Note: Descriptions are shown in the official language in which they were submitted.
CA 02607217 2007-11-05
PCT/EP2006/003760
Method of cultivating a cell culture in an automated cell culture system as
well as an
automated cell culture system
The present invention relates to a method of cultivating a cell culture in an
automated cell
culture system and to an automated cell culture system. In particular, the
present invention
relates to a device and a method of regulating cell culturing conditions and
cell cultivation
operating steps subject to the condition of the biological cells.
Cell cultures are often used in pharmaceutical and biotechnological
industries. In particular
for ethical and economical reasons, animal experiments have been increasingly
replaced by
cell culture systems and cell culture technologies in recent years. For
example, the European
Union and the OECD also recommend the replacement of animal experiments by
cell tests
without animal experiments for testing medicine, chemicals and cosmetics. The
employment
of cell cultures in the search for new active principles and active agents in
the pharmaceutical
and pesticide sectors has therefore meanwhile become inevitable.
Moreover, a number of in-vitro test methods in the field of toxicology has
been increasingly
adopted in regulatory proceedings. Thus, in the pharmaceutical and pesticide
sectors, too, the
use of cell cultures is no longer dispensed with.
However, a significance of the data obtained with cell cultures is at present
still limited by the
existing analysis technique. Moreover, common manual methods in cell
cultivation are time
consuming and costly, and their results moreover highly depend on the user.
Due to this fact, initiatives have been started recently for defining minimum
requirements on
cell and tissue cultures and for thus ensuring the significance, comparability
and
reproducibility of in-vitro works. As a result, analogously to the Good
Laboratory Practice
(GLP) directives, principles for cell culture works have been passed with the
designation
Good Cell Culture Practice (GCCP).
These directives, however, have no influence on the fact that today, as a
rule, cell cultures are
still cultivated employing a lot of laboratory personnel. The cells are, as a
rule, cultivated in
CA 02607217 2007-11-05
2
Petri dishes, micro-titre plates or in cell culture bottles. In most cases,
the steps necessary for
cell cultivation, such as initial inoculation of the cells, change of the
medium or passage of the
cells, are performed merely manually. Moreover, the monitoring of the cells is
discontinuous.
Changes of the pH value of the medium are observed by added color indicators.
The medium
is changed at regular intervals, depending on the growth rate of the
respective cells of the cell
cultures (by manual control after removal from an incubator) to ensure
sufficient supply of the
cells with nutrients.
As one can draw conclusions from the results of cell cultivation, i.e. from
the development of
the cell culture under the influence of certain factors, to the efficacy of
medicine or to a toxic
effect of substances (e.g. in cosmetics), the reproducible cultivation and the
standardized
operation of the cell culture has a particular significance for the mentioned
fields of
application.
The possibility of standardizing the cell culture techniques is, due to its
high dependency on
manual (and thus user-dependent and not reproducible) methods and the
resulting high efforts,
at present not sufficient for the use of this culture in applications, such as
stem-cell research,
screening or tissue engineering.
A prerequisite for this are rather standardized cell culture methods, as only
these permit a
comparison between the substances and their active principles over years.
Correspondingly, the following individual problems exist:
The adaptation of the cell culturing conditions is performed, in existing
solutions, on the basis
of fixed protocols and, as a consequence, inflexible procedures in the cell
cultivation process,
these protocols being based on the manual cultivation by the laboratory
personnel and their
experience as well as the subjective perception of the operator and the
established daily
routine in the cell culture laboratory.
The control and documentation of the cell condition is normally effected only
at an interval of
hours or days as thus less efforts are involved. This results in long control
intervals. In the
cultivation of sensible cells, such as in particular stem cells, it is,
however, often necessary to
control the cells at shorter intervals.
CA 02607217 2007-11-05
3
In the characterization of the cells, the selection of the examined areas is
made subjectively
and arbitrarily by the operator. Here, even the assistance by software for the
optical analysis is
insofar manual and not reproducible.
The manual change of the cell culture media involves individual variations in
time and thus
variations in the supply for the cells. Equally, this results in stress for
the cells, for example
due to undefined flows.
In the characterization of the cells by the operator, the cells are moreover
influenced due to
great variations of the cell culturing conditions, for example changes of the
ambient
temperature of>IOK, that means, the culturing conditions are interrupted.
