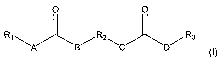Note: Descriptions are shown in the official language in which they were submitted.
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
TITLE
COATING COMPOSITIONS CONTAINING RHEOLOGY CONTROL
AGENTS
PRIORITY
This application claims priority from U.S. provisional Patent
Application Serial No. 60/643,440, filed January 13, 2005, and U.S.
provisional Patent Application Serial No. 60/643,482 filed January 13,
2005 both applications are incorporated herein by reference.
BACKGROUND OF THE INVENTION
This invention relates to solvent-borne and water-borne coating
compositions useful for finishing the exterior of automobiles and trucks,
and in particular to coating compositions containing rheology control
agents that provide the composition with improved rheology control to
facilitate a spray application that provides a high quality automotive finish.
DESCRIPTION OF THE PRIOR ART
The finish of choice currently being used on automobiles and trucks
is a clear coat/color coat finish in which a clear coating is applied over the
pigmented color coat or base coat to provide protection to the color coat
and improve the appearance of the overall finish particularly, gloss and
DOl (distinctness of image). Mono-coats of pigmented finishes are also
used without a clear coat on some automobiles and trucks, in particular,
older models. Primers, primer-surfacers, and sealers for many automotive
and truck applications are applied initially before one of the
aforementioned top coats are applied. All of the above compositions when
applied by conventional spraying techniques, have rheology control
problems, such as, running and sagging after application. Top coat
finishes containing flake pigments or special effect pigments have
problems with flake control and proper flake orientation for optimum
appearance.
Additional problems are caused by many localities having
regulations requiring the use of low VOC (volatile organic content) coating
i
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
compositions to reduce air pollution. Typically, these low VOC coating
compositions have a VOC of 2.1 pounds/gallon (252 g/1) or less and when
applied by conventional spray techniques often have problems with
running and sagging of the finish after application and also problems with
proper fiake orientation and control.
These low VOC coating compositions typically are used for OEM,
refinishing or repainting of automobiles and trucks or parts thereof and are
usually formulated using relatively low molecular weight polymers.
However, as pointed out above, such compositions generally have poor
rheology control and run and sag after spray application particularly when
applied to vertical surfaces, such as, door panels and body side panels
and have poor flake orientation and flake control. A rheology control agent
is needed to form a coating composition having improved rheology control
that prevent runs and sags after application and in general provides a
finish with an acceptable appearance with good gloss and DOI.
Rheology control is also very critical for the low solids lacquer
basecoats typically used in the refinishing or repainting of automobiles and
trucks. These lacquer basecoats are typically applied at very low solids,
as low as 10 percent by volume, using spray application. To achieve
adequate hiding in these coatings, a dry film thickness of around 15 to 65
microns is typically required. Because of the very low volume solids of
these coatings, the applied wet film thickness of these coatings can be
around 350 microns or more. This requires the use of a very effective
rheology control agent to prevent sagging and to give good flake
orientation. Another aspect of these lacquer coatings is that they typically
contain higher molecular weight binder components which can be
incompatible with many conventional rheology control agents.
Rheology control agents are shown in U.S. Patent 3,893,956, U.S.
Patent 4,311,622, U.S. Patent 4,314,924, U.S. Patent 4,677,028, U.S.
Patent 4,851,294, U.S. Patent 6,420,466 B1, U.S. Patent 6,617,468 B2,
and EP 0683214, EP 1162242, DE 10241853 B3 and WO 03037849.
These rheology control agents of the prior art in general cannot be
2
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
formulated into high solids compositions and do not provide the necessary
level of optical clarity to the resulting finishes and form finishes having
low
DOI levels, particularly when the coating compositions are ambient
temperature curing compositions. Some of these rheology control agents
have to be prepared in the presence of the binder of the coating
composition to achieve the desired level of rheology control, which adds to
the manufacturing costs of the composition by requiring additional
manufacturing steps and the use of specific and also expensive
equipment. Some rheology control agents, for example, taught by U.S.
6,617,468 B2 limit the weatherability of the resulting finish, which over time
negatively impacts the appearance of the finish. Some rheology control
agents, for example, taught by U.S. 4,311,622 are limited in their
compatibility with the resin system. These rheology control agents are
based on a symmetrical structure. Furthermore, some rheoiogy control
agents, for example, taught by U.S. 4,311,622 or WO 02064684 show
insufficient compatibility in the resin system of choice especially rheology
control agents prepared using hydroxyl functional monoamines.
U.S. Patent Publication 2002/059961, published October 31, 2002
shows gelling agents that are used to gel oils and in cosmetic
compositions, such as, antiperspirants but have not been suggested for
use in coating compositions.
Accordingly, there is still a need for coating compositions for a wide
variety of applications that contain a rheology control agent that will
provide an acceptable level of rheology control on application of the
composition without deteriorating the appearance, durability or
weatherability of both high solids and low solids coating compositions that
are often used in OEM automotive and truck manufacturing and to refinish
or repaint automobile and truck bodies or parts thereof.
SUMMARY OF THE INVENTION
The present invention is directed towards coating compositions
comprising a liquid carrier and film forming binder and a rheology control
agent having improved rheology control in both high and low solids
3
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
solvent-borne and water-borne coating compositions useful for OEM and
refinishing or repainting the exterior of automobile and truck bodies and
parts thereof. The rheology control agent used in the coating composition
of this invention comprises the following compound represented by the
following formula including isomers and mixtures of isomers thereof:
0 0
Rj'_'A B~R21-1 C D~R3
Formula (I)
wherein A, B, C and D equal CH2, CHR, NH, or 0, and A, B, C and D may
be the same or different and at least one of A and B equals NH and at
least one of C and D equals NH; and
wherein R1a R2, and R3 may be the same or different and represent a
linear, branched, hyper-branched, or dendritic ether, polyether or
hydrocarbon based chain, optionally forming at least one carbon-based
ring, being saturated or unsaturated and R2 represents linear or branched
alkylenes, ethers, polyethers, or polyester linkages and at least one of Ri,
R2, and R3 comprises an ester group which is branched off from the main
chain.
Substrates having adhered thereto a layer of the novel coating
composition containing the rheology control agent and the novel rheology
control agent are also part of this invention.
DETAILED DESCRIPTION OF THE INVENTION
The features and advantages of the present invention will be more
readily understood, by those of ordinary skill in the art, from reading the
following detailed description. It is to be appreciated those certain
features of the invention, which are, for clarity, described above and below
in the context of separate embodiments, may also be provided in
combination in a single embodiment. Conversely, various features of the
invention that are, for brevity, described in the context of a single
4
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
embodiment, may also be provided separately or in any sub-combination.
In addition, references in the singular may also include the plural (for
example, "a" and "an" may refer to one, or one or more) unless the context
specifically states otherwise.
The use of numerical values in the various ranges specified in this
application, unless expressly indicated otherwise, are stated as
approximations as though the minimum and maximum values within the
stated ranges were both preceded by the word "about." In this manner,
slight variations above and below the stated ranges can be used to
achieve substantially the same results as values within the ranges. Also,
the disclosure of these ranges is intended as a continuous range including
every value between the minimum and maximum values.
All patents, patent applications and publications referred to herein
are incorporated by reference in their entirety.
The coating compositions of this invention containing the novel
rheology control agent are solvent-borne or water-borne coating
compositions and in particular are clear and pigmented paint compositions
used for OEM, refinishing or repainting the exterior of automobiles and
trucks. The rheology control agent improves and controls the rheology of
the coating compositions to facilitate spray applications and provide a
Class A automotive finish having an excellent overall appearance and
good DOI. The resulting finish does not sag or run particularly when spray
applied to vertical surfaces. Under high shear conditions such as occur
when the coating composition is applied, for example, by spraying, the
viscosity of the composition is significantly reduced. After the coating is
applied, the shear conditions are low and the viscosity increases
significantly to eliminate runs and sags on a vertical surface and provides
a finish with an excellent appearance. Also, proper orientation of flake or
special effects pigments that are used in base coats and mono-coats is
controlled and the pigment and flake settling properties of such coatings
are also improved. The coating compositions of this invention can also be
used as primer, primer surfacers, and primer fillers.
5
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
A compound which can provide rheology control to a coating
composition must be compatible with the coating composition and not
deteriorate the properties of the resulting finish, such as, gloss and DOI or
the weatherability or durability of the finish. Small changes in the chemical
composition of a compound can significantly affect its use as a rheology
control agent. Rheological measurements are useful in characterizing the
effectiveness of a rheology control agent, but the final measure of the
ability of a compound to provide effective rheology control in a coating
composition, is to test the compound in a coating composition using
conventional application conditions, such as, spray application, optionally,
with subsequent drying or baking of the resulting finish and observe the
resulting appearance of the finish.
Typically, solvent-borne or water-borne coating compositions
containing the novel rheology control agent comprise 5 to 95 percent by
weight solvent or an aqueous carrier, based on the weight of the coating
composition, and 5 to 95 percent by weight of binder, which includes the
rheology control agent, also based on the weight of the coating
composition. Typically, the level of rheology control agent in such coating
compositions is in the range of 0.1 to 30 percent by weight, based on the
weight of the binder, and preferably, 0.1 to 10 percent by weight based on
the weight of the binder.
The coating compositions can be 100 percent binder solids
compositions and the rheology control agents are used in the ranges
shown above.
The term "binder" as used herein refers to the film forming
constituents of the composition and includes any crosslinking components,
such as, polyisocyanates, optional polymeric and/or oligomeric
components, and optional reactive diluents. Solvents, pigments, catalysts,
antioxidants, U.V. absorbers, light stabilizers, leveling agents, antifoaming
agents, anti-cratering agents and adhesion promoting agents are not
included in the term.
6
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
Molecular weight (both number and weight average) is determined
by gel permeation chromatography utilizing a high performance liquid
chromatograph supplied by Hewlett-Packard, Palo Alto, California and
unless otherwise stated the liquid phase used was tetrahydrofuran and the
standard was polymethyimethacrylate or polystyrene.
"Tg" (glass transition temperature) is in C and determined by
Differential Scanning Calorimetry or calculated according to the Fox
Equation.
"Lacquer" is a coating composition, which dries via evaporation of
the carrier, such as, a solvent, water or a mixture of solvent and water
without any substantial crosslinking of the binder of the coating
composition.
The rheology control agent used in the coating composition of this
invention is a compound having following formula
0 0
Rj__"A s1-1 R21_~ C D1__~ R3
Formula (I)
wherein A, B, C and D equal CH2, CHR, NH, or 0, and A, B, C and
D may be the same or different and at least one of A and B equals NH and
at least one of C and D equals NH; and
wherein Rl, R2, and R3 may be the same or different and represent
a linear, branched, hyper-branched, or dendritic ether, polyether or
hydrocarbon based chain, optionally forming at least one carbon-based
ring, being saturated or unsaturated and R2 represents linear or branched
alkylenes, ethers, polyethers, or polyester linkages and at least one of Ri,
R2, and R3 comprises an ester group which is branched off from the main
chain.
7
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
Novel rheology control agents of this invention comprise the above
Formula (1) having the various constituents defined as above with the
exception that excluded from Formula (1) is a compound wherein R2 is
CH2-CH2-CH2-CH2-CH(C(O)OCIH3), A, B, C and D are NH and R, and R3
are both equal to a linear octyl hydrocarbon chain.
The following formulas iilustrate particulariy useful rheology control
agents used in solvent-borne coatings, water-borne coatings or 100
percent solids coatings of this invention that provide the coating
compositions with excellent rheology control on application and form
finishes that have excellent overall appearance, good DOI, do not sag or
run on application, and have good flake orientation. Further, these
rheology control agents can be used in conjunction with a wide variety of
coating compositions containing as the binder, polyacrylates, linear
poly(meth)acry)ates, branched, grafted or segmented poly(meth)
acrylates,acrylic alkyd resins, polyesters, branched copolyesters,
carbamates, oligomers or polyesterurethanes. These coating
compositions may also utilize crosslinking agents, such as,
polyisocyanates, alkylated melamines, melamine derivatives, and
epoxides and other crosslinking agents.
