Note: Descriptions are shown in the official language in which they were submitted.
CA 02590813 2007-06-04
13196-38CA
EVANESCENT WAVE MULTIMODE OPTICAL WAVEGUIDE AND SENSOR WITH
HIGH MODE COUPLING
BACKGROUND OF THE INVENTION
1. Field of the Invention
The present invention relates to evanescent wave optical
waveguide sensors for measuring chemical or physical
parameters. More particularly, the present invention relates
to chemical sensors and more specifically to ion-selective
sensors.
2. Background Art
Chemical sensors are used in various applications including
environmental emission control, agri-food industry, and other
industrial applications. They are also used in biomedical
applications and clinical analysis for determining the pH, the
presence of specific ions or the oxygen or alcohol
concentration in a sample solution for example. Optical
chemical analysis methods include, for example, Fourier
transform infrared spectroscopy.
Optical fiber based optodes generally use a sensing membrane
deposited at the fiber tip which was previously cleaved and
polished. Alternatively, evanescent wave spectroscopy uses an
optical waveguide that is immersed into the sample solution.
Light is guided in the waveguide by internal reflection at
the waveguide-solution interface. The refractive index of the
waveguide is higher than that of the solution so that the
solution acts as a cladding for the optical waveguide. Light
is mostly propagated in the waveguide but part of the light,
namely the evanescent wave, propagates in the solution (acting
-1-
CA 02590813 2007-06-04
13196-38CA
in a way similar to a waveguide cladding) and can then be
absorbed by the analyte. Analysis of the measured absorption
spectra provides an indication of the presence of given
chemicals.
The use of a polymer membrane cladding on an optical waveguide
was also proposed as an alternative design of the evanescent
wave sensor. In this case, the analyte diffuses in the
membrane when the optical waveguide is immersed in the sample
solution. As light is guided by the core, the evanescent wave
propagates in the polymer membrane and the optical absorbance
of the analyte which diffused in the cladding is measured.
In conventional multimode optical waveguides, distribution of
the optical power between the core and the cladding is
different for each mode. Low-order modes are much confined in
the core of the fiber compared to high-order modes, the latter
interacting more strongly with the cladding or surrounding
sample solution. The high-order modes are then depleted
rapidly by evanescent wave absorption compared to the low-
order modes. The waveguide optical absorbance is therefore not
proportional to that of the cladding, the absorbance being
defined as minus the logarithm of the light transmittance of a
material or a device. Accordingly, in Payne, F. P. and Z. M.
Hale, "Deviation from Beer's law in multimode optical fiber
evanescent field sensors.", International Journal of
Optoelectronics, 8, 743, 1993, it was demonstrated that the
Beer-Lambert law, which linearly relates the absorbance of an
optical waveguide to the concentration of an absorbing species
in the cladding or in a surrounding solution is inapplicable
in the case of evanescent wave spectroscopy with multimode
fibers. Reliable quantification of the concentration of the
absorbing species is therefore not straightforward. It is
-2-
CA 02590813 2007-06-04
13196-38CA
noted that the Beer-Lambert law usually applies to light
propagating through a flat medium and says that the absorbance
of the medium is proportional to the concentration of the
absorbing chemicals it contains and to the light propagation
length in the medium.
SUMMARY OF INVENTION
It is therefore an aim of the present invention to provide an
evanescent wave sensing optical waveguide and sensor that
overcome some of the drawbacks of the prior art.
According to one aspect of the invention, there is provided an
evanescent wave multimode optical waveguide for use as an
optode. The sensing waveguide combines a multimode optical
waveguide with high mode coupling and with a cladding having
an optical absorption varying with a chemical or a physical
parameter to be sensed and interrogated by means of the
evanescent wave. The proposed optode allows absorbance-based
chemical analysis not only in colorless and transparent
solutions but also in colored and turbid ones without any need
to provide mechanical filtration of the sample in a close
proximity to the sensing element (e.g. dialysis membranes).
According to another aspect of the invention, there is
provided an evanescent wave multimode optical waveguide
sensitive to a chemical species or to a physical parameter.
The optical waveguide comprises a core and a cladding having a
cladding refractive index lower than that of the core for
guiding light to be propagated in the optical waveguide. The
cladding defines with the core an optical waveguide providing
mode coupling. A chemical indicator is provided in the
cladding for causing a variation of the optical absorption of
-3-
CA 02590813 2007-06-04
13196-38CA
the cladding as a function of the chemical species or the
physical parameter. The cladding is interrogated by the
evanescent wave of the propagated light. The mode coupling
causes unabsorbed light power to be redistributed among the
multiple modes while light propagates along the optical
waveguide.
For example, an ion-selective optical fiber sensor is prepared
from a polymer optical fiber (POF) and a cladding of a
conventional polymeric ion-selective coating. A high mode
coupling arises from micro-heterogeneities of the POF core
material (poly(methyl methacrylate) (PMMA) for example). The
variable optical absorption property is provided by the
sensing cladding containing a specific chemical indicator
(i.e., a dye) directly or indirectly selectively sensitive to
the target ion to be analyzed.
