Note: Descriptions are shown in the official language in which they were submitted.
CA 02607353 2007-10-22
METHOD OF TREATING WATER USING PETROLEUM COKE
FIELD OF THE INVENTION
The present invention relates to a method of treating water using petroleum
coke. More particularly, water produced during the recovery of bitumen from
oil
sands (hereinafter referred to as oil sands process-affected water or OSPW) is
treated with petroleum coke produced in coker reactors to remove dissolved
organics
therein.
BACKGROUND OF THE INVENTION
The demands for water in oil sands operations are high and therefore most
operations must rely on recycling process water. However, during oil sands
processing, a significant amount of dissolved inorganic (e.g., salts) and
organic (e.g.,
carboxylic acids, hydrocarbon) constituents are released into process waters.
Recycling of the oil sands process-affected water (OSPW) only serves to
increase
the levels of dissolved inorganic and organic content. Currently no OSPW is
released from the operations.
In order to meet water quality criteria for release, it is necessary to treat
the
OSPW to reduce the dissolved organics, such as naphthenic acids and other
hydrocarbons. Naphthenic acids have been demonstrated to be toxic to aquatic
biota (Alberta Environment Protection. 1996. Naphthenic acids background
information discussion report. Edmonton, Alberta, Alberta Environment,
Environmental Assessment Division). Thus, the concentration of naphthenic
acids
present in OSPW must be reduced to levels that are not detrimental to the
biological
community of a receiving aquatic system. Removal of naphthenic acids may be
accomplished with either natural bioremediation or treatment methods to remove
them from the OSPW.
Naphthenic acids (NAs) are natural constituents in many petroleum sources,
DMSLega1\053707\00209\ 2728294vl
CA 02607353 2007-10-22
2
including bitumen in the oil sands of Northern Alberta, Canada. NAs are
complex
mixtures of predominately low molecular weight (<500 amu), fully saturated
alkyl-
substituted acyclic and cycloaliphatic (one to more than six rings) carboxylic
acids
(Brient, J. A., Wessner, P. J., and Doyle, M. N. 1995. Naphthenic acids. In
Encyclopedia of Chemical Technology, 4th ed.; Kroschwitz, J. I., Ed.; John
Wiley &
Sons: New York, 1995; Vol. 16, pp 1017-1029). They are described by the
general
empirical formula CnH2n+ZO2, where n indicates the carbon number and Z is zero
or a
negative, even integer that specifies the hydrogen deficiency resulting from
ring
formation (i.e. Z=-2 indicates 1-ring, Z=-4, 2-rings etc.). While some of
naphthenic
acids will biodegrade rapidly, a fraction of the naphthenic acids associated
with the
OSPW have been shown to be more recalcitrant (Scoff, A. C., M.D. MacKinnon,
and
P.M. Fedorak. 2005. Naphthenic acids in Athabasca oil sands tailings waters
are
less biodegradable than commercial naphthenic acids. ES&T 39: 8388-8394). In
order to facilitate release of OSPW, it is desirable to find options for more
rapid
removal of NAs from OSPW that is effective, targeted to the dissolved organics
and
economically viable.
In surface oil sands mining operations for recovery of bitumen, also referred
to
as open-pit oil sands operations, hot or warm water, to which a process aid,
such as
caustic (NaOH) may be added, is mixed with the oil sand ore (about 1.5-2m3 of
water
per barrel of oil extracted) in order to separate the bitumen from the oil
sand. The
resulting oil sand slurry goes through a series of separators to produce lean
bitumen
froth. The tailings stream produced during bitumen extraction, which comprises
water, sand and un-recovered bitumen, is transported to settling basins, where
the
solids settle by gravity, and the resulting release water (OSPW) is recycled
for reuse
in the extraction process. Also included as recycle or release water is
seepage water
from sand structures containing settling basins. It is during this extraction
process
that leaching of both inorganic and organic constituents will occur.