Their impact on
cell development is not predictable.
Furthermore, there is a risk of contamination by a plurality of manual
processes as well as by
automation solutions of poor hygienic quality.
Another disadvantage is that the decision of the next step in the cell
cultivation process is
made according to a subjective assessment (for example depending on the
operator, the
weekday or time of day, or on imaging settings of the microscope) of the cell
culture (for
example evaluations, such as "degree of confluence approx. 70 or 80%", "medium
approx.
consumed", or "cell during passage now approximately all detached") and
according to the
planning of the cell cultivation personnel or their work schedule (it is, for
example, considered
to be sufficient to passage or change the medium "tomorrow" or "on Monday
after the
weekend", or "after the holidays", and the personnel/the operator therefore
does not have to
perform the cell cultivation process outside his/her regular working hours).
In systems known on the market, cell culture chambers are used as self-
sufficient system
components besides automation systems, where the automation systems are, for
example,
exclusively usable for the passage or the medium change. These automation
systems are no
integral part of the cell culture process as the available systems, e.g. cell
sowing, washing
steps, medium change and cell harvest, are performed independently. Depending
on the
system, further steps, such as transfection or determination of the cell
number, can be also
carried out.
CA 02607217 2007-11-05
, ~ ' =
4
In the characterization of cells by biochemical-biomolecular methods, at
present one can only
obtain snap-shots as the cells are often destroyed by the representation
methods. The
corresponding measuring methods are time consuming and not suited for
monitoring cells
over a relatively long period. A continued and consistent control and
monitoring of the cells is
not possible with the existing techniques.
The culturing conditions for the cells are often shaped by experience, such
as, for example, by
the intervals at which the culture medium is changed. This renders a possible
standardization
of the cell cultures difficult.
In contrast, systems which are capable of performing a regular monitoring of
the cells at short
intervals would be advantageous. Moreover, on the basis of the acquired data,
the cultivation
conditions would have to be readjusted if necessary.
Commercially available image analysis software for the analysis of cell
microscopy images is
either tuned to very special tasks (e.g. counting colored cells in
suspensions), or it requires
very good imaging skills of the user to exactly adapt the algorithms and
parameters to the
respective analysis task. In the image analysis of cell cultures, such an
adaptation is absolutely
necessary as different cell types can have very different appearances, and
even cells of one
type greatly vary depending on the cultivation stages. However, users in
biology often do not
have the required technical background for correspondingly adjusting the
software.
Starting from the above statements, it is an object of the present invention
to provide a method
of cultivating a cell culture in an automated cell culture system and an
automated cell culture
system, wherein the automation is part of the cell culture process, to obtain
optimized and
standardized cell culturing conditions.
The object according to the invention is achieved with respect to the method
aspect by a
method of cultivating a cell culture in an automated cell culture system,
wherein data
concerning the condition of cells are acquired in the cell culture and at
least one culturing
condition is regulated in accordance with the acquired condition of the cells
in the cell culture,
and/or at least one operating step determined to be necessary in the cell
cultivation process is
initiated or carried out.
CA 02607217 2007-11-05
With respect to the apparatus aspect, the object according to the invention is
achieved by an
automated cell culture system with at least one actoric element for handling a
cell culture, at
least one sensory element for acquiring data concerning the condition of the
cells in the cell
culture, an apparatus for evaluating the data concerning the condition of the
cells, and an
apparatus for regulating cell culturing conditions and/or for initiating
and/or carrying out
further operating steps in the cultivation.
In the method according to the invention and the cell culture system according
to the
invention, optimized and standardized cell culturing conditions are achieved
in that the culture
is held in an automated cell culture system which acquires data (concerning
the condition) of
the cell and moreover, depending on their condition, regulates the cell
culturing conditions
(preferably directly) upon the acquisition of the condition of the cell
culture and/or
initiates/triggers necessary operating steps in the cell cultivation. Thereby,
the influence of the
human operator is reduced and replaced by a more precise automatic solution.
Moreover, a
reproducible procedure is implemented which considers the natural variations
of the living
cell cultures.
Preferred embodiments of the method according to the invention and the
automated cell
culture system according to the invention are subject of the depending claims.
