Note: Descriptions are shown in the official language in which they were submitted.
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
BENZOFURANONE DERIVATIVES AS NONSTEROIDAL PROGESTERONE RECEPTOR MODULATORS
This invention relates to nonsteroidal progesterone receptor modulators, a
process for their production, the use of progesterone receptor modulators for
the
production of pharmaceutical agents as well as pharmaceutical compositions
that contain
these compounds.
The steroid hormone progesterone regulates the reproductive process in the
female organism in a decisive way. During the cycle and in pregnancy,
progesterone is
secreted in large amounts from the ovary or the placenta. By interaction with
estrogens,
progesterone produces cyclic changes of the uterine mucous membrane
(endometrium)
in the menstrual cycle. Under the influence of elevated progesterone levels
after
ovulation, the uterine mucous membrane is converted into a state that allows
the
nidation of an embryo (blastocyte). In pregnancy, progesterone controls the
relaxation
of the myometrium and retains the function of the decidual tissue.
In addition, it is known that progesterone inhibits the endometrial
proliferation
by the suppression of the estrogen-mediated mitosis in the uterus tissue (K.
Chwalisz, R.
M. Brenner, U. Fuhrmann, H. Hess-Stumpp, W. Elger, Steroids 65, 2000, 741-
751).
An important role of the progesterone and the progesterone receptors is also
known in pathophysiological processes. Progesterone receptors are detected in
foci of
endometriosis, but also in tumors of the uterus, the breast and the CNS. In
addition, it is
known that uterus leiomyomas grow in a progesterone-dependent manner.
The actions of progesterone in the tissues of genital organs and in other
tissues
are carried out by interactions with progesterone receptors, which are
responsible for the
cellular effects.
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
2
Progesterone receptor modulators are either pure agonists or partially or
completely inhibit the action of progesterone. Consequently, substances are
defined as
pure agonists, partial agonists (SPRMS) and pure antagonists.
According to the ability of the progesterone receptor modulators to influence
the
action of the progesterone receptor, these compounds have a considerable
potential as
therapeutic agents for gynecological and oncological indications as well as
for obstetrics
and birth control.
Pure progesterone receptor antagonists completely inhibit the action of
progesterone in the progesterone receptor. They have antiovulatory properties
as well as
the ability to inhibit estrogen effects in the endometrium up to full atrophy.
They are
therefore especially suitable for intervening in the female reproductive
process, e.g., in
post-ovulation, to prevent nidation; in pregnancy, to increase the reactivity
of the uterus
to prostaglandins or oxytocin or to ensure the opening and softening
("maturation") of
the cervix as well as to make the myometrium highly prepared for labor.
In foci of endometriosis or in tumor tissue, which are (is) equipped with
progesterone receptors, an advantageous influence of the disease process is
expected
after application of pure progesterone receptor antagonists. Special
advantages for
influencing pathologic conditions, such as endometriosis or uterus leiomyomas,
could
then be given if in addition an inhibition of the ovulation can be achieved by
the
progesterone receptor antagonists. When ovulation is inhibited, a portion of
the ovarian
hormone production and thus the stimulative effect that is due to this portion
are also
due to the pathologically altered tissue.
A large number of analogs with varying degrees of progesterone receptor-
antagonistic activity followed the first described progesterone receptor
antagonist RU
486 (also mifepristone). While RU 486, in addition to the progesterone
receptor-
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
3
antagonistic action, also shows an antiglucocorticoidal action, compounds
synthesized
later are distinguished primarily by a more selective action than progesterone
receptor
antagonists.
From the literature, in addition to steroidal compounds such as onapristone or
lilopristone, which are distinguished from progesterone-receptor-antagonistic
action to
antiglucorticoidal action relative to RU 486 by a better dissociation of
action, various
nonsteroidal structures, whose antagonistic action on the progesterone
receptor is
examined, are also known [see, e.g., S. A. Leonhardt and D. P. Edwards, Exp.
Biol.
Med. 227: 969-980 (2002) and R. Winneker, A. Fensome, J. E. Wrobel, Z. Zhang,
P.
Zhang, Seminars in Reproductive Medicine, Volume 23: 46-57 (2005)]. Previously
known compounds, however, have only moderately antagonistic activity compared
to
the known steroidal structures. The most effective nonsteroidal compounds are
described as having in vitro activities of 10% of the activity of RU 486.
The antiglucocorticoidal activity is disadvantageous for a therapeutic
application
in which the inhibition of the progesterone receptors is a primary focus of
therapy. An
antiglucocorticoidal activity causes undesirable side effects in the case of
therapeutically
necessary dosages. This can prevent the application of a therapeutically
useful dose or
lead to termination of the treatment.
The partial or complete reduction of the antiglucocorticoidal properties is
therefore an important requirement for the therapy with progesterone receptor
antagonists, in particular for those indications that require a treatment
lasting weeks or
months.
In contrast to the pure antagonists, progesterone receptor partial agonists
(SPRMs) show a residual agonistic property, which can be strongly pronounced
to
different degrees. This leads to the fact that these substances show potential
agonistic
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
4
actions of the progesterone receptor in specific organ systems (D. DeManno, W.
Elger,
R. Garg, R. Lee, B. Schneider, H. Hess-Stumpp, G. Schuber, K. Chwalisz,
Steroids 68,
2003, 1019-1032). Such an organ-specific and dissociated action can be of
therapeutic
use for the indications described.
It is therefore the object of this invention to make available additional
nonsteroidal progesterone receptor modulators. These compounds are to have a
reduced
antiglucocorticoidal action and are therefore suitable for the therapy and
prophylaxis of
gynecological diseases such as endometriosis, leiomyomas of the uterus,
dysfunctional
bleeding and dysmenorrhea. In addition, the compounds according to the
invention are
to be suitable for the therapy and prophylaxis of hormone-dependent tumors,
for
example breast, endometrial, ovarian and prostate cancers. In addition, the
compounds
are to be suitable for use in female birth control and for female hormone
replacement
therapy.
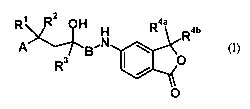
The object is achieved according to this invention by the preparation of non-
steroidal compounds of general formula I
R R Rab
A 'N
g
R3 O
O
(I),
in which
R' and R2, independently of one another, mean a hydrogen atom, a straight or
nonstraight, branched or unbranched Ci-C5-alkyl group, also together
with the C atom of the chain forming a ring with a total of 3-7 members,
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
R3 means a radical C=C-Ra, whereby
Ra means a hydrogen or a C1 -Cg-alkyl, C2-C8-alkenyl, C2-C8-alkinyl,
C3-Cto-cycloalkyl, or heterocycloalkyl that optionally is
substituted in one or more places, in the same way or differently,
with K, or an aryl or heteroaryl that optionally is substituted in one
or more places, in the same way or differently, with L,
K is a cyano, halogen, hydroxy, nitro, -C(O)Rb, CO2Rb,
-O-Rb, -S-Rb, SO2NRcRd, -C(O)-NR Rd, -OC(O)-NR Rd,
or -C=NORb -NRcRd or a C3-CI o-cycloalkyl that optionally
is substituted in one or more places, in the same way or
differently, with M, heterocycloalkyl, or aryl or heteroaryl
that optionally is substituted in one or more places with L,
L means CI-Cg-alkyl, C2-C8-alkenyl, C2-C8-alkinyl, CI-C6-
perfluoroalkyl, C l-C6-perfluoroalkoxy, C l-C6-alkoxy-C i -
C6-alkoxy, (CH2)P-C3-Cjo-cycloalkyl, (CH2)P
heterocycloalkyl, (CHZ)PCN, (CH2)pHal, (CH2)PNO2,
(CH2)p C6-C12-aryl, (CHZ)P-heteroaryl, -(CH2)PPO3(Rb)2,
-(CHZ)PNR Rd, -(CH2)PNReCORb,
-(CHZ)PNReCSRb, -(CH2)PNReS(O)Rb,
-(CH2)PNReS(O)2R6, -(CHz)PNReCONRcRd
-(CHz)PNReCOORb, -(CH2)PNReC(NH)NR Rd,
-(CH2)PNReCSNR Rd, -(CH2)PNReS(O)NRcRd,
-(CHz)PNReS(O)2NR Rd, -(CH2)pCORb,
-(CHz)pCSRb, -(CH2)PS(O)Rb,
b b
-(CH2)PS(O)(NH)R, -(CH2)pS(O)2R,
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
6
-(CH2)pS(O)2NR'Rd, -(CH2)pSO2ORb,
-(CH2)pCO2R', -(CH2)pCONRcRd,
-(CHZ)pCSNRcRd, -(CH2)pORb, -(CH2)pSRb,
-(CH2)pCR'(OH)-Re, -(CH2)p-C=NOR',
-O-(CHZ)õO-, -O-(CH2)n-CH2-, -O-CH=CH-
or -(CH2)õ+2-, whereby n = 1 or 2, and the terminal
oxygen atoms and/or carbon atoms are linked to
directly adjacent ring-carbon atoms,
M means C1 -C6-alkyl or a group -CORb, COZRb, -O-Rb, or
NRcRd, whereby
Rb means a hydrogen or a CI -C6-alkyl, C2-C8-alkenyl,
C2-C8-alkinyl, C3-Clo-cycloalkyl, C6-C12-aryl or Cl-
C3-perfluoroalkyl, and
R and Rd, independently of one another, mean a hydrogen,
C 1-C6-alkyl, C2-C8-alkenyl, C2-C8-alkinyl, C3-C i o-
cycloalkyl, C6-C12-aryl, C(O)Rb or a hydroxy
group, whereby if
Rc is a hydroxy group, Rd can be only one hydrogen, a
CI-C6-alkyl, C2-C8-alkenyl, C2-C8-alkinyl, C3-C Io-
cycloalkyl or C6-C ]2-aryl and vice versa, and
Re means a hydrogen, CI -C6-alkyl, C2-C8-alkenyl,