In one embodiment of the above Formula I, A, B, C and D are NH
and R2 comprises an ester group which is branched off from the main
chain of the molecule. Specific examples of this embodiment include, but
are not limited to the following structures:
H H H H
Ny N\ N~
O ~ O
OCH3
(1)
H H H H
NuN N~N
IOI 0 O
OCH3
(2)
8
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
H H H H
N ~ N\-~E
(3)
HJ'NH'~'..'~~
H H \ /
(4)
H~ H~H
II
0
(5)
N' ~hiN NH~N '
H H
(6)
H H H H
oN~ 0
M,Y--C
(7)
CH3
N NI.,~ H
O O
(8)
9
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
O HN
N~NN~O
H H C02Me H
(9)
HN
H
0y N\~/~HO
NH COZMe
(10)
0
H OMe N N
aHN~N --(
O \'O
O
(11)
In a second embodiment of the above Formula I, A, B, C and D are
NH, R2 comprises an ester group which is branched off from the main
chain of the molecule, and R, and R3 each comprises at least one ester
group. Specific examples of this embodiment include, but are not limited
to the following structures:
H H H H
O O
O N)rN N~N Oi
O " O
OCH3
(12)
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
/~O~NUN N~N~Oi\
IOI " O
OCH3
(13)
0 O O
4O
O NH-YN NyN
O ~ O
O 00 O
(14)
COOEt
EtOOC'
~~\NH EtOOC
O/ NH HN COOEt
MeOOC N--kO
H
(15)
O O'~00
-/4O'i~HN~NH~~/NA N------O\1~
O H H 0
(16)
In a third embodiment of the above Formula I, A, B, C and D are
NH and R2 comprises an ester group which is branched off from the main
chain of the molecule, and Ri and R3 each comprises at least one
11
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
urethane group. Specific examples of this embodiment include, but are
not limited to the following structures:
H
N~
N~O O COOMe HNfo
O
~ ~ ~
O'~ N~NJv
v N~O
H H H
(17)
0 000 _
/ \ HN~O~~HNxI~lH" NxN,O~NH \ !
O H H O
(18)
0 ao
y O.i-- HNxNH" NxN'-"Oy NHtiw-
O H H 0
(19)
In yet another embodiment of the above Formula I wherein A and D
are 0 and B and C are NH and R2 comprises an ester group which is
branched off from the main chain of the molecule. Specific examples of
this embodiment include, but are not limited to the following structure:
H H
OuN N~O
IOI 0 O
OCH3
(20)
In another embodiment of the above Formula I wherein A and D are
0 and B and C are NH, R2 comprises an ester group which is branched off
from the main chain of the molecule, and R, and R3 comprise at least one
12
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
urea group. Specific examples of this embodiment include, but are not
limited to the following structures:
aH H H
Ny N,/"O-rN N-r~,Ny N_O
O O O O
Me0 O
(21)
H H
NuN~-O~N~NNU
'OI O
MeO OO O
(22)
In another embodiment of the above Formula I wherein A, B, C, and
D are NH and R, and R3 each comprise at least one ester group which is
branched off the main chain. Specific examples of this embodiment
include, but are not limited to the following structures:
0
EtO 0 H H 0
Et0 HJX H NuN OEt
O IOI OEt
0
(23)
O
NH~ NyH O
H O
(24)
13
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
O 0
EtO 0 O OEt
Et0 /'h 1-1 H JI ~' H OEt
0 H H 0
(25)
COOEt
EtOOC""-~ EtOOC
NH
O~NH HN COOEt
N-~O
H
(26)
0 H N
0
ON'J~NN,f
H H O
rl O O
/O O1OJ 0
(27)
0 H N
O~NxNNV
O H I/ 0 O
f 0 O1O O
(28)
14
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
O ~-{
NN
0 pH H p p ~
f O p~
O p
(29)
H
NxN NN
p OH H O O
O O p
(30)
p H H
N)~ NNN
~ H H O O
O p ~
OO 0 10
(31)
In an embodiment of the above Formula I wherein A, C, and D are
NH, B is 0, R2 comprises at least one ester group which is branched off
the main chain. Specific examples of this embodiment include, but are not
limited to the following structures:
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
H
(32)
H H
N~N
H p
~
(33)
In an embodiment of the above Formula i, wherein A and D are 0,
and B and C are NH, and R, and R3 each comprises at least one urea
group and one ester group which is branched off from the main chain of
the molecule. Specific examples of this embodiment include, but are not
limited to the following structure:
Q pp~ H H H
HH Ipl ~ I 0
(34)
The rheology control agents shown in Formulas 2 to 34 are novel
compounds.
The branched rheology control agents of this invention may be
formed using a variety of synthetic methods. Synthesis of structures (2-
16, 23-31) for example, may comprise reacting an amine with a
diisocyanate in a suitable reaction vessel generally at a temperature
between 0 C and 120 C, preferably, from 10 C to 80 C and optionally, in
the presence of a diluent.
For example, the synthesis of the branched rheology control agent
of this invention comprises adding a solution of an organic diisocyanate in
16
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
a dry solvent, such as chloroform, under an inert atmosphere, such as
nitrogen, to a solution of a corresponding amino acid ester typically in a
1:2 molar ratio. The resulting reaction mixture is held under relatively cold
conditions, such as 10 C, and then after addition of the diisocyanate
solution the reaction mixture is allowed to warm and to continue, for
example, about 1-3 hours with agitation or until the IR (infra red)
isocyanate peak (about 2258 cm 1) disappears as monitored by an IR
spectrophotometer. At the completion of the reaction, the rheology control
agent that was formed is isolated, for example, by conventional
techniques, like filtration or evaporation of solvent and the agent is washed
with appropriate solvent and dried, for example, in a vacuum at room
temperature.
Alternatively, structures 2-16 and 23-31 may be obtained by
reacting an isocyanate with a diamine in a suitable reaction vessel,
generally at a temperature between 0 C and 120 C, preferably, from 10 C
to 80 C and optionally, in the presence of a diluent.
Suitable amines or diamines for the formation of subject invention
include, but are not limited to, 2-amino-4-methylpentanoate alkyl ester,
aminoethanoate alkyl ester, 2-amino-3-phenylpropanoate alkyl ester, 2-
aminopropanoate alkyl ester, 2-amino-3-carbamoylpropanoate alkyl ester,
diethyl 2-aminobutanedioate alkyl ester, 2-amino-4-carbamoylbutanoate
alkyl ester, 2-aminopentanedioate alkyl ester, 2-amino-3-
methylpentanoate alkyl ester, 2-amino-4-(methylthio)butanoate ester, 2-
amino-3-hydroxypropanoate alkyl ester, 2-amino-3-hydroxybutanoate alkyl
ester, 2-amino-3-(4-hydroxyphenyl)-propanoate alkyl ester, 2-amino-3-
methylbutanoate alkyl ester, 2,6-diaminohexanoate alkyl ester, di(ethylene
glycol) methyl ether 2-amino-4-methylpentanoate ester, di(ethylene glycol)
methyl ether aminoethanoate ester, di(ethylene glycol) methyl ether 2-
amino-3-phenylpropanoate, ethyl 2-aminobutanedioate, 2-amino-2-
(hydroxyethyl)propane-1,3-diol, 1,3-diamino-2-hydroxypropane, butyl
amine, octyl amine, hexyl amine, decyl amine, undecyl amine, dodecyl
amine, cyclohexyl amine, cyclododecylamine, methyl 1,5-diamino-2-
17
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
panetanoate, methyl 6-aminocaproate, ethyl 4-aminobutyrate,
hexamethylene diamine, cyclohexyl diamine, xylyiene diamine,
polyoxyalkylene monoamines (Jeffamine M), polyoxyalkylene diamines
(Jeffamine D), tetramethylene diamine, nobornene diamine, L-ornithine
methyl ester, L-lysine ethyl ester, L-lysine butyl ester, 3-amino-alanine
ethyl ester or mixtures thereof. The alkyl groups in the aforementioned
section refer to methyl, ethyl, propyl, butyl, isoproyl, and isobutyl groups.
Suitable isocyanates or diisocyanates for the formation of subject
invention include, but are not limited to, hexamethylene diisocyanate, 1,4-
diisocyanatobutane, toluene 2,4-diisocyanate, toluene 2,6-diisocyanate,
4,4'-diphenyl methane diisocyanate, 2,2'-diphenyl methane diisocyanate,
2,4'-diphenyl methane diisocyanate, isophorone diisocyanate, m-
tetramethylxylene diisocyanate, dicyclohexylmethane 4,4'-diisocyanate,
naphthalene 1,5-diisocyanate, p-phenylene diisocynate, methyl 2,6-
diisocyanatohexanoate, octyl isocyanate, cyclohexyl isocyanate, butyl
isocyanate, hexyl isocyanate, decyl isocyanate, undecyl isocyanate, or
mixtures thereof.
The synthesis of branched rheology control agents (17, 18, 19, 21,
and 22) of this invention may be formally separated into several steps.
These steps may be carried out sequentially in one reaction vessel or in
different reaction vessels followed by separation and/or purification steps.
Certain rheology control agents (21 and 22) of this invention may be
formed by first reacting an amino alcohol component with a mono-
isocyanate component. This intermediate is further reacted with a
diisocyanate component to form the rheology control agent. The other
rheology control agents (17, 18 and 19) are formed by first reacting an
amino alcohol component with a diisocyanate component. The reaction
temperature and reactant concentration is selected to favor the formation
of the intermediate addition product. Further reaction with a mono-
isocyanate component then forms the rheology control agent of this
invention.
18
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
Suitable aminoalcohols for the formation of subject invention
include but are not limited to 2-amino-2-(hydroxyethyl)propane-1,3-diol,
1,3-diamino-2-hydroxypropane, 2-hydroxyethylamine, 3-
hydroxypropylamine, or mixtures thereof.
Suitable mono-isocyanates or diisocyanates for the formation of
subject invention include but are not limited to, methyl 2,6-
diisocyanatohexanoate, octyl isocyanate, cyclohexyl isocyanate, butyl
isocyanate, hexyl isocyanate, undecyle isocyanate, or mixtures thereof.
The branched rheology control agents of this invention may be
formed using a variety of synthetic methods. Synthesis of structure (20),
for example, may comprise reacting a diisocyanate with an alcohol in the
presence of a urethane catalyst such as dibutyl tin dilaurate at a
temperature from 25 C to 120 C, preferably from 40 C to 90 C.
Suitable diisocyanates include but are not limited to methyl 2,6-
diisocyanatohexanoate.
Suitable alcohol include but are not limited to butanol, hexanol,
octanol, decyl alcohol, undecyl alcohol, monomethyl ether polyethylene
glycol, and mixtures thereof.
The synthesis of branched rheology control agents (32 & 33) of this
invention may be formally separated into several steps. These steps may
be carried out sequentially in one reaction vessel or in different reaction
vessels followed by separation and/or purification steps. Certain rheology
control agents (32 & 33) of this invention may be formed by first reacting
an amino acid ester with a mono-isocyanate component at 1:1 molar ratio
in a dry solvent. This intermediate is further reacted with another or the
same mono-isocyanate component to form the rheology control agent.
Suitable amino acid ester for the formation of subject invention
include but are not limited to 3-hydroxy- aspartic acid dimethyl ester,
serine benzylester, serine t-butylester, serine ethylester, serine
methylester, serine isopropyl ester, P-hydroxy-phenylalanine methyl ester,
threonine methyl ester, or mixtures thereof.
19
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
Suitable mono-isocyanates for the formation of subject invention
include but are not limited to, octyl isocyanate, cyclohexyl isocyanate,
butyl isocyanate, hexyl isocyanate, undecyle isocyanate, decyle
isocyanate, or mixtures thereof.
Suitable diisocyanates for the formation of subject invention
include, but are not limited to, hexamethylene diisocyanate, 1,4-
diisocyanatobutane, toluene 2,4-diisocyanate, toluene 2,6-diisocyanate,
4,4'-diphenyl methane diisocyanate, 2,2'-diphenyl methane diisocyanate,
2,4'-diphenyl methane diisocyanate, isophorone diisocyanate, m-
tetramethylxylene diisocyanate, dicyclohexylmethane 4,4'-diisocyanate,
naphthalene 1,5-diisocyanate, p-phenylene diisocynate, methyl 2,6-
diisocyanatohexanoate, or mixtures thereof.
