Note: Descriptions are shown in the official language in which they were submitted.
CA 02592695 2007-06-29
Respiratory Device for Treating Obstructive Sleep Apnea and
Method for Controlling Said Device
The invention relates to a respiratory device for treating
patients who suffer from obstructive sleep apnea having the
characterizing features disclosed in the preamble to claim 1, and
a method for controlling said device.
Respiratory devices for treating obstructive sleep apnea generate
an artificial atmosphere, the pressure of which is higher than
the natural atmosphere, and which is supplied continuously, via a
respiratory tube and a face mask, during sleep to the patient
being treated.
During sleep, as muscle tone generally decreases, the soft
tissues of the respiratory passages in the area around the
pharynx can collapse, causing the sleeping patient to begin
choking. This respiratory arrest is known as obstructive sleep
apnea. The reason for the collapse of the soft respiratory
passages is a drop in pressure in them, caused by the high rate
of respiratory airflow. As a result, the respiratory passages in
Park Case # 3650
CA 02592695 2007-06-29
2
the area around the base of the tongue and the soft palate are no
longer able to withstand the difference in pressure to the
exterior, normal surrounding atmosphere. They collapse against
one another and obstruct the airway. The continued suction force
of the lungs further intensifies the obstruction, and the patient
is no longer able to take in any air for several tens of seconds.
This process can occur several hundred times during sleep every
night. The long-term effects are a decrease in quality of life,
diseases of the cardiovascular system, and an overall decrease in
life expectancy.
When a patient with obstructive sleep apnea breathes from an
artificial atmosphere, the pressure of which is greater than the
pressure of the natural surrounding atmosphere, at least by the
level of the flow-based drop in pressure in the area around the
pharynx, then the soft tissue in the area around the pharynx can
no longer collapse. The patient is again able to breathe freely
and spontaneously, and the number of sleep apneas is reduced to
that of a normal person. The artificial atmosphere required for
this is generated by a respiratory device, and is supplied to the
patient via a respiratory tube and a breathing mask.
Respiratory devices that generate an artificial atmosphere at a
continuously constant treatment pressure are known as CPAP
systems (CPAP = Continuous Positive Airway Pressure).
The airflow in the respiratory passages of a patient is not free
from resistance. Laminar and turbulent resistance components
distributed throughout the passages produce an obstructive
effect. Resistance components are those that resist flow in the
nasal passages, the soft-walled respiratory passages in the
Park Case # 3650
CA 02592695 2007-06-29
3
pharynx, and the bronchial system. The degree of turbulent
resistance is dependent upon the flow rate.
The airway resistances of a patient and the flow resistances of a
device are arranged in series. The internal resistance of a
device can be influenced via a corresponding device controller.
A low level of strain on the respiratory musculature is achieved
when the flow resistance of the device is negatively configured,
thereby counteracting the effect of the positive airway
resistances of the patient. A negative resistance has a reversed
pressure/flow behavior. Referred to the internal flow resistance
of a CPAP device, said device would then generate a higher
pressure when acted upon by an air flow in the direction of the
patient, and would generate a lower pressure when the patient
exhales air in the direction of the device.
CPAP devices, which generate a higher treatment pressure during
inspiration and a lower treatment pressure during expiration, are
known as bi-level devices (e.g. a device under the name BIPAP of
the RESPIRONICS firm). To determine the point of transition from
inspiration to expiration and vice versa, these devices have a
processor, called the breath trigger. Bi-level devices are
always used if the patient already has generally elevated airway
resistance due to other illnesses, or if treatment using a CPAP
device would be successful only at a very high treatment
pressure.
The control of the pressure in bi-level systems is comparable to
the control of the pressure in respiratory devices for artificial
respiration. One drawback consists in the fact that only two
pressure levels exist, which are preset and cannot be adjusted,
Park Case # 3650
CA 02592695 2007-06-29
4
regardless of the respective depth of respiration. The shifts in
pressure initiated via the breath trigger create a rectangular
respiratory pressure pattern, and therefore no optimal
compensation of the resistances of the respiratory passages.
Thus the transition from the lower to the higher pressure level
effects a brief period of artificial respiration, in that air is
forced into the lungs. During the transition from the higher to
the lower pressure level, a constriction of the small respiratory
passages can occur due to the high expiration flow at the start,
making expiration even harder. These effects are uncomfortable
for the patient and can be mitigated by rounding off the
transition from one pressure level to the other, rather than
structuring it in a precisely rectangular pattern.
