Note: Descriptions are shown in the official language in which they were submitted.
CA 02608714 2007-11-16
WO 2006/130133 PCT/US2005/018851
TITLE OF THE INVENTION
Catheter Port Assembly for Extracorporeal Treatment
FIELD OF THE INVENTION
[0001] The present invention relates to a catheter port assembly and a method
of inserting
the catheter port assembly.
BACKGROUND OF THE INVENTION
[0002] Catheters for extracorporeal blood purification may be located in
various venous
locations and cavities throughout the body of a patient for administration of
solutes and for removal
of toxins and fluids from the body via an extracorporeal blood circulation.
Such venous
catheterization may be performed by using a single catheter having multiple
lumens. A typical
example of a multiple lumen catheter is a dual lumen catheter in which one
lumen serves to aspirate
blood (arterial line) and the other lumen serves to restitute cleaned blood
(venous line). An
example of such a dual lumen catheter assembly is the SPLIT CATH catheter,
manufactured by
Medical Components, Inc. of Harleysville, Pennsylvania. Catheterization may
also be performed
by using separate, single lumen catheters inserted through the same incision
into the deep vein to be
catheterized. Such dual catheter assemblies are also manufactured by Medical
Components, Inc. of
Harleysville, Pennsylvania. An example of a dual single lumen catheter
assembly is the Tesio
catheter system, sold by Medical Components, Inc.
[0003] Generally, to insert any catheter into a deep vein or other blood
vessel, the vessel is
identified by aspiration with a long hollow needle in accordance with the well
known Seldinger
technique. When blood enters a syringe attached to the needle, indicating that
the vessel has been
1
CA 02608714 2007-11-16
WO 2006/130133 PCT/US2005/018851
found, a thin guide wire is then introduced, typically through the syringe
needle or other introducer
device into the interior of the vessel. The introducer device is then removed,
leaving the distal end
portion of the guide wire that has been inserted into the vessel within the
vessel and the opposing
proximal end of the guide wire projecting beyond the surface of the skin of
the patient. At this
point, several options are available to a physician for catheter placement.
The simplest option is to
pass a semi-rigid catheter into the vessel directly over the guide wire. The
guide wire is then
removed, leaving the catheter in position within the vessel. If the catheter
to be inserted is
significantly larger than the guide wire or is constructed from soft, flexible
polymer material, a vein
dilator device, generally within a sheath, is passed over the guide wire to
enlarge the guidewire
entrance site and to facilitate the introduction of the catheter. The
guidewire and dilator are
removed and the catheter is inserted through the sheath, into the vein. The
sheath is then removed,
leaving the catheter in place.
[00041 For chronic catheterization, in which the catheter is intended to
remain inside the
patient for an extended period of time, such as for weeks or even months, it
is typically desirable to
subcutaneously tunnel the catheter into the patient using various tunneling
techniques. The
proximal end of the catheter may be tunneled after the catheter is inserted
into the patient's vein.
The subcutaneous tunnel provides a stable anchor to prevent the proximal end
of the catheter from
moving and possibly becoming dislodged, which could result in patient
discomfort and risk of
injury, such as infection, inflammation, or accidental withdrawal. Currently
available products do
not provide a catheter port that facilitates a secure connection with the exit
site of the patient.
Furthermore, current products do not provide for a compact port for the
administration of
extracorporeal treatment.
2
CA 02608714 2011-12-15
[00051 It would be beneficial to provide a catheter port assembly that
provides a self-
contained flow restricting valve. Additionally, it would be beneficial to
provide a catheter port
assembly that is adapted to be partially inserted into the exit site of a
subcutaneous tunnel,
thereby sealing the exit site and retaining the assembly partially within the
subcutaneous tunnel
through the ingrowth of flesh around the adapter.
BRIEF SUMMARY OF THE INVENTION
[00061 According to the present invention, there is provided a catheter port
assembly
comprising: a catheter having a proximal catheter portion concluding in a
proximal catheter end
and being adapted for implantation into vasculature of a patient and
subcutaneous tunneling of
the proximal portion; and a port body having an open distal end, an open
proximal port end and
a longitudinal channel extending therethrough between the open distal end and
the open
proximal port end, the open proximal port end adapted to be mechanically
releasably coupled
to an extracorporeal device; the port body further comprising a valve disposed
within the
longitudinal channel; wherein the valve is adapted to restrict flow in at
least one direction;
wherein the distal end is adapted to be connected to the proximal end of the
subcutaneously
tunneled proximal catheter portion; wherein the distal end is further adapted
to be at least
partially inserted into an exit of the subcutaneous tunnel of the patient
after being connected to
the catheter; and wherein the proximal port end is externally exposed at the
exit to be coupled
to the extracorporeal device.
[0007] According to another aspect of the invention, there is provided a
catheter port
assembly comprising: a catheter having a proximal catheter portion concluding
in a proximal
catheter end and being adapted for implantation into vasculature of a patient
and subcutaneous
tunneling of the proximal portion; a tubular body having an open first end, an
open second end,
3
CA 02608714 2011-12-15
a center portion and a longitudinal passageway extending between the open
first end and the
open second end, and the open first end being adapted to be mechanically
releasably coupled to
an extracorporeal device; a valve disposed within the longitudinal passageway;
wherein the
second end of the assembly comprises at least one barb adapted to be inserted
within a lumen
of the catheter, thereby engaging the catheter lumen; wherein the center
portion is wider than
the catheter lumen; wherein the second end further comprises a distal cover
adapted to further
engage the catheter lumen with the second end and provide a transition between
the catheter
lumen and the center portion; and wherein the second end is adapted to be
partially
subcutaneously inserted into a patient at an exit site of the subcutaneous
tunnel while the open
first end remains externally exposed for coupling to the extracorporeal
device.
3a
CA 02608714 2007-11-16
WO 2006/130133 PCT/US2005/018851
[0008] The present invention also discloses a method of inserting a catheter
port assembly.
The method comprises providing a catheter, having at least one lumen, the at
least one lumen of the
catheter comprising at least one distal end and at least one proximal end. A
trocar is also provided.
The trocar includes a distal end and a proximal end. A suture having a first
end and a second end,
and a port assembly are also provided. The port assembly comprises a body
having a distal end and
a valve, and a distal cover. The method further includes inserting the distal
end of the at least one
lumen into a vessel of a patient, then connecting the proximal end of the at
least one lumen and the
first end of the suture, to the distal end of the trocar. The method further
includes tunneling the
trocar, the proximal end of the at least one lumen and the first end of the
suture through the flesh of
the patient to an exit site and pulling the proximal end of the at least one
lumen and the first end of
the suture at least partially through the exit site. Additionally, the method
includes attaching the
proximal end of the at least one lumen and the first end of the suture to the
distal end of the body
and pulling the second end of the suture until at least a portion of the
assembly is disposed within
the patient.
BRIEF DESCRIPTION OF THE DRAWINGS
[0009] The accompanying drawings, which are incorporated herein and constitute
part of
this specification, illustrate the presently preferred embodiments of the
invention, and, together with
the general description given above and the detailed description given below,
serve to explain the
features of the invention. In the drawings:
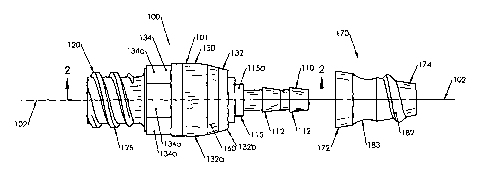
[0010] Fig. 1 is an exploded side view of a preferred embodiment of a catheter
port
assembly according to the present invention.
4
CA 02608714 2007-11-16
WO 2006/130133 PCT/US2005/018851
[0011] Fig. 2 is a sectional view of a body of the catheter port assembly
according to a
preferred embodiment of the present invention.
[0012] Fig. 3 is an enlarged sectional view of a distal cover of the catheter
port assembly
according to a preferred embodiment of the present invention.
[0013] Fig. 4 is an exploded side view of a catheter port assembly, including
a distal cover
and catheter lumen, partially in section.
[0014] Fig. 5 is a front view of a bracket according to the preferred
embodiment of the
present invention.
[0015] Fig. 5a is a sectional view of the bracket of Fig. 5, taken along the
line 5a-5a.
[0016] Fig. 5b is a front view of a bracket according to an alternative
embodiment of the
present invention.
[0017] Fig. 5c is a front view of the bracket of Fig. 5b, in an open position.
[0018] Fig. 5d is a front view of a bracket according a second alternative
embodiment of
.the present invention.
[0019] Fig. 5e is a front view of a bracket according to a third alternative
embodiment of
the present invention.
[0020] Fig. 5f is a bottom plan view of a portion of the bracket of Fig. 5e.
[0021] Fig. 6 is a partially broken away diagrammatic view of two catheter
port
assemblies subcutaneously tunneled and inserted into a patient according to
the preferred
embodiment of the present invention.
[0022] Fig. 7 is a side view of a catheter port assembly, including a catheter
and a suture,
according to the present invention.
CA 02608714 2007-11-16
WO 2006/130133 PCT/US2005/018851
[0023] Fig. 7a is a side view of the catheter port assembly of Fig 7, further
including a
distal cover, according to the present invention.
DETAILED DESCRIPTION OF THE INVENTION
[0024] In the drawings, like numerals indicate like elements throughout.
Certain
terminology is used herein for convenience only and is not to be taken as a
limitation on the present
invention. The words "proximal" and "distal" refer to directions away from and
closer to,
respectively, the insertion tip of the catheter in a catheter port assembly
100 according to the present
invention. The terminology includes the words above specifically mentioned,
derivatives thereof,
and words of similar import. The following describes preferred embodiments of
the invention.
However, it should be understood, based on this disclosure, that the invention
is not limited by the
preferred embodiments described herein.
[0025] Referring now to Fig. 1, a catheter port adapter assembly 100 according
to an
embodiment of the present invention is shown. The assembly 100 has a body 101,
comprising a
distal portion 110 and a proximal portion 120. A longitudinal axis 102 extends
between the distal
portion 110 and the proximal portion 120. A central portion 130 is disposed
along the longitudinal
axis 102 between the distal portion 110 and the proximal portion 120. The
assembly further
comprises a distal cover 170.
[0026] In the preferred embodiment shown here, the distal portion 110
preferably is
adapted to be inserted into a proximal end of a catheter lumen (not shown in
Fig. 1). The distal
portion 110 is adapted to engage the catheter lumen when the proximal end of
the lumen is disposed
about at least a portion of the distal portion 110. Preferably, the distal
portion 110 comprises at
least one barb 112 adapted to restrict the movement of the body 101 in a
proximal direction relative
6
CA 02608714 2007-11-16
WO 2006/130133 PCT/US2005/018851
to the lumen. While the at least one barb 112 is shown here comprises two
barbs 112, those skilled
in the art will recognize that movement of the catheter in relation to the
body 101 may be restricted
in other ways without departing from the scope of the present invention.
Examples of such other
means of attachment are a Tesio style connection (disclosed in U.S. Patent
No. 5,624,413), a
screw thread connection or any suitable combination of clips, couplings or
fittings known to those
skilled in the art to connect luers or adapters to catheter lumens.
Alternatively, if the catheter being
inserted is not retrograde subcutaneously tunneled, the catheter may be bonded
to the assembly 100
or insert molded to the assembly 100.
[0027] Referring to Fig. 2, the distal portion 110 of the body 101 has a
distal opening 114
and a distal passageway 116. The distal passageway 116 is adapted to
facilitate the flow of liquids,
preferably blood or medicaments, therethrough. Referring back to Fig. 1,
preferably, a proximal
part 115 of the distal portion 110, has a squared cross section, comprised of
four flat sides 115a
(only one side 115a being shown). Although the present embodiment discloses a
proximal part 115
of the distal portion 110 having four sides, those skilled in the art will
recognize that the proximal
.part 115 of the distal portion 110 may have any number of sides, or
alternatively be rounded or any
other suitable shape.
[0028] Referring to Figs. 1 and 2, preferably, the proximal portion 120 is
adapted to
releasably connect to an extracorporeal treatment device, such as a
hemodialysis machine (not
shown), or a cap (not shown). In the present embodiment, the means for
connection is a luer
connection 122, which is well known to those skilled in the art. The luer
connection 122 has a
proximal opening 124 with a tapered inner wall 123. Preferably, male threads
125 are disposed on
the outer surface of the proximal portion 120. Although male threads 125 and a
luer connection
7
CA 02608714 2007-11-16
WO 2006/130133 PCT/US2005/018851
122 are shown here, those skilled in the art will recognize that the proximal
end 120 may comprise
any other suitable means of connecting the assembly 100 to an extracorporeal
device.
[00291 Preferably, the central portion 130 has a larger cross section than
either of the
proximal or distal portions 120, 110 when taken along a plane perpendicular to
the paper.
Preferably, the central portion 130 has a generally circular cross section.
The central portion 130 is
sized to accommodate a valve 150 disposed therein along the longitudinal axis
102. Preferably, at
least a portion of the central portion 130 has a tapered outer surface 132
that tapers from wider, at a
proximal point 132a on the tapered outer surface 132, to narrower, at a distal
point 132b on the
tapered outer surface 132.
[00301 Referring back to Fig. 2, in construction, preferably the body 101
comprises at
least a first body section 103, comprising the proximal portion 120 and a
portion of the center
portion 130, and a second body section 104, comprising the distal section 110
and a portion of the
center portion 130. Preferably, the first body section 103 is adapted to
engage and connect to the
second body section 104. The connection may be welding, threads, a press fit
or any other suitable
means that is known to those skilled in the art. Preferably, a circumferential
reveal 156 is formed
between the first body section 103 and the second body section 104 when the
first body section 103
and the second body section 104 are connected together. Preferably, during
assembly, the valve
150 is disposed between the first body section 103 and the second body section
104 before the first
body section 103 and the second body section 104 are connected together,
thereby retaining the
valve 150 therein. While the valve 150 is shown here retained between the
first body section 103
and the second body section 104, those skilled in the art will recognize that
many other means for
retaining the valve 150 within the body 101 maybe used without departing from
the scope of the
present invention.
8
CA 02608714 2007-11-16
WO 2006/130133 PCT/US2005/018851
[0031] Preferably, an indicator ring 160 is disposed about the outer surface
of the central
portion 130. In the preferred embodiment, the indicator ring 160 is disposed
about the tapered outer
surface 132 between the proximal point 132a and the distal point 132b of the
tapered outer surface
132. Preferably, the indicator ring 160 is also tapered so that there is a
smooth surface between the
proximal point 132a and the distal point 132b of the tapered outer surface
132. Preferably, the
indicator ring 160 is colored to indicate whether the lumen that the assembly
100 is attached to is a
venous lumen, an arterial lumen, or used for some other purpose, such as
delivering medicaments to
the bloodstream. A color coded indicator ring 160 is desirable for the present
invention because,
when the assembly is properly installed in a patient, the lumen may be
completely covered by the
patient's flesh or the distal cover 170. The color coding system is well known
to those skilled in the
art. In the marking system, a blue marked lumen generally represents the
venous lumen, or the
lumen that facilitates the return of blood to the body, and the red marked
lumen generally represents
the arterial lumen, or the lumen that facilitates the withdrawal of blood from
the body. Although an
indicator ring 160 is used in the present embodiment to indicate the type of
lumen that the assembly
100 is connected to, those skilled in the art will recognize that any other
suitable means of
identifying the lumens may be used as well.
[0032] The central portion 130 further preferably includes a flattened portion
134.
Preferably, the outer surface of the flattened portion 134 has a hexagonal
cross section comprised of
six flattened sides 134a (only three sides 134a being shown). Preferably, the
flattened portion 134
is disposed proximally of the tapered outer surface 132. While the preferred
embodiment shown
here discloses a flattened portion 134 having a hexagonal cross section, those
skilled in the art will
recognize that flattened portions 134 having any number of sides may be used
without departing
9
CA 02608714 2007-11-16
WO 2006/130133 PCT/US2005/018851
from the scope of the present invention. Alternatively, the flattened portion
134 may be oblong or
some other shape as known to those skilled in the art.
[0033] Referring to Fig. 2, the central portion 130 and the proximal portion
120 define a
central passageway 136 that extends from the proximal opening 124 to the
distal passageway 116.
Preferably, the central passageway 136 is generally tubular and extends along
the longitudinal axis
102. Preferably, the valve 150 is disposed within the central passageway 136
along the longitudinal
axis 102. Preferably, the valve 150 includes a retaining ridge 152. In the
preferred embodiment,
the retaining ridge 152 is at least partially disposed within the
circumferential reveal 156 that
extends around the central passageway 136. While in the present embodiment,
the retaining ridge
152 is at least partially disposed within the circumferential reveal 156,
thereby retaining the valve
150 within the assembly 100, those skilled in the art will recognize that the
valve 150 may be
retained within the assembly 100 by any other suitable means.
[0034] Preferably, the body 101 is constructed of stainless steel, titanium or
some other
suitable material.
[0035] Preferably, the valve 150 restricts flow in a first direction and
facilitates flow in a
second direction. It is also preferable that the valve 150 provides sufficient
resistance to flow in all
directions to reduce the occurrence of leakage of blood out of the patient or
air or contaminants into
the patient. The orientation of the valve 150 may be altered so that, in a
pair of assemblies 100, the
valve 150 of a first assembly 100 would restrict flow in a first direction and
the valve 150 of a
second assembly 100 would restrict flow in a second direction. The valve 150
shown in the present
embodiment is a bi-directional pressure relief valve. Preferably, the valve
150 is constructed
according to the teachings of U.S. Patent No. 4,434,810. However, those
skilled in the art will also
CA 02608714 2007-11-16
WO 2006/130133 PCT/US2005/018851
recognize that the valve 150 may be a bidirectional valve (not shown), or any
other suitable type of
valve. Preferably, the valve 150 is constructed from silicone, a polymer or
some other material.
[0036] Referring to Figs. 3 and 4, the assembly 100 further comprises the
distal cover 170
having a proximal end 172, a distal end 174 and a longitudinal passageway 176
extending
therethrough between the proximal end 172 and the distal end 174. The proximal
end 172 of the
distal cover 170 comprises a proximal opening 178. The distal end 174 of the
distal cover 170
comprises a distal opening 180. The distal cover 170 is preferably conical in
shape, having a widest
part nearest the proximal end 172 and a narrowest part nearest the distal end
174.
[0037] The distal cover 170 is adapted to be connected to the body 101 by
inserting the
distal portion 110 of the body 101 through the proximal opening 178 of the
distal cover 170.
Preferably, the longitudinal passageway 176 of the distal cover 170 is sized
to engage the body 101
and the outside of a catheter lumen 200 that is disposed over the distal
portion 110 of the body 101.
[0038] Preferably, the proximal end 172 of the distal cover 170 is adapted to
engage the
four flat sides 115a of the proximal part 115 of the distal portion 110 of the
assembly. The
longitudinal passageway 176 of the distal cover 170 preferably comprises four
flats 184a (only one
flat 184a being shown) that frictionally engage the four flat sides 115a of
the distal portion 110 of
the body 101. Those skilled in the art will recognize that although four flat
sides 115a and four flats
184a are shown here, any number of flat sides 115a and flats 184a may be used
and that preferably,
there is the same number of flat sides 115a and flats 184a.
[0039] Referring to Fig. 1, preferably the proximal end 172 of the distal
cover 170 is sized
so that widest part of distal cover 172 has a similar cross sectional size as
the portion of the central
portion 130 located at the distal point 132b on proximal outer surface 132.
This facilitates a smooth
11
CA 02608714 2007-11-16
WO 2006/130133 PCT/US2005/018851
transition between the proximal outer surface 132 and the distal cover 170
when the assembly 100
is assembled.
100401 Referring now to Figs. 1, 3 and 4, the distal cover 170 preferably
comprises at least
one retaining ridge 182. In the preferred embodiment shown here, the retaining
ridge 182 is
generally helical in shape and extends approximately 360 degrees around the
distal cover 170.
While a generally helical retaining ridge 182 is shown here, those skilled in
the art will recognize
that retaining ridges having various configurations may also be used without
departing from the
scope of the present invention. Preferably, the retaining ridge 182 is adapted
to retain at least a
portion of the assembly 100 within the flesh of a patient. Preferably, a
rounded indentation 183 is
circumferentially disposed around the distal cover 170 between the retaining
ridge 182 and the
proximal end 172. The rounded indentation 183 is preferably positioned along
the distal cover 170
so that a part of the rounded indentation 183 will be disposed within the
patient when the assembly
is inserted and a portion of the rounded indentation 183 will remain outside
of the patient after
insertion. Preferably, the distal cover 170 is constructed from silicone, a
polymer or some other
material.
[00411 Referring to Figs. 5, 5a and 6, a plurality of assemblies 100, 100' may
be held
together with a stabilizing bracket 190. Preferably, the bracket 190 is
adapted to engage at least one
assembly 100. The present embodiment shows a bracket 190 adapted to engage two
assemblies
100, 100'. Preferably, the adapter 190 has a first passageway 191 and a second
passageway 192,
through which at least a portion of the assemblies 100 is to pass before the
bracket 190 engages the
assemblies 100. Preferably, the first and second passageways 191, 192 comprise
first and second
circular portions 193, 194 and first and second hexagonal portions 195, 196.
Preferably, when the
passageways 191, 192 of the bracket 190 engage the assemblies 100, 100', the
circular portions
12
CA 02608714 2007-11-16
WO 2006/130133 PCT/US2005/018851
193, 194 are located distally of the hexagonal portions 195, 196 so that the
circular portions 193,
194 engage the central portion 130 of the body 101 on each assembly 100, 100'.
Preferably, when
the bracket 190 engages the assemblies 100, 100', the hexagonal portions 195,
196 frictionally
engage the flattened portion 134 of the body 101 on each assembly 100, 100'.
The bracket 190
preferably engages the assemblies 100, 100' after the assemblies 100, 100' are
inserted into the
patient 10 preferably, after insertion, the bracket 190, with the side of the
openings comprising the
circular portions 193, 195 facing the patient, is slid distally about the
assemblies 100, 100' until the
circular portions 193, 194 engage each respective center portion 130 and the
hexagonal portion
engages each respective flattened portion 134.
[0042] Referring now to Figs 5b and 5c, an alternative bracket 290 is shown.
Preferably,
the bracket 290 has a first bracket portion 297 and a second bracket portion
297'. The first bracket
portion 297 is preferably connected to the second bracket portion 297' by a
hinge 298. Preferably,
when the bracket 290 is disposed in the closed position, as shown in Fig. 5a,
a first clasp portion
299 engages a second clasp portion 299', thereby retaining the bracket 290 in
the closed position.
Preferably, the first clasp portion 299 and second clasp portion 299' comprise
a tang and a keeper
respectively. Alternatively, the first and second clasp portions 299, 299' may
comprise opposing
tangs, a tab and a recess or any other configuration known to those skilled in
the art to releasably
retain the hinged bracket 290 in the closed position.
[0043] Like the bracket 190, the bracket 290 preferably comprises first and
second
passageways 291, 292 adapted to engage the assemblies 100, 100'. Preferably
the passageways
291, 292 comprise first and second circular portions 293, 294 and first and
second hexagonal
portions 295, 296. Preferably, after the assemblies 100, 100' are inserted
into the patient, the
bracket 290 engages the assemblies 100, 100' by closing the bracket 290 around
the assemblies
13
CA 02608714 2007-11-16
WO 2006/130133 PCT/US2005/018851
100, 100'. Preferably, like the bracket 190, when the bracket 290 engages the
assemblies 100, 100',
the hexagonal portions 295, 296 engage each respective flattened portion 134
and the circular
portions 293, 294 engage each respective center portion 130 of the assemblies
100, 100'.
[00441 A third embodiment of a bracket 390 is shown in Fig. 5d. The bracket
390
comprises a first and second openings 391, 392 respectively. Preferably, at
least two edges of the
first opening 391 and the second opening 392 respectively comprise first and
second flattened
bracket portions 395, 396. In the preferred embodiment shown in Fig. 5d, three
sides of each of the
first and second flattened bracket portions 395, 396 are generally similar to
the upper half of a
hexagon. Preferably, two opposing sides of each of the flattened bracket
portions 395, 396 are
generally parallel to each other and extend along the each side of the
openings 391, 392 in a
direction that is generally vertical when viewing Fig. 5d. The first and
second flattened bracket
portions are preferably adapted to engage each respective flattened portion
134 of the assemblies
100, 100'. Preferably, a first receiving end 393 and a second receiving end
394 are disposed on the
opposite.side of the first and second openings 391, 392 of the bracket 390
from the flattened bracket
portions 395, 396. Preferably, the bracket is engaged to the assemblies 100,
100' by inserting the
assemblies 100, 100' in the first and second receiving ends 393, 394,
respectively, and then sliding
the bracket 390 around the assemblies 100, 100' so that the first and second
flattened bracket
portions 395, 396 engage the flattened portion 134 of the assemblies 100,
100'.
[00451 A fourth embodiment of a bracket 490 is shown in Figs. 5e and 5f. The
bracket 490
comprises a first bracket portion 497 and a second bracket portion 497'.
Preferably the first and
second bracket portions 497, 497' are similar to the first and second bracket
portions 297, 297' of
Figs. 5b and 5c in that each of the first and second bracket portions 497,
497' define a portion of a
first passageway 491 and a second passageway 492. Preferably the passageways
491, 492 comprise
14
CA 02608714 2007-11-16
WO 2006/130133 PCT/US2005/018851
first and second circular portions 493, 494, first and second hexagonal
portions 495, 496 and first
and second narrowed portions 488, 489. Preferably, like the bracket 290, when
the bracket 490
engages the assemblies 100, 100', the hexagonal portions 495, 496 engage each
respective flattened
portion 134 and the circular portions 493, 494 engage each respective center
portion 130 of the
assemblies 100, 100'. Preferably, the first and second narrowed portions 488,
489 are adapted to
engage each respective proximal portion 120 of the assemblies 100, 100'.
Preferably, the first and
second narrowed portions 488, 489 engage the assemblies 100, 100' distally of
each of the
respective male threads 125 of each respective proximal portion 120 and
proximally of the central
portion 130.
[0046] Preferably, the first and second bracket portions 497, 497' are
releasably
connectable to each other via at least two tangs 484, 484' and clips 485,
485'. In the fourth
embodiment shown in Figs. 5e and 5f, the tangs 484, 484' extend outwardly from
the sides of the
first bracket portion 497 and the clips 485, 485' extend outwardly and towards
the first bracket
portion 497 from the second bracket portion 497. Preferably, the clips 485,
485' are adapted
engage the tangs 484, 484' when the bracket 490 is disposed to frictionally
engage the assemblies
100, 100'. The first bracket portion 497 is preferably disengaged from the
second bracket portion
497' by pushing the clips 485, 485' away from each other and the first bracket
portion 497.