The synthesis of branched rheology control agent (34) of this
invention may be formally separated into several steps. These steps may
be carried out sequentially in one reaction vessel or in different reaction
vessels followed by separation and/or purification steps. Rheology control
agent (34) of this invention may be formed by first reacting an amino acid
ester with a mono-isocyanate component at 1:1 molar ratio in a dry
solvent. This intermediate is further reacted with another diisocyanate
component to form the rheology control agent at 2:1 molar ratio.
The rheology control agent can be formulated, dissolved, or
dispersed in an organic solvent or a mixture of solvents. More preferably,
the solvent is a ketone, ester, acetate, blend of ester and aicohol, aprotic
amide, aprotic sulfoxide, organic acid with a pKa less than 5.5, blend of
organic acids with the above solvents or aprotic amine. Examples of other
useful solvents include methyl ethyl ketone, methyl isobutyl ketone, methyl
amyl ketone, amyl acetate, ethylene glycol butyl ether acetate, propylene
glycol monomethyl ether acetate, xylene, N-methylpyrrolidone, 1-ethyl-2-
pyrrolidinone, 1-(2-hydroxyethyl)-2-pyrrolidinone, 1-cyclohexyl-2-
pyrrolidone, 1-ethyl-2-pyrrolidinone, 1 -vinyl-2-pyrro li done, 2-pyrrolidone-
5-
carboxylic acid, 1,5-dimethyl-2-pyrrolidinone, 1-benzyl-2-pyrrolidinone,
acetic acid, dodecylbenzene sulfonic acid, alkyl sufonic acids, aryl sulfonic
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
acids, formic acid, phosphoric acid, blends of acidic acid and N-
methylpyrrolidone, blends of butyl acetate and butanol, blends of aromatic
hydrocarbons, or mixtures thereof.
The rheology control agent of this invention can be combined with
the film forming coating system using a variety of methods. For example,
the rheology control agent can be added to the film forming coating
mixture as a solid in powder form. Agitation methods, known to those
skilled in the art, may be used to disperse, dissolve or distribute the
rheology control agent. The use of a high speed disperser has been found
to be a particularly effective dispersing technique at dispersing these
rheology control agents in a binder component and solvent of the coating
formulation. This dispersion is subsequently added to the other
components of the coating formulation to make the final coating.
Alternatively, the rheology control agent can be prepared directly using the
binder system of the film forming coating mixture as the reaction medium
using the general synthesis procedures outlined above.
Conventional rheology control agents have been produced in the
presence of a binder resin as shown, for example, in GB 1,454,414,
wherein a urea adduct is prepared in situ in the presence of the binder.
The rheology control agents of this invention may also be produced in the
presence of a binder to directly form a desired fibril structure in the binder
resin. The length of the fibrils can be adjusted as known to those skilled in
the art using, for example, a shear treatment, or by modifying the mixing
conditions.
The rheology control agents of this invention may also be prepared
following the outlined synthesis procedures directly from the starting
materials described above by using a non-solvent, which has a limited
solubility for the product. This strategy results in a precipitate that can be
used as such, be milled in order to reduce the average length, or be re-
crystallized, for example to increase the purity or to change the structural
morphology or fibril length. In the preparation process of the rheology
control agent the dosing conditions or the stirrer speed can be changed to
21
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
influence the average structure build or fibril formation.
Alternatively, the rheology control agents of this invention may also
be utilized in the form of their solutions at temperatures between 0 and
150 C and in a polar solvent optionally containing 0-3.0 mols of an
inorganic or organic acid compounds per urea group. The rheology
control agents of this invention as defined above have a solids content of
- 75 wt. % and preferably of 15 - 40 wt. %. These solutions of the
rheology control agent can be used as additives to a coating formulation.
Suitable solvents for this purpose are, for example, N-methyl pyrrolidone,
10 dimethyl acetamide, n-butanol, aliphatic diols, butyl glycol, acetic acids,
1-
ethyl-2- pyrrolidinone, 1-(2-hydroxyethyl)-2-pyrrolidinone, 1-cyclohexyl-2-
pyrrolidone, 1-ethyl-2-pyrrolidinone, 1-vinyl-2-pyrrolidone, 2-pyrrolidone-5-
carboxylic acid, 1,5-dimethyl-2-pyrrolidinone, 1-benzyl-2-pyrrolidinone and
mixtures thereof.
Optionally, inorganic or organic acid compounds can be added to
maximize the solids content and stability of these solutions. By stability is
meant no significant precipitation or gelation upon aging either at room
temperature or at elevated temperature (up to 50 C) storage. Preferred
inorganic compounds used in these solutions and are selected from LiCi,
LiBr, NaCI, KCI, CaC12, LiNO3, lithium acetate, lithium acetylacetonate,
tetraalkylammonium phosphate, organophosphate, LiOC(O)Me or other Li-
salts of carboxylic acids, sulfonic acids, benzoic acids or substituted
benzoic acids, with LiCi as the preferred inorganic compound. Preferred
organic acid compounds used in these solutions are selected from
dodecylbenzene sulfonic acid in isopropanol, p-toluene sulfonic acids and
trifluoromethane sulfonic acid. Surprisingly, it has been found that some
of these rheology control agents can be dissolved at high solids (>10% by
weight) in solvent without the use of these inorganic compounds or
organic acids.
The solvent-borne coating compositions and water-borne coating
compositions of this invention containing rheology control agents are
useful in a wide variety of applications, such as, clear coating
22
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
compositions, base coating compositions, pigmented mono coating
compositions, primer surfacers and primer fillers. Typical binders used in
these compositions are acrylic polymers, such as, poly(meth)acrylates,
meaning both polyacrylates and polymethacrylates, linear, branched,
grafted, or segmented poly(meth)acrylates, polyacrylourethanes,
polyesters, branched copolyesters, oligomers, e.g., urethane oligomers,
polyester urethanes, polyepoxides and carbamate functional polymers.
Typical crosslinking agents that may be used in these compositions are
polyisocyanates, blocked polyisocyanates, melamine crosslinking agents,
alkylated melamines, silanes, benzoguanamines and other crosslinking
agents known to those skilled in the art.
The acrylic polymers used to form coating compositions containing
the novel rheology control agent of this invention may be random polymers
or structured copolymers, such as, block or graft copolymers. Particularly
useful structured copolymers are the branched acrylics with segmented
arms as disclosed in U.S. Serial No.: 10/983,462 filed November 8, 2004
and U.S. Serial No. 101983,875 filed November 8, 2004, both of which are
incorporated herein by reference.
A block copolymer used in the present invention may have an AB
diblock structure, or ABA or ABC triblock structure, for example, Graft
copolymers can be used in the present invention having a backbone
segment and a side chain segment(s). Random copolymers that can be
used have polymer segments randomly distributed in the polymer chain.
Acrylic AB, ABA or ABC block copolymers can be prepared by
using a stepwise polymerization process such as anionic, group transfer
polymerization (GTP) taught in U.S. Patent No. 4,508,880, Webster et al.,
""Living" polymers and process for their preparation", atom transfer radical
polymerization (ATRP) taught in U.S. Patent No. 6,462,125, White et al.,
and radical addition fragmentation transfer (RAFT) taught in U.S. Patent
No. 6,271,340, Anderson, et al. "Method of controlling polymer molecular
weight and structure". Polymers so produced have precisely controlled
23
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
molecular weight, block sizes and very narrow molecular weight
distributions.
Aqueous coating compositions containing AB block copolymers as
pigment dispersants disclosed in Houze et al. U.S. Pat. No. 6,204,319,
which is hereby incorporated by reference, can utilize the novel rheology
control agents of this invention.
Graft copolymers may be prepared by a macromonomer approach
using the special cobalt chain transfer (SCT) method reported in U.S.
Patent, No. 6,472,463, Ma, the disclosure of which is herein incorporated
by reference.
Random copolymers can be prepared using conventional free
radical polymerization techniques as described in U.S. Patent, No.
6,451,950, Ma. The disclosure of which is herein incorporated by
reference.
Typically useful acrylic polymers have a number average molecular
weight of about 1,000 to 100,000, a Tg of 10 to 100 C and contain
moieties, such as, hydroxyl, carboxyl, glycidyl and amino groups.
Typically useful acrylic polymers are known in the art and the following are
typical examples of monomers used to form such polymers: linear alkyl
(meth)acrylates having 1 to 12 carbon atoms in the alkyl group, cyclic or
branched alkyl (meth)acrylates having 3 to 12 carbon atoms in the alkyl
group including isobornyl (meth)acrylate, hydroxy alkyl (meth)acrylates
having 1 to 4 carbon atoms in the alkyl group, glycidyl (meth)acrylate,
hydroxy amino alkyl (meth)acrylates having 1 to 4 carbon atoms in the
alkyl group, and the polymers can contain styrene, alpha methyl styrene,
vinyl toluene, (meth)acrylonitrile (meth)acryl amides, (meth)acrylic acid,
(meaning both acrylic acid and methacrylic acid) trim ethoxys i lyl propyl
(meth)acrylate and the like.
Examples of (meth)acrylic acid esters useful for forming these
acrylic polymers are methyl acrylate, ethyl acrylate, isopropyl acrylate,
tert.-butyl acrylate, n-butyl acrylate, isobutyl acrylate, 2-ethylhexyl
acrylate,
lauryl acrylate, stearyl acrylate and the corresponding methacrylates.
24
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
Examples of (meth)acrylic acid esters with cyclic alcohols are cyclohexyl
acrylate, trimethylcyclohexyl acrylate, 4-tert.-butylcyclohexyl acrylate,
isobornyl acrylate and the corresponding methacrylates.
Additional unsaturated monomers that do not contain additional
functional groups useful for forming the acrylic polymers are, for example,
vinyl ethers, such as, isobutyl vinyl ether and vinyl esters, such as, vinyl
acetate, vinyl propionate, vinyl aromatic hydrocarbons, preferably those
with 8 to 9 carbon atoms per molecule. Examples of such monomers are
styrene, alpha-methylstyrene, chlorostyrenes, 2,5-dimethylstyrene, p-
methoxystyrene, vinyl toluene. Styrene is preferably used.
Small proportions of olefinically polyunsaturated monomers may
also be used. These are monomers having at least 2 free-radically
polymerizable double bonds per molecule. Examples of these are
divinylbenzene, 1,4-butanediol diacrylate, 1,6-hexanediol diacryiate,
neopentyl glycol dimethacrylate, glycerol dimethacrylate.
Hydroxy-functional (meth)acrylic polymers generally are formed by
free-radical copolymerization using conventional processes well known to
those skilled in the art, for example, bulk, solution or bead polymerization,
in particular by free-radical solution polymerization using free-radical
initiators.
Suitable hydroxyl-functional unsaturated monomers that are used to
introduce hydroxyl groups into the acrylic polymer are, for example,
hydroxyalkyl esters of alpha,beta-olefinically unsaturated monocarboxylic
acids with primary or secondary hydroxyl groups. These may, for
example, comprise the hydroxyalkyl esters of acrylic acid, methacrylic
acid, crotonic acid and/or isocrotonic acid. The hydroxyalkyl esters of
(meth)acrylic acid are preferred. Examples of suitable hydroxyalkyl esters
of alpha,beta-olefinically unsaturated monocarboxylic acids with primary
hydroxyl groups are hydroxyethyl (meth)acrylate, hydroxypropyl
(meth)acrylate, hydroxybutyl (meth)acrylate, hydroxyamyl (meth)acrylate,
hydroxyhexyl (meth)acrylate. Examples of suitable hydroxyalkyl esters
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
with secondary hydroxyl groups are 2-hydroxypropyl (meth)acrylate, 2-
hydroxybutyl (meth)acrylate, 3-hydroxybutyl (meth)acrylate.
Preferred are hydroxy functional acrylic polymers having a hydroxy
equivalent weight of 300 to 1300 and are polymers of hydroxy alkyl
(meth)acrylates and one or more of the aforementioned monomers. The
hydroxyl equivalent weight is the grams of resin per equivalent of hydroxyl
groups. The following are typically preferred acrylic polymers:
styrene/methyl methacrylate/isobutyl methacrylate/hydroxyethyl
(meth)acrylate; styrene/methyl methacrylate/isobutyl methacrylate/2-
ethylhexyl methacrylate/isobornyl methacrylate/hydroxyethyl
(meth)acrylate and styrene/isobornyl methacrylate/2-ethylhexyl
methacrylate/hydroxy propyl methacrylate/hydroxyethyl (meth)acrylate.