It is noted that the indicator refers herein to any compound
that changes its optical absorption spectrum according to the
chemical equilibrium it establishes with its surrounding
environment. It can be organic, organometallic or inorganic.
Quantum dots can also be considered as indicators as well as
polymer semiconductors.
The proposed sensing principle is extended further for
measuring various chemical species or physical parameters
detected optically through a change of the optical absorption
spectrum of the sensing cladding by thermochromism
(temperature), solvatochromism (solvent vapor detection),
electrochromism (current), ionochromism (ion), halochromism
(pH), and piezochromism (pressure), etc.
While light propagates in the optical waveguide with a sensing
cladding, it remains confined in the core and the evanescent
-4-
CA 02590813 2007-06-04
13196-38CA
wave propagates in the sensing cladding generating the optode
response. Thus, the proposed evanescent wave optical waveguide
can be used in colored and turbid sample solutions since light
does not propagate in the non-transparent solution.
In conventional low mode coupling waveguides, high-order modes
interact more strongly with the sensing cladding or
surrounding sample solution than the low-order modes,
resulting in the high-order modes to be depleted rapidly along
the waveguide due to evanescent wave absorption. As a
consequence, the waveguide optical absorbance is not
proportional to that of the cladding. On the contrary, a
strong mode coupling in the optical waveguide results in the
replenishing of the high-order modes via mode mixing, i.e. the
optical power is continuously redistributed among the modes
while light propagates along the waveguide. This in turn leads
to a linear dependence of the absorbance of the sensing
waveguide on that of the sensing cladding, the latter
following a Beer-Lambert law. Consequently, the absorbance of
the cladding being proportional to the concentration of a
given state of the indicator within the cladding, there is a
relation similar to a Beer-Lambert law between the
concentration of the dye (in this state) and the optical
waveguide absorbance, and the sensing waveguide can be used
reliably for quantitative analysis.
Moreover, a strong mode coupling results in an absorbance
independence on the light injection conditions at the input of
the waveguide, which provides stability of the sensor to light
injection conditions between the light source and the sensing
waveguide and between the sensing waveguide and the detector.
Similarly, the sensor is less sensitive to vibrations,
movements and bending of the sensing optical fiber.
-5-
CA 02590813 2007-06-04
13196-38CA
There is provided an evanescent wave multimode optical
waveguide sensitive to a chemical species or to a physical
parameter. The optical waveguide comprises an optical
waveguide core having a core refractive index and a cladding
surrounding the core and having a cladding refractive index
lower than the core refractive index for guiding light to be
propagated in the optical waveguide such that an evanescent
wave of light propagates in the cladding. The cladding defines
with the core an optical waveguide providing mode coupling
such that multiple modes of the propagated light are coupled
while light propagates along the optical waveguide. A chemical
indicator is provided in the cladding. The indicator causing a
variation of the optical absorption of the cladding as a
function of the chemical species or the physical parameter.
The evanescent wave propagating in the cladding resulting in
light to be at least partly absorbed by the indicator. The
mode coupling causing unabsorbed light power to be
redistributed among the multiple modes while light propagates
along the optical waveguide.
According to another aspect of the invention, mode coupling
provides that the absorbance of the sensing waveguide depends
linearly on the concentration of the absorbing species within
its cladding, leading to a relation similar to a Beer-Lambert
law.
According to another aspect of the invention, there is
provided an evanescent wave multimode optical waveguide sensor
for measuring a chemical species or a physical parameter. The
sensor comprises a portion of an optical waveguide having an
optical waveguide core and a cladding surrounding the core
having a refractive index lower than that of the core for
guiding light to be propagated in the optical waveguide such
-6-
CA 02590813 2007-06-04
13196-38CA
that an evanescent wave of the light propagates in the
cladding. The cladding has an optical absorption varying with
the chemical species or the physical parameter. The evanescent
wave is partially absorbed by the cladding from the optical
absorption. The optical waveguide has a mode coupling length
which is substantially less than a sensing length of the
optical waveguide for providing mode coupling of light power
between multiple modes of the propagated light. A light source
unit is connected to the optical waveguide for providing the
light to be propagated in the optical waveguide. A detection
unit is connected to the optical waveguide for detecting the
light propagated in the optical waveguide for determining an
optical absorbance of the light in the optical waveguide. A
processing unit is associated with the detection unit for
determining the chemical species or the physical parameter
from the determined optical absorbance.
According to another aspect of the invention, there is
provided a method for determining a concentration of a
chemical species in a sample solution or a physical parameter.
The method comprises : providing an optical waveguide having
an optical waveguide core and a cladding for guiding light to
be propagated in the optical waveguide; propagating light in
the optical waveguide such that mode coupling occurs in the
optical waveguide while the light propagates, an evanescent
wave of the light propagating in the cladding to be partially
absorbed by the cladding; exposing the optical waveguide to
the sample solution or to the physical parameter to produce a
variation of an optical absorption of the cladding; detecting
light propagated in the optical waveguide; determining an
optical absorbance in the optical waveguide from the detected
light; quantifying the concentration of the chemical species
or the physical parameter from the determined optical
-7-
CA 02590813 2007-06-04
13196-38CA
absorbance; and outputting the quantified concentration of the
chemical species or the quantified physical parameter.
