Note: Descriptions are shown in the official language in which they were submitted.
CA 02595717 2007-07-24
WO 2006/080369 PCT/JP2006/301177
DESCRIPTION
CATALYST FOR PURIFYING EXHAUST GASES
AND
EXHAUST-GAS PURIFICATION CONTROLLER USING THE SAME
TECHNICAL FIELD
[0001] Thepresentinvention relates to a catalyst for purifying
exhaust gases, such as a three-way catalyst for purifying HC,
CO and NOx in exhaust gases, and an exhaust-gas purification
controller using the same. In particular,it relates to a catalyst
for purifying exhaust gases, catalyst which is good in terms of
the HC purifying performance in low-temperature regions, such
as at the time of starting engine, and an exhaust-gas purification
controller using the same, exhaust-gas purification controller
which can control the combustion of internal combustion engine
optimally and can accordingly demonstrate high NOx purifying
performance.
BACKGROUND ART
[0002] As a catalyst for purifying automotive exhaust gases,
a three-way catalyst has been used extensively conventionally.
The three-way catalyst comprises a porous support, such as alumina,
and a noble metal, such as Pt, loaded on the porous support, and
can purify CO, HC and NOx efficiently at around the theoretical
air-fuel ratio.
[0003] Among the noble metals, Pt and Pd contribute to the
oxidation purification of CO and HC mainly, and Rh contributes
to the reduction purification of NOx mainly, and at the same Rh
acts to inhibit the sintering of Pt or Pd. Therefore, it has
1
CA 02595717 2007-07-24
WO 2006/080369 PCT/JP2006/301177
been understood that, by using Pt or Pd with Rh combinedly, it
is possible to suppress the drawback that the activity of Pt or
Pd has been lowered by the decrease of active sites in Pt or Pd
resulting from the sintering of Pt or Pd, and that it is accordingly
possible to improve the heat resistance of Pt or Pd.
[0004] The noble metal loaded on the three-way catalyst does
not effect the catalytic reaction at temperatures lower than the
activation temperature. Accordingly, there has been a drawback
that the emission of HC is abundant, because the three-way catalyst
does not function suf f iciently in exhaust gaseswhose temperature
falls in low-temperature region, such as at the time of starting
engine. Moreover, the following fact is another cause of the
drawback. That is, the air-fuelratio has often become fuel-rich
atmospheres so that the HC content is abundant when an engine
is cold started.
[0005] Hence, as disclosed in Japanese Unexamined Patent
Publication (KOKAI) No. 6-205, 983, it has been often carried out
to increase the loading amount of noble metal on the exhaust-gas
flow upstream side of catalyst. On the exhaust-gas f low upstream
side of catalyst, since exhaust gases, which have not been turned
into the laminar flow, collide with the cellular walls of catalyst,
the temperature increment of catalyst is so quick that the noble
metal reaches the activation temperature quickly relatively.
After the noble metal reaches the activation temperature, the
temperature of catalyst is increased furthermore by the reaction
heat of the noble metal. Accordingly, the temperature increment
of catalyst is facilitated on the exhaust-gas flow downstream
2
CA 02595717 2007-07-24
WO 2006/080369 PCT/JP2006/301177
side. Consequently, the purifying performance of catalyst is
improved in low-temperature region.
[00061 However, when increasing the loading amount of Pt, for
instance, the loading density of Pt heightens. Accordingly, the
sintering between Pt particles has facilitated. Consequently,
there has been a drawback that that the activity of Pt is likely
to lower.
[00071 Moreover, it has been known to use Pd whose HC oxidizing
activity is high especially as the noble metal. For example,
Japanese Unexamined Patent Publication (KOKAI) No. 8-24,644
proposes a catalyst in which Pd is loaded over the entire length
of the catalyst and at the same time Pt is loaded on the exhaust-gas
flow upstream side of the catalyst. This catalyst demonstrates
high purifying performance, because the balance between the
characteristics of Pd, whose three-way activity is good at around
the stoichiometric point, and the characteristics of Pt, whose
NOXpurifyingperformance is goodon fuel-lean sides, is optimized.
[00081 In addition, Japanese Unexamined Patent Publication
(KOKAI) No. 8-332, 350 proposes a catalyst in which Pd and Rh are
loaded on the exhaust-gas flow up-stream side and Pt and Rh are
loaded on the downstream side with respect to Pd and Rh. This
catalyst is good in terms of the HC purifying performance in
low-temperature region and the durability at high temperatures,
because Pd is loaded in a higher concentration on the upstream
side. Moreover, this catalyst demonstrates high NOX purifying
performance, because the upstream-side reaction enhances the
activity of the downstream-side Pt.
3
CA 02595717 2007-07-24
WO 2006/080369 PCT/JP2006/301177
[0009] However, there is a problem that the NOx purifying
performance of catalyst is lower when Pd and Rh coexist than when
Pt and Rh coexist. Moreover, the alloying of Pd with Rh is more
likely to develop than the alloying of Pt with Rh. Accordingly,
there is a drawback that the alloying has lowered the
characteristics of Rh. In addition, since Rh is extremely scarce
as resource, it has been desired to make use of Rh efficiently
and at the same time to enhance the durability of Rh by suppressing
the degradation.
