Note: Descriptions are shown in the official language in which they were submitted.
CA 02582717 2007-04-03
}
Description
PROCESS FOR PRODUCING AGLYCON AND FLAVOR-IMPROVED FOOD
CONTAINING THE AGLYCON BY DIGLYCOSIDASE, AND CONVERTING
AGENT TO BE USED IN THE PROCESS
This application is a division of Canadian Application
Serial Number 2,401,268, which is the national phase
application of PCT International Application PCT/JP01/02656,
filed March 29, 2001.
Technical Field
The present invention relates to a process for
producing an aglycon, a process for producing a protein having
an increased aglycon content or a food containing said protein,
a process for producing a flavour-improved protein or a food
containing said protein, and a process for forming an
isoflavone in a living body. The invention may be utilized,
for example, for producing processed foods, health foods,
dietary supplements, and medicaments.
Background Art
Glycosides are chemical substances wherein a
saccharide is bonded to a non-saccharide part called aglycon,
and are widely present in nature.
Some glycosides are known to exhibit physiological activity
through decomposition into aglycons by a glycosidase such as
glucosidase produced by enteric bacteria though the glycosides
themselves do not exhibit the physiological activity because of their
poor enteric absorption, as phytochemicals (phytogenic functional
1
CA 02582717 2007-04-03
ingredients) such as polyphenols having antioxidation
action and phytoestrogens having a weak estrogenic action.
Thus, in view of preventing and symptom-alleviating effects
of life-style related diseases such as cancer,
arteriosclerosis, osteoporosis, and climacteric disorder,
and diseases owing to aging, attention has been focused on
soybean protein concentrates, soybean-processed materials,
and food containing the soybean protein concentrates.
Among the phytochemicals, glucosides that contain
isoflavones as aglycons as represented by the following
general formula (hereinafter, sometimes referred to as
isoflavone glucosides) contain aglycons such as daidzein,
genistein, glycitein, and it is revealed from cellular
level investigations and epidemiological surveys that they
inhibit growth of breast cancer and prostatic cancer cells,
alleviate arteriosclerosis and osteoporosis, and also
alleviate climacteric disorder owing to the female hormone-
like estrogenic action.
CH2OR3
O O O
OH
I OH R2
OH O
1 ~ OH
2
CA 02582717 2007-04-03
= (
=
(wherein R1 and R2 each is independently selected from the
group consisting of H, OH, and OCH3, and R3 is selected
from the group of H, COCH3, COCH2COOH, and COCH2CH2COOH)
However, there is a possibility that a sufficient
amount of glycosidase cannot be produced by enteric
bacteria in elderly persons, sick persons, and antibiotics-
administered patients, and also a glycosidase is difficult
to decompose glycosides modified with acetyl or malonyl
group and disaccharide or trisaccharide glycosides, so that
it cannot be expected to absorb a sufficient amount of
phytochemicals contained in soybean protein concentrates
and the like.
Moreover, it is avoided to take foods containing
soybean-processed materials such as soybean protein
concentrates especially in Western countries owing to the
distinctive smell and bitterness, and thus some limitation
exists as sources of aglycon isoflavones.
Accordingly, a process for forming phytochemicals
efficiently in a living body, and an improvement of flavor
of a soybean-processed material rich in aglycon
isoflavones, which are highly efficiently absorbed and
capable of ingesting a sufficient amount of isoflavones by
taking small amount of them, and having less smell and
bitterness distinctive of soybean or of a food containing
the soybean-processed material have been desired.
3
CA 02582717 2007-04-03
On the other hand, the method for converting an
isoflavone glucoside into an aglycon isoflavone is known as
described in JP-A-10-117792. In this method, a phytogenic
protein extract is treated with an alkali to convert a
modified glucoside isoflavone into a glucoside isoflavone,
which is then subjected to a treatment with glycosidase.
Such a treatment is carried out because conventional
glycosidase cannot act directly on the modified glucoside
isoflavone. Thus, the method is accompanied by the
problems of requirement of two steps, enhancement of
bitterness by the alkali-treatment, change in physical
propgrties and ingredients, formation of by-products, waste
liquid after the alkali-treatment, and the like. Also,
glucosidase which takes charge of a main part of the action
tends to be influenced by free glucose, so that the kind
and concentration of the material used for the production
may be limited.
Moreover, JP-A-8-214787 describes a process for
converting a glucoside isoflavone into an aglycon
isoflavone by fermentation using a microorganism. However,
there are possibilities of decomposing the resulting
aglycon isoflavone by the microorganism and of forming
unexpected by-products, and therefore many problems may
arise at the actual production.
Furthermore, a method of hydrolysis with an acid
such as hydrochloric acid may be a candidate, but the
4
CA 02582717 2007-04-03
. . ~
decomposition of proteins, phospholipids, neutral lipids,
and other ingredients may occur along with formation of by-
products because of the severe conditions. Especially, the
formation of chlorinated compounds such as MCP
(monochloropropanol) and DCP (dichloropropanol) whose
carcinogenicity has been reported cannot be avoided.
Therefore, an object of the invention is to
provide a process for producing a phytogenic
physiologically active substance of aglycon type
efficiently without resort of any acid/alkali treatment or
fermentation and substantially without changing the
physical properties of a material. Moreover, other objpcts
of the invention are to enhance the aglycon content in a
protein or a protein-containing food by using diglycosidase
and/or a specific enzyme preparation and to improve the
flavor. These objects and other objects will be further
clarified by the following detailed explanations.
Disclosure of the Invention
As a result of extensive studies, we have found
that diglycosidase discovered from origins of various
microorganisms efficiently decompose glycosides which are
difficult to decompose by conventional glycosidase and also
acts even in a living body, and thus accomplished the
invention. Furthermore, we have found that the invention
can be conducted using any diglycosidase from any source.
5
CA 02582717 2007-04-03
, . ~ .
Namely, the invention relates to the following.
(1) A process for producing an aglycon which comprises
forming an aglycon by treating, with diglycosidase, a glycoside
containing a compound selected from the group consisting of
phytoestrogens, polyphenols, isoflavones, biochanin A,
formononetin, cumestrol, and lignans as the aglycon.
(2) The process for producing an aglycon according
to (1), wherein the aglycon is an isoflavone.
(3) The process for producing an aglycon as described
above, wherein the glycoside containing an isoflavone as the
aglycon is one.or more selected from the group consisting of
daidzin, genistin, or glycitin and acetyl derivatives, succinyl
derivatives, or malonyl derivatives thereof.
(4) The process for producing an aglycon as described
above, wherein the diglycosidase is a glucose-tolerant one.
(5) The process for producing an aglycon as described
above, wherein the diglycosidase is diglycosidase produced by
Penicillium multicolor IAM7153.
(6) A process for producing a protein having an increased
aglycon content or a food containing the protein, which
comprises a step of treating a protein or protein-containing food with
diglycosidase.
6
CA 02582717 2007-04-03
=
(7) The process for producing a protein having an
increased aglycon content or a food containing the protein
as described above, wherein the protein or protein-
containing food contains a glycoside containing an
isoflavone as the aglycon.
(8) The process for producing a protein having an
increased aglycon content or a food containing the protein
as described above, wherein the protein or protein-
containing food to be produced is a further flavor-improved
one.
(9) The process for producing a protein having an
increased.aglycon content or a food containing.the protein
as described above, wherein the glycoside containing an
isoflavone as the aglycon is one or more selected from the
group consisting of daidzin, genistin, or glycitin and
acetyl derivatives, succinyl derivatives, or malonyl
derivatives thereof.
(10) The process for producing a protein having
an increased aglycon content or a food containing the
protein as described above, which further comprises a step
of treating with an enzyme preparation containing mainly at
least one enzyme selected from the group consisting of
amylases, proteases, lipases, a-glucosidases, and yeast-
dissolving enzymes.
(11) The process for producing a protein having
an increased aglycon content or a food containing the
7
CA 02582717 2007-04-03
. , '
protein as described above, wherein the improvement of
flavor is reduction of bitterness and/or astringency.
(12) A process for producing a flavor-improved
protein or a food containing the protein, which comprises a
step of treating with an enzyme preparation containing
mainly at least one enzyme selected from the group
consisting of amylases, cellulases, pectinases, proteases,
lipases, a-glucosidases, a-galactosidase, and yeast-
dissolving enzymes.
(13) The process for producing a flavor-improved
protein or a food containing the protein as described
above, wherein the protnin or protein-containing food
contains a glycoside containing a flavonoid as the aglycon.
(14) The process for producing a flavor-improved
protein or a food containing the protein as described
above, wherein the protein or protein-containing food
contains a glycoside containing an isoflavone as the
aglycon.
(15) A method of administering diglycosidase
orally to form an aglycon from a glycoside in a living
body.
(16) The method as described above, wherein
diglycosidase is orally administered to form an aglycon in
a living body from a glycoside containing an isoflavone as
the aglycon.
8
CA 02582717 2007-04-03
. - 1
.
(17) A method of converting a physiologically active
substance of glycoside type into a physiologically active
substance of aglycon type, which comprises treating the
physiologically active substance of glycoside type with
diglycosidase.
(18) A process for producing a composition rich in a
phytogenic physiologically active substance of aglycon type, which
comprises treating a phytogenic material containing a phytogenic
physiologically active substance of glycoside type with
diglycosidase.
(19) A method of accelerating a bioabsorption of a
physiologically active substance, which comprises administering
diglycosidase orally before, during, or after the ingestion of a
food containing a physiologically active substance of glycoside
type.
(20) An agent converting a physiologically active substance
of glycoside type into the physiologically active substance of
aglycon type, which contains at least diglycosidase.
In another aspect, the present invention provides a process
for producing an aglycon which comprises forming an aglycon by
treating, with diglycosidase, a glycoside containing an
isoflavone as the aglycon, wherein said diglycosidase has
activity to act upon a disaccharide glycoside to release
saccharides in a disaccharide unit, wherein said diglycosidase is
isolated from a micoroorganism, wherein said diglycosidase is not
inhibited by free glucose, wherein said diglycosidase has an
9
CA 02582717 2007-04-03
=
optimum temperature of about 55 C, wherein said diglycosidase has
an optimum pH of about 3.5 to 5, and wherein the diglycosidase is
diglycosidase produced by Penicillium multicolor IAM7153 or a
mutant strain thereof.
In another aspect, the present invention provides a method
of converting a physiologically active substance of glycoside
type into a physiologically active substance of aglycon type,
which comprises treating the physiologically active substance of
glycoside type with diglycosidase, wherein said physiologically
active substance of glycoside type comprises an isoflavone as the
aglycon, wherein said diglycosidase has activity to act upon a
disaccharide glycoside to release saccharides in a disaccharide
unit, wherein said diglycosidase is isolated from Penicillium
multicolor IAM7153 or a mutant strain thereof, wherein said
diglycosidase is not inhibited by free glucose, wherein said
diglycosidase has an optimum temperature of about 55 C, and
wherein said diglycosidase has an optimum pH of about 3.5 5.
In another aspect, the present invention provides a process
for producing a composition rich in a phytogenic physiologically
active substance of aglycon type, which comprises treating a
phytogenic material containing a phytogenic physiologically
active substance of glycoside type with diglycosidase, wherein
the phytogenic physiologically active substance of glycoside type
comprises an isoflavone as the aglycon, wherein said
diglycosidase has activity to act upon a disaccharide glycoside
to release saccharides in a disaccharide unit, wherein said
diglycosidase is isolated from Penicillium multicolor IAM7153 or
9a
CA 02582717 2007-04-03
'. (
.
a mutant strain thereof, wherein said diglycosidase is not
inhibited by free glucose, wherein said diglycosidase has an
optimum temperature of about 55 C, and wherein said diglycosidase
has an optimum pH of about 3.5 to S.
In another aspect, the present invention provides a process
for producing an aglycon which comprises forming an aglycon by
treating, with diglycosidase, a glycoside containing an
isoflavone as the aglycon, wherein said diglycosidase has
activity to act upon a disaccharide glycoside to release
saccharides in a disaccharide unit, and wherein said
diglycosidase is prepared by a process comprising: (a) culturing
Penicillium multicolor IAM 7152 or a mutant strain thereof in a
nutrient medium under aerobic conditions with a pH from 3 to 8 to
effect production of said diglycosidase in a culture mixture;
(b) isolating said diglycosidase by a combination of
centrifugation, ultrafiltration concentration, and salting out of
said diglycosidase by 50 to 80% ammonium sulfate from said
culture mixture to produce an isolate diglycosidase fraction; and
(c) purifying said diglycosidase from said isolated diglycosidase
fraction by hydrophobic chromatography.
These embodiments and other embodiments of the invention will
be further clarified by the following detailed explanations.
Diglycosidase described in the invention efficiently acts on
the glycosides that contain a compound selected from the group
consisting of phytoestrogens, polyphenols, isoflavones, biochanin
A, formononetin,
9b
CA 02582717 2007-04-03
cumestrol, and lignans as the aglycon (hereinafter, also
referred to as aglycon glycoside), can very efficiently act
especially on the glycosides containing an isoflavone as
the aglycon (hereinafter, also referred to as isoflavone
glycosides), and is hardly influenced by free glucose.
Therefore, the process can be advantageously carried out in
the case that the isoflavone glycoside is daidzin,
genistin, or glycitin, or an acetyl derivative, succinyl
derivative, or malonyl derivative thereof. By the way, the
isoflavone formed from the isoflavone glycoside is also
referred to as aglycon isoflavone.
Moreov9r, by administering the diglycosidasg, the
preventive effect on various diseases can be enhanced
through the formation of an isoflavone from an aglycon
glycoside or a food containing the same which is orally
ingested. Diglycosidase produced by Penicillium multicolor
IAM7153 is preferably used as diglycosidase. (3-
Galactosidase derived from Penicillium multicolor is an
enzyme which is described in Food Additives List and whose
safety is recognized, and thus diglycosidase produced by
such highly safety bacterium is estimated to be highly
safe.
Furthermore, in the case of using soybean as a
starting material, although the benefit of soybean protein
concentrates and soybean-processed materials to health is
reported, ingestion of them are avoided especially in
CA 02582717 2007-04-03
Western countries owing to the distinctive smell and
bitterness. The above-mentioned acetylglycoside
isoflavones and malonylglycoside isoflavones have a strong
bitterness but the aglycon isoflavones have less
bitterness. Therefore, a food wherein bitterness of
soybean protein concentrate or soybean-processed material
is efficiently reduced can be provided by the conversion
into aglycon isoflavones by diglycosidase.
When such soybean protein concentrate or soybean-
processed material wherein isoflavones are concentrated as
aglycons having higher absorption efficiency is used, a
sufficient amount of isoflavones can be ingested through a
little intake, and also the form can be changed to a form
easily ingested by people who avoid the smell and strange
taste distinctive of soybean.
Moreover, owing to the smell and strange taste of
soybean protein concentrates or soybean-processed
materials, the amount used is limited in the case of using
them as food materials, and therefore soybean protein
concentrates or soybean-processed materials have a
limitation as isoflavone sources for foods. According to
the invention, use of the soybean protein concentrates or
soybean-processed materials, wherein isoflavone glycosides
are digested with diglycosidase and concentrated as
isoflavone aglycons which have higher absorption efficiency
and less bitterness, enables supply of isoflavones to foods
11
CA 02582717 2007-04-03
through a little use of the concentrates or materials, so
that they can be utilized for many kinds of foods as
isoflavone sources.
Diglycosidase for use in the invention is
characterized in that it has an activity of acting on a
disaccharide glycoside, which is difficult to utilize as a
substrate by conventional glucosidase, to isolate a
saccharide as a two-saccharide unit from the disaccharide
glycoside and also to form an aglycon. Herein, an enzyme
having the above activity is referred to as
"diglycosidase".
. The diglycosidase in the invention,is an enzyme
which is classified into a saccharide-chain hydrolase but
has a property different from the properties of
conventional a- and (3-glycosidases. Diglycosidase can
utilize, as a substrate, so-called a glycoside wherein a
linear or branched saccharide chain composed of single or
two or more kinds of saccharides is bonded to a compound
other than a saccharide through hydroxyl group in the
saccharide chain, and recognizes the substrate at the two-
saccharide unit to cleavage it, whereby corresponding
disaccharide and an aglycon having a saccharide chain with
two saccharide-smaller chain length are formed successively
and finally, an aglycon is formed. Additionally, it also
decomposes modified glucosides such as acetyl derivatives,
succinyl derivatives, and malonyl derivatives, which are
12
CA 02582717 2007-04-03
difficult to decompose by conventional glucosidase, into
saccharides and aglycons. As representative examples of
saccharides present in nature, starch, cellulose,
polysaccharides constituting cell walls, and the like may
be mentioned. Many kinds of saccharide chains may be
suitable for the saccharide chains of glycosides, and
examples thereof include 6-O-R-D-xylopyranosyl-P-D-
glucopyranoside (R-primeveroside), 6-O-a-L-
arabinopyranosyl-R-D-glucopyranoside (vicianoside), 6-O-a-
L-arabinofuranosyl-R-D-glucopyranoside, 6-O-a-L-
rhamnopyranosyl-R-D-glucopyranoside (rutinoside), 6-O-R-D-
apiofuranosyl-R-D-glucopyranoside, 6-O-R-D-glucopyranosyl-
~-D-glucopyranoside (gentiobioside), 4-O-a-glucopyranosyl-
R-D-glucopyranoside (maltose), 2-O-a-L-rhamnopyranosyl-p-D-
galactopyranoside (rhamninose), 6-O-a-L-rhamnopyranosyl-R-
D-galactopyranoside (robinobioside), 2-O-R-D-xylopyranosyl-
R-D-glucopyranoside (xylosylglucose), 4-O-R-D-
glucopyranosyl-R-D-glucopyranoside (cellobioside),
xylobioside, and the like. Other than the above-mentioned
compounds, any combination of saccharides can be recognized
as a substrate for the reaction as far as the combination
has a disaccharide structure. Aglycon means a compound to
be obtained from a glycoside by eliminating a saccharide of
the glycoside. Aglycons of glycosides are widely present
in nature, and examples thereof include volatile compounds
in plants such as linalool, geraniol, citronellal,
13
CA 02582717 2007-04-03
phenethyl alcohol, citronellol, jasmones, limonene,
terpinene, citral, nerol, pinene, borneol, terpineol,
methyl jasmonate, hexanol, hexenol, hexanal, hexenal,
vanillin, benzaldehyde, eugenol, methyl salicylate,
linalool oxide, benzyl alcohol, and vomifomitol; pigments
in plants such as alizarin, purpurin, anthocyanidin
including pellagonidin, cyanidin, deiphinidin, peonidin,
petunidin, and malvidin; and flavonoids such as nariltin,
naringenin, hesperetin, neohesperetin, diosmetin,
quercetin, campherol, myricetin, isorhamnetin, and
syringenin; and the like. Other than the compounds
mentioned herein, various compounds may be present as
aglycons of glycosides or may become aglycons of
glycosides.
Furthermore, diglycosidase can utilize so-called
monosaccharide glycosides, wherein one molecule of
saccharide is bonded to an aglycon, as substrates to form
corresponding monosaccharides and aglycons, other than
above-mentioned disaccharide-isolating activity. In
particular, it is a characteristic that diglycosidase can
act on monosaccharide glycosides which is resistant to
hydrolysis by conventional (3-glucosidase.
Diglycosidase for use in the invention can be
obtained from microorganisms having ability of producing
diglycosidase without requiring undue experimental burden
14
CA 02582717 2007-04-03
from those skilled in the art (For example, cf.
