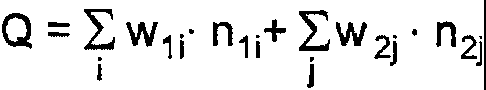Note: Descriptions are shown in the official language in which they were submitted.
CA 02588539 2007-05-15
Clariant International Ltd 2006DE421 Dr. KM/sch
Description
Cold flow improvers for vegetable or animal fuel oils
The present invention relates to an additive, to its use as a cold flow
improver for
vegetable or animal fuel oils and to correspondingly additized fuel oils.
In view of decreasing world crude oil reserves and the discussion about the
environmentally damaging consequences of the use of fossil and mineral fuels,
there
is increasing interest in alternative energy sources based on renewable raw
materials. These include in particular natural oils and fats of vegetable or
animal
origin. These are generally triglycerides of fatty acids having from 10 to 24
carbon
atoms and a calorific value comparable to conventional fuels, but are at the
same
time regarded as being less harmful to the environment. Biofuels, i.e. fuels
derived
from animal or vegetable material, are obtained from renewable sources and,
when
they are combusted, therefore generate only as much CO2 as had previously been
converted to biomass. It has been reported that less carbon dioxide is formed
in the
course of combustion than by the equivalent amount of crude oil distillate
fuel, for
example diesel fuel, and that very little sulfur dioxide is formed. In
addition, they are
biodegradable.
Oils obtained from animal or vegetable material are mainly metabolism products
which include triglycerides of monocarboxylic acids, and generally correspond
to the
formula
H H H
H-C C C-H
O C R O C R O C R
O O O
where R is an aliphatic radical which has from 10 to 25 carbon atoms and may
be
CA 02588539 2007-05-15
2
saturated or unsaturated.
In general, such oils contain glycerides from a series of acids whose number
and
type vary with the source of the oil, and they may additionally contain
phosphoglycerides. Such oils can be obtained by processes known from the prior
art.
As a consequence of the sometimes unsatisfactory physical properties of the
triglycerides, the industry has applied itself to converting the naturally
occurring
triglycerides to fatty acid esters of lower alcohols such as methanol or
ethanol.
A hindrance to the use of triglycerides and also fatty acid esters of lower
monohydric
alcohols as a replacement for diesel fuel, alone or in a mixture with diesel
fuel, has
been found to be their flow behavior at low temperatures. The cause of this is
the
high uniformity of these oils in comparison to mineral oil middle distillates.
For
example, rapeseed oil methyl ester (RME) has a cold filter plugging point
(CFPP) of
-14 C. It has hitherto been impossible using the prior art additives to
reliably attain a
CFPP value of -20 C required for use as a winter diesel in Central Europe, or
of
-22 C or lower for special applications. This problem is worsened when oils
are used
which comprise relatively large amounts of saturated fatty acid esters, as are
present, for example, in sunflower oil methyl ester, used oil methyl ester or
soybean
oil methyl ester.
EP-A-0 665 873 discloses a fuel oil composition which includes a biofuel, a
fuel oil
based on crude oil and an additive which comprises (a) an oil-soluble ethylene
copolymer or (b) a comb polymer or (c) a polar nitrogen compound or (d) a
compound in which at least one substantially linear alkyl group having from 10
to 30
carbon atoms is bonded to a nonpolymeric organic radical, in order to provide
at least
one linear chain of atoms which includes the carbon atoms of the alkyl groups
and
one or more nonterminal oxygen atoms, or (e) one or more of components (a),
(b),
(c) and (d).
EP-A-0 629 231 discloses a composition which comprises a relatively large
proportion of oil which consists substantially of alkyl esters of fatty acids
which are
derived from vegetable or animal oils or both, mixed with a smali proportion
of
CA 02588539 2007-05-15
3
mineral oil cold flow improvers, which comprises one or more of the following:
(I) comb polymer, the copolymer (which may be esterified) of maleic anhydride
or
fumaric acid and another ethylenically unsaturated monomer, or polymer or
copolymer of a-olefin, or fumarate or itaconate polymer or copolymer,
(II) polyoxyalkylene ester, ester/ether or a mixture thereof,
(III) ethylene/unsaturated ester copolymer,
(IV) polar, organic, nitrogen-containing paraffin crystal growth inhibitor,
(V) hydrocarbon polymer,
(VI) sulfur-carboxyl compounds and
(VII) aromatic pour point depressant modified with hydrocarbon radicals,
with the proviso that the composition does not comprise any mixtures of
polymeric
esters or copolymers of esters of acrylic and/or methacrylic acid which are
derived
from alcohols having from 1 to 22 carbon atoms.
EP-A-0 543 356 discloses a process for preparing compositions having improved
low
temperature performance for use as fuels or lubricants, starting from the
esters of
naturally occurring long-chain fatty acids with monohydric C1-C6-alcohols
(FAE),
which comprises
a) adding PPD additives (pour point depressants) known per se and used for
improving the low temperature performance of mineral oils in amounts of from
0.0001 to 10% by weight, based on the long-chain fatty acid esters FAE and
b) cooling the nonadditized long-chain fatty acid esters FAE to a temperature
below the cold filter plugging point and
c) removing the resulting precipitates (FAN).
