Note: Descriptions are shown in the official language in which they were submitted.
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
OCT USING SPECTRALLY RESOLVED BANDWIDTH
FIELD OF THE INVENTICN
The present invention is related to a system for
optical coherence tomographic imaging of turbid (i.e.,
scattering) materials utilizing multiple channels of
information. The multiple channels of information may be
comprised and encompass spatial, angle, spectral and
polarization domains. More specifically, the present
invention is related to methods and apparatus utilizing
optical sources, systems or receivers capable of providing
(source), processing (system) or recording (receiver) a
multiplicity of channels of spectral information for optical
coherence tomographic imaging of turbid materials. In these
methods and apparatus the multiplicity of channels of
spectral information that can be provided by the source,
processed by the system, or recorded by the receiver are used
to convey simultaneously spatial, spectral or polarimetric
information relating to the turbid material being imaged
tomographically.
The multichannel optical coherence tomographic
methods can be incorporated into an endoscopic probe for
imaging a patient. The endoscope comprises an optical fiber
array and can comprise a plurality of optical fibers adapted
to be disposed in the patient. The optical fiber array
transmits the light from the light source into the patient,
and transmits the light reflected by the patient out of the
patient. The plurality of optical fibers in the array are in
optical communication with the light source. The multichannel
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-2-
optical coherence tomography system comprises a detector for
receiving the light from the array and analyzing the light.
The methods and apparatus may be applied for imaging a
vessel, biliary, GU and/or GI tract of a patient.
BACKGROUND OF THE INVENTION
Myocardial infarction or heart attack remains the
leading cause of death in our society. Unfortunately, most
of us can identify a family member or close friend that has
suffered from a myocardial infarction. Until recently many
investigators believed that coronary arteries critically
blocked with atherosclerotic plaque that subsequently
progressed to total occlusion was the primary mechanism for
myocardial infarction. Recent evidence from many
investigational studies, however, clearly indicate that most
infarctions are due to sudden rupture of non -critically
stenosed coronary arteries due to sudden plaque rupture. For
example, Little and coworkers (Little, WC, Downes, TR,
Applegate, RJ. The underlying coronary lesion in myocardial
infarction: implications for coronary angiography. Clin
Cardiol 1991; 14: 868-874, incorporated by reference'herein)
observed that approximately 70% of patients suffering from an
acute plaque rupture were initiated on plaques that were less
than 50% occluded as revealed by previous coronary
angiography. This and similar observations have been
confirmed by other investigators (Nissen, S. Coronary
angiography and intravascular ultrasound. Am J Cardiol 2001;
87 (suppl): 15A - 20A, incorporated by reference herein).
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-3-
The development of technologies to identify these
unstable plaques holds the potential to decrease
substantially the incidence of acute coronary syndromes that
often lead to premature death. Unfortunately, no methods are
currently available to the cardiologist that may be applied
to specify which coronary plaques are vulnerable and thus
prone to rupture. Although treadmill testing has been used
for decades to identify patients at greater cardiovascular
risk, this approach does not have the specificity to
differentiate between stable and vulnerable plaques that are
prone to rupture and frequently result in myocardial
infarction. Inasmuch as a great deal of information exists
regarding the pathology of unstable plaques (determined at
autopsy) technologies based upon identifying the well
described pathologic appearance of the vulnerable plaque
offers a promising long term strategy to solve this problem.
The unstable plaque was first identified and
characterized by pathologists in the early 1980's. Davis and
coworkers noted that with the reconstruction of serial
histological sections in patients with acute myocardial
infarctions associated with death, a rupture or fissuring of
atheromatous plaque was evident (Davis MJ, Thomas AC. Plaque
fissuring: the cause of acute myocardial infarction, sudden
death, and crescendo angina. Br Heart J 1985; 53: 363-373,
incorporated by reference herein). Ulcerated plaques were
further characterized as having a thin fibrous cap, increased
macrophages with decreased smooth muscle cells and an
increased lipid core when compared to non-ulcerated
atherosclerotic plaques in human aortas (Davis MJ, Richardson
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-4-
PD, Woolf N, Katz DR, Mann J. Risk of thrombosis in human
atherosclerotic plaques: role of extracellular lipid,
macrophage, and smooth muscle cell content, incorporated by
reference herein). Furthermore, no correlation in size of
lipid pool and percent stenosis was observed when imaging by
coronary angiography. In fact, most cardiologists agree that
unstable plaques progress to more stenotic yet stable plaques
through progression via rupture with the formation of a mural
thrombus and plaque remodeling, but without complete luminal
occlusion (Topol EJ, Rabbaic R. Strategies to achieve
coronary arterial plaque stabilization. Cardiovasc Res 1999;
41: 402-417, incorporated by reference herein). Neo-
vascularization with intra-plaque hemorrhage may also play a
role in this progression from small lesions (<50% occluded)
to larger significant plaques. Yet, if the unique features
of unstable plaque could be recognized by the cardiologist
and then stabilized, a dramatic decrease may be realized in
both acute myocardial infarction and unstable angina
syndromes, and in the sudden progression of coronary artery
disease.
The present invention uses depth-resolved light
reflection or Optical Coherence Tomography (OCT) to identify
the pathological features that have been identified in the
vulnerable plaque. In OCT, light from a broad band light
source or tunable laser source is input into an
interferometer with a portion of light directed to the vessel
wall and the other portion directed to a reference surface.
The distal end of the optical fiber is interfaced with a
catheter for interrogation of the coronary artery during a
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-5-
heart catheterization procedure. The reflected light from
the plaque is recombined with the signal from the reference
surface forming interference fringes (measured by an
photovoltaic detector) allowing precise depth-resolved
imaging of the plaque on a micron scale.
OCT uses narrow linewidth tunable laser source or
a superluminescent diode source emitting light over a broad
bandwidth (distribution of wave length) to make in situ
tomographic images with axial resolution of 10-20 pm and
tissue penetration of 2-3 mm. OCT has the potential to image
tissues at the level of a single cell. In fact, the
inventors have recently utilized broader band width optical
sources such as femto-second pulsed lasers, so that axial
resolution is improved to 4 microns or less. With such
resolution, OCT can be applied to visualize intimal caps,
their thickness, and details of structure including fissures,
the size and extent of the underlying lipid pool and the
presence of inflammatory cells. Moreover, near infrared
light sources used in OCT instrumentation can penetrate into
heavily calcified tissue regions characteristic of advanced
coronary artery disease. With cellular resolution,
application of OCT may be used to identify other details of
the vulnerable plaque such as infiltration of monocytes and
macrophages. In short, application of OCT can provide
detailed images of a pathologic specimen without cutting or
disturbing the tissue.
One concern regarding application of this
technology to image atherosclerotic plaques within the
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-6-
arterial lumen is the strong scattering of light due to the
presence of red blood cells. Once a catheter system is
positioned in a coronary artery, the blood flow between the
OCT optical fiber and artery can obscure light penetration
into the vessel wall. One proposed solution is the use of
saline flushes. Saline use is limited in duration, however,
since myocardial ischemia eventually occurs in the distal
myocardium. The inventors have proposed the use of
artificial hemoglobin in the place of saline. Artificial
hemoglobin is non-particulate and therefore does not scatter
light. Moreover, artificial hemoglobin is about to be
approved by the United States Food and Drug Administration as
a blood substitute and can carry oxygen necessary to prevent
myocardial ischemia. Recently, the inventors demonstrated
the viability of using artificial hemoglobin to reduce light
scattering by blood in mouse myocardium coronary arteries
(Villard JW, Feldman MD, Kim Jeehyun, Milner TE, Freeman GL.
Use of a blood substitute to determine instantaneous murine
right ventricular thickening with optical coherence
tomography. Circulation 2002; Volume 105: Pages 1843-1849,
incorporated by reference herein).
The first prototype of an OCT catheter to image
coronary plaques has been built and is currently being tested
by investigators in Boston at Harvard - MIT (Jang IK, Bouma
BE, Kang DH, et al. Visualization of coronary
atherosclerotic plaques in patients using optical coherence
tomography: comparison with intravascular ultrasound. JACC
2002; 39: 604-609, incorporated by reference herein) in
association with Light Lab Co. The prototype catheter
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-7-
consists of a single light source and is able to image over
a 360 degree arc of a coronary arterial lumen by rotating a
shaft that spins the optical fiber. Because the rotating
shaft is housed outside of the body, the spinning rod in the
catheter must rotate with uniform angular velocity so that
the light can be focused for equal intervals of time on each
angular segment of the coronary artery. Mechanical drag in
the rotating shaft can produce significant distortion and
artifacts in recorded OCT images of the coronary artery.