One particularly preferred hydroxy containing acrylic polymer contains 35
to 50 percent by weight styrene, 15 to 25 percent by weight ethylhexyl
methacrylate and 15 to 20 percent by weight isobornyl methacrylate and
to 30 percent by weight hydroxyethyl methacrylate.
Additional useful hydroxy-functional unsaturated monomers are
reaction products of alpha,beta-unsaturated monocarboxylic acids with
glycidyl esters of saturated monocarboxylic acids branched in alpha
20 position, for example with glycidyl esters of saturated alpha-
alkylalkanemonocarboxylic acids or alpha,alpha'-
dialkylalkanemonocarboxylic acids. These preferably comprise the
reaction products of (meth)acrylic acid with glycidyl esters of saturated
alpha,alpha-dialkylalkanemonocarboxylic acids with 7 to 13 carbon atoms
per molecule, particularly preferably with 9 to 11 carbon atoms per
molecule. These reaction products may be formed before, during or after
the copolymerization reaction.
Further usable hydroxy-functional unsaturated monomers are
reaction products of hydroxyalkyl (meth)acrylates with lactones.
Hydroxyalkyl (meth)acrylates which may be used are, for example, those
stated above. Suitable lactones are, for example, those that have 3 to 15
carbon atoms in the ring, wherein the rings may also comprise different
26
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
substituents. Preferred lactones are gamma-butyrolactone, delta-
valerolactone, epsilon-caprolactone, beta-hydroxy-beta-methyl-delta-
valerolactone, lambda-laurolactone or mixtures thereof. Epsilon-
caprolactone is particularly preferred. The reaction products preferably
comprise those prepared from 1 mole of a hydroxyalkyl ester of an
alpha,beta-unsaturated monocarboxylic acid and 1 to 5 moles, preferably
on average 2 moles, of a lactone. The hydroxyl groups of the hydroxyalkyl
esters may be modified with the lactone before, during or after the
copolymerization reaction.
Suitable unsaturated monomers that can be used to provide the
acrylic polymer with carboxyl groups are, for example, olefinically
unsaturated monocarboxylic acids, such as, for example, acrylic acid,
methacrylic acid, crotonic acid, isocrotonic acid, itaconic acid. Acrylic acid
and methacrylic acid are preferably used.
Suitable unsaturated monomers that can be used to provide the
acrylic polymer with glycidyl groups are, for example, allyl glycidyl ether,
3,4-epoxy-1-vinylcyclohexane, epoxycyclohexyl (meth)acrylate, vinyl
glycidyl ether and glycidyl (meth)acrylate. Glycidyl (meth)acrylate is
preferably used.
Free-radically polymerizable, olefinically unsaturated
monomers which, apart from at least one olefinic double bond, do not
contain additional functional groups that can be used to form the acrylic
polymer are, for example, esters of unsaturated carboxylic acids with
aliphatic monohydric branched or unbranched as well as cyclic alcohols
with I to 20 carbon atoms. The unsaturated carboxylic acids, which may
be considered, are acrylic acid, methacrylic acid, crotonic acid and
isocrotonic acid. Esters of (meth)acrylic acid are preferred.
The acrylic polymer can contain (meth)acrylamides. Typical
examples of such acrylic polymers are polymers of (meth)acrylamide and
alkyl (meth)acrylates, hydroxy alkyl (meth)acrylates, (meth)acrylic acid and
or one of the aforementioned ethylenically unsaturated polymerizable
monomers.
27
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
Acrylic oligomers having a number average molecular weight of
300 to 3,000 of the aforementioned monomeric components also can be
used as an optional polymeric component. Useful acrylic oligomers are
disclosed in U.S. Serial No. 10/617,585 filed July 11, 2003. By using
monomers and reactants well known to those skilled in the art, these
oligomers can have the one or more of the following groups that are
reactive with isocyanate: hydroxyl, carboxyl, glycidyl, amine, aldimine,
phosphoric acid and ketimine.
Acrylourethanes also can be used to form the novel coating
composition of this invention. Typical useful acrylourethanes are formed by
reacting the aforementioned acrylic polymers with an organic
polyisocyanate. Generally, an excess of the acrylic polymer is used so that
the resulting acrylourethane has terminal acrylic segments having reactive
groups as described above. These acrylourethanes can have reactive end
groups and/or pendant groups such as hydroxyl, carboxyl, amine, glycidyl,
amide, silane or mixtures of such groups. Useful organic polyisocyanates
are described hereinafter as the crosslinking component but also can be
used to form acrylourethanes useful in this invention. Typically useful
acrylourethanes are disclosed in Stamegna et al. U.S. Patent 4,659,780,
which is hereby incorporated by reference.
Polyesters can also be used, such as, hydroxyl or carboxyl
terminated or hydroxyl or carboxyl containing polyesters. The following
are typically useful polyesters or ester oligomers: polyesters or oligomers
of caprolactone diol and cyclohexane dimethylol, polyesters or oligomers
of tris-hydroxy ethylisocyanurate and caprolactone, polyesters or
oligomers of trimethylol propane, phthalic acid or anhydride and ethylene
oxide, polyesters or oligomers of pentaerythritol, hexahydrophthalic
anhydride and ethylene oxide, polyesters or oligomers of pentaerythritol,
hexahydrophthalic anhydride and butylene oxide as disclosed in U.S.
6,221,484 B1.
The aforementioned polyesters and oligomers can be reacted with
an organic isocyanate to form polyesterurethane polymers and oligomers
28
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
that can be used in the novel composition.
One useful polyesterurethane that can used in the composition is
formed by reacting an aliphatic polyisocyanate with an aliphatic or
cycloaliphatic monohydric alcohol and subsequently reacting the resulting
composition with a hydroxy functional aliphatic carboxylic acid until all of
the isocyanate groups have been reacted. One useful polyurethane
oligomer comprises the reaction product of the isocyanurate of hexane
diisocyanate, cyclohexanol and dimethylol propionic acid.
Useful branched copolyesters polyols and the preparation thereof
are described in WO 03/070843 published August 28, 2003, which is
hereby incorporated by reference.
The branched copolyester polyol has a number average molecular
weight not exceeding 30,000, alternately in the range of from 1,000 to
30,000, further alternately in the range of 2,000 to 20,000, and still further
alternately in the range of 5,000 to 15,000. The copolyester polyol has
hydroxyl groups ranging from 5 to 200 per polymer chain, preferably 6 to
70, and more preferably 10 to 50, and carboxyl groups ranging from 0 to
40 per chain, preferably 1 to 40, more preferably 1 to 20 and most
preferably I to 10. The Tg (glass transition temperature) of the
copolyester polyol ranges from -70 C to 50 C, preferably from - 65 C to
40 C, and more preferably from -60 C to 30 C.
The branched copolyester polyol is conventionally polymerized from
a monomer mixture containing a chain extender selected from the group
consisting of a hydroxy carboxylic acid, a lactone of a hydroxy carboxylic
acid and a combination thereof; and one or more hyper branching
monomers.
The following additional ingredients can be included in the coating
composition, particularly when the coating composition is useful as a
lacquer, in amounts of 0.1 % to 98% by weight and alternately in the range
of 50% to 95% by weight, all based on the weight of the binder of the
coating composition.
29
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
Useful acrylic alkyd polymers having a weight average molecular
- weight ranging from 3,000 to 100,000 and a Tg ranging from 0 C to 100 C
are conventionally polymerized from a monomer mixture that can include
one or more of the following monomers: an alkyl (meth)acrylate, for
example, methyl (meth)acrylate, butyl (meth)acrylate, ethyl (meth)acrylate,
2-ethyl hexyl (meth)acrylate; a hydroxy alkyl (meth)acrylate, for example,
hydroxy ethyl (meth)acrylate, hydroxy propyl (meth)acrylate, hydroxy butyl
(meth)acrylate; (meth)acrylic acid; styrene; and alkyl amino alkyl
(meth)acrylate, for example, diethylamino ethyl (meth)acrylate or t-butyl
aminoethyl methacrylate; and one or more of the following drying oils: vinyl
oxazoline drying oil esters of linseed oil fatty acids, tall oil fatty acids
or
tung oil fatty acids.
One preferred polymer is polymerized from a monomer mixture that
contains an alkyl (meth)acrylate, hydroxy alkyl acrylate, alkylamino alkyl
acrylate and vinyl oxazoline ester of drying oil fatty acids.
Suitable iminiated acrylic polymers can be obtained by reacting
acrylic polymers having carboxyl groups with an alkylene imine, such as
propylene imine.
Suitable cellulose acetate butyrates are supplied by Eastman
Chemical Co., Kingsport, Tennessee under the trade names CAB-381-20
and CAB-531-1 and are preferably used in an amount of 0.1 to 20 percent
by weight based on the weight of the binder.
A suitable ethylene-vinyl acetate co-polymer (wax) is supplied by
Honeywell Specialty Chemicals - Wax and Additives, Morristown, New
Jersey, under the trade name A-Ce 405 (T) Ethylene - Vinyl Acetate
Copolymer.
Suitable nitrocellulose resins preferably have a viscosity of about
1/2-6 seconds. Preferably, a blend of nitrocellulose resins is used.
Optionally, the lacquer can contain ester gum and castor oil.
Suitable alkyd resins are the esterification products of a drying oil
fatty acid, such as linseed oil and tall oil fatty acid, dehydrated castor
oil, a
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
polyhydric alcohol, a dicarboxylic acid and an aromatic monocarboxylic
acid. Typical polyhydric alcohols that can be used to prepare the alkyd
resin used in this invention are glycerine, pentaerythritol, trimethylol
ethane, trimethylol propane; glycols, such as ethylene glycol, propylene
glycol, butane diol and pentane diol. Typical dicarboxylic acids or
anhydrides that can be used to prepare the alkyd resin are phthalic acid,
phthalic anhydride, isophthalic acid, terephthalic acid maleic, and fumaric
acid. Typical monocarboxylic aromatic acids are benzoic acid, paratertiary
butylbenzoic acid, phenol acetic acid and triethyl benzoic acid. One
preferred alkyd resin is a reaction product of an acrylic polymer and an
alkyd resin.
Useful carbamate containing polymers are disclosed in U.S. Patent
Application Publication 2003/0050388, which is hereby incorporated by
reference and in particular discloses a carbamate polymer comprises the
reaction product of an aliphatic polyisocyanate, a monohydric alcohol, a
hydroxyfunctional aliphatic carboxylic acid and a polyalkylene ether glycol
and has a number average molecular weight in the range of 100 to 2000.
Other useful carbamate functional polymers are disclosed in Ramesh et al.
U.S. Patent 6,462,144 B1, which is hereby incorporated by reference and
shows a carbamate functional polymer having a hyperbranched or star
polyol core, a first chain extension based on a polycarboxylic acid or
anhydride, a second chain extension based on an epoxy containing
compound, and having carbamate functional groups on the core, the
second chain extension or both. Acrylic polymers having primary
functional carbamate functionality are useful and are disclosed in U.S.
Patent 5,866,259, which is hereby incorporated by reference.
Suitable plasticizers include butyl benzyl phthalate, dibutyl
phthalate, triphenyl phosphate, 2-ethylhexylbenzyl phthalate, dicyclohexyl
phthalate, diallyl toluene phthalate, dibenzyl phthalate, butylcyclohexyl
phthalate, mixed benzoic acid and fatty oil acid esters of pentaerythritol,
poly(propylene adipate) dibenzoate, diethylene glycol dibenzoate,
tetrabutylthiodisuccinate, butyl phthalyl butyl glycolate, acetyltributyl
31
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
citrate, dibenzyl sebacate, tricresyl phosphate, toluene ethyl sulfonamide,
the di-2-ethyl hexyl ester of hexamethylene diphthalate, and di(methyl
cyclohexyl) phthalate. One preferred plasticizer of this group is butyl
benzyl phthalate.
