Note: Descriptions are shown in the official language in which they were submitted.
CA 02596032 2007-08-02
SOIL AND WATER REMEDIATION METHOD AND APPARATUS
BACKGROUND
1. Field of Invention
The invention relates to methods and apparatuses for the remediation of
contaminated
water and/or soil and, in particular, to the reduction of the concentration of
organic compounds in
water and/or soil.
2. Discussion of Related Art
Both State and Federal governments have issued regulations governing hazardous
organic
and inorganic contaminants in the environment. Subsurface soil and groundwater
contamination
with organic and inorganic contaminants has been a concern since the 1970's.
Action levels and
clean-up standards have been promulgated by both State and Federal government
for numerous
organic and inorganic contaminants.
Regulated organic contaminants in the subsurface environment include, but are
not limited
to: polychlorinated biphenyls (PCBs); chlorinated volatile organic compounds
(CVOCs) such as
tetrachloroethene (PCE), trichloroethene (TCE), trichloroethane (TCA),
dichloroethene
(DCE), vinyl chloride; fuel constituents such as benzene, ethylbenzene,
toluene, xylene, methyl
tert butyl ether (MTBE), tertiary butyl alcohol (TBA), polynuclear aromatic
hydrocarbons
(PAHs), ethylene dibromide (EDB); pesticides such as (but not limited to) DDT;
and
herbicides such as (but not limited to) silvex. Regulated inorganic
contaminants in the subsurface
environment include, but are not limited to: heavy metals, such as lead,
arsenic, chromium,
mercury and silver. State and Federal regulations that govern these subsurface
contaminants outline
a protocol for subsurface investigation to identify the extent of
contamination, identification of the
1
CA 02596032 2007-08-02
human health and ecological risk posed by the contaminants, development of
remedial action
alternatives for reducing or eliminating any significant risk posed by the
contaminants, and
selection and implementation of remedial measures to achieve the remediation
goals.
In situ (ISCO) or ex situ (ESCO) chemical oxidation technology has emerged as
a
prominent remedial measure due to its cost-effectiveness and timeliness in
achieving remediation
goals. ISCO technology can be used alone or in combination with other
complementary
technologies, such as soil vapor extraction (SVE) for removal of volatile
organic compounds from
the unsaturated zone, multi-phase extraction for removal of organic
contaminant from the
unsaturated and saturated zones, or vertical recirculation systems in the
saturated zone.
The literature regarding ISCO or ESCO reports the use of a strong oxidizing
agent to treat
contaminated soil and water by chemically degrading recalcitrant and hazardous
chemicals. Such
oxidants include hydrogen peroxide, Fenton's reagent, ozone, permanganate,
persulfates, and other
peroxygens.
SUMMARY OF INVENTION
The subject matter of this application may involve, in some cases,
interrelated products,
alternative solutions to a particular problem, and/or a plurality of different
uses of a single system
or article.
In one aspect, a method of reducing the concentration of an organic
contaminant in soil is
provided, the method comprising introducing persulfate and ozone or
persulfate, ozone and
hydrogen peroxide into a saturated zone to oxidize at least a portion of the
organic contaminant.
In another aspect a method of reducing the concentration of an organic
contaminant in soil
and/or groundwater is provided, the method comprising introducing a first
oxidant into a saturated
zone to produce a radius of influence, introducing a second oxidant into a
region of a smear zone,
the region being vertically aligned with a portion of the radius of influence,
and oxidizing at least
a portion of the organic contaminant in the saturated zone.
In another aspect a system for remediating contaminated soil and/or
groundwater is
provided, the system comprising a first well comprising at least a first
injection port, the injection
port constructed and arranged to inject an oxidant into the saturated zone to
form a radius of
2
CA 02596032 2007-08-02
influence in the saturated zone, and a second well comprising at least a
second injection port, the
second injection port constructed and arranged to inject an oxidant into the
smear zone in a region
vertically aligned with at least a portion of the radius of influence.
In another aspect a method of reducing the concentration of an organic
contaminant in
water is provided, the method comprising introducing persulfate and ozone
concurrently or
persulfate, ozone and hydrogen peroxide concurrently into the water to oxidize
at least a
portion of the organic contaminant.
In another aspect a method of remediating contaminated soil and/or groundwater
is
provided, the method comprising injecting a first oxidant into the smear zone,
injecting a second
oxidant into the saturated zone under pressure to produce a mounded
groundwater table in the
smear zone, and mixing the first and second oxidants in the mounded
groundwater table to oxidize
contaminants in the smear zone.
BRIEF DESCRIPTION OF DRAWINGS
In the drawings, FIG. I provides a plan view of an embodiment of a groundwater
treatment
system;
FIG. 2 provides an underground cutaway view of the system of FIG. 1;
FIG. 3 provides cross-sectional views of two of the wells shown in FIG. 2;
FIG. 4 provides a cross-sectional side view of the manifold system
accompanying
the wells of FIG. 3;
FIG. 5 provides a cross-sectional top view of the manifold system of FIG. 4;
and
FIG. 6 is a bar graph showing experimental results.
DETAILED DESCRIPTION
A variety of oxidizers are known to be useful in remediating groundwater and
soil
contaminated with organic compounds. Typically, however, an operator chooses a
single oxidizer
based on, for example, the soil type or contaminant class. Preferred oxidizers
in the field are those
that have an ability to permeate through the subsurface either above the
groundwater table
(unsaturated zone) or below the groundwater table (saturated zone) while
interacting with target
3
CA 02596032 2007-08-02
compounds throughout the entire zone of contamination. Oxidizing species, such
as peroxide,
ozone, and hydroxyl radicals can provide powerful oxidation but have
relatively short life times
within the subsurface. Persulfate radicals typically persist for greater time
periods in the
environment.
