Note: Descriptions are shown in the official language in which they were submitted.
CA 02609780 2007-11-26
DYSTAR TEXTILFARBEN GMBH & CO. DEUTSCHLAND KG 2005/D508 Dr.My
Blue disperse dyes colorfast to light at high temperatures
The present invention relates to the field of disperse dyes.
Polyester fibers for use in automotive fabrics are generally dyed blue using
dyes
of the class of the anthraquinones. However, the dyes of this type which are
used in present-day commercial practice do not fully satisfy high requirements
lo with regard to lightfastness, especially the colorfastness to light at high
temperatures. This applies in particular to combinations (known as
trichromats)
comprising yellow and red disperse dyes colorfast to light at high
temperatures,
where it is important that the individual components of the trichromat fade at
the same rate in order that there are no hue changes under the action of
light.
Anthraquinoneacridones and their use as dyes are already known from the
literature. For instance, DE239543, CH56472, CH144867, DE579326,
DE665598, US2,185,140, DE652773 describe vat dyes of this type for dyeing
cotton.
2o But anthraquinoneacridones have also already been described for dyeing
polyester fibers. See DE 1 176 775 B, but in particular W002/051942,
W002/051924, DE 1 171 101 B and DE 1 278 391 B. The latter describes a
process for dyeing and printing fiber composed of high molecular weight
polyesters with, for example, anthraquinonone-3,4-benzacridones bearing a
butyrylamino or 9-chloropropionylamino radical in position 1. The dyes do
indeed
provide strong dyeings having excellent fastness properties, but are deficient
with regard to affinity for polyester.
It is an object of the present invention to provide blue-dyeing disperse dyes
that
are superior to existing dyes with regard to colorfastness to light at high
temperatures, especially in admixtures with other dyes in a trichromat, and
also
with regard to their affinity.
CA 02609780 2007-11-26
2
We have found that this object is achieved, surprisingly, by specifically
selected
representatives from the series of the anthraquinoneacridones.
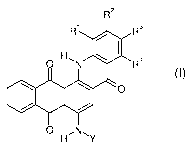
The present invention thus provides dyes of the general formula I
R2
R' R3
H, R4
O N
O
O HN, Y
(I)
where
R'to R4 are independently hydrogen, (C,-C4)-alkyl, (C,-C,)-alkoxy, CF3, NOZ,
CN,
halogen, CORS, COORS, CONR6R', S0ZR5 or SO2NR6R',
io where R5, R6 and R' are each hydrogen or (C,-C4)-alkyl, but R6 and R'
cannot both be hydrogen; and
Y is -CO(CH2)3C1 or -S02R8,
where R8 is (C,-C8)-alkyl, (C,-C$)-alkyl, substituted by NO2, CN, halogen or
phenyl, phenyl, phenyl substituted by one or more substituents selected
from (C,-C4)-alkyl, (C,-C4)-alkoxy, CF3, NO2, CN, halogen, CORS, COORS,
CONR6R7, S02R5 and S02NR6R', or is naphthyl or naphthyl substituted by
one or more substituents selected from
(C,-C4)-alkyl, (C,-C4)-alkoxy, CF3, NOz, CN, halogen, COR5, COORS,
CONR6R7, S02R5 and S02NR6R',
although R8 cannot be 4-methylphenyl when R' to R4 are all hydrogen and cannot
be phenyl or 4-methylphenyl when R' and R3 are both chlorine and R2 and R4 are
both hydrogen.
(C,-C4)-Alkyl R' to R' may each be linear or branched and are for example
methyl, ethyl, n-propyl, i-propyl, n-butyl, i-butyl, sec-butyl or tert-butyl.
(C,-Cg)-
Alkyl R$ may additionally be selected from pentyl, hexyl, heptyl and octyl.
Methyl and ethyl are particularly preferred alkyl. The same holds mutatis
CA 02609780 2007-11-26
3
mutandis for (C,-C4)-alkoxy groups, for which methoxy and ethoxy are
accordingly particularly preferred.