Unfortunately, because the catheter will always be forced to
make several bends between the entry point in the femoral
artery to the coronary artery (e.g., the 180 degree turn
around the aortic arch), uneven mechanical drag will result
in OCT image artifacts. As the application of OCT is shifted
from imaging gross anatomical structures of the coronary
artery to its capability to image at the level of a single
cell, non-uniform rotation of the single fiber OCT prototype
will become an increasingly problematic source of distortion
and image artifact.
Essentially, current endoscope type single channel
OCT systems developed by Light Lab Co. suffers by
non-constant rotating speed that forms irregular images of a
vessel target. See U.S. Patent 6,134,003, incorporated by
reference herein. Their approach of a rotary shaft to spin
a single mode fiber is prone to produce artifact. The
catheter will always be forced to make several bends from its
entry in the femoral artery, to the 180 degree turn around
the aortic arch, to its final destination in the coronary
artery. All these bends will cause uneven friction on the
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-8-
rotary shaft, and uneven time distribution of the light on
the entire 360 degree arch of the coronary artery. As the
application of OCT is shifted from gross anatomical
structures of the coronary artery to its capability to image
at the level of a single cell, then non-uniform rotation of
the single fiber OCT will become even a greater source of
greater artifact.
The present invention solves rotational distortion
and related artifactual problems by developing a multiphase
array OCT catheter. By incorporating 10-60 individual OCT
fibers within a single catheter, rotation of the optical
fiber or similar element (e.g., micro-motor driven mirror)
and associated image distortion and artifacts are eliminated
and spatial resolution may be improved. The catheter will
allow 10-60 individual sources of light to independently
image the 360 degree arc of the coronary arterial lumen. An
additional advantage of the multiphase array is provision of
greater spatial resolution of the object being interrogated
in comparison to single fiber designs. Many investigators
recognize that a single rotating fiber or micro-motor driven
mirrors utilized in current designs will not allow imaging at
the level of a single cell while the multiphase array
approach can provide cellular resolution.
The construction of a multiphase array OCT catheter
requires resolution of a number of problems using innovative
design solutions. Successful design and demonstration of the
catheter requires the development of an optical channel
containing 10-60 individual fibers in a 1.5 mm diameter.
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-9-
Each fiber requires a lens to focus the light, and a mirror
fabricated using nanotechnology to redirect light from each
fiber by 90 degrees from the catheter to the luminal surface
of the coronary artery. Further, each of the 10-60 light
paths has to be split again for both reference and artery
paths. The present invention provides design solutions to
both the catheter and multichannel interferometer.
SUMMARY OF THE INVENTION
The present invention pertains to an endoscope for
a patient. The endoscope comprises a light producing means,
such as a light source. The endoscope comprises an optical
fiber array comprising a plurality of optical fibers adapted
to be disposed in the patient. The optical fiber array
transmits the light from the light producing means into the
patient, and transmits the light reflected by the patient out
of the patient. The plurality of the optical fibers of the
array in optical communication with the light producing
means. The endoscope comprises a detector for receiving the
light from the array and analyzing the light. The plurality
of the optical fibers of the array in optical communication
with the detector. I
The present invention pertains to a method for
imaging a patient. The method comprises the steps of
transmitting light from a light source into an optical fiber
array comprising a plurality of optical fibers in the
patient. There is the step of transmitting the light
reflected by the patient out of the patient. There is the
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-10-
step of receiving the light from the array at a detector.
There is the step of analyzing the light with the detector.
The present invention pertains to an apparatus for
studying an object. The apparatus comprises means for
producing light. The apparatus comprises means.for analyzing
the light that has reflected from the object based on
polarization, space, position or angle.
The present invention pertains to an apparatus for
studying an object. The apparatus comprises means for
producing light. The apparatus comprises means for analyzing
the light that has reflected from the object based on
polarization.
The present invention pertains to an apparatus for
studying an object. The apparatus comprises means for
producing light. The apparatus comprises means for analyzing
the light that has reflected from the object based on space.
The present invention pertains to an apparatus for
studying an object. The apparatus comprises means for
producing light. The apparatus comprises means for analyzing
the light that has reflected from the object based on angle.
The present invention pertains to a method for
studying an object. The method comprises the steps of
producing light. The method comprises the steps of analyzing
the light that has reflected from the object based on
polarization, space, position or angle.
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-11-
The present invention pertains to a method for
studying an object. The method comprises the steps of
producing light. The method comprises the steps of analyzing
the light that has reflected from the object based on
polarization.
The present invention pertains to a method for
studying an object. The method comprises the steps of
producing light. The method comprises the steps of analyzing
the light that has reflected from the object based on space.
The present invention pertains to a method for
studying an object. The method comprises the steps of
producing light. The method comprises the steps of analyzing
the light,that has reflected from the object based on angle.
BRIEF DESCRIPTION OF THE DRAWINGS
In the accompanying drawings, the preferred
embodiment of the invention and preferred methods of
practicing the invention are illustrated in which:
Figure 1 is a schematic representation of an
overview of the present invention.
Figure 2 is a top view of an input arm (light
source) of the present invention.
Figure 3 is a schematic representation of a side
view of the input arm (light source).
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-12-
Figure 4 is a schematic representation of a fiber
based solution for the input arm.
Figure 5 is a schematic representation of a side
view of the sample arm.
Figure 6 is a schematic representation of an axial
view of the sample arm.
Figure 7 is a schematic representation of a top
view of an axicon lens.
Figure 8 is a schematic representation of an
optical fiber array of the sample arm.
Figure 9 is a schematic representation of a
perspective view of a probe tip of the sample arm emphasing
the mirrors to refocus the light on the tissue of interest.
Figure 10 is a schematic representation of a side'
view of a groove of the tip with an attached fiber ending
with a 45 angled mirror (reflection).
Figure 11 is a schematic representation of a top
view of the tip with an attached fiber.
Figure 12 is a schematic representation of a first
step of manufacture of each fiber lens of the sample arm.
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-13-
Figure 13 is a schematic representation of a second
step in the manufacture of each fiber lens of the sample arm.
Figure 14 is a schematic representation of a
reference arm of the present invention.
Figure 15 is a schematic representation of a top
view of a detection arm of the present invention.
Figure 16 is a schematic representation of a side
view of the detection arm.
Figure 17 is an alternative schematic
representation of a scanning probe of the sample arm.
Figures 18a and 18b are schematic representations
of an hydraulic mechanism.
Figures 19a and 19b are schematic representations
of exploded views of the hydraulic mechanism.
Figures 20a-20d are schematic representations of
different views of a twisted shaft of the hydraulic
mechanism.
Figures 21a and 21b are schematic representations
of the fiber-shaft holder.
Figures 22a-22c are schematic representations of
fiber grooves.
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-14-
Figure 23 is a side view of the micro-mirror.
Figure 24 is a perspective view of the
micro-mirror.
Figure 25 is a perspective view of the micro-mirror
with a portion irradiated by the laser beam.
Figure 26 is a perspective view of the micro-mirror
having a deformation generated from being irradiated by a
laser beam as shown in figure 25.
Figure 27 is a schematic representation of the
micro-mirror being continuously heated by a laser beam
shining on different locations of the micro-mirror.
Figure 28 is a schematic representation of the
resulting changing of the tilting direction of the
micro-mirror because of the changing location of the laser
beam on the micro-mirror.
Figure 29 is a schematic representation of the
micro-mirror in the probe cover relative to the fibers.
Figure 30 is a schematic representation of the
micro-mirror movement relative to the fiber.
Figure 31 is a schematic diagram of single channel
fiber-based polarization sensitive spectral domain optical
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-15-
coherence tomography with a fiber optic spectral polarimetry
instrument (FOSPI).
Figure 32 is a schematic representation of a
fiber-based spatially multiplexed swept source optical
coherence tomography.
Figure 33 is a schematic representation of a
multi-fiber angle-domain OCT.
Figures 34 and 35 are images recorded with a
spatially multiplexed OCT system.
Figures 36 and 37 are phase retardation due to
birefringence and fast-axis angle, respectively.
