Note: Descriptions are shown in the official language in which they were submitted.
CA 02594634 2014-04-22
4
Early Stage Peritonitis Detection Apparatus and Methods
Background of the Invention
The invention relates to apparatus and methods for medical diagnostic testing.
It has application, inter alia, in detecting the onsite of peritonitis, for
example, during
continuous ambulatory peritoneal dialysis (CAPD) and automated peritoneal
dialysis
(APD) procedures.
Peritoneal dialysis (PD) is a medical procedure for removing toxins from the
blood that takes advantage of the semi-permeable membrane surrounding the
walls of
the abdomen or peritoneal cavity. During a PD procedure, a solution is
introduced into
the patient's abdomen, where it remains for up to several hours, removing
blood
toxins via osmotic transfer through that membrane. At completion of the
procedure,
the solution is drained from the body along with the toxins. CAPD is the
manual form
of this procedure, requiring that the patient manually drain fresh PD solution
into, and
spent PD solution out from, the peritoneum. In APD, the entire procedure is
handled
by automated equipment.
Peritonitis is a common complication of both CAPD and APD. Often caused
by introduction of bacteria (e.g., from the tubing, connectors and other
apparatus that
make up the PD transfer set) to the peritoneum during dialysis, this swelling
of the
peritoneum can cause vomiting, abdominal tenderness and a host of other
symptoms.
Although responsive to antibiotics, peritonitis can end a patient's ability to
stay on
APD and CAPD therapies. In extreme cases, it can be fatal.
Standard tests for peritonitis, usually conducted on occurrence of acute
clinical
symptoms, include the Gram stain procedure, performing a cell count on the
peritoneal fluid, culturing that fluid, and/or performing a blood culture.
Largely, these
tests can only be done in the lab, after a patient has presented with
symptoms. By that
time, the peritonitis may well
- 1 -
CA 02594634 2007-07-24
have set in, resulting in undue patient distress and potentially necessitating
more extensive
treatment.
More recently, reagent test strips have become available, making it possible
for phy-
sicians or patient's themselves to perform more immediate diagnosis. However,
test strips
have a limited time window of utility and have generally not been successful
in early stage
detection.
CAPD and APD patients are typically counseled to maintain a keen eye for
another
symptom of peritonitis: a turbid or cloudy effluent bag. This can be late-
developing, unfor-
tunately, and is further compounded if the PD solution remains in the body for
a long period
before expulsion (as is the case, for example, during daytime dwells of APD
patients). De-
tection of turbid effluent is further complicated in APD equipment with long
drain lines,
since patients may only see the effluent lines and not the effluent bag (where
the turbidity is
more readily apparent). Moreover, patients who are blind or have poor eyesight
must rely on
friends, family and/or caregivers to inspect the spent PD fluid for turbidity.
The prior art suggests that such cloudiness might be detected automatically,
e.g.,
within APD equipment, by detecting the overall amount of non-coherent,
polychromatic
light that passes through a vessel of PD effluent by use of a source of such
light positioned on
one side of the vessel and a detector positioned at an opposing side.
Implementations of this
technique have generally not proven reliable because of poor signal-to-noise.
An object of the invention is to provide improved methods and apparatus for
medical
diagnosis, testing and/or treatment in the home or lab.
A further object of the invention is to provide improved methods and apparatus
for
PD therapy.
A still further object of the invention is to provide improved methods and
apparatus
for detecting the onset of peritonitis, e.g,. in connection with peritoneal
dialysis.
- 2 -
CA 02594634 2007-07-24
Yet a still further object of the invention is to provide such methods and
apparatus as
can be implemented at reasonable cost, yet, produce efficacious results.
- 3 -
CA 02594634 2007-07-24
Summary of the Invention
The foregoing are among the objects attained by the invention which provides,
in one
aspect, automated medical testing methods and apparatus that detect the onset
of peritonitis
from optical characteristics of PD effluent resolved at cellular scales in the
flow path.
For example, according to one aspect of the invention, an APD machine
includes, in
an effluent flow path, apparatus for early stage peritonitis detection
comprising an illumina-
tion source and a detector. The source is arranged to illuminate peritoneal
effluent in a
chamber that forms part of the flow path, and the detector is arranged to
detect illuminant
scattered by the effluent. The detector detects that scattered illuminant at a
cellular scale of
resolution, e.g., on a scale such that separate cellular-sized biological (or
other) components
in the effluent can be distinguished from one another based on scattering
events detected by
the detector.