DE-A-40 40 317 discloses mixtures of fatty acid lower alkyl esters having
improved
cold stability comprising
a) from 58 to 95% by weight of at least one ester within the iodine number
range
from 50 to 150 and being derived from fatty acids having from 12 to 22 carbon
atoms and lower aliphatic alcohols having from 1 to 4 carbon atoms,
CA 02588539 2007-05-15
4
b) from 4 to 40% by weight of at least one ester of fatty acids having from 6
to 14
carbon atoms and lower aliphatic alcohols having from 1 to 4 carbon atoms
and
c) from 0.1 to 2% by weight of at least one polymeric ester.
EP-A-O 153 176 discloses the use of polymers based on unsaturated dialkyl C4-
C8-
dicarboxylates having an average alkyl chain length of from 12 to 14 as cold
flow
improvers for certain crude oil distillate fuel oils. Mentioned as suitable
comonomers
are unsaturated esters, in particular vinyl acetate, but also a-olefins.
EP-A-0 153 177 discloses an additive concentrate which comprises a combination
of
I) a copolymer having at least 25% by weight of an n-alkyl ester of a
monoethylenically unsaturated C4-C8 mono- or dicarboxylic acid, the average
number of carbon atoms in the n-alkyl radicals being 12 - 14, and another
unsaturated ester or an olefin, with
II) another low temperature flow improver for distillate fuel oils.
EP-A-1 491 614 discloses oils of vegetable or animal origin and their blends
with
crude oil distillate fuel oils, which comprise an ethylene-vinyl ester
copolymer which
contains at least 17 mol% of vinyl ester and has a degree of branching of 5 or
more
alkyl branches per 100 methylene groups to improve their low temperature
properties.
With the known additives, it is often impossible to reliably adjust fatty acid
esters,
especially those which comprise a total of more than 7% by weight of paimitic
acid
methyl ester and stearic acid methyl ester, to a CFPP of -10 C which is
required for
use as winter diesel in Southern Central Europe and of -20 C in Northern
Central
Europe, and of -22 C and lower for specific applications. An additional
problem with
the existing additives is a lack of cold transition stability of the additized
oils, i.e. the
set CFPP value of the oils rises gradually when the oil is stored for a
prolonged
period at varying temperatures in the region of its cloud point or lower.
Moreover,
especially oils having a high content of palmitic acid methyl ester and
stearic acid
methyl ester exhibit a strong tendency to sedimentation in the course of
storage at
low temperatures. It is known from practice that sedimentation of the
additized fatty
CA 02588539 2007-05-15
acid esters which occurs in laboratory experiments under cold conditions, in
spite of
the CFPP being attained, can lead to filter blockages in the engine and the
fuel is
thus not suitable for use in transport.
5 It was therefore an object of the invention to provide additives for
improving the cold
flow behavior of fatty acid esters which are derived, for example, from
rapeseed oil,
used oil, sunflower oil and/or soybean oil and which comprise, at least 7% by
weight
of palmitic acid methyl ester and stearic acid methyl ester, to set CFPP
values of
-10 C or -20 C or lower which remain constant even in the course of prolonged
storage of the oil in the region of its cloud point and lower. Moreover, these
additives
should contribute to preventing the sedimentation tendency of these oils, such
that,
even after storage of the fatty acid esters for several days, they remain
homogeneous and free-flowing and their CFPP does not change either.
It has now been found that, surprisingly, an additive comprising ethylene
copolymers
and comb polymers is an outstanding flow improver for such fatty acid esters.
The invention provides an additive comprising
A) a copolymer of ethylene and from 13 to 17 mol% of at least one acrylic
ester or
vinyl ester having a Cl-C18-afkyl radical and a melt viscosity V140 of not
more
than 80 mPas, and
B) a comb polymer comprising structural units formed from
131) at least one olefin as monomer 1, which bears at least one C8-C18-alkyl
radical
on the olefinic double bond, and
B2) at least one ethylenically unsaturated dicarboxylic acid as monomer 2,
which
bears at least one C8-C16-alkyl radical bonded via an amide and/or imide
group,
in which the parameter Q
Q = Ew, ;= n1+Ew2j = n2j
i j
CA 02588539 2007-05-15
6
in which
wl is the molar proportion of the individual chain lengths n, in the alkyl
radicals of
monomer 1,
w2 is the molar proportion of the individual chain lengths n2 in the alkyl
radicals of
the amide and/or imide groups of monomer 2,
ni are the individual chain lengths in the alkyl radicals of monomer 1,
n2 are the individual chain lengths in the alkyl radicals of the amide and/or
imide
groups of monomer 2,
i is the serial variable for the chain lengths in the alkyl radicals of
monomer 1,
and
j is the serial variable for the chain lengths in the alkyl radicals of the
amide
and/or imide groups of monomer 2
assumes values of from 23 to 27.
The invention further provides a fuel oil composition comprising a fuel oil of
animal or
vegetable origin and the above-defined additive.
The invention further provides for the use of the above-defined additive for
improving
the cold flow properties of fuel oils of animal or vegetable origin.
The invention further provides a process for improving the cold flow
properties of fuel
oils of animal or vegetable origin by adding the above-defined additive to
fuel oils of
animal or vegetable origin.
In a preferred embodiment of the invention, Q assumes values of from 24 to 26.
Chain length of olefins is understood here to mean the chain length of the
monomeric
olefin minus the two olefinically bonded carbon atoms. In olefins with
nonterminal
double bonds, for example olefins with vinylidene moiety, the chain length is
equal to
the total chain length of the olefin minus the two olefinically bonded carbon
atoms.
