Note: Descriptions are shown in the official language in which they were submitted.
CA 02589651 2007-04-20
MULTI-WAVELENGTH FLUOROMETRIC SYSTEM AND PROBE
FOR MONITORING OF BIOPROCESSES
FIELD OF THE INVENTION
[001] This invention relates to systems for measuring properties of certain
samples, and more particularly, to fluorometric systems for on-line monitoring
of
biological processes.
BACKGROUND OF THE INVENTION
[002] External perturbations as well as population variability often result in
broad fluctuations of growth and production rates of microorganisms. Close
monitoring of a bioprocess is required to maximize process efficiency. Because
of a
lack of reliable on-line monitoring techniques, most often on-line bioprocess
monitoring is limited to biogas analysis for oxygen and carbon dioxide
content,
while such key process parameters as substrate and product concentrations are
only
measurable off-line. Consequently, the results are available with a
significant delay
from the time of sampling. This delay leads to untimely process diagnosis as
well as
limits process control to pre-programmed feed strategies.
[003] Recently developed on-line monitoring methods use flow injection
analysis
(FIA) techniques as well as near- and mid- infrared spectrometry (Tosi et al,
Biotechnol. Prog., 19, 1816-1821 (2003)). While these techniques have been
used
successfully at the laboratory scale, high equipment cost is prohibitive for
most
industrial applications.
[004] The use of fluorometry for rapid detection of fermentation imbalances
and metabolic activities has already been demonstrated. Most often,
fluorescence is
measured by illuminating the sample at one wavelength and measuring
fluorescence
at another (higher) wavelength, i.e. a single excitation - single emission
technique is
used. In particular, NADPH-dependent fluorometry has been used for monitoring
fermentation as well as aerobic and anaerobic wastewater treatment processes.
1
CA 02589651 2007-04-20
However, bioreactor broth contains large amounts of proteins, amino acids, and
other
fluorescent compounds that interfere with NADPH-related fluorescence thus
limiting
industrial applications of single excitation-single emission fluorometry. The
quality
of monitoring can be improved by using multiple-excitation multiple-emission
fluorescence measurements (e.g. Tartakovsky, B.; Lishman, L. A.; Legge, R. L.,
Water Research, 30 (12), 2941-2948 (1996)). In this technique, both excitation
and emission wavelengths are varied to obtain two-dimensional spectra. For
this
reason, this technique of fluorometric measurement, also employed herein, is
often
called two-dimensional fluorometry. The spectra are often processed using
multivariate statistical analysis methods, such as Partial Least Square (PLS)
regression, which provides a linear relationship between analytical
measurements
and multi-wavelength spectra.
[005] To select a desired excitation wavelength, the light should pass through
a monochromator or a filter wheel. The fluorescence signal (emission spectrum)
can be measured by using a second monochromator or a filter wheel followed by
a
spectrometer. Alternatively, a close caption detector (CCD) array spectrometer
can be used. Notably, the use of a monochromator or a filter wheel increases
the
setup cost and dimensions as well as it increases the scan time.
[006] Light emitting diodes (LEDs) produce high intensity light in a narrow
range (20 -30 nm) of wavelengths. Thus, LEDs can be used for sample
illumination at a fraction of the cost of conventional light sources equipped
with
monochromators or filter wheels. Indeed, some LED light sources are
commercially available (LS-450, Ocean Optics Inc., Dunedin, Florida, USA).
While LEDs are often used for illumination in the visible range of
wavelengths,
the use of LEDs for UV applications is relatively new. The UV LEDs are
constantly improving with some newer LEDs emitting light at 350 nm (RLT350-
30, ROITHNER, LASERTECHNIK, Vienna, Austria).
[007] Fluorometers or similar devices have been described in patent
literature as well. Some of the devices use LEDs. US Patents 6,825,927
(Goldman et al.) and 6,873,417 (Bahatt et al.) are examples of the prior art
in this
respect.
2
CA 02589651 2007-04-20
=
[0081 It is also known to use various optical waveguide arrangements to
transfer light from the light source to the sample to be illuminated and to
transfer
the light (also fluorescence light) emitted by the sample to the measuring
instruments. Exemplary patents are US 6,791,687 and 6,166,804.