C2-C8-alkinyl, C3-Clo-cycloalkyl or C6-CI2-aryl,
and
p can be a number from 0- 6,
or
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
7
R3 is a radical C = C-RgRh, whereby
Rg and Rh, independently of one another, are a hydrogen or a CI -C8-alkyl,
C2-C8-alkenyl or C2-C8-alkinyl that optionally is substituted in one
or more places, in the same way or differently, with X, in which
X is a cyano, halogen, hydroxy, nitro, -C(O)Rb, CO2Rb,
-O-Rb, -C(O)-NR Rd, -NR Rd with the meanings already
further mentioned above for Rb, Rc and Rd, and
R4aand R4b, independently of one another, mean a hydrogen atom, a C, -C4-
alkyl,
a C2-C4-alkenyl or together with the ring-carbon atom forming a 3- to 6-
membered ring,
A means a monocyclic or bicyclic, carbocyclic or heterocyclic aromatic
ring, which optionally can be substituted in one or more places with CI -
C8-alkyl, C2-C8-alkenyl, C2-C8-alkinyl, Cl-C6-perfluoroalkyl, Ci-C6-
perfluoroalkoxy, C I-C6-alkoxy-C j-C6-alkyl, C 1-C6-alkoxy-C I-C6-alkoxy,
(CH2)P-C3-Cjo-cycloalkyl, (CH2)P-heterocycloalkyl, (CH2)PCN,
(CH2)PHal, (CHZ)pNOz, (CH2)P-C6-C12-aryl, (CHZ)P heteroaryl,
-(CH2)pPO3(Rb)2, -(CH2)pNRcRd, -(CH2)pNReCORb, -(CH2)pNReCSRb,
-(CH2)pNReS(O)Rb, -(CH2)pNReS(0)2Rb, -(CHZ)PNReCONR Rd,
-(CHz)PNReCOOR', -(CH2)PNReC(NH)NRcRd, -(CH2)PNReC SNRcRd,
-(CHZ)PNReS(O)NRcRd, -(CH2)PNReS(O)2NRcRd, -(CH2)PCORb,
-(CHZ)PCSRb, -(CHZ)p S(O)Rb, -(CH2)PS(O)(NH)Rb, -(CH2)PS(O)2Rb,
-(CH2)PS(O)2NRcRd, -(CH2)PSO2OR6, -(CH2)PCOZRb, -(CH2)PCONR Rd,
-(CH2)pCSNRcRd, -(CH2)pORb, -(CH2)PSRb, -(CHZ)pCRb(OH)-Rd,
-(CH2)P C=NORb, -O-(CH2)n-O-, -O-(CH2),-CH2-, -O-CH=CH- or
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
8
-(CH2)õ+2-, whereby n= 1 or 2, and the terminal oxygen atoms and/or
carbon atoms are linked to directly adjacent ring-carbon atoms, or
A means a radical -CO2Rb, C(O)NR Rd, CORb,
or
A means an alkenyl group -CR5 = CR6R7, whereby
R , R are the same or different and, independently of one
56 and R~
another, mean hydrogen atoms, halogen atoms, aryl radicals or an
unsubstituted or partially or completely fluorinated C,-CS alkyl
group, or
A means an alkinyl group -C=CRS, with the meaning cited above for R5, and
B means a carbonyl group or a CH2 group
as well as their pharmaceutically acceptable salts.
The compounds of general formula I according to the invention can be present
as
different stereoisomers because of the presence of asymmetry centers. Both the
racemates and the separately present stereoisomers are part of the subject of
this
invention.
In addition, this invention comprises the new compounds as pharmaceutical
active ingredients, their production, their therapeutic application and
pharmaceutical
dispensing forms that contain the new substances.
The compounds of general formula (I) according to the invention or their
pharmaceutically acceptable salts can be used for the production of a
pharmaceutical
agent, especially for treatment and prophylaxis of gynecological diseases,
such as
endometriosis, leiomyomas of the uterus, dysfunctional bleeding and
dysmenorrhea. In
addition, the compounds according to the invention can be used for the
treatment and
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
9
prophylaxis of hormone-dependent tumors, such as, for example, for breast,
prostate and
endometrial cancers.
The compounds of general formula (I) according to the invention or their
pharmaceutically acceptable salts are suitable for use for female birth
control or for
female hormone replacement therapy.
A process for the production of the compounds of general formula (I),
moreover,
is also a subject of this invention. Substituent R3 is introduced to a keto
group by
selective addition reaction of organometallic compounds such as lithium
alkinylene or
magnesium haloalkinylene. This results, either directly or after implementing
additional
modifications, in the compounds of general formula (I) according to the
invention.
A R g~N ~ O R3-Li oder R3-MgHal R' 2 H H R4a R4b
1\~
(/ A B,N I~ O
Q R 3
O
[or]
The production of the compounds according to the invention is carried out by
selective addition of organometallic compounds to ketoamides, which were
described in,
e.g., laid-open specifications WO 200375915 and WO 9854159. The organometallic
compounds can be, for example, lithium alkinyl compounds or magnesium
haloalkinyl
compounds. The latter are produced by, e.g., reaction of the corresponding
alkines with
butyllithium or Grignard compounds. Analogously to this, the corresponding
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
organometallic alkenyl compounds can also be produced. The reactivity of the
keto
group in comparison to amidocarbonyl or to phthalide is in this case
significantly higher,
such that with suitable selection of the reaction conditions, a selective
addition is
achieved. As an alternative, the alkinyl or alkenyl radicals that are
introduced as R3 can
also be further modified later. For these modifications, reactions that have
become
known to one skilled in the art, such as oxidation, reduction, substitution,
alkylation, or
palladium-catalyzed reaction, are suitable. Optionally present protective
groups are
cleaved off at a suitable time.
The nonsteroidal compounds of general formula I according to the invention
have a strongly antagonistic or strongly partially agonistic action on the
progesterone
receptor. They exhibit a strong dissociation of action with respect to their
bonding
strength on the progesterone receptor and on the glucocorticoid receptor.
While known
progesterone receptor antagonists, such as Mifepristone (RU 486), in addition
to the
desired high binding affinity for the progesterone receptor likewise show a
high affinity
for the glucocorticoid receptor, the compounds according to the invention are
distinguished by a very low glutocorticoid receptor bond with simultaneously
present
high progesterone receptor affinity.
The substituents of the compounds of general formula I according to the
invention that are defined as groups can have the meanings below in each case:
C i-C5-, C i-C6- or CI -C8-alkyl groups are defined as straight or
nonstraight,
branched or unbranched alkyl radicals. In this case, for example, this is a
methyl, ethyl,
n-propyl, iso-propyl, n-, iso-, tert-butyl, an n-pentyl, 2,2-dimethylpropyl, 3-
methylbutyl,
hexyl, heptyl or octyl group.
In terms of Ra, in this case, the methyl, ethyl, n-propyl or n-butyl group as
well as
an n-pentyl group are preferred.
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
11
In terms of R' and RZ, methyl or ethyl is preferred.
According to the invention, a hydrogen is preferred for R4a and R4b.
Alkenyl is defined as straight or nonstraight, branched or unbranched alkenyl
radicals. In terms of the invention, a C2-C8-alkenyl group is defined, for
example, as
follows: vinyl, allyl, 3-buten-1-yl- or 2,3-dimethyl-2-propenyl. If aromatic
compound A
is substituted with a C2-Cg-alkenyl radical, this is preferably a vinyl group.
Alkinyl is defined as straight or nonstraight, branched or unbranched alkinyl
radicals. For example, an ethinyl, propinyl, butinyl, pentinyl, hexinyl or
octinyl group,
but preferably an ethinyl or propinyl group, is to stand for a C2-C8-alkinyl
radical.
For C3-CI o-cycloalkyl, for example, cyclopropane, cyclobutane, cyclopentane
and cyclohexane can be mentioned. Cyclopropyl, cyclopentyl and cyclohexyl are
preferred.
In terms of Ra, K or L, heterocycloalkyl is defined as 3- to 8-membered
heterocycloalkyl radicals. Examples of heterocycloalkyl are morpholine,
tetrahydrofuran, pyran, piperazine, piperidine, pyrrolidine, oxirane, oxetane,
aziridine,
dioxolane and dioxane. In this case, the position of the heteroatom in
relation to the
point of linkage can be any chemically possible position.
For example, methoxymethoxy, ethoxymethoxy or 2-methoxyethoxy can stand
for a C i-C6-alkoxyl-C I -C6-alkoxy group.
In terms of the invention, a radical ORb is a hydroxy, methoxy, ethoxy, n-
propoxy, iso-propoxy, n-, iso-, or tert-butoxy group, or an n-pentoxy, 2,2-
dimethylpropoxy or 3-methylbutoxy group. Hydroxy, methoxy and ethoxy are
preferred.
For a partially or completely fluorinated Ci-C5-alkyl group, the
perfluorinated
alkyl groups that appear above are considered. Of the latter, primarily the
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
12
trifluoromethyl group or the pentafluoroethyl group as well as as partially
fluorinated
alkyl groups, for example the 5,5,4,4-pentafluoropentyl group or the
5,5,5,4,4,3,3-
heptafluoropentyl group, are preferred.
A fluorine, chlorine, bromine or iodine atom can stand for a halogen atom.
Preferred here is fluorine, chlorine or bromine.
If Rl and R2 together with the C atom of the chain form a 3- to 7-membered
ring,
this is, for example, a cyclopropyl, cyclobutyl, cyclopentyl or cyclohexyl
ring. The
cyclopropyl ring as well as the cyclopentyl ring are preferred.
The monocyclic or bicyclic carbocyclic aromatic ring A, which can be
substituted in several places, is a carbocyclic or heterocyclic aryl radical.
In the first case, it is, for example, a phenyl or naphthyl radical,
preferably a
phenyl radical.
As a heterocyclic radical, for example, a monocyclic heterocyclic radical, for
example the thienyl, furyl, pyranyl, pyrrolyl, imidazolyl, pyrazolyl, pyridyl,
pyrazinyl,
pyrimidinyl, pyridazinyl, thiazolyl, oxazolyl, furazanyl, pyrrolinyl,
imidazolinyl,
pyrazolinyl, thiazolinyl, triazolyl, or tetrazolyl radical, and specifically
all possible
isomers relative to the positions of the heteroatoms, can be used.
