Note: Descriptions are shown in the official language in which they were submitted.
CA 02606332 2007-10-22
WO 2006/115916 PCT/US2006/014639
GAS STREAM PURIFICATION METHOD
Field of the Invention
[0001] The present invention relates to a method of
purifying a gas stream in which the gas stream is
introduced into a series of electrically driven oxygen
separation zones having one or more oxygen ion
conducting electrolytes to separate oxygen from the gas
stream. More particularly, the present invention
relates to such a purification method in which voltages
are applied to each of the separation zones to induce
an oxygen ion current that approaches a limiting value
beyond which a further increase in voltage will not
produce an increase in oxygen separation.
Background of the Invention
[0002] Electrically driven oxygen transport
membranes are utilized to purify feeds by separating
oxygen. Such membranes employ an electrolyte which is
normally an ionic conductor such as gadolinium doped
ceria or yttrium stabilized zirconia. If the
electrolyte is subjected to an elevated temperature and
an electric potential is applied to electrodes
sandwiching the electrolyte, oxygen will ionize at an
electrode that serves as a cathode to produce oxygen
ions. The oxygen ions will permeate through the
membrane and emerge at the anode electrode. The oxygen
ions combine to form elemental dioxygen and in so
doing, give up excess electrons to the anode electrode.
[0003] Electrically driven oxygen separation devices
have been utilized to purify crude argon streams.
Crude argon is produced by the cryogenic distillation
CA 02606332 2007-10-22
WO 2006/115916 PCT/US2006/014639
- 2 -
of air. In cryogenic distillation, an air stream.is
compressed, purified and cooled to at or near its dew
point. The cooled air stream is introduced into a
double column arrangement having higher and lower
pressure columns connected to one another in a heat
transfer relationship by a condenser-reboiler. The
compressed and purified air is successively refined in
the higher and lower pressure columns into oxygen and
nitrogen rich fractions. A vapor stream, comparatively
rich in argon, is withdrawn from the lower pressure
column and introduced into a crude argon column to
produce an argon rich tower overhead. The argon column
is refluxed by condensing tower overhead and part of
the liquid condensate is withdrawn as the crude argon
stream.
[0004] In US 5,557,951 a crude liquid argon stream
is vaporized and heated. The resultant heated stream
is then introduced into an electrically driven oxygen
transport membrane unit to remove oxygen from the
heated stream and thereby to produce a purified argon
stream.
[0005] In US 5,035,726 low pressure crude argon
stream is warmed and compressed. The compressed stream
is further heated and fed to an electrically driven
oxygen transport membrane unit to remove the bulk of
the oxygen. The resultant purified gas stream is then
introduced into a distillation column for removal of
nitrogen. Liquefied oxygen is withdrawn from the
bottom of such column.
[0006] US 5,454,923 illustrates a further inert gas
purification system utilizing an electrochemical cell
CA 02606332 2007-10-22
WO 2006/115916 PCT/US2006/014639
- 3 -
having a non porous electrolyte membrane coated with
electrodes and heated to high temperatures.
[0007] The electrical power usage of any
electrically driven oxygen transport membrane device is
important in determining whether such operations will
be economically feasible for purification purposes.
This problem is particularly critical in purifying
crude argon because a major expense in the cryogenic
rectification of air arises from electrical power
consumed, principally in the compression of the air.
Therefore, the power consumption of an electrically
driven oxygen separator and its use in purifying crude
argon is critical to the profitability of an air
separation plant making argon. The same problem
pertains to other gas purification systems, for
example, purification of crude nitrogen streams
produced by a pressure swing adsorption unit utilizing
an adsorbent bed or a membrane system employing a
polymeric membrane. Both types of units also consume
electricity in compressing air and hence, power
consumption is an important factor in the utilization
of an electrically driven oxygen separator used for
purifying crude nitrogen streams obtained from such
sources. As will be discussed, the present invention
provides a purification method that minimizes the use
of electrical power for a specific size of an
electrically driven oxygen separator.
Summary of the Invention
[0008] The present invention provides a method of
purifying a gas stream by removing oxygen from the gas
stream. In accordance with the method, the gas stream
CA 02606332 2007-10-22
WO 2006/115916 PCT/US2006/014639
- 4 -
is introduced into a series of electrically driven
oxygen separation zones that operate at an elevated
temperature to separate the oxygen from the gas stream.
