Note: Descriptions are shown in the official language in which they were submitted.
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
TABLET FORMULATION FOR SUSTAINED DRUG-RELEASE
FIELD OF THE INVENTION
The present invention relates to a sustained-release drug formulation. More
specifically, the invention relates to a pharmaceutical formulation
maintaining
the integrity of a hydrophilic tablet comprising substituted amylose as a
matrix
for sustained release of the drug contained in the tablet.
BRIEF DESCRIPTION OF THE PRIOR ART
Controlled drug-release systems and their characteristics
For many years, increased attention has been given to drug administration
characteristics, which has led to the development of new pharmaceutical
dosage forms allowing the control of drug release.
Among the many oral dosage forms that can be used for controlled drug-
release, tablets are of major interest in the pharmaceutical industry because
of
their highly efficient manufacturing technology.
Matrix tablets obtained by direct compression of a mixture of a drug and a
polymer would be the simplest way to orally achieve controlled release of the
active ingredient. Of course, these tablets should also show good mechanical
qualities (i.e. tablet hardness and resistance to friability) to meet
manufacturing
process, subsequent handling and packaging requirements.
Furthermore, matrix polymers should be easily obtained, biocompatible and
non-toxic, with the proviso that biodegradable synthetic polymers have the
disadvantage of possible toxicity after absorption of the degraded products.
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
2
Polysaccharide matrices
Several types of polymers have been proposed so far as matrices for the
controlled release of drugs. Examples of such polymers used in hydrophilic
matrices are some cellulose derivates like hydroxypropylmethylcellulose, non-
cellulosic polysaccharides like guar gum or alginate derivates, acrylic acid
polymers like Carbopol [Buri P. and Doelker E., Pharm. Acta Helv., 55, 189-
197 (1980)]. Poly(vinylpyrrolidone) has also been proposed in addition to the
above-mentioned polymers [Lapidus H. and Lordi N.G., J. Pharm. Sci., 57,
1292-1301 (1968)]. Other polymers, for example ethylcellulose or
polyvinylchloride, have been deployed in inert matrices [Salomon J.-L. and
Doelker E., Pharm. Acta Helv., 55, 174-182 (1980)].
Polysaccharide biodegradable matrices for tablets are of interest because the
degradation of a natural product like starch occurs naturally in the human
body
[Kost J. et al., Biomaterials, 11, 695-698 (1990)].
Starch is composed of two distinct fractions: (1) amylose, a non-ramified
fraction containing about 4,000 glucose units, and (2) amylopectin, a branched
fraction containing about 100,000 glucose units [Biliaderis C., Can. J.
Physiol.
Pharmacol., 69, 60-78 (1991)].
Unmodified, modified, derivatized and cross-linked starches have been
proposed as binders, disintegrants or fillers in tablets [Short et al., U.S.
Pat.
Nos. 3,622,677 and 4,072,535; Trubiano, U.S. Pat. No. 4,369,308; McKee, U.S.
Pat. No. 3,034,911], but no controlled release properties have been described.
Thus, these patents are not relevant when considering the present invention.
Some works have disclosed the use of physically- and/or chemically-modified
starches for sustained drug-release. The authors of these papers have
presented the usual types of starches, i.e. those containing low amounts of
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
3
amylose, and have not even mentioned the role of amylose, nor amylose itself
[Nakano M. et al., Chem. Pharm. Bull., 35, 4346-4350 (1987); Van Aerde P. et
al., Int. J. Pharm., 45, 145-152 (1988)]. Some works have even attributed a
negative role to amylose present in thermally-modified starches used in
sustained drug-release tablets [Hermann J. et al., Int. J. Pharm., 56, 51-63 &
65-70 (1989) and Int. J. Pharm., 63, 201-205 (1990)].
Physical modifications of amylose for pharmaceutical formulations have also
been disclosed: non-granular amylose as a binder-disintegrant [Nichols et al.,
U.S. Pat. No. 3,490,742], and glassy amylose as a coating for oral, delayed-
release composition due to enzymatic degradation of the coating into the colon
[Alwood et al., U.S. Pat. No. 5,108,758]. These patents are not related to
substituted amylose as a matrix excipient for sustained drug-release and,
accordingly, are not related to the present invention.
Wai-Chiu C. et al. [Wai-Chiu et al., U.S. Pat. No. 5,468,286] disclosed a
starch
binder and/or filler useful in manufacturing tablets, pellets, capsules or
granules.
The tablet excipient is prepared by enzymatically debranching starch with
alpha-
1,6-D-glucanohydrolase to yield at least 20% by weight of "short chain
amylose". No controlled release properties are claimed for this excipient.
Moreover, starch (unmodified, modified or cross-linked) must be enzymatically
treated with alpha-1,6-D-glucanohydrolase to be debranched and to yield so-
called "short chain amylose". Thus, starch with a high content of amylopectin
is
obviously preferred, and amylose is rejected as being unsuitable because
debranching is impossible since it has no branching. The role of amylose is
not
only ignored but also considered negatively.
In connection with this reference, it must also be emphasized that "short-
chain
amylose" does not exist. In the present specification and appended claims,
when the term "amylose" is used, it refers only to amylose having a long chain
consisting of more than 250 glucose units (between 1,000 and 5,000 units
according to most of the scientific literature), joined by alpha-1,4-D glucose
links, in a linear sequence. This is totally different from short chains of 20
to 25
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
4
glucose units. Consequently, this work is not related to the present
invention,
which regards a particular pharmaceutical formulation to maintain the
integrity of
a substituted amylose matrix tablet.
Several patents relate to the use of cross-linked amylose in tablets for
controlled drug-release or as a binder-disintegrant in certain cases [Mateescu
et
al., U.S. Pat. No. 5,456,921; Mateescu et al., U.S. Patent No. 5,603,956;
Cartilier et al., U.S. patent No. 5,616,343; Dumoulin et al., U.S. Pat. No.
5,807,575; Chouinard et al., U.S. Pat. No. 5,885,615; Cremer et al., U.S. Pat.
No. 6,238,698].
Lenaerts V. et al. [U.S. Pat. No. 6,284,273] disclosed cross-linked high
amylose starch rendered resistant to amylase. Such amylase-resistant starches
are obtained by co-cross-linking high amylose starch with polyols. Suitable
agents that could be used as additives to high amylose starch for controlled
release prior to cross-linking of the high amylose starch include, but are not
limited to, polyvinyl alcohol, beta-(1-3) xylan, xanthan gum, locust bean gum
and guar gum.
Lenaerts V. et al. [U.S. Pat. No. 6,419,957] disclosed cross-linked high
amylose
starch having functional groups as a matrix for the slow release of
pharmaceutical agents. This matrix tablet excipient is prepared by a process
comprising the steps of: (a) reacting high amylose starch with a cross-linking
agent cross-linked at a concentration of about 0.1 g to about 40 g of cross-
linking agent per 100 g of high amylose starch to afford cross-linked amylose;
and (b) reacting the cross-linked high amylose starch with a functional group-
attaching reagent at a concentration of about 75 g to about 250 g of
functional
group-attaching reagent per 100 g of cross-linked amylose to afford cross-
linked
amylose having a functional group.
Lenaerts V. et al. [U.S. Pat. No. 6,607,748] disclosed cross-linked high
amylose
starch for use in controlled-release pharmaceutical formulations and
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
manufacturing processes. Such cross-linked high amylose starch is prepared by
(a) cross-linking and chemical modification of high amylose starch, (b)
gelatinization, and (c) drying to obtain a powder of said controlled-release
excipient.
5
All these patents disclose only cross-linked amylose and some of its variants,
which are to be distinguished from linearly-substituted amylose used in the
present invention.
As already mentioned above, carboxymethyl starch has been disclosed as a
tablet disintegrant [McKee, U.S. Pat. No. 3,034,911]. This is explainable as
all
starches used or disclosed in this patent contain low levels of amylose, and
one
knows today that high amylose content is an essential feature to obtain
sustained drug-release properties [Cartilier et al., U.S. Patent No.
5,879,707,
Substituted amylose as a matrix for sustained drug release].
For example, Mehta A. et al. [U.S. Pat. No. 4,904,476] disclosed also the use
of
sodium starch glycolate as a disintegrant. This patent considers only
carboxymethyl starch having a low content in amylose as opposed to the
present invention which considers high amylose starch, but also discloses a
disintegrant, which is the opposite of a sustained-release system.
Staniforth J. et al. [U.S. Pat. No. 5,004,614] disclosed a controlled-release
device with a coating that is substantially impermeable to the entrance of an
environmental fluid and substantially impermeable to the exit of the active
agent
during a dispensing period and having an orifice for drug release. Cross-
linked
or un-cross-linked sodium carboxymethyl starch is proposed among other
materials for coating.
The coated controlled-release system described herein is totally different
from a
matrix tablet when considering the structural aspects and the release
mechanisms involved. Also, the necessary presence of an orifice through the
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
6
coating distinguishes it from the present invention. Furthermore, a
hydrophilic
matrix system, as described in the present invention, necessarily implies that
water penetrates the tablet, contrary to the U.S. Pat. No. 5,004,614
invention,
which requires the coating to be impermeable to an aqueous environment.
Finally, there is no mention of the necessity of having high amylose content,
an
essential feature of the present invention.