In terms of R3, an aryl radical is an optionally substituted phenyl, 1- or 2-
naphthyl radical, whereby the phenyl radical is preferred. Examples of a
heteroaryl
radical are the 2-, 3- or 4-pyridinyl radical, the 2- or 3-furyl radical, the
2- or 3-thienyl
radical, the 2- or 3-pyrrolyl radical, the 2-, 4- or 5-imidazolyl radical, the
pyrazinyl
radical, the 2-, 4- or 5-pyrimidinyl radical or the 3- or 4-pyridazinyl
radical.
The number p for the (CH2)P radical can be a number from 0 to 6, preferably 0
to
2. "Radicals" are defined according to the invention as all functional groups
that are
presented in connection with (CH2)p.
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
13
In the case that the compounds of general formula I (B = -CH2-) are present as
salts, this can be, for example, in the form of hydrochloride, sulfate,
nitrate, tartrate,
citrate, fumarate, succinate or benzoate.
If the compounds according to the invention are present as racemic mixtures,
they can be separated into the pure, optically active forms according to
methods of
racemate separation that are familiar to one skilled in the art. For example,
the racemic
mixtures can be separated into pure isomers by chromatography on an even
optically
active carrier material (CHIRALPAK AD ). It is also possible to esterify the
free
hydroxy group in a racemic compound of general formula I with an optically
active acid
and to separate the diastereomeric esters that are obtained by fractionated
crystallization
or by chromatography and to saponify the separated esters in each case to form
the
optically pure isomers. As an optically active acid, for example, mandelic
acid,
camphorsulfonic acid or tartaric acid can be used.
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
14
The compounds that are mentioned below as well as the use thereof are
preferred
according to the invention:
No. Racemic or R3 ,
Enantiomer R OH
1 rac " H
2 +
3 - ~ ~ o
4 rac o
+
6 -
7 rac ~
8 +
9 - f
rac
11 +
12 - r
13 rac o"
14 + r~
-
16 rac OH
17
18 - f
19 rac "
+ f~
21 -
22 rac ll ~
23 + o
24 - ''
rac 0
26 + OH
27 - /
28 rac
29 + ~OH
- f
31 rac
32
33 - /
34 rac
+
36 - -~'
37 rac CF
38 + ,
39 -
rac N
+
41
/
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
42 -
43 rac
44 +
45 - "~
46 rac
47 +
48
49 rac "
50 + .~%
51 -
52 rac , o
53 + ~~~ lr
54 -
55 rac OH
56 +
57 -
58 rac , i 0
59 +
60 -
No. Racemic or R3
Enantiomer ,
H
61 rac H R OH
62 + /~ H
63
- ~ " ~
64 rac o I~
65 + o\ o
66 -
67 rac
68 +
69 - ~
70 rac ~
71 +
72 - f
73 rac o"
74 + f~
75 -
76 rac oH
77 +
78 - r
79 rac H
80 + r/
81 -
82 rac
83 + / ~o~
84 -
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
16
85 rac
86 + OH
87 -
88 rac
89 + J~OH
90 - r
91 rac
92 +
93 - -~ ~
94 rac
95 +
96 - -~'
97 rac CF
98 +
99 -
100 rac N
101 + /~ \
102 -
103 rac
104 +
105 - "~
106 rac -
107 +
108 - '
109 rac "
110 +
111 -
112 rac O
113 + ~~~l-
114 -
115 rac OH
116 +
117 -
118 rac
119 + O'
120 -
No. Racemic or R3
Enantiomer ,
121 rac H R oH
122 + =~ ~ H
123 - ~ " ~
124 rac 0
(~ o
125 + o
126 -
127 rac ~
/
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
17
128 +
129 -
130 rac
131 +
132 - r
133 rac o"
134 + r~
135 -
--OM
136 rac /
137 +
138 -
139 rac
140 + r~
141 -
142 rac u
143 +
144 - ''
145 rac op
146 + OH
147 -
148 rac
149 + OH
150 - r
151 rac
152 +
153 - ~
154 rac
155 +
156 - -~'
157 rac
158 + 159 -
160 rac
161 +
162
163 rac
164 +
165 - '~
166 rac
167 +
168 - ~
169 rac "
170 +
171 -
172 rac
173 +
.~
174 -
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
18
175 rac OH
176 +
177 -
178 rac 0
OH
179 +
180 -
No. Racemic or R3
Enantiomer ,
181 rac -+ R OH
182 + /~ H
183 - ~ " ~
184 rac o I~ 0
185 + o
186
187 rac
188
189 - '~
190 rac
191 +
192 - r
193 rac o"
194 + r~
195 -
196 rac oH
197 + ~
198 - f
199 rac H
200 + r~
201 -
202 rac u o~
203 +
204 - ''
205 rac op
206 + OH
207 -
208 rac
V OH
209 +
210 - r
211 rac
212 +
213 - ~
214 rac
215 +
216 - ~~
217 rac CF
218 + // \ ,
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
19
219 -
220 rac
221 + /~~i~
222 -
223 rac
224 +
225 -
226 rac
227 + ~ -
228 - "~
229 rac "
230
231 -
232 rac r- o
233 + l~
234 -
~i
~
235 rac
236 +
237 -
238 rac
239 oM
240 -
No. Racemic or R3
Enantiomer R'
OH
241 rac H
242 +
243 - ( ~ o o
244 rac
245 + o
246 -
247 rac
248 +
249 - f
250 rac
251 +
252 - f
253 rac H
254 + f~
255 -
256 rac oH
257 + ~
258 - r
259 rac OH
260 + f~
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
261 -
262 rac u o
263 + / \
264 - ''
265 rac 0
266 + OH
267 -
268 rac
OH
269 + l/
270 - r
271 rac
272 +
273
274 rac
275 +
276 - -~'
277 rac CF,
278 +
279 - ~~
280 rac N
281 + //\~,
282 -
283 rac
284 +
285 - "~
286 rac
287 +
288 - "~
289 rac "
290 +
291 -
292 rac ::-~
293 + lr
294 -
295 rac M
296 +
297 -
298 rac
299 + m
300 -
No. Racemic or R3
Enantiomer R,
301 rac H o"
302 + .-1 H
303 - ~ " ~
ci o ( / 0
0
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
21
304 rac
305 +
306 -
307 rac ~
308 +
309 - ''
310 rac
311 +
312 - r
313 rac H
314 + f~
315 -
316 rac oH
317 + ~
318 - f
319 rac OH
320 + f~
321 -
322 rac u Q~
323 +
324 - ''
325 rac 0
326 + //'oH
327 -
~OH
~j
328 rac r
329 +
330 -
331 rac
332 + ~ -
333 - ~
334 rac
335 +
336 - -~'
337 rac CF
338 + f~ ~ -
339 -
340 rac
341 + /~~i '
342 -
343 rac
344
345 - '~
346 rac
347
348 - "~
349 rac "
350 +
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
22
351 -
352 rac
353 +
354 -
355 rac 'OK
356 +
357 -
358 rac
359 + /~ ' i oN
360 -
No. Racemic or R3
Enantiomer ,
361 rac -+ R OH
362 + o H
363
364 rac o o
365 + o
366 -
367 rac
368 +
369 - r
370 rac f
371 +
372 -
373 rac o"
374 +
375 -
376 rac oH
377 +
378 - f
379 rac ,-~ OH
380 + r/
381 -
382 rac u
383 + o
384 - ''
385 rac o
386 + -~IIA oH
387 -
388 rac
389 + >OH
390 -
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
23
391 rac
392 + ~ -
393 - -~'
394 rac
395 +
396 - -~'
397 rac CF
398 + // \ ,
399 -
400 rac
401 + /~ ~
402 -
403 rac
404 +
405 - "~
406 rac
407 +
408 - '~
409 rac "
410 +
411 -
412 rac
413 +
414 -
415 rac OH
416 +
417 -
418 rac
419 + "
420 -
No. Racemic or R3
Enantiomer ,
421 rac -+ R OH
H
422 + /~ ~cq0
423
424 rac o 425 + 0
426 -
427 rac ~
428 + ~j
429 - f
430 rac
431 +
432 - r
433 rac OH
d~
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
24
434 +
435 -
436 rac oH
437
438 -
439 rac "
440 + f~
441 -
442 rac u
443 +
444 -
445 rac 0
446 + /~oH
447 -
448 rac
449 + OH
450 - r
451 rac
452 +
453 - f
454 rac
455 +
456 - -~'
457 rac CF,
458 +
459 - ~~
460 rac N
461 +
462 -
463 rac
464
465 - "~
466 rac
467 +
468 - "~
NO,
469 rac
470 +
471 -
472 rac
473 + o
474 -
475 rac '0õ
476 +
477 -
478 rac
479 ON
480 -
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
No. Racemic or R3
Enantiomer ,
481 rac H R OH
482 + =~~ o H
483
484 rac Br o o
485 + o
486 -
487 rac
488 + f~
489 -
490 rac
491 +
492 - f
493 rac o"
494 + f~
495 -
496 rac OH
497 +
498 - r
499 rac OH
500 + f~
501 -
502 rac u
503 + o
504
505 rac op
506 + /OH
507 -
508 rac
509 + ~OH
510 - r
511 rac
512
513 - ~
214 rac
515 +
516 - -~~
517 rac
518 + /~ 519 -
520 rac
521 +
522
523 rac
524 +
525 - "~
526 rac
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
26
227 +
528 -
529 rac "
530 + .~%
531 -
532 rac
533 + lr
534 -
535 rac H
536 +
537 -
538 rac
539 + OH
540 -
No. Racemic or R3
Enantiomer R'
OH
541 rac H
542 + =~ ~ o r~
543 o I~ o
544 rac
545 + o
