Note: Descriptions are shown in the official language in which they were submitted.
CA 02603426 2007-09-27
WATER TREATMENT AND METHOD OF WATER TREATMENT
BACKGROUND OF THE INVENTION
1. Field of the Invention
The present invention relates to a water treating agent and
a water treatment method. More specifically, the present invention
relates to a water treating agent capable of preventing the general
corrosion and local corrosion of the heat transfer surface of a
boiler tube and of providing excellent heat transfer property, and
a water treatment method using the water treating agent.
2. Description of the Related Art
A boiler, which is widely used as an energy supply facility
forheating, power generation, or the like, is a device that generates
steam. The inner surface portion of a water pipe for generating
steam in a boiler (the heat transfer surface of a boiler tube) is
in a high-temperature, high-pressure environment. A component such
as calcium in water (boiler feed water) supplied to the boiler adheres
as a scale to the heat transfer surface (scaling) , or the heat transfer
surfaces is corroded by the boiler feed water.
When a scale adheres to the heat transfer surface, the scale
prevents heat transfer, with the result that heat transfer property
such as bcU-ler efficiency reduces. In addition, when the heat
~ransfer surface is corroded, the corrosion damages the boiler tube.
The operation of the boiler may be stopped depending on the degree
1
CA 02603426 2007-09-27
of the damage to the boiler tube.
In view of the foregoing, a chemical such as a scale inhibitor
or a pH adjustor has been conventionally added as a water treating
agent to boiler feed water in order to prevent (suppress) the scaling
or corrosion of the heat transfer surface of a boiler tube. However,
conventional water treating agents have been used individually for
preventing the scaling of the heat transfer surface of a boiler
tube and preventing the corrosion of the heat transfer surface,
so it has been difficult to perform the prevention of the scaling
of the heat transfer surface of the boiler tube and the prevention
of the corrosion of the heat transfer surface simultaneously in
a balanced manner.
That is, the addition of a water treating agent to boiler feed
water for the purpose of preventing the scaling of the heat transfer
surface of a water pipe has been unable to sufficiently prevent
the corrosion of the heat transfer surface although the addition
has been able to prevent the scaling of the heat transfer surface.
On the other hand, the addition of a water treating agent to the
boiler feed water for the purpose of preventing the corrosion of
the heat transfer surface has been unable to sufficiently prevent
the scaling of the heat transfer surface although the addition has
been able to prevent the corrosion of the heat transfci arr-zce.
In view of the foregoing, there has been proposed a water
treating agent containing silica, apHadjustor, and a scale inhibitor
2
CA 02603426 2007-09-27
as a water treating agent capable of performing the prevention of
the scaling of a heat transfer surface and the prevention of the
corrosion of the heat transfer surface simultaneously in a balanced
manner in order to suppress the corrosion of a heat transfer surface
and the generation of a scale caused by an influence of water (see
JP 2003-159597 A).
The water treating agent described in JP 2003-159597 A forms
a silica layer and an iron hydroxide layer (for example, iron
oxyhydroxide) on the heat transfer surface of a boiler tube by means
of a silica component and the pH adjustor in order to prevent the
corrosion of the heat transfer surface. However, when the thickness
of each of the silica layer and the iron hydroxide layer formed
on the heat transf er surf ace is insuf f icient, no anticorrosive action
is exerted, so the heat transfer surface may be corroded.
In addition, when the heat transfer surface is covered with
the corrosionproduct, there arises a problem in that the heat transfer
property of a boiler deteriorates owing to the low thermal
conductivity of the corrosion product. In addition, when the entire
heat transfer surface cannot be uniformly covered with the silica
layer and the iron hydroxide layer, local corrosion (pitting
corrosion) may occur on the heat transfer surface. When the heat
transfer surface is locally corroded, a roie -c,ci.etratina the boiler
tube may be opened, so there aris-s a problem in that water leaks
from the hole into the furnace of the boiler.
3
CA 02603426 2007-09-27
SUMMARY OF THE INVENTION
An obj ect of the present invention is to provide a water treating
agent capable of preventing the general corrosion and local corrosion
of the heat transfer surface of a boiler tube and of providing
excellent heat transfer property, and a water treatment method using
the water treating agent.
The object of the present inventionmaybe achievedby providing
a water treating agent including: water as a main component; a coating
film forming agent of which a coating film is formed on a heat transfer
surface of a boiler tube; a oxygen scavenger; a scale inhibitor;
and a pH adjustor.
With such constitution, a coating film capable of suppressing
corrosion is formed of the coating film forming agent on the heat
transfer surface of the boiler tube. Dissolved oxygen in boiler
feed water is removed by the oxygen scavenger. Furthermore, the
scaling of the heat transfer surface of the boiler tube is prevented
bythe scale inhibitor, andthepHof theboiler feedwater is adjusted.
Therefore, the general corrosion and local corrosion of the heat
transfer surface of the boiler tube can be prevented, and excellent
heat transfer property can be obtaired.
The coating film fnr,::l:.r; 3aent includes at least one kind
selected from the a~_oup consisting of silica, sodium silicate,
potassium silicate, an orthosilicate, and a polysilicate.
