Note: Descriptions are shown in the official language in which they were submitted.
CA 02598208 2007-08-16
Tetrahydronaphthalene derivatives,
processes for their preparation
and their use as antiinflammatory agents
Introduction
The invention relates to tetrahydronaphthalene
derivatives, processes for their preparation and their
use as antiinflammatory agents.
Open-chain non-steroidal antiinflammatory agents are
known in the art (DE 100 38 639 and WO 02/10143). These
compounds show experimentally dissociations between
antiinflammatory and unwanted metabolic effects and are
superior to non-steroidal glucocorticoids described to
date or exhibit at least as good an effect.
The present invention provides further non-steroidal
antiinflammatory agents.
Brief description of the invention
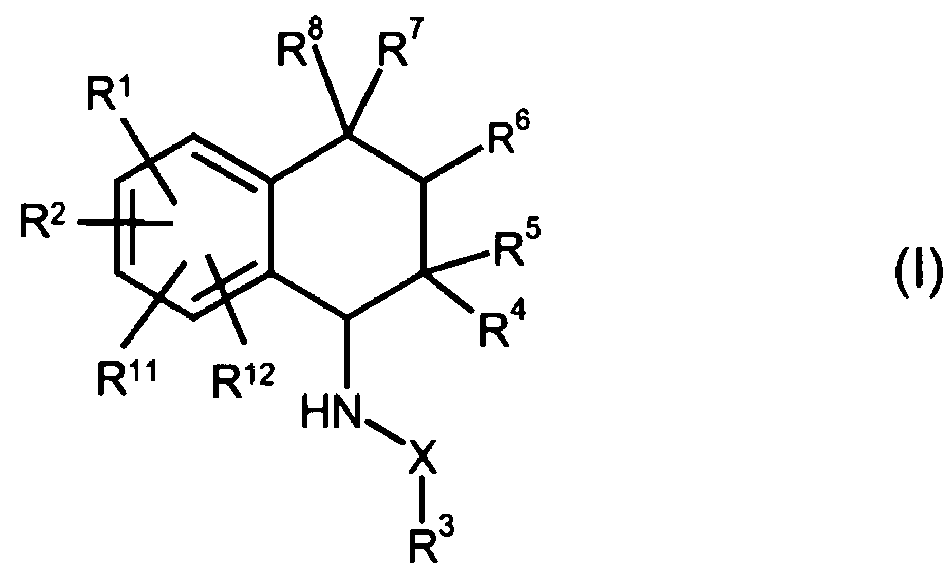
The present invention relates to compounds of the
general formula (I)
RB R'
R'
R 8
~
RZ Rs
R4
R" R'2
HN~
X
13
R
in which
R 1 and R2 are independently of one another a hydrogen
atom, a hydroxy group, a halogen atom, an
CA 02598208 2007-08-16
- 2 -
optionally substituted (C1-C10)-alkyl group, a
(C1-Clo) -alkoxy group, a (C1-Clo) -alkylthio group, a
(C1-CS)-perfluoroalkyl group, a cyano group, a
nitro group, or an -NR9R9a group,
or R' and R 2 together form a group selected from the
groups -0- (CH2) n-0-, -0- (CH2) n-CH2-, -O-CH=CH-,
- (CH2) n+2-r -NH- (CH2) n+i-, -N (C1-C3-alkyl) - (CH2) n+i-
and -NH-N=CH-,
where n is 1 or 2, and the terminal oxygen atoms
and/or carbon atoms and/or nitrogen atoms are
linked to directly adjacent ring carbon atoms,
R11 is a hydrogen atom, a hydroxy group, a halogen
atom, a cyano group, an optionally substituted
(C1-Clo) -alkyl group, a (C1-Clo) -alkoxy group, a
(C1-Clo) -alkylthio group, or a (C1-C5) -
perfluoroalkyl group,
R12 is a hydrogen atom, a hydroxy group, a halogen
atom, a cyano group, an optionally substituted
(CI-Clo) -alkyl group, or a (C1-Clo) -alkoxy group,
R3 is a C1-Clo-alkyl group which is optionally
substituted by 1 to 3 hydroxy groups, 1 to 3
halogen atoms, and/or 1 to 3(C1-CS)-alkoxy groups,
an optionally substituted (C3-C7) -cycloalkyl group,
an optionally substituted heterocyclyl group,
an optionally substituted aryl group, or
a mono- or bicyclic heteroaryl group which is
optionally substituted by one or more groups which
are selected independently of one another from
(C1-C5)-alkyl groups which themselves may
optionally be substituted by 1 to 3
hydroxy or 1 to 3-C00R13 groups,
(C1-C5) -alkoxy groups,
halogen atoms, hydroxy groups, -NRgR9a groups,
and
exo methylene groups
and optionally comprises 1 to 4 nitrogen atoms
and/or 1 to 2 oxygen atoms and/or 1 to 2 sulfur
atoms and/or 1 to 2 keto groups, this group being
linked by any position to the group X and possibly
CA 02598208 2007-08-16
- 3 -
being optionally hydrogenated at one or more
positions,
R4 is a hydroxy group, an -OR10 group or an -0 (CO) Rl0
group,
R5 is a (Cl-C1 ) -alkyl group which is optionally
partly or completely fluorinated, a
(C3-C7) cycloalkyl group, a (C1-C8) alkyl-
(C3-C7) cycloalkyl group, a (C2-C$) alkenyl-
(C3-C7) cycloalkyl group, a heterocyclyl group, a
(C1-C$) alkylheterocyclyl group, a (C2-CB) -
alkenylheterocyclyl group, an aryl group, a
(C1-C$) alkylaryl group, a(C2-C8) alkenylaryl group,
a (CZ-C8) -alkynylaryl group,
a mono- or bicyclic heteroaryl group which is
optionally substituted by 1 to 2 keto groups, 1 to
2 (C1-C5) -alkyl groups, 1 to 2 (C1-C5) -alkoxy
groups, 1 to 3 halogen atoms, and/or 1 to 2 exo
methylene groups and comprises 1 to 3 nitrogen
atoms and/or 1 to 2 oxygen atoms and/or 1 to 2
sulfur atoms,
a (C1-C8) alkylheteroaryl group, a
(CZ-C8) alkenylheteroaryl group, or a
(Cz-C8)alkynylheteroaryl group,
this group being linked by any position to the
tetrahydronaphthalene system and possibly being
optionally hydrogenated at one or more positions,
R6 is a hydrogen atom, a halogen atom, or an
optionally substituted (C1-C10)alkyl group,
R7 and R8 are independently of one another a hydrogen
atom, a halogen atom, an optionally substituted
(C1-C10) -alkyl group, a cyano group, together a
(Ci-Cio) -alkylidene group or together with the
carbon atom of the tetrahydronaphthalene system an
optionally substituted (C3-C6) -cyclalkyl ring;
or
R6 and R7 together form a fused five- to eight-membered
saturated or unsaturated carbocycle or heterocycle
which is optionally substituted by 1 to 2 keto
groups, 1 to 2 (C1-C5) -alkyl groups, 1 to 2
CA 02598208 2007-08-16
- 4 -
(C1-C5)-alkoxy groups, and/or 1 to 4 halogen atoms;
or
R1 and R8 together form a fused five- to eight-membered
saturated or unsaturated carbocycle or heterocycle
which is optionally substituted by 1 to 2 keto
groups, 1 to 2 (C1-C5) -alkyl groups, 1 to 2
(C1-C5)-alkoxy groups, and/or 1 to 4 halogen atoms;
R9 and R9a are independently of one another a hydrogen
atom, (C1-C5) -alkyl or - (CO) - (C1-C5) -alkyl,
R10 is a(C1-C10) -alkyl group or any hydroxy protective
group,
R13 is a hydrogen atom or a(C1-C5) -alkyl group, and
X is a group -C(=O)-, -C(=S)-, -C(=O)-NH-,
-C (=S) -NH-, -S ( O ) m (where m = 1 or 2 ) , -C (=0) -0-,
-C (=S) -0- or a group -(CHZ) P- (where p = 1, 2 or
3), where if X comprises a carbonyl or
thiocarbonyl function, this function is linked to
the group -NH- in the general formula (I),
with the proviso that if R3 is an optionally
substituted (C1-C10) -alkyl group, X cannot be a
- ( CH2 ) p- group;
in the form of any stereoisomer or of a mixture of
stereoisomers; or as pharmacologically acceptable salt
or derivative.
The present invention further relates to processes for
preparing compounds of the general formula (I) as
described herein.
The present invention further relates to pharmaceutical
compositions which include one or more compounds of the
general formula (I) in combination with one or more
pharmaceutical carriers or excipients.
The present invention additionally relates to the use
of the compounds of the general formula (I) for
manufacturing pharmaceutical compositions having an
antiinflammatory effect.
CA 02598208 2007-08-16
-
The present invention further relates to compounds of
the general formula (IV)
Ra R'
R'
R6
~
R2 Re
R4
R" R72
NHZ (IV)
5
in which the substituents R1 to RlZ have the
abovementioned meanings, and to the use of these
compounds for preparing compounds of the general
formula (I) as described above.
Detailed description of the invention
Definitions
The term halogen atom or halogen means a fluorine,
chlorine, bromine or iodine atom. A fluorine, chlorine
or bromine atom is preferred.
The alkyl groups mentioned in the definitions of the
general formula (I) may be straight-chain or branched
and are for example a methyl, ethyl, n-propyl,
isopropyl, n-butyl, isobutyl, tert-butyl or n-pentyl,
2,2-dimethylpropyl, 2-methylbutyl or 3-methylbutyl
group, and the hexyl, heptyl, nonyl, decyl group and
derivatives thereof branched in any way. Alkyl groups
which comprise 1 to 10, 1 to 8 or 1 to 5 carbon atoms
are preferred. A methyl or ethyl group is particularly
preferred.