Cartilier L. et al. [U.S. Patent No. 5,879,707] disclosed a pharmaceutical
sustained-release tablet for oral administration, consisting of a compressed
blend of at least two dry powders including a powder of at least one
pharmaceutical drug and a powder of a sustained-release matrix for the drug.
The sustained-release matrix consists essentially of non-crystalline, un-cross-
linked, substituted amylose prepared by reacting, in a basic medium, amylose
with at least one organic substituent that reacts functionally with the
hydroxyl
groups of the amylose molecule.
SUMMARY OF THE INVENTION
Substituted amylose is known to be an interesting excipient for the
preparation
by direct compression of drug sustained release hydrophilic matrix tablets.
It has now been discovered that high amylose starch substituted with organic
groups comprising at least one carboxyl group can be advantageously
combined to electrolytes in order to maintain the swollen hydrophilic matrix
tablet integrity when it is immersed in a medium undergoing pH changes
simulating the pH evolution of the environment surrounding an oral
pharmaceutical dosage form transiting along the gastrointestinal tract.
In the absence of an electrolyte, such a swollen tablet produces cracks and/or
partial or complete separation of the tablet parts in order to relieve the
tablet
internal stress, which forbids their normal and safe therapeutic use.
Surprisingly,
the addition of an electrolyte provides a complete stabilization of the
swollen
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
7
matrix structure or at least significantly delays the apparition of the
abovementioned problems and/or decreases their intensity.
More particularly, it has been found that high amylose carboxymethyl starch
matrix tablets can be advantageously improved by the addition of electrolytes.
Such an addition permits to maintain the integrity of the swollen matrix
tablets
while allowing a controlled and sustained release of the drug with a
remarkable
close-to-linear release profile.
A first object of the present invention is thus to provide a pharmaceutical
sustained release tablet with an improved integrity for oral administration of
at
least one drug, wherein the tablet consists of a compressed blend of at least
three dry powders including
a powder of said at least one drug,
a powder of a sustained release matrix for the drug, the sustained
release matrix consisting of an un-cross-linked high amylose starch wherein
said high amylose is substituted by at least one organic substituent
comprising
at least one carboxyl group, and
a powder of at least one electrolyte.
Preferably, the high amylose starch is substituted by at least one organic
substituent which is a carboxyalkyl containing 2 to 4 carbon atoms, a salt of
this
carboxyalkyl or a mixture thereof. More preferably, the organic substituent is
carboxymethyl, sodium carboxymethyl or mixture thereof.
Advantageously, the degree of substitution of the substituted amylose starch,
which is expressed as the ratio of numbers of moles of the at least one
organic
substituent per kg of the high amylose starch, is equal to or higher than 0.1.
More advantageously, the degree of substitution ranges from 0.1 to 0.4.
The electrolytes used in accordance with the present invention may be found in
a dry powder form or in a liquid form adsorbed on a dry powder.
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
8
Preferably, the electrolytes consist of low molecular weight electrolytes
which
may be selected from strong or weak acids, strong or weak bases and salts that
are strong or weak electrolytes. They also may consist of a buffer.
Preferably also, the electrolytes are selected from weak organic bases and
weak organic acids. More preferably, they consist of salts.
Strong electrolytes are preferred to weak electrolytes.
The most preferred electrolytes that may be used in accordance with the
invention are sodium chloride, potassium chloride, calcium chloride, calcium
lactate, sodium sulfate, citric acid, arginine hydrochloride, urea, sodium
acid
phosphate and disodium phosphate.
The drug present in the tablet may have a solubility ranging from very soluble
to
very slightly soluble. It can be in any pharmaceutically suitable form like a
salt, a
free base or a free acid. The tablet of the present invention may also include
more than one drug.
The tablet according to the invention can also include at least one other
excipient liker those commonly used in the pharmaceutical area. By way of
examples, the excipient may consist of hydroxypropylmethylcellulose (HPMC),
lubricants such as magnesium stearate, colorants, anti-oxydants and/or
fillers.
Surprisingly, it has also been discovered that high amylose carboxymethyl
starch matrix tablets containing very soluble ionic drugs show excellent
performance regarding release rates and matrix integrity, particularly when
they
contain high concentrations of that drug. In such cases, the drug also acts as
the electrolyte.
It is thus another object of the present invention to provide a pharmaceutical
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
9
sustained release tablet with an improved integrity for oral administration of
at
least one drug, consisting of a compressed blend of at least two dry powders
including
a powder of said at least one drug, the drug being a very soluble ionic
drug representing at least 20 % by weight of the total weight of the tablet,
and
a powder of a sustained release matrix for the drug, the sustained
release matrix consisting of an un-cross-linked high amylose starch wherein
said high amylose is substituted by at least one organic substituent selected
from the group consisting of carboxymethyl, sodium carboxymethyl and mixture
thereof, said substituted amylose starch having a degree of substitution,
expressed as the ratio of the number of moles of carboxymethyl substituents
per kg of the high amylose starch, that is equal to or higher than 0.1.
Preferably, the degree of substitution of the substituted amylose starch
ranges
from 0.1 to 0.4.
The tablet according to this other object of the invention can also include at
least one other excipient. Suitable excipients are excipients well known in
the
pharmaceutical area and include, without being limited to,
hydroxypropylmethylcellulose (HPMC), lubricants such as magnesium stearate,
colorants, anti-oxydants and fillers.
The invention and its advantages will be better understood upon reading the
following non-restrictive detailed description and examples, with reference
being
made to the accompanying drawings.
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
DESCRIPTION OF THE DRAWINGS
FIG. 1 represents the different types of cracks being observed for high
amylose
carboxymethyl starch matrix tablets: a) C1; b) nCl; c) C2.
5
FIG. 2 represents the different types of bursting being observed for high
amylose carboxymethyl starch matrix tablets: a) DC=double cone (note that
some C2 cracks also appear in this particular case); b) M*=mushroom type, but
on one face only. The second tablet has been dried but nevertheless retains
its
10 characteristic structure.
FIG. 3 is a diagram showing the percentage (%) of acetaminophen released in
acidic and moderately alkaline media from SA, CA.lab-1.55 matrix tablets in
function of time (hours) for 400-mg tablets containing 10% of drug.
FIG. 4 is a diagram showing the percentage (%) of acetaminophen released
from SA, CA.lab-1.8, SA, CA.lab-1.55 and SA, G-2.7 matrix tablets in function
of
time (hours) for 400-mg tablets containing 10% of drug. SA, CA.lab matrix
tablets were immersed for 1 hour in acidic medium (pH=1.2), and then in
moderately alkaline medium (pH=7.4). The data for SA, G-2.7, extracted from
U.S. Patent No. 5,879,707, were obtained under experimental conditions
exactly similar to the SA, CA.lab tablets testing except that a constant pH
medium was used (pH=7.4).
FIG. 5 is a diagram showing the percentage (%) of acetaminophen released
from SA, CA-0.05 matrix tablets in function of time (hours) for 400-mg tablets
containing 10% of drug and different sodium chloride loadings (0, 10 and 15%).
FIG. 6 is a diagram showing the percentage (%) of acetaminophen released
from SA, CA-0.05 matrix tablets in function of time (hours) for 400-mg tablets
containing 10% of drug and 10% of sodium chloride when they are immersed
for 0.5, 1 or 2 hours in an acidic medium.
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
11
FIG. 7 is a diagram showing the percentage (%) of pseudoephedrine
hydrochloride (PE) released from SA, CA-0.05 matrix tablets in function of
time
(hours) for 800-mg tablets containing different drug loadings (20, 37.5, 50
and
60%).
FIG. 8 is a diagram showing total drug-release time (hours) in function of the
pseudoephedrine hydrochloride percentage (%) in SA, CA-0.05 matrix tablets of
different weights (400, 600 and 800 mg).
DETAILED DESCRIPTION OF THE INVENTION
Preliminary considerations
Cartilier L. et al. [U.S. Patent No. 5,879,707] disclosed a pharmaceutical
sustained-release tablet for oral administration, consisting of a compressed
blend of at least two dry powders, including a powder of at least one
pharmaceutical drug and the powder of a sustained-release matrix for the drug.
The sustained-release matrix essentially consists of non-crystalline, un-cross-
linked, substituted amylose prepared by reacting, in a basic medium, amylose
with at least one organic substituent having a function that reacts with the
hydroxyl groups of the amylose molecule. Typical substituted amylose tablets
swell in water, but differ from customary swellable hydrophilic matrices by a
surprisingly high mechanical strength in the swollen state. As a consequence,
it
is possible to create tablets that do not show any disintegration, even if
mechanical stresses occur, such as, for example, after administration in the
gastrointestinal tract. Various aspects of this invention have also been
covered
in the scientific literature [Chebli C. and Cartilier L., J. Pharm. Belg.,
54(2), 51-
53 & 54-56 (1999); Chebli C. et al., Pharm. Res., 16(9), 1436-1440 (1999);
Chebli C. and Cartilier L., Int. J. Pharm., 193(02), 167-173 (2000); Chebli C.
et
al., Int. J. Pharm., 222(2), 183-189 (2001), Cartilier L. et al., Proceedings
of
ISAB2-2005, page 102, 3rd International Symposium on Advanced
Biomaterials/Biomechanics, April 3-6, 2005].
