Note: Descriptions are shown in the official language in which they were submitted.
CA 02605200 2007-11-02
PITCH-BASED CARBON FOAM HEAT
SINK WITH PHASE CHANGE MATERIAL
This is a divisional application of Canadian Patent Application Serial No.
2,334,583 filed on
Apri12, 1999.
The present invention relates to porous carbon foam filled with phase change
materials
and encased to form a heat sink product, and more particularly to a process
for producing them.
It should be understood that the expression "the invention" and the like
encompasses the subject-
matter of both the parent and the divisional applications.
BACKGROUND OF THE INVENTION
There are currently many applications that require the storage of large
quantities
of heat for either cooling or heating an object_ Typically these applications
produce heat
so rapidly that normal dissipation through cooling fins, natural convectiori,
or radiation
cannot dissipate the heat quickly enough and, thus, the object over heats_ To
alleviate
this problem, a material with a large specific heat capacity, such as a heat
sink, is placed
in contact with the object as it heats. During the heating process, heat is
transferred to
the heat sink from the hot object, and as the heat sink's temperature rises,
it "stores" the
heat more rapidly than can be dissipated to the environment through
convection.
Unfortunately, as the temperature of the heat sink rises the heat flux from
the hot object
decreases, due to a smaller temperature difference between the two objects.
Therefore,
although this method of energy storage can absorb large quantities of heat in
some
applications, it is not sufficient for all applications
Another method of absorbing heat is through a change of phase of the material,
rather than a change in temperature. Typically, the phase transformation of a
material
absorbs two orders of magnitude greater thermal energy than the~teat
capacity'of the
material. For example, the vaporization of I gram of water at 100 C absorbs
2,439
joules of energy, whereas changing the temperature of water from 99 C to 100 C
only
absorbs 4.21 Joules of energy. In other words, raising the temperature of 579
grams of
water from 99 C to 100 C absorbs the same amount of heat as evaporating 1 gram
of
water at 100 C. The same trend is found at the melting point of the material.
This
1
CA 02605200 2007-11-02
phenomenon has been utilized in some applications to either absorb or evolve
tremendous amounts of energy in situations where heat sinks will not work.
Although a solid block of phase change material has a very large theoretical
capacity to absorb heat, the process is not a rapid one because of the
difficulties of heat
S transfer and thus it cannot be utilized in certain applications. However,
the utilization of
the high thennal conductivity foam will overcome the shortcomings described
above. If
the high conductivity foam is filled with the phase change material, the
process can
become very rapid. Because of the extremely high conductivity in the struts of
the foam,
as heat contacts the surface of the foam, it is rapidly transmitted throughout
the foam to
a very large surface area of the phase change material. Thus, heat is very
quickly
distributed throughout the phase change material, allowing it to absorb or
emit thermal
energy extremely quickly without changing temperature, thus keeping the
driving force
for heat transfer at its maximum.
Heat sinks have been utilized in the aerospace community to absorb energy in
applications such as missiles and aircraft where rapid heat generation is
found. A
material that has a high heat of melting is encased in a graphite or metallic
case, typically
aluminum, and placed in contact with the object creating the heat. Since most
phase
change materials have a low thermal conductivity, the rate of heat transfer
through the
material is limited, but this is offset by the high energy absorbing
capability of the phase
change. As heat is transmitted through the metallic or graphite case to the
phase change
material, the phase change material closest to the heat source begins to melt.
Since the
temperature of the phase change material does not change until all the
material melts,
the flux from the heat source to the phase change material remains relatively
constant.
However, as the heat continues to melt more phase change material, more liquid
is
formed. Unfortunately, the liquid has a much lower thermal conductivity, thus
hampering heat flow further. In fact, the overall low thermal conductivity of
the solid
and liquid phase change materials limits the rate of heat absorption and,
thus, reduces
the efficiency of the system.
Recent developments of fiber-reinforced composites, including carbon foams,
have been driven by requirements for improved strength, stiffness, creep
resistance, and
toughness in structural eneineering materials. Carbon fibers have led to
significant
2
CA 02605200 2007-11-02
advancements in these properties in composites ofvarious polymeric, metal, and
ceramic
matrices.
