Note: Descriptions are shown in the official language in which they were submitted.
CA 02593558 2007-06-22
June 22, 2007
Canadian Patent Application
Vaccine against Clostridium perfringens
Field of Invention
The invention relates to the production of a vaccine. More specifically, the
invention
provides a vaccine for controlling Clostridium perfringens in animals.
Background of the Invention
Clostridium are characterized as spore-forming, anaerobic, Gram positive
bacilli. The
species, Clostridium perfringens, can be subdivided into subspecies. Five
subspecies have
been described. These subspecies are generally known as "type" A-E. All
subspecies produce
several toxins, both major and minor toxins. The four major toxins are the
alpha, beta,
epsilon and iota toxin. All C. perfringens types produce the alpha-toxin. The
beta-toxin is
produced by C. perfringens types B and C. In addition, a range of minor toxins
is produced
by all C. perfringens types.
One or more of these various toxins can play a role in C. perfringens related
pathogenesis. Type A is known to be pathogenic for various birds, man, cows
and pigs. Type
B is mainly pathogenic for lamb, sheep and goat, and causes "lamb dysentery"
and
haemorrhagic enteritis. Type C is pathogenic for man, sheep, calf, lamb, pig,
and bird. C.
perfringens can cause of "struck", haemorrhagic enteritis, necrotic enteritis
and
enterotoxemia.
Necrotic enteritis (NE) is an economically important enteric disease of birds,
for
example poultry, caused by Clostridium perfringens. The disease is usually
controlled by
antimicrobial drugs administered at prophylactic doses either in water or
feed. However,
there is concern about the routine prophylactic use of antimicrobial drugs in
food animal
production because of their contribution to resistance problems. If
antimicrobial drugs were
banned for such purposes in North America, there might be an increase in NE in
poultry, for
example chicken flocks, as has happened in Scandinavia (12).
Although vaccination offers an alternative approach to antimicrobial drugs in
control
of the disease, very little is known about immunity to NE. However, there has
been
considerable work on immunity to C. perfringens in other circumstances, since
it is a cause
of gas gangrene in people. This has identified the alpha-toxin, a
phospholipase C
CA 02593558 2007-06-22
2
exoenzyme, both as a major virulence factor and as an important immunogen. For
example,
a genetically engineered vaccine inducing alpha-toxin (amino acids 247-370)
serum
antibodies was shown by Williamson and Titball (34) to neutralize hemolytic
activity of the
toxin and to provide protection against C. perfringens in mice. Bennett et al.
(5) showed that
a recombinant Vaccinia virus vector expressing the non-toxic C-domain region
of the alpha-
toxin protein provided antibody-mediated protection against experimental toxin
challenge.
More recently, Stevens et al. (30) showed significant prevention of gas
gangrene in mice by
immunization using the C-terminal domain of the alpha -toxin (amino acids 247-
370). In
addition, there is evidence based on naturally occurring antibodies or
maternal vaccination
that antibodies to alpha-toxin are involved in immunity to NE (10,19).
However, the
importance of alpha-toxin or any other protein in immunity to NE in birds, for
example
chickens, remains to be demonstrated, and one study has shown the immunizing
effects of
alpha-toxin minus mutants (32). A recent study also demonstrated that an alpha-
toxin
minus mutant produced NE experimentally in chickens, demonstrating that
factors other
than alpha-toxin are important in the pathogenesis of NE (14). Other studies
have shown
that the immunizing ability to protect against NE was associated with virulent
rather than
with avirulent C. perfringens (32).
While the prior art has demonstrated some immunizing effect of whole-cell C.
perfringens in chickens, the basis of this immunity is poorly understood. NE
is usually
controlled by antimicrobial drugs but, if these are unavailable or not used,
there is currently
no other simple way to control infection. Therefore, there is a need for novel
vaccine for
controlling Clostridium perfringens in birds.
An object of an aspect of the present invention is to provide a novel vaccine
for
controlling Clostridium perfringens in birds.
Summary of the Invention
In an aspect, there is provided a vaccine for controlling C. perfringens in an
animal
comprising an isolated nucleic acid molecule which comprises a nucleic acid
sequence that
encodes a C. perfringens secreted antigenic polypeptide or a variant thereof.
In another aspect, there is provided a vaccine for controlling C. perfringens
in an
animal comprising an isolated C. perfringens secreted antigenic polypeptide or
a variant
thereof.
=
CA 02593558 2015-12-10
3
In yet another aspect, there is provided a vaccine for controlling C.
perfringens in
an animal comprising a recombinant cell producing an isolated C. perfringens
secreted
antigenic polypeptide or a variant thereof.
In accordance with another aspect, there is provided a vaccine for controlling
C.
perfringens in an animal comprising an isolated C. perfringens secreted
antigenic
polypeptide comprising an amino acid sequence of Figure 1 (SEQ ID NO:1) or a
variant
thereof conferring an immunoprotective effect against C. perfringens, wherein
the variant
comprises an amino acid sequence having at least 80 % identity with the amino
acid
sequence of Figure 1 (SEQ ID NO:1).
In accordance with another aspect, there is provided a vaccine for controlling
C.
perfringens in an animal comprising a recombinant cell producing an isolated
C.
perfringens secreted antigenic polypeptide comprising an amino acid sequence
of Figure
1 (SEQ ID NO:1) or a variant thereof conferring an immunoprotective effect
against C.
perfringens, wherein the variant comprises an amino acid sequence having at
least 80 %
identity with the amino acid sequence of Figure 1 (SEQ ID NO:1).
In accordance with another aspect, there is provided a use of an isolated C.
perfringens secreted antigenic polypeptide comprising an amino acid sequence
of Figure
1 (SEQ ID NO:1) or a variant thereof conferring an immunoprotective effect
against C.
perfringens for preparation of a vaccine, wherein the variant comprises an
amino acid
sequence having at least 80 % identity with the amino acid sequence of Figure
1 (SEQ
ID NO:1).
Other features and advantages of the present invention will become apparent
from
the following detailed description. It should be understood, however, that the
detailed
description and the specific examples while indicating embodiments of the
invention are
given by way of illustration only, since various changes and modifications
within the
scope of the invention will become apparent to those skilled in the art from
the detailed
description.
Brief Description of the Drawings
The present invention will become more fully understood from the detailed
description given herein and from the accompanying drawings, which are given
by way of
illustration only and do not limit the intended scope of the invention.
Figure 1 shows an amino acid sequence of Hypothetical Protein (HP) of C.
perfringens Strain 13, GenBank Accession # 18144943.
CA 02593558 2015-12-10
3a
Figure 2 shows an amino acid sequence of Pyruvate ferredoxin oxidoreductase
(PFOR) of C. perfringens Strain 13, GenBank Accession # 18311043. The
underlined
portion corresponds to the amino acid sequence of truncated PFOR (tPFOR).
Figure 3 shows an amino acid sequence of Elongation factor-G (EF-G) of C.
perfringens Strain 13, GenBank Accession # 18311390.
Figure 4 shows an amino acid sequence of Perfringolysin 0 of C. perfringens
Strain
13, GenBank Accession # 18143820.
Figure 5 shows an amino acid sequence of Glyceraldehyde 3-phoshate
dehydrogenase (GPD) of C. perfringens Strain 13, GenBank Accession # 18144966.
Figure 6 shows an amino acid sequence of Fructose bi-phosphate aldolase (FBA)
of C. perfringens Strain 13, GenBank Accession # 18310332.
CA 02593558 2007-06-22
4
Figure 7 shows recombinant C. perfringens histidine-tagged proteins purified
from
Escherichia coli cells. (A) Coomassie stained purified proteins (B) Reactivity
of purified
proteins to immune serum from chickens immune to necrotic enteritis. In each
panel, Lane
1- Alpha-toxin (45 kDa), Lane 2- GPD of Figure 5 (40 kDa), Lane 3- FBA of
Figure 6 (35
kDa), Lane 4- tPFOR of Figure 2 (67 kDa), Lane 5- HP of Figure 1 (90-100 kDa)
and Lane M-
Molecular mass standards.
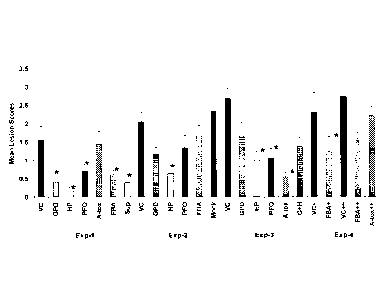
Figure 8 shows a summary of mean lesion scores of immunized broiler chicken
groups challenged with C. perfringens infected feed, together with the
concurrent
unimmunized controls. VC-vehicle-only controls, A-tox- alpha-toxin, FBA-
Fructose 1,6-
biphosphate aldolase, GPD- Glyceraldehyde 3-phosphate dehydrogenase, tPFOR-
Truncated
pyruvate: ferredoxin oxidoreductase, HP- Hypothetical protein, Sup- culture
supernatant of
C. perfringens, G+H- combination of GPD and HP, Exp- Experiment. + -birds in
this group
were challenged for 3 days and autopsied on day 6. ++ -birds in this group
were given a
severe challenge. * -immunized group that had significantly fewer chickens
with lesions
compared to unimmunized vehicle-only controls; Fisher's exact test, p< 0.05.
Figure 9 shows serum IgY ELISA titres of broiler chickens immunized
intramuscularly
with C. perfringens purified proteins. Serum collected at three time-points;
Day 0- Pre-
immunization titre, Day 10- Mid-experiment, Day 20- Pre-challenge titre. FBA-
Fructose 1,6-
biphosphate aldolase, GPD- Glyceraldehyde 3-phosphate dehydrogenase, tPFOR-
Truncated
pyruvate: ferredoxin oxidoreductase, HP-Hypothetical protein, Exp- Experiment.
* Significant titre values when compared to pre-immunization titres, p < 0.05.
Figure 10 shows intestinal IgY and IgA ELISA titres of broiler chickens
immunized
intramuscularly with C. perfringens purified proteins. Samples analyzed were
from pooled
intestines collected from at least 10 chickens in each group. FBA-Fructose 1,6-
biphosphate
aldolase, GPD- Glyceraldehyde 3-phosphate dehydrogenase, tPFOR- Truncated
pyruvate:
ferredoxin oxidoreductase, HP- Hypothetical protein, Exp-Experiment..