[009] While the use of LEDs has reduced the cost of the fluorometric
apparatus, there is still a room for improvement of the accuracy and
reliability of
the on-line monitoring of bioprocesses, e.g. food processing or wastewater
treatment.
SUMMARY OF THE INVENTION
[0010] The invention attempts to meet the above improvement objectives. In
particular, simultaneous fluorescence of several components, which limits
application of fluorescence-based measurements in bioreactor monitoring, can
be
dealt with by acquiring fluorescence spectra at various excitation wavelengths
and
at different combination of excitation wavelengths. This increases total
amount of
information on fluorescence and reflection properties of the sample and
improves
accuracy of simultaneous measurements of culture broth components such as
microorganisms, substrates, intermediates, and products.
[0011] In accordance with one aspect of the invention, there is provided a
fluorometric system comprising
an excitation light source for illuminating a sample to generate emission
light
therein, the excitation light comprising at least two diverse light sources
with
different spectral width,
means for activating the diverse light sources sequentially,
a detector for detecting the emission light and producing spectral input from
the
emission light, and
a processor for evaluating properties of the sample based on the combined
spectral input.
3
CA 02589651 2007-04-20
[0012] In an embodiment of the invention, the system uses a novel probe which
is normally placed such that one end of the probe is disposed adjacent to or
proximate to the sample. In contrast to standard optical probes, the probe of
the
invention comprises the excitation light source disposed at the end of the
probe
adjacent to the sample. For the purpose of fluorometric measurements as
described
herein, the probe comprises a broadband light source and a monochromatic light
source, both sources disposed proximate to the sample for transmitting
unguided
light energy to the sample.
[0013] In one embodiment of the invention, the excitation light source is a
set of
light emitting diodes. Energy emitted from the sample in response to the
excitation
energy is transmitted to a detector, typically a spectrometer, via an optical
fiber that
is partly disposed in the probe to transmit light emitted from the sample to
the light
detector
[0014] In accordance with another aspect of the present invention, there is
provided a fluorometric system suitable for monitoring biological processes,
the
system comprising:
an excitation light source disposed to illuminate a sample to generate
emission of
light from the sample, the excitation light source comprising a filter-free
broadband
light source preferably including a UV/VIS wavelength range, and at least one
monochromatic light source,
a light detector for detecting the at least fluorescent light emitted from the
sample
and for acquiring spectra of the emitted light from the broadband light source
and
from the at least one monochromatic light source,
a processor coupled to the detector for evaluating the sample based on
analysis of
the spectra acquired by the light detector, and
control means for activating sequentially or simultaneously the broadband
light
source and the monochromatic light source or one of the monochromatic light
sources,
wherein the processor comprises means for combining and analyzing a plurality
of spectra produced by the light detector by detecting spectral input produced
sequentially or simultaneously by the broadband light source, the
monochromatic
light source or sources and a combination of the monochromatic light sources.
4
CA 02589651 2007-04-20
[0015] The excitation light is in part scattered (reflected) by solid
particles
within a culture broth (cells, solids, etc). This reflected light is measured
by the
spectrometer. Another part of the excitation light is absorbed by the
fluorescent
particle and the light is emitted at a higher wavelength, also measured by the
spectrometer. Because of the use of a "broadband" (UV/VIS) excitation light,
at
each wavelength the signal measured by the spectrometer consists of two
components: reflection (same wavelength as excitation light) and fluorescence
(coming from the excitation at a lower wavelength). In the absence of solid
particles, the reflection signal (scattered light) measured by a fiber-optic
reflection
probe with coaxial excitation and emission fibers (i.e. at an excitation-
emission angle
of 180 ) will be negligible.
[0016] In some embodiments, the filter-free broadband light source has a
wavelength range from about 200 nm to about 800 nm, and the excitation light
source encompasses a plurality of monochromatic light sources, at least some
of
them being in the UV range. The monochromatic light sources can be LEDs. The
"broadband" may denote a combination of light from several LEDs and then the
range may be non-continuous i.e. may consist of several ranges, each defined
by a
single LED.