However, current applications of carbon fibers have evolved from structural
reinforcement to thermal management in application ranging from high-density
electronic modules to conununication satellites. This has stimulated research
into novel
reinforcements and composite processing methods. High thermal conductivity,
low
weight, and low coefficient of thermal expansion are the primary concerns in
thermal
management applications. See Shih, Wei, "Development of Carbon-Carbon
Composites
for Electronic Thermal Management Applications," IDA Workshop, May 3-5, 1994,
supported by AF Wright Laboratory under Contract Number F33615-93-C-2363 and
AR Phillips Laboratory Contract Number F29601-93-C-0165 and Engle, G.B., "High
Thermal Conductivity C/C Composites for Thermal Management," IDA Workshop,
May 3-5, 1994, supported by AF Wright Laboratory under Contract F33615-93-C-
2363
and AR Phillips Laboratory Contract Number F29601-93-C-0165. Such applications
are
striving towards a sandwich type approach in which a low-density structural
core
material (i.e. honeycomb or foam) is sandwiched between a high thermal
conductivity
facesheet. Structural cores are limited to low density materials to ensure
that the weight
limits are not exceeded. Unfortunately, carbon foams and carbon honeycomb
materials
are the only available materials for use in high temperature applications
(>1600 C).
High thermal conductivity carbon honeycomb materials are extremely expensive
to
manufacture compared to low conductivity honeycombs, therefore, a performance
penalty is paid for low cost materials. High conductivity carbon foams are
also more
expensive to manufacture than low. conductivity carbon foams, in part, due to
the
starting materials.
In order to produce high stiffness and high conductivity carbon foams,
invariably, a pitch must be used as the precursor. This is because pitch is
the only
precursor which forms a highly aligned graphitic structure which i$a
requirement for
high conductivity. Typical processes utilize a blowing technique to produce a
foam of
the pitch precursor in which the pitch is melted and passed from a hioh
pressure region
to a low pressure reeion. Thermodynamicalty, this produces a "Flash," thereby
causing
the low molecular weight compounds in the pitch to vaporize (the pitch boils),
resultinQ
3
CA 02605200 2007-11-02
in a pitch foam. See Hagar, Joseph W. and Max L. Lake, "Novel Hybrid
Composites
Based on Carbon Foams," Mat. Res. Soc. Symp -, Materials Research Society,
270:29-
34 (1992); Hagar, Joseph W. and Max L. Lake, "Formulation of a Mathematical
Process
Model Process Ivlodel for the Foaming of a Mesophase Carbon Precursor," Mat.
Res.
Soc. Symp_, Materials Research Society, 270:35-40 (1992); Gibson, L.J. and
M.F.
Ashby, Cellular Solids' Structures & Properties, -Pergamon Press, New York
(1988);
Gibson, L.J., Mat. Sci. and Eng A 110, 1(1989); Knippenberg and B. Lersmacher,
Phillips Tech_ Rev., 36(4), (1976); and Bonzom, A., P. Crepaux and E. J.
Moutard,
U.S. patent 4,276,246, (1981). Then, the pitch foam must be oxidatively
stabilized by
heating in air (or oxygen) for many hours, thereby, crosslinking the structure
and
"stabilizing" the pitch so it does not melt during carbonization. See Hagar,
Joseph W.
and Max L. Lake, "Formulation of a Mathematical Process Model Process Model
for
the Foanung of a Mesophase Carbon Precursor, Mat. Res. Soc. Symp., Materials
Research Society, 270:35-40 (1992); and Wlute, J_L., and P.M. Shaeffer.
Carbon,
27:697 (1989). This is a time consuming step and can be an expensive step
depending
on the part size and equipment required. The "stabilized" or oxidized pitch is
then
carbonized in an inert atmosphere to temperatures as high as I 100 C. Then,
graphitization is performed at temperatures as high as 3000 C to produce a
high thermal
conductivlty graphitic structure, resulting in a stiff and very thermally
conductive foam.
Other techniques utilize a polymeric precursor, such as phenolic, urethane, or
blends of these with pitch. See Hagar, Joseph W. and Max L. Lake, "Idealized
Strut
Geometries for Open-Celled Foams," Mat. Res. Soc. Symp., Materials Research
Society,
270:41-46 (1992); Aubert, J. W., MRS Symposiurr Proceedings, 207:117-127
(1990);
Cowlard, F.C. and J.C. Lewis, J. of Mat. Sci., 2:507-512 (1967); and Noda, T.,
Inagaki
and S. Yamada, J. of Non-Crystalline Solids, 1:285-302, (1969). High pressure
is
applied and the sample is heated. At a specified temperature, the pressure is
released,
thus causing the liquid to foam as volatile compounds are release'd. The
polymeric
precursors are cured and then carbonized without a stabilization step.
However, these
precursors produce a"glassy" or vitreous carbon which does not exhibit
graphitic
structure and, thus, has low thermal conductivity and low stiffness. See
HaQar, Joseph
4
CA 02605200 2007-11-02
W. and Max L. Lake, "Idealized Strut Geometries for Open-Celled Foams," Mat.
Res.
Soc. Symp., Materials Research Society, 270: 41-46 (1992).
In either case, once the foam is formed, it is then bonded in a separate step
to the
facesheet used in the composite. This can be an expensive step in the
utilization of the foam.