Detailed Description of the Preferred Embodiments
A vaccine is provided for controlling Clostridium perfringens in animals. The
vaccine
may comprise a C. perfringens antigenic polypeptide or variant thereof, a
nucleic acid
molecule encoding the C. perfringens antigenic polypeptide or variant thereof,
or a
recombinant cell producing the C. perfringens antigenic polypeptide or variant
thereof.
CA 02593558 2007-06-22
Administration of the vaccine to a subject can confer an immunoprotective
effect to the
subject against C. perfringens. The vaccine may be for prophylactic,
therapeutic, or both
prophylactic and therapeutic treatment.
The vaccine will typically comprise an isolated C. perfringens secreted
antigenic
5 polypeptide or variant thereof, an isolated nucleic acid molecule
encoding the C. perfringens
secreted antigenic polypeptide or variant thereof, or a recombinant cell
producing the C.
perfringens secreted antigenic polypeptide or variant thereof.
An antigenic polypeptide may be provided by any source or method, for example,
natural isolate or recombinant or synthetic origin or suitable combinations
thereof.
Administration of the antigenic polypeptide to a subject can confer an
immunoprotective
effect to the subject against C. perfringens. The antigenic polypeptide may be
of any length
provided that the immunoprotective activity is maintained. The sequence of the
antigenic
polypeptide may be based on a complete or partial naturally occurring amino
acid sequence
of a polypeptide that naturally occurs in virulent C. perfringens type A. An
antigenic
polypeptide may be used either singly or in combination with other
polypeptides, antigenic
or otherwise, in the preparation of a vaccine. A polypeptide refers to a chain
of amino acids,
for example peptides, oligopeptides, or proteins, having a biological
function, and does not
refer to a specific length of the chain.
An isolated C. perfringens antigenic polypeptide is a polypeptide that has
been
identified and separated and/or recovered from at least one component of its
natural
environment. The isolated polypeptide will typically have been purified by at
least one
purification step, and, in some embodiments purification may be achieved (1)
to a degree
sufficient to obtain at least 15 residues of N-terminal or internal amino acid
sequence by use
of a sequenator, or (2) to homogeneity by SDS-PAGE under non-reducing or
reducing
conditions using Coomassie blue or, preferably, silver stain. Isolated
polypeptide includes
polypeptide in situ within recombinant cells, since at least one component of
the C.
perfringens antigenic polypeptide natural environment will not be present. An
isolated
polypeptide may be produced by synthetic or recombinant techniques, for
example as
described in J. Sambrook, E. F. Fritsch, and T. Maniatis, 1989, Molecular
Cloning: A
Laboratory Manual, Second Edition, Books 1-3, Cold Spring Harbor Laboratory
Press. An
isolated polypeptide produced as a result of recombinant techniques may be
referred to as a
recombinant polypeptide.
A nucleic acid encoding an antigenic polypeptide may be any nucleic acid
molecule of,
for example. cDNA, genomic DNA, synthetic DNA or RNA origin or suitable
combinations
thereof. Administration of the nucleic acid encoding an antigenic polypeptide
to a subject
CA 02593558 2007-06-22
6
can confer an immunoprotective effect to the subject against C. perfringens.
The nucleic acid
may be of any length provided that the immunoprotective activity is maintained
by the
encoded antigenic polypeptide. The sequence of the nucleic acid encoding an
antigenic
polypeptide may be based on a complete or partial naturally occurring nucleic
acid sequence
found in virulent C. perfringens type A. A nucleic acid sequence encoding an
antigenic
polypeptide may be used either singly or in combination with other nucleic
acid sequences,
encoding antigenic polypeptides or encoding any other desired polypeptide, in
the
preparation of a vaccine.
An isolated nucleic acid molecule encoding a C. perfringens antigenic
polypeptide is a
nucleic acid molecule that is identified and separated from at least one
contaminant nucleic
acid molecule with which it is ordinarily associated in the natural source of
the nucleic acid.
Such an isolated nucleic acid molecule is other than in the form or setting in
which it is found
in nature. Isolated nucleic acid molecules therefore are distinguished from
the nucleic acid
molecule as it exists in natural cells. An isolated nucleic acid molecule
encoding a C.
perfringens antigenic polypeptide includes nucleic acid molecule encoding a C.
perfringens
antigenic polypeptide contained in cells that ordinarily express the C.
perfringens antigenic
polypeptide where, for example, the nucleic acid molecule is in a chromosomal
or
extrachromosomal location different from that of natural cells. The isolated
nucleic acid
molecule may be referred to as a recombinant nucleic acid molecule where the
isolated
nucleic acid molecule has been manipulated using recombinant techniques, for
example, as
described in 3. Sambrook, E. F. Fritsch, and T. Maniatis, 1989, Molecular
Cloning: A
Laboratory Manual, Second Edition, Books 1-3, Cold Spring Harbor Laboratory
Press.
Variants include, without limitation, analogs, derivatives, fragments,
truncations,
mutants, deletions, substitutions, insertions, fusions and the like.
An antigenic polypeptide or a nucleic acid encoding an antigenic polypeptide
may be
mutated or changed or derivatised in any manner desired (for example, any
number or
combination of deletions, insertions, or substitutions) to produce a
corresponding variant.
Use of such variants in producing vaccines and in vaccinating a subject is
contemplated, and
such a variant nucleic acid or variant polypeptide may be mutated or changed
or derivatised
in any manner in comparison to a naturally occurring nucleic acid or
polypeptide sequence,
respectively, found in virulent C. perfringens (type A), provided that the
capability of
conferring an immunoprotective effect against C. perfringens is maintained.
Similarly,
nucleic acids or polypeptides having varying degrees of sequence identity to a
corresponding
naturally occurring nucleic acid or polypeptide sequence found in virulent C.
perfringens
(type A) may be tolerated without eliminating an immunoprotective activity
against C.
CA 02593558 2007-06-22
7
perfringens. For example, a vaccine may comprise an antigenic polypeptide
having a
sequence that is identical to a naturally-occurring form of the antigenic
polypeptide or a
variant thereof that has a sequence that is at least 80% identical to a
naturally-occurring
form of the antigenic polypeptide. As another example, a vaccine may comprise
a nucleic
acid molecule having a coding sequence that is identical to a naturally-
occurring form of the
coding sequence or a variant thereof that has a sequence that is at least 70%
identical to a
naturally-occurring form of the coding sequence. Determination of sequence
identity of
proteins and nucleic acids by computer based methods, as well as nucleic acid
hybridization
techniques using high stringency conditions for determining or identifying
nucleic acid
sequences that share high (eg., at least 70%) sequence identity are well known
to the
skilled person.
Stringency of hybridization reactions is readily determinable by one of
ordinary skill in
the art, and generally is an empirical calculation dependent upon probe
length, washing
temperature, and salt concentration. In general, longer probes require higher
temperatures
for proper annealing, while shorter probes need lower temperatures.
Hybridization generally
depends on the ability of denatured DNA to reanneal when complementary strands
are
present in an environment below their melting temperature. The higher the
degree of
sequence identity between the probe and hybridizable sequence, the higher the
relative
temperature which can be used. High stringency conditions may be identified by
those that:
(1) employ low ionic strength and high temperature for washing, for example
0.015 M
sodium chloride/0.0015 M sodium citrate/0.1% sodium dodecyl sulfate at 50 C.;
(2) employ
during hybridization a denaturing agent, such as formamide, for example, 50%
(v/v)
formamide with 0.1% bovine serum albumin/0.1% Fico11/0.1%
polyvinylpyrrolidone/50 mM
sodium phosphate buffer at pH 6.5 with 750 mM sodium chloride, 75 mM sodium
citrate at
42 C.; or (3) employ 50% formamide, 5xSSC (0.75 M NaCI, 0.075 M sodium
citrate), 50 mM
sodium phosphate (pH 6.8), 0.1% sodium pyrophosphate, 5x Denhardt's solution,
sonicated
salmon sperm DNA (50 pg/ml), 0.1% SDS, and 10% dextran sulfate at 42 C., with
washes
at 42 C. in 0.2xSSC (sodium chloride/sodium citrate) and 50% formamide at 55
C., followed
by a high-stringency wash consisting of 0.1xSSC containing EDTA at 55 C.
Hybridization and
wash times should be sufficient for achieving equilibrium.
Percent (%) sequence identity of amino acid or nucleic acid sequences with
respect to
antigenic polypeptides as, for example in Figures 1 to 6, and nucleic acid
sequences
encoding antigen polypeptides is the percentage of residues in a candidate
sequence that
are identical with the antigenic polypeptide amino acid sequence or the
antigenic
polypeptide-encoding nucleic acid sequence, as the case may be, after aligning
the
CA 02593558 2007-06-22
8
sequences and introducing gaps, if necessary, to achieve the maximum percent
sequence
identity. Alignment for purposes of determining percent amino acid sequence
identity or
percent nucleic acid sequence identity can be achieved in various ways that
are within the
skill in the art, for instance, using publicly available computer software
such as BLAST,
BLAST-2, ALIGN, ALIGN-2 or Megalign (DNASTAR) software. Those skilled in the
art can
determine appropriate parameters for measuring alignment, including any
algorithms
needed to achieve maximal alignment over a desired length of sequence, for
example, at
least 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140, 150, 160, 170,
180, 190, or
200 residues or even the full-length of the sequences being compared.
When considering an antigenic polypeptide or variant thereof, the variant
antigenic
polypeptide will typically have an amino acid sequence that is at least 80,
82, 84, 86, 88, 90,
92, 94, 96, or 98 percent identical to the corresponding antigenic
polypeptide.
When considering a nucleic acid sequence encoding an antigenic polypeptide or
variant thereof, the variant nucleic acid sequence will typically be at least
70, 72, 74, 76, 78,
80, 82, 84, 86, 88, 90, 92, 94, 96, or 98 percent identical to the
corresponding nucleic acid
encoding the antigenic polypeptide.
