-
pyran-
3-ylamino]-methyl}-4H-be nzo [1,4] oxazin-3-one:
Starting from intermediate 127.iii (0.100 g, 0.34 mmol) and 3-oxo-3,4-dihydro-
2H-benzo[1,4]oxazine-6-carbaldehyde (0.067 g, 0.38 mmol), the title compound
(0.032 g)
was prepared as a white solid according to the procedure described in Example
88, step 88.iv.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-161-
1HNMR (CDC13) S: 10.66 (s, 1H); 8.59 (s, 1H); 7.55 (dt, J = 1.4, 15.9 Hz, 1H,
overlapped);
7.54 (dd, J= 1.4, 15.9 Hz, 1H, overlapped); 7.28 (dd, J= 1.4, 7.7 Hz, 1H);
6.89 (d,
J= 1.3 Hz, IH); 6.87 (s, 2H); 4.53 (s, 2H); 4.15 (AB, J= 5.9, 15.0, 37.0 Hz,
2H); 4.03 (s,
3H); 3.97 (ddd, J= 1.9, 4.2, 10.8 Hz, 1H); 3.69 (m, 3H); 3.01 (t, J= 10.6 Hz,
IH); 2.07 (m,
1H); 1.88 (m, 2H); 1.49 (m, 1H); 1.28 (m, 1H).
MS (ESI, m/z): 451.2 [M+H+].
Example 141: 6-{(3R,6S)-[6-(3-methoxy-quinoxalin-5-yloxymethyl)-tetrahydro-
pyran-
3-yl amino]-methyl}-4H-be nzo [ 1,4] thiazin-3-one :
Starting from intermediate 127.iii (0.100 g, 0.34 mmol) and 3-oxo-3,4-dihydro-
2H-benzo[1,4]thiazine-6-carbaldehyde (0.073 g, 0.38 mmol), the title compound
(0.020 g)
was prepared as a white solid according to the procedure described in Example
88, step 88.iv.
MS (ESI, m/z): 467.3 [M+H+].
Example 142: 3-oxo-3,4-dihydro-2H-pyrido[3,2-b] [1,4]thiazine-6-carboxylic
acid
(3R, 6S)-[6-(3-methoxy-quinoxalin-5-yloxymethyl)-tetrahydro-pyran-3-yl]-amide:
To a solution of intermediate 127.iii (0.1 g, 0.34 mmol) in DMF (6 ml) were
added 3-oxo-
3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid (0.074 g, 0.35
mmol),
HATU (0.16 g) and DIPEA (0.18 ml). The reaction mixture was stiTred overnight
at rt. The
solvent was removed in vacuo. The residue was taken up in water a.nd the solid
was filtered
off. The solid was purified by chromatography (DCM-MeOH 19-1 containing 1% aq.
NH4OH) to afford the title compound (0.068 g) as a white solid.
MS (ESI, m/z): 482.2 [M+H+].
Example 143: 3-oxo-3,4-dihydro-2H-pyrido[3,2-b] [1,4]thiazine-6-carboxylic
acid
(3R,6S)-[6-(2-methoxy-quinolin-8-yloxymethyl)-tetrahydro-pyran-3-yl]-amide:
Tha title compound (0.068 g, 0.14 mmol) was obtained as a white solid starting
from
intermediate 133.ii (0.1 g, 0.34 mmol) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-
b][1,4]thiazine-
6-carboxylic acid (0.080 g, 0.38 mmol), carrying out the procedure described
in Example 142.
1H NMR (d6-DMSO) S: 10.98 (s, 1H); 8.20 (d, J= 8.9 Hz, 1H); 7.97 (d, J = 7.9
Hz, 1H);
7.97 (overlapped m, 1H); 7.60 (d, J = 7.9 Hz, 1H); 7.46 (dd, J = 1.2, 7.8 Hz,
1H); 7.33 (app t,
J= 7.8 Hz, 1H); 7.22 (dd, J= 1,2, 7.8 Hz, 1H); 7.02 (d, J= 8.9 Hz, 1H); 4.25
(dd, J= 5.9,
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 162 -
10.7 Hz, 1H); 4.16 (dd, J = 4.4, 10.7 Hz, 1H); 4.01 (s, 3H); 4.00-3.86 (m,
2H); 3.80 (m, 1H);
3.64 (s, 2H); 3.23 (t, J= 10.1 Hz, 1 H); 2.08 (m, 1 H); 2.01 (m, 1 H); 1.71-
1.62 (m, 2H).
MS (ESI, m/z): 481.3 [M+H+].
Example 144: 4-{(2S,5R)-5-[(2,3-dihydro-[1,4]dioxino[2,3-c]pyridin-7-ylmethyl)-
amino]-tetrahydro-pyran-2-ylmethoxy}-quinoline-6-carbonitrile:
144.i. 4-((2R, 5S)-5-amino-tetrahydro-pyran-2-ylmethoxy)-quinoline-6-
carbonitrile:
The title amine (1.40 g) was obtained as a colourless foam starting from 4-
hydroxy-quinoline-
6-carbonitrile (1.5 g, 8.8 mmol) and intermediate 78.i (2.04 g, 8.8 rnrnol)
using the procedure
described in Example 135, step 135.i. The compound was purified by
chromatography
(DCM-MeOH 47-3 and 1% concentrated NH4 H).
1H NMR (d6-DMSO) 6: 8.88 (d, J = 5.3 Hz, 1H); 8.62 (m, 1H); 8.1 1-8.02 (m,
2H); 7.19 (d,
J = 5.3 Hz, 1H); 4.26 (m, 2H); 3.78 (ddd, J= 2.0, 4.4, 10.6 Hz, 1H); 3.76 (m,
1H); 2.97 (t,
J = 10.5 Hz, 1H); 2.64 (m, 1H); 1.95 (m, 1H); 1.85 (m, 1H); 1.51 (rn, 1H);
1.40 (br s , 2H);
1.27 (m, 1H).
MS (ESI, m/z): 284.3 [M+H+].
144.ii. 4-{(2S, 5R)-5-[(2, 3-dihydro-[1, 4]dioxino[2, 3-cJpyridin-7 ylinethyl)-
aminoJ-
tetrahydro pyran-2 ylmethoxyJ-quinoline-6-caNbonitrile:
Starting from intermediate 144.i (0.114 g, 0.4 mmol) and 2,3-dihydro-
[1,4]dioxino[2,3-c]pyridine-7-carbaldehyde (0.066 g, 0.4 mmol), the title
compound (0.103 g,
59% yield) was prepared as a white solid according to the procedure described
in Example 88,
step 88.iv.
1H NMR (CDC13) S: 8.86 (d, J= 5.3 Hz, 1H); 8.64 (m, 1H); 8.14 (s, 1H); 8.10
(d, J= 8.7 Hz,
1H); 7.84 (dd, J = 1.9, 8.7 Hz, 1H), 6.89 (s, 1H); 6.84 (d. J = 5.3 Hz, 1H);
4.37-4.17 (m, 7H),
3.94 (dd, AB system, J = 13.8 Hz, A= 0.058, 2H); 3.88 (m, 1H); 3.41 (t, J =
10.7 Hz, 1H);
3.01 (br s, 1H); 2.91 (m, 1H); 2.32 (m, 1H); 1.95 (m, 1H); 1.74-1.62 (m, 2H).
MS (ESI, m/z): 433.3 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-163-
Example 145: 4-{(2S,5R)-5-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-
tetrahyd ro-pyran-2-ylmethoxy}-quinoline-6-carbonitrile:
Starting from intermediate 144.i (0.1 g, 0.35 mmol) and 2,3-dihydro-
benzo[1,4]dioxine-
6-carbaldehyde (0.063 g, 0.38 mmol), the title compound (0.095 g) was prepared
as a white
foam according to the procedure described in Example 88, step 88. iv.
MS (ESI, m/z): 432.4 [M+H+].
Example 146: 4-{(2S,5R)-5-[(3-oxo-3,4-dihydro-2H-benzo[1,4] thiazin-6-
ylmethyl)-
amino]-tetrahyd ro-pyran-2-ylmethoxy}-quinoline-6-carbonitrile:
Starting from intermediate 144.i (0.1 g, 0.35 mmol) (0.1 g, 0.35 mmol) and 3-
oxo-
3,4-dihydro-2H-benzo[1,4]thiazine-6-carbaldehyde (0.075 g, 0.38 mmol), the
title compound
(0.035 g) was prepared as a yellowish foam according to the procedure
described in
Example 88, step 88.iv.
MS (ESI, m/z): 461.3 [M+H}].
Example 147: 3-oxo-3,4-dihydro-2F7-pyrido[3,2-b] [1,4]thiaziae-6-carboxylic
acid
(3R,6S)-[6-(6-cyano-quinolin-4-yloxymethyl)-tetrahydro-pyram-3-yl]-amide:
The title compound (0.027 g, 14% yield) was obtained as a orange solid
starting from
intermediate 144.i (0.114 g, 0.4 mmol) and 3-oxo-3,4-dihydro-2F-I-pyrido[3,2-
b][1,4]thiazine-
6-carboxylic acid (0.092 g, 0.44 mmol), carrying out the procedure described
in Example 142.
MS (ESI, m/z): 476.2 [M+H+].
Example 148: 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazime-6-carboxylic acid
(4R,7,S)-[4-(6-methoxy-quinolin-4-yloxymethyl)-cis-(4RS,5RS)-2,2-dimethyl-
tetrahydro-
[1,3] dioxolo [4,5-c] pyran-7-yl]-amide:
148.i. (3R, 6S)-(6-hydroxymethyl-tetrahydro pyran-3 yl)-carbamic acid benzyl
ester:
A solution of intermediate 78.i (5.45 g, 23.8 mmol) in TFA (20 ml) was stirred
at rt for 30
min. The volatiles were removed under reduced pressure. The residue was taken
up in water
(100 ml). NaHCO3 was added carefully until gas evolution ceased. Additional
NaHCO3 (2 g)
was added and the mixture was cooled to 0 C. Cbz-Cl (4.1 ml, 28.6 mmol) was
added. The
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-164-
reaction proceeded for 3 h with warming to rt. The solid formed was diluted
with water and
filtered off. The solid was further washed with water and Hex. After drying
under HV, the
title compound (5.4 g, 86% yield) was obtained as a white solid.
MS (ESI, m/z): 264.3 [M+H+].
148.ii. (3R, 6S)-[6-(6-methoxy-quinolin-4 yloxymethyl)-3, 6-dihydro-2H pyran-
3ylJ-carbamic
acid benzyl ester:
This compound (1.99 g, 231/'o yield) was obtained as a yellowish solid frorn
intermediate 148.i
(5.4 g, 20.5 mmol) and 6-methoxy-quinolin-4-ol (5.4 g, 30.7 mmol), according
to the
procedure of Example 77, step 77.ii.
MS (ESI, m/z): [M+H+].
148.iii. (3S, 4RS, 5RS, 6R)-[4, 5-dihydroxy-6-(6-methoxy-quinolin-4
yloxyrnethyl)-tetrahydro-
pyran-3 ylJ-carbamic acid benzyl ester:
To a solution of intermediate 148.ii (1.99 g, 4.73 mmol) in DCM (35 ml) and
water (3.5 ml)
were added NMO (1.89 g, 14.2 mmol) and potassium osmate dihydrate (0.086 g,
0.23 mmol)
and the thick mixture was stirred overnight. The solvent was removed under
reduced pressure.
The residue was partitioned between water and EA, and sodium bisulfite (5 g)
was added. The
solids were filtered off and dried in vacuo to give the title diol (1.88 g,
87% yield).
MS (ESI, m/z): 455.5 [M+H-'].
148.iv. (4R, 7S)-[4- (6-methoxy-quinolin-4yloxymethyl)-cis-(4RS, 5RS)-2, 2-
dimethyl-
tetrahydro-[l, 3Jdioxolo[4, S-cJpyran-7 ylJ-carbamic acid benzyl ester:
To a solution of intermediate 148.iii (1.88 g, 4.14 mmol) in DMF (20 ml) was
added PTSA
(0.95 mg, 5 mmol) and 2,2-dimethoxypropane (3.2 ml, 26 mmol). The solution was
stirred for
4 d. Water (10 ml) and solid NaHCO3 (0.42 g) were added. After evaporation to
dryness, the
residue was purified by column chromatography over silica gel (DC1VI-MeOH 97-3
then
DCM-MeOH 19-1) to afford the title compound (1.97 g, 3.98 mmol) as a pale
yellow oil. The
compound was recovered as a 3:1 mixture of diastereomers.
'H NMR (CDC13) 8 main diastereomer: 8.57 (d, J= 3.8 Hz, 1H); 7.92 (d, J= 9.2
Hz, 1H);
7.45 (d, J= 2.8 Hz, 1H); 7.37-7.31 (s, 5H); 7.35 (m overlapped, 1H); 6.75 (d,
J= 5.3 Hz,
1H); 5.27 (m, 1H); 5.10 (s, 2H); 4.46 (m, 1H); 4.39 (dd, J= 2.6, 11.0 Hz, 1H);
4.28 (m, 1H);
4.10 (m, 1H); 3.92 (s, 3H); 3.60 (m, 1H); 3.35 (t, J= 11.1 Hz, 1H); 1.52 (s,
3H); 1.36 (s, 3H).
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 165 -
148.v. (4R,7S)-4-(6-methoxy-quinolin-4-yloxymethyl)-2,2-dimethyl-tetrahydro-
[ 1,3 ] dioxolo[4, 5-c]pyran-7-ylamine:
To a solution of intermediate 148.iv (1.97 g, 3.98 mmol) in EA (60 ml) was
added 10%
palladium on charcoal (1.7 g). The reaction was stirred under hydrogen
atmosphere for 5 h
before being filtered. The filtrate was concentrated in vacuo. The residue was
purified by
column chromatography over silica gel (DCM-MeOH 9-1 containing 1% concentrated
NH4OH) to afford the title amine as a white foam (0.885 g, 61% yield). The
compound was
obtained as a 3:1 mixture of diastereomers.
MS (ESI, m/z): 361.3 [M+H+].
148 . vi. 3-oxo-3, 4-dihydro-2H pyNido[3, 2-b][1, 4]thiazine-6-carboxylic acid
(4R, 7S)-[4-(6-methoxy-quinolin-4 yloxynaethyl)-cis-(4RS, 5RS)-2, 2-dimethyl-
tetral7ydro-
[1,3]dioxolo[4,5-cJpyran-7 ylJ-anaide:
To a mixture of intermediate 148.v (0.34 g) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid (0.218 g) in DMF (9 ml) and
DCM (6 ml)
were added DIPEA (0.493 ml) and HATU (0.430 g). The reaction mixture was
stirred
overnight at rt and concentrated to dryness. The residue was purified by
column
chromatography over silica gel (DCM-MeOH 97-3 with 1% concentrated NH4OH then
DCM-
MeOH 19-1 with 1% concentrated NH4OH) to afford a yellow solid (0.206 g, 0.37
mmol).
The compound was obtained as a 3:1 mixture of diastereomers.
MS (ESI, m/z): 553.4 [M+H+].
Example 149: 6-{(4R,7S)-[4-(6-methoxy-quinolin-4-yloxymethyl)-(4S,5S)-2,2-
dimethyl-
tetrahyd ro- [ 1,3] dioxolo [4, 5-c] pyran-7-ylamino] -methyl}-4H-pyrido [3,2-
b] [ 1, 4] thiazin-
3-one:
Starting from intermediate 148.v (0.539 g, 1.5 mmol) and 3-oxo-3,4-dihydro-
2FI-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.319 g, 1.64 mmol), the title
compound
(0.425 g, 52% yield) was prepared as a white solid according to the procedure
described in
Example 88, step 88.iv (DCM-MeOH 19-1 with 1% concentrated NH40H being however
used as chromatography eluent).
MS (ESI, m/z): 539.0 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 166 -
Example 150: 6-{(4R,7S)-([4-(6-methoxy-quinolin-4-yloxymethyl)-
(4R,5R)-2,2-dimethyl-tetrahydro-[1,3]dioxolo [4,5-c]pyran-7-ylamino]-methyl}-
4.FI-pyrido[3,2-b] [1,4]thiazin-3-one:
Starting from intermediate 148.v (0.539 g, 1-5 mmol) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.319 g, 1.64 mmol), the title
compound
(0.105 g, 13% yield) was prepared as a brown solid according to the procedure
described in
Example 88, step 88.iv (DCM-MeOH 19-1 with 1% concentrated NH4OH being however
used as chromatography eluent).
MS (ESI, m/z): 539.3 [M+H+].
Example 151: 6-{(3S,4S,5S,6R)-[4,5-dihydroxy-6-(6-methoxy-quinolin-4-
yloxymethyl)-
tetrahydro-pyran-3-ylamino]-methyl}-4H-pyrido[3,2-b] [1,4]thiazin-3-one:
A solution of the compound of Example 149 (0.418 g, 0.77 mmol) in a TFA-water
mixture
(3-1, 15 ml) was stirred at rt for 1.5 h, concentrated and basified with 1M
aq. NaOH A solid
precipitated which was filtered, washed with water and dried under HV to yield
the title
compound as a beige solid (0.337 g, 0.67 mmol).
MS (ESI, m/z: 499.1 [M+H+].
Example 152: 6-{(3S,4R,5R,6R)-[4,5-dihydroxy-6-(6-methoxy-quinolin-4-
yloxymethyl)-
tetrahydro-pyran-3-ylamino]-methyl}-4H-pyrido[3,Z-b] [1,4]thiazin-3-one:
A solution of the compound of Example 149 (0.094 g, 0.17 mmol) in a TFA-water
mixture
(3-1, 3.5 ml) was stirred at rt for 1.5 h, concentrated and basified with 1M
aq. NaOH. The
mixture was concentrated to dryness and the residue purified by column
chromatography
(DCM-IVIeOH 9-1 with 1% concentrated NH4OH) to afford a beige solid (0.056 g,
0.11 mol).
MS (ESI, m/z): 499.1 [M+H+].
Example 153: 8-{(2S,5R)-5-[(3-oxo-3,4-dihydro-2H-pyrido[3,2-b] [1,4]oxazin-
6-ylmethyl)-amino]-tetrahydro-pyran-2-ylmethoxy}-quinoline-2-carbonitrile:
Starting from (2S,5R)-8-(5-amino-tetrahydro-pyran-2-ylmethoxy)-quinoline-2-
carbonitrile
(0.179 g, 0.63 mmol) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]oxazine-6-
carbaldehyde
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 167 -
(0.118 g, 0.66 mmol), the title compound (0.040 g, 14% yield; purity 70%) was
prepared as a
yellowish foam according to the procedure described in Example 88, step 88.iv.
MS (ESI, m/z): 446.0 [M+H+].
Example 154: 6-{[(3S,6R)-6-(6-methoxy-quinazolin-4-yloxym ethyl)-tetrahydro-
pyran-
3-ylamino]-methyl}-4H-pyrido[3,2-b][1,4]thiazin-3-one:
154.i. (S)-(1-hydroxymethiyl pent-4-enyl)-carbamic acid tert-butyl ester:
To a suspension of LiBH4 (1.15 g, 53 mmol) in THF (300 ml) at rt was added a
solution of
(S)-2-tert-butoxycarbonylamino-hex-5-enoic acid methyl ester (12.9 g, 53 mmol,
prepared
according to J. Org. Chern. (1995), 60, 2210) in THF (100 ml). The mixture was
stirred at rt
for 4 h, poured into water and extracted with EA. The organic layer was washed
with brine,
dried over MgSO4 and concentrated to give the title alcohol (11.4 g, 99%
yield) as a
colourless oil.
1H NMR (CDC13) S: 5.75 -5.65 (m, 1H), 5.00-4.90 (m, 2H), 4.5 (br, 1H, OH),
3.70-3.45 (m,
3H), 2.10-2.00 (m, 2H), 1.60-1.35 (m, 2H), 1.38 (s, 9H).
154.ii. (IS,3RS,4RS)-(1-Fiydroxymethyl-3-oxiranyl propyl)-carban2ic acid tert-
butyl ester:
Intermediate 154.i (11.4 g, 53 mmol) was dissolved in 1,2-DCE (300 ml) and
water (250 ml)
and 1Mphosphate buffer pH 8 (150 ml) was added. MCPBA (14.3 g, 1.1 eq, 70%)
was added
and the mixture vigorously stirred overnight. The two phases were separated
and the aqueous
phase was extracted once more with DCM. The combined organic layers were
washed with a
sat. NaHCO3 solution, dried over MgSO4, filtered and concentrated to dryness.
The residue
was purified by chromatography on silica gel (Hex:EA 1:1 then EA) to give the
title epoxide
(7.74 g, 63% yield, mixture of diastereomers) as a colourless oil.
1H NMR (CDC13) 8: 4.90-4.85 (m, 1H), 3.75-3.50 (m, 3H), 3.00-2.90 (m, 1H),
2.80-2.51 (m,
IH), 2.6 (br, 1H, OH), 2.55-2.50 (m, 1H), 1.80-1.40 (m, 4H), 1.42 (s, 9H).
154.iii. (3S,6R)-(6-hydro.xymethyl-tetrahydropyran-3 yl)-carbarnic acid tert-
butyl ester:
A solution of intermediate 154.ii (2.3 g, 10 mmol) in DCM (50 ml) was treated
with
D,L-10-camphor sulfonic acid (0.1 eq). The reaction was slightly exothermic.
The mixture
was stirred at rt for 3 h, concentrated and purified by chromatography on SiO2
(EA) to give
the title tetrahydropyran (0.874 g, 37% yield) as a colourless solid.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-168-
1H NMR (CDC13) 8: 4.28 (br, 1H), 4.20-10 (m, 1H), 3.70-3.30 (m, 5H), 3.04 (t,
1H,
J=10.6 Hz), 2.20-2.00 (m, 2H, 1.75-1.20 (m, 11H).
154.iv. [(3S,6R)-6-(6-methoxy-quinazolin-4 yloxymetlryl)-tetrahydro pyran-3
ylJ-carbamic
acid tert-butyl ester:
NaH (0.036 g, 0.82 mmol, 55% dispersion in oil) was added to a solution of 4-
chloro-
8-methoxyquinazoline (0.160 g, 0.822 mmol) arnd intermediate 154.iii (0.190 g,
0.822 mmol)
in DMF (3 ml) at rt. The mixture was stirred at rt for 2 h, then partitioned
between EA and
water. The organic layer was washed with water and brine, dried over MgSO4,
filtered and
concentrated in vacuo. The residue was purified by chromatography on silica
gel (Hex:EA 1:1
then EA) then further purified by crystallisation from ether. The title ether
(0.1 g, 3 1 fo yield)
was obtained as a colourless solid.
MS (ESI, m/z): 389.9 [M+].
154.v. (3S,6R)-6-(6-methoxy-quinazolin-4 yloxymethyl)-tetrahydropyran-3
ylamine:
A solution of intermediate 154.iv (0.1 g, 0.25 rnmol) in DCM (10 ml) was
treated with TFA
(2 ml). The mixture was stirred at rt for 3 h, concentrated in vacuo and
partitioned between
DCM and NH4OH. The organic layer was dried over MgSO4, filtered and
concentrated to
dryness to give the title amine (0.071 g, 96% yield) as a solid, which was
used without further
purification.
MS (ESI, m/z): 290.3 [M+H+].
154.vi. 6-{[(3S, 6R)-6-(6-methoxy-quinazolin-4 yloxymethyl)-tetrahydro pyran-3
ylarninoJ-
rnethyl)-4H-pyrido[3, 2-b][l, 4Jthiazin-3-one:
A solution of intennediate 154.v (0.072 g, 0.24 mmol) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.048 g) in a 1,2-DCE:MeOH
mixture (1:1,
4 ml) was stirred at rt overnight. NaBH4 (exce ss) was added and stirring
continued for 2 h.
The mixture was partitioned between DCM and concentrated NH4OH, the organic
layer dried
over MgS04 and concentrated to dryness. The residue was purified by
chromatography on
silica gel (EA:MeOH 9:1 + 1% concentrated NH4OH) to give the title comp ound
as a
yellowish foam (0.085 g, 73% yield).
'H NMR (d6-DMSO) 6: 8.66 (s, 1H), 7.78 (d, 1H, J=9.1 Hz), 7.73 (d, 1H, J=7.8
Hz),
7. 5 9(dd, 1H, J=9.1 Hz, J=2.9 Hz), 7.3 7(d, 1 H, J=2.9 Hz), 7.10 (d, 1 H,
J=7. 8 Hz), 4.52 (d,
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 169 -
1H, ..T=5.5 Hz), 4.05-3.90 (m, IH), 3.92 (s, 3H), 3.80-3.60 (m, 3H), 3.53 (s,
2H), 3.02 (t, IH,
J=1 0.4 Hz), 2.20-2.00 (m, 2H), 1.85-1.75 (m, 1H), 1.60-1.20 (m, 2H).
MS (ESI, m/z): 468.2 [M+H+].
Example 155: (2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-[(3S,6R)-6-(3-methoxy-
quinolin-5-yloxymethyl)-tetrahydro-pyran-3-yl]-amine:
155. i. [(3S, 6R)-6-(3-methoxy-quinolin-5yloxymethyl)-tetrahydropyran-3 ylJ-
carbamic acid
tert-butyl ester:
This compound (0.709 g, 50% yield) was obtained as a yellowish foam from
intermediate 154.iii (0.845 g, 3.65 mmol) and 3-methoxy-quinolin-5-ol (2.62 g,
15 mmol)
according to the procedure of Example 77, step 77.ii.
MS (ESI, m/z): 389.0 [M+H+].
155. ii. (3S, 6R)-6-(3-methoxy-quinolin-5 yloxymethyl)-tetrahydro pyran-
3ylamine:
Starting from intermediate 155.i (0.709 g, 1.82 mmol), the title compound
(0.550 g, quant.)
was prepared as a yellowish oil according to the procedure of Exainple 77,
step 77.iii.
MS (ESI, m/z): 289.2 [M+H+].
155 . iii. (2, 3-dihydro-benzo[1, 4]dioxin-6 ylmethyl)-[(3S, 6R)-6-(3-methoxy-
quinolin-5-
yloxymethyl)-tetrahydro pyran-3 ylJ-amine:
A solution of intermediate 155.ii (0.173 g, 0.6 mmol) and 2,3-dihydro-
benzo[1,4]dioxine-
6-carbaldehyde (0.0985 g, 0.6 mmol) in a DCE:MeOH mixture (2:1, 6 ml) was
stirred at rt
overnight. NaBH4 (1 eq) was added and stirring continued for 2 h. The mixture
was
partitioned between DCM and concentrated NH4OH. The organic layer was dried
over
MgSO4 and concentrated. Chromatography over silica gel (EAJMeOH 9:1 + 1%
N114OH)
gave the title compound as a colourless oil (167 mg, 63%).
MS (ESI, m/z): 437.3 [M+H+].
Example 156: 6-{[(3S,6R)-6-(3-methoxy-quinolin-5-yloxymethyl)-tetrahydro-pyran-
3-ylamino]-methyl}-4H-pyrido[3,2-b] [1,4]oxazin-3-one:
A solution of intermediate 155.ii (173 mg, 0.6 mmol) and 3-oxo-3,4-dihydro-
2FI-pyrido[3,2-b][1,4]oxazine-6-carbaldehyde (107 mg, 0.6 mmal) in 1,2-
DCE/MeOH (2:1,
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 170 -
6 ml) was stirred at rt overnight. NaBH4 (1 eq) was added and stirring
continued for 2 h. The
mixture was partitioned between DCM and NH4OH and the organic layer dried over
MgSO4
and concentrated. Chromatography over silica gel (EA/MeOH 9:1 + 1% NH4OH) ga
ve the
title compound as a colourless solid (139 mg, 51%).
MS (ESI, m/z): 451.2 [M+H+].
Example 157: 6-{[(3S,6R)-6-(3-methoxy-quinolin-5-yloxymethyl)-tetrahydro-pyra_
n-
3-ylamino]-methyl}-4Il-pyrido [3,2-b] [1,4]thiazin-3-one:
A solution of intermediate 155.ii (0.173 g, 0.6 mmol) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.117 g, 0.6 mmol) in a 1,2-
DCE:1V1eOH
mixture (2:1, 6 ml) was stirred at rt overnight. NaBH4 (1 eq) was added and
stirring continued
for 2 h. The mixture was partitioned between DCM and concentrated NH4OH. The
organic
layer was dried over MgSO4, filtered and concentrated. Chromatography over
silica gel
(EA:MeOH 9:1 +1% concentrated NH4OH) gave the title compound (0.146 g, 52%
yield) as
a colourless oil.
MS (ESI, m/z): 467.1 [M+H' ].
Example 158: 6-({(3R,6R)-6-[2-(6-methoxy-[1,5]naphthyridin-4-yl)-ethyl]-
tetrahy-dro-
pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4]thiazin-3-one:
158.i. (3R, 6S)-(6 formyl-tetrahydro pyran-3 yl)-carbamic acid tert-butyl
ester:
To a solution of oxalyl chloride (3.5 ml) in DCM (25 ml) cooled to -78 C, was
added
dropwise a solution of DMSO (3.5 ml) in DCM (25 ml). After 15 min stirring, a
solution of
(3R,6S)-(6-hydroxymethyl-tetrahydro-pyran-3-yl)-carbamic acid tert-butyl ester
(prepared as
described in Eur. J. Org. Chem. (2003), 2418-2427) in DCM (25 ml) was added
dropwise.
The reaction was stirred 1 h and a solution of TEA (15 ml) in DCM (15 ml) was
added
dropwise. The reaction proceeded for 1 h, with warming to 0 C. Saturated
NaHCO3 (50 ml)
was added. The organic layer was separated, dried over Na2SO4, filtered and
concentxated in
vacuo. The residue was chromatographed (Hex-EA 1-2) to afford the title
aldehyde (2.5 g) as
a colourless solid.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-171-
158.ii. (3R,6S)-(6-ethynyl-tetrahydro pyran-3 yl)-carbamic acid tert-butyl
ester:
To a solution of p-toluenesulfonyl azide (3.08 g) in MeCN (200 mL) were added
K2C03
(5.38 g) and dimethyl-2-oxophosphonate (2.13 ml). The nzixture was stirred 2 h
at rt and a
solution of intermediate 158.i (2.5 g) in MeOH (30 ml) was added. The mixture
was stirred
overnight at rt. After concentration to dryness, the residue was partitioned
between water
(50 ml) and EA (100 ml). The aq. layer was extracted with EA (2 x 100 ml). The
combined
organic layers were washed with brine, dried over Na2SO4, filtered and
concentrated to
dryness. The residue was filtered through silica gel (EA-Hex 1-3) to afford
the title alkyne
(1.9 g) as a white solid.
1H NMR (CDC13) S: 4.78 (m, 1H); 4.39 (m, 1H); 4.14 (dd, J= 3.0, 11.4 Hz, 1H);
3.71 (m,
IH); 3.34 (m, 1 H); 2.50 (d, J= 2.2 Hz, 1 H); 2.11-1.99 (m, 2H); 1.73 (m, 1
H); 1.60 (m, 1 H);
1.46 (s, 9H).
MS (ESI, m/z): 226.2 [M+H+].
158.iii. [(3R,6S)-6-(6-methoxy-[1,5]naphthyridin-4 ylethynyl)-tetrahydro pyran-
3 ylJ-
carbamic acid tert-butyl ester:
To a solution of intermediate 158.ii (0.885 g) and trifluoromethanesulfonic
acid 6-methoxy-
[1,5]naphthyridin-4-yl ester (1.1 g) in DMF (5 ml) and TEA (3 ml) were added
successively
copper iodide (0.065 g) and bis(triphenylphosphine)palladium(II) chloride
(0.125 g). The
reaction mixture was stirred at rt for 90 min. The solvents were removed under
reduced
pressure and the residue was partitioned between EA (100 ml) and water (50
ml). The aq.
layer was extracted twice more (2 x 100 ml). The combined organic layers were
washed with
brine, dried over Na2SO4, filtered and concentrated to dryness. The residue
was
chromatographed over silica gel (Hex-EA 1-2) to afford the title alkyne (0.975
g) as a beige
solid.
MS (ESI, m/z): 384.5 [M+H+].
158.iv. {(3R, 6R)-6-[2-(6-methoxy-[1, 5]naphthyridin-4 yl) -ethyl]-tetrahydro
pyran-3yl}-
carbamic acid tert-butyl ester:
To a solution of intermediate 158.iii (0.975 g) in MeOH (20 ml) was added 10%
palladium on
charcoal (0.5 g). The reaction was stirred under hydrogen for 90 min. After
dilution with EA
(200 ml), the catalyst was removed by filtration and the filtrate was
concentrated to dryness to
afford the title compound as a beige solid (0.97 g).
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-172-
MS (ESI, ni/z): 388.4 [M+H+].
158.v. (3R, 6R)-6-[2-(6-methoxy-[1, 5]naphthyridin-4 yl)-ethylJ-tetrahydro
pyrcrn-3 ylamine:
The title amine (0.6 g) was obtained as a white solid starting from
interrrnediate 158.iv
(0.97 g) and using the procedure described in Example 77, step 77.iii. The c
ompound was
purified by chromatography (DCM-MeOH 9-1 containing 1% concentrated NH4OH).
'H NMR (CDC13) b: 8.66 (d, J = 7.5 Hz, 1H); 8.20 (d, J = 9.0 Hz, 1H); 7.40
4(d, J = 7.5 Hz,
1H); 7.11 (d, J= 9.0 Hz, 1H); 4.09 (s, 3H); 4.00 (ddd, J= 2.2, 4.4, 10.6 Hz,
1H);
3.34-3.17 (m, 3H); 3.02 (t, J= 10.6 Hz, 1H); 2.86 (m, 1H); 2.08-1.92 (m, 3H),
1.74 (m, IH);
1.62 (br s, 2H); 1.46 (m, 1H); 1.26 (m, 1H).
MS (ESI, m/z): 288.3 [M+H+].
8.vi. 6-({(3R, 6R)-6-[2-(6-methoxy-[1, 5]naphthyridin-4 yl)-ethylJ-tetrahydro
pyran-3-
ylezmino}-methyl)-4H-pyrido[3, 2-bJ[1, 4]thiazin-3-one:
To a solution of intermediate 158.v (0.1 g) in 1,2-DCE (6 ml) and MeOH (2 ml)
were added
po-wdered 3 A molecular sieves (2 g) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-
b][1,4]thiazine-
15 6-carbaldehyde (0.074 g). The reaction mixture was stirred at 50 C
overnight_ After cooling
to rt, NaBH4 (0.1 g) was added. After 2 h, the reaction mixture was filtered
through a plug of
Hydromatrix (pretreated with NaHCO3). After concentration in vacuo, ttie
residue was
chromatographed over silica gel (DCM-MeOH 19-1 with 1% concentrated a.q.
NH4OH) to
afford the title compound (0.045 g) as a white foam.
lIi NMR (d6-DMSO) S: 10.87 (s, 1H); 8.66 (d, J = 4.4 Hz, 1H); 8.23 (d, J = 9.0
Hz, 1H); 7.73
(d, J = 7.8 Hz, 1H); 7.52 (d, J 4.4 Hz, 1H); 7.24 (d, J = 9.0 Hz, 1H); 7.08
(d, J = 7.8 Hz,
IH); 4.06 (s, 3H); 3.95 (ddd, J 1.6, 4.4, 12.5 Hz, 1H); 3.72 (br s, 2H); 3.53
(s, 2H); 3.21-
3.09 (m, 3H); 2.93 (t, J = 10.4 Hz, 1H); 2.48 (partially overlapped with DMSO,
m, 1H); 2.09
(br s, 1H); 1.99 (m, 1H); 1.84 (m, 2H); 1.73 (m, 1H); 1.28-1.15 (m, 2H).
