Note: Descriptions are shown in the official language in which they were submitted.
CA 02595686 2011-01-17
SOLUBLE DIVERTING AGENTS
[0001]
FIELD OF THE INVENTION
[0002] The present invention provides methods and compositions for treating
subterranean wells
and, more specifically, provides methods and compositions for stimulating
multiple intervals in
subterranean wells. In particular, this invention provides methods and
compositions for diverting
well trealment fluids into multiple intervals by introducing propping
materials coated with a
water soluble polymer e.g. collagen, polyvinyl acetate/polyvinyl alcohol,
polyalkyl oxides,
poly(lactic acid), periodic chart elements of group I or II (alkali metal or
alkaline earth metal)
silicate polymer, or combinations thereof with materials that are slowly water
soluble for use in
redirecting the flow of stimulation fluids from a tubing string into the
subterranean environment.
DESCRIPTION OF RELATED ART
[0003] Well treatments, such as acid and fracture treatments of subterranean
formations are
routinely used to improve or stimulate the recovery of hydrocarbons. In many
cases, a
subterranean formation may include two or more intervals having varying
permeability and/or
injectivity. Some intervals may possess relatively low injectivity, or ability
to accept injected
fluids, due to relatively low permeability, high in-situ stress, and/or
formation damage. Such
intervals may be completed through preparations in a cased wellbore and/or may
be completed
open hole. In some cases, such formation intervals may be present in a highly
deviated or
horizontal section of a wellbore, for example, a lateral open hole section. In
any case, when
treating multiple intervals having variable injectivity it is often the case
that most, if not all, of
the introduced well treatment fluid will be displaced into one, or only a few,
of the intervals
having the highest injectivity. Even if there is only one interval to be
treated, the tendency for the
growth of the fracture can be either up or down. This depends on the in situ
formation stress and
the permeability variation in the formation layer. Below the created fracture
can be a water zone.
If the created fracture breaks into this zone, the well can be ruined due to
excess water and a cut
off of the petroleum components of the productive interval. Above the created
fracture zone a
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-2-
gas cap may exist which would cause harm to the production of the well because
of gas
bypassing the liquid petroleum components of the well.
[0004] In an effort to more evenly distribute displaced well treatment fluids
into each of the
multiple intervals being treated, methods and materials for diverting
treatment fluids into
intervals of lower permeability and/or injectivity have been developed.
However, conventional
diversion techniques may be costly and/or may achieve only limited success. In
this regard,
mechanical diversion techniques are typically complicated and costly.
Furthermore, mechanical
diversion methods are typically limited to cased hole environments and depend
upon adequate
cement and tool isolation for achieving diversion.
[0005] The efficient and simultaneous treatment of multiple sets of
perforations over an
extended vertical section has thus been a problem in well stimulation for
numerous years.
Numerous treatment diversion methods, such as oil-soluble calcium soap,
sulfuric acid, and
Dowell's "Fixafrac" (a mixture of lime, kerosene, a graded calcium chloride
soap, and a gelling
agent, and Dowell's FLAX-2TM as described by Harrison in his comprehensive
review Journal of
Petroleum Technology, pp. 593-598 (1972), have been used to treat multiple
zones with a wide
variety of effectiveness. A great variety of chemical based diverting agents
have been used in
attempts to plug formation openings and divert treating fluids to other zones
of the formation.
For example, wax beads have been used as diverting agents. However, the wax
beads have
limited melting points, from about 138 F to about 192 F, making them useless
if the formation
temperature exceeds their melting point.
[0006] Naphthalene (moth balls) and sodium chloride particles have also been
described to be
useful as effective diverting agents. Naphthalene particles are readily
soluble in oil, but melt at
about 180 F, thereby limiting their use to applications in lower-temperature
formations. Sodium
chloride, having a melting point of about 1,470 F, while useful at high
temperatures, requires
that the well be cleaned with water or dilute acid after the formation has
been treated in order to
fully remove the sodium chloride particles. Furthermore, sodium chloride
cannot be used with
hydrofluoric acid to treat subterranean wells due to the formation of
insoluble precipitates which
can problematically block the wellbore.
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-3-
[0007] Alternatively, diversion agents such as polymers, suspended solid
materials and/or foam
have been employed when simultaneously treating multiple intervals of variable
injectivity. Such
diversion agents are typically pumped into a subterranean formation prior to a
well treatment
fluid in order to seal off intervals of higher permeability and divert the
well treatment fluid to
intervals of lower permeability. However, the diverting action of such
diversion agents is often
difficult to predict and monitor, and may not be successful in diverting
treatment fluid into all
desired intervals. These problems may be further aggravated in open hole
completions,
especially in highly deviated completions having large areas of a formation
open to the wellbore.
The presence of natural fractures may also make diversion more difficult.
[0008] Several attempts to address the issues of areas of differing
permeability within a wellbore
have been addressed over the years. U.S. Patent No. 2,803,306 to Hower offers
a process for
increasing the permeability of an underground formation having several zones
of varying
permeability. The steps described include introducing into a well bore a
treatment fluid
containing hydrochloric acid which has oil-soluble particles dispersed
therein, the material being
selected from gilsonite, naphthalene, para-dichlorobenzene, anthracene, and [i-
naphthol. Upon
treatment, the particles provide a partial blockage of the more permeable
zones of the
subterranean formation, allowing the treatment fluid to enter the less-
permeable zones.
[0009] U.S. Patent No. 3,797,575 assigned to Halliburton discloses diverting-
forming additives
comprised of relatively water insoluble solid material dissolved in a solvent
such as methanol or
isopropanol. When the additive is combined with an aqueous treatment fluid,
the solid material,
dissolved in the additive, is precipitated in the aqueous treating fluid into
a finally divided form,
which then act as a diverting agent. U.S. Patent No. 3,724,549, also assigned
to Halliburton,
describes a diverting agent material for diverting aqueous treatment fluids
into progressively less
permeable subterranean formations. The material is composed of a carrier
liquid and graded
particles of cyclic or linear hydrocarbon resins having between about 20 and
about 1,400 carbon
atoms, and a melting point of about 200 F. This material is described as
being largely water and
acid insoluble, but soluble in oil, such that the resin can be removed by the
produced oil after the
completion of the oil treatment operation.
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-4-
[0010] The use of radiation-induced polymers as either temporary or permanent
diverting agents
has been described by Knight, et al. in U.S. Patent No. 3,872,923. According
to the
specification, temporary or permanent reductions in permeability can be
obtained by injecting an
aqueous solution containing a water-soluble polymer obtained by radiation-
induced
polymerization of acrylamide and/or methacrylamide and acrylic acid,
methacrylic acid, and/or
alkali metal salts of such acids. The resultant polymeric diverting agent has
properties, such as
temperature and pH stability, so as to effect a reduction of permeability of
the porous medium.
Permeability within the formation can be restored by subsequent treatment with
a chemical to
break down the polymer, such as hydrazine hypochlorite solution or strong
mineral acids.
[0011] U.S. Patent Nos. 3,954,629 and 4,005,753 to Scheffel, et al., offer
polymeric diverting
agents, and methods of treating subterranean formations with such polymeric
diverting agents,
respectively. The polymeric composition is described to comprise solid
particles of a
homogenous mixture of polyethylene, ethylene-vinyl acetate copolymer, a
polyamide, and a
softening agent such as long chain aliphatic diamides. These polymeric
diverting agents are
reported to be suitable for use in subterranean formations where formation
temperatures are 350
F or greater.
[0012] Methods of temporarily plugging a subterranean formation using a
diverting material
comprising an aqueous carrier liquid and a diverting agent comprising a solid
azo component and
a methylenic component are described by Dill, et al. in U.S. Patent No.
4,527,628. The diverting
agent is preferably Hansa Yellow G (Fanchon Yellow YH-5707 pigment) or Fast
Yellow 4RLF
dye, both of which have an azo component and a methylenic component and are
further
characterized as having a melting point of at least 332.6 F, a degree of
solubility in water at a
temperature of water from about 200 to about 425 F, and a degree of
solubility in kerosene at a
temperature of from about 200 F to about 425 F.
[0013] In U.S. Patent No. 6,367,548, Purvis, et al. describes methods and
compositions for
stimulating multiple intervals in subterranean wells by diverting well
treatment fluids into
multiple intervals. According to the specification, this is accomplished by
alternately displacing
diverting agent from the annulus of the weilbore into a subterranean formation
and displacing
treatment fluid from a tubing string into the subterranean formation.