DETAZLE,D DESCRIPTION
Referring now to the drawings wherein like
reference numerals refer to similar or identical parts
throughout the several views, and more specifically to
figures 1-5, 15 and 16 thereof, there is shown an endoscope
for a patient. The endoscope 10 comprises means 102 for
producing light, such as a light source 51. The endoscope 10
comprises an optical fiber array 28 comprising a plurality of
optical fibers 8 adapted to be disposed in the patient. The
optical fiber array 28 transmits the light from the producing
means, preferably including a light source 51, into the
patient, and transmits the light reflected by the patient out
of the patient. The plurality of the optical fibers 8 of the
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-16-
array 28 is in optical communication with the light producing
means 102. The endoscope 10 comprises a detector D for
receiving the light from the array 28 and analyzing the
light. The plurality of the optical fibers 8 of the array 28
is in optical communication with the detector D.
Preferably, the endoscope 10 includes a tube 53
about which the plurality of optical fibers 8 are disposed.
The tube 53 preferably has grooves 54 that extend
longitudinally along the tube 53, as shown in figure 10. One
of the plurality of optical fibers 8 is disposed in each of
the grooves 54. Preferably, the endoscope 10 includes a
probe tip 55, as shown in figure 11, having a reflector 56
disposed in each groove which reflects light from the optical
fiber 8 in the groove when the reflector 56 is in the patient
and reflects light from the patient to the optical fiber 8
when the array 28 is in the patient.
The light source 51 preferably includes a coherent
light source 51 and means 57 for guiding the light from the
light source 51 to the plurality of optical fibers 8 of the
array 28. Preferably, the optical fiber 8 is single mode,
has a core 118 with cladding 120 disposed about the core 118,
and has a lens 122 at its tip which focuses the light from
the core 118 to the reflector 56 and light from the reflector
56 to the core 118, as shown in figures 12 and 13. The array
28 preferably includes a transparent cover 7.
Preferably, the light source 51 comprises an input
arm 58, the array 28 comprises a sample arm 59, the detector
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-17-
D comprises a reference arm 60 and a detector arm 61; and the
input arm 58, the detector arm 61, the sample arm 59 and the
reference arm 60 together form an interferometer. The
reference arm 60 preferably uses RSOD to introduce depth
scanning and dispersion compensation to the interferometer.
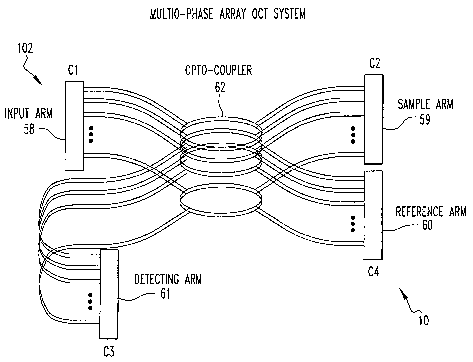
Preferably, the endoscope 10 includes an opto-
coupler 62 which optically couples corresponding optical
fibers 8 of the input arm 58, sample arm 59, reference arm 60
and detecting arm together. The detector D preferably
determines structural information about the patient from the
intensity of an interference signal from reflected light from
corresponding fibers of the sample arm 59 and the reference
arm 60 having a same bypass length.
Preferably, the probe tip 55 includes a scanning
head 1 which holds N optical fibers 8, where N is greater
than or equal to 2 and is an integer, as shown in figures 17-
22c. The N optical fibers 8 are preferably arranged around
the scanning head 1 in parallel and equal spacing.
Preferably, the probe tip 55 includes a mechanism 134 for
moving the scanning head 1 so each of the optical fibers 8
scan an angular range of N/360 degrees. The moving mechanism
134 preferably includes a mechanism 9 for linear motion which
causes the scanning head 1 to rotate.
Preferably, the linear motion mechanism 9 includes
a fiber shaft holder having a shaft channel 31 extending
axially along the holder, and N fiber channels 32 are
arranged around the holder in parallel with the shaft channel
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-18-
31, and a twisting shaft that fits in and conforms with the
shaft channel 31, as the shaft moves in the channel, the
holder rotates.
The scanning head 1 preferably has a socket head
that conforms with the shaft and causes the scanning head 1
to rotate. Preferably, the probe tip 55 includes a guide
wire holder 2 disposed on the scanning probe 50 which
receives and follows a guide wire when the guard wire is in
a blood vessel, biliary tract, and possible GU tract. A
guide wire is not necessary in the GI tract. Preferably, the
endoscope 10 includes a spring disposed between the scanning
head 1 and the fiber shaft holder which forces the shaft back
after the shaft has moved forward.
The present invention pertains to a method for
imaging a vessel, GU, GI or biliary tract of a patient. The
method comprises the steps of transmitting light from a light
source 51 into an optical fiber array 28 comprising a
plurality of optical fibers 8 in the patient. There is the
step of transmitting the light reflected by the patient out
of the patient. There is the step of receiving the light
from the array 28 at a detector D. There is the step of
analyzing the light with the detector D. ,
Preferably, there are the steps of reflecting light
from each optical fiber 8 with a corresponding reflector 56
associated with the fiber, and reflecting light from the
patient to the associated fiber with a reflector 56. There
is preferably the step of moving each of N optical fibers 8
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-19-
comprising the optical fiber array 28 an angular range of
N/360 degrees. Preferably, there is the step of applying a
linear motion to cause each of the N optical fibers 8 of the
optical fiber array 28 to move the angular range.
The step of applying the linear motion preferably
includes the step of moving axially forward.in parallel with
the N optical fibers 8 a twisting shaft through a shaft
channel 31 extending axially along a fiber shaft holder
having N fiber channels 32 arranged around the holder in
parallel with the shaft channel 31 which causes the holder to
rotate. Each of the N optical fibers 8 is disposed in a
respective fiber channel 32 of the N fiber channels 32. The
twisting shaft fits in and conforms with the shaft channel
31, as the shaft moves in the channel. Preferably, there is
the step of guiding the optical fiber array 28 along a guide
wire which is received by a guide wire holder 2 when the
guide wire is in a blood vessel, biliary tract, and possibly
GU system, but not in the GI tract.
The present invention pertains to an apparatus for
studying an object. The apparatus comprises means for
producing light. The apparatus comprises means for analyzing
the light that has reflected from the object based on
polarization, space, position or angle.
The means for analyzing is preferably described in
the figures, where polarization is found in figure 31,
position in figures 1-30, space in figure 32, and angle in
figure 33.
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-20-
The present invention pertains to an apparatus for
studying an object. The apparatus comprises means for
producing light. The apparatus comprises means for analyzing
the light that has reflected from the object based on
polarization.
The present invention pertains to an apparatus for
studying an object. The apparatus comprises means for
producing light. The apparatus comprises means for analyzing
the light that has reflected from the object based on space.
The present invention pertains to an apparatus for
studying an object. The apparatus comprises means for
producing light. The apparatus comprises means for analyzing
the light that has reflected from the object based on angle.
The present invention pertains to a method for
studying an object. The method comprises the steps of
producing light. The method comprises the steps of analyzing
the light that has reflected from the object based on
polarization, space, position or angle.
The present invention pertains to a method for
studying an object. The method comprises the steps of
producing light. The method comprises the steps of analyzing
the light that has reflected from the object based on
polarization.
The present invention pertains to a method for
studying an object. The method comprises the steps of
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-21-
producing light. The method comprises the steps of analyzing
the light that has reflected from the object based on space.
The present invention pertains to a method for
studying an object. The method comprises the steps of
producing light. The method comprises the steps of analyzing
the light that has reflected from the object based on angle.
In the operation of the invention, a near infrared
broadband light source 51 sends a light beam into the input
arm 58 of the array 28 type interferometer. The beam profile
from the light source 51 is a circular gaussion. The optics
before connector 1 makes the beam profile linear and focuses
it into the connector 1. The array 28 type interferometer
consists of multiple fiber-based interferometer that has four
fiber arms connected to an opto-coupler 62. Incoming light
into the input arm 58 is divided to the sample and reference
arms 59, 60, respectively. In the sample arm 59, optical
fibers 8 are distributed like an annular ring, and light will
be focused at the target vessel perpendicular to the optical
axis. In the reference arm 60, RSOD introduces depth
scanning and dispersion compensation. When the reflected
light from both arms have the same light path length,
strictly speaking within a coherence length, interference
occurs. The intensity of the interference signal represents
the structural information of a sample.
More specifically, in regard to the input arm 58,
and referring to figures 1, 2 and 3, a single beam comes out
of S1 and will be collimated by Ll. At this point, the beam
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-22-
diameter is big enough to project across all of C1's area,
but the beam is still circular. CL1 and CL2, circular
lenses, change the beam profile to a linear shape, which
means that the beam is not circular anymore, but it looks
narrow from figure 2 and the same shape with the beam after
L1 on figure 3. ML1 focuses all light onto Cl.