BRIEF DESCRIPTION OF THE DRAWINGS
Further features and advantages of the present invention will
become apparent from the following detailed description, taken
in combination with the appended drawings, in which:
Fig. 1 is a schematic view illustrating an evanescent wave
multimode optical fiber, in accordance with one embodiment of
the invention;
Fig. 2 is a graph showing the simulation of the relationship
between the intrinsic absorbance of the cladding and the
absorbance of a sensing optical fiber of increasing mode
coupling;
Fig. 3 is a graph showing the optical absorption spectra of a
Ca2+-selective optical fiber with varying concentrations of
Ca2+ ions;
Fig. 4 is a graph showing the calibration curve of the Ca2+-
selective optical fiber of Fig. 3;
Fig. 5 is a graph showing the time response of the Ca2+-
selective optical fiber of Fig. 3;
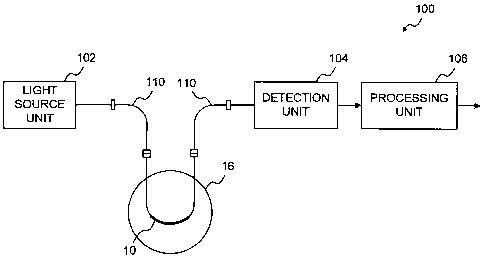
Fig. 6 is a block diagram illustrating an evanescent wave
multimode optical waveguide sensor comprising the optical
fiber of Fig. 1, wherein the sensing optical fiber is used in
transmission;
Fig. 7 is a block diagram illustrating an evanescent wave
multimode optical waveguide sensor comprising the optical
-8-
CA 02590813 2007-06-04
13196-38CA
fiber of Fig. 1, wherein the sensing optical fiber is used in
reflection; and
Fig. 8 is a flow chart illustrating a method for sensing a
chemical species or a physical parameter.
It will be noted that throughout the appended drawings, like
features are identified by like reference numerals.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
Now referring to the drawings, Fig. 1 illustrates an
evanescent wave multimode optical fiber 10. The optical fiber
10 has a multimode core 12 and a sensing cladding 14
surrounding the core 12. The core 12 is made of a
substantially heterogeneous material so as to provide high
mode coupling. In this embodiment, the optical fiber 12 is a
plastic optical fiber (POF) and the core 12 is made of
poly(methyl methAcrylate) (PMMA), such as Plexiglas . In this
case, mode coupling is provided in the optical fiber by
optical scattering within the core material. Any other
material providing a high structural or microscopic
heterogeneity resulting in high mode coupling in the multimode
optical fiber 10 could be used as well in the fiber core 12.
Instead of using a heterogeneous material, high mode coupling
in the multimode optical fiber 10 can be otherwise achieved by
providing structural irregularities along the fiber in the
core 12 or the cladding 14.
Different mechanisms can be used to induce a mode coupling
within an optical fiber. For example, high mode coupling can
be produced by providing inhomogeneities, such as material
impurities associated with the manufacturing process, multiple
micro-deformations in the core material, micro-bends of the
-9-
CA 02590813 2007-06-04
13196-38CA
optical fiber, an irregular core-cladding interface,
refractive index fluctuations, or macrodefects, curvatures or
macrocraks caused by fiber aging, and/or long-duration bending
stress or high pressures. Creating such inhomogeneities in the
cladding can also provide high mode coupling. As such,
irregularities in the optical fiber 10 can be provided by any
means which causes the refractive indices of the optical fiber
to vary along the fiber 10 to provide mode coupling. It is
noted that the numerical aperture of the optical fiber also
10 influences the mode coupling length. A higher numerical
aperture implies a larger number of propagation modes, and a
longer fiber length is then required to complete the coupling
between all of the modes.
The sensing cladding 14 comprises a chemical indicator.
Therefore, in addition to stable intrinsic core attenuation
losses, light propagating in the fiber is absorbed via the
evanescent field which interacts with the cladding 14. In this
embodiment, the fiber 10 is sensitive to a given chemical or
chemicals in a sample solution 16 in which the optical fiber
10 is immersed, but it is noted that in other embodiments the
indicator could be sensitive to a physical parameter to be
measured.
The sensing cladding 14 has a refractive index lower than that
of the core 12 so that light propagated in the optical fiber
10 is guided by total internal reflection at the core-cladding
interface 20. The sensing cladding 14 is also selected to have
sufficient mechanical adhesion to the material of the core 12.
When the ion-selective sensing optical fiber 10 is immersed
into a sample solution 16, a reversible chemical equilibrium
is established between the cladding 14 and the sample solution
16. As will be discussed hereinbelow, for ions sensing, the
-10-
CA 02590813 2007-06-04
13196-38CA
absorption spectrum of the optical fiber 10 is related to the
relative concentration of protonated and deprotonated forms of
the dye indicator. Accordingly, the sensing optical fiber 10
can be used as a quantitative sensor by allowing determination
of the degree of deprotonation (1-x) of the indicator.