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
12
Several observations can, however, be made about the invention disclosed in
U.S. Patent No. 5,879,707:
- All the examples and experimental results provided in the above-mentioned
references present the use of non-ionic substituted amylose polymers
although Patent No. 5,879,707 does not restrict itself to non-ionic
substituents and mentions the possibility of grafting a carboxylic (-COOH)
substituent to protect the hydrophilic matrix from enzymatic degradation.
- In vitro drug release tests were done in an aqueous medium maintained at a
constant pH (pH=7.34). Since the substituted amylose polymers were non-
ionic, the gelification properties were not pH-dependent, and, thus, there
was no need to conduct release experiments in a pH gradient simulating the
pH evolution of the gastrointestinal tract.
- Also, preferably, the substituted amylose has a substituent-to-amylose ratio
(expressed in mole of substituent per kg of amylose) that is equal to or
higher than 0.4. More preferably, such a ratio ranges from 0.4 to 7Ø
- Furthermore, when the pharmaceutical drug(s) used in the tablet is (are)
very slightly soluble, the powder of such drug(s) may represent up to 80% by
weight of the tablet. When, however, the pharmaceutical drug(s) is(are)
highly soluble, the powder of such drug(s) should not exceed 40% by weight
of the tablet. Furthermore, the results obtained for sodium salicylate, a
freely
soluble drug (see example 5 and Figure 16), show a time for release of 95%
of the drug of 6.5 hours for a 400-mg tablet containing 10% of drug,
demonstrating controlled release but rather poor performance in terms of
sustained release.
On the other hand, carboxymethyl starch, i.e. starch containing a low amount
of
amylose and which has been reacted with chloroacetic acid or sodium
chloroacetate, is used as a disintegrant in immediate-release tablets to
promote
their fast disintegration and subsequent fast dissolution of the active
ingredient
now dispersed in an aqueous environment [Bolhuis G.K. et al., Drug Develop.
Ind. Pharm. 12(4), 621-630 (1986); Bolhuis G.K et al., Acta Pharm. Tech.,
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
13
30(1), 242-32 (1984)]. Such a product has been commercialized among others
under the trademark Explotab . It targets the opposite goal of a hydrophilic
matrix system, which tries to maintain the integrity of the dosage form in
order to
release slowly the active ingredient. Note that erodible matrices are just a
special case where physical and/or chemical matrix degradation is progressive
and controlled to allow controlled release of the active ingredient.
Despite the fact that carboxymethyl starch is used as a disintegrant, it would
be
interesting to evaluate the sustained-release properties of
carboxymethylamylose, more precisely high amylose carboxymethyl starch as
low amylose carboxymethyl starch has served patients for decades and has
thereby proved its safety. Also, since carboxymethylamylose is an ionic
polymer
that will be used for oral sustained drug-release, in vitro release tests now
need
to consider the pH changes occurring in the gastrointestinal tract.
The problem
Matrix tablets comprising high amylose carboxymethyl starch and drug have
been obtained and tested for their release properties according to U.S. Patent
No. 5,879,707. Their release properties also have been evaluated in a pH
gradient simulating the pH evolution of the tablet environment when traveling
along the gastrointestinal tract, i.e. from a strongly acidic to a moderately
alkaline environment.
Matrix tablets containing high amylose starch substituted through an
etherification reaction with chloracetic acid or sodium chloracetate showed
good
sustained drug-release properties in a moderately alkaline medium, i.e. pH=7.4
aqueous solution. Surprisingly, the tablets selected for their best sustained-
release properties and good mechanical resistance to stress, when swollen in
the pH=7.4 aqueous solution, presented cracks, separated into two parts
loosely attached at their centre or even split into several parts when they
were
evaluated with a pH gradient. Some tablets containing high amylose
carboxymethyl starch with a very low substitution degree presented the same
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
14
problems whatever the aqueous medium in which they were immersed.
Such poor mechanical behavior of these tablets forbids their normal
therapeutic
use. Indeed, when the stomach churns, thereby exerting significant physical
force on the formulation, there is a strong risk of the tablet breaking apart,
which
could lead to a burst of drug release, especially when the drug is freely or
very
soluble. Furthermore, the integrity of the matrix tablet must be strictly
maintained in cases such as dry or press-coated tablets, double or multi-
layered
tablets, and geometry-controlled-release tablets.
The solution
The swelling of the various substituted amylose tablets reported in the above-
mentioned references can be described as moderate when compared to the
usual hydrophilic matrices like hydroxypropylmethylcellulose. This is
particularly
true for the high amylose carboxymethyl starch tablets of the present
invention,
which contain a very rigid gel. It is hypothesized that the rigidity of the
gel and its
tight network hinder water penetration into the tablet, but more importantly
strongly decrease diffusion of the dissolved drug out of the matrix tablet.
Consequently, the drug accumulates in a dissolved state inside the tablet,
thereby increasing the internal osmotic pressure, which in the end produces
cracks and/or partial or complete separation of the tablet parts to relieve
the
tablet internal stress.
The easiest logical approach to solve the above-mentioned problems would be
to increase the drug concentration to decrease the polymer concentration,
thereby reducing the tightness of the gel network. Indeed, it was hoped that
an
increase in tablet porosity would resolve the problem of the lack of gel
elasticity,
but such an approach was unsuccessful.
The next logical approach to solve the problem described above would be to
add swelling polymers (ionic or non-ionic) to maintain the integrity of the
high
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
amylose carboxymethyl starch tablet, these polymers hopefully combining with
high amylose carboxymethyl starch to create a more elastic and less dense
network to facilitate drug diffusion, thus relieving the internal stress of
the matrix
tablet. This approach failed totally as well as did approaches based on adding
5 similar substances (pregelatinized starches with high or low amylose
content,
soluble starch derivates). Despite the fact that the approach was similar to
increasing drug concentration, adding non-ionic soluble filler was also tried
but
proved to be unsuccessful. Finally, adding insoluble ionic filler failed as
well.
10 When electrolytes are dissolved in water, the solute exists in the form of
ions in
the solution. Strong electrolytes like NaCI or HCI exist almost completely in
the
ionic form in moderately concentrated aqueous solutions. Inorganic acids such
as HCI, HNO3i H2SO4, inorganic bases such as NaOH and KOH of the alkali
metal family, Ba(OH)2 and Ca(OH)2 of the alkaline earth group, and most
15 inorganic and organic salts are highly ionized and belong to the class of
strong
electrolytes. For weak electrolytes like acetic acid, equilibrium exists
between
the molecules and ions. Most organic acids and bases and some inorganic
compounds, such as H3B03 and NH4OH, belong to the class of weak
electrolytes. Even some salts and complex ions are weak electrolytes [Martin
A.
et al., Physical Pharmacy, 1983a].
The theory of electrolytes has found several applications in the
pharmaceutical
field. Often, when an organic drug is poorly soluble, one synthesizes a salt
thereof to increase the water solubility of the drug. Also, electrolytes are
added
to adjust the tonicity of injectable solutions to make them isotonic. Osmotic
pumps are a well-known type of drug-delivery device where the use of salts
allows the generation of a driving force, i.e. osmotic pressure, permitting
constant drug release through a hole drilled in the semi-permeable membrane
surrounding the core [Martin A. et al., Physical Pharmacy, 1983b].
Electrolytes
may be employed as osmotic agents although non-ionic substances may be
deployed too: "Osmagents useful as release modifying agents in the present
invention include, for example, sodium chloride, calcium chloride, calcium
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
16
lactate, sodium sulfate, lactose, glucose, sucrose, mannitol, urea, and many
other organic and inorganic compounds known in the art" [U.S. Pat. No.
5,004,614]. This is indeed a classical application of osmotic agents promoting
or
accelerating drug release. All these applications are based on the fact that
electrolytes are generally highly soluble and because they generate osmotic
pressure resulting from their dissolution in aqueous media.
Adding an electrolyte to the high amylose carboxymethyl starch matrix
formulation, typically sodium chloride, is the opposite of what should be done
logically. Indeed, sodium chloride should pump more water and faster inside
the
tablet, thereby increasing internal osmotic pressure, and making the tablet
present cracks, separate into two parts loosely attached at their centre or
even
burst into several parts, and all that more quickly and at a higher level than
in
the absence of the said electrolyte. Surprisingly, the addition of an
electrolyte
provides complete stabilization of the swollen matrix structure or at least
significantly delays the appearance of the above-mentioned problems and/or
decreases their intensity, thereby allowing its use in oral drug delivery.
Nevertheless, strong electrolytes are preferred to weak electrolytes like weak
organic acids and bases.
Surprisingly, it was observed that high amylose carboxymethyl starch matrix
tablets containing very soluble ionic drugs like pseudoephedrine hydrochloride
showed excellent performance regarding release rates and matrix integrity,
especially when they contained high concentrations of that drug. In cases of
very soluble ionic drugs, it might be useful to replace, at least partially,
current
fillers like lactose by an electrolyte when formulations containing low drug
concentrations are requested.
Pharmaceutical sustained-release tablets, according to the invention, are
prepared by compressing, as is known per se, a blend of dry powders, including
at least a pharmaceutical drug powder, at least a powder of high amylose
carboxymethyl starch used as a sustained-release matrix and an electrolyte. If
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
17
desired, the tablets may also include a small amount of lubricant, and one or
more fillers, also in powder form. If desired, a mixture of two or more drugs
may
be used instead of one. Once the drug and the other ingredients have been
blended, generally by conventional means, including, but not limited to,
powder
blending, dry or wet granulation, the resulting blend is compressed to form a
tablet. The method of preparing such tablets is well-known in the art and need
not be described further. Pharmaceutical sustained-release tablets according
to
the invention can also be of the dry-coated type, prepared by direct
compression for example. Once again, the methods of preparing dry-coated
tablets are well-known and need not be described further.