Halogen is for example fluorine, chlorine or bromine, with chlorine and
bromine
being preferred.
R' to R4 are each preferably hydrogen,
Examples of R8 are particularly ethyl, n-propyl, i-propyi, n-butyl, 1 -
naphthyl,
2-naphthyl, phenyl, 4-methylphenyl, 4-chlorophenyl, 2-bromophenyl, 4-
bromophenyl, 2-nitrophenyl, 3-nitrophenyl, 4-nitrophenyl, phenylmethyl, 4-
to chloro-3-nitrophenyl, 3-trifluoromethyiphenyl, 3,4-dimethoxyphenyl and 4-
methoxyphenyl.
The present invention's dyes of the general formula I may be utilized together
with one or more dyes of the kind typically used for dyeing polyesters fibers
or
polyester textile materials for automotive fabrics.
The present invention accordingly also provides dye mixtures comprising at
least
one dye of the general formula I and at least one dye useful for dyeing
polyester
textile materials for automotive fabrics.
2o Dyes useful for dyeing polyester textile materials for automotive fabrics
are in
particular azo, disazo, anthraquinone, nitro and naphthalimide dyes, which
will be
well known to those skilled in the art.
Preferred yellow and orange dyes of this kind are for example the Colour Index
listings C.I. Disperse Yellow 23, 42, 51, 59, 65, 71, 86, 108, 122, 163, 182
and 211, C.I. Solvent Yellow 163, C.I. Disperse Orange 29, 30, 32, 41, 44, 45,
61 and 73, C.I. Pigment Orange 70, C.I. Solvent Brown 53, and also dyes of the
formulae II and III
CI
OZN ~OH
~ ~N ~ -
C! N ~ ~ N
(II)
CA 02609780 2007-11-26
4
R Ri0
E
N
-1 R11
N _
S
N-H R1z
\ OR 13
N/ O
(III)
where
R9 to R12 are independently hydrogen, chlorine, methyl, ethyl, isopropyl, tert-
butyl, cyclohexyl, methoxy, ethoxy, n-propoxy, n-butoxy, methoxyethyl,
ethoxyethyl, butoxyethyl or phenoxy, and
R13 is methyl, ethyl, propyl, isopropyl, allyl, n-butyl, isobutyl, n- and
isopentyl, hexyl, octyl, 2-ethylhexyl, methoxyethyl, ethoxyethyl,
butoxyethyl, butoxyethoxyethyl.
Preferred red dyes of this kind are for example the Colour Index listings C.I.
Disperse Red 60, 82, 86, 91, 92, 127, 134, 138, 159, 167, 191, 202, 258,
279, 284, 302 and 323, C.I. Solvent Red 176, and also dyes of the formulae IV,
V and V!
CN
CN
j \
O2N N~ ,R 15
- N N
N H
R14 N
H (IV)
CN
CN
~ ~ N.\ /Rn
NC S N ~ N
N H
R16 N
H (V)
CA 02609780 2007-11-26
0
O H~N~R1s
Rzo
I \
/
o S
(R19
(VI)
where
R14 and R15 are independently hydroxyethoxyethyl or phenyl,
R 16 and R" are independently hydrogen, hydroxyethoxyethyl,
5 hydroxybutoxypropyl, acetoxyethoxyethyl or acetoxybutoxypropyl,
R'$ is (C,-C8)-alkyl, phenyl or phenyl substituted by (C,-C4)-alkyl, hydroxyl
or
halogen, and
R19 and R20 are independently hydrogen or halogen, and also
n is 0, 1 or 2.
Preferred blue and violet dyes of this kind are for example the Colour Index
listings C.I. Blue 27, 54, 56, 60, 73, 77, 79, 79:1, 87, 266, 333 and 361,
C.I.
Disperse Violet 27, 28, 57 and 95 and also the dyes of the formula VII
0
H,
23 0 N (Rz1)v
(R
O
0 HN
'R22 /m ( V I I )
where
R21, R22 and R23 are independently (C,-C$)-alkyl, halogen or hydroxyl, and
m, o and p are independently 0, 1 or 2.