When the optical fiber 10 having a thin ion-selective cladding
14 is immersed in an aqueous sample solution 16 (refractive
index of about 1.333), the resulting configuration can be
viewed as a double-clad fiber, the cladding 14 providing a
first clad layer and the water solution 16 providing a second
clad layer. In terms of geometrical optics, some of the rays
are guided via total internal reflections at the cladding-
solution interface 18. Those rays are rapidly attenuated as
they suffer strong absorption due to multiple passages through
the cladding 14. Accordingly, the optical power detected at
the output of the optical fiber 10 only comes from optical
modes guided by total internal reflection at the core-cladding
interface 20. For optical wavelengths within the absorption
spectrum of the dye indicator, a model assuming a cladding 14
of infinite thickness is thus justified. The optical fiber 10
is therefore considered as a single-clad fiber.
The optical fiber 10 is largely multimode. This implies that
either a ray tracing or a modal analysis model can be used.
According to both models, the Beer-Lambert law that relates,
for instance, the concentrations of the protonated and
deprotonated states of the dye indicator in the cladding to
the optical absorbance of the optical fiber at a given
wavelength would not be valid if the optical fiber showed low
mode coupling, as it is the case for high optical quality
optical fibers. When light propagates along such low mode
coupling optical fibers, higher-order modes show an evanescent
-11-
CA 02590813 2007-06-04
13196-38CA
wave that extends further into the cladding and they are
consequently more attenuated than the low-order modes. The
proportion of optical power propagating in high-order modes
should decrease and the absorption rate should decrease
accordingly. On the contrary, the Beer-Lambert law assumes a
uniform absorption rate. However, it can be shown that the
Beer-Lambert law is justified in the case of strong mode
coupling such as mode coupling provided by the heterogeneous
core 12 of the optical fiber 10.
Payne and Hale's model
Now referring to Fig. 2, a theory for multimode evanescent
fiber sensors was proposed in Payne, F. P. and Z. M. Hale,
"Deviation from Beer's law in multimode optical fiber
evanescent field sensors.", International Journal of
Optoelectronics, 8, 743, 1993. It considers an ideal step-
index single-clad fiber with an absorbing cladding of infinite
thickness. Based on analytical approximations developed for
weakly guiding multimode fibers, the absorbance of the sensing
optical fiber 10 at a given wavelength X is related to the
intrinsic unit length Neperian absorbance a(A) of the material
of the cladding 14. The graph of Fig. 2 shows this
relationship according to the model of Payne and Hale in a
specific case where A= 650 nm and the numerical aperture NA
and the core diameter of the fiber are respectively 0.2 and
400 pm.
It is noted that the model of Payne and Hale assumes no
significant mode coupling and a uniform optical power
distribution in the propagation modes at the input of the
optical fiber 10. Sensing optical waveguides with no
significant mode coupling may behave according to this model
but are sensitive to input light conditions. These assumptions
-12-
CA 02590813 2007-06-04
13196-38CA
are not needed in the case of high mode coupling sensing
optical fibers.
Mode coupling model
An approximate model of mode coupling along the optical fiber
10 can be provided by dividing the optical fiber 10 into n
sections of sub-lengths L/n, where L is the total length of
the optical fiber 10. According to a simplified model, it is
then assumed that the output power of each section is
redistributed uniformly between the modes before entering the
next section. Hence, each fiber section can be described by
the Payne and Hale's model applied for a fiber of length L/n.
The modified overall transmittance T' (X) is then simply given
by the multiplication of the n individual transmittances:
T'(11,L)=[T(A,L/n)]" . (1)
The higher the value of n, the more important the mode
coupling is. For high values of n, it can be demonstrated that
the transmittance of the multimode evanescent wave optical
fiber 10 is given by a modified Beer-Lambert law, namely:
T'(11) ;z~ e a(A)n(A)L (2)
where a(X) is the unit length absorption spectrum of the
cladding 14 and rl(1~) is the fraction of the total optical
power propagating in the cladding.
Fig. 2 shows the relationship between the intrinsic absorbance
of the cladding 14 and the absorbance (defined as -log(T'(X)))
of the sensing optical fiber 10 with an increasing number of
sections n, i.e. for increasing mode coupling. Both the
abscissa and the ordinate of the graph of Fig. 2 have been
normalized for better understanding. It shows that in the case
-13-
CA 02590813 2007-06-04
13196-38CA
of strong mode coupling, there is a linear relationship
between the intrinsic absorbance of the cladding 14 and the
absorbance of the sensing optical fiber 10. The modified Beer-
Lambert law (eq. 2) is then valid. Accordingly, contrarily to
the requirements for most fiber optic applications, high mode
coupling is preferable in the case of the present evanescent
wave sensing optical fiber and sensor.