4
CA 02603426 2007-09-27
With such constitution, a coating film capable of suppressing
corrosion is formed of at least one kind or two or more kinds of
silica, sodium silicate, potassium silicate, an orthosilicate, and
a polysilicate each serving as the coating film forming agent on
the heat transfer surface of theboiler tube. Therefore, the general
corrosion and local corrosion of the heat transfer surface of the
boiler tube can be additionally prevented, and excellent heat
transfer property can be obtained.
The oxygen scavenger includes at least one kind selected from
the group consisting of vitamin C and a salt thereof, tannin, a
saccharide type oxygen scavenger, erythorbic acid and a salt thereof,
and sulfite.
Here, for example, vitamin C and a salt thereof are used for
the oxygen scavenger because oxygen dissolved into boiler feed water
can be removed owing to the strong reducing power of the oxygen
scavenger and the oxygen scavenger has no toxicity unlike hydrazine.
The boiler feed water from which dissolved oxygen has been removed
exhibits a reduced corrosive action on the heat transfer surface
of the boiler tube.
With such constitution, dissolved oxygen in the boiler feed
water is removed by a oxygen scavenger having no toxicity such as
a oxygen scavenner composed of at least one kind or two or more
kinds c-r vitamin C and a salt thereof, tannin, a saccharide type
oxygen scavenger, erythorbic acid and a salt thereof, and sulfite.
CA 02603426 2007-09-27
Therefore, the general corrosion and local corrosion of the heat
transfer surface of the boiler tube can be additionally prevented,
and excellent heat transfer property can be obtained.
The scale inhibitor includes at least one kind selected from
the group consisting of citric acid, ethylenediaminetetraacetic
acid and a salt thereof, polyacrylic acid and a salt thereof, and
polymaleic acid and a salt thereof.
Here, the scale inhibitor is used because it can prevent the
adhesion of a scale to the heat transfer surface of the boiler tube
(scaling). That is, when citric acid, and
ethylenediaminetetraacetic acid and a salt thereof are used for
the scale inhibitor, a calcium ion or a magnesium ion in the boiler
feed water is chelated by the scale inhibitor, so it cannot adhere
as a scale to the heat transfer surface of the boiler tube . Inaddition,
when polyacrylic acid, polymaleic acid, or the like is used for
the scale inhibitor, the growth of the crystal nucleus of a scale
formed of a calcium ion or a magnesium ion is prevented, so such
ion cannot adhere as a scale to the heat transfer surface of the
boiler tube.
With such constitution, the scaling of the heat transfer
surface of the boiler tube is preventedby the scale inhibitor composed
cf -;t least one kind or two or more kinds of citric
ethylenediaminetetraacetic acid and a salt thereof, pjiyacrylic
acid and a salt thereof, and polymaleic acid and a salt thereof.
6
CA 02603426 2007-09-27
As described above, the scaling of the heat transfer surface of
the boiler tube is prevented by, for example,
ethylenediaminetetraacetic acid and a salt thereof each serving
as the scale inhibitor. Therefore, the general corrosion and local
corrosion of the heat transfer surface of the boiler tube can be
prevented, and additionally excellent heat transfer property can
be obtained.
The pH adjustor includes at least one kind selected from the
group consisting of hydroxides of alkali metals such as sodium
hydroxide and potassium hydroxide.
Here, the pH adjustor is used for adjusting the pH of the boiler
feed water to an alkali side so that the corrosion of the heat transfer
surface of the boiler tube is prevented.
With such constitution, the pH of the boiler feed water is
adjusted by the pH adjustor composed of at least one kind or two
or more kinds of hydroxides of alkali metals such as sodium hydroxide
and potassium hydroxide. As described above, the pH of the boiler
feed water is adjusted by the pH adjustor such as sodium hydroxide.
Therefore, the general corrosion and local corrosion of the heat
transfer surface of the boiler tube can be prevented, and excellent
heat transfer property can be obtained.
Inaddition, the obj ect of the present inver.t achieved
bymeans of a water treatment method incluc? i::y the steps of: adjusting
the concentration of the above-described water treating agent to
7
CA 02603426 2007-09-27
apredetermined concentration; detecting the concentration of silica
dissolved into a water supply tank and a dissolved oxygen amount
in the tank; and controlling the amount of the above-described water
treating agent to be supplied to the water supply tank.
With such constitution, when a silica concentration or a
dissolved oxygen amount in boiler feed water fluctuates, the amount
of a water treating agent, which contains a coating film forming
agent of which a coating film is formed on the heat transfer surface
of a boiler tube, a oxygen scavenger, a scale inhibitor, and a pH
adjustor, and the concentration of which is adjusted to a
predetermined concentration, to be supplied to the water supply
tank can be controlled in correspondence with the fluctuation. As
a result, even when a silica concentration or a dissolved oxygen
amount in the boiler feed water fluctuates, the general corrosion
and local corrosion of the heat transfer surface of the boiler tube
can beprevented,andexcellentheat transfer property can be obtained
by adjusting the concentration of a water treating agent to a
predetermined concentration and by controlling the amount of the
water treating agent the concentration of which has been adjusted
to be supplied to the water supply tank in correspondence with the
fluctuation.
