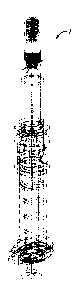Note: Descriptions are shown in the official language in which they were submitted.
CA 02611543 2007-11-22
STICK-RESISTANT MEDICATION ADMINISTRATION SYSTEM
RELATED APPLICATION DATA
The instant application claims priority to prior provisional application no.
60 / 933,191,
filed June 5, 2007, still pending.
FIELD OF THE INVENTION
The present invention relates generally to medication administration systems,
and in a
particular though non-limiting embodiment, to a syringe-like device admitting
to medication
administration via intravenous, intramuscular, and/or subcutaneous routes in a
safe and
controlled manner, and without the danger of a needle-stick injury.
BACKGROUND OF INVENTION
As will be readily appreciated by those of skill in the pertinent arts,
contaminated needle-
stick injuries constitute a longstanding and very serious problem in the
healthcare industry.
There are currently more than 20 known serious and potentially fatal blood-
borne diseases
transferable via needle-stick injury, the most serious being HIV and
Hepatitis. According to one
recent worldwide confidential survey, the problem remains widespread despite
healthcare
industry efforts to prevent accidental needle-sticks, with as many as one in
four nurses in the
United States reporting that they had received at least one needle-stick
injury in the past 12
months. In Sub-Sahara Africa, where the spread of HIV/AIDS is an epidemic,
nearly two-thirds
of nurses reported accidental needle sticks.
Needle-sticks do not generally occur out of ignorance or because nurses do not
know how
to safely give an injection, but rather because presently employed methods of
injection utilize
exposed needles that are inherently very sharp and dangerous. In short,
anytime a needle or
1
CA 02611543 2007-11-22
other sharp object is exposed to a healthcare worker or another person, there
is a risk of
accidental needle-stick injury, often times depending on entirely
unforeseeable conditions and
circumstances, such as a bump from a co-worker, temporary loss of control of
the device, patient
movement, recapping, and so on. Many of the unforeseen circumstances that
create needle-stick
injuries are difficult, if not impossible, to anticipate or control. For these
reasons and others, a
better solution is required.
Prior efforts to prevent needle sticks have proven inadequate primarily
because the
needlepoint remains exposed to the healthcare worker during use of the device.
It is generally
only after an injection is fully performed that currently known devices offer
a safety mechanism
to cover or remove the needle from exposure in some fashion. According to the
best figures
available at this time, such designs have achieved a maximum reduction of no
more than one-
half of all needle-stick injuries, and offer no solution for the remaining one-
half of needle-sticks
that occur between the time a syringe is opened and completion of the
medication injection.
2
CA 02611543 2007-11-22
SUMMARY OF THE INVENTION
A medication administration system is provided whereby a user can administer
medication via intravenous, intramuscular, or subcutaneous routes from a
single device in
a safe and controlled manner, and without the danger of a needle-stick injury.
The
system essentially consists of a syringe-like device that is a closed system
having an
aseptic fluid path. The device includes a syringe body having an integrally
disposed
needle assembly and a plunger; a hollow casing with a syringe flange holder
disposed at
one end; a hollow needle-guard that is moveable both coaxially and
telescopically within
the casing and over the syringe assembly; an end cap which joins with the
casing and
holds the syringe assembly in a fixed position; an elastic means to urge the
needle guard
towards its most forward position; a locking means to lock the device while at
rest; and a
coupling adapter which joins at one end of the needle guard, limits the
forward travel of
the needle, creates a closed and aseptic fluid path to an IV port, and
includes a means to
join to the IV port, thereby completing the closed system without ever
exposing the
needle.
3
CA 02611543 2007-11-22
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a side view of an uncapped needle-stick resistant medication
administration
system according to the invention, depicted in an assembled state.
FIG. 2 is an exploded side view of a needle-stick resistant medication
administration
system according to the invention.
FIG. 3 is a syringe assembly as is already known in the art.
FIGs. 4a - 4d depict various portions of the claimed device as would be
suitable for
practice in accord with the invention.