For the sensing optical fiber 10 to respond according to the
Beer-Lambert law, i.e. to provide a linear relationship
between the absorbance of the sensing optical fiber 10 and the
absolute concentration of any state of the indicator in the
cladding, the optical fiber 10 should follow a strong mode
coupling regime.
The mode coupling length (Lc) of a multimode optical fiber is
the length over which an equilibrium mode distribution is
achieved. This length is inversely proportional to the
coupling coefficient D as defined by the following power-flow
equation describing the evolution of the angular content
P(6,z) of a multimode optical beam propagating in a optical
fiber that shows mode coupling:
aP(9,z) D aP(B,z) + D a2P(9,z)
az - Y(e) P(B, Z) + 0 ae aez (3)
where e is the angle of a ray with respect to the propagation
axis Z and y(8) is the angle-dependent attenuation. As the
beam propagates along the fiber, the different mechanisms
causing mode coupling induce a diffusion of the angular
content as suggested by the light diffusion equation (3)
above. Beyond the mode coupling length Lc, the angular
distribution P(6) becomes essentially independent of the
initial angular components injected into the fiber. The
-14-
CA 02590813 2007-06-04
13196-38CA
coupling length is determined from the mode coupling
coefficient D which can be measured as described in S. Savovic
and A. Djordjevich, "Method for calculating the coupling
coefficient in step-index optical fibers", Applied Optics, 46,
1477 (2007).
The coupling length Lc should be small for the absorption of
the sensor described herein to follow a simple Beer-Lambert
law. More specifically, the coupling length should be much
shorter than the sensing length of the sensing optical fiber
of the sensor. Fig. 2 suggests, for example, a factor of more
than twenty or so, and, if possible, of more than one hundred,
for a Beer-Lambert-like behavior of the sensor.
Coupling lengths of mode coupling optical fibers typically
range from a few millimeters to a few meters, depending on the
coupling coefficient D which itself depends on the particular
coupling mechanism(s) involved. Considering typical sensing
optical fiber lengths of 2 to 10 centimeters for in-solution
ion detection sensors, coupling lengths shorter than about 5
millimeters or so are suitable for such applications. Coupling
lengths shorter than about 1 millimeters is preferable for
sensing lengths in the low range. It is noted that in other
applications, as will be discussed herein below, the sensing
optical fiber can be longer and so can be the coupling length
Lc.
Ion-selective sensing cladding
As explained hereinabove, in the present embodiment, the
sensing cladding 14 of the sensing optical fiber 10 comprises
a light-absorbing indicator directly or indirectly providing
the optical response to a given chemical or to given
chemicals. Direct sensitivity refers to an indicator which is
-15-
CA 02590813 2007-06-04
13196-38CA
directly in a chemical equilibrium with the analyte. The
simplest example of a direct sensitivity is a pH indicator in
a pH optode. Indirect sensitivity refers to a chemical
equilibrium between the indicator and the analyte which is
carried on by many chemical intermediates which are in
equilibrium with each other. Such a more complex mechanism is
schematized by the example below.
The matrix material of the ion-selective cladding 14 is
prepared from a plasticized or plasticizer-free polymer having
thermoplastic properties. For example, the cladding 14 can be
prepared from plasticized polyvinyl chloride (PVC). The
cladding 14 further contains a chromoionophore/indicator C,
i.e. a lipophilic pH indicator, an ion-selective ionophore L,
and ionic sites R . The chromoionophore C provides the optical
response of the sensing cladding 14, the ionophore L provides
the chemical selectivity, and the ionic sites provide
electroneutrality by providing charge conservation in the
cladding material. The working principle of this membrane is
based on a reversible ionic-exchange between analyte ions M+
in solution and hydrogen ions H+ in the sensing cladding 14,
and follows:
K
nL(m) + CH+(m) + R-(m) + M+(aq) 4 C(m) + [MLn ]+(m) + H+(aq) + R-(m) , ( 4 )
F--
where n is the stoichiometry of the ion-ligand complex [MLn]+,
(m) means that the substance is the in cladding 14 and (aq),
in the aqueous sample solution. K is the chemical equilibrium
constant. This ionic exchange directly affects the equilibrium
between the two chromoionophore states in the cladding 14,
namely the protonated state CH+ and the deprotonated state C.
Since CH+ and C have different colors, a change in their
-16-
CA 02590813 2007-06-04
13196-38CA
relative concentration can be quantified using spectroscopic
techniques. The relative concentration of the deprotonated
state is referred to as the degree of deprotonation (1-x) and
is given by
1-x= [C] . (5)
[C]+[CH+]
The degree of deprotonation (1-x) is related to the
concentration - or, to be exact, to the activity - of the
target ion in the sample solution 16. This relation depends on
the chemical equilibriums in the cladding 14. For instance,
for the example given in Figs. 3-5, the activity a of the
analyte and the degree of deprotonation are related by this
equation :
a- 1 xIO-pH [R]-(1-xX[C]+[CH+]) (6)
zK 1-x n ~
[L]-([R]-(1-xX[C]+[CH+])~ZJ
where [X] is the concentration of compound X, n=z=2 in this
particular case and K is the equilibrium constant obtained by
calibration. More details can be found in K. Seiler, "Ion-
Selective Optode Membranes", Fluka Chemie AG, Buch, 1993,
p. 16-24.
