Note: Descriptions are shown in the official language in which they were submitted.
CA 02597963 2007-08-20
METHOD OF PROVIDING READILY AVAILABLE CELLULAR MATERIAL
DERIVED FROM PERIPHERAL BLOOD, AND A COMPOSITION THEREOF
FIELD OF THE INVENTION
The present invention is directed to adult stem cells from peripheral blood
prepared in
a TVEMF-bioreactor, and to a process for such preparation, compositions
thereof, and
methods of treating a mammal with the cells or compositions.
BACKGROUND OF THE INVENTION
Regeneration of mammalian, particularly human, tissue has long been a desire
of the
medical community. Thus far, repair of human tissue has been accomplished
largely by
transplantations of like tissue from a donor. Beginning essentially with the
kidney transplant
from one of the Herrick twins to the other and later made world famous by
South African
Doctor Christian Barnard's transplant of a heart from Denise Darval to Louis
Washkansky on
December 3, 1967, tissue transplantation became a widely accepted method of
extending life
in terminal patients.
Transplantation of human tissue, from its first use, encountered major
problems,
primarily tissue rejection due to the body's natural immune system. This often
caused the use
of tissue transplantation to have a limited prolongation of life (Washkansky
lived only 18
days past the surgery).
In order to overcome the problem of the body's immune system, numerous anti-
rejection drugs (e.g. Imuran, Cyclosporine) were soon developed to suppress
the immune
system and thus prolong the use of the tissue prior to rejection. However, the
rejection
problem has continued creating the need for an alternative to tissue
transplantation.
Bone marrow transplantation has also been used, and is still the procedure of
choice
for treatment of some illnesses, such as leukemia, to repair certain tissues
such as bone
marrow, but bone marrow transplantation also has problems. It requires a match
from a donor
(found less than 50% of the time); it is painful, expensive, and risky.
Consequently, an
altemative to bone marrow transplantation is highly desirable. Transplantation
of tissue stem
cells such as the transplantation of liver stem cells found in U.S. Patent No.
6,129,911 have
similar limitations rendering their widespread use questionable.
In recent years, researchers have experimented with the use of pluripotent
embryonic
stem cells as an alternative to tissue transplant. The theory behind the use
of embryonic stem
1
CA 02597963 2007-08-20
cells has been that they can theoretically be utilized to regenerate virtually
any tissue in the
body. The use of embryonic stem cells for tissue regeneration, however, has
also encountered
problems. Among the more serious of these problems are that transplanted
embryonic stem
cells have limited controllability, they sometimes grow into tumors, and the
human embryonic
stem cells that are available for research would be rejected by a patient's
immune system
(Nature, June 17, 2002: Pearson, "Stem Cell Hopes Double", news@nature.com,
published
online:21 June 2002). Further, widespread use of embryonic stem cells is so
burdened with
ethical, moral, and political concerns that its widespread use remains
questionable.
The pluripotent nature of stem cells was first discovered from an adult stem
cell found
in bone marrow. Verfaille, C.M. et al., Pluripotency of mesenchymal stem cells
derived from
adult marrow. Nature 417, published online 20 June; doi:10.1038/nature00900,
(2002) cited
by Pearson, H. Stem cell hopes double. news@nature.com, published online:21
June 2002;
doi: 10.103 8/news020617-11.
Boyse et al., U.S. Pat. No. 6,569,427 B1, discloses the cryopreservation and
usefulness of cryopreserved fetal or neonatal blood in the treatment or
prevention of various
diseases and disorders such as anemias, malignancies, autoimmune disorders,
and various
immune dysfunctions and deficiencies. Boyse also discloses the use of
hematopoietic
reconstitution in gene therapy with the use of a heterologous gene sequence.
The Boyse
disclosure stops short, however, of expansion of cells for therapeutic uses.
CorCell, a cord
blood bank, provides statistics on expansion, cryopreservation, and
transplantation of
umbilical cord blood stem cells. "Expansion of Umbilical Cord Blood Stem
Cells",
Information Sheet Umbilical Cord Blood, CorCell, Inc. (2003). One expansion
process
discloses utilizing a bioreactor with a central collagen based matrix.
Research Center Julich:
Blood Stem Cells from the Bioreactor. Press release May 17, 2001.
Research continues in an effort to elucidate the molecular mechanisms involved
in the
expansion of stem cells. For example, the CorCell article discloses that a
signal molecule
named Delta-1 aids in the development of cord blood stem cells. Ohishi K. et
al.: Delta-1
enhances marrow and thymus repopulating ability of human CD34+/CD38- cord
blood cells.
Clin. Invest. 110:1165-1174 (2002).
2
CA 02597963 2007-08-20
Throughout this application, the term "peripheral blood" means blood that
circulates,
or has circulated, systematically in a maminal. The term "peripheral blood
cells" means cells
found in peripheral blood.
While adult stem cells can be found in numerous mature tissues, they are found
in
lesser quantities and are harder to locate. Also, stem cells found in tissues
may be dedicated
to that tissue, and less able to function as a truly pluripotent cell.
Peripheral blood cells,
however, are more readily available than stem cells in tissues.
There is a need, therefore, to provide a method and process of repairing human
tissue
that is not based on organ transplantation, bone marrow transplantation, or
embryonic stem
cells, and yet provides a composition of expanded peripheral blood stem cells,
preferably in a
therapeutic condition and dosage and unlikely to elicit an immune response,
for use in a
matter of hours rather than days.
SUMMARY OF THE INVENTION
The present invention relates in part to peripheral blood stem cells from a
mammal,
preferably human, preferably wherein said stem cells are TVEMF-expanded. The
present
invention also relates to peripheral blood stem cells from a mammal,
preferably human, wherein
said stem cells are expanded in a rotating bioreactor, preferably to at least
seven times greater
than the number before expansion. The present invention also relates to
introducing to the
mammal a therapeutically effective amount of expanded adult stem cells, or
TVEMF-expanded
adult stem cells repair tissue and/or tissue function. The invention also
relates to compositions
comprising these cells, with other components added as desired, including
pharmaceutically
acceptable carriers, cryopreservatives, and cell culture media.
The present invention also relates to a process for preparing expanded,
preferably
TVEMF-expanded, peripheral blood stem cells and stem cell compositions for
repairing tissue
and/or function by placing a peripheral blood mixture in a culture chamber of
a rotatable
bioreactor, preferably a TVEMF-bioreactor; and rotating the bioreactor. In a
preferred
embodiment, the rotating bioreactor is a TVEMF bioreactor which is provided
with the
additional step of subjecting the peripheral blood stem cell mixture to a
TVEMF and TVEMF-
expanding the peripheral blood stem cells in the TVEMF bioreactor to prepare
TVEMF-
expanded peripheral blood stem cells and a stem cell composition. The present
invention also
3
CA 02597963 2007-08-20
relates to a method of cryopreserving the expanded stem cells by lowering
their temperature to -
120 C to -196 C for one year or longer.
BRIEF DESCRIPTION OF THE DRAWINGS
In the drawings,
Figure 1 schematically illustrates a preferred embodiment of a culture carrier
flow loop of a
bioreactor;
Figure 2 is an elevated side view of a prefened embodiment of a TVEMF-
bioreactor of the
invention;
Figure 3 is a side perspective of a preferred embodiment of the TVEMF-
bioreactor of Figure 2;
Figure 4 is a vertical cross sectional view of a preferred embodiment of a
TVEMF- bioreactor;
Figure 5 is a vertical cross sectional view of a TVEMF- bioreactor;
Figure 6 is an elevated side view of a time varying electromagnetic force
source that can house,
and provide a time varying electromagnetic force to, a bioreactor;
Figure 7 is a front view of the TVEMF source shown in Figure 6;
Figure 8 is a front view of the TVEMF source shown in Figure 6, further
showing a bioreactor
therein,
Figure 9 is the orbital path of a typical cell in a non-rotating reference
frame;
Figure 10 is a graph of the magnitude of deviation of a cell per revolution;
Figure 11 is a representative cell path as observed in a rotating reference
frame of the culture
medium;
Figure 12 illustrates the expansion pattern of total nucleated cells in a
rotating bioreactor versus a
dynamic moving culture;
Figure 13 illustrates the expansion pattern of CD 133+ cells in a rotating
bioreactor versus a
dynamic moving culture;
Figure 14 illustrates the expansion pattern of CD34+ cells in a rotating
bioreactor versus a
dynamic moving culture;
Figure 15 is a graphic illustration of the expansion (increase in number) from
day 0 to day 6 of
CD34+ cells cultured in a rotating TVEMF-bioreactor; and
Figure 16 illustrates the number of CD34+ cells at day 6 in a TVEMF-expansion
culture as
compared with and a non-TVEMF expansion culture.
4
CA 02597963 2007-08-20
DETAILED DESCRIPTION OF THE DRAWINGS
In the simplest terms, a rotatingbioreactor comprises a cell culture chamber
and a time
varying electromagnetic force source. In operation, ablood mixture is placed
into the cell culture
chamber. The cell culture chamber is filled so as to create a three-
dimensional environment
wherein each individual non-adherent peripheral blood cell is suspended. The
cell culture
chamber is rotated in one direction, 360 degrees, over a period of time during
which a time
varying electromagnetic force is generated in the chamber by the time varying
electromagnetic
force source. During their time in the rotating bioreactor, the cells are
suspended in discrete
microenvironments in the essentially quiescent three-dimensional environment
created therein.
In a preferred embodiment, the rotating bioreactor is a TVEMF-bioreactor
wherein, in addition
to being suspended by rotating, the cells are exposed to a time varying
electromagnetic force to
provide additional unique characteristics to the cells and to enhance the
expansion process.
Upon completion of thetime, the expanded blood mixture is removed from the
chamber. In a
more complex TVEMF- bioreactor system, the time varying electromagnetic force
source can be
integral to the TVEMF- bioreactor, as illustrated in Figures 2-5, but can also
be adjacent to a
bioreactor as in Figures 6-8. The TVEMF source preferably comprises a TVEMF
generating
device, which may preferably be a coil, more preferably at least one loop.
Furthermore, a fluid
carrier such as cell culture media or buffer (preferably similar to that media
added to ablood
mixture, discussed below), which provides sustenance to the cells, can be
periodically refreshed
and removed. Preferred TVEMF- bioreactors are described herein. However, it is
also
contemplated that tissue and/or function can be repaired by using a non-TVEMF
rotating
bioreactor to expand peripheral blood stem cells.
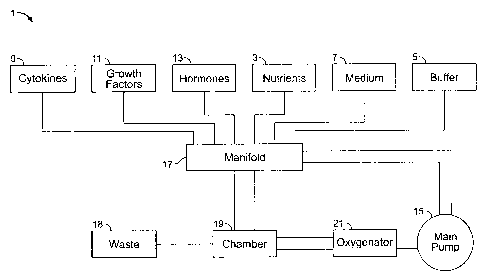
Referring now to Figure 1, illustrated is a preferred embodiment of a culture
carrier flow
loop 1 in an overall bioreactor culture system for growing mammalian cells
having a cell culture
chamber 19, preferably a rotating cell culture chamber, an oxygenator 21, an
apparatus for
facilitating the directional flow of the culture carrier, preferably by the
use of a main pump 15,
and a supply manifold 17 for the selective input of such culture carrier
requirements as, but not
limited to, nutrients 3, buffers 5, fresh medium 7, cytokines 9, growth
factors 11, and hormones
13. In this preferred embodiment, the main pump 15 provides fresh fluid
carrier to the
oxygenator 21 where the fluid carrier is oxygenated and passed through the
cell culture chamber
5
CA 02597963 2007-08-20
19. The waste in the spent fluid carrier from the cell culture chamber 19 is
removed and
delivered to the waste 18 and the remaining cell culture carrier is returned
to the manifold 17
where it receives a fresh charge, as necessary, before recycling by the pump
15 through the
oxygenator 21 to the cell culture chamber 19.
In the culture carrier flow loop 1, the culture carrier is circulated through
the living cell
culture in the chamber 19 and around the culture carrier flow loop 1, as shown
in Figure 1. In
this loop 1, adjustments are made in response to chemical sensors (not shown)
that maintain
constant conditions within the cell culture reactor chamber 19. Controlling
carbon dioxide
pressures and introducing acids or bases corrects pH. Oxygen, nitrogen, and
carbon dioxide are
dissolved in a gas exchange system (not shown) in order to support cell
respiration. The closed
loop 1 adds oxygen and removes carbon dioxide from a circulating gas
capacitance. Although
Figure 1 is one preferred embodiment of a culture carrier flow loop that may
be used in the
present invention, the invention is not intended to be so limited. The input
of culture carrier
elements such as, but not limited to, oxygen, nutrients, buffers, fresh
medium, cytokines, growth
factors, and hormones into a bioreactor can also be performed manually,
automatically, or by
other control means, as can be the control and removal of waste and carbon
dioxide.
Figures 2 and 3 illustrate a preferred embodiment of a TVEMF- bioreactor 10
with an
integral time varying electromagnetic force source. Figure 4 is a cross
section of a rotatable
TVEMF-bioreactor 10 for use in the present invention in a preferred form. The
TVEMF-
bioreactor 10 of Figure 4 is illustrated with an integral time varying
electromagnetic force
source. Figure 5 also illustrates a preferred embodiment of a TVEIVIF-
bioreactor with an
integral time varying electromagnetic force source. Figures 6-8 show a
rotating bioreactor with
an adjacent time varying electromagnetic force source.
Turning now to Figure 2, illustrated in Figure 2 is an elevated side view of a
preferred
embodiment of a TVEMF-bioreactor 10 of the present invention. Figure 2
comprises a motor
housing 111 supported by a base 112. A motor 113 is attached inside the motor
housing 111 and
connected by a first wire 114 and a second wire 115 to a control box 116 that
has a control
means therein whereby the speed of the motor 113 can be incrementally
controlled by turning the
control knob 117. The motor housing I 11 has a motor 113 inside set so that a
motor shaft 118
extends through the housing 111 with the motor shaft 118 being longitudinal so
that the center of
6
CA 02597963 2007-08-20
the shaft 118 is parallel to the plane of the earth at the location of a
longitudinal chamber 119,
preferably made of a transparent material including, but not limited to,
plastic.