This is known as an open optic solution:
Light source Sl has a fiber tip from which light departs
into air.
L1 is a collimating lens 122, so the fiber tip of the
light source 51 should be located at the back of the
focal point of Ll in order to collimate the light.
CL1, 2 are cylindrical lenses. Separation between two is
the sum of each cylindrical lens 122 focal length. They
work as a telescope which decrease beam size only in one
direction. In other words, the size of the beam does
not change from figure 3.
ML1 is a micro lens array 28, which has a lot of small
lenses. Each of the small lenses is positioned to have
a focal point at each fiber entrance of Cl. Cl should
be located at the focal point of ML1. All micro lenses
have same focal length. Cl is a linear fiber array 28.
In an alternative embodiment of the input arm 58,
as shown in figure 4, known as a fiber based solution:
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-23-
Light source Sl is connected to a single mode fiber,
which is connected to fiber splitter (50:50), S1.
The first fiber splitter is 1 by 2. Each output end of
the 1*2 fiber splitter is connected to 1*4 splitter,
SP1.
Each output end of the 1*4 splitter, 2nd layer, is
connected to another 1*4 splitter, 3rd layer, SP2.
At the output of the 3d layer, the number of fiber is
32. 32 fiber comprises a linear fiber array 28, SP3.
Linear fiber array 28:
Each fiber is a single mode fiber, which can have a
different cutoff frequency. The cutoff frequency is
dependent on the center wavelength of the light source
51. Usually, 850nm or 1300nm of center wavelength for
the light source 51 are used.
Each fiber is attached to another so that all together
they form a linear fiber array 28.
Cl is connected to multiple interferometers. Each
interferometer consists of four fiber arms and opto-coupler
62. At each end of each arm, there is a linear array 28
fiber connector(C1, C2, C3 C4). Incoming light will be
divided by the opto-coupler 62 into the sample and reference
arms 59, 60, respectively.
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-24-
With respect to the sample arm 59, this sample arm
59, as shown in figures 5, 6, 7, 8 and 17, goes into the
target vessel. C2 is connected to a linear fiber array 28
which is of an annular shape at the other end. The total
length of the arm will be around 2-3m. When the light leaves
the annular tip F, it will be collimated by L1 and then
reflected by L2 outward from the probe.
Reflected light from tissue will follow back to L2
and L1 and be gathered by the fiber tip. Later, two
reflected lights from the sample and reference arms 59, 60,
respectively, will make interference, which will be detected
by the array 28 detector D at the detection arm.
The sample arm 59 is supposed to go through a
target vessel, GI, GU or biliary tract. C2 is connected to
a linear fiber array 28 which has an annular shape at the
other end (probe tip 55) ( f igure 8). Total length of the
sample arm 59 is about 1.5m. The fiber array 28 will be
molded by a transparent cover 7 material (ex: silicon resin
or polymers).
At the annular probe tip F shown in figure 9, each
fiber is glued at a groove of a cylindrical polymer tube 53.
The shape of each groove is shown at figures 10 and 11. Each
groove end has a reflector 56 which is 45 oblique to axial
direction. The groove will be made by micro fabrication
technique. Each fiber has a lens 122 at the tip, which can
be manufactured by splicing a multimode fiber with the same
diameter of the cladding 120 of the single mode fiber and
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-25-
then melting the end of multimode fiber in order to get
curvature (figures 12 and lS). When the light leaves the
fiber tip, the light will be reflected outward by the
reflector 56 at the end of the groove, and then will be
focused at the target tissue area. Reflected light from the
tissue will follow back the same path as the incoming light,
and go to the detection arm.
Micromachining or micro-electro-mechanical systems
(MEMS) and nanotechnology are becoming increasingly popular
for the development of improved biomaterials and devices
(Macilwain C., "US plans large funding boost to support
nanotechnology boom," Nature, 1999; 400:95, incorporated by
reference herein) . Similar to manufacturing methods used for
computer microchips, MEMS processes combine etching and/or
material deposition and photolithographic-patterning
techniques to develop ultrasmall devices (Madou, M.,
"Fundamentals of microfabrication," CRC Press: Boca Raton,
2002, incorporated by reference herein). MEMS has been
proven promising in medicine for its small mass and volume,
low cost, and high functionality. Successful MEMS devices in
medicine include smart sensor for cataract removal, silicon
neurowells, microneedles for gene and drug delivery, and DNA
arrays (Polla, D. L., Erdman, A. G., Robbins, W. P., Markus,
D. T., Diaz-Diaz, J., Rizq, R., Nam, Y., Brickner, H. T.,
Wang, A., Krulevitch, P., "Microdevices in Medicine," Annu.
Rev. Biomed. Encr., 2000; 02:551-76; McAllister et al., 2000,
both of which are incorporated by reference herein).
However, most of the MEMS processes are planar in nature for
two-dimension (2D) micro-features and primary for processing
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-26-
silicon material. Other micromachining processes include
laser beam micromachining (LBM), micro-electrical discharge
machine (micro-EDM), and electron beam machining (EBM)
(Madou, M., "Fundamentals of microfabrication," CRC Press:
Boca Raton, 2002), incorporated by reference herein. Micro-
fabrication and micro-device development using metals, metal
alloys, silicon, glass, and polymers are described in the
following. (Chen, S. C., Cahill, D. G., and Grigoropoulos,
C. P., "Transient Melting and Deformation in Pulsed Laser
Surface Micro-modification of Ni-P Disks," J. Heat Transfer,
vol. 122 (no. 1), pp. 107-12, 2000; Kancharla, V. and Chen,
S. C., "Fabrication of Biodegradable Microdevices by Laser
Micromachining of Biodegradable Polymers," Biomedical
Microdevices, 2002, Vol. 4(2): 105-109; Chen, S. C.,
Kancharla, V., and Lu, Y., "Laser-based Microscale Patterning
of Biodegradable Polymers for Biomedical Applications," in
press, International J. Nano Technolocty, 2002; Zheng, W. and
Chen, S. C., "Continuous Flow, nano-liter Scale Polymerase
Chain Reaction System," Transactions of NAMRC1 SME, Vol. 30,
pp. 551-555, 2002; Chen, S. C., "Design and Analysis of a
Heat Conduction-based, Continuous Flow, Nano-liter Scale
Polymerase Chain Reaction System," BECON, 2002, all of which
are incorporated by reference herein).
For the array 28, a stainless steel cylinder is
chosen with a diameter of 1.5 mm as the base material. The
diameter is 1.0 mmm for vascular applications, larger for GU,
GI and biliary applications, up to 3.0 mm, if desired. Both
the micro-grooves 54 (or micro-channels of 200 microns wide)
and the reflecting surfaces are machined by micro-electrical
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-27-
discharge machining (micro-EDM) or micro-milling using
focused ion machined tool. To enhance the reflectivity of
the reflecting surface, the stainless steel cylinder are
coated with evaporated aluminum using electron-beam
evaporation.
In regard to the reference arm 60, shown in figure
14, light is collimated by L1 after leaving connector C4, and
be spectrally distributed by a grating (Gl) and will be
focused to a mirror (GAl). By vibrating GA1, the light path
length will be changed in order to achieve depth scanning.
There are many options to build the reference arm
60 applying existing techniques. A very simple form of the
reference arm 60 has just a mirror attached onto a voice coil
that is driven by a function generator with sine wave. The
light reflects back by the mirror and the mirror position
changes the light path length. This path length change
provides depth scanning of the target tissue because
interference occurs only when both arms have the same light
path length. Preferably, the reference arm 60 is more
complicated than the simple one. That is called Rapid-
Scanning Optical Delay (RSOD) which can provide fast depth
scanning and dispersion compensation.
Linear array type beam launches from C4, and is
collimated by Li. A mirror (Ml) reflects the beam to a
grating (Gi) which spectrally distributes the broadband
source light. Spectrally distributed light will be focused
on a Galvono-scanning mirror (GAl) by a lens (L2).
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-28-
Separation between G1 and L2 determines the amount of
chromatic dispersion degree so any material dispersion can be
compensated for usually caused by fibers. The beam offset
from the scanning mirror center determines the fringe
frequency that will show up after interfering two reflected
lights. The reflected light from the GAl goes to L2, G1, and
to' M2. And then the light reflected following back incoming
path and will be coupled back to C4.
Referring to the detection arm, as shown in figures
15 and 16, light is collimated by L1 after leaving connector
C3, and is circular. Combination of CL1 and CL2 makes the
beam look linear in one plane (horizontal) . Micro-lens array
ML1 makes the light focus on the array 28 detector D.
As shown in figures 17, 19a, and 19b, the scanning
probe 50 is comprised of a scanning head 1, a fiber-shaft
holder 3, a twisted shaft 4, a transparent cover 7, a guide
wire holder 2, and a mechanism 9 for linear motion. In this
embodiment, the scanning head 1 is adapted to hold a fiber
bunch that contain 20 optical fibers 8, which are arranged
around the scanning head 1 in parallel and equal spacing. In
operation, each of the fibers is set to scan an angular range
of 18 degrees (360 - 20 = 18 ). Reflective surfaces 11 are
formed on the scanning head 1 and are oriented 45 degrees to
the central axis of each respective optical fibers 8, such
that they would guide the light from the fiber bunch and
direct the light through the transparent cover 7.
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-29-
The scanning head 1 is designed to provide an 18
degrees' back-and-forth rotation. The back-and-forth
rotation realizes the scanning function required by the OCT
system. The mechanism of this back-and forth rotation is
described below.
The fiber-shaft holder is substantially a
multi-tubular structure. It is formed with one shaft channel
31 extending along the central axis of the fiber-shaft holder
and 20 fiber channels 32 arranged around the fiber-shaft
holder 3 in parallel. The optical fibers 8 extend through
respective fiber channels 32. The shaft channel 31 has a
round cross-sectional area. At the upper end of the shaft
channel 31, the shaft channel 31 is an opening, but the
geometry of the opening is reduced from the round
cross-sectional area to a rectangular cross-sectional hole
311. The reason for this structural design will be described
along with the description of the twisted shaft 4.
The twisted shaft 4 has a rectangular cross-section
area, which is identical in geometry to the rectangular
cross-sectional hole of the fiber-shaft holder 3. Indicated
by its name, the shaft 4 is partially twisted along the shaft
central axis and can be divided into a non-twisted part 41
and a twisted part 42. In assembly, the shaft 4.is passed
through the rectangular cross-sectional hole of the
fiber-shaft holder 3, and it is enabled to slide
back-and-forth via the rectangular cross-sectional hole. The
relative motion of the surfaces of the rectangular
cross-sectional hole and the twisted shaft 4 form the
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-30-
mechanism that realizes a back-and-forth rotation. The
reason is that when the twisted part 42 of the shaft 4 slides
through the rectangular cross-sectional hole, the shaft 4
itself is forced to rotate along the shaft central axis to
fit the matching of both the surfaces of the rectangular
cross-sectional hole and the twisted shaft 4. Particularly,
the shaft 4 and the holder 3 compose a mechanism 9 that can
transmit a linear motion into a rotational motion.
The description is now focused on the scanning head
1. The scanning head 1 has a rectangular socket 12, which has
a cross-section area identical to that of the twisted shaft
4. The rectangular socket 12 provides a channel covering the
non-twisted part 41 of the twisted shaft 4 and lets the
non-twisted part 41 exert the back-and forth motion inside
the rectangular socket 12. The moving range of the shaft 4 is
constrained such that the twisted part 42 does not pass into
the scanning head's rectangular socket 12 (that will result
in a geometric mismatch), but the twisted part 42 only
interacts with the fiber-shaft holder's rectangular
cross-sectional hole. According to the description above, the
motion of the shaft 4 is comprised of a linear component (V)
and an angular component (w). Referring to the geometry of
the rectangular socket 12 and non-twisted part 41 of the
shaft 4, the shaft motion's linear component (V) would not
contribute to the motion of the scanning head 1(regardless
of the friction between the surfaces), but the angular
component (cw) does. The scanning head 1 rotates back and
forth with the rotational motion of the twisted shaft 4,
which in turn results from the twisted shaft's linear
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-31-
back--and-forth movement relative to the fiber-shaft holder 3.
As a result, the scanning head 1 provides a back-and-forth
rotational motion transmitted from the back and forth linear
motion provided by the twisted shaft 4.
A guide wire holder 2 is a module used to guide the
scanning probe 50 toward the investigated section of the
detected blood vessel, biliary duct, and possibly GU
application. For the GI tract, a guide wire is generally not
used. In operation, a guide wire 01, or "guide tissue", is
previously disposed along a specific route of human vessels,
such that a track for the scanning probe 50 of the OCT system
can be formed. The guide wire holder 2 constrains the
scanning probe 50 such that it can only slide along the track
formed by the guide wire 01. The scanning probe 50 is
therefore guided to the patient section to be investigated.
Guide wire holder 2 and holder 5 function as
bearings of the scanning head 1. They constrain the movement
of the scanning head 1 and stabilize it. As well, a
compressive spring 6 is disposed between the scanning head 1
and the fiber-shaft holder 3. The spring 6 is mildly
compressed in assembly, such that it pushes the scanning head
1 against the holder 5 and eliminates any potential axial
movement of the scanning head 1 that may result in axial
positioning errors (od). It is preferable that the spring 6
supplies torque between the scanning head 1 and the
fiber-shaft holder 3. The spring 6 has its both ends,
respectively, fixed on the scanning head 1 and the
fiber-shaft holder 3. The spring 6 is mildly twisted in
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-32-
assembly. By this means, the spring can provide a torque to
the back-and-forth rotational mechanism, such that the
backlash (resulting from, for example, the tolerance between
the rectangular cross-sectional hole and the shaft) of the
rotational mechanism, as well as the resultant angular
positioning errors (,~e), are eliminated.
Note that, the cross-section geometry of the shaft
channel 31 is circular. With respect to the shaft channel
31, the twisted shaft 4 is formed with a cylinder part 43 at
its end of the twisted part 42. The cylinder part 43 and the
shaft channel 31 performs a motion like a piston. In an
upward movement of the twisted shaft 4, due to the geometric
difference, the cylinder part 43 would be blocked at the edge
33 of the rectangular cross-sectional hole of the fiber-shaft
holder 3 and provide an upper stopper for the twisted shaft
4. On the other hand, a lower stopper 34 is placed to block
the cylinder part 43 in a downward movement. The function of
the upper and lower stoppers is helpful in controlling the
movement of the twisted shaft 4, as well as controlling the
angular motion of the scanning head 1.
There are many methods in the prior art that are
able to provide the power for the mechanism to push and pull
the twisted shaft 4 to generate the linear movement.
However, hydraulic force, particularly fluidic pressure, is
preferred due to the following advantages:
1. Electricity is not required to be transmitted
into the scanning head 1 to energize a
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-33-
hydraulic linear mechanism 9. Some of the
mechanisms, such as electromagnetic systems
(or more particularly, some micro-motors),
require not only electricity to be energized,
but also additional components, e.g., coils
or magnets, installed to the scanning head 1
to transform the electrical energy into
mechanical momentum. The use of electricity
is not preferable for medical issues; and the
requirement of additional components would
increase the technical difficulty in
manufacturing and the complexity of the whole
system. Some of the other mechanisms, like
those comprising piezoelectric materials, can
be composed with little space and simple
structure, but they still need to receive a
large voltage to generate the required
momentum.
2. A hydraulic mechanism 9 takes little space.
The structure of the hydraulic mechanism 9 is
illustrated in figures 18a and 18b. The hydraulic mechanism
9 can be simply a liquid conduit that guides liquid, such as
water, to push or pull the piston system comprised of the
cylinder part 43 and the shaft channel 31. Considering that
leakage through the gap of a piston system may result in
undesirable problems, the hydraulic mechanism 9 is,
preferably, comprised of a micro-balloon 91 made by a
polymeric thin film. As shown in figures 18a and 18b, the
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-34-
twisted shaft 4 is in its lower position when the balloon 91
is flat (figure 18a) As water is pumped into the piston
system, the balloon 91 becomes turgid, and the twisted shaft
4 is pushed toward its upper position with an 18 degree spin
(figure 18b). The required back-and forth motion can be
generated by switching the flat and turgid states of the
micro-balloon 91.
For a single fiber OCT system, a scan rate of 6
rev/sec (6 Hz) is satisfactory [Andrew M. Rollins et al.,
"Real-time in vivo imaging of human gastrointestinal
ultrastructure by use of endoscopic optical coherence
tomography with a novel efficient interferometer design",
OPTICS LETTERS, Vol. 24, No. 19, Oct 1, 1999, incorporated by
reference herein]. That means in one second the OCT system
should be able to provide at least 6 pictures illustrating
the cross-sectional data of the vessel. The scanning probe 50
has 20 fibers, so the satisfactory scan rate can be reduced
to 0.3 Hz (6=20 = 0.3), which is much slower and much easier
to be realized by the hydraulic actuating system. Ideally,
15 pictures/sec. is required for optimal image resolution.
