Note: Descriptions are shown in the official language in which they were submitted.
CA 02591994 2012-12-18
RADIATION DETECTABLE AND PROTECTIVE ARTICLES
FIELD OF THE INVENTION
[0001] The present invention relates to radiation detectable and protective
articles. The
radiation detectable articles of the present invention can be easily detected
through the use of x-
rays and other radioactive emissions. The processes and compositions for
producing such
radiation detectable articles can also be applied to creating articles which
protect against radiation
as well as other types of hazards, such as fire, chemical, biological and
projectile hazards.
BACKGROUND OF THE INVENTION
[0002] Radiation has been used by humans in numerous ways. The most well known
destructive application of radiation is atomic bombs. The electromagnetic
radiation released by
an atomic bomb can penetrate deeply into human tissue to damage human cells.
The threat posed
by atomic bombs has arguably increased in recent years with the growth of
terrorism and the very
real possibility that a "dirty bomb" can be made by terrorists through use of
readily available
nuclear waste materials. The destructive threat to humanity of such nuclear
bombs has given rise
to a need for cost-effective radiation protection, including the need for
lightweight radiation
protective garments. Ideally, such lightweight radiation protective garments
would
simultaneously provide protection against other types of hazards, such as
fire, chemical,
biological, projectile hazards and other forms of electromagnetic radiation.
In this way, first
responders, such as firemen, paramedics, policemen or the military, could use
a single garment to
provide them with protection against any type of hazard they might foreseeably
confront. Such
"universal" protective garments are addressed in Applicants' patent number
6,841,791 granted
January 11, 2005, entitled "Multiple Hazard Protection Articles And Methods
For Making
Them."
[0003] A number of constructive uses have also been developed for harnessing
radiation. These
constructive uses include medical x-rays and nuclear power plants. Other
constructive uses of
radiation, though, remain undiscovered. For example, in many industries,
automated, high-speed
machines are used to manufacture products quickly and inexpensively. The food
industry is one
such industry. For example, machines largely do the manufacture and packaging
of the many
popular brands of breakfast cereals. To market this mass-produced breakfast
cereal, the breakfast
cereal manufacturers often include a prize or "premium" inside the cereal box,
such as a model of
1
CA 02591994 2012-12-18
-
a popular superhero. This premium is typically inserted and sealed into the
box by machine
during the packaging process.
[0004] Where high speed, automated manufacturing processes are used, there is
a need for
quality control procedures. Returning to the cereal box example, if the cereal
box assembly
machine runs out of premiums or has its premium insertion apparatus jammed, a
number of
cereal boxes might be sealed, shipped and sold without the premium. Since, for
children's cereals,
the cereal box is often purchased for the primary purpose of receiving the
premium inside, the
manufacturer's failure to include the premium in the cereal box can lead to
angry and
disillusioned customers.
[0005] As such, there is a need, particularly in the high speed manufacturing
art, to be able to
quickly check to see if the manufactured product is made in full compliance
with the company's
manufacturing standards (e.g., including any premium) and that the product is
also free of foreign
contaminants. In the case of cereal boxes, this includes making sure that all
of the cereal boxes
which are supposed to have premiums actually have them and lack foreign
contaminants, such as
stones and metals, which can inadvertently enter the final assembly.
[0006] While visual inspection by humans is often performed to maintain
quality control, visual
inspection is difficult to effectively perform for products manufactured on a
high-speed assembly
line. One problem with visual inspection is giving the human inspector enough
time to perform a
proper inspection without slowing down the manufacturing process. In the case
of trying to detect
premiums in cereal boxes, this problem is compounded by the fact that the
cereal box is visually
opaque and, as such, not amenable to visual inspection of items, such as
premiums, which are
inside the cereal box.
BRIEF SUMMARY OF THE INVENTION
[0007] The present invention includes compositions and processes for forming
radiopaque
polymeric articles. When these radiopaque polymeric articles are used in high
speed, automated
manufacturing processes, their attributes and presence can be easily confirmed
through the use of
radiation inspection apparatuses.
[0008] A radiopaque polymeric article of the present invention can be created
by mixing a
radiopaque material, such as barium, bismuth, tungsten or their compounds,
with a powdered
polymer, palletized polymer or liquid solution, emulsion or suspension of a
polymer in solvent or
2
CA 02591994 2012-12-18
water. The polymer may advantageously be selected from a broad range of
plastics including, but
not limited to, polyurethane, polyamide, polyvinyl chloride, polyvinyl
alcohol, natural latex,
polyethylene, polypropylene, ethylene vinyl acetate, polyester, acrylonitrile-
butadiene-styrene,
acrylic, polycarbonate, polyoxymethylene, acetal, polytetrafluoroethylene
(TEFLONTm),
ionomers, celluloses, polyetherketones, silicones, epoxy, elastomers, polymer
foams and other
polymer compounds.
[0009] The radiopaque polymeric mixture can then be used to form a radiopaque
polymeric
article through a number of existing commercial processes, such as injection
molding, extrusion
and thermoforming. For example, in the case of injection molding, the
radiopaque polymeric
mixture can be heated in an extruder and then injected into a mold until it
assumes the shape of
the mold. After the radiopaque polymeric mixture has hardened into the
appropriate molded
shape, it is removed from the mold. In the case of a superhero model, the
molded model can then
be wrapped in cellophane and inserted, as a premium, into a cereal box.
[00101 The radiopaque article may also be advantageously formed by spraying,
adhering or
coating a radiopaque adhesive mixture onto a pre-existing article. For
example, mixing a
lightweight radiopaque material with an adhesive, such as a gum adhesive or a
liquid polymer,
can form the radiopaque adhesive mixture. The radiopaque adhesive mixture may
then be applied
to the pre-existing article either by spraying the radiopaque adhesive mixture
onto the article or
dipping the article in the radiopaque adhesive mixture.
[00111 During the manufacturing process, a radiation inspection apparatus can
be used to detect
the presence and attributes of a radiopaque polymeric article. In one
embodiment, x-rays are
passed through the radiopaque polymeric article itself or a radiation
transmissible package
containing the radiopaque polymeric article. An x-ray detector is then
positioned on the opposite
side of the radiopaque polymeric article to detect where the radiation has
been attenuated and
where it has been transmitted. Through this x-ray detector, the presence of
the radiopaque
polymeric article can be confirmed and, if desired, the attributes of the
radiopaque polymeric
article (e.g., proper dimensions, quantity, lack of defects etc.) can be
ascertained. This x-ray
detector can also make sure that undesired foreign contaminants, such as
stones or metal debris,
are not included in the finished product.
[0012] A number of the processes and compositions used for creating radiopaque
detectable
objects may also be used to provide protection against a wide spectrum of
ionizing radiation,
3
CA 02591994 2012-12-18
such as neutron, ultraviolet, gamma and radio frequency radiation. For
example, in U.S. patent
6,841,791, the radiopaque polymeric compounds of the present invention are
used to create
radiation protective garments, which, in some cases, can also provide
protection against other
hazards (e.g., fire, chemical, biological, projectile etc.). Similarly, in the
same way an adhesive
mixture of radiation protective materials can be sprayed onto a pre-existing
object to make it
radiation detectable, the same type of mixture can be sprayed onto a garment
to make it attenuate
radiation.
[0013] As another part of the present invention, recent advances in
nanotechnology can be used
to create better radiation detectable and radiation attenuating articles. In
certain embodiments,
these radiation attenuating articles can also provide protection against other
types of hazards,
such as fire, chemical, biological, projectile hazards, and a wide range of
electromagnetic
radiation energies. Owing to their small size and high surface area to volume
ratio, these nano-
materials have demonstrated unique electrical, mechanical and optical
properties. In this
invention, various types of nano-materials can be utilized to enhance
mechanical, thermal,
attenuating and barrier protection properties of a product.
[0014] In the present invention, nano-materials are used in at least three
different ways. In one
embodiment, nano-materials are added to the previously disclosed radiation
protective polymeric
mixtures to either enhance the radiation protection or provide additional
protections, such as fire,
chemical, biological and/or projectile protection. In a second embodiment,
nanoparticles formed
from radiopaque materials (e.g., barium, bismuth, tungsten etc.) are used in
the radiation
protective mixture instead of more bulky forms of the same or similar
radiopaque materials. Use
of such radiopaque nano-materials allows more even dispersion of radiopaque
materials in the
polymeric mixture, with the attendant possibility of allowing higher
concentrations of radiopaque
materials before the polymer becomes embrittled. In a third embodiment, the
nano-materials are
formed into a discrete nano-material layer. Such a discrete nano-material
layer could either be
added to a product or formed into a stand alone product.