[0010] Note that the three-way catalyst oxidizes HC and CO and
reduces NOX to purify them in exhaust-gas atmospheres at around
the stoichiometric point. Accordingly, it is essential to
control the air-fuel ratio of engine so that the exhaust-gas
atmospheres are at around the stoichiometric point. It is
possible to carry out such a control by detecting a physical
quantity, such as the oxygen concentration in exhaust gases
emitted from engine, which relates to the catalyst-inlet gas
atmosphere, and carrying out the feed back control of the air-fuel
ratio (A/F) of engine depending on the physical quantity. However,
even when air-fuel mixtures with fuel-rich air-fuel ratios are
combusted to produce exhaust gases, the catalyst-outlet gas
atmospheres might be turned into the stoichiometric atmosphere
or fuel-lean atmospheres, because HC have been consumed in the
three-way catalyst. Consequently, the exhaust-gas atmospheres
immediately downstream to engine might differfrom the exhaust-gas
atmospheres at the outlet of the three-way catalyst.
[0011] Hence, a first sensor for detecting a physical quantity,
4
CA 02595717 2007-07-24
WO 2006/080369 PCT/JP2006/301177
which relates to the three-way catalyst-inlet gas atmospheres,
and a second sensor for detecting a physical quantity, which
relates to the three-way catalyst-outlet gas atmospheres, have
been disposed in an exhaust system of engine conventionally. The
output difference between the first and second sensors has been
judged to change the fuel injection volume. Thus, it is possible
to control the air-fuel ratio optimally depending on the degree
of three-way catalyst' s activity, and accordingly it is possible
to secure high conversions. Moreover, when there should be a
difference between both atmospheres, which the first and second
sensor detect, and when the difference is detected so that it
is lower than a predetermined range, it is possible to know the
replacement timing of three-way catalyst explicitly.
(00121 One of the inventors of the present invention proposed
a novel catalyst in Japanese Patent Application No. 2004-262, 301.
The catalyst has a catalytic loading layer, which comprises: a
coexistence area on which Rh and Pt are loaded over an area extending
from the exhaust-gas inlet-end surface of a support substrate
to a location of 4/10 or less of the overall length of the support
substrate; and an Rh area which is formed from the coexistence
area to the exhaust-gas flow downstream side, and on which Rh
is loaded uniformly in the exhaust-gas flow direction. In this
catalyst, since the coexistence area with Pt and Rh loaded is
formed on the exhaust-gas flow upstream side, which is more likely
to become high temperatures than the exhaust-gas flow downstream
side, Rh suppresses the sintering of Pt in the coexistence area.
Accordingly, the activity of Pt is inhibited from lowering.
CA 02595717 2007-07-24
WO 2006/080369 PCT/JP2006/301177
Moreover, even if Pt is alloyed with Rh in the coexistence area
to degrade the characteristics of Rh, Rh, which is loaded on the
Rh area, shows the characteristics fully, and additionally the
length of the coexistence area is 4/10 or less of the overall
length of the support substrate. Consequently, it is possible
to make use of Rh efficiently.
[0013] However, when trying to control the air-fuel ratio of
engine depending on the output values from the first and second
sensors as described above, using the novel catalyst proposed
in Japanese Patent Application No. 2004-262,301 as a three-way
catalyst, there has been a problem that large errors arise in
the output values from the second sensor. That is, immediately
after combusting an air-fuel fuel mixture with a fuel-rich A/F
ratio in engine, the outlet gas from the three-way catalyst should
be a fuel-lean atmosphere because HC have been consumed to reduce
NOX. However, there occurs a drawback that the output value from
the second sensor has indicated that the outlet gas from the
three-way catalyst is a fuel-rich atmosphere. If such is the
case, since an engine control unit controls the air-fuel ratio
to turn it into the stoichiometric air-fuel ratio, not only the
NOX conversion of the three-way catalyst has degraded, but also
the accuracy of air-fuel ratio control has deteriorated.
Eventually, the accuracy of grasping the deterioration degree
of three-way catalyst has lowered.
[0014] One of the causes that bring out such a problem is believed
to be as follows . When the inlet gas to the above-described novel
catalyst is in a fuel-rich atmosphere, the Rh area of the novel
6
CA 02595717 2007-07-24
WO 2006/080369 PCT/JP2006/301177
catalyst facilitates the steam reforming reaction to generate
H2. The resulting H2 has fluctuated the sudden output change-over
point (or threshold value) of the second sensor.
DISCLOSURE OF THE INVENTION
(0015] The present invention has been developed in view of the
aforementioned circumstances. It is therefore an object of the
present invention to control the air-fuel ratio of internal
combustion engine optimally by inhibiting the unnecessary
fluctuation of the sudden output change-over point of the second
sensor, unnecessary fluctuation which results from H2 generated
in the Rh area of the novel catalyst.
10016] A catalyst according to the present invention for
purifying exhaust gases achieves the aforementioned object, and
comprises:
a support substrate having an exhaust-gas flow passage;
and
a catalytic loading layer formed on a surface of the
exhaust-gas flow passage, and composed of a porous oxide support
and a catalytic ingredient, the catalytic loading layer
comprising:
an Rh area on which rhodium is loaded as the catalytic
ingredient; arld
an oxidizing area which is formed on an exhaust-gas flow
downstream side with respect to the Rh area, and on which a catalytic
ingredient exhibiting an oxidizing activity at least is loaded.
