Note: Descriptions are shown in the official language in which they were submitted.
CA 02602062 2007-10-10
a s
Docket No. 0004.0039 1/24
APPARATUS FOR AND METHOD OF DENITRIFYING A SOLUTION
BACKGROUND OF THE INVENTION
[0001] Denitrification of solutions is useful for many reasons, such as
limiting the total
nitrogen discharged in wastewater to comply with local permits. Other reasons
include:
improving freshwater quality; controlling alkalinity and oxygen recovery,
producing
stabilized effluent, and reducing issues stemming from sludge accumulation in
the
clarifier.
[0002] Removing nitrogen from wastewater requires understanding the different
forms
of nitrogen and some commonly referred to terms:
[0003] Total Nitrogen (TN) is the sum of all nitrogen forms or:
Total Nitrogen = TKN + NO2- NO3-
where:
TKN stands for Total Kjeldahl Nitrogen, which is the sum of: NH3 + Organic
Nitrogen;
NH3 stands for Ammonia Nitrogen or Ammonium ion (NH4-);
Organic Nitrogen is derived from amino acids, proteins, urea, uric acid, etc.;
NO2 represents a Nitrite ion;
NO3 represents a Nitrate ion; and
N2 represents Nitrogen Gas.
[0004] Refractory Nitrogen cannot be decomposed biologically.
[0005] Alkalinity is defined as the ability to resist a drop in pH. For every
part
ammonia (NH3) converted to nitrate (NO3-), 7.1 parts of alkalinity are
depleted, and for
every part nitrate (NO3-) removed, 3.6 parts of alkalinity are recovered.
[0006] An anoxic zone is a basin, or portion that is mixed, but not aerated.
The
dissolved oxygen levels must be less than 1.0 mg/L, and avoid as low as 0.0
mg/L. In
an anoxic zone, denitrifying bacteria derive oxygen from the nitrate (NO3-)
compounds.
CA 02602062 2007-10-10
Docket No. 0004.0039 2/24
[0007] Nitrification and denitrification are two terms that are commonly
misunderstood.
Both are individually distinct processes. Nitrification is the conversion of
ammonia
(NH3) to nitrate (NO3-). This is a two-step process involving oxygen and two
types of
bacteria, Nitrosomonas and Nitrobacter, known collectively as nitrifiers,
represented as
follows:
Ammonia (NH3) + Oxygen (02) + Alkalinity + Nitrosomonas =
Nitrite (NO2-) Oxygen (02) + Alkalinity + Nitrobacter =
Nitrate (NO3-)
Nitrite (NO2-) is unstable and is easily converted into nitrate. The total
conversion of
ammonia (NH3) to nitrate (NO3-) requires 4.6 parts oxygen and 7.1 parts
alkalinity to
convert 1 part ammonia (NH3).
[0008] Denitrification is the conversion of nitrate (NO3) to nitrogen gas
(N2).
Heterotrophic bacteria use nitrate (NO3) as an oxygen source under anoxic
conditions
to break down organic substances as follows:
Nitrates (NO3-) Organics + Heterotrophic bacteria =
Nitrogen Gas + Oxygen + Alkalinity
[0009] In practice, only certain forms of nitrogen are monitored in wastewater
treatment facilities with specialized testing equipment. Testing for TKN
involves a test
that many wastewater treatment facility laboratories are not equipped to
perform. If
testing for TKN is not possible, other methods are used for monitoring the
nitrogen
cycle.
[0010] Typically, ammonia (NH3) values are approximately 60% of the TKN
values,
and the organic nitrogen generally is removed to the settled sludge. Also,
total Kjeldahl
nitrogen (TKN) generally equals 15 - 20 % of the Biochemical Oxygen Demand
(BOD)
of the raw sewage. Testing the following aid in monitoring and controlling the
nitrogen
cycle: pH, alkalinity, ammonia (NH3), nitrite (NO2) and nitrate (NO3-). All
major
CA 02602062 2007-10-10
Docket No. 0004.0039 3/24
laboratory supply companies sell field test kits that are inexpensive, easy to
use, and
provide quick relatively accurate results.
[0011] Having a good understanding of the form and extent of nitrogen in a
wastewater treatment facility requires a good sampling program that gives a
complete
profile of the system. The first sampling point should test the raw influent,
or primary
effluent if the system has a primary clarifier. Typically, what enters the
system is high
in alkalinity and ammonia (NH3) with little to no nitrite (N02-) or nitrate
(NO3-). A quick
way to determine if additional alkalinity may be needed is to multiply the
amount of
ammonia (NH3) by 7.1 mg/L. If this number exceeds the influent alkalinity
concentration, sodium hydroxide or lime may be needed to be added to the
aeration
tank.