When the polymers formed from the olefins 131) and the dicarboxamidesrmides
B2)
rather than the monomeric olefins are considered, the chain length is the
length of
the alkyl radicals which - introduced into the polymer by the olefin - depart
from the
CA 02588539 2007-05-15
7
polymer backbone.
Suitable ethylene copolymers A) are preferably those which contain from 13 to
17 mol% of one or more vinyl esters and/or (meth)acrylic esters and from 83 to
87%
by weight of ethylene. Particular preference is given to ethylene copolymers
having
from 15 to 17 mol% of at least one vinyl ester. Suitable vinyl esters derive
from fatty
acids having linear or branched alkyl groups having from 1 to 30 carbon atoms.
Preferred ethylene copolymers have a melt viscosity V140 of at least 5 mPas,
preferably from 10 to 80 mPas, in particular from 20 to 60 mPas.
Examples of suitable vinyl esters are vinyl acetate, vinyl propionate, vinyl
butyrate,
vinyl hexanoate, vinyl heptanoate, vinyl octanoate, vinyl laurate and vinyl
stearate,
and esters of vinyl alcohol based on branched fatty acids, such as vinyl
isobutyrate,
vinyl pivalate, vinyl 2-ethylhexanoate, vinyl isononanoate, vinyl
neononanoate, vinyl
neodecanoate and vinyl neoundecanoate. Likewise suitable as comonomers are
esters of acrylic acid and methacrylic acid having from 1 to 20 carbon atoms
in the
alkyl radical, such as methyl (meth)acrylate, ethyl (meth)acrylate, propyl
(meth)acrylate, n- and isobutyl (meth)acrylate, hexyl (meth)acrylate, octyl
(meth)acrylate, 2-ethylhexyl (meth)acrylate, decyl (meth)acrylate, dodecyl
(meth)acrylate, tetradecyl (meth)acryiate, hexadecyl (meth)acrylate, octadecyl
(meth)acrylate. Also suitable are mixtures of two, three, four or even more of
these
comonomers.
Further preferred copolymers contain, in addition to ethylene and from 13 to
17 mol%
of vinyl esters, also from 0.5 to 10 mol% of olefins having from 3 to 10
carbon atoms,
for example propene, butene, isobutylene, hexene, 4-methylpentene, octene,
diisobutylene and/or norbornene.
The copolymers A preferably have weight-average molecular weights Mw, measured
by means of gel permeation chromatography (GPC) against polystyrene standards
in
THF of from 1000 to 10 000 g/mol, in particular from 1500 to 5000 g/mol. Their
degrees of branching determined by means of'H NMR spectroscopy (400 MHz with
CDC13 as the solvent) are preferably less than 6, in particular less than
CA 02588539 2007-05-15
8
CH3/100 CH2 groups. The methyl groups stem from the short-chain and long-chain
branches, and not from copolymerized comonomers.
The copolymers A can be prepared by customary copolymerization processes, for
5 example suspension polymerization, solution polymerization, gas phase
polymerization or high pressure bulk polymerization. Preference is given to
carrying
out the high pressure bulk polymerization at pressures of from 50 to 400 MPa,
preferably from 100 to 300 MPa, and temperatures from 100 to 300 C, preferably
from 150 to 250 C. In a particularly preferred preparation variant, the
polymerization
is effected in a multizone reactor in which the temperature difference between
the
peroxide feeds along the tubular reactor is kept to a minimum, i.e. < 50 C,
preferably
< 30 C, in particular < 15 C. The temperature maxima in the individual
reaction
zones preferably differ by less than 30 C, more preferably by less than 20 C
and
especially by less than 10 C.
The reaction of the monomers is initiated by free radical-forming initiators
(free
radical chain initiators). This substance class includes, for example, oxygen,
hydroperoxides, peroxides and azo compounds, such as cumene hydroperoxide, t-
butyl hydroperoxide, dilauroyl peroxide, dibenzoyl peroxide, bis(2-ethylhexyl)
peroxodicarbonate, t-butyl perpivalate, t-butyl permaleate, t-butyl
perbenzoate,
dicumyl peroxide, t-butyl cumyl peroxide, di(t-butyl) peroxide, 2,2'-azobis(2-
methyl-
propanonitrile), 2,2'-azobis(2-methylbutyronitrile). The initiators are used
individually
or as a mixture of two or more substances in amounts of from 0.01 to 20% by
weight,
preferably from 0.05 to 10% by weight, based on the monomer mixture.
The high-pressure bulk polymerization is carried out in known high-pressure
reactors,
for example autoclaves or tubular reactors, batchwise or continuously; tubular
reactors have been found to be particularly useful. Solvents such as aliphatic
and/or
aromatic hydrocarbons or hydrocarbon mixtures, benzene or toluene may be
present
in the reaction mixture. Preference is given to the substantially solvent-free
procedure. In a preferred embodiment of the polymerization, the mixture of the
monomers, the initiator and, if used, the moderator, is fed to a tubular
reactor via the
reactor entrance and also via one or more side branches. Preferred moderators
are,
for example, hydrogen, saturated and unsaturated hydrocarbons, for example
CA 02588539 2007-05-15
9
propane or propene, aldehydes, for example propionaldehyde, n-butyraidehyde or
isobutyraidehyde, ketones, for example acetone, methyl ethyl ketone, methyl
isobutyl
ketone, cyclohexanone, and alcohols, for example butanol. The comonomers and
also the moderators may be metered into the reactor either together with
ethylene or
else separately via sidestreams. The monomer streams may have different
compositions (EP-A-0 271 738 and EP-A-0 922 716).