The extraordinary mechanical properties of commercial carbon fibers are due to
the
unique graphitic morphology of the extruded filaments: See Edie, D. D., "Pitch
and
Mesophase Fibers," in Carbon Fibers, Filaments and Composites, Figueiredo
(editor), Kluwer
Academic Publishers, Boston, pp. 43-72 (1990). Contemporary advanced
structuial
composites exploit these properties by creating a disconnected network of
graphitic filaments
held together by an appropriate matrix. Carbon foam derived from a pitch
precursor can be
considered to be an interconnected network of graphitic ligaments or struts,
as shown in
Figure 1. As such interconnected networks, they represent a potential
alternative as
reinforcement in structural composite materials.
The process'of this invention overcomes current manufacturing limitations by
avoiding a "blowing" or "pressure release" technique to produce the foam.
Furthermore, an
oxidation stabilization step is not required, as in other methods used to
produce pitch based
carbon foams with a highly aligned graphitic structure. This process is less
time consuming,
and therefore, will be lower in cost and easier to fabricate. The foam can be
produced with
an integrated sheet of high thermal conductivity carbon on the surface of the
foam, thereby
producing a carbon foam with a smooth sheet on the surface to improve heat
transfer.
SUMMARY OF THE INVENTION
The invention includes a process of producing a carbon foam heat sink
comprising:
selecting an appropriate mold shape; introducing pitch to an appropriate level
in said mold;
purging air from said mold to form a vacuum; heating said pitch to a
temperature sufficient to
coalesce said pitch into a liquid; releasing said vacuum and backfilling an
inert fluid at a
static pressure up to about 6.89 x 106 Pa (about 1000 psi); heating said pitch
to a temperature
sufficient to cause gases to evolve and form a carbon foam; heating said
carbon foam *to a
temperature sufficient to coke the pitch; cooling said carbon foam to room
temperature and
CA 02605200 2007-11-02
simultaneously releasing said inert fluid; at least partiaTly encasing said
carbon foam in an
encasement material; and at least partially filling porous regions of said
carbon foam with a
phase change material.
The invention also includes a carbon foam heat sink product as produced by the
process that is set forth in the preceding paragraph.
In addition, the invention includes a process of producing a carbon foam heat
sink
comprising: selecting an appropriate mold shape and a mold composed of a
material that
molten pitch does not wet; introducing said pitch to an appropriate level in
the mold;
purging air from said mold to form a vacuum; heating said pitch to a
temperature sufficient to
coalesce said pitch into a liquid; releasing said vacuum and backfilling an
inert fluid at a
static pressure up to about 6.89 x 106 Pa (about 1000 psi); heating said pitch
to a temperature
sufficient to coke the pitch and yield a carbon foam; cooling said foam to
room temperature
and simultaneously releasing said inert fluid; at least partially encasing
said foam in an
encasement material; and at least partially filling porous regions of said
foam with a phase
change material.
Furthermore, the invention includes a carbon foam heat sink product as
produced by
the process that is set forth in the preceding paragraph. -
The invention also includes a process of producing a carbon foam heat sink
comprising: selecting an appropriate mold shape; introducing pitch to an
appropriate level in
said mold; purging air from said mold to form a vacuum; heating said pitch to
a temperature
sufficient to coalesce said pitch into a liquid; releasing said vacuum and
backfilling an inert
fluid at a static pressure up to about 6.89 x IU6. Pa (about 1000 psi);
heating said pitch to a
temperature sufficient to cause gases to evolve and form carbon foam; heating
said carbon
foam to a temperature sufficient to coke the pitch; cooling said carbon foam
to room
temperature and simultaneously releasing said inert fluid; placing facesheets
on.the opposite
sides of said carbon foam; adhering the facesheets to said carbon foam; at
least partially
encasing said carbon foam and facesheets in an encasement material; and at
least partially
filling porous regions of said carbon foam with a phase change material.
5A
CA 02605200 2007-11-02
In addition, the invention includes a composite carbon foam heat sink product
produced bythe process that is set forth in the preceding paragraph.
Furthermore, the invention includes an open cellular, graphitic
carbon foam containin.g porous regionsat least partially filled with a phase
change
material.
Moreover, the invention includes the carbon foam as described in the
preceding paragraph, wherein the carbon foam is produced by the process
comprising:
(1) heating a liquified pitch under non-oxidizing conditions below 5000 C.
while
applying a static superatmospheric pressure sufficient to produce a carbon
foam; (2)
coking said carbon foam by heating said carbon foam at a temperature of 500 -
1000
C. under non-oxidizing conditions while applying a static superatmospheric
pressure;
(3) heating the coked carbon foam under conditions sufficient to produce'
graphitic carbon foam; and (4) at least partially filling porous regions in
the graphitic
carbon foam with said phase change material.