Rather than continuous rotation, the scanning probe
50 operates in a back-and-forth manner, so that the angular
speed of the scanning head 1 will not be constant even when
the whole system reaches its steady state. During operation,
therefore, detecting the angle of the scanning head 1, as
well as figuring out the angular position that the scanned
data belongs to, are important issues. The angle of the
scanning head 1 can be simply approximated by comparing the
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-35-
output effort of the pumping system with a reference curve
obtained from previous experiments. More precise detection
can be reached by the analysis of the feedback of the optical
signals. For example, analyzing the Light Doppler Effect
[Volker Westphal at a1.., "Real-time, high velocity-resolution
color Doppler optical coherence tomography", OPTICS LETTERS,
Vol. 27, No. 1, Jan 1, 2002, incorporated by reference
herei.n] of the feedback signals is another method.
The twisted shaft 4 can be formed by precise CNC
machining that is well known in the industry. A thin round
shaft, minimum diameter 1.0 mm, may be used as the intrinsic
material before the machining. For production, two ends of
the round shaft are clamped, its central portion is precisely
milled and four orthogonal planes on the central portion are
generated. The planes define the rectangular cross-section
of the twisted shaft 4 (forming a long shaft in this step),
as shown in figure 20a. Following the milling, one of the two
clamps holding the shaft is rotated relative to the other
clamp to twist the shaft a specific angle about its central
axis. The twisted part of the twisted shaft 4 being formed.
Following the twisting step, the rotated clamp is
released to free the elastic distortion of the shaft (with
its plastic distortion remaining), and then the clamp is
tightened again. At the next step, as shown by figure 20b,
the shaft is milled again at one side of its still-round
portion, thereby generating another rectangular portion that
is untwisted.
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-36-
The cylindrical portion (serves as a piston) is
formed from the round portion of the shaft. A precise
lathering could further be used to fix the central axis and
diameter of the cylindrical part. As shown in figure 20c,
only a short portion of the shaft is required. The excess
portion of the shaft part is cut off.
As shown in figure 21a, the fiber-shaft holder 3
can be combined with two parts, A and B. The part A is
actually the body of the catheter. The cross-section of the
catheter is shown in figure 21b; the catheter could be
manufactured by the cable extrusion technique that generally
is applied in fiber optics industry [Refer to the homepage of
Optical Cable Corporation.] Note that the central channel of
the catheter is used to be the conduit for the guidance of
actuating liquid mentioned previously. There are also
several conduits used to guide air flowing in and out the
probing tip to balance the air pressure inside the OCT system
(during operation, the free volume inside the probing tip
changes while the twi.sted shaft 4 is moving). The diameter
of the conduit is equal to that of the cylinder part 43 of
the twisted shaft 4.
Part B in figure 21a is simply a plate having fiber
holding edges (B1) and a rectangular central opening (B2).
This part could be made from metal by using punching
technology as is commonly applied in the industry. In
assembly, Part A and Part B are connected with glue such as
epoxy. The lower stopper, which is required to constrain the
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-37-
twisted shaft 4 at its lower position, is formed together
with the formation of the micro-balloon.
Micro-molding with polymeric material (such as SBS)
could be used to fabricate the scanning head 1. The process
of micro-molding requires a set of micro-molds. In this
case, the fiber grooves 54 and the reflective surface 11 at
the end of the fiber grooves 54 can be realized by a set of
micro-molds comprised of 18 edges (figure 22a), each of which
has the geometry shown in figure 6b. As well, the central
rectangular channel could be molded by a rectangular shaft
made by the equipment for the fabrication of the twisted
shaft 4. For the convenience of assembly, the scanning head
1 could be previously provided with the geometry shown in
figure 22c. The excess parts of the scanning head 1 would
provide guidance and help with the alignment for the optical
fibers 8. UV glue could be used to fix the position of the
optical fibers 8. The excess portion of the scanning head 1
could be cut off after the assembly of the optical fibers 8.
In another embodiment, laser beams heat at least
three different locations on the surface of the micro-mirror
210, which is shown as a disk in figures 23-25, successively.
The micro-mirror 210 will provide a wabling corresponding to
this kind of un-symmetric heating process, and an incident
light (other than the heating laser) can be redirected in a
swaying manner.
The heating process corresponds to the rotation
period of the micro-mirror 210 as required.
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-38-
The micro-mirror 210 comprises two layers: a first
layer 212 and a second layer 214 (figure 23). At least one
of the two layers can generate structural deformation
(contraction or expansion) by the application of laser light.
If the case is that both of the layers are deformable by
laser light, the sensitivities of the two layers to a same
laser light would be set different to each others. Figure 24
shows the perspective view of the micro-mirror 210.
When the micro-mirror 210 is irradiated with a
laser beam, there will be expansion or contraction in the
layers. Because the expansion or contraction within the
layers is of different degrees (only one layer is deformed or
the two layers are deformed with different degrees), the
structure of the whole micro-mirror 210 will be twisted.
For example, in figure 25, when the section marked
with the pie is irradiated with a laser beam, there is a
deformation generated as shown in figure 26.
The material of the first and second layers 212,
214 could be metals or photosensitive polymers.
In the case of metal layers, for example, the first
layer 212 is poly-silicon and the second layer 214 is gold.
The mechanism of the expansion or contraction within the
layers is thermal expansion. The metals will absorb the
energy of a laser beam and be heated. Due to different
thermal expansion coefficients of the two layers, the
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-39-
structure will be twisted or bent. This will result in
turning the mirror, as shown in figure 26.
In the case of photosensitive polymers, for
example, liquid crystal materials, the mechanism of the
expansion or contraction inside the layers is a phase change
of the materials. Under the irradiation of a laser beam, the
molecules of the polymeric materials will undergo phase
change, wherein the chemical structures of the materials are
deformed, and a structural deformation occurs. Next, similar
to the case of metal layers, the degrees of deformation of
the two layers are different, and there will be a twisting or
bending effect in the structure of the micro-mirror 210, and
the effect in figure 26 is reached.
When the structure is twisted or bent by the
application of laser energy, the surface of the mirror, shown
in figure 24, can be tiled to a specific direction.
Therefore, one can control the direction of the micro-mirror
210 by controlling the laser energy input.
The way to control the application of the laser
light is to select the location on the micro-mirror 210 to be
irradiated by the laser beam, and control the intensity of
the laser. By controlling the location, one can control the
tilting direction of the mirror; and by controlling the
intensity, one can control the tilting angle of the
micro-mirror 210.
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-40-
Referring to figure 25 and figure 26, by
continuously changing the laser-shining location (figure 27),
the tilting direction of the micro-mirror 210 can be
continuously changed (figure 28). That is, the micro-mirror
210 could be rotated by changing the location of the
laser-shining.
This is the mechanism for the rotation of the
laser-actuated micro-mirror 210.
As to the assembly of the whole OCT system (figure
29), the micro-mirror 210 is mounted on a base 21b connected
to the tip end of the probe cover. There is no object between
the fibers and the mirror. Fiber 1, which is used to guide
the detecting light, is the same fiber used in other
embodiments of the OCT probe. The detecting light is
redirected by the tilting surface of the micro-mirror 210,
such that it can scan around by means of the tilting and
rotating mirror. The fibers 2 are used to guide the
actuating-laser light. As shown, at least three fibers 2 are
needed. The fibers 2 fire lasers in turns, such that they can
generate continuous tilting effect as shown in figure 27 and
figure 28.
The other features of the laser-actuating OCT probe
are the same as those described in other embodiments. For
instance, the fiber, and fibers 2 are disposed in a fiber
shaft holder 3.
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-41-
After the fabrication by semiconductor technique,
which is well known by those skillful in the art, the mirror
is formed on a substrate (usually silicon substrate) . The
substrate material forms the base. Then a small piece is cut
from the base that carries the mirror from the substrate with
a dicer. The small piece is mounted on to the tip's end by
glue (EPOXY, for example).
Only one fiber 1 is enough to transmit the
detecting light in this embodiment. During operation, a
circular scanning profile of the detecting laser is realized.