The abovementioned alkyl groups may optionally be
substituted by 1 to 5, preferably 1 to 3, groups which
are selected independently of one another from hydroxy,
cyano, nitro, -COOR13, (C1-C5) -alkoxy groups, halogen
CA 02598208 2007-08-16
- 6 -
atoms, -NR9R9a, a partly or completely fluorinated
(C1-C3) -alkyl group. The alkyl groups may preferably be
substituted by 1 to 3 halogen atoms and/or 1 to 3
hydroxy and/or 1 to 3 cyano and/or 1 to 3-COOR13
groups. Fluorine atom, hydroxy, methoxy and/or cyano
groups represent a particularly preferred subgroup of
substituents.
1 to 3 hydroxy and/or 1 to 3-COOR13 groups are a
further particularly preferred group of substituents
for the alkyl groups. Hydroxy groups are particularly
preferred in this connection.
Examples of a suitable partly or completely fluorinated
alkyl group are the following partly or completely
fluorinated following groups: fluoromethyl,
difluoromethyl, trifluoromethyl, fluoroethyl,
1,1-difluoroethyl, 1,2-difluoroethyl, 1,1,1-trifluoro-
ethyl, tetrafluoroethyl, pentafluoroethyl. Of these,
the trifluoromethyl or the pentafluoroethyl are
preferred. The completely fluorinated group is also
called perfluoroalkyl group. The reagents which are
optionally employed during the synthesis can be
purchased, or the published syntheses of the
corresponding reagents belong to the prior art, or
published syntheses can be applied analogously.
The alkenyl groups have at least one C=C double bond
and may be straight-chain or branched. Alkenyl groups
having 2 to 8 carbon atoms are preferred.
The alkynyl groups have at least one C=C triple bond
and may be straight-chain or branched. Alkynyl groups
having 2 to 8 carbon atoms are preferred.
The alkoxy groups mentioned in the definitions of the
general formula (I) may be straight-chain or branched
and be for example a methoxy, ethoxy, n-propoxy,
isopropoxy, n-butoxy, isobutoxy, tert-butoxy or
CA 02598208 2007-08-16
- 7 -
n-pentoxy, 2,2-dimethylpropoxy, 2-methylbutoxy or
3-methylbutoxy group. C1-C5- and C1-C3-, C1-C$-, and
Cl-Clo-alkoxy groups are preferred. A methoxy or ethoxy
group is particularly preferred.
The alkylthio groups mentioned in the definitions of
the general formula (I) may be straight-chain or
branched and are for example a methylthio, ethylthio,
n-propylthio, isopropylthio, n-butylthio, isobutylthio,
tert-butylthio or n-pentylthio, 2,2-dimethylpropylthio,
2-methylbutylthio or 3-methylbutylthio group.
C1-C5-Alkylthio groups are preferred. A methylthio or
ethylthio group is particularly preferred.
The alkoxy and alkylthio groups described above may
have on their alkyl groups the same substituents which
have been described hereinbefore for the alkyl groups
in general. Preferred substituents for alkoxy and
alkylthio groups are selected independently of one
another from halogen atoms (especially fluorine and/or
chlorine), hydroxy and cyano groups.
The substituent -NR9R9a means for example -NH2, -NH (CH3) ,
-N ( CH3 ) 2, -NH ( C2H5 ) , -N ( C2H5 ) 2, -NH (C3H-7) ~ -N (C3H7) 2,
-NH (C4H9) , -N (C4H9) 2, -NH (C5H11) , -N (C5H11) 2, -NH (CO) CH3r
NH ( CO ) C2H5, -NH ( CO ) C3H7, -NH ( CO ) C4H9r -NH ( CO ) C5H11.
The cycloalkyl group means a saturated cyclic group
which is optionally substituted by one or more groups
selected from hydroxy groups, halogen atoms, (C1-C5)-
alkyl groups, (C1-C5) -alkoxy groups, -NR9R9a groups,
-COOR13 groups, -CHO, cyano and has 3 to 7 ring carbon
atoms such as, for example, cyclopropyl, methyl-
cyclopropyl, cyclobutyl, methylcyclobutyl, cyclopentyl,
methylcyclopentyl, cyclohexyl, methylcyclohexyl,
cycloheptyl, methylcycloheptyl.
A (C1-C$) alkyl- (C3-C7) cycloalkyl group R5 means a
cycloalkyl group (as defined above) which is linked via
CA 02598208 2007-08-16
- 8 -
a straight-chain or branched (C1-C$)-alkyl unit (as
defined above) to the ring system. Examples of such
groups are - (CH2) -cycloalkyl, - (C2H4) -cycloalkyl,
- (C3H6) -cycloalkyl, - (C4H8) -cycloalkyl, - (C5H10-cyclo-
alkyl, where cycloalkyl is defined as described above.
A (C2-C8) alkenyl- (C3-C7) cycloalkyl group R5 means a
cycloalkyl group (as defined above) which is linked via
a straight-chain or branched (CZ-C8)-alkenyl unit to the
ring system. Examples of such groups are -(CH=CH)-
cycloalkyl, - [C (CH3) =CH] -cycloalkyl, -[CH=C(CH3)]-
cycloalkyl, -(CH=CH-CH2)-cycloalkyl, -(CH2-CH=CH)-
cycloalkyl, - (CH=CH-CH2-CH2) -cycloalkyl, - (CHZ-CH=CH-
CH2) -cycloalkyl, - (CH2-CH2-CH=CH) -cycloalkyl,
- (C (CH3) =CH-CHZ) -cycloalkyl, - (CH=C (CH3) -CH2) -cyclo-
alkyl.
An alkylidene or exo alkylidene group means a group
having 1 to 10 carbon atoms which is bonded via an exo
double bond to the system (ring or chain) . (C1-C5) - and
(C1-C3)-alkylidene is preferred, and exo methylene is
particularly preferred.
The heterocyclyl group is a cyclic, non-aromatic group
comprising one or more heteroatoms and may be for
example pyrrolidine, imidazolidine, pyrazolidine,
piperidine. Perhydroquinoline and perhydroisoquinoline
are also included in the heterocyclyl groups of the
invention.
Examples of suitable substituents for heterocyclyl and
heteroaryl groups are substituents from the following
group: optionally substituted C1-C5-alkyl groups,
hydroxy, (C1-C5) -alkoxy, -NR9R9a, halogen, cyano,
-C00R13, -CHO. The substituents may optionally also be
bonded to the nitrogen atom of the heterocyclyl or
heteroaryl group; N-oxides are also included in the
definition.
CA 02598208 2007-08-16
- 9 -
Aryl groups in the context of the invention are
aromatic or partly aromatic carbocyclic groups having 6
to 14 carbon atoms which have one ring, such as, for
example, phenyl or phenylene, or a plurality of fused
rings, such as, for example, naphthyl or anthranyl.
Examples which may be mentioned are phenyl, naphthyl,
tetralinyl, anthranyl, indanyl, and indenyl. The
optionally substituted phenyl group and the naphthyl
group are preferred.
The aryl groups may be substituted at any suitable
position leading to a stable compound by one or more
radicals from the group of hydroxy, halogen, C1-C5-alkyl
which is optionally substituted by 1 to 3 hydroxy
groups or -COOR13 groups, or C1-C5-alkoxy, cyano, -CF3
and nitro.
The aryl groups may be partly hydrogenated and then, in
addition or as alternative to the substituents detailed
above, also carry keto andlor exo alkylidene. Partly
hydrogenated phenyl means for example cyclohexadienyl,
cyclohexenyl, cyclohexyl. A partly hydrogenated
substituted naphthalene system is for example
1-tetralone or 2-tetralone.
A(C1-C8)alkylaryl group is an aryl group as already
described above which is linked via a straight-chain or
branched (C1-C8)-alkyl unit (as defined above) to the
ring system.
A(C2-C&)alkenylaryl group is an aryl group as already
described above which is linked via a straight-chain or
branched (C2-C8) -alkenyl unit (as defined above) to the
ring system.
A(CZ-C8)alkynylaryl group is an aryl group as already
described above which is linked via a straight-chain or
branched (C2-C8) -alkynyl unit (as defined above) to the
ring system.
CA 02598208 2007-08-16
- 10 -
The mono- or bicyclic heteroaryl group may optionally
comprise 1 to 9 groups selected from nitrogen atoms,
oxygen atoms, sulfur atoms or keto groups, of which a
maximum of 4 nitrogen atoms, a maximum of 2 oxygen
atoms, a maximum of 2 sulfur atoms and/or a maximum of
2 keto groups may be present. Every subcombination of
these groups is possible. The heteroaryl group may be
hydrogenated at one or more positions.
Monocyclic heteroaryl groups may be for example
pyridine, pyrazine, pyrimidine, pyridazine, triazine,
azaindolizine, 2H- and 4H-pyran, 2H- and 4H-thiopyran,
furan, thiophene, 1H- and 4H-pyrazole, 1H- and
2H-pyrrole, oxazole, thiazole, furazan, 1H- and
4H-imidazole, isoxazole, isothiazole, oxadiazole,
triazole, tetrazole, thiadiazole.
Bicyclic heteroaryl groups may be for example
phthalidyl, thiophthalidyl, indolyl, isoindolyl,
dihydroindolyl, dihydroisoindolyl, indazolyl,
benzothiazolyl, indolonyl, dihydroindolonyl,
isoindolonyl, dihydroisoindolonyl, benzofuranyl,
benzimidazolyl, benzo[b]thienyl, benzo[c]thienyl,
dihydroisoquinolinyl, dihydroquinolinyl,
benzoxazinonyl, phthalazinonyl, dihydrophthalazinonyl,
quinolinyl, isoquinolinyl, quinolonyl, isoquinolonyl,
quinazolinyl, quinoxalinyl, cinnolinyl, phthalazinyl,
dihydrophthalazinyl, 1,7- or 1,8-naphthyridinyl,
coumarinyl, isocoumarinyl, indolizinyl,
isobenzofuranyl, azaindolyl, azaisoindolyl,
pyrazolo[1,5-a]pyridinyl, furanopyridyl,
furanopyrimidinyl, furanopyrazinyl, furanopyridazinyl,
dihydrobenzofuranyl, dihydrofuranopyridyl,
dihydrofuranopyrimidinyl, dihydrofuranopyrazinyl,
dihydrofuranopyridazinyl, dihydrobenzofuranyl group.