(0017] In the present catalyst, the catalytic loading layer
can desirably further comprise a coexistence area, which is f ormed
7
CA 02595717 2007-07-24
WO 2006/080369 PCT/JP2006/301177
on an exhaust-gas flow upstream side with respect to the Rh area,
and on which rhodium and platinum are loaded as the catalytic
ingredient. Moreover, it is preferable that the support
substrate can have a predeterminedoverall length; the coexistence
area of the catalytic loading layer can have a length which is
4/10 times or less as short as the predetermined overall length
of the support substrate; and the coexistence area can comprise
rhodium and platinum in a proportion of Pt with respect to Rh
falling in a range of 10 :_!5 Pt/Rh _"~ 60 by weight ratio. Inaddition,
the porous support can preferably include ceria at least.
[0018] An exhaust-gas purification controller according to the
present invention achieves the aforementioned object, and
comprises:
the present catalyst, and disposed in an exhaust channel
of an internal combustion engine;
a first sensor disposed on an exhaust-gas flow upstream
side with respect to the present catalyst, and detecting a physical
quantity relating to a catalyst-inlet gas atmosphere;
a second sensor disposed on an exhaust-gas flow downstream
side with respect to the present catalyst, and detecting a physical
quantity relating to a catalyst-outlet gas atmosphere; and
a control device for receiving detection signals, which
are output from the first sensor and the second sensor, and
controlling an air-fuel ratio of the internal combustion engine.
[0019] Since the present catalyst comprises the oxidizing area,
which is disposed on a downstream side with respect to the Rh
area, H2, which is generated in the Rh area, is oxidized in the
8
CA 02595717 2007-07-24
WO 2006/080369 PCT/JP2006/301177
oxidizing area. Accordingly, it is possible to inhibit the sudden
output change-over point of the second sensor from fluctuating.
Consequently, the present exhaust-gas purification controller
can minimize the error in the output values from the second sensor
remarkably. Therefore, not only the present exhaust-gas
purification controller can have the present catalyst exhibit
an improved NOx conversion, but also it can upgrade the accuracy
of air-fuel control greatly. Moreover, the present exhaust-gas
purification controller exhibits enhanced accuracy for grasping
the degradation degree of catalyst.
[00201 Moreover, when the present catalyst comprises the
coexistence area with Pt and Rh loaded, coexistence area which
is formed on an exhaust-gas flow upstream side being more likely
to become high temperatures than an exhaust-gas flow downstream
side, Rh inhibits the sintering of Pt in the coexistence area
so that the activity of Pt is prevented from lowering. Moreover,
even if Pt is alloyed with Rh to lower the characteristics of
Rh, Rh, which is loaded on the Rh area, shows the characteristics
fully. In addition, when the length of the coexistence area is
controlled to be 4/10 times or less as short as the overall length
of the support substrate, and when the proportion of Pt with respect
to Rh is controlled to fall in a range of 10 c Pt/Rh c 60 by
weight ratio in the coexistence area, Rh, which is alloyed with
Pt, is so less that it is possible to make use of Rh efficiently.
BRIEF DESCRIPTION OF THE DRAWINGS
100211 A more complete appreciation of the present invention
and many of its advantages will be readily obtained as the same
9
CA 02595717 2007-07-24
WO 2006/080369 PCT/JP2006/301177
becomes better understood by reference to the following detailed
description when considered in connection with the accompanying
drawings and detailed specification, all of which forms a part
of the disclosure.
[0022] Fig.lisa perspectivediagram for illustrating a catalyst
of Example No. 1 according to the present invention.
[0023] Fig. 2 is a cross-sectional diagram for illustrating
the catalyst of Example No. 1 according to the present invention.
[0024] Fig. 3 is a block diagram for illustrating an exhaust-gas
purification controller of Example No. 1 according to the present
invention.
[0025] Fig.4isa flowchartforillustrating how the exhaust-gas
purification controller of Example No. 1 according to the present
invention carries out controlling the combustion of engine.
[0026] Fig. 5 is a time chart for illustrating the relationships
between the A/F value and the output value from a second sensor
when the air-fuel ratio is switched from fuel-lean atmosphere
to fuel-rich atmosphere.
BEST MODE FOR CARRYING OUT THE INVENTION
[0027] Having generally described the present invention, a
further understanding can be obtained by reference to thespecific
preferred embodiments which are provided herein for the purpose
of illustration only and not intended to limit the scope of the
appended claims.
[0028] The present catalyst comprises the oxidizing area which
is further formed on a downstream side with respect to the Rh
area, in addition to the Rh area. Therefore, even if
CA 02595717 2007-07-24
WO 2006/080369 PCT/JP2006/301177
fuel-rich-atmosphere exhaust gases flow into the present catalyst
to facilitate the steam reforming reaction in the Rh area so that
H2 is generated, the resulting H2 is oxidized in the oxidizing
area, and accordingly hardly contacts with the second sensor.