Examples of suitable co- or terpolymers include:
ethylene-vinyl acetate copolymers having 10 - 40% by weight of vinyl acetate
and
60 - 90% by weight of ethylene;
the ethylene-vinyl acetate-hexene terpolymers known from DE-A-34 43 475;
the ethylene-vinyl acetate-diisobutylene terpolymers described in EP-A-0 203
554;
the mixture of an ethylene-vinyl acetate-diisobutylene terpolymer and an
ethylene/vinyl acetate copolymer known from EP-A-0 254 284;
the mixtures of an ethylene-vinyl acetate copolymer and an ethylene-vinyl
acetate-
N-vinylpyrrolidone terpolymer known from EP-A-0 405 270;
the ethylene/vinyl acetatersobutyl vinyl ether terpolymers described in
EP-A-0 463 518;
the ethylene/vinyl acetate/vinyl neononanoate or vinyl neodecanoate
terpolymers
which, apart from ethylene, contain 10 - 35% by weight of vinyl acetate and 1-
25%
by weight of the particular neo compound, known from EP-A-0 493 769;
the terpolymers of ethylene, a first vinyl ester having up to 4 carbon atoms
and a
second vinyl ester which is derived from a branched carboxylic acid having up
to 7
carbon atoms or a branched but nontertiary carboxylic acid having from 8 to 15
carbon atoms, described in EP-A-0 778 875;
the terpolymers of ethylene, the vinyl ester of one or more aliphatic C2- to
C20-
CA 02588539 2007-05-15
monocarboxylic acids and 4-methylpentene-1, described in DE-A-196 20 118;
the terpolymers of ethylene, the vinyl ester of one or more aliphatic C2- to
C20-
monocarboxylic acids and bicyclo[2.2.1]hept-2-ene, disclosed in DE-A-196 20
119;
5
the terpolymers of ethylene and at least one olefinically unsaturated
comonomer
which contains one or more hydroxyl groups, described in EP-A-0 926 168.
Preference is given to using mixtures of identical or different ethylene
copolymers.
10 The polymers on which the mixtures are based more preferably differ in at
least one
characteristic. For example, they may contain different comonomers, different
comonomer contents, molecular weights and/or degrees of branching. The mixing
ratio of the different ethylene copolymers is preferably between 20:1 and
1:20,
preferably from 10:1 to 1:10, in particular from 5:1 to 1:5.
The copolymers B are derived from the amides and imides of ethylenically
unsaturated dicarboxylic acids. Preferred dicarboxylic acids are maleic acid,
fumaric
acid and itaconic acid. Particularly suitable comonomers are monoolefins B1
having
from 10 to 20, in particular having from 12 to 18, carbon atoms. These are
preferably
linear and the double bond is preferably terminal, as, for example, in
dodecene,
tridecene, tetradecene, pentadecene, hexadecene, heptadecene and octadecene.
The molar ratio of dicarboxamide/imide to olefin or olefins in the polymer is
preferably
in the range from 1:1.5 to 1.5:1, and is especially equimolar.
It is possible for copolymer B also to contain minor amounts of up to 20 mol%,
preferably < 10 mol%, especially < 5 mol%, of further comonomers which are
copolymerizable with ethylenically unsaturated dicarboxamidesrimides and the
olefins mentioned, for example olefins having from 2 to 50 carbon atoms, allyl
polyglycol ethers, Cl-C30-alkyl (meth)acrylates, vinylaromatics or Cl-CZO-
alkyl vinyl
ethers. Equally, minor amounts of poly(isobutylenes) having molecular weights
of up
to 5000 g/mol are used, preference being given to highly reactive variants
having a
high proportion of terminal vinylidene groups. These further comonomers are
not
taken into account in the calculation of the parameter Q which is critical for
the
effectiveness.
CA 02588539 2007-05-15
11
Allyl polyglycol ethers correspond to the general formula
R'
CH2 C
H2C O (I
R2
where
R' is hydrogen or methyl,
R2 is hydrogen or Cl-C4-alkyl,
m is a number from 1 to 100,
R3 is Cl-C24-alkyl, C5-C20-cycloalkyl, C6-C18-aryl or -C(O)-R4,
R4 is Cl-C40-alkyl, C5-Clo-cycloalkyl or C6-C18-aryl.
The inventive copolymers B) are prepared preferably at temperatures between 50
and 220 C, in particular from 100 to 190 C. The preferred preparation process
is
solvent-free bulk polymerization, but it is also possible to carry out the
polymerization
in the presence of aprotic solvent such as benzene, toluene, xylene or of
higher-
boiling aromatic, aliphatic or isoaliphatic solvents or solvent mixtures such
as
kerosene or Solvent Naphtha. Particular preference is given to polymerizing in
a
small amount of moderating, aliphatic or isoaliphatic solvents. The proportion
of
solvent in the polymerization mixture is generally between 10 and 90% by
weight,
preferably between 35 and 60% by weight. In the solution polymerization, the
reaction temperature may be adjusted particularly simply by the boiling point
of the
solvent or by working under reduced or elevated pressure.