[0015] Nano-materials for use in the present invention include nanoparticles,
nanotubes and
nano-platelets. Nanoparticles are predominantly formed as solid grains, but
may also consist of
hollow nanospheres, nano shells, hemi-spheres, parabolas and so forth.
Nanoparticles can be
formed of various metal/non-metal powders including oxides, sulphides and
ceramic powders.
Nano-platelets are layered nano-materials which include natural nanoclays and
synthetic nano-
4
CA 02591994 2012-12-18
clays, such as silicic acids and transition-metal dichalcogenides (i.e.
tantalum dichalcogenides
interacted with lithium). Nanotubes are tube like nano-materials that have a
diameter of a few
nanometers but yet could be several microns in length.
[0016] To attenuate electromagnetic radiation such as radio waves, ultra-
violet rays and
ionizing radiation, nano-particles can be formed of conventional radiopaque
materials such as
tungsten, tantalum, barium or their compounds, shell structures such as metal
coated magnetic
particles like Fe203/Au, Si02/ Au or other coated semiconducting particles
like PbS/CdS. Hollow
metal, metal oxide/sulphides nanospheres or nanospheres of other compounds;
nanoparticles
having shapes of parabolas, hemi-spheres and shell structures can also be used
in this current
invention. Shaped nanoparticles (e.g., nanoparabolas, nano hemispheres,
nanospheres etc.) are
believed to deflect, reflect and capture radiation in a manner similar to the
way mirrors deflect,
reflect and capture lightwaves. Since these shaped nanoparticles are believed
to attenuate
radiation differently than powdered radiopaque nanoparticles, these shaped
nanoparticles do not
need to be formed from radiopaque materials, but may instead be formed from
such materials as
metal/semiconductor hybrid particles. For example, the hybrid CdS-coated Ag
nanoparticles
exhibit red-shifted plasmon resonance absorption. This resonance absorption
band of the metal
nanoparticles is a function of particle size. As the particle size decreases,
the theoretical
wavelength of maximum absorption intensity can be approached. By creating
these nano-spheres,
nano-hemispheres, and nano-parabolic structures in specific shapes and
curvatures, the optical
properties can be used against smaller wavelengths of the light spectrum to
attenuate
electromagnetic radiation in the radio waves, ultraviolet rays and ionizing
radiation frequencies.
Rather than absorbing the electromagnetic radiation as in the case of heavy
metals, the
electromagnetic radiation is effectively redirected, shifted, or reflected to
allow its energy to be
reduced to a lower level or converted to heat.
[0017] To enhance the chemical and fire-retardant properties of a polymeric
mixture, nano-
materials of suitable composition can be evenly dispersed in the polymeric
mixture. For instance,
nanoclays when properly dispersed in a polymer enhances its chemical
properties by creating a
tortuous path in the polymer matrix, which makes hard for the harmful
chemicals, biological
agents and other gases, such as oxygen, to penetrate the polymer. To increase
the fire-retardant
properties of the polymer, a small percentage of nanoclays or other nano
platelets in the range of
2 to 10% could be added along with conventional fire retardants, such as
alumina trihydrate,
5
CA 02591994 2012-12-18
magnesium hydroxide or other organic brominated and organic chlorinated
compounds either in
the nanoscale or micron range. Nanotubes can be used to enhance the mechanical
properties such
as tensile strength, flexibility, modulus and electrical conductivity of a
polymeric mixture.
[0018] There are three general ways of dispersing nano-materials into the
polymeric mixture.
The first is direct mixing of the polymer and the nano-materials either as a
discrete phase or in
solution. The second is in-situ polymerization in the presence of a nano
material, and the third is
in-situ particle processing which involves both in-situ formation of the nano-
materials and in-situ
polymerization. Also, nanomaterials could be coated on a number of substrates
by several
techniques such as evaporization, sputtering (glow-discharge, ion-beam,
laser), ion-plating,
chemical vapor deposition (CVD), plasma enhanced CVD, thermal spraying, dip
coating,
fluidized bed and atomized liquid spray.
[0019] It should also be noted that nano-materials tend to agglomerate to
reduce their surface
area and, therefore, without proper dispersion and distribution in the polymer
matrix the desired
properties of the resulting nano-composite cannot be achieved. In order to
disperse nano-
materials into a polymer and process the resulting mixture by standard
manufacturing techniques,
they should preferably be surface modified. For instance, in the case of
nanoclays, the clay
surface is modified by a process known as compatibilization so that they are
attracted to the resin
matrices and thus get thoroughly dispersed. The two most common
compatibilization methods
known are onium ion modification and the ion-dipole interaction.
[0020] By incorporating nano-materials into polymeric mixtures of the present
invention or
creating a pure nanolayer, a radiation protective shield can be created to
either make an article
radiation detectable, radiation protective (ultraviolet, radiofrequency,
electromagnetic, x-
radiation, or gamma radiation) or "universally" protective (i.e., protective
against one or more
hazards such as neutron radiation, fire, chemical, biological, or projectile
hazards). The resultant
radiopaque polymeric mixture, with or without the nano-materials, can
additionally be laminated
to a chemical film, anti-ballistics fabric, woven or non-woven, or flame
retardant material as
described in our previously referenced patent applications.
BRIEF DESCRIPTION OF THE DRAWINGS
[0021] FIG. 1 shows a front view of a radiopaque polymeric article of the
present invention.
6
CA 02591994 2012-12-18
[0022] FIG. 2 shows a side view of an injection molding apparatus for creating
radiopaque
polymeric articles of the present invention.
[0023] FIG. 3 shows a perspective view of an apparatus for detecting the
presence and attributes
of a radiopaque polymeric article on a high-speed assembly line.
[00241 FIG. 4 shows a front view of radiation protective full body suit.
[0025] FIG. 5 A shows a side view of a nano-hemisphere for attenuating
radiation.
[0026] FIG. 5B shows a side view of a nanosphere for attenuating radiation.
[00271 FIG. 6 illustrates a cross-section view of a composite material which
can provide
multiple forms of hazard protection.
[0028] FIG. 7 illustrates a vest formed of multiple hazard protecting layers.
[00291 FIG. 8 illustrates an exploded view of protective clothing which can be
worn as
undergarments.
[0030] FIG. 9 illustrates a pocket and a hazard protecting insert for that
pocket.
[0031] FIG. 10 shows a perspective view of a radiation attenuating bomb
containment vessel.
DETAILED DESCRIPTION OF THE INVENTION
[0032] Referring now to FIG. 1, an example of a radiopaque polymeric article
10 of the present
invention is shown. In this case, the radiopaque polymeric article 10 is a
premium which can be
inserted into cereal boxes taking the form of a plastic toy model. The
radiopaque polymeric
article 10 is preferably formed from a polymeric mixture, which includes one
or more radiopaque
materials and one or more polymers. The inclusion of one or more radiopaque
materials is
important for this polymeric mixture because polymers themselves are largely
transparent to
many forms of radiation, such as x-rays, and, as such, using a polymer alone
will not produce an
effective radiopaque polymeric article.
[0033] For the radiopaque materials, barium sulfate, tungsten and bismuth are
preferred choices
for the present invention because, as compared with lead, for example, they
have fewer known
heath hazards. Other radiopaque materials can also be used, including, but not
limited to, barium,
other barium compounds (e.g., barium chloride), tungsten compounds (e.g.,
tungsten carbide and
7
CA 02591994 2012-12-18
tungsten oxide), bismuth compounds, tantalum, tantalum compounds, tin,
titanium, titanium
compounds, Diatrizoate Meglumine Jnj. USP (sold by Nycomed Corporation under
the trade
name HYPAQUETm), Acetrizoate Sodium, boron, boric acid, boron oxide, boron
salts, other
boron compounds, beryllium, beryllium
compounds, Bunamiodyl Sodium, Diatrizoate Sodium, Ethiodized Oil, Iobenzamic
Acid,
Iocarmic Acid, Iocetamic Acid, Iodipamide, Iodixanol, Iodized Oil,
Iodoalphionic Acid, o-
Iodohippurate Sodium, Iodophthalein Sodium, Iodopyracet, Ioglycamic Acid,
Iohexol,
Iomeglamic Acid, Iopamidol, Iopanoic Acid, Iopentol, Iophendylate, Iophenoxic
Acid,
Iopromide, Iopronic Acid, Iopydol, Iopydone, Iothalamic Acid, Iotrolan,
Ioversol, Ioxaglic Acid,
Ioxilan, Ipodate, Meglumine Acetrizoate, Meglumine Ditrizoate Methiodal
Sodium,
Metrizamide, Metrizoic Acid, Phenobutiodil, Phentetiothalein Sodium,
Propyliodone, Sodium
Iodomethamate, Sozoiodolic Acid, Thorium Oxide and Trypanoate Sodium. These
radiopaque
materials can be purchased from a variety of chemical supply companies, such
as Fisher
Scientific, P.O. Box 4829, Norcross, Georgia 30091 (Telephone: 1-800-766-
7000), Aldrich
Chemical Company, P.O. Box 2060, Milwaukee, Wisconsin (Telephone: 1-800-558-
9160) and
Sigma, P.O. Box 14508, St. Louis, Missouri 63178 (Telephone: 1-800-325-3010).