EXAMPLES
EXAMPLE 1
Preparation of matrix tablets
Considering that the present invention concerns with a pharmaceutical
sustained-release tablet for oral administration, consisting essentially of a
compressed blend of at least three dry powders, including the powder of at
least
one pharmaceutical drug, the powder of a sustained-release matrixforthe drug
and the powder of at least one electrolyte, preparation of the said matrix
tablets
will be explained below. The drug(s), high amylose-substituted starches,
electrolytes and various excipients used in matrix formulations are presented
here as is the tablet preparation procedure.
To illustrate the advantages of the present invention, various drugs have been
selected as models for the evaluation of swollen matrix tablet integrity or
for
release profile study. Note that some other drugs were simply discussed in the
description of the present invention. For clarity, certain descriptive terms
reported in Table 1 will be used. They allude to the ranges of solubility
given in
the pharmacopeia monographs ["The United States Pharmacopeia XXIII-The
National Formulary XVIII", 1995]. These terms are defined in a table on page
2071, entitled "Description and Solubility', which gives the corresponding
parts
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
18
of solvent required for one part of solute. The solubility of the various
drugs
reported in the examples and/or simply discussed herein is described as
follows:
Table 1. U.S.P. XXIII Solubility Specifications
Parts of solvent
Descriptive terms required to solubilize Examples of drugs
1 part of drug
Very soluble Less than 1 Pseudoephedrine
hydrochloride, Sodium
salicylate (in boiling water)
Freely soluble From 1 to 10 Sodium salicylate,
bupropion hydrochloride
Soluble From 10 to Acetaminophen
30 (in boiling water)
Sparingly soluble From 30 to Acetaminophen (room
100 temperature)
Slightly soluble From 100 to 1,000 Theophylline
Very slightly soluble From 1,000 to 10,000
Practically insoluble 10,000 and
or insoluble over
First, substituted amylose (SA) was prepared by reacting high amylose starch
(Hylon VII , The National Starch and Chemical Company, Bridgewater, NJ,
U.S.A.) with sodium chloracetate (Aldrich Chemical Company, Saint Louis, MO,
U.S.A.), in a strongly basic medium [see U.S. Patent No. 5,879,707]. Different
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
19
degrees of substitution are obtained by simply varying the substituent/high
amylose starch ratio. The products prepared according to this example are
referred to as SA, CA.lab-n, where "SA" means high amylose substituted starch,
"CA" defines the substituent used, herein chloracetate, ".Iab" means batches
obtained at the laboratory scale, and "n" represents the degree of
substitution
(DS) expressed as the ratio "mole of substituent/kg of high amylose starch".
It is
worth remembering that Hylon VII contains approximately 70% of amylose
chains and 30% of amylopectin. Note that SA, CA.lab-0.00, which will be used
in Example 10, is high amylose starch treated in the same way except that no
reactant was added to the reacting medium.
Second, high amylose sodium carboxymethyl starch was obtained directly from
Roquette Freres S.A. (Lestrem, France). However, SA, CA pilot batches were
dried with alcohol in place of acetone. The DS is expressed in anotherway than
for laboratory scale batches: it is defined as the number of moles of reactant
divided by the number of moles of anhydroglucose; the number of moles of
anhydroglucose is obtained by dividing the starch dry weight by 162
(162=molecular weight of one unit of anhydroglucose). SA, CA-0.05 (more
precisely 0.046) and 0.07 (more precisely 0.067) are used in the present
invention.
Some electrolytes were also included in the present invention: sodium
chloride,
sodium acid phosphate, disodium phosphate, arginine hydrochloride, and citric
acid.
Finally, when specific excipients were part of the formulation evaluated,
their
role was explained in the appropriate example.
Tablets have been prepared by direct compression, i.e. dry mixing of drug, SA,
CA.lab-n or SA, CA-n, electrolytes, if any, and excipients, if any, followed
by
compression. The drug and the other ingredients of the formulation were mixed
manually in a mortar. For swollen matrix integrity evaluation, tablets
weighing
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
400 mg each were compressed for 20 seconds at 2.5 ton/cmZ pressure on an
IR 30-ton press (C-30 Research & Industrial Instruments Company, London,
U.K.). The diameter of the tablets was 1.26 cm. For drug release evaluation,
tablets weighing 400, 600 or 800 mg each were compressed for 20 seconds at
5 2.5 ton/cm2 pressure on an IR 30-ton press (C-30 Research & Industrial
Instruments Company). The diameter of the tablets was 1.26 cm.
EXAMPLE 2
Evaluation of swollen tablet integrity
10 The applicants have observed that high amylose carboxymethyl starch matrix
tablets presented cracks, separated into two parts loosely attached at their
centre or even split into several parts when swollen in aqueous solution,
particularly when going through a pH gradient. Surprisingly, it was noted that
the
addition of an electrolyte provides complete stabilization of the swollen
matrix
15 structure or at least significantly delays the appearance of the above-
mentioned
problems and/or decreases their intensity, thereby allowing its application in
oral
drug delivery. Thus, a standardized method has been designed to describe the
modifications occurring during the tablet's immersion in an aqueous solution
as
well as the moment of their appearance.
Tablets prepared as disclosed hereinabove in Example 1 were placed
individually in 900 ml of a hydrochloric acid solution medium (pH=1.2), at 37
C,
in U.S.P. XXIII dissolution apparatus No. 2 equipped with a rotating paddle
(50
rpm). After remaining in the acidic solution for a period of 1.5 hours, the
tablets
were transferred to a phosphate buffer solution medium (pH=7.4), at 37 C, in
the same U.S.P. XXIII dissolution apparatus No. 2 equipped with a rotating
paddle (50 rpm) until the end of the test. All formulations were tested in
triplicate.
The observation of macroscopic transformations has been standardized with
specific qualitative terms describing them and recording the moment of their
appearance (hours) in a table. A sequence of two events, cracks followed by
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
21
bursting, was noted as the appearance of crack(s) in the tablet was often
followed by more drastic modification of the matrix structure, the bursting
being
partial or total. The following terms have been employed: C1=crack type 1;
nC1=multiple cracks type 1; C2=cracks type 2; DC=double cone; M=mushroom;
M*=mushroom type, but on one face only; flakiness; erosion. Cl represents a
single crack appearing along the radial surface of the cylinder. nCl
represents
multiple cracks appearing along the radial surface of the tablet. C2 means
that
one or more cracks appear on one or both facial surfaces of the tablet. DC
means that the tablet separates longitudinally into two parts loosely attached
at
their centre; each part adopts a convex shape due to internal tension of the
shrinking gel. M* and M represent partial bursting of the tablet where all the
parts remained well attached to the main part of the tablet; the shape looks
like
a mushroom or like dry earth in some desert areas. These structural
modifications have been schematically represented in Figures 1 and 2.
Some empirical rules may be drawn from the analysis of Examples 4 to 25: Cl
leads to DC; C2 leads to M* or M; nC1 leads to flakiness; C1+C2 leads to DC,
M, M*, DC+M* or DC+M; the erosion process is not linked to the appearance of
cracks. However, the addition of electrolytes smoothes the transformation
process as some crack phenomena do not necessarily lead to the bursting
appearance; electrolytes may also hinder the appearance of DC, leading to the
appearance of a M structure. This allows the consideration of a rather semi-
quantitative approach, keeping in mind that the more the tablet fully splits
apart,
the higher are the risks of undesired burst release in vivo.
EXAMPLE 3
Drug release evaluation
The drug release properties of some typical matrix tablets were assessed by an
in vitro dissolution test. Acetaminophen was used as drug model with
solubility
intermediate between soluble and sparingly soluble; its solubility is not
influenced by pH in physiological conditions. Pseudoephedrine hydrochloride
was used as a very soluble ionic drug model.
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
22
Two types of experimental conditions were tested: a) constant pH (pH=7.4), a
method exactly similar to that in U.S. Patent No. 5,879,707; b) pH gradient,
where the pH progressed from an acidic value (pH=1.2) to a moderately alkaline
one (pH=7.4), simulating roughly physiological conditions.
a) Constant pH
Tablets prepared as disclosed hereinabove in Example 1 were placed
individually in 900 ml of a phosphate buffer solution medium (pH=7.4), at 37
C,
in U.S.P. XXIII dissolution apparatus No. 2 equipped with a rotating paddle
(50
rpm). The amount of acetaminophen released at predetermined time intervals
was followed spectrophotometrically (acetaminophen: 242 nm). All formulations
were tested in triplicate. The drug release results are expressed in terms of
cumulative % or mg released in function of time (hours).
b) pH gradient
Tablets prepared as disclosed hereinabove in Example 1 were placed
individually in 900 ml of a hydrochloric acid solution medium (pH=1.2), at 37
C,
in U.S.P. XXIII dissolution apparatus No. 2 equipped with a rotating paddle
(50
rpm). After remaining in the acidic solution for a period of 0.5, 1 or 2
hours, the
tablets were transferred to a phosphate buffer solution medium (pH=7.4), at
37 C, in the same U.S.P. XXIII dissolution apparatus No. 2 equipped with a
rotating paddle (50 rpm) until the end of the test. The amount of
acetaminophen
or pseudoephedrine hydrochloride released at predetermined time intervals was
followed spectrophotometrically (acetaminophen: 242 nm and pseudoephedrine
hydrochloride: 257 nm). All formulations were tested in triplicate. The drug
release results are expressed in terms of cumulative % or mg released in
function of time (hours).