The present invention will be illustrated below with reference to preferred
embodiments in
connection with the corresponding drawing in which an optical arrangement for
obtaining
quantifiable spatial information of the cells and cell cultures is shown.
A preferred embodiment of the automated cell culture system comprises as
actoric elements
an automated handling unit for transporting cell culture vessels (for example
cell culture
bottles or micro-titre plates), an automated handling apparatus for liquids
(for example the cell
suspensions, fluorescent dyes, and nutrient media), and a storage for
temporarily not used cell
culture vessels.
The present automated cell culture system moreover comprises a conditioning
unit for
preparing the exterior cell culturing conditions, in particular a conditioned
vertical circulation
air unit with defined environmental conditions.
CA 02607217 2007-11-05
6
The present embodiment furthermore comprises a unit for generating a laminar
flow, a
medium supply, devices for the sterilization of surfaces, and a centrifuge. By
a corresponding
design of the surfaces as well as the choice of the material, decontamination
properties are
achieved by which the risk of cross-contamination is minimized. These include,
for example,
the prevention of unsealed threaded joints, of dead spaces or plane
construction surfaces
which would hinder the drainage of optional rinsing liquids or production
residues.
Moreover, the condition of the cells in the cell culture in the present
embodiment of the
automated cell culture system is acquired with one or several devices for
obtaining
information from the culture. For this, an optical image acquisition and/or
electrical/electrochemical sensors are provided (as sensory elements).
For example, an optical examination of the cell culture with various imaging
microscopic
methods, such as transmitted light, phase contrast or differential
interference contrast, "DIC",
are possible.
Possibly, an imaging fluorescent optic is added by which the spatial
distribution of fluorescent
dyes in the cell culture is determined.
These imaging methods are connected to an automatic image analysis which is
suited for
drawing conclusions to the condition of individual cells or the complete
culture. Here, the
spatial structure of the cells and cell cultures provides interesting
additional information.
For obtaining such quantifiable information about a spatial structure of the
cells and cell
cultures, the conventional methods, such as phase contrast and differential
interference
contrast (DIC), are, however, not suited. These visually give an impression of
the third
dimension (in the observation direction, z-direction in the figure). This
information, however,
is not in all cases a quantitative information that can be further processed
in an image analysis
software. In particular, the images which are obtained with the mentioned
conventional
contrast method highly depend on the adjustment of the optics.
CA 02607217 2007-11-05
7
Objective, quantifiable information, however, is obtained by an
interferometric method in
which, in addition to the microscopic beam path (measurement beam path), an
additional
reference beam path is added.
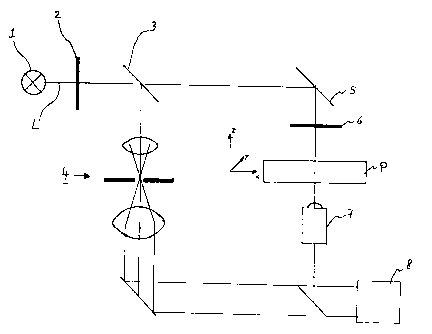
In the (only) figure, such an optical arrangement for obtaining quantifiable
spatial information
about the cells and cell cultures is shown.
Here, a beam of light L is generated by a light source 1 and passed over a
polarization filter 2.
The polarized beam with a wavelength of V2 is divided into two partial beams
L1, L2 by
means of a beam splitter 3. The partial beam LI, which forms the reference
beam, passes
through an assembly of a telescope and a spatial filter (designated with
reference numeral 4).
The partial beam L2, which forms the measurement beam, is passed to sample P
via a
deflection means 5 and a further polarization filter 6. After the passage
through the sample P,
the measurement beam L2 passes through a microscope objective 7. The
measurement beam
exiting from the microscope objective 7 is superposed by the reference beam L1
on an
evaluation optics 8 (in particular a CCD field).
The beam division to the beam parts L1 and L2 is uniform in the present case,
i.e. the
intensity L 1 to L2 is 50% to 50%.
As can be seen in the (only) figure, the reference beam L 1 is superposed by
the measurement
beam L2 exiting from the objective. The interference pattern formed on the
optical evaluation
means 8 contains the information about the phase shift of the light at any
location in the x-y-
axis on the sample (in the figure, a Cartesian system of coordinates is
shown). The optical
phase is equivalent to the product of the thickness and the index of
refraction of the observed
object P. Assuming that the indices of refraction within the cells are
homogenous and
identical from cell to cell, one can calculate the thickness of the cells at
any location in the x-y
plane therefrom.
