Note: Descriptions are shown in the official language in which they were submitted.
CA 02590771 2007-06-05
WO 2006/061603 PCT/GB2005/004685
1
ANKLE-FOOT ORTHOSIS
This invention relates to anlde-foot orthoses.
Orthoses are mechanical devices which impose forces upon a limb of a patient
-- and which can be used for a variety of different purposes. For example,
orthoses can
be provided for supportive, functional, corrective or protective purposes, or
indeed for
a combination of these.
Ankle-foot orthoses are typically used to provide protection to the ankle and
foot of a patient as well as to provide support against excessive
plantarflexion, or
-- "foot-drop" as it is more colloquially known. Foot plantarflexion is a
medical
condition that results from disease, trauma or congenital abnormality.
Patients affected
by the condition typically experience difficulty in walking as their feet tend
to drop
when lifted off the ground, and to avoid stumbling they typically have to lift
their foot
higher than they would otherwise have to. It is also not atypical for patients
to have
-- problems during the swing-through phase of their gait cycle, as a typical
sufferer will
tend also to exhibit poor, or impaired, dorsiflexion.
The primary function of an ankle-foot orthosis is to provide a resistance to
plantarflexion which helps keep the patient's foot in the correct position
when the foot
is lifted off the ground. As well as this resistive function, a good anlde-
foot orthosis
-- should also provide a degree of assistance to dorsiflexion during the swing-
through
phase of the patient's gait.
A variety of different ankle-foot orthoses have previously been proposed for
resisting plantarflexion, and in some cases for additionally assisting
dorsiflexion.
One previously proposed device is commonly known in the art as an "under
-- foot" orthosis. As this colloquial name suggests, the orthosis fits under
the foot, and in
this case outside of a shoe. This particular device cannot be worn without a
shoe, and
as such the shoe is an integral component of the orthosis. The orthosis
comprises a pair
of supporting metal rods, one connected to either side of the shoe in the
region of the
heel by means of a pl antarflexion stop that prevents further foot drop. The
upper ends
-- of the rods are connected to a supporting band which is secured about the
calf of a
patient.
Another previously proposed "under foot" orthosis (which must also be used
with a shoe) comprises a rigid one-piece plastics moulding composed of
integral sole-
abutting and calf-abutting regions. The top of the calf-abutting region is
provided with
CA 02590771 2007-06-05
WO 2006/061603 PCT/GB2005/004685
2
a closure mechanism that enables the device to be secured to the calf of a
patient, and
the sole abutting region acts in conjunction with the shoe to support the foot
of the
patient.
Another previously proposed device is known colloquially as an "over foot"
orthosis, meaning that the orthosis fits over the front (dorsal) aspect of the
foot, rather
than under it as in the abovementioned previously proposed devices. This "over
foot"
orthosis comprises a rigid plastics shell which is worn up against the shin,
and which
is secured around the calf by means of an appropriate securing band. The
orthosis
includes a stirrup which fits over the foot in the region of the instep to
provide the
patient with a resistance to plantarflexion.
All of the aforementioned orthoses provide the patient with a device which is
capable of resisting plantarflexion. However, it is also the case that each of
them has a
number of attendant disadvantages.
To alleviate these problems, we have previously provided (see granted UK
Patent No. 2330309) a sock-like structure which is formed of a resiliently
flexible
material - such as silicone for example. The sock-like structure, by virtue of
the
inherent resilience of the material from which it is made, provides a
resistance to
plantarflexion and also stores energy which can subsequently be released to
assist
dorsiflexion. The orthosis can be coloured to mimic the colour of the
patient's' skin
(and as such is cosmetically pleasing), can comfortably be worn in a normal
off-the-
shelf shoe, and need not be worn with a shoe in order to provide a beneficial
effect.
The part of the sock-like structure which envelops the patient's ankle and
lower leg in use includes an opening (to permit the user to put on the
device), and in
the preferred arrangement the opening is closed (to secure the orthosis in
place on the
foot of a patient) by means of respective parts of a mechanical hook and loop
closure
(such as velcro ) which are embedded in the sock-like structure. Typically,
one part
of the closure is provided on the outside of the orthosis adjacent one side of
the
opening and the other part is provided on the inside surface of a tab
extending from the
other side of the opening, the closure on the tab being attachable to the
closure
adjacent the one side of the opening to close the opening, and secure the
orthosis in
place.