If the heteroaryl groups are partly or completely
hydrogenated, the present invention includes compounds
CA 02598208 2007-08-16
- 11 -
of the general formula (I) in which R3 is for example
tetrahydropyranyl, 2H-pyranyl, 4H-pyranyl, piperidyl,
tetrahydropyridyl, dihydropyridyl, 1H-pyridin-2-onyl,
1H-pyridin-4-onyl, 4-aminopyridyl, 1H-pyridin-4-
ylideneaminyl, chromanyl, isochromanyl, thiochromanyl,
decahydroquinolinyl, tetrahydroquinolinyl, dihydro-
quinolinyl, 5,6,7,8-tetrahydro-lH-quinolin-4-onyl,
decahydroisoquinolinyl, tetrahydroisoquinolinyl,
dihydroisoquinolinyl, 3,4-dihydro-2H-benz[1,4]oxazinyl,
1,2-dihydro[1,3]benzoxazin-4-onyl, 3,4-dihydrobenz-
[1,4]oxazin-4-onyl, 3,4-dihydro-2H-benzo[1,4]thiazinyl,
4H-benzo[1,4]thiazinyl, 1,2,3,4-tetrahydroquinoxalinyl,
1H-cinnolin-4-onyl, 3H-quinozolin-4-onyl, 1H-
quinazolin-4-onyl, 3,4-dihydro-lH-quinoxalin-2-onyl,
2,3-1,2,3,4-tetrahydro[1,5]naphthyridinyl, dihydro-1H-
[1,5]naphthyridyl, 1H-[1,5]naphthyrid-4-onyl , 5,6,7,8-
tetrahydro-lH-naphthyridin-4-onyl, 1,2-
dihydropyrido[3,2-d][1,3]oxazin-4-onyl, octahydro-1H-
indolyl, 2,3-dihydro-lH-indolyl, octahydro-2H-
isoindolyl, 1,3-dihydro-2H-isoindolyl,
1,2-dihydroindazolyl, 1H-pyrrolo[2,3-b]pyridyl,
2,3-dihydro-lH-pyrrolo[2,3-b]pyridyl, 2,2-dihydro-lH-
pyrrolo[2,3-b]pyridin-3-onyl.
The mono- or bicyclic heteroaryl group may optionally
be substituted by one or more substituents selected
from C1-C5-alkyl groups which are optionally substituted
by 1 to 3 hydroxy groups or 1 to 3-COOR13 groups, or
Cl-C5-alkoxy groups, halogen atoms, and/or exo methylene
groups. The substituents may, if possible, optionally
also be bonded directly to the heteroatom (e.g. to the
nitrogen atom). The present invention also includes
N-oxides.
A(C1-C8) alkylheteroaryl group is a heteroaryl group as
already described above which is linked via a straight-
chain or branched (C1-C8) -alkyl unit (as defined above)
to the ring system.
CA 02598208 2007-08-16
- 12 -
A(CZ-C8)alkenylheteroaryl group is a heteroaryl group
as already described above which is linked via a
straight-chain or branched (CZ-C8)-alkenyl unit (as
defined above) to the ring system.
A(C2-C$)alkynylheteroaryl group is a heteroaryl group
as already described above which is linked via a
straight-chain or branched (C2-Ca)-alkynyl unit (as
defined above) to the ring system.
A(C1-Ca)alkylheterocyclyl group is a heterocyclyl group
as already described above which is linked via a
straight-chain or branched (C1-C$)-alkyl unit (as
defined above) to the ring system.
A(CZ-C8)alkenylheterocyclyl group is a heterocyclyl
group as already described above which is linked via a
straight-chain or branched (CZ-C8)-alkenyl unit (as
defined above) to the ring system.
Suitable hydroxy protective groups are all conventional
hydroxy protective groups known to the skilled worker,
in particular silyl ethers or esters of organic C1-Clo
acids, C1-C5 ethers, benzyl ethers or benzyl esters.
Conventional hydroxy protective groups are described in
detail in T.W. Greene, P.G.M. Wuts "Protective Groups
in Organic Synthesis", 2nd edition, John Wiley & Sons,
1991). The protective groups are preferably alkyl-,
aryl- or mixed alkylaryl-substituted silyl groups, e.g.
the trimethylsilyl (TNIS), triethylsilyl (TES), tert-
butyldimethylsilyl (TBDMS), tert-butyldiphenylsilyl
(TBDPS) or triisopropylsilyl groups (TIPS) or other
customary hydroxy protective groups (e.g.
methoxymethyl, methoxyethoxymethyl, ethoxyethyl,
tetrahydrofuranyl, tetrahydropyranyl groups).
The compounds of the invention of the general formula
(I) may, owing to the presence of centers of asymmetry,
exist as stereoisomers. The present invention relates
CA 02598208 2007-08-16
- 13 -
to all possible diastereoisomers both as racemates and
in enantiopure form. The term stereoisomers also
includes all possible diastereoisomers and regioisomers
and tautomers (e.g. keto-enol tautomers) in which the
stereoisomers of the invention may exist, and to which
the invention therefore likewise relates.
The compounds of the invention may also be in the form
of salts with pharmacologically acceptable anions, for
example in the form of the hydrochloride, sulfate,
nitrate, phosphate, pivalate, maleate, fumarate,
tartrate, benzoate, mesylate, citrate or succinate.
The invention also includes pharmacologically suitable
derivatives or prodrugs of the compounds of the general
formula (I). Derivatives or prodrugs refers for example
to esters, ethers or amides of the compounds of the
general formula (I) or other compounds which metabolize
in the body to compounds of the general formula (I).
Suitable compounds are listed for example in Hans
Bundgaard (Editor), Design of Prodrugs, Elsevier,
Amsterdam 1985.
Preferred embodiments
A subgroup of compounds of the invention of the general
formula (I) are those compounds in which R' and R8 are
independently of one another a hydrogen atom, a halogen
atom, an optionally substituted (C1-Clo)-alkyl group, a
cyano group, together a(C1-Clo)-alkylidene group or
together with the carbon atom of the tetrahydro-
naphthalene system an optionally substituted (C3-C6)-
cycloalkyl ring.
A further subgroup of compounds of the invention of the
general formula (I) are those compounds in which R6 and
R' together form a fused five- to eight-membered
saturated or unsaturated carbocycle or heterocycle
which is optionally substituted by 1 to 2 keto groups,
CA 02598208 2007-08-16
- 14 -
1 to 2 (C1-C5) -alkyl groups, 1 to 2 (C1-C5) -alkoxy
groups, and/or 1 to 4 halogen atoms.
A further subgroup of compounds of the invention of the
general formula (I) are those compounds in which R1 and
R8 together form a fused five- to eight-membered
saturated or unsaturated carbocycle or heterocycle
which is optionally substituted by 1 to 2 keto groups,
1 to 2 (C1-C5) -alkyl groups, 1 to 2 (C1-C5) -alkoxy
groups, and/or 1 to 4 halogen atoms.
A further subgroup of compounds of the invention of the
general formula (I) are those compounds in which R' and
R2 are independently of one another a hydrogen atom, a
hydroxy group, a halogen atom, an optionally
substituted (C1-C10) -alkyl group, a (C1-C10) -alkoxy
group, a (C1-C10) -alkylthio group, a (C1-CS) -
perfluoroalkyl group, a cyano group, a nitro group, or
an -NR9R9a group.
A further subgroup of compounds of the invention of the
general formula (I) are those compounds in which or R1
and R2 together form a group selected from the groups
-0- (CH2 ) n-0, -0- (CH2 ) n-CH2-, -O-CH=CH-, - ( CH2 ) n+2- r
-NH- (CH2) n+l-, -N (C1-C3-alkyl) - (CH2) õ+1- and -NH-N=CH-,
where n = 1 or 2, and the terminal oxygen atoms and/or
carbon atoms and/or nitrogen atoms are linked to
directly adjacent ring carbon atoms.
A preferred group of compounds of the general formula
(I) are those compounds in which X is a group -C (=0) -,
-C (=0 ) -NH-, -SO2- or -CH2- .
A further preferred group of compounds of the general
formula (I) are those compounds in which R4 is a
hydroxy group or a group -OR10. Those compounds in which
R 4 is a hydroxy group are particularly preferred.
CA 02598208 2007-08-16
- 15 -
A further preferred group of compounds of the general
formula (I) are those compounds in which R5 is a
(C1-C5) -alkyl group or a partly or completely
fluorinated (Cl-C5) -alkyl group, an aryl group, a
(C1-C8) alkylaryl group, a (CZ-C8) alkenylaryl group, a
(C3-C7) cycloalkyl gorup, a (C1-C8) alkyl (C3-C-,) cycloalkyl
group or a (C2-C8) alkenyl (C3-C7) cycloalkyl group.
Compounds which are preferred in this connection are
those in which R5 is a(C1-C5)-alkyl group or a partly
or completely fluorinated (C1-C5)-alkyl group.
Particularly preferred compounds in this connection are
those in which R5 is a trifluoromethyl or a
pentafluoroethyl group.
A further preferred group of compounds of the general
formula (I) are those compounds in which R' is a
halogen atom or an optionally substituted methyl or
ethyl group.
A further preferred group of compounds of the general
formula (I) are those compounds in which R' and R8 are
each a methyl group, or together with the carbon atom
of the tetrahydronaphthalene system form a cyclopropyl
group. Particularly preferred compounds are those in
which R7 and R8 are each a methyl group.
A further preferred group of compounds of the general
formula (I) are those compounds in which R3 is an
optionally substituted aryl or heteroaryl group.