In this preferred embodiment, the longitudinal chamber 119 is connected to the
shaft 118
so that in operation the chamber 119 rotates about its longitudinal axis with
the longitudinal axis
parallel to the plane of the earth. The chamber 119 is wound with a wire coil
120. The size of the
wire coil 120 and number of times it is wound are such that when a square wave
current
preferably of from 0.1mA to 1000rnA is supplied to the wire coil 120, a time
varying
electromagnetic force preferably of from 0.05 gauss to 6 gauss is generated
within the chamber
119. The wire coil 120 is connected to a first ring 121 and a second ring 122
at the end of the
shaft 118 by wires 123 and 124. These rings 121, 122 are then contacted by a
first
electromagnetic delivery wire 125 and a second electromagnetic delivery wire
128 in such a
manner that the chamber 119 can rotate while the current is constantly
supplied to the coil 120.
An electromagnetic generating (TVEMF source) device 126 is connected to the
wires 125, 128.
The electromagnetic generating device 126 supplies a square wave to the wires
125, 128 and coil
120 by adjusting its output by turning an electromagnetic generating device
knob 127.
Figure 3 is a side perspective view of the TVEMF-bioreactor 10 shown in Figure
2 that
may be used in the present invention.
Turning now to the rotating TVEMF-bioreactor 10 illustrated in Figure 4 with a
culture
chamber 230 which is preferably transparent and adapted to contain ablood
mixture therein,
further comprising an outer housing 220 which includes a first 290 and second
291 cylindrically
shaped transverse end cap member having facing first 228 and second 229 end
surfaces arranged
to receive an inner cylindrical tubular glass member 293 and an outer tubular
glass member 294.
Suitable pressure seals are provided. Between the inner 293 and outer 294
tubular members is an
annular wire heater 296 which is utilized for obtaining the proper incubation
temperatures for
cell growth. The wire heater 296 can also be used as a time varying
electromagnetic force source
to supply a time varying electric field to the culture chamber 230 or, as
depicted in Figure 5, a
separate wire coil 144 can be used to supply a time varying electromagnetic
force. The first end
cap member 290 and second end cap member 291 have inner curved surfaces
adjoining the end
surfaces 228, 229 for promoting smoother flow of the mixture within the
chamber 230. The first
end cap member 290, and second end cap member 291 have a first central fluid
transfer journal
member 292 and second central fluid transfer journal member 295, respectively,
that are
7
CA 02597963 2007-08-20
rotatably received respectively on an input shaft 223 and an output shaft 225.
Each transfer
journal member 294, 295 has a flange to seat in a recessed counter bore in an
end cap member
290, 291 and is attached by a first lock washer and ring 297, and second lock
washer and ring
298 against longitudinal motion relative to a shaft 223, 225. Each joumal
member 294, 295 has
an intermediate annular recess that is connected to Iongitudinally extending,
circumferentially
arranged passages. Each annular recess in a journal member 292, 295 is coupled
by a fiust
radially disposed passage 278 and second radially disposed passage 279 in an
end cap member
290 and 291, respectively, to first input coupling 203 and second input
coupling 204. Carrier in a
radial passage 278 or 279 flows through a first annular recess and the
longitudinal passages in a
journal member 294 or 295 to permit access carrier through a journal member
292, 295 to each
end of the journal 292, 295 where the access is circumferential about a shaft
223, 225.
Attached to the end cap members 290 and 291 are a first tubular bearing
housing 205,
and second tubular bearing housing 206 containing ball bearings which
relatively support the
outer housing 220 on the input 223 and output 225 shafts. The first bearing
housing 205 has an
attached first sprocket gear 210 for providing a rotative drive for the outer
housing 220 in a
rotative direction about the input 223 and output 225 shafts and the
longitudinal axis 221. The
first bearing housing 205, and second bearing housing 206 also have provisions
for electrical
take out of the wire heater 296 and any other sensor.
The inner filter assembly 235 includes inner 215 and outer 216 tubular members
having
perforations or apertures along their lengths and have a first 217 and second
218 inner filter
assembly end cap member with perforations. The inner tubular member 215 is
constructed in two
pieces with an interlocking centrally located coupling section and each piece
attached to an end
cap 217 or 218. The outer tubular member 216 is mounted between the fnst 217
and second
inner filter assembly end caps.
The end cap members 217, 218 are respectively rotatably supported on the input
shaft
223 and the output shaft 225. The inner member 215 is rotatively attached to
the output shaft 225
by a pin and an interfitting groove 219. A polyester cloth 224 with a ten-
micron weave is
disposed over the outer surface of the outer member 216 and attached to 0-
rings at either end.
Because the inner member 215 is attached by a coupling pin to a slot in the
output drive shaft
225, the output drive shaft 225 can rotate the inner member 215. The inner
member 215 is
coupled by the first 217 and second 218 end caps that support the outer member
216. The output
8
CA 02597963 2007-08-20
shaft 225 is extended through bearings in a first stationary housing 240 and
is coupled to a first
sprocket gear 241. As illustrated, the output shaft 225 has a tubular bore 222
that extends from a
first port or passageway 289 in the first stationary housing 2401ocated
between seals to the inner
member 215 so that a flow of fluid carrier can be exited from the inner member
215 through the
stationary housing 240.
Between the first 217 and second 218 end caps for the inner member 235 and the
journals
292, 295 in the outer housing 220, are a first 227 and second 226 hub for the
blade members 50a
and 50b. The second hub 226 on the input shaft 223 is coupled to the input
shaft 223 by a pin
231 so that the second hub 226 rotates with the input shaft 223. Each hub 227,
226 has axially
extending passageways for the transmittal of carrier through a hub.
The input shaft 223 extends through bearings in the second stationary housing
260 for
rotatable support of the input shaft 223. A second longitudinal passageway 267
extends through
the input shaft 223 to a location intermediate of retaining washers and rings
that are disposed in a
second annular recess 232 between the faceplate and the housing 260. A third
radial passageway
272 in the second end cap member 291 permits fluid carrier in the recess to
exit from the second
end cap member 291. While not shown, the third passageway 272 connects through
piping and a
Y joint to each of the passages 278 and 279.
A sample port is shown in Figure 4, where a first bore 237 extending along a
first axis
intersects a corner 233 of the chamber 230 and forms a restricted opening 234.
The bore 237 has
a counter bore and a threaded ring at one end to threadedly receive a
cylindrical valve member
236. The valve member 236 has a complimentarily formed tip to engage the
opening 234 and
protrude slightly into the interior of the chamber 230. An 0-ring 243 on the
valve member 236
provides a seal. A second bore 244 along a second axis intersects the first
bore 237 at a location
between the 0-ring 243 and the opening 234. An elastomer or plastic stopper
245 closes the
second bore 244 and can be entered with a hypodermic syringe for removing a
sample. To
remove a sample, the valve member 236 is backed off to access the opening 234
and the bore
244. A syringe can then be used to extract a sample and the opening 234 can be
reclosed. No
outside contamination reaches the interior of the TVEMF-bioreactor 10.
In operation, carrier is input to the second port or passageway 266 to the
shaft
passageway and thence to the first radially disposed 278 and second radially
disposed
passageways 279 via the third radial passageway 272. When the carrier enters
the chamber 230
9
CA 02597963 2007-08-20
via the longitudinal passages in the journals 292, 294 the carrier impinges on
an end surface 228,
229 of the hubs 227, 226 and is dispersed radially as well as axially through
the passageways in
the hubs 227, 226. Carrier passing through the hubs 227, 226 impinges on the
end cap members
217, 218 and is dispersed radially. The flow of entry fluid carrier is thus
radially outward away
from the longitudinal axis 221 and flows in a toroidal fashion from each end
to exit through the
polyester cloth 224 and openings in filter assembly 235 to exit via the
passageways 266 and 289.
By controlling the rotational speed and direction of rotation of the outer
housing 220, chamber
230, and inner filter assembly 235 any desired type of carrier action can be
obtained. Of major
importance, however, is the fact that a clinostat operation can be obtained
together with a
continuous supply of fresh fluid carrier.
If a time varying electromagnetic force is not applied using the integral
annular wire
heater 296, it can be applied by another preferred time varying
electromagnetic force source. For
instance, Figures 6-8 illustrate a time varying electromagnetic force source
140 which provides
an electromagnetic force to a cell culture in a bioreactor which does not have
an integral time
varying electromagnetic force, but rather has an adjacent time varying
electromagnetic force
source. Specifically, Figure 6 is a preferred embodiment of a time varying
electromagnetic force
source 140. Figure 6 is an elevated side perspective of the time varying
electromagnetic force
source 140 which comprises a support base 145, a cylinder coil support 146
supported on the
base 145 with a wire coil 147 wrapped around the support 146. Figure 7 is a
front perspective of
the time varying electromagnetic force source 140 illustrated in Figure 6.
Figure 8 is a front
perspective of the time varying electromagnetic force source 140, which
illustrat.es that in
operation, an entire bioreactor 148 is inserted into a cylinder coil support
146 which is supported
by a support base 145 and which is wound by a wire coil 147. It is not
necessary that the
TVEMF source comprise a coil, but may also preferably comprise at least one
loop, each of
which emit a TVEMF signal. Since the time varying electromagnetic force source
140 is
adjacent to the bioreactor 148, the time varying electromagnetic force source
140 can be reused.
In addition, since the time varying electromagnetic force source 140 is
adjacent to the bioreactor
148, the source 140 can be used to generate an electromagnetic force in all
types of bioreactors,
preferably rotating.
Furthermore, in operation a preferred embodiment of the present invention
contemplates
that an electromagnetic generating source is turned on and adjusted so that
the output generates
CA 02597963 2007-08-20
the desired electromagnetic field in the blood mixture-containing chamber. One
embodiment of
the TVEMF source is that it can be configured to emit a TVEMF signal
exhibiting a relatively
high magnetic field amplitude (between about 10 to 100 Gauss) and exhibiting a
magnetic slew
rate greater than 1000 Gauss per second. Another embodiment of the TVEMF
source is that it
can be configured to emit a TVEMF signal exhibiting a relatively low magnetic
field amplitude
(between about 0.1 to 10 Gauss) along a bipolar square wave function at a
frequency of between
1 to 100 Hz. Yet another embodiment of the TVEMF source is that it can also be
configured to
emit a TVEMF signal exhibiting relatively low magnetic field amplitude
(between about 0.1 to
Gauss) along a square wave function having a duty cycle between about 0.1 to
99.9 percent.
10 Still another embodiment of the TVEMF source is that it can also be
configured to emit a
TVEMF signal exhibiting a magnetic field having a magnetic slew rate greater
than about 1000
Gauss per second that has a active duty pulse duration of less than 1 ms.
Still yet another
embodiment of the TVEMF source is that it can also be configured to emit a
TVEMF signal
exhibiting a magnetic field having a magnetic slew rate greater than about 50
Gauss per second
exhibiting a bipolar pulses having an active duty cycle of less than 1%. Even
still yet another
embodiment of the TVEMF source is that it can also be configured to emit a
TVEMF signal
exhibiting a magnetic field between about 1 to 100 Gauss peak-to-peak and
having a magnetic
slew rate bipolar pulses with an active duty cycle of less than 1%. Still
another embodiment of
the TVEMF source, for instance comprising a solenoid coil, is that it can also
be configured to
emit a TVEMF signal exhibiting a time-dependent magnetic field exhibiting a
relatively uniform
(not varying by more than 5%) magnetic field strength throughout the cell
mixture contents.
However, these parameters are not meant to be limiting to the TVEMF of the
present
invention, and as such may vary based on other aspects of this invention.
TVEMF may be
measured for instance by standard equipment such as an EN131 Cell Sensor Gauss
Meter.
As various changes could be made in rotating bioreactors subjected to a time
varying
electromagnetic force as are contemplated in the present invention, without
departing from the
scope of the invention, it is intended that all matter contained in the above
description be
interpreted as illustrative and not limiting.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS OF THE
INVENTION
11
CA 02597963 2007-08-20
The present invention is related to a method of expanding peripheral blood
stem cells
without substantial differentiation. This invention may be more fully
described by the preferred
embodiment as hereinafter described, but is not intended to be limited
thereto. Peripheral blood
cells are removed from a patient. A subpopulation of these cells is currently
referred to as adult
stem cells. The peripheral blood cells, or any subset thereof, are placed in a
bioreactor as
described herein. The bioreactor vessel is rotated 360 about a substantially
horizontal
longitudinal central axis at a speed that provides for suspension of the
peripheral blood cells to
maintain each cell in a discrete microenvironment essentially without any
turbulence, and with
low shear stress. During the time that the cells are in the reactor, they may
be fed nutrients,
exposed to hormones, cytokines, or growth factors, and/or genetically
modified, and toxic
materials are preferably removed. The toxic materials typically removed are
from peripheral
blood cells comprising the toxic granular material of dying cells and the
toxic material of
granulocytes and macrophages. In addition to providing a rotating bioreactor
for the suspension
and expansion of cells, the present invention also contemplates the addition
of TVEMF to the
rotating bioreactor.
The following definitions are meant to aid in the description and
understanding of the
defined terms in the context of the present invention. The definitions are not
meant to limit these
terms to less than is described throughout this application. Furthermore,
several definitions are
included relating to TVEMF - all of the definitions in this regard should be
considered to
complement each other, and not construed against each other.
As used throughout this application, the term "rotating bioreactor" refers to
a bioreactor
that can be rotated about a substantially horizontal axis, horizontal to the
plane of the earth, and
about the culture chambers longitudinal axis. In addition, the rotating
bioreactor is rotated 360
degrees in one direction so that the cells contained therein are suspended in
discrete
microenvironments with very little, if any, turbulence and low shear stress. A
short recess is
permitted wherein culture media can be refreshed, samples taken, or for other
reasons, without
disturbing the suspension of the cells in the rotating bioreactor. The
bioreactors of the present
invention, with and without TVEMF, provide a three-dimensional environment
wherein the
entire volume of the culture chamber is filled so as to provide essentially
zero headspace. In
addition, the rotating bioreactor essentially mimics a microgravity situation.