For definitely determining the phase, in the present embodiment, a component
(telescope +
spatial filter) for the defined phase shift of the reference beam is included.
From several
images which have been taken with various reference phases, a clear difference
is made
between phase and intensity information. At the same time, one obtains a phase
value that is
definite within a range of 27r. Beyond an interference order (phase change of
2n), the phase
CA 02607217 2007-11-05
8
can be corrected by means of well-known "unwrapping" techniques.
Apart from the above-mentioned imaging methods, moreover optical methods are
important
by which the optical transmission or the fluorescence can be averaged and
acquired over
relatively large areas of the culture. With this, one can e.g. acquire the
coloration of indicators
which are added to the culture medium. By this, methods for averaging the cell
properties can
be made available.
Furthermore, electrical or electrochemical measuring methods can be employed
by which, for
instance, the local temperature, the pH value or ion concentrations are
determined.
To be able to automatically determine the condition of the cell culture,
software for the
evaluation of image data and sensor signals and for controlling the complete
course of the cell
cultivation are provided. With this software, the image data of the cultivated
cells acquired by
the system are processed via an image analysis. The same supplies quantitative
analysis
results, such as for example cell density, number of dead cells, number of
mitosis and number
of morphologically modified cells, which give an image of the culture which
can result,
together with its history, i.e. data acquired earlier from this culture, and
together with the
protocols for the cultivation of the special culture, in a very exact
evaluation of the present
condition of the cell culture. This very exact evaluation of the condition of
the cell culture is a
solid basis for the determination of a possible intervention in the culturing
conditions and/or
for the initiation of an operating step in the cultivation process. This
intervention can then be
effected directly by the system, or it can lead to a message to the exterior.
In this case, the
system can give a message, possibly with a suggestion for an action, which is
then triggered
by the operator.
The processing of the cell images and the sensor signals is designed as
trainable software.
Thus, the system can be easily adapted to modified tasks (such as other cell
cultures or
modified basic conditions). The user presets, e.g. by means of images, a
classification of cell
types or of defined conditions of the culture, which are subsequently
identified by the image
processing during the cultivation.
In particular, here the image analysis software for the detection of relevant
image structures,
such as in particular healthy cells, shattered cells, detached cells, can be
trained by the user.
CA 02607217 2007-11-05
9
To this end, in a training phase, examples of the structures to be identified
are marked in some
images. The image analysis software calculates a set of features (intensity,
morphological
features, textures) for these images and determines those features which
characterize the
selected examples, and in particular criteria which discriminate the
structures searched for
from other structures in the feature space. After the termination of the
training phase, these
calculated parameters can be used to automatically identify the desired
structures in further
images.
Apart from the automatic data acquisition and regulation, the documentation
(e.g. the
regulation parameter, the measured values of the regulation, the condition of
the cell culture
or the medium at different points of time, the measured values of the
cultivation parameters
and the condition of the cell culture on the basis of which a regulation or an
operating step has
been performed) is very important. In this way, the history of the culture and
cultivation can
be traced. Thus, a time controlled, more precise condition description is
provided, and in case
of a not optimal result, troubleshooting is facilitated.
In a first example of use of the present automated cell culture system, for an
optimal cell
differentiation, the composition of the differentiation medium is adapted to
the degree of
differentiation of the cell culture. For this, image data of the cell culture
are continuously
taken and evaluated by means of the evaluation software. In case of certain
conditions of the
cell which are identified by the software either the medium present in the
cell culture vessel is
automatically replaced by a new medium of a different composition, or growth
factors are
added to the present medium. Preferably, for the image analysis, the above
described trainable
software is used. The condition of the cell culture is acquired, for example,
by means of
morphological features or by means of minimally invasive marking substances. A
training of
the image analysis software can be made in preliminary tests by experienced
personnel or else
by means of the invasive marking substances.
In a second example of use, the replacement of the culture medium is made in
response to the
cell function. In this case, a certain cell function, e.g. a contraction rate
in case of
cardiomyocytes, is quasi continuously observed. As soon as the parameters of
the selected cell
function leave a previously defined range, a replacement of the cell culture
medium is caused.
