Note: Descriptions are shown in the official language in which they were submitted.
CA 02591168 2007-05-14
WO OQr74764 PCT/U$pQ115612
METHOD OF MANUFACTURING AN
IIVTRACUTANEOUS MICROlVEEDLE ARRAY
TECHMCAI.. F(E1,D
"I'hc present invention relates generally to medical devices and is
particularly
direeted to a fluid dispensing device and a fluid sampling device of the typa
which, in one
t5 embodiment penetratcs the stratum comeum and epidermis, but not into the
dermis of
siQn, and in another embodiment peneteates into the detrnis so as to interface
with blood
or other biological fluids. The invention is specificalEy disclosed as an
array of
microneedles which painlessly and with minimaJ trauma to the skin cnable fluid
transfer
either into a body as a dispenaing device, or fmm the body to sample body
fluid.
BACKGROUND OF THE INV61V77ON
Topical delivcry of drugs is a very useful method for achieving systemic or
Incalized pharmacological effects. The main challenge in transcutaneous drug
delivcry is
providing sufficient dntg penetration across the skin. The skin cansists of
rnuldple layers
starting with a stratean corneurn layer about (for humans) twemy (20) microns
in
thickness (comprising dead cells), a viable epidermal tissuc layer about
eeventy (70)
microns in thiclaness, and a dermal tissue layer about two (2) mm in
t}ticlauss.
Tlze thin layer of stratum corneuin represents a major barrier for chemical
penetration through skin. 'l'he stratum corneum is responmble for 50%a to 90%
of the skin
barrier property, depending upon the drug material's water solubility and
molecular
weight. The epidermis comprises living tissue with a high concentration of
water. This
layer presents a lesser barrier for drug peaetration. The dermis contains a
rich capillary
network close to the derrnat/epidermal j-unctivn, and once a drug reaches the
derrnal depth
CA 02591168 2007-05-14
wo oor74764 PCT/LtS0Ul15612
it diffuses rapidly to doelr tissue layer6 (such as hair follicles, muscles,
and internal
organs), or systemically via blood circulation.
Current topical drug delivery methods are based upon the use of penetration
enhanciag methods, which often cause skin irritation, and the nse of occlusive
patches
that hydrate the stratelm comeum to reduce its barrier properties. Only small
fraetions of
tapically applied drug penetrates thraugh alan, with very poor efficiency.
Convention methods of biological fluid sampling and non-oral drug delivery are
normally iu1vasive. That is, the skin is lanced in order to extract blood and
measure
various components when performing fluid sampling, or a drug delivery
procedure is
normally parfoimed by injection, which causes pain and requires special
medical training.
An alternative to drug delivery by injection has been proposed by I3enry,
McAllister,
Allen, and Prausnitz, of Georgia Institute of Technology (in a paper titled
"Micromachined Needles for the Transdannal Delivery of Drugs), in which an
array of
solid microneedles is used to penetrate through the stratum corneum and into
the viable
cpida7na1 layer, but not to the demtal layer. In this Georgia Tech design,
however, the
fluid is prone to leak,age around th array of microneedles, since the fluid
is on the
exterior surface of the structure holding the micnoneedies.
Another alternative to dtug delivery by injoction is disclosed in U.S. Patent
No.
3,964,4482 (by Gerstel), in which an array of eitlter solid or hollow
microneedles is used to
penetrate through the stratum aorneum, into the epidermal layer, but not to
the dermal
layer. Fluid is to be dispensed either through hollow microneedles, through
permeable
solid projections, or around non-permeable solid projections that are
surrounded by a
permeable material or an apertnre. A membrane materiad is used to contro] the
rate of
drug release, and the drug transfer mechanism is absorption. The microneedle
size is
disclosed as having a diameter of 15 gauge through 40 gauge (using stendard
medical
gauge needle dimensions), and a length in the range of 5-100 microns. The
permeable
material may be filled with a liquid, hydrogel, sol, gel, of the like for
trmsporting a drug
ttunugh the projectinns and through the stratum corncum.
Another structure is disclosed in WO 98/00193 (by Altea TcchnoIogics, Inc.) in
the farm of a drug delivery system, or analyte monitoring system, that uses
pyranridal-
shaped projections that have channels along their outer surfaces. These
projections have a
2
CA 02591168 2007-05-14
WO 00l74764 PCTI[JSOO/15612
length in the range of 30-50 microns, and provide a trans-dermal or trans-
mucous delivery
system, which can be enltsttocd with ultrasound.
Another structure, disclosed in WO 97/48440, WO 97/4844I , and WO 97/48442
(by ALZA Corp.) is in the form of a device for cnhaztcing transdelmal agent
delivery or
sampling. It employs a plurality of solid metallic microblades and anchor
elatnents,
etched from a metal sheet, with a length of 25400 mm. WO 96/37256 (by Silicon
Microdeviccs, Inc.) disclosed another silicon niicroblade structure with blade
lengths of
10-20mm. For enhancing tran$dermal delivery.
Most of the other conventional drug delivery systems involve an invasive
needlc
or plurality of needles. An example of this is U.S. Patent Number 5,848,991
(by Gross)
which uses a hollow needle to penetrate through the epidermis and into the
deamis of the
subject's skin when the housing containing an expansible%antractible chamber
holding a
reservoir of fluidic drug is attached to the skin. Anot.her example of this is
U.S. Patent
Number 5,250,023 (by Lee) which administers fluidic drugs using a plurality of
solid
needles that penetrate into the demtis. The Lee drug delivery system ionizes
the drug to
help tranafer the drug into thc sbn by an electric charge. The needles are
disclosed as
being within the range of200 microns through 2,000 microns.
Another exatnple of a needle that penetrates into the dennis is provided in
U.S.
5,591,139, WO 99/00155, and U.S. 5,855,801 (by Lin) in which the needle is
processed
using integrated circuit fabrication techniques. The needles am disclosed as
having a
length in the range of 1,000 nticrons through 6,000 microns,
The use of microneedles has great advantages in that intracutancous drug
delivery
can be accomplished without paiut and without bleeding. As used herein, the
term
"mieroneedles" refers to a plurality of elongated structures that are
sufficiently long to
penetzate ehrough the stratum corneum skin layer and into the epidermal layer,
yet are also
sufficiently short to not penetrate to the deimal layer. Of course, if the
dead cells have
been completely or mostly removed from a portion of skin, then a very minute
length of
microneedle could be used to reach the viable epidenmal tissue.
Since microneedle technology shows much promise for drug delivery, it would be
a further advantage if a microneedle apparatus could be provided to sample
fluids within
skin tissue. Fmthermore, it would be a further advantage to provide a
microneedle array
3
CA 02591168 2007-05-14
WO 00l74764 PGTIUS00l1S612
in which the individual mieroneedles were of a hollow structure so as to allow
fluids to
pass from an internal chamber through the hoilow microneedles and into the
skin, and
wm of snfficient length to ensure that they will reach into the epidermis,
entirely through
the stratum corneum.
SUMMARY OF'i'HE INVENTION
Acoordingly, it is a primary advantage of the present invention to provide a
microneedle atray in the form of a patch which caa perform intracutancous drug
delivery.
It is another advantage of the present invention to provide a microneedle an-
ay in the fotm
of a patch that can perform biological body-fluid testing and/or sampling
(including
interstitial fluids and/or blood). It is a further advantage of the present
invention to
provide a microneedle array as part of a closed-loop system to control dtug
delivery,
basad on feedback info,mation that analyzes body fluids, which can aahieve
real titne
continuous dosing and monitoring of body activity. It is yet another advantage
of the
present invention to provide an electrophoretically/mieroneedle-erihanced
transdermal
drug delivery system in order to achieve high-rate drug delivery and to
achieve saAnpling
of body fluids. It is a yet further advantage of the present invention to
provide a method
for raanufacturing an array of microneedles using miorofabric,ation
techniques, including
standard semiconductor fabrication tec.hniques. It is still another advantage
of the present
invention to provide a method of manufacturing an array of ntieroneedles
comprising a
plastic rnaterial by a"self-molding" method, amicromolding method, a
nzicroembossing
method, or a niicroinjeotion method. It is stil} another advantage of the
present invantion
to provide an array of edged miGroneedles tbat, in one configuration are
hollow and have
at least one blade with a substantially sbarp edge that assists in penetration
of the stratum
corneum of skin, and in another configuration the microneedlcs are solid and
have at least
one blade with a substantially sharp edge to assist in penetrating the strahtm
comeum. It
is still a further advantage of the present invention to provide a nucroneedle
array that has
sufficient separation distanee between the individual rnicroneedles so as to
ensure
pettatration of the stratum cometun of skin to achieve greater transderrnal
flux. It is still
another advantage of the pre.aent invention to provide a method of
manufacturing an array
of microneedles in which a metal ruold is initially manufactured for use in a
4
CA 02591168 2007-05-14
WO 00/74764 PG'r/C1500f15612
microembossing procedure, while allowing a sufiicient sepaistion distance
between
individual microneodies of the array, then use a procedure for creating hollow
chambers
and through-holes in the substzate of the microneedle array. It is yet another
advantage of
the present invention to provide a microneedle array that has sensing
capabilities using
optical, spectroscopic, colorimetric, cloctrochemical, thenaial, gravimctaic,
and light
scattering sensing means. It is still another advantage of the present
invention to provide
a mcthod for manufacturing an array of rnicroneedles that uses shear forces
during a de--
molding procedure to create aharp hollow microneedies.
Additional advantages and other novel features of the invention will be sct
forth in
part in the description that follows and in part will become apparent to those
skilled in the
art upon examination of the following or may be learned with the practice of
the
invention.
To achieve the foregoing and other advantages, and in accordance with one
aspect
of the present invention, a first embodiment of an improved micraneedte array
is
consftvcted of silicon and silicon dioxide eompotmds using MEMS (i.e., Micro-
Eleetro-
Mechanical-$ystems) teclDnology and standard microfabrication techniques. The
microneedle array may be fabricated from a silicon die which can be etched in
a
microfabrication process to create hollow or solid individual microneedles.
The resulting
array of microneedles can penctrate with a small pressure through the stratum
corneum of
sion (including sldn of animals, reptiles, or other creatures---typically slan
of a living
organism) to either deliver drugs or to facilitate biological fluid sampling
(e.g., sampling
interstitial fluids and/or blood) through the hollow microneedies or pores
made through
skin via solid microneed]cs. The drug resersroir, and/or the chemical analysis
components
for sampling body fluid, may be fabricated inside the silicon die, or an
additional thick
film layer can be bonded or othrrwise attached over the silicon substrate to
create the
reservoir. The delivery of drugs and sampling of fluids can be performed by
way of
passive diffusion (c.g., time release), instantaneous injection, pressure,
vacuuln,
ultrasound, or electrophoresis (e.g., iontophoresis). A complete closed-loop
system can
be manufactared including active elements, such as micro-machined pumps,
heaters, and
mixers, as well as passive elements such as sensors. A"smart patch" can
thereby be
fabricated that samples body fluids, performs chemistry to decide on the
appropriate drug
5
CA 02591168 2007-05-14
w0 00/74764 PCTI[)500l15612
dosage, and then administers the conmsponding amount of drug. Such a system
oan be
made disposablc, including one with an on-board power supply,
In a second anbadiment, an array of hollow (or solid) micronccdles can be
constructed of plastic or some other type of molded or cast material. When
using plastic,
a micro-machining technique is used to fabricate the molds for a plastic
microfomiing
process. The nnblds are detachable and can be re-used. Since this procedure
requires only
a one-time investment in the mold micro-machining, the resulting plastic
microstructure
should be much less expensive than the use of microfabrication techniques to
construct
microneedle arra.ys, as well as being able to manufscture plastic microneedle
arrays much
more quickly and accv.rately. It will be undeTstood that such hollow
microneedles may
also be referred to herein as "hollow elements," or "hollow prmjections,"
including in the
claims. It will also be understood that such solid microneedles rnay also be
referrcd to
herein as "solid elements," or "sokid projections" (or merely "projections"),
including in
the clsim$.
Molds used in the seeand embodiment of the present invention can contain a
micropillar array and microhole array (or both), which are fabricated by micro-
machining
methods. Such micro-machining methods may include micro electrode-discharge
machining to make the molds from a variety of inetals, including staialess
steel,
aluminum, copper, iron, tungsten, and their alloys. The molds alternatively
can be
fabricated by microfabrication techniques, including deep reactive etching to
make
silicon, silicon dioxide, and silicon carbide molds. Also, LIGA or deep W
processes can
be usad to make molds and/or elcctroplated metal molds.
The manufacturing procedures for creating plastic (or other moldable material)
arrays of micrvneedles include: "seif-molding," micromolding, microembossing,
aad
microinjection techniques. In the "self-molding" method, a plastic film (such
as a
polymer) is placed on a micropillar atray, the plastic is then heated, and
plastic
deformation due to gravitational force causes the plastic film to deform and
create the
microneedle structure. Using this procedure, only a single mold-half is
required. When
using the micromolding technique, a similar micropillar array is used along
with a second
mold-half, which is then closed over the plastic film to form the microneedle
structure,
The micro-embossing method uses a single mold-half that contains an array of
6
CA 02591168 2007-05-14
WO 00f14764 PCTlVS00115612
m.icxopillars and eonieal cut-outs (mieroholes) which is pressad against a
flat surface
(which esstntially acts as the second mold-half) upon which the plastic film
is initially
piaced. In the microinjection method, a melted plastic substance is injected
between two
micno-rnacluned molds that contain microhole and micropillar arrays.
Of course, instead of molding a plastic material, the micronecdle arrays of
the
present invention could also be construeted of a mctallic niateriai by a die
casting method
using some of the same structures as are used in the molding techniques
discussed above.
Since meral is somewhat more expensive and more difficult to work with, it is
probably
not the prefecred material except for some very stringent requireznents
involving unusual
chemicals or unusual application or placement circurnstances. The use of
chemieal
enhancers, ultrasound, or electric fields may also be used to increase
transdermal flow rate
when used with the microneedle arrays of the present invention.
In the dispensing of a liquid drug, the present invention can be effectively
combined with the application of an electric field between an anode and
cathode attached
to the skin which causes a low-level electric current. The present invention
combines the
microneedle array with clectrophoretic (e.g., iontopboresis) or electroosmotic
enhancemcnt, which provides the necessary means for molecules to travel
through the
thicker derntis into or from the body, thereby increasing the pcnneability of
both the
stratum corncum and deeper laycrs of sldn. While the tra.nsport intprovement
through the
stratum corneum is mostly due to microneedle piercing, electrophoresis (e.g.,
iontopboresis) provides higher transport tates in epidermis and dermis.
The present invention can thereby be used with medical devices to dispense
drugs
by clecttophoretic/microneedle enhancement, to sample body fluids (while
provid'v-g an
electrophoretically/microneedle-enhanced body-fluid sensor), and a drug
delivery systern
with fluid sampling feedback using a combination of the other two devices. For
example,
the body-fluid sensor can be used for a continuous or periodic sampling
noninvasive
measurement of blood glucose level by extracting glucose through the skin by
reverse
iontophoresis, and measuring its concentration using a bioelectrochemieal
sensor. The
dxug delivery portion of this inventaon uses the niicroneedle array to provide
electrodes
that apply an electric potential between the electrodes. One of tthe
electtodes is also filled
7
CA 02591168 2007-05-14
WO oN74764 PCT/US0O/15612
with an ionizod drug, and the charged drug molecules move into the body due to
the
applied electric potential.
in an alternative erabodiment of hollow micmnoedlcs, an edged microneodle is
provided that includes at least one longitudinal blade that raw to the top
surface or tip of
the microneedle to aid in penctration of the stratum corneum of skin. The
blade at the top
surface provides a shmp tip that incroases the likelihood of pe,nctrating the
skin whan
coming into contact therewith. In a prefemred mode of the edged hollow
microneedles,
there are two such longitudinal blades that are constnteted on opposite
surfaces at
approximately a 180 angle along the cylindrical side wall of the microneedle.
Each
edged blade has a cross-section that, when viewed from abovc the microneedle
top, has a
profile that is approximately that of an isosceles triangle. The blade's edge
can run the
entire length of the microneedle from its vcry top surface to its bottom
surface where it is
mounted onto the substrate, or the edge can be discontinued pariway down the
length of
the microneedle as thc micraneedlc outer surfaee approaches the substrate. The
orientation of the blades in the microneedle array can be random, in which the
blades of
variaus individual microneedles point in all different directions.
