Note: Descriptions are shown in the official language in which they were submitted.
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
DIBENZODIAZEPINONE ANALOGUES, PROCESSES FOR THEIR PRODUCTION
AND THEIR USE AS PHARMACEUTICALS
FIELD OF THE INVENTION
This invention relates to dibenzodiazepinone analogues having improved
properties, which are chemical derivatives of Compound 1. The invention
further relates
to their pharmaceutically acceptable salts, solvates and prodrugs, and to
methods for
obtaining the compounds. One method of obtaining the derivatives involves post-
io biosynthesis chemical modification of Compound 1. The present invention
further
relates to the use of dibenzodiazepinone analogues, and their pharmaceutically
acceptable salts, solvates and prodrugs as pharmaceuticals, in particular to
their use as
inhibitors of cancer cell growth, mammalian lipoxygenase, and'for treating
acute and
chronic inflammation, and to pharmaceutical compositions comprising a
dibenzodiazepinone analogue, or a pharmaceutically acceptable salt, solvate or
prodrug thereof.
BACKGROUND OF THE INVENTION
The euactinomycetes are a subset of a large and complex group of Gram-
20 positive bacteria known as actinomycetes. Over the past few decades these
organisms, which are abundant in soil, have generated significant commercial
and
scientific interest as a result of the large number of therapeutically useful
compounds,
particularly antibiotics, produced as secondary metabolites. The intensive
search for
strains able to produce new antibiotics has led to the identification of
hundreds of new
species. Many of the euactinomycetes, particularly Streptomyces and the
closely related
Saccharopolyspora genera, have been extensively studied. Both of these genera
produce a notable diversity of biologically active metabolites. Because of the
commercial significance of these compounds, much is known about the genetics
and
30 physiology of these organisms. Another representative genus of
euactinomycetes,
Micromonospora, has also generated commercial interest. For example, U.S.
Patent
No. 5,541,181 (Ohkuma et al.) discloses a dibenzodiazepinone compound,
specifically
5-farnesyl-4,7,9-trihydroxy-dibenzodiazepin-1 1 -one (named "BU-4664L"),
produced by
1
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
a known euactinomycetes strain, Micromonospora sp. M990-6 (ATCC 55378). ECO-
4601 (Compound 1) and Micromonospora sp. strains 046-ECO11 and [S01]046 are
disclosed in CA 2,466,340. Its use for the treatment of cancer is disclosed in
PCT/CA2005/000751.
0
N
N O-OH
OH I HO
ECO-4601 (Compound 1)
Synthetic dibenzodiazepinone analogs were disclosed in the published Canadian
patent application 2,248,820 as having anti-histamine properties.
Although many biologically active compounds have been identified from
bacteria,
lo there remains the need to obtain novel compounds with enhanced properties.
Thus,
there exists a considerable need to obtain pharmaceutically active compounds
in a
cost-effective manner and with high yield. The present invention solves these
problems
by providing new therapeutic compounds and methods to generate these novel
compounds by post-biosynthetic chemical modifications.
SUMMARY OF THE INVENTION
In one aspect of the invention, the dibenzodiazepinone analogue is represented
by a compound of Formula I as defined below, or a hydrogenated or
hydroalkoxylated
farnesyl derivative, or a pharmaceutically acceptable salt, solvate or prodrug
of a
20 compound of Formula I. In another embodiment, the dibenzodiazepinone
analogue is
represented by any one of Compounds 2 to 27 as defined below, or a
pharmaceutically
acceptable salt, solvate or prodrug, or salt of a prodrug of any one of
Compounds 2 to
25. In a further embodiment, the dibenzodiazepinone analogue is represented by
any
one of Compounds 2 to 5, 7, 13, 14, 15, 18, 21, and 22 as defined below, or a
pharmaceutically acceptable solvate or prodrug of any one of Compounds 2 to 5,
7, 13,
14, 15, 18, 21, and 22.
In yet another aspect of the invention, the dibenzodiazepinone analogue is
represented by any one of Compounds 23 to 25 as defined below, or a
2
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
pharmaceutically acceptable solvate or prodrug of any one of Compounds 23 to
25.
The invention further encompasses a dibenzodiazepinone analogue obtained by
a method comprising the steps of: (a) providing an isolated form of ECO-4601,
(b)
chemically modifying Compound 1. In another embodiment, the dibenzodiazepinone
analogue is a compound of Formula I, or a hydrogenated or hydroalkoxylated
farnesyl
derivative, or a pharmaceutically acceptable salt, solvate or prodrug thereof.
In another
embodiment the chemical modification step (b) is one or more steps selected
from N-
alkylation, 0-triacetylation, aryl bromination, and farnesyl hydrogenation or
hydroalkoxylation. In another embodiment the dibenzodiazepinone analogue is
selected
lo from Compounds 2 to 27. In another embodiment the dibenzodiazepinone
analogue is,
selected from Compounds 2 to 5, 7, 13, 14, 15, 18, 21, and 22. In another
embodiment
the dibenzodiazepinone analogue is selected from Compounds 23 to 25.
The invention further encompasses a method for making a dibenzodiazepinone
compound, comprising chemically modifying the farnesyl dibenzodiazepinone
Compound 1, and optionally isolating and purifying the dibenzodiazepinone
compound
produced. In one embodiment, the chemical modification step comprises at least
one
step selected from N-alkylations, O-acylations, aryl bromination and
modifications of the
double bonds of the farnesyl side chain including, hydrogenation, and
hydroalkoxylation. In a subclass of this embodiment, the farnesyl side chain
20 modification reaction is partial (one or two double bonds modified) or
complete (all three
double bonds modified).
The invention further encompasses the use of a compound of Formula I or a
hydrogenated or hydroalkoxylated farnesyl derivative, or a pharmaceutically
acceptable
salt, solvate or prodrug thereof as an antitumor agent for the treatment of a
pre-
cancerous or cancerous condition in a mammal. In one embodiment, the compound
is
selected from Compounds 2 to 27. In another embodiment, the compound is
selected
from Compounds 2 to 5, 7, 13, 14, 15, 18, 21, and 22. In another embodiment
the
compound is selected from Compounds 23 to 25.
The invention further encompasses the use of a compound of Formula I or a
3o hydrogenated or hydroalkoxylated farnesyl derivative, or a pharmaceutically
acceptable
salt or prodrug thereof as an antineoplastic agent for the treatment of a
proliferative
disorder in a mammal. In one embodiment, the compound is selected from
3
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
Compounds 2 to 27. In another embodiment, the compound is selected from
Compounds 2 to 5, 7, 13, 14, 15, 18, 21, and 22. In another embodiment the
compound
is selected from Compounds 23 to 25.
The invention further encompasses the use of a compound of Formula I or a
hydrogenated or hydroalkoxylated farnesyl derivative, or a pharmaceutically
acceptable
salt or prodrug thereof in the preparation of a medicament for the treatment
of a pre-
cancerous or cancerous condition in a mammal. In one embodiment, the compound
is
selected from Compounds 2 to 27. In another embodiment, the compound is
selected
from Compounds 2 to 5, 7, 13, 14, 15, 18, 21, and 22. In another embodiment
the
io compound is selected from Compounds 23 to 25.
The invention further encompasses a commercial package comprising a
compound of Formula I or a hydrogenated or hydroalkoxylated farnesyl
derivative, or a
pharmaceutically acceptable salt or prodrug thereof, together with
instructions for use in
the treatment of a neoplasm or a pre-cancerous or cancerous condition. In one
embodiment, the compound is selected from Compounds 2 to 27. Iri another
embodiment, the compound is selected from Compounds 2 to 5, 7, 13, 14, 15, 18,
21,
and 22. In another embodiment the compound is selected from Compounds 23 to
25.
In one embodiment, the cancer cell, neoplastic, pre-cancerous or cancerous
condition, in the above-mentioned uses, is selected from leukemia, melanoma,
breast
20 cancer, lung cancer, pancreatic cancer, ovarian cancer, renal cancer, colon
or
colorectal cancer, prostate cancer, and CNS cancer. In another embodiment, the
cancer cell, and pre-cancerous or cancerous condition, in the above-mentioned
methods and uses, is selected from leukemia, breast cancer, prostate cancer,
and CNS
cancer.
BRIEF DESCRIPTION OF THE FIGURES
Figure 1: shows inhibition of tumor growth resulting from bolus administration
of 10 to
30 mg/kg of Compound I to C6 glioblastoma-bearing mice one day after tumor
cell
inoculation.
3o Figure 2: shows inhibition of tumor growth resulting from bolus
administration of 20-30
mg/kg of Compound I to glioblastoma-bearing mice ten days after tumor cell
inoculation.
4
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
Figure 3: shows micrographs of tumor sections from mice bearing glioblastoma
tumors
and treated with saline or Compound 1. The cell density of tumor treated with
Compound 1 appears decreased and nuclei from tumor cells are larger and
pycnotic
suggesting a cytotoxic effect.
Figure 4: inhibition of tumor growth resulting from bolus administration of 20
to 75
mg/kg of Compound 2 to C6 glioblastoma-bearing mice from day 11 to day 20 of
treatment.
Figure 5: shows the mean ( SD) plasma concentrations of Compound 1 in Swiss
mice
following 30 mg/kg intravenous (iv), intraperitoneal (ip), subcutaneous (sc)
and oral (po)
io bolus administrations.
Figure 6: shows the mean ( SD) plasma concentrations of Compounds 1 and 2 in
CD-1
mice following 30 mg/kg intravenous (iv) and intraperitoneal (ip) bolus
administrations.
Figure 7: shows the mean concentration of Compound 1 in various tissues, 30
minutes
after 30mg/kg intravenous (iv), intraperitoneal (ip) and subcutaneous (sc)
bolus
administrations.
DETAILED DESCRIPTION OF THE INVENTION
The present invention relates to novel dibenzodiazepinone analogues having
improved properties, herein referred as the compounds of Formula I and
hydrogenated
20 or hydroalkoxylated farnesyl derivatives, and Compounds 2 to 27. The
invention further
relates to pharmaceutically acceptable salts, solvates and prodrugs of
dibenzodiazepinone compounds. The invention further relates to processes of
obtaining
the analogs by chemical modification of Compound 1. Compound 1 is isolated
from
strains of actinomycetes, Micromonospora sp. 046-ECO11 (also as 046(ECO11)) or
[SOI]046, as described in PCT/CA04/000069.
The invention also relates to a method for producing novel dibenzodiazepinone
analogs, by chemical modification of the farnesyl dibenzodiazepinone obtained
from
fermentation and isolation. In a subclass of this embodiment, the compound
produced
is a compound of Formula I or a hydrogenated or hydroalkoxylated farnesyl
derivative
30 thereof, or a compound selected from Compounds 23 to 27.
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
The present invention also relates to pharmaceutical compositions comprising a
compound selected from the compounds of Formula I, and Compounds 2 to 27, and
their pharmaceutically acceptabl.e salts, solvates and derivatives. The
compounds are
useful as pharmaceuticals, in particular for use as inhibitors of neoplastic
cell growth.
The following detailed description discloses how to make and use the
dibenzodiazepinone analogs and compositions containing these compounds to
inhibit
tumor growth.
Accordingly, certain aspects of the present invention relate to pharmaceutical
compositions comprising the dibenzodiazepinone compounds of the present
invention
io together with a pharmaceutically acceptable carrier, and methods of using
the
pharmaceutical compositions to treat pre-cancererous or cancerous conditions.
1. Definitions
All technical and scientific terms used herein have the same meaning as
commonly understood by one of ordinary skill in the art to which this
invention belongs.
For convenience, the meaning of certain terms and phrases used in the
specification,
examples, and appended claims, are provided below.
As used herein, the term "farnesyl dibenzodiazepinone" refers to Compo"und 1,
namely 10-farnesyl-4,6,8-trihydroxy-5,10-dihydrodibenzo[b,e][1,4]diazepin-11-
one, also
2o referred to as ECO-4601.
As used herein, the terms "compound(s) of the invention", "dibenzodiazepinone
analogue(s)", "dibenzodiazepinone compound(s)", and equivalent expressions
refer to a
class of dibenzodiazepinone compounds containing a farnesyl moiety or being
derived
from a farnesyl moiety, and pharmaceutically acceptable salts, solvates and
prodrugs
thereof. The term includes a compound of Formula I, a compound selected from
Compounds 2 to 27, or the exemplified compounds of the present invention,
Compounds 2 to 5, 7, 13, 14, 15, 18, and 21 to 25, or a pharmaceutically
acceptable
salt, solvate or prodrug of any of the above compounds. As used herein, the
term
"dibenzodiazepinone analogues" includes compounds of this class that can be
used as
30 intermediates in chemical syntheses and variants containing different
isotopes than the
most abundant isotope of an atom (e.g, D replacing H, 13C replacing 12C, etc).
The
6
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
compounds of the invention are also sometimes referred as "active
ingredients".
As used herein, the term "chemical modification" refers to one or more steps
of
modifying a dibenzodiazepinone compound,'referred to as "starting material",
by
chemical synthesis. Preferred compound used as starting materials in a
chemical
modification process is Compound 1. Examples of chemical modification steps
include
N-alkylations, 0-acylations, aromatic bromination, and modifications of the
double
bonds of the farnesyl side chain including, hydrogenation and
hydroalkoxylation.
Farnesyl side chain modification reaction can be partial (one or two double
bonds
modified) or complete (three double bonds modified). Chemical modification
steps are
lo also defined in the Schemes of Section IIIB, and exemplified in Examples 4
to 8.
The term "ester" refers to a dibenzodiazepinone analogue obtained by the
replacement of a hydrogen atom from an alcohol by a C(O)R" replacement group
by an
0-acylation reaction as defined in Scheme 1(b) below, wherein C(O)R" can also
be a C-
coupled amino acid. The term ester also encompasses ester equivalents
including,
without limitation, carbonate, carbamate, and the like. More particularly, the
term "ester"
encompasses esters of the alcohols in positions 4, 6, and 8 (see Exampes 3-8
for atom
numbering).
The term "N-alkylated derivative" refers to a dibenzodiazepinone analogue
obtained by the replacement of a hydrogen atom of a nitrogen atom by an R
2o replacement group by an N-alkylation reaction as defined in Scheme 2(a)
below. More
particularly, the term "N-alkylated derivative" encompasses substituted
derivatives at
the nitrogen in position 5 (see Exampes 3-8 for atom numbering).
As used herein, the term "hydrogenated or hydroalkoxylated farnesyl
derivative"
refers to a compound having a modified farnesyl side chain at one to three
positions by
either saturation (addition of two hydrogen atoms) or by addition of a
molecule of
alcohol (H and OC1_6alkyl) produced respectively by the procedures generally
defined in
Schemes 3(a) and (b) of Section IIIB, and more specifically in Examples 4 and
7.
As used herein, abbreviations have their common meaning. Unless otherwise
noted, the abbreviations "Ac", "Me", "Et", "Pr", "i-Pr", "Bu", "Bz", "Bn" and
"Ph",
3o respectively refer to acetyl, methyl, ethyl, propyl (n- or iso-propyl), iso-
propyl, butyl (n-,
iso-, sec- or tert-butyl), benzoyl, benzyl and phenyl. Abbreviations in the
specification
correspond to units of measure, techniques, properties or compounds as
follows: "RT"
7
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
and "Rt" mean retention time, "min" means minutes, "h" means hour(s), "pL"
means
microliter(s), "mL" means milliliter(s), "mM" means millimolar, "M" means
molar,
"mmole" means millimole(s), "eq" means molar equivalent(s). "High Pressure
Liquid
Chromatography" or "High Performance Liquid Chromatography" are abbreviated
HPLC.
The term "alkyl" refers to linear, branched or cyclic, saturated hydrocarbon
groups. Examples of alkyl groups include, without limitation, methyl, ethyl, n-
propyl,
isopropyl, n-butyl, pentyl, hexyl, heptyl, cyclopentyl, cyclohexyl,
cyclohexylmethyl, and
the like. Alkyl groups may optionally be substituted with substituents
selected from
io acyl, amino, acylamino, acyloxy, carboalkoxy, carboxy, carboxyamido, cyano,
halo,
hydroxyl, nitro, thio, alkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl,
aryl, heteroaryl,
alkoxy, aryloxy, sulfinyl, sulfonyl, oxo, guanidino and formyl.
The term "Cl_nalkyl", wherein n is an integer from 2 to 12, refers to an alkyl
group
having from 1 to the indicated "n" number of carbons. The Ci_õalkyl can be
cyclic or a
straight or branched chain.
The term "linear Cl_nalkyl", wherein n is an integer from 2 to 10, refers to
an alkyl
group having from 1 to the indicated "n" number of carbons and being liear,
i.e. not
cyclic or branched in the vicinity of the attached atom (herein the nitrogen).
The Cl_
nalkyl can optionally be substituted with groups such as amino, cyano, halo,
hydroxyl,
2o nitro, thio, and alkoxy.
The term "alkenyl" refers to linear, branched or cyclic unsaturated
hydrocarbon
groups containing, from one to six carbon-carbon double bonds. Examples of
alkenyl
groups include, without limitation, vinyl, 1-propene-2-yl, 1-butene-4-yl, 2-
butene-4-yl, 1-
pentene-5-yl and the like. Alkenyl groups may optionally be substituted with
substituents selected from acyl, amino, acylamino, acyloxy, carboalkoxy,
carboxy,
carboxyamido, cyano, halo, hydroxyl, nitro, thio, alkyl, alkenyl, alkynyl,
cycloalkyl,
heterocycloalkyl, aryl, heteroaryl, alkoxy, aryloxy, sulfinyl, sulfonyl,
formyl, oxo and
guanidino. The double bond portion(s) of the unsaturated hydrocarbon chain may
be
either in the cis or trans configuration.
30 The term "C2_nalkenyl", wherein n is an integer from 3 to 12, refers to an
alkenyl
group having from 2 to the indicated "n" number of carbons. The C2_nalkenyl
can be
cyclic or a straight or branched chain.
8
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
The term "alkynyl" refers to linear, branched or cyclic unsaturated
hydrocarbon
groups containing at least one carbon-carbon triple bond. Examples of alkynyl
groups
include, without limitation, ethynyl, 1-propyne-3-yl, 1-butyne-4-yl, 2-butyne-
4-yl, 1-
pentyne-5-yl and the like. Alkynyl groups may optionally be substituted with
substituents selected from acyl, amino, acylamino, acyloxy, carboalkoxy,
carboxy,
carboxyamido, cyano, halo, hydroxyl, nitro, thio, alkyl, alkenyl, alkynyl,
cycloalkyl,
heterocycloalkyl, aryl, heteroaryl, alkoxy, aryloxy, sulfinyl, sulfonyl,
formyl, oxo and
guanidine.