FIGs. 5a - 5b depict raised views of a body portion suitable for practice in
accord with the
invention.
FIGs. 6a - 6b are side views of an annular IV port-coupling device according
to the
invention.
FIGs. 7a - 7c are various views of a threaded needle guard equipped with
notched
regions, suitable for engagement with matching protruding teeth contained in
the threads of an
anti-reuse cap.
4
CA 02611543 2007-11-22
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
An object of the present invention is to provide a system in which a user can
safely
dispense medication in 3 of the 4 primary routes of administration (the main
exception being
inhalation), namely, by means of intravenous, intramuscular, or subcutaneous
injection, without
ever exposing the user to a needlepoint. In short, since at no point in time
during operation or
storage is the needlepoint exposed to the external environment, there is also
no time during
which an accidental needle-stick injury can occur.
Another object of the invention is to provide a medication administration
system that can
be safely used across a range of medication routes, including subcutaneous,
intravenous, and
intramuscular routes, all from the same device or device system.
A further object of the invention is to provide both passive and automatic
means of
returning a medication administration device to a safe-locked position any
time the device is not
in active use, even if an accidental loss of control of the device occurs.
It is a further object of the invention to provide a medication administration
system
design that lends itself to easy, cost-effective production tooling, as well
as inexpensive and
reliable manufacturing and assembly processes.
A further object of the present invention is to provide a medication
administration device
that can be fully disabled after completion of use, so that the device cannot
be reused, in order to
prevent the spread of secondary infections associated with the reuse of used
needles.
In one particular, though non-limiting, embodiment, the objects of the
invention
described above and others are accomplished by a device comprising a
conventional hypodermic
syringe and needle assembly equipped with a plunger, surrounded by one or more
of the
following associated components: a primary assembly of a casing; a needle
guard; an elastic
5
CA 02611543 2007-11-22
means to constantly urge the needle guard to the forward most or most extended
position; a
locking means that automatically engages in the needle guard's forward-most
position; and an
end cap to hold the assembly together. Further embodiments comprise a
secondary coupling
device assembly present to maintain a closed system with an IV line utilizing
a luer-type port
connection, and to prevent exposure of the needle when using the device for
intravenous
medication administration.
In one embodiment, the mentioned casing substantially surrounds the syringe
and needle
assembly, and is open at each end. A first open end functions to engage with
the needle guard
telescopically and coaxially between a fully extended resting position, a
plurality of partially
retracted positions, and a fully retracted position. A second end of the
casing is made to accept
the flange of a syringe assembly, and to engage with the end cap in a locking
frictional manner
so that the assembly is securely held together.
In a further embodiment, the needle guard engages with the casing in a
telescopic and
coaxial manner through one end, and further comprises a locking means so that
the whole device
rests in an automatically locked position whenever it is not in active use.
The needle guard also
provides a surface against which an elastic pressure means applies either
forward or rearward
pressure by either pressing or pulling so as to constantly urge the needle
guard towards a
forward-most position.
In another example embodiment, the needle guard has an opening formed at
either end.
At one end, there is an opening to allow injection of the needle directly into
an IV port made
from a semi-permeable material, or instead into the coupling device of an IV
line equipped with
a luer-type valve configuration, which is commonly used in the industry.
Additionally, the
needle guard has, disposed at the same open end, a threaded means for securely
attaching to the
6
CA 02611543 2007-11-22
coupling device if desired, thereby achieving a closed system without exposure
of the needle
once coupled.
At the other end of the needle guard, there is a second open end dimensioned
to accept,
pass through, and clear the surface of the syringe and needle assembly. The
needle guard also
carries a means for keeping the needle guard and casing in relatively stable
coaxial and
telescopic alignment with a minimum of rotational movement. If the device is
used for either
intramuscular or subcutaneous injection, the coupling device is unnecessary,
and the primary
assembly is used to perform the injection directly, thereby establishing a
closed system in which
the needle is never exposed at any time during use to the healthcare worker.