In an alternative embodiment of a solid microneedle, a star-shaped solid
nnicroneedlc is provided having at least one blade with a relatively sharp
edge to assist in
penetrating the stratum corneum of ekia. In a preferred embodiment of a bladed
or edged
solid microneedle, a three pointed star-shaped solid rnicroneedle is provided
in which
each blade has a triangular cross-section when viewed from the top of the
microneedle,
and each of these triangles approximates that of an isosceles triangle. The
base of each of
the isosceles tniangles meets at a oenter of the microneedle to fom a star-
shaped structure
when seen &om the top of the microneedle. At loast one hole through the
substrate
preferably is located near the side sarfaces of at least one pair of blades of
the solid
micxoneodle, and preferably a through-holc would be located near each pair of
such
blades. In this preferred etnbodiment, there would be three edged blades and
three
adjaeent through-holes in the snbstrate for each mieroneedle.
In a further alternative embodiment, a porous polymer, such as a hydrogel or
solgel matrix can be impregnated with active material and depasited in the
inside comers
between the blades of the star, This provides an additional delivery
mechanism.
8
CA 02591168 2007-05-14
w0 00/74760 PCTIU50W15612
The micronoedlt arrays of the present invention are signifiCantly improved by
using a proper separation distance between each of the individual
microneedles. A very
useful range of separation distanccs between microneedles is in the range of
100-300
microns, and more prefcrably in the range of 100-200 microns. The outer
diameter and
nzicroneedlo length is algo rrery important, and in combination with the
separation
distance will be crucial as to whether or not the microneedles will actually
penetrate the
stratnnl corneum of sldn. For hollow circular microneedles, a useful outer
diameter range
is from 20-100 microns, and more prcferably in the range of 20-50 microns. For
circular
nixcroneedles that do not have sharp edges, a useful length for use with
interstitial fluids is
in the range of 50-200 mici+ans, and more preferabty in the range of 100-150
micnun; for
use with other biological fluids, a useful length is in the range of 200
micrans - 3 mm,
and more preferably in the range of 200-400 microns.
For circular trollow microneedles having sbarp edges (such as those having the
blades with triangular shaped edges), a useful length for use with
interstitial fluids is in
the range of 50-200 microns, and more preferably in the range of 80-150
microns; for use
with other biological fluids, a usefill length is again in the range of 200
microns - 3 mm,
and more preferably in the range of 200-400 miamns. An example of a"sharp
edge" as
used herein is where the tip of the blade edge exhibits a dimension at its
angular vertex
that is as narrow or nanrower than 0.5 microns. For solid rnicroneedlcs having
a star-
2o shaped profile with sharp edges for its star-shapcd blades, a useful length
is in the range
of 50-200 microns, and nwre preferably in the range of 80-150 microns, while
the radius
of each of its blades is in the range of 10-50 microns, and more preferably in
the range of
10-15 mivrons.
The present invention ean be manufactured with an alternative methodology
using
a mold preparation procedare that begins by placing an optical mask over a
layer of
PMMA material, then exposing the PMMA material that is not masked to x-rays or
enother type of high energy radiation (e.g., neutrons, electrons), and
developing that
PMMA material in a photoresist process. The remaining PMMA material is then
coated
(e.g., electroplated) with metal, such as nickel. When tlle coating has
reached the
appropriate thickness, ii is detached to become a metal mold to create polymer
or other
type of moldable plastic material. This metal mold is then used in a
microcmbossir+g
9
CA 02591168 2007-05-14
w0 oo/74764 PCT/USO0/15612
procedure, in which the metai mold is presstd against a heated layer of
polymer or other
plastic material. Once the mold is pressol down to its proper distance, the
plastic or
polymer material is cooled to be solidified, and the mold is then detached,
thercby leaving
behind an array of nricroneedles, If the microneedles are hollow, then
altemative
procedures to create through-holes alI the way through the microneedles and
its
emderlying substrate material uses a methodology such as, for example, laser
ablation,
water jet erosion, electric discharge machining, plasma etahing, and particle
bombardment
Another alternative procedure to create polymer or plastic microneedles is to
begin
with a two-layer laminate structure of biocompatible material. A metallic mold
created
by any process is then pressed down all the way through the top layer of tllis
laminate,
and partially into the bottom layer to ensure that the top layer is entirely
penetratcd. This
occurs while the laminate material has been heated to its plastic, deformable
t.emperature.
Once the laminate material has then been cooled, the mold is removed and the
top layer is
ddached frorn the bottom layer. This top layer will now have holes that will
be further
operated upon by a microembossing procedure using a different mold. This
diffcre+it
mold creates hollow microneedles, in which the through-holes that norm-ally
need to be
latcr created in the substrate have already been created in advance by the
first pressing or
molding pzocedure.
Another refinement of the present invention is to create a microneedle array
that
has sensing capabilities. In this structure, the tips or side gruoves of the
niiaroneedles are
coated with a particular chemical that aids in detecting a particular chemical
or biological
structure or fluid that come into contact with the tips of the microneedles. A
sensing
means is performed by the use of optical energy, for example such as a laser
light source
that is directed through the microneedle structure, in which the microneedles
themselves
are made of substantially transparent material. Other sensing mechanisms also
could be
used, as discussed hcreinbelow.
A further alternative manufactuiring process for hollow or solid microneedles
is to
create shear forces along the outer surfaces of the distal or tip portion of
the hollow or
solid microneedle during its molding or embossing process. The shear forces
are actually
created during the de-molding step while the microneedle array material is
being cooled.
lo
CA 02591168 2009-03-18
The amount of shear can be controlled by the cool-down temperature, and if
properly done will result in microneedles having sharp edges (rather than
smooth
edges) along their upper surfaces at their tips.
According to a further aspect of the invention, there is provided a method
of manufacturing a microneedle array, comprising:
(a) providing a semiconductor wafer;
(b) creating a plurality of annular oxide patterns on the top surface of said
wafer;
(c) forming a plurality of indentations in the bottom surface;
(d) forming, by etching away material, a plurality of needle-like projections
in the top surface of said wafer, said needle-like projections having
locations that
are aligned with said indentations; and
(e) forming a plurality of through holes in said plurality of needle-like
projections, thereby creating an array of hollow microneedies.
Still other advantages of the present invention will become apparent to
those skilled in the art from the following description and drawings wherein
there
is described and shown a preferred embodiment of this invention in one of the
best modes contemplated for carrying out the invention. As will be realized,
the
invention is capable of other different embodiments, and its several details
are
capable of modification in various, obvious aspects all without departing from
the
invention. Accordingly, the drawings and description will be regarded as
illustrative in nature and not as restrictive.
BRIEF DESCRIPTION OF THE DRAWINGS
The accompanying drawings incorporated in and forming a part of the
specification illustrate several aspects of the present invention, and
together with
the description and claims serve to explain the principles of the invention.
In the
drawings:
Figure 1 is an elevational view in partial cross-section of a bottom mold
provided at the initial step of a "self-molding" method of manufacturing an
array
-11-
CA 02591168 2009-03-18
of plastic microneedies, as constructed according to the principles of the
present
invention.
Figure 2 is an elevational view in partial cross-section of the mold of
Figure 1 in a second step of the self-molding procedure.
Figure 3 is an elevational view in partial cross-section of the mold of
Figure 1 in a third step of the self-molding procedure.
Figure 4 is an elevational view in partial cross-section of the mold of
Figure 1 in a fourth step of the self-molding procedure.
Figure 5 is an elevational view in partial cross-section of the mold of
Figure 1 in a fifth step of the self-molding procedure.
Figure 6 is an elevational view in cross-section of an array of hollow
microneedies constructed according to the self-molding procedure depicted in
Figures 1-5.
Figure 7 is a cross-sectional view of a top mold-half used in a
micromolding procedure, according to the principles of the present invention
-lla-
CA 02591168 2007-05-14
WO 00/74764 PCT/U$00/15612
Figure 8 is an clevational view of the bottom half of the mold that mates to
the top
mold-half of Figure 7, and which is used to form plastie microneedles
according to the
micromolding procedure.
Figure 9 is an elevationai view in pattial cross-sectyon of one of the method
steps
in the micromolding procedure using the mold halves of Figures 7 and 8.
Figure 10 is an clevational view in partial cross-section of the mold of
Figure 9
depicting the next step in the micromolding procedure.
Figure 11 is a cross-sectional view of an array of plastic mieronoedles
constructed
according to the micromolding procedure depictcd in Figwxs 7-10.
Figure 12 is an clevational view in partial cross-section of a top mold-half
and a
bottom planar surface used in creating an array of molded, plastic
microneedles by a
microernbossing procedure, as constructed according to the principles of the
preaent
invention.
Figure 13 is an elevational view in partial cross-section of the mold of
Figure 12
in a subsequent process st.ep of the rnicroembossing mcthod.
Figure 14 is an elevational view in partial cross-section of the mold if
Figure 12
showing a later step in the microembossing procedure.
Figure 15 is a cross-sectional view of a microneedlc array of hollow
microneedles
constYUCted by the mold of Figures 12-14.
Figure 15A is a cross-sectional view of an array of microneedles which are not
hollow, and are constructed according to the mold of Figures 12-14 without the
micropillars.
Figure 16 is an elevational view in partial cross-sectYon of a two-piecc mold
used
in a mioroinjection method of manufacturing plastic microneedles, as
constructed
according to the principles of the present invention.
Figure 17 is a cross-scctional view of amicroneedle array of hollow
microneedles
constructed by the mold of Figure 16.
Figure 18 is a cross-sectional view of the initial semiconductor wafer that
will be
formed into an array of rnicroneedles by a microfabrication procedure,
according to the
principles of the present invention.
12
CA 02591168 2007-05-14
WO YOl14764 PCTIUS00115612
Figure 19 is a cross-sectionai view of the semiconductor wafer of F'igure 18
after a
hole pattem has been established, and a#ter a silicon nitride layer has been
deposited.
Figure 20 is a cross-sectional view of the wafer of Figure 18 after a
photoresist
mask operation, a deep reactive ion etch operation, and an oxidize operation
have been
pexformed
Figure 21 is a cross-sectional view of the wafbr of Figure 20 after the
silicon
nitride has been removed, and after a deep reactive ion etch has created
through holes,
theaeby resulting in a hollow microneedle.
Figurc 22 is a perspective view of a microneedle array on a semiconductor
substrate, including a magn}1'ied view of individual cylindrical microneedles.
p'igure 23 is a cross-scctional view of an electrophoretically enhanced body-
fluid
sasor, based upon a hollow microneedle array, as constructed according to the
principles
of the present invention.
Figure 24 is a cross-sectional view of an electrophomtxcally enhanced body-
fluid
sensor, based upon a solid microneedle array, as constructed according to the
principles of
the preeept invention.
Figure 25 is a cross-sectional view of an electrode, based upon a hollow
microneedle array, as constructed according to the principles of the present
invention.
Figure 26 is a cross-sectional view of an electr+ode, basecl upon a solid
niicroneedle
array, es constructed according to the principles of the present invpltion.
Figure 27 is a perspective view of a sansing system attached to a human hand
and
for=m, which includes an electrophoretically enhanced body-fluid sensor as per
Figure
23 and an electande as per Figure 25.
Figure 28 is a cross-sectional view of an electrophoretically enhanced drug
delivery system, based upon a hollow niicrorleedle array, as constructed
according to the
principles of the present invention. '
Figure 29 is a cross-sectional view of an eleotrophoretically enhanced drug
delivery system, based upon a solid microneedle array, as constructed
aeoording to the
principles of the present invention.
,13
CA 02591168 2007-05-14
WO OOV74764 PCIYC3S0Ul15612
Figure 30 is a perspective view of a closed-loop drug-delivery system, as
viewed
from the side of a patch that makes contact with the skin, as conshucted
according to the
principles of the pTesont invention.
Figure 31 is a perspective view of the olosod-loop drug-delivery system of
Figure
30, as seen from the opposite side of the patch.
Figure 32 is a perspective view of an aiternative embodiment hollow
microneedle
haviag sharp edges for greater penetration into skin.
Figure 33 is a top plan view oft,he edged hollow microneedle ofF'igure 32.
Figure 34 is a perspective view of an altternative construction for an edged
hollow
microneedle as seen in Figure 32.
Figure 35 is a perspective view of an alternative embodimea-t eolid
micrnneedle
having a star-shaped sct of skarp blades.
Figure 36 is a top plan view of the star-shaped solid miamneedle of Figare 35.
Figum 37 is a table of nlicronoedle penetration data for an array of circular
hollow
niicroneedles at a separation distance of 50 microns.
Figure 38 is a table of rnicroncedle penetraiion data for an array of circular
hollow
microneedles at a separation distance of 100 mierons.
Fig= 39 is a table of rrm,icroneedle penetration data for an array of circular
hollow
microneedles at a sepwation distance of 150 microns.
Figure 40 is a table of microneedlc penetration data for an array of circular
hollow
micrnneedles at a separation diatancc of 200 microns.
Figpre 41 is a table of tnioroneedle penctration data for an array of circular
hollow
microneedles at a separation distance of 250 microns.
Figure 42 is a table of micronc.edle penetration data for an array of circular
hollow
microneedles at a separa.tion distance of 300 microns.
Figure 43 is a table of microneedle penctration data for an array of edged
hollow
microneedles at a separation distance of 50 microns.
Figure 44 is a table of microneedle penetration data for an array of edged
hollow
microneedlcs at a sepatation distaace of 100 microns.
Figeu+e 45 is a table of rnicroneodlc penetration data for an array of edged
hollow
microneedles at a separation distance of 150 microns.
14
CA 02591168 2007-05-14
WO 00R4764 PCT/U800l1s612
Figure 46 is a table of microneedle penetration data for an array of edgcd
hollow
microueedles at a separation distanoe of 200 microns.
Figure 47 is a table of microneedle penetn3tion data for an array of edged
hollow
microneadles at a separation distance of 250 microns,
Figure 48 is a table of microneedle penatration data for an array of edged
hollow
microneedles at a separation distance of 300 microns.
Figure 49 is a graph showing the effect of microneedle separation versus
firansdennal tlux.
Figure 50 is a gaph showing the effect of microneedle length versus
transdermal
flux for two different nlicroneedle separation distances.
Figur+e 51 is a graph showing the effect of microneedle length versus a ratio
of
transdermal flux versus skin damage, for two differ+ent niicroncedle
separation distanees.
Figure 52 is a graph showing the effect of applied pressure of a fluid versos
transdermai flux for a particular microneedle array.
Figures 53A-53E are elevatioaal views in cross-section illustrating steps for
preparing a mold for a micromolding pmcMure to create hollow circular
microneedles.
Figures 54A-54F are cleva#ional views in cross-section of process steps for a
microembossing procedure to creatc hollow microneedles, as well as
micromaclvning and
laser buming steps to cswte hollow ehambers and through-holes in the bottom of
the
substrate structure.
Figures 55A-55F are elevational views in cross-section of further process
steps for
creating hollow micconcedles.
Figure 56A-56B are an elevational views in cross-section of microneedle arrays
that have sensing capabilities using optical devices or chemical coatings.
Figures 57A-57B are side elevational views of a de-molding procedure to ereate
sharp hollow microneedles.
DETAILED l)ESCRIPTION OF TEiE PREPtAItED EMBODIMENT
Reference will now be made in detail to the present preferred embodimen[ of
the
irnvention, an example of wlzich is illustrated in the accompanying drawings,
wherein like
numerals indicate the same elements throughout the views.
CA 02591168 2007-05-14
wa o0/7a7d4 Pr1YUSUOns612
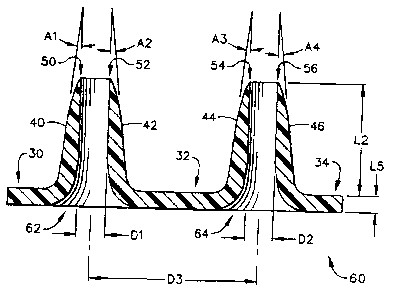
Referring now to the drawings, Figure 1 shows a mold generally designated by
the
referenoe numeral 10 that comprises a plurality of micropillars, including
rnicropillars 12
and 14, that are mounted to a base 16 having a planar upper surface 18,
Micropillar 12
preferably is cylindrical in shape, 8nd has an outer diameter designated "Dl,"
whereas
micropillar 14 (which also preferably is cylindrical in shape) has a diameter
designated
"I)2." The centerlines of micropillars 12 and 14 are separated by a distanoc
"133;" and the
vertical height ofmicropillars t2 and 14 is designated by the letter "Ll."
In a preferred configuration, the diameters D1 and D2 are in the range of 1-49
microns, more preferably about ten (10) microns (i.e., 10 microns = 10
micrometers), the
height L1 in the range of 50-3000 microns, whdvas the separation distance D3
is in the
range of 50-1 000 microns, more preferably from 50-200 microns.