The term "C2_nalkynyl", wherein n is an integer from 3 to 12, refers to an
alkynyl
lo group having from 2 to the indicated "n" number of carbons. The C2_nalkynyl
can be
cyclic or a straight or branched chain.
The term "cycloalkyl" or "cycloalkyl ring" refers to an alkyl group, as
defined
above, further comprising a saturated or partially unsaturated carbocyclic
ring in a
single or fused carbocyclic ring system having from three to fifteen ring
members.
Examples of cycloalkyl groups include, without limitation, cyclopropyl,
cyclobutyl,
cyclopentyl, cyclopenten-1-yl, cyclopenten-2-yl, cyclopenten-3-yl, cyclohexyl,
cyclohexen-1-yl, cyclohexen-2-yl, cyclohexen-3-yl, cycloheptyl,
bicyclo[4,3,0]nonanyl,
norbornyl, and the like. Cycloalkyl groups may optionally be substituted with
substituents selected from acyl, amino, acylamino, acyloxy, carboalkoxy,
carboxy,
20 carboxyamido, cyano, halo, hydroxyl, nitro, thio, alkyl, alkenyl, alkynyl,
cycloalkyl,
heterocycloalkyl, aryl, heteroaryl, alkoxy, aryloxy, sulfinyl, sulfonyl and
formyl.
The term "C3_ncycloalkyl", wherein n is an integer from 4 to 15, refers to a
cycloalkyl ring or ring system or having from 3 to the indicated "n" number of
carbons.
The term "heterocycloalkyl", "heterocyclic" or "heterocycloalkyl ring" refers
to a
cycloalkyl group, as defined above, further comprising one to four hetero
atoms (e.g. N,
0, S, P) or hetero groups (e.g. NH, NR", P02, SO, SO2) in a single or fused
heterocyclic
ring system having from three to fifteen ring members (e.g. tetrahydrofuranyl
has five
ring members, including one oxygen atom). Examples of a heterocycloalkyl,
heterocyclic or heterocycloalkyl ring include, without limitation,
pyrrolidino,
30 tetrahydrofuranyl, tetrahydrodithienyl, tetrahydropyranyl,
tetrahydrothiopyranyl,
piperidino, morpholino, thiomorpholino, thioxanyl, piperazinyl, azetidinyl,
oxetanyl,
thietanyl, homopiperidinyl, oxepanyl, thiepanyl, oxazepinyl, diazepinyl,
thiazepinyl,
9
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
1,2,3,6-tetrahydropyridinyl, 2-pyrrolinyl, 3-pyrrolinyl, indolinyl, 2H-
pyranyl, 4H-pyranyl,
dioxanyl, 1,3-dioxolanyl, pyrazolinyl, dithianyl, dithiolanyl, dihydropyranyl,
dihydrothienyl,
dihydrofuranyl, pyrazolidinyl, imidazolinyl, imidazolidinyl, 3-
azabicyclo[3,1,0]hexanyl, 3-
azabicyclo[4,1,0]heptanyl, 3H-indolyl, and quinolizinyl. The foregoing
heterocycloalkyl
groups, as derived from the compounds listed above, may be C-attached or N-
attached
where such is possible. Heterocycloalkyl, heterocyclic or heterocycloalkyl
ring may
optionally be substituted with substituents selected from acyl, amino,
acylamino,
acyloxy, oxo, thiocarbonyl, imino, carboalkoxy, carboxy, carboxyamido, cyano,
halo,
hydroxyl, nitro, thio, alkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl,
aryl, heteroaryl,
io alkoxy, aryloxy, sulfinyl, sulfonyl and formyl.
The term "C3_nheterocycloalkyl", wherein n is an integer from 4 to 15, refers
to an
heterocycloalkyl group having from 3 to the indicated- "n" nuniber of atoms in
the cycle
and at least one hetero group as defined above.
The terms "halo" or "halogen" refers to bromine, chlorine, fluorine or iodine
substituents.
The term "aryl" or "aryl ring" refers to common aromatic groups having "4n+2"
Tr(pi) electrons, wherein n is an integer from 1 to 3, in a conjugated
monocyclic or
polycyclic system and having from five to fourteen ring atoms. Aryl may be
directly
attached, or connected via a C1_3alkyl group (also referred to as aralkyl).
Examples of
2o aryl include, without limitation, phenyl, benzyl, phenethyl, 1-phenylethyl,
tolyl, naphthyl,
biphenyl, terphenyl, and the like. Aryl groups may optionally be substituted
with one or
more substituent group selected from acyl, amino, acylamino, acyloxy, azido,
alkythio,
carboalkoxy, ca,rboxy, carboxyamido, cyano, halo, hydroxyl, nitro, thio,
alkyl, alkenyl,
alkynyl, cycloalkyl, heterocyclyl, aryl, heteroaryl, alkoxy, aryloxy,
sulfinyl, sulfonyl and
formyl.
The term "C5_naryl", wherein n is an integer from 5 to 14, refers to an aryl
group
having from 5 to the indicated "n" number of atoms, including carbon,
nitrogen, oxygen
and sulfur. The C5_naryl can be mono or polycyclic.
The term "heteroaryl" or "heteroaryl ring" refers to an aryl ring, as defined
above,
30 further containing one to four heteroatoms selected from oxygen, nitrogen,
sulphur or
phosphorus. Examples of heteroaryl include, without limitation, pyridyl,
imidazolyl,
pyrimidinyl, pyrazolyl, triazolyl, tetrazolyl, furyl, thienyl, isooxazolyl,
thiazolyl, oxazolyl,
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
isothiazolyl, pyrrollyl, quinolinyl, isoquinolinyl, indolyl, benzimidazolyl,
benzofuranyl,
cinnolinyl, indazolyl, indolizinyl, phthalazinyl, pyridazinyl, triazinyl,
isoindolyl, pteridinyl,
purinyl, oxadiazolyl, thiadiazolyl, furazanyl, benzofurazanyl,
benzothiophenyl,
benzothiazolyl, benzoxazolyl, quinazolinyl, quinoxalinyl, naphthyridinyl, and
furopyridinyl
groups. Heteroaryl may optionally be substituted with one or more substituent
group
selected from acyl, amino, acylamino, acyloxy, azido, alkythio, carboalkoxy,
carboxy,
carboxyamido, cyano, halo, hydroxyl, nitro, thio, alkyl, alkenyl, alkynyl,
cycloalkyl,
heterocyclyl, aryl, heteroaryl, alkoxy, aryloxy, sulfinyl, sulfonyl and
formyl. Heteroaryl
may be directly attached, or connected via a CI_3alkyl group (also referred'to
as
io heteroaralkyl). The foregoing heteroaryl groups, as derived from the
compounds listed
above, may be C-attached or N-attached where such is possible.
The term "C5_nheteroaryl", wherein n is an integer from 5 to 14, refers to an
heteroaryl group having from 5 to the indicated "n" number of atoms, including
carbon,
nitrogen, oxygen and sulphur atoms. The C5_nheteroaryl can be mono or
polycyclic.
The term "amino acid" refers to an organic acid containing an amino group. The
term includes both naturally occurring and synthetic amino acids; therefore,
the amino
group can be but is not required to be, attached to the carbon*next to the
acid. A C-
coupled amino acid substituent is attached to the heteroatom (nitrogen or
oxygen) of
the parent molecule via its carboxylic acid function. C-coupled amino acid
forms an
2o ester with the parent molecule when the heteroatom is oxygen, and an amide
when the
heteroatom is nitrogen. Examples of amino acids include, without limitation,
alanine,
valine, leucine, isoleucine, proline, phenylalanine, tryptophan, methionine,
glycine,
serine, threonine, cysteine, asparagine, glutamine, tyrosine, histidine,
lysine, arginine,
aspartic acid, glutamic acid, desmosine, ornithine, 2-aminobutyric acid,
cyclohexylalanine, 'dimethylglycine, phenylglycine, norvaline, norieucine,
hydroxylysine,
allo-hydroxylysine, hydroxyproline, isodesmosine, allo-isoleucine,
ethylglycine, beta-
alanine, aminoadipic acid, aminobutyric acid, ethyl asparagine, and N-methyl
amino
acids. Amino acids can be pure L or D isomers or mixtures of L and D isomers.
The compounds of the present invention can possess one or more asymmetric
30 carbon atoms and can exist as optical isomers forming mixtures of racemic
or non-
racemic compounds. The compounds of the present invention are useful as single
isomers or as a mixture of stereochemical isomeric forms. Diastereoisomers,
i.e.,
nonsuperimposable stereochemical isomers, can be separated by conventional
means
11
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
such as chromatography, distillation, crystallization or sublimation. The
optical isomers
can be obtained by resolution of the racemic mixtures according to
conventional
processes, including chiral chromatography (e.g. HPLC), immunoassay
techniques, or
the use of covalently (e.g. Mosher's esters) or non-covalently (e.g. chiral
salts) bound
chiral reagents to respectively form a diastereomeric ester or salt, which can
be further
separated by conventional methods, such as chromatography, distillation,
crystallization
or sublimation. The chiral ester or salt is then cleaved or exchanged by
conventional
means, to recover the desired isomer(s).
The invention encompasses isolated or purified compounds., An "isolated" or
"purified" compound refers to a compound which represents at least 10%, 20%,
50%,
80% or 90% of the mixture by weight, provided that the mixture comprising the
compound of the invention has demonstrable (i.e. statistically significant)
biological
activity including cytostatic, cytotoxic, enzyme inhibitory or receptor
binding action when
tested in conventional biological assays known to a person skilled in the art.
The term "pharmaceutically acceptable salt" refers to nontoxic salts
synthesized
from a compound which contains a basic or acidic moiety by conventional
chemical
methods. Generally, such salts can be prepared by reacting the free acid or
base forms
of these compounds with a stoichiometric amount of the appropriate base or
acid in
water or in an organic solvent, or in a mixture of the two; generally,
nonaqueous media
like ether, ethyl acetate, methanol, ethanol, isopropanol, or acetonitrile are
preferred.
Another method for the preparation of salts is by the use of ion exchange
resins. The
term "pharmaceutically acceptable salt" includes both acid addition salts and
base
addition salts, either of the parent compound or of a prodrug or solvate
thereof. The
nature of the salt is not critical, provided that it is pharmaceutically
acceptable.
Exemplary acids used in acid addition salts include, without limitation,
hydrochloric,
hydrobromic, hydroiodic, nitric, carbonic, sulfuric, sulfonic, phosphoric,
formic, acetic,
citric, tartaric, succinic, oxalic, malic, glutamic, propionic, glycolic,
gluconic, maleic,
embonic (pamoic), methanesulfonic, ethanesulfonic, 2-hydroxyethanesulfonic,
pantothenic, benzenesulfonic, toluenesulfonic, sulfanilic, mesylic,
cyclohexylaminosulfonic, stearic, algenic, P-hydroxybutyric, malonic,
galactaric,
galacturonic acid and the like. Suitable pharmaceutically acceptable base
addition salts
include, without limitation, metallic salts made from aluminium, calcium,
lithium,
magnesium, potassium, sodium and zinc or organic salts, such as those made
from
12
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
N,N'-dibenzylethylenediamine, chloroprocaine, choline, diethanolamine,
ethylenediamine, N-methylglucamine, lysine, procaine and the like. Additional
examples of pharmaceutically acceptable salts are listed in Berge et al
(1977), Journal
of Pharmaceutical Sciences, vol 66, no 1, pp 1-19, the content of which is
incorporated
herein, by reference in its entirety.
The term "solvate" refers to a physical association of a compound of this
invention with one or more solvent molecules, whether organic or inorganic.
This
physical association includes hydrogen bonding. In certain instances the
solvate will be
capable of isolation, for example when one or more solvent molecules are
incorporated
io in the crystal lattice of the crystalline solid. "Solvate" encompasses both
solution-phase
and isolable solvates. Exemplary solvates include hydrates, ethanolates,
methanolates,
hemiethanolates, and the like.
The term "pharmaceutically acceptable prodrug" means any pharmaceutically
acceptable ester, salt of an ester or any other derivative of a compound of
this
invention, which upon administration to a recipient, is capable of providing,
either
directly or indirectly, a compound of this invention or a biologically active
metabolite or
residue thereof. Particularly favored salts or prodrugs are those with
improved
properties, such as solubility, efficacy, or bioavailability of the compounds
of this
invention when such compounds are administered to a mammal (e.g., by allowing
an
20 orally administered compound to be more readily absorbed into the blood) or
which
enhance delivery of the parent compound to a biological compartment (e.g., the
brain or
lymphatic system) relative to the parent species. As used herein, a prodrug is
a drug
having one or more functional groups covalently bound to a carrier wherein
metabolic or
chemical release of the drug occurs in vivo when the drug is administered to a
mammalian subject. Pharmaceutically'acceptable prodrugs of the compounds of
this
invention include derivatives of hydroxyl groups such as, without limitation,
acyloxymethyl, acyloxyethyl and acylthioethyl ethers, esters, amino acid
esters,
phosphate esters, sulfonate and sulfate esters, and their metal salts, and the
like.
30 II. Compounds of the invention
13
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
In one aspect, the invention relates to novel dibenzodiazepinone analogues,
referred to herein as the compounds of the invention, and to pharmaceutically
acceptable salts, solvates and prodrugs thereof.
The compounds of the invention, Compounds 2 to 5, 7, 13, 14, 15, 18, and 21 to
25, may be characterized by any one of their physicochemical and spectral
properties,
such as mass and NMR spectra, detailed in Example 4 through Example 8.
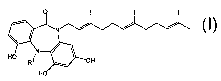
In another aspect, compounds of the invention are characterized by Formula I:
0
N ~ \
P
Ho
Ri _ OH
Ho Formula I
wherein,
R' is a linear Cl_loalkyl;
or a farnesyl derivative thereof, wherein said farnesyl derivative has one,
two or
three hydrogenated or hydroalkoxylated double bonds;
or a pharmaceutically acceptable salt or prodrug thereof.
In one embodiment, R' is a linear Cl_loalkyl, and all other groups are as
previously disclosed. In a subclass of this embodiment, the alkyl group is
optionally
substituted with a substituent selected from halo, fluoro, amino and carboxy,
or
pharmaceutically acceptable salt thereof. In another embodiment, R, is methyl.
In
another erribodiment, R' is ethyl. In another embodiment, R' is n-propyl. In
another
2o embodiment, R' is n-butyl. In another embodiment, R' is n-pentyl. In
another
embodiment, R' is n-hexyl. ,In another embodiment, R' is methyl, and the
farnesyl is
fully hydrogenated (i.e. saturated). In another embodiment, R' is methyl, and
one
double bond of the farnesyl is hydrogenated. In another embodiment, R' is
methyl, and
two double bonds of the farnesyl are hydrogenated. In another embodiment, R'
is a
linear Cl_loalkyl, and one double bond of the farnesyl is hydrogenated. In
another
embodiment, R' is a linear Cl_loalkyl, and two double bonds of the farnesyl
are
hydrogenated. In another embodiment, R' is a linear Cl_loalkyl, and the
farnesyl is fully
hydrogenated (i.e. saturated). In another embodiment, R' is a linear
Cl_loalkyl, and one
14
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
double bond of the farnesyl is hydromethoxylated. In another embodiment, R, is
a
linear Cl_loalkyl, and two double bonds of the farnesyl are hydromethoxylated.
In
another embodiment, R' is methyl, and one double bond of the farnesyl is
hydroalkoxylated. In another embodiment, R' is methyl, and two double bonds of
the
farnesyl are hydroalkoxylated. In another embodiment, R' is ethyl, and one
double
bond of the farnesyl is hydroalkoxylated. In another embodiment, R, is ethyl,
and two
,double bonds of the farnesyl are hydroalkoxylated. The invention encompasses
all
esters or N-alkylated- derivatives, and pharmaceutically acceptable salts,
solvates and
prodrugs of the foregoing compounds. -
The following are exemplary compounds of the invention, such named
compounds are not intended to limit the scope of the invention in any way:
A. N-Alkylated products:
O O
N N
I/ N / \ OH I/ N / \ OH
OH Me OH ')
HO HO
Compound 2; Compound 3;
O
o N
\ / / / I \
N
I \ N O-OH
/ N _ / oH OH
HO OH HO
Compound 4; Compound 5;
0 0
N N
/ \ OH I / N OH OH
HO HO
Compound 6; Compound 7;
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
0 0
N N
N / \ OH N O-OH
OH OH
HO HO
Compound 8; Compound 9;
0
O N
N
\ /
NO-OH
OH OH /CFZ F2C HO
OH CF3 . I
HO CF3
Compound 10; Compound 11;
0 0
N N
OH OH
N N
OH OH Cp3
F3C HO HO
Compound 12; Compound 13;
B. Hydrogenated derivatives of N-alkylated products:
0 0
~ N ~ N
N OH N / \ OH
OH C~"{3 OH CH3 _
HO HO
Compound 14; Compound 15;
0
N
OH
N
OH CIH3 -
HO
Compound 16;
C. Hydroalkoxylated derivatives of N-alkylated products:
16
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
o O
\ N / / \ N
OCH3 OCH3
N*OH / \ OH
OH ~OH CH3
HO HO
Compound 17; Compound 18;
o O
N / N / e
OCH3 OCH3 OCH3
I/ / \ OH e / \ OH
N NI
OH ~H3 OH J _
HO / HO
Compound 19; = Compound 20;
0 0
N / \ N
OCH3 OCH3 OCH3
N OH N OH
OH OH
HO HO
Compound 21; Compound 22;
D. Other selections:
0 0
N N
N *OH N OH
OH I OH I
HO HO Br
Compound 23; Compound 24;
O O
N N
H
N / \ OAc N P O
7
oAc I OH Me Br
AcO HO
Compound 25; Compound 26; and
O
/ / \ OH
OH Me
HO Br
Compound 27;
17
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
or a pharmaceutically acceptable salt, solvate of prodrug of any one of
Compounds 2 to
27.
The invention further provides esters and N-alkylated derivatives of any of
the
foregoing compounds. Certain embodiments expressly exclude one or more of the
compounds of Formula I.