If desired, the coating composition can include metallic driers,
chelating agents, or a combination thereof. Suitable organometallic driers
include cobalt naphthenate, copper naphthenate, lead tallate, calcium
naphthenate, iron naphthenate, lithium naphthenate, lead naphthenate,
nickel octoate, zirconium octoate, cobalt octoate, iron octoate, zinc
octoate, and alkyl tin dilaurates, such as dibutyl tin dilaurate. Suitable
chelating agents include aluminum monoisopropoxide monoversatate,
aluminum (monoiospropyl)phthalate, aluminum diethoxyethoxide
monoversatate, aluminum trisecondary butoxide, aluminum diisopropoxide
monoacetacetic ester chelate and aluminum isopropoxide.
Also, polytrimethylene ether diols may be used as an additive
having a number average molecular weight (Mn) in the range of from 500
to 5,000, alternately in the range of from 1,000 to 3,000; a polydispersity in
the range of from 1.1 to 2.1 and a hydroxyl number in the range of from 20
to 200. The preferred polytrimethylene ether diol has a Tg of -75 C.
Copolymers of polytrimethylene ether diols are also suitable. For
example, such copolymers are prepared by copolymerizing 1,3-
propanediol with another diol, such as, ethane diol, hexane diol, 2-methyl-
1,3-propanediol, 2,2-dimethyl-1,3-propanediol, trimethylol propane and
pentaerythritol, wherein at least 50 percent of the copolymer results from
1,3-propanediol. A blend of a high and low molecular weight
polytrimethylene ether diol can be used wherein the high molecular weight
diol has an Mn ranging from 1,000 to 4,000 and the low molecular weight
diol has an Mn ranging from 150 to 500. The average Mn of the diol
should be in the range of 1,000 to 4,000. It should be noted that, the
polytrimethylene ether diols suitable for use in the present invention can
include polytrimethylene ether triols and other higher functionality
polytrimethylene ether polyols in an amount ranging from 1 to 20%, by
32
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
weight, based on the weight of the polytrimethylene ether diol. It is
believed that the presence of polytrimethylene ether diols in the
crosslinked coating composition of this invention can improve the chip
resistance of a coating resulting therefrom.
Additional details of the foregoing additives are provided in U.S.
Patent, 3,585,160, U.S. Patent 4,242,243, U.S. Patent 4,692,481, and
U.S. Re 31.309, which are incorporated therein by reference.
Crosslinking Acgents
Lacquer coating compositions can be formulated without the use of
a crosslinking agent. Typical crosslinkable compositions that utilize the
novel rheology control agents are solvent borne or water borne
compositions having a binder containing in the range of 25-95 percent by
weight of the aforementioned film forming polymer and 5-75 percent by
weight of a crosslinking agent. Preferably, the binder contains in the
range of 40-90 percent by weight of the film forming polymer and 10-60
percent by weight of the crosslinking agent. Useful crosslinking agents
include organic polyisocyanates, blocked organic polyisocyanates,
melamines, alkylated melamines, benzoquanamines, epoxides and
silanes
Typically useful organic polyisocyanates crosslinking agents that
can be used in the novel composition of this invention include aliphatic
polyisocyanates, cycioaliphatic polyisocyanates and isocyanate adducts.
Typical polyisocyanates can contain within the range of 2 to 10, preferably
2.5 to 8, more preferably 3 to 5 isocyanate functionalities. Generally, the
ratio of equivalents of isocyanate functionalities on the polyisocyanate per
equivalent of all of the functional groups present ranges from 0.5/1 to
3.0/1, preferably from 0.7/1 to 1.8/1, more preferably from 0.8/1 to 1.3/1.
Examples of suitable aliphatic and cycloaliphatic polyisocyanates
that can be used include the following: 4,4'dicyclohexyl methane
diisocyanate, ("H12MDI"), trans-cyclohexane-1,4-diisocyanate, 1,6-
hexamethylene diisocyanate ("HDI"), isophorone diisocyanate,("IPDI"),
other aliphatic or cycloaliphatic di-, tri- or tetra-isocyanates, such as, 1,2-
33
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
propylene diisocyanate, tetramethylene diisocyanate, 2,3-butylene
diisocyanate, octamethylene diisocyanate, 2,2,4-trimethyl hexamethylene
diisocyanate, dodecamethylene diisocyanate, omega-dipropyl ether
diisocyanate, 1,3-cyclopentane diisocyanate, 1,2 cyclohexane
diisocyanate, 1,4 cyclohexane diisocyanate, 4-methyl-1,3-
diisocyanatocyclohexane, dicyclohexylmethane-4,4'-diisocyanate, 3,3'-
dimethyl-dicyclohexylmethane 4,4'-diisocyanate, polyisocyanates having
isocyanurate structural units, such as, the isocyanurate of hexamethylene
diisocyanate and the isocyanurate of isophorone diisocyanate, the adduct
of 2 molecules of a diisocyanate, such as, hexamethylene diisocyanate,
uretidiones of hexamethylene diisocyanate, uretidiones of isophorone
diisocyanate and a diol, such as, ethylene glycol, the adduct of 3
molecules of hexamethylene diisocyanate and 1 molecule of water,
allophanates, trimers and biurets of hexamethylene diisocyanate,
allophanates, trimers and biurets of isophorone diisocyanate and the
isocyanurate of hexane diisocyanate.
Tri-functional isocyanates also can be used, such as, Desmodur
N 3300, trimer of hexamethylene diisocyanate, Desmodur 3400, trimer of
isophorone diisocyanate, Desmodur 4470 trimer of isophorone
diisocyanate, these trimers are sold by Bayer Corporation. A trimer of
hexamethylene diisocyanate sold as Tolonate HDT from Rhodia
Corporation is also suitable.
An isocyanate functional adduct can be used, such as, an adduct of
an aliphatic polyisocyanate and a polyol. Also, any of the aforementioned
polyisocyanates can be used with a polyol to form an adduct. Polyols,
such as, trimethylol alkanes, particularly, trimethylol propane or ethane
can be used to form an adduct.
The melamine crosslinking agents are generally partially alkylated
melamine formaldehyde compounds and may be monomeric or polymeric
or mixtures thereof. Some of the suitable monomeric melamines include
low molecular weight melamines which contain, on an average, three or
more methylol groups etherized with a C, to C5 monohydric alcohol such
34
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
as methanol, n-butanol, or isobutanol per triazine nucleus, and have an
average degree of condensation up to about 2 and preferably in the-range
of about 1.1 to about 1.8, and have a proportion of mononuclear species
not less than about 50 percent by weight. By contrast the polymeric
melamines have an average degree of condensation of more than 1.9.
Some such suitable monomeric melamines include alkylated
melamines, such as methylated, butylated, isobutylated melamines and
mixtures thereof. Many of these suitable monomeric melamines are
supplied commercially. For example, Cytec Industries Inc., West
Patterson, New Jersey supplies Cymel 301 (degree of polymerization of
1.5, 95% methyl and 5% methylol), Cymel" 350 (degree of polymerization
of 1.6, 84 percent methyl and 16 percent methylol), 303, 325, 327 and
370, which are all monomeric me)amines. Suitable polymeric melamines
include high amino (partially alkylated) melamine known as Resimene
BMP5503 (molecular weight 690, polydispersity of 1.98, 56 percent butyi,
44 percent amino), which is supplied by Solutia Inc., St. Louis, Missouri, or
Cymel 1158 provided by Cytec Industries Inc., West Patterson, New
Jersey. Cytec Industries Inc. also supplies Cymel 1130 @ 80 percent
solids (degree of polymerization of 2.5), Cymel 1133 (48 percent methyl,
4 percent methylol and 48 percent butyl), both of which are polymeric
melamines.
If desired, appropriate catalysts may also be included in the
activated compositions to accelerate the curing process of a potmix of the
coating composition.
When the activated compositions include melamine as the
crosslinking agent, it also preferably includes a catalytically active amount
of one or more acid catalysts to further enhance the crosslinking of the
components on curing. Generally, catalytically active amount of the acid
catalyst in the coating composition ranges from about 0.1 percent to about
5 percent, preferably ranges from 0.1 percent to 2 percent, more
preferably ranges from 0.5 percent to 1.2 percent, all in weight percent
based on the weight of the binder. Some suitable acid catalysts include
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
aromatic sulfonic acids, such as dodecylbenzene sulfonic acid, para-
toluenesulfonic acid and dinonylnaphthalene sulfonic acid, all of which are
either unblocked or blocked with an amine, such as dimethyl oxazolidine
and 2-amino-2-methyl-l-propanol, n,n-dimethylethanolamine or a
combination thereof. Other acid catalysts that can be used, such as
phosphoric acids, more particularly, phenyl acid phosphate, benzoic acid,
oligomers having pendant acid groups, all of which may be unblocked or
blocked with an amine.
When the activated compositions include a polyisocyanate as the
crosslinking agent, the coating composition preferably includes a
catalytically active amount of one or more tin or tertiary amine catalysts for
accelerating the curing process. Generally, catalytically active amount of
the catalyst in the coating composition ranges from about 0.001 percent to
about 5 percent, preferably ranges from 0.005 percent to 2 percent, more
preferably ranges from 0.01 percent to 1 percent, all in weight percent
based on the weight of the binder. A wide variety of catalysts can be
used, such as, tin compounds, including dibutyl tin dilaurate and dibutyl tin
diacetate; tertiary amines, such as, triethylenediamine. These catalysts
can be used alone or in conjunction with carboxylic acids, such as, acetic
acid. One of the commercially available catalysts, sold under the
trademark, Fastcat 4202 dibutyl tin dilaurate by Elf-Atochem North
America, Inc. Philadelphia, Pennsylvania, is particularly suitable.
Carrier Medium
The liquid carrier medium comprises an organic solvent or blend of
solvents or an aqueous carrier comprising water and optionally,
compatible organic solvents. The coating compositions contain about 5-
95 percent, more typically 10-85 percent by weight of solvent, and about
5-95 percent, more typically 15-90 percent by weight, of an organic liquid
carrier (based on the weight of the coating composition). The selection of
organic solvent depends upon the requirements of the specific end use
application of the coating composition of this invention, such as the VOC
emission requirements, the selected pigments, binder and crosslinking
36
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
agents. Representative examples of organic solvents which are useful
herein include alcohols, such as methanol, ethanol, n-propanol, and
isopropanol; ketones, such as acetone, butanone, pentanone, hexanone,
and methyl ethyl ketone, methyl isobutyl ketone, diisobutyl ketone, methyl
amyl ketone; alkyl esters of acetic, propionic, and butyric acids, such as
ethyl acetate, butyl acetate, and amyl acetate; ethers, such as
tetrahydrofuran, diethyl ether, and ethylene glycol and polyethylene glycol
monoalkyl and dialkyl ethers, such as cellosolves and carbitols; and
glycols, such as ethylene glycol and propylene glycol and mixtures
thereof, and aromatic hydrocarbon solvents, such as xylene, toluene.
Typically, aqueous carriers comprise water and a blend of organic
solvents suited for the requirements of the coating composition.
Pigments
The novel coating composition may be used as a base coat or as a
pigmented mono-coat topcoat. Both of these compositions require the
presence of pigments. Typically, a pigment-to-binder ratio of 0.1/100 to
200/100 is used depending on the color and type of pigment used. The
pigments are formulated into mill bases by conventional procedures, such
as, grinding, sand milling, ball milling, high speed mixing, attritor grinding
and two or three roll milling. Generally, the mill base comprises pigment
and a dispersant in a liquid carrier. The mill base is added in an
appropriate amount to the coating composition with mixing to form a
pigmented coating composition.
Any of the conventionally-used organic and inorganic pigments,
such as, white pigments, like, titanium dioxide, color pigments, metallic
flakes, such as, aluminum flake, special effects pigments, such as, coated
mica flakes, coated aluminum flakes and the like, azo, anthraquinone,
thioindigo, oxazine, quinacridone, lakes and toners of acidic dye stuffs,
copper phthalocyanine and its derivatives, and various mixtures and
modifications thereof and extender pigments can be used.
The novel coating composition may be used as a primer, primer
surfacer, or sealer in which case typical pigments used in primers would
37
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
be added, such as, carbon black, barytes, silica, iron oxide and other
pigments that are commonly used in primers in a pigment-to-binder ratio of
10/100 to 300/100.