Microelectrode-discharge machining can be used to fabricate the mold 10 from
metals, such as stainless steel, aluu,inum, copper, iron, tungsten, or other
metal alloys.
Mold 10 could also be fabricated from silicon or silicon carbide using
integrated cxrcuft
processing, or photolithographic pmcessing.
Figure 2 depicts the mold 10 and a thin layer of plastic, such as a polymer
film,
designated by the reference numeral 20, which is placed on the micropillars 12
and 14,
thereby making contact at the refercnce ntunerals 22 and 24, respectively.
Once the
polymer film is placed on the micropillars, the polymer is heated to just
above the melting
temperature of the plastic material. Micropillara 12 and 14 are heated to
above the glass
transition terrnperature of the plastic material, but are preferably held
below the melting
ten,perat,ure of the plastic material. This eatablishes a teinperatvx+e
gradieat within the
plastic film, after which the plastic 51m is subjeeted to nat ral
gravitational forces, or
placed in a centrifuge. Furthermore, an air-pressure gradicnt also can be
establishod
across the deforming plastic film, by applying pressure from above, or by
applying a
vacuum from below the film level. The overall effect on the plastic fiim is
that it will
undergo a "self-molding" operation, by way of the gravitational force or
centrifugal force,
and the air-pressnre gradient can be used to accelerate the self-molding
process.
Figure 3 depicts the mold 10 at a further step in the processing of the
plastic flm,
showing the result of the temperatute gradient. This result is that the areas
contacting the
micropillars (at the rEfemce mumerals 22 and 24) will have a smallcr
deformation as
16
CA 02591168 2007-05-14
WO 00/74764 PGTIUS00/15612
eomparcd to the remaining portions of the plastic film 20 that are between the
pillars 12
and 14. Therefore, the portions 30, 32, and 34 of the plastic material will
undergo greater
dcformation, as viewed on Figure 3.
Figaro 4 depicts the mold 10 at yet a latcr steg in the seif-molding procesa,
showing the initial stage in which the umold (including micropiliars 12 and
14) is heated
above the malting teanperatsue of the plsstic materia120. During this latter
stage of the
self-molding process, the plastic material will continue to melt and to be
removed from
the tops of the pillars 12 and 14. As viewed in Figure 4, the remaining
portions not in
contact with micrapiilars 12 and 14 will continue to deform downward (as
viewed on
t0 Figure 4) at the reference numerals 30, 32, and 34.
Figure 5 depicts the mold 10 at the final stage of self-rnolding, which
illustrat,es
the fact that the plastic material has completely melted down and away firom
the tops 22
and 24 of the nucropillars 12 and 14. At this point the mold and the plastic
material are
both cooled down, thereby f'arming the final shape that will become the
microneedles.
i5 This final -9hape includes an outer wa1140 and 42 for the microneedle being
formed by
micropiliar 12, and an outer wall at 44 and 46 fdr the microneedle being
fotxned at the
micropillar 14.
Figure 6 illustrates the cross-sectional shape of the microneedle array,
genera.lly
designated by the reference numeral 60, after it has been detached from the
mold 10. The
20 left hand microneedle 62 has a relatively sharp upper edge, which appears
as points 50
and 52. Its outer wall is illustrated at 40 and 42, which are sloped with
respeet to the
vcrtical, as designated by the angles "Al" and "A2," The right-hand side
microneedle 64
exhibits a similar sharp top edge, as in.dicated by the points 54 and 56, and
also exhibits a
sloped outer wall at 44 arx146. The angle of this outer wall is indicated at
the angles
25 "A3" and "A4." The preferred value of angles Al-A4 is in the range of zero
(0) to forty-
five (45) degrees.
The inner diameter of the left-hand microneedie 62 is indicated by the
distance
"D ]," and the inner diameter of the rigbt-hand microneedle 64 is indicated by
the distance
"D2." These distances D 1 and D2 are substantially the same distance as the
diameter of
30 nnicropillars 12 and 14, as indicated in Figure I. Furthermore, the
distance D3 between
the centerlines of the rnicroneedles on Figure 6 is essentially the same as
the distance D3
17
CA 02591168 2007-05-14
WO ppR4764 PCTlUSOD/iS612
between the mieropillars on Figure 1. The Icngth "L2" of the microneedles on
Figure 6 is
somewhat less than the length Y.l on Figure 1, although this length L2 could
theoretically
be a maximum distance of Ll .
It will be understood that the plastic material (also referred to herein as
the
"polyme,r film") may consist of any type of permAnently deformable material
that is
capable of undergoing a gradual deformation as its melting point is reacheci
or sligbtly
exceeded. This "plastic matcrial" could even be some type of inetallic
substence in a
situation where the metallic material would deform at a low enough
temperatttre so as to
not harm the mold itself. The preferred material is a polyamide such as nylan,
although
many other types of polymer material catainly could be used to advantage.
Other
potential mateiials include: polyesters, vinyls, polystyrenes, polycarbonates,
acrylics such
as PMMA, polyorethanes, epoxides, phenolics, and acrylonitriles like
acrylonitrilebutadienestyrene (ABS). Of course, one important criterion is
that the
material which makes up the microneedles does not chemically react with sldn,
or with
the iuidic substance that is being transported through the hollow interiors
of the
microncedle array.
Figure 7 depicts a top mold-half, generally designated by the reference
numeral
110, of a second embodiment of the prescnt invenAtion in which the
mattufacturing method
for creating an arrsy of hollow miccnneedles is performed by a mioromolding
procedure.
The top mold-half 110 includes two "microholes" that have sloped side walls,
designated
by the referenae numerals 112 and 114 for the left-hand microhole 113, and by
the
reforence numerals 116 and 118 for the right-hand microhole 117. The
microholes 113
and 117 have a vettical (in Figurc 7) dimension referred to herein as a
distaace "L11".
Microholes 113 and 117 correspond to a pair of micropillars 122 and 124 that
aro part of a
bottom mold-half, generally designated by the reference number 120, and
illustrated in
Figure 8,
Referring back to Figure 7, the sloped side walls of the microhole 113 are
depicted
by the angles "Al l" and "A12," with respect to the veiYical. The side walls
of microhole
117 are also sloped with respect to the vertical, as illustrated by the angles
"Al3" and
"A14" on Figure 7. Since microhole 113 preferably is in a conical overall
shape, the
angle All will be equal to the angle A12; similarly for microhole 117, the
angle A13 will
1B
CA 02591168 2007-05-14
Wp 00174764 PCT/tJS00115612
be equal to the angle A14. It is preferred that all microholes in the top mold-
half 110
exhibit the same angle with respect to the vertical, which means that angles
Al l and A13
are also equal to one another. A preferred value for angles A11-A14 is in the
range of
zero (0) through forty-five (45) degsees. The larger the angle from the
vcrtical, the
S greater the trawna to the skin tissue when a nlicroneedle is pressed against
the skin. On
Figure 7, the illustrated angle Al 1 is apprnxitnately twelve (12) degrees.
Referring now to Figure 8, the bottom mold-half 120 includes a base 126 having
a
substantially planar top surface 128, upon which the two micropillars 122 and
124 are
mounted. Y"hese inicropillars ac+e preferably cylindrical in shape, and have a
diameter of
DI 1 and D12, respectively. The distance between the centerlines of these
micropillars is
designated as D13. Diameters Dll and D12 preferably are in the range 1-49
microns,
more preferably about 10 microns. The distance "D13" represents the separation
distance
between the center lines of micropillars 122 and 124, which preferably is in
the range 50-
1000 microns, more preferably in the range of 100-200 microns.
The two mold-halves 110 and 120 can be fabricated from metals using
microelectrnde-discharge machining techniques. Alternativoly, the molds could
be
fabricated from silicon or silicon carbide using integrated circuit processing
or
lithographic processing.
On Figtua 8, a thin plastic film, generally designatad by the reference
numeral
130, is placed on top of the micropillars and heated above the glass
transition temperature
of the plastic nmerial while the plastic material 130 rests upon the tops of
the pillars at
132 and 134, thereby causing the plastic material to become sufficient pliable
or "soft" for
pwposes of pennanently deforming the naterial's shape. Preferably, the
telnperaturc of
the plastic material will not be raised above its melting temperature,
although it would not
inhibit the method of the present invention for the plastic material to become
molten just
before the next step of the procedure, In Figure 9, the top mold-half 110 is
presscd
downward and begins to deFotnl the plastic film 130. While a pottion of the
plastic
material 130 temporarily resides above the micropillars at 132 and 134, a
larger anyount
of the plastic material is pressed downward directly by the mold top-half 110
at 140, 142,
and 144. As can be seen in Figure 9, the two mold halves 110 and 120 are
aligned so that
the microholes 113 and 117 correspond axially to the micropillars 122 and 124,
19
CA 02591168 2007-05-14
WO 00174764 PC?/USOO/15612
respectively. The two mold halves now begin to operate as a single mold
assembly,
generally designated by the reference numeml 100.
In Figure 10, the two mold halves 110 and 120 have completely closed, thereby
squeezing all of the plastic material 130 away from the tops of the
micropillars 122 and
124. At this point, the plastic mieroneedles are formed, and the mold and the
plastic
material are both cooled down.
The wall 112 and 114 of the first microhole 113 causes a side outer wall to be
formed out of the plastic rnatetial at 150 and 152. The corresponding inner
wall of the
tnieroneedle 182 is depicted at 160 and 162, which is caused by the shape of
the
micropillar 122. Since the outer wall is sloped, it will converge with the
inner wall 160
and 162, near the top points at 170 and 172, A similar outer wall 154 and 156
is formed
by the inner wall 116 and 118 of niiccohole 117. The itmer wall of the
microneedle 184 is
depicted at 164 and 166, aaW these inner and outer walls convcrge near points
174 and
176.
is Figurc 11 illushntes the microneedle array, geturally designaterl by the
reference
numeml 180, after the mold is removed from the plastic material 130. A lower
relatively
planar base remains, as illustrated at 140, 142, and 144. On Figure 11, two
different
microneedles are formed at 182 and 184. The angles formed by the walls are as
follows:
angle Al l by walls 150 and 160, angle A12 by walls 162 and 152, angle A13 by
walls
154 and 164, and angle A14 by walls 166 and 156. The points at the top if the
microneedles (designated at 170, 172, 174, and 176) are fairly sharp, and this
sharpness
can be adjusted by the ahape of the mold with respect to the microholes and
micropillar
Orientatlons.
The inner diameter ofmicroneedle 182 is designated by the distauce Dl l, and
the
inner diarneter of the microneedle 184 is designated by the distance J312, The
distance
between the centerlines of these microneedles is designated as D13. These
distances
correspond to those illustrated on Figure 8.
It is prefenred that all of the angles Al 1-A14 are equal to one another, and
that the
angles fall within the range of zero (0) to forty-five (45) degrees. The
preferred angle
really depends upon the strength of the matcrial being used to construct the
microneed3es,
CA 02591168 2007-05-14
WO 00/74764 PC"3YUSOO115612
in which a greater angle (e.g., angle A11) provides greater strcrogth.
However, this
amgular increase also causes greater trauma to the skin.
->~l Microneedle array 180 also includes a relatively flat base strueture, as
indicated at
the reference numerals 140, 142, and 144. This base sttucture has a vertical
thickness as
designated by the dimension L15 (see Figure 11). The micraneedle heig,ht is
designated
by the dimeitsion L12 on Figure 11. The haigbt must be su.flicient to
pcnetrate the sldn
through the stratuin ooraerun and into the epidermis, and a prefcnred
dimension for height
L12 is in the range of 50-3000 microns (although, certainly microneedles
shorter than 50
microns in length could be constructed in this manner-for use with skin
cosmetics, for
example). The thickness L15 can ba of any size, however, the important
criterion is that
it be thick enough to be mechanically sound so as to retain the microneedle
structure as it
is usad to penetrate the skin.
Referring now to Figure 12, a top mold-half 210 is combined with a planar
bottom
mold-half 240 to create an entire mold, generally dcsignated by the reference
nurneral
200. The top mold-half 210 contains an array of microholes with micropillars
at the
center of each of the microholes. For example, a microltole 213, having its
oonical wall at
212 and 214, is preferably concentric with a micropillar 222, and a microbole
217, having
its conical wall at 216 and 218, is pceferably concentric with a micrnpillar
224.
The tabricacion method used in conjunction with the mold 200 is referred to
herein
as "microembossing" for the reason that the bottom mold-half 240 is simply a
flat or
planar surface. This greatly simpl3fies the construction of this particular
mold. A thin
plastic til.m at 230 is placed upon the top surface 242 of this bottom mold-
half 240. In the
later steps, it will be seen that the plastic materia1230 is heated while the
top mold-half
210 is pressed down against the bottom mold-half 240.
Microhole 213 and micropillar 222 have an angular relationship as illustrated
by
the angles "A21" and "A22." A similar angular relationship exists for
microhole 217 and
micropillar 224, as ilhistrated by the angles "A23" and "A24." These angles
A21-A24
will preferably be in the range of zero (0) to forty-five (45) degrees from
the vertical. As
noted hereinabove, the greater the angle, tlae greater the transport rate,
however, also the
greater tr-auma to the skin tissue when used.
21
CA 02591168 2007-05-14
WO 00174764 PC'rlUSOOrl5612
Micropillar 222 prcferably has a cylindrical diape with an outer diameter
des-gnated at "D21," and micropillar 224 similarly has a preferred cylindrical
shape
having a diameter "D22." Diameters 1721 and D22 preferably are in the range 1-
49
micrans, more preferably about 10 microns. The distance "D23" raprescnts tho
sepamtion
distance between the center lines of mieropillars 222 and 224, which
preferably is in the
range 50-1000 microns, more preferably in the range of 100-200 microns.
The length of the micropillars from the bottom surface 228 of the top mold-
half
210 to the closed end of the microhol+;s at 215 and 225, respectively, is
designated as the
length "L21." The micropillars 222 and 224 are somewhat longer than this
length L21,
10. since they are to mate agai.nst the upper surfaae 242 of the bottom mold-
half 240, and
thcrefore are longer by a distance designated as "L25." In this rnanncr, the
microneedles
will be hollow throughout their entire length. The combined length of
dimensions L21
and L25 preferably will be approximately 150 microns.
The molds 210 and 240 will preferably be made from a metal, in which
microelectr+ode-diseharge machining can be used to fabricate such metallic
molds.
Alternatively, the molds could be fabrica#ed from silicon or silicon carbide,
for cxample,
using integrated circuit processing or lithographic procesaing.
Referring now to Figure 13, after the plastio material is heated above its
glass
transition temperature, thereby causing the plasstic material to become
sufficient pliable or
soft" for purposes of permanently defonning the material's shape. Preferably,
the
temperature of the plastic material will not be raised above its melting
temporature,
although it woWd not inhibit the method of the present invention for the
plastic material
to become molten just before the top mold 210 begins to be pressed down
against the
plastic material 230. This top mold movement begins to deform that plastic
material 230
such that it begins to fiil the microhoics, as illustrated at 232 and 234 (for
microhole 213)
and at 236 and 238 (for microhole 217).
Xn Figure 14, the top mold-half 210 has now been completely closed agairLst
the
bottom planar mold-half 240, and the plastic material 230 has now completely
filled the
microholes, as illustrated at 232, 234, 236, and 238. The shape of the plastic
material
now has a conical outer wall at 250 and 252, and a corresponding cylindrical
inner wall at
260 and 262, for the left-hand microneedle 282 on Figure 14. Conespondingly
for the
22
CA 02591168 2007-05-14
WO 00174764 PC'T/CTS0D/15612
right-hand microneedle 284, the plastic material shapG has an outer conical
wall at 254
and 256, as well as a cylindrical inner wall at 264 and 266. The conical outer
walls and
the cylindrical inner walls converge at the top points 270 and 272, and 274
and 276. The
bottom surface 228 of the top mold-half 210 causes a base to be formed in the
plastic
material 230 at the locations indicatcd by the reference numerals 244, 246,
and 248. Once
this shape has been formed, the mold and the plastic material are cooled down,
and then
the molds are separated so that the pl.astic microneedle array is dctached to
form the shape
as illustrated in Figure 15.
In Figure 15, a microneedle array 280 has been formed out of the plastic
material
230, which as viewed on Figure 15 depicts two microneedles 282 and 284. The
left-hand
mieroneedle 282 comprises an outer conical wall es viewed at 250 and 252, and
a hollow
interior cylindrical wall at 260 and 262. These walls converge at the top
points (as
viewed on this Figure) at 270 and 272, and the convergence angle is given as
"A21" and
"A22." The right-hand microneedle 284 comprises an outer conical wall 254 and
256 and
a hollow interior cylindrical wall 262 and 264. These walls converge at the
top points (on
this Figure) at 274 and 276, and the convergence angle is given as "A23" and
"A24."