Prodrugs of the compounds of the invention include compounds wherein one or
more of the 4, 6 and 8-hydroxy groups, or any other hydroxyl group on the
molecule is
bounded to any group that, when administered to a mammalian subject, is
cleaved to
io form the free hydroxyl group. Examples of prodrugs include, but are not
limited to,
acetate, formate, hemisuccinate, benzoate, dimethylaminoacetate and
phosphoryloxycarbonyl derivatives of hydroxy functional groups;
dimethylglycine esters,
aminoalkylbenzyl esters, aminoalkyl esters or carboxyalkyl esters of hydroxy
functional
groups. Carbamate and carbonate derivatives of the hydroxy groups are also
included.
Derivatizations of hydroxyl groups also encompassed, are (acyloxy)methyl and
(acyloxy)ethyl ethers, wherein the acyl group contains an alkyl group
optionally
substituted with groups including, but not limited to, ether, amino and
carboxylic acid
functionalities, or where the acyl group is an amino acid ester. Also included
are
phosphate and phosphonate esters, sulfate esters, sulfonate esters, which are
in
2o alkylated (such as bis-pivaloyloxymethyl (POM) phosphate trimester or -
P(O)O2Et2) or
in the salt form (such as sodium phosphate ester (-P(O)O-2Na+2)). For further
examples
of prodrugs used in anticancer therapy and their metabolism, see Rooseboom et
al
(2004), Phamacol. Rev., vol 56, 53=102, incorporated herein by reference. When
the
prodrug contains an acidic or basic moiety, the prodrug may also be prepared
as its
pharmaceutically acceptable salt.
The compounds of this invention may be formulated into pharmaceutical
compositions comprised of a compound of the invention, in combination with a
pharmaceutically acceptable carrier, as discussed in Section IV below.
III. Methods of Producing Dibenzodiazepinone Analogues
18
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
A. Fermentation
The terms "farnesyl dibenzodiazepinone-producing microorganism" and
"producer of farnesyl dibenzodiazepinone," as used herein, refer to a
microorganism
that carries genetic information necessary to produce a farnesyl
dibenzodiazepinone
compound, whether or not the organism naturally produces the compound. The
terms
apply equally to organisms in which the genetic information to produce the
farnesyl
dibenzodiazepinone compound is found in the organism as it exists in its
natural
environment, and to organisms in which the genetic information is introduced
by
recombinant techniques.
io Compound is produced by isolation of the fermentation broth of
Micromonospora strains 046-ECO11 and [S01]046 as described in USSN 10/762,107,
incorporated herein by reference in its entirety. It is to be understood that
the production
of Compound 1 is not limited to the use of the particular strains 046-ECO11
and
[S01]046: Rather, other ECO-4601 producing organisms may be used, such as
mutants or variants of 046-ECO11 and [SO1]046 that can be derived from this
organism
by known means such as X-ray irradiation, ultraviolet irradiation, treatment
with nitrogen
mustard, phage exposure, antibiotic resistance selection and the like; or
through the
use of recombinant genetic engineering techniques. For examples, see Manual of
Industrial Microbiology and biotechnology, Demain and Solomon, American
Society for
20 Microbiology, Washington D.C., 1986; Hesketh et al. (1997), J. Antibiotics,
vol 50, no 6,
532-535; and Hosoya et al. (1998), Antimicrobial Agents and Chemotherapy, vol
42, no 8,
2041-2047), the content of which are incorporated herein by reference in their
entirety.
The farnesyl dibenzodiazepinone compound may be biosynthesized by various
microorganisms. Microorganisms that may synthesize the farnesyl
dibenzodiazepinone
compound include but are not limited to bacteria of the order Actinomycetales,
also
referred to as actinomycetes. Non-limiting examples of members belonging to
the
genera of Actinomycetes include Nocardia, Geodermatophilus, Actinoplanes,
Micromonospora, Nocardioides, Saccharothrix, Amycolatopsis, Kutzneria,
Saccharomonospora, Saccharopolyspora, Kitasatospora, Streptomyces,
Microbispora,
30 Streptosporangium, and Actinomadura. The taxonomy of actinomycetes is
complex
and reference is made to Goodfellow, Suprageneric Classification of
Actinomycetes
(1989); Bergey's Manual of Systematic Bacteriology, Vol. 4 (Williams and
Wilkins,
Baltimore, pp. 2322-2339); and to Embley and Stackebrandt, "The molecular
phylogeny
19
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
and systematics of the actinomycetes," Annu. Rev. Microbiol. (1994) 48:257-
289, for
genera that may synthesize the compounds of the invention. The content of
which
refrences is incorporated by reference in their entirety.
Farnesyl dibenzodiazepinone-producing microorganisms are cultivated in culture
medium containing known nutritional sources for actinomycetes. Such media
having
assimilable sources of carbon, nitrogen, plus optional inorganic salts and
other known
growth factors, at a pH of about 6 to about 9. Suitable media include, without
limitation,
the growth media provided in Table 1. Microorganisms are cultivated at
incubation
temperatures of about 18 C to about 40 C for about 3 to about 40 days.
Table I
Examples of Fermentation Media for Compound 1 Production
Component QB MA KH RM JA FA XX CL
pH*' 7.2 7.5 7 6.85 7.3 7.0 7.0 7.0
Glucose 12 10 10 10
Sucrose 100
Cane molasses 15
Corn starch -30
Soluble starch 10 25
Potato dextrin 20 40 20 20
Corn steep solid 5
Corn steep liquor 5 15
Dried yeast 2
Yeast extract 5 8.34
Malt extract 35
PharmamediaTM 10 15
Glycerol 30 20
NZ-Amine A 5 10
Soybean powder 15
Fish meal 10
Bacto-peptone 2.5 5
MgSOd.7H2O I
CaCO3 4 1 2 2 3 2
NaCI 5 (NH4)2 S04 2 2
K2 SO4 0.25
MgC12.6H20 10
Na2HPO4 3
Casamino acid 0.1
Proflo oilTM (mL/L) 4 0.05
Silicon defoamer
oil (mL/L) 0.3
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
Table I
Examples of Fermentation Media for Compound 1 Production
Component QB MA KH RM JA FA XX CL
MOPS 21
Trace element
solution *2 mI/L 2
Unless otherwise indicated all the ingredients are in g/L.
*1 The pH is to adjusted as marked prior to the addition of CaCO3.
*2 Trace elements solution contains: ZnCI2 40 mg; Fe CI3 6H2O (200 mg);
CuC12.2H20 (10 mg);
MnCI2.4H20; Na2B4O7.10Hz0 (10mg); (NH4) 6 M07024 .4H20 (10 mg) per litre.
The culture media inoculated with a farnesy dibenzodiazepinone-producing
microorganism may be aerated by incubating the inoculated culture media with
agitation, for example, shaking on a rotary shaker, a shaking water bath, or
in a
fermentor. Aeration may also be achieved by the injection of air, oxygen or an
io appropriate gaseous mixture to the inoculated culture media during
incubation.
Following cultivation, the farnesyl dibenzodiazepinone compound can be
extracted and
isolated from the cultivated culture media by techniques known to a person
skilled in
the art and/or disclosed herein, including for example centrifugation,
chromatography,
adsorption, filtration. For example, the cultivated culture media can be
optionally
acidified and mixed with a suitable organic solvent such as methanol, ethanol,
n-
butanol, ethyl acetate, n-butyl acetate or 4-methyl-2-pentanone. The organic
layer can
be separated from the mycelial cake for example, by centrifugation and
decantation or
filtration. The mycelial cake is further optionally extracted with an organic
solvent, and
the organic extracts combined. The organic layer is further optionally
treated, for
2o example by: aqueous washings, precipitation, filtration and the like,
followed the
removal of the solvent, for example, by evaporation to dryness under vacuum.
The
resulting residue can optionally be reconstituted with for example water,
ethyl ether,
ethanol, ethyl acetate, methanol or a mixture thereof, and re-extracted in a
two-phase
system with a suitable organic solvent such as hexane, carbon tetrachloride,
methylene
chloride or a mixture thereof. After removal of the solvent, the compound can
be
further purified by the use of standard techniques such as normal and reverse-
phase
liquid chromatography, crystallization, sublimation, adsorption, mass
exclusion
chromatography, and the like.
21
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
B. Chemical Modifications:
The farnesyl dibenzodiazepinone Compound 1 is biosynthesized by
microorganisms and isolated as described herein, and in Canadian patent
2,466,340
(and PCT/CA04/000069). Compound 1 is subjected to random and/or directed
chemical modifications to form compounds that are derivatives or structural
analogues.
Such derivatives or structural analogues having similar functional activities
are within
the scope.of the present invention. The farnesyl dibenzodiazepinone may be
modified
by one or more chemical modification steps, using methods known in the art and
described herein. Examples of chemical modifications procedures are also
provided in
io Examples 4 to 8.
Dibenzodiazepinone analogues that are derivatives of Compound 1, for example
those identified herein as the compounds of Formula I and their derivatives,
and
Compounds 2 to 27, are generated by standard organic chemistry approaches.
General principles of organic chemistry required for making and manipulating
the
compounds described herein, including functional moieties, reactivity and
common
protocols are described, for example, in "Advanced Organic Chemistry," 4th
Edition by
Jerry March (1992), Wiley-Interscience, USA, incorporated herein by reference
in its
entirety. In addition, it will be appreciated by one of ordinary skill in the
art that the
synthetic methods described herein may use a variety of protecting groups,
whether or
2o not they are explicitly described. A "protecting group" as used herein
means a moiety
used to block one or more functional moieties such as reactive groups
including
oxygen, sulfur or nitrogen, so that a reaction can be carried out selectively
at another
reactive site in a polyfunctional compound. General principles for the use of
protective
groups, their applicability to specific functional groups and their uses are
described for
example in T. H. Greene and P. G. M. Wuts, Protective Groups in Organic
Synthesis,
3rd Edition, John Wiley & Sons, New York (1999), incorporated herein by
reference in
its entirety.
Alcohols and phenols are protected, if necessary with, for example: silyl
ethers
(TMS: trimethylsilyl, TIPS: triisopropylsilyl), acetals (MOM: methyloxymethyl,
BOM:
3o benzyloxymethyl), esters (acetate, benzoyl) and ethers (Bn: benzyl).
Alcohols are
deprotected by conditions such as: TBAF (tetrabutylammonium fluoride) for
silyl ethers,
aqueous acid catalysis for acetals and esters, saponification for esters, and
hydrogenolysis for Bn and BOM. Amine is protected, if necessary, using
standard
22
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
amino acid protecting groups, for example, carbamates (such as t-butyl (BOC)
and
benzyl (CBZ)), fluorene derivatives (such as FMOC: N-(9-
fluorenylmethoxycarbonyl)-),
etc. Amine is deprotected by conditions such as: acid hydrolysis for BOC,
hydrogenolysis for CBZ, or base treatment for FMOC. All protection and
deprotection
conditions are demonstrated in the Greene et al reference above.
Those skilled in the art will readily appreciate that many synthetic chemical
processes may be used to produce derivatives of Compound 1. The following
schemes
are exemplary of the routine chemical modifications that may be used to
produce
compounds of the invention. Any chemical synthetic process known to a person
skilled
io in the art providing the structures described herein may be used and are
therefore
comprised in the present invention.
Scheme 1: Alcohol(s) modifications (0-acylations)
OH ~ O R"
(a) \ yy
O
wherein, R" is an acetate.
In Scheme 1, phenolic alcohols are converted to esters when reacted with
activated carboxylic acids (R"C(O)X) such as an acid halide, anhydride, N-
hydroxysuccinimide ester, or a carboxylic acid activated by a coupling agent
(e.g.: EDC
(1-(3-dimethylaminopropyl)-3-d'iisopropylethylcarbodiimide hydrochloride); or
HATU (0-
20 (7-azabenzotriazol-l-yl)-N,N,N;N=tetramethyluronium hexafluorophosphate))
with a
base (e.g., pyridine or N,N-diisopropylethylamine (DIPEA)) and optional
acatalysts such
HOBt (1-hydroxybenzotriazole hydrate) and/or DMAP (4-(dimethylamino)pyridine).
The
same reactions may be accomplished on alcohols formed by farnesyl modification
reactions (Scheme 3).
Scheme 1 is used to obtain, for example, Compounds 25 from Compound 1; and
to produce esters of the Compounds of the invention.
23
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
Scheme 2: Amine modifications (N-alkylations)
~'NH (a) NR
I
rw~r .rv~n~
wherein, R is selected an optionally substituted linear Cl_loalkyl.
In Scheme 2, amine group in position 5 (for position, see Example 3) is
alkylated. In Scheme 2(a), an amine is alkylated using an RX alkylating agent
such as
dialkyl sulfates and alkyl halides, preferably in the presence of a base
(e.g., sodium
bicarbonate, pyridine and the like).
Scheme 2 is used to prepare, for example, Compounds 2 to 13 from Compound
1, and Compound 14 from Compound 23; and to produce any of the Compounds of
io Formula I comprising an N-alkyl group.
Scheme 3: Double bond(s) modifications
H
(a)
H H
H Rz
(b)
H H
wherein R' is OCI_6alkyl.
In Scheme 3, double bond is modified by: (a) hydrogenation; and (b)
hydroalkoxylation. In (a) hydrogenation is carried out using a hydrogen source
(e.g.
hydrogen, formic acid) and a catalyst (such as rhodium, platinum, or
palladium). In (b),
electrophilic addition to the double bond is achieved by the formation of a
carbocation
from addition of a proton in ac'idic conditions (e.g., p-toluene sulfonic
acid, alkyl
20 sulfate/NaHCO3/MeOH, and the like), and trapping of the carbocation with an
alcohol
(C1_6alkyl alcohol, hydroalkoxylation).
Scheme 3 is used to obtain, for example: in (a) Compound 23 from Compound
1, and Compounds 14 to 16 from Compound 2; in (b) Compounds 17 to 19 from
Compound 2, Compounds 20 to 22 from Compound 3. Schemes 3 (a) and (b) are also
24
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
used to produce any hydrogenated and hydroalkoxylated farnesyl derivative of
the
Compounds of Formula I.
Scheme 4: Aromatic substitutions
H X
Jv1nn Jv~nr
wherein, X is Br.
In Scheme 4, the aryl group is modified by aromatic substitutions
(bromination).
brominating agents include bromine, N-haloamides (e.g, N-bromosuccinimide
(NBS),
tetraalkylammonium polyhalides).
Scheme 4 is used to prepare Compound 24 from Compound 1, Compound 26
from Compound 2 and Compound 27 from Compound 14.
Prodrugs are prepared by routine chemical modifications such as described in
Jerry March, supra, including esterification (Scheme 1) and alkylation
reactions, i.e.,
use of activated acids or mixed anhydrides (acyl halides, use of coupling
reagents, etc),
and by the use of alkylating agents (R-X, wherein X is a leaving group, suchas
diazo,
and R is the desired group). Phosphate prodrugs are prepared by
phosphorylation, for
example, with dialkyl phosphites using procedure such as described in
Silverberg et al.
(1996), Tet. Lett., Vol. 37, 711-774, U.S. patent 5,561,122 (Pettit et al) and
in Hwang
2o and Cole (2004), Org. Lett., vol 6, no 10, 1555-1556 ((POM)2phosphate
triester from
(POM)2phosphoryl chloride), the content of which is incorporated herein by
reference in
their entirety.
IV. Pharmaceutical compositions comprising the compounds of the invention
The invention provides a pharmaceutical composition comprising a compound of
the invention, or a pharmaceutically acceptable salt, solvate or prodrug
thereof, in
combination with a pharmaceutically acceptable carrier. The pharmaceutical
composition comprising a dibenzodiazepinone analogue is useful for treating
diseases
and disorders associated with uncontrolled cellular growth and proliferation,
such as a
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
neoplastic condition. The pharmaceutical composition is also useful in
treating other
diseases and disorders, including inflammation, autoimmune diseases,
infections,
neurodegenerative diseases and stress. The pharmaceutical composition
comprising a
dibenzodiazepinone analogue may be packaged into a convenient commercial
package
providing the necessary materials, such as the pharmaceutical composition and
written
instructions for its use in treating a neoplastic condition, in a suitable
container.
The compounds of the present invention, or pharmaceutically acceptable salts,
solvates or prodrugs thereof, can be formulated for oral, sublingual,
intranasal,
intraocular, rectal, transdermal, mucosal, topical or parenteral
administration for the
io therapeutic or prophylactic treatment of neoplastic and proliferative
diseases and
disorders. Parenteral modes of administration include without limitation,
intradermal,
subcutaneous (s.c., s.q., sub-Q, Hypo), intramuscular (i.m.), intravenous
(i.v.),
intraperitoneal (i.p.), intra-arterial, intramedulary, intracardiac, intra-
articular (joint),
intrasynovial (joint fluid area), intracerebral or intracranial, intraspinal,
intracisternal, and
intrathecal (spinal fluids). Any known device useful for parenteral injection
or infusion of
drug formulations can be used to effect such administration. For oral and/or
parental
administration, compounds of the present invention can be mixed with
conventional
pharmaceutical carriers and excipients and used in the form of solutions,
emulsions,
tablets, capsules, soft gels, elixirs, suspensions, syrups; wafers and the
like. The
20 compositions comprising a compound of the present invention will contain
from about
0.1 % to about 99.9%, about 1% to about 98%, about 5% to about 95%, about 10%
to
about 80% or about 15% to about 60% by weight of the active compound.
The pharmaceutical preparations disclosed herein are prepared in accordance
with standard procedures and are administered at dosages that are selected to
reduce,
prevent, or eliminate cancer. (See, e.g., Remington's Pharmaceutical Sciences,
Mack
Publishing Company, Easton, PA; and Goodman and Gilman, Pharmaceutical Basis
of
Therapeutics, Pergamon Press, New York, NY, the contents of which are
incorporated
herein by reference, for a general description of the methods for
administering various
agents for human therapy).
30 As used herein, the term "unit dosage" refers to physically discrete units
suitable
as unitary dosages for human subjects and other mammals, each unit containing
a
predetermined quantity of dibenzodiazepinone analogue calculated to produce
the
desired therapeutic effect, in association with a suitable pharmaceutically
acceptable
26
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
carriers. In one embodiment, the unit dosage contains from 10 to 3000 mg of
active
ingredient. In another embodiment, the unit dosage contains 20 to 1000 mg of
active
ingredient. The compositions of the present invention can be delivered using
controlled
(e.g., capsules) or sustained release delivery systems (e.g., bioerodable
matrices).
Exemplary delayed release delivery systems for drug delivery that are suitable
for
administration of the compositions of the invention are described in U.S.
Patent Nos
4,452,775 (issued to Kent), 5,039,660 (issued to Leonard), and 3,854,480
(issued to
Zaffaroni), incorporated herein by reference in their entirety.