Coating Compositions and Additives to Improve Weatherability
The coating composition formulated with the novel rheology control
agent of this invention can be used as a clear coat that is applied over a
pigmented base coat that may be a pigmented version of the composition
of this invention or another type of a pigmented base coat. The clear
coating can be in solution or in dispersion form.
Typically, a clear coating is applied over the base coating before
the base coating is fully cured. This is a so called "wet-on-wet process".
In this process, a base coating is applied to a substrate and flash dried
and then the clear coating is applied and both layers are then fully cured
either at ambient temperatures or cured by heating to elevated
temperatures, for example, of 50 C to 150 C for 15 to 45 minutes to form
a clear coat/base coat finish. When used in combination with a primer or
primer-surfacer, the primer or primer-surfacer is also flash dried and then
the base coating and clear coating are applied as above. This is a so-
called "wet on wet on wet" process. The base coating and clear coating
preferably have a dry coating thickness ranging from 25 to 75 microns and
to 100 microns, respectively.
When refinishing automobile and truck bodies, the original OEM
topcoat is usually sanded and a primer or sealer coat applied and then a
mono coat or a basecoat/clear coat is applied. These coatings are usually
25 cured at ambient temperatures or at slightly elevated temperatures, such
as, 40 to 100 C.
To improve the weathering properties of clear coatings, the novel
coating composition contains about 0.1 to 5 percent by weight, based on
the weight of the binder, of ultraviolet light absorbers. Typically useful
ultraviolet light absorbers include hydroxyphenyl benzotriazols, such as, 2-
(2-hyd roxy-5-m ethyl phenyl)-2H -benzotrazole, 2-(2-hydroxy-3,5-di-
38
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
tert.amyl-phenyl)-2H-benzotriazole, 2[2-hydroxy-3,5-di(1,1-
dimethy{benzyl)phenyl]-2H-benzotriazole, reaction product of 2-(2-
hydroxy-3-tert.butyl-5-methyl propionate)-2H-benzotriazole and
polyethylene ether glycol having a weight average molecular weight of
300, 2-(2-hydroxy-3-tert.butyl-5-iso-octyl propionate)-2H-benzotriazole;
hydroxyphenyl s-triazines, such as, 2-[4((2,-hydroxy-3-
dodecyloxy/tridecyloxypropyl)-oxy)-2-hydroxyphenyl]-4, 6-b is(2,4-
dimethylphenyl)-1,3,5-triazine, 2-[4(2-hydroxy-3-(2-ethylhexyl)-oxy)-2-
hydroxyphenyl]-4,6-bis(2,4-dimethylphenyl)1,3,5-triazine, 2-(4-octyloxy-2-
hydroxyphenyl)-4,6-bis(2,4-dimethylphenyl)-1,3,5-triazine;
hydroxybenzophenone U.V. absorbers, such as, 2,4-
dihydroxybenzophenone, 2-hydroxy-4-octyloxybenzophenone, and 2-
hydroxy-4-dodecyloxybenzophenone.
These clear coating compositions also may contain about 0.1 to 5
percent by weight, based on the weight of the binder, of a di- substituted
phenol antioxidant or a hydroperoxide decomposer. Typically useful
antioxidants include tetrakis[methylene(3,5-di-tert-butylhydroxy
hydrocinnamate)]methane, octadecyl 3,5-di-tert-butyl-4-
hydroxyhydrocinnamate, tris(2,4-di-tert-butylphenyl) phosphite, 1,3,5-
tris(3,5-di-tert-butyl-4-hydroxybenzyl)-1,3,5-triazine-2,4,6(1 H,3H,5H)-trione
and benzenepropanoic acid, 3,5-bis(1,1-dimethyl-ethyl)-4-hydroxy-C7-C9
branched alkyl esters. Typically useful hydroperoxide decomposers
include Sanko HCA (9,10-dihydro-9-oxa-10-phosphenanthrene-10-
oxide), triphenyl phosphate and other organo-phosphorous compounds,
such as, Irgafos TNPP from Ciba Specialty Chemicals, Irgafos 168,
from Ciba Specialty Chemicals, Ultranox 626 from GE Speciafty
Chemicals, Mark PEP-6 from Asahi Denka, Mark HP-10 from Asahi
Denka, Irgafos P-EPQ from Ciba Specialty Chemicals, Ethanox 398 from
Albemarle, Weston 618 from GE Specialty Chemicals, Irgafos 12 from
Ciba Specialty Chemicals, Irgafos 38 from Ciba Specialty Chemicals,
Ultranox 641 from GE Specialty Chemicals and Doverphos S-9228
from Dover Chemicals.
39
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
These clear coating compositions also may contain about 0.1-5
percent by weight, based on the weight of the binder, of hindered amine
light stabilizers. Typically useful hindered amine light stabilizers include N-
(1,2,2,6,6-pentamethyl-4-piperidinyl)-2-dodecyl succinimide, N(1 acetyl-
2,2,6,6-tetramethyl-4-piperidinyl)-2-dodecyl succinimide, N-
(2hydroxyethyl)-2,6,6,6-tetramethylpiperidine-4-ol-succinic acid copolymer,
1,3,5 triazine-2,4,6-triamine, N,N"'-[1,2-ethanediybis[[[4,6-
bis[butyl(1,2,2,6,6-pentamethyl-4-piperidinyl)amino]-1,3,5-triazine-2-
yl]imino]-3,1-propanediyl]]bis[N, N"'-dibutyl-N',N"'-bis(1,2,2,6,6-
pentamethyl-4-piperidinyl)], poly-[[6-[1,1,3,3-tetramethylbutyl)-amino]-
1,3,5-trianzine-2,4-diyl][2,2,6,6-tetramethylpiperidinyl)-imino]-1,6-hexane-
diyl[(2,2,6,6-tetramethyl-4-piperidinyl)-imino]), bis(2,2,6,6-tetramethyl-4-
piperidinyl)sebacate, bis(1,2,2,6,6-pentamethyl-4-piperidinyl)sebacate,
bis(1-octyloxy-2,2,6,6-tetramethyl-4-piperidinyl)sebacate, bis(1,2,2,6,6-
pentamethyl-4-piperidinyl)[3,5bis(1,1-dimethylethyl-4-hydroxy-
phenyl)methyl]butyl propanedioate, 8-acetyl-3-dodecyl-7,7,9,9,-
tetramethyl-1,3,8-triazaspiro(4,5)decane-2,4-dion, dodecyl/tetradecyl-3-
(2,2,4,4-tetramethyl-2l-oxo-7-oxa-3,20-diazal dispiro(5.1.11.2)henicosan-
20-yl)propionate.
Other Additives
In addition, coating composition utilizing the rheology control agent
of this invention may contain a variety of other optional ingredients,
including fillers, plasticizers, antioxidants, surfactants and flow control
agents.
For example, the novel composition can contain 0.1 to 30 percent
by weight, based on the weight of the binder, of acrylic NAD (non-aqueous
dispersed) resins. These NAD resins typically are high molecular weight
resins having a crosslinked acrylic core with a Tg between 20 to 100 C
and attached to the core are low Tg stabilizer segments. A description of
such NADs is found in Antonelli et al. U.S. Patent 4,591,533 and in
Barsotti et al. U.S. Patent 5,763,528 which patents are hereby
incorporated by reference.
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
Also, these coating composition may include other conventional
formulation additives known to those skilled in the art, such as, wetting
agents, leveling and flow control agents, for example, Resiflow S
(polybutylacrylate), BYK 320 and 325 (high molecular weight
polyacrylates), BYK 347 (polyether-modified siloxane), rheology control
agents, such as, fumed silica, defoamers, surfactants and emulsifiers to
help stabilize the composition. Other additives that tend to improve mar
resistance can be added, such as, silsesquioxanes and other silicate-
based micro-particles.
One particularly useful additive is a blend of the novel rheology
control agent and finely divided silica in a weight ratio of 0.1:1 to 1:0.1.
Other particularly useful additive is a blend of the novel rheology control
agent and bis-urea crystals as mentioned in U.S. 4,311,622 in a weight
ratio of 0.1:1 to 1:0.1.
The rheology control agent may be incorporated into one of the
components of a typical two component (2K) coating composition. For
example, in a typical 2K acrylic/isocyanate system, the rheology control
agent may be incorporated with the acrylic polymer component which is
then blended with the isocyanate component just before application.
Application
Coating composition containing the novel rheology control agent
can be applied by conventional techniques, such as, spraying,
electrostatic spraying, dipping, brushing, and flow coating. Spraying and
electrostatic spraying are preferred methods of application.
In OEM applications, the composition is typically baked at 60 -
150 C. for about 15-30 minutes to form a coating about 2.5 - 75 microns
thick. When the composition is used in a base coat/clear coat system, the
basecoat may be dried to a tack-free state and cured or preferably flash
dried for a short period before the clear coat is applied (wet-on-wet). The
base coat/clear coat finish is then baked as mentioned above to provide a
dried and cured finish. The novel coating composition can also be
41
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
formulated with the 3-wet (wet-on-wet-on-wet) coating process, where the
primer, basecoat and clearcoat are applied in sequential steps without
baking process in between each layer. The final coating is then baked to
provide a dried and cure finish. The present invention is also applicable to
non-baking refinish systems, as wili be readily appreciated by those skilled
in the art.
If used in refinishing vehicles, the base coat may be allowed to "dry
to the touch" at ambient temperature conditions or under warm air before
the clear coating is applied. The base coating and clear coating preferably
have a dry coating thickness ranging from 25 to 75 microns and 25 to 100
microns, respectively. These coatings are usually cured at ambient
temperatures or at slightly elevated temperatures, such as, 40 to 100 C.
These coating compositions are particularly useful for the repair
and refinish of automobile bodies and truck bodies and parts as a clear
coat, pigmented base coat, as a primer surfacer or primer filler. The novel
composition has uses for coating any and all items manufactured and
painted by automobile sub-suppliers, frame rails, commercial trucks and
truck bodies, including but not limited to beverage bottles, utility bodies,
ready mix concrete delivery vehicle bodies, waste hauling vehicle bodies,
and fire and emergency vehicle bodies, as well as any potential
attachments or components to such truck bodies, buses, farm and
construction equipment, truck caps and covers, commercial trailers,
consumer trailers, recreational vehicles, including but not limited to, motor
homes, campers, conversion vans, vans, large commercial aircraft and
small pleasure aircraft, pleasure vehicles, such as, snow mobiles, all
terrain vehicles, personal watercraft, motorcycles, and boats. The novel
composition also can be used as a coating for industrial and commercial
new construction and maintenance thereof; cement and wood floors; walls
of commercial and residential structures, such as, office buildings and
homes; amusement park equipment; concrete surfaces, such as parking
lots and drive ways; asphalt and concrete road surface, wood substrates,
marine surfaces; outdoor structures, such as bridges, towers; coil coating;
42
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
railroad cars; printed circuit boards; machinery; OEM tools; signs;
fiberglass structures; sporting goods; and sporting equipment.
The present invention is further defined in the following Examples.
It should be understood that these Examples are given by way of
illustration only. From the above discussion and these Examples, one
skilled in the art can ascertain the essential characteristics of this
invention, and without departing from the spirit and scope thereof, can
make various changes and modifications of the invention to adapt it to
various uses and conditions. As a result, the present invention is not
limited by the illustrative examples set forth herein below, but rather is
defined by the claims contained herein below.
The following Examples illustrate the invention. All parts and
percentages are on a weight basis unless otherwise indicated. All
molecular weights disclosed herein are determined by LC/MS (Liquid
Chromatography/Mass Spectroscopy).
EXAMPLES
Example 1
The following reaction was carried out under a blanket of nitrogen.
An organic diisocyanate of 2.28 g (0.0107 mol) of lysine diisocyanate
methyl ester was added drop-wise into a flask equipped with a stirrer
containing 2.75 g (0.0212 mol) octyl amine in 30 mL of chloroform and the
reaction mixture was held at room temperature for about 2 hours with
constant stirring. The reaction mixture was monitored with an IR
spectrophotometer and when the isocyanate peak (about 2258 cm"1)
disappeared the reaction was considered completed and the reaction
mixture was filtered to remove the product formed and any residual
solvent was removed under vacuum. The yield was 4.21 g, 84 percent
yield. The resulting rheology control agent had the following formula:
H H H H
Ny N\ V/ N___rN
O " O
OCH3
43
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
Example 2
The following reaction was carried out under a blanket of nitrogen.