A rotating
bioreactor can be made a rotating TVEMF bioreactor with the addition of TVEMF.
12
CA 02597963 2007-08-20
As used throughout this application, the term "adult stem cell" refers to a
pluripotent,
totipotent, and/or multipotent cell that is undifferentiated and that may give
rise to more
undifferentiated cells and is also capable to giving rise to differentiated
cells, but only if directed
to. With regard to the present invention, an adult stem cell is preferably a
CD133+ cell, more
preferably a CD34+, and most preferably a non-terminally differentiated
peripheral blood stem
cell.
As used throughout this application, the term "blood" refers to peripheral
blood.
"Peripheral blood" is systemic blood; that is, blood that circulates, or has
circulated, systemically
in a mammal. The mammal is not meant to be a fetus. For the purposes of the
present invention,
there is no reason to distinguish between peripheral blood located at
different parts of the same
circulatory loop.
As used throughout this application, the term "peripheral blood cell" refers
to a cell from
blood. Blood cells capable of replication may undergo expansion, preferably
TVEMF-expansion
in a TVEMF bioreactor, and may be present in compositions of the present
invention.
As used throughout this application, the term "peripheral blood stem cell"
refers to an
adult stem cell from peripheral blood. Peripheral blood stem cells are adult
stem cells, which as
mentioned above are also known as somatic stem cells, and are not embryonic
stem cells derived
directly from an embryo. Preferabty, a peripheral blood stem cell of the
present invention is a
CD34+ cell, more preferably a CD 133+ cells, and most preferably a non-
terminally
differentiated cell.
As used throughout this application, the term "peripheral blood stem cell
composition",
or reference thereto, refers to peripheral blood stem cells and a carrier of
some sort, whether a
pharmaceutically acceptable carrier, plasma, peripheral blood, albumin, cell
culture medium,
growth factor, copper chelating agent, hormone, buffer, cryopreservative, or
some other
substance. Reference to naturally-occurring peripheral blood is preferably to
compare peripheral
blood stem cells of the present invention with their original peripheral blood
source. However, if
such a comparison is not available, then naturally-occurring peripheral blood
may refer to
average or typical characteristics of such peripheral blood, preferably of the
same mammalian
species as the source of the peripheral blood stem cells of this invention.
A "pharmaceutical peripheral blood stem cell composition" of this invention is
a
peripheral blood stem cell composition that is suitable for administration
into a mammal,
13
CA 02597963 2007-08-20
preferably into a human. Such a composition has a therapeutically effective
amount of expanded
(preferably TVEMF-expanded) peripheral blood stem cells. A therapeutically
effective amount
of expanded peripheral blood stem cells is (also discussed elsewhere herein)
preferably at least
1000 stem cells, more preferably at least 104 stem cells, even more preferably
at least 105 stem
cells, and even more preferably in an amount of at least l07 to 109 stem
cells, or even more stem
cells such as 1012 stem cells. Administration of such numbers of expanded stem
cells may be in
one or more doses. As indicated throughout this application, the number of
stem cells
administered to a patient may be liniited to the number of stem cells
originally available in
source peripheral blood, as multiplied by expansion according to this
invention. Without being
bound by theory, it is believed that stem cells not used by the body after
administration will
simply be removed by natural body systems. It should also be noted that
another preferred
embodiment provides for the culturing of cells wherein the cells are expanded
for a time without
regard to the number of cells in the culture, but instead, where the expanded
cells are cultured
and thereby have unique characteristics that are suitable to the repair of
mammalian tissue and/or
function.
As used throughout this application, the term "peripheral blood mixture"
refers to a
mixture of peripheral blood/peripheral blood cells with a substance that helps
the cells to expand,
such as a medium for growth of cells, that may be placed in a bioreactor (for
instance in a cell
culture chamber). The peripheral blood cells may be present in the peripheral
blood mixture
simply by mixing whole peripheral blood with a substance such as a cell
culture medium. Also,
the peripheral blood mixture may be made with a cellular preparation from
peripheral blood, as
described throughout this application, such as a `buffy coat," containing
peripheral blood stem
cells. Preferably, the peripheral blood mixture comprises peripheral blood
stem cells and
Dulbecco's medium (DMEM). Preferably, at least half of the peripheral blood
mixture is a cell
culture medium such as DMEM.
As used throughout this application, the term ` TVEMF" refers to "Time Varying
Electromagnetic Force".
As used throughout this application, the term ` TVEMF-bioreactor" refers to a
rotating
bioreactor to which TVEMF is applied, as described more fully in the
Description of the
Drawings, above. The TVEMF applied to a bioreactor is preferably as disclosed
herein. See for
instance Figures 2, 3, 4 and 5 herein for examples (not meant to be limiting)
of a TVEMF-
14
CA 02597963 2007-08-20
bioreactor. In a simple embodiment, a TVEMF-bioreactor of the present
invention provides for
the rotation of an enclosed peripheral blood mixture at an appropriateTVEMFand
allows the
peripheral blood cells (including stem cells) therein to expand. Preferably, a
TVEMF-bioreactor
allows for the exchange of growth medium (preferably with additives) and for
oxygenation of the
peripheral blood mixture. The TVEMF-bioreactor provides a mechanism for
expanding cells for
several days or more. The TVEMF-bioreactor subjects cells in the bioreactor to
TVEMF, so that
TVEMF is passed through or otherwise exposed to the cells, the cells thus
undergoing TVEMF-
expansion. The rotation of the TVEMF-bioreactor during TVEMF-expansion is
preferably at a
rate of 5 to 120 rpm, more preferably 10 to 30 rpm, to foster minimal wall
collision frequency
and intensity so as to maintain the bloodstream cell three-dimensional
geometry and cell-to-cell
support and cell-to-cell geometry.
As used throughout this application, the term "expanded peripheral blood
cells" refers to
peripheral blood cells increased in number (ie concentration) and/or cultured
after being placed
in a rotating bioreactor. "TVEMF expanded peripheral blood cells" refers to
peripheral blood
cells TVEMF-expanded in a TVEMF bioreactor wherein the cells are increased in
number and/or
cultured in the rotating TVEMF-bioreactor and subjected to a TVEMF. The
increase in number
of cells is the result of cell replication in the bioreactor, so that the
total number of cells in the
bioreactor increases. The increase in number of cells is expressly not due to
a simple reduction
in volume of fluid, for instance, reducing the volume of peripheral blood from
70 ml to 10 ml
and thereby increasing the number of cells per ml. By increasing in number it
is intended that the
cells replicate (and thereby grow in number). Substantially all peripheral
blood stem cells
(preferably CD34+, more preferably CD133+, and most preferably non-terminally
differentiated
stem cells) preferably expand without undergoing further differentiation.
"Substantially all" is
meant to refer to at least 70%, preferably at least 80%, more preferably at
least 90%, even more
preferably at least 95%, even more preferably at least 97%, and most
preferably at least 99% of
the stem cells do not differentiate.
In a preferred embodiment, the number of cells expanded not important. In such
an
embodiment, it is contemplated that by culturing the cells in the rotating
bioreactor (with or
without TVEMF), the cells will have enhanced repairing and regenerating
capabilities. For
instance, the cells may have enhanced tissue repairing characteristics or
tissue function repairing
characteristics by being cultured in the rotating bioreactor. If the
preference is to culture the
CA 02597963 2007-08-20
cells then a user may not focus on the number of cells expanded. For instance,
if the culture in
the rotating bioreactor is the focus of the method, then zero additional
cells, less than the number
that were placed in the rotating bioreactor, and at least one more than number
that were placed in
the bioreactor may all be acceptable numbers.
As used throughout this application, the term "expansion" refers to the
process of
increasing the number of peripheral blood cells in a bioreactor and/or
culturing the peripheral
blood cells, preferably peripheral blood stem cells, in a rotating bioreactor,
preferably a
TVEMF-bioreactor whereing the cells are subjected to a TVEMF. Preferably, the
increase in
number of peripheral blood stem cells is at least 7 times the number of
peripheral blood stem
cells that were placed into the rotating bioreactor, preferably TVEMF, for
expansion. The
expansion of peripheral blood stem cells in a rotating bioreactor according to
the present
invention provides for peripheral blood stem cells that maintain, or have
essentially the same,
three-dimensional geometry and cell-to-cell support and cell-to-cell geometry
as peripheral
blood stem cells prior to expansion, and also have a unique phenotypic
expression due to the
three-dimensional culture.
Other aspects of expansion may also provide the exceptional characteristics of
the
peripheral blood stem cells of the present invention, which is why the number
or expanded
cells may not be the focus of the expansion process, but rather, the three-
dimensional culture
environment in the rotating system. Not to be bound by theory, in one
embodiment,
expansion provides for high concentrations of peripheral blood stem cells that
maintain their
three-dimensional geometry and cell-to-cell support while at the same time
adopt a unique
phenotypic expression as a result of the culture environment in which they are
expanded.
TVEMF may affect some properties of stem cells during TVEMF-expansion, for
instance up-
regulation of genes promoting growth, or down regulation of genes preventing
growth.
Overall, expansion results in promoting growth in number and/or culture but
not
differentiation overall. It is also contemplated that before expansion, the
cells may preferably
be cultured in a two-dimensional or preferably in a three-dimensional system
for a preferred
amount of time before placing the cells in the rotating system for expansion.
Some genes that are up regulated may preferably include, but are not limited
to, those
coding for membrane proteins such as proteoglycan 3, CYP1B1, IL9R, HBA1, and
RHAG;
coding for cytoskeletal proteins such as SPTA1, ANK1; enzymes such as NCALD,
LSS,
16
CA 02597963 2007-08-20
PDE4B, SPUB, ELA2, HGD, ADAMDEC1, HMGCS1, COVA1, and PFKB4;
nuclear/transcription factors such as Pirin; and others such as S 100A8, A9.
Some genes that
are down regulated may preferably include, but are not limited to, membrane
proteins such as
IL2R, I1.17 R, EVI27, TGFR3, FCGRIA, MRCI, CCRI, CRL4, FER1L3, EMP1, and
THBD; transport proteins such as ABC1A and ABCG1; glycoproteins/cell surface
proteins
such as Versican, CDIc, CD 14, areg, z39iG, hm12, and CLECSF5; cytoskeletal
transduction
proteins such as SKG1; secreted proteins such as SCYA3, gro3, and galectin3;
nuclear
transcription factors sucha s KRML, LOC51713, KLF4, and EGR 1; and HMOX1 and
BPHL.
Preferably, the up regulated genes are up regulated up to 2 fold, and
preferably the down
regulated genes are down regulated up to four fold.
As used throughout this application, the term "TVEMF-expanded cell" refers to
a cell
that has been subjected to the process of TVEMF-expansion. A TVEMF-expanded
cell retains
some core properties of the same cell in vivo, but also has a unique
phenotypic expression as a
result of the TVEMF-expansion process including suspension in the rotating
TVEMF-bioreactor.
As afore mentioned, in a preferred embodiment, the expanded cell may
preferably be cultured
rather than expanded in number. In another preferred embodiment, the cells are
expanded
without the addition of TVEMF.
Throughout this application, the terms "repair", "replenish" and "regenerate"
are used.
These terms are not meant to be mutually exclusive, but rather related to
overall tissue repair.
Throughout this application, reference to the repair of tissue, treatment of
disease or
condition, are not meant to be exclusive but rather relate to the objective of
overall tissue repair
where improvement in tissue results from adminitstration of stem cells as
discussed herein.
While the present invention is directed in part to diseases or conditions that
are symptomatic, and
possibly life-threatening, the present invention is also meant to include
treatment of minor repair,
and even prevention/prophylaxis of disease/condition by early introduction of
expanded stem
cells, before symptoms or problems in the mammal's (preferably human's) health
are notice.
As used throughout this application, the term "toxic substance" or related
terms may refer
to substances that are toxic to a cell, preferably a peripheral blood stem
cell; or toxic to a patient.
In particular, the term toxic substance refers to dead cells, macrophages, as
well as substances
that may be unique or unusual in peripheral blood (for instance, sickle cells
in peripheral blood,
17
CA 02597963 2007-08-20
or other tissue or waste). Other toxic substances are discussed throughout
this application.
Removal of these substances from peripheral blood is well-known in the art.
As used throughout this application, the term "apheresis of bone marrow"
refers to
inserting a needle into bone and extracting bone marrow. Such apheresis is
well-known in the
art.
As used throughout this application, the term "autologous" refers to a
situation in
which the donor (source of peripheral blood stem cells prior to expansion) and
recipient are
the same mammal. The present invention includes autologous tissue repair and
replenishment.
As used throughout this application, the term "allogeneic" refers to a
situation in
which the donor (source of peripheral blood stem cells prior to expansion) and
recipient are
not the same mammal. The present invention includes allogeneic tissue repair
and
replenishment.
As used throughout this application, the term "cell-to-cell geometry" refers
to the
geometry of cells including the spacing, distance between, and physical
relationship of the
cells relative to one another. For instance, expanded stem cells of this
invention stay in
relation to each other as in the body. The expanded cells are within the
bounds of natural
spacing between cells, in contrast to for instance two-dimensional expansion
containers,
where such spacing is not kept.
As used throughout this application, the term "cell-to-cell support" refers to
the
support one cell provides to an adjacent cell. For instance, healthy tissue
and cells maintain
interactions such as chemical, hormonal, neural (where applicable/appropriate)
with other
cells in the body. In the present invention, these interactions are maintained
within normal
functioning parameters, meaning they do not for instance begin to send toxic
or damaging
signals to other cells (unless such would be done in the natural peripheral
blood environment).
As used throughout this application, the term "three-dimensional geometry"
refers to
the geometry of cells in a three-dimensional state (same as or very similar to
their natural
state), as opposed to two-dimensional geometry for instance as found in cells
grown in a Petri
dish, where the cells become flattened and/or stretched.
For each of the above three definitions, relating to maintenance of cell-to-
cell support
and geometry and three dimensional geometry of stem cells of the present
invention, the term
18
CA 02597963 2007-08-20
"essentially the same" means that normal geometry and support are provided in
expanded
cells of this invention, so that the cells are not for instance changed in
such a way as to
bedisfunctional, unable to repair tissue or toxic or harmful to other cells.