The ionic sites R present in the cladding 14 allows an ionic
exchange providing cationic/anionic selectivity of the
cladding due to the salt structure, i.e., lipophilic anion or
cation remaining in the cladding 14 and exchangeable counter
ion, cation or anion. The selectivity of ionic exchange of the
ionic sites R is dictated by the lipophilicity of the
exchangeable ions. Due to the cladding electroneutrality, the
number of cations/anions that can enter the cladding 14 does
-17-
CA 02590813 2007-06-04
13196-38CA
not exceed the concentration of the respective ionic sites. In
turn, the ionophore L modulates the selectivity of the ionic
exchange provided by the ionic sites R by means of reversible
selective binding/complexation of the target ion. In order to
provide dominating complexation-determined cladding
selectivity, the concentration of the ionophore is selected to
be higher than that of the ionic sites.
The ion-selective sensing cladding 14 can be prepared from
either plasticized or plasticizer-free polymers. Example of
suitable polymeric matrices are: poly(vinyl chloride) (PVC)
plasticized with bis(2-ethylhexyl) adipate (DOS), i.e.
PVC:DOS; a copolymer of methyl methacrylate (MMA) and n-decyl
methacrylate(DMA-MMA); or a copolymer of methyl methacrylate
and n-butyl acrylate (BA-MMA). PVC-DOS can be prepared from
commercially available products. DMA-MMA can be prepared as
described in U.S. Patent Application Pub. No. 2003/0217920 Al
to Peper et al. and BA-MMA can be prepared as described in
International Publication No. WO 00/54039 to Hall.
One example of suitable chromoionophore C is ETH 5294.
Examples of suitable ionophores L are CaZ+-selective ionophore
ETH 1001 and K+-selective ionophore valinomycin. Finally,
ionic sites can be provided by, for example, sodium tetrakis
[3,5-bis (trifluoromethyl) phenyl] borate (NaTFPB). An
evanescent wave optical fiber 10 sensitive to a different ion
can be obtained by selecting a different appropriate
ionophore.
Manufacturing of the fiber
An evanescent wave optical fiber 10 can be manufactured using
different processes. One possible process consists of coating
a layer of cladding 14 on an available optical fiber core 12.
-18-
CA 02590813 2007-06-04
13196-38CA
Coating can be integrated to the drawing process of the core
12 by coating the core 12 continuously during fiber drawing.
The coating may also be deposited after the fiber's core has
been drawn. The coating can also be applied later on, on an
available core 12 of optical fiber. For example, the existent
cladding of an available plastic optical fiber can be removed
along a given section. The bare core is then coated with a
sensing cladding 14 along that section. Coating of the core 12
is preferred when the materials used for making the core 12
and the cladding 14 have incompatible glass transition
temperatures, or drawing temperatures. It is however noted
that the obtained cladding 14 should be thin enough to provide
a suitable time response. Indeed, the evanescent wave
interacts with the few micrometers of cladding that are close
to the core 12. The thinner the cladding 14, the faster the
chemical equilibrium will be achieved between the cladding 14
and the sample solution 16 upon a variation in the composition
of the sample solution 16 and the faster will be the response
of the sensing optical fiber 10.
In one embodiment, a coating of ion-selective polymeric
cladding 14 is deposited on the optical fiber core 12 from a
tetrahydrofuran (THF) solution of the selected cladding
composition. Drying of the coating solution provides a plastic
optical fiber 10 coated with the sensing cladding 14.
If the cladding and core materials have the same thermoplastic
properties, the optical fiber 10 can be drawn in a manner
similar to that used for standard manufacturing of optical
fibers. The optical fiber 10 can then be directly drawn from
an optical fiber preform consisting of an optical core rod and
a sensitive optical cladding layer. For example, a layer of
sensing cladding can be coated on a PMMA rod in order to
-19-
CA 02590813 2007-06-04
13196-38CA
provide the fiber preform. PMMA being a thermoplastic polymer,
an optical fiber can be drawn at a drawing temperature low
enough to avoid degradation of the components of the ion-
selective cladding material.
Drawing a fiber from a sensing cladding-coated preform allows
the production of a large quantity of optode sensing parts in
a single step. The preparation of the sensor is then reduced
to the connection of the sensing fiber 10 to the remaining
components of the optical sensing system. From a 10 cm-long
preform having a diameter of 1 cm, one can produce 62.5 meters
of optical fiber having a diameter of 400 pm, or 625 10 cm-
long optode sensing parts. Due to the short length of optical
fiber required to make an optode, low optical quality material
can be used as, for example, commercially available Plexiglas
with typical intrinsic attenuation of 1.5 to 5 dB/m in the
wavelength range of 400 to 700 nm. The resulting 10 cm-long
optodes have a core attenuation of about 0.2 to 0.5 dB which
is fine for most practical applications.