The comfort of a bi-level device is decisively influenced by the
proper functioning of the breath trigger. Precisely at the end
of the expiration phase, when the respiratory air flow has nearly
died down, additional air fluctuations occur, because the stroke
volume rhythmically influences the volume of the compressed lungs
and the flow of air exiting them. Therefore a breath trigger
cannot precisely identify the end of an expiration, and the
patient has the feeling that the change in pressure frequently
takes place at the incorrect time.
It is thus the object of the invention to provide a respiratory
device for treating sleep apnea, which, when used, substantially
reduces the strain on the respiratory musculature of the patient,
makes the course of treatment more pleasant, and decreases
potential secondary effects of the treatment. A further aspect
of the invention is to provide a method for controlling such a
device.
Park Case # 3650
CA 02592695 2007-06-29
This object is attained with a respiratory device having the
characterizing features of patent claim 1. Advantageous further
improvements on such a respiratory device are disclosed in the
dependent claims 2 through 5.
A further feature of the attainment of the object consists in a
method for controlling such a respiratory device, as is disclosed
in patent claim 6, with advantageous further improvements
according to claims 7 and 8.
The respiratory device according to the invention comprises a
pressure generator that is capable of providing any level of
pressure up to a freely selectable threshold limit, and is
controlled via a pressure regulator. With the pressure
generator, for example a ventilator, the respiratory device
generates an artificial atmosphere, which is supplied to the
patient through the respiratory tube and the breathing mask.
The control mechanism contained in the respiratory device
according to the invention, which in one advantageous further
improvement can be a servo processor (see claim 3), detects a
pressure loss from the airway resistance and adjusts the
treatment pressure course essentially proportionally to the
pattern of the detected pressure loss.
In one advantageous further improvement, the control mechanism is
equipped to calculate the pressure loss from the airway
resistance using the measured variables of the respiratory
airflow and airway resistance of the patient. For this purpose,
the respiratory device is equipped with a device for determining
respiratory airflow. The patient's airway resistance can be
determined externally and communicated to the respiratory device
in the form of an input, or, as disclosed in an advantageous
further improvement according to claim 4, this resistance can
automatically be permanently or intermittently determined with
Park Case # 3650
CA 02592695 2007-06-29
6
the help of a measuring device that is integrated into the
respiratory device.
As an advantageous further improvement, a possible continuous or
intermittent measurement of airway resistance can be effected,
for example, with the help of a modulated pressure or airflow
signal. In this manner, changes in airway resistance are always
immediately accounted for in the inventive control mechanism of
the respiratory device. Because air is ordinarily supplied from
the respiratory device via a nasal mask, and the flow resistance
in the nose is subject to fairly major changes, the continuous
measurement of airway resistance is a good way of compensating
for the varying degrees of impairment of nasal respiration.
A further advantageous embodiment of the respiratory device of
the invention involves combining the inventive properties with
the operating mode for devices (see claim 4) that function on the
basis of the auto-adjust principle (auto-CPAP) for determining an
optimal basic pressure, which are known in the prior art and
described in the patent literature, for example in EP 0705 615
Al. A respiratory device equipped with this combination
according to the invention then automatically determines the most
favorable basic pressure, which can also change with the physical
constitution of the patient.
Dispensing with the above-described advantageous embodiments,
both basic pressure and airway resistance can be determined via
external means and permanently entered into the respiratory
device of the invention.
The overall effect achieved with the respiratory device of the
invention is that of a respiratory force amplifier, which
Park Case # 3650
CA 02592695 2007-06-29
7
supports inspiration effort with a matched pressure increase and
expiration effort with a likewise matched pressure decrease. This
effect is comparable to that of a power steering mechanism or a
power braking mechanism in an automobile.
Comfortable breathing is achieved in that both the pressure
increase during inspiration and the pressure decrease during
expiration are nearly proportional to the pressure loss from
airway resistance. To accomplish this, with known airway
resistance, the pressure loss is calculated as the product of the
airway resistance and a portion of the square of the respiratory
airflow, since turbulent resistance increases in a linear fashion
with the flow rate. The size of this portion determines the
degree of respiratory force amplification.