The pharmaceutically-acceptable compositions of the present invention comprise
lo one or more compounds of the present invention in association with one or
more non-
toxic, pharmaceutically-acceptable carriers and/or diluents and/or adjuvants
and/or
excipients, collectively referred to herein as "carrier" materials, and if
desired other
active ingredients. Pharmaceutically acceptable carriers include, for example,
solvents,
vehicles or medium such as saline, buffered saline, dextrose, water, glycerol,
ethanol,
propylene glycol, polysorbate 80 (Tween-80T"'), poly(ethylene) glycol 300 and
400
(PEG 300 and 400), PEGylated castor oil (E.g. Cremophor EL), poloxamer 407 and
188, hydrophobic carriers, and combinations thereof. Hydrophobic carriers
include, for
example, fat emulsions, lipids, PEGylated phopholids, polymer matrices,
biocompatible
polymers, lipospheres, vesicles, particles, and liposomes. The term
specifically
2o excludes cell culture medium.
Excipients or additives included in a formulation have different purposes
depending, for example on the nature of the drug, and the mode of
administration.
Examples of generally used excipients include, without limitation: stabilizing
agents,
solubilizing agents and surfactants, buffers, antioxidants and preservatives,
tonicity
agents, bulking agents, lubricating agents, emulsifiers, suspending or
viscosity agents,
inert diluents, fillers, disintegrating agents, binding agents, wetting
agents, lubricating
agents, antibacterials, chelating agents, sweetners, perfuming agents,
flavouring
agents, coloring agents, administration aids, and combinations thereof.
The compositions may contain common carriers and excipients, such as
30 cornstarch or gelatin, lactose, sucrose, microcrystalline cellulose,
kaolin, mannitol,
dicalcium phosphate, sodium chloride and alginic acid. The compositions'may
contain
crosarmellose sodium, microcrystalline cellulose, sodium starch glycolate and
alginic
acid.
27
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
Formulations for parenteral administration can be in the form of aqueous or
non-
aqueous isotonic sterile injection solutions, suspensions or fat emulsions,
comprising a
compound of this invention, or a pharmaceutically acceptable salt or prodrug
thereof.
The parenteral form used for injection must be fluid to the extent that easy
syringability
exists. These solutions or suspensions can be prepared from sterile
concentrated
liquids, powders or granules. The compounds can be dissolved in a carrier such
as a
solvent or vehicle, for example, polyethylene glycol, propylene glycol,
ethanol, corn oil,
benzyl alcohol, glycofurol, N,N-dimethylacetamide, N-methylpyrrolidone,
glycerine,
saline, dextrose, water, glycerol, hydrophobic carriers, and combinations
thereof.
Excipients used in parenteral preparations also include, without limitation,
stabilizing agents (e.g. carbohydrates, amino acids and polysorbates),
solubilizing
agents (e.g. cetrimide, sodium docusate, glyceryl monooleate,
polyvinylpyrolidone
(PVP) and polyethylene glycol (PEG)) and surfactants (e.g. polysorbates,
tocopherol
PEG succinate, poloxamer and CremophorT"'), buffers (e.g. acetates, citrates,
phosphates, tartrates, lactates, succinates, amino acids and the like),
antioxidants and
preservatives (e.g. BHA, BHT, gentisic acids, vitamin E, ascorbic acid and
sulfur
containing agents such as sulfites, bisulfites, metabisulfites, thioglycerols,
thioglycolates
and the like), tonicity agents (for adjusting physiological compatibility),
suspending or
viscosity agents, antibacterials (e.g. thimersol, benzethonium chloride,
benzalkonium
chloride, phenol, cresol and chlorobutanol), chelating agents, and
administration aids
(e.g. local anesthetics, anti-inflammatory agents, anti-clotting agents, vaso-
constrictors
for prolongation and agents that increase tissue permeability), and
combinations
thereof.
Parenteral formulations using hydrophobic carriers include, for example, fat
emulsions and formulations containing lipids, lipospheres, vesicles, particles
and
liposomes. Fat emulsions include in addition to the above-mentioned
excipients, a lipid
and an aqueous phase, and additives such as emulsifiers (e.g. phospholipids,
poloxamers, polysorbates, and polyoxyethylene castor oil), and osmotic agents
(e.g.
sodium chloride, glycerol, sorbitol, xylitol and glucose). Liposomes include
natural or
3o derived phospholipids and optionally stabilizing agents such as
cholesterol.
In another embodiment, the parenteral unit dosage form of the compound can be
a ready-to-use solution of the compound in a suitable carrier in sterile,
hermetically
sealed ampoules or in sterile pre-loaded syringes. The suitable carrier
optionally
28
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
comprises any of the above-mentioned excipients.
Alternatively, the unit dosage of the compound of the present invention can be
in
a concentrated liquid, powder or granular form for ex tempore reconstitution
in the
appropriate pharmaceutically acceptable carrier at the time of delivery. In
addition the
above-mentioned excipients, powder forms optionally include bulking agents
(e.g.
mannitol, glycine, lactose, sucrose, trehalose, dextran, hydroxyethyl starch,
ficoll and
gelatin), and cryo or lyoprotectants.
For example, in intravenous (IV) use, a sterile formulation of the compound of
formula I and optionally one or more additives, including solubilizers or
surfactants, can
io be dissolved or suspended in any of the commonly Used intravenous fluids
and
administered by infusion. Intravenous fluids include, without limitation,
physiological
saline, phosphate buffered saline, 5% glucose or Ringer'STM solution.
In another example, in intramuscular preparations, a sterile formulation of
the
compound of the present invention or suitable soluble salts or prodrugs
forming the
compound, can be dissolved and administered in a pharmaceutical diluent such
as
Water-for-Injection (WFI), physiological saline or 5% glucose. A suitable
insoluble form
of the compound may be prepared and administered as a suspension in an aqueous
base or a pharmaceutically acceptable oil base, e.g. an ester of a long chain
fatty acid
such as ethyl oleate.
20 For oral use, solid formulations such as tablets and capsules are
particularly
useful. Sustained released or enterically coated preparations may also be
devised.
For pediatric and geriatric applications, suspension, syrups and chewable
tablets are
especially suitable. For oral administration, the pharmaceutical compositions
are in the
form of, for example, tablets, capsules, suspensions or liquid syrups or
elixirs, wafers
and the like. For general oral administration, excipient or additives include,
but are not
limited to inert diluents, fillers, disintegrating agents, binding agents,
wetting agents,
lubricating agents, sweetening agents, flavoring agents, coloring agents and
preservatives.
The oral pharmaceutical composition is preferably made in the form of a unit
3o dosage containing a therapeutically-effective amount of the active
ingredient.
Examples of such dosage units are tablets and capsules. For therapeutic
purposes,
the tablets and capsules which can contain, in addition to the active
ingredient,
29
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
conventional carriers such as: inert diluents (e.g., sodium and calcium
carbonate,
sodium and calcium phosphate, and lactose), binding agents (e.g., acacia gum,
starch,
gelatin, sucrose, polyvinylpyrrolidone (Providone), sorbitol, or tragacanth
methylcellulose, sodium carboxymethylcellulose, hydroxypropyl methylcellulose,
and
ethylcellulose), fillers (e.g., calcium phosphate, glycine, lactose, maize-
starch, sorbitol,
or sucrose), lubricants or lubricating agents (e.g., magnesium stearate or
other metallic
stearates, stearic acid, polyethylene glycol, waxes, oils, silica and
colloical silica, silicon
fluid or talc), disintegrants or disintegrating agents (e.g., potato starch,
corn starch and
alginic acid), flavouring, coloring agents, or acceptable wetting agents.
Carriers may
io also include coating excipients such as glyceryl monostearate or glyceryl
distearate, to
delay absorption in the gastrointestinal tract.
Oral liquid preparations, generally in the form of aqueous or oily solutions,
suspensions, emulsions, syrups or elixirs, may contain conventional additives
such as
suspending agents, emulsifying agents, non-aqueous agents, preservatives,
coloring
agents and flavoring agents. Examples of additives for liquid preparations
include
acacia, almond oil, ethyl alcohol, fractionated coconut oil, gelatin, glucose
syrup,
glycerin, hydrogenated edible fats, lecithin, methyl cellulose, methyl or
propyl para-
hydroxybenzoate, propylene glycol, sorbitol, or sorbic acid.
For both liquid and solid oral preparations, flavoring agents such as
peppermint,
20 oil of wintergreen, cherry, grape, fruit flavoring or the like can also be
used. It may also
be desirable to add a coloring agent to make the dosage form more aesthetic in
appearance or to help identify the product. For topical use the compounds of
present
invention can also be prepared in suitable forms to be applied to the skin, or
mucus
membranes of the nose and throat, and can take the form of creams, ointments,
liquid
sprays or inhalants, lozenges, or throat paints. Such topical formulations
further can.
include chemical compounds such as dimethylsulfoxide (DMSO) to facilitate
surface
penetration of the active ingredient. For application to the eyes or ears, the
compounds
of the present invention can be presented in liquid or semi-liquid form
formulated in
hydrophobic or hydrophilic bases as ointments, creams, lotions, paints or
powders. For
3o rectal administration the compounds of the present invention can be
administered in the
form of suppositories admixed with conventional carriers such as cocoa butter,
wax or
other glyceride.
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
V. Medical Use in the Treatment of Neoplasms
In one aspect, the invention relates to a method for inhibiting growth and/or
proliferation of cancer cells in a mammal. In another aspect, the invention
provides a
method for treating neoplasms in a mammal. Mammals include ungulates (e.g.
sheeps,
goats, cows, horses, pigs), and non-ungulates,'including rodents, felines,
canines and
primates (i.e. human and non-human primates). In a preferred embodiment, the
mammal is a human.
The dibenzodiazepinone analogues of the present invention may bind to or
interact with other cancer-associated proteins and polypeptides, including,
without
io limitation, polypeptides encoded by oncogenes, polypeptides that induce
angiogenesis,
proteins involved in metastasizing and/or invasive processes, and proteases
that
regulate apoptosis and the cell cycle. Regardless of the mechanism of action,
the
dibenzodiazepinone analogues of the invention have been demonstrated to
exhibit anti-
cancer activity both in vitro and in vivo. Based on these discoveries,
applicants have
developed methods for treating neoplasms.
As used herein, the terms "neoplasm", "neoplastic disorder", "neoplasia"
"cancer," "tumor" and "proliferative disorder" refer to cells having the
capacity for
autonomous growth, i.e., an abnormal state of condition characterized by
rapidly
proliferating cell growth which generally forms a distinct mass that show
partial or total
20 lack of structural organization and functional coordination with normal
tissue. The
terms are meant to encompass hematopoietic neoplasms (e.g. lymphomas or
leukemias) as well as solid neoplasms (e.g. sarcomas or carcinomas), including
all
types of pre-cancerous and cancerous growths, or oncogenic processes,
metastatic
tissues or malignantly transformed cells, tissues, or organs, irrespective of
histopathologic type or stage of invasiveness. Hematopoietic neoplasms are
malignant
tumors affecting hematopoietic structures (structures pertaining to the
formation of
blood cells) and components of the immune system, including leukemias (related
to
leukocytes (white blood cells) and their precursors in the blood and bone
marrow)
arising from myeloid, lymphoid or erythroid lineages, and lymphomas (relates
to
30 lymphocytes). Solid neoplasms include sarcomas, which are malignant
neoplasms that
originate from connective tissues such as muscle, cartilage, blood vessels,
fibrous
tissue, fat or bone. Solid neoplasms also include carcinomas, which are
malignant
neoplasms arising from epithelial structures (including external epithelia
(e.g., skin and
31
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
linings of the gastrointestinal tract, lungs, and cervix), and internal
epithelia that line
various glands (e.g., breast, pancreas, thyroid). Examples of neoplasms that
are
particularly susceptible to treatment by the methods of the invention include
leukemia,
and hepatocellular cancers, sarcoma, vascular endothelial cancers, breast
carcers,
central nervous system cancers (e.g. astrocytoma, gliosarcoma, neuroblastoma,
oligodendroglioma and glioblastoma), prostate cancers, lung and bronchus
cancers,
larynx cancers, esophagus cancers, colon cancers, colorectal cancers, gastro-
intestinal
cancers, melanomas, ovarian and endometrial cancer, renal and bladder cancer,
liver
cancer, endocrine cancer (e.g. thyroid), and pancreatic cancer.
The dibenzodiazepinone analogue is brought into contact with or introduced
into
a cancerous cell or tissue. In general, the methods of the invention for
delivering the
compositions of the invention in vivo utilize art-recognized protocols for
delivering
therapeutic agents with the only substantial procedural modification being the
substitution of the dibenzodiazepinone analogue of the present invention for
the
therapeutic agent in the art-recognized protocols. The route by which the
dibenzodiazepinone analogue is administered, as well as the formulation,
carrier or
vehicle will depend on the location as well as the type of the neoplasm. A
wide variety
of administration routes can be employed. The dibenzodiazepinone analogue may
be
administered by intravenous or intraperitoneal infusion or injection. For
example, for a
solid neoplasm that is accessible, the compound of the invention may be
administered
by injection directly into the neoplasm. For a hematopoietic neoplasm the
compound
may be administered intravenously or intravascularly. For neoplasms that are
not
easily accessible within the body, such as metastases or brain tumors, the
compound
may be administered in a manner such that it can be transported systemically
through
the body of the mammal and thereby reach the neoplasm and distant metastases
for
example intrathecally, intravenously or intramuscularly or orally.
Alternatively, the
compound can be administered directly to the tumor. The compound can also be
administered subcutaneously, intraperitoneally, topically (for example for
melanoma),
rectally (for example colorectal neoplasm) vaginally (for example for cervical
or vaginal
3o neoplasm), nasally or by inhalation spray (for example for lung neoplasm).
The dibenzodiazepinone analogue is administered in an amount that is
sufficient
to inhibit the growth or proliferation of a neoplastic cell, or to treat a
neoplastic disorder.
The term "inhibition" refers to suppression, killing, stasis, or destruction
of cancer cells.
32
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
The inhibition of mammalian cancer cell growth according to this method can be
monitored in several ways. Cancer cells grown in vitro can be treated with the
compound and monitored for growth or death relative to the same cells cultured
in the
absence of the compound. A cessation of growth or a slowing of the growth rate
(i.e.,
the doubling rate), e.g., by 50% or more at 100 micromolar, is indicative of
cancer cell
inhibition (see Anticancer Drug Development Guide: preclinical screening,
clinical trials
and approval; B.A. Teicher and P.A. Andrews, ed., 2004, Humana Press, Totowa,
NJ).
Alternatively, cancer cell inhibition can be monitored by administering the
compound to
an animal model of the cancer of interest. Examples of experimental non-human
lo animal cancer models are known in the art and described below and in the
examples
herein. A cessation of tumor growth (i.e., no further increase in size) or a
reduction in
tumor size (i.e., tumor volume by least a 58%) in animals treated with the
compound
relative to tumors in control animals not treated with the compound is
indicative of
significant tumor growth inhibition (see Anticancer Drug Development Guide:
preclinical
screening, clinical trials and approval; B.A. Teicher and P.A. Andrews, ed.,
2004,
Humana Press, Totowa, NJ).
The term "treatment" refers to the application or administration of a
dibenzodiazepinone analogue to a mammal, or application or administration of a
dibenzodiazepinone analogue to an isolated tissue or cell line from a mammal,
who has
2a a neoplastic disorder, a symptom of a neoplastic disorder or a
predisposition toward a
neoplastic disorder, with the purpose to cure, heal, alleviate, relieve,
alter, ameliorate,
improve, or control the disorder, the symptoms of disorder, or the
predisposition toward
disorder. The term "treating" is defined as administering, to a mammal, an
amount of a
dibenzodiazepinone analogue sufficient to result in the prevention, reduction
or
elimination of neoplastic cells in a mammal ("therapeutically effective
amount"). The
therapeutically effective amount and timing of dosage will be determined on an
individual basis and may be based, at least in part, on consideration of the
age, body
weight, sex, diet and general health of the recipient subject, on the nature
and severity
of the disease condition, and on previous treatments and other diseases
present.
30 Other factors also include the route and frequency of administration, the
activity of the
administered compound, the metabolic stability, length of action and excretion
of the
compound, drug combination, the tolerance of the recipient subject to the
compound
and the type of neoplasm or proliferative disorder. In one embodiment, a
33
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
therapeutically effective amount of the compound is in the range of about 0.01
to about
750 mg/kg of body weight of the mammal. In another embodiment, the
therapeutically
effective amount is in the range of about 0.01 to about 300 mg/kg body weight
per day.
In yet another embodiment, the therapeutically effective amount is in the
range of 10 to
about 50 mg/kg body weight per day. The therapeutically effective doses of the
above
embodiments may also be expressed in milligrams per square meter (mg/m) in the
case of a human patient. Conversion factors for different mammalian species
may be
found in:Freireich et al, Quantitative comparison of toxicity of anticancer
agents in
mouse, rat, dog, monkey and man,. Cancer Chemoth. Report, 1966, 50(4): 219-
244,
lo incorporated herein by reference in its entirety. When special requirements
may be
needed (e.g. for children patients), the therapeutically effective doses
described above
may be outside the ranges stated herein. Such higher or lower doses are within
the
scope of the present invention.
To monitor the efficacy of tumor treatment in a human, tumor size and/or tumor
morphology is measured before and after initiation of the treatment, and
treatment is
considered effective if either the tumor size ceases further growth, or if the
tumor is
reduced in size, e.g., by at least 10% or more (e.g., 20%, 30%, 40%, 50%, 60%,
70%,
80%, 90% or even 100%, that is, the absence of the tumor). Prolongation of
survival,
time-to-disease progression, partial response and objective response rate are
surrogate
20 measures of clinical activity of the investigational agent. Tumor shrinkage
is considered
to be one treatment-specific response. This system is limited by the
requiremenf that
patients have visceral masses that are amenable to accurate measurement.
Methods of
determining the size of a tumor in vivo vary with the type of tumor, and
include, for
example, various imaging techniques well known to those in the medical imaging
or
oncology fields (MRI, CAT, PET, etc.), as well as histological techniques and
flow
cytometry. For certain types of cancer, evaluation of serum tumor markers are
also
used to evaluate response (eg prostate-specific antigen (PSA) for prostate
cancer, and
carcino-embryonic antigen (CEA), for colon cancer). Other methods of
monitoring
cancer growth include cell counts (e.g. in leukemias) in blood or relief in
bone pain (e.g.
30 prostate cancer).