546 -
547 rac
548 +
549 - r
550 rac
551 +
552 - r
553 rac o"
554 + r~
555 -
556 rac oH
557 +
558 - r
559 rac OH
560 + i~
561 -
562 rac u
563 +
564 -
565 rac o
566 + /~oH
567 -
568 rac
569 + aH
570 - ~
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
27
571 rac
572 + -
573 - f
574 rac
575 +
576 - -~'
577 rac
578 + 579 -
580 rac
581 +
582 -
583 rac
584 +
585 - "~
586 rac
587 +
588 - "~
589 rac "
590 +
591 -
592 rac
593 + 0
594 -
595 rac H
596 +
597 -
598 rac om
599 +
600 -
No. Racemic or R3
Enantiomer R,
601 rac OH
602 + H
603 - ~ " ~
604 rac o I~ o
605 + cF, 0
606 -
607 rac
608 +
609 - r
610 rac
611 +
612 -
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
28
613 rac OH
614 +
615 -
616 rac oH
617 +
618 - f
619 rac OH
620 + f~
621 -
622 rac u
623 + o
624 -
625 rac op
626 + f/'oH
627 -
628 rac
629 + ~H
//
630 - f
631 rac
632 + ~ -
633 - f ~
634 rac
635 + ~ ~ I
636 - -~'
637 rac ~,
638 + /~ 639 -
640 rac
641 +
642 -
643 rac
644 +
645 - '~
646 rac ,
647
648 - "~
649 rac "
650 + .,%
651
652 rac
653 + 0
654 -
655 rac OH
656 +
657 -
658 rac ~
659 +
660 -
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
29
No. Racemic or R3
Enantiomer ,
661 rac H R OH
662 + /~ o H
663 - ~ " ~
664 rac o I~
665 + F 0
666 -
667 rac
668 + f~
669 -
670 rac
671 +
672 - r
673 rac H
674 + r~
675 -
676 rac OH
677 + ~
678 - r
679 rac OH
680 + f~
681 -
682 rac u
683 + /\
684 - ''
685 rac 0
686 + //'OH
687 -
688 rac
OH
689 +
690 - f
691 rac
692 +
693 - ~'
694 rac
695 +
696 - -~'
697 rac CF
698 + 699 -
700 rac N
701 +
702 -
703 rac
704
705 - "~
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
O\
706 rac
707 +
708 -
709 rac "
710 +
711 -
712 rac o
713 +
714 -
715 rac ~ ~ OH
716 +
717 -
718 rac
719 + oõ
720 -
No. Racemic or R3
Enantiomer ,
721 rac /H R OH
722 + / ~ H
723 - o
724 rac <o o o
725 + o
726 -
727 rac
728 +
729 - f
730 rac
731
732 - f
733 rac H
734 + r~
735 -
736 rac oH
737 +
738 - r
739 rac ,-~ H
740 + r/
741 -
742 rac u
743 + /\
744 -
745 rac o
746 + OH
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
31
747 -
748 rac
749 + ~oH
750 - r
751 rac -~
752 +
753 -
754 rac
755 +
756 - -~'
757 rac CF
758 + f~ ~ I ~
759 -
760 rac
761 + // \ ~ ,
762 -
763 rac
764 + 'o-10
765 -
766 rac
767 +
768 -
769 rac "
770 +
771 -
772 rac
773 + lr
774 -
775 rac OH
776 +
777 -
778 rac
779 + oM
780 -
No. Racemic or R3 R3 oH
Enantiomer H
781 rac H " ~
782 + o TIIo
783 - cF 0
784 rac 3
785 +
786 -
787 rac
788 +
r
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
32
789 -
790 rac 791 +
792 - r
793 rac OH
794 + r-
795 -
796 rac OH
797 +
798 - f
799 rac OH
800 + f/
801 -
802 rac u o~
803 +
804 -
805 rac O
806 + /~OH 807 -
808 rac
809 + ~OH
810 - f
811 rac
812 + ~ -
813 - f
814 rac
815 +
816 - -~'
817 rac CF
818 +
819 -
820 rac
821 + /~ ~ \
822 -
823 rac
824 + ~ .
825 -
826 rac
827 +
828 -
829 rac "
830 +
831 -
832 rac
833 + /~ ~ ~
834
835 rac OH
836 +
837 -
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
33
838 rac
OH
839 +
840 -
No. Racemic or R3 R'
OH
Enantiomer o
841 rac -+ ni
842 + /~ I~ o
o
843 - 0
CF3
844 rac
845 +
846 -
847 rac
848 +
849 - ''
850 rac ~
851 +
852 - f
853 rac o"
854 + f~
855 -
856 rac oH
857 +
858 - r
859 rac OH
860 + r~
861 -
862 rac u
863 +
864 - ''
865 rac 0
866 + //'oH
867 -
868 rac
869 + ~oH
870 - r
871 rac
872 + -
873 - ~'
874 rac
875 +
876 - -~'
877 rac CF
878
879 -
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
34
880 rac
881 + /~ - ~
882 -
883 rac
884 +
885 - "~
886 rac
887 + ~
888 - "~
889 rac "
890 +
891 -
892 rac
893 + /~ lr
894 -
O"
/ ~ ~
/ /
895 rac
896 +
897 -
898 rac
899 + /~ O
900 -
No. Racemic or R3
Enantiomer R,
901 rac -+ OH
902 + H
903
904 rac o
o
905 + CF3 O
906 -
907 rac
908 +
909 - ''
910 rac
911 +
912 - f
913 rac o"
914 +
915 -
916 rac oH
917 + ~
918 - r
919 rac "
920 + f/
921 -
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
922 rac u
923 +
924 -
925 rac op
926 + 1111'OH
927 -
928 rac
OH
929 +
930 - f
931 rac
932 +
933 -
934 rac
935 +
936 - -~'
937 rac F
938 + .
939 -
940 rac
941 +
942 -
943 rac
944 +
945 - '~
946 rac
947 +
948 - "~
949 rac "
950 + .~%
951 -
952 rac
953 +
954 -
955 rac OH
956 +
957 -
958 rac
959 + "
960 -
No. Racemic or R3
Enantiomer ,
961 rac /-+ \ R OH
962 + =~' o H
N ~~
F ~ / o
n
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
36
963 -
964 rac
965 +
966 -
967 rac
968 +
969 - f
970 rac
971 +
972 - f
973 rac o"
974 +
975 -
976 rac OH
977 + ~
978 - f
979 rac ,--~ OH
980 + r/
981 -
982 rac u
983 +
984 - ''
0
OH
985 rac
986 +
987 -
988 rac
989 + ~OH
990 - r
991 rac
992 +
993 - -~'
994 rac
995 +
996 - -~'
997 rac F
998 + f~ 999 -
1000 rac
1001 + /~ \
1002 -
1003 rac
1004 +
1005 - "~
1006 rac ,
1007 +
1008 - '~
1009 rac "
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
37
1010 +
1011 -
1012 rac
1013 +
1014 -
1015 rac OM
1016 +
1017 -
1018 rac ~
1019 +
1020 -
No. Racemic or R3
Enantiomer ,
1021 rac H R OH
1022 + .~~ o H
1023
1024 rac o o
1025 + F 0
1026 -
1027 rac
1028 +
1029 - '~
1030 rac f
1031 +
1032 -
1033 rac o"
1034 + f~
1035 -
1036 rac oH
1037 +
1038 - ~
1039 rac OH
1040 +
1041 -
1042 rac u
1043 +
1044 - ''
1045 rac 0
1046 + /~oH
1047 -
1048 rac
V oH
1049 +
1050 - f
1051 rac
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
38
1052 +
1053 -
1054 rac
1055 +
1056 - -~'
1057 rac F
1058 + ~
1059 -
1060 rac N
1061 + /~ i '
1062 -
1063 rac
1064 +
1065 -
1066 rac \
1067 + .
1068 -
1069 rac "
1070 +
1071 -
1072 rac
1073 + / ~
1074 -
1075 rac 1076 +
1077 -
1078 rac
1079 +
1080 -
No. Racemic or R3 R OH
Enantiomer 0 H
1081 rac " ~
1082 + o o I ~ o
1083 - I o
1084 rac
1085 +
1086 -
1087 rac
1088
1089 -
1090 rac ~
1091 +
1092 - f
1093 rac o"
1094 + 1~
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
39
1095 -
1096 rac OH
1097 + ~
1098 - f
1099 rac H
1100 + r~
1101 -
o~
1102 rac 0
1103 +
1104 - ''
1105 rac 0
1106 + //\OH
1107 -
1108 rac
1109 + ~OH
1110 - f
1111 rac
1112 +
1113 - f '
1114 rac
1115 +
1116 - -~'
1117 rac
1118 +
1119 -
1120 rac
1121 + /~ ~ '
1122 -
1123 rac
1124 +
1125 - "~
1126 rac
1127 +
1128 - "~
~NOr
-/
1129 rac
1130 +
1131 -
1132 rac
1133 + 0
1134 -
1135 rac ~ ~ OH
1136 +
1137 -
1138 rac ~
1139 + N
1140 -
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
No. Racemic or R3
Enantiomer R'
OH
1141 rac H o
1142 +
1143 - o 105~ o
1144 rac
1145 + f / o
1146 -
1147 rac
1148 +
1149 - r
1150 rac
1151 +
1152 - f
1153 rac OH
1154 + f-
1155 -
1156 rac oH
1157 + ~
1158 - f
1159 rac "
1160 + f~
1161 -
1162 rac u
1163 + o
1164 - ''
1165 rac 0
1166 + oH
1167 -
1168 rac
1169 + oH
1170 - r
1171 rac
1172 +
1173 - f
1174 rac
1175 +
1176 - -~~
1177 rac CF,
1178 +
1179
1180 rac
1181 + /~ '
1182 -
1183 rac
1184 +
1185 -
1186 rac
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
41
1187 +
1188 -
1189 rac "
1190 +
1191 -
1192 rac
1193 + 0
1194 -
1195 rac OH
1196 +
1197 -
1198 rac
1199 + "
1200 -
No. Racemic or R3
Enantiomer R,
1201 rac -+ OH
1202 + o H
1203
1204 rac ci o o
1205 + o
1206 -
1207 rac
1208 +
1209 - f
1210 rac
1211
1212 - f
1213 rac o"
1214 + f~
1215 -
1216 rac oH
1217 + ~
1218 - r
1219 rac OH
1220 + f~
1221 -
0
1222 rac
1223 +
1224 -
1225 rac op
1226 + //~oH
1227 -
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
42
1228 rac
OH
1229 +
1230 - r
1231 rac
1232 +
1233 - f
1234 rac
1235 +
1236 - -~~
1237 rac CF,
1238 +
1239 - ~~
1240 rac
1241 +
1242 -
1243 rac
1244 + .
