Note: Descriptions are shown in the official language in which they were submitted.
CA 02584258 2007-03-29
BREATHING SOUND ANALYSIS FOR ESTIMATION OF AIRFLOW RATE
This invention relates to an apparatus for use in breathing sound
analysis for estimation of airflow rate.
This application is related to a co-pending Application filed on the
same day as this application under Attomey Docket No. 84201-1402 and entitled
BREATHING SOUND ANALYSIS FOR DETECTION OF SLEEP
APNEA/HYPOPNEA EVENTS.
BACKGROUND OF THE INVENTION
Acoustical respiratory flow estimation has drawn much attention in
recent years due to dilflculties in airflow measurement, In clinical
respiratory and/or
swallowing assessment, flow is usually measured by spirometry devices, such as
pneumotachograph, nasal cannulae connected to a pressure transducer, heated
thermistor or anemometry. Airflow is also measured by indirect means, i.e.,
detection of chest and/or abdominal movements using respiratory inductance
plethysmography (RIP), strain gauges, or magnetometers. The most reliable
measurement of airflow is achieved by a mouth piece or facemask connected to a
pneumotachograph. However, this device cannot be used during the swallowing
assessment. Therefore, when recording sound dufing a swallow, flow is usually
measured by nasal cannulae connected to a pressure transducer. Potentially,
this
method could be an inaccurate measure of airflow because the air leaks around
the
nasal cannulae. In addition, if the subject breathes through the mouth, flow
is not
registered at all.
,, ~õ,
CA 02584258 2007-03-29
2
For these reasons, the combined use of nasal cannulae connected to a
pressure transducer and the measurement of respiratory inductance
plethomogoraphy to monitor volume changes has been recommended as the best
approach in recording flow to assess respiratory and swallowing pattems.
However,
application of these techniques has some disadvantages, especially when
studying
young children or patients with neurological impairments, where the study of
swallowing is clinically important. Although the application of nasal cannulae
may
seem a minor intrusion, it can produce agitation in chikiren and patients with
neurological impairment. In addition, applying the RIP devices is difficult in
children
with neurological impairment as their poor postural control and physical
deformities
can make it challenging to ensure stable positioning,
In one of the ttrst attempts at acoustical flow estimation, researchers
attempted to estimate flow from tracheal sound by investigating eight
different
methods in the two categories of "reference eurve" and "hierarchical
clustering
analysis". The results showed a mean error between 13-15% of the measured flow
for seven of the methods, with 31% for the eighth method. In the works by
another
group, flow estimation using either tracheal or lung sounds was achieved by
investigating different models with about 90% overall accuracy over different
flow
rates from low to high flow rates. In these studies the exponential model
between
flow and average power of tracheal sound was found to be superior to other
models.
In another study, the tracheal sound envelope was investigated for flow
estimation. The tracheal sound was band-pass filtered in the range of 200-1000
Hz
iõ~,
CA 02584258 2007-03-29
3
and then a Hilbert transform was applied to the filtered signal. The
transformed
signal was used to calculate the tracheal sound envelope and to estimate the
flow
froim the calculated envelope by a linear model. The estimated flow was then
used
to measure ventilation, but the flow estimation error was not reported. The
flow rate
in that study was constant at tidal flow and haff of the reCorded flow signal
was used
to calibrate the model.
All of the above mentioned methods assume that at least some
samples of breath sound with known flow at each flow rate were available to
derive
the model co-efficients for flow estimation. Capturing respiratory sounds at
different
flow rates for cafibration may not always be possible prior to assessment
especially
when assessing young children, patients with neurological impairments and/or
patients in emergency conditions.
Analysis of breathing sounds from a patient for determination of sleep
apnea and/or hypopnea is proposed in a paper entitled "Validation of a New
System
of 'T'racheal sound Analysis for the diagnosis of Sleep Apnea-Hypopnea
Syndrome"
by Nakano et al in "SLEEP" Vo127 No. 6 published in 2004. This constitutes a
research paper postulating that sleep apnea can be detected by breathing sound
analysis but providing no practical details for a system which may be used in
practise. It is believed that no further work has been published and no
commercial
machine has arisen from this paper.
US Patent 6,241,683 (Macklem) issued June 5th 2001 discloses a
method for estimating air flow from breathing sounds where the system
determines
CA 02584258 2007-03-29
4
times when sounds are too low to make an accurate determination and uses an
interpolation method to fill in the information in these times. Such an
arrangement is
of course of no value in detecting apnea or hypopnea since i# accepts that the
information in these times is inaccurate.
SUMMARY OF THE INVENTION
It is one object of the invention to provide an apparatus for use in
analysis of breathing of a patient.
According to a first aspect of the invention there is provided an
apparatus comprising:
a microphone arranged to be located on the patient for detecting
breathing sounds;
a detector module for receiving and analyzing the signals to extract
data relating to the breathing;
the detector module being arranged to analyze the signals to generate
an estimate of air flow;
and a display for displaying for a clinician the estimated air flow rate
relative time;
wherein the detector module is arranged to calculate a function
representing the range of the signal or the entropy of the signal providing an
estimate of air flow during breathing.