Those of skill
in the art will readily recognize that other radiopaque materials
incorporating the same metals can
be used interchangeably with the ones listed.
[0034] The polymer used in the polymeric mixture of the present invention may
preferably be
selected from a broad range of plastics including, but not limited to,
polyurethane, polyamide,
polyvinyl chloride, polyvinyl alcohol, natural latex, polyethylene,
polypropylene, ethylene vinyl
acetate, polyester, polyisoprene, polystyrene, polysulfone, acrylonitrile-
butadiene-styrene,
acrylic, polycarbonate, polyoxymethylene, acetal, polytetrafluoroethylene
(TEFLONTm),
ionomers, celluloses, polyetherketone, silicones, epoxy, elastomers, polymer
foams and other
polymer compounds.
[0035] Conventional additives may be included in the polymeric mixture to
improve the
flexibility, strength, durability or other properties of the end product
and/or to help insure that the
polymeric mixture has an appropriate uniformity and consistency. These
additives might be, in
appropriate cases, plasticizers (e.g., epoxy soybean oil, ethylene glycol,
propylene glycol, etc.),
emulsifiers, surfactants, suspension agents, leveling agents, drying
promoters, adhesives, flow
enhancers, and flame retardants.
8
CA 02591994 2012-12-18
[0036] The proportions of these various polymeric mixture ingredients can
vary. Using a greater
proportion of conventional sized radiopaque materials will generally allow the
presence and
attributes of the radiopaque polymeric article to be more easily ascertained
through radiation
detection techniques. Nonetheless, if the proportion of conventional sized
radiopaque materials
compared to the polymer is too high, the polymeric mixture will become brittle
when dried or
cooled and easily crumble apart. The inventors have found from their work that
over 50% of the
polymeric mixture, by weight, can be barium sulfate, tungsten, bismuth or
other conventional
sized radiation protective materials, with most of the rest of the mixture
consisting of the
polymer.
[0037] In one preferred embodiment, the polymeric mixture contains
approximately 85% by
weight of conventional sized radiopaque materials and approximately 15% by
weight of polymer.
In this preferred embodiment, the radiopaque materials used in the polymeric
mixture are
tungsten (75%), barium sulfate (20%) and bismuth (5%). The currently preferred
polymers for
this preferred embodiment are a mixture of ethyl vinyl acetate (EVA) and
polyethylene.
10038] It may be appropriate to consider the use of lead as one of the
radiopaque materials for
the polymeric mixture. While, because of its potential health hazards, lead
would not be as
preferred as many of the other radiopaque materials previously listed, lead
nonetheless might
have a role in some radiopaque polymeric mixtures.
[0039] A number of known manufacturing processes may advantageously be used to
create the
radiopaque polymeric articles of the present invention. For example, the
radiopaque polymeric
mixture of the present invention can first be melted in an extruder and then
pushed by a piston in
molten form into the mold of an injection-molding machine. FIG. 2 illustrates
such an injection-
molding machine 20. In the FIG. 2 embodiment, the radiopaque polymeric mixture
24 is inserted
into a hopper 26. The hopper 26 then feeds the radiopaque polymeric mixture 24
into an extruder
30, which, through use of extrusion heaters 32, melts the polymeric mixture 24
into dough like
consistency. The extrusion screw 34 moves the melted polymeric mixture toward
the mold 40. As
the melted polymeric mixture leaves the extruder 30, it is injected under
pressure through
extruder nozzle 36 into mold 40. When the polymeric mixture has cooled inside
the mold 40, it
can be popped out of the mold 40 in finished form. A further example of an
injection molding
apparatus and process is described in Walter's U.S. Patent No. 6,572,801 Bl.
9
CA 02591994 2012-12-18
[0040] Thermoplastics, thermosets and elastomers can all be injection molded.
By using
multiple inlets to the mold 40 (not shown), a co-injection molding process
allows molding of
components with different materials, colors and/or features. Moreover, other
types of molding
techniques can be used depending on the shape, thickness, weight range,
allowable tolerance,
surface roughness and economic batch size of the injection-molded articles.
These other types of
molding techniques include, but are not limited to, rotational molding for
large hollow closed or
semi-closed structures, blow molding, foam molding, compression molding, resin
transfer
molding, die-casting, sand casting, investment casting, polymer casting, shape
rolling, die
forging, extrusion, press forming, roll forming, spinning, thermoforming, lay-
up methods,
powder methods, laser prototyping and deposition.
[0041] Many other known plastic forming techniques can be used to form the
radiopaque
polymeric articles of the present invention. For example, the polymeric
mixture of the present
invention could again be put into the hopper of an extruder, heated and, in
this case, deposited in
molten form as a thin film on a conveyor belt. Vacuum pressure could then be
applied to the thin film so as to draw the molten film into intimate contact
with a mold
impression to form the thin film into its desired shape. An example of such
vacuum forming
techniques is described in greater detail in Gilbert's U.S. Patent No.
6,319,456 Bl. Alternatively,
instead of drawing the thin film into a vacuum mold, the thin film sheet could
simply be cut into
a desired planar shape.
[0042] As a further alternative, the article, such as the superhero, could be
pre- formed and
subsequently made radiopaque through the application of a thin radiopaque
layer. In such case,
mixing a lightweight radiopaque material with an adhesive, such as a gum
adhesive or a liquid
polymer, could advantageously form the radiopaque layer. The radiopaque
adhesive mixture
could then be applied to the pre- formed article either by spraying the
radiopaque adhesive
mixture onto the outside of the article or dipping the article in a solution
of the radiopaque
adhesive mixture.
[0043] The mold used in the injection molding process shown in FIG. 2 might,
for example, be
in the shape of the superhero model 10 illustrated in FIG. 1. In addition to
premiums, injection
molding is used today to produce many other types of plastic articles, which
could benefit from
becoming radiopaque polymeric articles of the present invention. For example,
a plastic straw is
often attached to children's juice cartons in order to allow the child to
drink the juice without
0
CA 02591994 2012-12-18
=
spilling. Similarly, a plastic utensil, such as a spoon or fork, might be
attached to a serving of
food embedded in a plastic container. If the straw or other utensil is missing
from the food
container, the user will either have to throw the product away or manually try
to eat from the
container, thereby dealing with an attendant mess. If the straw or other
utensil in this example
were made of a radiopaque polymer of the present invention, a radiation
inspection apparatus
could be used to make sure that all the containers leaving the assembly line
have radiopaque
straws or other utensils attached.
[0044] Since the straw or other utensil in this example touches the mouth of
the user, it would
be important to choose a non-toxic radiopaque material for the polymeric
mixture, such as barium
sulfate, iodine, bismuth or some combination of them or their compounds,
rather than a toxic
material, such as lead. A further factor to be considered in selecting a
suitable radiopaque
material is the degree of radiation attenuation which the material would
provide. For example, in
the straw and juice box example, the cardboard juice box freely transmits
radiation. As such, one
would not need a radiopaque compound with strong attenuating properties to
create a sufficient
radiation contrast between the straw and the box. More specifically, a
radiopaque compound with
relatively weaker attenuation properties, such as iodine compounds, could be
used for the straw
and juice box example or a radiopaque compound with stronger attenuation
properties, such as a
bismuth compound, could be used in lower concentrations.
[0045] Other packaging industries would also benefit from the principles of
the present
invention. In disposable medical products, most of the devices are made of
plastic and, if any of
the contents were missing, the entire device would likely fail. Using
radiopaque plastics of the
present invention, an x-ray inspection of the sealed medical package at the
factory could insure
that all the contents of the medical device were present. Also in the field of
medicine, a catheter
could be manufactured with radiopaque materials using the principles of the
present invention,
which would allow the catheter's insertion into the human body to be carefully
monitored using
an x-ray machine. By so monitoring the catheter insertion, the doctor could
make sure that the
catheter reaches the correct position in the patient's body.
[0046] As a further application, guns, knives and explosives are now being
produced from
plastics, which cannot be detected by the x-ray scanning machines used at
airports. If such plastic
guns, knives and/or explosives were in the hands of terrorists, they could be
used to pose a threat
to airplane crews and passengers. Using the principles of the present
invention, the government
11
CA 02591994 2012-12-18
could require that all plastic guns, knives and explosives incorporate one and
more radiopaque
materials of the present invention so that they would be readily detectable by
the x-ray scanning
machines used at airports. Given the great importance of detecting guns,
knives and explosives at
airports, the government would likely want to require that radiopaque
compounds with high
attenuating properties, such as bismuth compounds, be used for these
applications.
