Note: Descriptions are shown in the official language in which they were submitted.
CA 02610345 2007-11-29
WO 2006/128302
PCT/CA2006/000906
SYSTEM AND METHOD OF COMPUTER-AIDED DETECTION
Field of Invention
[0001] The invention relates generally to the field of computer-aided
detection ("CAD")
and analysis of abnormalities. In particular, the invention relates to
automatic detection of
abnormalities in and analysis of medical images and automated assessment
thereof
Background of Invention
[0002] With the emphasis on early detection of cancer, more and more people
are taking
part in early screening programs, such as mammography screening and in some
parts of the
world ultrasound screening for breast cancer. Some recent studies suggest that
diagnostic
breast ultrasonography may successfully help distinguish many benign from
malignant solid
lesions or nodules. For example, in "Solid breast nodules: use of sonography
to distinguish
between benign and malignant lesions," by Stavros, A. T., et al., Radiology
196:123-134,
1995 ("Stavros"), it was suggested that sonography may be used to accurately
classify some
solid lesions as benign, allowing imaging follow-up rather than biopsy.
Stavros provides a
general method of reviewing lesions by detecting and evaluating
characteristics of
sonographic images corresponding to a set of pre-defined characteristics and
their
description ("Stavros characteristics"). Such local characteristics may
include local
spiculation, local branch pattern, local duct extension and local micro-
lobulation, among
others.
[0003] In general, successful early detection of abnormalities and
diagnosis of cancer
requires a radiologist to successfully and correctly identify and evaluate
characteristics of
masses seen in individual medical images in order to distinguish benign from
malignant
solid nodules. Medical images are not limited to those obtained from
mammography or
ultrasound screenings, namely X-ray images (or digitized X-ray images) or
sonographic
images, but may include medical images obtained from any suitable medical
scanning
device utilizing any underlying image acquisition technology. Some examples of
such
medical images include sonographic images, Doppler images, spectral Doppler
images, X-
ray images, computed tomography (CT) images, positron emission tomography
(PET)
images, PET-CT images and magnetic resonance imaging (MRI) images.
[0004] The experience and expertise of an examining radiologist plays an
important role
in correctly identifying the characteristics so that a well-informed diagnosis
may be
CA 02610345 2007-11-29
WO 2006/128302
PCT/CA2006/000906
- 2 -
established. Computer-aided detection has become an increasingly essential
problem-
solving tool in detecting and diagnosing cancer and other diseases. Modern
technology has
been advancing in many different ways to aid a radiologist to automatically
identify and
evaluate a battery of characteristics of masses seen in medical images. For
example,
technology has been developed to aid a radiologist to automatically identify
and evaluate
sonographic characteristics, to distinguish benign features in medical images
from
sonographic findings of malignancy, and to combine individual benign findings
and
malignant findings to classify a nodule as either benign or malignant in order
to make a
diagnosis. It is also known to automatically detect and mark candidate lesions
or potential
abnormalities within the image and thereby assist radiologists in the
interpretation of
medical images. General availability or accessibility of digitized medical
imaging further
facilitates the computerized image processing and computer-aided detection.
[0005] However, while computerized pattern recognition has seen
tremendous advances
in the past decade or so, sometimes, a computer application may still have
difficulty in
identifying most or all abnormalities. It is desirable not to miss a malignant
lesion in the
early stage of disease. As a radiologist may not place too high a confidence
in results of
automated detection, biopsy may be ordered, which sometimes turn out to be
unnecessary.
Further, even if successful detection of all relevant characteristics in a
medical image were
possible, automated diagnosis may not always provide a correct diagnosis due
to, for
example, inadequacy or lack of sophistication of models underlying a diagnosis
engine.
[0006] The foregoing creates challenges and constraints for all CAD
systems for
extracting, i.e., identifying characteristics and medical features in medical
images and
suggesting diagnosis based on characteristics automatically detected in the
medical image.
There is therefore a need for a CAD system and method as compared to the
existing art. It
is an object of the present invention to mitigate or obviate at least one of
the above
mentioned disadvantages.
Summary of Invention
[0007] The invention relates to computer-aided automatic detection and
identification of
abnormalities in and analysis of medical images. Computer assisted assessment
of detected
abnormalities is also provided. Features within a medical image relevant to
diagnosing
diseases are identified and presented to a user for review. Advantageously,
the medical
CA 02610345 2007-11-29
WO 2006/128302
PCT/CA2006/000906
- 3 -
image is first segmented to provide one or more segmentation candidates to
facilitate further
image processing. A segmentation candidate is confirmed or selected from the
segmentation
candidate or candidates, either manually by a user or automatically detected
or identified by
the system. The segmented medical image is analyzed to extract and identify
features in the
image relevant to a diagnosis, based on which the system computes an initial
diagnosis by
combining the identified features with a diagnosis model. The user is provided
with an
annotation tool to confirm or modify a list of identified features presented
to the user. Upon
modification of the list of features, a revised diagnosis is dynamically re-
computed. Upon a
user having selected a diagnosis, either confirming or modifying the computed
diagnosis, a
diagnosis report is generated reflecting the features present in the medical
image as
validated by the user and the diagnosis confirmed or modified by the user.
[0008] In a first aspect of the invention, there is provided a system
for providing
interactive computer-aided detection of abnormalities present in one medical
image or
multiple medical images. The system includes an image processor for processing
a medical
image and extracting features within the medical image relevant to diagnosing
the
abnormalities, the extracted features satisfying descriptions of a set of pre-
defined features,
a decision engine for generating a computed diagnosis from the extracted
features, and an
annotation and modification tool for a user to identify a set of features
within the medical
image aided with the extracted features and to establish a diagnosis based on
the set of
identified features and the computed diagnosis.
[0009] In one feature of this aspect of the invention, the plurality of
rules are calibrated
from a pool of diagnosed medical images. In another feature of this aspect of
the invention,
the system includes a lesion locator for analyzing the medical image and
identifying a
suspect lesion within the medical image. In yet another feature, the image
processor
segments the medical image, identifies a plurality of segmentation candidates
of the medical
image for user selection, and receives an indication from a user to process
one of the
segmentation candidates as a segmented image.
[0010] Optionally, a user is able to reject any of the displayed
segmentation candidates
and review the complete set of intermediate segmentation results leading to
the displayed
candidates with the objective of selecting another candidate. The user can
also refine a
selected candidate by modifying segmentation results, for example, by editing
existing
CA 02610345 2007-11-29
WO 2006/128302
PCT/CA2006/000906
- 4 -
control points or defining additional control points on a segmentation
outline, thereby obtain
a modified segmentation outline.
[0011]
In a second aspect of the invention, there is provided a system for providing
interactive computer-aided detection of abnormalities captured in a medical
image. The
system includes a display for presenting the medical image; input devices for
receiving user
input; an analytic engine for identifying image characteristics from the
medical image and
providing an initial set of identified image characteristics for user review;
and an annotation
and modification tool for a user to modify said initial set of identified
image characteristics
to obtain a modified set of identified image characteristics. The system
computes an initial
diagnosis from the initial set and a set of pre-defined criteria, provides the
initial set and the
initial diagnosis to the user for review, receives the modified set from the
user, and re-
computes a diagnosis from the modified set and the set of pre-defined criteria
for user
validation.
[0012]
In another aspect of the invention, there is provided a system for providing
computer-aided diagnosis of abnormalities in a plurality of medical images.
The plurality
of medical images are different views of a region of a patient's body. The
system includes
an image acquisition module for acquiring the plurality of medical images, an
image
processor for processing each of the plurality of medical images and
identifying an initial
set of features within the each medical image relevant to diagnosing the
abnormalities, a
decision engine for computing an initial diagnosis from the plurality of the
initial sets of
identified features, and an annotation and modification tool for a user to
modify the initial
set of identified features to obtain a modified set of identified features.
The decision engine
re-computes a computed diagnosis for user validation from the modified set of
identified
features.
[0013] In one feature of this aspect of the invention, the system is
configured for
processing medical images obtained from multiple modalities. These multiple
modalities
include at least two of sonographic images, Doppler images, spectral Doppler
images, X-ray
images, CT images, PET images, PET-CT images and MRI images.
[0014]
In yet another aspect of the invention, there is provided a method of
providing
interactive computer-aided detection of abnormalities captured in a medical
image. The
method includes the steps of obtaining a digitized medical image; processing
the digitized
CA 02610345 2007-11-29
WO 2006/128302
PCT/CA2006/000906
- 5 -
medical image to identify an initial set of image features within the
digitized medical image,
the initial set of identified image features satisfying descriptions of a set
of pre-defined
characteristics; providing the initial set of identified image features for
user review;
receiving a modified set of image features modified by the user from the
initial set of
identified image features; computing a diagnosis from the modified set for
user validation;
and producing a diagnosis report upon receiving a validated diagnosis from the
user.