Other statemeats referring to the above-defined terms or other terms used
throughout
this application are not meant to be limited by the above definitions, and may
contribute to the
definitions. Information relating to various aspects of this invention is
provided throughout
this application, and is not meant to be limited only to the section to which
it is contained, but
is meant to contribute to an understanding of the invention as a whole.
This invention may be more fully described by the preferred embodiment(s) as
hereinafter described, but is not intended to be limited thereto.
Operative Method - Preparing a Peripheral Blood Mixture
Blood is collected from a mammal, preferably a primate mammal, and more
preferably a human, for instance as described throughout this application, and
preferably
according to the syringe method as well known in the art. Peripheral blood may
be collected,
for instance, and expanded immediately and used, or cryopreserved in expanded
or
unexpanded form for later use. Peripheral blood would only be removed from a
human in an
amount that would not be threatening to the subject. Preferably, about 10 to
about 500 ml
peripheral blood is collected; more preferably, 100-300 ml, even more
preferably, 150-200
ml. The collection of peripheral blood according to this invention is not
meant to be limiting,
but can also include for instance other means of directly collecting mammalian
peripheral
blood, pooling peripheral blood from one or more sources, indirectly
collecting blood for
instance by acquiring the blood from a commercial or othersource, including
for instance
cryopreserved peripheral blood from a` blood bank", or blood otherwise stored
for later use.
Typically, when directly collected from a mammal, peripheral blood is drawn
into one
or more syringes, preferably containing anticoagulants. The blood may be
stored in the
syringe or transferred to another vessel. Blood may then be separated into its
parts; white
blood cells, red blood cells, and plasma. This is either done in a centrifuge
(an apparatus that
spins the container of blood until the blood is divided) or by sedimentation
(the process of
injecting sediment into the container of blood causing the blood to separate).
Second, once the
blood is divided with the red blood cells (RBC) on the bottom, white blood
cells (WBC) in
the middle, and the plasma on top, the white blood cells are removed for
storage. The middle
19
CA 02597963 2007-08-20
layer, also known as the "buffy coat" contains the blood stem cells of
interest; the other parts
of the blood are not needed. For some blood banks, this will be the extent of
their processing.
However, other banks will go on to process the buffy coat by removing the
mononuclear cells
(in this case, a subset of white blood cells) from the WBC. While not everyone
agrees with
this method, there is less to store and less cryogenic nitrogen is needed to
store the cells.
Another method for separating blood cells is to subject all of the collected
peripheral
blood to one or more (preferably three) rounds of continuous flow
leukapheresis in a separator
such as a Cobe Spectra cell separator. Such processing will separate blood
cells having one
nucleus from other blood cells. The stem cells are part of the group having
one nucleus.
Other methods for the separation of blood cells are known in the art.
It is preferable to remove the RBC from the peripheral blood sample. While
people
may have the same HLA type (which is needed for the transplanting of stem
cells), they may
not have the same blood type. By removing the RBC, adverse reactions to a stem
cell
transplant can be minimized. By eliminating the RBC, therefore, the stem cell
sample has a
better chance of being compatible with more people. RBC can also burst when
they are
thawed, releasing free hemoglobin. This type of hemoglobin can seriously
affect the kidneys
of people receiving a transplant. Additionally, the viability of the stem
cells are reduced when
RBC rupture.
Also, particularly if storing blood cryogenically or transferring the blood to
another
mammal, the peripheral blood may be tested to ensure no infectious or genetic
diseases, such
as HIV/AIDS, hepatitis, leukemia or immune disorder, is present. If such a
disease exists, the
blood may be discarded or used with associated risks noted for a future user
to consider.
In still another embodiment of this invention, peripheral blood cells may be
obtained
from a donor. Prior to collection, the donor istreated with G-CSF (preferably
in an amount of
0.3ng to 5ug, more preferably 1 ng/kg to l00ng/kg, even more preferably 5
ng/kg to 20 ng/kg,
and even more preferably 6 ng/kg) every 12 hr over 3 days and then once on day
4. In a
preferred method, a like amount of GM-CSF is also administered. Other
alternatives are to
use GM-CSF alone, or other growth factor molecules, interleukins. Blood is
then collected
from the donor, and may be used whole in a peripheral blood mixture or first
separated into
cellular parts as discussed throughout this application, where the cellular
part including stem
ceilsis used to prepare the peripheral blood mixture to be expanded. Cells may
be separated,
CA 02597963 2007-08-20
for instance, by subjecting the donor's total blood volume to 3 rounds of
continuous-flow
leukapheresis through a separator, such as a Cobe Spectra cell separator.
Preferably, the
expanded stem cells are reintroduced into the same donor, where the donor is
in need of tissue
repair as discussed herein. However, allogeneic introduction may also be used,
as also
indicated herein. Other pre-collection administrations will also be evident to
those skilled in
the art.
Preferably, red blood cells are removed from the peripheral blood and the
remaining
cells including peripheral blood stem cells are placed with an appropriate
media in a
bioreactor (see "peripheral blood mixture") such as that described herein. In
a more preferred
embodiment of this invention, only the "buffy coat" (which includes peripheral
blood stem
cells, as discussed throughout this application) described above is the
cellular material placed
in the bioreactor. Other embodiments include removing other non-stem cells and
components
of the peripheral blood, to prepare different peripheral blood preparation(s).
Such a peripheral
blood preparation may even have, as the only remaining peripheral blood
component,
peripheral blood stem cells. Removal of non-stem cell types of peripheral
blood cells may be
achieved through negative separation techniques, such as but not limited to
sedimentation and
centrifugation. Many negative separation methods are well-known in the art.
However,
positive selection techniques may also be used, and are preferred in this
invention. Methods
for removing various components of the peripheral blood and positively
selecting for, but not
limited to, CD34+ and/or other markers such as CD 133+, are known in the art,
and may be
used so long as they do not lyse or otherwise irreversibly harm the desired
peripheral blood
stem cells. For instance, an affinity method selective for CD34+ may be used.
Preferably, a
"buffy coat" as described above is prepared from peripheral blood, and the
peripheral blood
stem cells therein separated from the buffy coat forexpansion.
The collected peripheral blood, or desired cellular parts as discussed above,
must be
placed into a rotating bioreactor for expansion, or preferably a TVEMF-
bioreactor for
TVEMF-expansion, to occur. As discussed above, the term "peripheral blood
mixture"
comprises a mixture of peripheral blood (or desired cellular part, for
instance peripheral blood
without red blood cells) with a substance that allows the cells to expand,
such as a medium for
growth of cells that will be placed in a bioreactor. Cell culture media, media
that allow cells
to grow and expand, arewell-known in the art. Preferably, the substance that
allows the cells
21
CA 02597963 2007-08-20
to expand is cell culture media, more preferably Dulbecco's medium. The
components of the
cell media must, of course, not kill or damage the stem cells. Other
components may also be
added to the peripheral blood mixture prior to or duringexpansion. For
instance, the
peripheral blood may be placed in the bioreactor with Dulbecco's medium and
further
supplemented with 5% (or some other desired amount, for instance in the range
of about 1%
to about 10%) of human serum albumin. Other additives to the peripheral blood
mixture,
including but not limited to growth factor, copper chelating agent, cytokine,
hormone and
other substances that may enhance expansion may also be added to the
peripheral blood
outside or inside the bioreactor before being placed in the bioreactor.
Preferably, the entire volume of a peripheral blood collection from one
individual
(preferably human peripheral blood in an amount of about 10 ntl to about 500
ml, more
preferably about 100 ml to about 300 nil, even more preferably about 150 to
about 200 ml
peripheral blood) is mixed with a cell culture medium such as Dulbecco's
medium (DMEM)
and supplemented with 5% human serum albumin to prepare a peripheral blood
mixture for
expansion. For instance, for a 50 to 100 ml peripheral blood sample,
preferably about 25 to
about 100 ml DMEM/5% human serum albumin is used, so that the total volume of
the
peripheral blood mixture is about 75 to about 200 ml when placed in the
bioreactor. As a
general rule, the more peripheral blood that may be collected, the better;if a
collection from
one individual results in more than 100 ml, the use of all of that peripheral
blood is preferred.
Where a larger volume is available, for instance by pooling peripheral blood
(from the same
or different source), more than one dose may be preferred. The use of a
perfusion bioreactor
is particularly useful when peripheral blood collections are pooled and
expanded together.
A copper chelating agent of the present invention may be any non-toxic copper
chelating agent, and is preferably Penicillamine or Trientine Hydrochloride.
More preferably,
the Penicillamine is D(-)-2-Amino-3-Mercaptor-3-Methylbutanic Acid (Sigma-
Aldrich),
dissolved in DMSO and added to the peripheral blood mixture in an amount of
about 10 ppm.
The copper chelating agent may also be administered to a mammal, where
peripheral blood
will then be directly collected from the mammal. Preferably such
administration is more than
one day, more preferably more than two days, before collecting peripheral
blood from the
mammal. The purpose of the copper chelating agent, whether added to the
peripheral blood
mixture itself or administered to a peripheral blood donor mammal, or both, is
to reduce the
22
CA 02597963 2007-08-20
amount of copper in the peripheral blood prior to expansion. Not to be bound
by theory, it is
believed that the decrease in amount of available copper may enhance
expansion, including
TVEMF-expansion.
The term "placed in a bioreactor" is not meant to be limiting and also applies
to
peripheral blood "placed in a TVEMF-bioreactor"- the peripheral blood mixture
may be
made entirely outside of the bioreactor and then the mixture placed inside the
bioreactor.
Also, the peripheral blood mixture may be entirely mixed inside the
bioreactor. For instance,
the peripheral blood (or a cellular portion thereof) may be placed in the
bioreactor and
supplemented with Dulbecco's medium and 5% human serum albumin either already
in the
bioreactor, added simultaneously to the bioreactor, or added after the
peripheral blood to the
bioreactor.
A preferred peripheral blood mixture of the present invention comprises the
following:
peripheral blood stem cells isolated from the buffy coat of a peripheral blood
sample and
Dulbecco's medium which, with the cells, is about 150-250 ml, preferably about
200 ml total
volume. Even more preferably, G-CSF (Granulocyte-Colony Stimulating Factor) is
included
in the peripheral blood mixture. Preferably, G-CSF is present in an amount
sufficient to
enhance expansion of peripheral blood stem cells. Even more preferably, the
amount of G-
CSF present in the peripheral blood mixture prior to TVEMF-expansion is about
25 to about
200 ng/ml peripheral blood mixture, more preferably about 50 to about 150
ng/nal, and even
more preferably about 100 ng/ml.
Operative method- Expansion
In use, the rotation of a bioreactor (TVEMF or otherwise) provides a
stabilized culture
environment into which cells may be introduced, suspended, maintained, and
expanded with
improved retention of delicate three-dimensional structural integrity by
simultaneously
minimizing the fluid shear stress, providing three-dimensional freedom for
cell and substrate
spatial orientation, and increasing localization of cells in a particular
spatial region for the
duration of the expansion (hereinafter referred to as "three criteria "). The
rotating TVEMF-
bioreactor also provides these three criteria, and at the same time, exposes
the cells to a TVEMF.
Of particular interest to the present invention is the dimension of the
culture chamber, the
sedimentation rate of the cells, the rotation rate, the external gravitational
field, and the TVEMF.
23
CA 02597963 2007-08-20
The stabilized culture environment referred to in the operation of present
invention is that
condition in the culture medium, particularly the fluid velocity gradients,
prior to introduction of
cells, which will support a nearly uniform suspension of cells upon their
introduction thereby
creating a three-dimensional culture upon addition of the cells. In a
preferred embodiment, the
culture medium is initially stabilized into a near solid body horizontal
rotation 360 degrees about
an axis within the confines of a similarly rotating chamber wall of a
rotatable bioreactor. The
rotating continues in the same direction about the axis. The chamber walls are
set in motion
relative to the culture medium so as to initially introduce essentially no
fluid stress shear field
therein. Cells are introduced to, and move through, the culture medium in the
stabilized culture
environment thus creating a three-dimensional culture. The cells move under
the influence of
gravity, centrifugal, and coriolus forces, and the presence of cells within
the culture medium of
the three-dimensional culture induces secondary effects to the culture medium.
The motion of
the culture medium with respect to the culture chamber, fluid shear stress,
and other fluid
motions, is due to the presence of these cells within the culture medium.
In most cases the cells with which the stabilized culture environment is
primed sediment
at a slow rate preferably under 0.1 centimeter per second. It is therefore
possible, at this early
stage of the three-dimensional culture, to select from a broad range of
rotational rates (preferably
of from about 2 to about 30 RPM) and chamber diameters (preferably of from
about 0.5 to about
36 inches). Preferably, the slowest rotational rate is advantageous because it
minimizes
equipment wear and other logistics associated with handling the three-
dimensional culture. The
preferred speed of the present invention is of from 5 to 120 RPM, and more
preferably from 10
to 30 RPM.
Not to be bound by theory, rotation about a substantially horizontal axis with
respect to
the external gravity vector at an angular rate optimizes the orbital path of
cells suspended within
the three-dimensional culture. The progress of the three-dimensional culture
is preferably
assessed by a visual, manual, or automatic determination. An increase in the
density of cells
may require appropriate adjustment of the rotation speed in order to optimize
the particular
paths. An increase in density is related to an increase in the number of cells
in the culture
chamber. The rotation of the culture chamber optimally controls collision
frequencies, collision
intensities, and localization of the cells in relation to other cells and also
the limiting boundaries
of the culture chamber of the rotatable bioreactor. In order to control the
rotation, if the cells are
24
CA 02597963 2007-08-20
observed to excessively distort inwards on the downward side and outwards on
the upwards side
then the revolutions per minute ("RPM") may preferably be increased. If the
cells are observed
to centrifugate excessively to the outer walls then the RPM may preferably be
reduced.
Optimally, the zero-head space of the three-dimensional culture provides a
space wherein cells
may preferably be distributed throughout the volume of culture medium
effectively utilizing the
full culture chamber capacity.