The invention includes the carbon foam that is described in the two preceding
paragraphs, wherein the carbon foam is at least partially encased.
The invention also includes a temperature control apparatus attached to a
spacecraf3 in an external location thereof, said apparatus comprising an
encased
carbon foam as described in the preceding paragraph containing in at least
some of its
pores a phase change material that will (1) undergo a phase change at a
temperature
induced by solar radiant energy when in space and (2) revert to its former
state when
not exposed to solar radiant energy when in space.
In addition, the invention includes arr apparatus for thawing frozen food
comprising the above-described encased carbon foam.
5B
CA 02605200 2007-11-02
Furthermore, the invention includes a temperature control apparatus for aiding
in maintaining a temperature of an object in contact therewith below 1800 C.
comprising the above-described encased carbon foam, which contains in at least
some
of its pores a phase change material that melts at an elevated temperature
above 800
C. but does inot vaporize below 1800 C.
Moreover, the invention includes a method for transferring heat comprising
transferring heat from an object to the above-described encased carbon foam,
which
contains in at least some of its pores a phase change material that undergoes
phase
change while said object is in contact with said encased carbon foam.
The invention also includes a method for transferring heat comprising
transferring heat to an object from the above-described encased carbon foam,
which
contains in at least some of its pores a phase change material that undergoes
phase
change while said object is in contact with said encased carbon foam.
In addition, the invention includes a method for reducing temperature rise of
an object when heat is generated due to conditions in a surrounding
environment, said
method comprising contacting said object in said surrounding environment with
the
above-described encas.ed carbon foam, which contains in at least some of its
pores a
phase change material that changes phase when said heat is generated in the
surrounding environment.
Furthermore, the invention includes a process for producing an open cellular
graphitic carbon foam comprising: (1) heating a liquified pitch under non-
oxidizing conditions below 500 C, while applying a static superatmospheric
pressure
sufficient to produce a carbon foam; (2) coking said carbon foam by heating
said
carbon foam at a temperature of 500 - 1000 C. under non-oxidizing conditions
while
applying a static superatmospheric pressure; (3) heating the coked carbon foam
under
5C
CA 02605200 2007-11-02
conditions sufficient to produce a graphitic carbon foam; and (4) at least
partially filling porous regions in the graphitic carbon foam with a phase
change material to produce the carbon foam as described in the seventh
paragraph of
this "Summary of the Invention" section.
Moreover, the invention includes a process comprising: (1) heating a liquified
pitch under non-oxidizing, superatmospheric conditions below about 500 C.
sufficient to produce a carbon foam; (2) coking said carbon foam by heating
said
carbon foam at a temperature above about 500 C. under non-oxidizing
superatmospheric conditions;,(3) heating the coked carbon foam under
conditions
sufficient to produce a substantially graphitic carbon foam; and (4) at least
partially
filling porous regions in the substanUn, graphitic carbon foam with a phase-
change
material.
According to an aspect of the present invention there is provided a process
comprising at least partially filling the porous region of a thermally
conductive, non-
oxidatively stabilized, mesophase pitch-derived carbon foam with a phase-
change
material.
An object of the present invention is production of encased high thermal
conductivity porous carbon foam filled with a phase change material wherein
tremendous amounts of thermal energy are stored and emitted very rapidly. The
porous foam is filled with a phase change material (PCM) at a temperature
close to
the operating temperature of'the device. As heat is added to the surface, from
a heat
source such as a computer chip, friction due to xe-entry through the
atmosphere, or
radiation such as sunlight, it is transmitted rapidly and uniformly throughout
the foam
and then to
SD
CA 02605200 2007-11-02
the phase change material_ As the material changes phase, it absorbs orders of
magnitude more energy than non-PCM material due to transfer of the latent heat
of
fusion or vaporization_ Conversely, the filled foam can be utilized to emit
energy
rapidly when placed in contact with a cold object.
Non-limiting embodiments disclosed herein are a device to rapidly thaw
frozen foods or freeze thawed foods, a design to prevent overheating of
satellites or
store thermal energy as they experience cyclic heating during orbit, and a
design to
cool leading edges during hypersonic flight or re-entry from space.
Another object of the present invention is to provide carbon foam and a
composite from a mesophase or isotropic pitch such as synthetic, petroleum or
coal-tar based pitch. Another object is to provide a carbon foam and a
composite
from pitch which does not require an oxidative stabilization step.
These and other objectives are accomplished by a method of producing
carbon foam heat sink wherein an appropriate mold shape is selected and
preferably an appropriate mold release agent is applied to walls of the mold.