Bitumen can also be recovered from oil sands in situ (in the geological
formation) using the Steam Assisted Gravity Drainage process (the "SAGD"
DMSLegal\053707\00209\ 2728294v1
CA 02607353 2007-10-22
3
process). SAGD requires the generation of large amounts of steam in steam
generators, which steam is injected via injection wells to fluidize the
bitumen for
recovery. A bitumen/water mixture results and the mixture is pumped to'the
surface
where the bitumen is separated from the water. The produced water stream (oil
sands process-affected water) is then reused to produce more steam for
extraction.
As in surface mining operations, the produced water stream contains dissolved
organics that need to be removed. The produced water in SAGD must be treated
to
meet requirements for once-through steam generators, and the retentate from
this
preparation will contain elevated NAs.
Bitumen produced from either surface mining operations or SAGD can be
further upgraded by thermal cracking using either a delayed or fluid coker
reactor, as
are known in the art, to take the highly viscous bitumen (API gravity of about
8 ) to a
less viscous hydrocarbon product (API gravity of about 30 ). During coking, an
excess amount of petroleum coke is produced, which excess coke is currently
disposed of as waste product. Thus, petroleum coke produced from coking
operations is a readily available commodity.
There is a need for an effective, selective and economical water treatment
process for the OSPW produced during bitumen oil extraction processes so that
the
water can be reused in the operation or released into the environment.
SUMMARY OF THE INVENTION
The present invention is based on the surprising discovery that petroleum
coke can be used to treat process water from oil sands extraction operations
to
remove a substantial portion of dissolved organics without having to activate
the
petroleum coke first. The present invention is particularly effective in
treating oil
sands process-affected water (OSPW) produced during surface oil sands mining
operations, and, particularly, when fresh product coke (FPC) produced during
fluid
coking operations is used.
DMSLegal\053707\00209\ 27282941
CA 02607353 2007-10-22
4
In one broad aspect of the invention, a process for treating water containing
dissolved organics is provided, comprising:
= removing petroleum coke from a coking operation;
= forming a petroleum coke/water slurry by adding the water to be treated
to the petroleum coke; and
= mixing the petroleum coke/water slurry for a sufficient time in a carbon
adsorption reactor to allow the petroleum coke to adsorb a substantial
portion of the dissolved organics from the water.
The water containing dissolved organics can be oil sands product water
generated during bitumen extraction processes used in either oil sands surface
mining or in situ mining operations. For example, but not meaning to be
limiting,
OSPW can be from obtained from tailings settling basins (fresh release water
from
extraction tailings) or from reclamation components (aged OSPW) such as end-
pit
lakes, sand dyke seepage, etc. However, it is understood that the present
invention
can be used to treat any water source that has a substantial amount of
dissolved
organics such as naphthenic acids and hydrocarbons, for example, which could
be
present in ground water.
There are two main types of petroleum coke that can be produced depending
on the type of coker reactor used, namely, fluid coke and delayed coke. A
typical
fluid coke comprises particles having an average particle size of about 200 pm
in
diameter with an onion-like layered structure (Chung, K. H., L.C.G. Janke, R.
Dureau,
E. Furimsky. 1996. Leachability of cokes from Syncrude stockpiles. ES &T (3):
50-
53). Preferably, hot fresh petroleum coke is used, which has been removed
directly
from the coker burner of the coking operation.
Delayed coke from delayed coking operations can also be used. However,
delayed coke is produced in the form of larger lumps. Thus, when delayed coke
is
DMSLegal\053707\00209\ 2728294v1
CA 02607353 2007-10-22
used in the present invention, the lumps of coke are preferably first
pulverized to give
a fine powder having an average particle size comparable to fluid coke.
Contrary to conventional thinking (see, for example, U.S. Patent No.
6,932,956), hot fresh petroleum coke removed from a fluid coking operation is
not
5 unreactive, although the activity of hot fresh petroleum coke is relatively
low in
comparison with commercial particle or granular activated carbon (e.g., on
average
about 5-10 m2/g for fluid coke versus about 500-1500 m2/g for activated
carbon).