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-5-
[0014] Other methods for diverting a fracture treatment include the limited-
entry technique
described by LaGrone, et al.,SPE 530, pp. 695-702 (1963), and the Technique of
Multi-Fracture
Fracturing Using a Diverting Agent (TMFUD) suggested by Dingxiang, et al., SPE
30816, pp.
80-86 (1988), the latter of which has shown an average oil production
improvement of 15.0 t/d
for each well, and a cumulative production improvement of 340.3 x 104 tons. A
viscoelastic
surfactant-based diverting agent for use in acid stimulations has also been
described (Alleman,
D., et al., SPE 80222 (2003)), which is a VES gel (polyQuat) characterized by
a distinctive
vesicle structure stable at high pH and a thermal stability of about 250 F.
This gel-type
diversion agent is typically pumped into a subterranean formation prior to a
well stimulation
fluid in order to seal off intervals of high permeability and divert the well
treatment fluid to
intervals of low permeability.
[0015] In light of all these advances and new techniques, the diverting action
of diverting agents
is often difficult to predict and monitor, and may not be successful in
diverting treatment fluid
into all the desired intervals, thereby failing to allow maximum benefit from
the fracture
procedure. These problems can be further aggravated in open hole completions,
especially in
highly-deviated completions having large areas of a formation open to the
wellbore. The
presence of natural fractures within the subterranean formation can also serve
to make diversion
more challenging. Thus, there exists a need for new compositions and methods
for diverting
well treatment fluids into multiple intervals of varying permeability within a
subterranean
formation.
SUMMARY OF THE INVENTION
[0016] The present invention provides a method of using particles having a
soluble outer coating
as diverting agents in subterranean formations. The soluble outer coating will
dissolve after a
desired period of time at downhole temperatures and pressures in the presence
of standard
downhole fracturing fluids and breaker compositions. Examples of the soluble
outer coating
include collagen, poly(alkylene) oxides, poly(lactic acid), polyvinylacetate,
polyvinyl alcohol,
polyvinylacetate/polyvinylalcohol, polylactone, polyacrylate, latex,
polyester, group I or II
silicate polymer or mixtures thereof.
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-6-
[0017] The present invention provides water soluble polymer coated proppants
as diverting
agents and methods of using such diverting agents for treating a subterranean
formation. The
diverting agent together with a carrier liquid is introduced into a
subterranean formation. The
liquid carrier flows into fractures and/or intervals within the subterranean
formation. The
fractures or intervals present varying degrees of permeability. In accordance
with the methods of
the present invention, the liquid carrier with diverting agent will flow to
the most permeable
interval first. The temperature of the formation will cause the water soluble
polymer coating of
the diverting agent to soften and swell, thereby plugging the fracture.
[0018] In one embodiment, a diverting agent suitable for diverting well
treatment fluids into a
single or a multiple interval is described, wherein the diverting agent is
comprised of a
particulate substrate and a water-soluble outer layer. Such water soluble
outer layer polymer is
exemplified, without limitation, by collagen, poly(alkylene) oxides,
poly(lactic acid)
polyvinylacetate, polyvinylalcohols, polyvinylacetate/polyvinylalcohol,
polymeric lactones,
water-soluble acrylics, latex, polyester, group I or II silicate polymer, and
admixtures thereof.
[0019] In a further embodiment, a diverting agent suitable for diverting well
treatment fluids into
a single or a multiple interval is described, wherein the diverting agent is
comprised of a
particulate substrate an intermediate water insoluble layer and a water
soluble polymer outer
layer. The water soluble outer layer polymer is exemplified, without
limitation, by collagen,
poly(alkylene) oxides, poly(lactic acid), polyvinylacetate, polyvinylalcohols,
polyvinylacetate/polyvinylalcohol, polymeric lactones, water-soluble acrylics,
latex, polyester,
group I or II silicate polymer and admixtures thereof. The water insoluble
intermediate layer is
exemplified by phenol-aldehyde novolac polymers and phenol-aldehyde resole
polymers.
[0020] In yet another embodiment, a diverting agent suitable for diverting
well treatment fluids
into a single or a multiple interval within a wellbore is described, wherein
the diverting agent is
substantially a water-soluble polymer particle such as a collagen bead or
granular particles of
poly(alkylene) oxide, poly(lactic acid), polyvinylacetate, polyvinylalcohol,
polyvinylacetate/polyvinylalcohol, polymeric lactones, water-soluble acrylics,
latex, polyester,
group I or II silicate polymer, or mixtures thereof
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-7-
[0021] In a further embodiment, a method of stimulating individual intervals
of a subterranean
formation is disclosed, the method including the steps of introducing a
diverting agent having a
water-soluble component on its outer layer into an inner pipe of a wellbore in
combination with a
low viscosity fluid or a fracturing fluid; displacing the diverting agent and
fracturing fluid into
the subterranean formation, allowing the diverting agent to progressively plug
portions of the
formation being treated; and repeating the process as necessary, adding the
diverting agent to the
carrier fluid in slugs during the fracturing operation.
DESCRIPTION OF THE FIGURES
[0022] The following figures form part of the present specification and are
included to further
demonstrate certain aspects of the present invention. The invention may be
better understood by
reference to one or more of these figures in combination with the detailed
description of specific
embodiments presented herein.
[0023] FIG. 1 shows an elevational cross-sectional view of a downhole portion
of a subterranean
formation having a vertical casing and a single treatment interval, wherein
variously coated
diverting agents are being injected into the hydrocarbon-bearing formation in
accordance with an
aspect of the present disclosure.
[0024] FIG. 2 illustrates the elevational cross-sectional view of the
subterranean formation of
FIG. 1, wherein proppants are being injected into a hydrocarbon-bearing
formation having
diverting agents of the present invention injected.
[0025] FIG. 3 shows a well with a vertical casing and multiple treatment
intervals 58, 60 and 62
and variously coated diverting agents being injected, in accordance with an
aspect of the present
disclosure.
DEFINITIONS
[0026] The following definitions are provided in order to aid those skilled in
the art in
understanding the detailed description of the present invention.
[0027] The term "carrier liquid" as used herein refers to oil or water based
liquids that are
capable of moving particles (e.g., proppants) that are in suspension. Low
viscosity carrier fluid
have less carrying capacity and the particles can be affected by gravity so
that they either rise if
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-8-
they are less dense than the liquid or sink if they are more dense than the
liquid. High viscosity
liquids can carry particles with less settling or rising since the viscosity
overcomes gravity
effects.
[0028] The term "crosslinker" or "cross-linking agent", as used herein, refers
to those
compounds used to covalently modify proteins, such as collagen, and includes
both
homobifunctional crosslinkers that contain two identical reactive groups, and
heterobifunctional
crosslinkers which contain two different reactive groups.
[0029] The term "diverting agent", as used herein, means and refers generally
to an agent that
functions to prevent, either temporarily or permanently, the flow of a liquid
into a particular
location, usually located in a subterranean formation, wherein the agent
serves to seal the
location and thereby cause the liquid to "divert" to a different location.
[0030] The term "proppant", as used herein, refers to those sized particles
that are used in well
work-overs and treatments, such as hydraulic fracturing operations, to hold
fractures open
following the treatment. Such sized particles are often mixed with fracturing
fluid(s) to hold
fractures open after a hydraulic fracturing treatment or similar downhole well
treatment. In
addition to naturally occurring sand grains and nut hulls, the term "proppant"
includes man-made
or specially engineered proppants, such as resin-coated sand or high-strength
ceramic materials
like sintered bauxite. Resin coated proppants are typified by those that are
coated with phenol-
aldehyde novolac polymers or phenol-aldehyde resole polymers. Typically, but
not necessarily,
proppant materials are carefully sorted for size and sphericity to provide an
efficient conduit for
production of fluid from the reservoir to the wellbore.
[0031] In embodiments described and disclosed herein, the use of the term
"introducing"
includes pumping, injecting, pouring, releasing, displacing, spotting,
circulating, or otherwise
placing a fluid or material within a well, wellbore, or subterranean formation
using any suitable
manner known in the art. Similarly, as used herein, the terms "combining",
"contacting", and
"applying" include any known suitable methods for admixing, exposing, or
otherwise causing
two or more materials, compounds, or components to come together in a manner
sufficient to
cause at least partial reaction or other interaction to occur between the
materials, compounds, or
components.