[0047] Preferably the brackets 190, 290, 390, 490 are constructed from
silicone, a polymer
or some other suitable material. Alternatively, the brackets 190, 290, 390,
490 may be constructed
of a combination of steel, titanium or some other rigid material and silicone,
a polymer or some
other suitable semirigid material.
[0048] Referring back to Fig. 6, two catheters 200, 200' are shown in an
inserted position
in a patient 10. The insertion is preferably performed one catheter at a time,
however those skilled
CA 02608714 2007-11-16
WO 2006/130133 PCT/US2005/018851
in the art will recognize that the catheters 200, 200' may be inserted
simultaneously as well. The
insertion of a catheter 200 according to the methods disclosed herein is
performed in iterations. A
first iteration comprises inserting a first catheter 200 and assembly 100 and
a second iteration
comprises inserting a second catheter 200' and assembly 100'. For each
iteration, separate tools,
equipment and accessories to complete the insertion are preferably provided.
The separate tools,
equipment and accessories preferably comprise at least the catheter 200, a
trocar (not shown), a
suture 220 and the port assembly 100. Those skilled in the art will recognize
that more or less than
two iterations may be performed without departing from the scope of the
present invention.
[0049] For insertion, it is preferable to insert a distal end 202, 202' of the
catheters into the
patient's vessel 18, such as the patient's internal jugular vein. Insertion is
preferably performed
according to methods that are well known to those skilled in the art. After
the distal ends 202, 202'
are inserted into the vessel 18, the proximal ends 201, 201' are preferably
connected to the distal
(non pointed) end of the trocar using methods known to those skilled in the
art. Preferably, a
suture 220, 220' is attached to each of the trocars and subcutaneously
tunneled with the catheters
200, 200' from an entrance site 13, located near where the distal end 202,
202' of the catheters 200,
200' enter the vessel 18, to exit sites 14, 14'. Preferably the catheters 200,
200' are tunneled
through separate subcutaneous tunnels 16, 16'. Preferably, the sutures 220,
220' are attached to the
trocars by tying the suture around the trocar, or by some other method known
to those skilled in the
art. Preferably, as shown in Fig. 6, the catheters 200, 200' are tunneled
above the clavicle 12.
Tunneling the catheters 200, 200' above the clavicle 12 provides additional
anchoring and support,
as is well known to those skilled in the art.
[0050] Preferably, the catheters 200, 200' are subcutaneously tunneled to a
point where
the proximal ends 201, 201' of the catheters 200, 200' exit the patient at
exit sites 14, 14'.
16
CA 02608714 2007-11-16
WO 2006/130133 PCT/US2005/018851
Preferably, the exit sites 14, 14' are larger than the outer diameter of the
catheter 200, 200' but
smaller than the outer diameter of the central portion 130 of the body 101.
[0051] Referring to Figs. 4, 7 and 7a, once the catheter lumen 200 is tunneled
through the
patient 10, and a portion of the catheter lumen 200 extends from the catheter
exit site, the distal
cover 170 is slid over a proximal end 201 of the catheter lumen 200, as shown
in Fig. 4. Preferably,
after the distal cover 170 (shown in Figs 4 and 7a) is placed over the
catheter lumen 200, the
proximal end 201 of the catheter lumen 200 is disposed around the distal
portion of the body 101, as
shown in Fig. 7. The proximal end 201 of catheter lumen 200 is preferably
disposed about the
barbs 112 when disposed around the distal portion of the body 101. Although
the present
embodiment shows a distal cover 170 that is slid over the lumen 200 prior to
engaging the lumen
200 with the distal portion 110 of the assembly 100, those skilled in the art
will recognize that,
without departing from the scope of the present invention, the distal cover
170 may have some other
configuration that allows the attachment of the distal cover 170 to the
assembly at a time before or
after said attachment occurs with the present embodiment.
[0052] Referring to Fig. 7, preferably, after the proximal end 201 of the
catheter lumen
200 is slid over the distal portion 110 of the body 101, the suture 220 is
tied, and knotted in a knot
222, around the proximal end 201 of the catheter lumen 200. Preferably, the
suture 220 is tied
around the catheter lumen 200 at a position along the distal portion 110 of
the body 101 between
two barbs 112. The catheter lumen 200 is further restricted from moving
relative to the assembly
100 when the suture 220 is tightened around the catheter lumen 200, thereby
squeezing the catheter
lumen 200 around the distal portion 110 of the body 101.
