Note: Descriptions are shown in the official language in which they were submitted.
.CA 02599083 2009-11-13
1
SYSTEMS AND METHODS FOR MAPPING AND MARKING THE THICKNESS OF
BIOPROSTHETIC SHEET
The present is a divisional of Canadian Patent no. 2,398,513 which has a
filing date of
February 16, 2001.
Field of the Invention
The present invention relates to systems and methods for measuring the
thickness of sheet-
like bio-materials and, in particular, to an improved pericardial tissue
mapping and marking system
and methods therefore, especially for measuring tissue to be used for making
prosthetic heart valve
leaflets.
Background of the Invention
Prosthetic heart valves are used to replace damaged or diseased heart valves.
In vertebrate
animals, the heart is a hollow muscular organ having four pumping chambers:
the left and right atria
and the left and right ventricles, each provided with its own one-way valve.
The natural heart
valves are identified as the aortic, mitral (or bicuspid), tricuspid, and
pulmonary valves. Prosthetic
heart valves can be used to replace any of these natural valves. The two
primary types of prosthetic
heart valves known in the art are mechanical valves and bio-prosthetic valves.
Bio-prosthetic
valves may be formed from an intact, multi-leaflet porcine (pig) heart valve,
or by shaping a
plurality of individual leaflets out of bovine pericardial tissue or other
materials, and combining the
leaflets to form the valve. The present invention provides systems and methods
for assessing and
preparing material for leaflets in bio-prosthetic valves.
The pericardium is a sac around the heart of vertebrate animals, and bovine
(cow)
pericardium is commonly used to make individual leaflets for prosthetic heart
valves. The bovine
pericardium is first harvested from the animal and then chemically fixed to
crosslink collagen and
elastin molecules in the tissue and increase the tissue durability, before
being cut into leaflets.
Various physical characteristics of the tissue may be examined before or after
fixation. One
drawback faced by a patient having an implanted bio-prosthetic heart valve is
the potential for
calcification of the leaflets if the valve remains in place for an extended
period of time (more than
ten years). Calcification tends to make the leaflets less flexible. A
significant amount of research
has been accomplished in mitigating calcification of bovine pericardial
leaflets to lengthen the
useable life of
CA 02599083 2007-09-10
2
the heart valve. Calcification may reduce the performance of the heart valve,
and
thus, the highest quality materials and design in the heart valve is required
to
forestall a failure of the valve from excessive calcium deposits.
One aspect of designing heart valves which is very important in improving
their performance is the selection of the pericardial tissue used in the
leaflets. In all
heart valves, the natural action of the flexible heart valve leaflets, which
seal against
each other, or co-apt, is desirable. The difficulty in simulating the leaflet
movement
of an actual heart valve (especially a mitral valve) in a prosthetic valve is
that the
leaflets used are "inanimate." There are no muscular attachments to the
leaflets as in
the natural valve, and the prosthetic leaflets must co-apt to function
properly solely
in response to the fluid pressures within the heart chambers. Indeed, natural
coaptation of the leaflets in bio-prosthetic valves comprising a plurality of
individual
leaflets sewn together is particularly difficult, even when compared to
inanimate but
intact valves, such as harvested porcine valves.
Despite the drawbacks of artificial heart valve material, over twenty years of
clinical experience surrounding implanted artificial heart valves has produced
a
proven track record of success. Research in extending the useful life of the
bio-
prosthetic valves continues, however. Much of this research involves the
mechanical properties of fresh or fixed bovine pericardium.
A good discussion of the various physical properties of fixed bovine
pericardium is given in Simionescu, et al, Mapping of Glutaraldehyde-Treated
Bovine Pericardium and Tissue Selection For Bio-prosthetic Heart Valves,
Journal
of Bio-Medical Materials Research, Vol. 27, 697-704, John Wiley & Sons, Inc.,
1993. Simionescu, et al., recognized the sometimes striking variations in
physical
properties of the pericardial tissue, even in the same pericardial sac. Their
research
mapped out areas in individual pericardial sacs and tested those areas for
various
properties to determine the optimum area on the tissue from which to cut heart
valve
leaflets. Simionescu, et al. measured the thickness of the pericardial sacs at
5 mm
increments and plotted the resulting values on a paper template identical in
shape
and size to the sac. On other templates, parameters such as the suture holding
power, fiber orientation, and shrinkage temperature were mapped. After
superimposing all of the templates, optimum areas from which to cut leaflets
were
CA 02599083 2007-09-10
3
identified. Simionescu, et. al., utilized a manual thickness measuring tool
similar to
that described below with respect to Figure 1.
A number of steps in a typical commercial process for preparing pericardial
tissue for heart valve leaflets is illustrated in Figure 1. First, a fresh
pericardial sac
20 is obtained from a regulation slaughterhouse. The sac 20 is then cut open
along
predetermined anatomical landmarks, as indicated at 22. The sac is then
flattened at
24 and typically cleaned of excess fat and other impurities. After trimming
obviously unusable areas, a window 26 of tissue is fixed, typically by
immersing in
an aldehyde to cross-link the tissue, and then quarantined for a period of
about two
weeks. Rough edges of the tissue window 26 are removed and the tissue bio-
sorted
to result in a tissue section 28. The process of bio-sorting involves visually
inspecting the window 26 for unusable areas, and trimming the section 28
therefrom.
Subsequently, the section 28 is further cleaned as indicated at 30.
The section 28 is then placed flat on a platform 32 for thickness
measurement using a contact indicator 34. The thickness is measured by moving
the
section 28 randomly around the platform 32 while a spindle 36 of the indicator
34
moves up-and-down at various points. The thickness at each point is displayed
at 38
and recorded mentally by the operator. After sorting the measured sections 28
by
thickness, as indicated at 40, leaflets 42 are die cut from the sections, with
thinner
leaflets 42 generally being used for smaller valves, and thicker leaflets
being used for
larger valves. Of course, this process is relatively time-consuming and the
quality of
the final leaflets is dependent at several steps on the skill of the
technician.
Moreover, the number of leaflets obtained from each sac is inconsistent, and
subject
to some inefficiency from the manual selection process.
More recently, Baxter International Inc. has added a sophisticated leaflet
selection method into its tissue valve manufacturing process. The method
includes
applying a load to each leaflet, as opposed to pericardial tissue in bulk, and
recording
the strain response. The results of the load test in combination with a droop
test can
be used to group similar leaflets. Such a method is disclosed in U.S. Patent
No.
5,961,549 to Huynh, issued October 5, 1999, and entitled, "PROSTHETIC HEART
VALVE LEAFLET SELECTION METHOD AND APPARATUS". Although this
method improves the quality of the resulting combination of leaflets, because
of the
existing inefficiencies in the process of supplying tissue from which to cut
the
CA 02599083 2007-09-10
4
leaflets, the subsequent filter of leaflet selection further reduces the total
usable
leaflet output such that costs are increased.
Despite much research into the characteristics of bovine pericardium and
leaflets, there remains a need for a system and method for rapidly and
reliably
characterizing material, especially pericardial tissue, for use in fabricating
heart
valve leaflets.
Summary of the Invention
The present invention provides a method of measuring the thickness of a
bio-material sheet for use in bioprostheses, such as heart valves, grafts, and
the
like. The method involves mapping the thickness of the sheet and marking the
sheet into areas or zones of similar thickness. The measuring, mapping, and
marking steps can all be carried out automatically with a system that receives
the
sheet and translates it under a measurement head and a marking head, with the
mapping function being performed by a connected computer and associated
software. In a preferred embodiment, the bio-material sheet is bovine
pericardium
and from which heart valve leaflets are to be cut. The method further may
include
providing input as to a preferred thickness needed, and selecting the zones
based
on that input to maximize the preferred thickness marked.