1245 - "~
1246 rac
1247 +
1248 - "~
1249 rac "
1250 +
1251 -
1252 rac
1253 + r
0
1254 -
1255 rac OH
1256 +
1257 -
1258 rac
1259 + oH
1260 -
No. Racemic or R3
Enantiomer ,
1261 rac /H \ R OH
1262 + o H
1263 - ~ ~ " () o
1264 rac o
1265 + F 0
1266 -
1267 rac
1268 +
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
43
1269 -
1270 rac
1271 +
1272 - '(
1273 rac OH
1274 +
1275 -
1276 rac oH
1277 +
1278 - f
1279 rac OH
1280 + f/
1281 -
1282 rac u
1283 +
1284 - ''
1285 rac op
1286 + //'aH
1287 -
1288 rac
1289 + aH
1290 - f
1291 rac
1292 +
1293 - ~
1294 rac
1295 +
1296 - -~'
1297 rac
1298 + /~ ~ 1299 -
1300 rac
1301 +
1302 -
1303 rac
1304 + ol~
1305 - '~
1306 rac
1307 + ~ . ~
1308 - "~
1309 rac "
1310
1311 -
1312 rac
1313 + 0
1314 -
1315 rac ~
1316
1317 -
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
44
0
O+
1318 rac
1319 +
1320 -
No. Racemic or R3
Enantiomer ,
1321 rac -" R OH
1322 + c~ H
1323
1324 rac 0 o
1325 + F 0
1326 -
1327 rac
1328 +
1329 - f
1330 rac ~
1331 +
1332 - f
1333 rac o"
1334 + r~
1335 -
1336 rac oH
1337 +
1338 - r
1339 rac ,-~ OH
1340 + r/
1341 -
1342 rac u
1343 +
1344 - ''
1345 rac 0
1346 + //'oH
1347 -
1348 rac
V OH
1349 +
1350 - r
1351 rac ~
1352 +
1353 - -~
1354 rac
1355 +
1356 - -~'
1357 rac CF
1358 + ~~ ~ ~ ~
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
1359 -
1360 rac
1361 + /~~i '
1362 -
1363 rac
1364 +
1365 -
1366 rac
1367 +
1368 - "~
1369 rac "
1370 +
1371 -
1372 rac
1373 +
1374 -
1375 rac OH
1376 +
1377 -
1378 rac 0
1379 +
1380 -
No. Racemic or R3 R3
OH
Enantiomer o
1381 rac r~
1382 + o
1383
1384 rac 0
1385 +
1386 -
1387 rac
1388 +
1389 - f
1390 rac
1391 +
1392 - f
1393 rac o"
1394 + f~
1395 -
1396 rac oH
1397 +
r
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
46
1398 -
1399 rac OH
1400 + /~
1401 -
1402 rac
1403 + o
1404 -
0
1405 rac 1-1-~ oH
1406 +
1407 -
1408 rac
1409 + ~OM
1410 - f
1411 rac
1412 +
1413 - f '
1414 rac
1415 + ~ ~ I
1416 - -~'
1417 rac ~
1418 + /~ ~
1419 -
1420 rac
1421 + /~ ~ i '
1422 -
1423 rac
1424 +
1425 - "~
1426 rac
1427 +
1428 - "~
1429 rac "
1430 +
1431 -
1432 rac
1433 + 0
1434 -
1435 rac "
1436 +
1437 -
1438 rac
1439 + OH
1440 -
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
47
No. Racemic or R3
Enantiomer R3
OH
1441 rac H
1442 + r~
1443 - I~ o o
1444 rac 0
1445 +
1446 -
1447 rac
1448 +
1449 - ''
1450 rac r
1451 +
1452 -
1453 rac o"
1454 + f~
1455 -
1456 rac oH
1457 +
1458 - r
1459 rac OH
1460 + f~
1461 -
1462 rac u
1463 +
1464 -
1465 rac o
1466 + / p
I'aH
1467 -
1468 rac
1469 + OH
1470 - r
1471 rac
1472 +
1473 - -~~
1474 rac
1475 +
1476 - -~~
1477 rac CF
1478 + /~ ~ 1479 -
1480 rac
1481 +
1482 -
1483 rac
1484 +
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
48
1485 -
1486 rac
1487 + -
1488 - "~
1489 rac "
1490 +
1491 -
1492 rac
1493 +
1494 -
1495 rac H
1496 +
1497 -
1498 rac
1499 + om
1500 -
No. Racemic or R3
Enantiomer R,
1501 rac H oH
1502 + / ~ /-o H
1503 - o ~ " ~
1504 rac 0
I~ o
1505 + 0
1506 -
1507 rac
1508
1509 - f
1510 rac
1511 +
1512 - r
1513 rac o"
1514 + r~
1515 -
1516 rac oH
1517 + ~~
1518 - r
1519 rac OH
1520 + f~
1521 -
1522 rac ll Q
1523 \
1524 -
1525 rac O
1526 + OH
1527 -
1528 rac \/
7'-OH
/ ~/
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
49
1529 +
1530 -
1531 rac
1532 +
1533 - f
1534 rac
1535 +
1536 - f
1537 rac CF,
1538 +
1539 - -~~
1540 rac N
1541 + /~~~'
1542 -
1543 rac
1544 + 'o-10
1545 - "~
1546 rac
1547 +
1548 -
NO,
1549 rac
1550 +
1551 -
1552 rac
1553 +
1554 -
1555 rac OH
1556
1557 -
1558 rac
1559 + oN
1560 -
No. Racemic or R3
Enantiomer ,
1561 rac /-+ \ R OH
1562 + / ~ o H
1563
o
1564 rac o
1565 + f 0
1566 -
1567 rac
1568
1569 -
1570 rac
%~
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
1571 +
1572 -
1573 rac o"
1574 + r~
1575 -
1576 rac oH
1577 +
1578 - f
1579 rac OH
1580 + r~
1581 -
1582 rac u
1583 + o
1584 - ''
1585 rac o
1586 + 1-1~oH
1587 -
1588 rac OH
1589 +
1590 - f
1591 rac
1592 +
1593 - ~
1594 rac -~
1595 +
1596 -
1597 rac CF,
1598 +
1599
1600 rac
1601 + /~ ~
1602 -
1603 rac
1604 +
1605 - "~
1606 rac
1607 +
1608 -
1609 rac "
1610 + .~%
1611 -
1612 rac
1613 +
1614 -
1615 rac - "
1616 +
1617 -
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
51
1618 rac 0
~,
1619
1620 -
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
52
Biological Characterization of the Compounds According to the Invention
The identification of progesterone receptor modulators can be performed using
simple methods, test programs that are known to one skilled in the art. To
this end, for
example, a compound that is to be tested can be incubated together with a
gestagen in a
test system for progesterone receptors, and it can be examined whether the
progesterone-
mediated action in this test system is altered in the presence of modulators.
The substances of general formula I according to the invention were tested in
the
following models:
Progesterone Receptor Binding Test
Measurement of the Receptor Binding Affinity:
The receptor binding affinity was determined by competitive binding of a
specifically binding 3H-labeled hormone (tracer) and the compound to be tested
on
receptors in the cytosol from animal target organs. In this case, receptor
saturation and
reaction equilibrium were sought.
The tracers and increasing concentrations of the compound to be tested
(competitor) were co-incubated with the receptor-containing cytosol fraction
at 0-4 C
over 18 hours. After separation of the unbonded tracer with carbon-dextran
suspension,
the receptor-bonded tracer portion was measured for each concentration, and
the ICs0
was determined from the concentration sequence. As a quotient of the IC50
values of the
reference substance and the compound to be tested (x 100%), the relative molar
binding
affinity (RBA) was calculated (RBA of the reference substance = 100%).
For the receptor types, the following incubation conditions were selected:
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
53
Progesterone Receptor:
Uterus cytosol of the estradiol-primed rabbit, homogenized in TED buffer (20
mmol of Tris/HCI, pH 7.4; 1 mmol of ethylenediamine tetraacetate, 2 mmol of
dithiothreitol) with 250 mmol of saccharose; stored at -30 C. Tracer: 3H-ORG
2058, 5
nmol; reference substance: progesterone.
Glucocorticoid Receptor:
Thymus cytosol of the adrenalectomized rat, thymi stored at -30 C; buffer:
TED. Tracer: 3H-Dexamethasone, 20 nmol; reference substance: dexamethasone.
The relative receptor binding affinities (RBA values) of the compounds of
general formula (I) according to the invention on the progesterone receptor
are between
3 and 100% relative to the progesterone. On the glucocorticoid receptor, the
RBA
values are in the range of 3 to 30% relative to dexamethasone.
The compounds according to the invention accordingly have a high affinity to
the
progesterone receptor but only a low affinity to the glucocorticoid receptor.
Antagonism of Progesterone Receptor PR-B
The transactivation assay is performed as described in WO 02/054064.
Agonism of Progesterone Receptor PR-B
The transactivation assay is performed as described in Fuhrmann et al.
(Fuhrmann,U.; Hess-Stump, H.; Cleve, A.; Neef, G.; Schwede, W.; Hoffmann, J.;
Fritzemeier, K.-H., Chwalisz, K.; Journal of Medicinal Chem., 43, 26, 2000,
5010-
5016).
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
54
Antagonistic activity Agonistic activity
No.
IC50 [nM] Efficacy [%] EC50 [nM] Efficacy [%]
9 3 96 n.b. 3
13 3 92 n.b. 7
3b 0,4 97 n.b. 2
7 4 82 2 11
Dosage
For use according to the invention, the progesterone receptor modulators can
be
administered orally, enterally, parenterally or transdermally.
In general, satisfactory results can be expected in the treatment of the above-
mentioned indications if the daily doses encompass a range of 1 g to 500 mg
of the
compound according to the invention.
Suitable dosages of the compounds according to the invention in humans for the
treatment of endometriosis, leiomyomas of the uterus and dysfunctional
bleeding as well
as for use in birth control as well as for hormone replacement therapy are 50
g to 500
mg per day, depending on age and constitution of the patient, whereby the
necessary
daily dose can be administered one or more times.