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-9-
[0032] The term "water soluble" as used herein refers to resins, polymers, or
coatings which are
stable (do not dissolve) under ambient, surface conditions, but which become
soluble after a
given time (usually over several hours or several days) when placed in a
subterranean
environment.
[0033] The term "treatment", as used herein, refers to any of numerous
operations on or within
the downhole well, wellbore, or reservoir, including but not limited to a
workover type of
treatment, a stimulation type of treatment, such as a hydraulic fracturing
treatment or an acid
treatment, isolation treatments, control of reservoir fluid treatments, or
other remedial types of
treatments performed to improve the overall well operation and productivity.
[0034] The term "stimulation", as used herein, refers to productivity
improvement or restoration
operations on a well as a result of a hydraulic fracturing, acid fracturing,
matrix acidizing, sand
treatment, or other type of treatment intended to increase and/or maximize the
well's production
rate or its longevity, often by creating highly conductive reservoir flow
paths.
DETAILED DESCRIPTION OF THE INVENTION
[0035] In embodiments of the disclosed diverting agent, single and multiple
intervals of a
subterranean formation can be treated or stimulated in stages by successively
introducing the
diverting agent comprising a particulate substrate and a slowly water-soluble
outer coating
comprising collagen or a combination of collagen and a slowly water-soluble,
non-collagenous
material.
[0036] The invention provides particle compositions comprising soluble
material coatings
comprising collagen, as well as processes for preparing such compositions.
These compositions
are useful in subterranean formations for diverting well treatment fluids in a
single interval to
increase the fracture length or in multiple intervals of a subterranean
formation having varying
permeability and/or injectivity during a hydraulic fracturing operation. In
using the diverting
agents of the present invention in fracturing processes, the proppant (or
particulate substrate)
coated with a slowly water-soluble coating such as a collagen alone or in
combination with a
non-collagenic water-soluble, plastic coating material acts to divert the
fracture, as the coatings
on the proppants act as the defining boundaries of the initial fracture.
Following the fracturing
treatment, the coating can be removed due to the slow-dissolution
characteristics of the coating,
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-10-
leaving standard propping agents with high permeability to flow into the
fracture and act as
proppants.
[0037] While compositions and methods are described in terms of "comprising"
various
components or steps, the compositions and methods can also "consist
essentially of' or "consist
of the various components and steps.
A. Substrate
[0038] Particulate material, also referred to herein as substrate material,
suitable for use with the
present invention includes a variety of particulate materials known to be
suitable or potentially
suitable propping agents which can be employed in downhole operations. In
accordance with the
present invention, the particulate material (or substrate material) which can
be used include any
propping agent suitable for hydraulic fracturing known in the art. Examples of
such particulate
materials include, but are not limited to, natural materials, silica
proppants, ceramic proppants,
metallic proppants, synthetic organic proppants, mixtures thereof, and the
like.
[0039] Natural products suitable for use as proppants include, but are not
limited to, nut shells
such as walnut, brazil nut, and macadamia nut, as well as fruit pits such as
peach pits, apricot
pits, olive pits, and any resin impregnated or resin coated version of these.
Typical resin
coatings or impregnations include bisphenols, bisphenol homopolymers, blends
of bisphenol
homopolymers with phenol-aldehyde polymer, bisphenol-aldehyde resins and/or
polymers,
phenol-aldehyde polymers and homopolymers, modified and unmodified resoles,
phenolic
materials including arylphenols, alkyiphenols, alkoxyphenols, and
aryloxyphenols, resorcinol
resins, epoxy resins, novolak polymer resins, novolak bisphenol-aldehyde
polymers, and waxes,
as well as the precured or curable versions of such resin coatings.
[0040] Silica proppants suitable for use with the present invention include,
but are not limited to,
glass spheres and glass microspheres, glass beads, silica quartz sand, and
sands of all types such
as white or brown. Typical silica sands suitable for use include Northern
White Sands
(Fairmount Minerals, Chardon, OH), Ottawa, Jordan, Brady, Hickory, Arizona,
St. Peter,
Wonowoc, and Chalfort, as well as any resin coated version of these sands. In
the case of silica
fibers being used, the fibers can be straight, curved, crimped, or spiral
shaped, and can be of any
grade, such as E-grade, S-grade, and AR-grade. Examples of suitable resin-
coated silica
CA 02595686 2011-01-17
-11-
proppants for use with the present invention include deformable proppants such
as FLEXSAND
LSTM and FLEXSAND MSTM (available from BJ Services, Inc., Houston, TX) and
Tempered
HS , Tempered LC , Tempered DC , and Tempered TF tempered proppants, all
available
from Santrol, Fresno, TX.
[0041] Ceramic proppants suitable for use with the methods of the present
invention include, but
are not limited to, ceramic beads; spent fluid-cracking catalysts (FCC) such
as those described in
U.S. Patent No. 6,372,378; ultra lightweight porous ceramics; economy
lightweight ceramics
such as "ECONOPROPTM" (Carbo Ceramics, Inc., Irving, TX); lightweight ceramics
such as
"CARBOLITETM"; intermediate strength ceramics such as "CARBOPROPTM" (available
from Carbo Ceramics, Inc., Irving, TX); high strength ceramics such as
"CARBOHSPTM" and
"Sintered Bauxite" (Carbo Ceramics, Inc., Irving, TX), and "HYPERPROP G2TM"
DYNAPROP G2TM, or OPTIPROP G2TM encapsulated, curable ceramic proppants
(available
from Santrol, Fresno, TX) as well as any resin coated or resin impregnated
versions of these,
such as described above.
[00421 Metallic proppants suitable for use with the embodiments of the present
invention
include, but are not limited to, aluminum shot, aluminum pellets, aluminum
needles, aluminum
wire, iron shot, steel shot, and the like, as well as any resin coated
versions of these metallic
proppants.
[0043] Synthetic proppants are also suitable for use with the present
invention. Examples of
suitable synthetic proppants include, but are not limited to, plastic
particles or beads, nylon
beads, nylon pellets, SDVB (styrene divinyl benzene) beads, carbon fibers such
as PANEXTM
carbon fibers from Zoltek Corporation (Van Nuys, CA), and resin agglomerate
particles similar
to "FLEXSAND MSTM" (BJ Services Company, Houston, TX), as well as resin coated
versions
thereof.
[0044] Additionally, soluble materials suitable for use as proppants are also
envisioned to be
useful with the methods of the present invention. For example, soluble
proppants which are
placed in the channels of the created perforations include, but are not
limited to, marble or
limestone chips or any other suitable carbonate particulates. Additionally,
wax, plastic, or resin
particles, either coated or uncoated, which are either soluble through contact
with a treatment
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-12-
chemical or can melt and flowback from the fracture are suitable for use as
proppants with the
present invention.
[0045] Suitable with the present invention, propping agents are typically used
in concentrations
from about 1 to about 18 pounds per gallon (about 120 g/L to about 2,160 g/L)
of fracturing fluid
composition, but higher or lower concentrations may also be used as required.
[0046] Similarly, the particulate substrate suitable for use with the present
invention has a
particle size in the range of USA Standard Testing screen numbers from about 4
to about 200
(i.e., screen openings of about 0.18 inch to about 0.003 inch). More
particularly, particulate
substrate sizes suitable for use with the present invention include size
ranges from about 4 mesh
(4750 microns) to about 200 mesh (75 microns). Also suitable for use with the
present invention
are particulate materials or proppants having size designations of 6/12, 8/16,
12/18, 12/20, 16/20,
16/30, 20/40, 30/50, 40/70 and 70/140, although any desired size distribution
can be used, such
as 10/40, 14/20, 14/30, 14/40, 18/40, and the like, as well as any combination
thereof (e.g., a
mixture of 10/40 and 14/40). The preferred mesh size, in accordance with the
present invention,
is 20/40 mesh.
B. Soluble Coating
[0047] The soluble coatings used in accordance with the present invention can
be any number of
known soluble agents that are slowly soluble in downhole, subterranean
formations over a period
of time. Soluble polymer materials used in accordance with the present
invention should be
soluble (that is, capable of dissolving in) in brines, water, oil, organic
solvents, acid or acidic
media, and/or in fluids having a pH in the range from about 1 to about 14, as
well as mixtures
thereof under the conditions found in downhole, subterranean formation.