[0015]
In yet another aspect of the invention, there is provided a method of
acquiring a
medical image aided by a computer-aided detection system, the computer-aided
detection
system having a medical imaging device for generating a medical image and an
analytic
engine for processing the medical image, the method includes the steps of
acquiring a
plurality of medical images from a patient using the medical imaging device,
analyzing each
of the plurality of medical image using the analytic engine; and adjusting
acquisition
conditions to obtain an optimal image from the plurality of medical images.
[0016]
In other aspects the invention provides various combinations and subsets of
the
aspects described above.
Brief Description of Drawings
[0017]
For the purposes of description, but not of limitation, the foregoing and
other
aspects of the invention are explained in greater detail with reference to the
accompanying
drawings, in which:
[0018] Figure 1 is a schematic diagram showing a CAD system that implements
an
embodiment of the present invention;
[0019]
Figure 2 illustrates schematically functional components and architecture of a
software system for controlling the CAD system shown in Figure 1;
[0020]
Figures 3A shows an exemplary screen display presented to a user by the system
shown in Figure 1, from which the user can select an initial segmentation
candidate and
define a region of interests ("ROI") for further study;
[0021]
Figures 3B shows another exemplary screen display for a user to enter
identification parameters to define a region of interests for further study;
CA 02610345 2007-11-29
WO 2006/128302
PCT/CA2006/000906
- 6 -
[0022] Figure 4A shows an exemplary screen display presented to a user
by the system
of Figure 1, from which the user may select one of several segmentation
candidates for
further processing and study;
[0023] Figure 4B illustrates schematically a segmentation candidate of
Figure 4A
showing only its segmentation boundary outline and its control points;
[0024] Figure 5 shows a suspect lesion being tagged as a type DCIS
lesion in an
exemplary screen display generated by the system shown in Figure 1;
[0025] Figure 6 shows an exemplary screen display presented to a user of
the system
shown in Figure 1, that displays initial results for further evaluation by the
user, the display
being the result of processing of the segmentation candidate selected by the
user from one
of the segmentation candidates shown in Figure 6;
[0026] Figure 7 shows an exemplary screen display that a radiologist may
use for
modifying and saving a summary text on findings generated from a build-in
template and
results shown in Figure 6;
[0027] Figures 8A and 8B show steps of a workflow implemented by the
software
system shown in Figure 2, wherein Figure 8A shows the first half of the
workflow and
Figure 8B shows the second half;
[0028] Figure 9 shows a process modified from that shown in Figures 8A
and 8B for
processing multiple images for a single lesion in a loop;
[0029] Figure 10 shows steps of another process modified from that shown in
Figures
8A and 8B for segmenting multiple lesions per image, or several lesions on
multiple
images;
[0030] Figures 11A to 11D are some exemplary screen displays produced by
the system
as a user follows the steps shown in Figure 10;
[0031] Figure 12 shows schematically a CAD system implemented differently
from that
shown in Figure 1;
[0032] Figure 13A and 13B show an exemplary screen display that a user
of the system
shown in Figure 12 may use to enter location and orientation information of a
probe or
transducer and a report incorporating such location and orientation
information; and
CA 02610345 2007-11-29
WO 2006/128302
PCT/CA2006/000906
- 7 -
[0033]
Figure 14 shows schematically a process that an operator uses the CAD system
shown in Figure 12 to obtain optimal imaging results and make a diagnosis.
Detailed Description of the Invention
[0034]
The description which follows and the embodiments described therein are
provided by way of illustration of an example, or examples, of particular
embodiments of
the principles of the present invention. These examples are provided for the
purposes of
explanation, and not limitation, of those principles and of the invention. In
the description
which follows, like parts are marked throughout the specification and the
drawings with the
same respective reference numerals.
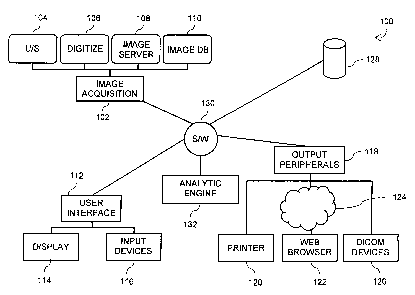
100351 Figure 1 shows a CAD system 100 that is controlled by a software
system for
automatically analyzing medical images, detecting, identifying and classifying
physical,
textural and morphological characteristics or other features of masses within
medical
images, providing computer-aided detection and assessment of suspected lesions
for user
selection, and allowing interactive feedback from a user to dynamically modify
a list of
detected features and the diagnosis computed therefrom. The user may be a
technician, a
radiologist, or a physician. The user may also be an operator of the CAD
system 100, for
example, a staff member, who receives instructions from a radiologist or a
physician from a
remote location. The CAD system 100 may be used by a user to acquire medical
images
from a medical scanning device and analyze the images in real-time. The user
may also
load a previously acquired medical image from a database for further analysis.
Alternatively, a user, such as a radiologist or physician, may share an image,
whether
acquired in real-time or previously acquired, with other radiologists or
physicians to
collectively evaluate and analyze the image and establish a diagnosis.
100361
The CAD system shown in Figure 1 has an image acquisition subsystem 102.
The image acquisition subsystem 102 can acquire medical images in real-time
when
connected to one or multiple medical image scanning devices. The CAD system
provides
in general a multi-modality platform. Which modality is selected depends on
the image
type. For example, the system may be implemented or configured to support
ultrasound
images, X-ray images, or CT, PET, PET-CT, Nuclear, MRI images, or images from
other
imaging modalities that is connected to the CAD system. The system itself may
also be
included in a console or workstation for review of some or all medical imaging
modalities.
CA 02610345 2007-11-29
WO 2006/128302
PCT/CA2006/000906
=
-8-
100371 In one implementation, the image acquisition subsystem is
connected to a
medical scanning device 104 for acquiring medical images from a patient in
real-time. As
noted, the medical scanning device 104 can be an ultrasound machine that
includes an
ultrasonic source and a transducer or transducers. The medical scanning device
may also be
X-ray based, consisting of an X-ray source and an X-ray imager. The medical
scanning
device may also be a CT, PET, Nuclear or MIZI scanner. Any suitable imaging
device for
acquiring medical images of a patient's tissue, bones or organs may be used.
[0038] The image acquisition subsystem 102 may also load previously
acquired images
for further study or for sharing with other users, such as radiologists,
technician or
physicians. For example, the image acquisition subsystem 102 may include a
digitizer 106
for digitizing a previously acquired image that is recorded on a film.
Alternatively, the
image acquisition subsystem 102 may retrieve an image from a remote image
server 108 or
from an image database 110 accessible to the CAD system.
[0039] The CAD system 100 includes a user interface 112 that allows a
user of the
system to view an image, to manipulate its presentation, and to interact with
the system.
The user interface 112 includes a display 114. The display 114 may be a
monitor, a
projector, or any other suitable display device that is capable of visually
presenting a
medical image to the user and is capable of presenting graphical and textual
contents. The
user interface 112 also includes input devices 116 for the user to interact
with the system
and to identify to the system particular regions of interest in the displayed
medical image.
The input device 116 may include a keyboard, for example, for the user to
enter any textual
input. A voice recognition module may be provided for voice-to-text
transcription. It may
also include a mouse or some other pointing device for the user to identify a
particular pixel
or region of the medical image to the system. Display 114 and input device 116
may be
physically combined into a single piece of hardware unit, such as a touch
screen that is
capable of both displaying graphic and textual output and receiving user
input.
[0040] The system 100 also provides a number of output peripherals 118.
A user may
use the output peripherals 118 to reproduce or record results of an analysis
session or other
output of the system. For example, the output peripherals may include a
printer 120. The
printer may be, for example, film based or paper based. A film-based printer
may be used
to transfer the medical images, either the original image or the processed
image to a film for
CA 02610345 2007-11-29
WO 2006/128302
PCT/CA2006/000906
- 9 -
use with more traditional display devices that require a filmed image. A paper-
based printer
may also be used to produce hard copy reports for sharing with other
physicians or for
archiving purposes. The output peripherals 118 may also include a web browser
122, for
sharing results with other radiologists or physicians over a telecommunication
network 124.
The telecommunication network 124 may be a local area network (LAN) or the
Internet.
This allows a physician to remotely review images obtained by an operator from
a patient
and make any modification in real-time to results automatically produced by
the system
100. In addition, the output peripherals 118 may include DICOM-compliant
devices 126
for transferring or storing processed results, namely composite images
generated by the
system together with associated reports.