The separation of oxygen produces a purified gas
stream. Each of the electrically driven separation
zones has an electrolyte and cathode and anode
assemblies for applying a voltage to the electrolyte
such that the oxygen ions are transported through the
electrolyte and emerge therefrom to recombine into
elemental oxygen. This separates the oxygen from the
gas stream to produce the purified gas stream.
[0009] The oxygen is separated from the gas stream
at a successively lower partial pressure due to the
separation of the oxygen within the successive
electrically driven oxygen separation zones. Each of
the electrically driven separation zones is capable of
separating the oxygen as an increasing function of the
voltage applied to the cathode and anode assemblies up
to a level that induces an oxygen ion current that
approaches a limiting oxygen ion current within the
electrically driven oxygen separation zones. At the
limiting oxygen ion current, a further increase in
voltage fails to produce an increase in oxygen
separation. The limiting oxygen ion current is a
function of the successively lower partial pressure
such that the voltage applied decreases for each of the
successive electrically driven oxygen separation zones.
The voltage is applied to each of the electrically
driven oxygen separation zones in an amount selected
such that the oxygen ion current approaches the
limiting oxygen ion current applicable thereto.
CA 02606332 2007-10-22
WO 2006/115916 PCT/US2006/014639
- 5 -
[0010] In the prior art, constant voltage is applied
to the electrically driven oxygen separation zone.
However, as the gas stream traverses the device, it
becomes evermore dilute in oxygen. As a result, the
oxygen partial pressure decreases. Thus, a voltage is
not optimally being applied because as the gas to be
treated passes through the membrane, the partial
pressure of the gas decreases and the same voltage is
not necessary. Separately applying voltages so that
the oxygen ion current approaches the limiting value in
downstream zones of separation thereby reduces the
overall power consumption of the membrane for a
specific size of membrane required for a particular
application.
[0011] The present invention has application to the
purification of crude argon streams by removing oxygen
from such streams. If the gas stream is a crude argon
stream, the gas stream is formed by vaporizing a crude
liquid argon stream withdrawn from a crude argon column
of a cryogenic air separation plant and containing the
oxygen in a range of between about 0.1 percent and
about 3 percent by volume. Preferably, the crude
liquid argon stream contains between 0.5 percent and
about 2 percent by volume.
[0012] A further application of the present
invention relates to the purification of gas streams
that constitute crude nitrogen streams withdrawn from
pressure swing adsorption units or membrane separation
units. In such case, the crude nitrogen stream can
contain between about .05 percent and about 2 percent
by volume oxygen. More preferably, the crude nitrogen
stream contains between about .1 percent and about 1
CA 02606332 2007-10-22
WO 2006/115916 PCT/US2006/014639
- 6 -
percent by volume oxygen and most preferably, the crude
nitrogen stream contains between about .15 percent and
about .5 percent by volume oxygen.
[0013] The oxygen ion current can be between about
80 percent and about 99.99 percent of the limiting
oxygen ion current. In preferred modes of operation of
the present invention, the oxygen ion current is at
least about 95 percent of the limiting oxygen ion
current.
[0014] If the electrolyte is formed from YSZ, the
elevated operational temperature of the electrically
driven oxygen separation zones is between about 600 C
and about 900 C. A temperature range of between about
650 C and about 800 C is more preferred and a range of
between about 700 C and about 800 C is particularly
preferred.
[0015] The electrolyte can be a common electrolyte
extending through each of the electrically driven
oxygen separation zones. In such case each of the
electrically driven oxygen separation zones is defined
between the particular cathode and anode assemblies at
which the voltage is applied. It is understood,
however, that the electrically driven oxygen separation
zones could be separate devices or separated from one
another in the same device. As will be discussed,
where the electrically driven oxygen separation zones
are separated, advantageously, the initial zone could
be formed from 8YSZ. The term "8YSZ" means yttria
doped or stabilized zirconia containing about 8 percent
by mole yttria. The successive zones could be formed
from 6YSZ or 3YSZ.
CA 02606332 2007-10-22
WO 2006/115916 PCT/US2006/014639
- 7 -
[0016] In an application of the invention to the
purification of crude argon, a crude liquid argon
stream is vaporized through indirect heat exchange with
an air stream, thereby to liquefy the air stream and
thereby to form the crude argon gas stream to be
purified. The crude argon gas stream is heated through
indirect heat exchange with the purified gas stream and
the purified gas stream is liquefied through indirect
heat exchange with a liquid air stream.