EXAMPLE 4
Effect of tablet drug loading on in vitro tablet integrity
Tablets were prepared according to the procedure described in Example 1 and
evaluated according to the test conditions described in Example 2. The active
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
23
ingredient (A.1.) was acetaminophen, a non-ionic drug, sparingly soluble to
soluble, depending on the temperature (from room temperature to boiling
water), with its solubility uninfluenced by pH at physiological conditions.
The
formulations and their evaluation are presented in Table 2.
Table 2. Evaluation of the integrity of swollen tablets containing increasing
acetaminophen concentrations and SA, CA-0.05 or SA, CA-0.07
SA, SA,
A.I. Cracks Bursting
No. CA-0.05 CA-0.07
(%) (%) (%) Time Type Time Type
1 10 90 4.6 C1 8.0 DC
2 10 90 1.5 C1 4.5 DC
3 20 80 4.6 C1 8.0 DC
4 20 80 1.5 C1 2.0 DC
5 30 70 3.0 C1 4.0 DC
6 50 50 Disintegration
7 60 40 Disintegration
8 80 20 Impossible to obtain compressed
tablets
Matrix tablets containing acetaminophen and high amylose carboxymethyl
starch are not practically useful, whatever their substitution degree, as they
quickly show major cracks (type Cl) followed by split-up in the worst form
(DC).
Increased drug loading accelerates the process and/or amplifies it and does
not
constitute a valid approach to solve the problem.
EXAMPLE 5
Effect of adding a non-ionic hydrophilic polymer
Tablets were prepared according to the procedure described in Example 1 and
evaluated according to the test conditions described in Example 2. The A.I.
was
acetaminophen. The formulations and their evaluation are presented in Table 3.
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
24
Table 3. Evaluation of the integrity of swollen tablets containing
acetaminophen, SA, CA-0.05 and increasing concentrations of HPMC K4M
HPMC
A.I. SA, Methocel Cracks Bursting
No. CA-0.05
K4M
( /a) (%) (%) Time Type Time Type
1 10 0 90 No
2 10 10 80 No
3 10 25 65 No
4 10 45 45 6 nCl 2.0 Flakiness
10 50 40 6 nCl 4.0 Flakiness
6 10 60 30 6 nCl Flakiness
7 10 65 25 2 nCl Flakiness
8 10 70 20 2 nCl Flakiness
9 10 72 18 4.5 C1+C2 M+DC
10 75 15 5 C1 DC
5
Matrix tablets containing a high percentage of high amylose carboxymethyl
starch SA, CA-0.05 and a low percentage of HPMC K4M still present the same
problems, i.e. crack Cl and bursting DC. Increasing the HPMC K4M
concentration brings another type of problem as all tablets now demonstrate
10 flakiness. When the percentage of HPMC is higher than that of SA, CA-0.05,
the tablets do not present structural problems anymore, but are outside the
scope of this invention. Thus, the addition of a non-ionic hydrophilic polymer
is
not a valuable solution to the matrix integrity problem of high amylose
carboxymethyl starch matrix tablets.
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
EXAMPLE 6
Effect of adding a non-ionic hydrophilic polymer (cont.)
Tablets were prepared according to the procedure described in Example 1 and
evaluated according to the test conditions described in Example 2. The A.I.
was
5 acetaminophen. The formulations and their evaluation are presented in Table
4.
Table 4. Evaluation of the integrity of swollen tablets containing
acetaminophen, SA, CA-0.07 and increasing concentrations of HPMC K4M
HPMC
A.I. SA, Methocel Cracks Bursting
No. CA-0.07
K4M
(%) (%) (%) Time Type Time Type
1 10 60 30 4.5 nCl Flakiness
2 10 70 20 3 C1+C2 M+DC
One can make the same observations regarding the addition of HPMC K4M to
SA, CA-0.07 as in the case of SA, CA-0.05. A low percentage of HPMC K4M is
not able to avoid the problems of cracking and bursting. Increasing the HPMC
K4M percentage starts flakiness phenomena. Changing the substitution degree
and adding a non-ionic hydrophilic polymer is not a valuable solution to the
above-mentioned problems.
EXAMPLE 7
Effect of adding a non-ionic hydrophilic polymer (but less hydrophilic than
HPMC K4M)
Tablets were prepared according to the procedure described in Example 1 and
evaluated according to the test conditions described in Example 2. The A.I.
was
acetaminophen. The formulations and their evaluation are presented in Table 5.
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
26
Table 5. Evaluation of the integrity of swollen tablets containing
acetaminophen, SA, CA-0.05 and increasing concentrations of HPMC E4M
HPMC
A.I. SA, Methocel Cracks Bursting
No. CA-0.05
E4M
(%) (%) (%) Time Type Time Type
1 10 0 90 No
2 10 45 45 6 nCl Flakiness
3 10 50 40 6 nCl Flakiness
4 10 60 30 6 nCl Flakiness
Matrix tablets containing high amylose carboxymethyl starch SA, CA-0.05 and a
percentage of HPMC E4M varying from 30 to 45% still present the same
problems of flakiness. When the percentage of HPMC E4M is higher than that
of SA, CA-0.05, tablets do not present structural problems anymore, but are
outside the scope of this invention. Thus, the addition of a non-ionic polymer
less hydrophilic than HPMC K4M is not a valuable solution to the matrix
integrity
problem of high amylose carboxymethyl starch matrix tablets. The small gain in
time before the first cracks appear in the tablet may be explained by the fact
that HPMC E4M is less hydrophilic.
EXAMPLE 8
Effect of adding an ionic hydrophilic polymer
Tablets were prepared according to the procedure described in Example 1 and
evaluated according to the test conditions described in Example 2. The A.I.
was
acetaminophen. The formulations and their evaluation are presented in Table 6.
Note that in the case of formulations 2 and 3, the tablets were immersed for
only 30 minutes in acidic medium.
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
27
Table 6. Evaluation of the integrity of swollen tablets containing
acetaminophen, SA, CA-0.05and increasing concentrations of Carbopol
SA, Carbopol Magnesium
A.I. Cracks Bursting
No. CA-0.05 974 P NF stearate
(%) (%) (%) (%) Time Type Time Type
1 10 75 15 7 C1 DC
2 10 74.5 15 0.5 Significant
No erosion
3 10 79.5 10 0.5 Significant
No erosion
The addition of an ionic hydrophilic polymer to SA, CA-0.05 matrix tablets
does
not stop the appearance of cracks and bursting phenomena. Furthermore,
adding a hydrophobic lubricant like magnesium stearate to such compositions
starts a significant erosion process. This adds to the impossibility of using
such
polymers in the formulation of SA, CA-0.05 matrix tablets to solve the above-
mentioned problems.
EXAMPLE 9
Effect of adding pregelatinized starch (with low amylose content)
Tablets were prepared according to the procedure described in Example 1 and
evaluated according to the test conditions described in Exarriple 2. The A.I.
was
acetaminophen. The formulations and their evaluation are presented in Table 7.
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
28
Table 7. Evaluation of the integrity of swollen tablets containing
acetaminophen, SA, CA-0.05 or SA, CA-0,07 and increasing concentrations
of Lycatab PGS
SA, Lycatab
A.I. SA, Cracks Bursting
No. CA-0.05 CA-0.07 PGS
(%) ( /a) (%) (%) Time Type Time Type
1 10 89.75 0.25 4.5 C1 7.0 DC
2 10 88.75 1.25 4.5 C 1 7.0 DC
3 10 87.50 2.50 4.5 C1 8.0 DC
4 10 85.00 5.00 5.0 C1 -8.0 DC
10 80.00 10.00 5.0 C1 8.0 DC
6 10 75.00 15.00 4.5 C1 8.0 DC
7 10 89.75 0.25 1.0 C1 3.5 DC
8 10 88.75 1.25 1.0 C1 3.5 DC
9 10 87.50 2.50 1.0 C1 3.5 DC
10 85.00 5.00 1.0 C1 3.5 DC
11 10 80.00 10.00 1.0 C1 3.5 DC
5
The addition of pregelatinized starch with low amylose content did not help to
maintain the integrity of SA, CA-0.05 high amylose carboxymethyl starch
matrices. Increasing the degree of substitution of high amylose carboxymethyl
starch while adding the said pregelatinized starch accelerated the process as
10 Cl cracks and DC bursting appeared slightly sooner than in the absence of
pregelatinized starch (see Example 4).
EXAMPLE 10
Effect of adding pregelatinized high amylose starch
Tablets were prepared according to the procedure described in Example 1 and
evaluated according to the test conditions described in Example 2. The A.I.
was
acetaminophen. The formulations and their evaluation are presented in Table 8.