In one aspect of the invention, a method of measuring the thickness of a
bio-material sheet comprises first flattening the sheet on a sanitary surface,
simultaneously measuring the thickness of a plurality of points on the
flattened
sheet, and automatically recording the measured thicknesses of the plurality
of
points. The step of simultaneously measuring desirably includes measuring at
least
three points, and more preferably at least ten points, on the flattened sheet.
Further, the step of simultaneously measuring may occur more than once,
wherein
the plurality of points in each step of simultaneously measuring is arrayed
along a
line, and wherein each line is spaced from the line in a preceding or
subsequent
step of measuring so as to obtain a two-dimensional array of measured points
on
the sheet.
In another aspect of the invention, the method may further include
providing a measurement head positioned normal to the surface, and relatively
displacing the surface and measurement head in a direction parallel to the
surface
between each successive step of simultaneously measuring. A base may be
CA 02599083 2007-09-10
provided upon which both the surface and measurement head are mounted, and the
step of relatively displacing may comprise translating the measurement head
relative to the base between each successive step of a simultaneously
measuring.
Desirably, a programmable controller controls movement of the measurement
5 head.
The step of simultaneously measuring may include simultaneously
contacting a plurality of points on a surface of the sheet facing away from
the
surface, preferably with a plurality of coil-driven shafts and monitoring the
position of each shaft. Or, the step of simultaneously contacting includes
simultaneously contacting the surface of the sheet with a plurality of free-
sliding
pins and monitoring the position of each pin.
In another aspect, the present invention provides a method of mapping the
topography of a bio-material sheet by first providing a measuring system
including
a sanitary surface and a measurement head positioned normal to and spaced from
the surface, wherein the measurement head includes a plurality of sensors
adapted
to measure distance along spaced axes normal to the surface. The sheet of bio-
material is flattened on the surface, and the thickness of the sheet at a
plurality of
points is measured using the sensors. The thickness data is then used to
create a
topographical map of the sheet. The method, further may include marking the
sheet to indicate the thickness of the plurality of points corresponding to
the
topographical map. Also, areas of different thickness may be marked on the
sheet.
In a preferred embodiment, the sheet is bovine pericardium and the step of
marking
areas of different thicknesses includes identifying discrete zones of similar
thickness that are large enough from which to cut a heart valve leaflet. The
method may involve controlling the marking with a computer, supplying the
computer with information regarding a preferred thickness of heart valve
leaflet,
and controlling the marking based on the preferred leaflet thickness
information so
as to maximize the number of discrete zones of the preferred leaflet thickness
that
are marked.
In a still further aspect, the invention provides a method of automated
mapping of a bio-material sheet to indicate discrete zones from which to cut
heart
valve leaflets, comprising measuring the thickness of a plurality of points on
a
flattened sheet, automatically recording the measured thicknesses of the
plurality
.CA 02599083 2009-11-13
6
of points, and using the recorded thicknesses to mark discrete zones of the
sheet that are large
enough from which to cut heart valve leaflets. The method desirably includes
determining an
acceptable thickness range for each of a number of sizes of heart valve
leaflets; and determining
an acceptable minimum size of the discrete zones for each of a number of sizes
of heart valve
leaflets. Where the plurality of points is a two-dimensional array, a
plurality of planar units are
each centered on one of the measured points, and each discrete zone comprises
a plurality of
contiguous planar units. Each discrete zone may be selected so that at least
some of the planar
units within that discrete zone have a measured thickness within the
acceptable thickness range
for the corresponding heart valve leaflet. Finally, the method further may
include marking the
discrete zones on the sheet so as to maximize the number of discrete zones of
the preferred leaflet
thickness that are marked.
The present invention in particular provides a method of inventory control of
a bio-material for
use in implants, comprising: providing an inventory supply of a bio-material;
measuring the
thickness of a plurality of points on a plurality of flattened elements of bio-
material from the
inventory supply; inputting the measured thicknesses to a computer, providing
the computer with
data regarding a preferred thickness of the flattened elements of bio-material
for the implant; and
cutting the flattened elements of bio-material into discrete zones based on
the measured thickness
and preferred thickness data so as to control the number of discrete zones of
the preferred
thickness that are cut from the inventory supply.
In accordance with the present invention a method is provided which may
further include:
providing a human-machine interface enabling the computer to be manually
supplied with a value
of a preferred thickness of the flattened elements of bio-material for the
implants; and providing
software loaded on the computer to analyze the measured thickness information
and identify
discrete areas of similar thickness on the flattened elements of bio-material,
the software being
configured to maximize the number of discrete zones of preferred thickness
identified.
The present invention also provides a method of measuring a bio-material for
use in an implant,
comprising: providing a bio-material; flattening the biomaterial on a sanitary
reference surface;
measuring the thickness of a plurality of points on the flattened bio-
material; inputting the
measured thicknesses to a computer; and running software on the computer that
identifies
preferred areas on the bio-material to use in the implant based on the
measured thicknesses.
'CA 02599083 2009-11-13
6a
In accordance with the present invention a method is provided which may
further include:
providing a human-machine interface enabling the computer to be manually
supplied with a value
of a preferred thickness of the bio-material for the implant; and wherein the
preferred areas are of
similar thickness, and the software is configured to control the number of
preferred areas
identified.
A system for measuring the thickness of a bio-material sheet is also provided,
comprising a base
adapted to be fixed with respect to a support floor, a sanitary platen mounted
on the base, and a
measurement head mounted on the base and positioned normal to and spaced from
the platen. The
measurement head includes a plurality of sensors adapted to measure distances
along spaced
measurement axes disposed normal to the platen, and the sensors are adapted to
measure the
thickness of a bio-material sheet that has been placed on the platen. The
system may further
include a movable carriage on which is defined the platen, and a first
mechanism configured to
relatively displace the platen and measurement head across the platen to
enable each sensor to
measure the thickness of the sheet at more than one point. Desirably, the
platen defines a planar
surface on which the bio-material sheet is measured, and the first mechanism
enables relative
linear translation of the planar surface and measurement head, preferably
relative to the base
along a first axis parallel to the planar surface. A second mechanism may be
provided to
relatively displace the planar surface and measurement head along a second
axis parallel to the
planar surface and perpendicular to the first axis, and desirably the second
mechanism translates
the planar surface relative to the base along the second axis. A third
mechanism may permit
relative displacement of each of the sensors on the measurement head along the
respective
parallel measurement axes disposed normal to the planar surface.
CA 02599083 2007-09-10
7
In the system as described above, the sensors each preferably include a tip
for contacting a surface of the sheet facing away from the platen. Further,
the third
mechanism desirably includes a plurality of coil-driven shafts, one per
sensor, with
the tips positioned at the end of the shafts, and a position detector for
monitoring
the position of each shaft.
Still another aspect of the invention is a system for topographically
mapping the thickness of a bio-material sheet, comprising:
a measurement head adapted to measure the thickness of a plurality
of points on the sheet;
a computer connected to receive data corresponding to the thickness
of the sheet at the plurality of points; and
software loaded on the computer and configured to analyze the data
and identify discrete areas of similar thickness on the sheet.
The system may also include a marking head for marking the discrete areas
of similar thickness directly on the bio-material sheet. Where the bio-
material
sheet is suitable for forming heart valve leaflets therefrom, the system
further
includes a human-machine interface enabling the computer to be supplied with a
value of a preferred thickness of heart valve leaflet. The software is
configured to
control the marking head to maximize the number of discrete zones of the
preferred leaflet thickness that are marked. Preferably, the human-machine
interface comprises a touch-screen monitor, and the marking head comprises an
ink jet type of dye dispenser.