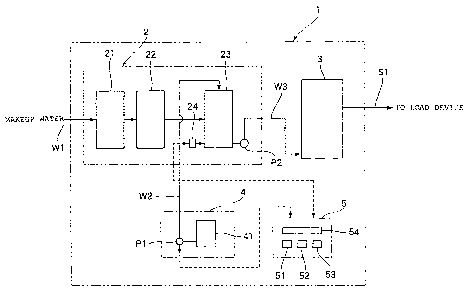
BRIEF DESCp.Tf~TION OF THE DRAWING
Fig. 1 is a schematic view showing the constitution of a steam
8
CA 02603426 2007-09-27
boiler device according to Example 2 of the present invention.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present invention is realized by a water treating agent
constituted by blending water as a main component with a coating
film forming agent of which a coating film is formed on the heat
transfer surface of a boiler tube, a oxygen scavenger, a scale
inhibitor, and a pH adjustor.
At least one kind or two or more kinds of known coating film
forming agents such as silica (silicicanhydride), sodium silicate,
potassium silicate, an orthosilicate, and a polysilicate are used
for the coating film forming agent of the water treating agent.
The coating film forming agent is adsorbed to the heat transfer
surface of a boiler tube to form a coating film with which the heat
transfer surface of the boiler tube is covered. The coating film
formed of the coating film forming agent covers the heat transfer
surface of the boiler tube to serve as a barrier layer, thereby
acting as a protective coating film against corrosion. Therefore,
the corrosion of the heat transfer surface of the boiler tube covered
with the protective coating film is suppressed.
At least one kind or two or:~ore kinds of known oxygen scavengers
such as vitamin C; ~~It thereof, tannin, a saccharide type
oxygen scave=yer, erythorbic acid and a salt thereof, and sulfite
are used for the oxygen scavenger to be used together with the coati ng
9
CA 02603426 2007-09-27
film forming agent. The oxygen scavenger has no toxicity unlike
hydrazine, and can remove oxygen dissolved into boiler feed water.
Oxygen dissolved into the boiler feed water serves as an oxidant
to exhibit a corrosive action on the heat transfer surface of a
boiler tube. Therefore, the removal of oxygen dissolved into the
boiler feed water by means of the oxygen scavenger prevents the
general corrosion of the heat transfer surface of the boiler tube
because an oxidant concentration in the boiler feed water reduces.
In addition, the removal of oxygen dissolved into the boiler feed
water reduces the nonuniformity of an oxygen concentration on the
heat transfer surface of the boiler tube. As a result, an oxygen
concentration cell is hardly formed, and the local corrosion of
the heat transfer surface of the boiler tube is prevented. Here,
detailed description will be given of the prevention of the local
corrosion of the heat transfer surface of a boiler tube by means
of the water treating agent according to an embodiment of the present
invention.
In general, when a coatingfilm capable of preventing corrosion
is formed on the heat transfer surface of a boiler tube, a material
(such as carbon steel) used for the heat transfer surface of the
boiler tube i_s hardly corroded by boiler feed water. The coating
film T::=:-med on the heat transfer surface of the boiler tube is a
result of, for example, the adsorption of a silica component zo
the surface of carbon steel or the formation of iron oxyhydroxide
CA 02603426 2007-09-27
on the surface of carbon steel. However, the surface of the heat
transfer surface of the boiler tube may have a portion where the
formation of a coating film is insufficient owing to a factor
inhibiting the formation of a coating film (coating film deficient
portion) . There are various factors inhibiting the formation of
a coating film including: the presence of a chloride ion inhibiting
the formation of a coating film in the boiler feed water; the
nonuniformity of a surface due to the surface segregation of sulfur
(sulfide) or the like in carbon steel; and the nonuniformity of
the concentrationof a coating film forming agent due to the nonuni f orm
flow rate of the boiler feed water flowing in the boiler tube. When
a factor inhibiting the formation of a coating film is present,
the surface of the heat transfer surface of the boiler tube has
a coating film portion formed of silica or iron oxide (sound coating
film portion) and a portion where the formation of a coating film
is insuf f icient (coating f ilm deficientportion). The sound coating
film portion and the coating film deficient portion form a local
cell, so local corrosion occurs in some cases.
By the way, it is generally known that local corrosion such
as gap corrosion or pitting corrosion (which refers to the corrosion
of a metal surface, the corrosion not being uniform, and the cor.roCion
occurring locally in a concentrated manner) is apt to in an
environment where general corrosion (which rei-ers to corrosion
occurring nearly uniformly on a metal surface) hardly occurs. A
Il
CA 02603426 2007-09-27
metal surface is not locally corroded because general corrosion
occurs nearly uniformly on the metal surface. In contrast, local
corrosion is apt to occur in a coating film deficient portion in
the case where the corrosion of the metal surface is entirely
suppressed by a passive coating film or a protective coating film
(a sound coating film is formed to suppress general corrosion).