The cell sedimentation rate and the external gravitations field place a lower
limit on the
fluid shear stress obtainable, even within the operating range of the present
invention, due to
gravitationally induced drift of the cells through the culture medium of the
three-dimensional
culture. Calculations and measurements place this minimum fluid shear stress
very nearly to that
resulting from the cells' terminal sedimentation velocity (through the culture
medium) for the
external gravity field strength. Centrifugal and coriolis induced motion
[classical angular
kinematics provide the following equation relating the Coriolis force to an
object's mass (m), its
velocity in a rotating frame (v,) and the angular velocity of the rotating
frame of reference (0):
Fco,;oiis = -2 m (w x v,)] along with secondary effects due to cell and
culture medium interactions,
act to further degrade the fluid shear stress level as the cells expand.
Not to be bound by theory, but an environment that is substantially similar to
microgravity may be obtained in the rotating bioreactor. In order to obtain
the minimal fluid
shear stress level it is preferable that the culture chamber be rotated at
substantially the same rate
as the culture medium. Not to be bound by theory, but this minimizes the fluid
velocity gradient
induced upon the three-dimensional culture. It is advantageous to control the
rate of expansion
in order to maintain the cell density (and associated sedimentation rate)
within a range for which
the rate of expansion is able to satisfy the three criteria. In addition,
transient disruptions of the
expansion process are permitted and tolerated for, among other reasons,
logistical purposes
during initial system priming, sample acquisition, system maintenance, and
culture termination.
Rotating cells about an axis substantially perpendicular to gravity can
produce a variety
of sedimentation rates, all of which according to the present invention remain
spatially localized
in distinct regions for extended periods of time ranging from seconds (when
sedimentation
characteristics are large) to hours or days (when sedimentation differences
are small). Not to be
bound by theory, but this allows these cells sufficient time to interact and
associate as necessary
with each other in a three-dimensional culture. Preferably, cells undergo
expansion for at least 4
CA 02597963 2007-08-20
days, more preferably from about 7 days to about 14 days, most preferably from
about 7 days to
about 10 days, even more preferably about 7 days. Expansion may continue in a
bioreactor
(TVEMF or otherwise) for up to 160 days. While expansion may occur for even
longer than 160
days, such a lengthy expansion is not a preferred embodiment of the present
invention.
Preferably, expansion may continue in a rotatable bioreactor to produce a
number of cells that is
at least 7 times the original number of cells that were placed in the
rotatable bioreactor.
Culture chamber dimensions also influence the path of cells in the three-
dimensional
culture of the present invention. A culture chamber diameter is preferably
chosen which has the
appropriate volume, preferably of from about 15ml to about 2L for the intended
three-
dimensional culture and which will allow a sufficient seeding density of
cells. Not to be bound
by theory, but the outward cells drift due to centrifugal force is exaggerated
at higher culture
chamber radii and for rapidly sedimenting cells.
The path of the cells in the three-dimensional culture has been analytically
calculated
incorporating the cell motion resulting from gravity, centrifugation, and
coriolus effects. A
computer simulation of these governing equations allows the operator to model
the process and
select parameters acceptable (or optimal) for the particular planned three-
dimensional culture.
Figure 9 shows the typical shape of the cell orbit as observed from the
external (non-rotating)
reference frame. Figure 10 is a graph of the radial deviation of a cell from
the ideal circular
streamline plotted as a function of RPM (for a typical cell sedimenting at 0.5
cm per second
terminal velocity). This graph (Figure 10) shows the decreasing amplitude of
the sinusoidally
varying radial cells deviation as induced by gravitational sedimentation.
Figure 10 also shows
increasing radial cell deviation (per revolution) due to centrifugation as RPM
is increased. These
opposing constraints influence carefully choosing the optimal RPM to
preferably minimize cell
impact with, or accumulation at, the chamber walls. A family of curves is
generated which is
increasingly restrictive, in terms of workable RPM selections, as the external
gravity field
strength is increased or the cell sedimentation rate is increased. This family
of curves, or
preferably the computer model which solves these governing orbit equations, is
preferably
utilized to select the optimal RPM and chamber dimensions for the expansion of
cells of a given
sedimentation rate in a given external gravity field strength. Not to be bound
by theory, but as a
typical three-dimensional culture is expanded the number of cells and
therefore the cell density
26
CA 02597963 2007-08-20
effects the sedimentation rate, and therefore, the rotation rate may
preferably be adjusted to
optimize the same.
In the three-dimensional culture, the cell orbit (Figure 9) from the rotating
reference
frame of the culture medium is seen to move in a nearly circular path under
the influence of the
rotating gravity vector (Figure 11). Not to be bound by theory, but the two
pseudo forces,
coriolis and centrifugal, result from the rotating (accelerated) reference
frame and cause
distortion of the otherwise nearly circular path. Higher gravity levels and
higher cell
sedimentation rates produce larger radius circular paths which correspond to
larger trajectory
deviations from the ideal circular orbit as seen in the non- rotating
reference frame. In the
rotating reference frame it is thought, not to be bound by theory, that cells
of differing
sedimentation rates will remain spatially localized near each other for long
periods of time with
greatly reduced net cumulative separation than if the gravity vector were not
rotated; the cells are
sedimenting, but in a small circle (as observed in the rotating reference
frame). Thus, in
operation the present invention provides cells of differing sedimentation
properties with
sufficient time to interact mechanically and through soluble chemical signals
thereby effecting
their cell-to-cell interactions including geometry and support. In operation,
the present invention
provides for sedimentation rates of preferably from about 0 cm/second up to 10
cm/second.
Furthermore, in operation the culture chamber of the present invention has at
least one
aperture preferably for the input of fresh culture medium and a cell mixture
and the removal of a
volume of spent culture medium containing metabolic waste, but not limited
thereto. Preferably,
the exchange of culture medium can also be via a culture medium loop wherein
fresh or recycled
culture medium may be moved within the culture chamber preferably at a rate
sufficient to
support metabolic gas exchange, nutrient delivery, and metabolic waste product
removal. This
may slightly degrade the otherwise quiescent three-dimensional culture. It is
preferable,
therefore, to introduce a mechanism for the support of preferred components
including, but not
limited to, respiratory gas exchange, nutrient delivery, growth factor
delivery to the culture
medium of the three-dimensional culture, and also a mechanism for metabolic
waste product
removal in order to provide a long term three-dimensional culture able to
support significant
metabolic loads for periods of hours to months.
It is expected that expansion in a rotating bioreactor provides a unique
environment that
effects the cell phenotype, as gauged by RNA expression levels. The cells
adapt to the unique
27
CA 02597963 2007-08-20
three-dimensional environment in which they are suspended. Cells expanded in
the three-
dimensional environment of a rotating bioreactor express different gene
expression patterns, and
therefore, different membrane and surface protein configurations, and
different cytoskeletal
details. This feature of the ceIls is a result of the three-dimensional
environment in which the
cells are suspended and expanded, referred to as genetic expression modified.
It is expected that
the cell exposure to TVEMF in a TVEMF-bioreactor provides even more
exceptional
characteristics to the expanding peripheral blood cell than those detected by
rotation alone.
During the time that the cells are in the rotating bioreactor (with or without
TVEMF),
they are preferably fed nutrients and fresh media (DMEM and 5% human serum
albumin),
exposed to hormones, cytokines, and/or growth factors (preferably G-CSF); and
toxic
materials are removed. The toxic materials removed from peripheral blood cells
in a
bioreactor include the toxic granular material of dying cells and the toxic
material of
granulocytes and macrophages.
Preferably, expansion is carried out in a rotating bioreactor at a temperature
of about
26 C to about 41 C, and more preferably, at a temperature of about 37C.
One method of monitoring the overall expansion of cells undergoing expansion
is by
visual inspection. Peripheral blood stem cells are typically dark red in
color. Once the
bioreactor begins to rotate, and in a preferred embodiment the TVEMF is
applied, the cells
that are distributed throughout the full volume of media preferably cluster in
the center of the
bioreactor vessel as they become greater in number (denser), with the medium
surrounding
the colored cluster of cells. Oxygenation and other nutrient additions often
do not cloud the
ability to visualize the cell cluster through a visualization (typically clear
plastic) window
built into the bioreactor. Formation of the cluster is important for helping
the stem cells
maintain their three-dimensional geometry and cell-to-cell support and cell-to-
cell geometry;
if the cluster appears to scatter and cells begin to contact the wall of the
bioreactor vessel, the
rotational speed is increased (manually or automatically) so that the
centralized cluster of cells
may form again. A measurement of the visible diameter of the cell cluster
taken soon after
formation may be compared with later cluster diameters, to indicate the
approximate number
increase in cells in the bioreactor. Measurement of the increase in the number
of cells during
expansion may also be taken in a number of ways, as known in the art. An
automatic sensor
could also be included in the bioreactor to monitor and measure the increase
in cluster size.
28
CA 02597963 2007-08-20
The expansion process may be carefully monitored, for instance by a laboratory
expert, who will check cell cluster formation to ensure the cells remain
clustered inside the
bioreactor and will increase the rotation of the bioreactor when the cell
cluster begins to
scatter. An automatic system for monitoring the cell cluster and viscosity of
the peripheral
blood mixture inside the bioreactor may also monitor the cell clusters. A
change in the
viscosity of the cell cluster may become apparent about 2 days after beginning
the expansion
process, and the rotational speed of the bioreactor may be increased around
that time. The
bioreactor speed may vary throughout expansion. Preferably, the rotational
speed is timely
adjusted so that the cells undergoing expansion do not contact the sides of
the rotating
bioreactor vessel.
Also, the laboratory expert may, for instance once a day, or once every two
days,
manually (for instance with a syringe) insert fresh media and preferably other
desired
additives such as nutrients and growth factors, as discussed above, into the
bioreactor, and
draw off the old media containing cell wastes and toxins. Also, fresh media
and other
additives may be automatically pumped into the bioreactor during expansion,
and wastes
automatically removed.
Peripheral blood stem cells may increase to at least seven times their
original number
about 7 to about 14 days after being placed in the bioreactor and expanded.
Preferably, the
expansion lasts about 7 to 10 days, and more preferably about 7 days.
Measurement of the
number of stem cells does not need to be taken during expansion therefore. As
indicated
above and throughout this application, expanded peripheral blood stem cells of
the present
invention have essentially the same three-dimensional geometry and cell-to-
cell support and
cell-to-cell geometry as naturally-occurring, non-expanded peripheral blood
stem cells due to
the essentially non-turbulent and low shear stress culture regime. The
expanded peripheral
blood cell retains fundamental properties of the non-expanded peripheral blood
cells. The
gentle free drifting of the cells through soluble molecular species which
control cell function
and are substrates and products of cell metabolism allows the rotating
bioreactor systems to
produce a unique living product cell in terms of transcribed RNA pattern
coding for multiple
cell structural and functional proteins and cell sub organelles.
Another embodiment of the present invention relates to an ex vivo mammalian
peripheral
blood stem cell composition that functions to assist a body system or tissue
to repair, replenish
29
CA 02597963 2007-08-20
and regenerate tissue, for example, the tissues described throughout this
application. The
composition comprises expanded peripheral blood stem cells, preferably with
TVEMF. The
peripheral blood cells in the composition are preferably expanded to at least
seven times the
number that were placed in the culture chamber of the rotatable bioreactor.
For instance,
preferably, if a number X of peripheral blood stem cells was placed in a
certain volume into a
bioreactor, then after expansion, the number of peripheral blood stem cells
from that same
volume of peripheral blood stem cells place into the bioreactor will be at
least 7X. While this at-
least-seven-times-expansion is not necessary for this invention to work, this
expansion is
preferred for therapeutic purposes. For instance, the expanded cells may be
only in amount of 2
times the number of peripheral blood stem cells placed in the rotating
bioreactor, if desired.
Preferably, expanded cells are in a range of about 4 times to about 25 times
the number of
peripheral blood stem cells placed in the bioreactor. In another preferred
embodiment, the
expanded cells number in an amount that is at least one cell more than the
number that were
placed in the culture chamber of the rotatable bioreactor. In this embodiment,
the phenotypic
expression of the cells after expansion is the preferred focus for repairing a
body function or
tissue.
The present invention is also directed to a composition comprising peripheral
blood stem
cells from a mammal, wherein said peripheral blood stem cells are expanded in
a rotating
TV'BMF-bioreactor while suspending the cells therein to up or down regulate
genes as effected
by the cells environment, interactions, and three-dimensional geometry. A
composition of the
present invention may include a pharmaceutically acceptable carrier; plasma,
peripheral blood,
albumin, cell culture medium, growth factor, copper chelating agent, hormone,
buffer or
cryopreservative. "Pharmaceutically acceptable carrier" means an agent that
will allow the
introduction of the stem cells into a mammal, preferably a human. Such carrier
may include
substances mentioned herein, including in particular any substances that may
be used for
peripheral blood transfusion, for instance peripheral blood, plasma, albumin,
preferably from the
mammal to which the composition will be introduced. The term "introduction" of
a composition
to a mammal is meant to refer to "administration" of a composition to an
animal. "Acceptable
carrier" generally refers to any substance the peripheral blood stem cells of
the present invention
may survive in, ie that is not toxic to the cells, whether after TVEMF-
expansion, prior to or after
cryopreservation, prior to introduction (administration) into a mammal. Such
carriers are well
CA 02597963 2007-08-20
known in the art, and may include a wide variety of substances, including
substances described
for such a purpose throughout this application. For instance, plasma,
peripheral blood, albumin,
cell culture medium, buffer and cryopreservative are all acceptable carriers
of this invention.
The desired carrier may depend in part on the desired use.
Expanded peripheral blood stem cells have essentially the same, or maintain,
the
three-dimensional geometry and the cell-to-cell support and cell-to-cell
geometry as the
peripheral blood from which they originated. A preferred composition comprises
expanded
peripheral blood stem cells, preferably in a suspension of Dulbecco's medium
or in a solution
ready for cryopreservation. The composition is preferably free of toxic
granular material, for
example, dying cells and the toxic material or content of granulocytes and
macrophages. The
composition may be a cryopreserved composition comprising expanded peripheral
blood stem
cells by decreasing the temperature of the composition to a temperature of
from -120 C to -
196 C and maintaining the cryopreserved composition at that temperature range
until needed
for therapeutic or other use. As discussed below, preferably, as much toxic
material as is
possible is removed from the composition prior to cryopreservation.