Pitch
is introduced to an appropriate level in the mold, and the mold is purged of
air by
applying a vacuum, for example. Alternativeiy, an inert fluid could be
employed. The
pitch is heated to a temperature sufficient to coalesce the pitch into a
liquid which
preferably is of about 50 C to about 100 C above the softening point of the
pitch.
The vacuum is released and an inert fluid applied at a static pressure up to
about
6.89 x 106 Pa (about 1000 psi). The pitch is heated to a temperature
sufficient to
cause gases to evolve and foam the pitch. The pitch is further heated to a
temperature sufficient to coke the pitch and the pitch is cooled to room
temperature
with a simuftaneous and gradual release of pressure. The foam is then filled
with
a phase change material and encased to produce an efficient heat storage
product.
In another aspect, the previously described steps are employed in a mold
composed of a material such that the molten pitch does not adhere to the
surface
of the mold.
In yet another aspect, the objectives are accomplished by the carbon foam
product produced by the methods disclosed herein including a foam product with
a
smooth integral facesheet.
6
CA 02605200 2007-11-02
In still another aspect a carbon foam composite product is produced by
adhering
facesheets to the carbon foam produced by the process of this invention.
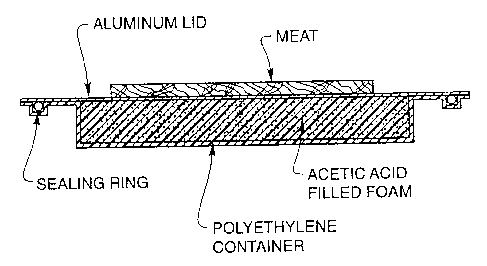
Fig. 1 is section cut of a heat sink device for thawing food using acetic acid
as
the phase change material.
Fig. 2 is a section cut of a heat sink to prevent overheating of sateliites
during
cyclic orbits.
Fig. 3 is a section cut of a heat sink used on the leading edge of a shuttle
orbiter.
Fig. 4 is a micrograph illustrating typical carbon foam with interconnected
carbon ligaments and open porosity.
Fig. 5-9 are micrographs of pitch-derived carbon foam graphitized at 2500 C
and at various magnifications.
Fig. 10 is a SEM rnicrograph of carbon foam produced by the process of this
invention.
Fig. 11 is a chart illustrating cumulative intrusion volume versus pore
diameter.
Fig. 12 is a chart illustratine log differential intrusion volume versus pore
diameter.
Fig. 13 is a graph illustrating the temperatures at wluch volatiles are given
off
from raw pitch.
Fig. 14 is an X-ray analysis of the graphitized foam produced by the process
of
th,is invention.
Figs. 15 A-C are photographs illustrating foam produced with alurninum
crucibles and the smooth structure or face sheet that develops.
Fig_ I 6A is a schematic view iiiustrating the production of a carbon foam
composite made in accordance with this invention.
Fig. 16B is a perspective view of the carbon foam composite of this invention.
DETAILED DESCRIPTION OF THE INVENIION
In order to illustrate the carbon foam heat sink product of this invention,
the
following examples are set forth. They are not intended to limit the invention
in any way_
7
CA 02605200 2007-11-02
Example 1: Device for Thawing Food
Acetic acid has a heat of melting of 45 J/g at a melting point of 11 C. The
heat of melting of food, primarily ice, is roughly 79 Jlg at 0 C. Therefore,
take a
block of foam and fill it with liquid acetic acid at room temp. The foam will
be
encased in a box made from an insulating polymer such as polyethylene on atl
sides except the top. The top of the foam/acetic acid block will be capped
with a
high thermal conductivity aluminum plate that snaps into place thus sealing
the
foam/acetic acid inside the polymer case (illustrated in figure 1). If the
foam block
is 25.4cm. x 38.1 cm. x 1.27 cm. (10-in. x 15-in. x 0.5-in.) thick, the mass
of foam is
614 grams. The mass of acetic acid that fills the foam is roughly 921 grams.
Therefore, when a piece of frozen meat is placed in contact with the top of
the
aluminum block, the foam will cool to the freezing point of the acetic acid
(11 C). At
this point, the heat given off from the acetic acid as it freezes (it also
remains at 11
C) will be equivalent to 49 KJ. This heat is rapidly transferred to the frozen
meat as
it thaws (it also remains at 0 C). This amount of heat is sufficient to melt
roughly
500 grams (1 lb.) of meat.
Example 2: Heat Sink To Prevent Overheating Of Satellites During Cyclic
Orbits.
