Note: Descriptions are shown in the official language in which they were submitted.
CA 02606208 2007-10-16
WO 2006/115284 PCT/JP2006/308918
1
DESCRIPTION
BIOFUEL CONVERSION PROCESS
Technical Field
The present invention is directed to a process,
method, apparatus and materials for efficient conversion of
waste vegetable oils into biofuel that does not use
methanol as a reactant or catalyst.
Background Art
Biofuel is a type of fuel made using non-petroleum
based oils converted to allow combustion within power
plants and engines as a replacement for heavy oils and
diesel fuel. Biofuels have desirable burning
characteristics and are derived from renewable resources.
For example, biofuels may be derived from vegetable oils
processed from crops.
Vegetable oils are used extensively in food
preparation in restaurants, hotels, hospitals and other
large institutions. The use of vegetable oils in food
preparation generates substantial waste product that must
be appropriately disposed of. Processing of these waste
vegetable oils into biofuel thus serves two beneficial
purposes, creation of a clean and environmentally friendly
CA 02606208 2007-10-16
WO 2006/115284 PCT/JP2006/308918
2
fuel source and the elimination of waste disposal
requirements.
To understand the scope of the potential use of the
present invention it is beneficial to appreciate the extent
of the generation of waste vegetable oils in our society.
As an example, the city of Okinawa, Japan, the home of the
inventor,'has about 1.2 million people. It is estimated
that Okinawa generates about 400,000 liters of waste
vegetable oils, primarily tempura cooking oils, every month,
but that amount may be only about 14 of the actual annual
production. Of the known amount, approximately 90% is
recoverable and available for reprocessing. In addition,
Okinawa has a very large US military base having 50,000
military and civilian personnel that is estimated to
generate about 100,000 liters of waste vegetable oil per
month.
There are several known processes relating to the
field of conversion of waste vegetable oils into biofuel.
In one practice, the waste vegetable oils are gathered and
stored in large drums, for example 55 gallon drums. The
waste vegetable oils are allowed to sit for thirty days so
that the sediments in the waste oil settle to the bottom of
the container. After the settling process, the top
clarified oil is removed for processing in a column
catalytic reaction chamber while the bottom layer of oil
having retained sediments and oil are sent for disposal.
CA 02606208 2007-10-16
WO 2006/115284 PCT/JP2006/308918
3
This process is inefficient as it requires extensive
storage periods and there is still a significant amount of
sediment contaminated waste oil that is sent for disposal.
It is also a common process in the convQrsion of vegetable
oils, whether virgin or waste oils, to use methanol as a
reactant or catalyst. The methanol based processes have as
an advantage that the resulting biofuel can be used in
engines without being mixed with other fuels. A volume of
vegetable oil is mixed with a solution of Methanol and
Sodium hydroxide. Approximately 800 of the oil volume
becomes fuel, and byproducts are glycerin, fatty acids.
Engines running on biodiesel sometimes register an
increase in Nitrous Oxide (NOx) emissions. The range of
increasing in NOx emissions resulting from biodiesel can be
anywhere between 1-15% but is generally around 5%. The
complete lack of sulfur in biodiesel fuel allows the use of
powerful NOx breaking catalysts that had been unusable.
The present invention is directed to a process,
method, apparatus and materials for efficient conversion of
waste vegetalale oils into biofuel that does not use
methanol as a reactant or catalyst. Engines running on
biofuel register a decrease in Nitrous Oxide (NOx)
emissions.
However, the methanol based processes result in a
biofuel that does not mix well with other fuels, such as
diesel fuel, so a small quantity of methanol derived
CA 02606208 2007-10-16
WO 2006/115284 PCT/JP2006/308918
4
biofuels must be mixed with other fuels, such as diesel
fuel for automobiles.
It would therefore be beneficial to have a biofuel
conversion process that does not require the storage and
settling of solids from the waste oils and that does not
waste a percentage of the oil contaminated with sediments,
and that results in a biofuel that can be mixed in a tank
with other fuels such as diesel fuel.
Disclosure of Invention
The present invention is directed to a process,
method, apparatus and materials for efficient conversion of
waste vegetable oils into biofuel that does not use
methanol as a reactant or catalyst. The resulting biofuel
is mixed with kerosene or heavy oil to form a stable diesel
fuel grade fuel that is mixable with diesel fuel. In
addition, the process and apparatus are also applicable to
the conversion of virgin vegetable oils and other waste or
virgin oils, such as used motor oil, into fuels or fuel
additives. In the process, the waste oils are mixed with a
blend of catalyst and absorption powders in a tank and
heated to about 80 degrees centigrade for 40 to 60 minutes.