[0041]
The system 100 has a data warehouse 128. The data warehouse may include its
own modules for retrieving and managing data, or may simply provide storage
space for
storing data therein. The data warehouse 128 is generally for storing system
related or
generated data, including archiving processed medical images. For example, the
data
warehouse 128 may be used for storing pre-diagnosed images, modeling
parameters, and
other pertinent data used by the system for providing automated detection.
Preferably, the
data warehouse 128 supports archiving DICOM-compliant images but other forms
of
images such as JPEG, BITMAP etc. may also be processed. Annotations, comments,
results of image processing all can be archived as part of a DICOM-compliant
file. Audit
information, such as user ID, date or time stamp of processed images, and user
addition or
modification of detected features all can be recorded for each archived
instance of a
processed image, as well.
100421
The system 100 shown in Figure 1 is controlled by a software system 130.
Referring to Figure 2, software system 130 coordinates and controls the flow
of data and the
processes implemented by the CAD system 100. The software system 130 has a
number of
components. These software components do not have to reside in a single
computer
hardware unit. They may be dedicated software systems stored at different
locations and
executing on different processors of the hardware units, or even as
independent modules
executing on different computers. The software components can also be provided
by
different manufacturers. For example, a medical scanning device manufacturer
may
provide its own software component for image processing or feature extraction.
These
software components can be combined together to provide the functionality of
system 100
CA 02610345 2007-11-29
WO 2006/128302 PCT/CA2006/000906
- 10 -
as described herein. These software components may also be combined in such a
way as to
form different subsystems to deliver dedicated sub-functionalities.
For ease of
convenience, in the following, these software components will be considered
conceptually
as part of the software system 130 that has all of its components stored on
one computer
readable medium, such as a hard disk, and executing on one processor. As will
be
appreciated, the CAD system provides in general a multi-modality platform.
This may be
achieved, for example, by providing a modality-specific component in each
component of
the software system 130, where required, to implement the supported
modalities.
100431
The software system 130 has an analytical engine 132 for analysing medical
images and deriving a diagnosis for user review and validation. For example,
in one
implementation, the analytical engine 132 processes images obtained by the
image
acquisition subsystem 102 to identify regions of interests for further feature
extraction,
extracts features presented in an image, such as physical or morphological
characteristics,
prepares the resulting information for display and review, and maps the set of
detected
features to a diagnosis for user review and confirmation.
100441
Figure 2 shows schematically components of the software system 130. The
software system 130 has a central control module 202 for controlling and
coordinating data
flow between and processes of various component modules of software system
130.
Software system 130 has individual modules for interacting, directing and
monitoring
corresponding hardware units or subsystems. Its image loader 204 interacts
with and directs
the operation of image acquisition subsystem 102 of the CAD system 100.
Conceptually
part of the analytical engine 132, an image display and manipulation module
206 is
provided for a user to adjust and manipulate the display of images. Also
provided as part of
the analytical engine 132 are an image processing module 208, a decision
module 210, and
an annotation and modification module 212. A report module 214 is provided for
producing reports and generating output.
100451
When a medical image is required for processing or viewing, the image loader
204 directs the image acquisition subsystem 102 to load, i.e., to retrieve or
obtain the
medical image. Once the medical image is retrieved or obtained, the image
display and
manipulation module 206 sends the image to the display 114 for displaying the
image to a
user. The user can use the input devices 116 to further manipulate or adjust
the display of
CA 02610345 2007-11-29
WO 2006/128302 PC T/CA2006/000906
- 11 -
the image on the display 114. A user may manipulate the displaying of image,
for example,
by changing its contrast, brightness level, or panning or zooming in or out of
a particular
region of the image. The user may also select a region of the image for
further processing.
[0046] The image processing module 208 or image processor is responsible
for pattern
recognition and feature extraction and performs various computerized image
processing and
pattern recognition operations. The image processing module 208 computes,
i.e., extracts
and identifies physical, texture, morphological as well as modality-specific
characteristics
associated with a mass defined by the boundary of an abnormal region, such as
a lesion or a
nodule, that has been identified in a segmentation process. In general, the
image processing
module needs to be implemented or configured differently to process images
obtained from
different modalities. For example, when an ultrasound image is loaded, the
features are
generally those defined for ultrasound images. The features may be those
associated with
the interior of a suspect lesion as well as those identified from regions
outside but adjacent
the boundary of an abnormal region, such as posterior shadowing in an
ultrasound image.
The features to be extracted or identified are generally pre-defined and
considered by the
medical profession as being relevant to diagnosing diseases, such as cancer.
The
descriptions of these features are generally provided together with the
definitions of these
features. One such set of pre-defined characteristics and lexicon is that
developed by
American College of Radiology (ACR) for use with Breast Imaging Reporting and
Data
systems (BI-RADS0). For different applications, different pre-defined sets and
standards
may be used. For example, as part of a standard, BI-RADS lexicon is primarily
used for
radiology, while the Bethesda System for Reporting Cervical Cytologic
Diagnoses is
primarily used for cytology. It will be understood that while the examples
provided herein
relate to diagnosing cancer, they are for illustration only and the system and
the process and
method described herein are applicable to diagnosing diseases in general, and
not restricted
to diagnosing cancer.
[0047] The required image processing operations may include segmentation
(i.e.,
selecting and delineating a region of an image for further study and
processing), pattern
recognition (i.e., analyzing and classifying patterns in an image) and feature
extraction (i.e.,
analyzing and identifying features or characteristics that may be relevant to
diagnosing
abnormal or normal conditions in the tissues represented by the image). Figure
2 shows
CA 02610345 2007-11-29
WO 2006/128302 PCT/CA2006/000906
- 12 -
three modules for segmentation, pattern recognition and feature extraction,
though it will be
appreciated that other modules may be included for other image processing
needs.
[0048]
The image processing module 208 is shown to have a segmentation module 216.
The segmentation module 216 analyzes a region of interest ("ROI") identified
by a user and
delineates the boundary of an abnormal region such as a nodule within the ROI.
The ROI
may be identified manually by a user, or automatically by the system and
suggested to a
user. In one implementation, the user selects and identifies the ROI to the
system by first
selecting a segmentation "seed point", i.e., a point in the interested region.
Figure 3A
shows an exemplary screen display from which a user may select an ROI.
Typically, the
seed point 302 is selected at a point near the general center of the
interested region, such as
a suspected solid nodule. The user may select the segmentation seed point by,
for example,
using a mouse and clicking a point in the central region of the nodule (see
Figure 3A). ROI
is defined by selecting the seed point and dragging the cursor away from that
point. A
circle appears constraining the region into which the segmentation algorithm
shall work.
The user releases the mouse button until the ROT 304 is sufficiently large as
to enclose the
entire nodule.
100491
Alternatively, a user may identify the ROT by providing a set of coordinate
values of the "seed point" and an estimated size of the lesion. This approach
may be further
refined, where the lesion appears to be an elongated mass, by providing an
orientation of an
axis generally aligned with the elongated mass and an aspect ratio. Figure 3B
shows a
location identification window 306 for a user to enter lesion identification
parameters 308,
which may include, for example, any one of a lesion identification number 310,
a lesion size
parameter 312, lesion coordinates 314, a lesion feature indicator 316, a
lesion depth
indicator 318, among others, and a combination thereof. Here, the lesion
identification
number 310 refers to an identification number, for example, a first lesion, a
second lesion, a
third lesion, and so on, among several lesions identified in the image. The
lesion size
parameter 312 provides an estimate of the lesion size, for example, 1 cm. The
location of
the lesion may be defined using a suitable coordinate system through lesion
coordinates
314, such as depth from skin, distance from nipple and azimuth angle from a
vertical
direction. The lesion feature indicator 316 refers to a feature type, for
example, features
related to mass, shape, orientation, calcification of a suspect lesion, among
others. The
CA 02610345 2007-11-29
WO 2006/128302 PCT/CA2006/000906
- 13 -
lesion depth indicator 318 provides an estimate of a depth of the lesion from
skin as a
relative measure, e.g., relative to the size of breast base.
100501
Advantageously, once a suspect lesion is identified, the image may be
segmented to delineate a boundary contour of the suspect lesion, or
segmentation outline.
This may facilitate further image processing, as image patterns and features
relevant to a
diagnosis of the suspected lesion are more likely those inside or outside but
adjacent the
segmentation outline. Different algorithms may be implemented for segmenting
an ROI.