In a particularly preferred embodiment, therefore, the present invention
provides a three-axis computer-controlled positioning system, an array of
programmable linear actuators, a high-performance dispenser for tissue
marking, a
PC-based data acquisition and processing system, a human-machine interface
(HMI), and a central programmable logic controller (PLC) to control the
overall
system. A thickness measurement is made by placing the tissue sample on a flat
stainless-steel measurement plate. Mechanical holders may or may not be used
to
retain the tissue sample on the plate. The thickness of the tissue sample is
determined by touching the tissue with the actuator rod in a raster pattern
across
the surface of the sample. A three-axis motion system is used to translate the
CA 02599083 2007-09-10
8
linear actuators in one direction (X) while the position of the meaurement
plate
(and thus the sample) is incremented along a second axis (Y). The actuators
and
the dispensing head translate along the third axis (Z) with respect to the
plate for
tissue measurement and/or marking. At each point in the measurement, the
positions of the actuator rods are digitized and stored. Following data
collection,
this information is processed to calculate the thickness of the tissue at each
point in
the measurement process. Based on these measurements, a thickness map is
generated and used to identify tissue thickness areas for tissue zone marking
and
cutting.
A further understanding of the nature and advantages of the invention will
become apparent by reference to the remaining portions of the specification
and
drawings.
Brief Description of the Drawings
Figure 1 illustrates a sequence of prior art steps for preparing and measuring
the thickness of bovine pericardial tissue prior to forming leaflets from the
tissue;
Figures 2A-2F illustrate a sequence of steps of the present invention for
preparing, measuring and mapping the thickness of bovine pericardial tissue
prior to
forming leaflets from the tissue;
Figure 3 shows a series of plan views of a number of sizes of heart valve
leaflets with grid patterns used in the present invention superimposed
thereover;
Figure 4 shows three rectangular areas that are suitable for forming different
sized leaflets;
Figure 5 is a perspective view of an apparatus of the present invention for
measuring and mapping the thickness of sheet-like bio-materials;
Figure 6 is a perspective view of the apparatus of Figure 5 with a number of
upper components removed to illustrate a base portion;
Figures 7A-7C are plan and elevational views of the apparatus of Figure 5;
Figure 8 is a perspective view of an exemplary thickness measuring tool used
in the apparatus of Figure 5;
Figures 9A-9B are front and side elevational views, respectively, of the
thickness measuring tool of Figure 8;
Figure 10 is a perspective view of a platen on which sheet-like bio-materials
are positioned for measurement in the apparatus of Figure 5;
CA 02599083 2007-09-10
9
Figures 11A-11B are plan and elevational views, respectively, of the platen
of Figure 10, with Figure 11 A illustrating a pericardial sac positioned flat
thereon;
Figure 12 is a perspective exploded view of an exemplary measurement tool
cleaning apparatus;
Figure 13 is a schematic view of the various components and
interconnections of the apparatus of Figure 5;
Figure 14 is an image of the main touch screen display for use in operating
the apparatus of the present invention;
Figure 15 is an image of a touch screen display for optimizing the tissue
mapping function of the apparatus of the present invention; and
Figure 16 is an image of a touch screen display for calibrating the apparatus
of the present invention.
Description of the Preferred Embodiments
The present invention provides systems and methods for measuring, mapping
and marking the thickness of a bio-material, in particular a sheet bio-
material. The
term "bio-material" pertains to any material that is suitable for implant in
the human
body, and is synonymous with bioprosthetic material. For example, suitable bio-
materials include, but are not limited to, bovine or other mammalian
pericardium,
biocompatible material such as polyester, synthetic matrices having
collagenous
growth thereon, etc.. Although the invention is described and illustrated in
terms of
an automated system for measuring, mapping and marking a bio-material, various
aspects of the invention could be accomplished by manual means. For example,
existing manual measurement methods could be used to compile the thickness
data
needed for the mapping and marking functions of the system. Indeed, the
measuring, mapping, and marking techniques described herein could all be
accomplished manually. Finally, although the invention is described
specifically in
terms of assessing a sheet of bovine pericardium for forming heart valve
leaflets, the
invention is also suitable for forming other bioprosthetic implants or
components,
including ventricular patches, skin grafts, etc..
CA 02599083 2007-09-10
Measuring and Mapping Steps
With reference to Figures 2A-2F, a sequence of steps in the preparation,
thickness measurement, and mapping of a sac 50 of bovine pericardium is shown.
First, as seen in Figure 2A, the sac 50 is harvested at a regulation
slaughterhouse.
5 Although each sac 50 is unique, certain anatomical characteristics are
shared;
including an apex 52 and a pair of sternopericardial ligaments 54. The sac 50
arrives
from the slaughterhouse in a three-dimensional sac shape, and must be severed
along
a cut line 56 using a scalpel 58, as seen in Figure 2B. The sac is opened up,
as
indicated by the arrows 60 in Figure 2B, and flattened into the configuration
shown
10 in Figure 2C. Figure 2B also illustrates a base 62 which will be used in
conjunction
with the apex 52 to define a base-apex line 64 seen on the flattened sac 50.
The
base-apex line 64 provides an approximate indication of the fiber orientation
of the
sac 50, which will be important during the ultimate step of cutting heart
valve
leaflets (or other structure) from the sac. The pericardial sac 50 is
desirably fixed
with a buffered solution of glutaraldehyde or other fixative, quarantined and
then
cleaned.
Figure 2D illustrates the flattened sac 50 having a measurement grid pattern
66 superimposed thereon. The measurement grid pattern 66 shown comprises a two-
dimensional rectangular array of square units 68, although other grid patterns
could
be used. As will be explained in detail below, a thickness measurement of the
sac 50
is taken at the center point of each of the square units 68 so as to
topographically
map the entire sac. The center-to-center spacing S is seen in Figure 2D and
can be
varied depending on the map resolution desired. In an exemplary embodiment,
the
spacing S is approximately 9.5 mm (0.375 in). The grid pattern 66 shown
encompasses a majority of the sac 50, but does not extend much beyond the
outlines
of sac. Again, as will be seen below, the grid pattern 66 can be widened
further
beyond the sac as desired.
After the thickness of the sac 50 is measured at the center point of each of
the
square units 68, a two-dimensional data grid 70 having a topographical
thickness
map 72 of the sac 50 is produced, as seen in Figure 2E. Again, this data grid
70 and
map 72 can be produced by hand or automatically using computer logic.
Preferably,
as will be explained, the data grid 70 and map 72 are automatically generated
by
software on a computer associated with the physical measurement apparatus. The
CA 02599083 2007-09-10
11
thickness of the sac 50 in each of the square units 68 is transposed on to the
data grid
70 as a color within one of the grid units 74. An exemplary topographical map
72 is
shown, with the various thicknesses of the pericardial sac 50 indicated by
different
color symbols, as explained by the legend. There are four different colors
used
(other than the white border) corresponding to a different thickness or range
of
thickness. Of course, the number of different thicknesses or ranges indicated
could
be more or less than four. The specific thicknesses or ranges corresponding to
each
of the different colors will be further detailed below.
Figure 2F illustrates a subsequent step in the process of mapping the
pericardial sac 50 in preparation for cutting heart of leaflets therefrom.
Specifically,
zones 80a, 80b, 80c are depicted corresponding to contiguous grid units 74 of
the
same or similar thickness. Each of the zones 80 is delineated by a zone border
82
and by a zone indicator 84. In the illustrated example, the zone indicators 84
are the
letter symbols A-C corresponding to the three usable thicknesses.
With reference to Figure 3, a number of 2 x 4 or 4 x 5 arrays of grid units 74
are shown with the outlines 86 of various sizes of heart valve leaflets
superimposed
thereupon. Each leaflet outline 86 includes an arcuate cusp edge 88, a
coapting edge
90, and a pair of oppositely-directed commissure tabs 92 separating the cusp
and
coapting edges.
These illustrations show how many grid units 74 are needed to form an area
from which a particular size leaflet may be cut. For example, a 27 mm leaflet
requires an area defined within a 4 x 5 array of grid units 74. Of course, as
explained
above, the size of the grid units 74 can be varied, and thus the number of
grid units
within each required array can be varied. In the present embodiment, each of
the
grid units 74 is a square with sides of about 9.5 mm (0.375 in.), and thus the
array of
grid units needed to form a 27 mm leaflet has size of about 38.0 mm x 47.5 mm
(1.5
in. x 1.875 in.).