[0017] Alternatively, the crude liquid argon stream,
the purified gas stream and a liquid nitrogen stream
are subjected to indirect heat exchange, thereby to
vaporize the crude liquid argon stream to form the
crude argon stream to be purified, to vaporize the
liquid nitrogen stream and to liquefy the purified gas
stream. The resultant crude argon gas stream is heated
through further indirect heat exchange with the
purified gas stream prior to the purified gas stream
engaging in the heat exchange involving the purified
gas stream and the crude liquid argon stream. Further
pressure is imparted to the crude argon stream by a
blower to raise the pressure thereof above that of the
purified gas stream to provide a better match of
heating and cooling curves for the heat exchange
between the liquid and gas streams.
[0018] In yet a further alternative, the present
invention provides vaporizing the crude liquid argon
stream and liquefying the product stream in a main heat
exchanger of a cryogenic air separation plant. The
crude argon stream can be heated through indirect heat
exchange with the purified gas stream prior to
liquefaction of the purified gas stream within the main
CA 02606332 2007-10-22
WO 2006/115916 PCT/US2006/014639
- 8 -
heat exchanger. A compressed and purified air stream
to be rectified in the cryogenic air separation plant
can be cooled within the main heat exchanger and oxygen
and nitrogen product streams of the cryogenic air
separation plant can be warmed within the main heat
exchanger.
[0019] In an application of the present invention to
the purification of crude nitrogen streams, the gas
stream and the purified gas stream are subject to
indirect heat exchange to heat the gas stream and cool
the purified gas stream. During start-up or
maintenance of the electrically driven oxygen
separation unit, the pressure swing adsorption unit or
the membrane separation unit can be operated at a lower
capacity to produce the crude nittogen stream at a
higher purity than operations conducted at full
capacity.
[0020] In either of the above applications for
purifying crude argon or purifying crude nitrogen, a
trim heater can be employed to further heat the gas
stream prior to treatment within the electrically
driven oxygen separation zones.
[0021] In any embodiment of the present invention,
the oxygen separated from the gas stream can be
extracted with a purge stream. In case of an
application of the present invention involving the
purification of a crude nitrogen stream, the purge
stream can be formed from part of the gas stream to be
purified.
CA 02606332 2007-10-22
WO 2006/115916 PCT/US2006/014639
- 9 -
Brief Description of the Drawings
[0022] While the specification concludes with
claims distinctly pointing out the subject matter that
Applicants regard as their invention, it is believed
that the invention will be better understood when
taken in connection with the accompanying drawings in
which:
[0023] Fig. 1 is a schematic illustration of an
oxygen separation device in accordance with the present
invention;
[0024] Fig. 2 is a graphical representation of
applied voltage versus ionic current as a fraction of
the limiting ionic current in an electrically driven
oxygen separation device;
[0025] Fig. 3 is a schematic illustration of the use
of the oxygen separation device illustrated in Fig. 1
applied to the purification of crude argon;
[0026] Fig. 4 is an alternative embodiment of Fig.
3;
[0027] Fig. 5 is an alternative embodiment of Fig.
4; and
[0028] Fig. 6 is a schematic illustration of the use
of the oxygen separation device illustrated in Fig. 1
applied to the purification of crude nitrogen.
[0029] In order to avoid needless repetition in the
explanation of the accompanying Figures, the same
reference numerals were used to indicate like
components and streams not requiring a different
explanation or description.
Detailed Description
CA 02606332 2007-10-22
WO 2006/115916 PCT/US2006/014639
- 10 -
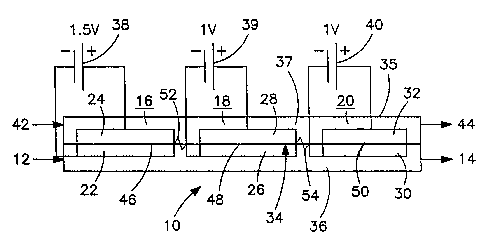
[0030] With reference to Fig. 1 an electrically
driven oxygen separation device 10 is illustrated that
is designed to purify a gas stream 12 to produce a
purified gas stream 14 through separation of oxygen
from gas stream 12. The oxygen is separated within
electrically driven oxygen separation zones 16, 18 and
20 that are defined by three cathode and anode
assemblies having cathode and anode electrodes 22, 24;
26, 28 and 30, 32, respectively. Although not
illustrated, but as would be known to those skilled in
the art, the cathode and anode assemblies would also be
provided with conventional current collectors. Said
electrodes and current collectors are all porous and
sandwich an electrolyte 34, which as will be discussed
may have separate sections. The cathode and anode
assemblies and electrolyte 34 are housed within a
casing 35 to define passageways 36 and 37 on opposite
sides of electrolyte 34. It is to be noted as used
herein and in the claims, the term "cathode and anode
assemblies" means porous cathodes and anodes and
associated porous current collectors.