Ozone may be applied to the unsaturated zone using vent wells for ozone
injection and
SVE technology whereby a vacuum is induced in the subsurface to distribute the
ozone
throughout the area of contamination. Ozone can also be applied to the
saturated zone using
sparging techniques whereby ozone is diffused into the groundwater directly or
added to air and
sparged into the groundwater.
In one aspect of the invention, a method for reducing the concentration of
organic
compounds in soil, water andlor groundwater is provided. Contaminated soil in
the saturated zone,
smear zone and/or unsaturated zone can be remediated to concentrations that
meet local, federal or
other mandated or chosen levels. Water and/or soil may be decontaminated in
situ or ex situ. The
method may involve the co-introduction of two or more oxidants, for example,
persulfate and
ozone, into any of the saturated, unsaturated and smear zones. An additional
oxidant such as
hydrogen peroxide may also be used. Results show that the co-introduction of
these oxidants
provides greater benefits than using them independently. Strong oxidizing
compounds can exhibit
greater persistence in the groundwater when used concurrently with other
oxidizers.
Different types of soils may be treated including, for example, sand, rock,
sediment, loam
and clay. Waters that can be treated include, for example, groundwater, waste
water, process
water and runoff.
Organic contaminants that can be remediated include, but are not limited to,
volatile
organic compounds, semi-volatile organics (SVOC's) polychlorinated biphenyls
(PCBs);
chlorinated volatile organic contaminants (CVOCs), benzene, ethylbenzene,
toluene, xylene
(BTEX), methyl tert butyl ether (MTBE), tertiary butyl alcohol (TBA),
polynuclear aromatic
hydrocarbons (PAHs), ethylene dibromide (EDB); pesticides and herbicides such
as DDT and
silvex, tetrachloroethene (PCE), trichloroethene (TCE), trichloroethane (TCA),
dichloroethane
(DCA), methylene chloride, carbon tetrachloride, dichloroethene (DCE), vinyl
chloride, light non-
aqueous phase liquids (LNAPL) and fuels such as gasoline, diesel fuel, fuel
oils (including #2, #4
and #6) and jet fuels (e.g., JP4 and JP5).
4
CA 02596032 2007-08-02
In another aspect a method and system are provided for reducing the
concentration of
organic compounds in soil and/or groundwater. An oxidant mixture such as
persulfate and ozone
or persulfate, ozone, and hydrogen peroxide may be introduced into the
saturated zone, resulting in
a radius of influence in which organic contaminants are oxidized and reduced
in concentration.
Above the radius of influence, another oxidant (which may be the same as the
first) is introduced
into the smear zone. This second oxidant can attack any contaminants present
in the smear zone
and may also prevent contaminants from escaping through the smear zone if and
when they are
volatilized in the saturated zone. For instance, heat and/or the introduction
of gases may remove
some contaminants from the saturated zone rather than destroy them; however,
the formation of a
gaseous oxidant blanket in the smear zone can trap and destroy these escaped
compounds before
the compounds can emerge from the saturated zone into the smear zone or the
unsaturated zone.
"Persulfate" includes both monopersulfate and dipersulfate. Typically,
persulfate is in the
form of aqueous sodium, potassium or ammonium dipersulfate or sodium or
potassium
monopersulfate or a mixture thereof.
"Saturated zone" refers to the region of the soil profile that is consistently
below ground
water level.
"Unsaturated zone" refers to the region of the soil profile that is
consistently above
ground water level.
"Smear zone" refers to the region of the soil profile through which the ground
water
level fluctuates, typically on a seasonal basis. The smear zone is the region
that when the
ground water is at its highest would be considered saturated and when the
ground water is at its
lowest would be considered unsaturated.
"Organic contaminant" is an organic compound that is not native to the soil or
water in
which it is found. Organic compounds may include, for example, hydrocarbon-
based fuels,
solvents, pesticides, herbicides, PCBs, volatile hydrocarbons, semi-volatile
hydrocarbons,
chlorinated volatile hydrocarbons, BTEX and MTBE.
"Radius of influence" describes the radius around a well or other injection
point defining
an area throughout which an adequate amount of reactant can be introduced to
oxidize at least
some of the organic contaminants present.
In one embodiment, a method is provided for reducing the amount of organic
contaminants
5
CA 02596032 2007-08-02
in a soil or water sample either in situ or ex situ. At least a portion of
organic contaminant present
can be oxidized. "At least a portion" means at least some of the molecules
present in the sample
being treated will be oxidized. It does not mean that a portion of a specific
molecule is
oxidized. "Soil" as used herein includes soil, sediment, clay and rock.
It has been found that a combination of the two water soluble reagents,
persulfate and
ozone, provides a level of compound destruction that is superior to that of
either one of the reagents
used without the other, even at much greater concentrations. Persulfate is a
preferred oxidant for
remediating soil for several reasons including that it has minimal reactivity
with natural soil
components and therefore all, or most, of the oxidizing power of the reagent
is available to oxidize
organic contaminants. Persulfate may be a long-lived oxidant, and this
increased longevity can
result in an increased radius of influence and can help to minimize the
required number of injection
points throughout the contaminated area. Persulfate may be introduced to water
or soil as a liquid,
typically in the form of an aqueous solution of sodium persulfate. Ozone may
be provided as a gas
or as a liquid, for example, an aqueous solution. In some embodiments, a third
reagent, hydrogen
peroxide, may be added as well. Hydrogen peroxide is typically used in
solution form and in some
embodiments may be mixed with persulfate.