The dibenzodiazepinone compound may be administered once daily, or the
compound may be administered as two, three, four, or more sub-doses at
appropriate
intervals throughout the day. In that case, the dibenzodiazepinone compound
34
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
contained in each sub-dose must be correspondingly smaller in order to achieve
the
total daily dosage. The dosage unit can also be compounded for delivery over
several
days, e.g., using a conventional sustained release formulation which provides
sustained
release of the dibenzodiazepinone compound over a several day period.
Sustained
release formulations are well known in the art. In this embodiment, the dosage
unit
contains a corresponding multiple of the daily dose. The effective dose can be
administered either as a single administration event (e.g., a bolus injection)
or as a slow
injection or infusion, e.g. over 30 minutes to about 24 hours. The compound
may be
administered as a treatment, for up to 30 days. Moreover, treatment of a
subject with a
lo therapeutically effective amount of a composition can include a single
treatment or a,
series of treatments (e.g., a four-week treatment repeated 3 times, with a 2
months
interval between each treatment). Estimates of effective dosages, toxicities
and in vivo
half-lives for the dibenzodiazepinone compounds encompassed by the invention
can be
made using conventional methodologies or on the basis of in vivo testing using
an
appropriate animal model.
The dibenzodiazepinone compound may be administered in conjunction with or
in addition to known other anticancer treatments such as radiotherapy, or
other known
anticancer.compounds or chemotherapeutic agents. Such agents include, but are
not
limited to, 5-flurouracil, mitomycin C, methotrexate, hydroxyurea,
cyclophosphamide,
2o dacarbazine, mitoxantrone, anthracyclines (Epirubicin and Doxurubicin),
etopside,
pregnasome, platinum compounds such as carboplatin and cisplatin, taxanes such
as
PaclitaxelT"' and DocetaxelT"'; hormone therapies such as tamoxifen and anti-
estrogens; antibodies to receptors, such as herceptin and Iressa; aromatase
inhibitors,
progestational agents and LHRH analogues; biological response modifiers such
as IL2
and interferons; multidrug reversing agents such as the cyclosporin analogue
PSC
833.
Toxicity and therapeutic efficacy of dibenzodiazepinone compounds can be
determined by standard pharmaceutical procedures in cell cultures or
experimental
animals. Therapeutic efficacy is determined in animal models as described
above and
30 in the examples herein. Toxicity studies are done to determine the lethal
dose for 10%
of tested animals (LD10). Animals are treated at the maximum tolerated dose
(MTD):
the highest dose not producing mortality or greater than 20% body weight loss.
The
effective dose (ED) is related to the MTD in a given tumor model to determine
the
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
therapeutic index of the compound. A therapeutic index (MTD/ED) close to 1.0
has
been found to be acceptable for some chemotherapeutic drugs, a preferred
therapeutic
index for classical chemotherapeutic drugs is 1.25 or higher.
The data obtained from cell culture assays and animal studies can be used in
formulating a range of dosage for use 'in humans. The dosage of compositions
of the
invention will generally be within a range of circulating concentrations that
include the
MTD. The dosage may vary within this range depending upon the dosage form
employed and the route of administration utilized. For any compound used in
the
method of the invention, the therapeutically effective dose can be estimated
initially
io from cell culture assays. A dose may be formulated in animal models to
achieve a
circulating plasma concentration range of the compound. Such information can
be
used to more accurately determine useful doses in humans. Levels in plasma may
be
measured, for example, by HPLC.
Animal models to determine antitumor efficacy of a compound are generally
carried out in mice. Either murine tumor cells are inoculated subcutaneously
into the
hind flank of mice from the same species (syngeneic models) or human tumor
cells are
inoculated subcutaneously into the hind flank of severe combined immune
deficient
(SCID) mice or other immune deficient mice (nude mice) (xenograft models).
Advances in mouse genetics have generated a number of mouse models for the
20 study of various human diseases including cancer. The MMHCC (Mouse models
of
Human Cancer Consortium) web page (emice.nci.nih.gov), sponsored by the
National
Cancer Institute, provides disease-site-specific compendium of known cancer
models,
and has links to the searchable Cancer Models Database
(cancermodels.nci.nih.gov ),
as well as the NCI-MMHCC mouse repository. Mouse repositories can also be
found
at: The Jackson Laboratory, Charles River Laboratories, Taconic, Harlan,
Mutant
Mouse Regional Resource Centers (MMRRC) National Network and at the European
Mouse Mutant Archive. Such models may be used for in vivo testing of
dibenzodiazepinone compounds, as well as for determining a therapeutically
effective
dose.
30 In addition to the compounds of the invention, pharmaceutically acceptable
salts,
solvates or prodrugs of said compounds may also be employed incompositions to
treat
or prevent the above-identified disorders.
36
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
EXAMPLES
Unless otherwise noted, all reagents were purchased from Sigma Chemical Co.
(St. Louis, MO), Aldrich.
All NMR spectra were collected in deuterated solvent on a Varian 500T"'
Spectrometer (1H NMR at 500 MHz, 13C NMR at 125 MHz). UV and mass spectra were
collected by Waters 2690T"" HPLC using a photodiode array detector (PDA, 210-
400nm) coupled to a Waters MicromassTM ZQTM mass detector.
Unless otherwise indicated, all numbers expressing quantities of ingredients,
io properties such as molecular weight, reaction conditions, molar equivalents
(eq),
percentage of binding and/or inhibition, G150, IC50 and so forth used in the
specification
and claims are to be understood as being modified in all instances by the term
"about".
Accordingly, unless indicated to the contrary, the numerical parameters set
forth in the
present specification and attached claims are approximations. At the very
least, and
not as an attempt to limit the application of the doctrine of equivalents to
the scope of
the claims, each numerical parameter should at least be construed in light of
the
number of significant figures and by applying ordinary rounding techniques.
Notwithstanding that the numerical ranges and parameters setting forth the
broad
scope of the invention are approximations, the numerical values set in the
examples,
2o Tables and Figures are reported as precisely as pbssible. Any numerical
values may
inherently contain certain errors resulting from variations in experiments,
testing
measurements, statistical analyses and such.
In the following section, examples describe in detail the chemical synthesis
of
representative compounds of the present invention. The procedures are
illustrations,
and the invention should not be construed as being limited by chemical
reactions and
conditions they express. No attempt has been made to optimize the yields
obtained in
these reactions, and it would be obvious to one skilled in the art that
variations in
reaction times, temperature, solvent and/or reagents could increase the
yields.
In addition, the materials, methods, and examples, including in vitro and in
vivo
30 efficacy, bioavailability, toxicity and pharmacological properties are
illustrative only and
not intended to be limiting. Although methods and materials similar or
equivalent to
those described herein can be used in the practice or testing of the present
invention,
37
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
suitable methods and materials are described below. All publications, patent
applications, patents, and other references mentioned herein are incorporated
by
reference in their entirety. In case of conflict, the present specification,
including
definitions, will control.
EXAMPLE 1: PRODUCTION OF COMPOUND 1 BY FERMENTATION
Examplary fermentation procedures:
a) Fermentation procedure
Micromonospora sp. (deposit accession number IDAC 070303-01) was
lo maintained on agar plates of ISP2 agar (Difco Laboratories, Detroit, MI).
An inoculum
for the production phase was prepared by transferring the surface growth of
the
Micromonospora sp. from the agar plates to 125-mL flasks containing 25 mL of
sterile
medium comprised of glucose 10g, potato dextrin type IV (Sigma) 20 g, yeast
extract 5
g, N Z Amine-A 5 g, 1 g CaCO3 made up to one liter with tap water (pH 7.0).
The
culture was incubated at about 28 C for approximately 70-72 hours on a rotary
shaker
set at 250 rpm. Following incubation, 10 mL of culture was transferred to a 2L
baffled
flask containing 600 mL of sterile production medium containing 20 g/L potato
dextrin
type IV (sigma), 30 g/L glycerol, 2.5 g/L Bacto-peptone, 8.34 g/L yeast
extract, 3 g/L
CaCO3, pH 7Ø Fermentation broth was prepared by incubating the production
culture
2o at 28 C in a rotary shaker sef at 250 rpm for 5 days.
b) Alternate procedure:
The fermentation was accomplished as a I x 10L batch in a 14.5 L fermentor
(BioFlo, 110T"" Fermentor, New Brunswick Scientific, Edison, NJ, USA) using an
improved procedure described in CA patent application 2,466,340, filed January
21,
2004.
Micromonospora sp. (deposit accession number IDAC 070303-01) was
maintained on agar plates of ISP2 agar (Difco Laboratories, Detroit, MI). An
inoculum
for the production phase was prepared by transferring the surface growth of
the
Micromonospora sp. from the agar plates to 2-L flasks containing 500 mL of
sterile
30 medium comprised of 10 g glucose, 20 g potato dextrin, 5 g yeast extract, 5
g NZ-
Amine A, and I g CaCO3 made up to one liter with tap water (pH 7.0). The
culture was
38
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
incubated at about 28 C for approximately 70 hours on a rotary shaker set at
250 rpm.
Following incubation, 300 mL of culture was transferred to a 14.5 L fermentor
containing 10 L of sterile production medium. Each liter of production medium
was
composed of 20 g potato dextrin, 30 g glycerol, 2.5 g Bacto-peptone, 8.34 g
yeast
extract, 0.3 mL Silicone defoamer oil (Chem Service), 0.05 ml Proflo oilTM
(Traders
protein) and 3 g CaCO3 made to one liter with distilled water and adjusted to
pH 7Ø
The culture was incubated at 28 C, with dissolved oxygen (dO2) controlled at
25% in a
cascade loop with agitation varied between 320-600 RPM and aeration set at a
fixed
rate of 0.5 v/v/m.
In addition to the above medium, other preferred media for the production of
Compound 1 by fermentation are provided in Table 1(QB, MA, KH, RM, JA, FA,
CL).
Any one of Micromonospora sp. 046-ECO11 or [S01]046 may be used in these
exemplified methods.
EXAMPLE 2: ISOLATION OF COMPOUND 1
Examplary isolation procedures:
a) Isolation procedure 1:
500 mL ethyl acetate was added to 500 mL of fermentation broth prepared as
described in Example I above. The mixture was agitated for 30 minutes on an
orbital
shaker at 200 rpm to create an emulsion. The phases were separated by
centrifugation
and decantation. Between 4 and 5 g of anhydrous MgSO4 was added to the organic
phase, which was then filtered and the solvents removed in vacuo.
An ethyl acetate extract from 2 L fermentation was mixed with HP-20 resin (100
mL; Mitsubishi Casei Corp., Tokyo, Japan) in water (300 mL). Ethyl acetate was
removed in vacuo, the resin was filtered on a Buchner funnel and the filtrate
was
discarded. The adsorbed HP-20 resin was then washed successively with 2 x 125
mL
of 50% acetonitrile in water, 2x125 mL of 75% acetonitrile in water and 2 x
125 mL of
acetonitrile.
Fractions containing Compound 1 were evaporated to dryness and 100 mg was
3o digested in the 5 mL of the upper phase of a mixture prepared from
chloroform,
cyclohexane, methanol, and water in the ratios, by volume, of 5:2:10:5. The
sample
39
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
was subjected to centrifugal pa,rtition chromatography using a High Speed
Countercurrent Chromatography (HSCC) system (Kromaton Technologies, Angers,
France) fitted with a 200 mL cartridge and prepacked with the upper phase of
this two-
phase system. The HSCC was run with the lower phase mobile and Compound 1 was
eluted at approximately one-half column volume. Fractions were collected and
Compound 1 was detected by TLC of aliquots of the fractions on commercial
Kieselgel
60F254 plates. Compound could be visualized by inspection of dried plates
under UV
light or by spraying the plates with a spray containing vanillin (0.75%) and
concentrated
sulfuric acid (1.5%, v/v) in ethanol and subsequently heating, the plate.
Fractions
io contained substantially pure Compound 1, although highly colored. A buff-
colored
sample could be obtained by chromatography on HPLC as follows.
6 mg of sample was dissolved in acetonitrile and injected onto a preparative
HPLC column (XterraTM ODS (10 m), 19x150mm, Waters Co., Milford, MA), with a 9
mL/min flow rate and UV peak detection at 300 nm. The column was eluted with
acetonitrile/buffer (5 mM of NH4HCO3) according to the following gradient
shown in
Table 2.
Table 2
Preparative HPLC gradient
Time (min) Water (%) Acetonitrile (%)
0 50 50
0 100
0 100
50 50
50 50
Fractions containing Compound 1 were combined, concentrated and lyophilized
20 to give a yield of 3.8 mg compound.
b) Isolation procedure 2:
Compound I was also isolated using the following alternative protocol. At the
end of the incubation period, the fermentation broth from the baffled flasks
of Example
I was centrifuged and the supernatant decanted from the pellet containing the
bacterial
mycelia. 100 mL of 100% MeOH was added to the mycelial pellet and the sample
was
stirred for 10 minutes and centrifuged for 15 minutes. The methanolic
supernatant was
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
decanted and saved. 100 mL of acetone was then .added to the mycelial pellet
and
stirred for 10 minutes then centrifuged for 15 minutes. The acetonic
supernatant was
decanted and combined with the methanolic supernatant. Finally, 100 mL of 20%
MeOH/H20 was added to the mycelial pellet, stirred for 10 minutes and
centrifuged for
15 minutes. The supernatant was combined with the acetonic and methanolic
supernatants.
The combined supernatant was added to 400 ml of HP-20 resin in 1000 mL of
water and the organics were removed in vacuo. The resuJting slurry was
filtered on a
Buchner funnel and the filtrate was discarded. HP-20 resin was washed
successively
io with 2x500mL of 50% MeOH/H20, 2x500mL of 75% MeOH/H20 and 2a~500mL of
MeOH.
The individual washes were collected separately and analyzed by TLC as
described above. Those fractions containing Compound 1 were evaporated to near
dryness and lyophilized. The lyophilizate was dissolved in methanol and
injected onto a
preparative HPLC column (XterraTM ODS (10 m), 19x150mm, Waters Co., Milford,
MA)
with a flow rate of 9 mL/min and peak detection at 300 nm.
The column was eluted with acetonitrile/buffer (5 mM of NH4HCO3) according to
gradient shown in Table 3.
Table 3
Preparative HPLC gradient
Time (min) Buffer (%) Acetonitrile (%)
0 95 5
15 45 55
20 5 95
30 5 95
35 95 5
20 Fractions containing Compound I were combined, concentrated and lyophilized
to yield about 33.7 mg of compound.
c) Isolation procedure 3:
liters of the whole broth from Example 1 was extracted twice with equal
volumes of ethyl acetate and the two extracts were combined and concentrated
to
dryness. The dried extract was weighed, and for every gram of dry extract, 100
mL of
41
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
MeOH-H20 (2:1 v/v) and 100 mL of hexane was added. The mixture was swirled
gently
but well to achieve dissolution. The two layers were separated and the aqueous
layer is
washed with 100 mL of hexane. The two hexane layers were combined and the
combined hexane solution was washed with 100 mL methanol:water (2:1, v/v). The
two
methanol:water 'layers were combined and treated with 200 mL of EtOAc and 400
mL of
water. The layers were separated and the aqueous layer extracted twice further
with
200 mL portions of EtOAc. The EtOAc layers are combined and concentrated. The
residue obtained (220 mg) was suitable for final purification, either by HSCC
or by
HPLC as described above. This extraction process achieved a ten-fold
purification
1o when compared with the extraction protocol used in (a) or (b).
EXAMPLE 3: ELUCIDATION OF THE STRUCTURE OF COMPOUND 1
1 õ 2" 31'
O
1 11 10 2' 31 q.11 5 67, 8' 9. 1~ 1- 12'
2 \1a N
9a 9
3 4a 8 OH
4 5 i 5a C28H34N204
7 Mol. Wt.: 462.25
OH H 6
HO
The calculated molecular weight of the major isotope (462.25) and formula
(C28H34N204) of Compound I was confirmed by mass spectral analysis: negative
ionization gave an (M-H)- molecular ion of 461.2 and positive ionization gave
an (M+H)+
molecular ion of 463.3. UVmax was determined to be 230nm with a shoulder at
290
nm.
Proton and carbon NMR spectral analysis is shown in Table 4. NMR data were
20 collected dissolved in MeOH-d4 including proton, carbon and
multidimensional pulse
sequences gDQCOSY, gHSQC, gHMBC, and NOESY. A number of cross peaks in the
2D spectra of Compound I are key in the structural determination. For example,
the
farnesyl chain is placed on the amide nitrogen by a strong cross peak between
the
proton signal of the terminal methylene of that chain at 4.52 ppm and the
amide
carbonyl carbon at 170 ppm in the gHMBC experiment. This conclusion is
confirmed
by a cross peak in the NOESY spectrum between the same methylene signals at
4.52
ppm and the aromatic proton signal at 6.25 ppm from one of the two protons of
the tetra
42
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
substituted benzenoid ring. Assignment of proton and carbon signals are shown
in
Table 4.
Table 4
'H and13C NMR (8H, ppm) Data of Compound 1 in MeOH-D4
Assignment 'H 13C Group
1 7.15 122.3 CH
2 6.74 121.0 CH
3 6.83 116.9 CH
4 - 146.0 C-OH
4a - 142.0 C
5a - 126.0 C
6 - 148.2 C-OH
7 6.20 100.0 CH
8 - 153.0 C-OH
9 6.25 101.0 CH
9a - 135.0 C
11 - 170.0 C(O)
11 a - 125.0 C
1' 4.52 48.7 CHZ
2' 5.35 121.1 CH
3' - 138.5 C
4' 2.03 39.5 CH2
5' 2.08 26.7 CH2
6' 5.09 124.1 CH
7' - '135.0 C
8' 1.95 39.6 CH2
9' 2.02 26.3 CH2
10' 5.06 124.4 CH
11' - 130.9 C
12' 1.64 24.8 CH3
1" 1.72 15.5 CH3
2" 1.59 14.9 CH3
3" 1.55 16.5 CH3
Based on the mass, UV and NMR spectroscopy data, the structure of the
compound was determined to be the structure of Compound 1 shown above.