According to further embodiments, the end cap has an outer surface resembling
the
dimensions of the casing end that accepts the flange of a syringe and needle
assembly. The end
cap has a center opening that allows placement of the end cap over and past
the end of the
plunger contained in the syringe and needle assembly. The end cap has one
surface that engages
and holds the flange of the syringe assembly firmly against the flange-
accepting end of the
casing. When fully assembled, an opposite surface lies approximately flush
with the back-most
surfaces of the casing. The end cap also has one or more teeth that bend
inwardly during
assembly, and snap outwardly upon reaching a set of receiving grooves or holes
in the flange-
receiving end of the casing, which, once snapped in place, are not easily
disassembled, but which
securely hold the entire assembly together.
Disposed between the needle guard and the casing is an elastic tension-
delivering means
suitable for either pushing or pulling against the two parts in such a manner
that the needle guard
is always urged forward along the inside of the casing towards a forward-most
(i.e., fully
extended) position within the casing. Typically, the elastic means will
comprise a spring or other
7
CA 02611543 2007-11-22
similar tension-delivering object, though other means of advancing the needle
guard forward will
also suffice provided said means satisfies the basic function of constantly
urging the needle
guard toward the front-most, fully extended, position within the casing body.
For example, the elastic means could also be accomplished with an elastic
rubber band or
other similar device without departing from the scope of the invention.
In other embodiments, the secondary assembly is formed in such a manner that
there are
at least two threaded portions, one disposed at either end, capable of
engaging and attaching to
external devices equipped with similar matching threads. The aforementioned
devices might
commonly include an IV port or the like, so that the threaded end of the
primary assembly can be
threaded and affixed to the IV port.
Disposed between the two threaded ends is an elastic, semi-permeable compound
disposed in a fixed location such as is commonly used in IV ports. On the
distal side of the
elastic, semi-permeable compound is a threaded end that engages the primary
assembly via a
mechanism that limits the forward travel of the needle after passing through
the elastic, semi-
permeable compound to a predetermined fixed point, and which also forms one
ore more vents
through which medication may pass within the closed aseptic system towards an
IV port (for
example, through a conduit designed to engage the luer-type port of an IV
line, in a secure and
liquid-tight seal).
The above-described medication administration system achieves many important
and
unexpected beneficial results. For example, the system enables the user to
draw up and
administer medication in a manner that maintains a continuous fluid path
within the syringe and
needle assembly in a closed, aseptic manner that effectively guards both the
medication and the
patient from introduction of foreign matter or bacteria.
8
CA 02611543 2007-11-22
By contrast, traditional syringes (including even safety syringes), provide
little if any
safeguards against the needle and medication becoming contaminated with
bacteria or other
foreign matter, since the needles are commonly exposed during use. Since
infected injection
sites are a relatively common occurrence in healthcare settings, and can
produce very serious,
long lasting, and difficult to treat infections, the present invention is
suitably disposed to
immediately fill a long-standing need within the industry for sterility that
can only be achieved in
closed systems, and safety that can only be achieved in a system in which the
healthcare worker
is never exposed to a needle.
Referring now to the attached drawings for greater detail, the example
embodiment in
FIG. 1 depicts a complete needle-stick resistant medication administration
system in an
assembled state, with the exception of an anti-reuse cap, which is designated
numeral 60 in FIG.
7c.
The example embodiment in FIG. 2 is an exploded side view of a needle-stick
resistant
medication administration system comprising a casing 10, a needle guard 20, an
end cap 30, an
IV port coupling device 40, and a spring 50. Depicted in FIG. 3 is closer view
of known syringe
100, presented to show how the present invention can be designed around any
conventional
hypodermic syringe by simply adjusting the dimensions of the product, since
there are many
such syringes presently on the market.
Referring now to the example embodiments depicted in FIGs. 4a - 4d and FIGs.