[0047] Referring now to FIG. 3, a radiation inspection apparatus 50 and
process is illustrated
for detecting radiopaque polymeric articles 10 of the present invention. In
the process shown in
FIG. 3, boxes or other containers 52 incorporating the radiopaque polymeric
articles 10 are
moving along a conveyor belt 60 in a high speed manufacturing process. In this
preferred
embodiment, an x-ray tube 70 is used to generate radiation for detecting the
presence or absence
of the radiopaque polymeric articles 10 in the box or other container 52. The
x-ray tube 70 is
controlled by an x-ray controller 72, which sends control signals to a high-
voltage generator 74.
The high voltage generator 74 applies a high voltage
between the anode and the cathode of the x-ray tube 70 to produce x-rays 76. A
lead plate 78
with a slit 79 is interposed between the x-ray tube 70 and the box or other
container 52. This lead
plate 79 serves to focus the x-rays on the box or other container 52 being
inspected and prevent
extraneous x-rays from harming manufacturing workers.
[0048] After the x-rays pass through the box or other container 52 being
examined, the x-rays
are detected by an x-ray detector 80. The x-ray detector 80 can include a
scintillator and one or
more MOS image sensor(s). In such an arrangement, incident x-rays are
converted by the
scintillator into visual light, which is detected by the MOS image sensor(s).
The MOS image
sensor, in turn, outputs a detection signal 82 whose characteristics
correspond to the amount of
incident x-ray radiation detected.
[0049] A data processor 84 is used to analyze the detection signal 82 received
from the x-ray
detector 80. Since the box 52 itself would evenly transmit x-ray radiation, a
detection signal with
no discontinuities would indicate that the radiopaque polymeric article 10 is
missing from the box
52. By contrast, since the radiopaque polymeric article 10 would block a
portion of the radiation,
a detection signal with sharp discontinuities would usually indicate the
presence of the
radiopaque polymeric article 10 in the box.
[0050] Further confirmation that the radiopaque polymeric article 10 is
actually in the box 52
can be made by measuring the level or pattern of x-rays detected by the x-ray
detector 80. For
12
CA 02591994 2012-12-18
example, the amount of radiation detected by the x-ray detector 80 for a box
52 having a
radiation detectable article 10 can be measured and loaded into the memory of
the data processor
84 as a template. The data processor 84 could then compare the level for each
subsequent box 52
on the conveyor 60 with the memorized template value and, if the two values
match within a pre-
determined tolerance, the data processor 84 could conclude that the box 52
indeed contains the
radiopaque polymeric article 10. If, by contrast, the data processor 84
concludes that the box 52 is
missing the radiopaque polymeric article 10, it could send a signal to alarm
86 to alert an
attendant to the defective box. Alternatively, the data processor 84 could
direct an operation unit
88 to either stop the assembly line or eject the defective box from the
assembly line.
[0051] For even greater precision of inspection, the x-ray detector 80 could
have a pattern of
detection pixels which each would detect the transmission of x-ray radiation
over a small defined
area. To establish a template, a box 52 with a radiopaque polymeric article 10
could be x-rayed
with the x-rays being detected by the pattern of pixels. The level of detected
x-
rays for each pixel would then be stored in the memory of the data processor
84 as a template for
future inspections. The data processor 84 could then, for each pixel, compare
the level of detected
radiation for each box 52 inspected during the manufacturing process with the
memorized
template to first determine, within a predetermined tolerance, whether the
inspected box 52
contains a radiopaque polymeric article 10. The data received from the
detailed detection pixels
could then be used to determine the shape (e.g., outside contours) of the
radiopaque polymeric
article. Moreover, use of the detection pixels also allows analysis of whether
there is a crack, nick
or other defect in the radiopaque polymeric article 10. The use of pixel data
in a radiation
inspection apparatus to detect the presence of cracks or nicks in an article
is described in greater
detail in Sawada's U.S. Patent No. 6,574,303 B2.
[0052] In addition to detecting the presence and attributes of a desirable
object in a box, the
radiation inspection apparatus 50 shown in FIG. 3 could simultaneously, or
alternatively, detect
unwanted contaminants, such as stones, dirt or metal debris. Since such
contaminants are likely
to attenuate radiation differently from both the box and the radiopaque
polymeric article, the data
processor 84 could use a suspicious difference in detected radiation
attenuation to either sound
alarm 86 or use operation unit 88 to stop the conveyor 60.
[0053] Thus far, the focus has been on methods and compositions for forming
radiopaque
detectable articles. Nonetheless, many of the same principles can be applied
to making articles
13
CA 02591994 2012-12-18
which protect against the harmful effects of radiation, including ultraviolet,
electromagnetic,
radiofrequency, neutron, x-ray and gamma radiation, as well as other hazards
(e.g., fire, chemical,
biological, and ballistic). For example, in U.S. patent 6,841,791, the
radiopaque polymeric
compounds of the present invention are used to create garments which protects
against radiation
and other hazards. Similarly, in the same way an adhesive mixture of
lightweight radiation
protective materials can be sprayed, adhered or coated onto a pre-formed
object to make it
radiation detectable in the previous examples, the same type of mixture can
also be sprayed,
adhered or coated onto a garment to make it radiation protective.
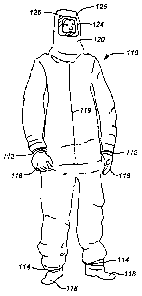
[00541 FIG. 4 shows a full body suit 100, which is constructed from radiation
protecting
polymeric mixtures of the present invention. To provide complete surface
protection, the full
body suit 110 should preferably be a one-piece jumpsuit, which covers every
portion of the
human body. Elastic bands 112, 114 can be used around the hand and foot areas
to help insure a
tight fit. Alternatively, the gloves 116, booties 118 and hood 120 can be
separate pieces, which
overlap with the rest of the jumpsuit so as to leave no skin surface exposed.
The full body suit
110 can also include hook and loop fasteners or a zipper flap 119 to allow the
user to easily enter
the full body suit 110. A transparent eye shield 124 is preferably included
with the full body suit
110 to provide protection for the face. For convenience, the eye shield 124
could be hinged, such
as with corner rivets 126, in order to allow the user to flip the shield 124
up and down.
Alternatively, the eye protection can be a stand alone device, such as safety
glasses (not shown).
To provide radiation protection, the eye shield 124 preferably incorporates
lead or other
radiopaque compounds that are capable of attenuating radiation.
[0055] Turning to FIG. 6, a composite material cross-section 200 is
illustrated which, when
fashioned into an article, can provide protection against numerous life
threatening hazards,
including toxic chemicals, infectious biological agents, fire and metal
projectile hazards, in
addition to the hazards posed by radiation,. As part of this multiple hazard
protection composite
material, there can be two layers of fabric 204, 208 with a radiation
protective polymer mixture
206 sandwiched between them. Added to these three layers 204, 206, 208 can be
additional layers
210, 220, 230 to protect against different hazards. For example, a nonporous
chemical protective
layer 210 and/or 220 can be added to the three radiation protective layers
204, 206, 208. This
nonporous chemical layer can either be a polymer film 210 laminated onto the
three radiation
14
CA 02591994 2012-12-18
protective layers 204, 206, 208 and/or a chemical protective fabric 220 which
is sewn or
otherwise adhered onto the three radiation protective layers.
[0056] This chemical protective layer 210, 220 can be constructed of known
chemical
protective polymers and/or fabrics. For example, one known class of chemically
protective
fabrics are non- woven textiles, such as the flashspun polyethylene fabric
sold by DuPont under
the tradename Tyvek0, polypropylene fabrics such as Kimberly-Clark's
KleenguardTM, Kappler's
Proshield 1TM, Lakeland's Safeguard 76TM, fabrics mixing polyethylene with
polypropylene and
cellulose based fabrics such as DuPont's SontaraTM and Kimberly Clark's
PrevailTM. A similar
type of non-woven textile would be the class of plastic films laminated onto
one or both sides of
a nonwoven fabric including DuPont's TyChem0 series of fabrics, Kimberly
Clark's HazardGard
I, JJTM fabrics, Kappler's CPFTM and Responder series of fabrics and ILC
Dover's Ready 1
fabricTM. These non- woven textiles would typically be combined with the three
radiation
protective layers 204, 206, 208 by sewing or otherwise adhering the fabrics
together.
[0057] Chemical protection can also be imparted by using polyvinyl chloride
and/or
chlorinated polyethylene films, such as ILC Dover's ChemturionTm. These films
could be
laminated or extruded onto the three radiation protective layers 204, 206,
208.