EXAMPLE 4: DIALKYLSULFATE REACTIONS
a) Synthesis and structural elucidation of Compounds 2 and 18
43
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
O
2" 3"
2 \1a 1 10,,1 23, 4~ 5~ 6~7 8' g 1~1, 12'
9a 9
3 4a \8 OH
4 5N 5a C29H36N204
1 6 7 Mol. Wt.: 476.27
OH CH3
HO
Compound 2, namely 10-farnesyl-4,6,8-trihydroxy-5-methyl-5,10-dihydro-
dibenzo[b,e][1,4]diazepin-1l-one, and
1õ 2" 3"
0
1 11 10 2- 31 4- 5' 6' 7 8' 91 1ol 12'
\1a N 2 ga 9 H3CO
4a 8 oH C H020
4 5 N 5a 30 5
Mol. Wt.: 508.29
OH I H3 6 7
HO
Compound 18, namely 10-(7-methoxy-3,7,11-tr.imethyldodeca-2,10-dienyl)-4,6,8-
trihydroxy-5-methyl-5,10-dihydro-dibenzo[b,e][1,4]diazepin-11-one, were
prepared and
identified as follows:
Preparation:
Compound 1 (500.0 mg) was dissolved in methanol (MeOH, 20 mL) and stirred
io with dimethyl sulfate (0.5 mL) and NaHCO3 (250 mg) at room temperature for
48 hrs.
The reaction mixture was diluted to 200 mL by adding water and extracted with
ethyl
acetate (EtOAc, 300 mL x 3). The organic layer was separated and dried under
vacuum, re-dissolved in MeOH and filtered through a 0.45 pm 13 mm AcrodiscTM
GHP
syringe filter. The filtrate was subjected for isolation on a Waters HPLC
coupled to a
photodiode array detector. Compound 18 (12.1 mg) and Compound 2 (308.5 mg)
were
isolated by the multiple injections on Nova-PackT"' HR C18 6pm 25 x 200 mm
column
(20 mL/min, H20/CH3CN gradient 80:20-30:70, 0-8 min; 30:70-0:100, 8-18 min),
eluting
at 14.5 and 16.8 min, respectively.
Structural elucidation of Compounds 2 and 18:
20 The calculated molecular weight for the major isotope (476.27) and formula
(C29H36N204) of Compound 2 was confirmed by mass spectral analysis: negative
ionization gave an (M-H)" molecular ion of 475.6 and positive ionization gave
an
(M+Na)+ molecular ion of 499.4. Proton and carbon NMR spectral analysis is
shown in
Table 5. Signals were easily assigned based on Compound I structure knowledge.
The
44
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
calculated molecular weight for the major isotope (508.29) and formula
(C30H4oN205) of
Compound 18 was confirmed by mass spectral analysis: negative ionization gave
an
(M-H)- molecular ion of 507.3 and positive ionization gave an (M+H)+ molecular
ion of
509.3. The characteristic N-methyl (signal 5), methoxy (signal 7'-OMe) and the
methylene group (6'), from the addition of methanol on the farnesyl chain were
easily
assigned as shown in Table 5.
Table 5
NMR (S, ppm) Data of Compounds 2 and 18 in MeOH-D4
Compound 2 Compound 18
Assignment 'H '3C ,H Group
1 7.21 122.1 7.21 CH
2 7.14 127.3 7.14 CH
3 7.02 118.4 7.02 CH
4 - 152.6 - C-OH
4a - 139.3 - C
5-N-Me 2.92 41.1 2.93 N-CH3
5a - 125.4 - C
6 - 154.8 - C-OH
7 6.22 99.6 6.20 CH
8 - 156.8 - C-OH
9 6.34 101.4 6.34 CH
9a - 142.0 - C
11 - 168.2 - C(O)
11 a - 133.5 - C
1' 4.83, 4.58 47.7 4.89, 4.57 CH2
2' 5.44 119.8 5.42 CH
3' - 139.3 - C
4' 2.07 b 39.5 2.06 CH2
5' 2.12 b 26.2 1.42 CH2
6' 5.10 123.8 1.42 CH (CH2)a
7' - 135.1 - C
7' OMe N/A N/A 3.13 OCH3
8' 1.95 b 39.8 1.42 CH2
9' 2.04 b ' 26.8 1.93 CH2
10' 5.07 124.3 5.12 CH
11' - 130.8 - C
12' 1.65 24.9 1.68 CH3
1" 1.78 15.8 1.77 CH3
2" 1.60 15.1 1.10 CH3
3" 1.55 16.6 1.60 CH3
N/A: not applicable, group not present in the molecule
a. CH in Compound 2, CH2 in Compound 18
b. Signals for 4', 5', 8' and 9' are very close; assignment was based on
Compound I
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
b) Synthesis and structural elucidation of Compounds 3, 21, and 22
2õ 3õ
O
\1 a 1 1N 2- 3, 4, 5, 6' 7, 8' g 1~
2 1, 12'
ga 9
3 4a / \8 OH
4 5N 5a C'30H38N204
Mol. Wt.: 490.28
OH 6 7
HO
Compound 3: 10- farnesyl-4,6,8-trihydroxy-5-ethyl-5,1 0-dihydro-
dibenzo[b,e][1,4]diazepin-1 1 -one;
1õ 2õ
O
1 11 10 2- 3, 4, 5 6' 8' g, 10' 11 12'
\1a N
2 9a 9 H3CO
4a / \$ OH C31H42N205
4 5 N 5a Mol. Wt.: 522.31
OH 6 7
HO
Compound 21: 10-(11-methoxy-3,7,11-trimethyl-2,6-dodecadienyl)-4,6,8-
trihydroxy-5-
ethyl-5,10-dihydro-dibenzo[b,e][1,4]diazepin-1 1-one;
1õ 2õ 3,.
0
7, 11,
\1 a11 1N 2~3, 4, 5, 6' g 9, 10' 12'
2 ga g H3CO H3CO
( /I
3 4a 8 OH C32H46N205
4 5 N 5a Mol. Wt.: 554.34
OH 6 7
HO
Compound 22: 10-(7,11 -dimethoxy-3,7,1 1 -trimethyl-2-dodecenyl)-4,6,8-
trihydroxy-5-
lo ethyl-5,10-dihydro-dibenzo[b,e][1,4]diazepin-l1-one;
Preparation:
Compound 1 (85.3 mg) was stirred for 72 hrs at room temperature in a mixture
of
diethyl sulfate (2.0 mL) and NaHCO3 (99.9 mg) in MeOH (2 mL). The resulting
mixture
was filtered through a 0.45 pm 13 mm AcrodiscTM. GHP syringe filter. The
solution- was
purified by preparative HPLC (multiple injections on a NovaPackT"' HR C-18
25x200
mm column (20 mL/min, H20/CH3CN gradient 80:20-30:70, 0-8 min; 30:70-0:100, 8-
18
min) to give four major peaks: Compound 3 (20.0 mg with some impurities, RT:
16.6
min), Compound 21 (17.54 mg, RT:.14.3 min) and Compound 22 (7.82 mg, RT: 12.6
min) were obtained. Fractions containing non-ethylated analogs of Compounds 21
and
46
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
22 were also isolated respectively in 5.65 mg (RT: 11.6 min) and 2.20 mg (RT:
10.3
min) quantities. The fraction containing Compound 3 was further purified by
HPLC
using the same column (20 mL/min, H20/CH3CN gradient 80:20-30:70, 0-8 min;
30:70-
0:100, 8-18 min, curve 7), to give substantially pure Compound 3 (13.85 mg,
RT: 17.9
min).
Structural elucidation of Compounds 3, 21 and 22:
The calculated molecular weight of the major isotope (490.28) and formula
(C30H38N204) of Compound 3 was confirmed by mass spectral analysis: negative
ionization gave an (M-H)- molecular ion of 489.3 and positive ionization gave
an (M+H)+
io molecular ion of 491.3. Proton NMR signals were easily assigned based on
Compounds I and 2 structures knowledge. The characteristic N-ethyl group (5-N-
Et)
was easily assigned as shown in Table 6.
The calculated molecular weight of the major isotope (522.31) and formula
(C31H42N205) of Compound 21 was confirmed by mass spectral analysis: negative
ionization gave an (M-H)- molecular"ion of 521.3 and positive ionization gave
an (M+H)+
molecular ion of 523.5, and a fragment having an (M+H-HOCH3)+ molecular ion of
491.3. The characteristic N-ethyl group (5-N-Et), and the methoxy (11'-OMe)
and
methylene (10') groups from the addition of methanol on the farnesyl chain
were easily
assigned as shown in Table 6.
20 The calculated molecular weight of the major isotope (554.34) and formula
(C32H46N206) of Compound 22 was confirmed by mass spectral analysis: negative
ionization gave an (M-H)" molecular ion of 553.4 and positive ionization gave
an (M+H)+
molecular ion of 555.4, and fragments having respectively (M+H-HOCH3)+ and
(M+H-
HOCH3-HOCH3)+ molecular ion of 523.5 and 491.3. The characteristic N-ethyl
group (5-
N-Et), and methoxy (7'-OMe and 11'-OMe) groups from the addition of two
molecules of
methanol on the farnesyl chain were easily assigned as shown in Table 6. The
methylene groups (5', 6', 8', 9' and 10') were all found to have similar
chemical shifts,
which is consistent with the saturated alkyl group.
Table 6
'H NMR (8H, ppm) Data of Compounds 3, 21 and 22 in MeOH-D4
Assignment 3 21 22 Group
1 7.20 7.20 7.20 CH
2 7.13 7.14 7.14 CH
47
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
Table 6
1H NMR (8H, ppm) Data of Compounds 3, 21 and 22 in MeOH-D4
Assignment 3 21 22 Group
3 7.02 7.03 7.03 CH
5-N-Et (Cl) 3.23, 3.15 3.23, 3.15 3.24, 3.16 CH2
5-N-Et (C2) 1.07 1.09 1.09 CH3
7 6.22 6.22 6.22 CH
9 6.34 6.34 6.34 CH
1' 4.58, 4.56 4.82, 4.56 4.91, 4.54 CH2
2' 5.41 5.41 5.38 CH
4' 2.06 2.07 2.05 CH2
5' 2.11 2.13 ** CH2
6' 5.10 5.11 ** CH (CH2) a
7'-OMe N/A N/A 3.17 OCH3
8' 1.95 1.95 ** CH2
9' 2.04 1.38 ** CH2
10' 5.07 1.38 ** CH (CH2) b
11'-OMe N/A 3.13 3.12 OCH3
12' 1.65 1.08 1.14 CH3
1" 1.77 1.78 1.78 CH3
2" 1.60 1.10. 1.10 CH3
3" 1.55 1.08 1.14 CH3
N/A: not applicable, group not present in the molecule
** signal 1.22-1.49 ppm, 10 protons
a. CH in Compounds 3 and 21, CH2 in Compound 22
b. CH in Compound 3, CH2 in Compounds 21 and 22
c) Synthesis and structural elucidation of Compound 4
1 23.
O
2 \1 a 1 1N 23 4 5' 6~7 $ 9' 1~ 1' 12'
9a 9
I 4a 8 OH C31H40N204
4 5 N 5a Mol. Wt.: 504.30
OH 6 7
HO
Compound 4, namely 10-farnesyl-4,6,8-trihydroxy-5-n-propyl-5,10-dihydro-
dibenzo[b,e][1,4]diazepin-1l-one, was prepared and identified as follows:
lo Preparation:
Compound 1 (46.7 mg) was stirred for 72 hrs at room temperature in a mixture
of
dipropyl sulfate (0.5 mL) and NaHCO3 (46.3 mg) in MeOH (3 mL). The resulting
mixture
was filtered through a 0.45 pm 13 mm AcrodiscTM GHP syringe filter. The
solution was
48
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
purified by preparative HPLC (multiple injections on a NovaPackT"' HR C-18
25x200
mm column: 20 mL/min, H2O/CH3CN gradient 80:20-30:70, 0-8 min; 30:70-0:100,. 8-
18
min) to give substantially pure Compound 4 (18.0 mg, RT: 17.3 min).
Structural elucidation of Compound 4:
The calculated molecular weight of the major isotope (504.30) and formula
(C31H40N204) of Compound 4 was confirmed by mass spectral analysis: negative
ionization gave an (M-H)" molecular ion of 503.4 and positive ionization gave
an (M+H)+
molecular ion of 505.5. Proton NMR signals were easily assigned based on
Compounds 1 and 2 structures knowledge. The characteristic N-Propyl group (5-N-
Pr
io (Cl to C3)) was easily assigned as shown in Table 7 below.
d) Synthesis and elucidation of Compound 13
2" 3"
O
1 11 10 1 23t 4 5' 6~7' $ 9' 1~ 112'
2 \1a N
9a 9
3 \ $ OH
~a
4 5N 5a C29H33D3N204
OH I D3 6_ 7 Mol. Wt.: 479.29
HO
Compound 13: 10-Farnesyl-4,6,8-trihydroxy-5-(trideuteriomethyl)-5,10-dihydro-
dibenzo[b,e][1,4]diazepin-11-one, was prepared and identified according to the
following procedure:
Preparation:
Compound 1 (121.3 mg) was dissolved in MeOH (3.0 mL), dimethyl sulfate-d6
20 (150 pL, CDN isotopes Inc.) and NaHCO3 (58.1 mg) were added, and the
reaction was
stirred at room temperature overnight. The reaction mixture was filtered and
the filtrate
was subjected to Waters HPLC purification (multiple injections on Nova PackT"'
HR C-
18 25 x 200 mm column: 20 mL/min, H20/CH3CN gradient 70:30-20:80, 0-4 min;
20:80-
0:100, 4-9 min, 100% CH3CN, 9-12 min) to give Compound 13 (82.7 mg, RT 9.4
min).
Structural elucidation:
The calculated molecular weight of the major isotope (479.29) and formula
(C29H33D3N204) of Compound 13 was confirmed by mass spectral analysis:
negative
ionizatiori gave an (M-H)- molecular ion of 478.5, and positive ionization
gave an
49
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
(M+H)+ molecular ion 'of 480.6. The structure was further confirmed by proton
NMR
spectrum as shown in Table 7 below.
Table 7
'H NMR (8H, ppm) Data of Compounds 4 and 13 in MeOH-D4
Assign Compound 4 Compound 13 Group
1 7.19 7.21 CH
2 7.12 7.14 CH
3 7.01 7.02 CH
5-N-Pr (C1) 3.04, 3.15 - CH2 a
5-N-Pr (C2) 1.50 N/A CH2
5-N-Pr (C3) 0.91 N/A CH3
7 6.22 6.22 CH
9 6.34 6.34 CH
1' 4.54, 4.88 4.83, 4.59 CH2
2' 5.40 5.44 CH
4' 2.07 2.07 CH2
5' 2.12 2.12 CH2
6' 5.09 5.10 CH
8' 1.96 1.95 CH2
9' 2.03 2.03 CH2
10' 5.07 5.07 CH
12' 1.65 1.65 CH3
1 " 1.78 1.77 CH3
2" 1.60 1.60 CH3
3" 1.55 1.55 CH3
N/A: not applicable, group not present in the molecule
a. CH2 in Compound 4, CD3 in Compound 13.
EXAMPLE 5: ALKYL HALIDE REACTIONS
a) Synthesis and structural elucidation of Compound 5
O
1 2 3..
0 23, 4~ 5 6~7 8' 9 1~ 1, 12'
1 11 10,123,1
\1a N
2 9a 9
3 I ~a / \ $ OH C32H42N204
4 5 N 5a Mol. Wt.: 518.31
OH 6 7
HO
Compound 5, namely 10-farnesyl-4,6,8-trihydroxy-5-n-butyl-5,10-dihydro-
lo dibenzo[b,e][1,4]diazepin-1l-one, was prepared and identified as follows:
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
Preparation:
Compound 1 (43.5 mg) was stirred in 1-bromobutane (2.0 mL) with pyridine (50
pL) at 80 C dvernight. The reaction mixture was diluted with MeOH (1.0 mL),
filtered
and subjected for Waters HPLC as described above (in Example 4(c)) to give a
semi-
purified Compound 5 (RT: 18.1 min). The semi-purified compound was further
purified
using the same conditions (except with curve 7) to give substantially pure
Compound 5
(10.5 mg, RT: 17.9 min).
Structure elucidation:
The calculated molecular weight of the major isotope (518.31) and formula
(C32H42N204) of Compound 5 was confirmed by mass spectral analysis: negative
ionization gave an (M-H)- molecular ion of 517.4 and positive ionization gave
an (M+H)+
molecular ion of 519.5. The characteristic N-n-butyl group (5-N-alkyl (C1-C4))
was
easily assigned as shown in Table 8 below.
b) Synthesis and structural elucidation of Compound 7
O
1 2 3..
1 11 10 23, 4' 5' 6~T 891 111 12.
\1a N
2
9a 9
3 I ~a / \ OH C34H46N204
4 5 N 5a Mol. Wt.: 546.35
OH 6 ~
HO
Compound 7, namely 1 0-farnesyl-4,6,8-trihydroxy-5-n-hexyl-5,1 0-dihydro-
dibenzo[b,e][1,4]diazepin-1 1 -one, was prepared and identified as follows:
Preparation:
Compound 1 (39.2 mg) was stirred in 1-bromohexane (2.0 mL) with pyridine (50
pL) at 80 C overnight. The reaction mixture was diluted with MeOH (1.0 mL),
filtered
and subjected for Waters HPLC (multiple injections on a NovaPackT"' HR C-18
25x200
mm column: 20 mL/min, H20/CH3CN gradient 80:20-30:70, 0-8 min; 30:70-0:100, 8-
18
min; isocratic CH3CN 18-24 minutes) to give substantially pure Compound 7
(14.0 mg,
RT: 20.1 min).
Structural elucidation:
51
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
The calculated molecular weight (546.35) and formula (C34H46N204) of
Compound 7 was confirmed by mass spectral analysis: negative ionization gave
an (M-
H)" molecular ion of 545.6 and positive ionization gave an (M+H)+ molecular
ion of
547.6. Proton NMR signals were easily assigned based on Compounds I and, 2
structures knowledge. The characteristic N-n-hexyl group (5-N-alkyl (C1-C6))
was easily
assigned as shown in Table 8 below.
c) Synthesis of Compounds 2 to 4, 6, and 8 to 12
Compounds 2 to 4, 6, and 8 to 12 are produced by the procedure described in a)
lo and b), by substituting the alkylating agent used in these procedures
respectively by the
following alkylating agents: iodomethane, bromoethane, 1-bromopropane, 1-
bromopentane, 1-bromoheptane, 1-chlorooctane, trifuoromethyl iodide,
heptafluoro-l-
iodopropane, and 2-iodo-1,1,1-trifluoroethane.