It is believed that use of ozone in conjunction with persulfate may result in
a high rate of
conversion to persulfate radicals that can provide for a wider, more intense,
radius of influence. If
hydrogen peroxide is employed along with ozone, a high rate of conversion to
hydroxyl radicals
may result and may also contribute to a wide radius of influence. Known
processes may initiate a
site clean-up by injecting large quantities of a single oxidant such as an
aqueous solution of
persulfate or hydrogen peroxide. Persulfate and hydrogen peroxide, when
injected individually,
however, do not react sufficiently fast enough relative to the rate of
injection, and it is believed that
the large volume of the solution that is typically injected simply displaces
much of the
contaminated ground water before the persulfate or hydrogen peroxide can react
with any
contaminants which the groundwater may contain. By including ozone prior to,
or concurrently
with, the injection of the aqueous persulfate or hydrogen peroxide, it has
been found that much of
the contaminant mass can be oxidized before it is displaced. Ozone itself does
not show great
persistence and cannot be provided, by itself, in molar quantities great
enough to destroy significant
levels of contaminants, such as MTBE in soil or groundwater, in a short period
of time. When
6
CA 02596032 2007-08-02
ozone by itself is diffused or sparged into groundwater, treatment occurs over
several months as
opposed to several days. In combination with persulfate or hydrogen peroxide,
however, ozone
provides improved levels of contaminant destruction. It has also been found
that a discontinuous
pumping procedure that allows for "rest periods" when no solution is injected
can provide for
greater destruction levels and less displacement of contaminated water.
In some embodiments, the persulfate and, optionally, hydrogen peroxide, may be
injected
into the water, ground water (saturated zone), smear zone or unsaturated zone
via a first injector.
Ozone may be injected via a second injector in the same region (or another
region) as the first
injector. Ozone may be formed on site and in many cases may be generated at a
concentration
from about 1% to 10% by volume. Ozone and air may be sparged at rates that
provide for a
preferred radius of influence and in some cases the radius of influence may be
at least as broad as
that of a co-oxidant that may be introduced concurrently to the site. Ozone
can be diffused into
groundwater or the smear zone at flow rates of up to or greater than 80 scfh.
In preferred
embodiments, sparge rates may be, for example, 0.1-20 scfm per injection well.
Together, the
ozone and persulfate and, optionally, hydrogen peroxide, can provide a
combined radius of
influence that provides greater destruction of compounds over a greater area
than is realized using
either compound independently, even when used independently at greater
concentrations.
When treating ex situ samples such as excavated soil, waste water or process
water,
methods of introducing reagents may be simplified and reagents such as
oxidants, pH buffers
and/or surfactants may simply be added to the processor at the desired time in
the process.
Nonetheless, it is often preferred to include both persulfate and ozone to
provide desired results.
Hydrogen peroxide may also be included to improve destruction rates and
increase the spectrum of
compounds that can be destroyed in many ex situ samples.
Destruction rates, either in situ or ex situ, may also be aided by raising the
temperature of
the reaction. For instance, the temperature may be raised to greater than 30
OC, greater than 40 OC,
0
greater than 50 C, greater than 70 C or greater than 90 C. However, cooler
temperatures may also
be used with the method when, for example, volatilization of compounds should
be minimized or
when mobile compounds such as MTBE are being targeted. In these lower
temperature
applications, effective destruction levels can be obtained at temperatures
less than 40 OC, less than
0
30 C or less than or equal to 20 C.
7
CA 02596032 2007-08-02
Reagents may be introduced into a soil or ground water sample using a well
that may be
vertically; horizontally or otherwise oriented. Wells may be temporary, semi
permanent or
permanent and may be sealed in the bore hole using substances known to those
skilled in the art
such as bentonite, grout or cement. A well may be telescoping and may include
one or more
conduits for transporting reagents from above-ground supplies to the target
site, such as the
saturated zone or the smear zone. Conduits for different reagents may be
coaxial with each other
or may run through distinct conduits in the well. Conduits may be made of, or
coated with, a non-
corrosive material such as stainless steel, alloys, PTFE, PVC or CPVC. A
second reagent may be
introduced through a different well than the first and may deliver the reagent
at a different
depth than the first. However, the second well may be positioned so that the
radius of influence
of the second injection point substantially overlaps the radius of influence
of the first injection
point. For example, with vertically installed wells, the vertical axis of the
second well may be
close to the vertical axis of the first well. In some embodiments the two
wells may be within 20',
15', 10', 5', 2' or 1' of each other. These two wells form a couplet.
Persulfate and ozone may be used at approximately equal molar ratios or the
molar ratio of
persulfate to ozone may be, for example, greater than or equal to 10:1, 100:1,
200:1, 500:1,
1000:1, 2000:1 or 5000:1. If hydrogen peroxide is used, the molar ratio of
peroxide to ozone may
also be, for example, greater than or equal to 1:1, 1:2, 2:1, 5:1 or 10:1. The
reagents may be
supplied at any effective concentration that may be determined, in part, from
the type of soil,
groundwater characteristics, type of contaminant, concentration of
contaminant, and the vehicle
used to transport the reagent. In some preferred embodiments, persulfate may
be used at a
concentration of from 500 mg/L to 250,000 mg/L; soluble ozone may be used at a
concentration
range of from I mg/L to 300 mg/L; and hydrogen peroxide may be used at a
concentration of from
500 mg/L to 250,000 mg/L. Ozone gas may be diffused in pure oxygen over an
effective range,
typically about 2-10 %.
