Note: Descriptions are shown in the official language in which they were submitted.
CA 02598927 2007-08-22
WO 2006/090118 PCT/GB2006/000528
Safety needle accessory
This invention relates to a safety needle accessory and particularly to a
safety needle
accessory sealed with in a pack suitable for sealing pre-filled syringes.
Needle stick injuries carry a significant risk of spreading infection such as
HIV and
hepatitis, and are commonplace among healthcare worlcers. The USA has led the
way
in introducing legislation that obliges healthcare providers to use the safest
devices
when giving injections, intravenous drug administration and similar invasive
procedures. Other countries are following, and even without legislation, the
ever-
present risk of litigation has alerted pharmaceutical companies and health
authorities
to seek suitable safe devices.
As a result of the heightened awareness of needle stick injuries, there have
been a
large number of inventions purporting to solve the problem. Most take the form
of a
protective sleeve which covers the needle tip after the injection has been
given, or
means for retracting the needle rapidly into the syringe barrel. In the former
case, a
weakness of the designs has been the need for the user to perform an action to
render
the needle safe; thus if the step is omitted, the risk remains. In the second
case, the
needle retraction mechanism requires that the syringe plunger is pushed to the
end of
its stroke in order to activate the retraction mechanism. In other words,
virtually none
of the devices are "fail safe". In many real-life situations, the patient can
involuntarily react to the pain of the injection and pull away from the
needle, exposing
the sharp tip, and therefore presenting a risk of a needle stick.
Another drawback of prior art safety needles (which in the present context
includes
safety syringes) is that they are not compatible with current accepted
practice. The
problems includes drug incompatibility with the device construction materials,
difficulty in using standard sterilising methods, difficulty in fitting to the
syringe,
large size, difficulty in filling, and very high cost.
A cornrnon requirement is for pre-filled syringes, and for reasons of drug
compatibility and long-term storage, the syringe barrel is often made from
glass, with
the hypodermic needle bonded into the delivery end of the syringe barrel.
1
CA 02598927 2007-08-22
WO 2006/090118 PCT/GB2006/000528
Alternatively, a few drugs are compatible with plastics, and there are
available plastic
syringe barrels with moulded or bonded hypodermic needles. Hitherto, there
have not
been any successful combinations of pre-filled syringe with a bonded-in needle
and a
safety device to protect the user from suffering a needle-stick injury, and it
is to this
requirement the present invention is directed. One of the main reasons for the
lack of
commercial success is that the proposed new devices often include drug contact
materials which do not have a safety and compatibility record, or have clumsy
operating procedure, are too big, or incompatible with common filling
techniques and
so forth.
As stated hereinabove, there have been no successful safety needle and pre-
filled
syringe combinations, and the challenge is to meet the strict requirements of
various
sterilising methods, maintaining sterility of the drug and needle during
storage,
preventing loss of drug through thermal expansion, ease of use, and low cost.
Accordingly, the present invention provides a safety needle accessory
comprising a
hub for surrounding a hollow needle having a tip and having a connector for
attachment to a syringe, a slidable sleeve adapted to slide over the needle in
a first
longitudinal direction from a first position in which the needle is fully or
partially
covered by the sleeve to a second position in which the needle is exposed, and
in a
second longitudinal direction from the second position to a third position in
which the
needle is fully covered by the sleeve, and a pack surrounding the hollow
needle, hub
and slidable sleeve having a closed end covering the needle and an open end
exposing
the connector of the hub, wherein the safety needle accessory further
comprises a first
seal attached to the connector of the hub and a second seal between the hub
and the
paclc.
The present invention also provides an injection device comprising a syringe
having a
hollow needle attached thereto and a safety needle accessory as defined
herein.
In a first preferred embodiment, a safety device as described in our co-
pending patent
application WO 2004/071560 is assembled to a syringe (i.e. barrel and plunger)
having a bonded hypodermic needle, over which is fitted the safety device. A
seal
between the syringe and hub of the safety needle accessory is provided by a
soft
2
CA 02598927 2007-08-22
WO 2006/090118 PCT/GB2006/000528
polymer. A pack encloses the syringe and safety device assembly, and has a
rubber
seal which seals against the periphery of the safety device hub, and further
has a
rubber seal which seals the opening of the hypodermic needle.
In a second preferred embodiment, which is similar to the first, the seal
between the
hub of the safety needle accessory and connector also seals directly to the
pack, and
the hypodermic needle seal is provided as before.
As an alternative to the previous embodiments, in a third embodiment, the
safety
device is bonded to the outlet connector of a syringe.