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
29
Table 8. Evaluation of the integrity of swollen tablets containing
acetaminophen, SA, CA-0.05 and increasing concentrations of SA, CA-0.00
SA, SA,
A.I. Cracks Bursting
No. CA-0.05 CA-0.00
(%) (%) (%) Time Type Time Type
1 10 80 10 5.5 C1+C2 8.0 DC
2 10 70 20 6.0 C1+C2 8.0 DC
3 10 60 30 6.0 C1+C2 8.0 DC
4 10 50 40 6.0 C1+C2 8.0 DC
The addition of pregelatinized starch with high amylose content did not help
to
maintain the integrity of SA, CA-0.05 high amylose carboxymethyl starch
matrices. The said pregelatinized starch slightly delayed the apparearance of
cracks and C2 showed in addition to Cl. DC bursting appeared at the same
moment as in the absence of pregelatinized starch (see Example 4).
EXAMPLE 11
Effect of adding dextrin, an oligosaccharide derived from starch
Tablets were prepared according to the procedure described in Example 1 and
evaluated according to the test conditions described in Example 2. The A.I.
was
acetaminophen. The formulations and their evaluation are presented in Table 9.
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
Table 9. Evaluation of the integrity of swollen tablets containing
acetaminophen, SA, CA-0.05 or SA, CA-0.07 and increasing concentrations
of dextrin
Dextrin
SA, SA,
A.I. Type 2 Cracks Bursting
No. CA-0.05 CA-0.07
Sigma
(%) (%) (%) (%) Time Type Time Type
1 10 87 3 4.66 C1 7.66 DC
2 10 85 5 4.66 C1 7.66 DC
3 10 80 10 4.66 C1 7.66 DC
4 10 75 15 4.0 C1 5.66 DC
5 10 70 20 3.75 C1 5.66 DC
6 10 87 3 1.5 C1 4.5 DC
7 10 85 5 1.5 C1 4.0 DC
8 10 80 10 1.5 C1 4.0 DC
9 10 75 15 1.0 C1 2.0 DC
10 10 70 20 1.0 C1 2.0 DC
5
Increasing concentrations of an oligosaccharide like dextrin did not modify
the
nature of the problems associated with the use of SA, CA-0.05 or 0.07 matrix
tablets, but they progressively decreased the time before their appearance.
Thus, adding similar polymers of lower molecular weight does not constitute a
10 valid solution to resolve the above-mentioned problems.
EXAMPLE 12
Effect of adding a non-ionic soluble filler, sugar
A non-ionic soluble filler, sugar, was added to the formulation of SA, CA-0.05
15 matrix tablets containing acetaminophen. Tablets were prepared according to
Example I and evaluated as described in Example 2. However, this strategy
proved to be ineffective too.
For example, a tablet containing 10% of acetaminophen, 80% of SA, CA-0.05
and 10% of saccharose showed cracks (C1+C2 type) after 2 hours only and
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
31
split apart (DC+M type) after 4 hours only. The same kind of observations as
in
the case of dextrin can be made. Increasing the solubility of a non-ionic
saccharidic excipient (saccharose>dextrin>starch) has a negative effect on the
integrity of the matrix tablet (see also Example 4 for comparison).
EXAMPLE 13
Effect of adding an insoluble ionic filler
.Considering that increasing the solubility of the non-ionic excipient added
to the
formulation negatively affected the integrity of SA, CA matrix tablets, some
trials
were performed with an insoluble ionic filler, EmcompressO (a certain type of
calcium phosphate). Again, it did not resolve the problems, but even increased
them. For example, a tablet containing 10% of acetaminophen, 75% of SA, CA-
0.05 and 15% of Emcompress showed a Cl type crack after only 1.5 hours,
leading quickly to DC bursting.
EXAMPLE 14
Effect of adding a low molecular weight organic acid
Tablets were prepared according to the procedure described in Example 1 and
evaluated according to the test conditions described in Example 2. The A.I.
was
acetaminophen. The formulations and their evaluation are presented in Table
10.
Table 10. Evaluation of the integrity of swollen tablets containing
acetaminophen, SA, CA-0.05 and increasing concentrations of citric acid
SA, Citric
A.I. Cracks Bursting
No. CA-0.05 acid
(%) (%) (%) Time Type Time Type
1 10 85 5 5.0 C2 7.0 DC+M*
2 10 80 10 6.0 C2 8.0 DC+M*
The addition of a low molecular weight organic acid like citric acid, a weak
electrolyte, helped to partially resolve the problems, i.e. cracks and
bursting.
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
32
Indeed, the nature of cracks changed from C1 to C2, and the moment of their
appearance was delayed slightly.
EXAMPLE 15
Effect of adding a salt formed from a low molecular weight organic base and
an acid
Tablets were prepared according to the procedure described in Example I and
evaluated according to the test conditions described in Example 2. The A.I.
was
acetaminophen. The formulations and their evaluation are presented in Table
11.
Table 11. Evaluation of the integrity of swollen tablets containing
acetaminophen, SA, CA-0.05 and increasing concentrations of arginine
hydrochloride
SA, Arginine
A.I. Cracks Bursting
No. CA-0.05 hydrochloride
(%) (%) (%) Time Type Time Type
1 10 80 10 5.0 C1 12.0 DC
2 10 75 15 4.5 C2 M
3 10 70 20 5.0 C2 M*
4 20 70 10 4.5 C2 M
5 30 60 10 4.5 C1 DC
The addition of arginine hydrochloride helped to partially resolve the above-
mentioned problems. Indeed, not only the nature of the cracks changed from
Cl to C2 when arginine hydrochloride concentration was increased, but also the
nature of the bursting changed from DC to M or M*, thus preserving the general
shape of the tablet. However, too large an increase in drug concentration
while
retaining the same arginine hydrochloride concentration made the problems
reappear. Thus, the concentration of the electrolyte added to the formulation
needs to be adapted to the concentration and solubility of the A.I. present.
Note
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
33
that tablet No. 1 showed significant swelling (300%), but demonstrated
shrinking
after 5 hours of testing.
EXAMPLE 16
Effect of adding a phosphate buffer (pH = 7.4)
Tablets were prepared according to the procedure described in Example 1 and
evaluated according to the test conditions described in Example 2. The A.I.
was
acetaminophen. The formulations and their evaluation are presented in Table
12.
Table 12. Evaluation of the integrity of swollen tablets containing
acetaminophen, SA, CA-0.05 or SA, CA-0,07 and increasing concentrations
of phosphate buffer (pH = 7.4)
SA, SA, ,
No. A.I. CA-0.05 CA-0.07 Na2HPO4 Cracks Bursting
(pH=7.4)
(mg) (mg) (mg) (mg/mg) Time Type Time Type
1 40 359.0 0.2/0.8 5 C1+C2 7.0 DC
2 40 355.2 1.113.8 5 C1+C2 7.0 M
3 40 350.3 2.1/7.6 5 C1+C2 6.0 M
4 40 340.6 4.2/15.2 4.5 C1+C2 6.0 M
5 40 321.2 8.4/30.4 3.5 C1 5.0 M
6 40 359.0 0.2/0.8 1.5 C1 3.0 DC
7 40 355.2 1.1/3.8 1.5 C1 4.0 DC
8 40 350.3 2.1/7.6 1.5 C1 4.0 DC
9 40 340.6 4.2/15.2 1.5 C1 5.0 DC
10 40 321.2 8.4/30.4 1.0 C1 2.0 DC
Regarding SA, CA-0.05 matrix tablets, the addition of a buffer (pH=7.4) did
not
prolong the time before cracks or bursting appeared, but 1% of such a buffer
was sufficient to favorably change the nature of the cracks, especially the
bursting, which was now partial (=M). Such an improvement was not noted for
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
34
SA, CA-0.07. A higher DS means that more carboxylic functions will be grafted
on the polymer, thus changing the behavior of the matrix in the presence of a
buffer. The addition of a buffer may positively affect the integrity of
swollen high
amylose carboxymethyl starch matrices, provided the nature and concentration
of the buffer (pH value) are carefully selected in function of DS of the
polymer
and the nature and concentration of the drug included in the tablet.
EXAMPLE 17
Effect of adding a phosphate buffer (pH = 6.0)
Tablets were prepared according to the procedure described in Example 1 and
evaluated according to the test conditions described in Example 2. The A.I.
was
acetaminophen. The formulations and their evaluation are presented in Table
13.
Table 13. Evaluation of the integrity of swollen tablets containinq
acetaminophen, SA, CA-0.05 or SA, CA-0.07 and increasing concentrations
of phosphate buffer (pH = 6.0)
SA, SA, ,
No. A.I. CA-0.05 CA-0.07 NaZHPO4 Cracks Bursting
(pH=6.0)
(mg) (mg) (mg) (mg/mg) Time Type Time Type
1 40 358.8 1.0/0.1 5 C1 6.0 DC
2 40 354.2 5.2/0.6 5 C1 6.0 DC
3 40 348.4 10.4/1.2 5 C1 6.0 DC
4 40 336.8 20.8/2.4 4.75 C1 6.0 DC
5 40 313.6 41.6/4.8 4.5 C1+C2 6.0 DC
6 40 358.8 1.0/0.1 1 C1 3.5 DC
7 40 354.2 5.2/0.6 1 C1 3.5 DC
8 40 348.4 10.4/1.2 1 C1 3.5 DC
9 40 336.8 20.8/2.4 1 C1 3.5 DC
10 40 313.6 41.6/4.8 1 C1 3.5 DC
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
For SA, CA-0.05 matrices, the addition of a phosphate buffer (pH=6.0) very
slightly increased the time before cracks appeared. Regarding SA, CA-0.07, no
improvement was noticed for the range of concentrations investigated. The
remarks in Example 16 about the choice of the buffer nature and concentration
5 as well as the effect of drug nature and concentration apply equally here.