This is because the coating film deficient portion is more likely
to be corroded than the sound coating film portion, so the coating
film def icient portion is locally corroded in a concentrated manner.
That is, the reason for the foregoing is as described below.
A local cell is formed between the coating film deficient portion
andthesound coatingfilm portion,the coatingfilm deficient portion
serves as the anode electrode of the local cell (the negative electrode
of the cell where an oxidation reaction occurs and a metal is dissolved,
that is, corroded), and the sound coating film portion serves as
the cathode electrode of the local cell (the positive electrode
of the cell where a reduction reaction occurs and a metal is not
dissolved, that is, not corroded) . The local cell has an oxygen
concentration cell formed therein so that a cell reaction proceeds.
It is generally known that an oxygen concentration cell shows a
larger corrosion rate as a ratio between th~ cxygen amount
(concentration) of a sound coating film --f which a cathode
is formed and that of a coating riim deficient portion of which
an anode is formedbecomes larger (a cell electromotive force becomes
12
CA 02603426 2007-09-27
larger) In other words, the fact means that local corrosion due
to the coating film deficient portion can be prevented (suppressed)
by reducing the ratio between the oxygen amount (concentration)
of the sound coating film portion and that of the coating film
deficient portion.
The water treating agent of the present invention prevents
local corrosion because the agent reduces the amount (concentration)
of oxygen dissolved into boiler feed water by means of a oxygen
scavenger so that an oxygen concentration cell is hardly formed
even when the coating film formed of the above coating film forming
agent on the heat transfer surface of a boiler tube forms a coating
film deficient portion owing to a certain reason. That is, a
reduction in amount (concentration) of oxygen dissolved into the
boiler feed water reduces the absolute amount of the oxygen amount
(concentration) of a sound coating filmportion. Therefore, a ratio
between the oxygen amount (concentration) of the sound coating film
portion and that of the coating film deficient portion reduces,
so the electromotive force of the oxygen concentration cell reduces
and local corrosion can be prevented.
In addition, the water treating agent of the present invention
is blended with a scale inhibitor a: well as the oxygen scavenger.
At least one kind or two nr _acre kinds of known scale inhibitors
such as citric acid; ethylenediaminetetraacetic acid and a salt
thereof, polyacrylic acid, and polymaleic acid are used for the
13
CA 02603426 2007-09-27
scale inhibitor. When citric acid, andethylenediaminetetraacetic
acid and a salt thereof (EDTA-Na) are used for the scale inhibitor,
a calcium ion or a magnesium ion in boiler feed water is chelated
by the scale inhibitor, so a scale hardly adheres to the heat transfer
surface of a boiler tube. In addition, when polyacrylic acid and
a salt thereof, polymaleic acid and a salt thereof, and the like
are used for the scale inhibitor, the growth of the crystal nucleus
of a scale formed of a calcium ion or a magnesium ion is prevented,
so a scale hardly adheres to the heat transfer surface of the boiler
tube. As described above, when a scale hardly adheres to the heat
transfer surface of the boiler tube, a boiler can operate while
maintaining its excellent heat transfer property.
Furthermore, the water treating agent is blended with a pH
adj ustor. At least one kind or two or more kinds of known pH adj ustors
such as hydroxides of alkali metals such as sodium hydroxide and
potassium hydroxide are used for the pH adjustor. The pH adjustor
adjusts the pH of boiler feed water to an alkali side so that the
corrosion of the heat transfer surface of a boiler tube is prevented.
By the way, each of the coating film forming agent, the oxygen
scavenger, the scale inhibitor, and the pH adjustor in the present
invention can be dissolved as an individual chemical into water
before it is However, in consideration of the labor of
loadin,,= (injecting) a water treating agent, they are desirably
dissolved together into water to prepare a single preparation.
14
CA 02603426 2007-09-27
[Examples]
Hereinafter, an example (any one of Examples 1 to 3) of a water
treating agent according to an embodiment of the present invention
will be described in detail on the basis of Table 1.
Table 1
Example Comparative
example
1 2 3 1 2
Sodium silicate [g] 1.26 5.46 9.66 0.04 21.5
Vitamin C [g] 2.5 5.0 7.5 0.0 0.0
EDTA-2Na [g} 0.4 0.4 0.4 0.4 0.4
Sodium hydroxide 4.0 4.0 4.0 4.0 4.0
[g]
Presence or absence
of general Absent Absent Absent Absent Absent
corrosion
Presence or absence Slightly Absent Absent Present Absent
of local corrosion present
Maximum depth of
pitting corrosion 80 33 2 130 2
[um]
Presence or absence Absent Absent Absent Absent Pre-
of adhesion of scale sent
Ca solubility of
boiler water ->10 _10 ?10 ?10 8.2
[mg/liter]
(1) Example 1
The water treating agent of Example 1 shown in Table 1 is obtained
by blending pure water with a coating film forming agent, a oxygen
~~.-:Venger, a scale inh il~ito~ , ai:c:.~ a r~ ~~ l:-t:,r C" r~?"G" ~,' ,,;
~or!