Preferably the detector module is arranged to cancel heart sounds
from the function.
Ir.
CA 02584258 2007-03-29
In one preferred method, the function is the range of the signal which is
defined as the log of the difference between minimum and maximum of the signal
within each short window (i.e. 100 ms) of data.
In another preferred method, the function is the entropy of the signal
5 which is defined by the following formula:
H(P)n,lQgnõ
M
where p, is the probability distribution function of the r' event.
Preferably the extraneous sounds are removed by the detector module
prior to flow estimation.
Preferably the display is arranged to display the estimated air flow
versus time in any desired time length being chosen by the user.
Preferably the display is capable of zoom-in and zoom-out functions in
the same window.
Preferably the display is capable of playing the breathing sounds in
any data window,
Preferably the microphone is arranged to be located in the ear of the
patient.
Preferably the microphone in the ear includes a transmitter arranged
for wireless transmission to a receiver.
Preferably the apparatus is arranged such that inspiration and
expiration are monitored by an initial calibration wherein the patient is
instructed to
initialize the system by taking a deep breath, hold it, start up of the
monitoring
, , ~.
CA 02584258 2007-03-29
6
system, then exhale and continue breathing.
Preferably an estimate of flow rate is calibrated using a look-up table of
previously measured flow-sound relationship data that is sorted based on
characteristics of the subjects.
Preferably the characteristics in the look-up table include BMI, gender,
height, neck circumferenoe, and smoking history of the subject.
Preferably the detector module is arranged to cancel heart sounds.
According to a second aspect of the invention there is provided an
apparatus for use in use in analysis of breathing of a patient during sleep
comprising:
a microphone arranged to be located on the patient for detecting
breathing sounds;
a detector module for receiving and analyzing the signals to extract
data relating to the bn?athing;
the detector module being arranged to analyze the signais to generate
an estimate of air flow during inspiration and expiration;
and a display for displaying the data for a clinician;
wherein the apparatus is arranged such that inspiration and expiration
are imonitored by an initlal calibration wherein the patient is instructed to
initialize the
system by taking a deep breath, hold it, start up of the monitoring system,
then
exhale and continue breathing.
According to a third aspect of the invention there is provided an
, N ,11
CA 02584258 2007-03-29
7
apparatus for use in use in analysis of breathing of a patient during sleep
comprising:
a microphone arranged to be located on the patient for detecting
breathing sounds;
a detector module for receiving and analyzing the signals to extract
data relating to the breathing;
the detector module being arranged to analyze the signals to generate
an estimate of air flow during inspiration and expiration;
and a display for displaying the data for a clinician;
wherein an estimate of flow rate is calibrated using a look-up table of
previously measured flow-sound relationship data that is sorted based on
characteristics of the subjects.
This proposal aims to develop a prototype of an integrated system to
acquire, de-noise, analyze the tracheal respiratory sounds, estimate airflow
acoustically.
Long distance monitoring and diagnostic aid tools provide large
financial saving to both the health care system and families. This proposal
will
provide a novel system to both developing a new and yet simple diagnostic tool
for
sleep apnea disorder, and also a new way to connect the specialists and
physicians
with patients either in remote areas or even at their homes. Aside from its
obvious
benefit for covering the remote areas with equal opportunity for health care,
it also
reduces the long waiting list for sleep studies. From a public health
perspective,
CA 02584258 2007-03-29
8
non-invasive and inexpensive methods to determine airway responses across all
ages and conditions would present a major step forward in the management of
sleep
apnea disorders.
Long distance monitoring and diagnostic aid tools provide large
frnaricial savings to both the health care system and the patient's families.
From a
public health perspective, non-invasive and inexpensive methods to determine
airway responses across all ages and conditions would present a major step
forward
in the management of sleep apnea disorders.
BRIEF DESCRIPTION OF THE DRAWINGS
One embodiment of the invention will now be described in conjunction
with the accompanying drawings in which:
Figure 1 is a schematic illustration of a sleep apnea detection
apparatus according to the present invention.
Figure 2 is an illustration of a typical screen displaying the data to the
physician.
Figure 3(a) is a graphical representation of Tracheal sound entropy:
Figure 3(b) is a graphical representation of entropy after applying
nonlinear median fitter (star marks represents the estimated apnea segments)
Figure 3(c) is a graphical representation of flow signal (solid line) along
with the estimated (dotted line) and real (dashed line) apnea segments for a
typical
subject.
CA 02584258 2007-03-29
9
Figure 4 is a graphical representation of Mean and standard deviation
values of errors in estimating apnea periods for different subjects
Figure 5 is a block diagram illustrating the adaptive filtering scheme for
removing the snoring sounds from the signal using the signal recorded by the
auxiliary microphone in the vicinity of the patient.