The reagents used, for example ozone, persulfate and/or hydrogen peroxide, may
be
introduced to the target site simultaneously or sequentially. When introduced
sequentially, the time
between sequential injections should preferably not be so great that the
activity of the first-injected
reagent has been significantly reduced before providing the second reagent.
Improved results are
apparent in many cases when oxidants are concurrently active at the site. In
some preferred
8
CA 02596032 2007-08-02
embodiments, the temperature at the reaction site is kept at or below 20 OC.
This may be done by
limiting oxidant selection to persulfate and ozone or by limiting the supply
of hydrogen peroxide to
a threshold that keeps the reaction temperature at or below about 20 C.
In another aspect of the invention, a system and method are provided for
reducing the
concentration of organic contaminants in soil, water and groundwater. Reagents
may be applied to
different soil zones to provide for more complete destruction of contaminants.
With remediation systems that utilize sparging with either air or other gases
in the saturated
zone there is the potential to volatilize some organics into the unsaturated
zone before they can be
oxidized. In addition, when adding oxidants to the saturated zone, heat may be
produced, causing
volatile organics to be driven from the saturated zone into the smear zone
and/or unsaturated zone.
Some of these contaminants may be removed using soil vapor extraction (SVE)
techniques, but
these methods require use of an induced vacuum and associated piping network
over a large
surface area with above ground off-gas treatment such as granular activated
carbon or thermal
oxidation. The system described herein can trap and destroy many or all of
these volatile organics
with or without the addition of SVE.
In some embodiments, the saturated zone, smear zone, and/or unsaturated zone
may be
pre-oxidized with a first oxidant prior to applying a second oxidant for the
purpose of destroying
contaminants. This step may help to improve the completeness of chemical
destruction in later
steps.
The pH of an oxidant solution may be controlled to enhance, for example,
stability
and/or reactivity. In some embodiments a preferred pH range is 5.0 - 9.0 and
in many cases 5.0-
7Ø In some cases, a more acidic pH may be used during the reaction but it is
usually preferable to
restore the pH to above about 5.0 at the end of the project. The pH of a
hydrogen peroxide solution
may be controlled using, for example, a phosphate buffer. Once a target soil
is chosen, an optimal
pH for various oxidant solutions can be determined in the field or lab by
those of skill in the art.
In addition to the desire to have longer lived reactive species to promote
greater radial
influence from the point of injection, there is also a desire to reduce the
number of injection events
required to achieve cleanup standards. Typically, using known techniques, two
or more injection
events are required to achieve the required reduction in contaminant
concentration to meet target
clean-up goals. There are at least two reasons for this: 1) contaminants
trapped in the "smear zone"
9
CA 02596032 2007-08-02
are not targeted by existing ISCO technology, and 2) contaminants and oxidants
are slow to
diffuse into and out of micro-pores within the saturated zone, especially in
fine grained soils. The
system described herein can address these issues, as well as others.
In one set of embodiments a first reagent is introduced into the saturated
zone. The
reagent may be any compound or combination of compounds that can reduce the
concentration of
organic contaminants. The reagent may be an oxidant. Oxidants may include, for
example,
persulfate, hydrogen peroxide, permanganate, peroxygens, Fenton's reagent,
ozone, and other
compounds capable of destroying the target contaminant.
This reagent, or combination of reagents, may be introduced as a liquid, a gas
or an
atomized suspension. The reagent typically produces a radius of influence
within which
contaminants may be destroyed at efficiencies of >80%, >90%, >95% or >99%.
Some
contaminants may escape the saturated zone and may even be driven from the
saturated zone by
the chemical treatment. A second reagent may be injected into the smear zone
above the zone
formed by the radius of influence of the first reagent to produce a secondary
blanket. SVE
techniques may also be employed but may not be necessary.
By introducing a second reagent (which may be the same or a different compound
or
compounds) into the smear zone, a blanket of reagent is formed above the
groundwater that can
capture and destroy contaminants (typically volatile and semi-volatile
compounds) that may
emerge from the saturated zone before the first reagent has been able to break
them down. In this
manner, these contaminants may never reach the unsaturated zone or surface,
and most or all of
the escaping compounds can be destroyed in situ. This may result in lower
disposal costs
compared to SVE and may also result in a reduction of volatilized materials
that might otherwise
escape to the atmosphere. In addition, when an oxidant is applied in excess of
the oxidant demand
to the smear zone, the excess oxidant may infiltrate the saturated zone at a
later time to provide
additional oxidation of saturated zone contaminants. Furthermore, the
technology can be used to
directly destroy contaminants that are resident in the smear zone. As the
groundwater level
moves up and down through the smear zone over time, some classes of
contaminants, such as
light non-aqueous phase liquids (LNAPL), may float on top of the water and
move with it. This
can result in a high concentration of these contaminants in the smear zone,
making this region an
important target for remediation.
CA 02596032 2007-08-02
Another advantage of injecting a layer of an oxidant, such as ozone, into the
smear zone is
that it can result in a state of "hypersaturation" in groundwater. While ozone
typically will diffuse
out of solution and leave a less effective aqueous solution behind, the
presence of a gaseous ozone
blanket will, according to Henry's Law, reduce diffusion of ozone from
adjacent aqueous ozone
solutions and will thus result in a higher concentration of oxidant (ozone) in
solution (and in the
groundwater) that would otherwise be present. This means a higher rate of
contaminant
destruction, extended reaction time and/or a wider radius of influence.