In the dye mixtures of the present invention, the fractions of dye or dyes of
the
general formula I and of dye or dyes useful for dyeing polyester textile
materials
for automotive fabrics depend solely on the hue to be achieved, and thus may
vary within wide limits. In general, the amounts of dye or dyes of the general
formula I range from 1 % to 99% by weight and the amounts of dye or dyes
CA 02609780 2007-11-26
6
useful for dyeing polyester textile materials for automotive fabrics from 99%
to
1 % by weight.
The present invention's dyes of the general formula I are obtainable in a
conventional manner.
For instance, they are obtainable by reacting a compound of the general
formula
VIII
O NH2
I / O
O HN Ra
R~ Rs
RZ (VIII)
where R' to R4 are each as defined above, with a compound of the general
io formula IX
Hal-Y (IX)
where Hal is halogen, particularly chlorine, and Y is as defined above.
This reaction can be carried out with or without the assistance of acid-
binding
agents familiar to one skilled in the art.
The compounds of the general formula VIII are obtainable for example by
reacting bromamine acid of the formula X
O NHZ
I \ I \ S03H
O Br (X)
with a substituted anthranilic acid of the general formula XI
R4
HOOC \ R3
1
H2N RZ
R~ (XI)
where R' to R4 are each as defined above, to form the compound of the general
CA 02609780 2007-11-26
7
formula XII
O NH2
S03H
COOH
0 HINI Ra
R' R3
R2 (XII)
and cyclizing the latter by means of chlorosulfonic acid to form the compound
of
the general formula (XI11)
O NH2
S03H
O
O HN Ra
R~ Rs
s Rz (XIII)
Finally, the sulfonic acid group is removed, for example with sodium
dithionite.
The reaction of bromamine acid of the formula X with a substituted anthranilic
acid of the general formula X1 preferably takes place in the presence of
copper
to powder and of a base under otherwise well-known reaction conditions. The
other
reaction steps mentioned are also carried out under well-known reaction
conditions.
The present invention's dyes and dye mixtures are very useful for dyeing and
15 printing hydrophobic synthetic materials, the dyeings and prints obtained
having
a remarkably high lightfastness and colorfastness to light at high
temperatures,
so that the textiles thus dyed can be used for automotive interiors.
The dyes of the present invention particularly exhibit a better build-up
performance than the dyes of W002/051942 and are also superior to those dyes
20 in terms of colorfastness in pale shades when exposed to light at high
temperatures.
CA 02609780 2007-11-26
8
The present invention thus also provides for the use of dyes of the general
formula I
R2
R1 g Rs
O H,N R4
~ I O H~N, Y
(I)
where
R'to R4 are independently hydrogen, (C,-C4)-alkyl, (C,-C,)-alkoxy, CF3, NO2,
CN,
halogen, CORS, COOR5, CONR6R7, S02R5 or SO2NR6R',
where R5, R6 and R' are each hydrogen or (C,-C4)-alkyl, but R6 and R'
cannot both be hydrogen; and
Y is -CO(CH2)3C1 or -S02R8,
where R8 is (C,-C8)-alkyl, (C,-C81-alkyl, substituted by NO2, CN, halogen or
phenyl, phenyl, phenyl substituted by one or more substituents selected
from (C,-C4)-alkyl, (C,-C4)-alkoxy, CF3, NO2, CN, halogen, CORS, COORS,
CONR6R7, S02R5 and S02NR6R', or is naphthyl or naphthyl substituted by
one or more substituents selected from
(C,-C4)-alkyl, (C,-C4)-alkoxy, CF3, NO2, CN, halogen, COR5, COORS,
CONR6R7, S02R5 and S02NR6R7
,
for dyeing and printing hydrophobic synthetic materials.
Useful hydrophobic synthetic materials include for example secondary cellulose
2o acetate, cellulose triacetate, polyamides and, in particular, high
molecular weight
polyesters. Materials composed of high molecular weight polyesters are in
particular those based on polyethylene glycol terephthalates.