Particularly preferred compounds in this connection are
those in which the aryl or heteroaryl group is selected
from the group consisting of naphthyl, benzofuranyl,
pyrazolo[1,5-a]pyridinyl, phenyl, phthalidyl,
isoindolyl, dihydroindolyl, dihydroisoindolyl,
dihydroisoquinolinyl, thiophthalidyl, benzoxazinonyl,
phthalazinonyl, quinolinyl, isoquinolinyl, quinolonyl,
isoquinolonyl, indazolyl, benzothiazolyl, chromanyl,
isochromanyl, quinazolinyl, quinoxalinyl, cinnolinyl,
phthalazinyl, 1,7- or 1,8-naphthyridinyl,
CA 02598208 2007-08-16
- 16 -
dihydroindolonyl, dihydroisoindolonyl, benzimidazole or
indolyl. Particularly preferred groups in this
connection are naphthyl, benzofuranyl, quinoxalinyl,
and pyrazolo[1,5-a]pyridinyl.
A subgroup of compounds of the general formula (I) are
those compounds in which the substituents R11 and R' 2
are each independently of one another a hydrogen atom,
a halogen atom, in particular fluorine, a cyano or a
methoxy group.
In a preferred subgroup, the substituents R" and R12
are each a hydrogen atom.
A particularly preferred group of compounds of the
general formula (I) as defined above are those
compounds in which R1, R2, R11, and R12 are independently
of one another a hydrogen atom, a halogen atom, a
hydroxy group, an optionally substituted (C1-C10)-alkyl
group, or a (C1-C10) -alkoxy group, R3 is an optionally
substituted aryl or heteroaryl group, R4 is a hydroxy
group, an -OR10 group or an -O (CO) Rl0 group, R5 is a(C1-
C1 )alkyl group which is optionally partly or completely
fluorinated, R6 is a hydrogen atom, a halogen atom or
an optionally substituted (C1-C10) -alkyl group, R7 and Ra
are independently of one another an optionally
substituted (C1-C10) -alkyl group, together a (C1-C10) -
alkylidene group or together with the carbon atom of
the tetrahydronaphthalene system an optionally
substituted (C3-C6) -cycloalkyl ring, R10 is a (Cl-C10) -
alkyl group, and X is the group -C(=O)-, -C(=O)-NH-, -
S(O)m- (where m equals 1 or 2), or -(CH2)p- (where p
equals 1, 2 or 3).
A very particularly preferred group of compounds of the
general formula (I) as defined above are those
compounds in which R1 and R 2 are each independently of
one another a hydrogen atom, a hydroxy group or a
methoxy group, R" and R12 are each a hydrogen atom, R3
CA 02598208 2007-08-16
- 17 -
is a naphthyl, benzofuranyl, quinoxalinyl or
pyrazolo[1,5-a]pyridinyl group, R4 is a hydroxy group,
R5 is a trifluoromethyl group, R6 is a hydrogen atom, R'
and R8 are each a methyl group, and X is one of the
groups -C(=O)-, -C(=0)-NH-, -SOZ- and -CH2-.
Every further possible combination of the
abovementioned subgroups and of substituents indicated
as preferred with their general and/or specific
meanings is likewise to be regarded as encompassed by
the present invention.
Preparation processes
The compounds of the invention of the general formula
(I) can be obtained in various ways. The preparation
processes described below likewise form a part of the
present invention.
Unless indicated otherwise, the substituents used in
the process descriptions below have the same meaning as
above in the section "Brief description of the
invention", including the definitions stated in the
section "Detailed description of the invention".
One process according to the invention (process A) for
preparing compounds of the general formula (I) is
characterized in that
a) an open-chain carbonyl compound of the
general formula (II)
R8 R' R5 R O
R~
R 4
z ~
R6 H
Ri~ R~2
(II)
CA 02598208 2007-08-16
- 18 -
is cyclized where appropriate with addition
of an inorganic or organic acid or Lewis
acid to a compound of the general formula
(III)
R8 R '
R R6
R2 R5
4
R~ ~ R12 R
OH (III) ,
b) the compound of the general formula (III)
is converted by processes known in the art
for replacing the hydroxy group by the
amino group into a compound of the general
formula (IV)
R$ R 7
R R6
R2 R5
4
RR12 R
NH2
(IV);
and
c) the compound of the general formula (IV) is
converted by reaction with a compound
selected from compounds of the general
formulae R3-X-Nu (where Nu is a nucleofugic
group), R3-N=C=O or R3-N=C=S, and where
appropriate subsequent replacement of a
carbonyl oxygen atom by sulfur by processes
known in the art, into a compound of the
general formula (I).
The substituents R1 to R12 have the meanings indicated
above.
CA 02598208 2007-08-16
- 19 -
Replacement of the hydroxy group by the amino group in
step b) indicated above can be achieved for example by
converting the compound of the general formula (III) by
processes known in the art (nucleophilic substitution)
into the corresponding azide, which can be reduced
under suitable conditions to the primary amine of the
general formula (IV).
A further possibility for introducing the amino group
consists of reacting the compound of the general
formula (III) with Burgess' reagent (Tetrahedron Lett.
2002, 43, 3887-3890) and subsequent cleavage of the
resulting heterocycle, which is obtainable by processes
known in the art.
The optional replacement of a carbonyl oxygen atom by
sulfur which is described in step c) is known in the
art and can be achieved for example by reaction with
Lawesson's reagent or phosphorus pentasulfide.
Examples of suitable nucleofugic groups Nu in the
compound R3-X-Nu used in step c) are halogen atoms or
leaving groups such as, for example, the acetate,
tosylate, mesylate or triflate group. The compounds
R3-X-Nu thus belong for example to the classes of the
halides of carboxylic acids, sulfonic acids, or
sulfinic acids, or of mixed anhydrides of these acids,
and to the esters of chloroformic acid, of
toluenesulfonic acid, of methylsulfonic acid and of
trifluoromethylsulfonic acid.
A further process according to the invention
(process B) for preparing compounds of the general
formula (I) consists of reacting a compound of the
general formula (IV) as described above with a compound
of the general formula R3-CHO, and reducing the
resulting imine.
CA 02598208 2007-08-16
- 20 -
Finally, a further process according to the invention
(process C) for preparing compounds of the general
formula (I) consists of reacting a compound of the
general formula (IV) as described above with phosgene
or thiophosgene, and subsequently reacting the
resulting isocyanate or isothiocyanate with compounds
of the general formula R3-OH or R3-NH2 to give a
compound of the general formula (I), where X has the
meaning -C(=O)-NH-, -C(=S)-NH- or -C(=O)-O- indicated
in claim 1.
A further process according to the invention
(process D) for preparing a compound of the general
formula (I) is characterized in that
a) a compound of the general formula (VI)
R~ R 8
\ R6
R2
R R 12
(VI)
where R8 is a (C1-Clo) -alkyl group, is
reacted with an a-keto carboxylic acid or
an a-keto carboxylic ester of the general
formula R5-C(=O)-COOR13 in an ene reaction
in the presence of an optionally chiral
Lewis acid to give a compound of the
general formula (VII)
R R$ R' OH
OR13
R
2 R 5
11 R6 O
R R12
(VII)
in which R7 and R$ together have the
meaning of a (C1-Clo) -alkylidene group,
CA 02598208 2007-08-16
- 21 -
b) the compound of the general formula (VII)
is reduced to an aldehyde of the general
formula (IIa),
R R$ R7 OH
O
R R 6
R~ R12
(IIa)
in which R' and R8 together have the
meaning of a(C1-Clo)-alkylidene group, and
c) the aldehyde (IIa) is converted in analogy
to process A indicated above into a
compound of the general formula (I) in
which R7 and R8 together have the meaning
of a (Cl-Clo) -alkylidene group.
The substituents R' to R' 3 have the meanings indicated
above.
Processes for selective reduction of a carboxylic acid
or of a carboxylic ester to the aldehyde, as used in
step b) of process D, are known in the art.
It is optionally possible to include in process D
further reaction steps to modify R7 and R8. Thus, for
example, the (C1-Clo)-alkylidene group in intermediate
(IIa) can be hydrogenated, thus providing compounds of
the general formula (I) in which one of the radicals R7
and R 8 has the meaning of a hydrogen atom, whereas the
other radical is a(C1-Clo) -alkyl group.
The (C1-Clo)-alkylidene group in intermediate (IIa) can
also be employed as substrate for a hydrohalogenation.
The final products of the synthesis resulting in this
case are compounds of the general formula (I) in which
one of the radicals R7 and R8 has the meaning of a
CA 02598208 2007-08-16
- 22 -
halogen atom, or else the other radical is a(C1-Clo) -
alkyl group.
Finally, it is also possible to carry out cycloaddition
reactions on the (C1-Clo)-alkylidene group in
intermediate (IIa). Particularly preferred in this
connection are cyclopropanation reactions which afford
as final products of the synthesis compounds of the
general formula (I) in which the radicals R' and R8
have, together with the ring carbon atom, the meaning
of an (optionally substituted) cyclopropane ring.
It is directly evident to the skilled worker that these
modifications to the radicals R' and R8 in process D do
not necessarily have to be carried out on intermediate
(IIa) but can also where appropriate be carried out at
a later time in the complete synthesis. These
variations in the sequence of the reaction steps are
likewise included in the present invention.
Biological activity
The antiinflammatory effect of the compounds of the
general formula (I) is tested in an animal experiment
by testing in the croton oil-induced inflammation in
the rat and mouse (J. Exp. Med. (1995), 182, 99-108).
For this purpose, croton oil is applied in ethanolic
solution topically to the ears of the animals. The test
substances are likewise administered topically or
systemically at the same time as or two hours before
the croton oil. After 16-24 hours, the ear weight is
measured as a measure of the inflammatory edema, the
peroxidase activity is measured as a measure of the
migration in of granulocytes and the elastase activity
is measured as a measure of the migration in of
neutrophilic granulocytes. In this test, the compounds
of the general formula (I) inhibit the three
abovementioned parameters of inflammation both after
topical and after systemic administration.