[0058] Another class of chemical protective layers are polymer films with
microscopic pores
laminated onto fabrics such as Gore-tax or polypropylene based fabrics such
as DuPont's
NexGenTM, Kimberly Clark's Kleenguard UltraTM, Lakeland's Micro-MaxTm and
Kappler's
Proshield 2TM. Chemical protection can further be provided by materials
incorporating an
absorbent layer, such as the carbon/fabric combinations sold by Blucher GmbH
and Lanx.
Another class of chemically protective fabrics are woven fabrics coated with
rubber or plastic on
one or both sides. These coated chemically protective fabrics include
polyvinyl chloride and
nylon composites, polyurethane/nylon composites, neoprene/aramid composites,
butyl/nylon
composites, chlorinated polyethylene/nylon composites, polytetrafluoroethylene
(i.e.,
Teflon )/fiberglass composites and chlorobutyl/aramid composites.
[0059] Because the chemical protective layers 210, 220 is preferably
nonporous, it will also
provide protection against infectious biological agents.
[0060] While the fabric shown in FIG. 6 can provide a broad measure of
protection with only
the addition of one or more chemical protective layers 210, 220 to the three
radiation protective
layers 204, 206, 208, further or alternative layers 210, 220, 230 can
nonetheless also be chosen to
CA 02591994 2012-12-18
protect against additional hazards or promote heat dissipation. For example,
where the chemically
protective layer 210 is a plastic laminate, layer 220 in FIG. 6 could be
another woven or
nonwoven fabric layer and layer 230 could be a fire protection layer, such as
a layer produced
from the Nomex fire resistant aramid fabric manufactured by DuPont. Other
types of fire
resistant materials include combinations of the Nomex and Kevlar aramid
fabrics such as that
sold by Southern Mills, combinations of melamine resin with aramid fibers,
combinations of
polytetrafluoroethylene (i.e., Teflon ) with aramid fibers, combinations of
rayon with aramid
fibers, combinations of polybenzimidazole with aramid fibers, combinations of
polyphenylenebenzobisoxazole with aramid fibers, combinations of polyimide
with aramid fibers
and MylarTM plastic films. Moreover, traditional fire retardant additives
include aluminum
trihydrate (ATH), magnesium hydroxide or organic brominated or chlorinated
compounds.
Alternatively, layer 230 could be a bullet or shrapnel resistant layer
produced from bullet
stopping aramid and/or polyethylene fibers.
[0061] It may alternatively be prudent to form layer 230 of a heat dissipation
material. One way
of forming such a heat dissipation layer is to mix compounds with high thermal
conductivity,
such as silver, copper, gold, aluminum, beryllium, calcium, tungsten,
magnesium, zinc, iron,
nickel, molybdenum, carbon and/or tin, with a polymer in the same way that the
radiation
protective materials are mixed with polymers to form radiation protective
layer 206.
[0062] While a six layer hazard protecting fabric 200 is illustrated in FIG.
6, those of skill in the
art will readily recognize that a multiple hazard protecting fabric can be
created with more or less
than six layers. For example, the woven or non-woven fabric layers 204, 208
illustrated in FIG. 6
can be omitted. It is also possible to combine different hazard protecting or
heat dissipating layers
together into a single layer. For example, while the radiation protective
layer 206 of the present
invention has been found to provide superior heat dissipating properties on
its own, these heat
dissipating properties can be enhanced by adding strong thermal conductors,
such as silver,
copper and/or aluminum, to the mixture of radiopaque materials in the
radiation protective layer
206.
[0063] Turning now to FIG. 7, a bullet proof vest 300 is illustrated which has
additional hazard
protecting properties. Most of the bullet proof vest 300 is of conventional
design, similar to that
shown in Borgese's U.S. Patent No. 4,989,266. The bullet proof protection is
primarily provided
by layers of polyethylene fibers 314 and/or aramid fibers 316. Commercially
available
16
CA 02591994 2012-12-18
=
polyethylene fabrics used for bulletproof vests include Honeywell's SpectraTM
series of ultra high
molecular weight polyethylene fabrics and Honeywell's SpectraguardTM ultra
high molecular
weight polyethylene fabrics which also include fiberglass. Commercially
available aramid fabrics
used in bulletproof vests include DuPont's Kevlar series of aramid fabrics
and Akzo's
TwaronTm series of aramid fabrics. In this preferred example, the bullet proof
vest has one or
more layers of aramid fibers 316 sandwiched between layers of polyethylene
fibers 314. To
obtain greater levels of protection against bullets and shrapnel, one
typically creates a greater
number of layers of aramid fibers 314 and/or polyethylene fibers 316.
Additional strength can be
created by laying plies of the bulletproof material at 90 degree orientations
to one another and
encapsulating them between layers of thermoplastic. Ceramics and plates can be
added to provide
even higher levels of protection. The bullet proof vest 300 shown in FIG. 7 is
preferably held
together by a fabric insert casing 312.
100641 To add additional hazard protection to the bullet proof vest 300 shown
in FIG. 7, an
additional layer 320 can be inserted. This additional layer 320 can, in one
embodiment, be a
radiation protecting layer. By adding such a radiation protecting layer to the
bullet proof vest, the
bullet proof vest would achieve protection against radiation as well as
bullets and shrapnel.
Similarly, one could impart fire, chemical and/or biological protection by
using a multiple layer
material of the type described in connection with FIG. 6. In the case of
radiation protection alone,
one would usually want the added layer 320 to be situated close to the user's
body in order to take
advantage of the superior heat dissipation properties of the radiation
protective layer. By contrast,
in the case of a fabric imparting fire, chemical and/or biological protection,
one would typically
want that layer near the outside of the bullet proof vest in order to prevent
those contaminants
from permeating into the bullet proof vest 300.
100651 Turning now to FIG. 8, a multipiece protective garment 400 is
illustrated which can be
used as an undergarment. In certain applications, it is best to disguise the
fact that one may be
wearing a protective garment. For example, a policeman or other first
responder may want to be
protected against radiation and other hazards while not alarming others that
such hazards may be
present. Similarly, the attendant who operates an x-ray inspection machine at
an airport would
want to be protected against continuous exposure to radiation throughout the
work day while not
causing airline passengers to panic about their own incidental contact with
the same x-ray
inspection machine.
17
CA 02591994 2012-12-18
[0066] In the FIG. 8 embodiment, this multipiece protective garment includes a
vest 410, two
shoulder flaps 420, a rear groin flap 430, a front groin flap 440 and two
thigh flaps 450. Through
a head hole 412, the vest 410 would fit over the user's head so that the front
vest panel 414 would
cover the user's chest and the rear vest panel 415 would cover the user's
back. To achieve a snug
fit, the front vest panel 414 is attached to the rear vest panel 415 using
straps 416. The straps 416
can be fastened in a number of well known ways, including snap buttons,
VELCROTM fasteners,
tie straps, buckles are the like. Rear groin flap 430 and front groin flap 440
are used to protect the
waist and groin area of the user. The rear groin flap 430 would be fitted over
the user's buttocks
while the front groin flap 440 would be fitted over the user's groin. Upper
straps 434, 444 are
provided so that the rear groin flap 430 and front groin flap 440 can be
attached to the bottom of
the vest 410 so that they can hang from the vest. For a snug fit, lower straps
432, 442 are
provided on both groin flaps 430, 440 which can be pulled under the groin to
connect with the
lower straps 432, 442 from the mating groin flap 430, 440. To protect the
user's thighs, two thigh
flaps 450 are provided. These thigh flaps 450 are curved so that they can wrap
around the user's
left and right thighs. Four straps 452, 454 are provided for each of these
thigh flaps. The lower
thigh flap straps 452 would wrap around the user's upper leg and fasten onto
the mating lower
thigh flap strap 452. By contrast, the upper thigh flap straps 454 could
either be fastened to the
lower portion of the front groin flap 440 or, like the lower thigh flap straps
452, could wrap
around the user's upper leg and fasten onto the mating upper thigh flap strap
454. The user's
shoulders are protected by shoulder flaps 420. These shoulder flaps 420 are
used to cover the
user's left and right shoulders while being attached to the upper portions 418
of the vest 410. By
leaving the sides of the vest 410 open and using shoulder flap 420
attachments, the multipiece
protective garment 400 of the present invention allows for free arm movement
while providing
protection for vital organs. Similarly, by separating the vest 410 from the
groin flaps 430, 440
and thigh flaps 450, the user is allowed to freely move his legs and torso
while again obtaining
protection for vital organs.