[0031] Electrodes 22, 24; 26, 28; and 30, 32 are so
named in that they consist of electrically conductive
materials. Conventional current collectors form outer
layers of the electrodes and are in turn connected to
direct current electrical power sources 38, 39 and 40.
As illustrated, different voltages are applied to
cathode electrodes and anode electrodes 22, 24; 26, 28;
and 30, 32 by electrical power sources 38, 39 and 40
such that the oxygen within gas stream 12 passing
through passageway 36 ionizes at the juncture of pores
within cathodes 22, 26 and 30 and the electrolyte 34
CA 02606332 2007-10-22
WO 2006/115916 PCT/US2006/014639
- 11 -
and the resultant oxygen ions are transported through
the electrolyte 34 to anode electrodes 24, 28 and 32.
The oxygen ions combine at the juncture of pores within
anode electrodes 24, 28 and 32 and electrolyte 34 to
form elemental dioxygen. The resultant free electrons
are conducted within anodes 24, 28 and 32 back to
electrical power sources 38, 39 and 40. The oxygen is
extracted by way of a purge gas stream 42 introduced
into passageway 37 to sweep and remove the oxygen from
electrically driven oxygen separation device 10. Purge
gas stream 42 can be nitrogen or other inert gas
containing less than about 21 percent oxygen. Other
known extraction means can be employed such as vacuum
extraction. Purge gas stream 42 is discharged as
oxygen containing purge gas stream 44.
[0032] In the illustrated embodiment, the
electrolyte 34 is of tubular configuration, about 0.5
mm thick, about 6.3 mm in outside diameter and has an
overall length of about 91 cm to provide a surface area
of about 160 cm2. The cathodes 22, 26 and 30 are
fabricated from a mixture of 50wt% lanthanum strontium
manganite (Lao,8Sro,2MnO3 s) and 50wt% yttria-stabilized
zirconia (Zro.asYo.1501.925) . Each are between about 10
and about 30 microns thick, have an average pore size
of 10 microns and a porosity of about 40 percent. In
this regard, average pore size is measured by image
analysis of scanning electron micrographs. The current
collector can be silver and have a thickness of between
about 50 and about 100 microns, a pore size of about 10
microns and a porosity of about 40 percent. The anodes
24, 28 and 32 are fabricated from a mixture of 50wto
CA 02606332 2007-10-22
WO 2006/115916 PCT/US2006/014639
- 12 -
lanthanum strontium manganite (Lao.8Sro,2MnO3 s) and 50
wt% yttria-stabilized zirconia (Zro,$sYo.ls01.925) . Each
of the anodes are about 20 micron thick, have pores
with a pore size of about 5 micron and a porosity of
about 40 percent.
[0033] The assemblies of cathode and anode electrode
22, 24; 26, 28 and 30, 32, divide the electrolyte into
three equal portions 46, 48 and 50 with gaps 52 and 54
of about 1 cm between each of the same. In this
regard, the gaps are spacers that can be fabricated of
YSZ and connected to portions 46, 48 and 50 by glass
sealing, brazing or other bonding and sealing
technique. As can be appreciated, casing 35 could thus
be fabricated from three separate sections with flow
connection between said sections for the passage of gas
stream 12 and purge gas stream 42 on opposite sides of
the electrolyte. Moreover, although electrolyte 34 is
shown as broken up into three portions 46, 48 and 50,
46, in a possible embodiment of the present invention,
electrolyte 34 could be common to all electrically
driven oxygen separation zones 16, 18 and 20 with no
gaps.