CA 02598208 2007-08-16
- 23 -
The binding of the substances to the glucocorticoid
receptor (GR) and further steroid hormone receptors
(mineral corticoid receptor (MR), progesterone receptor
(PR) and androgen receptor (AR)) is examined with the
aid of recombinantly prepared receptors. Cytosol
preparations of Sf9 cells which had been infected with
recombinant baculoviruses which code for the GR are
employed for the binding studies. Compared with the
reference substance [3H]-dexamethasone, the substances
show a high affinity for the GR. Thus, an IC50(GR) =
36 nM and IC50 (PR) > 1 uM was measured for the compound
of example 3L.
The essential molecular mechanism for the
antiinflammatory effect of glucocorticoids is regarded
as the GR-mediated inhibition of the transcription of
cytokines, adhesion molecules, enzymes and other pro-
inflammatory factors. This inhibition is brought about
by an interaction of the GR with other transcription
factors, e.g. AP-1 and NF-kappa-B (for review, see Cato
ACB and Wade E, BioEssays 18, 371-378 1996).
The compounds of the invention of the general formula
(I) inhibit the secretion, induced by
lipopolysaccharide (LPS), of the cytokine IL-8 in the
human THP-1 monocyte cell line. The concentration of
the cytokines was determined in the supernatant using a
commercially available ELISA kit. The compound of
example 3E exhibited an IC50 ( IL8 ) 1}.imol inhibition at
an 11% efficiency with regard to [3H]-dexamethasone as
standard.
One of the commonest unwanted effects of a
glucocorticoid therapy is the so-called "steroid
diabetes" [cf. Hatz, HJ, Glucocorticoide:
Immunologische Grundlagen, Pharmakologie und
Therapierichtlinien, Wissenschaftliche
Verlagsgesellschaft mbH, Stuttgart, 1998]. The cause of
CA 02598208 2007-08-16
- 24 -
this is stimulation of gluconeogenesis in the liver by
induction of the enzymes responsible therefor and by
free amino acids resulting from the breakdown of
proteins (catabolic effect of the glucocorticoids) . A
key enzyme in catabolic metabolism in the liver is
tyrosine aminotransferase (TAT) . The activity of this
enzyme can be determined by photometry on liver
homogenates and represents a good measure of the
unwanted metabolic effects of glucocorticoids. To
measure the TAT induction, the animals are sacrificed
8 hours after administration of the test substances,
the livers are removed, and the TAT activity in the
homogenate is measured. In this test, the compounds of
the general formula (I) induce, in doses in which they
have antiinflammatory activity, tyrosine
aminotransferase to only a small extent or not at all.
Medical indications
Owing to their antiinflammatory and additional anti-
allergic, immunosuppressive and antiproliferative
effect, the compounds of the invention of the general
formula (I) can be used as medicaments for the
treatment or prophylaxis of the following pathological
states in patients, especially mammals and preferably
humans.
In this connection, the term "DISORDER" stands for the
following indications:
(i) pulmonary disorders associated with
inflammatory, allergic and/or proliferative
processes:
- chronic obstructive lung disorders of any
origin, especially bronchial asthma
- bronchitis of varying origin
- all types of restrictive lung disorders,
especially allergic alveolitis,
CA 02598208 2007-08-16
- 25 -
- all types of pulmonary edema, especially
toxic pulmonary edema
- sarcoidoses and granulomatoses, especially
Boeck's disease
(ii) rheumatic disorders/autoimmune diseases/joint
disorders associated with inflammatory, allergic
and/or proliferative processes:
- all types of rheumatic disorders, especially
rheumatoid arthritis, acute rheumatic fever,
polymyalgia rheumatica
- reactive arthritis
- inflammatory soft tissue disorders of other
origin
- arthritic symptoms associated with
degenerative joint disorders (arthroses)
- traumatic arthritides
- collagenoses of any origin, e.g. systemic
lupus erythematosus, scleroderma,
polymyositis, dermatomyositis, Sjogren's
syndrome, Still's syndrome, Felty's syndrome
(iii) allergies associated with inflammatory and/or
proliferative processes:
- all types of allergic reactions, e.g.
angioedema, hayfever, insect bite, allergic
reactions to drugs, blood derivatives,
contrast agents etc., anaphylactic shock,
urticaria, contact dermatitis
(iv) vessel inflammations (vasculitides)
- polyarteritis nodosa, temporal arteritis,
erythema nodosum
(v) dermatological disorders associated with
inflammatory, allergic and/or proliferative
processes:
- atopic dermatitis (especially in children)
- psoriasis
- pityriasis rubra pilaris
- erythematous disorders induced by various
noxae, e.g. radiation, chemicals, burns, etc.
- bullous dermatoses
CA 02598208 2007-08-16
- 26 -
- lichenoid disorders
- pruritus (e.g. of allergic origin)
- seborrheic eczema
- rosacea
- pemphigus vulgaris
- erythema multiforme exudativum
- balanitis
- vulvitis
- hair loss such as alopecia areata
- cutaneous T-cell lymphomas
(vi) renal disorders associated with inflammatory,
allergic and/or proliferative processes:
- nephrotic syndrome
- all nephritides
(vii) liver disorders associated with inflammatory,
allergic and/or proliferative processes:
- acute liver cell necrosis
- acute hepatitis of varying origin, e.g.
viral, toxic, drug-induced
- chronic aggressive and/or chronic
intermittent hepatitis
(viii) gastrointestinal disorders associated with
inflammatory, allergic and/or proliferative
processes:
- regional enteritis (Crohn's disease)
- ulcerative colitis
- gastritis
- reflux esophagitis
- gastroenteritides of other origin, e.g.
indigenous sprue
(ix) proctological disorders associated with
inflammatory, allergic and/or proliferative
processes:
- anal eczema
- fissures
- hemorrhoids
- idiopathic proctitis
(x) ocular disorders associated with inflammatory,
allergic and/or proliferative processes:
CA 02598208 2007-08-16
- 27 -
- allergic keratitis, uveitis, iritis,
- conjunctivitis
- blepharitis
- optic neuritis
- chorioditis
- sympathetic ophthalmia
(xi) ear-nose-throat disorders associated with
inflammatory, allergic and/or proliferative
processes:
- allergic rhinitis, hayfever
- otitis externa, e.g. caused by contact exema,
infection etc.
- otitis media
(xii) neurological disorders associated with
inflammatory, allergic and/or proliferative
processes:
- cerebral edema, especially tumor-related
cerebral edema
- multiple sclerosis
- acute encephalomyelitis
- meningitis
- various types of seizures, e.g. infantile
spasms
(xiii) blood disorders associated with inflammatory,
allergic and/or proliferative processes:
- acquired hemolytic anemia
- idiopathic thrombocytopenia
(xiv) neoplastic disorders associated with
inflammatory, allergic and/or proliferative
processes:
- acute lymphatic leukemia
- malignant lymphomas
- lymphogranulomatoses
- lymphosarcomas
- extensive metastases, especially associated
with breast, bronchial and prostate
carcinomas
CA 02598208 2007-08-16
- 28 -
(xv) endocrine disorders associated with
inflammatory, allergic and/or proliferative
processes
- endocrine orbitopathy
- thyrotoxic crisis
- de Quervain's thyroiditis
- Hashimoto's thyroiditis
- Basedow's disease
(xvi) organ and tissue transplantations, graft-versus-
hose disease
(xvii) severe states of shock, e.g. anaphylactic shock,
systemic inflammatory response syndrome (SIRS)
(xviii) emesis associated with inflammatory, allergic
and/or proliferative processes:
- e.g. in combination with a 5-HT3 antagonist
in cytostic-related vomiting.
(xix) pain of inflammatory origin, e.g. lumbago
(xx) replacement therapy for:
- congenital primary adrenal insufficiency,
e.g. congenital adrenogenital syndrome
- acquired primary adrenal insufficiency, e.g.
Addison's disease, autoimmune adrenalitis,
post-infection, tumors, metastases etc.
- congenital secondary adrenal insufficiency,
e.g. congenital hypopituitarism
- acquired secondary adrenal insufficiency,
e.g. post-infection, tumors etc.
Medicaments comprising stereoisomers of the general
formula I show a particular efficacy for the following
disorders:
1. lung disorders
2. rheumatic disorders/autoimmune diseases
3. dermatological disorders
4. degenerative joint disorders
5. vessel inflammations
6. graft versus host disease
7. severe states of shock
CA 02598208 2007-08-16
- 29 -
8. emesis associated with inflammatory, allergic
and/or proliferative processes
9. inflammation-related pain.
In addition, the compounds of the invention of the
general formula (I) can be employed for the therapy and
prophylaxis of further pathological states which are
not mentioned above but for which synthetic
glucocorticoids are currently used (concerning this,
see Hatz, HJ, Glucocorticoide: Immunologische
Grundlagen, Pharmakologie und Therapierichtlinien,
Wissenschafliche Verlagsgesellachaft mbH, Stuttgart,
1998).
All the aforementioned indications are described in
detail in Hatz, HJ, Glucocorticoide: Immunologische
Grundlagen, Pharmakologie und Therapierichtlinien,
Wissenschafliche Verlagsgesellachaft mbH, Stuttgart,
1998).
The suitable dose for a therapeutic effect in the
abovementioned pathological states varies and depends
for example on the potency of the compound of the
general formula (I), the patient (e.g. height, weight,
gender, etc.), the mode of administration and the
nature and severity of the conditions to be treated,
and the use as prophylactic or therapeutic agent.
The invention relates to the use of the claimed
compounds for manufacturing a pharmaceutical
composition.