MS (ESI, m/z): 466.3 [M+H].
Example 159: 3-oxo-3,4-dihydro-2H-pyrido[3,2-b] [1,4]thiazine-6-carboxylic
acid
{(3R,6R)-6-[2-(6-methoxy-[1,5] naphthyridin-4-yl)-ethyl]-tetrahydro-pyran-3-
yl}-amide:
To a suspension of intermediate 158.v (0.1 g, 0.348 mmol) and 3-oxo-3,4-
dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid (0.081 g, 0.38 mmol) in DIMF
(4 ml) were
added DIPEA (0.18 ml, 1.04 mmol) and HATU (0.15 g, 0.39 mmol). The reaction
mixture
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-173-
was stirred at rt for 5 h and then concentrated to dryness. The residue was
chromatographed
(DCM-MeOH 19-1) to afford the title amide (0.081 g, 0.17 mmol) as a white
solid.
MS (ESI, m/z) : 480.2 [M+H+].
Example 160: 6-({(3R,6R)-(6-[2-(6-methoxy-[1,5]naphthyridin-4-yl)-ethyl]-
tetrahydro-
pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b][1,4]oxazin-3-one:
This compound was prepared from intermediate 158.v (0.1 g, 0. 348 mmol) and 3-
oxo-
3,4-dihydro-2H-pyrido[3,2-b][1,4]oxazine-6-carbaldehyde (0.068 g, 0.38 mmol)
according to
the procedure of Example 88, step 88.iv. The title compound (0.038 g, 0.084
mmol) was
obtained as a foam.
'H NMR (d6-DMSO) 8: 11.17 (s, 1H); 8.66 (d, J= 4.4 Hz, 1H); 8-23 (d, J= 9.1
Hz, 1H);
7.51 (d, J= 4.4 Hz, 1 H); 7.30 (d, J = 8.0 Hz, 1 H); 7.24 (d, J = 9.1 Hz, 1
H); 7.01 (d, J= 8.0 Hz,
1H); 4.61 (s, 2H); 4.01 (s, 3H); 3.94 (m, 1H); 3.69 (dd, AB system' J = 15.0
Hz, 0= 0.056,
2H); 3.26-3.07 (m, 3H); 2.92 (t, J = 10.4 Hz, 1H); 2.48 (partially overlapped
m, 1H); 1.98 (m,
2H); 1-87-1.80 (m, 2H); 1.73 (m, 1H); 1.32-1.15 (m, 2H).
MS (ESI, m/z): 450.4 [M+H+].
Example 161: 6-((3R,6R)-{6-[2-(6-methoxy-[1,5]naphthyridin-4-yl)-ethyl]-
tetrahydro-
pyran-3-ylaniino}-methyl)-4H-benzo [1,4]thiazin-3-one:
Starting from intermediate 158.v (0.100 g) and 3-oxo-3,4-dihydro-2H-
benzo[1,4]thiazine-
6-carbaldehyde (0.074 g), the title compound (0.094 g) was prepared as a
yellow foam
according to the procedure described in Example 88, step 88.iv.
'H NMR (CDC13) 8: 8.65 (d, J = 4.5 Hz, 1H); 8.40 (br. s, 1H); 8-18 (d, J = 9.0
Hz, 1H);
7.37 (d, J= 4.5 Hz, 1H);7.25(d,J=7.9Hz, 111);7.10(d,J=9.0Hz,
1H);6.96(dd,J=1.5,
7.9 Hz, 1H); 6.85 (d, J= 1.5 Hz, 1H); 4.10 (m, 1H); 4.06 (s, 3H); 3.77 (AB, J=
3.77, 3.0,
30.1 Hz, 2H); 3.41 (s, 2H); 3.25 (m, 3H); 3.06 (t, J= 10.6 Hz, 1H); 2.68 (m,
1H); 2.07 (m,
1H); 1.93 (m, 2H); 1.74 (m, 1H); 1.39 (m, 1H), 1.28 (m, 11-1).
MS (ESI, m/z): 465.2 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 174 -
Example 162: 6-({(3R,6R)-6-[2-(6-methoxy-quin azolin-4-yl)-ethyl]-tetrahydro-
pyran-
3-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4]thiazin-3-one:
162.i. [(3R, 6S)-6-(6-methoxy-quinazolin-4 ylethynyl)-tetrahydro pyran-3 ylJ-
carbamic acid
tert-butyl ester:
Starting from 4-chloro-6-methoxy-quinazoline (0.564 g) and intermediate 158.ii
(0.65 g) and
using the procedure of Example 158, step 158.iii, the title alkyne (0.49 g)
was recovered as an
orange solid.
MS (ESI, m/z): 384.4 [M+H+].
162.ii. {(3R, 6R)-6-[2-(6-methoxy-quinazolin-4 yl)-ethylJ-tetrahydro pyran-3
yl)-carbezmic
acid tert-butyl ester:
The title compound (0.39 g) was obtained as a solid starting from intermediate
162.i 4(0.49 g)
and using the procedure described in Example 157, step 157.iv.
MS (ESI, m/z): 388.4 [M+H+]
162.iii. (3R, 6R)-6-[2-(6-methoxy-quinazolin-4 yl)-ethylJ-tetrahydro pyran-3
ylamine:
The title amine (0.175 g) was obtained as a white solid starting from
intermediate 162.ii
(0.39 g) and using the procedure described in Example 77, step 77.iii. The
compound was
purified by chromatography over silica gel (DCM-MeOH 9-1 containing 1%
concentrated
NH4OH).
MS (ESI, m/z): 288.3 [M+H+].
162.iv. 6-({(3R, 6R)-6-[2-(6-methoxy-quinazolin-4 yl)-ethylJ-tetrahydro pyran-
3 ylam ino}-
methyl)-4.H pyrido[3, 2-b][1, 4]thiazin-3-one:
This compound was prepared from intermediate 162.iii (0.1 g) and 3-oxo-3,4-
dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.074 g) according to the
procedure described
in Exarnple 88, step 88.iv. The title compound (0.054 g) was obtained as a
foam.
MS (ESI, m/z): 466.5 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-175-
Example 163: 6-((3R,6R)-{6-[2-(6-methoxy-quinazolin-4-yl)-ethyl] -tetrahydro-
pyran-
3-ylamino}-methyl)-4H'-benzo[1,4]thiazin-3-one:
This was prepared from intermediate 162.iii (0.072 g) and 3-oxo-3,4-dihydro-
2H-benzo[1,4]thiazine-6-carbaldehyde (0.053 g) according to the procedure
described in
Example 88, step 88.iv. The title compound (0.062 g) was obtained as a foam.
MS (ESI, m/z): 465.5 [M+H+].
Example 164: 6-({(3R,6,S)-6-E-[2-(3-methoxy-quinolin-5-yl)-vinyl]-tetrahydro-
pyran-
3-ylamino}-methyl)-4H-pyrido [3,2-b] [1,4]thiazin-3-one:
164.i. (3R, 6S)-[6-(1 phenyl-lH-tetrazol-5ylsulfarrylmethyl)-tetrahydY-opyran-
3 ylJ-carbamic
acid tert-butyl ester:
To an ice-chilled solution of intermediate 78.i (5.12 g), in THF (200 ml),
were added
successively dropwise PPh3 (11.40 g), phenyltetrazole thiol (5.52 g) and DIAD
(6.6 ml). The
resulting solution was stirred at rt overnight. The reaction mixture was
concentrated to
dryness and the residue was purified by column chromatography over silica gel
(EA-Hex 1-9
to 1-1) to afford the title compound as a white solid (8.16 g).
MS (ESI, m/z): 392.5 [1VI+H+].
164.ii. (3R,6S)-[6-(1 phenyl-lH-tetrazole-5-sulfonylmethyl)-tetrahyd-o pyran-3
ylJ-carbamic
acid tert-butyl ester:
To a stirred solution of intermediate 164.i (8.1 g, 20.7 mmol) in EtOH (180
ml) was added at
rt a solution of ammonium molybdate (3.200 g) in 30% aqueous hydrogen peroxide
(27 ml)
The reaction mixture was stirred at rt for 2 h. Saturated sodium thiosulfate
(180 ml) was
carefully added, cooling with an ice-bath. The solvent was removedt under
reduced pressure
and the residue extracted three times with EA. The combined organic layers
were washed
with brine, dried over Na2SO4, filtered and concentrated under reduced
pressure. The residue
was purified by coluinn chromatography (EA-Hex 1-2 then 1-1) to afford a white
foam
(4.39 g).
MS (ESI, m/z): 4245 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 176 -
164.iii. {(3R, 6S)-6-[2-(3-methoxy-quinolin-5yl)-vinylJ-tetralhydro pyran-3
yl}-carbamic acid
tert-butyl ester:
To a solution of intermediate 164.ii (4.39g, 10.3mmol) in DMF-HMPA (3-1, 60
ml) cooled to
-35 C was added dropwise LiHMDS (1M in THF, 12.44 ml) and, after the addition
was
complete, 3-methoxy-quinoline-5-carbaldehyde (1.9 g) in DMF-HMPA (3 -1, 120
ml). The
reaction was allowed to warm slowly to rt and stirred overnight. Water and
ether were added.
The layers were separated and the aqueous layer was extracted three times with
ether. The
organic layers were washed with water and brine, dried over MgSO4, filtered
and
concentrated under reduced pressure. 'The residue was purified by column
chromatography
over silica gel (EA-Hex 1-2 to 2-1) to afford a yellow solid (2 g) consisting
of a 2:1 E:Z
mixture.
MS (ESI, m/z): 385.3 [M+H+]
164.iv. (3R,6S)-6-[2-(3-methoxy-quinolin-S yl)-vinylJ-tetrahydro pyran-3
ylamine:
A solution of intermediate 164.iii (0.536 g) in TFA (5 ml) was stirred for 5
min. The reaction
mixture was concentrated to dryness. The residue was basified with a 3MNaOH
solution and
the mixture extracted five times with DCM-MeOH 9-1. The combined organic
layers were
washed with brine, dried over Na2SO4, filtered and evaporated. The residue was
purified by
column chromatography over silica gel (DCM-MeOH 19-1 containing 1 1o aq.
NH4OH) to
afford a yellow gum (0.187 g) consisting of a 2:1 E:Z mixture.
MS (ESI, m/z): 285.3 [M+H+].
164.v. 6-({(3R,6S)-6-E-[2-(3-methoxy-quinolin-5yl)-vinyl]-tetrahydro pyrcin-3
ylamino}-
methyl)-4Fl-pyrido[3, 2-bJ[1, 4]thiazin-3-one:
Starting from intermediate 164. iv (0.100 g) and 3-oxo-3,4-dihydro-
2FI-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.072 g), the title compound
(0.059 g) was
obtained as a white foam according to the procedure described in Example 88,
step 88.iv. The
compound was obtained as an equimolar mixture of E and Z isomers.
MS (ESI, m/z): 463.5 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 177 -
Example 165: 6-({(3R,6R)-6-[2-(3-methoxy-quinolin-5-y1)-ethyl]-tetrahydro-
pyran-
3-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4]thiazin-3-one:
165.i. {(3R,6R)-6-[2-(3-rnethoxy-quinolin-S yl)-ethylJ-tetrahydro pyran-3 yl}-
cap-bamic acid
tert-butyl ester:
To a solution of intermediate 164.iii (1 g), in MeOH (40 ml) under nitrogen
was added 10%
palladium on charcoal (0.5 g). The resulting mixture was stirred under
hydrogern atmosphere
for 1 h_ The reaction mixture was diluted with EA, filtered and the filtrate
concentrated under
reduced pressure. The product was used in the next step without further
purification.
MS (ESI, m/z): 387.4 [M+H+].
165.ii. (3R,6R)-6-[2-(3-methoxy-quinolin-5 yl)-ethylJ-tetrahydropyran-3
ylamine:
A solution of crude intermediate 165.i (2.55 rnmol) in TFA (5 ml) was stirred
f r 5 min and
concentrated to dryness. The residue was basified with 1MNaOH solution and
extracted three
times with DCM-MeOH mixture (9-1, 3 x 50 ml). The combined organic layers
yvere washed
with brine, dried over Na2SO4, filtered and concentrated in vacuo. The residue
was purified by
column chromatography over silica gel (DCM-MeOH 19-1 containing 1% aq 1VH4OH
then
DCM-1VIeOH 9-1 containing 1% aq. NH4OH) t afford the title amine (0.583 g) as
a yellow
oil.
MS (ESI, m/z): 287.2 [M+H+].
165.iii_ 6-({(3R,6R)-6-[2-(3-methoxy-quinolin-S yl)-ethylJ-tetrahydNO pyran-3
ylamino}-
methyl~-4H-pyrido[3, 2-bJ[1, 4]thiazin-3-one:
Starting from intermediate 165.ii (0.100 g) and 3-ox(>-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde c0.075 g), the title compound
C0.075 g) was
obtained as a foam according to the procedure described in Example 88, step
88.iv.
MS (ESI, m/z): 465.4 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 178 -
Example 166: 3-oxo-3,4-dihydro-2H-pyrido[3,2-b] [1,4]thiazine-6-carboxylic
acid
(3R,6R)-{6-[2-(3-methoxy-quinolin-5-yl)-ethyl]-tetrahydro-pyran-3-yl}-amide:
This compound was prepared from intermediate 165.ii (0.1 g, 0.35 mmol) and 3-
oxo-
3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid (0.074 g, 0.35
mmol) according
to the procedure of Example 159. The title compound (0.022 g) was obtained as
a white solid.
MS (ESI, m/z): 479.3 [M+]H+].
Example 167: 6-((3R,6R)-{6-[2-(3-methoxy-quinolin-5-yl)-ethyl]-tetrahydro-
pyran-
3-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4]oxazin-3-one:
This compound (0.072 g) was obtained as a white foam from intermediate 165.ii
(0.1 g,
0.35 mmol) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]oxazine-6-carbaldehyde
(0.0068 g,
0.38 mmol) according to the procedure described in Example 88, step 88.iv.
MS (ESI, m/z): 449.4 [M+H+].
Example 168: 6-((3R,6R)-{6-[2-(6-methoxy-quinolin-4-yl)-ethyl]-tetrahydro-
pyran-
3-ylamino}-methyl)-4H-pyrido [3,2-b] [1,4]thiazin-3-one:
168.i. [(3R, 6S)-6-(6-methoxy-quinolin-4 ylethynyl)-tetrahydro pyran-3 ylJ-
carbamic aczd
tert-butyl ester:
This compound was prepared from intermediate 158.ii (0.942 g) and
trifluoromethanesulfonic
acid 6-methoxy-quinolin-4-yl ester (1.194 g, prepared as in WO 00/405 54)
according to the
procedure of Example 158, step 158.iii. A yellow solid (1.35 g) was obtained.
MS (ESI, m/z): 383.5 [M-+H+].
168.ii. {(3R, 6R)-6-[2-(6-methoxy-quinolin-4 yl)-ethylJ-tetrahydro pyran-3 yl}-
carbamic acid
tert-butyl ester:
This compound was prepared from intermediate 168.i (1.35 g) using the
procedure described
in Example 158, step 158. iv. The title product (1.35 g) was obtained as a
white foam.
MS (ESI, m/z): 387.4 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 179 -
168.iii. (3R,6R)-6-[2-(6-methoxy-quinolin-4yl)-ethylJ-tetrahydno pyran-3
ylamine:
The title amine (0.898 g) was obtained as a yellow oil starting frorrn
intermediate 168.ii
(1.35 g) and using the procedure described in Example 77, step 77.iii- The
compound was
purified by chromatography (DCM-MeOH 9-1 containing 1% concentrated NH4OH.
'H NMR (CDC13) 8: 8.65 (d, J= 4.4 Hz, 1H); 8.00 (d, J= 9.0 Hz, 1H); 7.35 (dd,
J= 2.8, 9.0 Hz, 1H); 7.32 (d, J= 2.8 Hz, 1H); 7.19 (d, J= 4.4 Hz, 1H); 3.99
(m, 1H); 3.95 (s,
3H); 3.22 (m, 2H); 3.09 (t, J= 7.8 Hz, 1 H); 3.0 (t, J=10.6 Hz, 1 H); 2_ 83
(m, 1 H); 2.02 (m,
1H); 1.88 (m, 2H); 1.64 (m, 1H); 1.48 (br. s, 2H); 1.40 (m, 1H); 1.23 (m, 1H).
MS (ESI, m/z): 287.3 [M+H+].
168.iv. 6-((3R, 6R)-[6-[2-(6-methoxy-quinolin-4 yl)-ethylJ-tetrahydro pyf=an-3
ylamino}-
methyl)-4HI pyrido[3,2-bJ[1,4Jthiazin-3-one:
Starting from intermediate 168.iii (0.100 g) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.066 g, 1.1 eq.), the title
compound (0.062 g)
was obtained as a beige foam according to the procedure described in Ea--ample
88, step 88.iv.
'H NMR (CDC13) b: 8.66 (d, J= 4.4 Hz, 1H); 8.34 (br. s, 1H); 8.01 (d, J= 9.1
Hz, 1H);
7.58 (d, J= 7. 8 Hz, 1H); 7.36 (dd, J = 2.7, 9.1 Hz, 1H); 7.31 (d, J = 2.7 Hz,
1H); 7.19 (d,
J= 4.4 Hz, 1H); 6.96 (d, J= 7.8 Hz, 1H); 4.13 (ddd, J= 2.3, 4.5, 10.9 THz,
1H); 3.94 (s, 3H);
3.87 (AB, J= 5.6, 42.8 Hz, 2H); 3.47 (s, 2H); 3.25 (m, 111); 3.13 (t, J = 10.7
Hz, 1 H,
overlapped); 3.12 (m, 2H, overlapped); 2.71 (m, 1H); 2.09 (m, 1H); 1.88 (m,
2H), 1.69 (m,
1H), 1.37 (m, 2H).
MS (ESI, m/z): 465.5 [M+H+].
Example 169: (3R,6R)-6-({6-[2-(6-methoxy-quinolin-4-yl)-ethyl]-tetrahydro-
pyran-
3-ylamino} -in ethyl)-4H-benzo [ 1,4] thiazin-3-one :
Starting from intermediate 168.iii (0.100 g) and 3-oxo-3,4-dihydro-2H-
benzo[1,4]thiazine-
6-carbaldehyde (0.074 g), the title compound (0.085 g) was obtained as a
yellow foam
according to the procedure described in Example 88, step 88.iv.
'H NMR (CDC13) S: 8.67 (d, J= 4.4 Hz, 1H); 8.44 (br. s, 1H); 8.01 (d, J= 9.1
Hz, 1H);
7.36 (dd, J= 2.7, 9.1 Hz, 1H); 7.31 (d, J= 2.7 Hz, 1H); 7.26 (d, J= 7.9 Hz,
1H); 7.19 (d,
J= 4.4Hz, 1 H); 6.97 (dd, J= 1. 5, 7.9 Hz, 1H); 6.86 (d, J= 1.5 Hz, 1 H); 4.10
(ddd,
J= 2.0, 4.2, 10.9 Hz, 1H); 3.94 (s, 3H); 3.78 (AB, J= 4.6, 31.6 Iiz, 2H); 3.41
(s, 2H);
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 180 -
3.22 (m, 2H); 3.10 (t, J= 10.3 Hz, 1H, overlapped); 3.06 (m, 1H, overlapped);
2.70 (m, 1H);
2.07 (m, IH); 1.86 (m, 2H), 1.66 (m, IH), 1.39 (m, 1H), 1.25 (m, IH).
MS (ESI, m/z): 464.4 [M+H+].
Example 170: 6-((3R,6R)-{6-[2-(6-methoxy-quinolin-4-y1)-ethyl]-tetrahydro-
pyran-
3-ylamino}-methyl)-4H-benzo[1,4]oxazin-3-one:
This compound (0.034 g) was obtained as a white solid from intermediate
168.iii (0.100 g,
0.345 mmol) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]oxazine-6-carbaldehyde
(0.068 g,
0.38 mmol) according to the procedure described in Example 88, step 88.iv.
MS (ESI, m/z): 449.6 [M+H+].
Example 171: 6-({3R,6S')-6-[(IR,2R)-1,2-dihydroxy-2-(3-methoxy-quinolin-5-yl)-
ethyl]-
tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4]thiazin-3-one:
171.i. (2R,5S)-toluene-4-sulfonic acid S-tert-butoxyearbonylamino-tetrahydro
1ayran-
2 ylmetlzyl ester:
To an ice-chilled solution of intermediate 78.i (10.3 g, 44.53 mmol) in DCM
(250 ml) were
added TEA (12.4 ml, 99 mmol), DMAP (0.5 g) and p-toluenesulfonyl chloride (9.4
g,
49 mmol). The reaction was stirred at rt for 4 h. Saturated NaHCO3 (100 ml)
was added. The
volatiles were removed under reduced pressure and the residue was taken up in
EA (400 ml).
The organic layer was washed with saturated copper sulfate (2 x 150 ml), water
(3 x 150 ml)
and brine (100 ml). The organic layer was dried over Na2S04, filtered and
concentrated to
dryness to afford after drying the title tosylate (17.1 g, 100%) as a white
solid.
171.ii. (3R,6S)-(6-iodonmethyl-tetrahydro pyran-3 yl)-carbamic acid tert-butyZ
ester:
To a solution of intermediate 171.i (17.1 g, 44.5 mmol) in acetone (150 ml)
was added NaI
(20 g, 133.8 mmol). The mixture was heated at 65 C for 20 h. After cooling,
water (200 ml)
was added and the volatiles were removed under reduced pressure. The residue
was filtered
and the solid was thoroughly washed with water, Hex and water. The solid v~ras
collected and
dried in vacuo to afford the title iodide (13.72 g, 90.1% yield) as a beige
solid_
MS (ESI, m/z): 342.2 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-181-
171.iii. (3R, 6S)-[6-(1 phenyl-lFl-tetrazol-5 ylsulfanylmethyl)-letrahydro
pyran-3 ylJ-
carbamic acid tert-butyl ester:
To a solution of 1-phenyl-lH-tetrazole-5-thiol (7.9 g) in EtOH (90 ml) was
added KOl3
(2.8 g, 49.9 mmol). The reaction mixture was refluxed for 1 h and intermediate
171.ii (13.7 gõ
40.2 mmol) was added. The mixture was refluxed overnight. Water (150 ml) was
added and
the volatiles were removed under reduced pressure. The solid was filtered off
and thoroughly
washed with water. After drying, the title sulphide (15.6 g, 39.8 mmol) was
obtained as a
white solid.
MS (ESI, rn/z): 342.2 [M+H+].
171.iv. (312,6S)-[6-(1 phenyl-IH-tetrazole-5-sulfonylrnethyl)-tetrahydropyran-
3 ylJ-
carbamic czcid tert-butyl ester:
To a solution of intermediate 171.iii (15.6 g, 39.8 mmol) in EtOH (300 ml)
were adde=d
ammoniurn molybdate (11 g, 8.8 mmol) and then 30% aq. hydrogen peroxide (50
ml). Tlie
mixture was heated at 65 C for 4 h. The reaction mixture was diluted with
water (11) arnd
EtOH was removed under reduced pressure. The aqueous layer was extracted twice
with E-A
(2 x500 rnl). The combined extracts were washed with brine, dried over Na2SO4,
filtered arnd
concentrated in vacuo. The title compound was crystallized from EA (50 ml) and
Hex (1 1).
The solid was then further washed with water and Hex and dried in vacuo to
yield the title
sulfone (15.2 g, 35.89 mmol) as a beige solid.
MS (ESI, m/z): 424.4 [M+H+].
171.v. {(3R, 6S)-trans-6-[2-(3-methoxy-quinolin-5 yl)-vinyl]-tetrahydro pyran-
3 yl}-
carbamic acid tert-butyl ester:
To a solution of 3-methoxy-quinoline-5-carbaldehyde (0.64 g, 3.41 mmol) axid
intermediate 171.iv (1.9 g, 4.48 mmol) in 1,2-DME (20 mL), cooled to -60 C,
was add_ed
dropwise over 15 min, potassium bis(trimethylsilyl) amide (0.5M in toluene, 14
ml). T'he
mixture was warmed gradually to rt over 2 h and water (20 rnl) and EA (100 ml)
were added.
The two layers were decanted and the aq. layer was extracted once more with EA
(100 n-Zl).
The combined organic layers were dried over Na2SO4, filtered and concentrated
to dryne:ss.
The residue was chromatographed over silica gel (EA) to afford the title that
was further
triturated in Hex to afford the title alkene (0.55 g, 1.43 mmol) as an off-
white solid.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-182-
1H NMR (d6-DMSO) 8: 8.64 (d, J = 2.8 Hz, 1H); 7.87 (d, J= 8.1 Hz, 1H); 7.81
(d, J = 2.8 Hz,
1H); 7.72 (d, J= 6.6 Hz, 1H); 7.53 (dd, J= 6.6, 8.1 Hz, 1H); 7.36 (d, J= 15.7
Hz, 1H);
6.82 (d, J= 8.1 Hz, IH); 6.32 (dd, J= 6.2, 15.7 Hz, IH) ; 4.00 (m, 1H); 3.97
(s, 3H); 3.88 (m,
1H); 3.38 (br s, 1H); 3.08 (t, J = 10.7 Hz, 1H); 1.88 (m, 21-1); 1.51 (m, 2H);
1.38 (s, 9H).
MS (ESI, m/z): 385.0 [M+H+].
171.vi. {(3R,6S)-6-[(1R,2R)-1,2-dihydroxy-2-(3-methoxy-quinolin-5 yl)-ethylJ-
tetrahydro-
pyran-3 yl}-carbamic acid tert-butyl ester:
To a mixture of intermediate 171.v (0.55 g, 1.43 mm(>l) in 2-methyl-2-propanol
(7 mL),
EA (2 mL) and water (8 mL) were added AD mix P (2.5 g) and methanesulfonamide
(0.163 g, 1.71 mmol). The reaction mixture was stirred vigorously over 3 d.
Sodium bisulfite
(2.7 g) was added carefully and the mixture was stirred 30 min. EA (50 ml) was
added and the
two layers were decanted. The aq. layer was extracted with EA twice more. The
combined
extracts were washed with brine, dried over Na2SO4, filtered and concentrated
to dryness. The
residue was chromatographed (DCM-MeOH 19-1) to afford the title diol (0.52 g,
86% yield)
as a white foam.
1H NMR (CDC13) 6: 8.69 (d, J = 2.4 Hz, 1H); 8.04 (d, J= 8.4 Hz, 1H); 7.82 (d,
J = 2.4 Hz,
1H); 7.72 (d, J = 6.3 Hz, 1H); 7.58 (dd, J = 6.3, 8.1 Hz, 1H); 5.44 (d, J =
6.9 Hz, 1H); 4.82 (br
s, 2H); 4.13 (m, 2H); 3.96 (s, 3H); 3.67 (d, J= 6Hz, 1H); 3.59 (br s, 1H);
2.94 (m, 1H);
2.79 (t, J = 10.8 Hz, 1H); 2.02 (m, 1H); 1.88 (qd, J= 4.2, 11.7 Hz, 1H); 1.50
(partially
overlapped m, 1H); 1.43 (s, 9H); 1.15 (qd, J= 5.1, 12.6 Hz, IH).
MS (ESI, m/z): 419.2 [M+H+].
171.vii. (IR,2R)-1-((2S,5R)-5-amino-tetrahydro pyran-2 yl)-2-(3-methoxy-
quinolin-S yl)-
ethane-1, 2-diol:
A solution of intermediate 171.vi (0.52 g, 1.24 mmol) in TFA (5 ml) was
stirred at rt for
15 min. After evaporation to dryness the residue was partitioned between IN
aq. NaOH
(20 ml) and DCM-MeOH (9-1, 60 ml). The aq. layer was extracted three times
with the same
mixture. The combined organic layers were washed with brine, dried over
Na2SO4, filtered
and concentrated to dryness. The residue was chromatographed over silica gel
(DCM-MeOH
6-1 with 1% aq. NH4OH) to afford the title amine (0.250 g, 0.78 mmol) as a
white foam.
MS (ESI, m/z): 319.2 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-183-
171.viii. 6-((3R, 6S)-6-[(1 R, 2R)-1, 2-dihydroxy-2-(3-metlaoxy-quinolin-5yl)-
ethyl]-
tetrahydro pyran-3 ylamino}-methyl)-4H-pyrido[3,2-bJ[1,4]thiazin-3-one:
To a solution of intermediate 171.vii (0.1 g) in MeOH (1.5 ml) and DCE (5 ml)
vvere added
3 A molecular sieves (2 g) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1
,4]thiazine-
6-carbaldehyde (0.067 g). The reaction mixture was heated at 50 C overnight.
NaBH4 (0.1 g)
was added and the reaction proceeded for 2 h. The reaction mixture was then
filtered through
Hydromatrix and the filtrate was concentrated to dryness. The residue was
chrotnatographed
over silica gel (DCM-MeOH 9-1 containing 1% aq. NH40H) to afford the title:
compound
(0.015 g) as a beige foam.
MS (ESI, m/z): 497.2 [M+H+].
Example 172: 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic
ztcid
{(3R,6S)-6-[(1R,2R)-1,2-dihyd roxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-tetrahy
dro-pyran-
3-yl}-amide:
To a solution of intermediate 171.vii (0.052 g) in DMF (3 ml) were added 3-oxo-
3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid (0.04 g), HATU (0.081 g) and
DIPEA
(0.09 ml). The reaction mixture was stirred overnight at rt. The solvent was
removed in vacuo.
The residue was taken up in water and the solid was filtered off. The solid
was purified by
chromatography over silica gel (DCM-MeOH 19-1 with 1% aq. NH4OH) to afford the
title
compound (0.010 g, 12%) as a beige solid.
MS (ESI, m/z): 511.0 [M+H+].
Example 173: 6-({(3R,6S)-6-[(1R,2R)-1,2-dihydroxy-2-(6-methoxy-
[1,5]naphthyridin-
4-yl)-ethyl]-tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4]thiazin-
3-one:
173.i. cis, trans-[(3R, 6S)-6-(2-ti~ibutylstannanyl-vinyl)-tetrahydro pyran-3
ylJ-caY-bamic acid
tert-butyl ester:
To a solution of intermediate 158.ii (1.95 g, 8.65 mmol) in THF (26 ml) was
added
bis(triphenylphosphine) palladium dichloride (0.12 g, 0.17 mmol) and then
tributyltin hydride
(2.75 ml, 10.38 mmol). The mixture was stirred at rt for 20 min. The reaction
mixture was
concentrated to dryness and the residue was chromatographed (Hex then Hey--EA
9-1) to
afford the title stannane (3.4 g) as an equimolar mixture of cis and trans
isomers.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-184-
173.ii. trans-{(3R,6S)-6-[2-(6-methoxy-[1,5]naphthyridin-4 yl)-vinylJ-
tetrahydro pyr=ean-
3 yl}-carbamic acid tert-butyl ester:
To a solution of intermediate 173.i (3.4 g, 6.58 mmol) and
trifluoromethanesulfanic acid
6-methoxy-[1,5]naphthyridin-4-yl ester (1.91 g, 6.2 mmol) in 1,4-dioxane (30
ml) werre added
successively LiCI (0.78 g, 18.6 mmol), 2,6-di-tert-butyl-4-methylphenol (few
seeds) and
tetrakis(triphenylphosphine) palladium (0.14 g, 0.12 mmol). The reaction
mixture was heated
at 100 C overnight. After cooling, the solids were filtered off and the
filtrate was corncentra.ted
to dryness. The residue was chromatographed (Hex-EA 1-1) to afford a white
solid that was
triturated in Hex to yield the title (E)-alkene (1.1 g,) as a white solid.
1H NMR (d6-DMSO) S: 8.72 (d, J = 4.7 Hz, 1H); 8.25 (d, J = 9.0 Hz, 1H); 7.84
(d, J = 4.7 Hz,
1H); 7.55 (d, J = 16.5 Hz, 1 H); 7.28 (d, J = 9.0 Hz, 1 H); 6.93 (dd, J= 5.3
Hz, 16.5 Hz, 1 H);
6.85 (d, J= 7.7 Hz, IH); 4.04 (s, 3H); 4.01 (partially overlapped m, 1H); 3.90
(m, 1H);
3.39 (br s, 1H); 3.10 (t, J= 10.6 Hz, 1H); 1.89 (m, 2H); 1.50 (m, 2H); 1.39
(s, 9H).
MS (ESI, m/z): 386.1 [M+13+].
173.iii. (3R,6S)-{6-[(IR,2R)-1,2-dihydroxy-2-(6-methoxy-[1,5]naphthyridin-4
yl)-ethylJ-
tetrahydro pyran-3 yl}-carbamic acid tert-butyl ester:
This compound was obtained as a white foam (1.1 g, 92%) from intermediate
173.ii (1.1 g,
2.85 mmol) according to the procedure of Example 171, step 171.vi.
'H NMR (d6-DMSO) 6: 8_76 (d, J= 4.5Hz, 1H); 8.25 (d, J= 9.0Hz, 1H); 7.74 (d,
J= 4.5Hz,
1H); 7.25 (d, J= 9.0Hz, 1H); 6.81 (br s, 1H); 6.75 (d, J= 7.8Hz, 1H); 5.65 (br
d, J= 6.6Hz,
1H); 5.28 (d, J= 6.8Hz, 1H); 4.41 (d, J= 6.4Hz, 1H); 4.00 (s, 3H); 3.79 (m,
2H); 3.3 3 (m, 1H);
2.92 (t, J= 10.7Hz, 1H); 1.96 (m, 2H); 1.47 (m, 2H); 1.38 (s, 9H).
MS (ESI, m/z): 420.2 [M+]H+].
173.iv. (IR,2R)-1-[(2S,5R)-(5-amino-tetrahydro pyran-2 yl)-2-(6-methoxy-
[1,5]naphthyridin-4 yl)-ethane-1,2-diol:
This compound was obtained as a yellowish foam (0.53 g, 80% yield) from
intermediate
173.iii (0.872 g, 2.08 mmol) according to the procedure of Example 171, step
171.vii.
1H NMR (CDC13) S: 8.78 (d, J = 4.5 Hz, IH); 8.25 (d, J= 9.3 Hz, 1H); 7.56 (d,
J = 4.5 Hz,
1H); 7.14 (d, J = 9.0 Hz, 1H); 5.59 (d, J = 3.3 Hz, 1H); 4.04 (s, 3H); 3.95
(partially
overlapped m, 2H); 3.38 (br d, J = 11.4 Hz, 1H); 3.01 (t, J = 10.8 Hz, 1H);
2.56 (m, 1H);
2.08 (m, 1H); 1.85 (m, lIi); 1.63 (m, 1H); 1.28 (br s, 4H); 1.26 (overlapped
m, 1Hj.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-185-
MS (ESI, m/z): 320.2 [M-+-H+].
173.v. 6-(((3R, 6S)-6- j(I R, 2R)-1, 2-dihydroxy-2-(6-methoxy-[1,
5]naplitltyridin-4 yl)-ethylJ-
tetrahydro pyran-3 ylaminoJ-methyl)-4Fl-pyrido[3,2-bJ[l,4]thiazin-3-vne:
To a solution of interrnediate 173.iv (0.1 g, 0.31 mmol) in MeOH (1.5 ml) and
1,2-DCE
(5 ml) were added 3 A molecular sieves (2 g) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.067 g, 0.35 mmol). The mixture
was heated
at 50 C overnight. After cooling, NaBH4 (0.1 g) was added and the reaction
proceeded for
2 h. The reaction mixture was filtered through Hydromatrix (treated with
saturated aq.