[0012] pH is significant because, when ammonia (NH3) begins converting to
nitrate
(NO3-) in the aeration tank, many hydrogen ions are released. When alkalinity
drops
below 50 mg/L, pH can drop dramatically. The pH of the aeration tank should
never
drop below 6.5, otherwise desired biological activity will be inhibited and
toxic ammonia
(NH3) can bleed through the system to the environment.
[0013] Ammonia (NH3) should have extremely low concentrations. Nitrite (NO2-)
should be very low to non-detectable, with the majority of the nitrogen in the
nitrate
(NO3-) form. If a suitable environment is maintained in the aeration tank,
most of the
ammonia (NH3) will be converted to nitrate (NO3-) by the time it leaves the
tank.
[0014] All tested nitrite (NO2-) levels should be very low. High levels of
nitrite (NO2-)
in the system indicate an existing or anticipate problem with the
nitrification cycle.
[0015] Nitrosomonas bacteria are hardier than Nitrobacter bacteria. If the
Nitrobacter
bacteria die off, the Nitrosomonas bacteria will continue working on the
ammonia (NH3)
and the cycle will overload with high levels of nitrite (NO2_). An effluent
with high nitrite
(NO2) is difficult to disinfect because of the tremendous chlorine
demand it poses.
CA 02602062 2007-10-10
t I
Docket No. 0004.0039 4/24
[0016] Other problems also can occur during nitrification. A decrease in the
aeration
tank pH due to insufficient alkalinity causes ammonia (NH3) to bleed through
the
system, which causes decreased microbiological activity. Other factors that
prevent
complete nitrification include: a lack of dissolved oxygen; high mixed liquor
suspended
solids; low mean cell retention time; and cold temperatures.
[0017] All of these factors can inhibit the nitrification cycle. High ammonia
(NH3)
discharges can affect toxicity testing. High nitrite (NO2-) levels will cause
a tremendous
chlorine demand making disinfection difficult, jeopardizing fecal coliform
limits. Leaving
sludge that is high in nitrate (NO3) too long in a secondary clarifier can
cause it to rise
to the surface when the nitrogen gas is released. This is messy and
jeopardizes TSS
limits.
[0018] Although problematic, nitrifying wastewater is important for many
reasons.
Aside from permit limits, ammonia (NH3) is toxic to fish and other aquatic
life. Ammonia
(NH3) discharges also place a very high oxygen demand on the receiving
streams.
Nitrification also aids in producing a highly stabilized effluent.
[0019] When all of the ammonia (NH3) is converted to nitrate (NO3-), it is
removed
from the system or denitrified. Denitrification requires an anoxic zone within
the
wastewater treatment facility. Regardless of where and how it is done, the
principles of
operating an anoxic zone are always the same. First, dissolved oxygen levels
must be
as low as possible without reaching 0.0 mg/L. A safe target point to avoid
septicity
while starting an anoxic zone is 0.5 mg/L. A good operating point is 0.2 mg/L.
[0020] Second, a carbon source must exist for denitrification to occur. A
"carbon
source" supplies life energy to the bacteria. A carbon source compound may
include
additional elements to carbon, such as hydrogen and oxygen. The bacteria also
must
have oxygen to be able to utilize the carbon. They obtain oxygen from the
easiest
sources in the order of: (1) free and dissolved oxygen; (2) nitrate (NO3-);
and then (3)
sulfate (SO4--). If the environment has no free or dissolved oxygen, the
bacteria obtain
oxygen by breaking down nitrate (NO3-) returned to the anoxic zone in the form
of
activated sludge. As the bacteria use the nitrate (NO3) as an oxygen source to
break
CA 02602062 2007-10-10
Docket No. 0004.0039 5/24
down the carbon, their food source, nitrogen gas is released to the atmosphere
as
follows:
bacteria + Carbon Source + Nitrate (NO3-) _
Nitrogen Gas (N2) + Carbon Dloxide(C02) + 3.6 parts Alkalinity + Water (H20)
When all of the nitrate (NO3) is used up, the bacteria look for oxygen from
available
sulfate (SO4-). As the sulfates are used up, the free sulfides will combine
with
hydrogen to form hydrogen sulfide, which has a characteristic "rotten egg"
odor. Thus,
treatment plant operators are can always tell when all of the nitrate (NO3) is
being
converted into nitrogen gas (N2)-
[0021] Raw influent can be used as a carbon source. However, most treatment
plants
supplement the carbon source, for example, by injecting methanol, ethanol or
other like
carbon sources. Roughly 2.0 - 2.5 parts methanol is required for every part
nitrate
(NO3-) that is denitrified.