Angles A21-A24 are preferably in the range of zero (0) to forty-five (45)
degrees.
Microneedle array 280 also includes a relatively flat base structure, as
indicated at
the referct-cti numerals 244, 246, and 248. This baso structure has a vertical
tlliclanm as
designated by the dimension L25. The tnicroneedle height is designated by the
dimcnsion
L22. The height must be sufticient to penetrate the skin through the stratum
comcum and
into the epidermis, and has a preferred dimension for use with inte,rstitial
fluids in the
raugc of 50=200 nzicrons (although, as noted above, much shorter microneedles
could be
constructed in this manner). The height L22 could also be a greater distance
for use with
other biological fluids, preferably in the range of 200-3000 microns. The
thickness L25
can be of any size, however, the important criterion is that it be thick
enough to be
mechanically sound so as to retain the microneedle structure as it is used to
penetrate the
skin,
The inside diameter of the hollow microneedles is illustrated as D21 and D22,
which correspond to the diameters of a cylindrical hollow opening. The
distance D23
23
CA 02591168 2007-05-14
Wo Qo/74764 PCTJUS00J15612
represents the separation distance between the centerlines of the two
rnioroneedles 282
and 284, in this array 280.
Figure l SA represents an alternative embodiment in which a rnic,roneedle
array
290 comprises "solid" microneedles 292 and 294, rather than hollow
microneedles as seen
at 282 and 284 on Figure 15. These solid microneedles 292 and 294 are fonned
by a
similar mold as viewed on Figure 12, but with the micropillars 222 and 224
removed
fmm this mold, and a change in shape of the rnicroholes 213 and 217. This
simple
change allows the solid microneedles to be fonnod within conical microholes
(not shown
on Figure 12), and produces a pointed conical shape, as exhibited by the outer
conical
wall 250 and 252 for microneedle 292, with a top pointed surface at 296.
Similarly, the
nsicraneedle 294 has a conical outer wall 254 and 256, with a similar top
pointed surface
at 298. The other dimensions and features of the solid microneedle array 290
can be
exactly the same as those features of the hollow rcticroneedle array 280 of
p'igure 15, or
the dimensions may be different since this is for a different application.
The holes 251, 253, 255, can be fabricated during the rpicnnstautping or
microembossing procedure via inclusion of appropriate micrapilIars located
adjacent to
the microholcs 213 and 217 in Figure 12.
Refcning to Figure 16, a mold 300 consists of two mold-halves 310 anci 340.
These mold-halves 310 and 340 are vittually identical in shape, and probably
in size, as
compared to the mold-halves 210 and 240 of the mold 200 on Figure 12. The main
dift'etence in Figure 16 is that these mold-halves are to be used in a
microinjection
procedure in which molten plastic material is injected from the side at 330
into the
opening between the mold-halves formed by the bottom surface 328 of the top
mold-half
310 and the top surface 342 of tiie bottom mold-half 340.
The mold struchn=e 300 is preferab2y made of a metallic materiai by a micro-
machining process, although it could be made of a scmiconductor material such
as silicon
or silicon carbide, if desired. On Figure 16, the plastic material 330 is
being filted from
the left-hand side in this view, and has already fifled a first rnicrohole 313
with plastic
material. The plastic material is illustrated as it is advancing, and has
reached the point at
the reference numeral 336. As tirne proceeds, the plastic material will reach
and fill the
24
CA 02591168 2007-05-14
WO oaV74764 PCT1USOUt156t2
second microhole 317, whicb has a conicai inner wall at 316 atad 318, and a
coaesponding nsicropillar 324. -
At the first microhole 313, the plastic material has fiiled the shape around a
micropillar 322 and within the conical walls of this rnicrohole 313, to form a
hollow cone
having an outer wall at 332 and 334. The piastic material will be forced
upward until it
reaches a top point as soen at the reference numerals 370 and 372. The outcr
conical
shape at 332 and 334 will converge with the interior shape of the micropillar
322 at an
angle designated by the angles "A31" and "A32," Microhole 317 also exhibits a
converging angular shape at "A33'" and "A34," which is the convergence angle
between
the conical walls 316 and 318 and the outer cylindrical shape of the
micropillar 324.
The sepsration betweeb the surl`aces 328 and 342 is given by the length
dimension
"L35," which will become the thickness of the planar face material that will
remain once
the mold is opened. The vertical dimension (in Figure 16) of the microholes is
given by
the dimension "L31" which prefcrably will create microneedles long enough to
penetrate
through the stratum corneum and into the epidermis, but not so long as to
penetrate all the
way to tho demzis when used with interstitial fluids, On the other hand, for
use with other
biological fluids, the microneedle lengtb will be grcater, prefcrably in the
range of 200-
3000 microns, so as to penetrate into the dermis.
Figure 17 illustrates the microneedle array, generally designated by the
reference
numeral 380. On Figure 17, two microneedles are illustrated at 382 and 384.
These
microneedies have a length "L32," which in theory should be exactly the same
as thc
dimension L31 on Figure 16, asswning the mold was prope,rly filled with
material, A
preferred distance for L32 is in the range of 50-200 niicrons.
The plastic maleria1330 }aas a planar base structure, as illustrated at 344,
346, and
348. The thicknGss of this base structure is the dimension L35. The
rnicroncxdies
themselves exhibit a conical outer wall at 350 and 352 fvr the left-hand
microneedle 382,
and at 354 and 356 for the right-hand microneedle at 384. Each microneedle has
a hollow
interior, as illustrated by the cylindrical surface 360 and 362 for
microneedle 382, and 364
and 366 for micrnneedle 384. These surfaces converge to form points (as
illustrated on
Figure 17) at 370 and 372 for microneedle 382, and at 374 and 376 for
rnicroncedle 384.
CA 02591168 2007-05-14
=
WO 00/74764 PC'X7USU0/15612
The convergcncc angle of thesc walls is designated by the angles A31-A34, and
preferably will be in the range of zero (0)-to forty-five (45) degrees.
The inner diameter of microncedle 382 is given by the dimension D31, and for
microneedle 384 is given by dimension D32. These dimensions preferably are in
the
range 1-49, more preferably about 10 microns. The separation distance between
the
center lines of the microneedles is given at ri33, which preferably is in the
range 50-1000
microns, more preferably in the range of 100-200 microns. The height L32 is
preferably
in the range of 50-3000 microns and, depending upon the convergence angle A31-
A34,
the bottom width of the conica] microneedics will vary depending upon the
exact
applicatioo for usage. In one preferred embodiment, this bottom dimension,
designated
by "D34" and "D3S," will be approximately twenty (20) microns. The vertical
thickness
at L35 will likely be made as thin as possible, howcver, the important
criterion is that it is
sufficiently thick to be mechanically sound to hold the rnicroneedle array 380
together as
a single stfucture during actual usage. It is likely that, for most plastic
materiais that
i5 might be used in this molding procedure, the dimension L35 will be in the
range of ten
(10) microns through two (2) mm, or greater.
The angular relationship between the microneedles and the corresponding planar
base surfa~ is preferably perpendicular, although an exaat right aagle of 90
degrees is not
required. This applies to all microneodle embodiments herein dosaribed,
including
microncedles 62, 64 and planar surfaces 30, 32, 34 of Figure 6, microneedles
182, 184
and plmar surfaaes 140, 142, 144 of Figure 11, nticroneedles 282, 284 and
planar
surfaces 244, 246, 248 of Figure 15, microneedles 292, 294 and plaaar surfaces
244, 246,
248 of Figure 15A, anicrnneedles 382, 384 and planar surfaces 344, 346, 348 of
Figure 17,
and microneedie 470 and planar surfaces 440, 446 of figure 21.
It will be understood that other methais of forming plastic micronecdles could
be
utilixed to create hollow microneedles in an array, without departitng from
the principles
of the present invention. It witl also be wnderstood that various types of
materials could
be used for such molding procedures, including metallic materials that might
be cast
using higher temperature dies of a similar shape and size, without departing
from the
principles of the present invention.
26
CA 02591168 2007-05-14
WO 00174764 PG"T/USOdl15612
It will be further undarstood that variations in dimensions and angular
rclatior-ships could be utilized to consttuct an array of hollow microneedles,
without
departing from the principles of the present invention. It will be still
farther understood
that the angular relationship between the microneedles and their planar basa
surface need
not be precisely p,crpcndieular (although tbat eoniiguration is preferred),
but could have
some variation without departing from the principles of the present invention;
the
microneedles also need not bc exactly parallel with one anothcr, even though
that
configurati,on is preferred.
It wili be yet firther understood that other nucroneedle shapes oould be usod
than
a cylir-drical shape, if desired, without departing from the principles of the
present
invention, p'or example, the nhspc for hoAow microneedles could prescribe a
circle,
ellipse, square, triangle, cresccnt or other arcuate path, or some other
goometric structure
for either the inner opcning or the outer peruneter. p'urthermore, the inner
opening's
shape could be different frorn the outer perimeter's shape.
Moreover, it will be unde,rstood that, with only simple modifications to the
molds,
an array of solid tnicroneedles could bc fabr;cated using the molding
techniques described
hareGin, without depafing from the principles of the present inventson. The
outer shape
for such solid microneedles could prescribe a circle, ellipse, square,
triangle, crescent or
other arcuate path, a star or other jagged perimeter, or some other geqmetric
structure.
Referring now to Figure 18, a procedure for forming dry etched miicroncedles
will
be described using an example of microfabrication (e.g., semiconductor
fabrication)
tecbniques. Starting with a single crystal silicon wafer at rcference numeral
400, it is
preferred to nse a double side po[ish wafer and to grow an oxide layer on the
entire outer
surface. In Figure 18, a cross-omtion of this wafer appears as a substrate
410, a top oxide
layer 412, and a bottom oxide layer 414. Any single crystal silicon wafer will
suffice,
although it is preferred to use a crystal structure l0Q=type wafer, for
re.asons that will be
explained below. A 110-type wafer could be used, however, it would create
different
angles at certain etching steps.
To create the sttvcture depicted in Figure 19, oertaan praaess steps musL
first be
performed, as described below. The first step is a pattern oxide stcp which is
performed
on the top side only to remove much of the top oxide layer 412, The pattern
used will
27
CA 02591168 2007-05-14
WO 00/74764 PCTR7S00115612
create multiple annular regions comprisRng two concentric circles each, of
which the
cross-section will appear as the rectangles 416 and 418 on Figure 19. In
perspective,
these annular-shaped features will have the appearance as illustrated on the
perspective
view of Fxgure 22 at the reference numerals 416 and 418. These ann.ular oxide
patterQs
are the initial stagea of the array locations of the muitiple micruneedles
that will be
formed on this substrate 410.
The next step is to deposit a layer of silicon nitride using a low pressure
vapor
deposition step, which will form a silicon nitride layer on both the top and
bottom
surfaces of the substrate 410. This appears as the uppernost layer 420 and the
bottommost layer 422 and 424. It will be undorukood that the bottommost layer
422 and
424 is one continuous layer at this step, although it is not illustrated as
such on Figure 19,
since a later stcp etches out a portion of the bottom side of the substrate
between the
layars 422 and 424.
Next in the process is a pattem bottom procedure in which a square hole is
patterned beneath the annulus 416, 418, which is not, directly visible on
Figure 19. The
square holes placed by the pattern bottom procedure are now used in a KOH
etching step
that is applied to the bottom side only of the substrate 410. This KOH etching
step
creates a window along the bottom of the substrate as viewed along the
surfaces 432, 430,
and 434 on p'igure 19. This window interrupts the oxide layer 414 along the
bottom of
substtate 410, and divides it (on Figure 19) into two segments 413 and 415.
This window
(or hole) also interrupts the silicon nitride layer into two segments (on
Figure 19) 422 and
424.
The slope angle of the etched window along surfaces 432 and 434 is 54.7
degrees,
due to the prefetred 100-type silicon material. Iftype-110 silicon material
was used, then
this slope would be 90 degrees. That would be fute, however, erystalline
silicon 100-type
material is less expensive than silicon 110-type mateiial. Affter the KOH time
etching
stcp has been completed, the silieon wafer will have the appearance as
depicted in Figure
19.
The next fabricatdon opexation is to perform a pattern top nitride procedure
using a
photoresist mask. This removes the entire upper silicon nitride layer 420
except where
the photoresist mask was located, which happens to be aligned with the upper
oxide
28
CA 02591168 2007-05-14
WO 00174764 PCTNSOO/15612
annulus at 416 and 418. Tbe remaining upper silicon nitride is indicated at
the reference
numeral 426 on Figure 20, although at this stage in the fabrication procedure,
the upper
surfaoc will still be a planar surPace at the level of the oxida layer 416 and
418, across the
entire horizontal dimension of Figure 20.
The next fabrication step is to pc,rform a deep roactive ion etch (DRIE)
operation
on the top surface of the substrate 410, which will etch away a relatively
deep portion of
the upper substratc except at locations where the silicon nitride layer still
remains, i.e., at
426. In this DRIE procedure, it is pt+eferred to remQve approximataly 50-70
microns of
material. After that has occurred, the remaining photoresist mask material is
removed.
This now exposes the top silicon nitride layer 426.
The next fabrication stcp is to oxidize all of the bare silicon that is now
exposed
along the outer surfaoes. This will form a layer of silicon dioxide at
locations on >;'igure
20, such as at 440, 442, 444, 446, 452, 450, and 454. 'The outer silicon
nitride layers at
426, 423, and 425 are not oxidized. The outer silicon nitride layers 423 and
425 are
essentially the sarne stn-etures as layers 422 and 424 on Figm 19, although
the silicon
dioxide layers 452 and 454 are now formed above these "pads" 423 and 425. It
is
preferred that this oxidation be a*n' im$l amount, just enough for a future
DRIE masking
procedure, and that the oxidized thiclrness be approximately 5,000 Angstroms.
At this
point in the fabrication procedure, the silicon wafer has the appearance of
that depicted in
Figure 20.
The next step in the fabrication procedure is to retnove the silicon nitride
layer on
the top, which will remove the layer at 426 as seen on h'igure 20. T7iis will
expose a
circular regiort in the very center of the annulus such that pure silicon is
now the
outermost material on the top side of the wafer, ARar that has occutrcd, a
deep reactive
ion etch operation is perfonned to create a through-hole at the rcference
numeral 460 on
Figure 21. After this step has been performed, there will be pure silicon
exposed as the
inner wall of the through-hole 460. Therefore, the next step is to oxidize the
entire wafer,
which will placc a thin cylindrical shell of silicon dioxide around the inner
diameter of
thraugh-hole 460, and this oxidized layer is viewed on Figure 21 at 462 and
464.
After these steps bave been performod, a microneedle 465 is the result, having
an
outer diameter at "D41," and an inner diameter through-hole at "A42." It is
preferred that
29
CA 02591168 2007-05-14
WO OOr14764 PCTlUSeo/15617
the inner diameter D42 have a distmwe in the range of 5-10 microns. The height
of the
micronacdle is given at the dimension "I.41," which has a preferred dimension
in the
range of 50-200 rnicrorts. On Figure 21, the substrate 410 has been divided
into halves at
410A and 410B. Tn addition, the bottom oxide layer 450 has been dividcd in
halves at
450A and 450B.
The bottom chamber formed by the slopod surfam 452 and 454, in combination
with the horizontal surfaces 450A and 450B, act as a smsll, recessed storage
tank or
chamber generally indicated by the reference numeral 470. This chamber 470 can
be used
to store a fluid, such as insulin, that is to be dispensed through the
cylindrical opening 460
in the hollow microneedle 465. At the seale of Figure 21, this chamber is not
very large
in overall physical volucne, and it normally would be preferred to
interconnect all of such
chambers for each of the microneedles in the overall array so that a common
fluid source
could be ttsed to dispense fluid to each of these chambers 470. Furthermore,
there may be
a need to dispense a physically much larger volume of fluid, and it also may
be desirable
to provide a pressure source, such as a pump. In such situations, it may be
preferable to
have an extennal storage tank that is in conununication with each of the fluid
chambers
470 on the wafer that is used to make up the array of microneedles, such as
niicroncedle
465.
Figure 22 depicts an array of microneedles an subsirate 410, and also
illustrates a
magnified view of some of these microneedles 465. Each microneedle 465
exhibits a
cylindrical shape in the vertical direction, and has an outer diameter D41, an
aimular
shaped upper surface at 416 and 418, and a through-hole at 460. Each of the
microneedles 465 extends out from the planar surface 440,ofthe substrate 410.