Our previous orthosis represented a quantum leap in the field and alleviated
most (if not all) of the disadvantages mentioned above (the bulk of which had
long
been associated with previously proposed devices), and has proved to be
extremely
CA 02590771 2007-06-05
WO 2006/061603 PCT/GB2005/004685
3
commercially successful. Despite this it is still more expensive to
manufacture (and
hence more expensive for patients to purchase) than we would otherwise like.
One particular problem we have noted in this regard is that it is difficult to
embed the respective parts of the velcro closure in the silicone sock-like
structure,
and that once embedded the closures can sometimes come away from the sock-like
structure thereby causing the device to fail. These problems tend to lengthen
the time
taken to make any one device, and to reduce the yield of the production
process as a
whole. The effect of this is, ultimately, to make the orthoses more expensive
to make,
and hence more expensive to purchase, than they otherwise would be.
It is an aim of the present invention to provide an orthosis which avoids, or
at
least alleviates, these problems. In particular, it is an aim of the invention
to provide
an orthosis which can more easily be manufactured and hence can be
manufactured
(and ultimately sold) for less.
In pursuit of the above mentioned aims, a presently preferred embodiment of
the invention provides an ankle-foot orthosis comprising: a sock-like
structure formed
of first and second tubular sections set at an angle to one another, wherein
the second
tubular section is capable of being opened for insertion of the patient's foot
and lower
leg into respective ones of said first and second tubular sections, the
orthosis further
comprising a closure member that can be passed round at least part of the
periphery of
the second tubular section and secured in place to urge the second tubular
section
towards a closed position in which the second tubular section is closely
fitted to the
lower leg of the patient.
The principal advantages of this orthosis over that which we previously
proposed, is that it can be manufactured more quickly (albeit at the cost of a
slight
reduction in its aesthetic appearance), and with fewer failures. By reducing
the time
taken to make any one given orthosis and the number of failed devices, it is
possible to
significantly increase the yield of the manufacturing process and hence to
reduce the
manufacturing cost (and ultimately the sale price) of each of the orthoses. In
tests we
have found that the manufacturing time for an orthosis as described herein can
be
reduced by approximately 40 percent as compared to the manufacturing time for
an
orthosis as described in our previous GB patent. This reduction in
manufacturing time
allows us to sell orthoses of the type described herein for about half the
price of
orthoses of the type described in our earlier GB patent.
CA 02590771 2007-06-05
WO 2006/061603 PCT/GB2005/004685
4
In a highly preferred embodiment, the closure member is located outside of the
periphery of the second tubular section when the second tubular section is in
,the
aforementioned closed position.
Further objects, features and advantages of embodiments of the present
invention will be apparent from the detailed description that follows.
Various preferred embodiments of the invention will now be described, by way
of illustrative example only, with reference to the accompanying drawings, in
which:
Fig. 1 is a schematic front perspective view of an orthosis in accordance with
a
preferred embodiment of the present invention in a closed configuration;
Fig. 2 is a rear perspective view of the orthosis depicted in Fig. 1;
Fig. 3 is a side elevation of the orthosis depicted in Figs. 1 and 2;
Fig. 4 is a rear perspective view of the orthosis depicted in Fig. 1 in an
open
configuration; and
Fig. 5 is a rear perspective view of the orthosis depicted in Fig. 4 in a
partly
closed configuration.
Fig. 6 is a schematic front perspective view of an orthosis in accordance with
a
second embodiment of the invention;
Fig. 7 is a schematic side view of an orthosis in accordance with a third
embodiment of the invention; and
Fig. 8 is a schematic side view of an orthosis in accordance with a fourth
embodiment of the invention.
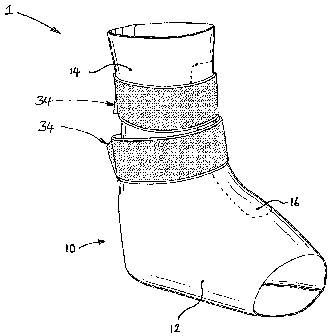
Fig. 1 is a perspective view of an embodiment of the invention in a closed
configuration. The orthosis 1 depicted in Fig. 1 is shown as it would appear
when
worn by a patient (the patient's foot, ankle and lower leg having been omitted
for
clarity), although it worth noting that the orthosis is sufficiently resilient
to keep its
three dimensional shape (generally as shown in Fig. 1) when not being worn by
the
patient.