As a result, not only it is possible to inhibit the sudden output
change-over point of the second sensor from fluctuating, but also
it is possible to improve the detection accuracy of the secorid
sensor. Thus, the NOxconversion of the present catalyst upgrades
as well as the air-fuel control accuracy of the present exhaust-gas
purification controller enhances. In addition, the accuracy of
the present exhaust-gas purification controller for grasping the
degradation degree of catalyst improves as well.
100291 The Rh area of the catalytic loading layer can preferably
comprise Rh in a loading amount of from 0.05 to 5 g with respect
to 1-L volume of the support substrate. When the loading amount
of Rh is less than the lower limit of the range, the resulting
Rh area exhibits insufficient purifying performance. When the
loading amount of Rh is more than the upper limit of the range,
the effect of Rh addition has saturated so that it is impossible
to utilize Rh effectively. Note that the loading density of Rh
in the Rh region can differ from the loading density of Rh in
the coexistence area. However,from the viewpoint of production,
it is convenient to control the loading density of Rh in the Rh
region identical with the loading density of Rh in the coexistence
area.
[00301 The forming range of the oxidizing area is not limited
in particular, as far as the oxidizing area is formed on an
11
CA 02595717 2007-07-24
WO 2006/080369 PCT/JP2006/301177
exhaust-gas flow downstream side with respect to the Rh area.
However, it is desirable to form the oxidizing area over the entire
exhaust-gas flow downstream side of the catalytic loading layer
with respect to the Rh area. On the oxidizing area, a catalytic
ingredient, which exhibits an oxidizing activity at least, is
loaded. As for such a catalytic ingredient, it is possible to
exemplify Pt, Pd, Ni, and Co. Among them, it is especially
preferable to use at least one member selected from the group
consisting of Pt and Pd. Note that the other noble metals, or
transition metals other than noble metals can be loaded on the
oxidizing area in such a loading amount that does not impair the
oxidizing activity of the catalytic ingredient. Note that the
oxidizing area of the catalytic loading layer can preferably
comprise the catalytic ingredient in a loading amount of from
0.05 to 100 g, further preferably from 1 to 40 g with respect
to 1-L volume of the support substrate.
[0031] The present catalyst can desirably further comprise a
coexistence area, which is disposed on an exhaust-gas flow
upstream side with respect to the Rh area and on which Rh and
Pt are loaded. When the present catalyst comprises the
coexistence area, low-temperature exhaust gases, which are
produced when starting an engine,first pass the coexistence area
after they collide with the exhaust-gas inlet-end surface of the
present catalyst in such a state that they have not yet turned
into laminar flow. Accordingly, the heat of the exhaust gases
increases the temperature of the present catalyst quickly so that
Pt with good ignitability, which is loaded on the coexistence
12
CA 02595717 2007-07-24
WO 2006/080369 PCT/JP2006/301177
area, reaches the activation temperature in a short period of
time relatively. Then, the reaction heat further increases the
temperature of the present catalyst so that the temperature
increment is facilitated as well on the exhaust-gas flow
downstream side of the present catalyst. Consequently, the
present catalyst demonstrates improved purifying performance for
HC and NOX.
[00321 On the other hand, even when the coexistence area becomes
high temperatures, since Rh inhibits the sintering of Pt, the
activity of Pt is prevented from lowering so that the durability
of the present catalyst enhances. Note that, even if Pt is alloyed
with Rh to degrade the characteristics of Rh in the coexistence
area, Rh, which is loaded on the Rh area, show the characteristics
fully. Moreover, the coexistence area can preferably have a
length, which is 4/10 times or less, further preferably from 0/10
to 4/10 times furthermore preferably from 2/10 to 4/10 times,
as short as the overall length of the support substrate. Thus,
it is possible to make the amount of Rh, which is alloyed with
Pt, less. Accordingly, it is possible to use expensive Rh
efficiently. When the coexistence areaisformed to havealength,
which is more than 4/10 times as short as the overall length of
the support substrate, the proportion of Rh alloying with Pt
increases so that the resulting catalyst has shown insufficient
purifying performance for HC and NOx. The coexistence area can
be formed continuously from the exhaust-gas inlet-end surface
of the present catalyst. However, it has been known thatcatalytic
ingredients, such as noble metals, which are loaded in a range
13
CA 02595717 2007-07-24
WO 2006/080369 PCT/JP2006/301177
of 5 mm from the exhaust-gas inlet-end surface of catalyst,
contribute to catalytic reactions in lesser degrees relatively.
Consequently, it is advisable to dispose the coexistence area
by 5 mm or more on an exhaust-gas flow downstream side with respect
to the exhaust-gas inlet-end surface of the present catalyst.
[0033] Note that, when the coexistence area is formed to have
a length, which is 4/10 times or less as short as the overall
length of the support substrate, the oxidizing area can desirably
have a length, which is 1/5 time or less, further desirably from
1/10 to 1/5 time, as short as the overall length the support
substrate: and the balance can desirably be assigned to the Rh
area. When the Rh area has a length, which is more than 4/10
times as short as the overall length of the support substrate,
the resulting catalyst has showed lowered purifying performance
for NOx. Since it is sufficient for the oxidizing area to have
a function of oxidizing H2, it is satisfactory that the oxidizing
area has a length, which is 1/5 time or less as short as the overall
of length of the support substrate.