amount per 100 g in total of the water treating agent. That is,
the water treating agent of Example 1 is obtained by blending 1.26
CA 02603426 2007-09-27
g ofsodiumsilicate(WakoPureChemicalIndustries,Ltd.,guaranteed
reagent) as a coating film forming agent, 2.5 g of vitamin C
(L-ascorbic acid) (Wako Pure Chemical Industries, Ltd., guaranteed
reagent) as a oxygen scavenger, 0. 4 g of EDTA-2Na (Wako Pure Chemical
Industries, Ltd., guaranteed reagent) as a scale inhibitor, and
4.0 g of sodium hydroxide (Wako Pure Chemical Industries, Ltd.,
guaranteed reagent) as a pH adjustor. 500 mg of the water treating
agent were loaded into per 1 liter of softened water. Here, softened
water of Osaka City that has been artificially adjusted is used
as softened water to serve as boiler feed water. The adjusted
softened water has properties of water including a pH of 7.5, an
electric conductivity of 25 mS/m, an M alkaline strength of 20
mg/liter-CaC03, and a hardness of 1 mg/liter-CaC03. The softened
water of Osaka City that has been artificially adjusted is used
for softened water to be used for each of examples and comparative
examples of the water treating agent, and description about the
softened water is omitted hereinafter.
(2) Example 2
The water treating agent of Example 2 is obtained by blending
pure water with a coating film forming agent, a oxygen scavenger,
a scale inhibitor, and a pH adjustor each in a predetermined amount
per 100 g in total of the water treating agent, ~ -, _~? -;uTne manner
as in the water treating agent of Examplt:~ I. That is, the water
treating agent of Example 2 is obtained by blending 5.46 g of sodium
16
CA 02603426 2007-09-27
silicate (Wako Pure Chemical Industries, Ltd., guaranteed reagent)
as a coating film forming agent, 5.0 g of vitamin C (L-ascorbic
acid) (Wako Pure Chemical Industries, Ltd., guaranteed reagent)
as a oxygen scavenger, 0. 4 g of EDTA-2Na (Wako Pure Chemical Industries,
Ltd., guaranteed reagent) as a scale inhibitor, and 4.0 g of sodium
hydroxide (Wako Pure Chemical Industries, Ltd., guaranteed reagent)
as a pH adjustor. 500 mg of the water treating agent were loaded
into per 1 liter of softened water.
(3) Example 3
The water treating agent of Example 3 is obtained by blending
pure water with a coating film forming agent, a oxygen scavenger,
a scale inhibitor, and a pH adjustor each in a predetermined amount
per 100 g in total of the water treating agent, in the same manner
as in the water treating agent of Example 1. That is, the water
treating agent of Example 3 is obtained by blending 9. 66 g of sodium
silicate as a coating film forming agent, 7.5 g of vitamin C
(L-ascorbic acid) (Wako Pure Chemical Industries, Ltd., guaranteed
reagent) as a oxygen scavenger, 0.4 g of EDTA-2Na (Wako Pure Chemical
Industries, Ltd., guaranteed reagent) as a scale inhibitor, and
4.0 g of sodium hydroxide (Wako Pure Chemical Industries, Ltd.,
guaranteed reagent) as a pH adjustor. 500 -mg ;:f the water treating
agent were loaded into per 1 1ite- o' softened water.
[Comparative ExamplPs]
(1) Comparative Example 1
17
CA 02603426 2007-09-27
The water treating agent of Comparative Example 1 is obtained
by blending pure water with a coating film forming agent, a scale
inhibitor, and a pH adjustor each in a predetermined amount per
100 g in total of the water treating agent. Thatis, the water treating
agent of Comparative Example 1 is obtained by blending 0.04 g of
sodium silicate (Wako Pure Chemical Industries, Ltd., guaranteed
reagent) as a coating film forming agent, 0.4 g of EDTA-2Na (Wako
Pure Chemical Industries, Ltd., guaranteed reagent) as a scale
inhibitor, and 4.0 g of sodium hydroxide (Wako Pure Chemical
Industries, Ltd., guaranteed reagent) as a pH adjustor. 500 mg of
the water treating agent were loaded into per 1 liter of softened
water.
(2) Comparative Example 2
The water treating agent of Comparative Example 2 is obtained
by blending pure water with a coating film forming agent, a scale
inhibitor, and a pH adjustor each in a predetermined amount per
100 g in total of the water treating agent, in the same manner as
in the water treating agent of Comparative Example 1. That is, the
water treating agent of Comparative Example 2 is obtained by blending
21.5 g of sodium silicate (Wako Pure Chemical Industries, Ltd.,
guaranteed reagent) as a coating film forming agent, 0. 4 g of EDTA-2Na
(Wako Pure Chemical ~ r_i ~ctr~ es, Ltd. , guaranteed reagent) as a scale
inhibitor, ar_-~_ 4.0 g of sodium hydroxide (Wako Pure Chemical
Industries, Ltd., guaranteed reagent) as a pH adjustor. 500 mg of
18
CA 02603426 2007-09-27
the water treating agent were loaded into per 1 liter of softened
water.