5a - 5b
together, and starting with the casing assembly 10, a body portion 2 is formed
in an elongated,
approximately annular shape, with an irregularly-shaped end 3 designed to
accept a syringe
flange in such a manner as to align the syringe coaxially with the casing 10
and an associated
needle-guard 20. In this particular embodiment, irregular end 3 further
comprises one or more
9
CA 02611543 2007-11-22
grooves designed to engage with one or more teeth 31 of end cap 30 in order to
help center and
locate end cap 30 just prior to assembly. As shown, irregular end 3 further
comprises one or
more holes 5 through which the teeth 31 of end cap 30 extend into and snap in
place in a secure
manner. Irregular end 3 also comprises one or more grooves 6 shaped to receive
and align one
or more locking tabs 21 disposed on needle guard 20.
In this particular embodiment, the casing 10 has one or more surfaces 14
formed on an
inner portion of irregular end 3 disposed perpendicular to body portion 2,
said surfaces 14 shaped
to securely engage the forward flat surface of a syringe flange (see, for
example, FIG. 3, item
102). Irregular end 3 also has one or more outer surfaces 15 disposed parallel
to and capable of
mating with surface 14, which are used by the operator to help hold the device
in place. Irregular
end 3 also has an opening or void space 17 defined by the inner outline of
irregular end 3. Along
the body 2 of casing 10, one or more rings 7 are formed, which are thicker
areas along the body 2
to provide added stiffness, gripping surface, and to guard the locking tab(s)
21 from prolonged
compression during packaging and shipment.
Formed within the length of body portion 2 are one or more holes 8, which pass
through
the body from an outside surface toward the inside surface. Hole(s) 8 are
positioned to receive
locking tab(s) 21 whenever needle-guard 20 is extended to a forward-most
position, and which
then prevent any unintentional back-out movement of the needle-guard 20. A
second end 11 of
casing 2 is approximately annular, and disposed in mechanical communication
with a
correspondingly shaped opening or void space 12. In this embodiment, opening
12 further
comprises one or more grooves 9 shaped to receive one or more annular ribs 22
reciprocally
located on needle-guard 20 in a coaxial, telescopic engagement that prevents
rotation of needle-
CA 02611543 2007-11-22
guard 20 with respect to casing 10, thereby maintaining coaxial alignment of
locking tab(s) 21
with holes 8.
Near an end portion of groove 6 closest to hole 8, a sloped section 13 is
shaped so as to
engage a locking tab arm 23 at a corresponding angle. This feature helps to
uniformly maintain
the centering of needle-guard 20 between each inside surface of casing 10,
which assists in the
proper function and reliability of the device. Casing 10 comprises a top
surface 16 disposed near
round opening 12, which is angled slightly upward off the horizontal plain
looking from the
outer rim towards the inner rim. This feature aids in manufacturing by
allowing the injection
mold manufacturer to form a preferred tapered shut-off area in this locality,
which greatly
increases the reliability and longevity of the injection mold.
According to further embodiments, end cap 30 is formed having a left and right
outer
edge surface 32 that matches irregular end 3 of casing 10. End cap 30 also
comprises a top and
bottom outer edge surface 33 that matches the inner outline of irregular end 3
of casing 10.
During assembly (and in particular, after a syringe 100 and spring 50 have
first been inserted),
irregular end 3 is faced upward, and then end cap 30 is put into position and
located in irregular
end 3, with teeth 31 facing downward toward irregular end 3. End cap 30 is
then pressed into
opening 3, and the teeth of end cap 30 bent inward until they reach the
hole(s) 5 of irregular end
3, at which point the teeth 31 again snap outward due to memory of the
material, and lock within
hole(s) 5, and within opening 17, and cannot be again removed without
substantial force. This
latter aspect is the action that holds the full assembly together.
However, in most embodiments (for example, as the system is depicted in Figure
4d with
4 such teeth 31 and four such holes 5), the device will still function even if
only a single such
tooth 31 and hole 5 are engaged. As shown, end cap 30 has a central opening
34, shaped to
11
CA 02611543 2007-11-22
receive, pass through and allow operation of syringe plunger 103 (FIG. 3)
after assembly is
completed. During actual manufacturing, it has been found that syringe 100
usually has a
varying thickness of syringe flange 102 that must be compensated for.
Accordingly, end cap 30
also comprises one or more crush ribs 35, shaped to provide a portion that
extends between the
end cap inner surface 36 and the back surface 104 of syringe flange 102, and
which will contact
both surfaces, even when the thickness of syringe flange 102 varies slightly.
In further embodiments, crushed rib 35 is shaped in a manner that permits
slight
compression of any material disposed between inner surface 36 and syringe
flange surface 104.
This feature allows for each of the constituent assemblies to be firmly
together in proper place
and alignment, even with the variable thickness of syringe flange 102. This
aspect of the system
helps provide consistent and reliable functionality, with a low rejection rate
of the devices during
manufacturing, since free play of the syringe 100 within assembly 1 should
generally be avoided.
Around each tooth 31, there is an opening 36, shaped to match the outline of
tooth 31, which
allows free flexure of tooth 31 back and forth within the opening 36.
Referring now again briefly to FIGs. 1 and 2, once the device is assembled, a
compression spring 50 is compressably disposed within the assembly between end
cap 30 and
the back end of needle-guard 20. Compression spring 50 supplies the constant
forward urging
force needed to push needle-guard 20 towards a fully extended and locked
position. This
arrangement achieves a fully automatic, though passive, safety feature of the
system, whereby
the device remains in a constantly state safety-locked position when not in
use, or when disposed
in an active though temporary rest position, or even during an instant in time
when an operator
may lose control of while performing an injection.
12
CA 02611543 2007-11-22
Referring now to FIGs. 5a - 5b, it is seen that in this embodiment a needle-
guard 20 is
formed having a tapered rear arched surface 24 shaped to facilitate the
assembly and centering of
spring 50 in coaxial alignment with needle-guard 20 (and also with the
aforementioned casing
assembly 1). This embodiment also comprises a second arched surface 25, which
matches
closely the inside diameter contours of spring 50, upon which spring 50 rests
during use, so that
the spring compresses inwardly and outwardly as the device is used. Finally,
needle-guard 20
has a slanted surface 26 against which a forward end 51 of spring 50 abuts,
and thereby
preventing additionally forward.
In one example embodiment, surface 26 contains a groove 27, shaped to receive
a first
coil end of spring end 51 in order to prevent misalignment of spring 50 within
the assembly 1. In
other embodiments, needle-guard 20 has a hollow passage between the rear and
front of the part,
beginning near a larger opening 28, and terminating near a smaller opening 29
formed near the
front-most opening of the part.
In this embodiment, second arched surface 25 is joined with a third arched
surface 18,
which serves as a spacer in order to keep spring 50 pushed far enough back in
the assembly so
that the locking tab arm(s) 23 will freely flex both upwardly and downwardly
within the
assembly without interference of spring 50. An opening 19 surrounds locking
tab arm 23, which
also allows free flexure of locking tab arm 23 upwardly and downwardly without
interference.
Locking tab arm 23 generally comprises a memory material, such as a plastic,
so that it will
readily return back to its original position when flexed downward, away from
its initial position,
while in proximity of hole 8 in casing 10.
In a further embodiment, needle-guard 20 has a forward, elongated, hollowed
section 37,
shaped to accept the syringe barrel 105, syringe hub 106, and hollow bore
needle assembly 107
13
CA 02611543 2007-11-22
within the hollow area. Preferably, hollow bore needle assembly 107 will pass
through or past
opening 29 whenever the device is not in use. The front end of needle-guard 20
is supplied with
a threaded section 38 disposed near a front portion of the part, which is
shaped to accept and
engage matching threads 41 of an IV port coupling device 40 (see, for example,
FIG. 7).
Near the forward-most end of needle-guard 20, a front end surface 39 is
formed, which,
due to the constant urging of needle-guard 20 toward a forward-most position,
remains at all
times in contact with the medication vial surface (or patient skin surface)
while in use, thus
preventing any exposure whatsoever of the dangerous and sharp needle-point 108
to the operator.