The proposed site can be investigated using soil borings or monitoring wells
to assess the
horizontal and vertical extent of any contamination to the subsurface soil and
groundwater using
lo methods known to those skilled in the art. Soil core samples can be taken
to determine the extent
of the smear zone which represents the area in which the groundwater height
fluctuates from high
to low over time. Soil core samples may be kept for determination of soil
properties which may be
particularly useful when direct-push technology is to be used. For example,
see U.S. Patent
Application Serial No. 10l931,012 titled IN SITU REMEDIAL ALTERNATIVE AND
AQUIFER PROPERTIES EVALUATION PROBE SYSTEM which is hereby incorporated by
reference herein. A screening analysis can be performed on site using, for
example, test kits, a
photo-ionization detector (PID) or a gas chromatograph (GC) equipped with
various detectors.
Hydraulic conductivity of the soil in the saturated zone can be estimated
after a soil sieve
analysis is performed. Soils may also be analyzed for total organic content,
iron content and
20 pH. Organic contamination in the smear zone can also be assessed to
determine at what level
the groundwater may be affected by the presence of organic contaminants in the
smear zone.
In many cases it is helpful to understand the groundwater hydraulic properties
prior to
remediating a site. To determine these properties the groundwater elevation is
gauged in one or
more monitoring wells and the groundwater hydraulic conductivity is measured
using slug tests or
pumping tests. From the groundwater elevation and hydraulic conductivity and
the estimated soil
porosity, the groundwater flow direction and velocity may be calculated. The
presence and extent
of any light non-aqueous phase liquid (LNAPL) and any dense non-aqueous liquid
(DNAPL) can
be determined and may be used to select a specific injector and design. The
hydraulic conductivity
over both the horizontal and vertical spatial area of contamination may also
be determined and can
30 be used to choose the injector design, placement and depth.
I1
CA 02596032 2007-08-02
It may be preferred to evaluate the chemical oxidant dosage requirements prior
to
commencing large scale remediation. This may be done, for example, on site
using a field push-
pull test or in a laboratory using a bench scale test. Depending on the
determined oxidant demand,
an oxidant or group of oxidants may be chosen. For example, a combination of
ozone and
persulfate has been shown to be useful when a moderate oxidant demand is
indicated and a
combination of ozone, persulfate and hydrogen peroxide may be used when
oxidant demand is
high or when a wider spectrum of contaminants are targeted. A field scale push-
pull test can
provide the chemical oxidant demand as well as the mass transfer and hydraulic
effects under
actual field conditions. For example, see U.S. Patent Application Serial No.
10/931,012 titled IN
to SITU REMEDIAL ALTERNATIVE AND AQUIFER PROPERTIES EVALUATION PROBE
SYSTEM which describes a mobile push-pull testing system.
Injection of oxidants or other materials into the saturated zone may result in
"groundwater
mounding" where the pressure of the injected oxidants forces the ground water
up into the smear
zone. The profile of this groundwater mound may be essentially that of a dome
centered around
the injection well. Contaminant destruction may be most efficient when the
groundwater mound is
forced up to the upper boundary of the smear zone. In this manner, organic
compounds in the
smear zone may be more readily exposed to oxidants and aqueous based oxidants
may be more
efficiently transported to the sites of contamination in the smear zone. In
many cases, the height
of the groundwater mound may be limited to the upper boundary of the smear
zone to avoid
20 transporting contaminants (e.g., LNAPL) to the unsaturated zone that may
already be substantially
free of these contaminants.
The measured hydraulic properties of the soil may provide some guidance as to
the
pressure and flow rates necessary to provide a desired groundwater mound. The
height, width and
profile of the groundwater mound may be empirically determined by measuring
the groundwater
height in injection wells or piezometers as the oxidant pressure and/or flow
rate are adjusted.
Pressures and flows may be adjusted, or cycled, to produce a preferred
groundwater mound. The
peak of a groundwater mound is typically directly above the point of
injection. In many
embodiments, the height of a preferred mound is at, but not above, the upper
boundary of the
smear zone. The cross-sectional profile of a groundwater mound typically shows
the height of the
12
CA 02596032 2007-08-02
mound falling off as the horizontal distance from the point of injection
increases. See FIG. 2. A
substantially flat profile may be preferred, as this mound formation may
encompass a greater
volume of the smear zone and therefore lead to greater levels of contaminant
destruction.
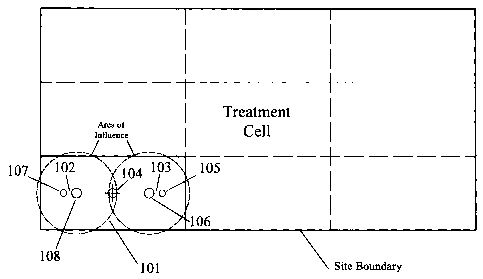
FIGS. 1-5 illustrate a specific embodiment useful in remediating contaminated
soil and/or
groundwater in situ. FIG. 1 provides a plan view illustrating the hypothetical
division of a
remediation site into treatment cells. Rectangular treatment cell 101 can be
treated efficiently by
using injection couplets 102 and 103. The injection couplets may be the same
or different, and in
this case they each include a pair of injection wells. Injection wells 105 and
107 are constructed
and arranged to inject reagents into the smear zone. Injection wells 106 and
108 are constructed
lo and arranged to inject reagents into the saturated zone within a
substantially circular area of
influence. Reagents may be injected as liquids, gases or as atomized liquids.