The average molecular mass Mw of the inventive copolymers B is generally
between
1200 and 200 000 g/mol, in particular between 2000 and 100 000 g/mol, measured
by means of gel permeation chromatography (GPC) against polystyrene standards
in
THF. Inventive copolymers B have to be oil-soluble in doses relevant in
practice, i.e.
they have to dissolve without residue at 50 C in the oil to be additized.
CA 02588539 2007-05-15
12
The reaction of the monomers is initiated by free radical-forming initiators
(free-
radical chain starters). This substance class includes, for example, oxygen,
hydroperoxides and peroxides, for example cumene hydroperoxide, t-butyl
hydroperoxide, dilauroyl peroxide, dibenzoyl peroxide, bis(2-ethylhexyl)
peroxodicarbonate, t-butyl perpivalate, t-butyl permaleate, t-butyl
perbenzoate,
dicumyl peroxide, t-butyl cumyl peroxide, di(t-butyl) peroxide, and also azo
compounds, for example 2-2'-azobis(2-methylpropanonitrile) or 2,2'-azobis(2-
methylbutyronitrile). The initiators are used individually or as a mixture of
two or more
substances in amounts of from 0.01 to 20% by weight, preferably from 0.05 to
10%
by weight, based on the monomer mixture.
The copolymers may be prepared either by reacting maleic acid, fumaric acid
and/or
itaconic acid or their anhydrides with the corresponding amine and
subsequently
copolymerizing, or by copolymerizing olefin or olefins with at least one
unsaturated
dicarboxylic acid or derivative thereof, for example itaconic anhydride and/or
maleic
anhydride and subsequently reacting with amines. Preference is given to
carrying out
a copolymerization with anhydrides and converting the resulting copolymer to
an
amide and/or an imide after the preparation.
In both cases, the reaction with amines is effected, for example, by reacting
with from
0.8 to 2.5 mol of amine per mole of anhydride, preferably with from 1.0 to 2.0
mol of
amine per mole of anhydride, at from 50 to 300 C. When approx. 1 mol of amine
is
used per mole of anhydride, monoamides are formed preferentially at reaction
temperatures of from approx. 50 to 100 C and additionally bear one carboxyl
group
per amide group. At higher reaction temperatures of from approx. 100 to 250 C,
imides are formed preferentially from primary amines with elimination of
water. When
larger amounts of amine are used, preferably 2 mol of amine per mole of
anhydride,
amide-ammonium salts are formed at from approx. 50 to 200 C and diamides at
higher temperatures of, for exampie, 100-300 C, preferably 120-250 C. The
water of
reaction may be distilled off by means of an inert gas stream or removed by
means of
azeotropic distillation in the presence of an organic solvent. To this end,
preferably
20-80%, in particular 30-70%, especially 35-55% by weight of at least one
organic
solvent is used. Here, copolymers (diluted to 50% in solvent) having acid
numbers of
30-70 mg KOH/g, preferably of 40-60 mg KOH/g, are regarded as monoamides.
CA 02588539 2007-05-15
13
Corresponding copolymers having acid numbers of less than 40 mg, especially
less
than 30 mg KOH/g, are regarded as diamides or imides. Particular preference is
given to monoamides and diamides.
Suitable amines are primary and secondary amines having one or two C8-C16-
alkyl
radicals. They may bear one, two or three amino groups which are bonded via
alkylene radicals having two or three carbon atoms. Preference is given to
monoamines. In particular, they bear linear alkyl radicals, but may also
contain minor
amounts, for example up to 30% by weight, preferably up to 20% by weight and
especially up to 10% by weight of branched amines (in the 1- or 2-position).
Either
shorter- or longer-chain amines may be used, but their proportion is
preferably below
mol% and especially below 10 mol%, for example between 1 and 5 mol%, based
on the total amount of the amines used.
15 Particularly preferred primary amines are octylamine, 2-ethylhexylamine,
decylamine,
undecylamine, dodecylamine, n-tridecylamine, isotridecylamine,
tetradecylamine,
pentadecylamine, hexadecylamine and mixtures thereof.
Preferred secondary amines are dioctylamine, dinonylamine, didecylamine,
20 didodecylamine, ditetradecylamine, dihexadecylamine, and also amines having
different alkyl chain lengths, for example N-octyl-N-decylamine, N-decyl-
N-dodecylamine, N-decyl-N-tetradecylamine, N-decyl-N-hexadecylamine, N-dodecyl-
N-tetradecylamine, N-dodecyl-N-hexadecylamine, N-tetradecyl-N-hexadecylamine.
Also suitable in accordance with the invention are secondary amines which, in
addition to a C8-C16-alkyl radical, bear shorter side chains having from 1 to
5 carbon
atoms, for example methyl or ethyl groups. In the case of secondary amines, it
is the
average of the alkyl chain lengths of from C8 to C16 that is taken into
account as the
alkyl chain length n for the calculation of the parameter Q. Neither shorter
nor longer
alkyl radicals, where present, are taken into account in the calculation,
since they do
not contribute to the effectiveness of the additives.
Particularly preferred copolymers B contain monoamides and diamides of primary
monoamines as monomer 2.
CA 02588539 2007-05-15
14
The use of mixtures of different olefins in the polymerization and mixtures of
different
amines in the amidation or imidation allows the effectiveness to be further
adapted to
specific fatty acid ester compositions.