WO00/18931) .
The microorganisms producing diglycosidase of the
invention can be obtained by the following screening, for
example. That is, an enrichment culture is carried out by
inoculating a soil suspension to a liquid medium for
separation containing eugenyl primeveroside or the like as
sole carbon source, applying the culture liquid onto a
similar plating agar medium for separation and selecting
colonies grown. These strains are cultured in a suitable
liquid medium and strains having pNP-isolating activity can
be selected through cleavage of disaccharide from pNP-
primeveroside or the like.
On these strains thus selected, microorganisms
producing diglycosidase can be screened using pNP-
primeveroside or the like as a substrate and disaccharide
isolation as a measure.
The producing ability has been already confirmed
on Aspergillus niger IF04407 (available from Institute of
Fermentation, 2-17-85, Juso-honmachi, Yodogawa-ku, Osaka),
Aspergillus niger IAM 2020, Aspergillus fumi.gatus IAM2046,
Penici113.um multicolor IAM7153 (available from Institute of
Molecular Cell Biology, the University of Tokyo, 1-1-1,
Yayoi, Bunkyo-ku, Tokyo), and the like.
Additionally, in other various microorganisms, the
diglycosidase activity has been confirmed on various
CA 02582717 2007-04-03
microorganisms such as the genus Aspergillus, the genus
Penicillium, the genus Rhizopus, the genus Rhizomncor, the
genus Talaromyces, the genus Mortierella, the genus
Cryptococcus, the genus Microbacterium, the genus
Corynebacterium, the genus Actinoplanes, and the like.
Any strain can be used in the invention as far as
it has an ability of producing diglycosidase, and the
strain is not limited to the above-mentioned strains.
Furthermore, the process for producing diglycosidase usable
in the invention includes mutant strains of the strains
having a diglycosidase-producing ability, or various
microorganisms or various cells (e.g., yeast cells,
bacterial cells, higher plant cells, and animal cells)
modified so as to be capable of producing diglycosidase by
recombinant DNA method, and particularly preferred are
those modified so as to be capable of producing
diglycosidase with high productivity. In the case that a
diglycosidase-producing ability is imparted by introducing
a diglycosidase gene, the microorganism used as a host may
not have a diglycosidase-producing ability.
For producing diglycosidase using the above
various microorganisms, a method and conditions suitable
for the culture of the microorganism can be set, and the
method and conditions are not particularly limited. For
example, any of liquid culture and solid culture may be
used for culturing the above various strains, but liquid
16
CA 02582717 2007-04-03
culture is preferably used. The liquid culture may be
carried out as follows, for example.
The medium to be employed may be any medium as far
as the microorganism producing diglycosidase is capable of
growing in the medium. For example, there may be used
media to which carbon sources such as glucose, sucrose,
gentiobiose, soluble starch, glycerol, dextrin, molasses,
and organic acids; further nitrogen sources such as
ammonium sulfate, ammonium carbonate, ammonium phosphonate,
ammonium acetate, or peptone, yeast extract, corn steep
liquor, casein hydrolysate, bran, and meat extract; and
further inorganic salts such as.potassium salts, magnesium
salts, sodium salts, phosphonates, manganese salts, iron
salts, and zinc salts are added. Furthermore, for
accumulating diglycosidase, various inducing substances may
be added to the medium. As the inducing substances,
saccharides may be used, for example, and there may be
preferably used gentose (e.g., gentose #80, Nihon Shokuhin
Kako Co., Ltd.), gentiobiose, genti-oligosaccharide (e.g.,
gentiologo etc., Wako Pure Chemical Industries, Ltd.),
galactomannan, and the like. The adding amount of these
inducing substances is not particularly limited as far as
the productivity of aimed diglycosidase is enhanced, but
the substance is preferably added in an amount of 0.01 to
10%.
17
CA 02582717 2007-04-03
' i.
The pH of the medium is adjusted to from about 3 to 8,
preferably from about 5 to 6, and culture is carried out at a
temperature of about 10 to 50 C, preferably about 25 to 30 C for 1
to 15 days, preferably 4 to 7 days under aerobic conditions. As
the culturing method, a shaking culture or an aerobic submerged
culture by means of a jar fermenter may be utilized. However, the
above various culturing conditions may be optionally changed, of
course, depending on the microorganism or cell to be cultured, and
the conditions are not particularly limited as far as diglycosidase
of the invention is produced.
For isolation and purification of diglycosidase from the
culture liquid obtained, using a diglycosidase activity as a
measure, purified diglycosidase can be obtained by combining
centrifugal separation, UF concentration, salting out, and various
chromatography such as ion exchange resins, and treating in a usual
manner (Referential document: Tanpakusitsu=Kouso no Kisojikkenhou,
Basic experimental methods for proteins and enzymes, edited by
Takeichi Horio, 2na edition, issued in Japan on November 10, 2004
by Nankodo Co., Ltd., ISBN: 9784524401239).
A culture liquid obtained by culturing the above microorganism
may be utilized as such as the enzyme composition of the
invention. Of course, the culture liquid may be optionally changed
in the degree of purification according to the purpose used in the
invention.
18
CA 02582717 2007-04-03
The invention provides a process for producing an
aglycon which comprises forming an aglycon by treating,
with diglycosidase, a glycoside containing a compound
selected from the group consisting of phytoestrogens,
polyphenols, isoflavones, biochanin A, formononetin,
cumestrol, and lignans as the aglycon. The producing
process includes the reaction of a phytogenic material
containing the above compound as the aglycon with a
sufficient amount of diglycosidase under weakly acidic
conditions at an appropriate temperature and pH for a
sufficient period of time so as to convert at least most of
the glycoside in the starting material into an aglycon,
whereby an aglycon is produced. The invention provides a
producing process wherein diglycosidase is added to a plant
extract in order to produce a plant extract rich in an
aglycon.
The novel process is a one-step process of
converting most of an aglycon glycoside into free aglycon
by an enzyme preparation containing a hydrolase of
disaccharide glycosides, i.e., diglycosidase. The process
is effective for the aglycon glycosides present in
phytogenic materials, preferably proteins or protein foods.
Since the process is found to be capable of substantially
complete conversion of modified glucoside isoflavones and
glucoside isoflavones into aglycon isoflavones, it includes
the conversion of modified glucoside isoflavones and
19
CA 02582717 2007-04-03
glucoside isoflavones into aglycon isoflavones. In some
phytogenic protein materials, particularly soybean protein
materials, substantial part of total isoflavone contents in
the phytogenic protein materials is present in the form of
isoflavone glycosides. Therefore, not only the conversion
of glycoside isoflavones into aglycon isoflavones but also
the conversion of modified glycoside isoflavones into
aglycon isoflavones are necessary for maximum increase of
the amount of aglycon isoflavones obtainable from the
phytogenic protein materials.
The starting material in a preferred embodiment is
any protein or protein-containing food (more preferably a
phytogenic material, phytogenic protein, or phytogenic
protein-containing food) containing a physiologically
active substance of glycoside type. Some processes in the
following explanations are described using soybean products
as examples, but the process of the invention can be
generally applied to a wide range of proteins or protein-
containing foods other than soybean and soybean products.
In the invention, the "protein or protein-
containing food" preferably contains a physiologically
active substance of glycoside type but is not particularly
limited.
The "phytogenic material" in the invention means a
whole plant body which is edible or used as a medicine, or
a part thereof such as leaf, flower, fruit, stem, or root,
CA 02582717 2007-04-03
or a processed product thereof. Examples thereof include
whole plant bodies harvested, or parts thereof such as
leaf, flower, fruit, stem, and root, and plant extracts and
processed products thereof. Specific examples of the
phytogenic material include the following materials:
phytogenic proteins such as soybean protein, soymilk,
juices (orange juice, grape juice, apple juice, pomegranate
juice), herb tea, plant extracts such as herb extract, and
processed products of the above materials such as juice
drinks, wine, tea, black tea, and cocoa.
The "phytogenic protein" means a protein
obtainable from the above "phytogenic material", and may be
a mixture with other ingredients derived from the
phytogenic material.
In the invention, the "compound selected from the
group consisting of phytochemicals, phytoestrogens,
polyphenols, isoflavone, biochanin A, formononetin,
cumestrol, and lignans" is not particularly limited as far
as the compound falls within these conceptual range, but it
is preferably a compound which exhibits a physiological
activity or enhances a physiological activity in a living
body (preferably a warm-blooded animal, more preferably
human). The compound is preferably a flavonoid, more
preferably an isoflavone, most preferably an isoflavone
represented by the above structural formula.
21
CA 02582717 2007-04-03
The "physiologically active substance" in the
invention means a substance, most of which is preferably
present as a glycoside in a plant body and which exhibits a
physiological activity or enhances a physiological activity
in a living body upon the conversion into the aglycon type.
Specifically, the physiologically active substance includes
phytochemicals, phytoestrogens, polyphenols, isoflavone,
biochanin A, formononetin, cumestrol, and lignans as
mentioned above, and preferred are isoflavones.
The "physiologically active substance of glycoside
type" means that the aglycon of the above glycoside is a
physiologically active substance, and the saccharide chain
is composed of one or more saccharide, preferably two or
more saccharides. The two-saccharide chains include those
mentioned above and the like.
The "enzyme preparation containing mainly at least
one enzyme selected from the group consisting of amylases,
proteases, lipases, a-glucosidases, and yeast-dissolving
enzymes" is not particularly limited as far as it mainly
contains these enzymes, and commercially available enzymes
may be employed. The following will illustrates those
manufactured by Amano Enzyme Inc. Examples of amylase
include Amylase AD "Amano" 1(optimum pH: 6.0, optimum
temperature: 70 C), Gluczyme NL 4.2 (optimum pH: 4.5,
optimum temperature: 65 C), Transglucosidase L"Amano"
(optimum pH: 5.0, optimum temperature: 60 C), and the like.
22
CA 02582717 2007-04-03
Examples of cellulase include Cellulase A"Amano" 3
(optimum pH: 4.5, optimum temperature: 55 C), Cellulase T
"Amano" 4, Hemicellulase "Amano" 90G (optiiaum pH: 4.5,
optimum temperature: 50 C), Hemicellulase GM "Amano", and
the like. Examples of pectinase include Pectinase PL
"Amano" (optimum pH: 4.55-0, optimum temperature: 60-55 C)
and the like. Examples of protease include Umamizyme,
Newlase F3G, Papain W-40, Pancreatin F, Protease B,
Protease A"Amano" G, and the like, and examples of lipase
include Lipase A"Amano" 6 (optimum pH: 6.5, optimum
temperature: 45 C), and the like. A yeast-dissolving
enzyme preparation YL-15 (optimum pH: 7.0, optimum
temperature: 50-55 C) is mentioned as a yeast-dissolving
enzyme, and ADG-S-DS (optimum pH: 4.5-5, optimum
temperature: 50-60 C) and the like are mentioned as an a-
galactosidase.
The above enzymes and enzyme preparations can be
produced by known methods. For example, an enzyme can be
obtained by screening a microorganism producing a specific
enzyme mentioned above in a similar manner to the
production of diglycosidase and culturing the resulting
enzyme-producing strain in a suitable medium. Examples of
the above enzyme-producing strain include Bacillus
subtillis, Aspergillus niger, Aspergillus oryzae,
Trichoderma viride, Rhizopus nivenus, Pseudomonas sp., and
the like.
23
CA 02582717 2007-04-03
The following will explain the invention in
further detail with regard to the process for producing a
protein having an increased aglycon content or a food
containing the protein, which comprises a step of treating
a protein or protein-containing food with diglycosidase, by
way of illustration of a phytogenic physiologically active
ingredient (especially an isoflavone glycoside) derived
from a phytogenic material, but the invention can be
conducted using any above compound other than the
phytogenic physiologically active ingredient derived from a
phytogenic material. By the way, the term of soybean
material used herein means any type of soybean or variants
of soybeans.
Some different embodiments are possible as
specific processes for carrying out the invention.
In the first embodiment, a phytogenic
physiologically active substance of glycoside type is
converted into a phytogenic physiologically active
substance of aglycon type while the phytogenic
physiologically active substance is left in the phytogenic
material. Therefore, the formed phytogenic physiologically
active substance of aglycon type may be left in the
phytogenic material or may be suitably removed. The
aglycon form of the phytogenic physiologically active
substance may be generally removed by a solvent,
hydrophobic effluence or extraction. The solvent suitable
24
CA 02582717 2007-04-03
for the operation includes acetone, ethanol, and other
similar organic solvents, but is not limited thereto.
In the second embodiment, a phytogenic
physiologically active substance of glycoside type (e.g.,
isoflavone modified glycoside or isoflavone glucoside) in a
phytogenic material is removed from the phytogenic material
by aqueous effluence or extraction. The aqueous effluence
is carried out through the effluence of relatively soluble
phytogenic physiologically active substance of glycoside
type by immersing the phytogenic material or by exposing
the phytogenic material to or dipping it in water or a
mixture of hydrophilic solvents.such as ethanol or other
alcohols. The pH of the resulting aqueous solution is from
about pH 2 to about pH 5, preferably about pH 4. After
removal, the phytogenic physiologically active substance of
glycoside type is converted into the phytogenic
physiologically active substance of aglycon type.
In the third embodiment, prior to all the
operations for conversion, a phytogenic physiologically
active substance of glycoside type is removed from a
phytogenic material.
Depending on the type of phytogenic material
containing a phytogenic physiologically active substance of
glycoside type, in some cases, the phytogenic material is
preferably processed to a finely crushed form. This
operation is desirable for bringing a phytogenic
CA 02582717 2007-04-03
physiologically active substance in the phytogenic material
into contact with a reagent (diglycosidase) employed in the
step which will be described in detail in the following.
The material may be subjected to grinding, crushing, or
other processing. When the phytogenic material is in
condition that isoflavone compounds in the phytogenic
material easily come into contact with an external reagent
or reactant, e.g., a small leaf part in a plant, it is not
necessary to subject the phytogenic material to the above
processing.
The conversion of a phytogenic physiologically
active substance of glycoside type into the phytogenic
physiologically active substance of aglycon type is
sometimes partially carried out by enzymes present in the
mixture depending on the phytogenic material used. These
enzymes may be present naturally in phytogenic protein
materials or may be derived from microorganisms grown in
the materials. Such enzymes are called as residual
enzymes. However, there is a possibility that the
conversion of the phytogenic physiologically active
substance of glycoside type into the phytogenic
physiologically active substance of aglycon type cannot be
carried out sufficiently depending on the nature and
concentration of the residual enzyme in the phytogenic
protein materials. By adding an enzyme preparation
containing an external enzyme, i.e., diglycosidase, maximum
26
CA 02582717 2007-04-03
converting efficiency of the phytogenic physiologically
active substance of aglycon type can be achieved.
In the invention, the amount of the enzyme to be
added depends on various factors including the type of
enzyme present, the distribution of enzyme concentration,
the pH of reaction system, the activity of enzyme present,
and temperature. In the case of adding an enzyme,
typically preferred enzyme amount is preferably from 28 to
2800 AU, usually from 10 to 10000 AU relative to 100 g of a
phytogenic material as total concentration of enzyme
present based on dry weight thereof. When a sufficient
concentration of enzym~s including a residual enzyme, an
additional enzyme or both enzymes are present in the
system, a phytogenic physiologically active substance of
glycoside type is brought into contact with the enzymes at
an appropriate temperature and pH for a sufficient period
of time so as to convert substantially all the phytogenic
physiologically active substance of glycoside type in the
mixture into the phytogenic physiologically active
substance of aglycon type.
The conversion-production step is preferably
carried out at a pH of about 2 to about 6. More preferred
pH range for the conversion-production step is from about 3
to about 5. Depending on the phytogenic material used, the
pH may be adjusted with an acidic reagent such as
hydrochloric acid, phosphoric acid, acetic acid, or
27
CA 02582717 2007-04-03
sulfuric acid, or an alkaline reagent such as sodium
hydroxide. In many cases, it is assumed to use an acidic
or alkaline reagent of food grade. The temperature to be
used in the conversion-production step is preferably from
about 25 C to about 65 C. More preferred temperature is
from about 30 C to about 55 C. Throughout the reaction,
the temperature is usually constant, but the temperature
may be elevated or lowered according to the successive step
and final intended use. Namely, it may be relatively
freely changed according to the various circumstances of
the situation.
The period of time necessary,for the conversion
and production may be determined depending on complicated
relationship between various factors of the kind,
concentration, and physical properties of the material to
be reacted, the concentration of the enzyme added, and
further the temperature and pH of the reaction system. In
most cases, the conversion-production can be substantially
completely achieved within 6 to 12 hours. The period of
time for the conversion-production can be shortened
depending on the concentration of the diglycosidase
preparation added. At the conversion-production step, most
of the isoflavone glycoside in the mixture can be converted
into the aglycon isoflavone. The efficiency of the
conversion is usually at least about 50% or more,
preferably about 70% or more. By adopting the above
28
CA 02582717 2007-04-03
. (.
preferable reaction conditions, nearly complete conversion
can be achieved.
By adopting conditions similar to the above, it is
possible to carry out a process of the invention for
producing an aglycon which comprises forming an aglycon by
treating, with diglycosidase, a glycoside containing a
compound selected from the group consisting of
phytoestrogens, polyphenols, isoflavones, biochanin A,
formononetin, cumestrol, and lignans as the aglycon.
In addition to the above step, in the invention,
the process may further comprise a step of treating with an
enzyme preparation containing mainly at least one enzyme
selected from the group consisting of amylases, proteases,
lipases, a-glucosidase, and yeast-dissolving enzymes. This
step may be conducted before or after the step of treating
with diglycosidase, or the treatment with diglycosidase and
the enzyme preparation may be carried out at the same time.
In this case, the treatment with diglycosidase and the
enzyme preparation at the same time is carried out under
the conditions similar to those in the case of using
diglycosidase solely. Moreover, when the treatment with an
enzyme preparation is carried out before or after the
treatment with diglycosidase, the pH, temperature, period
of time, and the like may be selected in consideration of
optimum pH and optimum temperature of the above each enzyme
preparation. This process is also accompanied by the
29
CA 02582717 2007-04-03
effects of increasing aglycon content in a protein or
protein-containing food and of improving flavor through the
reduction of bitterness and/or astringency.
Additionally, in the invention, during the process
of finding the above effects of the combined use with
diglycosidase, it was found that flavor is improved by
treating a protein or protein-containing food with a
specific enzyme preparation alone, i.e., an enzyme
preparation containing mainly at least one enzyme selected
from the group consisting of amylases, cellulases,
pectinases, proteases, lipases, a-glucosidase, a-
galactosidases, and yeast-dissolving enzymes. With regard
to the treating conditions in this case, the pH is
preferably from 3 to 8, more preferably from 5 to 7.5, and
the treating temperature and treating time are similar to
the case of the combined use with diglycosidase. By the
way, the treated product may be optionally adjusted to a
desired pH.
By treating a protein or protein-containing food
as mentioned above, the flavor of the protein or protein-
containing food can be improved and particularly,
bitterness and/or astringency can be reduced.