Planar or channel sensing optical waveguides can also be
manufactured by spin-coating a planar substrate with the
selected cladding cocktail dissolved in a suitable solvent.
Thin cladding membrane, 4 pm-thick for example, can be
achieved using such methods.
Example 1 - manufacturing by preform drawing
In one embodiment, the core of the preform is made from a
commercial rod of Plexiglas having a diameter of 11 mm with a
refractive index n20D of 1.491 and a glass transition
temperature Tg of about 108-109 C. The preform is prepared by
multiple constant-speed immersions of the Plexiglas rod into
a solution of the selected cladding material compound until
-20-
CA 02590813 2007-06-04
13196-38CA
the desired layer thickness is reached on the perform. In this
case, the cladding matrix is made of a copolymer poly(methyl
methacrylate-co-decyl methacrylate) (pMMA-DMA) (n20D = 1.476)
synthesized in benzene. The pMMA-DMA-based cladding cocktail
also comprises chromoionophore ETH 5294, anionic sites
tetrakis [3,5-bis(trifluoromethyl) phenyl] borate sodium
(NaTFPB), and potassium-selective ionophore valinomycin using
tetrahydrofuran as the solvent. Fiber drawing is performed at
a furnace temperature of 200 C. In those conditions, preform
temperature does not exceed 180 C but maintains this
temperature for a few minutes.
Example 2
Fig. 3 shows experimental optical absorption spectra
illustrating the sensitivity of an optical fiber having a Ca2+-
]5 selective nBA-MMA cladding painted on a bare PMMA fiber core.
The different curves show the optical spectra of the fiber
obtained when immersed in solutions of various concentrations
of CaC12. The spectra are baseline-corrected and normalized to
the isobestic wavelength of 571 pm. Fig. 4 shows the
calibration curve of the Ca2+-selective optical fiber and
Fig. 5 shows the time response of the Ca2+-selective optical
fiber.
The Ca2+-selective optical fiber under test is made from a
Plexiglas fiber core drawn, using drawing techniques known in
the art, from a 10 cm Plexiglas (PMMA) rod having a 10 mm
diameter. A 400 pm optical fiber core is obtained. A 10 cm-
long section of optical fiber core is then coated by painting
a solution of the polymeric cladding mixture dissolved in
tetrahydrofuran to provide the sensing cladding. A dried
coating of approximately 4}zm is obtained. In this example,
the cladding mixture contains a matrix nBA-MMA, ionic sites
-21-
CA 02590813 2007-06-04
13196-38CA
NaTFPB 12.26 mmol/kg, ionophore ETH 1001 40,23 mmol/kg and
chromoionophore I ETH 5294 9.47 mmol/kg.
Figs. 3-5 show experimental results obtained with aqueous
solutions of 1M HC1 and 1M KOH (absorbance spectra of all
protonated and all deprotonated chromoionophore, respectively)
and sample solutions containing 10-6 M to 10-2 M CaC12, and a
constant buffer background of 0.05 M TRIS-HC1 pH 7.
Fig. 6 illustrates an evanescent wave multimode optical fiber
sensor 100 using the sensing fiber 10 in a transmission
configuration while Fig. 7 illustrates an optical fiber sensor
200 using the sensing fiber 10 in a reflection configuration.
Both configurations comprise a light source unit 102 and a
detection unit 104 for measuring the transmission spectrum of
the sensing optical fiber 10 over a useful bandwidth for
detection of a variation in the absorption of the sensing
optical fiber 10 due to the change of concentration of a
chemical species in the sample solution 16. The light source
unit 102 provides the light to be propagated in the optical
fiber 10 and the detection unit 104 detects the light
propagated in the optical fiber 10 after a one-way
transmission in the case of the configuration of Fig. 6 and
after one back and forth transmission in the case of the
configuration of Fig. 7. The absorption spectrum of the
optical fiber 10 is then measured.
In one embodiment, the light source unit 102 is a broadband
white light source and a fiber optics spectrophotometer is
used as the detection unit 104 but one should appreciate that
a tunable broadband light source and an optical detector could
be used instead. A broadband analysis provides the absorption
spectrum of the optical fiber 10 but it is noted that the
absorbance of the optical fiber 10 could also be measured at a
-22-
CA 02590813 2007-06-04
13196-38CA
single wavelength representative of the variation of the
absorbance of the fiber 10 related to the concentration of the
analyte (or to the physical parameter) to be measured. A
measurement at a second wavelength can additionally be
provided as a reference. For example, the light source unit
102 comprises two or more single-wavelength sources combined
before light injection to the sensing optical fiber 10. The
detection unit may then comprise an optical wavelength
division coupler for splitting the two wavelengths from light
collected from the sensing optical fiber 10 and two optical
detectors for detecting the optical power at each split
wavelength.
In all cases, both sensors 100 and 200 comprise a processing
unit 106 for determining the concentration of the chemical
species to be sensed from the measured optical absorbance of
the sensing optical fiber 10 according to prior calibration.