In a preferred embodiment, the additives, as well as constituents A and B, may
also
comprise polymers and copolymers based on C1Q-C24-alkyl acrylates or
methacrylates (constituent C). These poly(alkyl acrylates) and methacrylates
have
molecular weights Mw of from 800 to 1 000 000 g/mol, and derive preferably
from
capryiic alcohol, capric alcohol, undecyl alcohol, lauryl alcohol, myristyl
alcohol, cetyl
alcohol, paimitoleyl alcohol, stearyl alcohol or mixtures thereof, for example
coconut
alcohol, palm alcohol, tallow fat alcohol or behenyl alcohol.
In a preferred embodiment, mixtures of different copolymers B are used, the
mean
(weight average) of the parameter Q of the mixture components assuming values
of
from 23 to 27 and preferably values of from 24 to 26.
The mixing ratio of the inventive additive constituents A and B is (in parts
by weight)
from 20:1 to 1:20, preferably from 10:1 to 1:10, in particular from 5:1 to
1:5. The
proportion of component C in the formulations composed of A, B and C may be up
to
40% by weight; it is preferably less than 20% by weight, in particular between
1 and
10% by weight, based on the total weight of A, B and C.
The inventive additives are added to oiis in amounts of from 0.001 to 5% by
weight,
preferably from 0.005 to 1% by weight and especially from 0.01 to 0.6% by
weight.
They may be used as such or else dissolved or dispersed in solvents, for
example
aliphatic and/or aromatic hydrocarbons or hydrocarbon mixtures, for example
toluene, xylene, ethylbenzene, decane, pentadecane, petroleum fractions,
kerosene,
naphtha, diesel, heating oil, isoparaffins or commercial solvent mixtures such
as
Solvent Naphtha, Hydrosol A 200 N, Shellsol A 150 ND, Caromax 20 LN,
Shellsol AB, Sofvesso 150, Solvesso 150 ND, Solvesso 200, Exxsol, Isopar
and Shellsol D types. They are preferably dissolved in fuel oil of animal or
vegetable
origin based on fatty acid alkyl esters. The inventive additives preferably
comprise
1- 80%, especially 10 - 70%, in particular 25 - 60%, of solvent.
CA 02588539 2007-05-15
In a preferred embodiment, the fuel oil, which is frequently also referred to
as
biodiesel or biofuel, comprises fatty acid alkyl esters composed of fatty
acids having
from 12 to 24 carbon atoms and alcohols having from 1 to 4 carbon atoms.
Typically,
a relatively large portion of the fatty acids contains one, two or three
double bonds.
5
Examples of oils which are derived from animal or vegetable material and in
which
the inventive additive can be used are rapeseed oil, coriander oil, soya oil,
cottonseed oil, sunflower oil, castor oil, olive oil, peanut oil, maize oil,
almond oil,
palm kernel oil, coconut oil, mustardseed oil, bovine tallow, bone oil, fish
oils and
10 used cooking oils. Further examples include oils which are derived from
wheat, jute,
sesame, shea tree nut, arachis oil and linseed oil. The fatty acid alkyl
esters also
referred to as biodiesel can be derived from these oils by processes disclosed
by the
prior art. Preference is given to rapeseed oil, which is a mixture of fatty
acids partially
esterified with glycerol, since it is obtainable in large amounts and is
obtainable in a
15 simple manner by extractive pressing of rapeseeds. In addition, preference
is given
to the likewise widely available oils of used oil, palm oil, sunflowers and
soya, and
also to their mixtures with rapeseed oil.
Particularly suitable biofuels are lower alkyl esters of fatty acids. These
include, for
example, commercially available mixtures of the ethyl, propyl, butyl and in
particular
methyl esters of fatty acids having from 14 to 22 carbon atoms, for example of
lauric
acid, myristic acid, paimitic acid, paimitoleic acid, stearic acid, oleic
acid, elaidic acid,
petroselic acid, ricinolic acid, elaeostearic acid, linoleic acid, linolenic
acid, eicosanoic
acid, gadoleic acid, docosanoic acid or erucic acid, each of which preferably
has an
iodine number of from 50 to 150, in particular from 90 to 125. Mixtures having
particularly advantageous properties are those which comprise mainly, i.e.
comprise
at least 50% by weight of, methyl esters of fatty acids having from 16 to 22
carbon
atoms, and 1, 2 or 3 double bonds. The preferred lower alkyl esters of fatty
acids are
the methyl esters of oleic acid, linoleic acid, linolenic acid and erucic
acid.
Commercial mixtures of the type mentioned are obtained, for example, by
hydrolyzing and esterifying, or by transesterifying, animal and vegetable fats
and oils
with lower aliphatic alcohols. Equally suitable as starting materials are used
cooking
oils. To prepare lower alkyl esters of fatty acids, it is advantageous to
start from fats
CA 02588539 2007-05-15
16
and oils having a high iodine number, for example sunflower oil, rapeseed oil,
coriander oil, castor oil, soya oil, cottonseed oil, peanut oil or bovine
tallow.
Preference is given to lower alkyl esters of fatty acids based on a novel type
of
rapeseed oil, more than 80% by weight of whose fatty acid component is derived
from unsaturated fatty acids having 18 carbon atoms.