NaHCO3). The filtra.te was concentrated to dryness and the residue
chromatographed
(DCM-MeOH 9-1 with 1% aq. NH4OH) to afford the title compound (0.043 g, 27%
yield) as
a white solid.
MS (ESI, m/z): 498.2 [M+H+].
Example 174: 3-oxo-3,4-dihydro-2H-pyrido[3,2-b] [1,4]thiazine-6-carboxylic
acid
{(3R,6S)-6-[(1R,2R)-1,2-dihydroxy-2-(6-methoxy-[1,5] naphthyridin-4-yl)-ethyl]-
tetrahydro-pyran-3-yl}-amide:
The title compound (0.04 g, 25%) was produced as a white solid from
intermediate 173.iv
(0.1 g, 0.314 mmol) using the procedure of Example 172.
IH NMR (d6-DMSO) 6: 10.95 (s, 1H); 8.77 (d, J= 4.5 Hz, lI-L); 8.27 (d, J= 9.1
Hz, 1H);
7.97 (d, J = 7.9 Hz, 1 H); 7.97 (overlapped br s, 1 H); 7.76 (d, J = 4.5 Hz, 1
H); 7.60 (d,
J= 7.9 Hz, 1H); 7.26 (d, J= 9.1 Hz, 1H); 5,70 (dd, J= 1.6, 6.7 IRz, 1H); 5.32
(d, J= 6.7 Hz,
1H); 4.48 (d, J = 6.3 Hz, 1H); 4.02 (s, 3H); 3.93 (m, 2H); 3.82 (td, J= 2, 7.0
Hz, 1H); 3.64 (s,
2H); 3.46 (m, 11-1); 3.11 (t, J= 10.4 Hz, IH); 2.09 (m, 2H); 1.62 (m, 211).
MS (ESI, m/z): S 12.2 [M+H+].
Example 175: (3R,6S)-6-({6-[(1R,2R)-1,2-dihydroxy-2-(2-inethoxy-quinolin-8-yl)-
ethyll-
tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4]thiazin-3-one:
1751 trans-{(3R, 6S)-6- f2-(2-methoxy-quinolin-8 yl)-vinylJ-te trahydro pyran-
3 yl}-carbannic
acid tert-butyl ester:
This compound (0.23 g, 11% yield) was prepared as a white solid, starting from
intermediate 173.i (3.0 g, 5.8 mmol) and trifluoromethanesulfonic acid 2-
methoxy-quinoli-n-8-
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 186 -
yl ester (1.69 g, 5.5 mmol; prepared as in WO 2004/002490) and using the
procedure of
Example 173, step 173.ii.
'H NMR (d6-DMSO) S: 8.23 (d, J = 8.9 Hz, 1H); 7.90 (d, J = 6.5 Hz, 1H); 7.79
(d, J = 8.0 Hz,
1H); 7.61 (d, J = 16.0 Hz, 1H); 7.40 (dd, J = 6.5, 8.0 Hz, 1H); 7.03 (d, J =
8.9 Hz, 1H);
6.83 (d, J= 7.8 Hz, 1H); 6.58 (dd, J = 5.9, 16.0 Hz, 1H); 4.02 (s, 3H); 3.99
(m, 1H); 3.88 (dd,
J = 3.4, 10.7 Hz, 1H); 3.40 (br s, 1H); 3.09 (t, J= 10.5 Hz, 1H); 1.88 (m,
2H); 1.51 (m, 2H).
MS (ESI, m/z): 385.3 [M+H+].
175.ii. {6-[(1R,2R)-1,2-dihydroxy-2-(2-methoxy-quinolin-8 yl)-ethylJ-
tetrahydro pyran-3 yl}-
carbamic acid tert-butyl ester:
This compound (0.23 g, 92% yield) was prepared as a white solid, starting from
intennediate 175.i (0.23 g, 0.6 mrnol) and using the procedure of Example 173,
step 173.iii.
MS (ESI, m/z): 419.2 [M+H+].
175.iii. (IR,2R)-1-[(2S,5R)-amino-tetrahydro pyran-2 yl)-2-(2-methoxy-quinolin-
8 yl)-
ethane-1, 2-diol:
This compound (0.15 g, 85% yield) was obtained as a colourless solid starting
from
intermediate 175.ii (0.23 g, 0.55 rnmol) and using the procedure of Example
173, step 173.iv.
MS (ESI, m/z): 319.2 [M+H+].
175.iv. (3R,6S)-6-({6-[(IR,2R)-I,2-dihydroxy-2-(2-methoxy-quinolin-8yl)-ethylJ-
tetrahydro-
pyran-3 ylamino}-methyl)-4H-pyrido[3,2-bJ[1,4]thiazin-3-one:
This compound (0.055 g, 350K yield) was prepared as a beige solid, starting
from
intermediate 175.iii (0.1 g, 0.315 mmol) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.067 g, 0.35 mmol) and using
the procedure
of Example 173, step 173.v.
'H NMR (d6-DMSO) 8: 10.87 (s, 1H); 8.21 (d, J = 9.0 Hz, 1H); 7-79-7.72 (m,
3H); 7.41 (app
t, J = 7.6 Hz, 1H); 7.09 (d, J = 7.6 Hz, 1H); 7.00 (d, J = 8.8 Hz, 1H); 5.68
(dd, J = 2.6, 6.6 Hz,
1H); 5.02 (d, J= 6.6 Hz, 111); 4.19 (d, J = 6.2 Hz, 111); 3.96 (s, 3H); 3.95
(overlapped m, 1H);
3.74 (dd, AB system, J = 2.7, 15.0 Hz, 2H); 3.67 (td, J = 2.7, 6.4 Hz, 1H);
3.53 (s, 2H);
3.28 (m, 1H); 2.87 (t, J= 10.5 Eiz, 1H); 2.07 (m, 2H); 1.89 (br d, J= 12.2 Hz,
1H); 1.49 (m,
1H); 1.30-1.11 (m, 3H).
MS (ESI, m/z): 497.1 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-157-
Example 176: 3-oxo-3,4-dihydro-2H-pyrido- [3,2-b] [1,4]thiazine-6-carboxylic
acid
{(3R,6S)-6-[(1R,2R)-1,2-dihydroxy-2-(2-meth oxy-quinolin-8-yl)-ethyl]-
tetrahydro-pyran-
3-yl}-amide:
The title compound (0.045g, 54% yield) was obtained as a white solid starting
from
intermediate 175.iii (0.052 g, 0.164 mmol) and using the procedure of Example
172.
'H NMR (d6-DMSO) 8: 10.93 (s, 1H); 8.23 (d, J = 9.0 Hz, 1H); 7.96 (two
overlapped d,
J= 7.9Hz and J = 8.2 Hz, 2 x 1H); 7.81-7.74 (m, 2H); 7.59 (d, J = 7.9 Hz, 1H);
7.43 (t,
J = 7.5 Hz, 1 H); 7.02 (d, J = 8.8 Hz, 1 H); 5.72 (dd, J = 2.5, 6.6 Hz, 1 H);
5.08 (d, J = (5.6 Hz,
1H); 4.31 (d, J= 6.4 Hz, 1H); 4.00 (s, 3H); 3.9 8-3.86 (m, 2H); 3.75 (td, J=
2.6, 6.5 Hz, 1H);
3.64 (s, 2H); 3.78 (m, 1H); 3.10 (t, J= 10.2 Hz, 1H); 2.04 (m, 2H); 1.70-1.54
(m, 2H).
MS (ESI, m/z): 511.2 [M+H+].
Example 177: (3R,6S)-6-({6-[(1R,2R)-1,2-ditiydroxy-2-(3-methoxy-quinoxalin-5-
yl)-
ethyl] -tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrido [3,2-b] [ 1,4] thiazin-3-
one:
177.i. trans-{(3R, 6S)-{6-[2-(3-methoxy-quinoxczlin-5 yl)-vinylJ-tetrahydro
pyran-3 yl}-
carbamic acid tert-butyl ester:
The title (E)-alkene (2.01 g, 57% yield) was prepared as a beige solid,
starting from
intermediate 104.vii (1.5 g, 7.97 mmol) and iritermediate 164.ii (3.81 g, 9
mmol) and using
the procedure of Example 171, step 171.v.
'H NMR (d6-DMSO) &: 8.62 (s, 1H); 7.99 (d, J= 7.4 Hz, 1H); 7.91 (dd, J = 1.2,
8.2 Hz, 1H);
7.60 (dd, J = 7.4, 8.2 Hz, 1H); 7.53 (d, J = 15.8 Hz, 1H); 6.84 (d, J = 8.0
Hz, 1H); 6.63 (dd,
J = 5.7, 15.8 Hz, 1H); 4.08 (s, 3H); 3.99 (m, 1H); 3.89 (br dd, J = 3.0, 10.2
Hz, 1H); 3_38 (br
s, 1H); 3.09 (t, J= 10.5 Hz, 1H); 1.91-1.86 (m, 2H); 1.53-1.45 (m, 2H); 1.39
(s, 9H).
MS (ESI, m/z): 386.2 [M+H+].
177.ii. (3R,6S)-{6-[(IR,2R)-1,2-dihydroxy-2-(3-methoxy-quinoxalin-5 yl)-ethylJ-
tetrahydro-
pyran-3yl}-carbamic acid tert-butyl ester:
The title diol (1.89 g, 86%) was prepared as a white foam starting from
intermediate 177.i
(2.01 g, 5.2 mmol) and using the procedure of Example 171, step 171.vi.
MS (ESI, m/z): 419.2 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-188-
177.iii. (1R,2R)-1-[(2S,5R)-(5-amino-tetrahydro pyran-2 yl)-2-(3-methoxy-
qzsinoxalin-5 yl)-
ethane-l,2-diol:
This compound (0.252 g) was prepared as a yellowish foarn, starting from
intermediate 177.ii
(0.315 g, 0.79 mmol) and using the procedure of Example 173, step 173.iv.
'H NMR (d6-DMSO) S: 8.60 (s, 1H); 7.90-7.85 (m, 2H); 7.62 (dd, J = 7.7, 7.8
Hz, 1H);
5.68 (br s, 1H); 5.11 (br d, J= 4.3 Hz, 1H); 4.27 (br s, 1 H); 4.03 (s, 3H);
3.77 (ddd, J= 1.7,
4.3, 10.4 Hz, 1H); 3.62 (dd, J = 2.3, 6.1 Hz, 1H); 3.23 (ddd, J = 1.7, 6.4,
11.0 Hz, 1H); 2.75 (t,
J= 10.4 Hz, 1H); 2.56 (m, 1H); 1.95-1.84 (m, 2H); 1.52 (m, 1H); 1.45 (br s,
2H);
1.13 (m, 1H).
MS (ESI, m/z): 320.2 [M+H+].
177.iv. (3R,6S)-6-([6-[(IR,2R)-1,2-dihydroxy-2-(3-methoxy-qtsinoxalin-5 yl)-
ethylJ-
tetrahydro pyran-3 ylanaino}-methyl)-4H-pyrido[3,2-bJ[],4Jthiazin-3-one:
Starting from intermediate 177.iii (0.100 g) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.067 g), the title compound
(0.087 g) was
prepared as a yellow foam according to the procedure of Example 88, step
88.iv.
MS (ESI, m/z): 498.2 [M+H+].
Example 178: 6-({(3R,6S)-6-[(1S,2,S)-1,2-dihydroxy-2-(6-methoxy-
[1,5]naphthyridin-
4-yl)-ethyl]-tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4]thiazin-
3-one:
178. i. 2-methoxy-8-styryl-[1, 5]naphthyridine:
Trifluoromethanesulfonic acid 6-methoxy-[1,5]naphthyridin-4-yl ester (1.5 g,
4.86 mmol),
trans-phenylvinyl boronic acid (0.8 g, 5.35 mmol) and K2C03 (0.9 g, 6.32 mmol)
were
introduced in a two-neck flask. The atmosphere was flushed with nitrogen.
Dioxane (20 ml)
and water (5 ml) were added. The mixture was stirred at rt for 5 min and
tetrakis(triphenylphosphine) palladium (0.28 g, 0.24 mmol) was added. The
mixture was
heated at reflux for 5 h. After cooling, the reaction mixture was diluted with
EA (10 ml) and
water (50 ml). The aqueous layer was extracted with EA (2 K 100 ml). The
combined extracts
were concentrated to dryness. The residue was chromatographed (Hex-EA 1-1) to
afford the
title alkene (1.26 g, 4.8 mmol) as an oil that crystallized on standing.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 189 -
IH NMR (d6-DMSO) 8: 8.77 (d, J= 4.7 Hz, 1H); 8.28 (d, J= 9.0 Hz, 1H); 8.19 (d,
J = 16.7 Hz, 1 H); 8.01 (d, J= 4.7 Hz, 1H); 7.91 (d, .T = 16.7 Hz, 1 H); 7.74
(m, 2H);
7.40-7.34 (m, 3H); 7.30 (d, J = 9.0 Hz, 1H); 4.12 (s, 3H).
178.ii. 1-(6-methoxy-[1,5]naphthyridin-4 yl)-2 phenyl-ethane-1,2-diol:
To a mixture of intermediate 178.i (1.26 g, 4.8 mmol) in 2-methyl-2-propanol
(24 ml) and
water (24 ml) were added methanesulfonamide (0.52 g) and AD mix [3 (7 g). The
mixture
was stirred at rt for 12 h. Sodium bisulfite (7.5 g) was added carefully and
stirring was
continued 20 min. The two layers were decanted and the aqueous layer was
extracted with EA
(2 x 100 ml). The combined organic layers were dried over NazSO4, filtered and
concentrated
to dryness. The residue was triturated in Hex-EA (1-3, 30 ml) and the
resulting solid was
filtered off and dried in vacuo to afford the title diol (1.3 g) as a white
solid.
MS (ESI, m/z): 297.1 [M+H}].
178. iii. 6-methoxy-[1, 5]naphthyridine-4-carbaldehyde:
To a solution of intermediate 178.ii (1.3 g, 4.4 mmol) in acetone (15 ml) was
added a solution
of sodium periodate (2.35 g, 10.96 mmol) in water (5 ml). The reaction mixture
was stirred at
rt for 30 min. The reaction mixture was diluted with THF (100 ml) and the
solids were filtered
off. The filtrate was concentrated to dryness and the residue was resuspended
in water
(100 ml), ether (10 ml) and Hex (100 ml). The slurry was stirred at rt for 15
min and filtered.
The solids were washed with water and Hex. After drying, the title aldehyde
(0.42 g) was
recovered as a white solid.
'H NMR (d6-DMSO) S: 11.25 (s, 1H); 9.02 (d, J = 4.4 Hz, 1H); 8.42 (d, J = 9.1
Hz, 1H);
7.92 (d, J = 4.4 Hz, 1H); 7.40 (d, J = 9.1 Hz, 1H); 4.11 (s, 3H).
178.iv. {(3R,6S)-6-[2-(6-methoxy-[1,5]naphthyridin-4 yl)-vznylJ-tetrahydro
pyran-3 yl}-
carbamic acid tert-butyl ester:
Starting from intermediate 178.iii (0.4 g, 2.12 mmol) and intermediate 164.ii
(0.9 g,
2.2 mmol), the title alkene (0.44 g, 51% yield) was obtained as a white solid
using the
protocol of Example 171, step 171.v.
MS (ESI, m/z): 386.0 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 190 -
178.v. (3R,6S)-{6-[(1S,2S)-1,2-dihydroxy-2-(6-methoxy-[1,5]naphthyridin-4-yl)-
ethyl]-
tetrahydro-pyran-3-yl}-carbamic acid tert-butyl ester:
The title diol (0.47 g, 98%) was obtained as a white foam starting from
intermediate 178.iv
(0.44 g, 1.14 mmol) and using the protocol of Example 171, step 171.vi, except
that
AD mix a was used instead of AD mix (3 .
'H NMR (d6-DMSO) S: 8.75 (d, J = 4.5 Hz, 1H); 8.24 (d, J 9.0 Hz, 1H); 7.74 (d,
J = 4.5 Hz,
1H); 7.23 (d, J = 9.0 Hz, 1 H); 6.81 (br s, 1 H); 6.75 (br d, J 8.3 Hz, 1 H);
5.83 (d, J = 6.1 Hz,
1H); 5.25 (d, J = 6.6 Hz, 114); 4.49 (d, J = 9.1 Hz, 1H); 4.00 (s, 3H); 3.76
(m, 111); 3.67 (app t,
J= 6.6 Hz, 1H); 3.36 (m, 1H); 2.99 (t, J= 10.9 Hz, 1H); 1.99-1.86 (m, 2H);
1.38 (s, 9H);
1.35-1.13 (m, 2H).
MS (ESI, m/z): 420.2 [M+H+].
178.vi. (IS,2S)-1-[(2S,5R)-(5-amino-tetrahydro pyran-2yl)-2-(6-methoxy-
[1,5Jnaphthyridin-
4 yl)-ethane-l,2-diol:
This was obtained as a yellowish foam (0-17 g, 47% yield) from intermediate
178.v (0.47 g,
2.08 mmol) according to the procedure of Example 77, step 77.iii.
'H NMR (d6-DMSO) S: 8.75 (d, J = 4.5 Hz, 114); 8.24 (d, J = 9.0 Hz, IH); 7.74
(d, J = 4.5 Hz,
1 H); 7.23 (d, J = 9.0 Hz, 1 H); 5.84 (d, J = 5.3 Hz, 1 H); 5.21 (d, J = 6.5
Hz, 1 H); 4.46 (d,
J= 8.3 Hz, 1H); 3.99 (s, 3H); 3.76 (ddd, J = 2.5, 4.6, 8.8 Hz, 1H); 3.65 (td,
J = 1.5, 8.0 Hz,
1H); 3.32 (m, 1H); 2.86 (t, J = 10.4 Hz, lI3); 2.54 (partially overlapped m,
1H); 1.93 (m, 2H);
1.55, br s, 2H); 1.2-1.09 (m, 2H).
MS (ESI, m/z): 320.2 [M+H+].
178.vii. 6-({(3R, 6S)-6-[(1 S, 2S)-1, 2-dihydroxy-2-(6-methoxy-[1,
5]naphthyridin-4 yl)-ethylJ-
tetrahydro pyNan-3ylamino}-methyl)-4H-pyrido[3,2-bJ[1,4]thiazin-3-one:
This compound (0.06 g, 22% yield) was obtained as a white solid from
intermediate 178.vi
(0.17 g, 0.53 mmol) and 3-oxo-3,4-dilhydro-2H-pyrido[3,2-b][1,4]thiazine-6-
carbaldehyde
(0.113 g, 0.58 mmol), using the procedure of Example 173, step 173.v.
MS (ESI, m/z): 498.2 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-191-
Example 179: 3-oxo-3,4-dihydro-2H-pyrido[3,2-b] [1,4]thiazine-6-carboxylic
acid
(3R,6S)-{6-[(1R,2R)-1,2-dihydroxy-2-(3-methoxy-quinoxalin-5-yl)-ethyl]
tetrahydro-
pyran-3-yl}-amide:
The title compound (0.051 g) was obtained as a yellowish solid, starting from
interrnediate 177.iii (0.100 g) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-
b][1,4]thiazine-
6-carboxylic acid (0.072 g) and using the procedure of Example 172.
MS (ESI, m/z): 512.2 [M+H+].
Example 180: 6-({(3R,6S)-6-[(1R,2R)-1,2-dihydroxy-2-(3-rnethoxy-quinoxalin-
5-yl) ethyl]-tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrido [3,2-b] [1,4]
oxazin-3-one:
Starting from intermediate 177.iii (0.096 g) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]oxazine-6-carbaldehyde (0.059 g), the title compound
(0.038 g) was
obtaiined as a yellow solid according to the procedure of Example 88, step
88.iv.
MS (ESI, m/z): 482.2 [M+H+].
Exainple 181: 8-((1R,2R)-1,2-dihydroxy-2-{(2S,5R)-[(3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazin-6-ylmethyl)-amino]-tetrahydro-pyran-2-yl}-ethyl)-
quinoline-2-carbonitrile:
181. i. trans-(3R, 6S)-{6-[2-(2-cyano-quino1in-8 yl)-vinyl]-tetrczhydro pyran-
3 yl}-carbamic
acid tert-butyl ester:
The title (E)-alkene (1.80 g, 71%) was obtained as a white solid, starting
from
intermediate 173.i (5.0 g, 9.7 mmol) and trifluoromethanesulfonic acid 2-cyano-
quinolin-8-yl
ester (2.0 g, 6.6 mmol) and using the procedure of Example 173, step 173.ii.
1H NMR (d6-DMSO) S: 8.89 (d, J = 8.5 Hz, 1H); 8.16 (d, J = 6.3 Hz, 1H); 8.06
(d, J = 8.5 Hz,
1H); 8.03 (dd, J = 1.2, 7.1 Hz, IH); 7.78 (dd, J = 6.3, 7.1 Hz, 1H); 7.67 (d,
J = 16.3 Hz, 1H);
6.85 (d, J = 7.7 Hz, IH); 6.61 (dd, J = 5.3, 16.3 Hz, 111); 4.03 (m, 1H); 3.93
(dd, J = 2.7,
10.7 Hz, 1H); 3.41 (br s, 1H); 3.12 (t, J= 10.5 Iiz, 1H); 1.92-1.87 (m, 2H);
1.54-1.46 (partially overlapped m, 2H); 1.40 (s, 9H).
MS (ESI, m/z): 380.3 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-192-
181.ii. (3R,6S)-{6-[(IR,2R)-2-(cyano-quinolin-8 yl)-1,2-dihydroxy-ethylJ-
tetrahydro pyran-
3 yl}-ca.-bamic acid tert-butyl ester:
The title diol (1.60 g, 81%) was obtained as a white solid starting from
intermediate 181.i
(1.80 g, 4.74 mmol) and using the procedure of Exarriple 173, step 173.iii.
MS (ESI, m/z): 419.2 [M+H+].
181.iii. 8-[(1R,2R)-2-((2S,5R)-5-amino-tetrahydro pyran-2 yl)-1,2-dihydroxy-
ethylJ-
quinoline-2-carbonitrile:
The title amine (0.62 g, 74% yield) was obtained as a colourless solid
starting from
intermediate 181.ii (1.1 g, 2.61 mmol) and using the procedure of Example 173,
step 173.iv.
MS (ESI, m/z): 314.2 [M+H+].
181.iv. 8-((1 R, 2R)-1, 2-dihydroxy-2-{(2S, 5R)-[(3-oxo-3, 4-dihydro-
2H-pyrido[3,2-b][1,4]thiazin-6 ylmethyl)-aminoJ-tel-rahydropyran-2 yl}-ethyl)-
quinoline-
2-carbo.-ritrile:
The title compound (0.142 g, 28%) was obtained as white flakes, starting from
intermediate
181.iii (0.314 g, 1 mmol) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-
6-carbaldehyde (0.214 g, 1.1 mmol) and using the procedure of Example 173,
step 173.v.
1H NMR (d6-DMSO) 8: 10.87 (s, 1H); 8.65 (d, J = 8.5 Hz, 1H); 8.04-7.99 (m,
3H); 7.81 (dd,
J = 7.0, 8.3 Hz, 1H); 7.73 (d, J = 7.8 Hz, 1H); 7.09 (d, J = 7.9 Hz, 1H); 5.77
(dd, J = 2.6,
6.8 Hz, 1H); 5.19 (d, J= 6.3 Hz, 1H); 4.35 (d, J= 6.3 Hz, 1H); 3.92 (dd, J=
2.4, 10.8 Hz,
1H); 3.77 (br s, 2H); 3.60 (td, J = 2.9, 6.3 Hz, 1H); 3.53 (s, 2H); 3.24 (dd,
J = 6.3, 9.6 Hz,
1H); 2.81(t, J = 10.5 Hz, 1H); 2.50 (overlapped m, 1H); 2.08 (m, 2H); 1.89 (br
d, J = 13.9 Hz,
1H); 1.50 (m, 1H); 1.19 (m, 1H).
MS (ESI, m/z): 492.1 [M+H+].
Example 182: 3-oxo-3,4-dihydro-2H-pyrido[3,2-b] [1,4]thiazine-6-carboxylic
acid
{(3R,6S)-6-[(1R,2R)-2-(2-cyano-quinolin-8-yl)-1,2-dihydroxy-ethyl]-tetrahydro-
pyran-
3-yl}-arnide:
This compound (0.045 g) was obtained as a yellowish solid, starting from
intermediate 181.iii
(0.092 g, 0.29 mmol) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-
carboxylic acidl
(0.068 g, 0.32 mmol) and using the procedure of Example 172.
MS (ESI, m/z): 506.2 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-193-
Example 183: 8-((IR,2R)-1,2-dihydroxy-2-{(2S,5R)-5-[(3-oxo-3,4-dihydro-
2H-pyrido[3,2-b] [1,4]oxazin-6-ylmethyl)-amino]-tetrahydro-pyran-2-yl}-ethyl)-
quinoline-2-carbonitrile:
This compound (0.064 g, 21 % yield) was obtained as a white solid, starting
from
intermediate 181.iii (0.2 g, 0.64 rnmol) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-
b][1,4]oxazine-
6-carbaldehyde (0.12 g, 0.67 mmol) and using the procedure of Example 88, step
88.iv.
MS (ESI, m/z): 476.2 [M+H+].
Example 184: 6-((3R,6S)-{6-[ts-ans-(1RS,2RS)-1,2-dihydroxy-2-(3- methoxy-
quinolin-
5-yl)-ethyl]-tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4]thiazin-
3-one:
184.i. (3R, 6S)-(6-[((2S, 3R)-3-(3-naethoxy-quinolin-5 yl)-oxiranylJ-tet.-
ahydro pyran-3 yl}-
carbamic acid tert-butyl ester:
To a solution of intermediate 171.vi (0.504 g, 1.21 mmol) and triethyl
orthoacetate (0.264 ml,
1.45 mmol) in DCM (3.6 ml), cooled to 0 C, was added trimethylsilyl chloride
(0.185 ml,
1.45 mmol). The solution was stirred for 1 h and the reaction mixture
concentrated to dryness.
The residue was dissolved in MeOH (2.4 ml). K2C03 (0.416 g, 3.01 nzmol) was
added and the
suspension was vigorously stirred for 3 h. The suspension was concentrated to
dryness and
the residue was partioned between in EA and water. The phases were separated
and the aq.
layer extracted once with EA. The combined organic layers were dried over
Na2SO4, filtered
and evaporated under reduced pressure. The residue was purified by column
chromatography
over silica gel (DCM-MeOH 19-1) to afford the title epoxide (0.254 g, 52%
yield) as a white
foam.
'H NMR (CDC13) S: 8.63 (d, J = 2.8 Hz, 1H); 7.94 (dd, J = 1.7, 7.9 Hz, 1H);
7.61 (d,
J = 2.5 Hz, 1H); 7.47-7.39 (m, 2H); 4.28 (br d, J = 1.3 Hz, 1H); 4.23 (br s,
1H); 4.12 (ddd,
J = 2.1, 5.0, 10.8 Hz, 1H); 3.90 (s, 3H); 3.63 (br s, 1H); 4.89 (ddd, J= 2.3,
5.0, 11.2 Hz, 1H);
3.11 (dd, J= 2.3, 5.0 Hz, 1H); 3.05 (t, J= 10.6 Hz, 1H); 2.15 (m, 1H); 1.83
(m, 1H); 1.67 (m,
1H); 1.38 (s, 9H); 1.35 (overlapped m, 1H).
MS (ESI, m/z): 401.0 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 194 -
184.ii. trans-(IRS,2RS)-1-((2S,SR)-5-amino-tetrahydro pyran-2 yl)-2-(3-methoxy-
quirtolin-
S yl)-ethane-1,2-diol:
A solution of intermediate 184.i (0.253 g, 0.63 mmol) in TFA (5 ml) was
stirred at rt for
min. The volatiles were removed in vacuo and 1M aq, NaOH was added until pH 9
was
5 reached. The mixture was extracted eight times with a DCM-MeOH (9-1)
mixture. The
combined organic layers were dried over NaZSO4, filtered and concentrated
under reduced
pressure. The residue was purified by column chromatography over silica gel
(DCM-MeOH
9-1 containing 1% concentrated NH4OH to DCM-MeOH 4-1 containing 1% NH4OH) to
afford the title diol (0.136 g, 67% yield, 1.5:1 mixture of diastereomers) as
a white foam.
MS (ESI, m/z): 319.2 [M+H+].
184. iii. 6-((3R, 6S)-{6-[trans-(1 RS, 2RS)-1, 2-dihydroxy-2-(3-methoxy-
quinolin-5 yl)-ethylJ-
tetrahydro pyran-3ylamino}-methyl)-4H-pyrido[3,2-b][1,4]thiazin-3-one:
Starting from intermediate 184.ii (0.136 g, 0.43 mrnol) and 3-oxo-3,4-dihydro-
2H-pyrido [3,2-b] [ 1,4]thiazine-6-carbaldehyde (0.092 g, 1.1 (-- q.), the
title compound (0.113 g,
57% yield, 1.5:1 mixture of diastereomers) was obtained as a yellowish foam.
MS (ESI, m/z): 497.2 [M+H+].
Example 185: 6-((3R,6S)-{6-[(1R,2R)-1,2-dihydroxy-2-(6-methoxy-quinolin-4-yl)-
ethyl]-
tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4]thiazin-3-one:
185.i. trans-(3R, 6S)-{6-[2-(6-methoxy-quinolin-4 yl)-vinylJ-tetrahydro pyran-
3 yl}-carbanaic
acid tert-butyl ester:
The title (E)-alkene (3.16 g, 41%) was obtained as a yellowish solid, starting
from
intermediate 164.ii (8.47 g, 20 mmol) and 6-methoxy-quinoline-4-carbaldehyde
(5.5 g,
22 mmol) and using the procedure of Example 164, step 164_ iii.
MS (ESI, m/z): 385.3 [M+H}].
185.ii. (3R,6S)-{6-[(IR,2R)-1,2-dihydroxy-2-(6-naethoxy-quinolin-4 yl)-ethylJ-
tetrahydro-
pyran-3 yl}-carbamic acid tert-butyl ester:
The title diol (0.814 g, 38%) was obtained as a white foam, starting from
intermediate 185.i
(1.96 g, 5.1 mmol) and using the procedure of Example 173, step 173.iii.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-195-
1H NMR (CDC13) S: 8.72 (d, J = 4.5 Hz, 1H); 8.03 (d, J = 9.2 Hz, 1H); 7.57 (d,
J = 4.5 Hz,
1 H); 7.3 8(dd, J= 2.7, 9.2 Hz, 1 H); 7.32 (d, J = 2.7 Hz, 1 H); 5.51 (d, J=
5.3 Hz, 1 H);
4.21 (br d, partially overlapped, J= 6.2 Hz, 1H); 4.18 (ddd, J = 1.8, 4.6,
10.5 Hz= 1H); 3.94 (s,
3H); 3.69 (dd, J = 2.6, 5.2 Hz, 1 H); 3.63 (br s, 1 H); 3.15 (td, J = 2.2,
11.4 Hz, 1 H); 2.91 (t,
J= 10.6 Hz, 1 H); 2.07 (m, 1 H); 1.91 (qd, J = 4.0, 13.4 Hz, 1H); 1.90 (br s,
2H); 1.62 (m, 1H);
1.45 (s, 9H); 1.19 (qd, J = 4.2, 12.5 Hz, IH).
MS (ESI, m/z): 419.4 [M+H+].
185.iii. (IR,2R)-1-((2S,SR)-S-amino-tetrahydro pyran-2 yl)-2-(6-methoxy-
quinvlin-4 yl)-
ethane-1, 2-diol:
The title amine (0.605 g) was obtained as a white foam, starting from
interrrnediate 185.ii
(0.810 g, 1.94 mrnol) and using the procedure of Example 173, step 173.iv.
MS (ESI, m/z): 3 19.1 [M+H+].
185. iv. 6-((3R, 6S)-{6-[(I R, 2R)-1, 2-dihydroxy-2-(6-methoxy-quinol in-4-y1)-
ethyZ]-tetrahydro
pyran-3 ylamino}-methyl)-4I pyrido[3,2-b][1,4]thiazin-3-one:
This compound (0.064 g, 21%) was prepared as a white solid, starting from
intermediate 185.iii (0.105 g, 0.33 mmol) and 3-oxo-3,4-dihydro-2:.H-
pyrido[3,2-
b][1,4]thiazine-6-carbaldehyde (0.07 g, 0.36 mmol) and using the procedure
described in
Example 88, step 88.iv.
'H NMR (DMSO) S: 10.87 (s, 1H); 8.71 (d, J= 4.5 Hz, 1H); 7.93 (d, J= 9.1 Hz,
1H);
7.73 (d, J= 7.9 Hz, 1H); 7.54 (d, J = 4.5 Hz, 1H); 7.44 (d, J= 2.6 Hz, 1 H);
7.40 (dd,
J= 2.6, 9.1 Hz, 1H); 7.07 (d, J= 7.9 Hz, 1H); 5.41 (d, J= 5.2 Hz, 1H); 5.35
<t, J= 5.2 Hz,
1H); 4.79 (d, J = 6.4 Hz, 1H); 3.94 (m, 1H); 3.88 (s, 3H); 3.72 (br. d, J =
4.0 Hz, 1H); 3.53 (s,
2H); 2.92 (m, 1 H); 2.71 (t, J= 10.8 Hz, 1H); 2.01 (m, 1H); 1.63 (m, 2H); 1.23
(m, 1H);
1.06 (m, 1H).
MS (ESI, m/z): 497.2[M+W].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-196-
Example 186: 6-((3R,6S)-{6-[(1S,2R)-1,2-dihydroxy-2-(6-methoxy-quin olin-4-yl)-
ethyl]-
tetrahydro-pyran-3-ylamino}-metliyl)-4H-pyrido[3,2-b] [1,4]thiazin-3-one:
186.i. (3R, 6S)-{6-[(1 S, 2S)-1, 2-dihydroxy-2-(6-methoxy-quinolin-4-y1)-
ethytJ-tetrahydro-
pyran-3 yl}-carbamic acid tert-butyZ ester:
To a solution of intermediate 185.i (1.04 g, 2.71 mmol) in DCM (20 ml) and
water (2 ml) was
added NMO (1.08 g, 8.13 mmol) and potassium osmate (0.05 g, 0.14 mrrrol, 5
mol%). The
reaction was stirred for 3.5 h at rt. Water (30 ml) and sodium bisulfite (1.5
g) were added. The
mixture was stirred at rt for 15 min and the phases separated. The aq. layer
was extracted once
more with EA. The combined organic layers were dried over Na2 SO4, filtered
and
concentrated. The residue was purified by column chromatography (DCIVI-MeOH 19-
1 then
9-1) to afford the title compound as a white solid (0.850 g, 75% yield).
MS (ESI, m/z): 419.3 [M+H+].
186.ii. (IS,2S)-1-((2S,5R)-5-amino-tetrahydro pyran-2yl)-2-(6-methoxy-quinolin-
4yl)-
ethane-1, 2-diol:
This compound (0.537 g, 83% yield) was obtained as a white foam, starting from
intermediate 186.ii (0.810 g, 1.94 mmol) and using the procedure of Example
173,
step 173.iv.