For treatment of breast cancer, the dosage range for the compounds according
to
the invention comprises 10 mg to 1000 mg daily.
The formulation of the pharmaceutical preparations based on the new compounds
is carried out in a way that is known in the art, by the active ingredient
being processed
with the vehicles, fillers, substances that influence decomposition, binding
agents,
moisturizers, lubricants, absorbents, diluents, flavoring correctives, dyes,
etc., that are
commonly used in galenicals and being converted into the desired form of
administration.
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
In this case, reference is made to Remington's Pharmaceutical Science, 15" Ed.
Mack
Publishing Company, East Pennsylvania (1980).
For oral administration, in particular tablets, film tablets, coated tablets,
capsules,
pills, powders, granulates, lozenges, suspensions, emulsions or solutions are
suitable.
For parenteral administration, injection and infusion preparations are
possible.
For intraarticulate injection, correspondingly prepared crystal suspensions
can be
used.
For intramuscular injection, aqueous and oily injection solutions or
suspensions and
corresponding depot preparations can be used.
For rectal administration, the new compounds can be used in the form of
suppositories, capsules, solutions (e.g., in the form of enemas) and ointments
both for
systemic therapy and for local therapy.
In addition, agents for vaginal application can also be mentioned as
preparations.
For pulmonary administration of the new compounds, the latter can be used in
the
form of aerosols and inhalants.
For transdermal administration, patches are possible, or for topical
application,
formulations in gels, ointments, fatty ointments, creams, pastes, powders,
milk and
tinctures are possible. The dosage of the compounds of general formula I
should be
0.0 1%- 20% in these preparations to achieve an adequate pharmacological
action.
Corresponding tablets can be obtained by, for example, mixing active
ingredient
with known adjuvants, for example inert diluents such as dextrose, sugar,
sorbitol,
mannitol, polyvinylpyrrolidone, explosives such as corn starch or alginic
acid, binders such
as starch or gelatin, lubricants such as magnesium stearate or talc and/or
agents for
achieiving a depot effect such as carboxylpolymethylene, carboxyl methyl
cellulose,
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
56
cellulose acetate phthalate or polyvinyl acetate. The tablets can also consist
of several
layers.
Accordingly, coated tablets can be produced by coating cores, produced
analogously to the tablets, with agents that are commonly used in tablet
coatings, for
example polyvinylpyrrolidone or shellac, gum arabic, talc, titanium oxide or
sugar. In this
case, the coated tablet shell can also consist of several layers, whereby the
adjuvants that
are mentioned above in the tablets can be used.
Solutions or suspensions of the compounds of general formula I according to
the
invention can contain additional taste-improving agents such as saccharine,
cyclamate or
sugar, as well as, e.g., flavoring substances, such as vanilla or orange
extract. In addition,
they can contain suspending adjuvants such as sodium carboxy methyl cellulose
or
preservatives such as p-hydroxybenzoates.
The capsules that contain compounds of general formula I can be produced by,
for
example, the compound(s) of general formula I being mixed with an inert
vehicle such as
lactose or sorbitol and encapsulated in gelatin capsules.
Suitable suppositories can be produced by, for example, mixing with vehicles
that
are provided for this purpose, such as neutral fats or polyethylene glycol, or
derivatives
thereof.
The compounds of general formula (I) according to the invention or their
pharmaceutically acceptable salts can be used based on their antagonistic or
partial
agonistic action for the production of a pharmaceutical agent, in particular
for treatment
and prophylaxis of gynecological diseases, such as endometriosis, leiomyomas
of the
uterus, dysfunctional bleeding and dysmenorrhea. In addition, they can be used
to
counteract hormonal irregularities, to trigger menstruation and alone or in
combination with
prostaglandins and/or oxytocin to induce birth.
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
57
In addition, the compounds of general formula (I) according to the invention
or
their pharmaceutically acceptable salts are suitable for the production of
preparations for
contraception for women (see also WO 93/23020, WO 93/21927).
In addition, the compounds according to the invention or their
pharmaceutically
acceptable salts can be used alone or in combination with a Selective Estrogen
Receptor
Modulator (SERM) for female hormone replacement therapy.
In addition, the above-mentioned compounds exert an antiproliferative action
in
hormone-dependent tumors. They are therefore suitable for the therapy of
hormone-
dependent carcinomas, such as, for example, for breast, prostate or
endometrial carcinomas.
The compounds according to the invention or their pharmaceutically acceptable
salts can be used for the treatment of hormone-dependent carcinomas, both in
first-line
therapy and in second-line therapy, in particular after tamoxifen failure.
The compounds of general formula (I) according to the invention that have an
antagonistic or partial agonistic action or their pharmaceutically acceptable
salts can also
be used in combination with compounds that have an antiestrogenic action
(estrogen
receptor antagonists or aromatase inhibitors) or Selective Estrogen Receptor
Modulators
(SERM) for the production of pharmaceutical preparations for treating hormone-
dependent tumors. For the treatment of endometriosis or leiomyomas of the
uterus, the
compounds according to the invention can also be used in combination with
SERMs or
an antiestrogen (estrogen receptor antagonists or aromatase inhibitors). In
the treatment
of hormone-dependent tumors, the progesterone receptor modulator and the
antiestrogen
(estrogen receptor antagonists or aromatase inhibitors) or the SERM can be
provided for
simultaneous or else for sequential administration. In sequential
administration,
preferably first the antiestrogen (estrogen receptor antagonists or aromatase
inhibitor) or
SERM is administered, and then the progesterone receptor modulator is
administered.
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
58
In this case, in the combination with the nonsteroidal progesterone receptor
modulators according to the invention, for example, the following
antiestrogens
(estrogen receptor antagonists or aromatase inhibitors) or SERMs are
considered:
tamoxifen, 5-(4-{5-[(RS)-(4,4,5,5,5-pentafluoropentyl)sulfinyl]-
pentyloxy}phenyl)-6-phenyl-8,9-dihydro-7H-benzocyclohepten-2-ol (WO 00/03979),
ICI 182 780 (7alpha-[9-(4,4,5,5-pentafluoropentylsulfinyl)nonyl]estra-
1,3,5(10)-triene-
3,17-beta-diol), 1lbeta-fluoro-7alpha-[5-(methyl{3-[(4,4,5,5,5-
pentafluoropentyl)sulfanyl]propyl } amino)pentyl] estra-1,3,5(10)-triene-
3,17beta-diol
(W098/07740), 1lbeta-fluoro-7alpha-{5-[methyl(7,7,8,8,9,9,10,10,10-
nonafluorodecyl)amino]pentyl}estra-1,3,5(10)-triene-3,17beta-diol (WO
99/33855),
11 beta-fluoro-17alpha-methyl-7alpha- {5 -[methyl(8,8,9,9,9-pentafluorononyl)-
amino]pentyl}estra-1,3,5(10)-triene-3,17beta-diol (WO 03/045972), clomifene,
raloxifene as well as other antiestrogenically active compounds, and aromatase
inhibitors, such as, for example, fadrozole, formestane, letrozole,
anastrozole or
atamestane.
Finally, this invention also relates to the use of the compounds of general
formula I, optionally together with an antiestrogen or SERM, for the
production of a
pharmaceutical agent.
This invention also relates to pharmaceutical compositions that contain at
least
one compound according to the invention, optionally in the form of a
pharmaceutically/pharmacologically compatible salt, without or together with
pharmaceutically compatible adjuvants and/or vehicles.
These pharmaceutical compositions and pharmaceutical agents can be provided
for oral, rectal, vaginal, subcutaneous, percutaneous, intravenous or
intramuscular
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
59
administration. In addition to commonly used vehicles and/or diluents, they
contain at
least one compound according to the invention.
The pharmaceutical agents of the invention are produced in a known way with
the commonly used solid or liquid vehicles or diluents and the usually used
pharmaceutical-technical adjuvants corresponding to the desired type of
administration
with a suitable dosage. The preferred preparations exist in a dispensing form
that is
suitable for oral administration. Such dispensing forms are, for example,
tablets, film
tablets, coated tablets, capsules, pills, powders, solutions or suspensions or
else depot
forms.
The pharmaceutical compositions that contain at least one of the compounds
according to the invention are preferably administered orally.
Parenteral preparations, such as injection solutions, are also considered.
In addition, for example, suppositories and agents for vaginal application can
also be mentioned as preparations.
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
The following examples are used for a more detailed explanation of the subject
of the invention, without intending that it be limited to these examples.
The production of the starting compound 5-{3-[1-(2-fluoro-5-
trifluoromethylphenyl)-cyclopropyl]-2-oxopropionylamino}phthalide is described
in
Patent WO 200375915, and the production of 5-{3-[1-phenyl-cyclopropyl]-2-
oxopropionylamino}phthalide is described in WO 9854159.
rac-5- {2-Ethinyl-2-hydroxy-3-[ 1-(2-fluoro-5-trifluoromethylphenyl)-
cyclopropyl]-
propionylamino}phthalide 1
F HO / N
0
0
I ~
0
CF,
Ethinyl magnesium bromide (6 ml, 0.5 M in tetrahydrofuran) was added to an
ice-cold solution that consists of 5-{3-[1-(2-fluoro-5-trifluoromethylphenyl)-
cyclopropyl]-2-oxopropionylamino}phthalide (632 mg) in THF (4 ml). The
reaction
solution under argon was allowed to come to room temperature within 3 hours.
Then,
the reaction mixture was poured into ice-cold, saturated ammonium chloride
solution. It
was extracted with ethyl acetate. The combined organic phases were washed with
saturated sodium chloride solution and dried on sodium sulfate. The crude
product that
is obtained was chromatographed on silica gel. 2.2 g of product was obtained.
'H-NMR (ppm, CDC13, 400 MHz): 0.83 (1 H), 0.92-1.10 (2H), 2.37 (1 H), 2.56
(IH), 2.59 (IH), 3.10 (1H), 5.28 (2H), 7.02 (1H), 7.31 (1H), 7.37 (1H), 7.58
(1H), 7.86
(1 H), 7.94 (1 H), 8.70 (1 H).