DETiAiLED DESCRIPTION
One of the reasons to record many signals in a sleep study is the
inaccuracy of those recorded signals in sleep apnea detection when they are
used
as a single measure. For example, nasal cannulae are used to measure airflow;
however, when the patient breathes through the mouth, the nasal cannuiae
register
nothing and hence give a false positive detection error for apnea. Therefore,
combination of nasal pressure plus thermistor and End-tidal carbon dioxide
concentration In the expired air (ETCOz) is used to have a quaiitative measure
of
respiratory eirflow. The abdominal movement recordings are mainly used to
detect
respiratory effort and hence to distinguish between central and obstn,ctive
sleep
apnea. The ECG signals are also being used for detecting heart rate
variability and
another measure for apnea detection as well as monitoring patient's heart
condition
during the night. The combination of EOG (Electrooculogram), EEG
(Electroencephalogram), and EMG (Electromyogram) signals are used for
assessing
the rapid eye movement (REM) sleep stage that is characterized by
desynchronization of the EEG and loss of muscle tone, Recording these signals
are
necessary if insight in sleep quality is saught for diagnosis of certain sleep
disorders.
, ,y
CA 02584258 2007-03-29
The most important information that doctors seek from a complete sleep study
is the
duration and frequency of apnea and/or hypopnea and the blood's Oxygen
saturation (Sa02) level of the patient during the apnea. Oxygen level usually
drops
during the apnea and will rise quickly with awakenings. However, oximetry
alone
5 does not detect all cases of sleep apnea.
As the first and most important information of a sleep study is an
accurate measure of duration and frequency of apnea during sleep, the present
arrangement provides a fully automated system to detect apnea with only one
single
sensor that can also easily be applied by the patient at home and detect apnea
10 acoustically; hence reducing the need for a complete laboratory sleep
study.
The apparatus provides an integrated system for remote and local
monitoring and assessment of sleep apnea as a diagnostic aid for physicians
and
allows the following:
To record the Sa02 data simultaneously with respiratory sound signals
through either a neck band with a microphone mounted in a chamber placed over
the supra-sternal notch, or by a microphone from inside the ear, followed by a
signal
conditioning unit.
To screen the raw data, separate snoring and other adventitious
sounds from breath sounds, estimate flow from the sounds and detect apnee
and/or
hypopnea episodes, determine the duration and frequency of the apnea episodes
and finally display the raw data, estimated airflow and display the estimated
airflow
,. , ,,,
CA 02584258 2007-03-29
11
with marked detected apnea/hypopnea along with related information (duration,
frequency and the corresponded Sa02 data).
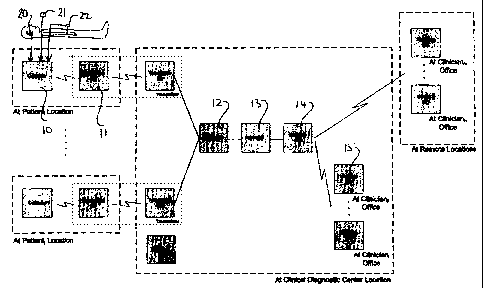
Figure 1 shows the apparatus for sleep apnea detection that can also
be used as a home-care device while being connected to a clinical diagnostic
center
for online monitoring.
From a public health perspective, non-invasive and inexpensive
methods to determine airway responses across all ages and conditions would
present a major step forward in the management of sleep apnea disorders
The apparatus consists of six modules that permit sleep apnea
detection diagnosis. The clinical diagnosis can be performed either locally
(e.g. at a
clinical diagnostic center) andlor remotely (e.g. at clinician's office/home).
The
apparatus will support several clinicians simultaneously carrying out clinical
work on
different patients. Likewise, patients can be monitored either locally (e.g.
at a clinical
diagnostic center) and/or remotely (e.g. at patient's home). The apparatus
will also
support many patients being ooncurrently monitored.
The apparatus has the following modules
Coti!ector module 10,
Transmitter module 11,
Organizer module 12,
Detector module 13,
Interface module 14, and
Manager module 15.
, , ~.
CA 02584258 2007-03-29
12
Collector module 10 captures physiological signals from different body
parts. The body parts include a microphone and transmitter 20 at the ear or
over the
neck by a wireless microphone mounted in chamber with a neckband for recording
sounds, a sensor 22 at the fingers of the patient for reoording oximetry data
and an
external microphone 21 for recording sound from the environment around the
patient. Other signals can be detected in some cases from other body parts if
the
physicians request other biological signals, but this is not generally
intended herein.
The collector module locally transfers wirelessly the signais to the
Transmitter
module 11,
Transmitter module 11 receives biological signals from the Collector
module 10, securely transmits those signals and reoeives the signais at the
diagnostic center for Its delivery to the Organizer module 12.
The Transmitter module 11 consists of two components: The
Transmitter Sender (S) and the Transmitter Rece+ver (R). The Transmitter
Sender
together with the Collector module resides at the patient location. The
Transmitter
Sender receives and store temporally signals from the Coiiector, and securely
and
reliably transfers the signals to the Transmitter Receiver. The Transmitter
Receiver
resides at the diagnostic center iocation. The Transmitter Receiver securely
and
reliably aompts the signals from the Transmitter Sender, and forwards the
signals to
the Organizer for the signal management and processing. There is one pair of
collector - transmitter modules per patient being monitored.