In addition, by administering the phytogenic
physiologically active substance of aglycon type produced
as above or a composition rich in the phytogenic
physiologically active substance of aglycon type as such or
CA 02582717 2007-04-03
as a mixture with a food or drink, the effect derived from
the phytogenic physiologically active substance can be
attained. The effects of the phytogenic physiologically
active substance include effects of preventing various
diseases (cancer, life-style related diseases,
osteoporosis, a burning sensation in climacteric disorder,
and the like), and of regulation of intestinal function,
inununostimulation, and biophylactic action. Moreover,
other than the administration of the phytogenic
physiologically active substance which is converted into
aglycon type beforehand, by administering orally a
phytogenic physiologically active substance of glycoside
type and/or a phytogenic material containing a phytogenic
physiologically active substance of glycoside type together
with diglycosidase, the phytogenic physiologically active
substance of glycoside type is converted into the
phytogenic physiologically active substance of aglycon type
in a living body, for example in stomach or intestines and
the absorption of the phytogenic physiologically active
substance of aglycon type and the migration into blood are
accelerated, whereby preventive effect of the phytogenic
physiologically active substance to various diseases can be
enhanced.
The method of accelerating a bioabsorption of a
physiologically active substance according to the
invention, which comprises administering diglycosidase
31
CA 02582717 2007-04-03
orally before, during, and/or after the ingestion of a food
containing physiologically active substances of glycoside
type, (preferably, a method of forming an isoflavone from a
glycoside containing an isoflavone as the aglycon in a
living body), is conducted as follows.
The target is a warm-blooded animal, preferably
human or livestock.
As far as the phytogenic physiologically active
substance of glycoside type comes into contact with
diglycosidase in stomach and intestines, diglycosidase may
be administered at any time before, during, or after the
ingestion of a food containing physiologically active ,
substances of glycoside type. Preferred is between just
after a meal and one hour after the meal.
The dose of diglycosidase is not particularly
limited as far as the conversion of the phytogenic
physiologically active substance of glycoside type into the
phytogenic physiologically active substance of aglycon type
occurs in a living body, but diglycosidase is orally
administered in an amount of usually from 10 mg/day to 500
mg/day, preferably from 30 mg/day to 300 mg/day, more
preferably 100 mg/day to 200 mg/day. The number of dose is
not particularly limited but is preferably from once per
several days to several times per day, particularly
preferably three times per day (i.e., after every meal).
32
CA 02582717 2007-04-03
Moreover, the ingesting amount of the
physiologically active substance is not particularly
limited as far as its effect is attained, but the substance
is ingested in an amount of preferably 10 mg/day or more,
more preferably 50 mg/day or more, further preferably 50
mg/day to 100 mg/day.
Furthermore, diglycosidase may be administered
solely, as an enzyme preparation, and/or as a mixture with
conventional glycosidase (e.g., glucosidase, galactosidase,
etc.).
Diglycosidase may be used as an enzyme
preparation. In this case, the enzyme preparation contains
diglycosidase as the essential ingredient, and may further
contain various enzymes, stabilizers, and the like.
Additionally, in the case of the administration as
an enzyme preparation mixed with conventional glycosidase,
examples of the conventional glycosidase include
glucosidase, galactosidase, xylosidase, and rhamnosidase,
and the dose of diglycosidase is preferably from 10 mg/day
to 50 mg/day.
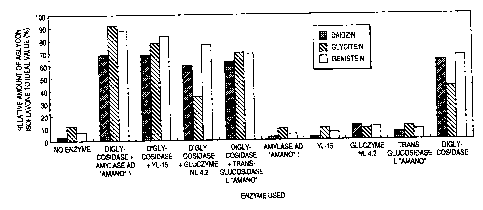
Brief Description of the Drawings
Figure 1 is a graph showing the results of Example
7. Figure 2 is a graph showing the results of Example S.
Figure 3 is a graph showing the results of Example 14.
Figure 4 is a graph showing the results of Example 15.
33
CA 02582717 2007-04-03
Best Mode for Carrying Out the Invention
The invention will be explained in detail with
illustrating Examples using soybean materials as phytogenic
materials. Examples are illustrated for the purpose of
explanation only and they by no means restrict the scope of
the invention.
The phytogenic materials, for example defatted
soybean, soymilk, concentrated soybean protein, and various
soybean products contain 12 kinds of isoflavone compounds.
Specifically, they contain aglycons of glycitein, daidzein,
and genistein; glucoside glycosides of glycitin, daidzin,
and genistin; acetylglycitin, acetyldaidzin, and
acetylgenistin having 0-acetyl group at 6-position of the
glucose residue; and malonylglycitin, malonyldaidzin, and
malonylgenistin having 0-malonyl group at 6-position of the
glucose residue. The existing ratio of these compounds is
characteristic to each of the dif.ference of varieties of
soybean and the difference of treatment in the production
steps.
Unless otherwise stated, ratio, part(s), percent,
and the like are herein based on weight.
By the way, the measured activities of various
enzymes herein are shown as values obtainable by the method
described below unless otherwise stated.
Diglycosidase activity
34
CA 02582717 2007-04-03
The activity was measured on an automatic chemical
analyzing apparatus (TBA-30R manufactured by Toshiba
Corporation). Thirty L of an enzyme sample was mixed with
200 L of 2 mM solution of p-nitrophenyl (pNP)
primeveroside used as a substrate of disaccharide
glycoside, which is obtained by dissolving the compound in
an acetate buffer (pH 5.5), followed by reaction at 40 C
for 9.75 minutes at the cycle time of 22.5 seconds. Then,
250 L of sodium carbonate was added thereto and then
absorbance at 412 nm was measured. A blank derived from
the sample was measured similarly using 20 mM acetate
buffer (pH 5.5) instead of the substrate solution.
The enzyme amount increasing the absorbance by 1
under the conditions is defined as 1 AU.
The pNP-primeveroside used herein can be
synthesized, for example, by reacting pNP-glucoside
(manufactured by Merck) with xylo-origosaccharide
(manufactured by Wako Pure Chemical Industries, Ltd.) using
an enzyme, xylosidase (manufactured by Sigma) to bond
xylose to pNP-glucoside in 0-1,6-manner via one residue
transfer.
CA 02582717 2007-04-03
[0001]
Example 1(Production of diglycosidase by Penicillium
multicolor IAM7153)
Culture of diglycosidase
A medium for growth (pH 5.6) containing 2.0% of defatted
soybean, 3.0% of glucose, 0.5% of potassium dihydrogen phosphate,
0.4% of ammonium sulfate, 0.3% of dry yeast was sterilized at
121 C for 20 minutes. To 100 mL of the sterilized medium was
inoculated 1 oese of Penicillium multicolor IAM7153, followed by
pre-culture at 27 C at the shaking rate of 140 min-1. After 5 days,
L of a main medium of pH 4.9 containing 1.0% of Sunfiber R,
2.0% of potassium dihydrogen phosphate, 1.0% of ammonium sulfate,
and 3.13% of meast PIG was sterilized in a 30 L jar fermenter at
121 C for 20 minutes while stirring at 150 min-l. The pre-medium
15 was inoculated at a rate of 1.5% and the whole was cultured at a
stirring number of 250 min-1, an aeration of 0.75 vvm (15 L/min),
an inner pressure of 0.5 kg/cm2 (48 kPa), and a temperature of
27 1 C for 8 days.
Purification of diglycosidase
20 To the culture broth were added 2% by weight each, based on
* *
total liquid amount, of Zemlite Super 56M and Fineflow A as
filtration aids and filtration through diatomaceous earth was
carried out. The filtrate was concentrated by a factor of 20 using
an ultrafiltration
*
Trade-mark
36
CA 02582717 2007-04-03
~".
membrane UF AIP-2020 (MW 6,000) and also the substitution by 20 mM
acetate buffer of pH 4.7 was conducted. Ammonium sulfate was added
to the above ultrafiltration concentrate to conduct 50% ammonium
sulfate-salting out. The resulting precipitate was removed and
ammonium sulfate was further added to the supernatant to conduct
80% ammonium sulfate-salting out. The precipitate was recovered
and dissolved in 20 mM acetate buffer of pH 4.7. The solution was
passed through a 10-DG column (BioRad Co.) to exchange the buffer
for 20 mM acetate buffer of pH 4.7 containing 30% saturated
ammonium sulfate (this solution is also referred to as "crude
diglycosidase"). This solution was applied to a hydrophobic
* *
chromatography (HiLoad 16/10 Phenyl Sepharose High Performance
(Pharmacia)) to separate a fraction showing diglycosidase activity
from fractions showing P-glucosidase and P-xylosidase activities.
Elution was started at room temperature with 20 mM acetate buffer
containing 30% saturated ammonium sulfate at a flow rate of 2
mL/min and elution was carried out by linear gradient of 30 to 0%.
A fraction showing diglycosidase activity was eluted at 10 to
12.5% saturated ammonium sulfate concentration. The diglycosidase
fraction recovered was concentrated and a centrifuged supernatant
was charged onto 10-DG column to exchange the solution for 25 mM
tris-hydrochioride buffer of pH 7.1. This liquid was applied to an
*
isoelectric chromatography (Mono-P HR5/20 (Pharmacia))
*
Trade-mark
37
CA 02582717 2007-04-03
and elution was started at room temperature with polybuffer
74 of pH 5.0 at 1 mL/min. The aimed diglycosidase activity
was eluted from pH 6.2 to pH 6.3. Since a single band was
obtained on an SDS electrophoresis of the fraction, it was
proved that diglycosidase (hereinafter, also referred to as
"purified diglycosidase") could be purified.
Example 2 (Reactivity of diglycosidase toward various
isoflavone glycosides)
Diglycosidase was diluted with an acetate buffer
of pH 4.0 to prepare a 0.75 AU/mL enzyme solution. As
references, a similar operation was conducted using ~3-
glucosidase (manufactured by Fluka) derived from
Aspergillus niger, (3-glucosidase (manufactured by Sigma)
derived from almond, P-xylosidase derived from pectinase G
(manufactured by Amano Enzyme Inc.). Each of purified
products of isoflavone glycosides (glycitin,
acetyiglycitin, malonylglycitin, daidzin, acetyldaidzin,
malonyldaidzin, genistin, acetylgenistin, malonylgenistin,
all manufactured by Nacalai Tesque, Inc.) was dissolved in
methanol to prepare each 2mM substrate solution. The
reaction was carried out by mixing 10 L of a substrate
solution, 200 L of 20 mM acetate buffer (pH 4.0), and 40
L of each purified enzyme solution at a total liquid
volume of 250 L. The reaction was carried out at 55 C and
isolation of an aglycon isoflavone from an isoflavone
38
CA 02582717 2007-04-03
glycoside in the reaction mixture was detected by HLPC at
0, 1, 3, and 6 hours of the reaction.
HLPC analysis
To the reaction mixture was added 700 L of
ethanol, followed by stirring and ultrasonication. After
centrifugation at 15,000 rpm and 4 C for 10 minutes, the
supernatant was filtered through a filter and then the
filtrate was applied to HPLC.
The isoflavone glycoside and aglycon isoflavone
contained in the filtrate was separated and detected by a
high performance liquid chromatography (HPLC, Shimadzu
CLASS LC-10 system) using TOSOH TSK ggl ODS-80TM column
(manufactured by Tosoh Corporation). The filtrate
containing an isoflavone glycoside and an aglycon
isoflavone was injected into the column by means of an
auto-injector (Shimadzu, SIL-lOAXL) and elution was started
with a solution containing 2% of eluting solution A
(acetonitrile) and 98% of eluting solution B(10$ acetic
acid solution), and after 5 minutes, continued by a linear
concentration gradient finishing with a solution of 50% of
eluting solution A and 50% of eluting solution B. Total
flow rate was 0.8 mL/min and 12 kinds of isoflavone
glycosides and aglycon isoflavones, i.e., glycitin,
daidzin, genistin, 6"-O-acetylglycitin, 6"-O-acetyldaidzin,
6"-0-acetylgenistin, 6"-0-malonylglycitin, 6"-0-
malonyldaidzin, 6"-0-malonylgenistin, glycitein, daidzein,
39
CA 02582717 2007-04-03
- . (.
and genistein can be separated. The absorbance at 260 nm
was detected by a UV detector (Shimadzu, SPD-10AV). Using
purified products (manufactured by Nacalai Tesque, Inc.) of
the above isoflavone glycosides and aglycon isoflavones,
the isoflavone glycoside and aglycon isoflavone were
quantitatively determined according to a calibration curve
method. By the way, unless otherwise stated, the measuring
conditions of HPLC herein mean those described in the
example.
As a result, 0-glucosidase derived from
Aspergillus niger could act well on 3 types of glucoside
isoflavones of glycitin, daidzin, and genistin, but the
reactivity on modified glycosides was very low. 0-
Glucosidase derived from a plant exhibits a low efficiency
of decomposing glycoside isoflavones and no action was
observed on modified isoflavones. On the other hand,
diglycosidase could cleave all the isoflavone glycosides at
a high efficiency. Of these, a high efficiency was
observed toward acetylglucoside isoflavones. From the
above results, diglycosidase was found to isolate aglycon
isoflavones from isoflavone glycosides through cleavage
very efficiently (Table 1).
In addition, it was found that the efficiency of
decomposing isoflavone glucosides, especially genistin
could be further enhanced by the combined use of ~3-
glucosidase in addition to the enzyme.
Table 1
Reaction time (h)
Purified enzyme Substrate o ~ 3 6
glucoside a l con glucoside aglycon glucoside a 1 con glucoside a 1 con
lycitin 100 0 2.1 97.9 0.2 99.8 0.3 99.7
daidzin 99.1 0.9 29.9 70.1 18.2 81.8 11.5 88.5
genistin 100 0 100 0 100 0 100 0
malonylglycitin 100 0 21.7 78.3 15.5 84.5 14 86
Diglycosidase derived from
multicolor alonyldaidzin 100 0 49.2 50.8 39.9 60.1 38.5 61.5
malonylgenistin 100 0 25.8 74.2 17.9 82.1 17.7 82.3
acetylglycitin 100 0 2 98 2.1 97.9 2.5 97.5
acetyldaidzin 100 0 10.4 89.6 3.8 96.2 3.8 96.2
acetylgenistin 100 0 0.2 99.8 0.1 99.9 0.2 99.8
lycitin 100 0 99.5 0.5 100 0 100 0
daidzin 99.1 0.9 92.9 7.1 85.3 14.7 75.2 24.8
enistin 100 0 100 0 100 0 100 0 N
malonylglycitin 100 0 99.6 0.4 99.2 0.8 98.6 1.4
Diglycosidase derived from
. fumigatus alonyldaidzin 100 0 90 10 79.5 20.5 67.7 32.3 N
malonylgenistin 100 0 97.6 2.4 94.8 5.2 91.8 8.2
acetylglycitin 100 0 94 6 86.5 13.5 77.3 22.7
acetyldaidzin 100 0 73.9 26.1 52.8 47.2 33.5 66.5
acetylgenistin 100 0 87.4 12.6 77.1 22.9 64.8 35.2
glycitin 100 0 99.2 0.8 97.6 2.4 95.5 4.5
daidzin 99.1 0.9 90.9 9.1 76 24 57.9 42.1
genistin 100 0 98.5 1.5 98.2 1.8 98.2 1.8
-Xylosidase derived from malonylglycitin 100 0 100 0 100 0 99.6 0.4
~
pectinase G alonyldaidzin 100 0 99.6 0.4 98.4 1.6 96.7 3.3
malonylgenistin 100 0 100 0 99.4 0.6 98 2
acetylglycitin 100 0 100 0 100 0 100 0
acetyldaidzin 100 0 90.3 9.7 98.7 1.3 97.7 2.3
acetylgenistin 100 0 100 4- 0 99.8 0.2 99.5 0.5
Table l(continued)
Reaction time (h)
Purified enzyme Substrate 0 1 3 6
glucoside aglycon glucoside aglycon glucoside aglycon glucoside aglycon
ycitin 100 0 53.5 49.5 11.7 88.3 4.5 95.5
daidzin 100 0 0 100 0 100 0 100
genistin 100 0 0 100 0 100 0 100
alonylglycitin 100 0 98.8 1.2 95.6 4.4 90.9 9.1
S-Glucosidase derived from
niger malonyldaidzin 100 0 95.5 4.5 90.7 9.3 84 16
malonylgenistin 100 0 96.9 3.1 93 7 87.9 12.1
0
acetylglycitin 100 0 100 0 99 1 98.1 1.9
acetyldaidzin 100 0 95.6 4.4 93.6 6.4 91 9 Ln
acetylgenistin 100 0 98.6 1.4 96.7 3.3 93.8 6.2 N
glycitin 100 0 98.4 1.6 98.1 1.9 98.1 1.9 ~
daidzin 99.1 0.9 90.9 9.1 89.2 10.8 88.7 11.3 0
genistin 100 0 90.6 9.4 88.6 11.4 88.2 11.8 0
malonylglycitin 100 0 100 0 100 0 100 0 ~
,6 -Glucosidase derived from malonyidaidzin 100 0 100 0 100 0 100 0 W
almond
alonylgenistin 100 0 100 0 100 0 100 0
acetylglycitin 100 0 100 0 100 0 100 0
acetyldaidzin 99.8 0.2 99.5 0.5 99.4 0.6 99.4 0.6
acetylgenistin 100 0 100 0 100 0 100 0
CA 02582717 2007-04-03
' = !
Example 3 (Influence of free glucose on diglycosidase
activity)
Purified diglycosidase was diluted with 20 mM
acetate buffer of pH 4.0 to prepare a 0.75 AU/mL enzyme
solution.
With a glucose solution was mixed 10 L of each 2
mM substrate solution described in Example 2, and 20 mM
acetate buffer of pH 4.0 was added thereto to be a liquid
volume of 210 L. Further, 40 L of the enzyme solution
was added and reaction was carried out at a final liquid
volume of 250 L. Adjustment of the glucose solution to be
added allows glucose to exist in the reaction mixture in
the range of 0 to 20%. The reaction was carried out at
55 C and the existence of isoflavone glycosides and aglycon
isoflavones was detected by HLPC at 0, 0.5, 1, and 3 hours
of the reaction.
When the results were compared assuming that
isolated aglycon amount at the glucose concentration of 0%
and the reaction time of 0.5 hour is 100% using each
isoflavone glycoside as the substrate, the converting
efficiency of diglycosidase into aglycon isoflavone is
hardly inhibited by the increase of free glucose
concentration. To the contrary, increase of the converting
efficiency was observed until 8% glucose concentration.
Therefore, in the conversion into aglycon isoflavone by
43
CA 02582717 2007-04-03
diglycosidase, no inhibition by glucose was observed up to
20% concentration (Table 2).
Moreover, 0-glucosidase used in the conventional
converting method into aglycon isoflavone is inhibited by
glucose and large decrease of the reaction efficiency was
observed, but diglycosidase of the invention was found to
be hardly inhibited by glucose. Therefore, the amount,
kind, and usage of phytogenic materials, which are starting
materials for converting into aglycon isoflavone, are
restricted in the case of the conventional glycosidase, but
there is no such restriction in the case of diglycosidase
of the invention and the efficiency of,the conversion into
isoflavone aglycon is remarkably enhanced.