[0034] The coexistence area can preferably comprise Rh and Pt
in a proportion of Pt with respect to Rh falling in a range of
c Pt/Rh 60 by weight ratio. It is especially desirable
that the proportion of Pt with respect to Rh can fall in a range
of 15 c Pt/Rh :_5 50 by weight ratio. The proportion of Pt with
respect to Rh is smaller than the lower limit of the preferable
range, the resulting catalyst exhibits lowered ignitability so
that it has shown degraded HC purifying performance at low
temperatures. When the proportion of Pt with respect to Rh is
14
CA 02595717 2007-07-24
WO 2006/080369 PCT/JP2006/301177
larger than the upper limit of the preferable range, the sintering
of Pt is likely to occur at high temperatures. Specifically,
the coexistence area can preferably comprise Pt in a loading amount
of from 0. 5 to 40 g, further preferably from 5 to 40 g, furthermore
preferably from 10 to 40 g, with respect to 1-L volume of the
support substrate. When the loading amount of Pt is less than
the lower limit of the preferable range, the resulting catalyst
is poor in terms of the ignitability at low temperatures so that
it has shown insufficient purifying performance for HC and NOx.
When the loading amount of Pt is more than the upper limit of
the preferable range, not only the effect of Pt addition has
saturated but also the sintering of Pt is likely to occur at high
temperatures. Moreover, the coexistence area can comprise Rh
in such a loading amount that can inhibit loaded Pt from sintering.
For example, the coexistence area can preferably comprise Rh in
a loading amount of from 0.05 to 5 g, further preferably from
0. 1 to 5 g, with respect to 1-L volume of the support substrate.
When the loading amount of Rh is less than the lower limit of
the preferable range, the sintering of Pt is likely to occur at
high temperatures. Note that the other noble metalsorbase metals
can be loaded on the coexistence area in such a loading amount
that does not impair the advantages resulting from the coexistence
area. However, it is desirable that only Pt and Rh can be loaded
on the coexistence area.
[0035] The present catalyst can be formed as pellet shapes,
honeycomb shapes, and foam shapes. The support substrate can
be made of heat-resistant ceramic, such as cordierite, or metallic
CA 02595717 2007-07-24
WO 2006/080369 PCT/JP2006/301177
foil. On the inner peripheral surface of a plurality of cells,
which are demarcated in the support substrate, or on the surface
of the support substrate, the catalytic loading layer, which is
composed of the porous oxide support and catalytic ingredient,
is formed.
[00361 As for the porous oxide support, it is possible to use
a single species or a plurality of species which are selected
from the group consisting of A1203, Si02, Zr02, CeO2 and Ti02.
Moreover, it is possible to use composite oxides which are composed
of a plurality of the simple oxides. Among such composite oxides,
it is preferable use composite oxides including CeO2. That is,
it is possible to inhibit the exhaust-gas atmospheres from
fluctuating by means of the oxygen absorbing-and-releasing
ability of Ce02. Moreover, when the porous oxide support is
composed of a Ce02-ZrO2 composite oxide, the porous oxide support
made of Ce02-ZrO2 and with Pt loaded exhibits more upgraded oxygen
absorbing-and-releasing ability than CeO2 with Pt loaded does.
In addition, the porous oxide support made of Ce02-ZrO2 and with
Rh loaded shows more enhanced hydrogen generating ability as well
as NOX purifying performance than Ce02 with Rh loaded does.
100371 The porous oxide support of the catalytic loading layer
can preferably have a uniform composition over the entire length
of the support substrate in view of production process. However,
depending on actual cases, it is possible to use different porous
oxide support for the Rh area and the oxidizing area, or further
for the coexistence area. For example, the porous oxide support
can be composed of A1203 in the coexistence area and oxidizing
16
CA 02595717 2007-07-24
WO 2006/080369 PCT/JP2006/301177
area; and can be composed of a Ce02-ZrO2 composite oxide in the
Rh area. If such is the case, since the characteristics of
catalytic ingredients furthermore enhance in all of the three
areas, the present catalyst shows much better purifying
performance.
1 0038 ] The present exhaust-gas purification controller
comprises the present catalyst, a first sensor, a second sensor,
and a control device. The present catalyst is disposed in an
exhaust channel of an internal combustion engine. The first
sensor is disposed on an exhaust-gas flow upstream side with
respect to the catalyst, and detects a physical quantity relating
to a catalyst-inlet gas atmosphere. The second sensor is disposed
on an exhaust-gas flow downstream side with respect to the catalyst,
and detects a physical quantity relating to a catalyst-outlet
gas atmosphere. The control device is for receiving detection
signals, which are output from the first sensor and the second
sensor, and controlling an air-fuel ratio of the internal
combustion engine.
[0039] As for the first and second sensors, it is possible to
use A/F sensors, oxygen sensors, and the like, which have been
used conventionally. As for the control device, it is possible
to use engine control units (hereinafter abbreviated to as"ECU").
In the second sensor at least, the sudden output change-over point
might be fluctuated by H2. The control subj ects, which the control
device carries out, can be the same as those of the conventional
ones. By using the present catalyst, it is possible to prevent
the sudden output change-over point of the second sensor from
17
CA 02595717 2007-07-24
WO 2006/080369 PCT/JP2006/301177
fluctuating in fuel-rich-atmosphere exhaust gases, which are
produced by combusting fuel-rich air-fuelmixtures. Asa result,
it is possible to carry out the air-fuel ratio control with high
accuracy.