[0034] The actual voltage that is applied across the
electrolyte 34 has to be greater than the Nernst
potential due to a difference in the oxygen partial
pressure on the anode and cathode side in order for
oxygen to be removed from gas stream 10. The Nernst
potential is described by the following equation:
R= T p02 on anode side of OTM membrane
UNe~"St - 4= F ioge pO2 on argon side of OTM membrane
CA 02606332 2007-10-22
WO 2006/115916 PCT/US2006/014639
- 13 -
In this equation, R is the gas constant, T is the
temperature in Kelvin, F is Faraday's constant and
"P02" is the partial pressure of oxygen.
[0035] The upper limit of the voltage that can be
possibly applied is a voltage that will cause failure
of electrolyte 34 due to electrochemical reduction.
Electrochemical reduction can induce electronic
conductivity into electrolyte 34 rendering it
electrically inefficient for oxygen ion conduction.
Additionally, such reduction can also cause isothermal
expansion which will generate stresses in electrolyte
34 that can be sufficient to cause it to fracture.
Typically, the maximum voltage is 2 volts. However, as
will be discussed, the voltage that should be applied
in each of the electrically driven oxygen separation
zones 16, 18 and 20 and portions 46, 48 and 50 of
electrolyte 34 should be much less than the ultimate
limit.
[0036] With reference to Fig. 2, a graphical
representation is illustrated of applied voltage versus
ionic current as a fraction of a limiting ionic current
that is induced within electrolyte 34. The ionic
current is a measure of the ionic flux passing through
electrolyte 34 per unit area and is analogous of the
flux of electrons passing through a conductor to
measure an electrical current. The behavior of applied
voltage illustrated in Fig. 2 is governed by the
following equation:
_ R -T &nic n
VaPplied - lOge 1
q' -F ~limit
CA 02606332 2007-10-22
WO 2006/115916 PCT/US2006/014639
- 14 -
In this equation, R is the gas constant, T is the
temperature in Kelvin, F is the Faraday constant, and I
is current.
[0037] As the applied voltage is increased, a point
is reached in which a limiting ionic current exists. A
further increase in voltage will not increase the
degree of separation. This limit to the ionic current
is a function of partial pressure. As such, as the
partial pressure decreases, the limiting ionic current
decreases and therefore, in accordance with the present
invention, the voltage to be applied should decrease.
For example, in case of an argon stream containing 2
percent oxygen, a voltage of 2 volts can be applied
without reaching a limiting current. However, as
oxygen is removed from the argon stream down to 100
parts per million, then a limiting current can be
observed when a voltage of approximately 0.6 volts is
applied. The particular value of the limiting current
and the applied voltage that results in the limiting
current is a complicated function of the temperature,
the oxygen partial pressure on either side of the
electrolyte, the composition of the atmosphere on
either side of the electrolyte, the electrochemical
performance, the size of the electrolyte, the
microstructure of electrodes on either side of the
electrolyte and the particular electrochemical
mechanism that is giving rise to the limiting current
behavior.
[0038] Due to the complexity in accurately
determining the limiting voltage, in electrically
driven oxygen separation device 10, particular values
of voltage applied to each of electrically driven
CA 02606332 2007-10-22
WO 2006/115916 PCT/US2006/014639
- 15 -
oxygen separation zones 16, 18 and 20 is best
determined by experiment. In such an experiment, an
oxygen analyzer is placed downstream of the particular
zone and successively higher voltages are applied until
a decrease in oxygen concentration is not observed.
The accuracy of such experiment will of course
determine the number or size of step increases in
voltage that are applied until the limiting voltage is
achieved. For example, .01 voltage steps will
determine the limiting voltage within one percent while
.001 voltage steps will determine the limiting voltage
within .1 percent.
[0039] Preferably the applied voltage can be chosen
as to result in an oxygen ion current that is between
about 80 percent and about 99.9 percent of the oxygen
ion limiting current. In any embodiment it is
preferred, however, that the oxygen ion current induced
in an electrolyte is at least about 95 percent of the
oxygen ion limiting current.
[0040] By way of a calculated example, power source
38 applies 1.5 volts between cathode electrode 22 and
anode electrode assembly 24 within zone 16. Assuming
that an argon stream or a crude nitrogen stream is to
be purified that contains roughly 1.8 percent oxygen, a
significant amount of the oxygen will be removed within
zone 16 so that the stream exiting 16 and entering 18
should contain approximately 1000 parts per million
oxygen. Power source 39 supplies about 1 volt between
cathode and anode electrodes 26 and 28 within zone 18
and the stream exiting zone 18 should contain roughly
100 parts per million oxygen. Power source 40 applies
0.5 volts between cathode and anode electrodes 30 and
CA 02606332 2007-10-22
WO 2006/115916 PCT/US2006/014639
- 16 -
32 so that purified gas stream 14 has an oxygen
concentration of about 10 parts per million. As can be
appreciated, had 1.5 volts been applied throughout
electrochemical device 10 much more power would have
been consumed.