[0048] Preferably, the soluble coating is a structural protein such as
collagen or atelocollagen, a
vegetable protein such as found in wheat, maize, oat or almonds, or a collgen
originating from a
marine environment. The latter type of collagen can be extracted from fish,
algae, plankton,
micro-plankton, and the like. More preferably, the soluble coating is
collagen, including Type I
collagen, Type II collagen, Type III collagen, Type IV, or Type V collagen, as
well as
combinations thereof. Most preferably, in accordance with the present
invention, the soluble
coating is a Type I collagen or an atelocollagen.
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-13-
[0049] Type I collagens or atelocollagens suitable for use as soluble coatings
in accordance with
the present invention are those collagens containing at least one
hydroxyproline residue. Such
Type I collagens or atelocollagens include collagens found in tendons, skin,
bone, scar tissue,
and the like, such as tropocollagens, as well as products derived from the
controlled, enzymatic
or chemical reduction of collagen proteins. Such collagens preferably have a
molecular weight
from about 10,000 Daltons to about 500,000 Daltons, and more preferably from
about 100,000
Daltons to about 300,000 Daltons. Suitable molecular weights of about 100,000
daltons,
125,000 daltons, 150,000 daltons, 175,000 daltons, 200,000 daltons, 225,000
daltons, 250,000
daltons, 275,000 daltons, 300,000 daltons, as well as molecular weights
between any two of
these values, e.g., collagens having a molecular weight from about 225,000 to
about 275,000
daltons. For example, a preferred Type I collagen suitable for use with the
present invention is
tropocollagen with a molecular weight of about 250,000 as supplied by
Milligans and Higgins,
Inc. (Johnstown, NY).
[0050] Collagens suitable for use within the present invention have Bloom
strengths from about
100 psi to about 900 psi, and more preferably from about 300 psi to about 700
psi. Most
preferably, collagens suitable for use with the present invention have Bloom
strengths from
about 400 psi to about 600 psi. Suitable Bloom strengths, in accordance with
the present
invention, are about 400 psi, about 410 psi, about 420 psi, about 430 psi,
about 440 psi, about
450 psi, about 460 psi, about 470 psi, about 480 psi, about 490 psi, about 500
psi, about 510 psi,
about 520 psi, about 530 psi, about 540 psi, about 550 psi, about 560 psi,
about 570 psi, about
580 psi, about 590 psi, and about 600 psi, as well as Bloom strengths between
any two of these
values, e.g., from about 400 psi to about 520 psi, such as 512 psi.
[0051] Bloom strength, as used herein, refers to the measured value of the
strength and/or
rigidity of a gelatinous substance, such as collagen, formed by a standard
solution of definite
concentration that has been retained at a constant temperature for a specified
period of time, in
accordance with standardized bloom testing procedures, such as BS757:1975,
GMIA Testing
Standard B5757, International Standard IS09665 for testing adhesive animal
glues, or similar
standards as described in "Official Methods of Analysis of AOAC INTERNATIONAL
(OMA) ",
17th Edition, Volume II; AOAC International Publications (2003). Bloom
strength values are
typically given in "pounds per square inch" (psi) or grams, reflecting the
force required to
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-14-
depress a chosen area of the surface of the sample a distance of 4 mm. In a
typical procedure, a
gel product, such as collagen or gelatin, is formed to a specified consistency
(e.g., 6 and 2/3 %
solution) and kept at a constant temperature in a constant temperature bath at
10 C for 18 hours.
A device called a Texture Analyzer (e.g., the TA.XT2i Texture Analyzer,
Scarsdale, NY) then
measures the weight in grams (or the pressure, in psi) required to depress a
standard AOAC
[Associateion of Official Analytical Chemists] gelometer plunger having a
sharp, lower edge 4
mm into the gel; alternatively, a BS plunger which has a bottom edge rounded
to a radius of 0.4
mm can be used as the plunger. For example, if this procedure requires 200
grams to depress the
plunger, then the gelatin has a Bloom strength of 200.
[0052] Type I collagens suitable for use within the present invention have a
sieve distribution
/size designation of 6/12, 8/16, 12/18, 12/20, 16/20, 16/30, 20/40, 30/50,
40/70 and 70/140, as
well as sieve distributions between any two of these designations, although
any desired size
distribution can be used, such as 8/40, 10/40, 14/20, 14/30, 14/40, 18/40, and
the like, as well as
any combination thereof (e.g., a mixture of 10/40 and 14/40). The preferred
mesh size, in
accordance with the present invention, is 8/40 mesh.
[0053] Collagens, as used herein as soluble coatings, can be either cross-
linked, uncross-linked,
or a combination of both, and the type and degree of cross-linking will depend
upon the specific
application of the collagen-based soluble coating. There are four fundamental
strategies for
fixing collagenous materials and materials constructed of processed collagen
fibers or purified
collagen. These include exogenous chemical cross-linking using agents that
covalently couple
neighboring collagen fibrils using targeted reactive moieties in the collagen
fibrillar system and
the cross-linking molecules themselves; physiochemical cross-linking
techniques such as photo-
oxidation, microwave irradiation, dehydration and dehydrothermal treatment,
that covalently join
collagen fibrils via the naturally occurring reactive amino acid side chains;
chemical catalysis of
intramolecular cross-links between amino acid side chains on the collagen
fibrils; and
polymerizing compounds mixed with collagenous assemblies and forming polymeric
non-
covalent or covalent interactions that do not chemically react with collagen
fibrils [Koob, T.J.,
"Collagen Fixation", in Encyclopedia of Biomaterials and Biomedical
Engineering, Wnek, G.E.,
Bowlin, G.L., Eds., 2004]. In accordance with the present invention, the
collagen used as a
soluble coating is preferably cross-linked using chemical cross-linking
techniques. These
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-15-
include, but are not limited to, aldehyde-based cross-linking techniques,
polyepoxy compound-
based cross-linking techniques, the use of isocyanates, carbodiimide cross-
linking, and acyl azide
based crosslinking. More preferably, the collagen is cross-linked using
aldehyde-based cross-
linking techniques, such as by using glutaraldehyde or formaldehyde.
[0054] Aldehyde-based cross-linking techniques includes those techniques using
a reagent
containing two reactive aldehyde groups to form covalent cross-links between
neighboring
collagen proteins, especially the e-amino groups of lysine residues in
collagen [Khor, E.,
Bioraterials, Vol. 18: pp. 95-105 (1997)]. Aldehydes suitable for use with the
present invention
include but are not limited to glutaraldehyde, formaldehyde, propionaldehyde,
and
butyraldehyde.
[0055] Polyepoxy based cross-linking techniques and agents include the use of
compounds, such
as short, branched polymers, terminating in reactive epoxy functionalities.
Polyepoxy
compounds suitable for use as cross-linking agents in the present invention
include but are not
limited to glycerol ethers, glycol, and glycerol polyglycidyl ethers.
[0056] Isocyanates are also suitable for use as cross-linking agents in the
present invention.
Generally, the isocyanates (R-NCO) react with primary amines to form a urea
bond (R-H-
CO-NH-R); difunctional isocyanates therefore have the ability to cross-link
collagen via its
lysine side chains. Isocyanates suitable for use as cross-linking agents in
the present invention
are preferably diisocyanates, including biphenyl diisocyanate, dimethoxy-4,4'-
biphenyl
diisocyanante, dimethyl-4,4'-biphenyl diisocyanate, 1,3-
bis(isocyanatomethyl)benzene, phenyl
diisocyanate, toluene diisocyanate, tolylene diisocyanate, diisocyanato
hexane, diisocyanato
octane, diisocyanato butane, isophorone diisocyanante, xylene diisocyanate,
hexamethylene
diisocyanante, octamethylene diisocyanante, phenylene diisocyanate, and
poly(hexamethylene
diisocyanate). Preferably, the isocyanate used as a cross-linking agent of the
collagen molecules
of the present invention is hexamethylene diisocyanate.