The orthosis comprises an orthotic structure 10 which is comprised of a first
tubular section 12 and a second tubular section 14. The first and second
tubular
sections are contiguous, and in most instances the second tubular section 14
will have
been integrally formed with the first tubular section 12. The first and second
tubular
portions are set at an angle to one another to provide, at least in use, a
generally L-
shaped internal cavity 16 (Fig. 4) which is sized such that the orthosis
accepts and fits
closely about the foot, ankle and lower leg of a patient (not shown) in use.
CA 02590771 2007-06-05
WO 2006/061603 PCT/GB2005/004685
In a highly preferred arrangement the sock-like structure is formed not so
that
the first section is set perpendicular to the second, but so that the
structure exhibits
approximately 3 to 10, preferably 5, degrees of dorsiflexion. This
dorsiflexion drops
to zero when the orthosis is being worn by a patient and the weight of their
foot is
5 applied to the orthosis during the swing through phase of their gait.
The orthotic structure 10 may be described as being generally "sock-like", or
in
other words akin to a sock, in that when worn by a patient the second section
14
envelops a portion of the patient's lower leg including the medial malleolus
(the inside
of the ankle) and the lateral malleolus (the outside of the ankle), and the
first section
12 envelops at least a portion of the plantar aspect of the foot (the sole of
the foot) and
at least a portion of the dorsal aspect of the foot (the front of the foot).
In the preferred arrangement (as depicted) the orthotic structure is
configured
so that the patient's toes and calcaneum (heel) are exposed. This is because
we have
found that an orthosis which exposes the heel and toes is significantly more
comfortable for the patient to wear, whilst also giving the patient a greater
sense of
confidence when walking (particularly when barefoot) on a given surface due to
the
fact that their toes and heel can grip that surface. It will be appreciated,
however, that
the orthosis may instead be configured to envelop the toes and/or heel if
desired.
As a further alternative for those patients with particularly sensitive
anldes, the
first and second sections may be configured so that the walls of the first and
second
sections are thinned or include apertures in those regions of the structure
which would
normally overlie the medial and lateral malleolae.
The resilience of the orthotic structure 10 is chosen, and may be varied, in
dependence upon the degree to which the patient suffers from plantarflexion.
In
particular, the resilience of the orthotic structure 10 is chosen to provide a
resistance to
plantarflexion that is sufficient for correcting the particular degree of
excessive
plantarflexion experienced by the patient who is going to be using the
orthosis.
Variations in the resilience of a given orthotic structure 10 (as may be
required
for patients experiencing a lesser or greater degree of excessive
plantarflexion) may be
effected by changing the material from which the orthotic structure is formed,
by
changing the thickness of the orthotic structure, or by incorporating
reinforcing means
¨ such as a resilient rib - into the orthosis (or indeed by means of a
combination of
these).
CA 02590771 2007-06-05
WO 2006/061603 PCT/GB2005/004685
6
As an illustrative example, the orthotic structure could include reinforcing
means in the form of a resilient rib 16 (shown in ghost in Fig. 1) that
extends partway
along the dorsal aspect of the foot. The rib 16 could be formed integrally
with the
orthosis, or could be removably insertable into a pocket provided on the
dorsal aspect
of the orthotic structure. The latter arrangement would be particularly useful
in that it
would allow for the stiffness of the rib and/or the angle of support (by
inserting
differently shaped ribs) to be changed if desired. The rib may be of the same
material
as the remainder of the orthotic structure 10, or may be of a different ¨
preferably
more resilient ¨ material such as plastics, metal or carbon fibre.
The first 12 and second 14 tubular sections are formed of a resiliently
flexible
material, and in the preferred embodiment this material is a silicone
elastomer.
Preferably the silicone elastomer has a Shore A durometer of 30 to 80,
preferably of
40 to 70, and most preferably of 50 or 65.
The use of a resilient material for the orthotic structure 10 (and optionally
for
the rib, if provided) is a fundamental departure from "under foot" or "over
foot"
devices of the type described above.