Table 8
'H NMR (8H, ppm) Data of Compounds 5 and 7 in MeOH-D4
Assignment 5 7 Group
1 7.20 7.20 CH
2 7.13 7.12 CH
3 7.02 7.02 CH
5-N-alkyl (Cl) 3.-18, 3.09 3.18, 3.08 CH2 ~
.(C2) 1.46 1.48 CH2 -
(C3) 1.35 1.30 CHZ
(C4) 0.89 1.30 b
(C5) N/A 1.30 CH2
(C6) N/A 0.89 CH3
7 6.22 6.23 CH
9 6.34 6.33 CH
1' 4.89, 4.54 4.92, 4.52 CH2
2' 5.40 5.39 CH
4' a 2.06 2.06 CH2
5' a 2.12 2.11 CH2
6' 5.09 5.09 CH
8' a 1.96 1.96 CH2
9' a 2.04 2.04 CH2
10' 5.07 5.07 CH
12' 1.65 1.64 CH3
1" 1.78 1.78 CH3
2" 1.60 1.60 CH3
52
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
Table 8,
1H NMR (8H, ppm) Data of Compounds 5 and 7 in MeOH-D4
Assignment 5 7 Group
3" 1.55 1.56 CH3
N/A: not a,pplicabie, group not present in the molecule
a. Signals at 4', 5', 8' and 9' are very close; assignment was based on
Compound 1.
b. CH3 in Compound 5, CH2 in Compound 7.
EXAMPLE 6: O-ACYLATION
Synthesis and structural elucidation of Compound 25 (O-acet lay tion):
2" 3"
O
1 ~1 a 1 1N 2- 3 4. 5 6' 7 8' g 1~ 1 12'
9a g
3 4a 8 OAc
4 5 i 5a _ C34H40N207
OAc H 6 7 Exact Mass: 588.28
AcO
Compound 25: 4,6,8-triacetoxy-l0-farnesyl-5,10-dihydro-
dibenzo[b,e][1,4]diazepin-ll-
io one, was prepared and identified according to the following procedure:
Preparation:
Compound 1 (120.5 mg) was stirred overnight with acetic anhydride (720 pL, 29
eq, Aldrich) in presence of 6 drops of pyridine (Aldrich). The reaction
mixtures
submitted to HPLC separation. Purification by multiple injection on a WatersTM
RCM
Nova-Pak HRTM C18, 6pm, 60A 25 x 200 mm column (20 mL/min H20/CH3CN 80:30-
70:75, 0-8 min; 30:70-0:100, 8-18 min, 100% CH3CN, 18-20 min) gave Compound 25
(91.2 mg) with a retention time of 18.5 min. The 4,8-diacetoxy (11.4 mg), 4,6-
diacetoxy
(9.2 mg), and 6,8-diacetoxy (11.4 mg) compounds were also isolated at
retention times
of 16.2, 17.6, and 18.0 respectively.
20 Structural elucidation:
The calculated molecular weight of the major isotope (588.28) and formula
(C34H40N207) of Compound 25 were confirmed by mass spectral (MS) analysis. MS
of
Compound 25 gave a(M-H)- molecular ion of 587.6 by negative ionization and a
(M+Na)+ molecular ion of 611.5 by positive ionization. Proton NMR spectral
analysis for
Compound 25 is shown in Table 9. Signals were easily assigned based on
comparison
53
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
with the spectra of Compound 1.
Table 9
1H NMR (8H, ppm) Data of Compound 25 in CDCI3
Assignment 25 Group
1 7.75 CH
2 7.10 CH
3 7.16 CH
4-OAc 2.41 CH3
6.15 NH
6-OAc 2.40 CH3
7 6.96 CH
8-OAc 2.27 CH3
9 6.84 CH
1' 4.58 CH2
2' 5.42 CH
4 a 2.06 CH2
5 a 2.09 CH2
6' 5.10 CH
8' a 1.98 CH2
9' a 2.06 CH2
10' 5.10 CH
12' 1.68 CH3
1" 1.72 CH3
2" 1.61 CH3
3" 1.60 CH3
N/A: Not applicable, group not present in the molecule.
a. Signals of 4', 5', 8' and 9' are very close; assignment was based on
Compound 1.
EXAMPLE 7: FARNESYL SIDE CHAIN MODIFICATIONS
a) Synthesis and structural elucidation of Compound 14
1 2 3.
O
1 11 10 2' 3, 5, 6712
' 7 8'9 10' 11, 12'
1a N
2
9a 9
4a $ OH C29H42N204
4 5 N 5a Mol. Wt.: 482.31
OH I CH3 6 7
HO
Compound 14, namely 10-(3,7,11-trimethyldodecyl)-4,6,8-trihydroxy-5-methyl-
5,10-
io dihydro-dibenzo[b,e][1,4]diazepin-1l-one, was prepared and identified as
follows:
Preparation:
54
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
A solution of Compound 2 (23.7 mg) in MeOH (2.0 mL) was stirred under
hydrogen gas overnight in presence of platinum oxide (Pt02, 10 mg, catalyst)
as a
catalyst. The reaction mixture was filtered and concentrated in vacuo to give
21.6 mg of
Compound 14.
Structural elucidation:
The calculated molecular weight (482.31) and formula (C29H42N204) of
Compound 14 was confirmed by mass spectral analysis: negative ionization gave
an
(M-H)- molecular ion of 481.3 and positive ionization gave an (M+H)+ molecular
ion of
483.3. The farnesyl olefinic protons on the NMR spectra were replaced by
aliphatic
io proton signals in the region of around 0.76-1.86 ppm, integrating for 17
protons, 3CH,
7CH2. The characteristic N-methyl group (5-N-Me) was easily assigned as shown
in
Table 10 below..
b) Synthesis and structural elucidation of Compound 15
1õ 2"
O
\1 a 1N 2~3 4. 5 6' g 9 10' 11' 12'
2
1 9a 9
4a g OH C29H0204
4 5 N 5a Mol. Wt.: 480.30
OH I H3 6 7
HO
Compound 15, namely 10-(3,7,11-trimethyl-2-dodecenyl)-4,6,8-trihydroxy-5-
methyl-
5,10-dihydro-dibenzo[b,e][1,4]diazepin-11-one, was prepared and identified as
follows:
Preparation:
A solution of Compound 2 (308.5 mg) in MeOH (220 mL) was stirred under
2o hydrogen gas overnight in presence of platinum oxide (Pt02, 10 mg) as
catalyst. The
reaction mixture was filtered and purified by HPLC according to the procedure
described in Example 4(c) to give pure Compound 15 (82.3 mg, RT: 17.0 min).
Structural elucidation:
The calculated molecular weight (480.30) and formula (C29H40N204) of
Compound 15 was confirmed by mass spectral analysis: negative ionization gave
an
(M-H)- molecular ion of 479.3 and positive ionization gave an (M+H)+ molecular
ion of
481.6. The NMR spectrum analysis was based on the structural elucidation of
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
Compounds 2 and 14, and the results presented in Table 10. The farnesyl
olefinic
protons of positions 6'-7' and 10'-11' on the NMR spectra were replaced by
aliphatic
proton signals and, together with the aliphatic protons of positions 5', 8'
and 9', are
observed in the region of about 1.07 to 1.51 ppm, integrating for 12 protons,
2CH,
5CH2. The farnesyl olefinic proton at position 2'(CH) was shown on the NMR to
have a
chemical shift of 5.41 ppm. The methylene group at positions 4' was shown at
2.03
ppm. The characteristic 5-N-methyl group was also easily assigned to a
chemical shift
of 2.93 ppm.
lo c) Synthesis and structural elucidation of Compound 23
2" 3
O
1 10 3, 41 51 6' 7' 10' 11'
2' 11 2 \1a N
9a 9
3 4a 8 OH
4 5N 5a
C2sH4oN204
OH i 6 7 Mol. Wt.: 468.30
HO
Compound 23: 10-(3,7,11 -trimethyldodecyl)-4,6,8-trihydroxy-5,1 0-dihydro-
dibenzo[b,e][1,4]diazepin-1 1 -one, was prepared and identified according to
the
following procedure:
Preparation:
A solution of Compound 1 (51.1 mg in 3.0 mL MeOH) was stirred under
hydrogen gas overnight in presence of platinum oxide (Pt02,10 mg, 0.4 eq) as a
catalyst. The reaction mixture was filtered and purified by direct preparative
HPLC using
a Phenomenex SynergiT"' MAX RP 21.2 x 200 mm column (20 mL/min, H20/CH3CN
20 gradient 30:70-30:70, 0-2 min; 30:70-0:100, 2-20 min). Fractions having a
retention
time of 12.8 min were combined to give 45.2 mg of Compound 23.
Structural elucidation:
Calculated molecular weight of the major isotope (468.30) and formula
(C28H40N204) were confirmed by mass spectral analysis. Compound 23 mass
spectra
gave a(M-H)" molecular ion of 467.4 by negative ionization and a(M+H)+
molecular ion
of 469.4 by positive ionization. Proton NMR spectral analysis of Compound 23
is shown
in Table 10 below.'Signals were easily assigned based on Compound 1 structure
56
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
knowledge. As expected, aliphatic proton signals at positions 2'-11' all have
very close
chemical shifts ranging from about I to 1.75 ppm (integrating for 17 protons),
methyl
protons at positions 12' and 1"-3" are all very close as well (shifts 0.8-0.95
ppm,
integrating for 12 protons). These signals are also complex from the fact that
2
diastereomers of positions 3' and 7' are present in the mixture, and in
different
proportions. Labile protons were not observed since NMR was done in deuterated
methanol. Table 10
'H NMR (8H, ppm) Data of Compounds 14, 15 and 23 in CD3OD
Assignment 14 15 23 Group
1 7.18 7.21 7.15 CH
2 7.12 7.14 6.76 CH
3 7.01 7.02 6.84 CH
5-N-Me 2.96, 2.95' 2.93 N/A CH3
7 6.23 6.21 6.24 CH
9 6.33, 6.35 b, 6.34 6.26 CH
1' 4.42, ,3.86 4.56, 4.87 4.16, 3.99 CH2
21-11' a -0.76-1.86 c -1.00-1.75
12' and 1"-3" a -0.87-1.00 d -0.8-0.95 d
N/A: not applicable, group not present in the molecule
a. Signals are very close.
b. Mixture of isomers.
c. Compounds 14 and 23: 3CH, 7CH2 (17H); Compound 15: 2'(CH) at 5.41 ppm,
4'(CH2) at 2.03
ppm, 5'-11' (12H) at 1.07-1.51 ppm.
d. Compounds 14 and 23: 4CH3 (12H); Compound 15: 1" (CH3) at 1.75 ppm, 2", 3"
and 12' (9H) at
0.88-0.80 ppm.
EXAMPLE 8: AROMATIC SUBSTITUTION REACTION
a) Synthesis and structural elucidation of Compound 24 by bromination
2"
O
1 11 10 2- 3, 41 5, 67- 8' 91 10~ 2'
1a N
2 9a
I 9
3 4a 8 OH
4 5N 5a C28H33BrN2O4
7 Mol. Wt.: 540.16 (M)
OH H 6 Mol. Wt.: 542.16 (M+2)
HO Br
Compound 24: 10-(farnesyl)-7-bromo-4,6,8-trihydroxy-5,10-dihydro-
dibenzo[b,e][1,4]diazepin-1 1-one, was prepared and identified according to
the
57
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
following procedure:
Preparation:
Compound 1(116.0 mg) and N-bromosuccinimide, (NBS, 45.5 mg) were
dissolved in tetrahydrofuran (THF, 3.0 mL) and stirred at room temperature for
4 days.
The reaction mixture was filtered and subjected to Waters HPLC purification
(Nova-
PackT"' HR C-18 25 x 200 mm column: 20 mL/min, H20/CH3CN gradient 80:20-30:70,
0-8 min; 30:70-0:100, 8-18 min) to give Compound 24 (13.6 min) together with
some
impurities. The semi-purified sample was further purified by HPLC (SymmetryTM
C-18
25 x 100 mm column: 20 mL/min, H20/CH3CN gradient 70:30-30:70, 0-15 min), to
give
io pure Compound 24 (9.5 mg, RT 13.0 min).
Structural elucidation:
The calculated molecular weight of the major isotopes (540.16 and 542.16) and
formula (C28H33BrN2O4) of Compound 24 was confirmed by mass spectral analysis:
negative ionization gave (M-H)- molecular ions of 539.2 and 541.1, and
positive
ionization gave (M+H)+ molecular ions of 541.3 and 543.2. The presence of the
two
molecular ions in each mass spectrum confirmed the presence of a bromine group
in
the molecule. The structure was further confirmed by the absence of the
aromatic
(position 7) signal in the proton NMR spectrum as shown in Table 11 below.
Table 11
'H NMR (8H, ppm) Data of Compound 24 in CD3OD
Assignment Compound 24 Group
1 7.18 CH
2 6.79 CH
3 6.87 CH
9 6.49 CH
1' 4.56 CH2
2' 5.34 CH
4' 2.06 CH2
5' 2.09 CH2
6' 5.10 CH
8' 1.96 CH2
9' 2.04 CH2
10' 5.08 CH
12' 1.66 CH3
1" 1.74 CH3
2" 1.60 CH3
3" 1.57 CH3
58
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
b) Synthesis of Compound 26
Compound 2 and N-bromosuccinimide are dissolved in tetrahydrofuran and
stirred at room temperature for 4 days (conditions as described in (a)). The
reaction
mixture is filtered and subjected to HPLC purification to provide pure
Compound 26.
c) Synthesis of Compound 27
Compound 14 and N-bromosuccinimide are dissolved in tetrahydrofuran and
stirred at room temperature for 4 days (conditions as described in (a)). The
reaction
io mixture is filtered and subjected to HPLC purification to provide pure
Compound 27.
EXAMPLE 9. IN VITRO PROFILING OF THE COMPOUNDS OF THE INVENTION
(a) In vitro anticancer activity of the compounds of Formula I against four
cell lines:
In vitro cytotoxic activities of exemplified Compounds are shown in Table 12,
along with hemolytic activity of each compound. Compounds were tested in four
cell
lines: HT-29 (colorectal carcinoma), SF268 (CNS), MDA-MB-231 (mammary gland
adenocarcinoma) and PC-3 (prostate adenocarcinoma). Procedures used for each
series of tests are described below.
Table 12
In vitro Cytotoxic Activities
Compound HT-29 SF-268 PC-3 MDA-MB-231 Average
No: (GI50 pM) (G150 pM) (Gie0 ~ (GieO p M) (Gi60 pM)
1a/b 11.2/9.33 1.96/1.55 1.95/3.76 1.79/3.18 4.23/4.45
2 b 0.65 0.12 0.45 0.24 0.36
3 b 2.04 0.76 1.15 2.16 1.53
4 b 2.57 0.89 1.25 2.27 1.74
b 2.50 0.56 1.14 1.39 1.40
7 b 2.44 0.53 1.33 1.92 1.55
13 b 0.69 0.16 0.82 0.51 0.54
14 b 0.29 0.07 0.23 0.24 0.21
b 0.69 0.23 0.65 0.37 0.49
18 b 1.43 0.33 1.80 1.02 1.14
21 b 1.89 1.73 1.08 2.19 1.72
22 b 1.83 0.91 1.39 2.40 1.63
23 a 4.26 0.72 0.90 0.59 1.62
24 b 2.02 2.04 1.19 2.02 1.82
25a 9.33 1.95 1.20 2.79 3.82
a. Results obtained by method (a) below
b. Results obtained by method (b) below
c. Average G150 values in pM.
59
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
All compounds described in Table 12 were shown to have better anticancer
activity than Compound 1. These compounds include Compounds 2 to 5, 7, 13, 14,
15,
18, and 21 to 25. Compounds Compounds 2 to 5, 7, 13, 14, 15, 18, 21, and 22
are N-
linear alkyl derivatives of Compound 1, encompassed by Formula I, and their
hydrogenated and hydrornethoxylated farnesyl derivatives. The hydrogenated
farnesyl
derivative Compound 23, brominated aryl derivative Compound 24, and
triacetylated
Compound 25 were also found to be generally more active than Compound 1.
io Method (a):
Cytotoxic activities were determined in vitro for Compo,unds 1, 23 and 25 to
determine the concentration of each compound needed to obtain a 50% inhibition
of
cell proliferation (G150). The G150 value emphasizes the correction for the
cell count at
time zero and, using the seven absorbance measurements [time zero, (Tz),
control
growth, (C), and test growth in the presence of drug at the five concentration
levels
(Ti)], G150 is calculated as [(Ti-Tz)/(C-Tz)] x 100 =-50, which is the drug
concentration
resulting in a 50% reduction in the net DNA content intreated relative to
control cells
during the drug incubation.
Compounds were dissolved at 10 mM in DMSO. Dilution in vehicle to
20 concentrations of 30, 10, 3, 1 and 0.3 pM were prepared immediately before
assays.
Depending on the cell line's growth characteristics, 4000-10000 cells were
plated in two
96-wells pates (day 0) and incubated for 16 hours. The following day,
propidium iodide
ws added to one of the two plates and fluorescence measured (Tz). Test
compounds
were added to the second plate, as well as vehicle control, and cells further
incubated
for 96 hours. Each compound was tested at each concentration and in
triplicates. The
equivalent cell number was determined after adding propidium iodide by
measuring the signal by fluorescence (C for control). G150 results were
calculated using the formula
above and are shown in Table 12.
30 Method (b):
In vitro cytotoxic activities (GI50) of Compounds 1, 2 to 5, 7, 13, 14, 15,
18, 21,
22, and 24 were determined using propidium iodide (PI) as being the
concentration of
drug resulting in 50% growth inhibition, and by using the following method.
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
Two 96-well plates were seeded in duplicate with each cell line at the
appropriate
inoculation density (HT29: 3,000; SF268: 3,000; PC-3: 3,000; and MDA-MB-231:
7,500
cells) and according to the technical data sheet of each cell line (rows A-G,
75 pL of
media per well). Row H was filled wih medium only (150 pL, negative control-
medium).
The plates were incubated at appropriate temperature and CO2 concentration for
24
hrs.
Test Compounds were prepared as 15X stock solutions in appropriate medium
and corresponding to 450, 45, 0.45, 0.045, and 0.0045 pM (prepared the day of
the
experiment). An aliquot of each was diluted 7.5-fold in appropriate test
medium to give
io a set of six 2X concentration solutions (60, 6, 0.6, 0.06, 0.006, and
0.0006 pM). A 75 pL
aliquot of each concentration was added to each corresponding well (rows A to
F) of
the second plate. Row G was filled with 75 pL of medium/0.6% DMSO (negative
control-cells). The second plate was incubated at appropriate temperature and
CO2
concentration for 96 hrs.