[0067] The multipiece protective garment 400 is constructed from the same type
of radiation
and hazard protecting materials previously described. For radiation protection
alone, a radiation
protective polymeric film can be applied to fabric in the manner described in
U.S. patent
6,841,791 and then cut into the shapes illustrated in FIG. 8. Alternatively, a
multilayer material of
the type shown in FIG. 6 or a multilayer composite of the type shown in FIG. 7
could be cut into
the shapes illustrated in FIG. 8 to provide protection against multiple
hazards including radiation,
18
CA 02591994 2012-12-18
chemical, biological, fire and projectile hazards. In addition to
undergarments, these same
principles could be applied to producing a hazard protection blanket, "dirty"
or nuclear bomb
suppression blanket, jacket, pants, shirt, drape, x-ray apron, vest, cap,
glove and similar
protective articles. These same principles could also be applied to the
manufacture of liners or
coatings for vehicles, walls, vessels, airplanes, spacecraft, house
foundations and containers to
shield against a wide spectrum of electromagnetic and ionizing radiation.
[0068] FIG. 9 shows an alternate embodiment to constructing components of the
multipiece
protective garment 400. In this embodiment, the rear groin flap 530 is
constructed from standard
fabric in the form of a pocket. The protective layer or layers are then made
in the
form of an insert 540 which can fit into the top of the pocket. Straps 536 are
sewn into the
bottom of the fabric pocket 530 in order to prevent the insert 540 from
falling out of the pocket
after insertion. This pocket 530 and insert 540 approach allows different
types of inserts to be
used depending upon the expected hazard. For example, if the user is likely to
encounter only a
radiation hazard, an insert can be used which protects only against radiation
hazards. On the other
hand, if projectile, chemical or biological hazards are also possible, a more
bulky insert can be
used which would provide protection against these additional hazards. This
type of pocket 530
and insert 540 may also be used to form a pocket on the back of vest 410 (see,
FIG. 8), for
example, to provide additional protection for the spine or as a belt loop to
accept a belt or lumbar
support brace.
[0069] Additionally, recent advances in nanotechnology can be used to create
better radiation
detectable and protective articles In certain embodiments, these radiation
attenuating articles can
also provide protection against other types of hazards, such as fire,
chemical, biological and
projectile hazards as well as against a wide range of electromagnetic
radiation energies.
[0070] Nano-materials are materials that have structural features (particle
size or grain size, for
example) in the range of 1-100 nanometers in at least one dimension. Owing to
their small size
and high specific surface area to volume ratio, these materials demonstrate
unique mechanical,
electrical, electronic and optical properties. In addition, nano-materials,
unlike conventional
micron-sized materials, are less likely to create large stress concentrations,
which in turn
increases their yield strength, tensile strength and Young's modulus.
[0071] In the present invention, nano-materials are used in at least three
different ways. In one
embodiment, nano-materials are added to the previously disclosed radiation
protective polymeric
19
CA 02591994 2012-12-18
mixtures to either enhance the radiation protection or provide additional
protections, such as fire,
chemical, biological and/or projectile protection. In a second embodiment,
nanoparticles formed
from radiopaque materials (e.g., barium, bismuth, tungsten etc.) or other
hazard protecting
materials are used in the polymeric mixture instead of more bulky forms of the
same or similar
protective materials. Use of radiopaque nano-materials allows more even
dispersion of
radiopaque materials in the polymeric mixture with the attendant possibility
of allowing higher
concentrations of radiopaque materials before the polymer becomes embrittled.
In a third
embodiment, the nano-materials are formed into a discrete nano-material layer.
Such a discrete
nano-material layer could either be added to a product or formed into a stand
alone product.
[0072] Nano-materials used in the present invention include nanoparticles,
nanotubes and nano
platelets.
[0073] The first type of nanomaterials used in the present invention are
nanoparticles. Suitable
nanoparticles include nanopowders of conventional radiopaque materials, nano
ceramics, nano
shells, nanospheres and other nanoparticles in the shape of hemispheres and
parabolas. Relative
proportions of radiopaque materials could be increased in a polymeric mixture
by replacing bulky
radiopaque materials with radiopaque nanopowders or by incorporating a mixture
of both nano-
sized and micron-sized radiopaque powders. By incorporating a greater
proportion of radiopaque
nanopowders into the polymeric mixture, the resultant product could have
enhanced
electromagnetic radiation attenuating capabilities.
[0074] Nanopowders of radiopaque materials are commercially available and
could be
incorporated into a polymer using standard compounding techniques. The types
of radiopaque
nano-powders that could be used include: tungsten, barium, boron, lead, tin,
bismuth, depleted
uranium, cerium, yttrium, tantalum, lanthanum, neodymium and their compounds.
Tungsten
(APS: 100nm) and tantalum (APS: 100nm) nanopowders can be purchased, for
example, from
Argonide Nanomaterial Technologies, Sanford, Florida. Rare earth radiopaque
nanomaterials of
cerium oxide, yttrium oxide or neodymium oxide can be purchased at
NanoProducts Corporation,
Longmont, CO.
[0075] Moreover, nanoparticles formed in the shape of hollow nanospheres, nano-
hemi spheres,
nano parabolas and nano shells could be used in the present invention to
achieve radio
pacification and attenuation of a wide spectrum of electromagnetic radiation.
These shaped
nanoparticles are believed to deflect, reflect and capture radiation in a
manner similar to the way
CA 02591994 2012-12-18
mirrors deflect, reflect and capture lightwaves. Since these shaped
nanoparticles are believed to
attenuate radiation differently than powdered radiopaque nanoparticles, these
shaped
nanoparticles do not need to be formed from radiopaque materials, but may
instead be formed
from such materials as metal/semiconductor hybrid particles. For example,
hybrid CdS coated
gold nanoparticles have been found to exhibit red-shifted plasmon resonance
absorption. This
resonance absorption band of the metal nanoparticles is a function of the
particle size. As the
particle size decreases, the theoretical wavelength of maximum absorption
intensity could be
approached. By creating these metal nano spheres, hemi spheres
and parabola structures in specific shapes and curvatures, the optical-like
properties can be used
to attenuate against smaller wavelengths of the electromagnetic spectrum
including radio waves,
ultraviolet rays, and ionizing radiation, such as x-rays and gamma rays.
Unlike conventional
heavy metals, which absorb or scatter the electromagnetic radiation, these
nanoparticles
effectively redirect, shift or reflect the electromagnetic radiation, later
converting it into a lesser
energy or heat.
[0076] Referring to FIG. 5 A, the deflection of radiation 132 by the concave
inner surface 134
of a nano-hemisphere 130 is illustrated. In FIG. 5B, radiation 142 passes
through the convex
outer surface 144 of a nanosphere 140, but is internally reflected and,
thereby, captured by the
concave inner surface 146 of the same nanosphere 140.
[0077] As is known in the art, resonating antennas in a parabolic or semi-
spherical shape have
very sharp directional characteristics. By analogy, when creating similar
resonating
characteristics for radiation at a nano level, one would preferably want to
orient the position of
the nano-materials in space to create a layer which would block radiation
coming from a
particular direction (e.g., the outside of a garment). Nonetheless, by
applying a coating of
randomly positioned particles in hundreds of layers, one can effectively
achieve shielding from
all directions. For better performance, such a coating of nano-materials
should have a minimum
of voids. As such, when making a mixture of nano-materials with a binder, the
nano-materials
should preferably be the bulk of the coating, for example, over 70% and, more
preferably,
between 85% and 95% by weight.
[0078] Ceramic nanoparticles could also be added to the radiopaque polymeric
mixture to
enhance not only mechanical strength, like tensile strength and creep
resistance, but also enhance
heat resistance, anti-ballistic, electromagnetic attenuation and neutron
emission attenuation.
21
CA 02591994 2012-12-18
Ceramic nano-powders, which include, but are not limited to, oxides of
aluminum, zirconium,
silicon, titanium, mullite and spinel as well as carbides/nitrides such as
boron carbide, silicon
carbide, titanium carbide, tungsten carbide, boron nitride, silicon nitride,
titanium diboride,
zirconium diboride and other intermetallics like nickel aluminide, titanium
aluminide and
molybdenum disilicate could be advantageously incorporated into the polymeric
mixture to
provide radiation attenuation. These ceramic nanomaterials can be prepared by
a number of
methods including chemical vapor deposition, pulsed laser deposition,
conventional powder
processing (i.e. sol-gel processing), plasma synthesis, pyrolysis,
carbothermal reduction,
hydrothermal processes, emulsion processes, combustion
synthesis, NIST process, precipitation, electrical arc, and ball milling.
Adding metallic second
phase particles into ceramics can also be done to enhance mechanical, thermal
and
electromagnetic attenuation properties. Metals such as tungsten, molybdenum,
nickel, copper,
cobalt and iron can be added to the ceramics using conventional powder
metallurgical techniques
and solution chemical processes like sol-gel, as well as co-precipitation
methods.