CA 02584258 2007-03-29
13
Inter-Transmitter components signal transmission can occurred locally
for those cases when the Collector-Transmitter Sender resides in the same
center
(e.g. at a diagnostic facilityr) or remotely for those cases when the
Collector-
Transmitter Sender resides externally (e.g. at a patient home). The
transmission
can be wireless or wired (e.g. through the internetrntranet).
Organizer module 12 receives all captured signals from the Transmitter
module, organizes and classifies received signals per patient/physician and
prepares the signals for its processing by the Detector module. The Organizer
module simultaneously supports receiving many signals from difFerent patients
that
is signals from collector - transmitter module pairs.
Detector module 13 pre-processes and analyzes the patient biological
signals, and performs the sleep apnea detection. The Detector performs snoring
sound detection and separation prior to the apnea/hypopnea detection. The
Detector has self-calibrated acoustical respiratory airflow estimation and
phase
detection uhlized in respiratory and sleep apnea assessments.
Interface module 14 provides the graphical user interface to the
clinicians. The Interface module gives a secure, reliable, user-friendly,
interactive
access to the analysis performed by the Detector and it is organized per
patient/physician. The Interface module consists of two main components: the
Interface Master (M) and the Interface Client (C). The Interfaoe Master serves
the
information to the Interface Client(s), while the Interface Client provides
the access
to the clinicians. Several Interface Clients can run concurrently giving out
resuits to
CA 02584258 2007-03-29
14
several clinicians. The Interface Client can be executed locally (e.g.
intranet) or
extemally (e.g, internet).
Manager module 15 provides the application management functions. It
provides the graphical user interface to the application administrator at the
diagnostic center location.
All system/application parameters are setup at the Manager module.
The systemlappiication parameters configure the apparatus for its proper
operation.
The collector module microphone may comprise a neck band with a
microphone mounted in a chamber placed over the supra-stemal notch. However
the preferred arrangement as shown in Figure 1 schematically comprises a
wireless
microphone inside the ear or by a microphone mounted in a chamber with a neck
banci to record respiratory sounds followed by a suitable signal conditioning
unit
depending on the type of the used sensor. The second sensor 21 oollects sound
from the environment around. The third sensor 22 collects the conventional
Sa02
data or other oximetry data. The three sensors allow from the patient
simultaneous
data acquisition of the sound signals and the Sa02 data.
There are two options for recording respiratory sounds: using the ear
microphone or the neck microphone. The very small miniature ear microphone is
inserted into a piece of foam which has open ends and inserted to inside the
microphone. The small preamplifier of the microphone is placed behind the ear
similar to a hearing aid device. The ear microphone includes a wireless
transmitter
which is placed behind the ear, the miniature microphone and the foam for
securing
,.,,1,
CA 02584258 2007-03-29
the imicrophone inside the ear. In case of neck microphone, it is inserted in
a
chamber (with the size of a loony) which aikwvs about 2 mm distance between
the
microphone and the skin when the chamber is piaoed over supra-sternal notch of
the
trachea of the patient with double sided adhesive ring tapes. The neck
microphone
5 will come with a neck band mainly for the comfort of the patient and also to
keep the
wire of the microphone free of touching the skin. In either case, the
preamplifier and
transmitter of the wireless microphone can be placed in the pocket of the
subject.
Alternatively the whole element mounted in the ear canal includes the pro-
ampiifier
and transmitter for complete wireless operation.
10 The detector module pre-processes and analyzes the recorded signal
in order to provide a user friendly, smart and interactive interface for the
physician as
a monitoring and diagnostic aid tool. The software in this part will de-noise
the
recorded sound, separate snoring sounds, estimate the flow acoustically,
detect
apnea and/or hypopnea episodes, count the duration and the frequency of their
15 occurrence, display the estimated flow with marked apnea episodes as shown
in
Figure 3 along with the related information.
The respiratory sounds either from the ear or from the neck of the
patient will be recorded by a small wireless microphone. A Transmitter -
Sender
Module DSP board is designed to receive the analog signal, amplify and filter
the
signal, digitize it with a minimum of 5120 Hz sampling rate and store it as a
binary
fife,
CA 02584258 2007-03-29
16
The Sa02 data simultaneously with the respiratory sounds is digitized
with 5120 Hz sampling rate and stored in a binary file for the entire duration
of the
sleep at the collector module.
The detector module signal processing of the sound signals has three
stages. First an automated aigorithm finds the artifacts (that normally appear
as
impulses in the signal) and removes them from further analyses. Secondly, the
snoring sounds, if they exist, are identified and separated from the
respiratory
sounds. Finally, from the cleaned respiratory sounds the entropy of the signal
is
calculated, the effect of heart sounds is removed, and apnea episodes are
detected
by the technique as described hereinafter. The average duration of the apnea
episodes, their frequency of occurrence and whether they are associated with
snoring, is presented as part of the information in the GUI interface for the
physician.