However, at the applicant's operation, about 0.02-0.03t of coke per barrel of
bitumen
upgraded is produced. Thus, a large quantity of petroleum coke is readily
available.
Thus, in one embodiment, OSPW/coke slurry is formed and pipeling of the
slurry performs as the reactor for adsorption. Thus, when slurry densities of
about 15
to about 30% by wt, are used, no enhanced activation is required. At the rates
of
fresh petroleum coke production in a normal coking operation, there is a
sufficient
supply of the coke to treat more than 10 million m3 annually. Hence, the
present
invention offers an economical way to treat OSPW, as producing activated
carbon
from petroleum coke (that seen in the coke storage deposits) can be very
costly and
time consuming. Furthermore, because of petroleum coke's unique properties, in
particular, fluid coke, it is expected that the activation process could be
problematic
as a result of environmental issues such as energy consumption and fugitive
emissions. Currently, most of the excess fluid coke produced is stored in
special
cells or in beaches within current settling basins so it is available for
future needs.
Hence, the present invention utilizes a product that has previously been
considered
in the industry to be a waste product.
In one embodiment, the petroleum coke is hot fresh fluid coke produced during
fluid coking, where coke is produced at high enough rates such that the
concentration of the coke in the resulting coke/water slurry can be expected
to range
from about 10% to about >40% by weight. It has been shown that optimum dosages
will range between about 15% to about 30% by weight.
DMSLega1\053707\00209\ 2728294v1
CA 02607353 2007-10-22
6
The present invention is particularly effective in reducing the concentration
of
naphthenic acids. For example, when using OSPW produced during oil sands
mining
operations, for example, OSPW from extraction tailings, a reduction of the
naphthenic
acid concentrations ranging from 70% to more than 90% is routinely obtained.
The
efficiency of organic carbon removal is dependent on the petroleum coke
content in
the slurry.
The carbon adsorption reactor of the present invention can be any stirred tank
reactor known in the art, such as a continuous flow stirred tank reactor. In
the
alternative, the carbon adsorption reactor can be a plug flow reactor, such as
a
pipeline of sufficient length to provide proper mixing and residence time.
The process for treating water containing dissolved organics may further
comprise the step of separating the petroleum coke from the treated water. One
embodiment takes advantage of the rapid settling characteristics of the coke
in the
transport slurries. Thus, gravity settling and collection of release waters,
or design of
deposit cells with bottom drainage will produce treated water with dissolved
organics
such as NAs removed. Passive separation methods that use open cells have the
added benefit of reducing suspended solids contents by increasing the
residence
time of the slurry and when allowed to percolate through the petroleum coke,
further
improving this aspect of water quality.
Another embodiment involves more proactive treatments to reduce turbidity of
treated waters, which include filtration or ultrafiltration using filtration
membranes
such as ZeeWeedTM Ultrafiltration Membranes. Once the treated water has been
separated from the petroleum coke, the treated water can be recycled for
operations
needs or can be evaluated for potential release into the environment, either
directly
or after a further treatment option.
In a preferred embodiment, the treated water that is not recycled for
operation needs but is being returned into the environment may be further
treated
using advanced oxidation methods such as ozonation, biological reactors such
as
DMSLega1\053707\00209\ 2728294v1
CA 02607353 2007-10-22
7
engineered or natural aquatic systems, or membrane methods such as
nanofiltration
and reverse osmosis. These methods would further remove remaining dissolved
organics, specifically the naphthenic acids, so that release into the
environment
would likely be possible.