First Plate: PI (30 pL, 50 pg/mL) was added to each well of the first plate
without
removing the culture medium. The plate was centrifuged (Sorvall Legend-RT,
swinging
bucket) at 3500 rpm/10 min. Fluorescence intensity (Thermo, Varioskan, /\eX:
530 nm;
Aem: 620 nm) was measured to give the first measurement, dead cells (DC at To;
before
freezing). Two round of Freeze (-80 C)/Thaw (37 C) were done. Fluorescence
intensity
20 was determined to give the second measure, total cells (TC at To; after
freeze/thaw)
Second plate was processed as the first one, except there were three rounds of
freeze/thaw instead of two. First measurement gave the treated dead cells
value (TDC),
and the second measurement gave the treated total cells value (TTC). Both
values
were collected for each treated well and control (CTC and CDC).
Each value (DC, TC, TDC, TTC, CTC and CDC) was corrected by removing the
background value (medium only) to give the value (FUDC(T=o), FUTC(T=0), FUTDC,
FUTTC,
FUcTc and FUCDC) used in the calculation of the T/C (%) (Treated/Control) for
each
concentration. T/C (%) for each concentration is calculated using the
following formula:
(FUTTC - FUTDC) - (FUTC(7==0) - FUDC(-r=p)) X 100
T/C (%)=
(FUCTC - FUCDC) - (FUTC(T=0) - FUDc(T=0))
30 The G150 value emphasizes the correction for the cell count at time zero
for cell
survival. The T/C values are transposed in a graph to determine G150 values,
the
concentration at with the=T/C is 50%.
61
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
(b) Anticancer activity profiling of Compound 2 against 36 cell lines (IC50):
Culture conditions and activity evaluations of Compound 2 against 36 cancer
cell
lines were done as described in Method(a) of Example 9(a), except that results
were
expressed as the concentration of drug which inhibits 50% of the cell growth
(IC50,
calculated using the formula: [Ti/C] x 100 = -50). The low micromolar to
nanomolar
levels of IC50 values shown in Table 13 demonstrated a pharmacologically
relevant
cytotoxic activity of Compound 2 against a variety of 36 tumor types including
melanomas, pancreatic, lung, colon, gastric, bladder, renal, CNS, head and
neck,
io prostate; uterus, ovarian and breast carcinomas.
Table 13
In vitro profiles of Compound 2 (IC50)
# Type Cell line Origin Histology in nude mice IC50 (pM)
I Bladder T24 ATCC Transitional cell ca 0.127
2 Bladder 1218L Xenograft Urothelial adeno ca 0.166
3 Colon HCT116 NCI Adeno ca, pd 0.156
4 Colon HT29 NCI Adeno ca, pd 0.223
CNS 498NL Xenograft Glioblastoma 0.176
6 CNS SF268 NCI Nd 0.010
7 Gastric 251L Xenograft Adeno ca, pd 0.105
8 Head & Neck 536L Xenograft Hypopharynx ca 0.181
9 Lung 1121L Xenograft Large cell ca 0.125
Lung 289L Xenograft Adeno ca 1.553
11 Lung 526L Xenograft Adeno ca 0.104
12 Lung 629L Xenograft Adeno ca 0.164
13 Lung 529L Xenograft Large cell ca, 0.127
14 Lung H460 NCI Large cell ca 0.366
Mammary 401 NL Xenograft Pap adeno ca, wd 0.194
16 Mammary MCF7 NCI Mamma ca 0.276
17 Melanoma 276L Xenograft Mm, amelanotic 1.948
18 Melanoma 394NL Xenograft mm, amelanotic,pd 0.020
19 Melanoma 462NL Xenograft Mm, amelanotic 0.978
Melanoma 514L Xenograft Mm, melanotic 0.110
21 Melanoma 520L Xenograft Mm, melanotic 0.085
22 Ovarian 1619L Xenograft Adeno ca, md 0.579
23 Ovarian 899L Xenograft Pap serous ca, md 0.238
24 Ovarian OVCAR3 NCI Adeno ca, md 0.139
Pancreas 1657L Xenograft Adeno ca, md 1.777.
26 Pancreas PANC1 ATCC nd 0.125
27 Prostate 22RV1 ATCC Adeno ca, md 0.142
28 Prostate DU145 NCI Adeno ca, md 0.158
29 Prostate LNCAP DSMZ Adeno ca, md 0.485
62
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
Table 13
In vitro profiles of Compound 2(IC5o)
# Type Cell line Origin Histology in nude mice IC50 (iaM)
30 Prostate PC3M NCI Adeno ca, pd ' 0.114
31 Pleuramesot. 1752L Xenograft pleuromesothelioma 1.503
32 Renal 1781L Xenograft renal ca 0.172
33 Renal 393NL Xenograft Hypernephroma, wd 0.527
34 Renal 486L Xenograft Hypernephroma, pd 1.144
35 Renal 944L Xenograft Hypernephroma 0.230
36 Uterus 1138L Xenograft Carcinosarcoma, wd 0.139
Mean of all cell lines: 0.407
ca=carcimoma; pd=poorly differentiated; pap=papillary; md=moderately
differentiated; wd=well differentiated;
mm=malignant melanoma; nd=not determined
EXAMPLE 10: IN VIVO EFFICACY OF COMPOUNDS 1 AND 2
In vivo efficacy of Compounds 1 and 2 in a glioma model:
The aim of this study was to test whether Compounds 1 and 2 prevent or delay
tumor growth in C6 glioblastoma cell-bearing mice, and to determine an
effective
dosage regimen.
Animals: A total of 60 six-week-old female mice (Mus musculus nude mice),
ranging
between 18 to 25 g in weight, were observed for 7 days before treatment.
Animal
io experiments were performed according to ethical guidelines of animal
experimentation
(Charte du comite d'ethique du CNRS, juillet 2003) and the English guidelines
for the
welfare of animals in experimental neoplasia (WORKMAN, P., TWENTYMAN, P.,
BALKWILL, F., et al. (1998). United Kingdom Coordinating Committee on Cancer
Research (UKCCCR) Guidelines for the welfare of animals in experimental
neoplasia
(Second Edition, July 1997; British Journal of Cancer, 77:1-10). Any dead or
apparently
sick mice were promptly removed and replaced with healthy mice. Sick mice were
euthanized upon removal from the cage. Animals were maintained in rooms under
controlled conditions of temperature (23 2 C), humidity (45 5%),
photoperiodicity (12
hrs light / 12 hrs dark) and air exchange. Animals were housed in
polycarbonate cages
20 (5/single cage) that were equipped to provide food and water. Animal
bedding consisted
of sterile wood shavings that were replaced every other.day. Food was provided
ad
libitum, being placed in the metal lid on the top of the cage. Autoclaved tap
water was
provided ad libitum. Water bottles were equipped with rubber stoppers and
sipper
tubes. Water bottles were cleaned, sterilized and replaced ohce a week. Two
different
63
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
numbers engraved on two earrings identified the animals. Each cage was labeled
with a
specific code.
Tumor Cell Line: The C6 cell line was cloned from a rat glial tumor induced by
N-
nitrosomethyurea (NMU) according to Premont et al. (Premont J, Benda P, Jard
S., [3H]
norepinephrine binding by rat glial cells in culture. Lack of correlation
between binding
and adenylate cyclase activation. Biochim Biophys Acta. 1975 Feb 13;381(2):368-
76.)
after series of alternate culture and animal passages. Cells were grown as
adherent
monolayers at 37 C in a humidified atmosphere (5% C02, 95% air). The culture
medium was DMEM supplemented with 2 mM L-glutamine and 10% fetal bovine
io serum. For experimental use, tumor cells were detached from the culture
flask by a 10
min treatment with trypsin-versen. The cells were counted in a hemocytometer
and their
viability assessed by 0.25% trypan blue exclusion.
Preparation of the Test Article: For the test article, the following procedure
was followed
for reconstitution (performed immediately preceding injection). The vehicle
consisted of
a mixture of benzyl alcohol (1.5%), ethanol (8.5%), propylene glycol (27%),
PEG 400
(27%), dimethylacetarriide (6%) and water (30%). The vehicle solution was
first
vortexed in order to obtain a homogeneous liquid. 0.6 mL of the vortexed
vehicle
solution was added to each vial containing the test article (Compound 1).
Vials were
mixed thoroughly by vortexing for 1 minute and inverted and shaken vigorously.
Vials
20 were mixed again prior to injection into each animal.
Animal Inoculation with tumor cells: Experiment started at day 0 (Do). On Do,
mice.
received a superficial intramuscular injection of C6 tumor cells (5 x 105
cells) in 0.1 mL
of DMEM complete medium into the upper right posterior leg.
Treatment regimen and Results:
First series of experiments:
In a first series of experiments, treatment started 24 hrs following
inoculation of
C6 cells. On the day of the treatment, each mouse was slowly injected with 100
pL of
test or control articles by the i.p. route. For all groups, treatment was
performed until the
tumor volume of the saline-treated mice (group 1) reached approximately 3 cm3
(around
3o day 16). Mice of group I were treated daily with a saline isosmotic
solution for 16 days.
Mice of group 2 were treated daily with the vehicle solution for 16 days. Mice
of group 3
were treated daily with 10 mg/kg of Compound 1 for 16 days. Mice of group 4
were
64
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
treated every two days with 30 mg/kg of Compound I and received 8 treatments.
Mice
of group 5 were treated every three days with 30 mg/kg of Compound I and
received 6
treatments. Measurement of tumor volume started as soon as tumors became
palpable
(>100 mm3; day 11 post-inoculation) and was evaluated every second day until
the end
of the treatment using callipers. As shown in Table 14 and Figure 1, the mean
value of
the tumor volume of all Compound 1 treated groups (6 mice/group) was
significantly
reduced as demonstrated by the one-way analysis of variance (Anova) test
followed by
the non-parametric Dunnett's multiple comparison test comparing treated groups
to the
saline group. An asterisk in the P value column of Table 14 indicates a
statistically
lo significant value, while "ns" signifies not significant.
Table 14
Compound 1 in vivo antitumor efficacy against C6 glioblastoma
Treatment Treatment Tumor volume (mm ) 00 Inhibition P value
regimen (mean SEM)
Saline Q1 x 16 3,004.1 249.64 - -
Vehicle solution Q1 x 16 2,162.0 350.0 28.0% >0.05 ns
Compound 1(10 mg/kg) Q1 x 16 1,220.4 283.46 59.4% <0.01 *
Compound 1 (30 mg/kg) Q2 x 8 1,236.9 233.99 58.8% <0.01 *
Compound 1(30 mg/kg) Q3 x 6 1,184.1 221.45 60.6% <0.01 *
Second series experiments:
In a second series of experiments, treatment started at day 10 following
inoculation of C6 cells when tumors became palpable (around 100 to 200 mm).
Treatment was repeated daily for 5 consecutive days. On the day of the
treatment,
each mouse was slowly injected with 100 pL of Compound 1 by i.p. route. Mice
of
group 1 were treated daily with saline isosmotic solution. Mice of group 2
were treated
daily with the vehicle solution. Mice of group 3 were treated daily with 20
mg/kg of
Compound 1. Mice of group 4 were treated daily with 30 mg/kg of Compound 1.
Mice
20 were treated until the tumor volume of the saline-treated control mice
(group 1) reached
around 4 cm3. Tumor volume was measured every second day until the end of the
treatment using callipers. As shown in Table 15 and Figure 2, the mean value
of the
tumor volume of all Compound 1 treated groups (6 mice/group) was significantly
reduced as demonstrated by the one-way analysis of variance (Anova) test
followed by
the non-parametric Dunnett's multiple comparison test comparing treated groups
to the
saline group. An asterisk in the P value column of Table 15 indicates a
statistically
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
significant value, while "ns" signifies not significant.
Histological analysis of tumor sections showed pronounced morphological
changes between tumors from Compound 1-treated mice and those from mice in the
control groups. In tumors from mice treated with Compound 1 (20 - 30 mg/kg),
cell
density was decreased and the nuclei of remaining tumor cells appeared larger
and
pycnotic while no such changes were observed for tumors from vehicle-treated
mice
(Figure 3).
Table 15
Compound 1 in vivo antitumor efficacy against C6 glioblastoma
Treatment Treatment Tumor volume (mm ) % Inhibition P value
regimen (mean SEM)
Saline Q1 x 5 4,363.1 614.31 - -
Vehicle solution Q1 x 5 3,205.0 632.37 26.5% >0.05 ns
Compound 1 (20 mg/kg) Q1 x 5 1,721.5 374.79 60.5% <0.01 *
Compound 1(30 mg/kg) Q1 x 5 1,131.6 525.21 74.1% <0.01 *
In vivo antitumor efficacy of Compound 2:
lo Antitumor efficacy of Compound 2 against rat glioblastoma tumor (C6)
xenografts in
female swiss nude mice was accomplished as described above. The results and
dosage regimen are summarized in Figure 4. Significant efficacy was shown
following
intravenous administration, at a dosage regimen of 75 mg/kg (qd5/2/qd5).
EXAMPLE 11: Pharmacokinetic profiles
Compounds I and 2 were separately dissolved in ethanol (5%), Polysorbate 80
(15%), PEG 400 (5%) and dextrose (5%) at a final concentration of 6 mg/mI (for
all
parenteral administration routes). For oral administration, Compound 1 was
solubilized
in Cremophor EL/ Ethanol (50%:50%) at a final concentration of 6 mg/mI. Prior
to
2o dosing, animals (female Crl: CDI mice; 6 weeks of age, 22-24g) were
weighed,
randomly selected and assigned to the different treatment groups. Compound 1
was
administered by the intravenous (iv), subcutaneous (sc), intraperitoneal (ip),
or oral (po)
route to the assigned animals. Compound 2 was administered by the intravenous
(iv),
or intraperitoneal (ip) route to the assigned animals. The dosing volume of
Compounds
I and 2 was 5 mL per kg body weight. Animals were anesthetized with 5%
isoflurane
66
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
prior to bleeding. Blood was collected into microtainer tubes containing the
anticoagulant K2EDTA by cardiac puncture from each of 4 animals per bleeding
timepoint (2 min, 5 min, 15 min, 30 min, 1 h, 2h, 4h and 8h). Following
collection, the
samples were centrifuged and the plasma obtained from each sample was
recovered
and stored frozen (at approximately -80 C) pending analysis. At the 5 min and
30 min
time points, the following organs were harvested from each animal: brain,
lungs,
skeletal muscle, fat tissue, kidneys, spleen, thymus and liver. Tissues were
frozen (at
approximately -80 C) pending analysis. Samples were. analysed by LC/MS/MS.
Standard curve ranged from 25 to 2000 ng/mL with limit of quantitation (LOQ)
<_ 25
io ng/mL and limit of detection (LOD) of 10 ng/mL.
Plasma values of Compounds I and 2 falling below the limit of quantitation
(LOQ) were set to zero. Mean concentration valuesand standard deviation (SD)
were
calculated at each timepoints of the pharmacokinetic study (n=4
animals/timepoint).
The following pharmacokinetic parameters were calculated: area under the
plasma
concentration versus time curve from time zero to the last measurable
concentration -
time point (AUCo_t), area under the plasma concentration versus time curve
extrapolated to infinity (AUC;nf), maximum observed plasma concentration
(Cmax), time
of maximum plasma concentration (tmax), apparent first-order terminal
elimination rate
constant (kei), apparent first-order terminal elimination half-life will be
calculated as
20 0.693/kel (tii2). The systemic clearance (CL) of Compound I after
intravenous
administration was calculated using Dose/AUCinf. Pharmacokinetic parameters
were
calculated using KineticaTM 4.1.1 (InnaPhase Corporation, Philadelphia, PA).
Results:
Mean plasma concentrations of Compound I following intravenous (iv),
intraperitoneal (ip), subcutaneous (sc), and oral (po) administrations at 30
mg/kg are
presented in Figure 5. Mean plasma concentrations of Compound 2 following iv
and ip
administrations at 30 mg/kg, compared with Compound I via the same routes of
administration, are presented in Figure 6. When administered iv, Compound 2
had an
AUC of 92.08 pM=h and an observed Cmax of 105 pg/mL, compared to an AUC of
40.4
30 pM=h and an observed Cmax of 130 pg/mL for Compound 1. When administered
ip,
Compound 2 had an AUC of 58.75 pM=h and an observed Cmax of 5.8 pg/mL,
compared to an AUC of 9.5 pM=h and an observed Cmax of 2.25 pg/mL for Compound
1.
67
CA 02581658 2007-03-26
WO 2006/034574 PCT/CA2005/001467
Mean ( SD) plasma concentrations of Compound 1 following I.V. administration
of a 30 mg/kg dose declined rapidly in a biexponential manner resulting in
very short
half lives (t1/2 (x and R of 4.6 min and 2.56 h, respectively). The
pharmacokinetics of
Compound I following intraperitoneal and subcutaneous administrations, and
Compound 2 following intraperitoneal and intravenous administration, showed a
PK
profile suggestive of slow release. With these routes of administration, the
compound
plasma concentration was sustained and maintained at therapeutically relevant
levels
for over 8 hours. Compound 2 showed a half life (t1i2) of more than 40 hours
following
both IP and IV administrations. Oral administration of Compound 1 resulted in
io moderate but sustained drug levels. These data indicated that Compound I
was orally
bioavailable at a 5-8% level.
Mean tissue concentrations of Compound 1, 30 min after intravenous (iv),
intraperitoneal (ip) or subcutaneous (sc) administrations at 30 mg/kg are
presented in
Figure 7. The 30 min time point was chosen since plasma concentrations were
similar
with all three routes of administration. Compound I is well distributed
following iv and
ip dosing. Surprisingly, although ip and sc administrations resulted in a
similar PK
profile, tissue levels were significantly lower following sc dosing. This
could be
explained by the absence of peak levels following sc administration compared
with iv
';and ip administrations.
20 Acute toxicity studies in CD-1 nu/nu mice for Compound 2, using the same
formulation, gave an MTD _ 50 mg/kg (ip, NOAEL: 30 mg/kg) and ? 100 mg/kg (iv,
NOAEL: 75 mg/kg), with weight losses of about 7% for several days post-
injection.
Compound 1 had an MTD of 150 mg/kg when administered iv. Acute toxicity
studies
with Compound 46 gave an MTD of 30 mg/kg (ip).
All patents, patent applications, and published references cited herein are
hereby incorporated by reference in their entirety. While this invention has
been
particularly shown and described with references to preferred embodiments
thereof, it
will be understood by those skilled in the art that various changes in form
and details
30 may be made therein without departing from the scope of the invention
encompassed,
by the appended claims.
68