The desired thickness of pericardium for heart valve leaflets varies with the
size of the leaflets, with smaller leaflets generally being thinner than
larger leaflets.
Although the overall area on the pericardial sac 50 needed to cut a particular
size
leaflet is seen in Figure 3, the entire area need not be the desired thickness
of the
leaflet. Figure 4 illustrates preferred patterns 94 of pericardium from which
to cut
various sized leaflets. Specifically, the left pattern is for 19, 21, and 23
mm leaflets,
CA 02599083 2007-09-10
12
and the right pattern is for 25, 27, 29, 31, and 33 mm leaflets. These
patterns are
derived by superimposing the leaflet shapes over the arrays of grid units as
indicated
in Figure 3 and determining the size of the mid-portion of each leaflet
relative to the
respective array. It is believed that as long as the mid-portion of each
leaflet is the
desired thickness that it will perform adequately. That is, the mid-portion of
each
leaflet is generally defined by the area within the cusp edge 88 and by
extension of
the cusp edge to the coapting edge 90. The commissure tabs 92 are typically
folded
and sutured around structural commissure posts within the heart valve, and the
thickness thereof is deemed less important.
An interior region 96 of each pattern 94 comprises a regular array of whole
grid units 74, while a peripheral region 98 need not correspond to whole grid
units.
The interior region 96 has a thickness that corresponds to the preferred
thickness of
the particular leaflet being cut, while the thickness of the peripheral region
98 may or
may not be the same thickness. The dimensions x1, x2, yl, and y2 for the
interior
regions 96 and overall pattern 94, are illustrated, and exemplary values are
given
below in TABLE I.
TABLE I -
PREFERRED PATTERN DIMENSIONS FOR DIFFERENT SIZED LEAFLETS
LEAFLET THICKNESS THICKNESS X1 X2 Y1 Y1
SIZE RANGE OF RANGE OF mm, mm, mm, mm,
(mm) INTERIOR PERIPHERAL (inch) (inch) (inch) (inch)
REGION 96 REGION 98
mm, (inch) mm, (inch)
19, 21, 23 0.345-0.470, 0.318-0.648, 19.05, 38.1, 19.05, 19.05,
(Aortic) (.0136-.0185) (.0125-.0255) (0.75) (1.5) (0.75) (0.75)
25, 27, 29 0.447-0.546, 0.419-0.648, 28.58, 47.63, 19.05, 28.58,
(Aortic (.0176-.0215) (.0165-.0255) (1.125) (1.875) (0.75) (1.125)
and
Mitral)
31, 33 0.523-0.597, 0.495-0.648, 28.58, 47.63, 19.05, 28.58,
(Mitral) (.0206-.0235) (.0195-.0255) (1.125) (1.875) (0.75) (1.125)
CA 02599083 2007-09-10
13
Exemplary Measuring and Mapping System
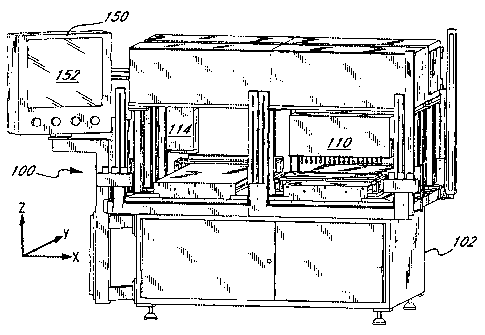
Figures 5, 6 and 7A-7C illustrate an exemplary automated system 100 for
measuring, mapping, and marking a sheet of bio-material in accordance with the
present invention. The system is designed to receive sheet-like bio-material
in a
variety of configurations, such as the flattened sac 50 seen in Figure 2C, and
output a
sheet having specific markings thereon corresponding to the implant or
prosthetic
component being produced. Alternatively, as mentioned above, one or more of
the
measuring, mapping, and marking functions may be performed elsewhere either
manually or with the assistance of a further automated mechanism.
The system 100 generally comprises a base 102 and a plurality of
mechanical, electrical, and optical subsystems mounted thereon. The base 102
is a
relatively sturdy rectilinear structure, and is illustrated separately in
Figure 6 with a
number of operating components removed therefrom. The base 102 defines a
horizontal table 104 over which the bio-material sheets translate and are
measured.
The table 104 is rectangular and a plurality of upstanding light curtain
columns 106
are mounted at each corner, and at a midpoint along one side. The columns 106
generate planar optical safety curtains when the system 100 is in operation
which,
when broken, trigger an automatic shutoff function. In this manner, the system
100
will not operate when a user's hand is within the rectilinear volume defined
within
the columns 106. Figure 6 also illustrates a plurality of on/off operating
switches
108 conveniently disposed at each corner of the table 104. Finally, coordinate
axes
are shown in Figure 6 corresponding to the three primary orthogonal
directions. The
two-dimensional illustrations of Figures 7A-7C also include their respective
coordinate axes.
The system 100 has two main operating subsystems, a measurement
subsystem and a marking subsystem. The measurement subsystem comprises a
measurement head 110 supported to translate above the table 104 and along the
X-
axis, as indicated by the arrow 112 in Figure 7A. The marking subsystem
comprises
a marking head 114 also supported to translate above the table 104 and along
the X-
axis, as indicated by the arrow 116. The respective mechanisms for supporting
and
linearly translating the measurement head 110 and marking head 114 are
contained
within housings 118, 120, as seen in Figures 7A and 7C. The mechanisms within
the housings 118, 120 are not shown, and may take a number of conventional
forms,
CA 02599083 2007-09-10
14
including a preferred form of a linear slide and a motorized threaded rod
combination. For example, a motor 122 shown in Figure 7B projecting from the
left
side of the housing 118 has an output shaft that rotates about the X-axis and
turns a
threaded rod for translation of the measurement head 110. Likewise, a motor
124
extends from the left side of the housing 120 and turns a threaded rod to
translate the
marking head 114. The housings 118,120 are, in turn, supported above the table
104
on legs 126. The particular structure and functions of the measurement and
marking
subsystems will be described in more detail below.
The table 104 includes a pair of channels 130 as seen in Figure 6 extending
from one edge to the other of the table along the Y-axis. The channels 130
receive
mechanisms for linear translation of a pair of workstations or carriages 132,
which
are described in detail below with respect to Figures 10 and 11. The carriages
132
each defined thereon a work surface 134 that serves as a work platform for
measuring the thickness of the bio-material sheet. Figure 7B illustrates one
of the
work surfaces 134 and its direction of movement 136 along the Y-axis. Again,
the
mechanisms for translation of the carriages 132 are not shown, although a
preferred
form includes a linear slide and motorized threaded rod combination. In this
regard,
a pair of motors 138 are shown projecting from the side of the base 102, which
motors include output shafts that rotate about the Y-axis and turn threaded
rods for
translation of the carriages 132.
With reference now to the plan view of Figure 7B, a first track 140a and a
second track 140b are defined, respectively, for the two carriages 132 along
the
extent of their travel in the Y-axis. Both tracks 140a, 140b extend the entire
width of
the table 104 and intersect three distinct workstations. Specifically, a load
station
142 is defined at the lower portion of Figure 7B by a portion of the table 104
that is
exposed from underneath either housing 118 or 120. In addition, a measurement
station 144 is defined below the measurement head 110, and a marking station
146 is
defined below the marking head 114, for each track 140. The carriages 132
shuttle
along their respective tracks 140a, 140b from the load station 142 to the
measurement station 144, from there to the marking station 146, and then back
to the
load station.