Artifacts (usually due to movement) appear as very short duration
pulses in the recorded signal. Wavelet analysis is a highly reliable method
with high
accuracy to automatically detect these artifacts. On the other hand, snoring
sounds
are musical sounds which appear with harmonic components in the spectrogram of
the recorded signal. Detection of snoring sounds is similar to detection of
crackle
sounds in the lung sounds. Multi-scale product of the wavelet coefficients is
used to
detect and separate the snoring sounds. Techniques for the application of
digital
signal processing techniques on biological signals including noise and
adventitious
sounds separation are known.
CA 02584258 2007-03-29
i7'
Once the respiratory sound signal is pre-processed and cleaned of
extra sounds, the entropy of the signal is calculated. As heart sounds have
overlap
with respiratory sounds at low frequencies and this is more pronounced at very
low
flow rate (the case of hypopnea), the effect of heart sounds has to be
cancelled from
the entropy or the range parameter of the signals prior to apnea detection.
This is
described in more detail hereinafter.
Then, from the entropy or the range parameter of the signal, the apnea
episodes are identified using Otsu's thresholding method as described
hereinafter.
The flow estimation method as described hereinafter is enhanced to
make the method self-cafibrated. That enables the apparatus to estimate the
actual
amount of flow. Finally, the episodes of hypopnea and apnea are marked; their
duration and frequency of occurrence during the entire sleep is presented on
the
interface module GUI display as a diagnosfic aid to the physician,
Depending on the type of microphone used, both sounds result in the
same apnea detection episodes and flow estimation while the tuning of the
algorithm
for each sound signal requires slight modification, i.e. the threshold or the
parameters of the flow estimation model are different.
The apnea detection aigorithm requires a snoring separation algorithm.
Ttiis can use one or more of the following prinoipies:
Appiying Wavelet analysis to detect and mark the snoring sounds in
the time-frequency domain.
, ,.,~,
CA 02584258 2007-03-29
18
Applying the adaptive filter cancellation technique to remove the
snoring sounds from the signal using the signal recorded by the auxiiiary
microphone in the vicinity of the patient.
An automated aigorithm can be provided to clean the recorded breath
sound signal from all extra plausible noises such as cough sounds, swallowing
sounds, vocal noise (in case the patient talks while dreaming), and arqfacts
due to
movements following the apnea detection algorithm on the cleaned signal and
validate the results. These extraneous sounds will be removed using wavelet
analysis for localization and several different filter banks to remove each
type of
noises either automatically or at the users command.
Display
The interface module 14 provides a display of the detected
apnea/hypopnea episodes and related information for a clinician. The display
includes a display 30 of airflow vetsus time is plotted with apnea and
hypopnea
episodes marked on the screen.
The display inciudes oximetry data 31 plotted in association with the
estimated airflow.
The display has touch screen controls 32, 33 providing zoom-in and
zoom-out functions in the same window for both airflow and oximetry data
simultaneously.
The display is capable of playing the breathing and snoring sounds in
any zoomed-in or zoomed-out data window, that is the sounds are stored to
allow an
CA 02584258 2007-03-29
19
actual rendition of those sounds to the clinician to study the sounds at or
around an
apnea event.
The display is capable of displaying the extracted information about the
frequency and duration of apnealhypopnea episodes, and their association with
the
level of oximetry data in a separate window for the ciinician.
Annea Qatection
Referring now to Figures 4(a), 4(b) and 4(c), further detail of the Sleep
Apnea detection components is now described.
In order to smooth the calculated entropy or range parameter, it is
segmented into windows of 200ms with 50% overiap between adjacent windows.
Each window was then presented by its median value which Is not sensitive to
jerky
fluctuation of the signal.
Next, the smoothed entropy or the smoothed range signal is classified
into two groups of breathing and apnea using a nonparametric and unsupervised
method for automatic threshold selection using the principies of OTSU.
In Otsu's method the threshold is chosen such that the variance
between dasses is maximized. The between-dass variance is defined as the sum
of variances of all classes respect to the total mean value of all classes:
Qa="'o(iia -itr)2 a."'i(/4 -Nr)~+ ~1 ~
, . ,,~ ,
CA 02584258 2007-03-29
where wr,1ul (i =1,2) are the probability and mean values of the
classes, respectively and r is the average of total values.
and the optimum threshold k' is selected so as:
ae (kF ) ~ai 6e (k). (2)
5 The average of entropy or range values is another statistical measure
that can be used to detect apnea segments. In this study both the Otsu and the
average value of entropy or range value were used to define the classification
threshold as:
15 Thr=min*,M~
(3)
where k' is the Otsu threshold and m is the average of the entropy or
range values.
Figure 4 presents (a) Tracheal sound entropy, (b) entropy after
applying nonlinear median filter (star marks represents the estimated apnea
segments) and c) flow signal (solid line) along with the estimated (dotted
line) and
real (dashed line) apnea segments for a typical subject. Comparing the results
depicted in Figure 4(a) and Figure 4(b), the effect of applying median filter
is evident.