These previously proposed devices
recommended the use of a non-resilient, i.e. rigid, material. The primary
advantage of
using a resilient material as opposed to a rigid material is that the material
can flex to
store energy during certain phases of the patient's gait, and release that
energy during
other phases of the patient's gait (in particular the swing-through phase) to
thereby
actively assist the walking process as a whole, and dorsiflection in
particular.
Dorsiflexion and plantarflexion of a foot is predominantly controlled by the
tibialis anterior muscle and tendon, and the structure of the orthosis
functions to assist
the operation of this muscle - in particular for those patients who experience
persisting
foot drop resulting from a neurological impairment caused, for example, by
trauma,
disease or genetics. This bio-mechanical function of the device of the
invention is
fundamentally different to so-called athletic support stockings, for example
those of
the tubigrip type, which provide no means for assisting the operation of the
tibialis
anterior muscle and tendon (to resist plantarflexion and assist dorsiflexion
of a
patient's foot), and are instead wholly concerned with resisting abnormal
lateral
movement of the foot.
As aforementioned, the orthotic structure 10 is closely fitted to the foot and
lower leg of the patient and as such may be configured to provide a
compressive force
to the foot and lower leg of the patient. In a preferred embodiment, the
compressive
CA 02590771 2007-06-05
WO 2006/061603 PCT/GB2005/004685
7
force may be tailored to help treat other conditions such as the effects and
symptoms
of venous insufficiency, for example varicose veins. It is also conceivable
for
different regions of the orthotic structure to provide different compressive
forces. For
example, the orthotic structure may exert a greater compressive force on the
foot of the
patient than on the ankle in order to provide a pumping effect to assist blood
flow to
and from the foot.
Referring now to Figs. 4 & 5, the second tubular section 14 of the orthotic
structure is configured so that it can be opened to permit the orthosis to be
put on by a
patient. In this embodiment opening of the second tubular section 14 is
achieved by
configuring the second tubular section so that it comprises a second part 20
that is
arranged to overlap a first part 18 when the orthosis 1 is in a closed
position fitted
about the lower leg of the patient - the second part 20 being moveable (in a
direction A
indicated generally in Fig. 5) away from the first part 18 to open the second
tubular
section 14.
In the preferred arrangement, as depicted, the first part 18 comprises a part
of
the second tubular section 14 which extends generally from a part 22 of the
orthotic
structure 10 which overlies the medial malleolus when the orthosis is worn by
a
patient, and terminates at a line running longitudinally from an uppermost
edge 24 of
the second tubular section 14 (i.e. that edge which is in the vicinity of the
patient's
lower leg in use) to a lowermost edge 26 of the second tubular section 14
(i.e. that
edge which is in the vicinity of the patient's calcaneum in use) generally
midway
between the part 22 of the second tubular section that is arranged to overlie
the
patient's medial malleolus in use and a part 28 (Fig. 4) of the second tubular
section
that is arranged to overlie the patient's lateral malleolus in use. The second
part 20
comprises a part of the second tubular section 14 which extends generally from
the
part 28 (Fig. 4) of the orthotic structure 10 which overlies the patient's
lateral
malleolus to form a tab portion 30 which overlaps the first part 18 when the
second
tubular section 14 is closed. In the preferred arrangement the tab portion 30
overlaps
the first part 18 (when the second tubular section 14 is closed) to an extent
whereby
the tab portion 30 terminates at an axial line 32 running longitudinally down
the
second tubular section and terminating at or about that portion 22 of the
orthosis which
overlies the patient's medial malleolus in use.
As depicted in Figs. 4 & 5, in the preferred embodiment the orthosis includes
closure members 34 which are fixedly attached to the aforementioned tab
portion 30
CA 02590771 2007-06-05
WO 2006/061603 PCT/GB2005/004685
8
and which can be used ¨ in a manner described in detail below ¨ to secure the
second
tubular portion 14 in the aforementioned closed position about the lower leg
of the
patient. In the preferred arrangement two closure members are provided, but it
will be
appreciated that a single closure member or more than two closure members may
be
provided if desired.