For example, in one implementation, a front propagation type of region growing
algorithm
is implemented for segmenting lesions in an ultrasound image. A "seed point"
within the
suspect lesion is first selected. Adaptive thresholds are selected for
determining the
boundary outline. Region growing from seed point based on adaptive thresholds
may
further take into account local information and is constrained by domain
knowledge. Initial
region outlines are defined based on local information. When equilibrium is
reached,
defined region outlines are refined by deformable model driven by domain
constraints. It
will be appreciated that any suitable algorithm can be used for segmenting an
ROI.
Different applications may require different suitable algorithms. For example,
algorithms
best suited for segmenting images for diagnosing breast cancer may not be
optimal for
segmenting images obtained from a CT scan; as another example, a segmentation
algorithm
developed for ultrasound images will need to be re-tuned and/or modified to
process MRI
data.
100511 Each
algorithm can produce several segmentation candidates, i.e., segmentation
outlines that may correctly delineate the suspect lesion. Based on certain pre-
established
criteria, the system can present one as the best candidate and the rest as
second-best
candidates. The segmentation module 216 may present only the best candidate
produced by
the most suitable algorithm. Preferably, the segmentation module 216 presents
the best
candidate along with several second-best candidates for user selection. Where
several
algorithms are available, candidates identified by other algorithms may also
be presented for
user selection.
[0052] In one
implementation, the segmentation module 216 presents for user selection
6 segmentation candidates in a temporary window, as shown in Figure 4A. Each
candidate
image 402 is a composite image with the original image superimposed thereon a
possible
CA 02610345 2007-11-29
WO 2006/128302 PCT/CA2006/000906
- 14 -
lesion boundary 404. What is considered the best candidate 406 of the
segmentation
process is identified, e.g., by highlighting it, and made active for further
processing. Along
with the best candidate 406 are displayed several second-best results 408.
Only these six
candidates, instead of all segmentation candidates, are provided to the user
for selection. A
user may select one the system determined to be the best result. The user may
also select a
segmented image from one of the other candidate images 408. The user may
identify a
selection to the system by, for example, double-clicking a segmentation
candidate.
Optionally, a user can reject any or all of the displayed candidates and
review the complete
set of segmentation results. This allows the user to visually examine all
segmentation
results and pick one suitable candidate based on the user's own experience and
judgment.
Alternatively, the system may also be configured to select the best candidate
generated
using the most suitable algorithm for further processing, without any user
intervention.
100531 The user can also refine a selected candidate by editing the
segmentation outline
404. To do this, a user may edit existing control points or defining
additional control points
on a segmentation outline. The user may modify a displayed segmentation
candidate by
editing one or several control points 410 of the segmentation outline 404 to
manually
segment an ROI (see Figure 4B). The user may also modify a displayed candidate
by
defining new control point(s). After the user finishes editing existing
control point(s) or
adding new control point(s), the system displays a modified segmentation
outline for the
user to confirm. Once the system receives a selection from the user, the
system starts its
computerized pattern recognition and feature extraction process.
100541 The image processing module 208 shown in Figure 2 has a pattern
recognition
module 218. Pattern recognition module 218 analyzes an image, in particular an
ROI
delineated by the segmentation module 216, to identify and index morphological
and
texture patterns or features in the image. Pixels both inside and outside the
segmentation
outline are scanned to identify patterns or local features of a suspect lesion
and modality-
specific features such as sonographic characteristics. Local characteristics
such as local
spiculation, local branch pattern, local duct extension and local micro-
lobulation, may be
identified. The segmentation outline itself also can be analyzed to identify
features of the
suspect lesion that may be relevant to the diagnosis. Patterns, local
features, modality-
specific characteristics, features identified from the segmentation outline,
among other
features, are compared with descriptions of a set of pre-defined features,
such as
CA 02610345 2007-11-29
WO 2006/128302 PCT/CA2006/000906
- 15 -
sonographic characteristics defined by ACR-BIRADS lexicon or Stavros
characteristics, to
generate a list of features as identified from the set of the standard.
Pattern recognition
module 218 analyzes the image to identify these patterns and local features.
Pattern
recognition module 218 may also analyze the image to identify features such as
clustering
and contrast of pixels in a segmented ROI, or analyze the image to incorporate
some notion
of domain knowledge including surrounding information in order to better
identify specific
local features.
[0055] The image processing module 208 shown in Figure 2 has a feature
extraction
module 220 for extracting from these locally identified patterns special
features that may be
relevant to diagnosing cancer. Some of these features may include shape,
orientation,
angular margin, lesion boundary, and calcification. The features may also
include those
unique to a specific detection technology. For example, for an ultrasonic
image, the
features may include echo patterns and posterior acoustic features.
[0056] In one implementation, the feature extraction module 220 detects
features
matching descriptions of a set of pre-defined sonographic characteristics
combined with
ACR-BIRADS lexicon. In other words, a feature is considered to be identified
and detected
if characteristics of an object in the image satisfy the corresponding
description of the
feature in the set of pre-defined characteristics. The feature extraction
module 220 uses a
set of pre-defined characteristics and the characteristics' description, for
example, the ACR-
B1RADS lexicon, to make automated feature identification and extraction. The
feature
extraction module 220 uses detection performance thresholds to determine if
any feature
can be identified from the indexed local characteristics recognized by the
pattern
recognition module 218. The indexed characteristics are each assigned a
probability based
on a goodness-of-fit indicator against the description of the matched feature,
to provide a
statistical measure of the likelihood of their presence in the image. A
characteristic is
considered to exist in the image or is detected when the probability is above
that threshold.
Conveniently, all characteristics may be assigned the same threshold.
Preferably, these
thresholds may be based on Stavros' performance thresholds obtained from
calibrating a set
of diagnosed images. Such thresholds then depend on each characteristics and
are
determined from the results of calibrating the set of already diagnosed
images.
CA 02610345 2007-11-29
WO 2006/128302 PCT/CA2006/000906
- 16 -
[0057]
The software system 130 has a decision module 210 for computing an automated
diagnosis or assessment and suggesting the computed diagnosis to a user. The
decision
module 210 examines all features identified, including properties such as the
statistical
likelihood each feature may present and the extent or size of the feature,
ranks the
importance of each feature relating to a diagnosis, and computes an assessment
or diagnosis.
In general, a range of discrete assessments are possible. The particular set
of possible
assessments depends on the standard used. For example, the assessment may be
one of
benign, malignant, or indeterminate, or may be one to which BI-RADS category
the lesion
belongs. In other words, the decision module 210 maps the findings, or set of
features
extracted, to an assessment of the lesion based on the underlying model. As
will be
appreciated, different models may be employed for assessing suspected lesions.
The
modality of the software system 130 permits different models to be applied to
the same set
of features extracted to arrive at an assessment. As will be further
appreciated, the
operation of the decision module 210 is independent of the feature extraction
module 220.
The decision module 210 will provide an automated assessment whether the set
of features
provided to it as input is automatically identified, entirely identified
manually by a user, or a
combination hereof. In other words, the decision module 210 may be considered
as a sub-
system that provides the dedicated sub-functionality, namely, computing an
assessment, as
mentioned earlier.
[0058] Different modules may be provided for providing different diagnosing
functions.
Assessments obtained by applying different models may not necessarily be the
same.
Results from different models are combined, preferably with appropriate
weights, to arrive
at a computed diagnosis. The decision module 210 in Figure 2 is shown to have
an Al rule
module 222 and an assessment module 224, though it will be understood that the
modular
design of the software system 130 allows the substitution or addition of
diagnosis modules
where desirable.
[0059]
The Al rule module 222 makes use of knowledge gained in the past, such as
from diagnosis of a pool of image data, the corresponding biopsy results and
collective
knowledge of radiologists and physicians. In one implementation, the knowledge
is
summarized as a set of artificial intelligence (AI) rules. From the set of Al
rules, the
findings made from pattern recognition and feature extraction can be mapped to
an
automated assessment. As will be described in detail later, not all features
detected may be
CA 02610345 2007-11-29
WO 2006/128302 PCT/CA2006/000906
- 17 -
of equal importance. The importance of each detected and identified features
will be
incorporated in the set of Al rules. Al rule module 222 computes a preliminary
diagnosis
assessment based on the set of features detected and their relative importance
to a particular
diagnosis.
[0060] The following example outlines the steps of one method of producing
a set of Al
rules, in this case, to build a statistical model. A pool of diagnosed images,
together with
their corresponding biopsy results, is first selected. Characteristics
identified from these
images as well as the known diagnosed results are compiled. From these data, a
statistical
model based on mutivariate adaptive regression splines (MARS) technology can
be built,
Y = CO + I Ci * BFi
where CO and Ci are coefficients and BFi are the i-th basis functions. Each
basis function
takes as input a defined combination of defined set of characteristics and
potentially defined
set of basis functions. For example, a basis function may have the form BF240
= (ECHO
20 [0061] Once such a model is built, it can be incorporated into the
Al rule module 222
for computing a diagnosis, namely an overall likelihood that a lesion may be
benign or
malignant, based on the set of characteristics identified in the diagnosed
images. It will be
appreciated that the computation of an assessment is not limited to using a
statistical model.