[0053] Referring now to Figs. 4, 7 and 7a, preferably, after the suture 220 is
tightened
around the catheter lumen 200, the suture 220 is placed along the catheter
lumen 200 and the distal
17
CA 02608714 2007-11-16
WO 2006/130133 PCT/US2005/018851
cover 170 is slid proximally along the catheter lumen 200, over the suture
220, toward the body
101. Preferably, the distal cover 170 is slid proximally until the distal
cover 170 engages the body
101, thereby covering the proximal end 201 of the catheter lumen 200 and the
knot 202 of the
suture 200. As discussed previously, preferably, when the distal cover 170 is
slid towards the body
101, the flats 184a of the distal cover 170 engage the flat sides 115a of the
body 101.
[0054] Referring back to Fig. 6, after the assemblies 100, 100' are connected
to the
catheters 200, 200', the assemblies 100, 100' are then placed at least
partially through the exit sites
14, 14' and into subcutaneous tunnels 16, 16'. When the assemblies 100, 100'
are at least partially
disposed within the exit sites 14, 14', preferably the retaining ridge 182,
182' of each assembly 100,
100' engages the flesh of the patient 10 so that the flesh of the patient 10
heals around the retaining
ridge 182, thereby further securing the assemblies 100, 100' at least
partially within the patient 10.
[0055] Referring to Figs 6, 7 and 7a, preferably, the catheter assemblies 100,
100' are
pulled distally, back into the patient 10 until the assemblies 100, 100' are
at least partially within
the exit sites 14, 14'. Preferably, the assemblies 100, 100' at least
partially plug the exit sites 14,
14' when pulled distally back into the patient 10. This is performed by
pulling on the sutures 220,
220' at the entrance site 13. Preferably, the assemblies are pulled back into
the patient to a point
where the rounded indentation 183 is disposed at the skin level, leaving part
of the rounded
indentation 183 outside of the patient 10 and part of the rounded indentation
183 within the patient
10. Preferably, the retaining ridge 182 is disposed inside of the patient 10,
thereby allowing the
patient's 10 flesh to heal around the retaining ridge 182 and further
restricting the movement of the
assemblies 100, 100' relative to the patient 10. Preferably, the knot 222 of
the suture 220 (shown in
Fig. 7) is a first end of the sutures 220, 220' and second ends 224, 224' of
the sutures 220, 220' are
disposed near the entrance site 13 of the subcutaneous tunnels 16, 16' after
the assemblies 100, 100'
18
CA 02608714 2007-11-16
WO 2006/130133 PCT/US2005/018851
are tunneled. Preferably, after the inserting physician pulls on the second
ends 224, 224' of the
sutures 220, 220' thereby disposing the assemblies 100, 100' at least
partially within the exit sites
14, 14', the second ends 224, 224' of the sutures 220, 220' are knotted
together in a second knot
210, located near the entrance site 13. Knotting the sutures 220, 220'
together near the entrance site
13 serves to further secure the assemblies 100, 100' within the patient 10.
[00561 Preferably, after the assemblies 100, 100' are inserted into the
patient, the
assemblies 100, 100' are secured to each other using the bracket 190.
Preferably the bracket 190
engages the assemblies 100, 100' as discussed previously herein, although
those skilled in the art
will recognize that the bracket 190 may engage the assemblies 100, 100' in
other ways without
departing from the scope of the present invention.
[00571 In use, preferably the arterial assembly 100 is connected to the
arterial blood line of
an extracorporeal treatment device, such as a hemodialysis machine (not
shown). The venous
assembly 100' is then preferably connected to the venous blood line of an
extracorporeal treatment
device, such as a hemodialysis machine (not shown). During treatment, it is
preferable that blood is
withdrawn from the arterial lumen 200 and treated blood is returned to the
vessel 18 via the venous
lumen 200'. Preferably, the respective valves 150 are adapted to facilitate
the direction of blood
flow preferred for each lumen 200, 200'. Preferably, each respective valve 150
is adapted to
facilitate flow therethrough even if the assemblies 100, 100' are not
connected to the proper
extracorporeal bloodlines. In a situation involving improper connections, it
is preferable that each
respective valve 150 would allow flow at decreased levels, and that in a case
of disconnection, each
respective valve 150 would restrict the flow of blood, air or contaminants
therethrough.
[00581 It will be appreciated by those skilled in the art that changes could
be made to the
embodiments described above without departing from the broad inventive concept
thereof. It is
19
CA 02608714 2007-11-16
WO 2006/130133 PCT/US2005/018851
understood, therefore, that this invention is not limited to the particular
embodiments disclosed, but
it is intended to cover modifications within the spirit and scope of the
present invention as defined
by the appended claims.