As can be seen in Figure 22, substrate 410 can either be made much larger in
height so as to have a very large internal volume for holding a fluid
substance, or the
substrate itself could be mounted onta a different material that has some type
of fluidic
opening that is in communication with the chambers 470 of the individual
rnieroneedles
465.
lt will be understood that other semiconductor substances besides silicon
could be
used for the fa'brication of the array of microneedles depicted on Figure 22,
without
departing from the principles of the present invention. Furthermore, the
microneedles
CA 02591168 2007-05-14
WO 00R4764 FGT/US{lOf15612
could be coated with materials such as silican carbide to impart additional
strength.
Moreovcr, other microneedle shapes could be used tban a cylindrical shape with
an
annular top surface, and in fact, the top surfaace of such microneedles could
be sloped to
create a sbarper edge, if desired, without departing from the principles of
the present
s invcntaon.
It will also be understood that the pref'erred dimensions discussed
hereinabove are
only preferred, and any microneedle length or diameter t.bat is appropriate
for a particular
chemical fluidic compound and for a particular skin structure could be used
without
departing frvm the principtes of the present invention. As discussed above,
for use with
interstitial body fluids it is preferred that the microneedle penetrate t1-
rough the stratum
corneum into the epidettnis, but not penetrate into the den,nis itself. This
means that sucb
micraneedles would typically be no longer than two hundred (200) microns,
though they
must typically be at least fifty (50) microns in length. However, for use with
other
biological fluids, a useful length is in the range of 200 microns - 3 nam, and
more
preferably in the range of 200-400 microns. Of course, if cosmetic
applications were
desired, then the microneedle could be much shorter in length, even as short
as one (1)
micron. Finally, it will be understood that any size or shape of fluid-holding
chamber
could be used in a drug-detivery system, which will be further discussed
hcreinbelow. In
addition, for a body-fluid sampling system, a fluid-holding chamber would also
preferably be in communication with the through-holes 460 of each of the
microncedles
465.
Figure 23 depicts an electrophoretieally enhanced body-fluid sensor that is
based
upon a hollow microneedle array, generally designated by the reference numera]
500.
Sensor 500 includes a plurality of microneedles 530, which are each hollow,
having a
vertical operting throughout, as indicated at 532. A fluid chamber 510 is in
commtmication with the hollow portions 532 of the array of microneedles 530.
Of
course, other fluid driving mechanisms could be used as well, such as passive
diffusion
(e.g., time release), instantaneous injection, pressure, vacuum, or
ultrasound.
Fluid chamber 510 is constructed of a bottom (in Figurc 23) planar surface 512-
-
which has openings that are aligned with the micr+aneedles 530--a left
vertical waIl 514,
and a right vertical wall 516. The top (or ceiling) of the fluid chamber 510
is made up of
3]
CA 02591168 2007-05-14
WO 00/74764 PC'Tl1J800/15512
a planar material which is divided into individual electrodes. The middle
electe+ode 525 is
part of the fluid sensor, and makes it possible to measure a cunvnt or voltage
within the
fluid cbamber 510. Elcctrodes 520 and 522 are electrically connected to one
another (and
can be of a single gtructure, such as an annular ring) so as to act as the
electrophoretic
electrodes (i.e., as eithe.r an anode or a cathode) that facilitate the
transport of fluid
through the hollow microneedies 530 from the skin into the fluid chamber 510.
The height of the fluid chamber stracture is designated as "L50," which could
be
any reasonabic diineasion that is large enough to hold a sufficient volumc of
fluid for a
particuler applieation, Of course, if desinod, the fluid chamber 510 could be
connccte.d to
a much larger external reservoir (not shown), and a pump could even be used if
pressure
or vacuum is desired for a particular application.
The layer 540 represents the stratum corneum, the layer 542 represents the
viable
epidermis, and the largest layer 544 represents the dermis, which contains
nerves and
capillaries.
1S The applicution of mioroneedles 530 into the stratum corneutn 540 and
epidcrrnis
$42 decreases the electrical resistance of the strariun eorneum by a factor of
approximately fifty (50). The applied voltage, therefore, during
electropboresis (e.g.,
iontophoresis) or electroosmosis can be greatly reduced, thereby resulting in
low power
eonsumption and improved safety. lontophoresis provides the necassary means
for
molecules to travel through the thicker dermis into or from the body. The
combination of
the microneedles and the eleetric field that is applied bctween the electrodes
520 and 522
(acting as an anode, for example) and a remotely placed electrode (e.g.,
electrode
assembly 505, viewed on Figure 25, and acting as a cathode, for example)
provides for an
incresse in permrahility for both the strahnn corneum and the deeper layers of
skin.
While the transport improvement in stratum corneurn is mostly due to
microneedle
piercing, the electrophoresis provides higher transport rates in the epidermis
and dermis.
This is not only true for small sized molecules, but also for the larger and
more complex
useful molecules.
The body-fluid sampling senror 500 can be used for a continuous non-invasive
measurcment of blood glucose level, for example. Glucose is extracted through
the skin
by reverse iontophoresis, and its concentr8tion is then characterized by a
32
CA 02591168 2007-05-14
WO 00174764 PCT/17S0U115612
bioelcctrochemical sensor. The sensor comprises the chamber 510 that is filled
with
hydrogel and glucose oxidase, and the electrode 525, The glucose znolecules
are moved
from the body by the flow of sodium and chloride ions caused by the applied
electric
potential. The detection of the glucose concentration in the hydrogel pad is
performed by
the bioeiectrochemical sensor.
An alternative embodiment 550 is depicted in Figure 24, in which the
microneedles 580 am solid, rather than hollow. A lluid-filled chamber 560 is
provided
and also comprises hydrogel i"illed with glucose oxidase. The chamber 560 is
made of a
bottom wall 562 that has openings proximal to the individual microneedles 580,
in which
these openings are designated by the reference numerai 585. Chatnbcr 560 also
includes
side walls 564 and 566, as weli as electrodes S70, 572, and 575.
The electrode 575 is constructed as part of the bioelecerochemical sensor. The
electrodes 570 and 572 act as the electrophoretic el ctrodes, acting either as
an anode or
cathode to set up an electric current through the skin which flows to a
semotely-attached
(to the skin) electrode (e.g., electrode assembly 555, viewed on Figure 26).
As in the sensor 500 of p'igurc 23, the tmnsport rate of fluids is enhancad by
not
only the piercing effect of the microneedles 580, but also tlhe electric field
inducing a
curt-cnt through the skin. In the glucose sampling example, glucose is
attracted into the
chamber 560, and its concentration is measured by the bioelectrachomical
sensor.
The height of the fluid chamber stxucture is designated as "L55," which could
be
any reasonable dimension that is large cnough to hold a sufficient volume of
fluid for a
particular application. Of course, if desired, the fluid chamber 560 could be
connected to
a much larger external reservoir (not ahown), and a pump could even be used if
pressure
or vacuum is desired for a partioular applicatiorn,
Figure 25 depicts an electrophoretic electrade assembly that is based upon a
hollow microneedle array, generally designated by the reference numeral 505.
Electrode
assembly 505 includes a plurality of microneedles 531, each being hollow and
having a
vertical opening throughout, as indicated at 533. A fluid chamber 511 is in
communication with the hollow portions 533 of thc array of microneedles 531.
Fluid chamber 511 is constructed of a bottom planar surface 513-which has
openings that are aligned with the microneedles 531--a left vertical wa11515,
and a right
33
CA 02591168 2007-05-14
WO 00174764 FCTlCI$00/15622
vertical wal15 17. The top (or ceiling) of fluid chamber 511 is made of a
planar clectrode
material 526. The eleetrode 526 is to be electrically connected to a low-
current voltage
source (not shown on Figure 25), either through a subsirate pathway (such as a
integrated
circuit trace or a printed circuit foil path) or a wire (also not shown on
Figure 25).
The height of the fluid chamber 511 is given by the dimension "L52," which can
be of any practical9ize to hold a suffficient amount of hydrogel, for example,
to aid in the
conduction of current while actirlg as the electrode. In electrode assembly
505, the fluid
within chambcr 511 preferably would not be electrically charged.
As can be seen in Figure 25, the hollow microneedles 531 penetrate the stratum
corneum 540 and into the viable epidermis 542. The mieroneedles 531 preferably
will
not be sufficiently loug to penetrate all the way to the dermis 544.
An attcrnative embodiment 555 is depicted in Figure 26, in which the
microneedles 581 are solid, rather than hollow. A fluid chamber 561 is
provided and
preferably is filled with hydrogel (which is not electirically charged).
Chamber 561 is
y5 made of a bottom wall 563 that has openings proximal to the individual
microneedles
581, in which these openings are designated by the reference numeral 586.
Chamber 561
also includes side walls 565 and 567, as well as a top (or ceiling) electrode
576. The
electrode 576 may act as a cathode, for example, in a situation where
electrode assembly
555 is being used in conjunction with a body-fluid sensor, such as sensor
assembly 550
viewed on Figure 24, in which its electrodes $70 and 572 may act, for
exxample, as an
anode. The heig,ht "L57" of fluid chamber 561 could be any rcasonable
dirnension that is
large enough to hold a sufficient volume of the hydrogel to enhance the fluid
flow via the
electrie field between the respective anode and cathode of the system.
Figure 27 illustrates a portion of a human arrn and hand 590, along with a
drug
delivery electrode assembly 500 atYd a second electrode assembly 505. Both
electrodes
are attachod to the sldn of the human user, via their microneedles, such as
the hollow
microneedles 530 (viewed on Figure 23) and the hollow microneedles 531 (viewed
on
Figure 25).
Since an electrical voltage is applied between the two electrode assemblies
500
and 505, it is prefcrred to use a low current power supply, generally
designated by the
re#'erence numeral 596, that is connecred to each of the electrodes via a wire
592 or a wire
34
CA 02591168 2007-05-14
WO 00114764 PGTIL1S0D115612
594, respectively. It will be understood that any type of physical electrical
circuit could
be used to provide the electrical conductors and power supply necessary to set
up an
appropriate eleotrical potential, without departing &om the priaciples of the
present
invention. In fact, the elcctrode assemblies and wiring, along with an
associated power
supply, could all be contained on a single apparatus within a substrate, such
as that
viewed on Figures 30 and 31 herein, or by use of printed circuit boards.
Figure 28 depicts an electrophoretically enhanced fluidic drug delivery
apparatus
that is based upon a hollow microneedle array, genarally designated by the
reference
numeral 600. Diug-delivery apparatus 600 inehmdes a plurality of microneedles
630,
which are each hollow, having a vertical opening throughout, as indicated at
632. A fluid
chamber 610 is in communication with the hollow portions 632 of the array of
microneedles 630.
p'luid cbamber 610 is canstructed of a bottom (in Figetre 28) planar surface
612-
which has openings that ara aligned with the microneedles 630- a left vertical
wall 614,
and a right vertical wa11616. The top (or ceiling) of the fluid chamber 610 is
nlade up of
a planar material 620 that acts as an electrode. Electrode 620 is part of the
drug delivery
apparatus, and makes it possible to induce a currcnt flow through fluid
chamber 610.
Electrodes 620 and 622 are connected so as to act as the electrophoretic
electrodos (i.e., as
eithcr an anode or a cathode) that facilitate the transport of fluid thrQugh
the hollow
microneedles 630 from the fluid chamber 610 into the skin.
The height of the fluid chamber strnet.ure is designated as "L60," which could
be
any reasonable dimension that is large ennugh to hold a sufficient vohmle of
fluid for a
particular drug delivery application. Of course, if desired, the fluid chamber
510 could be
connected to a much iarger external reservoir (not shown), and a pump could
even be
used if pressure or vacuum is desired for a particular application.
The layer 540 represents the stratum corneum, the layer 542 represents the
viable
epidermis, and the largest layer 544 represcnts the dermis, which contains
netves and
capillaries.
The appiication of microneedles 630 into the stratum corncum 540 and
epidernlis
542 decresaes the electrical resistance of the stratum corneurn by a factor of
approximately fifty (50). The applied voltage, themfore, dur;ng
electroghoresis (e.g.,
CA 02591168 2007-05-14
WO 00l14764 pCTNS00115612
lontophoresis) ean be greatly reduced, thercby resulting in ]ow power
consumption and
improved safety, Iontophoresis provides the ncccssary means for molecules to
travel
thraugh the thicker dermis into or from the body. The combination of the
tnicronerdles
and the electric field that is applied between the electrodes 620 and 622
(acting as anodes,
for example), and another electrodc (e.g., electrode assembly 505, acting as a
cathode)
that is attached elsewhore on the skin of the user, provides for an increase
in penmeability
for both the stratum eomeum and the deeper layoxs of s1dn. While the transport
improvement in stratum corneum is mostly due to microneedle pieroing, the
electrophoresis provides higher transport rates in the epiderniis and d=rris.
This is not
only true for snn,al] sized molecules, but also for the larger and more
complex useful
rnolecules.
The drug delivery appamatus 600 can be used for a continuaus non-invasive
medical device that caa eontinuously deliver a fluidic drug through the sk'vn
and into the
body. For example, insulin could be delivered to the blood st7cam via the
micronoedles
531, through the stratum corneum 540 and epidermis 542, and also into the
dermis 544
where the insulin would be absorbed into the capillaries (not shown).
An aLternative embodiment 650 is depicted in Figure 29, in which the
miaroneedles 680 are solid, rather than hollow. A fluid-fxlled chamber 660 is
provided
and also Gontains hydrogcl. Chamber 660 is madc of a bottom wall 662 that has
openings
proximal to the individual micrbneodles 680, in which these openings are
designated by
the reference numcral 685. Chamber 660 also includes side walls 664 and 666,
as well as
electrodes 670, 672, and 675.
The electrode 675 is constructed as part of the bioelectrochemical sensor. The
electnides 670 and 672 act as the electrophoredc electrodes, acting either as
the anode or
cathode to set up an electric current through the skin, in conjtmction with
another
electrode assembly (such as electrode assembly 655, viewed on Figure 26)
placed
eUwwhere on the user's skin.
As in the drug delivery apparatus 600 of Figure 28, the transport rate of
fluids is
enhanced by not only the piercing effect of the microneedles 680, but also the
electric
field inducing a current through the skin. In the insulin dispensing example,
insulin is
36
CA 02591168 2007-05-14
WO 06114764 PCTJU8015612
repelled from the chamber 660, and therafore, flows out through openings 685
proximal
to microncedles 680, then into the usea's skin.
The height of the fluid chamber structuro is designatod as "L65," which could
be
any reasonable dimension that is large enough to hold a sufficient volume of
fluid for a
particular application. Of cotase, if desired, the fluid chamber 660 could be
oonnected to
a much larger external reservoir (not shown), and a pump could even be used if
pressure
or vacuum is desired for a particular application.
Figure 30 depicts a closed-loop drug-delivery system gtnerally dcsignsted by
the
reference numeral 700. This closed-loop system 700 includes a pair of
electrophoretic
pads, gcnerally designated by the reference nuinecals 500 and 505, which each
include an
array of microneedles for fluid sampling. Pad 500 comprises a sensor assembly
(as
described heeinabove with respect to Figure 23), and pad 505 comprises an
eleetmde
assembly (as described hereinabove with respect to Figure 25).
Closed-loop system 700 also includes a pair of clectrophoretic pads, generally
t5 designated by the reference numerals 600 and 605, that each include an
array of
microneedles for drug delivery. Pad 600 comprises a drug dclive,ry apparatus
(as
described horeinabove with reVect to Figure 28), aad pad 505 comprAes an
electrode
assembly (as described hereinabove with respect to Figure 25). Of course,
electrophoretic
pads having solid rnicroneedies could instead be used, such that pads 500 and
600 (with
hollow micronoedies) could be replaced by pads 550 and 650 (with solid
microneedles),
and pad 505 (with hollow microneedles) could be replaced by a pad 555 (with
solid
taicroneedles).
Pads 500 and 600 are mounted to a substrate 710, which can be made of either a
solid or a somewhat flexible material. Within substrate 710 preferably resides
a reservoir
712 (within the Substrate 710) that holds the fluid which is to be dispensed
through the
microneedles of pads 600. Reservoir 712 could be made up of individual "small"
chambers, such as a largc number of chambers 610 that are connected to a
source of
fluidic drug.
It will be understood that the reservoir 712 preferably is completely
contained
within substrate 710, and carueot be sean fmm this view of Figure 31. As an
alternative,
however, a fluid channel (such as a flexible tube at 730) could be con3nected
into substratc
37
CA 02591168 2007-05-14
WO 001741164 PCT/US00/15612
710 and, by use of a pump (not shown), fiuther quantities of the fluid could
be providod
and dispensed through the microneedies of pads 600, using fluidic pressure.