[0017] In accordance with yet another aspect of the invention, there is
provided
a method for fluorometric analysis of biological processes, the method
comprising
providing an excitation light source comprising a broadband (multiwavelength)
light source and at least one monochromatic light source, for illuminating a
sample,
activating sequentially the broadband light source and the at least one
monochromatic light source to cause sequential emission of light,
detecting the sequential emission of light to generate sequential spectral
input,
and
analyzing the sequential spectral input to produce combined spectra
representative of fluorescence compounds present in the sample.
[0018] In an embodiment of the invention, the step of sequential activation
may
include activation of a combination of monochromatic lights.
CA 02589651 2007-04-20
BRIEF DESCRIPTION OF THE DRAWINGS
[0019] The invention will be explained in more detail by way of the following
description in conjunction with the drawings, in which
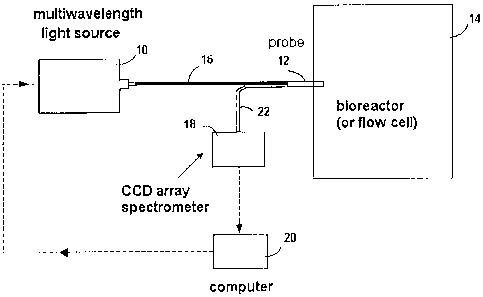
[0020] Fig. 1 represents schematically an embodiment of the fluorometric
system of the invention,
[0021] Fig. 2a, 2b and 2c represent various configurations of probe attachment
in the system,
[0022] Fig. 3a illustrates a LED assembly of the excitation light source,
[0023] Fig. 3b illustrates an exemplary arrangement of the components of the
excitation light source,
[0024] Fig. 3c is a front view of an exemplary LED assembly of Fig. 3a,
[0025] Fig. 3d is a longitudinal sectional view of an embodiment of a probe
for
fluorescence measurements,
[0026] Fig. 3e is a cross-sectional view of the probe of Fig. 3d,
[0027] Fig. 4 is a schematic diagram of spectrum acquisition and processing,
[0028] Fig. 5 illustrates liquid-gas separation for fluorescence measurements,
[0029] Fig. 6a is a graph representing results of calibration of a Partial
Least
Squares (PLS) model of VSS measurements in the recirculation loop of an
anaerobic reactor,
[0030] Fig. 6b is a graph representing VSS measurements in the same location
as in Fig. 6a, and
[0031] Fig. 6c is a graph representing COD measurements in the same location
as in Fig. 6a.
DETAILED DESCRIPTION OF THE INVENTION.
[0032] In general terms, the fluorometric system for on-line bioprocess
monitoring, as represented in Fig. 1, employs a multiple light source 10, an
optical
probe 12 shown as mounted on a bioreactor 14 and coupled optically to the
light
source 10 via an optical waveguide 16, a CCD (close caption detector)
spectrometer
18 (USB 2000 from Ocean Optics Inc., FL, USA) for acquiring fluorescence and
6
CA 02589651 2007-04-20
other emission light data, and a computer-based data processing unit 20
operatively
connected to the spectrometer 18 and to the light source 10. The probe 12 is
coupled
to the spectrometer 18 through another optical waveguide 22.
[0033] The probe 12 may be mounted directly on the bioreactor 14 as shown in
detail in Fig. 2a. Alternatively, it may be mounted on an external
recirculation line
24 of the bioreactor 14 (Fig. 2b) or in a flow ce1126 installed on the
recirculation line
24 (Fig. 2c). If a flow cell is used, fluorescence is measured using two
windows
positioned at an angle to each other (e.g. 450 or 90 ).
[0034] The multiple (multiwavelength) light source 10 combines several light
sources in a single unit (Figs. 3a-3c). It includes a broadband light source
28 that
emits light in an ultra-violet and visible (UV/VIS) range of wavelengths, e.g.
a
pulsed xenon lamp (200-750 nm). It also includes several light emitting diodes
(LEDs) 30, each of which emits light in a narrow range of wavelengths,
approximately 30 nm. For instance, a set of LEDs with peak wavelengths at 375,
400, 420, and 450 nm can be used. A schematic representation of the LED-based
part of the multiwavelength (MW) light source 10 is shown in Figs. 3a and 3c.