Another embodiment of the present invention relates to a method of
regenerating
tissue and/or function with a composition of expanded peripheral blood stem
cells, either
having undergone cryopreservation or soon after expansion is complete. The
cells may be
introduced into a mammalian body, preferably human, for instance injected
intravenously,
directly into the tissue to be repaired, into the abdominal cavity, attaching
to the
peritoneum/peritoneal cavity, allowing the body's natural system to repair and
regenerate the
tissue. Preferably, the compositionintroduced into the mammalian body is free
of toxic
material and other materials that may cause an adverse reaction to the
administered expanded
peripheral blood stem cells.
An expanded peripheral blood stem cell composition of the present invention
should
be introduced into a mammal, preferably a human, in an amount sufficient to
achieve repair of
tissue and/or function, or to treat a desired disease or condition.
Preferably, at least 20 ml of
an expanded peripheral blood stem cell composition having 10' to 109 stem
cells per ml is
used for any treatment, preferably all at once, in particular where a
traumatic injury has
occurred and immediate tissue repair needed. This amount is particularly
preferred in a 75-80
kg human. The amount of expanded peripheral blood stem cells in a composition
being
31
CA 02597963 2007-08-20
introduced into the source mammal is inherently related to the number of cells
present in the
source peripheral blood material. A preferred range of expanded peripheral
blood stem cells
introduced into a patient may be, for instance, about 10 ml to about 50 ml of
an expanded
peripheral blood stem cell composition having 107 to 109 stem cells per ml, or
potentially
even more. While it is understood that a high concentration of any substance,
administered to
a mammal, may be toxic or even lethal, it is unlikely that introducing all of
a mammal's
peripheral blood stem cells, for instance after expansion, will cause an
overdose in expanded
peripheral blood stem cells. Where peripheral blood from several donors is
used, the number
of peripheral blood stem cells introduced into a mammal may be higher.
Therefore, it should
be realized that the expanded cells may be introduced to the mammal from an
allogeneic
source or an autologous source. Also, the dosage of cells that may be
introduced to the
patient is not limited by the amount of peripheral blood provided from
collection from one
individual; multiple administrations, for instance once a day or twice a day,
or once a week, or
other administration time frames, may more easily be used. Also, where a
tissue is to be
treated, the type of tissue may warrant the use of as many expanded peripheral
blood stem
cells as are available.
Example #1- Qualitative and Quantitative comparison between a Rotating
Bioreactor
and a Dynamic Moving Culture.
An experiment was conducted to demonstrate the qualitative differences between
two
cultures and the differences in the rates of expansion. To illustrate the
differences a
comparison was made between gene expression levels as assayed by abundance of
mRNA
transcripts in two samples of blood stem cells cultured in two different
methods: (A) shaken
Petri plate (dynamic moving culture) (B) rotating bioreactor. The cultures
were set up, refed,
harvested and otherwise manipulated in the identical manner. The test was
documented using
techniques well accepted in the art including Affymetrix Gene Array to prove
the differences
in genetic expression levels. All conditions and manipulations were the same
for the two
cultures except for the type of culture vessel in which they were expanded.
Culture A serves as the baseline on which to determine increase or decrease of
transcript levels in culture B. There are several differences in membrane
composition
between the 2 cultures, as far as cell surface receptors are concerned. In
addition, several of
32
CA 02597963 2007-08-20
the other genes that are altered in the rotating bioreactor culture (mostly
the `decreased' ones)
have a role in innate and adaptive immunity. Also, some transcripts of genes
involved in cell-
to-cell contacts and cytoskeletal structures are significantly changed. Some
of the altered
genes are involved in cell proliferation.
Below is a summary of the most relevant functions of a subset of the array
data. Included
in this summary are only those genes that show at least a 200% (1-fold)
difference in expression
levels between samples, either decreased (T) or increased (lI). The data are
further clustered
based on cellular localization and/or function.
"Decreased" Genes(Range of change is 4-to-1 fold)
A. MEMBRANE PROTEINS
1. Receptors
- lL2R: aka CD25, expressed in regulatory T cells and macrophages and
activated T-
and B-cells; involved in cytokine-cytokine receptor interactions and role in
cell proliferation
- IL17R: receptor for IL17, and essential cytokine that acts as an immune
response
modulator
- EVI27: truncated precursor of IL17 receptor homolog
- TGFR3: (aka beta-glycan) also has a soluble form; involved in cell
differentiation, cell
cycle progression, migration, adhesion, ECM production
- FCGRIa: (aka CD64, human Fc- receptor) expressed in macrophages/monocytes,
neutrophils; involved in phagocytosis, the immune response and cell signal
transduction
- MRC 1: (aka CD206; Mannose Receptor; lectin-family) expressed in
macrophages/
monocytes (where expression increasing during culture), and dendrtitic cells;
involved in
innate and adaptive immunity
- CCR 1: (chemokine receptor, aka CD 191, MIP 1 receptor, RANTES receptor);
multipass protein expressed in several hematopoietic cells that transduces a
signal in response
to several chemokines by increasing intracellular calcium ions level;
responsible for affecting
stem cell proliferation; role in cell adhesion, inflammation and immune
response
- CRL4: putative cytokine receptor precursor with role in signal transduction
and
proliferation
33
CA 02597963 2007-08-20
- FER1L3: (myoferlin) single-pass protein at nuclear and plasma membranes;
involved
in membrane regeneration and repair; expressed in cardiac and skeletal muscle
- EMP1: (aka TMP) mutli-pass protein of claudin family involved in formation
of tight
junctions, and cell-to-cell contact
- THBD: (thrombomodulin aka CD141); single pass endothelial cell receptor with
lectin
and EGF-like domains; complexes with tbrombin to activate the coagulation
cascade (factor
Va and VIIIa)
2. Transporters
- ABCA1: multipass protein involved in cholesterol trafficking (efflux);
expressed in
macrophages and keratinocytes
- ABCG1: multi-pass transporter involved in macrophage lipid homeostasis;
expressed
in intracellular compartments of macrophages mostly; found in the endoplasmic
reticulum
membrane and Golgi apparatus;
3. Glycoproteins/Cell Surface
- Versican (aka CSPG2, chondroitin sulfate proteoglycan 2); involved in
maintaining
ECM integrity, and has a role in cell proliferation, migration, and cell-cell
adhesion (also
interacts with tenascinR)
- CDIc: expressed in activated Tcells; involved in mounting immune response
- CD14: cell surface marker expressed in monocytes/macrophages
- AREG: (amphiregulin) involved in cell-to-cell signaling and proliferation;
growth-
modulating glycoprotein. Inhibits growth of several human carcinoma cells in
culture and
stimulates proliferation of human fibroblasts and certain other tumor cells
- Z391g: a membrane spanning immunoglobulin with a role in mounting the immune
response; expressed in monocytes and dendritic cells
- HML2: (aka CLEClOA, CD301) single pass lectin expressed in macrophages;
Probable role in regulating adaptive and innate immune responses. Binds in a
calcium-
dependent manner to terminal galactose and N-acetylgalactosamine units, linked
to serine or
threonine.
34
CA 02597963 2007-08-20
- CLECSF5: single pass myeloid lectin; involved in proinflammatory activation
of
myeloid cells via TYROBP-mediated signaling in a calcium-dependent manner
B. CYTOSOLIC/SIGNAL TRANSDUCTION:
- SKG1: expressed in granulocytes; has a role in response to oxidative stress
and in
cellular communication; part of the proteasome - ubiquitin pathway
C. SECRETED
- SCYA3 (aka CCL3, MIP1): secreted by macrophages/monocytes; soluble monokine
with inflammatory and chemokinetic properties involved in mediating the
inflammatory
response; a major HIV-suppressive factor produced by CD8+ T-cells.
- GRO3: (aka CKCL3, MIP2); secreted by PB monocytes; chemokine with
chemotactic
activity for neutrophils and a role in inflammation and iminunity
- Galectin3: soluble protein secreted by macrophages/monocytes; can bind the
ECM to
activate cells or restrain mobility; involved in other processes including
inflammation,
neoplastic transformation, and innate and acquired immunity by binding IgE;
also has a
nuclear form; inhibited by MMP9.
D. NUCLEAR/TRANSCRIPTION FACTORS
- KRML; LOC51713; KLF4: three gene members of Kreisler/Krox family of nuclear
transcription factors involved in bone and inner ear morphogenesis, epithelial
cell
differentiation and/or development of the skeleton and kidney
- EGR 1: (aka KROX24) expressed in lymphocytes and lymphoid organs; involved
in
macrophage differentiation, and inflammation/apoptosis pathways; activates
genes in
differentiation
E. ENZYMES
- HMOX1: (heme oxygenase) microsomal (ER); highly expressed in spleen;
involved in
heme turnover; ubiquitously expressed following induction by several stresses,
potent anti-
inflammatory proteins whenever oxidation injury takes place
CA 02597963 2007-08-20
- BPHL: mitochondrial serine hydrolase that catalyzes the hydrolytic
activation of
amino acid ester prodrugs of nucleoside analogs; may play a role in
detoxification processes
"Increased" Genes (range of change is 2-to-1 fold)
A. MEMBRANE PROTEINS
- Proteoglycan 3: expressed in eosinophils and granulocytes, highly expressed
in bone
marrow; involved in immune response, neutrophil activation and release of II.8
and histamine
- CYP 1 B 1: Cytochromes P450 are a group of heme-thiolate monooxygenases
involved
in an NADPH-dependent electron transport pathway. It oxidizes a variety of
structurally
unrelated compounds, including steroids, fatty acids, and xenobiotics
- IL9R: single pass interleukin receptor, involved in cell proliferation and
signaling,
expressed in hemaotpoietic cells
- HBA1: (CD31) binds heme and iron involved in oxygen transport, specific to
RBCs
- RHAG (aka CD241) expressed in erythrocytes, Rh blood group protein multipass
protein ammonium transporter; binds ankyrin, a component of the RBC
cytoskeleton
B. CYTOSKELETAL PRTOEINS
- SPTA1; ANK1: both proteins are located on cytoplasmic face of plasma
membrane of
erythrocytes (RBC) and act to anchor transmembrane proteins to the
cytoskeleton; together
with actin and other proteins they form the RBC cytoskeleton superstructure
and are
responsible for keeping its shape
- NCALD: neurocalcin; cytosolic; involved in vesicle-mediated transport; binds
actin,
tubulin and clathrin; can bind Ca2+; expressed in neural tissues and testes
C. ENZYMES (cytosolic)
- LSS: cholesterol metabolism-steroid biosynthesis
- PDE4B: involved in anti-inflammatory response, high in CNS; purine
metabolism
- SPUVE: a secreted serine protease (unknown function)
- ELA2: serine protease expressed in leukocytes/neutrophoils, involved in
protein
hydrolysis including elastin; serves to modify the function of NK cells,
monocytes and
granulocytes; inhibits chemotaxis in anti-inflammatory response, high in BM
36
CA 02597963 2007-08-20
- HGD: iron binding oxygenase involved in tyrosine metab and phenylalalnine
catabolism
- ADAMDEC 1: expressed in macrophages; a secreted zinc binding serum protease
involved in immune response; up-regulated during primary monocyte to
macrophage and/or
dendritic cell differentiation
- HMGCS 1: soluble co-enzyme A synthase involved in cholesterol biosynthesis
- COVA1 hydroquinone oxidase (X-linked) extracellular and trans plasma
membrane
associated (secreted factor) has copper as a cofactor has several properties
associated with
prions; naturally is glycosylated ; involved ultradian rhythm maintenance,
cell growth
regulation, electron transport
- PFKB4: glycolytic enzyme
D. NUCLEAR/TRANSCRIPTION FACTORS
- Pirin: iron-binding nuclear transcription factor; DNA replication and
transactivation
(X-linked); interacts with SMAD signaling cascade
E. OTHER
S100A8, A9: secreted, calcium binding proteins (isoforms A8, A9 expressed in
epithelial cells) expressed by monocytes/macrophages and granulocytes as part
of the
inflammatory response; inhibitor of protein kinases. Also expressed in
epithelial cells
constitutively or induced during dermatoses. May interact with components of
the
intermediate filaments in monocytes and epithelial cells; highly expressed in
bone marrow.
Figures 12, 13, and 14 illustrate that the cells in a rotating bioreactor
expand to a
significantly greater number than cells in a dynamic moving culture. The
expansion of
CD 133+ cells, total nucleated cells and CD34+ ceUs were analyzed.
These results demonstrate that cells expanded in a rotating system, such as a
TVEMF-
bioreactor are qualitatively unique. The non-turbulent regime in the rotating
bioreactor allows
the cells to expand in a low shear environment so that the input cell is not
disturbed as much
as it would be in other three-dimensional systems. However, as a result of the
expansion
process, the expanded blood stem cells have a unique phenotypic expression to
support their
37
CA 02597963 2007-08-20
suspension in the three-dimensional environment. That expression is fostered
and maintained
without differentiation and over a high rate of expansion.
Example #2-TVEMF-Expansion in a TVEMF-bioreactor
CD 1 33-selected cells were pre-cultured in a two-dimensional culture system
for three
days prior to placing the cells in a rotating bioreactor with and without
TVEMF. Samples V 1
and V2 were cultured without TVEMF and V 1T and V2T were cultured with TVEMF,
while
all other conditions stayed the same. The cells were placed in a 10nil
rotating TVEMF-
bioreactor at a density of about 0.2 x 106 cells/ml, and the entire bioreactor
volume was filled.
The culture medium used for this experiment was IMDM. The bioreactors were
rotated at
approximately 20 rpm. The following data refers to the culture period in the
rotating
TVEMF-bioreactor, and does not reflect the two-dimensional pre-culture. The
cultures were
expanded at 37 C, and in 5% CO2. All other culture conditions remained the
same for each
sample, V l, V2, V 1T and V2T.
Figure 15 illustrates the results of the TVEMF-expansion (numbers of cells).