The invention further provides:
(i) the use of one of the compounds of the
invention of the general formula (I) or mixture
thereof for manufacturing a pharmaceutical
composition for the treatment or prevention of
inflammatory processes, and especially for the
treatment of a DISORDER (as defined above);
CA 02598208 2007-08-16
- 30 -
(ii) a method for the treatment or prevention of
inflammatory processes, especially for the
treatment of a DISORDER (as defined above),
which method includes administration of a
pharmaceutically effective amount of a compound
of the general formula (I), where the amount
alleviates or suppresses the disease or the
symptoms, and where the compound is given to a
patient, preferably a mammal, in particular a
human, requiring such a treatment;
(iii) a pharmaceutical composition having an
antiinflammatory effect, in particular for the
treatment of a DISORDER (as defined above),
where the composition includes one of the
compounds of the invention of the general
formula (I) or mixtures thereof and, where
appropriate, at least one pharmaceutical
excipient and/or carrier.
Satisfactory results are generally to be expected in
animals when the daily doses include a range from 1 pg
to 100 000 pg of the compound of the invention per kg
of body weight. For larger mammals, for example humans,
a recommended daily dose is in the range from 1 pg to
100 000 pg per kg of body weight. A dose of 10 to
000 pg per kg of body weight is preferred, and a
dose of 10 to 10 000 pg per kg of body weight is more
preferred. This dose is for example expediently
administered more than once a day. For the treatment of
30 acute shock (e.g. anaphylactic shock) it is possible to
give single doses which are distinctly higher than the
abovementioned doses.
The pharmaceutical products based on the novel
compounds are formulated in a manner known per se by
processing the active ingredient with the carrier
substances, fillers, substances influencing
disintegration, binders, humectants, lubricants,
absorbents, diluents, masking flavors, colorants etc.
CA 02598208 2007-08-16
- 31 -
which are in use in pharmaceutical technology, and
converting into the desired administration form.
Reference should be made in this connection to
Remington's Pharmaceutical Science, 15th ed. Mack
Publishing Company, East Pennsylvania (1980).
Particularly suitable for oral administration are
tablets, coated tablets, capsules, pills, powders,
granules, pastilles, suspensions, emulsions or
solutions.
Preparations for injection and infusion are possible
for parenteral administration.
Appropriately prepared crystal suspensions can be used
for intraarticular injection.
Aqueous and oily solutions for injections or
suspensions and corresponding depot preparations can be
used for intramuscular injection.
The novel compounds can be used for rectal
administration in the form of suppositories, capsules,
solutions (e.g. in the form of enemas) and ointments
both for systemic and for local therapy.
The novel compounds can be used in the form of aerosols
and inhalations for pulmonary administration thereof.
For local use on eyes, the external auditory canal,
middle ear, nasal cavity and paranasal sinuses, the
novel compounds can be used as drops, ointments,
tinctures and gels in appropriate pharmaceutical
preparations.
Formulations possible for topical application are gels,
ointments, greasy ointments, creams, pastes, dusting
powders, suspensions, emulsions and solutions. The
dosage of the compounds of the general formula (I) in
CA 02598208 2007-08-16
- 32 -
these preparations should be 0.01% - 20% in order to
achieve an adequate pharmacological effect.
The invention likewise includes the compounds of the
invention of the general formula (I) as therapeutic
active ingredient. The invention further includes the
compounds of the invention of the general formula (I)
as therapeutic active ingredient together with one or
more pharmaceutically suitable and acceptable
excipients and/or carriers.
The compounds of the invention of the general formula
(I) can also where appropriate be formulated and/or
administered in combination with further active
ingredients.
The invention therefore also relates to combination
therapies or combined compositions in which a compounds
of the general formula (I) or a pharmaceutically
acceptable salt thereof, or a pharmaceutical
composition comprising a compound of the general
formula (I) or a pharmaceutically acceptable salt
thereof, is administered either simultaneously (where
appropriate in the same composition) or successively
together with one or more medicaments for the treatment
of one of the pathological states mentioned above. For
the treatment of rheumatoid arthritis, osteoarthritis,
COPD (chronic obstructive pulmonary disorder), asthma
or allergic rhinitis, for example, it is possible to
combine a compound of the general formula (I) of the
present invention with one or more medicaments for the
treatment of such a condition. Where such a combination
is administered by inhalation, the medicament to be
combined can be selected from the following list:
= a PDE4 inhibitor including an inhibitor of the
PDE4D isoform;
CA 02598208 2007-08-16
- 33 -
= a selective (3.sub2.adrenoceptor agonist such as,
for example, metaproterenol, isoproterenol,
isoprenaline, albuterol, salbutamol, formoterol,
salmeterol, terbutaline, orciprenaline, bitolterol
mesylate, pirbuterol or indacaterol;
= a muscarine receptor antagonist (for example an
Ml, M2 or M3 antagonist, such as, for example, a
selective M3 antagonist) such as, for example,
ipratropium bromide, tiotropium bromide,
oxitropium bromide, pirenzepine or telenzepine;
= a modulator of chemokine receptor function (such
as, for example, a CCR1 receptor antagonist); or
= an inhibitor of p38 kinase function.
For another aspect of the present invention, such a
combination with a compound of the general formula (I)
or a pharmaceutically acceptable salt thereof is
employed for the treatment of COPD, asthma or allergic
rhinitis and can be administered by inhalation or
orally in combination with xanthine (such as, for
example, aminophylline or theophylline), which can
likewise be administered by inhalation or orally.
Examples
The cis/trans nomenclature used in the examples below
refers to the position of the substituents in position
1 and 2 of the saturated ring of the
tetrahydronaphthalene system. In this connection, cis
means that the highest priority substituent (according
to the Cahn-Ingold-Prelog definition) on carbon atom 1
is located in the axial position and the highest
priority substituent on carbon atom 2 is located in the
equatorial position; or that the highest priority
substituent (according to the Cahn-Ingold-Prelog
definition) on carbon atom 1 is located in the
CA 02598208 2007-08-16
- 34 -
equatorial position and the highest priority
substituent on carbon atom 2 is located in the axial
position. Correspondingly, trans means that the two
highest priority substituents in each case on carbon
atom 1 and carbon atom 2 are located either both in the
axial position or both in the equatorial position.
Example 1: Synthesis of compounds of the general
formula (III)
6-Fluoro-5-methoxy-4,4-dimethyl-2-trifluoromethyl-
1,2,3,4-tetrahydronaphthalene-l,2-diol (cis and trans
isomers)
1.75 g (5.67 mmol) of 4-(3-fluoro-2-methoxyphenyl)-2-
hydroxy-4,4-dimethyl-2-trifluoromethylpentanal are
dissolved in 20 ml of dichlormethane, and 2.6 ml of
trifluoroacetic acid are added. The reaction is stirred
at room temperature under a nitrogen atmosphere for
24 h. For workup, the reaction solution is evaporated
with toluene in vacuo three times and then purified by
chromatography: 1.28 g of cis-6-fluoro-5-methoxy-4,4-
dimethyl-2-trifluoromethyl-1,2,3,4-tetrahydro-
naphthalene-1,2-diol and 300 mg of trans-6-fluoro-
5-methoxy-4,4-dimethyl-2-trifluoromethyl-1,2,3,4-
tetrahydronaphthalene-1,2-diol (900).
cis-6-Fluoro-5-methoxy-4,4-dimethyl-2-trifluoromethyl-
1,2,3,4-tetrahydronaphthalene-1,2-diol: 1H-NMR (300
MHz, CDC13) : b/ppm = 1. 45 (s, 3H) , l, 59 (s, 3H) , 1.81 (d,
1H) , 2.08 (d, 1H), 2.56 (d, 1H), 3.18 (d, 1H), 3.94 (d,
3H), 5.03 (d, 1H), 7.03 (dd, 1H), 7.31 (ddd, 1H).
trans-6-Fluoro-5-methoxy-4,4-dimethyl-2-trifluoro-
methyl-1,2,3,4-tetrahydronaphthalene-1,2-diol: 1H-NMR
(300 MHz, CDC13): b/ppm = 1.52 (s, 3H), 1.55 (s, 3H),
1.83 (dd, 1H), 1.87-1.90 (m, 2H), 2.42 (d, 1H), 3.95
(d, 3H), 4.68 (dd, 1H), 6. 99-7 . 06 (m, 2H).
The following compounds are obtained in analogy to the
above method:
CA 02598208 2007-08-16
- 35 -
6-Fluoro-5-methoxy-4,4-dimethyl-2-trifluoromethyl-
1,2,3,4-tetrahydronaphthalene-1,2-diol (cis and trans
isomer mixture)
7-Methoxy-4,4-dimethyl-2-trifluoromethyl-1,2,3,4-
tetrahydronaphthalene-1,2-diol (cis and trans isomer
mixture)
Example 2: Synthesis of compounds of the general
formula (IV):
Example 2A: cis-l-Amino-6-fluoro-5-methoxy-4,4-di-
methyl-2-trifluoromethyl-1,2,3,4-tetrahydro-
naphthalen-2-ol
a) Benzyl 7-fluoro-6-methoxy-5,5-dimethyl-2,2-dioxo-3a-
trifluoromethyl-3a,4,5,9b-tetrahydro-3-oxy-2lambda*6*-
thia-l-aza-cyclopenta[a]naphthalene-l-carboxylate
2.00 g (6.48 mmol) of 6-fluoro-5-methoxy-4,4-dimethyl-
2-trifluoromethyl-1,2,3,4-tetrahydronaphthalene-1,2-
diol and 5.12 g (16.2 mmol) of Burgess's reagent are
dissolved in 50 ml of THF and stirred under a nitrogen
atmosphere at 65-70 C for 7 h and then at room
temperature for 12 h. The reaction solution is
evaporated in vacuo, and the residue is purified by
chromatography: 1.66 g (51%).