An overlap in the
respective areas of influence of each couplet may result in more complete
levels of contaminant
destruction. Monitoring well 104 can be used to perform an initial evaluation
of the site. Soil gas
and/or groundwater can be used to monitor ongoing progress, and can be used to
determine the
level of a groundwater mound. By placing the monitoring well equidistant from
both injection
couplets, contaminant destruction can be monitored at a spot most likely to
have the least exposure
to high oxidant levels.
FIG. 2 provides a cutaway view of the system illustrated in FIG. 1. Wells 106
and 108
are positioned with injection ports in the saturated zone while wells 105 and
107 are installed with
20 injection ports in the smear zone. The height of the smear zone is
dependent on the amount of
movement of the water table but in many cases is between 2 and 10 feet. Thus,
oxidant injected
directly into the smear zone may not only destroy resident contaminants in the
smear zone but may
also destroy contaminants that migrate upward from the saturated zone either
naturally or due to
remediation activity. In alternative embodiments, both wells of a couplet may
be positioned with
injection ports in the saturated zone. In these cases, well 107 may be used to
supply air
and/or an oxidant directly to the saturated zone while well 108 may be used to
supply oxidants
such as persulfate and/or hydrogen peroxide to the saturated zone. When these
injection ports are
lowered into the groundwater (saturated zone), contaminants in the ground
water may be directly
targeted with a combination of persulfate, hydrogen peroxide and ozone. Well
107 may also be
30 used for air jetting to increase the radius of influence of the oxidants
provided.
13
CA 02596032 2007-08-02
FIG. 3 provides a cutaway cross-section view of an injection couplet of FIGS.
1 and 2.
Injection wells 107 and 108 are fixed in road box 301 which has been inserted
into the ground.
Injection well 108 includes outer conduit 314 that may be 2 inch diameter
stainless steel well
pipe or other non-corrosive material. Sand backfill 312 and bentonite seal 305
secure and seal the
well pipe in bore hole 306. Central conduit 303 may pass through the center of
conduit 314 and
may be held in place by welded perforated centralizer 316. Conduit 303 may be
made of a non-
corrosive material capable of withstanding constant flow of pressurized ozone,
for example,
PTFE. Locknut 318 secures conduit 303 to a 1 inch Schuma diffuser 317 via
threaded connector
319. Well pipe 314 is extended by 2 inch #10 SS slot well screen 320 and
conduit 304 is
lo terminated by threaded stainless steel end cap 321. End cap 321 forces any
material entering
conduit 304 to exit through well screen 320. Any material passing through
diffuser 317 is also
forced to pass through well screen 320 before entering the saturated zone.
Injection well 107 terminates in the smear zone (although in other embodiments
it may
enter the saturated zone) and includes a corrosion resistant 1 inch stainless
steel tube 307 that
forms conduit 302 which can transport corrosive oxidizers such as ozone,
persulfate and/or
hydrogen peroxide. Seal 309 may be bentonite or an inflated borehole packer,
for example, and
forms a seal between borehole 308 and corrosion resistant pipe 307. The steel
tube is terminated
by threaded end cap 315. Nozzle 310 can be used to deliver oxidants at a
preset or variable rate
and may also be used to deliver a burst of air during an air jetting step to
produce a soil fissure
20 311. Repeated air jetting may improve the migration of any oxidants (e.g.,
hydrogen peroxide or
persulfate) that are injected after the jetting procedure. This procedure may
aid in mixing
oxidants provided via well 108 with ozone that is provided via well 107. A
similar mixing
process can occur when both injectors (310 and 317) are positioned in the
saturated zone, which
may be used, for instance, when low permeability soil is encountered.
FIGS. 4 and 5 provide illustrations of the valving and control mechanisms to
operate the
system shown in FIGS. 1-3. Any or all reagent flows may be computer
controlled, for instance,
by using a Programmable Logic Controller (PLC) and appropriately selected
valves and gauges.
Well pipe 107 is joined to injector inlet 425 by threaded connection 423 and
in turn is joined to
injector inlet 418 by threaded connector 420. Check valves 419 and 424 prevent
backflow of
30 fluids injected into the well. For instance, air may be delivered through
inlet 418 while ozone
14
CA 02596032 2007-08-02
is delivered at inlet 425. The two fluids may then be mixed in conduit 302 and
delivered to
nozzle 310.
Well 108 includes two coaxial conduits for carrying multiple reagents to the
saturated
zone. The well casing is joined to stainless steel pipe 422 by well thread
joint 427. Mixing
chamber 417 provides a region for the mixing of oxidants and/or air. Sight
chamber 416
provides visual access to the mixing process. Pipe 415 joins chamber 417 to
cross 401. Pipe
402 connects inlet 405 to cross 401 while check valve 404 and pressure gauge
403 can be used to
monitor and control the flow of fluid into cross 401. Similarly, inlet 411 is
connected to cross 401
by pipe 412. Check valve 410 and pressure gauge 409 serve to monitor and
control the flow of
io fluid into the system from inlet 411. Inlet 408 provides feed to conduit
303 via pipe 413 and is in
line with check valve 407 and pressure gauge 406.
As shown in FIG. 5, inlet 411 (FIG. 4) may be plumbed to two additional inlets
501 and
513 that may be used to feed multiple oxidants to cross 401. Check valves 516
and 514 control
backflow through these two inlets. Pipe 515 feeds the fluids from inlets 501
and 513 to pipe 412
and then to cross 401.