[0022] The mixed liquor suspended solids concentration must be kept in balance
with
the carbon source supply. In other words, the carbon source-to-microorganisms
ratio
should be in the proper range, on the lower end, for the type of process
operating. The
pH of the anoxic zone should be close to neutral (7.0) and never drop below
6.5.
[0023] Optimal denitrification occurs when as much as possible of the nitrate
(NO3-) is
converted into nitrogen gas (N2). Achieving this requires a sufficient amount
of a
carbon source so that the indigenous heterotrophic bacteria will consume all
of the
dissolved oxygen as well as the oxygen from the nitrate (NO3-), thereby
converting as
much as possible of the nitrate (NO3-) into nitrogen gas (N2).
[0024] Many carbon sources for denitrification have been studied and utilized
in
wastewater treatment systems. The most popular include the simple alcohols
methanol
[15] and ethanol [3]. Acetate in the form of either acetic acid [1] or some
acetate salt,
e.g. sodium acetate [7], has also been used. "Acetate" refers to either the
ion, as in
sodium acetate, or the substituent group, as in ethyl acetate [6]. The studies
frequently
CA 02602062 2007-10-10
Docket No. 0004.0039 6/24
indicate acetate [7] as the most effective of these listed, and the many other
compounds subjected to these studies.
H H H H O
11
H-C-O-H H-C-C-O-H H-C-C-OH I ACETIC [15] H METHANOL [3] H H c2Hso ETHANOL H
Ij] C2H402
ACID
H O H H O H
H-C-C-O- H+M Na+ H-C-C-O-8-C-H
H ACETATE ION [7] H H H
C2H302-
E76MACETAiE
$N EB1Eitj
C4H102
[0025] However, these compounds leave much to be desired for use as
denitrification
carbon sources for wastewater treatment units, especially on-site wastewater
treatment
units. Acetic acid is a solid and corrosive in the pure state. When diluted to
safer
levels, it becomes very bulky. Acetate salts also are hazardous solids, and
face the
same fate on adequate dilution. Since acetate salts of sodium or potassium are
solids,
they must be dissolved for pumping by metering devices. These solutions are
bulky,
and leave solid residue on drying that can foul the equipment. The residual
from
utilization by the bacteria is an increase in alkalinity that is impractical
to control in an
unattended system.
[0026] Among the other compounds used for larger plants are simple alcohols,
like
ethanol [3] and methanol [15], depicted above, and polyalcohols like glycerol
[2].
These alcohols also have their own limitations with respect to on-site use.
H
H-C-O-H
H-C-O-H
H-C-O-H
H
{2] GLYCEROL
C3H803
[0027] Fatty acids, monoglycerides, and diglycerides derived from the
saponification
of fats also can be used as carbon sources. Short-chain fatty acids are water
soluble,
CA 02602062 2007-10-10
Docket No. 0004.0039 7/24
while longer-chain fatty acids reduce solubility so that they become
surfactants, with
soap being the classic, example. Their esters are insoluble.
[0028] Fats and oils are esters of glycerin and 3 long chain fatty acids, and
are also
known as triglycerides [8]. Fatty acids that have carbon-to-carbon double
bonds are
referred to as "unsaturated fatty acids" [5].
ii
O -C 18]
-C C57 H110Q6
91
FAT MOLECULE; TRtGLYCER!DE;
HHH H O
H-C-C=C- =C- -C-C-H
I I
H H x
FATTY ACID 5]
WITH UNSATURATED LINK
OR DOUBLE BOND
[0029] These traditional supplementary carbon sources, methanol and ethanol,
have
undesirable characteristics, especially for on-site use, including acute
toxicity; volatile;
flammable; and form explosive vapor mixtures with air in confined spaces.
Ethanol,
while grain derived in its natural form is highly regulated and expensive.
Cheaper,
unregulated denatured ethanol, in excess amounts, inhibits decomposition. It
also,
when decomposed, yields byproducts including benzene, ethylene, toluene, and
xylene, which should not be released into the environment. Since an excess of
carbon
source is needed to ensure that a sufficient amount of heterotrophic bacteria
will locate
and convert as much as possible of the nitrate (NO3) into nitrogen gas (NA),
using
denatured ethanol causes less and less conversion and could build up in the
treatment
tank and stifles decomposition. Although ethanol is a good carbon source, it
must be
converted to acetaldehyde [14], and then acetate before the bacteria can
utilize it.