1H-NMR (300 MHz, CDC13) : 6/ppm = 1. 54 (s, 3H) , 1.59 (s,
3H), 2.01 (d, 1H), 2.29 (d, 1H) , 3.95 (d, 3H), 5.36 (d,
1H) , 5.44 (d, 1H), 5.95 (s, 1H) , 6.97 (dd, 1H) , 7.19
(dd, 1H), 7.35-7.42 (m, 5H).
b) cis-l-Amino-6-fluoro-5-methoxy-4,4-dimethyl-2-tri-
fluoromethyl-1,2,3,4-tetrahydronaphthalen-2-ol
1.2 g (2.38 mmol) of benzyl 7-fluoro-6-methoxy-5,5-
dimethyl-2,2-dioxo-3a-trifluoromethyl-3a,4,5,9b-
tetrahydro-3-oxy-2lambda*6*-thia-l-aza-
cyclopenta[a]naphthalene-l-carboxylate are dissolved in
12 ml of dioxane and, after addition of 8 ml of 4N HC1
solution, treated in a microwave at 250 watt and 140 C
CA 02598208 2007-08-16
- 36 -
for 20 minutes twice. For workup, the solution is
evaporated in vacuo, and the residue is adjusted to
pH 14 with 4N sodium hydroxide solution at 0 C and
extracted three times with ethyl acetate. The combined
organic phases are washed with saturated sodium
chloride solution, dried over sodium sulfate, filtered
and concentrated in vacuo: 41% of the desired product.
1H-NMR (300 MHz, CDC13) : b/ppm = 1.36 (s, 3H), 1.49 (s,
3H), 1.78 (s, 1H), 1.95 (d, 1H), 3.83 (d, 3H), 4.03 (s,
1H) , 7.12 (dd, 1H), 7.51 (dd, 1H).
Example 2B: trans-l-Amino-6-fluoro-5-methoxy-4,4-
dimethyl-2-trifluoromethyl-1,2,3,4-tetrahydro-
naphthalen-2-ol
a) 1-Azido-6-fluoro-5-methoxy-4,4-dimethyl-2-trifluoro-
methyl-1,2,3,4-tetrahydronaphthalen-2-ol
420 mg (1.27 mmol) of tetrabromomethane and 454 mg
(1.14 mmol) of 1,2-bis(diphenylphoshino)ethane (DiPhos)
are added to a solution of 130 mg (0.422 mmol) of
6-fluoro-5-methoxy-4,4-dimethyl-2-trifluoromethyl-
1,2,3,4-tetrahydronaphthalene-l,2-cis-diol in 4.5 ml of
dichlormethane at room temperature under a nitrogen
atmosphere. It is possible analogously to start this
reaction also with the corresponding 1,2-cis/trans-diol
mixture instead of the 1,2-cis-diol. After stirring at
room temperature for 3 h, 20 ml of ether are added and,
after stirring for 5 min, the resulting precipitate is
filtered off and washed with ether, and the solvent is
evaporated in vacuo: 200 mg of crude product which is
employed without further purification in the next
reaction.
190 mg (0.512 mmol) of crude product are mixed with
2 ml of a sodium azide-containing DMSO solution (150 mg
of sodium azide in 5 ml of dimethyl sulfoxide are
stirred at room temperature for 24 h). The reaction
solution is stirred at room temperature for 3 h and at
40-45 C for 3 h under a nitrogen atmosphere. For
workup, 5 ml of water are added to the reaction mixture
CA 02598208 2007-08-16
- 37 -
and it is extracted three times with ethyl acetate. The
combined organic phases are washed with saturated
sodium chloride solution, dried over sodium sulfate,
filtered, concentrated in vacuo and purified by column
chromatography: 80 mg of the title compound.
1H-NMR (300 MHz, CDC13) : 6/ppm = 1.52 (s, 3H), 1.56 (s,
3H), 1.84 (dd, 1H), 1.97 (s, 1H), 2.25 (d, 1H), 3.98
(d, 3H), 4.48 (d, 1H), 6.93 (dd, 1H), 7.07 (dd, 1H).
b) trans-l-Amino-6-fluoro-5-methoxy-4,4-dimethyl-
2-trifluoromethyl-1,2,3,4-tetrahydronaphthalen-2-ol
311 mg (4.76 mmol) of zinc powder are added to a
solution of 1.22 g (3.66 mmol) of l-azido-6-fluoro-
5-methoxy-4,4-dimethyl-2-trifluoromethyl-1,2,3,4-
tetrahydronaphthalen-2-ol and 450 mg (8.42 mmol) of
ammonium chloride in 9.7 ml of ethanol and 3.3 ml of
water, and the mixture is then stirred at 90 C under a
nitrogen atmosphere for 30 min. After cooling to room
temperature, 25 ml of ethyl acetate and 1.22 ml of
ammonia solution are added, and the mixture is stirred
for 5 min and filtered. The filtrate is washed with
saturated sodium chloride solution, dried over sodium
sulfate, filtered and concentrated in vacuo. The crude
product is purified by column chromatography: 950 mg of
the title compound.
1H-NMR (300 MHz, CDC13) : 6/ppm = 1. 42 (s, 3H) , 1.46 (s,
3H), 1.61 (dd, 1H), 1.77 (bs, 2H), 2.20 (d, 1H), 3.84
(d, 3H), 3.88 (s, 1H), 5.63 (s, 1H), 6.98 (dd, 1H),
7.10 (dd, 1H).
Example 3: Synthesis of compounds of the general
formula (I)
Example 3A: N-(cis-6-fluoro-2-hydroxy-5-methoxy-4,4-
dimethyl-2-trifluoromethyl-1,2,3,4-tetrahydro-
naphthalen-1-yl)naphthalene-2-carboxamide
A solution of 4-dimethylaminopyridine (73 mg,
0.60 mmol) in DMF (0.4 ml) and a solution of
CA 02598208 2007-08-16
- 38 -
2-naphthalenecarbonyl chloride (84 mg, 0.44 mmol) in
DMF (0.8 ml) are successively added to a solution of
cis-l-amino-6-fluoro-5-methoxy-4,4-dimethyl-2-tri-
fluoromethyl-1,2,3,4-tetrahydronaphthalen-2-ol (100 mg,
0.40 mmol) in DMF (0.8 ml), and the resulting mixture
is stirred at room temperature overnight. For workup,
sodium bicarbonate solution (3 ml, half-saturated
solution in water) and ethyl acetate (6 ml) are added
and, after extraction, the organic phase is isolated
and concentrated. One third of the resulting crude
product is purified by HPLC-MS and affords 28 mg of the
title compound.
F
F
F
= OH F
0 NH
HPLC: 3.4 min (method A).
MS (ESI) : m/z 461.
1H-NMR (CDC13, 400 MHz): 8/ppm = 1.52 (s, 3H), 1.67 (s,
3H), 2.12 (m, 2H), 3.30 (broad s), 3.97 (s br, 3H),
5.67 (d, 1H), 6.93-7.07 (m, 3H), 7.57 (m, 2H), 7.91 (m,
4H), 8.36 (s, 1H).
Example 3B: N-(cis-6-fluoro-2,5-dihydroxy-4,4-dimethyl-
2-trifluoromethyl-1,2,3,4-tetrahydronaphthalen-
1-yl)naphthalene-2-carboxamide
The remaining two thirds of the crude product from the
synthesis described above in example 3A are dissolved
in dichloromethane (0.5 ml) and cooled to -40 C and, at
this temperature, boron tribromide solution (1 ml, 1.OM
in dichloromethane, 1.0 mmol) is added, and the mixture
is warmed to room temperature while stirring overnight.
CA 02598208 2007-08-16
- 39 -
For workup, potassium carbonate solution (1.5 ml, half-
saturated solution in water) is added in an ice bath,
the resulting mixture is stirred in the ice bath for
15 min and diluted and extracted with ethyl acetate
(3 ml), and the organic phase is isolated and
concentrated. The residue is purified by HPLC-MS and
affords 88 mg of the title compound.
OH
F
F
F
= OH F
0 NH
HPLC: 3.1 min (method A).
MS (ESI) : m/z 447.
1H-NMR (CDC13, 400 MHz) : b/ppm = 1. 56 (s, 3H) , 1. 69 (s,
3H), 2.13 (s, 2H), 5.43 (d, 1H), 5.66 (d, 1H), 6.88-
7.03 (m, 3H), 7.57 (m, 2H), 7.92 (m, 4H), 8.37 (s, 1H).
The following compounds of the general formula (I) were
obtained in analogy to examples 3A and 3B described
above:
CA 02598208 2007-08-16
- 40 -
Ex. Structure Name MS HPLC
(ESI)
3C o N-(cis-6-fluoro-2- 461 3.3 min.
F F hydroxy-5-methoxy-4,4-
(method A)
F dimethyl-2-trifluoro-
OH F
o NH methyl-1,2,3,4-
tetrahydronaphthalen-
~
1-yl)naphthalene-l-
carboxamide
3D o N-(cis-6-fluoro-2- 463 2.9 min.
F
F hydroxy-5-methoxy-4,4- (method A)
dimethyl-2-trifluoro-
OH F
o NH methyl-1,2,3,4-
tetrahydronaphthalen-
I 1-yl)quinoxaline-6-
N
N carboxamide
3E 1-cis-6-fluoro-2- 476 3.4 min.
F
F hydroxy-5-methoxy-4,4- (method A)
oH F dimethyl-2-trifluoro-
0 NH
y methyl-1,2,3,4-
/ NH
tetrahydronaphthalen-
~ 1-yl)-3-naphthalen-2-
ylurea
3F 0 1-(cis-6-fluoro-2- 476 3.3 min.
F
F hydroxy-5-methoxy-4,4- (method A)
dimethyl-2-trifluoro-
= OH F
oyNH methyl-1, 2, 3, 4-
NH tetrahydronaphthalen-
~ I 1-yl)-3-naphthalen-l-
~ ylurea
CA 02598208 2007-08-16
- 41 -
3G o N-(cis-6-fluoro-2- 451 3.2 min.
F F hydroxy-5-methoxy-4,4-
(method A)
F dimethyl-2-trifluoro-
OH F
O NH methyl-1,2,3,4-
tetrahydronaphthalen-
1-yl)benzofuran-5-
carboxamide
3H o N-(cis-6-fluoro-2- 451 3.3 min.