EXAMPLES
[0040] The present invention will be hereinafter described in
detail with reference to examples, a comparative example and a
conventional example.
(Example No. 1)
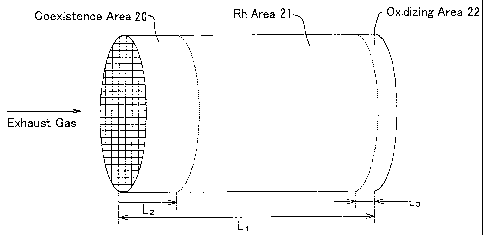
[0041] Fig. 1 and Fig. 2 illustrate a catalyst according to
Example No. 1 of the present invention for purifying exhaust gases.
The catalyst comprises a cylinder-shaped honeycomb substrate 1,
and a catalytic loading layer 2. The honeycomb substrate 1
comprises a large number of square-shaped cells, and has an overall
length of 130 mm (L1) . The catalytic loading layer 1 is formed
on the surface of the cells. A coexistence area 20 is formed
by a length of 20 mm (L2) from the exhaust-gas inlet-end surface
of the catalyst to an exhaust-gas flow downstream side; an Rh
area 21 is formed by a length of 100 mm from the coexistence area
20 to an exhaust-gas flow downstream side; and an oxidizing area
22 is formed by a length of 10 mm (L3) from the Rh area 21 to
the exhaust-gas outlet-end surface of the catalyst.
[0042] Hereinafter, the production process of the catalyst will
be described instead of describing the construction thereof in
detail.
[0043] 120 parts by weight of a Ce02-ZrO2 solid solution powder,
80 parts by weight of an activated alumina powder, and an alumina
18
CA 02595717 2007-07-24
WO 2006/080369 PCT/JP2006/301177
binder were mixed with a predetermined amount of water. Note
that the Ce02-ZrO2 solid solution powder was composed of Ce02r
Zr02 and Y203 in a proportion of CeO2 : Zr02 : Y203 = 65 : 30 :
15 by molar ratio. Moreover, the alumina binder was composed
of alumina hydrate in an amount of 3 parts by weight, and 40%
aluminum nitrate aqueous solution in an amount of 44 parts by
weight. The resulting mixture was milled to prepare a slurry.
The resultant slurry was wash coated onto the honeycomb substrate
1. Note that honeycomb substrate 1 was made of cordierite; and
had a volume of 1.1 L, cells in a quantity of 600 cells/inch2,
an average cellular wall thickness of 75 ,um, an overall length
of 130 mm, and a diameter of 103 mm. Thereafter, the excessive
slurry was blown off with air. After drying the honeycomb
substrate 1 at 120 C, the honeycomb substrate 1 was calcined
at 650 C for 3 hours. Thus, a coating layer was formed on the
entire cellular surfaces of the honeycomb substrate 1. Note that
the coating layer was formed in an amount of 210 g with respect
to 1-L volume of the honeycomb substrate 1.
[00441 Then, the entire coating layer was immersed into an RhC13
aqueous solution having a predetermined concentration (that is,
the honeycomb substrate 1 was immersed into it over the entire
overall length) to load Rh by means of adsorption. After drying
the honeycomb substrate 1 at 120 C, the honeycomb substrate 1
was calcined at 500 C for 1 hour. Thus, Rh was loaded on the
coating layer. Note that Rh was loaded in an amount of 0.4 g
with respect to 1-L volume of the honeycomb substrate 1.
[0045) Subsequently, the coating layer was impregnated with
19
CA 02595717 2007-07-24
WO 2006/080369 PCT/JP2006/301177
a Pt(N02)2(NH3)Z aqueous solution having a predetermined
concentration by a length of 20 mm from the exhaust-gas inlet-end
surface of the honeycomb substrate 1 to an exhaust-gas flow
downstream side thereof. After drying the honeycomb substrate
1 at 120 C, the honeycomb substrate 1 was calcined at 650 C for
3 hours to load Pt on the coating layer. Thus, the coexistence
area 20 was formed. Note that Pt was loaded on the coexistence
area 20 in an amount of 10 g with respect to 1-L volume of the
honeycomb substrate 1.
(0046] Finally, the coating layer was impregnated with a
Pt(N02)2(NH3)2 aqueous solution having a predetermined
concentration by a length of 10 mm from the exhaust-gas outlet-end
surface of the honeycomb substrate 1 to an exhaust-gas flow
upstream side thereof. After drying the honeycomb substrate 1
at 120 C, the honeycomb substrate 1 was calcined at 650 C for
3 hours to load Pt on the coating layer. Thus, the oxidizing
area 22 was formed. Note that Pt was loaded on the oxidizing
area 22 in an amount of 5 g with respect to 1-L volume of the
honeycomb substrate 1.
[0047] The catalyst of Example No. 1 prepared as described above
was installed to an exhaust system of an automobile immediately
below the 2.4-L displacement engine to make an exhaust-gas
purification controller illustrated in Fig. 3.