An organic diisocyanate of 23.18 g(0.1092 mol) of lysine diisocyanate
methyl ester was added drop-wise into a flask equipped with a stirrer
containing 37.05 g (0.2163 moI) undecyl amine in 700 mL of chloroform
and the reaction mixture was held at room temperature for about 2 hours
with constant stirring. The reaction mixture was monitored with an IR
spectrophotometer and when the isocyanate peak (about 2258 cm"1)
disappeared the reaction was considered completed and the reaction
mixture was filtered to remove the product formed and any residual
solvent was removed under vacuum. The yield was 53.89 g, 89.8 percent
yield. The resulting rheology control agent had the following formula:
H H H H
NuN N\,N
IOI " leJ
OCH3
Example 3
The following reaction was carried out under a blanket of nitrogen.
An organic diisocyanate of 14.53 g (0.068 mol) of lysine diisocyanate
methyl ester was added drop-wise into a flask equipped with a stirrer
containing 25 g (0.1334 mol) N-cyclohexyl-(2-hydroxyethyl)urea and 0.08
g of dibutyltindilaurate in 340 mL of acetonitrile and the reaction mixture
was refluxed at 78 C for about 2 hours with constant stirring. The reaction
mixture was monitored with an IR spectrophotometer and when the
isocyanate peak (about 2258 cm"1) disappeared the reaction was
considered completed and the reaction mixture was filtered to remove the
product formed and any residual solvent was removed under vacuum.
The yield was 36.79 g, 94.3 percent yield. The resulting rheology control
agent had the following formula:
H H
aNyN--~O-IrNH N-F C,,~iNUN
O O O IOI ~
~O
44
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
Example 4
The following reaction was carried out under a blanket of nitrogen.
An organic diisocyanate of 2.01 g (0.0095 mol) of lysine diisocyanate
methyl ester was added drop-wise into a flask equipped with a stirrer
containing 4.03 g (0.0186 mol) N-hexyl-(2-hydroxyethyl)urea and 0.01 g of
dibutyltindilaurate in 50 mL of acetonitrile and the reaction mixture was
refluxed at 78 C for about 2 hours with constant stirring. The reaction
mixture was monitored with an IR spectrophotometer and when the
isocyanate peak (about 2258 cm"1) disappeared the reaction was
considered completed and the reaction mixture was filtered to remove the
product formed and any residual solvent was removed under vacuum.
The yield was 5.24 g, 87.3 percent yield. The resulting rheology control
agent had the following formula:
H H
NuN,./'~O-r N N---ONyN
IOI O p O
Me0 O
Example 5
The following reaction was carried out under a blanket of nitrogen.
An organic diisocyanate of 2.15 g (0.0101 mol) of lysine diisocyanate
methyl ester was added drop-wise into a flask equipped with a stirrer
containing 3.61 g(0.0199 mol) methyl 6-aminocaproate and 2.21 g of
triethylamine (0.0219 mol) in 50 mL of methylene chloride and the reaction
mixture was held at room temperature for about 2 hours with constant
stirring. The reaction mixture was monitored with an IR
spectrophotometer and when the isocyanate peak (about 2258 cm"1)
disappeared the reaction was considered completed and the reaction
mixture was filtered to remove the product formed and any residual
solvent was removed under vacuum. The yield was 4.23 g, 84.6 percent
yield. The resulting rheology control agent had the following formula:
O H H H H O
Ny N N__~, N O
O O
OCH3
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
Example 6
The following reaction was carried out under a blanket of nitrogen.
An organic diisocyanate of 2.27 g (0.0107 mol) of lysine diisocyanate
methyl ester was added drop-wise into a flask equipped with a stirrer
containing 3.52 g (0.0210 mol) ethyl 4-aminobutyrate hydrochloride and
2.34 g of triethylamine (0.0231 mol) in 50 mL of methylene chloride and
the reaction mixture was held at room temperature for about 2 hours with
constant stirring. The reaction mixture was monitored with an IR
spectrophotometer and when the isocyanate peak (about 2258 cm"1)
disappeared the reaction was considered completed and the reaction
mixture was filtered to remove the product formed and any residual
solvent was removed under vacuum. The yield was 3.67 g, 73.7 percent
yield. The resulting rheology control agent had the following formula:
N N N N
o " 0
OCH3
Example 7
The following reaction was carried out under a blanket of nitrogen.
An organic diisocyanate of 2.05 g (0.0097 mol) of lysine diisocyanate
methyl ester was added drop-wise into a flask equipped with a stirrer
containing 1.22 g (0.0095 mol) octylamine in 30 mL of acetonitrile and the
reaction mixture was held at room temperature for about 2 hours with
constant stirring. 10 mL of N-methylpyrolidinone (NMP) was added to the
reaction mixture followed by the addition of a solution containing 10 mL
NMP and 1.76 g N-cyclohexyl-(2-hydroxyethyl)urea (0.0095 mol). The
reaction mixture was refluxed at 80 C for 2 hours with constant stirring.
The reaction mixture was monitored with an lR spectrophotometer and
when the isocyanate peak (about 2258 cm"1) disappeared the reaction
was considered completed. The reaction mixture was precipitated out in
water and filtered to remove the product formed and any residual soivent
46
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
was removed under vacuum. The yield was 3.17 g, 63.4 percent yield.
The resulting rheology control agent had the following formula:
H H
NUN~j-O~N N~N
'OI O O
Me0 O
Example 8
The following reaction was carried out under a blanket of nitrogen.
1.60 g (9.995 mmol) of 1,6-diisocyanatohexane in 10 mL of chloroform
was added drop-wise into a flask containing 3.88 g (19.1 mmol) diethyl 2-
aminopentanedioate in 50 mL of chloroform. The flask was held at about
10 C during the addition and was equipped with a stirrer. Then the
reaction mixture was allowed to warm to room temperature and stirred for
about 2 hours. The reaction mixture was monitored with an IR
spectrophotometer and when the isocyanate peak (about 2258 cm"1)
disappeared the reaction was considered completed. The product was
collected by filtration and subsequently drying under vacuum to give a
white powder (5.4 g, 94.1 percent yield). The resulting rheology control
agent had the following formula:
N H
N H H~/~/~'
O
Example 9
The following reaction was carried out under a blanket of nitrogen.
12.61 g (75.0 mmol) of 1,6-diisocyanatohexane in 20 mL of toluene was
47
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
added dropwise into a flask containing 40.10 g 2-(2-methoxyethoxy)ethyl
2-amino-3-phenylpropanoate in 80 mL of toluene. The flask was held at
about 10 C during the addition. The flask was equipped with a stirrer.
The reaction mixture was allowed to warm to room temperature and stirred
for overnight. The reaction mixture was monitored with an IR
spectrophotometer and when the isocyanate peak (about 2258 cm"1)
disappeared the reaction was considered completed. The product was
collected by filtration and subsequently drying under vacuum to give a
white powder (45.1g, 85.6 percent yield). The resulting rheology control
agent had the following formula:
- N~ H H
MY N
H H O
O
r0
Example 10
The following reaction was carried out under a blanket of nitrogen.
1.26 g (7.50 mmol) of 1,6-diisocyanatohexane in 20 mL of toluene was
added drop-wise into a flask containing 3.5 g 2-(2-methoxyethoxy)ethyl 2-
amino-3-phenylpropanoate in 80 mL of toluene. The flask was held at
about 10 C during the addition. The flask was equipped with a stirrer.
The reaction mixture was allowed to warm to room temperature and stirred
for overnight. The reaction mixture was monitored with an IR
spectrophotometer and when the isocyanate peak (about 2258 cm"')
disappeared the reaction was considered completed. The product was
collected by filtration and subsequently drying under vacuum to give a
white powder (5.1 g, 96.8 percent yield). The resulting rheology control
agent had the following formula:
48
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
O H H
NN
O H H O
? 1 O O 0
I-)
0-rO 0
Example 11
The following reaction was carried out under a blanket of nitrogen.
2.94 g (0.0375 mol) of acetyl chloride was added drop-wise into a flask
containing 5 g (0.0125 mol) N,N"-2-hydroxyl-1,3-propanediylbis(N'-octyl)
urea above intermediate in 33.3g of I M LiCI/NMP. The flask was held at
about 10 C during the addition. The flask was equipped with a stirrer.
The reaction mixture was allowed to warm to room temperature and stirred
for overnight. The reaction mixture was monitored with a NMR
spectrometer and when the chemical shift (CH-) corresponded to the
desired structure and the integration was correct, the reaction was
considered completed. The product was precipitated by water, collected
by filtration and subsequently drying under vacuum to give a white powder
(2.55 g, 46.1 percent yield). The resulting rheology control agent had the
following formula:
N~HN'Y'NH~
H O~ H
Example 12
The reaction was carried out under a blanket of nitrogen. 4.52 g
(0.0375 mol) of valeryl chloride was added drop-wise into a flask
containing 5 g (0.0125 mol) N,N"-2-hydroxyl-1,3-propanediylbis(N'-octyl)
urea in 33.3g of I M LiCI/NMP. The flask was held at about 10 C during
the addition. The flask was equipped with a stirrer. The reaction mixture
49
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
was allowed to warm to room temperature and stirred for overnight. The
reaction mixture was monitored with a NMR spectrometer and when the
chemical shift (CH-) corresponded to the desired structure and the
integration was correct, the reaction was considered completed. The
product was precipitated by water, collected by filtration and subsequently
drying under vacuum to give a white powder (3.11 g, 51.3 percent yield).
The resulting rheology control agent had the following formula:
' 'H N'Y'N H"
H Oir---N
0
Example 13
The following reaction was carried out under a blanket of nitrogen.
4.77 g (31.0 mmol) of octylisocyanate in 20 mL of chloroform was added
dropwise into a flask containing 3.75 g (15.5 mmol) lysine ethylester
hydrochloride and 3.49 g (35.0 mmol) triethylamine in 80 mL of
chloroform. The flask was held at about 10 C during the addition. The
flask was equipped with a stirrer. The reaction mixture was monitored with
an IR spectrophotometer and when the isocyanate peak (about 2258 cm"1)
disappeared the reaction was considered completed. The reaction
mixture was allowed to warm to room temperature and stirred for
overnight. The product was collected by filtration and subsequently drying
under vacuum to give a white powder (6.2 g, 86.1 percent yield). The
resulting rheology control agent had the following formula:
H H H H
O 0
OEt
Example 14
The following reaction was carried out under a blanket of nitrogen.
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
An organic isocyanate of 3.27 g (0.020 mol) of octyl isocyanate was added
drop-wise into a flask equipped with a stirrer containing 3.175 g (0.020
mol) of L-serine methyl ester hydrochloride and 3.04 g (0.030 mol) of
triethylamine in 100 mL acetonitrile. The flask was held at about 10 C
during the addition. The flask was equipped with a stirrer. The reaction
mixture was allowed to warm to room temperature and stirred for 2 hours.
The reaction mixture was monitored with an LC/MS. When the
corresponding peaks of the starting materials disappeared and the
spectrum showed the expected intermediate mass peak of methyl 3-
hydroxy-2-(3-octylureido)propanoate, the reaction was considered
completed. In the same reaction flask, 0.08 g of dibutyltindilaurate and
1.68 g (0.010 mol) of 1,6-diisocyanatohexane were added. The reaction
mixture was refluxed at 78 C for about 2 hours with constant stirring. The
reaction mixture was monitored with an LC/MS. When the corresponding
peaks of the starting materials disappeared and the spectrum showed the
expected mass peak of final product, the reaction was considered
completed. The reaction mixture was filtered and washed with water to
remove the product formed and any residual solvent was removed under
vacuum to give a white powder (5.16 g, 77.0 percent yield). The resulting
rheology control agent had the following formula:
I
_.N JL H H
H H O H O O
Example 15 I
The following reaction was carried out under a blanket of nitrogen.
An organic isocyanate of 3.27 g (0.020 mol) of octyl isocyanate was added
drop-wise into a flask equipped with a stirrer containing 3.175 g (0.020
mol) of L-serine methyl ester hydrochloride and 3.04 g (0.030 mol) of
triethylamine in 100 mL acetonitrile. The flask was held at about 10 C
during the addition. The flask was equipped with a stirrer. The reaction
mixture was allowed to warm to room temperature and stirred for 2 hours.