The various inlets and pathways can be used to carry a variety of oxidants and
carrier
fluids. In a preferred embodiment, inlet 408 can be used to provide ozone to
the lower injector,
inlet 405 can be used to provide air, inlet 501 can provide hydrogen peroxide
and inlet 513 can
provide persulfate. Thus, a mixture of persulfate and hydrogen peroxide can be
delivered to pipe
20 412 and subsequently to cross 401 where it can be mixed with air entering
via inlet 405. Ozone
entering inlet 408 can be carried to diffuser 317 via conduit 303 without
mixing with the
air/persulfate/hydrogen peroxide mixture.
The system described in FIGS. 1-5 was used to remediate a site contaminated
with
gasoline including MtBE. The site had been previously treated using hydrogen
peroxide only
and high residual concentrations of MtBE remained. The following procedure was
used:
The injection rate and radius of hydraulic influence were estimated for the
site so that the
site could be divided into treatment cells as shown in FIG. 1. In this case,
treatment cells measured
15 ft x 30 ft. A pair of injector couplets was installed at the site as shown
in FIG. 1. The couplets
were placed approximately 15 feet from each other with a monitoring well
positioned between
30 the two couplets. The terminal end of well 108 was placed in the saturated
zone while the
CA 02596032 2007-08-02
terminal end of well 107 was positioned in the smear zone. The depths of each
zone had been
previously determined using soil and water samples, as describe above.
The smear zone was air jetted using blasts of high volume and high pressure
(e.g., 100 psi)
air through injector nozzle 310. This was repeated periodically throughout the
remediation. Ozone,
at a rate of 3.21bs/day was then flowed via inlet 302 to nozzle 310 and
blanketed the smear zone.
The parameters for sequential air jetting steps are provided in Table 1,
below.
Table 1 - Air Jetting
Location Flow
Step Procedure in FIGS Duration Rate Pressure
Collect VOC concentration daily 104
1 in Monitoring Point
Review geotechnical parameters
2 for air jefting feasibility
3 Air pulse 105,310 20 sec s80m 100 psi
Read pressure pulse at 104
4 monitoring point
Air pulse 107,310 20 sec s~~ 100 psi
Read pressure pulse at 104
6 monitorin point
7 Assess fissure extent
8 Introduce ozone gas 310 Continuous 40 scfh 42 psi
9 Repeat air pulse periodically 105, 107, 310 20 sec s80m 100 psi
Start system injection in
groundwater and optionally
continue air pulse to smear zone
and constant O3 gas flow to 105, 106,
smear zone. 107,108
10 After the blanket of ozone was resident in the smear zone, the lower
injector (in the
saturated zone) was activated by adding liquid oxidants persulfate and
hydrogen peroxide through
inlets 513 and 501, respectively. Persulfate was provided at a concentration
of 35 g/L and ozone at
3.21bs/day. Subsequently, hydrogen peroxide was provided at 3.5% solution with
ozone at 3.2
lbs/day. The flow of persulfate and hydrogen peroxide was adjusted to produce
a groundwater
mound extending to, but not above, the upper boundary of the smear zone.
Groundwater level
was monitored in injection well 107 which was equipped with water level sensor
330 positioned
16
CA 02596032 2007-08-02
at the top of the smear zone. When the groundwater mound reached the sensor,
the flow of
persulfate and peroxide and air was attenuated to maintain the groundwater
mound at that level.
Ozone was pumped into inlet 408 and passed through Schuma diffuser 317 before
exiting through
well screen 320 into the groundwater. The ozone exited the diffuser in bubbles
having a diameter
of about 20 m. Pressurized air was provided to inlet 405 and was mixed with
persulfate and
hydrogen peroxide in cross 401. The air/persulfate/hydrogen peroxide mixture
was delivered
through annular conduit 304 and passed through well screen 320 into the ground
water. The ozone
was supplied to conduit 303 at a pressure of 42 psi while the air was provided
to conduit 304 at a
pressure of about 40 psi, slightly less than that of the ozone. By operating
conduit 303 at a
slightly higher pressure than conduit 304, the fluid carried by central
conduit 303 can exit the
system without backflow issues that might occur in the absence of this
pressure difference. The
pressurized air may also help to prevent the ozone from diffusing out of the
water in which it is
carried. It may be preferred to program the system so that the air flow to the
saturated zone must
be turned on when ozone is flowing in the central conduit. In this way, ozone
is prevented from
entering the annular conduit and instead is directed outwards through the
injector screen. Air and/or
ozone may be cycled or pulsed in order to achieve desired destruction levels
and a desired
groundwater mound. Preferably, the ozone and/or air are supplied at a constant
rate that results in
groundwater mound that is constantly near the upper boundary of the smear
zone. An example of a
60 minute injection cycle is summarized in table 2, below. An "X" means that
the indicated
reagent was turned on. The absence of an X indicates that the flow was turned
off for the indicated
period of time. This resting step is believed to provide time to allow the
oxidizers to react with the
contaminants without simply displacing the contaminated groundwater.
Continuous injection of
aqueous reagent without a resting step may move more contaminated groundwater
than it
remediates. In most cases, this movement, or displacement, is to be avoided.
The injection cycle
shown in Table 2 resulted in a groundwater mound consistently close to the
upper boundary of the
smear zone.