[Evaluation]
(1) Experimental conditions
Boiler feed water added with a predetermined amount of each
of the water treating agents shown in Examples 1 to 3 and Comparative
Examples 1 and 2 was used to evaluate the heat transfer surface
of a boiler tube for scale adhesion property and corrosion. The
evaluation for scale adhesion property and corrosion was performed
by means of an experimental once-through boiler having an amount
of evaporation of 1.35 kg/hr. Softened water added with a
predetermined amount of each of the water treating agents shown
in Examples 1 to 3 and Comparative Examples 1 and 2 intended for
boiler feed water was supplied to the experimental once-through
boiler, and the boiler was operated in such a manner that the blow
rate of the boiler feed water would be 10% while steam having a
pressure of 0.3 MPa was continuously generated. The experimental
once-through boiler was operated continuously for 48 hours in such
a manner that the evaluation of the heat transfer surface of the
boiler tube for scale adhesion property and corrosion would be as
close as possible to the evaluation of an actual device. The heat
transtei ;,urface of the boiler tube was evaluated for scale adhesion
property by: sampling boiler water (referring to water in the boiler
tube which had received heating) 24 hours after the initiation of
19
CA 02603426 2007-09-27
the operation of the boiler; measuring the Ca concentration in the
boiler water; evaluating the boiler water for Ca solubility;
extracting a boiler tube for evaluation from the experimental
once-through boiler after the completion of the operation for 48
hours; and observing the heat transfer surface of the boiler tube.
Inaddition, the heat transfer surface of the boiler tube was evaluated
for corrosion by observing the heat transfer surface of the boiler
tube for evaluation.
(2) Evaluation on scale adhesion property
The heat transfer surface of a boiler tube is evaluated for
scale adhesion property through the following procedure.
At first, boiler water is sampled 24 hours after the initiation
of the operation of the boiler. The Ca concentration of the sampled
boiler water is measured by means of an ICP light emission analyzer.
The boiler water is evaluated for Ca solubility on the basis of
the measured Ca concentration. Then, the heat transfer surface of
the boiler tube is evaluated for scale adhesion property. Here,
the evaluation for scale adhesion property based on the evaluation
for Ca solubility is performed by paying attention to the fact that
the precipitation of Ca as a scale on the heat transfer surface
of theboiler tube reduces the Ca concentration, that is, Ca soluhil i_ty
of theboilerwater. Tobe specific, if the experimental o.., e- _'s r~ah
boiler is operated at a blow rate of the boiler .Vater containing
Ca at a concentration of 1. 0 mg/liter-CaC03 and Ca does not precipitate
CA 02603426 2007-09-27
as a scale on the heat transfer surface of the boiler tube, the
Ca solubility of the boiler water does not reduce, and the Ca
concentration is detected while maintaining its value of about 10
mg/liter. On the other hand, if Ca precipitates as a scale on the
heat transfer surface of the boiler tube, the Ca solubility of the
boiler water reduces, and a Ca concentration lower than 10 mg/liter
is detected.
Next, the operation of the experimental once-through boiler
is stopped 48 hours after the initiation of the operation of the
boiler. A boilertubeisextractedfromthestopped boiler. Whether
Ca adheres as a scale to the heat transfer surface of the boiler
tube is observed with the eyes and a loupe, followed by evaluation
for scale adhesion property. The evaluation for scale adhesion
property can also be performed on the basis of the thickness of
a scale adhering to the heat transfer surface of the boiler tube,
the thickness being measured by means of a thicknessmeter.
Thus, Table 1 shows the Ca solubility of the boiler feed water
added with a predetermined amount of each of the water treating
agents shown in Examples 1 to 3 and Comparative Examples 1 and 2
as a Ca concentration 24 hours after the initiation of the operation
of the boiler.
According to the results shown in 1, the boiler feed
water added with a predetermined amc,,~. nc of each of the water treating
agents shown in Examples 1 to 3 and Comparative Example 1 had a
21
CA 02603426 2007-09-27
Ca solubility of 10 mg/liter or more. On the other hand, the boiler
feed water added with a predetermined amount of the water treating
agent shown in Comparative Example 2 had a Ca solubility of 10 mg/liter
or less . The Ca solubility in the water treating agent of Comparative
Example 2 was small because a thick layer of a silica component
in sodium silicate bound to Ca adhered to the heat transfer surface
of the boiler tube owing to a large sodium silicate concentration
in the boiler feed water.
(3) Evaluation for corrosion
The heat transfer surface of a boiler tube was evaluated for
corrosion through the following procedure. At first, the operation
of the boiler is stopped 48 hours after the initiation of the operation.
Aboiler tube is extracted fromthe stoppedboiler. Ascale is removed
from the surface of the tube through water washing or acid cleaning.
Next, the surface of the boiler tube from which a scale has been
removed is visually observed with the eyes, a loupe, or the like
for investigation into the presence or absence of general corrosion.
Finally, whether local corrosion such as pitting corrosion (also
referred to as pitting) occurs on the surface of the boiler tube
from which a scale has been removed is visually observed with the
eyes, a loupe, or the like . The depth of a site where pitting corrosion
occurs is measureci with~ an on~ ic-~;rl displacement gauge so that the
maximum depth of pitti.g corrosion is determined.