The various subsystems of the automated system 100 are actuated, monitored
and coordinated through a programmable controller, as will be more fully
explained
CA 02599083 2007-09-10
below. Various inputs to the controller are supplied via a human-machine
interface
150, which in the illustrated embodiment comprises a computer monitor having a
touch screen 152. The monitor 150 is conveniently mounted on a stanchion 154
at
one corner of the base 102.
5 Figures 8 and 9A-9B illustrate various details of the measurement head 110
of the present invention. The operational portion of the measurement head 110
comprises a plurality of sensors 160 arrayed in a line and directed downward
in the
Z-axis. The sensors 160 may take a variety of forms, but can generally be
categorized into those sensors that contact the bio-material and those that do
not.
10 That is, contact sensors are designed to produce a signal upon contact with
the bio-
material that, in combination with knowledge of the relative height of the
sensor
above the work surface 134, produces the thickness of the bio-material. Non-
contact
sensors, such as infrared or laser sensors, emit an electromagnetic wave or
optical
beam toward the bio-material and detect the thickness thereof from the
reflected
15 wave or beam. The present invention encompasses any sensor that can detect
the
thickness of a material relative to a reference surface on which the material
is placed.
In a presently preferred embodiment of the invention, the sensors 160
comprise linear actuators 162 that displace a shaft 164 having a tip 166 into
contact
with the bio-material. With knowledge of the position of the shaft 164 upon
contact
of the tip 166 with the bio-material, the linear actuator produces an
electronic signal
corresponding to the thickness of the bio-material at that point. The linear
actuators
162 are supported on a platform 168 having apertures therethrough for the
shafts
164. The platform 168 is suspended on a frame 170 underneath a mechanism for
translating the measurement head 110. Specifically, a slide plate 172 is
adapted to
translate within a corresponding groove (not shown) fixed with respect to the
base
102 and an internally threaded screw block 174 travels along the
aforementioned
motorized threaded rod actuated by the motor 122 (Figure 7B). The moving
measurement head 110 communicates with the rest of the system 100 via a cable
carrier 176, or similar expedient.
As mentioned, the sensors 160 are aligned in a linear array in parallel with
the X-axis to form a row of sensors. Desirably, there are at least two sensors
160 to
speed up the measurement and mapping function of the system 100, and
preferably
there are at least three sensors, with at least ten being most preferred. The
illustrated
CA 02599083 2007-09-10
16
embodiment includes eighteen sensors 160 spaced apart a distance S1. In this
configuration, therefore, eighteen separate points on a bio-material spaced
apart a
distance S 1 can be simultaneously measured by the measurement head 110 (a row
of
measurements). As will be explained below, relative displacement between the
bio-
material and the measurement head 110 in the Y-axis enables measurement of a
second row and subsequent rows of eighteen points, which results in a two-
dimensional array of thickness measurements. Each sensor 160 thus measures a
column of points in the Y-direction.
The distance S 1 between the sensors 160 may be equal to or greater than the
center-to-center spacing S of the grid units 68 in the grid pattern 66 shown
in Figure
2D. Desirably, the distance Si is an even multiple of the spacing S so that
more than
one column of measurements along the Y-axis is made, each column being offset
from the adjacent column by the grid spacing S. In a preferred embodiment, the
distance S1 is 28.6 mm (1.125 in) and the spacing S is 9.5 mm (0.375 in), so
that
three columns of offset measurements are made.
Of course, other arrangements of sensors 160 may be used to produce a two-
dimensional array of thickness measurements. For instance, the relative
displacement between the measurement head 110 and the bio-material may be
other
than linear as disclosed herein, such as rotational. Alternatively, the
sensors 160
may be arranged in a two-dimensional array, as opposed to being in line. In
the
latter arrangement, a single measurement taken by the measurement head results
in a
two-dimensional array. Those of skill in the art will therefore understand
that there
are variety of sensor configurations and measurement techniques within the
scope of
the present invention for producing a two-dimensional array of thickness
measurements.
It should also be noted at this point that although the system 100 is
illustrated
as being especially suitable for measuring and mapping a planar sheet of bio-
material, it is contemplated that the bio-material may be other than planar,
such as
tubular. Also, in this respect, the term "flatten" the sheet on the work
surface should
not be construed to imply a planar work surface. As an example of an other
than
planar work surface, the tubular bio-material may be mounted on a cylindrical
mandrel with the measurement head 110 adapted to rotate therearound to measure
the thickness of the tube and produce a three-dimensional topographical map.
CA 02599083 2007-09-10
17
Likewise, mapping of bioprosthetic surfaces that are defined on three-
dimensional
objects other than sheet substrates is also possible with modification of the
apparatus
of the present invention. For example, the free-sliding pin type of sensor may
be
used to accurately measure more pronounced topographical changes, much like
the
familiar desktop novelty having an array of free-sliding pins mounted in a
frame. In
short, other arrangements are possible, and the invention should not be
considered
limited to measuring planar or even sheet substrates.
Figures 10 and 11A-11B illustrate details of the carriage 132 of the present
invention for supporting the sheet-like bio-material, such as a flattened
bovine
pericardium sac 180. The carriage 132 comprises a generally hollow frame 182
supporting a rectilinear platen 184 thereon. The upper surface of the platen
184
defines the work surface 134 previously mentioned. The work surface 134 on
which
the sheet-like bio-material is measured is microbiologically clean and
sanitary to
inhibit contamination of the material. The sheet-like bio-material may be
clamped to
the surface 134 to prevent movement using conventional clamps (not shown), but
in
a preferred embodiment, the bio-material is simply laid flat on the surface
and
smoothed down with a wiper device, such as a rubber squeegee-like device. If
bovine pericardium is used, it has been found that the wiping method works
adequately, which reduces the setup time and equipment needed, and also
reduces
the foreign surfaces contacting the pericardium.
An internally threaded screw block 186 is seen underneath the frame 182 in
Figure 11B, which block travels along a motorized threaded rod driven by one
of the
motors 138 (Figure 7B). A calibration bar 188 is secured at one side of the
frame
182 and is generally aligned along the X-axis. The calibration bar 188
includes a
number of stepped calibration surfaces 190, also extending along the X-axis.
The
calibration surfaces 190 provide precision measurements for the sensors 160
during
a calibration process. That is, a series of surfaces 190, including a zero
reference
surface, having known relative elevations is provided on the calibration bar
188.
The elevation values of the surfaces as measured by the array of sensors 160
permits
the user and/or system to detect any non-calibrated or otherwise faulty
sensors. If
such a condition exists, the faulty sensor may be reprogrammed, repaired to
replace a
malfunctioning part, or replaced altogether.
.CA 02599083 2009-11-13
18
The X-axis and Y-axis are indicated in the plan view of Figure 11A. The bovine
pericardium sac 180 is shown oriented with the base-apex line 192 parallel to
the X-axis. In
this manner, the sac 180 is desirably be measured, mapped, and then marked in
a grid pattern
that is either parallel to or perpendicular to the base-apex line 192. Because
the fiber
orientation of the sac 180 is generally known with respect to the base-apex
line 192, cutting
the individual heart valve leaflets with respect to the marked grid pattern is
facilitated.
Figure 12 illustrates a tip cleaning tray 194 and associated tip cleaning
cover 196. A
pair of end mounts 198 permit the cleaning tray 194 to be secured with respect
to the
carriage 132 for cleaning the tips 166 of the sensors 160. That is, each tip
166 extends
through an aperture in the cover 196 into a cleaning solution provided within
the tray 194. A
preferred cleaning regimen will be described below.