In
this setup, the LEDs are attached in a circular arrangement around a central
LED.
The light output of each LED is transferred to the light source output by
means of a
collimating lens, e.g. 25 mm collimating lens 32. The output of the light
source is
connected to an optical fiber 16 (Fig. 1) using a connector, e.g. SMA-type
connector.
The LEDs are controlled either manually by switches placed on the light source
cover or automatically by computer using external TTL-level signal unit 34.
The
LEDs can be grouped, e.g. in groups of two identical LEDs, 2, 3 and 4 around a
central LED 1, as shown in Fig. 3c, to increase light intensity.
Alternatively, up to 3
LEDs can be connected using a bifurcated or a trifurcated fiber with a
sufficiently
large diameter (e.g. 600 pm) to reduce light losses.
[0035] Modern UV LEDs emit light starting from 350 nm (e.g. RLT350-30 by
ROITHNER, LASERTECHNIK, Vienna, Austria). While the technology is
constantly improving, a lower wavelength UV light is desired for detecting
proteins
and other components with a maximum of fluorescence at the excitation
wavelengths
7
CA 02589651 2007-04-20
below 350 nm. This can be achieved by adding another xenon or a deuterium lamp
34 equipped with a low-pass optical filter (below 350 nm) to the light source
setup
10. The outputs of all lamps can be combined by a trifurcated fiber 36 (e.g.
an
optical fiber with a three-way coupler) as shown in Fig. 3b.
[0036] Instead of a pulsed xenon lamp, a broadband light source for the
purpose
of the invention can be realized by combining several LEDs with excitation
peaks in
the UV and VIS range of wavelengths. The outputs of the various light sources
can
be combined using a n-furcated (e.g. bifurcated or trifurcated) fiber or a
collimating
lens thus providing a multi-wavelength light source instead of a single UV/VIS
broadband lamp. Thus, the term "broadband light source" should be understood
quite liberally as this term encompasses both a UV/VIS light source producing
a
continuous broadband signal and a combination of several LEDs producing light
in a
non-continuous wavelength range.
[0037] The multiple light source 10 and the spectrometer 18 of the
fluorometric
system are controlled by a computer in order to acquire fluorescence spectra
at
different excitation wavelengths. The lights are turned on sequentially and
corresponding spectra are acquired by the spectrometer and stored in the
computer
memory. As explained previously, a simultaneous irradiation by several LEDs
can
be used to obtain a combined fluorescence/scattering spectrum, similar to that
obtained using a UVlVIS lamp. This technique can be used either to reduce the
number of lamps in the MW light source or to obtain an additional spectra at a
different profile of excitation wavelengths.
[0038] In operation, a computer-based algorithm controls the light source 10
to
activate sequentially, in intervals ranging from several milliseconds to
several
seconds, the broadband light source (which, as explained above, may itself be
a
combination of light from several light sources) and one or more of the
remaining
"monochromatic" light sources (LEDs, deuterium lamp with filter etc.). The
light
beams thus sequentially produced are passed to the probe 12 to illuminate a
biological sample in the reactor 14 as shown for example in Fig. 2a. If the
sample
contains fluorescent compounds, e.g. proteins, light emission produced by the
sample and passed to the CCD spectrometer 18 will provide a fluorescence
8
CA 02589651 2007-04-20
spectrum. If the light beam is a broadband light, the spectrum produced by the
sample may contain both fluorescence and scattered light.
[0039] The spectra sequentially detected by means of the spectrometer 18 are
then analyzed by the computer 20. To this end, spectra derived from various
components (broadband or monochromatic) of the light source 10 are combined by
the algorithm to carry out the analysis. The computer synchronizes the
multiple
light source 10 and the CCD array spectrometer 18 via the control unit 34. The
computer 20 also carries out data storage and processing functions. A more
detailed
description of the algorithm follows.
[0040] In the arrangement illustrated in Fig. 1, the probe 12 is a
conventional
optical probe (reflection probe) providing excitation energy to the sample and
receiving emission from the sample. Typically, the conventional probe consists
of
two fibers joined in an enclosed stainless steel ferule on one end and split
at the
other end (bifurcated fiber). One of the fibers 16 is connected to the light
source
and is used to deliver excitation light to the sample (illumination fiber).
The
second fiber 22 (read fiber) is used to transmit the fluorescence to a
spectrometer
18. The diameter of the probe is 5 mm or less. One drawback of such reflection
probe is a significant light loss in the illumination fiber at the light
source-air-
glass interface. Depending on the fiber diameter and other parameters, 80-95%
of
the light source signal can be lost. While this performance is acceptable for
many
applications, in other cases higher illumination intensity may be required. In
particular, novel UV LEDs can emit light at a wavelength of 250 nm, however
the
light intensity is almost an order of magnitude lower than in LEDs with an
excitation wavelength of 350 nm and above. Consequently, the application of
250
nm LEDs in the reflection probe setup is limited to highly fluorescent media.
In
addition, the intensity of the fluorescence signal is directly proportional to
the
intensity of the light source and therefore measurement time and the interval
between the measurements can be reduced if light losses are reduced.
[0041] In accordance with another aspect of the invention, it is proposed to
use a novel electro-optical probe which incorporates the functionality of both
the
light source 10 and probe 12 of Fig. 1. As shown in Fig. 3d, the probe 37 has
a
9
CA 02589651 2007-04-20
number of LEDs 38 mounted in a holder at the tip of the probe, therefore the
illumination fiber 16 is not required for sample illumination. The
fluorescence
signal of the sample is transmitted to a spectrometer by an optical (read)
fiber 22,
as in the arrangement of Fig. 1.
[0042] As shown in Figs 3d and 3e, the probe 37 of the invention has a
housing 39 which is terminated with a quartz window 41. The LEDs 38 (only
three shown for clarity) are assembled substantially concentrically around the
optical fiber 22 (read fiber) using a holder 40 and are controlled via
electrical
wiring 42. The LEDs 38 and the read fiber 22 are attached to the holder 40
using
bolts 44. A threaded cover 46 is used to attach the holder and the quartz
window
to the probe. The maximal number of LEDs depends on the diameter of the probe,
the typical LED diameter being 5 mm.
[0043] As mentioned above, the probe 37 of Figs. 3d-3e functions both as a
light source 10 and a probe 12 of Fig. 1. The read fiber 22 provides emission
signals from the sample to the spectrometer 18. The functionality of diodes 38
is
the same as that of the light source 10 of Fig. 1.
[0044] It will be appreciated that one of the differences between a standard
optical probe (e.g. R400-7, Ocean Optics Inc., Dunedin, Florida, USA) and the
electro-optical probe of Fig. 3d and 3e is that the optical energy transmitted
to the
sample from the source (LEDs or equivalents) is unguided and the waveguide
(illumination fiber 16) between the source and the sample is eliminated.
[0045] For the purpose of illustration, assuming that the multiple light
source of
the invention includes a UV/VIS light source and five LEDs (Fig. 3b), an
exemplary
spectrum acquisition run is as follows:
l. UV/VIS read spectrum #1
2. LED 1 read spectrum #2
3. LED 2 read spectrum #3
... ........ ...................
6. LED 5 read spectrum #6
7. LEDs 1-5 read spectrum #7
CA 02589651 2007-04-20
[0046] At the end of the run, spectra #1-#7 are processed together i.e.
combined
by the algorithm and analyzed to generate data representative of sample
properties.
100471 At a given instant in time, the sample is characterized by several
fluorescence spectra, each obtained at a different excitation wavelength or
wavelength range. These two-dimensional fluorescence spectra are processed
using
multivariate statistical methods, such as partial least squares (PLS) and
principal
component analysis (PCA) algorithms. While PLS regression is used to evaluate
concentrations of target components, principal component analysis is used to
estimate process trends for diagnostic purposes. In addition, spectral areas
can be
used to correlate fluorescence signals with such process parameters as
chemical
oxygen demand (COD), and biological oxygen demand (BOD). Notably, COD and
BOD concentrations reflect total content of complex organic materials in the
liquid.
An overall functional diagram of spectral analysis, including the software-
executed
steps, is shown in Fig. 4 in which the fluorometric system (generally
designated as
48) generates fluorescence spectra for use in regression model training (left
column,
steps C1-C4) and measurements and diagnosis (right column, steps M1-M5).
Analytical measurements (box 49) are carried out periodically in parallel with
the
fluorometric measurements for comparison. The output of the calibration steps
(box
50) is provided to steps M4 and M5 of the measurement sequence. The software
carries out the functions of data collection, data storage, regression model
calibration, and measurements.
[0048] The software function of model calibration infers analytical
measurements with fluorescence spectra acquired at the time of sampling. The
calibration procedure requires several (e.g. ten or more) measurements for
successful
calibration. The following sequence of calculations is used for model
calibration:
Step C-1: Fluorescence spectra acquired using at least UV/VIS (broadband) and
monochromatic light sources are normalized by computing each spectrum area and
dividing each element of the corresponding spectrum by the area (e.g. the
spectra are
normalized to 1).
Step C-2. Fluorescence spectra are truncated so that only areas containing
significant fluorescence and/or reflection signals are retained.
11
CA 02589651 2007-04-20
Step C-3. The spectra acquired at each data acquisition interval are combined
in
a linear array representing one acquisition cycle.
Step C-4. A calibration procedure is carried out by inferring the spectra with
available analytical measurements using a regression model (e.g. PLS
regression
model). Simultaneously, the normalized and combined spectra are used to
calculate
the principal components (PCs) for principal component analysis (PCA). The
output
of the calibration procedure is a set of regression model parameters, which
can be
used to carry out the measurements and process diagnostics.
[0049] The following sequence of calculations is used for MW fluorescence-
based measurements:
Step M-1: At each data acquisition step, the normalization procedure is
carried
out in agreement with step C-1 (see above) by computing each spectrum area and
dividing each element of the corresponding spectrum by the value of the area.
Step M-2: Fluorescence spectra are truncated as described in step C-2.
Step M-3. The spectra are combined in a linear array representing one
acquisition cycle.
Step M-4: Concentrations of process components are computed using model
parameters estimated in the calibration procedure (step C-4).
Step M-5: Process trends are estimated using principal components (PCs)
computed in step C-4.
[00501 Notably, steps C-3 and C-4 are compulsory for obtaining regression
models, while steps C-1 and C-2 can be omitted. Accordingly, steps M-1 and M-2
should be omitted if steps C-1 and C-2 are not carried out during the
calibration
procedure.
[0051] It will be understood that each light source provides partial
characterization of the sample. A combination of several spectra obtained at
different excitation wavelengths allows for more accurate measurements. It can
be noted that the spectra acquired using an UV/VIS (200-700 nm) light source
contain both fluorescence and scattered light. Because light scattering
depends on
the amount and size distribution of solid particles, the spectra obtained with
the
UV/VIS light source can be inferred with such parameters as cell density
and/or
12
CA 02589651 2007-04-20
total (soluble and solid materials) chemical oxygen demand (COD). Usually,
bioreactor broths as well as wastewaters contain large amounts of fluorescent
materials. Consequently, sequential spectra acquisition using several light
sources
is required for accurate measurements of various components, as illustrated in
the
example below.
Example
[0052] Measurements of total CODs, volatile fatty acids (VFAs), and volatile
suspended solids (VSS) were carried out in a 5 L anaerobic reactor. The
fluorometric setup was equipped with a multi-wavelength light source
containing
Xenon (200-700 nm) and UV LED (380 nm) connected by a bifurcated fiber with
a reflection probe installed in the external recirculation line of the
reactor.
[0053] The fluorometric measurements according to the invention were
carried out for a period of 25 days. Chemical oxygen demand and volatile
suspended solids (VSS) were measured periodically according to Standard
Methods (APHA, AWWA and WEF. (1995) Standard Methods for examination of
water and wastewater. American Public Health Association. Washington). VFA
concentrations were measured using a gas chromatograph. Fluorescence spectra
were acquired in 10 min intervals with background acquisition prior to each
fluorescence measurement.