Produce a carbon-carbon composite with the foam in which the foam is a
core material with carbon-carbon face sheets (figure 2). Fill the foam core
with a
suitable phase change material, such as a paraffin wax, that melts around the
maximum operating temperature of the satellite components. One method to
perform this would be to drill a hole in one surface of the carbon-carbon face
sheets and vacuum fill the phase change material in the liquid state into the
porous foam. Once filled, the sample can be cooled (the phase change material
solidifies) and the hole can be plugged with an epoxy or screw-type cap. The
epoxy
and any other sealant must be able to withstand the operating temperature of
the
application. The foam-core composite will then be mounted on the side of the
satellite that is exposed to the sun during orbit. As the satellite orbits the
earth and
is exposed to the sun, the radiant energy from the sun will begin to heat the
composite panel to the melting point of the phase change material. At this
point,
the panel will not increase in temperature as the phase change material melts.
The amount of radiant energy the panel can absorb will be dependent on the
thickness
8
CA 02605200 2007-11-02
and outer dimensions of the panel. This can be easily calculated and designed
through
knowledge of the orbit times of the satellite such that the material never
completely
melts and, thus, never exceeds the melt temperature. Then, when the satellite
leaves the
view of the sun, it will begin to radiate heat to space and the phase change
material will
begin to freeze. The cycle will repeat itself once the satellite comes into
view of the sun
once again.
Example 3: Heat Sink for Leading Edees
Currently, the shuttle orbiter experiences extreme heats during reentry.
Specifically, the leading edges of the craft can reach 1800 C and the betly of
the craft
can reach temperatures as high as 1200 C. If a foam core composite panel is
placed at
the surface of the leading edges and at the surface of the belly (Figure 3),
it would be
able to absorb enough energy to dramatically reduce the maximum~ temperature
of the
hot areas. This also would permit a faster re-entry or (steeper glide slope)
and maintain
the current maximum temperatures. In this case the phase change material would
most
likely be an alloy, e_g, germanium-silicon, which melts around 800-900C and
does not
vaporize until much higher than the maximum temperature of the craft_
For example, Germanium has a heat of formation (heat of melting) of 488 ]/g.
This would require 1.0 Kg of Germanium to reduce the temperature of 1 Kg of
existing
carbon/carbon heat-shield by 668 C. In other words, if the existing carbon-
carbon were
replaced pound-for-pound with germanium filled foam, the maximum temperature
of the
heat shield would only be about 113 1 C instead of about 1800 C during re-
entry,
depending on the duration of thermai loading.
Example 4
Pitch powder, granules, or pellets are placed in a mold with the desired final
shape of the foam. These pitch mateiials can be solvated if desired, In this
example,
Mitsubishi ARA-24 mesophase pitch was utilized. A proper mold release agent or
film is
applied to the sides of the mold to allow removal of the part. In this case,
Boron Nitride
spray and Dry Graphite Lubricant were separately used as a mold release agent.
If the
mold is made from pure aluminum, no mold release agent is necessary since the
molten
9
CA 02605200 2007-11-02
pitch does not adhere to the aluminum and, thus, will not stick to the mold.
Similar mold
materials may be found that the pitch does not adhere and, thus, they will not
need mold
release. The sample is evacuated to less than 1 torr and then heated to a
temperature
approximately 50 to 100 C above the softening point. In this case where
Mitsubishi ARA24
mesophase pitch was used, 300 C was sufficient. At this point, the vacuum is
released to a
nitrogen blanket and then a pressure of up to 6.89 x 106 Pa (1000 psi is
applied). The
temperature of the system is then raised to 800 C, or a temperature sufficient
to coke the
pitch which is 500 C to 1000 C. This is performed at a rate of no greater than
5 C/min. and
preferably at about 2 C/min. The temperature is held for at least 15 minutes
to achieve an
assured soak and then the fumace power is turned off and cooled to room
temperature.
Preferably the foam was cooled at a rate of approximately 1.5 C/min. with
release of
pressure at a rate of approximately 3.38 x 104 Pa/min (approximately 2
psi/min). Final foam
temperatures for three product runs were 500 C, 630 C and 800 C. During the
cooling cycle,
pressure is released gradually to atmospheric conditions. The foam was then
heat treated to
1050 C (carbonized) under a nitrogen blanket and then heat treated in separate
runs to
2500 C and 2800 C (graphitized) in Argon.
Carbon foam produced with this technique was examined with photomicrography,
scanning electron microscopy (SEM), X-ray analysis, and mercury porisimetry.
As can be
seen in the Figures 5-10, the isochromatic regions under crosspolarized light
indicate that the
struts of the foam are completely graphitic. That is, all of the pitch was
converted to graphite
and aligned along the axis of the struts. These struts are also similar in
size and are
interconnected throughout the foam. This would indicate that the foam would
have high
stiffness and good strength. As seen in Fig. 10 by the SEM micrograph of the
foam, the foam
is open cellular meaning that the porosity is not closed. Figures 11 and 12
are results of the
mercury porisimetry tests. These tests indicate that the pore sizes are in the
range of 90-200
microns.