Table 2: Isolation of isoflavone aglycons by diglycosidase
in presence of glucose
Glu- Isoflavone glycoside
cose
Concen- gly- daidzin acetyl- acetyl- acetyl- malonyl- malonyl- malonyl-
tration citin glycitin genistin daidzin glycitin genistin daidzin
%
0 100 100 100 100 100 100 100 100
2 96 128.1 100.9 104.1 108.1 121.4 152 125.3
4 99.7 126 100.9 104.1 112.2 127.6 164.7 132.1
8 100.1 129.1 100.9 104.1 115.8 128.6 163.5 125.4
92.8 111.9 100.9 104.1 114.5 116.4 139.5 98.1
44
CA 02582717 2007-04-03
Example 4 (Examination of temperature in the conversion
into isoflavone aglycons by diglycosidase using soybean
materials)
Into 400 L of 20 mM acetate buffer of pH 4.0 was
suspended 50 mg of each of various soybean materials
(roasted soy flour (manufactured by Fuji Shokuryo K.K.),
soymilk (manufactured by Gitoh Shokuhin K.K.), defatted
soybean (manufactured by Fuji Seiyu K.K.), concentrated
soybean protein (manufactured by Fuji Seiyu K.K.)), whereby
a substrate solution was prepared. Crude diglycosidase was
diluted with 20 mM acetate buffer of pH 4.0 to be the
glycosidase activity of 1.88 AU/mL. Fifty L of the,enzyme
solution was mixed with the substrate solution and the
whole was reacted at 80, 65, 55, 45, 37, or 30 C at the
total volume of 500 L. To the reaction mixture was added
700 L of ethanol after 0, 1, 3, and 6 hours of the
reaction. After stirring and ultrasonication, the mixture
was subjected to centrifugal separation at 15,000 rpm and
4 C for 10 minutes. The supernatant was filtered through a
filter and the existence of isoflavone glycosides and
aglycon isoflavones contained in the reaction mixture was
detected by HLPC.
The decomposition efficiency from isoflavone
glycosides into aglycon isoflavones by an enzyme
preparation containing diglycosidase activity was
investigated during the treatment of 4 kinds of soybean
CA 02582717 2007-04-03
materials (roasted soy flour, soymilk, concentrated soybean
protein, defatted soybean) with the enzyme, the isoflavone
compounds being separated into three groups. Namely, the
conversion efficiency was analyzed upon three groups of
glycitin family (glycitin, malonylglycitin, acetylglycitin,
glycitein), genistin family (genistin, malonylgenistin,
acetylgenistin, genistein), and daidzin family (daidzin,
malonyldaidzin, malonyldaidzin, acetyldaidzin, daidzein
(Tables 3 to 14).
46
CA 02582717 2007-04-03
Table 3: Decomposition efficiency of glycitin family
in roasted soy flour
Reaction Heat Reaction
tempera- treatment time Glycoside Aglycon
ture of enzyme
( C) (h) glycitin malonylglycitin acetylglycitin glycitein
30 no 0 50.9 not detected 38.7 10.4
1 34.8 not detected 33.8 31.5
3 not detected not detected 33.2 66.8
6 not detected not detected 19.1 80.9
...............................................................................
...........................................................
...................................................................
yes 6 51.4 not detected 37.5 11.1
45 no 0 51.3 not detected 38.3 10.4
1 12.4 not detected 27.4 60.2
3 12.4 not detected 20.3 67.4
6 5.9 not detected 9.1 85.0
............................................. ....
...............................................................................
...................................................... ....
yes 6 49.8 not detected 36.8 13.4
55 no 0 50.5 not detected 39.1 10.4
1 not detected not detected 30.1 69.9
3 not detected not detected 12.1 87.9
6 not detected not detected 6.0 94A
....
yes 6 51.7 not detected 37.3 11.0
65 no 0 50.9 not detected 38.7 10.4
1 not detected not detected 34.5 65.5
3 not detected not detected 31.0 69.0
6 not detected not detected 29.0 71.0
yes .........._..___..._.._...._........._........._-_._-
__.__._.._.__.__........._._.._........~...M._....._...._..__.._. ...
_._.._..._
6 50.3 not detected 39.3 10.4
80 no 0 51.3 not detected 38.3 10.4
1 19.2 not detected 40.1 40.8
3 20.5 not detected 38.4 41.1
6 33.6 not detected 35:~................................30:~.............
.............. ............
............................................................
.................................... _............................... _. .
yes 6 50.6 not detected 39.4 9.9
47
CA 02582717 2007-04-03
Table 4: Decomposition efficiency of genistin family
in roasted soy flour
Reaction Reaction
tempera- Heat Glycoside Aglycon
ture treatment time
of enzyme malonyl- acetyl-
( C) (h) genistin genistein
genistin genistin
30 no 0 49.7 not detected 45.5 4.9
1 25.7 not detected 45.3 29.0
3 12.0 not detected 43.8 44.2
6 5.5 not detected 40.6 53.8
...._m.... Y....._.._._...._.
................ .....
yes 6 50.3 not detected 43.8 5.8
45 no 0 50.2 not detected 45.3 4.4
1 3.7 not detected 43.6 52.7
3 5.6 not detected 38.5 55.9
6 1.2 not detected 31.7 67.1
yes
....._......_............_......_._.....__..._......_....._.._......._...__..._
._...__.........._....w............_........_..._..._._......._..._-~___..__
6 51.7 not detected 39.5 8.8
55 no 0 49.7 not detected 45.7 4.6
1 1.2 not detected 42.5 56.2
3 not detected not detected 34.0 66.0
.6 not detected not detected 27: 3 _72.7
..................................................................._...........
................................................... ... ...................._
yes 6 54.0 not detected 39.9 6.2
65 no 0 49.7 not detected 45.5 4.9
1 not detected not detected 43.6 56.4
3 not detected not detected 40.5 59.5
.6 not detected not detected 38.8 61.2 _
...............................................................................
...._.........._._..........__. .... ................................
yes 6 50.7 not detected 44.3 5.0
80 no 0 50.2 not detected 45.3 4.4
1 7.8 not detected 48.5 43.6
3 9.1 not detected 48.2 42.7
6 18.4 not detected 47.7 33.9
yes 6 52.2 not detected 43.4 4.3
48
CA 02582717 2007-04-03 ~
Table 5: Decomposition efficiency of daidzin family in
roasted soy flour
Reaction Heat Reaction
tempera- treatment time Glycoside Aglycon
ture of enzyme
( C) (h) daidzin malonyldaidzin acetyldaidzin daidzein
35 no 0 48.6 not detected 47.8 3.6
1 11.7 not detected 46.9 41.4
3 not detected not detected 45.3 54.7
6 not detected not detected 39.6 60.4
....... ........
............................................................................_..
.................. .....................
............................_._.................
yes 6 50.3 not detected 43.8 5.8
45 no 0 48.6 not detected 47.7 3.7
1 3.6 not detected 42.9 53.5
3 2.7 not detected 37.9 59.4
6 5.4 not detected 28.5 66.2
..........
e6 49.4 not detected 42.5 8.1
yes
55 no 0 48.8 not detected 47.5 3.7
1 not detected not detected 42.8 57.2
3 not detected not detected 32.5 67.5
6 not detected not detected 26.7 73.3
................. _,.............................. _.........................
........................................................._.....................
.................. .........
yes 6 51.1 not detected 43.6 5.3
65 no 0 48.6 not detected 47.8 3.6
1 not detected not detected 43.7 56.3
3 not detected not detected 40.2 59.8
6 not detected not detected 37.8 62.2
y _...__......._._.__._......___......__..._._ _.. ..._._.. ._ ._.....__.._
__..._.._ .._.__ _ w.
.............
es 6 49.7 not detected 46.1 4.2
80 no 0 48.6 not detected 47.7 3.7
1 3.2 not detected 49.1 47.7
3 4.4 not detected 47.9 47.7
.6 12.5 not detected ................................ 46.7 40.8
...............................................................................
.....................
................._........._......._.........._..........
yes 6 51.3 not detected 45.0 3.8
49
CA 02582717 2007-04-03 ~
Table 6: Decomposition efficiency of glycitin family in
soymilk
Reaction Heat Reaction Glycoside Aglycon
temperature treatment time
( C) of enzyme (h) glycitin malonylglycitin acetylglycitin glycitein
30 no 0 43.9 48.0 not detected 8.1
1 not detected 51.0 not detected 49.0
3 not detected 39.6 not detected 60.4
6 not detected 28.4 not detected 71.6
y __........_... ..._. .___._._.._ ... .......__.._ .............._. _ .__. ~
..m.
.......----_-_.
yes 6 44.5 47.7 not detected 7.9
45 no 0 42.6 49.1 not detected 8.2
1 not detected 46.2 not detected 53.8
3 not detected 29.9 not detected 70.1
6 not detected 15.3 not detected 84.7
yes ....._
............._..__.._.._........_.....__....~....._..__.._..___...~....
6 45.4 47.2 not detected 7.4
55 no 0 47.8 44.6 not detected 7.6
1 not detected 39.1 not detected 60.9
3 not detected 27.7 not detected 72.3
6 not detected 16.5 not detected 83.5
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . .
............................................
yes 6 53.5 39.9 not detected 6.7
65 no 0 43.9 48.0 not detectetl 8.1
1 not detected 42.4 not detected 57.6
3 not detected 34.3 not detected 65.7
6 not detected 25.5 not detected 74.5
............ ....................
...............................................................................
............... .....................................
.............................__...........................
..................... _....
yes 6 56.8 36.0 not detected 7.2
80 no 0 42.6 49.1 not detected 8.2
1 10.7 43.6 not detected 45.7
3 22.5 32.6 not detected 44.9
6 44.2 21.3 not detected 34.4
..._..-.m........___._....._...
...__..........._..__....._..__....._.........._......_...---
..._...._....._._......._._...._..._._..
.....__.._......_.._..._............_..
yes 6 71.4 19.3 not detected 9.3
CA 02582717 2007-04-03
Table 7: Decomposition efficiency of genistin family in
soymilk
Reaction Heat Reaction Glycoside Aglycon
temperature treatment time
( C) of enzyme (h) genistin malonylgenistin acetylgenistin genistein
30 no 0 30.7 58.5 0.9 10.0
1 not detected 56.5 not detected 43.5
3 not detected 47.9 not detected 52.1
6 not detected
............................................................... . ..
...................................................3 6:.1
............................ not , detected ......................... 63:
9............
yes 6 31.2 57.7 0.9 10.1
45 no 0 31.0 57.8 0.9 10.3
1 not detected 49.4 not detected 50.6
3 not detected 34.7 not detected 65.3
6 not detected 20.7 not detected 79.3
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . ..........................................
yes 6 34.4 57.2 0.6 7.9
55 no 0 31.6 59.1 0.6 8.7
1 not detected 46.9 not detected 53.1
3 not detected 32.5 not detected 67.5
6 not detected 20.4 not detected 79.6
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . .
yes 6 37.4 53.3 0.7 8.6
65 no 0 30.7 58.5 0.9 10.0
1 not detected 48.9 not detected 51.1
3 not detected 40.6 not detected 59.4
6 not detected 31.8 not detected 68.2
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . ............................... _
.......................................................
yes 6 44.4 44.6 1.1 9.9
80 no 0 31.0 57.8 0.9 10.3
1 10.6 50.4 0.9 38.0
3 24.3 37.7 1.0 37.0
6 43.9 24.1 2.3 29.6
...................................... . ................. . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
yes 6 64.5 22.9 2.5 10.1
51
CA 02582717 2007-04-03
Table 8: Decomposition efficiency of daidzin family in
soymilk
Reaction Heat Reaction
tempera- treatment time Glycoside Aglycon
ture of enzyme
( C) (h) daidzin malonyldaidzin acetyldaidzin daidzein
30 no 0 34.1 56.8 not detected 9.1
1 not detected 58.2 not detected 41.8
3 not detected 52.8 not detected 47.2
6 not detected 46.2 not detected 53.8
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . _ . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . .
yes 6 34.8 56.1 not detected 9.1
45 no 0 33.9 57.2 not detected 8.9
1 6.8 50.2 not detected 43.0
3 6.9 41.1 not detected 52.0
6 6.9 30.2 not detected 62.8
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . .
yes 6 36.0 55.8 not detected 8.2
55 no 0 34.2 57.3 not detected 8.6
1 not detected 50.9 not detected 49.1
3 not detected 41.1 not detected 58.9
6 not detected 31.1 not detected 68.9
.........................................................................._....
......_................................................._......................
...._............._............................................................
_........
yes 6 40.7 50.8 not detected 8.6
65 no 0 34.1 56.8 not detected 9.1
1 not detected 51.7 not detected 48.3
3 not detected 43.9 not detected 56.1
6 not detected 35.3 not detected 64.7
.......................... . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . _
.....
yes 6 48.7 42.4 not detected 8.8
80 no 0 33.9 57.2 not detected 8.9
1 8.9 50.8 not detected 40.3
3 23.9 36.4 not detected 39.7
6 42.9 23.0 not detected 34.1
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . .
yes 6 69.5 21.8 not detected 8.8
52
CA 02582717 2007-04-03 ~
Table 9: Decomposition efficiency of glycitin family in
concentrated soybean protein
Reaction Heat Reaction
tempera- treatment time Glycoside Aglycon
ture of enzyme
( C) (h) glycitin malonylglycitin acetylglycitin glycitein
30 no 0 52.2 0.4 36.0 11.4
1 47.1 0.5 35.0 17.5
3 39.1 0.5 33.4 26.9
6 28.4 0.5 30.8 40.3
...............................................................................
...............................................................................
...............................................................................
......... ......
yes 6 52.8 0.4 35.4 11.3
45 no 0 52.3 0.4 35.9 11.4
1 30.2 0.6 34.1 35.2
3 18.8 0.6 31.0 49.6
6 7.5 0.6 27.1 64.9
...............................................................................
...............................................................................
...............................................................................
........._..__._...
yes 6 53.2 0.5 35.0 11.3
55 no 0 52.3 0.4 36.0 11.2
1 14.2 0.5 33.9 51.4
3 not detected 0.5 29.8 69.7
6 not detected 0.5 26.8 72.7
...............................................................................
...............................................................................
...............................................................................
.._...._._.._.._
yes 6 52.9 0.4 35.3 11.3
65 no 0 52.2 0.4 36.0 11.4
1 5.2 0.5 35.7 58.6
3 not detected 0.5 35.5 64.0
6 not detected 0.4 35.8 63.8
...............................................................................
................._.............................................................
...............................................................................
....._._
yes 6 52.5 0.4 35.7 11.4
80 no 0 52.3 0.4 35.9 11.4
1 39.7 0.4 36.0 23.8
3 40.5 0.4 35.7 23.4
6 41.7 0.3 35.2 22.8
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . _ ........ _ .......
yes 6 53.6 0.3 34.8 11.4
53
CA 02582717 2007-04-03
Table 10: Decomposition efficiency of genistin family in
concentrated soybean protein
Reaction Heat Reaction
tempera- Glycoside Aglycon
treatment time
ture of enzyme
( C) (h) genistin malonylgenistin acetylgenistin genistein
30 no 0 49.8 not detected 43.1 7.1
1 41.1 not detected 43.2 15.7
3 30.3 not detected 43.0 26.7
..........................................6..............................19:~..
............................. not.detected..............................42
:5.................................... 37.8.._.......
yes 6 51.8 not detected 41.5 6.7
45 no 0 49.7 not detected 43.0 7.2
1 22.2 not detected 42.8 35.0
3 15.6 not detected 41.0 43.4
..........................................6
:3.................................. not . detected
.............................. 39.6 .................................... 55:1
.... .
.._ ......
yes 6 54.5 not detected 38.9 6.5
55 no 0 50.1 not detected 42.9 7.0
1 7.0 not detected 43.1 49.8
3 0.8 not detected 41.1 58.1
..........................................6.................._ not detected
..................... .. ..not.... . .. ..detected
..............................39...
:....................................61:~_
yes 6 52.3 not detected 41.1 6.6
65 no 0 49.8 not detected 43.1 7.1
1 not detected not detected 45.2 54.8
3 not detected not detected 44.0 56.0
6 not detected not detected 43.4 56.6
... . ...._ ............................................
............._.................................................................
......... _...........................
yes 6 50.8 not detected 42.3 6.9
80 no 0 49.7 not detected 43.0 7.2
1 29.6 not detected 44.0 26.4
3 30.3 not detected 43.6 26.1
6 .............................32.:3................................
...not.........................detected....... ..........................43...
:4..................................._?4:3...._ .....
yes 6 51.9 not detected 41.4 6.7
54
CA 02582717 2007-04-03
Table 11: Decomposition efficiency of daidzin family
in concentrated soybean protein
Reaction Heat Reaction
tempera- t~eatment time Glycoside Aglycon
ture of enzyme
( C) (h) daidzin malonyldaidzin acetyldaidzin daidzein
30 no 0 52.3 not detected 44.6 3.0
1 29.5 not detected 44.4 26.2
3 13.4 not detected 43.9 42.7
6 4.0 not detected 43.5 52.5
yes .._._..__....._....._m.._ .__..._ ...............~.
.._.__.....__.._....._.............
6 54.5 not detected 42.3 3.2
45 no 0 52.4 not detected 44.5 3.1
1 10.6 not detected 44.1 45.3
3 9.6 not detected 41.5 48.8
..............6................................1.7 not detected
42.9...................................._55.5
...............................................................................
................... . ... . ........_.....
yes 6 94.3 not detected 0.0 5.7
55 no 0 52.5 not detected 44.5 3.0
1 1.0 not detected 44.0 55.0
3 not detected not detected 40.6 59.4
6 not detected not detected 37.7 62.3
...............................................................................
..................................................................
.........................................................................
yes 6 54.6 not detected 42.3 3.2
65 no 0 52.3 not detected 44.6 3.0
1 1.8 not detected 44.3 53.9
3 1.7 not detected 42.9 55.4
.....................................6 1.7 not detected 41.6 56.7
................................. ...................
_..........................
..._.....
yes 6 53.2 not detected 43.6 3.2
80 no 0 52.4 not detected 44.5 3.1
1 22.6 not detected 45.5 31.9
3 23.3 not detected 44.7 32.0
6 25.5 not detected 43.8 30.7
_
..._._..__...__ .................._.. ..._._..._.
..._..._...__................._._........_...._.... ... ......_.__......
..._........................___._..........._._......_.
yes 6 54.5 not detected 42.3 3.2
CA 02582717 2007-04-03
Table 12: Decomposition efficiency of glycitin family in
defatted soybean
Reaction Heat
Reaction
tempera- treat- time Glycoside Aglycon
ture ment
( C) of (h) glycitin malonyl- acetylglycitin glycitein
enzyme glycitin
30 no 0 not detected 61.3 not detected 38.7
1 not detected 48.3 not detected 51.7
3 not detected 44.6 not detected 55.4
..........................................6....................... ..
..not..... .. . ......detected .......................... 45
:6......................... not detected ...................................
54:4...........
yes 6 not detected 45.1 not detected 54.9
45 no 0 not detected 65.2 not detected 34.8
1 not detected 44.3 not detected 55.7
3 not detected 44.8 not detected 55.2
6 not detected 4
:.......................... not detected ............................_.....
59:~...........
................................................................. . .. .. .
.............................
yes 6 not detected 44.2 not detected 55.8
55 no 0 not detected 61.3 not detected 38.7
1 not detected 44.8 not detected 55.2
3 not detected 42.6 not detected 57.4
..........................................6 ............. not detected
..........................42:......................... not detected
...................................5.~ :3...........