The reaction mixture was monitored with an LC/MS. When the
51
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
corresponding peaks of the starting materials disappeared and the
spectrum showed the expected intermediate mass peak of methyl 3-
hydroxy-2-(3-octylureido)propanoate, the reaction was considered
completed. In the same reaction flask, 0.08 g of dibutyltindilaurate and
2.61 g (0.020 mol) of cyclohexylisocynate were added. The reaction
mixture was refluxed at 78 C for about 2 hours with constant stirring. The
reaction mixture was monitored with an LC/MS. When the corresponding
peaks of the starting materials disappeared and the spectrum showed the
expected mass peak of final product, the reaction was considered
complete. The reaction mixture was filtered and washed with water to
obtain the product and any residual solvent was removed under vacuum to
give a white powder (5.80 g, 72.5 percent yield). The resulting rheology
control agent had the following formula:
~ H H
N
H
Example 16
The following reaction was carried out under a blanket of nitrogen.
An organic isocyanate of 2.71 g (0.021 mol) of cyclohexyl isocyanate was
added drop-wise into a flask equipped with a stirrer containing 3.24 g
(0.021 mol) of L-serine methyl ester hydrochloride and 4.21 g (0.042 mol)
of triethylamine in 100 mL acetonitrile. The flask was held at about 10 C
during the addition. The flask was equipped with a stirrer. The reaction
mixture was allowed to warm to room temperature and stirred for 2 hours.
The reaction mixture was monitored with an LC/MS. When the
corresponding peaks of the starting materials disappeared and the
spectrum showed the expected intermediate mass peak of methyl 2-(3-
cyclohexylureido)-3-hydroxypropanoate, the reaction was considered
completed. In the same reaction flask, 0.08 g of dibutyltindilaurate and
3.40 g (0.021 mol) of octylisocyanate were added. The reaction mixture
was refluxed at 78 C for about 2 hours with constant stirring. The reaction
52
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
mixture was monitored with an LC/MS. When the corresponding peaks of
the starting materials disappeared and the spectrum showed the expected
mass peak of final product, the reaction was considered completed and
the reaction mixture was filtered and washed with water to obtain the
product and any residual solvent was removed under vacuum to give a
white powder (3.59 g, 43.5 percent yield). The resulting rheology control
agent had the following formula:
~ H_. H
H 0"~ 0~~ '-'
Example 17
An acrylic or polyester based coating resin was combined with 2
percent of the rheology control agent and diluted with appropriate amount
of solvent. After thorough mixing, the mixture was aged for at least one
hour before viscosity was measured. The results on various rheology
control agents are shown in Table 1.
Table 1.
Rheology Solid wt % Viscosity Viscosity Viscosity Viscosity Viscosity
Agent Resin Rheology Cps Cps cps cps cps
agent 0.5 rpm 5 rpm 50 rpm 100 rpm 250 rpm
Example 1 High Tg Acrylic 2 41800 9240 2200 1100 440
Resin
Example 2 High Tg Acrylic 2 28200 4560 668 496 416
Resin
Example 4 High Tg Acrylic 2 6400 1580 446 345 262
Resin
Example 7 High Tg Acrylic 2 2000 560 264 232 198
Resin
Example 9 High Tg Acrylic 2 28400 2240 616 497 409
Resin
Example 4 Low Mw 2 44600 2000 720 553 358
Polyester
Example 7 Low Mw 2 10600 2100 392 260 170
Polyester
Example 9 Low Mw 2 11200 1440 340 270 236
Polyester
Control Low Mw 0 60 60 68 65 63
Polyester
53
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
*High Tg Acrylic Resin- 58% solids in organic solvents of an acrylic polymer
of
SIMMA/IBMA/HEMA having a Mn of 6200 and Mw 12,000 and prepared according to
Example (Col. 5) U.S. 5,279,862.
*Low Mw Polyester is prepared according to the teachings in WO 2003/070843
S - styrene, MMA- methyl methacrylate, IBMA - isobutylmethacrylate, HEMA - 2-
hydroxy
ethyl methacrylate.
Example 18
Two aluminum paints were prepared by mixing the following
ingredients (weight basis). The paint mixtures were sprayed onto primed
steel panels. A clear composition of an acrylic urethane polymer was then
sprayed over the aluminum paint on the panels and dried at ambient
temperature for about 8 hours. Readings were taken on the panel with an
absolute colorimeter described in U.S. Patent 4,412,744.
Ingredient Control This Invention
Example
Aluminum flake (60% solid in solvent, from Silberline, 16.67 16.67
MFG. Co. Inc.)
Graft Acrylic Copolymer* 1.97 1.97
Acrylic resin #1** 6.02 6.02
CAB Blend (15% soln. in butyl acetate/MAK 70:30) 100.00 100.00
Polyester*** 38.46 38.46
Acrylic resin #2**** 75.50 90.60
70/30 butyl acetate/methyl amyl ketone 110.74 260.10
Example 1 (25% solid in NMP with 2.25% LiCI) 0 4.00
Wax dispersion (6% solid in 40% xylene/54% butyl 167.06 0
acetate)
Near spec L 123.63 127.43
Flop 10.80 11.79
*Graft Acrylic Copolymer: Prepared in accordance with the procedure described
in
Example #6 of U.S. 6,472,463 but using methyl toluene sulfonate versus benzyl
chloride.
**Acrylic resin#1: 66% solid in butyl acetate of an acrylic polymer of
S/MMA/IBMA/HEMA.
***Acrylic resin #2: 59.6% solids in organic solvents of an acrylic polymer of
S/MMA/IBMA/HEMA having a Mn of 6200 and Mw 12,000 and prepared according to
Example (Col, 5) U.S. 5,279,862.
54
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
****Polyester: 65% solid in organic solvents of a branched co-polyester polyol
(prepared
according to the teachings WO 2003/070843)
The above example indicates that a higher Flop Index and higher
near spec. L were achieved with the coating composition of this invention
in comparison to the control example where a wax dispersion is used for
rheology control. To achieve the similar Flop index and near spec. L,
much higher amount of wax dispersion has to be used which limits the
overall formulation latitude. Furthermore, another disadvantage of using a
wax dispersion is that xylene, which is on HAPS list, is in the composition.
Examgle 19
Dispersion Preparation #1 #2
Add in order with mixing
Highly Branched Copolyester Polyol* 58.68 58.68
Butyl acetate/Butanol (80/20 weight % blend) 147.48 147.48
Add slowly with mixing at high speed (approximately 5000 RPM) on a lab
top high speed disperser using a blade with a diameter of approximately 6
cm.
Rheology control agent of Example 3 12.6 16.8
(35 percent in NMP)
Mix at high speed (approximately 5000 RPM) on a lab top high speed
disperser, using a blade with a diameter of approximately 6 cm. for 30
minutes.
Add with low speed mixing
Butyl acetate/Butanol (80/20 weight percent blend) 25.0 25.0
*Same composition as Solution 5 of WO 03/070843 but made in methyl
amyl ketone as the solvent vs. propylene glycol monomethyl ether acetate.
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
Basecoat Preparation
Solvent Blend A
Component Grams
Acetone 162
Isobutyl alcohol 234
lsopropanol 180
Methyl Isobutyl Ketone 108
Aliphatic hydrocarbon (bp=90-110C) 270
Xylene 216
Aromatic hydrocarbon (bp=150-190C) 18
Total 1188
Solvent Blend B
Component Grams
Butyl acetate 7964.60
Methyl amyl ketone 3413.40
Total 11378.00
A CAB Solution, shown below, was produced by slowly adding
cellulose acetate butyrate to solvent while mixing on an air mixer:
Component Description Grams
Solvent Blend B Solvent Blend 5055.57
CAB-381-2* cellulose acetate butyrate 669.12
CAB-531-1* cellulose acetate butyrate 223.04
Total 5947.73
*Supplied by Eastman Chemical Co., Kingsport, Tennessee.
Silver Metallic Tinting Composition
Acrylic resin* 46.02
Sparkle Silver 5745 Aluminum Paste from Silberline 25.47
56
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
Solvent blend A 24.91
*A random acrylic copolymer Sty/IBOMA/EHA/HEMA/BMA/MMA
(10/10/15/30/10/25% by weight) at 66.40% wt solids in n-butyl acetate was
prepared with the standard free radical polymerization procedure.
A basecoat lacquer coating composition was made by adding the
components listed in Table 2 in order on an air mixer:
Table 2
Component Coating Coating
Exhibit 1 Exhibit 2
Dispersion preparation #1 246.76
Dispersion preparation #2 247.96
Graft acrylic copolymer prepared in accordance with 2.82 2.82
the procedure described in Example #6 of U.S.
6,472,463 but using methyl toluene sulfonate versus
benzyl chloride
Acrylic resin* 9.2 9.2
Graft copolymer (Example #1 of U.S. Serial No. 79.7 79.7
10/983,462)
CAB solution (prepared above) 152.78 152.78
Silver Metallic tinting composition 96.4 96.4
Solvent blend A 219.55 219.55
* A random acrylic copolymer Sty/1BOMA/EHA/HEMA/BMAIMMA (10/10/15/30/10/25 Jo
by weight) at 66.40% wt solids in n-butyl acetate was prepared with the
standard free
radical polymerization procedure.
BMA - butyl methacrylate, IBOMA - isobornyl methacrylate.
Panel Preparation
57
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
The silver basecoats were sprayed per the application instructions
used for DuPont TM ChromaPremier Basecoat specified in the DuPont
ChromaSystem Tech Manual. The basecoats were sprayed to hiding over
ACT APR10288 cold rolled steel panels which were wiped with DuPont
First Klean 3900STM, sanded with 80 grit sand paper, wiped again with
DuPont First Klean 3900STM, then primed with DuPontTM Variprime
615STM/625STM Self-Etching Primer as per the instructions in the DuPont
ChromaSystem Tech Manual. The basecoats were clearcoated with
DuPontTM ChromaClear V-7500STM Multi-Use as per the instructions in
the DuPont ChromaSystem Tech Manual. Basecoat/clearcoat panels
were flashed and then baked in a 140 F (60 C) oven for 30 minutes.
Topcoated panels were allowed to air dry for an additional 7 days prior to
testing.
Below in Table 3 are tabulated the Head-Brightness (HOB) and flop
values for these coatings. Measurements were taken with a DuPont
ChromaVision Custom Color MA 100B meter manufactured by X-Rite, Inc.
of Grandville, Michigan:
Table 3
Basecoat Near spec Lightness: HOB Flop
Exhibit 1 118.67 9.21
Exhibit 2 132.46 12.82
This data shows that the use of the rheology control agent of this
invention gave exception flake control in a refinish lacquer basecoat. This
is even more remarkable in coating Exhibit 2 (with an HOB of 132.96),
which uses 4 percent on binder of the rheology control agent. This level of
rheology control agent is much lower than the typical level of traditional
rheology control agents such as wax, which are used at around 10 percent
on binder in these types of coatings. Thus, the rheology control agents of
58
CA 02594735 2007-07-11
WO 2006/076715 PCT/US2006/001547
this invention give excellent coating appearance at much lower levels than
traditional rheology control agents.
Example 20
The above prepared rheology control agents of Examples 3, 5, 9,
10 and 15 were tested for rheological activity in a waterborne based
coating composition, Aquacryi 514, described in US Patent 6,204,319.
Each of the waterborne coating compositions was blended with 5% of
various rheology control agents and diluted with an appropriate amount of
aqueous carrier. After mixing vigorously, the mixture was checked for
gelation / viscosity increase after certain times by inverting the container.
The results on various rheology control agents are shown in
following Table 4:
Table 4
Rheology
RCA Solid wt% Gel time (hh:mm: ss)
Control Agents
Ex. 3 5 3:00:00
Ex.5 5 12:00:00
Ex. 9 5 Viscous
Ex. 10 5 00:10:00
Ex. 15 5 12:00:00
The above test results shown in Table 4 show that 5 wt% of the
rheology control agent of this invention thickens a water borne coating
composition effectively. Gel activity depends on the structure of a
rheology control agent and the resin used in the aqueous coating
composition.
59