Techniques and strategies for producing variants are well known in the art. In
one
example, with regard to polypeptides, an antigenic polypeptide may be modified
in vivo or in
vitro by, glycosylation, amidation, phosphorylation, carboxylation,
truncation, fragmentation,
substitution, and the like without eliminating an immunoprotective activity
against C.
perfringens. In another example, with regard to nucleic acids, substitution
mutations can be
made in a nucleic acid encoding an antigenic polypeptide such that a
particular codon is
changed to a codon which codes for a different amino acid. A substitution
mutation of this
sort can be made to change an amino acid in the resulting protein in a non-
conservative
manner (i.e., by changing the codon from an amino acid belonging to a grouping
of amino
acids having a particular size or characteristic to an amino acid belonging to
another
grouping) or in a conservative manner (i.e. by changing the codon from an
amino acid
belonging to a grouping of amino acids having a particular size or
characteristic to an amino
acid belonging to the same grouping). Such a conservative change generally
leads to less
change in the structure and function of the resulting protein. A non-
conservative change is
more likely to alter the structure, activity or function of the resulting
protein. Groupings of
amino acids are known to the skilled person. For example, the nonpolar
(hydrophobic) amino
acids include alanine, leucine, isoleucine, valine, proline, phenylalanine,
tryptophan and
methionine. Amino acids containing aromatic ring structures are phenylalanine,
tryptophan,
and tyrosine. The polar neutral amino acids include glycine, serine,
threonine, cysteine,
CA 02593558 2007-06-22
9
tyrosine, asparagine, and glutamine. The positively charges (basic) amino
acids include
arginine, lysine and histidine. The negatively charged (acidic) amino acids
include aspartic
acid and glutamic acid. Any number of such substitutions or any other type of
alteration
(eg., deletion or insertion) or modification may be tolerated provided that
the
immunoprotective effect of the antigenic polypeptide is not eliminated.
Recombinant cells, comprising an antigenic polypeptide or a nucleic acid
sequence
that encodes an antigenic polypeptide may be used as vaccines for controlling
C.
perfringens. Recombinant cell types may include any cell type that is
compatible with the
physiology of an intended vaccination subject. Cells of eukaryotic or
prokaryotic origin may
be used. Prokaryotic cells that can survive within the gastrointestinal system
of an intended
vaccination subject may be particularly useful for preparation of oral or
enteral vaccines. For
example, cells that form part of the intestinal flora of an intended
vaccination subject (such
as Eschercichia coli or Lactobacillus species) may be used. In another
example, aviru lent
Salmonella or Listeria or other attenuated invasive bacterial cells may be
used.
A cell may be altered or modified to comprise a nucleic acid sequence that
does not
naturally occur in the cell, and as such the cell will be considered
recombinant. In other
examples, a cell may be altered or modified to comprise an additional copy of
a nucleic acid
sequence that naturally occurs in the cell, and such cells will also be
considered
recombinant. As is understood by one of skill in the art, a nucleic acid
encoding an antigenic
polypeptide may be introduced into a cell using any known technique, for
example,
microinjection, electroporation, viral transfection, lipofectamine
transfection, calcium
phosphate precipitation and the like. In certain non-limiting examples, a
bacterial cell may
be modified by introduction of a nucleic acid molecule encoding an antigenic
polypeptide,
and then the modified cells may be administered to a subject. In certain other
examples, a
nucleic acid molecule encoding an antigenic polypeptide may be incorporated
into an
appropriate construct or vehicle, for example a viral construct, and
administered to a subject
such that the nucleic acid molecule encoding the antigenic polypeptide is
introduced and
expressed in at least a portion of the cells of the subject.
A nucleic acid encoding an antigenic polypeptide may be operably linked to
control
sequences, typically in the context of a suitable vector. A useful control
sequence may be
any nucleic acid element that is necessary or advantageous for expression of
the coding
sequence of the nucleic acid sequence. Each control sequence may be native or
foreign to
the nucleic acid sequence encoding the antigenic polypeptide. Such control
sequences
include, but are not limited to, a leader, a polyadenylation sequence, a
propeptide sequence,
a promoter, a signal sequence, or a transcription terminator. Alternatives for
incorporating
CA 02593558 2007-06-22
control sequences are readily available to the skilled person. For example, a
nucleic acid
encoding an antigenic polypeptide may be under the control of an endogenous
upstream
promoter, or it may be put under control of a heterologous upstream promoter.
Examples of
suitable promoters for directing the transcription of the modified nucleotide
sequence, such
5 as PS4 nucleic acids, in a bacterial host include the promoter of the lac
operon of E. coli, the
Streptomyces coelicolor agarase gene dagA promoters, the promoters of the
Bacillus
licheniformis alpha-amylase gene (amyL), the promoters of the Bacillus
stearothermophilus
maltogenic amylase gene (amyM), the promoters of the Bacillus
amyloliquefaciens alpha-
amylase gene (amyQ), the promoters of the Bacillus subtilis xylA and xylB
genes, the
10 promoter of the Bacillus subtilis aprE gene and a promoter derived from
a Lactococcus sp.--
derived promoter including the P170 promoter. When the gene encoding the PS4
variant
polypeptide is expressed in a bacterial species such as E. coli, a suitable
promoter can be
selected, for example, from a bacteriophage promoter including a T7 promoter
and a phage
lambda promoter.
For transcription in a fungal species, examples of useful promoters are those
derived
from the genes encoding the, Aspergillus oryzae TAKA amylase, Rhizomucor
miehei aspartic
proteinase, Aspergillus niger neutral .alpha.-amylase, A. niger acid stable
.alpha.-amylase,
A. niger glucoamylase, Rhizomucor miehei lipase, Aspergillus oryzae alkaline
protease,
Aspergillus oryzae triose phosphate isomerase or Aspergillus nidulans
acetamidase.
Examples of suitable promoters for the expression in a yeast species include
but are
not limited to the Gal 1 and Gal 10 promoters of Saccharomyces cerevisiae and
the Pichia
pastoris A0X1 or A0X2 promoters.
Still further suitable promoters are available to the skilled person, for
example,
cytomegalovirus, Rous Sarcoma Virus, synthetic pox viral promoter, pox
synthetic late
promoter 1, pox synthetic late promoter 2 early promoter 2, pox 01L promoter,
pox 14L
promoter, pox 13L promoter; pox 12L promoter, pox IlL promoter, pox DIOR
promoter, PRV
gX, HSV-1 alpha 4, chicken beta-actin promoter, HCMV immediate early, MDV gA,
MDV gB,
MDV gD, ILT gB, BHV-1.1 VP8 and ILT gD and internal ribosomal entry site
promoter.
A suitable vector may be any vector (for example, a plasmid or virus) which
can
incorporate a nucleic acid sequence encoding an antigenic polypeptide and any
desired
control sequences and can bring about the expression of the nucleic acid
sequence . The
choice of the vector will typically depend on the compatibility of the vector
with a host cell
into which the vector is to be introduced. In certain examples, the vector may
exist as an
extrachromosomal entity, with replication being independent of chromosomal
replication, for
example, a plasmid, an extrachromosomal element, a minichromosome, or an
artificial
CA 02593558 2007-06-22
11
chromosome. In other examples, the vector may be one which, when introduced
into the
host cell, is integrated into the genome and replicated together with the
chromosome(s) into
which it has been integrated. Still other examples of vectors and techniques
for manipulating
vectors will be known and apparent to the skilled person.
Recombinant cells may comprise an antigenic polypeptide or a nucleic acid
sequence
encoding an antigenic polypeptide, either singly or in combination, with other
desired
polypeptide or nucleic acid molecules, respectively, to optimize vaccination
efficacy.
Furthermore, a nucleic acid sequence may be mutated or altered prior to
introduction into
the cells as desired, for example for codon optimization for expression in a
particular cell
.. type. In addition, a nucleic acid sequence may be altered to encoded a
fusion of an
antigenic polypeptide with one or more other polypeptide as desired in an
application, for
example fusion with a targeting polypeptide or a carrier polypeptide.
As is understood by the skilled person, administration of a vaccine can be
done in a
variety of manners. For example, administration may be done intramuscularly,
subcutaneously, intravenously, intranasally, intradermaly, intrabursally, in
ovo, ocularly,
orally, intra-tracheally or intra-bronchially, as well as combinations of such
modalities. The
dose of the vaccine may vary with the size of the intended vaccination
subject. Methods of
administration are known to the skilled person, for example, U.S. Pat. Nos.
5,693,622;
5,589,466; 5,580,859; and 5,566,064. The amounts of polypeptide, nucleic acid
sequence,
or recombinant cell needed for preparation of a vaccine is well understood by
one of skill in
the art.
An antigenic polypeptide, a nucleic acid encoding an antigenic polypeptide, or
a
recombinant cell, may be used in combination with a pharmaceutically
acceptable carrier for
preparation of a vaccine. Pharmaceutically acceptable carriers for vaccines
are well known
.. to those skilled in the art and include but are not limited to proteins,
sugars, and the like.
One example of such a suitable carrier is a physiologically balanced culture
medium
containing one or more stabilizing agents such as hydrolyzed proteins,
lactose, and the like.
Another example of an acceptable carrier is 0.01-0.1M, and preferably 0.05M,
phosphate
buffer or 0.8% saline. Acceptable carriers may be aqueous or non-aqueous
solutions,
.. suspensions, and emulsions. Examples of non-aqueous solvents are propylene
glycol,
polyethylene glycol, vegetable oils such as olive oil, and injectable organic
esters such as
ethyl oleate. Examples of aqueous carriers are water, alcoholic/aqueous
solutions, emulsions
or suspensions, including saline and buffered media. Preservatives and other
additives for
vaccines are also well know to the skilled person, for example antimicrobials,
antioxidants,
chelating agents, inert gases, organic acids and the like.