.........,. .................. ... .. ...........
yes 6 not detected 45.5 not detected 54.5
65 no 0 not detected 65.2 not detected 34.8
1 not detected 45.3 not detected 54.7
3 not detected 42.1 not detected 57.9
..........................................6....................... not
detected ..........................40 :~......................... not detected
60.0
...... ........ ... ........_.............
yes 6 not detected 48.8 not detected 51.2
80 no 0 not detected 65.2 not detected 34.8
1 not detected 46.1 not detected 53.9
3 not detected 45.0 not detected 55.0
..........................................6.......................not
detected..........................40:3..........................not
detected...................................59:.~........._
yes 6 not detected 48.1 not detected 51.9
56
CA 02582717 2007-04-03
Table 13: Decomposition efficiency of genistin family in
defatted soybean
Reaction Heat Reaction Glycoside Aglycon
temperature treatment time
( C) of enzyme (h) genistin malonylgenistin acetylgenistin genistein
30 no 0 38.0 47.0 1.7 13.3
1 not detected 51.3 1.3 47.4
3 not detected 49.1 0.7 50.2
6 7.6 45.6 0.1 46.6
_...._y_ ............._._..._..._.................. ___._.
_...................._. ._....._....._._............... _. ..._. _
............._....... ~ ...._.._.
yes 6 not detected 49.5 0.4 50.2
45 no 0 37.0 47.0 1.5 14.5
1 0.9 49.0 0.8 49.2
3 1.0 46.8 0.3 51.8
..................................
.............6........................................1Ø.....................
...............44.:~ .................................... 0.2 .......54.6
... ................................ _...
yes 6 1.3 48.1 0.2 50.4
55 no 0 38.0 47.0 1.7 13.3
1 0.0 48.8 0.7 50.5
3 0.0 46.1 0.2 53.7
6 0.0 43.4 0.1 56.5
.......................................... . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . .
................................................ yes 6 0.0 47.8 0.4 51.9
65 no 0 37.0 , 47.0 1.5 14.5
1 0.0 48.9 1.2 49.9
3 1.8 45.2 1.2 51.8
6 3.3 41.11.1 54.5
__..... _._. __.._..__. _._~ ............._..__.__.._..._.____.........._.
............. .....
6 28.2 39.7 1.3 30.7
yes
80 no 0 37.0 47.0 1.5 14.5
1 9.6 43.8 1.8 44.9
3 22.1 32.5 2.1 43.3
6 ................................... 35.3
.................................... 20.9 2.5 41.3
... ....... ..... .....................................
........................................
yes 6 57.8 20.1 2.4 19.6
57
CA 02582717 2007-04-03 ~
Table 14: Decomposition efficiency of daidzin family in
defatted soybean
Reaction Heat Reaction
tempera- treatment time Glycoside Aglycon
ture of enzyme
( C) (h) daidzin malonyldaidzin acetyldaidzin daidzein
30 no 0 42.2 43.3 not detected 14.5
1 not detected 46.6 not detected 53.4
3 not detected 45.2 not detected 54.8
6 not detected 45.1 not detected 54.9
.__.__.._...._.........._...._..___...._..._....._.._._...._...__~........_..._
.._..._....._... .._._........._.._. ...._....._._.._._.._...__ . ........_.
yes 6 not detected 45.4 not detected 54.6
45 no 0 40.5 43.3 not detected 16.2
1 not detected 45.5 not detected 54.5
3 not detected 43.7 not detected 56.3
6 not detected 41.7 not detected
..........................................._ ......... ....................
.................................. ........................................
............................................................5g:3............
yes 6 not detected 44.3 not detected 55.7
55 no 0 42.2 43.3 not detected 14.5
l not detected 45.0 not detected 55.0
3 not detected 42.7 not detected 57.3
6not detected 40.3 not detected 59.7 yes
6 not detected 44.1 not detected 55.9
65 no 0 40.5 43.3 not detected 16.2
1 not detected 44.7 not detected 55.3
3 not detected 41.6 not detected 58.4
6 not detected 37.3 not detected 62.7
................... .........
_................................................................
..,................, ........................_.
...................................................._..........................
......
yes 6 32.8 35.2 not detected 32.0
80 no 0 40.5 43.3 not detected 16.2
1 7.8 39.8 not detected 52.4
3 19.2 28.8 not detected 52.0
6 30.4 17.8 not detected 51.7
_..__............. ............ yes 6 60.9 17.4 not detected 21.7
58
CA 02582717 2007-04-03
All three groups of isoflavone glucosides were
decomposed at all the temperature ranges tested, and
promptly at 37 to 65 C, particularly 55 C. Furthermore, in
defatted soybean, endogenous 0-glucosidase also
participates in the decomposition. =It is revealed that the
decomposition of modified glucoside glycosides, most of
which is considered to occur by the action of
diglycosidase, easily occurs at a temperature of 37 to
55 C. Among three groups of aglycon isoflavones, maximum
isolation of aglycons was observed in the reaction at 55 C
for 6 hours. In the case of soy flour, glycitein was about
94%, genistein about 74%, and daidzein about 73%. In the
case of soymilk, glycitein was about 84%, genistein about
80%, and daidzein about 70%. In the case of concentrated
soybean protein, glycitein was about 73%, genistein about
61%, and daidzein about 62%. In the case of defatted
soybean, glycitein was about 57%, genistein about 57%, and
daidzein about 60%.
From these results, it was revealed that the
decomposition of modified glucoside glycosides by
diglycosidase efficiently occurred at a temperature of 37
to 65 C, particularly around 55 C.
Example 5 (Examination of pH in the conversion into
isoflavone aglycons by diglycosidase using soybean
materials)
59
CA 02582717 2007-04-03
A substrate solution of 450 L was prepared by
suspending 50 mg of each soybean material (roasted soy
flour (manufactured by Fuji Shokuryo K.K.), soymilk
(manufactured by Gitoh Shokuhin K.K.), defatted soybean
(manufactured by Fuji Seiyu K.K.), concentrated soybean
protein (manufactured by Fuji Seiyu K.K.)), and adjusting
the pH to 2 to 11 with hydrochloric acid or sodium
hydroxide. Each enzyme solution of pH 2-11 wherein
diglycosidase activity of crude diglycosidase was adjusted
to 1.88 AU/mL was added thereto in an amount of 50 L and
the whole was reacted at 55 C at the total volume of 500
.L. To the reaction mixture was added 700 L of ethanol
after 0, 1, 3, and 6 hours of the reaction. After stirring
and ultrasonication, the mixture was subjected to
centrifugal separation at 15,000 rpm and 4 C for 10
minutes. The supernatant was filtered through a filter and
the existence of isoflavone glycosides and aglycon
isoflavones contained in the reaction mixture was detected
by HLPC (Tables 15 to 26).
CA 02582717 2007-04-03
Table 15: Decomposition efficiency of glycitin family in
roasted soy flour
Reac- Heat treat- Reaction
Glycoside Aglycon
tion ment time
pH of en2yme (h) glycitin malonylglycitin acetylglycitin glycitein
pH2 no 0 56.8 not detected 35.3 7.9
56.6 not detected 34.0 9.4
3 56.3 not detected 34.2 9.5
6 54.4 not detected 35.5 10.1
...............................................................................
.. ... .
...................................................................:5..........
............................ 10.1
.................
yes 6 54.4 not detected 35.2 10.4
pH3 no 0 56.8 not detected 35.3 7.9
1 not detected not detected 38.7 61.3
3 not detected not detected 30.8 69.2
6 not detected not detected 31.7 68.3 ..._............. ......... ._ .......
_._..__ _.. _ _._._......._...__ . .____-__....w....... . _ ......__
..........
yes 6 55.1 not detected 35.6 9.3
pH4 no 0 56.6 not detected 34.8 8.6
not detected not detected 18.8 81.2
3 not detected not detected 8.2 91.8
6 not detected not detected not detected 100.0 y
es 6 55.1 not detected 35.9 9.0
pH5 , no 0 56.6 not detected 34.R 8.6
1 not detected not detected 22.9 77.1
3 not detected not detected 6.5 93.5
6 not detected not detected not detected 100.0
.... ............ ..._...-__............ yes 6 55.4 not detected 35.2 9.4
pH6.5 no 0 55.9 not detected 35.4 8.8
not detected not detected 35.0 65.0
3 not detected not detected 19.7 80.3
6 not detected not detected 11.9 88.1
yes . ~_ . 6 54.8 not detected 35.4 9.8
pH8.5 no 0 59.4 not detected 31.0 9.6
39.4 not detected 32.4 28.2
3 29.3 not detected 30.6 40.1
.......6 2not detected ....................30:
.....................................4.~:4..........
..................... .................................... .. ... . .
yes 6 59.2 not detected 31.7 9.1
61
CA 02582717 2007-04-03
Table 16: Decomposition efficiency of genistin family
in roasted soy flour
Reac- Heat Reaction
Glycoside Aglycon
tion treat- time
ment
pH of (h) genistin malonylgenistin acetylgenistin genistein
enzyme
pH2 no 0 50.0 not detected 45.2 4.8
1 49.7 not detected 45.0 5.3
3 50.7 not detected 43.1 6.2
6 52.7 not detected 40.7 6.5
~_-
_..._ .........
_.
yes 6 52.8 not detected 41.2 6.0
pH3 no 0 50.0 not detected 45.2 4.8
1 3.4 not detected 45.2 51.4
3 not detected not detected 41.9 58.1
....................6 .....................not detected..................._not
detected ................43.:5...................................56:5........
.................... ........................................
yes 6 49.9 not detected 45.1 5.0
pH4 no 0 49.9 not detected 45.4 4.7
not detected not detected 36.3 63.7
3 not detected not detected 23.4 76.6
6 not detected not detected 13.4 86.6
...............................................................................
....
....._..........................................._.............................
..................,... . ............................. .... .................
yes 6 50.2 not detected 45.4 4.4
pH5 no 0 49.9 not detected 45.4 4.7
not detected not detected 39.9 60.1
3 not detected not detected 26.9 73.1
6 not detected not detected 17.6 82.4
.........y
.............._..._...............__..._..........m.__....~.._..__.._......_.__
_.w___.__.._........_..
es 6 52.6 not detected 42.0 5.3
pH6.5 no 0 49.4 not detected 45.8 4.8
not detected not detected 45.3 54.7
3 not detected not detected 38.8 61.2
6 n........................................................... t detected not
detected
........................................................ . .. . ... _
............................................................. ..............
66.0
....................
yes 6 54.8 not detected 39.2 6.0
pH8.5 no 0 55.8 not detected 39.5 4.6
1 28.7 not detected 39.5 31.8
3 19.2 not detected 39.2 41.6
6 14.5 not detected 37.3 48.2
....................................... . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . _ .......................
yes 6 59.5 not detected 35.2 5.3
62
CA 02582717 2007-04-03 ~
Table 17: Decomposition efficiency of daidzin family
in roasted soy flour
Heat Reac-
Reaction treat- tion Glycoside Aglycon
ment time
pH enzyme (h) daidzin malonyldaidzin acetyldaidzin daidzein
pH2 no 0 49.1 not detected 47.4 3.5
1 47.6 not detected 46.9 5.4
3 50.3 not detected 45.0 4.8
6 53.1 not detected 42.4 4.5 _._....._.........
_...._.
yes 6 53.8 not detected 42.4 3.8
pH3 no 0 49.1 not detected 47.4 3.5
not detected not detected 46.0 54.0
3 not detected not detected 39.6 60.4
6 not detected not detected 40.7 59.3
...............................................................................
...............................................................................
...................... .........._.........
yes 6 49.0 not detected 47.4 3.7
pH4 no 0 48.7 not detected 47.7 3.5
not detected not detected 34.4 65.6
3 not detected not detected 20.5 79.5
6 not detected not detected 11.1 88.9
.............................................
..................................................._..............
........................................
..............._..._..........
yes 6 48.6 not detected 47.9 3.5
pH5 no 0 48.7 not detected 47.7 3.5
not detected not detected 38.5 61.5
3 not detected not detected 24.7 75.3
,6 not detected not detected 15.2 84.8
.....................
............................................................................__.
........._.................................................................
yes 6 50.6 not detected 45.0 4.4
pH6.5 no 0 48.4 not detected 48.2 3.4
not detected not detected 45.4 54.6
3 not detected not detected 39.2 60.8
6 not detected not detected 34.4 65.6
._._...__._.....__.._.._..._.__..._.....__...._...._...__........._...__......_
_.._._.._........_.._...__...._ .. ............._....._.___....~.
yes 6 51.9 not detected 43.4 4.7
pH8.5 no 0 54.1 not detected 42.1 3.8
1 20.4 not detected 42.7 36.9
3 13.3 not detected 40.3 46.4
6 .................................10, 2 not detected 38.0 51; 8
........_.
...............................................................................
......................................... ..................
yes 6 58.8 not detected 37.3 3.9
63
CA 02582717 2007-04-03
Table 18: Decomposition efficiency of glycitin
family in soymilk
Reac- Heat Reaction
treat- Glycoside Aglycon
tion tiine
ment
pH of (h) glycitin malonylglycitin acetylglycitin glycitein
enzyme
pH2.3 no 0 50.2 42.6 not detected 7.2
not detected 51.3 not detected 48.7
3 not detected 47.7 not detected 52.3
6 not detected 46.5 not detected 53.5
...............................................................................
...............................................................................
................... ........................................
yes 6 51.7 41.3 not detected 6.9
pH3.5 no 0 50.2 42.6 not detected 7.2
1 not detected 21.9 not detected 78.1
3 not detected not detected not detected 100.0
6 not detected not detected not detected 100.0
...............................................................................
...............................................................................
....................
yes 6 54.2 38.2 not detected 7.6
pH4.8 no 0 50.7 43.2 not detected 6.2
1 not detected 37.0 not detected 63.0
3 not detected 21.1 not detected 78.9
6 not detected 8.3 not detected 91.7
.... ..... .. ...
y_
yes 6 54.3 39.2 not detected 6.5
pH6.2 no 0 50.7 43.2 not detected 6.2
1 not detected 46.4 not detected 53.6
3 not detected 34.5 not detected 65.5
6 not detected 29.6 not detected 70.4
e6 54.2 37.6 not detected 8.2
yes
pH7.2 no 0 50.0 43.9 not detected 6.1
not detected 48.8 not detected 51.2
3 not detected 46.2 not detected 53.8
6 not detected 41.6 not detectod 58.4
...............................................................................
......................................................_.._ ~
..........................................
yes 6 56.9 37.5 not detected 5.6
pH 11.6 no 0 54.3 39.4 not detected 6.2
not detected 50.4 not detected 49.6
3 not detected 46.5 not detected 53.5
.......................................b...,.................. .not detected
44Ø........................... not detected .................. 56.0
..........
... ................................................_...........
............_~._.
yes 6 59.7 34.4 not detected 5.8
64
CA 02582717 2007-04-03
Table 19: Decomposition efficiency of genistin family
in soymilk
Reac- Heat Reaction
tion treat- time Glycoside Aglycon
ment
pH of (h) genistin malonylgenistin acetylgenistin genistein
enzyme
pH2.3 no 0 31.5 58.2 0.6 9.8
not detected 57.5 0.7 41.8
3 not detected 54.1 0.8 45.1
6 not detected 52.1 0.6 47.3
yes 6 35.5 54.3 0.6 9.6
pH3.5 no 0 31.5 58.2 0.6 9.8
I not detected 27.9 not detected 72.1
3 not detected 7.5 not detected 92.5
6 not detected .............~:~................................. _ not
,detected..............................97:3.............
.............................................. .
yes 6 37.0 52.7 0.5 9.8
pH4.8 no 0 31.7 58.6 0.5 9.1
1 not detected 41.0 not detected 59.0
3 not detected 23.9 not detected 76.1
6 not detected 11.3 not detected 88.7 ........_y
_.........._~..._............_.___...._._......._....._..._....._.._..._.
..._. ....._.._._._._._
es 6 37.8 51.9 0.6 9.7
pH6.2 no 0 31.7 58.6 0.5 9.1
not detected 52.6 not detected 47.4
3 not detected 41.2 not detected 58.8
6 not detected 35.8 not detected 64.2
yes 6 38.3 51.5 0.6 9.5
pH7.2 no 0 31.2 57.0 0.7 11.1
not detected 56.9 not detected 43.1
3 not detected 51.5 not detected 48.5
6 not detected 46.4 not detected 53:6.. .......
......................................................
............................................................................
yes 6 38.9 51.1 0.7 9.3
pH11.6 no 0 34.8 54.2 0.5 10.5
1 not detected 57.7 not detected 42.3
3 not detected 52.6 not detected 47.4
6 not detected 49.5 not detected 50.5
yes 6 41.3 47.7 0.9 10.1
CA 02582717 2007-04-03
Table 20: Decomposition efficiency of daidzin family
in soymilk
Heat Reaction
Reaction treat- time Glycoside Aglycon
ment
pH of (h) daidzin malonyidaidzin acetyldaidzin daidzein
enzyme
pH2.3 no 0 34.4 55.1 not detected 10.5
not detected 54.7 not detected 45.3
3 not detected 51.1 not detected 48.9
.......................................6...................... not . detected
.........................48 :5...................... not detected
....................s.~ :5...........
yes 6 38.8 51.0 not detected 10.2
pH3.5 no 0 34.4 55.1 not detected 10.5
not detected 35.6 not detected 64.4
3 not detected 15.1 not detected 84.9
6 not detected 7.4 not detected 92.6
...............................................................................
...............................................................................
......... .........................
yes 6 40.4 49.1 not detected 10.6
pH4.8 no 0 34.9 56.2 not detected 9.0
not detected 47.4 not detected 52.6
3 not detected 33.9 not detected 66.1
6 not detected 20.3 not detected 79.7
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . _ _ . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . .
yes 6 41.2 49.7 not detected 9.1
pH6.2 no 0 34.9 56.2 not detected 9.0
not detected 54.1 not detected 45.9
3 not detected 46.4 not detected 53.6
......................................_6......................not.detected.....
....................41:9 not detected....................58.:1............
yes 6 40.6 49.7 not detected 9.7
pH7.2 no 0 34.9 56.1 not detected 9.0
t not detected 56.4 not detected 43.6
3 not detected 52.4 not detected 47.6
6 not detected 48.2 not detected 51.8
...................................
_..................................................
_....................................................................... ....
..........................................................
yes 6 41.5 48.3 not detected 10.2
pHl 1.6 no 0 38.8 52.5 not detected 8.7
1 not detected 56.4 not detected 43.6
3 not detected 50.4 not detected 49.6
6 not detected 47.7 not detected 52.3
....................................... . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
_ ..................................................
yes 6 44.6 46.1 not detected 9.3
66
CA 02582717 2007-04-03
Table 21: Decomposition efficiency of glycitin
family in concentrated soybean protein
Reac- Heat Reaction
tion treat- time Glycoside Aglycon
ment
pH of (h) glycitin malonylglycitin acetylglycitin glycitein
enzyme
pH 1.6 no 0 52.3 0.4 35.9 11.3
1 52.9 0.4 35.1 11.6
3 54.3 0.4 33.5 11.7
6 ............................ 56. ...1 ....................................
0.4 31.8 ................................... ..1:.~...............
.. ....
yes 6 57.9 0.4 30.9 10.8
pH2.7 no 0 52.3 0.4 35.9 11.3
1 38.0 0.5 36.6 25.0
3 37.2 0.5 36.5 25.7
6..__.._..__._.._....33.7 0.5 .._..___~ 36.6 29.2
....._.._y _...._..._.._. ..._._..____..._.
...........................W....._............_...._.
es 6 52.7 0.4 35.4 11.5
pH3.7 no 0 52.4 0.4 36.1 11.1
1 not detected 0.5 32.9 66.7
3 not detected 0.4 26.1 73.5
.......................................6 not detected
0.4................................._22.1...................................77.