BRIEF DESCRIPTION OF THE DRAWINGS
The present invention, both as to its organization and manner of operation,
may best be understood by reference to the following descriptions, and the
accompanying drawings of various embodiments wherein like reference numerals
are
used throughout the several views, and in which:
FIG. 1 is a simplified schematic of a known fluid coking circuit; and
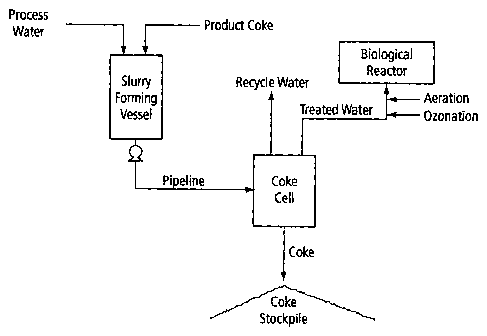
FIG. 2 is a simplified schematic of an embodiment of the water treatment
process of the present invention.
FIG. 3 is a graph showing the % naphthenic acids removed versus the mass
% of petroleum coke (fresh product coke) used.
FIG. 4 is a graph showing the concentration of naphthenic acids (ppm) at
various time intervals during continuous operation of a pipeline reactor.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
The detailed description set forth below in connection with the appended
drawings is intended as a description of various embodiments of the present
invention and is not intended to represent the only embodiments contemplated
by the
applicant. The detailed description includes specific details for the purpose
of
providing a comprehensive understanding of the present invention. However, it
will
be apparent to those skilled in the art that the present invention may be
practiced
without these specific details.
DMSLegal\053707\00209\ 2728294'I
CA 02607353 2007-10-22
8
A fluid coking operation is illustrated in FIG. 1. It involves a fluidized bed
coker
reactor working in tandem with a fluidized bed coke burner. In the reactor,
incoming
feed oil contacts a fluidized bed of hot coke particles and heat is
transferred from the
coke particles to the oil. The reactor is conventionally operated at a
temperature of
about 530 C. Hot coke entering the reactor is conventionally at a temperature
of
about 600-650 C to supply the heat requirement of the coker. "Cold" coke is
continuously removed from the reactor and returned to the burner. The cold
coke
leaving the reactor is at a temperature of about 530 C. In the burner, the
cold coke is
partially combusted with air, to produce hot coke. Part of the hot coke is
recycled to
the reactor to provide the heat required. The balance of the hot coke is
removed
from the burner as product coke. The burner is conventionally operated at a
temperature of 650 C. The burner temperature is controlled by the addition of
air.
When petroleum coke exits the coker burner, it is either recycled back to the
coker reactor (referred to as "hot coke") or disposed of as waste product
(referred to
as "product coke" or "fresh product coke"). The fresh product coke can be
temporarily stored in coke silos or it can be used directly to form an
OSPW/coke
slurry. Surprisingly, the fresh product coke was found to be effective in
removing
dissolved organics such as naphthenic acid from oil sands process water when
an
OSPW/coke slurry is formed and the slurry is subsequently pipelined.
FIG. 2 is a schematic of a water treatment process of the present invention.
In
this embodiment, process water obtained from a bitumen extraction operation is
first
slurried with product coke in a vessel. Routinely, process water present as
the
release water for recycle in the settling basins from open pit oil sands
operations will
contain elevated dissolved organic carbon content (50-70mgC/L), of which
naphthenic acids are the dominant constituent (concentrations range from 50-
80mg/L). Typically, the coke/water slurry is formed such that the coke
concentration
averages between about 20 to about 30% by wt or about 2 to about 3x105mg/L.
The coke/water slurry is then pumped through a pipeline (a plug-flow reaction
vessel) using a slurry pump where the adsorption of dissolved organics by the
DMSLegal\053707\00209\ 2728294vI
CA 02607353 2007-10-22
9
petroleum coke primarily occurs. The use of a pipeline will not only result in
adsorption of dissolved organics, but will also allow the product coke to be
transported to a suitable area for stockpiling. The pipeline length will vary;
however,
routinely the pipeline is approximately 5 km or more in length to give the
slurry
sufficient residence time (>20 minutes) for the adsorption process to occur.
As
previously mentioned, instead of a pipeline as the carbon adsorption vessel,
any
stirred vessel can be used as a carbon adsorption reactor, where adsorption of
the
dissolved organics to the petroleum coke can occur. When using a stirred
vessel, the
slurry may be formed directly in the vessel, eliminating the need for a slurry-
forming
vessel.