CA 02593558 2007-06-22
12
Acceptable adjuvants, for example as described in US Patent No. 6,908,620, may
be
used to enhance the immune response to an antigenic polypeptide. Acceptable
adjuvants
include, without limitation: polymers of acrylic or methacrylic acid, maleic
anhydride and
alkenyl derivative polymers; immunostimulating sequences (ISS), such as
oligodeoxyribonucleotide sequences having one ore more non-methylated CpG
units
(W098/16247); oil in water emulsion; cation lipids containing a quaternary
ammonium salt;
cytokines; aluminum hydroxide or aluminum phosphate; or any combinations or
mixtures
thereof.
An oil in water emulsion adjuvant can be based on, for example, light liquid
paraffin
oil (European pharmacopoeia type), isoprenoid oil such as squalane, squalene,
oil resulting
from the oligomerization of alkenes, e.g. isobutene or decene, esters of acids
or alcohols
having a straight-chain alkyl group, such as vegetable oils, ethyl oleate,
propylene glycol,
di(caprylate/caprate), glycerol tri(caprylate/caprate) and propylene glycol
dioleate, or esters
of branched, fatty alcohols or acids, especially isostearic acid esters. The
oil is used in
combination with emulsifiers to form an emulsion. The emulsifiers may be
nonionic
surfactants, such as: esters of on the one hand sorbitan, mannide (e.g.
anhydromannitol
oleate), glycerol, polyglycerol or propylene glycol and on the other hand
oleic, isostearic,
ricinoleic or hydroxystearic acids, said esters being optionally ethoxylated,
or
polyoxypropylene-polyoxyethylene copolymer blocks, such as Pluronic, e.g.,
L121.
Examples of adjuvant polymers crosslinked acrylic or nnethacrylic acid,
especially
crosslinked by polyalkenyl ethers of sugars or polyalcohols. U.S. Pat. No.
2,909,462,
provides examples of such acrylic polymers crosslinked by a polyhydroxyl
compound having
at least three hydroxyl groups, preferably no more than eight such groups, the
hydrogen
atoms of at least three hydroxyl groups being replaced by unsaturated,
aliphatic radicals
having at least two carbon atoms.
An example of a cationic lipid adjuvant is DMRIE (N-(2-hydroxyethyl)-N,N-
dimethy1-
2,3-bis(tetradecyloxy)-1-propane ammonium; W096/34109), either alone or
associated with
a neutral lipid, for example, DOPE (dioleoyl-phosphatidyl-ethanol amine), to
form DMRIE-
DOPE.
Examples of cytokine adjuvants are granulocyte colony stimulating factor (G-
CSF),
granulocyte/macrophage colony stimulating factor (GM-CSF), interferon alpha
(IFN alpha),
interferon beta (TEN beta), interferon gamma, (IFN gamma), interleukin-1alpha
(IL-1alpha),
interleukin-1beta (IL-1beta), interleukin-2 (IL-2), interleukin-3 (IL-3),
interleukin-4 (IL-4),
interleukin-5 (IL-5), interleukin-6 (IL-6), interleukin-7 (IL-7), interleukin-
8 (IL-8),
interleukin-9 (IL-9), interleukin-10 (IL-10), interleukin-11 (IL-11),
interleukin-12 (IL-12),
CA 02593558 2007-06-22
13
tumor necrosis factor alpha (TNF alpha), tumor necrosis factor beta (TNF
beta), and
transforming growth factor beta (TGF beta). A cytokine adjuvant can be in form
of a
cytokine polypeptide or a nucleic acid sequence encoding a cytokine
polypeptide.
Still further adjuvants known to the skilled person include, without
limitation,
complete Freund's adjuvant, incomplete Freund's adjuvant, saponin, mineral
gels such as
aluminum hydroxide, surface active substances such as lysolecithin, pluronic
polyols,
polyanions, peptides, keyhole limpet hemocyanins, dinitrophenol, and the like.
Adjuvants may be co-administered or sequentially administered with a vaccine.
The vaccine described herein can be useful for controlling C. perfringens in
an animal,
for example a bird, a cow or a pig. The vaccine may be useful in any bird,
wild,
domesticated or commercially farmed, for example, chicken, turkey, goose,
duck, pheasant,
quail, pigeon and ostrich.
When introducing elements disclosed herein, the articles "a", "an", "the", and
"said"
are intended to mean that there are one or more of the elements unless the
context dictates
otherwise. For example, the term "a compound" and "at least one compound" may
include a
plurality of compounds, including mixtures thereof. The terms "comprising",
"having",
"including" are intended to be open-ended and mean that there may be
additional elements
other than the listed elements.
The above disclosure generally describes preferred embodiments. A more
complete
understanding can be obtained by reference to the following specific Examples.
These
Examples are described solely for purposes of illustration and are not
intended to limit the
scope of the invention. Changes in form and substitution of equivalents are
contemplated as
circumstances may suggest or render expedient. Although specific terms have
been
employed herein, such terms are intended in a descriptive sense and not for
purposes of
limitation.
Examples
Example 1: Clostridium perfringens Antigens Recognized by Broiler Chickens
Immune to
Necrotis Enteritis
Four strains of C. perfringens (CP1, CP4, CP5, and CP6) used in this study are
clinical isolates from field cases of NE. Strains CP1 and CP4 are virulent,
and CP5 and CP6
avirulent, isolates, as assessed by their abilities to cause NE (32).
Clostridium perfringens
cells were grown anaerobically in an empirically formulated medium (tryptic
soy broth
[Difco] 50%, nutrient broth [Difco] 25% and peptone water [Difco] 25%) for 24
h at 37 C,
and the cells and culture supernatant were collected thereafter. The cells
were lysed by
CA 02593558 2015-01-15
,
14
eight freeze-thaw cycles with liquid nitrogen to obtain whole-cell proteins.
The culture
supernatant was dialyzed and concentrated by use of 10-kDa cutoff AmiconTM
filters
(Millipore Inc., Billerica, MA) to obtain secreted proteins. The protein
concentration was
determined using a PlusOneTM 2-D QuantTM kit (Amersham Biosciences, San
Francisco,
CA). The protein contents of concentrated secreted and whole-cell protein
samples were
3 to 4 mg/ml. For sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-
PAGE) analysis under reducing conditions, 100 pg of protein sample was
applied.
The secreted and whole-cell proteins were separated by one-dimensional
SDS-PAGE in a 12.5 /0 acrylamide gel under reducing and nonreducing conditions
(16).
The gels were visualized by Coomassie R-250 staining. The proteins from the
gel were
transferred to a nitrocellulose membrane of 0.45-pm pore size (Bio-Rad
Laboratories) by
use of a Hoefer tank buffer system (Amersham Biosciences) followed by reaction
with
primary antibodies (serum or intestinal washing) at 1:1,000 and 1:500
dilutions,
respectively. Serum (source of immunoglobulin Y [IgY]) used in this study was
pooled
from broiler chickens immune to virulent C. perfringens challenge in infection-
immunization experiments (32). The pooled small intestinal washings made from
these
birds by use of phosphate-buffered saline were dialyzed, concentrated, and
used as the
source of primary antibody (IgA and IgY) in Western blotting and
neutralization
experiments. Anti-chicken IgY (heavy plus light chains) and anti-chicken IgA
were used
as secondary antibodies at 1:2,000 and 1:1,000 dilutions, respectively.
Specific
immunoreactive protein bands were visualized using an alkaline phosphatise-
conjugated
substrate kit (Bio-Rad Laboratories).
Several protein bands from strain CP4 showed reactivity to immune serum, but
similar reactivity was not observed for secreted proteins from CP5. This lack
of reactivity
was also observed when secreted proteins from avirulent strain CP6 were
reacted with
immune serum. Secreted proteins from another virulent strain, CP1, showed
reactivity
similar to that seen for CP4. The secreted protein bands of CP4 that showed
reactivity to
immune serum were consistently reactive in multiple gels run at different
times.
Although there was little reactivity of CP4- and CP5-secreted proteins to
intestinal IgA,
the reactivity of these secreted proteins to intestinal IgY was similar to
that of Western
blots done with immune serum. Therefore, it seems that both intestinal and
serum IgY
antibodies are important in immunity to this infection. No differences in the
whole-cell
protein reactivities to serum or intestinal washings between virulent and
avirulent strains
were observed, suggesting that the trait of immune protection against NE lies
in the
secreted components of virulent C. perfringens.
CA 02593558 2015-01-15
Six immunoreactive secreted proteins unique to virulent strains, of which
five were highly antigenic, were identified in the parallel-run Coomassie-
stained gels by
use of the coordinates of molecular-weight-marker hands and the distance of
migration.
The gels from the centers of these bands were excised, in-gel digested, and
identified by
5 mass spectrometric techniques, namely, matrix-assisted laser desorption
ionization mass
spectrometry (MALDI-MS) and electrospray ionization mass spectrometry (ESI-
MS/MS).
The peptide masses and sequence data from MS analysis were searched against
the
National Center for Biotechnology Information (NCBI) protein database by MS-
Fit and
Matrix-Science Mascot search to identify the protein that had the highest
homology
10 percentage match. Of the six antigenic secreted proteins unique to the
two virulent,
innmunoprotective strains identified by MS (Figures 1 to 6), three
(perfringolysin 0,
fructose 1,6 biphosphate aldolase [FBA], and elongation factor G [EF-G]) are
regulated
by the VirR-VirS virulence regulon of C. perfringens (3). In addition to
virulence genes,
this regulon controls genes involved in energy metabolism, such as those
encoding FBA
15 and NAD-dependent 13-hydroxybutyryl coenzyme A dehydrogenase, as well as
others that
may be indirectly involved in bacterial virulence (3,13, 28). It therefore
seems possible
that the marked difference in the innmunoreactivities of the secreted proteins
between
the two virulent and the two avirulent strains is the result of a mutation in
this regulatory
region in the avirulent strains and that the quantity of these proteins
produced is too low
to be detected in SDS-PAGE and Western blotting experiments. The avirulent
strains do
produce alpha-toxin; however, the amount produced was not quantified.
Example 2: Antigenic Epitopes of alpha-Toxin
Purified C. perfringens alpha-toxin (Sigma Laboratories) was separated by SDS
PAGE and Western blotting performed using chicken immune serum and intestinal
antibodies, as well as alpha-toxin antiserum (I G. Songer, University of
Arizona) raised
in goats. As expected, alpha-toxin antiserum detected purified alpha toxin (43
kDa).