5
........._ .................................................
.......................
yes 6 52.4 0.4 35.9 11.3
pH5.1 no 0 52.4 0.4 36.1 11.1
1 4.1 0.6 33.2 62.1
3 not detected 0.6 26.0 73.4
6 not detected 0.6 21.0 78.5
_..._._.._.__......._. ~ ..._... _ _..__. _ .._._.......... ..__._.._ __._
...._...... ..~__..__...._._.. .
...................
yes 6 52.3 0.4 35.8 11.5
pH6.6 no 0 52.2 0.4 36.1 11.3
1 22.6 0.4 34.8 42.2
3 6.9 0.4 32.4 60.3
.......................................6...............................2.:~....
................................0;4 29.5 68.1
I .............................................................
yes 6 53.3 0.4 35.2 11.1
pH8.6 no 0 54.9 0.4 33.0 11.7
1 51.6 0.4 32.6 15.4
3 45.9 0.4 32.6 21.0
.....................................6.............................36:?........
........................._0:4..._............................32
:~..................................30:9...........
yes 6 56.8 0.3 31.6 11.3
67
CA 02582717 2007-04-03 ~
Table 22: Decomposition efficiency of genistin family in
concentrated soybean protein
Reac- Heat Reaction
tion treat- time Glycoside Aglycon
ment
pH of (h) genistin malonylgenistin acetylgenistin genistein
enzyme
pH1.6 no 0 49.7 not detected 42.5 7.8
1 50.2 not detected 42.2 7.6
3 51.6 not detected 40.6 7.8
6 53.3 not detected 3
.........................................
..................................................................
............ _ ..............................................8:9
................................................ 7.9
...........
yes 6 54.4 not detected 37.7 7.9
pH2.7 no 0 49.7 not detected 42.5 7.8
1 23.1 not detected 44.3 32.6
3 22.1 not detected 43.7 34.2
6 17.5 not detected.._.__. 44:0......_.. 38.4 _..Y.._._......_.
_...~...._........._ ............._ ............_._. . _. _.._.
yes 6 50.5 not detected 42.5 7.0
pH3.7 no 0 50.7 not detected 43.1 6.2
1 not detected not detected 43.5 56.5
3 not detected not detected 36.9 63.1
6 not detected not detected 30.7 69.3
- ~
.._......y...__...._..._._..w......_............_.._.___...~.~
..............._._._..~....__......._...._.._._. ... _.
es 6 50.6 not detected 43.0 6.4
pH5.1 no 0 50.7 not detected 43.1 6.2
1 not detected not detected 45.2 54.8
3 not detected not detected 40.2 59.8
6 not detected not detected 35.6 .64.4
........................................ ............
_................................... ................ ............ _._-
.........._.. .........................._.
yes 6 51.4 not detected 42.4 6.2
pH6.6 no 0 50.4 not detected 43.0 6.5
1 13.9 not detected 43.6 42.4
3 3.8 not detected 42.8 53.3
.6 1.7 not detected 41.0 57.2
.................
...............................................................
........................................ .........
yes 6 53.2 not detected 40.6 6.2
pH8.6 no 0 53.8 not detected 39.7 6.5
1 50.4 not detected 38.8 10.8
3 45.1 not detected 39.0 15.8
6 36.3 not detected w _. 38.5 . 25.2
...___._
yes ....__.......__. ...._ ...........__......._.__._......._.._..._. .
...~..._. ............._..._._...._. .
e6 56.2 not detected 37.6 6.2
68
CA 02582717 2007-04-03
Table 23: Decomposition efficiency of daidzin family
in concentrated soybean protein
Reac- Heat Reaction
tion treat- time Glycoside Aglycon
ment
pH of (h) daidzin malonyldaidzin acetyldaidzin daidzein
enzyme
pHI.6 no 0 52.5 not detected 44.5 3.0
1 53.0 not detected 43.7 3.3
3 55.0 not detected 41.7 3.3
.................................6...........................5.~.:2............
........... not
detected........................._39:4.....................................3:4.
...............
yes 6 59.0 not detected 38.1 2.9
pH2.7 no 0 52.5 not detected 44.5 3.0
1 12.9 not detected 46.4 40.7
3 12.5 not detected 46.0 41.6
..... .................. .......9:3.........................not
detected..........................45:g..................................45:~...
.............
.....................
yes 6 53.0 not detected 43.9 3.2
pH3.7 no 0 52.5 not detected 44.6 3.0
1 not detected not detected 41.6 58.4
3 not detected not detected 33.0 67.0
.......................6 not detected not
detected..........................25:2.................................74 .:8
................
........................................ _....... _ _.............
yes 6 52.5 not detected 44.4 3.1
pH5.1 no 0 52.5 not detected 44.6 3.0
1 not detected not detected 43.4 56.6
3 not detected not detected 37.0 63.0
6 not detected not detected. 31.2 68:8.............
...................................................... _.._.
yes 6 53.4 not detected 43.4 3.2
pH6.6 no 0 52.1 not detected 44.8 3.0
1 5.0 not detected 43.9 51.0
3 not detected not detected 42.9 57.1
...... ....................................... not detected not
detected..........................41.:2. ..............58.:8................
.................................................._...._......._.
yes 6 55.1 not detected 41.8 3.2
pH8.6 no 0 56.1 not detected 40.7 3.2
1 50.8 not detected 39.2 9.9
3 45.0 not detected 39.0 15.9
6 ...........................34:5....................... not.
detected.......................... 3 g:.
~...................................?.~.:4................
yes 6 59.1 not detected 37.7 3.2
69
CA 02582717 2007-04-03
Table 24: Decomposition efficiency of glycitin
family in defatted soybean
Reac- Heat Reaction
tion treat- time Glycoside Aglycon
ment
pH of (h) glycitin malonylglycitin acetylglycitin glycitein
enzyme
pH2.6 no 0 not detected 100.0 not detected not detected
1 not detected 100.0 not detected not detected
3 not detected 100.0 not detected not detected
6 not detected 100.0 not detected not detected
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . ................ . . . . . . . . . .
yes 6 not detected 100.0 not detected not detected
pH3.4 no 0 not detected 100.0 not detected not detected
1 not detected 100.0 not detected not detected
3 not detected 100.0 not detected not detected
6 not detected 100.0 not detected not detected
..........................................................................-
.............................................._................................
.......__....................................................................
yes 6 not detected 100.0 not detected not detected
pH4.8 no 0 not detected 64.0 not detected 36.0
not detected 40.1 not detected 59.9
3 not detected 37.5 not detected 62.5
6 not detected 31.9 not detected 68.1
...............................................................................
.......... ............................ .............................. .......
-.............................................................................
yes 6 not detected 56.2 not detected 43.8
pH5.4 no 0 not detected 64.0 not detected 36.0
not detected 45.1 not detected 54.9
3 not detected 100.0 not detected not detected
6 not detected 32.3 not detected 67.7
.................................................
_........................................................................
_.._........................._...._.._..........._.............................
...........................
yes 6 not detected 39.3 not detected 60.7
pH6.6 no 0 not detected 39.6 not detected 60.4
not detected 52.9 not detected 47.1
3 not detected 53.6 not detected 46.4
6 not detected 58.1 not detected 41.9
......
.............................................................................._
................................._.............................................
.................................................................._.
yes 6 not detected 50.3 not detected 49.7
pH7.8 no 0 not detected 66.8 not detected 33.2
1 not detected 57.5 not detected 42.5
3 not detected 53.2 not detected 46.8
6 not detected 49.1 not detected 50.9
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . - . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . ................
yes 6 not detected 55.8 not detected 44.2
CA 02582717 2007-04-03
Table 25: Decomposition efficiency of genistin
family in defatted soybean
Reac- Heat Reaction
treat- Glycoside Aglycon
tion time
ment
pH of (h) genistin malonylgenistin acetylgenistin genistein
enzyme
pH2.6 no 0 42.0 43.8 1.8 12.4
1 36.3 44.4 1.7 17.7
3 20.3 45.4 1.7 32.7
6 20.1 43.5 1.9 34.6
..............................................
...................................... . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . .
yes 6 44.3 42.0 1.6 12.1
pH3.4 no 0 42.0 43.8 1.8 12.4
not detected 41.6 1.5 56.9
3 not detected 36.4 1.1 62.5
6 not detected 30.4 0.8 68.8
6 44.3 39.8 1.8 14.1
yes
pH4.8 no 0 40.5 43.2 1.8 14.5
not detected 42.8 1.0 56.1
3 not detected 37.5 0.6 61.9
6 not detected 31.1 0.4 68.5
yes ._..._.........._.
....._....._._.._._..._.__._....._.._.._.._..__.__.._..._._....__._..........._
. ....._.._..-.. ..r.__
e6 31.8 41.7 1.3 25.2
pH5.4 no 0 40.5 43.2 1.8 14.5
not detected 48.0 0.5 51.5
3 not detected 44.6 not detected 55.4
-6 not detected 39.not detected 60.3
............................................................._.................
.... ........ ..........................
yes 6 6.2 45.8 not detected 48.0
pH6.6 no 0 39.2 47.1 1.7 12.0
not detected 50.1 0.6 49.3
3 not detected 48.3 0.3 51.4
..................................6 not detected 46.1
0:2.....................................53.7
.........._....... .....................................
yes 6 7.7 45.7 0.2 46.4
pH7.8 no 0 40.4 45.8 1.6 12.2
1 15.7 46.6 1.0 36.7
3 12.3 44.4 0.8 42.5
6 11.4 42.2 0.7 45.7
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. .
yes 6 37.5 39.9 0.6 22.0
71
CA 02582717 2007-04-03 ~
Table 26: Decomposition efficiency of daidzin family
in defatted soybean
Heat Reaction
Reaction treat- time Glycoside Aglycon
ment
pH of (h) daidzin malonyldaidzin acetyldaidzin daidzein
enzyme
pH2.6 no 0 45.8 41.0 not detected 13.2
1 37.5 41.2 not detected 21.3
3 17.3 41.8 not detected 40.9
.......................................6..............................~
6:9..................................40 :2....................... not detected
........................42 :9..........
yes 6 48.0 38.9 not detected 13.0
pH3.4 no 0 45.8 41.0 not detected 13.2
not detected 39.4 not detected 60.6
3 not detected 34.5 not detected 65.5
6 not detected 29.0 not detected 71
................................... ..
..........................................................................:0
. . . . ........ ... . . . . . .. . . .
..............
yes 6 47.8 37.1 not detected 15.1
pH4.8 no 0 43.5 40.7 not detected 15.7
not detected 40.5 not detected 59.5
3 not detected 36.7 not detected 63.3
not detected
...............................................................................
... .......................30:5.......................... ...not ..
...detected ............................................................. _
yes 6 37.1 38.4 not detected 24.5
pH5.4 no 0 43.5 40.7 not detected 15.7
not detected 44.2 not detected 55.8
3 not detected 41.5 not detected 58.5
6 not detected 36.9 not detected 63.1
............................................................
_.................
_..............................................................................
........ _................................
.................................
yes 6 not detected 44.9 not detected 55.1
pH6.6 no 0 43.6 43.1 not detected 13.2
not detected 45.9 not detected 54.1
3 not detected 44.0 not detected 56.0
6 not detected 41
....................................... ................................. 8 ..
..not ... .. .detected ........................ 58.2
.........._..
yes 6 9.3 41.2 not detected 49.5
pH7.8 no 0 44.7 42.0 not detected 13.3
1 13.3 43.6 not detected 43.1
3 10.1 41.4 not detected 48.4
.......................................6................................9:4....
..............................35:~..........................not
detected........................s i :9 _.......
yes 6 41.6 36.7 not detected 21.7
72
CA 02582717 2007-04-03
From these results, maximum pH was found to be in
the range of 3.5 to 5. Specifically, in the case of
roasted soy flour, after the reaction at pH 4 for 6 hours,
the existing ratio of aglycon of each isoflavone family was
as follows: glycitein 100%, genistein 87%, and daidzein
89%. in the case of soymilk, almost complete decomposition
of isoflavone glycosides occurred after the reaction at pH
3.5 for 6 hours, and glycitein was 100%, genistein 97%, and
daidzein 93%. In the case of concentrated soybean protein,
after the reaction at pH 3.7 for 6 hours, glycitein was
78%, genistein 69%, and daidzein 75%. In the case of
defatted soybean, maximum isolation of aglycons was
observed after the reaction at pH 3.4 for 6 hours, and
glycitein was 68%, genistein 69%, and daidzein 71%.
Example 6 (Examination of substrate concentration in the
conversion into isoflavone aglycons by diglycosidase using
soybean materials)
Into 20 mM acetate buffer of pH 4.0 was suspended
0.1 g, 0.25 g, 0.5 g, 1.0 g, or 1.5 g of each of various
soybean materials (roasted soy flour (manufactured by Fuji
Shokuryo K.K.), soymilk (manufactured by Gitoh Shokuhin
K.K.), defatted soybean (manufactured by Fuji Seiyu K.K.),
concentrated soybean protein (manufactured by Fuji Seiyu
K.K.)). The pH of the suspension was measured and the
liquid volume was adjusted to 4.5 mL while the pH was
73
CA 02582717 2007-04-03 {
adjusted to 4.0 with 1N hydrochloric acid. An enzyme
solution wherein diglycosidase activity of crude
diglycosidase was adjusted to 1.88 AU/mL was added thereto
in an amount of 0.5 mL, whereby the final liquid volume was
5.0 mL. Namely, the ratio of the soybean material in the
reaction mixture was 2%, 5%, 10%, 20%, or 30% (w/v). The
whole was reacted at 55 C under shaking. To 5 mL of the
reaction mixture was added 7 mL of ethanol after 0, 1, 3,
and 6 hours of the reaction. After ultrasonication, the
mixture was thoroughly mixed. It was subjected to
centrifugal separation at 2,000 rpm and room temperature
for 5,minutes. Then, 1 L of the supernatant was placed in
a 1.5 mL microtube and was subjected to centrifugal
separation at 15,000 rpm and 4 C for 10 minutes. The
supernatant was filtered through a filter and each sample
was suitably diluted by a factor of 1 to 6 depending on the
substrate concentration. Fifty L of the diluted solution
was analyzed by HLPC (Tables 27 to 38).
74
CA 02582717 2007-04-03 ~
Table 27: Decomposition efficiency of glycitin family in
roasted soy flour
Substrate Reaction
concentra- Heat Glycoside Aglycon
tion treatment time
of enzyme acetyl-
(%) (h) glycitin ma{onylglycitin glycitin glycitein
2 no 0 50.2 not detected 33.6 16.2
1 not detected not detected not detected 100.0
3 not detected not detected not detected 100.0
6 not detected not detected not detected 100.0 _..... yes
.._._.._._._...._...__._._..._....__._..._...w.__.......___......__.._._._.....
_._._____.__._._.
6 53.4 not detected 34.4 12.2
no 0 54.8 not detected 33.8 11.4
1 not detected not detected 11.8 88.2
3 not detected not detected 7.2 92.8
6 not detected not detected not detected 100.0
_._._.___._.
____...._.._._._._.__...._.___..._..._.__.._._...____~._...___.....__..._....__
.__..__._..__..____._..._.._....._...._.....__...._
yes 6 54.8 not detected 33.8 11.4
no 0 70.3 not detected 24.2 5.5
1 not detected not detected '16.7 83.3
3 not detected not detected 9.7 90.3
6' not detected not detected not detected 100.0'
................... _...... ...... .................................. _.......
_................................................... __....
............................................. _.........
_..........................................................
_.._
yes 6 55.0 not detected 35.1 9.9
no 0 59.6 not detected 31.8 8.6
1 not detected not detected 22.9 77.1
3 not detected not detected 13.8 86.2
6 not detected not detected 10.0 90.0
..............
_.....................................................................
_..................................
.._.__.........................................................................
......................................................
_....
yes 6 56.0 not detected 35.0 9.1
no 0 55.9 not detected 35.3 8.8
1 not detected not detected 28.4 71.6
3 not detected not detected 24.4 75.6
6 not detected not detected 13.7 86.3
...................._.
...............................................................................
.......................................
.........._......_..._............._..._._......_
yes 6 56.9 not detected 34.4 8.7
CA 02582717 2007-04-03 ~
Table 28: Decomposition efficiency of genistin family in
roasted soy flour
Substrate
Reaction
concentra- Heat Glycoside Aglycon
tion treatment time
of enzyme acetyl-
(%) (h) genistin malonylgenistin genistin genistein
2 no 0 33.2 not detected 44.7 22.1
1 not detected not detected 10.6 89.4
3 not detected not detected 5.0 95.0
6 not detected not detected 1.5 98.5
...............................................................................
...............................................................................
...............................................................................
....................._...-
yes 6 48.4 not detected 45.0 6.7
no 0 47.8 not detected 44.7 7.5
1 not detected not detected 22.1 77.9
3 not detected not detected 15.2 84.8
6 not detected not detected 9.2 90.8
................._.._..........................................................
...............................................................................
._.............................................................................
.............._........_.
yes 6 47.8 not detected 44.7 7.5
no 0 59.5 not detected 38.2 2.3
1 not detected not detected 33.3 66.7
3 not detected not detected 25.3 74.7
6 not detected not detected 18.7 81.3
...............................................................................
....................._.........................;
........................._.....................................................
..............................................__...._...
yes 6 49.5 not detected 45.9 4.6
no 0 50.5 not detected 46.1 3.4
1 not detected not detected 44.1 55.9
3 not detected not detected 35.8 64.2
6 not detected not detected 28.6 71.4
.......................................
.................................................
..._._.............................................................
_..............................................................................
. _..................... -_
yes 6 49.5 not detected 46.2 4.3
no 0 49.4 not detected 46.7 3.8
1 5.7 not detected 46.6 47.7
3 9.6 not detected 41.4 48.9
6 2.0 not detected 37.8 60.2
.......................................... . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . _ _
yes 6 50.1 not detected 46.1 3.8
76
CA 02582717 2007-04-03
~
Table 29: Decomposition efficiency of daidzin family in
roasted soy flour
Substrate Heat Reaction
concentra- treatment time Glycoside Aglycon
tion of enzyme
(%) (h) daidzin malonyldaidzin acetyldaidzin daidzein
2 no 0 30.2 not detected 47.6 22.3
1 not detected not detected 9.2 90.8
3 not detected not detected not detected 100.0
6 not detected not detected not detected 100.0
...............................................................................
................ _.. .. . ............................ ..
yes 6 46.6 not detected 47.2 6.2
no 0 46.1 not detected 46.1 7.7
1 not detected not detected 20.9 79.1
3 not detected not detected 12.5 87.5
6 not detected not detected.. 6:8.... 93.
...............................................................................
............. ............................................. ...........
6 46.1 not detected 46.1 7.7
no 0 60.1 not detected 38.5 1.4
1 not detected not detected 30.5 69.5
3 not detected not detected 21.5 78.5
6 not detected not detected 14.9 85.1
...............................................................................
......._.........................................................._............
............................................_...._.............................
........._
yes 6 47.5 not detected 47.7 4.8
no 0 50.3 not detected 46.1 3.7
1 not detected not detected 40.7 59.3
3 not detected not detected 32.6 67.4
6 not detected not detected 25.1 74.9
..........................................................
_..............................................................................
......
_............................................................................
_............. _.................... __..._
yes 6 48.6 not detected 47.2 4.2
no 0 47.7 not detected 47.6 4.7
1 not detected not detected 46.4 53.6
3 not detected not detected 42.6 57.4
6 not detected not. detected .34 :3 65 : _
7_.