The present invention will now described with reference to the following
drawings, in
which:
Fig. 1 shows a longitudinal section on the centreline of a syringe and safety
needle
accessory in accordance with an embodiment of the present invention;
Figs. 2 and 3 show an overall view of the syringe and safety needle accessory
in
accordance with the present invention with the pack attached and detached,
respectively;
Fig. 4 shows a longitudinal section on the centreline of a syringe and safety
needle
accessory in accordance with a further embodiment of the present invention;
and
Fig. 5 shows a similar assembly to those illustrated in the other drawings in
which the
safety device is bonded to the syringe with an adhesive.
Unless stated otherwise, like parts are given lilce notation.
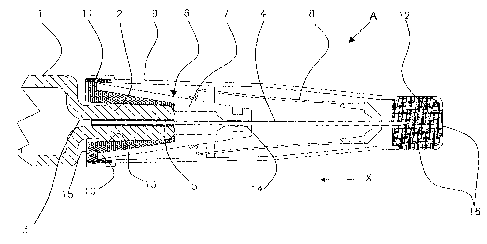
Fig. 1 shows an injection device A having a syringe 1 (only part of the
syringe barrel
is shown) having a connector 2. Connector 2 has a hole 3, into which is bonded
with
an adhesive 5 a hollow needle 4 permitting fluid communication between the
syringe
and the hollow needle 4. The syringe is often made from borosilicate glass,
and the
stainless steel needle may be bonded with an ultra-violet cured adhesive, this
being a
typical construction in common use. The safety needle accessory 6, which is
more
fully described in WO 2004/071560, has a hub 7 which surrounds the connector
2,
and is fitted with slidable sleeve 8, although any other safety shield could
be used.
The sleeve 8 is arranged to operate when the needle device is pushed onto the
patient's
3
CA 02598927 2007-08-22
WO 2006/090118 PCT/GB2006/000528
skin, by sliding along the hub in the direction of arrow X. When the device is
withdrawn from the patient, the sleeve 8 slides back down the hub 7 until it
locks in a
position to prevent a needle stick injury. There are a number of devices which
have
been disclosed which achieve the same objective of preventing needle stick
injuries,
and the present invention may be adapted to suit the features peculiar to
those devices,
to achieve the same end.
In the present invention, the hub 7 has a hole 14 through which the needle 4
passes
with clearance. Hub 7 has an elastic seal 10 which engages sealingly with the
connector 2. The connector 2 has a ridge 13 around its circumference, which
locally
increases the sealing force on the seal 10, and preferably prevents easy
removal of the
hub 7 and seal 10 from the connector 2. Thus, the elasticity of the seal
provides
frictional contact between the seal and the other component (here the
connector on the
syringe) such that a barrier to the ingress of bacteria and pyrogens is
created. To
achieve low manufacturing cost, the hub 7 may be made from a plastics
material, and
seal 10 is preferably co-injected moulded into the hub 7. This process will
ensure
bonding of the seal 10 to the hub 7, and thus prevent the passage of harmful
bacteria
at the junction. Alternatively, and not shown, the seal 10 may be moulded as a
separate component, and may feature suitable ribs to engage the hub 7. To
complete
the protection of the drug and needle, a paclc 9 is fitted over the safety
device/syringe
assembly. The pack 9 has an elastic seal 11 which seals onto the rim 15 of
seal 10. In
addition, a third seal 12 may be located in the closed end of the pack 9, i.e.
the end
distal to the syringe, and seals the tip of the needle 4. The third seal 12 is
typically an
elastic seal and sealing is effected by the needle being position such that
the tip
penetrates the seal 12. The third seal prevents loss or contamination of the
injectate.
Preferably, the pack 9 has one or more holes 16 sealed by the elastic seal 12,
but
through which sterilising gas may permeate. Seal 12 prevents the contents of
the
. syringe 1 from leaking during thermal expansion. The pack 9 is preferably
moulded
in an inexpensive pharmaceutical grade of polyethylene or polypropylene, and
the two
seals 11 and 12 are preferably co-injection moulded to the pack 9.
Alternatively, and
not shown, both seals 11 and 12 may be made as separate components with the
necessary ribs and retaining features to ensure sealing and retention when
fitted to the
pack 9.