Table 1 shows the presence or absence of general corrosion,
22
CA 02603426 2007-09-27
the presence or absence of local corrosion, and the maximum depth
of pitting corrosion in the heat transfer surface of a boiler tube
for boiler feed water added with a predetermined amount of each
of the water treating agents shown in Examples 1 to 3 and Comparative
Examples 1 and 2.
According to the results shown in Table 1, the general corrosion
and local corrosion of the heat transfer surface of a boiler tube
were prevented in case of the boiler feed water added with a
predetermined amount of each of the water treating agents shown
in Examples 1 to 3 and Comparative Example 2. On the other hand,
the general corrosion of the heat transfer surface of a boiler tube
was prevented, but the local corrosion of the heat transfer surface
was not prevented in case of the boiler feed water added with a
predetermined amount of the water treating agent shown in Comparative
Example 1.
As can be seen from the foregoing, according to any one of
the water treating agents according to Examples 1 to 3, a coating
film capable of suppressing corrosion is formed of the coating film
forming agent on the heat transfer surface of the boiler tube.
Dissolved oxygen in boiler feed water is removed by the oxygen
scavenger. Furthermore, the scaling of the heat transfer surface
of :-i:e s;eiier tube is prevented by the scale inhibitor, and the
pH of th,~ boiler feed water is adjusted. Therefore, the general
corrosion and local corrosion of the heat transfer surface of the
23
CA 02603426 2007-09-27
boiler tube can be prevented, and excellent heat transfer property
can be obtained.
Next, an example of a water treatment method using the water
treating agent of the present invention for a steam boiler device
will be describedwith reference to Fig. 1. Fig. 1 shows the schematic
constitution of a steam boiler device.
In Fig. 1, a steam boiler device 1 is constituted by a water
supply device portion 2, a steam boiler 3, a water treating agent
supply portion 4, and a control portion S.
The water supply device portion 2 is constituted by various
pretreatment devices supplyingboiler feed feedwatthe.steam boiler
3, and is equipped with a water softener 21, a deaeration device
22, and a water supply tank 23. The water softener 21 is a device
for subjecting makeup water such as tap water or industrial water
supplied from the outside via a makeup water supplying path W1 to
a softening treatment (softening) . The makeup water is subjected
to a softening treatment (softened) for removing a calcium ion or
a magnesium ion in the makeup water. Here, the calcium ion or the
magnesium ion is removed for preventing such ion from adhering as
a scale to the heat transfer surface of a boiler tube to reduce
heat transfer property. The makeup water (soft water) softened by
rile water softener 21 is deaerated by the deaeration device 2-_
The deaeration device 22 is a device mainly int,:~~,ded for
removing dissolved oxygen in soft water in advance because the
24
CA 02603426 2007-09-27
presence of dissolved oxygen in soft water makes the heat transfer
surface of a boiler tube prone to corrode with the oxygen. For example,
a device for continuously removing oxygen by means of a gas separable
membrane or through heating, or a batch type device utilizing reduced
pressure or ultrasonic wave is used for the deaeration device 22.
Of those, a device using a gas separable membrane that is a membrane
through which a gas passes and no liquid passes is a preferable
device because the device can be easily handled, can be continuously
operated stably, and is inexpensive. That is, a device using a gas
separable membrane can deaerate soft water by: causing water
subjected to a softening treatment (soft water) to flow into the
gas separable membrane; and establishing a vacuum state outside
the membrane so that a gas in the soft water passes through the
membrane to be evacuated to the outside of the membrane.
The soft water from which dissolved oxygen has been removed
by the deaeration device 22 is reserved in the water supply tank
23. The water supply tank 23 reserves boiler feed water to be supplied
to the steam boiler 3, and is equipped with a detection portion
24 for measuring and detecting a silica concentration and a dissolved
oxygen amount in the boiler feed water. The soft water deaerated
by the deaeration device 22 and condensed water that has received
heat exchange with steam generated by the steam'~c3 in a load
device (not shown) are supplied to the w'-er supply tank 23 via
a condensed water collecting path (not shown) . In addition, a water
CA 02603426 2007-09-27
treating agent reserved in a water treating agent tank 41 to be
described later is supplied by a chemical supplying pump P1 via
a water treating agent supplying path W2 to the water supply tank
23 for treating the boiler feed water with a chemical. The water
treating agent is a water treating agent described in the above
embodiment of the present invention, and is obtained by blending
water as a main component with a coating film forming agent of which
a coating film is formed on the heat transfer surface of a boiler
tube, a oxygen scavenger, a scale inhibitor, and a pH adjustor.
Therefore, supplying the water treating agent to the boiler feed
water can prevent the corrosion of the heat transfer surface of
the boiler tube and the adhesion of a scale to the heat transfer
surface. The boiler feed water is supplied by a water supplying
pump P2 from the water supply tank 23 to the steam boiler 3 via
a water supplying path W3.