.........................................
_..............................................................................
............... ..
yes 6 49.0 not detected 47.4 3.6
77
CA 02582717 2007-04-03 (
Table 30: Decomposition efficiency of glycitin family in
soymilk
Substrate Heat
Reaction
concentra- treatment time Glycoside Aglycon
tion of
(%) enzyme (h) glycitin malonylglycitin acetyl- glycitin glycitein
2 no 0 71.8 28.2 not detected not detected
1 not detected not detected not detected 100.0
3 not detected not detected not detected 100.0
6 not detected not detected not detected 100.0
.. ... ... . .. ..
.............................................................._..........__....
........................................................_......._......
yes 6 51.8 48.2 not detected not detected
no 0 49.4 50.6 not detected not detected
1 not detected 24.6 not detected 75.4
3 not detected 7.5 not detected 92.5
6 not detected not detected not detected 100.0
.. ... ... .. ..
................................................................._........_....
............................................._...........__._._.._
yes 6 56.7 43.3 not detected not detected
no 0 52.5 41.1 not detected 6.4
1 not detected 26.4 not detected 73.6
3 not detected 10.5 not detected 89.5
6 not detected 4.2 not detected 95.8
.......................................
...................._...._.....................................................
............................ ............................... ....._......
_......
yes 6 56.8 37.0 not detected 6.2
no 0 52.4 41.3 not detected 6.3
1 not detected 27.8 not detected 72.2
3 not detected 9.4 not detected 90.6
6 not detected 5.9 not detected 94.1
_
........._.........._
...............................................................................
.......................... ..
yes 6 55.5 38.8 not detected 5.7
no 0 50.9 43.0 not detected 6.1
1 not detected 33.1 not detected 66.9
3 not detected 15.9 not detected 84.1
6 not detected ~
detected _.._........
.............................................................. ....... . ..
..........................:.~ ........................... .. not ......... _
........ _ .................................................
yes 6 55.8 37.5 not detected 6.7
78
CA 02582717 2007-04-03 ~
Table 31: Decomposition efficiency of genistin family in
soymilk
Substrate Heat Reaction
concentra- treatment time G lycoside Aglycon
tion of enzyme
(%) (h) genistin malonylgenistin acetyl- genistin genistein
2 no 0 54.5 35.0 not detected 10.5
1 not detected 15.3 not detected 84.7
3 not detected 3.5 not detected 96.5
6 not detected 0.0 not detected...... 100.0
.................................................. ................ .. . . .
............................ .......................................
yes 6 38.0 49.5 not detected 12.5
no 0 31.5 58.2 not detected 10.3
1 not detected 24.9 not detected 75.1
3 not detected 6.9 not detected 93.1
6 not detected 2
....................................................................... .. .
............................ _ .......................:1 _._
......................................................... not detected 97.9
_._......_............................_._._.........
yes 6 37.9 52.5 not detected 9.7
no 0 32.9 56.5 0.5 10.1
1 not detected 31.5 not detected 68.5
3 not detected 11.9 not detected 88.1
6 not detected 4.8 not detected 95.2
....................................................................... ...
..................................................._
............................. _.................................... _.......
yes 6 37.6 51.2 0.5 10.6
no 0 33.3 56.3 0.6 9.9
1 not detected 37.6 not detected 62.4
3 not detected 15.7 not detected 84.3
6 not detected
10................................................._..................... ....
... .... ...........................:~ _..................... not _ detected
................................, 89.3
yes 6 38.6 51.0 0.6 9.8
no 0 33.6 55.9 0.6 9.9
1 not detected 42.5 not detected 57.5
3 not detected 25.4 not detected 74.6
6 not detected
....................................................................... ..
....................................................1.~:~___...................
.. not . detected 82.9
_.... _....._._._
yes 6 38.6 50.0 0.6 10.9
79
CA 02582717 2007-04-03 r
Table 32: Decomposition efficiency of daidzin family in
soymilk
Substrate Heat Reaction
concentra- treatment time Glycoside Aglycon
tion of enzyme
(%) (h) daidzin malonyldaidzin acetyl- daidzin daidzein
2 no 0 53.5 32.2 not detected 14.4
1 not detected 27.5 not detected 72.5
3 not detected 9.9 not detected 90.1
.6.................. not detected.........................2:9 not detected
97.1
.. ... .. .. . ... . ..
..................................._...........................................
........................._._.
yes 6 39.8 47.0 not detected 13.1
no 0 34.2 53.6 not detected 12.2
1 not detected 34.2 not detected 65.8
3 not detected 15.9 not detected 84.1
6 not detected 6
.......................................................................
...................................................
yes 6 40.5 47.6 not detected 12.0
no 0 34.6 53.4 not detected 12.0
1 not detected 37.4 not detected 62.6
3 not detected 20.5 not detected 79.5
6 not detected l~
...............................................................................
...............................................................................
...............................................................................
............
yes 6 39.4 47.8 not detected 12.8
no 0 34.1 53.6 not detected 12.3
1 not detected 41.2 not detected 58.8
3 not detected 21.5 not detected 78.5
6 not detected 16.4 not detected 83
.......................................................... .. ...... . . ... .
.... .................. ........................... .... ...... . .... . ..
.......................:6............
yes 6 39.8 48.1 not detected 12.1
no 0 34.9 53.5 not detected 11.6
1 not detected 43.8 not detected 56.2
3 not detected 29.7 not detected 70.3
6 not detected 21.9 not detected 78.1
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . _ . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . _ .
. . . . . . . . . . . . . . . .
yes 6 40.0 46.9 not detected 13.1
CA 02582717 2007-04-03
Table 33: Decomposition efficiency of glycitin family in
concentrated soybean protein
Substrate Reaction
concentra- Heat Glycoside Aglycon
tion treatment time
of enzyme 1-
(%) (h) glycitin malonylglycitin ace1 t tm glycitein
gYc
2 no 0 52.2 0.4 35.8 11.6
1 not detected 0.4 14.3 85.3
3 not detected 0.2 5.3 94.4
6 not detected not detected 0.0 100.0
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . .
......................................... .
.................................................... yes 6 53.4 0.4 35.0 11.2
no 0 52.9 0.4 35.6 11.2
1 not detected 0.5 27.1 72.4
3 not detected 0.4 17.8 81.9
6 not detected 0.2 12.3 87.4
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . .
yes 6 54.0 0.4 34.4 11.3
no 0 52.9 0.3 35.7 11.0
1 1.6 0.4 31.2 66.8
3 not detected 0.3 26.2 73.5
6 not detected 0.3 23.9 75.8
...............................................................................
..............;
.............................................._................................
.............................................................._._._..........._
...
yes 6 53.4 0.3 35.2 11.1
no 0 53.1 0.2 35.9 10.8
1 13.2 0.3 35.4 51.2
3 not detected 0.2 33.6 66.2
6 not detected 0.3 30.5 69.2
...............................................................................
...............................................................................
...............................................................................
-.__..........._._
yes 6 52.8 0.4 35.8 11.1
no 0 57.5 0.4 33.9 8.2
1 2.0 0.6 35.9 61.5
3 not detected 0.5 30.7 68.9
6 not detected 0.3 28.3 71.4
.........................
...............................................................................
...............................................................................
.............................. _......... _.... __..__._....... yes 6 58.0 0.4
33.4 8.2
81
CA 02582717 2007-04-03
Table 34: Decomposition efficiency of genistin family in
concentrated soybean protein
Substrate Reaction
concentra- Heat Glycoside Aglycon
tion treatment time
of enzyme 1-
(%) (h) genistin malonylgenistin acetygenistein
genistin
2 no 0 50.5 not detected 42.9 6.6
1 1.2 not detected 20.0 78.7
3 not detected not detected 8.3 91.7
6 not detected not detected 3.3 96.7
...........
...............................................................................
..
_..............................................................................
...............................................................................
........ .... _.....
yes 6 51.6 not detected 41.3 7.0
no 0 51.2 not detected 43.1 5.8
1 not detected not detected 36.4 63.6
3 not detected not detected 26.3 73.7
6 not detected not detected 17.8 82.2
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . _ . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . _ . . . . . .
yes 6 52.2 not detected 41.7 6.2
no 0 50.6 not detected 43.4 5.9
1 1.3 not detected 42.8 55.8
3 not detected not detected 37.3 62.7
6 not detected not detected 33.2 66.8
_
...............................................................................
..................................................................._......;....
.._............................................................................
...................__...._.
yes 6 50.9 not detected 42.8 6.3
no 0 50.4 not detected 43.7 5.9
1 6.0 not detected 47.0 46.9
3 not detected not detected 44.6 55.4
6 not detected not detected 42.3 57.7
...................................
_..................................................................
_..................
.........._............._..........................................._..........
.................................................. _.......... _...
yes 6 50.8 not detected 43.2 6.1
no 0 54.6 not detected 43.5 1.9
1 2.8 not detected 65.3 31.9
3 not detected not detected 61.0 39.0
6 not detected not detected 55.9 44.1
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . ..................... .....
yes 6 55.6 not detected 42.4 2.0
82
CA 02582717 2007-04-03
Table 35: Decomposition efficiency of daidzin family in
concentrated soybean protein
Substrate Heat Reaction
concentra- treatment time Glycoside Aglycon
tion of enzyme
(%) (h) daidzin malonyldaidzin acetyl- daidzin daidzein
2 no 0 52.8 not detected 44.0 3.2
1 not detected not detected 17.8 82.2
3 not detected not detected 5.3 94.7
6 not detected not detected not detected 100.0
._..... _..._ ................. ...._.
yes 6 52.6 not detected 43.2 4.2
no 0 53.0 not detected 44.0 3.1
1 not detected not detected 32.6 67.4
3 not detected not detected 20.5 79.5
..................................................6 not detected not detected
12.0 88.0
...............................................................................
._.......................................... _...............
.............
yes 6 53.5 not detected 43.0 3.5
no 0 52.8 not detected 44.2 3.0
1 2.2 not detected 39.7 58.1
3 not detected not detected 32.9 67.1
6 not detected not detected 26.9 73.1
__._..__.___.._....._.._. .._.....___.___.~....._..-
....._......._._.........__....._._..õ_...~.____.._.._....._......_._._........
..__...._..___.._...-..._._.._
yes 6 53.3 not detected 43.4 3.3
no 0 52.9 not detected 44.1 3.0
1 0.7 not detected 46.4 52.9
3 not detected not detected 41.5 58.5
..................................................6 not detected not detected
37.9................................ 62:1....
...............................................................................
............_._..................._.........
.............
yes 6 52.8 not detected 44.0 3.2
no 0 49.2 not detected 49.2 1.7
1 2.7 not detected 56.6 40.7
3 not detected not detected 49.9 50.1
.6 not detected not detected 42.8 ................................
57:2................
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . _ .................. ................ .
. . . . . . . . . . .
yes 6 49.8 not detected 48.4 1.8
83
CA 02582717 2007-04-03 ~
Table 36. Decomposition efficiency of glycitin family in
defatted soybean
Substrate Heat Reaction
concentra- treatment time Glycoside Aglycon
tion of enzyme
(%) (h) glycitin malonylglycitin acetylglycitin glycitein
2 no 0 not detected not detected not detected not detected
1 not detected not detected not detected not detected
3 not detected not detected not detected not detected
6 not detected not detected not detected not detected
...............................................................................
........................................................................_..._..
....................................._............_....
yes 6 not detected not detected not detected not detected
no 0 not detected 58.4 not detected 41.6
1 not detected 41.6 not detected 58.4
3 not detected 31.8 not detected 68.2
6 not detected 19.5 not detected 80.5
__
...... _...... -. ..........
y -..
yes 6 not detected 58.5 not detected 41.5
no 0 not detected 53.0 not detected 47.0
1 not detected 54.7 not detected 45.3
3 not detected 36.6 not detected 63.4
6 not detected 27.9 not detected 72.1
_._........... _ .............
...............................................................................
....._......................................................................._.
..._................ ..................................
yes 6 not detected 57.6 not detected 42.4
Table 37: Decomposition efficiency of genistin family in
defatted soybean
Substrate Heat Reaction
concentra- treatment time Glycoside Aglycon
tion of enzyme
(%) (h) genistin malonylgenistin acetylgenistin genistein
2 no 0 37.7 45.4 1.8 15.1
1 not detected 31.4 not detected 68.6
3 not detected 17.0 not detected 83.0
6 not detected 11.4 ry not detected 88.6
....y___.......... _.... _....__._.._...._.___.......___.._......... _...___
yes 6 38.7 41.8 1.5 18.0
5 no 0 40.0 43.9 1.9 14.2
1 not detected 38.0 0.8 61.2
3 not detected 29.6 0.5 69.9
................6 not detected 19Ø.......................0:3
80:.~................ .
..........................................................................
...............
yes 6 41.8 41.1 1.8 15.4
10 no 0 42.4 41.6 1.9 14.1
1 not detected 41.5 1.3 57.2
3 not detected 35.1 0.9 64.0
6 not detected 29.1 ..0
.................................................:6....... 70.3
................................................. ........................
......................._..............
.................................
yes 6 44.3 39.4 1.7 14.5
5
84
CA 02582717 2007-04-03 ~
Table 38: Decomposition efficiency of daidzin family in
defatted soybean
Substrate Heat Reaction
concentra- treatment time Glycoside Aglycon
tion of enzyme
(%) (h) daidzin malonyldaidzin acetyldaidzin daidzein
2 no 0 42.3 42.2 not detected 15.5
1 not detected 35.2 not detected 64.8
3 not detected 21.2 not detected 78.8
6 not detected 16, 5 not detected 83.5
.....................................
........................................................
yes 6 43.7 38.1 not detected 18.2
no 0 44.0 41.1 not detected 14.9
1 not detected 37.7 not detected 62.3
3 not detected 30.6 not detected 69.4
6 not detected 21.0 not detected 79.0
... _... ............. ._ _.._...~.._.._M. ._... __......_.._..
_._.__..__..._.._. _..._. ...... _ _ _ .._.__.~ ._.._ _ __. _ .
_.................. _ .................. ..
yes 6 45.8 38.1 not detected 16.1
no 0 45.6 39.7 not detected 14.7
1 not detected 39.5 not detected 60.5
3 not detected 34.2 not detected 65.8
6 not detected 29.0 not detected 71.0
._............_.
.................................................
................................................................._.............
.................
................................................................
yes 6 47.5 37.0 not detected 15.5
By combining maximum reaction temperature and pH,
5 in all the materials examined, isolation of each isoflavone
glucoside was found to be 70% or more when the material
concentration is 10% or less. In particular, when the
material concentration ranges from 2% to 5%, it was
revealed that almost 100% conversion of isoflavone
10 glycosides into aglycons occurred.
Example 7 (Influence of commercially available enzyme
preparations accelerating the conversion into aglycon
isoflavones by diglycosidase)
Soyaflavone (manufactured by Fuji Seiyu K.K.) was
suspended into 0.1 M sodium acetate of pH 3.0 and the
CA 02582717 2007-04-03 ~
substrate concentration was adjusted to 30% (w/v) and pH of
the solution to 5Ø The suspension was pre-incubated at
50 C for 1 hour, whereby the temperature of the suspension
was elevated to 50 C. To the suspension was added each
commercially available enzyme preparation (Amylase AD
"Aznano" 1, YL-15, Gluczyme NL4.2, Transglucosidase L
"Amano", all manufactured by Amano Enzyme Inc.) solely or
in combination with diglycosidase (0.3 AU) so as to be 0.1%
(w/v). The whole was reacted at 50 C for 6 hours and the
change of the composition of isoflavone glycosides and
aglycon isoflavones was analyzed by HPLC. Isoflavone
glycosides and aglycon isoflavones were quantitatively
determined. Among them, relative values of the aglycon
isoflavones were shown in Figure 1, ideal values of the
aglycon isoflavones being 100%.
The ideal value of each aglycon isoflavone was
calculated as follows based on the content of each
isoflavone glycoside and aglycon isoflavone in the case
that no enzyme was added.
Ideal value of aglycon isoflavone
= AG + Gl x MAr,/M6l + G2 x MAG/N62 + = = = = =
AG; amount of aglycon isoflavone, G1; amount of isoflavone
glycoside, blAG; molecular weight of aglycon isoflavone, M;
molecular weight of isoflavone glycoside
86
CA 02582717 2007-04-03
Each commercially available enzyme preparation it
self could hardly hydrolyze glycosides but the combination
with diglycosidase obviously increased the amount of
isolated aglycon isoflavones. For example, the combination
of diglycosidase with Amylase AD "Amano" 1 resulted in 2.1-
fold increase of glycitein and 1.3-fold increase of
genistein as compared with the action of diglycosidase
alone. However, about 1.1-fold increase was observed for
daidzein. It was revealed that the combination of
diglycosidase with YL-15 resulted in 1.8-fold increase of
glycitein, 1.2-fold increase of genistein, and 1.1-fold
increase of daidzein, the combination of diglycosidase with
Gluczyme NL-4.2 resulted in 0.8-fold increase of glycitein,
1.1-fold increase of genistein, and 1.0-fold increase of
daidzein, and the combination of diglycosidase with
Transglucosidase L"Amano" resulted in 1.6-fold increase of
glycitein, 1.0-fold increase of genistein, and 1.0-fold
increase of daidzein.
Example 8 (Examination of effective amount of Amylase AD
"Amano" 1 accelerating the conversion into aglycon
isoflavones by diglycosidase)
Roasted soy flour (manufactured by Fuji Shokuhin
K.K.) was suspended into 0.1 M sodium acetate adjusted to
pH 3.15, the substrate concentration was adjusted to 30%
87
CA 02582717 2007-04-03 r
(w/v), and the pH of the solution to 5Ø The suspension
was pre-incubated at 50 C for 1 hour, whereby the
temperature of the suspension was elevated to 50 C. To the
suspension were added diglycosidase so as to be a final
concentration of 0.1% (w/v) (0.3 AU) and Amylase AD "Amano"
1 (manufactured by Amano Enzyme Innc.) so as to be 0.1,
0.05, 0.01, 0.005, and 0.0001% (w/v). The whole was
reacted at 50 C for 3 hours and the change of isoflavone
composition was analyzed by HPLC.
As shown in Figure 2, it is found that the
isolated amount of aglycon rather increased at a
concentration lower than 0.1% (w/v). An effect was
observed even at a mall amount of 0.0001% (w/v). At 0.005%
(w/v), glycitein and genistein increased by a factor of
1.23 and 1.20, respectively. In the case of daidzein, 1.2-
fold increase was observed. By the way, 0% means the
results in the case of diglycosidase alone.
Example 9 (Flavor improvement of soybean protein by
diglycosidase or each commercially available enzyme
preparation)
To 10 g of Fujipro (separated soybean protein,
manufactured by Fuji Seiyu K.K.) was added 90 mL of water,
and the whole was thoroughly mixed to obtain a soybean
protein solution. Thereto was added diglycosidase or each
of commercially available enzyme preparation (Amylase AD
88
CA 02582717 2007-04-03
"Amano" 1, ADG-S-DS, Lipase A"Amano" 6, Lactase F-DS,
Lactase F, Cellulase A"Amano" 3, Hemicellulase "Amano"
90G, Protease B, YL-15, Pectinase PL "Amano",
Transglucosidase L"A:nano", Gluczyme NL4.2, all
manufactured by Amano Enzyme Inc.) so that diglycosidase
(0.5 AU) or each enzyme preparation was contained in the
soybean protein solution in a concentration of 0.25% (w/v).