A biofuel is therefore an oil which is obtained from vegetable or animal
material or
both or a derivative thereof which can be used as a fuel and in particular as
a diesel
or heating oil. Although many of the above oils can be used as biofuels,
preference is
given firstly to vegetabie oil derivatives, particularly preferred biofuels
being alkyl
ester derivatives of rapeseed oil, cottonseed oil, soya oil, sunflower oil,
olive oil or
palm oil, and very particular preference is given to rapeseed oil methyl
ester,
sunflower oil methyl ester, palm oil methyl ester and soya oil methyl ester.
Owing to
the high demand for biofuels, ever more manufacturers are switching from fatty
acid
methyl esters to other raw material sources with higher availability. Mention
shouid
be made here particularly of used oil, which is used in the form of used oil
methyl
ester as biodiesel alone or in a blend with other fatty acid methyl esters,
for example
rapeseed oil methyl ester, sunflower oil methyl ester, palm oil methyl ester
and
soybean oil methyl ester. Mention should also be made of mixtures of rapeseed
oil
methyl ester with soybean oil methyl ester or rapeseed oil methyl ester with a
mixture
of soybean oil methyl ester and palm oil methyl ester.
The additive can be introduced to the oil to be additized by processes known
in the
prior art. When more than one additive component or coadditive component is to
be
used, such components can be introduced into the oil together or separately in
any
combination.
The inventive additives allow the CFPP value of biodiesel to be adjusted to
values of
-10 C and below -20 C and in some cases to values of below -25 C, as required
for
marketing for use especially in winter. Equally, the pour point of biodiesel
is lowered
by the addition of the inventive additives. The inventive additives are
particularly
advantageous in problematic oils which have a high proportion of esters of the
saturated fatty acids palmitic acid and stearic acid of more than 7% by
weight, as
present, for example, in fatty acid methyl esters obtained from used oil,
sunflowers
CA 02588539 2007-05-15
17
and soybean. It is thus also possible with the inventive additives to adjust
mixtures of
rapeseed oil methyl ester and/or used oil methyl ester and/or sunflower oil
methyl
ester and/or soybean oil methyl ester to CFPP values of -10 C or -20 C and
lower. It
is thus also possible with the inventive additives to adjust used oil methyl
ester or
sunflower oil methyl ester or soybean oil methyl ester to CFPP values of -10 C
or
-20 C and lower. In addition, the oils thus additized have a good cold
transition
stability, i.e. the CFPP value remains constant even in the case of storage
under
winter conditions, and do not tend to sediment at constant low temperatures
(e.g.
-10 C or -22 C).
To produce additive packages for specific solutions to problems, the inventive
additives may also be used together with one or more oil-soluble coadditives
which,
even alone, improve the cold flow properties of crude oils, lubricant oils or
fuel oils.
Examples of such coadditives are polar compounds which bring about paraffin
dispersancy (paraffin dispersants) and oil-soluble amphiphiles.
The inventive additives may be used in a mixture with paraffin dispersants.
Paraffin
dispersants reduce the size of the paraffin crystals and have the effect that
the
paraffin particles do not settle out but rather remain dispersed in colloidal
form with
significantly reduced sedimentation tendency. Useful paraffin dispersants have
been
found to be both low molecular weight and polymeric oil-soluble compounds
having
ionic or polar groups, for example amine salts and/or amides. Particular
preferred
paraffin dispersants comprise reaction products of secondary fatty amines
having
from 20 to 44 carbon atoms, in particular dicoconut amine, ditallow fat amine,
distearylamine and dibehenylamine with carboxylic acids and their derivatives.
Particularly useful paraffin dispersants have been found to be those which are
obtained by reacting aliphatic or aromatic amines, preferably long-chain
aliphatic
amines, with aliphatic or aromatic mono-, di-, tri- or tetracarboxylic acids
or their
anhydrides (cf. US 4 211 534). Equally suitable as paraffin dispersants are
amides
and ammonium salts of aminoalkylenepolycarboxylic acids, such as
nitrilotriacetic
acid or ethylenediaminetetraacetic acid, with secondary amines (cf. EP 0 398
101).
Other paraffin dispersants are copolymers of maleic anhydride and a,p-
unsaturated
compounds which may optionally be reacted with primary monoalkylamines and/or
aliphatic alcohols (cf. EP 0 154 177), and the reaction products of alkenyl-
spiro-
CA 02588539 2007-05-15
18
bislactones with amines (cf. EP 0 413 279 B1), and, according to
EP-A-0 606 055 A2, reaction products of terpolymers based on a,R-unsaturated
dicarboxylic anhydrides, a,p-unsaturated compounds and polyoxyalkylene ethers
of
lower unsaturated alcohols.
The mixing ratio (in parts by weight) of the inventive additives with paraffin
dispersants is from 1:10 to 20:1, preferably from 1:1 to 10:1.
The oils treated with the inventive additive may also be added to middle
distillates
obtained from crude oil. The mixtures of biofuel and middle distillate thus
obtained
can in turn be admixed with cold additives such as flow improvers or wax
dispersants, and also performance packages.
The mixing ratio between biofuel and middle distillates may be between 1:99
and
99:1. Particular preference is given to mixing ratios of biofuel:middle
distillate = 3:97
to 30:70.