[0041] Where significant quantities of oxygen are
present within gas stream 12, the total electrochemical
performance of electrolyte 34 is governed by the
performance of electrolyte 34 and assemblies of the
cathodes and anodes. Preferably, under such conditions
the electrolyte 34 should have a high oxygen ion
conductivity. 8 mole percent yttria doped zirconia
(8YSZ, Zro.852Y0.14801.926) is preferred. Other electrolyte
material such as gadolinium doped ceria and lanthanum
strontium gadolinium magnesium oxide are possible
materials because they possess even higher ionic
conductivity than 8YSZ. However the increased cost and
lower strength of these materials outweigh their
improved electrochemical performance in a practical
device.
[0042] The resistance of the electrolyte is a
function of the oxygen partial pressure and the
resistance in the cathode is proportional to the oxygen
partial pressure according to the following equation:
Rcathode C POZYl; where n is an experimentally
determined number between 1 and 4, inclusive.
As the oxygen content of the argon stream is reduced,
then the electrochemical performance becomes
increasingly dependent upon the resistance of the
electrodes, particularly the cathode which is in direct
contact with the crude argon and less dependent on the
CA 02606332 2007-10-22
WO 2006/115916 PCT/US2006/014639
- 17 -
performance of the electrolyte. Under these conditions
it is advantageous to use an electrolyte material with
a lower ionic conductivity but improved strength such
as 6 mole percent yttria doped zirconia (6YSZ,
Zr0.886Y0.11401.943) or 3 mole percent yttria doped zirconia
(3YSZ, Zro.942Yo.o5801.971) . Thus, in electrically driven
oxygen separation device 10 applied to purifying crude
argon, portion 46 of electrolyte 34 can be fabricated
from 8 mole percent yttria doped zirconia ("8YSZ") and
portions 48 and 50 can be fabricated from either 6 or 3
mole percent yttria doped zirconia.
[0043] If a YSZ electrolyte is used, then,
preferably, the membrane will operate somewhere between
600 C and about 900 C, more preferably between about
650 C and about 850 C and most preferably between 700 C
and about 800 C.
[0044] With reference to Figs. 3, electrically
driven oxygen separation device 10 is shown as an
oxygen purifier to purify a crude liquid argon stream
60 obtained from an air separation unit. In such an
air separation unit, an air stream is compressed,
purified and cooled to at or near its dew point. The
cooled air stream is introduced into the bottom of a
higher pressure column to produce a crude oxygen column
bottoms and a nitrogen rich overhead. The resulting
tower overhead is condensed and further refined in a
lower pressure column that produces an oxygen rich
column bottoms and a nitrogen rich tower overhead. The
oxygen rich liquid column bottoms serves to condense
the tower overhead produced in the higher pressure
column and the crude liquid oxygen produced in the
higher pressure column is expanded and used to condense
CA 02606332 2007-10-22
WO 2006/115916 PCT/US2006/014639
- 18 -
tower overhead in the lower pressure column. A vapor
stream having an argon concentration within the range
of between about 5 and about 25 percent is withdrawn
from the lower pressure column and introduced into the
bottom of a crude argon column. The crude argon column
operates as a stripping column so that the argon
content of the vapor increases as the vapor rises
within the column and contacts a descending liquid
phase. The liquid phase thereby becomes evermore
enriched in oxygen during its descent within the
column. A product stream can be withdrawn from the top
of the argon column as a liquid to form the crude
liquid argon stream 60. The liquid column bottoms is
returned to the lower pressure column.
[0045] Crude liquid argon stream 60 preferably has
an oxygen concentration of between about 0.1 percent
and about 3 percent by volume. More preferably, crude
liquid argon stream 60 has an oxygen concentration of
between about 0.5 percent and about 2 percent by
volume. The reason for such ranges is that typically
crude liquid argon has an oxygen concentration of about
3 percent by volume and with limited added expense, the
oxygen concentration in the crude liquid argon stream
can be reduced by a slight increase in the number of
stages. The reduced oxygen content reduces the amount
of power required for electrically driven oxygen
separation device 10.