In this embodiment, illustrated in figure 30, the detecting
laser is not centered to the mirror's center. Instead, the
following remain constant: (1) d, the distance between the
mirror center and the axis of the detecting light. (2) alfa,
the angle between the mirror surface and the axis of the
detecting light. An open-loop system is used for position
feedback to properly arrange the periodical change of the
laser powers from the three fibers 2 to realize the constant
alfa and d.
The position control is more complex than
single-fiber 2 actuation. Particularly, the micro-mirror 210
needs a period of time to respond mechanically to the laser
energy coming from the fiber 2. Even though it is known when
and which of the fibers 2 are firing the laser power, the
exact direction of the mirror surface information cannot be
assured.
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-42-
The absolute position of the mirror is actually not
necessary. Instead, speed-control is used to control the
rotation of the scanning mirror. For example, in the case of
the mirror driven by a transmission cable rotated from
outside, the exact position of the mirror (which may be
affected by a delay of cable transmission due to the cable's
compliance) is not of concern; the rotation period of the
mirror is controlled so that the "relative position" of the
mirror is known. After receiving a continuous data stream
from the reflected detecting laser, the cross-section image
of the vessel is constructed by simply matching the data
series to the rotating period.
In this embodiment, the operation will be similar.
What is different is that the micro-mirror 210 is not
actuated by a rotator but by three bimorph heat-deformable
cantilever beams. This makes the control more complex. If
only one of the fiber 2 fires at one time, it will be very
different if not impossible for the mirror to scan a circular
profile needed. Instead, the three fibers 2 are needed to
fire together, with different powers, to bend the three
cantilevers at different status at one time to match a
circular scanning profile. The three cantilevers are
actuated individually by the three fibers 2 such that they
cooperate with specific bending patterns that realize a
circular scanning profile on the wall of the vessel.
In an alternative embodiment regarding the
micro-mirror 210, the fibers 1 and the fibers 2 are reversed
so healing energy comes from a single fiber 2 disposed
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-43-
preferably along the central axis of the tube. The plurality
of fibers 1 are disposed about the circumference of the tube.
When the micro-mirror 210 is irradiated by the laser beam
from the fiber 2, the laser energy causes the mirror to bend.
By changing the intensity of the laser or pulsing the laser,
motion can be imported to the micro-mirror 210 which wires
the probe tip to which it is attached, to move back and
forth, and thus the plurality of fibers 1 for scanning the
interior of the area of the patient in question.
Thermal expansion material normally can generate
-5% of elongation for a temperature rise of 100 C. The
length of the material inside the OCT is originally 20 mm,
which can therefore generate a thermal elongation of 1 mm.
Polymers, including photosensitive polymers and shape memory'
polymers are able to generate > 100% of photo-induced
elongations or shrinkages. The material inside the OCT is
originally 1 mm, which can therefore generate a thermal
elongation of another 1 mm.
Generally:
Optical tomographic instrumentation may be
specified by spectrally resolved bandwidth, which is
equivalent to number of spectrally resolvable cells. Each
spectrally resolvable cell has a width Sv, such that number
of cells resolvable by the instrument is Nzhstrument=AV/cSV, where
Av is the available optical bandwidth of source light. The
range of group-time delays the optical tomographic instrument
can resolve is given by: A'Cinstrument ' 1I 5V. The smallest
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-44-
resolvable group-time delay the optical tomographic
instrument can resolve is Oticoherence ' a-/AV = Number of
spectrally resolvable cells the optical tomographic
instrument may resolve is given by:
Ni.nstrument-0zinstrument/Oticoherence =
For 1 OCT A-scan into the object being imaged, the
requirement for number of spectrally resolvable cells is -
NA-scan -Az/Z'cr Lc - cq/dvr Az = imaging depth, Lc
(coherence length), and cg is the group velocity of light in
the object.
NA-scan ~ ArA-scan dV
Where ACA scan = AZ/cg is the round-trip propagation time for
light to propagate from the most superficial and deepest
position (to be imaged) in the object.
For some optical tomographic imaging instruments
(e.g., those that employ narrow linewidth tunable laser
sources or high resolution spectrometers),
Ninstrument/NA-scan QZinstrument/ATA-scan - AV/8V >> ].
The above condition can be stated in three manners:
a) the number of spectrally resolvable cells for the
instrument (Ninstrument) is much greater than that required for
one A-scan (NA-scan)% 2) the range of group time delays the
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-45-
instrumentation is capable of resolving (Azinstrument) is much
greater than the group-time delay for a single A-scan (AzA_
s,an); 3) available optical bandwidth of source light (Av) is
much greater than spectral width of each resolvable cell of
the instrumentation (8v).
Because the instrument can resolve many more cells
than that required for one A-scan, multiplexing techniques
are presented here to efficiently utilize the information
carrying capacity (bandwidth) afforded by optical tomographic
imaging instruments.
Selection criteria of multiplexing techniques
employed may be derived in part by the ratio NinstrumentjNA-scan -
16Zinstrument~A'rA-scan = AV/SV. Larger ratios provide a wider
selection of possible multiplexing techniques and more
candidate domains (polarization, space, angle, temporal) to
multiplex into. Moreover, multiplexing spectral information
into just one domain (e.g. spatial) is not the only
envisioned approach. Generally, additional spectral
information may be resolved into multiple domains (e.g.,
polarization and spatial).
Specific Implementations:
A. Polarization: The additional spectral cells may
be used to record information in the polarization domain
using a system indicated in figure 31. At least two incident
polarization states 90y apart on the Poincare sphere are
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-46-
input into the interferometer. The polarization signature of
the light reflected from the sample, such as a vessel wall or
nerve fiber layer, is compared to known polarization
signatures of materials, such as plaques or a diseased nerve
fiber layer. The reflected light and thus the material from
which it was reflected is then identified. The fiber
delivery system described in PCT patent application number
PCT/US2004/012773, incorporated by reference herein, can be
used.
The theory of operation of this approach is
described using Mueller matrices or the spectrally-resolved
Jones calculus. By inserting a FOSPI in the detection path
of the spectral domain optical coherence tomography (SD-OCT)
instrumentation, the full set of Stokes parameters of light
backscattered at the specific depth in the specimen can be
obtained without any other polarization controlling
components in reference/sample/detection path of the
interferometer and the prior knowledge of the polarization
state of the light incident on the sample. In this
configuration, two factors determine the spectral modulation.
One is optical path length difference between the reference
and sample surface, (Lf(v)), introduced by the common-path
SDOCT and the other is phase retardations, cp1 (v) and (~2 (v)
generated by the retarder system in the FOSPI. Therefore,
output from the presented single channel polarization
sensitive (PS)SD-OCT in the time-delay domain is the
convolution of the output from FOSPI and that from SD-OCT.
i
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-47-
The Stokes parameters of light at the output of the
interferometer are
Sj = IJl 1 + I.J1,2 +Sl,l
where the first two terms are the Stokes parameters of light
from the reference and sample path, respectively, and the
last term is the contribution of interference. Consider the
birefringent sample with phase retardation b and fast-axis
oriented at angle of cx. Then, the Stokes parameters of the
light from the sample (Si,z) and interference (Si,l) are
calculated in terms of the Stokes parameters of light, from
the reference, So,l, S1,1, S2,11 S3,1.
2
So,2 = r,. So,1
SI,2 = r,? (cos2 2a +cos 8 sin2 2a)Sl,l +1;2 (1- cos b) sin 2a cos 2aSz 1- r2
sin S sin 2aS3,1
(i)
S2,2=r?(1-cos8)sin2acos2aS11 +1;?(sin2 2a+cos,5cos2 2a)S21 +a,2 sincSsin2aS3,1
S32 =Yy S1T1C5s1112aSIi-7,,2 sinlScos2aS2,1 +1.2 cos&31
So,j = 2 r s , cos cos 2 So,l + 2rS sin 0 sin ~(cos 2 aSl j + sin 2 aS2,1)
Sl,; = 2s s, cos A(cos 2 Sz,l -sin ~ sin 2aS3,1) + 2~ s sin A sin ~ cos 2aSo,1
(2)
S2 ; 2rS cos A(cos ~ S2,1 + sin ~ sin 2 aS'31) + 2rs, sin A sin ~ cos 2 aSo,l
S3 ;= 2rs, cos 0(sin ~ sin2aS1,1- sin ~ cos 2aS2,1 + cos ~ S31)
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-48-
with a reflection coefficient of the sample rs and an optical
path length difference between the sample and reference path
o. Here, the terms including trigonometric functions of o
represent the interference between the light from reference
and sample paths.