The composition is then passed through a filter to remove
the added powders, any sediments and certain contaminates
in the oil including most carbon solids, as well as certain
fatty acids and consti'tuents of the waste oil. The mixing
CA 02606208 2007-10-16
WO 2006/115284 PCT/JP2006/308918
and filtering process clarifies the resulting biofuel and
enhances the energy content from 4,000 to 5,000
calories/gram to 9,000 to 10,000 calories/gram.
The clarified biofuel resulting from vegetable oils
5 may then be blended with kerosene and filtered through the
filter bed containing the removed sediments from the first
filtering process or a powder mixture of absorption powders
is added to the blended biofuel and kerosene, mixed for 40
to 60 minutes and then filtered. A similar process can be
used to recover and generate fuel grade oil from used or
waste motor oil, with a resulting product that can be mixed
with the biofuel derived from vegetable oils as a
replacement for the kerosene additive to produce a li'ght
grade or heavy grade fuel oil.
Brief Description of Drawings
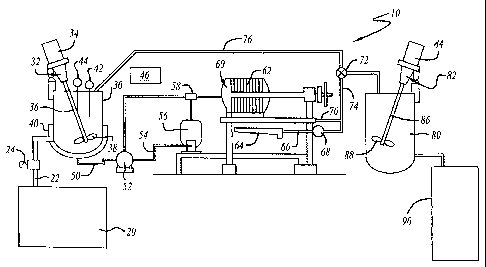
Figure 1 schematically depicts the processing
apparatus of the present invention.
Figure 2 schematically depicts an alternate
configuration of the processing apparatus of the present
invention.
Figure 3 schematically depicts the fuel blending
filter assembly and process used with the apparatus of
Figure 2.
Best Mode for Carrying'Out of the Invention
CA 02606208 2007-10-16
WO 2006/115284 PCT/JP2006/308918
6
Figure 1 schematically depicts a basic biofuel
conversion apparatus 10 according to a first aspect of the
present invention. The apparatus 10 includes an oil tank
20 that is either a large storage tank or plurality of
storage drums. Oil from tank 20 is routed via pipe 22 and
pump 24 to a catalyst tank 30. The catalyst tank 30
includes a mixing apparatus 32, for example a motor 34,
shaft 36 and impeller 38. The lower section of the
catalyst tank 30 includes a heating assembly 40, for
example a steam heat exchange system. The catalyst tank 30
also includes a temperature sensor 42 and may include a
level sensor 44. The temperature sensor 42 is connected to
a controller 46 that controls the pump 24 and the timing of
the process as well as the temperature control for the
heating assembly 40.
Upon completion of a reaction period in the catalyst
tank 30, the mixture is delivered via a pipe 50 to pump 52.
The output of pump 52 is directed to a pipe 54. Pipe 54
delivers the mixture to a high pressure filter assembly 60.
An air compressor 56 provides compressed air via pipe 50 to
a junction valve 58 in pipe 54 upstream of the filter
assembly 60. The operations of the pump 52 and the
compressor 56 are controlled by the controller 46. The air
compressor 56 is used at the end of a filtering process
step to force oils in the pipes and falter assembly 60
through the filter in assembly 60.
CA 02606208 2007-10-16
WO 2006/115284 PCT/JP2006/308918
7
The high pressure filter 60 filters the mixture from
the catalytic tank 30 through a filter media 62. The high
pressure filter 60 may include a plurality of filter
chambers assembled in series and fed via an axial flow path
aligned with the inlet connection to pipe 54. Preferably,
the filter media 62 has a 150 mesh fiberglass filter
although a range about this mesh size is potentially
applicable.
The primary output of high pressure filter 62 is
provided to pipe 70. A secondary output of high pressure
filter 60 caused by leakage along the edges of the filter
media 62 is captured in a tray 64 and delivered via pipe 66
to pump 68 which preferably directs the secondary output
back to the inlet of the high pressure filter 60.
Pipe 70 from the high pressure filter 62 is connected
to a valve 72 that directs the flow to a pipe 74 or
recirculation pipe 76. The output of pipe 74 is a fuel
combination tank 80. The output from recirculation pipe 76
is a return into the catalyst tank 30.