Electrical Component Interfaces
Figure 13 schematically illustrates the main electrical components of the
system 100
of present invention, and their interconnections. The system 100 is controlled
primarily
through a programmable logic controller (PLC) 200 that transfers information
back and forth
to a human-machine interface 202 through an ethernet connection 204. The human-
machine
interface 202, in turn, communicates with a plurality of measurement sensors
within a
measurement head 206. Specifically, a communication line 208 (denoted COMI)
from the
human-machine interface 202 connects directly to a code operated switch (COS)
210, which
connects via a plurality of RS 232 cables 212 to each sensor within the
measurement head
206. A digital input/output (1/0) cable 214 transfers information to and from
the PLC 200
and a marking head 216. One or more remote input/output (1/0) cables 218
transfer
information to and from the PLC 200 and a plurality of servo drives 220 used
to translate the
measurement head 206, marking head 216, and workpiece carriages (not shown in
Figure
13). A digital input/output (1/0) cable 222 transfers information to and from
the servo drives
220 and the marking head 216 to turn on and off the ink jet.
Specific examples of these various electrical components will now be given,
with
the understanding that alternative equipment and/or manufacturers could be
substituted. The
programmable logic controller 200 may be an Allen Bradley 5/40E
CA 02599083 2009-11-13
19
(series 5 model 40) with an ethernet port. The HMI 202 may include an IBM-
compatible
computer and a Christensen 18 inch touch-screen monitor model number LSX 18T,
with
ELO* touch screen software. The code operated switch (COS) 210 is available
from Black
Box Corp., of Lawrence, PA. That has 16 serial input communication ports and 1
serial
output port connected to the HMI 202. The sensors 160 within the measurement
head 206
are desirably servo feedback displacement actuators, such as are available
from SMAC
(Carlsbad, CA) as model LAL-37-050-50-DC-MOD, and controlled by SMAC model LAC-
25 two-axis controllers, or their equivalent. The marking head 216 desirably
comprises a
BioDot' (Irvine, CA) ink jet marking pen having a dispensing platform model
BioJet Quanti
3000* and a dispensing head model BioJet BLJ4000*. The "ink" dispensed is
desirably a
toxicity-free reagent or dye. The servo drives that control movement of the
workpiece
carriages, the sensors within the measurement head 206, and the marking head
216, are
desirably made by Allen Bradley of Milwaukee, WI, and include model 1326AB-
B410G-21
servo motors. The system 100 is supplied with 480 volts from the power grid
for the servo
drives 220, which power is transformed to 120 volts for those components,
including the
PLC 200, requiring such standard power supply. The sensors within the
measurement head
206 may require DC power, and thus 24 volt DC power supplies may be provided.
The HMI 202 desirably includes a touch-screen monitor that is mounted directly
to
the physical components of the system 100, as explained above. This
configuration enables
close monitoring of the system and rapid modification to the operation thereof
by a user
having a first-hand view. The touch-screen monitor is relatively more sanitary
than, say, a
keyboard, and is thus preferred for clean manufacturing practices. However,
the HMI 202
could be located outside a "clean room" in which the physical components of
the system are
placed, and thus could take the form of a number of such interfaces.
Various software applications are preferably utilized in conjunction with the
aforementioned electrical components to operate, monitor, and coordinate the
various system
actions. For example, the HMI 202 desirably includes a supervisory, control,
and data
acquisition (SCADA) software package that uses Visual Basic in the background
and for
configuration, such as a program sold under the brand-name Fix Dynamics from
Intellution
of Norwood, MA. The relay ladder logic of the
* trademark
CA 02599083 2007-09-10
controller 200 controls the general machine functions, including receiving
commands from the HMI 202 concerning when and where to move the servo drives
220, checking the safety conditions, relaying the movement information to the
servo
drives 220, and telling the marking head 216 when and where to dispense dye.
The
5 preferred Allen Bradley servo drives 220 are programmed using GML software
from
Allen Bradley. Logic associated with the marking head 216 is pre-programmed
with
a dye pump speed to assure that the dye supply will not run out during any
marking
cycle.
The preferred sensors within the measurement head 206 include a linear
10 actuator and a controller. Each controller may be associated with one or
more linear
actuators, typically two. Therefore, in the preferred embodiment illustrated
above,
there are 18 linear actuators and 9 controllers. Each controller is
programmable,
preferably via the HMI 202. In the exemplary embodiment, the SMAC linear
actuators and controllers permit the position, speed, acceleration, torque and
force of
15 a coil-driven shaft to be programmed.
There are four programs associated with the servo drives 220. One program
is associated with the movement of each of the workpiece carriages 132, a
third
program is associated with movement of the measurement head 206, and a fourth
program is associated with movement of the marking head 216. Again, each of
these
20 programs is adjustable using the Allen Bradley GML software, preferably via
a
laptop computer.
The exemplary marking head 216 is also programmable, although the
program is edited using a BioDot hand-held terminal. Once edited, however, the
marking head 216 program may be downloaded to a personal-computer as a backup.
Overall Pericardial Tissue Processing and Measurement
In the present invention, the pericardial sac 50 is desirably fixed with a
buffered solution of glutaraldehyde or other fixative. After fixation, the sac
50 is
quarantined and then cleaned prior to the thickness measurement as described
herein.
The thickness of the entire tissue surface of the sac 50, or portion thereof,
is
automatically measured at a resolution of 3/8 inches center-to-center and
mapped.
Data from these measurements is then used to generate a complete tissue
thickness
mapping profile. The thickness map is used to identify and mark tissue
thickness
CA 02599083 2007-09-10
21
areas or tissues zones from which to cut leaflets. The marked tissue zones
will be
manually cut out and sorted per thickness ranges. The tissue zones will be
visually
inspected per bio-sort criteria before transferring to a cutting operation
where
acceptable tissue areas will be manually die cut into leaflets. In an
alternative
sequence, the quarantine step occurs after the measurement, mapping, marking,
and
cutting steps.
Measuring and Mapping Operation
An example sequence includes:
1. Load bio-material sheet onto first measurement platen corresponding to
first workpiece track;
2. Initiate measurement/marking cycle by pushing start button;
3. Advance platen in Y-direction along first workpiece track to
measurement station;
4. Translate measurement head in X-direction to position sensor array
above platen in first workpiece track;
5. Contact sensor array to top surface of bio-material sheet with controlled
light force to measure a row of points;
6. Transfer data corresponding to thickness of bio-material sheet to control
system;
7. Advance platen in Y-direction and measure another row of points;
8. Repeat steps 5-7 until the bio-material sheet has been measured along
the Y-direction;
9. Optionally, offset measurement head in X-direction and repeat steps 5-8
to obtain a grid of measurements;
10. Generate a thickness map using the software algorithm in the control
system;
11. Advance platen in Y-direction along first workpiece track to marking
station;
12. Translate both measurement head and the marking head in the X-
direction so as to switch places above workpiece tracks, with marking
head positioned above platen in first workpiece track;
13. Mark bio-material sheet on platen in first workpiece track into thickness
CA 02599083 2007-09-10
22
zones using marking head and thickness map instructions from control
system;
14. Advance platen in first workpiece track in Y-direction to load station to
enable removal of the measured and marked bio-material sheet.
The above sequence corresponds to the measurement marking of a bio-
material sheet on one of the platens and workpiece tracks in the system of the
present
invention. As described above, however, there are desirably two platens and
workpiece tracks operating in parallel. Therefore, the following general
sequence
may also be followed to increase throughput of the system:
1. Load sheet on platen 1 and translate along track 1 to measurement
station;
2. Measure and map sheet on platen 1;
3. Translate platen 1 to marking station;
4. Translate measurement head over track 2;
5. Load sheet on platen 2 and translate along track 2 to measurement
station;
6. Simultaneously measure and map sheet on platen 2 while marking sheet
on platen 1;
7. Translate platen 1 to load station and remove sheet;
8. Translate platen 2 to marking station;
9. Map sheet on platen 2;
10. Translate platen 2 to load station and remove sheet.
Thickness Measurement Alternatives
As mentioned above, various means can be used to measure the thickness of
bio-material sheet in accordance with the present invention. If a contact
measurement method is used, the following parameters are preferred;
a sampling increment center-to-center distance of 9.5 mm (0.375 inches)
a flat contact tip of a diameter of approximately 7.0 mm (0.275 inches)
a vertical measuring force equivalent to the force applied by a Mitutoyo
low-pressure model 543 measurement gauge; i.e., with the spring attached
CA 02599083 2007-09-10
23
and the weight removed, a force of less than 0.42 N or 43 g
a measurement table dimension in the X-Y plane of 8 inches by 20 inches
a linear actuator accuracy of about 0.0 13 mm (0.0005 inches) or less
an X-Y positioning accuracy of about 0.13 mm (0.005 inches) or less
scan time for thickness measurement of a pericardial sac of 2 minutes or
less
a range of sheet thickness measurements of 0.356-0.584 mm (0.014-0.023
inches)
Other non-contact measurement approaches include laser or ultrasound
scanning. For best results using such devices, extensive testing should be
undertaken to determine the level of accuracy, repeatability, and reliability.