Middle distillate refers in particular to those mineral oiis which are
obtained by
distilling crude oil and boil within the range from 120 to 450 C, for example
kerosene,
jet fuel, diesel and heating oil. Preference is given to using those middle
distillates
which contain 0.05% by weight of sulfur and less, more preferably less than
350 ppm
of sulfur, in particular less than 200 ppm of sulfur and in special cases less
than
50 ppm of sulfur. They are generally those middle distillates which have been
subjected to refining under hydrogenating conditions, and which therefore
contain
only small proportions of polyaromatic and polar compounds. They are
preferably
those middle distillates which have 95% distillation points below 370 C, in
particular
350 C and in special cases below 330 C. Suitable middle distillates are also
synthetic fuels, as made available, for example, by the Fischer-Tropsch
process.
The additives may be used alone or else together with other additives, for
example
with other pour point depressants or dewaxing aids, with antioxidants, cetane
number
improvers, dehazers, demulsifiers, detergents, dispersants, defoamers, dyes,
corrosion inhibitors, conductivity improvers, sludge inhibitors, odorants
and/or
additives for lowering the cloud point.
CA 02588539 2007-05-15
19
Examples
Table 1 Characterization of the ethylene copolymers used
Example Comonomer(s) V140 CH3/100 CH2 Content of
vinyl ester
Al (C) Ethylene / VAC / 110 mPas 4.2 13.3 mol%
vinyl neodecanoate
A2 Ethylene / VAC 35 mPas 3.9 16.6 mol%
A3 (C) Ethylene / VAC 154 mPas 3.0 16.7 mol%
A4 (C) Ethylene / VAC 125 mPas 3.0 13.8 mol%
VAC = vinyl acetate
The vinyl ester content was determined by means of pyrolysis and subsequent
titration.
The viscosity (V140) was measured with a Haake Reostress 600 viscometer.
The degree of branching (CH3/100CH2) was measured on a1 H NMR unit at 400 MHz
in CDCl3, and calculated by means of integration of the individual signals.
CA 02588539 2007-05-15
Table 2 Characterization of the comb polymers used
Example Comonomers Amine Q Acid number [mg
KOH/g]
BI MSA-co-C14/,6-a-oiefin (1:0.5:0.5) C12 amine 25 2
B2 MSA-co-C14/,6-a-olefin (1:0.5:0.5) C14 amine 25.0 57
5 Table 3 Acrylates
C1 Poly(octadecyl acrylate), K value 32
C2 Poly(behenyl acrylate), K value 18
Table 4 Characterization of the test oils
Oil No.: CFPP [ C] Composition
El -8 SoyaME RME 30:70
E2 -7 RME/PME85:15
E3 -11 RME / AME 60:40
E4 -10 RME / AME 50:50
E5 -8 RME / AME 40:60
E6 -8 RME / AME 45:55
SoyaME = soya methyl ester
RME = rapeseed oil methyl ester
PME = palm oil methyl ester
AME = used oil methyl ester
CA 02588539 2007-05-15
21
Table 5 Methyl ester distribution of the test oils
El E2 E3 E4 E5 E6
C16:0 6.53 6.21 6.74 7.33 8.13 7.76
C18:0 1.19 2.36 2.71 2.97 3.32 3.16
C18:1 45.19 48.73 52.56 50.84 45.36 46.77
C18:2 36.33 28.77 26.45 28.17 32.27 31.00
C18:3 8.37 9.18 6.36 5.49 5.45 5.89
C20:1 /2/3 0.79 1.25 1.14 1.04 0.97 1.03
C20:0 0.39 0.57 0.58 0.56 0.53 0.57
C22:0 0.15 0.39 0.48 0.51 0.50 0.49
In the tables which follow, the mixing ratio according to weight of the
additives A, B
and C is A:B = 4:1, or, when C is present in the mixtures, A:B:C = 4:1:0.2.
The total
amount of additive is evident from the top row of the table.
Table 6 CFPP testing in test oil El
Ex. Comb polymer Ethylene copolymer Polyacrylate 2000 3000 4000
ppm ppm ppm
1 B1 A2 - -18 -22 -20
2 (C) BI Al - -12 -16 -10
3 B1 A2 C3 -18 -21 -21
Table 7 CFPP testing in test oil E2
Ex. Comb polymer Ethylene copolymer Polyacrylate 2000 ppm 3000 ppm 4000 ppm
4 (C) B1 A4 - -6 -10 -10
5 B1 A2 C1 -8 -10 -11
CA 02588539 2007-05-15
= 22
Table 8 CFPP testing in test oil E3
Ex. Comb polymer Ethylene copolymer Polyacrylate 2000 3000
ppm ppm
6 B1 A2 -- -20 -23
7(c) B1 A4 - -17 -19
Table 9 CFPP testing in test oil E4
Ex. Comb polymer Ethylene copolymer Polyacrylate 2000 3000
ppm ppm
8 B1 A2 - -17 -22
9(C) B2 A3 -- -14 -17
Table 10 CFPP testing in test oil E5
Ex. Comb polymer Ethylene copolymer Polyacrylate 2000 3000
ppm ppm
10 B1 A2 - -13 -18
11 B 1 A2 C2 -13 -18
12 (C) B1 A3 -- -11 -13
Table 11 CFPP testing in test oil E6
Ex. Comb polymer Ethylene copolymer Polyacrylate 4000 ppm
13 B1 A2 - -19
14 B1 A2 C2 -19
15(C) B1 A3 -- -16