[0048] The exhaust-gas purification controller comprises an
engine 3, a catalytic converter 30, a catalyst 31, a first sensor
32, a second sensor 33, and a control device 4. The catalytic
converter 30 is placed in the exhaust pipe of the engine 3. The
CA 02595717 2007-07-24
WO 2006/080369 PCT/JP2006/301177
catalyst 31 is installed within the catalytic converter 30. The
first sensor 32 comprises an A/F sensor, which is placed between
the engine 3 and the catalytic converter 30 and detects the A/F
equivalent value of inlet exhaust gases to the catalyst 31. The
second sensor 33 comprises an oxygen sensor, which is placed on
an exhaust-gas flow downstream side with respect to the catalytic
converter 30 and detects an oxygen gas concentration in outlet
exhaust gases from the catalyst 31. The controller device 4,
to which detection signals are input from the first sensor 32
and second sensor 33, controls the air-fuel ratio of the engine
3 based on the input values. [0049] Fig. 4 illustrates how the
control device 4 carries out the control subj ects . When the engine
3 is started, the first sensor 32 first detects the catalyst-inlet
gas atmosphere at step 100. At step 101, the control device 4
judgesthe deviation of the detected catalyst-inlet gas atmosphere
from the stoichiometric A/F ratio. When the control device 4
judges that the A/F ratio falls in a range of 14.6 0.05, the
stoichiometric atmosphere, the control device 4 does not do
anything and returns the programmed control process to step 100.
On the other hand, when the control device 4 judges that the A/F
ratio deviates from 14. 6, the theoretical stoichiometric value,
by more than 0.05, the control device 4 judges whether the
catalyst-inlet gas atmosphere derives from fuel-lean atmosphere
or not at step 102. When the control device 4 judges that the
catalyst-inlet gas atmosphere derivesfrom fuel-lean atmosphere,
the control device 4 controls the fuel injection volume so as
to make the A/F ratio fall in a range of 14. 6 0.05 at step 103.
21
CA 02595717 2007-07-24
WO 2006/080369 PCT/JP2006/301177
Then, the control device 4 returns the programmed control process
to step 100. On the contrary, when the catalyst-inlet gas
atmosphere does not derive from fuel-lean atmosphere, the control
device 4 judges that the catalyst-inlet gas atmosphere derives
from fuel-rich atmosphere. Then, the control device 4 has the
second sensor 33 detect the oxygen concentration of the
catalyst-outlet gas atmosphere at step 104.
[00501 At step 105, the control device 4 judges whether the
catalyst-outlet gas atmosphere derives f rom fuel-rich atmosphere
or not. When the catalyst-outlet gas atmosphere derives from
fuel-rich atmosphere, the control device 4 controls the fuel
injection volume so as to make the A/F ratio fall in a range of
14. 6 0. 05 at step 103. Thereafter, the control device 4 returns
the programmed control process to step 100. On the contrary,
when the control device 4 does not judge that the catalyst-outlet
gas atmosphere derives from fuel-rich atmosphere, the control
device 4 refers to records, such as the accumulated service time
and thermal history of the catalyst 31, to judge whether the
catalyst 31 has been degraded or not based on a map, which is
stored separately, at step 106.
[00511 When the control device 4 does not judge that the catalyst
31 has been degraded, the control device 4 returns the programmed
control process to step 104 to have the second sensor 33 re-detect
the oxygen concentration of the catalyst-outlet gas atmosphere.
On the other hand, when the control device 4 judges that the catalyst
31 has been degraded, the control device 4 displays a replacement
symbol to call the driver's attention to the replacement of the
22
CA 02595717 2007-07-24
WO 2006/080369 PCT/JP2006/301177
catalyst 31. Moreover, at step 103, the control device 4 controls
the fuel injection volume so as to make the A/F ratio fall in
a range of 14.6 0.05. Thereafter, the control device 4 returns
the programmed control process to step 100.
[0052] Using the above-described exhaust-gas purification
controller of Example No. 1, the catalyst of Example No. 1 was
first subjected to a degradation treatment at an inlet gas
temperature of 950 C (or a catalyst bed temperature of 1, 000 C)
for 100 hours. After completing the degradation treatment, the
engine 3 was operated under the following conditions: a revolving
speed of 1, 600 rpm; and an exhaust-gas flow rate of 10 g/second.
Meanwhile, the output values of the second sensor 33 were measured
with time after the target value of the engine 3's A/F ratio,
which the control device 4 judged according to the detected value
of the first sensor 32, was switched from 14. 8 to 14.4. In this
instance, note that the air-fuel ratio control was carried out
so as to keep the target value of the engine 3' s A/F ratio constant
at 14.8 or 14.4 as illustrated in Fig. 5, without performing the
programmed control shown in Fig. 4. Fig. 5 illustrates the result
of measuring the output values of the second sensor 33 with time.
[0053] Moreover, the engine 3 was operated under a steady driving
condition, 60 km/hour, while performing the programmed control
shown in Fig. 4. Meanwhile, the NOX emission was measured with
time. Table 1 below summarizes the result of measuring the NO,,
emission.
(Example No. 2)
[0054] Except that the catalytic ingredient loaded on the
23
CA 02595717 2007-07-24
WO 2006/080369 PCT/JP2006/301177
oxidizing area 22 was changed from Pt to Pd, a catalyst of Example
No. 2 was prepared in the same manner as set forth in Example
No. 1. Moreover, the output values of the second sensor 33 and
the NOX emission under a steady driving condition were measured
in the same manner as described in Example No. 1. Fig. 5
illustrates the result of measuring the second sensor 33' s output
values, and Table 1 below summarizes the result of measuring the
NOx emission.