The star marks in Figure 4(b) show the estimated apnea segments. Investigating
CA 02584258 2007-03-29
21
the results depicted in Figure 4(c) it is clear that the proposed method
detects all the
apnea segments and classifies them correctiy from the breath segments.
In this arrangement a new acoustical method for apnea detection is
proposed which is based on tracheal sound entropy or range vafue. The method
is
fast and easy to be implemented, which makes it suitable for on-line
applications.
Removal of snoring sounds by time-frequency filtering techniques may
have some problems due to the fact that snoring sounds also have strong low
frequency components, in which the acoustical apnea detection is based on, As
an
alternative, the snoring sounds can be recorded by another auxiliary
microphone in
the vicinity of the subject. This signal will not have breathing sounds and
can be
used as a noise referenoe. The apparatus then uses adaptive fiitering for
noise
(snore) cancellation.
Snoring sounds are musical sounds which appear with harmonic
components in the spectrogram of the recorded signal. We record the snoring
sounds by an auxiliary microphone in the vicinity of the patient. Using the
source of
noise (recorded by the auxiliary microphone) adaptive filtering will cancel
the snoring
sounds from the breath and snoring sounds recorded over the neck or inside the
ear
of the patient.
Figure 5 illustrates the block diagram of the adaptive filtering scheme.
The filter has two inputs, the primary input and the reference signal. The
primary
input, x(t), (the microphone over the neck or inside the ear) contains an
interference,
m(n), (snoring sounds) along with the infprmation bearing signal, b(n),
(tracheal
~ ,r,.
CA 02584258 2007-03-29
22
sound). The reference input, r(n), (the au)iiiary microphone) represents a
version of
interference with undetectable information bearing (tracheal sounds) signal.
The
output of the RLS FIR filter, y(n), is close to the interi'erence component of
the
primary signal. Therefore, the output af the adaptive filter, e(n), is the
minimum mean square error estimate of the information bearing signal,
õ
L(n).
Computational demand of the smart, automated algorithm to run 8
hours of sleep data can be high, As an altemative, the algorithms are written
in C++
code that increases the speed of the algorithms compared to a high level
signal
processing software such as MATLAB. With fast, state-of-art new computers,
this
will not be a problem eonsidering that this system will replace the 4 hours
labor work
of an sleep lab technician (the usual time to analyze one PSG patient's data)
with a
few minutes of processing time.
Flow Estimation
Flow calibration
In order to provide an effective flow estimation method it desirable to
provide another sound channel recorded over the lung to be used for
respiratory
phase detection and second it is desimble to provide one breath with known
flow
from the patient to calibrate (tune) the model to that patient. This
caiibration (tuning)
is necessary because there is a wide variation of flow-sound reiationship
between
the subjects due to their different chest size, lung capacity, gender, age,
etc.
CA 02584258 2007-03-29
23
Respiratorv phase detection
Thus the present arrangement provides a method of respiratory phase
detection with only one channel breath sound (Tracheal sound signal).
In this method the patient is required to have a deep breath, hold it,
start the program and then exhale and keep breathing normally but with
different
flow rates from low to high for 30 seconds. This 30 second data that starts
with
expiration phase is used by the program to derive the necessary information
for
phase detection of the rest of breath sounds. The phase detection aigorithm
is:
1. Sequester the 30 second initialization data into 100 ms
segments with 50% overiap between the suocessive segments.
2. Calculate the average power (in dB) of each segment over the
range of 150-450 Hz. The valleys of the resultant signal, which looks like a
rectified
sinusoid, determine the onsets of the breaths.
3. Knowing that the first phase is the exhalation, label the
initialization data as inspiration/expiration phases. Also by comparing the
max
power in each phase, label them as low, tidal and high flow rates.
4. Calculate the mean value of the average power (this time
calculated over the range of 500-1200 Hz of each segment) of the top 20% of
each
phase and store it for inspiration and expiration phases separately.
5. Calculate the ratio of the mean of the average power calculated
in Step 4 between the inspiration and expiration phases.
, , ,,Cõ
CA 02584258 2007-03-29
24
6. Apply this ratio as a threshold to the rest of the data to
determine respiratory phases. For example, if the ratio of inspiration and
expiration
Is {calculated as 1.2, and the ratio of any known phase respect to the
adjacent phase
(calculated with the same method) is equal to 0.8, it means that the first
known
phase Is expiration and the second one is inspiration.
Automatic Self Calibration
Since havi:ng one breath with known flow defeats the purpose of
eliminating the flow measurement, in this arrangement is provided a method of
automatic self calibration using a data bank. The concept includes a very
large data
bank of breathing sounds (tracheal sound) of people. This data bank is sorted
based on body-mass-index (BMI), age, gender, and smoking history of the
subjects.
This data is used to match the patient's BMI and other information to suggest
the
known flow-sound relationship required for calibration.
De-noising and Adventitiaus Sound Removal
Since the patient might have some respiratory diseases that may
cause some adventitious sounds, i.e., crackle sounds or wheezes, an algorithm
is
required to be run by the choice of the user (the clinician) to remove all
adventitious
sounds prior to flow estimation.