The treatment was carried out at 50 C for 5 hours. By the
way, the reaction pH was 7.1. For a sensory test, pH was
not adjusted for avoiding the influence of a buffer (the
same shall apply to Examples 10 to 12).
Sensory test (Flavor improvement of separated soybean
protein treated with diglycosidase or each commercially
available enzyme preparation)
For carrying out a sensory test, each soybean
protein treated with diglycosidase or each commercially
available enzyme preparation was subjected to centrifugal
separation at 1500xg and 4 C for 20 minutes. The
precipitate was removed and pH of the supernatant was
adjusted to 6.0 with hydrochloric acid. Using a solution
obtained by two-fold dilution of the solution, the test was
carried out. The evaluation was conducted by five expert
panelists, and the deliciousness, bitterness=astringency,
and aftertaste were compared with those of Control
untreated with the enzyme (Table 39).
89
CA 02582717 2007-04-03 (
Table 39: Flavor-improving effect of enzyme preparation on
separated soybean protein
Panelist A Panelist B Panelist C
bitter- deli- bitter- af-
name deli- ness = after- ness = - deli- ness = after-
cious- cious- ter- cious-
ness ~~in- taste ness astrin- taste ness astrin- taste
gency gency gency
Untreated (Control) t t t t f
Diglycosidase - - + - + t - - t
Amylase AD ~ - _ + ~ - - t -
"Amano" 1
ADG-D-DS - - + :1_- - t - -{- +
Lipase A "Amano" 6 t t + t - + t -I-
Lactase F-DS t f t t - t f t
t ~ f t t
Lactase F "Amano"
Cellulase A +
"Amano" 3
Hemicellulase ~ - _ +
- - - t
"Amano" 90G
Protease B t + t t t t
f
YL-15 t +++ + t +++ t +++
Pectinase PL + . t f
Trans- glucosidase L t t t t
Gluczyme f f t t f
Panelist D Panelist E
bitter- deli- bitter-
Enryme name deli- ness = after- cious ness = after-
cious
- -
ness astrin- taste ness astrin- taste
gency gency
Untreated (Control) t t
Diglycosidase + t + + -
Amylase AD "Amano" I - f
-
ADG-D-DS + t +
t t
Lipase A "Amano" 6 t +
Lactase F-DS
t t t
Lactase F "Amano"
Cellulase A "Amano" 3 + - +
- - ~
Hemicellulase "Amano" 90G t - t -{-
Protease B t
YL-15 t -f- -f- + t +
Pectinase PL t t ~-
Transglucosidase L t t t f
Gluczyme t t t
; No change is observed as compared with Control.
+; This means that "deliciousness" and "aftertaste" are improved and
"bitterness = astringency" is strengthened.
-; This means that "deliciousness" and "aftertaste" become worse and
"bitterness = astringency" is reduced.
The increase in number of + and - means that the tendency becomes strong.
CA 02582717 2007-04-03 {
As a result, the bitterness=astringency was reduced
or disappeared by diglycosidase, Amylase AD "Amano" 1, ADG-
S-DS, Lipase A"Amano" 6, Cellulase A"Amano" 3, or
Hemicellulase "Amano" 90G.
Also, it was revealed that the treatment with
diglycosidase, Amylase AD "Amano" 1, ADG-S-DS, Lipase A
"Amano" 6, Cellulase A"Amano" 3, Hemicellulase "Amano"
90G, YL-15, or Pectinase PL "Amano" was effective for
improving aftertaste. It was revealed that these enzyme
preparations improve overall flavor, for example,
appearance of sweetness and reduction of smelling of grass,
other than the examined articles.
Example 10 (Flavor improvement of soybean protein by
diglycosidase and each commercially available enzyme
preparation)
To 10 g of Fujipro (separated soybean protein,
manufactured by Fuji Seiyu K.K.) was added 90 mL of water,
and the whole was thoroughly mixed to obtain a soybean
protein solution. Thereto was added diglycosidase and each
of commercially available enzyme preparation (Amylase AD
"Amano" 1, ADG-S-DS, Lipase A"Amano" 6, Lactase F-DS,
Lactase F, Cellulase A"Amano" 3, Hemicellulase "Aiaano"
90G, Protease B, YL-15, Pectinase PL "Amano",
Transglucosidase L "Amano", Gluczyme NL4.2, all
manufactured by Amano Enzyme Inc.) so that diglycosidase
91
CA 02582717 2007-04-03 ~
was contained 6.5 AU in the soybean protein solution and
each enzyme preparation in a concentration of 0.25% (w/v).
The treatment was carried out at 50 C for 5 hours to obtain
an enzyme-treated soybean protein. By the way, the
reaction pH was 7.1.
Sensory test (Flavor improvement of separated soybean
protein treated with diglycosidase and each commercially
available enzyme preparation)
For carrying out a sensory test, each soybean
protein treated with enzymes was subjected to centrifugal
separation at 1500xg and,4 C for 20 minutes. The
precipitate was removed and pH of the supernatant was
adjusted to 6.0 with hydrochloric acid. Using a solution
obtained by two-fold dilution of the solution, the test was
carried out. The evaluation was conducted by five expert
panelists, and the deliciousness, bitterness=astringency,
and aftertaste were compared with those of Control
untreated with the enzyme (Table 40).
92
CA 02582717 2007-04-03 ~
Table 40: Flavor improvement of separated soybean protein
by combination of enzymes
= Panelist A Panelist B Panelist C
bitter- deli- bitter- deli bitter-
Enzyme name del~- ness- after- ness= after- - ness= after-
cious- cious- cious
ness astrin- taste ness astrin- taste _ ness astrin- taste
gency gency gency
Untreated (Control) ~ f
Diglycosidase+ Amylase AD ~ _ - - + + - - - + "Amano" 1 - -
Diglycosidase+ ADG-S-DS + - - - + - ++ - - ++
Diglycosidase+ Lipase A + --- t - + f +
"Amano"6
Diglycosidase+ Lactase F- - -
+
DS f
Diglycosidase+ Lactase F :L + + - + f +
"Amano"
Diglycosidase+ Cellulase A 4- + + + - - - + + - t
---
"Amano"3
Diglycosidase+ + - - - +-Iõ + - - + -
Hemicellulase "Amano"90G
Diglycosidase+ Protease B - - - t + - - -
Diglycosidase+YL-15 t + + + + + + +
Diglycosidase+ Pectinase PL + - + + + - +
- -
"Amano"
Diglycosidase+
Transglucosidase L - f - f -
"Amano"
Diglycosidase+ Gluczyme + - # :
NL4.2
Panelist D Panelist E
deli- bitter- deli- bitter-
Enzyme name ness = after- ness = after-
cious- cious-
ness astrin- taste ness astrin- taste
gency gency
Untreated (Control) f t t t
Diglycosidase+ Amylase AD "Amano" I ++ + - - + +
Diglycosidase+ ADG-S-DS ++ - ++ t - - +
Diglycosidase+ Lipase A "Amano"6 ++ + - -}-
Diglycosidase+ Lactase F-DS t + - f
Diglycosidase+ Lactase F "Amano" + + t - - + -f-
Diglycosidase+ Cellulase A "Amano"3 + + - + - - - +
Diglycosidase+ Hemicellulase "Amano" 90G + + - ++ + -
Diglycosidase+ Protease B +-1- t t - +
Diglycosidase+ YL-15 + + + + t t +
Diglycosidase+ Pectinase PL "Amano" + + + t - -1-
Diglycosidase+ Transglucosidase L "Amano" + + - t
Diglycosidase+ Gluczyme NL4.2 + ; t - t
93
CA 02582717 2007-04-03 ~
As a result, it was revealed that effects, for
example, appearance of sweetness, reduction of bitterness=
astringency, or improvement of aftertaste were observed in
all the combinations of diglycosidase and each commercially
available enzyme preparation (diglycosidase and Amylase AD
"Amano" 1, diglycosidase and ADG-S-DS, diglycosidase and
Lipase A"Amano" 6, diglycosidase and Lactase F-DS,
diglycosidase and Lactase F "Amano", diglycosidase and
Cellulase A"Amano" 3, diglycosidase and Hemicellulase
"Amano" 90G, diglycosidase and Protease B, diglycosidase
and YL-15, diglycosidase and Pectinase PL "Amano",
diglycosidase and Transglucosidase L"Amano", diglycosidase
and Gluczyme NL4.2) shown in the table. From these facts,
it was evident that flavor-improving effect was stronger in
the combination of diglycosidase and each commercially
available enzyme preparation than in the case of
diglycosidase alone.
Example 11 (Flavor improvement of soymilk by diglycosidase
or each commercially available enzyme preparation)
To 20 mL of an ingredient-unadjusted soymi.lk
(Gitoh Shokuhin K.K.) was added diglycosidase or each of
commercially available enzyme preparation (ADG-S-DS,
Amylase AD "Amano" 1, Cellulase A"Amano" 3, Hemicellulase
"Amano" 90G, all manufactured by Amano Enzyme Inc.) so that
diglycosidase was contained 6.5 AU in the soymilk or each
94
CA 02582717 2007-04-03
enzyme preparation in a concentration of 0.25% (w/v). The
treatment was carried out at 55 C for 1.5 hours.
Thereafter, the enzymes were inactivated by heat treatment
at 70 C for 1 hour. By the way, the reaction pH was 6.6.
Sensory test (Flavor improvement of soymilk treated with
diglycosidase or each commercially available enzyme
preparation)
The enzyme-treated liquid thus obtained was
diluted with water by a factor of 3, and then subjected to
a sensory test. The evaluation was conducted by five
expert panelists, and the deli.ciousness, bitterness=
astringency, and aftertaste were compared with those of
Control untreated with the enzyme (Table 41).
CA 02582717 2007-04-03
Table 41: Flavor-improving effect of enzyme preparation
on soymilk
Panelist A Panelist B Panelist C
bitter- bitter- bitter-
Enzyme name sweet- ness = after- sweet- ness - after- sweet- ness - after-
ness astrin- taste ness astrin- taste ness astrin- taste
gency gency gency
Untreated (Control) f t
Diglycosidase - f + + - +
ADG-D-DS + - + - + -
Amylase AD + - - + + - + + -
"Amano" 1
Cellulase A + - +
t -
"Amano"3
Hemicellulase + + + + - +
"Amano"90G
Panelist D Panelist E
bitter- bitter-
Enzyme name sweet- ness = after- sweet- ness = after-
ness astrin- taste ness astriit- taste
gency gency
Untreated (Control) f
Diglycosidase + +
ADG-D-DS + +
Amylase AD "Amano" 1
Cellulase A "Amano"3 + - +
Hemicellulase "Amano" 90G + - -}-
It was revealed that appearance of sweetness,
reduction of bitterness-astringency, and improvement of
aftertaste were effected by diglycosidase or a commercially
available enzyme preparation.
Example 12 (Flavor improvement of soymilk by diglycosidase
and each commercially available enzyme preparation)
To 20 mL of an ingredient-unadjusted soymilk
(Gitoh Shokuhin K.K.) was added diglycosidase and each of
96
CA 02582717 2007-04-03
commercially available enzyme preparation (ADG-S-DS,
Amylase AD "Amano" 1, Cellulase A"Amano" 3, or
Hemicellulase "Annano" 90G, all manufactured by Amano Enzyme
Inc.) so that diglycosidase was contained 6.5 AU in the
soymilk and each enzyme preparation in a concentration of
0.25$ (w/v). The treatment was carried out at 55 C for 1.5
hours. Thereafter, the enzymes were inactivated by heat
treatment at 70 C for 1 hour. By the way, the reaction pH
was 6.6.
Sensory test (Flavor improvement of soymilk treated with
diglycosidase and each commercially available enzyme
preparation)
The enzyme-treated liquid thus obtained was
diluted with water by a factor of 3, and then subjected to
a sensory test. The evaluation was conducted by five
expert panelists, and the deliciousness, bitterness=
astringency, and aftertaste were compared with those of
Control untreated with the enzyme (Table 42).
97
CA 02582717 2007-04-03
Table 42: Flavor improvement of soymilk by combination of
enzymes
Panelist A Panelist B Panelist C
bitter- bitter- bitter-
Enzyme name sweet- ness = after- sweet- ness - after- sweet- ness - after-
ness astrin- taste ness astrin- taste ness astrin- taste
gency gency gency
Untreated (Control) t t f
Diglycosidase+ ADG-S- + - - + - + + + - - +
DS
Diglycosidase+ Amylase + - - - + + - - t + - -
AD "Amano" 1
Diglycosidase+ Cellulase A + - ~ + - + + + + - - +
"Amano" 3
Diglycosidase+
Hemicellulase "Amano" + - + - + + -
90G
Panelist D Panelist E
bitter- bitter-
Enzyme name sweet- - ness = after- sweet- ness - after-
ness astrin- taste ness astrin- taste
gency gency
Untreated (Control) f f :k
f
Diglycosidase+ ADG-S-DS + - + +
Diglycosidase+ Amylase AD "Amano" I = - + +
Diglycosidase+ Cellulase A "Amano" 3 + - + + + - +
Diglycosidase+Hemicellulase "Amano" 90G + f + + - +
As a result of the sensory test, it was revealed
that effects, for example, appearance of sweetness,
reduction of bitterness-astringency, or improvement of
aftertaste were observed in all the combinations of
diglycosidase and each commercially available enzyme
preparation (diglycosidase and ADG-S-DS, diglycosidase and
Amylase AD "Amano" 1, diglycosidase and Cellulase A"Amano"
3, diglycosidase and Hemicellulase "Amano" 90G) shown in
the table. Furthermore, it was revealed that such a
98
CA 02582717 2007-04-03
. , ~
treatment with the enzymes improves overall flavor, for
example, reduction of smelling of grass. From these facts,
it was evident that flavor-improving effect was stronger in
the combination of diglycosidase and each commercially-
available enzyme preparation than in the case of
diglycosidase alone or a commercially available enzyme
preparation alone.
Example 13 (Formation of aglycon isoflavones from defatted
soybean protein by diglycosidase at the pH in a stomach)
Into 20 mM acetate buffer of pH 4.0 was suspended
0.05 g of defatted soybean protein (Fuji Seiyu R.R. ). The
pH of the suspension was measured and the liquid volume was
adjusted to 4.5 mL while the pH was adjusted to 4.0 with iN
hydrochloric acid. An enzyme solution wherein the
diglycosidase activity of crude diglycosidase was adjusted
to 1.88 AU/mL was added thereto in an amount of 0.5 mL,
whereby the final liquid volume was 5.0 mL (concentration
of defatted soybean protein: 1% (w/v)), followed by
treatment at 37 C for 3 hours. After the treatment, 75 L
of methanol and 500 L of water were added to 25 L of the
reaction mixture. The whole was filtered through a 0.2 m
filter and then the filtrate was further diluted with water
by a factor of 2.5, followed by HPLC analysis.
As a result of treating defatted soybean protein
at pH 4 which was the pH range in a stomach during a meal
99
CA 02582717 2007-04-03
as described above, no isolation of aglycon isoflavones was
observed in the treatment at pH 4 without adding the
enzyme, but isolation of aglycon isoflavones was observed
in the product treated with diglycosidase. Therefore, it
was proved that diglycosidase could convert isoflavone
glycosides into aglycon isoflavones under the pH condition
in a stomach.
Example 14 (Formation of aglycon isoflavones from roasterd
soy flour by diglycosidase at the pH in a stomach)
Into 100 mL of 50 mM acetate buffer (pH 5) was
suspended 2.5 g of roasted soy flour (manufactured by
Kakudai Sangyo).
To 3 mL of the suspension was added 0.001, 0.002,
0.005, 0.01, 0.025, 0.05, 0.075, 0.15, 0.374, 0.75, or 1.5
mg of diglycosidase (290 AU/g), followed by incubation
under shaking at 37 C for 30 minutes. After the reaction,
10 mL of methanol was added thereto, and aglycon
isoflavones were extracted and analyzed by HPLC.
As described above, the formation of aglycon
isoflavones from isoflavone glycosides contained in soy
flour was investigated after the reaction at pH 5 and 37 C
for 30 minutes on the assumption of the environment in a
stomach. As a result, as shown in Table 43 and Figure 3,
80% or more of the isoflavone glycosides could be converted
into aglycon isoflavones. This result suggests that
100
CA 02582717 2007-04-03
aglycon isoflavones may be formed from isoflavone
glycosides in a stomach when diglycosidase is orally
administered.
Table 43
Relationship between added amount of diglycosidase and
formation (%) of aglycon isoflavones
aglycon
diglycosidase isoflavones
M
1.500 86.1%
0.750 81.3%
0.374 74.7%
0.150 65.4%
0.075 56.3%
0.050 51.1%
0.025 44.3%
0.010 31.0%
0.005 20.3%
0.002 12.8%
0.001 9.2%
0 5.0%
Example 15 (Formation of aglycon isoflavones from an
isoflavone preparation by diglycosidase at the pH in a
stomach)
Into 100 mL of 50 mM acetate buffer (pH 5) was
suspended 150 mg of an isoflavone preparation (manufactured
by Nature's Bountry, USA). To 3 mL of the suspension was
added 0, 0.00045, 0.00113, 0.00225, 0.0045, 0.009, 0.0225,
0.045, 0.09, 0.18, 0.45, or 0.9 mg of diglycosidase (290
AU/g), followed by incubation under shaking at 37 C for 30
101
CA 02582717 2007-04-03
. , ,
minutes. After the reaction, 10 mL of methanol was added
thereto, and aglycon isoflavones were extracted and
analyzed by HPLC.
As described above, the formation of aglycon
isoflavones from isoflavone glycosides contained in the
isoflavone preparation was investigated after the reaction
at pH 5 and 37 C for 30 minutes on the assumption of the
environment in a stomach. As a result, as shown in Table
44 and Figure 4, 80% or more of the isoflavone glycosides
could be converted into aglycon isoflavones. This result
suggests that aglycon isoflavones may be formed from
isoflavone glycosides in a stomach when diglycosidase,is
orally administered.
Table 44
Relationship between added amount of diglycosidase and
formation (%) of aglycon isoflavones
diglycosidase aglycon isoflavones
(mg) (1h)
0.90000 79.4%
0.45000 78.7%
0.18000 75.3%
0.09000 70.7%
0.04500 64.6%
0.02250 59.2%
0.00900 47.5%
0.00450 38.9%
0.00225 30.3%
0.00113 25.6%
0.00045 21.5%
0.00000 20.0%
102
CA 02582717 2007-04-03
. '~
Industrial Applicability
A physiologically active substance of aglycon type
can be efficiently produced, without resort to any
acid/alkali treatment or fermentation and substantially
without changing the physical properties of a material.
Since diglycosidase has a nature of well acting on
6"-O-acetyl and 6"-O-malonylglucosides which are resistant
to decomposition by conventional glucosidase, the process
can be conducted at one-step without requiring the process
of converting decomposition-resistant isoflavone glycosides
into isoflavone glycosides decomposable by glucosidase, the
process being described in JP-A-10-117792. Moreover, the
present process hardly causes change of physical properties
of a starting material derived from decomposition of
proteins or phospholipids, the decomposition being caused
during the process of hydrolysis with a strong acid.
Furthermore, by using diglycosidase and/or a specific
enzyme preparation, the aglycon content in a protein or
protein-containing food can be increased and also the
flavor thereof can be improved.
103