[0079] Alternatively, nanoparticles could be synthesized by several additional
techniques. One
such technique is colloidal templating in which an inner removable template
particle, such as
silica or polymer beads, are coated with metal materials in a multi-step
colloidal or vapor-phase
assembly and can later be removed to create empty metallic shells. Creating
uniform coating on a
particle template by colloidal self-assembly is based on the concept of self-
assembled organic
molecular species. The two ends of the molecules to be joined have specific
functional groups
(i.e. Thiols, amines, carboxylic groups) that can be targeted for specific
interactions with the
template and the clusters that are used to make the coatings. Uniform dense
packing of the
molecules around the templates leads to close packing of the clusters that
form a porous but space
filled shell around the template. Non-interacting metal-coated magnetic
particles, which include
Si02/ Au, Fe304ZAu, NiO/Co, silver, platinum, tantalum, tungsten, aluminum and
copper or
coated semi-conducting particles, such as PbS/CdS, are examples of such
composite particle
structures. Alternatively PbS-coated CdS nanocomposite particles that are a
few nanometers in
diameter can be synthesized by ion displacement in inverse micro emulsions.
The refractive
nonlinearity in these nanocomposite particles may be attributed to the optical
Stark effect and to
strong interfacial and inter nano-particle interactions.
22
CA 02591994 2012-12-18
[0080] Hollow nanospheres could be synthesized by taking advantage of the
nanoscale
Kirkendall effect. When nano-crystals, such as cobalt, are exposed to sulfur,
there is a differential
in diffusion in which the cobalt atoms move outward more quickly that then
sulfur atoms thus
creating a hollow nanosphere of cobalt sulfide. Cobalt oxide and cobalt
selenide could also be
synthesized by this technique. Similarly, it may be possible to synthesize
other metals such as
silver, gold, platinum, aluminum, copper and tungsten using this technique.
[0081] Nanoparticles could also be made via in-situ particle formation/in-situ
polymerization. In this method, a stable suspension of metal particles is
prepared in the presence
of a polymer. Once in solution, the composite can be cast, or additional
monomers of the same or
different polymer type can be added to form a nano-composite. The reaction
occurs in the
presence of a protective polymer, which limits the size of the resultant nano-
composite. Particle
size is also controlled by the choice of metal precursor and the metal/polymer
interaction. For
example, if PdC12 is compared with (NH4)2PdC14, the former tends to form
halogen-bridged
complexes and thus tends to form agglomerates of nano-particles, but the
latter does not. The
interaction of the metal precursor with the polymer is also important in
controlling particle size.
If the polymer has a stronger interaction with the precursor, then the
particle size tends to be
reduced because the metal precursors are prevented from phase separating.
Using this technique,
nanoparticles could also be formed through the use of micelles formed from
amphiphilic block
polymers or cross-linked gelled matrices. Using copolymers to form micelles,
metal salts are
introduced that can either penetrate the micelle or are stable in the micelle
corona. A reducing
agent can be added and metal particles form either within the micelles or in
the corona resulting
in several morphologies.
[0082] In general , nanoparticle size is controlled in several ways depending
on the synthesizing
technique used. For instance, in gas phase, synthesis particle size is
controlled by varying the
system parameters such as temperature, gas flow rate and system pressure. In
other methods such
as sol-gel technique, the particle size can be varied by changing the
concentration of the solutions
and temperature. In mechanical milling, the particle size depends more on the
speed of the
grinding media and milling time.
[0083] Hollow nano-crystals can be commercially obtained from the Molecular
Foundry at the
Berkeley National Laboratory in Berkeley California, which specializes in
synthesizing hollow
metal, metal oxide/sulphide nano-crystals.
23
CA 02591994 2012-12-18
[0084] The second type of nano-materials used in the present invention are
nano-tubes. Nano-
tubes are typically formed from carbon. When added to a polymeric mixture,
nano-tubes
represent another way to enhance mechanical properties like modulus, chemical
resistance, flame
resistance, strength and also electrical conductivity of the mixture. Carbon
nano-tubes have
unique electrical properties because the electronic conduction process in nano-
tubes is confined
in the radial direction and, as a result, they can also be used to attenuate
electromagnetic
radiation. The methods to produce these nanotubes includes chemical vapor
deposition
techniques using catalysts and hydrocarbon precursors to grow the nano-tubes.
Nano-tubes can
also be made by electric arc, laser ablation, chemical vapor deposition and
high pressure carbon
monoxide conversion (HiPC0). HiPCO uses high-pressure disproportionation of
carbon
monoxide gas in the presence of iron carbonyl catalyst vapor to produce nano-
tubes of 80%
purity in large quantities. Other types of nano-tubes include the hexagonal
boron nitride nano-
tubes, nano-tubes made of dichalcogenides (i.e. MoS2, WS2), nano-tubes of
oxides (i.e. V20,
Mo03,), gold nanotubes and organic nano-tubes. Nano-tubes may be commercially
purchased
from Materials and Electrochemical Research Corporation of Tucson, Arizona.
[0085] Once produced, nano-tubes should undergo purification procedures before
they can be
incorporated into a radiopaque polymeric mixture of the present invention.
Methods of
purification and processing include preliminary filtration, dissolution, micro-
filtration, settling
and chromatography. The resultant nano-tube product is then preferably
dispersed in the
polymeric mixture with a surfactant, such as sodium dodecyl sulfate.
[0086] The third type of nano-materials that could be added to the radio
opaque polymeric
mixture are nano platelets (i.e., plate-like nano-fillers). Nano platelets are
layered materials that
typically have a high aspect ratio and a thickness on the order of about 1 nm.
[0087] When added to a polymeric mixture in quantity, nano-platelets would
enhance the
mixture's chemical, ballistic, fire, electromagnetic radiation and neutron
resistance. Nano-
platelets include nano-clays, such as montmorillonites clays. Montmorillonite
clays belong to the
smectite group which also includes clays like bentonite, hectorite,
pyrophyllite, talc, vermiculite,
sauconite, saponite and nontronite, layered silicic acids (i.e. kanemite and
makatite) and layered
double hydroxides. Clays from other groups, such as kaolinites and chlorites,
and other
phyllosilicates, such as mica, could also be used. Transition-metal
dichalcogenides (i.e. tantalum
dichalcogenides intercalated with lithium) could also be dispersed in a
polymer mixture not only
24
CA 02591994 2012-12-18
to provide the mixture with nanoclay like properties (because of its similar
layered structure), but
also to enhance the electromagnetic radiation attenuation.
[0088] Natural nano-clays, such as smectites clays, are highly layered weakly
bonded materials.
Each layer consists of two sheets of silica tetrahedra with an edge shared
octahedral sheet of
either alumina or magnesia. Due to the isomorphic substitution of alumina into
the silicate layers
or magnesium for aluminum, each unit cell has a negative charge. The natural
nano-clay layers
are held together with a layer of charge compensating cations such as Lithium
(Li+), Sodium
(Na+), Potassium (K+), and Calcium (Ca+). These charge-compensating cations
provide a route
to the rich intercalation chemistry and surface modification that is required
to disperse nanoclays
into the polymer. Synthetic clays, such as hydrotalcite, carry a positive
charge on the platelets.
For these layered nano-clays to become useful within the radiopaque polymeric
mixture, the
layers should be separated and dispersed properly within the mixture. In the
case of nano-clays,
such as silicate clays, they are inherently hydrophilic while the polymers
tend to be hydrophobic.
To get intercalation and exfoliation of these clays, the galleries or layers
of these clays must be
opened and the polarities of the resultant clay must match the polarity of the
polymer so that the
polymer will intercalate between the layers. This is done by exchanging an
organic cation for an
inorganic cation. The larger organic cation will swell the layers and increase
the hydrophobic
properties of the clay. The organically modified clay can then be intercalated
with the polymer by
several routes. For positively charged clays, such as hydrotalcite, an anionic
surfactant can be
used. Other types of clay modifications can be used depending on the choice of
polymer. These
include ion-dipole interactions, the use of silane coupling agents and the use
of block polymers.
An example of ion-dipole interactions is the intercalation of a small
molecule, such as
dodecylpyrrolidone, into the clay. Unfavorable interactions of clay edges with
polymers can be
overcome by the use of silane coupling agents to modify the edges.
Alternatively,
compatibilization of clays and polymers can be done through the use of
copolymers where one
component of the copolymer is compatible with the clay and the other component
of the
copolymer is compatible with the polymer.