The fuel combination tank 80 includes a mixing
assembly 82, including a motor 84 driving a shaft 86 having
an impeller 88. In fuel combination tank 80, the reacted
and filtered oil from the high pressure filter 60 is
blended with a base fuel, either kerosene or light oil
(heating oil). An absorption powder composition may also
be added to the blend to enhance the chemical bonding of
CA 02606208 2007-10-16
WO 2006/115284 PCT/JP2006/308918
8
the blend and to remove additional contaminants such as ash
and carbon particles.
After a sufficient retention and mixing period in the
fuel combination tank 80, the blend is routed through high
pressure filter 60, or a different high pressure filter of
essentially the same design, which filters out the
absorption powder from the blend. The-output of the high
pressure filter 60 after filtering the blend is directed to
a product tank 90.
Figure 2 schematically depicts a second more advanced
biofuel conversion apparatus 100 according to a second
aspect of present invention. Apparatus 100 includes oil
tank 110 that is a large oil tank or plurality of storage
drums. Oil from tank 110 is routed via pipe 112 to a pump
114 and the output of pump 114 is directed to pipe 116
having a control valve 118. Pipe 116 directs oil to a
centrifugal separator 120. The separator 120 has an outlet
to pipe 122 and waste outlet to waste 124. The separator
120 includes a motor 126 driving a centrifugal impeller
assembly 128, to separate particulate matter from the oil
in the centrifugal separator 120.
Pipe 122 delivers the cleaned oil to a holding tank
130. Oil from the holding tank 130 is pumped via pump 132
and pipe 134 into a catalytic mixing tank 140. The
catalytic mixing tank 140 includes a motor driven mixing
assembly 142 with temperature sensor 144 and level sensor
CA 02606208 2007-10-16
WO 2006/115284 PCT/JP2006/308918
9
146 similar to that described above for Figure 1. Further,
the catalytic mixing tank 40 includes a heating assembly
148, for example a steam heat exchange system, to heat
materials in the catalytic mixing tank 140.
Catalytic mixing tank 140 is also configured to
receive powders from powder tank 150 via pipe 152 and auger
screw feed 154. The output of mixing tank 140 is directed
to a pipe 160 to a high pressure pump 170. The high
pressure pump delivers the mixture via pipe 172 to a high
pressure filter assembly 180. Pipe 172 preferably also
includes pressure sensor 174 and a junction valve 176 with
junction valve 176 also being connected to an air
compressor 178 for cleaning the pipes and filters at the
end of a batch.
The high pressure filter assembly 180 is configured
similar to that of high pressure filter assembly 60 of
Figure 1. Accordingly, the high pressure filter assembly
180 includes a plurality of filter chambers 182 and filter
media 184 to separate particulates and powder materials
from the mixed oil composition passing therethrough. The
outlet product from high pressure filter assembly 180 is
delivered via pipe 190 to a secondary filter assembly 194.
The secondary filter assembly 194 includes a directional
valve 196 to separate the flow to one of two filters 198.
Each of the filters 198 includes a paper filter having a
250 to 300 mesh filter'media. The output side of each of
CA 02606208 2007-10-16
WO 2006/115284 PCT/JP2006/308918
filters 198 is directed to a pipe 200 to deliver the
filtered fuel to a storage tank 220.
The high pressure assembly 180 also produces a
secondary output to a tray 202. The flow from the tray 202
5 is directed through pipe 204 to a pump 206. The output of
pump 206 is directed to pipe 208. Pipe 208 terminates in
a directional valve 210 which separate the flow into one of
two pipes 212 each having a filter 214. Each filter 214
includes a paper filter media having a mesh size of 250 to
10 300. The output of the filters 214 is directed to a pipe
216 which delivers the output to the storage tank 220.
Figure 3 depicts the blending system for blending the
processed oil with a base fuel such as kerosene or light
oil. The process starts with the storage tank 220 having
filtered processed oil from the system of Figure 2. The
system also includes a fuel tank 222 for containing
kerosene or light oil. Each of the tanks 220 and 222 are
configured to deliver oil or fuel via pipes 224 and 226,
respectively, to a catalytic blending tank 230. Optionally,
a high pressure filter may be included in pipe 224 to
filter out any retained particulate matter. Also, as
depicted, there may be two catalytic blending tanks 230 to
allow for multiple batch processing.