CA 02602062 2007-10-10
Docket No. 0004.0039 8/24
H O
1 II
H-C-C-H [14]
i
H ACETALDEHYDE
C2H40
[0030] What is needed is a carbon source compound that can deliver the
effectiveness
of acetate with none of the above-mentioned issues, and has only residuals
that can be
assimilated by the denitrifying bacteria.
[0031] One such compound class could be the acetate esters of glycerol. Other
polyalcohols, such as ethylene glycol [16], propylene glycol [17] and butylene
glycol
[19]-[22] also might serve as carriers of acetate in the form of esters, which
are
combinations of alcohols and organic acids. One example might be 1,2-propylene
glycol diacetate [18]. Ethanol and acetic acid combine to form ethyl acetate
[6],
depicted above.
OH
[OH [16] OH [17] OH OH OH OH OH
ETHYLENE GLYCOL PROPYLENE GLYCOL
1,2-Butylene glycol [19] 1,3 Butylene glycol [201
OH OH
O
O
OH [181
O
OH "'~
1,3-Butane diol[21] 1,4-Butane diol [22j PROPYLENE GLYCOL
DIACETATE
[0032] Many wastewater treatment facilities perform single-tank
denitrification by
creating and utilizing anoxic zones. Some examples are:
(1) Constructing a dedicated anoxic zone at the head of the aeration tank by
installing a baffle and mechanical mixers;
(2) Utilizing the first 1/4 to 1/3 of the aeration basin as an anoxic zone by
throttling the aeration system diffusers valves to allow mixing without
transferring
CA 02602062 2007-10-10
Docket No. 0004.0039 9/24
dissolved oxygen. A dissolved oxygen probe in the aeration tank tied into a
variable frequency drive that sends a signal to the blowers, providing a
continuous dissolved oxygen level as determined by the set points; and
(3) Utilizing timers to cycle the aeration system on and off which allows the
whole aeration basin to be used intermittently as an anoxic zone.
These approaches do not completely denitrify the wastewater so treated.
[0033] What are needed, and not taught or suggested in the art, are an
apparatus for
and method of denitrifying a solution that employs an inexpensive, non-toxic,
unregulated carbon source for heterotrophic bacteria to reduce all nitrate
(NO3) in
solution.
SUMMARY OF THE INVENTION
[0034] The invention overcomes the disadvantages noted above by providing
apparatus for and method of denitrifying a solution that employs an
inexpensive, non-
toxic, unregulated carbon source that promotes activity of heterotrophic
bacteria that
reduce all nitrate (NO3-) in solution.
[0035] To that end, an embodiment of an apparatus for denitrifying solution
configured
according to principles of the invention includes a sensor configured to
measure a
parameter of the solution and define a measurement and a controller configured
to be
operably connectable with the sensor and to compare the measurement with a
predetermined value. When a predetermined relationship between the measurement
and the predetermined value exists, the controller causes an amount of a
carbon
source to be introduced into the solution for a duration and at a frequency.
One or
more of the amount, duration and frequency are determined so that indigenous
heterotrophic bacteria deplete dissolved oxygen in the solution and decompose
oxygen
from nitrate in the solution to obtain its combined oxygen.
[0036] Another embodiment of an apparatus for denitrifying solution configured
according to principles of the invention includes a denitrification tank
configured to
receive a portion of the solution for a period of time wherein controlling an
amount,
CA 02602062 2009-09-17
duration and frequency of introduction of a carbon source into the solution
promotes
indigenous heterotrophic bacteria depletion of dissolved oxygen in the
solution and
decomposition of oxygen from nitrate in the solution to obtain its combined
oxygen.
[0037] An embodiment of a compound for denitrifying a solution configured
according to
principles of the invention includes a backbone selected from a polyol, a
simple
sugar and combinations thereof and a substituant selected from: a low
molecular
weight organic acid radical attached with ester linkages or an alkyl group
from a simple
alcohol attached with ether linkages.
[0038] An embodiment of a method of denitrifying a solution configured
according to
principles of the invention includes introducing into the solution an amount
of a carbon
source within a duration and at a frequency so that indigenous heterotrophic
bacteria
deplete dissolved oxygen in the solution and decompose oxygen from nitrate in
the
solution to obtain its combined oxygen.