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
61
rac-5- {2-Hydroxy-3- [1-(2-fluoro-5-trifluoromethylphenyl)-cyclopropyl]-2-
propinyl-
propionylamino}phthalide 2
F HO // N
~ O
~/ Q
0
o
CF3
Analogously to Example 1, 145 mg of product was obtained from 1-
propinylmagnesium bromide (2 ml of 0.5 M solution in tetrahydrofuran) and 210
mg of
6- [4-(2-chloro-4-fluorophenyl)-4-methyl-2-oxovaleroylamino]-4-methyl-2,3 -
benzoxazin-l-one.
'H-NMR (ppm, CDC13, 400 MHz): 0.86 (1H), 0.90-1.05 (3H), 1.72 (3H), 2.35
(1H), 2.49 (1H), 2.96 (1H), 5.27 (2H), 7.03 (1H), 7.30 (1H), 7.36 (1H), 7.58
(1H), 7.85
(1H), 7.98 (1H), 8.73 (1H).
(+)-5-{2-Hydroxy-3-[1-(2-fluoro-5-trifluoromethylphenyl)-cyclopropyl]-2-phenyl-
propionylamino}phthalide 3a and
(-)-5-{2-Hydroxy-3-[1-(2-fluoro-5-trifluoromethylphenyl)-cyclopropyl]-2-
(phenylethinyl)-propionylamino}phthalide 3b
F HO N
~11 O
O
CF,
n-Butyllithium (625 l, 1.6 M in hexane) was added at -78 C to a solution of
110
l of phenylacetylene in tetrahydrofuran. Stirring was allowed to continue at
this
temperature for 30 minutes, and then a solution of 5-{3-[1-(2-fluoro-5-
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
62
trifluoromethylphenyl)-cyclopropyl]-2-oxopropionylamino}phthalide (210 mg) in
5 ml
of tetrahydrofuran was added in drops. Then, it was allowed to come to 23 C
over about
3 hours and then stirred for 10 more hours. Then, the reaction mixture was
poured into
ice-cold, saturated ammonium chloride solution. It was extracted with ethyl
acetate.
The combined organic phases were washed with saturated sodium chloride
solution and
dried on sodium sulfate. The crude product was chromatographed on silica gel.
The
racemic mixture obtained was then separated by preparative chiral HPLC
(Chiralpak AD
column, 250x10 mm) into enantiomers 3a (46 mg) and 3b (47 mg).
3a and 3b:
'H-NMR (ppm, CDC13, 300 MHz): 0.88 (1H), 0.95-1.11 (3H), 2.46 (1H), 2.65
(1 H), 3.10 (1 H), 5.27 (2H), 7.00 (1 H), 7.24-7.42 (7H), 7.61 (1 H), 7.84 (1
H), 7.98 (1 H),
8.80 (1 H).
3a :[a] ZO: + 12.9 (CHC13, 1.06 g/100 ml; X=589 nM)
3b :[a] 20: - 14.4 (CHC13, 1.03 g100 ml; a,=589 nM)
Analogously to Example 3, compounds 4 and 5 were produced from 5-{3-[1-(2-
fluoro-5-trifluoromethylphenyl)-cyclopropyl]-2-oxopropionylamino}phthalide and
the
respective lithium aryl acetylide.
rac-5-{2-Hydroxy-3-[1-(2-fluoro-5-trifluoromethylphenyl)-cyclopropyl]-2-[(4-
trifluoromethylphenyl)ethinyl]propionylamino}phthalide 4
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
63
CF,
F HO 0
Q
o
CF3
'H-NMR (ppm, CDC13, 300 MHz): 0.92 (1H), 0.99-1.16 (3H), 2.55 (1H), 2.68
(1 H), 3.27 (1 H), 5.30 (2H), 7.03 (1 H), 7.30-7.52 (4H), 7.55-7.62 (2H), 6.67
(1 H), 7.99
(1 H), 8.03 (1 H), 8.84 (1 H).
(+)-5-{2-Hydroxy-3-[1-(2-fluoro-5-trifluormethylphenyl)-cyclopropyl]-2-[(4-
trifluormethylphenyl)ethinyl]propionylamino}phthalide 4a and
(-)-5-{2-Hydroxy-3-[1-(2-fluoro-5-trifluormethylphenyl)-cyclopropyl]-2-[(4-
trifluormethylphenyl)ethinyl]propionylamino}phthalide 4b
The racemic mixture (150 mg) which was described in example 4 was separated by
preparative chiral HPLC (column Chiralpak AD 250x 10 mm) into the enantiomers
4a
(51 mg) and 4b (62 mg).
4a :[a] 20: + 6.3 (CHC13, 1.07 g/100 ml; X=589 nM)
4b :[a] 20: - 5.3 (CHC13, 1.09 g100 ml; X=589 nM)
rac-5-{2-Hydroxy-3-[1-(2-fluoro-5-trifluoromethylphenyl)-cyclopropyl]-2-[(4-
methylphenyl)ethinyl]propionylamino}phthalide 5
4I ~
F HO // N
I 0
0
CF3
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
64
'H-NMR (ppm, CDC13, 300 MHz): 0.87 (1H), 0.93-1.15 (3H), 2.38 (3H), 2.45
(1 H), 2.66 (111), 3.11 (1 H), 5.25 (2H), 6.99 (11-1), 7.10 (2H), 7.18-7.3
8(4H), 7.61 (1 H),
7.86 (1H), 8.00 (1H), 8.80 (1H).
(+)-5-{2-Hydroxy-3-[1-(2-fluoro-5-trifluoromethylphenyl)-cyclopropyl]-2-[(4-
methylphenyl)ethinyl]propionylamino}phthalide 5a and
(-)-5-{2-Hydroxy-3-[1-(2-fluoro-5-trifluoromethylphenyl)-cyclopropyl]-2-[(4-
methylphenyl)ethinyl] propionylamino} phthalide 5b
The racemic mixture (109 mg) which was described in example 5 was separated by
preparative chiral HPLC (colunm Chiralpak AD 250x10 mm) into the enantiomers
5a
(41 mg) and 5b (28 mg).
5a :[a] 20: + 14.8 (CHC13, 1.07 g/100 ml; k=589 nM)
5b :[a] 20: - 16.3 (CHC13, 1.13 g100 ml; a,=589 nM)
rac-5- {2- [(4-Acetoxyph enyl) ethinyl] -2-hyd roxy-3- [ 1-(2-flu o ro-5-
trifluoromethylphenyl)-cyclopropyl]-propionylamino}phthalide 6
0
0
F HO H
O
O
CF3
A suspension of the compound (104 mg) described under Example 1,
triphenylphosphine (12.2 mg), copper iodide (8.9 mg), 4-iodophenyl acetate (92
mg),
palladium acetate (5.3 mg) in THF (5 ml) and triethylamine (5 ml) was reacted
for I
hour in an ultrasound bath at 25 C under argon. Then, it was poured into
saturated,
aqueous ammonium chloride solution. It was extracted with ethyl acetate and
washed
with water and saturated sodium chloride solution. The combined organic phases
were
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
dried on sodium sulfate. After column chromatography of the crude product on
silica
gel, 55 mg of product was obtained.
'H-NMR (ppm, CDC13, 400 MHz): 0.88 (1H), 0.95-1.10 (3H), 2.29 (3H), 2.45
(1H), 2.63 (1H), 3.17 (1H), 5.29 (2H), 6.97-7.07 (3H), 7.28-7.37 (4H), 7.60
(1H), 7.84
(1H), 7.98 (1H), 8.80 (1H).
rac-5- {2-Hydroxy-2- [(4-hydroxyphenyl)ethinyl]-3- [1-(2-fluoro-5-
trifluoromethylphenyl)- cyclopropyl]-propionylamino}phthalide 7
OH
F HO H
0
o
CF3
A solution of the compound described under 6 (45 mg) in 5 ml of methanol was
mixed with sodium bicarbonate (130 mg). Stirring was continued for 2 more
hours at
23 C. Then, the reaction mixture was diluted with ethyl acetate. Then, it was
washed
twice with saturated sodium chloride solution. After drying on sodium sulfate,
the crude
product was purified on silica gel by column chromatography. 38 mg of product
was
obtained.
1 H-NMR (ppm, CDC13, 300 MHz): 0.87 (1H), 0.92-1.11 (3H), 2.43 (1H), 2.64
(1 H), 3.11 (1 H), 5.27 (2H), 5.67 (1 H), 6.73 (2H), 6.98 (1 H), 7.14 (2H),
7.28-7.3 8(2H),
7.60 (1H), 7.85 (1H), 7.97 (1H), 8.84 (1H).
rac-5-{2-[(4-Carboxyphenyl)ethinyl]-2-hydroxy-3-[1-(2-fluoro-5-
trifluoromethylphenyl)- cyclopropyl]-propionylamino}phthalide 8
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
66
CO,H
F HO N
O
0
o
CF3
Analogously to Example 6, compound 8 was produced from the compound
described under Example 1 and 4-iodobenzoic acid.
1H-NMR (ppm, CDC13/MeOD (5%), 400 MHz): 0.82 (1H), 0.89-1.05 (3H), 2.37
(1 H), 2.65 (1 H), 5.24 (2H), 6.97 (1 H), 7.3 5(1 H), 7.44 (2H), 7.50-7.65
(2H), 7.72 (1H),
7.80 (1 H), 7.92 (2H).
rac-5- {2-Hydroxy-3- [ 1-(2-fluoro-5-trifluoromethylphenyl)-cyclopropylJ-2-
(pentin-
1-yl)-propionylamino}phthalide 9
F HO // N
O
0
CF,
A solution that consists of 1-pentyne (0.94 ml) in THF (9 ml) was mixed at-78
C
with nBuLi (0.6 ml, 1.6 M in hexane). It was allowed to stir for 30 minutes at
-78 C,
and then a solution of 5-{3-[1-(2-fluoro-5-trifluoromethylphenyl)-cyclopropyl]-
2-
oxopropionylamino}phthalide (200 mg) in 3 ml of tetrahydrofuran was added.
Then, it
was allowed to come to 23 C over about 3 hours, and it was stined for 10 more
hours at
this temperature. Then, the reaction mixture was poured into ice-cold,
saturated
ammonium chloride solution. It was extracted with ethyl acetate. The combined
organic phases were washed with saturated sodium chloride solution and dried
on
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
67
sodium sulfate. The crude product was chromatographed on silica gel. 130 mg of
product was obtained.