In a third example of use, the cultivation of a first cell type is assisted by
means of a further
CA 02607217 2007-11-05
cell type (feeder cells). If in the mixed cultivation with these two cell
types, the first cell type
is present in a sufficient amount, the present culture medium is automatically
replaced by a
culture medium, which leads to the destruction of the second cell type. When
all cells of the
second type are dead, the culture medium is automatically replaced by normal
medium.
In a fourth example of use, an operating step is initiated on the basis of a
condition of the cell
culture, such as a passage of the cell culture, when an intended degree of
coverage/confluence
is achieved, to further cell culture bottles or other cell containers,
dispensing the cells in
micro-titre plate wells, or individual cell withdrawal/handling/further
processing.
In a fifth example of use, cells are cultivated in containers (e.g. Petri
dishes, MTP, cell culture
bottles), test substances (e.g. in case of active agent tests or toxicity
tests) are added, and the
cell culture in its property (e.g. growth rate, morphology, nucleus-plasma
ratio, type of
growth, such as for example criss-cross growth, formation of the cell
function, cell counting
in certain areas, for example colony center/colony edge) is documented in a
time controlled
manner, and a suggestion for the classification (e.g. normal, modified,
transformed, cell
number in the area, medium nucleus size, medium cytoplasm area, medium number
of
neutrites, etc.) is made to the system.
A sixth example of use relates to the optimization of the cell culture process
in primary cell
cultures, e.g. ceratinocytes from different biopsies, and/or the maintenance
of the cell culture
without further differentiating out.
A seventh example of use relates to an autonomous cultivation process of a
robust routine cell
culture (e.g. HeLa, HEK, COS), including change of medium, passage, sowing
based on the
optical and/or electrical/electrochemical sensory mechanism (e.g. for
screening experiments
with cells of the same quality within one cell culture) independent of regular
working times of
the laboratory personnel (e.g. over night, over the weekend, over holidays).
An eighth example of use relates to the standardization of a cell culture
(definition of the
process parameters for the cell cultivation, e.g. point of time for passage or
change of
medium, seeding density, planting efficiency (how many cells grow?)) with the
present
automated cell culture system on the basis of the documentation already
illustrated above,
before it is transferred to a system with rigid protocols or the cultivation
is established in the
CA 02607217 2007-11-05
11
bioreactor/fermenter.
In a ninth example of use, certain areas which have formed during the
cultivation and
comprise characteristic features are withdrawn from a cell culture. As soon as
these areas
have formed, this is detected by means of an image analysis, and the cells of
the areas are
automatically transferred to a new culture vessel by means of a pipette.
In a tenth example of use, aliquots from a cell suspension with a very low
cell concentration
are placed into wells of a micro-titre plate. In the regulated cell culture
system, only cells of
those wells are processed further in which there initially was exactly one
cell.
From the above description, one can take in particular an automated cell
culture system,
consisting of actoric elements (for handling cell culture vessels, cell
suspensions, fluorescent
dyes and nutrient media), a conditioned vertical circulation air unit with
defined
environmental conditions, sensory elements (optical image acquisition and/or
electrical
sensory mechanism), and software for the evaluation of image data and sensor
signals and for
controlling the complete process, where the culturing conditions are regulated
and/or
operating steps are initiated and/or carried out in the cultivation depending
on the situation.
The image acquisition is in this case made, among others, with an
interferometric method.
Here, in addition to the microscopic beam path, a reference beam is introduced
which is
superposed by the measurement beam which exits from the microscope objective.
The processing of the cell images and the sensor signals is designed as
trainable software in
the present case. Thus, the system can be easily adapted to modified tasks
(other cell cultures,
modified basic conditions).
The system components are designed with a highly hygienic quality and thus
prevent the
contamination of the product. This is in particular true for the design of the
surface roughness
of smaller than 20 m, corresponding sealing elements of PTFE or similar
materials, as well
as a corresponding constructive design.
CA 02607217 2007-11-05
12
The planning of the automated cell cultivation is made on the basis of the
desired end time of
the production. Starting from a point of time at which the cell lines are to
be available, a
backward planning is carried out stating when a change of the medium and
passages are to be
carried out.