Laser
scanning in particular offers the advantages of being faster and cleaner than
contact
methods. In addition, a laser scanner has a relatively simple moving mechanism
and
can be purchased at a reasonable cost. Unfortunately, a laser will be more
sensitive
to vibration, moisture, surrounding lighting, surface finish condition, and
dust/particles in the air.
One specific example of the use of lasers is in conjunction with free-sliding
pins. The pins contact the top surface of the sheet being measured and a laser
measures the locations of the tops of the pins. Another contact-type
measurement
system utilizes a multi-axis servo controller encoder from Axima. The
measurements involve using free-sliding pins to touch the bio-material sheet
while
the position of each pin is determined by the encoder. The positions of the
pins may
be monitored by pairs of photo or smart fiber-optic sensors which provides
small
beams in a range of 0.002-0.004 mm with low hysteresis for quick detection.
The
photo eyes are constantly monitored by the controller through programmable
control
logic for break continuity. The position of the pins is determined by the
count or
number of turns of the built-in encoder. The pin height accuracy of the Axima
encoder is in the range of 0.0076 mm (0.0003 inches).
Marking Method Preferences
The system 100 maps and then marks the zones 80a, 80b, 80c depicted in
Figure 2F corresponding to contiguous grid units 74 of the same or similar
thickness.
CA 02599083 2007-09-10
24
As mentioned elsewhere herein, the zones are desirably cut out, inspected, and
sorted, and leaflets are then cut from the zones using templates, or a similar
expedient. Of course, it is also possible to mark not just the zones 80 with
the
system 100, but also the leaflet shapes themselves.
A non-contact printing method is desirably used for marking the bio-
compatible sheet. In a preferred embodiment, the non-contact marking system is
a
high-performance dispenser utilizing ink jet technology and a toxicity-free
reagent or
dye. The marking system is constructed from stainless-steel, PTFE, and similar
materials for corrosion resistance and biological compatibility.
Monitoring and Control Screens
Figures 14-16 depict several images of an operator monitor and control
screen, such as the touch-screen 152 seen in Figure 5. Although the preferred
embodiment utilizes touch-screen technology, the images in Figures 14-16 may
be
solely for monitoring purposes, with the actual control being accomplished via
a
different or remote device (i.e. a keyboard).
Figure 14 illustrates a system status screen 250 that will be displayed during
a majority of the operating sequence of the system 100. In effect, the system
status
screen is the default. The name of the particular screen is indicated in the
middle top
portion thereof, as seen in the display window 240. Just below the screen name
240,
a display 242 indicates the particular vendor of the biocompatible sheet being
measured and mapped (important for regulatory purposes when biological
material is
the workpiece).
In the upper left corner, the system status screen includes four mode buttons
252 providing overall control of the operating mode of the equipment. The four
operating modes correspond to an automatic mode, a manual mode, a calibration
mode, and a clean mode. It should be noted that each of the mode buttons 252,
and
indeed all of the various screen buttons, is a bordered icon to indicate its
function as
a button, with the ability to switch the button ON and OFF. Only one of the
mode
buttons 252 can be ON at one time, with the corresponding border typically
being
illuminated or colored differently to indicate its status in contrast with the
other three
buttons which are OFF. In addition, the particular mode selected is preferably
CA 02599083 2007-09-10
indicated in textual form, as shown above the buttons 252 with the example
"MANUAL MODE."
The operator typically actuates the calibration mode button prior to a
production run, or at convenient intervals thoughout a run. A calibration
sequence
5 wherein each of the sensors 160 is calibrated against the calibration bar
188 will be
described in more detail below.
It should be noted here that the status screen 250 duplicates a number of
buttons and displays on the left and right side corresponding to the two
workpiece
carriages 132, beginning with a zero platen position button 254 entitled "ZERO
10 SMACS," located just below the mode buttons 252, and continuing downward to
a
full pattern button 262. Therefore, the separate carriages can be monitored
and
controlled in parallel.
The zero platen position button 254 establishes a zero reference position of
the sensors 160 against the work surface 134, from which sheet thickness
15 measurements are taken. (The acronym "SMACS" refers to a particular vendor
for
the measurement sensors 160). That is, the operator presses the button 254
which
causes the array of sensors 160 to contact the work surface 134 at multiple
locations
to establish a 2-dimesional array of reference heights across the platen 134.
Typically, the platen 134 will be precision surfaced, but minor irregularities
may
20 exist or develop over time.
The display box 256 indicates the length of the last cycle for the respective
left and right carriages 132. The length of the cycle generally corresponds to
the size
of the workpiece, and whether the full pattern button 262 has been actuated.
Cycle
start and stop buttons 258 function as toggle switches, and duplicate
functions of the
25 physical operator control buttons 108 provided at the corners of the table
104, as
seen in Figure 6. A display 260 indicates the percent completion of the
current cycle
for the two carriages 132.
The full pattern button 262, when actuated, programs the system 100 to read
a full pattern covering the entire work surface 134 regardless of the actual
workpiece
size. At times it may be necessary to measure more than one sheet on the
platen 134,
and thus there may be irregular spaces between the sheets. The full pattern
button
262 prevents the control system from prematurely discontinuing the measurement
process, which otherwise occurs if the button 262 is not actuated and no sheet
is
CA 02599083 2007-09-10
26
sensed. In the normal situation, where only one relatively cohesively shaped
sheet is
being measured, the full pattern button 262 is not actuated. In that instance,
the
system 100 will measure a partial platen pattern; that is, the system will
take
measurements until all of the sheet on the work surface 134 has been measured
and
then stop. Specifically, the platen 134 translates in the Y-direction under
the
measurement head until all of the sensors 160 read zero elevation from the
platen
height (on the first pass, two additional zero measurements beyond the edge of
the
sheet are required to ensure the edge of the sheet has been reached).
In the upper right portion of the screen 250, a production requirements
display 264 indicates the number of leaflets needed in each size (small,
medium, or
large), the number already mapped and marked, and, after a subtraction
operation,
the number of leaflets that remain to be mapped and marked. This display is
important in keeping the operator apprised of the size of leaflet needed so
that the
system can be programmed to favor a particular size of leaflet.
Towards the bottom of screen 250, a series of navigational buttons 266
enable access to other screens in the program. As will be seen in Figures 15
and 16,
the system status screen 250 appears as one of these navigational buttons 266.
Again, these buttons 266 toggle one another so that only one can be actuated
at any
one time. Below the navigational buttons 266, a fault display 268 is provided
along
the entire bottom portion of the screen 250. The fault display 268 indicates
the most
recent alarm condition. Desirably, only those alarm conditions requiring
immediate
attention to continue production are displayed. In Figure 14, the fault
display 268
indicates that the right side light curtain has failed, which is a serious
condition
requiring immediate attention.
In the center of the system status screen 250, a schematic plan view 270 of
the moving parts of the system 100 is displayed. The plan view 270 indicates,
at
272, the operational status of each of the servo drives, including the two
servo drives
for the parallel carriages 132, a servo drive for the movement of the
measurement
head 110 (indicated as SMACS), and a servo drive for the movement of the
marking
head 114 (indicated as BIO DOT, which is a particular vendor for the marking
head).