In the preferred embodiment, the closure members are fixedly attached to the
tab portion by means of tubular double-headed rivets 36, also known as "speed
rivets",
(or equivalent fixings) the like of which are available from Evans and Evans
(Unit 24,
Red Lion Business Park, Red Lion Road, Tolworth, Surbiton, Surrey, UK), or
Algeos
(Sheridan House, Bridge Industrial Estate, Speke Hall Road, Liverpool, L249HB
United Kingdom). Whilst it is undoubtedly the case that a variety of different
fixings
will be immediately evident to those persons skilled in the art, speed rivets
are
nevertheless preferred as they permit the closure member attachment stage of
the
orthosis production process to be accomplished more quickly than if the
closure
members were, for example, to be sown onto the tubular section.
In the preferred embodiment where the tab portion 30 of the second tubular
section 14 overlaps the first part 18 it is preferred that the closure members
34 are
attached to the tab portion 30 in such a manner that the fixings 36 also
overlap the
aforementioned first part 18 of the second tubular section 14. This is highly
= advantageous as it means that the fixings do not bear on the patient's lower
leg (where
they could cause discomfort) but instead bear upon the first part of the
second tubular
section.
In the preferred arrangement, the closure members comprise a first strip of
material 38 carrying on the outside 38a thereof (i.e. the side facing away
from the
second tubular section) one part of a mechanical hook and loop securing system
(such
as velcro(3). A second strip of material 40 carrying on the inside 40a thereof
(i.e. the
side facing towards the second tubular section) the other part of the
mechanical hook
and loop securing system is attached (for example sown onto) the free end of
the first
strip of material 38. In a highly preferred arrangement the first strip of
material 38
carries a set of hoops (and the second strip 40 carries the hooks) so that the
closure
members do not interfere with any clothes that the patient might be wearing.
As mentioned above, the closure members are provided to enable the second
tubular section to be secured in the aforementioned closed position. To this
end, the
closure members can be wound (in the direction B indicated in Fig. 4 ¨ i.e. in
a
CA 02590771 2007-06-05
WO 2006/061603 PCT/GB2005/004685
9
continuation of the direction A (Fig. 5) in which the tab portion 30 may be
moved to
open the second tubular section 14) round the periphery of the second tubular
section
14 until the hooks or loops on the second strip of material 40 overlie the
loops or
hooks on the first strip of material 38, whereupon the second strip of
material 40 can
be secured to the first 38 to secure the second tubular section 14 in the
closed position.
As an alternative, the closure members 34 could be affixed to the tab section
30
in such a way that they are wound in the opposite direction (i.e. in a
direction which
would move the tab portion to close the second tubular portion) and then
secured as
aforementioned. However, such an arrangement is less preferred as pulling the
closure
member(s) in a direction which acts to close the tubular section 14 may
encourage the
patient to overtighten the second tubular section about their lower leg,
perhaps to a
point where the second tubular section is secured so tightly that it impacts
adversely
on the circulation in the patients lower leg and foot. This is particularly
the case for
those patients who have suffered nerve damage and might not immediately be
aware
that the orthosis has been overtightened.
Whilst the above arrangement is preferred, it will be apparent that a number
of
other alternatives are possible. For example, it is not necessary for the
closure
members to be attached to the second tubular section. The closure members
could be
detachable from the second tubular member, and simply secured around it. The
second tubular section 14, as depicted schematically in Fig. 6, could also be
provided
with a number of hoops 42 through which fully detachable closure members 34
could
be threaded and secured (as depicted).
As a further alternative, depicted schematically in Fig. 7, the closure member
could comprise a strip of material (not shown) that is fully detachable from
the
orthosis and which carries hooks or loops of a mechanical hook and loop
engagement
mechanism. The orthosis has two strips of material 44 per closure member
fixedly
attached (for example by means of speed rivets 46 or equivalent fixings) to
the second
tubular section 14 ¨ the first being attached to the outside of the
aforementioned tab
portion and the second being attached to the outside of a region of the
aforementioned
first part of the second tubular section which is not overlapped by the tab
portion when
the second tubular section is closed. Each of the strips of material carry
loops or
hooks of the mechanical hook arid loop engagement mechanism, so that the
detachable
strip of material can be secured to each of the two attached strips of
material to hold
the second tubular section in the closed position.