Also advantageous is an adjustment to the different stages of
sleep, which are known to vary according to different respiratory
parameters, such as breathing rate and tidal volume. In this
manner, at low respiratory intensity, a proportional artificial
respiration is prevented in that the difference between maximum
inspiration pressure and minimum expiration pressure
automatically becomes smaller. At high respiratory intensity,
however, the difference between maximum inspiration pressure and
minimum expiration pressure increases accordingly.
From a predetermined intensity it can also be established with
the help of the control mechanism whether the entire pressure
loss from respiratory resistance or only a portion of it should
be compensated for via the respiratory device (see claim 6). To
accomplish this, the output signal from the control mechanism and
the predetermined basic pressure signal are added together and
Park Case # 3650
CA 02592695 2007-06-29
8
supplied to the pressure regulating device as a command variable
(see claim 7).
Below, the invention will be described in greater detail with
reference to an exemplary embodiment. The attached set of
drawings shows:
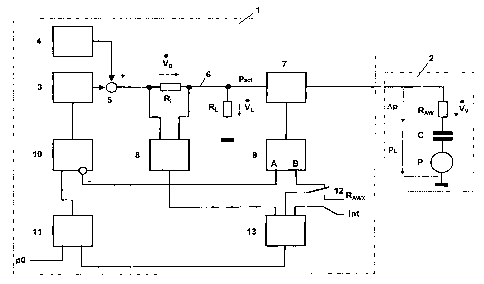
Fig. 1 a functional block diagram of an exemplary embodiment
of a respiratory device according to the invention and
Fig. 2 a chronological representation of the signal patterns
for respiratory airflow Vv,pressure loss Lp,
treatment pressure pa,t and intrapulmonary pressure PL
for different proportions of the pressure loss Lp
taken into account in determining the treatment
pressure pact.
The functional block diagram in Figure 1 shows a variant that
represents one possible implementation of a respiratory device 1
according to the invention.
The respiratory device 1 comprises a pressure generator 3 for
generating pressure, here in the form of a ventilator, and an
oscillation pump 4, which generates an oscillating pressure
pattern with a low amplitude. The two pressure levels are
combined in a summation unit 5 to form a total pressure, which is
supplied via a flow resistance R; and a respiratory tube 6 to the
breathing mask 7. At the flow resistance Ri, this creates a
pressure decrease that is dependent upon the airflow from the
device Vo. A flow processor 8 uses the signal from the device
airflow Vo to determine the signal pattern of the respiratory
airflow Vv that ventilates the lungs of the patient 2. This is
Park Case # 3650
CA 02592695 2007-06-29
9
achieved by calculating the pattern of the leakage airflow VL and
subtracting this from the pattern of the device airflow VI. A
leakage airflow is a desired airflow through an expiration valve
RL, which ordinarily is simply an opening into the surrounding
air, and a possible additional parasitic escape of air through
portions of the mask that are not airtight.
The treatment pressure pa,t is measured in the breathing mask 7,
which ordinarily is simply a nasal mask. The pressure measured
there is supplied to a pressure processor 9, which generates two
output signals. One signal at the output A represents the
current constant pressure portion and is supplied to the pressure
regulating device 10 as the actual pressure. Said device
compares the actual pressure with a target pressure, which is
supplied by the summation unit 11 and contains as a component the
predetermined basic pressure po of the respiratory device 1. The
difference between the target pressure and the actual pressure
determines the level of pressure to be generated by the pressure
generator 3 in such a way that, to the greatest possible extent,
no permanent control deviation occurs.
The other signal at the output B of the pressure processor 9
represents the positive-negative pressure in the breathing mask
7. This is generated by the oscillation pump 4. However, its
amplitude is dependent upon the size ratios of all existing
resistances, the flow resistance Ri, the airway resistance RAW of
the patient 2 and the flow resistance RL of an expiration
opening. For purposes of clarity the figure shows the compliance
C and the respiratory pump P of the patient, however these do not
contribute to the functioning of the respiratory device 1
according to the invention.
The variables of flow resistance Ri and flow resistance RL of the
expiration opening are known, so that the pressure processor can
Park Case # 3650
CA 02592695 2007-06-29
calculate the differential pressure over the airway resistance
RAW, which is a measurement of the magnitude of this resistance.
The measurement result is supplied via the reversing switch 12 to
a servo processor 13, which uses the determined respiratory
airflow Vv to calculate the pressure loss Ap from the airway
resistance RAW, and from the predetermined intensity int
determines whether the total pressure loss Ap that is consumed
via the airway resistance RAW, or only a part of it, should be
compensated for via the respiratory device 1.