[0057] Carbodiimide cross-linking agents and techniques can also be used
within the scope of
the present invention. These agents react with the carboxyl groups of aspartic
and glutamic acid
side chains within the collagen to form isoacylurea derivatives/iso-peptide
bonds [Khor, E.,
ibid.]. Carbodiimides suitable for use as cross-linking agents with the
collagen of the present
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-16-
invention include but are not limited to N,N'-dicyclohexylcarbodiimide (DCC);
N,N'-
diisopropylcarbodiimide (DIC); N,N'-di-tert-butylcarbodiimide; 1-ethyl-3-(3-
dimethylaminopropyl)carbodiimide (EDC; EDAC); water-soluble EDC (WSC); 1-tert-
butyl-3-
ethylcarbodiimide; 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide;
bis(trimethylsilyl)-
carbodiimide; 1,3-bis(2,2-diinethyl-1,3-dioxolan-4-ylmethyl)carbodiimide
(BDDC, as described
in U.S. Patent No. 5,602,264); N-cyclohexyl-N'-(2-morpholinoethyl)
carbodiimide; N,N'-
diethylcarbodiimide (DEC); 1-cyclohexyl-3-(2-morpholinoethyl)carbodiimide
methyl-p-
toluenesulfonate [e.g., Sheehan, J.C., et al., J. Org. Chem., Vol. 21: pp. 439-
441 (1956)];
oligomeric alkyl cyclohexylcarbodiimides, such as those described by Zhang, et
al. [J. Org.
Chem., Vol. 69: pp. 8340-8344 (2004)]; polymer bound DCC; and polymer bound
EDC, such as
cross-linked N-ethyl-N'-(3-dimethylaminopropyl)carbodiimide on JANDAJELTM.
Additionally,
N-hydroxysuccinimide (NHS), 1-hydroxy-7-azabenzotriazole (HOAt), or similar
reagents can be
utilized in conjunction with the carbodiimide to minimize internal
rearrangement of the activated
isoacylurea derivative and provide more efficient cross-linking.
[0058] As with carbodiimide treatment, acyl azide crosslinking agents produce
covalent bonds
between the carboxylic acid side chains of aspartic acid and glutamic acids
and the E-amino
groups of the lysines of collagen [Petit, H., et al., J. Biomed. Mater. Res.,
Vol. 24: pp. 179-187
(1990)]. Following esterification of the carboxyl groups in which a methyl
group is added to the
acid, the biomaterial is treated with hydrazine to form the corresponding
hydrazide; sodium
nitride is then added to react with the hydrazide and form the acyl azide. Any
number of
hydrazines known in the art can be used in this method, including
maleimidopropionic acid
hydrazide (MPH).
[0059] Other chemical cross-linking agents suitable for use in the present
invention to provide
cross-linked collagen molecules which act as soluble coatings on proppant
particles include but
are not limited to homobifunctional cross-linkers such as BMME, BSOCOES, DSP
(a thio-
cleavable cross-linker), DSS, EGS, water-soluble EGS, and SATA, as well as
heterobifunctional
cross-linking agents including GMB, NMS, PMPI, SMCC, SPDP, and MPH
(maleimidopropionic acid hydrazide), MCH, EMCH (maleimidopaprionic acid
hydrazide),
KMUH (N-(k-Maleimidoundecanoic acid)hydrazide), and MPBH (4-(4-N-
MaleimidoPhenyl)butyric acid hydrazide), all available from Interchim (Cedex,
France).
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-17-
[0060] Other techniques suitable for crosslinking the collagen fibers for use
as soluble proppant
coatings include but are not limited to dehydration, UV irradiation at 254 nm,
glucose-mediated
cross-linking (glycation) in conjunction with UV irradiation, and biological
cross-linking. The
latter technique includes using natural products such as genipin and its
related iridoid compounds
which are isolated from the fruits of the gardenia plant (Gardenia j
asminoides), which are
dialdehydes in aqueous solution and thereby can react with the c-amino groups
on lysine side
chains of neighboring collagen molecules to provide a cross-link. Other
biological cross-linking
systems suitable for use with the present invention include catechol-quinone
tanning systems,
such as 3,4-dihydroxytyrainine, and nordihydroguaiaretic acid (NDGA), isolated
from the
creosote bush, which acts as a cross-linking agent via the two catechols on
NDGA [Koob, T.J.,
Comp. Biochem. Physiol., Part A, Vol. 133: pp. 1171-1192 (2002)].
[0061] The slowly water-soluble coatings on the particulate substrates, in
accordance with the
present disclosure, can also be non-collagenic materials such as synthetic
polymers that are
slowly water soluble. Such non-collagenic materials include but are not
limited to: polyethylene
oxides, polypropylene oxides, polycaprolactones; grafts of
polyethylene/polypropylene and
polycaprolenes; grafts of polyethylene/polypropylene oxides and
polycaprolactones; water
soluble or water reducible acrylics; water reducible phenoxy resin; latex;
polyesters; soluble
block copolymers; grafts of polyvinyl alcohol (PVA) and polyvinyl acetates;
polyactides and
derivatives of polylactic acid; polyglycolic acid (PGA); polyglycoliclactic
acid (PGLA). Also
useful for a water soluble coating are periodic chart elements of group I or
II (alkali metal or
alkaline earth metal) silicate polymers, e.g. SOLOSILTM (Foseco International,
Ltd., Great
Britain), a sodium silicate polymer.
C. Method of Using
[0062] In embodiments of the disclosed method, single or multiple intervals of
a subterranean
formation may be treated or stimulated in stages by successively introducing
diverting agent of
the present invention into the formation followed by introduction of well
treatment fluid into the
formation. As used herein, "wellbore" includes cased and/or open hole sections
of a well, it
being understood that a wellbore may be vertical, horizontal, or a combination
thereof. The term
"pipe string" refers to any conduit suitable for placement and transportation
of fluids into a
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-18-
wellbore including, but not limited to, tubing, work string, drill pipe, coil
tubing, etc.
Furthermore, it will be understood with benefit of this disclosure that the
disclosed diversion
agents and diversion treatment techniques are suitable for use with any type
of well treatment
fluid including, but not limited to, acid treatments, condensate treatments,
hydraulic fracture
treatments, and the like. Furthermore, it will be understood that the benefits
of the disclosed
methods and compositions may be realized with well treatments performed below,
at, or above a
fracturing pressure of a formation.
[0063] First: WELLBORE USE: In this aspect of the invention, the use of fully
soluble particles
in the wellbore (such as collagen or other water soluble polymer plastics or
mixtures of these) to
divert fluid flow from one zone to another and then dissolve is disclosed. The
use of collagen (in
both the uncrosslinked and crosslinked form) and soluble plastics are useful
in diverting the flow
of fluids in the well. These diverting materials should be in the range of 1
to 100 mesh size,
preferably 4 to 50 mesh size and can be used in combination with other
additives or plastic
materials to enhance performance by diverting the flow of fluids from one zone
to another.
[0064] These materials have been used as diverting ball sealers but recent
tests have shown that
the material could be used as a diverting agent to block fluid from flowing
into one zone and into
another of either higher pore pressure or lower permeability.
[0065] The present invention provides a method treating a cased wellbore to
divert flow of
fluids from one zone to another. The method involves pumping into a wellbore a
diverting fluid
that is made up of an aqueous carrier liquid having dispersed therein a
particulate form of a water
soluble polymer and wherein the particulate polymer has a density greater than
or less than the
density of the carrier liquid. As the diverting fluid is pumped into the
wellbore the particulate
polymer settles into zones of the wellbore and thereby diverts flow of a
treating fluid from one
zone to another. Generally the treating fluid is diverted or blocked from
flowing into a zone of
higher pore pressure or lower permeability.
[0066] In the methods of this invention relating to wellbore use, the water-
soluble particulate
polymer is collagen, poly(alkylene) oxide, poly(lactic acid),
polyvinylacetate, polyvinylalcohol,
polyvinylacetate/polyvinylalcohol, polylactone, polyacrylate, latex,
polyester, periodic chart
elements of group I or II (alkali metal or alkaline-earth metal) silicate
polymer or mixtures
thereof. Typically the particulate polymer is present in the carrier liquid in
an amount from
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-19-
about 0.001 pounds per gallon to about 10 pounds per gallon of the carrier
liquid.
Advantageously, the particulate polymer is comprised of varying densities
greater or less than
the density of the carrier fluid. Typically, the carrier liquid is water,
brine, aqueous acid
solutions, or gelled acid solutions.
[0067] Second: GENERATED FRACTURE USE: In this aspect of the invention, the
use of
coated particles of various propping agents (coated with either fully soluble
or a mix of soluble
and insoluble collagen or polymeric plastic materials) can be pumped into the
fractured
formations to prevent fractures from diverting out of the producing zone. For
example, a dense
sintered bauxite particle with a soluble or partially soluble coating would
fall to the bottom of the
fracture and divert the fracture from the lower strata or a water zone. Also,
a low- density walnut
shell with a soluble or partially soluble coating would tend to rise inside
the fracture to divert the
fracture from upward growth into a gas or water zone. The coating can be
either fully or
partially soluble since the proppant will remain in place in the fracture and
provide conductivity
in the fracture after the frac job is completed. Some of the coating on the
proppant should be
soluble but a mixture of both soluble and insoluble plastics or collagen is
desirable to prevent
movement of the propping agent in the fracture.
[0068] The use of diverting agents in fractures is that a proppant or propping
agent would be
coated with a soluble or partially soluble coating - using a collagen and/or
polymeric plastic
coating material or any mixture of these. The fracture would be diverted by
using these soluble
coatings on proppants as the defining boundaries of the initial fracture.
After the fracturing
treatment, the coating would disappear and the previously coated particles
would return to
normal propping agents, which have high permeability. Coatings on various
density proppants
could cause the fracture boundaries to be set early in the fracture process
since a low viscosity
fluid would allow a high density coated proppant to settle or fall inside the
fracture to make a
lower boundary on the fracture and divert it out from the wellbore to make a
longer fracture and
increase the productivity of the well. Likewise, a low density coated proppant
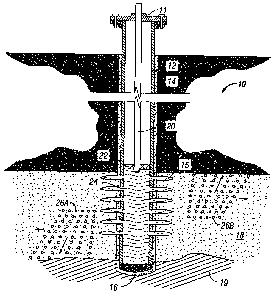
would tend to rise
to the top part of the growing fracture to form a top boundary and divert the
growing fracture
away from upper zones that may harm the production of the well. With the
fracture contained at
top and bottom the fracture could grow outward and a longer contained fracture
would improve
the well potential productivity.
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-20-
[0069] Figure 1 illustrates a well with a vertical cased wellbore section and
a single interval
formation that is to be treated in accordance with one embodiment of the
present disclosure. The
well 10 of FIG. 1 has a casing 12 extending from the wellhead 11 for at least
a portion of its
length and is cemented around the outside with cement sheath 14 to hold the
casing 12 in place
and isolate the penetrated formation or intervals. The cement sheath 14
extends upward from the
bottom of the wellbore in the annulus between the outside of the casing 12 and
the inside wall of
the wellbore at least to a point above the producing strata/hydrocarbon
bearing formation 18.
The reasons for the inclusion of this sheath are many, but essentially the
cement sheath 14 helps
to ensure the integrity of the well-bore (i.e., so it does not collapse), or
to isolate specific,
different geologic zones (i.e., an oil-bearing zone from an (undesirable)
water-producing zone).
The wellbore is also optionally equipped with a casing or liner shoe 16 so as
to help guide the
casing string 12 past ledges or obstacles during its placement in the
wellbore. For the
hydrocarbons in the producing strata 18 to be produced, it is necessary to
establish fluid
communication between the producing strata 18 and the interior of casing 12.
This is
accomplished by perforations 15 made through casing 12 and the cement sheath
14 by means
known to those of ordinary skill in the art. Such means include, but are not
limited to,
perforation guns, shaped charge devices, and phase charge devices, such as
those described in
U.S. Patent Nos. 6,755,249, 5,095,099, and 5,816,343; Horizontal Oriented
Perforating Systems
(HOPS), such as those manufactured by Owen Oil Tubes, Inc. (Ft. Worth, TX);
mechanical
perforating devices such as laterally movable punches (U.S. Patent No.
2,482,913), needle punch
perforators, and toothed wheel perforators such as those described in U.S.
Patent No. 4,220,201;
and shearable plugs such as described in U.S. Patent No. 4,498,543. The
perforations 15 form a
flow path for fluid from the formation into the casing 12, and vice-versa.
[0070] The hydrocarbons flowing out of the producing strata 18 through the
perforations 15 and
into the interior of the casing 12 can be transported to the surface through a
production tubing 20.
A production packer, 22, can optionally be installed near the lower end of the
production tubing
20 and above the highest perforation 15 in order to achieve a pressure seal
between the
production tubing 20 and the casing 12. Optionally, and equally acceptable in
accordance with
the present invention, production tubings 20 need not be used, in which case
the entire volume of
casing 12 is used to conduct the hydrocarbons to the surface of the earth.
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-21-
[0071] When diversion is needed during a well treatment operation, heavy
weight proppant
diverting agents 26a and/or light weight proppant diverting agents 26b, both
of which are
substantially coated with a soluble coating in accordance with the present
invention (i.e., have a
collagen-containing coating), are used to substantially seal the upper and
lower sections of the
producing strata 18. This substantial sealing, or border formation, occurs
when the temporary
diverting agents 26a and/or 26b are introduced into the casing 12 at a
predetermined time during
the treatment. When the diverting agents 26a and/or 26b are introduced into
the fluid upstream
of the perforated parts of the casing 12, they are carried down the production
tubing 20 or casing
12 by the treating fluid 24 flow. Once the treating fluid 24 arrives at the
perforated interval in
the casing, it flows outwardly through the perforations 15 and into the strata
18 being treated.
The flow of the treating fluid 24 through the perforations 15 carries the
temporary diverting
agents 26a and/or 26b through the perforations and out into the strata 18. At
this point, the heavy
weight proppant diverting agents 26a, having a density greater than that of
the treating fluid 24,
settle to the bottom of the created fracture (as indicated by the arrows),
forming a temporary
"lower border" between the fracture and, for example a sand, shale or clay
layer 19 or other area
to which it is desirable to seal off from the producing strata. Similarly,
light weight proppant
diverting agents 26a, having a density less than that of treating fluid 24,
rise to the top of the
created fracture (as indicated by the arrows), thereby forming another
temporary "upper border"
between the fracture and an undesirable layer, such as a shale or clay band of
strata.
[0072] Figure 2 illustrates the next step of this aspect of the present
invention. Once the
temporary diverting agents 26a and 26b are seated at the top and/or bottom of
the created
fracture, respectively, the fluid flow rate and viscosity of the treating
fluid 24, containing regular
proppant particles 28, is increased. In this manner, the fracture can grow
outward, away from the
wellbore (in the direction of the arrow) and in doing so increase the overall
length of the fracture,
thereby aiding in increasing the stimulation and/or longevity of the well. At
the completion of
the well treatment, the soluble coating on the temporary diverting agents 26a
and 26b will
dissolve, allowing the remaining proppant particles to be removed with the
treating fluid 24
through perforations 15, or to remain and act as additional proppants in
propping open the
fractured strata.
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-22-
[0073] FIG. 3 illustrates a further embodiment of the present invention. A
well 50 having a
vertical cased wellbore with a casing 54 extended from the wellhead 52 for at
least a portion of
the length of the wellbore, and a cement sheath 56 extending upwards from the
bottom of the
wellbore in the annulus between the outside of the casing 54 and the inside
wall of the wellbore,
at least to a point above the existing strata, similar to that shown in FIG.
1. Exposed within the
open hole section of the wellbore is a subterranean formation having multiple
treatment intervals
58, 60 and 62. Although three separated intervals are illustrated in FIG. 3,
it will be understood
with benefit of this disclosure that anywhere from two treatment intervals up
to any number of
treatment intervals can be treated using the presently disclosed methods and
compositions.
Furthermore, it will be understood that such treatment intervals can be
contiguously disposed
rather than separated by relatively impermeable areas such as shale breaks.
Although FIG. 3
illustrates a fully cased wellbore, it will also be understood that disclosed
treatment methods may
be utilized with virtually any type of wellbore completion scenario. For
example, the disclosed
methods may advantageously be employed to treat well configurations including,
but not limited
to, vertical wellbores, fully cased wellbores, horizontal wellbores, wellbores
having multiple
laterals, and wellbores sharing one or more of these characteristics.
[0074] In FIG. 3, treatment intervals 58, 60 and 62 represent identified
intervals of a
subterranean formation that have been identified for treatment. In this
regard, any number of
intervals or only a portion thereof present in the subterranean formation may
be so identified.
Alternatively, such intervals may also represent perforated intervals in a
cased wellbore. As
shown in FIG. 3, perforations 66 extend through casing 54 and cement sheath 56
by means
known to those of skill in the art, and in doing so form a flow path for fluid
from the formation
into the casing 54, and vice-versa.