The position of each of the carriages 132 is indicated at 274. The cumulative
status
of the four ON/OFF switches 108 around the table 104 is indicated at 276. That
is,
the indicator 276 will only illuminate the green light if all four of the
ON/OFF
CA 02599083 2007-09-10
27
switches 108 are in the ON position. Finally, a series of bars 278 around the
periphery of the plan view 270 display the operational status of the light
curtains
around the physical system 100.
Prior to describing the system parameter screen shown in Figure 15, the
reader is referred back to the navigational buttons 266 in Figure 14 in which
the
second button from the left selects the system parameter screen. In an
exemplary
embodiment of the present invention, wherein the system 100 is utilized for
measuring and mapping biocompatible sheet for use in heart valve leaflets, a
leaflet
thickness priority display and control table 280 is provided in the upper left
corner of
the parameter screen. The table 280 includes a left column 282 that displays a
series
of priorities. A number of buttons 284 in the right three columns 286a, 286b,
286c
can be actuated to order the leaflet thickness priority. The three primary
choices in
the left column 282 correspond to three rows 288a, 288b, 288c in the table
280.
Because of their toggling relationship, only one button 284 in each column
286, and
only one button in each row 288 can be actuated at any one time.
In the illustrated embodiment, the leaflet sizes (generally corresponding to
leaflet thickness) are grouped into small (19, 21, and 23 mm), medium (25 and
27
mm), and large (29, 31, and 33 mm). Therefore, based on the initial production
requirements, as modified during a production cycle and indicated in the
display box
264 in Figure 14, the operator can favor either small medium or large
leaflets. For
example, if small leaflets are desired, the upper left button 284
corresponding to row
286a (priority 1 - high) and column 288a (large leaflets) is actuated. If
there is a
secondary preference for medium sized leaflets, then the button 284
corresponding
to row 286b (priority 2 - medium) and column 288b (medium leaflets) is
actuated.
By default, therefore, the large leaflets column 286c will be relegated to
priority 3
(low), and the button corresponding to row 286ac and column 288c will be
actuated.
The upper right portion of the parameter screen in Figure 15 includes a
leaflet size needed display and control box 290. As indicated above with
respect to
Figures 3 and 4 in the discussion of leaflet sizes relative to measured sheet
thickness,
there are different leaflet sizes associated with each thickness range. That
is,
differently sized leaflets can be formed from a particular portion of sheet
having a
measured thickness. Specifically, in the illustrated embodiment there are
three
leaflet sizes (19, 21, and 23 mm) for the small thickness range, two sizes (25
and 27
CA 02599083 2007-09-10
28
mm) for the medium thickness range, and three sizes (29, 31, and 33 mm) for
the
large thickness range. Without the display and control box 290, the system 100
might produce an excessive number of any one particular sized leaflet while
neglecting another size.
The three columns 292a, 292b, and 292c each correspond to one of the
thickness ranges, with the different sized leaflets separated within each
column in the
rows 294a, 294b, and 294c. At the intersection of each column 292 and row 294,
a
display indicating the number of leaflets needed for a particular size is
provided. For
example, the number of size 19 mm leaflets that are needed is indicated as
100. To
alter the number needed for any of the sizes, the operator need only touch
that
particular button on the screen and a small keypad (not shown) will appear
permitting modification thereof. In Figure 15, therefore, the displays
indicate that
100 leaflets are needed for each of the sizes in the small thickness range,
500 leaflets
are needed for each of the sizes in the medium thickness range, and 300
leaflets are
needed for each of the sizes in the large thickness range.
Display and control buttons 296 below each of the columns 292 indicate the
percent yield adjust for each thickness range. When measuring and mapping
biological tissue material, such as pericardial sac, the system 100 may not
recognize
visual defects. Therefore, an adjustment must be made to compensate for sheet
material that is subsequently discarded based on visual inspection. For
example, the
large size range column indicates the percent yield adjust button 296 at 90%.
That
90% corresponds to a discard level from subsequent visual inspection of 10%.
Consequently, because 900 total zones within the large thickness range are
required
for leaflet cutting, the system will actually map and mark a total of about
1000
zones. In turn, the display of the number of zones actually marked will exceed
the
number needed as long as the percent yield adjust is less than 100%.
Subsequently,
10% (i.e. 100) of the 1000 zones actually mapped and marked will be discarded,
leaving 900 usable zones.
Just below the display and control box 290, a production values display 298
is provided which mirrors the production requirements display 264 of Figure
14.
Again, the production values display 298 helps the operator adjust the leaflet
size
needed display and control box 290 "on-the-fly." A vendor select button 300,
and a
vendor display 302 are seen on the left side of the system parameter screen. A
reset
CA 02599083 2007-09-10
29
counter 304 enables the operator to zero out the "marked" values in the
production
values display 298. The values in the column for leaflets "needed" default to
those
values entered in the leaflet size needed display and control box 290. When
pressing
the reset counter 304, a separate pop-up window (not shown) asks for
confirmation
that this action is desired.
Towards the bottom of the system parameter screen of Figure 15, a display
306 of the number of leaflets found in the three size ranges in the last sac
that was
measured is provided. The navigational buttons 308 and the fault display 310
are
essentially the same as those described for Figure 15.
Figure 16 illustrates a calibration screen, with the title of the screen
displayed
at 320. The mode buttons 322 are repeated here and have the same function as
was
described for the same buttons in Figure 14. On both the left and right sides
of the
screen, a series of five buttons 324, 326, 328, 330, and 332 are provided to
select the
calibration operation. Again, two sets of buttons on the left and right are
provided
corresponding to the two workpiece carriages 132. The lineup button 324
performs
set up for the marking head 114. The individual calibration button 326
performs an
individual calibration on all the sensors 160. The values for each of the
sensors are
displayed along the display line 340 (exemplary values are omitted for
clarity). The
next three buttons, 328, 330, 332, perform calibrations on the sensors 160,
with the
corresponding values being displayed along the display lines 342, 344, and
346,
respectively. Each of these calibration operations causes the array of sensors
160 to
collectively contact a different elevational surface 190 on the calibration
bar 188.
Specifically, the button 328 causes the sensors 160 to contact the surface 190
corresponding to the high end of the high thickness range, the button 330
causes the
sensors 160 to contact the surface 190 corresponding to the low end of the low
thickness range, and the button 332 causes the sensors 160 to contact the
surface 190
corresponding to the zero reference on the calibration bar 188 (typically
performed
first).
The calibrate button 334 performs all four calibration procedures in sequence
automatically. The mode button 322 corresponding to CAL MODE must be actuated
for this operation. Actuating the INK CONFIRM button 336 sequences the marking
head 114 to insure dye is present for mapping. Again, the navigational buttons
348,
and fault display 350 are as described above.
CA 02599083 2007-09-10
General Advantages
Certain advantages of the present invention are listed below:
improved process control -- reduced operator judgment; consistent
5 identification of bio-material sheet thickness from which to locate leaflet
cut out sites;
systematic automated mapping/marking process: enables the
inclusion of all possible leaflet cut out sections and reduces the number of
intermediate steps required to produce a leaflet (i.e., subsectioning, tissue
10 sorting);
inventory control -- better control on selectivity of leaflet sizes
required;
multiple points within a bio-material sheet can be measured for
thickness by an array of programmable linear actuators and a three-axis
15 computer-controlled positioning system;
sheet thickness is measured by an automatic "height" gauge using a
linear actuator with programmable control of position, speed, acceleration
and force;
after the thickness measurement, the bio-material sheet is marked
20 by a high-performance dispenser with a biocompatible and toxicity-free
reagent.
While the foregoing is a complete description of the preferred embodiments
of the invention, various alternatives, modifications, and equivalents may be
used. It
25 will be obvious that certain other modifications may be practiced within
the scope of
the appended claims.