This algorithm has two parts: adventitious sound Iocalization and
removal. For adventitious localization the arrangement herein uses multi-scale
(level 3) product of wavelet coefficients and applies a running threshold of
mean plus
three times of standard deviation to detect and localize the adventitious
sounds.
CA 02584258 2007-03-29
Then, the segments including artefacts will be removed in time-frequency
domain,
the signal will be interpolated by spline interpolation and the breath sound
signal will
be reconstructed in time domain by taking the inverse of the spectrogram.
Flow Estimation Using entropy or range parameter
5 1. Band-pass filter the tracheal sounds in the frequency range of
75 to 600 Hz and nomnalize the signal.
2. Sequester the band-pass filtered signal into segments of 50 ms
(512 samples) with 75% overlap between the suocessive segments.
3. Let x(t) be the signal in each segment. The range value in each
10 segment can be defined as:
L, =1og[mean(x I x >[max(x) *(1- r/100)D-mean(x I x <[rnax(x) * r/100D ],
(1)
where x is the tracheal sound signal in each window and mean() is the average
value, and r =1,
15 or:
L, =1og[var(x)],
(2)
where var(x) is the variance of the signal in each segment.
4. The other feature that can be used for flow estimation is the entropy
20 of the signal in each segment. Let {X, ,.=., XN } represent the values of
signal x in
each segment. Estimate the probability density function (pdf) of signal x(t),
p(x), in
each window using the Normal kernel estimator.
. I I , , III
CA 02584258 2007-03-29
26
A.t (x) N
= N ,.i h ~ h (3)
where N is the number of samples (205), K is the Gaussian kemel function
( K'(x) =(2r)-"Z exp(-x2 / 2) ) and h is the kemel bandwidth. For Gaussian
kernel the
optimum h is approximated as:
6 hoP, =1.05 Q(x) N-o.z (4)
where r(x) is the estimated standard deviation of the signal x(t) in each
window.
Calculate the Shannon entropy in each segment:
N
L, pf l~(pi}' (5)
(~1
5. Use the modified linear model (6) to estimate flow from tracheal
sounds entropy or range (Eq. 1, 2 or 5) feature:
meantL~, )
~esi -C~ mea L~ LPh +C2 ,
(6)
where C, and Cz are the model coefficients derived from the one breath with
known
flow, L~, =[h, =, Lw] is a vector representing the entropy or range value of
the signal
in the upper 40% values of each respiratory phase (inspiration or expiration),
w is
the number of segments in the upper 40% values of each respiratory phase and
L;
is the entropy or range values of tracheal sound in each segment (Eq. 1, 2 or
5).
Similarly, L,,,, is the same vector that is calculated using the base
respiratory phase
CA 02584258 2007-03-29
27
signal. Base respiratory phase is the one breath that is assumed to be
available
with known flow to calibrate the model.
Heart sounds localization
1. Band-pass filter the tracheal sound records in the range of 75-
2501) Hz to remove motion artifacts and high-frequency noises.
2. Divide the filtered signal into segments of 20 ms (205 samples)
with 50% overlap between successive segments.
3. Let x(t) be the signal in each segment. The range value in each
segment can be defined as:
Lr =1og[mean(x I x>[max(x) *(1- r/ l 00)D- mean(x I x<[max(x) * r/ 100D ],
(7)
where x is the tracheal sound signal in each window and meanQ is the
average value, and r =1,
or:
L. = Ilog[var(x)], (8)
where var(x) is the variance of the signal in each segment
The other feature that can be used for heart sounds localization is
entropy of the signal. Let {X,,.=.,X,,} represent the values of signal x in
each
segment. Estimate the probability density function (pdf} of signal x(t), p(x),
in each
window using the Normal kemel estimator:
1 N 1 K ~x-X;
Pk(x)=N~h h
(9)
CA 02584258 2007-03-29
28
where N is the number of samples (205), K is the Gaussian kemel
furiction (K(x) -(2")-l1Z eXQ(_x2 /2) ) and h is the kernel bandwidth. For
Gaussian
kernel the optimum h is approximated as:
h,Pu =1.Q6 d(x) N-0.2
(10)
where er(x) is the estimated standard deviation of the signal x(t) in
each window.
4. Calculate the Shannon entropy in each segment:
N
H(P) ~ -~ Pr log(pr ) . (11)
r=~
5. Define average plus standard deviation value (lr+a) of the
calculated entropy or range value as the threshold for heart sounds
localization.
6. Mark the segments with entropy or range values of higher than
this threshold as heart sounds-included segments.
Removina the effects of heart oun
1. Localize heart sounds with the method mentioned above.
2. Calculate the range or entropy values for the segments void of
heart sounds.
3. Apply spline interpotation to estimate the values of the entropy
or range value in the segments including heart sounds. This technique
effectively
cancels the effect of heart sounds on the entropy or range values of the
tracheal
sound.