Figtue 31 illustrates the opposite side of the closed-loop system 700. A
controller
720 is mounted to the upper surface (in this view) of substrate 710.
Controller 720
preferably comprises a typc of microchip tbat contains a central pr+ocessing
unit that can
perform numeric calculations and logical operations. A microprocessor that
executes
sottware instructions in a sequential (or in a parallel) maaner would be
sufficient. A
microcontroller integrated circuit would also suffice, or an ASIC that
contains a
microprocessor circuit.
Adjacent to contraller 720 is an electrophoretic power supply with a battery,
the
combination being generally designated by the reference numeral 722. In
addition, a
visual indicator can be placed on the surface of the subsirate, as at 730.
This visual
indicator could give a dirccf reading of the quantity of interest, sach as
glucose
conaentration, or some other body-fluid parameter. The visual indicator
preferably
comprises a liquid crystal display that is capable of displaying atphanumeric
characters,
including nutnbers.
While a pumping system that creates fluid pressure could be used for
dispensing a
fluidic drug into a body through hollow microneedles, such as emplaced on pads
600, in
many instances it is preferred to use an electrophoresis method to enhance the
delivery of
the drugs through the microneedles. As discussed hereinabove, application of
microneedles can decrease the electrical resistance of the stratum corneum by
a faetor of
fifl,y (50), and so the voltage necessary to facilitate electrophoresis ean be
greatly reduced,
improving safety and requiring much less power conaumption. By use of the
electrophoresis, the molecules making up the fluid drug will travel through
the tlvicker
derrnis into or from the body, and the cornbination of both transport-
enhancing methods
provides an increase in permcability for both the sirat.urn comeum and the
deeper layers
of the skin. The transport improvement in the st<-atum comeum is mostly due to
microneedle piercing, although the electrophoresis provides higher transport
rates in the
e.pidexnnis and dermis.
The closed-loop drug-deiivery system and fluid-sampling system 700 can be used
for continuous noninvasive maasurement of blood glucose level by extracting,
via reverse
39
CA 02591168 2007-05-14
WO 0o114764 pcrnrsoa15612
iontophoresis, glucose through the skin and measuring its concentration by the
bioelectrochemical sensor (such as tht sensor constructed of the hydrogel
chamber S 10
and sensor electrode 525, along with the controller 720). The hydrogel pads
containing
microncedles (i.e., pads 500) enhance the reverse iontophoresis to move
glucose
molecules from the body by the flow of sodium and chloride ions, which are
causeA by
the applied electric potential via electrodes 520 and 522. Once the glucose
concentration
is measured within the hydrogel pads 500, the proper amount of insulin, for
example, can
be dispensed through the other pair of pads 600 that make up part of the
closed-loop
systern 700.
As discussed hereinabove, drug delivery is performed by applying an electric
potential between two rnicroneedle array electrodes. One of the electrodes is
filled with
an ionized drug (such as insulin), and the clsarged drug molecules move into
the body due
to the electric poteYttial. Controller 720 will detemtine how much of a drug
is to be
dispensed through the microncedle array 600 at any particular time, theroby
making the
closed-loop system 700 a"arnart" drug-delivery system.
This smart drug-deiivery system can be used as an artificial pancreas for
diabetes
patieuts, as a portable hormonatlierapy device, as a portablo system for
conti.nuous out-
patiGnt ehemothei'apy, as a site-spoeifac analgesic patch, as a teraporary
andlor rate-
controlled nicotine patcli, or for many other types of drugs. Such systcrns
could be made
as a disposable design, or as a refillable design.
It will be undergtood that the closod-loop system 700 cari be used in many
applications, including as a painless and convenient trsnsdennal drug-delivcry
system for
continuous and controlled ouipatient therapies, a painless and convenient body-
fluid
sampling system for continuous and progcwnmed outpatient body-fluid
monit.vring, as a
high-rate transdermal drug delivery system, or as a high-accuracy transdermal
body-fluid
sampling system. More specifically, the closed-loop system 700 of the prosent
invention
can be used as a portable high-accuracy painless sonsor for outpatient blood
glucose-level
monitoring, as a portsble system for continuous or rate controlled outpatient
chemotherapy, as a temporary and rate controlled nicotine patch, as a site-
spec'if"ic
controlled analgesic patch, as an exter,nalty attached artificial panereas, as
extemally
attached artifieial endocrine glands, as tomperature-controlled fever-reducing
patches, as
39
CA 02591168 2007-05-14
WO 00174764 P'CT/US00/15612
heart rute-controlled nitroglycerin high-rate transdeimal p8tohes, as
temporari3y controlied
hormonal high-rate transdermal patches, as ereatile dysfunction treatment high-
rate
transdermal patches, and as a concinuous eocurate blood-analysis system.
Another use of
the olnsed-loop system 700 of the present invention is to fonm a portable drug
dclivery
systern for outpatient delivery of the following drugs and thc.rapeutic
agcnts, for example:
centrgl nervous system therapy agents, psychic cnergizing drugs,
tranquilizers,
antieonvulsants, muscle relaxants and anti-parkinson agents, smoking cessation
agents,
analgetics, antipyretics and anti-inflammatory agents, antispasmodics and
antiulcer
agents, antimicrobials, antimalarias, sympathomimetric patches, antiparasitYc
agents,
neoplastic agents, nutritional agents, and vitamins,
It will be understood that various materials other than those disclosed
hereinabove
can be used for constructing the closed-loop system 700, and for construct#ng
individual
body-fluid sampling sensors and individual drug-delivery systems. Such other
materials
could include diamond, bio-compatible metals, eeramics, polymers, and polymer
composites, including 1'Y'REX , It will yet be further understood tlv.t the
electrophoretically/mieroneedle-enhanced transdormal method of transport of
the present
invention can also be combined with ultrasound and electraporation, in order
to achieve
high-rate drug delivcry into individual cclls.
It will also be understood that tho length of the individual microneed.les is
by far
the most important dimension with regard to providing a painless and bloodless
drag-
dispensing systetn, or a painless and bloodless body-fluids sampling systan
using the
opposite diicection of fluid flow. While the dimensions discussed heceinabove
are
preferred, and the ranges discussed are normal for human skin, it will further
be
understood that the cnicroneedle arrays of the present invention can be used
on skin of any
other farm of living (or even dead) creatures or organisms, and the prefcmd
dimensions
may be quite different as compared to those samc dimensions for use with human
skin, all
without departing from the principles of the presealt invention.
It yet will be understood that the chemicals and materials used in the molds
and
dies can be quite differerrt than those discussed hereinabove, without
departing from the
principles of the present inventiaa Further, it will be understood that the
chemicals used
in etching and layering operations of microfabrication discussed above could
be quite
CA 02591168 2007-05-14
WO 90/74764 PCY/i7B00/15612
diffcrcait than those discussed hereinabove, without departing fram the
principles of the
present invention. -
Figure 32 illustrates another alternative embodiment of a hollow microneedle,
generally designated by the reference n,umeral 800. The main body of the
niicroneedle
800 has a generally cylindrical shape, as indicated by its outer surface at
802, A generally
circular opening c.reates a hole at 806 thrQugh which fluids can pass. The
cylindrical
shape is preferably maintained throughout the length of microneedle 800, so
that its
bottom profile would also maintain a generally circular shape, as depicted at
810. Of
course, minor variances in this shape could be utilized without departing from
the
principles of the present invention, such as an elliptical shape for its cross-
section (rather
than a circular shape), for example.
The genera.l cylindrical shape is preferably maintained also at the top
portion, as
seen by the outer wall at 808. The top surfaca at 804 will have the fonn of a
pair of
conccntric circles, in situations where the opening 806 is circular. The
bottom portion at
iS 810 of microneedle 800 is abutted to a base element having a geuerally
planar surface ai
805. In a preferred mode of construction, microneedle 800 and the surfacc 805
would be
of a unitary construction, i.e., it would be formed froin a single piece of
material. Tlus
single piece of material would preferabiy be a molded plastic or like
materiai, or a cast
metal or like material. Of course, composite materials could also be utilized.
One primary advantage of the shape of microneedle 800 is that it has a pair of
shatp edged projections at 820 and 830 that aid the penetration of the outer
surface (i.e.,
stratum corneum) of the skin, thereby requiring less forecr to be applied when
using an
axray of such microneedles 800. Each edgod projection or blade 820, 830 has a
cross-
sectional shape that is genarally triangular when viewed from the top of
microncedte 800
(see Figure 33). The oxaot shape of the triangle will depend upon the strength
requirements of each of the blades 820, 830, the material used to construet
rtticroneedle
800, and the amount of skin damage that is allowable in a partieular usage
application.
The prcferred cross-sectional shape is that of an isosceles triangle having a
base angle in
the range between I and 45 . Of course, a rounded contour could be used
instead of
straight walls for the blade snrfaces, without departing from the principles
of the prescnt
invention.
41
CA 02591168 2007-05-14
WO 00/74764 PCTIpS00115612
The illustrated blade 820 has an upper generally ttiargular surface at 822,
and onc
of its side walls is represcnted by the planar surfaee at 824, as seezi on
p'igurc 32. A
sin2ilar planar wall is on the opposite side at 836 (sce Figure 33), and the
junction of these
two planar walls 824, 826 forms a generally sharp edge, as depicted at the
reference
numeral828.
The second protrusion or blade 830 is similarly formed of two generally planar
side walls at 834 and 836 (see Figure 33), wiiich also join at a generally
sharp edge at
838. The upper surface of the blade 830 is depicted at 832 as having a
generally
triangular shape, in the illustrated embodiment.
It will be understood that either less or more than two sharpened blade
projcctions
could be utilized in the microneedle 800 of Figure 32 without depardng from
the
principles of the present invention, although the two blades 820 and 830 ara
an optimal
design.
As illustrated on Figure 33, the inner diameter of the opening 806 is depicted
at
the referenee numeral 842, and the outer diameter of the microneedle 800 is
depicted at
the reference numeral. 844. The size of the outer diameter of microneedle 800
is very
important as to its pCnetratitig capabilities into the skin, whereas the inncr
diameter 842 is
of lesser importance in that regard. However, the inner diameter 842 tnust be
large
enough to easily pass the desired molecules of the fluid to be passod
therothrough.
Figure 34 illtisttates a similar hollow microneedle, generaliy designated by
the
reference tttuneral 850. This alternative embodiment microneedle 850 also
includes two
longitudinal blade stiucturos at 870 and 880, and also is of a generally
cyliudrical shape
tiu+oughout most of its length from its base element's i>ottom surface at 855
to its top
surface at 554. The opening at 856 is also generally circular in situations
where the
microneedle 850 is of eylindrie,al shape. Of course, the overall outer shape
of the
microneedle 850 and the inner shape of the opening 856 could be somewhat non-
circular
(such as an ellipse) witbout departing from the principles of the present
invention.
In Figure 34, microncedle 850 could be constructed of a moldccl plastic or a
cast
metal material, but in this patticular representation the microneedle 850 is
constiucted
using semiconductor fabrication techniques. The first blade 870 has a
generally planar
side wall at 874, aiid in conjunction with a siniilar side wall not shown on
Figure 34,
42
CA 02591168 2007-05-14
WO W4764 rCr/tasoon5612
forms a generally sharp edgc at 878. The cross-section profile of this blade
stractxve 870
is seen at 872, as having a generally isosceles triangular shape, although
more rounded
side walls could bc utilized without departing from the principles of the
present invention.
On Figare 34, this sharp edge 878 does not continue all the way to the bottom
surface 855
of the microncedle base sttucture, but instead continues down to a point where
the blade
structure discontinues, as illustrated at 862. This could be utilized to
create a greater yield
of microneedle stiucttucs using seniiconductor fabrication techniques, or
could be utiiized
to create a structure having greater mechanical strength near the bottom areas
(e.g., at the
side wall area 864) of the microneedie 850. When using this type of shape for
the
structure oi'microneedle 850, the outer diameter of the microneedle has the
form shown at
860 as it joins the planar bottom surface 855. This shape at 860 could be
generally semi-
circular, but also could be of a larger diameter to provide greater mechanical
strength than
the outer diameternear the top surface 854 of rnioronoedle 850.
The second blade 880 has a similar top profile at 882, and a simiiar sharp
odge at
ZS 888. The side wall structare near the bottom of the second blade 880 is not
viewable in
Figtue 34, but can be inferred from the shape of the bottom sidewall at 864.
Other variatioms in shape of the microneedle structures depicted in Figures 32
and
34 could be utilixed without departing from the principles of the preseat
invention. The
primary goals are to create machanically sound structures that can penetrate
the stratum
corneum of human skin (or other type of animal or even plant slan), and the
sharp
Iongitudinal blade structures are a great improvement over such hollow
mioroneedles that
do not have these side blades, enhancing penetration of drugs through the
skin. Tt wili be
understood that the microneedle sttvcttues depicted in Figures 32 and 34 cauld
be
constructed of any materials and by any type of fabrication techniques,
without departing
from the principlcs of the present invention.
Another variation in the hollow microneedles depicted on Figures 32 and 34
would be to have a top surface that is not generally flat, but instead has a
arcuate or
parabolic top surfacc as seen from orie of the sides of the microneedle
structure. This type
of structure could either be machined, or could be generated during de-
molding, as
illuserated in Figures 57A and 57B, discussed hereinbelow.
An altcrnative solid mieronecdle shape is depicted in Figares 35 a.nd 36, in
which
43
CA 02591168 2007-05-14
WO UOf14764 PCT/[1S00115612
the solid microneedle is generally star-ahapod in profile, As viewed from its
top surface
(see Figure 36), the solid microneedle 900 is a generally three-pointed star
shape, having
three Iongitudinal blades at 910, 920, and 930. The top surface of each of
these star-
shaped blades is depicted at 914, 924, and 934, and as can be seen from
Figures 35 and
36, a major portion of these top surfaces is generally triangular in shape.
The preferred
shape is that of an isosceles triangle, in which the base angle of this
triangle is in the
range of 1-450. Of course, the smalier this base angla, the smalt.er ihc
amount of skin
damsge done when the nzicroneedle 900 is inserted into the straturm cwneum.
Each blade 910, 920, and 930 bas a pair of generally planar side walls at 912,
913,
922, 923, 932, and 933 (although these side walls could be somewhat curved in
contour,
if desired). These side walls converge to farm a ganetally sharp point at 918,
928, and
938, respectively. In the illustrated embodiment of Figure 35, niicr+aneedle
900 continnes
this star-shaped profile from its top surfaces at 914, 924, and 934 down to
its bottom
edges at 916, 926, and 936, where the microneedle structure ,loins its top
plaaar base
siructure at 905. Of course, the very upper smrfacGs are most key as far as
makin.g a
penetraiton into the skin through the stratnm corneum, and the precise shape
of the blades
910, 920, and 930 may somewhat vary along the longitudinal length of
micronsedle 900
without departing from the priacaples of the present invention. The major
benefit of this
shape is its small cross-sectional area allowing easy insertion into the skin,
yet a large
surfar.e arta providing high rates of active penetration through the skin.
Since microneedle 900 is solid, for liquid to be dispensed into the skin or to
be
sampled from the skin, a set of openings is provided in the base element or
substrate at
908. It is preferred that a single opening be located along each pair of
projections or
blades, as illustrated on Figure 36, in which an opening 940, 942, and 944 is
provided
between the blades 910-920, 920-930, and 930-910, respectively. Of cowse,
different
sized holes and different hole locations, as well as differcnt numbers of
holes for that
matter, could be utilized with the solid microneedle 900, without departing
from the
principles of the present invention.
Microneeclle 900 could be constructed of virta.ally any material that is
biocompatible with human siout (or other amimal or plant skin). This includes
molded
plastic or cast metal, or perltaps a silicon or silicon-dioxide structure that
is manufactured
44
CA 02591168 2007-05-14
WO OOR4764 PCT/U5U0115612
using semiconductor and plastic fabrication techniques. The top surfacc at
914, 924, and
934 is illustrated as being generally planar, although this could be changed
easily enough
to cause the mid-portions of the rnicroneedle 900 to be somewhat lower than
the points of
the three blades at their top edges 918, 928, and 938. Such a construction
would have a
similar side appearance to the hollow microeedle 1420 dapicted on Figure S7B.