Interestingly, in the Western immunoblot no antibodies to alpha-toxin were
detected
either in serum or intestinal washings of immune birds. However, further study
showed
antibodies to alpha toxin were detected in serum at titres of 5000 when native
(and not
denatured) alpha toxin was used in a lecithinase inhibition assay. In
addition, there was
immunoreactivity when a Western immunoblot was run against alpha toxin
electrophoresed in a non-denaturing gel. This suggests that neutralizing
antibodies to
alpha toxin are present in immune birds but these may be to conformational
rather than
linear protein epitopes.
CA 02593558 2007-06-22
16
Achieving conformational but non-toxic alpha-toxin epitopes in a vaccine may
prove challenging. A structure-function analysis of alpha-toxin suggests that
the toxicity is
associated with the N-terminus whereas the immunogenicity is associated with
the C-
terminal domain (1, 7, 33). However, the hemolytic activity was found to
result from an
interaction of both the domains (1). Although many studies have emphasized the
importance
of non-toxic C-terminal domain in protection against experimental gas gangrene
(5, 30, 34),
some have shown the neutralizing epitopes to be on the N-terminus (18). It
seems likely
that the positioning of protective, neutralizing, conformational epitopes of
alpha-toxin is
subtle and that these epitopes are shared upon folding between both domains,
which play a
key role in toxicity and also in protective innmunogenicity, thus making them
hard to access
by antibodies.
ExaMple 3: Cloning, Expression and Purification of Secreted Antigenic
Polypeptides from
virulent C. Derfrincens:
The chromosomal DNA of the virulent, protective, C. perfringens strain CP4 was
used
as the source of DNA for cloning of secreted antigens: alpha-toxin,
glyceraldehyde 3-
phosphate dehydrogenase (GPD), pyruvate: ferredoxin oxidoreductase (PFOR),
fructose 1,6-
biphosphate aldolase (FBA) and a Hypothetical Protein (HP). PCR was performed
using the
proofreading DNA polymerase (Qiagen, Mississauga, ON) and primers designed to
specifically amplify DNA fragments in the genes. The primers used to amplify
the genes are
given in Table 1.
CA 02593558 2015-01-15
17
Table 1. List of primers used to amplify genes encoding proteins used in
immunization
experiments
Gene Sequences 5'-3' Amplicon
size (bp)
Alpha-toxin
Forward-ccgctcgagttgggatggaaaaattgat 1100
Reverse-ccggaattctttatattataagttgaattt
Hypothetical protein Forward- 5400
ccgctcgaggaataagagaaaaatagcag
Reverse-
ccgggtaccacgttaaataaatagaacat
Glyceraldehyde 3- Forward- 1000
phosphate ccgctcgagggtaaaagtagctattaacgg
dehydrogenase
Reverse-
ccgggtaccttagaaactaagcattttaaa
Fructose 1,6- Forward- 900
biphosphate aldolase ccgcggatccatggcattagttaacgcaaa
Reverse-
ccgcctcgagagctctgtttactgaaccga
Truncated pyruvate: Forward- 1600
ferredoxin ccgcctcgagcacttcattagaaccagttg
oxidoreductase
Reverse-
ccgcggatcctagctaagtagtcttggtct
After purification (PCR Purification Kit, QiagenTM, Mississauga, ON), the PCR
products were cloned into plasmid expression vectors so as to generate
proteins fused
with histidine residues (6-His) either at the N-terminus or at the C-terminus
of the
protein sequence. Two plasmid vectors, pBAD (for cloning alpha-toxin, GPD and
HP) and
pET-28 (for cloning FBA and tPFOR), were used in this study.
The resulting plasmids were introduced into E. coil LMG 194 or BL21 Star (DE3)
(Invitrogen, Carlsbad, CA), following the manufacturer's instructions. For
protein
expression, overnight cultures were used to inoculate a fresh Luria broth (LB)
medium
supplemented with ampicillin or kanamycin (100 pginn1). Bacteria were grown at
37 C
under aerobic conditions and L-arabinose (St. Louis, MO) (0.2% final) or
isopropyl-beta-
D-thiogalactopyranoside (IPTG) (Qbiogene, Vista, CA) was added (1 mM) to the
bacterial
culture in exponential growth phase (0D500-0.5). After a further incubation
for 4 h, cells
were harvested by centrifugation.
Purification of recombinant proteins was performed by affinity chromatography
on
nickel-nitrilotriacetic acid (Ni-NTA) agarose following the manufacturer's
instructions
CA 02593558 2007-06-22
18
(Qiagen). Briefly, when the proteins were expressed as soluble proteins,
bacterial pellets
were resuspended in a buffer (50mM NaH2PO4, 300 mM NaCI) containing lyzozyme
(1
mg/ml) and incubated for 60 min in ice. The bacterial cells were lysed using a
French
pressure cell (3-4 cycles of 1000 psi). The supernatant was collected by
centrifugation and
added to Ni-NTA agarose. The washing and elution steps were performed using
buffers
containing increasing concentration of imidazole. Finally, imidazole was
removed from the
eluted material by dialysis against phosphate buffered saline, pH 7.2, (PBS)
and the
recombinant proteins were concentrated using Amicon filter -10kD (Millipore,
Billerica, MA)
and the protein concentration was determined using PlusOne' 2-D Quant kit
(Amersham
Biosciences, San Francisco, CA).
Purified recombinant proteins were separated by one-dimensional sodium dodecyl
sulfate polyacrylamide gel electrophoresis (SDS-PAGE) in a 12.5% acrylamide
gel under
denaturing conditions as described by Laemmli (16). Proteins were transferred
to
nitrocellulose membrane of 0.45 pm pore size using a mini-gel transfer
assembly (Bio-Rad
Laboratories, Hercules, CA). After completion of transfer, non-specific
binding sites on the
membranes were blocked with blocking buffer containing 1% casein (Bio-Rad
Laboratories)
and incubated with primary antibodies (immune serum collected from infection-
immunized
birds in a previous study (32) at 1:1000 dilution. Anti-chicken IgY (H+L)
(Cedarlane
Laboratories, Hornby, ON) was used as secondary antibody at 1:2000 dilution.
The blots
were developed and specific irnmunoreactive protein bands were visualized
using an alkaline
phosphatase-conjugated substrate kit (Bio-Rad Laboratories) (see Figure 7).
All five genes selected for the immunization study were successfully cloned,
expressed and purified to homogeneity.
Cloning of the full length pfor gene was not successful, possibly because of
homologous recombination. However, a portion of the gene that encoded a
truncated protein
(tPFOR) of 67 kDa size (see underlined portion of Figure 2) that contained the
Iron- Sulphur
(Fe-S) active sites of this enzyme was successfully cloned and purified in
large quantities.
Although alpha-toxin was successfully cloned, expressed and purified, the
quantity
obtained was insufficient for immunization, possibly because of toxicity for
the E. coil host.
Hence, commercially available purified alpha-toxin (Sigma Laboratories, St.
Louis, MO) was
used for immunization experiments.
Hypothetical Protein (190 kDa) was found to be cleaved into two bands of 90-
100
kDa size upon expression. Attempts to express the entire protein by changing
E. coil
expression hosts were unsuccessful. Since these two bands reacted strongly to
anti-histidine
antibodies as well as to immune serum collected from infection-immunized birds
(Figure 7)
CA 02593558 2007-06-22
19
from a previous study (32) these two bands were further purified in large
quantities and
used in immunization experiments.
Example 4: Immunization Experiments in Chickens
Experiments with chickens and conditions for their use were approved by the
University of Guelph Animal Care Committee in accordance to the Canadian
Council on
Animal Care's Guidelines. Commercial 1-day-old male White Plymouth Rock
broiler chickens
(Bonnie's Chick Hatchery, Elmira, Ont., Canada) were fed an antibiotic-free
chicken starter
containing 20% protein for 13 days followed by a formulated wheat-based grower
feed,
containing 28% protein (Arkell Research Station, University of Guelph). Birds
were
immunized intramuscularly in the pectoral muscle in a volume of 0.2 ml per
bird with
purified recombinant proteins at different concentrations and frequencies.
In all experiments, the number of birds in each group was between 10 and 20
and all
birds were identified individually. Blood was collected from the wing vein
from all the groups
at three times: pre-immunization, mid-experiment and pre-challenge. Intestinal
washings
were collected using PBS at autopsy.
For the experimental infection of birds, virulent C. perfringens CP4 was grown
in
cooked meat medium (Difco) (CMM) for 24 h at 37 C. Fluid thioglycolate medium
(FTG,
Difco) was then inoculated with a 3% (v/v) inoculum from the C. perfringens
infected CMM
and incubated at 37 C for 24 h. The growth at 24 h was log,o 8.24 0.09 C.
perfringens
CFU/ml. The inoculated FTG was then mixed with feed at a ratio of 2:1 (v/w).
Inoculated
feed was prepared freshly twice per day and fed to chickens for 3-5 days as
described.
Autopsy
Chickens were euthanized with carbon dioxide gas and their small intestines
(duodenum to ileum) examined for grossly visible lesions. Any chickens that
had reached a
pre-determined severity of clinical illness prior to autopsy were euthanized
and later
autopsied. Intestinal lesions in the small intestine (duodenum to ileum) were
scored as
follows: 0 = no gross lesions; 1 = thin or friable wall; 2 = focal necrosis or
ulceration; 3 =
large patches of necrosis; 4 = severe extensive necrosis; 5 = chickens that
died during
experiment, having 4+ lesions (25). Blind scoring was employed to avoid scorer
bias. A
pathological change of mild generalized and superficial intestinal necrosis,
not previously
included in this scoring system (25), was assigned a score of 1+.
CA 02593558 2015-01-15
A visual summary of mean lesion scores of birds from all immunized groups that
received different doses of antigens and challenge across different
experiments, together
with the concurrent unimmunized controls, is shown in Figure 8.
5 Measurement of the antibody titers in chicken sera and intestinal
washings
The specific antibody titers were determined by the end point dilution method
using
TM
an enzyme-linked immunosorbent assay (ELISA). Microtiter plates (Immulon-2,
Chantilly,
VA) were coated with recombinant proteins (5pg/m1 in 0.1 M carbonate buffer pH
9.6) for 60
min at 37 C followed by an overnight incubation at 4 C. After blocking coated
plates for 60
10 min at 37 C with PBS containing 3% of bovine serum albumin (BSA Sigma),
immune sera
were serially diluted in PBS containing BSA 1% and incubated for 2 h with
recombinant
protein-coated plates at room temperature. After washing with PBS containing
Tween 20
0.1% (PBST), alkaline phosphatase (AP)- coupled anti-chicken IgY (H+L)
(1:5,000 in PBS-
Tween 20 0.1%-BSA 1%) was added to the microplates and incubated for 60 min at
room
15 temperature. After extensive washing with PBST, color reaction was
developed using alkaline
phosphate substrate kit (Bio-Rad Laboratories) following the manufacturer's
instructions. The
reaction was stopped by adding 0.4 M NaOH. The absorbance was measured at 405
nm in an
ELISA spectrophotometer. The specific antibody titer of immune serum was
expressed as the
reciprocal of the serum dilution (log2 OD) that gave an A405 value above the
cut-off, defined
20 as twice the absorbance value of the un-immunized and mock control
wells.
Intestinal antibody response was also measured by ELISA following the
procedure
described above for serum. Intestinal washings from at least 10 chickens per
group were
pooled and the total protein content was measured using PlusOneTM 2-D Quant
kit
(Amersham Biosciences) and used as source of primary antibody after keeping
the protein
content of the initial dilution (1:10) constant across all the groups.
Alkaline phosphatase
conjugated anti-chicken IgY and IgA were used as secondary antibodies at
dilutions of
1:4000 and 1:2000 respectively. The end-point titres were determined as
described above
for serum ELISA.
Although purification of histidine tagged recombinant proteins through Ni-NTA
agarose column should yield eluted protein free of LPS, using mock-immunized
(as well as
non-immunized control bird) serum to determine the cut-off value further
strengthened the
interpretation of results. All the proteins used to immunize birds in the
immunization
experiments described produced significant antigen-specific serum antibody
titres (Figure 9)
compared to their pre-immunization titres. Alpha-toxoid immunized but non-
protected birds
CA 02593558 2007-06-22
21
as shown in Experiment-1 below had higher titres than toxoid-active toxin
immunized birds
that were highly protected in Experiment-3 below.
Intestinal antibody responses to all the immunized proteins showed higher IgY
than
IgA titers (Figure 10). However, unlike serum, such a difference was not
observed between
alpha-toxoid (Experiment-1) and toxoid-active toxin boosted (Experiment-3)
birds.
Statistical analysis
Statistical analysis was performed to determine whether there was a
significant
difference between the number of birds with lesions from immunized groups and
birds from
the unimmunized, vehicle-only controls. A two-tailed Fisher's exact test
determined whether
the two groups differed in the proportion with which they fell into the two
classifications of
either lesions or no lesions, under the null hypothesis that the proportions
were the same.
Data was analyzed in a 2 x 2 contingency table with the unimmunized control
group in one
column and immunized groups in the other column. Lesion scores were ranked
from 0 to 5+,
however, a "protective" response was given to birds with <1+ lesions. The null
hypothesis
was rejected at a = 0.05. For serum ELISA, a one-way ANOVA (Mini-tab 14
software) was
used to determine significant differences the antibody titres between pre-
immunized and
immunized time points across all the groups. Statistical analysis could not be
made on
intestinal ELISA, since the washings collected were pooled per group.
A summary of the immunization experiments is shown in Table 2.
Table 2. Summary of experimental design
Experiment Immunization Dosage of Frequency of Oral
Groups vaccine/ administration challenge
Bird
1 VC, Supa, Alpha- 20 pg Three times; day 3 days
toxoid, GPD, HP, 7, 14 and 21 (mild)
FBA and tPFOR
2 VC, MCb, GPD, HP, 40 pg Two times; day 7 5 days
FBA and tPFOR and 14 (moderate)
3 VC, Alpha-toxoid/ 20 pg Three times; day 5 days
toxin`, GPD, HP, 7, 14 and 21 (severe)
tPFOR, combination
of GPD and HP
4A VC and FBA 20 pg Three times; day 3 days
7, 14 and 21 (mild-
moderate)
CA 02593558 2007-06-22
22
48
VC, Alpha-toxin d and 20 pg Three times; day 5 days
FBA 7, 14 and 21 (severe)
VC- Vehicle-only controls, GPD- glyceraldehyde 3-phosphate dehydrogenase,
tPFOR-
truncated pyruvate: ferredoxin oxidoreductase, FBA- fructose 1,6-biphosphate
aldolase, HP-
Hypothetical protein, Sup- crude culture supernatent of virulent C.
perfringens and MC-
mock-immunized controls
a Birds received 60 pg/inj of culture supernatant that was processed and
concentrated
following a protocol described earlier (15).
b Birds were mock immunized with an unrelated protein that was cloned,
expressed and
purified from E. coli in the same manner as C. perfringens related proteins.
C Birds received alpha-toxoid in the first two injections followed by active
alpha-toxin in the
third.
d Birds in this group received three injections of alpha-toxin where in the
first and the third
injections were with 20 pg and the second was reduced to 10 pg.
In Experiment-1, birds were immunized with alpha-toxoid, HP, GPD, FBA and
tPFOR.
Each bird received three injections of 20 pg of recombinant protein and 50 pg
of Quil-A
adjuvant (Superfos Biosector, Vedbaek, Denmark) on days 7, 14 and 21 of age. A
week
later, following a 20 h fast, birds were orally challenged 2-3 times a day
with virulent C.
perfringens. This challenge was considered 'mild' since the duration of
challenge was 3 days
and lesions produced in non-immunized birds were relatively mild. Post mortem
examination
was performed on all birds on day 31, or on birds that died earlier, and the
intestinal lesions
were scored. A group of birds also received crude culture supernatant of CP4
obtained,
processed and concentrated following a protocol described earlier (15) at a
dose of 60 pg/
bird/ injection. The unimmunized controls received only Quil-A adjuvant
followed by a
challenge similar to immunized groups. Purified alpha-toxin was toxoided
following the
protocol described (11) and used for immunization.
HP, GPD, tPFOR and FBA showed significant protection against a mild infection
challenge (Table 3). Immunization with crude culture supernatant that
contained all secreted
proteins including those purified also showed significant protection. Alpha-
toxoid did not
protect birds against challenge. Hypothetical Protein showed the greatest
protection.
CA 02593558 2015-01-15
23
Table 3. Intestinal lesion scores of birds immunized with three injections
intramuscularly,
then infected with a mild challenge by C. perfringens
No. of Lesion scores
Protein Mean
chickens
1
0 1 2 3 4 5
+ + + + +
Vehicle-only controls 10 1 3 4 1 1 0 1.55
Culture
8 1 1 0 0 , 0 0.4
supernatant*
Alpha-toxoid 12 3 4 3 1 1 0 1.41
HP* 12 10 2 0 0 0I 0 0.16
GPD* 10 7 2 1 0 0 0 0.4
tPFOR* 10 4 5 1 1 0 0 0 ____________ 01 I
FBA* 10 4 6 010 010
I 0.6
* Immunized groups that had significantly fewer chickens with lesions compared
5 to unimmunized vehicle-only controls; Fisher's exact test, p< 0.05.
In Experiment-2, birds were immunized with HP, GPD and tPFOR. Each bird
received two injections of 40 pg of recombinant protein and 50 pg of QuilATM
adjuvant
on days 14 and 21 of age. A week later, following 20 h fasting birds were
orally
10 challenged with virulent C. perfringens twice a day for five days, from
days 28-32 of age.
Since feeding was restricted to twice daily and birds ran out of feed on some
occasions,
and in light of the moderate lesion scores in unimmunized birds, this
challenge was
considered moderate'. Post mortem examination was performed on day 33 and
intestinal lesions were scored. The unimmunized controls received only QuilATM
adjuvant
followed by a challenge similar to immunized groups. A group of birds were
mock
immunized with an unrelated protein that was cloned, expressed and purified
from E. coli
in the same manner as C. perfringens related proteins.
HP alone showed significant protection against a moderate challenge, whereas
GPD, tPFOR and FBA did not (Table 4). Immunization (mock) with an unrelated
purified
recombinant fusion protein showed a mean lesion score similar to that of
unimmunized
controls. The increased mean lesion score of controls compared to Experiment-1
indicated the effect of increased length of challenge.
CA 02593558 2007-06-22
24
Table 4. Intestinal lesion scores of birds immunized with two injections
intramuscularly, then
infected with a moderate challenge by C. perfringens
1 ___________________________________________________________________
No. of Lesion scores
Protein Mean
chickens
3. 2 3 1 4 5
0
+ + + + +
Vehicle-only controls , 18 1 4 9 2 1 1 2.05
Mock controls 10 0 1 6 3 0 0 2.20
HP* 17 10 3 4 0 0 0 0.64
GPD 17 3 8 6 0 0 0 1.17
tPFOR 18 7 4 3 3 0 1 1.33
FBA 18 2 7 6 I 2 0 1 1.66
,
*Immunized group had significantly fewer chickens with lesions compared to
unimmunized
vehicle-only controls; Fisher's exact test, p< 0.05.
In Experiment-3, the immunization schedule was same as in the Experiment-1 but
birds were challenged orally for 5 days. This challenge was considered
'severe' since the
birds were fed 2 to 3 times a day to ensure that they always had infected feed
available.
Birds in this experiment were immunized with alpha-toxoid/toxin, HP, GPD, and
tPFOR. A
group of birds received alpha-toxoid in the first two injections followed by
active, non-
toxoided, alpha-toxin in the third. This modification was made since
injections with alpha-
toxoid alone in the Experiment-1 did not show protection. The unimmunized
controls
received only Quil-A adjuvant followed by a challenge similar to immunized
groups. A group
of birds were immunized with a combination GPD and HP (20 pg each) along with
Quil-A and
challenged similarly as other groups.
This infection challenge was characterized as severe, since lesions scores in
non-
immunized chickens were greater than in Experiments- 1 and 2, an enhancement
of severity
attributed to constant challenge with infected feed. Alpha-toxin immunized
birds, which
received two initial injections of alpha-toxoid but a third injection with
native, non-toxoided,
alpha-toxin showed the greatest protection against heavy challenge (Table 5).
Birds
immunized with either HP or tPFOR also showed significant protection against
severe
=
CA 02593558 2007-06-22
challenge. Although birds immunized with GPD, FBA and the combination of GPD
and HP had
mean lesion scores lower than non-immunized controls, no statistical
significance was
observed.
Table 5. Intestinal lesion scores of birds immunized with three injections
intramuscularly,
5 then infected with a severe challenge by C. perfringens.
No. of Lesion scores
Protein Mean
chickens
0 1 2 3 4 5
++ + + +
Vehicle-only controls 22 0 5 5 6 4 2 2.68
Alpha toxoid/toxin*
a 19 10 8 1 0 0 0 0.53
HP* 20 8 6 4 2 0 0 1.0
GPD 18 4 4 6 1 1 1 , 1.64
tPFOR* 19 9 2 6 2 0 0 1.05
GPD + HP 19 5 5 7 1 I 1 0 I 1.36
*Immunized groups that have significantly fewer chickens with lesions compared
to
unimmunized vehicle-only controls; Fisher's exact test, p< 0.05.
a Birds in this group received alpha-toxoid in the first two injection and
toxin in the third.
In Experiment-4, the immunization schedule was same as in the Experiment-1 and
3,
but birds were challenged orally for either 3 ("mild-moderate") or 5 days
("severe"). One
group of birds was immunized with FBA and challenged for 3 days and autopsied
on the day
6 together with the 3-day challenged unimmunized controls. Two other groups
were
immunized with either FBA or active (non-toxoided) alpha-toxin, followed by a
severe
challenge for 5 days. In the active alpha-toxin group, birds received three
injections of active
alpha-toxin where in the first and the third injections were with 20 pg and
the second was
reduced to 10 pg since 20 pg dose in the first injection was toxic, causing
the death of some
birds.
The mean lesion scores in birds of either immunized or unimmunized groups that
were challenged for 3 days and autopsied on day 6 (Table 6) were higher than
observed in
birds of Experiment-1 that were challenged for 3 days and autopsied on day 4
(Table 2). The
CA 02593558 2007-06-22
26
FBA immunized birds showed significant protection compared to unimmunized
controls. In
the 5-day-challenged (heavy dose) groups, neither FBA nor the alpha-toxin
immunized
birds were protected (Table 6). Mean lesion scores of unimmunized controls
were
comparable to the scores of unimmunized controls in Experiment-3 that also
received a
severe challenge.
Table 6. Intestinal lesion scores of birds immunized with three injections
intramuscularly,
then infected with mild-moderate or severe challenge by C. perfringens
Protein
=No. of Lesion scores
Mean
chickens
I 1 2 3 4 5
0
+ + + + +
A ¨
Groups infected with mild-moderate challenge
Vehicle-only controls 10 0 5 2 0 1 2 2.3
FBA* 13 4 6 1 1 0 1 1.23
Groups infected with severe challenge
Vehicle-only controls 11 0 3 2 3 1 2 2.72
Alpha-toxina 10 0 2 4 4 0 0 2.2
FBA 14 1 4 9 0 0 0 1.57
*Immunized group that had significantly fewer chickens with lesions compared
to
unimmunized vehicle-only controls; Fisher's exact test, p< 0.05.
a- Birds in this group received three injections of alpha-toxin where in the
first and the third
injections were with 20 pg and the second was reduced to 10 pg.
Experiments 1 to 4 show that a degree of immunity to C. perfringens infection
in
broiler chickens can be produced by immunization with several different
secreted C.
perfringens proteins. All the proteins used in immunization, including alpha-
toxin, showed
significant protection depending on the severity of challenge. Of the five
secreted proteins
used for immunization experiments, three proteins namely alpha-toxin, HP and
tPFOR
significantly protected chickens against a heavy challenge, whereas the other
two proteins,
CA 02593558 2007-06-22
27
GPD and FBA, significantly protected against mild challenge. Nevertheless a
degree of
protection was apparent with these latter proteins even against severe
challenge.
Priming with alpha-toxoid and boosting with active toxin showed significant
protection, whereas immunization with three injections of either alpha-toxoid
or of active
.. toxin did not protect (Tables 3, 6). The failure of active toxin to protect
birds against a heavy
challenge may have resulted from the toxin's activity on immune system cells.
The failure of
alpha-toxoid to protect in the Experiment-1 may be the result of a degradation
effect of
toxoiding on the protein that was observed on SDS-PAGE gel (data not shown).
However, it
is clear from Figure 9 that toxoiding was adequate to induce antibodies
sufficient for the
.. birds to tolerate the active toxin given as a booster in the Experiment-3.
The findings
suggest that antibodies to conformational (rather than linear) epitopes of
alpha-toxin are
needed in protection against NE.
Perfringolysin 0 (also known as theta toxin), a 52 kDa protein is a potent
hemolytic
cytolysin that mediates necrosis in the pathogenesis of clostridial gas
gangrene (29) and is
an important protective immunogen in mouse and guinea-pig gas gangrene models
(8). The
purified alpha-toxin (Sigma Laboratories) used to immunize birds had traces of
perfringolysin 0, identified using mass spectrometry (data not shown).
However, the relative
amounts in the otherwise apparently pure alpha-toxin preparation (assessed by
SDS-PAGE)
were not quantified. It is possible that the protection observed in alpha-
toxoid/toxin
immunized birds (Table 5), can be partly attributed to perfringolysin 0 and
that a synergistic
effect on the induction of neutralizing antibodies against both the toxins may
have
contributed to better protection. A synergistic effect of alpha-toxin and
perfringolysin 0 in
the pathogenesis of C. perfringens- mediated gas gangrene has been observed
(2). Proteins
identified as important in immunity to NE, may also be involved in the
pathogenesis of, and
immunity to, gas gangrene.
Among proteins other than alpha-toxin used for immunization, HP showed
significant
protection against all severities of challenge (Tables 3 - 5). The truncated
PFOR protein also
produced significant protection against a heavy challenge in Experiment-3. The
tPFOR did
not produce significant protection when administered only twice (Experiment-
2), possibly
because this dosage produced a lower antibody response than observed in birds
immunized
three times, albeit with a lower dose (Figure 9). Because of the inconsistency
in the
protective effect observed with FBA in Experiments 1 and 2, a group of birds
in Experiment-4
was immunized with FBA and challenged for 3 or 5 days, then necropsied on day
6 together
with the concurrent unimmunized controls. The study confirmed that
immunization with FBA
provided some protection against mild-moderate challenge. The mean lesion
scores in 3-
CA 02593558 2007-06-22
28
day- challenged birds of either immunized or unimmunized groups (Experiment-
4A) were
higher than the birds of Experiment-1 that were challenged for 3 days and
necropsied on
day 4, suggesting that delay to necropsy of birds following 3 day challenge
was associated
with higher lesion scores.
Experiments 1 to 4 shows that immunization with secreted C. perfingens
proteins
provides some immunity to birds against C. perfringens infection. Both alpha-
toxin and
perfringolysin 0 are regulated in C. perfringens by the VirR-VirS two-
component regulon. (4,
26) a regulon that also controls genes involved in energy metabolism such as
FBA and NAD-
dependent 8-hydroxybutyryl co-enzyme-A dehydrogenase, as well as others that
may be
indirectly involved in bacterial virulence (3, 13, 28). There is growing
evidence that certain
enzymes, such as GPD and FBA, that are conventionally regarded as metabolic or
"house-
keeping" enzymes, may have a 'dual role' in both the pathogenesis of, and
immunity to,
other infections (17, 23). For example, recent studies of the virulence of
Group A
streptococci (GAS) indicate that GPD assists in attachment of GAS to host cell
plasmin and
fibronectin receptors (6, 20, 35) and also plays an important role in cellular
communication
by activating host protein phosphorylation mechanisms (24). Furthermore, GPD
has been
suggested to be a putative virulence factor in staphylococcal and neisserial
infections (9,
22). Interestingly, a recent study showed that antibodies to FBA and GPD of
Streptococcus
pneumoniae showed age-dependent increased serum titers in children of
different ages.
Immunization of mice with recombinant GPD and FBA showed significant
protection against
respiratory challenge with virulent S. pneumoniae (17). A role for FBA in
immunity to
Onchocerca volvulus, a filarid nematode causing River Blindness in humans, has
also been
suggested (21). Similarly, PFOR, an enzyme crucial for anaerobic energy
metabolism, has
been suggested to have a role in immunity to invasive amoebiasis (31).
Hypothetical Protein
is a novel protein of C. perfringens of unknown function identified in its
genome (27) that
may have protease activity (zinc- nnetallopeptidase) based on the analysis of
its protein
structure (15).
Serum ELISA responses suggested that protection against NE is antibody
mediated,
since failure of protection in immunized groups in Experiment- 2 was
associated with low
antibody titers (Figure 8). However, alpha-toxoid/ toxin immunized- protected
birds had
lower antibody titers than toxoid- immunized birds that were not protected,
suggesting the
importance of conformational epitope- specific neutralizing antibodies,
despite in low titers,
in mounting a protective immune response. The intestinal antibody response, as
expected,
was biased towards IgY, since systemic immunization results in more antigen-
specific IgY
than IgA (Figure 9). Immunization with HP, that significantly protected birds
against all
CA 02593558 2007-06-22
29
severities of challenge doses, produced higher IgA titers in all three
experiments compared
to other immunized groups. However, this association of IgA titers to
protection was not
evident in either alpha-toxoid/ toxin or tPFOR immunized groups that were also
significantly
protected birds against a heavy challenge in Experiment- 3.
Experiment 1 to 4 have demonstrated the immunizing ability of C. perfringens
secreted proteins in protecting against C. perfringens in broiler chickens.
CA 02593558 2007-06-22
References
1. Alape-Giron, A., M. Flores-Diaz, I. Guillouard, C. E. Naylor, R. W.
Titball, A. 2000.
Identification of residues critical for toxicity in Clostridium perfringens
phospholipase C, the
5 key toxin in gas gangrene. Eur. J. Biochem. 267:5191-5197.
2. Awad, M. M., D. M. Ellemor, R. L. Boyd, J. J. Emmins, and J. I. Rood.
2001.
Synergistic effects of alpha-toxin and perfringolysin 0 in Clostridium
perfringens-imediated
gas gangrene. Infect. Immun. 69:7904-7910.
3. Banu, S., K. Ohtani, H. Yaguchi, T. Swe, S. T. Cole, H. Hayashi, and T.
Shimizu.
10 2000. Identification of novel VirRIVirS-regulated genes in Clostridium
perfringens. Mol.
Microbiol. 35:854-864.
4. Ba-Thein, W., M. Lyristis, K. Ohtani, I. T. Nisbet, H. Hayashi, J. I.
Rood, and T.
Shimizu. 1996. The virR/virS locus regulates the transcription of genes
encoding
extracellular toxin production in Clostridium perfringens. J. Bacteriol.
178:2514-2520.
15 5. Bennett, A. M., T. Lescott, R. J. Phillpotts, M. Mackett, and R.
W. Titball. 1999.
Recombinant vaccinia viruses protect against Clostridium perfringens alpha-
toxin. Viral
Immunol. 12:97-105.
6. Boel, G., H. Jin, and V. Pancholi. 2005. Inhibition of cell surface
export of group A
Streptococcal anchorless surface dehydrogenase affects bacterial adherence and
20 antiphagocytic properties. Infect. Immun. 73:6237-6248.
7. Eaton, J. T., C. E. Naylor, A. M. Howells, D. S. Moss, R. W. Titball,
and A. K. Basak.
2002. Crystal structure of the C. perfringens alpha-toxin with the active site
closed by a
flexible loop region. J. Mol. Biol. 319:275-281.
8. Efirnova, M. G., V. A. Blagoveshchenskii, and B. V. Khatuntseva. 1982.
Protective
25 properties of theta-hemolysin obtained by affinity chromatography. Zh.
Mikrobiol. Epidemiol.
Immunobiol. 12:87-92.
9. Grifantini, R., E. Bartolini, A. Muzzi, M. Draghi, E. Frigimelica, J.
Berger, F. Randazzo,
and G. Grandi. 2002. Gene expression profile in Neisseria meningitidis and
Neisseria
lactamica upon host-cell contact: From basic research to vaccine development.
Ann. N. Y.
30 Acad. Sci. 975:202-216.
10. Heier, B. T., A. Lovland, K. B. Soleim, M. Kaldhusdal, and J. Jarp.
2001. A field study
of naturally occurring specific antibodies against Clostridium perfringens
alpha-toxin in
Norwegian broiler flocks. Avian Dis. 45:724-732.
11. Ito, A. 1968. Alpha-toxoid of Clostridium perfringens. I. purification
and toxoiding of
alpha-toxin of C. perfringens. Jpn. J. Med. Sci. Biol. 21:379-391.
CA 02593558 2007-06-22
31
12. Kaldhusdal, M., and A. Lovland. 2000. Necrotic enteritis: The
economical impact of
Clostridium perfringens is greater than anticipated. World Poultry 16:50-51.
13. Kawsar, H. I., K. Ohtani, K. Okumura, H. Hayashi, and T. Shimizu. 2004.
Organization and transcriptional regulation of myo-inositol operon in
Clostridium perfringens.
FEMS Microbiol. Lett. 235:289-295.
14. Keyburn, A. L., S. A. Sheedy, M. E. Ford, M. M. Williamson, M. M. Awad,
3. I. Rood,
and R. J. Moore. 2006. The alpha-toxin of Clostridium perfringens is not an
essential
virulence factorin necrotic enteritis in chickens. Infect. Immun.74:6496-6500.
15. Kulkarni, R. R., V. R. Parreira, S. Sharif, and J. F. Prescott. 2006.
Clostridium
perfringens antigens recognized by broiler chickens immune to necrotic
enteritis. Clin.
Vaccine Immunol. 13:1358-1362.
16. Laemmli, U. K. 1970. Cleavage of structural proteins during the
assembly of the head
of bacteriophage T4. Nature 227:680-685.
17. Ling, E., G. Feldman, M. Portnoi, R. Dagan, K. Overweg, F. Mulholland,
V. Chalifa-
Caspi, J. Wells, and Y. Mizrachi-Nebenzahl. 2004. Glycolytic enzymes
associated with the cell
surface of Streptococcus pneunnoniae are antigenic in humans and elicit
protective immune
responses in the mouse. Clin. Exp. Immunol. 138:290-298.
18. Logan, A. J., E. D. Williamson, R. W. Titball, D. A. Percival, A. D.
Shuttlewo th, 3. W.
Conlan, and D. C. Kelly. 1991. Epitope mapping of the alpha-toxin of
Clostridium
perfringens. Infect. Immun. 59:4338-4342.
19. Lovland, A., M. Kaldhusdal, K. Redhead, E. Skjerve, and A. Lillehaug.
2004. Maternal
vaccination against subclinical necrotic enteritis in broilers. Avian Pathol.
33:83-92.
20. Madureira, P., M. Baptista, M. Vieira, V. Magalhaes, A. Camelo, L.
Oliveira, A. Ribeiro,
D. Tavares, P. Trieu-Cuot, M. Vilanova, and P. Ferreira. 2007. Streptococcus
agalactiae
GAPDH is a virulence-associated immunomodulatory protein. J. Immunol. 178:1379-
1387.
21. McCarthy, J. S., M. Wieseman, 3. Tropea, D. Kaslow, D. Abraham, S.
glycolytic enzyme fructose-1,6-bisphosphate aldolase as a target for a
protective immune
response in humans. Infect. Immun. 70:851-858.
22. Modun, B., and P. Williams. 1999. The staphylococcal transferrin-
binding protein is a
cell wall glyceraldehyde-3-phosphate dehydrogenase. Infect. Immun. 67:1086-
1092.
23. Pancholi, V., and G. S. Chhatwal. 2003. Housekeeping enzymes as
virulence factors
for pathogens. Int. J. Med. Microbiol. 293:391-401.
24. Pancholi, V., and V. A. Fischetti. 1997. Regulation of the
phosphorylation of human
pharyngeal cell proteins by group A streptococcal surface dehydrogenase:
Signal
transduction between streptococci and pharyngeal cells. J. Exp. Med. 186:1633-
1643.
CA 02593558 2007-06-22
32
25. Prescott, J. F. 1979. The prevention of experimentally induced necrotic
enteritis in
chickens by avoparcin. Avian Dis. 23:1072-1074.
26. Shimizu, T., W. Ba-Thein, M. Tamaki, and H. Hayashi. 1994. The virR
gene, a
member of a class of two-component response regulators, regulates the
production of
perfringolysin 0, collagenase, and hemagglutinin in Clostridium perfringens.
J. Bacteriol.
176:1616-1623.
27. Shimizu, T., K. Ohtani, H. Hirakawa, K. Ohshima, A. Yamashita, T.
Shiba, N.
Ogasawara, M. Hattori, S. Kuhara, and H. Hayashi. 2002. Complete genome
sequence of
Clostridium perfringens, an anaerobic flesh-eater. Proc. Natl. Acad. Sci. U.
S. A. 99:996-
1001.
28. Shimizu, T., K. Shima, K. Yoshino, K. Yonezawa, T. Shimizu, and H.
Hayashi. 2002.
Proteome and transcriptome analysis of the virulence genes regulated by the
VirR/VirS
system in Clostridium perfringens. 3. Bacteriol. 184:2587-2594.
29. Stevens, D. L., and A. E. Bryant. 1993. Role of theta toxin, a
sulfhydryl-activated
cytolysin, in the pathogenesis of clostridial gas gangrene. Clin. Infect. Dis.
16 Suppl 4:S195-
9.
30. Stevens, D. L., R. W. Titball, M. Jepson, C. R. Bayer, S. M. Hayes-
Schroer, and A. E.
Bryant. 2004. Immunization with the C-domain of alpha-toxin prevents lethal
infection,
localizes tissue injury, and promotes host response to challenge with
Clostridium
perfringens. J. Infect. Dis. 190:767-773.
31. Thammapalerd, N., D. Kotimanusvanij, M. Duchene, J. A. Uperoft, R.
Mitchell, A.
Healey, N. Samarawickrema, S. Tharavanij, G. Wiedermann, and P. Uperoft. 1996.
Pyruvate: Ferredoxin oxidoreductase from Entamoeba histolytica recognized by a
monoclonal
antibody. Southeast Asian J. Trop. Med. Public Health 27:63-70.
32. Thompson, D. R., V. R. Parreira, R. R. Kulkarni, and J. F. Prescott.
2006. Live
attenuated vaccine-based control of necrotic enteritis of broiler chickens.
Vet. Microbiol.
113:25-34.
33. Titball, R. W., A. M. Fearn, and E. D. Williamson. 1993. Biochemical
and
immunological properties of the C-terminal domain of the alpha-toxin of
Clostridium
perfringens. FEMS Microbiol. Lett. 110:45-50.
34. Williamson, E. D., and R. W. Titball. 1993. A genetically engineered
vaccine against
the alpha-toxin of Clostridium perfringens protects mice against experimental
gas gangrene.
Vaccine 11:1253-1258.
CA 02593558 2015-01-15
33
35. Winram, S. B., and R. Lottenberg. 1996. The plasmin-binding protein
plr of group
A streptococci is identified as glyceraldehyde-3-phosphate dehydrogenase.
Microbiology
142 (8):2311-2320.
The above-described embodiments are intended to be examples and alterations
and modifications may be effected thereto, by those of skill in the art,
without departing
from the scope of the invention.