186.iii. 6-((3R, 6S)-{6-[(1S, 2R)-1, 2-dihydroxy-2-(6-methoxy-quinolin-4yl)-
ethylJ-tetrahydro-
pyran-3 ylamino}-methyl)-4H-pyrido[3,2-bJ[1, 4]thiazin-3-one:
The title compound (0.047 g, 15 1o yield) was obtained as a yellowish solid,
starting from
intermediate 186.ii (0.20 g, 0.63 mmol) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-
b][1,4]thiazine-
6-carbaldehyde (0.134 g, 0.69 mmol), using the procedure of Example 88, step
88.iv. The title
compound was the major isomer in a 1.5 to 1 mixture with the compound of
Example 185.
MS (ESI, m/z): 497.2 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-197-
Example 187: (1RS)-1-{(2S,5R)-5-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-
amino]-
tetrahyd ro-pyran-2-yl}-2-(6-methoxy-q uinolin-4-yl)-ethanol:
187.i. (3R,6S)-6-[(IRS)-1-hydroxy-2-(6-methoxy-quinolin-4 yl)-ethyl.j-tet-
rahydro pyran-
3yl)-carbamic acid tert-butyl ester:
To a solution of N,N-diisopropylamine (2 ml) in THF (9.25 ml), cooled to -78
C, was added
n-BuLi (1.6 M in Hex, 8.75 ml). The mixture was stirred 10 min at this
temperature, then
min at 0 C. To a solution of 6-methoxy-4-methyl-quinoline (2.23 g) in THF (24
ml),
cooled to -78 C, was added LDA (20 ml). The mixture was stirred 15 rnin at -78
C and 15
min at 0 C. After cooling to -78 C, a solution of intermediate 158.i (1 g) in
THF (5 ml + 3 ml
10 rinse) was added. The mixture was then stirred 10 min at -78 C and 30 min
at 0 C. Water
(20 ml) and EA (50 ml) were added, the two layers were decanted and the aq.
layer was
extracted with EA (2 x 50 ml). The combined organic extracts were washed with
brine, dried
over Na2 SO4, filtered and concentrated to dryness. The residue was
chromatographed over
silica gel (EA) to afford the title alcohol (0.78 g, 1.93 mmol) as a white
foam. The compound
15 was obtained as 1.2:1 mixture of diastereomers.
MS (ESI, m/z): 403.1 [M+H+]
187.ii. (1RS)-1-((2S,5R)-5-amino-tetrahydro-pyran-2-yl)-2-(6-methoxy-quinolin-
4-yl)-
ethanol:
This cornpound (0.49 g) was obtained as a white solid, starting frorn
intermediate 187.i
(0.78 g, 1.93 mmol) and using the procedure of Example 173, step 173.iv.
MS (ESI, m/z): 303.2 [M+H+].
187.iii. (1 RS)-1-{(2S, 5R)-5-[(2, 3-dihydro-benzo[1, 4]dioxin-6 ylmethyl)-
crminoJ-tetrahydro-
pyran-2 yl}-2-(6-methoxy-quinolin-4 yl)-ethanol:
This cornpound (0.085 g) was obtained as a white foam, starting from
intermediate 187.ii
(0.1 g, 0.33 mmol) and 2,3-dihydro-benzo[1,4]dioxine-6-carbaldehyde (0.06 g,
0.36 mmol),
and using the procedure of Example 88, step U.N.
MS (ESI, m/z): 451.2 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-198-
Example 188: 6-({(3R,6S)-6-[(1RS)-1-hydroxy-2-(6-methoxy-quinolin-4-yl)-ethyl]-
tetrahyd ro-pyran-3-ylamino}-methyl)-4H-pyrido[1,4]thiazin-3-one:
This cornpound (0.087g, 57% yield) was obtained as a white foam, starting from
intermediate 187.ii (0.1 g, 0.33 mmol) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-
b][1,4]thiazine-
6-carbaldehyde (0.070 g, 0.36 mmol) and using the procedure of Example 88,
step 88.iv.
MS (ESI, m/z): 481.5 [M+H+].
Example 189: 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid
{(3R,6S)-6-[(1RS)-1-hydroxy-2-(6-methoxy-quinolin-4-yl)-ethyl]-tetrahydro-
pyran-3-yl}-
amide:
The title compound (0.030 g) was obtained as a white solid, starting from
intermediate 187.ii
(0.115 g, 0.38 mmol) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-
carboxylic acid
(0.095 g, 0.45 mmol) and using the procedure of Exarriple 172. The compound
was obtained
with a purity of 85% (as judged by 1H NMR).
MS (ESI, m/z): 493.2 [M-H+].
Example 190: 6-({(3R,6S)-6-[(1S')-1-hydroxy-2-(6-methoxy-quinolin-4-yl)-ethyl]-
tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4]thiazin-3-one:
190.i. (3R, 6S)-{6-[(4R, 5R)-5-(6-naethoxy-quinolin-4 yl)-2-oxo-[1, 3]dioxolan-
4 ylJ-
tetrahydro pyran-3 yl}-carbamic acid tert-butyl ester:
To a solution of intermediate 185.ii (3.06 g) in DCM (40 ml) was added TEA
(2.04 ml). The
solution was cooled to 0 C and triphosgene (2.17 g) was added in one portion.
The reaction
mixture was stirred at this temperature for 30 min then concentrated to
dryness. The residue
was purified by column chromatography over silica gel (DCM-MeOH 19-1
containing 1%
NH~OI3) to afford the title product as a pale yellow foam (2.75 g).
'H NMR (CDC13) b: 8.82 (d, J= 4.5 Hz, 1H); 8.10 (d, J= 9.3 Hz, 11-1); 7.49-
7.43(m, 2H);
7.21 (br. s, 1H); 6.27 (d, J= 4.2 Hz, 1H); 4.61 (m, 1fI); 4.28 (ddd, J= 2.0,
4.7, 10.6 Hz, 2H);
3.95 (s, 3H); 3.70 (m, 2H); 3.18 (t, J= 10.7Hz, 1H); 2.27 (m, 1H); 1.84 (m,
211); 1.46 (s, 9H);
1.45 (m overlapped, 1H).
MS (ESI, m/z): 445.0 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-199-
190.ii. (3R,6S)-{6-[(IS)-1-hydroxy-2-(6-methoxy-quinolin-4 yl)-ethylJ-
tetrahydro pyran-
3 yl}-carbamic acid tert-butyl ester:
To a solution of intermediate 190.i (2.75 g) in EA (250 ml) under argon was
added Pd/C
(1.32 g). The resulting suspension was stirred under hydrogen atmosphere.
After 2.5 h, more
Pd/C (0.66 g) was added and the reaction was stirred overnight under hydrogen
atmosphere.
The catalyst was filtered off and the filtrate concentrated in vacuo. The
residue was purified
by column chromatography over silica gel (DCM-MeOH 19-1 containing 1%
concentrated
NH4OH) to afford the title compound as a white foam (1.23 g).
IH NMR (DMSO) 6: 8.62 (d, J= 4.4 Hz, 1H); 7.92 (d, J= 9.0 Hz, 1 H); 7.42 (d,
J= 2.5 Hz,
1 H); 7.40 (dd, J= 2.7, 9.0 Hz, 1 H); 7.33 (d, J= 4.4 Hz, 1 H); 6.76 (br. d,
J= 8.0 Hz, 1 H);
4.83 (d, J= 6.4 Hz, 1 H); 3.91 (s, 3H); 3.90 (overlapped m, 1 H); 3. 74 (m, 1
H); 3.3 8 (br. s,
1H); 3.29 (overlapped dd, visible J= 3.8 Hz, 1H); 3.12 (d, J= 10.4 Hz, 111);
2.98 (t,
J = 10.3 Hz, 1H); 2.97 (m overlapped, 1H); 1.90 (d, J= 9.2 Hz, 1H); 1.60 (m,
2H); 1.39 (s,
9H); 1.38 (m overlapped, 1H).
MS (ESI, m/z): 403.0 [M+H+].
190.iii. (IS)-1-((2S,SR)-(5-amino-tetrahydropyran-2 yl)-2-(6-methoxy-quinolin-
4yl)-
ethanol:
A solution of intermediate 190.ii (1.23 g) in TFA (15 ml) was stirred for 10
min. The solution
was concentrated to dryness, basified with a 2M NaOH solution, diluted with
DCM-MeOH
9-1 and the phases separated. The aqueous layer was extracted 6 time s with
DCM-MeOH 9-1.
The combined organic layers were dried over Na2SO4, filtered and concentrated
to dryness to
afford a white solid (0.768 g).
1H NMR (DMSO) 6: 8.62 (d, J= 4.4 Hz, 1H); 7.92 (d, J= 9.1 Hz, 1H); 7.44 (d, J=
2.7 Hz,
1H); 7.39 (dd, J= 2.8, 9.1 Hz, 1H); 7.32 (d, J= 4.4 Hz, 1H); 4.79 (d, J= 6.3
Hz, 1H);
3.91 (s, 3H); 3.88 (ddd, J= 1.8, 4.4, 10.5 Hz, 1H); 3.73 (m, 1H); 3.31 (dd, J=
4.0, 13.6 Hz,
1H); 3.10 (dt, J= 3.4, 10.4 Hz, 1H); 2.93 (m overlapped, 1H); 2.87 (t
overlapped,
J= 10.5 Hz, 1H); 2.61 (m, 1H); 1.92 (m, 1H); 1.56 (m, 2H); 1.39 (br s, 2H)1.13
(m, 1H).
MS (ESI, m/z): 303.2 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 200 -
190.iv. 6-({(3R, 6S)-6-[(1 S)-1-hydroxy-2-(6-methoxy-quinolin-4 yl)-ethylJ-
tetrahydropyran-
3 ylarraino}-methyl)-4Fl-pyrido[3,2-bJ[1,4]thiazin-3-one:
Starting from intermediate 190.iii (0.1 g, 0.33 minol) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.071 g, 0.36 mmol), the title
corrnpound
(0.095 g, 60% yield) was obtained as a foam using the procedure of Example 88,
step g 8.iv.
MS (ESI, m/z): 481.0 [M+H+].
Example 191: 6-((3R, 6S)-{6-[1-hydroxy-2-(6-methoxy-quinolin-4-yl)-ethyl]-
tetraliydro-
pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4)oxazin-3-one:
Starting from intermediate 190.iii (0.102 g) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]oxazine-6-carbaldehyde (0.066 g), the title compound
(0.034 g) was
obtained as a white foam using the procedure of Example 88, step 88.iv.
1H NMR (DMSO) 6: 11.19 (s, 1H); 8.62 (d, J= 4.4 Hz, 1H); 7.92 (d, J= 9.0 Hz,
113); 7.42 (d
overlapped, J= 2.4 Hz, 1H); 7.39 (dd overlapped, J = 2.7, 8.9 Hz, 1H); 7.32
(m, 2H); 7.03 (d,
J= 8.1 Hz, 1H); 4.78 (d, J= 6.3 Hz, 1H); 4.62 (s, 2H); 4.05 (m, 1H); 3.90 (s,
3H); 3.71 (m,
3H); 3.32 (dd, J= 9.7, 13.6 Hz, 1H); 3.14 (m, 1H); 2.97 (t overlapped, J=
10.413z, 1H);
2.93 (rn overlapped, 1H); 2.51 (overlapped m, 11H); 2.07 (m, 1H); 1.65 (m,
1H); 1.50 (m, 1H);
1.24 (br s, 1 H); 1.22 (m, 1 H).
MS (ESI, m/z): 465.4 [M+H+].
Exaniple 192: 7-((3R,6,S)-{6-[(1S)-l-hydroxy-2-(6-methoxy-quinolin-4-yl)-
ethyl]-
tetrahydro-pyran-3-ylamino}-methyl)-1H-pyrido[3,4-b] [1,4]oxazin-2-one:
This compound (0.005 g, 4% yield) was obtained as a white solid, startirig
from
intermediate 190.iii (0.07 g, 0.23 mmol) and 2-oxo-2,3-dihydro-
1H-pyrido[3,4-b][1,4]oxazine-7-carbaldehyde (0.037 g, 0.21 mmol; prepared as
described in
WO 03/087098) and using the procedure of Exa.mple 88, step 88.iv.
MS (ESI, m/z): 465.1 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 201 -
Example 193: 3-oxo-3,4-dihydro-2H-pyrido [3,2-b] [1,4]thiazine-6-carboxylic
acid
(3R,6S)-{6-[(LS)-1-hydroxy-2-(6-methoxy-quinolin-4-yl)-ethyl]-tetrahydro-pyran-
3-yl}-
amide:
This compound (0.060 g, 36% yield) was obtained as a white solid, starting
from
intermediate 190.iii (0.1 g, 0.33 mmol) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-
b][1,4]thiazine-
6-carboxylic acid (0.076 g, 0.36 mmol) and using the procedure of Example 172.
MS (ESI, m/z): 495.0 [M+H+].
Example 194: 6-({(3R,6S)-6-[(1R)-1-hydroxy-2-(6-methoxy-quinolin-4-yl)-ethyl]-
tetrahyd ro-pyran-3-ylami no}-m ethyl)-4H-pyrido [ 1,4] thiazin-3-o ne :
Starting from intermediate 190.iii (0.1 g, 0.33 mmD l) and 3-oxo-3,4-dihydro-
2H pyrido[3,2-b][1,4]oxazine-6-carbaldehyde (0.070 g, 0.36 minol), and using
the procedure
described in Example 88, step 88.iv, a mixture of compounds was obtained. The
two
diastereomers were separated by chromatography over silica gel (DCM-MeOH 9-1
1% aq.
NH4OH). The first eluting diastereomer (0.017g, 11% yield) was obtained as a
yellowish
foam. The second eluting diastereomer (0.007g, 4% yield) was prepared as a
yellowish foam.
First diastereomer:
Analytical data (lIH-NMR, MS) are identical to the ones recorded for the
compound of
Example 191.
Second diastereomer:
'H NMR (DMSO) 8: 11.19 (s, 1H); 8.62 (d, J= 4.4 Hz, lIH); 7.92 (d, J= 9.0 Hz,
1H);
7.42-7.35 (m, 2H); 7.32 (m, 2H); 7.03 (d, J= 8.1 Hz, 1H); 4.58 (d, J= 6.3 Hz,
IH); 4.62 (s,
2H); 4.03 (m, 1H); 3.91 (s, 3H); 3.72 (dd, AB system, J= 14.8 Hz, 0= 0.057,
2H); 3.59 (m,
1H); 3.51 (dd, J= 1.8, 12.0 Hz, 1H); 3.14 (m, 1H); 2.97 (t, J = 10.5 Hz, 1H);
2.84 (m, 1H);
2.51 (overlapped in, 1H); 1.95 (m, 2H); 1.69 (m, 1H); 1.08-0.97 (m, 2H).
MS (ESI, m/z): 465.5 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-202-
Example 195: 6-({(3R,6S)-6-[(1RS)-1-hydroxy-2-(6-methoxy-[1,5]naphthyridin-4-
yl)-
ethyl]-tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4]thia.zin-3-
one:
195.i. (3R,6S)-[6-(6-methoxy-[1,5]naphthyridin-4 ylethynyl)-tetrahydro pyran-3
ylJ-
carbamic acid benzyl ester:
A solution of intermediate 158.iii (0 - 307 g, 0.8 mmol) in TFA (5 ml) was
stirred at rt for
min. After evaporation to dryness, the residue was partitioned between aq.
NaOH (20 ml)
and a DCM-MeOH mixture (9-1, 50 rnl). The aq. layer was extracted with the
same mixture
(2 x 50 ml). The combined extracts were washed with brine, dried over Na2SO4,
filtered and
concentrated to dryness. The residue (0.24 g) was taken up in EA (4 ml) and
water (4 ml).
10 NaHCO3 (0.2 g) and benzyl chloroformate (0.14 ml, 0.9 mmol) were added. The
reaction
proceeded for 30 min. The two layers -were decanted and the aq. layer was
extracted once with
EA. The combined organic layers were concentrated to dryness and the residue
was
chromatographed over silica gel (Hex-EA 1-2) to afford the title alkyne as a
white solid
(0.24 g).
1H NMR (CDC13) S: 8.73 (d, J = 4.6 Hz, 1H); 8.23 (d, J = 9.0 Hz, 1H); 7.64 (d,
J= 4.6 Hz,
1H); 7.42-7.31 (m, 5H); 7.18 (d, J= 9.0 Hz, 1H); 5.22 (d, J= 8.2 Hz, 1H); 5.13
(s, 2H);
4.86 (t, J= 4.1 Hz, 1H); 4.43 (dd, J = 2.5, 11.7 Hz, 1H); 4.13 (s, 3H); 3.83
(m, 1H); 3.55 (dd,
J = 4.5, 12.1 Hz, 1H); 2.35-2.16 (m, 21H); 1.94-1.78 (m, 2H).
195.ii. (3R,6S)-{6-[2-(6-methoxy-[1,5]naphthyridin-4 yl)-acetylJ-tetrahydro
pyran-3 yl)-
carbamic acid benzyl ester:
To a solution of intermediate 195.i (0.24 g, 0.57 mmol) in MeOH (2 ml) and THF
(2 ml) was
added a solution of mercury oxide, (5.1 ml, prepared from 0.075 g of mercury
oxide in 12 ml
of a 4% wt-wt H2SO4 solution in water). The reaction mixture was heated at 55
C for 4 h,
then cooled to rt. NaHCO3 was added until pH 8 was reached. The aq. layer was
extracted
with EA (2 x 100 ml). The combined extracts were washed with brine, dried over
Na2SO~,
filtered and concentrated in vacuo. The residue was chromatographed over
silica gel (DCM-
MeOH 19-1) to afford the title ketone (0.17 g, 67% yield) as a semi-solid.
MS (ESI, m/z): 436.4 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 203 -
195.iii. (3R,6S)-{6-[(1RS)-1-hydroxy-2-(6-methoxy-[],5Jnaphthyr idin-4 yl)-
ethylJ-
tetrahydNO pyNan-3yl}-car=bamic acid benzyl ester:
To a solution of intermediate 195.ii (0.17 g, 0.39 mmol) in THF (2 ml) and
MeOH (2 ml) was
added, at rt, NaBH4 (0.077 g). The reaction was stirred 30 min and water (5
ml) was added.
The volatiles were removed under reduced pressure and the residue was
extracted twice with
EA (2 x 25 ml). The combined extracts were concentrated to dryness and the
residue was
chromatographed over silica gel (DCM-MeOH 19-1) to afford the title alcohol
(0.045 g, 27%
yield) as a pale yellow solid.
MS (ESI, m/z): 438.4 [M+H+].
195.iv. (1 RS)-1-((2S, 5R)-S-amino-tetrahydro pyran-2 yl)-2-(6-fnethoxy[1,
5]naphthyridin-
4 yl)-ethanol:
A solution of intermediate 195.iii (0.045 g, 0.10 mmol) in TFA (3 ml) was let
at rt for 3 days.
The volatiles were removed under reduced pressure and the residue was taken up
in THF
(2 ml) and 2M NaOH (2 ml). The mixture was stirred at rt 30 min and the
volatiles were
removed under reduced pressure. The residue was chrornatographed over silica
gel
(DCM-MeOH 6-1 with 1% aq. NH4OH) to afford the title amirie (0.029 g, 92%
yield) as a
colourless oil.
MS (ESI, m/z): 304.4 [M+H+].
195.v. 6-({(3R, 6S)-6-[(1 RS)-1-hydnoxy-2-(6-methoxy-[1, 5]naphthyridin-4yl)-
ethylJ-
tetrahydro pyran-3 ylamino}-methyl)-4Hpyrido[3,2-bJ[1,4]thiazin-3-one:
Starting from intermediate 195.iv (0.029 g, 0.096 mmol) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.020 g, 0.106 rnmol), the title
compound
(0.030 g, 59% yield) was obtained as a off-white solid according to the
procedure described in
Example 88, step 88.iv. The compound was obtained as an equiinolar mixture of
epimers.
1H NMR (DMSO) S: 10.87 (s, 1H); 8.66 (d, J = 4.5 Hz, 1H); 8.24 (d, J = 9.0 Hz,
1H); 7.73 (d,
J = 7.4 Hz, IH); 7.53 (two overlapped d, J = 4.5 Hz, 2 x 0.5I3); 7.23 (d, J =
9.0 Hz, 1H);
7.09 (d, J = 7.4 Hz, 1H); 4.70 (d, J = 6.4 Hz, 0.5H); 4.57 (d, J= 6.4 Hz,
0.5H), 4.00 (s, 3H);
3.99 (overlapped m, 1H); 3.88 (m, 1H); 3.74 (br s, 2H); 3.59 (dd, J= 3.1, 13.2
Hz, 0.5H);
3.53 (s, 2H); 3.44 (dd, J= 3.5, 10.8 Hz, 0.5H); 3.13 (m, 1H); 3.02-2.83 (m,
2H);
2.50 (overlapped m, 1H); 2.14-2.02 (m, 2H); 1.86 (rn, 0.5H); 1.65 (m, 0.5H);
1.57-1.13 (m, 3H).
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 204 -
MS (ESI, m/z): 482.4 [M+H+].
Example 196: 3-oxo-3,4-dihydro-2H-pyrido[3,2-b] [1,4]thiazine-6-carboxylic
acid
(3R,6S)- {6- [(1S)-1-hydroxy-2-(6-methoxy- [1,5] nap hthyridin-4-yl)-ethyl]-
tetrahydro-
pyran-3-yl}-amide:
196.i. {(3R, 6S)-6-[(4R, 5R)-5-(6-methoxy-[1, 5]naphthyridin-4 yl)-2-oxo-[1,
3]dioxolan-4 ylJ-
tetrahydro pyran-3 yl}-carbamic acid tert-butyl ester:
The title cyclic carbonate (0.75 g, 64%) was obtained as a foam, starting from
intermediate
173.iii (1.1 g, 2.62 mmol) and using the procedure of Example 191, step 191.i.
'H NMR (CDC13) 6: 8.84 (d, J = 4.5 Hz, IH); 8.39 (d, J = 9.1 Hz, IH); 7.60 (d,
J = 4.5 Hz,
IH); 7.21 (d, J= 9.0 Hz, 1 H); 6.22 (br d, J= 4.1 Hz, 1 H); 4.70 (dd, J= 1.8,
4.2 Hz, 1 H);
4.30 (ddd, J = 2.0, 4.6, 10.5 Hz, 1H); 4.30 (overlapped br s, 1H); 4.08 (s,
3H); 3.80 (td,
J = 2.4, 11.5 Hz, 1H); 3.69 (br s, 1H); 3.13 (q, J = 8.2, 10.4 Hz, 1H); 2.22
(m, 1H); L.89 (qd,
J = 3.8, 12.9 Hz, 1H); 1.71 (m, 1H); 1.47 (s, 9H); 1.32 (partially overlapped
m, 1H).
MS (ESI, m/z): 445.7 [M+H+].
196.ii. (3R,6S)-{6-[(IS)-1-hydroxy-2-(6-methoxy-[1,5]naphthyridin-4 yl)-ethylJ-
tetj'ahydro-
pyran-3 yl}-carbanaic acid tert-butyl ester:
The title alcohol (0.22 g, 32%) was obtained as a white solid starting from
intermediate 196.i
(0.75 g, 1.68 mmol) and using the procedure of Example 191, step 191.ii.
'H NMR (CDC13) S: 8.70 (d, J= 4.5Hz, 1H); 8.25 (d, J = 9.1 Hz, 1H); 7.49 (d,
J= 4.5 Hz,
1H); 7.14 (d, J = 9.0 Hz, 11-1); 4.26 (br s, 1H); 4.18 (ddd, J = 2.0, 4.7,
10.5 Hz, IH); 4.08 (s,
3H); 4.02 (m, lIi); 3.73 (br s, IH); 3.68 (overlapped br s, 1H); 3.41 (dd, J =
3.5, 13.2 liz, 111);
1 H); 3.31 (dd, J = 8.5, 13.2 Hz, 1 H); 3.24 (ddd, J = 2.3, 5.0, 11.2 Hz, 1H);
3.03 (t,
J= 10.6 Hz, 1H); 2.17 (m, 1H); 1.86 (m, 1H); 1.77-1.63 (m, 11-1); 1.46 (s,
9H); 1.31 (qd,
J = 4.3, 12.4 Hz, 1H).
MS (ESI, m/z): 403.9 [M+].
196.iii. (1S)-1-((2S,5R)-5-amino-tetrahydro pyran-2 yl)-2-(6-methoxy-
[1,5]naphthyy-idin-
4 yl)-ethanol:
The title amine (0.158 g, 95%) was obtained as a colourless foam, starting
from
intermediate 196.ii (0.22 g, 0.55 mmol) and using the procedure of Example
173, step 173.iv.
MS (ESI, m/z): 304.1 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 205 -
196.iv. 3-oxo-3, 4-dihydro-2Fl-pyrido[3, 2-bJ[l, 4Jthiazine-6-carboxylic acid
(3R, 6S)-{6-[(1 S)-1-hydroxy-2-(6-methoxy-[I , 5]naphthyridin-4 yl)-ethylJ-
tetrahydr- pyran-
3 yl}-amide:
This compound (0.016 g, 24%) was obtained as a white solid, starting from
intermediate 196.iii (0.04 g, 0.13 mmol) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid (0.033 g, 0.16 mmol) and using
the
procedure of Example 172.
MS (ESI, m/z): 496.2 [M+H+].
Example 197: (3R,6S)-6-({6-[(1S)-1-hydroxy-2-(6-methoxy-[1,5]naphthyridia-4-
yl)-
ethyl]-tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrido [3,2-b] [1,4]thiazin-3-
one:
Starting from intermediate 196.iii (0.055 g, 0.182 mmol) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.039 g, 0.2 mmol), the title
compound
(0.033 g, 37%) was obtained as an off-white solid using the procedure of
EXample 88,
step 88.iv.
'H NMR (DMSO) 8: 10.93 (s, 1H); 8.67 (d, J = 4.4 Hz, 1H); 8.25 (d, J = 9.0 Hz,
l H); 7.97 (d,
J = 7.8 Hz, 1H); 7.93 (d, J = 8.5 Hz, 1H); 7.60 (d, J = 7.8 Hz, 1H); 7.55 (d,
J = 4.4 Hz, 1H);
7.25 (d, J= 9.0 Hz, 111); 4.69 (d, J= 6.4Hz, 1H); 4.10 (s, 3H); 4-10-3.54
(partially
overlapped m, 3H); 3.64 (s, 2H); 3.50 (dd, J= 3.7, 12.6 Hz, 1H); 3.21 (m,
11I); 3.13 (t,
J = 10.4 Hz, 1H); 3.03 (dd, J= 8.9, 12.8 Hz, 1H); 2.03 (m, 1H); 1.79-1.50 (m,
311) _
MS (ESI, m/z): 482.2 [M+H+].
Example 198: 6-((3R, 6S)-{6-[(1S)-1-hydroxy-2-(6-methoxy-[1,5]naphthyridin-4-
yl)-
ethyl]-tetrahydro-pyran-3-ylamino}-metliyl)-4H-pyrido[3,2-b] [1,4]oxazin-3-
one:
Starting from intermediate 196.iii (0 _ 055 g, 0.18 mmol) and 3-oxo-3,4-
dihydro-
2H-pyrido[3,2-b][1,4]oxazine-6-carbaldehyde (0.036 g, 1.1 eq), the title
compound (0.017 g,
20% yield) was prepared as pale yellow solid according to the procedure of
Bxample 88,
step 88.iv.
MS (ESI, m/z): 466.3 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-206-
Example 199: (3S,6R)-(6-({6-[(IS,2S)-1,2-dihydroxy-2-(6-methoxy-
[1,5]naphthyridin-
4-yl)-ethyl] -tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrido[3,2-bI
[1,4]thiazin-3-one:
199.i. (3S,6R)-[6-(1 phenyl-lH-tetrazole-S-sulfonylmethyl)-tetrahydro pyran-
3ylJ-carbamic
acid tert-butyl ester:
This compound (5.9 g) was obtained as a colourless solid, starting from
intermediate 154.iii
(8.2 g, 35.5 mmol) and using the procedure of Example 171, steps 171.i to
171.iv.
MS (ESI, n/z): 424.4 [M+H}].
199.ii. ( 3S, 6R)-{6-[2-(6-methoxy-[1, 5]naphthyridin-4 yl)-vinylJ-tetrahydro
pyran-3 yl}-
carbanzic acid tert-butyl ester:
Julia coupling was carried out as described for Example 171, step 171.v,
starting from
intermediate 199.i (4.95 g, 11.7 mmol) and 6-methoxy-[1,5]naphthyridine-4-
carbaldehyde
(1.9 g, 10.9 mmol) to give 3.4 g (75.4%) of product as a colourless solid.
1H NMR (d6-DMSO) S: 8.70 (d, 1H, J=4.65 Hz), 8.23 (d, 1H, J=9.06 Hz), 7.82 (d,
1H,
J= 4.65 Hz), 7.54 (d, 1H, J= 16.3 Hz), 7.26 (d, 1H, J= 9.06 Hz), 6.91 (dd, 1H,
J= 16.3,
5.34 Hz), 6.88 (br, 1H, NH), 4.03 (s, 3H), 4.05-3.95 (m, 1H), 3.90-3.80 (m,
1H),
3.45-3.35 (in, 1H), 3.08 (t, 1H, J=10.6 Hz), 2.00-1.80 (m, 2H), 1.60-1.40 (m,
2H),
1.36 (s, 9Ii).
199.iii. (3S,6R)-{6-[(1S,2S)-1,2-dihydroxy-2-(6-methoxy-[I,5]naphthyridin-4
yl)-ethylJ-
tetrahydro pyran-3 yl}-carbamic acid tert-butyl ester:
Intermediate 199.ii (1.6 g, 4.15 mmol) was treated with AD mix U. according to
the procedure
of Example 171, step 171.vi to give 1.53 g (88%) of title diol as a colourless
solid.
MS (ESI, rn/z): 464.1 [M+H+].
199.iv. (1S,2S)-1-((2R,SS)-S-amino-tetrahydro pyran-2 yl)-2-(6-methoxy-
[1,5]naphthyridin-
4yl)-ethane-1, 2-diol:
A solution of interrnediate 199.iii (500 mg, 1.2 mmol) in DCM (10 ml) was
treated with TFA
(4 ml). The mixture was stirred at rt for 3 h, concentrated in vacuo,
paxtitioned between DCM
and NH40H. The organic layer was dried over MgSO4 and concentrated to give the
title
amine (243 mg, 64%) as a colourless foam.
MS (ESI, rn/z): 320.2 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-207-
199.v. (3S, 6R)-(6-({6-[(I S, 2S) -1, 2-dihydroxy-2-(6-methoxy-[I ,
5Jnaphthyridin-4 yl)-ethylJ-
tetrahydro pyrczn-3ylamino}-methyl)-4H-pyrido[3,2-b][1,4]tlaiazin-3-one:
A mixture of intermediate 199.iv (120 mg, 0.37 mrrnol) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (73 mg, 1 eq) in 1,2-DCE/MeOH 1:1
(5 ml)
was stirred at rt overnight. NaBH4 (excess) was added and stir-ring was
continued for 1 h. The
mixture was partitioned between DCM and NH4OH and the organic layer was dried
over
MgSO4 and concentrated in vacuo. The product was isolated in 32% yield (60 mg)
as a beige
foam.
MS (ESI, m/z): 498.1 [M+H+].
Example 200: 6-((3R,6S)-{6-[(1S)-1-hydroxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-
tetrahydro-py ran-3-ylamino}-methyl)-4H-pyrido [3,2-b] [1,4] oxazin-3-one:
200.i. (3R,6S)-{(4R,5R)-6-[5-(3-methoxy-quinolin-5 yl)-2-oxo-[1,3]dioxolan-
4ylJ-
tetrahydro pyrezn-3 yl}-carbamic acid tert-butyl ester:
The title carbonate (0.96 g, 30%) was prepared from intermediate 171.vi (3.0
g, 7.16 mmol)
according to the procedure of Example 191, step 191.i.
MS (ESI, m/z): 445.0 [M+H+].
200.ii. (3R,6S~-{6-[(IS)-1-hydroxy-2-(3-methoxy-quinolin-5 yl)-ethylJ-
tetrahydro pyran-
3 yl}-carbamic acid tert-butyl ester:
The title alcohol (0.52 g, 60% yield) was prepared from intermediate 200.i
(0.96 g,
2.16 mmol) according to the procedure of Example 191, step 191.ii.
MS (ESI, m/z): 403.1 [M+H+].
200.iii. (1 S)-1-((2S, 5R)-5-amino-tetrahydro pyran-2 yl)-2-(3-methoxy-
quinolin-5 yl)-
ethanol:
The title amine (0.39 g, 99% yield) was obtained frorn intermediate 200.ii
(0.52 g,
1.29 mmol) according to the procedure of Example 173, step 173.iv.
1H NMR (DMSO) 6: 8.64 (d, J = 2.8 Hz, 1H); 7.83-7.79 (rrn, 2H); 7.49 (dd, J =
7.0, 8.1 Hz,
1H); 7.42 (dd, J = 1.3, 7.0 Hz, 11-1); 4.70 (d, J = 6.2 Hz, 1H); 3.94 (s,
311); 3.88 (ddd, J= 2.0,
4.5, 10.5 Hz, 1H); 3.64 (m, 114); 3.31 (overlapped m, 1H); 3.03 (m, 111); 2.95
(dd, J = 8.0,
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 208 -
13.8 Hz, 1H); 2.84 (t, J = 10.4 Hz, 1H); 2.60 (m, 1H); 1.90 (m, 1H); 1.60-1.46
(m, 2H);
1.42 (br s, 2H); 1.11 (m, 1H).
MS (ESI, m/z): 303.1 [M+H+].
200.iv. 6-((3R,6S)-{6-[(1S)-1-hydroxy-2-(3-methoxy-quinolin-5 yl)-ethylJ-
tetrahydro pyran-
3 ylamino}-methyl~ -4H-pyrido[3, 2-bJ[I, 41oxazin-3-one:
Starting from intermediate 200.iii (0.042 g, 0.14 mmol) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]oxazine-6-carbaldehyde (0.027 g, 1.1 eq), the title
compound (0.015 g,
23% yield) was obtained as pale yellow solid using the procedure of Example
88, step 88.iv.
MS (ESI, m/z): 465.1 [M+H+].
Example 201: 6-((3R, 6S)-{6-[(1,S)-1-hydroxy-2-(3-methoxy-quinolin-5-yl)-
ethyl]-
tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4]thia2.Fin-3-one:
Starting from intermediate 200.iii (0.100 g, 0.33 mmol) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]oxazine-6-carbaldehyde (0.070 g, 1.1 eq), the title
compound (0.081 g,
50% yield) was obtained as a pale yellow solid using the procedure of Example
88, step 88.iv.
The purity of the compound was 90%.
'H NMR (DMSO) S: 10.87 (s, 1H); 8.64 (d, J= 2.8 Hz, 111); 7.:83-7.75 (m, 2H);
7.74 (d,
J= 7.8Hz, 1H); 7.49 (dd, J= 7.0, 8.2 Hz, 1H); 7.41 (dd, J= 1.3, 7.0 Hz, 1H);
7.09 (d,
J = 7.9 Hz, 1H); 4.70 (d, J = 6.3 Hz, 1H); 4.04 (m, 1H); 3.93 (s, 3H); 3.75
(d, AB system,
J= 14.9 Hz, 0= 0. 058, 2H); 3.65 (m, 1H); 3.53 (s, 2H); 3.30 (overlapped m,
1H); 3.09 (m,
1H); 2.99-2.90 (rn, 2H); 2.13 (br s, IH); 2.04 (m, 1H); 1.63-1.47 (m, 2H);
1.23 (br s, 1H);
1.19 (m, 1H).