It will be understood that more or less than three blades could be constructed
to
create a solid microneedle such as that of mieroneedle 900, without departing
from the
principles of the present invention. Even a single blade design could be used,
having
either one or two sharp edges. While the three-bladed solid microneedle 900 is
of an
optimal dasigin, certainly a four-bladed design could also be manufactured and
used, and
provide generally good results. In a four-bladed design, it would be preferrad
that each
pair of blade.s have a eorresponding through-hole in the substrate beneath the
bottom
portion of the solid microneedle, although such holes aro not necessarily
required between
each pair of blades. The size of each of the through-holes such as holes 940,
942, and 944
is up to the designer, although its inner diameter should be sufficiently
large to allow
useful molecules to pass therettuough.
Another very important attribute of arrays of rnicroneedles is the sepaiation
distauee between each of the microneedles with regard to their placement on
the substrate
or base strueture. On one hand, the more microneetllos per given area of a
substrate, the
greater the amount of "transdcrmal flux" (or transdermal flow) of a fluid that
will be
transported through the mirxoneedles (i.e., in the case of hollow
microneedles). On the
other hand, it has been determined that the closer the spacing of
microneadles, the less
likely that the microneedles will actually penetrate the stratum corneum layer
of slan due
to the olasticity characteristics and mechanical strength of skin. Therefare,
a dichotomy
exists that indicates the separation between microncedles is critical for a
useful device.
Figures 37-42 provide tabular data ilhistrating the effects of rnicroneedle
length,
microneedle outer diameter, and microneedle separation for circular hollow
microneedles,
such as those depicted in Figure 15, Figure 22, and p`igures 25 and 28. As
related
hereiuabove, the rnicronoedles illustrated in these figures arc hollow, having
internal
cylindrical openings, but are not edged or sharpened with respect to having
any type of
blade structure along their outer surfaces or tips. Furthermore, the tabular
data of Figures
CA 02591168 2007-05-14
WO 00/74764 PCT/r1SOO/15l12
37-42 are with respect to microneedles that aro an-anged in a hexagonal
eonfigurntion.
All diAlensions ori these Figures 37-42 are in microns (i.e., micrometers).
EaCh chart
shows ten rows that represent various microneedle lengths in the range of 30-
300
microns, and ten columns showing nnicraneedle outer diameters in the range of
10-100
microns. Each chart is for a different separation distance, starting with 50
microns, and
then incrementing by 50 microns to the funal chart of Figure 42 that shows a
separacion of
300 microns.
The table entries of "Y" represent a situation where the microneedle
penetrates the
skin. A table entry of "n" represents a configuration where the nficroneadle
will not
penetrate skin. Finally, the "diamond" shape represents a table entry in which
the
micronoedle will possibly penetrate the skin, however, it is not certain that
penetration
will occur.
Each table contains a dashad liae (such as line 1002 on Figure 37) that
roughly
indicates that table entries below the line will likely penetrate the skin,
wheress table
entries above the line will likely not penetrate the skin. These lines
represcnt
approximations to a certain extent, and a tolerance of at least plus or minus
10% should
be considerod when utilizing this data. In some circumstances, the tolerance
should be
more like plus or nsinus 20%.
On the varioas cbmrts, the lines are indicated at 1002 for Figure 37, 1004 for
Figure 38, 1006 for Figure 39, 1008 for Figure 40, 1010 for Figure 41, and
1012 for
Figure 42. Each of these lines can be approximately defined by an equation, in
which the
variables are microneedle length rc.presented by "L," and the outer diameter
represented
by the variable "D." For these equations, all dirnensions are in microns. In
Figure 37, the
equation is: L= 9D + 120; for Figure 38, the eqnaeion is: L= 5D + 50; for
Figume 39, thc
equation is: L = 2.77D + 72.3; for Figure 40, the equation is: L= 1.54D +
59.2; for
Figure 41, the equation is: L= 0.856D + 124; and for Figure 42, the equation
is: L
0.47D+ 133.
Figures 43-48 provide further tabular data, this time for edged or "sharp"
hollow
microneedles, such as those depicted in Fignres 32-34. These edged
neicroneedles are
also circular or cylindrical in overali shape, but, as described above,
include two
longitudinal blades with a relatively sharp edge to aid in pcnetrating the
stratwn corneum
46
CA 02591168 2007-05-14
WO 0&74764 PCT/USUO/iS612
of the skin. As will be seen as compared to the tables of Figures 37-42,
penetrating skin
is more easily accomplished using the edged microneedles. As noted
hereinabove, an
"edged" microneedle is one in which its tip has a radius less than or equal to
0.5 microns.
As before, a table entry of "Y" indicates that a penetration occurs, a tablc
entry of
"n" indicates that a penetration does not occur, and a table entry of a
diamond-shaped
symbol indicates that a penetratian of the skin may occur, but is not
definite. A dashed
line is drawn on Figures 43-48 to indicate the likelihood that entries above
the dashed line
will not succeed in penetrating the skin, whilc entries below the line will be
successful in
such penetration. The lines are indicated by the reference numGrals 1022 for
Figure 43,
1024 for Figure 44, 1026 for Figure 45, 1028 for Figure 46, 1030 for Figure
47, and 1032
f4r Figure 48.
Similar equations far these lines can be determined from this data, where
again the
variable L is equal to the uticroncedle length and the variable D is equal to
the outer
diameter of the miaroneedle. In Figure 43, the approximate equation is: T. =
9D + 30; in
Figure 44, the equation is: L 5D; in Figure 45, the equation is: L= 2.77D +
11.5; in
Figure 46, the equationn is: T. = 1.54D + 56; in Figure 47, the equation is:
L= 0.$56D +
64.4; and in Figure 48, the equation is: L= 0.47D + 96.5.
It can be easily seen from the tabulated data of Figures 37w48 that the
greater the
separation between mieroncedles, the more likely that the skin will be
penetrated at any
given length of microneedle. If relatively small microneedles having an outer
diameter of
twenty niicrons arc desired for use in a microneuile atray, then the tabular
data indicates
that the microneedle should be at least 100 microns in length, and either 250
or 300
microns separation distaacc (see Figures 41 and 42). On the other hand, the
same 20
micron outer diameter microneedles tbat include edges (as per Figure 32) will
likely
penetrato the skin at a needle length of at least 60 microns and a separation
of 150 or 200
microns. This is an obvious improvement in microneedlc density per unit arca
of the
substrate upon which the microncedle array is mounted, thereby allowing a
dramatic
increase in the amount of material delivered or extracted through the skdn.
Microneedle density is an important factor in dispensing fluids or sampling
fluids
ttuvugh the stratum corneum oftbe slan. This is clearly indicated in the graph
of Figure
49, in which the X-axxis represents rnicraneedle separation in microns, and
the Y-axis
47
CA 02591168 2007-05-14
W4 00/74764 PGT/tJ5i00/15612
reprosents the transdermal flux of an active fluid such as a niacinamide
solution, in units
of micrograrns per square centimatcr per 24 hours of time.
The base or reftrence line of Figure 49 is represented by the "intact skin"
line
1044, which is in essence the tramsdermal flux rate of normal skin without any
microneedles, in the above units of five (5) microgranYS per squarG centimeter
per 24
hours time. 'fhis base line 1044 is also indicated as being "1X" times a
nominal
ttmnsdermal flux rate. If the stratum corncuro layer of human skin is semoved,
then the
transdermal flux rate is increased by a factor of twenty-four (24), and is
represantod by the
line 1042, which indicates approximately one hundred twenty (120) micmgrans
per
square centimeter per 24 hours of tranadermat flux flow rate. This line is
also referred to
as "24X" on Figure 49.
If microneedles are used, the flow rate is variable, as per tho curve (or more
accurately, the segmented line) at 1040, which at 100 micrans of separation
provides a 46
times (or 46X) flow rate as compared to the intact skin flow rate of 1 X. This
flow rate
naturally decreases as the microneedle separation increases, since the density
of
niicr+oneedles is proportionate to the square root of separation distance. For
example, at a
nueroneedle separation of 400 microns, the transdermal flux rate is only 5
times (5X) the
flow rate of intact skin (at 1X).
Figure 49 assumes that the microneedla lengths are sufficiently long and have
a
sufficient shape to penctxate the skin at the separcations listed along the X-
axis.
Otherwise, the transdernmal flux rates will be significaatly reduced. However,
any
rnicroneedle usage that docs not actually penetrate the stratum conoeum will
likely cneate
a certain amount of indents and breaks in the sldn, which will provide a
certain incr+ease in
the transdennal flux rate. For example, if the microneedlc aaay is provided
having
microneedles of 40 micron$ in outer dimneter and 50 microns in length, it is
not Itkely
that microneedle penctration will occur ia very many places at virtually any
separation.
However, there will still be enough indents and breaks in the skin to provide
a four times
(i.e., 4X) increase in the tiransdennal flux of a drug or solution such as
niacin.amide in
water. To achieve the results of Figure 49, the microncedle length was 100
microns and
its outer diameter was 20 microns. It can be seen from Figure 49 that a
microneedle
separation of around 1?0-175 microns will provide results that are equal to
the rernoval of
49
CA 02591168 2007-05-14
WO 00174764 PCT/USd0U16612
the stratum corne;un layer of sldn.
Utilizing a passive diusion model of ltunn,an skin and microneedle
structures, the
inventors also provide the chart of Figure 50. The X-axis of Figure 50
represents the
mieroneedlle length in microns, while the Y'-axis represents the transdermal
flux of an
s active solution, in micrograms per square centimeter per 24 hours time
period. 'I'he
curves on the graph are depictcd with respect to a 5% niacinamide solution in
water.
The lower curve at 1052 represeats a nucroneadlc array in which thc needles
have
a 200 micron sepwation in a hexagonal pattem. '1`he upper curve at 1050
represents a
anicroneedle array in which the micrtmeedles have a 100 micron separation in a
hexaganal
to pattern. Very useful transdermal flux rates can be provided witb
mieroneed1e arrays
having a separation of 200 nticrons at a needle length of 100-110 microns, and
an outer
diameter of 20 microns. It can be seen from F'iguYe 46 that this range of
microneedle
lengths and outer diameters lies within a small tolerance of the line 1028
that indicates
whether or not micmneedle penetration will occur in skin. This table of,data
on Figure 46
15 represents edged hoDow microneedles, as described above.
Figure 51 provides another measure of usage for rnicronecdles. The X-axis
represents microneedle length in microns, while the Y-axis is a ratio of
iransdermal flux
using a solution of niacinamide in water versus skin damage whon using the
microneedle
array. A nominal figure of txansdermal flux versus skin daunage is provided at
the value
20 of one (1) along the Y-axis. The upper curve at 1060 depicts the ratio when
microneedles
have a 200 micron scparation. The lower curve 1062 shows a sinzilar
micronecdle array
having only a 100 micron separation. Whilo the twsdcnmal flux will typically
be much
greater when the microneedie separation is smaller, also the sldn damage will
be gmaicr.
As can be seen fr4m the curves 1060 and 1062, once the microneedle length
exceeds 100
25 micron.s, the transdermal flux versus skin damage ratio tends to incrcase
rather sharpay.
The microneedle outer diameter was 20 microns for the data of Figure 51.
Figure 52 is another graph representing information regarding passive
diffusion of
fluids using mieroneedles as compared to the use of rnicroneedles under
pressure to
increase the transdcrrnal flow. The X-axis is in units of pressure, gs per
square
30 centimeter. The Y-axis is the transdermal flux of an active solution in
micrograms per
square ecnticneter per 24 hours time period, and the values of this chart are
for a 5%
49
CA 02591168 2007-05-14
WO Dq174764 PC'TIUS00I15612
solution of niacinamide. In addition, the results of this chart were produced
using
microneedles of 100 microns length, 20 microrts outer diameter, and a
separation of 200
mlcro118.
For intact skin, the lowest horizontal line at 1076 indicates a retatively low
transdermal flux of the solution to the skin. If the stratum corneum of the
skin is
removai, this t.ransdermal flux greatly increases to the higher harizontal
jine at 1072.
Another horizontal line at 1074 indicates the transdermal flux rate using
microneedles
under passive ditf'usion.
If pre9sm is applied, then the flow rate changes as the pressure changos. This
is
indicated by the sloped line 1070. As can be seen, if the pressare is
increased by three
orders of magnitude, then the flow cate of the transdcrmal flux also increases
by
apptoximately three onders of magnitude.
Based upon the above information, it is preferred that the outer diameter of
circular micronoedles (without "sharp" edges) be in the range of 20-100
microns, more
prcferably about 20-50 microns. In addition, it is preferred that the height
(or length) of
the microneedles for use with inter$titial fluids be in the range of 50-200
microns, more
preferably about 100-150 microps; for use with other biological fluids, the
prefarred
length is in the range of 200 microns - 3 mrn, and more preferably in the
range of 200-
400 nYicrons. Finalty, it is preferted that the separation between
microneedles in the array
be in the range of 100-300 microns, moro preferably about 140--200 microns. Of
course,
dimensions outside the above-listed ranges will still be somewhat useful, even
for
micreneedle lengths and soparation distances as small as 50 microns, or as
large as 1000
microns.
For hollow circular microneedles having edges (e.g., see microneedle 800 in
2S Figure 32), it is prefetred that the outer diameter be in the range of 20-
100 microns, and
more preferably in the range of 20-50 microns. For use with interstitial
ttuids the length
will preferably be in the range of 50-200 microns, more preferably in the
range of 80-150
microns; for use with other biological fluids, the length will preferably be
in the range of
200 microns - 3 mm, and more preferably in the range of 200-400 microns.
Finally, the
separation will preferably be in the range of 100-300 microns, rnore
preferably in the
range of 100-200 microns.
CA 02591168 2007-05-14
WO 0rT4764 pcrrusuwyseiz
For solid microneedles of the star-shaped design depicted on Figures 35 and
36, it
is prefem:d that the radius of one of the spokes or edgod blades (e.g., blade
910), as
indicated by the radius 950 on Figure 36, be prefecably in the range of 10-50
microns, and
more preferably in the range of 10-15 micrcns. The length of the solid
micronecdles will
S prcferably fall in the range of 50-200 microns for use with interstitial
fluids, and more
preferably in the range of 80-150 rnicrons; for use with other biological
fluids, the length
wifl preferably be in the range of 200 microns - 3 mm, and more pre.ferably in
the range
of 200-400 microns. The separation distance will preferably fail in the range
of 100-300
microns, and more preferably in the range of 100-200 microns.
Figures 53A-53E illustrate the steps for preparing a mold to make hallow
micronecdles, according to the principles of the present invcntion. The first
step is
depicted in Figure 53A, in which a substrate 1100 is provided with a top layer
of positive
photoresist material at 1102. The substrate oan be spin coated, or an adhesive
can be used
ta attach the photoresist 1102 to the substrate 1100. The substrate can
consist of silicon,
l5 silicon-dioxide, plastic, metal, or other suitable compounds. The
photoresist material will
preferably comprise poly(methylmethaciylate), also known as "PMMA," alttiough
other
suitable compounds could be used, such as polyoxymethylene (POM),
polyalkcnsu]foxle
(PAS), polyrnethacrylimide (PMI), and poly(lactide-co-glycolide) (PLG).
In Figure 53B, a mask at 1104 is placed over the photoresist layer 1102, and
eleetramagnetie energy is direeted through the mask from a light source, in
which the
light energy moves in the direction as indicated at 1106 on Figure 53H. Tbe
mask 1004
preferably is made of gold metal, and in this instance, the eleatromagnetic
energy
comprises x-rays. It will be understood that many different types of
photoresist
procedures or the like could be used without depart.ing from the principles of
the present
inventio.n, and for example, high energy nuclear particles might be
substituted for
electromagnetic energy in some processes.
Figure 53C represents an expose and develop step, in which a chemical compound
is used to etch away the portions of the PMMA material that were not protected
by the
mask 1104 in the prior step at F'igure 538. On Figure 53C, the
three^dimerxsiona]
microneedle sbapes begins to become apparent. A pair of hollow microneedle
forms are
illustrated in Figure 53C at ] 110 and 1120. In cross-section, the microneedle
form 1 I 10
51
CA 02591168 2007-05-14
WO 00174764 PCTlUSoo115612
shows a first wall at 1112, a scoorid wail at 1114, and a hollow area or hole
at 1116.
Similarly, the micron+eedle form 1120 comprises a first wall at 1122, a seoond
wall at
1124, and hollow m=ea or kwle at 1126.
Soth microneedle forms 1110 and 1120 will be of the appropriate length and
outer
diameter to produce microneedle arrays as recommended hereinabove. The
separation
betweon micronccdles is depicted by the dimension 1105, and this also will
preferably be
of a distance as recommended hereinabove.
Figure 53D is a step where thc micronecdle forms arc electropla#ed with metal.