The blending tank 230 includes a mixing assembly 232
having a motor 234 having a shaft 236 driving impeller 238.
The blending tank 230 inay also include level sensor 240 and'
CA 02606208 2007-10-16
WO 2006/115284 PCT/JP2006/308918
11
temperature sensor 242 to provide signals to a system
controller 244 that monitors the level and temperature of
the blending composition. The blending tank 230 may also
receive powdered materials from the powder tank 246,
delivering powders via a pipe 248 to the catalytic blending
tank 230.
The blended output from the blending tank 230 is
delivered via a pipe 250 to a high pressure pump 260. The
output of the high pressure pump 260 is delivered via a
pipe 262 to a high pressure filter assembly 270. The pipe
262 preferably includes a pressure sensor 264 and a
junction valve 266, the junction valve 266 also being
connected to receive pressurized air from a compressor 268
used to clean out the pipes and high pressure filter
assembly 270.
The blended composition is delivered to the high
pressure filter assembly 270 to remove powder composition
and particulate matter from the blend. The output of the
high pressure filter assembly 270 is directed to a pipe 280
which delivers the output to a filter assembly 290. The
filter assembly 290 includes a pair of filters 292 that may
be used sequentially as discussed above with respect to the
filter assembly 198. The filters 292 include paper filter
media having a 250 to 300 mesh filter media. The output of
the filter assembly 290 is directed to a biofuel tank 300.
The high pressure filter tank assembly 270 may also
CA 02606208 2007-10-16
WO 2006/115284 PCT/JP2006/308918
12
have a secondary output captured by a tray 272 and
delivered to a pump 274 via a pipe 276. The output from
pump 274 is directed to filter assembly 278 having a pair
of filters 282, operated sequentially. Filter assembly 278
has an output pipe 284 delivering the filtered fuel'mixture
to biofuel tank 300.
The biofuel conversion process utilizing either the
apparatus of Figure 1 or Figure 2 relies on the use of a
catalytic material and absorption material composition.
The catalytic materials are blended and grounded to fine or
super fine powder having a particle size less than 500 pm
and preferably having a particle size of less than 100 pm.
The powdered catalytic materials are mixed with vegetable
oil or waste vegetable oil in the catalytic tanks. The
catalytic powders are preferably:
to 35 weight percent of an aluminum sludge zeolite
material (Na20, Si02, H20 ) and, either
65 to 75 weight percent of a composition of calcium
oxide, sodium monoxide, aluminum oxide, silicon dioxide and
20 water in the formula 0.75Ca0, 0.2Na20, A1203r 2Si02, 4.5H20
for processing vegetable oils, or 65 to 75 weight percent
of sodium monoxide, silicon dioxide and water in the
formula: Na20, nSiOZ, xH2O, for processing mineral or
petroleum based oils.
25 In addition to the powdered catalytic materials, the
powder composition added to the catalytic mixing tank
CA 02606208 2007-10-16
WO 2006/115284 PCT/JP2006/308918
13
includes powdered absorption materials, preferably selected
from the group consisting of one or more of the following
materials, w-ith the preferred composition including each of
the materials in the relative weight percentages indicated
below:
79.6 to 82 percent Silicon Dioxide (Si02),
11. 6 to 12 percent Aluminum Oxide (A1203) ,
2.7 to 3.1 percent Iron Oxide (Fe203),
3.3 to 4.1 percent Magnesium Oxide (MgO), and
0.7 to 0.9 percent Calcium Oxide (CaO).
,
While the process may not require all of these
constituent component absorption powders, the best results
have been obtained using all of these absorption powders.
Moreover, while the narrow ranges above have been found to
be effective an appropriate range for each of the component
materials is 75 to 85 weight percent of silicon dioxide
(Si02), 10 to 14 weight percent of aluminum oxide (A1203), 2
to 4 weight percent of iron oxide (Fe203), 2 to 5 weight
percent of magnesium oxide (MgO), and 0.5 to 2 weight
percent of calcium oxide (CaO).