A thermogravimetric study of the raw pitch was performed to determine the
temperature at which the volatiles are evolved. As can be seen in Figure 14,
the pitch loses
nearly 20% of its mass fairly rapidly in the temperature range between about
420 C and
about 480 C. Afthough this was performed at atmospheric pressure, the addition
of 6.89 x106
Pa (1000 psi) pressure will not shift this effect significantly. Therefore,
while the
CA 02605200 2007-11-02
pressure is at 6.89 x 106 Pa (1000 psi), gases rapidly evolved during heating
through the temperature range of 420 C to 480 C. The gases produce a foaming
effect (like boiling) on the molten pitch. As the temperature is increased
further to
temperatures ranging from 500 C to 1000 C (depending on the specific pitch),
the
foamed pifch becomes coked (or rigid), thus producing a solid foam derived
from
pitch. Hence, the foaming has occurred before the release of pressure and,
therefore, this process is very different from previous art.
Samples from the foam were machined into specimens for measuring the
thermal conductivity. The bulk thermal conductivity ranged from 58 Wlm= K to
106
W/m= K. The average density of the samples was 0.53 g/cm3. When weight is
taken into account, the specific thermal conductivity of the pitch derived
from foam
is over 4 times greater than that of copper. Further derivations can be
utilized to
estimate the thermal conductivity of the struts themselves to be nearly 700
W/m= K.
This is comparable to high thermal conductivity carbon fibers produced from
this
same ARA24 mesophase pitch.
X-ray analysis of the foam was performed to determine the crystalline
structure of the material. The x-ray results are shown in Figure 14. From this
data,
the graphene layer spacing (dOO2) was determined to be 0_336 nm. The coherence
length (La, oo) was determined to be 203.3 nm and the stacking height was
determined to be 442.3 nm.
The compression strength of the samples were measured to be 3.4 MPa
and the compression modulus was measured to be 73.4 MPa. The foam sample
was easily machined and could be handled readily without fear of damage,
indicating good strength.
It is important to note that when this pitch is heated in a similar manner,
but
only under atmospheric pressure, the pitch foams dramatically more than when
under pressure. In fact, the resulting foam is so fragile that it could not
even be
handled to perform tests. Molding under pressure serves to limit the growth of
the
cells and produces a usable material.
11
CA 02605200 2007-11-02
Example 5
An alternative to the method of Example 4 is to utilize a mold made from
aluminum. In this case two molds were used, an aluminum weighing dish and a
sectioned soda can. The same process as set forth in Example 4 is employed
except that the final coking temperature was only 630 C, so as to prevent the
aluminum from melting.
Figures 15 A-C illustrate the ability to utilized complex shaped molds for
producing complex shaped foam. In one case, shown in Fig. 15 A, the top of a
soda can was removed and the remaining can used as a mold. No release agent
was utilized. Note that the shape of the resulting part conforms to the shape
of the
soda can, even after graphitization to 2800 C. This demonstrates the
dimensional
stability of the foam and the ability to produce near net shaped parts.
In the second case, as shown in Figs. 15 B and C employing an aluminum
weight dish, a very smooth surface was formed on the surface contacting the
aluminum. This is directly attributable to the fact that the molten pitch does
not
adhere to the surface of the aluminum. This would allow one to produce complex
shaped parts with smooth surfaces so as to improve contact area for bonding or
improving heat transfer. This smooth surface will act as a face sheet and,
thus, a
foam-core composite can be fabricated in-situ with the fabrication of the face
sheet. Since it is fabricated together and an integral material no interface
joints
result, thermal stresses will be less, resulting in a stronger material.
The following examples illustrate the production of a composite material
employing the foam of this invention.
Example 6
Pitch derived carbon foam was produced with the method described in
Example 4. Referring to Fig. 16A the carbon foam 10 was then machined into a
block 5.08 cm x 5.08 cm x 1.27 cm (2" x 2"x 1/2"). Two pieces 12 and 14 of a
prepeg comprised of Hercules AS4 carbon fibers and ICI Fibirite
Polyetheretherkeytone thermoplastic resin also of 5.08 cm x 5.08 cm x 1.27 cm
(2" x 2" x 1/2") size were placed on the top and bottom of the foam sample,
and all
was placed in a matched graphite mold 16 for compression by graphite plunger
18. The composite sample was heated under an
12
CA 02605200 2007-11-02
applied pressure of 6.89 x 105 Pa (100 psi) to a temperature of 380 C at a
rate of
C/min. The composite was then heated under a pressure of 6.89 x 105 Pa (100
psi) to a temperature of 650 C. The foam core sandwich panel generally 20 was
then removed from the mold and carbonized under nitrogen to 1050 C and then
graphitized to 2800 C, resulting in a foam with carbon-carbon facesheets
bonded
to the surface. The composite generally 30 is shown in Figure 16B.