In
the prefcnod embodiment, this metal at 1130 will comprise nickel. As an
optional
intermediate step, the substrate I 100 and micronecdlc farms at 1112, 1114,
1122, and
1124 (wh4ch in combination comprise two circular or cylindrical mieraneedlG
forms) can
be chemically coated to aid in later release before the electroplating takes
place.
After the nickel electroplating has achieved tho appropriate thickness, the
step of
dctaching this rnctal form takes place in the step illustrated on Figttce 53E.
A"rcvetse
contour" mold will now exist, as generally depicted by the reference nusneral
1130.
Instead of a hollow area or hole, a cylindrical projection now appcttrs at
1132 and 1134 in
the metal mold. Similarly, instead of cylindrical or nearly cylindrieal
projections at 1112,
1114, and 1122, 1124 (as scen on Figures 53C and 53D), there are now hollow
cylindrical
shapes formed at 1140r1142, emd 1144-1146, which represent the areas where the
microneedle cylindrical walls will form.
Figures 54A-54C depict the steps of microembossing to forrn molded
microneedles that are hollow, as consmwted according to the principles of the
present
invention. The metal micrnneedle mold at 1130 is attached to a moveable press
ram
1152, to form a structure that will be impressed against a polymer or other
plastic
material. This znovcable stru.cture is indicated by the reference numeral
1150. The
polymer or other type of plastic material at 1 I60 is piaced on the surface of
a heated plate
1154. The microneedle material preferably will camprise a biocoxapatible
polymer
material, although other materials could be used including polycarbon, or even
PMMA,
The heated plate 1154 provides suffacient theanal energy to raise the
temperature
of the biocompatible polymcc material at 1160 until it becomes readily
deformable. In
other words, the polymer material is placed into its "plastic" stage by
raising its
52
CA 02591168 2007-05-14
WO 00174764 PCT/USOOl15612
temqerature substantially to its elastic working tcmperaturc. The moveable
press
assembly 1150 is now pressed down toward the heated plate 1154 and against the
biocornpatible polymer material 1160, It is prefen=ed to accomplish this task
within a
vacuum to preaerve the biocompatibility and sterilization characteristics of
the future
micronctdles.
A cool-down stage is next, as depicted by the final result in Figure 54B. The
heated plate 1154 now becomes a cooling plate, and the biocompatible polymer
material
is cooled to the point where it becomes solid and wiil not readily deform, The
moveable
press ram assembly 1150 is now raised, thereby leaving behind a microneedle
array
having a substrate at 1162. In the illastrated embodiment of Figure 54B, there
are two
hollow mieroneedles at 1170 and 1180, not yet having through-holes in the
substirate
1162. The microneedle at 1170 is depicted in cross-section as having a first
wall 1172
and a second wall 1174, which are generally cylindrical in shape. These wa11s
surround a
hollow area or hole at 1176. Similarly, microneedle 1180 shows a cross-section
of a pair
ts of walls at 1182 and 1184, containing a cylindrical hollow area at 1186.
After the cooiydown stage, the microneedle array is removed from the plaLe
1154,
tWeby leaving behind the structure as illustrated at Figure 54C. The
rnicronoedle
scparation is indicated at the dimension 1165. This dimension is equal to the
dimension
1105 depicted on Figure 53C.
It will be understood that other types of plastic forming proeesses can be
used than
embossing. In fact, virtually all types of inoiding or microtnolding processcs
can be
utiiized. Embossing is one subset of these types of moldings, and injection
molding is a
second subset, which was dcscrt'bed hcreinabove for other microneedle shapes.
The above structtire depicted in Figure 54C could be used as the "f1naP"
produot
for ecrtain uses with skin. This structure consists of substrate 1162 and two
hollow
microneedles 1170 and 1180, in which the hollow cavities 1176 and 11$6 each
form a
small cup-like volume that does not protrude completely through the substrate
1162. This
stencture could be used for drug delivery by filling the cup-like hollow
cavities 1176 and
1186 with a drug active that can slowly leach out into biological systems.
Figures 54D-54F illustrate various methods of forming charnbers beneath the
microneedle array, and forming through-holes. In Figure 54D, a hollow chamber
at 1190
53
CA 02591168 2007-05-14
WO 00174764 YCT/USU0115612
is formed on the opposite side of the substrate, thereby forrning a
microneedle array
structuYe 1192 that contains hollow microneedles 1170 and 1180, and a chamber
that can
hold some type of ftid, This chamber can be foxmed by micromachining, or
perhaps by
an oxygen plasma etching process. Other methodologies could be used without
departing
from the principles of the present invention.
In Figure 54E, a laser light source is used to finish the "drilling" process
to make
through-holes that are concentric or otherwise centered along the hollow
microneedles
1170 and 1180. On Figure 54E, a laser light source is used to burn away some
of the
substrato material along the lines at 1194 and 1196. The final result is shown
at Figure
54F, in which a final microneedlc array 1198 is iliustrated showing through-
holes from
the chamber 1190 to the top of the microneedles, in which the mioroneedle
openings 1176
and 1186 are aligned with the laser light bumed holes at 1195 and 1197,
respectively.
Figures 55A-SSF illustrxte an alternative methodology for constructing hollow
plastic microneedles. Starting with a laniinate material at 1200 and a
biocompatible
y5 polymer at 1202, these materials are joined along a planar surface at the
line 1204 on
Figure SSA. This joining can be performed by an adhesivc pracess, or other
temporary
mechaniaal means.
A mold 1210 is now provided, which preferably will be made of a metallic or
other suitable material. In Figure 55B, the biocompatible polymers are placed
on a heatcd
plate 1212, and the mold 1210 is placed upon a moveable press ram. After the
mold has
been pressed into the biocompatible polymers, the ram press is rcmoved and the
material
is cooled, thereby arriving at a structure illustrated in Figure 55C in which
holes 1224,
1226, and 1228 are formed all the way through the upper layer, now designated
as 1220.
These holes also continue part-way into the lower layer at 1222.
The laminate materials that were earlier giucd together are now detached from
one
another. This now provides a film structure 1220 that has the through-holes
1224, 1226,
and 1228, and is illustrated in Figure 55D. This film layer 1220 is now placed
upon a
heated plate 1230. A mold structure 1280 is now provided and will be pressed
against
film layer 1220 after the film layer 1220 has been heated to its plastic
stage. On Figure
55E, the cylindrical projeetiQns 1282, 1284, and 1286 are used to create the
through-holes
for three hollow microneedles.
54
CA 02591168 2007-05-14
WO 00/74764 PCTlU900l15612
In an alternativc configuration, thc cylindrical projections 1282, 1284, and
1286
can be somewhat shortened so that they rest against the planar top surface of
the hcatsd
plate 1230, i.e., along the horizontal (on Figure 55E) line 1235. The heated
plate 1230, in
this altemtive configuration, would be substantially flat along its top
surface at 1235,
such that the openangs 1232, 1234, and 1236 would be filled.
After the pressing process has oecurred and the material 1220 is cooled (by
piate
1230) to the point where it becomes solidified, the mold 1280 is removed and a
new
stnwtwe at 1240 is formed and removed from the plate 1230. This is illustrated
in Figure
55F. This new stnicture 1240 rapresents a microncedle attay having three
hollow
cylindYtical microncedles at 1242, 1252, and 1262. These micronecdles have
hollow
through-holes as illustratcd at 1244,1254, and 1264, respectively.
Another use for the microneedles of the present invention is to include a
sensing
capability by some type of optical means with a plastic naicroneedie array
structure that is
constructed of a substantially transparent material. This could be used with
both hollow
and solid microneedles, although it is prefecred that solid microncedles be
usod to prevent
contamination of the light souroe mechanism that is being utilized for this
sensing
capability. In Figure 56, a nticroneedle arra.y structure 1300 is depicted as
having a
substrate 1302, and three microneedles at 1310, 1320, and 1330. The upper
areas of these
microneedles near their tips are coated with a chemical material that aids in
detecting a
chemical or other biologieal process. This chemical coat3ng is indicated on
the threc
micxoneedles at 1312, 1322, and 1332.
Once the microneedle amy 1300 has been placed into the skin, a light source is
used to provide electromagnetic energy in the direction indicated by the
arrows 1350. It
is preferred that the light source be some type of laser source, so that the
electramagnetic
energy is coIlimated. The chemical coating at 1312, 1322, and 1332 will be of
a type that
will either change color or change its light passing characteristies when in
contact with
the target fluid or biological materials. In this methodology, the laser light
that is
reflected back toward the optical energy source wil! either be reduced in
intensity, as
compared to before any chernical cbanges were noted at the ends of the
microneedles, or
will have a color variation.
Another use for this configuration is to provide optical energy directly into
CA 02591168 2007-05-14
WO 0(4r14764 rCr/IU$00115612
portions of skin that can be directly affected or stimulated by certain
froquencies of light.
Tn this instance, the laser light may directly provide eithcr optical or
thermal energy into
slcin tissue, or could provide a methodology for transfernng such energy into
muscle
tissue at certain locations in an animal body.
Alternatively, the sensors can be integrated with the microneedle an=ay by
layering
the sensor components on the face of the device containing the protrusions
that will
perforate the skin. One or more layers can be used depending upon the
complexity of the
datection process. SiTnpie ccmduetivity measurements for analytes like sodizun
ions can
be made with only one conductive layer of a biocompatible material, such as
the layer
1312 on Figure 56A, or a layer 1372 on Figure 56B.
More complex analyses (e.g., glucosc) are accomplished by using several layers
of
sensing mateiials. To prepare an enzyme electrode, a biocompatible prapolymer
doped
with an enzyme, an enzyme modified with a polymerizable group, or an enzyme
modified
with a group that can be tethered or adsorbed to the electroconductive surface
is coated on
is top of the electrically conductive polymer and is polymeri2ed using a
curing agent or an
energy source such as light, or heat as necessary. This is illustrated in
Figure 56B where
the eoating constitutes an enzyme layer that is depicted at 1374. The
electrically
conductive layer is depicted at 1372. A single microneedle structune 1370 is
illustrated in
Figure 56B as a longitudinal element protruding from a substrate 1360,
however, it will
be understood that ntany such longitudinal elements can be constructed on the
substrate
1360 to create a mieroncedle array (siniilar to, e.g., the rnicroneedles 1310,
1320, and
1330 on Figure 56A).
The enzyme film can also be coated with additional layers of biocompatible
polymers (as depicted at 1376) that can be etnployed to protect the sensor
components
from leaching, reactions with biological entities, or to regulate the access
of analytes to
the cnzyme layer. As deQicted in Figure 56B, the electrically conductive layer
1372,
enzyme layer 1374, and "top" polymer layer 1376 are deposited on virtually the
entire
surface of the microneedle alray, except for an portion at the end of the
substrate
struature, as generally depicted by the reference numeral 1365. The side walls
of a
microneedle array comprising multiple microneedle devices such as the
microneedle
structure 1370 are not completely coated with the enzyme layer 1374 or second
polymer
56
CA 02591168 2007-05-14
WO OO174764 FC'T/[JSOOraS612
layer 1376, because those arcas will be used for electrical contact with an
eloetinchernical
analysis circuit. Therefore, only the electrically conduetive layer 1372 is
deposited
throughout the upper surface of the substrate 1360, including the portions
near the left (on
Figure 568) hand end, at the numeral 1365.
These sensor component layers 1372, 1374, 1376 can be deposited on
microneedles (e.g., microneedle 1370) by dipping the microneodle deviccs in
the
appropriate chemical reagents, spin-coating tcchniques, electro deposition,
stamping,
deposition of dry powders, and similar processes known by those sldllful in
the art. The
left-end portion near 1365 is preferably masked during the deposition
procedures for the
enzyme layer or second polymer layer, thereby leaving exposed the electrically
conductive layer 1372 in this region.
The first conductive layer 1372 deposited on the microneedlcs can consist of
tnany
available materials; metals are proferred and include: Au, Cr, Ti, Pt, Ag, Cu.
Conductive
polymer mixtures such as 7,7,8,8-tetracyanoquinodimethane with
tetrathiafulvalene or N-
methylphenazinium can also be used. Furthermore, conductive polymers such as
polyacetilene, polythiophene, polyparaphenylene, and polyphenylene vinylene
and
polyaniline can be used.
The enzyme coating can be eatrapped in any one of the following poiytners or
aopolymer mixtures in the seoond layer at 1374: glutaraldehyde, poly(ethylene
glycol)
diclycidy ether and poly[(1-vinylimidazole) asmium (4,4'dimethyl
bipryidine),.Cl), poly
N-nnethylpyrrole, poly [(viayl pyridine) Os(bipyridine),CI}, cyclodextrin
polymers, and
gelatin.
The outer biocompatible protection layer at 1376 can in.clud.e: siGcones,
tluorinated-ethylene propylene, nafion, cellulose, poiy(vinylpyriclinc)
acetaic, a2iridine,
polyurethanes, epoxies, fluorocarbons, acrylics, patylene, and polyimides.
Another use for this configuration is to provide electrical energy directly
into
portions of skin that can be directly affected or stimulated by a small
electrical current. In
this instance, the electricity is conducted via the conductive layer 1372. If
it is desirabie
to provide electrical current directly at the tips of the microneedles, then
the enzyme layer
1374 and protective polymer layer 1376 can be elirninated from the
manufacturing
process, leaving only the electrically eonductive layer 1372 covering the
entire substrate
57
CA 02591168 2007-05-14
WO 00174764 PCT/USOO/15612
1360 and micmneedle structure at 1370. In this manner, electrical energy may
be directly
provided into sldn tissue, or could ultimately be transfen+ed into muscle
tissue at oortain
locations in an animal body.
Figures 57A and 5713 illustrate a refinement of the embossing process that was
earlier described in relation to Figures 54A-54C. In Figure 57A, the
micronecdle
substrate at 1400 has been deformed by a metal (or other type of material)
mold at 1410.
A single hollow microneedlo structure is being formed in Figure 57A, as
indicated by the
crass-section cylindrical wall at 1402 and 1404. As the substrate material
1400 is cooled,
shear forces are generated during the de-molding procedure which occurs whcn
the mold
1410 is removed from the upper surface of the substrate 1400. These sbear
forces will
mainly occur along the inner surfaces of the walls 1402 and 1404, which
indicate the
inner diameter of the hoUow microneedle near its tip.
The amount of shear forces can be controlled by the cool-down temperature and
timing as to when the mold 1410 is released. If the shear forcc is maintained
at a
sufficicnt magnitude, the final structure will not have a perfectly flat
surface alang the top
of the ntieroneedle, but instead will have a shape similar to tltat of the
microaeedle 1420
depicted in Figure 57B. In this microneedle 1420, the upper surEace of the
tnicroneedle
has sharp points at 1422 and 1424, and a rather arcuate shape along two of its
semi-
circular edges at 1426. This shspe also can be parabolic or elliptical in
nahrc, and the
important aspect of this shape is to provide sharper edges at the points 1422
and 1424.
This is an alternative methodology for forming hollow circular microneadles
that can
more easily penetrate the strahun corneum of skin, and may not require the
edged blades
of the tnicroneedle stractares depicted in Figure 32.
The star-shaped solid microneodle stravtares can also be created using a
molding
process similar to that depictod in Figures 53A-53E, and 54A-54C. Of course,
the solid
micinneedles will not require through-holes that arc in alignrnent with the
center of each
micronoedle, but will instead require through-holes in the substrate material
at locations
that am substantially proximal to the pair of blade stractures near the top
surface of the
substrate.
It will be understood that all types of molding or casting procedures could be
utilized in conjunction with the present invention, so long as these molding
procedures
58
CA 02591168 2007-05-14
WO 00/74764 PCTlUSM11S612
can be utilized to create the v ry small sttuctw'cs required by the
microneed3es of the
prc=t invention. Furthermore, semiconductor fabrication tcchniques can be used
to
create the structures illustrated on Figures 32-36, using processes that were
desmbed
hereinabovc in reference to Figures 1$ 22. Certninly fluid reservoirs can be
constructed
S for use with the microneedle structurts of Figures 32-36, and fitrthermore
various
methods of use can be utiiized with these micronccdle struetures, such as
electmphoresis
or ultrasound.
The foregoing description of a preferncd cmbodiment of the invention has been
presented for purposes of illustration and description. It is not intended to
be exhaustive
or to limit the invention to the precise form disclosed. Obvioris
modifications or
variations are possible in light of the above teachings. The embodiment was
chosen and
described in order to best illustrate the principles of the invention and its
practical
application to thereby enable one of ordinary sldll in the art to best utilize
the invention in
various embodiments and with various modifications as are suited to the
particular use
contemplated. It is intended that the scope of the inventionk be defined by
the claims
appended hcreto.
59