17
CA 02596032 2007-08-02
Table 2- 60 Minute Injection Cycle
Hydrogen
Step ime FroDuration Air Ozone Persuifate Peroxide
T=O (min) Smear Saturated Smear Saturated Saturated Saturated
Zone Zone Zone Zone Zone Zone
1 0-10 min 10 X X X X X X
2 10-15 min 5 X X X X
3 15-25 min 10 X X X X X X
4 25-30 min 5 X X X X
30-40 min 10 X X X X X X
6 40-45 min 5 X X X X
7 45-55 min 10 X X X X X X
8 55-60 min 5 X X X X
njection
--------- Cycle 60 60 60 60 60 40 40
ime
All flow rates for air and oxidants and volume of oxidants were measured and
recorded. The ozone concentration at monitoring well 104 was measured by
collecting
vapor samples from the well and analyzing them for ozone concentration to
assure that an
adequate supply of ozone was blanketing the smear zone. Groundwater samples
were also periodically analyzed for temperature, pH, ORP, peroxides and
sulfate to
assess the distribution of oxidants in the groundwater. Based on these
results, volumes of
each oxidant were adjusted to assure continued destruction of resident organic
contaminants. After 20 days of steady state operation, the system was shut
down and after
7 days and 30 days, ground water samples from monitoring well 104 were
collected and
laboratory analyzed for MTBE. This process was repeated until contaminant
target levels
of less than 70 g/L in groundwater were achieved. Subsequent samples are
scheduled to
be taken at quarterly intervals to evaluate any contaminant "rebound" that may
occur. If
rebound does occur, the system may be re-started as described above.
Experimental Results -
To evaluate the effectiveness of one embodiment of the invention a bench top
experiment was designed and completed to determine the relative destruction
efficiency of a persulfate/ozone and a persulfate/hydrogen peroxide system as
well as
18
CA 02596032 2007-08-02
the combination of all three of these oxidants.
The experiments were conducted by charging a 40 mL VOA vial (zero
headspace) with a stock solution of persulfate, ozone, distilled-deionized
water (DDI)
hydrogen peroxide and contaminated groundwater from a site in Somerville,
Massachusetts. Each vial was spiked with MtBE to a concentration of 28.9 mg/L.
Persulfate was provided at a concentration of 40 g/L, ozone at 20 mg/L and
hydrogen
peroxide at 125 mg/L. Reagents were allowed to react with the sample at
ambient
temperature (20 C) for 24 hours and then the vials were quenched at 4 C.
The results are summarized in Table 3, below, and in FIG. 6. Under the
experimental conditions shown, at 30 C complete destruction of MtBE was
achieved
with each reagent set except for ozone and ozone! H202. However, at 20 C a
significant improvement in MtBE destruction was achieved by the combination of
Na2S208 + 03 and the combination of Na2S208 + 03 + H202 when compared to the
other reagents. This indicates that the use, in situ or ex situ, of one of
these
combinations of reagents will provide significantly improved results over any
one of
these reagents alone at a temperature of about 20 C.
Table 3
Degradation of MtBE with combinations of Na2S2Og, H202, and 03 at 20 C and 30
C
C 30 C
[MtBE]24
Vial [MtBE] hrs % [MtBE]24 hrs %
No. Oxidant s Degradation m,L Degradation
7 Na S2O8 28.9 16.8 42% 0 100%
8 Na S2O8 + H202 28.9 11.9 59% 0 100%
9 03 28.9 16.4 43% 25.5 12%
10 0+ H202 28.9 17.9 38% 24.1 17%
11 Na S2O8 + 03 28.9 5.94 79% 0 100%
Na2S2O8 + 03 +
12 H202 28.9 7.84 73% 0 100%
19
CA 02596032 2007-08-02
While several embodiments of the present invention have been described and
illustrated herein,
those of ordinary skill in the art will readily envision a variety of other
means and/or structures for
performing the functions and/or obtaining the results and/or one or more of
the advantages
described herein, and each of such variations and/or modifications is deemed
to be within the scope
of the present invention. More generally, those skilled in the art will
readily appreciate that all
parameters, dimensions, materials, and configurations described herein are
meant to be exemplary
and that the actual parameters, dimensions, materials, and/or configurations
will depend upon the
specific application or applications for which the teachings of the present
invention is/are used.
Those skilled in the art will recognize, or be able to ascertain using no more
than routine
experimentation, many equivalents to the specific embodiments of the invention
described herein.
It is, therefore, to be understood that the foregoing embodiments are
presented by way of example
only and that, within the scope of the appended claims and equivalents
thereto; the invention may
be practiced otherwise than as specifically described and claimed. The present
invention is directed
to each individual feature, system, article, material, kit, and/or method
described herein. In
addition, any combination of two or more such features, systems, articles,
materials, kits, and/or
methods, if such features, systems, articles, materials, kits, and/or methods
are not mutually
inconsistent, is included within the scope of the present invention. All
definitions, as defmed
and used herein, should be understood to control over dictionary definitions,
definitions in
documents incorporated by reference, and/or ordinary meanings of the defined
terms.
The indefinite articles "a" and "an," as used herein in the specification and
in the claims,
unless clearly indicated to the contrary, should be understood to mean "at
least one."
The phrase "and/or," as used herein in the specification and in the claims,
should be
understood to mean "either or both" of the elements so conjoined, i.e.,
elements that are
conjunctively present in some cases and disjunctively present in other cases.
All references, patents and patent applications and publications that are
cited or referred
to in this application are incorporated in their entirety herein by reference.
What is claimed is: