Note: Descriptions are shown in the official language in which they were submitted.
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
COMPOSITIONS AND METHODS FOR TREATING CONDITIONS
RELATED TO EPHRIN SIGNALING WITH CiJPREDOXINS
RELATED APPLICATIONS
This application claims priority under 35 U.S.C. 119 and 120 to U.S.
Provisional Patent Application Serial No. 60/764,749, filed February 3, 2006,
U.S.
Provisional Patent Application Serial No. 60/682,812, filed May 20, 2005, and
U.S.
Patent Application No. 11/244,105, filed October 6, 2005, which claims
priority to
U.S. Provisional Patent Application Serial No. 60/616,782, filed October 7,
2004, and
U.S. Provisional Patent Application Serial No. 60/680,500, filed May 13, 2005,
and is
a continuation-in-part of U.S. Patent Application Serial Number 10/720,603,
filed
November 11, 2003, which claims priority to U.S. Provisional Patent
Application
Serial No. 60/414,550, filed August 15, 2003, and which is a continuation-in-
part of
U.S. Patent Application Serial Number 10/047,710, filed January 15, 2002,
which
claims priority to U.S. Provisional Patent Application Serial Number
60/269,133,
filed February 15, 2001. The entire content of these prior applications is
fully
incorporated herein by reference.
STATEMENT OF GOVERNMENTAL INTEREST
The subject matter of this application has been supported by research grants
from the National Institutes of Health (NIH), Bethesda, Maryland, U.S.A.,
(Grant
Numbers Al 16790-21, ES 04050-16, Al 45541, CA09432 and N01-CM97567). The
government may have certain rights in this invention.
FIELD OF THE INVENTION
The present invention relates to cupredoxins and their use in modulating
cellular functions involving ephrins and ephrin receptors. The invention also
relates
to methods of treating ephrin-related conditions. More particularly, the
invention
relates to the use of a substantially pure cupredoxin in methods of slowing
growth and
metastasis of cancer cells and pathological conditions, and specifically those
related to
1
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
ephrin / ephrin receptor signaling, as well as other therapeutic methods
related to
ephrin / ephrin receptor signaling. The invention also relates to variants,
derivatives
and structural equivalents of cupredoxins that retain the ability to interfere
with the
ephrin signaling system in cells.
BACKGROUND
The ephrin receptors (Eph receptors) are a large family of receptor tyrosine
kinases that regulate a multitude of processes in developing and adult tissues
by
binding a family of ligands called ephrins. Eph receptors are divided into
either the
A- or B-type with ephrin ligands. There are currently nine known members of
the A-
type, EphAl-8 and EphA10, and four known members of the B-type, EphB 1-4 and
EphB6. In general, the A class receptors preferentially bind A-type ligands,
while the
B class receptors preferentially bind the B-type ligands. The Eph receptors
are like
other receptor tyrosine kinases, with a single transmembrane spanning domain,
with a
glycosylated extracellular region comprised of a ligand-binding domain with
immunoglobulin-like motifs, a cysteine rich region and two fibronectin type
III
repeats. (Surawska et al., Cytokine & Growth Factor Reviews 15:419-433
(2004)).
The ephrin ligands are divided into the A and B class depending on their
sequence
conservation. EphrinA ligands are glycosylphosphatidylinisotol anchored and
usually
bound by Eph-A type receptors, while ephrinB ligands contain a transmembrane
domain and a short cytoplasmic region and are usually bound by EphB-type
receptors.
Id.
The signaling process begins when Eph receptor dimerizes with an ephrin
ligand, causing the receptor to become phosphorylated. Aggregates of ephrin-
EphReceptor complexes are formed by higher-order clustering. Receptor
activation is
thought to depend on the degree of multimerization, but is not limited to the
tetrameric form as receptor phosphorylation is observed in both lower- and
higher-
order forms. Depending on the state of multimerization, distinct Eph receptor
complexes can induce biological effects. In addition to the "forward"
signaling
through the Eph receptor into the receptor-expressing cell, there is also
"backwards"
signaling through the ephrin into the ephrin-expressing cell. For example, the
cytoplasmic tail on the B-ephrins can become phosphorylated leading to the
2
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
recruitment of signaling effectors and a signal transduction cascade within
the ephrin-
signaling cell. Id.
Ephrins are now known to have roles in many cell-cell interactions, including
axon pathfinding, neuronal cell migration, and interactions in vascular
endothelial
cells and specialized epithelia. (Flanagan & Vanderhaeghen, Annu. Rev.
Neurosci.
21:309-345 (1998); Frisen et al., EMBO J. 18:5159-5165 (1999)). Eph receptors
have
also been implicated in a variety of pathological processes, including tumor
progression, pathological forms of angiogenesis, chronic pain following tissue
damage, inhibition of nerve regeneration after spinal cord injury, and human
congenital malformations. (Koolpe et al., J Biol Chem. 280:17301-17311
(2005)).
Eph receptors are also reported to play a role in the balance of stem cell
self-renewal
versus cell-fate determination and differentiation. Id.
Eph receptor and ephrin over-expression can result in tumorigenesis, and are
associated with angiogenesis and metastasis in many types of human cancer,
including lung, breast and prostate cancer, as well as melanoma and leukemia.
(Surawska et al., Cytokine & Growth Factor Reviews 15:419-433 (2004)). Over-
expression of the Eph receptor is thought not to affect the proliferation of
cells, but
changes their invasive behavior. According to one theory, in malignant cells
with
high levels of EphA2, the receptors are mislocalized, not able to bind their
ephrin
ligands, and therefore not phosphorylated, resulting in increased
extracellular matrix
adhesions and higher metastatic potential. (Ruoslahti, Adv. Cancer Res. 76:1-
20
(1999)). Angiogenesis is the formation of new blood vessels and capillaries
from pre-
existing vasculature and is an essential process for tumor survival and
growth.
Evidence exists that implicates Eph receptor/ephrin up-regulation during blood
vessel
invasion of tumors. (Surawska et al., (2004).) A-type ephrins in particular
are
associated with tumor angiogenesis, and EphA2-Fc and EphA3-Fc fusion proteins
decreased tumor vascular density, tumor volume and cell proliferation, and
also
increased apoptosis. (Brantley et al., Oncogene 21:7011-7026 (2002)).
The crystal structure of the EphB2 receptor-ephrinB2 complex indicates that
the ectodomain of the ephrinB2 folding topology is an eight-stranded barrel
that is a
variation on the common Greek key P-barrel fold, and shares considerable
homology
with the cupredoxin family of copper-binding proteins, although ephrinB2 does
not
3
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
bind copper. The main difference between ephrin and the cupredoxin-fold
proteins is
the unusual length of the ephrin G-HL and C-DL loops with are part of the
dimerization and tetramerization ligand receptor interfaces, respectively.
Crystallization studies further indicate that the G-HL loop is involved in
receptor
binding. (Himanen et al., Nature 414:933-938 (2001)). The extracellular domain
of
mouse ephrinB2 also has a topological similarity to plant nodulins and
phytocyanins.
(Toth et al., Developmental Cell, 1:83-92 (2001)).
Reports on regression of cancer in humans and animals infected with
microbial pathogens date back more than 100 years, originating with the
initial report
by Coley. (Clin. Orthop. Relat. Res. 262:3-12 (1.891)). Several subsequent
reports
have shown that microbial pathogens replicate at tumor sites under hypoxic
conditions and also stimulate the host's immune system during infection,
leading to
an inhibition of cancer progression. (Alexandrof et al., Lancet 353:1689-1694
(1999);
Paglia & Guzman, Cancer Immunol. Immunother. 46:88-92 (1998); Pawelek et al.,
Cancer Res. 57:4537-4544 (1997)). Bacterial pathogens such as Pseudomonas
aeruginosa and many others produce a range of virulence factors that allow the
bacteria to escape host defense and cause disease. (Tang et al., Infect.
Immun. 64:37-
43 (1996); Clark and Bavoil, Methods in Enzymology, vol. 235, Bacterial
Pathogenesis, Academic Press, Inc. San Diego Calif. (1994); Salyers and Whitt,
Bacterial Pathogenesis: A Molecular Approach, ASM Press, Washington D.C.
(1994)). Some virulence factors induce apoptosis in phagocytic cells such as
macrophages to subvert host defense. (Monack et al., Proc. Natl. Acad. Sci.
USA
94:10385-10390 (1997); Zychlinsky and Sansonetti, J. Clin. Investig. 100:493-
495
(1997)).
Two redox proteins elaborated by P. aeruginosa, the cupredoxin azurin and
cytochrome c551(Cyt cssi), both enter J774 cells and show significant
cytotoxic
activity towards the human cancer cells as compared to normal cells. (Zaborina
et al.,
Microbiology 146: 2521-2530 (2000)). Azurin can also enter human inelanoma
iJISO-Mel-2 or human breast cancer MCF-7 cells. (Yamada et al., PNAS 99:14098-
14103 (2002); Punj et al., Oncogene 23:2367-2378 (2004); Yamada et al., Cell.
Biol.
7:14181431 (2005)). In addition, azurin from P. aeruginosa preferentially
enters
J774 murine reticulum cell sarcoma cells, forms a complex with and stabilizes
the
4
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
tumor suppressor protein p53, enhances the intracellular concentration of p53,
and
induces apoptosis . (Yamada et al., Infection and Immunity, 70:7054-7062
(2002)).
Azurin also caused a significant increase of apoptosis in human osteosarcoma
cells as
compared to non-cancerous cells. (Ye et al., Ai Zheng 24:298-304 (2003)).
Cytochrome C551 (CYt C551) from P. aeruginosa enhances the level of tumor
suppressor protein p 161nk4a and inhibits cell cycle progression in J774
cells. (Hiraoka
et al., PNAS 101:6427-6432 (2004)). However, when colon cancer cells, such as
HCT 116 cells, or p53-null lung cancer H1299 cells were grown in presence of
wild
type azurin or wild type cytochrome c551 for 3 days, they inhibited the growth
of HCT
116 cells at a much lower concentration (IC50 = 17 g/ml for azurin; 12 g/ml
for
Cyt C) than H1299 cells (>20 g/ml). Id.
A cancer is a malignant tumor of potentially unlimited growth. It is primarily
the pathogenic replication (a loss of normal regulatory control) of various
types of
cells found in the human body. Initial treatment of the disease is often
surgery,
radiation treatment or the combination of these treatments, but locally
recurrent and
metastatic disease is frequent. Chemotherapeutic treatments for some cancers
are
available but these seldom induce long term regression. Hence, they are often
not
curative. Commonly, tumors and their metastases become refractory to
chemotherapy, in an event known as the development of multidrug resistance. In
many cases, tumors are inherently resistant to some classes of
chemotherapeutic
agents. In addition, such treatments threaten noncancerous cells, are
stressful to the
human body, and produce many side effects. Improved agents are therefore
needed to
prevent the spread of cancer cells.
SUMMARY OF THE INVENTION
The present invention relates to compositions and methods of use of
cupredoxins, and variants, derivatives and structural equivalents of
cupredoxins that
interfere with the ephrin signaling system in mammalian cells. Specifically,
the
invention relates to compositions and methods that use cupredoxins, such as
azurin
and plastocyanin, and variants, derivatives and structural equivalents
thereof, to treat
cancer in mammals.
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
One aspect of the invention relates to an isolated peptide that is a variant,
derivative or structural equivalent of a cupredoxin, and that can inhibit the
growth of
cancer in mammalian cells or tissues. This peptide may be an azurin,
plastocyanin,
pseudoazurin, plastocyanin, rusticyanin or auracyanin, and specifically an
azurin,
plastocyanin and rusticyanin. In some embodiments, the cupredoxin is from
Pseudomonas aeruginosa, Thiobacillus ferrooxidans, Phormidium laminosum,
Alcaligenes faecalis, Achromobacter xylosoxidan, Bordetella bronchiseptica,
Methylomonas sp., Neisseria meningitidis, Neisseria gonorrhoeae, Pseudomonas
fluorescens, Pseudoinonas chlororaphis, Xylellafastidiosa, Cucurnis sativus,
Chloroflexus aurantiacus, Vibrio parahaemolyticus or Ulva pertusa, and
specifically
Pseudomonas aeruginosa, Thiobacillusferrooxidans, Phormidium laminosum or Ulva
pertusa. The isolated peptide may be part SEQ ID NOS: 1-17 and 22-23.
Additionally, SEQ ID NOS: 1-17 and 22-23 may have at least about 90% amino
acid
sequence identity to the peptide.
In some embodiments, the isolated peptide may be a truncation of a
cupredoxin. In specific embodiments, the peptide is more than about 10
residues and
not more than about 100 residues. The peptide may comprise or, alternatively,
consist
of P. aeruginosa azurin residues 96-113, P. aeruginosa azurin residues 88-113,
Ulva
pertusa plastocyanin residues 70-84, Ulva pertusa residues 57-98, or SEQ ID
NOS:
22-30. In some embodiments, the isolated peptide comprises equivalent residues
of a
subject cupredoxin as a region of an object cupredoxin selected from the group
consisting of P. aeruginosa azurin residues 96-113, P. aeruginosa azurin
residues 88-
113, Ulvapertusa plastocyanin residues 70-84, Ulvapertusa residues 57-98, or
SEQ
ID NOS: 22-30.
Another aspect of the invention is a composition comprising at least one
cupredoxin, or variant, derivative or structural equivalent of a cupredoxin
that can
inhibit the growth of cancer in mammalian cells or tissues in a pharmaceutical
composition. In some embodiments, the pharmaceutical composition is formulated
for intravenous administration. The cupredoxin may be from Pseudomonas
aeruginosa, Thiobacillus ferrooxidans, Phormidiutn laminosum, Alcaligenes
faecalis,
Achromobacter xylosoxidan, Bordetella bronchiseptica, Methylomonas sp.,
Neisseria
meningitidis, Neisseria gonorrhoeae, Pseudomonas fluorescens, Pseudomonas
6
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
chlororaphis, Xylella fastidiosa, Cucunais sativus, Chloroflexus aurantiacus,
Vibrio
parahaemolyticus or Zllva pertusa, and specifically Pseudomonas aeruginosa,
Thiobacillusferrooxidans, Phorrnidiuna laminosum and Ulva pertusa. In some
embodiments, the cupredoxin may be SEQ ID NOS: 1-17 and 22-23.
Another aspect of the invention is a method to treat a mammalian patient
suffering a pathological condition related to the ephrin signaling system or
suffering
from cancer, comprising administering to the patient a'therapeutically
effective
amount of a composition comprising at least one cupredoxin, or variant,
derivative or
structural equivalent of a cupredoxin in a pharmaceutical composition. In some
embodiments, the patient is suffering from interstitial cystitis (IC), lesions
associated
with inflammatory bowel disease (IBD), HIV infection, cardiovascular disease,
central nervous system disorders, peripheral vascular diseases, viral
diseases,
degeneration of the central nervous system (Christopher Reeve's disease) or
Alzheimer's disease. In other embodiments, the patient is suffering from a
cancer,
such as breast cancer, liver cancer, gastrointestinal cancer, neuroblastoma,
neural
cancer, leukemia, lymphoma, prostrate cancer, pancreatic cancer, lung cancer,
melanoma, ovarian cancer, endometrial tumor, choriocarcinoma, teratocarcinoma,
thyroid cancer, all sarcomas including those arising from soft tissues and
bone, renal
carcinomas, epidermoid cancer or non-small cell lung cancer. In some
embodiments,
the patient is a human.
Another aspect of the invention is a composition comprising at least two
isolated polypeptides that are a cupredoxin, or a variant, derivative or
structural
equivalent of a cupredoxin can inhibit the growth of cancer in mammalian cells
or
tissues. In some embodiments, the composition is in a pharmaceutical
composition.
Another aspect of the invention is a kit comprising a composition with at
least
one cupredoxin, or variant, derivative or structural equivalent of a
cupredoxin in a
pharmaceutical composition in a vial. The kit may be designed for intravenous
administration.
Another aspect of the invention is a method, comprising contacting the
mammalian cancer cells with a cupredoxin, or variant, derivative or structural
equivalent thereof; and measuring the growth of the cancer cells. The cancer
cells
may be breast cancer, liver cancer, gastrointestinal cancer, neuroblastoma,
neural
7
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
cancer, leukemia, lymphoma, prostrate cancer, pancreatic cancer, lung cancer,
melanoma, ovarian cancer, endometrial tumor, choriocarcinoma, teratocarcinoma,
thyroid cancer, all sarcomas including those arising from soft tissues and
bone, renal
carcinomas, epidermoid cancer or non-small cell lung cancer. In some
embodiments,
the cancer cells are in vivo.
Another aspect of the invention is an expression vector which encodes a
variant, derivative or structural equivalent of a cupredoxin which can inhibit
the
growth of cancer in mammalian cells or tissues.
Another aspect of the invention is an isolated peptide that is a variant,
derivative or
structural equivalent of a cupredoxin; and that can bind an ephrin receptor,
such as
EphAl, EphA2, EphA3, EphA4, EphA5, EphA6, EphA7, EphA8, EphAlO, EphBl,
EphB2, EphB3, EphB4 and EphB6. In some embodiments, the peptide also binds an
ephrin, such as ephrinAl, ephrinA2, ephrinA3, ephrinA4, ephrinA5, ephrinBl,
ephrinB2, ephrinB3 and ephrinB4. In other embodiments, the isolated peptide
binds
an ephrin and its receptor, and specifically Ephrin2B and Eph2B. In a related
aspect,
an isolated peptide is a variant, derivative or structural equivalent of a
cupredoxin;
and that can bind an ephrin, such as ephrinAl, ephrinA2, ephrinA3, ephrinA4,
ephrinA5, ephrinBl, ephrinB2, ephrinB3 and ephrinB4.
Another aspect of the invention is a method comprising administering a
pharmaceutical composition containing at least one cupredoxin, or peptide of
the
invention to a mammalian patient to guide the growth of blood vessels in the
patient.
Another aspect of the invention is a method comprising administering a
pharmaceutical composition containing at least one cupredoxin, or peptide of
the
invention to a mammalian patient to decrease the growth of blood vessels in
the
patient.
Another aspect of the invention is a method comprising administering a
pharmaceutical composition containing at least one cupredoxin, or peptide of
the
invention to a mammalian patient to guide to growth of neurons in the patient.
Another aspect of the invention is a method comprising administering a
pharmaceutical composition containing at least one cupredoxin, or peptide of
the
invention to a mammalian patient to promote osteogenesis in the patient.
8
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
Another aspect of the invention is a method comprising substituting an
effective amount of a pharmaceutical composition containing at least one
cupredoxin,
or peptide of the invention to a mammalian patient for an ephrin in a
therapeutic
method requiring the use of the ephrin.
Another aspect of the invention is a method comprising administering a
pharmaceutical composition containing at least one cupredoxin, or peptide of
the
invention to a mammalian cell to inhibit the activity of an ephrin receptor
associated
with the cell.
Another aspect of the invention is a method comprising administering a
pharmaceutical composition containing at least one cupredoxin, or peptide of
the
invention to increase the activity of an ephrin receptor associated with the
cell.
Another aspect of the invention is a method comprising administering to a
human
patient having a tissue expressing an ephrin receptor, a pharmaceutical
composition
containing at least one cupredoxin, or peptide of the invention which
comprises a
derivative of cupredoxin, or a variant, derivative or structural equivalent
fused to a
pharmaceutical agent. In some embodiments, the tissues expressing an ephrin
receptor is cancer.
Another aspect of the invention is a method to detect tissues with ephrin
receptors which has the steps of administering to a human patient a
pharmaceutical
composition containing at least one cupredoxin, or peptide of the invention
fused to a
detectable probe and detecting the distribution of the probe within the
patient.
These and other aspects, advantages, and features of the invention will become
apparent from the following figures and detailed description of the specific
embodiments.
BRIEF DESCRIPTION OF THE SEQUENCES
SEQ ID NO: 1. Amino acid sequence of azurin from Pseudomonas aeruginosa.
SEQ ID NO: 2. Amino acid sequence of plastocyanin from Phorinidium laminosuni.
SEQ ID NO: 3. Amino acid sequence of rusticyanin from Thiobacillus
ferrooxidans.
SEQ ID NO: 4. Amino acid sequence of pseudoazurin from Achromobacter
cycloclastes.
SEQ ID NO: 5. Amino acid sequence of azurin from Alcaligenes faecalis.
9
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
SEQ ID NO: 6. Amino acid sequence of azurin from Achrornobacter xylosoxidans
ssp. denitrificans I.
SEQ ID NO: 7. Amino acid sequence of azurin from Bordetella bronchiseptica.
SEQ ID NO: 8. Amino acid sequence of azurin from Metlzylomonas sp. J.
SEQ ID NO: 9. Amino acid sequence of azurin from Neisseria meningitidis Z2491.
SEQ ID NO: 10. Amino acid sequence of azurin from Neisseria gonorrhoeae.
SEQ ID NO: 11. Amino acid sequence of azurin from Pseudomonasfluorescens.
SEQ ID NO: 12. Amino acid sequence of azurin from Pseudomonas chlororaphis.
SEQ ID NO: 13. Amino acid sequence of azurin from Xylellafastidiosa 9a5c.
SEQ ID NO: 14. Amino acid sequence of stellacyanin from Cucumis sativus.
SEQ ID NO: 15. Amino acid sequence of auracyanin A from Chloroflexus
aurantiacus.
SEQ ID NO: 16. Amino acid sequence of auracyanin B from Chloroflexus
aurantiacus.
SEQ ID NO: 17. Amino acid sequence of cucumber basic protein from Cucumis
sativus.
SEQ ID NO: 18. Amino acid sequence of 18-mer azurin peptide, Pseudomonas
aeruginosa azurin from residues 96-113.
SEQ ID NO: 19. Amino acid sequence of Pseudomonas aeruginosa azurin from
residues 88-113.
SEQ ID NO: 20. Amino acid sequence of Illva pertusa plastocyanin from residues
70-84.
SEQ ID NO: 21. Amino acid sequence of Vibrio parahaemolyticus azurin.
SEQ ID NO: 22. Amino acid sequence of Ulva pertusa plastocyanin.
SEQ ID NO: 23. Amino acid sequence of EphrinB2 ectodomain from human.
SEQ ID NO: 24. Amino acid sequence of G-H loop region of human EphrinB2.
SEQ ID NO: 25. Amino acid sequence of the P. aeruginosa azurin region
structurally
analogous to the EphrinB2 G-H loop region.
SEQ ID NO: 26. Amino acid sequence of the Thiobacillus (Acidithiobacillus)
ferrooxidans rusticyanin region structurally analogous to the EphrinB2 G-H
loop
region.
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
SEQ ID NO:'27. Amino acid sequence of the Chloroflexus aurantiacus auracyanin
B
region structurally analogous to the EphrinB2 G-H loop region.
SEQ ID NO: 28. Amino acid sequence of the Ulvapertusa plastocyanin region
structurally analogous to the EphrinB2 G-H loop region.
SEQ ID NO: 29. Amino acid sequence of the Cucunais sativus cucumber basic
protein region structurally analogous to the EphrinB2 G-H loop region.
SEQ ID NO: 30. Amino acid sequence of the Cucumis sativus stellacyanin region
structurally analogous to the EphrinB2 G-H loop region.
SEQ ID NO: 31. Amino acid sequence of human EphrinB2 residues 69-138.
SEQ ID NO: 32. Amino acid sequence of Ulvapertusa plastocyanin residues 57-98.
SEQ ID NO: 33. Amino acid sequence of human EphrinB2 residues 68-138.
SEQ ID NO: 34. Amino acid sequence of P. aeruginosa azurin residues 76-128.
BRIEF DESCRIPTION OF THE FIGURES
Figure 1. Figure 1 depicts a structural alignment of azurin with ephrinB2. The
G-H
loop of ephrin B-2 (region that mediates high-affmity interaction with the
EphB
receptors) is indicated by boxes. 17ZG A is the amino acid sequence of azurin
from
Pseudomonas aeruginosa (SEQ ID NO: 1). 1KGY E is the amino acid sequence of
ephrinB2 ectodomain from human (SEQ ID NO: 23).
Figure 2. Figure 2 depicts a structural alignment of cupredoxins with
ephrinB2. The
box indicates the G-H loop of ephrinB2 (15 aa), involved in Eph receptor
binding.
Conserved residues are indicated in bold and underlined. Capital letters are
super-
positions. Dashes are where there are no alignments. The EphrinB2 G-H loop
region
is SEQ ID NO: 24. The P. aeruginosa azurin region structurally analogous to
the
EphrinB2 G-H loop region is SEQ ID NO: 25. The Thiobacillus
(Acidithiobacillus)
ferrooxidans rusticyanin region structurally analogous to the EphrinB2 G-H
loop
region is SEQ ID NO: 26. The Chlorof exus aurantiacus auracyanin region
structurally analogous to the EphrinB2 G-H loop region is SEQ ID NO: 27. The
Phormidium laminosum plastocyanin region structurally analogous to the
EphrinB2
G-H loop region is SEQ ID NO: 28. The Cucumis sativus cucumber basic protein
region structurally analogous to the EphrinB2 G-H loop region is SEQ ID NO:
29.
11
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
The Cucumis sativus stellacyanin region structurally analogous to the EphrinB2
G-H
loop region is SEQ ID NO: 30.
Figure 3. Figure 3 depicts a comparison among the structures of the ephrinB2
ectodomain from human (lkgy_E), plastocyanin from Ulvapertusa (liuz), azurin
from Pseudomonas aeruginosa (ljzg_A) and rusticyanin from Thiobacillus
(Acidithiobacillus) ferrooxidans (lrcy). In Fig. 3A, the topology of each
protein is
shown using TOPS cartoons. TOPS cartoons represent the structure as a sequence
of
secondary structure elements (SSEs): 0-strands (depicted as triangles) and
helices
(alpha and 310) (depicted as circles), how they are connected in a sequence
from
amino to carboxyl terminus, and their relative spatial positions and
orientations. The
direction of the elements can be deduced from the connecting lines. "Up"
strands are
indicated by upward pointing triangles and "Down" strands by downward pointing
triangles. Fig. 3B, the pictures were drawn using the MolMol program (Koradi
et al.,
J. Mol. Graphics 14:51-55 (1996)).
Figure 4. Figure 4 depicts a surface plasmon resonance sensorgrams for the
association of cupredoxins with bound Eph-Fc. Selective binding of azurin
(Fig. 4A),
plastocyanin (Fig. 4B), and rusticyanin (Fig. 4C) with EphA-Fc and EphB-Fc
proteins
is represented.
Figure 5. Figure 5 depicts a schematic representation of various truncated
azurin
constructs derived from full length azurin (SEQ ID.NO: 1). Secondary structure
elements are illustrated as arrows for (3-sheets and helices (alpha and 310)
as
rectangles. Various segments of the gene encoding the 128 amino acid azurin
were
fused at the 3'-end of the gst gene (encoding glutathione S-transferase) in
frame,
cloned in E. coli, hyperexpressed and the fusion proteins purified as
described earlier
(Yamada et al., Cell Micro. 7:1418-1431 (2005)).
Figure 6. Figure 6 depicts the relative binding affinities of azurin and
selectively
constructed GST-Azu fusions for EphB2-Fc determined in surface plasmon
resonance
studies. In Fig. 6A, an initial screening experiment was performed to
determine
relative binding strengths of azurin or GST-Azu wherein the SPR traces were
recorded after injection of the cupredoxins (100 nM) onto EphB2-Fc-modified
CM5
sensor chips. Notably, azurin, GST-Azu 88-113, and GST-Azu 36-128 bind
stronger
than the native ligand (ephrinB2-Fc) to EphB2-Fc. In Fig. 6B, binding affinity
curves
12
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
for the interactions of azurin, GST-Azu 88-113, ephrinB2-Fc, and GST-Azu 36-89
to
immobilized EphB2-Fc after titrating.increasing concentrations (0.05-100 nM)
of the
cupredoxins. Equilibrium resonance signals (Req) were extrapolated from the
individual sensorgrams to construct the curves. Binding dissociation constants
(Kd)
were calculated (Table 6) after fitting the data to a Langmuir (1:1) binding
model
using the equation Req = Rmax/(1 + Kd/C) and the curve fits are shown
connecting
the data points in the titration curves. The quantitative data sets agree with
those from
the initial binding screen.
Figure 7. Figure 7 depicts the binding interactions of azurin and GST-Azu with
ephrinB2-Fc and EphB2-Fc determined in binding titrations and competition
assays.
In Fig. 7A, SPR binding curves for the interactions of azurin and GST-Azu with
ephrinB2-Fc for which binding affinities (Kd) were determined as previously
described. In Fig. 7B, SPR binding competition studies with EphB2-Fc
immobilized
on CM5 sensor chips.
Figure 8. Figure 8 depicts the structural alignment comprising the C-terminals
of
plastocyanin from Ulva pertusa (1IUZ) and azurin from P. aeruginosa (1JZG A),
(Fig. 8A and Fig. 8B respectively) with human ephrinB2 ectodomain (1KGY E) as
computed by the VAST algorithm. Superimposed secondary structure elements are
denoted by a bold capital letter. Dashes and lower-case lettering are where
there are
no alignments. Secondary structure elemerits according to the structures are
illustrated as arrows for (3-sheets and open rectangles for 310 helices.
Identical amino
acids are indicated by an asterisk. Amino acids highlighted in dark gray and
light
gray in the ephrin B2 sequence indicate residues involved in the interaction
between
ephrinB2 and EphB2 receptor and in the ligand dimerization respectively
(Himanen et
al., Nature 414:933-938 (2001); Toth et al., Dev. Cell. 1:83-92 (2001)). The
plastocyanin and azurin peptides (called Plc 70-84 (SEQ ID NO: 20) and Azu 96-
113
(SEQ ID NO: 18), respectively), corresponding to the G-H loop region of
ephrinB2,
which is the main region mediating high affinity binding of the ephrins to the
Eph
receptors are boxed and represented below each alignment. The G and H loop
regions
are marked with thick arrows on top of the amino acid sequences of ephrinB2.
1KGY E residues 69-138 and 68-138 are found in SEQ ID NOS: 31 and 33,
respectively. 1IUZ residues 57-98 is found in SEQ ID NO: 32. 1JZG A residues
76-
13
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
128 is found in SEQ ID NO: 34.
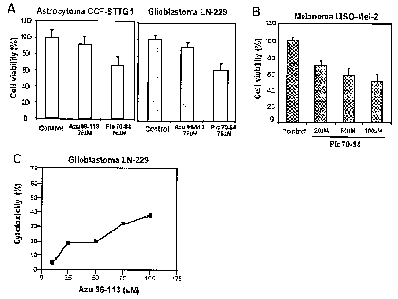
Figure 9. Figure 9 depicts the effects of cupredoxin peptides on cancer cell
viability.
(In Fig. 9A, effect of azurin (Azu 96-113) and plastocyanin (Plc 70-84)
synthetic
peptides on cell viability of Astrocytoma CCF-STTG1 and Glioblastoma LN-229
cancer cell lines. In Fig. 9B, effect of different concentrations of
plastocyanin (Plc
70-84) synthetic peptide on Melanoma UISO-Mel-2 cell viability. Cell viability
was
determined by MTT assay as described in Example 10. Cancer cells (2 x 104
cells per
well in 96-well plates) were treated with the synthetic peptides at different
concentrations for 24 h at 37 C. Data are presented as the percentage of cell
viability
as compared to that of untreated control (100% viability) In Fig. 9C,
cytotoxic
activity of Azu 96-113 synthetic peptide towards Glioblastoma LN-229 cells.
Cytotoxicity effects were determined by MTT assay. Cancer (2 x 104 cells per
well in
96-well plates) were treated with various concentrations of Azu 96-113 (10,
25, 50,
75, 100 M) for 24 h at 37 C. Percent cytotoxicity is expressed as percentage
of cell
death as compared to that of untreated control (0% cytotoxicity).
Figure 10. Effect of GST-Azu 36-128 and GST-Azu 88-113 on cell viability of
MCF-7 cells. GST-Azu peptides were added at increasing concentrations (1.25,
6.25
and 12.5 M) into 96 well plates containing 8 x 103 cancer cells per well,
incubated at
37 C for 48 h and subsequently analyzed using MTT assay. GST and GST-Azu 36-
89 at the same concentrations and untreated cells were run in parallel with
GST-Azu
36-128 and GST-Azu 88-113 as controls.
DETAILED DESCRIPTION OF THE INVENTION
Definitions
As used herein, the term "cell" includes both the singular or the plural of
the
term, unless specifically described as a "single cell."
As used herein, the terms "polypeptide," "peptide," and "proteift" are used
interchangeably to refer to a polymer of amino acid residues. The terms apply
to
amino acid polymers in which one or more amino acid residue is an artificial
chemical
analogue of a corresponding naturally occurring amino acid. The terms also
apply to
naturally occurring amino acid polymers. The terms "polypeptide," "peptide,"
and
"protein" are also inclusive of modifications including, but not limited to,
14
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
glycosylation, lipid att achment, sulfation, gamma-carboxylation of glutamic
acid
residues, hydroxylation and ADP-ribosylation. It will be appreciated that
polypeptides are not always entirely linear. For instance, polypeptides may be
branched as a result of ubiquitination and they may be, circular (with or
without
branching), generally as a result of post-translation events, including
natural
processing event and events brought about by human manipulation which do not
occur naturally. Circular, branched and branched circular polypeptides may be
synthesized by non-translation natural process and by entirely synthetic
methods as
well. Further, this invention contemplates the use of both the methionine-
containing
and the methionine-less amino terminal variants of the protein of the
invention.
As used herein, the term "pathological condition" includes anatomic and
physiological deviations from the normal that constitute an impairment of the
normal
state of the living animal or one of its parts, that interrupts or modifies
the
performance of the bodily functions, and is a response to various factors (as
malnutrition, industrial hazards, or climate), to specific infective agents
(as worms,
parasitic protozoa, bacteria, or viruses), to inherent defects of the organism
(as genetic
anomalies), or to combinations of these factors.
As used herein, the term "condition" includes anatomic and physiological
deviations from the normal that constitute an impairment of the normal state
of the
living animal or one of its parts, that interrupts or modifies the performance
of the
bodily functions.
As used herein, the term "inhibit cell growth" means the slowing or ceasing of
cell
division and/or cell expansion. This term also includes the inhibition of cell
development or increases cell death.
As used herein, the term "suffering from" includes presently exhibiting the
symptoms of a pathological condition, having a pathological condition even
without
observable symptoms, in recovery from a pathological condition, and recovered
from
a pathological condition.
A used herein, the term "treatment" includes preventing, lowering, stopping,
or reversing the progression or severity of the condition or symptoms
associated with
a condition being treated. As such, the term "treatment" includes medical,
therapeutic, and/or prophylactic administration, as appropriate.
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
A "therapeutically effective amount" is an amount effective to prevent or slow
the
development of, or to partially or totally alleviate the existing symptoms in
a
particular condition for which the subject being treated. Determination of a
therapeutically effective amount is well within the capability of those
skilled in the
art.
As used herein, the term "deficient in expression of the p53 tumor suppressor
gene" refers to a cell having a p53 tumor suppressor gene that is inactivated,
mutated,
lost or under produced. For example, such a deficiency may occur as a result
of
genetic aberrations within the p53 gene or due to epigenic reasons such as
hypermethylation of C residues in the CG islands upstream of the tumor
suppressor
genes or interaction with viral and cellular oncogenes.
The term "substantially pure", when used to modify the term "cupredoxin", as
used herein, refers to a cupredoxin, for example, a cupredoxin isolated from
the
growth medium or cellular contents, in a form substantially free of, or
unadulterated
by, other proteins and/or active inhibitory compounds. The term "substantially
pure"
refers to a factor in an amount of at least about 75%, by dry weight, of
isolated
fraction, or at least "75% substantially pure." More specifically, the term
"substantially pure" refers to a compound of at least about 85%, by dry
weight, active
compound, or at least "85% substantially pure." Most specifically, the term
"substantially pure" refers to a compound of at least about 95%, by dry
weight, active
compound, or at least "95% substantially pure." The substantially pure
cupredoxin
can be used in combination with one or more other substantially pure compounds
or
isolated cupredoxins.
The phrases "isolated," "purified" or "biologically pure" refer to material
which is substantially or essentially free from components which normally
accompany the material as it is found in its native state. Thus, isolated
peptides in
accordance with the invention preferably do not contain materials normally
associated
with the peptides in their in situ environment. An "isolated" region refers to
a region
that does not include the whole sequence of the polypeptide from which the
region
was derived. An "isolated" nucleic acid, protein, or respective fragment
thereof has
been substantially removed from its in vivo environment so that it may be
manipulated by the skilled artisan, such as but not limited to nucleotide
sequencing,
16
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
restriction digestion, site-directed mutagenesis, and subcloning into
expression
vectors for a nucleic acid fragment as well as obtaining the protein or
protein
fragment in substantially pure quantities.
The term "variant" as used herein with respect to a peptide, refers to amino
acid sequence variants which may have amino acids replaced, deleted, or
inserted as
compared to the wild-type polypeptide. Variants may be truncations of the wild-
type
peptide. Thus, a variant peptide may be made by manipulation of genes encoding
the
polypeptide. A variant may be made by altering the basic composition or
characteristics of the polypeptide, but not at least some of its fundamental
activities.
For example, a"variant" of azurin can be a mutated azurin that retains its
ability to
inhibit the growth of mammalian cancer cells. In some cases, a variant peptide
is
synthesized with non-natural amino acids, such as s-(3,5-dinitrobenzoyl)-Lys
residues. (Ghadiri & Fernholz, J. Am. Chem. Soc., 112:9633-9635 (1990)). In
some
embodiments, the variant has not more than 20, 19, 18, 17 or 16 amino acids
replaced,
deleted or inserted compared to wild-type peptide. In some embodiments, the
variant
has not more than 15, 14, 13, 12 or 11 amino acids replaced, deleted or
inserted
compared to wild-type peptide. In some embodiments, the variant has not more
than
10, 9, 8 or 7 amino acids replaced, deleted or inserted compared to wild-type
peptide.
In some embodiments, the variant has not more than 6 amino acids replaced,
deleted
or inserted compared to wild-type peptide. In some embodiments, the variant
has not
more than 5 or 4 amino acids replaced, deleted or inserted compared to wild-
type
peptide. In some embodiments, the variant has not more than 3, 2 or 1 amino
acids
replaced, deleted or inserted compared to wild-type peptide.
The term "amino acid," as used herein, means an amino acid moiety that
comprises any naturally-occurring or non-naturally occurring or synthetic
amino acid
residue, i.e., any moiety comprising at least one carboxyl and at least one
amino
residue directly linked by one, two, three or more carbon atoms, typically one
(a)
carbon atom.
The term "derivative" as used herein with respect to a peptide refers to a
peptide that is derived from the subject peptide. A derivation includes
chemical
modifications of the peptide such that the peptide still retains some of its
fundamental
activities. For example, a "derivative" of azurin can be a chemically modified
azurin
17
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
that retains its ability to inhibit the growth of mammalian cancer cells.
Chemical
modifications of interest include, but are not limited to, amidation,
acetylation,
sulfation, polyethylene glycol (PEG) modification, phosphorylation or
glycosylation
of the peptide. In addition, a derivative peptide maybe a fusion of a
polypeptide or
fragment thereof to a chemical compound, such as but not limited to, another
peptide,
drug molecule or other therapeutic or pharmaceutical agent or a detectable
probe.
The term "percent (%) amino acid sequence identity" is defined as the
percentage of amino acid residues in a polypeptide that are identical with
amino acid
residues in a candidate sequence when the two sequences are aligned. To
determine
% amino acid identity, sequences are aligned and if necessary, gaps are
introduced to
achieve the maximum % sequence identity; conservative substitutions are not
considered as part of the sequence identity. Amino acid sequence alignment
procedures to determine percent identity are well known to those of skill in
the art.
Often publicly available computer software such as BLAST, BLAST2, ALIGN2 or
Megalign (DNASTAR) software is used to align peptide sequences. In a specific
embodiment, Blastp (available from the National Center for Biotechnology
Information, Bethesda MD) is used using the default parameters of long
complexity
filter, expect 10, word size 3, existence 11 and extension 1.
When amino acid sequences are aligned, the % amino acid sequence identity
of a given amino acid sequence A to, with, or against a given amino acid
sequence B
(which can alternatively be phrased as a given amino acid sequence A that has
or
comprises a certain % amino acid sequence identity to, with, or against a
given amino
acid sequence B) can be calculated as:
% amino acid sequence identity = X/Y* 100
where
X is the number of amino acid residues scored as identical matches by
the sequence alignment program's or algorithm's alignment of A and B and
Y is the total number of amino acid residues in B.
If the length of amino acid sequence A is not equal to the length of amino
acid
sequence B, the % amino acid sequence identity of A to B will not equal the %
amino
acid sequence identity of B to A. When comparing longer sequences to shorter
sequences, the shorter sequence will be the "B" sequence. For example, when
18
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
comparing truncated peptides to the corresponding wild-type polypeptide, the
truncated peptide will be the "B" sequence.
General
Some aspects of the present invention provides compositions and methods that
use cupredoxins that have structural similarity to ephrin to interfere with
the ephrin
signaling system in various mammalian cells and tissues, and also inhibit the
growth
of mammalian cancer cells. Specifically, the present invention provides for
compositions and methods that use cupredoxins and variants, derivatives and
structural equivalents thereof, to interfere with the ephrin signaling system,
and also
inhibit the growth of mammalian cancer cells in vitro and in vivo.
The inventors previously discovered that pathogenic microorganisms secrete
ATP-independent cytotoxic factors, for example redox proteins such as azurin
from
P. aeruginosa, and that such factors cause J774 cell death by apoptosis,
particularly in
cancer cells. It was also known that azurin has a domain from about amino acid
residues 50-77 that facilitates the protein to enter preferentially into
cancer cells to
induce cytotoxicity.
Surprisingly, the inventors have now discovered that a C-terminal domain is
found in azurins and other cupredoxins that shows a structural similarity to
the
ephrins. See, Examples 2, 5 and 9. It is also now known that azurin and
plastocyanin,
and particular regions of these peptides that are structurally homologous to
the ephrin
G-H loop, bind competitively to ephrin receptors in a 1:1 ratio. See, Examples
6-8.
P. aeruginosa azurin, in particular, binds to ephrin receptors EphB2 and
EphA6. See,
Example 6. Phormidium laminosum plastocyanin shows specificity for binding
ephrin receptors EphAl, A3 and B2, and to a lesser extent ephrin receptors
EphA2
and A6. See, Example 6. Finally, rusticyanin shows weak binding to ephrin
receptors
EphA8 and B 1. See, Example 6. It is also now known that azurin region amino
acid
residues 88-113, which contains the structural homology to the ephrin G-H loop
region, binds to ephrin receptor EphB2. See, Example 7. Finally, it is now
known
that azurin and the 88-113 region of azurin bind to ephrinB2 as well as the
ephrin
receptor EphB2, and can compete with ephrinB2 for its receptor, EphB2. See,
Example 8.
19
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
The inventors have also discovered that cupredoxins with regions that have a
structural similarity to the ephrin G-H loop regions inhibit the growth of
mammalian
cancer cells in vitro. In general, Phormidium laminosum plastocyanin,
Thiobacillus
ferrooxidans rusticyanin and P. aeruginosa azurin inhibit the growth in vitro
of Mel-2
human melanoma cells and MCF-7 human breast cancer cells in a trypan blue
assay.
See, Example 4. Further, the 88-113 residue region of azurin is now known to
inhibit
cell growth of MCF-7 breast cancer cells. See, Example 10. Finally, an 18-mer
azurin peptide and a 15-mer Ulva pertusa plastocyanin peptide that correspond
to the
region of structural similarity to the ephrinB2 G-H loop region are now known
to
inhibit the growth of MCF-7 human breast cancer cells, CCF-STTG1 brain tumor
astrocytoma cells and LN-229 glioblastoma cells in vitro. See, Examples 3 and
9.
Surprising, cupredoxins with structural similarity to the G-H loop of ephrinB2
also affect ephrin-related development in vivo. It is now known from in vivo
studies
of ephrin-related development in C. elegans, that the cupredoxin rusticyanin
interferes
with tail muscle formation while cupredoxin azurin prevents embryonic
development,
both ephrin-related developmental processes. See, Example 1.
It is now appreciated that in addition to azurin, the cupredoxins rusticyanin
and plastocyanin also share a structural homology with the G and H regions of
ephrinB2. See, Examples 2 and 5. Further, it is known that the azurin and the
phytocyanins stellacyanin and cucumber basic protein also share a significant
structural homology with ephrins. Because of the structural conservation in
the
cupredoxin family of proteins in general, it is predicted that many other
cupredoxins
and cupredoxin-like proteins will also display a significant structural
homology to
ephrins. See, Example 2. It is therefore contemplated that cupredoxin family
proteins
in general can be used to treat the conditions related to ephrin-signaling,
and cancer,
using the compositions and methods of the invention. Specific cupredoxins of
interest
include, but are not limited to, azurin, rusticyanin, plastocyanin,
stellacyanin,
auracyanin, pseudoazurin and cucumber basic protein. Exemplary protein
sequences
are found herein as plastocyanin (SEQ ID NOS: 2 and 22), rusticyanin (SEQ ID
NO:
3), pseudoazurin (SEQ ID NO: 4), stellacyanin (SEQ ID NO: 14), auracyanin (SEQ
ID NOS: 15 and 16), and cucumber basic protein (SEQ ID NO: 17). As used
herein,
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
the term "cupredoxin" refers to any member of the cupredoxin family of
proteins,
including cupredoxin-like proteins, such as Laz from Neisseria.
Azurins are particularly specific, and exemplary protein sequences for these
cupredoxins are found herein, but not limited to, as those isolated from
Pseudomonas
aeruginosa (SEQ ID NO: 1); Alcaligenes faecalis (SEQ ID NO: 5); Achronzobacter
xylosoxidans ssp.denitrificans I(SEQ ID NO: 6); Bordetella bronchiseptica (SEQ
ID
NO: 7); Methylomonas sp. J (SEQ ID NO: 8); Neisseria nzeningitidis (SEQ ID NO:
9); Neisseria gonnorrhoeae (SEQ ID NO: 10), Pseudomonasfluorescens (SEQ ID
NO: 11); Pseudomonas chlororaphis (SEQ ID NO: 12); .Xylella fastidiosa 9a5c
(SEQ
ID NO: 13) and VibNio parahaemolyticus (SEQ ID NO: 21). In a most specific
embodiment, the azurin is from Pseudomonas aeruginosa. In other specific
embodiments, the cupredoxin is plastocyanin (SEQ ID NOS: 2 and 22),
rusticyanin
(SEQ ID NO: 3), pseudoazurin (SEQ ID NO: 4), stellacyanin (SEQ ID NO: 14),
auracyanin (SEQ ID NOS: 15 and 16), and cucumber basic protein (SEQ ID NO:
17).
In some embodiments, the cupredoxins have a Greek key beta-barrel structure.
The Greek key beta-barrel structure is a well 'known protein fold. See, for
example,
Zhang & Kim (Proteins 40:409-419 (2000)). The Greek Key topology was named
after a pattern that was common on Greek pottery. It has three up-and-down
beta
strands connected by hairpins, which are followed by a longer connection to a
fourth
beta strand, which lies adjacent to the first beta strand. In a specific
embodiment, the
cupredoxins and variants, derivatives and structural equivalents of
cupredoxins
comprise at least one Greek Key beta-barrel structure. In another specific
embodiment, the cupredoxins and variants, derivatives and structural
equivalents of
cupredoxins comprise a Greek Key structure of at least 4 beta strands. In
another
more specific embodiment, the cupredoxins and variants, derivatives and
structural
equivalents of cupredoxins comprise a Greek Key structure of at least eight
beta
strands. In another specific embodiment, the cupredoxin and variants,
derivatives and
structural equivalents of cupredoxins comprise more than one Greek Key beta-
barrel
structure.
21
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
Compositions of the Invention
The invention provides for peptides that are variants, derivatives or
structural
equivalents of cupredoxin. In some embodiments, the peptide is substantially
pure.
In other embodiments, the peptide is in a composition that comprises, consists
of or
consists essentially of the peptide. In other embodiments, the peptide is
isolated. In
some embodiments, the peptide is less that a full length cupredoxin, and
retains some
of the functional characteristics of the cupredoxin. In some embodiments, the
peptide
retains the ability to interfere with ephrin-signaling in mammalian cells and
tissues,
and/or inhibit the growth of mammalian cancer cells. In another specific
embodiment, the peptide does not raise an immune response in a mammal, and
more
specifically a human.
The invention also provides compositions comprising at least one, at least two
or at least three cupredoxin(s), or variant(s), derivative(s) or structural
equivalent(s) of
a cupredoxin. The invention also provides compositions comprising at least
one, at
least two, or at least three cupredoxin(s) or variant(s), derivative(s) or
structural
equivalent(s) of cupredoxin(s) in a pharmaceutical composition.
Because of the high structural homology between the cupredoxins, it is
contemplated that other cupredoxins of the family will be able to interfere
with ephrin
signaling, and specifically inhibit the growth of cancer in mammalian cells
and
tissues. In some embodiments, the cupredoxin is, but is not limited to,
azurin,
pseudoazurin, plastocyanin, pseudoazurin, rusticyanin or auracyanin. In
particularly
specific embodiments, the azurin is derived from Pseudomonas aeruginosa,
Alcaligenes faecalis, Achromobacter xylosoxidans ssp. denitrificans I,
Bordetella
bronchiseptica, Methylomonas sp., Neisseria meningitidis, Neisseria
gonorrhoeae,
Pseudomonasfluorescens, Pseudomonas chlororaphis, Xylella fastidiosa 9a5 or
Vibrio parahaemolyticus. In a very specific embodiment, the azurin is from
Pseudomonas aeruginosa. In other specific embodiments the cupredoxin is a
plastocyanin, and more specifically a plastocyanin derived from Phormidium
laminosum or Ulvapertusa. In other specific embodiments, the cupredoxin in a
rusticyanin, and more specifically a rusticyanin derived from Thiobacillus
22
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
ferrooxidans. In other specific embodiments, the cupredoxin comprises an amino
acid sequence that is SEQ ID NO: 1-17, 21-22.
The invention provides for amino acid sequence variants of a cupredoxin
which have amino acids replaced, deleted, or inserted as compared to the wild-
type
polypeptide. Variants of the invention may be truncations of the wild-type
polypeptide. As used herein, a "truncation" of a polypeptide is the peptide
that results
from the removal of at least one amino acid residue from at least one end of
the
polypeptide sequence. In some embodiments, the truncation peptide results from
at
least the removal of at least one amino acid reside, at least five amino acid
residues, at
least 10 amino acid residues, at least 50 amino acid residues, or about 100
amino acid
residues in total from either or both ends of the polypeptide sequence. In
some
embodiments, the composition comprises a peptide that consists of a region of
a
cupredoxin that is less that the full length wild-type polypeptide. In some
embodiments, the composition comprises a peptide that consists of more than
about
residues, more than about 15 residues or more than about 20 residues of a
truncated cupredoxin. In some embodiments, the composition comprises a peptide
that consists of not more than about 100 residues, not more than about 50
residues,
not more than about 40 residues or not more than about 30 residues of a
truncated
cupredoxin. In some embodiments, the variant is a peptide to which a
cupredoxin,
and more specifically to SEQ ID NOS: 1-17, 21-22 has at least about 90% amino
acid
sequence identity, at least about 95%a amino acid sequence identity or at
least about
99% amino acid sequence identity.
In specific embodiments, the variant of cupredoxin comprises P. aeruginosa
azurin residues 96-113 (SEQ ID NO: 18), 88-113 (SEQ ID NO: 19) or SEQ ID NO:
25. In other embodiments, the variant of cupredoxin consists of P. aeruginosa
azurin
residues 96-113 (SEQ ID NO: 18), 88-113 (SEQ ID NO: 19) or SEQ ID NO: 25. In
other specific embodiments, the variant of cupredoxin comprises Ulva pertusa
residues 70-84 (SEQ ID NO: 20), Ulva pertusa residues 57-98 (SEQ ID NO: 32),
or
Ulva pertusa sequence SEQ ID NO: 28. In other specific embodiments, the
variant of
cupredoxin consists of Ulva pertusa residues 70-84 (SEQ ID NO: 20),
Ulvapertusa
residues 57-98 (SEQ ID NO: 32), or Ulvapertusa sequence SEQ ID NO: 28. In
other
specific embodiments, the variant of cupredoxin comprises
Thiobacillusferrooxidans
23
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
rusticyanin sequence SEQ ID NO: 26, Chloroflexus aurantiacus auracyanin (SEQ
ID
NO: 27), Cucumis sativus sequences SEQ ID NOS: 29 and 30. In other specific
embodiments, the variant of cupredoxin consists of Thiobacillus ferrooxidans
rusticyanin sequence SEQ ID NO: 26, Chloroflexus aurantiacus auracyanin (SEQ
ID
NO: 27), Cucumis sativus sequences SEQ ID NOS: 29 and 30. In other specific
embodiments, the variant consists of the equivalent residues to the above
truncated
sequences from another cupredoxin. It is also contemplated that other
cupredoxin
variants can be designed that have a similar activity to any of the
aforementioned
variants. To do this, the subject cupredoxin amino acid sequence will be
aligned to
the object cupredoxin sequence, such as those that contain the truncated
variants
above, using BLAST, BLAST2, ALIGN2 or Megalign (DNASTAR), the relevant
residues of the truncated variant located on the object cupredoxin sequence,
and the
equivalent residues found on the subject cupredoxin sequence, and the
equivalent
truncated variant thus designed.
The variants also include peptides made with synthetic amino acids not
naturally occurring. For example, non-naturally occurring amino acids may be
integrated into the variant peptide to extend or optimize the half-life of the
composition in the bloodstream. Such variants include, but are not limited to,
D,L-
peptides (diastereomer), (Futaki et al., J. Biol. Chem. 276(8):5836-40 (2001);
Papo et
al., Cancer Res. 64(16):5779-86 (2004); Miller et al, Biochem. Pharmacol.
36(l):169-
76, (1987)).; peptides containing unusual amino acids (Lee et al., J. Pept.
Res.
63(2):69-84 (2004))., and olefin-containing non-natural amino acid followed by
hydrocarbon stapling (Schafineister et al., J. Am. Chem. Soc. 122:5891-5892
(2000);
Walenski et al., Science 305:1466-1470 (2004)). and peptides conprising 6-(3,5-
dinitrobenzoyl)-Lys residues.
In other embodiments, the peptide of the invention is a derivative of a
cupredoxin. The derivatives of cupredoxin are chemical modifications of the
peptide
such that the peptide still retains some of its fundamental activities. For
example, a
"derivative" of azurin can be a chemically modified azurin that retains its
ability to
interfere with ephrin-signaling, and specifically inhibit the growth of cancer
in
mammalian cells and tissues. Chemical modifications of interest include, but
are not
limited to, amidation, acetylation, sulfation, polyethylene glycol (PEG)
modification,
24
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
phosphorylation and glycosylation of the peptide. In addition, a derivative
peptide
maybe a fusion of a cupredoxin, or variant, derivative 'or structural
equivalent thereof
to a chemical compound, such as but not limited to, another peptide, drug
molecule or
other therapeutic or pharmaceutical agent or a detectable probe. Derivatives
of
interest include chemical modifications by which the half-life in the
bloodstream of
the peptides and compositions of the invention can be extended or optimized,
such as
by several methods well known to those in the art, including but not limited
to,
circularized peptides (Monk et al., BioDrugs 19(4):261-78, (2005); DeFreest et
al., J.
Pept. Res. 63(5):409-19 (2004))., N- and C- terminal modifications (Labrie et
al.,
Clin. Invest. Med. 13(5):275-8, (1990))., and olefin-containing non-natural
amino
acid followed by hydrocarbon stapling (Schafineister et al., J. Am. Chem. Soc.
122:5891-5892 (2000); Walenski et al., Science 305:1466-1470 (2004)).
It is contemplated that the peptides invention may be a variant, derivative
and/or structural equivalent of a cupredoxin. For example, the peptides may be
a
truncation of azurin that has been PEGylated, thus making it both a variant
and a
derivative. In one embodiment, the peptides of the invention are synthesized
with
a,a-disubstituted non-natural amino acids containing olefin-bearing tethers,
followed
by an all-hydrocarbon "staple" by ruthenium catalyzed olefin metathesis.
(Scharmeister et al., J. Am. Chem. Soc. 122:5891-5892 (2000); Walensky et al.,
Science 305:1466-1470 (2004)). Additionally, peptides that are structural
equivalents
of azurin may be fused to other peptides, thus making a peptide that is both a
structural equivalent and a derivative. These examples are merely to
illustrate and not
to limit the invention. Variants, derivatives or structural equivalents of
cupredoxin
may or may not bind copper.
In another embodiment, the peptide is a structural equivalent of a cupredoxin.
Examples of studies that determine significant structural homology between
cupredoxins and other proteins include Toth et al. (Developmental Cell 1:82-92
(2001)). Specifically, significant structural homology between a cupredoxin
and the
structural equivalent is determined by using the VAST algorithm. (Gibrat et
al., Curr
Opin Struct Biol 6:377-385 (1996); Madej et al., Proteins 23:356-3690 (1995)).
In
specific embodiments, the VAST p value from a structural comparison of a
cupredoxin to the structural equivalent is less than about 10"3, less than
about 10"5, or
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
less than about 10-7. In other embodiments, significant structural homology
between a
cupredoxin and the structural equivalent is determined by using the DALI
algorithm
(Holm & Sander, J. Mol. Biol. 233:123-138 (1993)). In specific embodiments,
the
DALI Z score for a pairwise structural comparison is at least about 3.5, at
least about
7.0, or at least about 10Ø Examples of these determinations are found in
Examples 2
and 9.
In some embodiments, the cupredoxin, or variant, derivative or structural
equivalent thereof has some of the functional characteristics of the P.
aeruginosa
azurin, Ulva pertusa plastocyanin, Phormidium laminosum plastocyanin or
Thiobacillus feNrooxidans rusticyanin. In a specific embodiment, the
cupredoxin or
variant, derivative or structural equivalent thereof inhibits interferes with
the ephrin-
signaling system, and/or inhibits the growth of cancer in mammalian cells and
tissues.
The inhibition of growth of mammalian cancer cells or tissues may or may not
be
related to any interference with the ephrin-signaling system by the
cupredoxin, or
variant, derivative or structural equivalent thereof. Methods that determine
whether
the cupredoxin, or variant, derivative or structural equivalent thereof
interferes with
ephrin signaling are well known in the art, and include the determining of
whether the
cupredoxin, or variant, derivative or structural equivalent thereof binds to a
component of the ephrin signaling pathway, such as, but not limited to, an
ephrin
and/or an ephrin receptor. The ephrin signaling system at its broadest
includes the
ephrin and associated ephrin receptor, and any molecules (or "components")
required
to transmit the signal both backward and forward. In a narrower view, the
ephrin
signaling system includes only the ephrin and related ephrin receptor
responsible for
the signaling. The term "interfere" when used in the context of the ephrin
signaling
system can result in either an increase or decrease of the associated ephrin
signaling,
in either or both of the "forward" and "backward" directions. Methods to
measure the
interference in the ephrin signaling system are well known in the art. One
method to
determine interference of ephrin signaling is the binding or competition of
the peptide
to or with an ephrin or ephrin receptor, or other component of the ephrin
signaling
system, as shown in Examples 6-8. In vivo systems can be used, such as the C.
elegans where it is now known that cupredoxins can interfere with ephrin
signaling to
alter tail muscle formation and embryonic development, as described in Example
1.
26
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
Other methods include, but are not limited to, the Eph receptor
phosphorylation assay
in Himanen et al. (Nat. Neurosci. 7:501-509 (2004)). and Koolpe et al. (J.
Biol.
Chem. 280:17301-17311 (2005)).
Because it is now known that cupredoxins and variants of cupredoxins can,
among other things, interfere with ephrin signaling and inhibit the growth of
cancer in
mammalian cells and tissues, it is now possible to design variants,
derivatives and
structural equivalents of cupredoxins that retain this activity, for example.
Such
variants, derivatives and structural equivalents can be made by, for example,
creating
a "library" of various variants, derivatives and structural equivalents of,
and then
testing each for anti-cancer activity, for example, using one of many methods
known
in the art, such the exemplary methods in Examples 3, 9 and 10. It is
contemplated
that the resulting variants, derivatives and structural equivalents of
cupredoxins with
anti-cancer activity can be used in the methods of the invention, in place of
or in
addition to cupredoxins.
In other embodiments, the cupredoxin, or variant, derivative or structural
equivalent thereof may have a significant structural homology with an ephrin.
In a
specific embodiment, the cupredoxins and variants and derivative of
cupredoxins
have a significant structural homology around the G-H loop region of ephrin.
Examples of studies that determine significant structural homology between
cupredoxins and ephrins include Toth et al. (Developmental Cell 1:82-92
(2001)). and
Example 2 herein. Specifically, significant structural homology between a
cupredoxin and an ephrin is determined by using the VAST algorithm. (Gibrat et
al.,
Curr Opin Struct Biol 6:377-385 (1996); Madej et al., Proteins 23:356-3690
(1995)).
In specific embodiments, the VAST p value from a structural comparison of a
cupredoxin to an ephrin is less than about 10-3, less than about 10"5, or less
than about
10-7. In other embodiments, significant structural homology between a
cupredoxin
and an ephrin is determined by using the DALI algorithm (Holm & Sander, J.
Mol.
Biol. 233:123-138 (1993)). In specific embodiments, the DALI Z score for a
pairwise
structural comparison is at least about 3.5, at least about 7.0, or at least
about 10Ø
In another embodiment, the cupredoxin, or variant, derivative or structural
equivalent thereof binds to either an ephrin and/or an Eph receptor. It is now
known
that several cupredoxins have a C-terminal region that is structurally similar
to
27
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
ephrinB2 ectodomain. See Examples 2, 5 and 9. In a specific embodiment, the
cupredoxin, or variant, derivative or structural equivalent thereof bind to an
ephrin. In
a particularly specific embodiment, the ephrin is, but is not limited to,
ephrinAl,
ephrinA2, ephrinA3, ephrinA4, ephrinA5, ephrinBl, ephrinB2, ephrinB3 and
ephrinB4. In a particularly specific embodiment, the ephrin is, but is not
limited to,
ephrinBl, ephrinB2, ephrinB3 and ephrinB4. In another specific embodiment, the
cupredoxin, or variant, derivative or structural equivalent thereof binds to
an Eph
receptor. In particularly specific embodiments, the Eph receptor is, but is
not limited
to, EphAl, EphA2, EphA3, EphA4, EphA5, EphA6, EphA7, EphA8, EphAlO,
EphBl, EphB2, EphB3, EphB4 and EphB6. In some embodiments, the cupredoxin,
or variant, derivative or structural equivalent thereof binds to both a ephrin
and an
ephrin receptor. In some embodiments, the cupredoxin, or variant, derivative
or
structural equivalent thereof binds to a ephrin and its receptor, specifically
EphrinB2
and EphB2. Methods for determining the binding of proteins to other proteins
are
well known in the art. Examples of methods determining binding to Eph
receptors
include Examples 6-8, as well as Koolpe et al. (J. Biol. Chem. 280:17301-17311
(2005)). and Himanen et al. (Nat. Neurosci. 7:501-509 (2004)).
In some specific embodiments, the cupredoxin, or variant, derivative or
structural equivalent thereof induces apoptosis in a mammalian cancer cell,
more
specifically a J774 cell. The ability of a cupredoxin or other polypeptide to
induce
apoptosis may be observed by mitosensor ApoAlert confocal microscopy using a
MITOSENSORTM APOLERTTm Mitochondrial Membrane Sensor kit (Clontech
Laboratories, Inc., Palo Alto, California, U.S.A.), by measuring caspase-8,
caspase-9
and caspase-3 activity using the method described in Zou et al. J. Biol. Chem.
274:
11549-11556 (1999))., and by detecting apoptosis-induced nuclear DNA
fragmentation using, for example, the APOLERTTM DNA fragmentation kit
(Clontech
Laboratories, Inc., Palo Alto, California, U.S.A.).
In another specific embodiment, the cupredoxin, or variant, derivative or
structural equivalent thereof induces cellular growth arrest in a mammalian
cancer
cell, more specifically a J774 cell. Cellular growth arrest can be determined
by
measuring the extent of inhibition of cell cycle progression, such as by the
method
found in Yamada et al. (PNAS 101:4770-4775 (2004)). In another specific
28
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
embodiment, the cupredoxin, or variant, derivative or structural equivalent
thereof
inhibits cell cycle progression in a mammalian cancer cell, more specifically
a J774
cell.
Cupredoxins
These small blue copper proteins (cupredoxins) are electron transfer proteins
(10-20 kDa) that participate in bacterial electron transfer chains or are of
unknown
function. The copper ion is solely bound by the protein matrix. A special
distorted
trigonal planar arrangement to two histidine and one cysteine ligands around
the
copper gives rise to very peculiar electronic properties of the metal site and
an intense
blue color. A number of cupredoxins have been crystallographically
characterized at
medium to high resolution.
The cupredoxins in general have a low sequence homology but high structural
homology. (Gough & Clothia, Structure 12:917-925 (2004); De Rienzo et al.,
Protein
Science 9:1439-1454 (2000).) For example, the amino acid sequence of azurin is
31% identical to that of auracyanin B, 16.3% to that of rusticyanin, 20.3 % to
that of
plastocyanin, and 17.3% to that of pseudoazurin. See, Table 1. However, the
structural similarity of these proteins is more pronounced. The VAST p value
for the
comparison of the structure of azurin to auracyanin B is 10"7.4, azurin to
rusticyanin is
10-5, azurin to plastocyanin is 10-5'6, and azurin to psuedoazurin is 10~. 1.
All of the cupredoxins possess an eight-stranded Greek key beta-barrel or
beta-sandwich fold and have a highly conserved site architecture. (De Rienzo
et al.,
Protein Science 9:1439-1454 (2000).), A prominent hydrophobic patch, due to
the
presence of many long chain aliphatic residues such as methionines and
leucines, is
present around the copper site in azurins, amicyanins, cyanobacterial
plastocyanins,
cucumber basic protein and to a lesser extent, pseudoazurin and eukaryotic
plastocyanins. Id. Hydrophobic patches are also found to a lesser extent in
stellacyanin and rusticyanin copper sites, but have different features. Id.
29
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
Table 1. Sequence and structure alignment of azurin (1JZG) from P. aeruginosa
to
other proteins using VAST algorithm.
PDB Alignment % aa P-value Score
lengthl identity RMSD4 Description
1AOZ A 2 82 18.3 10 e-7 12.2 1.9 Ascorbate oxidase
1 QHQ_A 113 31 10e-7.4 12.1
1.9 AuracyaninB
1V54 B 1 79 20.3 lOe-6.0 11.2 2.1 Cytocrome c oxidase
1GY2 A 92 16.3 lOe-5.0 11.1
1.8 Rusticyanin
3MSP A 74 8.1 10e-6.7 10.9 2.5 Motile Major Sperm
Protein5
1 IUZ 74 20.3 l Oe-5 .6 10.3
2.3 Plastocyanin
lKGY E 90 5.6 lOe-4.6 10.1
3.4 Ephrinb2
1 PMY 75 17.3 lOe-4.1 9.8
2.3 Pseudoazurin
lAligned Length: The number of equivalent pairs of C-alpha atoms
superimposed between the two structures, i.e. how many residues have been
used to calculate the 3D superposition.
2P-VAL: The VAST p value is a measure of the significance of the
comparison, expressed as a probability. For example, if the p value is 0.001,
then the odds are 1000 to 1 against seeing a match of this quality by pure
chance. The p value from VAST is adjusted for the effects of multiple
comparisons using the assumption that there are 500 independent and
unrelated types of domains in the MMDB database. The p value shown thus
corresponds to the p value for the pairwise comparison of each domain pair,
divided by 500.
3Score: The VAST structure-similarity score. This number is related to
the number of secondary structure elements superimposed and the quality of
that superposition. Higher VAST scores correlate with higher similarity.
4RMSD: The root mean square superposition residual in Angstroms. This
number is calculated after optimal superposition of two structures, as the
square root of the mean square distances between equivalent C-alpha atoms.
Note that the RMSD value scales with the extent of the structural alignments
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
and that this size must be taken into consideration when using RMSD as a
descriptor of overall structural similarity.
C. elegans major sperm protein proved to be an ephrin antagonist in
oocyte maturation (Kuwabara, 2003 "The multifaceted C. elegans major
sperm protein: an ephrin signalling antagonist in oocyte maturation" Genes
and Development, 17:155-161.
Azurin
The azurins are copper-containing proteins of 128 amino acid residues which
belong to the family of cupredoxins involved in electron transfer in certain
bacteria.
The azurins include those from P. aeruginosa (PA) (SEQ ID NO: 1), A.
xylosoxidans,
and A. denitrificans. (Murphy et al., J. Mol. Biol. 315:859-871 (2002)). The
amino
acid sequence identity between the azurins varies between 60-90%, these
proteins
showed a strong structural homology. All azurins have a characteristic 0-
sandwich
with Greek key motif and the single copper atom is always placed at the same
region
of the protein. In addition, azurins possess an essentially neutral
hydrophobic patch
surrounding the copper site. Id.
Plastocyanins
The plastocyanins are soluble proteins of cyanobacteria, algae and plants that
contain one molecule of copper per molecule and are blue in their oxidized
form.
They occur in the chloroplast, where they function as electron carriers. Since
the
determination of the structure of poplar plastocyanin in 1978, the structure
of algal
(Scenedesmus, Enteromorpha, Chlamydomonas) and plant (French bean)
plastocyanins has been determined either by crystallographic or NMR methods,
and
the poplar structure has been refined to 1.33 A resolution. SEQ ID NO: 2 shows
the
amino acid sequence of plastocyanin from Phormidiurn laminosum, a thermophilic
cyanobacterium. SEQ ID NO: 22 shows the amino acid sequence of plastocyanin
from Ulva petrusa.
Despite the sequence divergence among plastocyanins of algae and vascular
plants (e.g., 62% sequence identity between the Chlamydomonas and poplar
proteins),
the three-dimensional structures are conserved (e.g., 0.76 A rms deviation in
the C
31
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
alpha positions between the Chlamydomonas and Poplar proteins). Structural
features include a distorted tetrahedral copper binding site at one end of an
eight-
stranded antiparallel beta-barrel, a pronounced negative patch, and a flat
hydrophobic
surface. The copper site is optimized for its electron transfer function, and
the
negative and hydrophobic patches are proposed to be involved in recognition of
physiological reaction partners. Chemical modification, cross-linking, and
site-
directed mutagenesis experiments have confirmed the importance of the negative
and
hydrophobic patches in binding interactions with cytochrome f, and validated
the
model of two functionally significant electron transfer paths involving
plastocyanin.
One putative electron transfer path is relatively short (approximately 4 A)
and
involves the solvent-exposed copper ligand His-87 in the hydrophobic patch,
while
the other is more lengthy (approximately 12-15 A) and involves the nearly
conserved
residue Tyr-83 in the negative patch. (Redinbo et al., J. Bioenerg. Biomembr.
26:49-
66 (1994)).
Rusticyanins
Rusticyanins are blue-copper containing single-chain polypeptides obtained
from a Thiobacillus (now called Acidithiobacillus). The X-ray crystal
structure of the
oxidized form of the extremely stable and highly oxidizing cupredoxin
rusticyanin
from Thiobacillusferrooxidans (SEQ ID NO: 3) has been determined by
multiwavelength anomalous diffraction and refined to 1.9A resolution. The
rusticyanins are composed of a core beta-sandwich fold composed of a six- and
a
seven-stranded b-sheet. Like other cupredoxins, the copper ion is coordinated
by a
cluster of four conserved residues (His 85, Cys138, His143, Met148) arranged
in a
distorted tetrahedron. (Walter et al., J. Mol. Biol. 263:730-51 (1996)).
Pseudoazurins
The pseudoazurins are a family of blue-copper containing single-chain
polypeptide. The amino acid sequence of pseudoazurin obtained from
Achromobacter cycloclastes is shown in SEQ ID NO: 4. The X-ray structure
analysis
of pseudoazurin shows that it has a similar structure to the azurins although
there is
low sequence homology between these proteins. Two main differences exist
between
32
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
the overall structure of the pseudoazurins and azurins. There is a carboxy
terminus
extension in the pseudoazurins, relative to the azurins, consisting of two
alpha-helices.
In the mid-peptide region azurins contain an extended loop, shortened in the
pseudoazurins, which forms a flap containing a short a-helix. The only major
differences at the copper atom site are the conformation of the MET side-chain
and
the Met-S copper bond length, which is significantly shorter in pseudoazurin
than in
azurin.
Phytocyanins
The proteins identifiable as phytocyanins include, but are not limited to,
cucumber basic protein, stellacyanin, mavicyanin, umecyanin, a cucumber
peeling
cupredoxin, a putative blue copper protein in pea pods, and a blue copper
protein from
Arabidopsis thaliana. In all except cucumber basic protein and the pea-pod
protein,
the axial methionine ligand normally found at blue copper sites is replaced by
glutamine.
Auracyanin
Three small blue copper proteins designated auracyanin A, auracyanin B-1,
and auracyanin B-2 have been isolated from the thermophilic green gliding
photosynthetic bacterium Chloroflexus aurantiacus. The two B forms are
glycoproteins and have almost identical properties to each other, but are
distinct from
the A form. The sodium dodecyl sulfate-polyacrylamide gel electrophoresis
demonstrates apparent monomer molecular masses as 14 (A), 18 (B-2), and 22 (B-
1)
kDa.
The amino acid sequence of auracyanin A has been determined and showed
auracyanin A to be a polypeptide of 139 residues. (Van Dreissche et al;.
Protein
Science 8:947-957 (1999).) His58, Cys123, His128, and Met132 are spaced in a
way
to be expected if they are the evolutionary conserved metal ligands as in the
known
small copper proteins plastocyanin and azurin. Secondary structure prediction
also
indicates that auracyanin has a general beta-barrel structure similar to that
of azurin
from Pseudonaonas aeruginosa and plastocyanin from poplar leaves. However,
auracyanin appears to have sequence characteristics of both small copper
protein
33
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
sequence classes. The overall similarity with a consensus sequence of azurin
is
roughly the same as that with a consensus sequence of plastocyanin, namely
30.5%.
The N-terminal sequence region 1-18 of auracyanin is remarkably rich in
glycine and
hydroxy amino acids. Id. See exemplary amino acid sequence SEQ ID NO: 15 for
chain A of auracyanin from Chloroflexus aurantiacus (NCBI Protein Data Bank
Accession No. AAM12874).
The auracyanin B molecule has a standard cupredoxin fold. The crystal
structure of auracyanin B from Chloroflexus aurantiacus has been studied.
(Bond et
al., J. Mol. Biol. 306:47-67 (2001); the contents of which are incorporated
for all
purposes by reference.) With the exception of an additional N-terminal strand,
the
molecule is very similar to that of the bacterial cupredoxin, azurin. As in
other
cupredoxins, one of the Cu ligands lies on strand 4 of the polypeptide, and
the other
three lie along a large loop between strands 7 and 8. The Cu site geometry is
discussed with reference to the amino acid spacing between the latter three
ligands.
The crystallographically characterized Cu-binding domain of auracyanin B is
probably tethered to the periplasmic side of the cytoplasmic membrane by an N-
terminal tail that exhibits significant sequence identity with known tethers
in several
other membrane-associated electron-transfer proteins. The amino acid sequences
of
the B forms are presented in McManus et al. (J Biol Chem. 267:6531-6540
(1992).).
See exemplary amino acid sequence SEQ ID NO: 16 for chain B of auracyanin from
Chloroflexus aurantiacus (NCBI Protein Data Bank Accession No. 1 QHQA).
Stellacyanin
Stellacyanins are a subclass of phytocyanins, a ubiquitous family of plant
cupredoxins. An exemplary sequence of a stellacyanin is included herein as SEQ
ID
NO: 14. The crystal structure of umecyanin, a stellacyanin from horseradish
root
(Koch et al., J. Am. Chenz. Soc. 127:158-166 (2005)). and cucumber
stellacyanin
(Hart el al., Protein Science 5:2175-2183 (1996)). is also known. The protein
has an
overall fold similar to the other phytocyanins. The ephrin B2 protein
ectodomain
tertiary structure bears a significant similarity to stellacyanin. (Toth et
al.,
Developmental Cell 1:83-92 (2001).) An exemplary amino acid sequence of a
34
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
stellacyanin is found in the National Center for Biotechnology Information
Protein
Data Bank as Accession No. 1JER, SEQ ID NO: 14.
Cucumber basic protein
An exemplary amino acid sequence from a cucumber basic protein is included
herein as SEQ ID NO: 17. The crystal structure of the cucumber basic protein
(CBP), a type 1 blue copper protein, has been refined at 1.8 A resolution. The
molecule resembles other blue copper proteins in having a Greek key beta-
barrel
structure, except that the barrel is open on one side and is better described
as a "beta-
sandwich" or "beta-taco". (Guss et al., J. Mol. Biol. 262:686-705 (1996).) The
ephrinB2 protein ectodomian tertiary structure bears a high similarity (rms
deviation
1.5A for the 50 a carbons) to the cucumber basic protein. (Toth et al.,
Developmental
Cell 1:83-92 (2001).)
The Cu atom has the normal blue copper NNSS' co-ordination with bond
lengths Cu-N(His39) = 1.93 A, Cu-S(Cys79) = 2.16 A, Cu-N(His84) = 1.95 A, Cu-
S(Met89) = 2.61 A. A disulphide link, (Cys52)-S-S-(Cys85), appears to play an
important role in stabilizing the molecular structure. The polypeptide fold is
typical
of a sub-family of blue copper proteins (phytocyanins) as well as a non-
metalloprotein, ragweed allergen Ra3, with which CBP has a high degree of
sequence
identity. The proteins currently identifiable as phytocyanins are CBP,
stellacyanin,
mavicyanin, umecyanin, a cucumber peeling cupredoxin, a putative blue copper
protein in pea pods, and a blue copper protein from Arabidopsis thaliana. In
all except
CBP and the pea-pod protein, the axial methionine ligand normally found at
blue
copper sites is replaced by glutamine. An exemplary sequence for cucumber
basic
protein is found in NCBI Protein Data Bank Accession No. 2CBP, SEQ ID NO: 17.
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
Methods of the Invention
Another aspect of the present invention relates to the use of one or more
cupredoxins and variants, derivatives and structural equivalents thereof in a
method
to treat a mammalian patient suffering from a pathological condition. More
specifically, the condition is related to the ephrin signaling system.
Additionally, the
mammalian patient may be suffering from cancer.
In general, it is now known that cupredoxins and variants, derivatives and
structural equivalents thereof will interfere with the activity of the ephrin
signaling
system in a cell or tissue. In addition, it is now known that cupredoxins and
variants,
derivatives and structural equivalents thereof will inhibit the growth of
cancer in cells
and tissues. In specific embodiments, the cell or tissue is mammalian. In more
specific embodiments, the cell is human. In some embodiments, the cell is not
human. In one embodiment, the cupredoxin and variant, derivative or structural
equivalent thereof is administered to a cell to inhibit the activity of an
ephrin receptor
on the surface of the cell. In another embodiment, the cupredoxin and variant,
derivative or structural equivalent thereof is administered to increase the
activity of an
ephrin receptor on the surface of the cell. In other specific embodiments, the
cupredoxin and variants and derivatives of cupredoxin inhibit and/or increase
the
forward and/or backward ephrin signaling. Further, it is possible, for
example, for a
forward signal to be inhibited while a backwards signal is increased.
The pathological condition suffered by the mammalian patient is specifically
one that is related to the activity of the ephrin signaling system. It is now
appreciated
that cupredoxins comprise a certain structural homology to ephrins, and can
also act
like ephrins in vivo in relation to the ephrin signaling system. It is
contemplated that
cupredoxins can be used to treat pathological conditions that are related to
the ephrin
signaling system. In specific embodiments, the pathological condition is
accompanied by a higher than normal or lower than normal concentration of a
component of the ephrin signaling system. In other specific embodiments, the
pathological condition results from an over-expression or under-expression of
a
component of the ephrin signaling system. In other specific embodiments, the
pathological condition results from the excessive turnover or lack of turnover
of a
component of the ephrin signaling system. In other specific embodiments, the
36
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
pathological condition causes an over-expression or under-expression of a
component
of the ephrin signaling system. In another specific embodiment, the
pathological
condition causes excessive turnover or lack of turnover of a component of the
ephrin
signaling system. A"component" of the ephrin signaling system includes any
molecule that transmits or causes to be transmitted a signal resulting from
ephrin
binding to an ephrin receptor. In more specific embodiments, the component is
an
ephrin or an ephrin receptor.
Additionally, the pathological condition can be accompanied by an abnormal
distribution, either intracellular, intercellular, or tissue-specific, of a
component of the
ephrin signaling system. In a specific embodiment, the ephrin receptor is
found in a
higher concentration on the surface of a cell or cells. In a more specific
embodiment,
an ephrin receptor is up-regulated or down-regulated in a tissue. In another
more
specific embodiment, an ephrinis up-regulated or down-regulated in a tissue.
In another aspect of the invention, the cupredoxin, or variant, derivative or
structural equivalent thereof is administered to a patent with cancer. In some
embodiments, the patient is mammalian, and specifically human. In other
embodiments, the patient is not human. While not limiting the mechanism of
action
of this treatment method, many cancers are associated with increased or
decreased
concentrations of components of ephrin signaling system. Many ephrins and Eph
receptors have been shown to be up-regulated or down-regulated in tumors,
particularly in the more aggressive stages of tumor progression. For example,
EphA2
is up-regulated in breast, liver and prostate cancer, and glioblastoma,
esophageal
squamous cell cancer, ovarian cancer and melanoma. In a specific embodiment,
the
cancer is associated with up-regulated EphA2 receptor. However, it is known
that in
many cancers, different components of the ephrin signaling system are up-
regulated
or down-regulated, in particular, the ephrins and Eph receptors. Examples of
the
abnormal expression of ephrins and ephrin receptors in various tumors are well
known in the art, and described in Surawska et al. (Cytokine & Growth Factor
Reviews 15:419-433 (2004)). In other embodiments, the cancer is not associated
with
abnormal amounts of components or activities of the ephrin signaling system.
In
specific embodiments, the cancer is, but is not limited to, breast cancer,
liver cancer,
gastrointestinal cancer, neuroblastoma, neural cancer, leukemia, lymphoma,
prostrate
37
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
cancer, pancreatic cancer, lung cancer, melanoma, ovarian cancer, endometrial
tumor,
choriocarcinoma, teratocarcinoma, thyroid cancer, all sarcomas including those
arising from soft tissues and bone, renal carcinomas, epidermoid cancer and
non-
small cell lung cancer. In a specific embodiment, the cancer is deficient in
the
expression of p53 tumor suppressor gene.
In other embodiments, the cancer has various attributes related to the stage
of
the tumor growth. An early step in tumor development is vascularization, where
arteries are recruited to supply the tumor with blood. While not limiting the
mechanism of operation to any one means, it is known that the ephrin signaling
system is related to angiogenesis. In one embodiment, the cancer is one with
which
angiogenesis is associated. Another stage of tumor development is metastasis,
where
the tumor forms metastases, which are defined as tumor implants discontinuous
with
the primary tumor. While not limiting the mechanism of operation to any one
means,
it is thought that metastasis is also related to the ephrin signaling system.
In one
embodiment, the cancer is pre-metastatic. In another embodiment, the cancer is
metastatic. Method of measuring an effect of a compound on angiogenesis are
well-
known in the art. Specific methods for measuring angiogenesis include, but are
not
limited to, the assays found in the following articles, the contents of which
are
incorporated for all purposes by reference: Daniel et al., Kidney Int. Suppl.
57:S73-
S81 (1996); Myers et al., J.Cell Biol. 148:343-351 (2000); Pandey et al.,
Science
268:567-569 (1995); Brantley et al., Oncogene 21:7011-7026 (2002).
There are many pathological conditions in addition to tumors that are
associated with the ephrin signaling pathway. For example, pathological forms
of
angiogenesis (Adams & Klein, Trends Cardiov. Medicine 10:183-188 (2000);
Brantley-Sieders & Chen, Angiogenesis 7:17-28 (2004); Noren et al., Proc.
Natl.
Acad. Sci. USA 101:5583-558 (2004).), chronic pain following tissue damage
(Battaglia et al., Nat Neurosci. 6:339-340 (2003))., inhibition of nerve
regeneration
after spinal cord injury (Goldscmit et al., J. Neurosci. 6:339-340 (2003)).,
and human
congenital malformations (Twigg et al. Proc. Natl. Acad. Sci. USA 101:8652-
8657
(2004); Wieland et al., Am. J. Hum. Genet. 74:1209-1215 (2004))., and specific
embodiments of the invention use cupredoxin, or variants, derivatives or
structural
equivalents thereof to treat these pathological conditions. In specific
embodiments,
38
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
the pathological condition is interstitial cystitis (IC), lesions associated
with
inflammatory bowel disease (IBD), HIV infection, cardiovascular disease,
central
nervous system disorders, peripheral vascular diseases, viral diseases,
degeneration of
the central nervous system (Christopher Reeve's disease) and Alzheimer's
disease.
For methodology related to the treatment of patients with interstitial
cystitis and
inflammatory bowel disease, see, for example, U.S. Patent Application
Publication
20050049176 (published March 3, 2005, the contents of which are incorporated
for all
purposes by this reference.). For methodology related to the inhibition or
stimulation
of angiogenesis, see, for example, U.S. Patent Application Publication No.
20040136983 (published July 15, 2004, the contents of which are incorporated
for all
purposes by this reference.). For methodology related to therapies for
osteogenesis,
see, for example, U.S. Patent Application Publication No. 20040265808
(published
July 15, 2004, the contents of which are incorporated for all purposes by this
reference.).
The cupredoxin or variant, derivative or structural equivalent of cupredoxin
can be administered to the patient by many routes and in many regimens that
will be
well known to those in the art. In specific embodiments, the cupredoxin or
variant or
derivative of cupredoxin is administered intravenously, intramuscularly,
subcutaneously or by injection into a tumor. In a specific embodiment,
cupredoxin or
variant, derivative or structural equivalent of cupredoxin is administered
intravenously. In particularly specific embodiments, the cupredoxin or variant
or
derivative thereof is administered with chemotherapy to patients with cancer
or
recovering from cancer.
In addition to pathological conditions, the cupredoxin or variant, derivative
or
structural equivalent of cupredoxin can be used in therapeutic methods related
to other
conditions suffered by a patient. Such conditions can be the result of
accidents which
damage the nervous or vascular system, recovery from other therapies, such as
surgery, and conditions related to old age, among others. In one embodiment,
the
patient requires the growth or regrowth of blood vessels and the cupredoxin or
variant, derivative or structural equivalent of cupredoxin is administered to
guide the
growth of blood vessels. In another embodiment, the patient is in need of a
decrease
in the growth of blood vessels and the cupredoxin or variant, derivative or
structural
39
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
equivalent of cupredoxin is administered to inhibit the growth of blood
vessels. In
another embodiment, the cupredoxin or variant, derivative or structural
equivalent of
cupredoxin is administered to a patient in need of neuron generation or
regeneration
to guide the growth of neurons. In another embodiment the cupredoxin or
variant,
derivative or structural equivalent of cupredoxin is administered to a patient
to
promote osteogenesis.
Another aspect of the invention is a method to detect cells that display
specific
Ephrin receptors in vivo. It is now known that the cupredoxins contain a
region of
high structural homology to ephrin B2 and other ephrins, and that cupredoxins
can
bind with specificity to ephrin receptors in vitro. Therefore, cupredoxins
will localize
to the surface of cells expressing ephrin receptors. Accordingly, in some
embodiments, cupredoxins or variants derivatives or structural equivalents of
cupredoxins can be used to locate these Eph receptor-expressing cells and
tissues
amongst non-Eph receptor expressing cells or tissues. In addition, cupredoxins
or
variants derivatives or structural equivalents of cupredoxins that display a
binding
preference of a particular kind of Eph receptor can be used to locate cells or
tissues
specifically expressing that kind of Eph receptor. In one embodiment, the
cupredoxin
or variants or derivatives thereof are linked to a detectable probe and
administered to
a human patient, and the localization of the detectable probe is measured in
the patient
to determine the localization of the Eph receptor-expressing cells or tissues.
In a
particularly specific embodiment, the cell is a cancer cell. The detectable
probe may
be one of many currently known in the art. Probes of specific interest,
include but are
not limited to, fluorescent probes, radioactive probes, and iodine, gadolinium
and
gold.
In another embodiment of the invention, the cupredoxins or variants
derivatives or structural equivalents of cupredoxins are linked to a drug and
are
administered to a human patient in order to deliver the drug to Eph receptor
expressing cells or tissues. In another specific embodiment, the tissue is a
tumor. In a
particularly specific embodiment, the cell is a cancer cell. The drug may be
any kind
of chemical compound that has an effect on a cell expressing Eph receptors.
The drug
may be an organic or inorganic compound, including, but not limited to, a
peptide,
DNA molecule, RNA molecule, a pharmaceutical composition and derivatives of
any
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
of these. In specific embodiments, the drug is a toxin such as Pseudonzonas
exotoxin
A domain III, or a chemical compound such as taxol, or other drug that kills
cancer
cells.
A cupredoxin or variant, derivative or structural equivalent of cupredoxin can
be administered to a cell or a human patient by administering a DNA containing
a
coding region encoding the cupredoxin or variant, derivative or structural
equivalent
of cupredoxin operably linked to a promoter region that is expressed in the
desired
cell or tissue of the human patient. In a specific embodiment, a DNA encoding
a
cupredoxin or variant, derivative or structural equivalent of cupredoxin is
administered to a patient in order to treat a condition amenable to treatment
with
cupredoxin or variant, derivative or structural equivalent of cupredoxin.
Appropriate
vectors and methods to administer them to cells and human patients are well
known in
the art. Methodologies to express foreign proteins in human subjects are well
known
in the art and can be adapted to express a cupredoxin or variant, derivative
or
structural equivalent of cupredoxin in patients. Exemplary protocols are found
in the
following U.S. patents, among other sources: U.S. Pat. No. 6,339,068, issued
January
15, 2002; U.S. Pat. No. 6,867,000, issued March 15, 2005; U.S. Pat. No.
6,821,957,
issued November 23, 2004 ; U.S. Pat. No. 6,821,955, issued November 23, 2004,
U.S.
Pat. No. 6,562,376, issued May 13, 2003; the contents of all of which are
incorporated
for all purposes by this reference. In more specific embodiments, the DNA
encoding
a cupredoxin or variant, derivative or structural equivalent of cupredoxin is
injected
into a tumor of a patient suffering from cancer. In more specific embodiments,
the
DNA encoding a cupredoxin or variant, derivative or structural equivalent of
cupredoxin is injected into a tumor of a patient suffering from cancer.
In some embodiments, the pharmaceutical composition is administered to the
patient by intravenous injection, intramuscular injection, subcutaneous
injection,
inhalation, topical administration, transdermal patch, suppository, or oral,
and
specifically intravenous injection. The pharmaceutical composition may be
administered to the patient simultaneously or within 1 minute to 1 week or
more of
the administration of another drug known to treat specific pathological
conditions
related to ephrin signaling or cancer. The pharmaceutical composition may be
administered at about the same time as another anti-cancer drug.
41
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
Pharmaceutical Compositions Comprising Cupredoxins and Variants,
Derivatives and Structural Equivalents of Cupredoxins
Pharmaceutical compositions comprising at least one cupredoxin or variant,
derivative or structural equivalent of cupredoxin can be manufactured in any
conventional manner, e.g., by conventional mixing, dissolving, granulating,
dragee-
making, emulsifying, encapsulating, entrapping, or lyophilizing processes. The
substantially pure cupredoxins or variants, derivatives or structural
equivalents of
cupredoxins can be readily combined with a pharmaceutically acceptable carrier
well-
known in the art. Such carriers enable the preparation to be formulated as a
tablet,
pill, dragee, capsule, liquid, gel, syrup, slurry, suspension, and the like.
Suitable
excipients can also include, for example, fillers and cellulose preparations.
Other
excipients can include, for example, flavoring agents, coloring agents,
detackifiers,
thickeners, and other acceptable additives, adjuvants, or binders. General
methodology on pharmaceutical dosage forms is found in Ansel et al.,
Pharmaceutical Dosage Forms and Drug Delivery Systems (Lippencott Williams &
Wilkins, Baltimore MD (1999)).
The composition comprising a cupredoxin, or variant, derivative or structural
equivalent thereof used in the invention may be administered in a variety of
ways,
including by injection (e.g., intradermal, subcutaneous, intramuscular,
intraperitoneal
and the like), by inhalation, by topical administration, by suppository, by
using a
transdermal patch or by mouth. General information on drug delivery systems
can be
found in Ansel et al., Id.. In some embodiments, the composition comprising a
cupredoxin, or variant, derivative or structural equivalent thereof can be
formulated
and used directly as injectibles, for subcutaneous and intravenous injection,
among
others. The composition comprising a cupredoxin, or variant, derivative or
structural
equivalent thereof can also be taken orally after mixing with protective
agents such as
polypropylene glycols or similar coating agents.
When administration is by injection, the cupredoxin, or variant, derivative or
structural equivalent thereof may be formulated in aqueous solutions,
specifically in
physiologically compatible buffers such as Hanks solution, Ringer's solution,
or
physiological saline buffer. The solution may contain formulatory agents such
as
42
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
suspending, stabilizing and/or dispersing agents. Alternatively, the
cupredoxin, or
variant, derivative or structural equivalent thereof may be in powder form for
constitution with a suitable vehicle, e.g., sterile pyrogen-free water, before
use. In
some embodiments, the pharmaceutical composition does not comprise an adjuvant
or
any other substance added to enhance the immune response stimulated by the
peptide.
In some embodiments, the pharmaceutical composition comprises a substance that
inhibits an immune response to the peptide.
When administration is by intravenous fluids, the intravenous fluids for use
administering the cupredoxin, or variant, derivative or structural equivalent
thereof
may be composed of crystalloids or colloids. Crystalloids as used herein are
aqueous
solutions of mineral salts or other water-soluble molecules. Colloids as used
herein
contain larger insoluble moleCules, such as gelatin. Intravenous fluids may be
sterile.
Crystalloid fluids that may be used for intravenous administration include but
are not limited to, normal saline (a solution of sodium chloride at 0.9%
concentration), Ringer's lactate or Ringer's solution, and a solution of 5%
dextrose in
water sometimes called D5W, as described in Table 2.
Table 2. Composition of Common Crystalloid Solutions
Solution Other Name [Na+] [Cl"] [Glucose]
D5W 5% Dextrose 0 0 252
2/3 & 1/3 3.3% Dextrose 51 51 168
/ 0.3% saline
Half-normal 0.45% NaCI 77 77 0
saline
Normal saline 0.9% NaCI 154 154 0
Ringer's Ringer's 130 109 0
lactate* solution
*Ringer's lactate also has 28 mmol/L lactate, 4 mmol/L K+ and 3 mmol/L Ca2+.
When administration is by inhalation, the cupredoxin, or variant, derivative
or
structural equivalent thereof may be delivered in the form of an aerosol spray
from
pressurized packs or a nebulizer with the use of a suitable propellant, e.g.,
43
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
dichlorodifluoromethane, trichlorofluoromethane, carbon dioxide or other
suitable
gas. In the case of a pressurized aerosol, the dosage unit may be determined
by
providing a valve to deliver a metered amount. Capsules and cartridges of,
e.g.,
gelatin, for use in an inhaler or insufflator may be formulated containing a
powder
mix of the proteins and a suitable powder base such as lactose or starch.
When administration is by topical administration, the cupredoxin, or variant,
derivative or structural equivalent thereof may be formulated as solutions,
gels,
ointments, creams, jellies, suspensions, and the like, as are well known in
the art. In
some embodiments, administration is by means of a transdermal patch. When
administration is by suppository (e.g., rectal or vaginal), cupredoxin or
variants,
derivatives and structural equivalents thereof compositions may also be
formulated in
compositions containing conventional suppository bases.
When administration is oral, a cupredoxin, or variant, derivative or
structural
equivalent thereof can be readily formulated by combining the cupredoxin, or
variant,
derivative or structural equivalent thereof with pharmaceutically acceptable
carriers
well known in the art. A solid carrier, such as mannitol, lactose, magnesium
stearate,
and the like may be employed; such carriers enable the cupredoxin and
variants,
derivatives and structural equivalents thereof to be formulated as tablets,
pills,
dragees, capsules, liquids, gels, syrups, slurries, suspensions and the like,
for oral
ingestion by a subject to be treated. For oral solid formulations such as, for
example,
powders, capsules and tablets, suitable excipients include fillers such as
sugars,
cellulose preparation, granulating agents, and binding agents.
Other convenient carriers, as well-known in the art, also include multivalent
carriers, such as bacterial capsular polysaccharide, a dextran or a
genetically
engineered vector. In addition, sustained-release formulations that include a
cupredoxin, or variant, derivative or structural equivalent thereof allow for
the release
of cupredoxin, or variant, derivative or structural equivalent thereof over
extended
periods of time, such that without the sustained release formulation, the
cupredoxin,
or variant, derivative or structural equivalent thereof would be cleared from
a
subject's system, and/or degraded by, for example, proteases and simple
hydrolysis
before eliciting or enhancing a therapeutic effect.
44
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
The half-life in the bloodstream of the compositions of the invention can be
extended or optimized by several methods well known to those in the art,
including
but not limited to, circularized peptides (Monk et al., BioDrugs 19(4):261-78,
(2005);
DeFreest et al., J. Pept. Res. 63(5):409-19 (2004))., D,L-peptides
(diastereomer),
(Futaki et al., J. Biol. Chem. Feb 23;276(8):5836-40 (2001); Papo et al.,
Cancer Res.
64(16):5779-86 (2004); Miller et al., Biochem. Pharmacol. 36(1):169-76,
(1987)).;
peptides containing unusual amino acids (Lee et al., J. Pept. Res. 63(2):69-84
(2004))., N- and C- terminal modifications (Labrie et al., Clin. Invest. Med.
13(5):275-8, (1990))., and hydrocarbon stapling (Schaftneister et al., J. Am.
Chem.
Soc. 122:5891-5892 (2000); Walenski et al., Science 305:1466-1470 (2004)). Of
particular interest are d-isomerization (substitution) and modification of
peptide
stability via D-substitution or L- amino acid substitution.
In various embodiments, the pharmaceutical composition includes carriers and
excipients (including but not limited to buffers, carbohydrates, mannitol,
proteins,
polypeptides or amino acids such as glycine, antioxidants, bacteriostats,
chelating
agents, suspending agents, thickening agents and/or preservatives), water,
oils, saline
solutions, aqueous dextrose and glycerol solutions, other pharmaceutically
acceptable
auxiliary substances as required to approximate physiological conditions, such
as
buffering agents, tonicity adjusting agents, wetting agents and the like. It
will be
recognized that, while any suitable carrier known to those of ordinary skill
in the art
may be employed to administer the compositions of this invention, the type of
carrier
will vary depending on the mode of administration. Compounds may also be
encapsulated within liposomes using well-known technology. Biodegradable
microspheres may also be employed as carriers for the pharmaceutical
compositions
of this invention. Suitable biodegradable microspheres are disclosed, for
example, in
U.S. Patent Nos. 4,897,268; 5,075,109; 5,928,647; 5,811,128; 5,820,883;
5,853,763;
5,814,344 and 5,942,252.
The pharmaceutical compositions may be sterilized by conventional, well-
known sterilization techniques, or may be sterile filtered. The resulting
aqueous
solutions may be packaged for'use as is, or lyophilized, the lyophilized
preparation
being combined with a sterile solution prior to administration.
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
Administration of Cupredoxins
The cupredoxin, or variant, derivative or structural equivalent thereof can be
administered formulated as pharmaceutical compositions and administered by any
suitable route, for example, by oral, buccal, inhalation, sublingual, rectal,
vaginal,
transurethral, nasal, topical, percutaneous, i.e., transdermal or parenteral
(including
intravenous, intramuscular, subcutaneous and intracoronary) administration.
The
pharmaceutical formulations thereof can be administered in any amount
effective to
achieve its intended purpose. More specifically, the composition is
administered in a
therapeutically effective amount. In specific embodiments, the therapeutically
effective amount is generally from about 0.01-20 mg/day/kg of body weight.
The compounds comprising cupredoxin, or variant, derivative or structural
equivalent thereof are useful for the treatment of a condition related to
ephrin-
signaling, or cancer in mammalian cells and tissues, alone or in combination
with
other active agents. The appropriate dosage will, of course, vary depending
upon, for
example, the compound of cupredoxin, or variant, derivative or structural
equivalent
thereof employed, the host, the mode of administration and the nature and
severity of
the conditions being treated. However, in general, satisfactory results in
humans are
indicated to be obtained at daily dosages from about 0.01-20 mg/kg of body
weight.
An indicated daily dosage in humans is in the range from about 0.7 mg to about
1400
mg of a compound of cupredoxin, or variant, derivative or structural
equivalent
thereof conveniently administered, for example, in daily doses, weekly doses,
monthly doses, and/or continuous dosing. Daily doses can be in discrete
dosages
from 1 to 12 times per day. Alternatively, doses can be administered every
other day,
every third day, every fourth day, every fifth day, every sixth day, every
week, and
similarly in day increments up to 31 days or more. Alternatively, dosing can
be
continuous using patches, i.v. administration and the like.
The method of introducing cupredoxin and/or variant, derivative or structural
equivalent thereof to patients is, in some embodiments, co-administration with
other
drugs known to treat specific pathological conditions related to ephrin
signaling or
cancer, or other conditions or diseases Such methods are well-known in the
art. In a
specific embodiment, the cupredoxin and/or variant, derivative or structural
equivalent thereof are part of an cocktail or co-dosing containing or with
other
46
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
pathological conditions related to ephrin signaling or cancer. Drugs of
interest
include those used to treat inflammatory bowel disease, HIV infection, viral
diseases,
cardiovascular disease, peripheral vascular diseases, central nervous system
disorders,
degeneration of the central nervous system and Alzheimer's disease.
Drugs for treating with inflammatory bowel disease, include, but are not
limited to, aminosalicylates, such as, sulfasalazine (Azulfidine ), olsalazine
(Dipentum ), mesalamine (Asacol, Pentasa ), and balsalazide (Colazal );
corticosteroids, such as, prednisone, Medrol , methylprednisolone,
hydrocortisone,
Budesonide (Entocort EC); immunomodulators, such as, azathioprine (Imuran ), 6-
mercaptopurine (6-MP, Purinethol ) and cyclosporine A(Sandimmuneo, Neoral );
antibiotics, such as, metronidazole (Flagyl ) and ciprofloxacin (Cipro );
biologic
therapies, such as, infliximab (Remicade ); and miscellaneous therapies, such
as,
tacrolimus (FK506) and mycophenolate mofetil.
Drugs for treating HIV infection include, but are not limited to, reverse
transcriptase inhibitors: AZT (zidovudine [Retrovir ]), ddC (zalcitabine
[Hivid ],
dideoxyinosine), d4T (stavudine [Zerit ]), and 3TC (lamivudine [Epivir ]),
nonnucleoside reverse transcriptase inhibitors (NNRTIS): delavirdine
(Rescriptor )
and nevirapine (Viramune ), protease inhibitors: ritonavir (Norvir ), a
lopinavir and
ritonavir combination (Kaletra ), saquinavir (Invirase ), indinavir sulphate
(Crixivan ), amprenavir (Agenerase ), and nelfinavir (Viracept ).
Drugs for treating viral diseases include, but are not limited to, acyclovir ,
varicella
zoster immune globulin (VZIG ), peginterferon, ribavirin, acyclovir (Zovirax
),
valacyclovir (Valtrex ), famciclovir (Famvir ), amantadine, rimantadine,
zanamivir,
oseltamivir, and alpha interferon.
Drugs for treating cardiovascular disorders include, but are not limited to,
anticoagulants, antiplatelet agents, thrombolytic agents, adrenergic blockers,
adrenergic stimulants, alpha/beta adrenergic blockers, angiotensin converting
enzyme
(ACE) inhibitors, angiotensin converting enzyme (ACE) inhibitors with calcium
channel blockers, angiotensin converting enzyme (ACE) inhibitors with
diuretics,
angiotensin II receptor antagonists, calcium channel blockers, diuretics
(including
carbonic anhydrase inhibitors, loop diuretics, potassium-sparing diuretics,
thiazides
and related diuretics, vasodilators, vasopressors, etc.
47
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
Drugs for treating peripheral vascular disease include, but are not limited
to,
pentoxifylline (Trental ), an oral methylxanthine derivative, and cilostazol
(Pletal ), a
phosphodiesterase III inhibitor; antiplatelet/antithrombotic therapy such as
aspirin;
anticoagulants such as heparin and warfarin (Coumadin ); cholesterol lowering
drugs, such as, niacin, statins, fibrates, lopid tablets (gemfibrozil; Parke-
Davis);
tricor tablets (fenofibrate; Abbott) bile acid sequestrants, colestid tablets
(micronized
colestipol hydrochloride; Pharmacia and Upjohn); welchol tablets (colesevelam
hydrochloride; Sankyo); calcium channel blockers; vitamins and dietary
supplements,
such as, folate, B-6, B-12, L-arginine and omega-3 fatty acids; and HMG-COA
Reductase inhibitors, such as, advicor tablets (Niacin/Lovastatin; Kos);
altocor
extended-release tablets (lovastatin; Andryx labs); lescol capsules
(fluvastatin
sodium; Novartis & Reliant); lipitor tablets (atorvastatin; Parke-Davis and
Pfizer);
mevacor tablets (lovastatin; Merck); pravachol tablets (Pravastatin sodium;
Bristol-
Myers Squibb) pravigard PAC tablets (Buffered Aspirin and Pravastatin Sodium;
Bristol-Myers Squibb); zocor tablets (Simvastatin; Merck); nicotinic acid
agents,
such as, advicor tablets (Niacin/Lovastatin; Kos (also listed as a HMG-COA
Reductase inhibitor)).; niaspan (niacin; Kos); and miscellaneous agents, such
as,
zetia tablets (ezetimibe; Merck/Schering Plough).
Drugs for treating central nervous system disorders include, but are not
limited
to, psychotherapeutic agents, such as, various benzodiazepine preparations and
combinations, antianxiety agents, antidepressants (including monoamine oxidase
inhibitors (MAOI), selective serotonin reuptake inhibitors (SSRIs), tricyclic
antidepressants), antimanic agents, antipanic agents, antipsychotic agents,
psychostimulants, and obsessive-compulsive disorder management agents;
migraine
preparations, such as, beta adrenergic blocking agents, isometheptene and
serotonin
receptor agonists, as well as miscellarieous migraine preparations including
active
ingredients in depakote tablets (Divalproex sodium; Abbott) and excedrin
migraine
tablets (acetaminophen; BMS Products); sedatives and hypnotics;
anticonvulsants;
and pimozide . Drugs for treating Parkinson's disease include, but are not
limited to,
anticholinergic agents, catechol-o-methyltransferase inhibitors, dopamine
agents and
monoamine oxidase (MAO) inhibitors.
48
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
Drugs for treating CNS degeneration disorders include the following: Drugs
for treating Multiple Sclerosis include, but are not limited to, active
ingredients in
avonex (interferon beta-la; Biogen Neurology); Betaseron for SC injection
(modified form of Interferon beta-1b; Berlex); copaxone for injection
(Glatiramer
Acetate; Teva Neuroscience); depo-medrol injectable suspension
(Methylprednisolone acetate; Pharmacia & Upjohn); Novantrone for injection
concentrate (Mitoxantrone supplied as mitoxantrone hydrochloride; Serono);
Orapred oral solution (prednisolone sodium phosphate oral solution; Ascent);
and
Rebif injection (interferon beta-la; Pfizer & Serono). Drugs for treating
Huntington's Disease include, but are not limited to, tranquilizers such as
clonazepam
(Klonopin ); antipsychotic drugs such as haloperidol (Haldol ) and clozapine
(Clozaril ); fluoxetine (Prozac , Sarafem ), sertraline (Zoloft ),
nortriptyline
(Aventyl , Pamelor ), and lithium (Eskalith , Lithobid ).
Drugs for treating Alzheimer's disease include, but are not limited to,
aricept
tablets (Donepezil Hydrochloride; Eisai or Pfizer); exelori capsules
(rivastigmine (as
the hydrogen tartrate salt); Novartis); exelon oral solution (rivastigmine
tartrate;
Novartis); reminyl oral solution (galantamine hydrobromide; Janssen) or
reminyl
tablets (galantamine hydrobromide; Janssen).
The method of introducing compounds comprising a cupredoxin, or variant,
derivative or structurally equivalent thereof to patients is, in some
embodiments, co-
administration with other drugs known to treat cancer. Such methods are well-
known
in the art. In a specific embodiment, the compounds containing a cupredoxin,
or
variant, derivative or structurally equivalent thereof are part of an cocktail
or co-
dosing containing or with other drugs for treating cancer. Such drugs include,
for
example, those listed herein and specifically 5-fluorouracil; Interferon a;
Methotrexate; Tamoxifen; and Vincrinstine. The above examples are provided for
illustration only, many other such compounds are known to those skilled in the
art.
Other drugs suitable for treating cancer include, but not limited to,
alkylating
agents such as nitrogen mustards, alkyl sulfonates, nitrosoureas,
ethylenimines, and
triazenes; antimetabolites such as folate antagonists, purine analogues, and
pyrimidine
analogues; antibiotics such as anthracyclines, bleomycins, mitomycin,
dactinomycin,
and plicamycin; enzymes such as L-asparaginase; farnesyl-protein transferase
49
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
inhibitors; 5.alpha.-reductase inhibitors; inhibitors of 17.beta.-
hydroxysteroid
dehydrogenase type 3; hormonal agents such as glucocorticoids,
estrogens/antiestrogens, androgens/antiandrogens, progestins, and luteinizing
hormone-releasing hormone antagonists, octreotide acetate; microtubule-
disruptor
agents, such as ecteinascidins or their analogs and derivatives; microtubule-
stabilizing
agents such as taxanes, for example, paclitaxel (Taxol ), docetaxel (Taxotere
), and
their analogs, and epothilones, such as epothilones A-F and their analogs;
plant-
derived products, such as vinca alkaloids, epipodophyllotoxins, taxanes; and
topiosomerase inhibitors; prenyl-protein transferase inhibitors; and
miscellaneous
agents such as hydroxyurea, procarbazine, mitotane, hexamethylmelamine,
platinum
coordination complexes such as cisplatin and carboplatin; and other agents
used as
anti-cancer and cytotoxic agents such as biological response modifiers, growth
factors; immune modulators and monoclonal antibodies. The compounds of the
invention may also be used in conjunction with radiation therapy and surgery.
Representative examples of these classes of anti-cancer and cytotoxic agents
include but are not limited to mechlorethamine hydrochloride,
cyclophosphamide,
chlorambucil, melphalan, ifosfamide, busulfan, carmustin, lomustine,
semustine,
streptozocin, thiotepa, dacarbazine, methotrexate, thioguanine,
mercaptopurine,
fludarabine, pentastatin, cladribin, cytarabine, fluorouracil, doxorubicin
hydrochloride, daunorubicin, idarubicin, bleomycin sulfate, mitomycin C,
actinomycin D, safracins, saframycins, quinocarcins, discodermolides,
vincristine,
vinblastine, vinorelbine tartrate, etoposide, etoposide phosphate, teniposide,
paclitaxel, tamoxifen, estramustine, estramustine phosphate sodium, flutamide,
buserelin, leuprolide, pteridines, diyneses, levamisole, aflacon, interferon,
interleukins, aldesleukin, filgrastim, sargramostim, rituximab, BCG,
tretinoin,
irinotecan hydrochloride, betamethosone, gemcitabine hydrochloride,
altretamine, and
topoteca and any analogs or derivatives thereof.
Preferred members of these classes include, but, are not limited to,
paclitaxel,
cisplatin, carboplatin, doxorubicin, carminomycin, daunorubicin, aminopterin,
methotrexate, methopterin, mitomycin C, ecteinascidin 743, or pofiromycin, 5-
fluorouracil, 6-mercaptopurine, gemcitabine, cytosine arabinoside,
podophyllotoxin or
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
podophyllotoxin derivatives such as etoposide, etoposide phosphate or
teniposide,
melphalan, vinblastine, vincristine, leurosidine, vindesine and leurosine.
Examples of anticancer and other cytotoxic agents useful to co-administer
with the compositions of the invention include the following: epothilone
derivatives
as found in German Patent No. 4138042.8; WO 97/19086, WO 98/2246 1, WO
98/25929, WO 98/38192, WO 99/01124, WO 99/02224, WO 99/02514, WO
99/03848, WO 99/07692, WO 99/27890, WO 99/28324, WO 99/43653, WO
99/54330, WO 99/54318, WO 99/54319, WO 99/65913, WO 99/67252, WO
99/67253 and WO 00/00485; cyclin dependent kinase inhibitors as found in WO
99/24416 (see also U.S. Pat. No. 6,040,321); and prenyl-protein transferase
inhibitors
as found in WO 97/30992 and WO 98/54966; and agents such as those described
generically and specifically in U.S. Pat. No. 6,011,029 (the compounds of
which U.S.
patent can be employed together with any NHR modulators (including, but not
limited
to, those of present invention) such as AR modulators, ER modulators, with
LHRH
modulators, or with surgical techniques, especially in the treatment of
cancer).
The exact formulation, route of administration, and dosage is determined by
the attending physician in view of the patient's condition. Dosage amount and
interval can be adjusted individually to provide plasma levels of the active
cupredoxin, or variant, derivative or structural equivalent thereof which are
sufficient
to maintain therapeutic effect. Generally, the desired cupredoxin, or variant,
derivative or structural equivalent thereof is administered in an admixture
with a
pharmaceutical carrier selected with regard to the intended route of
administration and
standard pharmaceutical practice.
In one aspect, the cupredoxin, or variant, derivative or structural equivalent
thereof is delivered as DNA such that the polypeptide is generated in situ. In
one
embodiment, the DNA is "naked," as described, for example, in Ulmer et al.,
(Science
259:1745-1749 (1993)). and reviewed by Cohen (Science 259:1691-1692 (1993)).
The uptake of naked DNA may be increased by coating the DNA onto a carrier,
e.g.,
biodegradable beads, which are then efficiently transported into the cells. In
such
methods, the DNA may be present within any of a variety of delivery systems
known
to those of ordinary skill in the art, including nucleic acid expression
systems,
bacterial and viral expression systems. Techniques for incorporating DNA into
such
51
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
expression systems are well known to those of ordinary skill in the art. See,
e.g.,
W090/11092, W093/24640, WO 93/17706, and U.S. Pat. No. 5,736,524.
Vectors, used to shuttle genetic material from organism to organism, can be
divided into two general classes: cloning vectors are replicating plasmid or
phage with
regions that are essential for propagation in an appropriate host cell and
into which
foreign DNA can be inserted; the foreign DNA is replicated and propagated as
if it
were a component of the vector. An expression vector (such as a plasmid,
yeast, or
animal virus genome) is used to introduce foreign genetic material into a host
cell or
tissue in order to transcribe and translate the foreign DNA, such as the DNA
of a
cupredoxin. In expression vectors, the introduced DNA is operably-linked to
elements such as promoters that signal to the host cell to highly transcribe
the inserted
DNA. Some promoters are exceptionally useful, such as inducible promoters that
control gene transcription in response to specific factors. Operably-linking a
cupredoxin and variants, derivatives or structural equivalents thereof
polynucleotide
to an inducible promoter can control the expression of the cupredoxin and/or
cytochrome c and variants and derivatives thereof in response to specific
factors.
Examples of classic inducible promoters include those that are responsive to a-
interferon, heat shock, heavy metal ions, and steroids such as glucocorticoids
(Kaufinan, Methods Enzymol. 185:487-511 (1990)) and tetracycline. Other
desirable
inducible promoters include those that are not endogenous to the cells in
which the
construct is being introduced, but, are responsive in those cellswhen the
induction
agent is exogenously supplied. In general, useful expression vectors are often
plasmids. However, other forms of expression vectors, such as viral vectors
(e.g.,
replication defective retroviruses, adenoviruses and adeno-associated viruses)
are
contemplated.
Vector choice is dictated by the organism or cells being used and the desired
fate of the vector. In general, vectors comprise signal sequences, origins of
replication, marker genes, polylinker sites, enhancer elements, promoters, and
transcription termination sequences.
52
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
Kits Comprising Cupredoxin, Or Variant, Derivative Or Structural Equivalent
Thereof
In one aspect, the invention provides kits containing one or more of the
following in a package or container: (1) a biologically active composition
comprising
at least one cupredoxin, or variant, derivative or structural equivalent
thereof; (2) a
biologically active composition comprising a co-administered drug; (3) a
pharmaceutically acceptable excipient; (4) a vehicle for administration, such
as a
syringe; (5) instructions for administration. Embodiments in which two or more
of
components (1) - (5) are found in the same packaging or container are also
contemplated. The co-administered drug may be selected from those previously
mentioned.
When a kit is supplied, the different components of the composition may be
packaged in separate containers and admixed immediately before use. Such
packaging of the components separately may permit long-term storage without
losing
the active components' functions.
The reagents included in the kits can be supplied in containers of any sort
such
that the life of the different components are preserved and are not adsorbed
or altered
by the materials of the container. For example, sealed glass ampules may
contain
lyophilized cupredoxin and variants, derivatives or structural equivalents
thereof, or
buffers that have been packaged under a neutral, non-reacting gas, such as
nitrogen.
Ampules may consist of any suitable material, such as glass, organic polymers,
such
as polycarbonate, polystyrene, etc., ceramic, metal or any other material
typically
employed to hold similar reagents. Other examples of suitable containers
include
simple bottles that may be fabricated from similar substances as ampules, and
envelopes, that may comprise foil-lined interiors, such as aluminum or an
alloy.
Other containers include test tubes, vials, flasks, bottles, syringes, or the
like.
Containers may have a sterile access port, such as a bottle having a stopper
that can be
pierced by a hypodermic injection needle. Other containers may have two
compartments that are separated by a readily removable membrane that upon
removal
permits the components to be mixed. Removable membranes may be glass, plastic,
rubber, etc.
53
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
Kits may also be supplied with instructional materials. Instructions may be
printed on paper or other substrate, and/or may be supplied as an electronic-
readable
medium, such as a floppy disc, CD-ROM, DVD-ROM, Zip disc, videotape,
audiotape, flash memory device etc. Detailed instructions may not be
physically
associated with the kit; instead, a user may be directed to an internet web
site
specified by the manufacturer or distributor of the kit, or supplied as
electronic mail.
Modification Of Cupredoxin and Variants, Derivatives or Structural Equivalents
Thereof
A cupredoxin, or variant, derivative or structural equivalent thereof may be
chemically modified or genetically altered to produce variants and derivatives
as
explained above. Such variants and derivatives may be synthesized by standard
techniques.
In addition to naturally-occurring allelic variants of cupredoxin, changes can
be introduced by mutation into cupredoxin coding sequence that incur
alterations in
the amino acid sequences of the encoded cupredoxin that do not significantly
alter the
ability of cupredoxin to interfere with ephrin signaling or inhibit the growth
of cancer.
A "non-essential" amino acid residue is a residue that can be altered from the
wild-type sequences of the cupredoxin without altering biological activity,
whereas an
"essential" amino acid residue is required for such biological activity. For
example,
amino acid residues that are conserved among the cupredoxins are predicted to
be
particularly non-amenable to alteration, and thus "essential."
Amino acids for which conservative substitutions that do not change the
activity of the polypeptide can be made are well known in the art. Useful
conservative substitutions are shown in Table 3, "Preferred substitutions."
Conservative substitutions whereby an amino acid of one class is replaced with
another amino acid of the same type fall within the scope of the invention so
long as
the substitution does not materially alter the biological activity of the
compound.
54
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
Table 3. Preferred substitutions
Original residue Exemplary substitutions Preferred
substitutions
Ala (A) Val, Leu, Ile Val
Arg (R) Lys, Gln, Asn Lys
Asn (N) Gln, His, Lys, Arg Gln
Asp (D) Glu Glu
Cys (C) Ser Ser
Gln (Q) Asn Asn
Glu (E) Asp Asp
Gly (G) Pro, Ala Ala
His (H) Asn, Gln, Lys, Arg Arg
Ile (I) Leu, Val, Met, Ala, Phe, Leu
Norleucine
Leu (L) Norleucine, Ile, Val, Met, Ala, Ile
Phe
Lys (K) Arg, Gln, Asn Arg
Met (M) Leu, Phe, Ile Leu
Phe (F) Leu, Val, Ile, Ala, Tyr Leu
Pro (P) Ala Ala
Ser(S) Thr Thr
Thr (T) Ser Ser
Trp (W) Tyr, Phe Tyr
Tyr (Y) Trp, Phe, Thr, Ser Phe
Val (V) Ile, Leu, Met, Phe, Ala, Leu
Norleucine
Non-conservative substitutions that affect (1) the structure of the
polypeptide
backbone, such as a(3-sheet or a-helical conformation, (2) the charge, (3)
hydrophobicity, or (4) the bulk of the side chain of the target site can
modify the
cytotoxic factor function. Residues are divided into groups based on common
side-
chain properties as denoted in Table 4. Non-conservative substitutions entail
exchanging a member of one of these classes for another class. Substitutions
may be
introduced into conservative substitution sites or more specifically into non-
conserved
sites.
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
Table 4. Amino acid classes
Class Amino acids
hydrophobic Norleucine, Met, Ala, Val, Leu, Ile
neutral hydrophilic Cys, Ser, Thr
acidic Asp, Glu
basic Asn, Gln, His, Lys, Arg
disrupt chain conformation Gly, Pro
aromatic Trp, Tyr, Phe
The variant polypeptides can be made using methods known in the art such as
oligonucleotide-mediated (site-directed) mutagenesis, alanine scanning, and
PCR
mutagenesis. Site-directed mutagenesis (Carter, Biochem J. 237:1-7 (1986);
Zoller
and Smith, Methods Enzymol. 154:329-350 (1987)), cassette mutagenesis,
restriction
selection mutagenesis (Wells et al., Gene 34:315-323 (1985)) or other known
techniques can be performed on the cloned DNA to produce the cupredoxin
variant
DNA.
Known mutations of cupredoxins can also be used to create variant cupredoxin
to be used in the methods of the invention. For example, the C 112D and
M44KM64E
mutants of Pseudomonas aeruginosa azurin are known to have cytotoxic and
growth
arresting activity that is different from the native azurin, and such altered
activity can
be useful in the treatment methods of the present invention. One embodiment of
the
methods of the invention utilize cupredoxin and variants and derivatives
thereof
retaining the ability to interfere with ephrin signaling or inhibit the growth
of cancer
in mammalian cells. In another embodiment, the methods of the present
invention
utilize cupredoxin variants such as the M44KM64E mutant, having the ability to
cause cellular growth arrest.
A more complete understanding of the present invention can be obtained by
reference to the following specific Examples. The Examples are described
solely for
purposes of illustration and are not intended to limit the scope of the
invention.
Changes in form and substitution of equivalents are contemplated as
circumstances
may suggest or render expedient. Although specific terms have been employed
56
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
herein, such terms are intended in a descriptive sense and not for purposes of
limitations. Modifications and variations of the invention as hereinbefore set
forth
can be made without departing from the spirit and scope thereof, and,
therefore, only
such limitations should be imposed as are indicated by the appended claims.
EXAMPLES.
Example 1: In vivo studies of C. elegans development.
Some ephrins are known in Caenorhabditis elegans to be involved in muscle
formation in the tail and embryonic development. Our preliminary experiments
indicate that rusticyanin interferes with tail muscle formation (causing
paralysis of the
tail) while azurin prevents baby birth (embryonic development).
In these experiments, the azurin and rusticyanin genes were hyper-expressed in
E.
coli. A control E. coli without azurin or rusticyanin genes was maintained.
When C.
elegans worms were fed control E. eoli for up to 3 days, they were healthy and
produced babies. When C. elegans were fed azurin-expressing E. coli, they
seemed
healthy but produced very few babies. When C. elegans were fed rusticyanin-
expressing E. coli, about 30% could only move their heads or upper parts of
their
bodies but not their tails or the lower part of their body, demonstrating
paralysis.
Example 2: Azurin structural neighbors analysis
Protein structure neighbors analysis was determined by direct comparison of
3-dimensional protein structures in the Molecular Modeling DataBase with the
Vector
Alignment Search Tool (VAST) algorithm (Gibrat et al., Curr Opin Struct Biol
6:377-
385 (1996); Madej et al., Proteins 23:356-3690 (1995)). The Molecular Modeling
DataBase contains experimental data from crystallographic and NMR structure
determinations and is maintained by the National Center for Biotechnology
Information (Bethesda, MD, USA). A program that performs the VAST protein
structure neighbors analysis is available through the National Center for
Biotechnology Information.
Azurin showed a significant (Vast P value: 10 e -4.6; Alignment length: 90 aa;
% Identity: 6) (Fig. 1), structural superposition with the MMDB (Molecular
Modeling
Database) entry 1 KGY E (crystal structure of the EphB2-EphrinB2 complex). The
57
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
superposition alignment of azurin with the referred crystal structure is
related to the
chain E(138 aa), a protein denominated Ephrin (Ephrin-B2). This computational
analysis is in accordance with the structural data published in Himanen et al.
(Nature,
vo1414: 933-938 (2001)).
Further VAST analysis indicates that the cupredoxins rusticyanin, auracyanin,
plastocyanin, cucumber basic protein and stellacyanin also show significant
structural
homolgy to ephrinB2 in the G-H loop region. (Fig. 2)
Example 3: Efficacy of the synthetic peptides derived from azurin and
plastocyanin.
The efficacy of the synthetic peptides derived from azurin and plastocyanin
that are structurally similar to the human ephrin B-2 G-H loop has been
analyzed. An
18-mer azurin peptide with the following sequence has been synthesized by
standard
techniques:
TFDVSKLKEGEQYMFFCT SEQ iD NO: 18
MCF-7 breast cancer cells were incubated in 16-well plates with 5 and 50 ug/ml
of
the 18-mer azurin peptide for 0, 24, 48 and 72 hours, after which the number
of MCF-
7 cells were counted in a coulter counter. The peptide was seen at 50 ug/ml to
inhibit
MCF-7 cell growth by 50% in 48 to 72 hours, as compared to cells without the
synthetic peptide treatment. The extent of cell growth inhibition was about
25% at 5
ug/ml of the 18-mer synthetic peptide as compared to untreated control. This
experiment shows that the synthetic peptide designed solely on its structural
similarity
to the B-2 ephrin does in fact inhibit the cancer cell progression promoted by
the B-2
ephrin.
Example 4: In vitro measurement of effect of cupredoxins on the growth of Mel-
2
and MCF-7 cells
The growth of cells treated with cupredoxins was measured using a 16-well
plate. Mel-2 or MCF-7 cells (5 x 105 cells per well) were allowed to adhere to
multiwell (16-well, in this instance) plates for 24 hours. After adherence,
the growth
medium was siphoned off. PBS (phosphate-buffered saline) or various
cupredoxins/cytochromes at concentrations of 0.1 to 10 M in PBS were then
added
58
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
to the wells containing fresh growth media and the growth of the cancer cells
was
followed for 24, 48 and 72 hours. After the incubation period, trypan blue was
added
to the culture and the number of dead floating cells was counted. Both live
and dead
floating cells were counted to determine the IC50 at various cupredoxin doses.
The
IC50 is the concentration of protein that inhibits the cell culture growth by
50%.
At 500,000 cells per well at 24 hours of growth, enough cells were present for
reproducible counts. In the cupredoxin-minus control cell cultures, as the
cells grew,
they had less space to adhere to the bottom of the well, began to die and
became
floating cells. In plastocyanin-treated or rusticyanin-treated cell cultures,
the cells
also overgrew the surface area of the well and began to die and float but
their numbers
were less than the control. However, in the azurin-treated cell cultures, both
the Mel-
2 and MCF-7 cell line growth was inhibited leading to very few floating cells.
Example 5: Structural Similarity between EphrinB2 Ectodomain and
Cupredoxins
Structural similarities between ephrinB2 ectodomain and cupredoxins were
determined by using VAST and DALI algorithms (Holm and Sander, J. Mol. Biol.
233:123-138 (1993); Gibrat et al., Curr. Opin. Biol. 6"377-385 (1996)).
respectively
available through the U.S. National Institute of Heath and the European
Bioinformatics Institute. Structure-based pairwise sequence alignments were
calculated using the VAST algorithm. Protein structural diagrams are performed
in
two dimensions using TOPS (Topology Of Protein Structure) cartoons (Torrance
et
al., BioinfoNmatics 21:2537-2538 (2005)). The assessments of the structures
were
performed by using the program Mol Mol (Koradi et al., .I. Mol. Graphics 14:51-
55
(1996)).
Several homologs were found with both programs (data not shown), including
a small subset of monomeric cupredoxin proteins, plastocyanin, azurin and
rusticyanin. Specifically, the structural comparison between ephrinB2
ectodomain
and these three cupredoxins (which are chosen as representative proteins of
the
cupredoxin family), provided VAST and DALI alignments with significant and
quite
similar scores, respectively ranging from 11.0 to 9.7 (out of a maximum
possible
15.7) and 6.7 to 6.4. DALI Z scores <2.0 are structurally dissimilar (Table
5). The
59
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
most notable structural conservation exists between the ephrinB2 ectodomain
and
plastocyanin, superimposed with a root mean square deviation (r.m.s.d.) of 1.8
A
(calculated over 67 structurally equivalent Ca atoms) (Table 5).
Contrastingly, azurin,
plastocyanin and rusticyanin exhibit weaker primary sequence identity (less
than
10%) with the ephrinB2 ectodomain (Table 5).
Table 5 - Structural similarity of the human ephrin B2 ectodomain to three
members
(plastocyanin, azurin and rusticyanin) of the monodomain cupredoxin family.
PDB Name VAST DALI Z RMSD' Alignment %
scorea scoreb to length Identity
IKGY_E
lIUZ Plastocyanin 11.0 6.4 1.8 67 9.0
1JZG Azurin 10.1 6.7 3.4 90 5.6
1RCY Rusticyanin 9.7 6.1 3.1 87 8.0
a - VAST score - The VAST structure-similarity score; this number is related
to the number of secondary structure elements superimposed and
the quality of that superposition. Higher VAST scores correlate with higher
similarity.
b - DALI Z score - The Z scores are calculated using pairwise comparisions of
the ephrin ectodomain structure with other structures in the DALI
database. The higher the Z score, the less likely it is that the similarity
between the 3D
structures is random (pairs with Z<2.0 are structurally
dissimilar).
c - RMSD - Root-mean-square deviation of backbone residues in angstroms
between the aligned parts of the pair of structures.
Fig. 3 shows TOPS cartoons (A) and MolMol pictures (B) of the ephrinB2
ectodomain and each of the three cupredoxins under study. The topological
description showed that the proteins adopt a sandwich of two P sheets which
form the
core of the Greek-key fold and show remarkable structural similarity in the
way of the
number and orientation of the (3-strands ; 1KGY E(ephrinB2), lIUZ
(plastocyanin)
and 1JZG JZG-A (azu(Fig. 3). In contrast, the number and arrangement of a-
helices
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
are much less conserved. In JZG A, the helical structure is unique compared
with the
other proteins represented herein. As can be expected for proteins with
different
sizes, the loops that connect the elements of secondary structure showed
differences
in the lengths and their conformations. 1RCY (rusticyanin) has the lowest
structural
homology, with substantial differences in the lengths of shared secondary
structure
elements (SSEs) and the presence of non-shared SSEs
Example 6: Analysis of Eph-Fc Receptor-Cupredoxin Interactions by Surface
Plasmon Resonance
Specific interactions of the Eph receptors with cupredoxins were determined
by surface plasmon resonance (SPR) analyses. The human Astrocytoma CCF-
STTGl, Glioblastoma LN 229 and MCF-7 (breast cancer) cells were cultured in
RPMI medium 1640 containing 2 mM L-glutamine, 10 mM Hepes, 10% (vol/vol)
heat-inactivated FBS, 100 units/ml penicillin, and 100 g/mi streptomycin at
37 C in
a humidifier incubator with 5 % C02 as described earlier (Yamada et al., Cell
Microbiol. 7:1418-1431 (2005); Hiraoka et al., Proc. Natl. Acad. Sci. ZISA
101: 6427-
6432 (2004)). The human melanoma cells of UISO-Mel-2 (Yamada et al., Proc.
Natl.
Acad. Sci. USA 99:14098-14103 (2002)). were cultivated in MEM with Hank's
medium supplemented with 10% FBS. Escherichia coli JM109 and BL21 (DE3)
were used as host strains for hyperproduction of azurin, rusticyanin and
plastocyanin.
Azurin and rusticyanin were purified as described before (Yamada et al., Proc.
Natl.
Acad. Sci. USA 99:14098-14103 (2002); Yamada et al., Cell Cycle 3:1182-1187
(2004)). Construction and purification of GST-azurin fusion derivatives have
been
reported earlier (Yamada et al., Cell Microbiol. 7:1418-1431 (2005)).
Purification
and expression of plastocyanin from Phormidium laminosum was essentially
carried
out as described earlier (Schlarb et al., Gene 234:275-283 (1999))., except
that E. coli
strain BL21(DE3)Cd+(RIL) was used instead of BL21(DE3). The concentration of
fully oxidized protein was determined spectrophotometrically at 598 nm, using
an
extinction coefficient of 4700 M-lcm 1.
The Eph ectodomain Fc fusion proteins and ephrins were purchased as
lyopholized powders from R & D Systems; Minneapolis, MN. All other chemicals
used for surface plasmon resonance and growth experiments were purchased from
61
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
Biacore AB International or Sigma and were of high analytical grade. Direct
protein-
protein interactions between cupredoxins or GST-peptides with Eph-Fc or
ephrinB2-
Fc were determined with a Biacore X biosensor system (Biacore AB) which is
based
on surface plasmon resonance (SPR) technology.
In initial screening experiments immobilization of azurin, rusticyanin, or
plastocyanin to a single channel on the CM5 sensor chip was achieved using the
amine coupling=procedure. Sequential injections ofN-hydroxysuccinimide/N-(3-
dimethylaminopropy 1)-N-ethylcarbodiimide (0.05M/0.2M, 35 L), cupredoxin
protein (255 M, 50 L), 1M ethanolamine (50 L, pH 8.8), and 100 mM NaOH (10
uL) covalently linked the proteins to CM5 sensor chips with increases in
resonance
signals of 300 RU. Binding experiments were conducted via sequential
injections of
100 nM of Eph-Fc proteins in HBS-EP running buffer (0.01 M HEPES, pH 7.4, 0.15
M NaC1, 3 mM EDTA, 0.005% v/v Surfactant P20) over the sensor surfaces at flow
rates of 5 and 30 L/min for 2.3 min (70 L injection) with intermediate
injections of
100 mM NaOH (10 L pulse) to regenerate the cupredoxin-CM5 surface. All
binding
experiments were run against a bare Au CM5 sensor surface (negative channel)
to
correct for nonspecific binding.
The binding screens were conducted on cupredoxin modified sensor chips
with sequential injection of 100 nM of Eph-Fe receptors at a flow rate of 30
L/min
over 2.3 min. The curves represent the beginning of the association phase for
the
interactions of Eph-Fc with azurin, plastocyanin, and rusticyanin. Relative
binding
affinities were taken as a function of the saturating resonances (Req) which
varied
from 79 RU for rusticyanin binding to EphBl-Fc or EphA8-Fc to 1248 RU for
azurin
binding to EphB2-Fc. The cross-selective binding of cupredoxins to specific
EphA
and EphB receptor proteins is notable with the best interactions occurring
between
cupredoxin and EphB.
SPR sensorgrams for binding of immobilized cupredoxins with Eph-Fc
indicated selective recognition between these two subsets of proteins (Fig. 4A-
C). In
these measurements, Eph-Fc concentrations were kept constant at 100 nM so that
the
differences in the degree of association expressed in terms of Req reflect the
differences in affinities between the Eph-Fc receptor proteins and
cupredoxins.
Azurin showed the highest affinity for EphB2-Fc and A6 and also tightly bound
A4
62
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
and A7 (Fig. 4A). On the other hand, plastocyanin showed selectivity for EphAl-
Fc,
A3 and B2 and, to a lesser degree, A2 and A6 (Fig. 4B). Lastly, rusticyanin
recognized EphA8-Fc, and B 1, but only weakly (Fig. 4C). The associative
interactions of azurin with EphB2-Fc and EphA6-Fc were the highest with Req
values
1248 and 1200 RU respectively.
Example 7: Analysis of Binding Affinities of Azurin and GST-Azu Peptides with
EphB2-Fc -SPR Binding Measurements
Binding affinities of azurin and GST-azu peptides with EphB2-Fc -SPR
binding measurements were performed with azurin or GST-Azu peptides using the
immobilized EphB2-Fc to evaluate the relative binding affinity of the full
length
azurin or its various domains for the EphB2-Fc receptor. Azurin or GST-Azu
peptides were injected at increasing concentrations (0.05-100 nM) to EphB2-Fc
or
ephrinB2-Fc modified CM5 chips with 100 mM NaOH pulses in between injections.
The data were fit to a Langmuir (1:1) binding model [Req = Rmax/(1 + Kd/C) to
extrapolate equilibrium binding constants (Kd).
The experimental strategy for elucidating the structural determinant of azurin
for EphB2-Fc binding is shown in Fig. 5 which depicts the map of the
primary/secondary structural parameters of azurin and the GST-Azu constructs
as
described earlier (Yamada et al., Cell Microbiol. 7:1418-1431 (2005)). In
particular,
the GST-Azu 88-113 is coincident with the GH loop region of ephrinB2 (native
ligand) and therefore its binding efficacy with EphB2-Fc was of particular
interest.
Initial binding measurements of azurin and various GST-Azu constructs (all at
100
nM) for EphB2-Fc revealed their relative affinity: i.e., azurin > GST-Azu 88-
113 >
CST-Azu 36-128 > ephrinB2-Fc > GST-Azu 36-89 >> GST (Fig. 6A). Azurin and
GST-tagged azurin showed comparable affinities (data not shown). To quantify
the
binding affinities, the saturating response values (Req) for azurin or GST-Azu
were
measured as a function of their concentrations (0-1,00 nM) (Fig. 6B). The
binding
data were fit to a simple Langmuir (1:1) binding equation to determine
equilibrium
dissociation constants (Kd). Notably, azurin (Kd = 6 nM) and GST-Azu 88-113
(Kd
= 12 nM) had 5- and 2.5-fold higher affinities, respectively, for EphB2-Fc
than its
native ephrinB2-Fc ligand (Kd = 30 nM). Also, GST-Azu 36-128 (Table 6) showed
63
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
slightly higher affinity (Kd = 23 nM) than ephrinB2-Fc (Kd = 30 nM). In
contrast,
GST-Azu 36-89 and GST exhibited negligible binding (Table 6). These data
indicate
the significance of the Azu 88-113 region of azurin for high affinity EphB2-Fc
interaction. The Azu 88-113 region consists of the GH loop domain that is
structurally homologous to the GH loop found in ephrinB2.
Table 6. Relative binding affinities of azurin and GST-Azu constructs with
EphB2-Fc
Analyte Req in RU Molecular Weight Kd in nM
wt azurin 427 13,929 6 0.75
ephrinB2-Fc 159 130,200 30 =J= 5.1
GST 14 26,000 > 160
GST-Azu 36-128 244 36,076 23 ~ 1.5
GST-Azu 36-89 57 31,924 61 ~ 9.0
GST-Azu 88-113 314 28,943 12 ~ 1.5
The relative binding strengths of azurin and GST-Azu constructs with EphB2-
Fc were determined in SPR binding titration experiments and the extrapolated
datasets are summarized here and are compared to the binding of native
ephrinB2-Fc
ligand and GST. The data from an initial screening experiment were generated
upon
injection of 100 nM of analyte to the sensor surfaces and the saturating
signals (Req)
are listed in resonance units. Saturation in the binding was achieved at each
titration
point in the binding curves depicted in Fig 6b and these values plotted
against
[analyte] were used to calculate equilibrium dissociation constants (Kd). Kd
values
ranged from 6 to 61 nM for the interactions of azurin and GST-Azu while EphB2-
Fc
with the native ligand had an intermediate binding affinity within this range.
Example 8: Analysis of Competition Binding Studies for Azurin/ GST-Azu and
EphrinB2-Fc with EphB2-Fc
To better understand the physiological effects for the high-affinity azurin-
EphB2-Fc binding, we performed further binding measurements. Competition
binding titrations were conducted similar to the binding constant studies
except that
64
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
ephrinB2-Fc (246 nM) + competitor [azurin or GST-Azu (0-1020 nM)] were
injected
over the EphB2-Fc CM5 sensor surface.
Azurin + ephrinB2-Fc samples were added at different azurin concentrations
(0-1020 nM, [ephrinB2-Fc] is 246 nM) to the sensor surface and the data were
plotted
as a ratio of resonances, % total response [Req (azurin+ephrinB2-
Fc)/(Req/(ephrinB2-
Fc)).]. GST-Azu 88-113 and GST-Azu 36-89 were titrated with ephrinB2-Fc to
immobilized EphB2-Fc and analyzed in a similar manner. Competition data
suggest 1:
1 stoichiometry of binding between azurin and GST-Azu 88-113 with immobilized
EphB2-Fc.
We first measured the binding of azurin and GST-Azu constructs to the
EphB2-Fc ligand, ephrinB2-Fc. Fig. 7A shows that azurin indeed binds ephrinB2-
Fc
with high affinity (Kd = 8.5 + 0.8 nM), reflecting the structural similarities
between
these two proteins (Table 5). Relative high affinity of GST-Azu 88-113 (Kd =
39 +
6.5 nM) for ephrinB2-Fc indicates that the region between 88 and 113 is also
responsible for binding to ephrinB2-Fc. In contrast, GST and GST-Azu 36-89
showed no significant interactions with ephrinB2-Fc. Taken together, these
data
indicate that azurin can bind with high affinity to both EphB2-Fc and ephrinB2-
Fc via
its GH loop (88-113) region.
To further test this notion, we performed SPR analysis of binding of ephrinB2-
Fc to EphB2-Fc immobilized onto the CM5 sensor chip after ephrinB2-Fc is
incubated with varying concentrations of azurin and GST-Azu constructs. Fig.
7B
shows the inhibition of ephrinB2-Fc ([ephrinB2-Fc] = 246 nM) binding to EphB2-
Fc
by 0-1020 nM of azurin, GST-Azu 88-113, and GST-Azu 36-89, respectively. The
inhibition is expressed in terms of % total response (= [R eq (azurin +
ephrinB2-Fc) /
Req (ephrinB2-Fc alone)] * 100). The inhibition profiles indicate diminished
binding
of total protein to the immobilized EphB2-Fc when ephrinB2-Fc is preincubated
with
azurin or GST-Azu 88-113, with azurin being a more potent inhibitor than GST-
Azu
88-113. The preincubation of azurin or GST-Azu 88-113 with ephrinB2-Fc reduced
the total protein binding to the surface by up to 60%. GST-Azu 36-89, on the
other
hand, did not affect total protein binding (Fig. 7B), and this is consistent
with its weak
binding to either ephrinB2-Fc or EphB2-Fc. It appears that azurin (or GST-Azu
88-
113) forms a stoichiometric complex with ephrinB2-Fc because maximal
inhibition
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
was achieved with 1 to 1 ratio of ephrinB2-Fc and azurin (or GST-Azu 88-113).
The
fact that the inhibition by azurin or GST-Azu 88-113 levels off at 40 to 50%
indicates
that the putative azurin-ephrinB2-Fc complex has some affinity for the EphB2-
Fc
receptor. Collectively, these binding data indicate that azurin has high
affinity for
both ephrinB2-Fc and EphB2-Fc and that it can interfere with ephrinB2-Fc-EphB2-
Fc
binding by a dual mechanism of ligand sequestration and receptor occupation.
Example 9: Analysis of Cytotoxic Activity of Azu 96-113 and P1c70-84 Synthetic
Peptides and GST-Azu Fusion Derivatives Toward Various Cancer Cell Lines
Upon structure-based sequence alignment of azurin and plastocyanin with
human ephrinB2 ectodomain, we designed the peptides corresponding to the G-H
loop region of ephrinB2 (called Azu 96-113 (SEQ ID NO: 18) and Plc 70-84 (SEQ
ID
NO: 20))., which is the main region mediating high affinity binding of the
ephrins to
the Eph receptors (Himanen et al., Nature 414:933-938 (2001); Toth et al.,
Dev. Cell.
1:83-92 (2001)). In Fig. 8, the structural superimposition of the C-terminal
segments
of ephrinB2 ectodomain, azurin and plastocyanin can be seen. Structurally
based
sequence alignment of azurin and plastocyanin with human ephrinB2 ectodomain
was
used to design peptides based on the region mediating high affinity binding of
the
ephrinB2 to the EphB2 receptor (G strand -loop-H strand of the ephrinB2-Fc
ectodomain) of azurin, namely Azu 96-113 (96-TFDVSKLKEGEQYMFFCT -113)
and plastocyanin, namely Plc 70-84 (70-VRKLSTPGVYGVYCE-84) (Fig. 8). The
peptides were purchased from GenScript Corporation (Piscataway, NJ) as 99 %
pure.
They were purified by reverse phase high-pressure liquid chromatography and
their
identity verified by mass spectrometry. Peptides were dissolved in phosphate-
buffered saline (PBS) (lx) and stored in aliquots at -20 C until use.
In order to see if such domains of azurin may also play a role as antagonists
to
Eph signaling in cancer progression, we performed quantitative MTT assays in a
number of cancer cell lines normally known to hyperexpress EphB
receptors/ephrin
ligands. During a 24 h incubation at 37 C, both azurin and plastocyanin G-H
loop
peptides at 75 M showed induction of cell death in brain tumors astrocytoma
CCF-
STTG1 and glioblastoma LN-229 (Fig. 9A). The plastocyanin peptide Plc 70-84
showed somewhat higher cytotoxic activity than the azurin peptide Azu 96-113.
To
66
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
determine if such cytotoxicity is dose dependent and effective for other
cancer cell
lines, we evaluated the effect of several concentrations of these peptides on
melanoma
(Fig. 9B) or glioblastoma cells (Fig. 9C). In both cases, increasing peptide
concentrations led to increasing cytotoxicity (Fig. 9B and 9C).
Example 10: Analysis of the Ability of GST-Azu Fusion Peptides to Induce Cell
Death in Breast Cancer MCF-7 Cells
We have tested the ability of GST-Azu fusion peptides to induce cell death in
breast cancer MCF-7 cells. For measurement of the cytotoxicity of the azurin
and
plastocyanin synthetic peptides, the 3-(4,5-dimethylthiazol -2-yl-2,5-diphenyl
tetrazolium bromide) (MTT) (Sigma) assay (Mosmann, J. Immunol. Methods 65:55-
63 (1983)). was conducted. Approximately 2 x 104 cells per well were seeded
into
96-well culture plates in 100 gl of RPMI 1640 medium. After overnight growth,
the
supernatant was removed and new media containing azurin or plastocyanin
synthetic
peptides at various specified concentrations were added to the attached cells.
After 24
or 48 h treatment, 10 l of 5 mg/ml MTT solution was added to the culture and
incubated for 1 h at 37 C. The MTT reaction was terminated by the addition of
40
mM HCl in isopropanol. The MTT formazan formed was measured
spectrophotometrically as described earlier (Mosmann, 1983). Untreated control
cells
were compared to treated cells for determining viability and therefore a
measure of
cytotoxicity.
There was very little cytotoxicity (Fig. 10) triggered by GST or GST-Azu 36-
89 fusion protein similar to the control (without protein treatment). However,
GST-
Azu 36-128 or GST-Azu 88-113, harboring the azurin region capable of
interfering in
ephrinB2/EphB2 binding, showed significant cytotoxicity in a dose dependent
manner
confirming the role of the Azu 88-113 region in triggering MCF-7 cell death.
Example 11: Treatment Of Patients Suffering From A Pathological Condition
Related To Ephrin Signaling
A Phase UII clinical trial of a cupredoxin compound (Study Drug) is
performed in patients suffering from cancer. Specifically, the cupredoxin
compound
is Plc 70-84 (SEQ ID NO: 20).
67
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
Forty-nine adult patients with histologically verified cancers of the breast,
colon and melanoma who demonstrate clinical and radiographic progression or
recurrence following adequate treatment by currently available FDA-approved
chemotherapeutic drugs and regimen are enrolled in an open-label prospective
study
administering the Study Drug. To be eligible for enrollment in the study, all
patients
demonstrate increasing volume of measurable tumor after completion of approved
course of chemotherapy regimens. The evidence of persistent metastatic
deposits
and/or continued increase in size or volume must be histologically
established. This
histological proof can be obtained by a fine needle aspiration (FNA) biopsy.
The treatment program is instituted after obtaining informed consent from all
patients in accordance with the Institutional Review Board of the University
of
Illinois, Chicago and the FDA. The patients will have no intercurrent illness
such as
other malignancy, history of previous malignancy, blood dyscrasias, insulin
dependent diabetes or other serious cardiovascular diseases which might
interfere in
appropriate evaluation of the effects of the proposed therapy. Baseline blood
work
(Complete Blood Counts [CBC] and Serum Chemistry) including liver function
studies (LFT) is performed prior to initiation of therapy. All eligible
patients must not
receive any cancer chemotherapy concurrently during the period of the trial.
The study drug(s) is administered by daily intravenous injection of a
pharmaceutically acceptable preparation of the Study Drug for 12 weeks and the
subjects will be observed for any dose limiting toxicity. There will be 7 dose
levels
starting with 10 mg/kg/day and increasing by 5 mg/kg/day up to a maximum dose
of
50 mg/kg/day. The efficacy of each dose level will be recorded in 7 patients
with
advanced measurable cancer (breast, colon, and melanoma).
The response is estimated by measuring the measurable tumor in 2 dimensions
(a and b). 1) Total disappearance of the target metastatic tumors is
considered as
complete response (CR); 2) A 75% reduction is considered excellent, partial
response
(PR); and 3). A good response (PR) is post treatment reduction in size by 50%.
4)
Reduction of 25% in size is considered as stable disease (SD) and 5) < 25% is
considered as no response (NR). Patients demonstrating a progression of
disease
have their treatment discontinued but will be followed for an additional 12
weeks.
68
CA 02608512 2007-11-14
WO 2007/018671 PCT/US2006/019684
Total disappearance, and any reduction in size of the target metastatic tumors
will indicate that the azurin treatment is effective for treating cancer.
Other
indications that the Plc 70-84 treatment is effective are a decrease rate of
in the
appearance of new metastatic tumors and a decrease in the angiogenesis
associated
with tumors.
69