[0075] The hydrocarbons flowing out of the producing strata in treatment
intervals 58, 60 and 62
through the perforations 66 and into the interior of the casing can be
transported to the surface
through a production tubing, 64. Further, and as illustrated in FIG. 3, a
production packer 68 can
be optionally installed substantially near the lower end of the production
tubing 64 and above the
highest perforation 66 in order to achieve a pressure seal between the
production tubing 64 and
the casing 54. Production tubing 64 need not always be used, and in those
instances the entire
interior volume of casing 54 is used to conduct the hydrocarbons to the
surface to wellhead 52.
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-23-
[0076] When diversion is needed during a well treatment, diverting agents 72
are used to
substantially seal some of the perforations 66. Substantial sealing occurs
when flow through a
perforation 66 is significantly reduced, as often indicated by an increase in
wellbore pressure as a
diverting agent 72 blocks off one or more perforations 66. In accordance with
this aspect of the
present invention, diverting agents 72 are preferred to be substantially
spherical in shape,
although other geometries can be used. Using diverting agents 72 of the
present invention to
plug some of the perforations 66 is accomplished by introducing the diverting
agents 72 into the
casing 12 at a pre-determined time during the treatment. When the diverting
agents 72 are
introduced into the fluid upstream of the perforated parts (66) of the casing
12, they are carried
down the production tubing 64 or casing 12 by a flowing fracturing fluid 70.
Once the fracturing
fluid 70 arrives at the perforated interval in the casing, it flows outwardly
through perforations
66 and into the treatment intervals 58, 60, and 62 being treated. The flow of
the fracturing fluid
70 through the perforations 66 carries the diverting agents 72 toward the
perforations 66, causing
them to seat on the perforations 66. Once seated on the perforations 66,
diverting agents 72 are
held onto the perforations 66 by the fluid pressure differential which exists
between the inside of
the casing 54 and the treatment intervals 58, 60 and 62 on the outside of
casing 54. The
diverting agents 72 are preferentially sized to substantially seal the
perforations 66 when seated
upon them. The seated diverting agents 72 thereby serve to effectively close
those perforations
66 until such time as the pressure differential is reversed and the diverting
agents released, or the
diverting agents 72 dissolve over a period of time due to changes in their
environment (e.g., the
introduction of water).
[0077] The diverting agents 72 will tend to first seal the perforations 66
through which the
fracturing fluid 70 is flowing most rapidly. The preferential closing of the
high flow rate
perforations 66 tends to equalize treatment of the treatment intervals 58, 60
and 62 over the
entire, perforated interval. For maximum effectiveness in seating on
perforations 66, the
diverting agents 72 should have a density less than the density of the
treating fluid 70 in the
wellbore at the temperature and pressure conditions encountered in the
perforated area
downhole. Generally, and in accordance with this aspect of the present
invention, the diverting
agent 72 will have at least a substantial outer surface comprised of collagen
or a mixture of
collagens. The number of diverting agents 72 needed during a workover or well
treatment
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-24-
depends upon the objectives and characteristics of the individual well and the
stimulation
treatment to be used, and can be determined by one skilled in the art.
[0078] In the practice of disclosed methods, the diverting agent or medium
suitable for achieving
diversion of fluids into the identified treatment intervals that is employed
is the diverting agent
of the present invention comprising a particulate substrate and a slowly water-
soluble collagen
outer layer. In one embodiment, a neutrally buoyant variation of this collagen-
containing
diverting system can be employed, so as to reduce the chance of segregation of
the diverting
agent and particulate diverting agent carrier fluid. A "neutrally buoyant"
diverting system is a
system in which a particulate diverting agent is suspended in a carrier fluid
having sufficiently
close density or specific gravities to result in a mixture in which solid
components of the
diverting agent do not substantially settle or rise in the system under static
conditions. Such
segregation can result in, for example, accumulation of diverting agent at one
or more locations
in the wellbore and sticking of the pipe string within wellbore sections.
Furthermore,
segregation can result in loss of diversion action due to movement of the
diverting agent away
from the intervals to be treated. Neutrally buoyant diverting systems may be
of particular
advantage in highly deviated or horizontal wells, where gravity segregation of
a non-neutrally
buoyant diverting system may prevent efficient blockage or reduction in
permeability of the
entire circumference of formation face exposed in the wellbore due, for
example, to migration of
diverting agent upwards or downwards in the highly deviated or horizontal
section of the
wellbore.
[0079] Diverting agents which may be employed include the diverting agents of
the present
invention, having a slowly water-soluble outer coating, alone or in
combination with any
diverting agent (e.g., oil soluble, acid soluble, etc.) suitable for diverting
subsequent treatment
fluids into intervals having lower injectivity. One suitable diverting agent
in accordance with the
present invention is a diverting agent that is substantially collagen.
Examples of suitable
diverting agents which can be combined with the diverting agent of the present
invention
include, but are not limited to, benzoic acid flakes, wax (such as "Divert VI"
available from BJ
Services), cement grade gilsonite or unitaite, polymers (including, but not
limited to, natural
polymers such as guar, or synthetic polymers such as polyacrylate), rock salt,
and the like. Other
types of suitable diverting agents that can be employed include, but are not
limited to, acid
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-25-
soluble diverting agents such as those described in U.S. Pat. No. 3,353,874,
and phtalimide
particles such as those as described in U.S. Pat. No. 4,444,264.
[0080] In one embodiment of the present invention, any type of carrier fluid
having a density
suitable for forming a neutrally buoyant diverter system may be employed,
including natural or
synthetic brines (such as KCl water, etc.) and carrier fluids including
gelling agents (such as
normal or synthetic polymers) or other weighting materials known in the art.
Cement grade
gilsonite (also known as "Uintate") is a natural variety of asphalt that is
crushed and sorted into
multiple-size particles. This diverting agent composition may be blended at
the well site with
specific chemically-modified fresh water (water containing for example, about
0.05% to about
I% of a wetting surfactant) to disperse the gilsonite and optionally, a
weighting agent (including
but not limited to salts such as KC1, NH4Cl, NaCl, CaC12, etc.) for density
adjustment and/or
formation-clay control, and a gelling agent (a polymer such as guar gum,
hydroxy propylguar,
carboxy methylhydroxy proprylguar, carboxy methyl hydroxyethyl cellulose,
xanthan gum,
carboxy methyl cellulose, etc.) for viscosity adjustment and/or drag
reduction.
[0081] The diverting agent of the present invention is preferably present in
the carrier fluid in
concentrations of from about 0.001 pounds per gallon to about 10 pounds per
gallon of carrier
liquid but concentrations outside this range can also be used. The most
preferred concentrations
of diverting agents are from about 0.01 to about 6 pounds per gallon of
carrier fluid. Diverting
agent concentrations of less than about 0.001 pound per gallon will not as
readily plug
formations when used in carrier fluids volumes which are normally available at
an oil well site.
A progressively large volume of carrier fluid would be required to create
adequate formation
plugs at concentrations of less than 0.001 pounds per gallon.
[0082] Concentrations of diverting agent greater than about 10 pounds per
gallon would not
increase the diverting of the treating fluid to an appreciable extent and
therefore are not
particularly desirable in carrying out the present invention.
[0083] The carrier liquid is typically composed of water, brine, aqueous acid
solutions, or gelled
acid solutions. The acid solutions can be gelled with a celluloses, gums,
polysaccharides,
polyacrylamides, alkoxylated fatty amines and mixtures thereof.
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-26-
[0084] The diverting agent may be added to the carrier fluid as the treatment
is started,
continuously as the treating fluid is pumped into the well bore or may be
added in intervals in the
carrier fluid between stages of the treatment. For instance, in acidizing
procedures the diverting
agent may be added to the acidizing fluid continuously. Thus, the diverting
agent will
progressively plug portions of the formation being treated, thereby
frustrating the tendency of the
acid to flow only into the most permeable portions of the formation and,
instead, creating an
evenly acidized formation. When the treating fluid is pumped in stages, the
first stage is
followed by a volume of the diverting material composed of a carrier fluid,
usually gelled or
emulsified water or acid, containing the bridging agent. The diverting agent
seals off the portion
of the formation penetrated by the first stage of treating fluid. The second
stage of treating fluid
is then pumped into another portion of the formation. Alternating volumes of
treating fluid and
diverting material may be continued to provide a uniformly acidized formation.