The assessment may also be computed using a super vector machine (SVM) method
or may
[0062] Although an assessment may be provided in any manner, in general,
the
30 assessment module 224 provides a user with an assessment conforming with
a common
standard, such as providing a diagnosis as a BI-RADS assessment. A single
assessment
CA 02610345 2007-11-29
WO 2006/128302 PCT/CA2006/000906
- 18 -
may be presented to a user as an automatically computed diagnosis. Preferably,
a group of
possible BI-RADS assessments is presented to a user. In one implementation, a
user is
presented with one of two groups of assessments: "benign" which corresponds to
BI-RADS
1 to 3 and "malignant" which corresponds to BI-RADS 4a to 5. The user will
then have to
select a particular assessment from the suggested group of assessments or make
an
assessment selected from outside the suggested group. This tends to discourage
adopting an
automated diagnosis without evaluation by a user. Of course, other granularity
of the
grouping is possible. For example, the possible assessments may be divided
into "benign",
"intermediate, or possible benign", and "malignant."
[0063] After a
diagnosis is computed, the decision module 210 may also tag the lesion,
i.e., associate the lesion with a type. Some common types include fibroadenoma
(FA),
invasive ductal carcinoma plus DCIS component (II), invasive ductal (ID)
carcinoma, ductal
carcinoma in-situs (DCIS), and invasive lobular (IL) carcinoma. Generally, a
value of
confidence level that a suspected lesion may be of a particular type is first
computed. If the
value of confidence level falls within a defined confidence range, the lesion
is tagged as
belonging to that type. Figure 5 shows a suspected lesion being tagged as a
DCIS type 502.
[0064] Referring
to Figure 5, a controller 504, such as a knob-shaped activatable area on
a graphic user interface, allows a user to set a confidence range defined by
an upper
threshold 506 and a lower threshold 508. Figure 5 also can optionally display
values of
confidence level computed for different types on sliding rulers 510, to
indicate the
confidence level associated with lesion types. For example, Figure 5 shows
graphically
values of confidence level for types FA, II, ID, IL together with tagged type
DCIS. This
advantageously provides feedback to the user as to a likely type of the
suspect lesion.
Although Figure 5 shows only one type being associated with a lesion, it is
possible that
several types have the values of confidence level associated therewith falling
within the
confidence range. The system may then require a user to select a type, which
may be a type
with its value of confidence level falling within the defined range, or may be
one outside the
range. Alternatively, if a type has the largest value of confidence level, the
system may also
automatically tag the lesion to be of that type.
[0065] To
supplement the automated detection of characteristics, an annotation tool,
implemented as an annotation and modification module 212 is provided so that a
user may
CA 02610345 2007-11-29
WO 2006/128302 PCT/CA2006/000906
- 19 -
add annotations to an image or modify annotations already entered. Here,
annotation
generally refers to association of regions of a medical image, features or
characteristics of
the regions or patterns in or adjacent a region with features selected from a
pre-defined set
of features, such as that defined by BI-RADS. With the aid of annotation and
modification
module 212, a user can also add features that are not identified by the
software system 130
or remove false positives, i.e., features automatically detected by the system
but are
considered false detection. A user can also assign a different probability to
a feature or
modify a probability assigned by the system. Advantageously, as the list of
features and
their respective probabilities are modified by the user, the system
automatically re-computes
its automated assessment, to give the user an immediate feedback so the user
can make a
more accurate and improved diagnosis.
[0066] The annotation and modification module 212 provides a list of
detected
characteristics for a user to review and annotate. Such a list may be
presented to a user in a
results window 600 as shown in Figure 6. The results window 600 contains a
complete list
of a set of pre-defined characteristics, with the detected characteristics pre-
populated. Any
suitable set of pre-defined characteristics may be used. Some of them include
Stavros
characteristics and BI-RADS lexicon. In one implementation, the pre-defined
set is that of
BI-RADS. The results window 600 may be presented to a user on the display 114.
It may
also be made available to a web browser 122 connected to the system remotely.
The results
window 600 shown in Figure 6 has an image window 602 and a results panel 604.
A
composite image is displayed in the image window 602 along with an original
image.
Features detected 606 are indicated in the composite image where possible.
Some of the
features are annotated. An icon, symbol or other graphical representation may
be used to
indicate an annotation 608. In the bottom portion of the results window 600 is
a diagnosis
panel 610 for displaying computed diagnosis and for the user to select a
validated diagnosis.
Also shown at the bottom of the results window 600 is a comment window 612 for
entering
comments and annotations.
[0067] Together with the composite image displayed in the image window
602, features
detected automatically by the system are preferably presented to the user in
the results panel
604 in a tree-structured feature list. Referring to Figure 6, the results
panel 604 shows a
series of checkboxes 614 linked in a tree-structure to indicate their
interrelationship. Each
checkbox in Figure 6 corresponds to a characteristic of the Stavros
characteristics. Some of
CA 02610345 2007-11-29
WO 2006/128302 PCT/CA2006/000906
- 20 -
these checkboxes are activated, i.e., highlighted or checked. A checked
checkbox 616
indicates that the feature has been detected in the image displayed in the
image window 602
on the left hand side. An unchecked checkbox 618 indicates that the
corresponding
characteristic or feature is not detected in the image.
[0068] The user can modify the automated detection by removing a detected
characteristic from the list or add a characteristic to the list. If the
removed characteristic
can be traced back to a region in the image, the displayed image may be
automatically
updated to indicate the removal of the characteristic, for example, by
removing the
corresponding icon. Conversely, a user can add a characteristic that has not
been identified
by the system in an automated detection process, namely, to identify a
location in the
medical image as the site of the characteristic. A characteristic manually
added to an image
can be automatically added to the list of identified characteristics. The
annotation and
modification module 212 allows the user to verify and confirm the system
findings and
make any necessary modifications based on his or her judgment and expertise.
Annotations
can be applied multiple times to each image. Referring to Figure 6, if a user
unchecks a
characteristic that can be traced back to the image in the image window 602,
the unchecked
characteristic 620 is removed automatically from the image. To add a
characteristic, the
user may simply check the checkbox corresponding to the characteristic. The
user may also
use the annotation tool to drag a checkbox corresponding to the characteristic
to be added to
a desired location on the image and release it. A symbol or icon
representative of the
selected characteristic 622 will be dropped at the selected location. A user
can then enter
or edit a comment in the comment window 612 per added annotation. This step
can be
repeated for as many annotations and characteristics as desired or required.
Each time a
characteristic is added or removed, the image is updated where possible. In
other words, if
the characteristics may be represented by a symbol or icon image, that symbol
or icon is
also added or removed.
MOM
As the list of features (or characteristics) is modified or updated by the
user, the
system also updates its computed diagnosis at the same time. It will be
appreciated that
when a user adds a new feature, the user may also assign a probability to that
finding. In
one implementation, all user added features are assigned a probability of 100%
and all user
removed features are assigned a probability of 0%, but other values of a
probability can be
assigned, too.
CA 02610345 2007-11-29
WO 2006/128302 PCT/CA2006/000906
- 21 -
[00701 In one implementation of the system, nine different diagnosis
categories are
provided, namely, {Incomplete, 1, 2, 3, 4a, 4b, 4c, 5 and 6}. This set
corresponds to the
categories used in BI-RADS. According to this scheme, 1 is Negative, 2 is
Benign Finding,
3 is Probably Benign Finding, 4 is Suspicious Abnormality (which is further
sub-divided or
refined in the field by radiologists into 4a, 4b and 4c: 4a is finding with a
low suspicion of
being cancerous, 4b is finding with an intermediate suspicion of being
cancerous and 4c
finding of moderate concern of being cancerous, but not as high as Category
5), 5 is Highly
Suggestive of Malignancy and 6 is Known Cancer.
MOWN! These possible diagnosis are divided into groups, or buckets. Different
granularity, i.e., different number of buckets, may be implemented. In one
implementation,
a two-bucket approach is taken. In the diagnosis panel 610, the first bucket
624 is shown to
include diagnosis 1, 2 and 3 and the second bucket 626 includes diagnosis 4a,
4b, 4c and 5.
In the initial results displayed, the system will only highlight one of the
two groups instead
of any particular diagnosis. A user may select a diagnosis from the group,
making a
diagnosis. The user may also override the system and select a diagnosis
outside the group if
the user strongly disagrees with an automated diagnosis computed by the
system. As will
be described later, a user may be required to select a diagnosis before the
system will
produce any report.