The
number of CD34+ cells increased from between 20 x 104 cells/ml and 48 x 104
celis/ml by
day 6. Figure 16 illustrates the expansion rate (number of cells) in a
rotating TVEMF-
bioreactor as compared with a rotating non- TVEMF bioreactor. The results show
that on day
6, the cultures that were exposed to TVEMF had more cells than those that were
not. The
difference between expansion with and without TVEMF was between about 10 x 104
cells/ml
and about 15 x 104 cells/ml.
Example #3-TVEMF-Expansion of Cells in a TVEMF Bioreactor
Peripheral blood was collected and peripheral blood cells expanded as shown in
Table 1, and
described below.
A) Collection and maintenance of cells
Human peripheral blood (75 ml; about 0.75 x 106 cells/ml) was collected from
15
human donors by syringe as above; blood collected from 10 donors was suspended
in 75ml
Iscove's modified Dulbecco's medium (IMDM) (GIBCO, Grand Island, NY)
supplemented
with 20% of 5% human albumin (HA), 100 ng/ml recombinant human G-CSF (Amgen
Inc.,
Thousand Oaks, CA), and 100 ng/ml recombinant human stem cell factor (SCF)
(Amgen) to
prepare a peripheral blood mixture. Part of each peripheral blood sample was
set aside as a
38
CA 02597963 2007-08-20
"controP' sample. The peripheral blood mixture was placed in a TVEMF-
bioreactor as shown
in Figures 2 and 3 herein. TVEMF-expansion occurred at 37 C, 6% C02, with a
normal air
Oz/N ratio. The TVEMF-bioreactor was rotated at a speed of 10 rotations per
minute (rpm)
initially, and adjusted as needed, as described throughout this application,
to keep the
peripheral blood cells suspended in the bioreactor. A time varying current of
6mA was
applied to the bioreactor. The square wave TVEMF signal applied to the
peripheral blood
mixture was about 0.5 Gauss. (frequency: about 10 cycles/sec). Culture media
in the
peripheral blood mixture in the TVEMF-bioreactor was changed/freshened every
one to two
days. At day 10, the cells were removed from the TVEMF-bioreactor and washed
with PBS
and analyzed. The results are as set forth in Table 1. Control data refers to
a sample of human
peripheral blood that has not been expanded; Expanded Sample refers to the
respective
control sample after TVEMF-expansion.
39
CA 02597963 2007-08-20
Table 1
Control I Cell Count 300,000 Viability 98%
Control 2 Cell Count 325,000 Viability 100%
Control 3 Cell Count 350,000 Viability 98%
Control 4 Cell Count 300,000 Viability 98%
Control 5 Cell Count 315,000 Viability 99%
Control 6 Cell Count 320,000 Viability 98%
Control 7 Cell Count 310,000 Viability 98%
Control 8 Cell Count 340,000 Viability 100%
Control 9 Cell Count 300,000 Viability 98%
Control 10 Cell Count 320,000 Viability 98%
Expanded Sample 1 Cell Count 3,000,000 Viability 99%
Corresponding CD34+
increase: yes
Expanded Sample 2 Cell Count 3,500,000 Viability 100%
Corresponding CD34+
increase: yes
Expanded Sample 3 Cell Count 3,750,000 Viability 98%
Corresponding CD34+
increase: yes
Expanded Sample 4 Cell Count 3,250,000 Viability 98%
Corresponding CD34+
increase: yes
Expanded Sample 5 Cell Count 3,450,000 Viability 100%
Corresponding CD34+
increase: yes
Expanded Sample 6 Cell Count 3,400,000 Viability 98%
Corresponding CD34+
increase: yes
Expanded Sample 7 Cell Count 3,200,000 Viability 98%
Corresponding CD34+
increase: yes
Expanded Sample 8 Cell Count 3,500,000 Viability 100%
Corresponding CD34+
increase: yes
Expanded Sample 9 Cell Count 3,150,000 Viability 98%
Corresponding CD34+
increase: yes
Expanded Sample 10 Cell Count 3,500,000 Viability 99%
Corresponding CD34+
increase: yes
As may be seen from Table 1, TVEMF-expansion of peripheral blood cells
resulted in
roughly a 10-fold increase in the number of cells over 10 days, as compared to
non-expanded
CA 02597963 2007-08-20
control, with a corresponding increase in CD34+ cells. The culture media where
the cells
were growing was changed/freshened once every 1-2 days.
B) Analysis of TVEMF-expanded cells
Total cell counts of Control and Expanded Samples were obtained with a
counting
chamber (a device such as a hemocytometer used by placing a volume of either
the control
cell suspension or expanded sample on a specially-made microscope slide with a
microgrid
and counting the number of cells in the sample). The results of the total cell
counts in Control
samples and in Expanded Samples after 10 days of TVEMF-expansion are shown in
Table 1.
The indication of corresponding CD34+ increase in Table 1 was determined as
follows: CD34+ cells of the Expanded Samples were separated from other cells
therein with
a Human CD34 Selection Kit (EasySep positive selection, StemCell
Technologies), and
counted with a counting chamber as indicated above and confirmed with FACScan
flow
cytometer (Becton-Dickinson). CFU-GEMM and CFU-GM were counted by clonogenic
assay., Cell viability (where a viable cell is alive and a non-viable cell is
dead) was
determined by trypan blue exclusion test. The answer of "yes" in all Expanded
Samples
indicates that the number of CD34+ cells increased in amounts corresponding to
the total cell
count.
C) Increase in amount of hematopoletic colony-forming cells
Incubation of the donors' peripheral blood ceIls in this TVEMF-expansion
tissue
culture system significantly increases the numbers of hematopoietic colony-
forming cells. As
determined in a separate assay, a constant increase in the numbers of CFU-GM
(up to 7-fold)
and CFU-GEMM (up to 9-fold) colony-forming cells is observed up to day 7 with
no clear
plateau.
D) Increase in CD34+ cells
Incubation of MNCs from normal donors in this TVEMF-expansion tissue culture
system significantly increases the numbers of CD34+ cells. As determined in a
separate
assay, the average number of CD34+ cells increased 10-fold by day 6 of culture
and plateaus
on that same day.
Operative Method - Cryopreservation
As mentioned above, peripheral blood is collected from a mammal, preferably a
human. Red blood cells, at least, are preferably removed from the peripheral
blood. The
41
CA 02597963 2007-08-20
peripheral blood stem cells (with other cells and media as desired) are placed
in a bioreactor,
preferably a TVEMF-bioreactor and subjected to a time varying electromagnetic
force, and
expanded. If RBCs were not removed prior to expansion, preferably they are
removed after
expansion. The expanded cells may be cryogenically preserved. Further details
relating to a
method for the cryopreservation of expanded peripheral blood stem cells, and
compositions
comprising such cells are provided herein and in particular below.
After, for instance, TVEMF-expansion, the TVEMF-expanded cells, including
TVEIVIF-expanded peripheral blood stem cells, are preferably transferred into
at least one
cryopreservation container containing at least one cryoprotective agent. The
TVEMF-
expanded peripheral blood stem cells are preferably first washed with a
solution (for instance,
a buffer solution or the desired cryopreservative solution) to remove media
and other
components present during TVEMF-expansion, and then preferably mixed in a
solution that
allows for cryopreservation of the cells. Such solution is conunonly referred
to as a
cryopreservative, cryopreservation solution or cryoprotectant. The cells are
transferred to an
appropriate cryogenic container and the container decreased in temperature to
generally from
-120 C to -196 C, preferably about -130*C to about -150 C, and maintained at
that
temperature. Preferably, this decrease in temperature is done slowly and
carefully, so as to not
damage, or at least to minimize damage, to the stem cells during the freezing
process. When
needed, the temperature of the cells (about the temperature of the cryogenic
container) is
raised to a temperature compatible with introduction of the cells into the
human body
(generally from around room temperature to around body temperature), and the
TVEMF-
expanded cells may be introduced into a mammalian body, preferably human, for
instance as
discussed throughout this application.
42
CA 02597963 2007-08-20
Freezing cells is ordinarily destructive. Not to be bound by theory, on
cooling, water
within the cell freezes. Injury then may occur by osmotic effects on the cell
membrane, cell
dehydration, solute concentration, and ice crystal formation. As ice forms
outside the cell,
available water is removed from solution and withdrawn from the cell, causing
osmotic
dehydration and raised solute concentration that may eventually destroy the
cell. (For a
discussion, see Mazur, P., 1977, Cryobiology 14:251-272.)
Different materials have different freezing points. Preferably, a peripheral
blood stem
cell composition ready for cryopreservation contains as few contaminating
substances as
possible, to minimize cell wall damage from the crystallizaton and freezing
process.
These injurious effects can be reduced or even circumvented by (a) use of a
cryoprotective agent, (b) control of the freezing rate, and (c) storage at a
temperature
sufficiently low to minimize degradative reactions.
The inclusion of cryopreservation agents is preferred in the present
invention.
Cryoprotective agents which can be used include but are not limited to a
sufficient amount of
dimethyl sulfoxide (DMSO) (Lovelock, J. E. and Bishop, M. W. H., 1959, Nature
183:1394-
1395; Ashwood-Smith, M. J., 1961, Nature 190:1204-1205), glycerol,
polyvinylpyrrolidine
(Rinfret, A. P., 1960, Ann. N.Y. Acad. Sci. 85:576), polyethylene glycol
(Sloviter, H. A. and
Ravdin, R. G., 1962, Nature 196:548), albumin, dextran, sucrose, ethylene
glycol, i-erythritol, D-
ribitol, D-mannitol (Rowe, A. W., et al., 1962, Fed. Proc. 21:157), D-
sorbitol, i-inositol, D-
lactose, choline chloride (Bender, M. A., et al., 1960, J. Appl. Physiol.
15:520), amino acid-
glucose solutions or amino acids (Phan The Tran and Bender, M. A., 1960, Exp.
Cell Res.
20:651), methanol, acetamide, glycerol monoacetate (Lovelock, J. E., 1954,
Biochem. J. 56:265),
and inorganic salts (Phan The Tran and Bender, M. A., 1960, Proc. Soc. Exp.
Biol. Med.
104:388; Phan The Tran and Bender, M. A., 1961, in Radiobiology, Proceedings
of the Third
Australian Conference on Radiobiology, Ilbery, P. L. T., ed., Butterworth,
London, p. 59). In a
preferred embodiment, DMSO is used. DMSO, a liquid, is nontoxic to cells in
low
concentration. Being a small molecule, DMSO freely permeates the cell and
protects intracellular
organelles by combining with water to modify its freezability and prevent
damage from ice
formation. Adding plasma (for instance, to a concentration of 20-25%) can
augment the
protective effect of DMSO. After addition of DMSO, cells should be kept at 0 C
or below, since
DMSO concentrations of about 1% may be toxic at temperatures above 4 C. My
selected
43
CA 02597963 2007-08-20
preferred cryoprotective agents are, in combination with TVEMF-expanded
peripheral blood
stem cells for the total composition: 20 to 40% dimethyl sulfoxide solution in
60 to 80% amino
acid-glucose solution, or 15 to 25% hydroxyethyl starch solution, or 4 to 6%
glycerol, 3 to 5%
glucose, 6 to 10% dextran T10, or 15 to 25% polyethylene glycol or 75 to 85%
amino acid-
glucose solution. The amount of cryopreservative indicated above is preferably
the total amount
of cryopreservative in the entire composition (not just the amount of
substance added to a
composition).
While other substances, other than peripheral blood cells and a cryoprotective
agent, may
be present in a composition of the present invention to be cryopreserved,
preferably
cryopreservation of a TVEMF-expanded peripheral blood stem cell composition of
the present
invention occurs with as few other substances as possible, for instance for
reasons such as those
discussed regarding the mechanism of freezing, above.
Preferably, expanded peripheral blood stem cell composition of the present
invention
is cooled to a temperature in the range of about -120 C to about -196 C,
preferably about -
130 C to about -196 C, and even more preferably about -130 C to about -150 C.
A controlled slow cooling rate is critical. Different cryoprotective agents
(Rapatz, G.,
et al., 1968, Cryobiology 5(l):18-25) and different cell types have different
optimal cooling
rates (see e.g. Rowe, A. W. and Rinfret, A. P., 1962, Blood 20:636; Rowe, A.
W., 1966,
Cryobiology 3(1):12-18; Lewis, J. P., et al., 1967, Transfusion 7(1):17-32;
and Mazur, P.,
1970, Science 168:939-949 for effects of cooling velocity on survival of
peripheral cells (and
on their transplantation potential)). The heat of fusion phase where water
turns to ice should
be minimal. The cooling procedure can be carried out by use of, e.g., a
programmable
freezing device or a methanol bath procedure.
Progranunable freezing apparatuses allow determination of optimal cooling
rates and
facilitate standard reproducible cooling. Programmable controlled-rate
freezers such as
Cryomed or Planar permit tuning of the freezing regimen to the desired cooling
rate curve.
Other acceptable freezers may be, for example, Sanyo Modl MDF- 1 155ATN- 152C
and
Model MDF-2136ATN -135C, Princeton CryoTech TEC 2000. For example, for
peripheral
blood cells or CD34+ cells in 10% DMSO and 20% plasma, the optimal rate is 1
to 3 C
/minute from 0 C to -200 C.
44
CA 02597963 2007-08-20
In a preferred embodiment, this cooling rate can be used for the cells of the
invention.
The cryogenic container holding the cells must be stable at cryogenic
temperatures and allow
for rapid heat transfer for effective control of both freezing and thawing.
Sealed plastic vials
(e.g., Nunc, Wheaton cryules) or glass ampules can be used for multiple small
amounts (1-2
ml), while larger volumes (100-200 ml) can be frozen in polyolefm bags (e.g.,
Delmed) held
between metal plates for better heat transfer during cooling. (Bags of bone
marrow cells have
been successfully frozen by placing them in -80 C freezers that, fortuitously,
gives a cooling
rate of approximately 3 C /minute).
In an alternative embodiment, the methanol bath method of cooling can be used.