CA 02590771 2007-06-05
WO 2006/061603 PCT/GB2005/004685
As a yet further alternative depicted schematically in a partly closed
position in
Fig. 8, a strip of material 46 (carrying hooks or loops of a mechanical hook
and loop
engagement mechanism on its outwardly face) may be attached to the outside of
a
region of the second tubular section 14 which is not overlapped by the tab
portion 30
5 when the second tubular section 14 is closed A second strip 48 of
material, fixedly
attached at one end to the tab portion 30 and carrying on its underside (i.e.
the side
facing the second tubular section) loops or hooks of the mechanical engagement
mechanism may then be secured to the strip of material 46 fixedly attached to
the
second tubular section to secure the second tubular section in the
aforementioned
10 closed position.
In another modification of the preferred embodiment depicted in Figs. 4 & 5,
the closure member 34 does not necessarily need to comprise two strips of
material
(38, 40) attached to one another. Rather, the closure member could instead
comprise a
single strip of material provided with hooks or loops of the mechanical
engagement
mechanism on one side, and loops or hooks of the mechanism provided on the
other.
As a further modification, the mechanical hook and loop closure mechanism
could be
replaced by another type of fixing ¨ such as press studs for example.
In a further alternative arrangement not depicted in the drawings, the second
tubular portion could be longitudinally split, with respective edges of the
split being
joined by a section of elastic material ¨ the elastic material being sown or
otherwise
affixed to the resilient material of the second tubular portion, and
permitting the
second tubular section 14 to be opened for the insertion of a patient's foot
and lower
leg. Any of the abovementioned closure members could then be provided to
secure
the second tubular section around the patient's lower leg (suitable
modifications being
made to account for the absence of a tab portion).
As a further alternative arrangement it will be apparent to those persons
skilled
in the art that the tab portion 30 of the second tubular portion 14 need not
overlap the
first part 18. The second tubular portion 14 could simply comprise a
longitudinal slit,
one or more closure members being provided to bring opposed edges of the slit
towards one another to close the slit and secure the second tubular section
about the
lower leg of the patient. Such an arrangement, whilst eminently possible, is
less
preferred as the fixings used to attach the closure member(s) to the orthosis
would then
be in contact with the patient's lower leg.
CA 02590771 2007-06-05
WO 2006/061603 PCT/GB2005/004685
11
Yet further configurations which permit the second tubular section to be
opened will be apparent to those persons skilled in the art, but it remains
the case that
the configuration described in detail above with reference to Figs. 1 to 5 is
most
preferred as the resulting orthosis can more quickly be manufactured than an
orthosis
which included a strip of elastic material, for example.
As mentioned above, one advantage of the orthosis described herein is that it
can be manufactured much more quickly than the orthosis described in our prior
GB
patent. Fabrication of an orthosis in accordance with that prior patent
necessitated the
encapsulation of velcro strips in the material of the orthosis to provide a
means for
closing the insertion slit in the second tubular section. To manufacture an
orthosis in
accordance with the teachings provided herein all one need do is fixedly
attach the
closure member(s) to the second tubular section for example by means of speed
rivets,
a much faster and much more robust alternative to the encapsulation of strips
of
velcro in the body of the orthosis.
The orthotic structure of either embodiment may be coloured so that it can be
matched to the skin colour of the patient (as can the closure member(s)), and
may be
provided in a variety of different sizes and shapes. The orthotic structure
may be
manufactured by injection moulding but may alternatively be manufactured by
milling
(as described below) and subsequently building up layers of silicone elastomer
upon a
suitable cast (typically of a patient's foot and lower leg).
The orthotic structure, as mentioned above, may be manufactured from a
number of different resiliently flexible materials. Amongst these, silicone
elastomer is
a particularly preferred material. Two suitable elastomers are sold under the
product
names HCR9960 and MED4035 by Nusil Technology of 1050 Cindy Lane,
Carpinteria, California, USA. Another family of suitable elastomers are sold
by Nusil
Technology under the registered trademark VersaSi13 .
HCR9960 has a working time of approximately 1:2 hours and MED4035 has a
shorter working time of approximately 3 to 4 hours, after which the elastomer
cures.
The elastomers are thermo-setting and are strained through a 200 mesh screen
to
remove particulate contaminants.
The elastomers are supplied as A and B components which are preferably
combined in equal portions on a two roll mill, or other suitable device, prior
to use.
CA 02590771 2007-06-05
WO 2006/061603 PCT/GB2005/004685
12
A suggested sequence for blending the two components is to first soften part B
on the mill and then soften part A, after which an equal weight of part B
should be
added to part A and then thoroughly mixed. At this stage, it is recommended to
keep
the temperature of the material as low as possible so as to maximise the table
life of
the elastomer. The mixture may then be manually fitted to a plaster cast of a
patient's
foot, or more preferably supplied to injection moulding apparatus to mould a
suitably
shaped orthotic. Curing of the blended elastomer may be accelerated by heat
and can
take from 3 to 4 hours. The cure may be inhibited by any ambient traces of
organic
rubbers and other substances and thus it is important for the fabrication of
the orthotic
structure to be conducted in a thoroughly cleaned area.
The VersaSi13 family of elastomers include 3 base stocks which when
vulcanised produce tough, durable elastomers with Shore A durometers of 30, 50
and
70, and the base stocks can be blended to produce elastomers of intermediate
durometer. The three base stocks are compounded with CAT-40 and CAT-55 - CAT-
40 being an inhibitor, and CAT-55 being a platinum catalyst.
Each series (i.e. 30, 50 or 70 durometer) is supplied as a three part system
which must be compounded, for example on a two roll mill, prior to use.
Elastomers
of intermediate durometer can be produced by blending 30, 50 or 70 durometer
elastomers in a 1:1 ratio. For example, a 40 durometer elastomer can be
achieved by
blending VersaSi13 30 and VersaSi13 50 in a 1:1 ratio mix.
To produce a given elastomer it is suggested to soften approximately 25% of
the total required base stock on a cooled two roll mill. The entire required
quantity of
CAT-40 should then be added, and the resulting mixture milled until
homogeneous.
Whilst the base/CAT-40 mixture is turning on the mill, small increments of CAT-
55
should be added until the entire required amount has been added. Next the
remaining
base stock should added and milled. Once the elastomer has been produced, the
mixture may then be manually fitted to a plaster cast of a patient's foot or
more
preferably supplied to injection moulding apparatus to mould a suitably shaped
orthotic structure.
Cure of the resulting mixture is accelerated by heat. For example, the
elastomer will cure in a mould cross-section up to 0.075 inch (0.00195m) thick
in less
than ten minutes at 116 C. The vulcanisation rate can be increased by
increasing the
cure temperature, and an optional post cure, such as four hours at 177 C may
be
CA 02590771 2014-08-11
WO 2006/061603 PCT/GB2005/004685
13
implemented if desired. An important point to note is that cure of the
elastomer may
be inhibited by traces of amines, sulphur, nitrogen oxide, organo-tin
compounds and
carbon monoxide. As such it is important for manufacture of the orthotic
structure to
be conducted in a thoroughly clean environment.
The performance of the device described herein is expected to be at least on a
par with that provided by our previously proposed device (as described in
granted UK.
Patent No. 2330309). This device was found to provide a considerable
improvement
not only to the degree of plantarflexion, but also to the walking speed and
the effort
involved in walking (known as the Physiological Cost Index or PCI) of a group
of
patients. At an initial point in the study it was found that the orthosis
provided an
increase of roughly 10% in walking speed and a reduction of roughly 2% in the
PCI.
At the end of the study, roughly six months later, it was found that that same
group of
patients experienced an increase in walking speed of roughly 20% and a
reduction of
roughly 32% in the PCI as compared to when they were initially without the
orthosis.
It is anticipated that the orthosis of the present invention will provide
similar, and
hopefully, better results.
We have also found that the orthosis of the present invention improves the
patient's proprioception. When not wearing an orthosis as herein described,
patients
tend to stumble when walking. However, when the orthosis is worn, the pressure
exerted by the orthosis on the skin receptors sends a message to the brain
that helps the
patient determine where the foot is in space. This in turn helps the patients
to walk
faster and avoid stumbling.
It will be understood from the above that the orthoses herein described
provide
an effective means to tackle the problem of plantarflexion. Advantageously,
and in
addition to this function, the orthoses herein described can significantly
augment
dorsiflexion during the swing-through phase of a patient's gait cycle. The
principal
reason for this is believed to be that the orthotic structure (and resilient
rib, if
provided) store energy when compressed, and this energy is released during the
swing
through phase of the patient's gait cycle. It is anticipated, therefore, that
patients will
not only find that the orthoses of the embodiments tackle the problem of
plantarflexion
but also actively assist the walking process.