[0039] Another embodiment of a method of denitrifying a solution configured
according
to principles of the invention includes reducing a level of dissolved oxygen
in the
solution sufficiently so that indigenous heterotrophic bacteria deplete the
dissolved
oxygen in the solution and decompose oxygen from nitrate in the solution to
obtain its
combined oxygen.
[0040] The invention provides improved elements and arrangements thereof, for
the
purposes described, which are inexpensive, dependable and effective in
accomplishing
intended purposes of the invention.
[0040a] According to an aspect of the present invention there is provided an
apparatus
for denitrifying a solution comprising:a sensor configured to measure a
parameter of the
solution and define a measurement; and a controller configured to be operably
connectable with said sensor and to compare the measurement with a
predetermined
value; wherein: when a predetermined relationship between the measurement and
the
CA 02602062 2009-09-17
10a
predetermined value exists, said controller causes an amount of a carbon
source to be
introduced into the solution for a duration and at a frequency; and one or
more of the
amount, duration and frequency are determined so that indigenous heterotrophic
bacteria deplete dissolved oxygen in the solution and decompose oxygen from
nitrate in
the solution to obtain its combined oxygen.
[0040b] According to a further aspect of the present invention there is
provided an
apparatus for denitrifying a solution comprising a denitrification tank
configured to
receive a portion of the solution for a period of time wherein controlling an
amount,
duration and frequency of introduction of a carbon source into the solution
promotes
indigenous heterotrophic bacteria depletion of dissolved oxygen in the
solution and
decomposition of oxygen from nitrate in the solution to obtain its combined
oxygen.
[0040c] According to a further aspect of the present invention there is
provided a
compound for denitrifying a solution comprising: a backbone selected from a
polyol, a
simple sugar and combinations thereof; and a substituent selected from: a low
molecular
weight organic acid radical attached with ester linkages, an alkyl group from
a simple
alcohol attached with ether linkages and combinations thereof; wherein said
polyol is
selected from ethylene glycol, glycerin, propanediol, diethylene glycol,
triethylene glycol,
dipropylene glycol, tripropylene glycol, dibutyleneqlycol, and combinations
thereof.
[0040d] According to a further aspect of the present invention there is
provided a
method of denitrifying a solution comprising introducing into the solution an
amount of a
carbon source within a duration and at a frequency so that indigenous
heterotrophic
bacteria deplete dissolved oxygen in the solution and obtain oxygen from
nitrate in the
solution.
[0040e] According to a further aspect of the present invention there is
provided a
method of denitrifying a solution comprising reducing a level of dissolved
oxygen in the
solution sufficiently so that indigenous heterotrophic bacteria deplete the
dissolved
CA 02602062 2009-09-17
10b
oxygen in the solution and obtain oxygen from nitrate in the solution.
[0040f] According to a further aspect of the present invention there is
provided an
apparatus for denitrifying a solution comprising a controller that releases an
amount of a
carbon source into the solution for a duration at a frequency; wherein one or
more of
the amount, duration and frequency are determined so that indigenous
heterotrophic
bacteria deplete dissolved oxygen in the solution and decompose oxygen from
nitrate in
the solution to obtain its combined oxygen.
[0040g] According to a further aspect of the present invention there is
provided a
compound for denitrifying a solution comprising: a backbone selected from a
polyol, a
simple sugar and combinations thereof; and a substituent comprising an alkyl
group
from a simple alcohol attached with ether linkages; wherein said simple sugar
is
selected from glucose, fructose, mannose, and combinations thereof.
[0040h] According to a further aspect of the present invention there is
provided a
compound for denitrifying a solution comprising: a backbone selected from a
polyol, a
simple sugar and combinations thereof; and a substituent comprising an alkyl
group from
a simple alcohol attached with ether linkages.
[0040i] According to a final aspect of the present invention there is provided
a compound
for denitrifying a solution comprising: a backbone selected from a polyol, a
simple sugar
and combinations thereof; and a substituent selected from: a low molecular
weight
organic acid radical attached with ester linkages, an alkyl group from a
simple alcohol
attached with ether linkages and combinations thereof; wherein: said simple
sugar is
selected from glucose, fructose, mannose, and combinations thereof; and said
polyol is
selected from ethylene glycol, propanediol, diethylene glycol, triethylene
glycol,
dipropylene glycol, tripropylene glycol, dibutyleneglycol, and combinations
thereof.
[0041] Other features and advantages of the invention will become apparent
from the
following description of the preferred embodiments, which refers to the
accompanying
drawings.