1H-NMR (ppm, CDC13, 400 MHz): 0.82 (1H), 0.92-1.07 (6H), 1.45 (2H), 2.08
(2H), 2.30 (1H), 2.53 (1H), 2.83 (1H), 5.27 (2H), 7.02 (1H), 7.29 (1H), 7.36
(1H), 7.57
(1 H), 7.84 (1 H), 7.96 (1 H), 8.72 (1 H).
(+)-5-{2-Hydroxy-3-[1-(2-fluoro-5-trifluoromethylphenyl)-cyclopropyl]-2-
(pentin-l-
yl)-propionylamino}phthalide 9a and
(-)-5-{2-Hydroxy-3-[1-(2-fluoro-5-trifluoromethylphenyl)-cyclopropyl]-2-
(pentin-1-
yl)-propionylamino}phthalide 9b
The racemic mixture (120 mg) which was described in example 9 was separated by
preparative chiral HPLC (column Chiralpak AD 250x10 mm) into the enantiomers
9a
(46 mg) and 9b (47 mg).
9a :[a] 20: + 10.9 (CHC13, 1.01 g/100 ml; a,=589 nM)
9b :[a] ZO: - 10.6 (CHC13, 1.08 g100 ml; a,=589 nM)
rac-5-{2-Hydroxy-3-[1-(2-fluoro-5-trifluoromethylphenyl)-cyclopropyl]-2-(hexin-
l-
yl)-propionylamino}phthalide 10
F HC // H
~ O
0
0
CF,
Compound 10 was synthesized analogously to Example 9.
'H-NMR (ppm, CDC13, 400 MHz): 0.80-1.06 (7H), 1.30-1.50 (2H), 1.59 (2H),
2.10 (2H), 2.30 (1H), 2.52 (1H), 2.82 (1H), 5.28 (2H), 7.02 (1H), 7.30 (1H),
7.36 (1H),
7.57 (1H), 7.84 (1H), 7.95 (1H), 8.72 (1H).
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
68
rac-5- {2-Hydroxy-3-[1-(2-fluoro-5-trifluoromethylphenyl)-cyclopropyl]-2-[(4-
hydroxy)butin-1-yl]-propionylamino}phthalide 11
OH
F HO N
_~
0 O
0
CF3
Stage A: Reaction of 4-(tert-butyldimethylsilyloxo)but-1-yne (175 mg), nBuLi
(0.59 ml, 1.6 M in hexane) 5-{3-[1-(2-Fluoro-5-trifluoromethylphenyl)-
cyclopropyl]-2-
oxopropionylamino}-phthalide (200 mg) in tetrahydrofuran analogously to the
process
described under Example 9 yielded 165 mg of product.
Stage B: The product obtained under stage A (160 mg) was dissolved in 5 ml of
tetrahydrofuran. At 0 C, 270 l of a 1 molar solution of tetrabutylammonium
fluoride
in tetrahydrofuran was added and stirred for one hour at 0 C and for another 2
hours at
23 C. Then, the reaction mixture was poured into saturated, aqueous sodium
bicarbonate solution. It was extracted several times with ethyl acetate. The
combined
organic phases were washed with saturated sodium chloride solution and dried
on
sodium sulfate. After column chromatography on silica gel, 77 mg of product
was
obtained.
1H-NMR (ppm, CDC13, 400 MHz): 0.83 (1H), 0.90-1.03 (3H), 2.20-2.40 (3H),
2.50 (IH), 3.39 (1H), 3.68 (2H), 5.25 (2H), 7.01 (1H), 7.32 (2H), 7.57 (1H),
7.82 (1H),
7.93 (1 H), 8.91 (1 H).
Analogously to Example 11, compounds 12 and 13 were produced from 5-{3-[1-
(2-fluoro-5-trifluoromethylphenyl)-cyclopropyl]-2-oxopropionylamino }
phthalide:
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
69
rac-5- {2-Hydroxy-3- [ 1-(2-fluoro-5-trifluoromethylphenyl)-cyclopropyl]-2-[(5-
hydroxy)pentin-1-yl]-propionylamino}phthalide 12
OH
F HO
0
0
0
CF,
'H-NMR (ppm, CDC13, 400 MHz): 0.83 (1H), 0.90-1.03 (3H), 1.70 (2H), 2.24
(2H), 2.33 (1H), 2.50 (1H), 3.09 (1H), 3.71 (2H), 5.26 (2H), 7.02 (1H), 7.35
(2H), 7.57
(1H), 7.83 (1H), 7.97 (1H), 8.82 (1H).
rac-5- {2-Hydroxy-3-[1-(2-fluoro-5-trifluoromethylphenyl)-cyclopropyl]-2- [(3-
hydroxy)propin-1-yl]-propionylamino}phthalide 13
OH
F HO // N
0
0
CF,
'H-NMR (ppm, CDC13, 400 MHz): 0.84 (1 H), 0.90-1.03 (3H), 2.37 (1 H), 2.52
(1H), 3.25 (1H), 4.17 (2H), 5.27 (2H), 7.02 (1H), 7.30-7.40 (2H), 7.58 (1H),
7.83 (1H),
7.91 (1H), 8.77 (1 H).
(+)-5-{2-Hydroxy-3-[1-(2-fluoro-5-trifluoromethylphenyl)-cyclopropyl J-2-[(3-
hydroxy)propin-1-yl]-propionylamino}phthalide 13a and
(-)-5-{2-Hydroxy-3-[1-(2-fluoro-5-trifluoromethylphenyl)-cyclopropyl]-2-[(3-
hydroxy)propin-1-yl]-propionylamino}phthalide 13b
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
The racemic mixture (80 mg) which was described in example 13 was separated by
preparative chiral HPLC (column Chiralpak AD 250x10 mm) into the enantiomers
13a
(35 mg) and 13b (37 mg).
13a :[a] 20: + 28.3 (CHC13, 1.01 g/100 ml; X,=589 nM)
13b :[a] 20: - 29.3 (CHC13, 1.08 g100 ml; k=589 nM)
rac-5- {2-Hydroxy-3- [ 1-(2-fluoro-5-trifluoromethylphenyl)-cyclopropyl]-2-
[(4-
hydroxy-3-methyl)butin-1-yl]-propionylamino}phthalide 14
OH
F HO // N
0
0 Q
I ~ I
~
0
CF3
Stage A: Analogously to Example 11, 300 mg of 5-{3-[1-(2-fluoro-5-
trifluoromethylphenyl)-cyclopropyl]-2-oxopropionylamino}phthalide and 282 mg
of
tert-butyl-(1,1-dimethylprop-2-ynyl-oxy)-dimethylsilane are reacted. 15 mg of
product
A is obtained.
Stage B: 70 mg of the compound that is obtained under A was dissolved in 1 ml
of dichloromethane. 650 l of trifluoroacetic acid (20% in dichloromethane)
was added
at 0 C, and it was stirred for 3.5 hours at 0 C. Then, it was evaporated to
the dry state in
a vacuum, and the residue was purified by column chromatography on silica gel.
27 mg
of product was obtained.
'H-NMR (ppm, CDC13, 400 MHz): 0.82 (1 H), 0.90-1.00 (2H), 1.04 (1 H), 1.47
(6H), 2.28 (1H), 2.58 (1H), 3.08 (1H), 5.27 (2H), 7.03 (1H), 7.30 (1H), 7.36
(1H), 7.59
(1H), 7.83 (1H), 7.91 (1H), 8.78 (1H).
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
71
rac-5- {2-Hydroxy-3- [ 1-(2-fluoro-5-trifluoromethylphenyl)-cyclopropyl]-2-(2-
(tert-
butylcarboxy)ethin-1-yl)-propionylamino}phthalide 15
O y-
0
F HO H
0
0
o
CF,
Compound 15 was synthesized analogously to Example 9.
'H-NMR (ppm, CDC13, 400 MHz): 0.87 (1H), 0.93-1.05 (3H), 1.46 (9H), 2.42
(1H), 2.59 (1H), 3.39 (1H), 5.28 (2H), 7.03 (1H), 7.30-7.42 (2H), 7.57 (1H),
7.85 (1H),
7.92 (1H), 8.68 (1H).
rac-5- {2-Hydroxy-3- [ 1-(2-fluoro-5-trifluoromethylphenyl)-cyclopropyl]-2-(2-
carboxyethin-1-yl)-propionylamino}phthalide 16
0
OH
F HO N
~O
0 Q
0
il~ ~
CF,
50 mg of the compound that is described under Example 15 was dissolved in 5
ml of dichloromethane. 100 l of trifluoroacetic acid was added, and it was
stirred for
12 more hours at 23 C. Then, it was evaporated to the dry state in a vacuum,
and the
residue was purified by column chromatography on silica gel. 36 mg of product
was
obtained.
'H-NMR (ppm, DMSO-D6, 300 MHz): 0.48 (1H), 0.78 (1H), 0.86 (1H), 1.09
(1 H), 1.73 (1 H), 2.89 (1 H), 5.27 (2H), 6.62 (1 H), 7.13 (1 H), 7.31 (1 H),
7.41 (1 H), 7.53
(1H), 7.62 (1H), 7.68 (1H), 9.85 (1H).
CA 02611900 2007-12-11
WO 2006/136462 PCT/EP2006/006532
72
Analogously to Example 3, compound 17 was produced from 5-{3-[1-phenyl-
cyclopropyl] -2-oxopropionylamino } phthalide:
rac-5- {2-Hydroxy-3-11-phenyl-cyclopropyl]-2-(phenyl-ethinyl)propionylamino}-
phthalide 17
HO N
O
c/oo
'H-NMR (ppm, CDC13, 400 MHz): 0.78 (1H), 0.90 (1H), 1.10-1.21 (2H), 2.38
(1 H), 2.72 (1 H), 2.77 (1 H), 5.28 (2H), 7.18 (1H), 7.25-7.42 (6H), 7.41-7.52
(4H), 7.82
(1 H), 8.06 (1 H), 8.79 (1 H).