Here, the silica concentration detected by the detection
portion 24 is the concentration of silica (silicon dioxide), and
is measured and detected in accordance with molybdenum yellow
absorption photometry described in JIS K 0101. In addition, a
dissolved oxygen amount is measured and detected in accordance with
the industrial water testing method described in JIS K 0101.
The steam boiler 3 to which t-n_ iciler feed water has been
supplied generates steam, an= L-he steam is supplied to various load
devices (not shown) via a steam line Sl.
26
CA 02603426 2007-09-27
The steam boiler 3 heats the boiler feed water to generate
steam, and is in a high-temperature, high-pressure environment.
In particular, a boiler tube (not shown) is in a severe environment
because the outer surf ace side of the tube directly receives radiation
heat from a heating source and high-temperature, high-pressure
boiler feed water or steam flows on the inner surface side of the
tube (the heat transfer surface of the boiler tube) . When the boiler
feed water has poor water quality, the heat transfer surface of
the boiler tube is corroded, or a scale adheres to the heat transfer
surface. Accordingly, the steam boiler 3 may be unable to perform
a stable operation continuously for a long time period. Therefore,
the management of the properties of water of the boiler feed water
is an important item, and such water treatment method for boiler
feed water as shown in this example has been demanded.
The water treatment method of this example is a method involving
supplying the water treating agent the concentration of which has
been adjusted to a predetermined concentration in advance and which
is reserved in the water treating agent tank 41 to the water supply
tank 23 in correspondence with a fluctuation in concentration of
silica or amount of oxygen dissolved into the boiler feed water
reserved in the water supply t-ank 23 if such fluctuation occurs.
In this case, the amour n-' the water treating agent to be supplied
to the water sl,.r,ply tank 23 is controlled in correspondence with
a fluctuation in silica concentration or dissolved oxygen amount.
27
CA 02603426 2007-09-27
The amount of the water treating agent to be supplied is controlled
by controlling the ejection amount of the chemical supplying pump
P1 of the water treating agent supply portion 4 by means of the
control portion 5 to be described later.
The water treating agent supply portion 4 is constituted by
the above-described water treating agent tank 41 reserving the water
treating agent and the chemical supplying pump Pl for supplying
the water treating agent from the water treating agent tank 41 to
the water supply tank 23. The water treating agent tank 41 reserves
the water treating agent the concentration of which has been adjusted
to a predetermined concentration in advance.
A fluctuation in silica concentration or dissolved oxygen
amount in the boiler feed water is judged by the control portion
on the basis of the silica concentration or dissolved oxygen amount
in the boiler feedwater detectedby the detection portion 24 possessed
by the water supply tank 23.
As shown in Fig. 1, the control portion 5 is electrically
connected to each of the devices constituting the water supply device
portion 2 and the water treating agent supply portion 4. To be
specific, the control portion 5 is electrically connected to the
detection portion 24 possessed by the water supply tank 23 and the
chemic,~j_ supplying pump P1.
The control portion 5 is constituted as a logical circuit mai r~=y
composed of a microcomputer, and includes: a central processing
28
CA 02603426 2007-09-27
unit (CPU) 51; an RAM 52 for temporarily recording data; an ROM
53 on which a processing program is recorded; and an input/output
port 54 for inputting or outputting various signals. As described
above, information concerning a silica concentration or a dissolved
oxygen amount is inputted to the control portion 5 from the detection
portion 24, and the control portion outputs a signal for controlling
the ejection amount of the chemical supplying pump P1 on the basis
of the input, to thereby control the ejection amount of the chemical
supplying pump Pl.
An increase in amount of the water treating agent to be supplied
to the water supply tank 23 makes a scale apt to adhere to the heat
transfer surface of the boiler tube, so heat transfer property
deteriorates. The control of the ejection amount of the chemical
supplying pump Pl is intended for preventing the deterioration.
On the other hand, a reduction in amount of the water treating agent
to be supplied to the water supply tank 23 reduces the thickness
of a silica layer or of an iron hydroxide layer to be formed on
the heat transfer surface of the boiler tube, so the heat transfer
surfaceoftheboilertube mayundergo generalcorrosion. Thecontrol
is intended alsofor preventing the general corrosion. Inaddition,
when the distribution of the thickness of the silica layer or of
the iron hydroxide layer to be formed on the heat transtFr surface
of the boiler tube becomes nonuniform, the heat transfer surface
of the boiler tube is apt to undergo local corrosion. The control
29
CA 02603426 2007-09-27
is intended also for preventing the local corrosion.
As described above, according to the water treatment method
of this example, when a silica concentration or a dissolved oxygen
amount in boiler feed water fluctuates, the general corrosion and
local corrosion of the heat transfer surface of a boiler tube can
be prevented, and excellent heat transfer property can be obtained
by controlling the amount of a water treating agent, which is obtained
by blending a coating film forming agent of which a coating film
is formed on the heat transfer surface of the boiler tube, a oxygen
scavenger, a scale inhibitor, andapHadjustor, and the concentration
of which is adjusted to a predetermined concentration, to be supplied
from a water treating agent tank to a water supply tank in
correspondence with the fluctuation.