As described hereinabove, the absorption of the sensing
optical fiber 10 follows a law similar to the Beer-Lambert law
and a measurement of the sensed parameter can be retrieved
accordingly.
In one embodiment, light from the light source unit 102 and to
the detection unit 104 is injected and collected from the
sensing optical fiber 10 using a 400 pm silica fiber 110 with
a numerical aperture of 0.22. Coupling of the silica injection
and collection optical fibers 110 to the sensing optical fiber
10 is made by gluing it with UV-curable fluoroacrylate inside
a Teflon'r" tube tightly fitted about the silica and sensing
optical fibers.
In the embodiment of Fig. 6 both ends of the sensing fiber 10
are connected, while in the embodiment of Fig. 7, only one end
of the sensing fiber 10 has an optical connection. In the
- 23 -
CA 02590813 2007-06-04
13196-38CA
embodiment of Fig. 7, light is injected and collected in the
sensing fiber 10 from the same end, propagated light being
reflected at the opposed end of the sensing fiber 10 using a
reflection coating 212. Light is then directed from the light
source unit 102 to the sensing fiber 10 and from the sensing
fiber 10 to the detection unit 104 using an optical coupler
208, for example.
Fig. 8 illustrates a method for sensing a chemical or a
physical parameter using, for example, one of the evanescent
wave multimode optical fiber sensors described hereinabove. In
step 310, an optical waveguide is provided. The optical
waveguide has an optical waveguide core and a cladding for
guiding light to be propagated in the optical waveguide. For
example, the optical fiber 10 can be used. In step 320, light
is propagated in the optical waveguide such that mode coupling
occurs in the optical waveguide while light propagates. Mode
coupling is produced, for example, by using a waveguide core
made of an heterogeneous material. The evanescent wave of
light which propagates in the cladding is to be partially
absorbed by the cladding. In step 330, the optical waveguide
is exposed to a sample solution containing the chemical
species or to a physical parameter to be sensed to produce a
variation of the optical absorption of the cladding. As
described above, the optical absorption can be varied using an
indicator provided in the cladding and directly or indirectly
sensitive to the change of concentration of the chemical
species or to the physical parameter. The evanescent wave is
partially absorbed by the cladding according to the variable
optical absorption of the cladding. In step 350, light
propagated in the optical waveguide is detected using, for
example, the detection unit 104. In step 360, the optical
absorbance of the light in the optical waveguide is determined
-24-
CA 02590813 2007-06-04
13196-38CA
from the detected light. In step 370, the concentration of the
chemical species or physical parameter to be determined is
quantified from the optical absorption making use of a pseudo
Beer-Lambert law and the involved equilibrium between the
indicator and the analyte concentration or the physical
parameter to be determined. Finally, in step 380, the
quantified chemical concentration or physical parameter is
outputted for use in monitoring the chemical species or the
physical parameter. For example, the quantified value can be
simply displayed or can be numerically outputted to be
recorded or analyzed by an external device.
While the invention is illustrated herein with embodiments for
sensing a concentration of an analyte in a solution, a
physical parameter can also be sensed using an indicator
sensitive to temperature (thermochromism), solvent vapor
detection (solvatochromism), current (electrochromism), ion
(ionochromism), pH (halochromism), pressure (piezochromism),
etc. The sensing cladding is then made of a matrix material
such as a plasticized polymer, a plasticizer-free polymer or
any other suitable non-polymer material, to which the suitable
indicator is added.
It is noted that not only ions but also neutral chemical
species can be analyzed using an evanescent wave multimode
optical waveguide as described herein. For instance, a
concentration of an alcohol can be analyzed making use of
chromoreactant CR-546 from Fluka as the indicator.
It is also noted that, in addition to aqueous solutions, non-
aqueous solutions and gaseous mixtures can be analyzed using
an evanescent wave multimode optical waveguide as described
herein. Depending on the sample media and the sensor
exposure/lifetime requirements, the properties of the
-25-
CA 02590813 2007-06-04
13196-38CA
materials used in the core and the sensing cladding are
adjusted for the specific application.
It is also noted that the light absorption of the analyte that
absorbs in UV/Vis-range can be directly measured without
resort to an indicator, by rather using direct spectroscopy.
For example, the cladding can be made of a permeable material
which can be impregnated by a light absorbing neutral analyte
through partitioning. When the sensor is immersed in a
solution containing the chemicals, the concentration of the
chemicals can then be directly determined by direct
spectroscopy provided that the optical fiber shows mode
coupling strong enough to provide reproducible results.
One major application of the proposed sensing waveguide and
sensor is for chemical sensors, namely ion-selective sensors.
The proposed sensing waveguide and sensor can be used in the
preparation of disposable/exchangeable and inexpensive key
elements of the optical sensors. The proposed sensing
waveguide and sensor provides insensitivity to the color and
turbidity of the sample solution and fairly low cost per
sensing unit.
The embodiments of the invention described above are intended
to be exemplary only. The scope of the invention is therefore
intended to be limited solely by the scope of the appended
claims.
-26-