CA 02602062 2007-10-10
Docket No. 0004.0039 11/24
BRIEF DESCRIPTION OF THE DRAWINGS
[0042] The invention is described in detail below with reference to the
following
figures, throughout which similar reference characters denote corresponding
features
consistently, wherein:
[0043] Fig. 1 is a vertical, cross-sectional detail view of an apparatus
configured
according to principles of the invention incorporated in a wastewater
treatment system;
[0044] Fig. 2 is a plan view of the embodiment of Fig. 1;
[0045] Fig. 3 is a vertical, cross-sectional detail view of an apparatus
configured
according to principles of the invention; and
[0046] Fig. 4 is a schematic representation of an embodiment of a method
configured
according to principles of the invention.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
[0047] The invention is an apparatus for and method of denitrifying a solution
that
accepts nitrified solution and introduces a carbon source into the solution
that
promotes heterotrophic bacterial reduction of nitrate (N03-)-
[0048] Referring to Figs. 1 and 2, a denitrification apparatus 100 is shown
incorporated in a conventional wastewater treatment plant A. Wastewater
treatment
plant A includes a pre-treatment tank B, a treatment tank C and a holding tank
D.
Untreated solution flows into the pre-treatment tank B, into and through the
treatment
tank C, into and through denitrification apparatus 100, into and through the
holding
tank D, then is voided into the environment.
[0049] Pre-treatment tank B receives raw, untreated wastewater and initiates
the
aerobic phase of treatment during which aerobic bacteria break down the
wastewater.
Pre-treatment tank B also retains any non-biodegradables inadvertently
introduced into
CA 02602062 2007-10-10
= 1
Docket No. 0004.0039 12/24
the system, such as rags and plastic, which settle out prior to introduction
of the fluid
into the treatment tank.
[0050] Treatment tank C is where the bulk of the aerobic wastewater
decomposition
occurs. Treatment tank C includes walls E and a floor F. A hopper G mounted in
tank
C cooperates with walls E and floor F to define aerator zones H and an
interior clarifier
chamber I. Diffusers J in treatment tank C promote flow in aerator zones H,
which
enhances the oxygen content of the wastewater in tank C and aerobic breakdown
of
solid matter therein. In aerator zones H, aeration thoroughly mixes the
organic
materials of the wastewater with the bacterial population so that the bacteria
attack and
reduce the organic materials.
[0051] Aerated and reduced wastewater from aeration zones H passes into
clarifier
chamber I. The throat-like lower aperture of hopper G minimizes fluid flow
within
clarifier chamber I and encourages the settling out of particulate matter in
clarifier
chamber I back into aerator zones H for additional breakdown.
[0052] Before passing wastewater from clarifier chamber I into holding tank D,
the
invention provides for denitrification of the wastewater from clarifier
chamber I in
denitrification apparatus 100, described in greater detail below.
[0053] Holding tank D receives denitrified wastewater from denitrification
apparatus
100 where it remains for a period of time. Any remaining particulate matter in
the
wastewater settles out prior to being pumped by a pump K out of wastewater
treatment
system A into the environment.
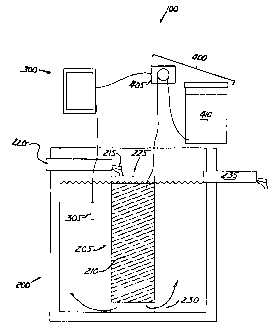
[0054] Referring to Fig. 3, denitrification system 100 preferably includes a
denitrification tank 200, a controller 300 and a doser 400. Denitrification
tank 200
receives nitrified solution. Controller 300 monitors parameters of the
solution in
denitrification tank 200 and regulates closer 400, which introduces a carbon
source into
denitrification tank 200 and cause conditions that are appropriate for
cellular
respiration and optimal for denitrification, as described in greater detail
below.
CA 02602062 2007-10-10
Docket No. 0004.0039 13/24
[0055] Denitrification tank 200 includes an anoxic media cell 205 in which
media 210
are suspended. Nitrified solution 215, preferably from pre-treatment tank C,
as shown
in Fig. 1, from inlet 220 enters the top 225 of anoxic media cell 205 and
passes through
media 210. From media 210, the solution passes out of the bottom 230 of anoxic
media cell 205. Media-treated solution is displaced by inflow and eventually
passes
from denitrification tank 200 through outlet 235.
[0056] Denitrification tank 200, while distinct from or selectably isolated
from the rest
of a wastewater treatment system, nevertheless may be structurally integral
therewith,
attached thereto or disposed therein.