The measured intensity from SDOCT passing through
the FOSPI for a birefringent sample, then, is
I otit i ( v ) =rs, cos 4 cos S 2 So 1+ Ns, sin A sin 8 2(cos 2aS1,1 + sin
2aS2I)
S[(cos 8 ~ 8 ~
+~ r 2 51 i- sin 2 sin2aS31) cos(r~ -- ~pz)+ sin 2 cos2a5o,1 sin(A - ~pz)
~ 8 Sl 8
2 f ,l - sin 2 sin 2aS3 I) cos(A + Tz )+ sin 2 cos 2aSo,1 sin(A + ~pz )
+ , (cos
+ 4rs I(cos S2,1 + sin ~ cos 2aS3,1) cos(d - Tz + Tl )
+ {sin ~ sin 2a(So,l + Sl,l )- sin 2 cos 2aSz,1 + cos 2 S311 } sin(d - ~p2 +
rpl
+ 4 t s~(cos Sz i+ sin Z cos 2aS3 i) cos(A + ~p2 - ~pl)= ( 3)
+ {sin 2 sin 2a(So,l - Sl,l )+ sin 2 cos 2aSz,i - cos ~ S31 } sin(A + ~pz -
(001
r cS S
-47.s l(cos 2 Sz,l + sin 2 cos2aS3,1) cos(A - V2 - ~p; )
+ {sin 8 2 sin 2a(So,l + S1,1) + sin 2 cos 2aSz,1 - cos 2 S31 } sin(t1- P2 -
~Pl)
-4 YS L(coS 2 521+ sin 2 cos 2aS3 1) cos(C4 +(~z + C~1)
+ {sin 2 sin 2a(So,l + SI,1) -- sin ~ cos 2aSz,1 + cos 2 S3,1 } sin(d + ~pz +
~pl )
1
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-49-
for the interference signal. Fourier transform of equation
(3) gives seven components in the positive optical path
length difference domain which are centered at o, o-_-cp2,
p ((~2-01}, p (02+q)1), respectively. Inverse Fourier
transforms of each component are as follows.
~ 9 So, - i siS ~
A:1Nse iA cos
~ 2 n 2(cos ZcrS11-~- sin 2aS2,1) (4)
a~ 1 r ei~zei4 ~cos ~ S sin ~
~Pz. 4 sin2txS3,i i sin - cos2aSo,l (5)
2 i,i - 2 ) - 2
A + CP2 - cp1 : $ jse'(Pz-4),)eiA [(COS ~ S2 1 + slll ~ cos2CYS3,2)
~ S S (6)
- i{sin 2 sin 2cr(Sa,l - Sl,l )+ sin 2 cos 2crSz,1 - cos ~ 53,1 }~
+Vz +o1~ 8 f:se'(mZ+PJe(cos 2 S21 + sin 2 cos2aS3 i)
LLS 8
- i{sin 2 sin2a(Sa,l + Sl l)- sin S 2 cos2aS2,i + cos ~ S3
(7)
Comparing with equation (2), real part of equation (4) gives
S0,iJ4 and real part of equation of (5) after shifting the
phase by -(p2 gives S1,1/8. Likewise, S2,iJ8 and S3,iJ8 can be
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-50-
obtained by taking the real part of subtraction of (7) from
(6) and the imaginary part of addition of (6) and (7) after
the appropriate phase shift, -(02-(~1) and -(02 +(P1) for (6)
and (7), respectively. Moreover, simple arithmetic gives
phase retardation due to the birefringence of the sample, 6,
without knowledge of incident polarization state. The real
part of (4), imaginary part of (5), the imaginary part of
subtraction of (7) from (6) are
~ rs cos ~ S ,1 (8)
-~rS,sinZcos2aSoi (9)
- 1 rS sin ~ sin 2crSa,j (10)
after the phase shift by -8, -(0+(~2) , -(A+~2-(~1) and
respectively. With a trigonometric identity, the
following can be obtained
tanS=2 (9)2+(10)2 (11)
2 (8)
Phase retardation due to birefringence [Fig. 36]
and fast-axis angle [Fig. 37] of the birefringent sample were
estimated from interference between the back surface of the
glass window and the back surface of the birefringent sample
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-51-
by using Eqs. above. For this measurement, the birefringent
sample was rotated in 5 increments from 0 to 90 . An
estimated single-pass phase retardation of 34.06 -!-2.68 is
consistent with a value deduced from the manufacturer's
specification (31.4 ). The estimated fast-axis angle is
shown in Fig. 4(b) and is plotted with respect to orientation
of the birefringent sample.
The results show practical demonstration of
polarization multiplexing.
B. Space or Lateral Position: The additional
spectral cells may be used. to record information in the space
or lateral position domain using a system indicated below.
1. Existing Multifiber Approach: (described above)
2. Spatially Scanned Light:
The schematic of the experimental setup of a
fiber-based spatially multiplexed swept source OCT
(SM-SS-OCT) system is depicted in Figure 32 using the system
described in PCT patent application number PCT/US2004/012773,
incorporated by reference herein, where the top is preferably
rotated at least 100 times for each position.
A tunable laser and spectrum analyzer (TLSA 1000,
Precision Photonics, Inc.) that operates in the 1520 - 1620
nm wavelength range (k0=1570 nm) with FWHM spectral line
width specified at 150 KHz is used as the illuminating source
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-52-
and is equipped with an optical isolator to protect the laser
from spurious reflections. The laser output is coupled into
one arm of a 2 x 2 fiber-based coupler (interferometer) The
50 0-50 o coupler splits this beam into two nearly equal parts,
used in the reference and sample arms, respectively. The
reference arm has a fixed path length, and simply consists of
a fixed mirror that reflects the entire light incident upon
it back into the fiber-based coupler. The light exiting the
sample arm of the interferometer is collimated, and scanned
across the sample by a scanning galvanometer and a focusing
lens. The scanning galvanometer and focusing lens is used to
rapidly scan the lateral positions of the tissue. The TLSA
1000 completes one complete wavelength sweep in approximately
one second. Within this time, the galvanometer is programmed
to sweep all lateral positions of the tissue several hundred
times. Light returning from the sample interferes with the
light from the fixed reference in the fiber-based
interferometer, and the resultant spectral interference
signal (due to path length variations between sample and
reference reflections) is detected by a photodetector placed
in the detection arm of the system. The electrical output is
digitized, and a non-uniform Fourier Transform (NUFT) of each
A-line spectral data gives the depth profile of the sample
reflectance. Figures 34 and 35 are images of a 100 micron
thick slide recorded with the spatially multiplexed OCT
system. The images are of the same object (microscope cover
glass) only for one image (figure 34) the intensity of the
light returning from the sample is displayed on a linear
greyscale while in the other image (figure 35) is displayed
according to logarithm of the intensity.
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-53-
C. Angle: The additional spectral cells may be
used to record information in the angle domain using a system
indicated in figure 33.
Figure 33 depicts a Multi Fiber Angle-domain OCT
system. The output of the frequency-swept source A is split
into n fibers through the splitter B. The light passes
through the circulators C, is collimated, focused through a
lens, contacts the tissue, and then is reflected into any of
the multiplicity of fibers. A reference reflector for each
path is introduced into each fiber segment. For example, the
reference reflector can be positioned at the terminal end of
each fiber segment. For each i'th input fiber segment,
interference is formed between light backscattered from the
tissue and into the j'th fiber and the reference reflection
from the j'th fiber. For N fibers, N2 interference fringes
are formed each corresponding to an incident (a;,) and
backscattered angle (pa). Light intensity in the spectral
domain is then converted to a voltage through a
photoreceiver, which outputs to an ADC board, which is read
into a computer. This system allows phase-sensitive angle
resolved imaging of discrete light paths in and out-of the
specimen. Using a space-spatial frequency transformation
(e.g., two-dimensional Fourier transformation) lateral
structures can be imaged with sub-wavelength resolution.
D. Space-Angle combinations (e.g. x dimension -
space, y dimension - angle) : The space and angle dimensions
may be combined to form systems that use the additional
spectral cells image both space and angles. For example,
CA 02610086 2007-11-28
WO 2006/133030 PCT/US2006/021629
-54-
additional spectral cells may be used to record position
information in one dimension (e.g. x) and angle information
in the orthogonal dimension (y).
Although the invention has been described in detail
in the foregoing embodiments for the purpose of illustration,
it is to be understood that such detail is solely for that
purpose and that variations can be made therein by those
skilled in the art without departing from the spirit and
scope of the invention except as it may be described by the
following claims.