Examole 1
CA 02584258 2007-03-29
29
Eight healthy subjects (3 males) aged 33.1t6.e years with body mass
index of 23.3 3.5 participated in this study. Tracheal sound was recorded
using
Siemens accelerometer (EMT25C) placed over supra-stemal notch using double
adhesive tapes. Respiratory flow signal was measured by a mouth pieoe
pneumotachograph (Fleisch No.3) connected to a differential pressure
transducer
(Validyne, Northridge, CA). The subjects were instructed to
breathe at very shallow flow rates wi#h different periods af breath hold (2,
4, 6 sec) to
simulate apnea. Tracheal sound and flow signals were reoorded and digitized
simuftaneously at a 10240 Hz sampling rate.
Feature Extraction
Among several features of tracheal sound such as the sound's mean
amplitude, average power and entropy used for flow estimation, entropy and the
range of signal have been shown to be the best features following flow
variation.
Therefore, in this study tracheal sounds entropy was used to detect apnea
(breath
hol(J in the experiments of this study) without the use of the measured flow
signal.
However, the recorded flow signal was used for validation of the acoustically
detected apnea.
Tracheal sound signal was band-pass filtered in the range of [75-600]
Hz, and then segmented into 50ms (512 samples) windows with 50% overlap
between the adjacent windows. !n each window the tracheal sound probabiiity
density function (pdf) was estimated based on kemel methods. Then, using the
= i I -~
CA 02584258 2007-03-29
method described eariier in this document Shannon entropy was calculated in
each
window that represents the changes in the signal's pdf. The effect of heart
sounds
which is most evident in the frequency range below 200 Hz was removed by the
method introduced earlier in this document.
5 Figure 3 shows the calculated entropy and its corresponding flow
signal for a typical subject. By comparing the signals depicted in Figure 1(a)
and
Figure 1(c) (solid line), it is evident that the values of entropy in the
breath hold
segments are smaller than those during breathing.
It should be noted that when localizing the segments including heart
10 sounds, it is nearly impossible to find out the exact boundaries of heart
sounds
segments. Therefore, there is always a trade off between the amount of heart
sounds interference in respiratory sounds and the amount of respiratory sounds
information missing during heart sounds cancellation. The high peaks in the
calculated entropy (Figure 3a) are related to the heart sounds components
remained
15 in the tracheal sound. Figure 4 displays the mean and standard deviation
values of
length and lag errors in estimating apnea periods for different subjects.
Example 2
In this study 10 healthy subjects of the previous participated. Subjects
were in two age groups: 5 adults (ail female) 29t8 years old and 5 children (3
20 . female) 9.6 1.7 years old. Respiratory sounds were recorded using Siemens
accelerometers (EMT25C) placed over supra-stemal notch and the upper right
lobe
lung. Respiratory flow was measured by a pneumotachograph (Fleisch No.3)
CA 02584258 2007-03-29
31
connected to a differential pressure transducer (Validyne, Northridge, CA).
Subjects
were instructed to breathe at 5 different flow rates with 5 breaths at each
target flow
followed by a 10s of breath hold at the end of experiment. In this study the
shallow
(< 6 mUs/kg), low (6-9 ml/s/kg), medium (12-18 mi/s/kg), high (18-27 ml/s/kg)
and
very high (? 27 mUs/kg) target flow rates were investigated. Tracheal sound
signals
were used for flow estimation while the lung sound signal in correspondence
with
tracheal sound signals were used for respiratory phase detection. The onsets
of
breaths were detected by running a threshold on the average power of the
tracheal
sounds and detecting the valleys of the signal. Since lung sounds are much
louder
during inspiration as opposed to expiration, then by comparing the average
power of
the lung and tracheal breath sounds it can easily and accurately be determined
which phases are inspiration or expiration.
As described above, the best performance for estimating flow from
tracheal sound entropy was achieved in the frequency range of 75-600 Hz.
Tracheal
sound signals were used for flow estimation while the lung sound signal in
correspondence with tracheal sound signal were used for respiratory phase
detection as mentioned above.
As described above, the best performance for estimating flow from
tracheal sound entropy was achieved in the frequency range of [75 600) Hz.
This is
in atx;ordance with the fact that the main energy components of tracheal sound
exists in the frequency range below 600-800Hz. Thus, tracheal sound was band-
pass fittered in this range followed by segmenting the band-pass filtered
signal into
CA 02584258 2007-03-29
32
segments of 50 ms (512 samples) with 75% overlap between the successive
segments.
When studying tracheal sound in the frequency range below 300 Hz,
heart sounds are the main source of interference that changes the time and
frequency characteristiCs of the tracheal sound. Therefore, the presence of
heart
sounds will cause an error which can become significant in flow estimation In
very
shallow breathing, when most of the signal's energy is ooncentrated at low
frequencies. Hence, in this study the effect of heart sounds on the extracted
parameters was cancelled by using the same method as described above.
Since various modifications can be made in my invention as herein
above described, and many apparently widely different embodiments of same made
within the spirit and scope of the claims without department from such spirit
and
scope, it is intended that all matter contained in the accompanying
specification shall
bei interpreted as illustrative only and not in a limiting sense.
.,,~.