[0054] The measurements were performed with a fluorometric system which
consisted of a Xenon (PX-2, Ocean Optics Inc., Dunedin, Florida, USA) and a
380
nm LED light sources connected by a bifurcated fiber to an R400-7 fiber optic
reflection probe with 6 illumination fibers and one read fiber, and an USB2000
CCD array fiber optic spectrometer (Ocean Optics Inc., Dunedin, Florida, USA).
The spectrometer used a UV/VIS grating with a spectral range of 250 to 800 nm
and a resolution of 0.9 nm. The fiber optic probe was inserted into the
external
recirculation loop of the reactor. Fluorescence was measured from the front
surface.
[0055] Two fluorescence spectra corresponding to excitation with UV/VIS
(200-700 nm) and LED UV (380 nm) were obtained at each acquisition period.
13
CA 02589651 2007-04-20
The data were divided into "calibration" and "validation" (18 days) sets. The
calibration data set was used to estimate parameters of PLS regression models
describing the dependence of COD and VFA concentrations on fluorescence
spectra. The validation data set was used to compare fluorescence-based and
analytical measurements. The quality of fluorescence-based measurements was
estimated using correlation coefficient (r2). A comparison of calibration and
validation accuracies using either both spectra (UV/VIS and UV at 380 nm), or
UV/VIS alone, or UV 380 nm is given in Table 1. This comparison shows that a
combination UV/VIS and UV 380 nm light sources provided better accuracy for
both calibration and validation measurements. Also, the advantage of using two
light sources was more pronounced for total COD and VSS measurements (higher
r2 values for validation with two light sources, see Table 1) because these
parameters include particulate organic materials. It can be noted, that
analytical
measurements of VSS strongly depend on the sampling procedure. Sample
inhomogeneity often results in large variations of the measurements as can be
seen
in Figs. 6a and 6b. Consequently, fluorescence-based measurements
significantly
improved the accuracy both for training (Fig. 6a) and validation (Fig. 6b and
6c)
data sets.
Table 1. Comparison of correlation coefficients obtained with different
combinations of light sources. Xenon lamp was used to obtain UV/VIS light in
200-700 nm and LED UV had a peak at 380 nm. To reduce noise, the emission
spectra were truncated to 400-600 nm for UV/VIS excitation and to 400-500 nm
for UV 380 nm excitation. Calculations were carried out using PLS regression
models with 4 latent variables (CODs and VFAs) and 3 latent variables (VSS).
light source total COD VFAs VSS
calibration validation calibration validation calibration validation
UV/VIS & UV 0.78 0.60 0.80 0.57 0.60 0.54
UV/VIS 0.73 0.23 0.65 0.29 0.60 0.42
UV 0.74 0.58 0.80 0.53 0.60 0.40
14
CA 02589651 2007-04-20
[0056] Overall, the use of multi-wavelength light source, which provided
sequential sample illumination in a broad range of wavelengths (UV/VIS, 200-
700
nm) and in a narrow UV range of wavelengths (370-390 nm) provided superior
accuracy of COD and VSS measurements in comparison with a single light
source.
[0057] According to another aspect of the invention, the amount of gas bubbles
in the external recirculation loop can be reduced by addition of a bypass line
(Fig. 5).
Notably, the presence of gas bubbles in the liquid sample considerably affects
fluorescence measurements. The bypass line essentially begins before the
fluorescence probe. At the bifurcation point 51, gas and liquid are separated
by
gravity. The probe 12 is installed at the lower branch 52 of the bifurcated
line, which
contains a minimal amount of gas bubbles. The upper branch 53 contains gas and
excess liquid. The two streams then merge after the probe. Flow distribution
between the two lines is controlled by means of valves #1 and #2.
[0058] Numerous modifications and alternative embodiments of the invention
will be apparent to those skilled in the art in view of the foregoing
description. For
example, LEDs can be replaced with laser diodes or other equivalents.
Accordingly,
this description is to be construed as illustrative only and is only for the
purpose of
teaching the best mode of carrying out the invention. It is intended that the
present
invention be limited only to the extent required by the appended claims and
the
applicable law.