Further, it has.been found that a preferred
composition contains a catalyst composition powder
consisting of: thirty weight percent of the aluminum sludge
zeolite material, and 70 weight percent of Na20, nSi02, xH2O,
or 70 weight percent of 0.75CaO, 0.2Na2O A1203, 2SiO2r
4.5H20; and an absorption composition powder consisting of:
CA 02606208 2007-10-16
WO 2006/115284 PCT/JP2006/308918
14
80.8 weight percent of silicon dioxide (Si02), 11.8 weight
percent of aluminum oxide (A1203), 2.9 weight percent of
iron oxide (Fe203), 3.7 weight percent of magnesium oxide
(Mg0), and 0.8 weight percent of calcium oxide (Ca0).
As described above, the powders forming the ionic
exchange or catalytic materials include an aluminum sludge
zeolite. This aluminum sludge zeolite material may be
processed to increase surface porosity by known methods
such as an acid wash, and then cleaned with a calcium wash
to leave a deposit of calcium within the porosity of the
zeolite.
In processing the vegetable oils in the processing
system described above, the amount of catalytic powder for
processing each 100 liters of vegetable oil is 250 to 750
grams with a preferred range of 450 to 550 grams. For most
processes, however, it has been found that an adequate
amount of catalytic powder without excess or waste is 500
grams for 100 liters of vegetable oil. By comparison, the
amount of the absorption powder for 100 liters of vegetable
oil is in a range of 1 to 2 kilograms for a resulting dark
oil or a range up to 3 kilograms-to produce a light or more
clarified oil.. For these processes, a preferred range for
the amount powders to yield a dark oil having a higher
carbon content would be 1.5 kilograms of absorption powder
to 100 liters of process oil to 3 kilograms of absorption
powder used to produce'a very clear or clarified oil.
CA 02606208 2007-10-16
WO 2006/115284 PCT/JP2006/308918
Adding in excess of 3 kilograms is not necessary and leads
to excess usage and waste of the absorption powders.
In the catalytic mixing tank, the powders in the
amounts described above are blended into the waste or
5 virgin vegetable oils and mixed for a period of 40 to 60
minutes as the mixture is heated to a reaction temperature
of 60 C to 80 C and, preferably, at the upper end of this
range such that the temperature is controlled to between
78 to 80 C by the heat exchange assembly and the
10 controller. It is also preferred that the material be
maintained at the reaction temperature for at least 20
minutes within the 40 to 60 minute interval.
Within the high pressure filter assemblies described
above, the materials are processed at a pressure of
15 approximately 0.5 MPa through the filter media. The
materials are processed at an initial flow rate of
approximately 90 liter per minute in a fresh filter media
although the processing rate will be reduced with the build
up of a filter cake on the filter media. It is preferred
that materials be recirculated at the beginning of any
filtering process, in particular if a new filter media has
been installed in the high pressure filter assembly. The
recirculation of the initial processed product is
beneficial to wet the filter media and to build a cake of
powder including catalytic powder and absorption powder on
the surface of the filter media. It is preferred that the
CA 02606208 2007-10-16
WO 2006/115284 PCT/JP2006/308918
16
thickness of the cake be 2 to 3 mm before the filter oil is
routed to the fuel combination tank 80 or tank 220. The
filter media can be used to process 2 to 3 batches of waste
oil before the filter requires cleaning, so the cake build
up on the surface of the filter media and within the high
pressure filter may be substantial. A cake buildup of 2 to
4 cm in thickness thus may increase the effectiveness of
the filter process.
While fatty acids retained in waste vegetable oils
may not be removed by the filter media in the high pressure
filter assembly, once the cake develops on the surface of
the filter media then it is believed that at least a
portion of the fatty acid from the vegetable oil can be
trapped in the cake and removed from the processed biofuel.
In this process, the absorption materials may allow fatty
acids to combine to be of a size that can be retained
either by the cake or by the filter media in the filtering
step.
When the filtered oil after the first processing
stage is blended with kerosene or light oil, the absorption
powders as discussed above may be added to and mixed with
the blend in a range of approximately 1 kilogram of
absorption powder to 200 liters of combined base oil and
kerosene. However, for a process which is going to be
filtered through a filter bed that has not been primed by
prior filtering of the'foregoing powders in the first stage;
CA 02606208 2007-10-16
WO 2006/115284 PCT/JP2006/308918
17
then preferred the range of absorption powders to 200
liters of fuel would be from 1 to 3 kilograms. By
comparison, if the blended materials are to be filtered
through a high pressure filter assembly that has previously
being used to filter either a mixture or blend from the
processes described above, and the filter has not been
cleaned, then the preferred range of absorption powder
would be 0 to 3 kilograms per 200 liters of combined
processed oil or kerosene.
It has been found that waste vegetable oil processed
according to the foregoing assembly and method provides a
biofuel component having the following properties:
pH 7.0
Flash point, PMCC 214.0 C
Kinematic Viscosity, 40 C 42.42 mm2/s
Pour Point .-7.5 C
Carbon (mass %) 0.31
Water 810 ppm
Ash (mass %) 0.002
Sulfur (mass %) 0.0002
Density, 15 C 0.9270 g/cm3
Calorie 39,310 J/g
The biofuel produced from waste vegetable oil
utilizing the foregoing technology is stable and, when once
mixed with the kerosene or light oil it can be combined
with other diesel fuel's or light oils to be burned in an
CA 02606208 2007-10-16
WO 2006/115284 PCT/JP2006/308918
18
engine or combustion process without chemical separation or
decomposition. In addition, the pH of the biofuel is
neutral and the biofuel has an energy content that is
consistent with the energy content of other fuels which
leads to the biofuel being stable when combined with other
fuels.
When processing the blended oil and kerosene through
the high pressure filter assembly, the blended composition
may not require absorption powder if the high pressure
filter assembly includes substantial cake deposits on the
surface of the filter media.
By the process according to the present invention,
the waste oil can be processed to remove particulates,
substantially all, if not all carbon, certain fatty acids,
and other contaminates. In addition, the pH of a waste oil
is neutralized to a pH of approximately 7.0, which occurs
because of compounds both within the catalytic powders and
the first three absorption powders.
The catalytic function of the catalytic powders
increases or ionizes oils to increase energy content and
combustibility and allow blending with kerosene or light
oils. In addition, by the process of mixing both catalytic
powders and absorption powders in the mixing tank, the
steps necessary to process waste oil into biofuel are
combined and the processing time for a batch to pass
through the entire process and system of the present
CA 02606208 2007-10-16
WO 2006/115284 PCT/JP2006/308918
19
invention may take less than two hours to per batch. Each
batch can be scalable within the foregoing system so that a
small facility could process a batch 100 liters while a
large facility could process a batch of several thousand
liters in each of the tanks.
While the present invention is particularly useful in
processing and cleaning waste vegetable oil, the process is
equally applicable to virgin vegetable oils to convert
these virgin oils to biofuels or biofuel additives.
Further, the apparatus described above may be used to
process petroleum based oil, such as used motor oil, to
convert the petroleum oil into a fuel grade oil or fuel
additive. To process petroleum oils, the same process
steps are carried out. However, the powdered catalytic
materials for petroleum based oils include 65 to 75 weight
percent sodium monoxide, silicon dioxide and water in the
formula: Na20, nSiO2, xH2O, mixed with 25 to 35 weight
percent aluminum sludge zeolite for processing mineral or
petroleum based oils.
When the high pressure filter assembly has been used
to process at least one and up to three or four batches,
the filter media may be cleaned to remove the cake build up.
The byproduct material in the cake build up primarily
consists of the catalytic powders and the absorption
powders as well as carbon, particulates, fatty acids, and
other contaminants as iaell as small amounts of oil. This
CA 02606208 2007-10-16
WO 2006/115284 PCT/JP2006/308918
byproduct material may be mixed with cement powder and
water and molded to form bricks or other configurations
which may be used for paving and construction materials.
It has been found that a mixture of 75% byproduct and 25%
5 cement powder is sufficie'nt to form a stable and hardened
brick. Other blends and ratios of byproduct to cement or
byproduct and binder materials can be used so as to form
useful construction materials. Accordingly, all products
and byproducts of the process according to this invention
10 are either useful biofuels or used as construction products.
Alternatively, the byproduct from the filter media may be
used as a fertilizer and soil conditioner, as opposed to
being formed into hardened materials, as the materials
contained in the byporoduct materials are primarily mineral
15 compositions and organic materials.
From the foregoing detailed description, it will be
evident that there are a number of changes, adaptations and
modifications of the present invention which come within
the understanding of those skilled in the art. The scope
20 of the invention includes any combination of the elements
from the different species or,embodiments disclosed herein,
as well as subassemblies, assemblies, and methods thereof.
However, it is intended that all such variations not
departing from the spirit of the invention be considered as
within the scope of the invention, and that the invention
is interpreted by the proper scope of the appended claims.