4
CA 02598927 2007-08-22
WO 2006/090118 PCT/GB2006/000528
The seal material for the first or second seal 10 and 11 may be low-density
polyethylene or a rubber, and the third seal 12 is preferably a pharmaceutical
grade of.
sealing rubber such as iso-butyl rubber. This material is often used because
it is a
gas-permeable material which permits the passage of a sterilising gas, such as
ethylene oxide, but prevents the passage of harmful bacteria and pyrogens. A
common way of bulk packing syringes for pre-f 11ing is to load them into holes
in a
plastic tray, so that the syringes hang by the finger flange. The loaded tray
is placed
into a plastic tub, and sealed by a permeable membrane. The sealed tub is
subjected
to a sterilising gas such as ethylene oxide, and then, after a period,
ordinary
atmosphere permeates through the membrane to displace the sterilising gas, and
the
membrane prevents the passage of bacteria. During this process, the
sterilising gas
also permeates through the rubber seals that protect the needle.
When it is preferable to avoid using ethylene oxide or other sterilising gas
sterilise the
assembly, other techniques such as by gamma radiation may be used, and since
the
sealing materials are not required to be permeable, may be made from other
resilient
or confonnable materials, such as polyester elastomers.
The assembly is shown complete in Fig. 2, and in use, the pack 9 is withdrawn
from
the syringe/safety needle assembly, Fig. 3, and the injection is given. By
incorporating the first and second seals 10 and 11, the ingress of
contaminants into the
pack 9 is prevented. The first seal 10 provides a seal between the connectors
of the
syringe 1 and hub 7 when the syringe is attached. The second seal 11 provides
a seal
between the hub 7 and the interior rim 15 of the open end of the pack 9 when
the pack
is positioned over the hub 7.
Fig. 4 shows a very similar assembly, but in this case, the first and second
seals 10
and 11 are formed of a unitary structure 10' which also seals on the inside of
the pack
9, thus reducing the cost of the seals, and reducing the number of potential
lealcage
paths. As an alternative to a unitary structure, the first and second seals
are formed
separately but are in mutual contact.
Where the accessory is supplied separately from the syringe, the open end of
the pack
may be covered by a releasable membrane or cap.
5
CA 02598927 2007-08-22
WO 2006/090118 PCT/GB2006/000528
Fig. 5 shows yet another alternative method of sealing the hub 7 to the
connector 2 by
an adhesive 17. Thus, in this embodiment the first seal 10 is an adhesive 17.
The
adhesive may be coated on the hub 7 and adheres to the syringe when the
syringe
connector is engaged with the hub connector. Preferably, the injection device
is
supplied with a pre-filled syringe adhered to the safety needle accessory. A
suitable
adhesive would be UV-cured or other rapidly setting adhesive compatible with
the
materials of construction.
The present invention is compatible with current pre-filled syringe
technology,
including pre-filled reconstitution syringes, whereby a solvent is caused to
mix with a
lyophilised drug prior to administration.
In all embodiments, when the pack 9 is removed, the safety device remains on
the
syringe. The present invention is not limited to a precise mechanism of
operation of
the slidable sleeve. However, the slidable sleeve adapted to slide over the
needle in a
first longitudinal direction from a first position in which the needle is
fully or partially
covered by the sleeve to a second position in which the needle is exposed, and
in a
second longitudinal direction from the second position to a third position in
which the
needle is fully covered by the sleeve. Preferably the slidable sleeve has an
elastically
deformable portion and/or further comprises an elastically deformable member
such
that as the slidable sleeve is caused to move in the first direction towards
the second
position as the needle is injected into a patient, a resultant force is
generated in the
deformable portion or deformable member which causes the slidable sleeve to
move
towards the third (and first) position when the needle is removed from the
patient, the
safety needle accessory further comprising a locking mechanism capable of
retaining
the slidable sleeve in the third position after removal of the needle from the
patient.
The accessory may also further comprise engageable portions on the slidable
sleeve
and the hub to hold the sleeve in the first position where the sleeve is
partially
retracted and, when assembled with a syringe having a hollow needle, the
needle is
partially exposed. This allows the tip of the needle to be seen by the user
prior to
injection. This assists the user in guiding the needle for placement on the
patient's
skin or aspirating excess drug or air. Preferably the injection device of the
present
invention is supplied prior to use with the needle tip partially exposed. The
pack is
6
CA 02598927 2007-08-22
WO 2006/090118 PCT/GB2006/000528
then removed and the needle injected into the patient. Further details are
described in
WO 2004/071560. Other safety devices intended to prevent or reduce needle-
stick
injuries, and which may employ a resiliently biased sliding sleeve (see, for
example
US 4,813,940 and US 5,104,384) may also be used with the accessory of the
present
invention.
7