[0046] Crude liquid argon stream 60 is vaporized
within a heat exchanger 62 through indirect heat
exchange with an air stream 64 that is thereby
liquefied. The resultant gaseous crude argon stream 66
is introduced into a heat exchanger 68 to form gas
CA 02606332 2007-10-22
WO 2006/115916 PCT/US2006/014639
- 19 -
stream 12 that is introduced into electrically driven
oxygen separation device 10 to produce purified gas
stream 14. Purified gas stream 14 exchanges heat with
gaseous crude argon stream 66 within heat exchanger 68
so that gas stream 12 to be purified possess a
temperature at or near the operational temperature of
electrically driven oxygen separation device 10.
Purified gas stream 14 after passage through heat
exchanger 68 is cooled to ambient or near ambient
temperatures as a result of such heat exchange.
Purified gas stream 14 is then introduced into a heat
exchanger 70 in which the purified gas stream 14
liquefies through indirect heat exchange with a liquid
air stream 72 that can be extracted from the higher
pressure distillation column. A trim heater 74 can be
provided so that gas stream 12 upon its introduction
into electrically driven oxygen separation device 10 is
exactly at operational temperature. As indicated
above, a purge gas stream 42 is introduced into
electrically driven oxygen separation device 10 to
extract the separated oxygen.
[0047] Fig. 4 is an alternative embodiment of Fig.
3. In order to avoid needless repetition of
explanation, the same reference numbers have been used
in Fig. 4 for elements that have the same description
as set forth above for Fig. 3. In Fig. 4, crude liquid
argon stream 60 vaporizes along with a liquid nitrogen
stream 76 within a heat exchanger 78 to in turn liquefy
purified gas stream 14. Purified gas stream 14 is at a
lower pressure than that of crude liquid argon stream
60 and therefore, a thermal mismatch exists that would
not permit purified gas stream 14 to otherwise vaporize
CA 02606332 2007-10-22
WO 2006/115916 PCT/US2006/014639
- 20 -
crude liquid argon stream 60. However, by adding a
blower 80 to slightly raise the pressure of the
resultant vaporized crude argon stream 66 and the
provision of liquid nitrogen stream 76, crude liquid
argon stream 60 will vaporize as required and purified
gas stream 14 will liquefy without an excessive
requirement for liquid nitrogen that would make'the
entire process unprofitable.
[0048] With reference to Fig. 5, In a yet further
embodiment, crude liquid argon stream 60 and purified
gas stream 14 are introduced into a main heat exchanger
82 of a cryogenic air separation plant. In main heat
exchanger 82 crude liquid argon stream 60 vaporizes and
product stream 14 after having been cooled in heat
exchanger 68 liquefies. Main heat exchanger 82 is
conventionally provided with passages for nitrogen and
oxygen product streams 84 and 86 to warm such streams.
For example, nitrogen product stream 84 could be made
up of tower overhead produced in a lower pressure
column of such cryogenic air separation plant and
oxygen product stream 86 could be liquid oxygen or
oxygen vapor made up of oxygen produced as column
bottoms within the lower pressure column. A further
passage is also provided for a compressed and purified
air stream 88 that is cooled within main heat exchanger
82 to a temperature at or near its dew point for
introduction into the bottom region of a higher
pressure column for rectification of the air. The flow
rates of crude liquid argon stream 60 and product gas
stream 14 are of such lesser magnitudes than the other
streams passing through main heat exchanger 82, that
the foregoing can be effectuated by a retrofit
CA 02606332 2007-10-22
WO 2006/115916 PCT/US2006/014639
- 21 -
modification of an existing main heat exchanger without
any significant effect on its performance. Optionally,
a blower 80 can be provided to provide a better match
between heating and cooling curves of product gas
stream 14 and crude liquid argon stream 60.
[0049] In the embodiments illustrated in Figs. 3, 4
and 5, the illustrated gas streams are sufficiently
pressurized to flow through the heat exchangers by
provision of a liquid head being built up within crude
liquid argon stream 60 by locating the argon column
from which crude liquid argon stream 60 is taken above
grade.