[0057] Media 210 encourage growth of denitrifying surface bacteria.
[0058] Controller 300 monitors one or more probes 305 in denitrification tank
200.
Probes 305 measure one or more of the following parameters: pH; dissolved
oxygen;
influent flow; effluent flow; conductivity; alkalinity; nitrates; and
oxidation reduction
potential. Based on one or more parameter values measured for one or more of
the
parameters, controller 300 causes doser 400 to dispense a carbon source in
denitrification tank 200 in an appropriate amount, for an appropriate duration
and at
appropriate frequencies so that denitrification tank 200 exhibits anoxic
conditions with
sufficient carbon, or otherwise promotes growth of denitrifying bacteria and
optimal
denitrification capabilities.
[0059] When controller 300 determines that an aerobic condition exists,
typically at
least 1 g/mL of 02, controller 300 instructs doser 400 to deliver an amount of
a carbon
source to denitrification tank 200. The carbon source supplies life energy to
the
bacteria. The bacteria then obtain oxygen from the easiest sources in the
order of: (1)
free and dissolved oxygen; (2) nitrate (NO3-); and then (3) sulfate (SO4--).
This
converts the aerobic conditions in denitrification tank 200 to anoxic.
Controller 300
also can ensure that denitrification tank 200 remains in an anoxic condition
for a
duration required for denitrification.
CA 02602062 2007-10-10
Docket No. 0004.0039 14/24
[0060] The denitrifying surface bacteria population increases more when
exposed to
cyclical aerobic-anaerobic conditions, rather than steady-state aerobic or
anaerobic
conditions. This is why it is preferable to cultivate the denitrifying surface
bacteria
population in a distinct denitrification tank 200 that may be selectably
placed, rather
than always in communication with the wastewater system, and in particular,
the
aeration tank or aerobic portion thereof. Carefully maintaining anoxic
conditions in
denitrification tank 200 ensures survival of the bacteria.
[0061] If the denitrification tank is aerobic all of the time, aerobes will
exist there, and if
it is anaerobic all of the time, anaerobes will exist there. Denitrifiers use
oxygen for
respiration and carbon for food. If the denitrifiers are already working under
anoxic
conditions, have food, but lack oxygen, they will use the closest thing
available for
respiration, which is Nitrate, which the denitrifiers convert into water and
N2 and CO2.
[0062] There also will exist an accumulation of biomass of living and dead
bacteria.
This biomass most likely uses some of the nitrate for amino acid and protein
formation.
Provisions must be made to periodically remove and dispose of this biomass.
One
method might be to return it to the aeration tank stage.
[0063] Utilizing a combination of the alcohols and acetate as a carbon source
eliminates all of the problems with carbon sources noted above. A combination
that is
particularly useful is glycerin and acetate in the form of a mixture of
glycerol acetates,
known in the bulk product industry as diacetin. This name derives from that of
the most
abundant component, 1,3-diacetin [9], but the mixture often contains
significant
amounts of 1,2-diacetin [10], triacetin [11], glycerol-1 -acetate [12], and
glycerol-2-acetate [13].
.H '0H
11 1
H - C-a -C-C- H
9
(~ -0,1 O -0, O OH O
H-C-O-H H 11 tt 11 O^ Oo OH O^
H-C-O-C-C- H t
1 11 OH ^ OH OH
H fl H GLYCEROL-1-ACETATE GLYCEROL-2-ACETATE
1,3-DtACETIN 1,2-DIACETIN TRIACTIN
[9] OR [10] [11] [12] [13]
1,3-GLYCEROL DIACETATE
C7H1205
CA 02602062 2007-10-10
Docket No. 0004.0039 1524
[0064] The preferred carbon source is diacetin or glycerol diacetate. Diacetin
is
preferred because it is rich in acetate substituants that have been shown to
be
exceptionally effective in the denitrification process. In addition, it is a
non-hazardous
material, non-toxic and non-flammable, and does not evaporate or form solids.
Diacetin is rich in available carbon. Also, unlike surplus amounts of ethanol
and
methanol, surplus amounts of diacetin do not inhibit the denitrification
process.
[0065] Acetate is superior to ethanol because ethanol must be converted
biologically
to acetaldehyde [14], and then to acetate before the denitrifying bacteria can
utilize it.
Providing a substance that is ready for use to the denitrifying bacteria
speeds up the
denitrification process by eliminating this conversion step.