By actuating a reversing switch 12, the resistance input to the
servo processor 13 can be switched over to a manual input of a
resistance value RAw,t. In this mode of operation, an externally
determined airway resistance can be permanently predetermined,
which can be practical for certain applications.
The output signal from the servo processor 13 and the
predetermined basic pressure po are added together by means of
the summation unit 11 to obtain the target treatment pressure Pact
for processing by the pressure regulating device 10.
Figure 2 shows the mode of operation of the respiratory device 1
of the invention in terms of signals. To facilitate
understanding of the processes, these are schematically
illustrated, and sinusoidal excitations are considered.
Once the respiratory pump P of the patient 2 is describing
inspiration and expiration cycles, the capacity (compliance) C of
the lungs is being loaded and unloaded via a respiratory airflow
Vv. In this, the respiratory airflow Vv flows through the airway
resistance RAW, whereby a portion of the treatment pressure Pact is
consumed. Between the pressure consumption Ap and the
Park Case # 3650
CA 02592695 2007-06-29
11
airflow, there is an approximately quadratic rather than a linear
connection. As the figure shows, the chronological pattern of
the pressure consumption 4p is therefore no longer sinusoidal in
shape.
With the respiratory device of the invention, the treatment
pressure Pact is continuously being adjusted by a portion of the
pressure loss Ap.
If this portion is zero (represented on the left of the figure),
the treatment pressure pa,t is unchanged over time. In this case,
the periodically fluctuating pattern of the pressure loss Ap that
is elicited by inspiration and expiration manifests itself
entirely in fluctuations in the intrapulmonary pressure PL. To a
person using the respiratory device, this means that he must
overcome the respiratory obstructions caused by his respiratory
resistance RAW using only his respiratory musculature.
With an increasing portion (represented at the center (portion =
0.5) and right (portion = 1) of the figure), the periodic pattern
of the pressure loss ~,p elicited by inspiration and expiration
increasingly causes fluctuations in the treatment pressure pact=
Consequently, fluctuations in the intrapulmonary pressure PL
decrease to the same degree. For the user, this means that his
respiratory efforts decrease. The respiratory effort required to
overcome the airway resistance RAW is taken over to an increasing
degree by the pressure generator. Even a user with an elevated
airway resistance RAW will then require no great effort from the
respiratory musculature for normal inspiration or expiration
processes. He can realize equal ventilation intensity with much
lower intrapulmonary suction or pressure.
Park Case # 3650
CA 02592695 2007-06-29
12
To determine the level of airway resistance RAW, the process of
oscillatory respiratory resistance measurement can be used, for
example.
Another possibility consists in determining the airway resistance
using external measuring processes and inputting the measured or
otherwise determined values.
With the respiratory device of the invention, obstructive sleep
apneas can be treated by generating an artificial atmosphere with
the help of the pressure generator 3, which is supplied to the
patient 2 via the respiratory tube 6 and the breathing mask 7.
In this treatment, the respiratory device 1 adjusts the pressure
of the artificial atmosphere such that the predetermined
treatment pressure pa~t of the artificial atmosphere is changed by
a proportional amount of the consumed pressure Ap at an airway
resistance RAW in the airway of the patient. Preferably, the
proportional amount by which the treatment pressure Pact of the
artificial atmosphere is adjusted, is separately predetermined
for an inspiration or an expiration.
Park Case # 3650
CA 02592695 2007-06-29
13
List of Reference Symbols
1 Respiratory device
2 Patient
3 Pressure generator
4 Oscillation pump
Summation unit
6 Respiratory tube
7 Breathing mask
8 Flow processor
9 Pressure processor
Pressure regulating device
11 Summation unit
12 Reversing switch
13 Servo processor
A Outlet
B Outlet
C Capacity of the lungs (compliance)
Int Intensity
P Respiratory pump
Po Basic pressure
Park Case # 3650
CA 02592695 2007-06-29
14
Pact Treatment pressure
pL Intrapulmonary pressure
RA, Airway resistance
RAWX Externally determined airway resistance
Ri Flow resistance
R, Flow resistance
VD Device airflow
VL Leakage airflow
vv Respiratory airflow
Ap Pressure loss
Park Case # 3650