MS (ESI, m/z) : 481.0 [M+H-'-].
Example 202: (IS)-1-((2S,5R)-5-heptylamino-tetrahydro-pyran-2-yl)-2-(6-methoxy-
quinolin-4-yl)-ethanol:
Starting from intermediate 190.iii (0.113 g, 0.375 mmol) and n-heptaldehyde
(0.057 ml,
0.412 mmol), the title compound (0.065 g, 43% yield) was obtained as a yellow
gum using
the procedure of Example 88, step 88.iv.
'H NMR (CDC13) 6: 8.66 (d, J= 4.4 Hz, 1H); 8.00 (d, J= 9.1 Hz, 1H); 7.36 (dd,
J= 2.7,
9.1 Hz, 1H); 7.28 (m, 2H); 4.15 (ddd, J= 2.2, 4.7, 11.0 Hz, 1H); 3.94 (s, 3H);
3.88 (m, 1H);
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 209 -
3.31 (dd, J= 5.6, 13.9 Hz, 1H); 3.17 (m, 2H); 3.06 (t, J= 10.6 Hz, IH); 2.64
(m, 3H);
2.09 (m, 1H); 1.66 (m, 2H); 1.45 (m, 2H); 1.23 (m, lOH); 0.88 (t, J = 6.9 Hz,
3H).
MS (ESI, mlz): 401.2 [M+H+].
Example 203: 6-((3R,6S)-{6-[(2R)-2-hydroxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-
tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b][1,4]tlbiazin-3-one and
6-((3R,6S)-{6- [(2S)-2-hydroxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-tetrahydro-
pyran-
3-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4]thiazin-3-one:
203.i. (3R,6S)-(6-vinyl-tetrahydro pyran-3 yl)-carbamic acid ten-butyl ester:
To a mixture of inethyltriphenylphosphonium bromide (11.65g, 32.71 mmol) in
THF
(100 ml) cooled to -78 C, was added n-BuLi (2.35Nin Hex, 13.6 ml). The mixture
was stirred
min at this temperature and then 45 min at 0 C. After cooling to -78 C, a
solution of
intermediate 1 58.i (3 g, 13.08 mmol) in THF-HMPA (2-1, 30 ml) was added. The
mixture
was stirred 30 min at this temperature and allowed to warm up to rt over 30
min. The mixture
was quenched by adding water (50 ml) and ether (100 ml). The two layers were
decanted and
15 the aq. layer was extracted twice with ether (2 x 150 ml). The combined
organic layers were
washed with brine and concentrated to dryness. The residue wa.s
chromatographed over silica
gel (Hex-EA 4-1) to afford the title alkene (1.47 g) as a white soalid.
IH NMR (CDC13) 8: 5.86 (dddd, J = 5.5, 10.7, 17.3 Hz, 1H); 5.26 (td, J = 1.5,
17.3 Hz, 1H);
513 (td, J= 1.5, 10.7 Hz, 1H); 4.32 (br s, 1H); 4.13 (ddd, J= 2-1, 4.6, 10.7
Hz, 1H), 3.76 (m,
1H); 3.63 (br s, 1H); 3.08 (t, J= 10.5 Hz, 1H); 2.12 (m, 1H); 1-80 (qd, J =
3.8, 13.6 Hz, 1H);
1.55 (partially overlapped m, 1H); 1.46 (s, 9H); 1.33 (m, 1H).
203.ii. (3R, 6S)-[6-(2-hydroxy-ethyl)-tetrahydropyran-3 yl]-ca.T-barnic acid
tert-butyl ester:
To an ice-chilled solution of intermediate 203.i (1.47 g, 6.46 mmol) in THF
(30 ml) was
added 9-borabicyclononane (2.37 g, 19.4 mmol). The mixture was then let
overnight at rt.
After cooling to 0 C, 3N aq. NaOH (25 ml) and 50% aq. hydrogen peroxide (12
ml) were
cautiously added. The resulting mixture was stirred at rt for 2 h- The two
layers were decanted
and the aq. layer was extracted with EA (2 x 200 ml). The combined organic
layers were
washed with 10% aq. sodium bisulfite (100 ml), water (50 ml) and dried over
Na2SO4. After
filtration and concentration to dryness, the residue was chromatographed over
silica gel
(Hex-EA 1-1 then 1-3) to afford the title alcohol (1.15 g, 4.68 nnmol) as a
white solid.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-210-
'H NMR (CDC13) b: 4.16 (br s, 1H); 4.01 (ddd, J= 2.2, 4.7, 10.7 Hz, 1FI); 3.69
(t, J = 5.2 Hz,
2H); 3.52 (br s, 1H); 3.39 (m, 1H); 2.93 (t, J = 10.7 Hz, 1H); 2.02 (br s,
1H); 2.00 (m, 1H);
1.72-1.58 (m, 3H); 1.45 (m, 1H); 1.37 (s, 9H); 1.24 (qd, J = 4.1, 12.2 Hz,
1H).
MS (ESI, m/z): 246.4 [M+H+].
203.iii. (3R, 6S)-[6-(2-oxo-ethyl)-tetrahydro pyran-3 yl]-carbamic acid tert-
butyl ester:
To a ice-chilled solution of intermediate 203.ii (1.15 g, 4.68 mmol) in DCM
(20 ml) was
added a solution of Dess-Martin periodinane (15wt% in DCM, 18 n-il). The
mixture was
stirred at 0 C for 15 min before warming to rt. The reaction proceeded for 2
h. The reaction
mixture was concentrated to dryness and the residue chromatographed (Hex-EA 1-
1) to afford
the title aldehyde (1.06 g, 4.35 rnmol) as a white solid.
'H NMR (CDC13) 6: 9.79 (t, J= 2.3 Hz, IH); 4.29 (br s, 1H); 4.07 (ddd, J =
2.1, 4.7, 10.7 Hz,
1H); 3.77 (m., 1 H); 3.61 (br s, 1 H); 3.04 (t, J = 10.7 Hz, 1 H); 2.65 (ddd,
J = 2.3, 7.6, 16.5 Hz,
1H); 2.49 (ddd, J= 2.3, 4.8, 16.5 Hz, 1H); 2.12 (m, 1H); 1.79 (m, 1H); 1.50
(overlapped m,
1H); 1.49 (s, 9H); 1.33 (qd, J= 4.1, 12.2 Hz, 1H).
203.iv. {(3R,6S)-6-[(2RS)-2-hydroxy-2-(3-methoxy-quinolin-5 yl)-ethyl.J-
tetrahydropyran-
3 ylJ-carbamic acid tert-butyl ester:
To a solution of 5-bromo-3-methoxy-quinoline (3.08 g, 12.94 rnmol; prepared as
in
DE 10316081) in THF (75 ml), cooled to -78 C, was added quickly n-BuLi (2.35N
in Hex,
5.4 ml). The mixture was stirred at the same temperature for 15 minutes and a
solution of
(3R,6S)-[6-(2-oxo-ethyl)-tetrahydro-pyran-3-yl]-carbamic acid tert-butyl ester
(1.05 g,
4.35 mmol) in THF (10 ml) was added. The mixture was stirred at the same
temperature for
5 min. 10% aq. NaHSO4 (20 ml) and EA (10 ml) were added. The two layers were
decanted
and the aq. layer was extracted with EA (2 x 100 ml). The combined organic
layers were
washed with brine, dried over Na2S04, filtered and concentrated to dryness.
The residue was
chromatographed over silica gel (Hex-EA 2-1 then 1-1 then 1-4) to yield the
title compound
(0.45 g, 1.11 mmol) as a yellowish foam. The compound was obtained as a 3.5:1
mixture of
epimers.
MS (ESI, m/z): 403.2 [M+H+] -
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 211 -
203.v. (2RS)-1-((2S,5R)-5-amino-tetrahydro pyrcrn-2 yl)-2-(3-methoxy-quinolin-
5 yZ)-
ethanol:
This compound was prepared starting from internzediate 203.iv (0.45 g, 1.11
mmol) and using
the procedure of Example 171, step 171.vii. A 3.5:1 mixture of epimers was
obta.ined as a
white foam (0.204 g, 0.67 mmol).
1H NMR (CDCl3) S: 8.70 (app d, J= 2.8 Hz, 1H); 7.99 (app d, J= 8.3 Hz, 1H);
7.82 (d,
J = 2.8 Hz, 0.77H); 7.74-7.67 (m, 1.23H); 7.60-7. 53 (2 overlapped dd, J =
7.5, 8.0 I3z, 0.23H,
J= 7.3, 8.3 Hz, 0.77 H); 5.69 (m, 0.23H); 5.57 (dd, J= 3.3, 9.2 Hz, 0.77H);
4.09-4.02 (m,
1H); 3.99 (s, 2.31H); 3.97 (s, 0.69H); 3.57 (m, 1 H); 3.09 (app t, J= 10.5 Hz,
1H); 2.87 (m,
1H); 2.19-1.99 (m, 2.77H), 1.95 (t, J= 3.0 Hz, 0.23H); 1.75-1.38 (m, 5H); 1.26
(app qd,
J = 4.0, 12.8 Hz, 1H).
MS (ESI, m/z): 303.0 [M+H}]
203.vi. 6-((3R,6S)-{6-[(2R)-2-hydroxy-2-(3-methoxy-quinolin-5 yl)-ethylJ-
tetrahydv-o pyran-
3 ylamino}-methyl)-4FI pyrido[3, 2-bJ[1, 4]thiazir,r-3-one and 6((3R, 6S)-{6-
[(2S)-2-hydroxy-
2-(3-methoxy-quinolin-5 yl)-ethylJ-tetrahydro pyp-an-3 ylamino}-methyl)-
4Hpyrido[3, 2-b][l, 41thiazin-3-one:
To a solution of intermediate 203.v (0.1 g, 0.33 rnmol) in 1,2-DCE (6 ml) and
MeOH (2 ml)
were added 3 A molecular sieves (2 g) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-
b][1,4]thiazine-
6-carbaldehyde (0.07 g, 0.364 mmol). The miKture was heated at 50 C overnight.
After
cooling to rt, NaBH4 (0.1 g) was added and the reaction proceeded 2 h. The
reaction mixture
was filtered through a plug of Hydromatrix (pretreated with NaHCO3). The
filtrate was
concentrated to dryness and the residue was chromatographed over silica gel
(DCM-MeOH
93-7 with 0.5% aq. NH40H) to afford a first diastereomer (0.062 g, 0.13 mrnol)
as a
yellowish foam. The second eluting diastereomer (0.020 g, 0.041 mmol) was then
obtained as
a yellowish foam.
First diastereomer:
1H NMR (d6-DMSO) S: 10.85 (s, 111); 8.65 (d, J 2.8 Hz, 1H); 7.86 (m, 2H'; 7.72
(d,
J = 7.9Hz, 1H); 7.65 (d, J = 7.3 Hz, 1H); 7.56 (dd, J 7.3, 8.2 Hz, 1H); 7.07
(d, J = 7.9 Hz,
1H); 5.38 (m, 2H); 3.93 (s, 3H); 3.92 (overlapp(--d m, 1H); 3.71 (dd, AB
system, J = 15.0 Hz,
A= 0.061, 2H); 3.52 (s, 2H); 3.01 (m, 1H); 2.77 (t, J = 10.5 Hz, 1H); 2.50
(overlapped m, 1H);
2.09-1.68 (m, 2H); 1.81-1.69 (m, 2H); 1.36-1.22 (m, 2H); 1.17-1.01 (m, 1H).
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-212-
MS (ESI, m/z): 481.2 [M+H+].
Second diastereomer:
'H NMR (d6-DMSO) S: 10.86 (s, 1H); 8.65 (d, J= 2.7 Hz, 1H); 7.86 (d, J= 2.7
Hz, 1H);
7.82 (d, J = 7.9 Hz, 1H); 7.74 (d, J = 7.7 Hz, 1H); 7.65 (d, J = 7.2 Hz, 1H);
7.54 (dd,
J = 7.2, 7.7 Hz, 1H); 7.09 (d, J = 7.9 Hz, 1H); 5.46-5.38 (m, 2H); 4.02 (m,
1H); 3.90 (s, 3H);
3.75 (dd, AB system, J = 14.9 Hz, A = 0.062, 2H); 3.58 (m, 1H); 3.53 (s, 2H);
3.07 (t,
J = 10.7 Hz, I H); 2.29 (br s, 1H); 1.98 (m, 1H); 1.78-1.47 (m, 3H); 1.40-
1.09 (m, 3H).
MS (ESI, m/z): 481.4 [M+H+].
Example 204: 3-oxo-3,4-dihydro-2H-pyrido[3,2-b] [1,4]thiazine-6-carboxylic
acid
(3R,6S)-{6-[(2RS)-2-hydroxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-tetrahydro-
pyran-3-yl}-
amide:
This compound was prepared from intermediate 203.v (0.1 g) and 3-oxo-3,4-
dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid (0.083 g), using the procedure
of
Example 159. The title compound (0.08 g, 49% yield) was obtained as a. white
solid in a 3.5:1
mixture of diastereomers.
MS (ESI, m/z): 493.2 [M+H+].
Example 205: rac-trans-(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)- [6-(6-
methoxy-
quinolin-4-yloxymethyl)-piperidin-3-yl]-amine:
205.i. rac-trans piperidine-1,2,5-tricarboxylic acid 1-benzyl ester 5-methyl
ester:
Cbz-Cl (3.75 g, 22 mmol) was added dropwise to a vigorously stirred mixture of
rac-trans-piperidine-2,5-dicarboxylic acid 5-methyl ester (3.74 g, 20 mmol,
prepared
according to J. Heterocycl. Chem. (1995), 32, 857), NaHCO3 (6.7 g), acetone
(80 ml) and
water (80 ml). The mixture was stirred until gas evolvement stopped. The
mixture was
acidified with 1N aq. HCl (160 ml) and extracted with EA (2 x 250 ml). The
combined
organic layers were washed with brine, dried over MgSO4, filtered and
concentrated in vacuo
to give the Cbz-protected compound (602 g, 96% yield) as a colourless oil,
which was used in
the next step without purification.
MS (ESI, m/z): 322.3 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-213-
205.ii. rac-trans-6-hydroxymethyl piperidine-1,3-dicarboxylic acid 1-benzyl
ester 3-methyl
ester:
A solution of borane in THF (1M, 60 ml, 3 eq) was added dropwise to a solution
of
intermediate 205.i (6.4 g, 20 mmol) in THF at 0 C. The mixture was stirred at
0 C for 1 h and
at rt for 3 h. Excess of reagent was destroyed by careful addition of MeOH and
the volatiles
were removed under reduced pressure. MeOH was added and again removed under
reduced
pressure. The residue was purified by chromatography on silica gel (EA) to
give the desired
alcohol as a colourless oil (3.16 g, 51% yield).
11H NMR (CDC13) 8: 7.40-7.20 (m, 5H), 5.20 (d, 1H, J=12.4 Hz), 5.10 (d, 1H,
J=12.4 Hz),
4.40-4.10 (m, 2H), 3.85-3.60 (m, 2H), 3.62 (s, 3H), 3.40-3.25 (m, 1H), 2.65-
2.55 (m, 1H),
2.46 (br, 1H), 2.05-1.50 (m, 4H).
205.iii. rac-trans-6-(6-methoxy-quinolin-4 yloxyz7aethyl) piperidine-1,3-
dicarboxylic acid
I -benzyl ester 3-methyl ester:
To a suspension of 6-methoxy-quinolin-4-ol (1.75 g, 10 mmol) and intermediate
205.ii
(3.07 g, 10 mmol) in THF (50 ml) at 0 C was added triphenylphosphine (3.15 g,
12 mmol),
and dropwise DIAD (2.43 g, 12 mmol). The mixture was gradually warmed to rt
and stirred at
this temperature for 2 d. Volatiles were removed under reduced pressure and
the residue was
chromatographed on silica gel (Hex:EA 2:1) to give 1.26 g (27% yield) of
product, which was
still contaminated with some triphenylphosphine but used as such in the next
step.
205.iv. rac-trans-6-(6-methoxy-quinolin-4 yloxynaethyl) piperidine-1,3-
dicarboxylic acid
1-benzyl ester:
Intermediate 205.iii (1.26 g, 2.72 mmol) was dissolved in THF-water-MeOH 3:1:1
(100 rnl)o
and LiOH hydrate (0.23 g, 2 eq) was added. The mixture was stirred at rt
overnight and at
50 C for 2 h. The mixture was concentrated in vacuo, neutralised with 1N aq.
HC1 and
extracted with EA. The organic extracts were washed with water and brine,
dried over
MgSO4, filtered and concentrated in vacuo. The residue was triturated with
ether and filtered
to give the desired acid (0.736 g, 60% yield) as a colourless solid.
1VIS (ESI, m/z): 451.3 [M-H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-214-
205.v. rac-trans-5-amino-2-(6-methoxy-quinolin-4 yloxymethyl) piperidine-l-
carboxylic acid
benzyl ester:
A solution of intermediate 205.iv (0.736 g, 1.63 mmol) in tert-butanol (10 ml)
was treated
with TEA (0.501 ml, 2.2 eq) and DPPA (0.494 g, 1.1 eq). The mixture was heated
at reflux
for 4 h, concerntrated and filtered over silica gel (EA then EA/MeOH 9:1 as
eluent). The
filtrate was concentrated in vacuo, taken up in DCM (10 ml) and treated with
TFA (2 ml).
After 2 h, the volatiles were removed under reduced pressure. The residue was
dissolved in
DCM, washed with NH4OH and water. After drying over MgSO4, filtration and
concentration
in vacuo, the residue was purified by chromatography over silica gel (EA:MeOH
9:1 + 1%
concentrated NIH4OH then MeOH) to give the title amine (0.410 g, 60 % yield)
as an oil.
MS (ESI, m/z): 422.5 [M+H+].
205.vi. rac-trans-5-[(2,3-dihydro-benzo[1,4]dioxin-6 ylmethyl)-aminoJ-2-(6-
methoxy-
quinolin-4 ylo.xymethyl) piperidine-l-carboxylic acid benzyl ester:
A mixture of intermediate 205.v (0.210 g, 0.5 mmol) and 2,3-dihydro-
benzo[1,4]dioxine-
6-carbaldehyde (0.082 g, 0.5 mmol) in a 1,2-DCE-MeOH mixture (1:1, 4 ml) was
stirred at rt
overnight. NaBH4 (excess) was added and stirring was continued for 1 h. The
mixture was
partitioned between DCM and concentrated NH4OH. The organic layer was dried
over
MgSO4, filtered and concentrated to dryness. The residue was purified by
chromatography
over silica gel (EA:MeOH 9:1 + 1% NH4OH) to give the product (0.125 g, 43%
yield) as a
colourless foam.
MS (ESI, m/z): 570.6 [M+H+].
205.vii. rac-tr-ans-(2, 3-dihydro-benzo[1, 4]dioxin-6 ylmethyl)-[6-(6-methoxy-
quinolin-
4 yloxymethyl) piperidin-3 ylJ-amine:
Intermediate 205.vi (0.121 g, 0.21 mmol) was dissolved in EtOH (5 ml). 10%
palladium on
charcoal (0.064 g) and cyclohexene (2 ml) were added and the mixture stirred
at rt for 5 h.
The catalyst was filtered off and the filtrate concentrated. The residue was
purified by
chromatography on silica gel (EA:MeOH 9:1 +1% NH4OH) to give the title
compound
(0.077 g, 83% yield) as a colourless foam.
'H NMR (d6-DMSO) 8: 8.55 (d, 1H, J=5.1 Hz), 7.86 (d, 11-1, J=9.2 Hz), 7.45 (d,
IH,
J=2.8 Hz), 7.38 (dd, 1H, J=9.2 Hz, J=2.8 Hz), 6.98 (d, 1H, J=5.1 Hz), 6.83 (s,
1H), 6.76 (s,
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-215-
2H), 4.24 (s, 4H), 4.20-4.00 (m, 3H), 3.90 (s, 3H), 3.20-3.05 (m, 3H), 3.00-
2.90 (m, IH),
2.45-2.15 (m, 3H), 2.00-1.90 (m, 1H), 1.90-1.80 (m, 1H)
MS (ESI, m/z): 436.2 [M+FI+].
Example 206: rac-trans-6-{[6-(6-methoxy-quinazolin-4-yloxymethyl)-piperidin-
3-ylamino]-methyl}-4H-pyrido[3,2-b][1,4]thiazin-3-one:
206.i. rac-transpiperidine-1,2,5-tricarboxylic acid 1-tert-butyl ester 5-
methyl ester:
A suspension of rac-trans-piperidine-2,5-dicarboxylic acid 5-methyl ester
(9.36 g, 50 mmol,
prepared according to J. Ileterocycl. Chem. (1995), 32, 857) in THF (100 ml)
was treated
with BOC2O (16.3 g, 1.5 eq) and 1M aq. NaOH (50 ml). The mixture was stirred
at rt
overnight, acidified with 1M aq. HCl and extracted with EA. The combined
organic extracts
were washed with water and brine, dried over MgSO4, filtered and concentrated.
The residue
was purified by chromatography on silica gel (Hex/EA 1:1) to give 7.8 g(54 fo
yield) of title
compound as a colourless o il.
MS (ESI, m/z): 286.4 [M-H-].
206.ii. rac-trans-6-hydroxymethyl piperidine-1, 3-dicarboxylic acid 1-tert-
butyl ester
3-methyl ester:
Intermediate 206.i (7.8 g, 27 mmol) was reduced with borane (1M in THF, 82
rrnl) using the
procedure of Exemple 205, step 205.ii. After chromatography on Si02 (Hex/EA
1:1), the title
alcohol (5.4 g, 73 % yield) was obtained as a colourless oil.
'H NMR (d6-DMSO) 8: 4.69 (t, 1H, J=5.5 Hz), 4.25-4.15 (m, 1H), 4.05-3.90 (m,
IH),
3.60 (s, 3H), 3.55-3.35 (rn, 2H), 3.05-2.95 (m, 1H), 2.65 (br, 1H), 1.90-1.50
(m, 4H),
1.38 (s, 9H).
206.iii. rac-trans-6-(tert-butyl-dimethyl-silanyloxymethyl)piperidine-l,3-
dicarboxylic acid
1-tert-butyl ester 3-methyl ester:
TBDMSCI (3.3 g, 22 mmol) was added to a solution of intermediate 206.ii (5.4
g, 20 mmol)
and imidazole (1.63 g, 24 minol) in THF (50 ml). The mixture was stirred at rt
overnight,
poured into a NH4C1 solution and extracted with ether. The combined organic
extracts were
washed with water and brine, dried over MgSO4 and concentrated to give 8.38 g
of a
colourless oil which was used without further purification.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-216-
'H NMR (d6-DMSO) 6: 4.25-4.15 (m, 1H), 4.05-3.90 (m, 1H), 3.50 (s, 3H), 3.55-
3.35 (m,
2H), 3.05-2.95 (m, 1H), 2.65 (br, 1H), 1.90-1.50 (in, 4H), 1.38 (s, 9H), 1.80
(s, 9H),
0.50 (s, 6H).
206.iv. f=ac-trans-6-(tert-butyl-dimethyl-silan.yloxyrnethyl) piperidine-1, 3-
dicarboxylic acid
1-tert-butyl ester:
A solution of intermediate 206.iii (7.7 g, 19.9 mmol) in MeOH/H20 3:1 (150 ml)
and THF
(10 mi) was treated with LiOH.H20 (1.7 g, 2 eq). The mixture was stirred at rt
for 4 h and
then concentrated in vacuo. Ether and 1M HCl (35 ml) were added and the two
phases
separated. The aqueous layer was extracted once more with ether and the
combined organic
extracts were washed with brine, dried over MgSO4 and concentrated. The acid
(5.47 g, 73 %
yield) was isolated after chromatography over silica gel (Hex/EA 4:1, 2:1) as
a colourless
solid.
'H NMR (d6-DMSO) 8: 12.20 (br, lOH), 4.25-4.15 (m, 1H), 4.05-3.90 (m, 1H),
3.70-3.45 (m, 2H), 3.05-2.95 (m, 1H), 2.65 (br, 1H), 1.90-1.50 (m, 4H), 1.38
(s, 9H),
1.80 (s, 9I3), 0.50 (s, 6H).
206.v. rac-trans-5-amino-2-(tert-butyl-dimethyl-silanyloxymethyl) piperidine-l-
carboxylic
acid tert-butyl ester:
A suspension of intermediate 206.iv (5.46 g, 14.6 mmol) in benzene (100 ml)
was treated
with TEA (1.62 g, 1.1 eq) and DPPA (4.4 g, 1.1 eq). The resulting solution was
heated at
reflux for 60 min, benzyl alcohol (4.5 ml, 3 eq) was added and heating
continued overnight.
The mixture was cooled to rt, diluted with 100 ml of EA and washed with NaHCO3
and brine,
dried over MgSO4 and concentrated in vacuo. Chromatography over silica gel
(Hex/EA 9:1,
4:1) separated the undesired isocyanate (700 mg) and the Cbz-protected
derivative (3.65 g,
52% yield). The isocyanate was dissolved in THF (20 ml) and treated with NaOH
(1M, 6 ml).
The mixture was vigorously stirred at rt for 1 h. The organic layer was
separated and the aq.
layer was once more extracted with EA. The combined organic phases were washed
with
NaHCO3, dried over MgSO4 and concentrated to give 270 mg of the title amine as
a
colourless solid.
MS (ESI, rn/z): 345.4 [M+W].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-217-
206.vi. rac-trans-2-(tert-butyl-dimethyl-silanyloxymethyl)-5-[(3-oxo-3, 4-
dihydro-
2H pyrido[3,2-bJ[1,4]thiazin-6 ylmethyl)-aminoJ piperidine-l-carboxylic acid
tert-butyl
ester:
A mixture of intennediate 206.v (270 mg, 0.78 mmol) and 3-oxo-3,4-dihydro-
2FI-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (152 mg, 078 mmol) in 1,2-
DCE/MeOH 1:1
(10 ml) was stirred at rt overnight. NaBH4 (excess) was added and stirring was
continued for
1 h. The mixture was partitioned between DCM and NH4OH and the organic layer
was dried
over MgSO4 and concentrated in vacuo. 400 mg (97% yield) of product were
obtained, which
were used without purification in the next step.
MS (ESI, m/z): 524.2 [M+H+].
206.vii. rac-trans-5-[tert-butoxycarbonyl-(3-oxo-3, 4-dihydro-2H-pyrido[3, 2-
b][1, 4]thiazin-
6ylmethyl)-aminoJ-2-hydroxymethylpiperidine-l-carboxylic acid tert-butyl
ester:
A solution of intermediate 206.vi (400 mg, 0.76 mmol) in THF (10 ml) was
trea_ted with
BOC2O (2 eq) and stirred at rt overnight. The mixture was concentrated in
vacuo ancl purified
by chromatography over silica gel (Hex /EA 4:1, 2:1, 1:1). This intennediate
(440 mg) was
dissolved in THF and treated with 1M TBAF solution (0.8 ml) and reaction was
allowed to
proceed for 4 h. The mixture was concentrated in vacuo and purified by
chromatogra_phy over
silica gel (Hex/EA 2:1, 1:1, EA) to give 290 mg of title alcohol (74%) as a
colourless foam.
MS (ESI, m/z): 623.2 [M+H+].
206.viii. rac-trans-5-[tert-butoxycarbonyl-(3-oxo-3, 4-dihydro-2H-pyrido[3,2-
bJ[1, 4Jthiazin-
6 ylmethyl)-aminoJ-2-(6-methoxy-quinazolin-4 yloxymethyl) piperidine-l-
carboxylic acid
tert-butyl ester:
This compound was prepared from 4-chloro-8-methoxyquinazoline (50 mg, 0.25
mniol) and
intermediate 206.vii (130 mg, 0.25 mmol), using the procedure of Example 154,
step 154.iv.
After chromatography over silica gel (Hex/EA 1:1, EA ), the title compound (90
ing, 53%
yield) was obtained as a yellowish foam.
MS (ESI, m/z): 667.1 [M+FI+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-218-
206.ix. rac-trans-6-{[6-(6-methoxy-quinazolin-4yloxymethyl)piperidin-3
ylamino]-methyl}-
4H-pyrido[3, 2-bJ[l, 4]thiazin-3-one:
A solution of intermediate 206.viii (90 mg, 0.135 mmol) in DCM (5 ml) was
treated with
TFA (1 ml). The rnixture was stirred at rt for 2 h, concentrated in vacuo and
partitioned
between DCM and NH4OH. The organic layer was dried over MgSO4 and the residue
purified
by chromatography over silica gel (EA/MeOH 9:1 + 1 0o NH4OH) to give 30 mg of
title
compound as a colourless foam.
MS (ESI, m/z): 467.2 [M+H+].
Example 207: rac-trafzs-(2,3-dihydro-benzo [1,4] dioxin-6-ylmethyl)-[6-(6-
methoxy-
quinazolin-4-yloxymethyl)-piperidin-3-yl]-amine:
207.i. rac-trans-6-(6-methoxy-quinazolin-4 yloxymethyl)piperidine-1,3-
dicarboxylic acid
1-tert-butyl ester 3-methyl ester:
This compound was prepared from 4-chloro-8-methoxyquinazoline (390 mg, 2 mmol)
and
intermediate 206.ii (547 mg, 2 mmol), using the procedure of Example 206, step
206.viii.
After chromatography over silica gel (Hex/EA 1:1, EA), the title compound (610
mg, 71%
yield) was obtained as a colourless foam.
MS (ESI, m/z): 43 2.5 [M+H+].
207.ii. rac-trans-6-(6-methoxy-quinazolin-4 yloxymethyl) piperidine-1,3-
dicarboxylic acid
1-tert-butyl ester:
Intermediate 207.i (600 mg, 1.4 mmol) was hydrolysed using the procedure of
Example 206,
step 206.iv. The title acid (180 mg, 31% yield) was obtained after
crystallisation from ether.
MS (ESI, m/z): 41 8.1 [M+H+].
207.iii. rac-trans-5-amino-2-(6-methoxy-quinazolin-4 yloxymethyl) piperidine-l-
carboxylic
acid tert-butyl ester:
To a suspension of intermediate 207.ii (170 mg, 0.4 mmol) in benzene (10 ml)
was added
TEA (1.2 eq) and DPPA (l.l eq). The mixture was heated to reflux for 2 h. The
mixture was
cooled to rt and partitioned between EA and water. The organic phase was dried
over MgSO4
and concentrated. The residue was dissolved in THF (6 m_1) and treated with
NaOH 1M (2 ml)
and the mixture vigorously stirred for 1 h at rt and 1 h at 50 C. The mixture
was diluted with
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-219-
water and extracted with EA. The cornbined organic phases were dried over
MgSO4 and
concentrated to give 100 mg (63% yield) of title amine as a colourless oil.
MS (ESI, m/z): 389.1 [M+H+].
207.iv. rac-trans-5-[(2, 3-dihydro-benza[1, 4]dioxin-6-ylmethyl)-aminoJ-2-(6-
methoxy-
quinazolin-4 yloxymethyl)piperidine-l-carboxylic acid tert-butyl ester:
A mixture of intermediate 207.iii (100 mg, 0.26 mmol) and 2,3-dihydro-
benzojl,4]dioxine-
6-carbaldehyde (42 mg, 0.26 mmol) in DCE:MeOH 1:1 (3 ml) was stirred at rt
overnight.
NaBH4 (excess) was added and stirrirng was continued for 1 h. The mixture wa.s
partitioned
between DCM and NH40H. The orgaanic layer was dried over MgSO4 and
concentrated in
vacuo. Purification by chromatography over silica gel (EA/MeOH 9:1 + 1% NH4OH)
gave
80 mg (58% yield) of product as a colourless oil.
MS (ESI, m/z): 537.3 [M+H+].
207.v. rac-trans-(2, 3-dihydro-benzo[I , 4Jdioxin-6 ylmethyl)-[6-(6-methoxy-
quinczzolin-
4 yloxymethyl) piperidin-3 ylJ-amine:
A solution of intermediate 207.iv (80 Ing, 0.15 mmol) in DCM (4 ml) was
treated with TFA
(1 ml). The mixture was stirred at rt for 2 h, concentrated in vacuo and
partitioned between
DCM and NH4OH. The organic layer was dried over MgSO4, concentrated in vacuo
and the
residue was purified by chromatography over silica gel (EA/MeOH 9:1 + 1%
NH4OH) to give
60 mg of title compound as a colourless oil.
MS (ESI, m/z): 437.4 [M+H+].
Example 208: rac-trafas-{2-(6-methoxy-quinazolin-4-yloxymethyl)-5-[(3-oxo-
3,4-dihydro-2H-pyrido[3,2-b] [1,4]thiazin-6-ylmethyl)-amino]-piperidin-1-yl]I -
acetic acid
tert-butyl ester:
208.i. rac-trans-5-benzyloxycarbonylczmino-2-hydroxymethyl piperidine-l-
carboxylic acid
tert-butyl ester:
5 -benzyloxycarbonylamino-2-(tert-butyl-dimethyl-silanyloxymethyl)-piperidine:
-1-carboxylic
acid tert-butyl ester (3.65 g, 7.625 mrrnol; obtained as secondary product at
Exarnple 206, step
206.v) was dissolved in THF (50 ml) and TBAF (1M in THF, 1.1 eq) was added.
The mixture
was stirred at rt for 1 h, concentrated in vacuo, partitioned between EA and
water. The
organic extracts were washed with water and brine, dried over MgSO4 and
concentrated in
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 220 -
vacuo. The residue was purified by chromatography over silica ge 1(Hex/EA 2:1,
1:1 then EA)
to afford the title alcohol (2.37 g, 85%) as a colourless oil.
MS (ESI, m/z): 365.2 [M+H+].
208.ii. S-benzyloxycarbonylarnino-2-(6-methoxy-quinazolin-4 ylvxymethyl)
piperidine-
1-carboxylic acid tert-butyl ester:
To a solution of 4-chloro-8-methoxyquinazoline (290 mg, 1.49 rnmol) and
intermediate 208.i
(544 mg, 1 eq) in DMF (5 ml) at 0 C was added NaH (60% dispersion in oil, 143
mg, 2 eq).
The rnixture was stirred at 0 C for 3 h, quenched with water and extracted
with ether. Organic
extracts were washed with water and brine, dried over MgSO4 and concentrated
in vacuo. The
residue was purified by chromatography over silica gel (Hex/EA 1:1, EA) to
give 422 mg
(54% yield) of coupling product as a colourless oil.
MS (ESI, m/z): 523.2 [M+H+].
208.iii. nac-trans-[6-(6-methoxy-quinazolin-4yloxymethyl) pipe7-idin-3ylJ-
carbamic acid
benzyl ester:
To a solution of intermediate 208.ii (422 mg, 0.808 mmol) in DCM (5 ml) was
added TFA
(2 m 1). The mixture was stirred at rt for 2 h, concentrated in vercuo and
partitioned between
DCM and NH4OH. The organic extracts were dried over MgSO4 and concentrated to
give the
title piperidine (340 mg, 99% yield), which was used without further
purification.
MS (ESI, m/z): 423.5 [M+H+].