The hydrophobic synthetic materials can be present in the form of sheet- or
threadlike constructions and can have been processed, for example, into yarns
or
into woven or knitted textile materials Fibrous textile materials are
preferred.
Polyester fibers and polyester textile materials for automotive fabrics are
very
particularly preferred.
CA 02609780 2007-11-26
9
A preferred embodiment of the use according to the present invention comprises
utilizing dye mixtures comprising at least one dye of the general formula I
and at
least one dye useful for dyeing polyester fibers and polyester textile
materials for
automotive fabrics.
The dyeing in accordance with the use provided by the present invention can be
carried out in a conventional manner, preferably from an aqueous dispersion,
if
appropriate in the presence of carriers, at between 80 to about 110 C by the
io exhaust process or by the HT process in a dyeing autoclave at 110 to 140 C,
and also by the so-called thermofix process, in which the fabric is padded
with
the dyeing liquor and subsequently fixed/set at about 180 to 230 C.
Printing of the materials mentioned can be carried out in a manner known per
se
by incorporating the dye or dye mixtures of the present invention in a print
paste
and treating the fabric printed therewith at temperatures between 180 to 230 C
with HT steam, high-pressure steam or dry heat, if appropriate in the presence
of
a carrier, to fix the dye.
2o The dyes and dye mixtures of the present invention are very particularly
useful
for dyeing and printing polyester fibers and polyester textile materials for
automotive fabrics. It is preferable for the dyeing and printing to be carried
out in
the presence of UV absorbers, for example UV absorbers based on
benzophenone or benzotriazole. Details concerning the dyeing and printing of
automotive fabrics are known to those skilled in the art and are described in
the
pertinent literature.
In addition, however, the dyes and dye mixtures of the present invention can
also be used for dyeing and printing hydrophobic synthetic materials envisaged
for other purposes, examples being alkalized polyester fibers, polyester
microfibers or materials that are not in fiber form.
CA 02609780 2007-11-26
The dyes and dye mixtures of the present invention shall be in a very fine
state
of subdivision when they are used in dyeing liquors, padding liquors or print
pastes.
The dyes are converted into the fine state of subdivision in a conventional
5 manner by slurrying the as-fabricated dye together with dispersants in a
liquid
medium, preferably in water, and subjecting the mixture to the action of
shearing
forces to mechanically comminute the original dye particles to such an extent
that an optimal specific surface area is achieved and sedimentation of the dye
is
minimized. This is accomplished in suitable mills, such as ball or sand mills.
The
lo particle size of the dyes is generally between 0.5 and 5,um and preferably
equal
to about 1 /im.
The dispersants used in the milling operation can be nonionic or anionic.
Nonionic dispersants include for example reaction products of alkylene oxides,
for example ethylene oxide or propylene oxide, with alkylatable compounds, for
1s example fatty alcohols, fatty amines, fatty acids, phenols, alkylphenols
and
carboxamides. Anionic dispersants are for example lignosulfonates, alkyl- or
alkylarylsulfonates or alkylaryl polyglycol ether sulfates.
The dye preparations thus obtained should be pourable for most applications.
2o Accordingly, the dye and dispersant content is limited in these cases. In
general,
the dispersions are adjusted to a dye content up to 50 percent by weight and a
dispersant content up to about 25 percent by weight. For economic reasons, dye
contents are in most cases not allowed to be below 15 percent by weight.
The dispersions may also contain still further auxiliaries, for example those
which
25 act as an oxidizing agent, for example sodium m-nitrobenzenesulfonate, or
fungicidal agents, for example sodium o-phenylphenoxide and sodium
pentachlorophenoxide, and particularly so-called "acid donors", examples being
butyrolactone, monochloroacetamide, sodium chloroacetate, sodium
dichloroacetate, the sodium salt of 3-chloropropionic acid, monosulfate esters
30 such as lauryl sulfate for example, and also sulfuric esters of ethoxylated
and
propoxylated alcohols, for example butylglycol sulfate.