Moving on now to FIG. 6, an annular IV port-coupling device 40 comprises a
rear
portion 42 having an opening shaped to receive the threads 38 of needle-guard
20 within a set of
threads 41. On the outside surface of the IV port-coupling device 40 is an
outer portion 43
serrated around the outer surface in order to facilitate gripping and twisting
of the device.
Near the inside end of threads 41 is an inner section 44 containing a semi-
permeable
thick elastic material that will permit insertion of needle-point 108
therethrough, while
simultaneously creating a tight liquid seal around needle assembly 107. Near
the forward end of
section 44 is an opening 45, formed inside the assembly on an opposite side,
in which the orifice
109 of hollow bore needle assembly 107 and needle-point 108 come to rest
during use. Near the
top of opening 45 is a top section 46 comprised of a solid material (for
example, a rigid,
inflexible plastic) that prevents any further forward travel of needle-point
107.
Disposed between the a beginning of opening 45 and solid material 46 is a
connecting
section, which also contains one or more vent openings 47, through which
liquid contents of
syringe 100 may pass within the assembly. Upon injection of the liquid
contents of syringe 100
into the IV port coupling device 40, the liquid fills an inside chamber 48,
and is then forced out
14
CA 02611543 2007-11-22
of the device via opposite end 54 through a hollow tubular portion 49, which
has been shaped to
enter into and conformingly seal with a matching IV line port with a spring
valve (not shown)
through front orifice 55. The forward section of IV port coupling device 40
also contains
forward threads 53, which are formed to engage the matching outside threads of
a typical IV port
and spring valve assembly, which is now commonly used in hospitals and
elsewhere in the
healthcare industry.
Referring now to FIGs. 7a - 7c, a threaded portion 38 of needle guard 20 is
equipped
with one or more notched areas 61 that are notched slightly inward. These
notched areas 61 are
shaped to engage with matching protruding teeth areas 62 contained in the
threads 63 of an anti-
reuse cap 60. The anti-reuse cap 60 can be made of any suitable solid
material, such as hard
plastic. One end of anti reuse cap 60 has opening 65, shaped to receive the
threads 38 of needle
guard 20. The opposite end of anti reuse cap 60 is closed and made of a hard
material, which will
not permit puncture, by needlepoint 108.
Protruding teeth areas 62 are formed through the wall of the part, so that
they flex
outwardly during attachment of the anti-reuse cap 60 to the threaded portion
38 of needle guard
20. Once the protruding teeth 62 pass over notched areas 61 of threads 38,
they cannot be
reversed, as teeth 62 lock within notched areas 61 if an attempt is made to
unscrew the anti-reuse
cap 60.
In this manner, and through use of the anti-reuse cap at the end of the
injection, the whole
system becomes permanently sealed and disabled, and cannot be reused. The
entire assembly
can then be promptly disposed of in the nearest sharps container.
Because of the automatic and passive safety feature of the device, the system
is foolproof
and safely operable not only throughout the entirety of the injection
procedure, but through all
CA 02611543 2007-11-22
points in time both before and after use. The system will greatly curb (or
even eliminate) needle
stick injuries to housekeeping personnel or other workers, and will also
eliminate injuries
resulting from overfilled sharps containers. In short, accidental needle-stick
injury of any kind is
virtually eliminated with use of the present invention.
Furthermore, through the use of the various features and parts, the claimed
system
provides an essentially closed medication administration system that prevents
the introduction of
foreign material and most bacteria during the injection procedure, and also
supplies a completely
aseptic path of travel for the injection of the medication, regardless of
which route of
administration is used.
The foregoing specification is provided for illustrative purposes only, and is
not intended
to describe all possible aspects of the present invention. Moreover, while the
invention has been
shown and described in detail with respect to several exemplary embodiments,
those of ordinary
skill in the pertinent arts will appreciate that minor changes to the
description, and various other
modifications, omissions and additions may also be made without departing from
either the spirit
or scope thereof.
16