[0050] With reference to Fig. 6, electrically driven
oxygen separation device 10 is shown as an oxygen
purifier to purify a crude nitrogen stream 90 obtained
from a pressure swing adsorption unit or a membrane
unit. As well known in the art, a pressure swing
adsorption unit has beds of adsorbent that can be
formed of carbon molecular sieve material. The air is
compressed and passed through an adsorbing bed to
adsorb oxygen contained in the air to produce the crude
nitrogen stream 82. While this is occurring at least
another bed is off-line and being regenerated by
allowing oxygen to desorb from the adsorbent contained
in such bed at a lower pressure. Part of the
regeneration can occur by pressure equalization with
yet other beds and various purge steps that can involve
purging the bed to be regenerated with some of the
product. After the bed is fully regenerated, it can be
brought back on line to produce a product. In membrane
separation units a compressed air stream is contacted
with a polymeric membrane that is made of material
CA 02606332 2007-10-22
WO 2006/115916 PCT/US2006/014639
- 22 -
selected to pass oxygen faster than nitrogen to produce
a permeate stream rich'in oxygen and a nitrogen
retentate stream that forms the crude nitrogen stream
82.
[0051] Crude nitrogen stream 90 is heated within a
heat exchanger 92 through indirect heat exchange with
product gas stream 14 to form gas stream 12 that is
introduced into electrically driven oxygen separation
device 10 to produce purified gas stream 14. Gas
stream 12 as a result of such heat exchange possesses a
temperature near the operational temperature of
electrically driven oxygen separation device 10. A
trim heater 94 can be used for final heating of gas
stream 12 to the operational temperature of
electrically driven oxygen separation device 10.
Purified gas stream 14 after passage through heat
exchanger 92 is cooled to ambient or near ambient
temperatures as a result of such heat exchange.
Additionally part of the gas stream 12 can be used to
form purge stream 42 to sweep and remove permeated
oxygen from electrically driven oxygen separation
device 10.
[0052] In an exemplary mode of operation, crude
nitrogen stream preferably contains about 0.5 volume
percent of oxygen and is heated to about 700 C within
heat exchanger 92. The electrically driven oxygen
separation device 10 operated at about 700 C requiring
a temperature rise of 50 C to account for atmospheric
heat loss or roughly 113 watts per 100 scfh of crude
nitrogen. Since, the electrically driven oxygen
separation device 10 requires roughly 93 watts per 100
scfh of crude nitrogen under such operational
CA 02606332 2007-10-22
WO 2006/115916 PCT/US2006/014639
- 23 -
conditions, about 20 watts per 100 scfh of crude
nitrogen is required for trim heater 94 to maintain
stable operating temperatures.
[0053] Crude nitrogen stream 90 preferably has an
oxygen concentration of between about .05 percent and
about 2 percent by volume oxygen. More preferably, the
oxygen concentration is between about .1 percent and
about 1 percent by volume oxygen. Most preferably, the
oxygen concentration is between about .15 percent and
about .5 percent by volume oxygen. It has been
calculated, that a minimum power usage for electrically
driven oxygen separation device 10 is achieved when
crude nitrogen stream contains about 1.5 percent by
volume oxygen. A minimum capital cost for the entire
system, including a pressure swing adsorption unit, is
realized when the crude nitrogen stream contains about
0.15 percent by volume oxygen. Overall, when the crude
nitrogen stream contains between about 0.15 percent and
about .5 percent oxygen by volume, the lowest total
cost will be achieved for a 10,000 scfh system.
[0054] During start-up conditions of the
electrically driven oxygen separation device 10, the
pressure swing adsorption unit or membrane separation
unit should be operated at a lower capacity and
therefore produce a crude nitrogen stream 90 at a lower
flow rate and therefore at a higher purity. This
allows the customer to receive the product while the
electrically driven oxygen separation device 10 is
being heated up. For most pressure swing adsorption
units, during start-up, such units should be operated
at about 30 percent of capacity to produce crude
nitrogen stream 90 with an oxygen concentration of
CA 02606332 2007-10-22
WO 2006/115916 PCT/US2006/014639
- 24 -
about 50 ppm. Also, the pressure swing adsorption unit
or membrane separation unit can be operated in such
manner during maintenance of any of the electrically
driven oxygen separation zones 16, 18 and 20.
[0055] While the present invention has been
described with reference to a preferred embodiment, as
will occur to those skilled in the art numerous
changes, omissions and additions can be made without
departing from the spirit and the scope of the present
invention.