CA 02609780 2007-11-26
11
The dye dispersions thus obtained are very advantageous for making up dyeing
liquors and print pastes.
There are certain fields of use where powder formulations are preferred. These
powders comprise the dye or dye mixture, dispersants and other auxiliaries,
for
example wetting, oxidizing, preserving and dustproofing agents and the
abovementioned "acid donors".
A preferred method of making pulverulent preparations of dye consists in
stripping the above-described liquid dye dispersions of their liquid, for
example
io by vacuum drying, freeze drying, by drying on drum dryers, but preferably
by
spray drying.
The dyeing liquors are made by diluting the requisite amounts of the above-
described dye formulations with the dyeing medium, preferably water, such that
a liquor ratio of 5:1 to 50:1 is obtained for dyeing. In addition, it is
generally
customary to include further dyeing auxiliaries, such as dispersing, wetting
and
fixing auxiliaries, in the liquors. Organic and inorganic acids such as acetic
acid,
succinic acid, boric acid or phosphoric acid are included to set a pH in the
range
from 4 to 5, preferably 4.5. It is advantageous to buffer the pH setting and
to
2o add a sufficient amount of a buffering system. The acetic acid/sodium
acetate
system is an example of an advantageous buffering system.
To use the dye or dye mixture in textile printing, the requisite amounts of
the
abovementioned dye formulations are kneaded in a conventional manner together
with thickeners, for example alkali metal alginates or the like, and if
appropriate
further additives, for example fixation accelerants, wetting agents and
oxidizing
agents, to give print pastes.
CA 02609780 2007-11-26
12
Example 1
8 parts of 6-aminoanthraquinone-2,1-acridone (general formula VIII where R'-R4
= hydrogen) are suspended in 100 parts of chlorobenzene. 4.5 parts of 4-
chlorobutyryl chloride are added dropwise at 80-100 C before stirring for 2 h
under reflux, cooling down to room temperature, filtering off with suction and
washing with chlorobenzene and then with methanol to obtain 10 parts of the
dye of the formula Ia
O H, N
C O 0 N
H' y-,~CI
O
Example 2
to 50 parts of 6-aminoanthraquinone-2,1-acridone (general formula VIII where
R'-R4
= hydrogen) are introduced as initial charge in 664 parts of chlorobenzene. 16
parts of pyridine and 23 parts of methanesulfonyl chloride are added at 100 C.
The mixture is stirred at 125 C for 15 h and is then admixed with a further
3.2
parts of pyridine and 4.6 parts of methanesulfonyl chloride. It is stirred at
125 C
for a further 12 h and then cooled down to room temperature. The product is
filtered off with suction and washed with chlorobenzene and then with methanol
to obtain 49 parts of the dye of the formula lb
H~
O N
I \ I \ O
O N ~S O
H
0
Example 2 is repeated to obtain the dyes in the table hereinbelow.
CA 02609780 2007-11-26
13
O H~N
~ ~ O
~ / ~ /
0 N O
H iS~R5
O
Example R5
3 Et
4 n-Pr
i-Pr
6 n-Bu
7 1-naphthyl
8 2-naphthyl
9 phenyl
4-methylphenyl
11 4-chlorophenyl
12 2-bromophenyl
13 4-bromophenyl
14 2-nitrophenyl
3-nitrophenyl
16 4-nitrophenyl
17 phenylmethyl
18 4-chloro-3-nitrophenyl
19 3-trifluoromethylphenyl
3,4-dimethoxyphenyl
21 4-methoxyphenyl
Example 22
5 30 g of the water-moist presscake of the dye obtained according to Example 2
in
200 ml of water are admixed with 63 g of sodium lignosulfonate and 3 g of a
nonionic dispersant (addition product of abietic acid and 50 mol equivalents
of
CA 02609780 2007-11-26
14
ethylene oxide) and adjusted to pH 7 with 25% sulfuric acid. This is followed
by
bead milling at room temperature for 1 h to 90% < 1,um, sieving and drying in
a
spray dryer. 2 g of the powder thus obtained are dispersed in 1000 g of water.