EXAMPLE.18
Effect of adding a phosphate buffer (pH = 5.4)
Tablets were prepared according to the procedure described in Example 1 and
10 evaluated according to the test conditions described in Example 2. The A.I.
was
acetaminophen. The formulations and their evaluation are presented in Table
14.
Table 14. Evaluation of the integrity of swollen tablets containing
15 acetaminophen, SA, CA-0.05 or SA, CA-0.07 and increasing concentrations
of phosphate buffer (pH = 5.4)
NaH2PO4.H20/
SA, SA,
A.I. NaaHPO4 Cracks Bursting
No. CA-0.05 CA-0.07
(pH=5.4)
(mg) (mg) (mg) (mg/mg) Time Type Time Type
1 40 358.8 1.2/0.1 4.0 C1 5.5 DC
2 40 353.8 6.0/0.2 4.5 C1+C2 7.0 DC
3 40 347.7 11.9/0.5 4.5 C1+C2 8.0 M
4 40 335.3 23.8/0.9 5.5 C2 9.0 M
5 40 310.6 47.6/1.8 5.5 C2 9.0 M
6 40 358.8 1.2/0.1 1 C2 3.5 DC
7 40 353.8 6.0/0.2 1 C2 3.5 DC
8 40 347.7 11.9/0.5 1 C2 3.5 DC
9 40 335.3 23.8/0.9 1 C2 3.5 DC
10 40 310.6 47.6/1.8 1 C2 3.5 DC
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
36
The addition of increasing concentrations of phosphate buffer (pH=5.4) to SA,
CA-0.05 matrix tablets shows an improvement in the swollen tablet's integrity,
in
the nature of the cracks and bursting as well as in the time of their
appearance.
Regarding SA, CA-0.07 matrix tablets, a minor improvement was only noticed in
the type of crack appearing. The remarks in Example 16 about the choice of
buffer nature and concentration as well as the effect of drug nature and
concentration apply equally here.
EXAMPLE 19
Effect of adding salt
Tablets were prepared according to the procedure described in Example 1 and
evaluated according to the test conditions described in Example 2. The A.I.
was
acetaminophen. The formulations and their evaluation are presented in Table
15.
Table 15. Evaluation of the integrity of swollen tablets containing
acetaminophen, SA, CA-0.05 or SA, CA-0.07 and increasing concentrations
of sodium chloride
SA, SA,
A.I. NaCi Cracks Bursting
No. CA-0.05 CA-0.07
(%) (%) (%) (%) Time Type Time Type
1 10 89.75 0.25 4.66 C1 7.0 DC
2 10 88.75 1.25 4.66 C1 7.0 DC
3 10 87.50 2.50 4.66 C1 7.0 DC
4 10 85.00 5.00 4.66 C1 7.0 DC
5 10 80.00 10.00 5.5 C1 8.0 M*
6 10 75.00 15.00 5.0 C1 8.0 M*
7 10 89.75 0.25 1.5 C1 4.0 DC
8 10 88.75 1.25 1.5 C1 4.5 DC
9 10 87.50 2.50 1.5 C1 4.5 DC
10 10 85.00 5.00 4.66 C1 7.0 DC
11 10 80.00 10.00 5.5 C1 M*
12 10 75.00 15.00 9.5 C1 M*
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
37
Adding sodium chloride in concentrations above 5% to SA, CA-0.05 matrix
tablets improved the swollen tablets integrity in the nature of the bursting
(M*)
and slightly increased the time before Cl cracks appeared. This trend was also
observed for SA, CA-0.07 matrix tablets, but with a dramatic increase of Cl
appearance time (almost 7 x). Again, there was a correlation between the
electrolyte nature and concentration on the one hand and the degree of
substitution of the polymer matrix on the other hand when trying to stabilize
a
high amylose carboxymethyl starch matrix.
EXAMPLE 20
Effect of adding salt (cont.)
Tablets were prepared according to the procedure described in Example 1 and
evaluated according to the test conditions described in Example 2. The A.I.
was
acetaminophen. The formulations and their evaluation are presented in Table
16.
Table 16. Evaluation of the integrity of swollen tablets containing SA, CA-
0.05 and increasing concentrations of acetaminophen and sodium chloride
A.I. SA, NaCI Cracks Bursting
No. CA-0.05
(%) (%) (%) Time Type Time Type
1 10 80 10 5.5 C1 8.0 M*
2 20 70 10 4.0 C1 12.0 DC
3 30 60 10 4.5 C1 M*
4 40 50 10 No
5 50 40 10 No
6 30 55 15 No
7 40 45 15 No
8 20 60 20 No
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
38
Increasing both drug and sodium chloride concentrations fully stabilized the
swollen matrix tablets. No cracks or bursting appeared, demonstrating the
surprising benefit of the addition of electrolytes.
EXAMPLE 21
Effect of adding salt (cont.)
Tablets were prepared according to the procedure described in Example 1 and
evaluated according to the test conditions described in Example 2. The A.I.
was
acetaminophen. The formulations and their evaluation are presented in Table
17.
Table 17. Evaluation of the integrity of swollen tablets containing
acetaminophen, SA, CA-0.05 and sodium chloride, compressed at different
compression forces
SA, Compression
A.I. NaCI Cracks Bursting
No. CA-0.05 force
(%) (%) (Tons) (%) Time Type Time Type
1 40 45 1.0 15 No
2 40 45 1.5 15 No
3 40 45 2.0 15 No
When drug and sodium chloride concentrations were carefully selected,
compression force, as usually implemented to manufacture substituted amylose
matrix tablets, did not influence the integrity of the swollen matrix tablets.
This
again demonstrates the usefulness of the invention.
EXAMPLE 22
Effect of adding salt (cont.)
Tablets were prepared according to the procedure described in Example 1 and
evaluated according to the test conditions described in Example 2. The A.I.
was
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
39
acetaminophen. The formulations and their evaluation are presented in Table
18.
Table 18. Evaluation of the integrity of swollen tablets containing
acetaminophen, SA, CA-0.05, sodium chloride and a hydrophilic non-ionic
polymer, HPMC K4M
SA, HPMC
A.I. NaCi Cracks Bursting
No. CA- 0.05 K4M
(%) (%) (%) (%) Time Type Time Type
1 10 70 10 10 6 C1+C2 24 M*
2 10 45 15 10 6 C1+C2 24 M*
3 10 45 10 15 6 C1+C2 24 M*
Other excipients like non-ionic hydrophilic polymers can be combined with high
amylose carboxymethyl starch when sodium chloride is used. The benefit of
sodium chloride addition remains as regards the improvement of matrix
integrity. When the SA, CA-0.05/HPMC K4M ratio is considered, one notices
that the flakiness phenomena have disappeared.
EXAMPLE 23
Effect of adding salt (cont.)
Tablets were prepared according to the procedure described in Example 1 and
evaluated according to the test conditions described in Example 2. The A.I.
was
acetaminophen. The formulations and their evaluation are presented in Table
19.
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
Table 19. Evaluation of the integrity of swollen tablets containing
acetaminophen, SA, CA-0.05, sodium chloride and a pregelatinized starch
with low amylose content
SA, Lycatab
A.I. NaCI Cracks Bursting
No. CA-0.05 PGS
( /a) (%) (%) (%) Time Type Time Type
1 10 70 10 10 4.5 Cl M*
2 10 75 7.5 7.5 8 C1 M*
3 10 75 5 10 5.5 C1 M*
5
In the same manner, excipients like pregelatinized starch can be combined with
high amylose carboxymethyl starch when sodium chloride is used. The benefit
of sodium chloride addition remains as regards the improvement of matrix
integrity.
EXAMPLE 24
Effect of adding electrolytes: application to other active ingredients
To illustrate the versatility and advantages of the present invention,
theophylline
was selected as another drug model for tablet integrity evaluation. Tablets
were
prepared according to the procedure described in Example 1 and evaluated
according to the test conditions described in Example 2. The formulations and
their evaluation are presented in Table 20.
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
41
Table 20. Evaluation of the integrity of swollen tablets containing
theophylline, SA, CA-0.05, and sodium chloride alone or a mixture of sodium
chloride and arginine hydrochloride
Theophylline SA, Arginine NaCI Cracks Bursting
No. CA-0.05 hydrochloride
(%) (%) (%) (%) Time Type Time Type
1 10 90 5.5 C2* M*
2 20 80 4.5 C1 DC
3 10 80 10 7 C2* No
4 20 70 10 4.5 C1 No
30 60 10 1.5 Cl 2 DC
6 10 70 10 10 No
5
Theophylline, a slightly soluble drug, appears to show the same problems as
acetaminophen with regard to matrix integrity. A moderate increase in drug
loading accelerates and amplifies the problems (see tablet No. 2). The
addition
of sodium chloride decreases the above-mentioned problems, but one notices
that an increase in drug loading needs to be accompanied by an increase in
electrolyte loading (see tablet No. 3 to 6) to maintain the benefit of
electrolyte
addition. Tablet No. 6 shows also the benefit of using a mixture of
electrolytes,
i.e. sodium chloride and arginine hydrochloride, to maintain the integrity of
high
amylose carboxymethyl starch matrix tablets.