[0111,11,10n one implementation, a user must validate a diagnosis by selecting
one
diagnosis from a default group, i.e., by selecting one diagnosis from either
the first bucket
624 or the second bucket 626. Without selecting a diagnosis, all possible
diagnosis in the
default group are highlighted. This tends to reduce the risk of accidentally
confirming a
diagnosis without a detailed examination of the results of automated
detection.
[0073] Using the annotation and modification module 212, a user can
annotate both
benign and malignant sonographic characteristics as described above.
Annotation and
modification module 212 also allows a user to add comments and notes related
to
annotations (annotation comment) or general notes related to the image
(general comments).
A general comment may be entered in the comment window 612. These comments and
notes may be entered as text, picked from a list of pre-defined comments, or
transcribed by
a voice-to-text module.
CA 02610345 2007-11-29
WO 2006/128302 PCT/CA2006/000906
-22-
100741 Conveniently, the annotation and modification module 212 may
include an
optional build-in template for generating a summary text, or summary note, as
part of the
general notes, reporting findings and the radiologist's assessment. The
template provides
the basic structure of a summary text, with suitable statements selectable by
the annotation
15 [0075] The report module 214 interacts with and directs the
operation of output
peripherals 118 of the system as well as communicating with the data warehouse
128. The
report module 214 also interacts with the user interface 112 for displaying
the processed
image or any report. Once an assessment is validated by a user, the report
module 214
produces a report for the current active image. The report may be printed on a
printer 120,
25 selected characteristics, as a part of a DICOM-structured report for
that processed image.
The same information may also be sent to a DICOM-compliant device 126 for
storing or
sharing with other physicians or radiologists.
[0076] The report contents are generally by default based on the data
available in the
processed image as annotated by the user and also contains other pertinent
information,
30 such as institution or patient identification information and the
patient's demographic
information. In other words, data available in the results window 600 are
generally
reflected in the report. The report may include detected features such as
sonographic
CA 02610345 2007-11-29
, WO 2006/128302 PCT/CA2006/000906
- 23 -
characteristics along with any annotations and comments and user
modifications. Original
medical image and its processed counterpart can be included as well. The
report can also
include other information such as institution information, patient demographic
information,
an overview of the software application and its algorithm settings. Finally,
the report may
contain the image findings and assessment of the radiologists, for example, in
a format
complying with the ACR-BIRADS Ultrasonic Lexicon Classification form.
[0077] The report can be provided as a form, with suitable boxes
checked to indicate
findings and an assessment. Conveniently, the report may include a summary
list, listing all
identified features. The report may also include a summary text, or
supplemented with a
summary text. The summary text may be based on findings and impressions
generated by
the annotation and modification module 212 and further modified by a
radiologist. The
summary text may also include a recommendation whether biopsy should be
performed.
[0078] A report may include identification information for traceability
and auditing
purposes. Identification information may include patient identification
number, study
identification number, unique report identifier, series number, time stamp,
namely the time
and date of the study or report, or other suitable identification information.
Conveniently, a
cryptographic module may be provided for signing the report digitally. An
electronic
signature generated by the cryptographic module may include some or all
identification
information to provide improved audit capability and to discourage accidental
modification
of the reports.
[0079] Multiple lesions from one image may be processed in one session,
in which case,
a single report containing all findings can be produced. Alternatively,
multiple images may
be processed in one session that leads to a single report containing all
findings about all
lesions in all images. The report can group the findings by lesion,
characteristics identified,
images processed or in some other fashion. An overall assessment, such as a BI-
RAD
assessment taking into account of findings about multiple lesions in a medical
image, a
single lesion seen in multiple images for the lesion, or multiple lesions in
multiple related
images, may also be provided.
[0080] Preferably, reports are archived as DICOM Secondary Capture.
Annotations,
comments, image processing results such as lesion boundaries and diagnosis
results are
archived as part of a DICOM-compliant file. A user can also save, for example,
a PDF
CA 02610345 2007-11-29
WO 2006/128302 PCT/CA2006/000906
- 24 -
version of the report locally in a patient's instantiated directory. This
facilitates easy future
reference. If an instance for that composite rendering already exists in the
archive for the
patient, a new instance is created. Audit information, such as user ID, date
or time stamp,
and user addition or modification of detected features, can be recorded for
each archived
instance.
[0081] With reference to Figure 8, steps of a workflow 800 are now
described in detail.
This is a work flow implemented by the system to match that of a radiologist
but with
further flexibility and user control built into the process. Images are first
acquired and
loaded by the image acquisition subsystem 102 under the control of image
loader 204 at the
image acquisition step 810. As described before, image loader 204 may load an
image from
a medical scanning device, load a medical image from the image database 110,
or receive a
medical image from a remote image server 108, among others.
[0082] Once the image is loaded, the image display and manipulation
module 206
displays the image on the display 114 at step 812. The user can manipulate the
presentation
of the image in a variety of ways in order to better view the image either as
a whole or focus
on a particular region. For example, a user can zoom or pan the image. The
user can adjust
brightness and contrast levels of the image as displayed on the display 114.
Thus, a user
can examine the image in great detail as well as to view any suspicious
regions in context.
In one implementation, the image acquisition subsystem 102 supports the
acquisition of
multiple images. Image display and manipulation module 206 provides a
predetermined
number (for example, 4) of images for selection at step 812. For example, the
image
scanning device may provide several images of cross-sections of an anatomical
part of a
patient, such as a breast, for viewing and selection by the radiologist. The
image display
and manipulation module 206 may display all cross-section images on a display
114, or it
may display only one of them, while displaying the rest as some thumbnail
views. The
user, such as a radiologist, may select one of the views for further
evaluation and study. In
case of breast ultrasound images, two views may be provided per case at the
same time (one
Radial and one Anti-Radial), also known as "R and AR views".
[0083] At a next step 814, the user may add annotations to the selected
image as
described in connection with the annotation and modification module 212. The
user may
also add annotations later after a results window 600 is pre-populated with
automatically
CA 02610345 2007-11-29
WO 2006/128302 PCT/CA2006/000906
- 25 -
detected features. Next, the user initiates CAD processing by identifying and
selecting an
ROI at step 816. Once the ROI is identified at step 816, the segmentation
module 216
begins processing the image and attempts to identify possible boundaries of an
abnormal
region such as a nodule.
[0084] During a segmentation step 818, a series of possible boundaries, or
contours of a
suspected nodule, are generated. Instead of selecting one boundary
automatically, the
segmentation module 216 requests the image display and manipulation module 206
to
overlay the possible boundaries with individual images to provide a series of
candidate
images and provide these candidate images for user selection.
[0085] At step 820, a user selects one of the candidates and communicates
that selection
to the system, for example, by pressing an "OK" button. Once the system
receives the
selection from the user at step 818, the system starts further processing at
step 822. At step
822, pattern recognition and feature extraction takes place. Optionally, a
user may
manually modify the selected contour by means of defining or modifying control
points 410
on the candidate contour and moving or editing them as shown in Figure 4B.
[0086] Features detected at step 822 are next provided to the decision
module 210 for
computing a computed diagnosis. The auto-diagnosis step may include an Al rule
mapping
824 step, during which the Al rule module 222 maps these characteristics to an
intermediate
result based on a set of pre-defined Al rules. The assessment module 224
combines the
result of Al rule mapping with the analysis of detected characteristics to
arrive at an
automated diagnosis at step 826.
[0087] At step 828, in a results window 600, the user is presented with
an initial result
from the automated detection process. The results window 600 is pre-populated
with all
detected features as well as with a group of suggested diagnosis.
[0088] A user can add or delete features by selecting or unselecting
checkboxes shown
in the results panel 604 (step 830). Based on this dynamically modified
feature list, as well
as their assigned probabilities, auto assessment module 224 dynamically
updates the
computed diagnosis. A different group of diagnosis may be dynamically
displayed if the
modification of the feature list is such that the automated diagnosis changes
from one group
to the other, such as from one of 4a, 4b and 5 to one of 1, 2 or 3, or vice
versa.
CA 02610345 2007-11-29
WO 2006/128302 PCT/CA2006/000906
- 26 -
[0089] Once a user is satisfied that features seen in the image are all
selected in the
feature panel and the checkboxes in the feature channel do not have any false
detection, the
user may confirm or select a diagnosis (step 832). After a diagnosis is
validated or selected
by the user, the reporting module 214 at step 834 automatically produces a
report. Results
from the analysis can be saved to a data warehouse 128, or shared with other
physicians or
radiologists. Audit trail of operations by the user, including selection of
ROI, selection of
segmentation candidates, annotation and modification of results and validation
of diagnosis,
can all be saved. This completes the image processing workflow 800.
[0090] As will be appreciated, although the workflow 800 described here
is for
processing one image at a time, with modification, the system may be used to
process
multiple lesions or multiple related images in one single session. For
example, the system
may be configured to permit the user to return to step 816 to select another
lesion or ROI at
the conclusion of step 832, instead or proceeding to the reporting step 834
directly. The
user may also return to step 810 to load another image for processing in the
same session.
The system may also be further configured to correlate the same lesion shown
in different
images, After all lesions in the same image or all images are processed, the
user can then
proceed to step 834 to produce a single report, containing results on all
lesions in all images
processed. Further, a global assessment based on all characteristics
identified in all lesions
(in all images) may also be produced and presented to the user for review and
validation.
[0091] In one implementation, the system is configured to assist a user to
process
multiple images for a single lesion. Referring to Figure 9, there is shown a
process for
processing multiple images for a single lesion in a loop 900. At step 910, one
of the
multiple images is first loaded. The loaded image may be segmented already, or
not
segmented as yet. Once loaded, an ROI is identified at step 920, for example
by identifying
its "seed point" and size using a graphical pointing device as shown in Figure
3A or through
identification parameters entered in a window as that shown in Figure 3B.
Next, the image
is examined at step 930 to determine whether the identified ROI is segmented.
If it is
already segmented, then segmentation 940 may be bypassed. A user may also
elect to
bypass segmentation even if an image is not segmented. As described earlier, a
user may
use the annotation tool to identify a list of features to the system, from
which the system
also can compute a diagnosis. If segmentation is to be bypassed, the system
proceeds to
step 950 for further processing, such as pattern recognition, feature
extraction and diagnosis
CA 02610345 2007-11-29
WO 2006/128302 PCT/CA2006/000906
- 27 -
computation. Alternatively, or if the ROT is to be segmented, the image is
forwarded to
segmentation module 216 for segmentation 940 and further processing.
[0092] After the image is processed, for example, following the
remaining steps 818 to
822 as described in reference to Figure 8, the process may return to the
beginning of the
[0093] In another implementation, the system is configured to assist a
user to process
multiple lesions per image, or several lesions on multiple images. Figure 10
shows a series
[0094] At step 1010, two images are loaded and shown to a user for
selection of lesion
candidates. Figure 11A shows a first image 1102 containing a first lesion 1104
and a
[0095] A lesion so identified may be marked with a circle or a generally
oval curve
[0096] Referring back to Figure 10, a lesion identified at step 1020 is
segmented. The
system may segment the image as described before, providing several
segmentation
CA 02610345 2007-11-29
WO 2006/128302 PCT/CA2006/000906
- 28 -
single segmentation candidate 1112 in a segmentation panel 1114 on the right
hand side and
an oval curve 1110 encircling the first lesion 1104 in the first image 1102 on
the left hand
side. In this example, only one segmentation candidate is provided by the
system although
it will be understood that multiple candidates are provided in general. Figure
11D shows a
segmentation candidate 1116 in a segmentation panel 1114 and the second lesion
1108 in
the first image 1102 on the left hand side.
100971 In another implementation, the system may take advantage of its
ability of
loading several images for a single lesion to perform segmentation in a three-
dimensional
space. As will be appreciated, a three-dimensional region can be represented
by a series of
slices. Each slice may be a two-dimensional image and contains a region
corresponding to
the lesion. As the series of images, or slices, are loaded, the representation
of the lesion in
each slice can be correlated with each other. The stack of slices thus
provides a three-
dimensional data set. As in a 2-dimensional segmentation process, the system
can also
segment the three-dimensional dataset and provides series of segmentation
candidates in the
three-dimension space for user selection, each segmentation candidate being a
three-
dimensional envelop enclosing the suspect lesion. A user can select one
envelop from the
candidates that best fits the boundary of the suspect lesion.
100981 In a further modified implementation, the CAD system displays in
a temporary
window, i.e., a temporarily allocated display region, a series of images for
user review and
selection. Advantageously, these images displayed in the temporary window can
be
"thumbnail" images. For example, at step 910, instead of loading one image,
several
thumbnail images may be loaded in the temporary window for selection. A
thumbnail
image is a version of a loaded medical image, generally with a reduced size,
for example, by
reducing its resolution. Because of its reduced size, a thumbnail image
generally permits
faster processing and manipulation. Images corresponding to these thumbnail
images can
be different slices of a three-dimensional data set, can be different versions
of a medical
image having different lesions highlighted, can be different medical images
showing the
same lesion, or images of the same region taken at different times, or a
combination thereof,
among others. These images can be images acquired in real-time or images
retrieved from
archives.
CA 02610345 2007-11-29
WO 2006/128302 PCT/CA2006/000906
- 29 -
[0099] These thumbnail images can be a series of images with suspect
lesion candidates
highlighted for user review and selection. Conveniently, these lesion
candidates can be
automatically identified by the system. For example, the system may image
processor may
be provided with a lesion locator for this purpose. The lesion locator first
scans all pixels in
each of the images corresponding to the thumbnail images and performs an image
analysis.
Regions that appear to have distinct features may be suggested as regions
corresponding to
lesion candidates to a user. Alternatively, regions having characteristics
like texture that
differs from the background may be suggested as lesion candidates to a user.
Thus, the
system can dynamically provide a number of lesion candidates for user
selection, without
requiring the user to identify or define a region of interest to the system
first. The system
may further segment each of the regions corresponding to lesion candidates and
present to
the user, along with each lesion candidate, the best segmentation candidate
for each lesion
candidate. Thus, the steps 920 to 940 may be automated, with minimum user
intervention.
This provides further assistance to a user in identifying lesions in medical
images.
[00100] It will be appreciated that lesion candidates can be identified using
any suitable
method, not restricted to examples described above. For example, in the case
of a three-
dimensional data set, lesions identified in one of the slices can provide
indication of lesions
in neighboring slices. As another example, an MRI data set may be a series of
contrast-
enhanced MRI images obtained at regular time intervals. Before or during the
exam, a
contrast enhancement agent is injected into a vein in a patient's arm.
Typically, a
gadolinium based contrast agent (e.g., Gd-DTPA) is used. The use of contrast
agents tends
to provide greater contrast between normal and abnormal tissues. Analyzing the
time-
variation of enhancement also facilitate delineating a sub-set, or sub-volume,
of imaged
region, or multiple sub-sets, as lesion candidates, which the system can
suggest to a user.
[00101] Advantageously, the temporary window for displaying thumbnail images
can be
configured for displaying thumbnail images that may be of interest to a user.
For example,
a user may select an image and place it in the temporary window for later
processing. The
image placed there may have been processed, partially processed, or not
processed at all. A
partially processed image may have a few lesions identified by the user but
have not been
processed to extract features from the lesions. Conveniently, the CAD system
may process
all newly acquired images to identify lesion candidates as described above and
place in the
temporary window those images that contain at least one suspect lesion. Thus,
the
CA 02610345 2007-11-29
WO 2006/128302 PCT/CA2006/000906
- 30 -
temporary window may have placed therein a series of thumbnail images
corresponding to
images that a user may wish to examine further. Preferably, the thumbnail
images are
ranked to assist a radiologist to prioritize them. Any suitable ranking system
may be used.
For example, the list of thumbnail images can be ranked by the likelihood that
an image
contains a lesion. The image with the most suspicious lesion is then placed on
the top of the
list. Or, a coloring system can be developed to indicate the likelihood. For
example, a red
outline can be used to indicate that a lesion candidate is most suspicious,
yellow for
significant suspicion, and blue for unprocessed images. Different ranking
system may also
be combined to provide better assistance to a radiologist.
1001021 Once a candidate lesion is identified, either selected by the user or
by the system,
the user may continue with the CAD process. For example, the CAD process may
continue
with extracting features associated with the candidate lesion and computing a
diagnosis
from the extracted features, the details of which have been described earlier.
Of course, the
user may also elect to bypass pattern recognition and feature extraction and
decide to select
manually features within the medical images, as described earlier. The CAD
software 130
is then used for computing a diagnosis from manually identified features
associated with the
lesion or lesions.
1001031 In operation, a user first initiates the CAD process by acquiring an
image or
several images so that the system 100 may load the image or images for review
and further
analysis. Such further review may be based on a user-identified ROI or a
general evaluation.
The system or software system initially displays a gallery of several, for
example 6,
candidates of segmented images or candidates of suspect lesions on the display
114. The
user may select any candidate and perform the interactive, controlled image
analysis in real-
time for further analysis of anatomy and pathology.