208. iv. rac-trans-[5-benzyloxycarbonylamino-2-(6-methoxy-quinazolin-
4yloxymethyl)-
piperidin-1 ylJ-acetic acid tert-butyl ester:
To a solution of intermediate 208.iii (340 mg, 0.8 mmol) in TIiF (5 ml) were
added DIPEA
(0.16 ml, 1.2 eq) and tert-butyl bromoacetate (0.141 ml, 1.2 eq) the mixture
was stirred at rt
for 2 d. The resulting suspension was poured into a sat. NH4CI solution and
extracted with
EA. The organic extracts were dried over MgSO4 and concentrated in vacuo. The
residue was
purified by chromatography over silica gel (Hex/EA 1:1, EA) to give 260 mg
(60% yield) of
desired compound as a colourless oil.
MS (ESI, m/z): 537.2 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 221 -
208.v. rac-trans-[5-amino-2-(6-methoxy-quinazolin-4 yloxymethyl) piperidin-1
ylJ-acetic
acid tert-butyl ester:
Intermediate 208.iv (260 rng, 0.48 mmol) was dissolved in EA (50 ml), degassed
several
times and treated with 10% Pd/C (170 mg). The reaction was stirred under
h_ydrogen
atmosphere (1 bar) for 5 h, filtered over Celite and concentrated in vacuo to
give 170 mg
(87% yield) of a colourless oil.
MS (ESI, m/z): 403.2 [M+]H+].
208.vi. rac-trans-{2-(6-methoxy-quinazolin-4 yloxymethyl)-5-[(3-oxo-3,4
dihydro-
2H-pyrido[3,2-bJ[1,4]thiazin-6 ylmethyl)-aminoJ piperidin-1 ylJ-acetic acid
tert-butyl ester:
Reductive amination was carried out as described in Example 206, step 206.vi,
starting from
intermediate 208.v (170 mg, 0.4 mmol) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-
b][1,4]-thiazine-
6-carbaldehyde (82 mg, 1 eq). The product (146 mg, 59% yield) was isolated as
a beige foam.
MS (ESI, m/z): 581.2 [M-+-H+]
Example 209: rac-trans- {2-(6-methoxy-quinazolin-4-yloxymethyl)-5-[(3-oxo-
3,4-dihydro-2H-pyrido[3,2-b] [1,4]thiazin-6-ylmethyl)-amino]-piperidin-1-yl}-
acetic acid:
A solution of the compound of Example 208 (100 mg, 0.17 mmol) in TFA (2 ml)
was stirred
at rt for 3 h. The mixture was concentrated in vacuo and crystallised from
ether to give the
title compound (130 mg) as its trifluoroacetate salt.
MS (ESI, m/z): 525.2 [M+H+].
Example 210: rac-trans-6-({6-[2-(6-methoxy-[1,5]naphthyridin-4-yl)-ethyl]-
piperidin-
3-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4]thiazin-3-one:
210.i. rac-trans-6 formyl piperidine-l,3-dicarboxylic acid 1-tert-butyl ester
3-methyl ester:
A solution of oxalyl chloride (4.5 ml, 52.9 mmol) in DCM (33 ml) was cooled to
-78 C. A
solution of DMSO (4.7 ml, 64.85 mmol) in DCM (33 ml) was added dropwise and
the
solution was stirred at -79 C for 15 min. A solution of intermediate 206.ii
(4.66 g, 17.1 mmol)
dissolved in DCM (33 nzl) was added dropwise. The solution was stirred at -78
C for 1 h
before dropwise addition of TEA (33 ml in 20 ml of DCM). The solution was
allowed to
warm to rt and was stirred for 3 h. Saturated aq. NaHCO3 (75 ml) was added.
The tvvo phases
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-222-
were separated and the aq. phase was extracted once more with DCM. Combined
organic
phases were washed with brine, dried over MgSO4 and concentrated in vacuo.
Chromatography over silica gel (Hex/EA 2:1) afforded the title aldehyde (1.14
g, 25 % yield)
as a yellowish oil in a 3:1 mixture of cis/trans isomers.
MS (ESI, m/z): 272.3 [M+H+].
210.ii. rac-trans-6-ethynyl piperidine-1, 3-dicarboxylic acid 1-tert-butyl
ester 3-naethyl ester:
To a solution of p-toluenesulfonyl azide (1.8 g, 9.08 mmol) in MeCN (115 ml)
was added
K2C03 (3.1 g, 22.1 mmol) and dimethyl acetylmethylphosphonate (1.2 ml, 8.87
mmol). The
mixture was stirred at rt for 2 h and a solution of intermediate 210.i (1.14
g, 4.2 mmol) in
MeOH (17 ml) was added. The mixture was stirred at rt overnight. The volatiles
were
removed under reduced pressure and the residue dissolved in water (50 ml) and
EA (100 ml).
The aqueous layer was extracted with EA (2 x 100 rn 1). The combined organic
layers were
washed with brine, dried over MgSO4, filtered and c ncentrated in vacuo. The
residue was
filtered through Si02 (Hex/EA 4:1) to afford the title alkyne (mixture of cis
and trans,
450 mg, 40%) as a yellowish oil.
MS (ESI, m/z): 268.5 [M+H*].
210.iii. rac-trans-6-(6-naethoxy-[1, 5]naphthyridin-4 ylethynyl) piperidine-1,
3-dicarboxylic
acid 1-tert-butyl ester 3-methyl ester:
To a solution of intermediate 210.ii (450mg, 1.68mmo1) and
trifluoromethanesulfonic acid
6-methoxy-[1,5]naphthyridin-4-yl ester (517 mg, 1 eq) in DMF (5 ml) and TEA
(1.4 ml) were
added successively copper iodide (35 mg) and
bis(triphenylphosphine)palladium(II) chloride
(59 mg). The reaction mixture was stirred at rt for 3 h- The mixture was
poured on water and
extracted with EA. The aq. layer was extracted twice more (2 x 100 ml). The
combined
organic layers were washed with water and bririe, dried over Na2SO4, filtered
and
concentrated to dryness. The residue was chromatographed over silica gel
(Hex/EA 1:2) to
afford the title alkyne (300 mg, 42 % yield) as a beige solid.
MS (ESI, m/z): 426.1 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 223 -
210.iv. rac-traazs-6-[2-(6-methoxy-[1,5]naphthyridin-4 yl)-ethylJ piperidine-
1,3-dicarboxylic
acid 1-tert-butyl ester 3-methyl ester:
Intermediate 210.iii (300 mg, 0.706 mmol) was hydrogenated over Pt02 (70 mg)
in EtOH
(20 ml) for 5 h(1 bar of H2). The mixture was filtered through Celite and
concentrated to give
225 mg (74% yield) of a colourless oil.
MS (ESI, m/z): 430.3 [M+H+]
210.v. rac-trans-6-[2-(6-methoxy-[1,5]naphthyridin-4 yl)-ethylJ piperidine-1,3-
dicarboxylic
acid 1-tert-butyl ester:
This compound (216 mg; 99% yield) was obtained as a colourless solid from
intermediate 210.iv (225 mg, 0.52 mmol), using the procedure of Example 206,
step 206.ii.
MS (ESI, m/z) : 416.3 [M+H+].
210.vi. rac-trezns-5-amino-2-[2-(6-methoxy-[1, 5]naphthyridin-4yl)-ethylJ
piperidine-
1-carboxylic acid tert-butyl ester:
Starting from intermediate 210.v (216 mg, 0.52 mmol), Curtius degradation was
carried out as
described in Example 207, step 207.iii, affording the target amine (137 mg,
68% yield) as a
colourless solid.
MS (ESI, m/z): 387.2 [M+H+].
210.vii. rac-tY-ans- 2-[2-(6-methoxy-[1,5]naphthyridin-4 yl)-ethylJ-5-[(3-o)co-
3,4-dihydro-
2H-pyrido[3,2-bJ[I, 4]thiazin-6-ylmethyl)-aminoJ piperidine-l-carboxylic czcid
tert-butyl
ester:
Starting from intermediate 210.vii (135 mg, 0.35 mmol) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (68 mg, 1 eq), reductive
amination was carried
out as described in Example 206, step 206.vi. The product (133 mg, 67% yield)
was isolated
as a yellowisli foam.
MS (ESI, m/z): 565.3 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 224 -
21O.viii. rac-trans-6-({6-[2-(6-methoxy-[1,5Jnaphthyridin-4 yl)-ethylJ
piperidin-3 ylamino}-
me thyl)-4Fl-pyrido[3, 2-bJ[l, 4Jthiazin-3-one:
Deprotection was carried out as described in Example 206, step 206.ix,
starting from
intermediate 210.vii (113 mg, 0.23 mmol) to give the title compound (100 mg,
91 % yield) as
an off-white foam.
MS (ESI, m/z): 465.4 [M+H}].
Example 211: rac-trans-5-[(3-oxo-3,4-dihydro-2H-pyrido [3,2-b] [1,4]thiazin-
6-ylmethyl)-amino]-piperidine-2-carboxylic mcid (6-methoxy-[1,5]naphthyridin-4-
yl)-
amide:
21 l.i. rac-trans-6-carbamoyl piperidine-1,3-dicarboxylic acid 1-tert-butyl
ester 3-methyl
ester:
Intermediate 206.i (1.7 g, 5.9 mmol) and NHS (715 mg, 1.05 eq) were added to a
solution of
DCC (1.28 g, 1.05 eq) in EA (25 ml). A precipitate formed and the mixture was
stirred at rt
overnight. The precipitate was filtered off and the filtrate concentrated in
vacuo. The residue
was dissolved in THF (100 ml) and NH3 (gas) was bubbled through for 15 min. A
white
precipitate formed which was filtered off. The filtrate was concentrated in
vacuo and the title
amide (800 mg, 47% yield; colourless solid) was obtained after chromatography
(Si02 / EA).
1H NMR (d6-DMSO) S: 7.31 (br, 1H), 7.04 (br, 1H), 4.4-4.30 (m, 1H), 4.20-4.10
(m, 1H),
3.61 (s, 3H), 3.40-3.20 (m, 1H), 2.70 (br, 1H), 2.00-1.40 (m, 4H), 1.37 (s,
9H).
21 I.H. rac-trans-6-(6-methoxy-[1,5]naphthyridin-4 ylcarbamoyl) piperidine-
1, 3-dicarboxylic acid 1-tert-butyl ester 3-methyl ester:
This compound (880 mg, 70% yield) was obtained as a colourless solid, starting
from
intermediate 211.i (800 mg, 2.8 mmol) and trifluoromethanesulfonic acid 6-
methoxy-
[1,5]naphthyridin-4-yl ester (861 mg, 1 eq) and using the procedure of Example
28, step 28.ii.
1VIS (ESI, m/z): 444.8 [M+H+].
211.iii. rac-trans-6-(6-methoxy-[1, 5]naphthyridin-4 ylcarbamoyl) piperidine-
1, 3-dicarboxylic acid 1-tert-butyl ester:
This compound (650 mg; 98% yield) was obtained as a colourless solid frorn
intermediate 21 Lii (680 mg, 1.53 mmol), using the procedure of Example 206,
step 206.ii.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 225 -
MS (ESI, m/z): 432.0 [M+H+].
211.iv. rac-trans-5-anaino-2-(6-methoxy-[1,5]naphthyridin-4 ylcarbamoyl)
piperidine-
1-carboxylic acid tert-butyl ester:
Starting from intermediate 211.iii (178 mg, 0.41 mmol), Curtius degradation
was carried out
as described in Example 207, step 207.iii, affording the target amine (136 mg,
82% yield) as a
colourless solid.
MS (ESI, m/z): 402.1 [M+H+].
211.v. rac-trans-2-(6-methoxy-[1, 5]naphthyridin-4 ylcarbamoyl)-5-[(3-oxo-3, 4-
dihydro-
2H-pyrido[3,2-bJ[1,4]thiazin-6 ylmethyl)-aminoJ piperidine-l-carboxylic cicid
tert-butyl
ester:
Starting from interrnediate 211.iv (136 mg, 0.34 mmol) and 3-oxo-3,4-dihydro-
2H-pyrido [3,2-b] [ 1,4]thiazine-6-carbaldehyde (66 mg, I eq), reductive am
ination was carried
out as described in EXample 206, step 206.vi. The product (110 mg, 56% yield)
was isolated
as a yellowish foam.
MS (ESI, m/z): 580.2 [M+H+].
211.vi. rac-trans-5-[(3-oxo-3, 4-dihydro-2H-pyrido[3, 2-bJ[1, 4]thiazin-6
ylmethyl)-aminoJ-
piperidine-2-carboxylic acid (6-methoxy-[1,5]naphthyridin-4yl)-amide:
Deprotection was carried out as described in Example 206, step 20fi.ix,
starting from
intermediate 211.v (110 mg, 0.19 mmol), to give after crystallisation from
ether/MeOH the
title compound (52 mg, 57 % yield) as an off-white solid.
'H NMR (d6-DMSO) S: 8.70 (d, 1H, J=5.04 Hz), 8.43 (d, 1H, J=5.04 Hz), 8.27 (d,
1H,
J=9.03 Hz), 7.70 (d, 1H, J=7.89 Hz), 7.32 (d, 1H, J=9.03 Hz), 7.07 (d, 1H,
J=7.89 Hz),
4.04 (s, 3H), 3.70 (s, 2H), 3.65-3.60 (m, 1H), 3.51 (s, 2H), 3.25-3.10 (m,
1H), 3.05-2.95 (m,
1H), 2.60-2.40 (m, 2H), 2.30-2.05 (in, 2H), 1.90-1.80 (m, 1H), 1.70-1.50 (m,
1H),
1.30-1.10 (m, 1H).
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-226-
Example 212: rac-trans-5-[(3-oxo-3,4-dihydro-2H-benzo[1,4]thiazin-6-ylmethyl)-
amiino]-piperidine-2-carboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-amide:
212A. rac-trans-2-(6-methoxy-[1, 5]naphthyridin-4 ylcarbamoyl)-5-[(3-oxo-3, 4-
dihydro-
2F7-benzo[1, 4]thiazin-6 ylmethyl)-ami7ioJ piperidine-l-carboxylic acid tert-
butyl ester:
Starting from intermediate 211.iv (110 mg, 0.27 mmol) and 3-oxo-3,4-dihydro-
2.H-benzo[1,4]thiazine-6-carbaldehyde (55 mg, 1 eq), reductive amination was
carried out as
described in Example 206, step 206.vi. The product (90 mg, 57% yield) was
isolated as a
yellowish foam.
MS (ESI, m/z): 579.2 [M+H+].
212.ii. rac-trans-5-[(3-oxo-3,4-dihyd.-o-2H-benzo[1,4]thiazin-6 ylmethyl)-
aminoJ-
piperidine-2-carboxylic acid (6-rnethoxy-[1,5]naphthyridin-4 yl)-amide:
Deprotection was carried out as described in Example 206, step 206.ix,
starting from
intermediate 212.i (90 mg, 0.19 mmol), to give after chromatography (EA/MeOH
9:1 -u 1%
NH4OH) the title compound (42 mg, 56 % yield) as an off-white solid.
'H NMR (d6-DMSO) b: 8.70 (d, 1H, J=5.04 Hz), 8.43 (d, 1H, J=5.04 Hz), 8.27 (d,
IH,
J=9.03 Hz), 7.34 (d, IH, J=9.03 Hz), 7.20 (d, IH, J=7.89 Hz), 7.00-6.85 (m,
2H), 4.04 (s,
311), 3.65-3.50 (m, 3H), 3.41 (s, 2H), 3.25-3.10 (m, IH), 3.05-2.95 (m, 1H),
2.60-2.40 (m,
2Ii), 2.30-2.05 (m, 2H), 1.90-1.80 (m, 1H), 1.70-1.50 (m, IH), 1.30-1.10 (m,
1H).
MS (ESI, m/z): 479.3 [M+H+].
Example 213: rac-trans-5-[(2,3-dihydro-benzo[1,4]dioxin-6-ylmethyl)-amino]-
piperidine-2-carboxylic acid (6-metboxy-[1,5]naphthyridin-4-yl)-amide:
213.i. rac-trans-5-[(2, 3-dihydro-benzo[1, 4]dioxin-6 ylmethyl)-anzinoJ-2-(6-
methoxy-
[1, S]naphthyridin-4 ylcarbamoyl)piperidine-l-carboxylic acid tert-butyl
ester:
Starting from intermediate 211.iv (1 10 mg, 0.27 mmol) and 2,3-dihydro-
benzo[1,4]dioxine-
6-carbaldehyde (45 mg, 1 eq), reductive amination was carried out as described
in
Example 206, step 206.vi. The product (100 mg, 66% yield) was isolated as a
colo-urless
foam.
MS (ESI, m/z): 550.3 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-227-
213.ii. rac-trans-5-[(2,3-dihydro-benzo[1,4]dioxin-6 ylmethyl)-aminoJ
piperidine-
2-carboxylic acid (6-methoxy-[], 5Jnaphthyridin-4 yl)-amide:
Deprotection was carried out as described in Example 206, step 206.ix,
starting from
intermediate 213.i (100 mg, 0.18 mmol), to give after chromatography (EA/NIeOH
9:1 + 1%
NH4OH) the title compound (40 mg, 49 % yield) as an off-white solid.
1H NMR (d6-DMSO) 8: 8.70 (d, 1H, J=5.04 Hz), 8.43 (d, 1H, J=5.041-Iz), 8.27
(d, 1H,
J=9.03 Hz), 7.34 (d, 1H, J=9.03 Hz), 6.80 (s, 1H), 6.74 (s, 2H), 4.18 (s,
4I1), 4.04 (s, 3H),
3.65-3.50 (m, 3H), 3.41 (s, 2H), 3.25-3.10 (m, 1H), 3.05-2.95 (m, 1H), 2.60-
2.40 (m, 2H),
2.30-2.05 (m, 2H), 1.90-1.80 (m, 1H), 1.70-1.50 (m, 1H), 1.30-1.10 (m, 1H).
MS (ESI, m/z): 450.2 [M+H+].
Example 214: cis-3-{[(3-oxo-3,4-dihydro-2H-pyrido[3,2-b] [1,4]thiazin-6-
ylmethyl)-
amino]-methyl}-cyclohexanecarboxylic acid (6-methoxy-[1,5]naphthyridiin-4-yl)-
amide:
214. i. cis- [(3-(6-methoxy-[1, 5Jnaphthyridin-4 ylcarbamoyl)-
cyclohexylmethiylJ-carbamic
acid tert-butyl ester:
This compound (1.08 g, 60% yield) was obtained as a white solid, starting from
intermediate 72.i (1.1 g, 4.3 mmol) and trifluoro-methanesulfonic a.cid 6-
methoxy-
[1,5]naphthyridin-4-yl ester (1.32 g, 4.3 mmol), using the procedure of
Example 59,
step 59.ii. After recrystallization in an EA-Hex mixture, the compound was
obtained as the
unique cis-isomer.
MS (ESI, m/z): 415.5 [M+H+].
214.ii. cis-3-aminomethyl-cyclohexanecarboxylic acid (6-methoxy-
[1,5]napAthyridin-4 yl)-
amide:
This compound (0.8 g, 97% yield) was prepared from intermediate 214.i (1.08 g,
2.6 mmol)
as described in Example 67, step 67.iii. The title amine was obtained as a
colourless oil.
MS (ESI, m/z): 315.3 [M+H+]-
214.iii. cis-3-[[(3-oxo-3,4-dihydro-2H-pyrido[3,2-bJ[1,4]thiazin-6 ylmethyl~-
aminoJ-
methyl}-cyclohexanecarboxylic acid (6-methoxy-[1,5Jnaphthyridin-4 yl)-anzide:
This compound (0.055 g, 30% yield) was obtained as a white solid, starting
from
intermediate 214.ii (0.1 g, 0.32 mmol) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-
b][1,4]thiazine-
6-carbaldehyde (0.072 g, 0.37 rnmol) and using the procedure of Example 63,
step 63.vii.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-228-
MS (ESI, m/z): 493.2 [M+H+].
Example 215: 3-{[(3-oxo-3,4-dihydro-2H-pyrido[3,2-b] [1,4]oxazin-6-ylmethyl)-
amino]-
methyl}-cyclohexanecarboxylic acid (6-methoxy-[1,5]naphthyridin-4-yl)-amide:
The title compound (0.059 g, 39% yield) was prepared as a white solid,
starting from
intermediate 69.ii (0.100 g, 0.32 mmol) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-
b][1,4]oxazine-
6-carbaldehyde (0.062 g, 0.35 mmol) and using the procedure of Example 63,
step 63.vii.
MS (ESI, m/z): 477.4 [M+H'].
Example 216: 6-((3R,6S)-{6-trans-[2-(3-fluoro-6-methoxy-[1,5]naphthyridin-4-
yl)-
vinyl]-tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4]thiazin-3-one
:
216.i. trans-7 fluoro-2-rnethoxy-8-styryl-[1,5]naphthyridine:
This compound (2.5 g, 89% yield) was obtained as a yellowish solid from 8-
bromo-7-fluoro-
2-methoxy-[1,5]naphthyridine (2.57 g, 10 mrnol; prepared as in WO 2004/058
144) and
trans-phenylvinyl boronic acid (1.62 g, 11 n-1mo1), using the procedure of
Example 178,
step 178.i.
MS (ESI, m/z): 281.0 [M+H}].
216.ii. 1-(3 fluoro-6-methoxy-[1,5]naphthyridin-4 yl)-2 phenyl-ethane-1,2-
diol:
The title diol (2.3 g, 81% yield) was obtained as a white foam, starting from
intermediate 216.i (2.5 g, 8.9 mmol) and using the procedure of Example 178,
step I 78.ii.
1H NMR (CDC13) 8: 8.42 (d, J = 0.7 Hz, 1H); 8.28 (d, J = 9.1 Hz, 1H); 7.24-
7.15 (m, 4H);
7.08 (m, 2H); 6.70 (br s, 1H); 5.28 (br s, 1H); 5.10 (d, J= 7.9Hz, 1H); 4.11
(s, 3H);
3.85 (br s, 1H).
216.iii. 3 fluoro-6-methoxy-[1,5]naphthyridine-4-carbaldehyde:
This compound (1.34 g, 89% yield) was obtained as a white solid, starting from
intermediate 216.ii (2.3 g, 7.25 mmol) and using the procedure of Example 178,
step 178.iii.
1H NMR (d6-DMSO) S: 11.08 (s, 1H); 9.01 (d, J= 1.3 Hz, 1H); 8.41 (d, J= 9.1
Hz, 1H);
7.37 (d, J 9.1 Hz, 1H); 4.09 (s, 3H).
MS (ESI, m/z): 206.9 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 229 -
216.iv. (3R, 6S)-{6-trans-[2-(3 fluoro-6-methoxy-[1,5]naphthyridin-4-y1)-
vinylJ-tetrahydro-
pyran-3 yl}-carbamic acid tert-butyl ester:
This compound (1.5 g, 59% yield) was obtained as a yellowish solid, starting
from
intermediate 164.ii (2.66 g, 6.3 mmol) and intermediate 216.iii (1.3 g, 6.3
mmol) and using
the protocol of Example 171, step 171.v.
MS (ESI, m/z): 404.0 [M+H+].
216.v. (3R, 6S)-6-trans-[2-(3 fluoro-6-methoxy-[1, 5]naphthyridin-4 yl)-vinylJ-
tetrahydro-
pyran-3 ylarnine:
This compound (0.090 g) was obtained as a yellowish foam, starting from
intermediate 216.iv
(0.2 g) and using the procedure of Example 164, step 164.iv.
MS (ESI, m/z): 304.1 [M+H+J.
216.vi. 6-((3R, 6S)-{6-trans-[2-(3 fluoro-6-methoxy-[1, 5]naphthyridin--l yl)-
vifrylJ-
tetrahydro pyran-3 ylamino}-methyl)-4Hpyrido[3,2-b][1,4Jthiazin-3-one:
This compound was obtained as a white solid (0.045 g, 31% yield) from
intermediate 216.v
(0.09 g, 0.3 mmol) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-
carbaldehyde
(0.063 g, 1.1 eq), using the procedure of Example 88, step 88.iv.
'H NMR (d6-DMSO) 8: 10.83 (s, 1H); 8.85 (s, 1H); 8.31 (d, J= 9.0 Hz, 1H); 7.70
(d,
J = 7.8Hz, 1 H); 7.26 (d, J = 9.0 Hz, 1H); 7.05 (d, J = 7.8 Hz, 1H); 6.72 (d,
J = 11.9 Hz, 1H),
6.04 (dd, J= 8.1, 11.9 Hz, 1H); 4.01 (s, 3H); 3.84-3.75 (m, 2H); 3.68 (br s,
2H); 3.42 (s, 2H);
2.83 (t, J= 10.8 Hz, 1H); 2.50 (overlapped m, 1H); 2.03-1.94 (m, 2H); 1.58 (m,
1H); 1.38 (m,
1H); 1.12 (m, 1H).
MS (ESI, m/z): 482.2 [M+H+]
Example 217: 6-(3R,6S)-{6-[(1R,2R)-2-(3-fluoro-6-methoxy-[1,5]naphthyridin-4-
yl)-
1,2-dihydroxy-ethyl]-tetrahydro-pyran-3-ylamino}-methyl)-
4H-pyrido[3,2-b] [1,4]thiazin-3-one:
217.i. (3R,6S)-{6-[(IR,2R)-2-(3 fluoro-6-methoxy-[1,5]naphthyridin-4 yl)-1,2-
dihydroxy-
ethylJ-tetrahydro pyran-3 yl}-carbamic acid tert-butyl ester:
This compound (0.525 g, 42% yield) was obtained as a brovvn oil, starting from
ntermediate 216. iv (1.13 g, 2.8 mmol) and applying the protocol of Example
171, step 171.vi.
The diol was obtained as a 6:1 mixture of diastereomers.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-230-
MS (ESI, m/z): 437.9 [M+H}].
217.ii. (IR,2R)-1-((2S5R)-5-amino-tetrahydro pyran-2 yl)-2-(3 fluoro-6-methoxy-
[1, 5Jnaphthyridin-4 yl)-ethane-1, 2-diol:
This compound (0.4 g, 98% yield) was obtained as a yellowish foam, starting
frotn
intermediate 217.i (0.525 g, 1_2 mmol) and applying the protocol of Example
171,
step 171.vii. It was obtained as a 6:1 mixture of diastereomers.
MS (ESI, m/z): 338.2 [M+H+].
217.iii. 6-(3R, 6S)-{6-[(1 R, 2R)-2- (3 fluoro-6-methoxy-[1, 5]naphthyridin-4
yl)-1, 2 dihydroxy-
ethylJ-tetrahydropyran-3 ylaminoJ-methyl)-4Hpyrido[3,2 bJ[1,41thiazin-3-one:
This compound (0.098 g, 32 0o yield) was obtained as a white solid, starting
frorin
intermediate 217.ii (0.2 g, 0.59 minol) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-
b][1,4]thiazine -
6-carbaldehyde (0.126 g, 1.1 eq) and applying the protocol of Example 171,
step 171.viii.
'H NMR (d6-DMSO) S: 10.83 (s, 1H); 8.75 (d, J = 1.5 Hz, 1H); 8.29 (d, J = 9.1
Hz, 1H>;
7.68 (d, J = 7.8 Hz, 1H); 7.22 (d, J = 9.1 Hz, 1H); 7.02 (d, J = 7.8 Hz, 1H);
5.70 (br t,
J = 7.1Hz, 1H); 5.45 (d, J = 7.1 Hz, 1H); 4.83 (d, J = 6 Hz, 1H); 4.00 (s,
3H); 3.88 (m, 1111)0;
3.73 (m, 1H); 3.65 (br s, 2H); 3.53 (s, 2H); 2.90 (m, 1H); 2.58 (overlapped m,
1H); 2.32 (in,
IH); 2.09-1.90 (m, 2H); 1.67-1.53 (m, 2H); 1.09 (m, 1H).
MS (ESI, m/z): 516.1 [M+H+].
Example 218: 6-({6-[2-(8-fluoro-6-methoxy-quinolin-4-yl)-vinyl]-tetrahydro-
pyran-
3-ylamino}-methyl)-4HHpyrido [3,2-b] [1,4]thiazin-3-one:
218.i. 4-bromo-8fluoro-6-methoxy-quinoline:
To a solution of 8-fluoro-6-rnethoxy-quinolin-4-ol (13.2 g, 68.3 mmol;
prepared as in
WO 2004/041210) in DMF (70 ml), heated to 40 C in a water-bath, was added PBr3
(7 nLl,
75 mmol). The mixture was stirred at this temperature for 1 h. The reaction
mixture wa.s
diluted with water (0.5 1) and saturated NaHCO3 was added until pH 8 was
reached. Tlae
solids were filtered off, taken up in EA and evaporated with silica gel (40
g). The material
was eluted (Hex-EA 3-1) to afford the title bromide (7.0 g, 40% yield) as a
yellow solid.
IH NMR (d6-DMSO) S: 8.60 (d, J = 4.6 Hz, 1H); 8.00 (d, J = 4.6 Hz, 1H); 7.48
(dd,
J= 2.6, 11.9 Hz, 1H); 7.24 (dd, J= 1.1, 2.6 Hz, 1H); 3.96 (s, 3H).
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 231 -
218.ii. trans-8 fluoro-6-methoxy-4-styryl-quinoline:
This compound (5.4 g, 99% yield) was obtained as a yellowish solid from
intermediate 218.i
(5 g, 19.52 mmol) and trans-phenylvinyl boronic acid (3.17 g, 1.1 eq), using
the procedure of
Example 178, step 178.i.
MS (ESI, rn/z): 279.9 [M+H+].
218.iii. 1- (8 fluoro-6-methoxy-quinolin-4 yl)-2 phenyl-ethane-1,2-diol:
This compound (5.4 g, 89% yield) was obtained as a beige solid, starting from
intermediate 218.ii (5.4 g, 19.4 mmol) and using the procedure of Example 178,
step 178.ii.
'H NMR (CDC13) S: 8.67 (d, J= 4.5 Hz, 1H); 7.56 (d, J= 4.5 Hz, 1H); 7.23-7.14
(m, 5H);
6.96 (d, J = 2.5, 11.5 Hz, 1H); 6.68 (d, J= 2.5 Hz, 1H); 5.34 (cl, J= 6.3 Hz,
IH); 4.97 (d,
J = 6.3 Hz, 1H); 4.83 (br s, 1H); 3.78 (s, 3H); 3.51 (br s, 1H).
218.iv. 8 fluoro-6-methoxy-quinoline-4-carbaldehyde:
This compound (2.67 g, 76% yield) was obtained as a white solid, starting from
intermediate 218.iii (5.3 g, 17 mmol) and using the procedure of Example 178,
step 178.iii.
'H NMR (d6-DMSO) 8: 10.51 (s, IH); 9.09 (d, J = 4.2 Hz, IH); 8.21 (dd, J =
1.1, 2.6 Hz,
1H); 8.10 (d, J = 4.2 Hz, 1H); 7.47 (dd, J = 2.6, 12.0 Hz, 1H); 3.96 (s, 3H).
218.v. {(3R,6S)-trans-6-[2-(8-fluoro-6-methoxy-quinolin-4-y1)-vinyl]-
tetrahydro-pyran-
3-yl}-carbamic acid tert-butyl ester:
This compound (4.08 g, 74% yield) was obtained as a yell wish solid, starting
from
intermediate 164.ii (5.82 g, 13.7 mmol) and intermediate 218.iv (2.67 g, 13.5
mmol) and
using the protocol of Example 171, step 171.v.
MS (ESI, m/z): 403.0 [M+H+].
218.vi. (3R, 6S)-trans-6-[2-(8 fluoro-6-methoxy-quinolin-4 yl)-vinylJ-
tetrahydro pyNan-
3 ylamine:
This compound (0.220 g, 69% yield) was obtained as a yellowish foam, starting
from
intermediate 218.v (0.425 g, 1.05 mmol) and using the protocol of Example 164,
step 164.iv.
MS (ESI, m/z): 303.0 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 232 -
218.vii. 6-({6-[2-(8 fluoro-6-methoxy-quinolin-4 yl)-vinylJ-tetrahydro pyran-3
ylam ino}-
methyl)-4H-pyrido[3, 2-b][l, 4]thiazin-3-one:
This compound (0.178 g) was obtained as a white solid, starting from
intermedia..te 218.vi
(0.220 g) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde
(0.155 g) and
using the procedure of Exarrmple 88, step 88.iv.
1H NMR (CDC13) 6: 10.88 (s, 1H); 8.70 (d, J= 4.5 Hz, 1H); 7.75 (d, J= 7.8 Hz,
1H>; 7.67 (d,
J= 4.5 Hz, 1 H); 7.3 6 (overlapped m, 2H); 7.31 (overlapped m, 1 H); 7.12 (d,
J= 7.9 Hz, 1 H);
6.59 (dd, J= 5.6, 15.8 Hz, 1 H); 4.08 (m, 2H); 3.95 (s, 3H); 3.77 (s, 2H);
3.54 (s, 2I-I); 3.11 (t,
J= 10.6 Hz, 1H); 2.56 (overlapped m, 1H); 2.18 (br. s, 1H); 2.09 (m, 1H); 1.90
(m, 1H);
1.40 (m, 2H).
MS (ESI, m/z): 481.3 [M+I][+].
Example 219: 6-((3R, 6S)-{6-[2-(8-fluoro-6-methoxy-quinolin-4-yl)-ethyl]-
tetral-ydro-
pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4]thiazin-3-one:
219.i. (3R, 6S)-{6-[2-(8 fluoro-6-methoxy-quinolin-4 yl)-ethylJ-tetrahydro
pyran-3 yl}-
carbamic acid tert-butyl ester:
To a solution of intermediate 218.v (0.899 g, 2.23 mmol) in EA (35 ml) under
nitrogen was
added 10% palladium on charcoal (0.54 g). The resulting mixture was stirred
under hydrogen
for 90 min.The catalyst was removed by filtration and the filtrate
concentrated to dryness. The
residue was purified by column chromatography over silica gel (EA-Hex 1-1) to
afford the
title product (0.553 g, 61% yield) as a white solid.
MS (ESI, m/z): 404.9 [M+] _
219.ii. 6-[2-(8 fluoro-6-methoxy-quinolin-4 yl)-ethyl]-tetraliydropyran-3
ylamine:
This amine (0.374 g, 91% yield) was obtained as a pale yellow solid, starting
from
intermediate 219.i (0.545 g, 1.35 mmol) and using the procedure of Example
164, step 164.iv.
MS (ESI, m/z): 305.0 [M+W].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-233-
219.iii. 6-((3R, 6S)-{6-[2-(8 fluoro-6-methoxy-quinolin-4yl)-ethylJ-tett--
ahydro pyran-
3 ylaminoJ-methyl)-4H-pyrido[3, 2-bJ[l, 4Jthiazin-3-one:
This compound (0.143 g) was obtained as a white foam, starting from
intermediate 219.ii
(0.150 g) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carba.ldehyde
(0.105 g) and
using the procedure of Example 88, step 88.iv.
'H NMR (CDCl3) b: 10.85 (s, 1H); 8.66 (d, J= 4.4 Hz, 1H); 7.73 (d, J= 7.8 Hz,
1H); 7.40 (d,
J= 4.4 Hz, 111); 7.32 (dd, J= 2.6, 12.1 Hz, 1 H); 7.23 (d, J= 2.0 Hz, 1H);
7.09 (d, J= 7.9 Hz,
1H); 3.98 (m, 1H); 3.93 (s, 3H); 3.73 (d, J= 2.1 Hz, 2H); 3.53 (s, 2H); 3.23
(m, 1H); 3.10 (m,
2H); 2.96 (t, J= 10.5 Hz, 1H); 2.51 (overlapped m, 1H); 2.09 (br. s, 114);
1.99 (m, 1H);
1.76 (m, 2H); 1.69 (m, 113); 1.22 (m, 2H).