(Comparative Example)
[00551 Except that no oxidizing area 22 was formed, that is,
the Rh area 21 was formed by a length of 110 mm from the coexistence
area 20 to the exhaust-gas outlet-end surface of the honeycomb
substrate 1, a catalyst of Comparative Example was prepared in
the same manner as set forth in Example No. 1. Moreover, the
output values of the second sensor 33 and the NO. emission under
a steady driving condition were measured in the same manner as
described in Example No. 1. Fig. 5 illustrates the result of
measuring the second sensor 33' s output values, and Table 1 below
summarizes the result of measuring the NO,, emission.
(Conventional Example)
[00561 Ahoneycomb substrate 1 was prepared. Note that a coating
layer was formed on the honeycomb substrate 1 in the same manner
as Example No. 1. Then, the entire coating layer was immersed
into an RhC13 aqueous solution having a predetermined
concentration (that is, the honeycomb substrate 1 was immersed
into it over the entire overall length) to load Rh by means of
adsorption. After drying the honeycomb substrate 1 at 120 C,
24
CA 02595717 2007-07-24
WO 2006/080369 PCT/JP2006/301177
the honeycomb substrate 1 was calcined at 500 C for 1 hour. Thus,
Rh was loaded on the coating layer. Note that Rh was loaded in
an amount of 0.4 g with respect to 1-L volume of the honeycomb
substrate 1. Subsequently, the coating layer was impregnated
with a Pt(N02)2(NH3)2 aqueous solution having a predetermined
concentration by the overall length of the honeycomb substrate
1. After drying the honeycomb substrate 1 at 120 C, the honeycomb
substrate 1 was calcined at 650 C for 3 hours to load Pt on the
coating layer. Note that Pt was loaded on the coating layer in
an amount of 1.5 g with respect to 1-L volume of the honeycomb
substrate 1.
[0057] Except that the resulting catalyst of Conventional
Example was used, the output values of the second sensor 33 and
the NOX emission under a steady driving condition were measured
in the same manner as described ip Example No. 1. Fig. 5
illustrates the result of measuring the second sensor33'soutput
values, and Table 1 below summarizes the result of measuring the
NOx emission.
<Evaluation>
TABLE 1
NOx Emission (g/km)
Ex. #1 0.0375
Ex. #2 0.0405
Comparative 0.0525
Ex.
Conventional 0.0591
Ex.
[0058] From Fig. 5, it is understood that the second sensor
33 exhibited the sudden output change-over point, which shifted
CA 02595717 2007-07-24
WO 2006/080369 PCT/JP2006/301177
greatly more on a shorter elapsed-time side, in the Comparative
Example than in the Example Nos. 1 and 2 as well as Conventional
Example. Specifically, the Comparative Example carried out the
judgement whether the catalyst-outlet exhaust gas was fuel-rich
or not in a shorter period of time than the Conventional Example
did. Accordingly, in the exhaust-gas purification control
illustrated in Fig. 4, the control device 4 carried out the
stoichiometric A/F control at an earlier time to make the air-fuel
ratio the theoretical stoichiometric value. Consequently, in
the Comparative Example, the air-fuel mixture was kept in
fuel-rich atmosphere in a shorter period of time than it was in
the Conventional Example. Therefore, the Comparative Example
is disadvantageous for purifying NOX.
(0059] However, in Example Nos. 1 and 2, the second sensor 33
exhibited the sudden output change-over point, which shifted by
lesser shift magnitude than that the second sensor 33 did in the
Conventional Example. Additionally, the sudden output
change-over point shifted more on a longer elapsed-time side.
Therefore, in Example Nos. 1 and 2, it was possible to secure
an ample time until the control device 4 judged that
catalyst-outlet exhaust-gas atmosphere derived from a fuel-rich
air-fuel mixture. As a result, Example Nos. 1 and 2 could upgrade
the NOx purifying performance. Moreover, from Table 1 above,
it is appreciated that the Comparative Example exhibited the
poorer NOX emission than Example Nos. 1 and 2 did. It is apparent
that the disadvantage resulted fromthe fact that the sudden output
change-over point of the second sensor 33 shifted more on a shorter
26
CA 02595717 2007-07-24
WO 2006/080369 PCT/JP2006/301177
elapsed-time side in the Comparative Example.
[00601 Note that, in the Conventional Example, the sudden output
change-over point of the second sensor 33 shifted more on a shorter
elapsed-time side than in Example Nos. 1 and 2 as shown in Fig.
5; but the NOx emission degraded more than that of the Comparative
Example as set forth in Table 1. It is believed that the following
action brought about the phenomena: Rh was alloyed with Pt in
the degradation treatment so that the reduction activity of Rh
had been degraded.
INDUSTRIAL APPLICABILITY
[00611 The present catalyst, and the present exhaust-gas
purification controller using the same can be applied to the
purification of exhaust gases emitted form internal combustion
engines, in particular to the purification of HC in
low-temperature regions, such as at the time of starting engine,
and the purification of NOx.
27