1001041 If the images are acquired in real-time, the system may be configured
to provide
feedback to the user and guide the user to adjust the medical scanning device
104 to acquire
a better image. This enables a radiologist to obtain an optimal image during
one
examination session, without having to recall a patient for another
examination due to,
suboptimal images being obtained. Suboptimal images may be caused by, for
example,
artificial shadowing due to improper orientation or positioning of an
ultrasonic transducer.
With real-time feedback, the user may adjust the orientation or position of
the ultrasonic
CA 02610345 2007-11-29
WO 2006/128302 PCT/CA2006/000906
-31 -
transducer to remove any artificial shadowing. Or, the user may move the
transducer to
sweep across a suspected region. A frame-grabbing technology can be
implemented to help
identify the best orientation or position of the instrument. As inappropriate
pressure applied
at the interface of transducer/gel/skin may degrade the quality of ultrasound
images
[00105] With the aid of a CAD system, a user can therefore obtain an optimal
image or
images for more clearly showing any abnormalities that may present in the
tissue. The
following steps can be followed. The image is first segmented if desirable,
with a number
15 characteristics. The detected characteristics are pre-populated
automatically in the results
window 600 as a list of detected characteristics.
[00106]
Further user control of the detection process is possible at this point. For
example, as described before, a user may add or remove any or all
characteristics originally
identified by the system. The Al rule module 222 and the assessment module 224
20 automatically computes or re-computes a diagnosis based on the
modification by the user
and then updates a BI-RADS assessment automatically. A report can be generated
upon a
diagnosis being validated by a user. Alternatively, the user may move or
adjust the medical
scanning device in order to obtain a better image, from which features are
identified or
extracted with a higher confidence level. The user can keep adjusting the
medical scanning
25 device and reviewing the results of image processing and analysis until
an optimal image is
obtained.
[00107] As described earlier, different medical imaging devices may be
integrated with a
CAD system. In one implementation as shown in Figure 12, the medical scanning
device 104
is an ultrasound machine 1202 that has a dedicated software application 1204
for indexing
30 image frames with positioning and orientation of ultrasound transducer.
The software
application 1204 is operatively connected to both the ultrasound machine 1202
and the CAD
CA 02610345 2007-11-29
WO 2006/128302 PCT/CA2006/000906
- 32 -
software system 130. Any medical images acquired by the ultrasound machine
1202 has an
index, which corresponds to a position coordinate and orientation angle of the
transducer when
taking the image. As an operator of the ultrasound machine 1202 moves the
transducer of the
ultrasound machine 1202 around a patient, a series of images 1206 are
produced, each having a
location and an orientation index. The operator may review the series of
images, and select
one that is considered of the best quality from the series of images for
further processing. For
example, once selected, the best image 1208 may be segmented and a gallery of
six
segmentation candidates may be displayed in a temporary window 1210 for user
selection and
that further feature extraction can be performed on the selected segmentation
candidate.
Alternatively, the operator may feed the series of images to the CAD software
system 130 to
initiate a CAD process on each of the acquired images.
[00108] As described earlier, the CAD software system 130 can be used to
identify and
extract a list of features from each of the images and compute an automated
diagnosis based on
the features extracted and identified. It may be possible that the operator
may decide, before
the CAD process is completed for all of the images, that the series of images
do not represent
optimal imaging. For example, it may be possible that because of improper
positioning or
orientation of the transducer, some artificial shadowing is introduced into
the images. The
artificial shadowing may cause difficulties for the CAD software system 130 to
correctly
identify true abnormalities in the images. Inappropriate pressure applied to
an ultrasound
transducer may also degrade the image quality. The early discovery of poor
quality of the
images allows an operator to adjust detection parameters such as position,
orientation or
pressure of the transducer, or even the position of the patient in order to
get optimal images.
This provides immediate feedback to the operator as to the quality of images
obtained so that
corrective actins, such as transducer repositioning, may be taken.
[00109] Once the operator is satisfied that the optimal images are
obtained, the operator
may select one best image 1208, so that the CAD software system 130 may
continue with the
CAD process, as described before. A list of automatically detected features as
well as an
automated diagnosis may be computed from the features once a segmentation
candidate is
selected by the user. The results are displayed in a results window 600. The
user, as described
before, may then confirm or modify the features automatically identified by
the system, and
then validate a diagnosis based on the suggested group of diagnosis presented
to the user. The
validated diagnosis, together with the medical images and other detection
results, may be
CA 02610345 2007-11-29
WO 2006/128302 PCT/CA2006/000906
- 33 -
saved, transmitted for sharing with other radiologists, or used for producing
a report, using the
output devices 1212.
[00110] Advantageously, when the CAD software system 130 is connected to a
transducer
for obtaining images in real-time, an operator may also enter the location and
orientation of the
transducer or probe through a probe/transducer location window for inclusion
in a report.
Figure 13A shows a graphical user interface for a user to enter the location
and orientation
information. The location may be entered by selecting a point in the wireframe
diagram 1302.
To facilitate entering orientation information, a rectangle 1304 for
representing a probe or
transducer is displaced superimposed onto the wireframe diagram. By rotating
the rectangle
1304, an orientation of the probe or transducer may be entered. Figure 13B
shows a page of
the report that provides the recorded location and orientation information.
[00111] Figure 14 is a flow chart summarizing the process 1400 described above
for
obtaining optimal images and then making a diagnosis based on the computed
results produced
by the CAD software system 130. Briefly, an operator initiates the process at
step 1402 by
acquiring images using a medical scanning device 104. Next, at step 1404, the
operator
initiates a CAD process to analyze the acquired image or images and extract
and identify
features relevant to a diagnosis. During the CAD process, the operator decides
whether the
image acquired is optimal, and adjusts accordingly image acquisition
conditions, such as
position and orientation of a transducer or positioning of the patient, at
step 1406 in order to
obtain optimal images. This process may be repeated until the operator is
satisfied that optimal
images are obtained. The operator then continues at step 1408 to make a
diagnosis based on
features identified and extracted from the optimal image as well as a
diagnosis computed from
the extracted features.
[00112] Variations to the process 1400 described is also possible. For
example, real-time
feedback may be provided during the process so that a user does not have to
complete the
CAD process on all images acquired. For example, each scan may produce a
series of images,
which may be displayed in a temporary window as a series of thumbnail images.
As described
above, the thumbnail images may all have different views of the same lesion
automatically
identified by the system, or may be the same initial image, with different
lesions identified in
each thumbnail images. Prior to proceeding further with steps 1404 to 1406, a
user can select
from the thumbnail images one or several images for further study and discard
the remaining
CA 02610345 2013-03-11
WO 2m16:1224302 PCT1CA20061000906
34 -
Ones Thus, instead of using the process 1400 for obtaining an optimal image, a
user can also
use a process modified from process 1400 for dynamically picking images for
studying a
particular suspect lesion or lesions.
(NI 131 As another example, the configuration shown in Figure 12 also allows
the operator
.5 to study the elasticity of a lesion, i e., to acquire elastogaphy
images. To initiate the process,
the operator starts by introducing some vibration into the region of tissues
under examination.
For example, the operator may apply some pressure to the tissues surrounding a
lesion and
then release the pressure. As will be appreciated, abnormal region such as a
lesion or nodule
may have different elasticity than its surrounding tissues. As the vibration
is introduced into
the tissue, elasticity of the abnormal region may be studied from the series
of frames, or
images. As will be appreciated, an abnormal region may have different
elasticity and therefore
may respond differently to the vibration than the surrounding tissues. The
series of images
captured, once indexed as a time sequence, can be used to identify legions or
nodules based on
elasticity variations. In one implementation, the segmentation module utilizes
these elasticity
differences as identified from a series of frames to provide a better
selection of segmentation
candidate.
1001141 As a further example, in another implementation, the medical scanning
device 104
shown in Figure 12 is a Doppler imager. As will be appreciated. Doppler
imaging is sensitive
to blood flows in blood vessels. If a transducer applies too much pressure on
the tissues,
thereby impeding blood flow inside the vessels, the image obtained may be of
poor quality.
The system provided by the configuration shown in Figure 12 provides an
immediate
feedback, such as an audible alert, to the operator, if the pressure applied
by the transducer is
too great As part of the step of adjusting detecting and acquisition
conditions, the operator
may adjust the pressure of the transducer applied on a patient's skin, in
order to obtain optimal
Doppler images.
100115) Various embodiments of the invention have now been described in detail
Those
skilled in the art will appreciate that numerous modifications, adaptations
and variations
may be made to the embodiments without departing from the scope of the
invention,