Example 7
Pitch derived carbon foam was produced with the method described in
Example 4. It was then machined into a block 5.08 cm x 5.08 cm x 1.27 cm
(2" x 2" x 1/2"). Two pieces of carbon-carbon material, 5.08 cm x 5.08 cm x
1.27 cm
(2" x 2" x 1/2 ), were coated lightly with a mixture of 50% ethanol, 50%
phenolic
Durez8 Resin available from Occidental Chemical Co. The foam block and
carbon-carbon material were positioned together and placed in a mold as
indicated in Example 6. The sample was heated to a temperature of 150 C at a
rate of 5 C/min and soaked at temperature for 14 hours. The sample was then
carbonized under nitrogen to 1050 C and then graphitized to 2800 C, resulting
in a
foam with carbon-carbon facesheets bonded to the surface. This is also shown
generally at 30 in Figure 16B.
Example.8
Pitch derived carbon foam was produced with the method described in Example
4_ The foam sample was then densified with carbon by the method of chemical
vapor
inftltration foi 100 hours. The density increased to 1.4 g/cm', the flexural
strength was
19.5 IvIPa and the flexural modulus was 2300 MPa_ The thermat conductivity of
the raw
foam was 58 W/m= K and the thermal conductivity of the densified foam was 94
W/m= K.
Exampte 9
Pitch derived carbon foam was produced with the inethod descnbed in Example
4. The foam sample was then densified with epoxy by the method of vacuum
impregnation. The epoxy was cured at 150 C for 5 hours_ The density increased
to 1.37
g/cm' and the flexuraf strenoth was measured to be 19.3 IviPa.
13
CA 02605200 2007-11-02
Other possible embodiments may include materials, such as metals, ceramics,
plastics, or fiber-reinforced plastics bonded to the surface of the foam of
this invention
to produce a foam core composite material with acceptable properties-
Additional
possible embodiments include ceramics, glass, or other materials impregnated
into the
foam for densification.
Based on the data taken to date from the carbon foam material, several
observations can be made outlining important features of the invention that
include:
1. Pitch-based carbon foam can be produced without an oxidative stabilization
step, thus saving time and costs.
2. High graphitic alignment in the struts of the foam is achieved upon
graphitization to 2500 C, and thus high thermal conductivity and stiffness
will be
exhibited by the foam, making them suitable as a core material for thermal
applications.
3. High compressive strengths should be achieved with mesophase pitch-based
carbon foams, making them suitable as a core material for structural
applications.
4. Foam core composites can be fabricated at the same time as the foam is
generated, thus saving time and costs.
5. Rigid monolithic preforms can be made with significant open porosity
suitable
for densification by the Chemical Vapor Infiltration method of ceramic and
carbon
infiltrants.
6. Rigid monolithic preforms can be made with significant open porosity
suitable
for activation, producing a monolithic activated carbon.
7. By varying the pressure applied, the size of the bubbles
formed during the foaming will change and, thus, the density, strength, and
other
properties can be affected.
The following alternative procedures and products can also be effected by the
process of this invention:
1. Fabrication of preforms with complex shapes for densrfication by CVI or
Melt
Impregnation.
2. Activated carbon monoliths with high thermal conductivity.
3. Optical absorbent.
4. Low density heating elements.
14
CA 02605200 2007-11-02
5. Firewall Material
6. Low secondary electron emission targets for high-energy physics
applications.
The present invention provides for the manufacture of pitch-based carbon foam
heat sink for structural and thermal composites. The process involves the
fabrication of a
graphitic foam from a mesophase or isotropic pitch which can be synthetic,
petroleum,
or coal-tar based. A blend of these pitches can also be employed. The
simplified process
utilizes a high pressure high temperature furnace and thereby, does not
require and
oxidative stabilization step. The foam has a relatively uniform distribution
of pore sizes
(-100 microns), very little closed porosity, and density of approximately 0.53
g/cm3.
The mesophase pitch is stretched along the struts of the foam structure and
thereby,
produces a highly aligned graphitic structure in the struts These struts will
exhibit
thermal conductivities and stiffness similar to the very expensive high
performance
carbon fibers (such as P-120 and K1100). Thus, the foam will exhibit high
stifFness and
thermal conductivity at a very low density (-0.5 g/cc). This foam can be
formed in place
as a core material for high temperature sandwich panels for both thermal and
structural
applications, thus reducing fabrication time. By utilizing an isotropic pitch,
the resulting
foam can be easily activated to produce a high surface area activated carbon.
The
activated carbon foam.will not experience the problems associated with
granules such as
attrition, channeling, and large pressure drops.