[0066] Diacetin is an excellent carbon source for on-site anoxic
denitrification of
solution because it provides a delivery system for the acetate moiety that
meets a
number of requirements. What makes glycerol particularly suited for
denitrification is
that it acts as a carrier for a readily available form of carbon. In layman's
terms,
glycerol is the carrier, and acetate is the container of the carbon food
source for the
denitrifying bacteria.
[0067] Diacetin is acetate attached to glycerin as a backbone. When the
acetate is
consumed, the bacteria also utilize the remaining glycerin, leaving only water
and
carbon dioxide as residuals. The bacteria tolerate inadvertent excesses of
diacetin
much better than excesses of other foods such as ethanol or especially
methanol. The
intermediate acetaldehyde and formaldehyde produced by these compounds are
known preservatives (antibacterial).
[0068] Diacetin is readily taken up by the facultative bacteria and held for
use until an
oxygen source of dissolved oxygen, nitrate, or nitrite becomes available,
keeping the
food away from the anaerobes. Facultative bacteria are those that can grow
with or
without oxygen.
CA 02602062 2007-10-10
Docket No. 0004.0039 16/24
[0069] Diacetin is a liquid, neutral, non-hazardous, very compact in its
carbon content.
It is used as a food additive and in the preparation of tablets from drugs in
the powder
form. Commercially, it is prepared from the reaction of acetic anhydride and
glycerin.
Environmental release is easily handled in small amounts.
[0070] While glycerol diacetate seems to be the most useful compound
structure,
other carbon sources also could be use that derive from fatty acid esters of
polyhydroxyl compounds so long as they fulfil the following conditions:
1. Liquid at all weather temperatures;
2. Readily miscible with water in the proportions of use;
3. Non volatile, nonflammable; and
4. Non toxic.
These requirements eliminate practically all of the class except the glyceryl
acetates.
[0071] Another class has ether groups as handles that are more likely to be
toxic
because they are rarely encountered in nature.
[0072] Polyhydroxyls have more than one hydroxy (-OH) group on the compound.
Ethylene glycol is the simplest member, with two groups (HOCH2CH2OH). Glycerin
has three. Simple sugars, like glucose, fructose, etc., have six.
[0073] Diacetin also is know as: Diacetylglycerol; Glycerin Diacetate;
Glycerine
Diacetate; Glyceryl Diacetate; Glycerol 1,3-diacetate; 2-(Acetyloxy)-1-
(hydroxymethyl)ethyl acetate.
[0074] Referring again to Fig. 3, doser 400 may include a peristaltic pump 405
or other
metering mechanism for delivering a predetermined volume of the carbon source
from
a container 410 into media cell 205 or influent stream 215. The carbon source
provides an energy source for the denitrifying bacteria, which consumes the
available
dissolved oxygen from the solution in denitrification tank 200, thereby
converting the
aerobic conditions denitrification tank to anoxic. The denitrifying bacterial
then
consume the oxygen in the remaining Nitrate and convert the nitrate into
water, N2 and
CO2.
CA 02602062 2007-10-10
Docket No. 0004.0039 17/24
[0075] Referring to Fig. 4, a method of denitrifying solution 500 configured
according
to principles of the invention includes: a step 505 of measuring a parameter
of the
solution and defining a measurement; a step 510 of comparing the measurement
with a
predetermined value; a step 515 introducing into the solution an amount of a
carbon
source, as described above, wherein the frequency and duration of the
introducing
and/or the amount is determined according to a relationship between the
measurement
and the predetermined value.
[0076] Step 505 may involve measuring one or more of: pH; dissolved oxygen;
influent flow; effluent flow; conductivity; alkalinity; nitrates; and
oxidation reduction
potential.
[0077] Step 510 may involve establishing data in a memory of the controller
against
which the controller may compare the measurement of step 505. Simple or
elaborate
conditions or logic statements may be defined for determining when an
appropriate
aerobic condition exists, following which anoxic conditions may be
appropriate.
[0078] Step 515 may involve instructing a doser to deliver an amount of a
carbon
source to the denitrification tank. Step 515 is timed and cycled so as to
cause
conditions in the solution to be anoxic, which promotes the growth of
indigenous
denitrifying bacteria. The amount of the carbon source also may be tailored to
create
conditions desired for optimal denitrification. The amount should be
sufficient and
within a short enough duration to remove the dissolved oxygen from the
solution
sufficiently so that the indigenous bacteria are forced to draw oxygen from
the
remaining nitrate.
[0079] The invention is not limited to the particular embodiments described
and
depicted herein, rather only to the following claims.