F F hydroxy-5-methoxy-4,4-
(method A)
F dimethyl-2-trifluoro-
OH F
O NH methyl-1,2,3,4-
tetrahydronaphthalen-
O
1-yl)benzofuran-2-
\ carboxamide
31 N-(cis-6-fluoro-2- 498 3.5 min.
F hydroxy-5-methoxy-4,4- (method A)
dimethyl-2-trifluoro-
0 F
01~s~NH methyl-1, 2, 3, 4-
tetrahydronaphthalen-
I 1-yl)naphthalene-2-
~ sulfonamide
3J o N-(cis-6-fluoro-2- 498 3.5 min.
F hydroxy-5-methoxy-4,4-
156___~F (method A)
F dimethyl-2-trifluoro-
O\~ NHOH F methyl-1, 2, 3, 4-
0=s
tetrahydronaphthalen-
I \ 1-yl)naphthalene-l-
/
sulfonamide
OH
3K F N-(cis-6-fluoro-2,5- 448 11.2 min.
F
dihydroxy-4,4- (method C)
dimethyl-2-trifluoro-
OH F
o NH methyl-1,2,3,4-
~ tetrahydronaphthalen-
I 1-yl)naphthalene-l-
carboxamide
CA 02598208 2007-08-16
- 42 -
OH
3L F N-(cis-6-fluoro-2,5- 438 10.3 min.
F dihydroxy-4,4- (method C)
F
= OH F dimethyl-2-trifluoro-
O NH
methyl-1,2,3,4-
tetrahydronaphthalen-
I 1-yl)benzofuran-5-
o carboxamide
3M N-(cis-6-fluoro-2,5- 438 10.8 min.
dihydroxy-4,4- (method C)
o
dimethyl-2-trifluoro-
0 MH methyl-1,2,3,4-
OH ~
= L tetrahydronaphthalen-
L
/ L 1-yl)benzofuran-2-
OH carboxamide
OH
3N F N-(cis-6-fluoro-2,5- 483 3.2 min.
F
dihydroxy-4,4- (method A)
OH F dimethyl-2-trifluoro-
O~\SNH methyl-1, 2, 3, 4-
I ~ tetrahydronaphthalen-
/ 1-yl)naphthalene-l-
sulfonamide
OH
30 F 1-(cis-6-fluoro-2,5- 463 10.7 min.
FF dihydroxy-4,4- (method C)
= OH F dimethyl-2-
OyNH
trifluoromethyl-
NH
1,2,3,4-tetrahydro-
naphthalen-l-yl)-3-
naphthalen-l-ylurea
OH
3P N-(cis-6-fluoro-2,5- 483 3.2 min.
F dihydroxy-4,4- (method A)
0 OH F dimethyl-2-trifluoro-
\ ~NH
0=S
methyl-1,2,3,4-
~ tetrahydronaphthalen-
1-yl)naphthalene-2-
sulfonamide
CA 02598208 2007-08-16
- 43 -
OH
3Q F N-(cis-6-fluoro-2,5- 450 8.3 min.
F
F dihydroxy-4,4- (method C)
= OH F dimethyl-2-trifluoro-
O NH
methyl-1,2,3,4-
I tetrahydronaphthalen-
NI 1-yl)quinoxaline-6-
carboxamide
OH
3R 1-(cis-6-fluoro-2,5- 463 9.5 min.
FF dihydroxy-4,4- (method B)
O NHOH F dimethyl-2-
trifluoromethyl-
/ trifluoromethyl-
NH
~ 1,2,3,4-tetrahydro-
~
naphthalen-l-yl)-3-
naphthalen-2-ylurea
OH
3S F N-(trans-6-fluoro-2,5- 447 2.9 min.
~
/ FF dihydroxy-4,4- (method A)
oH F dimethyl-2-trifluoro-
O NH
methyl-1,2,3,4-
~ tetrahydronaphthalen-
1-yl)naphthalene-2-
carboxamide
OH
3T F N-(trans-6-fluoro-2,5- 448 8.8 min.
\ F dihydroxy-4,4- (method B)
/
oH F dimethyl-2-trifluoro-
O NH
methyl-1,2,3,4-
I tetrahydronaphthalen-
/ 1-yl)naphthalene-l-
carboxamide
OH
3U F N-(trans-6-fluoro-2,5- 437 2.8 min.
FF dihydroxy-4,4- (method A)
1 6 o
O H F dimethyl-2-trifluoro-
NH methyl-1,2,3,4-
tetrahydronaphthalen-
0 1-yl)benzofuran-5-
carboxamide
CA 02598208 2007-08-16
- 44 -
OH
3V F N-(trans-6-fluoro-2,5- 438 3.0 min.
/ FF dihydroxy-4,4- (method A)
0 NHOH F dimethyl-2-trifluoro-
methyl-1,2,3,4-
o
tetrahydronaphthalen-
\ ~ 1-yl)benzofuran-2-
carboxamide
OH
3W F 1-(trans-6-fluoro-2,5- 463 9.2 min.
F dihydroxy-4,4- (method B)
Oy NHOH F dimethyl-2-
/ NH trifluoromethyl-
~ 1,2,3,4-tetrahydro-
naphthalen-1-yl)-3-
naphthalen-2-ylurea
OH
3X F 1-(trans-6-fluoro-2,5- 463 8.9 min.
F
F dihydroxy-4,4- (method B)
0~ NHOH F dimethyl-2-
NH trifluoromethyl-
~ 1,2,3,4-tetrahydro-
~ naphthalen-l-yl)-3-
naphthalen-1-ylurea
3Y \ N-(trans-6-fluoro-2- 462 2.8 min.
F
F hydroxy-5-methoxy-4,4- (method D)
oH F dimethyl-2-trifluoro-
O NH
methyl-1,2,3,4-
I tetrahydronaphthalen-
/ / 1-yl)naphthalene-l-
carboxamide
3Z 1-(trans-6-fluoro-2- 476 3.1 min.
F hydroxy-5-methoxy-4,4- (method A)
/ = F
OH dimethyl-2-
F
oyNH trifluoromethyl-
NH 1,2,3,4-tetrahydro-
~ naphthalen-1-yl)-3-
~ I naphthalen-1-ylurea
CA 02598208 2007-08-16
- 45 -
Example 4: Synthesis of further compounds of the
general formula (I)
Example 4: 6-Fluoro-5-methoxy-4,4-dimethyl-l-
[(pyrazolo[1,5-a]pyridin-3ylmethyl)amino]-2-
trifluoromethyl-1,2,3,4-tetrahydronaphthalen-2-ol, (cis
isomer)
~O
F F F
F
OH
NH
C ~
N-N
cis-l-Amino-6-fluoro-5-methoxy-4,4-
dimethyl-2-trifluoromethyl-1,2,3,4-
tetrahydronaphthalen-2-ol (62 mg, 0.20 mmol) and
pyrazolo[1,5-a]pyridine-3-carbaldehyde (30 mg (0.20
mmol) are dissolved in xylene (5.0 ml) and, after
addition of titanium tetraethoxide (0.085 ml, 0.40
mmol), stirred at 150 C for 3 hours. After removal of
the solvent, the residue is taken up in methanol
(1.0 ml) and tetrahydrofuran (1.0 ml), and sodium
borohydride (22 mg, 0.58 mmol) is added. The reaction
is stopped after 3 hours at room temperature by adding
water. Methanol and tetrahydrofuran are removed in
vacuo. The aqueous phase is extracted with
dichloromethane, and the organic phase is dried over
sodium sulfate. Removal of the solvent and subsequent
purification by chromatography (silica gel,
hexane/ethyl acetate 3:7) result in 26 mg of the title
compound.
1H-NMR (300 MHz, d6-DMSO): 6/ppm = 1.39 (s, 3H), 1.46
(s, 3H), 1.85 (d, 1H), 2.07 (d, 1H), 3.83 (d, 3H), 3.94
(s, 1H), 3.98 (d, 1H), 4.06 (d, 1H), 5.92 (br, 1H),
6.85 (td, 1H), 7.07-7.23 (m, 3H), 7.63 (d, 1H), 7.98
(s, 1H) , 8. 62 (d, 1H)
CA 02598208 2007-08-16
- 46 -
Description of the HPLC methods
Method A:
Waters Alliance 2795, Waters Photo Diode Array 2996
(200-320 nm), Micromass ZQ; column Micra NPS ODS 2
(33x4.6 mm, 1.5 um); gradient 0-90% acetonitrile (0.010
formic acid) in water (0.01% formic acid) (4.5 min.),
90% acetonitrile (0.01% formic acid) in water (0.01%
formic acid) (2 min); flow rate 0.8 ml/min.
Method B:
Waters Pump 515, Waters Dual Absorbance Detector 2487
(254 nm), Micromass ZQ; column X-Terra (150x4.6 mm,
5 pm); gradient 54-95% acetonitrile (0.01% formic acid)
in water (0.01% formic acid) (10 min); flow rate
1 ml/min.
Method C:
Waters Pump 616, Hitachi L-4000 (254 nm); column
Chromasil C8 (150x4.6 mm, 5 pm); gradient 30-95%
acetonitrile (0.01% formic acid) in water (0.01% formic
acid) (15 min.), 95% acetonitrile (0.01% formic acid)
in water (0.01% formic acid) (15 min); flow rate
1 ml/min.
Method D:
Hewlett-Packard 1100 Pump, HP1100 Detector (200-320
nm), Micromass PLCZ; column Micra NPS ODS 2 (33x4.6 mm,
1.5 pm); gradient 0-90% acetonitrile (0.01% trifluoro-
acetic acid) in water (0.01% trifluoroacetic acid)
(4.5 min), 90% acetonitrile (0.01% trifluoroacetic
acid) in water (0.01% trifluoroacetic acid) (2 min);
flow rate 0.8 ml/min.