The dispersion is admixed with 0.5 to 2 g per I of liquor of a commercially
available dispersant based on a condensation product of naphthalenesulfonic
acid
sodium salt and formaldehyde, 0.5 to 2 g per I of liquor of monosodium
phosphate and 2 g per I of liquor of a commercially available leveling
assistant
and adjusted to pH 4.5-5.5 with acetic acid. The dyeing liquor thus obtained
is
entered with 100 g of a woven fabric of textured polyester based on
lo polyethylene glycol terephthalate before dyeing at 130 C for 60 min. The
blue
dyeing obtained after reduction clearing possesses excellent lightfastness and
colorfastness to light at high temperatures and very good fastness to
sublimation.
Repeating this example with the dyes of Examples 1 and 3-21 likewise gives
blue dyeings of excellent colorfastness to light at high temperatures.
Example 23
0.176 g of the dye of the formula 1 a of Example 1 are dissolved in 10 ml of
2o DMF with heating, and the solution is admixed with 1 ml of conc. Levegal
DLP
(commercial product of Lanxess Deutschland GmbH) and also 290 ml of water.
While stirring, 0.318 g of the dye C.I. Disperse Yellow 71 (as 33.7% strength
finished material) and 0.300 g of the dye C.I. Disperse Red 86 (as 34.9%
strength finished material) are added. The pH is set to 4.5 with acetic
acid/sodium acetate and 1 g of Levegal DLP are added per 1 I of this liquor.
A volume containing 0.005 g of dye of the formula Ia of Example 1 is taken
from
this stock solution, made up to 100 ml with water and entered with 5 g of pile
polyester fabric. Dyeing is carried out at 135 C for 45 min using a heating-up
rate of 1 degree/min. Cooling is followed by hot and cold rinsing. The gray
3o dyeing obtained after reduction clearing possesses excellent colorfastness
to
light at high temperatures.
Repeating this dyeing in the presence of 0.100 g of a UV absorber based on
CA 02609780 2007-11-26
phenyltriazines or benzotriazoles likewise gives gray dyeings of excellent
colorfastness to light at high temperatures, with the colorfastness to light
at high
temperatures being somewhat higher in the case of the benzotriazole than
without use of a UV absorber.
5
Example 24
0.150 g of the dye of Example 9 and 0.150 g of the dye of Example 10 are
dissolved in 10 ml of DMF with heating, and the solution is admixed with 1 ml
of
conc. Levegal DLP and also 290 ml of water. While stirring, 0.388 g of the dye
io C.I. Disperse Yellow 71 (as 5.3% strength finished material), 0.388 g of
the dye
C.I. Solvent Yellow 163 (as 24.6% strength finished material) and 0.185 g of a
dye of the formula IV where R14 = phenyl and R15 = hydroxyethoxyethyl in the
form of the isomeric mixture with Rt4 and R15 (as 23.4% strength finished
material) are added. The pH is set to 4.5 with acetic acid/sodium acetate and
1 g
15 of Levegal DLP are added per 1 I of this liquor.
A volume containing 0.00425 g of dye of Example 9 is taken from this stock
solution, made up to 100 ml with water and entered with 5 g of pile polyester
fabric. Dyeing is carried out at 135 C for 45 min using a heating-up rate of
1 degree/min. Cooling is followed by hot and cold rinsing. The gray dyeing
obtained after reduction clearing possesses excellent colorfastness to light
at
high temperatures.
Repeating this dyeing in the presence of 0.150 g of a UV absorber based on
phenyltriazines or of 0.100 g of a UV absorber based on benzotriazoles
likewise
gives gray dyeings of excellent colorfastness to light at high temperatures.
In
both cases, the colorfastness to light at high temperatures is somewhat higher
than without use of a UV absorber, and with the phenyltriazine fading on tone
is
achieved in that the hue of the shade does not change in the course of fading.