[0089] The resistance of a polymeric mixture to harmful chemicals could be
improved
substantially by incorporating a small amount of nanoclays (about 2 to 5 % by
weight) into the
polymer mixture. The level of chemical resistance improvement depends on many
factors,
though, such as the degree of exfoliation of the nano platelets in the polymer
mixture, the
CA 02591994 2012-12-18
percentage of the nano-material filler, its aspect ratio, and the alignment of
the platelets. By
incorporating nano-clays within the polymeric mixture, oxygen transmission
through the mixture
is particularly reduced which then reduces polymer degradation by reducing
oxidation of the
resins and hence improving its flame retardancy property as well, hi addition,
the inorganic phase
can act as a sink to prevent polymer chains from decomposing.
[0090] To enhance flame retardance in polymeric mixtures of the present
invention, traditional
fire retardant additives, such as alumina trihydrate (ATH), magnesium
hydroxide or organic
brominated and chlorinated compounds are often added. Nonetheless, very high
levels of these
fire retardant additives are usually needed to achieve acceptable levels of
fire retardancy (e.g., for
cable or wires). These high additive levels make the manufacturing process
more difficult and
therefore embrittles the polymeric end product.
[0091] In the present invention, nanomaterials can be used in the polymeric
mixture to
overcome this fire retardant embrittlement problem. More specifically, a small
weight percent of
nanoclays (e.g., 2 to 10%) can be added with the traditional bulky fire-
retardant additives, such as
ATH or magnesium hydroxide, to drastically lower the additive loading levels
needed to achieve
the same or an improved level of flame resistance in a polymeric mixture.
Other nano sized fire-
retardant additives which could be added to the polymeric mixture are
nano/micron- sized oxides
such as antimony oxide, nano/micron-sized compounds of molybdenum, titanium,
zirconium and
zinc. Silicon carbide, silicon nitrate, aluminum nitride, silicon nano-tubes,
carbon nano-tubes,
boron nitride nano-tubes also could be used to enhance the fire resistant
properties of a polymer.
Moreover, conventional fire resistant additives, such as ATH or magnesium
hydroxide, could be
added in the nano-size range to more effectively achieve fire resistance in a
polymeric mixture.
Through use of these nanomaterials, the resultant polymeric mixture would be
more strong, light
and flexible.
[0092] One of the key limitations in the use of nano-materials in polymeric
compositions is
processing. More specifically, nanomaterials tend to agglomerate to reduce
their surface area and,
therefore, without proper dispersion and distribution in the polymer mixture,
the desired
properties of the resulting nano-composite cannot be achieved. In order to
effectively disperse
nanomaterials into a polymer and process the resulting mixture by standard
manufacturing
techniques, the nanomaterials should be surface modified. For instance, in the
case of nanoclays,
the clay surface can be modified by a process known as compatibilization so
that the nanoclays
26
CA 02591994 2012-12-18
are attracted to the polymeric resin matrices and thus get thoroughly
dispersed. The two most
common compatibilization methods are onium ion modification and the ion-dipole
interaction.
[0093] Once the nano-material agglomeration problem is overcome, there are
three general
ways of dispersing nano-materials into the polymeric mixture. The first is
direct mixing of the
polymer and the nano-materials either as a discrete phase or in solution. The
second is in-situ
polymerization in the presence of a nano-material, and the third is in-situ
particle processing
which involves both in-situ formation of the nano-materials and in-situ
polymerization.
[0094] For example, to prepare a chemical-fire resistant nano-composite, a
polymer, such as
ethyl vinyl acetate (EVA), can be directly mixed with nano-platelets such as
nano-clays, silicic
acids, or transitional metal dichalcogenides. Such a mixture can be made with
or without
conventional fire retardants, such as ATH and magnesium hydroxide. The
resultant polymeric
mixture can then be processed in a twin-screw extruder and formed into a
desired product using
blow molding, or injection molding. Compounding with twin-screw extruder
creates a great
amount of shear force, which helps exfoliate the nano-materials in the polymer
mixture. The
addition of nanoclays or nanotubes would increase the viscosity of the
polymeric mixture.
Therefore, the rheology of the mixture should be closely monitored and
controlled, though, by
adding rheological additives that are compatible to the polymer and filler
used (i.e. nanoclays or
nanotubes).
[0095] To produce the protective products of the present invention, nano-
materials can also be
coated on several different substrates, including polymeric substrates. Using
known techniques,
such as evaporation, sputtering (glow discharge/ion and beam/laser), ion
plating, chemical vapor
deposition (CVD), plasma enhanced CVD, thermal spraying, dip coating,
fluidized bed, and
atomized liquid spray, nano-composites can be coated on several different
substrates. Also, nano-
materials could be applied as a coat on different substrates by other
techniques like unassisted
spraying, spraying assisted by a high voltage electrical field, liquid coating
by such existing
technologies as roll stock, extrusion, coating and co-extrusion.
[0096] Alternatively, nano-materials can be applied to a flexible film, which
can then be coated
with a pressure sensitive adhesive to produce a self-adhering material with
shielding properties.
For protection of human skin from the sun's ultraviolet rays, for example,
nano-materials can be
mixed with binders to form a spray or ointment to be applied directly to the
skin. Further, since
27
CA 02591994 2012-12-18
=
the thickness of several hundred rows of nano-materials is on the order of a
single micrometer,
the nano shielding materials of the present invention can be made transparent
to visible light and
thereby allow its use in the manufacture of goggles and other clear shields,
with excellent optical
properties.
[0097] Turning now to FIG. 10, a bomb containment vessel 760 is shown which
includes bomb
containment sphere 762, front hatch 764, wheel assembly 766 and bomb tray 768.
The sphere
762 and front hatch 764 of the bomb containment vessel 760 are constructed out
of a hard
explosion resistant material, such as hardened steel. While existing bomb
containment vessels
760 are constructed to contain conventional bomb explosions, they are not
designed to also trap
or attenuate nuclear radiation, such as gamma and neutron emissions or rays.
Using the principles
of the present invention, though, the bomb containment vessel 760 can be
reconfigured to also
protect against the nuclear hazards produced by, for example, a "dirty" or
radiological bomb.
[0098] In the preferred embodiment, a radiation protective polymeric layer 770
is applied to the
outside of the bomb containment vessel 760. As before, the radiation
protective polymeric layer
770 is formed from a mixture, which includes one or more of the previously
mentioned
radiopaque materials and one or more of the previously mentioned polymers. In
the preferred
embodiment illustrated in FIG. 10, the radiopaque polymeric mixture is used to
form curved
radiopaque tiles 772. These radiopaque tiles 772 can be formed by any number
of known
manufacturing processes, including injection molding, extrusion, vacuum
forming, drape
forming, pressure forming and plug assisted forming. The radiopaque tiles 772
are then adhered
to the outside surface of the bomb containment vessel 760 and to each other.
To enhance the
appearance of the bomb containment vessel 760, a smooth decorative layer (not
shown) can then
be applied over the radiopaque tiles 772. Alternatively, a radiopaque
polymeric layer can be
formed in one piece, or can be evenly coated on the outside or within the bomb
containment
vessel through adhesive spraying, rotational molding, injection molding,
dipping in a liquid bath,
painting or other known coating and injection molding processes.
[0099] In operation, the hardened materials of the bomb containment vessel 760
will contain the
explosive force of the bomb while the radiation protective layer 770 of the
present invention will
contain any radiation emitted by the bomb. While the radiation protective
layer of the present
invention could also be applied to the inside of the bomb containment vessel
760, the inventors
28
CA 02591994 2012-12-18
. ,
believe that this would be less effective because of the damage an explosion
could do to the
radiation protective layer 770.
[0100] In the foregoing specification, a number of specific preferred
embodiments and methods
have been disclosed. It will, however, be evident to those of skill in the art
that various
modifications and changes may be made to these specific embodiments without
departing from
the broader spirit and scope of the invention as described above and set forth
in the appended
claims. For example, those of skill in the art will recognize that the
principles of the present
invention would apply to many types of articles besides the toys, utensils,
weapons and medical
devices previously described. More specifically, the relatively lightweight
radiopaque materials
of the present invention could be incorporated into virtually any type of
plastic product (e.g., auto
parts, phones, storage containers etc.) to allow the presence and/or
attributes of such products to
be assessed using x-rays. Further, the principles of the present invention
would apply to virtually
all types of manufacturing processes for plastic products. While x-ray
inspection has been
described in the preferred embodiments, other types of radiation, such as
alpha, beta or gamma
radiation could alternatively be used to detect the radiopaque polymeric
articles. In the case of
nano-materials, since many nano-materials have been found to have minimal
toxicity, nano-
composites could be added intravenously or orally to the human body to provide
enhanced tissue
contrast for use in radiography. The examples, accordingly, are to be regarded
in an illustrative,
rather than restrictive sense.
29