The
methanol bath method is well suited to routine cryopreservation of multiple
small items on a
large scale. The method does not require manual control of the freezing rate
nor a recorder to
monitor the rate. In a preferred aspect, DMSO-treated cells are precooled on
ice and
transferred to a tray containing chilled methanol that is placed, in turn, in
a mechanical
refrigerator (e.g., Harris or Revco) at -130 C. Thermocouple measurements of
the methanol
bath and the samples indicate the desired cooling rate of 1 to 3 C /minute.
After at least two
hours, the specimens will reach a temperature of -80 C and may be placed
directly into liquid
nitrogen (-196 C) for permanent storage.
After thorough freezing, expanded stem cells can be rapidly transferred to a
long-term
cryogenic storage vessel (such as a freezer). In a preferred embodiment, the
cells can be
cryogenically stored in liquid nitrogen (-196 C) or its vapor (-165 C). The
storage
temperature should be below -120 C, preferably below -130 C. Such storage is
greatly
facilitated by the availability of highly efficient liquid nitrogen
refrigerators, which resemble
large Thermos containers with an extremely low vacuum and intemal super
insulation, such
that heat leakage and nitrogen losses are kept to an absolute minimum.
The preferred apparatus and procedure for the cryopreservation of the cells is
that
manufactured by Thermogenesis Corp., Rancho Cordovo, CA, utilizing their
procedure for
lowering the cell temperature to below -130 C. The cells are held in a
Thermogenesis plasma
bag during freezing and storage.
Other freezers are commercially available. For instance, the "BioArchive"
freezer not
only freezes but also inventories a cryogenic sample such as peripheral blood
or cells of the
present invention, for instance managing up to 3,626 bags of frozen peripheral
blood at a
CA 02597963 2007-08-20
time. This freezer has a robotic arm that will retrieve a specific sample when
instructed,
ensuring that no other examples are disturbed or exposed to warmer
temperatures. Other
freezers commercially available include, but are not limited to, Sanyo Model
MDF-1 155
ATN-152C and Model MDF-2136 ATN-135C, and Princeton CryoTech TEC 2000.
After the temperature of the expanded peripheral blood stem cell composition
is
reduced to below -120 C, preferably below -130 C, they may be held in an
apparatus such as
a Thermogenesis freezer. Their temperature is maintained at a temperature of
about -120 C
to -196 C, preferably -130 C to -150 C. The temperature of a cryopreserved
expanded
peripheral blood stem cell composition of the present invention should not be
above
-120 C for a prolonged period of time.
Cryopreserved expanded peripheral blood stem ceLls, preferably TVEMF-expanded
peripheral blood stem cells, or a composition thereof, according to the
present invention may
be frozen for an indefinite period of time, to be thawed when needed. For
instance, a
composition may be frozen for up to 18 years. Even longer time periods may
work, perhaps
even as long as the lifetime of the peripheral blood donor.
When needed, bags with the cells therein may be placed in a thawing system
such as a
Thermogenesis Plasma Thawer or other thawing apparatus such as in the
Thermoline Thawer
series. The temperature of the cryopreserved composition is raised to room
temperature. In
another preferred method of thawing cells mixed with a cryoprotective agent,
bags having a
cryopreserved TVEMF-expanded peripheral blood stem cell composition of the
present
invention, stored in liquid nitrogen, may be placed in the gas phase of liquid
nitrogen for 15
minutes, exposed to ambient air room temperature for 5 minutes, and finally
thawed in a 37 C
water bath as rapidly as possible. The contents of the thawed bags may be
immediately
diluted with an equal volume of a solution containing 2.5% (weightlvolume)
human serum
albunun and 5% (weight/volume) Dextran 40 (Solplex 40; Sifra, Verona, Italy)
in isotonic salt
solution and subsequently centrifuged at 400 g for ten minutes. The
supernatant would be
removed and the sedimented cells resuspended in fresh albumin/Dextran
solution. See
Rubinstein, P. et al., Processing and cryopreservation of placental/umbilical
cord blood for
unrelated bone marrow reconstitution. Proc. Natl. Acad Sci. 92:10119-1012
(1995) for
Removal of Hypertonic Cryoprotectant; a variation on this preferred method of
thawing cells
can be found in Lazzari, L. et al., Evaluation of the effect of
cryopreservation on ex vivo
46
CA 02597963 2007-08-20
expansion of hematopoietic progenitors from cord blood. Bone Marrow Trans.
28:693-698
(2001).
After the cells are raised in temperature to room temperature, they are
available for
research or regeneration therapy. The thawed expanded peripheral blood stem
cell
composition may be introduced directly into a mammal, preferably human, or
used in its
thawed form for instance for desired research. The solution in which the
thawed cells are
present may be completely washed away, and exchanged with another, or added to
or
otherwise manipulated as desired. Various additives may be added to the thawed
compositions (or to a non-cryopreserved TVEMF-expanded peripheral blood stem
cell
composition) prior to introduction into a mammalian body, preferably soon to
immediately
prior to such introduction. Such additives include but are not limited to a
growth factor, a
copper chelating agent, a cytokine, a hormone, a suitable buffer or diluent.
Preferably, G-CSF
is added. Even more preferably, for humans, G-CSF is added in an amount of
about 20 to
about 40 micrograms/kg body weight, and even more preferably in an amount of
about 30
micrograms/kg body weight. Also, prior to introduction, the TVEMF-expanded
peripheral
blood stem cell composition may be mixed with the mammal's own, or a suitable
donor's,
plasma, peripheral blood or albumin, or other materials that for instance may
accompany
peripheral blood transfusions. The thawed peripheral blood stem cells can be
used for
instance to test to see if there is an adverse reaction to a pharmaceutical
that is desired to be
used for treatment or they can be used for treatment.
While the FDA has not approved use of expanded peripheral blood stem cells for
regeneration of tissue in the United States, such approval appears to be
imminent. Direct
injection of a sufficient amount of expanded peripheral blood stem cells
should be able to be
used to repair and regenerate tissue.
During the entire process of expansion, preservation, and thawing, blood stem
cells of the
present invention maintain the phenotypic characteristics maintained,
fostered, and developed as
a result of the expansion process and also TVEMF-expansion process.
An expanded, preferably TVEMF-expanded, blood stem cell composition of the
present invention should be introduced into a mammal, preferably a human, in a
"therapeutically effective" amount, sufficient to achieve tissue repair or
regeneration, or to
treat a desired disease or condition. Preferably, at least 20 ml of a TVEMF-
expanded blood
47
CA 02597963 2007-08-20
stem cell composition having 1W to 109 stem cells per ml is used for any
treatment, preferably
all at once, in particular where a traumatic injury has occurred and
innnediate tissue repair
needed. This amount is particularly preferred in a 75-80 kg human. The amount
of expanded
blood stem cells in a composition being introduced into a mammal depends in
part on the
number of cells present in the source blood material (in particular if only a
fairly limited
amount is available). A preferred range of TVEMF-expanded blood stem cells
introduced
into a patient may be, for instance, about 10 ml to about 50 ml of a TVEMF-
expanded blood
stem cell composition having 10' to 109 stem cells per ml, or potentially even
more. While it
is understood that a high concentration of any substance, administered to a
mammal, may be
toxic or even lethal, it is unlikely that introducing all of the expanded
blood stem cells, for
instance after expansion at least 7 times, will cause an overdose in expanded
blood stem cells.
Where blood from several donors or multiple collections from the same donor is
used, the
number of blood stem cells introduced into a mammal may be higher. Also, the
dosage of
cells that may be introduced to the patient is not limited by the amount of
blood provided
from collection from one individual; multiple administrations, for instance
once a day or
twice a day, or once a week, or other administration time frames, may more
easily be used.
Also, where a tissue is to be treated, the type of tissue may warrant the use
of as many
expanded blood stem cells as are available, or the use of a smaller dose.
It is to be understood that, while the embodiment described above generally
relates to
cryopreserving expanded blood stem cells, expansion may occur after thawing of
already
cryopreserved, non-expanded, or non-TVEMF-expanded, blood stem cells. Also, if
cryopreservation is desired, expansion may occur both before and after
freezing the cells.
Blood banks, for instance, have cryopreserved compositions comprising blood
stem cells in
frozen storage, in case such is needed at some point in time. Such
compositions may be
thawed according to conventional methods and then expanded as described
herein, including
variations in the process as described herein. Thereafter, such expanded
peripheral blood
stem cells are considered to be compositions of the present invention, as
described above.
Expansion prior to cryopreserving is preferred, for instance as if a traumatic
injury occurs, a
patient's peripheral blood stem cells have already been expanded and do not
require precious
extra days to prepare.
48
CA 02597963 2007-08-20
Also, while not preferred, it should be noted that expanded peripheral blood
stem cells
of the present invention may be cryopreserved, and then thawed, and then if
not used,
cryopreserved again. Prior to the cells being frozen, the cells are preferably
TVEMF-
expanded (that is, increased in number, not size). The cells may also be
expanded after being
frozen and then thawed, even if already expanded before freezing.
Expansion of peripheral blood stem cells may take several days. In a situation
where
it is important to have an immediate supply of peripheral blood stem cells,
such as a life-or-
death situation or in the case of a traumatic injury, especially if research
needs to be
accomplished prior to reintroduction of the cells, several days may not be
available to await
the expansion of the peripheral blood stem cells. It is particularly
desirable, therefore, to have
such expanded peripheral blood stem cells available from birth forward in
anticipation of an
emergency where every minute in delaying treatment can mean the difference in
life or death.
Also, it is to be understood that the expanded peripheral blood stem cells of
the
present application may be introduced into a mammal, preferably the source
manunal
(mammal that is the source of the peripheral blood), after expansion, with or
without
cryopreservation. However, such introduction need not be limited to only the
source mammal
(autologous); the expanded cells may also be transferred to a different mammal
(allogenic).
Also, it is to be understood that, while peripheral blood is the preferred
source of adult
stem cells for the present invention, adult stem cells from bone marrow may
also be
expanded, preferably TVEMF-expanded, and used in a manner similar to
peripheral blood
stem cells in the present invention. Bone marrow is not a readily available
source of stem
cells, but must be collected via apheresis or some other expensive and painful
method.
The present invention also includes a method of researching tissue, for
instance in
relation to a disease or condition. The method may include, for instance,
introducing a
peripheral blood stem cell composition into a test system for the disease
state. Such as system
may include, but is not limited to, for instance a mammal having the disease,
an appropriate
animal model for studying the disease or an in vitro test system for studying
the disease.
Expanded and TVEMF-expanded peripheral blood stem cells may be used for
research for
possible cures for the following diseases:
I. Diseases resulting from a failure or dysfunction of normal blood cell
production and
maturation, hyperproliferative stem cell disorders, aplastic anemia,
pancytopenia,
49
CA 02597963 2007-08-20
thrombocytopenia, red cell aplasia, Blackfan-Diamond syndrome due to drugs,
radiation, or
infection, idiopathic;
II. Hematopoietic malignancies, acute lymphoblastic (lymphocytic) leukemia,
chronic
lymphocytic leukemia, acute myelogenous leukemia, chronic myelogenous
leukemia, acute
malignant myelosclerosis, multiple myeloma, polycythemia vera, agnogenic
myelometaplasia,
Waldenstrom's macroglobulinemia, Hodgkin's lymphoma, non-Hodgkins's lymphoma;
III. Immunosuppression in patients with malignant, solid tumors, malignant
melanoma,
carcinoma of the stomach, ovarian carcinoma, breast carcinoma, small cell
lung, carcinoma,
retinoblastoma, testicular carcinoma, glioblastoma, rhabdomyosarcoma,
neuroblastoma, Ewing's
sarcoma, lymphoma;
IV. Autoimmune diseases, rheumatoid arthritis, diabetes type I, chronic
hepatitis, multiple
sclerosis, and systemic lupus erythematosus;
V. Genetic (congenital) disorders, anemias, familial aplastic, Fanconi's
syndrome,
Bloom's syndrome, pure red cell aplasia (PRCA), dyskeratosis congenital,
Blackfan-
Diamond syndrome, congenital dyserythropoietic syndromes I-N, Chwachmann-
Diamond
syndrome, dihydrofolate reductase deficiencies, formamino transferase
deficiency,
Lesch-Nyhan syndrome, congenital spherocytosis, congenital elliptocytosis,
congenital
stomatocytosis, congenital Rh null disease, paroxysmal nocturnal
hemoglobinuria, G6PD
(glucose-6-phosphate dehydrogenase), variants 1,2,3, pyruvate kinase
deficiency,
congenital erythropoietin sensitivity, deficiency, sickle cell disease and
trait, thalassemia
alpha, beta, gamma met-hemoglobinemia, congenital disorders of immunity,
severe combined
immunodeficiency disease, (SCID), bare lymphocyte syndrome, ionophore-
responsive
combined, immunodeficiency, combined immunodeficiency with a capping
abnormality,
riucleoside phosphorylase deficiency, granulocyte actin deficiency, infantile
agranulocytosis,
Gaucher's disease, adenosine deaminase deficiency, Kostmann's syndrome,
reticular dysgenesis,
congenital leukocyte dysfunction syndromes; and
VI. Others including osteopetrosis, myelosclerosis, acquired hemolytic
anemias, acquired
immunodeficiencies, infectious disorders causing primary or secondary
immunodeficiencies,
bacterial infections (e.g., Brucellosis, Listerosis, tuberculosis, leprosy),
parasitic infections (e.g.,
malaria, I,eishmaniasis), fungal infections, disorders involving
disproportions in lymphoid cell
sets and impaired immune functions due to aging phagocyte disorders,
Kostmann's
CA 02597963 2007-08-20
agranulocytosis, chronic granulomatous disease, Chediak-Higachi syndrome,
neutrophil actin
deficiency, neutrophil membrane GP-180 deficiency, metabolic storage diseases,
mucopolysaccharidoses, mucolipidoses, miscellaneous disorders involving immune
mechanisms,
Wiskott-Aldrich Syndrome, alpha 1-antitrypsin deficiency.
During the entire process of expansion, preservation, and thawing, peripheral
blood stem
cells of the present invention maintain their unique phenotypic expression
acquired, fostered, and
maintained as a result of the TVEMF-expansion process.
While preferred embodiments have been herein described, those skilled in the
art will
understand the present invention to include various changes and modifications.
The scope of the
invention is not intended to be limited to the above-described embodiments.
51