Related aspects of the invention provide apparatus as described above in which
the
detector is arranged such that separate white blood cells (WBCs) in the
effluent can be distin-
guished from one another based on reflection and scattering (collectively,
"scattering") of
illuminant. Apparatus with a detector so arranged can, by way of example,
count such WBCs
from scattering and can, further, signal the onset of peritonitis if those
counts change over
time and/or vary from a baseline.
Further related aspects of the invention provide apparatus as described above
in which
the detector is arranged such that cellular-sized biological (or other)
components of different
types in the effluent can be distinguished based on illuminant scattered by
them. Related as-
pects of the invention provide such apparatus in which the detector is so
arranged as to permit
WBCs in the effluent to be distinguished based on scattering from red blood
cells (RBCs),
fibrin and/or other components.
Other aspects of the invention provide apparatus as described above which
signal the
onset of peritonitis based on variance, e.g., over time and/or from a
baseline, in counts of se-
lected biological components in the effluent. Related aspects of the invention
provide such
apparatus as compute a trend of variance of those counts, e.g., with respect
to 'WBCs in the
- 4 -
CA 02594634 2007-07-24
effluent. Further related aspects of the invention provide such apparatus
which compute that
trend as a slope of a curve of those counts with respect to time and that
signals the onset of
peritonitis when that slope exceeds a selected amount.
Other related aspects of the invention provide such apparatus in which the
detector
counts scattering events ¨ i.e., events in which illuminant is reflected and
scattered from the
effluent to the detector ¨ based on intensity and/or location of the
scattering event. In one
such aspect of the invention, the detector comprises a pin diode that is
configured to count
scattering events, e.g., based on the intensity of illuminant detected from
the effluent. An
apparatus according to this aspect of the invention can, for example, signal
the onset of peri-
tonitis when the number of counts of a certain intensity (or range of
intensities, e.g., which
are based on cell size) varies, e.g., from a baseline and/or among drains of
spent PD solution
from the patient, and/or when a trend of that variance over time exceeds a
selected amount.
In other such aspects, the detector comprises a charge-coupled device (CCD)
that is
arranged to image the chamber ¨ that is, to record scattering events based on
both location
and (cumulative) intensity. Further related aspects of the invention provide
such apparatus in
which the detector generates a histogram of one or more such images, counting
scattering
events (e.g., based on intensity). Still further related aspects of the
invention provide such
apparatus which generates a histogram from multiple images taken, for example,
during a
drain of spent PD solution from the patient. As above, apparatus according to
these aspects
of the invention can, for example, signal the onset of peritonitis when the
number of counts
of a certain intensity (or range of intensities) varies over time, e.g., from
a baseline and/or
among successive drains of PD effluent from the patient.
Other related aspects of the invention provide such apparatus which the
histograms
are performed only with respect to selected scattering events recorded in the
images, e.g.,
scattering events of selected intensities and/or lengths. Apparatus accord to
these aspects of
the invention can, for example, signal the onset of peritonitis when the
number of counts
from scattering events likely caused by WBCs (and not, for example, RBCs or
fibrin) vary
over time from a baseline and/or among successive drains of PD effluent from
the patient.
- 5 -
CA 02594634 2007-07-24
Further aspects of the invention provide such apparatus in which the
illumination
source is a laser diode (or other source of coherent illuminant).
Related aspects of the invention provide such apparatus in which the detector
is ar-
ranged to detect side-scattering events, e.g., events detectable within a
field of view perpen-
dicular to a ray of illuminant sourced by the laser diode.
Further related aspects of the invention provide such apparatus in which
illuminant
sourced by the laser diode comprises a beam disposed ¨ and, specifically, for
example, cen-
tered ¨ within a portion of the flow path from which scattering events are
counted by the
detector.
Still further related aspects of the invention provide such apparatus in which
illumi-
nant sourced by the laser has a beam width selected based on size
characteristics of the bio-
logical (or other) components from which scattering events are to be counted.
Further related
aspects of the invention provide such methods in which the beam width has a
diameter of
about 1.5 times a size of components, e.g., WBCs. Yet still other aspects of
the invention
provide such apparatus in which the beam width has any of a circular and
gaussian cross-
section, or other beam size and/or shape.
Further aspects of the invention provide such apparatus in which the detector
com-
prises a lens arranged to resolve illuminant scattered from components of the
effluent at a cel-
lular scale of distances. Related aspects of the invention provide such
apparatus in which the
lens is arranged to provide a depth of field encompassing a substantive
portion of the flow
path within the detector field of view, e.g., a depth of field that
encompasses a flow chamber
from which scattering events are detected.
Other aspects of the invention provide apparatus as described above for use in
con-
nection with CAPD procedures.
Still other aspects of the invention provide such apparatus for use in
detecting the on-
set of peritonitis in fluid flows established independent of APD and/or CAPD
equipment in
which the PD fluid is collected. Such apparatus has application, for example,
in testing bags
- 6 -
CA 02594634 2014-04-22
=
(or other collections) of spent PD effluent, e.g., as they are being emptied
for disposal
or for further testing.
Yet still other aspects of the invention provide PD kits that include, in
addition
to conventional components (such as tubing, clamps, sterilization wipes, and
so forth),
a test apparatus as described above.
Still yet other aspects of the invention provide methods of testing PD
effluent
for the onset of peritonitis paralleling the operations described above.
Yet still other aspects of the invention provide apparatus and methods as
described above for use in detecting, in a PD effluent flow, blood (RBCs),
bubbles
and other undesirable byproducts of CAPD, APD and so forth. A related aspect
of the
invention is to provide such apparatus and methods for use in hemodialysis and
other
medical procedures.
In a further aspect, the present invention resides in an apparatus for testing
peritoneal dialysis (PD) effluent in a flow path, comprising an illumination
source that
illuminates peritoneal effluent in the flow path, a detector that includes a
lens that
resolves at a cellular scale of resolution illuminant reflected and/or
scattered
(collectively, "scattered") by the effluent, such that separate cellular-sized
components of a same type can be distinguished from one another within the
illuminant resolved by the lens, where those components are white blood cells,
red
blood cells, fibrin, bubbles, and/or other cellular-sized components of the
effluent, and
the detector detects and counts, in said illuminant resolved by the lens,
illuminant
scattered from separate cellular-sized components in the effluent, and the
detector
signals an onset of peritonitis if said counts change over time and/or vary
from a
baseline.
In yet a further aspect, the present invention resides in an apparatus for
testing
peritoneal dialysis (PD) effluent in a flow path, comprising an illumination
source that
illuminates peritoneal effluent in the flow path, a detector that includes a
lens that
resolves at a cellular scale of resolution illuminant any of reflected and/or
scattered
(collectively, "scattered") by the effluent,
- 7 -
CA 02594634 2014-04-22
. .
, .
such that at least selected separate cellular-sized components in the effluent
can be
distinguished from one another within the illuminant resolved by the lens,
where those
components are white blood cells, red blood cells, fibrin, bubbles, and/or
other
cellular-sized components of the effluent, and the detector signals an onset
of
peritonitis based on variance, over time and/or from a baseline, in counts of
at least
selected reflections and/or scatterings (collectively, "scatterings") resolved
by the lens
and detected by the detector from components in the effluent.
In yet an even further aspect, the present invention resides in an apparatus
for
testing peritoneal dialysis (PD) effluent in a flow path, comprising a laser
that
illuminates peritoneal effluent in the flow path, and a detector including a
lens that
resolves at a cellular scale of resolution illuminant reflected and/or
scattered
(collectively, "scattered") by the effluent, and a pin-diode configured to
signal the
detection of illuminant any of reflected and/or scattered (collectively,
"scattered") by
the effluent at a resolution resolvable at a scale such that at least selected
separate
cellular-sized components in the effluent can be distinguished from one
another
within the illuminant resolved by the lens, wherein the detector counts at
least
selected reflections and/or scatterings (collectively, "scatterings") signaled
by the pin-
diode from components in the effluent and signals an onset of peritonitis
based on
variance, over time and/or from a baseline, in counts of at least selected
scatterings.
- 7a -
CA 02594634 2007-07-24
Brief Description of the Drawings
A more complete understanding of the invention may be attained by reference to
the
drawings, in which:
Figures IA ¨ lE depict an automated peritoneal dialysis (APD) treatment system
ac-
cording to one practice of the invention and of the type with which the
invention can be prac-
ticed;
Figures 2A ¨ 2C depict a continuous ambulatory peritoneal dialysis (CAPD)
treat-
ment system according to one practice of the invention and of the type with
which the inven-
tion can be practiced;
Figures 3A ¨ 3B depict apparatus for testing PD effluent according to one
practice of
the invention;
Figure 4 depicts an image of the type generated by a charge coupled device in
an ap-
paratus according to one practice of the invention; and
Figures 5A ¨ 5C depict histograms of the type generated from images generated
by
charge coupled devices used in practice of the invention;
- 8 -
CA 02594634 2007-07-24
Detailed Description of the Illustrated Embodiment
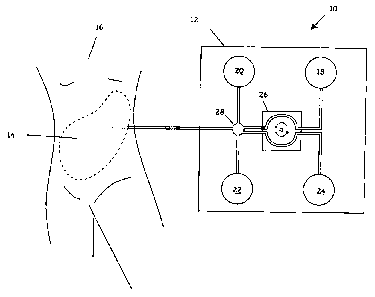
Figure 1 A depicts an automated peritoneal dialysis (APD) treatment system 10
ac-
cording to one practice of the invention and of the type with which the
invention can be prac-
ticed. The system 10 includes a cycler 12 or other apparatus to facilitate
introducing fresh
peritoneal dialysis (PD) solution into, and removing spent PD solution from,
the peritoneum
14 of a patient 16.
The system 10 includes a PD solution supply chamber 18, a heating chamber 20,
a
weigh chamber 22, and a disposal chamber 24, all constructed an operated in
the conven-
tional manner known in the art (albeit as adapted for inclusion of PD effluent
test apparatus
as discussed elsewhere herein). Thus, PD supply chamber 18 holds a supply of
fresh PD so-
lution for delivery to the patient 16; heating chamber 20 brings the fresh PD
solution to an
appropriate temperature for delivery to the peritoneum; weigh chamber 22 hold
spent PD so-
lution expelled from the peritoneum, e.g., for weighing; and, disposal chamber
24 holds spent
PD solution for disposal.
Pump 26 operates under control of a micro-controller (not shown) to move
solution
between the chambers 18 ¨ 24 in the conventional manner, e.g., as illustrated
in Figures 1B ¨
1E. Thus, for example, as shown in Figure 1B, pump 26 moves fresh PD solution
from sup-
ply chamber 18 to heating chamber 20 so that the latter can bring that
solution to temperature,
prior to its introduction into the patient's peritoneum 14. Once the desired
temperature is
achieved and treatment is to begin, the pump 26 opens a valve 28, allowing the
heated, fresh
PD solution to flow via gravity-assist into the peritoneum 14. See, Figure 1C.
Per Figure 1D, once the PD has dwelled for the desired period of time in the
perito-
neum 14, pump 26 opens valve 28 so that the spent PD solution can flow into
chamber 22 for
weighing (e.g., to insure that sufficient solution has be removed from the
peritoneum 14), as
per convention in the art. Pump 26 then moves the spent effluent from the
weigh chamber 22
to the disposal chamber 24 for collection prior to disposal by the patient,
health care worker,
or otherwise. See, Figure 1D.
- 9 -
CA 02594634 2007-07-24
The conventional aspects of system 10 shown and described here are merely by
way
of example. It will be appreciated that apparatus for testing PD effluent (as
discussed else-
where herein) may be used in connection with APD equipment of other
configurations and
modes of operation than those shown in Figures IA ¨ lE and described above.
Figure 2A depicts a continuous peritoneal dialysis (CAPD) treatment system 30
ac-
cording to one practice of the invention and of the type with which the
invention can be prac-
ticed. The system 30 includes a fresh PD solution supply bag 32, a spent PD
solution bag 34,
and a y-connector 36 for coupling those bags to peritoneal transfer set 38.
The system 30 is
constructed and operated in the conventional manner known in the art (albeit
as adapted for
inclusion of PD effluent test apparatus as discussed elsewhere herein). Thus,
for example,
the patient connects bags 32, 34 to the y-connector 36, as shown in Figure 2B,
for a brief ster-
ilizing flush of the connector 36. Then, as further shown in that drawing, the
patient config-
ures the connector 36 to permit fresh PD solution to flow, under gravity
assist, from bag 32
into the peritoneum. Once the PD solution has dwelled for the desired period,
the patient re-
configures the connector 36 to permit the spent PD solution to drain to bag 34
for disposal.
See, Figure 2C.
The conventional aspects of system 30 shown and described here are merely by
way
of example. It will be appreciated that apparatus for testing PD effluent (as
discussed else-
where herein) may be used in connection with CAPD equipment of other
configurations (e.g.,
with straight transfer tubing sets) and modes of operation than those shown in
Figures 2A ¨
2C and described above.
Figure 3A depicts an APD cycler 40 that is constructed and operated in the
manner of
cycler 12 (Figure 1), albeit including apparatus 42 according to the invention
for testing PD
effluent (i.e., PD solution drained from the peritoneum) in a flow path of
cycler 40 and/or
other APD system or components of which it is a part. The cycler 40 (with test
apparatus 42)
can be used in place of cycler 12 in the system 10 (Figure 1), as well as in
other APD treat-
ment systems. Likewise, the test apparatus 42 can be coupled into the effluent
flow path (i.e.,
drain lines) of the system 30 (Figure 2), as graphically depicted in inset
Figure 3B, as well as
in other CAPD systems. Moreover, the apparatus can be combined with kits for
APD and
CAPD procedures (e.g., kits that include tubing, clamps, sterilization wipes
and so forth).
Still further, the apparatus 42 can be coupled into fluid flow paths of
laboratory, doctor's of-
- 10 -
CA 02594634 2007-07-24
fice, hospital or home test equipment and it can be sold with kits for such
testing (e.g., kits
that include PD effluent sample phials, drop boxes, labeling and so forth).
For convenience,
operation of test apparatus 42 will be described with respect to cycler 40 of
Figure 3A,
though, it will be appreciated that apparatus 42 can be configured and
operated similarly in
the aforementioned and other environments in which it is used.
By way of overview, illustrated apparatus 42 tests PD effluent in a flow path
¨ here,
the path from peritoneum 14 to disposal chamber 24 ¨ for the onset of
peritonitis and/or
other conditions (e.g., the presence of blood and/or bubbles). To this end,
that apparatus in-
cludes an illumination source 44 and a detector 46. The source 44 is arranged
to illuminate
peritoneal effluent in a chamber 48 that forms part of the flow path, and the
detector 46 is ar-
ranged to detect illuminant scattered by that effluent, e.g., in a direction
normal to the illumi-
nant beam.
The source 44 and detector 46 are configured so that the detector detects
reflected
and/or scattered (collectively, "scattered") illuminant at a cellular scale of
resolution, e.g., on
a scale such that separate cellular-sized biological (or other) components in
the effluent can
be distinguished from one another. In applications such as those to which the
illustrated em-
bodiment is directed, i.e., early detection of the onset of peritonitis, this
permits separate
white blood cells (WBCs) in the effluent to be distinguished from one another
(as well as
from red blood cells, fibrin and other components of the effluent) so that
they can be counted
and so that the rate of change of those counts can be measured for purposes of
detecting and
signaling the onset of peritonitis. In other embodiments, this permits red
blood cells (or other
components, such as bubbles) in the effluent to be distinguished from one
another (as well as
from WBCs, fibrin, etc.) and counted; and so forth.
As noted, detector 46 is configured to detect illuminant scattered from the
chamber 48
at a cellular scale of resolution, e.g., on a scale such that separate
cellular-sized components
in the effluent can be distinguished from one another. As further noted, in
the illustrated em-
bodiment, this permits separate WBCs 54 in the effluent to be distinguished
from one another
(as well as from red blood cells 56, fibrin 58 and other components of the
effluent) so that
they can be counted and so that the rate of change of those counts can be
measured for pur-
poses of detecting and signaling the onset of peritonitis. In other
embodiments, this permits
-11-
CA 02594634 2014-04-22
other components- such as RBCs 56, fibrin 58, etc. ¨ to be detected in the
effluent for
purposes signaling other other conditions.
The illumination source of the illustrated embodiment comprises a low-power
laser diode generating a monochromatic collimated beam. Here, the wavelength
is
selected at 630 nm to coincide with an optical sensitivity of detector 46 and
for suitability
in reflection and scattering (collectively, as above, "scattering") from at
least selected
components (e.g., white blood cells) in the effluent. Other embodiments may
utilize lasers
of other wavelengths, monochromatic or otherwise, selected in accord with
foregoing or
other criterion, e.g., 830 nm and 780 nm lasers, to name but a few, as well as
other
illumination sources, monochromatic, polychromatic, coherent and/or otherwise.
The collimated beam generated by laser diode 44 of the illustrated embodiment
is
optionally shaped by lens or columinator 50 to result in a beam 52 of guassian
or circular
cross-section, though beams of other shapes may be used in other embodiments.
Lens 50 shapes the beam to optimize scattering from at least selected
components
in the effluent. In the illustrated embodiment, this means sizing the beam at
lx-2x and,
preferably, about 1.5x the average size of the effluent components to be
preferentially be
detected- here, WBCs. Given an average size of 12-15 1AM for neutrophils and
eosinophils, 8-10 gm for lymphocytes, and 16-20 gm for monocytes, beam 52 of
the
illustrated embodiment is accordingly sized between 10-40 gm and, preferably,
15-25 p.m
and, still more preferably, about 20 gm. This optimizes the apparatus 42 for
preferential
detection of WBCs over, for example, red blood cells 56, fibrin 58 and other
components
of the PD effluent. Other embodiments may use other beam sizes, e.g., for
reason of
preferential detection of other effluent components or otherwise.
The beam 52 of the illustrated embodiment is aimed to pass through chamber 48
in order to illuminate the effluent therein for purposes of evoking scattering
from
biological (and other) components in that fluid. Although in the illustrated
embodiment,
the beam is aimed to pass through a center of the chamber 48, as shown, in
other
embodiments the beam 52 may be directed otherwise.
- 12 -
CA 02594634 2007-07-24
Turning back to Figure 3A, detector 46 detects and counts scattering events ¨
i.e.,
events in which illuminant is scattered from the effluent in the chamber 48 to
the detec-
tor 46 based on the intensity and/or location of those events. In the
illustrated embodi-
ment, the detector 46 is, particularly, arranged to detect side-scattering,
e.g., events within a
field of view 64 centered on an axis 66 that is normal to the beam 52, as
shown. In other em-
bodiments, the detector may be arranged to detect other scattering events,
e.g., back-
scattering, forward-scattering, side-scattering at angles B other than normal.
Thus, while in
the illustrated embodiment, B is substantially 900, more generally, B is in
the range 300 ¨
1500; more preferably, between, 60 ¨ 1200; still more preferably, between 80
¨ 1001); and,
still more preferably, substantially 900, as illustrated.
In some embodiments, the detector 46 employs a single-cell (or few-celled)
photo di-
ode, i.e., pin-diode 68, for purposes of detecting and signaling the
occurrence of such scatter-
ing events. A lens 70 facilitates focusing the diode so that it detects those
events at a cellular
scale of resolution, e.g., on a scale such that separate cellular-sized
biological (or other) com-
ponents in the effluent can be distinguished (based on such scattering) from
one another. In
the illustrated embodiment, lens 70 is selected and arranged (vis-a-vis
chamber 48 and diode
68) to preferentially focus WBCs, though, in other embodiments, the lens 70
may be focused
otherwise. The lens 70 is further selected and arranged for a desired depth of
focus within the
field of view 64, e.g., in the illustrated embodiment, a depth of focus
matching the depth of
compartment 48, or a substantial portion thereof. The chamber 48 is configured
to match the
laser beam size and shape, e.g., so as to minimize or wholly avoid reflections
(or scattering)
of the beam 52 off the inner walls of the chamber itself
The laser diode 68 is selected and/or otherwise configured (e.g., through use
of ap-
propriate circuitry) to detect scattering from selected components of the
effluent ¨ here,
preferentially, WBCs, though, in other embodiments, RBCs, fibrin, bubbles
other compo-
nents of the effluent. Regardless, such selection and/or configuration can be
performed em-
pirically (e.g., by testing scattering detected from an effluent of known
composition) or oth-
erwise.
Scattering events detected and signaled by the diode 60 are routed to a
microprocessor
62 (or other suitable element) for analysis. In the illustrated embodiment,
this comprises
counting events signaled over time and generating an alert, e.g., when the
number of counts
- 13 -
CA 02594634 2007-07-24
of a certain intensity (or range of intensities) varies, e.g., (i) from a
baseline established for
patient 16, (ii) among successive drains of spent PD solution from that
patient 16, and/or (iii)
when a trend of that variance over time ¨ and, more particularly, a rate of
change of counts
over time (i.e., a "critical slope') ¨ exceeds a selected amount. Such an
alert can be in the
form of a visible and/or audible signal to the patient 16, health-care worker,
or otherwise; a
hardware or other interrupt to system 12 of which test apparatus 42 forms a
part; a software
function call to such system; or otherwise.
Other embodiments of the invention employ a charge-coupled device (CCD), in
place
of pin-diode 68, for purposes of detecting and signaling the occurrence of
scattering events.
As above, lens 70 facilitates focusing the CCD (and obtaining a desired depth
of focus) so
that it detects those events at a cellular scale of resolution and, in the
illustrated embodiment,
so that it preferentially focuses WBCs ¨ though, in other embodiments, the
lens 70 may be
focused otherwise. In the discussion that follows, elemental designation 68 is
used for the
CCD, as it was for the pin-diode, since the CCD is disposed in the same
functional place in
apparatus 42.
As with the pin-diode, the CCD 68 is selected and/or otherwise configured to
facili-
tate detection of scattering from selected components of the effluent (again,
here, preferen-
tially, WBCs). In this regard, the CCD 68 images the illuminated chamber 48,
recording both
the positions and intensities of scattering events (again, at a cellular scale
of resolution) so
that at least selected components (e.g., WBCs) in the effluent can be
distinguished from one
another and from other components of the effluent.
Figure 4 depicts such an image ¨ here, generated from a simulated effluent
incorpo-
rating, in lieu of WBCs, 80 glass beads (sized between 10 ¨ 30 microns) per
L.
Images generated by the CCD are routed to the microprocessor 62 (or other
suitable
element) for analysis. In the illustrated embodiment, this comprises taking a
histogram of
each image ¨ or, more preferably, from multiple such images generated during
drainage of
spent PD solution following a single PD treatment session ¨ with binning that
is based on
intensity. Depending on the number of counts in selected one(s) of the
histogram bins, the
microprocessor 62 can generate an alert, e.g., as discussed below.
- 14 -
CA 02594634 2007-07-24
Figures 5A ¨ 5C depict such histograms ¨ here, generated from a simulated
effluent
as described above with, respectively, 40 (Figure 5A), 80 (Figure 5B) and zero
(Figure 5C),
glass beads per L.
In the illustrated embodiment, it generates that alert, e.g., when the number
of counts
of a certain intensity (or range of intensities) varies, e.g., (i) from a
baseline established for
patient 16, (ii) among successive drains of spent PD solution from that
patient 16, and/or (iii)
when a trend of that variance over time (i.e., from PD treatment session to
session) ¨ and,
more particularly, a rate of change of counts over time (or "critical slope')
¨ exceeds a se-
lected amount. Again, such an alert can be in the form of a visible and/or
audible signal to
the patient 16, health-care worker, or otherwise; a hardware or other
interrupt to system 12 of
which test apparatus 42 forms a part; a software function call to such system;
or otherwise.
As will be appreciated, an advantage of taking histograms from multiple CCD
images
is that it tends to emphasize intensity counts in the critical range. This
improves the signal-
to-noise ratio and, thereby, increases the efficacy of detection (e.g., of
peritonitis or other
conditions reflected by the effluent). In embodiments of the invention using
this approach,
the CCD 68 can be controlled (e.g., by the microprocessor 62 or otherwise) to
acquire those
multiple images during PD solution drainage by successively entering
"acquisition" and
"read" modes: the former, for acquiring images of the illuminated chamber 48;
and the latter
for reading those images to the microprocessor.
In other embodiments of the invention, the microprocessor can perform image
pre-
processing prior to taking the histograms. Thus, for example, it can eliminate
pixel values
representing scattering from effluent components that are too long (e.g.,
fibrin) or too short
(e.g., RBCs) ¨ both, by way of example, with respect to embodiments intended
to count
WBCs for purposes of peritonitis detection. Further such preprocessing may be
selected de-
pending upon the specifics of the application to which the invention is
applied.
Described and shown herein are apparatus and methods for testing PD effluent
meet-
ing the objects set forth above. It will be appreciated that the embodiments
described here
are merely examples of the invention and that other embodiments, incorporating
changes
therein, fall within the scope of the invention. Thus, by way of non-limiting
example, it will
- 15 -
CA 02594634 2014-04-22
be appreciated that the apparatus and methods as described above for use in
detecting
peritonitis from PD effluent flow can be applied in detecting blood (RBCs),
bubbles
and other desirable or undesirable byproducts of CAPD, APD and so forth, all
by way
of non-limiting example. Further, it will be appreciated that such apparatus
and
methods can be applied in detecting bubbles and other byproducts of
hemodialysis.
- 16-