Although the
same technique of continuously introducing the diverting agent in the carrier
fluid may be used
for fracturing treatments, it is usual for the diverting agent to be added to
the carrier fluid in slugs
during fracturing operations.
[0085]A fracturing liquid is known to preferentially flow into the portion of
the subterranean
formation which most readily accepts the liquid. After this portion of the
formation is fractured,
the bridging agent may be added to the fracturing liquid so that it will plug
the already fractured
portion of the formation. Because the fracturing fluid is preferentially
flowing into the fracture
zone, it will carry the bridging agent with it. The fractured zone is thereby
plugged and the
fracturing fluid is diverted to the most permeable portion of the formation
that is still accepting
fluids.
[0086] This method of fracturing and diverting can, in one aspect of the
present invention, be
repeated to obtain multiple fractures.
[0087] The diverting agent is removed from the formation by means of
sublimation of the
diverting agent or by solubilization of the diverting agent by the produced
fluids. Increasing
formation temperatures result in a greater rate of dissolution or sublimation
of the diverting
agent. For instance, it has been found that at about 250 F, approximately 80
percent by weight
of the slightly water-soluble collagen sublimates in 24 hours, while at 300
F, about 95 percent
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-27-
by weight sublimates in 24 hours, and at a temperature of about 400 OF, about
99% of the
slightly water-soluble collagen sublimates/dissolves in about 24 hours. This
shows that the rate
of sublimation/dissolution of the diverting agent increases with increasing
formation
temperature.
[0088]The following examples are included to demonstrate preferred embodiments
of the
invention. It should be appreciated by those of skill in the art that the
techniques disclosed in the
examples which follow represent techniques discovered by the inventors to
function well in the
practice of the invention, and thus can be considered to constitute preferred
modes for its
practice. However, those of skill in the art should, in light of the present
disclosure, appreciate
that many changes can be made in the specific embodiments which are disclosed
and still obtain
a like or similar result without departing from the scope of the invention.
EXAMPLES
Example 1: Prophetic Example
[0089] The following prophetic example describes a method of how the soluble
coating on the
propping agent or agents of the present invention can be used to divert
fracture growth and
extend the fractures into the productive zone of an oil or gas well. The
primary purpose of the
soluble coated proppant is to define an upper and lower boundary in the
hydraulically generated
vertical fracture so that the main direction of growth continues to extend
outward in length away
from the wellbore. This additional length of the conductive fracture aids in
draining additional
areas of the productive formation, allowing oil, gas, and/or water recovery
production to be
improved and greater flow rates to be established as a result of longer
fracture length.
[0090] The following steps can be followed, using the soluble coated proppant
materials of the
present invention.
1. A fracture injection rate is established with a low viscosity fracturing
fluid.
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-28-
2. A soluble coated proppant, such as walnut hulls coated with a cross-linked
collagen, bauxite coated with cross-linked collagen, or a combination of both,
is
added at the blender tub in order to form a slurry in the fracturing fluid.
3. The fracturing fluid containing the soluble coated proppant is pumped
downhole.
The first part of the slurry enters the initial crack, taking the most fluid.
In doing
so, it slowly plugs the borders of the created fracture due to the use of a
soluble
diverting agent, such as collagen, that slowly softens and swells in the
fluid.
4. Once the flow rate is slowed or substantially reduced in the first crack,
pressure
builds up until another flow path, crack, or zone begins to take the soluble
coated
proppant-containing slurry.
5. In the instance that both the top and the bottom of the fracture need to be
contained by the soluble coated proppant, two different proppant densities are
preferably used. For example, a high density bauxite particle is coated with a
soluble, collagen coating that slowly softens and swells as it falls in the
fracture to
the bottom of the vertically-created fracture. To slow the growth upwards in
the
vertical fracture, a second proppant of low density, such as a soluble-coated
walnut hull, is added to the injection fluid. As the injection fluid enters
the
formation, the low-density, soluble-material coated proppant rises in the
vertical
fracture and slows down fluid loss and growth in an upward direction.
6. As the fracture is still being injected with fluid above the fracture rate
and
pressure, the fracture continues to grow away from the wellbore and control of
the
fracture growth is maintained by controlling the flow rate of the fracturing
fluid.
Injection is continued until the regular proppant fills the fracture, pressure
reaches a pre-set limit, or until the total planned volume is injected.
7. Standard, non-soluble coated proppants, such as Ottawa Sand (20/40),
ceramic, or
any number of resin-coated proppants, are injected into the formation, once
the
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-29-
top and bottom growth is diminished. Pumping is continued until the full
amount
of designated proppant (or proppants) are placed in the created fractures.
8. The well is shut in, and the pumping equipment is removed.
9. The well is returned to production, and the soluble collagen-coating on the
walnut
hulls or bauxite is removed as the water in the formation dissolves the
soluble
coating on the proppant over time.
Example 2: Procedure for determining the rate and degree of polymer
dissolution
[0091] Sand substrate was coated with various water-soluble polymers:
Chemical Name Trade Name Supplier
Poly(ethylene) oxide WSR 80 Dow Chemicals
Poly(propylene) oxide WSRN 750 Dow Chemicals
Poly(proplylene) oxide UCAR309 Dow Chemicals
Poly(lactic acid) PLA6551-D E&M specialties
Poly(lactic acid) PLA5600 E&M specialties
Poly(vinylacetate/alcohol) PVA/Hydrolene Idroplax Inc.
Collagen 1 Glue 512 Milligans and Higgins
Collagen 2 GM Bond Hormel foods
[0092] Thereafter, the following test procedure was used to determine rate and
degree of
solubility:
Determine the total mass of the polymer on the sand by regular LOI procedure.
Add 500
grams of coated sand in 1 liter of water. Take a 400mm filter paper and weight
it on an
analytical balance up to 4 decimal places. Prepare vacuum filtration apparatus
by using
400mm filter paper, perforated ceramic funnel, 2 liters Erlenmeyer flask with
side
opening connected to the vacuum pump by a rubber tube. Filter the coated sand
and
water slurry through 400mm filter paper after each one-minute interval.
Remember to
add coated sand back in the "filtered" water. Remove the filter paper from the
perforated
funnel after filtration is complete, and allow it to dry by keeping it in
desiccators. Weigh
the filter paper. This is the combined weight of dissolved polymer and filter
paper, and
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-30-
thus it should be greater than the weight of the filter paper before it was
used in filtration
process. Calculate the % of dissolved polymer by using the following formula:
X = ((C-B)/A))x 100
Where,
X = the percentage of dissolved polymer
A = mass (gms) of the polymer on the sand grains
B = mass (gms) of the filter prior to filtration process
C = mass (gms) of the filter after filtration process
Particles that swell and then dissolve Particles that dissolve without
swelling
UCAR309 WSR 80
COLLAGEN I. WSRN750
COLLAGEN 2 PLA 6551-D
PLA 5600
[0093] The results of this test procedure were that a polyethylene oxide(WSR
80 from Dow
Chemical) reach full dissolution at 80 F in about 300 minutes, at 150 F it
required about 180
minutes, and at 200 F it required about 90 minutes.
[0094] The same test was run using another polymer. These results showed that
the
polypropylene oxide polymer(WSRN 750 from Dow Chemicals) reached full
dissolution at 80 F
in about 390 minutes, at 150 F it required about 320 minutes, and at 200 F it
required about 245
minutes to fully dissolve.
[0095] Polymers that swell show 100% solubility within 30 minutes, but
microscopic analysis
shows retention on the filter paper due to swelling instead of dissolution.
Formation of
gelatinous mass and noticeable increase in the volume of the sand/water slurry
indicate polymer
swelling instead of polymer dissolution.
[0096] All of the compositions, methods, and/or processes disclosed and
claimed herein can be
made and executed without undue experimentation in light of the present
disclosure. While the
compositions and methods of this invention have been described in terms of
preferred
embodiments, it will be apparent to those of skill in the art that variations
may be applied to the
compositions, methods, and/or processes and in the steps or in the sequence of
steps of the
CA 02595686 2007-07-20
WO 2006/088603 PCT/US2006/001916
-31 -
methods described herein without departing from the concept and scope of the
invention. More
specifically, it will be apparent that certain agents which are both
chemically and physiologically
related may be substituted for the agents described herein while the same or
similar results would
be achieved. All such similar substitutes and modifications apparent to those
skilled in the art
are deemed to be within the scope and concept of the invention.