MS (ESI, m/z): 483.2 [M+H+].
Example 220: 6-((3R,6S)-{6-[(1R,2R)-2-(8-fluoro-6-methoxy-quinolin-4-yl)-
1,2-dihydroxy-ethyl]-tetrahydro-pyran-3-ylamino}-methyl)-
4H=pyrido[3,2-b] [1,4]thiazin-3-one:
220.i. {(3R,6S)-6-[(1R,2R)-2-(8 fluoro-6-methoxy-quinolin-4 yl)-1,2-dihydroxy-
ethylJ-
tetrahydro pyran-3 yl}-carbamic acid tert-butyl ester:
This diol (2.35 g, 79% yield) was obtained as a brown oil, starting from
intermediate 218.v
(2.73 g, 6.8 mmol) and applying the protocol of Example 171, step 171-vi.
MS (ESI, m/z): 437.3 [M+H+].
220.ii. (IR,2R)-1-((2S,5R)-(5-amino-tetrahydropyran-2yl)-2-(8 fluoro-6-methoxy-
quinolin-
4 yl)-ethane-1, 2-diol:
This amine (0.241 g, 72% yield) was obtained as a pale yellow solid, starting
from
intermediate 220.i (0.432 g, 1 mmol) and using the protocol of Example 164,
step 164.iv.
MS (ESI, m/z): 337.2 [M+H+].
220.iii. 6-((3R,6S)-{6-[(IR,2R)-2-(8 fluoro-6-methoxy-quinolin-4 yl)-z,2
dihydroxy-ethylJ-
tetrahydro pyran-3 ylamino}-methyl)-4Hpyrido[3,2 bJ[1,41thiazin-3-one:
This compound (0.063 g) was obtained as a pale yellow solid, starting from
intermediate 220.ii (0.105 g) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-
b][1,4]thiazine-
6-carbaldehyde (0.067 g) and using the procedure of Example 88, step 88.iv.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 234 -
'H NMR (CDC13) 8: 10.85 (s, 1H); 8.76 (d, J= 4.4 Hz, 1H); 7.73 (d, J= 7.8 Hz,
1H)O; 7.64 (d,
J= 4.5 Hz, 1 H); 7.32 (dd, J = 2.4, 11.9 Hz, 1 H); 7.28 (d, J = 2.6 Hz, 1 H);
7.07 (d, J = 7.9 Hz,
1H); 5.46 (d, J= 5.5 Hz, 1H); 5.33 (t, J= 4.9 Hz, 1 H); 5.17 (d, J= 5.8 Ilz, 1
H);
3.92 (overlapped m, 1H); 3.8 9 (s, 3H); 3.72 (d, J= 3.9 Hz, 2H); 3.53
(overlapped m, 1H);
3.52 (s, 2H); 2.96 (m, 1H); 2.74 (t, J= 10.4 Hz, 1H); 2.52 (overlapped m, 2H);
2.03 (m, 1H);
1.59 (m, 2H); 1.05 (m, 1H).
MS (ESI, m/z): 515.0 [M+H+].
Example 221: 6-((3R,6S)-{6-[(1R,2R)-2-(8-fluoro-6-methoxy-quinolin-4-y1)-
1,2-dihydroxy-ethyl]-tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b]
[1,4]oxazin-
3-one:
This compound (0.025 g) was obtained as a pale yellow solid, starting from
intermediate 220.ii (0.119 g) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-
b][1,4]oxazine-
6-carbaldehyde (0.069 g) and using the procedure of Example 88, step 88.iv.
1H NMR (CDC13) 6: 11.16 (s, 1H); 8.76 (d, J= 4.4 Hz, 1H); 7.63 (d, J= 4.4- Hz,
1H);
7.32 (dd, J= 2.4, 9.4 Hz, 1H); 7.29 (m, 2H); 7.00 (d, J= 8.1 Hz, 1H); 5.46 (d,
T= 5.5 Hz,
1H); 5.33 (t, J= 4.8 Hz, 1H); 4.80 (d, J= 6.4 Hz, 1H); 4.62 (s, 2H); 3.92
(overlapp(--d m, 1H);
3.89 (s, 3H); 3.69 (d, J= 4.2 Hz, 2H); 3.54 (m, 11-1); 2.96 (m, 1H); 2.73 (t,
J= 10.5 Hz, 1H);
2.51 (overlapped m, 2H); 2.00 (m, 1H); 1.64 (m, 2H); 1.17 (m, 1H).
MS (ESI, m/z): 499.2 [M+H-+].
Example 222: 6-(3R,6S)-{6-[(1R,2R)-2-(3-fluoro-6-methoxy-[1,5]naphthyridin-4-
yl)-
1,2-dihydroxy-ethyl]-tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b]
[1,4]oxazin-
3-one:
This compound (0.07 g, 22% yield) was obtained as a white solid, starting from
intermediate 217.ii (0.21 g, 0.62 mmol) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-
b][1,4]oxazine-
6-carbaldehyde (0.123 g, 1.1 eq) and applying the protocol of Example 171,
step 17 1.viii.
'H NMR (d6-DMSO) fi: 11.14 (s, 1H); 8.75 (d, J= 1.5 Hz, 1H); 8.29 (d, J= 9. 1
Hz, 1H);
7.27 (d, J = 8.1 Hz, 1H); 7.23 (d, J= 9.1 Hz, 1H); 6.96 (d, J = 8.1 Hz, 1H);
5.64 (br t,
J= 6.3Hz, 1H); 5.45 (d, J= 7.1 Hz, 1H); 4.83 (d, J= 6 Hz, 1H); 4.61 (s, 2H);
4.00 (s, 3H);
3.88 (m, 1H); 3.73 (m, 1H); 3.65 (br s, 2H); 2.95 (m, 1H); 2.58 (overlapped m,
111); 2.32 (m,
1H); 2.04-1.88 (m, 2H); 1.58-1.50 (m, 2H); 1.09 (m, 1H).
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-235-
MS (ESI, m/z): 500.1 [M+H+].
Example 223: (3S,6R)-6-({6-[(1S,2S)-1,2-dihydroxy-2-(6-methoxy-
[1,5]naphthyridin-
4-yl)-ethyl]-tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4]oxazin-
3-one:
This compound (38 mg, 21% yield) was obtained as a beige foam, starting from
intermediate 199.iv (120 mg, 0.37 mmol) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]oxazine-6-carbaldehyde (67 mg, 1 eq) and using the
procedure of
Example 206, step 206.vi.
MS (ESI, m/z): 482.2 [M+H+].
Example 224: (3S,6R)-(6-({6-[(1R,2R)-1,2-dihydroxy-2-(6-methoxy-
[1,5]naphthyrid in-
4-yl)-ethyl]-tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4]thiazin-
3-on e:
224.i. (3S,6R)-{6-[(IR,2R)-1,2-dihydroxy-2-(6-methoxy-[1,5]naphthyridin-4 yl)-
ethylJ-
tetrahydro pyran-3yl}-carbamic acid tert-butyl ester:
Interinediate 199.ii (1.6 g, 4.15 mmol) was treated with AD mix (3 , as
described for
Example 171, step 171.vi, to give 943 mg (54% yield) of the title diol as a
colourless solid.
MS (ESI, m/z): 464.1 [M+H+].
224.ii. (1R,2R)-1-((2R,5S)-5-amino-tetrahydro pyran-2 yl)-2-(6-methoxy-
[1,5]naphthyridin-
4 yl)-ethane-1,2-diol:
A solution of intermediate 224.i (283 mg, 0.675 mmol) in DCM (5 ml) was
treated with TFA
(2 ml) - The mixture was stirred at rt for 3 h, concentrated in vacuo,
partitioned between IDCM
and NH4OH. The organic layer was dried over MgSO4 and concentrated to give the
title
amine (200 mg, 92% yield) as a colourless foam.
MS (ESI, m/z): 320.2 [M+H+].
224.iii. (3S,6R)-(6-({6-[(1R,2R)-1,2-dihydroxy-2-(6-methoxy-[1,5]naphthyridin-
4 yl)-etlzylJ-
tetrahydro pyran-3 ylamino}-methyl)-4II pyrido[3, 2-bJ[1, 4Jthiazin-3-one:
This compound (93 mg, 60% yield) was obtained as a beige foam, starting from
intermediate 224.ii (100 mg, 0.31 mmol) and 3-oxo-3,4-dih_ydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (61 mg, 1 eq) and using the
procedure of
Example 206, step 206.vi.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-236-
MS (ESI, m/z): 498.1 [M+H+].
Example 225: (3S,6R)-6-({6-[(1R,2R)-1,2-dihydroxy-2-(6-methoxy-[1,5]
naphthyridin-
4-yl)-ethyl]-tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4]oxazin-
3-one:
This compound (67 mg, 44% yield) was obtained as a beige foam, starting from
intermediate 224.ii (100 mg, 0.31 mmol) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]oxazine-6-carbaldehyde (56 mg, 1 eq) and using the
procedure of
Example 206, step 206.vi.
MS (ESI, m/z): 482.2 [M+H+].
Example 226: (3S,6R)-6-({(6-[(IIZ)-l-hydroxy-2-(6-methoxy-[1,5]naphthyridin-4-
yl)-
ethyl]-tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b][1,4]thiazin-3-one:
226.i. {(3S,6R)-6-[(4S,5S)-5-(6-methoxy-[1,5]naphthyridin-4 yl)-2-oxo-
[1,3Jdioxotan-4 ylJ-
tetrahydro pyran-3 yl}-carbanaic eacid tert-butyl ester:
This compound (970 mg, 91% yield) was obtained as an orange foam, starting
from
intermediate 199.iii (1 g, 2.38 mmol) and using the procedure of Example 191,
step 191.i.
MS (ESI, m/z): 446.3 [M+H+].
226.ii. (3S,6R)-{6-[(1R)-1-hydroxy-2-(6-methoxy-[1,5]naphthyridin-4 yl)-ethyl]-
tezrahydro-
pyran-3 yl}-carbamic acid tert-butyl ester:
This compound (400 mg, 45% yield) was obtained as a yellowish foam, starting
from
intermediate 226.i (970 mg, 2.18 mmol) and using the procedure of Example 191,
step 191.ii.
MS (ESI, m/z): 403.9 [M+H+].
226.iii. (1R)-1-((2R,5S)-5-amino-tetrahydro-pyran-2-yl)-2-(6-methoxy-
[1,5]naphthyridin-
4-yl)-ethanol:
A solution of intermediate 226.ii (400 mg, 0.992 mmol) in DCM (6 ml) was
treated with TFA
(2 ml). The mixture was stirred at rt for 3 h, concentrated in vacuo,
partitioned bet-ween DCM
and NH4OH. The organic layer tivas dried over MgSO4 and concentrated to give
the title
amine (270 mg, 90%) as a colourless foam.
MS (ESI, m/z): 403.4 [M+H+]
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 237 -
226.iv. (3S,6R)-6-({(6-[(1R)-1-hydroxy-2-(6-methoxy-[1,5]naphthyridin-4 yl)-
ethylJ-
tetrahydropyran-3 ylamino}-methyl)-4H pyrido[3, 2-bJ[1, 4]thiazin-3-one:
A mixture of intermediate 226.iii (145 mg, 0.48 mmol) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (93 mg, 1 eq) in DCE/MeOH 1:1 (4
ml) was
stirred overnight at rt. An excess of NaCNBH3 was added and stirring continued
for 3 h- The
mixture was diluted with DCM and washed with aq. ammonia. The organic phases -
were
combined, dried over MgSO4 and concentrated. The residue was purified by
chromatography
over silica gel (EA/MeOH 9:1, 4:1 +1% NH4OH) and crystallised from ether to
give 82 mg
(36% yield) of title compound as a colourless solid.
1H NMR (d6-DMSO) 8: 8.66 (d, 1H, J=4.44 Hz), 8.23 (d, 1H, J=9.03 Hz), 7.73 (d,
1H,
J=7. 83 Hz), 7.53 (d, 1 H, J= 4.44 Hz), 7.24 (d, 1FI, J=9.03 Hz), 7.09 (d, 1H,
J=7. 83 Hz),
4.55 (d, IH, J=6.39 Hz), 4.00 (s, 3H), 4.05-3.95 (tn, 1H), 3.90-3.80 (m, 1H),
3.73 (br, 2H),
3.53 (s, 2H), 3.50-3.35 (m, 1H), 3.15-2.90 (m, 31-1), 2.10-1.80 (m, 2H), 1.80-
1.40 (m, 2H),
1.30-1.10 (m, 3H).
MS (ESI, m/z): 482.1 [M+H+].
Example 227: (3S,6R)-6-({6-[(1R)-1-hydroxy-2-(6-methoxy-[1,5]naphthyridin-4-
yl)-
ethyl]-tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4]oxazin-3-one:
This compound (78 mg, 35% yield) was obtained as a colourless solid, starting
from
intermediate 226.iii (145 mg, 0.48 rnmol) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]oxazine-6-carbaldehyde (85 nng, 1 eq) and using the
procedure of
Example 226, step 226.iv.
MS (ESI, m/z): 466.2 [M+H+].
Example 228: (3S,6R)-6-({(6-[(1S)-1-hydroxy-2-C6-methoxy-[1,5]naphthyridin-4-
y1)0-
ethyl]-tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4]thiazin-3-
one:
228.i. {(3S, 6R)-6-[(4R, 5R)-5-(6-methoxy-[1, 5]naphthyridin-4 yl)-2-oxo-[1,
3]dioxolan-,4rylJ-
tetrahydro pyran-3 yl}-carbamic acid tert-butyl este r:
This compound (540 mg, 771% yield) was obtained as an orange oil, starting
from
intermediate 224.i (1 g, 2.38 mmol) and using the procedure of Example 191,
step 191.i.
MS (ESI, m/z): 446.3 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-238-
228.ii. (3S,6R)-{6-[(IS)-1-hydroxy-2-(6-methoxy-[1,5]naphthyridin-4 yl)-ethylJ-
tetraliydro-
pyran-3 yl}-carbamic acid tert-butyl ester:
This compound (190 mg, 39% yield) was obtained as a yellowish foam, starting
from
intermediate 228.i (540 mg, 1.21 mmol) and using the procedure of Example 191,
step 191.ii.
MS (ESI, m/z): 403.9 [M+H+].
228.iii. (1S)-1-((2R,5S)-5-amino-tetrahydro pyran-2 yl)-2-(6-methoxy-
[1,5]naphthyridzn-
4yl)-ethanol:
A solution of intermediate 228.ii (190 mg, 0.47 mmol) ir1 DCM (6 ml) was
treated with TFA
(2 ml). The rriixture was stirred at rt for 3 h, concentrated in vacuo,
partitioned between DCM
and NH4OH. The organic layer was dried over MgSO4 and concentrated to give the
title
amine (130 mg, 90%) as a colourless foam.
MS (ESI, m/z): 403.4 [M+H+].
228.iv. (3S,6R)-6-({(6-[(1S)-1-hydroxy-2-(6-methoxy-[1,S]naphthyridin-4yl)-
ethylJ-
tetrahydro pyran-3-ylamino}-methyl)-4.H-pyrido[3,2-bJ[I , 4Jthiazin-3-one:
A mixture of intermediate 228.iii (120 mg, 0.4 mmol) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (77 mg, 1 eq) in DCE/MeOH 1:1 (4
m 1) was
stirred overnight at rt. An excess of NaCNBH3 was added and stirring continued
for 3 h. The
mixture was diluted with DCM and washed with aq. ammonia. The organic phases
were
combined, dried over MgSO4 and concentrated. The residue was purified by
chromatography
over silica gel (EA/MeOH 9:1, 4:1 + 1% NH4OH) and crystallised from ether to
give 30 mg
(16% yield) of title compound as a yellowish solid.
MS (ESI, m/z): 482.1 [M+H+].
Example 229: 6-({(3R,6,S)-6-[(1S,2S)-1,2-dihydroxy-2-(3-methoxy-quinoxalin-5-
yl)1-
ethyl]-tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrid o[3,2-b] [1,4]thiazin-3-
one:
229.i. (3R,6S)-{6-[(1S,2S)-1,2-dihydroxy-2-(3-methoxy-qziinoxalin-5 yl)-ethylJ-
tetrahydro-
pyran-3 yl}-carbamic acid tert-butyl ester:
This compound (0.304g, 69%) was obtained as a colourless foam starting from
intertnediate
177.i (0.401 g, 1.04 mmol) and using the protocol of EKample 171, step 171.vi,
exce;pt that
AD mix a was employed as reagent.
MS (ESI, m/z): 420.2 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-239-
229.ii. (IS,2S)-1-[(2S,5R)-(5-ezmino-tetrahydropyran-2yl)-2-(3-methoxy-
quinoxalin-5 yl)-
ethane-1, 2-diol:
This compound was obtained as a colourless solid (0.171 g, 75% yield) frorrn
intermediate 229.i (0.3 g, 0.71 mmol), using the protocol of Example 77, step
77.iii.
'H NMR (d6-DMSO) S: 8.59 (s, 1H); 7.87 (app d, J = 7.8 Hz, 2H); 7.62 (t, J =
7.8 Hz, 1H) ;
5.80 (br s, 1H); 5.09 (br d, J = 5.5 Hz, 1H); 4.42 (br d, J = 7.5 Hz, 1H);
4.03 (s, 3H);
3.74 (ddd, J = 2.8, 4.5, 8.9 Hz, 1 H); 3.54 (m, 1 H); 3.24 (m, 1 H); 2.81 (t,
J = 10.4 Hz, 1 H) ;
2.53 (overlapped m, 1H); 1.92 (m, 2H); 1.43 (br s, 2H); 1.26 (m, 1H); 1.07 (m,
1H).
MS (ESI, m/z): 320.0 [M+H+].
229.iii. 6-({(3R, 6S)-6-[(1S, 2S)-1, 2-dihydroxy-2-(3-methoxy-quinoxalin-5yl)-
ethylJ-
tetrahydropyran-3 ylarnino}-methyl)-4H pyrido[3, 2-bJ[1, 4]thiazin-3-one:
This compound was prepared from intermediate 229.ii (0.167 g, 0.52 mmol) and 3-
oxo-
3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.111 g, 0.57 mmol),
using ttie
procedure described in Example 171, step 171.v. The title compound (0.178 g,
68% yielci)
was obtained as a beige foarn.
'H NMR (d6-DMSO) 6: 10. 86 (s, 1H); 8.59 (s, 1H); 7.87 (app d, J = 7.8 Hz,
2H); 7.73 (d,
J = 7.8 Hz, 1H); 7.62 (t, J = 7.8 Hz, 1H); 7.08 (d, J = 7.8 Hz, 1H); 5.79 (dd,
J = 2.7, 6.3 I-Iz,
1H); 5.10 (d, J = 6.3 Hz, 1I-1); 4.43 (d, J = 7.8 Hz, 1H); 4.04 (s, 3H); 3.89
(dd, J = 2.2,
10.4 Hz, 1H); 3.75 (dd, AB system, J= 11.9 Hz, A= 0.055, 2H); 3.54 (overlapped
m, 1I-f);
3.53 (s, 2H); 3.28 (m, 1H); 2.90 (t, J = 10.5 Hz, IH); 2.46 (m, 1H); 2.05-1.91
(m, 31L);
1.31-1.11 (m, 2H).
MS (ESI, m/z): 498.2 [M+H+]
Example 230: 6-({(3S,6R)-6-[(1R,2R)-1,2-dihydroxy-2-(3-methoxy-quinoxalin-5-
yl)-
ethyl]-tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4]thiazin-3-
one:
230.i. trans-{(3S,6R)-{6-[2-(3-methoxy-quinoxalin-5 yl)-vinylJ-tetrahydro
pyran-3 yl}-
carbamic acid tert-butyl ester:
The title (E)-alkene (1.90 g, 99% yield) was obtained as a white solid,
starting fram
3-methoxy-quinoxaline-5-carbaldehyde (0.92 g, 4.88 mmol) and intermediate
171.iv (2.27 g,
3.4 mmol) and using the procedure described in Example 171, step 171.v. The
purity of tlle
compound was around 80%-
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 240 -
MS (ESI, m/z): 498.2 [M+H+].
230.ii. (3S,6R)-{6-[(IR, 2R)-1,2-dihydroxy-2-(3-methoxy-quinoxalin-5 yl)-
ethylJ-tetralhydro-
pyran-3 yl}-carbamic acid tert-butyl ester:
The title diol (0.29 g, 17% yield) was obtained as a colourless solid,
starting from
intermediate 230.i (1.5 g, 3.89 mmol) and using the protocol described in
Example 171,
step 171.vi.
'H NMR (CDC13) 6: 8.52 (s, 1H); 7.99 (dd, J = 1.3, 8.2 Hz, 1H); 7.85 (d, J =
7.1 Hz, 1H);
7.62 (dd, J = 7.1, 8.2 Hz, 1H); 5.84 (br s, 1H); 4.75 (br s, 1H); 4.28 (m,
1H); 4.20-4.07 (m,
1H); 4.07 (s, 3H); 3.93 (br s, 1H); 3.65 (br s, 1H); 3.43-3.34 (m, 2H); 3.00
(t, J= 10.6 Hz,
1H); 2.16 (m, 1H); 1.96 (m, 1H); 1.75 (m, 1H); 1.46 (s, 9H); 1.28 (rn, IH).
MS (ESI, m/z): 420.1 [M+H+].
230.iii. (1R,2R)-1-[(2R,5S)-(5-amino-tetrahydropyran-2 yl)-2-(3-methoxy-
quinoxalin-5 yl)-
etliane-1,2-diol:
This compound was obtained as a colourless solid (0.14 g, 75% yield) from
intermediate 230.ii (0.29 g, 0.68 mmol), using the protocol of Exairi-ple 77,
step 77.iii.
MS (ESI, m/z): 320.1 [M+H+].
230.iv. 6-({(3S,6R)-6-[(IR,2R)-1,2-dihydroxy-2-(3-methoxy-quinozalin-5 yl)-
ethylJ-
tetrahydro pyran-3ylamino}-methyl)-4H-pyrido[3,2-bJ[1,4]thiazin-3-one:
This compound (0.62 g, 28% yield) was obtained as a beige foarri from
intermediate 230.iii
(0.140 g, 0.44 mmol) and 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-
carbaldehyde
(0.093 g, 0.48 mmol), using the procedure of Example 173, step 173.v.
MS (ESI, m/z): 498.2 [M+H+].
Example 231: 6-({(3R,6S)-6-[(LS')-2-(8-fluoro-6-methoxy-quinolin-4-yl)-1-
hydroxy-
ethyl] -tetrahydro-pyr an-3-ylamino}-methyl)-4H-pyrido [3,2-b] [ 1,4] thiazin-
3-one:
231.i. {(3R, 6S)-6-[(4R, 5R)-5-(8 fluoro-6-methoxy-quinolin-4 yl)-2-oxo-[1,
3]dioxolan-4 yZJ-
tetrahydropyran-3 yl}-carbamic acid tert-butyl ester:
This compound (0.762 g, 37% yield) was obtained as a colourless foam, starting
from
intermediate 220.i (1.92 g, 4.41 mmol) and using the procedure described in
Example 190,
step 190. i.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 241 -
1H NMR (d6-DMSO) S: 8.84 (d, J = 4.5 Hz, 1H); 7.64 (d, J = 4.5 Hz, 1H); 7.44
(dd, J = 2.4,
12.0 Hz, 1 H); 7.13 (d, J= 1- 5 Hz, 1 H); 6.82 (br d, J= 8.1 Hz, 1H); 6.57 (d,
J= 4-.8 Hz, 1 H);
4.86 (dd, J = 2.5, 4.8 Hz, 1H); 3.97 (overlapped m, 1H); 3.96 (s, 3H); 3.67
(d, T= 11.2 Hz,
1 H); 3.57 (br s, 1H); 3.19 (t, J= 10.7 Hz, 1H); 1.92 (m, 1H); 1.69 (m, 1 H);
1.5 7(m, 1 H);
1.40 (overlapped m, 1H); 1.3 8 (s, 9H).
MS (ESI, m/z): 463.3 [M+H+].
231.ii. {(3R,6S)-6-[(1S)-2-(8 fluoro-6-methoxy-quinolin-4 yl)-1-hydroxy-ethylJ-
tetrahydro-
pyran-3 yl}-carbainic acid tert-butyl ester:
This compound (0.347 g, 50% yield) was obtained as a colourless foam, starting
from
intermediate 231.i (0.751 g, 1.62 mmol) and using the procedure of Example
190, step 190.ii.
MS (ESI, m/z): 421.2 [M+H+].
231.iii. (1S)-1-((2S, 5R)-5-ezmino-tetrahydropyran-2 yl)-2-(8 fluoro-6-methoxy-
q2cinolin-
4 yl)-ethanol:
This compound (0.21 g, 95% yield) was obtained as a colorless solid, starting
from
intermediate 231.ii (0.344 g, 0.819 mmol) and using the procedure of Example
173,
step 173.iv.
MS (ESI, m/z): 321.2 [M+H}].
231.iv. 6-({(3R,6S)-6-[(1S)-2-(8fluoro-6-methoxy-quinolin-4 yl)-1-hydroxy-
ethyt]-
tetrahydro pyran-3 ylamino}-methyl)-4Flpyrido[3,2-bJ[1,4]thiazin-3-one:
This compound (0.109 g, 64 % yield) was obtained as a colourless foam,
starting from
intermediate 231.iii (0.109 g, 0.34 mmol) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid (0.072 g, 0.37 mmol) and using
the
procedure of Example 172.
MS (ESI, m/z): 496.2 [M+H+].
Example 232: 6-{[6-(6-fluoro-quinolin-4-yloxymethyl)-tetrahydro-pyran-3-
ylamino]-
methyl}-4H-pyrido[3,2-b] [1,4]thiazin-3-one:
232.i. 6 fluoro-quinolin-4-al:
This compound (1.90 g, 83% yield) was obtained as a yellow solid, s-tarting
from
4-fluoroaniline (1.55 g, 14 rnmol) and using the procedure of Example 138,
step 138.i.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 242 -
MS (ESI, m/z): 164.1 [M+H+]
232.ii. (3R, 6S)-6-(6 fluoro-quinolin-4 yloxymethyl)-tetrahydro pyf=an-3
ylamine:
This compound (1.08 g, 3.9 mmol) was obtained as a colourless solid, starting
from
intermediate 78.i (2.55 g, 11 mmol) and intermediate 232.i (1.89 g, 11.6 mmol)
and using the
procedure described in Example 135, stepl35.i.
MS (ESI, m/z): 277.1 [M+H+].
232.iii. 6-{[6-(6 fluoro-quinolin-4 yloxymethyl)-tetrahydro pyran-3 ylezmino]-
methyl}-4H-
pyrido[3, 2-b][l, 4]thiazi72-3-one:
Starting from intermediate 232.ii. (0.135 g, 0.49 mmol) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.105 g, 1.1 eq.), the title
compound (0.133 g,
59% yield) was obtained as a pale yellow foam using the procedure of Example
88, step 88.iv.
'H NMR (CDC13) 8: 10. 87 (s, 1H); 8.71 (d, J= 5.2 Hz, 1H); 8.03 (dd, J= 5.4,
9.3 Hz, 1H);
7.75 (m, 2H); 7.66 (rn, 1 H); 7.09 (m, 2H); 4.22 (d, J= 4.9 Hz, 2H); 4.02 (m,
1 H);
3.76 (overlapped d, J = 2.4 Hz, 2H); 3.74 (overlapped m, 1H); 3.53 (s, 2H);
3.07 (t,
J= 10.5 Hz, 1H); 2.56 (overlapped m, 1H); 2.12 (m, 2H); 1.84 (ni, 1H); 1.48
(m, 1H);
1.32 (m, 1H).
MS (ESI, m/z) : 455.5 [1VI+H+].
Example 233: 6-{[(3R,6S)-6-(6,8-difluoro-quinolin-4-yloxymethyl)-tetrahydro-
pyran-
3-ylamino]-methyl}-4H-pyrido [3,2-b] [1,4]thiazin-3-one:
233.i. 6, 8-difluoro-quinolin-4-ol:
This compound (4.2 g, 29% yield) was prepared as a tan solid, starting from
2,4-difluoroaniline (10 g, 14 mmol) and using the procedure of Example 138,
step 138.i.
'H NMR (d6-DMSO) S: 7.87 (d, J= 7.4 Hz, 1H); 7.74 (ddd, J= Z.8, 8.6, 11.3 Hz,
1H);
7.58 (ddd, J= 1.5, 2.8, 9.1 Hz, 1H); 6.19 (d, J= 7.4 Hz, 1H).
MS (ESI, m/z): 164.1 [M+H+].
233.ii. (3R, 6S)-6-(6,8-dij7uoro-quinolin-4yloxymethyl)-tetrahydro pyrcrn-
3ylamine:
This compound (1.8 g, 61% yield) was prepared as a colourless foam, starting
from
intermediate 78.i (2.31 g, 10 mmol) and intermediate 233.i (1.90 g, 10. 5
mmol) and using the
procedure of Example 13 5, step 135.i. The purity of the product was 70-80%.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 243 -
MS (ESI, m/z): 295.3 [M+H}].
233.iii. 6-{[(3R,6S)-6-(6,8-difluoro-quinolin-4 yloxymethyl)-tetrahydro pyran-
3 ylcrminoJ-
methyl}-4H-pyrido[3, 2-bJ[l, 4Jthiazin-3-one:
Starting from intermediate 233.ii. (0.3 g, 1.02 mmol) and 3-oxo-3 ,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.188 g, 0.97 mmol), the title
compound
(0.230 g, 47% yield) was obtained as a colourless solid using the procedure of
Exainple 88,
step 88.iv.
MS (ESI, m/z): 473.3 [M+H+].
Example 234: 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]oxazine-6-carboxylic acid
(3R,6S)-{6-[(IS)-1-hydroxy-2-(3-methoxy-quinolin-5-yl)-ethyl]-tetrahydro-pyran-
3-yl}-
amide:
Starting from intermediate 203.iii. (0.155 g, 0.51 mmol) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]oxazine-6-carboxylic acid (0.118 g, 1.1 eq), the title
compound
(0.070 g, 27% yield) was obtained as a colorless solid using the procedure of
R.-cample 172.
Purification on silica gel was carried out using a DCM-MeOH 9-1 mixture
containing 1% aq.
NH4OH.
MS (ESI, m/z): 495.2 [M+H}].
Example 235: (LS')-1-{(2S,5R)-5-trans-[3-(2,5-difluoro-phenyl)-allylamino]-
tetrahydro-
pyran-2-yl}-2-(6-methoxy-quinolin-4-yl)-ethanol:
235.i. trans-3-(2,5-difluoro phenyl)-acrylic acid ethyl ester:
To an iced chilled suspension of NaH (1.13 g, 60% oil dispersion, 28.2 mmol)
in THF (32 ml)
was added triethylphosphonoacetate (5.6 ml, 28.2 mmol). The reaction mixture
v~ras stirred at
rt for 20 min. 2,5-difluoro-benzaldehyde (3.34 g, 23.5 mmol) was added
dropwise. After
min, 10% aq. NaHSO4 (100 ml) was added and the mixture was diluted with EA
(150 ml).
25 The two phases were separated and the aq layer was extracted twice (2 x 100
nil) with EA.
The combined organic layers were washed with brine (100 ml), dried over
Na2SO4, filtered
and concentrated to dryness. The residue was chromatographed over silica gel
(Hex-EA 19-1)
to afford the title unsaturated ester (5.0 g, 100%) as a colourless oil.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 244 -
'H NMR (CDC13): 7.76 (dd, J= 1, 16.1 Hz, IH); 7.26-7.21 (m, 1H); 7.13-7.03 (m,
2H);
6.52 (d, J= 16.1 Hz, 1H); 4.29 (q, J= 7.1 Hz, 2H); 1.36 (t, J= 7.1 Hz, 3H).
235.ii. trans-3-(2,5-d fluoro phenyl) prop-2-en-l-ol:
To a solution of intermediate 235.i (5.0 g, 23.5 mmol) in ether (100 ml),
cooled to 0 C, was
added a DIBAH (1M in hexanes, 60 ml, 60 mmol). The mixture was stirred at the
sarne
temperature for 40 min. Water (6 ml) was added and the mixture was stirred 30
min. The so lid
was filtered off and thoroughly washed with ether. The filtrate was
concentrated to dryness to
afford the title alcohol (4.0 g, 98% yield) as a colourless oil.
'H NMR (CDC13): 7.15 (ddd, J = 3.1, 5.9, 9.0 Hz, 1H); 7.00 (td, J = 4.6, 9.0
Hz, 1H);
6.95-6.87 (m, 1H); 6.75 (dd, J = 1.3, 16.1 Hz, 1 H); 6.45 (td, J = 5.3, 16.1
Hz, 1H); 4.38 (br d,
J = 5.3 Hz, 2H); 1.63 (s, 1H).
235.iii. trans-3-(2,5-difluorophenyl) propenal:
To a solution of intermediate 235.ii (1.70 g, 14 mmol) in DCM (20 ml) was
added, at rt, a
solution of Dess-Martin periodinane (15 wt% in DCM, 20 ml). The mixture was
stirred a-t rt
for 3 h. After concentration to dryness, the residue was chromatographed over
silica gel
(Hex-EA 9-1) to afford the title aldehyde (1.06 g, 63% yield) as a white
solid.
'H NMR (d6-DMSO): 9.74 (d, J = 7.6 Hz, 11H); 7.88-7.81 (m, 1H); 7.79
(overlapped dd,
J = 1.4, 16.0 Hz, 1H); 7.46-7.37 (m, 2H); 6.67 (dd, J = 7.6, 16.0 Hz, 1H).
235.iv. (1S)-1-{(2S,5R)-5-trans-[3-(2,5-difluorophenyl)-allylamino]-tetrahydro
pyran-2-y1}-
2-(6-methoxy-quinolin-4-yl)-ethanol:
Starting frorn intermediate 190.iii (0.105 g, 0.34 mmol) and intermediate
235.iii (0.064 g,
1.1 eq.), the title compound (0.126 g, 80% yield) was obtained as a colourless
foam accorcting
to the procedure described in Example 88, step 88.iv.
'H NMR (d6-DMSO): 8.62 (d, J= 4.4 Hz, lI-I); 7.92 (d, J= 9.1 Hz, 1H); 7.48
(ddd, J= 3.2,
6.1, 9.5 Hz, 1H); 7.43 (m, 1H); 7.39 (dd, J= 2.8, 9.1 Hz, 1H); 7.32 (d, J= 4.4
Hz, L H);
7.27 (m, 114); 7.11 (m, 1H); 6.62 (d, J = 16.2 Iiz, 1H); 6.49 (td, J = 5.3,
16.2 Hz, 1H); 4.78; (d,
J= 6.3 Hz, 1H); 4.08 (m, 1H); 3.90 (s, 3H); 3.74 (m, 1H); 3.40 (m, 2H); 3.30
(m, L H);
3.15 (m, lIi); 2.94 (m, 2H); 2.57 (m, 1H); 2.07 (m, 1H); 1.84 (br s, 1H); 1.58
(m, 2H);
1.18 (m, 1H).