EXAMPLE 25
Effect of adding electrolytes: application to other active ingredients (cont.)
To illustrate the versatility and advantages of the present invention,
bupropion
hydrochloride was selected as another drug model for tablet integrity
evaluation.
Tablets were prepared according to the procedure described in Example 1 and
evaluated according to the test conditions described in Example 2. The
formulations and their evaluation are presented in Table 21.
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
42
Table 21. Evaluation of the integrity of swollen tablets containing bupropion,
SA, CA-0.05, sodium chloride and arginine hydrochloride
Bupropion SA, Arginine. NaCI Cracks Bursting
No. hydrochloride CA-0.05 HCI
(%) (%) (%) (%) Time Type Time Type
1 10 90 5 C2 M*
2 .20 80 4 C1 DC
3 30 70 4 C1 DC
4 40 60 2 C1 2.5 DC
50 50 Disintegration after 2 h
6 25 50 16.67 8.33 No
5 Bupropion hydrochloride, a freely soluble drug, appears to show the same
problems as acetaminophen and theophylline as regards matrix integrity. Like
these two drugs, an increase in bupropion hydrochloride loading accelerates
and amplifies the problems (see tablet No. 2-5). The addition of a mixture of
sodium chloride and arginine hydrochloride resolves the above-mentioned
problems and shows the benefit of using a mixture of electrolytes to maintain
the integrity of high amylose carboxymethyl starch matrix tablets.
EXAMPLE 26
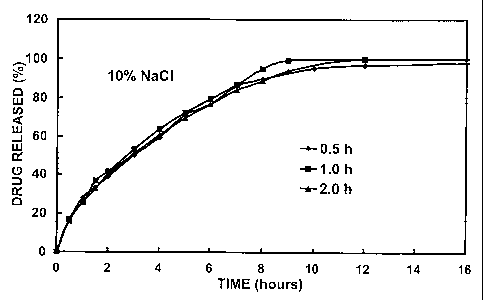
SA, CA.lab tablets were prepared according to the procedure described in
Example 1 and evaluated according to the test conditions described in Example
3 except that pH was maintained constant at 1.2 or 7.4. The A.I. was
acetaminophen. Figure 3 shows the percentage (%) of acetaminophen released
in acidic and moderately alkaline conditions from SA, CA.lab-1.55 matrix
tablets
in function of time (hours) for 400-mg tablets containing 10% of drug. The
burst
release rate was similar for both experiments, i.e. in vitro release in acidic
and
moderately alkaline environments. This part of the release profile is
essentially
due to dissolution of the drug present at the matrix surface and directly
exposed
to the aqueous environment. The solubility of acetaminophen being the same in
acidic and moderately alkaline environments, the results are not surprising.
The
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
43
second part of the release profile shows a significant difference where the
release rate in acidic medium is much slower than in the moderately alkaline
medium. It can be concluded that the high amylose carboxymethylstarch gel
structured differently depending on pH.
EXAMPLE 27
SA, CA.lab tablets were prepared according to the procedure described in
Example 1 and evaluated according to the test conditions described in Example
3 (pH gradient, 1 hour in pH=1.2). The A.I. was acetaminophen. Figure 4 shows
the percentage (%) of acetaminophen released from SA, CA.lab-1.8, SA,
CA.lab-1.55 and SA, G-2.7 matrix tablets in function of time (hours) for 400-
mg
tablets containing 10% of drug. SA, CA.lab matrix tablets were immersed for 1
hour in acidic medium (pH=1.2), and then transferred to a moderately alkaline
medium (pH=7.4). The data for SA, G-2.7, extracted from U.S. Patent No.
5,879,707, were obtained for experimental conditions exactly similar to SA, CA
tablet testing except that a constant pH medium was used (pH=7.4).
On the one hand, SA, CA.lab-1.55 tablets released the drug more slowly than
SA, CA.lab-1.8 matrices; on the other hand, SA, CA.lab-1.55 tablets showed
some significant cracking and bursting when going through a pH gradient. This
defect makes them useless when considering in vivo applications although they
demonstrated an in vitro performance equivalent to SA, G-2.7 tablets as
regards the release of a soluble drug like acetaminophen.
EXAMPLE 28
Tablets were prepared according to the procedure described in Example 1 and
evaluated according to the test conditions described in Example 3 (pH
gradient,
1 hour in pH=1.2). The A.I. was acetaminophen. Figure 5 shows the percentage
(%) of acetaminophen released from SA, CA-0.05 matrix tablets in function of
time (hours) for 400-mg tablets containing 10% of drug and different sodium
chloride loadings (0, 10 and 15%).
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
44
Surprisingly, a typical sustained-release profile is still observed despite
significant sodium chloride concentrations (10-15%) in tablets already
containing 10% of a soluble drug like acetaminophen. Also, no differences in
release rates could be reported between compositions containing 10 and 15%
of sodium chloride. The presence of sodium chloride does not even increase
the burst release of acetaminophen. Note that for tablets containing only
acetaminophen, a slight acceleration of drug release could be observed after 4
hours of evaluation. Indeed, a major crack appeared around that time and
promoted dissolution of the drug present at the surface generated by the
crack.
One must, however, consider that agitation in the dissolution apparatus is
quite
moderate compared to the stomach, which can churn during the digestion
process. In such conditions, the tablet would be broken apart, leading to more
dramatic consequences. Note also that, due to sodium chloride, only moderate,
partial bursting (M*) appeared after 8-9 hours of testing which was without
consequences on the release profile.
EXAMPLE 29
Tablets were prepared according to the procedure described in Example 1 and
evaluated according to the test conditions described in Example 3 (pH
gradient,
0.5, 1 or 2 hours in pH=1.2). The A.I. was acetaminophen. Figure 6 shows the
percentage (%) of acetaminophen released from SA, CA-0.05 matrix tablets in
function of time (hours) for 400-mg tablets containing 10% of drug and 10% of
sodium chloride when the tablets are immersed for 0.5, 1 or 2 hours in the
acidic medium.
It is noteworthy to note that the release profile from such matrices is not
influenced by acidic residence time although high amylose sodium
carboxymethylstarch is the sodium salt of an ionic polymer. Furthermore, no
modification of the release profile was observed when the tablet was
transferred
from an acidic to an alkaline medium.
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
EXAMPLE 30
Tablets were prepared according to the procedure described in Example 1 and
evaluated according to the test conditions described in Example 3 (pH
gradient,
0.5, 1 or 2 hours in pH=1.2). The A.I. was acetaminophen. All tablets
contained
5 10% of drug and 10% of sodium chloride. One batch of tablets contained 0.2%
of magnesium stearate and their release profile was compared to that of
tablets
without magnesium stearate. Magnesium stearate, a well-known tablet
lubricant, did not influence the release rate of acetaminophen from SA, CA-
0.05
matrix tablets.
EXAMPLE 31
Tablets were prepared according to the procedure described in Example 1 and
evaluated according to the test conditions described in Example 3 (pH
gradient,
1 hour in pH=1.2). The A.I. was pseudoephedrine hydrochloride (PE). Figure 7
shows the percentage (%) of PE released from SA, CA-0.05 matrix tablets in
function of time (hours) for 800-mg tablets containing different drug loadings
(20, 37.5, 50 and 60%). High amylose carboxymethylstarch matrices containing
a very soluble ionic drug like pseudoephedrine chloride presented a
surprisingly
excellent sustained-release performance when they were evaluated, regarding
release rates and matrix integrity, in a pH gradient simulating the pH
evolution
of the tablet environment when traveling along the gastrointestinal tract,
i.e.
from a strongly acidic to a moderately alkaline environment. Note that the
release profile corresponding to tablets containing 20% of drug displays a few
release rate accelerations corresponding to small cracks appearing in the
matrix. PE is very soluble, and slight secondary burst effects thus appear due
to
drug dissolution starting on the surface newly exposed to an aqueous
environment.
EXAMPLE 32
Tablets were prepared according to the procedure described in Example 1 and
evaluated according to the test conditions described in Example 3 (pH
gradient,
1 hour in pH=1.2). The A.I. was PE. Figure 8 shows the drug total release time
CA 02591806 2007-06-20
WO 2006/066399 PCT/CA2005/001934
46
(hours) in function of the PE percentage (%) in SA, CA-0.05 matrix tablets of
different weights (400, 600 and 800 mg). PE is a non-ionic, very soluble drug
and that makes it usually quite difficult to formulate, especially at high
loadings.
Figure 8 illustrates that high drug loading SA, CA-0.05 matrices with
excellent
performance are easily achieved. Note that, surprisingly, drug loadings lower
that 20% were not very successful as cracks appeared in the matrices. This is,
however, in good agreement with what has been observed when adding
electrolytes like sodium chloride to stabilize the matrix. Consequently, when
formulating low drug dosage forms, one has to add electrolytes, in place of
current fillers like lactose, to SA, CA-0.05 tablets to obtain a useful tablet
weight
and stable matrix. On the other hand, this makes it clear that in accordance
with
the invention, one may incorporate very large amounts of a very soluble ionic
drug in a tablet and still achieve very good release control.
Of course, numerous modifications could be made to the above invention, as
disclosed and exemplified, without departing from the scope of the appended
claims.