The petroleum coke can then be separated from the treated water using any
number of separation techniques or devices known in the art. For example, as
previously mentioned, the petroleum coke may be separated from the treated
water
by proactive methods involving filters or in a more passive manner using
sedimentation tanks or open pond fills, with either water release or
underfloor gravity
filtration through coke and sand beds. The remaining petroleum coke can then
be
stored in cells or stockpiles. Use of open pond fills has the added benefit of
increasing the residence time of the slurry and therefore one can collect
water that
has been allowed to percolate through the bed of petroleum coke.
The treated water that has been separated from the petroleum coke can now
be used as recycle water in further extraction operations or it can be
evaluated for
suitability for release to the environment. Depending upon the initial
dissolved
organics concentration of the water, the treated water might require further
treatment
such as with advanced oxidation or bioremediation reactor. Thus, additional
methods
for degradation or bioremediation of the remaining organics such as NAs may be
required prior to the release of treated water into the environment.
FIG. 3 is a graph which shows the % naphthenic acids removed from process
water containing about 50-80 ppm naphthenic acids as a function of the mass
percent of petroleum coke used in the preparation of the coke/water slurry. In
this
DMSLega1\053707\00209\ 2728294v1
CA 02607353 2010-06-02
instance, the petroleum coke was obtained from a fluid coking operation as is
routinely used in Fort McMurray, Alberta by the applicant. Slurries were
formed using
OSPW and increasing amounts of fluid coke. The slurries were mixed at room
temperature from several minutes to >48 hours using either a propeller stirrer
for
5 shorter times or a simple shaker for longer times. The coke was then allowed
to
settle out by gravity and the water analyzed for naphthenic acids content as
discussed below. It can be seen from the graph in FIG. 3 that a significant
amount of
the naphthenic acids were removed even when using only 10% by mass of fluid
coke. Beyond 40 mass % of coke in the slurry, the % of naphthenic acids
removed
10 started to level out.
FIG. 4 presents data obtained during the continuous operation of a coke slurry
pipeline as the carbon adsorption reactor, where samples were collected at the
point
of discharge at various times over the course of several months. Slurries were
formed using OSPW that was a mixture of process waters from two tailings
settling
basins and fluid coke (product coke), both obtained from applicant's oil sands
operation. Typically, the coke concentration in the slurry was about 20% by
mass or
about 200,000 ppm and the naphthenic acid concentration of the OSPW generally
ranged anywhere between 50 ppm and 80 ppm. The pipeline used was
approximately 5 km long. Slurry samples were taken at the end of the pipeline
and
the naphthenic acid concentrations measured by the technique of methylene
chloride
extraction/Fourier Transform Infrared Spectroscopy (FTIR) as described in
Syncrude
Analytical Methods Manual, 4th Edition, 1995. Syncrude Research Report
543.028S99R. Syncrude Canada Ltd., Edmonton, Alberta, Canada.
As mentioned, the naphthenic acid concentration in the process water ranged
anywhere between 50 ppm and 80 ppm. These values were used to represent the
concentration of naphthenic acids at the beginning of the pipeline. It can be
seen
from FIG. 4 that on average the naphthenic acid concentration at the end of
the
pipeline ranged from about 5 ppm to 15 ppm, representing a naphthenic acid
removal
WSLegaI\053707\0021516083009v1
CA 02607353 2007-10-22
11
efficiency of between 70% and 90%. The data shown is based on normal fluid
coker
operating conditions.
While the invention has been described in conjunction with the disclosed
embodiments, it will be understood that the invention is not intended to be
limited to
these embodiments. On the contrary, the current protection is intended to
cover
alternatives, modifications and equivalents, which may be included within the
spirit
and scope of the invention. Various modifications will remain readily apparent
to
those skilled in the art.
DMSLegal\053707\00209\ 2728294vI