MS (ESI, m/z): 455.5 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-245-
Example 236: 6-({(3R,6S)-6-[(2RS')-2-hydroxy-2-(6-methoxy-[1,5] naphthyridin-4-
yl)-
ethyl] -tetrahydro-pyran-3-ylamin o}-methyl)-4H-pyrido [3,2-b] [ 1,4] thiazin-
3-on e:
236.i. ((3R,6S)-6-[(2RS)-2-hydroxy-2-(6-methoxy-[1,5]naphthyridin-4 yl)-ethylJ-
tetrahydro-
pyran-3 yl}-carbamic acid tert-butyl ester:
Starting from 8-bromo-2-methoxy-[1,5]naphthyridine (5.88 g, 24.6 mmo 1) and
intermediate 203.iii (2.0 g, 8.22 mrnol), the title benzylic alcohol (1.6 g,
3.96 rnrrnol) was
obtained as a yellowish foam using the procedure described in Example 203,
step 203.iv. The
compound was recovered as a 1:1 mixture of epimers with a purity of 90%.
MS (ESI, m/z): 404.1 [M+H+].
236.ii. (2RS)-2-((2S,5R)-5-amino-tetrahydro pyran-2 yl)-1-(6-methoxy-
[1,5]naphthy7-idin-
4yl)-ethanol:
This amine (0.45 g, 74% yield) was prepared from intermediate 236.i (1.6 g,
3.96 mmol),
using the procedure described in Example 171, step 171.vii. A 1:1 mixture of
epirners was
obtained as a colourless foam.
MS (ESI, m/z): 304.2 [M+H+].
236.iii. 6-({(3R,6S)-6-[(2RS)-2-hydroxy-2-(6-methoxy-[1,5]naphthyridin-4 yl)-
ethyl.J-
tetrahydro pyran-3 ylamino}-methyl)-4Hpyrido[3,2-b][1,4]thiazin-3-one:
Starting from intermediate 236.ii (0.11 g, 0.36 mmol) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.077g, 1.1 eq), the title
compound (0.125 g,
71% yield) was obtained as a yellowish foam using the procedure of Example 88,
step 88.iv.
1H NMR (d6-DMSO) mixture of epimers : 10.86 (s, 1H); 8.77 (d, J = 4.6 Hz, 0.51-
1); 8.75 (d,
J = 4.6 Hz, 0.5H); 8.25 (d, J = 9.1 IFIz, 0.514); 8.24 (d, J = 9.1 Hz, 0.5H);
7.75-7.71 (m, 211);
7.25 (d, J= 9.1 Hz, 0.51-1); 7.23 (d, J= 9.1 Hz, 0.5H); 7.08 (d, J= 7.8 Hz,
0.5H); 7.06 (d,
J = 7.8 Hz, 0.5H); 5.74 (m, 0.5H); 5.63 (m, 0.5H); 5.39 (d, J = 4.9 Hz, 0.5H);
5.35 (d,
J = 5.2 Hz, 0.5H); 4.01 (s, 1.5H), 4.00 (s, 1.5H); 4.00-3.79 (m, 1H); 3.71 (br
s, 211); 3.52 (s,
2H), 3.52 (overlapped m, 0.5H); 3.43 (m, 0.51-1); 2.96 (t, J = 10.3 Hz, 0.5H);
2.90 (t,
J = 10.5 Hz, 0.5H); 2.46 (m, 1H); 2-08-1.74 (m, 4H); 1.64-1.55 (m, 1H); 1.31-
1.14 (tn, 2H).
MS (ESI, m/z): 482.1 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 246 -
Example 237: 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid
{(3R,6S)-6-[ (2RS)-2-hydroxy-2-(6-methoxy-[1,5]naphthyridin-4-yl)-ethyl]-
tetrahydro-
pyran-3-yl} -amide:
Starting from intermediate 236.ii (0.45 g, 1.48 mmol) and 3-o:P-,o-3,4-dihydro-
2.H-pyrido[3,2-b][1,4]oxazine-6-carboxylic acid (0.343 g, 1.1 eq), the title
compound (0.4 g,
54% yield) was obtained as a colourless solid according to the procedure
described in
Example 172. Purification on silica gel was carried out using a DCM-MeOH 93-7
mixture
containing 1 % aq. NH4OH.
MS (ESI, ni/z): 496.0 [M+H+].
Example 238: 2-{(2S,5R)-5-[trans-3-(2,5-difluoro-phenyl)-allylamino]-
tetrahydro-
pyran-2-yl}-1-(6-methoxy-[1,5] naphthyridin-4-yl)-ethanol:
Starting from intermediate 236.ii (0.15 g, 0.49 mmol) and intermediate 235.iii
(0.091 g,
1.1 eq), the title compound (0.160 g, 71% yield) was obtained as a colourless
semi-solid using
the procedure of Example 88, step 88.iv. Purification was carried out by
chror.natography over
silica gel (DCM-MeOH 19-1 containing 0.5% N1440H). The compound was btained
as a 1:1
mixture of epimers.
MS (ESI, rn/z): 456.2 [M+H+].
Example 239: trans-3-(2,5-difluoro-phenyl)-N-{(3R,6S)-6-[(2RS)-2-hydroxy-
2-(6-methoxy-[1,5] naphthyridin-4-yl)-ethyl]-tetrahydro-pyran-3-yl}-
acrylamide:
Starting from intermediate 236.ii (0.1 g, 0.33 mmol) and trans-3-(2,5-difluoro-
phenyl)-acrylic
acid (0.067 g, 1.1 eq), the title compound (0.116 g, 75% yield) was obtainecl
as a beige solid
using the procedure of Example 172. Purification was carried out by
chrornatography over
silica gel (DCM-MeOH 19-1 containing 1% aq. NH4OH). The compound was obtained
as a
1:1 mixture of epimers.
1H NMR (d6-DMSO) mixture of epimers 8.78 (d, J = 4.7 Hz, 0.5H); 8.76 (d, J =
4.7 Hz,
0.5H); 8.26 (d, J = 9.1 Hz, 0.5H); 8.25 (d, J 9.1 Hz, 0.5H); 8.14-8.10 (m,
13PI); 7.77-7.75 (m,
1H); 7.53-7.23 (m, 5H); 6.73 (d, J= 16.0 Hz, 0.5H); 6.72 (d, J= 16.0 Hz,
0.5H); 5.78 (m,
0.5H); 5.66 (m, 0.5H); 5.43 (d, J = 5.0 Hz, 0.5H); 5.40 (d, J = 5.2 Hz,
0.51L); 4.02 (s, 1.5H);
4.01 (s, 1.5H); 3.91-3.76 (m, 2H); 3.61 (m, 0.5H); 3.48 (m, 0.5H); 3.06 (t, J=
10.5 Hz, 0.5H);
2.98 (t, J = 10 Hz, 0.5H); 2.06-1.35 (m, 6H).
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 247 -
MS (ESI, m/z): 470.1 [M+H+].
Example 240: trans-3-(2,5-difluoro-phenyl)-N-{(3R,6S)-6-[(1S)-1-hydroxy-
2-(6-methoxy-quinolin-4-y1)-ethyl]-tetrahydro-pyran-3-yl}-acrylamide:
Starting from intermediate 190.iii (0.055 g, 0. 182 mmol) and trans-3-(2,5-
difluoro-phenyl)-
acrylic acid (0.064 g, 1.1 eq.), the title compound (0.070 g, 82% yield) was
obtained as a
colourless solid according to the procedure described in Example 172.
1H NMR (d6-DMSO) mixture of epimers : 8.63 (d, J = 4.4 Hz, 1H); 6.16 (d, J =
7.8 Hz, 1H);
7.92 (d, J = 9.1 Hz, 1H); 7.54-7.26 (m, 7H); 6.72 (d, J = 16.0 Hz, 1H); 4.86
(d, J = 6.4 Hz,
1H); 4.00 (m, 1H); 3.91 (s, 3H); 3.90-3.75 (m, 2H); 3.33 (m, 1H); 3.20 (m,
1H);
3.05 (t, J= 10.5 Hz, 1H); 2.97 (dd, J= 5.1, 13.7 Hz, 1H); 2.00 (m 1H); 1.72-
1.62 (m, 2H);
1.44 (m, 1H).
MS (ESI, m/z): 469.3 [M+H}].
Example 241: (1R,2S)-1-{(2S,5R)-5-[trans-3-(2,5-difluoro-phenyl)-allylamino]-
tetrahydro-pyran-2-yl}-2-(6-methoxy-[1,5] naphthyridin-4-yl)-ethane-1,2-diol:
Starting from intermediate 173.iv (0.054 g, 0.17 mmol) and intermediate
235.iii (0.031 g,
1.1 eq), the title compound (0.044 g, 56% yield) was obtained as a colorless
foam using the
procedure of Example 88, step 88.iv. Purification was carried out by
chromatography over
silica gel (DCM-MeOH 19-1 containing 1% aq. NH4OH).
Rf (TLC over Si02): 0.18 (DCM-MeOH 19-1 containing 1% aq. NH4OH).
MS (ESI, m/z): 472.2 [M+H+].
Example 242: 3-oxo-3,4-dihydro-2H-pyrido[3,2-b] [1,4]thiazine-6-carboxylic
acid
{(3R,6S)-6-[(1S,2R)-2-(3-fluoro-6-methoxy-[1,5] naphthyridin-4-yl)-1,2-
dihydroxy-ethyl]-
tetrahydro-pyran-3-yl}-amide:
Starting from intermediate 217.ii (0.3 g, 0.89 mmol) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]oxazine-6-carboxylic acid (0.205 g, 1.1 eq), the title
compound (0.3 g,
63% yield) was prepared as a light beige solid according to the procedure
described in
Example 172. Purification was carried out by trituration of the crude material
in ether.
MS (ESI, m/z): 530.3 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 248 -
Example 243: 3-oxo-3,4-dihydro-2H-pyrido[3,2-b] [1,4]oxazine-6-carboxylic acid
{(3R,6S)-6-[(IS,2R)-2-(3-fluoro-6-methoxy-[1,5]naphth-yridin-4-yl)-1,2-
dihydroxy-
ethylJ-tetrahyd ro-pyran-3-y1} amide:
243.i. 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]oxazine-6-carboxylic acid:
To a mixture of 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]oxazine-6-carbaldehyde
(1 g,
5.61 mrnol) in acetone (30 ml) and water (5 ml) was added potassium
permanganate (1.77 g,
2 eq.). The reaction proceeded 1 h at rt and THF (50 ml) and water (50 ml)
were added. The
reaction mixture was filtered and the filtrate concentrated in vacu . The pH
of the aq. residue
was brought to 1 adding 1N HC1. The solid that formed was filtered off, washed
with water
and dried in vacuo. The title acid (0.560 g, 52% yield) was obtained as a
orange solid.
MS (ESI, m/z): 195.1 [M+H+].
243.ii. 3-oxo-3, 4-dihydro-2H-pyrido[3,2-bJ[1, 4]oxazine-6-carboxylic acid
{(3R,6,S)-6-[(1S,2R)-2-(3 fluoro-6-methoxy-[1,5]naphth yridin-4yl)-1,2-
diliydroxy-ethylJ-
tetrahydro pyran-3 yl}amide:
Starting from intermediate 217.ii (0.32 g, 0.89 mmol) and intermediate 243.i
(0.202 g, 1.1 eq),
the title compound (0.007 g, 1.4% yield) was obtained as a colourless solid
according to the
procedure described in Example 172. Purification over silica gel was carried
out using
DCM-MeOH 9-1 1% aq. NH4OH.
MS (ESI, m/z): 514.1 [M+H}].
Example 244: (3,4-dichloro-benzyl)-{6-[2-(8-fluoro-6-methoacy-quinolin-4-yl)-
ethyl]-
tetrahydro-pyran-3-yl}-amine:
Starting from intermediate 219.ii (0.106 g, 0.34 mmol) and 3,4-
dichlorobenzaldehyde
(0.067 g, 1.1 eq.), the title compound (0.100 g, 62% yield) was obtained as a
colourless solid
according to the procedure described in Example 88, step 88.iv. The product
was purified by
chromatography over silica gel (DCM-MeOH 93-7 1% aq. NH4O1I).
'H N1VIR (d6-DMSO): 8.65 (d, J = 4.4 Hz, 1H); 7.60 (d, J= 1.9 ]EIz, 1H); 7.56
(d, J = 8.2 Hz,
1H); 7.39 (d, J = 4.4 Hz, IH); 7.34-7.23 (m, 3H); 3.97 (m, 1H); 3.92 (s, 3H);
3.73 (d,
J= 4.1 Hz, 2H); 3.21 (m, 111); 3.08 (m, 2H); 2.94 (t, J = 10.6 Hz, 114); 2.44
(m, 111); 2.23 (br.
s, 1I1); 1.97 (m 1H); 1.77 (m, 2H); 1.68 (m, 1H); 1.31-1.16 (m, 21U).
MS (ESI, m/z): 463.0 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 249 -
Example 245: 3-oxo-3,4-dihydro-2H-pyrido [3,2-b] [1,4]thiazine-6-carboxylic
acid
[(2R,3R,6S)-6-[(1S,2R)-1,2-dihydroxy-2-(6-methoxy-[1,5] naphthyridin-4-yl)-
ethyl]-
2-(2-hydroxy-ethyl)-tetrahydro-pyran-3-yl] -amide:
245.i. (2R, 3R, 6S)-(2-allyl-6-hydroxymethyl-3, 6-dihydro-2H-pyran-3 yl)-
carbamic acid
tert-butyl ester:
A solution of (2R,3R,6S)-[2-allyl-6-(tert-butyl-dimethyl-silanyloxymethyl)-3,6-
dihydro-
2H-pyran-3-yl]-carbamic acid tert-butyl ester (obtained as described in Eur.
J. Org.
Chem. (2003), 2418-2427; 26 g, 67.7 mmol) in AcOH (180 ml), water (60 ml) and
THF
(60 ml) was heated at 70 C for 7 h. The volatiles were removed in vacuo and
the residue was
partitioned between saturated NaHCO3 (200 ml) and EA (500 ml). The org. layer
was washed
with saturated NaHCO3 (2 x 100 ml), brine (100 ml), dried over Na2SO4,
filtered and
concentrated to dryness. The residue was purified over silica gel (Hex-EA 1-3)
to afford the
title alcohol (13.6 g, 74% yield) as a colourless oil.
IH NMR (CDC13): 6.07-5.76 (m, 3H); 5.22-5.12 (m, 211); 4.72 (br d, J = 10 Hz,
1H); 4.28 (qd,
J= 2.7, 6.6 Hz, 1H); 4.04 (m, 1H); 3.84 (rn, 1H); 3.73 (dd, J= 9.6, 11.7 Hz,
1H); 3.49 (m,
1H); 2.34 (m, 2H); 2.07 (br s, 1H); 1.47 (s, 91-1).
245. ii. (2R, 3R, 6S)-[2-((2RS)-2, 3-dihydroxy propyl)-6-hydroxymethyl-3, 6-
dihydro-2H-pyran-
3 ylJ-carbamic acid tert-butyl ester:
Intermediate 245.i (13.6 g, 50 mmol) was converted to the title triol (13.8 g,
90% yield,
colorless oil) using the protocol described in Example 171, step 171.vi.
MS (ESI, m/z): 304.5 [M+H+].
245.iii. (2R, 3R, 6S)-[2-((4RS)-2, 2-dirnethyl-[1, 3]dioxolan-4 ylmethyl)-6-
hydroxymethyl-
3, 6-dihydro-2H-pyran-3 ylJ-carbamic acid tert-butyl ester:
Intermediate 245.ii (13.8 g, 45.4 mmol) was converted into the title acetonide
(13.2 g,
38.4 mmol, colourless oil) using the protocol described in Example 148, step
148.iv.
Purification over silica gel was carried out using Hex-EA 1-2 mixture as an
eluent.
MS (ESI, m/z): [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 250 -
245.iv. (2R,3R,6S)- [2-((4RS)-2,2-dimethyl-[1,3]dioxolan-4 ylmethyl)-6-
hydroxymethyl-
tetrahydro pyran-3 ylJ-carbarnic acid tert-butyl ester:
To a solution of intermediate 245.iii (13.2 g, 38 mmol) in EA (190 ml) tivas
added platinum
oxide (1.5 g). The reaction was stirred under hydrogen atmosphere for tvvo
hours, filtered and
the filtrate was concentrated to dryness to afford the title pyran derivative
(12.57 g, 94%
yield) as a colourless foam.
MS (ESI, m/z): [M+H+].
245.v. (2R, 3R, 6S)-[2-((4RS)-2, 2-dimethyl-[1, 3]dioxolan-4 ylmethyl)-6-(1
phenyl-
IH-tetrazole-5-sulfonylmethyl)-tetrahydro pyran-3 ylJ-carbamic acid teY-t-
butyl ester:
Intermediate 245.iv (12.57 g, 36.2 mmol) was converted into the title sulfone
(4.07 g,
7.57 mmol, colourless foam) using the protocols of Example 164, steps 164.i.
and 164.ii.
MS (ESI, m/z): 538.1 [M+H+].
245.vi. (2R,3R,6S)-{2-(2,2-dimethyl-[1,3]dioxolan-4 ylmethyl)-6-trans-[2-(6-
methoxy-
[1,5]naphthyridin-4 yl)-vinylJ-tetrahydro pyran-3 yl}-carbamic acid te.-t-
butyl ester:
Starting from intermediate 245.v (4.0 g, 7.45 mmol) and 6-methoxy-
[1,5]naphthyridine-
4-carbaldehyde (1.7 g, 9 mmol), the title alkene (2.8 g, 62% yield) was
obtained as a
colourless foam.
MS (ESI, m/z): 500.4 [M+H+].
245.vii. (2R, 3R, 6S)-(2-(2-hydroxy-ethyl)-6-trans-[2-(6-methoxy-[],
S]naphthyridin-4 yl)-
vinyl]-tetrahyd~~opyran-3 yl}-carbamic acid tert-butyl ester and (2R,3R,6R)-{2-
(2-hydroxy-
ethyl)-6-[2-(6-methoxy-[1, 5]naphthyridin-4yl)-ethylJ-tetrahydro pyraac-3 yl}-
carbamic acid
tert-butyl ester:
A solution of intermediate 245.vi (2.8 g) in a THF-AcOH-water miKture (1-3-1,
50 ml) was
heated at 60 C for 6 h. The reaction mixture was concentrated to dryness and
the residue
partitioned between EA and saturated NaHCO3. If necessary, the pH was adjusted
to 7 by
adding solid NaHCO3. The aq. layer was extracted once with EA and the combined
org.
layers were washed with brine, dried over NaZSO4, filtered and concentrated to
dryness. The
residue was taken up in acetone (100 ml) and a warm solution of sodium
periodate (3 g) in
water (10 ml) was added. The mixture was stirred for lh. The reaction mixture
was filtered
through a pad of celite and the solvent removed in vacuo. The residue was
extracted twice
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 251 -
with EA. The combined org. layers were dried over Na2SO4, filtered and
concentrated in
vacuo. The residue was taken up in MeOH (30 ml) and NaBH4 (0.7 g) was added.
The
reaction proceeded 15 min. 10% aq. NaHSO4 (100 ml) was added. The volatiles
were
removed in vacuo and the aq. residue was extracted three times with EA (3 x
100 ml). The
combined org. layers were washed with brine, dried over Na2SO4, filtered and
concentrated to
dryness. The residue was chromatographed (DCM-MeOH 19-1) to afford a
colourless foam
(1.76 g), characterized as an unseparable mixture of alkane and alkene
derivatives. The
mixture was carried on without further purification.
MS (EI): 430.1 [M+H+] (alkene).
245.viii. (2R,3R,6S)-[6-[(1R,2R)-1,2-dihydroxy-2-(6-methoxy-[1,5]naphthyridin-
4yl)-ethylJ-
2-(2-hydroxy-ethyl)-tetrahydro pyran-3 ylJ-carbamic acid tert-butyl ester:
To a solution of intermediate 245.vii (1.76 g, mixture of compounds), was
added potassium
ferricyanide (4.04 g), K2C03 (1.7 g), methanesulfonamide (0.47 g), (DHQD)2PHAL
(0.035 g)
and potassium osmate dihydrate (0.005 g). The mixture was stirred 40 h at rt.
Sodium bisulfite
(6 g) was added. The two layers were decanted and the aq. layer was extracted
twice with EA
(2 x 150 ml). The combined org. layers were washed with brine, dried over
Na2SO4, filtered
and concentrated to dryness. The residue was chromatographed over silica gel
(DCM-MeO11[
19-1) to afford first the unreacted alkane (2R,3R,6R)-{2-(2-hydroxy-ethyl)-6-
[2-(6-methoxy-
[1,5]naphthyridin-4-yl)-ethyl]-tetrahydro-pyran-3-yl}-carbamic acid tert-butyl
ester (1.34 g,
3.10 mmol) as a colourless foam.
MS (El): 432.0 [M+PT']
Elution was continued with DCM-MeOlI 6-1 to give the desired triol (0.4 g,
0.86 mmol) as a-
colourless foam.
'H NMR (CDC13): 8.80 (d, J = 4.5 Hz, 1 H); 8.26 (d, J = 9.1 Hz, 1H); 7.64 (d,
J = 4.5 Hz, 1H);
7.16 (d, J = 9.1 Hz, 1H); 5.60 (br s, 1H); 4.96 (br d, J = 8.9 Hz, 1H); 4.46
(br s, 1H); 4.23 (m'
1H); 4.03 (s, 3H); 4.06-4.03 (m, 2H); ;3.88-3.75 (m, 4H);2.06-1.83 (m, 4H);
1.68-1.62 (m,
3H), 1.47 (s, 9H).
MS (El): 464.3 [M+H+].
245.ix. (1R,2R)-1-[(2R,5R)-5-amino-6-(2-hydroxy-ethyl)-tetrahydro pyran-2 ylJ-
2-(6-methoxy-[1,5]naphthyridin-4 yl)-ethane-1,2-diol:
Intermediate 245.viii (0.4 g, 0.86 mmol) was converted to the title amine
(0.13 g, 41% yield,
colourless foam) according to the procedure of Example 171, step 171.vii.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-252-
MS (EI): 364.2 [M+H+].
245.x. 3-oxo-3, 4-dihydro-2H pyrido[3,2-b][1, 4Jthiazine-6-carboxylic acid
[(2R, 3R, 6S)-6-[(1 S, 2R)-1, 2-dihydroxy-2-(6-methoxy-[], SJnaphthyridin-4
yl)-ethylJ-
2-(2-hydroxy-ethyl)-tetrahydro pyran-3 ylJ-amide:
Starting from intermediate 245.ix (0.13 g, 0.35 mmol) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid (0.082 g, 1.1 eq), the title
compound
(0.090 g, 45% yield) was obtained as a light beige solid according to the
procedure of
Example 172. Purification over silica gel was carried out using DCM-MeOH 9-1
containing
1% aq. NH4OH as an eluent.
IH NMR (d6-DMSO) : 11.24 (s, 1H); 8.78 (d, J=4.5 Hz, 1H); 8.28 d, J= 9.1 Hz,
1H);
8.27 (d, J = 8,8 Hz, 1H); 7.97 (d, J = 7.9 Hz, 1 H); 7.79 (d, J= 4.5 Hz, 1 H);
7.62 (d,
J= 7.8 Hz, 1 H); 7.27 (d, J = 9.0 Hz, 1 H); 5.67 (d, J = 6.9 Hz, 1 H); 5.40
(d, J= 6.9 Hz, 1 H);
4.41 (t, J= 5.2 Hz, 1H); 4.32 (d, J = 5.8 Hz, 1H); 4.05-3.95 (m, 4H); 3.99 (s,
3H); 3.65 (d, AB
system, J = 15.0 Hz, 0= 0.0577, 2H); 3.48 (m, 2H); 2.10-1.99 (m, 2H); 1.83-
1.66 (m, 3H);
1.47 (m, 11-1).
MS (ESI, m/z): 556.1 [M+H+].
Example 246: 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid
(2R,3R,6R)-{2-(2-hydroxy-ethyl)-6-[2-(6-methoxy-[l,5] naphthyridin-4-yl)-
ethyl]-
tetrahydro-pyran-3-yl}-amide:
246.i. 2-{(2R,3R,6R)-3-amino-6-[2-(6-methoxy-[1,S]nczphthyridin-4 yl)-ethylJ-
tetrahydro-
pyran-2yl}-ethanol:
(2R,3R,6R)-{2-(2-hydroxy-ethyl)-6-[2-(6-methoxy-[1,5 ]naphthyridin-4-yl)-
ethyl]-tetrahydro-
pyran-3-yl}-carbamic acid tert-butyl ester ester (collected from step
245.viii; 1.34 g,
3.1 mmol) was converted to the title amine (0.65 g, 63% yield, colourless
foam) according to
the procedure of Example 171, step 171.vii.
MS (El): 332.3 [M+H+].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 253 -
246.ii. 3-oxo-3,4-dihydro-2H pyrido[3,2-b][1,4Jthiazine-6-carboxylic acid
(2R, 3R, 6R)-{2-(2-hydroxy-ethyl)-6-[2-(6-methoxy-[1, 5]naphthyridin-4 yl)-
ethylJ-te trahydro-
pyran-3 yl}-arnide:
Starting from intermediate 246.i (0.2 g, 0.6 mmol) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carboxylic acid (0.082 g, 1.1 eq), the title
compound
(0.115 g, 36% yield) was obtained as a light beige solid using the procedure
of Example 172.
Purification over silica gel was carried out using DCM-MeOH 9-1 containing 1%
aq. NH4OH
as an eluent.
1H NMR (d6-DMSO) : 1 1.23 (s, 1H); 8.69 (d, J = 4.5 Hz, IH); 8.26 (d, J = 9. 0
Hz, 2H);
7.97 (d, J= 7.9 Hz, IH); 7.61 (d, J= 7.9 Hz, 1H); 7.58 (d, J= 4.5 Hz, 1H);
7.26 (d,
J = 9.0 Hz, 1H); 4.44 (t, J= 5.1 Hz, 1H); 4.05 (s, 3H); 4.06-3.99 (m, 2H);
3.8'7 (m, 1H);
3.65 (d, AB system, J = 15.0 Hz, A = 0.059, 2H); 3.49 (m, 2H); 3.24 (m, 1H);
3.09 (m, 1H);
2.27 (in, 1H); 1.99-1.83 (m-, 3H); 1.73-1.66 (m, 2H); 1.52-1.43 (m, 2H).
MS (ESI, m/z): 524.2 [M+]H+].
Example 247: 6-((2R,3R,6R)-{2-(2-hydroxy-ethyl)-6-[2-(6-methoxy-
[1,5]naphthyridin-
4-yl)-ethyl]-tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4]thiazin-
3-one:
Starting from intermediate 246.i (0.15 g, 0.45 mmol) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.097 g, 1.1 eq), the title
compouLind (0.08 g,
34% yield) was obtained as a colourless solid using the procedure of Example
88, step 88.iv.
The product was purified by chromatography over silica gel (DCM-MeOH 9-1 1%
aq.
NH4OH).
MS (ESI, m/z): 510.2 [M+I4+].
Example 248: trans-3-(2,5-difluoro-phenyl)-N-{(2R,3R,6R)-2-(2-hydroxy-eth)-1)-
6-[2-(6-methoxy-[1,5] nap bthyridin-4-yl)-ethyl]-tetrahydro-pyran-3-y1}-
acrylarnide:
Starting from intermediate 246.i (0.180 g, 0.54 mmol) and trans-3-(2,5-
difluoro-phenyl)-
acrylic acid (0.110 g, 1.1 eq.), the title compound (0.09 g, 33% yield) was
obtained as a
colourless solid using the procedure of Example 172. The product was purified
by
chromatography over silica gel (DCM-MeOH 9-1 1% aq. NH40H).
MS (ESI, m/z): 498.1 [M+V].
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 254 -
Example 249: trafts-3-(2,5-difluoro-phenyl)-N-{(2R,3R,6R)-6-[2-(6-methoxy-
quinolin-
4-yl)-ethyl]-tetrahydro-pyran-3-yl}-acrylamide:
Starting from intermediate 168.iii (0.098 g, 0.34 mmol) and trans-3-(2,5-
difluoro-phenyl)-
acrylic acid (0.071 g, 1.1 eq.), the title compound (0.02 g, 12% yield) was
obtained as a
colourless solid using the procedure of Example 172. The product was purified
by
chromatography over silica gel (DCM-MeOH 19-1 1% aq. NH4OH).
MS (ESI, m/z): 453.3 [M+H+].
Example 250: 6-({(3R,6b)-6-[(2R)-2-(3-fluoro-6-methoxy-quinolin-4-yl)-2-
hydroxy-
ethyl]-tetrahydro-pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4]thiazin-3-one
and
6-({(3R,6S)-6-[(2S)-2-(3-fluoro-6-methoxy-quinolin-4-yl)-2-hydroxy-ethyl]-
tetrahydro-
pyran-3-ylamino}-methyl)-4H-pyrido[3,2-b] [1,4]thiazin-3-one:
250.i. {(3R, 6S)-6-[(2RS)-2-(3fluoro-6-methoxy-quinolin-4 yl)-2-hydroxy-ethylJ-
tetrahydro-
pyran-3yl}-carbamic acid tert-butyl ester:
To a solution of diisopropylarnine (0.492 ml, 3.5 mmol) in THF (20 ml) cooled
to -78 C, was
added n-BuLi (1.4 ml, 2.5N in hexanes, 1 eq.). After stirring 20 min at this
temperature, a
solution of 3-fluoro-6-methoxy-quinoline (prepared as described in WO
2005/049575,
0.626 g, 3.55 mmol), in THF (6 ml) was added. The reaction mixture was kept 4
h at the same
temperature and a solution of intermediate 203.iii (0.43 g, 1.76 mmol) in THF
(3 ml) was
added. The reaction mixture was stirred 15 min at low temperature and allowed
to reach rt
over lh. Water (15 ml) was added. The two layers were decanted. The aq. layer
was extracted
twice with EA (2 x 50 ml). The combined organic layers were washed with brine,
dried over
Na2SO4, filtered and concentrated to dryness. The residue was purified by
chromatography
over silica gel (EA-Hex 3-1 then 1-0) to afford the title benzylic alcohol
(0.103 g, 13% yield)
as a yellowish foam. The compound was obtained as a equimolar mixture of
epimers.
MS (ESI, m/z): 421.0 [M+H+].
250.ii. (2RS)-2-((2S,SR)-S-amino-tetrahydro pyran-2 yl)-1-(3 fluoro-6-methoxy-
quinolin-
4yl)-ethanol:
This amine (0.078 g, 100% yield) was prepared from intermediate 250.i (0.103
g, 0.25 mmol)
using the procedure of Exarnple 171, step 171.vii. A 1:1 mixture of epimers
was obtained as a
colourless foam.
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
- 255 -
MS (ESI, m/z): 321.0 [M+Hi"].
250.iii. 6-({(3R,6S)-6-[(2R)-2-(3fluoro-6-methoxy-quinolin-4 yl)-2-hydroxy-
etlzylJ-
tetrahydro pyran-3-ylamino}-methyl)-4H-pyrido[3,2-bJ[1, 4]thiazin-3-one and
6-({(3R,6S)-6-[(2S)-2-(3fluoro-6-methoxy-quinolin-4 yl)-2-hydroxy-ethylJ-
tetrahydro pyran-
3 ylamino}-methyl)-4H pyrido[3, 2-b][1, 4]thiazin-3-one:
Starting from intermediate 250.ii (0.078 g, 0.25 mmol) and 3-oxo-3,4-dihydro-
2H-pyrido[3,2-b][1,4]thiazine-6-carbaldehyde (0.050 g, 1.1 eq), the two title
compoundswere
obtained using the procedure of Example 88, step 88.iv. Purification on silica
gel using
DCM-MeOH 9-1 containing 1% aq. NH4OH led to the separation of the two
epiiners.
First eluting epimer:
This epimer was obtained as a colourless foam (0.035 g, 30% yield).
Rf (TLC over Si02): 0.47 (DCM-MeOH 9-1 containing 1% aq. NH4OH).
MS (ESI, m/z): 499.0 [M+H+].
Second eluting epimer:
This epimer was obtained as a light orange foam (0.047 g, 39% yield).
Rf (TLC over Si02): 0.43 (DCM-MeOH 9-1 containing 1% aq. NH4OH)
MS (ESI, m/z): 499. 1 [M+H+].
Example 251: N-(2,5-difluoro-phenyl)-2-{(3R,6R)-6-[2-(6-methoxy-quinolin-4-yl)-
ethyl]-tetrahydro-pyran-3-ylamino}-acetamide:
251.i. 2-bromo-N-(2,5-difluoro phenyl)-acetamide:
To a mixture of 2.5-difluoroaniline (2.01 g, 15.6 mmol) and TEA (1.56 ml, 15.6
mmol) in
DCM (50 ml) cooled to 10 C, was added dropwise a mixture of bromo
acetylbromide (3.13 g,
15.6 mmol) in DCM (30 ml), After stirring two hours at rt, water (100 ml) was
added. The
organic layer was washed with saturated NaHCO3 (100 ml) and water (100 ml).
The org. layer
was washed over NazSO4, filtered and concentrated to dryness to afford the
title bromide
(2.74 g, 70% yield) as a reddish solid.
1H NMR (CDC13): 8.41 (br s, IH); 8.12 (ddd, J= 3.1, 6.2, 9.3 Hz, 1H); 7.08 (m-
, 1H); 6.80 (m,
1H); 4.04 (s, 2H).
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-256-
251.ii. N-(2,5-difluoro phenyl)-2-{(3R,6R)-6-[2-(6-methoxy-quinolin-4 yl)-
ethylJ-tetrahydro-
pyran-3 ylamino}-acetamide:
This compound (0.12 g, 13% yield) was obtained as a yellowish solid, starting
frorn
intermediate 251.i (0.49 g, 1.96 mmol) and intermediate 168.iii (0.48 g, 1.67
mmol) and using
the procedure of Example 103.
MS (ESI, m/z): 456.3 [M+H+].
Biological assays
In vitro assay
Experimental method:
These assays have been performed following the description given in "Methods
for dilution
Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 4th
ed.; Approvad
standard: NCCLS Document M7-A4; National Committee for Clinical Laboratory
StandarcLs:
Villanova, PA, USA, 1997". Minimal inhibitory concentrations (MICs; mg/1) were
determined in cation-adjusted Mueller-Hinton Broth (BBL) by a microdilution
method
following NCCLS guidelines (National Committee for Clinical Laboratory
Standarcis.
Methods for Dilution Antimicrobial Susceptibility). The pH of the test medium
was 7.2-7.3 -
All Examples were tested against several Gram positive and Gram negative
bacteria.
Typical antibacterial test results are given in the table hereafter (MIC in
mg/1).
Example No. S. aureus A798 S Pneunoniae 49619 M catarrizalis A89 4
5 0.5 1 <_ 0.063
8 0.125 0.25 <_ 0.031
28 0.063 0.5 0.125
35 < 0.031 0.25 0.031
53 < 0.063 0.5 1
70 <_ 0.031 0.25 < 0.031
CA 02580621 2007-03-16
WO 2006/032466 PCT/EP2005/010154
-257-
Example No. S. aureus A798 S Pneunoniae 49619 M catarrhalis A894
83 <_ 0.063 1 0.063
89 _ 0.031 0.063 0.03 1
95 0.125 0.5 0.063
100 0.5 0.25 0.063
101 0.031 1 0.031
108 < 0.031 0.25 0.03 1
116 0.125 1 0.25
131 0.25 1 0.03 1
139 0.03 1 0.5 0.03 1
153 0.031 0.125 0.031
174 :5 0.031 0.063 < 0.031
187 0.125 0.125 0.031
203 <_ 0.031 0.125 0.031
206 0.125 0.5 0.25
214 <_ 0.03 1 1 <_ 0.031